Abstract
Acute brain injury (ABI) occurs frequently in patients receiving venoarterial extracorporeal membrane oxygenation (VA-ECMO). We examined the association between peri-cannulation PaCO2 and ABI with granular blood gas data. We retrospectively analyzed adult patients who underwent VA-ECMO at a tertiary care center with standardized neuromonitoring. Pre- and post-cannulation PaCO2 were defined as the mean of all PaCO2 values in the 12 hours before and after cannulation, respectively. Peri-cannulation PaCO2 drop (△PaCO2) equaled pre- minus post-cannulation PaCO2. ABI included intracranial hemorrhage (ICH), ischemic stroke, hypoxic ischemic brain injury, cerebral edema, seizure, and brain death. Univariable logistic regression analysis was performed for the presence of ABI. Out of 129 VA-ECMO patients (median age=60, 63% male), 43 (33%) patients experienced ABI. Patients had a median of 11 (interquartile range: 8–14) peri-cannulation PaCO2 values. Comparing patients with and without ABI, pre-cannulation (39 vs. 42 mmHg, p=0.38) and post-cannulation (37 vs. 36 mmHg, p=0.82) PaCO2 were not different. However, higher pre-cannulation PaCO2 (OR=2.10, 95% confidence interval [CI]=1.10–4.00, p=0.02) and larger △PaCO2 (OR=2.69, 95%CI=1.18–6.13, p=0.02) were associated with ICH. In conclusion, in a cohort with granular ABG data and a standardized neuromonitoring protocol, higher pre-cannulation PaCO2 and larger △PaCO2 were associated with increased prevalence of ICH.
Keywords: Extracorporeal membrane oxygenation, Arterial carbon dioxide tension, Acute brain injury, Neurological injury, Neurological complication
Introduction
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is an increasingly utilized mode of circulatory support for patients with refractory cardiac or cardiopulmonary failure of reversible etiology(1). A high in-hospital mortality rate of 42–60% show the limitations of VA-ECMO therapy(2–4). As determined by the literature, acute brain injury (ABI) occurs in 11–20% (5,6) of patients, although this may be an underestimate since neuromonitoring protocols, not yet routinely applied, increase the sensitivity of ABI detection(7). Of importance, those with ABI have a nearly two-fold increase in mortality(6).
Despite its impact on mortality, the precise pathophysiology of ABI in patients with ECMO support remains to be elucidated. Recently, higher △ arterial carbon dioxide tension (PaCO2) in the peri-cannulation period was associated with higher prevalence of ABI in an analysis of the Extracorporeal Life Support Organization (ELSO) Registry(8). However, the ELSO registry only provides two arterial blood gas (ABG) data points, one pre and one post cannulation, and, furthermore, ABI is likely underdiagnosed in the registry due to an absence of standardized neuromonitoring and a central adjudication process for neurological diagnosis(6,7). Therefore, this finding would benefit from a further validation in ECMO centers where more frequent ABG collections and standardized neuromonitoring protocols are routinely implemented.
PaCO2 is an established driver of cerebral blood flow (CBF) autoregulation(9) and an important modulator for neuronal metabolic demand(10). Thus, a rapid reduction of PaCO2 after prolonged hypercapnia immediately after ECMO cannulation has mechanistic plausibility to be a cause of ABIs, resulting from disruptions in CBF autoregulation and sudden cerebral vasoconstriction. The purpose of this study was to better characterize the relationship between PaCO2 and ABI in a single institution VA-ECMO population with standardized neuromonitoring and granular ABG data, hypothesizing that PaCO2 levels before ECMO and the magnitude and rate of PaCO2 removal upon cannulation are associated with specific subtypes of ABIs.
Materials and Methods
Study design
This study was approved by the Johns Hopkins Hospital Institutional Review Board with a waiver of informed consent since this was an observational study (IRB00216321). We performed a retrospective analysis of a database containing patients undergoing ECMO at a tertiary care center between June 2016 and January 2021. This study derives from a multidisciplinary effort and a new initiative between the Cardiovascular Surgery Intensive Care Unit (CVSICU), Cardiac Critical Care Unit (CCU), and Division of Neurosciences Critical Care to improve overall clinical care and outcomes for patients treated with ECMO(7). Patients are housed in the CVSICU and CCU and all receive neurocritical care consultations according to our standardized neuromonitoring protocol(7). The ECMO attending physician rounds on both ICUs.
Participants
We included all adult patients (age ≥ 18 years) who received VA-ECMO. We excluded patients who underwent multiple runs to minimize the potential bias resulting from severe illness. This study was approved by Johns Hopkins Hospital Institutional Review Board and informed consent was waived as this was an observational study.
Data collection
For all patients in the study, we collected pre-cannulation characteristics including demographics, social history, past medical history, baseline neurologic function, cardiac diagnoses, pulmonary diagnoses, and laboratory values. ABGs were collected every 2–4 hours according to standard clinical protocol in our institution with more frequent collections if necessary. All patients underwent standard-of-care neurocritical care consultations and a standardized neuromonitoring protocol(7). At our institution, any significant neurologic issues or pre-existing brain injury, such as intracranial hemorrhage, is an exclusion criterion for VA-ECMO. All patients had a right radial arterial line for accurate and frequent ABG measurements and as a sensitive indicator of Harlequin syndrome. Baseline ABGs prior to ECMO cannulation and serial blood gases following cannulation were collected. Patients were routinely given sedation holidays, as deemed safe, so that neurologic examinations could be obtained.
Definitions
Pre-ECMO and post-cannulation PaCO2 were defined as the mean of all PaCO2 (mmHg) values collected in the 12 hours immediately preceding and following cannulation, respectively. These time intervals were selected to adequately capture the state of pre- and post-cannulation PaCO2, rather than choosing only one pre and post cannulation ABG value as recorded by the ELSO registry. Peri-cannulation PaCO2 drop (△PaCO2) was equal to the pre-cannulation PaCO2 minus post-cannulation PaCO2. In addition, relative PaCO2 drop percentage was calculated as: (△PaCO2 / pre-cannulation PaCO2)*100. The relative PaCO2 drop was arbitrarily categorized into five groups: −35% to −15%, −15% to 0%, 0 to 15%, 15 to 30%, and >30% drop. Negative drops represent a rise in post-cannulation PaCO2 compared to pre-cannulation. Pre- and post-cannulation rates of PaCO2 change were calculated as the slope of the linear regression line of all PaCO2 values in the 12 hours before and after cannulation. Hypercapnia and hypocapnia were defined as PaCO2 > 45 and < 35 mmHg, respectively. Good neurologic outcome was defined as a modified Rankin Score (mRS), at discharge, less than or equal to 3, while poor neurologic outcome was defined as mRS > 3.
Outcomes
The primary outcome was the presence of ABI. ABI, as a composite variable, was defined as ischemic stroke, intracranial hemorrhage (ICH), hypoxic ischemic brain injury, cerebral edema, seizure, or brain death. The secondary outcomes were individual ABI diagnoses and in-hospital mortality.
Statistical analysis
All data were presented median (interquartile range: IQR) for continuous variables and absolute numbers with percentages for binary and categorical variables. Demographic and clinical characteristics in patients with and without events were compared using Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test for binary or categorical variables. Paired pre- and post-cannulation PaCO2 values were compared using the Wilcoxon signed-rank test. A p value less than 0.05 was considered statistically significant. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by logistic regression analysis. All statistical analyses were performed using STATA 15.1 (StataCorp, College Station, TX, USA).
Results
Of 215 total ECMO runs during the study period, 129 patients underwent VA-ECMO support. The median age was 60 (IQR: 49–68) and 63% were male (Table 1). The indication for VA-ECMO included post-cardiotomy shock (n=53, 41%), cardiogenic shock (n=74, 57%), and cardiac arrest as extracorporeal cardiopulmonary resuscitation (n=37, 29%), though patients could have >1 indication. Patients were supported on VA-ECMO for a median of 94.8 hours (IQR: 58.3–189.4). The median Sequential Organ Failure Assessment (SOFA) score on day 1 of ECMO was 11 (10–13). Of 129 patients, 35 patients (27%) survived to discharge, 18 were discharged home (14%), 10 patients were discharged to acute rehabilitation (8%), 1 was discharged to a long-term facility (<1%), and 6 were discharged to a skilled nursing facility (5%). Ninety-four patients (73%) expired during the index hospitalization.
Table 1.
Baseline characteristics and clinical variables in patients with and without ABI.
| Variable | Total (n=129) | Patients without ABI (n=86) | Patients with ABI (n=43) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 60 (49–68) | 62 (50–68) | 55 (48–69) | 0.38 |
| Male | 81 (63%) | 56 (65%) | 25 (58%) | 0.44 |
| Body Mass Index, kg/m2 | 29 (25–34) | 29 (25–36) | 29 (25–33) | 0.62 |
| Ethnicity | ||||
| White | 83 (64%) | 58 (67%) | 25 (58%) | 0.30 |
| Black | 26 (20%) | 15 (17%) | 11 (26%) | 0.28 |
| Hispanic | 2 (2%) | 1 (1%) | 1 (2%) | 0.61 |
| Asian | 9 (7%) | 7 (8%) | 2 (5%) | 0.46 |
| Others | 9 (7%) | 5 (6%) | 4 (9%) | 0.46 |
| Past medical history | ||||
| Ischemic stroke | 10 (8%) | 6 (7%) | 4 (9%) | 0.64 |
| Intracranial hemorrhage | 2 (2%) | 0 (0%) | 2 (5%) | 0.04 |
| Hypertension | 97 (75%) | 63 (73%) | 34 (79%) | 0.47 |
| Hyperlipidemia | 71 (55%) | 47 (55%) | 24 (56%) | 0.90 |
| Heart failure | 44 (34%) | 30 (35%) | 14 (33%) | 0.79 |
| Chronic kidney disease | 18 (14%) | 11 (13%) | 7 (16%) | 0.59 |
| Atrial fibrillation | 38 (29%) | 26 (30%) | 12 (28%) | 0.78 |
| Antiplatelet therapy before index hospitalization | 68 (53%) | 49 (57%) | 19 (44%) | 0.17 |
| Anticoagulation before index hospitalization | 30 (23%) | 21 (24%) | 9 (21%) | 0.66 |
| Pre-cannulation variables (n = 126) | ||||
| Inotropic or vasoactive support | 106 (84%) | 73 (87%) | 33 (79%) | 0.23 |
| Cardiac arrest | 57 (45%) | 34 (40%) | 23 (55%) | 0.13 |
| Glasgow Coma Scale | 15 (6–15) | 15 (8–15) | 15 (3–15) | 0.55 |
| SOFA score | 11 (10–13) | 12 (10–13) | 11 (10–13) | 0.56 |
| Lactate (mmol/L) | 5.8 (2.8–10.1) | 5.4 (2.4–9.8) | 7.3 (3.3–13.1) | 0.10 |
| ECMO indications | ||||
| Cardiogenic shock | 74 (57%) | 51 (59%) | 23 (53%) | 0.53 |
| Post-cardiotomy shock | 53 (41%) | 32 (37%) | 21 (49%) | 0.21 |
| Cardiac arrest | 25 (19%) | 14 (16%) | 11 (26%) | 0.21 |
| Discharge location | ||||
| Home | 18 (14%) | 15 (17%) | 3 (7%) | 0.11 |
| Acute rehabilitation | 10 (8%) | 7 (8%) | 3 (7%) | 0.82 |
| Long-term facility | 1 (1%) | 1 (1%) | 0 (0%) | 0.48 |
| Skilled nursing facility | 6 (5%) | 4 (5%) | 2 (5%) | 1.00 |
| Mortality | 94 (73%) | 59 (69%) | 35 (81%) | 0.12 |
| ECMO duration (hours) | 94.8 (58.3–189.4) | 98.0 (54.3–201.8) | 94.6 (58.3–154.8) | 0.79 |
| Good neurologic outcome (mRS≤3) | 19 (15%) | 15 (17%) | 4 (9%) | 0.22 |
Data are presented as median (IQR) for continuous measures and n (%) for categorical measures. ABI: acute brain injury; SOFA: sequential organ failure assessment score.
Survivors were younger (54 vs. 62 years old, p=0.01), with fewer prior ischemic stroke (0% vs. 11%, p=0.045), hyperlipidemia (40% vs. 61%, p=0.04), and post-cardiotomy shock as indication (26% vs. 47%, p=0.03), and had a lower BMI (27 vs. 31 kg/m2, p<0.001) and lower lactate (4.8 vs. 6.6 mmol/L, p=0.02) (Supplementary Table 1). Pre- and post-cannulation PaCO2 and △PaCO2 were not associated with mortality (Supplementary Table 2).
Overall, 43 (33%) patients had at least one ABI during ECMO support (Table 1). The most common ABI was ischemic stroke (n=19, 15%), followed by HIBI (n=15, 12%) and seizure (n=8, 6%). Baseline clinical characteristics were similar in patients with ABI and without ABI, as described in Table1, except for a history of ICH, more commonly present in the ABI group (p=0.04).
A median of 4 (2–7) pre-cannulation and 6 (4–8) post-cannulation PaCO2 values were captured per patient. Overall, the median pre-cannulation PaCO2 was 40 (37–44) mmHg; the median post-cannulation PaCO2 was 36 (33–39) mmHg (pre- vs. post-cannulation, p<0.0001); and the median △PaCO2 was 5 (0–9) mmHg (Figure 1). Before ECMO support, hypercapnia and hypocapnia were present in 30 (23%) and 22 (17%) patients, respectively. In contrast, hypercapnia and hypocapnia were present in 5 (4%) and 48 (37%) patients, respectively, after ECMO cannulation. The presence of hypocapnia before or after cannulation was not associated with ABI. In univariable logistic regression, higher BMI (OR=1.06, 95% CI: 1.00–1.12, p=0.04) and cardiac arrest as an indication for VA-ECMO (OR=2.80, 95% CI: 1.01–7.15, p=0.03) were associated with greater odds of pre-cannulation hypercapnia.
Figure 1.
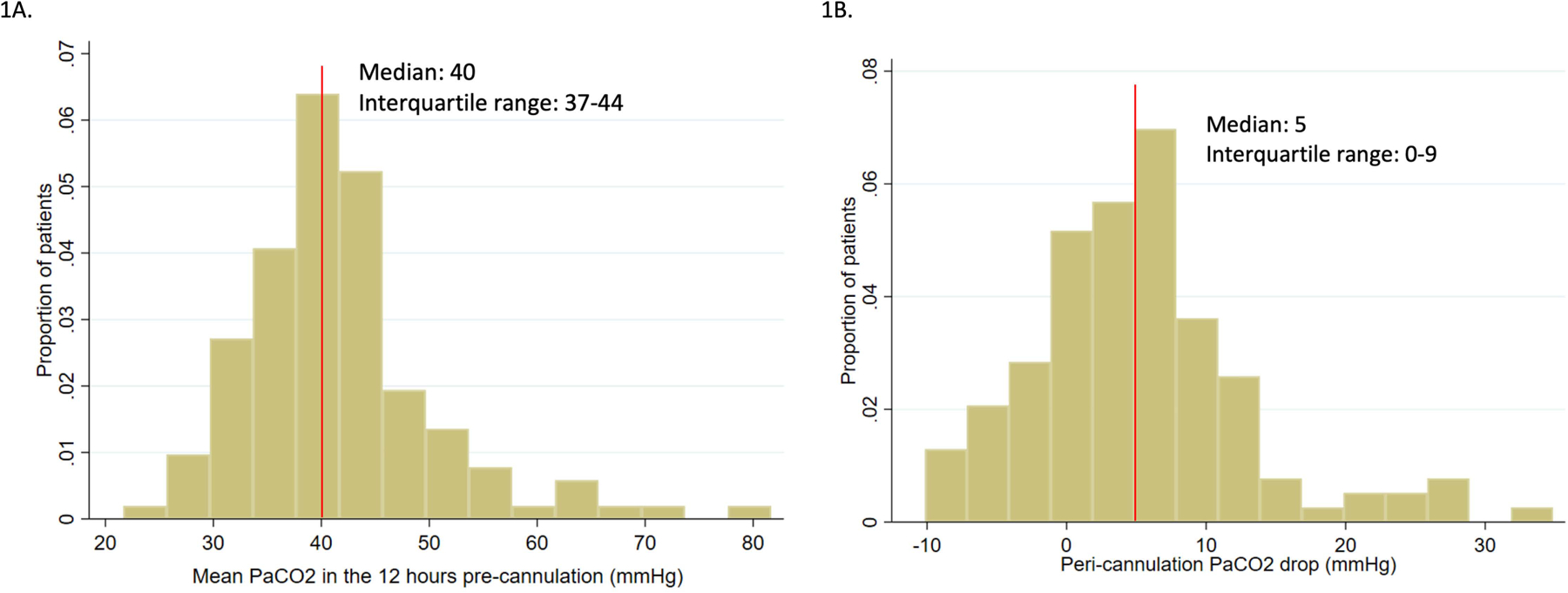
Histograms of pre-cannulation PaCO2 and peri-cannulation PaCO2 drop (△PaCO2).
In those with ABI vs. those without (Table 2), there was no significant difference in pre- and post-cannulation PaCO2 levels, rate of change of pCO2, or △PaCO2. There were also no differences in PaCO2 parameters when comparing ABI patients with good versus poor functional neurologic outcomes (Supplementary Table 3). However, in relating PaCO2 parameters with subtypes of ABI, patients with acute ICH had significantly higher pre-cannulation PaCO2 values than those who did not (47 vs. 40 mmHg, p=0.02) (Table 2). Furthermore, those with ICH had a greater △PaCO2 (8.8 vs. 4.5 mmHg, p=0.03) and decreases in post-cannulation PaCO2 (+0.23 vs. −1.12 mmHg/hour, p=0.02) compared to those without. Patients with cerebral edema also had a greater △PaCO2 than those without (10.4 vs. 4.5 mmHg, p=0.03) (Supplementary Table 2).
Table 2.
PaCO2 and incidence of ABI.
| PaCO2 Parameter | Patients without ABI (n=86) | Patients with ABI (n=43) | P value |
|---|---|---|---|
| Pre-cannulation | |||
| Mean | 39 (36–45) | 42 (37–44) | 0.38 |
| Slope | 0.28 (−1.15–1.87) | 0.33 (−0.67–2.90) | 0.78 |
| Highest | 46 (40–56) | 50 (43–54) | 0.27 |
| Lowest | 34 (28–39) | 34 (30–39) | 0.44 |
| Post-cannulation | |||
| Mean | 37 (33–39) | 36 (34–39) | 0.82 |
| Slope | 0.22 (−0.36–1.02) | 0.10 (−0.68–0.94) | 0.86 |
| Highest | 43 (39–46) | 43 (39–50) | 0.36 |
| Lowest | 30 (26–33) | 30 (25–32) | 0.48 |
| △PaCO2 | 4.8 (−0.4–8.5) | 4.5 (0.8–9.4) | 0.62 |
| Patients without ICH (n=122) | Patients with ICH (n=7) | P value | |
| Pre-cannulation | |||
| Mean | 40 (37–44) | 47 (41–56) | 0.02 |
| Slope | 0.31 (−0.78–1.93) | −0.11 (−0.67–1.11) | 0.57 |
| Highest | 47 (41–54) | 56 (48–65) | 0.05 |
| Lowest | 34 (29–39) | 39 (33–56) | 0.06 |
| Post-cannulation | |||
| Mean | 36 (33–39) | 36 (34–39) | 0.70 |
| Slope | 0.23 (−0.32–1.02) | −1.12 (−1.47–0.06) | 0.02 |
| Highest | 43 (39–48) | 48 (40–65) | 0.16 |
| Lowest | 30 (25–33) | 30 (28–32) | 0.67 |
| △PaCO2 | 4.5 (−0.1–8.3) | 8.8 (6.4–22.3) | 0.03 |
| Patients without Ischemic Stroke (n=110) | Patients with Ischemic Stroke (n=19) | P value | |
| Pre-cannulation | |||
| Mean | 40 (37–45) | 43 (38–43) | 0.86 |
| Slope | 0.28 (−0.78–2.56) | 0.37 (−0.55–1.31) | 0.90 |
| Highest | 48 (41–56) | 47 (43–54) | 0.89 |
| Lowest | 33 (29–39) | 34 (30–39) | 0.54 |
| Post-cannulation | |||
| Mean | 36 (33–38) | 37 (35–40) | 0.14 |
| Slope | 0.22 (−0.39–0.98) | 0.10 (−0.46–0.71) | 0.79 |
| Highest | 43 (38–48) | 46 (39–50) | 0.34 |
| Lowest | 30 (25–33) | 32 (26–34) | 0.27 |
| △PaCO2 | 5.2 (0.0–9.3) | 3.9 (0.3–7.6) | 0.31 |
P values are from Wilcoxon rank-sum test. PaCO2 values are represented as median (IQR). All values are expressed as mmHg, or mmHg/hour for slopes. ABI: acute brain injury; ICH: intracranial hemorrhage; HIBI: hypoxic ischemic brain injury.
In univariable logistic regression analysis, neither pre-cannulation PaCO2 nor △PaCO2 were associated with differential risk of in-hospital mortality or ABI as a composite variable. However, increased pre-cannulation PaCO2 was significantly associated with a higher risk of ICH (OR=2.10, 95% CI: 1.10–3.40, p=0.02) (Table 3), as was larger △PaCO2 (OR=2.69, 95%CI: 1.18–6.13, p=0.02). Additionally, patients with hypercapnia before cannulation were more likely to experience ICH (OR=4.92, 95%CI: 1.04–23.29, p=0.045). Rates of pre- and post-cannulation PaCO2 change were not significantly associated with outcomes in logistic regression models. Multivariable logistic regression analysis was not performed due to sample size limitations.
Table 3.
Univariate logistic regression for incidence of ICHs.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Pre-cannulation PaCO2* | 2.10 | 1.10 – 4.00 | 0.02 |
| △PaCO2** | 2.69 | 1.18 – 6.13 | 0.02 |
| Hypercapnia pre-cannulation | 4.92 | 1.04 – 23.39 | 0.045 |
| Neuromonitoring Protocol | 4.03 | 0.47 – 34.50 | 0.20 |
| Chronic Kidney Disease | 2.65 | 0.47 – 14.83 | 0.27 |
| Antiplatelet Therapy Pre-hospitalization | 0.34 | 0.06 – 1.82 | 0.21 |
| Male Sex | 0.42 | 0.09 – 1.98 | 0.27 |
| Body Mass Index | 0.97 | 0.87 – 1.09 | 0.63 |
Positive values for △PaCO2 represent drops (decreases) in PaCO2.
Represents OR for every 10-mmHg increase in pre-cannulation PaCO2.
Represents OR for every 10-mmHg increase in peri-cannulation PaCO2 drop.
Subgroup Analysis
For extracorporeal cardiopulmonary resuscitation (ECPR) patients, there were no differences in PaCO2 parameters between those who experienced ABI and those who did not. Furthermore, hypothermia, defined by a mean temperature in the first 24 hours of less than 36°C, was not associated with ABI, neurologic outcome, or mortality. Post-cardiotomy shock patients were older than those with other indications (68 vs. 55 years, p=0.002), however, other risk factors like BMI, sex, and pre-ECMO SOFA score were not statistically different. There were no significant differences in rates of ABI for post-cardiotomy shock survivors vs. non-survivors, however, survivors had a lower BMI (24 vs. 31 kg/m2, p=0.018) and much higher rates of having a good neurologic outcome (33% vs. 0%, p<0.001).
All collected PaCO2 values in the 24 hours peri-cannulation are plotted for those with and without ICH in Figure 2A. Those with the largest relative PaCO2 drop (>30% between pre- and post-cannulation) had a significantly increased risk of ICH compared to those with a 0–15% drop (OR=13.8, 95% CI: 1.30–146.78, p=0.03) (Figure 2B). Indeed, as the magnitude of peri-cannulation PaCO2 drop increases, so does the probability of ICH (Figure 2C). These relationships were not present for other ABI diagnoses.
Figure 2.
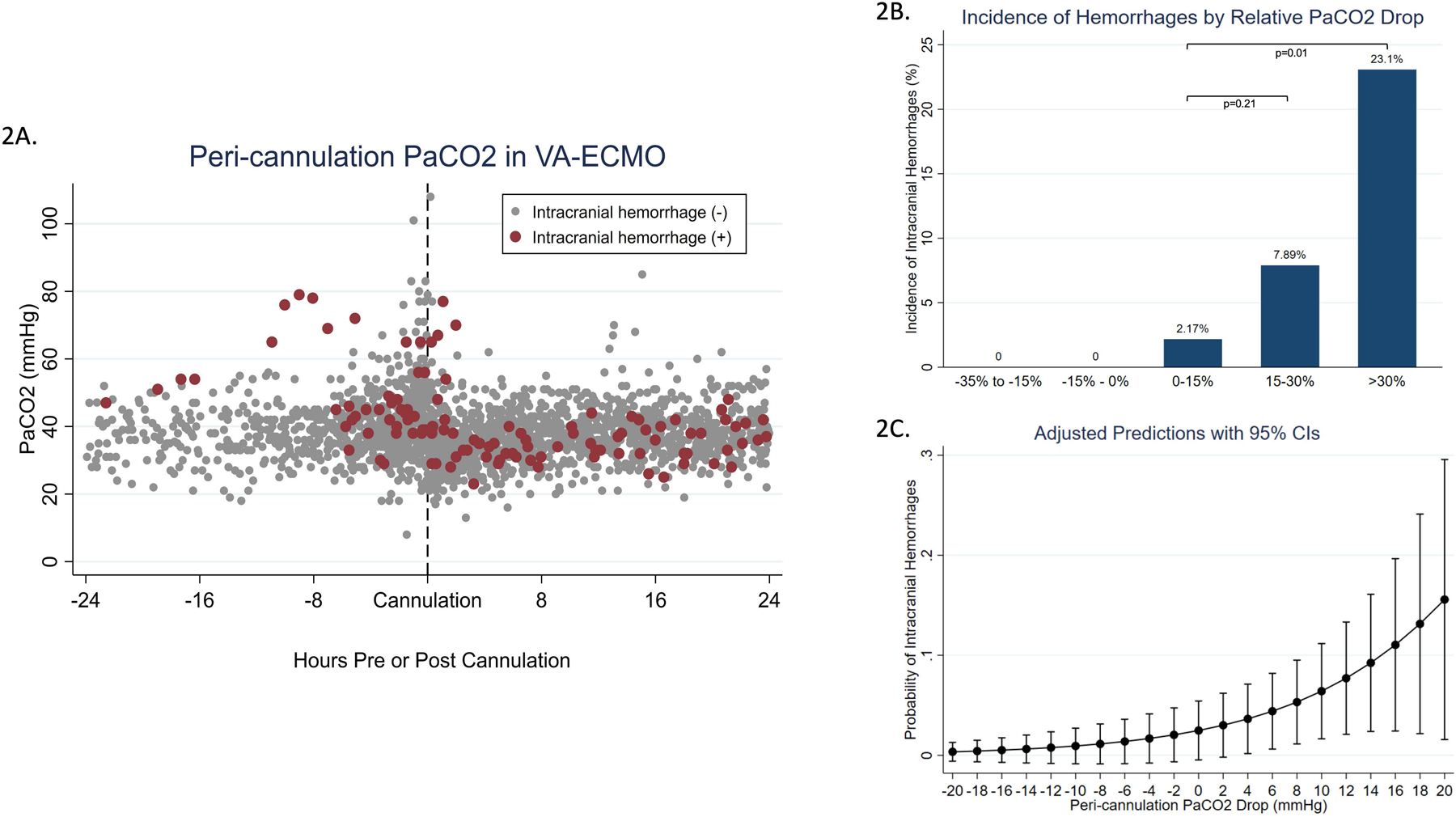
A) Peri-cannulation PaCO2 in VA-ECMO by presence or absence of intracranial hemorrhage. Red and gray dots represent PaCO2 values for patients with and without intracranial hemorrhages, respectively. B) Those with the largest peri-cannulation PaCO2 drop experience significantly more intracranial hemorrhages. P value by Pearson’s chi-square test. C) Probability of intracranial hemorrhage increases with increasing drops in PaCO2.
Discussion
In this single institution study of 129 patients undergoing VA-ECMO, higher pre-cannulation PaCO2 and larger peri-cannulation PaCO2 drops were associated with an increased odds of ICHs. However, we did not find PaCO2 before or after VA-ECMO cannulation, or peri-cannulation PaCO2 change, to be associated with mortality or composite ABI, as previously reported with ELSO registry data.
Arterial carbon dioxide tension may influence neurological outcomes through multiple mechanisms(9,11–13). It is well known to be a major driver in cerebral autoregulation. Hypocapnia induces vasoconstriction of cerebral vessels, which decreases CBF and increases the likelihood of ischemia(9,14,15). Additionally, acute hypocapnia can lead to ABI by increasing neuronal excitability and cerebral metabolic demand, which cannot be satisfied by the decreased CBF(10,16,17). However, these have not been studied extensively in the setting of continuous flow life support such as ECMO. Since carbon dioxide clearance is a highly effective and tunable setting, optimization of ABG parameters during ECMO has the potential to decrease ABI and improve survival.
Previous studies using single center data and the ELSO registry demonstrated that both hypo- and hypercapnia (PaCO2 < 30 mmHg and > 45 mmHg, respectively) prior to ECMO initiation were independently associated with mortality(8,18). There was also evidence suggesting that PaCO2 and its variability after ECMO initiation was associated with ABI, defined in the ELSO as ischemic stroke, ICH, seizure, and brain death(8,19–20). Further, Diehl et al.’s analysis of the ELSO registry revealed that neurological complications were also more common in VA-ECMO patients with >25 mmHg drops in PaCO2, and that hyper- and hypocapnia before initiation were associated with increased mortality(8). In the ELSO registry studies, PaCO2 prior to ECMO was not associated with composite ABI and degree of PaCO2 change after ECMO initiation was not associated with mortality(8,21).
However, studies using the ELSO registry are inherently limited by the type and timing of recorded data since there are only two time points of ABG data: one before (“pre-initiation”) and one after (“post-initiation”) ECMO initiation. Pre-initiation PaCO2 can be drawn at any time in the 6 hours prior to cannulation while post-initiation PaCO2 can be taken in the 6 hours surrounding the 24-hour mark after cannulation (i.e., between 18 and 30 hours after cannulation). For patients with multiple ABGs, only the one closest to before cannulation and the 24 hour-mark are recorded. Thus, it is difficult to determine if these two time points alone represent the true pre- and post-cannulation PaCO2 status. These discrete ABG values may fail to capture the extent of a patient’s dynamic hyper-, hypo-, or normo-carbic states over time. Furthermore, it has also been suggested that the true nadir PaCO2 following ECMO initiation is closer to six hours post-cannulation, not 24 hours(22). We echo this finding in our cohort as most patients had a nadir PaCO2 within the 8 hours immediately following cannulation.
There has been continued interest in targeted temperature management (TTM) and hypothermia for ECPR patients, with evidence both for and against targeted normothermia and hypothermia(23–26). A meta-analysis of ECPR studies has suggested that there is no difference in neurologic outcome between patients subjected to TTM vs non-TTM practices(27), and we echo this finding for our cohort’s neurologic outcome, ABI, and mortality. There is a paucity of multi-center studies examining PaCO2 and ABI specifically in ECPR patients, and a larger sample would permit multivariable analysis using temperature, PaCO2, and CPR practices. In our current ECPR cohort, there is no evidence to suggest that more conservative PaCO2 management will lead to better outcomes.
Our study provides strength in using a standardized neurological monitoring protocol. Current available literature on ABI in ECMO is limited by ill-defined guidelines for determining ABI and low sensitivity in ABI detection. Overall, the ABI proportion of 33% in our center is higher than previously reported by the ELSO studies(8,21,28,29). In our cohort, ABI was consistently diagnosed, and we captured two additional diagnoses, hypoxic ischemic injury and cerebral edema, which were not reported by the ELSO. These two diagnoses constituted 23% of all ABI occurrences in our cohort so their exclusion in the ELSO is an important consideration. Given these significant limitations, findings our study have important contribution to current literature regarding carbon dioxide tension and its impact on ICH.
Limitations
Our study was retrospective and only at a single institution, which may not reflect broader practice patterns. Additionally, our population was limited to 129 patients and small positive exposure groups, which restricts our statistical power. However, the sensitivity of ABI detection was high in our study with standardized neuromonitoring protocol, which strengthens the findings of the study. For patients with ECPR, we do not have an accurate duration of cardiac arrest, which may be an important covariate in ABI. However, ECPR is a subgroup (29%) of the entire study cohort. Finally, since we excluded those on venovenous ECMO support, our study may not reflect more vulnerable patients with primary respiratory failure who may have more severe blood gas derangements. Prospective, multicenter studies should be conducted to validate and generalize our findings.
Conclusions
Using granular ABG data and standardized neuromonitoring protocol, we demonstrated that arterial carbon dioxide before VA-ECMO and its change at cannulation were not associated with mortality or overall ABI occurrence, but higher values before cannulation and greater peri-cannulation drops were associated with ICH. A prospective multi-center study should be conducted to examine causal relationships between peri-cannulation PaCO2 and ABI.
Supplementary Material
Funding statement
SMC is supported by NHLBI (1K23HL157610).
Abbreviations and Acronyms
- ABI
acute brain injury
- HIBI
hypoxic ischemic brain injury
- BMI
body mass index (kg/m2)
- CI
confidence interval
- △PaCO2
peri-cannulation arterial carbon dioxide drop
- ELSO
Extracorporeal Life Support Organization
- ICH
intracranial hemorrhage
- IQR
interquartile range
- NMN
noninvasive multimodal neuromonitoring
- OR
odds ratio
- PaCO2
arterial carbon dioxide tension
- SOFA
Sequential Organ Failure Assessment score
- VA-ECMO
venoarterial extracorporeal membrane oxygenation
Footnotes
Conflict of interest statement
Conflict of interest: none declared.
References
- 1.McCarthy FH, McDermott KM, Kini V, Gutsche JT, Wald JW, Xie D, et al. Trends in U.S. Extracorporeal Membrane Oxygenation Use and Outcomes: 2002–2012. Semin Thorac Cardiovasc Surg. 2015;27(2):81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(1):60–7. [DOI] [PubMed] [Google Scholar]
- 3.Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology [Internet]. Vol. 7, Journal of Thoracic Disease. Pioneer Bioscience Publishing; 2015. [cited 2021 Jan 25]. p. E166–76. Available from: /pmc/articles/PMC4522501/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ECLS Registry Report [Internet]. 2018. [cited 2021 Jan 25]. Available from: https://www.elso.org/Portals/0/Files/Reports/2018/International Summary January 2018 First Page.pdf
- 5.Cho SM, Canner J, Chiarini G, Calligy K, Caturegli G, Rycus P, et al. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: Results from the extracorporeal life support organization registry. Crit Care Med [Internet]. 2020. Oct [cited 2021 Jan 25];48(10):E897–905. Available from: https://journals.lww.com/10.1097/CCM.0000000000004498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SM, Farrokh S, Whitman G, Bleck TP, Geocadin RG. Neurocritical Care for Extracorporeal Membrane Oxygenation Patients. Crit Care Med. 2019;47(12):1773–81. [DOI] [PubMed] [Google Scholar]
- 7.Cho SM, Ziai W, Mayasi Y, Gusdon AM, Creed J, Sharrock M, et al. Noninvasive Neurological Monitoring in Extracorporeal Membrane Oxygenation. ASAIO J. 2020;388–93. [DOI] [PubMed] [Google Scholar]
- 8.Diehl A, Burrell AJC, Udy AA, Alexander PMA, Rycus PT, Barbaro RP, et al. Association between Arterial Carbon Dioxide Tension and Clinical Outcomes in Venoarterial Extracorporeal Membrane Oxygenation. Crit Care Med. 2020;(5):977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HARPER AM, BELL RA. The effect of metabolic acidosis and alkalosis on the blood flow through the cerebral cortex. J Neurol Neurosurg Psychiatry [Internet]. 1963. [cited 2021 Mar 15];26(4):341–4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC495594/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron [Internet]. 2005. [cited 2021 Mar 15];48(6):1011–23. Available from: http://www.neuron.org/cgi/content/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huttunen J, Tolvanen H, Heinonen E, Voipio J, Wikström H, Ilmoniemi RJ, et al. Effects of voluntary hyperventilation on cortical sensory responses. Electroencephalographic and magnetoencephalographic studies. Exp Brain Res [Internet]. 1999. [cited 2021 Mar 7];125(3):248–54. Available from: https://link.springer.com/article/10.1007/s002210050680 [DOI] [PubMed] [Google Scholar]
- 12.Wirrell EC, Camfield PR, Gordon KE, Camfield CS, Dooley JM, Hanna BD. Will a Critical Level of Hyperventilation-Induced Hypocapnia Always Induce an Absence Seizure? Epilepsia [Internet]. 1996. May 1 [cited 2021 Mar 7];37(5):459–62. Available from: http://doi.wiley.com/10.1111/j.1528-1157.1996.tb00592.x [DOI] [PubMed] [Google Scholar]
- 13.Yoon SH, Zuccarello M, Rapoport RM. pCO 2 and pH regulation of cerebral blood flow. Front Physiol [Internet]. 2012. [cited 2021 Mar 15];3 SEP. Available from: /pmc/articles/PMC3442265/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godoy DA, Seifi A, Garza D, Lubillo-Montenegro S, Murillo-Cabezas F. Hyperventilation therapy for control of posttraumatic intracranial hypertension [Internet]. Vol. 8, Frontiers in Neurology. Frontiers Media S.A.; 2017. [cited 2021 Mar 15]. p. 250. Available from: /pmc/articles/PMC5511895/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rout MW, Lane DJ, Wollner L. Prognosis in Acute Cerebrovascular Accidents in Relation to Respiratory Pattern and Blood Gas Tensions. Br Med J [Internet]. 1971. Jul 3 [cited 2021 Mar 15];3(5765):7–9. Available from: /pmc/articles/PMC1800048/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Guo Q, Wang E. Hyperventilation in neurological patients. Curr Opin Anaesthesiol [Internet]. 2019. Oct 1 [cited 2021 Mar 15];32(5):568–73. Available from: https://journals.lww.com/10.1097/ACO.0000000000000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heffner JE, Sahn SA. Controlled Hyperventilation in Patients With Intracranial Hypertension: Application and Management. Arch Intern Med [Internet]. 1983. Apr 1 [cited 2021 Mar 15];143(4):765–9. Available from: https://jamanetwork.com/ [PubMed] [Google Scholar]
- 18.Luyt CE, Bréchot N, Demondion P, Jovanovic T, Hékimian G, Lebreton G, et al. Brain injury during venovenous extracorporeal membrane oxygenation [Internet]. Vol. 42, Intensive Care Medicine. Springer Verlag; 2016. [cited 2021 Mar 12]. p. 897–907. Available from: https://link.springer.com/article/10.1007/s00134-016-4318-3 [DOI] [PubMed] [Google Scholar]
- 19.Bembea MM, Lee R, Masten D, Kibler KK, Lehmann CU, Brady KM, et al. Magnitude of arterial carbon dioxide change at initiation of extracorporeal membrane oxygenation support is associated with survival. J Extra Corpor Technol [Internet]. 2013. Mar 1 [cited 2021 Mar 2];45(1):26–32. Available from: https://europepmc.org/articles/PMC4557460 [PMC free article] [PubMed] [Google Scholar]
- 20.Muellenbach RM, Kilgenstein C, Kranke P, Küstermann J, Kredel M, Roewer N, et al. Effects of venovenous extracorporeal membrane oxygenation on cerebral oxygenation in hypercapnic ARDS. Perfus (United Kingdom) [Internet]. 2014. Mar 25 [cited 2021 Mar 2];29(2):139–41. Available from: http://journals.sagepub.com/doi/10.1177/0267659113497073 [DOI] [PubMed] [Google Scholar]
- 21.Cavayas YA, Munshi L, Del Sorbo L, Fan E. The Early Change in PaCO2 after Extracorporeal Membrane Oxygenation Initiation Is Associated with Neurological Complications. Am J Respir Crit Care Med. 2020;201(12):1525–35. [DOI] [PubMed] [Google Scholar]
- 22.Kikutani K, Ohshimo S, Shime N. Early PaCO2 changes after initiating extracorporeal membrane oxygenation: Considerations for future research. Am J Respir Crit Care Med. 2020;202(11):1600–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullén S, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med [Internet]. 2021. Jun 16 [cited 2021 Sep 28];384(24):2283–94. Available from: https://www.nejm.org/doi/10.1056/NEJMoa2100591 [DOI] [PubMed] [Google Scholar]
- 24.Inoue A, Hifumi T, Sakamoto T, Kuroda Y. Extracorporeal Cardiopulmonary Resuscitation for Out‐of‐Hospital Cardiac Arrest in Adult Patients. J Am Hear Assoc Cardiovasc Cerebrovasc Dis [Internet]. 2020. Apr 9 [cited 2021 Jul 23];9(7):15291. Available from: /pmc/articles/PMC7428656/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lascarrou J-B, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N Engl J Med [Internet]. 2019. Oct 2 [cited 2021 Oct 19];381(24):2327–37. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1906661 [DOI] [PubMed] [Google Scholar]
- 26.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted Temperature Management at 33°C versus 36°C after Cardiac Arrest. N Engl J Med [Internet]. 2013. Dec 4 [cited 2021 Oct 19];369(23):2197–206. Available from: https://www.nejm.org/doi/full/10.1056/nejmoa1310519 [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Shoskes A, Ibrahim M, Amin M, Hasan L, Price C, et al. Does Targeted Temperature Management Improve Neurological Outcome in Extracorporeal Cardiopulmonary Resuscitation (ECPR)? [Internet]. Vol. 1, Journal of Intensive Care Medicine. SAGE PublicationsSage CA: Los Angeles, CA; 2021. [cited 2021 Oct 19]. Available from: https://journals.sagepub.com/doi/full/10.1177/08850666211018982 [DOI] [PubMed] [Google Scholar]
- 28.Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: Findings from the extracorporeal life support organization database. Crit Care Med. 2017;45(8):1389–97. [DOI] [PubMed] [Google Scholar]
- 29.Lorusso R, Barili F, Mauro M Di, Gelsomino S, Parise O, Rycus PT, et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results from the Extracorporeal Life Support Organization Registry. Crit Care Med. 2016;44(10):e964–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


