Abstract
Drug interactions involving benzodiazepines and related drugs (BZDs) are increasingly recognized as a contributor to increased risk of unintentional traumatic injury. Yet, it remains unknown to what extent drug interaction triads (3DIs) may amplify BZDs’ inherent injury risk. We identified BZD 3DI signals associated with increased injury rates by conducting high-throughput pharmacoepidemiologic screening of 2000–2019 Optum’s health insurance data. Using self-controlled case series design, we included patients aged ≥ 16 years with an injury while using a BZD + co-dispensed medication (i.e., base pair). During base pair-exposed observation time, we identified other co-dispensed medications as candidate interacting precipitants. Within each patient, we compared injury rates during time exposed to the drug triad versus to the base pair only using conditional Poisson regression, adjusting for time-varying covariates. We calculated rate ratios (RRs) with 95% confidence intervals (CIs) and accounted for multiple estimation via semi-Bayes shrinkage. Among the 65,123 BZD triads examined, 79 (0.1%) were associated with increased injury rates and considered 3DI signals. Adjusted RRs for signals ranged from 3.01 (95% CI = 1.53–5.94) for clonazepam + atorvastatin with cefuroxime to 1.42 (95% CI = 1.00–2.02, p = 0.049) for alprazolam + hydrocodone with tizanidine. These signals may help researchers prioritize future etiologic studies to investigate higher-order BZD interactions.
Subject terms: Therapeutics, Adverse effects, Drug therapy
Introduction
Benzodiazepines and related drugs (hereafter, BZDs) are increasingly prescribed to Americans to treat insomnia and anxiety1,2. The rate of outpatient visits that involved benzodiazepine prescribing more than doubled from 3.8% in 2003 to 7.4% in 20153. Between 2015–2016, 12.6% of United States (US) adults reported using prescription benzodiazepines annually4. Prevalence of past-month use of nonbenzodiazepine Z-drugs (i.e., eszopiclone, zaleplon, zolpidem) among US adults also quadrupled from 0.4% in 1999–2000 to 1.6% in 2013–20145. The increase in BZD use was accompanied by growth in related adverse events4, including traumatic injury6,7. By depressing the central nervous system (CNS), BZDs may cause drowsiness, light-headiness, ataxia, and impaired driving ability, leading to injurious falls and road accidents. BZD use has been consistently implicated in various types of unintentional traumatic injury, including hip fractures and motor vehicle crashes6,8–15.
BZD users commonly have multiple chronic conditions16 and thus are at high risk for polypharmacy. In a sample of commercially insured US adults, new BZD users were taking an average of six co-medications at the time of BZD initiation17. This is potentially concerning since co-dispensed drugs may have pharmacokinetic and/or pharmacodynamic interactions with BZDs and exacerbate their inherent injury risks. Indeed, concomitant use of an interacting drug has been associated with > two-fold risk of injury among BZD users9,18. Reducing these potentially preventable adverse outcomes is important to public health, as unintentional injury is a major cause of morbidity and mortality19. The US Senate Special Committee on Aging and the National Highway Traffic Safety Administration have emphasized identifying drug interactions involving psychoactive drugs like BZDs as a critical strategy to prevent fall-related injury and drug-impaired driving leading to motor vehicle crashes in older adults20,21.
Although prior etiologic studies have examined clinical consequences of pairwise BZD interactions, such as the BZD + opioid combination9,18, beyond pairwise BZD interactions such as drug-drug-drug interactions (3DIs) remain understudied18. While some 3DIs could be postulated based on known pairwise drug interactions, the complex interplay among the drug triads in the real world may necessitate independent examination22. Investigating BZD 3DIs is particularly relevant given the recent concern about the combination of BZDs, opioids, and skeletal muscle relaxants (SMRs)23. Indeed, patients on this triple therapy were found to have 1.3–2.0 times the risk of all-cause hospitalization compared with those on the BZD-opioid or BZD-SMR dual therapy23, raising concerns about 3DIs among these medications.
To address this knowledge gap, we conducted population-based pharmacoepidemiologic screening to identify BZD 3DI signals associated with increased rates of unintentional traumatic injury. By generating an evidence-based list of signals, we sought to help researchers prioritize future etiologic studies aimed to investigate higher-order BZD drug interactions.
Methods
Data source
We conducted pharmacoepidemiologic screening of Optum’s de-identified Clinformatics® Data Mart administrative data from May 1, 2000, through June 30, 2019. The database contains person-level information on enrollment status and healthcare billing records of a large US national sample of commercially insured and Medicare Advantage beneficiaries24 (see details in eMethods). As this study used secondary data routinely collected in healthcare, the University of Pennsylvania’s Institutional Review Board approved the study protocol (#831486) and waived the need to obtain consents from the participants. All methods were performed in accordance with the relevant guidelines and regulations.
Study design
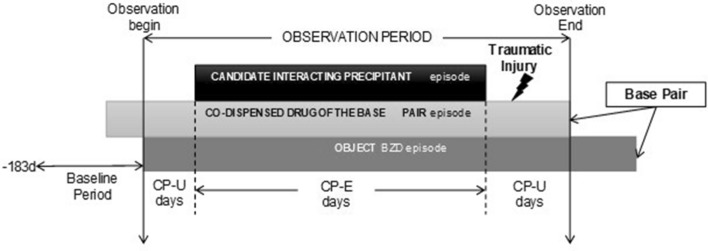
We performed a self-controlled case series (SCCS) for each drug triad comprised of a BZD (object drug, i.e., primary affected drug of the triad), a co-dispensed drug, and a candidate interacting precipitant drug (i.e., primary affecting drug of the triad) (see Fig. 1 for an illustration of the study design). We refer to the BZD + co-dispensed drug as the base pair. Within each study individual, we compared the injury rates during time exposed to the drug triad versus to the base pair only. We selected the SCCS design since this within-person epidemiologic approach cancels out time-invariant confounders automatically25, is highly computationally efficient25, and has been used widely for drug interaction research26–34.
Figure 1.
Example of benzodiazepine and related drug object + co-dispensed drug base pair exposure episode eligible for inclusion. BZD, benzodiazepine and related drug; CP-E, candidate precipitant-exposed; CP-U, candidate precipitant-unexposed.
Study samples
For each base pair, the study sample consisted of patients aged ≥ 16 years old who: (1) initiated a BZD of interest, defined as filling a prescription for BZD without filling one during the 183 days prior (i.e., baseline period); (2) were continuously enrolled during the baseline period; (3) had supply of the co-dispensed drug of the base pair while continuously using the BZD (i.e., were exposed to the base pair); (4) experienced an outcome event during the continuous exposure to the base pair; and (5) used at least one valid candidate interacting precipitant drug (see “Exposure”). BZDs under study were: (1) benzodiazepine receptor agonists, including anxiolytic benzodiazepines (alprazolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, lorazepam, oxazepam), hypnotic benzodiazepines (estazolam, flurazepam, quazepam, temazepam, triazolam), and nonbenzodiazepine Z-drugs (eszopiclone, zaleplon, zolpidem); and (2) related drugs, including dual orexin receptor antagonists (suvorexant) and melatonin receptor agonists (ramelteon, tasimelteon).
Follow-up
Patients were followed from the first day of base pair use until: (1) discontinuation of the base pair, defined as ≥ 1 day without supply of either the BZD or co-dispensed drug; to permit late prescription fills consistent with imperfect adherence, we extended BZD and co-dispensed drug days’ supply values by 20%; (2) switching to non-solid formulation of the BZD; (3) health plan disenrollment; or (4) June 30, 2019, whichever occurred first.
Exposure
The exposure of interest was use (versus non-use) of each candidate interacting precipitant, operationalized as any orally administered medication dispensed during the base pair-defined observation time. Using pharmacy claim dispensing dates and days’ supply, we categorized each observation day as exposed (if covered by the base pair + candidate interacting precipitant) and unexposed (if covered by the base pair only). Since co-dispensed drugs (A and B) given with BZD can be classified as either co-dispensed drug of the base pair or the candidate interacting precipitant, we examined both scenarios: (1) BZD + drug A (base pair) with drug B (candidate interacting precipitant) vs. without drug B; and (2) BZD + drug B (base pair) with drug A (candidate interacting precipitant) vs. without drug A. We used Lexicon Plus (Cerner Multum: Denver, CO, US) to assign co-dispensed drug of the base pair and the candidate interacting precipitants to pharmacologic and therapeutic classes.
Outcome
The primary outcome of interest was unintentional traumatic injury, defined as an emergency department (ED) visit with an any-position diagnosis or an inpatient hospitalization with the principal diagnosis indicative of injury. We followed the injury definition developed by the American College of Surgeon’s National Trauma Data Bank Data Standard35, and further excluded burns as they are unlikely to be attributable to BZD use36. Secondary outcomes included: (1) hip fracture, defined as having a principal inpatient discharge diagnosis indicative of typical open and closed hip fractures, excluding pathological hip fractures and atypical hip fractures; and (2) motor vehicle crash while the individual was driving, defined as having an unintentional injury plus an external cause of injury code for unintended traffic or nontraffic accident, excluding crashes of a self-inflicted, assault, or undetermined manner37. All outcomes were identified by the International Classification of Diseases, Ninth Revision, Clinical Modification, or Tenth Revision (ICD-9-CM or ICD-10) diagnostic codes on the medical claims within the Optum’s de-identified Clinformatics® Data Mart administrative data. We present algorithms, diagnostic codes, and their performance metrics in eTable 1.
Covariates
As the SCCS design inherently controls for time-invariant but not time-varying confounders25, we controlled for key covariates that may have changed during the observation time. We assessed the following variables on each observation day: (1) the average daily BZD dose, dichotomized based on the median; (2) follow-up month38, dichotomized based on two-month; and (3) ever having a prior any-position, any-claim type diagnosis of traumatic injury (yes/no)25.
Statistical analysis
We used conditional Poisson regression to estimate rate ratios (RRs) and 95% confidence intervals (CIs) comparing injury rates during candidate precipitant-exposed vs. candidate precipitant-unexposed observation days, i.e., , adjusting for the covariates named above. To avoid statistical instability, we refrained from estimating RRs if: (1) a base pair-candidate interacting precipitant combination had ≤ 5 exposed patients; (2) no events occurred during candidate interacting precipitant-exposed time; (3) the conditional Poisson regression model could not converge; or 4) the variance of the beta estimate for the parameter of interest was > 10. To reduce the chance for false-positive findings due to multiple testing, we adjusted RRs using semi-Bayes shrinkage39,40. See additional details in eMethods.
Results
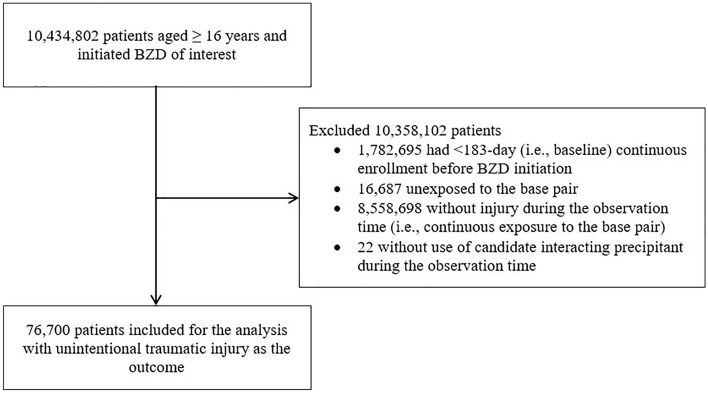
We included 76,700 BZD users in analyses of unintentional traumatic injury (see the flowchart of sample selection in Fig. 2). Table 1 summarizes patient characteristics by the BZD drug samples they contributed to. For the five most commonly used BZDs, these samples included 17,033, 16,851, 13,351, 13,004, and 9566 users of alprazolam, zolpidem, clonazepam, lorazepam, and diazepam, respectively. Most users were white (70.2–71.8%) and female (61.1–69.8%). In analyses of secondary outcomes, we included 2887 BZD users for typical hip fracture (eTable 2) and 629 BZD users for motor vehicle crash (eTable 3).
Figure 2.
Flowchart of sample selection for the analysis for unintentional traumatic injury. BZD, benzodiazepine and related drug.
Table 1.
Characteristics of benzodiazepine and related drug users experiencing an unintentional traumatic injury.
| Benzodiazepine and Related Drugs (N = 76,700 persons in totala) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Alprazolam | Chlordiazepoxide | Clobazam | Clonazepam | Clorazepate | Diazepam | Eszopiclone | Flurazepam | ||
| Persons | Count | 17,033 | 939 | 36 | 13,351 | 440 | 966 | 2891 | 254 |
| Days of observation period, per person | Median (Q1–Q3) | 64.0 (37.0–229.0) | 37.0 (20.0–100.0) | 48.0 (32.0–343.0) | 95.0 (37.0–250.0) | 53.0 (37.0–150.0) | 29.0 (10.0–77.0) | 68.0 (37.0–191.0) | 60.5 (37.0–164.5) |
| Days of observation | Count | 3,729,726 | 71,806 | 1734 | 2,529,898 | 59,172 | 851,794 | 374,719 | 14,403 |
| Age in years | Median (Q1–Q3) | 64.5 (50.0–76.3) | 60.2 (48.7–73.0) | 49.7 (29.5–60.7) | 60.6 (47.6–72.7) | 68.6 (56.1–77.6) | 57.9 (45.7–70.4) | 58.3 (47.8–71.7) | 58.4 (49.4–73.5) |
| Sex, count (%) | Female | 11,884 (69.8) | 441 (59.8) | 7 (63.6) | 7657 (66.5) | 259 (76.4) | 5061 (61.1) | 1457 (65.8) | 71 (68.3) |
| Race, count (%) | White | 12,075 (70.9) | 504 (68.4) | 4 (36.4) | 8165 (71.0) | 227 (67.0) | 5819 (70.2) | 1680 (75.8) | 71 (68.3) |
| African American | 1495 (8.8) | 53 (7.2) | 2 (18.2) | 1017 (8.8) | 18 (5.3) | 764 (9.2) | 159 (7.2) | 6 (5.8) | |
| Hispanic | 1360 (8.0) | 62 (8.4) | 3 (27.3) | 836 (7.3) | 34 (10.0) | 557 (6.7) | 147 (6.6) | 6 (5.8) | |
| Asian | 188 (1.1) | 6 (0.8) | 0 (0.0) | 124 (1.1) | 4 (1.2) | 94 (1.1) | 18 (0.8) | 3 (2.9) | |
| Unknown | 1915 (11.2) | 112 (15.2) | 2 (18.2) | 1366 (11.9) | 56 (16.5) | 1054 (12.7) | 211 (9.5) | 18 (17.3) | |
| Lorazepam | Oxazepam | Ramelteon | Suvorexant | Temazepam | Triazolam | Zaleplon | Zolpidem | ||
|---|---|---|---|---|---|---|---|---|---|
| Persons | Count | 13,004 | 6 | 550 | 527 | 4699 | 194 | 634 | 16,851 |
| Days of observation period, per person | Median (Q1–Q3) | 37.0 (25.0–137.0) | 92.0 (25.0–347.0) | 42.5 (37.0–121.0) | 63.0 (37.0–165.0) | 73.0 (37.0–192.0) | 65.0 (37.0–194.0) | 37.0 (37.0–99.0) | 62.0 (37.0–137.0) |
| Days of observation | Count | 2,025,647 | 1012 | 66,306 | 75,202 | 816,352 | 28,820 | 55,855 | 2,385,936 |
| Age in years | Median (Q1-Q3) | 72.0 (57.6–81.8) | 74.5 (58.3–83.6) | 67.2 (53.6–79.6) | 69.5 (59.8–77.9) | 70.1 (57.1–79.3) | 63.7 (50.2–76.5) | 56.9 (45.6–69.9) | 66.1 (52.3–76.5) |
| Sex, count (%) | Female | 9072 (69.8) | 5 (83.3) | 352 (64.0) | 376 (71.3) | 2992 (63.7) | 135 (69.6) | 427 (67.4) | 10,854 (64.4) |
| Race, count (%) | White | 9336 (71.8) | 5 (83.3) | 381 (69.3) | 353 (67.0) | 3171 (67.5) | 141 (72.7) | 466 (73.5) | 11,904 (70.6) |
| African American | 1105 (8.5) | 0 (0.0) | 63 (11.5) | 66 (12.5) | 416 (8.9) | 9 (4.6) | 44 (6.9) | 1516 (9.0) | |
| Hispanic | 884 (6.8) | 0 (0.0) | 39 (7.1) | 42 (8.0) | 484 (10.3) | 8 (4.1) | 31 (4.9) | 1203 (7.1) | |
| Asian | 160 (1.2) | 0 (0.0) | 9 (1.6) | 6 (1.1) | 64 (1.4) | 2 (1.0) | 9 (1.4) | 293 (1.7) | |
| Unknown | 1519 (11.7) | 1 (16.7) | 58 (10.5) | 60 (11.4) | 564 (12.0) | 34 (17.5) | 84 (13.2) | 1935 (11.5) |
Q, quartile.
aA person may have contributed to multiple drug episodes in the analysis.
Estazolam, quazepam, and tasimelteon were examined but did not have eligible samples with statistically stable models because none of the base pair-candidate interacting precipitant combinations had ≥ 5 exposed patients.
Table 2 provides summary data on RRs for unintentional traumatic injury, before and after covariate adjustment. We examined a total of 65,123 BZD drug triads in adjusted analyses, including 13,685, 12,887, 12,339, 12,231, and 6922 for alprazolam, clonazepam, lorazepam, zolpidem, and diazepam as object drugs, respectively. A total of 79 drug triads—28 for alprazolam, 23 for clonazepam, 19 for lorazepam, 8 for zolpidem, and 1 for diazepam—had statistically elevated RRs after semi-Bayes shrinkage and were thus considered potential 3DI signals. Volcano plots in eFigure 1 graphically depict semi-Bayes shrunk adjusted RRs for these five BZDs; corresponding sensitivity analyses using an alternative variance parameter for semi-Bayes shrinkage yielded similar findings (eFigure 2). We did not observe elevated RRs for base pairs including other BZDs. eTable 4 and eTable 5 provide summary data for typical hip fracture and motor vehicle crash, respectively. We did not observe elevated RRs for any BZD for either of these secondary outcomes. Visualization of all drug triads screened and their associated RRs can be accessed via GitHub and shinyApp links provided in eResults.
Table 2.
Summary data on rate ratios for unintentional traumatic injury, by benzodiazepine and related drug.
| Benzodiazepine and related drugsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alprazolam | Chlordiazepoxide | Clobazam | Clonazepam | Clorazepate | Diazepam | Eszopiclone | Flurazepam | |
| Unadjusted analyses | ||||||||
| Number of base pair-candidate interacting precipitant triads examined | 16,668 | 515 | 1 | 14,684 | 140 | 8206 | 3417 | 19 |
| Range of RRs after semi-Bayes shrinkage, min to max | 0.40–2.54 | 0.53–1.41 | 1.63–1.63 | 0.37–2.77 | 0.43–0.93 | 0.40–1.92 | 0.42–1.82 | 0.53–1.02 |
| Number of triads with statistically significantly elevated ratio of RRs | 17 | 0 | 0 | 21 | 0 | 0 | 0 | 0 |
| Confounder-adjusted analyses | ||||||||
| Number of base pair-candidate interacting precipitant triads examined | 13,685 | 154 | No valid modelsb | 12,887 | 9 | 6922 | 2262 | No valid modelsb |
| Range of RRs after semi-Bayes shrinkage, min to max | 0.38–2.48 | 0.64–1.50 | 0.33–3.01 | 0.46–0.58 | 0.37–2.10 | 0.42–1.89 | ||
| Number of triads with statistically significantly elevated ratio of RRs (i.e., potential 3DI signals) | 28 | 0 | 23 | 0 | 1 | 0 | ||
| Lorazepam | Oxazepam | Ramelteon | Suvorexant | Temazepam | Triazolam | Zaleplon | Zolpidem | |
|---|---|---|---|---|---|---|---|---|
| Unadjusted analyses | ||||||||
| Number of base pair-candidate interacting precipitant triads examined | 13,911 | 1 | 524 | 694 | 5225 | 34 | 390 | 14,508 |
| Range of RRs after semi-Bayes shrinkage, min to max | 0.38–2.66 | 6.09–6.09 | 0.46–1.12 | 0.49–1.36 | 0.41–2.09 | 0.59–0.86 | 0.50–1.58 | 0.36–2.40 |
| Number of triads with statistically significantly elevated ratio of RRs | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Confounder-adjusted analyses | ||||||||
| Number of base pair-candidate interacting precipitant triads examined | 12,339 | No valid modelsb | No valid modelsb | 292 | 4300 | 10 | 32 | 12,231 |
| Range of RRs after semi-Bayes shrinkage, min to max | 0.39–2.54 | 0.60–1.44 | 0.39–1.91 | 0.95–1.36 | 0.59–1.26 | 0.38–2.49 | ||
| Number of triads with statistically significantly elevated ratio of RRs (i.e., potential 3DI signals) | 19 | 0 | 0 | 0 | 0 | 8 | ||
max, maximum; min, minimum; RR, rate ratio.
aEstazolam, quazepam, and tasimelteon were also examined but no models were run because none of the base pair-candidate interacting precipitant combinations had ≥ 5 exposed patients.
bModels were run, but none had valid results (i.e., either model did not converge or variance estimate > 10).
Table 3 lists 3DI signals (N = 79) by BZD and therapeutic category of the co-dispensed drug of the base pair. The most common co-dispensed drugs for BZD signals included CNS with CNS agents (N = 13), cardiovascular with CNS agents (N = 11), and CNS with anti-infective agents (N = 7). Statistically elevated adjusted RRs for unintentional traumatic injury after semi-Bayes shrinkage ranged from 3.01 (95% CI = 1.53–5.94) for clonazepam + atorvastatin with cefuroxime to 1.42 (95% CI = 1.00–2.02, p = 0.049) for alprazolam + hydrocodone with tizanidine.
Table 3.
Benzodiazepine and related drugs (BZD) drug-drug-drug interaction signals of potential clinical concern given statistically significantly increased rates of unintentional traumatic injury, by commonly used BZD and therapeutic category of co-dispensed drug of the base pair.
| BZD | Therapeutic class of co-dispensed drug of the base pair | Co-dispensed drug of the base pair | Candidate Interacting Precipitant | Rate ratio, semi-Bayes shrunk and adjusteda | 95% confidence interval |
|---|---|---|---|---|---|
| ALPRAZOLAM | Cardiovascular | Fenofibrate | Ciprofloxacin | 2.15 | 1.01–4.57 |
| Amlodipine | Pregabalin | 2.02 | 1.10–3.69 | ||
| Diltiazem | Gabapentin | 1.94 | 1.06–3.52 | ||
| Carvedilol | Gabapentin | 1.69 | 1.04–2.75 | ||
| Lisinopril | Gabapentin | 1.42 | 1.03–1.97 | ||
| Central nervous system | Methadone | Ondansetron | 2.48 | 1.16–5.31 | |
| Meloxicam | Fluconazole | 2.34 | 1.13–4.85 | ||
| Aripiprazole | Ondansetron | 2.25 | 1.10–4.64 | ||
| Gabapentin | Fluconazole | 2.22 | 1.25–3.94 | ||
| Quetiapine | Levothyroxine | 2.05 | 1.06–3.95 | ||
| Oxycodone | Morphine | 1.80 | 1.09–2.96 | ||
| Quetiapine | Omeprazole | 1.79 | 1.00–3.18 | ||
| Gabapentin | Quetiapine | 1.72 | 1.02–2.91 | ||
| Oxycodone | Levothyroxine | 1.72 | 1.06–2.77 | ||
| Hydrocodone | Sulfamethoxazole | 1.60 | 1.02–2.50 | ||
| Hydrocodone | Trimethoprim | 1.60 | 1.02–2.49 | ||
| Hydrocodone | Gabapentin | 1.45 | 1.07–1.97 | ||
| Hydrocodone | Tizanidine | 1.42 | 1.00–2.02** | ||
| Endocrine and metabolic | Alendronate | Fluconazole | 2.16 | 1.04–4.49 | |
| Levothyroxine | Risperidone | 1.92 | 1.03–3.60 | ||
| Levothyroxine | Trimethoprim | 1.53 | 1.01–2.30 | ||
| Levothyroxine | Sulfamethoxazole | 1.51 | 1.00–2.28 | ||
| Gastrointestinal | Ranitidine | Furosemide | 2.26 | 1.20–4.24 | |
| Omeprazole | Propranolol | 2.12 | 1.02–4.41 | ||
| Hematological | Clopidogrel | Meloxicam | 2.22 | 1.11–4.45 | |
| Renal and genitourinary | Furosemide | Metolazone | 2.00 | 1.02–3.91 | |
| Furosemide | Sulfamethoxazole | 1.80 | 1.08–3.00 | ||
| Furosemide | Trimethoprim | 1.80 | 1.08–2.99 | ||
| CLONAZEPAM | Cardiovascular | Atorvastatin | Cefuroxime | 3.01 | 1.53–5.94 |
| Simvastatin | Clindamycin | 2.46 | 1.27–4.77 | ||
| Amlodipine | Pantoprazole | 2.00 | 1.16–3.45 | ||
| Metoprolol | Duloxetine | 1.93 | 1.13–3.29 | ||
| Central nervous system | Meloxicam | Furosemide | 2.96 | 1.54–5.69 | |
| Topiramate | Sumatriptan | 2.53 | 1.28–5.00 | ||
| Memantine | Tramadol | 2.24 | 1.15–4.36 | ||
| Donepezil | Metoprolol | 2.17 | 1.08–4.33 | ||
| Mirtazapine | Promethazine | 2.15 | 1.08–4.30 | ||
| Oxycodone | Hydroxyzine | 2.12 | 1.20–3.73 | ||
| Meloxicam | Prednisone | 2.07 | 1.19–3.61 | ||
| Escitalopram | Metoprolol | 2.00 | 1.06–3.78 | ||
| Sertraline | Gabapentin | 1.71 | 1.05–2.77 | ||
| Gabapentin | Duloxetine | 1.61 | 1.02–2.54 | ||
| Endocrine and metabolic | Metformin | Nitrofurantoin | 2.04 | 1.04–4.00 | |
| Metformin | Clindamycin | 2.03 | 1.02–4.04 | ||
| Levothyroxine | Omeprazole | 1.54 | 1.06–2.25 | ||
| Levothyroxine | Gabapentin | 1.49 | 1.08–2.04 | ||
| Gastrointestinal | Omeprazole | Atorvastatin | 1.68 | 1.02–2.78 | |
| Omeprazole | Gabapentin | 1.55 | 1.09–2.21 | ||
| Hematological | Clopidogrel | Furosemide | 1.68 | 1.02–2.78 | |
| Renal and genitourinary | Hydrochlorothiazide | Amlodipine | 1.66 | 1.02–2.70 | |
| Hydrochlorothiazide | Lisinopril | 1.58 | 1.02–2.44 | ||
| DIAZEPAM | Central nervous system | Fluoxetine | Tramadol | 2.05 | 1.03–4.08 |
| LORAZEPAM | Cardiovascular | Benazepril | Trimethoprim | 2.27 | 1.08–4.75 |
| Benazepril | Sulfamethoxazole | 2.15 | 1.02–4.55 | ||
| Fenofibrate | Hydrocodone | 1.90 | 1.06–3.40 | ||
| Amlodipine | Escitalopram | 1.84 | 1.05–3.22 | ||
| Digoxin | Hydrocodone | 1.77 | 1.02–3.07 | ||
| Metoprolol | Escitalopram | 1.75 | 1.04–2.97 | ||
| Amlodipine | Sulfamethoxazole | 1.66 | 1.02–2.72 | ||
| Central nervous system | Memantine | Gabapentin | 2.15 | 1.03–4.50 | |
| Venlafaxine | Hydrochlorothiazide | 2.06 | 1.07–3.98 | ||
| Escitalopram | Nitrofurantoin | 1.93 | 1.03–3.64 | ||
| Citalopram | Sulfamethoxazole | 1.87 | 1.07–3.28 | ||
| Citalopram | Trimethoprim | 1.77 | 1.01–3.11 | ||
| Hydrocodone | Levothyroxine | 1.74 | 1.05–2.90 | ||
| Endocrine and metabolic | Metformin | Escitalopram | 2.01 | 1.02–3.94 | |
| Levothyroxine | Atorvastatin | 1.69 | 1.08–2.64 | ||
| Gastrointestinal | Famotidine | Ondansetron | 2.54 | 1.24–5.24 | |
| Omeprazole | Nitrofurantoin | 2.09 | 1.25–3.50 | ||
| Renal and genitourinary | Furosemide | Clindamycin | 2.27 | 1.21–4.25 | |
| Hydrochlorothiazide | Escitalopram | 1.78 | 1.01–3.15 | ||
| ZOLPIDEM | Cardiovascular | Valsartan | Codeine | 2.05 | 1.04–4.04 |
| Amlodipine | Cyclobenzaprine | 2.01 | 1.21–3.34 | ||
| Amlodipine | Omeprazole | 1.68 | 1.05–2.70 | ||
| Central nervous system | Amitriptyline | Pantoprazole | 2.14 | 1.02–4.52 | |
| Endocrine and metabolic | Levothyroxine | Meloxicam | 1.63 | 1.01–2.61 | |
| Hematological | Warfarin | Azithromycin | 2.15 | 1.11–4.15 | |
| Clopidogrel | Gabapentin | 2.06 | 1.17–3.64 | ||
| Renal and genitourinary | Tamsulosin | Tramadol | 1.98 | 1.14–3.43 |
**p = 0.049.
aRate ratio was calculated as outcome rates during candidate precipitant-exposed person-time divided by outcome rates during candidate precipitant-unexposed days, i.e., , adjusting for the following time-varying covariates: average daily dose of BZD, follow-up month, and ever having a prior traumatic injury of interest.
Discussion
We conducted high-throughput pharmacoepidemiologic screening of real-world data to identify BZD 3DIs that were associated with unintentional traumatic injury. Among 65,123 BZD-base pairs coupled with a candidate interacting precipitant, we identified 79 potential 3DI signals in adjusted analyses, all involving one of the five most commonly dispensed BZDs—alprazolam, clonazepam, lorazepam, zolpidem, or diazepam. We also screened, but detected no signals, for hip fracture or motor vehicle crash, prespecified subsets of unintentional traumatic injury for which we had much smaller samples.
Although drug interactions are increasingly recognized as an important modifiable risk factor for BZD-related injuries9,18, few studies have examined to what extent higher-order interactions may contribute to such risks. One exception is the investigation of triads comprised of a BZD with two other CNS-active agents41, such as the combination of BZD, opioid, and SMR23. The present study yielded many expected results for this combination. For example, our observation that the addition of tizanidine to alprazolam + hydrocodone was associated with 1.40-fold increased rate of injury aligns with a recent investigation showing that combining these drug classes led to a higher risk of all-cause hospitalization23. Moreover, 16% of our identified 3DI signals comprised of BZDs and two other CNS agents. The finding that addition of two CNS agents (vs. one CNS agent) to BZD is associated with increased rate of injury seems to corroborate a previously demonstrated relationship between the total number of CNS medications and risk of injurious falls/fractures41. However, because many of these interacting CNS agents may cause injuries when used alone, whether our observed 3DI signals represent synergism or additivity remains to be determined.
Several other identified 3DI signals deserve further investigation, given their relatively high RRs and biological plausibility. We categorize these signals based on putative mechanisms. First is a candidate interacting precipitant that may pharmacokinetically interact with the BZD and/or the co-dispensed drug in the base pair, thereby compounding the pharmacodynamic interaction between these two within the base pair. For example, the RR = 2.15 for promethazine with clonazepam + mirtazapine may be potentially explained by promethazine’s inhibition of mirtazapine’s metabolism via cytochrome P450 (CYP) 2D6 that has been shown in in vitro studies42 and the resulting enhancement of the pharmacodynamic interaction between mirtazapine and clonazepam. Second is the CNS depressing effect of the candidate interacting precipitant may worsen that resulting from a pairwise pharmacokinetic interaction between BZD and the co-dispensed drug in the base pair. For example, the RR = 1.94 for gabapentin with alprazolam + diltiazem may be possible if gabapentin augments CNS depression arising from the pharmacokinetic interaction between alprazolam (a CYP3A4 substrate)43 and diltiazem (a moderate CYP3A4 inhibitor)44. Third is orthostatic hypotensive effects of the candidate interacting precipitant that may compound CNS depression from BZDs and/or the co-dispensed drug in the base pair. Examples include hydrochlorothiazide with lorazepam + venlafaxine (RR = 2.06) and metoprolol with clonazepam + escitalopram (RR = 2.00). As the resources to examine the clinical sequelae of 3DIs are likely limited, researchers may consider the above signals as targets for future etiologic studies.
We also identified numerous 3DI signals with an anti-infective as the candidate interacting precipitant, some of which may be biologically plausible. Examples include fluconazole with alprazolam + meloxicam (RR = 2.34) and alprazolam + gabapentin (RR = 2.22), respectively. By inhibiting45 alprazolam’s metabolism via CYP3A443, fluconazole may increase the concentration of alprazolam and enhance the pharmacodynamic interactions between alprazolam and meloxicam or gabapentin, potentially explaining the observed RRs. However, underlying mechanisms for most of the anti-infective signals remain unknown, including that with the highest RR—clonazepam + atorvastatin with cefuroxime (RR = 3.01). One possible explanation might be within-person confounding by indication, since infection may suppress drug metabolism via CYP450 pathways, elevating plasma concentrations of the BZD and/or the co-dispensed drug in the base pair (if they are metabolized via CYP450 pathways)46, and amplifying their inherent risks.
Our study has several strengths. First, using the healthcare records of millions of enrollees, we were able to study 3DIs that would be hard to study in smaller settings. Second, we used the analytic SCCS design, a rigorous approach that is not subject to confounding from time-invariant factors. Third, we examined signals associated with traumatic injury, a potentially preventable outcome of major public health importance. Fourth, we defined our study outcome using validated algorithms. Finally, we reduced false-positive signals and increased the specificity of our findings via semi-Bayes shrinkage.
Our study has several limitations. First, because we identified signals by comparing injury rates during person-time exposed to BZD-base pair with versus without a candidate interacting precipitant, some signals may represent inherent risk of the candidate interacting precipitant or pairwise interactions rather than true 3DIs. Future etiologic studies using negative control of each component of the drug triad may help elucidate the nature of the observed signals. Second, the pharmacy records capture only prescription fills without information on whether patients took the drug as recorded and therefore may introduce exposure misclassification, which may bias the results towards either direction. Third, our study may be susceptible to reverse causation. For example, if clinicians prescribed candidate interacting precipitants (such as anti-infectives) for early symptoms of an injury that later resulted in ED presentation or hospitalization, we may see elevated injury rates during the candidate precipitant-exposed time and consider the precipitant as interacting with the base pair. Future etiologic studies using active comparators for the candidate interacting precipitants may help address this limitation. Fourth, some unadjusted time-varying confounders, such as comorbidities, may have explained our findings. Given the high-throughput nature of our screening study, it was impractical to adjust for a comprehensive list of potential time-varying confounders. Yet, we accounted for the key covariates that were most likely to change during the observation period and explain the increased injury rates. Finally, we cannot rule out the possibility that some of our findings may be due to chance.
Conclusions
We identified 79 potential BZD drug-drug-drug interactions with elevated rates of unintentional traumatic injury. These signals included the five most commonly used BZDs—alprazolam, clonazepam, zolpidem, lorazepam, or diazepam. Our findings may provide important targets to guide hypothesis generation and prioritize future etiological investigations into higher-order BZD interactions and risk for unintentional traumatic injury.
Supplementary Information
Acknowledgements
The authors thank Mr. Willem van der Mei from the University of Pennsylvania for his help with data visualization.
Author contributions
C.E.L. and C.M.B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the conceptualization and design of the study and to the interpretation of the results. C.C. and C.E.L. drafted the manuscript. S.H., C.M.B., E.K.A., W.B.B., S.P.C., G.K.D., J.R.H., T.A.M. and T.P. revised the manuscript for important intellectual content. All authors gave final approval of the manuscript.
Funding
The United States (US) National Institutes of Health (R01AG060975, R01DA048001, R01AG064589, and R01AG025152) and the US Centers for Disease Control and Prevention (R01CE003347) supported this work.
Data availability
The data that support the findings of this study are available from Optum’s de-identified Clinformatics® Data Mart. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Optum.
Competing interests
Dr. Leonard is an Executive Committee Member of and Dr. Hennessy directs the University of Pennsylvania’s Center for Real-World Effectiveness and Safety of Therapeutics. The Center receives funds from Pfizer and Sanofi to support pharmacoepidemiology education. Dr. Leonard recently received honoraria from the American College of Clinical Pharmacy Foundation, HealthCanada, the University of Florida, the University of Massachusetts, the Scientific and Data Coordinating Center for the NIDDK-funded Chronic Renal Insufficiency Cohort Study, and the Consortium for Medical Marijuana Clinical Outcomes Research. Dr. Leonard is a Special Government Employee of the United States (US) Food and Drug Administration and consults for their Reagan-Udall Foundation. Dr. Leonard receives travel support from John Wiley & Sons. Dr. Leonard’s spouse is an employee of Merck; neither Dr. Leonard nor his spouse owns stock in the company. Dr. Horn is co-author and publisher of The Top 100 Drug Interactions: A Guide to Patient Management and a consultant to Urovant Sciences and Seegnal US. Dr. Bilker serves on multiple data safety monitoring boards for Genentech. Dr. Hennessy has consulted for multiple pharmaceutical companies. Dr. Pham Nguyen receives support from Acadia Pharmaceuticals Inc., unrelated to this project. All other authors declared no competing interests for this work.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19551-4.
References
- 1.Hwang CS, et al. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002–2014. Am. J. Prev. Med. 2016;51:151–160. doi: 10.1016/j.amepre.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann CN, Spira AP, Alexander GC, Rutkow L, Mojtabai R. Trends in prescribing of sedative-hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol. Drug Saf. 2016;25:637–645. doi: 10.1002/pds.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw. Open. 2019;2:e187399–e187399. doi: 10.1001/jamanetworkopen.2018.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr. Serv. 2019;70:97–106. doi: 10.1176/appi.ps.201800321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann CN, Spira AP, Depp CA, Mojtabai R. Long-term use of benzodiazepines and nonbenzodiazepine hypnotics, 1999–2014. Psychiatr. Serv. 2018;69:235–238. doi: 10.1176/appi.ps.201700095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–3307. doi: 10.1001/jama.1989.03430230088031. [DOI] [PubMed] [Google Scholar]
- 7.Maust DT, et al. Association of Medicare Part D benzodiazepine coverage expansion with changes in fall-related injuries and overdoses among Medicare advantage beneficiaries. JAMA Netw. Open. 2020;3:e202051–e202051. doi: 10.1001/jamanetworkopen.2020.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner AK, et al. Benzodiazepine use and hip fractures in the elderly: Who is at greatest risk? Arch. Intern. Med. 2004;164:1567–1572. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 9.Zint K, et al. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol. Drug Saf. 2010;19:1248–1255. doi: 10.1002/pds.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly K, Bracchi R, Hewitt J, Routledge PA, Carter B. Benzodiazepines, Z-drugs and the risk of hip fracture: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0174730. doi: 10.1371/journal.pone.0174730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbone F, et al. Association of road-traffic accidents with benzodiazepine use. Lancet. 1998;352:1331–1336. doi: 10.1016/S0140-6736(98)04087-2. [DOI] [PubMed] [Google Scholar]
- 12.Smink BE, Egberts AC, Lusthof KJ, Uges DR, De Gier JJ. The relationship between benzodiazepine use and traffic accidents. CNS Drugs. 2010;24:639–653. doi: 10.2165/11533170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Orriols L, et al. Benzodiazepine-like hypnotics and the associated risk of road traffic accidents. Clin. Pharmacol. Ther. 2011;89:595–601. doi: 10.1038/clpt.2011.3. [DOI] [PubMed] [Google Scholar]
- 14.Brandt J, Leong C. Benzodiazepines and Z-drugs: An updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17:493–507. doi: 10.1007/s40268-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tom SE, Wickwire EM, Park Y, Albrecht JS. Nonbenzodiazepine sedative hypnotics and risk of fall-related injury. Sleep. 2016;39:1009–1014. doi: 10.5665/sleep.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llorente MD, David D, Golden AG, Silverman MA. Defining patterns of benzodiazepine use in older adults. J. Geriatr. Psychiatry Neurol. 2000;13:150–160. doi: 10.1177/089198870001300309. [DOI] [PubMed] [Google Scholar]
- 17.Patorno E, Glynn RJ, Levin R, Lee MP, Huybrechts KF. Benzodiazepines and risk of all cause mortality in adults: Cohort study. BMJ. 2017;358:5. doi: 10.1136/bmj.j2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French DD, et al. Effect of concomitant use of benzodiazepines and other drugs on the risk of injury in a veterans population. Drug Saf. 2005;28:1141–1150. doi: 10.2165/00002018-200528120-00008. [DOI] [PubMed] [Google Scholar]
- 19.Haagsma JA, et al. The global burden of injury: Incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj. Prev. 2016;22:3–18. doi: 10.1136/injuryprev-2015-041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Senate Special Committee on Aging. Falls Prevention: National, State, and Local Solutions to Better Support Seniors. https://www.aging.senate.gov/imo/media/doc/SCA_Falls_Report_2019.pdf (2019).
- 21.U.S. Department of Transportation National Highway Traffic Safety Administration. Multiple Medications and Vehicle Crashes: Analysis of Databases. https://trid.trb.org/view/863878 (2008).
- 22.Horn, J. R. Triple Drug Interactions. https://www.pharmacytimes.com/view/interactions-0111 (2011).
- 23.Watanabe JH, Yang J. Hospitalization and combined use of opioids, benzodiazepines, and muscle relaxants in the United States. Hosp. Pharm. 2020;55:286–291. doi: 10.1177/0018578719894702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witenko C, et al. Considerations for the appropriate use of skeletal muscle relaxants for the management of acute low back pain. P T. 2014;39:427. [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: An alternative to standard epidemiological study designs. BMJ. 2016;354:5. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 26.Leonard CE, et al. Screening to identify signals of opioid drug interactions leading to unintentional traumatic injury. Biomed. Pharmacother. 2020;130:110531. doi: 10.1016/j.biopha.2020.110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard CE, et al. Population-based signals of antidepressant drug interactions associated with unintentional traumatic injury. Clin. Pharmacol. Ther. 2021;110(2):409–423. doi: 10.1002/cpt.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam YH, et al. Serious hypoglycemia and use of warfarin in combination with sulfonylureas or metformin. Clin. Pharmacol. Ther. 2019;105:210–218. doi: 10.1002/cpt.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam YH, et al. Sulfonylureas and metformin were not associated with an increased rate of serious bleeding in warfarin users: A self-controlled case series study. Clin. Pharmacol. Ther. 2020;108:1010–1017. doi: 10.1002/cpt.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hee NY, et al. Angiotensin-converting enzyme inhibitors used concomitantly with insulin secretagogues and the risk of serious hypoglycemia. Clin. Pharmacol. Ther. 2022;111(1):218–226. doi: 10.1002/cpt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X, et al. Biomedical informatics approaches to identifying drug-drug interactions: Application to insulin secretagogues. Epidemiology. 2017;28(3):459–468. doi: 10.1097/EDE.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou M, et al. Pharmacoepidemiologic screening of potential oral anticoagulant drug interactions leading to thromboembolic events. Clin. Pharmacol. Ther. 2020;108:377–386. doi: 10.1002/cpt.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard CE, et al. Thromboembolic and neurologic sequelae of discontinuation of an antihyperlipidemic drug during ongoing warfarin therapy. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen TPP, Brensinger CM, Bilker WB, Hennessy S, Leonard CE. Evaluation of serious bleeding signals during concomitant use of clopidogrel and hypnotic drugs. Biomed. Pharmacother. 2021;139:111559. doi: 10.1016/j.biopha.2021.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American College of Surgeons. National Trauma Data Standard: Data Dictionary. https://www.facs.org/quality-programs/trauma/tqp/center-programs/ntdb/ntds/archiveddictionary (2015)
- 36.Sears JM, Bowman SM, Rotert M, Hogg-Johnson S. A new method to classify injury severity by diagnosis: Validation using workers’ compensation and trauma registry data. J. Occup. Rehabil. 2015;25:742–751. doi: 10.1007/s10926-015-9582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Recommended framework for presenting injury mortality data. MMWR: Recommendations and reports: Morbidity and mortality weekly report. In Recommendations and reports/Centers for Disease Control, vol. 46, 1–30 (1997). [PubMed]
- 38.Whitaker HJ, Steer CD, Farrington CP. Self-controlled case series studies: Just how rare does a rare non-recurrent outcome need to be? Biom. J. 2018;60:1110–1120. doi: 10.1002/bimj.201800019. [DOI] [PubMed] [Google Scholar]
- 39.Greenland S. A semi-Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer-mortality study. Stat. Med. 1992;11:219–230. doi: 10.1002/sim.4780110208. [DOI] [PubMed] [Google Scholar]
- 40.Strömberg U. Empirical Bayes and semi-Bayes adjustments for a vast number of estimations. Eur. J. Epidemiol. 2009;24:737–741. doi: 10.1007/s10654-009-9393-0. [DOI] [PubMed] [Google Scholar]
- 41.Musich S, Wang SS, Slindee LB, Ruiz J, Yeh CS. Concurrent use of opioids with other central nervous system-active medications among older adults. Popul. Health Manag. 2020;23:286–296. doi: 10.1089/pop.2019.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He N, Zhang W-Q, Shockley D, Edeki T. Inhibitory effects of H1-antihistamines on CYP2D6-and CYP2C9-mediated drug metabolic reactions in human liver microsomes. Eur. J. Epidemiol. 2002;57:847–851. doi: 10.1007/s00228-001-0399-0. [DOI] [PubMed] [Google Scholar]
- 43.Venkatakrishnan K, Greenblatt DJ, von Moltke LL, Shader RI. Alprazolam is another substrate for human cytochrome P450–3A isoforms. J. Clin. Psychopharmacol. 1998;18:256. doi: 10.1097/00004714-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Yuan R, Flockhart DA, Balian JD. Pharmacokinetic and pharmacodynamic consequences of metabolism-based drug interactions with alprazolam, midazolam, and triazolam. J. Clin. Pharmacol. 1999;39:1109–1125. [PubMed] [Google Scholar]
- 45.Malhotra B, et al. Effects of the moderate CYP3A4 inhibitor, fluconazole, on the pharmacokinetics of fesoterodine in healthy subjects. Br. J. Clin. Pharmacol. 2011;72:263–269. doi: 10.1111/j.1365-2125.2011.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan ET. Impact of infectious and inflammatory disease on cytochrome P450–mediated drug metabolism and pharmacokinetics. Clin. Pharmacol. Ther. 2009;85:434–438. doi: 10.1038/clpt.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Optum’s de-identified Clinformatics® Data Mart. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Optum.