Abstract
Mitochondrial complex V plays an important role in oxidative phosphorylation by catalyzing the generation of ATP. Most complex V subunits are nuclear encoded and not yet associated with recognized Mendelian disorders. Using exome sequencing, we identified a rare homozygous splice variant (c.87+3A>G) in ATP5PO, the complex V subunit which encodes the oligomycin sensitivity conferring protein, in three individuals from two unrelated families, with clinical suspicion of a mitochondrial disorder. These individuals had a similar severe infantile and often lethal multi-systemic disorder that included hypotonia, developmental delay, hypertrophic cardiomyopathy, progressive epileptic encephalopathy, progressive cerebral atrophy, and white matter abnormalities on brain MRI consistent with Leigh syndrome. cDNA studies showed a predominant shortened transcript with skipping of exon 2 and low levels of the normal full-length transcript. Fibroblasts from the affected individuals demonstrated decreased ATP5PO protein, defective assembly of complex V with greatly reduced amounts of peripheral stalk proteins, and greatly reduced complex V hydrolytic activity. Further, expression of human ATP5PO cDNA without exon 2 (hATP5PO-Δex2) in yeast cells deleted for yATP5 (ATP5PO homolog) was unable to rescue growth on media which requires oxidative phosphorylation when compared to the wild type construct (hATP5PO-WT), indicating that exon 2 deletion leads to a non-functional protein. Collectively, our findings support the pathogenicity of the ATP5PO c.87+3A>G variant, which significantly reduces but does not eliminate complex V activity. These data along with the recent report of an affected individual with ATP5PO variants, add to the evidence that rare biallelic variants in ATP5PO result in defective complex V assembly, function and are associated with Leigh syndrome.
Keywords: ATP5PO, complex V, ATP synthase, Leigh syndrome, splice variant, mitochondrial disease, mitochondria, hypertrophic cardiomyopathy, seizure
INTRODUCTION
Mitochondria are essential organelles responsible for the production of cellular energy in the form of ATP molecules through oxidative phosphorylation. Primary mitochondrial respiratory chain diseases affect at least 1 in 4,300 live human births,1and mitochondrial dysfunction due to mutations in either mitochondrial or nuclear encoded genes has been implicated in a broad spectrum of human disease2.
In eukaryotes, the components of mitochondrial oxidative phosphorylation include the four multi-subunit protein complexes (I-IV) of the electron transport chain and complex V (FOF1ATP synthase) which catalyzes the last step in the generation of ATP. These transmembrane complexes are located on the inner membrane of mitochondria. In oxidative phosphorylation, complexes I-IV transfer electrons derived from oxidizable substrates and pump protons from the mitochondrial matrix to the intermembrane space to create a proton gradient. Complex V uses the energy created by the proton electromotive forces of the electrochemical gradient to phosphorylate ADP to ATP.
Human mitochondrial complex V or FOF1ATP synthase is primarily composed of two structurally connected sub-complexes F1 (factor 1) and FO (factor that confers sensitivity to oligomycin) which function as molecular rotors and are connected by a peripheral and a central stalk. F1 is located in the mitochondrial matrix and consists of 5 subunits, namely, α (ATP5F1A, MIM:164360), β (ATP5F1B, MIM:102910), γ (ATP5F1C, MIM:108729), δ (ATP5F1D, MIM 603150) and ε (ATP5F1E, MIM:606153). The α and β subunits form the catalytic core and the γ, δ and ε subunits constitute the central stalk of complex V. FO is bound to the inner mitochondrial membrane and consists of 10 unique subunits including a (MT-ATP6, MIM:516060), b (ATP5PB, MIM:603270), c (3 isoforms, ATP5MC1, MIM:603192; ATP5MC2, MIM:603193; ATP5MC3, MIM:602736), d (ATP5PD, MIM:618121), e (ATP5ME, MIM:601519), f (ATP5PF, MIM:600828), g (ATP5MG, MIM:617473), F6 (ATP5PF, MIM:603152), oligomycin sensitivity-conferring protein-OSCP (ATP5PO, MIM:600828), and A6L (MT-ATP8, MIM:516070). Eight c subunits assemble as a ring aided by the subunits e, f and g. The ATP5PO/OSCP, F6, b and d subunits together form the peripheral stalk. The proper functioning of complex V is also dependent on the minor subunit USMG5 involved in dimerization of complex V (ATP5MK, MIM:615204), assembly factors (TMEM70, MIM: 612418) working in conjunction with TMEM242; ATPAF1, MIM:608917; ATPAF2, MIM:608918) and regulatory factor ATPIF1 (ATPIF, MIM:614981). The majority of the complex V subunits are encoded by nuclear genes, except for the two FO subunits a and A6L, which are encoded by the mitochondrial genome. Mitochondrial oxidative phosphorylation is functionally conserved in eukaryotes, and the components of complex V are highly conserved between human and yeast3.
Until recently, pathogenic variants that result in complex V deficiency were only reported in 7 of the 18 genes encoding the complex V subunits (ATP5F1A, ATP5F1D, ATP5F1E, ATP5MK, ATP5MC3, MT-ATP6, MT-ATP8) and in 2 assembly factors (TMEM70, ATPAF2). A recent report described a single individual with compound heterozygous variants in ATP5PO (c.34C>T (p.Gln12Ter) and c.329-20A>G), who had seizures, hypotonia, elevated lactate in cerebrospinal fluid, acquired microcephaly, dystonia, global developmental delay, and progressive brain atrophy4. Here, we report three additional individuals from two unrelated families in whom we identified a rare, homozygous splice variant in ATP5PO. They had hypotonia, developmental delay, encephalopathy, seizures, hypertrophic cardiomyopathy, lactic acidosis, and MRI brain findings consistent with Leigh Syndrome. We demonstrate the molecular and biochemical effects of this variant with in vitro functional studies that evaluate splicing, activity and assembly of mitochondrial oxidative phosphorylation complexes and complementation, using human and yeast cells.
METHODS
Individual Ascertainment
Family 1: Written informed consents were obtained from the family for genetic analysis of the affected individual (II.3) and parents (I.1 & I.2) as per the French public health regulation. Parents also provided written consent for the analysis of the other unaffected family members (II.1, II.2 & II.4).
Family 2: This study was approved by the Institutional Review Board (IRB) of Columbia University and the Children’s Hospital of Philadelphia (CHOP). Written informed consents were obtained for all the participants. Additional written consent was obtained for publication of facial images of the affected individuals. Studies in fibroblasts were done under an IRB-approved study (COMIRB#18-1828). This family was also enrolled in the Undiagnosed Diseases Network Study at the Harvard Clinical Site at Massachusetts General Hospital. This study was approved by the NIH Intramural IRB, and written informed consent was obtained for all family members.
The remaining detailed materials and methods for all the experiments including genomic analysis, histochemistry, electron microscopy, cDNA analysis, mitochondrial enzyme activities, BN-PAGE analysis, growth assay and functional studies in budding yeast are available in the Supplemental Materials.
RESULTS
Clinical Findings
The demographic, clinical, and laboratory characteristics of the three affected individuals from the two independent families and with biallelic ATP5PO variants are summarized along with the previously reported individual in Table 1. Figures 1-2 and S1 show the pedigrees, results of muscle biopsy, MRI images of the brain for the affected individuals. Detailed medical histories of each individual are provided in the Supplemental Materials.
Table 1.
Clinical characteristics of individuals with biallelic ATP5PO (NM_001697) variants.
| Family 1 | Family 2 | Zech et al, 20224 | ||
|---|---|---|---|---|
| II.3 | II.1 | II.4 | patient 4 | |
| Age | 4 months (deceased) | 6 months (deceased) | 3 years (living) | 6 years (deceased) |
| Sex | Male | Male | Female | Female |
| Ethnicity | Moroccan | Turkish | European | |
| Variants | c.87+3A>G (homozygous) | c.34C>T (p.Gln12Ter) & c.329-20A>G (compound heterozygous) | ||
| Prenatal | IUGR CHD |
IUGR Oligohydramnios |
Gestational DM | NA |
| Neonatal | Respiratory distress Bradycardia |
Respiratory distress Bradycardia Generalized edema Bilateral hydronephrosis and renal insufficiency |
Respiratory distress | fever-induced partial seizures, hypotonia, elevated CSF lactate concentrations |
| Neurologic | Generalized hypotonia Seizure |
Generalized hypotonia Generalized hypertonia Increased reflexes |
Developmental delay Generalized hypotonia Epilepsy (infantile spasms, Lennox-Gestault, generalized tonic-clonic & myoclonic seizures) |
Developmental delay Muscular hypotonia Intellectual disability Seizures (focal seizures; generalized tonic-clonic seizures) Neurological regression |
| EEG | Severe diffuse epileptic without normal activity in the background | Diffuse background slowing with sharp waves and sharp and slow wave complexes over the frontal regions | Abnormal background rhythm consistent with severe diffuse epileptic encephalopathy with potentially epileptogenic foci and cerebral dysfunction | NA |
| Brain MRI | Periventricular cysts with mild ventriculomegaly Cavum septum pellucidum White matter hypersignal around the ventricles |
Cerebral atrophy with slight dilatation of the temporal horns Thin corpus callosum Arachnoid cysts in the posterior fossa and left anterior-inferior temporal lobe Bilateral old hemorrhage areas in the frontal and parietal lobes Cavum septum pellucidum Cavum vergae |
Diffuse supratentorial volume loss Restricted diffusion of the basal ganglia (predominantly globus pallidi and posterior putamina) and bilateral mesial temporal lobes Delayed myelination Thin corpus callosum Mild ventriculomegaly secondary to diffuse volume loss |
reduced brain volume delayed myelination and hypoplasia of the lower part of the cerebellar vermis (15 months) severe progressive global atrophy (5 years) |
| MR Spectroscopy | Long TE with decreased N-acetylaspartate (NAA) and elevated choline peaks | Not performed | Abnormal lactate peaks in the right frontal white matter (left was not able to be evaluated due to technical reasons) and bilateral basal ganglia. | NA |
| Cardiologic | Progressive HCM | Progressive HCM Ostium secundum ASD Pericardial effusion |
Progressive HCM | None |
| Muscle biopsy | Scattered hypotrophic fibers with mildly increased density of mitochondria Intracytoplasmic lipid was diffusely increased Near normal COX staining |
Variation in muscle fiber size Prominent lysosomes Weak staining of cytochrome c oxidase (COX) |
Scattered hypotrophic fibers, many of which contain variably increased intracytoplasmic lipid droplets and mitochondria that were mildly increased in size and density. (COX) levels = 55%b |
NA |
| Dysmorphic | NA (examined when 20 days old) |
High forehead Mildly depressed nasal bridge Bulbous nasal tip Full cheeks Deep philtrum (examined at 4 months of age) |
High forehead Prominent metopic suture Mildly depressed nasal bridge Hypertelorism Left ptosis Bulbous nasal tip Full cheeks Deep philtrum (examined at 13 months of age months of age) |
NA |
| Genitourinary | Hypospadias | Enlarged and echogenic kidneys without hydronephrosis at 5 months old Hypospadias Cryptorchidism |
None | None |
| Biochemical | Intermittent metabolic acidosis |
Blood Lactate ↑ Pyruvate ↑ CK ↓ Urine Lactate ↑ Pyruvate ↑ Ketones ↑ (mild) NBS Transiently ↑C4 |
Blood Lactate ↑ Pyruvate normal CSF Pyruvate normal Lactate normal Urine Lactate ↑ Pyruvate ↑ Ketones ↑ (severe while on ketogenic diet) 3-MGA ↑ NBS Transiently ↑C4 |
CSF Lactate ↑ |
| Other | None | None | None | Microcephaly Dystonia Restlessness Sleep disturbances Non-ambulatory Speech abnormality |
IUGR, intrauterine growth retardation; DM, diabetes mellitus; HCM, hypertrophic cardiomyopathy; ASD, atrial septal defect; CK, creatinine kinase; NBS, newborn screening, CHD, congenital heart defect; 3-MGA, 3-methyl glutaconic acid; CSF, cerebrospinal fluid, NA, not available
3-MGA was not assessed for individual II.1 from family 2
3-MGA was not elevated in the patient 4, Zech et al 2021
lactate, 3-MGA levels were not available for individual II.3 from family 1
Figure 1:
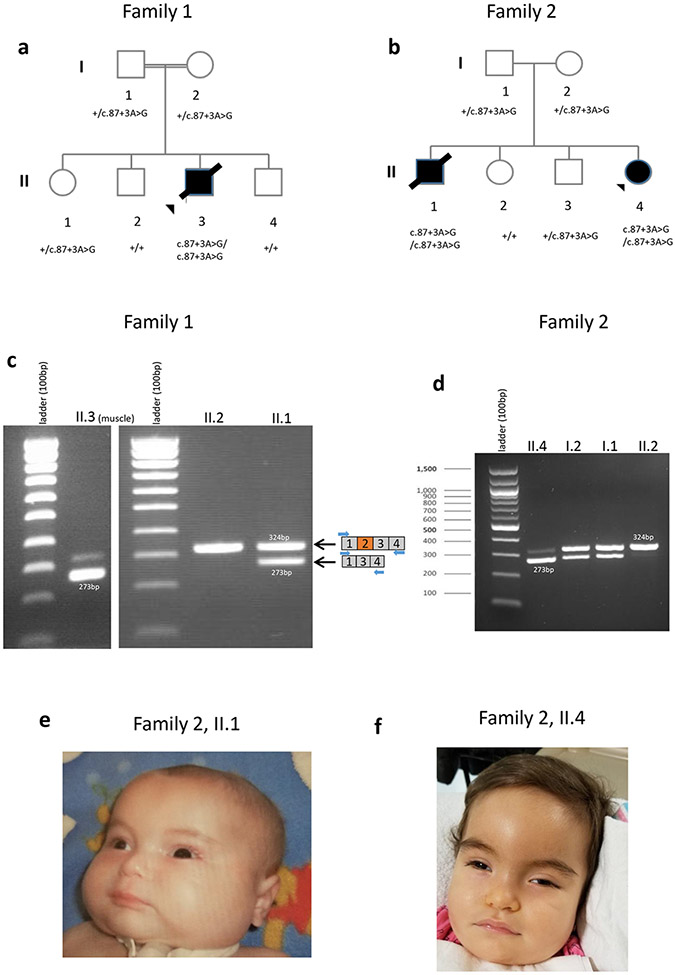
a-b) Pedigrees showing individuals from families 1 and 2. c) cDNA studies using a muscle sample of the affected individual in family 1 (II.3) showed ATP5PO exon 2 skipping and the presence of residual WT spliced product. Peripheral blood samples of unaffected siblings (II.2, II.1) were used for cDNA studies. d) cDNA studies using blood samples of the members of family 2 (I.1, I.2 (parents), II.2 (unaffected sibling), II.4 (affected) e,f) Photos of affected individuals of family 2 (II.1, II.4) showing high forehead, mildly depressed nasal bridge, bulbous nasal tip, full cheeks, deep philtrum. Additionally, II.4 individual (panel f) shows prominent metopic suture and hypertelorism.
Figure 2:
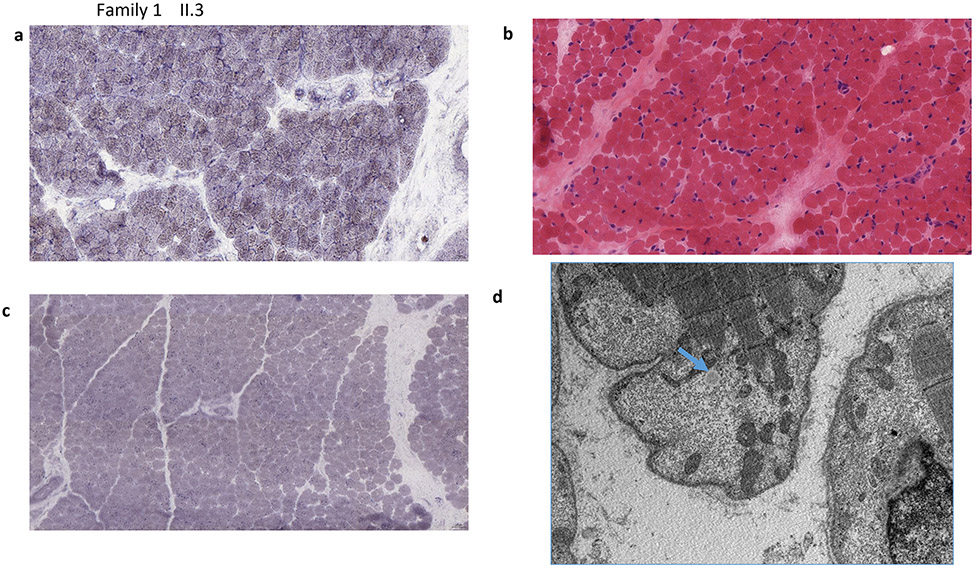
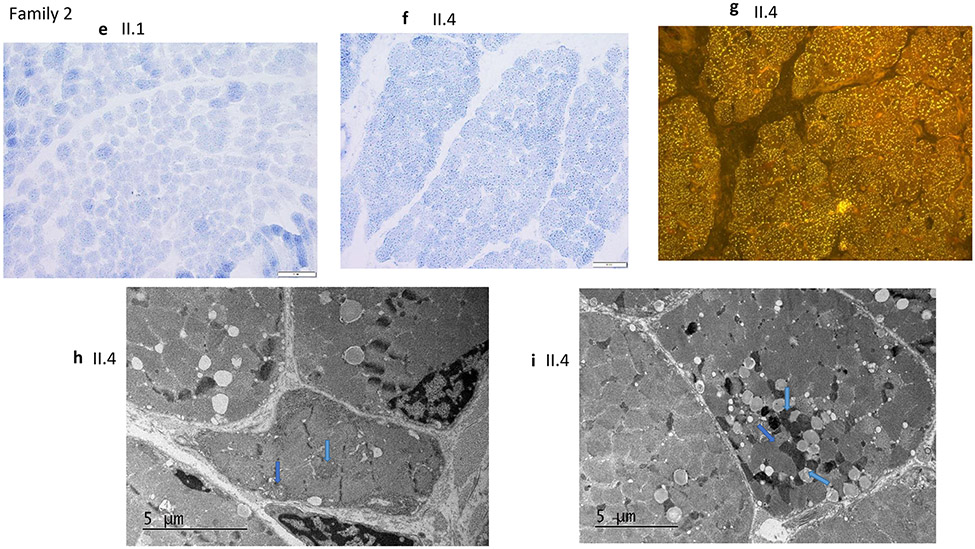
a-d) Muscle Biopsy results of affected individual (II.3) from family 1 . a) SDH (original magnification x400) Fiber size variation is increased. No ragged blue fibers were identified. b) SDH (original magnification x400) There are scattered hypotrophic fibers. No ragged blue fibers present, the staining intensity of SDH was mildly increased, suggesting increased density of mitochondria c) (Sudan Black, original magnification x400) A Sudan black stain revealed diffusely increased intracytoplasmic lipids. d) EM shows mitochondria are few, very irregular in shape and they are intermixed with glycogen not with lipid droplets (blue arrow). The increase of lipids seen with the Sudan Black stain is irregularly located and not present in this sub sarcolemnic area with some irregular-shaped mitochondria e-i) Muscle Biopsy results of the affected siblings (II.1, II.4) of family 2. e) II.1, SDH (original magnification x400) Fiber size variation is increased. No ragged blue fibers were identified. f)II.4, SDH (original magnification x400) There are scattered hypotrophic fibers. No ragged blue fibers present, while the staining intensity of SDH was felt to be mildly increased, suggesting increased density of mitochondria in fibers. g) II.4, (Nile Red, original magnification x400) A Nile red stain viewed under the fluorescent microscope reveals diffusely increased intracytoplasmic lipid. h,i) II.4, EM shows variably enlarged mitochondria intermixed with lipid droplets (arrows)
Molecular findings
For family 1, since there was a strong clinical suspicion of a mitochondrial disorder, enzyme activities of the mitochondrial respiratory chain complexes I-IV were assessed in a muscle biopsy sample (data not shown) and in fibroblasts (Table 2) from II.3, followed by BN-PAGE analysis (Figures 3a). These data suggested a complex V defect. Guided by the biochemical evidence, concomitant trio WES analysis focused on ultra-rare variants in stretches of homozygosity, identified a homozygous candidate variant in ATP5PO (Chr21-35286751-T-C, c.87+3A>G, NM_001697.3) in the affected individual (II.3; Table S1). The variant was confirmed by Sanger sequencing and segregated with disease in the family (Figures 1a and S2a). The variant was predicted by Human Splicing Finder (HSF) to significantly decrease the strength of the donor splice site of intron 2 (≥10%; 87.3-score for wild type vs 76.7- score for variant splice site).
Table 2:
Enzyme activities of the mitochondrial complexes in fibroblasts of individuals with pathogenic variants in ATP5PO
| Controls | Affected individual | Controls | Affected individual | |||
|---|---|---|---|---|---|---|
| Activity nmol.min−1.mg−1 mean (range) |
Activity nmol.min−1.mg−1 mean |
Z score | Ratio/CS mean (range) |
Ratio/CS mean |
Z score | |
| Family 1 (II.3) | ||||||
| Complex I | 245 (190-295) | 159 | −3.2 | 0.50 (0.36-0.62) | 0.22 | −4.0 |
| Complex II | 93 (65-130) | 72 | −1.3 | 0.17 (0.11-0.25) | 0.12 | −1.2 |
| Complex III | 706 (500-920) | 867 | +1.5 | 0.99 (0.64-1.70) | 1.23 | +1.2 |
| Complex IV | 626 (440-825) | 784 | +1.8 | 1.08 (0.72-1.63) | 1.11 | +0.2 |
| Complex V ATPase | 191 (132-280) | 26 | −4.9 | 0.35 (0.21-0.55) | 0.04 | −4.3 |
| Citrate synthase | 560 (350-840) | 708 | +1.5 | NA | NA | NA |
| Controls | Affected individual | Controls | Affected individual | |||
| Activity nmol.min−1.mg−1 mean (range) |
Activity nmol.min−1.mg−1 mean |
Z score | Ratio/CS x 1000 mean (range) |
Ratio/CS x 1000 mean |
Z score | |
| Family 2 (II.4) | ||||||
| Complex I | 90 (49-131) | 102 | +0.6 | 229 (145-396) | 375 | +1.7 |
| Complex II | 234 (131-364) | 252 | +0.4 | 595 (297-863) | 928 | +1.5 |
| Complex III | 15.3 (8.0-29.2) | 47.7 | +2.9 | 39 (19-56) | 176 | +3.7 |
| Complex II-III | 108 (62-159) | 208 | +2.1 | 248 (131-376) | 768 | +3.3 |
| Complex IV | 4.6 (2.2-7.1) | 5.5 | +0.6 | 12 (6-23) | 20 | +1.0 |
| Complex V ATPase | 279 (188-415) | 83 | −3.5 | NA | NA | NA |
| Citrate synthase | 407 (254-544) | 271 | −1.3 | NA | NA | NA |
The respiratory chain enzyme activities are shown for each affected individual with the control activities of the respective laboratory. The control enzyme activities, with the average and range specified, and the activities for individuals II.3 (family 1) and II.4 (family 2) are given in nmol.min−1.mg protein−1. The ratio of enzyme activities over citrate synthase (CS) is also shown. The ratios/CS are calculated for a CS in nmol/min/mg proteins in the case of patient II.3 (family 1), and for patient II.4 (family 2) the ratio values are multiplied by 1000. For both, the activity is expressed as the Z-score of the distribution of normal controls after log transformation. Abnormal values are shown in bold. NA denotes not applicable/not available
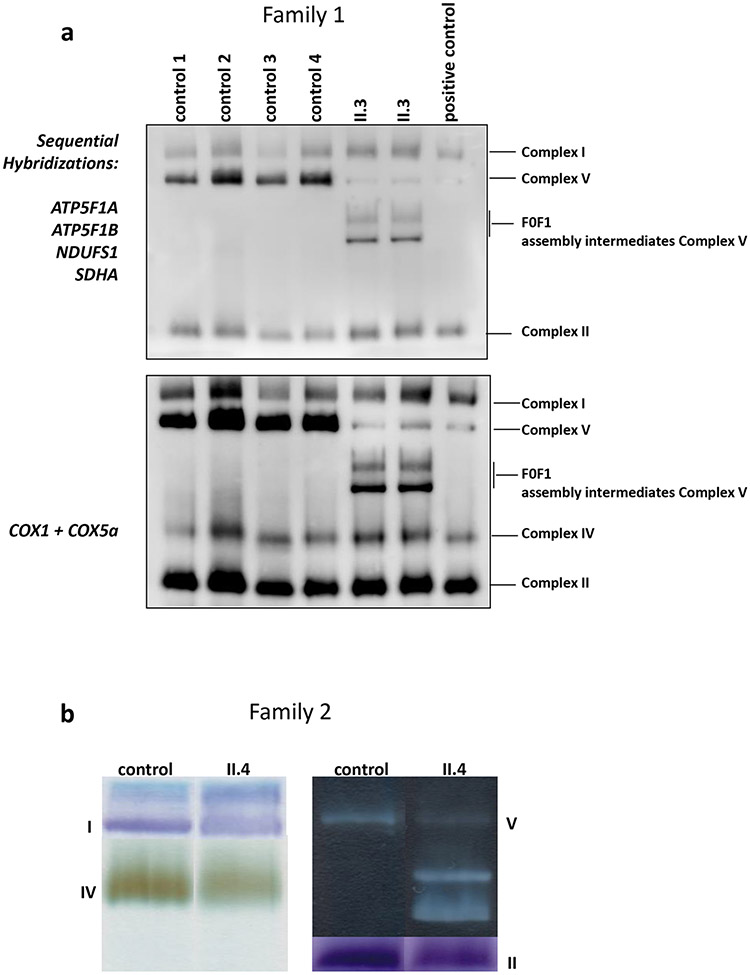
Figure 3:
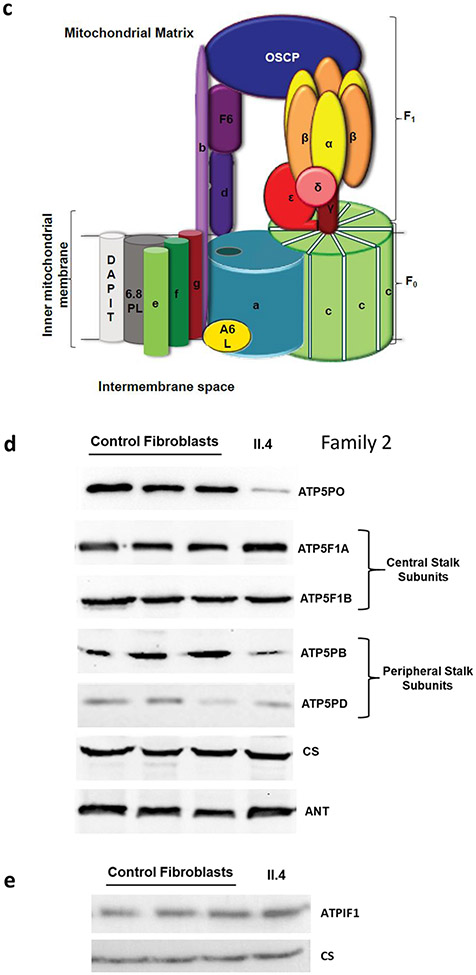
a) Mitochondrial complex assembly using family 1, individual II.3’s fibroblasts. Representative blue native PAGE following western blot analysis. Membranes were sequentially hybridized with different antibodies: ATP5F1A and ATP5F1B subunits for ATP synthase; NDUFS1 subunit for complex I; SDHA subunit for complex II, and COXI + COXVa subunits for complex IV. Experiments were performed on 4 different control fibroblast cell lines (Ctr1-4), fibroblast cells from the affected individual II.3 in duplicate and a patient fibroblast cell line carrying a homozygous pathogenic mutation (c.317-2A>G) in TMEM70 gene as a positive control for complex V assembly defect. Experiments were performed in duplicate on two biological replicates for each sample. b) Separation of the complexes on blue native PAGE followed by in-gel activity staining of a control and individual family 2, II.4’s fibroblasts, shows normal activities of complexes I, II, and IV, but complex V shows only lower molecular weight intermediates- F1,with near absent activity of the holocomplex. c) Cartoon representation of mitochondrial Complex V. ATP5PO encodes OSCP (blue oval), which stabilizes the F1 complex (yellow and orange ovals) with the peripheral stalk (purple subunits). d) Complex V proteins in individual II.4 from family 2. Western blot analysis showed reduced levels of the ATP5PO protein, stalk proteins b and d (ATP5PB and ATP5PD), whereas the α and the β subunits of the (F1) catalytic core (ATP5F1A and ATP5F1B) showed preserved steady state amounts. e) The amount of ATPIF1 protein is similar in individual II.4 of family 2 to controls.
For family 2, analysis after filtering of WES data, based on allele frequency (alternate allele frequency <1%), yielded a homozygous candidate variant in ATP5PO (Chr21-35286751-T-C, c.87+3A>G, NM_001697.3) in the affected individual (II.4; Table S1). This variant is absent in gnomAD (v2.1.1 and v3) and the Greater Middle East (GME) Variome Project, which is enriched for consanguineous populations. The variant was confirmed by Sanger sequencing and segregated with disease in the family (Figures 1b and S2b).
Families 1 and 2 were connected through GeneMatcher5, and notably these two unrelated families share the same homozygous ATP5PO splice variant. Analysis of a 3Mb region flanking the ATP5PO variant showed multiple distinct single nucleotide polymorphisms which were not shared between the two families but were shared within each family, suggesting that the ATP5PO variant seen in these unrelated families likely arose independently in the two families but was from a common ancestor within each of the two families (Table S2).
cDNA analysis of the ATP5PO c.87+3A>G splice region variant
Minigene assay and cDNA studies for the c.87+3A>G variant confirmed the aberrant splicing and the presence of residual full-length transcript (Figures 1c, d, S3-5, Supplemental data).
For family 1, RNA samples from the affected individual (II.3, muscle biopsy) and two unaffected siblings (II.1, II.2, peripheral blood) were used for cDNA studies. In individual II.2 who does not carry the variant, PCR using primers for exons 1 and 4 resulted in a single amplicon corresponding to the amplification of exons 1 to 4 (324bp; Figure 1c). For individual II.1, who is heterozygous for the variant, two bands were detected: a predominant band of 324bp corresponding to amplification of exons 1 to 4 and a smaller band of 273bp with lower intensity (Figure 1c). Sanger sequencing confirmed that the 273bp fragment corresponded to complete exon 2 skipping (Figure S5). The affected individual (II.3) predominantly expressed the aberrant, exon 2 skipped ATP5PO transcript (273bp), though a band of weak intensity corresponding to the wild type transcript (324bp) was also seen (Figures 1c, S3-5a; Supplemental data). The same primer pair was used for cDNA studies of family 2, leading to similar results (Figures 1d, S5b; Supplemental data).
Skipping of exon 2 is predicted to result in an in-frame deletion of 17 amino acids of coding exon 2. Western blot using cultured fibroblasts of the affected individual from family 2 (II.4) showed a strong depletion of the ATP5PO protein compared to controls (Figures 3c,d).
Assay for respiratory chain enzyme activities and blue native PAGE (BN-PAGE) showed defective complex V assembly
In fibroblasts from individual II.3 (family 1), the activities of respiratory chain enzyme complexes II, III and IV were within the normal range. However, the activities of oligomycin-sensitive ATPase and NADH ubiquinone reductase (complex I) were significantly decreased (Z score= −4.3 and −3.9 for complex V and complex I, respectively; Table 2). In fibroblasts from individual II.4 (family 2), the activities of complexes I, II, and IV were normal with increased activities of complex III and the combined II+III assay indicating possible upregulation in this sample (Table 2). The activity of the oligomycin-sensitive ATPase activity in fibroblasts was significantly decreased (Z score= −3.6; Table 2).
Using II.3’s (family 1) fibroblasts, the assembly of complex I analyzed by BN-PAGE was normal, without the accumulation of assembly intermediates (Figure S6a). In contrast, complex V revealed additional bands of lower molecular weight intermediates, corresponding to F1 sub-domains, with almost a complete absence of the complex V holoenzyme (Figures 3a, c).
BN-PAGE analysis showed normal activities of complexes I, II, and IV in II.4’s (Family 2) fibroblasts, but in-gel activity staining of complex V revealed the presence of additional bands of lower molecular weight with almost a complete absence of complex V holoenzyme (Figure 3b). Western blot analysis showed reduced levels of the ATP5PO protein, and reduced levels of protein subunits of the stalk as b and d, while the steady state protein levels of the α and the β subunits of the F1 catalytic core were maintained (Figure 3d). The ATPIF1 regulatory protein had preserved protein levels (Figure 3e). The assembly of complex I was also normal without accumulation of pathological intermediates (Figure S6b).
Reduced cell growth of affected individual’s fibroblasts in a glucose-free medium indicated defective mitochondrial oxidative phosphorylation
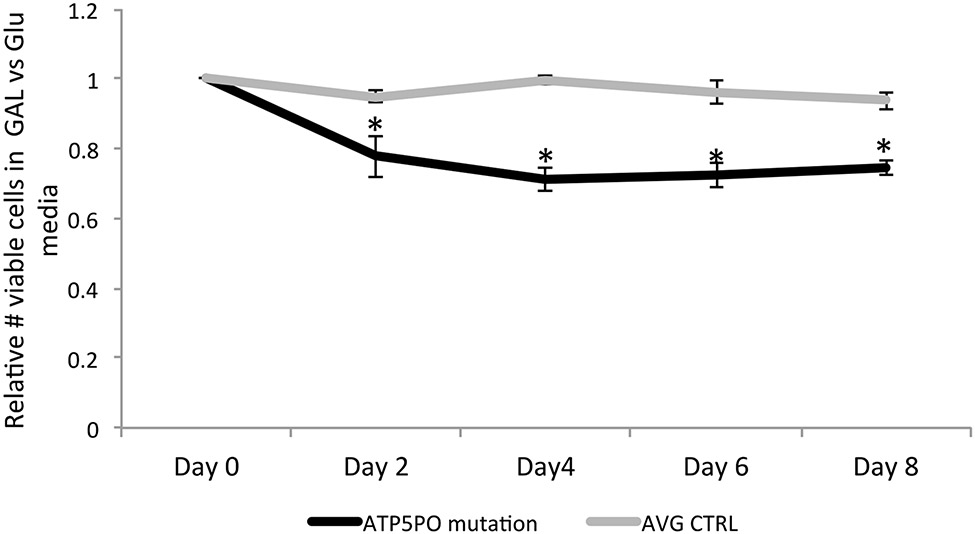
The growth rates of individual II.4’s (family 2) fibroblasts in media contain either glucose (permissive of glycolysis) or galactose (without glucose, therefore obligating mitochondrial oxidative phosphorylation) were compared with two control fibroblast lines. After 8 days, the II.4’s cells had expanded on an average 2.3 fold in galactose media, as compared to 3.1 fold in glucose, giving a relative growth rate of 74%. The average growth rates of the control fibroblasts in galactose were more similar to that in glucose media (1.9 fold vs 2.0 fold), giving a relative growth rate of 94% (Figure 4). The production of pyruvate from glucose yields 2 net ATP, whereas the production of pyruvate from galactose yields no net ATP, thus growth in galactose requires cells to shift their reliance on oxidative metabolism with increased oxygen consumption. The decreased relative growth of this II.4’s fibroblasts in galactose as compared to control fibroblasts is consistent with an impairment of mitochondrial OXPHOS function6,7.
Fig. 4.
Fibroblasts from family 2, individual II.4, demonstrate impaired growth in galactose media. The relative number of viable fibroblasts from individual II.4 in galactose vs glucose over a period of 8 days were compared to the average of that of two control fibroblast lines (n=3 biological replicates for each cell line and time point, * p<0.007)
Studies in yeast showed that the ATP5PO protein resulting from exon 2 skipping has impaired function
We used budding yeast to determine if the deletion of coding exon 2 has consequences on the mitochondrial oxidative phosphorylation function of ATP5PO protein. Yeast is a relevant model to assess the consequences of mutations in respiratory chain proteins as these S. cerevisiae mutants can be easily maintained on media where cells use fermentation to produce ATP (medium containing glucose), whereas they cannot grow on media where respiration is required8 (medium containing ethanol instead of glucose8). The ATP5 gene encodes the ATP5PO orthologue in yeast9 (Figure S7).
As shown in Figure S8a, diploid yeast cells heterozygous for ATP5 deletion (Y23657 atp5Δ/ATP5) are able to grow on glucose as well as on ethanol-containing medium, whereas haploid cells deleted for ATP5 (atp5Δ) are unable to grow on an ethanol-containing medium on which their ATP production only results from respiration, confirming the inability of these cells to perform functional oxidative phosphorylation. We expressed yeast ATP5 gene (yATP5), human wild-type (hATP5PO-WT) and mutant (hATP5PO-Δex2) ATP5PO cDNA in haploid atp5Δ yeast cells and assessed their ability to rescue the respiratory growth defect of atp5Δ cells. Strikingly, none of these constructs (including yATP5 used as a control) were able to rescue the oxidative phosphorylation defects of atp5Δ cells (Figure S8b). This observation is consistent with a previous report showing that atp5Δ cells exhibit a high level of genetic instability, leading to loss of their mtDNA10. Thus, our data show that the likely mtDNA loss in atp5Δ cells is too detrimental for oxidative phosphorylation and cannot be rescued.
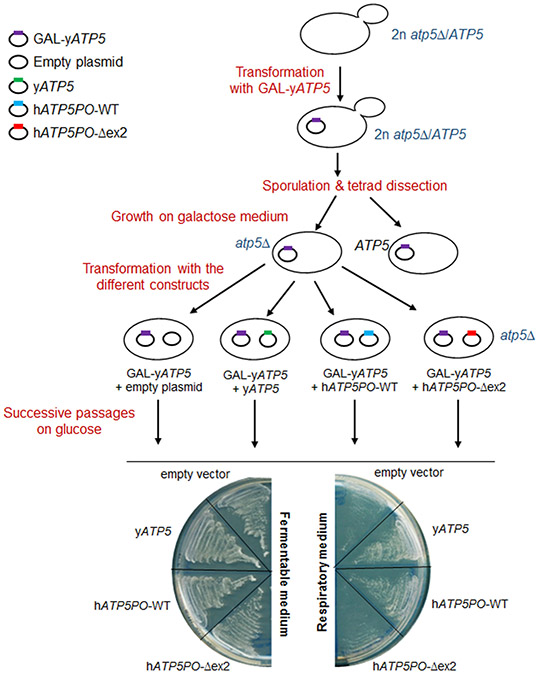
The study of hATP5PO-Δex2 functionality in atp5Δ cells thus required a progressive depletion strategy10,11. This strategy is based on the transient co-expression of the heterologous ATP5PO and yAtp5 proteins to maintain mtDNA, followed by the progressive depletion of yAtp5 protein, allowing the sole expression of the heterologous ATP5PO protein. Functional ATP5PO heterologous protein should allow cells to maintain respiration competency, whereas non-functional heterologous protein should lead to respiratory dysfunction. We used a diploid heterozygous atp5Δ/ATP5 strain that was transformed with yATP5 with expression driven by a galactose-inducible promoter (GAL1, Figure 5). Diploid cells were then led to sporulate (allows a single diploid cell to give rise to four haploid cells through meiosis) and atp5Δ haploid cells were grown on a galactose-containing medium to ensure the expression of the yATP5 transgene and thus avoid the loss of mtDNA. These atp5Δ haploid cells were subsequently transformed with either an empty plasmid or a plasmid constitutively expressing yeast wild type, human wild type, or mutant hATP5PO-Δex2. Transformants were passaged on glucose-containing media to switch off the expression of yATP5 gene, which was under the control of the GAL1 promoter, and to completely deplete cells of the yATP5 protein. Then, the ability of each construct to allow the growth of yeast cells on an ethanol-containing medium was assessed. As shown in Figure 5, cells expressing hATP5PO-Δex2 were unable to rescue respiratory growth unlike yATP5 and human wild-type ATP5PO. These results indicate that the deletion of exon 2 impairs the function of ATP5PO, causing respiratory defects.
Figure 5:
Functional assay to assess the capacity of human mutant hATP5PO-Δex2 to rescue respiratory growth defect of atp5Δ S.cerevisiae yeast cells. Heterozygous diploid cells atp5 Δ/ATP5 (Y23657 strain) were transformed with the yeast ATP5 gene (yATP5) under the control of the inducible GAL1 promoter (pRS416-GAL-yATP5 plasmid). Cells were then spread on a sporulation medium and tetrads were dissected to isolate the four haploid cells on galactose-containing medium to ensure the expression of yATP5 transgene and avoid the loss of mtDNA. atp5Δ haploid cells expressing yATP5 were subsequently transformed with an empty plasmid or plasmids expressing constitutively either yeast (yATP5) or human wild-type ATP5PO (hATP5PO-WT) and human mutant ATP5PO (hATP5PO-Δex2). ATP5PO has previously been shown to be a functional homolog of the Atp5p yeast protein, thus mtDNA is maintained in the presence of either Atp5p yeast protein or ATP5PO human protein. After 5 passages on 2% glucose to switch off GAL-yATP5 expression and eliminate yATP5 protein, cell growth was assessed on respiratory medium (2% ethanol). Whereas both yATP5 and wild-type human hATP5PO were able to rescue growth defect, the human mutant hATP5PO-Δex2 was, as the empty vector, unable to grow on respiratory medium, confirming that the deletion of exon 2 in hATP5PO leads to a non-functional protein.
Discussion
We identified a rare, homozygous, splice variant in ATP5PO which results in exon skipping and significantly decreased assembly of mitochondrial complex V (FOF1 ATP synthase) in three individuals from two unrelated families, with common clinical features of hypotonia, developmental delay, hypertrophic cardiomyopathy, lactic acidosis, progressive epileptic encephalopathy, brain MRI findings indicating progressive brain volume loss, and white matter disease consistent with Leigh syndrome. The two affected families are of Moroccan and Turkish ancestry, respectively. Haplotype analysis indicated that the same ATP5PO variant likely arose independently in these two families.
The two affected individuals of family 2 (II.1, II.4) showed brain atrophy with involvement of both cortical and subcortical structures, and hypoplastic corpus callosum, likely reflecting the white matter volume loss. In individual II.4, there were basal ganglia lesions, typical of Leigh disease. Similar clinical findings including hypotonia, epileptic encephalopathy, elevated lactate in cerebrospinal fluid (CSF), global developmental delay and progressive brain atrophy were described for the recently reported affected individual with biallelic ATP5PO variants4 (c.34C>T (p.Gln12Ter) and c.329-20A>G). Notably, infantile hypertrophic cardiomyopathy, which was diagnosed in the three affected individuals in our study, was not seen in the previously reported individual4.
Hypertrophic cardiomyopathy is common in individuals with complex V deficiency due to TMEM70 variants. It is less common in individuals with pathogenic MT-ATP6 or MT-ATP8 variants and was only seen in a small subset of individuals12 (7/31). It has also been reported in an individual with biallelic ATP5F1E variants. Transient dilated cardiomyopathy was reported in ATP5MK deficiency, but not in other nuclear complex V defects.
Epileptic encephalopathy seen in individual II.4 (family 2) is common in Leigh disease. Epilepsy is common in patients with complex V deficiency due to MT-ATP6 disease; it was noted in 10/31 individuals with MT-ATP6 variants13. Many of the nuclear complex V defects are distinguished by intractable epilepsy associated with severe encephalopathy, which is unusual in MT-ATP6 disease.
There were increased levels of 3-methylglutaconic acid (3-MGA) in the urine of individual II.4 (family 2), which has been noted in cases of complex V deficiency caused by defects of nuclear encoded genes such as TMEM70 and ATP5FD1, likely related to the essential function of complex V in maintaining the structure and cristae formation of the mitochondrial inner membrane. Notably the recently reported individual with biallelic ATP5PO variants did not show elevated 3-MGA. The elevation of C3 and 5-hydroxy-acylcarnitines described in individuals with MT-ATP6 pathogenic variants was not observed, but a transient increase in butyryl/isobutyrylcarnitine was seen in the two affected individuals of family 2 (II.1, II.4), without any corresponding abnormalities in urine organic acids.
ATP5PO is a nuclear gene, which encodes the oligomycin sensitivity conferring protein (OSCP), a complex V subunit in the mitochondrial respiratory chain. The ATP synthase complex is composed of two structural domains: F1, which contains the catalytic core and FO, a transmembrane domain that contains the proton channel. These two domains are held together by both the central stalk and peripheral stalk. ATP5PO is an elongated protein that is essential to the peripheral stalk, and connects this structure to the rotatory catalytic core. ATP5PO plays an important structural role in the assembly of complex V. The peripheral stalk also regulates rotation of F1, an essential step for ATP production. In addition, the ATP5PO/OSCP has been identified as having a moonlighting role as a major protein in the permeability of the transition pore14.
The assembly of complex V consists of the step-wise formation of specific sub-complexes, which then associate to form the holocomplex. The catalytic F1 complex associates with the central stalk and subsequently the c8 ring to form F1-c8 sub-complex. Prior to full assembly of the F1FO ATP synthase, the catalytic subunit is inhibited by the endogenous inhibitor Inhbitory Factor 1 (IF1). The peripheral stalk associates first as a sub-complex of b-e-g, which then associates with the F1-c8 sub-complex through addition of ATP5PO/OSCP and F6 before addition of subunit d15. The peripheral stalk can also associate directly with F1 and the central stalk prior to the insertion of the c-ring by the addition of the f subunit. In either case, the next intermediate is a subcomplex of the F1, central stalk, c-ring and peripheral stalk. In the absence of ATP5PO/OSCP and F6, the b-e-g-f sub-complex is unstable and rapidly degrades15. Deletion of ATP5PO/OSCP in human HAP13 cells results in an inactive F1-c8 ring bound by IF1 and the loss of most of the peripheral stalk proteins with only very low levels of the subunit proteins F6, e, f, and g. These cells have reduced protein levels of complexes I, III and IV and lower respiratory capacity3. This loss of stalk subunits is similar to that observed in cells with a loss of F6 but is different from the loss of subunit d, which still retains more stalk intermediates15. In contrast, partial downregulation of ATP5PO/OSCP with a shRNA did not change the expression of other subunits of ATPase or its structure16, indicating that profound loss of ATP5PO/OSCP expression is likely needed before it starts to affect the complex V assembly. Both the previously reported and our affected individuals lack proteins of the matrix part of the peripheral stalk (reflected in family 2, II.4 by the absence of subunits ATP5PO/OSCP, b and d, and in the reported individual in Zech et al., 20224, with the decrease in subunits d and f), and the membrane bound part of the peripheral stalk (in the reported individual in Zech et al., 20224 with the decrease in subunit e), showing that this variant profoundly impairs Complex V assembly. Thus, pathogenic variants in the critical subunits for early complex V assembly result in instability and loss of the peripheral stalk V resulting in its dysfunction. This is different from pathogenic variants in MT-ATP6 - a component which is inserted after the coalescence of the F1, c-ring, peripheral and central stalks, where the peripheral stalk is not lost. The inhibitory ATPIF1 protein remains intact, and likely bound to any subcomplexes and the impact of this on residual function remains under study.
To assess the functional consequences of the identified ATP5PO variant, a series of enzymatic assays were performed using cultured fibroblast cells from the affected individuals along with modelling the variant in yeast. cDNA studies showed that the splice variant results in skipping of coding exon 2, which leads to the significantly decreased full length ATP5PO protein and dysfunctional assembly of complex V.
The BN-PAGE analysis with in-gel activity staining confirmed a pathological pattern of lower molecular weight F1 subunits with sharply reduced holocomplex activity. This is different from what has been observed in individuals with other complex V disorders but is similar to that reported for the individual with the biallelic ATP5PO variants4.
In contrast, in individuals with MT-ATP6 and TMEM70 variants, active complex V assembly intermediates are present in addition to normal holocomplex enzyme17,18 whereas in individuals harboring ATP5F1D19 and ATPAF220 variants, a severe reduction in active complex V holocomplex was observed, as well as the presence of inactive lower molecular weight F1 subunits. The pattern observed in the current study, provides insight into the effect of pathogenic APT5PO variants on the assembly and ultimate structure of ATP synthase, whereby the lower molecular weight F1 subunits are present and have some residual activity, but the holocomplex is sharply reduced.
Fibroblasts from the affected individual II.4 (family 2) showed an isolated deficiency of the complex V ATPase activity, but not of the other complexes. In the affected individual II.3 (family 1), a decrease in complex I activity was found in association with the complex V deficiency. The decrease in complex V hydrolytic activity and the impaired complex V assembly, both strongly support the pathogenicity of the ATP5PO variant. On the contrary, we did not observe any complex I assembly defect in individual II.3, indicating a probable secondary decrease, related to the complex V deficiency. Impairment of complex I has been described in other complex V deficiencies, namely for subjects with variants in TMEM7021 and in MT-ATP617 (mt.8993T>G variant), and incomplete complex I assembly intermediates were seen in two patients with MT-ATP6 variant (Friederich and Van Hove, unpublished data). TMEM70 was shown to interact with the mitochondrial complex I assembly (the MCIA) complex22, which could explain the combined complex I and complex V deficiencies in TMEM70 mutants; however, this is not the case for MT-ATP6 mutants. Alternatively, complex V oligomerization plays a key role in the formation of mitochondrial cristae while complex V deficiencies induce their disorganization19,23. These findings indicated that complex V impairment could indirectly alter other respiratory chain complex activities by disrupting the mitochondrial cristae structure24. Additionally, given that family 1 is consanguineous, the presence of other deleterious homozygous variants in individual II.3, which are not detected by WES methodology (small copy number variants, deep intronic variants) and may directly or indirectly influence complex I activity, cannot be excluded. It is unclear whether the secondary complex I impairment might have modified the clinical presentation of the II.3 individual (family 1). However, the two unrelated affected individuals - II.3 and II.4 had common clinical features, as well as the previously reported individual with biallelic ATP5PO variants4, suggesting the central role of the complex V deficiency over decreased complex I activity in the individual II.3’s clinical presentation.
In yeast cells deleted for ATP5PO homolog, transformation of cDNA construct for human ATP5POΔex2 results in a decreased viability in obligate respiratory conditions needing functional oxidative phosphorylation when compared to wild type ATP5PO construct, further confirming the pathogenicity of the ATP5PO variant. Additionally, impaired growth of fibroblasts (II.4, family 2) when glucose was replaced with galactose, which requires a shift from glycolysis to oxidative phosphorylation to produce energy, also demonstrated a functional mitochondrial impairment in these cells. The substantial lactic acidosis in the patients with mitochondrial disorders implies a partial shift to glycolytic metabolism as seen in our affected individual II.4 as well.
The presence of the residual wild-type ATP5PO transcript likely resulted in the residual ATP5PO protein, the small amount of holocomplex V assembly and the residual complex V hydrolytic activity. This is similar to the recent report of an affected individual with bi-allelic ATP5PO variants, including a splice variant which also retained some normal splicing and residual activity4. Given these observations, we hypothesize that complete loss of function of ATP5PO is likely not compatible with life. This is supported by the absence of homozygous ATP5PO loss of function variants in human population databases (gnomADv2 & v3, TOPMed and GME Variome) and by the embryonic lethality of homozygous ATP5PO knockout mice (https://www.mousephenotype.org/data/genes/MGI:106341).
In summary, a homozygous splice variant in ATP5PO that significantly reduces but does not completely eliminate ATP5PO protein production, results in complex V deficiency and severe lethal infantile Leigh phenotype including hypertrophic cardiomyopathy, progressive epileptic encephalopathy and lactic acidosis. Pathogenic variants in APT5PO result in failure of assembly or loss of stability of the peripheral stalk resulting in OXPHOS dysfunction.
Supplementary Material
Acknowledgements & Funding
We thank the families for participation in this study. The authors acknowledge Dr. Michio Hirano Ilana Chilton and Alban Zeigler for assistance. Research reported in this manuscript was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Direction under award number U01HG007690 (D.A.S, M.A.W, F.A.H, E.T, L.C.B, C.A.R) and The Hill Family Fund for the Diagnosis and Management of Rare and Undiagnosed Diseases at Mass General (DS and ET). This study was also supported by K08DK113250 (R.G) and by a grant from the National Institutes of Health, NIH U54NS078059 for the North American Mitochondrial Disease Consortium (NAMDC) (JVH). NAMDC is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. This consortium is funded through collaboration between NCATS. Financial support was also received from the University of Colorado Foundation (mitochondrial research fund) and the Children’s Hospital Colorado Foundation (Riders for Samantha) (JVH and MWF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding sources had no role in the design or execution of the study, in the interpretation of data or the writing of the study.
Funding:
Research reported in this manuscript was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Direction under award number U01HG007690 (D.A.S, M.A.W, F.A.H, E.T, L.C.B, C.A.R) and The Hill Family Fund for the Diagnosis and Management of Rare and Undiagnosed Diseases at Mass General (DS and ET). This study was also supported by K08DK113250 (R.G) and by a grant from the National Institutes of Health, NIH U54NS078059 for the North American Mitochondrial Disease Consortium (NAMDC) (JVH). NAMDC is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. This consortium is funded through collaboration between NCATS. Financial support was also received from the University of Colorado Foundation (mitochondrial research fund) and the Children’s Hospital Colorado Foundation (Riders for Samantha) (JVH and MWF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding sources had no role in the design or execution of the study, in the interpretation of data or the writing of the study.
Footnotes
Conflict of interest statement: Johan Van Hove performed clinical trials and advisory function for Stealth Biotherapeutics, Inc and is employed in part by Children's Hospital Colorado, which performs clinical diagnostic studies on mitochondrial disorders. Rebecca Ganetzky is a consultant with Minovia Therapeutics. Mythily Ganapathi, Gaelle Friocourt, Naig Gueguen, Marisa W Friederich, Gerald Le Gac, Volkan Okur, Nadège Loaëc, Thomas Ludwig, Chandran Ka, Kurenai Tanji, Pascale Marcorelles, Evangelos Theodorou, Angela Lignelli-Dipple, Cécile Voisset, Melissa A. Walker, Lauren C. Briere, Amélie Bourhis, Marc Blondel, Charles LeDuc, Jacob Hagen, Cathleen Cooper, Colleen Muraresku, Claude Ferec, Armelle Garenne, Servane Lelez-Soquet, Cassandra A. Rogers, Yufeng Shen, Dana K. Strode, Peyman Bizargity, Alejandro Iglesias, Amy Goldstein, Frances A. High, David A. Sweetser,Vincent Procaccio, Cedric Le Marechal and Wendy K Chung declare that they have no relevant conflict of interest.
Details of ethics approval: This study was approved by the Institutional Review Board (IRB) of Columbia University and the Children’s Hospital of Philadelphia (CHOP). Studies in fibroblasts were done under an IRB-approved study (COMIRB#18-1828).
Informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Written informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article. Additional written consent was obtained for publication of facial images of the affected individuals.
Availability of data and material statement: Data supporting the results is available in the manuscript, supplemental materials and additional information is available upon request.
References
- 1.Gorman GS, Schaefer AM, Ng Y, et al. (2015) Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol 77: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace DC, Fan W, Procaccio V (2010) Mitochondrial energetics and therapeutics. Annu Rev Pathol 5: 297–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J, Carroll J, Ding S, Fearnley IM, Walker JE (2017) Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc Natl Acad Sci U S A 114: 9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zech M, Kopajtich R, Steinbrucker K, et al. (2022) Variants in Mitochondrial ATP Synthase Cause Variable Neurologic Phenotypes. Ann Neurol 91: 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobreira N, Schiettecatte F, Valle D, Hamosh A (2015) GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36: 928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson BH, Petrova-Benedict R, Buncic JR, Wallace DC (1992) Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem Med Metab Biol 48: 122–126. [DOI] [PubMed] [Google Scholar]
- 7.Aguer C, Gambarotta D, Mailloux RJ, et al. (2011) Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 6: e28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasserre JP, Dautant A, Aiyar RS, et al. (2015) Yeast as a system for modeling mitochondrial disease mechanisms and discovering therapies. Dis Model Mech 8: 509–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uh M, Jones D, Mueller DM (1990) The gene coding for the yeast oligomycin sensitivity-conferring protein. J Biol Chem 265: 19047–19052. [PubMed] [Google Scholar]
- 10.Prescott M, Bush NC, Nagley P, Devenish RJ (1994) Properties of yeast cells depleted of the OSCP subunit of mitochondrial ATP synthase by regulated expression of the ATP5 gene. Biochem Mol Biol Int 34: 789–799. [PubMed] [Google Scholar]
- 11.Prescott M, Higuti T, Nagley P, Devenish RJ (1995) The functional expression of a rat cDNA encoding OSCP in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 207: 943–949. [DOI] [PubMed] [Google Scholar]
- 12.Ng YS, Martikainen MH, Gorman GS, et al. (2019) Pathogenic variants in MT-ATP6: A United Kingdom-based mitochondrial disease cohort study. Ann Neurol 86: 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganetzky RD, Stendel C, McCormick EM, et al. (2019) MT-ATP6 mitochondrial disease variants: Phenotypic and biochemical features analysis in 218 published cases and cohort of 14 new cases. Hum Mutat 40: 499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carraro M, Jones K, Sartori G, et al. (2020) The Unique Cysteine of F-ATP Synthase OSCP Subunit Participates in Modulation of the Permeability Transition Pore. Cell Rep 32: 108095. [DOI] [PubMed] [Google Scholar]
- 15.He J, Carroll J, Ding S, Fearnley IM, Montgomery MG, Walker JE (2020) Assembly of the peripheral stalk of ATP synthase in human mitochondria. Proc Natl Acad Sci U S A 117: 29602–29608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KM, Chen X, Boitano A, Swenson L, Opipari AW Jr., Glick GD (2005) Identification and validation of the mitochondrial F1F0-ATPase as the molecular target of the immunomodulatory benzodiazepine Bz-423. Chem Biol 12: 485–496. [DOI] [PubMed] [Google Scholar]
- 17.Morava E, Rodenburg RJ, Hol F, et al. (2006) Clinical and biochemical characteristics in patients with a high mutant load of the mitochondrial T8993G/C mutations. Am J Med Genet A 140: 863–868. [DOI] [PubMed] [Google Scholar]
- 18.Knight KM, Shelkowitz E, Larson AA, et al. (2020) The mitochondrial DNA variant m.9032T > C in MT-ATP6 encoding p.(Leu169Pro) causes a complex mitochondrial neurological syndrome. Mitochondrion 55: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olahova M, Yoon WH, Thompson K, et al. (2018) Biallelic Mutations in ATP5F1D, which Encodes a Subunit of ATP Synthase, Cause a Metabolic Disorder. Am J Hum Genet 102: 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Meirleir L, Seneca S, Lissens W, et al. (2004) Respiratory chain complex V deficiency due to a mutation in the assembly gene ATP12. J Med Genet 41: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diodato D, Invernizzi F, Lamantea E, et al. (2015) Common and Novel TMEM70 Mutations in a Cohort of Italian Patients with Mitochondrial Encephalocardiomyopathy. JIMD Rep 15: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll J, He J, Ding S, Fearnley IM, Walker JE (2021) TMEM70 and TMEM242 help to assemble the rotor ring of human ATP synthase and interact with assembly factors for complex I. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonckheere AI, Huigsloot M, Lammens M, et al. (2011) Restoration of complex V deficiency caused by a novel deletion in the human TMEM70 gene normalizes mitochondrial morphology. Mitochondrion 11: 954–963. [DOI] [PubMed] [Google Scholar]
- 24.Ghezzi D, Zeviani M (2018) Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem 62: 271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.