Abstract
A series of novel 1,2,3-triazole derivatives of capsaicin and its structural isomer (new natural product hybrid capsaicinoid) were synthesized by exploiting one-/two-point modification of capsaicin without altering the amide linkage (neck). The newly synthesized compounds were screened for their antiproliferative activity against an NCI panel of 60 cancer cell lines at a single dose of 10 μM. Most of the compounds have demonstrated reduced growth between 55 and 95%, whereas capsaicin (10) has shown reduced growth between 0 and 24%. Compounds showing more than 50% growth inhibition were further evaluated for the IC50 value. Among the cell lines tested, lung cancer cell lines (A549, NCI-H460) were found to be more susceptible toward most of the synthesized compounds. Compounds 14g and 14j demonstrated good antiproliferative activity in NCI-H460 with IC50 values of 6.65 and 5.55 μM, respectively, while compounds 18b, 18c, 18f, and 18m demonstrated potential antiproliferative activity in A549 cell lines with IC50 values ranging between 2.9 and 10.5 μM. Among the compounds, compound 18f was found to demonstrate the best activity with an IC50 value of 2.91 μM against A549. Furthermore, 18f induces cell cycle arrest at the S-phase and disrupts the mitochondrial membrane potential, reducing cell migration potential by inducing cellular apoptosis and higher ROS generation along with a decrease in mitochondrial membrane potential in addition to surface and nuclear morphological alterations such as a reduction in the number and shrinkage of cells coupled with nuclear blabbing indicating the sign of apoptosis of A549 non-small cell lung cancer cell lines. Compound 18f has emerged as a lead molecule and may serve as a template for further discovery of capsaicinoid scaffolds.
Introduction
Despite the recent advances in therapies, cancer is still the second leading cause of death and a major cause of morbidity and mortality worldwide. Lung cancer is responsible for around 20% of all cancer deaths with an estimated 1.8 million new cases and 1.6 million deaths annually.1 Lung cancer represents one of the most malignant tumors, and non-small cell lung cancer (NSCLC, accounts for 80–85% of lung cancer cases) is the most aggressive type of lung cancer.2−4 Currently, chemotherapy is the standard treatment for patients with advanced non-small cell lung cancer. The absence of effective anti-lung cancer drugs makes the mortality of lung cancer still high. The high toxicity, low tumor specificity, and increasing resistance to available chemotherapeutic agents5,6 demand new drug candidates with high activity and efficacy against lung cancer.7−9
Due to the invasiveness, toxicity, and ineffectiveness of current therapeutic approaches, there has been renewed interest in using natural-product-based compounds for the treatment of cancer. Over the past few decades, natural products have gained a lot of interest in cancer drug discovery. More than 70% of anticancer drugs are directly derived from natural sources, developed through structural modifications, or inspired by natural products.10−12 Inspired by natural product scaffolds, structural modifications of natural products have evolved as a major area in drug development. A wide variety of secondary leads had emerged via structural modifications/semisynthetic modifications of natural products, and several of them are in the market.10,13
Capsaicin (10) is a major spicy component of chili peppers that are consumed as spices in many cultures worldwide. It has been used medicinally for centuries and is known for its analgesic,14,15 antioxidant,16 chemopreventive,17 chemotherapeutic,18 antidiabetic,19 anti-inflammatory,20 and antiobesity21 properties. Capsaicin and its derivatives were also found to be potent inhibitors of bacterial (Staphylococcus aureus SA-1199B) NorA efflux pumps.22 Capsaicin has demonstrated in vitro and in vivo anticancer activity against a variety of cancer types.23−25 Studies also revealed that capsaicin may act as a carcinogen or co-carcinogen.26,27 One of the broadly believed mechanisms is the interaction of capsaicin with transient receptor potential vanilloids (TRPVs). TRPVs lead to Ca2+-mediated mitochondrial damage and release cytochrome c that ultimately causes the cell apoptosis. It was found to be a robust apoptotic agent, but the low activity profile, toxicity at higher doses, and pungent nature limit its use as an anticancer agent.16,23 Capsaicin is approved as a topical treatment of neuropathic pain. Capsaicin selectively activates TRPV1, a Ca2+-permeable cationic ion channel that is enriched in the terminals of selected nociceptors. The limited analgesic potential for the use of systemically administered capsaicin studies in animals using local or topical application has yielded conflicting results. However, the side effects caused by capsaicin including pungency, rise in blood pressure, itching, musculoskeletal disorder, hyperalgesia, fatigue, vomiting, transient hypertension, stinging, and erythema at the application site limit its application as an oral therapeutic agent.28−30 Capsaicin, which inhibits VEGF, should be a key component in the development of novel anticancer therapies for NSCLC remission. Endothelial cells express VEGFR-2 (tyrosine kinase receptor), which is an effective target for suppressing tumor cell proliferation and metastasis, and it plays a crucial role in antiangiogenesis.23,31−33
On the other hand, the 1,2,3-triazole moiety is a key pharmacophore exhibiting a wide range of pharmacological activities.22,34−39 The 1,2,3-triazole moiety plays a significant role in medicinal chemistry owing to its capability of forming a hydrogen bond, which improves its solubility and ability to favorably interact with bimolecular targets.40−42 1,2,3-Triazoles are highly stable to metabolic degradation as compared to other heterocyclic compounds.43−45 Several 1,2,3-triazole tethered natural product scaffolds like oleanolic acid,46 quinolone,47 isatin,47 myrrhanone C,48 podophyllotoxin,49 artemisinin,3 coumarin,50 and curcumin51 with a hydrophobic character have demonstrated potential antiproliferative activities against lung cancer cell lines (Figure 1). Conjugation of the 1,2,3-triazole moiety evidenced to be one of the important strategies to improve the anticancer properties of natural scaffolds, and many secondary leads have been developed by this approach.
Figure 1.
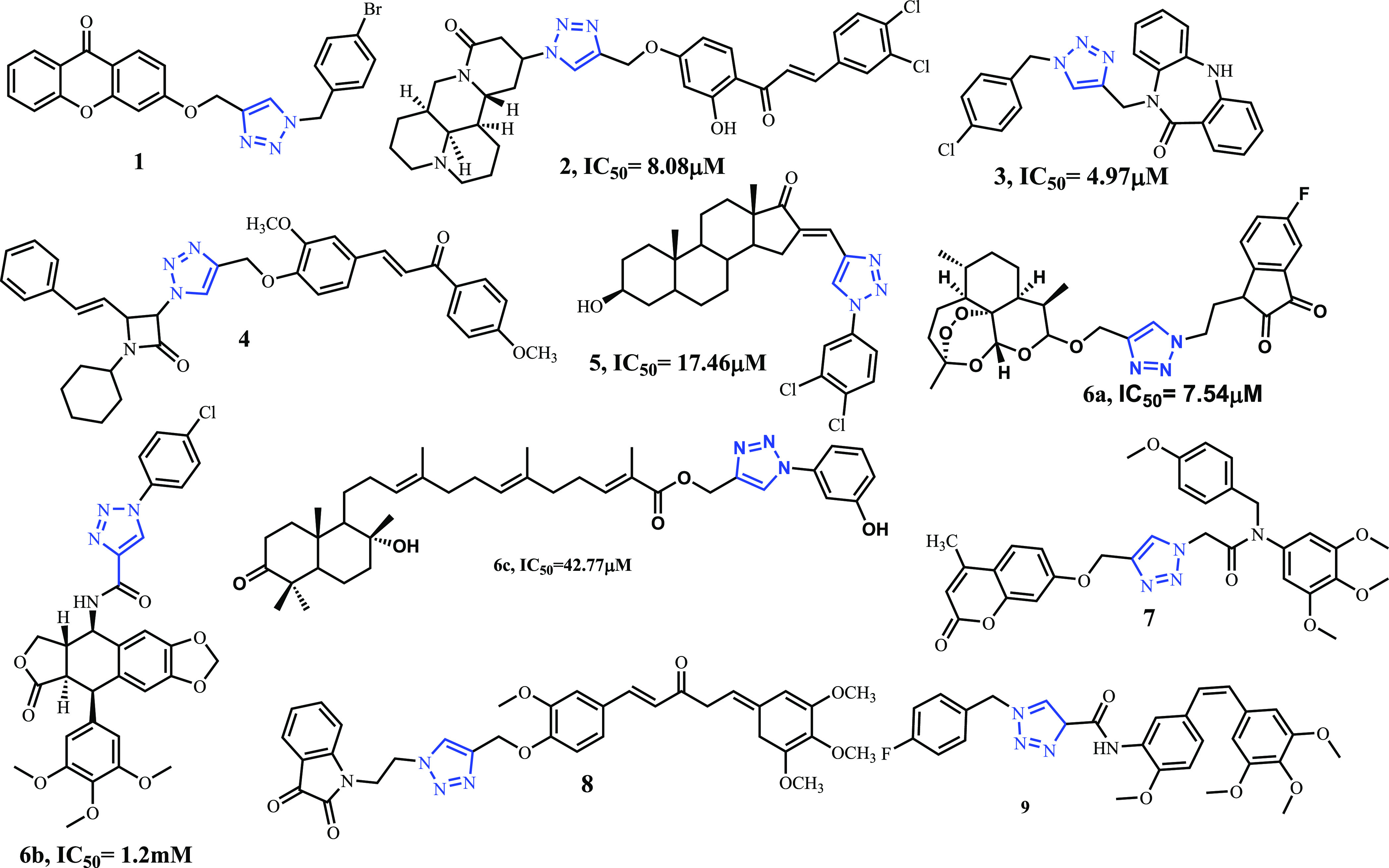
Rational for designing novel analogues embedded with 1,2,3-triazoles.
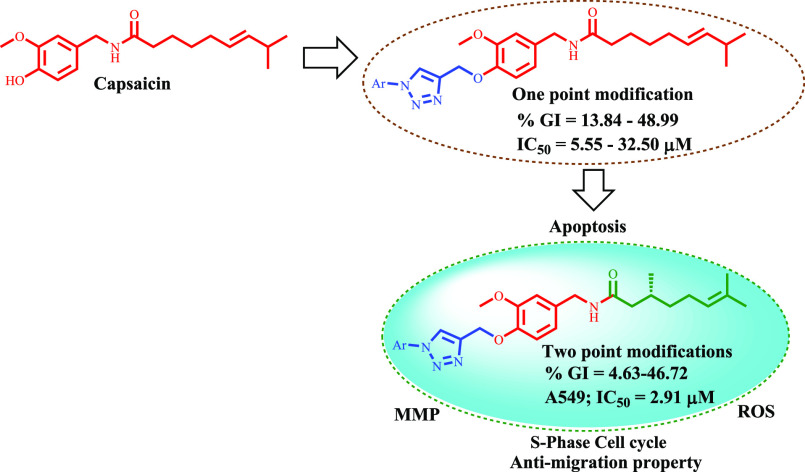
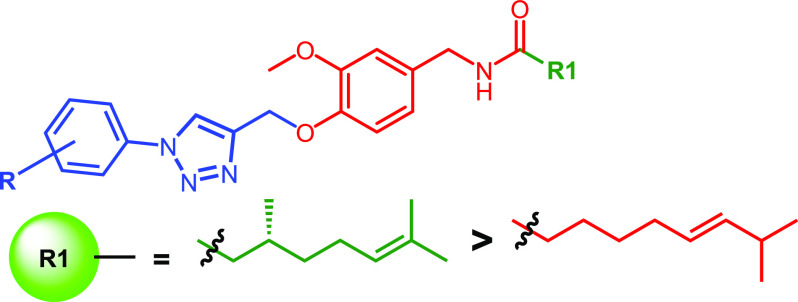
The anticancer properties of capsaicin as a robust apoptotic inducing agent and the long hydrophobic side chain present in capsaicin make it an ideal scaffold for the development of secondary leads against lung cancer.5,6,52,53 In view of the low anticancer activity profiles of capsaicin and the biological importance of the 1,2,3-triazole moiety toward the development of anticancer secondary leads against lung cancer, we aim to develop some new 1,2,3-triazole conjugates of capsaicin through one-/two-point modification of capsaicin as shown below (Figure 2).
Figure 2.
1,2,3-Triazole tethered capsaicinoids through one-/two-point modifications.
Results and Discussion
Chemistry
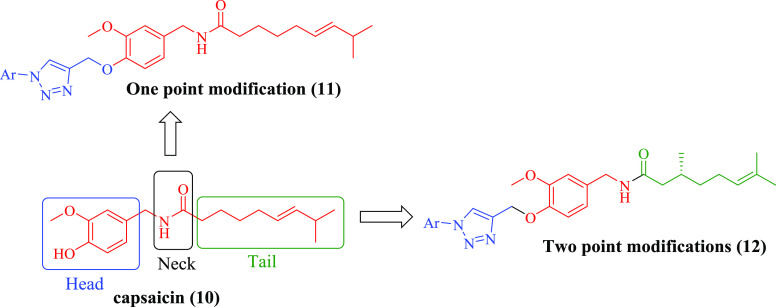
The designer molecules were synthesized through one-/two-point modification of capsaicin by employing a multistep synthetic strategy starting from vanillin as shown in Schemes 1 and 2. The one-point modification was carried at the vanillyl group (head) of capsaicin, while the two-point modification was carried out by varying the lipid group (tail) and vanillyl group (head).
Scheme 1. Synthesis of 1,2,3-Triazole Tethered Capsaicin Derivatives.
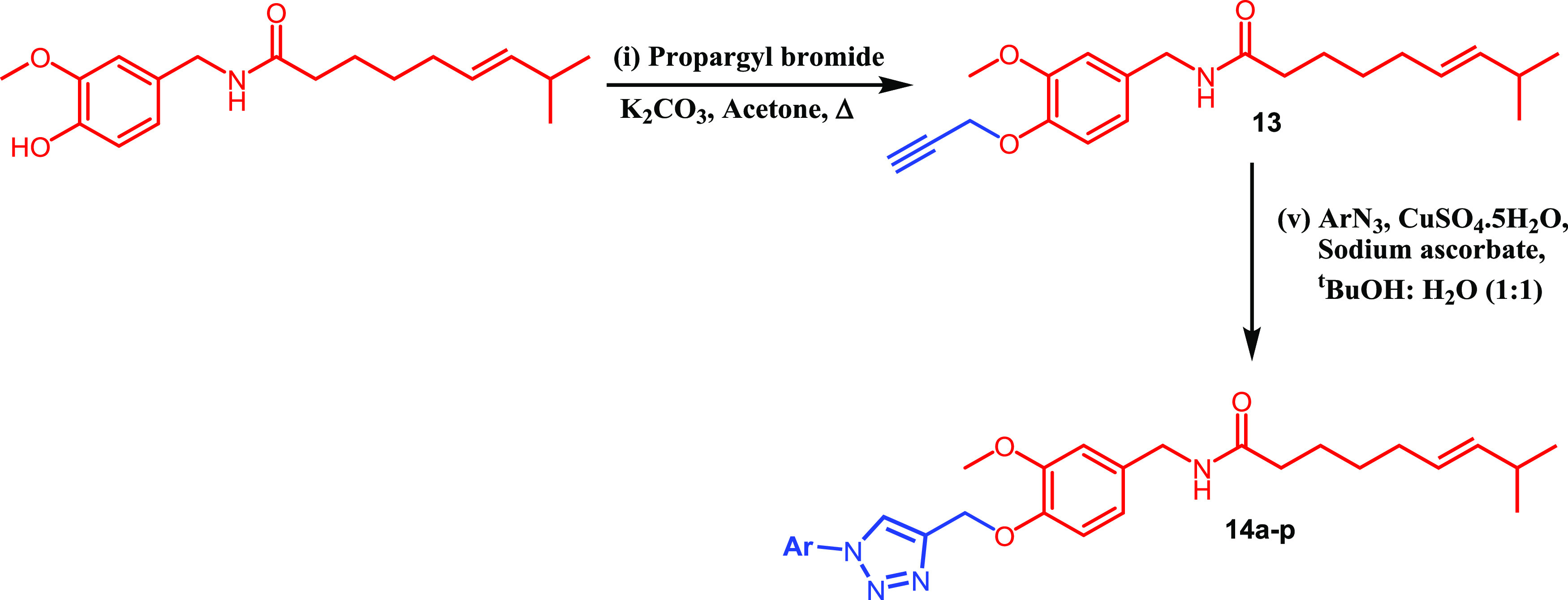
Scheme 2. Synthesis of 1,2,3-Triazole Tethered Natural Product Hybrid Capsaicinoids.
One-Point Modification
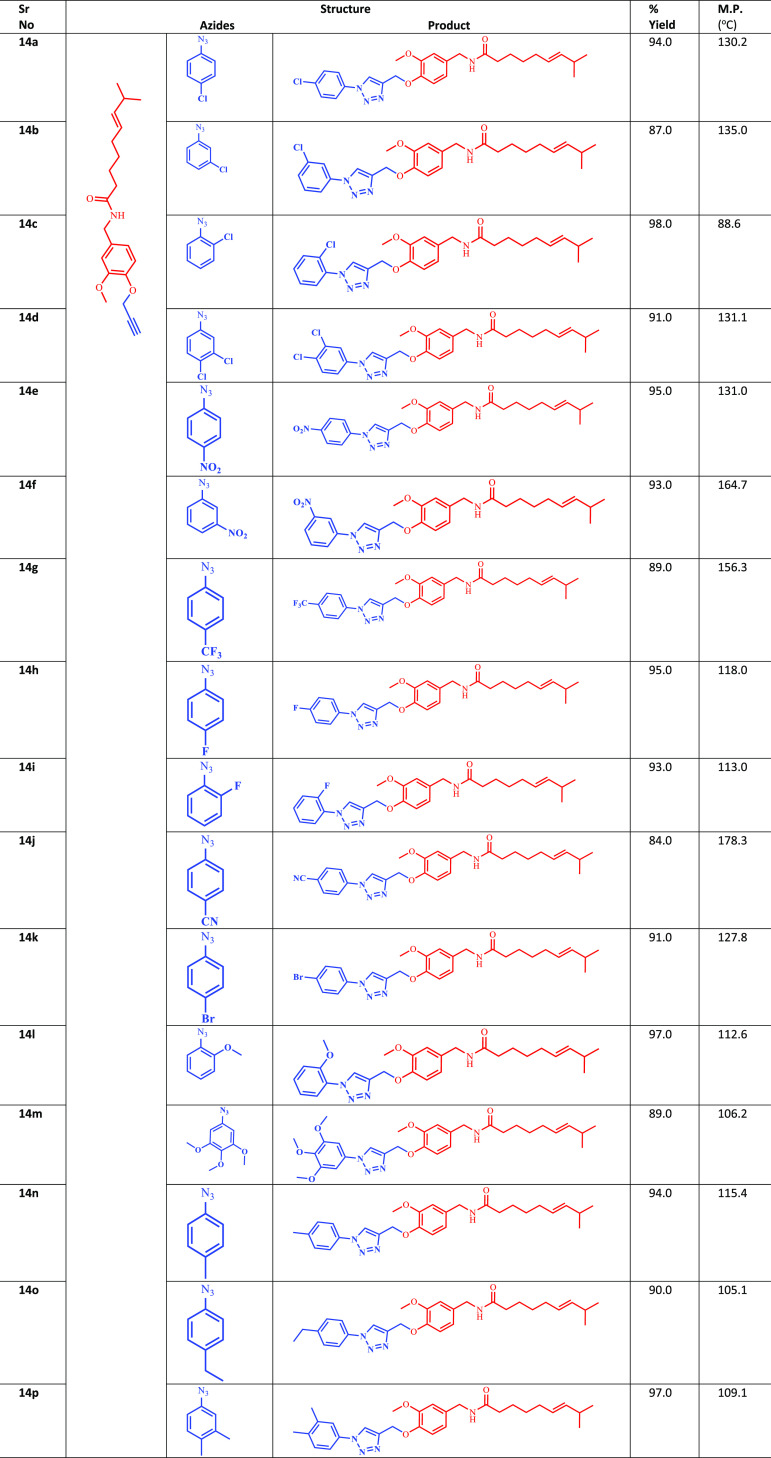
Natural capsaicin (10) was treated with propargyl bromide to give the propargylated intermediate (13). Finally, the propargylated intermediate was reacted with different substituted aromatic azides under Cu-I catalyzed click chemistry conditions to afford the desired molecules (14a–p) bearing the 1,2,3-triazole moiety (Scheme 1). All the synthesized compounds (14a–p) with their melting point are illustrated in Table 1.
Table 1. Chemical Structure of the Synthesized Compounds (18a–o).
Formation of propargylated capsaicin (13) was confirmed by the disappearance of a singlet corresponding to the phenolic −OH peak of capsaicin (10) at δ 8.83 ppm and the appearance of a doublet corresponding to −CH2– at δ 4.73 ppm and a singlet corresponding to alkynyl −CH (terminal alkyne) at δ 3.53 ppm in 1H NMR. Formation of 1,4-disubstituted 1,2,3-triazole derivatives (14a–p) through 1,3-dipolar cycloaddition between propargylated capsaicin (13) and aryl azides was confirmed by the presence of a singlet corresponding to the triazolyl proton in the range of δ 8.06–8.63 ppm, disappearance of the signal corresponding to alkynyl −CH at δ 3.53 ppm, and presence of an additional aromatic signal in 1H NMR. The appearance of signals corresponding to triazolyl and aromatic carbons in addition to the capsaicin signals54 in 13C NMR spectra further confirms the formation of target molecules. Finally, the formation of all the target molecules was confirmed by mass spectra.
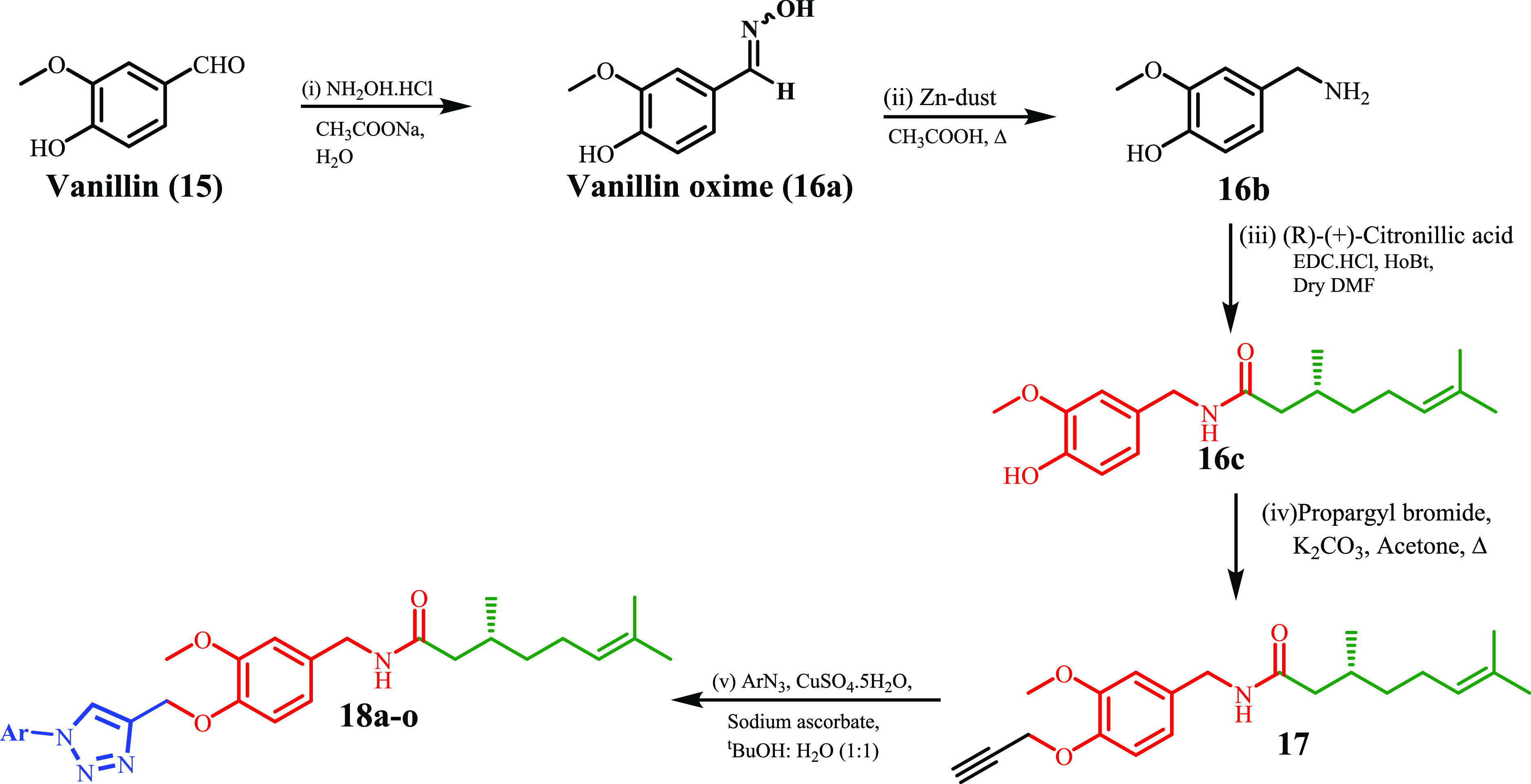
Two-Point Modification
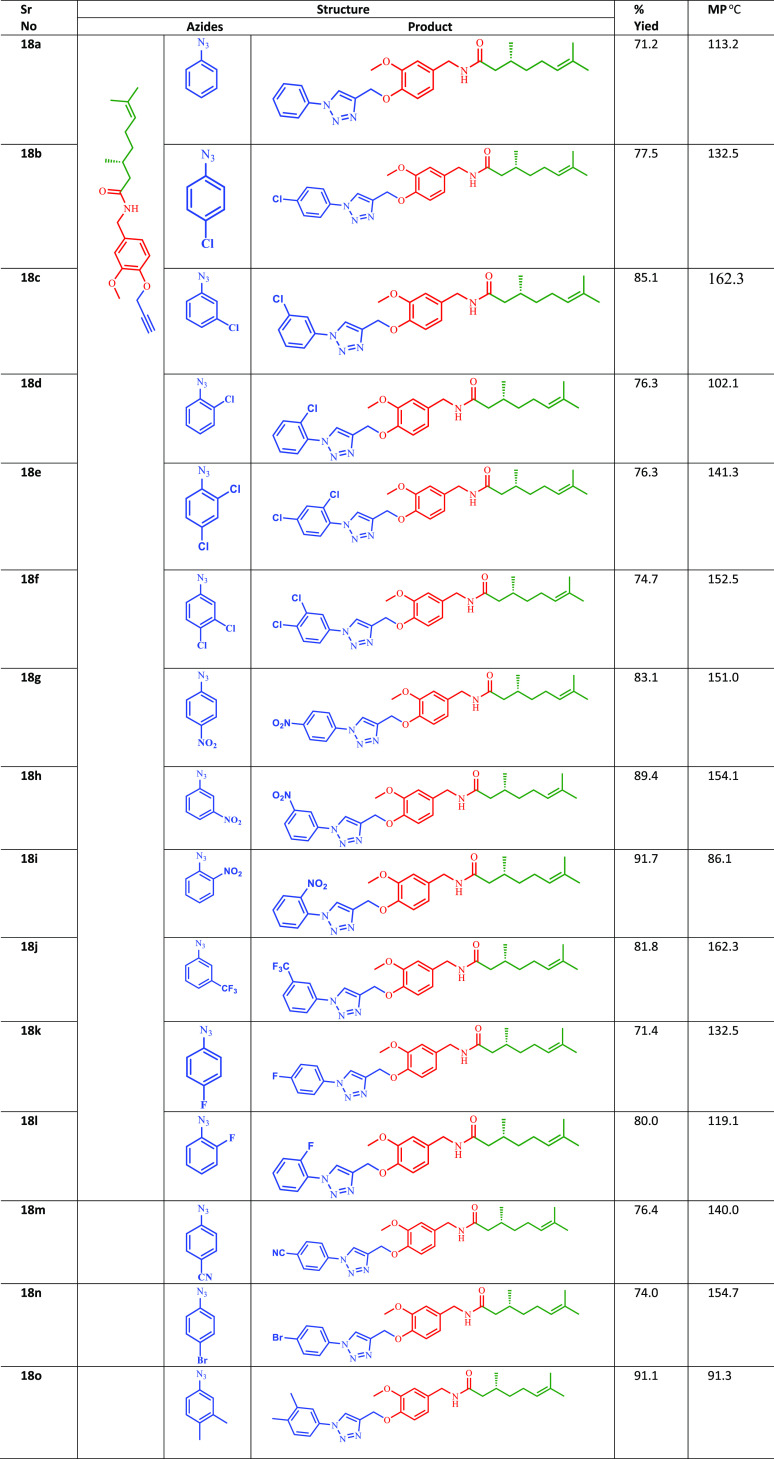
Vanillin (15) was reacted with hydroxylamine hydrochloride in the presence of sodium acetate trihydrate to afford the corresponding oxime (16a). The oxime (16a) was reduced to the corresponding benzyl amine (16b) by using Zn/CH3COOH. 3-Methoxy-4-hydroxy benzylamine was coupled with R-(+)-citronellic acid to afford the hybrid natural product conjugate under EDC·HCl coupling conditions. The natural product hybrid (16c) was further reacted with propargyl bromide to yield the propargylated intermediate (17). This propargylated intermediate was finally reacted with different substituted aromatic azides under Cu-I catalyzed click chemistry conditions to afford the new target molecules (18a–o) bearing the 1,2,3-triazole moiety (Scheme 2). All the synthesized compounds with their melting point are illustrated in Table 2.
Table 2. Chemical Structure of the Synthesized Natural Product Hybrid Compounds (18a–o).
Formation of vanillin oxime (16a) from vanillin was defined by the presence of a broad doublet corresponding to one proton at 7.75–7.71 ppm (−N–OH) and 5.90 ppm (−OH). Reduction of oxime to benzylamine (16b) was confirmed by the absence of peaks corresponding to aldoxime at 7.75 and 5.90 ppm and the presence of a triplet at δ 3.64–3.67 ppm corresponding to two protons (−CH2). Formation of amide (16c) from vanillyl amine and R-(+)-citronellic acid was confirmed by the appearance of two triplets at 5.47 and 5.07 ppm corresponding to an −NH of amide and an olefinic proton (HC=C) and other signals at the aliphatic region corresponding to R-(+)-citronellic acid. Propargylation of 16c to 17 was recognized by the presence of a triplet at δ 2.50 ppm corresponding to terminal alkyne and the presence of additional −CH2 protons at δ 4.73 ppm. Finally, formation of 1,2,3-triazoles (18a–o) from the propargylated intermediate (17) was affirmed by the presence of a singlet at δ 8.06–8.18 ppm (CDCl3, 1H NMR) corresponding to the −CH– proton of the 1,2,3-triazole moiety and the absence of signal at δ 2.50 ppm. Finally, formation of the compounds was confirmed by 1H NMR, 13C NMR, LC–MS, and HR-MS.
Biology
Antiproliferative Investigation against 60 Cell Lines
Newly synthesized 1,2,3-triazole conjugates of capsaicin (14a–p) and 1,2,3-triazole conjugates of the structural isomer of capsaicin (18a–n) were submitted to the Developmental Therapeutic Program-National Cancer Institute, Bethesda, USA (www.dtp.nci.nih.gov). These synthesized compounds were selected for the screening at a single dose of 10 μM and tested against 60 cancer cell lines under nine different cancer cell types (leukemia, lung, colon, CNS, melanoma, ovarian, renal, prostate, and breast cancers) with their subpanels. Screening results of in vitro antiproliferative activity of the tested compounds were reported as growth percent as shown in Tables 3 and 4. Compounds 18a, 18c, 18h, 18k, and 18o were screened against a panel of five human cancer cell lines, viz., breast (MCF-7), colon (HCT-116), lung (A549), pancreas (MiaPaCa), and prostate (PC-3) at 10 μM concentration, and the results are reported in percentage growth inhibition (GI) as depicted in Table 5.
Table 3. Antiproliferative Activity of Synthesized 1,2,3-Triazole Tethered Capsaicin (14a–p) against an NCI Panel of 60 Human Cancer Cell Lines.
| cancer panel | subpanel/comp no. | 14a | 14b | 14c | 14d | 14e | 14f | 14g | 14h | 14i | 14j | 14k | 14l | 14m | 14n | 14o | 14p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| leukemia | CCRF-CEM | 72.44 | 87.33 | 87.87 | 84.65 | 84.94 | 90.24 | 68.85 | 79.73 | 88.58 | 86.21 | 94.23 | 83.65 | 96.33 | 87.34 | 86.83 | 75.54 |
| K-562 | 66.66 | 84.87 | 76.84 | 77.13 | 74.53 | 81.49 | 76.27 | 68.74 | 82.35 | 75.37 | 59.18 | 76.13 | 79.71 | 79.98 | 78.64 | 74.64 | |
| MOLT-4 | 81.99 | 92.80 | 81.04 | 86.41 | 79.33 | 86.56 | 80.34 | 82.62 | 90.94 | 88.39 | 77.45 | 79.70 | 92.05 | 83.54 | 79.62 | 78.54 | |
| RPMI-8226 | 60.83 | 86.29 | 75.55 | 78.11 | 61.80 | 65.86 | 53.37 | 68.40 | 81.34 | 65.44 | 57.35 | 71.25 | 79.37 | 74.10 | 73.95 | 67.31 | |
| SR | 73.55 | 85.61 | 68.45 | 78.92 | 62.18 | 78.38 | 70.36 | 76.67 | 81.54 | 76.32 | 67.19 | 77.40 | 86.62 | 75.55 | 87.52 | 58.25 | |
| non-small cell lung cancer | A549/ATCC | 85.32 | 82.93 | 84.33 | 85.90 | 84.06 | 91.99 | 48.99 | 84.35 | 91.27 | 84.82 | 88.49 | 85.96 | 91.04 | 84.04 | 88.34 | 82.16 |
| EKVX | 72.02 | 80.81 | 82.11 | 76.21 | 75.28 | 95.11 | 88.25 | 72.71 | 79.11 | 90.82 | 72.66 | 89.40 | 95.93 | 84.83 | 82.11 | 79.28 | |
| HOP-62 | 83.97 | 71.45 | 81.95 | 90.38 | 80.87 | 97.38 | 64.56 | 84.07 | 84.95 | 102.9 | 88.21 | 88.73 | 90.68 | 86.39 | 94.51 | 97.43 | |
| HOP-92 | 77.10 | 62.44 | 80.14 | 78.72 | 90.32 | 70.38 | 13.84 | 66.16 | 78.66 | 80.48 | 68.79 | 72.06 | 82.53 | 83.54 | 86.50 | 80.41 | |
| NCI-H226 | 70.36 | 86.45 | 81.77 | 76.64 | 89.59 | 90.88 | 53.63 | 74.15 | 81.34 | 100.5 | 86.31 | 80.50 | 91.44 | 75.82 | 76.71 | 86.88 | |
| NCI-H23 | 78.67 | 87.17 | 93.56 | 93.19 | 83.21 | 87.96 | 94.56 | 81.41 | 95.32 | 72.14 | 70.17 | 96.02 | 90.08 | 87.91 | 89.51 | 74.91 | |
| NCI-H322M | 92.15 | 86.20 | 89.36 | 93.25 | 97.91 | 94.09 | 96.15 | 91.76 | 90.16 | 95.29 | 90.64 | 92.18 | 89.07 | 89.39 | 90.50 | 101.0 | |
| NCI-H460 | 79.92 | 84.09 | 101.3 | 101 | 99.76 | 91.79 | 16.74 | 75.71 | 103.5 | 92.96 | 72.09 | 104.4 | 101.5 | 93.21 | 102.7 | 86.45 | |
| NCI-H522 | 65.69 | 69.17 | 64.46 | 69.27 | 78.21 | 82.60 | 75.57 | 70.15 | 77.48 | 88.19 | 82.50 | 63.91 | 65.21 | 62.09 | 65.93 | 80.70 | |
| colon cancer | COLO205 | 108.5 | 102.4 | 106.4 | 110.6 | 117.9 | 106.8 | 72.59 | 119.9 | 115.7 | 117.3 | 103.1 | 116.8 | 106.2 | 115.2 | 120.9 | 106.5 |
| HCC-2998 | 112.7 | 104 | 110.5 | 98.1 | 107.5 | 101.2 | 94.75 | 110.2 | 97.17 | 94.51 | 88.79 | 117.3 | 91.82 | 108.4 | 99.66 | 100.6 | |
| HCT-116 | 63.68 | 76.84 | 100.3 | 106.8 | 77.19 | 41.96 | 2.97 | 65.61 | 97.81 | 15.41 | 49.09 | 82.68 | 87.14 | 92.44 | 90.69 | 48.16 | |
| HCT-15 | 91.77 | 91.31 | 94.11 | 93.61 | 96.24 | 77.90 | 59.70 | 89.61 | 95.97 | 83.47 | 94.02 | 106.1 | 103.2 | 103.9 | 94.42 | 71.18 | |
| HT29 | 88.41 | 99.25 | 98.23 | 99.43 | 98.13 | 88.86 | 78.99 | 106.0 | 106.9 | 95.56 | 98.09 | 98.69 | 87.19 | 100.1 | 109.4 | 97.85 | |
| KM12 | 95.56 | 98.56 | 97.72 | 99.98 | 90.03 | 91.20 | 81.18 | 94.49 | 98.88 | 91.46 | 94.30 | 98.21 | 102.6 | 96.05 | 100.3 | 97.54 | |
| SW-620 | 88.74 | 92.82 | 100.1 | 96.12 | 99.76 | 97.40 | 41.33 | 90.98 | 98.10 | 98.02 | 94.70 | 96.98 | 102.4 | 102.4 | 102.7 | 95.17 | |
| CNS cancer | SF-268 | 84.04 | 82.49 | 87.78 | 93.32 | 86.10 | 92.25 | 87.80 | 87.23 | 95.04 | 90.86 | 78.89 | 92.64 | 90.52 | 81.74 | 93.05 | 94.60 |
| SF-295 | 88.30 | 90.53 | 99.41 | 94.64 | 89.02 | 103.9 | 90.90 | 90.52 | 98.96 | 102.1 | 85.39 | 107.6 | 105.4 | 98.90 | 100.05 | 88.82 | |
| SF-539 | 87.11 | 81.97 | 104.2 | 96.14 | 104.1 | 94.44 | 81.86 | 92.18 | 104.8 | 94.55 | 92.40 | 106.3 | 92.01 | 100.3 | 103.68 | 105.0 | |
| SNB-19 | 87.72 | 59.97 | 89.74 | 87.31 | 97.39 | 68.23 | 76.10 | 81.35 | 84.84 | 81.91 | 80.91 | 91.56 | 85.13 | 77.94 | 85.68 | 95.85 | |
| SNB-75 | 76.44 | 75.84 | 87.34 | 79.08 | 83.94 | 91.95 | 49.67 | 83.46 | 88.08 | 94.17 | 90.92 | 103.3 | 95.25 | 99.45 | 92.63 | 90.98 | |
| U251 | 86.89 | 69.08 | 85.65 | 91.90 | 86.36 | 85.29 | 46.69 | 83.87 | 93.12 | 91.16 | 75.48 | 90.23 | 92.80 | 81.65 | 89.37 | 88.35 | |
| melanoma | LOX IMVI | 85.61 | 89.42 | 89.93 | 87.04 | 89.50 | 92.87 | 60.32 | 84.23 | 87.12 | 78.15 | 83.68 | 86.08 | 94.42 | 85.10 | 89.32 | 84.46 |
| MALME-3M | 97.85 | 98.65 | 107.4 | 105.3 | 98.44 | 103.1 | 99.71 | 103.2 | 107.7 | 102.3 | 102.7 | 106.2 | 98.49 | 109.8 | 107.95 | 107.2 | |
| M14 | 90.64 | 94.14 | 92.57 | 92.67 | 89.72 | 93.4 | 97.51 | 94.05 | 96.85 | 95.27 | 90.17 | 96.66 | 94.53 | 93.33 | 99.82 | 93.54 | |
| MDA-MB-435 | 99.29 | 98.61 | 102.6 | 94.22 | 99.74 | 101.2 | 107.0 | 98.80 | 100.6 | 107.7 | 106.9 | 109.6 | 106.5 | 109.0 | 107.0 | 104.3 | |
| SK-MEL-2 | 101.6 | 86.66 | 90.98 | 108.4 | 91.96 | 88.40 | 95.06 | 111.3 | 105.5 | 98.43 | 95.21 | 89.80 | 97.57 | 91.94 | 95.17 | 90.32 | |
| SK-MEL-28 | 106.5 | 105.7 | 105.8 | 104.0 | 102.4 | 105.9 | 107.0 | 102.5 | 107.3 | 104.6 | 100.4 | 115.6 | 108.1 | 113.7 | 114.9 | 112.1 | |
| SK-MEL-5 | 77.63 | 91.17 | 89.83 | 81.64 | 76.10 | 93.24 | 97.06 | 83.73 | 91.50 | 86.97 | 72.04 | 83.94 | 77.50 | 86.78 | 83.95 | 81.53 | |
| UACC-257 | 91.64 | 90.11 | 87.93 | 89.23 | 82.58 | 94.42 | 98.24 | 90.21 | 94.96 | 94.35 | 90.46 | 85.25 | 93.58 | 88.19 | 86.37 | 81.89 | |
| UACC-62 | 67.84 | 74.98 | 73.31 | 75.61 | 69.29 | 70.05 | 84.33 | 71.93 | 76.23 | 81.83 | 62.99 | 75.07 | 87.79 | 70.18 | 71.39 | 75.07 | |
| ovarian cancer | IGROV1 | 93.44 | 79.91 | 98.76 | 104.1 | 90.41 | 89.40 | 58.71 | 96.98 | 100.8 | 100.4 | 72.65 | 104.5 | 103.3 | 98.46 | 102.5 | 98.81 |
| OVCAR-3 | 104.9 | 84.40 | 101.1 | 92.4 | 92.69 | 104.9 | 106.8 | 96.37 | 97.52 | 108.9 | 100.1 | 103 | 103.1 | 91.85 | 101.0 | 100.4 | |
| OVCAR-4 | 82.64 | 67.25 | 87.87 | 69.52 | 87.18 | 94.19 | 98.59 | 82.15 | 84.19 | 94.94 | 89.23 | 99.97 | 103.4 | 88.79 | 88.97 | 84.94 | |
| OVCAR-5 | 100 | 99.78 | 103.5 | 102.4 | 99.15 | 107.3 | 96.56 | 97.57 | 102.6 | 95.30 | 116.2 | 112.8 | 109.1 | 108.4 | 112.13 | 107.4 | |
| OVCAR-8 | 78.97 | 57.20 | 89.57 | 91.05 | 82.87 | 76.93 | 41.06 | 71.55 | 94.5 | 63.28 | 79.60 | 82.16 | 88.18 | 76.53 | 91.14 | 83.35 | |
| NCI/ADR-RES | 78.12 | 76.59 | 92.04 | 91.11 | 28.98 | 81.79 | 48.24 | 70.62 | 89.77 | 79.67 | 71.97 | 94.61 | 91.69 | 87.43 | 87.71 | 80.64 | |
| SK-OV-3 | 91.64 | 78.80 | 91.28 | 97.24 | 76.69 | 93.55 | 91.30 | 86.36 | 97.84 | 88.22 | 74.85 | 99.76 | 89.94 | 94.38 | 101.6 | 98.61 | |
| renal cancer | 786-0 | 92.52 | 76.84 | 93.81 | 96.31 | 93.73 | 73.38 | 2.94 | 83.74 | 97.19 | 91.65 | 92.05 | 102.4 | 107.6 | 99.62 | 100.2 | 99.82 |
| ACHN | 59.66 | 72.99 | 74.08 | 81.98 | 78.92 | 81.18 | 82.95 | 87.69 | 72.01 | 68.45 | |||||||
| CAKI-1 | 88.99 | 79.62 | 93.42 | 58.30 | 90.66 | 81.67 | 85.84 | 88.42 | 90.63 | 74.59 | 74.31 | 79.93 | 79.48 | 88.54 | 77.39 | 94.35 | |
| RXF 393 | 97.98 | 91 | 104.9 | 100.6 | 99.73 | 92.69 | 90.23 | 95.34 | 103.1 | 90.77 | 105.4 | 101.0 | 104.4 | 102.5 | 101.0 | 98.20 | |
| SN12C | 80.18 | 84.24 | 94.47 | 112.6 | 91.67 | 89.51 | 65.57 | 82.38 | 92.65 | 98.27 | 83.0 | 98.02 | 101.6 | 89.3 | 98.54 | 88.40 | |
| TK-10 | 99.28 | 105.5 | 94.57 | 88.62 | 92.72 | 104.9 | 20.45 | 103.0 | 111.3 | 91.84 | 117.5 | 90.25 | 91.59 | 100.6 | 87.44 | 108.97 | |
| UO-31 | 76.58 | 72.21 | 75.19 | 102.7 | 60.63 | 74.73 | 92.75 | 86.04 | 77.11 | 111.8 | 67.93 | 105.0 | 116.9 | 75.81 | 93.93 | 75.67 | |
| prostate cancer | PC-3 | 79.23 | 83.59 | 80.60 | 77.08 | 77.97 | 82.16 | 70.50 | 84.14 | 82.05 | 79.46 | 73.69 | 73.46 | 80.91 | 84.17 | 70.87 | 78.43 |
| DU-145 | 98.92 | 95.88 | 99.19 | 86.53 | 104.7 | 108.7 | 62.20 | 96.08 | 105.8 | 44.39 | 98.83 | 83.12 | 85.40 | 96.95 | 93.77 | 107.95 | |
| breast cancer | MCF-7 | 84.88 | 86.88 | 97.24 | 95.33 | 83.45 | 74.06 | 80.73 | 89.31 | 97.12 | 103.0 | 76.17 | 98.16 | 102.3 | 96.29 | 102.2 | 86.88 |
| MDA-MB-231 ATCC | 79.50 | 64.80 | 80.85 | 87.29 | 75.32 | 88.39 | 75.16 | 69.06 | 78.71 | 90.83 | 71.32 | 95.76 | 92.66 | 72.21 | 88.05 | 89.27 | |
| HS 578T | 90.35 | 74.80 | 97.20 | 84.04 | 95.78 | 90.76 | 70.69 | 77.70 | 87.70 | 95.03 | 90.48 | 83 | 69.00 | 89.68 | 78.03 | 90.74 | |
| BT-549 | 86.83 | 99.35 | 90.81 | 91.78 | 80.40 | 96.27 | 80.41 | 91.05 | 89.25 | 91.24 | 88.08 | 93.12 | 79.12 | 105.0 | 99.09 | 93.65 | |
| T-47D | 71.04 | 81.08 | 74.30 | 87.05 | 63.99 | 72.34 | 84.51 | 73.75 | 83.08 | 76.82 | 68.37 | 92.18 | 95.75 | 77.41 | 111.0 | 73.17 | |
| MDA-MB-468 | 64.91 | 74.73 | 88.19 | 71.60 | 77.44 | 72.34 | 70.22 | 68.19 | 92.81 | 74.86 | 68.15 | 75.45 | 80.43 | 92.44 | 79.80 | 71.77 |
Table 4. Antiproliferative Activity of Synthesized 1,2,3-Triazole (18a–n) against an NCI Panel of 60 Human Cancer Cell Lines.
| PANELNME | comp no. | 16c | 18a | 18b | 18c | 18d | 18e | 18f | 18g | 18h | 18i | 18j | 18k | 18l | 18m | 18n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| leukemia | CCRF-CEM | 70.56 | 59.43 | 92.94 | 76.55 | 40.10 | 116.05 | 84.49 | 81.06 | 85.57 | 36.52 | 97.09 | 83.96 | 97.30 | 58.35 | 61.14 |
| HL-60(TB) | 76.37 | 93.01 | 87.80 | 83.86 | 41.06 | 95.54 | 83.95 | 110.2 | 100.27 | 47.68 | 96.30 | 89.26 | 91.54 | 70.85 | 88.43 | |
| K-562 | 89.43 | 79.40 | 53.52 | 60.82 | 44.62 | 81.37 | 51.89 | 44.88 | 83.08 | 51.53 | 59.00 | 72.74 | 81.62 | 47.60 | 66.32 | |
| MOLT-4 | 83.43 | 54.59 | 71.73 | 47.68 | 16.29 | 84.05 | 68.65 | 64.23 | 80.63 | 20.66 | 84.80 | 67.45 | 74.84 | 14.78 | 69.97 | |
| RPMI-8226 | 84.37 | 37.90 | 75.30 | 56.33 | 47.56 | 90.71 | 77.63 | 73.14 | 73.29 | 39.91 | 89.81 | 75.33 | 81.80 | 58.26 | 69.27 | |
| SR | 90.57 | 62.33 | 65.77 | 61.39 | 48.53 | 79.78 | 27.12 | 39.18 | 81.95 | 45.13 | 41.51 | 68.94 | 73.39 | 24.73 | 63.80 | |
| non-small cell lung cancer | A549/ATCC | 96.36 | 94.07 | 57.07 | 81.77 | 64.07 | 59.42 | 46.49 | 85.48 | 56.83 | 65.65 | 42.61 | 76.78 | 80.64 | 48.20 | 78.59 |
| EKVX | 86.35 | 74.29 | 75.34 | 51.21 | 54.00 | 81.06 | 77.24 | 75.25 | 69.05 | 58.81 | 92.14 | 65.19 | 78.32 | 81.03 | 79.03 | |
| HOP-62 | 85.81 | 120.28 | 61.55 | 89.31 | 79.25 | 11.33 | 95.67 | 103.84 | 82.74 | 78.10 | 88.76 | 67.99 | 46.72 | 88.63 | 65.10 | |
| HOP-92 | 83.48 | 103.88 | 53.02 | 71.92 | 79.43 | –3.27 | 51.60 | 86.49 | 76.24 | 57.55 | 86.10 | 55.58 | 4.63 | 65.16 | 45.94 | |
| NCI-H226 | 100.60 | 79.51 | 63.78 | 54.16 | 59.73 | 66.35 | 50.55 | 80.08 | 73.48 | 56.87 | 72.34 | 51.40 | 73.35 | 54.94 | 70.24 | |
| NCI-H23 | 94.13 | 91.88 | 64.87 | 72.88 | 64.51 | 56.64 | 68.51 | 45.49 | 66.55 | 62.45 | 79.35 | 74.89 | 68.25 | 64.54 | 60.06 | |
| NCI-H322M | 91.13 | 106.92 | 83.48 | 104.66 | 91.28 | 95.25 | 82.51 | 92.29 | 103.37 | 94.44 | 90.10 | 105.20 | 92.68 | 94.96 | 99.60 | |
| NCI-H460 | 103.61 | 97.03 | 65.04 | 88.73 | 78.43 | 75.06 | 45.51 | 78.17 | 62.92 | 84.19 | 14.61 | 79.49 | 89.03 | 45.78 | 74.17 | |
| NCI-H522 | 86.86 | 85.53 | 62.93 | 67.33 | 51.40 | 55.44 | 61.51 | 58.92 | 80.13 | 61.88 | 83.09 | 66.40 | 57.14 | 63.51 | 41.63 | |
| colon cancer | COLO 205 | 105.96 | 99.03 | 91.10 | 102.61 | 71.71 | 104.99 | 53.63 | 121.73 | 94.43 | 78.01 | 74.25 | 97.16 | 111.74 | 95.57 | 112.90 |
| HCC-2998 | 108.39 | 93.30 | 75.87 | 94.66 | 91.88 | 92.47 | 93.52 | 107.31 | 91.41 | 106.48 | 111.05 | 94.86 | 100.56 | 89.49 | 95.80 | |
| HCT-116 | 94.11 | 96.71 | 38.23 | 72.22 | 49.66 | 53.13 | 29.82 | 32.09 | 18.65 | 49.09 | 12.49 | 47.86 | 60.15 | 29.43 | 41.92 | |
| HCT-15 | 100.88 | 88.04 | 65.29 | 81.43 | 53.84 | 79.57 | 29.35 | 71.79 | 80.93 | 62.42 | 56.79 | 91.65 | 82.81 | 66.16 | 82.35 | |
| HT29 | 103.79 | 99.48 | 79.57 | 97.34 | 76.66 | 82.28 | 78.27 | 108.14 | 100.11 | 22.55 | 96.61 | 102.98 | 97.40 | 77.55 | 94.43 | |
| KM12 | 100.37 | 96.04 | 87.72 | 94.27 | 62.97 | 93.06 | 82.66 | 86.78 | 91.32 | 63.85 | 84.99 | 96.37 | 99.29 | 71.66 | 94.59 | |
| SW-620 | 98.70 | 76.75 | 97.41 | 80.24 | 89.26 | 29.90 | 86.83 | 80.92 | 92.93 | 38.71 | 101.33 | 92.72 | 71.03 | 91.70 | ||
| CNS cancer | SF-268 | 77.04 | 92.33 | 95.38 | 85.96 | 82.41 | 52.34 | 58.39 | 88.77 | 73.15 | 78.08 | 83.86 | 69.92 | 58.88 | 71.57 | 71.71 |
| SF-295 | 103.18 | 88.16 | 55.55 | 66.84 | 56.38 | 41.76 | 80.45 | 70.15 | 96.45 | 60.45 | 72.87 | 64.41 | 45.20 | 54.05 | 63.24 | |
| SF-539 | 93.56 | 96.00 | 67.72 | 73.07 | 75.23 | 66.88 | 5.30 | 43.12 | 69.79 | 66.99 | 16.35 | 68.43 | 44.60 | 59.74 | 51.16 | |
| SNB-19 | 95.32 | 74.44 | 78.63 | 67.87 | 61.27 | 49.98 | 81.31 | 84.82 | 73.39 | 66.43 | 97.17 | 53.11 | 55.68 | 83.59 | 57.66 | |
| SNB-75 | 56.78 | 41.60 | 60.00 | 7.99 | 47.20 | 78.02 | 56.57 | 65.15 | 10.58 | 54.57 | 47.32 | |||||
| U251 | 98.73 | 93.80 | 57.71 | 102.61 | 80.61 | 54.40 | 48.23 | 82.82 | 40.06 | 62.83 | 50.97 | 65.37 | 61.60 | 83.86 | 63.36 | |
| melanoma | LOX IMVI | 92.96 | 92.33 | 59.71 | 75.06 | 56.37 | 80.73 | 50.61 | 62.72 | 71.40 | 62.00 | 80.73 | 81.38 | 76.70 | 72.59 | 74.03 |
| MALME-3M | 90.29 | 88.78 | 68.35 | 87.23 | 68.22 | 79.26 | 77.44 | 76.19 | 71.40 | 70.30 | 80.81 | 96.02 | 78.28 | 75.36 | 85.41 | |
| M14 | 95.93 | 85.67 | 90.80 | 93.39 | 55.87 | 102.02 | 89.03 | 88.83 | 82.48 | 65.91 | 92.12 | 102.20 | 94.79 | 88.23 | 97.23 | |
| MDA-MB-435 | 102.18 | 97.17 | 84.94 | 93.75 | 66.50 | 98.16 | 91.28 | 88.25 | 99.53 | 74.94 | 74.88 | 103.84 | 100.50 | 81.26 | 98.30 | |
| SK-MEL-2 | 101.97 | 91.22 | 83.62 | 95.46 | 53.30 | 71.68 | 93.67 | 64.50 | 95.86 | 66.32 | 110.78 | 84.61 | 78.55 | 79.97 | 78.04 | |
| SK-MEL-28 | 106.36 | 99.70 | 86.95 | 76.60 | 69.78 | 96.28 | 89.28 | 87.77 | 98.47 | 85.31 | 91.55 | 89.56 | 94.93 | 85.39 | 96.54 | |
| SK-MEL-5 | 97.72 | 77.37 | 62.77 | 89.51 | 5.29 | 60.43 | 71.91 | 35.89 | 86.20 | 16.33 | 78.75 | 65.24 | 70.80 | 31.05 | 55.65 | |
| UACC-257 | 112.93 | 101.28 | 85.53 | 72.07 | 60.33 | 91.96 | 91.04 | 69.39 | 92.03 | 71.22 | 103.84 | 69.92 | 94.00 | 68.74 | 86.34 | |
| UACC-62 | 81.15 | 64.23 | 65.67 | 108.08 | 39.56 | 65.59 | 69.35 | 53.53 | 65.91 | 48.01 | 72.06 | 64.41 | 69.54 | 56.65 | 65.15 | |
| ovarian cancer | IGROV1 | 89.12 | 88.78 | 80.15 | 95.28 | 87.05 | 72.31 | 83.14 | 78.76 | 106.74 | 78.93 | 102.20 | 97.38 | 81.86 | 81.49 | 89.93 |
| OVCAR-3 | 94.85 | 116.94 | 82.26 | 70.95 | 83.57 | 101.33 | 60.12 | 107.92 | 98.44 | 80.34 | 102.85 | 107.56 | 105.46 | 94.05 | 104.69 | |
| OVCAR-4 | 78.72 | 95.26 | 52.67 | 106.44 | 33.26 | 49.69 | 53.41 | 63.75 | 63.65 | 45.26 | 45.13 | 73.07 | 71.43 | 55.29 | 49.55 | |
| OVCAR-5 | 93.28 | 69.23 | 80.72 | 72.31 | 93.08 | 91.71 | 86.36 | 101.94 | 98.00 | 95.61 | 97.48 | 101.77 | 94.92 | 97.97 | 105.21 | |
| OVCAR-8 | 99.92 | 107.77 | 49.14 | 57.30 | 49.81 | 29.30 | 46.30 | 63.55 | 23.70 | 48.57 | 28.11 | 52.29 | 66.47 | 59.99 | 47.52 | |
| NCI/ADR-RES | 94.46 | 81.94 | 47.96 | 99.18 | 24.02 | 32.86 | 36.55 | 17.58 | 3.67 | 36.03 | 44.05 | 45.40 | 54.77 | 14.02 | 52.06 | |
| SK-OV-3 | 97.77 | 47.99 | 72.13 | 75.06 | 82.97 | 26.10 | 99.00 | 80.08 | 95.87 | 68.44 | 88.99 | 85.05 | 43.56 | 82.43 | 64.02 | |
| renal cancer | 786-0 | 113.25 | 84.28 | 92.28 | 87.70 | 38.94 | 80.43 | 97.04 | 71.40 | 83.46 | 92.98 | 68.16 | 43.58 | 96.24 | 68.65 | |
| A498 | 103.70 | 92.16 | 98.87 | 141.89 | 106.80 | 58.98 | 79.95 | 92.59 | 91.34 | 107.83 | 90.96 | 136.18 | 103.28 | 92.30 | 104.93 | |
| ACHN | 96.18 | 110.84 | 44.83 | 80.22 | 67.59 | 61.09 | 71.52 | 72.71 | 111.72 | 80.79 | 77.61 | 69.02 | 65.32 | 72.75 | 56.39 | |
| CAKI-1 | 81.89 | 93.22 | 45.87 | 87.58 | 46.28 | 31.84 | 64.04 | 72.08 | 69.51 | 48.65 | 73.63 | 81.82 | 55.08 | 62.58 | 28.75 | |
| RXF 393 | 104.03 | 90.76 | 56.13 | 79.87 | 72.64 | 14.13 | 74.78 | 90.59 | 83.02 | 55.93 | 88.27 | 52.33 | 38.41 | 49.41 | 61.80 | |
| SN12C | 82.18 | 98.10 | 67.70 | 79.39 | 69.58 | 64.79 | 38.19 | 70.68 | 96.63 | 73.66 | 27.14 | 80.79 | 76.12 | 77.75 | 71.63 | |
| TK-10 | 120.42 | 88.46 | 95.63 | 99.80 | 93.11 | 68.70 | 90.74 | 104.96 | 72.43 | 113.60 | 101.93 | 91.77 | 95.83 | 105.47 | 108.92 | |
| UO-31 | 60.69 | 62.50 | 30.26 | 60.54 | 60.75 | 42.13 | 36.50 | 75.65 | 71.89 | 50.93 | 62.92 | |||||
| prostate cancer | PC-3 | 83.21 | 83.14 | 80.17 | 76.26 | 49.41 | 85.05 | 65.91 | 85.51 | 75.72 | 48.11 | 82.47 | 80.20 | 87.92 | 68.16 | 81.39 |
| DU-145 | 102.58 | 92.07 | 82.81 | 86.56 | 90.12 | 90.48 | 76.78 | 86.40 | 84.49 | 77.90 | 100.88 | 93.09 | 101.70 | 73.70 | 92.71 | |
| breast cancer | MCF7 | 97.19 | 80.61 | 51.57 | 63.32 | 40.61 | 67.54 | 49.58 | 63.96 | 74.54 | 50.45 | 56.77 | 77.07 | 66.90 | 42.30 | 60.21 |
| MDA-MB-231/ATCC | 79.57 | 59.59 | 72.60 | 59.66 | 75.74 | 76.76 | 71.94 | 87.20 | 66.80 | 81.38 | 80.58 | |||||
| HS 578T | 100.24 | 71.37 | 52.51 | 80.95 | 48.38 | 57.02 | 81.99 | 68.23 | 73.67 | 94.33 | 33.70 | 39.51 | 76.00 | 73.79 | ||
| BT-549 | 95.55 | 83.27 | 87.40 | 66.42 | 28.16 | 83.67 | 71.72 | 72.57 | 77.08 | 24.05 | 92.35 | 79.69 | 70.98 | 58.51 | 93.63 | |
| T-47D | 89.01 | 66.00 | 52.46 | 58.60 | 9.14 | 60.21 | 33.60 | 46.14 | 58.91 | 14.63 | 32.42 | 67.90 | 60.60 | 35.97 | 49.34 | |
| MDA-MB-468 | 99.02 | 66.15 | 41.27 | 43.43 | 28.48 | 50.44 | 54.99 | 41.44 | 56.62 | 31.66 | 64.90 | 64.96 | 57.69 | 30.23 | 46.73 |
Table 5. Percent GI on Breast, Colon, Lung, Pancreas, and Prostate Cell Lines.
| tissue cell line type | breast MCF-7 | colon HCT-116 | lung A549 | pancreas MiaPaCa | prostate PC-3 | |
|---|---|---|---|---|---|---|
| compounds | conc. (μM) | % GI | ||||
| 18a | 10 | 10 | 37 | 31 | 23 | 03 |
| 18b | 10 | 34 | 41 | 55 | 66 | 28 |
| 18c | 10 | 43 | 78 | 91 | 45 | 15 |
| 18d | 10 | 39 | 26 | 41 | 12 | 09 |
| 18e | 10 | 12 | 38 | 25 | 18 | 06 |
| 18f | 10 | 32 | 45 | 71 | 27 | 28 |
| 18g | 10 | 43 | 32 | 33 | 53 | 05 |
| 18h | 10 | 11 | 15 | 15 | 23 | 14 |
| 18i | 10 | 28 | 25 | 16 | 36 | 01 |
| 18j | 10 | 04 | 50 | 42 | 17 | 21 |
| 18k | 10 | 05 | 22 | 36 | 42 | 11 |
| 18l | 10 | 01 | 23 | 28 | 27 | 23 |
| 18m | 10 | 51 | 47 | 62 | 31 | 05 |
| 18n | 10 | 42 | 26 | 36 | 29 | 18 |
| 18o | 10 | 45 | 26 | 32 | 21 | 03 |
| capsaicin | 10 | 24 | 0.0 | 0.0 | 15 | 19 |
Among the capsaicin derivatives (14a–p), compounds 14f, 14p, 14j, 14g, and 14k illustrated susceptibility against the HCT-116 colon cancer cell line with 2.97–49.09% growth of cancer cell. Compound 14g demonstrated better antiproliferative activity against HOP-92, NCI-H460, HCT-116, 786-0, and SN12C cancer cell lined with growth of 2.97–20.45%. It also exhibited moderate antiproliferative activity with growth of 41.33–49.67% against A549/ATCC non-small cell lung cancer, SW-620 colon cancer, SNB-75 CNS cancer, U251 CNS cancer, OVCAR-5 ovarian cancer, and OVCAR-8 ovarian cancer cell lines. Herein, compounds 14e and 14j displayed potent activity against NCI/ADR-RES ovarian cancer and HCT-116 colon cancer with 28.98 and 15.41% growth, respectively. Compound 14j also demonstrated moderate antiproliferative activity against prostate PC-3 cancer cell line with a growth of 44.39%. Potent compounds (14g, 14j) that showed excellent activity from the preliminary screening (NCI antiproliferative data) were further evaluated for their IC50 values.
Inspired by the potent antiproliferative activity of 1,2,3-triazole tethered capsaicinoids, a series of (18a–o) 1,2,3-triazole derivatives of the structural isomer of capsaicin, which was derived by the hybrid conjugate of vanillyl amine and citronellic acid, were prepared and evaluated for their antiproliferative activity. Antiproliferative data revealed that compounds 18a–n exhibited cytotoxicity against various cancer cell lines. Compound 18b exhibited excellent activity against HCT-116, SNB-75, OVCAR-8, NCI/ADR-RES, ACHN, CAKI-1, and MDA-MB-468 with a range of growth of 38.23–49.14%. Compound 18d showed excellent activity against leukemia (CCRF-CEM, HL-60(TB), K-562, MOLT-4, RPMI-8226, SR), HCT-116, SK-MEL-5, UACC-62, OVCAR-4, OVCAR-8, NCI/ADR-RES, CAKI-1, UO-31, PC-3, MCF-7, BT-549, T-47D, and MDA-MB-468 with a growth range of 5.29–49.81%. Moreover, compound 18e also displayed moderate cytotoxicity against the non-small cell lung cancer HOP-62 cell line; CNS cancer SF-295, SNB-19, and SNB-75 cell lines; ovarian cancer OVCAR-4, OVCAR-8, NCI/ADR-RES, and SK-OV-3 cell lines; renal cancer CAKI-1 and RXF 393; and breast cancer HS 578T cell line with a growth range of 7.99–49.98%. Compound 18f exhibited moderate activity against SR, A549, NCI-H460, HCT-116, HCT-15, SW-620, SF-539, SNB-75, U251, OVCAR-8, NCI/ADR-RES, SN12C, MCF7, and T-47D cancer cell lines with a growth range of 5.30–49.58%. Compound 18g also exhibited good activity against SR, NCI-H23, HCT-116, SF-539, SK-MEL-28, NCI/ADR-RES, UO-31, T-47D, and MDA-MB-468 cancer cell lines with a growth range of 17.58–46.14%. Compound 18h has demonstrated promising activity against NCI/ADR-RES cell lines with a growth of 3.67%. Among all other synthesized compounds, only compound 18i demonstrated moderate activity against the leukemia (CCRF-CEM, HL-60(TB), K-562, MOLT-4, RPMI-8226, SR), colon cancer (HCT-116, HT29), melanoma (SK-MEL-5, UACC-62), ovarian cell (OVCAR-4, OVCAR-8, NCI/ADR-RES), renal cell (CAKI-1, UO-31), prostate cancer (PC-3), and breast cancer (BT-549, T-47D, MDA-MB-468) cell lines with a growth range of 14.05–48.65%. Compound 18f also showed good activity against SR, A549, NCI-H460, HCT-116, SW-620, SF-539, OVCAR-4, OVCAR-8, NCI/ADR-RES, SN12C, and T-47D cancer cell lines with a growth range of 10.58–45.13%. Compounds 18l, 18m, and 18n also showed good activity against K-562, MOLT-4, SR, A549, HOP-92, NCI-H460, NCI-H522, HCT-116, SNB-75, SF-295, SF-539, SNB-75, SK-OK-3, SK-MEL-5, OVCAR-4, OVCAR-8, NCI/ADR-RES, CAKI-1, 786–0, RXF 393, MCF7, HS 578T, T-47D, and MDA-MB-468 cancer cell lines with a growth range of 4.63–49.34%. Antiproliferative data revealed that compounds 18c and 18f exhibited excellent cytotoxicity against the lung (A549) cell line with a range of growth inhibition of 55–91%. Compound 18m showed moderate activity against breast (MCF-7) and lung (A549) cell lines with a growth inhibition range of 51–62% (Table 4).
Determination of IC50 Values
Compounds (14g, 14j, 18b, 18c, 18f, 18m) showing excellent % GI against non-small cell lung cancer (A549, NCI-H460), breast cancer (MCF-7), colon cancer (HCT-116), and ovarian cancer (SKOV-3) cell lines were further evaluated for their IC50 values using an SRB assay, and the results are depicted in Table 5.
Among the cell lines tested, lung cancer cell lines NCI-H460 and A549 were found to be more susceptible against these compounds. Compounds 18b, 18c, 18f, and 18m exhibited good to moderate cytotoxicity against the A549 cancer cell line with IC50 values ranging between 2.91 and 10.55 μM. Among the compounds tested, compounds 18c and 18f demonstrated the best antiproliferative activity against A549 with IC50 values of 3.68 and 2.91 μM, respectively, while compound 14g demonstrated moderate antiproliferative activity against NCI-H460. Compound 18m was found to be moderately active against both MCF-7 and A549 cell lines with IC50 values of 9.34 and 8.44 μM, respectively. Dose–response curve of compounds 18b, 18c, 18f, 18m doxorubicin and paclitaxel against A549 and MCF-7 cell lines is depicted in Supporting Information. The standard compounds doxorubicin and paclitaxel were screened as positive control, as shown in Table 6. Compound 18f was further evaluated for its toxicity against normal HEK-293 cells (normal kidney cells). The IC50 of compound 18f was 116-fold higher in HEK-293 (IC50 = 696 μM) as compared to A549 cells.
Table 6. IC50 (μM) Profile of Active Compounds.
| test compound | non-small
cell Lung cancer |
breast cancer | colon cancer | ovarian cancer | HEK-293 | |
|---|---|---|---|---|---|---|
| A549 | NCI-H460 | MCF-7 | HCT-116 | SKOV-3 | ||
| 14g | nt | 6.65 | nt | 8.90 | 10.63 | nt |
| 14j | nt | 5.55 | nt | 10.45 | 32.50 | nt |
| 18b | 10.554 | nt | nt | nt | nt | nt |
| 18c | 3.69 | nt | nt | nt | nt | nt |
| 18f | 2.91 | nt | nt | nt | nt | 696 |
| 18m | 8.44 | nt | 9.35 | nt | nt | nt |
| capsaicin | nt | 30.66 | nt | 40.16 | 22.03 | nt |
| paclitaxel | 0.03 nM | nt | nt | nt | nt | nt |
| doxorubicin | nt | 4.29 | 0.18 nM | 57.77 | 25.83 | nt |
Structure–Activity Relationship (SAR)
On the basis of the in vitro antiproliferative results (IC50) obtained, SAR of the synthesized compounds was developed based on the nature and position of the substituent present on the aromatic ring attached to the 1,2,3-triazole ring. Triazole conjugates in both series (14a–p and 18a–o) were found to be highly active when compared to the nonconjugated natural capsaicin and its structural isomer (16c). The lipophilic side chain of 1,2,3-triazole tethered capsaicinoids greatly influenced the antiproliferative activity. Replacing the lipophilic side chain of (R)-N-(4-hydroxy-3-methoxybenzyl)-3,7-dimethyloct-6-enamide with citronellic acid significantly enhanced the activity. Further, it has been observed that the nature of the substituents on the aromatic ring connected to the 1,2,3-triazole moiety also influenced the activity. Compounds with choro/bromo/cyano substituted aromatic moiety demonstrated better activity. Chloro-substitution led to better activity when compared to other substitutions. On the basis of the position of the substitution, the activity profile has been observed as 3,4-dichloro > 3-chloro > 4-chloro > 2-chloro. On the basis of SAR, the most active compound (18f) has been selected for further detailed studies (Figure 3).
Figure 3.
SAR for synthesized 1,2,3-triazoles against antiproliferative activity.
Reactive Oxygen Species (ROS) Generation Assay
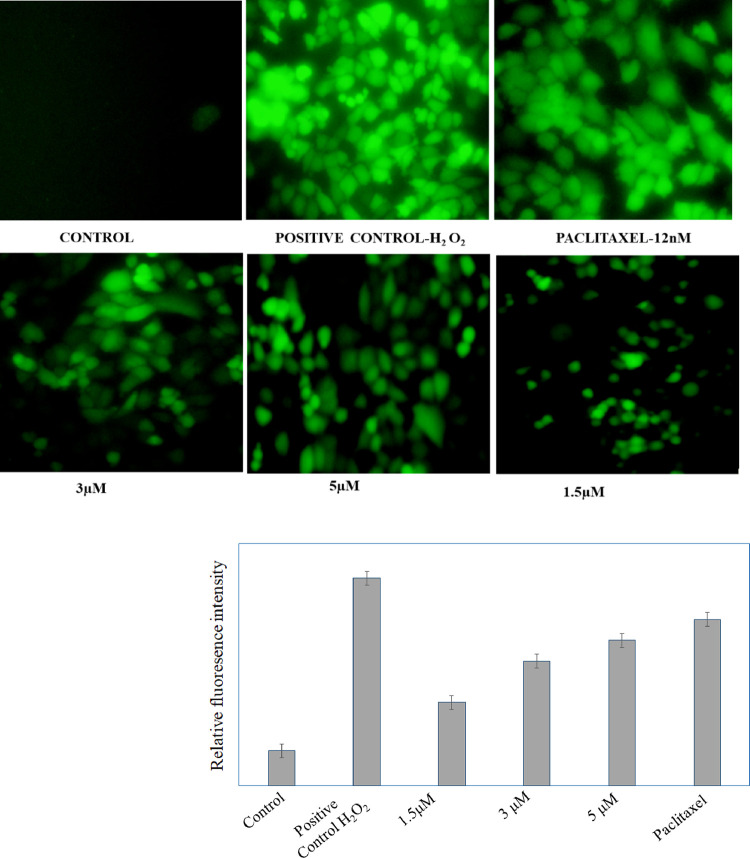
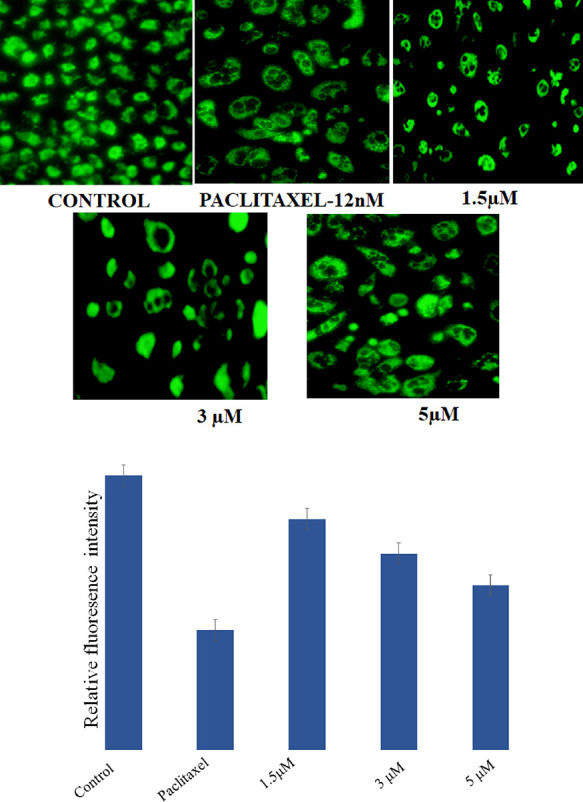
A higher level of ROS generation is a prime indication of apoptosis in cancerous cells. In the current study, A549 cells were incubated with DCFDA dye, and intracellular ROS was observed using a fluorescence microscope. A higher amount of ROS was produced in the positive control group. In a similar way, the amount of ROS was increased after the treatment with compound 18f at 5, 3, and 1.5 μM concentration. Observation through fluorescence microscope is a qualitative means of ROS generation in A549 lung cancer cells where a sharp increase in fluorescence intensity was observed (Figure 4). This study indicated that compound 18f triggered ROS generation in A549 cells, which is a key feature of apoptosis.
Figure 4.
Determination of reactive oxygen species in A549 cells by paclitaxel and 18f. H2O2 is used as positive control. Control group had no green fluorescence, and the ROS level was low in the control group but higher in the treated groups.
Apoptosis Assay through DAPI Staining
DAPI staining helped differentiate between normal and apoptotic cells by the nuclear morphological changes caused in a concentration-dependent manner after treatment with compound 18f at 5, 3, and 1.5 μM concentration against the A549 cell line. The morphological changes that have occurred can be visualized using fluorescence microscopy. The nuclei of untreated cells appeared as more or less rounded structures, while the treatment groups (18f) including paclitaxel showed chromatin condensation, nuclear blebbing, and formation of apoptotic bodies. The nuclear morphological changes, with the exception of the untreated control, clearly suggested that A549 cells had undergone apoptosis (Figure 5).
Figure 5.
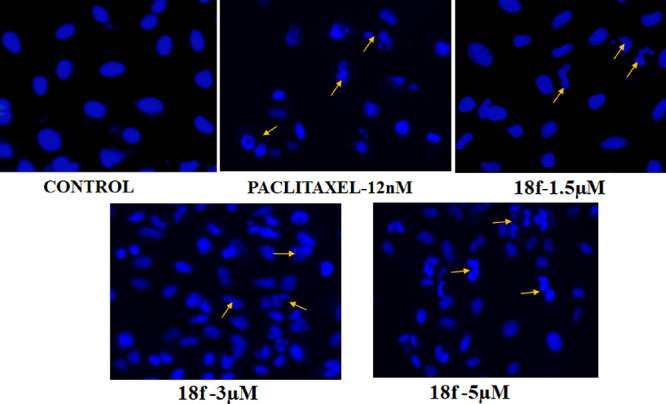
Fluorescence microscopy analysis of DAPI-stained cells was undertaken to study nuclear alterations and apoptotic body formation, both of which are also features of apoptosis. Effect of 18f on nuclear morphology of A549 lung cancer cells with varying concentrations and paclitaxel as positive control was assessed after DAPI staining. Arrows represent the changes in the nuclear structure such as chromatin condensation and nuclear damage.
Measurement of Loss of Mitochondrial Membrane Potential (ΔΨm)
Reduction of MMP (ΔΨm) is the main characteristic of apoptosis. To assess the effect of compound 18f, loss of MMP was observed through fluorescence microscopy using rhodamine-123 staining. After 48 h treatment with compound 18f against A549 cells, it was observed that the significant reduction in MMP at 3.0 μM was due to the decrease in fluorescence intensities that clearly justified the mitochondrial membrane destabilization in comparison to untreated cells. The result was quite similar in the case of the paclitaxel treatment group. The results obtained indicate the probable role of loss of MMP in the induction of apoptosis by 18f in the A549 cell line (Figure 6).
Figure 6.
Effect of paclitaxel and 18f on mitochondrial membrane potential using rhodamine-123. Exponentially growing A549 cells were treated with paclitaxel at its IC50 doses and 18f at varying concentrations for 48 h. Paclitaxel was used as positive control. Cells treated with paclitaxel and 18f at 5 μM show the maximum loss in the mitochondrial membrane potential.
Cell Cycle Analysis
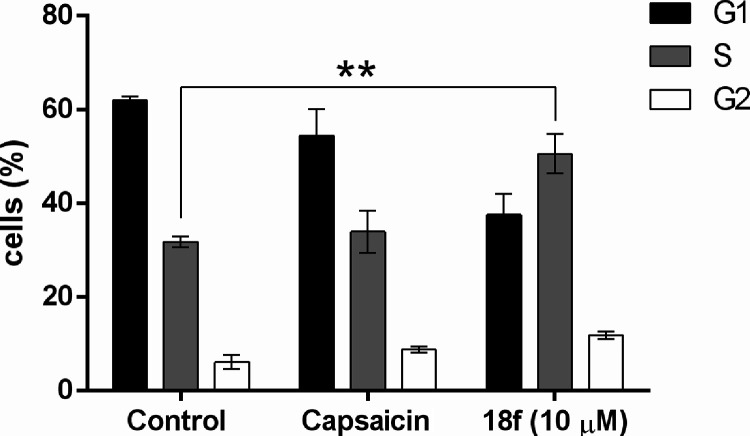
To determine the effect of compound 18f on the cell cycle of A549 cells, cell cycle analysis was performed using flow cytometry after 48 h of treatment. As shown in Figure 7, the treatment of compound 18f induced the accumulation of cells in the S-phase from 31.78% in control to 50.57%, with a concomitant decrease in G0–G1 phase reduction from 62.8% in control to 34.32% in compound 18f treated cells at 10 μM concentration (Figure 7).
Figure 7.
Compound 18f induces cell cycle arrest in A549 cells. Cell cycle analysis of A549 cancer cells treated with compound 18f for 48 h indicated that compound 18f induced cell cycle arrest at the S-phase. Data presented as mean ± SD of three independent experiments. **P < 0.01.
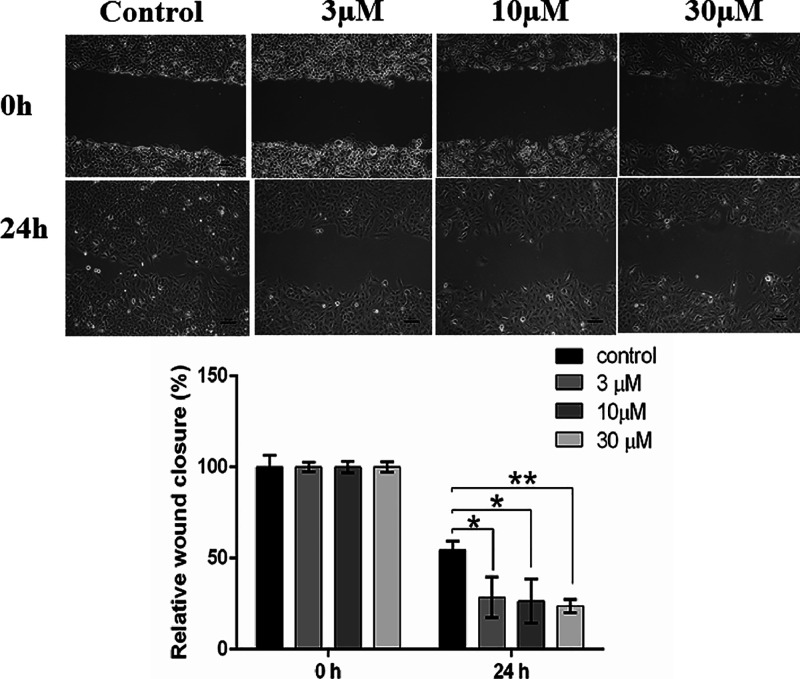
Migration Potential of NCI-H460 and A549 Lung Cancer Cells
To determine the effect of compounds 14j and 18f on the migration capacities of NCI-H460 and A549 cells, a wound healing assay was performed. Compound 14j showed moderate wound healing and inhibited the colony-forming ability of NCI-H460 cells (Figures S3 and S4). Our results demonstrated that doses of compound 18f treatment at different concentrations significantly inhibited the migration potential of A549 cells when compared to their respective control group after 24 h of scratching (Figure 8).
Figure 8.
Compound 18f inhibits the migration potential of A549 lung cancer cells. The migration potential of compound 18f treated cells at different concentrations was examined using a wound healing assay. Images were captured at different time points, and the wound area was quantified using the ImageJ software. Scale bar, 100 μm; magnification, 10×. *P < 0.05, **P < 0.01.
Conclusions
A series of thirty-one 1,2,3-triazole tethered capsaicinoids were synthesized by employing one-/two-point modifications around the capsaicin scaffold. All the newly synthesized compounds were evaluated for their antiproliferative activity against 60 cancer cell lines. Antiproliferative screening suggested that compounds 14g, 14j, 18b, 18c, 18f, and 18m showed good to moderate activity against cancer cell lines. The most potent compound (18f) showed good IC50 (2.92 μM) value against A549 non-small cell lung cancer. Compound 18f revealed triggered apoptosis, elevated intracellular ROS levels, disrupted mitochondrial membrane potential and reduced cell migration potential of A549 cells in a dose-dependent manner. Further, compound 18f was found to arrest the cell cycle at the S-phase and produced significant activity. As a result of the findings of this investigation, this compound may serve as a template for the further development of novel capsaicinoid-based anticancer agents against lung cancer.
Methods
Chemistry
General Procedure for Synthesis of Propargylation
A solution of 0.5 g of compound (10) was dissolved in 10 mL of dry acetone followed by the addition of activated K2CO3 (5 equiv). Then propargyl bromide (1.2 equiv) was added to it and was refluxed for 4–5 h. After the completion of the reaction (monitored by TLC), the reaction mixture was allowed to attain room temperature and was poured into ice-cold water to form a colorless solid that was afterward filtered off under a vacuum to afford the desired product.
(E)-N-{3-Methoxy-4-(prop-2-yn-1-yloxy)benzyl}-8-methylnon-6-enamide (13)
Pale yellow color solid, yield: 96%, 1H NMR (400 MHz, DMSO-d6, ppm) δ 8.24 (s,1H), 6.95 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 6.75 (d, J = 8.4 Hz, 1H), 5.40–5.27 (m, 2H), 4.73 (d, J = 1.6 Hz, 2H), 4.19 (d, J = 5.6 Hz, 2H), 3.73 (s, 3H), 3.53 (s, 1H), 2.12 (t, J = 7.6 Hz, 2H), 1.94 (q, J = 6.8 Hz, 1H), 1.55–1.48 (m, 2H), 1.33–1.23 (m, 4H), 0.93 (d, J = 6.4 Hz, 4H), 0.84 (d, J = 6.8 Hz, 2H); MS (ESI) m/z: [M + 1]+ 344.4; exact mass: 343.21; elemental analysis calculated for (C21H29NO3): C, 73.44; H, 8.51; N, 4.08; found: C, 73.39; H, 8.54; N, 4.10.
General Procedure for Synthesis of the 1,2,3-Triazole Ring (14a–p)
The solution of compound (13) bearing terminal alkyne was dissolved in 15 mL of t-butanol/water (1:1) at ambient temperature. Then 1.5 equiv of CuSO4·5H2O was added, and the reaction mixture was stirred for 10 min. Initially, the color of the reaction mixture was observed to be light blue. Then sodium ascorbate (4 equiv) was added to the reaction mixture and allowed to stir for15 min. The color of the reaction mixture changed from blue to dark brown. After 15 min, substituted aromatic azide (1.1 equiv) was added to the reaction mixture and was allowed to stir for a further 8 h at ambient temperature. After the completion of the reaction monitored by TLC, the reaction mixture was poured into the water and extracted with ethyl acetate (2 × 20 mL). The combined organic layer was dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure to obtain the final triazole derivative (14a–p), which was recrystallized with ethyl acetate/hexane (70–90% quantitative yields).
(E)-N-(4-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14a)
Creamish solid, 94.0% yield; m.p. = 130.2 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.08 (s, 1H), 7.72–7.69 (d, J = 8.4 Hz, 2H), 7.53–7.51 (d, J = 8.4 Hz, 2H), 7.05–7.03 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 6.83–6.81 (d, J = 8.4 Hz, 1H), 5.73 (s, 1H), 5.43–5.30 (m, 4H), 4.41–4.39 (d, J = 5.6 Hz, 2H), 3.89 (s, 3H), 2.25–2.21 (t, J = 6.8 Hz, 2H), 2.04–1.99 (q, J = 6.8 Hz, 1H), 1.72–1.65 (m, 2H), 1.45–1.38 (m, 4H), 0.98–0.97 (d, J = 6.8 Hz, 4H), 0.89–0.87 (d, J = 6.4 Hz, 2H); IR cm–1: 3481, 3445, 3358, 3274, 3212, 3125, 3061, 3023, 2919, 2857, 1636, 1549, 1506, 1460, 1418, 1379, 1251, 1137, 1095, 1029, 971, 804, 762, 726, 656, 607; MS (ESI) m/z: [M]+ 497.1; exact mass: 496.2241, elemental analysis calculated for (C27H33ClN4O3): C, 65.25; H, 6.69; N, 11.27; found: C, 65.22; H, 6.70; N, 11.25.
(E)-N-(4-((1-(3-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14b)
Light brown solid, 87.0% yield; m.p. = 135.0 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.10 (s, 1H), 7.81 (s, 1H), 7.66–7.64 (d, J = 7.6 Hz, 1H), 7.51–7.44 (m, 2H), 7.04–7.02 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 6.83–6.81 (d, J = 8.0 Hz, 1H), 5.73 (s, 1H), 5.43–5.28 (m, 4H), 4.41–4.40 (d, J = 5.6 Hz, 2H), 3.89 (s, 3H), 2.26–2.23 (t, J = 7.6 Hz, 2H), 2.04–1.99 (q, J = 6.8 Hz, 1H), 1.70–1.66 (m, 2H), 1.43–1.28 (m, 4H), 0.98–0.96 (d, J = 6.8 Hz, 4H), 0.89–0.87 (d, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.8(C=O), 149.8(triazolyl tertiary C), 146.8(vanillyl Ar C-OCH3), 145.3 (vanillyl Ar C-OCH2-), 138.1 (-CH=CH-CH(CH3)2), 137.8 (Ar C), 135.6(Ar C), 132.4(Ar C), 130.8 (vanillyl Ar C-CH2-NH-), 128.9 (Ar C), 126.4(-CH=CH-CH(CH3)2), 121.0(Ar C), 120.8 (vanillyl Ar C), 120.0(triazolyl -CH=C-N-), 118.5(Ar C), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.1(-OCH2-), 55.9(-OCH3), 43.3(-CH2NH), 36.7(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.2(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3421, 3288, 3180, 3121, 3091, 3006, 2955, 2924, 2893, 2852, 1629, 1593, 1506, 1456, 1322, 1217, 1137, 1027, 913, 846, 782, 666, 616, 531; MS (ESI) m/z: [M]+ 497.1; exact mass: 496.2241, elemental analysis calculated for (C27H33ClN4O3): C, 65.25; H, 6.69; N, 11.27; found: C, 65.22; H, 6.70; N, 11.25.
(E)-N-(4-((1-(2-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14c)
Creamish solid, 98.0% yield; m.p. = 88.6 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.09 (s, 1H), 7.63–7.58 (m, 2H), 7.50–7.44 (m, 2H), 7.05–7.03 (d, J = 12 Hz, 1H), 6.86 (s, 1H), 6.82–6.80 (d, J = 8.0 Hz, 2H), 5.86 (s, 1H), 5.42–5.29 (m, 4H), 4.39–4.38 (d, J = 5.6 Hz), 3.87 (s, 3H), 2.24–2.21 (t, J = 7.6 Hz, 2H), 2.01–1.98 (q, J = 6.8 Hz, 1H), 1.71–1.64 (m, 2H), 1.44–1.37 (m, 4H), 0.96 (d, J = 6.8 Hz, 4H), 0.87 (d, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm): 172.9(C=O), 149.9(vanillyl Ar C-OCH3), 146.9(vanillyl Ar C-OCH2-), 144.0(triazolyl tertiary C), 138.0(-CH=CH-CH(CH3)2), 130.8(Ar C), 130.7(vanillyl Ar C-CH2-NH-), 128.6(Ar C), 127.9(Ar C), 127.7(Ar C), 126.4(-CH=CH-CH(CH3)2), 125.1(Ar C), 120.0(triazolyl -CH=C-N-), 114.7(vanillyl Ar C), 111.7(Ar C), 63.2(-OCH2-), 55.9(-OCH3), 43.3(-CH2NH-), 36.6 (-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.2(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3486, 3448, 3400, 3310, 3157, 3116, 3075, 3034, 2927, 2868, 2800, 1633, 1511, 1458, 1418, 1383, 1332, 1257, 1216, 1135, 1003, 851, 815, 756, 641, 606, 570, 544; MS (ESI) m/z: [M]+ 497.1; exact mass: 496.2241, elemental analysis calculated for (C27H33ClN4O3): C, 65.25; H, 6.69; N, 11.27; found: C, 65.22; H, 6.71; N, 11025.
(E)-N-(4-((1-(3,4-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14d)
Brown solid, 91.0% yield; m.p. = 131.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.09 (s, 1H), 7.91 (s, 1H), 7.62 (s, 1H), 7.01–6.99 (d, J = 4.4 Hz, 1H), 6.86 (s, 1H), 6.82–6.80 (d, J = 8.4 Hz, 1H), 5.83 (s, 1H), 5.42–5.29 (m, 4H), 4.40–4.38 (d, J = 5.6 Hz, 2H), 3.88 (s, 3H), 2.25–2.21 (t, J = 7.6 Hz, 2H), 2.03–1.98 (q, J = 6.4 Hz, 1H), 1.71–1.64 (m, 2H), 1.44–1.28 (m, 4H), 0.97–0.96 (d, J = 6.8 Hz, 4H), 0.88–0.86 (d, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.9(C=O), 149.7(vanillyl Ar C-OCH3), 146.7(vanillyl Ar C-OCH2-), 145.5(triazolyl tertiary C), 138.1(-CH=CH-CH(CH3)2), 135.9(Ar C), 134.0(Ar C), 133.0(Ar C), 132.4(Ar C), 131.5(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 122.3(Ar C), 120.9(vanillyl Ar C), 120.0(triazolyl -CH=C-N-), 119.4(Ar C), 114.1(vanillyl Ar C), 111.6(vanillyl Ar C), 63.0(-OCH2-), 55.9(-OCH3-), 43.3(-CH2NH-), 36.6(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.3(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3436, 3343, 3306, 3276, 3209, 3113, 3075, 3017, 2928, 2888, 2819, 1742, 1699, 1638, 1515, 1456, 1420, 1393, 1318, 1232, 1136, 1026, 951, 811, 713, 664, 624, 591, 545; MS (ESI) m/z: [M + 2]+ 533.1; exact mass: 530.1851; elemental analysis calculated for (C27H32Cl2N4O3): C, 61.02; H, 6.07; N, 10.54; found: C, 61.05; H, 6.05; N, 10.58.
(E)-N-(3-Methoxy-4-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-8methylnon-6-enamide (14e)
Creamish yellow solid, 95.0% yield; m.p. = 131.0 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.45–8.23 (d, J = 8.8 Hz, 2H), 8.23 (s, 1H), 8.01–7.99 (d, J = 9.2 Hz, 2H), 7.04–7.02 (d, J = 8.0 Hz, 1H), 6.88 (s, 1H), 6.84–6.82 (d, J = 8.0 Hz, 1H), 5.75 (s, 1H), 5.43–5.30 (m, 4H), 4.41–4.40 (d, J = 5.6 Hz, 2H), 3.90 (s, 3H), 2.25–2.22 (t, J = 7.6 Hz, 2H), 2.04–1.99 (q, J = 6.8 Hz, 1H), 1.68–1.67 (m, 2H), 1.45–1.28 (m, 4H), 0.98–0.96 (d, J = 6.4 Hz, 4H), 0.89–0.87 (d, J = 6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 173.0(C=O), 149.7(vanillyl Ar C-OCH3), 148.7(Ar C-NO2), 147.2(vanillyl Ar C-OCH2-), 146.6 (triazolyl tertiary C), 141.5(Ar C), 138.1(-CH=CH-CH(CH3)2), 132.6(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 125.5(Ar C), 120.5(triazolyl -CH=C-N-), 119.9(Ar C), 114.1(vanillyl Ar C), 111.6(vanillyl Ar C), 63.0(-OCH2-), 55.9(-OCH3), 43.2(-CH2NH-), 36.6(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.2(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3415, 3316, 3246, 3201, 3102, 3049, 2988, 2923, 2860, 2829, 1672, 1635, 1599, 1551, 1515, 1463, 1341, 1258, 1229, 1136, 1021, 851, 816, 748, 684, 644, 600, 542; MS (ESI) m/z: [M + 1] + 508.1; exact mass: 507.2481; elemental analysis calculated for (C27H33N5O5): C, 63.89; H, 6.55; N, 13.80; found: C, 63.85; H, 6.58; N, 13.84.
(E)-N-(3-Methoxy-4-((1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-8-methylnon-6-enamide (14f)
Creamish brown, 93.0% yield; m.p. = 164.7 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.63 (s,1H), 8.33–8.32 (d, J = 7.2 Hz, 1H), 8.23–8.39 (m, 2H), 7.80–7.76 (m, 1H), 7.02–6.99 (d, J = 11.2 Hz, 1H), 6.88 (s, 1H), 6.83–6.82 (d, J = 6.4 Hz, 1H), 5.76 (s, 1H), 5.39 (s, 4H), 4.40–4.42 (d, J = 6.4 Hz 2H), 3.90 (s, 3H), 2.24 (s, 2H), 2.02–2.00 (m, 1H), 1.69 (s, 2H), 143–1.28 (m, 4H), 0.98–0.97 (d, J = 5.6 Hz, 4H), 0.89–0.88 (d, J = 5.2 Hz, 2H); IR cm–1: 3499, 3451, 3377, 3327, 3261, 3193, 3152, 3110, 3057, 2941, 2856, 2830, 1635, 1531, 1458, 1346, 1258, 1224, 1137, 1083, 1029, 971, 797, 732, 662, 589, 545; MS (ESI) m/z: [M + 1]+ 508.2; exact mass: 507.2481; elemental analysis calculated for (C27H33N5O5): C, 63.89; H, 6.55; N, 13.80; found: C, 63.92; H, 6.55; N, 13.78.
(E)-N-(3-Methoxy-4-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-8-methylnon-6-enamide (14g)
Creamish brown, 89.0% yield; m.p. = 156.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.17 (s, 1H), 8.05 (s, 1H), 7.97–7.96 (d, J = 8.0 Hz, 1H), 7.75–7.68 (m, 2H), 7.04–7.02 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 6.83–6.81 (d, J = 8.0 Hz, 1H), 5.79 (s, 1H), 5.42–5.30 (m, 4H), 4.404.39 (d, J = 6.0 Hz, 2H), 3.89 (s, 3H), 7.27–2.23 (t, J = 7.6 Hz, 2H), 2.03–1.98 (q, J = 6.8 Hz, 1H), 1.72–1.64 (m, 2H), 1.45–1.28 (m, 4H), 0.98–0.0.96 (d, J = 6.8 Hz, 4H), 0.88–0.87 (d, J = 6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.9(C=O), 149.7(vanillyl Ar C-OCH3), 146.7(vanillyl Ar C-OCH2-), 145.5(triazolyl tertiary C), 138.0(-CH=CH-CH(CH3)2), 137.2(Ar C), 132.5(Ar C), 130.5(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 125.4(vanillyl Ar C), 123.5(Ar C), 121.4(Ar C), 119.9(triazolyl -CH=C-N-), 117.4(Ar C), 114.2(vanillyl Ar C), 111.6(vanillyl Ar C), 63.0 (-OCH2-), 55.8 (OCH3), 43.2(CH2NH-), 36.6(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.2(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3492, 3415, 3373, 3274, 3209, 3178, 3151, 3128, 3089, 3046, 2926, 2856, 2809, 1638, 1546, 1512, 1459, 1326, 1260, 1229, 1167, 1119, 1029, 966, 891, 832, 795, 736, 690, 593, 547; MS (ESI) m/z: [M + 1]+ 531.1; exact mass: 530.2504; elemental analysis calculated for (C28H33F3N4O3): C, 63.38; H, 6.27; N, 10.56; found: C, 63.35; H, 6.25; N, 10.55.
(E)-N-(4-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14h)
Brown solid, 95.0% yield; m.p. = 118.0 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.11 (s,1H), 7.79–7.75 (m, 2H), 7.31–7.27 (t, J = 8.4 Hz, 2H), 7.10–7.04 (d, J = 8.0 Hz, 1H), 6.92 (s, 1H), 6.88–6.86 (d, J = 8.0 Hz, 1H), 5.83 (s, 1H), 5.47–5.35 (m, 4H), 4.45–4.44 (d, J = 5.6 Hz, 2H), 3.93 (s, 3H), 2.30–2.26 (t, J = 7.2 Hz, 2H), 2.08–2.03 (q, J = 6.8 Hz, 1H), 1.77–1.69 (m, 2H), 1.50–1.33 (m, 4H), 1.03–1.01 (d, J = 6.8 Hz, 4H), 0.93–0.92 (d, J = 6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.9(C=O), 163.7(Ar C-F), 149.7(vanillyl Ar C-OCH3), 146.8(vanillyl Ar C-OCH2-), 145.1(triazolyl tertiary C), 138.0(-CH=CH-CH(CH3)2), 133.2(Ar C), 132.3(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 122.6(Ar C), 122.5(Ar C), 120.0(triazolyl -CH=C-N-), 116.8(Ar C), 116.6(Ar C), 114.1(vanillyl Ar C), 111.6(vanillyl Ar C), 63.1(OCH2), 55.9(OCH3), 43.3(CH2NH), 38.9(COCH2), 36.6(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.6(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3472, 3418, 3393, 3326, 3282, 3181, 3147, 3076, 3036, 2922, 2864, 1635, 1512, 1458, 1416, 1259, 1224, 1136, 1017, 834, 801, 752, 709, 605, 543; MS (ESI) m/z: [M + 1]+ 481.1; exact mass: 480.2536; elemental analysis calculated for (C27H33FN4O3): C, 67.48; H, 6.92; N, 11.66; found: C, 67.45; H, 6.90; N, 11.69.
(E)-N-(4-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14i)
Creamish solid, 93.0% yield; m.p. = 113.0 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.21 (d, J = 2.4 Hz, 1H), 8.0–7.96 (dd, J = 7.6 and 1.6 Hz, 1H), 7.47–7.45 (m, 1H), 7.37–7.32 (m, 1H), 7.08–7.06 (d, J = 8.0 Hz, 1H), 6.87 (s, 1H), 6.84–6.82 (d, J = 8.0 Hz, 1H), 5.68 (s, 1H), 5.43–5.30 (m, 4H), 4.41–4.40 (d, J = 6.0 Hz, 2H), 3.89 (s, 1H), 2.27–2.21 (t, J = 7.2 Hz, 2H), 2.04–1.99 (q, J = 6.8 Hz, 1H), 1.73–1.65 (m, 2H), 1.46–1.28 (m, 4H), 0.98–0.97 (d, J = 6.8 Hz, 4H), 0.89–0.07 (d, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.9(C=O), 149.7(vanillyl Ar C-OCH3), 147.3(vanillyl Ar C-OCH2-), 146.7(triazolyl tertiary C), 141.0(vanillyl Ar C), 138.1(-CH=CH-CH(CH3)2), 132.6(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 125.5(Ar C), 120.9(Ar C), 120.5(triazolyl -CH=C-N-), 119.9(Ar C), 114.2(vanillyl Ar C), 111.6(vanillyl Ar C), 63.0(-OCH2-), 55.9(-OCH3-), 43.2(-CH2NH-), 36.8(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.6(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3476, 3414, 3367, 3293, 3245, 3212, 3083, 3046, 3016, 2918, 2860, 1672, 1633, 1515, 1462, 1419, 1359, 1261, 1234, 1135, 1038, 995, 853, 802, 751, 641; MS (ESI) m/z: [M + 1]+ 481.1; exact mass: 480.2536; elemental analysis calculated for (C27H33FN4O3): C, 67.48; H, 6.92; N, 11.66; found: C, 67.45; H, 6.95; N, 411.61.
(E)-N-(4-((1-(4-Cyanophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14j)
Light gray solid, 84.0% yield; m.p. = 178.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.18 (s, 1H), 8.00 (s, 2H), 7.86 (s, 1H), 7.10 (s, 1H), 7.04 (s, 1H), 6.88 (s, 1H), 6.83 (s, 1H), 5.75 (s, 1H), 5.39–5.34 (m, 4H), 4.40 (s, 2H), 3.89 (s, 3H), 2.23 (s, 2H), 2.01 (s, 1H), 1.68 (s, 2H), 1.46–1.28 (m, 4H), 0.98–0.97 (d, J = 6.4 Hz, 4H), 0.89–0.87 (d, J = 6.8 Hz, 2H); IR cm–1: 3470, 3390, 3343, 3302, 3257, 3150, 3079, 3029, 2956, 2923, 2857, 1628, 1513, 1454, 1414, 1365, 1257, 1220, 1134, 1018, 842, 793, 667, 619, 535; MS (ESI) m/z: [M + 1]+ 488.1; exact mass: 487.2583; elemental analysis calculated for (C28H33N5O3): C, 68.97; H, 6.82; N, 14.36; found: C, 68.98; H, 6.86; N, 14.35.
(E)-N-(4-((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14k)
Brown solid, 91.0% yield; m.p. = 127.8 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.09 (s, 1H), 7.68–7.62 (q, J = 7.2 Hz, 4H), 7.03–7.01 (d, J = 8.0 Hz, 1H), 6.86 (s, 1H), 6.81–6.79 (d, J = 8.0 Hz, 1H), 5.86 (s, 1H), 5.42–5.29 (m, 4H), 4.39–4.38 (d, J = 5.6 Hz, 2H), 3.87 (s, 3H), 2.24–2.21 (t, J = 7.2 Hz, 2H), 2.03–1.98 (q, J = 6.4 Hz, 1H), 1.71–1.63 (m, 2H), 1.44–1.28 (m, 4H), 0.97–0.96 (d, J = 6.8 Hz, 4H), 0.88–0.86 (d, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 173.0(C=O), 149.7(vanillyl Ar C-OCH3), 146.7(vanillyl Ar C-OCH2-), 138.0(triazolyl tertiary C), 135.8(-CH=CH-CH(CH3)2), 132.9(Ar C), 132.4(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 122.5(Ar C), 121.9(Ar C), 121.1(triazolyl -CH=C-N-), 119.9(Ar C), 114.1(vanillyl Ar C), 111.6(vanillyl Ar C), 63.0(-OCH2-), 55.8(-OCH3), 43.2(-CH2NH-), 36.6(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.2(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); , IR cm–1: 3461, 3410, 3371, 3291, 3227, 3159, 3056, 3025, 2957, 2924, 2863, 1638, 1593, 1508, 1459, 1415, 1325, 1258, 1231, 1137, 1012, 799, 727, 692, 613, 567, 519; MS (ESI) m/z: [M]+ 541, [M + 1]+ 542, [M + 2]+ 543; exact mass: 540.1736; elemental analysis calculated for (C27H33BrN4O3): C, 59.86; H, 6.14; N, 10.35; found: C, 59.85; H, 6.17; N, 10.38.
(E)-N-(3-Methoxy-4-((1-(2-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-8-methylnon-6-enamide (14l)
Light gray, 97.0% yield; m.p. = 112.6 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.23 (s, 1H), 7.80–7.78 (dd, J = 7.6 and 1.6 Hz, 1H), 7.47–7.42 (m, 1H), 7.14–7.07 (m, 3H), 6.86 (s, 1H), 6.83–6.81 (d, J = 8.0 Hz, 1H), 5.78 (s, 1H), 5.42–5.30 (m, 4H), 4.40–4.39 (d, J = 5.6 Hz, 2H), 3.90 (s, 3H), 3.88 (s, 3H), 2.26–2.23 (t, J = 7.2 Hz), 2.03–1.98 (q, J = 6.8 Hz, 1H), 1.72–1.64 (m, 2H), 1.45–1.28 (m, 4H), 0.98–0.696 (d, J = 6.8 Hz, 4H), 0.89–0.87 (d, J = 6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.9(C=O), 151.1(Ar C-OCH3), 149.8(vanillyl Ar C-OCH3), 147.0(vanillyl Ar C-OCH2-), 143.4(triazolyl tertiary C), 138.0(-CH=CH-CH(CH3)2), 132.1(Ar C), 130.1(vanillyl Ar C-CH2-NH-), 126.4(Ar C), 126.2(-CH=CH-CH(CH3)2), 125.4(Ar C), 125.3(Ar C), 121.2(Ar C), 120.0(triazolyl -CH=C-N-), 114.4(vanillyl Ar C), 112.2(vanillyl Ar C), 111.6(vanillyl Ar C), 63.2(OCH2), 55.9(OCH3), 43.3(CH2NH), 36.7(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.3(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3476, 3449, 3404, 3314, 3269, 3173, 3108, 3045, 3017, 2996, 2928, 2874, 2836, 1634, 1601, 1538, 1509, 1464, 1418, 1388, 1254, 1215, 1137, 1046, 1010, 852, 823, 754, 691, 639, 583, 549; MS (ESI) m/z: [M + 1]+ 493.1; exact mass: 492.2736; elemental analysis calculated for (C28H36N4O4): C, 68.27; H, 7.37; N, 11.37; found: C, 68.30; H, 7.34; N, 11.40.
(E)-N-(3-Methoxy-4-((1-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-8-methylnon-6-enamide (14m)
Brown solid, 89.0% yield; m.p. = 106.2 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.07 (s, 1H), 7.06–7.04 (d, J = 8.4 Hz, 1H), 6.96 (s, 2H), 6.87 (s, 1H), 6.83–6.81 (d, J = 8.0 Hz, 1H), 5.77 (s, 1H), 5.42–5.30 (m, 4H), 4.41–4.39 (d, J = 5.6 Hz, 2H), 3.95 (s, 9H), 3.91 (s, 3H), 3.95–3.89 (d, J = 8.4 Hz, 3H), 2.25–2.21 (t, J = 7.6 Hz, 2H), 2.04–1.98 (q, J = 6.8 Hz, 1H), 1.68–1.66 (d, J = 6.8 Hz, 2H), 1.43–1.39 (t, J = 8.0 Hz, 4H), 0.98–0.96 (d, J = 6.8 Hz, 4H), 0.88–0.87 (d, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.9(C=O), 153.9(triazolyl tertiary C), 149.7(vanillyl Ar C-OCH3), 146.9(vanillyl Ar C-OCH2-), 138.4(Ar C), 138.1(-CH=CH-CH(CH3)2), 132.8(Ar C), 132.3(vanillyl Ar C-CH2-NH-), 126.4(-CH=CH-CH(CH3)2), 121.4(Ar C), 120.0(triazolyl -CH=C-N-), 114.1(vanillyl Ar C), 111.6(vanillyl Ar C), 63.2(OCH3), 61.0(OCH2), 56.4(OCH3), 55.9(OCH3), 43.3(CH2NH), 36.6(-COCH2-), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.3(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2); IR cm–1: 3492, 3461, 3407, 3319, 3253, 3192, 3135, 3072, 3020, 2954, 2922, 2890, 2848, 1635, 1598, 1510, 1460, 1414, 1226, 1124, 1032, 1000, 861, 810, 737, 689, 644, 613, 558; MS (ESI) m/z: [M + 1]+ 553.1; exact mass: 552.2947; elemental analysis calculated for (C30H40N4O4): C, 65.20; H, 7.30; N, 10.14; found: C, 65.22; H, 7.35; N, 10.12.
(E)-N-(3-Methoxy-4-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-8-methylnon-6-enamide (14n)
Creamish solid, 94.0% yield; m.p. = 115.4 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.06 (s, 1H), 7.63–7.61 (d, J = 8.4 Hz, 2H), 7.35–7.33 (d, J = 8 Hz, 2H), 7.07–7.05 (d, J = 8.4 Hz, 1H), 6.87 (s, 1H), 6.83–6.81 (d, J = 8 Hz, 1H), 5.70 (s, 1H), 5.43–5.30 (m, 4H), 4.41–4.39 (d, J = 5.6 Hz, 2H), 3.89 (s, 3H), 2.45 (s, 3H), 2.25–2.21 (t, J = 7.2 Hz, 2H), 2.02–2.01 (q, J = 6.8 Hz, 1H), 1.68–1.65 (m, 2H), 1.45–1.38 (m, 4H), 0.98–0.97 (d, J = 6.8 Hz, 4H), 0.89–0.87 (d, J = 6.4 Hz, 2H); IR cm–1: 3461, 3411, 3377, 3306, 3233, 3173, 3146, 3123, 3025, 2957, 2926, 2870, 2809, 1632, 1516, 1460, 1414, 1258, 1218, 1161, 1134, 1096, 1037, 992, 847, 805, 709, 679, 637, 603, 573, 526; MS (ESI) m/z: [M + 1]+ 477.2; exact mass: 476.2787; elemental analysis calculated for (C28H36N4O4): C, 70.56; H, 7.61; N, 11.76; found: C, 70.55; H, 7.64; N, 11.8.
(E)-N-(4-((1-(4-Ethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14o)
Creamish brown solid, 90.0% yield; m.p. = 105.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.06 (s 1H), 7.65–7.63 (d, J = 8.4 Hz, 2H), 7.37–7.35 (d, J = 8.4 Hz, 2H), 7.06–7.04 (d, J = 8.0 Hz, 1H), 6.86 (s, 1H), 6.83–6.80 (d, J = 8 Hz, 1H), 5.76 (s, 1H), 5.42–5.30 (m, 4H), 4.40–4.39 (d, J = 5.6 Hz, 2H), 3.88 (s, 3H), 2.77–2.71 (q, J = 7.6 Hz, 2H), 2.25–2.21 (t, J = 7.6 Hz, 3H), 2.03–1.98 (q, J = 6.4 Hz, 2H), 1.72–1.64 (m, 2H), 1.45–1.37 (m, 1H), 1.32–1.28 (t, J = 7.6 Hz, 4H), 0.98–0.96 (d, J = 6.8 Hz, 4H), 0.89–0.87 ( d, J = 6.4 Hz, 2H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.8(C=O), 149.7(vanillyl Ar C-OCH3), 146.9(vanillyl Ar C-OCH2-), 145.3(triazolyl tertiary C), 144.7(vanillyl Ar C), 138.0(-CH=CH-CH(CH3)2), 134.8(Ar C), 132.2(vanillyl Ar C-CH2-NH-), 129.1(Ar C), 126.4(-CH=CH-CH(CH3)2), 121.2(Ar C), 120.6(Ar C), 120.0(triazolyl -CH=C-N-), 114.2(vanillyl Ar C), 111.6(vanillyl Ar C), 63.1(-OCH2-), 55.9(-OCH3), 43.3(-CH2NH-), 36.8(-COCH2-), 36.7(Ar CH2CH3), 32.2(-CH(CH3)2), 30.9(-CH2-CH=CH-CH(CH3)2), 29.3(-CH2-CH2-CH=CH-CH(CH3)2), 25.2(-COCH2-CH2-), 22.6(-CH(CH3)2), 15.4(Ar CH2CH3); IR cm–1: 3486, 3428, 3381, 3300, 3277, 3239, 3190, 3148, 3043, 2959, 2921, 2865, 1634, 1515, 1457, 1418, 1326, 1255, 1136, 1035, 993, 804, 733, 670, 603, 578, 541; MS (ESI) m/z: [M + 1]+ 491.1, exact mass: 490.2943; elemental analysis calculated for (C28H36N4O4): C, 70.99; H, 7.81; N, 11.42; found: C, 70.95; H, 7.82; N, 11.45.
(E)-N-(4-((1-(3,4-Dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-8-methylnon-6-enamide (14p)
Creamish solid, 97.0% yield; m.p. = 109.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.05 (s, 1H), 7.53 (s, 1H), 7.43–7.41 (d, J = 8.0 Hz, 1H), 7.29–7.28 (d, J = 2.4 Hz, 1H), 7.06–7.04 (d, J = 8.4 Hz, 1H), 6.87 (s, 1H), 6.83–6.81 (d, J = 8.0 Hz, 1H), 5.73 (s, 1H), 5.43–5.30 (m, 4H), 4.40–4.39 (d, J = 5.6 Hz, 2H), 3.89 (s, 3H), 2.37–2.34 (d, J = 8.4 Hz, 6H), 2.26–2.21 (t, J = 7.6 Hz, 2H), 2.04–1.99 (q, J = 6.8 Hz, 1H), 1.72–1.64 (m, 2H), 1.45–1.28 (m, 4H), 0.98–0.06 (d, J = 6.8 Hz, 4H), 0.89–0.87 (d, J = 6.8 Hz, 2H); IR cm–1: 3494, 3461, 3425, 3384, 3360, 3300, 3248, 3140, 3056, 3028, 2927, 2876, 1633, 1512, 1461, 1414, 1336, 1258, 1217, 1136, 1041, 990, 878, 848, 807, 699, 634, 596; MS (ESI) m/z: [M + 1]+ 493.1; exact mass: 490.2943; elemental analysis calculated for (C28H36N4O4): C, 70.99; H, 7.81; N, 11.42; found: C, 70.98; H, 7.85; N, 11.45.
4-Hydroxy-3-methoxybenzaldehyde Oxime (16a)
A solution of 1 g of hydroxylamine hydrochloride and sodium acetate trihydrate (2 equiv) was dissolved in 25 mL of water. Further on, vanillin (15, 0.9 equiv) was added to the reaction mixture and refluxed for 10 min. After the completion of the reaction monitored by TLC, the reaction mixture was brought to room temperature. The solid precipitate formed was filtered off under a vacuum and washed with 10 mL of cold water that led to the colorless solid in pure form in 76.4% quantitative yield. Melting point (m.p.) = 79.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 9.10 (s, 1H), 8.03 (s, 1H), 7.69 (s, 1H), 7.10–7.04 (m, 1H), 6.87 (d, J = 8.4 Hz, 1H), 3.86 (s, 3H); exact mass: 167.06, elemental analysis calculated for (C8H9NO3): C, 57.48; H, 5.43; N, 8.38; found: C, 57.47; H, 5.45; N, 8.37.
4-Hydroxy-3-methoxy-phenylamine (16b)
A solution of 1 g of 4-hydroxy-3-methoxy-benzaldehyde oxime (16a) in 5 mL of acetic acid was cooled to 10–15 °C. Then 4.71 g (4 equiv) of activated zinc dust was added to the reaction mixture and stirred at ambient temperature for a further 3 h. Completion of the reaction was monitored by TLC, and the reaction mixture was filtered off to remove excess zinc. Further on, the filtrate was collected and neutralized with ammonia to yield a white solid precipitate. The precipitate formed was filtered under a vacuum and washed with 20 mL of cold water to afford the required compound (16b) in pure form (74.0% yield) that was directly used for the next step without any further purification. 1H NMR (400 MHz, CDCl3, ppm) δ 6.83 (ddt, J = 8.8, 1.7, 0.9 Hz, 1H), 6.82–6.76 (m, 2H), 6.61 (s, 1H), 3.93 (tt, J = 6.2, 0.9 Hz, 2H), 3.85 (s, 2H), 1.39 (d, J = 12.5 Hz, 1H). Chemical formula: C8H11NO2, elemental analysis: C, 62.73; H, 7.24; N, 9.14. found C, 62.76; H, 7.29; N, 9.19.
General Procedure for N-(4-Hydroxy-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (16c)
To the DMF (10 mL) solution of R-(+)-citronellic acid (1.2 equiv), 2 equiv of (3-dimethylamino-propyl)-ethyl-carbodiimide hydrochloride (EDC·HCl) and 0.2 equiv of hydroxybenzotriazole (HOBt) were added, and the reaction mixture was stirred for 5 min under an inert atmosphere in a two-neck round-bottom flask. Finally, the compound (16b) was added in the reaction mixture to continue at room temperature overnight. The completion of the reaction was monitored by TLC; the reaction mixture was poured into ice-cold water and extracted with ethyl acetate (4 × 20 mL). The combined organic layers were dried over anhydrous sodium sulfate, filtered, and evaporated under a vacuum to afford the impure reaction mixture that was purified by column chromatography (silica gel) to obtain the pure natural product hybrid amide. 1H NMR (400 MHz, CDCl3, ppm) δ 6.86–6.81 (m, 2H), 6.78 (m, 1H), 6.57 (s, 1H), 5.12–5.04 (m, 1H), 4.38 (m, 2H), 3.85 (s, 2H), 2.49 (d, J = 16.2 Hz, 1H), 2.25 (d, J = 16.2 Hz, 1H), 2.10–2.00 (m, 1H), 2.03–1.80 (m, 2H), 1.66 (m, 3H), 1.53 (m, 1H), 1.27 (m, 1H), 0.97 (d, J = 8.1 Hz, 3H); exact mass: 305.20; elemental analysis calculated for (C18H27NO3): C, 70.79; H, 8.91; N, 4.59; O, 15.72; found: C, 70.77; H, 9.93; N, 4.57.
N-(3-Methoxy-4-(prop-2-yn-1-yloxy)benzyl)-3,7-dimethyloct-6-enamide (17)
A solution of 0.5 g compound (16c) was dissolved in 10 mL of dry acetone followed by the addition of activated K2CO3 (5 equiv). Then propargyl bromide (1.2 equiv) was added to it and refluxed for 4–5 h. After the completion of the reaction (monitored by TLC), the reaction mixture was allowed to attain room temperature and poured into ice-cold water to form a colorless solid that afterward was filtered off under a vacuum to afford the desired product. 1H NMR (400 MHz, CDCl3, ppm) δ 6.97–6.95 (d, J = 8.1 Hz, 1H), 6.95 (s, 1H), 6.83–6.79 (d, J = 18.2 Hz, 1H), 5.92–5.89 (d, J = 16.2 Hz, 1H), 5.07 (brs, 1H), 4.73 (s, 2H), 4.67 (s, 1H), 4.40–4.39 (d, J = 5.5 Hz , 1H), 4.37–4.36 (d, J = 5.4 Hz, 2H), 3.84 (s, 3H), 2.50 (s, 1H), 2.33 (s, 1H), 2.24–2.15 (m, 2H), 2.03–1.94 (m, 2H), 1.66 (s, 3H), 1.58 (s, 3H), 1.40–1.33 (m, 1H), 1.24–1.18 (m, 1H), 0.95–0.94 (d, J = 8.1 Hz, 3H); IR cm–1: 3280, 3075, 2964, 2924, 2851, 2116, 1638, 1596, 1553, 1513, 1453, 1420, 1378, 1265, 1219, 1140, 1022; exact mass: 343.21, elemental analysis calculated for (C21H29NO3): C, 73.44; H, 8.51; N, 4.08; O, 13.97; found: C, 73.43; H, 8.54; N, 4.06.
General Procedure of Formation of the 1,2,3-Triazole Ring (18a–o)
Same as the General Procedure for Synthesis of the 1,2,3-Triazole Ring (14a–p).
(R)-(+)-N-(3-Methoxy-4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-3,7-dimethyloct-6-enamide (18a)
Colorless solid, 114.5 mg, 79.3% yield; m.p. = 145.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.81 (s, 1H), 7.74–7.66 (m, 2H), 7.56–7.47 (m, 2H), 7.23–7.14 (m, 1H), 7.11–7.10 (t, J = 5.3 Hz, 1H), 6.89–6.88 (d, J = 8.1 Hz, 1H), 6.87–6.77 (m, 2H), 5.31 (d, J = 8.1 Hz, 2H), 5.08 (m, 1H), 4.38 (d, J = 8.1 Hz, 2H), 3.86 (s, 3H), 2.40 (d, J = 16.1 Hz, 1H), 2.18 (m, 1H), 2.12–2.01 (m, 1H), 2.01–1.85 (m, 2H), 1.66 (m, 3H), 1.63–1.52 (m, 4H), 1.30 (m, 1H), 0.96 (d, 8.1 Hz, 3H). 13C NMR (100 MHz, CDCl3, ppm) δ 173.9(C=O), 149.4(vanillyl Ar C-OCH3), 146.8(vanillyl Ar C-OCH2-), 146.7(triazolyl tertiary C), 136.1(Ar C), 133.2(vanillyl Ar C-CH2-NH-), 131.1(CH=C(CH3)2), 130.1(Ar C), 127.9(Ar C), 124.1(-CH=C(CH3)2), 121.0(vanillyl Ar C), 120.3(triazolyl -CH=C-N-), 119.2(Ar C), 114.8(vanillyl Ar C), 112.0(vanillyl Ar C), 58.1(-OCH2-), 55.1(-OCH3), 44.2(-CH2NH-), 36.4(-COCH2-), 30.1(-CH2CH(CH3)CH2-), 26.1(-CH2CHC(CH3)2), 24.6(CH=CHCH3), 21.6(-CH2CH2CHC(CH3)2), 21.1(-CH=CHCH3); IR cm–1: 3313, 3151, 3070, 2960, 2915, 2851, 2366, 1630, 1598, 1572, 1545, 1511, 1457, 1388, 1267, 1220, 1145, 1013; MS (ESI) m/z: [M + 1]+ 463.25, exact mass: 462.2630, elemental analysis calculated for (C27H34N4O3): C, 70.10; H, 7.41; N, 12.11; found: C, 70.13; H, 7.40; N, 12.14.
(R)-N-(4-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18b)
Light yellow solid, 112.3 mg, 77.5% yield; m.p. = 132.5 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.80 (s, 1H), 7.53–7.47 (m, 2H), 7.35 (d, J = 8.1 Hz, 2H), 7.11–7.10 (t, J = 5.3 Hz, 1H), 6.89–6.88 (s, 1H), 6.87–6.77 (m, 2H), 5.31 (s, 2H), 5.12–5.04 (brs, 1H), 4.38 (d, J = 8.1 Hz, 2H), 3.86 (s, 3H), 2.40 (d, J = 16.1 Hz, 1H), 2.18 (d, J = 16.1 Hz, 1H), 2.12–2.01 (m, 1H), 2.01–1.85 (m, 2H), 1.66 (m, 3H), 1.63–1.52 (m, 4H), 1.30 (m, 1H), 0.96 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 173.93(C=O), 149.6(vanillyl Ar C-OCH3), 146.6(vanillyl Ar C-OCH2-), 145.1(triazolyl tertiary C), 134.6(Ar C), 133.2(vanillyl Ar C-CH2-NH-), 131.3(C=C(CH3)2), 129.3(Ar C), 123.9(-CH=C(CH3)2), 121.8(Ar C), 121.2(vanillyl Ar C), 120.3(triazolyl -CH=C-N-), 114.2(vanillyl Ar C), 111.8(vanillyl Ar C), 60.2(-OCH2-), 56.2(-OCH3), 45.4(-CH2NH-), 37.5(-COCH2-), 31.8(-CH2CH(CH3)CH2-), 25.7(-CH=CHCH3), 24.1(-CH2CHC(CH3)2), 22.2(-CH=CHCH3); IR cm–1: 3265, 3144, 3061, 2978, 2958, 2866, 2363, 1665, 1583, 1574, 1540, 1501, 1455, 1386, 1255, 12,359, 1120, 1025; MS (ESI) m/z: [M + 1]+ 497.22, exact mass: 496.2241, elemental analysis calculated for (C27H33ClN4O3): C, 65.25; H, 6.69; N, 11.27; found: C, 65.27; H, 6.70; N, 11.27.
(R)-(+)-N-(4-((1-(3-Chlorophenyl)-1H-1,2,3-triazol-4-yl) methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18c)
White, 123.3 mg, 85.14% yield; m.p. = 137.0 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.07 (s, 1H), 7.78 (s, 1H), 7.63–7.61 (d, J = 7.9 Hz, 1H), 7.48–741(m, 2H), 7.01–6.99 (d, J = 8.1 Hz, 1H), 6.85(s, 1H), 6.80–6.78 (d, J = 8.1 Hz, 1H), 5.67 (brs, 1H), 5.35 (s, 2H), 5.09–5.05 (t, J = 7.0 Hz, 1H) 4.39–4.38 (d, J = 4.1 Hz, 2H), 3.86 (s, 3H), 2.25–2.21 (q, J = 8.1 Hz, 2H), 2.03–1.93 (m, 3H), 1.66 (s, 3H), 1.58 (s, 3H), 1.40–1.18 (m, 2H), 0.96–0.94 (d, 3H); 13C NMR (100 MHz, CDCl3 ppm) δ 171.9(C=O), 149.2(vanillyl Ar C-OCH3), 146.3(triazolyl tertiary C), 137.3(Ar C), 135.1(Ar C), 132.0(Ar C), 131.1(vanillyl Ar C-CH2-NH-), 130.4(-CH=C(CH3)2), 128.5(Ar C), 123.8(-CH=C(CH3)2), 120.3(triazolyl -CH=C-N-), 119.5(Ar C), 118.0(Ar C), 113.6(vanillyl Ar C), 111.1(vanillyl Ar C), 62.8(-OCH2-), 55.4(-OCH3), 44.1(-CH2NH-), 42.8(-COCH2-), 36.4(-CH2CH(CH3)CH2-), 30.0(-CH2CH(CH3)CH2-), 25.2(-CH2CHC(CH3)2), 25.0(-CH=CHCH3), 19.1(-CH2CH2CHC(CH3)2), 17.2(CH=CHCH3); IR cm–1: 3311, 3152, 3067, 2957, 2918, 2856, 2361, 1635, 1594, 1578, 1540, 1516, 1456, 1381, 1262, 1229, 1140, 1011; MS (ESI) m/z: [M + 1]+ 497.50; exact mass: 496.2241; elemental analysis calculated for (C27H33ClN4O3): C, 65.25; H, 6.69; Cl, 7.13; N, 11.27; found C, 65.21; H, 6.72; N, 11.26.
(R)-(+)-N-(4-((1-(2-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18d)
White solid, 110.5 mg, 76.30% yield; m.p. = 102.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.06 (s, 1H), 7.62–7.56 (m, 2H), 7.46–7.44 (m, 2H), 7.04 (d, J = 8.2 Hz, 1H), 6.84(s, 1H), 6.81–6.79 (d, J = 8.3 Hz, 2H), 5.65 (brs, 1H), 5.37 (s, 2H), 5.09–5.05 (t, J = 7.0 Hz, 2H), 4.39–4.38 (d, J = 4.4 Hz, 2H), 3.85 (s, 3H), 2.24–2.21 (q, J = 8.1 Hz, 2H), 2.03–1.93 (m; 3H), 1.66 (s, 3H), 1.57 (s, 3H), 1.40–1.18 (m, 2H), 0.96–0.94 (d, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 171.9(C=O), 149.4(vanillyl Ar C-OCH3), 146.4(vanillyl Ar C-OCH2-), 143.5(triazolyl tertiary C), 134.3(Ar C), 131.9(Ar C), 131.1(vanillyl Ar C-CH2-NH-), 130.4(-CH=C(CH3)2), 130.3(Ar C), 128.1(Ar C), 127.5(Ar C), 127.3(vanillyl Ar C), 124.7(-CH=C(CH3)2), 123.8(triazolyl -CH=C-N-), 119.6(Ar C), 114.1(vanillyl Ar C), 111.2(vanillyl Ar C), 62.8(-OCH2-), 55.4(-OCH3), 44.1(-CH2NH-), 42.8(-COCH2-), 36.4(-CH2CH(CH3)CH2-), 30.0(-CH2CH(CH3)CH2-), 25.2(-CH2CHC(CH3)2), 25.0(CH=CHCH3), 19.1(-CH2CH2CHC(CH3)2), 17.2(CH=CHCH3); MS (ESI) m/z: [M + 1]+ 497.50; exact mass: 496.2241; elemental analysis calculated for (C27H33ClN4O3): C, 65.25; H, 6.69; Cl, 7.13; N, 11.27; found C, 65.23; H, 6.71; N, 11.25.
(R)-(+)-N-(4-((1-(2,4-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18e)
White crystalline, 114.6 mg, 74.0% yield; m.p. = 1472.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.37 (s, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.40–7.39 (d, J = 8.1 Hz, 1H), 7.30 (s, 1H), 7.11–7.10 (t, J = 8.1 Hz, 1H), 6.89–6.87 (d, J = 8.1 Hz, 1H), 6.87–6.77 (m, 2H), 5.31 (d, J = 8.1 Hz, 2H), 5.12–5.04 (brs, 1H), 4.38–4.37 (d, J = 8.1 Hz, 2H), 3.86 (s, 3H), 2.40 (d, J = 16.1 Hz, 1H), 2.18 (d, J = 16.2 Hz, 1H), 2.12–2.01 (m, 1H), 2.01–1.85 (m, 2H), 1.66 (m, 3H), 1.63–1.52 (m, 4H), 1.30 (m, 1H), 0.96–0.94 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 173.3(C=O), 149.0(vanillyl Ar C-OCH3), 146.4(vanillyl Ar C-OCH2-), 146.0(triazolyl tertiary C), 134.1(Ar C), 133.5(Ar C), 131.2(vanillyl Ar C-CH2-NH-), 130.1(-CH=C(CH3)2), 129.7(Ar C), 128.0(Ar C), 124.2(-CH=C(CH3)2), 122.1(Ar C), 121.2(Ar C), 120.1(triazolyl -CH=C-N-), 114.4(vanillyl Ar C), 112.1(vanillyl Ar C), 59.5(-OCH2-), 55.7(-OCH3), 44.0(-CH2NH-), 36.0(COCH2-), 30.4(-CH2CH(CH3)CH2-), 26.1(-CH2CHC(CH3)2), 24.9(CH=CHCH3), 20.8(-CH2CH2CHC(CH3)2), 20.5(CH=CHCH3); IR cm–1: 3282, 3076, 2954, 2922, 2850, 2360, 1636, 1596, 1553, 1516, 1491, 1491, 1460, 1376, 1265, 1234, 1141, 1044, 1031; LC–MS m/z: [M + 1]+ 531.50; exact mass: 530.1851; elemental analysis calculated for (C27H32Cl2N4O3): C, 61.02; H, 6.07; Cl, 13.34; N, 10.54; found C, 61.01; H, 6.09; N, 10.55.
(R)-(+)-N-(4-((1-(3,4-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18f)
White crystalline, 115.6 mg, 74.7% yield; m.p. = 152.5 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.06 (s, 1H), 7.89 (s, 1H), 7.60 (s, 2H), 7.00–6.98 (d, J = 50.4 Hz, 1H), 6.85 (s, 1H), 6.80–6.78 (d, J = 7.9 Hz, 1H), 5.72 (brs, 1H), 5.33–5.31 (t, J = 8.0 Hz, 2H), 4.38–4.37 (d, J = 4.0 Hz, 2H), 3.86 (s, 3H), 2.24–2.21 (q, J = 6.0 Hz, 2H), 1.99–1.94 (m, 3H), 1.66 (s, 3H), 1.58 (s, 3H), 1.38–1.18 (m, 2H), 0.95–0.94 (d, J = 5.7 Hz, 3H); 13C NMR (100 MHz, CDCl3 ppm) δ 172.5(C=O), 149.8(vanillyl Ar C-OCH3), 146.8(vanillyl Ar C-OCH2-), 136.0(Ar C), 134.1(Ar C), 133.1(Ar C), 132.6(Ar C), 131.7(vanillyl Ar C-CH2-NH-), 131.6(-CH=C(CH3)2), 124.4(-CH=C(CH3)2), 122.4(Ar C), 121.1(vanillyl Ar C), 120.1(triazolyl -CH=C-N-), 119.5(Ar C), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.1(-OCH2-), 56.0(-OCH3), 44.7(-CH2NH-), 43.4(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.9(-CH2CHC(CH3)2), 25.5(-CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); IR cm–1: 3282, 3076, 2954, 2922, 2850, 2360, 1636, 1596, 1553, 1516, 1491, 1491, 1460, 1376, 1265, 1234, 1141, 1044, 1031; LC–MS m/z: [M+]+ 531.50; exact mass: 530.1851; elemental analysis calculated for (C27H32Cl2N4O3): C, 61.02; H, 6.07; Cl, 13.34; N, 10.54; found C, 61.05; H, 6.06; N, 10.56.
(R)-(+)-N-(3-Methoxy-4-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-3,7-dimethyloct-6-enamide (18g)
Light yellow solid, 122.8 mg, 83.1%; m.p. = 151.0 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.43–8.40 (d, J = 9.2 Hz, 2H), 8.19 (s, H), 7.98–7.96 (d, J = 9.0 Hz, 2H), 7.01–6.99 (d, J = 8.1 Hz, 1H), 6.86 (s, 1H), 6.81–6.79 (d, J = 8.1 Hz, 1H), 5.68 (brs, 1H), 5.36 (s, 2H), 5.08–5.05 (t, J = 7.2 Hz, 6.8 Hz, 1H), 4.39–4.38 (d, J = 4.4 Hz, 2H), 3.87 (s, 3H), 2.24–2.21 (m, 2H), 2.03–1.94 (m, 3H), 1.66 (s, 3H), 1.68 (m, 3H), 1.38–1.19 (m, 2H), 0.96–0.94 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 149.8(vanillyl Ar C-OCH3), 147.4(vanillyl Ar C-OCH2-), 146.7 (triazolyl tertiary C), 141.1(Ar C), 132.8(Ar C), 131.7(vanillyl Ar C-CH2-NH-), 125.7(-CH=C(CH3)2), 124.4(-CH=C(CH3)2), 121.2(Ar C), 120.6(vanillyl Ar C), 120.1(triazolyl -CH=C-N-), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.1(-OCH2-), 56.0(-OCH3), 44.6(-CH2NH-), 43.3(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.6(CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); IR cm–1: 3281, 3074, 2957, 2917, 2872, 2228, 1633, 1607, 1564, 1155, 1523, 1462, 1264, 1235, 1141, 1044, 1028; LC–MS m/z: [M + 1]+ 508.60; exact mass: 507.2482; elemental analysis calculated for (C27H32Cl2N4O3): C, 63.89; H, 6.55; N, 13.80; found C, 63.86, H, 6.53; N, 13.79.
(R)-(+)-N-(3-Methoxy-4-((1-(3-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-3,7-dimethyloct-6-enamide (18h)
Light yellow solid, 132.1 mg, 89.4%; m.p. = 154.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.59 (s, 1H), 8.32–8.30 (d, J = 8.2 Hz, 1H), 8.20 (s, 1H), 8.18–8.16 (d, J = 8.1 Hz, 1H), 7.77–7.73 (t, J = 8.0 Hz, 8.0 Hz, 1H), 7.01–6.99 (d, J = 8.1 Hz, 1H), 6.86 (s, 1H), 6.81–6.79 (d, J = 8.4 Hz, 1H), 5.70 (brs, 1H), 5.36 (s, 2H), 5.08–5.05 (t, J = 6.4 Hz, 6.6 Hz,1H), 4.39–4.38 (d, J = 4.4 Hz, 2H), 3.87 (s, 3H), 2.25–2.21 (d, J = 4.4 Hz, 2H), 2.05–1.94 (m, 3H), 1.66 (s, 3H), 1.58 (s, 3H), 1.40–1.18 (m, 2H), 0.96–0.94 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 149.8(vanillyl Ar C-OCH3), 149.0(vanillyl Ar C-OCH2-), 146.8 (triazolyl tertiary C), 137.7(Ar C), 132.7(Ar C), 131.7(vanillyl Ar C-CH2-NH-), 131.1(Ar C-NO2), 126.1(-CH=C(CH3)2), 124.4(-CH=C(CH3)2), 123.4(Ar C), 120.1(triazolyl -CH=C-N-), 115.4(Ar C), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.1(-OCH2-), 56.0(-OCH3), 44.6(-CH2NH-), 43.4(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.6(CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); IR cm–1: 3282, 3160, 3077, 2959, 2922, 2872, 1663, 1595, 1522, 1514, 1463, 1349, 1262, 1233, 1160, 1140, 1040; LC–MS m/z: [M + 1]+ 508.55; exact mass: 507.2482; elemental analysis calculated for (C27H32Cl2N4O3): C, 63.89; H, 6.55; N, 13.80; found C, 63.87; H, 6.57; N, 13.78.
(R)-(+)-N-(3-Methoxy-4-((1-(2-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzyl)-3,7-dimethyloct-6-enamide (18i)
Light yellow solid, 135.5 mg, 91.7% yield; m.p. = 86.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.09–8.07 (d, J = 7.0 Hz, 1H), 7.93 (s, 1H), 7.81–7.79 (t, J = 6.5 Hz, 1H), 7.72–7.68 (t, J = 7.7, 6.8 Hz, 1H), 7.63–7.61 (d, J = 8.1 Hz, 1H), 7.02–7.00 (d, J = 8.1 Hz, 1H), 6.85 (s, 1H), 6.81–6.79 (d, J = 8.2 Hz, 1H), 5.70 (brs, 1H), 5.37 (s, 2H), 5.09–5.05 (t, J = 6.8, 7.1 Hz, 1H), 4.39–4.37 (d, J = 3.9 Hz, 2H), 3.85 (s, 3H), 2.26–2.21 (d, J = 7.9 Hz, 2H), 2.03–1.94 (m, 3H), 1.66 (s, 3H), 1.58 (s, 3H), 1.39–1.18 (m, 2H), 0.96–0.94 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 149.9(vanillyl Ar C-OCH3), 146.8(vanillyl Ar C-OCH2-), 145.0(triazolyl tertiary C), 144.5(Ar C-NO2), 134.0(Ar C), 132.7(Ar C), 131.6(vanillyl Ar C-CH2-NH-), 131.0(Ar C), 130.3(-CH=C(CH3)2), 128.1(vanillyl Ar C), 125.7(Ar C), 124.7(Ar C), 124.4(-CH=C(CH3)2), 120.2(triazolyl -CH=C-N-), 114.8(vanillyl Ar C), 111.8(vanillyl Ar C), 63.2(-OCH2-), 56.0(-OCH3), 44.6(-CH2NH-), 43.4(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.6(CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); IR cm-1: 3082 2958, 2850, 1633, 1611, 1565, 1548, 1533, 1464, 1420, 1343, 1266, 1232, 1145, 1090, 1042, 1020, 1005; LC–MS m/z: [M + 1]+ 508.55; HRMS m/z: [M + 1]+ 508.55 exact mass: 507.2482; elemental analysis calculated for (C27H32Cl2N4O3): C, 63.82; H, 6.59; N, 13.88; found C, 63.86; H, 6.57; N, 13.91.
(R)-(+)-N-(3-Methoxy-4-((1-(3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methoxy) benzyl)-3,7-dimethyloct-6-enamide (18j)
White solid, 126.4 mg, 81.8% yield; m.p. =162.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.14 (s, 1H), 8.02 (s, 1H), 7.96–7.94 (d, J = 7.7 Hz, 1H), 7.72–7.65 (m, 2H), 7.02–7.00 (d, J = 8.1 Hz, 1H), 6.86 (s, 1H), 6.81–6.79 (d, J = 8.1 Hz, 1H), 5.67 (brs, 1H), 5.36 (s, 2H), 5.08–5.05 (t, J = 7.0 Hz, 1H), 4.39–4.38 (d, J = 3.9 Hz, 2H), 3.87 (s, 3H), 2.25 (d, J = 7.9 Hz, 2H), 2.03–1.93 (m, 3H), 1.66 (s, 3H) 1.58 (s, 3H), 1.38–1.19 (m, 2H), 0.96–0.94 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 149.8(vanillyl Ar C-OCH3), 146.8(vanillyl Ar C-OCH2-), 137.4(triazolyl tertiary C), 132.6(Ar C), 131.7(vanillyl Ar C-CH2-NH-), 130.7(-CH=C(CH3)2), 125.6(Ar C), 125.58(vanillyl Ar C), 124.4(-CH=C(CH3)2), 123.7(Ar C), 121.2(Ar C), 120.1(triazolyl -CH=C-N-), 117.6(Ar C), 117.57(Ar C), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.2(-OCH2-), 56.0(-OCH3), 44.7(-CH2NH-), 43.4(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.6(CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); LC–MS m/z: [M + 1]+ 531.60; HRMS m/z: [M + 1]+ 531.2582, exact mass: 530.2505; elemental analysis calculated for (C28H33F3N4O3): C, 63.38; H, 6.27; N, 10.56; found C, 63.41; H, 6.28; N, 10.59.
(R)-(+)-N-(4-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18k)
White solid, 126.4 mg, 81.8% yield; m.p. =162.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.81 (s, 1H), 7.62–7.55 (m, 2H), 7.15–7.07 (m, 2H), 6.89 (dt, J = 1.7, 0.8 Hz, 1H), 6.87–6.77 (m, 2H), 5.31–5.32 (d, J = 8.1 Hz, 2H), 5.08 (brs, 1H), 4.38–4.37 (d, J = 8.1 Hz, 2H), 3.86 (s, 3H), 2.40 (d, J = 16.1 Hz, 1H), 2.18 (d, J = 16.2 Hz, 1H), 2.12–2.01 (m, 1H), 2.01–1.85 (m, 2H), 1.66 (m, 3H), 1.63–1.52 (m, 2H), 1.30 (m, 1H), 0.96 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 165.1(Ar C-F), 163.3(Ar C), 148.4(vanillyl Ar C-OCH3), 147.5(triazolyl tertiary C), 146.1(vanillyl Ar C-OCH2-), 133.8(Ar C), 132.1(vanillyl Ar C-CH2-NH-), 131.4(-CH=C(CH3)2), 124.3(-CH=C(CH3)2), 122.9(Ar C), 121.5(triazolyl -CH=C-N-), 119.9(Ar C), 117.2(Ar C), 117.11(vanillyl Ar C), 114.6(vanillyl Ar C), 112.2(vanillyl Ar C), 59.1(-OCH2-), 55.8(-OCH3), 44.2(-CH2NH-), 36.2(-COCH2-), 30.1(-CH2CH(CH3)CH2-), 26.1(-CH2CHC(CH3)2), 24.6(CH=CHCH3), 20.6(-CH2CH2CHC(CH3)2), 20.17(CH=CHCH3); IR cm–1: 3325, 3122, 3075, 2969, 2904, 2841, 2365, 1639, 1595, 1569, 1533, 1505, 1447, 1393, 1261, 1214, 1145, 1010; MS (ESI) m/z: [M + 1]+ 481.26; exact mass: 480.2536; elemental analysis calculated for (C28H33F3N4O3): C, 63.38; H, 6.27; N, 10.56; found C, 63.41, H, 6.28; N, 10.59.
(R)-(+)-N-(4-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18l)
Pale yellow solid, 111.9 mg, 80.0% yield; m.p. = 119.1 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.18 (s, 1H), 7.96–7.93 (t, J = 7.4 Hz, 1H), 7.46–7.29 (m, 3H), 7.04–7.02 (d, J = 8.2 Hz, 1H), 6.84 (s, 1H), 6.80–6.78 (d, J = 8.1 Hz, 1H), 5.68 (brs, 1H), 5.36 (s, 2H), 5.07–5.05 (t, J = 6.7 Hz, 1H), 4.38–4.37 (d, J = 4.6 Hz, 2H), 3.85 (s, 3H), 2.25–2.21 (dd, J = 8.4 Hz, 2H), 2.01–1.93 (m, 3H), 1.66 (s, 3H) 1.58 (s, 3H), 1.42–1.18 (m, 2H), 0.95–0.94 (d, J = 6.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 154.7(Ar C-F), 152.2(triazolyl tertiary C), 149.9(vanillyl Ar C-OCH3), 146.9(vanillyl Ar C-OCH2-), 132.5(Ar C), 131.6(vanillyl Ar C-CH2-NH-), 130.5(Ar C), 130.4(-CH=C(CH3)2), 125.4(vanillyl Ar C), 125.3(Ar C), 125.2(Ar C), 124.9(Ar C), 124.4(-CH=C(CH3)2), 120.1(triazolyl -CH=C-N-), 117.2(Ar C), 117.0(Ar C), 114.5(vanillyl Ar C), 111.7(vanillyl Ar C), 63.2(OCH2), 55.9(-OCH3), 44.6(-CH2NH-), 43.4(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.5(CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.7(CH=CHCH3); IR cm-1: 3453, 3289, 3147, 3065, 2965, 2916, 2873, 2362, 1636, 1598, 1545, 1516, 1465, 1378, 1261, 1234, 1163, 1135, 1040; LC–MS m/z: [M + 1]+ 481.25; exact mass: 480.2537; elemental analysis calculated for (C27H33FN4O3): C, 67.48; H, 6.92; N, 11.66; found C, 67.47; H, 6.95; N, 11.65.
(R)-(+)-N-(4-((1-(4-Cyanophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18m)
White solid, 108.5 mg, 76.4% yield; m.p. = 140.0 °C, 1H NMR (400 MHz, CDCl3, ppm) δ 8.16 (s, 1H), 7.92–7.90 (d, J = 8.6 Hz, 1H), 7.84–7.82 (d, J = 8.5 Hz, 2H), 7.00–6.98 (d, J = 8.2 Hz, 1H), 6.85 (s, 1H), 6.80–6.78 (d, J = 8.1 Hz, 1H), 5.75 (brs, 1H), 5.34 (s, 2H), 5.08–5.05 (t, J = 6.9 Hz, 1H), 4.39–4.37 (d, J = 4.4 Hz, 2H), 3.86 (s, 3H), 2.25–2.21 (d, J = 8.4 Hz, 2H), 2.04–1.94 (m, 3H), 1.70 (s, 3H) 1.58 (s, 3H), 1.38–1.18 (m, 2H), 0.96–0.94 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.6(C=O), 149.8(vanillyl Ar C-OCH3), 146.7(vanillyl Ar C-OCH2-), 145.9(triazolyl tertiary C), 139.8(Ar C), 134.0(Ar C), 132.7(vanillyl Ar C-CH2-NH-), 131.7(-CH=C(CH3)2), 124.3(-CH=C(CH3)2), 121.0(vanillyl Ar C), 120.7(Ar C), 120.0(triazolyl -CH=C-N-), 117.8(Ar-CN), 114.2(vanillyl Ar C), 112.6(Ar C), 111.6(vanillyl Ar C), 63.0(-OCH2-), 56.0(-OCH3), 44.6(-CH2NH-), 43.3(-COCH2-), 37.0(-CH2CH(CH3)CH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.5(CH=CHCH3), 19.6(-CH2CH2CHC(CH3)2), 17.7(CH=CHCH3); IR cm–1: 3280, 3068, 2918, 2873, 1639, 1595, 1557, 1516, 1454, 1377, 1321, 1266, 1234, 1220, 1137, 1044; LC–MS m/z: [M + 1]+ 488.25; exact mass: 487.2583; elemental analysis calculated for (C28H33N5O3): C, 68.97; H, 6.82; N, 14.36; found C, 68.99; H, 6.84; N, 14.39.
(R)-(+)-N-(4-((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18n)
White solid, 116.8 mg, 74.0% yield; m.p. = 154.7 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.05 (s, 1H), 7.66–7.60 (d, J = 12.8 Hz, 4H), 7.02–7.00 (d, J = 8.1 Hz, 1H), 6.85 (s, 1H), 6.80–6.78 (d, J = 8.2 Hz, 1H), 5.66 (brs, 1H), 5.34 (s, 2H), 5.08–5.04 (t, J = 6.9 Hz, 1H), 4.38–4.37 (d, J = 4.2 Hz, 2H), 3.86 (s, 3H), 2.24–2.20 (d, J = 6.3 Hz, 2H), 2.03–1.93 (m, 3H), 1.66 (s, 3H), 1.58 (m, 3H), 1.38–1.18 (m, 2H), 0.95–0.94 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3 ppm) δ 172.5(C=O), 149.8(vanillyl Ar C-OCH3), 146.8(vanillyl Ar C-OCH2-), 145.4(triazolyl tertiary C), 136.0(Ar C), 133.1(Ar C), 132.5(vanillyl Ar C-CH2-NH-), 131.7(-CH=C(CH3)2), 124.4(-CH=C(CH3)2), 122.7(Ar C-Br), 122.0(Ar C), 121.1(vanillyl Ar C), 120.1(triazolyl -CH=C-N-), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.1(-OCH2), 56.0(-OCH3), 44.7(-CH2NH-), 43.4, 37.0(-COCH2-), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.6(CH=CHCH3), 19.7(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); IR cm–1: 3277, 3174, 3068, 2949, 2918, 2846, 2360, 1640, 1595, 1553, 1468, 1451, 1265, 1235, 1137, 1044, 1044, 1031, 1021; LC–MS m/z: [M + 3]+ 543.50; exact mass: 540.1736; elemental analysis calculated for (C27H33BrN4O3): C, 59.89; H, 6.14; N, 10.35; found C, 59.93; H, 6.11; N, 10.32.
(R)-(+)-N-(4-((1-(3,4-Dimethylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-3-methoxybenzyl)-3,7-dimethyloct-6-enamide (18o)
White solid, 130.2 mg, 91.1% yield; m.p. = 91.3 °C; 1H NMR (400 MHz, CDCl3, ppm) δ 8.03 (s, 1H), 7.50 (s, 1H), 7.40–7.38 (d, J = 7.9 Hz, 1H), 7.23 (s, 1H), 7.03–7.01 (d, J = 8.3 Hz, 1H), 6.84 (s, 1H), 6.80–6.78 (d, J = 8.2 Hz, 1H), 5.71 (brs, 1H), 5.33 (s, 2H), 5.08–5.05 (t, J = 6.9 Hz, 1H), 4.38–4.37 (d, J = 4.2 Hz, 2H), 3.85 (s, 3H), 2.33 (s, 3H), 2.31 (s, 3H), 2.25–2.20 (d, J = 16.3 Hz, 1H), 2.03–1.93 (m, 3H), 1.66 (s, 3H) 1.58 (s, 3H), 1.38–1.19 (m, 2H), 0.95–0.94 (d, J = 8.1 Hz, 3H); 13C NMR (100 MHz, CDCl3, ppm) δ 172.5(C=O), 149.8(vanillyl Ar C-OCH3), 146.9(vanillyl Ar C-OCH2-), 138.5(triazolyl tertiary C), 137.8(Ar C), 134.9(Ar C), 132.4(Ar C), 131.6(vanillyl Ar C-CH2-NH-), 130.7(-CH=C(CH3)2), 124.4(-CH=C(CH3)2), 121.8(vanillyl Ar C), 120.2(triazolyl -CH=C-N-), 117.9(Ar C), 114.2(vanillyl Ar C), 111.7(vanillyl Ar C), 63.3(-OCH2), 55.9(-OCH3), 44.6(-CH2NH-), 43.4(-COCH2-), 37.0(Ar CCH3), 30.6(-CH2CH(CH3)CH2-), 25.8(-CH2CHC(CH3)2), 25.6, 20.0(CH=CHCH3), 19.64(Ar CH3), 19.58(-CH2CH2CHC(CH3)2), 17.8(CH=CHCH3); IR cm–1: 3287, 2957, 2916, 2799, 2361, 1634, 1595, 1514, 1461, 1261, 1136, 1041; LC–MS m/z: [M + 1]+ 491.60; exact mass: 490.2944; elemental analysis calculated for (C29H38N4O3): C, 70.99; H, 7.81; N, 11.42; found C, 70.95; H, 7.84; N, 11.46.
Biological Assays
In Vitro Antiproliferative Activity at a Single Dose
All the synthesized compounds were screened for their antiproliferative activity against a panel of 60 cancer cell lines at the National Cancer Institute, Bethesda, MD, USA, as per the standard procedure given at http://www.dtp.nci.nih.gov. The RPMI 1640 medium (5% fetal bovine serum and 2 mM l-glutamine) was used to grow the human tumor cell lines. All the tumor cells were incubated into a 96-well microtiter plate. Then this plate was placed for incubation at 37 °C for 24 h. After those two plates of each cell line were fixed with TCA in situ, and optical density was measured at this point, which represented the cell population of each cell line at the time of compound addition (ODtzero). On the other hand, all the tested compounds were dissolved in DMSO to yield 400-fold desired final concentration and stored at −80 °C. These frozen compounds were thawed, and their aliquot part was diluted to 10–4 M concentration with the medium containing 50 μg/mL of gentamicin at the time of compound addition. The control sample was made with DMSO only. The tested compounds (100 μL) from the aliquot parts were added to the appropriate 96-well microtiter plate containing 100 μL of the medium resulting in the required final drug concentrations of 10–5 and 0 M (control). After the addition of tested compounds, the 96-well microtiter plate was incubated for 48 h at 100%, 5% CO2, 95% air, and 100% relative humidity. Cold TCA was used to stop the assay for adherent cells. Further on, 50 mL of 50% (w/v) TCA was used to fix the cell and incubated for 1 h at 4 °C. The supernatant was removed, and the 96-well microtiter plates were rinsed five times with water and air dried. A 100 mL solution of the protein binding dye Sulforhodamine B (SRB) was made at 0.4% (w/v) in 1% acetic acid and added to each well of the plates. These plates were placed at room temperature for incubation for 10 min and then washed with 1% acetic acid five times to remove unbound dye. Then the plates were treated with 10 mM Trizma base so that unbound dye was solubilized with the Trizma base. The absorbance was measured at a wavelength of 515 nm on an automated plate reader, and results for each tested compound were calculated as the percent of tumor growth of the treated cells in comparison with the untreated control cells. Optical density (OD) was recorded for the SRB-derived color just before exposing the cells to the test compound (ODtzero) and after 48 h exposure to the test compound (ODtest) or the control vehicle (ODctrl).
Cell Lines, Cell Culture, Growth Conditions, and Reagents
A panel of human cancer cell lines, namely, MCF-7 (breast), NCI-H460, A549 (lung), MiaPaCa (pancreas), HCT-116 (colon), and PC-3 (prostate), was purchased from ATCC. The cell lines were grown in T75 tissue culture flasks in a complete growth medium (RPMI-1640 and DMEM) added with 10% FBS,100 mg/mL streptomycin, as well as 100 U/mL penicillin in a humidified carbon dioxide incubator (New Brunswick, Galaxy 170R, Eppendorf) at 37 °C and 5% CO2 with 95% relative humidity. DAPI (4′ 6-diamidino-2-phenylindole) and Rhodamine-123, DCFDA (2′, 7′-dichlorofluorescein diacetate) were procured from Sigma-Aldrich (St. Louis, MO, USA). Sulforhodamine B dye was purchased from Hi Media. Monolayer cultures of the above cell lines were trypsinized using 0.25% trypsin/EDTA (1 mM) solution. After the cells got detached, the activity of trypsin/EDTA solution was stopped using the complete growth medium and centrifuged at 900 rpm for 5 min. Cells were again dispersed in the complete growth medium in tissue culture flasks and incubated in a CO2 incubator. When cells attained approx. 50–60% confluency, they were treated with target compounds dissolved in DMSO and the untreated control cultures with DMSO (<0.2%).
Cytotoxicity Activity against Different Cancer Cell Lines
The in vitro cytotoxicity activity of target compounds (18a–o) was carried out using the SRB (Sulforhodamine B) assay method reported. For preliminary screening, optimum inoculum densities per well of A549, NCI-H460, MCF-7, and HCT-116 cell lines were seeded in 96-well flat-bottom plates (NUNC). Briefly, 100 mL/well of cell suspensions was seeded in 96-well tissue culture plates and incubated for 24 h. When cells attained 50–60% confluency, then different concentrations of anticancer test compounds were incubated and kept for another 48 h. Next, after the completion of 48 h incubation, the cells were settled using 50% ice-cold trichloroacetic acid (TCA) and kept at 4 °C for 1 h, and the plates were washed thrice in an aqueous medium and air dried. Once the plates dried, 100 mL/well SRB dye was added and kept for half an hour at room temperature. Soon after, the plates were again washed thrice using 1% glacial acetic acid to eliminate excess unbound SRB, and the plates were further air dried. When the plates were completely dried, the bound SRB was solubilized by adding 100 mL/well 10 mM TRIS (tris(hydroxymethyl) aminomethane) buffer at pH 10.5, and plates were kept on an orbital shaker for 5 min. Lastly, absorbance was recorded at 540 nm in a microplate reader (Tecan Infinite M Nano). IC50 was determined by GraphPad Prism 6.55
Reactive Oxygen Species (ROS) Generation Assay
Dye 2′,7″-dichlorofluorescein diacetate (DCFH-DA) was used to measure intracellular ROS production. DCFH gets transformed into highly fluorescent 2′,7″-dichlorofluorescein (DCF) in the presence of an oxidant. In this study, 1 × 105 A549 cells were incubated for 24 h in six-well plates and 1.5, 3, and 5 μM concentration of molecule 18f and kept for a further 48 h incubation. H2O2 (0.05%) was used as the standard positive control and added 1 h before conclusion of the experiment. The cells were washed with ice-cold PBS, and 10 mM DCFH-DA was added to all the wells for 30 min in the dark. The plate was further washed with cold PBS, and the final volume of incomplete media was added to each well and finally observed under an inverted fluorescence microscope (Olympus IX70).56,57
Apoptosis Assay through DAPI Staining
To examine apoptotic cell death qualitatively, morphological variations in chromatin structure were detected using DAPI staining. Precisely, A549 cells at a density of 1 × 105 were seeded in six-well tissue culture plates and kept for 24 h to attain confluency of about 50–60%, treatment was given to the plate with the above-mentioned concentration of compound 18f and paclitaxel used as standard positive control drug, and cells were kept for 48 h incubation. Later, cells were washed with ice-cold PBS to remove dead cell moieties. Again, cells were fixed using 70% ethanol for 1 h at room temperature and washed with cold PBS. Finally, the cells were stained with 1 mg/mL DAPI in the dark for 5 min and washed with ice-cold PBS, the final volume of PBS was added to each well, and the plate was observed under an inverted fluorescence microscope (Olympus 1X70).58
Measurement of the Loss of Mitochondrial Membrane Potential (ΔΨm)
Mitochondrial perturbation due to the loss of membrane potential was studied using dye rhodamine-123 by a fluorescence microscope qualitatively. Non-small lung cancer A549 cells (1 × 105/mL/well) were seeded in six-well tissue culture plates; treated with 1.5, 3.0, and 5.0 μM concentration of compound 18f and paclitaxel used as the standard positive control drug; and incubated for 48 h. After treatment, cells were washed with ice-cold PBS; later, the final concentration of 0.2 mM rhodamine was incubated to all the wells and finally kept for 20–30 min in the dark inside the incubator. Further, cells were washed with cold PBS, and the final volume of incomplete media was added to all the wells and analyzed under a fluorescence microscope (Olympus 1X70).59
Cell Cycle Analysis
A549 cancer cells were treated with compound 18f and analyzed for the distribution of cell population in different phases of the cell cycle. After 48 h of treatment, the cells were harvested and washed with phosphate buffer saline (PBS). The cells were then fixed with ice-cold 70% ethanol. After overnight incubation at 4 °C, the cells were washed with PBS and incubated with propidium iodide solution (20 μg/mL) containing RNase (10 μg/mL) at 37 °C for half an hour. The cells were analyzed using a BD FACSverse cytometer. A total of 10, 000 events were analyzed for each sample, and the data were analyzed using the Modfit LT software.23
Wound Healing Assay
A549 cells (4 × 105) were seeded and grown within six-well plates until a monolayer was formed. Then, by using a 200 μL pipette tip, a scratch was given in the confluent monolayer. Cells were washed with 1× PBS to remove detached cells before incubating with fresh media with different compound 18f concentrations (3, 10, and 30 μM) or DMSO vehicle control. Cell migration across the wound area was captured at two time intervals (0 and 24 h) with an inverted microscope. The wound area was measured using the ImageJ software, and the percentage wound healed area during the course of the assay was calculated after normalizing the wound area.23
Acknowledgments
We thankfully acknowledge the technical and analytical support by DIF Chemistry-Jamia Hamdard and CIF-Jamia Hamdard. A.K. would like to thank CSIR New Delhi for providing fellowship under. 09/591(0148)/2017-EMR-1. S.S. is grateful to DST for providing project under DST-SERB-ECR/2017/001067/CS.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03325.
Dose–response curve, toxicity of most potent compound 18f, migration capacities (NCI-H460), colony-forming ability (NCI-H460), 1H NMR, 13C NMR, and mass spectral data of synthesized compounds were incorporated (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hirsch F. R.; Scagliotti G. V.; Mulshine J. L.; Kwon R.; Curran W. J. Jr.; Wu Y.-L.; Paz-Ares L. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet 2017, 389, 299–311. 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- Kuo T.-C.; Li L.-W.; Pan S.-H.; Fang J.-M.; Liu J.-H.; Cheng T.-J.; Wang C.-J.; Hung P.-F.; Chen H.-Y.; Hong T.-M.; Hsu Y.-L.; Wong C.-H.; Yang P.-C. Purine-Type Compounds Induce Microtubule Fragmentation and Lung Cancer Cell Death through Interaction with Katanin. J. Med. Chem. 2016, 59, 8521–8534. 10.1021/acs.jmedchem.6b00797. [DOI] [PubMed] [Google Scholar]
- Hou H.; Qu B.; Su C.; Hou G.; Gao F.. Design, Synthesis and Anti-Lung Cancer Evaluation of 1, 2, 3-Triazole Tethered Dihydroartemisinin-Isatin Hybrids. Front. Pharmacol. 2021, 12, 10.3389/fphar.2021.801580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławiński G.; Wrona A.; Dąbrowska-Kugacka A.; Raczak G.; Lewicka E. Immune Checkpoint Inhibitors and Cardiac Toxicity in Patients Treated for Non-Small Lung Cancer: A Review. Int. J. Mol. Sci. 2020, 21, 7195. 10.3390/ijms21197195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J. J.; Duscharla D.; Ummanni R.; Hanson P. R.; Malhotra S. V. Investigation on the Anticancer Activity of Symmetric and Unsymmetric Cyclic Sulfamides. ACS Med. Chem. Lett. 2021, 12, 202–210. 10.1021/acsmedchemlett.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y.; Yu B.; Huang H.; Peng Y.; Li E.; Yao Y.; Song C.; Yu W.; Zhu K.; Wang K.; Yi D.; Du J.; Chang J. Discovery of Dosimertinib, a Highly Potent, Selective, and Orally Efficacious Deuterated EGFR Targeting Clinical Candidate for the Treatment of Non-Small-Cell Lung Cancer. J. Med. Chem. 2021, 64, 925–937. 10.1021/acs.jmedchem.0c02005. [DOI] [PubMed] [Google Scholar]
- Arcaro A.; Fischer B. Current Status of Clinical Trials for Small Cell Lung Cancer. Rev. Recent Clin. Trials 2008, 3, 40–61. 10.2174/157488708783330503. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Guerrero D.; Tufman A. Rare Driver Alterations in Nonsmall Cell Lung Cancer: Novel Targeted Drugs. Curr. Opin. Oncol. 2022, 34, 77–82. 10.1097/CCO.0000000000000806. [DOI] [PubMed] [Google Scholar]
- Sankar K.; Bryant A. K.; Strohbehn G. W.; Zhao L.; Elliott D.; Moghanaki D.; Kelley M. J.; Ramnath N.; Green M. D. Real World Outcomes versus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer. Cancers 2022, 14, 614. 10.3390/cancers14030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumilak M.; Wiktorowska-Owczarek A.; Stanczak A. Hybrid Drugs—A Strategy for Overcoming Anticancer Drug Resistance?. Molecules 2021, 26, 2601. 10.3390/molecules26092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz F.; Haider M. R.; Shafi S.; Yar M. S. Anti-Tubulin Agents of Natural Origin: Targeting Taxol, Vinca, and Colchicine Binding Domains. Eur. J. Med. Chem. 2019, 171, 310–331. 10.1016/j.ejmech.2019.03.025. [DOI] [PubMed] [Google Scholar]
- Lichota A.; Gwozdzinski K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzadi I.; Zahoor A. F.; Rasul A.; Mansha A.; Ahmad S.; Raza Z. Synthesis, Hemolytic Studies, and In Silico Modeling of Novel Acefylline–1,2,4-Triazole Hybrids as Potential Anti-Cancer Agents against MCF-7 and A549. ACS Omega 2021, 6, 11943–11953. 10.1021/acsomega.1c00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganathan R.; Purushotham B.; Radhakrishnan N.; Swamy M. K. Capsaicin and Its Potential Anticancer Mechanisms of Action. Plant-derived Bioact. Chem. Mode Action 2020, 301–321. 10.1007/978-981-15-2361-8_14. [DOI] [Google Scholar]
- Walpole C. S. J.; Wrigglesworth R.; Bevan S.; Campbell E. A.; Dray A.; James I. F.; Masdin K. J.; Perkins M. N.; Winter J. Analogues of Capsaicin with Agonist Activity as Novel Analgesic Agents; Structure—Activity Studies. 2. The Amide Bond “B-Region.”. J. Med. Chem. 1993, 36, 2373–2380. 10.1021/jm00068a015. [DOI] [PubMed] [Google Scholar]
- Naaz F.; Khan A.; Kumari A.; Ali I.; Ahmad F.; Ahmad Lone B.; Ahmad N.; Ali Khan I.; Rajput V. S.; Grover A.; Shafi S. 1,3,4-Oxadiazole Conjugates of Capsaicin as Potent NorA Efflux Pump Inhibitors of Staphylococcus Aureus. Bioorg. Chem. 2021, 113, 105031 10.1016/j.bioorg.2021.105031. [DOI] [PubMed] [Google Scholar]
- Uarrota V. G.; Maraschin M.; de Bairros Â. D. F. M.; Pedreschi R. Factors Affecting the Capsaicinoid Profile of Hot Peppers and Biological Activity of Their Non-Pungent Analogs (Capsinoids) Present in Sweet Peppers. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–17. 10.1080/10408398.2020.1743642. [DOI] [PubMed] [Google Scholar]
- Jun H.-S.; Park T.; Lee C. K.; Kang M. K.; Park M. S.; Kang H. I.; Surh Y.-J.; Kim O. H. Capsaicin Induced Apoptosis of B16-F10 Melanoma Cells through down-Regulation of Bcl-2. Food Chem. Toxicol. 2007, 45, 708–715. 10.1016/j.fct.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Narang N.; Jiraungkoorskul W.; Jamrus P. Current Understanding of Antiobesity Property of Capsaicin. Pharmacogn. Rev. 2017, 11, 23. 10.4103/phrev.phrev_48_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richbart S. D.; Friedman J. R.; Brown K. C.; Gadepalli R. S.; Miles S. L.; Rimoldi J. M.; Rankin G. O.; Valentovic M. A.; Tirona M. T.; Finch P. T.; Hess J. A.; Dasgupta P. Nonpungent N-AVAM Capsaicin Analogues and Cancer Therapy. J. Med. Chem. 2021, 64, 1346–1361. 10.1021/acs.jmedchem.0c01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Zhou Y.; Fu J. Advances in Antiobesity Mechanisms of Capsaicin. Curr. Opin. Pharmacol. 2021, 61, 1–5. 10.1016/j.coph.2021.08.012. [DOI] [PubMed] [Google Scholar]
- Naaz F.; Ahmad F.; Lone B. A.; Pokharel Y. R.; Fuloria N. K.; Fuloria S.; Ravichandran M.; Pattabhiraman L.; Shafi S.; Shahar Yar M. Design and Synthesis of Newer 1,3,4-Oxadiazole and 1,2,4-Triazole Based Topsentin Analogues as Anti-Proliferative Agent Targeting Tubulin. Bioorg. Chem. 2020, 95, 103519 10.1016/j.bioorg.2019.103519. [DOI] [PubMed] [Google Scholar]
- Naaz F.; Ahmad F.; Lone B. A.; Khan A.; Sharma K.; IntzarAli; ShaharYar M.; Pokharel Y. R.; Shafi S. Apoptosis Inducing 1,3,4-Oxadiazole Conjugates of Capsaicin: Their In Vitro Antiproliferative and In Silico Studies. ACS Med. Chem. Lett. 2021, 12, 1694–1702. 10.1021/acsmedchemlett.1c00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapa-oliver A. M.; Mejía-teniente L.; Chili K.. Capsaicin : From ePlants to a Cancer-Suppressing Agent. 2016, 1–14, 10.3390/molecules21080931. [DOI] [PMC free article] [PubMed]
- Friedman J. R.; Richbart S. D.; Merritt J. C.; Brown K. C.; Denning K. L.; Tirona M. T.; Valentovic M. A.; Miles S. L.; Dasgupta P. Capsaicinoids: Multiple Effects on Angiogenesis, Invasion and Metastasis in Human Cancers. Biomed. Pharmacother. 2019, 118, 109317 10.1016/j.biopha.2019.109317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J.; LI T. Z.; XU G. H.; LUO B. B.; CHEN Y. X.; ZHANG T. Low-Concentration Capsaicin Promotes Colorectal Cancer Metastasis by Triggering ROS Production and Modulating Akt/MTOR and STAT-3 Pathways. Neoplasma 2013, 60, 364–372. 10.4149/neo_2013_048. [DOI] [PubMed] [Google Scholar]
- Surh Y.-J.; Lee S. S. Capsaicin in Hot Chili Pepper: Carcinogen, Co-Carcinogen or Anticarcinogen?. Food Chem. Toxicol. 1996, 34, 313–316. 10.1016/0278-6915(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Liao J.-C.; Deng J.-S.; Lin Y.-C.; Lee C.-Y.; Lee M.-M.; Hou W.-C.; Huang S.-S.; Huang G.-J. Antioxidant, Antinociceptive, and Anti-Inflammatory Activities from Actinidia Callosa Var. Callosa In Vitro and In Vivo. Evidence-Based Complementary Altern. Med. 2012, 2012, 1–14. 10.1155/2012/129152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarudin N.; Hisamuddin N.; Ong H.; Ahmad Azmi A.; Leong S.; Abas F.; Sulaiman M.; Shaik Mossadeq W. Analgesic Effect of 5-(3,4-Dihydroxyphenyl)-3-Hydroxy-1-(2-Hydroxyphenyl)Penta-2,4-Dien-1-One in Experimental Animal Models of Nociception. Molecules 2018, 23, 2099. 10.3390/molecules23092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J.; Bevan S.; Campbell E. A. Capsaicin and Pain Mechanisms. Br. J. Anaesth. 1995, 75, 157–168. 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Adhikary A.; Mazumdar M.; Mukherjee S.; Bhattacharjee P.; Guha D.; Choudhuri T.; Chattopadhyay S.; Sa G.; Sen A.; Das T. Capsaicin-Induced Activation of P53-SMAR1 Auto-Regulatory Loop Down-Regulates VEGF in Non-Small Cell Lung Cancer to Restrain Angiogenesis. PLoS One 2014, 9, e99743 10.1371/journal.pone.0099743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.; Lee S. H. Anticancer Properties of Capsaicin against Human Cancer. Anticancer Res. 2016, 36, 837–843. [PubMed] [Google Scholar]
- Zhang S.; Wang D.; Huang J.; Hu Y.; Xu Y. Application of Capsaicin as a Potential New Therapeutic Drug in Human Cancers. J. Clin. Pharm. Ther. 2020, 45, 16–28. 10.1111/jcpt.13039. [DOI] [PubMed] [Google Scholar]
- Mohammadi-Khanaposhtani M.; Safavi M.; Sabourian R.; Mahdavi M.; Pordeli M.; Saeedi M.; Ardestani S. K.; Foroumadi A.; Shafiee A.; Akbarzadeh T. Design, Synthesis, in Vitro Cytotoxic Activity Evaluation, and Apoptosis-Induction Study of New 9(10H)-Acridinone-1,2,3-Triazoles. Mol. Diversity 2015, 19, 787–795. 10.1007/s11030-015-9616-0. [DOI] [PubMed] [Google Scholar]
- Karan S.; Kashyap V. K.; Shafi S.; Saxena A. K. Structural and Inhibition Analysis of Novel Sulfur-Rich 2-Mercaptobenzothiazole and 1,2,3-Triazole Ligands against Mycobacterium Tuberculosis DprE1 Enzyme. J. Mol. Model. 2017, 23, 241. 10.1007/s00894-017-3403-z. [DOI] [PubMed] [Google Scholar]
- Naaz F.; Preeti Pallavi M. C.; Shafi S.; Mulakayala N.; Shahar Yar M.; Sampath Kumar H. M. 1,2,3-Triazole Tethered Indole-3-Glyoxamide Derivatives as Multiple Inhibitors of 5-LOX, COX-2 & Tubulin: Their Anti-Proliferative & Anti-Inflammatory Activity. Bioorg. Chem. 2018, 81, 1–20. 10.1016/j.bioorg.2018.07.029. [DOI] [PubMed] [Google Scholar]
- Arora B. S.; Shafi S.; Singh S.; Ismail T.; Kumar H. M. S. A Novel Domino-Click Approach for the Synthesis of Sugar Based Unsymmetrical Bis-1,2,3-Triazoles. Carbohydr. Res. 2008, 343, 139–144. 10.1016/j.carres.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Ismail T.; Shafi S.; Hyder I.; Sidiq T.; Khajuria A.; Alam S. M.; Halmuthur M. S. K. Design and Synthesis of Novel 1,2,3-Triazole- and 2-Isoxazoline-Based Bis -Heterocycles as Immune Potentiators. Arch. Pharm. (Weinheim) 2015, 348, 796–807. 10.1002/ardp.201400398. [DOI] [PubMed] [Google Scholar]
- Haider S.; Alam M. S.; Hamid H.; Shafi S.; Nargotra A.; Mahajan P.; Nazreen S.; Kalle A. M.; Kharbanda C.; Ali Y.; Alam A.; Panda A. K. Synthesis of Novel 1,2,3-Triazole Based Benzoxazolinones: Their TNF-α Based Molecular Docking with in-Vivo Anti-Inflammatory, Antinociceptive Activities and Ulcerogenic Risk Evaluation. Eur. J. Med. Chem. 2013, 70, 579–588. 10.1016/j.ejmech.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Lu H.; Zhou Q.; He J.; Jiang Z.; Peng C.; Tong R.; Shi J. Recent Advances in the Development of Protein–Protein Interactions Modulators: Mechanisms and Clinical Trials. Signal Transduction Targeted Ther. 2020, 5, 213. 10.1038/s41392-020-00315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehra N.; Tittal R. K.; Ghule V. D. 1,2,3-Triazoles of 8-Hydroxyquinoline and HBT: Synthesis and Studies (DNA Binding, Antimicrobial, Molecular Docking, ADME, and DFT). ACS Omega 2021, 6, 27089–27100. 10.1021/acsomega.1c03668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agouram N.; El Hadrami E. M.; Bentama A. 1,2,3-Triazoles as Biomimetics in Peptide Science. Molecules 2021, 26, 2937. 10.3390/molecules26102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorov K.; Zhao J.; Aisa H. A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. 10.1016/j.bmc.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonandi E.; Christodoulou M. S.; Fumagalli G.; Perdicchia D.; Rastelli G.; Passarella D. The 1,2,3-Triazole Ring as a Bioisostere in Medicinal Chemistry. Drug Discovery Today 2017, 22, 1572–1581. 10.1016/j.drudis.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Sahu A.; Sahu P.; Agrawal R. A Recent Review on Drug Modification Using 1,2,3-Triazole. Curr. Chem. Biol. 2020, 14, 71–87. 10.2174/2212796814999200807214519. [DOI] [Google Scholar]
- Wei G.; Luan W.; Wang S.; Cui S.; Li F.; Liu Y.; Liu Y.; Cheng M. A Library of 1,2,3-Triazole-Substituted Oleanolic Acid Derivatives as Anticancer Agents: Design, Synthesis, and Biological Evaluation. Org. Biomol. Chem. 2015, 13, 1507–1514. 10.1039/C4OB01605J. [DOI] [PubMed] [Google Scholar]
- Liang T.; Sun X.; Li W.; Hou G.; Gao F.. 1,2,3-Triazole-Containing Compounds as Anti–Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure–Activity Relationship. Front. Pharmacol. 2021, 12, 10.3389/fphar.2021.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekhar M.; Nayak V. L.; Ramakrishna S.; Mallavadhani U. V. Novel Triazole Hybrids of Myrrhanone C, a Natural Polypodane Triterpene: Synthesis, Cytotoxic Activity and Cell Based Studies. Eur. J. Med. Chem. 2016, 114, 293–307. 10.1016/j.ejmech.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Nerella S.; Kankala S.; Gavaji B. Synthesis of Podophyllotoxin-Glycosyl Triazoles via Click Protocol Mediated by Silver (I)- N -Heterocyclic Carbenes and Their Anticancer Evaluation as Topoisomerase-II Inhibitors. Nat. Prod. Res. 2021, 35, 9–16. 10.1080/14786419.2019.1610958. [DOI] [PubMed] [Google Scholar]
- Goud N. S.; Pooladanda V.; Mahammad G. S.; Jakkula P.; Gatreddi S.; Qureshi I. A.; Alvala R.; Godugu C.; Alvala M. Synthesis and Biological Evaluation of Morpholines Linked Coumarin–Triazole Hybrids as Anticancer Agents. Chem. Biol. Drug Des. 2019, 94, 1919–1929. 10.1111/cbdd.13578. [DOI] [PubMed] [Google Scholar]
- Seghetti F.; Di Martino R. M. C.; Catanzaro E.; Bisi A.; Gobbi S.; Rampa A.; Canonico B.; Montanari M.; Krysko D. V.; Papa S.; Fimognari C.; Belluti F. Curcumin-1,2,3-Triazole Conjugation for Targeting the Cancer Apoptosis Machinery. Molecules 2020, 25, 3066. 10.3390/molecules25133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-Y.; Chang Hsu Y.; Peng Y.-H.; Ke Y.-Y.; Lin W.-H.; Sun H.-Y.; Shiao H.-Y.; Kuo F.-M.; Chen P.-Y.; Lien T.-W.; Chen C.-H.; Chu C.-Y.; Wang S.-Y.; Yeh K.-C.; Chen C.-P.; Hsu T.-A.; Wu S.-Y.; Yeh T.-K.; Chen C.-T.; Hsieh H.-P. Discovery of a Furanopyrimidine-Based Epidermal Growth Factor Receptor Inhibitor (DBPR112) as a Clinical Candidate for the Treatment of Non-Small Cell Lung Cancer. J. Med. Chem. 2019, 62, 10108–10123. 10.1021/acs.jmedchem.9b00722. [DOI] [PubMed] [Google Scholar]
- Han C.; Huang Z.; Zheng C.; Wan L.; Zhang L.; Peng S.; Ding K.; Ji H.; Tian J.; Zhang Y. Novel Hybrids of (Phenylsulfonyl)Furoxan and Anilinopyrimidine as Potent and Selective Epidermal Growth Factor Receptor Inhibitors for Intervention of Non-Small-Cell Lung Cancer. J. Med. Chem. 2013, 56, 4738–4748. 10.1021/jm400463q. [DOI] [PubMed] [Google Scholar]
- Siudem P.; Paradowska K.; Bukowicki J. Conformational Analysis of Capsaicin Using 13C, 15N MAS NMR, GIAO DFT and GA Calculations. J. Mol. Struct. 2017, 1146, 773–781. 10.1016/j.molstruc.2017.05.142. [DOI] [Google Scholar]
- Sharma V.; Qayum A.; Kaul S.; Singh A.; Kapoor K. K.; Mukherjee D.; Singh S. K.; Dhar M. K. Carbohydrate Modifications of Neoandrographolide for Improved Reactive Oxygen Species-Mediated Apoptosis through Mitochondrial Pathway in Colon Cancer. ACS Omega 2019, 4, 20435–20442. 10.1021/acsomega.9b01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K.; Im G. J.; An Y. S.; Lee S. H.; Jung H. H.; Park S. Y. The Effects of the Antioxidant α-Tocopherol Succinate on Cisplatin-Induced Ototoxicity in HEI-OC1 Auditory Cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 9–14. 10.1016/j.ijporl.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Dheer D.; Behera C.; Singh D.; Abdullaha M.; Chashoo G.; Bharate S. B.; Gupta P. N.; Shankar R. Design, Synthesis and Comparative Analysis of Triphenyl-1,2,3-Triazoles as Anti-Proliferative Agents. Eur. J. Med. Chem. 2020, 207, 112813 10.1016/j.ejmech.2020.112813. [DOI] [PubMed] [Google Scholar]
- Kang Y.-H.; Lee E.; Choi M.-K.; Ku J.-L.; Kim S. H.; Park Y.-G.; Lim S.-J. Role of Reactive Oxygen Species in the Induction of Apoptosis by ?-Tocopheryl Succinate. Int. J. Cancer 2004, 112, 385–392. 10.1002/ijc.20424. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Singh B.; Mahajan G.; Sharma P. R.; Bharate S. B.; Mintoo M. J.; Mondhe D. M. A Novel Colchicine-Based Microtubule Inhibitor Exhibits Potent Antitumor Activity by Inducing Mitochondrial Mediated Apoptosis in MIA PaCa-2 Pancreatic Cancer Cells. Tumor Biol. 2016, 37, 13121–13136. 10.1007/s13277-016-5160-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.