Abstract
The good pathological response of primary tumors (PTs) to neoadjuvant immunotherapy has been acknowledged in non-small cell lung cancer (NSCLC), however, it remains unclear whether neoadjuvant immunotherapy shows consistent effects in metastatic lymph nodes (LNs). We compared the pathological response of PT and nodal downstaging using a pooled analysis to assess the effect of neoadjuvant immunotherapy on LNs. Original articles reporting the tumor major pathological response (ypT(MPR)), pathological complete response (ypT0) and nodal downstaging following neoadjuvant immunotherapy in NSCLC were retrieved. The OR and 95% CI were calculated by Review Manager V.5.3. Subgroup analysis was performed according to the neoadjuvant therapy regimen used. A total of 209 patients from 6 studies were included in this analysis. The frequency of nodal downstaging was comparable to that of ypT(MPR) (OR 1.31; 95% CI 0.84 to 2.05; p=0.24). Interestingly, ypN0 was observed more frequently than ypT0 (OR 3.26; 95% CI 2.06 to 5.16; p<0.0001). However, this difference was not observed in the subgroup of cN2 patients who underwent immune checkpoint inhibitor monotherapy (OR 1.58; 95% CI 0.56 to 4.48; p=0.39). Neoadjuvant immunotherapy results in satisfactory response in metastatic LN. Patients had a high probability of node clearance when ypT0 was confirmed, especially in patients treated with immunochemotherapy.
Keywords: immunotherapy, lung neoplasms
Introduction
The CheckMate 816 trial proved that neoadjuvant immunotherapy is superior to neoadjuvant chemotherapy in locally advanced non-small cell lung cancer (NSCLC).1 Immunotherapy has been associated with unprecedented high pathological response rates in recent trials, with major pathological response (MPR) and pathological complete response (pCR) rates ranging from 18% to 83% and 4.9% to 63%, respectively.2–7
MPR, defined as no more than 10% viable tumor remaining within the primary tumor (PT) bed and lymph node (LN) after neoadjuvant therapy, was used as a surrogate endpoint of overall survival (OS) and disease-free survival (DFS) in most neoadjuvant immunotherapy trials. However, except for the CheckMate 816 trial,1 the evaluation of the exact percentage of viable tumor cells in LNs is scarcely reported, and only some trials have reported the nodal downstaging rate, that is, evidence of the efficacy of neoadjuvant immunotherapy for LNs is lacking.
Indirect comparison can offer powerful evidence if direct evidence is absent. We compared the pathological response of PT and nodal downstaging using a pooled analysis to assess the effect of neoadjuvant immunotherapy for LNs.
Materials and methods
Data sources and search strategy
MeSH terms, including non-small cell lung cancer, neoadjuvant therapy, and immunotherapy, as well as their combinations, were used to search the PubMed, Embase, Cochrane Library electronic databases and conference abstracts for clinical trials. The detailed search strategy and inclusion criteria are presented in online supplemental materials.
jitc-2022-005160supp001.pdf (316.9KB, pdf)
Data extraction
In this study, we defined MPR in PT as ypT(MPR) and pCR in PT as ypT0. The following information was extracted: ypT(MPR), ypT0, clinical LN metastasis, clinical mediastinal LN metastasis (cN2), nodal downstaging, and nodal clearance (ypN0). Other details such as the immune checkpoint inhibitor (ICI) drug and dose and sample size are also shown in the information sheet.
Statistical analysis
Nodal downstaging, ypN0, ypT(MPR), and ypT0 were recoded into dichotomous variables, which were further compared using OR. All results were reported with 95% CIs. Statistical significance was considered when p<0.05. Heterogeneity was evaluated with χ2 and I2 tests. The fixed effects mode was used when I2<50%. Subgroup analyses were performed by dividing the neoadjuvant regimens into single-agent ICI and immunochemotherapy subgroups. Possible publication bias was examined by funnel plots and Egger’s test.
Risk of bias assessment
The Cochrane Handbook was used to assess the quality of randomized control trials and the quality of prospective phase I or phase II clinical trials was assessed using the Newcastle-Ottawa Scale.
Results
Eligible studies
Six studies,2–7 with a total of 209 enrolled patients, were included in the final analysis, (online supplemental figure 1). Details for the included studies are shown in online supplemental table 1 and table 1. The methodological quality of the included studies can be found in online supplemental table 2.
Table 1.
Pathological response of the primary tumor and lymph nodal downstaging of included studies
| First author | Sample size | Surgery completed | ypT (MPR) |
ypT0 | cN2 | cN1 | cN2 to ypN1 | cN2 to ypN0 | cN1 to ypN0 |
| Provencio2 | 46 | 41 | 34 | 26 | 30 | 3 | 1 | 25 | 3 |
| *Rothschild3 | 68 | 55 | 33 | 10 | 55 | NA | 11 | 26 | NA |
| Shu4 | 30 | 26 | 17 | 10 | 19 | – | 2 | 11 | – |
| Forde5 | 21 | 20 | 9 | 3 | 3 | 9 | 0 | 1 | 4 |
| Gao6 | 49 | 37 | 15 | 6 | 13 | 10 | 3 | 0 | 8 |
| Zhao7 | 33 | 30 | 20 | 15 | 24 | 5 | 1 | 15 | 5 |
*The study only recruited cN2 patients.
MPR, major pathological response; NA, not applicable.
Primary outcomes
ypT(MPR) versus node downstaging
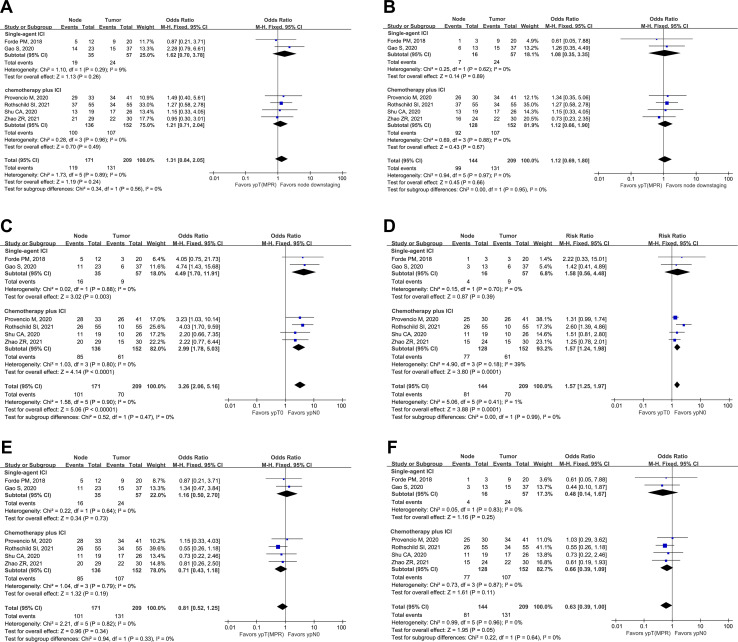
The overall ypT(MPR) rate was 62.7%, 24 of 57 (42.1%) patients in the ICI monotherapy subgroup and 107 of 152 (70.4%) patients in the immunochemotherapy subgroup achieved ypT(MPR). In the 171 patients diagnosed with LN metastases at baseline, nodal downstaging was found in 119 (69.6%) patients, 19 of 35 (54.3%) patients in the ICI monotherapy subgroup and 100 of 136 (73.5%) patients in the immunochemotherapy subgroup. There was a tendency for nodal downstaging to be more likely to occur than ypT(MPR), but no statistical significance was found (OR 1.31; 95% CI 0.84 to 2.05; p=0.24) in either the single-agent ICI subgroup (OR 1.62; 95% CI 0.70 to 3.78; p=0.26) or the immunochemotherapy subgroup (OR 1.21; 95% CI 0.71 to 2.04; p=0.49) (figure 1A).
Figure 1.
Forest plots showing ypT(MPR) versus node downstaging following neoadjuvant immunotherapy in the entire cohort (A) and cN2-only population (B). forest plots showing ypT0 versus ypN0 following neoadjuvant immunotherapy in the entire cohort (C) and cN2-only population (D). forest plots showing Ypt (MPR) versus ypN0 following neoadjuvant immunotherapy in the entire cohort (E) and cN2-only population (F). ICI, immune checkpoint inhibitor; MPR, major pathological response.
In patients with cN2, nodal downstaging was observed in 68.8% (99/144) of patients, 7 of 16 (43.8%) patients on single-agent ICIs, and 92 of 128 (71.9%) patients who underwent immunochemotherapy. The nodal downstaging trend was similar to that of ypT(MPR) for all patients (OR 1.12; 95% CI 0.69 to 1.80; p=0.66), the immunochemotherapy subgroup (OR 1.12; 95% CI 0.66 to 1.90; p=0.67), and the ICI monotherapy subgroup (OR 1.08; 95% CI 0.35 to 3.35; p=0.89) (figure 1B).
ypT0 versus ypN0
Nine of 57 (15.8%) patients achieved ypT0 in the single-agent ICI subgroup and 61 of 152 (40.1%) patients achieved ypT0 in the immunochemotherapy subgroup. The overall ypT0 rate for the 209 patients was 33.5%. In contrast, 101 of 171 (59.1%) patients with positive LN metastasis at baseline were confirmed to be at the ypN0 stage. Nodal downstaging to ypN0 was observed in 16 of 35 (45.7%) patients in the ICI monotherapy subgroup and 85 of 136 (62.5%) patients in the immunochemotherapy subgroup. In the single-agent ICI (OR 4.49; 95% CI 1.70 to 11.91; p=0.003) and in the immunochemotherapy (OR 2.99; 95% CI 1.78 to 5.03; p<0.0001) subgroup as well as in the total analysable cohort (OR 3.26; 95% CI 2.06 to 5.16; p<0.0001), ypN0 occurred more often than ypT0. (figure 1C).
In cN2 patients, neoadjuvant ICI monotherapy led to ypN0 in only 4 of 16 patients (25.0%), whereas neoadjuvant immunochemotherapy led to ypN0 in 77 of 128 (60.2%) patients. Thus, for all 144 cN2 patients combined, ypN0 was achieved in 56.3%. Nodal downstaging to ypN0 was also more likely to occur than ypT0 in both the entire cohort (OR 1.57; 95% CI 1.25 to 1.97; p=0.0001) and the immunochemotherapy subgroup (OR 1.57; 95% CI 1.24 to 1.98; p=0.0001) but not in the ICI monotherapy subgroup (OR 1.58; 95% CI 0.56 to 4.48; p=0.39) (figure 1D).
ypT(MPR) versus ypN0
The ability of neoadjuvant immunotherapy to induce ypT(MPR) was similar to that of ypN0 (OR 0.81; 95% CI 0.52 to 1.25; p=0.34) and this did not differ between the two subgroups (figure 1E). In cN2 patients, we found a tendency that ypT(MPR) was more likely to occur than nodal clearance, and the difference almost reached a significant threshold (OR 0.63; 95% CI 0.39 to 1.00; p=0.05) (figure 1F).
Publication bias test
The funnel plot (online supplemental figure 2) was symmetrically distributed, and Egger’s test showed no obvious publication bias in comparisons of ypT(MPR) versus nodal downstaging (p=0.671), ypT0 versus ypN0 (p=0.932), and ypT(MPR) versus ypN0 (p=0.203).
Discussion
To our knowledge, this study is the first to compare the pathological response in the PT and nodal downstaging of NSCLC following neoadjuvant immunotherapy. The findings indicate that neoadjuvant immunotherapy leads to a substantial response in metastatic LNs.
Nodal downstaging has been widely seen as a positive independent prognostic factor of NSCLC after neoadjuvant therapy. The long-term follow-up outcome of a multicenter phase II trial showed that mediastinal downstaging was associated with OS and DFS in cN2 NSCLC patients following neoadjuvant chemotherapy.8 Another study discovered that only MPRypN0 rather than MPRypN1-2 could accurately predict the DFS of NSCLC after complete resection.9 Based on the relative evidence for neoadjuvant chemotherapy, node downstaging or nodal clearance may play a critical role in long-term survival after neoadjuvant immunotherapy. Considering the better response of LNs to neoadjuvant immunotherapy, a reasonable ratiocination is that a certain number of patients might have nodal downstaging or nodal clearance despite failing to achieve an MPR status in their PTs. These patients might also benefit from neoadjuvant immunotherapy due to the downstaging of the disease. This phenomenon reminds us that a careful evaluation of the response in LNs is necessary for assessing the efficacy of neoadjuvant immunotherapy.
Although neoadjuvant immunotherapy was more effective in nodal downstaging, one may not simply conclude that ypN0 must be present in patients with PT pCR. In the trial of neoadjuvant sintilimab, only three of six patients with PT pCR had complete tumor clearance in LNs.6 In another trial of neoadjuvant nivolumab monotherapy, one patient with PT pCR had persistent hilar LN metastasis.5 Therefore, a complete LN dissection is warranted in any situation.
The other important note is that subgroup analysis in cases with cN2 showed that immunochemotherapy had a greater ability of nodal downstaging than ICI monotherapy (figure 1D). Based on this finding, we suggest immunochemotherapy as the preferred neoadjuvant regimen in patients with LN metastasis, especially cN2 patients. The study of Ling et al revealed a possible explanation for the unsatisfactory capability of mediastinal downstaging under ICI monotherapy. They assessed pathological responses in surgical specimens from 31 patients with squamous cell carcinoma who received neoadjuvant sintilimab monotherapy.10 Their results indicated a lower rate of the immune-activated phenotype in N2 LNs in N1/2-positive patients. Moreover, the percentage of inflamed morphological phenotypes in N2 LNs was lower than that in N1 LNs.
There are some limitations in this pooled analysis. First, this analysis enrolled only 6 studies consisting of 209 patients, and it is impossible to investigate the different efficacies of pathological responses among different ICIs. Second, the enrolled studies had no details about the pathologic responses of histological types. We cannot know whether there is a difference in pathological response and LN downstaging between different histological types. Finally, 22.2% of the enrolled cN2 patients did not have pathologically proven N2, which would exaggerate the ability of nodal downstaging via immunotherapy.
In conclusion, neoadjuvant immunotherapy leads to a substantial response in metastatic LNs, which further supports its application in resectable NSCLC. Preoperative immunochemotherapy may be particularly suitable for patients with LN metastasis, especially in the cN2 population.
Footnotes
W-YZ and Z-RZ contributed equally.
Contributors: W-YZ: data curation, formal analysis, methodology, software, visualization, roles/writing—original draft; Z-RZ: conceptualization, funding acquisition, investigation, roles/writing—original draft, writing—review and editing; SC: data curation, methodology, software; HY: resources, supervision, validation; Y-BL: validation, visualization; Y-ZW: data curation, formal analysis; HL: conceptualization, supervision, writing—review and editing.
Funding: This work was supported by the National Natural Science Foundation of China Youth Science Fund Project (grant number 82002407).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:1413–22. 10.1016/S1470-2045(20)30453-8 [DOI] [PubMed] [Google Scholar]
- 3.Rothschild S, Zippelius A, Eboulet EI, et al. SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC)—A multicenter single-arm phase II trial. Journal of Clinical Oncology 2020;38:9016. 10.1200/JCO.2020.38.15_suppl.9016 [DOI] [PubMed] [Google Scholar]
- 4.Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786–95. 10.1016/S1470-2045(20)30140-6 [DOI] [PubMed] [Google Scholar]
- 5.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816–26. 10.1016/j.jtho.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z-R, Yang C-P, Chen S, et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology 2021;10:1996000. 10.1080/2162402X.2021.1996000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betticher DC, Hsu Schmitz S-F, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099–106. 10.1038/sj.bjc.6603075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corsini EM, Weissferdt A, Pataer A, et al. Pathological nodal disease defines survival outcomes in patients with lung cancer with tumour major pathological response following neoadjuvant chemotherapy. Eur J Cardiothorac Surg 2021;59:100–8. 10.1093/ejcts/ezaa290 [DOI] [PubMed] [Google Scholar]
- 10.Ling Y, Li N, Li L, et al. Different pathologic responses to neoadjuvant anti-PD-1 in primary squamous lung cancer and regional lymph nodes. NPJ Precis Oncol 2020;4:32. 10.1038/s41698-020-00135-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005160supp001.pdf (316.9KB, pdf)