Abstract
Background/Aim: Since 2019, the COVID-19 pandemic has been a devastating disease affecting global health to a great extent. Some countries have added on herbal medicines as a complementary treatment for combating COVID-19 due to the urgency of stopping the spread of this viral disease. However, whether these herbal medicines are effective is uncertain. This systematic review and meta-analysis aimed to evaluate the effects of herbal medicine combined therapy in the treatment of COVID-19.
Methods: A literature search was performed following the PRISMA Statement and without language restrictions. Seven databases were searched from inception through December 2021. All selected studies were randomized clinical trials (RCTs). Comparing the effects of herbal medicine combined therapy with conventional western medicine, including improvement of clinical symptoms, chest CT images, viral conversion rate, C-reactive protein (CRP) and interleukin 6. Cochrane criteria were applied to examine the methodological quality of the enrolled trials; and meta-analysis software (RevMan 5.4.1) was used for data analysis.
Results: In total, the data of 5,417 participants from 40 trials were included in this systematic review; and 28 trials were qualified for meta-analysis. The trials had medium-to-high quality based on GRADE system. Meta-analysis showed that combining herbal medicine vs conventional treatment in 1) coughing (1.43 95% CI:1.21, 1.71, p = 0.0001), 2) fever (1.09 95% CI:1.00, 1.19, p = 0.06), 3) fatigue (1.21 95% CI:1.10, 1.33, p = 0.0001); 4) CT images (1.26 95% CI:1.19, 1.34, P ≤ 0.00001), 5) viral conversion rates (1.22 95% CI:1.06, 1.40, p = 0.005) and 6) viral conversion times (−3.72 95% CI: −6.05, −1.40, p = 0.002), 7) IL6 change (1.97 95% CI: −0.72, 4.66, p = 0.15) and 8) CRP change (−7.92 95% CI: −11.30, −4.53, P ≤ 0.00001).
Conclusion: Herbal medicine combined therapy significantly reduces COVID-19 clinical symptoms, improving CT images and viral conversion rates. Reported adverse events are mild. However, for certain biases in the included studies, and the need for further study on effective components of herbal medicine. Further large trials with better randomized design are warranted to definite a more definite role of herbal medicine.
Keywords: herbal medicine, COVID-19, systematic (Literature) review, meta-analysis, complementary therapy, traditional chinese medicine
1 Introduction
Since December 2019, the outbreak of the COVID-19 pandemic has had devastating effects on global health systems and economic growth, and has affected the lifestyle of human populations on a large scale (Sreepadmanabh et al., 2020). In March 2020, the World Health Organization (WHO) declared that COVID-19 was a global pandemic because the viral infection had spread rapidly within a growing number of countries.
The causative agent of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), distressed not only the respiratory tract system (Zhu et al., 2020), but also presented as a systemic disease associated with vascular inflammation; this affected multiple organs and eventually led to multi-organ failure in severely affected individuals (Ding et al., 2020; Smadja et al., 2021). Therefore, it became urgent to prevent or treat COVID-19 early and effectively.
Currently, treatment of COVID-19 includes administering antiviral and symptomatic support in mild cases; whereas no definitively effective drugs are available to treat this viral infection even though some non-specific therapeutic options exist and vaccination is ongoing (Mirtaleb et al., 2021). Since August 2021, the delta variant has exhibited high capability of invading the host’s immune system and appears to be more transmissible than other variants, making people more anxious (Harder et al., 2021; Shiehzadegan et al., 2021). In November 2021, the appearance of the Omicron (B.1.1.529) variant expressed high enhanced transmissibility and immune evasion and was shown to re-infect individuals previously infected with other SARS-CoV-2 variants (Kannan et al., 2021). In this scenario, beyond the conventional western medicine (CWM) approach, which includes antiviral drugs such as interferon, ribavirin, lopinavir-ritonavir, and chloroquine phosphate (Negrut et al., 2021; Wong et al., 2021), there is widespread use of antibacterial drugs, antitussives, expectorants and supportive therapy, while Herbal -medicine combined therapy is also flourishing in many countries. Since the origin of the COVID-19 outbreak in China in 2019, herbal medicine (HM) has been used to help combat COVID infection along with modern anti-infective agents because no vaccine or definitive antiviral treatment was available for COVID-19 at that time. In Asian countries such as China, Taiwan and Hong-Kong, HM has played a critical role in both treating and preventing COVID-19; the inclusion of traditional Chinese medicine (TCM) in the Chinese protocol is based on its long, successful historic experience in fighting against pestilence (Ni et al., 2020). To have a successful experience combating COVID-19, the TCM therapeutic schedule was included in the guidelines for diagnosis and treatment of COVID-19 (Jin Y. H. et al., 2020). Broadly speaking, many other countries such as India and Japan have also applied HM to alleviate the effects of infectious diseases in the context of SARS-CoV-2 (Ang et al., 2020a; Ang et al., 2020b). The volume of existing reports and systematic reviews provide irrefutable evidence that HM combined therapy possesses a potential antiviral capability against SARS-CoV-2 (Fan et al., 2020; Du et al., 2021), yet the lack of solid evidence-based trials makes the value of herbs vague (Panyod et al., 2020). Furthermore, no grading was performed on the certainty of evidence for their results also easily skew the result directions if not handled properly.
In addition to herbal medicine, some natural products were also under investigation, more and more studies applied network pharmacology in exploring the related pathways and mechanisms of these herbs and natural compounds (Fan et al., 2020; Ang et al., 2021). The more research focus on this field, the more appealing and potential of these herbs were found.
To date, many HM-related trials (not only TCM) have been published on aspects of COVID-19; however, the high heterogeneity and loose trial design made it hard for previous studies to draw definitive conclusions. To further understand the efficacy of HM combined therapy in treating the COVID-19, we conducted a systematic review and meta-analysis to evaluate the efficacy of HM in the treatment of COVID-19 objectively.
2 Methods
The protocol for this review and meta-analysis has been registered on the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42021287021. This review was reported according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021).
2.1 Data sources and search strategy
This systematic review was conducted in compliance with the PRISMA Statement ensure transparent and complete reporting. The following 7 databases were searched for relevant randomized clinical trials, with no language restrictions, from their inception dates to 30 December 2021: Embase (Elsevier), Medline (Ovid, including epub ahead of print, in-process, and other nonindexed citations), Cochrane Library (including clinical registers from WHO ICTRP and US ClinicalTrials.gov), CINAHL Complete (EBSCOhost), Scopus, China National Knowledge Infrastructure (CNKI) and Wanfang Data. The reference lists of eligible articles were also reviewed to identify additional studies for possible inclusion. We also manually retrieved relevant studies and clinical trials to acquire as many studies as possible. E-mail alerts were established to identify newly released studies from the different databases that fell within the scope of our review.
The key concepts – COVID-19 and Traditional Chinese Medicine–used in the search included their 216 synonyms in total and controlled vocabulary (12 Emtree terms, 13 MeSH terms, etc.). Highly sensitive search filters were applied to identify randomized clinical trials. Supplementary Appendix A1 displays the full search strategy for the individual databases.
2.2 Eligibility criteria and data extraction
All eligible studies examined studies that fulfilled the inclusion criteria, as follows: 1) Studies designed as randomized clinical trials (RCTs); 2) Adult patients (aged 18 years and older) with an established diagnosis of COVID-19 in evaluable status. The criteria of mild and moderate is according to the Clinical Spectrum of SARS -CoV-2 Infection from National Institutes of Health(Maier et al., 2021), which set mild illness as individuals who have any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but who do not have shortness of breath, dyspnea, or abnormal chest imaging; and moderate illness as individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥94% on room air at sea level. 3) The intervention group was treated with HM combined therapy. Patients in the control group were required to be treated with CWM or a combination of HM placebo and CWM. We excluded studies designed as retrospective studies, observational studies, repeated data studies and cross-sectional studies. Studies which set outcomes only with TCM syndromes evaluation, sample size less than 30; or if the full text cannot be obtained were also excluded. The selection of studies and data extraction were performed independently by two reviewers (Chien and Liu) according to the inclusion and exclusion criteria.
2.3 Risk of bias and quality assessment
Two reviewers (Chien and Liu) assessed the methodological quality of studies by using the Cochrane Collaboration’s tool (Higgins et al., 2022)and the new version of this tool, Risk of Bias version 2 (RoB 2). Six items of ROB 2 were evaluated as follows: Randomization, Deviations from intended interventions, Missing outcome data, Measurement of the outcome, Selective Outcome reporting, overall bias (Cochrane Collaboration, 2021). Once the disagreements were noted, discussions were held with the other investigators (Chang; Wu) to make a consensus decision.
2.4 Assessment of evidence certainty
We assessed the outcomes by using GRADE methodology (Guyatt et al., 2008). The overall evidence certainty was evaluated by using five downgrading domains which included considerations of study limitation, inconsistency, indirectness, imprecision, and publication bias. The level of evidence was classified as high, moderate, low, or very low. Grading was performed using GRADE pro software (Diekemper et al., 2018) (available from http://www.gradepro.org).
2.5 Outcome measures assessment
In the review, the outcome measures in the enrolled studies included chest CT scan; blood tests and cytokines, including CRP, interleukin 6, lymphocytes, etc; symptom evaluation; virus nuclei acid tests; hospitalization time, adverse events (AE) and mortality; and TCM syndrome score. In this SR, we chose data that were more objective, consistent in the unit and completeness for analysis. Therefore, we did not analyze the TCM syndrome score or hospitalization days since the method of evaluation of TCM syndrome is different, and factors affect hospitalization time might be bias. The targeted outcomes we chose were: 1) Clinical symptoms (fever; cough; fatigue, which were measured as percentage decreased %); 2) Chest CT manifestations (the percentage of consolidations in the whole lung or improved rate %); 3) Viral nucleic conversion rates (%) and duration (days); 4) Serum interleukin-6 (pg/ml) and CRP (mg/L), and the effect estimates were re-calculated using data extracted from the qualified studies.
2.6 Meta-analysis
To analyze the effects of combining herbal medicines on targeted outcomes after treatment compared with baseline values, we applied Review Manager software (RevMan, Version 5.4.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to analyze dichotomous and continuous outcome measures extracted from the original studies. Weighted mean difference (WMD) was utilized for data measurement of continuous outcomes, while risk ratio (RR) was used for dichotomous outcomes. Statistical heterogeneity was assessed using the Chi-square test (p < 0.1). The I2 statistic was also calculated, and we considered I 2 > 50% to indicate significant heterogeneity across studies (Higgins et al., 2003). A random-effects model was used if significant heterogeneity was shown among trials. Otherwise, results were obtained from a fixed-effects model. Funnel plot was also used to evaluate the publication bias (Sterne et al., 2011).
3 Results
3.1 Eligible studies
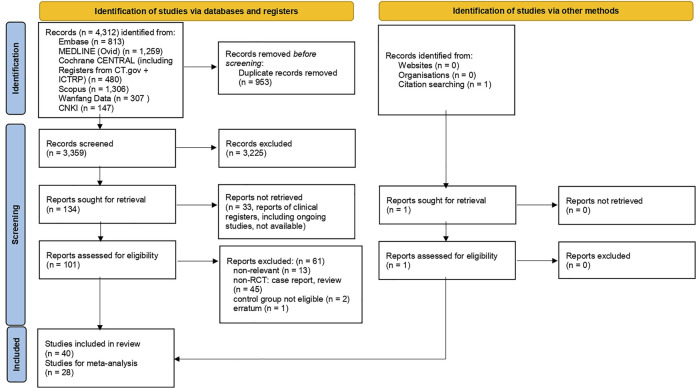
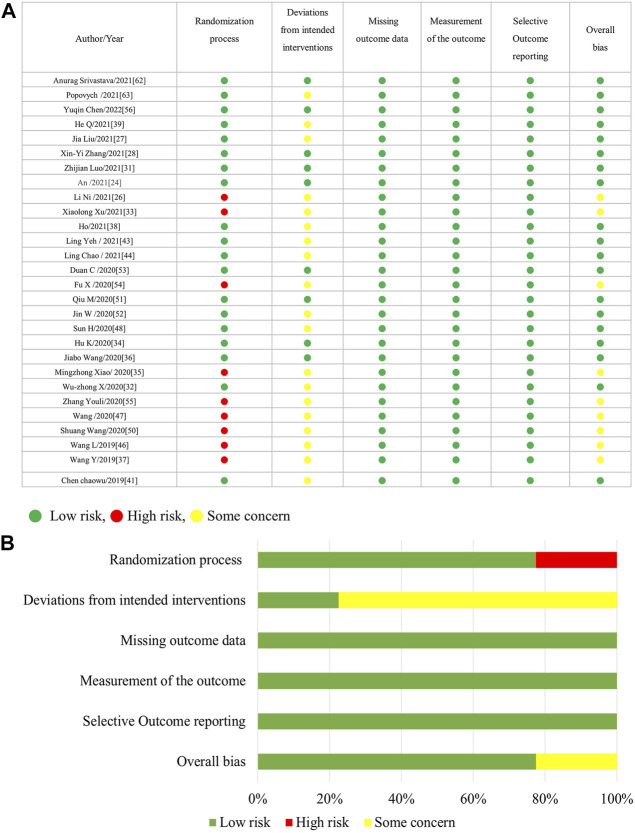
Figure 1 is the flow diagram of the literature search and screening, which complies with PRISMA guidelines (Page et al., 2021). Figure 2 demonstrates the risk of bias by applying low ROB, high ROB or some concern to each item. We also consulted the third reviewer if any disagreement occurred for risk of bias.
FIGURE 1.
Study selection flow diagram based on the PRISMA 2020 statement.
FIGURE 2.
(A): Risk of bias summary (ROB2). (B): Risk of bias graph.
3.2 Study characteristics
In total, 40 studies that fulfilled the inclusion criteria were included for systematic review; yet 28 randomized clinical trials were eligible for meta-analysis for targeted outcome measures; Table 1 summarizes the characteristics of the 40 randomized clinical trials.
TABLE 1.
The characteristics of the included study.
| References | Sample size (intervention: Control) | Age | Type of COVID 19 | Intervention | Control | Treatment days | Outcomes | Adverse report (V or no report--) |
|---|---|---|---|---|---|---|---|---|
| Mehrdad Karimi et al., 2021(Iran) | I:184 | I:48.7 | — | Persian medicine + c | Conventional western medicine | 7 | #131413 | V (Gastro-intestine events) |
| C:174 | C:50.8 | C, E,F | ||||||
| Morteza Kosari et al., 2021(Iran) | I:25 | I:43.5 | — | Propolis plus Hyoscyamus niger L. methanolic extract | Conventional western medicine | 6 | C | no report |
| C:25 | C:41.5 | |||||||
| Borujerdi et al., 2021(Iran) | I:59 | I:44.3 | Mild to moderate | Zufa syrup | Conventional western medicine + placebo | 10 | C,F | no AE |
| C:57 | C:44.0 | |||||||
| Kirti S Pawar, et al., 2021 (India) | I:70 | I:18–85 | Mild to severe | Curcumin and piperine | Conventional western medicine + placebo | 14 | A, B, C, E, F | no AE |
| C:70 | C:25–84 | |||||||
| Muhammed Majeed et al., 2021 (India) | I:50 | I:39.0 | — | ImmuActiveTM 500 mg capsule | Placebo (same appearance) | 28 | C, D, E, F | no AE |
| C:50 | C:37.3 | |||||||
| Anurag Srivastava et al., 2021 (India) | I1:40 | I1: 44.4 | Mild to moderate | I2:Nilavembu Kudineer + allopathy treatment | Placebo + allopathy treatment | 10 | B, C, D,E, F | V (vomiting and diarrhea) |
| I2:40 | I2: 42.8 | I3:Kaba Sura Kudineer | ||||||
| C:40 | I3: 39.5 | + allopathy treatment | ||||||
| Popovych et al., 2021 (Ukraine) | I:49 | I:33.1 | Mild | BNO 1030 (a standardized extract of seven medicinalplants (Imupret®) | Conventional western medicine | 14 | C, E, F | no AE |
| C:47 | C:3.6 | |||||||
| Yuqin Chen et al., 2022(China) | I:64 | I:54.2 | — | Bufei Huoxue capsules | Placebo | 90 | A, C, F, G | V (Abnormal liver function) |
| C:65 | C:52.5 | |||||||
| He Q, et al., 2021(China) | I:36 | — | Mild | Buzhong Yiqi decoction + c | Conventional western medicine | 10 | A, B, C,D,F | V (1 with arrthymia) |
| C:35 | ||||||||
| Jia Liu et al., 2021(China) | I:99 | I:56.0 | Mild to severe stages | Huashi Baidu granule + c | Conventional western medicine | 14 | A, B, C, D, F | V (diarrhea) |
| C:96 | C:56.5 | |||||||
| Xin-Yi Zhang et al., 2021(China) | I:65 | I:44.3 | Mild to moderate | Xiyanping injection + c | Conventional western medicine | 14 | C,D,F | V (mild) |
| C:65 | C:48.3 | |||||||
| Zhijian Luo et al., 2021(China | I:29 | I:60.3 | Severe | Xuebijing | Conventional western medicine | 14 | B,C,E,F | V (no AI) |
| C:28 | C:56.4 | |||||||
| Shuang Zhou et al., 2021(China) | I:57 | 66 (median) | Severe/Critical | Standard care (Table 1)+shenhuang granule | Conventional western medicine | 14 | B, F | V (Increased neutrophil) |
| C:54 | ||||||||
| An et al., 2021(China) | I:92 | I:50.2 | — | Jinhua Qinggan granules | Conventional western medicine | 14 | C,D, F | no AE |
| C:31 | C:44.7 | |||||||
| Li Ni et al., 2021(China) | I:56/61/59 | I:54/56/53 | — | Shuanghuanglian oral liquids | Conventional western medicine | 14 | A, C, D, F | No serious adverse events occurred |
| C:59 | C:51 | |||||||
| Xiaolong Xu et al., 2021(China) | I:77 | I:49.1 | — | Reduning injection | Conventional western medicine | 14 | C, D, E, F | V |
| C:80 | C:50.4 | |||||||
| Congcong Zeng et al., 2021(China) | I:30 | I:50.7 | Mild to moderate | Maxingshigan-Weijing Decoction | Conventional western medicine | 14 | B, C, D, E, G, F | no AE |
| C:29 | C:53.3 | |||||||
| Chen Zhao et al., 2021(China) | I:358 | I:52.0 | Mild | Huashibaidu Granule | Conventional treatment | 7 | C, E, F | V (Mild diarrhea) |
| C:384 | C:50 | |||||||
| Ping Xianghua et al., 2021(China) | I:30 | I:40.8 | Mild to severe | Jiawei Yupingfeng powder | Conventional western medicine | 14 | A, B, C, D, E, F | V (vomiting, chest distress diarrhea) |
| C:24 | C:41.2 | |||||||
| Ho et al., 2021(China) | I:34 | I:15–80 | Moderate to severe | Shengmai Powder | Conventional western medicine | 7 | A, B, D, G | no report |
| C:30 | C:15–80 | |||||||
| Zhang Dan et al., 2021(China) | I:240 | — | High risk | Fuzheng Gubiao Fangggan Decoction | Conventional western medicine | 14 | C | no report |
| C:240 | ||||||||
| Ling Yeh et al., 2021(China) | I:50 | I:43.3 | Ordinary | Modified Shengjiang Powder | Conventional western medicine treatment | 6 | B, C, F | no AE |
| C:50 | C:42.6 | |||||||
| Ling Chao et al., 2021(China) | I:51 | I:46.8 | Ordinary | Antivirus No. 1 + c | Conventional western medicine treatment | 9 | A, B, C, G | no report |
| C:45 | C:45.1 | |||||||
| Liu et al., 2021(China) | I:15 | I:41.6 | Mild to moderate | Jiawei Sang Ju drink | Conventional western medicine | 10 | A, B, C, D, F | V (no AI) |
| C:15 | C:44.5 | |||||||
| Duan C et al., 2020 (China) | I:82 | I:52.0 | Mild | jinhua qinggan granules | Conventional western medicine | 5 | C, G,F | V (diarrhea) |
| C:41 | C:50.3 | |||||||
| Fu X, et al., 2020(China) | I:37 | I:45.3 | Ordinary | Toujie Quwen granule | Conventional western medicine | 15 | B, C, F | no AE |
| C:36 | C:44.7 | |||||||
| Qiu M et al., 2020(China) | I:25 | I: 53.4 | Ordinary | Maxing Xuanfei Jiedu decoction | Conventional western medicine | 10 | A, C, G | no report |
| C:25 | C:51.3 | |||||||
| Jin W et al., 2020(China) | I:20; C:18 | I:43.6 | Ordinary | Compound Yin Chai granule + Qingqiao detoxification granule | Routine western medicine | 21 | A, B, C, E, F | no report |
| C:41.3 | ||||||||
| Sun H et al., 2020(China) | I:32 | I:45.4 | Mild, Ordinary | Lianhua Qingke granule + C | Antiviral medications | 14 | A, C, F | no AE |
| C:25 | C:42.0 | |||||||
| Hu K et al., 2021 (China) | 142:142 | I:50.4; C:50.8 | Ordinary | Lianhua Qingwen capsules (1.4 g,tid)+C | Antiviral medications | 14 | A, C, D, F | V (liver function) |
| Jiabo Wang et al., 2020(China) | I:24 | I:46.8 | — | Keguan-1 (ARDS-suppressing drug)+C | Antiviral drugs | 14 | A, D, F | V (diarrhea, anorexia, vomiting) |
| ; C:23 | C:51.4 | |||||||
| Mingzhong Xiao et al., 2020(China) | I:119 (58/61) | I:52.6/56.7 | — | 1)Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules or 2) Linahua granules | Antiviral drugs | 14 | C, F | no AE |
| ; C:63 | C:53.9 | Anti-infective drug | ||||||
| Wu-zhong Xiong et al., 2020(China) | I:22 | I:57.1; C:62.4 | Mild to ordinary | Xuanfei Baidu decoction + c | Conventional western medicine | 7 | B, C, F | no AE |
| C:20 | ||||||||
| Zhang Youli et al., 2020(China) | I:80 | I:53.4 | Ordinary | Jinyinhua Oral Liquid + C | Conventional western medicine | 10 | A, C, D | V (diarrhea) |
| C:40 | C:52.0 | |||||||
| Wang LQ et al., 2020(China) | I:58 | I:66 | Mild to severe | Gegen Qinlian pill | Routine treatment | — | A,B, C, D, F | no AE |
| C:60 | C:57.5 | |||||||
| Mou et al., 2020(China) | I:37 | I:47.4 | Mild to severe | Maxing Shigan Sanren Decoction | Conventional treatment | — | C | no report |
| C:37 | C:42.2 | |||||||
| Shuang Wang et al., 2020(China) | I:45 | I:43.8 | Ordinary | Tablets Combined with Sanren Decoction | Western medicine | 15 | B, C | no report |
| C:45 | C:42.6 | |||||||
| Wang L et al., 2020(China) | I:40 | — | Ordinary | Shengmai powder + Shenling Baizhu powder | Conventional western medicine | — | A, B, C, D, G, F | no AE |
| C:40 | ||||||||
| Wang Y et al., 2021(China) | I:70; C:70 | I:48.0 | Ordinary | Qingfei Paidu decoction + c | Conventional western medicine | 10 | B, E, F, G | V (fatigue) |
| C:49.4 | ||||||||
| Chen chaowu, et al., 2021(China) | I:28 | I:49.5 | Mild | Lianhua Qingwen capsule + c | Conventional western medicine | — | B, C, D, F | V (vomiting, diarrhea, liver) |
| C:29 | C:50.2 |
Outcomes: A: Chest CT, or other imaging; B: blood test and cytokine; C: symptom evaluation; D: virus nuclei acid tests; E: hospitalization time; F: AE, and mortality; G: TCM, syndrome score.
Among these 40 randomized clinical trials, 33 trials were conducted in China with Chinese herbal medicine; of these, 13 were published in English (Wang J. B. et al., 2020; Xiong W. Z. et al., 2020; Xiao et al., 2020; An et al., 2021; Zhao C. et al., 2021; Liu J. et al., 2021; Xu X. et al., 2021; Zhang X. Y. et al., 2021; Hu et al., 2021; Luo et al., 2021; Ni et al., 2021; Zeng et al., 2021; Zhou et al., 2021), while 20 were published in Chinese (Jin W. et al., 2020; Wang et al., 2020b; Wang et al., 2020c; Duan et al., 2020; Wang S. et al., 2020; Fu et al., 2020; Mou et al., 2020; Qiu et al., 2020; Sun et al., 2020; Zhang et al., 2020; Chen C. et al., 2021; Liu A. et al., 2021; Chen Y. et al., 2021; Zhao J. et al., 2021; Wang Y. et al., 2021; He et al., 2021; He and Zhang, 2021; Ping et al., 2021; Ye et al., 2021; Zhang, 2021). In addition, 3 studies were conducted in Iran (Karimi et al., 2021; Kosari et al., 2021; Borujerdi et al., 2022), 3 in India (Majeed et al., 2021; Pawar et al., 2021; Srivastava et al., 2021) and 1 in Ukraine (Popovych et al., 2021). Twelve randomized clinical trials (30%) were multi-center trials whereas others were conducted in a single site. In total, 5,417 study participants were included in this systematic review, with sample sizes ranging from 15 to 384. Treatment duration ranged from 6 to 90 days.
3.3 Assessment of methodological quality
Figure 2 shows that 33% of the included trials (13/40 randomized clinical trials) did not appropriately address the process of randomization, and more than 75% (31/40 trials) have the deviations from intended interventions. Regarding selective outcome reporting and incomplete outcome measures data, all included trials were within low risk; however, some trials were within unclear risk of allocation concealment. Generally speaking, the included trials were of medium quality, while details of randomization and blinding were the most frequent problems.
3.3.1 Grading of recommendations assessment development evaluation assessment
Table 2 summarizes the evidence certainty of outcomes. Considering that more than half of the enrolled randomized clinical trials were rated as some-concerned of bias (RoB), we downgraded the evidence certainty in the domain of study limitation. The domain of inconsistency was downgraded because of the varied heterogeneity in outcomes as how to evaluate the severity of cough and fever. Publication bias was not considered based on no asymmetry in funnel plots.
TABLE 2.
Grade evidence Profile.
| Certainty assessment | Risk difference 95% CI | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes (no of studies) | Study limitation | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | |
| Fever (12 randomized clinical trials) | No serious limitation | No serious inconsistency | No Serious indirectness | No serious | Undetected | Moderate | 1.09 (1.1.19) |
| ⊕⊕○○ | |||||||
| Cough (13) | Serious | Serious inconsistency | Serious indirectness | Serious | Undetected | Low | 1.43 (1.21, 1.71) |
| ⊕○○○ | |||||||
| Fatigue (11) | Serious | Serious inconsistency | Serious indirectness | Serious | Serious | Low | 1.21 (1.10.1.33) |
| ⊕○○○ | |||||||
| Chest CT (16) | No serious | No serious | No Serious indirectness | No serious | Undetected | High | 1.26 (1.19.1.34) |
| ○⊕⊕⊕ | |||||||
| Virus conversion time (10) | No serious | No serious | No Serious indirectness | No serious | Undetected | High | 1.22 (1.06.1.40) |
| ○⊕⊕⊕ | |||||||
| Virus conversion rate (5) | No serious | No serious | No Serious indirectness | No serious | Undetected | High | −3.72 (−6.05, −1.40) |
| ○⊕⊕⊕ | |||||||
| Interleukin -6 (4) | No serious | No serious | No Serious indirectness | No serious | Undetected | High | 1.97 (−0.72.4.66) |
| ○⊕⊕⊕ | |||||||
| CRP (10) | No serious | No serious | No Serious indirectness | No serious | Undetected | High | −7.92 (−11.3,−4.53) |
| ○⊕⊕⊕ | |||||||
GRADE: grading of recommendations assessment, Development, and Evaluation; RCT: randomized clinical trials; CI: confidence interval.
3.4 Publication bias
The funnel plot was used to explore potential publication bias (Figure 3). The funnel plot is symmetrical, indicating no obvious deviation and that publication bias is unlikely. Thirty-four trials (85%) reported “No” adverse events (AE), indicating little bias in this systematic review.
FIGURE 3.
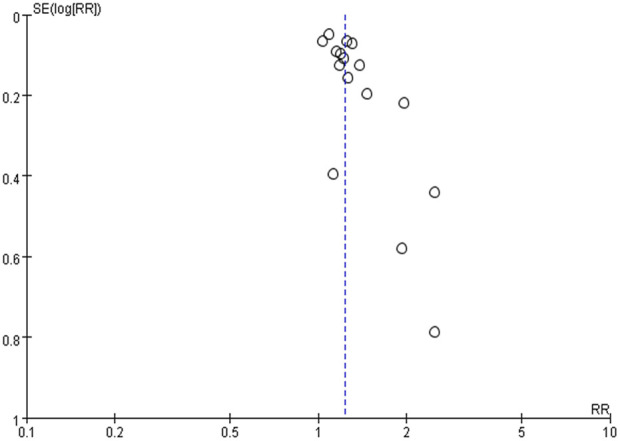
Funnel plots for included studies.
3.5 Main traditional chinese medicine and herbal components included in data analysis
This meta-analysis includes not only TCM but also HM, and 90% of the included trials used TCM formulas or granules. The complexity of TCM or HM components is evident; the most popular components are honeysuckle- and forsythia- based (which is a concept of couplet medicines in TCM theory), such as Jinhua qinggan granules (An et al., 2021), Jinyinhua liquid (Duan et al., 2020; Zhang et al., 2020), Toujie Quwen Granules, and Lianhua qingwen capsules (Duan et al., 2020; Yu et al., 2020; Chen C. et al., 2021; Hu et al., 2021). Another popular couplet medicines applied in these studies is ephedra sinica- and almond -based, such as Huashi Baidu granules (Zhao C. et al., 2021), Qingfei Paidu decoction (Wang Y. et al., 2021), Maxingshigan-Weijing decoction, Maxing Xuanfei Jiedu decoction (Qiu et al., 2020), Lianhua qingwen capsules (Chen et al., 2020; Yu et al., 2020; Chen C. et al., 2021; Hu et al., 2021) and Guangwenyilun. Other herbs are applied in India, Iran and Ukraine, including curcuminoids (Pawar et al., 2021), Propolis (Kosari et al., 2021), Rheum palmatum (Karimi et al., 2021) and polyherbal formulas (Borujerdi et al., 2022). The components of herbal medicine used in each trial are listed in Supplementary Appendix A2.
3.6 Outcomes and efficacy assessment
3.6.1 Effects of combined -herbal medicines on COVID-19 clinical symptoms (fever; cough; fatigue)
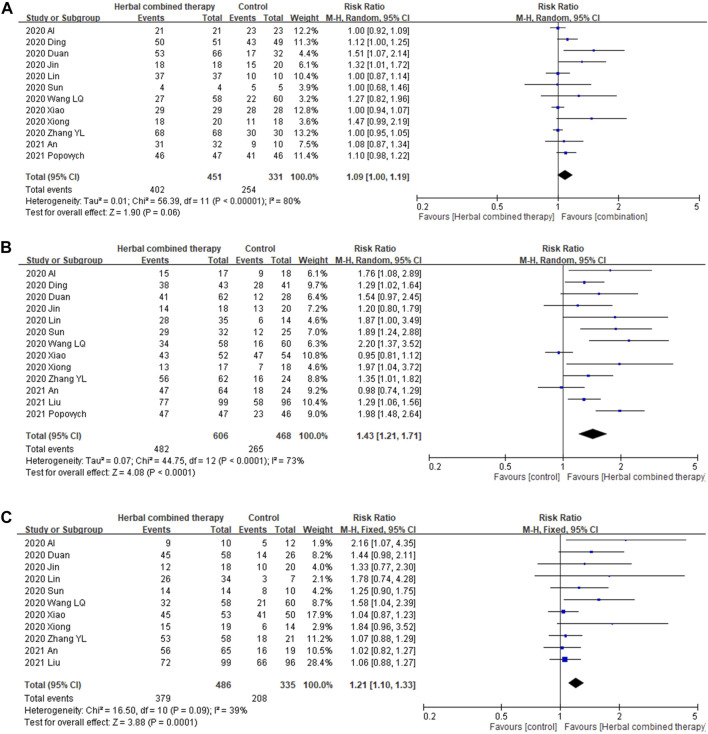
Significant between-study heterogeneity was observed in the effects of combined herbal medicines on COVID-19-related fever and cough (I2 = 80%, and 73%, respectively), but no significant between-study heterogeneity was noted in the effects of combined herbal medicines on fatigue (I2 = 39%). For the 12 trials that reported data on fever reduction cases, no significant differences were observed in subjects treated with combined HM and CWM (1.09 95% CI:1.00, 1.19, p = 0.06; Figure 4A) as compared with control intervention. In the 13 trials that reported data on cough reduction cases, significant improvement was observed in subjects treated with add-on herbs (1.43 95% CI:1.21, 1.71, p = 0.0001; Figure 4B) as compared with control subjects. In the 11 trials that reported data on fatigue reduction cases, significant differences in patient benefits were found when they were treated with combined HM and CWM (1.21 95% CI:1.10, 1.33, p = 0.0001; Figure 4C).
FIGURE 4.
Forrest plot of the effects of HM combined therapy in symptom relief. (A): comparison of HM combined therapy in fever reduction. (B): Comparison of HM combined therapy in cough reduction. (C): Comparison of HM combined therapy in fatigue reduction.
3.6.2 Effects of combined -herbal medicines on chest CT manifestations in COVID-19 patients
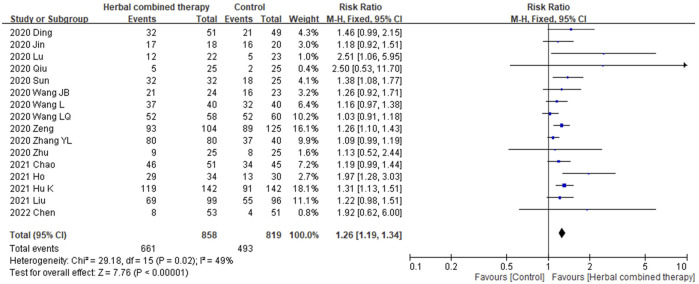
No significant between-study heterogeneity in chest CT manifestations were noted between studies in terms of combining HM and CWM (I2 = 49%). As for the 16 trials that reported chest CT data, significant improvement in CT images was observed in subjects receiving add-on herbal medicine (1.26 95% CI:1.19, 1.34, P ≤ 0.00001; Figure 5) as compared with those of control subjects. We provide additional random model effect in Supplementary Appendix A3.
FIGURE 5.
Forrest plot of the effects of HM combined therapy in chest CT improvement.
3.6.3 Effects of combined -herbal medicines on viral nucleic test, and viral negative conversion time (days)
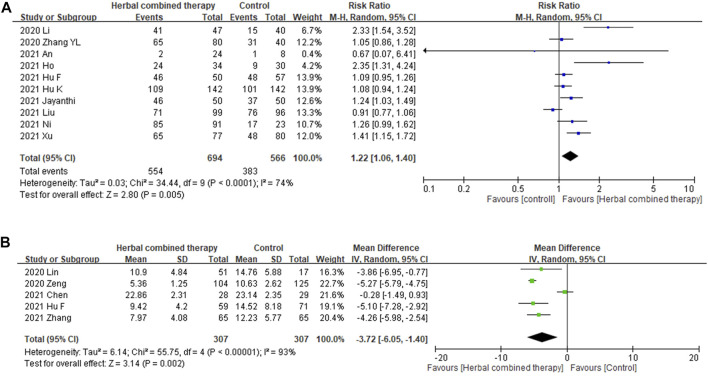
Significant between-study heterogeneity was observed in the effects of alternative medicines on viral conversion rate and viral conversion time (I 2 = 74%, and 93%, respectively). For the 10 trials that reported data on viral negative conversion rates, more significant improvements were observed in subjects whose treatment included with add-on herbs (1.22 95% CI:1.06, 1.40, p = 0.005; Figure 6A). In the 5 trials that reported data on viral negative conversion time (days), significant improvement was noted in subjects whose treatment included combined herbs (−3.72 95% CI: −6.05, −1.40, p = 0.002; Figure 6B) as compared with CWM alone.
FIGURE 6.
(A): Forrest plot of the effects of HM combined therapy in viral conversion rate. (B): Forrest plot of the effects of HM combined therapy in viral conversion time (day).
3.6.4 Effects of combined -herbal medicines on serum interleukin-6 (IL-6), and C reactive protein
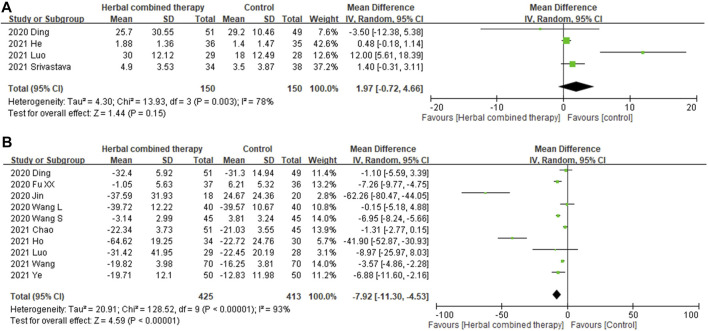
Significant between-study heterogeneity was observed in the effects of alternative medicines on serum interleukin-6 level and CRP level (I 2 = 78%, and 93%, respectively). In 4 trials that reported data on serum interleukin-6 level, no significant differences were observed in subjects treated with add-on herbs (1.97 95% CI: −0.72, 4.66, p = 0.15; Figure 7A). Regarding serum CRP levels, significant decreases were noted in the analysis of 10 enrolled trials that treated with combined HM (−7.92 95% CI: −11.30, −4.53, p = 0.000; Figure 7B) as compared with control subjects.
FIGURE 7.
(A): Forrest plot of the effects of HM combined therapy in interleukin-6 (ng/ml) in COVID19 patient. (B): Forrest plot of the effects of HM combined therapy in C-reactive protein (ng/ml)in COVID19 patient.
3.6.5 Safety concern
No serious adverse events were noted in all included randomized clinical trials, except few trials mentioned that participants might have some nausea or GI upset which not directly related to the herbal formula. Accordingly, no quantitative analysis was applied targeted the safety concern or adverse events.
4 Discussion
To our knowledge, this meta-analysis has the advantages of recruiting 2-years trials, and that outcomes selection was not only confined to subjective constitution scores or subjective symptom improvement but also included the objective measurement of changes in CT images, virus conversion rate and time and cytokines such as IL6, as well as changes in CRP levels. Except for IL6 changes, the results favored HM combined therapy by its add-on effect, which shortened virus conversion time and rate and improved clinical symptoms and CRP better than CWM alone. The mechanism of HM is hard to confirm since the components of these formulas are complicated, ranging from a single herb to more than ten herbs added into the treatment regimen in these trials.
Furthermore, the dosage and the course of treatment can always be adjusted according to the patient’s condition, for which sensitivity analysis shows increased heterogeneity. One study even tried to identify the most frequently-used herb associated with this issue (Xiong X. et al., 2020), but as each herb’s proportion varies between different formulas, and the use of HM or TCM emphasizes the synergistic effect between multiple herbs in individual formulas, it remains difficult to identify a specific single herb as a potential drug for COVID-19 treatment. However, Indian trials applied Zingiber officinale-based (Srivastava et al., 2021) or curcumin-based (Pawar et al., 2021) polyherbal formulas, which are also popularly used in Asian countries. Several TCM studies focused on “Lian-Hua Qing-Wen granules” (Hu et al., 2020; Shi M. et al., 2021) and Qingfei Paidu decoction (Li X. et al., 2021; Li Y. et al., 2021; Wang Q. et al., 2021; Wu et al., 2022), which are recognized as Chinese patent medicines and are now available in western countries. In the real world, HM emphasizes the synergistic effect based on holistic theory, which appeals to people who prefer natural products. In the academic field, some scholars have applied modern pharmacologic methods in analyzing TCM formulas and noted that they have similar mechanisms, including the regulation of apoptosis and immune response (Shi et al., 2021c), and anti-inflammatory effects, which tend to benefit lung function in patients with COVID-19 (Yan et al., 2020; Zhang F. et al., 2021). For example, a deep study proposed that “Rheum palmatum L (Da Huang),” a common component of these formulas, can directly block the viral life cycle (Shi et al., 2020), and inhibit viral transcription and replication (Galasiti Kankanamalage et al., 2018). The meta-analysis noted that combining HM therapy decreases CRP significantly, but does not decrease Il-6, indicating that HM helps to reduce the inflammatory status through an uncertain pathway. In a previous study, the CRP level correlated with the severity of lung involvement and prognosis (Beydogan and Yuruk Atasoy, 2021). In this context, it makes sense that many Chinese herbal medicines utilized in these trials targeted the lung meridian and anti-inflammatory effects are especially valuable. Similar studies investigated the potential herbs by utilizing information medicine and precision medicine (Balkrishna et al., 2021; Dzobo et al., 2021). While the present study has confirmed the efficacy of HM, further studies are needed to connect the potential drugs with precision medicine.
Additionally, some of the outcomes in the enrolled trials such as TCM syndrome, hospitalization days, or adverse events were not analyzed is due to the evaluation methods of TCM syndrome are not consistent or well addressed in original manuscripts. We didn’t analyze the hospitalization days owing to it was probably affected by some other medical, social, and medical rules problems which not directly related to COVID-19 infection. Furthermore, most studies address the adverse events in description without quantitative comparison; therefore, some outcomes were mentioned in the enrolled trials yet cannot be analyzed. Generally speaking, since the COVID-19 pandemic started in 2019, and researchers are still very much at a groping stage. Some consensuses are under modified during this period; thus, we only chose the presentive clinical symptom (fever, cough, fatigue), image improvements, and quantitative outcomes as cytokine change and reliable virus conversion times and rate for analysis. Further detailed analysis towards other items could be considered depend on how scholars treat the COVID related issues.
Regarding the basic mechanism about these herbs or potential of the plants’ extracts, more and more research focus on the network pharmacology analysis and cross-talks between signaling pathways (Qi et al., 2022). Take some formulas, which have been included in this meta-analysis, Lian Hua Qing Wen has been proved with regulating angiotensin converting enzyme 2 (ACE2) expression-disorder-caused symptoms and relieve the cytokine storm (Zheng et al., 2020). Some study revealed that acacetin, wogonin, and isorhamnetin were the main active ingredients in Qing Fei Pai Du decoction, (Li et al., 2022); while the target network model noted that some compounds such as licochalcone B acted on multiple targets, and multiple components interacted with the same target such as GPR35, reflecting the synergistic mechanism of Chinese medicine (Xu F. et al., 2021). Another special agent which not included in this trial is a potential mucosal topical agent “Ankaferd hemostat (ABS)”, which was approved with effect of antagonizing proteinase-activated receptors (PARs), mainly PAR-1. By activation of the PAR-1, mediators and hormones impact on the hemostasis, endothelial activation, alveolar epithelial cells and mucosal inflammatory responses which are the essentials of the COVID-19. The mucosal problem is an issue which has been ignored for most study focused on oral form and systematic treatment (Beyazit et al., 2020).
The basic research of herbs confronts the problems that in-depth studies are not available to precisely define bioactive compounds of plant origin and their mechanism of action as many cross-talks between target pathway network and protein-protein interaction (PPI) network (Wang L. et al., 2021); this underlines the need for studying the synthetic molecules to circumvent the viral load in the host system (Prasad et al., 2020). Additional trials or basic research may be designed on the hypothetically established plant extracts as add-on herbal medicines in the COVID-19 treatment field and there is a lot space for exploration.
Lastly, although some between -study heterogenicity was noted; we didn’t perform additional heterogeneity analysis as no more than 10 trials among these indicators are included and we still need to hold a conservative view point towards the results. Furthermore, no un-tolerable side effects were reported by 80% of the studies in the present meta-analysis. Among the enrolled trials, the treatment goals primarily targeted respiratory distress and symptoms. In the present review, the most common AEs were diarrhea or other gastrointestinal upset, which may also be associated with anti-inflammatory effects of the specific herbal medicines applied for treating COVID-19 (Luo et al., 2020). Therefore, the side effects were similar to those of antibiotics or associated with combining the herbs with the use of western medicines (anti-viral, antimicrobial agent); nevertheless, the events were mild and tolerable, we didn’t analyze since there were no quantitative report from the enrolled trials. However, another study advocated cautious use of HM in patients with prominent gastrointestinal symptoms (Shi et al., 2021b). We suggest that in the process of HM research, drug-drug interactions remain a concern in mainstream medicine, which prompts us to explore new drugs, not only those confined to herbal medicine. Therefore, the best way is to record AEs carefully, and the present review has revealed that HM is safe and the side effects are easily manageable. Accordingly, in this meta-analysis, patients with mild to moderate COVID-19 infection who received conventional therapy combined with HM benefited more than those receiving only CWM. From an epidemiological view, because COVID-19 is caused by rapid viral emission, shortening the disease is meaningful toward decreasing the spread of the pandemic and lowering the medical and economic burden (Pak et al., 2020; Zhang et al., 2022). Since the global population has paid a heavy price for this pandemic, it is worthwhile to make the best use of herbal medicines in treating infected patients worldwide.
4.1 Limitations
The present review has several limitations. First, many included trials lack details of methodology such as the randomization process, allocation or blinding. Secondly, the composition of herbal formulas varied considerably between studies and some even had overlapping components. Thirdly, most of the trials were conducted in a single center, which limits generalizability of results to other populations and may also compromise comparisons with multicenter studies. Lastly, more consensus about the reliable endpoints were in need to be reached about herbal medicine related study before we can make any further precise analysis and conclusion. As COVID-19 is an emergent infective disease, double blinding is difficult, yet in further study, more consistent herbal formulas and solid methodologies are required to make a powerful conclusion. We also expect more and more herbal medicine related COVID-19 research will published, and we might extract some studies which are qualified for sub-group analysis and provide valuable information for future scholars who has interests in mechanism exploration; and this is our preliminary meta-analysis on this issue.
5 Conclusion
This systematic review has demonstrated that herbal medicine is a viable complementary therapy for COVID-19 and the application of herbal medicine to COVID-19 patients in certain circumstances is recommended. It has the benefit of mitigating clinical symptoms (fever, cough, fatigue) and shortening the disease duration; yet for the complicated components in varied formulas, of which herb exert its effect need to be further investigated and clarified. The definitive herb or mechanism remains uncertain and related studies are ongoing. The merge of existing studies and further identify potential herb for following studies with scientific method is in emergent. Further large and rigorous multicenter trials or basic research integrating informative and precision medicine are warranted in order to clarify role of add-on herbal medicine in the COVID-19 regimen.
Acknowledgments
We thank all authors and participants in this study and the authors we have contacted who provided us with the information of their trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conception and design: T-JC Collection and assembly of data: T-JC; C-YL; C-JF Statistical analysis: J-HP; Y-IC literature research: Y-XW; S-WC Manuscript writing: All authors Final approval of manuscript.
Funding
The study is supported by Ministry of Science and Technology – MOST, Taiwan grant 108–2320-B-532–001 -MY3.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.950012/full#supplementary-material
Abbreviations
HM, Herbal medicine; CWM, conventional western medicine; WHO, World Health Organization; RoB 2, Risk of Bias version 2; RCTs, randomized controlled trials; AE, adverse events; RR, risk ratio; ACE2, angiotensin converting enzyme2.
References
- An X., Xu X., Xiao M., Min X., Lyu Y., Tian J., et al. (2021). Efficacy of Jinhua qinggan granules combined with western medicine in the treatment of confirmed and suspected COVID-19: A randomized controlled trial. Front. Med. 8, 728055. 10.3389/fmed.2021.728055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L., Lee H. W., Kim A., Choi J. Y., Lee M. S. (2021). Network analysis of herbs recommended for the treatment of COVID-19. Infect. Drug Resist. 14, 1833–1844. 10.2147/IDR.S305176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L., Lee H. W., Kim A., Lee M. S. (2020a). Herbal medicine for the management of COVID-19 during the medical observation period: A review of guidelines. Integr. Med. Res. 9, 100465. 10.1016/j.imr.2020.100465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L., Song E., Lee H. W., Lee M. S. (2020b). Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 9, 1583. 10.3390/jcm9051583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Verma S., Sharma P., Tomer M., Srivastava J., Varshney A. (2021). Comprehensive and rapid quality evaluation method for the ayurvedic medicine divya-swasari-vati using two analytical techniques: UPLC/QToF MS and HPLC-DAD. Pharm. (Basel) 14, 297. 10.3390/ph14040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyazit F., Beyazit Y., Tanoglu A., Haznedaroglu I. C. (2020). Ankaferd hemostat (ABS) as a potential mucosal topical agent for the management of COVID-19 syndrome based on its PAR-1 inhibitory effect and oestrogen content. Med. Hypotheses 143, 110150. 10.1016/j.mehy.2020.110150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydogan E., Yuruk Atasoy P. (2021). The relationship between CRP at admission and thorax CT findings in patients diagnosed with COVID-19. Int. J. Clin. Pract. 75, e14962. 10.1111/ijcp.14962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borujerdi R., Adeli S. H., Mohammadbeigi A., Aliasl F., Asghari A., Hormati A., et al. (2022). Effects of Iranian polyherbal syrup (zufa syrup) on oxygen saturation and clinical symptoms in suspected patients with COVID-19: A triple-blinded, randomized, placebo-controlled trial. Med. Gas. Res. 12, 44–50. 10.4103/2045-9912.325991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li X., Liu Y., Chen S. (2021a). [Clinical study of Lianhua qingwen capsule in the treatment of corona virus disease 2019]. Res. Integr. Tradit. Chin. West. Med. 13, 1–4. 10.3969/j.issn.1674-4616.2021.01.001 [DOI] [Google Scholar]
- Chen J., Zhou Y., Chen F., Liu X., Chen Y., Wang S. (2020). [Clinical study on treatment of COVID-19 in convalescent period treated with Lianhua Qingwen Capsule combined with Interferon α-2b]. Adv. Clin. Med. 10, 1144–1149. 10.12677/acm.2020.106174 [DOI] [Google Scholar]
- Chen Y., Liu C., Wang T., Qi J., Jia X., Zeng X., et al. (2021b). Efficacy and safety of bufei huoxue capsules in the management of convalescent patients with COVID-19 infection: A multicentre, double-blind, and randomised controlled trial. J. Ethnopharmacol. 284, 114830. 10.1016/j.jep.2021.114830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Collaboration (2021). Risk of bias 2 (RoB 2) tool. Available at: https://methods.cochrane.org/risk-bias-2 (Accessed July 15, 2022). [Google Scholar]
- Diekemper R. L., Patel S., Mette S. A., Ornelas J., Ouellette D. R., Casey K. R. (2018). Making the GRADE: CHEST updates its methodology. Chest 153, 756–759. 10.1016/j.chest.2016.04.018 [DOI] [PubMed] [Google Scholar]
- Ding Q., Lu P., Fan Y., Xia Y., Liu M. (2020). The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 92, 1549–1555. 10.1002/jmv.25781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Shi L., Cao W., Zuo B., Zhou A. (2021). Add-on effect of Chinese herbal medicine in the treatment of mild to moderate COVID-19: A systematic review and meta-analysis. PLoS One 16, e0256429. 10.1371/journal.pone.0256429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Xia W., Zheng C., Sun G., Li Z., Li Q., et al. (2020). [Clinical observation on Jinhua Qinggan Granule combined with conventional western medicine therapy in treating mild cases of Coronavirus Disease 2019]. J. Tradit. Chin. Med. 61, 1473–1477. 10.13288/j.11-2166/r.2020.17.001 [DOI] [Google Scholar]
- Dzobo K., Chiririwa H., Dandara C., Dzobo W. (2021). Coronavirus disease-2019 treatment strategies targeting interleukin-6 signaling and herbal medicine. OMICS 25, 13–22. 10.1089/omi.2020.0122 [DOI] [PubMed] [Google Scholar]
- Fan A. Y., Gu S., Alemi S. F., Research Group for Evidence-based Chinese M. (2020). Chinese herbal medicine for COVID-19: Current evidence with systematic review and meta-analysis. J. Integr. Med. 18, 385–394. 10.1016/j.joim.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Lin L., Tan X. (2020). [Clinical study on 37 case of COVID- 19 treated with integrated traditional Chinese and western medicine]. Tradit. Chin. Drug Res. Clin. Pharm. 31, 600–604. 10.19378/j.issn.1003-9783.2020.05.016 [DOI] [Google Scholar]
- Galasiti Kankanamalage A. C., Kim Y., Damalanka V. C., Rathnayake A. D., Fehr A. R., Mehzabeen N., et al. (2018). Structure-guided design of potent and permeable inhibitors of MERS coronavirus 3CL protease that utilize a piperidine moiety as a novel design element. Eur. J. Med. Chem. 150, 334–346. 10.1016/j.ejmech.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T., Kulper-Schiek W., Reda S., Treskova-Schwarzbach M., Koch J., Vygen-Bonnet S., et al. (2021). 2) variant: Second interim results of a living systematic review and meta-analysis, 1 january to 25 august 2021. Eff. COVID-19 vaccines against SARS-CoV-2 Infect. Delta (B.1.617.Euro Surveill 26, 2100920. 10.2807/1560-7917.ES.2021.26.41.2100920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Zhang Q. (2021). [Clinical efficacy analysis of ShengMai San in the treatment of Qi-Yin deficiency syndrome in convalescent stage of COVID-19]. ActaChin. Med. Pharm. 49, 84–86. 10.19664/j.cnki.1002-2392.210069 [DOI] [Google Scholar]
- He Q., Zhang Q., Gan X., Li X. (2021). [Clinical efficacy analysis of Buzhong Yiqi Decoction in the treatment of mild new coronavirus pneumonia]. J. Emerg. Tradit. Chin. Med. 30, 385–387. 10.3969/j.issn.1004-745X.2021.03.003 [DOI] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., et al. (2022). Cochrane handbook for systematic reviews of interventions version 6.3. Available at: https://training.cochrane.org/handbookrob (Accessed July 15, 2022). [Google Scholar]
- Hu K., Guan W. J., Bi Y., Zhang W., Li L., Zhang B., et al. (2021). Efficacy and safety of lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 85, 153242. 10.1016/j.phymed.2020.153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Yang M., Xie C. (2020). Efficacy and safety of lian-hua qing-wen granule for COVID-2019: A protocol for systematic review and meta-analysis. Med. Baltim. 99, e20203. 10.1097/MD.0000000000020203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., Lu Y., Zhao W., Tang S., Sang X., Zhang L., et al. (2020a). [The efficacy of recommended treatments with integrated Chinese and western medicine on coronavirus disease 2019 ( COVID-19) in sichuan: A clinical trial observation]. Pharm. Clin. Chin. Mat. Med. 36, 6–10. [Google Scholar]
- Jin Y. H., Cai L., Cheng Z. S., Cheng H., Deng T., Fan Y. P., et al. (2020b). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 7, 4. 10.1186/s40779-020-0233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S., Shaik Syed Ali P., Sheeza A. (2021). Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: A mini review. Eur. Rev. Med. Pharmacol. Sci. 25, 8019–8022. 10.26355/eurrev_202112_27653 [DOI] [PubMed] [Google Scholar]
- Karimi M., Zarei A., Soleymani S., Jamalimoghadamsiahkali S., Asadi A., Shati M., et al. (2021). Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytother. Res. 35, 6295–6309. 10.1002/ptr.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosari M., Noureddini M., Khamechi S. P., Najafi A., Ghaderi A., Sehat M., et al. (2021). The effect of propolis plus hyoscyamus Niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID-19: A clinical trial. Phytother. Res. 35, 4000–4006. 10.1002/ptr.7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang K., Bao J., Yang J., Wu C. (2022). Potential mechanism of action of Jing Fang Bai Du San in the treatment of COVID-19 using docking and network pharmacology. Int. J. Med. Sci. 19, 213–224. 10.7150/ijms.67116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xiang L., Lin Y., Tang Q., Meng F., Chen W. (2021a). Computational analysis illustrates the mechanism of Qingfei Paidu decoction in blocking the transition of COVID-19 patients from mild to severe stage. Curr. Gene Ther. 22, 277–289. 10.2174/1566523221666210907162005 [DOI] [PubMed] [Google Scholar]
- Li Y., Li B., Wang P., Wang Q. (2021b). Traditional Chinese medicine, Qingfei Paidu decoction and Xuanfei Baidu decoction, inhibited cytokine production via NF-kappaB signaling pathway in macrophages: Implications for coronavirus disease 2019 (COVID-19) therapy. Front. Pharmacol. 12, 722126. 10.3389/fphar.2021.722126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Wang W., Liao J., Wang X., Liu X., Zeng Y., et al. (2021a). [Observation on the curative effect of Jia Wei Sang Ju Decoction in the treatment of novel coronavirus pneumonia with wind-heat invading the lung (Jia Wei Sang Ju Yin zhi liao Feng Re Fan Fei zheng xin xing guan Zhuang bing du fei yan liao xiao guan cha)]. Mod. J. Integ Tradit. Chin. West Med. 30, 2395–2399. 10.3969/j.issn.1008-8849.2021.22.001 [DOI] [Google Scholar]
- Liu J., Yang W., Liu Y., Lu C., Ruan L., Zhao C., et al. (2021b). Combination of Hua Shi Bai du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine 91, 153671. 10.1016/j.phymed.2021.153671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C. H., Ma L. L., Liu H. M., Liao W., Xu R. C., Ci Z. M., et al. (2020). Research progress on main symptoms of novel coronavirus pneumonia improved by traditional Chinese medicine. Front. Pharmacol. 11, 556885. 10.3389/fphar.2020.556885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Chen W., Xiang M., Wang H., Xiao W., Xu C., et al. (2021). The preventive effect of xuebijing injection against cytokine storm for severe patients with COVID-19: A prospective randomized controlled trial. Eur. J. Integr. Med. 42, 101305. 10.1016/j.eujim.2021.101305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier H. E., Kuan G., Saborio S., Bustos Carrillo F. A., Plazaola M., Barilla C., et al. (2021). Clinical spectrum of SARS-CoV-2 infection and protection from symptomatic re-infection. Clin. Infect. Dis. 1, ciab717. 10.1093/cid/ciab717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M., Nagabhushanam K., Shah K., Mundkur L. (2021). A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of a nutritional supplement (ImmuActiveTM) for COVID-19 patients. Evid. Based Complement. Altern. Med. 2021, 8447545. 10.1155/2021/8447545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirtaleb M. S., Mirtaleb A. H., Nosrati H., Heshmatnia J., Falak R., Zolfaghari Emameh R. (2021). Potential therapeutic agents to COVID-19: An update review on antiviral therapy, immunotherapy, and cell therapy. Biomed. Pharmacother. 138, 111518. 10.1016/j.biopha.2021.111518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F., Gong X., Wei D., Gong X., Wang T., Xiong Y., et al. (2020). [Observation on curative effect of maxing shigan sanren decoction in the treatment of new coronavirus pneumonia]. J. Emerg. Tradit. Chin. Med. 36, 1259–1260. [Google Scholar]
- Negrut N., Codrean A., Hodisan I., Bungau S., Tit D. M., Marin R., et al. (2021). Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 21, 648. 10.3892/etm.2021.10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., et al. (2020). Combating COVID-19 with integrated traditional Chinese and Western medicine in China. Acta Pharm. Sin. B 10, 1149–1162. 10.1016/j.apsb.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Wen Z., Hu X., Tang W., Wang H., Zhou L., et al. (2021). Effects of shuanghuanglian oral liquids on patients with COVID-19: A randomized, open-label, parallel-controlled, multicenter clinical trial. Front. Med. 15, 704–717. 10.1007/s11684-021-0853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- Pak A., Adegboye O. A., Adekunle A. I., Rahman K. M., McBryde E. S., Eisen D. P. (2020). Economic consequences of the COVID-19 outbreak: The need for epidemic preparedness. Front. Public Health 8, 241. 10.3389/fpubh.2020.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyod S., Ho C. T., Sheen L. Y. (2020). Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Chin. Med. 10, 420–427. 10.1016/j.jtcme.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar K. S., Mastud R. N., Pawar S. K., Pawar S. S., Bhoite R. R., Bhoite R. R., et al. (2021). Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial. Front. Pharmacol. 12, 669362. 10.3389/fphar.2021.669362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping X.-H., Xu H.-L., Fu D.-F., Liu L., Xu H.-X. (2021). [Clinical observation of Jiawei Yupingfeng Powder combined with western medicine in the treatment of the novel coronavirus pneumonia]. Med. Forum 25, 149–151. [Google Scholar]
- Popovych V., Koshel I., Haman Y., Leschak V., Malofiichuk O., Kapustina N., et al. (2021). A randomized, open-label, multicentre, comparative study of therapeutic efficacy, safety, and tolerability of BNO 1030 extract, containing marshmallow root, chamomile flowers, horsetail herb, walnut leaves, yarrow herb, oak bark, dandelion herb, in the treatment of mild forms of COVID-19. Clin. Phytoscience 7, 72. 10.1186/s40816-021-00308-x [DOI] [PubMed] [Google Scholar]
- Prasad A., Muthamilarasan M., Prasad M. (2020). Synergistic antiviral effects against SARS-CoV-2 by plant-based molecules. Plant Cell Rep. 39, 1109–1114. 10.1007/s00299-020-02560-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J. H., Dong F. X., Wang K., Zhang S. Y., Liu Z. M., Wang W. J., et al. (2022). Feasibility analysis and mechanism exploration of Rhei Radix et Rhizome-Schisandrae Sphenantherae Fructus (RS) against COVID-19. J. Med. Microbiol. 71, 1. 10.1099/jmm.0.001528 [DOI] [PubMed] [Google Scholar]
- Qiu M., Li Q., Zhu D., Wang C., Sun Q., Qian C., et al. (2020). [Efficacy observation of maxing Xuanfei Jiedu decoction on moderate COVID-19 patients]. J Emerg Tradit Chin Med 29, 1129–1132. 10.3969/j.issn.1004-745X.2020.07.001 [DOI] [Google Scholar]
- Shi M., Peng B., Li A., Li Z., Song P., Li J., et al. (2021a). Broad anti-viral capacities of lian-hua-qing-wen capsule and jin-hua-qing-Gan granule and rational use against COVID-19 based on literature mining. Front. Pharmacol. 12, 640782. 10.3389/fphar.2021.640782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H., et al. (2020). Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study. Pharmacol. Res. 161, 105290. 10.1016/j.phrs.2020.105290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Wang F., Li J., Li Y., Li W., Wu X., et al. (2021b). The effect of Chinese herbal medicine on digestive system and liver functions should not be neglected in COVID-19: An updated systematic review and meta-analysis. IUBMB Life 73, 739–760. 10.1002/iub.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Wang F., Yao H., Kou S., Li W., Chen B., et al. (2021c). Oral Chinese herbal medicine on immune responses during coronavirus disease 2019: A systematic review and meta-analysis. Front. Med. (Lausanne) 8, 685734. 10.3389/fmed.2021.685734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiehzadegan S., Alaghemand N., Fox M., Venketaraman V. (2021). Analysis of the delta variant B.1.617.2 COVID-19. Clin. Pract. 11, 778–784. 10.3390/clinpract11040093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadja D. M., Mentzer S. J., Fontenay M., Laffan M. A., Ackermann M., Helms J., et al. (2021). COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 24, 755–788. 10.1007/s10456-021-09805-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreepadmanabh M., Sahu A. K., Chande A. (2020). COVID-19: Advances in diagnostic tools, treatment strategies, and vaccine development. J. Biosci. 45, 148. 10.1007/s12038-020-00114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Rengaraju M., Srivastava S., Narayanan V., Gupta V., Upadhayay R., et al. (2021). Efficacy of two siddha polyherbal decoctions, Nilavembu Kudineer and Kaba Sura Kudineer, along with standard allopathy treatment in the management of mild to moderate symptomatic COVID-19 patients-a double-blind, placebo-controlled, clinical trial. Trials 22, 570. 10.1186/s13063-021-05478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J. A., Sutton A. J., Ioannidis J. P., Terrin N., Jones D. R., Lau J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- Sun H., Xu F., Zhang L., Wei C., Chen J., Wang Q., et al. (2020). Study on clinical efficacy of Lianhua Qingke Granule in treatment of mild and ordinary COVID-19. Chin. J. Exp. Tradit. Med. Formulae 26, 29–34. 10.13422/j.cnki.syfjx.20201438 [DOI] [Google Scholar]
- Wang J. B., Wang Z. X., Jing J., Zhao P., Dong J. H., Zhou Y. F., et al. (2020a). Exploring an integrative therapy for treating COVID-19: A randomized controlled trial. Chin. J. Integr. Med. 26, 648–655. 10.1007/s11655-020-3426-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li W., Huang W., Zhou Z., Deng Y., Hu Y., et al. (2020b). [Clinical study of Gegen Qinlian pill in treating COVID-19]. Mod. Tradit. Chin. Med. Mater Mater-World Sci. Technol. 22, 3509–3514. 10.11842/wst.20200430002 [DOI] [Google Scholar]
- Wang L., Wang Y., Yang W., He X., Xu S., Liu X., et al. (2021a). Network pharmacology and molecular docking analysis on mechanisms of Tibetan Hongjingtian (Rhodiola crenulata) in the treatment of COVID-19. J. Med. Microbiol. 70, 001374. 10.1099/jmm.0.001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xu M., Wang Y., Li H.-B., Liu N., Zuo J.-L. (2020c). [Clinical study on shengmai powder combined with shenling baizhu powder in the treatment of common corona virus disease 2019]. China J. Traditl Chin. Med. Pharm. 35, 4268–4271. [Google Scholar]
- Wang Q., Zhu H., Li M., Liu Y., Lai H., Yang Q., et al. (2021b). Efficacy and safety of Qingfei Paidu decoction for treating COVID-19: A systematic review and meta-analysis. Front. Pharmacol. 12, 688857. 10.3389/fphar.2021.688857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liu X., Ran G., Liu M., Chen J. (2020d). [Clinical study on treatment of cases of COVID-19 treated with viable bifidobacterium tablets combined with sanren decoction]. Tradit. Chin. Med. 9, 288–292. 10.12677/tcm.2020.93043 [DOI] [Google Scholar]
- Wang Y., Chen L., Zheng L., Ku B.-Q., Yu R., Zhang X.-F. (2021c). [Clinical effects of Qingfei Paidu Decoction combined with conventional treatment on patients with coronavirus disease 2019]. Chin. Tradit. Pat. Med. 43, 656–659. 10.3969/j.issn.1001-1528.2021.03.017 [DOI] [Google Scholar]
- Wong C. K. H., Lau K. T. K., Au I. C. H., Xiong X., Lau E. H. Y., Cowling B. J. (2021). Clinical improvement, outcomes, antiviral activity, and costs associated with early treatment with remdesivir for patients with COVID-19. Clin. Infect. Dis. 7, 1450–1458. 10.1093/cid/ciab631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu L., Cao G., Min L., Dong T. (2022). Effect and mechanism of Qingfei Paidu decoction in the management of pulmonary fibrosis and COVID-19. Am. J. Chin. Med. 50, 33–51. 10.1142/S0192415X22500021 [DOI] [PubMed] [Google Scholar]
- Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., et al. (2020). Efficacy of huoxiang zhengqi dropping pills and Lianhua qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol. Res. 161, 105126. 10.1016/j.phrs.2020.105126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W. Z., Wang G., Du J., Ai W. (2020a). Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr. Med. Res. 9, 100489. 10.1016/j.imr.2020.100489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Wang P., Su K., Cho W. C., Xing Y. (2020b). Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis. Pharmacol. Res. 160, 105056. 10.1016/j.phrs.2020.105056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Hou T., Shen A., Jin H., Xiao Y., Yu W., et al. (2021a). Mechanism deconvolution of Qing Fei Pai Du decoction for treatment of Coronavirus Disease 2019 (COVID-19) by label-free integrative pharmacology assays. J. Ethnopharmacol. 280, 114488. 10.1016/j.jep.2021.114488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang J., Zheng W., Yang Z., Zhao X., Wang C., et al. (2021b). Efficacy and safety of reduning injection in the treatment of covid-19: A randomized, multicenter clinical study. Ann. Palliat. Med. 10, 5146–5155. 10.21037/apm-20-2121 [DOI] [PubMed] [Google Scholar]
- Yan H., Zou Y., Zou C. (2020). [Mechanism of Qingfei Paidu decoction for treatment of COVID-19: Analysis based on network pharmacology and molecular docking technology]. Nan Fang. Yi Ke Da Xue Xue Bao 40, 616–623. 10.12122/j.issn.1673-4254.2020.05.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Zhao H., Xu S., Chen W. (2021). [Clinical study of modified shengjiang powder in the treatment of ordinary COVID-19]. Chin. Foreign Med. Res. 19, 9–13. [Google Scholar]
- Yu P., Li Y. Z., Wan S. B., Wang Y. (2020). Effects of Lianhua qingwen granules plus arbidol on treatment of mild corona virus disease-19. Chin. Pharm. J. 55, 1042–1045. 10.11669/cpj.2020.12.014 [DOI] [Google Scholar]
- Zeng C., Yuan Z., Zhu J., Wang Y., Xie Y., Ye R., et al. (2021). Therapeutic effects of traditional Chinese medicine (Maxingshigan-Weijing Decoction) on COVID-19: An open-label randomized controlled trial. Integr. Med. Res. 10, 100782. 10.1016/j.imr.2021.100782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. (2021). [A randomized controlled study of Fuzheng Gubiao Fanggan Decoction intervention in high risk group of Corona Virus Disease 2019]. Pharm. Clin. Chin. Mat. Med. 12, 26–29. 10.3969/j.issn.1674-926X.2021.01.00710.1007/s12671-021-01618-4 [DOI] [Google Scholar]
- Zhang F., Huang J., Liu W., Wang C. R., Liu Y. F., Tu D. Z., et al. (2021a). Inhibition of drug-metabolizing enzymes by Qingfei Paidu decoction: Implication of herb-drug interactions in COVID-19 pharmacotherapy. Food Chem. Toxicol. 149, 111998. 10.1016/j.fct.2021.111998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li P., Zhang Z., Li W., Chen J., Song X., et al. (2022). Epidemic versus economic performances of the COVID-19 lockdown: A big data driven analysis. Cities 120, 103502. 10.1016/j.cities.2021.103502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Y., Lv L., Zhou Y. L., Xie L. D., Xu Q., Zou X. F., et al. (2021b). Efficacy and safety of xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 35, 4401–4410. 10.1002/ptr.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lei L., Xu Y., Wei D., Hu F. (2020). [Clinical efficacy of Jinyinhua oral liquid in the treatment of 80 patients with coronavirus disease 2019]. China Pharm. 29, 23–26. [Google Scholar]
- Zhao C., Li L., Yang W., Lv W., Wang J., Guo J., et al. (2021a). Chinese Medicine Formula Huashibaidu Granule early treatment for mild COVID-19 patients: An unblinded, cluster-randomized clinical trial. Front. Med. (Lausanne) 8, 696976. 10.3389/fmed.2021.696976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yang S., Ke D., Qiu L., Jiang L. (2021b). [Clinical observation of the Antiviral Formula-1 in the treatment of novel coronavirus pneumonia in early and middle stage with cold damp and depressed lung type]. Forum Tradit. Chin. Med. 36, 20–22. 10.13913/j.cnki.41-1110/r.2021.06.008 [DOI] [Google Scholar]
- Zheng S., Baak J. P., Li S., Xiao W., Ren H., Yang H., et al. (2020). Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in Corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW. Phytomedicine 79, 153336. 10.1016/j.phymed.2020.153336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Feng J., Xie Q., Huang T., Xu X., Zhou D., et al. (2021). Traditional Chinese medicine shenhuang granule in patients with severe/critical COVID-19: A randomized controlled multicenter trial. Phytomedicine 89, 153612. 10.1016/j.phymed.2021.153612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.