Abstract
Background
Uncomplicated urinary tract infections are among the commonest bacterial infections. Because antibiotic resistance is on the rise, there is growing interest in alternative, non-antimicrobial treatment options. This systematic review presents the current evidence on phytotherapy for the treatment and prevention of recurrent uncomplicated cystitis.
Methods
A systematic search of the relevant literature from January 2011 to August 2021 was carried out in the MEDLINE, Embase, and Cochrane Library databases and in two clinical trial registries. The trials included in the present review are randomized controlled trials (RCTs) of phytotherapeutic agents as monotherapy or combination therapy, in comparison to placebo, no treatment, non-pharmacological treatment, or drug treatment without any phytotherapeutic component. Two of the authors independently selected the publications, extracted the data, and estimated the risk of bias using the Cochrane Risk of Bias Tool.
Results
12 RCTs with a total of 1797 female patients were included. A trial of acute therapy with Chinese plant-based medicine revealed non-inferiority to antibiotic treatment. Six trials of prophylaxis with cranberry products yielded mixed results with regard to efficacy against recurrent urinary tract infections. A trial of Seidlitzia rosmarinus for the prevention of cystitis showed that its use was associated with a lower cystitis rate than placebo (at 6 months: 33 vs. 73%, p <0.001). In all trials but one, the risk of bias was unclear or high. No standardized assessment of adverse events was carried out.
Conclusion
Phytotherapeutic agents are an option for the treatment and prevention of recurrent cystitis in women. Given the heterogeneous state of the evidence on phytotherapy, no dependable recommendations can now be made for the clinical management of these patients with respect to phytotherapeutic agents.
There are an estimated 150 million urinary tract infections worldwide each year (1). Approximately 40% of all women have at least one UTI in their lifetime (2). Uncomplicated infections take a benign, self-limiting course; complicated ones are more likely to worsen or recur (3). By definition, UTIs are classified as uncomplicated when there are no relevant functional or anatomic abnormalities in the urinary tract, no relevant renal dysfunction, and no relevant pre-existing or concomitant diseases that promote UTI or serious complications (4). Uncomplicated urinary tract infections may be isolated, sporadic, or recurrent. The term “recurrent UTI” refers to two or more symptomatic episodes within six months or three or more symptomatic episodes within 12 months (4). The risk of recurrence in women is 30–50% per year for cystitis and 9% per year for pyelonephritis. Sexually active and postmenopausal women are particularly affected (5).
Because antibiotic resistance is increasing around the world, creating major challenges and costs for health care (6) and damage to the microbiome, there is growing public interest in alternative, non-antimicrobial treatment options and preventive measures (7, 8). A wide range of non-antibiotic alternatives is available (9, 10):
behavior modification
nutritional supplements
nonsteroidal anti-inflammatory drugs
probiotic agents
D-mannose
methenamine hippurate
estrogens
intravesical glycosaminoglycans
immune stimulants
hyaluronic acid
acupuncture
herbal medicines
Herbal medicines, also called phytotherapeutic agents, are already used to treat recurrent uncomplicated cystitis, but the published data are inconsistent in part (11, 12), making it difficult to derive any evidence-based recommendations for clinical practice.
In this review, we aim to pool the current evidence concerning phytotherapeutic agents in the treatment and prevention of recurrent uncomplicated cystitis in adults.
Methods
Research question and inclusion criteria
The research question was operationalized by means of a detailed a priori specification of the trial population, comparison arms, and endpoints to be considered. The inclusion and exclusion criteria are shown in Table 1. This systematic review was registered in the PROSPERO database (registration number CRD42021236008).
Endpoints
The following patient-related primary endpoints were defined:
cure or symptom relief
adverse events
recurrent cystitis during follow-up
health-related quality of life
Additional endpoints included restriction of everyday activities, symptom duration, antibiotic use in the intervention group, and patient satisfaction with treatment.
Search strategy
A systematic literature search was conducted in the MEDLINE, Embase, and Cochrane Library databases for the period January 2011 to August 2021 and was supplemented by a manual search. Searches were also performed for unpublished data and ongoing trials in clinical trials registries (clinicaltrials.gov; World Health Organization International Clinical Trials Registry Platform: www.who.int/ictrp). Detailed information on the search strategy can be found in eMethods.
Literatur screening
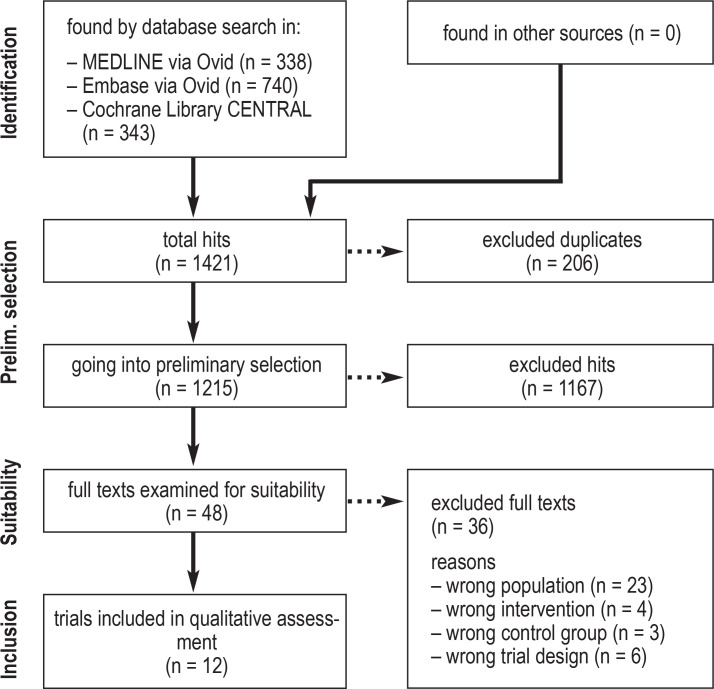
Two of the authors (JL, UK) independently screened the titles, abstracts, and full texts of the identified publications. Discrepant judgments were discussed with a third person (SS). Reasons for exclusion of full texts were documented for each of the publications that were not included (efigure).
eFigure.
PRISMA flowchart for the different phases of this systematic review.
For more information, see: www.prisma-statement.org.
Data extraction
Data from the included trials were entered into an a priori extraction table with the following categories: trial characteristics, patient characteristics, treatment groups, and endpoints. Data extraction was performed by one author (UK) and reviewed by another (JL). Discrepancies were discussed with a third author (SS). In case of incomplete data, the authors of the corresponding publication were contacted and asked to provide additional information.
Quality assessment
Two authors (JL, UK) independently assessed the risk of bias with the Cochrane risk-of-bias tool (13). Disagreements were resolved by involving a third author (SS).
Results
Trial selection
1421 references were identified by the database searches. Twelve RCTs involving a total of 1797 patients met the inclusion criteria (efigure). No trial included men or pregnant women. Chinese herbal medicines were studied in one trial (14), cranberry products in six (15– 20), Seidlitzia rosmarinus in one (21), and combined preparations in four (22– 25). Phytotherapeutic agents were used to treat acute episodes of recurrent cystitis in two trials (14, 23) and to prevent recurrent uncomplicated cystitis in ten (15– 22, 24, 25). Patient satisfaction and restrictions in daily living were not considered as endpoints in any of the included trials. The extracted trial data are given in Tables 2 and 3. More detailed information on the included trials can be found in the electronic supplement: the relevant trials that were identified from the trial registries are listed in eTable 1.
Tabbe 2. Evidence table for therapeutic trials.
| Reference | Study features | Treatment groups | Endpoints | Serious adverse events | |
|
Liu 2019 (14) |
• 122 patients •2010 – 2013 •Follow-up: 6 months |
Chinese herbal medicine (n = 61) 4 weeks Antibiotics (n = 61) 4 weeks |
Chinese herbal medicine vs. antibiotics | No clinically relevant side effects in either group | |
|
Symptom improvement • ≥=95% symptomatic improvement: 31% (19/61) vs. 30% (18/61) • ≥=70% symptomatic improvement: 48% (29/61) vs. 26% (16/61); p<0.05 • ≥=30% symptomatic improvement: 18% (11/61) vs. 39% (24/61); p<0.05. • <30% symptomatic improvement or no response: 3% (2/61) vs. 5% (3/61) |
Treatment efficacy: 90% (55/61) vs. 82% (50/61) rUTI: 13% (8/61) vs. 20% (12/61) rUTI in cured patients: 9% (5/55) vs. 14% (7/50) |
||||
|
Cai 2018 (23) |
•93 patients •2017 •Follow-up: 3 months |
400 mg L-methionine, 100 mg Hibiscus sabdariffa, 100 mg Boswellia serrata (n = 46) one week Antibiotics (n = 47) Short-term antibiotic therapy (according to the EAU guideline) |
Combined preparation vs. antibiotics |
Combined preparation: No side effects Antibiotics: 14.9% (7/47) discontinuation of drug because of drug-related adverse events. |
|
|
Clinical Improvement: •30 days: 96% (44/46) vs. 100% (47/47) •90 days: 96% (44/46) vs. 96% (45/47) |
Quality of life: •Baseline: 94.3 vs. 94.5 •30 days: 98.5 vs. 98.7 •90 days: 99.1 vs. 98.1 p<0.003 |
||||
EAU: European Association of Urology, n: number of patients in trial arm, UTI: urinary tract infection, rUTI: recurrent urinary tract infection.
Table 3. Evidence table for prevention trials.
| Reference | Study features | Treatment groups | Endpoints | Serious adverse events | |
| Cranberries | |||||
|
Maki 2016 (16) |
•N = 373 healthy female patients •2013 – 2015 •Follow-up only in the treatment period |
Cranberry drink (n= 185) 24 weeks Placebo (n = 188) 24 weeks |
Cranberry vs. placebo | Serious adverse events were probably not related to the treatments. | |
|
Reported symptomatic UTI episodes: •0: 82% (152/185) vs. 73% (138/188) •1: 15% (27/185) vs. 19% (36/188) •2: 3% (6/185) vs. 6% (11/188) •3: 0% (0/185) vs. 2% (3/188) • ≥1: 18% (33/185) vs. 27% (50/188) Total number of UTIs : 39 vs. 67 Total UTI with pyuria: 32 vs. 53 |
Incidence ratio •UTI: 0.61 (95% CI 0.41, 0.91); p = 0.016. •UTI with pyuria: 0.63 (95% CI 0.40, 0.97); p=0.037. |
||||
|
Vostalova 2015 (20) |
•N = 182 healthy female patients •2010 – 2011 •Follow-up only in the treatment period |
Cranberry capsules (n = 89) 6 months Placebo (n = 93) 6 months |
Cranberry vs. placebo | No adverse outcomes were reported | |
|
Within 6 months: • ≥1 UTI: 11% (9/83) vs. 26% (24/93); p=0.04. •2 UTI: 1% (1/83) vs. 6% (6/93). |
Relative risk reduction: 58% Cumulative incidence of UTI over 6 months: 9% vs. 19%. |
||||
|
Takahashi 2013 (19) |
•N = 213 patients with cystitis •2007 – 2009 •Follow-up only in the treatment period |
Cranberry juice (n = 82 with uncomplicated cystitis).24 weeks Placebo (n = 88 with uncomplicated cystitis). 24 weeks |
Cranberry vs. placebo | No serious adverse events | |
|
rUTI •total: 27% (22/82) vs. 39% (34/88); p=0.1300 •<50 years: 22% (6/27) vs. 12% (3/25); p=0.3623 • ≥50 years: 29% (16/55) vs. 49% (31/63); p=0.0425 |
Multivariate analysis (≥=50 years): HR: 1.037 (95% CI; 1.002–1.073); p=0.038 |
||||
|
Stapleton 2012 (18) |
•N = 176 healthy female patients •2005 – 2008 •Median follow-up: 168 days |
Cranberry juice (n = 120) No data on the duration of intake Placebo (n = 56) No data on the duration of intake |
Cranberry vs. placebo | No serious adverse events | |
|
UTI in follow-up: •total: 28% (33/120) vs. 30% (17/56); p=0.70 •>1 UTI: 8% (10/120) vs. 7% (4/56) |
Cumulative UTI rate at 6 months: 0.29 (95% CI, 0.21–0.38) vs. 0.37 (95% CI, 0.25–0.54); p=0.82 Adjusted HR for UTI: 0.68 (95% CI, 0.33–1.39); p=0.29 |
||||
|
Beerepoot 2011 (15) |
•N = 221 healthy female patients •2005 – 2007 •Follow up: 3 months |
Cranberry concentrate (n = 111) 12 months TMP-SMX (n = 110) 12 months |
TMP-SMX vs. cranberry | Serious adverse events· TMP-SMX: 0.91% (1/110) Stevens-Johnson syndrome (led to treatment discontinuation). Cranberry: none |
|
|
After 12 months Mean number of rUTIs (95% CI): 1.8 (0.8–2.7) vs. 4.0 (2.3–5.6); p=0.02. After 15 months Mean number of rUTIs (95% CI): 0.5 (0.3–0.7) vs. 0.7 (0.4–0.9); p=0.30. |
Median time to first rUTI (95% CI): 8 (6–10) vs. 4 (3–6) months; p = 0.03. Median number of antibiotic prescriptions during the intervention period (interquartile range): 0.5 (0–2) vs. 1 (0–2). |
||||
|
Sengupta 2011 (17) |
•N = 60 female patients with UTI •no data on study period and follow-up |
Cranberry capsules (n = 44) 90 days No treatment (n = 16) 90 days |
Symptom improvement: •Cranberry (total): 41% (18/44) complete resolution of urologic symptoms. •Cranberry (total): significant improvement from 10-day evaluation relative to baseline (data not shown). •Untreated: no improvement |
Use of the emergency drug •Cranberry (low dose): 10% (2/21) •Cranberry (high dose): 9% (2/23) •Untreated: 25 % (4/16) |
No serious adverse events |
| Seidlitzia rosmarinus | |||||
|
Kamalifard 2020 (21) |
•N = 126 healthy female patients •2017 – 2018 •Follow up: 4 months |
Seidlitzia rosmarinus (n = 63) 2 months Placebo (n = 63) 2 months |
Seidlitzia rosmarinus vs. placebo | No side effects were observed in either group | |
|
Cystitis incidence rate: •At 2 months: 19% (11/58) vs. 55% (32/58); OR: 0.19 (95% CI, 0.08, 0.43); p<0.001. •At 4 months: 22% (13/58) vs. 57% (33/58); OR: 0.21 (95% CI, 0.98, 0.49); p<0.001. •At 6 months: 33% (19/58) vs. 73% (43/59); OR: 0.18 (95% CI, 0.08, 0.40); p<0.001. |
Incidence of recurrent cystitis: 14% (8/58) vs. 66% (39/59); OR: 0.08 (95% CI, 0.03, 0.20); p<0.001 |
||||
| Combined preparations | |||||
|
Murina 2021 (25) |
•N = 55 healthy female patients •Trial period: no information •Follow-up: 150 days |
Cranberry, Lactobacillus paracasei LC11, D-mannose (n = 38) 90 days no treatment (n = 17) 90 days |
No UTI •Cranberry group 1: 65.8 % (12/19) •Cranberry group 2: 68.8 % (13/19) •Control group: 36.9% (6/17) p=0.05 |
A UTI •Cranberry group 1: 18.2 % (4/19) •Cranberry group 2: 15.6 % (3/19) •Control group: 10.2 % (2/17) Not significant ≥=2 UTI •Cranberry group 1: 16 % (3/19) •Cranberry group 2: 15.6 % (3/19) •Control group: 52.9% (9/17) p<0.01 |
No adverse events |
|
Bruyère 2019 (22) |
•N = 86 healthy female patients •2013 – 2015 •Follow-up only in the treatment period |
600 mg cranberry extract, 400 mg propolis, 5 mg zinc (n = 43) 6 months Placebo (n = 43) 6 months |
Combined preparation vs. placebo | Serious adverse events were probably not related to treatment | |
|
≥1 cystitis: 2.3 ± 1.8 vs. 3.1 ± 1.8; p=0.09 |
No clinically relevant change in quality of life | ||||
|
Koradia 2019 (24) |
•N = 90 healthy female patients •2016–2018 •Follow-up only in the treatment period |
18 mg cranberry extract (Vaccinium macrocarpon); 21 mg probiotics (Lactobacillus acidophilus, Lactobacillus plantarum); 160
μg vitamin A (retinyl acetate) (n = 45) 26 weeks Placebo (n = 45) 26 weeks |
Combined preparation vs. placebo | There were no serious adverse events or adverse events that led to dropping out of the trial | |
|
rUTI after 26 weeks: 9% (4/44) vs. 33% (15/45); p=0.0053 Duration of illness: mean (SD): 5 (0.8) vs. 12.2 (6.5) days; p=0.0095 |
Antibiotic treatment needed: 8% (3/40) vs. 27% (11/41); p= 0.0372 |
||||
KICI: confidence interval, HR: hazard ratio, rUTI:.recurrent UTI, SD: standard deviation, TMP-SMX: trimethoprim-sulfamethoxazole, UTI: urinary tract infection.
eTable 1. Findings of the trial register search.
| Intervention | Control | Trial topic | |
| Ongoing studies | |||
|
NCT03597152 Start: August 2020 Status: not yet recruited Estimated end of trial: December 2022 |
lactoferrin, D-mannose, cranberry fruit extract, hibiscus flower extract, vitamin C, vitamin D | placebo | treatment |
|
NCT02705495 Start: May 2015 Status: recruitment Estimated end of trial: May 2021 |
segmental and ear acupuncture + non-mandatory intake of cranberry products: bearberry leaf extract & cranberry concentrate and/or 2500 mg of dried cranberry extract (=225 mg proanthocyanidins) | recommended, but not mandatory: segmental and ear acupuncture + recommended use of cranberry products |
prevention |
| Terminated studies (without results)* | |||
|
NCT03042273** Start: May 2017 End of trial: December 2019 |
25 000 mg Vaccinium macrocarpon | placebo | prevention |
|
NCT03032003 Start: February 2017 End of trial: May 2018 |
240 mg proanthocyanidins | placebo | prevention |
|
NCT02454309 Start: July 2015 End of trial: March 2017 |
500 mg cranberry powder in a 36:1 concentration ratio | placebo | prevention |
|
NCT01219595*** Start: September 2010 End f trial: September 2012 |
42 g sweetened and dried cranberries | placebo (42 g strawberry fruit pieces) |
prevention |
|
EUCTR2013–004653–25-DE No data available |
garden nasturtium powder 200 mg & horseradish root powder 80 mg | placebo | prevention |
|
NTR7069 No data available |
live probiotic strains of L. pentosus W2 (KCA1), L. acidophilus W22, L. plantarum W21, L. salivarius W24, L. brevis W63, L. casei W56 and L. helveticus W74, cranberry extract (=36 mg proanthocyanidins) and D-mannose 1 g | placebo | treatment |
| Withdrawn trial | |||
|
NCT01881165 Start: September 2016 Lack of sponsoring |
500 mg cranberry powder in a 36:1 concentration ratio | placebo | prevention |
L. : Lactobacillus
*Whenever contact data were obtainable, the authors were contacted.
**Data analysis is not yet complete.
***The clinical trial revealed no significant changes in intestinal E . coli types after the consumption of dried cranberries
Risk of bias
One trial was assessed as having a low risk of bias in all domains (21), while all others were found to have a high or unclear risk of bias (table 4). In two trials, the subjects were not blinded (23, 25). In three, missing data on endpoints led to a high risk of bias. Data were missing for various reasons: a high dropout rate (15), a summarized presentation of data on patients taking different doses of medication (18), and the retrospective exclusion of trial subjects during the analysis (19). One trial was considered to be at high risk of bias because of selective endpoint reporting: symptom improvement was rated on a 5-point scale, but it was not explained how these scores were incorporated into the grouped rating of percent symptom improvement (14). Seven trials were rated at high risk of bias for other factors because UTIs and recurrent UTIs were defined only partially (16, 17, 22, 24) or not at all (19, 23), or the dosage of phytotherapeutic ingredients was not indicated (25).
Table 4. Assessment of the risk of bias (trials listed alphabetically).
|
Beerepoot 2011 (15) |
Bruyère 2019 (22) |
Cai
2018 (23) |
Kamalifard 2020 (21) |
Koradia 2019 (24) |
Liu
2019 (14) |
Maki 2016 (16) |
Murina 2021 (25) |
Sengupta 2011 (17) |
Stapleton 2012 (18) |
Takahasi 2013 (19) |
Vostalova 2015 (20) |
| Generation of the randomization sequence | |||||||||||
| low | low | unclear | low | low | low | low | low | low | low | unclear | low |
| Secrecy and unpredictability of group assignment | |||||||||||
| unclear | low | unclear | low | low | low | unclear | unclear | low | low | unclear | unclear |
| Blinding of trial staff / subjects | |||||||||||
| low | unclear | high | low | low | low | low | high | low | low | low | low |
| Blinding in endpoint ascertainment and evaluation | |||||||||||
| low | unclear | unclear | low | low | unclear | unclear | low | unclear | unclear | unclear | unclear |
| Missing endpoint data | |||||||||||
| high | low | low | low | low | low | low | low | low | high | high | low |
| Selective reporting of endpoints | |||||||||||
| low | low | low | low | low | high | low | low | low | low | low | low |
| Other causes of bias | |||||||||||
| unclear | high | high | low | high | unclear | high | high | high | unclear | high | low |
Therapeutic trials
Two therapeutic trials were found that compared phytotherapeutic agents with antibiotics. Liu et al. treated 122 women suffering from an acute episode of recurrent cystitis with either Chinese herbal medicine (a mixture of nine phytotherapeutic agents and one mineral agent) combined with a sham antibiotic (n = 61) or else antibiotics (levofloxacin 200 mg twice daily, amoxicillin/clavulanic acid 500 mg three times daily, or another test-matched antibiotic) combined with sham Chinese herbal medicine (n = 61) (14). Cai et al [23] compared an herbal combined preparation (400 mg L-methionine, 100 mg Hibiscus sabdariffa, 100 mg Boswellia serrata) with short-term antibiotic therapy according to the EAU guideline (23, 26) in 93 women with an acute episode of recurrent cystitis. The non-inferiority trial by Liu et al. revealed no inferiority of Chinese herbal medicine to antibiotics (14). The trial by Cai et al. also showed comparable results in terms of clinical improvement, but there was a statistically significant association of antibiotic treatment with a better quality of life at 90 days (23) (23) (Table 2).
eTable 2. Evidence table for therapeutic trials.
| Reference | Trial features | Definitions | Intervention | Control | Endpoints | Undesired events | Dropouts | Comments |
|
Liu 2019 (14) |
122 patients (age 18 – 75) 2010 – 2013 follow-up: 6 months trial centers: 3 China |
UTI: urine culture with >10⁵5 CFU/ml bacteria + dysuria, urinary frequency, and/or urinary urgency rUTI : ≥=3 episodes of uncomplicated UTI in the past year |
Chinese herbal medicine 61 patients 4 weeks Chinese herbal medicine granules (116 g) + antibiotic placebo Chinese herbal medicine : Anemarrhena asphodeloides Bunge (15 g), Platycladus orientalis (L.) Franco (10 g), Angelica sinensis (Oliv.) Diels (10 g), Rehmannia glutinosa (Gaertn.) DC. (15 g), Poria cocos (Schw.) Wolf (15 g), Salvia miltiorrhiza Bunge (10 g), Rheum palmatum L. (6 g), Polygonum aviculare L. (10 g), Dianthus superbus L. (10 g), Talcum (15 g). placebo: Identical form & taste b.i.d. |
Antibiotics 61 patients 4 weeks antibiotic granules (LVX: 200 mg; AMX: 500 mg) + Chinese herbal medicine placebo LVX : b.i.d. for one week or AMX : t.i.d. for one week AMX and LVX followed by placebo : 3 weeks |
Chinese herbal medicine vs. antibiotics symptomatic improvement: • ≥=95% improvement: 31% (19/61) vs. 30% (18/61) • ≥=70% improvement: 48% (29/61) vs. 26% (16/61); p<0.05 • ≥=30% improvement: 18% (11/61) vs. 39% (24/61); p<0.05. •<30% improvement or no response: 3% (2/61) vs. 5% (3/61) treatment efficacy : 90% (55/61) vs. 82% (50/61) rUTI : 13% (8/61) vs. 20% (12/61) rUTI in fully recovered patients : 9% (5/55) vs. 14% (7/50) |
no significant side effects in either group (data not shown) |
Chinese herbal medicine vs. antibiotics dropouts: •4 weeks: 3% (2/61) vs. 2% (1/61) •6 months: 22% (13/59) vs. 18% (11/60) rUTI analysis: 79% (48/61) vs. 82% (50/61) |
supported by the Chinese Academy of Traditional Chinese Medicine Joint Innovation Research Project, Traditional Chinese Medicine Dominant Disease Clinical Research Project and Capital Featured Clinical Application and Promotion Project, China no conflicts of interest |
|
Cai 2018 (23) |
93 patients (age 22–63) 2017 follow-up: 3 months Single-center country: no information |
UTI: no definition rUTI : no definition |
Combination preparation 46 patients one week 1 tablet containing 400 mg L-methionine, 100 mg Hibiscus sabdariffa, 100 mg Boswellia serrata b.i.d. |
Antibiotics 47 patients short-term antibiotic therapy (according to the EAU guideline) |
Combination preparation vs. antibiotics clinical Improvement: •30 days: 96% (44/46) vs. 100% (47/47) •90 days: 96% (44/46) vs. 96% (45/47) quality of life: •baseline: 94.3 vs. 94.5 •30 days: 98.5 vs. 98.7 •90 days: 99.1 vs. 98.1; p<0.003 |
Combination preparation: no side effects antibiotics : 14.9% (7/47) discontinuation of treatment due to drug side effects |
no dropouts | no conflicts of interest |
AMX: amoxicillin/clavulanic acid, b.i.d., twice daily; EAU: European Association of Urology, UTI: urinary tract infection, LVX: levofloxacin, rUTI: recurrent urinary tract infectio; t.i.d., three times daily
Prevention trials
Ten trials of preventive measures were identified (table 3). Five of them compared cranberry products with placebo for the prevention of recurrent cystitis (16– 20): two showed a significant lowering of the recurrence rate (16, 20), while two others showed no significant effects (18, 19). Takahashi et al. were able to show a significant lowering of the recurrence rate only in a subgroup of women aged 50 or older (cranberry: 49%, placebo: 29%; p = 0.04) (19). The report on one of these five trials contained no data on the recurrence rates in the two arms of the trial (17), and thus no conclusion can be drawn. In a Dutch trial conducted by Beerepoot et al. from 2005 to 2007, 221 healthy premenopausal women with a history of recurrent cystitis were treated with either antibiotics (480 mg trimethoprim-sulfamethoxazole [TMP-SMX] once daily) or cranberry capsules (500 mg twice daily) (15). Antibiotics were found to be significantly more effective than the cranberry product in preventing recurrent cystitis at the 12-month follow-up time point (1.8; 95% confidence interval [CI]: [0.8; 2.7] versus 4.0; [2.3; 5.6]; p = 0.02). The median number (with interquartile range) of antibiotic prescriptions in the intervention period was 0.5 (0–2) in the TMP-SMX group and 1 (0–2) in the cranberry group (15). TMP-SMX caused a serious adverse event (Stevens-Johnson syndrome) in one patient, after which the drug was discontinued (15). Three trials compared herbal combined preparations with placebo or no treatment for the prevention of recurrent cystitis in women (22, 24, 25). In a French trial conducted from 2013 to 2015, Bruyère et al. treated 42 women suffering from recurrent cystitis with a combined preparation of 400 mg propolis, 600 mg cranberry extract, and 5 mg zinc, 43 women with placebo. The recurrence rate during six months of follow-up was lower in the phytotherapy group (2.3 ± 1.8 versus 3.1 ± 1.8), but this difference was not statistically significant (p = 0.091). The groups did not differ significantly with respect to the quality of life or treatment side effects (22). In an Indian trial conducted from 2016 to 2018, a combined preparation with 18 mg of cranberry extract, 21 mg of probiotics, and 160 µg of vitamin A was compared to placebo in a cohort of 90 premenopausal women (45 women per group) (24): recurrent cystitis was significantly less common in women taking the combined drug (9% versus 33%; p = 0.005), and the combined drug also shortened the duration of acute cystitis (5 versus 12 days; p = 0.009) as well as lessening the need for antibiotics (8 versus 27%; p = 0.037) (24). Finally, in a single-center trial involving 55 premenopausal women, a combined preparation of cranberry, Lactobacillus paracasei LC11, and D-mannose was compared with no treatment. Two trial groups were defined (group 1: once daily for ten days per month for 90 days, group 2: once daily for 90 days), and episodes of recurrent cystitis up to 150 days after the start of treatment were tallied. Significantly more women in the control group developed cystitis during the trial period compared to the two treatment groups (group 1: 16%, group 2: 15.6%, control group: 52.9%; p < 0.01). There were no trial dropouts (25).
An Iranian multicenter trial conducted from 2017 to 2018 compared Seidlitzia rosmarinus with placebo for the prevention of recurrent cystitis (21). Recurrent cystitis was significantly less common in the intervention group than in the control group: 14% (8/58) versus 66% (39/59); odds ratio: 0.08; 95-% CI: [0.03; 0.20]; p < 0.001.
Adverse events
All phytotherapeutic agents had a good safety profile. In four trials, there were no adverse events in the phytotherapeutic intervention group (20, 21, 23, 25). Occasionally, serious events were reported that were considered unrelated or unlikely to be related to phytotherapeutic treatment (16, 22). Mild adverse events occurred sporadically, e.g., fever and weakness (17, 19). Gastrointestinal symptoms were most common (16– 19, 22, 24). For a detailed list of adverse events that occurred in the trials, see eTables 2n and 3.
Discussion
This systematic review of randomized controlled trials makes it clear that the available evidence on phytotherapy for the treatment and prevention of recurrent cystitis is highly heterogeneous. This review only included trials that were restricted to patients with recurrent urinary tract infections, yet the definition of recurrent cystitis among the included trials was far from uniform, varying from one or more (18) to four or more (22) cystitis episodes per year. The definition used in three of the trials (17, 19, 22, 23) was not stated at all. Moreover, the prevention trials included not only healthy women with a history of recurrent cystitis, but also women suffering from an acute episode of recurrent cystitis (17, 19). This makes it difficult to distinguish the preventive effect from the therapeutic effect.
The trials on the treatment of recurrent urinary tract infections are of limited informative value. The prevailing opinion at present is that acute UTIs should be treated in the same way regardless of whether they are recurrent or non-recurrent. Both of the treatment trials had short follow-up periods of six and three months, respectively (14, 23), and, in one of them, no definition of recurrent UTI was given (23). The data in these reports do not enable us to determine whether acute episodes of recurrent UTIs should be treated differently than non-recurrent UTIs, or whether phytotherapeutic agents are suitable in such cases. The trials concerning non-recurrent UTIs show that phytotherapeutic agents can be used for treatment instead of antibiotics (27, 28). In the prevention trials, too, the period of follow-up after therapy administered was often short: three months (15), a median of 168 days (18), or limited to the time that the patient was taking the drug (16, 17, 19, 20, 22, 24). Six of the ten prevention trials that were identified included cranberry-based single agents or combined preparations, with different mode of administration: beverages (16, 18, 19), capsules (15, 17, 20, 24), or sachets (25). The quantities of various ingredients were sometimes not stated (25) or varied widely across trials: for example, proanthocyanidin levels ranged from 1.4 mg per capsule (20) to 40 mg per drink (19). Because of this marked heterogeneity a meta-analysis for cranberry products was not possible. Most of the trials involving cranberry-based single or combination products that are included in the present review revealed a lower recurrence rate with cranberry products than with no treatment or placebo (16, 20, 24, 25). Two meta-analyses confirmed this preventive effect for women with recurrent UTIs (11, 29), although a Cochrane review published in 2012 did not find any such effect (12). The trials included in these three meta-analyses were markedly heterogeneous, and their results must therefore be interpreted with caution (11, 12, 29). One option discussed in a systematic review was to use cranberry products as an adjuvant preventive measure (29). More recent trials mainly used combined preparations of cranberries and lactobacilli (24, 25), which also lessen the recurrence rate (24, 25). The effect of lactobacilli alone on recurrent cystitis has already been reviewed in two meta-analyses, without any clear superiority to placebo or cranberry monotherapy (30, 31). The trials did not investigate whether the preventive effect is due to phytotherapeutic agents or, for example, to lactobacilli. In this review, a combined product (600 mg cranberry extract, 400 mg propolis, 5 mg zinc) without lactobacilli was considered: this was not associated with any difference in the incidence of recurrent cystitis compared to placebo, but it did prolong the mean time to the first cystitis episode to a statistically significant extent (69.9 ± 45.8 days versus 43.3 ± 45.9 days with placebo; p = 0.0258) (22).
Aside from the cranberry trials, a trial involving Seidlitzia rosmarinus was identified that had a low risk of bias; the incidence of cystitis was lower with this treatment than with placebo, even four months after the two months of treatment were over. Seidlitzia rosmarinus was previously studied in a pilot trial in men with benign prostatic hyperplasia, in which it led to a significant improvement of urologic symptoms (32).
The present review has a number of limitations. All of the included trials were restricted to women with recurrent cystitis, and their results cannot be extrapolated to men. The ten-year search period was chosen to take account of current developments, such as the use of combined drugs. Moreover, only RCTs that were published in either German or English were considered. One may assume, however, that these inclusion criteria succeeded in identifying the most relevant trials with the best available evidence on efficacy.
Overview
This systematic review shows that phytotherapeutic agents are a valid option for the prevention and treatment of recurrent cystitis in women, with few associated adverse events. The use of cranberry products as single agents or in combination was found to lower the recurrence rate in some trials. Because of the marked heterogeneity among trials, the evidence does not yet permit any conclusion about the efficacy of phytotherapeutic agents against recurrent cystitis. Further, methodologically improved trials should be conducted.
Supplementary Material
eMETHODS
Database searching strategies.
Search date: 23 August 2021
Cochrane Library
Embase
Medline
1) Urinary Tract Infection*:ti,ab,kw
2) [mh „Urinary Tract Infections“]
3) UTI?:ti,ab,kw
4) [mh „Cystitis“]
5) cystitis:ti,ab,kw
6) (recurren* or prophyla* or prevent* or therap* or treat*):ti,ab,kw
7) #1 or #2 or #3 or #4 or #5
8) #6 and #7
9) [mh „Phytotherapy“]
10) (phytotherap* or phyto-therap* or phytomedic* or phyto-medic* or phytopharma* or phyto-pharma* or phytodrug* or phyto-drug*):ti,ab,kw
11) [mh „Herbal Medicine“]
12) (herb* or plant*):ti,ab,kw
13) [mh „biological products“]
14) [mh „Plant Extracts“]
15) [mh „Plant Leaves“]
16) [mh „Plant Roots“]
17) [mh „Plants, Medicinal“]
18) [mh „Plant Oils“]
19) [mh „Medicine, Traditional“]
20) (bearberr* or birch* or nettle* or broken herb* or watercress* or cranberr* or strawberr* or ash* or haricot* or silverweed* or goldenrut* or hawkweed* or restharrow* or indigo root* or chestnut* or willowherb* or burdock* or tumeric* or cedar* or arborvitae* or lovage* or dandelion* or mate or horseradish* or orthosiphon* or parsely* or coneflower* or couch grass* or roselle* or hibiscus* or rosemar* or horsetail herb* or blackcurrant* or black currant* or cassis* or centaur* or knotweed* or juniper berr* or frankincense* or sandalwood*):ti,ab,kw
21) (uvae ursi or betula or urticae or herniaria or nasturtium officinale or vaccinium macrocarpon or fragaria or fraxinus or phaseolus vulgaris or potentilla anserine or solidaginis virgaureae or hieracium or ononis or baptisiae tinctoriae or aesculus hippocastanum or epilobium parviflorum or bardanae or curcuma or thuja occidentalis or levisticum officinale or taraxacum or ilex paraguariensis or brassicaceae or orthosiphon or petroselinum crispum or echinaceae purpureae or graminis rhizome or hibiscus or salvia rosmarinus or equisetum arvense or ribes nigrum or centaurium or polygonum aviculare or juniperus or boswellia or santalum album):ti,ab,kw
22) [mh „Arctostaphylos“] or [mh „Betula“] or [mh „Urtica dioica“] or [mh „Caryophyllaceae“] or [mh „Brassicaceae“] or [mh „Vaccinium macrocarpon“] or [mh „Fragaria“] or [mh „Fraxinus“] or [mh „Potentilla“] or [mh „Asteraceae“] or [mh „Fabaceae“] or [mh „Aesculus“] or [mh „Epilobium“] or [mh „Curcuma“] or [mh „Thuja“] or [mh „Levisticum“] or [mh „Ilex paraguariensis“] or [mh „Orthosiphon“] or [mh „Petroselinum“] or [mh „Agropyron“] or [mh „Salvia“] or [mh „Equisetum“] or [mh „Ribes“] or [mh „Centaurium“] or [mh „Polygonum“] or [mh „Juniperus“] or [mh „Santalum“] or [mh „Nasturtium“] or [mh „Taraxacum“] or [mh „Armoracia“] or [mh „Echinacea“] or [mh „Rosmarinus“] or [mh „hibiscus“] or [mh „boswellia“]
23) (b?rentraube* or birke* or brennnessel* or bruchkr?ut* or brunnenkresse* or moosbeer* or erdbeer* or esche or eschenbl?tt* or gartenbohnen* or g?nsefinger* or goldrute* or habicht* or hauhechel* or indigowurzel* or kastanie* or weidenr?s* or klette* or kurkuma* or lebensbaum* or liebst?ckel* or l?wenzahn* or matebl?tt* or meerrettich* or petersilie* or purpursonnenhut* or quecke* or hibiskus* or rosmarin* or schachtelhalm* or johannisbeer* or tausendg?lden* or kn?terich* or wacholder* or weihrauch* or sandelholz*):ti,ab,kw
24) #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
25) #24 and #8 with Publication Year from 2011 to 2021, with Cochrane Library publication date Between Jan 2011 and Dec 2021, in Trials
1) exp urinary tract infection/
2) urinary tract infection*.tw.
3) UTI?.tw.
4) Cystitis/
5) cystitis.tw.
6) exp Urethritis/
7) Urethritis.tw.
8) (recurren* or prophyla* or prevent* or therap* or treat*).tw.
9) 1 or 2 or 3 or 4 or 5 or 6 or 7
10) 8 and 9
11) exp Phytotherapy/
12) (phytotherap* or phyto-therap* or phytomedic* or phyto-medic* or phytopharma* or phyto-pharma* or phytodrug* or phyto-drug*).tw.
13) exp Herbal Medicine/
14) (herb* or plant*).tw.
15) exp biological products/ or exp Plant Extracts/ or exp Plant Leaves/ or exp Plant Roots/ or exp Plants, Medicinal/ or exp Plant Oils/ or exp Medicine, Traditional/
16) (bearberr* or birch* or nettle* or broken herb* or watercress* or cranberr* or strawberr* or ash* or haricot* or silverweed* or goldenrut* or hawkweed* or restharrow* or indigo root* or chestnut* or willowherb* or burdock* or tumeric* or cedar* or arborvitae* or lovage* or dandelion* or mate or horseradish* or orthosiphon* or parsely* or coneflower* or couch grass* or roselle* or hibiscus* or rosemar* or horsetail herb* or blackcurrant* or black currant* or cassis* or centaur* or knotweed* or juniper berr* or frankincense* or sandalwood*).mp.
17) (uvae ursi or betula or urticae or herniaria or nasturtium officinale or vaccinium macrocarpon or fragaria or fraxinus or phaseolus vulgaris or potentilla anserine or solidaginis virgaureae or hieracium or ononis or baptisiae tinctoriae or aesculus hippocastanum or epilobium parviflorum or bardanae or curcuma or thuja occidentalis or levisticum officinale or taraxacum or ilex paraguariensis or brassicaceae or orthosiphon or petroselinum crispum or echinaceae purpureae or graminis rhizome or hibiscus or salvia rosmarinus or equisetum arvense or ribes nigrum or centaurium or polygonum aviculare or juniperus or boswellia or santalum album).mp.
18) exp Arctostaphylos/ or exp Betula/ or exp Urtica dioica/ or exp Caryophyllaceae/ or exp Brassicaceae/ or exp Vaccinium macrocarpon/ or exp Fragaria/ or exp Fraxinus/ or exp Potentilla/ or exp Asteraceae/ or exp Fabaceae/ or exp Aesculus/ or exp Epilobium/ or exp Curcuma/ or exp Thuja/ or exp Levisticum/ or exp Ilex paraguariensis/ or exp Orthosiphon/ or exp Petroselinum/ or exp Agropyron/ or exp Salvia/ or exp Equisetum/ or exp Ribes/ or exp Centaurium/ or exp Polygonum/ or exp Juniperus/ or exp Santalum/ or exp Nasturtium/ or exp Taraxacum/ or exp Armoracia/ or exp Echinacea/ or exp Rosmarinus/ or exp Hibiscus/ or exp Boswellia/
19) (b?rentraube* or birke* or brennnessel* or bruchkr?ut* or brunnenkresse* or moosbeer* or erdbeer* or esche or eschenbl?tt* or gartenbohnen* or g?nsefinger* or goldrute* or habicht* or hauhechel* or indigowurzel* or kastanie* or weidenr?s* or klette* or kurkuma* or lebensbaum* or liebst?ckel* or l?wenzahn* or matebl?tt* or meerrettich* or petersilie* or purpursonnenhut* or quecke* or hibiskus* or rosmarin* or schachtelhalm* or johannisbeer* or tausendg?lden* or kn?terich* or wacholder* or weihrauch* or sandelholz*).mp.
20) 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21) 10 and 20
22) (randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.
23) 21 and 22
24) exp animals/ not humans/
25) 23 not 24
26) limit 25 to (english or german)
27) limit 26 to yr=“2011 -Current“
28) limit 27 to conference abstract status
29) 27 not 28
1) exp Urinary Tract Infections/
2) urinary tract infection*.tw.
3) UTI?.tw.
4) Cystitis/
5) cystitis.tw.
6) exp Urethritis/
7) Urethritis.tw.
8) (recurren* or prophyla* or prevent* or therap* or treat*).tw.
9) 1 or 2 or 3 or 4 or 5 or 6 or 7
10) 8 and 9
11) exp Phytotherapy/
12) (phytotherap* or phyto-therap* or phytomedic* or phyto-medic* or phytopharma* or phyto-pharma* or phytodrug* or phyto-drug*).tw.
13) exp Herbal Medicine/
14) (herb* or plant*).tw.
15) exp biological products/ or exp Plant Extracts/ or exp Plant Leaves/ or exp Plant Roots/ or exp Plants, Medicinal/ or exp Plant Oils/ or exp Medicine, Traditional/
16) (bearberr* or birch* or nettle* or broken herb* or watercress* or cranberr* or strawberr* or ash* or haricot* or silverweed* or goldenrut* or hawkweed* or restharrow* or indigo root* or chestnut* or willowherb* or burdock* or tumeric* or cedar* or arborvitae* or lovage* or dandelion* or mate or horseradish* or orthosiphon* or parsely* or coneflower* or couch grass* or roselle* or hibiscus* or rosemar* or horsetail herb* or blackcurrant* or black currant* or cassis* or centaur* or knotweed* or juniper berr* or frankincense* or sandalwood*).mp.
17) (uvae ursi or betula or urticae or herniaria or nasturtium officinale or vaccinium macrocarpon or fragaria or fraxinus or phaseolus vulgaris or potentilla anserine or solidaginis virgaureae or hieracium or ononis or baptisiae tinctoriae or aesculus hippocastanum or epilobium parviflorum or bardanae or curcuma or thuja occidentalis or levisticum officinale or taraxacum or ilex paraguariensis or brassicaceae or orthosiphon or petroselinum crispum or echinaceae purpureae or graminis rhizome or hibiscus or salvia rosmarinus or equisetum arvense or ribes nigrum or centaurium or polygonum aviculare or juniperus or boswellia or santalum album).mp.
18) (b?rentraube* or birke* or brennnessel* or bruchkr?ut* or brunnenkresse* or moosbeer* or erdbeer* or esche or eschenbl?tt* or gartenbohnen* or g?nsefinger* or goldrute* or habicht* or hauhechel* or indigowurzel* or kastanie* or weidenr?s* or klette* or kurkuma* or lebensbaum* or liebst?ckel* or l?wenzahn* or matebl?tt* or meerrettich* or petersilie* or purpursonnenhut* or quecke* or hibiskus* or rosmarin* or schachtelhalm* or johannisbeer* or tausendg?lden* or kn?terich* or wacholder* or weihrauch* or sandelholz*).mp.
19) exp Arctostaphylos/ or exp Betula/ or exp Urtica dioica/ or exp Caryophyllaceae/ or exp Brassicaceae/ or exp Vaccinium macrocarpon/ or exp Fragaria/ or exp Fraxinus/ or exp Potentilla/ or exp Asteraceae/ or exp Fabaceae/ or exp Aesculus/ or exp Epilobium/ or exp Curcuma/ or exp Thuja/ or exp Levisticum/ or exp Ilex paraguariensis/ or exp Orthosiphon/ or exp Petroselinum/ or exp Agropyron/ or exp Salvia/ or exp Equisetum/ or exp Ribes/ or exp Centaurium/ or exp Polygonum/ or exp Juniperus/ or exp Santalum/ or exp Nasturtium/ or exp Taraxacum/ or exp Armoracia/ or exp Echinacea/ or exp Rosmarinus/ or exp Hibiscus/ or exp Boswellia/
20) 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21) 10 and 20
22) (randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.
23) 21 and 22
24) exp animals/ not humans/
25) 23 not 24
26) limit 25 to (english or german)
27) limit 26 to yr=“2011 -Current“
Table 1. Inclusion and exclusion criteria.
| Study design | Randomized controlled trials |
| Languages | German, English |
| Period | January 2011 – August 2021 |
| Patients |
Inclusion: - healthy adults (>16 years) with a history of recurrent uncomplicated cystitis - adults (+16 years) with an acute episode of recurrent cystitis Exclusion: - patients with complicated urinary tract infections with relevant functional or anatomical abnormalities in the urinary tract (as defined in the AWMF S3 guideline on urinary tract infection [4]). |
| Intervention |
Inclusion: - phytotherapy as monotherapy or as combination therapy (any mode of administration). Exclusion: - combination therapies with antibiotics |
| Control |
Inclusion: - medication (e.g. antibiotics, analgesics) - non-pharmaceutical interventions (e.g., diet, lifestyle, acupuncture) - placebo - no treatment Exclusion: - phytotherapy - combination therapies with phytotherapeutics and non-pharmaceutical or pharmaceutical therapies. |
| Endpoints | - symptom improvement / resolution - symptom duration - adverse events - health-related quality of life - recurrent cystitis (during the follow-up period). - functional impairment in everyday life - antibiotic use in the intervention group - patient satisfaction |
AWMF, Association of the Scientific Medical Societies in Germany
eTable 3. Evidence table for prevention trials.
| Reference | Trial features | Definitions | Intervention | Control | Endpoints | Undesired events | Dropouts | Comments |
| Cranberries | ||||||||
|
Maki 2016 (16) |
373 healthy female patients (age 20–70) 2013 – 2015 Follow-up only in the treatment period Trial centers: 18 (1 in France) USA, France |
UTI: no definition rUTI : ≥=2 episodes of UTI requiring professional treatment in the past year, of which ≥=1 UTI was treated in the 6 months before trial entry (self-reported) |
Cranberry 185 patients 24 weeks Cranberry drink (240 ml): filtered water, cranberry juice from concentrate, fructose, natural flavors, pectin, sodium citrate, acesulfame potassium, sucralose q.d. |
Placebo n = 188 24 weeks Placebo drink (240 ml): filtered water, fructose, dextrose, citric acid, quinic acid, malic acid, natural flavors, pectin, potassium citrate, sodium citrate, Red 40, Blue 1, acesulfame potassium, sucralose q.d. |
Cranberry vs. placebo Reported symptomatic UTI episodes: •0: 82% (152/185) vs. 73% (138/188) •1: 15% (27/185) vs. 19% (36/188) •2: 3% (6/185) vs. 6% (11/188) •3: 0% (0/185) vs. 2% (3/188) • ≥=1: 18% (33/185) vs. 27% (50/188) Total number of UTIs : 39 vs. 67 Total UTI with pyuria: 32 vs. 53 Incidence ratio •UTI: 0.61 (95% CI 0.41, 0.91); p = 0.016. •UTI with pyuria: 0.63 (95% CI 0.40, 0.97); p=0.037 |
Cranberry vs. placebo Adverse events in ≥=5% of patients: •headache: 16/185 (9%) vs. 12/188 (6%) •sinusitis: 10/185 (5%) vs. 6/188 (3%) •upper respiratory tract infections: 13/185 (7%) vs. 13/188 (7%) Significant differences in nausea: more than usual: 3/185 (2%) vs. 11/188 (6%); p=0.044 Serious adverse events were probably not related to treatment. |
Cranberry vs. placebo Trial dropouts: 13.8% (26/188) vs. 13.5% (25/185) |
Supported by Ocean Spray; cranberry beverages were provided by Ocean Spray Cranberries Inc. Three authors received research grants and two authors are employees of Ocean Spray Cranberries Inc. |
|
Vostalova 2015 (20) |
182 healthy female patients (>18 years) 2010 – 2011 Follow-up only in the treatment period Single-center Czech Republic |
UTI: bacteriuria+ ≥=1 symptom: pollakisuria, burning on urination, hematuria, cloudy or foul-smelling urine, abdominal pain, itching, fever, and dysuria. rUTI : ≥=2 episodes of symptomatic UTI in the past 12 months |
Cranberry 89 patients 6 months Capsules with 100% cranberry fruit powder (250 mg) 1.4 mg proanthocyanidins per capsule b.i.d. |
Placebo n = 93 6 months Placebo capsules with the same appearance Low density maltodextrin, canola oil, Red 40 Lake, sodium aluminosilicate, Blue 1 Lake b.i.d. |
Cranberry vs. placebo Within 6 months: • ≥=1 UTI: 11% (9/83) vs. 26% (24/93); p=0.04. •2 UTI: 1% (1/83) vs. 6% (6/93). Relative risk reduction : 58 Cumulative incidence of UTI over 6 months:9% vs. 19%. |
No adverse outcomes were reported |
Cranberry vs. placebo Dropouts: 11% (10/89) vs. 8% (7/93) |
Financial support from Palacky University, Olomouc No conflicts of interest present |
|
Takahashi 2013 (19) |
213 women with cystitis (age 20–79) 2007 – 2009 Follow-up only in the treatment period Trial centers: 40 Japan |
UTI: no definition rUTI : no definition |
Cranberry 107 patients (82 with uncomplicated cystitis) 24 weeks Cranberry juice (125 ml) with proanthocyanidins (40 mg) q.d. |
Placebo n = 106 (88 with uncomplicated cystitis) 24 weeks Placebo juice (125 ml) Identical color & taste q.d. |
Cranberry vs. placebo rUTI •total: 27% (22/82) vs. 39% (34/88); p=0.1300 •<50 years: 22% (6/27) vs. 12% (3/25); p=0.3623 •≥=50 years: 29% (16/55) vs. 49% (31/63); p=0.0425 Multivariate analysis (≥=50 years): HR: 1.037 (95% CI; 1.002–1.073); p=0.038 |
Discomfort when drinking cranberry juice for the first time: 0.93% (1/107) | After an rUTI was diagnosed, subjects stopped taking the drink and were excluded from the trial | Subgroup analysis Target sample size not reached Data collection supported in part by Kikkoman Food Products Company and The Nisshin Oillio Group, Ltd, Tokyo, Japan |
|
Stapleton 2012 (18) |
176 healthy female patients (age 18 – 45 years, premenopausal) 2005 – 2008 Median follow-up: 168 days Study centers: 2 USA |
UTI: confirmed clinically or by urine culture rUTI : ≥=1 physician-diagnosed UTI in the past 12 months |
Cranberry (total) 120 patients No data on the duration of intake Cranberry juice: 27% cranberry juice and sucralose Cranberry (high dose) n = 60 Cranberry juice (≈237 ml) Cranberry (low dose) n = 60 Cranberry juice (≈118 ml) |
Placebo (total) 56 patients No data on the duration of intake Identical color & taste Placebo (high dose) n = 27 Placebo juice (≈237 ml) Placebo (low dose) n = 29 Placebo juice (≈118 ml) |
Cranberry (total) vs. placebo (total) UTI in follow-up: •total: 28% (33/120) vs. 30% (17/56); p=0.70 •>1 UTI: 8% (10/120) vs. 7% (4/56) Cumulative UTI rate at 6 months: 0.29 (95% CI, 0.21–0.38) vs. 0.37 (95% CI, 0.25–0.54); p=0.82 Adjusted HR for UTI: 0.68 (95% CI, 0.33–1.39; p=0.29) Dose-specific analyses were not performed because the combined dose comparisons were not significant. |
No serious adverse events Adverse events: Gastrointestinal symptoms (constipation, heartburn, soft stools), vaginal symptoms (itching, dryness), other symptoms (migraine) Cranberry (total) vs. placebo (total) Reported mild adverse events: 24% (29/120) vs. 13% (7/56); p=0.07 |
Cranberry high vs. low dose Study completed: 73% (44/60) vs. 68% (41/60) Placebo high vs. low dose Study completed: 66% (18/27) vs. 59% (17/29) |
The study was terminated before the target sample size could be reached because of administrative and budgetary problems. Supported by National Center for Complementary and Alternative Medicine, publication was made possible by Clinical and Translational Science Award grant from the National Center for Research Resources, a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research. |
|
Beerepoot 2011 (15) |
221 healthy female patients (≥=18 years, premenopausal) 2005 – 2007 Follow-up: 3 months Trial centers: 10 Netherlands |
UTI: Self-reportedr UTI : ≥=3 symptomatic UTIs in the year before trial entry |
Cranberry 111 patients 12 months Capsule with cranberry whole fruit concentrate 500 mg + placebo tablet 1x capsule (b.i.d.) + 1 x placebo tablet at night |
TMP-SMX 110 patients 12 months TMP-SMX tablet (480 mg) + placebo capsule 1 x TMP-SMX at night + 1 x placebo capsule (b.i.d.) |
TMP-SMX vs. cranberry After 12 months Mean number of rUTIs (95% CI): 1.8 (0.8–2.7) vs. 4.0 (2.3–5.6); p=0.02. Median time to first rUTI (95% CI): 8 (6–10) vs. 4 (3–6) months; p = 0.03. Median number of antibiotic prescriptions during the intervention period (interquartile range): 0.5 (0–2) vs. 1 (0–2). After 15 months Mean number of rUTIs (95% CI): 0.5 (0.3–0.7) vs. 0.7 (0.4–0.9); p=0.30. |
no statistical differences regarding adverse events Serious adverse events •TMP-SMX: 0.91% (1/110) Stevens-Johnson syndrome (led to treatment discontinuation) •Cranberry: none |
TMP-SMX vs. cranberry 89% (98/110) vs. 98% (109/111) Follow-up at 6 months: 61% (67/110) vs. 63% (70/111) Follow-up at 12 months: 52% (57/110) vs. 48% (53/111) Follow-up at 15 months: 49% (54/110) vs. 41% (45/111) |
Supported by Netherlands Organization for Health Research and Development Cranberry and Placebo Capsules from Springfield Nutraceuticals BV, Oud Beijerland, the Netherlands |
|
Sengupta 2011 (17) |
60 female patients with UTI (age 18 – 40) No data on study period, follow-up, or participating trial centers. India |
UTI: pain on urination and frequent urination, blood in urine, or suprapubic pain rUTI : no data |
Cranberry (total) 44 patients 90 days standardized whole cranberry powder (1.5% proanthocyanidins, carboxymethylcellulose as filler for the high dose). Cranberry (low dose) n = 21 Cranberry capsule (250 mg) b.i.d. Cranberry (high dose) n = 23 Cranberry capsule (500 mg) b.i.d. |
No treatment 16 patients 90 days |
Symptom improvement: •Cranberry (total): 41% (18/44) complete relief and remission of urologic symptoms •Cranberry (total): significant improvement from 10-day evaluation relative to baseline (data not shown). •Untreated: no improvement Use of emergency medication (norfloxacin 2x a day for 7 days) •Low dose: 10% (2/21) •High dose: 9% (2/23) •Untreated: 25 % (4/16) |
No serious adverse events General weakness, lower abdominal pain: •low dose: 14% (3/21) •high dose: 4% (1/23) Stomach burning, general weakness: without treatment: 13% (2/16) mild fever high dose: 13% (3/23) Heartburn after 60 days: high dose: 9% (2/23) |
None of the subjects dropped out of the trial because of side effects. |
Different baseline values for the presence of E. coli in urine •low dose: 67% (14/21) •high dose: 74% (17/23) •no treatment: 31% (4/13) |
| Seidlitzia rosmarinus | ||||||||
|
Kamalifard 2020 (21) |
126 healthy female patients (age 15 – 49) 2017 – 2018 Follow-up: 4 months multicenter Iran |
Cystitis: symptomatic acute or chronic cystitis + main symptoms: frequent urination, burning during urination, urinary urgency, oliguria and rarely suprapubic pressure pain Recurrent cystitis : ≥=2 cystitis in 6 months or ≥=3 in 1 year (as diagnosed by a physician) |
Seidlitzia rosmarinus 63 patients 2 months Capsule with ground Seidlitzia rosmarinus powder (500 mg) 3 capsules t.i.d. |
Placebo 63 patients 2 months Placebo capsule (500 mg strength) Same color, smell and taste 3 capsules t.i.d. |
Seidlitzia rosmarinus vs. placebo Cystitis incidence rate: •At 2 months: 19% (11/58) vs. 55% (32/58); OR: 0.19 (95% CI, 0.08, 0.43); p<0.001. •At 4 months: 22% (13/58) vs. 57% (33/58); OR: 0.21 (95% CI, 0.98, 0.49); p<0.001. •At 6 months: 33% (19/58) vs. 73% (43/59); OR: 0.18 (95% CI, 0.08, 0.40); p<0.001. Incidence of recurrent cystitis: 14% (8/58) vs. 66% (39/59); OR: 0.08 (95% CI, 0.03, 0.20); p<0.001 |
No side effects in either group |
Seidlitzia rosmarinus vs. placebo: Dropouts : 8% (5/63) vs. 6% (4/63) |
Funding: Tabriz University of Medical Sciences No conflicts of interest declared |
| Combined preparations | ||||||||
|
Murina 2021 (25) |
55 healthy female patients (age 20–46, premenopausal) No data on the study period Follow-up: 150 days monocentric Country: no information |
UTI: ≥=10³ CFU/ml in urine culture and lower urinary tract symptoms rUTI: ≥=3 urinary tract infections in the past year or two episodes in the past 6 months |
Combined preparation 38 patients 90 days sachet with cranberry, Lactobacillus paracasei LC11, D-mannose Group 1 n = 19 q.d. for 10 days a month for 90 days Group 2 n= 19 q.d. for 90 days |
No treatment 17 patients 90 days |
No UTI •Group 1: 65.8 % (12/19) •Group 2: 68.8 % (13/19) •Control group: 36.9% (6/17) p=0,05 1 UTI •Group 1: 18.2 % (4/19) •Group 2: 15.6 % (3/19) •Control group: 10.2 % (2/17) Not significant ≥=2 UTI •Group 1: 16 % (3/19) •Group 2: 15.6 % (3/19) •Control group: 52.9% (9/17) p<0.01 |
No adverse events | no dropouts | no sponsorship received No conflicts of interest declared Medical writing assistance was sponsored by Montefarmaco |
|
Bruyère 2019 (22) |
86 healthy female patients (>18 years) 2013 – 2015 Follow-up only in the treatment period Trial centers: 9 France |
Cystitis: no definition Recurrent cystitis : ≥=4 cystitis episodes in the past 12 months |
Combined preparation 43 patients 6 months Capsule with 600 mg cranberry extract, 400 mg propolis, 5 mg zinc First day: 4 capsules From the second day: 2 capsules daily |
Placebo 43 patients 6 months 1 placebo capsule First day: 4 capsules From the second day: 2 capsules daily |
Combined drug vs. placebo: ≥=1 cystitis: 2.3 ± 1.8 vs. 3.1 ± 1.8; p=0.09 No significant change in quality of life |
Adverse events: indigestion Serious adverse events were probably not related to treatment. |
Combined drug vs. placebo Received no intervention: 2% (1/43) vs. 0% (0/43) Dropouts: 2% (1/43) vs. 0% (0/43) |
Supported by Nutrivercell No conflicts of interest declared |
|
Koradia 2019 (24) |
90 healthy female patients (aged 18 – 55) 2016–2018 Follow-up only in the treatment period Study centers: 4 India |
UTI: no definition rUTI : ≥=2 episodes of uncomplicated acute UTI in the past 6 months or ≥= 3 episodes of uncomplicated acute UTI in the past 12 months |
Combined preparation 45 patients 26 weeks Capsule with 18 mg cranberry extract (Vaccinium macrocarpon); 21 mg probiotics (Lactobacillus acidophilus, Lactobacillus plantarum); 160 μg vitamin A (retinyl acetate) b.i.d. |
Placebo 45 patients 26 weeks 1 placebo capsule Same color (cellulose) b.i.d. |
Combined drug vs. placebo rUTI after 26 weeks: 9% (4/44) vs. 33% (15/45); p=0.0053 Duration of illness: mean (SD): 5 (0.8) vs. 12.2 (6.5) days; p=0.0095 Antibiotics needed: 8% (3/40) vs. 27% (11/41); p= 0.0372. |
Adverse events requiring treatment • Combined drug : 3 (6.8%) (abdominal distension and diarrhea) • Placebo: 0 There were no serious adverse events or adverse events that led to dropping out of the trial. |
Combined drug vs. placebo Trial completed: 89% (40/45) vs. 91% (41/45) |
Supported by ADM Protexin Ltd. Medical writing assistance: Dr Steve Clissold (Content Ed Net, UK) supported by ADM Protexi Ltd, Lopen Head, Somerset, UK; One author is an employee of ADM Protexin Ltd. |
b.i.d.: twice daily, E. coli: Escherichia coli, CI: confidence interval, HR: hazard ratio, LVX: levofloxacin, q.d.: once daily, rUTI: recurrent urinary tract infection, SD: standard deviation,t.i.d.: three times daily, TMP-SMX: trimethoprim-sulfamethoxazole, UTI: urinary tract infection.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. med. habil. Kranz has served as a paid consultant for, and received lecture honoraria from, Bionorica. Prof. Dr. med. Wagenlehner has served as a paid consultant for, and received lecture honoraria and reimbursement of travel expenses from, the same company, of whose advisory board he is a member.
The remaining authors state that they have no conflict of interest.
References
- 1.Harding GK, Ronald AR. The management of urinary infections: what have we learned in the past decade? Int J Antimicrob Agents. 1994;4:83–88. doi: 10.1016/0924-8579(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 2.Butler CC, Hawking MK, Quigley A, McNulty CA. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: a population-based survey. Br J Gen Pract. 2015;65:e702–e707. doi: 10.3399/bjgp15X686965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranz J, Wagenlehner FME, Schneidewind L. [Complicated urinary tract infections] Urologe A. 2020;59:1480–1485. doi: 10.1007/s00120-020-01343-1. [DOI] [PubMed] [Google Scholar]
- 4.Interdisziplinäre S3-Leitlinie. Epidemiologie, Diagnostik, Therapie, Prävention und Management unkomplizierter, bakterieller, ambulant erworbener Harnwegsinfektionen bei erwachsenen Patienten. AWMF Register-Nr.: 043/044 2017; Langversion 1.1-2 [Google Scholar]
- 5.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Wagenlehner FM, Bartoletti R, Cek M, et al. Antibiotic stewardship: a call for action by the urologic community. Eur Urol. 2013;64:358–360. doi: 10.1016/j.eururo.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8 doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ventola CL. The antibiotic resistance crisis: part 1. P T. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 9.Kranz J, Schmidt S, Schneidewind L. Current evidence on nonantibiotic prevention of recurrent urinary tract infections. Eur Urol Focus. 2019;5:17–19. doi: 10.1016/j.euf.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15:750–776. doi: 10.1038/s41585-018-0106-x. [DOI] [PubMed] [Google Scholar]
- 11.Fu Z, Liska D, Talan D, Chung M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: a systematic review and meta-analysis. J Nutr. 2017;147:2282–2288. doi: 10.3945/jn.117.254961. [DOI] [PubMed] [Google Scholar]
- 12.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD001321.pub5. Cd001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu SW, Guo J, Wu WK, Chen ZL, Zhang N. Treatment of uncomplicated recurrent urinary tract infection with chinese medicine formula: a randomized controlled trial. Chin J Integr Med. 2019;25:16–22. doi: 10.1007/s11655-017-2960-4. [DOI] [PubMed] [Google Scholar]
- 15.Beerepoot MA, ter Riet G, Nys S, et al. Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind noninferiority trial in premenopausal women. Arch Intern Med. 2011;171:1270–1278. doi: 10.1001/archinternmed.2011.306. [DOI] [PubMed] [Google Scholar]
- 16.Maki KC, Kaspar KL, Khoo C, Derrig LH, Schild AL, Gupta K. Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am J Clin Nutr. 2016;103:1434–1442. doi: 10.3945/ajcn.116.130542. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta K, Alluri KV, Golakoti T, et al. A randomized, double blind, controlled, dose dependent clinical trial to evaluate the efficacy of a proanthocyanidin standardized whole cranberry (Vaccinium macrocarpon) powder on infections of the urinary tract. Current Bioactive Compounds. 2011;7:39–46. [Google Scholar]
- 18.Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87:143–150. doi: 10.1016/j.mayocp.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi S, Hamasuna R, Yasuda M, et al. A randomized clinical trial to evaluate the preventive effect of cranberry juice (UR65) for patients with recurrent urinary tract infection. J Infect Chemother. 2013;19:112–117. doi: 10.1007/s10156-012-0467-7. [DOI] [PubMed] [Google Scholar]
- 20.Vostalova J, Vidlar A, Simanek V, et al. Are high proanthocyanidins key to cranberry efficacy in the prevention of recurrent urinary tract infection? Phytother Res. 2015;29:1559–1567. doi: 10.1002/ptr.5427. [DOI] [PubMed] [Google Scholar]
- 21.Kamalifard M, Abbasalizadeh S, Mirghafourvand M, et al. The effect of Seidlitzia rosmarinus (eshnan) on the prevention of recurrent cystitis in women of reproductive age: a randomized, controlled, clinical trial. Phytother Res. 2020;34:418–427. doi: 10.1002/ptr.6534. [DOI] [PubMed] [Google Scholar]
- 22.Bruyère F, Azzouzi AR, Lavigne JP, et al. A multicenter, randomized, placebo-controlled study evaluating the efficacy of a combination of propolis and cranberry (Vaccinium macrocarpon) (DUAB®) in preventing low urinary tract infection recurrence in women complaining of recurrent cystitis. Urol Int. 2019;103:41–48. doi: 10.1159/000496695. [DOI] [PubMed] [Google Scholar]
- 23.Cai T, Cocci A, Tiscione D, et al. L-methionine associated with hibiscus sabdariffa and boswellia serrata extracts are not inferior to antibiotic treatment for symptoms relief in patients affected by recurrent uncomplicated urinary tract infections: focus on antibiotic-sparing approach. Arch Ital Urol Androl. 2018;90:97–100. doi: 10.4081/aiua.2018.2.97. [DOI] [PubMed] [Google Scholar]
- 24.Koradia P, Kapadia S, Trivedi Y, Chanchu G, Harper A. Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: a controlled pilot study. Expert Rev Anti Infect Ther. 2019;17:733–740. doi: 10.1080/14787210.2019.1664287. [DOI] [PubMed] [Google Scholar]
- 25.Murina F, Vicariotto F, Lubrano C. Efficacy of an orally administered combination of lactobacillus paracasei LC11, cranberry and D-mannose for the prevention of uncomplicated, recurrent urinary tract infections in women. Urologia. 2021;88:64–68. doi: 10.1177/0391560320957483. [DOI] [PubMed] [Google Scholar]
- 26.European Association of Urology Guidelines on Urological Infections. www.uroweb.org/guideline/urological-infections (last accessed on 21 September 2021) [Google Scholar]
- 27.Rechberger E, Rechberger T, Wawrysiuk S, et al. A randomized clinical trial to evaluate the effect of canephron N in comparison to ciprofloxacin in the prevention of postoperative lower urinary tract infections after midurethral sling surgery. J Clin Med. 2020;9 doi: 10.3390/jcm9113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagenlehner FM, Abramov-Sommariva D, Höller M, Steindl H, Naber KG. Non-antibiotic herbal therapy (BNO 1045) versus antibiotic therapy (fosfomycin trometamol) for the treatment of acute lower uncomplicated urinary tract infections in women: a double-blind, parallel-group, randomized, multicentre, non-inferiority phase III trial. Urol Int. 2018;101:327–336. doi: 10.1159/000493368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia JY, Yang C, Xu DF, Xia H, Yang LG, Sun GJ. Consumption of cranberry as adjuvant therapy for urinary tract infections in susceptible populations: a systematic review and meta-analysis with trial sequential analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0256992. e0256992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2013;190:1981–1989. doi: 10.1016/j.juro.2013.04.142. [DOI] [PubMed] [Google Scholar]
- 31.Grin PM, Kowalewska PM, Alhazzan W, Fox-Robichaud AE. Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can J Urol. 2013;20:6607–6614. [PubMed] [Google Scholar]
- 32.Heidari M, Hosseinabadi R, Anbari K, Pournia Y, Tarverdian A. Seidlitzia rosmarinus for lower urinary tract symptoms associated with benign prostatic hyperplasia: a pilot randomized controlled clinical trial. Complement Ther Med. 2014;22:607–613. doi: 10.1016/j.ctim.2014.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMETHODS
Database searching strategies.
Search date: 23 August 2021
Cochrane Library
Embase
Medline
1) Urinary Tract Infection*:ti,ab,kw
2) [mh „Urinary Tract Infections“]
3) UTI?:ti,ab,kw
4) [mh „Cystitis“]
5) cystitis:ti,ab,kw
6) (recurren* or prophyla* or prevent* or therap* or treat*):ti,ab,kw
7) #1 or #2 or #3 or #4 or #5
8) #6 and #7
9) [mh „Phytotherapy“]
10) (phytotherap* or phyto-therap* or phytomedic* or phyto-medic* or phytopharma* or phyto-pharma* or phytodrug* or phyto-drug*):ti,ab,kw
11) [mh „Herbal Medicine“]
12) (herb* or plant*):ti,ab,kw
13) [mh „biological products“]
14) [mh „Plant Extracts“]
15) [mh „Plant Leaves“]
16) [mh „Plant Roots“]
17) [mh „Plants, Medicinal“]
18) [mh „Plant Oils“]
19) [mh „Medicine, Traditional“]
20) (bearberr* or birch* or nettle* or broken herb* or watercress* or cranberr* or strawberr* or ash* or haricot* or silverweed* or goldenrut* or hawkweed* or restharrow* or indigo root* or chestnut* or willowherb* or burdock* or tumeric* or cedar* or arborvitae* or lovage* or dandelion* or mate or horseradish* or orthosiphon* or parsely* or coneflower* or couch grass* or roselle* or hibiscus* or rosemar* or horsetail herb* or blackcurrant* or black currant* or cassis* or centaur* or knotweed* or juniper berr* or frankincense* or sandalwood*):ti,ab,kw
21) (uvae ursi or betula or urticae or herniaria or nasturtium officinale or vaccinium macrocarpon or fragaria or fraxinus or phaseolus vulgaris or potentilla anserine or solidaginis virgaureae or hieracium or ononis or baptisiae tinctoriae or aesculus hippocastanum or epilobium parviflorum or bardanae or curcuma or thuja occidentalis or levisticum officinale or taraxacum or ilex paraguariensis or brassicaceae or orthosiphon or petroselinum crispum or echinaceae purpureae or graminis rhizome or hibiscus or salvia rosmarinus or equisetum arvense or ribes nigrum or centaurium or polygonum aviculare or juniperus or boswellia or santalum album):ti,ab,kw
22) [mh „Arctostaphylos“] or [mh „Betula“] or [mh „Urtica dioica“] or [mh „Caryophyllaceae“] or [mh „Brassicaceae“] or [mh „Vaccinium macrocarpon“] or [mh „Fragaria“] or [mh „Fraxinus“] or [mh „Potentilla“] or [mh „Asteraceae“] or [mh „Fabaceae“] or [mh „Aesculus“] or [mh „Epilobium“] or [mh „Curcuma“] or [mh „Thuja“] or [mh „Levisticum“] or [mh „Ilex paraguariensis“] or [mh „Orthosiphon“] or [mh „Petroselinum“] or [mh „Agropyron“] or [mh „Salvia“] or [mh „Equisetum“] or [mh „Ribes“] or [mh „Centaurium“] or [mh „Polygonum“] or [mh „Juniperus“] or [mh „Santalum“] or [mh „Nasturtium“] or [mh „Taraxacum“] or [mh „Armoracia“] or [mh „Echinacea“] or [mh „Rosmarinus“] or [mh „hibiscus“] or [mh „boswellia“]
23) (b?rentraube* or birke* or brennnessel* or bruchkr?ut* or brunnenkresse* or moosbeer* or erdbeer* or esche or eschenbl?tt* or gartenbohnen* or g?nsefinger* or goldrute* or habicht* or hauhechel* or indigowurzel* or kastanie* or weidenr?s* or klette* or kurkuma* or lebensbaum* or liebst?ckel* or l?wenzahn* or matebl?tt* or meerrettich* or petersilie* or purpursonnenhut* or quecke* or hibiskus* or rosmarin* or schachtelhalm* or johannisbeer* or tausendg?lden* or kn?terich* or wacholder* or weihrauch* or sandelholz*):ti,ab,kw
24) #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
25) #24 and #8 with Publication Year from 2011 to 2021, with Cochrane Library publication date Between Jan 2011 and Dec 2021, in Trials
1) exp urinary tract infection/
2) urinary tract infection*.tw.
3) UTI?.tw.
4) Cystitis/
5) cystitis.tw.
6) exp Urethritis/
7) Urethritis.tw.
8) (recurren* or prophyla* or prevent* or therap* or treat*).tw.
9) 1 or 2 or 3 or 4 or 5 or 6 or 7
10) 8 and 9
11) exp Phytotherapy/
12) (phytotherap* or phyto-therap* or phytomedic* or phyto-medic* or phytopharma* or phyto-pharma* or phytodrug* or phyto-drug*).tw.
13) exp Herbal Medicine/
14) (herb* or plant*).tw.
15) exp biological products/ or exp Plant Extracts/ or exp Plant Leaves/ or exp Plant Roots/ or exp Plants, Medicinal/ or exp Plant Oils/ or exp Medicine, Traditional/
16) (bearberr* or birch* or nettle* or broken herb* or watercress* or cranberr* or strawberr* or ash* or haricot* or silverweed* or goldenrut* or hawkweed* or restharrow* or indigo root* or chestnut* or willowherb* or burdock* or tumeric* or cedar* or arborvitae* or lovage* or dandelion* or mate or horseradish* or orthosiphon* or parsely* or coneflower* or couch grass* or roselle* or hibiscus* or rosemar* or horsetail herb* or blackcurrant* or black currant* or cassis* or centaur* or knotweed* or juniper berr* or frankincense* or sandalwood*).mp.
17) (uvae ursi or betula or urticae or herniaria or nasturtium officinale or vaccinium macrocarpon or fragaria or fraxinus or phaseolus vulgaris or potentilla anserine or solidaginis virgaureae or hieracium or ononis or baptisiae tinctoriae or aesculus hippocastanum or epilobium parviflorum or bardanae or curcuma or thuja occidentalis or levisticum officinale or taraxacum or ilex paraguariensis or brassicaceae or orthosiphon or petroselinum crispum or echinaceae purpureae or graminis rhizome or hibiscus or salvia rosmarinus or equisetum arvense or ribes nigrum or centaurium or polygonum aviculare or juniperus or boswellia or santalum album).mp.
18) exp Arctostaphylos/ or exp Betula/ or exp Urtica dioica/ or exp Caryophyllaceae/ or exp Brassicaceae/ or exp Vaccinium macrocarpon/ or exp Fragaria/ or exp Fraxinus/ or exp Potentilla/ or exp Asteraceae/ or exp Fabaceae/ or exp Aesculus/ or exp Epilobium/ or exp Curcuma/ or exp Thuja/ or exp Levisticum/ or exp Ilex paraguariensis/ or exp Orthosiphon/ or exp Petroselinum/ or exp Agropyron/ or exp Salvia/ or exp Equisetum/ or exp Ribes/ or exp Centaurium/ or exp Polygonum/ or exp Juniperus/ or exp Santalum/ or exp Nasturtium/ or exp Taraxacum/ or exp Armoracia/ or exp Echinacea/ or exp Rosmarinus/ or exp Hibiscus/ or exp Boswellia/
19) (b?rentraube* or birke* or brennnessel* or bruchkr?ut* or brunnenkresse* or moosbeer* or erdbeer* or esche or eschenbl?tt* or gartenbohnen* or g?nsefinger* or goldrute* or habicht* or hauhechel* or indigowurzel* or kastanie* or weidenr?s* or klette* or kurkuma* or lebensbaum* or liebst?ckel* or l?wenzahn* or matebl?tt* or meerrettich* or petersilie* or purpursonnenhut* or quecke* or hibiskus* or rosmarin* or schachtelhalm* or johannisbeer* or tausendg?lden* or kn?terich* or wacholder* or weihrauch* or sandelholz*).mp.
20) 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21) 10 and 20
22) (randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.
23) 21 and 22
24) exp animals/ not humans/
25) 23 not 24
26) limit 25 to (english or german)
27) limit 26 to yr=“2011 -Current“
28) limit 27 to conference abstract status
29) 27 not 28
1) exp Urinary Tract Infections/
2) urinary tract infection*.tw.
3) UTI?.tw.
4) Cystitis/
5) cystitis.tw.
6) exp Urethritis/
7) Urethritis.tw.
8) (recurren* or prophyla* or prevent* or therap* or treat*).tw.
9) 1 or 2 or 3 or 4 or 5 or 6 or 7
10) 8 and 9
11) exp Phytotherapy/
12) (phytotherap* or phyto-therap* or phytomedic* or phyto-medic* or phytopharma* or phyto-pharma* or phytodrug* or phyto-drug*).tw.
13) exp Herbal Medicine/
14) (herb* or plant*).tw.
15) exp biological products/ or exp Plant Extracts/ or exp Plant Leaves/ or exp Plant Roots/ or exp Plants, Medicinal/ or exp Plant Oils/ or exp Medicine, Traditional/
16) (bearberr* or birch* or nettle* or broken herb* or watercress* or cranberr* or strawberr* or ash* or haricot* or silverweed* or goldenrut* or hawkweed* or restharrow* or indigo root* or chestnut* or willowherb* or burdock* or tumeric* or cedar* or arborvitae* or lovage* or dandelion* or mate or horseradish* or orthosiphon* or parsely* or coneflower* or couch grass* or roselle* or hibiscus* or rosemar* or horsetail herb* or blackcurrant* or black currant* or cassis* or centaur* or knotweed* or juniper berr* or frankincense* or sandalwood*).mp.
17) (uvae ursi or betula or urticae or herniaria or nasturtium officinale or vaccinium macrocarpon or fragaria or fraxinus or phaseolus vulgaris or potentilla anserine or solidaginis virgaureae or hieracium or ononis or baptisiae tinctoriae or aesculus hippocastanum or epilobium parviflorum or bardanae or curcuma or thuja occidentalis or levisticum officinale or taraxacum or ilex paraguariensis or brassicaceae or orthosiphon or petroselinum crispum or echinaceae purpureae or graminis rhizome or hibiscus or salvia rosmarinus or equisetum arvense or ribes nigrum or centaurium or polygonum aviculare or juniperus or boswellia or santalum album).mp.
18) (b?rentraube* or birke* or brennnessel* or bruchkr?ut* or brunnenkresse* or moosbeer* or erdbeer* or esche or eschenbl?tt* or gartenbohnen* or g?nsefinger* or goldrute* or habicht* or hauhechel* or indigowurzel* or kastanie* or weidenr?s* or klette* or kurkuma* or lebensbaum* or liebst?ckel* or l?wenzahn* or matebl?tt* or meerrettich* or petersilie* or purpursonnenhut* or quecke* or hibiskus* or rosmarin* or schachtelhalm* or johannisbeer* or tausendg?lden* or kn?terich* or wacholder* or weihrauch* or sandelholz*).mp.
19) exp Arctostaphylos/ or exp Betula/ or exp Urtica dioica/ or exp Caryophyllaceae/ or exp Brassicaceae/ or exp Vaccinium macrocarpon/ or exp Fragaria/ or exp Fraxinus/ or exp Potentilla/ or exp Asteraceae/ or exp Fabaceae/ or exp Aesculus/ or exp Epilobium/ or exp Curcuma/ or exp Thuja/ or exp Levisticum/ or exp Ilex paraguariensis/ or exp Orthosiphon/ or exp Petroselinum/ or exp Agropyron/ or exp Salvia/ or exp Equisetum/ or exp Ribes/ or exp Centaurium/ or exp Polygonum/ or exp Juniperus/ or exp Santalum/ or exp Nasturtium/ or exp Taraxacum/ or exp Armoracia/ or exp Echinacea/ or exp Rosmarinus/ or exp Hibiscus/ or exp Boswellia/
20) 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19
21) 10 and 20
22) (randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.
23) 21 and 22
24) exp animals/ not humans/
25) 23 not 24
26) limit 25 to (english or german)
27) limit 26 to yr=“2011 -Current“