Summary
Background
Harnessing CD8+ T cell responses is being explored to achieve HIV remission. Although HIV-specific CD8+ T cells become dysfunctional without treatment, antiretroviral therapy (ART) partially restores their function. However, the extent of this recovery under long-term ART is less understood.
Methods
We analyzed the differentiation status and function of HIV-specific CD8+ T cells after long-term ART initiated in acute or chronic HIV infection ex vivo and upon in vitro recall.
Findings
ART initiation in any stage of acute HIV infection promoted the persistence of long-lived HIV-specific CD8+ T cells with high expansion (P<0·0008) and cytotoxic capacity (P=0·02) after in vitro recall, albeit at low cell number (P=0·003). This superior expansion capacity correlated with stemness (r=0·90, P=0·006), measured by TCF-1 expression, similar to functional HIV-specific CD8+ T cells found in spontaneous controllers. Importanly, TCF-1 expression in these cells was associated with longer time to viral rebound ranging from 13 to 48 days after ART interruption (r =0·71, P=0·03). In contrast, ART initiation in chronic HIV infection led to more differentiated HIV-specific CD8+ T cells lacking stemness properties and exhibiting residual dysfunction upon recall, with reduced proliferation and cytolytic activity.
Interpretation
ART initiation in acute HIV infection preserves functional HIV-specific CD8+ T cells, albeit at numbers too low to control viral rebound post-ART. HIV remission strategies may need to boost HIV-specific CD8+ T cell numbers and induce stem cell-like properties to reverse the residual dysfunction persisting on ART in people treated after acute infection prior to ART release.
Funding
U.S. National Institutes of Health and U.S. Department of Defense.
Keywords: HIV, Antiretroviral therapy, CD8 T cells, Cell differentiation, TCF-1
Research in context.
Evidence before this study
Finding a therapeutic intervention that could lead to HIV remission is still challenging. Optimizing HIV-specific CD8+ T cell responses has been considered as one strategy since these cells play a critical role in killing HIV-infected cells. Treatment initiation in hyperacute HIV infection preserved the functionality of HIV-specific CD8+ T cells shortly after ART initiation compared with no treatment but these cells did not have enhanced proliferative and cytolytic potential. ART initiated later in infection only partially restores the function of HIV-specific CD8+ T cells exhausted by chronic HIV infection, but the extent of this remaining dysfunction and ways to revert it are still unknown.
Added value of this study
We showed here that long-term treatment initiated in acute infection leads to functional HIV-specific CD8+ T cells with enhanced proliferative and cytolytic potential regardless of the stage of acute infection at which treatment is initiated. In contrast, even if long-term treatment sharply reduces HIV-specific CD8+ T cells dysfunction in people treated later, these cells exhibit a residual dysfunction and are more prone to become dysfunctional again upon recall. Albeit at lower numbers, functional HIV-specific CD8+ T cells in people treated in acute infection exhibited more stemness, reflected by TCF-1 expression, that correlated with longer time to viral rebound after treatment interruption.
Implications of all the available evidence
HIV remission in people living with HIV on ART will likely require a combination of strategies aimed at reversing the residual dysfunction of HIV-specific CD8+ T cell under ART prior to boosting them, and reprograming their stemness through targeting TCF-1 could be a potential therapeutic strategy.
Alt-text: Unlabelled box
Introduction
According to UNAIDS (www.unaids.org/en/resources/fact-sheet), over 28 million people living with HIV are currently taking antiretroviral therapy (ART), with the majority achieving control of viral replication to levels under the limit of detection. However, the virus rebounds once ART is interrupted, even when ART was initiated in acute HIV infection (AHI),1, 2, 3, 4, 5, 6 therefore people living with HIV require ART for life.7,8 However, long-term ART-free HIV remission is possible as evidenced by a few individuals, called post-treatment controllers, who are able to control viral replication after discontinuation of ART.9, 10, 11 Boosting HIV-specific CD8+ T cell responses has been explored as a strategy to induce HIV remission since these cells play a critical role in limiting viral replication early in AHI.12,13 In non-human primate models, CD8+ T cell responses are responsible for viral control since their depletion causes an increase in viremia in spontaneous controller animals,14 suppressed ART-treated animals,15,16 and post-treatment controller animals.17 CD8+ T cells are also responsible for viral control in people who are spontaneous controllers.18, 19, 20 However in most people living with HIV, in the absence of treatment, HIV-specific CD8+ T cells become dysfunctional during chronic HIV infection (CHI), concomitant with the upregulation of immune checkpoint molecules including PD-1, because of continuous HIV antigen burden.21, 22, 23, 24
Dysfunction of HIV-specific CD8+ T cells begins after peak viremia in AHI, and includes metabolic dysfunction and an inability to survive and differentiate into long-lived memory T cells.24 ART initiation in AHI before peak viremia prevents loss of cytokine productivity and memory potential of HIV-specific CD8+ T cells.13,25 Previous studies also suggested that early ART initiation preserves T cell function on ART26,27 and reduces persistent immune activation.28, 29, 30 Post-treatment controllers are more frequently found in people treated from AHI31,32 than people treated from CHI.33,34 These characteristics make individuals treated from AHI the ideal group in which to study the role of CD8+ T cells in post-treatment viral control.35 However, most people treated in AHI rebound without significant delay compared to people treated in CHI,2,3,5,6 bringing to question the quality of their immune response. There is very limited knowledge on the preservation of long-term HIV-specific CD8+ T cells and immune correlates that induce post-treatment control or delayed viral rebound in people treated from AHI.1,10,36 Furthermore, data showing whether initiation of ART in AHI improves the quality of the HIV-specific CD8+ T cell recall response, either in vitro or in vivo after ART interruption, are lacking.
We and others have analyzed HIV-specific CD8+ T cells in people treated from CHI before and after ART and shown that ART normalizes the expression of checkpoint proteins and the IL-7 receptor, suggesting that ART restored cell survival and functionality of HIV-specific CD8+ T cells.37, 38, 39, 40, 41, 42 Other studies showed that HIV-specific CD8+ T cells people treated from CHI were less functional than cells from spontaneous controllers, suggesting that functional defects persisted during ART.18, 19, 20,43 Overall, the extent to which HIV-specific CD8+ T cells regain function or have persistent dysfunction in people treated from CHI is not clear due to these conflicting data. HIV-specific CD8+ T cell functionality has not yet been characterized in people treated from AHI. Spontaneous controllers do not experience sustained levels of viremia and have protective genetic backgrounds such as CCR5Δ32 or protective HLA alleles that are different from most people living with HIV.44, 45, 46, 47 Therefore, HIV-specific CD8+ T cells in spontaneous controllers might not be the best comparator to determine the level of functionality of HIV-specific CD8+ T cells in people on ART. Comparing the functionality of HIV-specific CD8+ T cells in people who initiated ART during AHI or CHI allows to compare individual with similar genetic backgrounds but having experienced different HIV antigen burden prior to ART.
To better understand the functional restoration or residual dysfunction of memory HIV-specific CD8+ T cells after initiation of ART, we investigated these cells after long-term ART in people who initiated treatment in AHI and CHI. The RV254/SEARCH010 AHI cohort follows a unique population of people in Bangkok, Thailand, who are enrolled upon diagnosis in different AHI stages, initiate ART immediately after diagnosis, and are followed after treatment.13,48,49 The RV304/SEARCH013 cohort is a matched cohort of people living with HIV in Bangkok, Thailand, who initiate ART during CHI and are followed after treatment. Here, we analyzed the quantity and quality of memory HIV-specific CD8+ T cells in people treated from AHI and CHI to define whether early initiation of ART in AHI confers the capacity to mount an efficient recall response in persisting memory HIV-specific CD8+ T cells after long-term ART and define the extent of the remaining dysfunction of HIV-specific CD8+ T cells in people treated from CHI.
Methods
Study design
Forty-four people living with HIV from the RV254/ SEARCH010 study48,49 treated from AHI and 28 participants treated in CHI from the RV304/ SEARCH01350 and SEARCH01151 studies, all conducted in Bangkok, Thailand, were included in these analyses (Tables 1 and 2). For all of the experiments, lab investigators were blinded to the participant ID and HIV infection stage at ART initiation (AHI stages or CHI) of specimens during procedures so as not to bias measurements. The specimen information was recovered to perform statistical analyses. No outliers were excluded from the analyses. All participants were pre-screened for HLA-A1101/ Cw0102 restricted HIV-specific CD8+ T cells.
Table 1.
Clinical characteristic of study participants longitudinally analyzed before and during ART.
| Pre-ART HIV infection stage | AHI (n=14) | Chronic (n=13) | P value |
|---|---|---|---|
| Percentage of male | 100% | 100% | |
| Pre-ART Age (years), median (IQR) | 26 (23-31) | 28 (27·5-33) | 0·07 |
| Pre-ART viral load (copies/mL), median (IQR) | 859,219 (443,546-8,460,425) | 28,962 (9,426-92,155) | <0·0001 |
| Pre-ART CD4 count cells/mm3 in plasma, median (IQR) | 366 (290-425) | 402 (319-505) | 0·28 |
| Pre-ART CD8 count cells/mm3 in plasma, median (IQR) | 592 (315-1202) | 1230 (831-1922) | 0·01 |
| Duration of ART (years), median (IQR) | 1·6 (1·59-1·61) | 1·9 (1·2-2·0) | 0·02 |
| On ART Age (years), median (IQR) | 27 (25-33) | 30 (29-35) | 0·10 |
| On ART CD4 count cells/mm3 in plasma, median (IQR) | 601 (517-785) | 680 (519-784) | 0·79 |
| On ART CD8 count cells/mm3 in plasma, median (IQR) | 736 (499-963) | 988 (569-1154) | 0·05 |
P values from Mann-Whitney are shown.
Table 2.
Clinical characteristic of study participants analyzed on ART.
| AHI 4G stage (S) upon ART initiation | AHI (n=44) | Chronic (n=28) | P value |
|---|---|---|---|
| Percentage of male | 93% | 79% | |
| Duration of ART (years), median (IQR) | 2.0 (1.6-2.5) | 1.9 (1.7-2.8) | 0·86 |
| Age (years), median (IQR) | 27 (25-33) | 33 (29-39) | 0·0005 |
| CD4 count cells/mm3 in plasma, median (IQR) | 613 (563-814) | 560 (464-726) | 0·14 |
| CD8 count cells/mm3 in plasma, median (IQR) | 654 (479-778) | 845 (699-1088) | 0·002 |
To identify correlations between CD8+ T cell characteristics and viral rebound (first detectable HIV RNA > 20 copies/mL) after ATI, PBMCs from ten RV254/ SEARCH010 participants who underwent ATI were analyzed. Three participants were enrolled in the RV411 study (ClinicalTrials.gov NCT02614950) in which all participants initiated ART during Fiebig stage I in AHI and underwent ATI without further intervention.2 Five participants were enrolled in the RV409/SEARCH019 study (ClinicalTrials.gov NCT02475915) in which participants initiated ART in Fiebig stages III and IV and were treated with vorinostat, hydroxychloroquine, and maraviroc (VHM) for 10 weeks prior to ATI to reactivate latent virus while maintaining ART.6 The VHM treatment did not show statistically significant effects on virologic and immunologic measures after ATI compared to the placebo group, including the time to viral rebound. One participant was in the placebo group of the RV397 study (ClinicalTrials.gov NCT02664415), initiated ART from Fiebig stage III, and received intravenous injections of placebo normal saline every 3 weeks during ATI instead of the broadly neutralizing Ab VRC01 received by the treatment group.3
HIV-1 sequence analysis
HIV-1 sequences were generated from samples collected prior to ART initiation from AHI and CHI participants via Sanger sequencing using endpoint dilution52; the corresponding HIV-1 Gag, Pol, Env and Nef sequences were translated. Circulating HIV-1 CRF01_AE sequences were downloaded from the Los Alamos National Labs HIV database (https://www.hiv.lanl.gov/) to create alignments of independent Gag (n=1049), Pol (n=323), Env (n=849) and Nef (n=548) sequences. CD8+ T cell epitopes were predicted for each amino acid (AA) sequence using NetMHCPan 4.1.53 Epitopes are defined as strong binders if the predicted eluted ligand percentile ranks (Rank EL (%)) are below 0.5.
Flow cytometry analysis
Thawed or cultured peripheral blood mononuclear cells (PBMCs) were first stained for peptide-MHC class I complex tetramer,54 cell surface markers, and then fixed/permeabilized for intracellular/intranuclear staining. Detailed methods and related reagents are found in Supplementary Methods.
In vitro memory CD8+ T cell recall assay
Thawed PBMCs from HIV-infected individuals were labeled with CellTrace Violet (Thermo Fisher Scientific) at 1μM according to the manufacturer's protocol. The cells were then plated at 5 × 106 cells/well in 24 well plate in CellGenix GMP DC Medium (CellGenix) containing recombinant human (rh) IL-7 (1ng/ml, PeproTech) to support minimal T cell survival and rh FLT3L (50ng/ml, PeproTech) to mobilize primary DCs. After 24 hours (day 1), cognate CD8 epitope peptides from HIV or EBV were added to the culture at 1μg/ml. On day 2, human serum (Access Biologicals) and rh IL-2 (Miltenyi Biotec) were added at final concentrations of 8% (volume/volume) and 20U/ml, respectively. Half of the medium was replaced with RPMI-1640 containing 8% human serum, rhIL-2 (20U/ml), and penicillin-streptomycin (100 U/ml, Quality Biological) on days 5, 7 and 9. HIV and EBV-specific CD8+ T cells in the culture were analyzed by flow cytometry on days 6 and 12.
Assay for cytotoxic activity
A cytotoxicity assay based on CFSE labeled target cells was used as previously described,55,56 with some modifications. Autologous PBMCs (1 × 106 cells) were expanded with phytohemagglutinin-L (1μg/ml, Sigma-Aldrich) in RPMI-1640 medium supplemented with 8% human serum, rhIL-2 (10 ng/ml), natural human IL-2/TCGF (5 B.M. U/ml, ZeptoMetrix), antiretrovirals (100 nM efavirenz, 180 nM zidovudine, and 200 nM raltegravir), and penicillin-streptomycin (100 U/) for 12 days. CD4+ T cells were isolated from the expanded PBMCs using magnetic negative selection (STEMCELL Technologies) and used as target cells. Autologous CD4+ T cells were labeled with two different concentrations of CFSE, 20 nM for CFSElow or 200 nM for CFSEhigh. CFSElow labeled target cells were pulsed with cognate epitope peptides (1µg/mL) for 45 min and mixed with CFSEhigh cells (no peptide pulse) at a ratio of 1:1. Twenty thousand each of CFSEhigh and CFSElow target cells were co-cultured for 4 hours with cells from day 13 of the in vitro memory CD8+ T cell recall assay at increasing E/T ratios (1, 2, 4, and 8) based on the frequency of HIV- or EBV-specific CD8+ T cells in the culture at day 12. The cells were analyzed by flow cytometry for the number of remaining live target cells and tetramer stained HIV or EBV-specific CD8+ T cells. The percentage of specific cell lysis was calculated as the proportion of live, peptide-pulsed cells (CFSElow) to live, non-peptide pulsed cells (CFSEhigh), and the accurate E/T ratio was determined by ratio of tetramer+ cell number to number of non-peptide pulsed target cells. The final percentage of specific cell lysis at E/T ratio 2 was determined by linear regression of the percentage specific cell lysis and observed E/T ratio calculated from the flow cytometry.
Ethics
All study participants provided written informed consent approved by the Chulalongkorn University and Walter Reed Army Institute of Research institutional review boards. The investigators have adhered to the policies for protection of human participants as prescribed in AR-70-25.
Statistical analysis
Statistical analyses were performed using the nonparametric Kruskal-Wallis test (more than 2 groups) or Mann-Whitney test (2 groups) for group comparisons, and the Wilcoxon matched-pairs signed-rank test for the longitudinal samples before and after ART initiation. The nonparametric Spearman test was used for all of the correlation analyses, and the Benjamini-Hochberg producedure was used to correct for multiple comparisons (FDR < 0.1). Original data unit from available data was used for all statistical analysis and no logarithm transformation was performed. Above statistical analysis was performed with GraphPad Prism 9. SPICE was used to perform a chi-square permutation test comparing the distribution of the CD8+ T cell subsets among the groups.57 P < 0.05 was considered significant.
Role of funders
The funding sources had no role in writing the manuscript nor in data collection, analysis and interpretation or any other aspect of the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Lower numbers of HIV-specific CD8+ T cells are sustained when ART is initiated in acute infection than in chronic infection
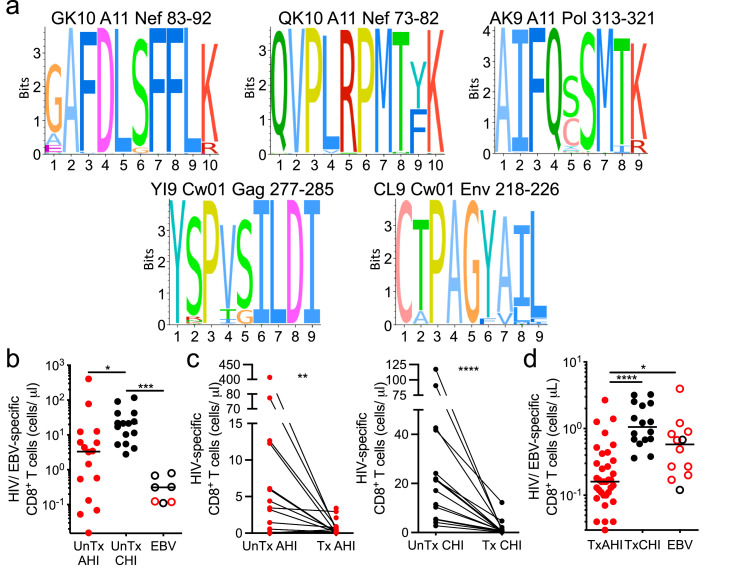
CD8+ T cells specific for five different HIV epitopes restricted by HLA-A*1101 and Cw*0102, dominant alleles in the Thai population, were analyzed in this study, and one EBV epitope restricted by HLA-A*1101 was analyzed as an internal comparator (Table S1).13,58,59 These five HIV-1 CD8+ T cell epitopes corresponded to highly conserved peptides in the HIV-1 proteome (Figure 1a). Viral sequences were available from a subset of participants (21 AHI, 8 CHI) and used to predict CD8+ T cell epitopes for each participant's sequences. Most predicted epitopes were identical to the epitopes tested, and a subset (7/30) showed one mutation with the tested epitope (Tables S2 and S3). Importantly, predicted epitopes with mutations had similar binding affinities as the tested epitopes (Table S4). Only one epitope in Nef (GK10 with the G83E mutation) was predicted to have weaker binding affinity but this variant epitope was only found in one of ten sequences in one participant (Tables S2 and S4). In previous work, the A11 QK10 variant and the original epitope were similarly recognized by HIV-specific CD8+ T cells within the same people carrying HLA-A11.60 The A11 AK9 variant was not found in our participants. These data indicate that the impact of virus variants on the HIV-specific CD8+ T cell response we analyzed was minimal.
Figure 1.
HIV-specific CD8+ T cell numbers before ART initiation in AHI and CHI and under long-term ART. (a) Sequence logo for the 5 HIV CD8+ T cell epitopes tested with tetramers: GK10, QK10, AK9, YI9, CL9. The sequence conservation for each epitope was measured using reference datasets of circulating CRF01_AE sequences. The relative size of each AA letter at a site represents the frequency of the residue across circulating viruses. The total height indicates the information content of the position. (b) Number of HIV- and EBV-specific tetramer+ CD8+ T cells per mL of peripheral blood in individuals prior to ART initiation in AHI (UnTx AHI, n=19) or CHI (UnTx CHI, n=15). (c) Number of HIV-specific CD8+ T cells before (UnTx) and during (Tx) long-term ART measured longitudinally in individuals who initiated treatment in AHI or CHI and were on ART for more than 1 year. (d) Number of HIV- and EBV-specific tetramer+ CD8+ T cells in individuals who initiated treatment in AHI (n=41) or in CHI (n=27) and were on ART for more than 1 year. Differences between UnTx AHI group, UnTx CHI group, and EBV-specific CD8+ T cells were analyzed by a Kruskal-Wallis test. Differences between samples collected before and after ART were analyzed by a Wilcoxon test. *P< 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001.
To understand the effect that reducing viral burden with ART has on HIV-specific CD8+ T cells, we analyzed longitudinally samples from before and after at least 1 year of treatment initiated in AHI in the RV254/ SEARCH010 cohort and in CHI in the RV304/ SEARCH013 cohort (Table 1). Before ART initiation, HIV-specific CD8+ T cells were at higher numbers in people in CHI (UnTx CHI) than in people in AHI (P = 0.02; UnTx AHI), and at higher numbers than EBV-specific CD8+ T cells (P = 0.0004; Figure 1b). ART initiated in both AHI (Tx AHI) and CHI (Tx CHI) resulted in a drastic reduction of cell numbers (AHI P = 0.003; CHI P< 0.0001 compared to UnTx) (Figure 1c). Participants treated from AHI had significantly lower numbers of HIV-specific CD8+ T cells in blood after ART than people who initiated ART during CHI (Table 2, P< 0.0001; Figure 1d). People who initiated treatment in the earliest detectable stage of AHI, Stage 1, exhibited the lowest numbers of HIV-specific CD8+ T cells, even lower than EBV-specific CD8+ T cells from the same individuals (P< 0.0001 against CHI, P = 0.004 against EBV; Figure S1).
HIV-specific CD8+ T cells exhibit phenotypes of effector cells in AHI and exhausted cells in CHI before ART initiation
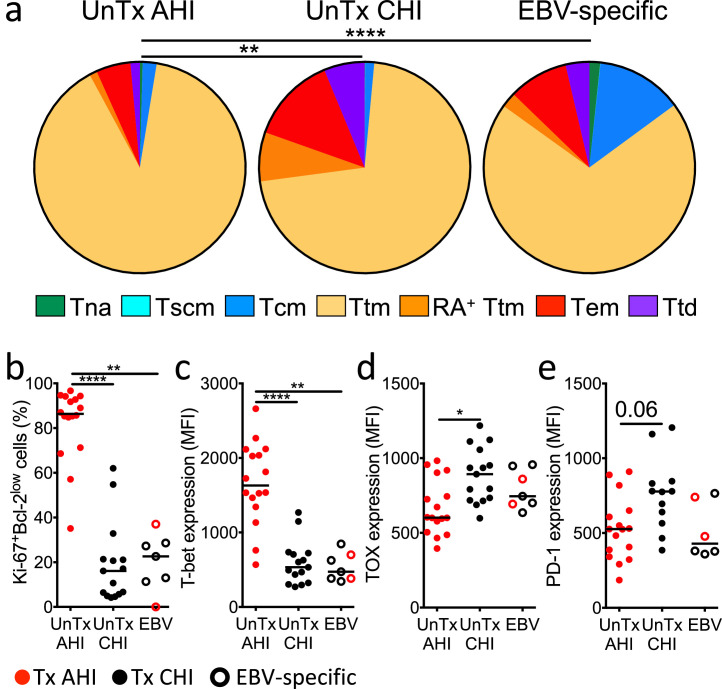
Before ART initiation, HIV-specific CD8+ T cells in both AHI and CHI were mostly found in short-lived transitional memory (Ttm) and effector memory (Tem) T cell subsets (Figure 2a, S2, and S3A), as previously reported.24,25,39,41 However, in AHI, the majority of them were Ki-67+Bcl-2low activated effector cells as we and others reported,12,13 and expressed the transcriptional factor T-bet, important for effector CD8+ T cell differentiation,61 at higher level than their counterparts in CHI and EBV-specific CD8+ T cells (Figure 2b and c). This was consistent with a trend toward higher expression of the cytolytic molecule Perforin (Figure S3b). We also confirmed that HIV-specific CD8+ T cells during CHI had an exhausted phenotype with higher expression of the transcription factor associated with T cell exhaustion, TOX, and a strong trend toward higher expression of the activation/check point protein PD-1 compared to those in AHI (Figure 2d and e). These data indicate that HIV-specific CD8+ T cells in AHI are effector T cells and those in CHI are exhausted cells.
Figure 2.
Differentiation status and phenotype of HIV-specific CD8+ T cells in people during acute and chronic HIV infection before ART initiation. (a) Frequencies of naïve-like (Tna), stem cell memory (Tscm), central memory (Tcm), transitional memory (Ttm), CD45RA+ transitional memory (RA+ Ttm), effector memory (Tem), and terminally differentiated cells (Ttd) in HIV/EBV-specific CD8+ T cells prior to ART initiation in CHI and AHI. Chi-square permutation test was used to compare the overall frequency distribution of the CD8+ T cell subsets among the groups. (b-e) Percentage of Ki-67+Bcl-2low cells (b) and expression of T-bet (c), TOX (d), and PD-1 (e) on HIV/EBV-specific CD8+ T cells. Differences between groups were analyzed by Kruskal-Wallis test. *P< 0.05; **P< 0.01; ****P< 0.0001.
Initiation of ART in AHI promotes the differentiation of HIV-specific CD8+ T cells with self-renewal and long-lived memory phenotypes
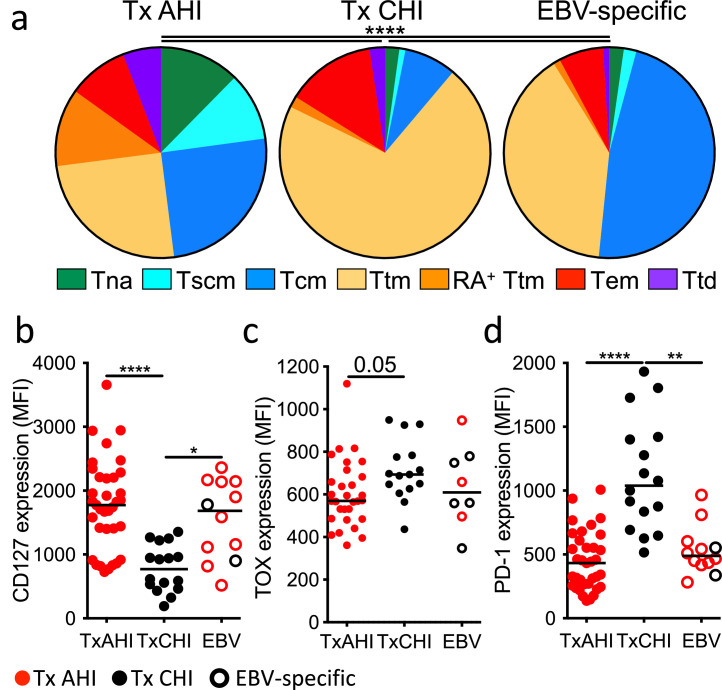
As numbers of HIV-specific CD8+ T cells drastically declined after ART initiation in both AHI and CHI (Figure 1c), we assessed their differentiation phenotype under long-term ART. The distribution of HIV-specific CD8+ T cell subsets was significantly different between AHI-treated and CHI-treated participants as well as with EBV-specific cells from the same individuals (P< 0.0001 for all combinations; Figure 3a). People treated from AHI had significantly higher percentages of T cell subsets endowed with potent self-renewal and long-lived capacities, such as stem cell memory (Tscm) and central memory (Tcm) cells, compared to people treated from CHI (P = 0.0002 and P = 0.004, respectively; Figure S4a). Participants treated from AHI also had higher frequencies of HIV-specific CD8+ T cells with naïve-like phenotype (Tna) (P = 0.03), which were similarly found in vaccine-induced yellow fever- and influenza-specific memory CD8+ T cells.62,63 In contrast, HIV-specific CD8+ T cells from individuals treated during CHI were still predominantly found in short-lived Ttm and Tem subsets as they were pre-ART (Figure 2a and Figure S3a). EBV-specific CD8+ T cells were predominantly composed of Tcm and Ttm subsets. Consistent with these T cell phenotypes, HIV-specific CD8+ T cells from participants treated from AHI showed significantly higher expression of CD127 than those from participants treated from CHI (P< 0.0001) but comparable to those of EBV-specific CD8+ T cells (Figure 3b), with a trend toward the highest expression of CD127 occurring in the group treated from AHI Stage 1 (Figure S4b). Consistent with their long-lived phenotype, HIV-specific memory CD8+ T cells in participants treated from AHI showed persisting T cell receptor (TCR) clonotypes within HIV Nef-specific CD8+ T cells between 2 visits during ART, as shown by the high Morisita-Horn index for both alpha and beta chains of the TCR (Table S5) and maintenance of dominant and subdominant clonotypes (Figure S4c) as seen in people treated in CHI.23,39,64,65 In addition, the frequency of HIV-specific CD8+ T cells was sustained at low but stable levels over 5 years of ART in this cohort.2 On the other hand, memory HIV-specific CD8+ T cells in people treated from CHI showed a trend toward higher expression of TOX and significantly higher PD-1 expression than cells in people treated from AHI (P > 0.0001 and P = 0.05, respectively; Figure 3c and d). These data indicate that early initiation of ART in AHI promotes differentiation of memory T cells with self-renewal and long-lived phenotypes and that the cells are stably sustained throughout treatment. Of note, ART initiation in any stage of AHI, including post-peak, led to the generation of long-lived HIV-specific memory cells.
Figure 3.
Differentiation status and phenotype of HIV-specific memory CD8+ T cells in people treated from acute and chronic HIV infection. (a) Average frequencies of naive-like (Tna), stem cell memory (Tscm), central memory (Tcm), transitional memory (Ttm), CD45RA+ transitional memory (RA+ Ttm), effector memory (Tem), and terminally differentiated cells (Ttd) in HIV/EBV-specific CD8+ T cells from individuals who initiated treatment in AHI (n=33) or in CHI (n=13) and were on ART for more than 1 year. Chi-square permutation test was used to compare the overall frequency distribution of the CD8+ T cell subsets among the groups. (b, c, and d) Expression of CD127 (b), TOX (c), and PD-1 (d) on/in HIV/EBV-specific CD8+ T cells. Differences between groups were analyzed by a Kruskal-Wallis test. *P< 0.05; **P< 0.01; ****P< 0.0001.
HIV-specific CD8+ T cells from people who initiated ART in AHI exhibit higher proliferative capacity than those treated from CHI
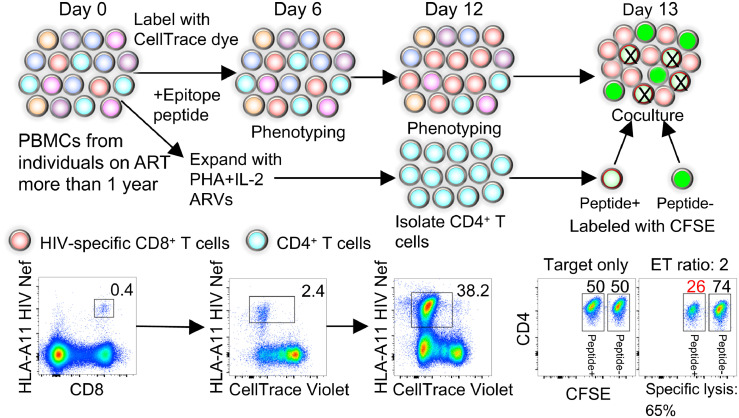
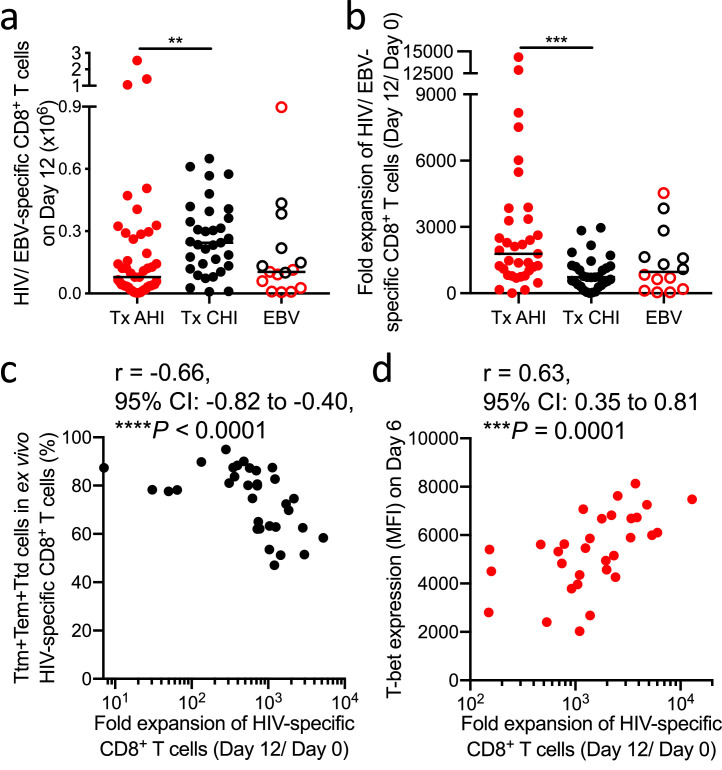
To further assess the quality of memory HIV-specific CD8+ T cells during long-term ART, we analyzed the quality of their response upon recall. For that, we designed an in vitro memory T cell recall assay in which we stimulate HIV-specific CD8+ T cells with a cognate HIV epitope peptide for 12 days, measuring their proliferation capacity at 6 and 12 days (Figure 4). The number of HIV-specific CD8+ T cells was significantly higher after recall when cells came from people treated in CHI compared to those treated from AHI (P = 0.003; Figure 5a), as the ex vivo number of memory HIV-specific CD8+ T cells was more than 7 times higher when ART was initiated in CHI (Figure 1d). However, the magnitude of cell expansion 12 days after stimulation was significantly higher when ART was initiated in AHI than in CHI (P = 0.0008; Figure 5b). The magnitude of expansion in both groups positively correlated with the cell number on day 12 (r = 0.46, P = 0.006 and r = 0.73, P< 0.0001, respectively; Figure S5a and b). The blunted expansion capacity of cells from people treated in CHI was associated with a higher combined frequency of short-lived T cell subsets in ex vivo HIV-specific CD8+ T cells (Ttm, Tem, and Ttd; r = -0.66, P< 0.0001; Figure 5c), as well as of the Ttm subset alone (r = -0.49, P = 0.004; Figure S5c), suggesting that skewed differentiation toward short-lived cells prevents cell expansion. Expression of T-bet positively correlated with cell expansion only when treatment was initiated in AHI (r = 0.63, P = 0.0001, Figure 5d). These data suggest that memory HIV-specific CD8+ T cells in people treated from AHI, though lower in number, have a higher proliferative capacity.
Figure 4.
Schematic of the proliferation and cytotoxicity assay of recalled memory HIV-specific CD8+ T cells. CellTrace dye labeled PBMCs were stimulated with cognate HIV epitope peptides. Tetramer+ HIV-specific CD8+ T cell phenotype and cell number were analyzed by flow cytometry after 6 and 12 days of stimulation. Autologous PBMCs were concurrently expanded with PHA and rhIL-2 for 12 days in the presence of ARVs. CD4+ T cells were purified from the PHA expanded PBMCs by magnetic negative selection. Autologous CD4+ T cells were labeled with low levels of CSFE and pulsed with HIV peptide or labeled with high levels of CFSE and not loaded with peptide. Cytotoxic capacity of CD8+ T cells was measured by co-culturing the peptide stimulated CD8+ T cells at day 13 post-stimulation at an E/T ratio of 2 with the peptide-pulsed and non-pulsed CFSE labeled autologous CD4+ T cells mixed 50/50 as targets.
Figure 5.
Magnitude of recalled memory CD8+ T cells from people treated in AHI and CHI. (a) Number of HIV- or EBV-specific CD8+ T cells in culture 12 days after peptide stimulation. Thirty-three individuals treated from AHI and 27 individuals treated from CHI were analyzed. (b) Fold expansion of HIV- or EBV-specific CD8+ T cells from day 0 (ex vivo) to day 12 after peptide stimulation. (c) Correlation between the fold expansion of HIV-specific CD8+ T cells and combined ex vivo frequency of Ttm, Tem, and Ttd in HIV-specific CD8+ T cells of people treated from CHI. (d) Correlation between the fold expansion of HIV-specific CD8+ T cells and expression of T-bet in day 6 recalled HIV-specific CD8+ T cells of people treated from AHI. Differences among groups were analyzed by Kruskal-Wallis test (**P< 0.01; ***P< 0.001; ****P< 0.0001). Correlations were analyzed by Spearman correlation with the Benjamini-Hochberg procedure for multiple comparisons (FDR < 0.1).
HIV-specific CD8+ T cells from people treated from AHI differentiate into functional effector CD8+ T cells with higher cytolytic capacity than those treated from CHI
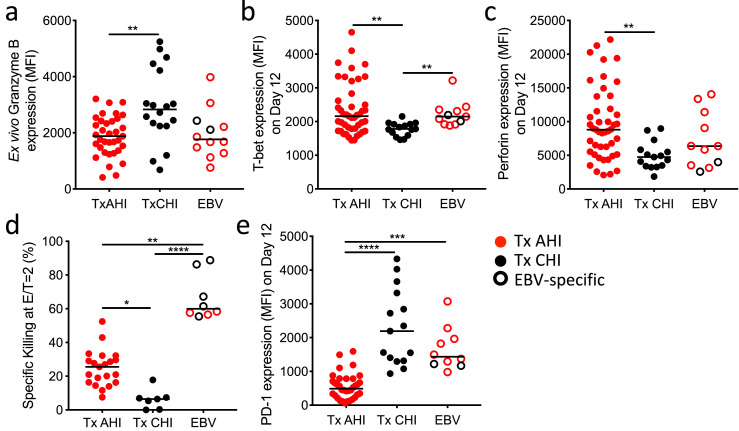
Before in vitro recall, HIV-specific CD8+ T cells from individual treated from CHI showed significantly higher expression of the cytolytic molecule Granzyme B than cells in people treated from AHI (P= 0.001, Figure 6a). To assess the cytolytic capacity after recall, we measured their specific killing on day 13 of the in vitro memory recall assay (Figure 4). We found a significantly higher expression of T-bet and the cytolytic molecule perforin at day 12 in recalled HIV-specific CD8+ T cells from participants treated from AHI compared to those treated from CHI (P= 0.005 and P= 0.002; Figure 6b and c, respectively). Consistent with this, cytolytic activity of the recalled HIV Nef GK10-specific CD8+ T cells was significantly higher in people treated from AHI than CHI (P= 0.02 at E/T2 and P= 0.04 at E/T1; Figure 6d and Figure S6a, respectively). A similar trend toward superior cytolytic capacity was confirmed for recalled HIV-specific CD8+ T cells specific for 4 other HIV epitopes (P= 0.11; Figure S6b). The blunted cytolytic capacity of HIV-specific CD8+ T cells from people treated in CHI was not due to a defect in antigen presentation or resistance of target cells to CD8+ T cell killing since EBV-specific CD8+ T cells from the same individuals showed superior cytolytic capacity than HIV-specific CD8+ T cells against the same autologous target cells loaded with peptides. We hypothesized that this dampened cytolytic activity in CD8+ T cells from people treated during CHI might be due to cell exhaustion, as PD-1 was expressed at the highest levels on ex vivo HIV-specific CD8+ T cells in people treated from CHI (Figure 3d). The elevated PD-1 expression was indeed maintained on these cells even after in vitro recall (Figure 6e, P< 0.0001 against Tx AHI. These data suggest that recalled HIV-specific CD8+ T cells in people treated from CHI exhibit residual dysfunction even after long-term ART whereas memory HIV-specific CD8+ T cells in people treated from AHI, though lower in number, have better capacity to differentiate into functional effector CD8+ T cells that can eliminate HIV-infected cells upon secondary antigen stimulation.
Figure 6.
Effector function of recalled memory CD8+ T cells from people treated in AHI and CHI. (a) Expression of Granzyme B in ex vivo HIV/EBV-specific CD8+ T cells from people treated from AHI and CHI. (b, c, and f) Expression of T-bet (b), perforin (c), and PD-1 (f) in/on HIV/EBV-specific CD8+ T cells 12 days after stimulation. (d) Cytotoxic capacity of day 13 recalled HIV Nef GK10- or EBV- specific CD8+ T cells cultured with autologous CD4+ T cells at an effector/ target ratio of 2. (e) Expression level of PD-1 on day 12 recalled HIV/EBV-specific CD8+ T cells from individuals treated from AHI and CHI. Differences among groups were analyzed by Kruskal-Wallis test (*P< 0.05; **P< 0.01; ***P< 0.001; ****P< 0.0001). Correlations were analyzed by Spearman correlation with the Benjamini-Hochberg procedure for multiple comparisons (FDR < 0.1).
TCF-1 expression associates with recall response and viral control after treatment interruption
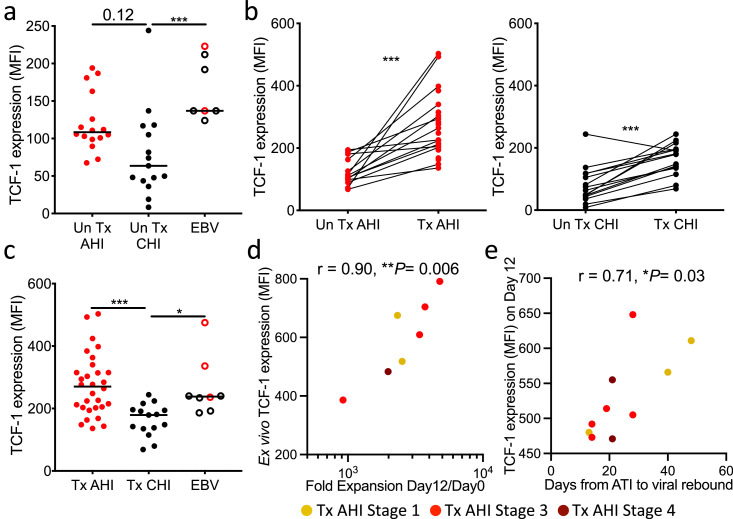
Since HIV-specific CD8+ T cells from people treated from AHI have characteristics of long-lived memory T cells (Figure 3), higher expansion potency (Figure 5b), and cytolytic capacity after recall (Figure 6d), we hypothesized that TCF-1, a transcription factor important for self-renewal capacity,66, 67, 68 maintains their functionality under long-term ART and supports a sustained effective immune response required to control rebounding viral replication after ART stop. Although TCF-1 is thought to be downregulated in effector cells, HIV-specific CD8+ T cells from prior to ART intiation in AHI showed TCF-1 expression comparable to that of EBV-specific CD8+ T cells and a trend toward higher TCF-1 expression than the cells in CHI (Figure 7a, P= 0.12). Initiation of ART recovered the expression of TCF-1 in both AHI and CHI (Figure 7b), but HIV-specific CD8+ T cells from people treated from AHI expressed significantly higher TCF-1 than those from participants treated from CHI (P= 0.0003, Figure 7c). Some RV254 participants treated from AHI underwent analytic treatment interruption (ATI) as part of 3 clinical trials although all participants experienced viral rebound ranging from 14 to 48 days (Figure S7a). In the participants who had detectable ex vivo memory HIV-specific CD8+ T cells, TCF-1 expression levels in these cells positively correlated with in vitro cell expansion in our memory recall assay (r = 0.90, P = 0.006; Figure 7d). There was no correlation between HIV reservoir size prior to the ATI and time to viral rebound (Figure S7b). However, we observed that TCF-1 expression 12 days after in vitro recall positively correlated with longer time to viral rebound during ATI (r = 0.71, P = 0.03; Figure 7e). These data suggest that early initiation of ART prevents the loss of TCF-1 expression and enables the maintenance of long-lived HIV-specific CD8+ T cells with effective recall response that can contribute to limiting viral rebound during ATI. These data further suggest that higher numbers of functional memory HIV-specific CD8+ T cells will be needed to impact durable viral control.
Figure 7.
Initiation of ART in AHI prevents loss of TCF-1 expression and the expression supports expansion of functional memory HIV-specific CD8+ T cells and associates with time to viral rebound after ATI. (a) Expression of TCF-1 in HIV/EBV-specific CD8+ T cells from people before ART initiation in AHI or CHI. (b) Expression of TCF-1 in HIV-specific CD8+ T cells before and during long-term ART initiated in AHI or CHI. (c) Expression of TCF-1 in HIV/EBV-specific CD8+ T cells from people treated from AHI or CHI. (d) Correlation between the fold expansion of HIV-specific CD8+ T cells and ex vivo expression of TCF-1 in HIV-specific CD8+ T cells from people who initiated treatment in AHI and subsequently underwent ATI. (e) Correlation between days to viral load rebound (VL>20 copies/mL) after ATI and expression of TCF-1 in HIV-specific CD8+ T cells 12 days after in vitro recall. Differences between samples collected before and after ART were analyzed by a Wilcoxon test. Differences between groups were analyzed by a Kruskal-Wallis test. Correlations were analyzed by Spearman correlation with the Benjamini-Hochberg procedure for multiple comparisons (FDR < 0.1). *P< 0.05; ***P< 0.001.
Discussion
In this study, we analyzed how initiation of ART in AHI or in CHI affects the quantity and quality of memory HIV-specific CD8+ T cells and their recall response after long-term viral suppression. We compared memory HIV-specific CD8+ T cells after ART in both groups in contrast to previous reports that studied people with viremia or spontaneous controllers off ART as comparators. In people who initiated ART during CHI, HIV-specific CD8+ T cells mainly displayed a Ttm phenotype before ART initiation, and even though ART resulted in decreased expression of PD-1 and increased expression of survival markers on these cells, as previously reported,37, 38, 39, 40, 41, 42 it did not induce their differentiation into long-lasting memory subsets, such as Tscm. This Tscm subset, endowed with stem cell-like capacity, is important as it was shown to be associated with improved prognosis of HIV infection.69 In contrast to people treated in CHI, initiation of ART in AHI induced higher frequencies of Tna, Tscm, and Tcm within HIV-specific CD8+ T cells, similar to the distribution observed with successful preventive vaccines such as the yellow fever vaccine.62,63 This finding is in agreement with lower expression of PD-1 and higher expression of CD127 on memory HIV-specific CD8+ T cells when ART is initiated in AHI. A previous study reported that early treatment in AHI Fiebig Stages I and II promoted the differentiation of HIV-specific CD8+ T cells into a Tem subset.25 Although these data are different from our observations, they can be explained by the fact that this previous study analyzed HIV-specific CD8+ T cell responses shortly after ART initiation whereas ours analyzed them after long-term ART. Indeed, in acute infection or successful live-vaccine immunization, only a minor portion of effector CD8+ T cells survive through the contraction phase after pathogen clearance and differentiate into long-lived memory T cells.63,70
We also showed that memory HIV-specific CD8+ T cells under long-term ART initiated in AHI not only exhibited a long-lived phenotype but also greater cell expansion and cytotoxic effector function after recall than those from people treated during CHI. These data contrast with data generated shortly after ART initiation in AHI, where it was found that HIV-specific CD8+ T cells did not have restored proliferation potency and cytolytic activity compared to those in untreated CHI.25 These contrasting results can also be explained by differences in the timing of analysis after ART initiation. Importantly, the gain of function seen after long-term ART was similar between individuals treated at the different AHI stages from Stage 1 to 5, suggesting that these cells are able to generate functional memory cells during ART independent of their differentiation state during AHI. These data indicate that intervention studies focused on boosting CD8+ T cell responses in early treated individuals could enroll people who initiated ART in all stages of AHI rather than only selecting people who initiated ART before peak viremia in AHI.
We demonstrated here that although PD-1 expression levels were significantly lower after ART initiation, they remained higher on memory HIV-specific CD8+ T cells in people treated from CHI than those treated from AHI. Interestingly, PD-1 was still overexpressed on HIV-specific CD8+ T cells from people treated in CHI 12 days after antigen exposure and these PD-1 expression levels negatively correlated with cytolytic capacity of the recalled HIV-specific CD8+ T cells. These data support the hypothesis that epigenetic modifications at the PD-1 promoter during CHI allow for a higher and more sustained upregulation of PD-1 when these cells are activated. These data suggest that interventions aimed at blocking the PD-1 pathway at the time of HIV-specific CD8+ T cell recall might be beneficial in people treated from CHI to allow them to achieve better viral control.
Transcription factors play a crucial role in the differentiation of memory CD8+ T cells and their recall responses.66, 67, 68,71 We showed here that a superior expansion capacity of HIV-specific CD8+ T cells correlated with expression of TCF-1. Lack of TCF-1 dampened not only the generation of functional memory CD8+ T cells but also secondary expansion in the mouse model,67 and its expression positively correlated with proliferative capacity of hepatitis C virus (HCV)-specific CD8+ T cells.72 Human CD8+ T cells expressing TCF-1 serve as memory cells with self-renewal capacity which can give rise to TCF-1low short-lived cells,68 consistent with HIV-specific CD8+ T cells in spontaneous controllers having higher proliferative capacity18 and elevated TCF-1 expression compared to non-controllers.43,73 Pharmacological induction of TCF-1 in HIV-specific CD8+ T cells from people treated from CHI increased spontaneous controller-like features in these cells including high metabolic plasticity, survival capacity, homeostatic proliferation, and antiviral capacity.74,75 Therefore, sustained expression of TCF-1 during the recall response could play an important role in limiting viral rebound after ATI, as evidenced by the correlation between higher expression of TCF-1 and longer time to viral rebound after ATI, although it is not sufficient for post-treatment control since all participants experienced virus rebound within 48 days.
We describe here that memory HIV-specific CD8+ T cells from people treated in AHI are superior in terms of long-lived phenotype, proliferation, and cytotoxic capacity upon recall compared to those from people treated in CHI, yet, they do not have an impact on viral rebound after ART interruption. This was also observed in the non-human primate model where CD8+ T cells reduced the viral set point post-ART interruption but not viral rebound.76 This might be partially explained by the very low absolute numbers of memory HIV-specific CD8+ T cells in people who initiated ART in AHI, putting them at a disadvantage against a fast-replicating virus even if they do have high proliferative capacity. One explanation for the very low absolute numbers of memory HIV-specific CD8+ T cells in people who initiated ART in AHI is that they have been exposed to a limited amount of antigen before ART initiation also leading to a limited HIV reservoir size.77 Indeed, the number of ex vivo HIV-specific CD8+ T cells in spontaneous controllers is significantly higher (2 to 20-fold) than people treated from CHI,19,43,78,79 who have even 7 times higher HIV-specific CD8+ T cells than people treated from AHI. Thus HIV-specific CD8+ T cells need to be boosted prior to treatment discontinuation in people treated from AHI to increase their numbers in order to enhance control of viral replication post-ART. Therapeutic vaccination has so far only induced at most a nominal delay of viral rebound after ATI,5,80,81 and novel therapeutic interventions that can significantly increase the numbers of HIV-specific CD8+ T cells might need to be explored. HIV-specific CD8+ T cells in people treated from CHI showed higher absolute cell numbers before and after recall than those treated from AHI, but these cells were still endowed with an exhausted phenotype and function after in vitro recall, providing some explanation for the inability of previous interventions aimed at boosting HIV-specific CD8+ T cells in people treated from CHI to induce post-ART control. HIV remission strategies will likely require a combination of strategies aimed at reversing the remaining T cell dysfunction and improving immune boosting prior to ATI in order to achieve higher quantities of HIV-specific CD8+ T cells with sustainable effector functions, targeting TCF-1 might be a good candidate to evaluate in future studies.
This study has several limitations. It would have been very informative to perform a direct comparison of HIV-specific CD8+ T cells from spontaneous controllers and people treated from AHI as spontaneous controllers have effective HIV-specific CD8+ T cell responses associated with viral control. Unfortunately, we do not have spontaneous controllers in our Thai cohorts. This might be in part due to the fact that HIV CRF01_AE, largely predominant in Thailand, is associated with higher viral set point compared to other HIV-1 subtypes.82 Although we were unable to make this direct comparison, previous studies have shown that HIV-specific CD8+ T cells from spontaneous controllers expressed higher levels of TCF-1 compared to people treated from CHI43 and that induction of TCF-1 in HIV-specific CD8+ T cells of people treated from CHI increased spontaneous controller-like features in these cells.74 These data are in line with our observation that early ART induces preserved HIV-specific CD8+ T cells expressing higher levels of TCF-1, similar to HIV-specific CD8+ T cells in spontaneous controllers. A second limitation of this study was the very strict ART resumption criteria of the ATI studies (two consecutive viral load measurements >1,000 copies/mL2,3,6). In addition to their role in delaying or controlling viral rebound, CD8+ T cell can also play a role in decreasing the viral set point after rebound.76,83 However, as all the participants analyzed in the ATI studies reached ART resumption criteria before reaching viral set point, we were unable to assess the potential effect of CD8+ T cell responses on the establishment of viral set point after rebound and could not provide an in vivo correlate of protection for HIV-specific CD8+ T cells endowed with stemness properties. Additional studies would be needed to demonstrate their role in viral control post ATI.
Contributors
H.T. designed and performed the experiments, analyzed the data, and wrote the manuscript. J.C.K. and J.L.M performed the experiments, helped analyze the data and write the manuscript. E.K., D.C., C.S., J.I., S.T., D.S., P.P., and T.C., and N.C managed the participant recruitment and follow-up in the studies. P.K.E., A.G., and R.T. performed the single cell RNA sequencing, and analyses. H.B. and M.R. performed the viral sequence analysis. S.B. performed participants screening for the tetramers. S.P. provided help in statistical analyses. N.P., M.D.S., M.L.R., and N.L.M. provided support for the clinical studies. N.C., E.K.H., and D.C.D. provided conceptual advice, and edited the manuscript. J.A., S.V., V.G.V., and T.A.C. designed the clinical study, provided conceptual advice, and edited the manuscript. L.T. designed the experiments, analyzed the data, and wrote the manuscript. H.T. and L.T. have accessed and verified the data. All authors read and approved the final version of the manuscript.
Data sharing statement
All relevant data in the manuscript will be shared by the lead contact upon request.
Declaration of interests
All the other authors declare that they have no competing interests.
Acknowledgments
We thank our study participants and staff from the Thai Red Cross AIDS Research Centre (TRC-ARC), Chulalongkorn University, and Armed Forces Research Institute of Medical Sciences (AFRIMS) for their valuable contributions to this study. We want to thank M. Creegan, J. Degler, and K. Lal, for sorting all the samples and D. Brooks and B. Colton for technical assistance. We thank V. Assawadarachai, A. Bates, M. Bose, A. Bradfield Raab, J. Buahen, M. DiGiorgio, E. Engeman, S. Howell Miller, M. Lazzaro, S. LePore, E. Lewitus, Y. Li, R. Loney, K. Okada, A. Marie O'Sullivan, C. Ogega, M. Pheko, K. Poltavee, E. Sanders-Buell, D. Silas, S. Tovanabutra, N. Tseng, and M. Waters for their HIV sequencing and data analysis. We are grateful to the Government Pharmaceutical Organization of Thailand, ViiV Healthcare, Gilead Sciences, and Merck for providing the antiretrovirals for this study. We also thank the RV254/SEARCH010, SEARCH011, RV304/SEARCH013, RV409/SEARCH019, RV411, and RV397 study group members at TRCARC, AFRIMS and MHRP. All MHC class I peptide monomers were obtained through the NIH Tetramer Core Facility. This study was supported by the following sources: NIH grant R01AI108433, R01MH095613, R21MH086341, R01NS061696 and a cooperative agreement (W81XWH‐07‐2‐0067, W81XWH‐11‐2‐0174, W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine Inc. and the U.S. Department of Defense. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above, the U.S. Department of the Army or the U.S. Department of Defense, the Henry M. Jackson Foundation for the Advancement of Military Medicine, the National Institutes of Health, the Department of Health and Human Services, or the United States government, nor does mention of trade names, commercial products, or organizations imply endorsement by the Thai Red Cross AIDS Research Centre.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104253.
Contributor Information
Lydie Trautmann, Email: trautmyl@ohsu.edu.
The RV254/SEARCH010, RV304/ SEARCH013, and SEARCH011 study groups:
Nipat Teeratakulpisarn, Supanit Pattanachaiwit, Somchai Sriplienchan, Ponpen Tantivitayakul, Ratchapong Kanaprach, Kiat Ruxrungtham, Netsiri Dumrongpisutikul, Ponlapat Rojnuckarin, Suthat Chottanapund, Kultida Poltavee, Tassanee Luekasemsuk, Hathairat Savadsuk, Suwanna Puttamsawin, Khunthalee Benjapornpong, Nisakorn Ratnaratorn, Kamonkan Tangnaree, Chutharat Munkong, Rommanus Thaimanee, Patcharin Eamyoung, Sasiwimol Ubolyam, Sukalya Lerdlum, Sopark Manasnayakorn, Rugsun Rerknimitr, Sunee Sirivichayakul, Phandee Wattanaboonyongcharoen, Jessica Cowden, Alexandra Schuetz, Siriwat Akapirat, Nampueng Churikanont, Saowanit Getchalarat, Denise Hsu, Ellen Turk, Oratai Butterworth, Mark Milazzo, Leigh Anne Eller, Julie Ake, Leigh Anne Eller, Serena Spudich, CAPT Lawrence Fox, Silvia Ratto-Kim, Victor DeGruttola, Yotin Chinvarun, Pasiri Sithinamsuwan, James Fletcher, Bruce Shiramizu, and Alexandra Schuetz
Appendix. Supplementary materials
References
- 1.Hurst J, Hoffmann M, Pace M, et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun. 2015;6:8495. doi: 10.1038/ncomms9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colby DJ, Trautmann L, Pinyakorn S, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowell TA, Colby DJ, Pinyakorn S, et al. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV. 2019;6(5):e297–e306. doi: 10.1016/S2352-3018(19)30053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannus P, Rutsaert S, De Wit S, et al. Rapid viral rebound after analytical treatment interruption in patients with very small HIV reservoir and minimal on-going viral transcription. J Int AIDS Soc. 2020;23(2):e25453. doi: 10.1002/jia2.25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colby DJ, Sarnecki M, Barouch DH, et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat Med. 2020;26(4):498–501. doi: 10.1038/s41591-020-0774-y. [DOI] [PubMed] [Google Scholar]
- 6.Kroon E, Ananworanich J, Pagliuzza A, et al. A randomized trial of vorinostat with treatment interruption after initiating antiretroviral therapy during acute HIV-1 infection. J Virus Erad. 2020;6(3) doi: 10.1016/j.jve.2020.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann N, von Siebenthal C, Vongrad V, et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun. 2019;10(1):3193. doi: 10.1038/s41467-019-10884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouzioux C, Hocqueloux L, Saez-Cirion A. Posttreatment controllers: what do they tell us? Curr Opin HIV AIDS. 2015;10(1):29–34. doi: 10.1097/COH.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 10.Cockerham LR, Hatano H, Deeks SG. Post-treatment controllers: role in HIV “cure” research. Curr HIV/AIDS Rep. 2016;13(1):1–9. doi: 10.1007/s11904-016-0296-x. [DOI] [PubMed] [Google Scholar]
- 11.Etemad B, Esmaeilzadeh E, Li JZ. Learning from the exceptions: HIV remission in post-treatment controllers. Front Immunol. 2019;10:1749. doi: 10.3389/fimmu.2019.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndhlovu ZM, Kamya P, Mewalal N, et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity. 2015;43(3):591–604. doi: 10.1016/j.immuni.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takata H, Buranapraditkun S, Kessing C, et al. Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med. 2017;9(377) doi: 10.1126/scitranslmed.aag1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartwright EK, Spicer L, Smith SA, et al. CD8(+) lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity. 2016;45(3):656–668. doi: 10.1016/j.immuni.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBrien JB, Mavigner M, Franchitti L, et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8(+) cells. Nature. 2020;578(7793):154–159. doi: 10.1038/s41586-020-1946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura Y, Gautam R, Chun TW, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543(7646):559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 19.Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migueles SA, Weeks KA, Nou E, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83(22):11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192(1):63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 23.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 24.Trautmann L, Mbitikon-Kobo FM, Goulet JP, et al. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120(17):3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ndhlovu ZM, Kazer SW, Nkosi T, et al. Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection. Sci Transl Med. 2019;11(493):eaau0528. doi: 10.1126/scitranslmed.aau0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streeck H, Jessen H, Alter G, et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis. 2006;194(6):734–739. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 27.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97(7):3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208(8):1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowell TA, Fletcher JL, Sereti I, et al. Initiation of antiretroviral therapy before detection of colonic infiltration by HIV reduces viral reservoirs, inflammation and immune activation. J Int AIDS Soc. 2016;19(1):21163. doi: 10.7448/IAS.19.1.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sereti I, Krebs SJ, Phanuphak N, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64(2):124–131. doi: 10.1093/cid/ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24(10):1598–1601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 32.Goujard C, Girault I, Rouzioux C, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther. 2012;17(6):1001–1009. doi: 10.3851/IMP2273. [DOI] [PubMed] [Google Scholar]
- 33.Van Gulck E, Bracke L, Heyndrickx L, et al. Immune and viral correlates of “secondary viral control” after treatment interruption in chronically HIV-1 infected patients. PLoS One. 2012;7(5):e37792. doi: 10.1371/journal.pone.0037792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assoumou L, Weiss L, Piketty C, et al. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS. 2015;29(15):2003–2007. doi: 10.1097/QAD.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 35.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254(1):326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehr M, Cahenzli J, Haas A, et al. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol. 2008;82(7):3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streeck H, Brumme ZL, Anastario M, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5(5):e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janbazian L, Price DA, Canderan G, et al. Clonotype and repertoire changes drive the functional improvement of HIV-specific CD8 T cell populations under conditions of limited antigenic stimulation. J Immunol. 2012;188(3):1156–1167. doi: 10.4049/jimmunol.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youngblood B, Noto A, Porichis F, et al. Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191(2):540–544. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vigano S, Negron J, Ouyang Z, et al. Prolonged antiretroviral therapy preserves HIV-1-specific CD8 T cells with stem cell-like properties. J Virol. 2015;89(15):7829–7840. doi: 10.1128/JVI.00789-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahnke YD, Fletez-Brant K, Sereti I, Roederer M. Reconstitution of peripheral T cells by tissue-derived CCR4+ central memory cells following HIV-1 antiretroviral therapy. Pathog Immun. 2016;1(2):260–290. doi: 10.20411/pai.v1i2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutishauser RL, Deguit CDT, Hiatt J, et al. TCF-1 regulates HIV-specific CD8+ T cell expansion capacity. JCI Insight. 2021;6(3) doi: 10.1172/jci.insight.136648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997;127(10):882–890. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 45.Walli R, Reinhart B, Luckow B, et al. HIV-1-infected long-term slow progressors heterozygous for delta32-CCR5 show significantly lower plasma viral load than wild-type slow progressors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(3):229–233. doi: 10.1097/00042560-199807010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Claireaux M, Robinot R, Kervevan J, et al. Low CCR5 expression protects HIV-specific CD4+ T cells of elite controllers from viral entry. Nat Commun. 2022;13(1):521. doi: 10.1038/s41467-022-28130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blankson JN. Effector mechanisms in HIV-1 infected elite controllers: highly active immune responses? Antiviral Res. 2010;85(1):295–302. doi: 10.1016/j.antiviral.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ananworanich J, Fletcher JL, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology. 2013;10:56. doi: 10.1186/1742-4690-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ananworanich J, Sacdalan CP, Pinyakorn S, et al. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J Virus Erad. 2016;2:43–48. doi: 10.1016/S2055-6640(20)30688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12) doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiramizu B, Ananworanich J, Chalermchai T, et al. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J Neurovirol. 2012;18(1):69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rolland M, Edlefsen PT, Larsen BB, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490(7420):417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48(W1):W449–W454. doi: 10.1093/nar/gkaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 55.Mbitikon-Kobo FM, Bonneville M, Sekaly RP, Trautmann L. Ex vivo measurement of the cytotoxic capacity of human primary antigen-specific CD8 T cells. J Immunol Methods. 2012;375(1-2):252–257. doi: 10.1016/j.jim.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Noto A, Ngauv P, Trautmann L. Cell-based flow cytometry assay to measure cytotoxic activity. J Vis Exp. 2013;(82):e51105. doi: 10.3791/51105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buranapraditkun S, Hempel U, Pitakpolrat P, et al. A novel immunodominant CD8+ T cell response restricted by a common HLA-C allele targets a conserved region of Gag HIV-1 clade CRF01_AE infected Thais. PLoS One. 2011;6(8):e23603. doi: 10.1371/journal.pone.0023603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gavioli R, Kurilla MG, de Campos-Lima PO, et al. Multiple HLA A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J Virol. 1993;67(3):1572–1578. doi: 10.1128/jvi.67.3.1572-1578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rolland M, Frahm N, Nickle DC, et al. Increased breadth and depth of cytotoxic T lymphocytes responses against HIV-1-B Nef by inclusion of epitope variant sequences. PLoS One. 2011;6(3):e17969. doi: 10.1371/journal.pone.0017969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci USA. 2003;100(26):15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuertes Marraco SA, Soneson C, Cagnon L, et al. Long-lasting stem cell-like memory CD8+ T cells with a naive-like profile upon yellow fever vaccination. Sci Transl Med. 2015;7(282):282ra48. doi: 10.1126/scitranslmed.aaa3700. [DOI] [PubMed] [Google Scholar]
- 63.Akondy RS, Fitch M, Edupuganti S, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–367. doi: 10.1038/nature24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer-Olson D, Brady KW, Bartman MT, et al. Fluctuations of functionally distinct CD8+ T-cell clonotypes demonstrate flexibility of the HIV-specific TCR repertoire. Blood. 2006;107(6):2373–2383. doi: 10.1182/blood-2005-04-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Conrad JA, Ramalingam RK, Duncan CB, et al. Antiretroviral therapy reduces the magnitude and T cell receptor repertoire diversity of HIV-specific T cell responses without changing T cell clonotype dominance. J Virol. 2012;86(8):4213–4221. doi: 10.1128/JVI.06000-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kratchmarov R, Magun AM, Reiner SL. TCF1 expression marks self-renewing human CD8(+) T cells. Blood Adv. 2018;2(14):1685–1690. doi: 10.1182/bloodadvances.2018016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro SP, Milush JM, Cunha-Neto E, et al. The CD8(+) memory stem T cell (T(SCM)) subset is associated with improved prognosis in chronic HIV-1 infection. J Virol. 2014;88(23):13836–13844. doi: 10.1128/JVI.01948-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Best JA, Blair DA, Knell J, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14(4):404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wieland D, Kemming J, Schuch A, et al. TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun. 2017;8:15050. doi: 10.1038/ncomms15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sekine T, Perez-Potti A, Nguyen S, et al. TOX is expressed by exhausted and polyfunctional human effector memory CD8(+) T cells. Sci Immunol. 2020;5(49) doi: 10.1126/sciimmunol.aba7918. [DOI] [PubMed] [Google Scholar]
- 74.Perdomo-Celis F, Passaes C, Monceaux V, et al. Reprogramming dysfunctional CD8+ T cells to promote properties associated with natural HIV control. J Clin Invest. 2022;132(11) doi: 10.1172/JCI157549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takata H, Trautmann L. Transforming dysfunctional CD8+ T cells into natural controller–like CD8+ T cells: can TCF-1 be the magic wand? J Clin Invest. 2022;132(11) doi: 10.1172/JCI160474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okoye AA, Duell DD, Fukazawa Y, et al. CD8+ T cells fail to limit SIV reactivation following ART withdrawal until after viral amplification. J Clin Invest. 2021;131(8) doi: 10.1172/JCI141677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leyre L, Kroon E, Vandergeeten C, et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med. 2020;12(533) doi: 10.1126/scitranslmed.aav3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emu B, Sinclair E, Hatano H, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008;82(11):5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vieira VA, Millar J, Adland E, et al. Robust HIV-specific CD4+ and CD8+ T-cell responses distinguish elite control in adolescents living with HIV from viremic nonprogressors. AIDS. 2022;36(1):95–105. doi: 10.1097/QAD.0000000000003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baden LR, Walsh SR, Seaman MS, et al. First-in-human randomized, controlled trial of mosaic HIV-1 immunogens delivered via a modified Vaccinia Ankara vector. J Infect Dis. 2018;218(4):633–644. doi: 10.1093/infdis/jiy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barouch DH, Tomaka FL, Wegmann F, et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19) Lancet. 2018;392(10143):232–243. doi: 10.1016/S0140-6736(18)31364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med. 2016;374(22):2120–2130. doi: 10.1056/NEJMoa1508952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Namazi G, Fajnzylber JM, Aga E, et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis. 2018;218(12):1954–1963. doi: 10.1093/infdis/jiy479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.