Abstract
Background
Allergy to cow's milk is the most common food allergy in infants and it is usually outgrown by 5 years of age. In some individuals it persists beyond early childhood. Oral immunotherapy (OIT, oral desensitization, specific oral tolerance induction) has been proposed as a promising therapeutic strategy for persistent IgE-mediated cow's milk allergy. We previously published the systematic review of OIT for cow's milk allergy (CMA) in 2010 as part of the World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines.
Objective
To systematically synthesize the currently available evidence about OIT for IgE-mediated CMA and to inform the updated 2022 WAO guidelines.
Methods
We searched the electronic databases including PubMed, Medline, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and the websites of selected allergy organizations. We included all studies irrespective of the language of the original publication. The last search was conducted in February 2021. We registered the protocol on Open Science Framework (10.17605/OSF.IO/AH2DT).
Results
We identified 2147 unique records published between 2010 and 2021, including 13 randomized trials and 109 observational studies addressing cow's milk OIT. We found low-certainty evidence that OIT with unheated cow's milk, compared to elimination diet alone, increased the likelihood of being able to consume ≥150 ml of cow's milk in controlled settings (risk ratio (RR): 12.3, 95% CI: 5.9 to 26.0; risk difference (RD): 25 more per 100, 95% CI 11 to 56) as well as accidently ingest a small amount (≥5 ml) of cow's milk (RR: 8.7, 95% CI: 4.7 to 16.1; RD: 25 more per 100, 95% CI 12 to 50). However, 2–8 weeks after discontinuation of a successful OIT, tolerance of cow's milk persisted in only 36% (range: 20%–91%) of patients. OIT increased the frequency of anaphylaxis (rate ratio: 60.0, 95% CI 15 to 244; rate difference 5 more anaphylactic reactions per 1 person per year, 95% CI: 4 to 6; moderate evidence) and the frequency of epinephrine use (rate ratio: 35.2, 95% CI: 9 to 136.5; rate difference 268 more events per 100 person-years, 95% CI: 203 to 333; high certainty). OIT also increased the risk of gastrointestinal symptoms (RR 6.9, 95% CI 1.6–30.9; RD 28 more per 100, CI 3 to 100) and respiratory symptoms (RR 49.0, 95% CI 3.12–770.6; RD 77 more per 100, CI 62 to 92), compared with avoidance diet alone. Single-arm observational studies showed that on average 6.9% of OIT patients (95% CI: 3.8%–10%) developed eosinophilic esophagitis (very low certainty evidence). We found 1 trial and 2 small case series of OIT with baked milk.
Conclusions
Moderate certainty evidence shows that OIT with unheated cow's milk in patients with IgE-mediated CMA is associated with an increased probability of being able to drink milk and, at the same time, an increased risk of serious adverse effects.
Keywords: Milk allergy, Oral immunotherapy, Systematic review, Meta-analysis, GRADE
Introduction
Description of the condition
IgE-mediated cow's milk allergy (CMA) is the most common food allergy in infants, affecting approximately 2% of the population under 4 years of age worldwide.1 International studies have highlighted significant between-country differences, with a reported incidence of IgE-mediated CMA ranging from less than 1% to about 2.3% in children aged up to 2 years.2,3 Up to 50% of individuals suffering from CMA outgrow it by 3 to 5 years of age,4, 5, 6 with further reduction during childhood and adolescence.7,8 The prevalence of challenge-confirmed CMA in adults varies depending on the methods of data collection and outcome ascertainment,9 ranging between 0.1% and 0.3%.1,3,10,11
Cow's milk is the third most common food, after peanut and tree nuts, to elicit food-induced anaphylaxis in pediatric and mixed-age populations responsible for 10 to 19% of cases,12, 13, 14 even though recent report from the United Kingdom suggests a more prominent role for cow's milk.15
The mainstay of therapy is strict avoidance of cow's milk and using rescue epinephrine in the event of anaphylaxis.16, 17, 18 However, eliminating cow's milk from the diet can be difficult and can pose nutritional as well as quality of life concerns since cow's milk is a ubiquitous food in many cultures and diets and is a prominent nutritional source in early childhood.19 This adds to the already significant anxiety and quality of life impairment associated with the unpredictability of allergic reactions.20
Due to the scarcity of therapeutic options and the difficulty in implementing them, there is a growing public, medical, and commercial interest in the therapeutic potential of oral immunotherapy (OIT).21, 22, 23
Description of the intervention
OIT for CMA involves a titrated administration of cow's milk orally at regular intervals to modulate specific immune response to milk proteins in patients with CMA.
Despite the differences among published protocols,24 they share some common key features. Usually, an OIT schedule begins with a build-up phase in which an increasing amount of milk is administered by a physician in a specialized clinical setting properly equipped in case of anaphylaxis. Thereafter, the highest tolerated dose is taken daily at home. The doses are usually increased either weekly or every other week until a determined threshold dose is reached. At this stage, a maintenance phase begins, during which patients usually consume a daily consistent dose of cow's milk and dairy products (often the maximum tolerated amount at the end of a build-up phase). Schedules differ in number of doses, administered product (fresh or baked milk, mixed in different excipient types), amount of milk proteins per dose, interval in-between doses, and maintenance dose. Furthermore, multiple protocols have been developed for OIT either for single-food or for multiple-foods simultaneously.
The previous World Allergy Orgnization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines in 2010 were informed by a systematic review, which found an increased probability to safely consume milk following OIT treatment (ie, desensitization) as compared with avoidance diet alone.25 Furthermore, the review highlighted how benefits in milk desensitization were associated with some safety concerns, namely a significant increase in the risk of allergic reactions and a greater necessity for rescue therapy. The aim of this review is to update our previous evidence synthesis and inform the novel WAO DRACMA guidelines.
Methods
Search strategy and selection criteria
This is an update of the systematic review we performed in 2010.25 A systematic literature search has been performed from January 2010 to February 2021. We ran separate searches for fresh milk and baked milk OIT. In both cases we have conducted searches for systematic reviews, randomized controlled trials (RCTs), and non-randomized studies (NRSs), in the data repositories described below:
-
a)
Systematic reviews: PubMed (NLM), Cochrane Reviews, Database of Abstracts of Reviews of Effects, National Institute for Health and Care Excellence (NICE), Canadian Agency for Drugs and Technologies in Health (CADTH), Agency for Healthcare Research and Quality (AHRQ), and Epistemonikos;
-
b)
RCTs and NRSs: OVID Embase and MEDLINE, Cochrane Central Register of Controlled Trials, and PubMed (NLM).
We searched for published and unpublished data in any language comparing cow's milk OIT with no OIT (placebo or strict milk avoidance) for the treatment of CMA. A professional librarian in collaboration with clinical and methodology experts developed specific search strategies without any methodology filters or language restrictions. We also reviewed the references of identified studies and selected narrative review articles.
Searching for systematic reviews we also included guidelines reporting a systematic review investigating cow's milk OIT for IgE-mediated CMA. Searching for individual studies we included both RCTs and NRSs (with at least 5 patients) of cow's milk OIT (either fresh or baked) in patients with confirmed IgE-mediated CMA. We present a detailed description of the search strategies and the inclusion and exclusion criteria in the online Supplemental Appendices 1 and 2.
Types of outcome measures
The WAO DRACMA guideline panel members specified a priori all outcomes of interest for this review. Panel members also rated the relative importance of all outcomes for decision-making following the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.26,27 We sought information for outcomes classified as either critical or important. Outcomes critical to the decision were: anaphylaxis; use of intramuscular (IM) epinephrine, adverse events leading to treatment discontinuation; severe gastrointestinal symptoms; severe respiratory symptoms; generalized erythema or urticaria-angioedema; ability to drink cow's milk and eat dairy products without a reaction; ability to accidently consume a small amount of cow's milk without a reaction; tolerance of cow's milk when it is reintroduced after a period of not consuming milk and milk products (i.e. sustained unresponsiveness); and emergency department visits. We accepted study authors' definitions of severe adverse effects. The outcomes deemed important but not critical for the decision were: hospital admission; death; mild respiratory symptoms; lip or mouth pruritus; angioedema; eosinophilic esophagitis; quality of life of pediatric patients; quality of life of caregivers. The same set of outcomes and respective importance ratings were considered for OIT with both fresh and baked cow's milk.
Data collection, evaluation, and analysis
We screened titles and abstracts of the identified records and subsequently reviewed full-text articles in duplicate using standardized pre-piloted forms on Rayyan online software.
Any disagreements were resolved by consensus. We corresponded with investigators of primary studies, where appropriate, to clarify study eligibility and to request further information about missing results. We extracted data about methodological quality, characteristics of a trial, setting, eligibility criteria, population studied, intervention, comparator and outcomes of interest using a standardized data collection form in Microsoft Excel.
We used the Newcastle-Ottawa Scale (NOS) to assess the risk of bias (RoB) in NRSs and the revised Cochrane RoB tool for RCTs (RoB 2.0) separately for each outcome.28,29 We classified studies as high RoB if at least 1 domain suggested the high risk. We evaluated the certainty of evidence (ie, quality of evidence or confidence in the estimates of effects) for each outcome following the GRADE methodology.26,27
For the assessment of treatment effects, we analyzed outcomes following the intention-to-treat principle.30 When a single study was published in multiple reports, we used relevant data from all reports and analyzed them as a single study. When appropriate, we combined the results across studies using meta-analysis. We used DerSimonian-Laird random-effects model unless specifically stated otherwise.30
For single-arm observational studies we used the restricted Maximum-Likelihood random-effects model to estimate the pooled weighted proportion for dichotomous data (ie, outcome that occurred only once in a single person) and the pooled incidence rate for count data (ie, outcome that could occur more than once in a single person). For studies with a comparison group, we estimated a pooled risk ratio (RR) for dichotomous data and a pooled incidence rate ratio23 for count data. For outcomes with zero events in 1 or both groups in any study we employed a continuity correction either by adding 0.5 at the numerator as described by Yates, or by adding the reciprocal of the opposite group arm size.31,32 For frequency data, we estimated person-time of follow-up by multiplying the number of participants by the reported duration of observation. We combined logarithmically transformed data and converted the results back to counts (nota bene: the numbers in the attached forest plots are natural logarithms of the outcome data). We estimated the absolute effects for dichotomous data by multiplying pooled RR by the baseline risk in the control groups and for the count data by an incidence rate difference (IRD) meta-analysis.
We evaluated the heterogeneity among studies by visual examination of forest plots and using χ2 and I2 statistics. We evaluated the publication bias visually by inspecting funnel plots if at least 10 studies were available for a given outcome.
We did all statistical analyses using jamovi version 1.6.33 We used GRADEpro to create the summary of findings tables.34
We conducted and reported this systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), GRADE, and Cochrane standards.30,35, 36, 37 We registered the protocol for this review in Open Science Framework (10.17605/OSF.IO/AH2DT). All decisions regarding the research question, analytic approach and RoB assessment were defined a priori.
Results
Existing systematic reviews
We found 8 systematic reviews published after the previous WAO DRACMA guidelines in 2010 that included studies of cow's milk OIT.25,38, 39, 40, 41, 42, 43, 44 No review was recent enough and/or reported outcomes of interest to be used for the WAO DRACMA guidelines without an update.
Included studies
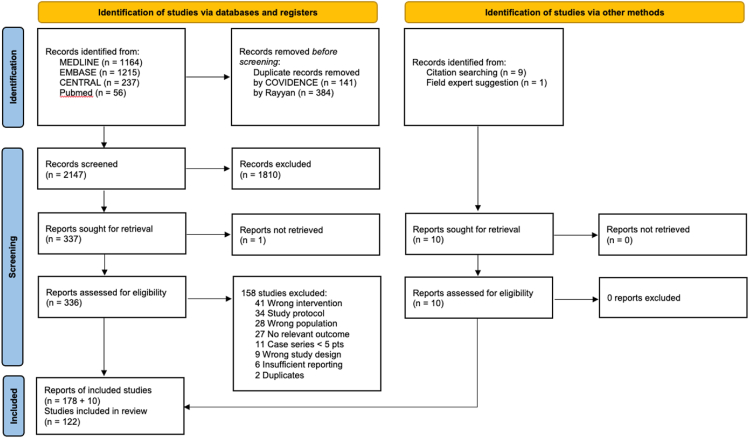
Searching for individual studies we identified 2672 records and reviewed 336 full-text articles (Fig. 1). The most recent update of the search was in February 2021. We included 188 reports of 13 RCTs and 109 NRS. Of the included studies, 1 trial and 2 NRSs reported OIT with baked milk.45, 46, 47 All reports on the same study were regarded as a single study in the analyses. Online Supplemental Table 1 provides a thorough description of the included studies; while Online Supplemental Table 2 shows the records excluded during full-text screening together with reasons for exclusion.
Fig. 1.
PRISMA diagram for the flow of records/studies through the study selection process
Randomized studies enrolled 454 participants (mean across trials: 35, range: 11 to 60), aged 8.4 years on average (mean range, 2–14 years), undergoing OIT (whole milk: 10 trials, pasteurized milk: 2 trials, baked milk: 1 trial) compared with no OIT (placebo: 5 trials, avoidance diet only: 7 trials). One trial48 failed to disclose data regarding the comparator and was therefore excluded from any subsequent analysis. A total of 6131 participants was enrolled in the included NRSs (median 35; range 5–710), with a median age of 7.5 years (range 3 months–32 years). Milk allergy was confirmed by oral food challenge (OFC) at study entry in 11 RCTs and 69 NRS. The trials presented a marked heterogeneity in terms of subjects’ baseline milk tolerance, which ranged from 0.5 ml to 200 ml of whole milk. The median target dose for desensitization was set to 200 ml whole milk, ranging from 76 ml49 to 300 ml. RoB was high in 4 trials.50, 51, 52, 53 There were “Some Concerns” about the RoB in 6 of the included RCTs.47,49,54, 55, 56, 57 The other trials reporting relevant outcomes were judged to be at low RoB.58,59 We have deemed the RoB to be not significantly different across the individual reported outcomes. The full assessment of the RoB for RCTs is available in the Online Supplemental Table 3. We did not detect any strong evidence of publication bias for all outcomes. We judged the RoB in the observational studies as high since all were single-arm studies requiring implicit comparison with historical controls (Online Supplemental Table 4).
Effects of interventions
OIT with fresh milk
Ability to drink cow's milk and eat dairy products without a reaction
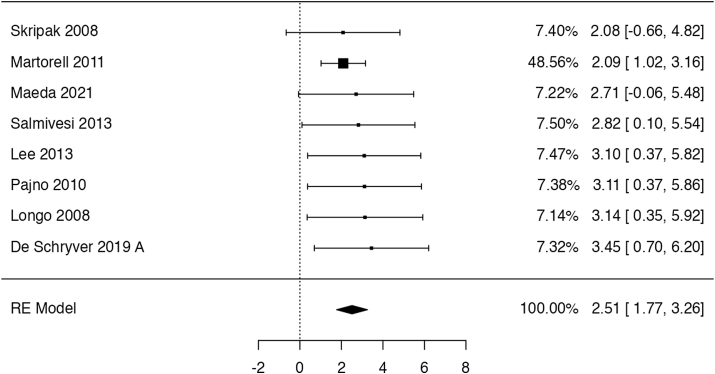
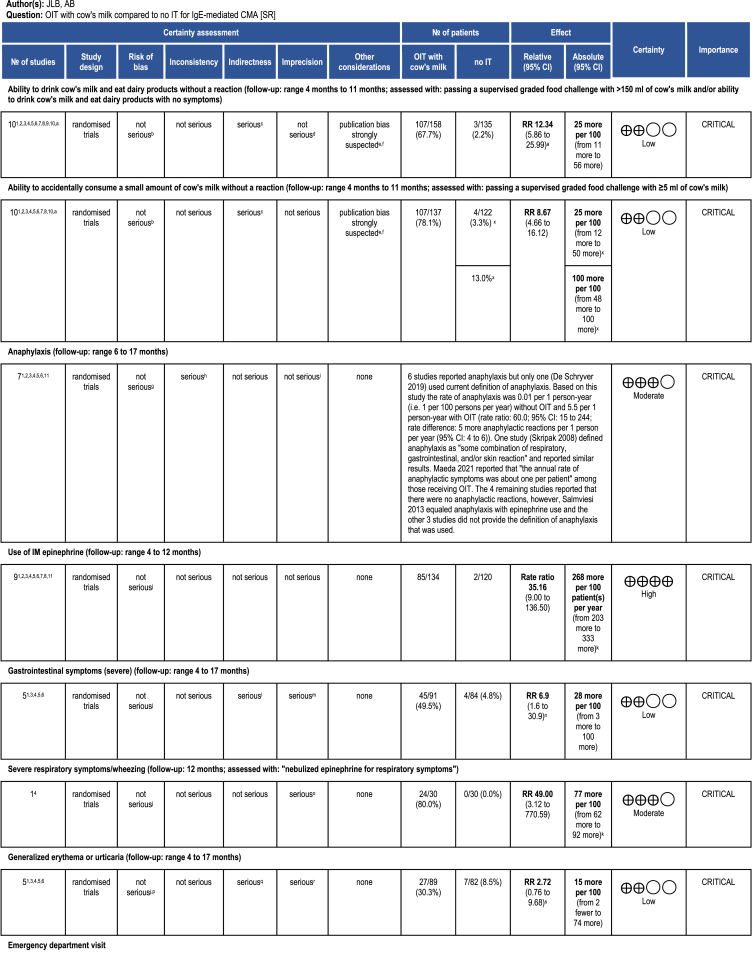
In 8 trials49,51,54, 55, 56, 57, 58, 59 (283 participants; follow-up: 4–11 months), OIT might have increased the probability of completing a food challenge with ≥150 ml of cow's milk (risk ratio: 12.34, 95% CI: 5.86 to 25.99; risk difference: 25 more per 100 patients, 95% CI: 11 to 56 more; Fig. 2). One additional study52 explicitly included only children that could tolerate at least 60 ml of milk at baseline and found a smaller effect of OIT (risk ratio: 1.44, 95% CI: 0.98 to 2.11). Another RCT50 published as a conference abstract reported desensitization in 4/11 children receiving OIT but failed to disclose outcome data of the control group (n = 4). We have considered the evidence for this outcome to be of low certainty due to serious indirectness, as it is not certain to what extent passing a graded food challenge represents the ability to drink milk in the real-world. There is also some risk of publication bias, as all studies were small, showing very large effects.
Fig. 2.
Logarithm of the risk ratio of consuming at least 150 ml of milk in the RCTs
Ability to accidently consume at least a small amount of cow's milk (5 ml) without a reaction
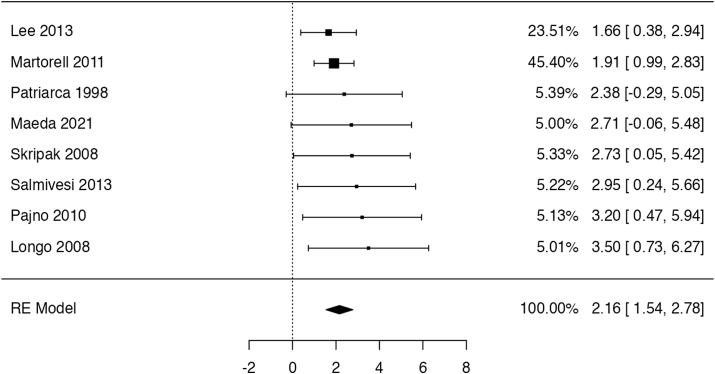
Eight trials49,51,53,55, 56, 57, 58, 59 completing a controlled oral food challenge with at least 5 ml of unheated milk (259 participants; follow-up: 4–11 months). The pooled results showed that OIT led to an increased probability of completing the oral food challenge with at least 5 ml of unheated milk (risk ratio: 8.67, 95% CI: 4.66 to 16.12; risk difference: 25 more per 100 patients, 95% CI: 12 to 50 more; Fig. 3). One study51 did not report the outcome data for the control group. We have considered the study to have 0 cases among controls, then performed sensitivity analysis assuming the effect to be equal to the largest observed in other studies, which showed no difference in results or interpretation. We rated the certainty of the evidence as low due to serious indirectness as well as suspect of publication bias.
Fig. 3.
Logarithm of risk ratio for consuming at least 5 ml of milk in the RCTs
Desensitization to more than 5 ml of milk after an avoidance period
We identified 13 observational studies60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72 (582 participants, with an avoidance period ranging from 2 to 8 weeks) with relevant data. Out of the cohort studies, only 4 reported usable data for both the intervention arms.61,65, 66, 67, 68 The analysis showed that OIT might increase the probability of not having an allergic reaction upon allergen reintroduction by oral food challenge (risk ratio: 3.35, 95% CI: 1.26 to 8.87; risk difference: 21 more per 100 patients, 95% CI: 2 to 72 more), but the evidence is very uncertain due to serious concerns of risk of bias and estimates’ imprecision.
Anaphylaxis
Seven trials49,51,54, 55, 56,58,59 reported either the number of patients who experienced anaphylaxis or the number of events of anaphylaxis per each group of patients. However, only 1 study54 applied an accepted definition of anaphylaxis, considered as “the involvement of 2 organ systems and/or hypotension in response to a cow's milk protein exposure”. The rate of anaphylaxis in this study was 1 per 100 patient-years with elimination diet alone and 550 per 100 person-years with OIT (rate ratio: 60.0, 95% CI: 15 to 244). In another study, Skripak and colleagues49 defined anaphylaxis as “some combination of respiratory, gastrointestinal, and/or skin reaction” and reported the effect estimates pointing in the same direction (rate ratio: 790, 95% CI: 0 to 3 × 109). The remaining 5 studies reported no anaphylactic reactions; however, they either did not provide the definition of anaphylaxis that they used or equated it with epinephrine use (Table 1).
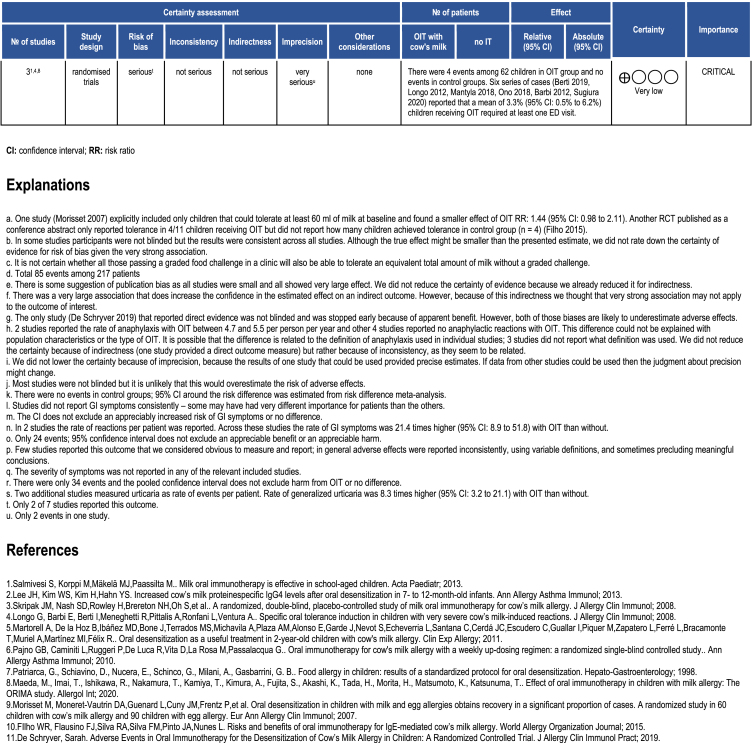
Table 1.
Evidence Profile of fresh milk OIT compared to no OIT
Use of intramuscular (IM) epinephrine (adrenaline)
Nine trials49,51,53, 54, 55, 56, 57, 58, 59 reported data on intramuscular (IM) epinephrine injection, with a follow-up range of 4–12 months. In 8 studies,49,51,53, 54, 55, 56, 57, 58 OIT increased need for IM epinephrine (rate ratio: 35.16, 95% CI: 9 to 136.5; rate difference: 268 more per 100 patient -years 95% CI: 203 to 333 more per 100 patient-years; Fig. 4). The finding was supported by the combined relative estimates from studies49,51,53,55,57,59 reporting dichotomous outcomes both for the induction and maintenance phases (risk ratio: 2.2, 95% CI: 0.57 to 8.58 for induction; 3.1, 95% CI: 0.85 to 11.17 for maintenance).
Fig. 4.
Logarithm of the rate ratio of IM epinephrine injections per patient-month in the RCTs
Adverse effects leading to the discontinuation of treatment
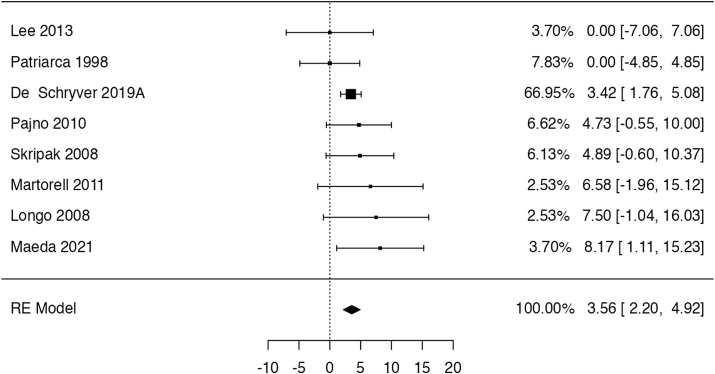
In 8 trials,49,51,54, 55, 56, 57, 58, 59 with 306 patients, OIT likely increased the risk of discontinuing treatment due to adverse effects, but the estimates are imprecise (risk ratio: 1.92, 95% CI: 0.92 to 3.99; risk difference: 6 more per 100 patients, 95% CI: from 0 to 18 more; Fig. 5). One study54 did not report whether 4 discontinuations in the control group were due to AE. We assumed they were, but if we had assumed the discontinuations to be not AE-related, still there would have been no difference in the effect estimate (risk ratio: 2.52, 95% CI: 0.9 to 7.1; risk difference: 9 more per 100 patients, 95% CI: from 1 fewer to 38 more). The certainty of evidence for this outcome was rated as moderate, given the limited number of events among both groups (only 31), and that the confidence interval includes both appreciable harm with OIT and no difference.
Fig. 5.
Logarithm of the risk ratio of AE leading to discontinuation of treatment in the RCTs
Severe gastrointestinal symptoms
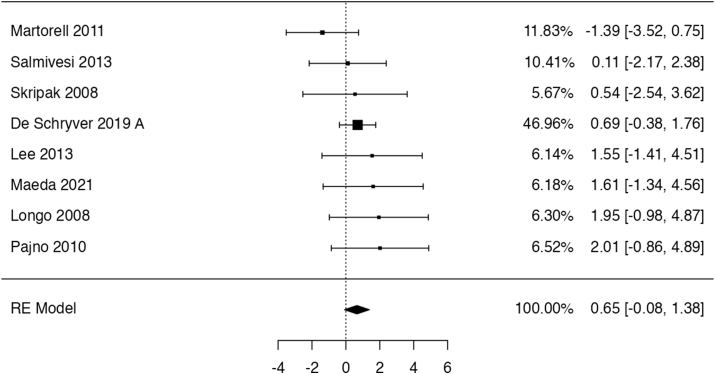
Five trials49,55,56,58,59 reported data on gastrointestinal symptoms (175 participants; follow up: 4–17 months), however no study provided information about their severity. Four studies55,56,58,59 reported the risk of any GI symptoms. In these trials OIT might have increased the risk for developing any GI symptoms (risk ratio: 6.9, 95% CI: 1.6 to 30.9; risk difference: 28 more per 100 patients, 95% CI: 3 to 100 more; Fig. 6). The rate of GI reactions per patient was reported in 2 studies49,55 and was 21.4 times higher with OIT than without (95% CI: 8.9 to 51.8). The certainty of evidence for this outcome was low, because of serious indirectness (no information about severity of symptoms) and imprecision.
Fig. 6.
Logarithm of the risk ratio of gastrointestinal AE in the RCTs
Severe respiratory symptoms/wheezing
Only 1 trial55 reported relevant data on severe respiratory symptoms, defined as “nebulized epinephrine for respiratory symptoms” (60 participants, 12 months follow-up). The risk of severe respiratory symptoms was likely higher among those who received OIT, compared with those who did not (risk ratio: 49, 95% CI: 3.1 to 770.6; risk difference: 77 more per 100 patients, 95% CI: 62 to 92 more). The certainty of evidence has been rated as moderate, due to the fragility of the results (only 24 events in 1 group) and some suspicion of reporting bias (only 1 study reported severe respiratory symptoms). Remaining studies either did not report this outcome or reported explicitly mild/moderate symptoms.
Generalized erythema and/or urticaria
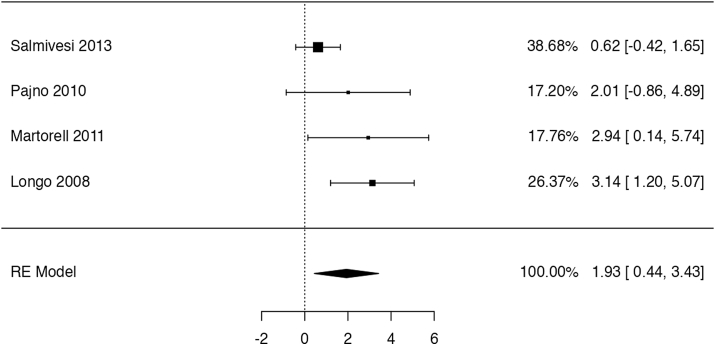
We identified 5 studies reporting relevant data, but severity of symptoms was not described in any. In 4 trials55,56,58,59 with a total of 171 participants and 4–17 months of follow-up OIT might have increased the risk of generalized erythema or urticaria, but the confidence interval did not exclude appreciable harm or no effect (risk ratio: 2.72, 95% CI: 0.76 to 9.68; risk difference: 15 more per 100 patients, 95% CI: 2 fewer to 74 more; Fig. 7). Two studies49,55 reported the rate of events per patient, which was found to be 8.3 times higher (95% CI: 3.2 to 21.1) with OIT than without. We considered this outcome to be of low certainty due to serious imprecision and indirectness.
Fig. 7.
Logarithm of the risk ratio of generalized erythema or urticaria in the RCTs
Angioedema
Four included trials55,56,58,59 (111 participants, with follow-up from 4 to 17 months) reported the occurrence of angioedema. One study55 failed to disclose data for both the intervention arms, hence we excluded it from this analysis. The pooled estimate showed that OIT probably increases the risk of angioedema (risk ratio: 4.66, 95% CI: 0.85 to 25.85; risk difference: 12 more per 100 patients, 95% CI: 2 to 22 more). Considering the imprecision in the resulting effect estimate, we have judged the evidence to be of moderate certainty.
Emergency department visit
Only 3 RCTs55,57,59 reported emergency department visits. There were 4 events among 62 children receiving OIT, and none in the control group. Since there were no events in any control group, we did not calculate a pooled risk ratio but rather estimated a pooled proportion of emergency department visits in those receiving OIT which was 5% (95% CI: 0 to 10). The certainty of the evidence for this outcome has been rated as very low due to very serious imprecision and risk of bias. Because of the very low certainty evidence from RCTs we also reviewed 6 single-arm studies73, 74, 75, 76, 77, 78 in which the pooled proportion of emergency department visits was 3.3% (95% CI: 0.5%–6.2%).
Hospital admission
No RCT reported hospital admissions. Six non-randomized studies73,74,76,79, 80, 81 reported that there were no hospitalizations among the 264 patients.
Lip or perioral pruritus
We found 4 trials51,55,56,59 (144 participants, with follow-up from 4 to 17 months) reporting on this outcome, yet 1 study51 failed to disclose the control group data, hence it has been excluded from this analysis. The quantitative analysis showed that OIT might increase the risk to present perioral pruritus (risk ratio: 12.76, 95% CI: 2.5 to 65.4; risk difference: 17 more per 100 patients, 95% CI: 2 to 95 more; Fig. 8). The certainty of the evidence for this outcome has been rated as low due to imprecision in effect estimates as well as concerns about risk of bias.
Fig. 8.
Logarithm of risk ratio of manifesting perioral pruritus in the RCTs
Eosinophilic esophagitis
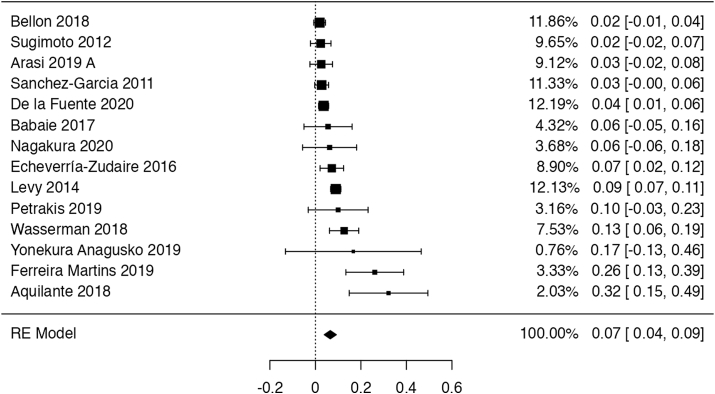
Fourteen series of cases82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95 (1545 participants with 2 years follow-up) reported that, on average, 6.9% (95% CI: 3.8%–10%) of patients receiving OIT developed eosinophilic esophagitis (EoE) (Fig. 9). The certainty of the evidence has been rated as very low due to indirectness (EoE was not confirmed with biopsy in most studies) and very serious concerns about risk of bias. The synthesis from studies with biopsy sampling yielded a slightly more conservative mean percentage of 5.0% (95% CI: 4.5%–5.4%).
Fig. 9.
Weighted mean proportion of patients developing EoE after OIT in the NRS
Death
Seven trials (follow-up: 4–11 months) reported no deaths in either intervention or control groups, with an overall study population of 277 children between 2 and 14 years of age.48,50,51,54, 55, 56,58 The certainty of the evidence has been rated as high.
Impact on quality of life
Five observational studies96, 97, 98, 99, 100 reported relevant data, with the outcome being measured after a median of 5 months following the OIT (range 1–43 months). In 4 of these studies, the authors employed either the parent or patient forms of the food allergy quality of life (FAQL) questionnaires. Epstein Rigbi and colleagues asked parents to assess the QoL of their children (n = 82; mean age 6 years) using the food allergy quality of life questionnaire parent form (FAQLQ-PF; minimal important difference: 0.5 point).96 Authors found that after 4 months of OIT total FAQLQ score improved in 37% of children, did not change in 38% and deteriorated in 26%. Carraro and colleagues also used FAQLQ-PF to assess QoL in 30 children (age: 3–12 years) 2 months after completion of OIT.97 The authors did not report a total score or the proportion of children who improved or deteriorated by ≥ 0.5 points. However, they noted that a difference between the median pre and post-OIT scores of −0.7 point for emotional impact, −0.94 point for food-related anxiety, and −1.5 point for social and dietary limitations, which might show a potential overall improvement in QoL. Hayashi and colleagues reported their results only as a conference abstract.98 Forty-six children with prior history of anaphylaxis underwent rush OIT. Study authors reported a consistent reduction in the percentage of both children and parents fearing the consequences of accidental allergen exposure or experiencing severe anxiety due to the allergic condition. Katz and colleagues assessed the QoL in 192 children 25 months after completion of OIT.99 They also reported their results only as a conference abstract. The authors did not report how quality of life was measured but stated that 88.9% of children improved.
Kauppila and colleagues used both the FAQLQ and the generic health-related quality of life (HRQoL) questionnaires (15D, 16D, and 17D).100 The authors measured the outcome post OIT in 295 patients (response rate for FAQLQ 48% and for generic HRQoL 54%). No difference in generic HRQoL was observed between OIT patients and the age- and gender-standardized general population. There was also no difference in the HRQoL scores between those who achieved desensitization and those who did not. However, mean FAQLQ scores were lower (better QoL) in those who achieved desensitization, compared with those who did not (FAQLQ-children, n = 47, score 1.7 vs 2.8; FAQLQ-teenager, n = 64, score 2.1 vs 2.9; FAQLQ-adult, n = 31, score 2.3 vs 2.7). We rated the certainty of the evidence as very low, due to very serious concerns of risk of bias and imprecision. Additionally, we noticed a major heterogeneity in the outcome measurement modalities across the included studies.
We have not found any eligible study measuring the impact of OIT specifically on caregivers’ quality of life.
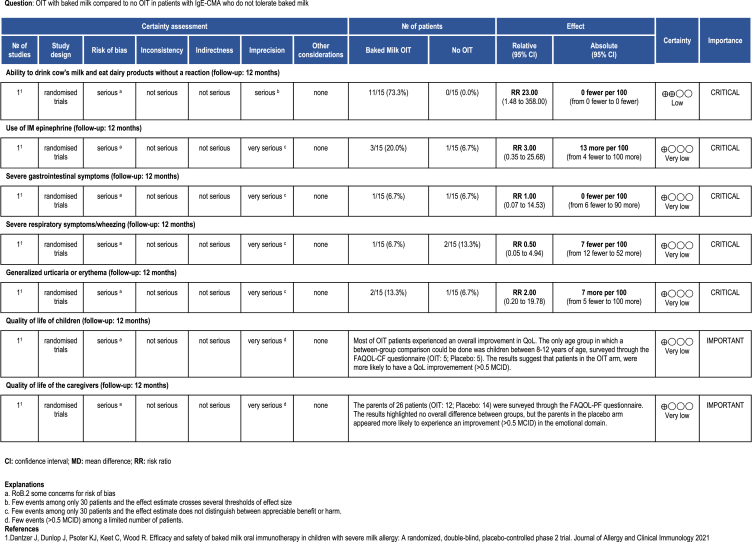
OIT with baked milk
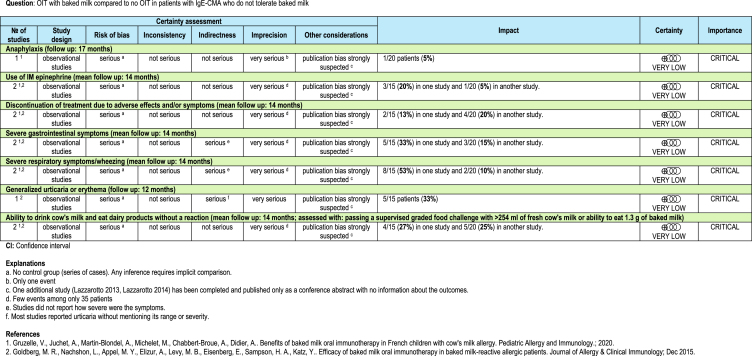
We found 1 randomized controlled trial and 2 series of cases of OIT with baked milk among patients who did not tolerate both unheated and baked milk.45, 46, 47 The mean follow-up for the 2 studies was 14 months. Tables 2A and 2B present the results of the studies.
Table 2B.
Evidence Profile from the included non-randomized studies.
Table 2A.
Evidence profile from the included controlled randomized trials.
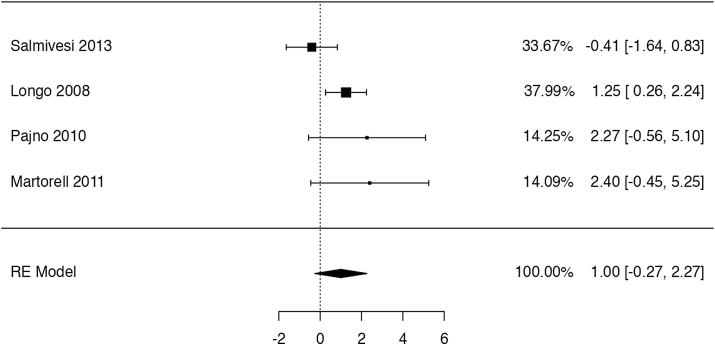
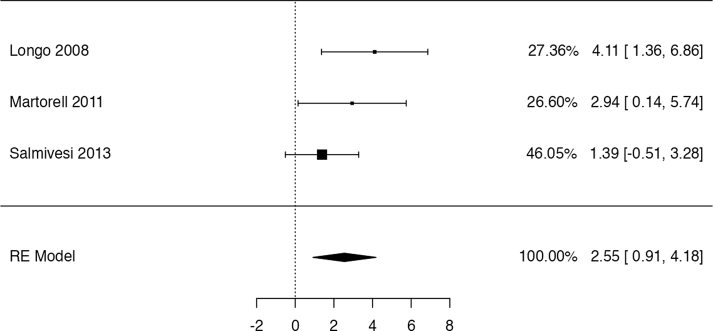
The identified trial by Dantzer et al47 shows that OIT with baked milk might increase the probability to ingest dairy products without a reaction (risk ratio: 23, 95% CI: 1.48 to 358; risk difference: 0 fewer per 100 patients). On the other hand, the study provides evidence of very low certainty for the remainder for the reported outcomes, not allowing to discriminate any appreciable harm or benefit associated to OIT with baked milk (Table 2). The trial reported data on QoL for both patients and caregivers, highlighting no major difference between the age-stratified group score changes. Still, when considering a 0.5 change in score as MID for improvement, the authors found that parents in the placebo group were more likely to improve with the respect to the “emotional impact domain” of the FAQOL-PF questionnaire, adn that patients in the OIT arm, were more likely to have a QoL improvement in at least 1 domain.
With respect to the case series studies, 1 study reported data on anaphylaxis, which occurred in 5% (1/20) participants.46 Both studies45,46 reported the need for intramuscular (IM) epinephrine in 20% (3/15) and 5% (1/20) of patients (5%) respectively. The percentage of patients who discontinued treatment owing to adverse effects was 13% (2/15) in 1 study and 20% (4/20) in the other. Severe gastrointestinal symptoms occurred in 33% (5/15) and 15% (3/20) of participants, while severe respiratory symptoms/wheezing in 53% (8/15) and 10% (2/20). One study reported generalized urticaria or erythema in 33% (5/15) of patients. After 1 year 4/15 patients (27%) in 1 study were able to eat 1.3 g of baked milk and 5/20 (25%) in another study were able to drink 254 ml of fresh cow's milk without a reaction. No other outcome of interest was reported by studies assessing OIT with baked milk.45,46
For all outcomes the certainty of the evidence was very low because of serious risk of bias (all studies were single-arm series of cases) and serious imprecision (few events among only 35 patients). There was also a strong suspicion of publication bias.
Discussion
In this systematic review we identified a body of evidence suggesting that the current OIT approaches likely increase the ability to ingest milk, as assessed by in-clinic supervised food challenge, while also increasing the risk of severe allergic reactions, such as anaphylaxis and other serious adverse events. The data on quality of life is very uncertain due to very high risk of bias and major lack of standardization in outcome measurement and reporting. We found only 2 small series of cases describing the effects of OIT with baked milk. Given the absence of data from non-OFC setting, the choice of outcomes has primarily focused on OFC setting, making the possibly strong assumption this might act as a surrogate of real-world scenario.
Strengths and limitations
The major strength of this review lies in the extensive and comprehensive search for both RCTs and NRSs. Furthermore, we have conducted a transparent appraisal of the evidence following the GRADE approach and summarized all findings in the evidence profiles. On the other hand, the study limitations are the limited search of the gray literature, and the employment of the NOS rather than the ROBINS-I tool to assess the risk of bias for observations studies. The decision about the assessment of the studies’ quality was to account for the fact that most studies lacked a control arm, while the lack of a systematic gray literature search was justified by the assistance of the WAO DRACMA guideline panel, which informed us of the absence of any relevant information outside of the indexed data repositories.
In light of this, most of the limitation of our research stems from the shortcoming in the identified body of the evidence, with the main one being the heterogeneity of the included populations, OIT protocols, and measured outcomes. Specifically, study authors have either employed diverging outcome definitions (e.g., anaphylaxis occurrence and severity) or have failed to report them altogether.
Secondly, a significant portion of the current literature consists of observational studies, either with a single-arm design or the use of historical comparison between groups, which are not properly adjusted for confounding or equally monitored. Also, we could not have full access to data from some studies. Still, these usually had a limited sample size, hence even if included, it is unlikely they would contribute significant information or meaningfully alter the overall results. Lastly, we found limitations in the reporting by study authors, who failed to provide results stratified by potentially relevant modifiers of effect (eg, age groups), which made it impossible to account for them in the evidence synthesis process.
Research implications
There is a current debate about the aim of OIT, with most studies viewing the ability to sustain a food challenge, the current diagnostic standard for CMA, as a measure of success.
In our view, there are some limitations, coming from both the assessment of OIT success and how the procedure is implemented in research:
-
A)
This review has highlighted a dire need for a standardization in outcome assessment and reporting, as this profoundly impacts the heterogeneity in the evidence, hence hampering our ability to estimate OIT effect.
-
B)
The oral challenge, despite being the current golden standard, lacks a standardized interpretation, further aggravating evidence heterogeneity.
-
C)
The assumption of OIT being successful upon completion of an oral food challenge needs to be further validated as a predictor of patients' future risk and frequency of allergic reactions in the real world (i.e., outside the clinic setting).101
-
D)
The assessment of quality of life requires to be standardized through the employment of robust and comprehensive food-allergy specific tools in future RCTs.
Consistently with guidance from GRADE and other institutions,26,102 future research on the topic should include qualitative studies investigating patients' and families’ knowledge about CMA and OIT as well as their values and preferences so to set the primary measures of therapeutic efficacy and safety as better tailored patient-centered outcomes.
Also, given the scarcity of high-quality studies, the scientific community should conduct more large, randomized trials recruiting patients with moderate and severe CMA (including those with previous severe anaphylaxis). These future studies should investigate patient important outcomes and acceptability on a longer time window and carry out detailed stratifications in the analyses to account for differences in OIT protocols or type of allergen. Furthermore, a greater effort should be made to study the effect of baked milk employed in OIT setting. The necessity to intensify research to better define these last points is further highlighted by the recent death of a young patient following a BM-OIT protocol103 and a child who suffered cardio-respiratory arrest with resulting brain damage while undergoing a OIT clinical trial.104
Finally, given the absence of proper economic evidence, further research is needed to understand the resource requirements and cost-effectiveness of OIT. The same necessity applies to understand how immunotherapy outcomes translate into real-world patient's prognosis, so to provide better-tailored treatment strategies based on solid decision-making frameworks.
Clinical implications
This systematic review provides secondary evidence that current approaches to OIT promote desensitization while also increasing the risk of adverse reactions. These data advocate for the investigation and eventual introduction in clinical practice of next-generation cow-milk immunotherapy regimens with an enhanced safety profile, either in the form of additional medications (eg, anti-IgE or anti-IL4R), change in allergen amount64 or administration modality.105 Also, our findings highlight the need for an unequivocal definition of relevant outcomes is needed, as well as consensus on the definition of food allergy.106 Considering the current view of OIT as a potential future model for the treatment of food allergies, combined with the prevalence of food allergy (7.5%), these findings are of major importance to the ongoing development of food allergy therapeutics and enhance patient health.
Role of the funding source
The World Allergy Organization contributed funding to this study. The corresponding author had full access to all data and had the final responsibility for the decision to submit for publication.
Availability of data and materials
Data and materials are provided in the supplementary documents.
Authors’ contributions
JLB, AF, HJS originally conceived this work. AB, DKC, RT, SA, JB wrote its first draft. SW, AB and JLB did the literature search. AB, RF and JLB screened records, evaluated full texts, and extracted data. AB and RF evaluated risk of bias. JLB and AB did the statistical analyses. HJS, DKC, SA, and SW provided critical methodological input. All authors reviewed the manuscript and provided critical intellectual contributions to the analysis and interpretation of the data, and the revision of the manuscript.
Ethics approval
Not applicable.
Members of the WAO DRACMA Guideline Group
Ignacio J. Ansotegui, MD, PhD (Department of Allergy & Immunology, Hospital Quironsalud Bizkaia, Erandio, Bilbao, Spain); Stefania Arasi, MD, PhD (Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy); Amal H. Assa’ad, MD (Division of Allergy and Immunology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA); Sami L. Bahna, MD, DrPH (Allergy/Immunology Section, Louisiana State University Health Sciences Center, Shreveport, LA, USA); Roberto Berni Canani, MD, PhD (Department of Translational Medical Science, University of Naples Federico II, Naples, Italy); Antonio Bognanni, MD (Department of Health Research Methods, Evidence and Impact - HEI, McMaster University, Hamilton, ON, Canada); Martin Bozzola, MD (Department of Pediatrics, Pediatric Allergy/Immunology Section, British Hospital, Buenos Aires, Argentina); Jan Brozek, MD, PhD (Department of Medicine, Division of _ Clinical Immunology and Allergy, Department of Clinical Epidemiology & Biostatistics, McMaster University Health Sciences Centre, Hamilton, ON, Canada); Derek K. Chu, MD, PhD (Department of Medicine, Division of Clinical Immunology and Allergy; Department of Clinical Epidemiology & Biostatistics, McMaster University Health Sciences Centre, Hamilton, ON, Canada); Lamia Dahdah, MD (Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy); Christophe Dupont, MD, PhD (Paris Descartes University, Pediatric Gastroenterology, Necker Hospital, Paris, Clinique Marcel Sembat, Boulogne-Billancourt, France); Motohiro Ebisawa, MD, PhD (Clinical Research Center for Allergy and Rheumatology, National Hospital Organization Sagamihara National Hospital, Kanagawa, Japan); Alessandro Fiocchi, MD (Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy); Ramon Targino Firmino MD (Faculty of Medical Sciences of Campina Grande, UNIFACISA University Centre, Campina Grande, Paraiba, Brazil); Elena Galli, MD, PhD (Pediatric Allergy Unit, Research Center, San PietroFatebenefratelli Hospital, Rome, Italy); Rose Kamenwa, MD (Department of Pediatrics and Child Health, Aga Khan University Hospital, Nairobi, Kenya); Gideon Lack, MBBCh (Department of Women and Children’s Health/Peter Gorer Department of Immunobiology, School of Life Course Sciences, Faculty of Life Sciences & Medicine, King’s College London, UK; Evelina London Children’s Hospital, Guy’s and St Thomas’ Hospital NHS Foundation Trust, London, UK), Haiqi Li, MD (Pediatric Division Department of Primary Child Care, Children’s Hospital, Chongqing Medical University, Chongqing, China); Alberto Martelli, MD (Italian Society of Pediatric Allergy and Immunology, Milano, Italy); Anna H. Nowak-Wegrzyn, MD, PhD (Department of Pediatrics, New York University Langone Health, New York, NY, USA; Department of Pediatrics, Gastroenterology and Nutrition, Collegium Medicum, University of Warmia and Mazury, Olsztyn, Poland); Nikolaos G. Papadopoulos, MD, PhD (Allergy Unit, 2nd Pediatric Clinic, University of Athens, Athens, Greece; Division of Infection, Immunity & Respiratory Medicine, University of Manchester, UK); Ruby Pawankar, MD, PhD (Department of Pediatrics, Nippon Medical School, Bunkyo-Ku, Tokyo, Japan); Maria Said, RN (Allergy & Anaphylaxis Australia (A&AA), Castle Hills, New South Wales, Australia); Mario Sánchez-Borges MD (Department of Allergy and Clinical Immunology, Centro Médico-Docente La Trinidad Caracas, Venezuela); Holger J. Schünemann, MD, MSc, PhD (Department of Health Research Methods, Evidence and Impact (HEI), McMaster University, Hamilton, ON, Canada, and Cochrane Canada and McMaster GRADE Centre, Hamilton, ON, Canada); Raanan Shamir, MD, PhD (Institute of Gastroenterology, Nutrition and Liver Disease, Schneider Children’s Medical Center, Petach-Tikva, Israel; Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel); Jonathan M. Spergel, MD, PhD (Division of Allergy and Immunology, Department of Pediatrics, The Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania, Philadelphia, PA, USA), Hania Szajewska, MD (The Medical University of Warsaw - Department of Paediatrics, Warsaw, Poland); Luigi Terracciano, MD (Italian NHS and Italian Society of Social and Preventive Pediatrics, Milano, Italy); Yvan Vandenplas, MD, PhD (Department of Pediatrics, UZ Brussel, Vrije Universiteit Brussel, Brussels, Belgium); Carina Venter, PhD, RD (Section of Allergy & Immunology, University of Colorado Denver School of Medicine, Children’s Hospital Colorado, Aurora, CO, USA); Amena Warner, RN, SN (PG Dip) (Allergy UK, Planwell House, Sidcup, Kent, UK); Susan Waserman, MD, MSc (Division of Clinical Immunology and Allergy, Department of Medicine, McMaster University, Hamilton, ON, Canada); Gary W. K. Wong, MD (Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China)
Authors’ consent for publication
All authors agreed to publication of this work in World Allergy Organization Journal.
Declaration of competing interest
H Schünemann and J Brożek, on behalf of McMaster University, received a research grant from the World Allergy Organization to conduct this review that was deposited into the university research account. S Waffenschmidt, A Agarwal, P Dziechciarz, A Horvath, R Jebai, H Mihara, and Y Roldan have nothing to disclose. S Arasi, S Bahna, A Bognanni, JL Brożek, DK Chu, L Dahdah, E Galli, R Kamenwa, H Li, A Martelli, R Pawankar, HS Schünemann, R T Firmino, L Terracciano, and A Warner have no conflicts to disclose. IJ Anstotegui – Abbott, Amgen, Astra Zeneca, Bayer, Bial, Faes Farma, Hikma, Menarini, Merck, Mundipharma, Roxall, Sanofi, Stallergenes, UCB. A Assa'ad – Aimmune Therapeutics, DBV Technologies, Astella, ABBVIE, Novartis, Sanofi, FARE, NIH and an intellectual property patent licensed to Hoth. R Berni Canani – Nutricia, Ch. Hansen, Danone, DVB, Humana, iHealth, Kraft Heinz, Mead Johnson, Nestlè, Novalac, Sanofi. M Bozzola – Danone. C Dupont – Nestle Health Science, Nestle France, Nutricia, Novalac, Sodilac, Abbott, Danone, and stock ownership at DBV Technologies. M Ebisawa – DBV Technologies, Mylan, ARS Pharmaceuticals, Novartis. A Fiocchi – Abbott, Danone. G Lack – FARE, National Peanut Board (NPB), The Davis Foundation, Action Medical Research, UK Food Standards Agency, Medical Research Council, DBV Technologies, Mission Mighty Me, Novartis, Sanofi-Genyzme, Regeneron, ALK-Abello, Lurie Children's Hospital. A Nowak-Wegrzyn – Nestle, Nutricia, Novartis, Gerber, Aimmune. N Papadopoulos – Novartis, Nutricia, HAL Allergy, Menarini/Faes Farma, Sanofi, Mylan/Meda, Biomay, AstraZeneca, GSK, MSD, ASIT Biotech, Boehringer Ingelheim, Gerolymatos International SA, Capricare. M Said – Nestle, Nutricia, Abbott, Bayer for Anaphylaxis Australia. J Spergel – DBV Technologies, Regeneron, Sanofi, and Aimmune. H Szajewska – Ausnutria, Cargill, Danone, Else Nutrition, Hipp, Nestle, and Nestle Nutrition Institute. Y Vandenplas – Abbott Nutrition, Biogaia, Biocodex, By Heart, CHR Hansen, Danone, ELSE Nutrition, Friesland Campina, Hero, Hypocrata, Nestle Health Science, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Orafti, Phacobel, Phathom Pharmaceuticals, Sari Husada, United Pharmaceuticals (Novalac), Wyeth, Yakult. C Venter – Reckitt Benckiser, Nestle Nutrition Institute, Danone, Abbott Nutrition, Else Nutrition, and Before Brands, DBV Technologies. S Waserman – Novartis-basic science work on peanut allergy, Aimmune-peanut OIT trial, Medical Advisor to Food Allergy Canada, and Pfizer, Bausch, Kaleo-consultant for epinephrine autoinjectors. GWK Wong – Nestle, Danone.
Acknowledgements
We would like to acknowledge the contribution of Dr. Julia Kreis, who was not able to become a co-author owing to the agreement with her employer. We thank the WAO DRACMA guideline panel members for their input.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100682.
Contributor Information
Jan L. Brożek, Email: jan.brozek@mcmaster.ca.
WAO DRACMA Guideline Group:
Ignacio J. Ansotegui, Stefania Arasi, Amal H. Assa'ad, Sami L. Bahna, Roberto Berni Canani, Antonio Bognanni, Martin Bozzola, Jan Brozek, Derek K. Chu, Lamia Dahdah, Christophe Dupont, Motohiro Ebisawa, Alessandro Fiocchi, Ramon Targino Firmino, Elena Galli, Rose Kamenwa, Gideon Lack, Haiqi Li, Alberto Martelli, Anna H. Nowak-Wegrzyn, Nikolaos G. Papadopoulos, Ruby Pawankar, Maria Said, Mario Sánchez-Borges, Holger J. Schünemann, Raanan Shamir, Jonathan M. Spergel, Hania Szajewska, Luigi Terracciano, Yvan Vandenplas, Carina Venter, Amena Warner, Susan Waserman, and GaryW.K. Wong
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rona R.J., Keil T., Summers C., et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Nwaru B.I., Hickstein L., Panesar S.S., et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 3.Schoemaker A.A., Sprikkelman A.B., Grimshaw K.E., et al. Incidence and natural history of challenge-proven cow's milk allergy in European children–EuroPrevall birth cohort. Allergy. 2015;70(8):963–972. doi: 10.1111/all.12630. [DOI] [PubMed] [Google Scholar]
- 4.Spergel J.M. Natural history of cow's milk allergy. J Allergy Clin Immunol. 2013;131(3):813–814. doi: 10.1016/j.jaci.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Hill D.J., Firer M.A., Ball G., Hosking C.S. Natural history of cows' milk allergy in children: immunological outcome over 2 years. Clin Exp Allergy. 1993;23(2):124–131. doi: 10.1111/j.1365-2222.1993.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 6.Santos A., Dias A., Pinheiro J.A. Predictive factors for the persistence of cow's milk allergy. Pediatr Allergy Immunol. 2010;21(8):1127–1134. doi: 10.1111/j.1399-3038.2010.01040.x. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer S.H. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Skripak J.M., Matsui E.C., Mudd K., Wood R.A. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–1177. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Woods R.K., Abramson M., Bailey M., Walters E.H. International prevalences of reported food allergies and intolerances. Comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991-1994. Eur J Clin Nutr. 2001;55(4):298–304. doi: 10.1038/sj.ejcn.1601159. [DOI] [PubMed] [Google Scholar]
- 10.Jansen J.J., Kardinaal A.F., Huijbers G., Vlieg-Boerstra B.J., Martens B.P., Ockhuizen T. Prevalence of food allergy and intolerance in the adult Dutch population. J Allergy Clin Immunol. 1994;93(2):446–456. doi: 10.1016/0091-6749(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 11.Osterballe M., Mortz C.G., Hansen T.K., Andersen K.E., Bindslev-Jensen C. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20(7):686–692. doi: 10.1111/j.1399-3038.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 12.Colver A.F., Nevantaus H., Macdougall C.F., Cant A.J. Severe food-allergic reactions in children across the UK and Ireland, 1998-2000. Acta Paediatr. 2005;94(6):689–695. doi: 10.1111/j.1651-2227.2005.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 13.Järvinen K.M., Sicherer S.H., Sampson H.A., Nowak-Wegrzyn A. Use of multiple doses of epinephrine in food-induced anaphylaxis in children. J Allergy Clin Immunol. 2008;122(1):133–138. doi: 10.1016/j.jaci.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Uguz A., Lack G., Pumphrey R., et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy. 2005;35(6):746–750. doi: 10.1111/j.1365-2222.2005.02257.x. [DOI] [PubMed] [Google Scholar]
- 15.Baseggio Conrado A., Ierodiakonou D., Gowland M.H., Boyle R.J., Turner P.J. Food anaphylaxis in the United Kingdom: analysis of national data, 1998-2018. BMJ. 2021;372 doi: 10.1136/bmj.n251. n251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chafen J.J., Newberry S.J., Riedl M.A., et al. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303(18):1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 17.National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Food and Nutrition Board. Committee on Food Allergies: Global Burden, Causes, Treatment, Prevention, and Public Policy . In: Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. Oria M.P., Stallings V.A., editors. National Academies Press (US); Washington (DC): 2016. [PubMed] [Google Scholar]
- 18.Sampson H.A., Muñoz-Furlong A., Campbell R.L., et al. Second symposium on the definition and management of anaphylaxis: summary report–second national Institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 19.Fiocchi A., Schünemann H.J., Brozek J., et al. Diagnosis and Rationale for action against cow's milk allergy (DRACMA): a summary report. J Allergy Clin Immunol. 2010;126(6):1119–1128. doi: 10.1016/j.jaci.2010.10.011. e12. [DOI] [PubMed] [Google Scholar]
- 20.Dunn Galvin A., Hourihane J.O. Psychosocial mediators of change and patient selection factors in oral immunotherapy trials. Clin Rev Allergy Immunol. 2018;55(2):217–236. doi: 10.1007/s12016-018-8700-5. [DOI] [PubMed] [Google Scholar]
- 21.Couzin-Frankel J. Toxin or treatment? Science. 2018;362(6412):278–282. doi: 10.1126/science.362.6412.278. [DOI] [PubMed] [Google Scholar]
- 22.Feuille E., Nowak-Wegrzyn A. Allergen-specific immunotherapies for food allergy. Allergy Asthma Immunol Res. 2018;10(3):189–206. doi: 10.4168/aair.2018.10.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu D.K., Wood R.A., French S., et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393(10187):2222–2232. doi: 10.1016/S0140-6736(19)30420-9. [DOI] [PubMed] [Google Scholar]
- 24.Pajno G.B., Fernandez-Rivas M., Arasi S., et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73(4):799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 25.Brożek J.L., Terracciano L., Hsu J., et al. Oral immunotherapy for IgE-mediated cow's milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012;42(3):363–374. doi: 10.1111/j.1365-2222.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Schünemann H.J., Guyatt G., Oxman A., editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2010/12/28. ed Apr. 380–2 pp. [Google Scholar]
- 28.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 29.Wells G., Shea B., O'Connell D., et al. 2000. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-randomized Studies in Meta-Analysis. [Google Scholar]
- 30.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane, vol. 2021. Available from www.training.cochrane.org/handbook.
- 31.Sweeting M.J., Sutton A.J., Lambert P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 32.Yates F. Contingency tables involving small numbers and the χ2 test. J Roy Stat Soc Suppl. 1934;1(2):217. [Google Scholar]
- 33.https://www.jamovi.org/TjpjVCSRf
- 34.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime Afgo.
- 35.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santesso N., Glenton C., Dahm P., et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Yeung J.P., Kloda L.A., McDevitt J., Ben-Shoshan M., Alizadehfar R. Oral immunotherapy for milk allergy. Cochrane Database Syst Rev. 2012;11(11):Cd009542. doi: 10.1002/14651858.CD009542.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Silva D., Geromi M., Panesar S.S., et al. Acute and long-term management of food allergy: systematic review. Allergy. 2014;69(2):159–167. doi: 10.1111/all.12314. [DOI] [PubMed] [Google Scholar]
- 40.Lucendo A.J., Arias A., Tenias J.M. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113(6):624–629. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Martorell Calatayud C., Muriel García A., Martorell Aragonés A., De La Hoz Caballer B. Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow's milk allergy in children: systematic review and meta-analysis. J Investig Allergol Clin Immunol. 2014;24(5):298–307. [PubMed] [Google Scholar]
- 42.Nurmatov U., Devereux G., Worth A., Healy L., Sheikh A. Effectiveness and safety of orally administered immunotherapy for food allergies: a systematic review and meta-analysis. Br J Nutr. 2014;111(1):12–22. doi: 10.1017/S0007114513002353. [DOI] [PubMed] [Google Scholar]
- 43.Nurmatov U., Dhami S., Arasi S., et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72(8):1133–1147. doi: 10.1111/all.13124. [DOI] [PubMed] [Google Scholar]
- 44.O'Keefe A.W., De Schryver S., Mill J., Mill C., Dery A., Ben-Shoshan M. Diagnosis and management of food allergies: new and emerging options: a systematic review. J Asthma Allergy. 2014;7:141–164. doi: 10.2147/JAA.S49277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg MR, Nachshon L, Appel MY, et al. Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J Allergy Clin Immunol.136(6):1601–1606. [DOI] [PubMed]
- 46.Gruzelle V., Juchet A., Martin-Blondel A., Michelet M., Chabbert-Broue A., Didier A. Benefits of baked milk oral immunotherapy in French children with cow's milk allergy. Pediatr Allergy Immunol. 2015;31(4):364–370. doi: 10.1111/pai.13216. [DOI] [PubMed] [Google Scholar]
- 47.Dantzer J., Dunlop J., Psoter K.J., Keet C., Wood R. Efficacy and safety of baked milk oral immunotherapy in children with severe milk allergy: a randomized, double-blind, placebo-controlled phase 2 trial. J Allergy Clin Immunol. 2022;149(4):1383–1391. doi: 10.1016/j.jaci.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Gabrielli S., Miles B.T., Langlois A., et al. Allergy, Asthma and Clinical Immunology Conference: Canadian Society of Allergy and Clinical Immunology Annual Scientific Meeting, CSACI. 2018. Skin prick test in milk allergic patients undergoing oral immunotherapy; p. 15. [Google Scholar]
- 49.Skripak J.M., Nash S.D., Rowley H., et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–1160. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filho W.R., Flausino F.J., Silva R.A., Silva F.M., Pinto J.A., Nunes L. World Allergy Organization Journal Conference: 3rd WAO International Scientific Conference, WISC. 2014. Risks and benefits of oral immunotherapy for IgE-mediated cow's milk allergy; p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.H., Kim W.S., Kim H., Hahn Y.S. Increased cow's milk protein-specific IgG4 levels after oral desensitization in 7- to 12-month-old infants. Ann Allergy Asthma Immunol. 2013;111(6):523–528. doi: 10.1016/j.anai.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Morisset M., Moneret-Vautrin D.A., Guenard L., et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. Euro Ann Allergy Clin Immunol. 2007;39(1):12–19. [PubMed] [Google Scholar]
- 53.Patriarca G., Schiavino D., Nucera E., Schinco G., Milani A., Gasbarrini G.B. Food allergy in children: results of a standardized protocol for oral desensitization. Hepato-Gastroenterol. 1998;45(19):52–58. [PubMed] [Google Scholar]
- 54.De Schryver S., Mazer B., Clarke A.E., et al. Adverse events in oral immunotherapy for the desensitization of cow's milk allergy in children: a randomized controlled trial. J Allergy Clin Immunol Pract. 2019;7(6):1912–1919. doi: 10.1016/j.jaip.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Longo G., Barbi E., Berti I., et al. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol. 2008;121(2):343–347. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Martorell A., De la Hoz B., Ibanez M.D., et al. Oral desensitization as a useful treatment in 2-year-old children with cow's milk allergy. Clin Exp Allergy. 2011;41(9):1297–1304. doi: 10.1111/j.1365-2222.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 57.Maeda M., Imai T., Ishikawa R., et al. Effect of oral immunotherapy in children with milk allergy: the ORIMA study. Allergol Int. 2021 Apr;70(2):223–228. doi: 10.1016/j.alit.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Pajno G.B., Caminiti L., Ruggeri P., et al. Oral immunotherapy for cow's milk allergy with a weekly up-dosing regimen: a randomized single-blind controlled study. Ann Allergy Asthma Immunol. 2010;105(5):376–381. doi: 10.1016/j.anai.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 59.Salmivesi S., Korppi M., Makela M.J., Paassilta M. Milk oral immunotherapy is effective in school-aged children. Acta Paediatr. 2013;102(2):172–176. doi: 10.1111/j.1651-2227.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- 60.Wood R.A., Kim J.S., Lindblad R., et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. 2016;137(4):1103–1110. doi: 10.1016/j.jaci.2015.10.005. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi M., Taniuchi S., Soejima K., Hatano Y., Yamanouchi S., Kaneko K. Two-weeks-sustained unresponsiveness by oral immunotherapy using microwave heated cow's milk for children with cow's milk allergy. Allergy Asthma Clin Immunol. 2016;12(1):44. doi: 10.1186/s13223-016-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato S., Noriyuki Y., Motohiro E. 2016. XXIV World Allergy Congress 2015: Seoul, Korea; pp. 14–17. October 2015. [Google Scholar]
- 63.Ogura K., Yanagida N., Sato S., et al. Evaluation of oral immunotherapy efficacy and safety by maintenance dose dependency: a multicenter randomized study. World Allergy Organ J. 2020;13(10) doi: 10.1016/j.waojou.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogura K., Yanagida N., Sato S., et al. Evaluation of oral immunotherapy efficacy and safety by maintenance dose dependency: a multicenter randomized study. World Allergy Organ J. 2020;13(10):100463. doi: 10.1016/j.waojou.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogura K, Komata T, Hasegawa M, et al. Efficacy of slow oral immunotherapy for cow's milk allergy. World Allergy Organ J 5:S41–S42.
- 66.Ogura K, Imai T, Iikura K, et al. Variable efficacy of oral immunotherapy for hen's egg, cow's milk and wheat allergy: compared to their natural courses. Allergy 67:412.
- 67.Miura Y., Nagakura K.I., Nishino M., et al. Long-term follow-up of fixed low-dose oral immunotherapy for children with severe cow's milk allergy. Pediatr Allergy Immunol. 2021;32(4):734–741. doi: 10.1111/pai.13442. [DOI] [PubMed] [Google Scholar]
- 68.Miura Y, Nagakura K, Yanagida N, Sato S, Ebisawa M. Efficacy and safety of low-dose oral immunotherapy for patients with severe cow's milk allergies: a 3-year follow-up. Allergy.73:54.
- 69.Meglio P., Bartone E., Plantamura M., Arabito E., Giampietro P.G. A protocol for oral desensitization in children with IgE-mediated cow's milk allergy. Allergy. 2004;59(9):980–987. doi: 10.1111/j.1398-9995.2004.00542.x. [DOI] [PubMed] [Google Scholar]
- 70.García-Lirio E., Gonzalez Diaz C., Gonzalez Hermosa A., Gamboa P., Aranguren R., Sanz M.L. Oral immunotherapy with egg and milk: changes in peripheral serum cytokines are not predictive factors for severe adverse reactions or for the final report. J Investig Allergol Clin Immunol. 2018;28(1):24–28. doi: 10.18176/jiaci.0212. [DOI] [PubMed] [Google Scholar]
- 71.El Badawy N.E., Abdel-Latif R.S. Food specific IgE as A biomarker of oral immunotherapy efficacy in comparison to double blind food challenge test. Egypt J Immunol/Egyptian Association of Immunologists. 2017;24(2):109–125. [PubMed] [Google Scholar]
- 72.Ebrahimi M., Gharagozlou M., Khalili A., Magaji Hamid K., Azizi G., Movahedi M. Induction of tolerance by oral immunotherapy in patients with cow's milk allergy. J Investig Allergol Clin Immunol. 2016;26(5):341–343. doi: 10.18176/jiaci.0094. [DOI] [PubMed] [Google Scholar]
- 73.Berti I., Badina L., Cozzi G., et al. Early oral immunotherapy in infants with cow's milk protein allergy. Pediatr Allergy Immunol. 2019;30(5):572–574. doi: 10.1111/pai.13057. [DOI] [PubMed] [Google Scholar]
- 74.Ono M, Takasato Y, Matsui T, Sugiura S, Ito K. The efficacy and safety of tailored oral immunotherapy for patients with mild food allergies. Allergy.73:321.
- 75.Mantyla J., Thomander T., Hakulinen A., et al. The effect of oral immunotherapy treatment in severe IgE mediated milk, peanut, and egg allergy in adults. Immun Inflamm Dis. 2018;6(2):307–311. doi: 10.1002/iid3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Longo G., Berti I., Barbi E., et al. Diagnosed child, treated child: food challenge as the first step toward tolerance induction in cow's milk protein allergy. Euro Ann Allergy Clin Immunol. 2012;44(2):54–60. [PubMed] [Google Scholar]
- 77.Sugiura S., Kitamura K., Makino A., et al. Slow low-dose oral immunotherapy: threshold and immunological change. Allergol Int. 2020;69(4):601–609. doi: 10.1016/j.alit.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Barbi E., Longo G., Berti I., et al. Adverse effects during specific oral tolerance induction: in home phase. Allergol Immunopathol. 2012;40(1):41–50. doi: 10.1016/j.aller.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Martinez-Botas J., Rodriguez-Alvarez M., Cerecedo I., et al. Identification of novel peptide biomarkers to predict safety and efficacy of cow's milk oral immunotherapy by peptide microarray. Clin Exp Allergy. 2015;45(6):1071–1084. doi: 10.1111/cea.12528. [DOI] [PubMed] [Google Scholar]
- 80.Patriarca G., Buonomo A., Roncallo C., et al. Oral desensitisation in cow milk allergy: immunological findings. Int J Immunopathol Pharmacol. 2002;15(1):53–58. doi: 10.1177/039463200201500107. [DOI] [PubMed] [Google Scholar]
- 81.Patriarca G., Nucera E., Pollastrini E., et al. Oral specific desensitization in food-allergic children. Dig Dis Sci. 2007;52(7):1662–1672. doi: 10.1007/s10620-006-9245-7. [DOI] [PubMed] [Google Scholar]
- 82.Aquilante B.P., Faion M.C.N., Marcelino F., et al. Eosinophilic esophagitis during milk oral immunotherapy: a growing concern. J Allergy Clin Immunol. 2018;141:AB135. [Google Scholar]
- 83.Babaie D., Nabavi M., Arshi S., et al. Cow's milk desensitization in anaphylactic patients: a new personalized-dose method. Iran J Allergy, Asthma Immunol. 2017;16(1):45–52. [PubMed] [Google Scholar]
- 84.Bellon S., Sanchez L., Gonzalez L., et al. Clinical and Translational Allergy Conference: 5th Pediatric Allergy and Asthma Meeting, PAAM. 2017. Evolution of immunologic parameters in children subjected to food oral immunotherapy with cow's milk during a 10-year follow-up; p. 8. [Google Scholar]
- 85.Levy M.B., Elizur A., Goldberg M.R., Nachshon L., Katz Y. Clinical predictors for favorable outcomes in an oral immunotherapy program for IgE-mediated cow's milk allergy. Ann Allergy Asthma Immunol. 2014;112(1):58–63. doi: 10.1016/j.anai.2013.10.001. e1. [DOI] [PubMed] [Google Scholar]
- 86.Wasserman Richard L., Sugerman Robert W., Ain Kamili Qurat Ul, Pence Dena M., Hague Angela R., Herbert Morley., editors. vol. 141. 2018. Eosinophilic esophagitis like oral immunotherapy related syndrome (ELORS) p. AB258. (2018 American Academy of Allergy, Asthma and Immunology, AAAAI and World Allergy Organization, WAO Joint Congress United States). (2 Supplement 1) [Google Scholar]
- 87.Yonekura Anagusko C.L., Mendonca J., Lopes M.M., et al. Specific IgG4 to milk proteins during oral immunotherapy for milk allergy: relationship to eosinophilic esophagitis. J Allergy Clin Immunol. 2017;143:AB138. [Google Scholar]
- 88.Arasi S., Caminiti L., Crisafulli G., et al. The safety of oral immunotherapy for food allergy during maintenance phase: effect of counselling on adverse reactions. World Allergy Organ J. 2019;12(1):100010. doi: 10.1016/j.waojou.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De La Fuente A, Morales-Cabeza C, Aparicio V, Infante S, Cabrera-Freitag P, Alvarez-Perea A. Eosinophilic esophagitis in cow's milk and egg oral immunotherapy (OIT). J Allergy Clin Immunol.145:AB139.
- 90.Echeverria-Zudaire L.A., Fernández-Fernández S., Rayo-Fernández A., et al. Primary eosinophilic gastrointestinal disorders in children who have received food oral immunotherapy. Allergol Immunopathol (Madr) 2016;44(6):531–536. doi: 10.1016/j.aller.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Ferreira Martins C.S., Torres P., Zanandrea A., et al. EoE, OIT and Anaphylaxis: an unsolved puzzle. J Allergy Clin Immunol. 2019;143(Supplement):AB136. [Google Scholar]
- 92.Nagakura K.I., Yanagida N., Nishino M., Takahashi K., Sato S., Ebisawa M. Randomized controlled trial of oral immunotherapy for children with severe cow's milk allergy: heated milk vs. unheated milk. World Allergy Organ J. 2020;13(8) [Google Scholar]
- 93.Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez M, Fermin I, Ibanez M. Three cases of eosinophilic esophagitis (EoE) among 110 milk-allergic children treated with milk oral immunotherapy. Allergy.66:409–410.
- 94.Sugimoto M., Nagao M., Kondo M., Kainuma K., Fujisawa T. World Allergy Organization Journal Conference: 2nd WAO International Scientific Conference, WISC. 2012. Food allergy and anaphylaxis-2041. Rush oral immunotherapy for severe food allergy: one year follow up; p. 6. [Google Scholar]
- 95.Petrakis D, Xepapadaki P, Galani M, et al. Oral immunotherapy (OIT): efficacy and safety in a group of children with cow's milk allergy. Allergy.74:797–798.
- 96.Epstein-Rigbi N., Goldberg M.R., Levy M.B., Nachshon L., Elizur A. Changes in quality of life of food-allergic children from initiation of oral immunotherapy, through updosing, upon reaching maintenance and after 6 months of follow-up. J Allergy Clin Immunol. 2018;141:AB240. [Google Scholar]
- 97.Carraro S., Frigo A.C., Perin M., et al. Impact of oral immunotherapy on quality of life in children with cow milk allergy: a pilot study. Int J Immunopathol Pharmacol. 2012;25(3):793–798. doi: 10.1177/039463201202500329. [DOI] [PubMed] [Google Scholar]
- 98.Hayashi N, Yanagida N, Goto M, et al. Improvement of quality of life of food induced anaphylactic children after rush oral immunotherapy. Allergy.66:.
- 99.Katz Y, Appel MY, Goldberg MR, Elizur A, Nachshon L, Levy MB. Long term follow-up of patients successfully completing oral immunotherapy for IgE-mediated cow's milk allergy. J Allergy Clin Immunol.2015 135(2):AB25.
- 100.Kauppila T.K., Pelkonen A.S., Roine R.P., et al. Health-related quality of life in patients who had partaken in milk oral immunotherapy and comparison to the general population. Allergy. 2021;76(1):387–390. doi: 10.1111/all.14525. [DOI] [PubMed] [Google Scholar]
- 101.Pettersson M.E., Koppelman G.H., Flokstra-De Blok B.M.J., Kollen B.J., Dubois A.E.J. Prediction of the severity of allergic reactions to foods. Allergy. 2018;73(7):1532–1540. doi: 10.1111/all.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Plaut M., Sawyer R.T., Fenton M.J. Summary of the 2008 national Institute of allergy and infectious diseases-US food and drug administration workshop on food allergy clinical trial design. J Allergy Clin Immunol. 2009;124(4):671–678. doi: 10.1016/j.jaci.2009.05.027. e1. [DOI] [PubMed] [Google Scholar]
- 103.Mondello W. Girl with milk allergy dies of severe reaction related to desensitization food allergy, milk & egg, news. December 20, 2021. https://www.allergicliving.com/2021/12/20/girl-with-milk-allergy-dies-of-severe-reaction-related-to-desensitization/ Available from:
- 104.Bloom D. 2017. Study: Nine Children Suffer Severe Symptoms in OIT Trial: SnackSafely.Com. [Google Scholar]
- 105.Nagakura K.I., Sato S., Miura Y., et al. A randomized trial of oral immunotherapy for pediatric cow's milk-induced anaphylaxis: heated vs unheated milk. Pediatr Allergy Immunol. 2021;32(1):161–169. doi: 10.1111/pai.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arasi S., Nurmatov U., Dunn-Galvin A., et al. Consensus on DEfinition of Food Allergy SEverity (DEFASE) an integrated mixed methods systematic review. World Allergy Organization Journal. 2021;14(3):100503. doi: 10.1016/j.waojou.2020.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials are provided in the supplementary documents.