Abstract
The ςB-dependent stress regulon in gram-positive bacteria might fulfill a physiological role in stress response and virulence similar to that of the ςS regulon in Escherichia coli and other gram-negative bacteria. In order to obtain evidence for the function of the ςB regulon of Staphylococcus aureus, especially in virulence control, ςB-dependent stress genes were identified. The two-dimensional protein pattern of wild-type cells of S. aureus COL was compared with that of an isogenic sigB mutant. By this approach, we found that the synthesis of about 27 cytoplasmic proteins seemed to be under the positive control of ςB. N-terminal sequencing of 18 proteins allowed the identification of their genes on the almost finished genome sequence of S. aureus COL and the analysis of the promoter structure. Transcriptional analyses of 11 of these genes confirmed their ςB dependency, and moreover, about 7 additional ςB-dependent genes were found which are cotranscribed with the newly detected genes, forming operons. Altogether, we identified 23 ςB-dependent genes and their corresponding proteins. Among them are proteins probably involved in the generation of NADH or in membrane transport mechanisms. Furthermore, at least one clpC-homologous gene was localized on the S. aureus sequence solely transcribed by ςB. In contrast, a second clpC-homologous gene in S. aureus forming an operon with ctsR, yacH, and yacI was ςB independently expressed.
Staphylococcus aureus is an important human pathogen. Its pathogenesis is very complex and probably involves the synthesis of cell wall-associated adhesins and the secretion of extracellular toxins with damaging effects on host cells, including those of the immune system (48). Nevertheless, even the ability of S. aureus to survive suboptimal growth conditions within the host should be a significant property which contributes to the virulence of this organism and is closely connected with the expression of stress genes (14).
In the gram-positive bacterium Bacillus subtilis, the alternative sigma factor ςB regulates a large number of general stress genes (2, 7, 9, 47, 61; A. Petersohn et al., submitted for publication). Some of these genes are involved in the protection of DNA, membranes, and proteins against oxidative damage, which might represent an important component within the stress response of glucose-starved cells (4, 21, 55). Moreover, ςB-dependent proteins contribute to survival under extreme environmental conditions such as heat or osmotic stress, repeated freezing and thawing, and acid or alkaline shock of starving B. subtilis (23, 63). In summary, the ςB regulon is expected to provide multiple stress resistance to starving B. subtilis cells in anticipation of future stress (26, 60).
A similar physiological role has been postulated for the RpoS (ςS) regulon in Escherichia coli and Salmonella enterica serovar Typhimurium (27, 39). In this context, it is interesting that orthologues of the ςB-dependent genes like katE, dps, opuE, and osmC in B. subtilis are regulated by RpoS in E. coli (4, 20, 39, 57, 62). Since rpoS mutants of gram-negative pathogens show significantly reduced virulence (45, 51, 66), it has been suggested that in pathogenic gram-positive bacteria, the ςB regulon also has a function in the ability of bacteria to interact with host defense mechanisms and persist during infection.
Over the last few years, ςB was identified in the gram-positive pathogens S. aureus (34, 67), Mycobacterium tuberculosis (16), and Listeria monocytogenes (6, 65). As expected, S. aureus and L. monocytogenes ςB mutant cells showed diminished stress tolerance compared with wild-type cells (10, 35, 44, 65). Recent results concerning the involvement of ςB in the virulence of these bacteria do not support the idea that ςB plays a significant role in infection processes (10, 35, 44). However, the question arises of whether the infection models analyzed until now really reflect the natural situation in the host.
In order to elucidate the function of ςB in the pathogenesis of S. aureus, it is necessary to know the genes which are under the control of this alternative sigma factor. Until now, only a few proteins have been identified that belong to the ςB regulon in S. aureus, among them asp23 and coa (25, 35, 43). It has also been demonstrated that the transcription of sar, encoding a global regulator which controls the synthesis of a variety of extracellular and cell surface proteins involved in the pathogenesis of S. aureus, is partly regulated by ςB (17, 41). Therefore, it was very surprising that a sigB mutation is associated with an enhanced SarA level (13). The overproduction of alpha-hemolysin, thermonuclease, and some other extracellular proteins might be the consequence of the up-regulation of SarA in the mutant (13, 35). The role of ςB in the regulation of SarA remains obscure and needs to be further analyzed.
The discovery and functional characterization of new ςB-dependent proteins should improve our understanding of the physiological role of the ςB regulon in S. aureus. High-resolution two-dimensional (2-D) protein gel electrophoresis is an excellent technique for visualizing a very large set of proteins synthesized by a bacterial cell. Looking for proteins that are no longer induced in a regulatory mutant is a good strategy with which to define the structure of regulons. In this study, we used 2-D protein gel electrophoresis and N-terminal sequencing of proteins to detect new members of the ςB regulon to get a more comprehensive view of the physiological role of the general stress response of S. aureus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. aureus strains used in this study were wild-type S. aureus COL and the isogenic sigB mutant (35). S. aureus strains were cultivated in LB (53) or in a synthetic medium described earlier (25). Heat stress conditions were provoked as follows. Cells were cultivated in LB to an optical density at 540 nm of 0.5 and transferred to 48°C. The time of the shift was regarded as zero. Samples were taken during exponential growth immediately prior to the shift or at the time indicated in the relevant figure legends.
Preparative 2-D gel electrophoresis and N-terminal microsequencing.
For preparation of cell extracts, bacteria were grown in the synthetic medium mentioned above. At an optical density at 500 nm of 1.0, cells were harvested by centrifugation of 50 ml of the culture, washed twice with Tris-EDTA buffer, and resuspended in Tris containing 2 mM phenylmethylsulfonyl fluoride. After incubation for 10 min on ice with lysostaphin (50 μg/ml), cells were disrupted using a French press. The lysate was centrifuged (10 min, 10,000 rpm [Heraeus 12148]) at 4°C; the supernatant fluid was stored frozen. Preparative 2-D gel electrophoresis and N-terminal microsequencing of proteins were carried out as described earlier (56) by using immobilized ptt gradients of 4 to 7 and 3 to 10. For microsequencing, the Coomassie-stained protein spots were cut from several 2-D gels and the collected gel pieces were concentrated as previously described (50, 54). The proteins or peptides generated by treatment with cyanogen bromide were blotted onto a polyvinylidene difluoride membrane, stained, and sequenced as previously described (56).
Analysis of transcription.
Total RNA of the S. aureus strains was isolated from exponentially growing or stressed cells by the acid phenol method described by Majumdar et al. (40) with modifications described previously (25, 61).
Northern blot analyses were carried out as described earlier (64). Chemiluminescent signals were detected by the Lumi-Imager from Boehringer Mannheim and analyzed by using the program LumiAnalyst (Boehringer Mannheim).
The specific RNA probes were prepared by in vitro translation with T7 polymerase and with the appropriate PCR fragments as templates. The PCR fragments were generated by using chromosomal DNA of S. aureus COL which was purified with a chromosomal DNA isolation kit in accordance with the protocol of the manufacturer (Promega) and the oligonucleotides listed in Table 1.
TABLE 1.
Oligonucleotides used in this study
| Designationa | Sequence (5′ → 3′) |
|---|---|
| CSB4F | AAACAAAGAAGACGCGGCTG |
| CSB4R | CTAATACGACTCACTATAGGGAGA-ACTTACCTTCGATTGCAGCG |
| CSB7F | CAAATGCCGTATAATTACAAG |
| CSB7R | CTAATACGACTCACTATAGGGAGA-ATATTTAATCTGTTCCAACCG |
| CSB9F | GGTTATAGGTGCTAATGGCG |
| CSB9R | CTAATACGACTCACTATAGGGAGA-CTTTAATGTCCTGATCACCAC |
| CSB10F | CCTACATGTGTCTATTGAGG |
| CSB10R | CTAATACGACTCACTATAGGGAGA-AATGCACCAAAGTTTTCCCC |
| CSB12F | TGTAGTAAATGACACTGGCG |
| CSB12R | CTAATACGACTCACTATAGGGAGA-CTAAGCTTTGGGACCTTTAG |
| CSB16F | TTATATGGCCGAGGCACTAC |
| CSB16R | CTAATACGACTCACTATAGGGAGA-TGTTACAGGTCGGTGATTGC |
| CSB22F | TGCTGATGTAATGGCAGAGC |
| CSB22R | CTAATACGACTCACTATAGGGAGA-AACACGACCTAAGCTTGACC |
| CSB28F | ACAAGAGGTACCGGGTTTAC |
| CSB28R | CTAATACGACTCACTATAGGGAGA-AACTCAACAGGTTGTCCTGC |
| CSB29F | TGGTGTCCTCTTTTACCATG |
| CSB29R | CTAATACGACTCACTATAGGGAGA-TCCAATTCATGCTATCACGC |
| CSB33F | TGTAGCAGAATATGCTGCTG |
| CSB33R | CTAATACGACTCACTATAGGGAGA-AAGCAAAGCGTGACGTAAAG |
| CSB35F (SarAF) | TAGGGAGGTTTTAAACATGG |
| CSB35R (SarAR) | CTAATACGACTCACTATAGGGAGA-GTTGTTTGGTTCAGTGATTC |
| CLPC2392F | CAATTAGAAACACCAAGACCG |
| CLPC2392R | CTAATACGACTCACTATAGGGAGA-ATCTAATGTACCGTCTTTGG |
| CLPC2161F | AAAAATAACACACAATATTC |
| CLPC2161R | CTAATACGACTCACTATAGGGAGA-CTCAACCGATAATTTGATGG |
Oligonucleotides with the letter R contain the recognition sequence for T7 at the 5′ end (29).
The oligonucleotides complementary to the C-terminal region of the genes contain the T7 recognition sequence (29) at the 5′ end (25).
Sequence analyses.
Preliminary sequence data was obtained from The Institute for Genomic Research (TIGR) through the website at http://www.tigr.org. Database searches were carried out using the BLAST program (3).
RESULTS
Identification of proteins belonging to the ςB regulon on 2-D protein gels.
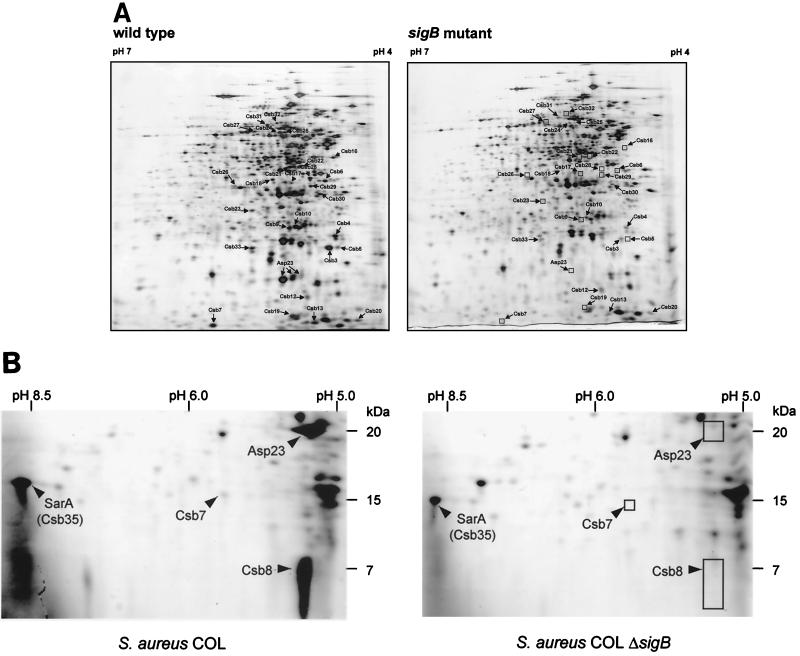
First, we looked for conditions that allowed induction of ςB-dependent stress proteins only in the wild type. Because ςB is active in cells growing in a synthetic medium (25), the protein synthesis patterns of exponentially growing cells of S. aureus COL and its isogenic sigB mutant cultivated in a synthetic medium were compared. This allowed us to identify 27 proteins belonging to the ςB regulon (Fig. 1A and B). These proteins, designated Csb (controlled by sigma B), were not or hardly detectable in the sigB mutant and might be under the positive control of ςB. The N-terminal sequences of 18 of these proteins were determined, and they are listed in Table 2. By using the uncompleted DNA sequence of S. aureus COL kindly provided by TIGR (updated May 1999 and August 1999), we were able to find the open reading frames coding for the majority of the proteins (Table 2). A protein database search was done with the deduced amino acid sequences of the newly identified ςB-dependent genes (Table 3).
FIG. 1.
ςB-dependent proteins of S. aureus. (A) 2-D pattern of cytoplasmic proteins from S. aureus COL and its isogenic sigB mutant. The proteins from 100 (A) or 500 (B) μg of crude cell extract of exponentially growing cells were separated by preparative 2-D polyacrylamide gel electrophoresis. Proteins were stained with silver nitrate (A) or Coomassie blue (B). The protein spots identified are indicated by arrows. Comparison of the protein synthesis patterns of wild-type and sigB mutant S. aureus COL grown in synthetic medium allowed the identification of proteins belonging to the ςB regulon. These proteins, designated Csb (controlled by sigma B), were not or hardly detectable in the mutant strain. (B) Sectors of 2-D gels covering the region where SarA (Csb35) is located. Cytoplasmic protein extracts of wild-type S. aureus COL and of its isogenic sigB mutant grown in synthetic medium were separated.
TABLE 2.
New ςB-dependent proteins in S. aureus
| Protein (length [amino acids]) | N-terminal sequence | Molecular mass (kDa); pIb | Distance (bp) from −10 to ATGd | Suggested −35 promoter sequenced | No. of intervening nucleotides | Suggested −10 promoter sequenced |
|---|---|---|---|---|---|---|
| Csb3 (171) | TKKVAIILANEFEDIEYSSP | 18.6; 4.6 | 38 | GTTTAA | 14 | GTCTAT |
| Csb4 (210) | MELQLAIDLLNKEDAA | 22.4; 4.6 | 43 | GTTTAA | 14 | GGGAAA |
| Csb5 | TKLVAa | |||||
| Csb7 (14) | PYNYKKQNGELM | 16.5; 5.6 | 184 | GTGTGA | 14 | GGGTAG |
| Csb8 (64) | ADESLFEQAL | 7.0; 5.2 | 42 | GTTTAG | 13 | GGGTAA |
| Csb9 (222) | TNILVIGANGGVGSLXVQQL | 24.0; 4.9 | 34 | GTTTTA | 14 | TGGTAT |
| Csb10 (253) | ASGLEIKDLEVE | 28.3; 4.9 | 109 | GTTTAA | 13 | AGGTAT |
| Csb12 (135) | ADITNXNDTGEDRNA | 15.1; 4.9 | 43 | GTTTTA | 14 | GGGTAA |
| Csb13 | MKVVTDVYIa | |||||
| Csb16 (407) | MTFSEKEQIQ | 45.1; 4.6 | 36 | GTTTAA | 13 | GTTTAT |
| Csb19 (140) | SNSQXIQAIENVLATSKVGVL | 15.8; 4.6 | 25 | GTTTAG | 14 | CGCTAT |
| Csb22 (360) | MKIAVGHGNGAVTAXV | 40.7; 4.8 | 106 | GTTTAA | 14 | GGTTAT |
| Csb24 (475) | MYDYTKQRLNGESA | 52.0; 5.0 | 36 | GTTTAT | 14 | GGATAA |
| Csb28 (293) | AAQDPKTKFK | 31.7; 4.7 | 36 | GATTAA | 15 | GGGTAA |
| Csb29 (305) | MENKYTH | 33.6; 4.7 | 40 | GTATTA | 12 | GGGTAT |
| Csb33 (199) | AMNILVFDNSQLVAEYAADI | 22.3; 5.3 | 59 | GTTTGA | 14 | GGGTAT |
| Csb34 | MATTEKPEGNXGAL | |||||
| Csb35 | AITKINDCFELLSMVT | GTGATA | 14 | GGGTATc |
No corresponding DNA sequence was found in the unfinished S. aureus COL database.
The theoretical molecular mass and pI were computed by using Expasy tools.
The promoter sequence was published previously (5).
The translational start sites and promoter sequences were determined by sequence analyses. The consensus sequences of ςB-dependent promoters in B. subtilis, with 13 to 15 intervening nucleotides, are as follows: −35, GTTTAT; −10, GGGTAT. Boldface letters indicate nucleotides essential for promoter activity.
TABLE 3.
Similarities of ςB-dependent S. aureus proteins to proteins in the database
| Protein (length [amino acids]) | Similar protein(s); function (length [amino acids]) | % Identity (no. of amino acids identical/total) |
|---|---|---|
| Csb3 (171) | S. aureus Yly1; hypothetical 18.6-kDa protein (171) | 100 (171/171) |
| B. subtilis YraA; unknown (154) | 56 (86/151) | |
| B. subtilis YfkMa; GS18, unknown (172) | 49 (84/171) | |
| Csb4 (210) | B. subtilis YckG; hexulose-6-phosphate synthase (210) | 55 (115/207) |
| Methanobacterium thermoautotrophicum MTH129; orotidine 5-phosphate decarboxylase (228) | 28 (58/207) | |
| B. subtilis PyrF; orotidine 5-phosphate decarboxylase (239) | 22 (41/185) | |
| Csb7 (141) | B. subtilis YdfG; unknown (147) | 36 (46/126) |
| Dehalospirillum multivorans Orf1; hypothetical protein (177) | 30 (28/93) | |
| Csb8 (64) | B. subtilis YwmGa; unknown (62) | 42 (20/47) |
| Csb9 (222) | S. aureus hypothetical protein (212) | 99 (211/212) |
| B. subtilis YhfKa; unknown (214) | 42 (91/213) | |
| Csb10 (253) | B. subtilis YurI; unknown, V296 vegetative protein, similar to ABC transporter (261) | 80 (200/249) |
| Csb12 (135) | B. subtilis YtrI; unknown (167) | 22 (20/87) |
| B. subtilis YopM; unknown (66) | 28 (13/46) | |
| Csb16 (407) | L. monocytogenes DapE; succinyl-diaminopimelate desuccinylase (379) | 42 (171/403) |
| B. subtilis ArgE; acetylornithine deacetylase (436) | 29 (99/337) | |
| Csb19 (140) | B. subtilis YdaGa; GS26, unknown (140) | 38 (54/139) |
| Csb22 (360) | Arthrobacter sp. ODH; opine dehydrogenase, norvalin dehydrogenase (359) | 25 (90/351) |
| Csb24 (475) | Staphylococcus xylosus CudA; glycine betaine aldehyde dehydrogenase (497) | 36 (175/477) |
| B. subtilis YcnHa; unknown, similar to succinate-semialdehyde dehydrogenase (462) | 37 (173/457) | |
| B. subtilis AldYa; aldehyde dehydrogenase (485) | 33 (155/468) | |
| Csb28 (293) | B. subtilis YhxDa | 60 (176/289) |
| B. subtilis YdaDa; GS39, unknown, similar to alcohol dehydrogenase (286) | 60 (129/267) | |
| B. subtilis YhdFa; unknown, similar to glucose-1-dehydrogenase (289) | 46 (129/279) | |
| Csb29 (305) | B. subtilis BmrUa; multidrug resistance protein cotranscribed with Bmr (297) | 32 (95/292) |
| Csb33 (199) | None found | |
| Csb35b | S. aureus RN450 SarA; staphylococcal accessory regulator (124); | 100 (16/16) |
| S. aureus RN6390 SarA; staphylococcal accessory regulator (113) | 100 (16/16) |
Only three proteins, Csb3, Csb9, and Csb35, have been described in S. aureus so far. Whereas Csb3 and Csb9 are identical to so far hypothetical proteins of S. aureus (8, 38), the N-terminal sequence of Csb35 resembles regulatory protein SarA of S. aureus (12) (Fig. 1B; Table 3). The transcription of the sar locus was already reported to be partly controlled by ςB (17, 41). In our experiments, we showed that the amount of SarA is diminished in a sigB mutant (Fig. 1B). Unfortunately, the function of Csb3 and Csb9 is not known. However, it is interesting that Csb3 is similar to YfkM in B. subtilis, which is also regulated by ςB (46).
The putative functions of nine of the newly identified proteins were derived from similarities to known proteins of other organisms. However, we did not confirm the physiological function of any of these proteins by experiments. Among them are three with similarities to various dehydrogenases: Csb22, Csb24, and Csb28 (Table 3). Interestingly, Csb24 and Csb28 shared similarities with proteins described to be ςB dependent in B. subtilis (46, 47) (Table 3).
Csb10 resembles various ATP-binding cassette transport (ABC transporter) proteins, and Csb29 is very similar to ςB-dependent BmrU in B. subtilis. Proteins encoded by the bmrRU operon in B. subtilis, such as Bmr (transporter) and BmrR (regulator), are known to be responsible for the drug resistance (1). However, BmrU itself did not show any significant similarities to any known proteins in the database and its function remains a matter of speculation.
For the Csb4 protein, we observed weak similarities to YckG of B. subtilis (22), which might encode a hexulose-6-phosphate synthase. Proteins Csb7, Csb8, Csb12, and Csb19 did not display significant similarities to proteins with known functions in the database. Open reading frames coding for proteins Csb5 and Csb13 could not be identified in the databases, and their N termini showed no similarities to known proteins.
Promoter characterization and transcriptional analyses of the newly identified ςB-dependent genes.
In B. subtilis, the recognition sequences for the ςB-containing RNA polymerase are strongly conserved and a consensus of all of the ςB-dependent promoter sequences currently available was derived: GTTTaa and GGG(A/T)A(A/T) for the −35 and −10 regions, respectively, which are separated by 13 to 15 nucleotides (47). The known ςB-dependent promoters in S. aureus are very similar to the consensus sequence in B. subtilis (17, 25, 34, 67). Recently, we have shown that the ςB promoter of asp23 is recognized by EςB in B. subtilis (25). Therefore, we used the consensus of B. subtilis to search for ςB-dependent promoters in front of the identified genes. As a result, we could find similar promoter structures immediately upstream of the translational start codon of 15 of these genes (Table 2).
For transcriptional analyses, we selected 11 genes and in all cases we confirmed their ςB dependency by Northern blots. These results implied that the transcription of the newly identified genes really depends on ςB-containing RNA polymerase in vivo. In all cases, the synthesis of the ςB-dependent transcripts was heat inducible in complex medium and the induction failed in the sigB mutant. Furthermore, we can distinguish between genes controlled solely by ςB and genes regulated in a more complex way.
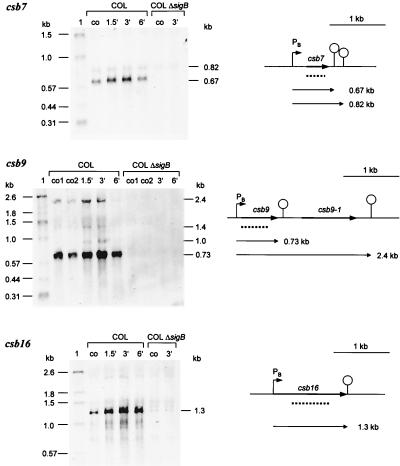
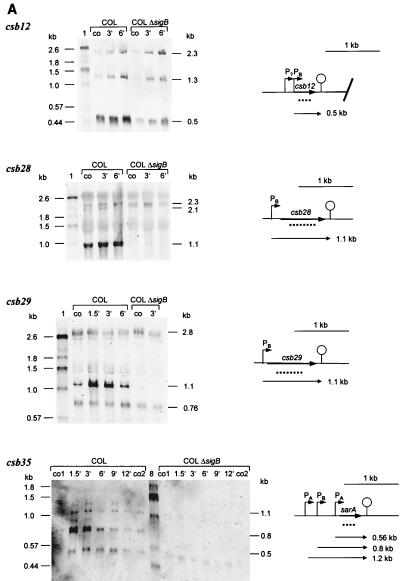
Only csb7, csb9, and csb16 are transcribed solely by ςB under the conditions tested so far (Fig. 2). For the genes csb7 and csb16, we detected one monocistronic transcript, respectively. Both genes were heat inducible. In the case of csb9, two main heat-inducible transcripts were found (0.73 and 2.4 kb), which are synthesized in a ςB-dependent manner. While the 0.73-kb transcript is a monocistronic transcript of csb9, the 2.4-kb transcript contains, in addition to the csb9 message, the mRNA of the open reading frame downstream of csb9, whose product (Csb9-1) is very similar to ManA (mannose-6-phosphate isomerase) in B. subtilis (49) (Table 4).
FIG. 2.
Northern blot analyses of solely ςB-dependent genes. RNA was isolated from S. aureus COL and its isogenic sigB mutant growing in LB at 37°C (lanes co) and at various times after a shift to 48°C. The membrane was hybridized with digoxigenin-labeled RNA probes for the respective genes. Relevant transcripts are indicated. Schematic representations of the gene loci based on sequences of S. aureus COL (TIGR, unpublished data) are shown (PB, ςB-dependent promoter). The broken lines represent the RNA probes used in the experiments whose results are shown. The operon structure of the csb9 locus was verified by using an RNA probe specific for csb9-1.
TABLE 4.
Similarities of new ςB-dependent S. aureus proteins whose genes are cotranscribed with the newly detected genes, forming operons
| Protein (length [amino acids]) | Similar protein(s); function (length [amino acids]) | % Identity (no. of amino acids identical/total) |
|---|---|---|
| Csb4-1 (183) | B. subtilis YckF; hypothetical protein (185) | 43 (76/176) |
| Csb9-1 (338) | S. aureus hypothetical protein (160) | 93 (150/160) |
| Bacillus halodurans mannose-6 phosphate isomerase (315) | 41 (129/311) | |
| Streptococcus mutans ManA; mannose-6-phosphate isomerase (316) | 44 (139/311) | |
| B. subtilis ManA; mannose-6-phosphate isomerase (316) | 42 (134/312) | |
| Csb22-1 (466) | Staphylococcus carnosus NhaC; putative Na+/H+ antiporter (231) | 61 (130/210) |
| Haemophilus influenzae YB07; hypothetical N+/H+ antiporter (468) | 30 (139/458) | |
| B. subtilis YqkI; unknown, similar to Na+/H+ antiporter (468) | 34 (160/460) | |
| B. subtilis YheL; unknown, similar to Na+/H+ antiporter (453) | 31 (140/450) | |
| Csb10-1 (435) | B. subtilis YurX; unknown, similar to unknown proteins (437) | 56 (245/430) |
| Synechocystis sp. hypothetical 52.8-kDa protein SLR0074, ABC transporter subunit (480) | 24 (106/438) | |
| E. coli YnhE; hypothetical protein (508) | 22 (103/450) | |
| B. subtilis YurU; unknown, similar to unknown proteins (465) | 24 (101/419) | |
| Csb10-2 (290) | B. subtilis YurW; similar to NifS protein homolog (406) | 60 (175/290) |
| Csb10-3 (155) | B. subtilis YurV; similar to NifU protein homolog (147) | 72 (101/140) |
| Mycobacterium leprae nitrogen fixation homolog NifU (165) | 38 (54/140) | |
| Csb10-4 (466) | B. subtilis YurU; unknown, similar to unknown proteins (465) | 84 (394/465) |
| Synechocystis sp. hypothetical 52.8-kDa protein SLR0074, ABC transporter subunit (480) | 43 (205/468) | |
| Guillardia theta ABC transporter (483) | 43 (202/467) | |
| E. coli YnhE; hypothetical protein (508) | 39 (190/478) |
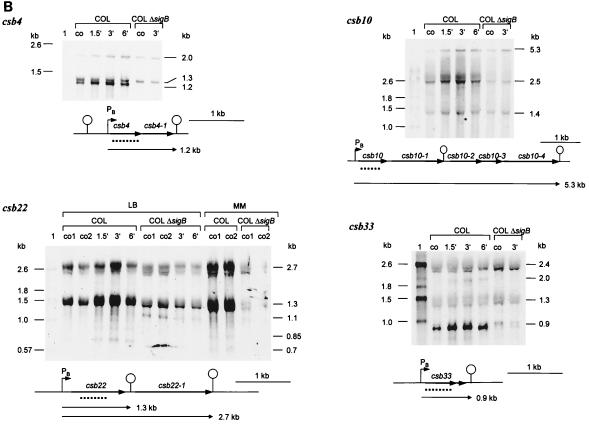
Transcription of csb4, csb10, csb12, csb22, csb28, csb29, csb33, and csb35 (sar) is only partly regulated by ςB (Fig. 3A and B). Transcription still occurred in the mutant, indicating that, in addition to ςB, a second sigma factor is involved. However, with the exception of csb22, in all cases, the main contribution to transcription was that of ςB. Only a low basal level of transcription was found in the sigB mutant which is not heat induced.
FIG. 3.
Northern blot analyses of genes partly regulated by ςB. RNA was isolated from S. aureus COL and its isogenic sigB mutant growing in LB at 37°C (lanes co) and at various times after a shift to 48°C. The membrane was hybridized with digoxigenin-labeled RNA probes for the respective genes. Relevant transcripts are indicated. Schematic representations of the gene loci based on sequences of S. aureus COL (TIGR, unpublished data) are shown (PB, ςB-dependent promoter; PA, ςA-dependent promoter). The broken lines represent the RNA probe used in the experiments whose results are shown. (A) ςB-dependent genes monocistronically transcribed. (B) ςB-dependent operons. We verified the operon structures of the csb10 and csb22 loci by using additional probes (csb10-1, csb10-4, and csb22-1). MM, synthetic medium.
According to the sizes of their transcripts, csb12, csb28, csb29, and csb35 (sar) seem to be monocistronically transcribed by ςB (Fig. 3A). In contrast, the detected ςB-dependent transcripts of the genes csb4, csb10, csb22, and csb33 should contain additional messages of open reading frames located downstream of the genes (Fig. 3B). These data indicate that additional genes belong to the ςB regulon whose products are very similar to subunits of ABC transporters (Csb10-1 and Csb10-4); to NifS (Csb10-2) and NifU (Csb10-3) in B. subtilis (36); to nhaC in S. carnosus, which might encode an Na+/H+ antiporter protein; and to YckF in B. subtilis (Csb4-1).
Transcriptional regulation of two different clp-like genes in S. aureus.
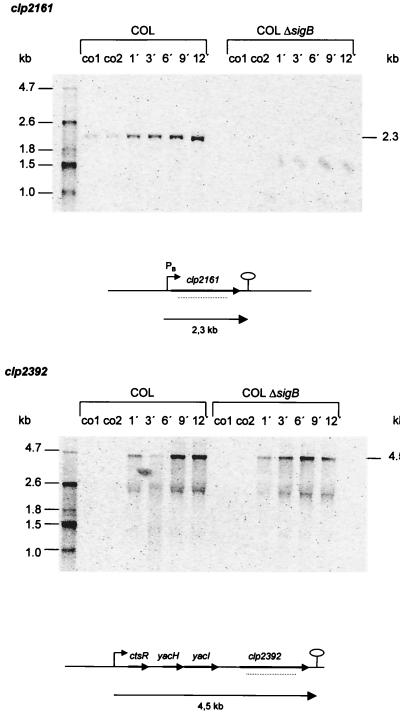
Recently, we have started a second approach in order to identify ςB-dependent genes in S. aureus by looking for genes homologous to ςB-dependent genes of B. subtilis. By this approach, we found at least two genes homologous to clpC in B. subtilis. The corresponding gene products show 44% (Clp2161) and 69% (Clp2392) identity to B. subtilis ClpC. Both proteins contain two nucleotide binding regions that are highly conserved among the Clp ATPases and are separated by a spacer of 60 amino acids in Clp2161 and 67 amino acids in Clp2392. The lengths of the spacers are typical for ClpC proteins (58). The gene order of the clp2392 operon supports the assumption that clp2393 encodes a ClpC protein. The other ClpC-related protein, Clp2161, also shows similarities to ClpE in Lactococcus lactis (54% identical amino acids); however, there is no putative zinc binding domain typical for ClpE proteins.
In Northern blot experiments for clp2161, we detected one heat-inducible transcript of about 2.3 kb, corresponding to the size of the proposed open reading frame only in the wild type and not in the sigB mutant. We found a promoter sequence very similar to the consensus of promoters recognized by ςB in front of the gene (GTTTTA N14 TGGAAA). Computer analysis predicted the presence of a terminator structure at the end of the putative gene (Fig. 4). In contrast, the transcription of clp2392 did not appear to be influenced by a mutation in sigB. We found a 4.5-kb heat-inducible transcript probably containing four genes homologous to ctsR, yacH, yacI, and clpC of B. subtilis (32, 33). Upstream of ctsR, we found a promoter region possibly recognized by the vegetative sigma factor ςA. Furthermore, three CtsR boxes were localized around the promoter region.
FIG. 4.
Northern blot analyses of clpC-homologous genes in S. aureus. RNA was isolated from S. aureus COL and its isogenic sigB mutant growing in LB at 37°C (lanes co) and at various times after a shift to 48°C. The membrane was hybridized with digoxigenin-labeled RNA probes for clp on contig 2161 (236099…21954) and contig 2392 (2866…1966). Relevant transcripts are indicated. Schematic representations of the gene loci based on sequences of S. aureus COL (TIGR, unpublished data) are shown (PB, ςB-dependent promoter). The broken lines represent the RNA probes used in the experiments whose results are shown. We used additional probes to find out which genes are cotranscribed with clp2392.
DISCUSSION
Our data on the ςB-dependent general stress regulon in B. subtilis suggested that this stress response might fulfill a physiological role similar to that of the ςS-dependent response in E. coli and other gram-negative bacteria, thus providing nonspecific, multiple, and general stress resistance to nongrowing cells (26, 27, 39, 63). Moreover, some ςS-dependent genes control virulence genes of gram-negative bacteria (66). Therefore, it was tempting to speculate that ςB is also involved in the control of virulence gene expression in gram-positive bacteria. This suggestion was supported by the finding that ςB initiates transcription at one of the three promoters of sarA encoding one of the important regulators of virulence-associated genes in S. aureus (41).
Only a few genes under ςB-control have been identified so far; among them is that for alkaline shock protein Asp23. However, the identification of Asp23 as a ςB-dependent protein did not make any progress in the functional analysis of the regulon since nothing was known about the function of Asp23 itself (25, 35, 37). In order to obtain a more comprehensive picture of the role of ςB in stress or starvation survival in general, including evidence for the suggested function of the ςB regulon in virulence control, new members of the general stress regulon in S. aureus should be identified. The putative functions of these new proteins should allow a preliminary prediction of the physiological role of the entire regulon. In the present study; we showed that the synthesis of about 27 proteins visible on 2-D gels of crude protein extracts of S. aureus COL are under the positive control of alternative sigma factor ςB. Eighteen of these proteins were identified by N-terminal sequencing, and the open reading frames coding for the proteins were localized on the uncompleted DNA sequence of S. aureus COL kindly provided by TIGR (updated May 1999 and August 1999). By transcriptional analyses of these new genes, about eight additional ςB-dependent genes were found which are cotranscribed with the newly detected genes, forming operons. Comparison of all of the newly identified genes with the B. subtilis genome showed that 20 of them are homologous to B. subtilis genes, only 7 of which are known to be regulated by ςB (Tables 3 and 4). The proteins belonging to the ςB regulon in S. aureus but not in B. subtilis may provide evidence about additional functions of the regulon in gram-positive pathogens. Moreover, two of the genes (csb12 and csb22) in S. aureus did not have any orthologues in B. subtilis. These genes are particularly interesting because they may form a reservoir of ςB-dependent genes whose products could interact in a specific manner with the host. Unfortunately, the function of these genes is still unknown.
For most of the genes described so far, at least a second sigma factor, in addition to ςB, is involved in gene expression. Obviously, the corresponding proteins seem to be necessary even under conditions under which ςB is not active. However, particularly in response to heat shock, the ςB-dependent promoter seemed to be the strongest one in all of the cases tested so far. This complex regulation was also described for many ςB-dependent proteins in B. subtilis (2, 19, 31, 55). Only csb7, csb9, and csb16 seem to be transcribed solely by ςB under the conditions tested so far.
The newly identified genes allow first conclusions on the physiological role of the ςB regulon in S. aureus. Protection of starved B. subtilis cells against oxidative damage could be the most essential component of ςB-mediated stress resistance (21). In accordance with this, a sigB mutant of S. aureus showed increased sensitivity to hydrogen peroxide (10, 35). Furthermore, maintenance of intracellular redox balance under stress and starvation might be very important and requires the reduction of oxidized biological molecules by using NAD(P)H. Therefore, a sufficient level of NAD(P)H seems to be a prerequisite for the cell to face oxidative stress. Among the newly identified genes, three are possible dehydrogenases which could be involved in the generation of reduction equivalents like NAD(P)H and FADH. In B. subtilis, the nifS gene product might contribute to NAD biosynthesis by generating the Fe-S clusters required for NadA activity (59). Interestingly, among the newly identified S. aureus genes there is one nifS-homologous gene.
Prokaryotic, as well as eukaryotic, organisms possess multidrug resistance efflux transporters whose expression is induced by various structurally divergent compounds such as antibiotics, inhibitors, and other toxic substances. In B. subtilis, the bmrUR operon, which encodes proteins that may contribute to resistance to multidrug compounds, is regulated by ςB. Very recently, it was shown that ςB is activated in mycobacterial cells after exposure to rifampin, streptomycin, and cycloserine (42). In S. aureus, methicillin resistance is widespreaded; however, the mechanism of this phenomen is not fully understood. Interestingly, it was reported that the sigB mutant of S. aureus COL showed a drastic reduction in methicillin resistance (15). Among the newly identified ςB-dependent proteins of S. aureus, we found proteins with significant similarities to Na+/H+ antiporters or ABC transporters. The Na+/H+ antiporters are widely distributed in cell membranes from bacteria to mammals. The antiporters play important roles in the Na+ cycle across the cytoplasmic membrane of all living cells. In bacteria, the antiporter extrudes Na+ or Li+ in exchange for H+. Besides their function in antibiotic resistance, they may play a role in (i) the establishment of an electrochemical potential of Na+ across the cytoplasmic membrane, (ii) the extrusion of Na+ and Li+, (iii) intracellular pH regulation under alkaline conditions, and (iv) cell volume regulation (11, 28).
 Besides the identification of ςB-dependent genes by proteomics, we started a second approach. In B. subtilis, we know of more than 150 genes belonging to the regulon (Petersohn et al., submitted for publication); among them are Clp proteins with essential functions in stress resistance (24, 30).
Besides the identification of ςB-dependent genes by proteomics, we started a second approach. In B. subtilis, we know of more than 150 genes belonging to the regulon (Petersohn et al., submitted for publication); among them are Clp proteins with essential functions in stress resistance (24, 30).
Recently, ClpC was shown to be involved in the virulence of L. monocytogenes (52). Therefore, we looked for clp-homologous genes in the S. aureus genome sequence. As a result, we localized two open reading frames possibly encoding ClpC proteins. It is a remarkable finding that at least one clpC-homologous gene seemed to be controlled solely by alternative stress sigma factor ςB and the other is probably controlled by the global regulator of class III general stress genes CtsR; however, experimental evidence for this is still lacking (18, 33). The Clp proteins are involved in several physiological processes, such as proteolysis, stress tolerance, competence, cell division, and virulence. The presence of at least two clpC-homologous genes in S. aureus implies an essential role of the protein in the physiology of this organism.
The identification of new members of the ςB regulon is a preliminary but essential step toward a more comprehensive understanding of the role of this large regulon in stress adaptation and virulence. No evidence has previously been presented for a role of ςB in the infection process and virulence (10, 44). Analysis of the effects of promising mutations in individual ςB-dependent genes on stress adaptation and infection is another approach to the problem of whether and to what extent ςB-dependent proteins contribute to survival within the host. These studies will provide an essential contribution to the understanding of the cell physiology of S. aureus.
ACKNOWLEDGMENTS
We are indebted to P. Bednarski for critical reading the manuscript. We are very grateful to Ines Kullik for providing S. aureus strain COL ΔsigB and Markus Bischoff for fruitful discussion. Elke Krüger and Ulf Gerth are acknowledged for a helpful discussion of Clp proteins. Furthermore, we thank Renate Gloger for excellent technical assistance. Preliminary sequence data was obtained from TIGR through the website at http://www.tigr.org.
Sequencing of S. aureus COL was accomplished with support from National Institute of Allergy and Infectious Diseases and The Merck Genome Research Institute. This work was supported by a grant from the Fonds der chemischen Industrie to M.H.
REFERENCES
- 1.Ahmed M, Borsch C M, Taylor S S, Vazquez-Laslop N, Neyfakh A A. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269:28506–28513. [PubMed] [Google Scholar]
- 2.Akbar S, Lee S Y, Boylan S A, Price C W. Two genes from Bacillus subtilis under the sole control of the general stress transcription factor ςB. Microbiology. 1999;145:1069–1078. doi: 10.1099/13500872-145-5-1069. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Lipman D J. Protein database searches for multiple alignments. Proc Natl Acad Sci USA. 1990;87:5509–5513. doi: 10.1073/pnas.87.14.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antelmann H, Engelmann S, Schmid R, Sorokin A, Lapidus A, Hecker M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor ςB in Bacillus subtilis. J Bacteriol. 1998;179:7251–7256. doi: 10.1128/jb.179.23.7251-7256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer M G, Heinrichs J H, Cheung A L. The molecular architecture of the sar locus in Staphylococcus aureus. J Bacteriol. 1996;178:4563–4570. doi: 10.1128/jb.178.15.4563-4570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker L A, Cetin M S, Hutkins R W, Benson A K. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 8.Borchardt S A, Babwah A V, Jayaswal R K. Sequence analysis of the region downstream from a peptidoglycan hydrolase-encoding gene from Staphylococcus aureus NCTC8325. Gene. 1993;137:253–258. doi: 10.1016/0378-1119(93)90016-v. [DOI] [PubMed] [Google Scholar]
- 9.Boylan S, Redfield A, Price C. Transcription factor ςB of Bacillus subtilis controls a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng J, Hicks D B, Krulwich T A. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/H+ and Na+(K+)/H+ exchange. Proc Natl Acad Sci USA. 1996;93:14446–14451. doi: 10.1073/pnas.93.25.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung A L, Projan S J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung A L, Chien Y T, Bayer A S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67:1331–1337. doi: 10.1128/iai.67.3.1331-1337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements M O, Foster S J. Stress resistance in Staphylococcus aureus. Trends Microbiol. 1999;7:458–462. doi: 10.1016/s0966-842x(99)01607-8. [DOI] [PubMed] [Google Scholar]
- 15.De Lencastre H, Wu S W, Pinho M G, Ludovice A M, Filipe S, Gardete S, Sobral R, Gill S, Chung M, Tomasz A. Antibiotic resistance as a stress response: complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb Drug Resist. 1999;5:163–175. doi: 10.1089/mdr.1999.5.163. [DOI] [PubMed] [Google Scholar]
- 16.DeMaio J, Zhang Y, Ko C, Young D B, Bishai W R. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deora R, Tseng T, Misra T K. Alternative transcription factor ςSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J Bacteriol. 1997;179:6355–6359. doi: 10.1128/jb.179.20.6355-6359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derré I, Rapoport G, Msadek T. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol Microbiol. 1999;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 19.Drzewiecki K, Eymann C, Mittenhuber G, Hecker M. The yvyD gene of Bacillus subtilis is under dual control of ςB and ςH. J Bacteriol. 1998;180:6674–6680. doi: 10.1128/jb.180.24.6674-6680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelmann S, Lindner C, Hecker M. Cloning, nucleotide sequence, and regulation of katE encoding a ςB-dependent catalase in Bacillus subtilis. J Bacteriol. 1995;177:5595–5605. doi: 10.1128/jb.177.19.5598-5605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelmann S, Hecker M. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol Lett. 1996;145:63–69. doi: 10.1111/j.1574-6968.1996.tb08557.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujishima Y, Yamane K. A 10 kb nucleotide sequence at the 5′ flanking region (32 degrees) of srfAA of the Bacillus subtilis chromosome. Microbiology. 1995;141:277–279. doi: 10.1099/13500872-141-2-277. [DOI] [PubMed] [Google Scholar]
- 23.Gaidenko T A, Price C W. General stress transcription factor ςB and sporulation transcriptionfactor ςH each contribute to survival of Bacillus subtilis under extreme growth conditions. J Bacteriol. 1998;180:3730–3733. doi: 10.1128/jb.180.14.3730-3733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerth U, Krüger E, Derré I, Msadek T, Hecker M. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol Microbiol. 1998;28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 25.Gertz S, Engelmann S, Schmid R, Ohlsen K, Hacker J, Hecker M. Regulation of ςB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol Gen Genet. 1999;261:558–566. doi: 10.1007/s004380051001. [DOI] [PubMed] [Google Scholar]
- 26.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the ςB regulon. Mol Microbiol. 1998;29:1129–1137. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 27.Hengge-Aronis R. Survival of hunger and stress—the role of rpoS in early stationary phase gene regulation in Escherichia coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 28.Hiramatsu T, Kodama K, Kuroda T, Mizushima T, Tsuchiya T. A putative multisubunit Na+/H+ antiporter from Staphylococcus aureus. J Bacteriol. 1998;180:6642–6648. doi: 10.1128/jb.180.24.6642-6648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgensen E D, Durbin R K, Risman S S, McAllister W T. Specific contacts between the bacteriophage T3, T7, and SP6 RNA polymerases and their promoters. J Biol Chem. 1991;266:645–651. [PubMed] [Google Scholar]
- 30.Krüger E, Völker U, Hecker M. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J Bacteriol. 1994;176:3360–3367. doi: 10.1128/jb.176.11.3360-3367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krüger E, Msadek T, Hecker M. Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol Microbiol. 1996;20:713–723. doi: 10.1111/j.1365-2958.1996.tb02511.x. [DOI] [PubMed] [Google Scholar]
- 32.Krüger E, Msadek T, Ohlmeier S, Hecker M. The Bacillus subtilis clpC operon encodes DNA repair and competence proteins. Microbiology. 1997;143:1309–1316. doi: 10.1099/00221287-143-4-1309. [DOI] [PubMed] [Google Scholar]
- 33.Krüger E, Hecker M. The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes. J Bacteriol. 1998;180:6681–6688. doi: 10.1128/jb.180.24.6681-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kullik I, Giachino P. The alternative sigma factor sigmaB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 35.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 37.Kuroda M, Ohta T, Hayashi H. Isolation and the gene cloning of an alkaline shock protein in methicillin resistant Staphylococcus aureus. Biochem Biophys Res Commun. 1995;207:978–984. doi: 10.1006/bbrc.1995.1281. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda M, Hayashi H, Ohta T. Chromosome-determined zinc-responsible operon czr in Staphylococcus aureus strain 912. Microbiol Immunol. 1999;43:115–125. doi: 10.1111/j.1348-0421.1999.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 39.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar D, Avissar Y J, Wyche J H. Simultaneous and rapid isolation of bacterial and eukaryotic DNA and RNA—a new approach for isolating DNA. BioTechniques. 1991;11:94–101. [PubMed] [Google Scholar]
- 41.Manna A C, Bayer M G, Cheung A L. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180:3828–3836. doi: 10.1128/jb.180.15.3828-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michele T M, Ko C, Bishai W R. Exposure to antibiotics induces expression of the Mycobacterium tuberculosis sigF gene: implications for chemotherapy against mycobacterial persistors. Antimicrob Agents Chemother. 1999;43:218–225. doi: 10.1128/aac.43.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyazaki E, Chen J M, Ko C, Bishai W R. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J Bacteriol. 1999;181:2846–2851. doi: 10.1128/jb.181.9.2846-2851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholas R O, Li T, McDevitt D, Marra A, Sucoloski S, Demarsh P L, Gentry D R. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect Immun. 1999;67:3667–3669. doi: 10.1128/iai.67.7.3667-3669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersohn A, Antelmann H, Gerth U, Hecker M. Identification and transcriptional analysis of new members of the sigmaB regulon in Bacillus subtilis. Microbiology. 1999;145:869–880. doi: 10.1099/13500872-145-4-869. [DOI] [PubMed] [Google Scholar]
- 47.Petersohn A, Bernhardt J, Gerth U, Höper D, Koburger T, Völker U, Hecker M. Identification of ςB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J Bacteriol. 1999;181:5718–5724. doi: 10.1128/jb.181.18.5718-5724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Projan S J, Novick R P. The molecular basis of pathogenicity. In: Crossley A B, Archer G L, editors. The staphylococci in human disease. Edinburgh, Scotland: Churchill Livingstone, Ltd.; 1997. pp. 55–81. [Google Scholar]
- 49.Rashid M H, Mori M, Sekiguchi J. Glucosaminidase of Bacillus subtilis: cloning, regulation, primary structure and biochemical characterization. Microbiology. 1995;141:2391–2404. doi: 10.1099/13500872-141-10-2391. [DOI] [PubMed] [Google Scholar]
- 50.Rider M H, Puype M, Van Damme J, Gevaert K, De Boeck S, D'Alayer J, Rasmussen H H, Celis J E, Vanderkerchove J. An agarose-based gel-concentration system for microsequence and mass spectrometric characterization of proteins previously purified in polyacrylamide gels starting at low picomole levels. Eur J Biochem. 1995;230:258–265. doi: 10.1111/j.1432-1033.1995.0258i.x. [DOI] [PubMed] [Google Scholar]
- 51.Robbe-Saule V, Coynault C, Norel F. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol Lett. 1995;126:171–176. doi: 10.1111/j.1574-6968.1995.tb07412.x. [DOI] [PubMed] [Google Scholar]
- 52.Rouquette C, Ripio M T, Pellegrini E, Bolla J M, Tascon R I, Vazquez-Boland J A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 54.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 55.Scharf C, Riethdorf S, Ernst H, Engelmann S, Völker U, Hecker M. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmid R, Bernhardt J, Antelmann H, Völker A, Mach H, Völker U, Hecker M. Identification of vegetative proteins for a two-dimensional protein index of Bacillus subtilis. Microbiology. 1997;143:991–998. doi: 10.1099/00221287-143-3-991. [DOI] [PubMed] [Google Scholar]
- 57.Spiegelhalter F, Bremer E. Osmoregulation of the opuE proline transport gene from Bacillus subtilis: contributions of the sigma A- and sigma B-dependent stress-responsive promoters. Mol Microbiol. 1998;29:285–296. doi: 10.1046/j.1365-2958.1998.00929.x. [DOI] [PubMed] [Google Scholar]
- 58.Squires C, Squires C L. The Clp proteins: proteolysis regulators or molecular chaperones? J Bacteriol. 1992;174:1081–1085. doi: 10.1128/jb.174.4.1081-1085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun D, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis nadB gene and a nifS-like gene, both of which are essential for NAD biosynthesis. J Bacteriol. 1993;175:1423–1432. doi: 10.1128/jb.175.5.1423-1432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijay K, Brody M S, Fredlund E, Price C W. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the SigmaB transcription factor of Bacillus subtilis. Mol Microbiol. 2000;35:180–188. doi: 10.1046/j.1365-2958.2000.01697.x. [DOI] [PubMed] [Google Scholar]
- 61.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 62.Völker U, Andersen K K, Antelmann H, Devine K M, Hecker M. One of two OsmC homologs in Bacillus subtilis is part of the ςB-dependent general stress regulon. J Bacteriol. 1998;180:4212–4218. doi: 10.1128/jb.180.16.4212-4218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Völker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiedmann M, Arvik T J, Hurley R J, Boor K J. General stress transcription factor ςB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson J A, Gulig P A. Regulation of the spvR gene of the Salmonella typhimurium virulence plasmid during exponential-phase growth in intracellular salts medium and at stationary phase in L broth. Microbiology. 1998;144:1823–1833. doi: 10.1099/00221287-144-7-1823. [DOI] [PubMed] [Google Scholar]
- 67.Wu S, de Lencastre H, Tomasz A. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J Bacteriol. 1996;178:6036–6042. doi: 10.1128/jb.178.20.6036-6042.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]