Abstract
Protein tyrosine kinases (PTKs) are a class of proteins with tyrosine kinase activity that phosphorylate tyrosine residues of critical molecules in signaling pathways. Their basal function is essential for maintaining normal cell growth and differentiation. However, aberrant activation of PTKs caused by various factors can deviate cell function from the expected trajectory to an abnormal growth state, leading to carcinogenesis. Inhibiting the aberrant PTK function could inhibit tumor growth. Therefore, tyrosine kinase inhibitors (TKIs), target-specific inhibitors of PTKs, have been used in treating malignant tumors and play a significant role in targeted therapy of cancer. Currently, drug resistance is the main reason for limiting TKIs efficacy of cancer. The increasing studies indicated that tumor microenvironment, cell death resistance, tumor metabolism, epigenetic modification and abnormal metabolism of TKIs were deeply involved in tumor development and TKI resistance, besides the abnormal activation of PTK-related signaling pathways involved in gene mutations. Accordingly, it is of great significance to study the underlying mechanisms of TKIs resistance and find solutions to reverse TKIs resistance for improving TKIs efficacy of cancer. Herein, we reviewed the drug resistance mechanisms of TKIs and the potential approaches to overcome TKI resistance, aiming to provide a theoretical basis for improving the efficacy of TKIs.
Subject terms: Cancer, Cancer
Introduction
Malignant tumors are the second leading cause of death and a public health concern worldwide. Anti-tumor therapy is the core means of reducing tumor mortality, and related research mainly focuses on molecular level1. Oncogenic mutations of cell signaling molecules lead to abnormal clonal proliferation, as the main characteristic of tumor cells. Some signaling proteins and pathways are susceptible to oncogenic mutations in normal cells, particularly molecules that control cell growth, differentiation, and developmental signals, one of which is protein tyrosine kinases (PTKs).1 PTKs are a class of proteins with tyrosine kinase activity whose function is tightly regulated in normal cells. Perturbation of PTKs function by mutations and other genetic alterations can lead to malignant transformation1,2. Previous studies have shown that more than 80% of oncogenes and proto-oncogenes finally increase PTKs expression.2 Therefore, inhibition of PTK overactivity is a major strategy for treating cancer.
Tyrosine kinase inhibitors (TKIs) are target-specific inhibitors of abnormal PTKs. As homologs of adenosine triphosphate (ATP), TKIs competitively occupy the ATPs-binding site of PTKs and block PTK-mediated signaling pathways in cancer cells, thereby inhibiting their growth and proliferation. TKIs have significant advantages over traditional chemotherapeutic agents, including high efficiency, low toxicity, and high specificity. Presently, TKIs are widely used for treating leukemia, non-small-cell lung cancer (NSCLC), renal cell carcinoma (RCC), gastrointestinal stromal tumor (GIST), breast cancer, and hepatocellular carcinoma (HCC), and their clinical application is rapidly growing.3–5
Tumor cells gradually develop resistance during TKIs therapy; however, the time frame for developing acquired drug resistance is not long. For instance, the effective time frame for treating NSCLC with first-generation epidermal growth factor receptor (EGFR) TKIs does not exceed one year.6 In addition, a small proportion of tumor cells are primarily resistant to TKIs. NSCLCs with Bcl-2-interacting mediator of cell death (BIM) deletion polymorphisms are primarily resistant to osimertinib.7 TKI resistance is a leading cause of recurrence, progression, treatment failure, low compliance and mortality among cancer patients. The mechanisms of TKIs resistance are complex. Previous studies have affirmed the relationship between TKI resistance and target-gene mutations. A growing body of evidence suggests that other factors, such as tumor microenvironment (TME) and epigenetics, are also involved in TKI resistance.8
Despite the remarkable effectiveness of TKIs in targeted therapy, TKIs resistance is increasingly growing. Here, we summarize the underlying mechanisms of TKIs resistance and discuss the potential approaches to overcome it. We aim to provide a theoretical basis for future research and management of TKIs resistance.
PTK, TKI, and cancer
PTK and cancer
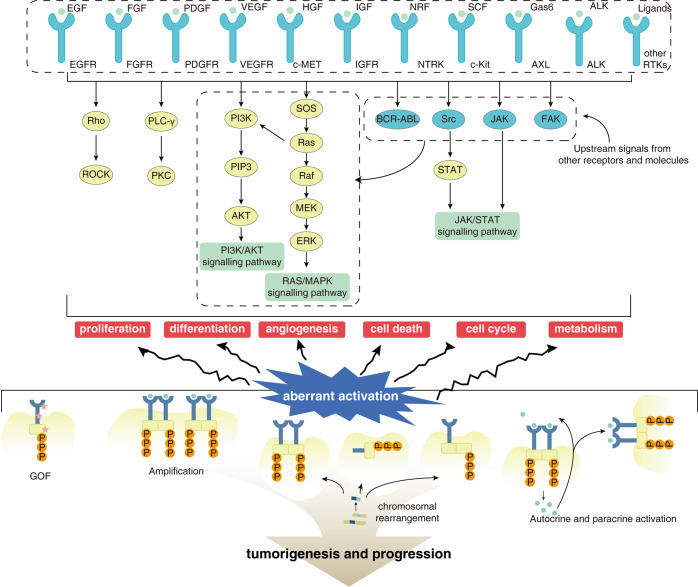
PTK activity is critical for the proper function of signal transduction pathways associated with cell proliferation, differentiation, and survival.9 Based on PTK location in the cell, PTKs are classified into receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (NRTKs). Located in the cell membrane, RTKs are divided into subfamilies based on the molecular characteristics of their extracellular structural domains. Previous studies classified 58 known human RTKs into 20 subfamilies.10 However, based on an in-depth functional analysis, three RTKs of the lemur tail kinase (LMTK) family were found to phosphorylate serine/threonine residues instead of tyrosine residues, resulting in the classification of 55 RTKs into 19 subfamilies.11 In addition to RTKs, there are a large number of NRTKs in cells, including ABL Proto-Oncogene (Abl), FES Proto-Oncogene (Fes), Janus Kinase (JAK), Focal adhesion kinase (Fak), and SRC Proto-oncogene (Src).9 NRTKs regulate various cellular functions, including cell proliferation, differentiation, adhesion, migration, and apoptosis by coupling with RTKs or other membrane receptor proteins such as G protein-coupled receptors. Moreover, NRTKs are also involved in T- and B-cell activation signaling pathways, thereby modulating immune response (Fig. 1).9,12
Fig. 1. The relationship between PTK and tumors.
The normal activation and inactivation of PTK is essential to maintain normal cellular function. PTK activation mutations include gain-of-function mutations, genomic amplification and overexpression, chromosomal rearrangements (gene fusions), and ligand autocrine/paracrine loops
Some PTKs mutations can keep PTKs in a persistently “active” state, causing overactivation of downstream pathways, and everlasting proliferation, differentiation, angiogenesis, and tumorigenesis.1 PTKs overactivation is mediated by four major types of mutations:13 (I) Gain-of-function (GOF) mutations include anaplastic lymphoma receptor tyrosine kinase (ALK), R1275Q, and F1147L mutations in neuroblastoma (NB).14 Some GOF mutations can increase the sensitivity of TKI and signaling transduction. For instance, deletion of four highly conserved amino acids, LREA, in EGFR exon 19 and the L858R point mutation in exon 21 of lung cancer improves therapeutic response to EGFR-TKIs.15,16 (II) Genomic amplification and overexpression is the second major type of mutation. For example, overexpression of EGFR and vascular endothelial growth factor receptor 2 (VEGFR2) is associated with medullary thyroid carcinoma.17 (III) Chromosomal rearrangements (gene fusions). The Philadelphia chromosome (BCR-ABL fusion) is a well-known example of gene fusion causing chronic myeloid leukemia (CML). MASC (Mammary analog secretory carcinoma) has an ETS variant transcription factor 6 (ETV6)-neurotrophic receptor tyrosine kinase 3 (NTRK3) gene translocation.18 In addition, partial duplications within genes are also a type of chromosomal rearrangement, and kinase domain duplication (KDD) of RTKs may be a novel mechanism for constitutive activation of RTKs.19,20 In a genomic analysis of 114,200 solid tumors, KDD was found in 0.62% of tumors, particularly brain tumors, predominantly in EGFR, platelet-derived growth factor receptor alpha (PDGFRA), and fibroblast growth factor receptor 3 (FGFR3).21 (IV) Ligand autocrine/paracrine loop. For example, fibroblasts can release hepatocyte growth factor (HGF), which binds to MET proto-oncogene (MET) in cancer cells and provokes a continuous survival benefits.
Protein tyrosine phosphatases (PTPs) are the physiological antagonists of PTKs. PTPs are responsible for attenuating the effects of PTKs by dephosphorylating target proteins. The subtle antagonistic function of PTKs and PTPs strictly regulates the phosphorylation level of intracellular proteins and is pivotal for maintaining normal signaling. However, antagonistic function of PTPs cannot completely attenuate PTKs overactivity caused by the above mechanisms.13 As endogenous PTPs cannot inhibit the sustained activation of PTKs, exogenous PTK inhibition is necessary.
TKI and cancer
The kinase structural domains of PTKs are responsible for transferring the phosphate group of ATP to the tyrosine residue of downstream signaling molecules. Regarding the structural similarity of TKIs and ATPs, TKIs can competitively bind to the kinase structural domains to prevent downstream pathway activation and inhibit tumor growth. Kinase inhibitors, including TKIs, are often classified into six types based on the mechanism of action, but there is no uniformity.22–24 Type I kinase inhibitors, including cabozantinib, crizotinib, and gefitinib, compete with substrates and bind to the ATP-binding pocket of the active conformation. Type II kinase inhibitors such as imatinib, sorafenib, and nilotinib, bind to the inactive conformation of protein kinases. The binding sites of types III and IV are not in the ATP pocket and function through allosteric mechanisms. However, due to the complexity of the allosteric mechanisms, there are few approved TKIs except for asciminib approved for CML.25 Types IV and V kinase inhibitors can form covalent bonds with kinase sites, thus irreversibly altering target activity. These kinase inhibitors such as osimertinib, afatinib, ibrutinib, and acalabrutinib possess better pharmacokinetic properties than reversible inhibitors.
TKIs bind to abnormal PTKs to inhibit downstream pathways. The PI3K/AKT and RAS/MAPK signaling pathways are two major PTKs-related pathways that can induce proliferation, inhibit apoptosis, promote angiogenesis, and regulate various cellular functions. Consistently, TKIs can reverse these alterations. Additionally, some TKIs can also modulate the immunosuppressive microenvironment of tumors,26,27 alter the molecules expression level of immune cell surface through the TME and promote the antitumor immune response. Interestingly, a clinical trial (NCT0301333) demonstrated that TKIs and antibiotics influenced the therapeutic response of RCC to immunotherapy by affecting the composition of the gut microbiota.28 A recent study in glioblastoma (GBM) cell lines provided new insights into the function of TKIs, other than the PTKs inhibitory role. The study revealed that some TKIs can activate general control nonderepressible 2 (GCN2), which activated integrated stress response (ISR), resulting in cell death.29
Although different TKIs have similar mechanisms of action, they differ in terms of targeting kinase profiles, pharmacokinetics (PK), and side effects.30 For example, osimertinib is a third-generation TKI targeting EGFR for NSCLC with specific mutations. Imatinib is highly selective for BCR-ABL mutation and is the best choice for CML. However, multiple PTK abnormalities exist in some tumors, and single-target therapy is sometimes inadequate. Multi-target TKIs that cover a broader range of PTK abnormalities can provide more potent inhibitory effects and have a broader range of indications. For instance, sorafenib can target various RTKs, including VEGFR and PDGFR, thereby inhibiting cancer cell proliferation and angiogenesis. Sorafenib is effective in HCC, RCC, and thyroid cancer (TC).31 As of 2021, 76 TKIs have been administered for treating various types of cancer, and more are in the phase of clinical trials.32 Entering the human body with a clear target, the small molecule TKIs have only limited nonspecific toxicity. The favorable safety allows TKIs to be easily combined with other treatment modalities, such as chemotherapy, immunotherapy, and radiotherapy. TKIs significantly prolong survival and improve the quality of life among cancer patients.
Although TKIs are highly effective in cancer, some issues such as adverse events and tumor resistance to TKIs still require the vigilance of clinicians. The most common adverse events of TKIs are dermatologic complications (such as folliculitis, paronychia, and periorbital edema) and hematologic side effects (such as neutropenia, gastrointestinal symptoms, and hypothyroidism).30 Management of TKI-related adverse events is of clinical significance for cancer patients. In addition, some patients weakly respond to TKIs or gradually become insensitive to TKIs as the manifestations of TKIs resistance.33,34 TKIs resistance leads to recurrence or rapid tumor progression, which seriously affects the survival of patients. Therefore, finding solutions for TKI resistance is an urgent priority.
Mechanisms of TKI resistance
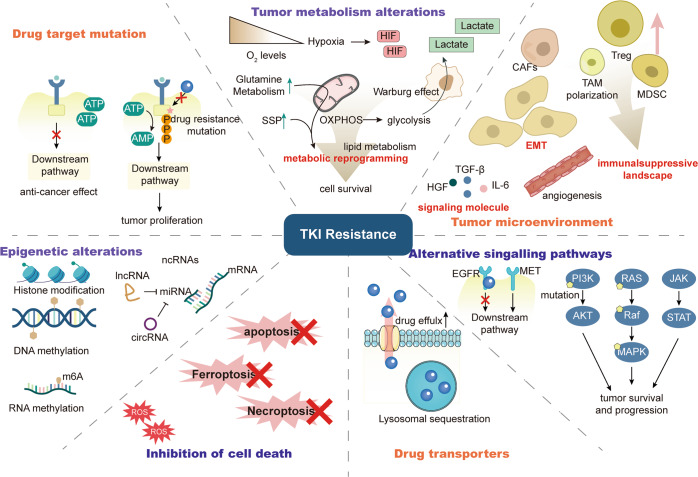
TKIs resistance mechanisms are highly complex, including abnormal drug metabolism, cell death, epigenetics, etc. Multiple factors lead to resistance to TKIs (Fig. 2). The mechanisms of drug resistance extensively vary among individuals. Identification of drug resistance mechanisms can improve the clinical efficacy of TKIs. Here, we elaborately review the current mechanisms of TKIs resistance.
Fig. 2. Overview of the mechanisms of tumor resistance to TKIs.
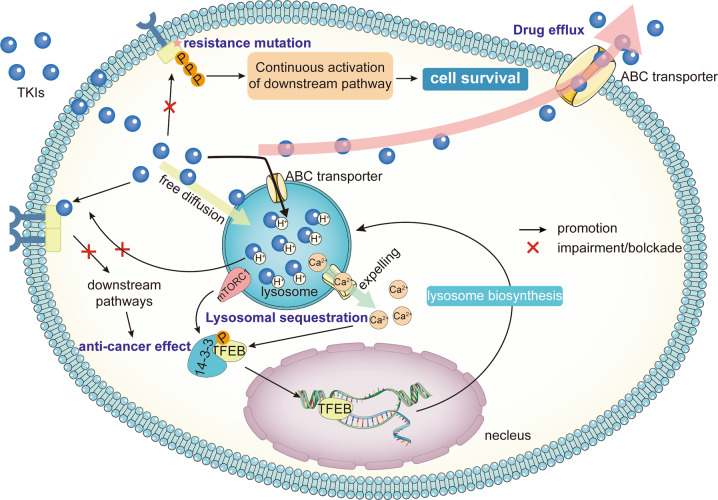
The mechanism of tumor resistance to TKIs is complex. The most direct mechanism is the mutation of TKI drug targets, which will lead to the inability of TKIs to bind and function, causing drug resistance. Tumors can also cause resistance by reducing intracellular TKI concentrations, such as enhanced drug efflux and lysosomal isolation. There are also other factors outside the tumor cell that can affect the tumor response to TKI. The tumor microenvironment is important for TKI resistance, with crosstalk of various cellular components within the TME, which on the one hand can complement the growth signal of cancer cells, and on the other hand, the immunosuppression caused by the TME prevents immune cells from killing tumors. In addition, tumor cells within the TME can undergo a series of adaptive changes, such as metabolic reprogramming and EMT, to resist cell death. Tumor cells can also activate downstream pathways through signaling bypass or without relying on PTK, leading to drug resistance. Finally, epigenetics permeates almost all drug resistance mechanisms through unique regulatory mechanisms
Reduced PTK-binding capacity of TKI
TKIs can generally enter the cell and bind to abnormally activated PTKs. However, extensive mutations in some PTKs decrease TKI binding capacity as a direct mechanism of TKI resistance (Table 1). On the one hand, intrinsic resistance occurs due to changes in kinase conformation and activity caused by specific mutations in PTK. For example, EGFR exon 20 insertion is observed in 3% of patients with lung adenocarcinoma and 9% of patients with EGFR mutations, leading to third-generation EGFR-TKI resistance.35 Deletions of exon 16 of human epidermal growth factor receptor 2 (HER2) splice variant (Δ16HER2) lead to primary resistance to lapatinib in breast cancer cell lines. It is also associated with acquired resistance to Src-TKI, saracatinib.36 In addition, KIT Proto-Oncogene (KIT) exon 9 mutation and PDGFRA D842V substitution are associated with intrinsic resistance to imatinib in GIST.37 Similarly, ALK L1196 M/G1202R causes primary resistance of NSCLC to lorlatinib.38 PDGFRB C843G can also lead to Ph-like acute lymphoblastic leukemia (ALL) resistance to all ABL TKIs, which is a classic example of this mechanism.39
Table 1.
On-target gene mutations of TKI resistance
| RTKs | Other mutations before gatekeeper | Gatekeeper mutations | Other mutations after gatekeeper | solvent-front mutations | covalent binding site mutations | Gene fusion mutation | |
|---|---|---|---|---|---|---|---|
| EGFR | L692V, E709A/K, L718Q/V, L719A, S768, | T790M | G796S, L792X/F/H/R/Y/V/P, G796R/S | G196S/R | C797S/X | – | |
| FGFR | FGFR1 | N546K/H | V561F/M | — | — | — | |
| FGFR2 | M536I, M538I, K462, I548V, N549K/H, N550H | V564F/I | E565A, I567, N568, V581, E584G, S587, L617V, L618M, V652L, K659M, K660E/M, K678M, H682L, K714R, E719G | — | — | — | |
| FGFR3 | — | V355M | V555M | — | — | — | |
| FGFR4 | — | V550L/E/M | C552 | — | — | — | |
| ALK | L1122, 1146 K, L1152V/Q, C1156Y, I1171T/N, L1174V, V1180 | L1196M | L1198F, F1245V, G1269A/S | G1202R, D1203N | — | EML4-ALK | |
| ROS1 | L1174F, L1951, S1986F/Y, L1986F/L, F2004C/I/V | L2026M, G2026M | L2086F, L2155S | G2032R/K, D2033N | — | — | |
| RET | E732K, V738A | V804L/M | Y806N, G807V, G810S, V871, M918T, S904F, F998V | L730V/I, G810A/C/S/R, | — | — | |
| NTRK | NTRK1 | V573, G595R | F589L | G667C/S, A608D | G595R/A | — | |
| NTRK2 | V601 | F633L | G709C | G639R | — | ||
| NTRK3 | — | F617L | G696A | G623R | — | ||
| BTK | Less frequently | — | — | — | C481S | — | |
| BCR-ABL | M244, L248, G250, Q252, Y253, E255, V299 | T315I | F317, A337, M351, M355, F359, H396, W464, P465, V468, I502 | — | — | — | |
| FLT | D200N, K429F, Y572C, L601F, | F691I/L | D835Y/V, Y842C/H | — | — | — | |
| KIT | V653A, V654A, T669I | T670I | D816H, D820A, N822K, Y823D, A829P | — | — | — | |
EGFR epidermal growth factor receptor, FGFR fibroblast growth factor receptor, ALK anaplastic lymphoma receptor tyrosine kinase, ROS1 ROS proto-oncogene 1, RET RET proto-oncogene, NTRK neurotrophic receptor tyrosine kinase 1, BTK bruton tyrosine kinase, FLT Fms related receptor tyrosine kinase 1, KIT KIT proto-oncogene, EML4 EMAP like 4.
PTK can develop secondary mutations during treatment with TKI, leading to acquired drug resistance. The gatekeeper mutation is one of the first identified acquired resistance mutations of TKI targeted kinase.40 The most typical gatekeeper mutation is substituting a smaller amino acid with an amino acid with a larger hydrophobic residue. This mutation results in a spatial blockade that hinders the formation of a hydrogen bond between PTK and TKI.41 EGFR T790M mutation in exon20 causes spatial blockade to the first- and second-generation EGFR TKIs. In the coincidence of activating mutations like L858R, T790M lowers Km [ATP], the ATP concentration to achieve a half-maximal reaction rate. Consequently, the affinity of ATP increases and the efficacy of TKI decreases.42,43 FGFR V564F, KIT T670I, and Ret Proto-Oncogene (RET) V804M are also gatekeeper mutations,44,45 but they are not as common as T790M and still lack of enough studies.
Solvent-front mutation can be observed in RET. The solvent-front refers to the kinase residues exposed to the solvent or the region of the kinase surface to which TKIs bind. L730 lies at the top of the solvent-front of RET and binds to nintedanib by hydrophobic interactions with piperazine, phenylamino, and phenyl groups.46 Thus, the RET L730V mutation results in nintedanib resistance. Another highly mutated site of RET solvent-front is at G810. The G810A mutation has an additional methyl group, which can form new hydrophobic bonds with the methyl and phenyl rings in nintedanib. Despite vandetanib, nintedanib has these methyl and phenyl rings, so G810 only causes resistance to vandetanib but no effects to nintedanib. Substitution of G810 by a more polar amino acid like serine prevents its TKI binding capacity and improves resistance to vandetanib and nintedanib.46 Moreover, selpercatinib and pralsetinib are specific RET TKIs that were recently approved. G810S/C/R mutation causes selpercatinib resistance, and L730 V/I mutation leads to pralsetinib resistance.47,48 In addition to RET, solvent-front mutations also exist in EGFR, ALK, ROS1, and NTRK and contribute to drug resistance, such as EGFR G796S/R, ALK G1202R, NTRK1-G595R, NTRK2-G639R, NTRK3-G623R, ROS1 G2032R, and ROS1 D2033N.49,50
Except for classic mutations of the kinase structural domain, some newly identified mutations can also weaken drug-target interactions by a spatial blockade. L792X/F, G796S, L718Q, and S768I, are some of the acquired mutations of EGFR and E565A, L617V, K641R, and K659M are the structural domain mutations of FGFR2 kinase.35,51,52 ALK resistance mutations include point mutations such as G1269A, I1171T/N, L1196M, C1156Y, F1245V, and F1174V, and compound mutations like L1196M/G1202R, and L1152V/Q1146K.53,54 As for ROS1, G2032R, S1896F, and L2000V lead to lorlatinib resistance,55,56 G2032R, L2086F, G2026M, D2033N, S1986F/Y, and L1174F cause crizotinib resistance.57 L2086F can cause resistance to lorlatinib, crizotinib, and entrectinib but not cabozantinib.56 F2004C/I/V contributes to the resistance of entrectinib, crizotinib, and cabozantinib.58 Among RET kinase mutations, L730I, V738A, V804 L/M, Y806N, and G810S mutations are pan-resistant to cabozantinib, lenvatinib, vandetanib, and nintedanib. Additionally, l730V, E732K, A807V, G810A, V871I, M918T, and F998V mutations cause resistance to one or some of these drugs. Interestingly, the V871I, M918T, and F998V mutations are far from the ATP-binding pocket but can also inhibit TKI binding.59 In addition, NSCLC patients with positive ALK carrying the EML4-ALK fusion gene can rapidly develop alectinib resistance within three months.60 The KIT V654A and D820A mutations can cause GIST resistance to imatinib.61,62 The NTRK G623R mutation can lead to MASC resistance to entrectinib, but the next-generation NTRK-TKI selitrectinib is still effective in MASC with this mutation.18,63
Mutation in the drug target site still decreases the efficacy of irreversible inhibitors. Osimertinib, a third-generation EGFR covalent inhibitor, binds to the C797 residue in the ATP-binding site of EGFR and potently inhibits receptor activation, which can be applied to positive T790M EGFR. The missense mutation in exon 20 C797S prevents covalent bond formation with EGFR and leads to osimertinib resistance.35 Bruton tyrosine kinase (BTK) TKIs like ibrutinib, acalabrutinib, and zanubrutinib, constitute another class of covalent inhibitors. They irreversibly and covalently bind to the C481 site of BTK. Therefore, drug resistance can occur in the presence of C481 mutation.64
The resistance mechanisms are not limited to spatial interference. RET S904F mutation increases the affinity of RET to ATP and leads to RET-TKI vandetanib resistance.65 FGFR2 N550H attenuates the auto-inhibitory mechanism of the kinase hinge region and allows the kinase to activate, preventing dovitinib from binding its target.66,67 Studying the role of ABL mutations in imatinib resistance showed that individual resistance mutations do not significantly affect drug binding capacity. Alterations in ABL kinase activity and the affinity for ATP and its kinase substrates can be observed together. The coincidence of these alterations changes the energy landscape of the kinase, which can be observed in imatinib resistance.68 This “resistance accumulation” mechanism may apply to a broader scope.
There are four possibly effective countermeasures to resistance mutations. For instance, the standard treatment is platinum-based chemotherapy in patients with T790M-negative tumors at the time of acquired resistance. For T790M mutation, irreversible inhibitors are still effective, and osimertinib is recommended by several countries based on clinical trials.43 Although C797S secondary mutation decreases the efficacy of osimertinib, recent findings from clinical studies show that a combination of first and third-generation EGFR TKI is an effective option if the T790M and T797S mutation loci are on different chromosomes, known as trans mutation. But EGFR with T790M and T797S mutations on the same chromosome, known as cis mutation, is insensitive to all EGFR-TKIs. In addition, in GIST with KIT T670I, cabozantinib can overcome imatinib resistance.69 As previously discussed, some resistance mutations may cause resistance to only one TKI and have little effect on other TKIs. In this case, changing to a more sensitive TKI may be effective. Second, TKI combined with other treatments will be discussed in the following sections. Third, switching to other treatments by identifying other potentially targetable mechanisms of resistance or participating in clinical trials might be suitable choice.
Finally, developing new TKIs or finding new sites of action is a promising solution for all mutation-related resistance. A Phase I clinical trial showed an unprecedented and durable clinical benefit when treating PDGFRA D842V-mutant GIST with avapritinib.70 The mice model showed that the next-generation ROS/ALK inhibitor SAF-189S had comparable efficacy to lorlatinib against crizotinib-resistant lung cancer with ROS G2032R-mutation.71 The next-generation ALK-TKI XMU-MP-5 led to marked cancer regression in mice with EML-ALK mutations.72 Ripretinib broadly inhibited activating mutations in KIT and PDGFRA and the efficacy was shown in preclinical studies.73 LY2874455 is a newly discovered pan-FGFR inhibitor overcoming FGFR gatekeeper mutations.74 Moreover, fourth-generation EGFR-TKIs are assumed to target PTKs with drug-resistance mutation by allosteric mechanisms. Some of them, such as DDC-01-1163, JBJ-04-125-02, and 4-(1-ethylsulfonyl-3-indolyl)-2-phenylaminopyrimidines are currently used in preclinical studies.75–77 Fourth-generation EGFR-TKIs combined with other drugs are promising, but more clinical trials are needed to consolidate the effectiveness.
In addition, most of the previously developed TKIs have tumor-specific indications, but new-generation TKIs have mutation-specific indications. For example, larotrectinib can effectively treat all solid tumors carrying NTRK fusion genes,78–81 and repotrectinib (TPX-0005) is a next-generation broad-spectrum inhibitor of ALK/ROS/NTRK that can treat a variety of solid tumors carrying ALK, ROS1, and NTRK mutations.50 TPX-0046 is a next-generation RET/SRC TKI with a three-dimensional macrocyclic structure distinct from selpercatinib and pralsetinib. It has potent activity against multiple RET mutations. Repotrectinib is also a next-generation multitarget inhibitor that overcomes resistance in ALK/ROS/NTRK-positive solid tumors with solvent-front mutations.50 However, repotrectinib can overcome gatekeeper and solvent-front mutations, it remains insensitive to NTRK1 G667C and NTRK3 G696C in DGF motifs.82
In general, resistance mutations may overlap and vary between TKIs, which may be related to the types of the kinase. Therefore, if a resistance mutation occurs during TKI therapy, changing the TKI may be helpful. However, it is not a long-term solution. Cancer cells gradually develop resistance mutations even with new-generation TKIs. Moreover, PTK resistance mutations can be associated with abnormal activation of non-target PTKs. Herein, EGFR mutation accompanied by MET amplification attenuates the efficacy of a single TKI to inhibit the proliferation caused by abnormal activation of other PTKs. A combination of other drugs, such as MET-TKIs, can partly overcome the resistance in such cases. Because of irreversible resistance mutations, proper pre-drug resistance administration and regular monitoring of drug-resistant mutations are pivotal to circumventing drug resistance. Studies on different EGFR-TKIs and pazopanib indicated that compared to continuous dosing, a higher dose of TKI or intermittent pulse known as “drug holidays” can increase the efficacy of targeted therapy, delay treatment resistance, and reduce toxicity.83–85
Early monitoring of drug resistance mutations can determine the best treatment regimen, particularly with the development of high-quality detection methods in recent years. Earlier, the biopsy was an effective method to detect mutations. However, tissue samples are not often enough to systematically represent the mutational status of the entire tumor, and the sample amount is also insufficient for molecular detection. In contrast, cell-free DNA (cf-DNA) and next-generation sequencing (NGS) are now available to detect circulating tumor DNA (ctDNA) for comprehensive detection of molecular alterations.43,86 Several studies support the potential of ctDNA-NGS for rapid selection of new drugs after TKI treatment failure and predicting the clinical outcomes.87,88 In addition, the Zebrafish patient-derived xenograft (ZTX) platform, which is based on patient-derived xenograft (PDX), is a high-efficacy preclinical model for studying resistance mechanisms for targeted therapies. It estimated the erlotinib treatment outcome and tumor invasion in patients with lung cancer within three days, and found it had high sensitivity (91%) and specificity (62%).89 It also effectively predicted individualized tumor regression.90 More importantly, we should understand when TKIs must be used to treat tumors with RTK mutations. There is heterogeneity in the initial response to TKIs for different types of mutation.91 Taking EGFR as an example, patients with typical L858R and EX19del mutations sufficiently respond to TKIs, while patients with some atypical EGFR mutations inadequately respond to these TKIs. For atypical PTK mutations, some existing TKIs may not be suitable. It is impossible to predict how much a patient will benefit from TKI therapy before TKIs are administered. However, the locus of the mutation can help to predict treatment outcomes. Accordingly, it is necessary to establish a classification system of PTK mutation to predict TKI sensitivity.91
Abnormal activation of PTK-related signaling pathways
The function of TKIs is to inhibit the activation of downstream pathways caused by abnormally activating PTKs. The PI3K/AKT, RAS/MAPK/ERK, and JAK/STAT signaling pathways are the most important pathways. However, PTK inhibited by TKI cannot suppress a signaling pathway activated by downstream molecule mutation. Therefore, RTK-independent overactivation of the downstream pathway is unresponsive to TKI. Sometimes several PTKs activate the same downstream pathway, and the inability of a TKI to inhibit all those PTKs leads to persistent activation of the pathway and drug resistance. On the other hand, activation of PTK-related downstream pathways by other signaling pathways can bypass the TKI and cause drug resistance. Downstream signaling pathway activation by other PTKs or other signaling pathways is named bypass resistance. Furthermore, many proteins can move into the nucleus in response to signals that regulate gene expression. Nuclear translocation, as part of the signaling pathway, has also been associated with TKI resistance. Here, we have summarized several types of aberrant activation of PTK-related signaling pathways which can be resist to TKIs.
Abnormal RTK-independent activation of downstream pathways
PI3K/AKT and RAS/MAPK pathways can be activated without upstream stimulation when the downstream signaling molecules are abnormally activated. In addition, each pathway has its specific negative regulators. Without the effective function of these negative regulators, TKIs-mediated inhibition of upstream molecules will be less effective. These two states are defined as abnormal RTK-independent activation of downstream pathways.
Mutations of downstream members of the pathway can activate the pathway, independent of the upstream molecules. Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) encodes the catalytic subunit of PI3K. Mutations of PIK3CA were found in EGFR-TKI-resistant NSCLC cell lines, MET-TKI capmatinib-resistant NSCLC cell lines, regorafenib-resistant CRC cell lines, KIT/PDGFR-TKI-resistant GIST cell lines, imatinib-resistant Ph+ ALL cell lines, and HER-TKI neratinib-resistant HER2+ breast cancer cell lines,92–97 resulted in enhancing PI3K kinase activity. Mechanistic target of rapamycin kinase (mTOR) L1433S mutation activates AKT and leads to EGFR-independent osimertinib resistance.98 Furthermore, the serum- and glucocorticoid-regulated kinase 1 (SGK1) is functionally and structurally similar with AKT. Despite crenolanib-mediated inhibition of AKT, breast cancer cells could maintain PI3K/SGK1 signal transduction.99 A similar scenario can be seen in the RAS/RAF/MAPK signaling pathway. Studies have shown that KRAS proto-oncogene (KRAS) mutation, BRAF proto-oncogene (BRAF) V600E mutation, and mitogen-activated protein kinase 1 (MAPK1) gene amplification are involved in EGFR-TKI-resistance.92,100 KRAS G12C mutation, KRAS amplification, NRAS proto-oncogene (NRAS) mutations, and MAP2K1 mutations have been detected in lorlatinib-resistant tumors.56 KRAS mutations are also associated with GIST resistance to KIT/PDGFR-TKIs.94 Furthermore, MEK1 and MEKK1 in-frame deletions have been detected in ROS-TKI-resistant LUAD (lung adenocarcinoma) patients.101 A-kinase anchoring protein 9 (AKAP9)-BRAF fusion leads crizotinib resistance.54 All of these genes encode key signaling molecules of the RAS/MAPK signaling pathway, and their mutations cause continuous ERK activation and transcription of downstream genes, lead to sustained cell proliferation.
A potential therapeutic approach is to inhibit downstream members of pathway.102 For the PI3K/AKT pathway, everolimus, a specific mTOR inhibitor, can block the PI3K/AKT pathway. Several phase II randomized clinical trials have shown that patients with VEGFR-TKI-resistant RCC can take advantage of lenvatinib in combination with everolimus.103–105 Everolimus also effectively induces CML cell death in monotherapy or combined with imatinib.106 However, one study showed that everolimus has limited efficacy in combination with EGFR-TKIs.107 Another clinical study showed no significant clinical benefit of erlotinib in combination with everolimus for neck squamous cell carcinoma (HNSCC).108 Consequently, the effectiveness of everolimus in other cancers remains to be explored. The next-generation mTOR inhibitor, Rapalink-1, combined with sunitinib, produced a better therapeutic response in RCC.109 PIK3CA inhibitors can theoretically block the PI3K/AKT pathway, but their clinical effectiveness in combination with TKIs was not assessed yet. In addition, direct inhibition of AKTs is also rational. For example, FGFR-TKIs combined with AKT inhibitors can overcome FGFR1 amplification-mediated EGFR-TKI resistance.110 AKT inhibitors such as MK2206 and capmatinib can overcome sorafenib resistance in HCC with MET mutations.111
For the RAS/MAPK signaling pathway, promising KRAS inhibitors have been introduced in recent years, and some of them, such as sotorasib have shown clinical efficacy.112 But the efficacy against TKI resistance is yet undetermined, and the clinical trial of their combination with TKI (NCT04185883) is underway. Fortunately, TKIs combined with RAF inhibitors, MEK inhibitors, or ERK inhibitors have been effective. RAF inhibitors combined with asciminib can treat acute myeloid leukemia (AML) with BCR-ABL-TKI resistance.113 The ERK inhibitor, ulixertinib, combined with dasatinib synergistically expedited NB cell death.114 Furthermore, the combination of MEK inhibitors and TKIs receives the most attention. MEK inhibitors in combination with TKI is effective in EGFR-TKI-resistant NSCLC cell lines, undifferentiated thyroid cancer (UTC) cell lines, FGFR-TKI-resistant gastric cancer cell lines, NTRK-TKI-resistant multiple tumor cell lines, and EPH receptor A2 (EphA2) TKI-resistant uterine cancer cell lines.115–119 However, the efficacy of combination therapy is not supported by all studies.120 Clinical trials demonstrated that the MEK inhibitor, trametinib, combined with the multitargeted TKI midostaurin can improve the prognosis of patients with FLT3-TKI-resistant AML.121 In addition, P21 (RAC1)-activated kinase 2 (PAK), whose overexpression is related to lenvatinib resistance in some types of TC is also a downstream molecule of RAS. PAK-mediated resistance can be reversed by the combination of PAK inhibitors and lenvatinib.122 In vitro studies have shown that dual inhibition of both pathways is an effective approach.123,124 The optimal dose is generally determined in phase I clinical trials by balancing efficacy and safety for clinical use.
As mentioned previously, TKIs cannot suppress the sustained activation of pathways due to the downregulation of negative regulators. Mutated in multiple advanced cancers 1 (MMAC1/PTEN) is a typical negative regulator of PI3K/AKT. Its low expression or loss of function is associated with resistance to EGFR-TKIs in LUAD, lapatinib in gastric cancer,125 and sunitinib and sorafenib in RCC.126,127 Similarly, low expression or loss of dual-specificity phosphatase 6 (DUSP6), a negative regulator of RAS/MAPK, is associated with resistance to FGFR-TKI and EGFR-TKI in lung cancer cell lines.128,129 PH domain leucine-rich repeat protein phosphatase (PHLPP), a common negative regulator of PI3K/AKT and MAPK/ERK pathways, is downregulated in EGFR-TKI-resistant NSLCL cell lines.130 Incomplete dephosphorylation of signaling molecules due to low expression of negative regulators can lead to TKI resistance. Apart from loss-of-function mutations, epigenetic modifications may be the main reason for the downregulation of negative regulators.
OTU deubiquitinase 1 (OTUD1) is a deubiquitinating enzyme that interacts with PTEN and regulates its stability. Downregulation of OTUD1 can lead to VEGFR-TKI resistance in clear cell renal carcinoma (ccRCC) via the OTUD1-PTEN axis.131 Glutaminase 2 (GT2) is also a posttranslational modifying enzyme that induces PTEN degradation, and its high expression is associated with resistance to erlotinib and gefitinib in NSCLC cell lines.132 Retinol-binding protein 2 (RBP2) is a histone demethylase that directly downregulates PTEN expression. RBP2 increases CML resistance to TKIs and shifts CML to the acute phase.133 In addition, the activated AKT pathway can activate testis-specific Y-encoded-like protein 5 (TSPYL5) and prevent its ubiquitination and subsequent degradation. Upregulation of TSPYL5 inhibits PTEN transcription, leading to drug resistance. Consistently, TSPYL5 inhibition improves drug resistance.134 The reasons for the low expression of DUSP1 and PHLPP have not been found, but it was elucidated that high expression levels of miR-452-5p in CRC can negatively regulate DUSP1 and lead to apoptosis resistance, suggesting a role of epigenetics in its regulation.135 Thus, low expression of negative regulators can be reversed by epigenetics manipulation.
Additionally, PTPs are physiological antagonists of RTKs, functioning as negative regulators of PTK. Studies have shown that PTPN1 and PTPN2 deficiency in anaplastic large cell lymphoma (ALCL) leads to ALK-TKI resistance in ALCL. PTPN1 is the PTP for ALK and Src homology region 2 containing protein tyrosine phosphatase 2 (SHP2), and oncogenic ALK mutations can inhibit its transcription. Low PTPN1 cannot dephosphorylate SHP2 tyrosine residues, resulting in the overactivation of downstream pathways. Dual blockade of ALK and SHP2 enhances ALK TKI efficacy.136 In addition, protein tyrosine phosphatase receptor type O (PTPRO) is a PTP for EGFR and Erb-b2 receptor tyrosine kinase 2 (ERBB2). Low expression of PTPRO enhances EGFR function, leading to lapatinib resistance. The lapatinib sensitivity was restored via upregulation of PTPRO by reducing cellular methylation by 5-azacytidine. It suggests that epigenetics is responsible for PTPRO downregulation.137
Interestingly, protein phosphatase 2 (PP2A) is a serine/threonine phosphatase and negatively regulates several signaling pathways. Previously it was shown that the expression levels of cellular inhibitors of PP2A (CIP2A) and novel splice variants of CIP2A are associated with sensitivity to imatinib in myeloid leukemia. Another study reported that decreased CIP2A expression restores the sensitivity of RCC to sunitinib, but the mechanism was unclear and may be related to the overactivation of the control pathway. However, CIP2A levels did not affect the sensitivity to second-generation TKIs such as dasatinib and nilotinib.138,139 Therefore, CIP2A levels may contribute to TKI selection.
Bypass mechanisms of resistance
As mentioned earlier, several RTKs can activate PI3K/AKT and RAS/MAPK pathways. The efficacy of TKI decreases by simultaneous mutation of several PTKs in tumor cells.140 For example, PTKs overactivation mutations other than EGFR mutations such as MET amplification, IGF-1R overexpression, HER2 amplification, AXL overexpression, ROS1 rearrangement, ALK rearrangement, and coiled-coil domain containing 6 (CCDC6)-RET fusion gene exist in EGFR-TKI-resistant cell lines.92,141–145 IGF-1R and MET also confer resistance to ALK and VEGFR-targeted therapies.146–148 Studying several cancer tissues with ALK-TKI-resistance showed that KIT D820E, MET E1012, EGFR P26_C291del, and MET amplification can lead to crizotinib resistance, and EGFR P753S can lead to alectinib resistance.149,150 Moreover, nerve growth factor (NGF)-mediated RAS/MAPK pathway activation can cause resistance to several TKIs in NB.114 The upregulation of AXL leads to FLT3-TKI quizartinib resistance in CML and imatinib resistance in GIST.151,152 The upregulation of KIT ligand SCF causes ErbB-TKI pyrotinib resistance.153 Moreover, EphA2 upregulation induces ccRCC resistance to VEGFR-TKI sunitinib.154 Overexpression of EphB family members induces lapatinib resistance in breast cancer cell lines and sorafenib resistance in HCC cell lines.155,156 Upregulated receptors or ligands can enhance the signaling, while point mutations and gene rearrangements lead to continuous activation of RTK without ligands, activating the PI3K/AKT and RAS/MAPK pathways and bypassing TKIs. In addition, the crosstalk between different RTKs, such as the crosstalk between MET, EGFR, and HER-3, leads to more complicated abnormal signaling.157,158 In addition to co-existing mutation of several PTKs, downstream signaling molecules can conduct compensatory signaling. For instance, upregulation of FOXK2 leads to VEGFA overexpression in UTC. VEGFA binded to VEGFR1 could promote angiogenesis and the transcription of FOXK2, thereby preventing the VEGFR2 inhibition by apatinib.159
In addition to RTKs, other receptor signals on the cell membrane or in the cell can activate PI3K/AKT and RAS/MAPK pathways. CD73, a membrane-bound nucleotidase of cancer cells, can activate the AKT pathway and cause lenvatinib resistance in HCC. CD73-related AKT overactivity enhances c-myc function. Subsequently, c-myc promotes SRY-Box transcription factor 9 (SOX9) expression and inhibits glycogen synthase kinase three beta (GSK3β) expression to prevent SOX9 degradation.160 The role of SOX9 has not been fully understood. SOX9 overexpression may be associated with stem cell features such as self-renewal and high proliferation capacity. SOX9 overexpression may also induce EMT by activating TGF-β/SMAD pathway.161 Moreover, CD73 increases the production of adenosine, an immunosuppressive metabolite that binds to the G protein-coupled adenosine A2a receptor (A2aR) on immune cells, and inhibits the immune response.162
Integrins are involved in cellular recognition and adhesion, and link the extracellular stimulus with the intracellular alterations. Integrin-mediated signal transduction has been implicated in many cellular processes. Integrins rely on some NRTKs, such as Src and FAK, to activate the downstream pathways and maintain signal transduction. Osteopontin (OPN) promotes tumor cell proliferation by binding integrin avβ3 and activating downstream FAK/AKT and ERK signaling pathways. Overexpression of OPN in NSCLC treated with EGFR-TKI compensated for the blockade of proliferation signals, causing EGFR-TKI-resistance.163 In the Ph+ CML cell lines, integrin β3 activated integrin-linked kinase (ILK) in response to extracellular signals, leading to imatinib resistance.164
Numerous cytokines and chemokines in TME can bind to their receptors on the cancer cell membrane and activate their downstream pathways. It was revealed that interleukin 6 (IL-6) leads to osimertinib resistance by binding to its receptors and activating LAMA5/FAK signaling.165 Tumor necrosis factor (TNF) is a mediator of intrinsic resistance to EGFR-TKIs. TNF binds to TNFR and activates the c-Jun N-terminal kinase (JNK) to increase growth arrest-specific 6 (Gas6) expression, which binds to AXL, activates ERK signaling, and resists EGFR inhibition. Disruption of the TNF-JNK-Axl-ERK axis at any level increases the sensitivity of GB to EGFR-TKIs.166 In CRC, CCR2 activates PI3K/AKT/GSK3β signaling and maintains β-linked protein stability, leading to regorafenib resistance.167 Ribonucleotide reductase subunit M2 (RRM2) competes with ubiquitin-protein ligase E3A (UBE3A) in RCC to bind to ANXA1, prevent ANXA1 degradation, and activate the AKT pathway, resulting in sunitinib resistance.168 In addition, Src, as an NRTK, also activates the PI3K/AKT pathway, leading to ALK-TKIs resistance in NSLCL.169
The JAK/STAT pathway is also a downstream of PTKs. It was shown that NGF/ tropomyosin receptor kinase A (TrkA) can alternatively activate the JAK/STAT3 pathway to induce EMT and erlotinib resistance in HNSCC.170 The Pim-1 proto-oncogene (PIM) is a downstream of JAK/STAT pathway. High PIM expression is associated with ALK-TKIs resistance in NB.171 Simultaneous inhibition of several signaling molecules in the JAK/STAT pathway could effectively overcome drug resistance.171–173
The combination of TKIs and inhibitors of downstream molecules or chemotherapeutic agents can effectively mitigate drug resistance.174 Administration of multitargeted TKIs and combinations of different TKIs are more effective. For instance, cabozantinib is an oral multikinase inhibitor targeting VEGFR1/2/3, MET, and AXL and has an acceptable efficacy in patients with advanced HCC and GIST.175,176 Repotrectinib is a next-generation ROS1/TRK/ALK multitarget inhibitor that effectively treats crizotinib-resistant lung cancer with ROS1 rearrangements. It also effectively treats brain metastases of lung cancer, thanks to its ability to cross the blood-brain barrier.177 Lenvatinib contributes to overcoming sorafenib resistance in HCC with FGFR4 expression.178 Gilteritinib is an FLT3/AXL-TKI that can treat ALK-TKI-resistant NSCLC and AML with FLT internal tandem duplication (ITD).179,180 Entrectinib is a multitargeted TKI that has been recently approved for multiple solid tumors with ROS fusion genes and NTRK1/2/3 fusion genes.181
The combination of TKIs has also been promising. Herein, the combination of gefitinib and apatinib has been more effective in NSCLC cell lines compared with each separately.182 Gefitinib combined with IGF1R-TKI may be effective against GBM with IGFR activation.183 Several MET-TKI and EGFR-TKI combinations have been effective in NSCLC patients with MET amplification and drug-resistant mutations.184,185 Lenvatinib in combination with gefitinib improves lenvatinib resistance in patients with HCC.186 FAK-TKI defactinib (VS-6063) could restore gefitinib sensitivity in gefitinib-resistant cell lines by blocking the downstream pathway.187 The triple combination of repotrectinib, EGFR-TKI, and MEK inhibitor was effective in cancer cells with NTRK1-G595R resistance, aberrant EGFR activation, and ERK reactivation.188 Some drugs other than TKIs can inhibit PTK. For instance, aspirin inhibits IGF-1R. Regorafenib, in combination with aspirin, had similar efficacy. As an anti-inflammatory agent, aspirin also alleviated inflammation.189 Berberine is a natural MET inhibitor with similar efficacy to MET-TKIs, which can be used in combination with TKIs.190
Interestingly, PTK can be indirectly targeted. Heat shock protein 90 (HSP90) acts as a molecular chaperone, maintaining the stability of RTKs (HER2, KIT, MET, etc.), AKT, ERK, and other signaling proteins. Therefore, HSP90 may indirectly inhibit PTK and other signaling molecules. In GIST cell lines with aberrant KIT activation and imatinib resistance, HSP90 is necessary for proper KIT folding, and HSP90 inhibitor TAS-116 reduces autophosphorylation-activated KIT and inhibits tumor growth.191 HSP90 inhibition combined with lapatinib has also been effective in breast cancer.192
HGF binds to MET after MET has been integrated with GRB2-associated binding protein 1 (Gab1). Metformin prevents MET from integrating with Gab. Studies have shown that metformin combined with alectinib may improve HGF/MET-induced resistance to alectinib in NSCLC cell lines.193 TKI, in combination with chemotherapy, is also effective. Crizotinib, combined with CHOP chemotherapy regimens, had a strong synergistic effect on ALCL in animal models and prevented drug resistance.194
Nuclear translocation
Nuclear translocation is a subcellular process and a part of the signal transduction pathway. Activated cytoplasmic proteins are transported to the nucleus to alter gene expression in response to external stimuli.195 Membrane EGFR translocation to the cytoplasm/nucleus is involved in resistance to gefitinib and osimertinib. EGFR translocation is associated with inhibition of the Hippo pathway, which may be linked to TKI resistance.196 Hippo signaling pathway prevents cell growth by sensing upstream stimuli and inhibiting the transcriptional function of YAP.197 Mechanistically, TKI can stimulate membrane EGFR (mEGFR) to translocate into the cytoplasm. By altering the molecular interaction of the central kinase complex of the hippo pathway, cytoplasmic EGFR (cEGFR) liberates YAP from cytoplasmic sequestration and increases its nuclear translocation. cEGFR can also accompany YAP into the nucleus. Nuclear EGFR (nEGFR) and YAP increase the transcription of downstream target genes, leading to cell proliferation and drug resistance.196
TIP30 is a tumor suppressor that negatively regulates both PI3K/AKT and RAS/MAPK pathways in NSCLC and prevents EGFR nuclear translocation from inhibiting nEGFR-mediated c-myc transcription. Low expression of TIP30 is associated with gefitinib resistance in NSCLC cell lines, while upregulation of TIP30 improves drug resistance by attenuating EGFR signaling pathway.198 In NSCLC cell lines, TKI-bounded EGFR can dimerize with other RTKs like HER and AXL to promote nuclear translocation of protein kinase C δ (PKCδ), a member of the PKC family.199 Nuclear PKCδ phosphorylates key nuclear proteins to regulate apoptosis. The intranuclear enrichment of PKCδ in gefitinib-resistant NSCLC promotes cell survival due to the transcription of anti-apoptotic molecules.200 The combination of sotrastaurin, a PKC inhibitor, and gefitinib significantly restored tumor sensitivity to gefitinib. However, the exact mechanism needs to be further investigated.
Although most of the previous studies have focused on typical RTK signaling pathways neighboring the cell membrane, recent findings suggest that at least 12 families of RTKs can be transferred to the nucleus, referred to as “membrane receptors in the nucleus (MRINs)”. High nuclear levels of MRINs are associated with poor prognosis and possess novel noncanonical functions in transcriptional regulation, cell proliferation, and malignant transformation.201 The nuclear translocation of EGFR family members contributes to drug resistance. Therefore, it is crucial to determine the mechanisms of nuclear translocation to overcome drug resistance.
In general, TKI resistance due to abnormal signaling pathways is not just attributed to abnormal membrane receptors. Abnormal activity and localization of signaling molecules and their regulatory molecules can also lead to sustained activation of the pathway. In addition, epigenetic modifications can affect the expression of signaling molecules, and recognition of these epigenetic alterations contributes to developing new drugs (Table 2).
Table 2.
Clinical studies of TKI in combination with other drugs to reverse drug resistance
| Brief information of RCT | Clinical Validity | Grade 3/4 treatment-related adverse events | ||||
|---|---|---|---|---|---|---|
| TKI | Co-drugs | NCT number (phase) | Experimental VS Controlling | Main indicators(month) | AE incidence | Major AE |
| Non-small-cell lung carcinoma | ||||||
| Erlotinib | Ramucirumab (anti-VEGFR) | NCT02411448 (III) | Erlotinib + Ramucirumab (224) VS Erlotinib + Placebo (225) | PFS: 19.4 VS 12.4 | 72% VS 54% |
Hypertension (24%) Dermatitis acneiform (15%) |
| Erlotinib | Bevacizumab (anti-VEGFR) | UMIN000017069 (III) | Erlotinib + Bevacizumab (114) VS Erlotinib alone (114) |
PFS: 16.9 VS 13.3 OOR: 72% VS 66% |
88% VS 46% | Rash (21%) |
| Gefitinib | Capmatinib (MET-TKI) | NCT01610336 (Ib/II) | Capmatinib + Gefitinib |
ORR: Cross phase:27% In MET copy > 6: 47% |
– | Increased amylase and lipase levels (6%) |
| Osimertinib | Savolitinib (MET-TKI) | NCT02143466 (Ib) | B1: Previous 3rd EGFR TKI (69) |
PFS: 5.4; OOR: 30% DOR: 7.9 |
57% | |
| B2: No previous 3rd EGFR TKI, T790M-(51) |
PFS: 9; OOR: 65% DOR: 9.0 |
|||||
| B3: No previous 3rd EGFR TKI, T790M+ (18) |
PFS: 11; OOR: 67% DOR: 12.4 |
– | – | |||
| D: No previous 3rd EGFR TKI, T790M+ (42). Half dosage of Savolitinib in B. |
PFS:9.1; OOR: 64% DOR: 8.0 |
38% | – | |||
| Afatinib | Cetuximab (anti-EGFR) | NCT02438722 (II)# | Afatinib + Cetuximab (83) VS Afatinib alone (85) | PFS*: 11.9 VS 13.4 | 72% VS 40% |
Acneiform rash (27%) Maculopapular rash (13%) Diarrhea (15%) |
| Osimertinib | Selumetinib (MEK1/2i) | NCT02143466 (Ib) | Osimertinib + Selumetinib (36) | OOR: 42% | 58% | |
| Savolitinib (MET-TKI) | Osimertinib + Savolitinib (18) | OOR:44% | 600 mg 75% 800 mg 83.3% | – | ||
| Durvalumab (anti-PD-1) | Osimertinib + Durvalumab (23) # | OOR: 43% |
3 mg/kg 60% 10 mg/kg 38.5% |
– | ||
| Osimertinib | Bevacizumab (anti-VEGFR) | NCT03133546# (II) | Osimertinib + Bevacizumab VS Osimertinib alone |
PFS*: 15.4 VS 12.3 OS:24 VS 24.3 OOR:55%VS 55% TTF: 8.2VS10.8 |
47% VS 18% |
Hypertension (24%) Lipase increased (7%) |
| Gefitinib | Fulvestrant (antiestrogen) | NCT01556191# (II) | Gefitinib + Fulvestrant (100)VS Gefitinib alone (104) |
PFS*: 9.9 VS 9.4 OS*: 22.1 VS 28.6 |
21.4%VS 24.2% |
Investigations (8.7%) Skin and subcutaneous tissue disorders (6.1%) |
| Erlotinib | Erlotinib+ Fulvestrant (88) VS Erlotinib alone (87) |
PFS*: 1.8 VS 2.0 OS*: 10.3 VS 7.3 |
13.8% VS 15.9% | Gastrointestinal disorders (3.4%) | ||
| Osimertinib | Ramucirumab (anti-VEGFR) | NCT02789345 (I) | Osimertinib + Ramucirumab |
PFS: 11 OOR: 76% DOR: 13.4 |
28% |
Hypertension (8%) Platelet count decreased (16%) |
| Gefitinib | Pemetrexed (chemo) |
- (II) |
Gefitinib + Pemetrexed (126) VS Gefitinib alone (65) |
PFS:16.2 VS 11.1 PFS in different TS level: High TS (12.6 VS 9.9) Low TS (22.6 VS 11.0) OS: 43.3 VS 36.8 |
25.4% VS 9.2% |
Fatigue (5.6%) Anorexia (4.0%) Mucositis oral (4.0%) |
| Gefitinib | Carboplatin plus pemetrexed (chemo) | UMIN000006340 (III) | Gefitinib + chemotherapy (170) VS Gefitinib alone (172) |
PFS:20.9 VS 11.2 OS: 50.9 VS 38.8 ORR: 84% VS 67% |
65.3% VS 31% |
Neutropenia (31.2%) Leukopenia (21.2%) Anemia (21.2%) Thrombocytopenia (17.1%) |
| Osimertinib | Durvalumab (anti-PD-1) | NCT02143466 (Ib) | A (previous TKI treated): Osimertinib + Durvalumab (23) |
OOR: 43% DOR:20.4 m |
Durvalumab 3 mg/kg:60% 10 mg/kg:38% |
Both Interstitial lung disease |
| B: Osimertinib + Durvalumab (11) |
PFS:6 OOR: 82% DOR:7.1 m |
82% | Interstitial lung disease (27%) | |||
| Gefitinib | S49076 (MET/AXL TKI) | - (I)# | Gefitinib+S49076(14) | limited anti-tumor activity | ||
| Osimertinib | Carboplatin- pemetrexed (Chemo) | jRCTs071180062 (II)# | Osimertinib + carboplatin-pemetrexed VS Single osimertinib |
PFS*: 14.6 VS 15.8 OS: NR OOR*: 53.6% VS 71.4% |
83.8%VS 45.2%* |
Neutropenia (51.6%) Leukopenia (38.7% |
| Gefitinib | Vorinostat (HDACi) | NCT02151721 (I) | Gefitinib + vorinostat (12) |
PFS: 5.2 OS: 22.4 DOR:83.3% |
50% | Hypokalemia (16%) |
| Apatinib | Pemetrexed –platinum (Chemo) | ChiCTR1800015920 (II) | Apatinib + Pemetrexed platinum (20) |
PFS: 7.7 OS: 20.1 OOR: TKI-pretreated:90% TKI naive:70% |
35% | Neutropenia (25%) |
| Afatinib | Anti-VEGF vaccination (anti-EGFR) | NCT03623750 (Ib) | Apatinib + anti-EGF vaccination (23) |
PFS: 14.8 OS: 26.9 OOR: 78.30% DOR:13.7 |
30% | Diarrhea (26%) |
| Alectinib | Bevacizumab (anti-VEGFR) | NCT04181060 (I/II) | Initial treatment: Alectinib + Bevacizumab (6) |
PFS: NR OS: 9 OOR: 100% DCR:100% |
– | – |
| Prior ALK TKI treatment: alectinib +bevacizumab (5) |
PFS: 9.5 1 year OS rate: 63.6% OOR: 60% DCR:100% |
27% |
Hypertension (9%) Proteinuria (9%) |
|||
| Erlotinib | Nivolumab (anti-PD-1) | NCT01454102 (I) |
Erlotinib + nivolumab (21): TKI-treated (20) TKI-naive (1) |
PFS: 5.1 OS:18.7 1 year OS rate:70% OOR: 15% |
52.4% |
Diarrhea (10%) AST increased (10%) |
| Tepotinib | Gefitinib (TKI) | NCT01982955 (Ib/II) | Tepotinib + Gefitinib (31) |
PFS:17.3 OS: 17.3 OOR: 45% |
62% |
Amylase concentration increased (16%) Lipase concentration increased (13%) |
| Chemo (24) |
PFS:18.7 OS: 18.7 OOR: 33% |
52% |
Anemia (30%) Neutrophil count decreased (13%) |
|||
| Renal Cell Carcinoma | ||||||
| Axitinib/Sunitinib | Pembrolizumab (anti-PD-1) | NCT02853331 (III) | Pembrolizumab + Axitinib (432) VS Sunitinib (429) |
PFS: 15.1 VS 11.1 OS: 89.9% VS 78.3% OOR: 59.3% VS 35.7% |
75.8% VS 70.6% |
Hypertension (22.1%) ALT decreased (13.3%) |
| Lenvatinib/ sunitinib | Pembrolizumab (anti-PD-1) Everolimus (mTORi) | NCT02811861 (III) | Lenvatinib +Pembrolizumab (355) |
PFS: 23.9 1 year OS rate: 79.2% OOR: 71.00% DOR:25.8 m |
82.40% |
Hypertension (27.6%) Diarrhea (9,7%) |
| Lenvatinib + Everolimus (357) |
PFS: 14.7 1 year OS rate: 66.1% OOR: 53.50% DOR:16.6 m |
83.10% |
Hypertension (22.5%) Diarrhea (11.5%) |
|||
| Sunitinib (356) |
PFS: 9.2 1 year OS rate: 70.4% OOR: 36.10% DOR: DOR:14.6% |
71.80% | Hypertension (18.8%) | |||
| Lenvatinib | Everolimus (mTORi) | NCT01136733 (II) | Lenvatinib + Everolimus (51) |
PFS: 14.6 OS: 24 OOR: 43% |
71% |
Constipation (37%) Diarrhea (20%) |
| Single lenvatinib (52) |
PFS: 7.4 OS: 19.1 OOR: 27% |
79% |
Hypertension (17%) Diarrhea (12%) |
|||
| Single everolimus (50) |
PFS: 5.5 OS: 15.4 OOR: 6% |
50% |
Anemia (12%) Hypertriglyceridaemia (8%) |
|||
| Lenvatinib | Everolimus (mTORi) | NCT02915783 (II) | Lenvatinib +everolimus (31) |
PFS: 9.2 OS: 15.6 OOR: 26% |
68% |
Hypertension (16%) Malignant progression (13%) |
| VEGFR-TKI | Everolimus (mTORi) | NCT01266837 (IV) | Everolimus (63) |
PFS: 3.8 OS: 16.8 OOR: 7.90% DOR: 60.3% |
57.10% |
Anemia (17.5%) hyperglycaemia(7.9%) fatigue(4.8%) |
| Hepatocellular Carcinoma | ||||||
| Lenvatinib Hcc | Pembrolizumab (anti-PD-1) | NCT03006926 (Ib) | Lenvatinib + Pembrolizumab (104) |
PFS: 9.3 OS: 22 OOR: 46% DOR:8.6 |
67% |
Hypertension (17%) AST increased (11%) |
| Sorafenib | Trametinib (MEK1/2i) | NCT02292173 (I) # | Sorafenib + Trametinib (17) |
PFS: 3.7 OS: 7.8 |
– |
Elevated AST (37%) hypertension (24%) |
| Cabozantinib (multi-target TKI) | NCT01908426 (III) | Cabozantinib (407) VS placebo (237) |
PFS: 5.2 VS 1.9 OS:10.8 VS 8 OOR: 4% |
68% VS 36% |
Palmar-plantar erythrodysesthesia (17%) hypertension (16%) increased ALT (12%) |
|
| Endometrial Carcinoma | ||||||
| Lenvatinib | Pembrolizumab (anti-PD-1) | NCT02501096 (II) | Lenvatinib +Pembrolizumab (108) |
PFS: 7.4 OS: 16.7 OOR: 63.6 DOR 21.2 |
66.90% |
Hypertension (31.5%) fatigue (7.3%) diarrhea (6.5%) |
| Lenvatinib | Pembrolizumab (anti-PD-1) | NCT03517449 (III) | Lenvatinib + Pembrolizumab (411) VS Chemo (416) |
PFS: 7.2 VS 3.8 OS: 18.3 VS 11.4 |
88.9% VS 72.7% |
Hypertension (37.9%) weight decrease (10.3%) |
| Breast Cancer | ||||||
| Tucatinib | Ado-Trastuzumab Emtansine (TDM1) (anti-HER2) | NCT01983501 (Ib) | Tucatinib +T-DM1(57) |
PFS: 8.2 OOR: 47% DOR:6.9 |
52% |
ALT increased (12%) AST increased (12%) |
| Cervical Cancer | ||||||
| Apatinib | Camrelizumab (anti-PD-1) | NCT03816553 (II) | Apatinib + Camrelizumab (45) |
PFS: 8.8 OS:NR OOR: 55.60% DMR:NR |
71.10% |
Hypertension (24.4%) anemia (20%) |
| Head And Neck Squamous Cell Carcinomas | ||||||
| Erlotinib | Everolimus (mTORi) | NCT00942734 (II) | Erlotinib + Everolimus (35) |
PFS: 2.9 OS: 10.3 OOR: 2.80% |
– | – |
| Multiple Solid Tumors | ||||||
| Afatinib | Sotorasib (RAFi) | NCT04185883 (I) | Recruiting | |||
| Lung Adenocarcinoma | ||||||
| Osimertinib | Bevacizumab (anti-VEGFR) | UMIN000023761 (II) | Osimertinib + Bevacizumab VS Single osimertinib |
PFS*: 9.4 VS 13.5 OS*: NR VS 22.1 OOR: 68% VS 53% |
80% |
Proteinuria (23%) hypertension (20%) |
| Chronic Myeloid Leukemia | ||||||
| imatinib CML | HCQ (Autophagy inhibitor) | NCT01227135 (II) | Imatinib + HCQ (30) VS Imatinib (32) |
Success Rate (BCR-ABL1 qPCR level reduction≥0.5 log after 12 m): 12 m: no difference; 24 m: 20.8% higher than single imatinib MMR: 12 m (66.7% VS 71.9%); 24 m (63.3% VS 68.8%). CMR: 12 m (0% VS 0%); 24 m (3.3% VS 6.3%). |
||
| Gastrointestinal Stromal Tumor | ||||||
| Cabozantinib (multi-target TKI) GIST | NCT02216578 (II) | Cabozantinib (50) |
PFS: 5.5 OS: 18.2 OOR: 60% DOR: 82% |
68% |
Hypertension (36%) diarrhea (26%) |
|
*No statistical difference. #: The trial shows limited clinical benefit or no benefit from combination therapy.
chemo: chemotherapy; MEK1/2: mek1/2 inhibitor; mTORi: mTOR inhibitor; HCQ: hydroxychloroquine; HDACi: histone deacetylases inhibitors; TDM1: trastuzumab emtansine; RAFi: RAF inhibitor; PFS: progression-free survival; OS: overall survival; ORR: objective response rate; DOR: duration of response; DMR: deep molecular response; TTF: time to treatment failure; AE: adverse event; NR: not reached; MMR: major molecular response; CMR: complete molecular response; TS: thymidylate synthase.
Tumor microenvironment
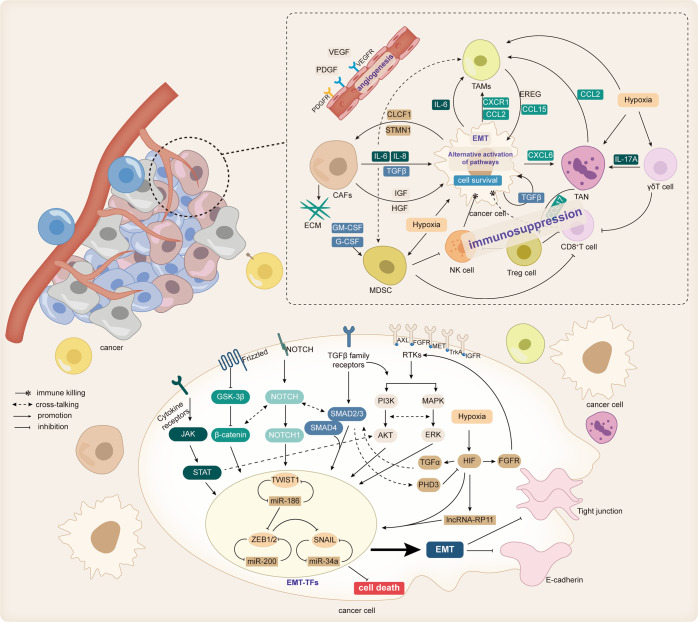
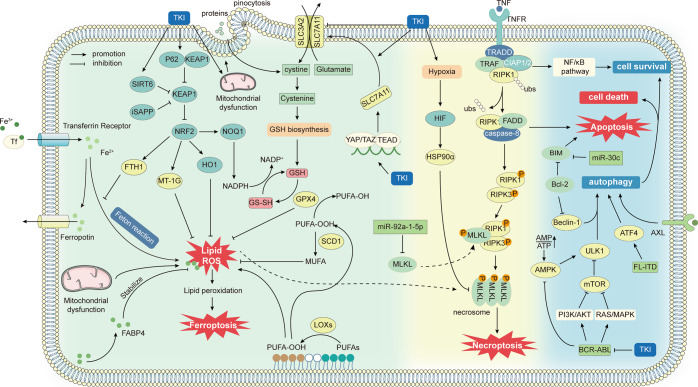
TME is enormously complex and includes various cell types, signaling molecules, cytokines, extracellular matrix (ECM), and blood vessels. TME is characterized by hypoxia, immunosuppression, and chronic inflammatory response. TME interacts with tumor cells, supports tumor cell proliferation and survival, and influences therapeutic response by modulating biological behaviors, such as tumor cell growth and proliferation. TKI therapy may induce TME remodeling and improve therapeutic response, but TME alterations can also induce drug resistance and maintain tumor progression. Uncontrolled expression of bioactive substances by cellular components of TME can compensate for suppressed signal transduction in tumor cells, induce immunosuppression, and promote epithelial-mesenchymal transition (EMT) and stemness of tumor cells, all of which are closely related to TKI resistance. In addition, the dysregulated metabolism of cellular components of TME can also affect cancer cells and promote TKI resistance (Fig. 3).
Fig. 3. Cellular composition within the TME and the effect of EMT on TKI resistance.
Cells within the TME include tumor cells, cancer-associated stromal cells, and immune cells. These cells crosstalk with other cells through cytokines and chemokines, promoting tumor progression. Some cell-secreted ligands, such as TGF-β, IL-6, HGF, IGF, etc., can bind to the corresponding receptors on the surface of tumor cells and affect the biological behavior of tumor cells, one of which is EMT. EMT can be mediated by the TGFβ/Smad classical pathway. Some cytokine receptors, such as IL-6 and some RTKs (AXL, FGFR, MET, IGFR, TrkA, etc.) can also activate the PI3K/AKT, RAS/MAPK, and JAK/STAT pathways in combination with the corresponding ligands to induce EMT. The Notch signaling pathway and wnt/β-catenin pathway are also involved in EMT, and there is crosstalk between these pathways. Tumor cells that develop EMT have a more aggressive phenotype and metastatic ability and are resistant to cell death and TKI resistance. In addition, the immunosuppressive state within the TME impairs the killing of tumor cells by immune cells and promotes tumor survival
Cellular component
Cellular components of TME include tumor cells and resident components, and recruited host cells known as cancer-associated stromal cells and immune cells. These cells promote tumor progression and induce drug resistance by releasing numerous cytokines and chemokines. Cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) are among these cells, and play important roles in the development of TKI resistance.
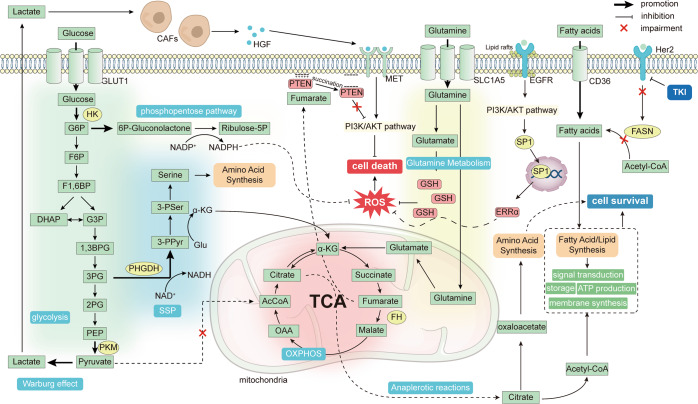
CAFs can release various cytokines, suppress immune function, shape a drug-resistant microenvironment, and interact with tumor cells. CAFs are of great importance for developing drug resistance.202 CAFs-derived HGF and IGF-1 conduct primary resistance to TKIs targeting different oncogenes.203 CAFs in osimertinib-resistant NSCLC cell lines release higher IL-6, IL-8, and HGF and express more CAF markers like α-smooth muscle agonists-α (α-SMA), fibroblast activation protein (FAP), and PDGFR.204 The same scenario can be seen in patients with metastatic RCC.205 HGF can bind to MET on cancer cells to activate MEK/ERK signaling pathway. IL-6 interacts with transforming growth factor-beta (TGF-β) to induce EMT.206,207 Through the PI3K/Akt/hypoxia-inducible factor 1 subunit alpha (HIF-1α) signaling pathway, IL-8 upregulates forkhead box C1 (FoxC1) expression to promote the expression of C-X-C motif chemokine receptor 1 (CXCR-1) and C-C motif chemokine ligand 2 (CCL2) to induce TAM infiltration and immunosuppression. In HCC, hepatic stellate cell (HSC)-derived CAFs release more HGF, which activates MET pathway and increases STMN1 expression in HCC cells. STMN1 promotes PDGF-BB expression, inducing HSC to acquire CAF characteristics and further elevate HGF level.208 On the contrary, HCC cells release B-cell activating factor (BAFF) to bind BAFF receptors on CAFs, activate the NF-κB pathway, and increase the expression of IL-6 and IL-8, causing sorafenib resistance.209 Apart from HGF, CAFs can also secrete IGF-1, which binds to IGF-1R on cancer cells, thereby promoting Annexin A2 (ANXA2) expression.210 The exact mechanism underlying ANXA2 effects was not uncovered. The current evidence suggests that ANXA2 plays a role in EMT and ECM degradation.211
CAFs-derived cardiotrophin-like cytokine factor 1 (CLCF1) stimulates HCC cancer cells to release TGF-β and C-X-C motif chemokine ligand 6 (CXCL6). TGF-β induces the stem cell characteristics, and CXCL6 induces N2-like polarization of TANs, forming a TME proper for cell stemness and immunosuppression. Meanwhile, CXCL6 induces more CLCF1 secretion, forming a positive feedback loop.212 Moreover, CAFs in luminal-like HER2+ breast cancer cell lines can release NRG1β, which binds to HER3 and stabilizes the HER2-HER3 dimer to maintain everlasting activation of downstream pathways, resulting in lapatinib resistance.213 In refractory tumors, CAFs promote tumor angiogenesis and progression despite administrating VEGFR TKIs,214 suggesting the existence of VEGFR-independent PDGFR-related angiogenesis pathways. Moreover, pericytes may contribute to this observation by increasing pericyte coverage and pericyte-derived VEGF.215
TAMs are abundant in the TME and are divided into two subgroups, M1 and M2. Tumor cells can promote TAM M2 polarization during treatment, which provokes drug resistance through multiple mechanisms. By secreting VEGF and IL-6, HNSCC cancer cells recruit macrophages and promote the M2 polarization. M2-polarized TAMs can secret CCL15 to bind C-C motif chemokine receptor 1 (CCR1) on tumor cells and activate the NF-κB pathway, thereby inducing gefitinib resistance.216 Metformin, an inhibitor of CCL15 expression, maintains gefitinib sensitivity. Moreover, high TAM-derived epiregulin (EREG) induces the formation of EGFR/ERBB2 heterodimers on cancer cells, induces AKT phosphorylation, and attenuates TKI-induced apoptosis, thereby reducing erlotinib therapeutic response in NSCLC patients.217 However, overexpression or knockdown of cancer cell-derived EREG does not affect the TKI sensitivity, indicating the importance of compensatory signaling pathways in TKI resistance. Upregulation of TAM-derived EREG may attribute to the activation of the JNK pathway by IL-1β in the TME.218 In erlotinib-resistant HNSCC, EREG/EGFR was found to upregulate the c-myc expression to stimulate cell proliferation.219 Furthermore, M2 TAMs can release HGF to bind to MET receptors in HCC tumor cells and induce sorafenib resistance. It also helps recruit more TAMs from the circulation to TME, deteriorating the situation.220
Similar to TAMs, TANs can also be polarized in response to TME signaling, and N2 TANs contribute to tumorigenesis. In an animal study, a high dose of VEGF-TKI induced γδ T cells to secrete IL-17A. IL-17A contributed to the N2 polarization of TANs and depletion of CD8+ T cells, forming an immunosuppressive microenvironment, resulting in VEGFR-TKI resistance.221 Similarly, sorafenib treatment for HCC was accompanied by a progressive increase in TAN infiltration, and depletion of TAN reversed sorafenib resistance. Mechanistically, sorafenib-related antiangiogenic therapy-induced hypoxia-activated HIF-1α/NF-κB signaling increases chemokine CXCL5 secretion in HCC cells, thereby recruiting TAN and inhibiting apoptosis. TANs further provoked TAM and Treg cell infiltration by releasing CCL2 and CCL17. These alterations weakened immune response but stimulated angiogenesis, making tumor cells insensitive to VEGFR-TKIs.222
Treg cells, which express CD25 and forkhead box P3 (FOXP3), induce immunosuppression, antigen tolerance, and immune evasion. In HCC, CCL22, which can induce CCR4+ Treg cells migration into the TME, was upregulated under sorafenib administration. Its upregulation was mediated by TNFα-related receptor-interacting serine/threonine-protein kinase 1 (RIPK1)/NF-κB pathway,223 and TGF-β can also upregulate CCL22 through TGF-β-miR-34a-CCL22 axis.224 Upregulating T-cell factor 1 (TCF1), programmed cell death protein 1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and releasing immunosuppressive cytokines like IL-10, Treg cells convey sorafenib resistance through an immunosuppressive landscape. Under such conditions, CCL22 or CCR4 blockade reduces the Treg population and improves sorafenib resistance.225 CCR4 blockade may be more effective because CCL17 also activates CCR4.223,224
MDSCs proliferation can be induced by cytokines and chemokines, and activated MDSCs enhance angiogenesis and suppress T-cell function by secreting various cytokines, playing a pro-tumorigenic and immunosuppressive role.226 In RCC, sunitinib can strongly inhibit MDSCs in the peripheral blood and spleen, but MDSCs in TME are highly resistant to sunitinib.227 Mechanistically, despite the partial inhibition of MDSCs’ proliferation ability through sunitinib-mediated STAT3 inhibition, MDSCs can maintain their proliferation in the presence of proliferative stimuli such as IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF),228 among which disordered GM-CSF can bypass the sunitinib-mediated STAT3 inhibition through STAT5 activation, providing alternative survival signals for MDSCs, exacerbating the immunosuppression, and increasing VEGF expression, leading to VEGFR-TKIs resistance. A novel role of MDSCs was found in EGFR-mutated LUAD. A subset of MDSCs-CD14+S100A9+ monocytic MDSCs differentiated to TAM. S100A9+ MDSCs-derived TAMs, which express S100A9+ and M2 marker CD206, attenuate the cytotoxic effect of EGFR-TKIs.229 Novel RELB alternative NF-κB pathway activation may be the reason, whose function in cell cycle arrest and death inhibition was shown by previous studies.230,231 In HCC, MDSCs-derived IL-6 were found to induce HCC cells to secret FGF-1. FGF-1 can activate CAFs and induce sorafenib resistance,232 suggesting the crosstalk between cellular components. These findings show the interaction in different cell types.
Cellular components of TME can aggravate immunosuppression, necessitating combination therapy with TKI and immune checkpoint inhibitors (ICIs). Theoretically, ICIs alleviate the immunosuppressive microenvironment and promote antitumor immune responses. TKIs can also activate immune cells, and there is no significant overlap in the toxicity of TKIs and ICIs.233 A phase III clinical trial showed that axitinib combined with pembrolizumab significantly prolonged the survival of RCC patients, compared with sunitinib alone.234 Clinical trials of lenvatinib combined with pembrolizumab for endometrial cancer, HCC, and RCC have also shown encouraging safety and efficacy.235–239 Lapatinib combined with camrelizumab, erlotinib combined with nivolumab, and osimertinib combined with bevacizumab have been promising in NSCLC.240–242 But nivolumab combined with EGFR-TKIs had a higher incidence of interstitial pneumonia although effectively treated NSCLC.243 However, despite improving immune function, lenvatinib combined with anti-PD-1 did not lead to durable tumor regression in TC,244 and these findings need to be validated with further studies.
To sum up, extensive interactions between various cellular components of TME contribute to tumorigenesis and immunosuppression. TKIs, especially anti-angiogenesis-related TKI (VEGFR-TKI, PDGFR-TKI, etc.), profoundly modulate TME to undermine tumor cell viability, but TME remodeling sometimes promotes drug resistance. The immunosuppressive TME markedly promotes drug resistance and tumor progression. Removing an immunosuppressive TME may attenuate cellular component-mediated TKI resistance.
Histological phenotypic transformation
EMT is a reversible process of the trans-differentiation of polarized epithelial cells to mesenchymal cells mediated by EMT transcription factors (EMT-TFs), such as snail family transcriptional repressor (SNAIL), zinc finger e-box binding homeobox (ZEB), and twist family BHLH transcription factor (TWIST). The process is associated with the removal of epithelial cell markers like E-cadherin and upregulation of mesenchymal cell markers such as N-cadherin and vimentin. In this process, tumor cells gradually lose their epithelial cell phenotype and intercellular adhesion junctions. Then, they obtain stem cell-like characteristics and possess greater mobility and migration ability.245,246 TKI-induced TME remodeling can induce abnormal activation of intracellular EMT-TFs,247 leading to the development of EMT, and promoting tumor progression, metastasis, and resistance to TKIs.
The mechanism of TKI resistance mediated by EMT is complex. Recent findings suggested that EMT may be an independent resistance mechanism. High ZEB1 expression without resistance mutations has been reported in erlotinib-resistant NSCLC cells, and knockdown of ZEB1 may restore the erlotinib sensitivity to the same level as the parental cells.248 A similar scenario has been observed in NSCLC cell lines resistant to gefitinib or osimertinib.249 Furthermore, EMT-related molecules such as SNAIL 1/2, ZEB 1/2, Claudin 1 (CLDN1), and CD44 were remarkably upregulated in tumor biopsies from sunitinib-resistant ccRCC patients.250 Moreover, dovitinib-treated surviving cell count in bladder cancer cell lines positively correlated with EMT level (p = 0.0483).244,251 These findings suggest that EMT can induce tumor cell resistance to TKI-induced apoptosis. Highly expressed EMT-TFs such as ZEB1 and TWIST1 can directly bind to the intron region and promoter of BIM, in mesenchymal NSCLC cancer cells and repress its transcription.248,252 In addition, EMT-TFs can also activate the ATR serine/threonine kinase (ATR)- checkpoint kinase 1 (CHK1)-AuroraB signaling cascade, and Aurora B can phosphorylate and degrade BIM.253 In the absence of BIM, TKIs cannot induce apoptosis despite the effective inhibition of oncogenic pathways.
The precipitating factor of EMT may be also associated with TKI resistance. TGF-β is a typical inducer of EMT, and its level increases with increasing gefitinib concentrations in NSCLC,254 a feedback regulation that promotes EMT and EGDR-TKI resistance established. Mechanistically, TGF-β binds to TβR (TGFR) to induce EMT-TFs expression to downregulate epithelial-related proteins and initiate EMT via the SMAD pathway or non-SMAD pathways such as PI3K/AKT and RAS/MAPK pathway.246 In addition, TGF-β can also induce EMT by inducing several EMT-triggering signaling pathways, such as the Notch, Wnt, and integral protein signaling pathways. Moreover, TGFβR/SMAD pathway downregulates phosphohydroxylase 3 (PHD3). PHD3 can be also downregulated by promoter methylation. PHD3 is a negative regulator of EMT, whose depletion stabilizes HIF-1/2 to upregulate the EGFR ligand TGFα. PHD3 downregulation also enhances EMT and spontaneous metastasis. In return, TGFα stimulates EGFR, thereby potentiating SMAD signaling, and reinforcing EMT and metastasis.255 Besides, studies in GB have shown that TGF-β also promotes YAP nuclear translocation and upregulates SNAIL2.256
Hypoxia is also a key trigger of EMT, which relies on HIF to regulate EMT. Hypoxia upregulates FGFR1, thereby increasing ERK phosphorylation, and upregulating ZEB1 to promote EMT and induce erlotinib resistance.100 Furthermore, HIF-2 targets the hypoxia-response element (HRE) of the Polo-like kinase 1 (Plk1) promoter. Plk1 induces EMT and leads to sunitinib resistance in ccRCC patients.257 Similarly, HIF-1α directly binds to the HRE of TWIST to regulate its expression and induce EMT.258 In addition, HIF can also induce overexpression of lncRNA-RP11-390F4.3 to regulate the expression of multiple EMT-TFs.259 Hypoxia can directly lead to EMT or expedite EMT by interacting with EMT-related pathways such as TGFβ, wnt, notch, and hedgehog through HIF.260
Some RTKs can also induce the expression of EMT-TFs through the ERK, JNK, and MAPK pathways. Some PTKs and their ligands, such as FGF/FGFR1, HGF/MET, IGF/IGFR, Gas6/AXL, and NGF/ TrkA have been implicated in EMT and TKI resistance.170,261–264 Among them, AXL has received more attention. AXL-mediated EMT is one of the resistance mechanisms of NSCLCs to EGFR-TKIs and ALK-TKIs, and HCCs to sorafenib.265–267
Since EMT is a reversible process, blocking EMT-related pathways in every stage can improve TKI resistance. Combination therapy with erlotinib and TGF-β type I receptor inhibitors effectively inhibited metastasis in erlotinib-resistant NSCLC cells.268 However, it is worth noting that dual inhibition of EGFR and TGFβR did not completely prevent acquired gefitinib resistance. Despite the reversal of EMT by TGFβR blockade, EGFR-resistant mutations like T790M became the predominant cause of the resistance after using TKI. These findings suggest that TKI resistance mechanisms may differ based on tumor type and stage of treatment.254 Replacement of EGFR-TKI is helpful to target the T790M mutation.
Targeting aberrantly activated AXL effectively attenuates TGF-β- and hypoxia-induced EMT.269,270 The AXL inhibitor, bemcentinib, and the MEK inhibitor, selumetinib specifically target mesenchymal and epithelial cells respectively, and their combination significantly inhibits the growth of tumors with both EMT and drug-resistant mutations. It successfully restores TKI sensitivity, making AXL a promising target for drug therapy.271 Since high expression of Aurora kinase family members is associated with EMT and drug resistance, inhibition of these proteins also restores drug sensitivity. TKI with alisertib, an AuroraA inhibitor, partly reverses the EMT and improves EGFR-TKIs resistance.272 Combining an AuroraB inhibitor with osimertinib can helpfully prevent EMT and improve TKI resistance. Mechanistically, the AuroraB inhibitor stabilizes BIM by reducing its ser87 phosphorylation and activates BIM-mediated mitotic catastrophe. Besides, it can transactivate the p53 upregulated modulator of apoptosis (PUMA), a molecule that binds and inhibits members of Bcl-2 anti-apoptotic protein and induces apoptosis through FOXO1/3.253
EMT is a critical biological process through which epithelial-origin malignant tumor cells acquire a high capacity for migration and invasion. EMT also allows tumor cells to escape apoptosis, degrade extracellular matrix, and resist TKIs. TME and EMT are closely related, as TME stimuli such as hypoxia and disordered extracellular signals cause abnormal intracellular EMT signals and induce EMT.
In fact, the underlying mechanism of EMT is markedly complex because of the following reasons. First, EMT inducers are various. It’s worth considering whether some factors dominate the EMT process compared to other factors, which can be called “determinant EMT inducers”. The predominant inducers have the potential to be drug targets. Second, EMT-related pathways are various, and interactions among which are even unclear. Third, epigenetic modifications can also regulate EMT and cause drug resistance. Fourth, EMT can also occur in normal cells, which needs to be distinguished from pathological EMT. Fifth, EMT is a dynamic and reversible process, and EMT-related TKI resistance may be a progressive step. It’s worthwhile to distinguish the cancer features in different EMT stages.
Discovering the molecular mechanisms of EMT and TKI resistance and exploring key molecules in EMT is crucial for understanding EMT-related TKI resistance.
Cellular metabolism
Tumorigenesis and tumor progression require metabolic reprogramming of tumor cells to meet the increased energy and nutrient demands and to reduce oxidative stress. And tumor cells compete with other cell types for nutrients and survival.273 Recent findings suggest the role of tumor metabolic reprogramming in TKI resistance induction and provide new insight into drug resistance improvement (Fig. 4).
Fig. 4. Impact of tumor metabolic reprogramming on TKI drug resistance.
Under the pressure of TKI treatment, tumor cells undergo metabolic reprogramming to adapt to the altered environment caused by TKI, but the metabolic reprogramming of tumor cells is not the same in different contexts. Some drug-resistant cells mostly exhibit elevated activity of enzymes related to the glycolytic pathway to enhance glycolysis, and the Warburg effect also tends to be enhanced, leading to increased lactate production, which can stimulate CAFs to secrete HGF and activate MET on cancer cells. In addition, serine metabolism, the pentosephosphate pathway, and glutamine metabolism in tumor cells are also reprogrammed. The intermediates of these pathways can enter the tricarboxylic acid cycle to assist lipid and protein synthesis and promote cell survival, and some antioxidant substances, such as GSH and NADPH, produced midway can also reduce ROS levels to avoid cell death. The reprogramming of lipid metabolism can promote cell survival and resist cell death by affecting multiple processes, such as lipid composition of cell membranes, intracellular signaling, and sensitivity to oxidative stress, leading to TKI resistance
A transcriptomic and proteomic analysis of imatinib-, nilotinib-, and dasatinib-resistant CML cell lines revealed their common metabolic profile. Compared to TKI-sensitive cells, which rely on mitochondrial oxidative phosphorylation (OXPHOS) for ATP production, the resistant cell lines significantly induced hypoxic signaling and had higher levels of the glycolytic pathway pentose phosphate pathway (PPP), and fatty acid metabolic pathway.274 Increased glycolysis was also observed in sunitinib-resistant RCC cell lines and EGFR-TKIs-resistant NSCLC cell lines.275,276 Increased glycolysis may be related to the upregulation of glycolysis-related enzymes and increased glucose uptake. Hexokinase (HK), the first glycolytic pathway enzyme, can be upregulated by hypoxia, which is associated with HCC resistance to regorafenib.277 Mechanistically, hypoxia stabilizes HIF-1α by ubiquitin-specific peptidase (USP29) and then transcriptionally activates HK.278 Pyruvate kinase M2 (PKM2), another rate-limiting enzyme of glycolysis, is overexpressed in TKI treatment, accompanied by high levels of lactate production. PKM2 overexpression is associated with imatinib resistance in CML cell lines and gefitinib resistance in colorectal cancer.279,280 In addition to inducing lactate production, PKM2 can directly activate the PI3K/AKT pathway and STAT3-related pathway to develop drug resistance. TKIs can induce IL-6 release, thereby upregulating glucose transporter type 1 (GLUT1) and increasing glucose uptake for proliferation. In addition, IL-6 can further increase its own levels by activating the NF-κB pathway and forming a positive feedback loop.276
Increased levels of glycolysis are accompanied by a shift from the pyruvate-mediated tricarboxylic acid (TCA) cycle to Warburg metabolism with lactate production increase.274 Increased lactate levels lead to the upregulation of lactate transporter proteins, glycolytic enzymes, and negative regulators of the TCA cycle in CAFs. High lactate levels induce CAFs to overexpress HGF in an NF-κB-dependent manner, which binds to and activates MET on cancer cells, and leads to gefitinib resistance in NSCLC. Therefore, inhibition of glycolysis may restore drug resistance.275 Higher lactate concentrations are also accompanied by upregulation of carbonic anhydrase 1 (CA1) and α-synuclein (α-Syn) in some cell lines.274 Some studies suggest that CA1 may be involved in fatty acid de novo synthesis,281 while α-Syn alters the metabolite into mitochondrial flux to attenuate oxidative stress and apoptosis.282 All of which are the manifestation of metabolic reprogramming.
However, it should be noted that elevated levels of glycolysis and downregulated levels of OXPHOS may not apply to all TKI-resistant cells. Metabolic profiling of erlotinib-resistant pancreatic ductal adenocarcinoma (PDAC) cells showed decreased levels of glycolysis and increased levels of PPP enzyme. PPP increases the NADPH/NADP+ ratio to protect cells against reactive oxygen species (ROS).283 Another study on EGFR-TKI-resistant NSCLC cell lines showed that resistant cell lines reduce glucose uptake and glycolytic capacity but enhance mitochondrial OXPHOS function. Inhibition of mitochondrial respiration by metformin, which targets OXPHOX complex I, may inhibit cell proliferation.284 Metabolic changes in different drug-resistant cancers are not all the same. Thus, individualized metabolic profiling is necessary.
Metabolomic analysis of sunitinib-resistant RCC cell lines showed a significant increase in glutamine transporter protein (SLC1A5) expression apart from the same increase in glycolytic enzymes and lactate.285 In addition, ROS1-TKI crizotinib-resistant NSCLC cell lines also showed increased glutamine metabolism and fatty acid synthesis dependence.286 High levels of intracellular glutamine can be converted into glutamate and then α-ketoglutarate (αKG) into TCA, which not only meets the increased energy demand of tumors but also helps the synthesis of amino acids and lipids through the anaplerotic reaction. Furthermore, glutamine is a precursor of glutathione (GSH), which is a powerful antioxidant that inhibits oxidative stress-induced cell death. Tumor cells initially rely on glutamine metabolism for energy, and the reliance situation even becomes severe in the development of resistance, and its role in antioxidant defense is more important in drug resistance. However, the role of glutamine metabolism in tumor biology goes beyond the two aforementioned aspects,287 which has the potential to reverse TKI resistance.
Lipid metabolic reprogramming can also confer tumor TKI resistance, among which lipid peroxidation is closely related. Fatty acids are classified into saturated fatty acids, monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs). Normal cells tend to meet fatty acids demands through exogenous uptake, while tumor cells rely on the de novo synthesis of fatty acids to meet their needs. Tumor cells can also increase membrane lipid saturation to reduce the sensitivity to oxidative stress and resist cell death.288 Sterol regulatory element-binding protein 1 (SREBP1) is responsible for the de novo synthesis, whose high expression can be seen in gefitinib-resistant cells. Inhibition of SREBP1 elevates ROS and lipid peroxidation levels, suggesting that SREBP1-induced de novo synthesis of fatty acids can prevent cell death.289 However, in HER2-TKI lapatinib-resistant breast cancer cell lines, lapatinib upregulated CD36 to increase exogenous fatty acid uptake instead of fatty acid de novo synthesis. Mechanistically, fatty acid synthase (FAS), which is responsible for de novo synthesis, can interact with HER2, so HER2 blockade inhibits FAS and fatty acid de novo synthesis. However, tumor cells can develop a compensatory mechanism to maintain fatty acid supply through exogenous fatty acid uptake, which is also a manifestation of metabolic reprogramming.290
Cholesterol metabolism is also one of the lipid metabolism pathways. Studies have shown that gefitinib downregulates the expression of peroxisome proliferator-activated receptor alpha (PPARα), LXRα, and ABCA1, leading to dysregulation of cholesterol efflux and induction of drug resistance in NSCLC cell lines.283 The exact relationship between cholesterol accumulation in cancer cells and drug resistance is unclear, but the combination of fenofibrate, a lipid-lowering drug that can upregulate the PPARα expression, and gefitinib can promote apoptosis in resistant cells. Activation of the PPARα/AMPK/AKT/FoxO1 pathway was involved in this effect. FoxO1 regulates the expression of several molecules involved in the cellular oxidative stress response, suggesting a role for PPARα in lipid peroxidation.291 Another study on osimertinib-resistant NSCLC cell lines revealed a possible role of cholesterol in drug resistance. Cholesterol accumulation in lipid rafts promotes EGFR-Src interaction, activates ERK/SP1 signaling via Src and enhances estrogen-related receptor alpha (ERRα) expression, which regulates ROS levels and maintains cell proliferation, leading to drug resistance.292 Phospholipid metabolism is also associated with drug resistance. An elevated level of lysophosphatidylcholine acyltransferase 1 (LPCAT1), an important enzyme of phospholipid metabolism, was observed in LUAD-resistant cell lines. LPCAT1 induced lysophosphatidylcholine to convert to a saturated state from an unsaturated one, remodeling the cell membrane lipids’ constitution. Cell membrane lipids remodeling led to activation of the EGFR/PI3K/AKT pathway, expedited cell proliferation and LPCAT1 expression, forming a positive feedback.293,294
There are also several amino acid metabolic pathways associated with drug resistance. First, phosphoglycerate dehydrogenase (PHGDH), a key enzyme of the serine synthesis pathway (SSP), is a major driver of sorafenib resistance in HCC. Inhibition of PHGDH can inhibit SSP, reduce the production of αKG, serine, and NADPH II, and induce cell death by increasing ROS production. Sorafenib induces PHGDH expression,295 leading to tumor cell survival and drug resistance. Second, methionine metabolism, or the whole sulfur amino acid (SAA) metabolism, which is involved in protein synthesis, sulfur metabolism, redox homeostasis, epigenetic modification, and signal transduction. Methionine metabolism is also associated with sorafenib resistance in HCC. Hepatocyte nuclear factor 4 alpha (HNF4α) is a regulator of SAA and is negatively correlated with EMT in HCC. Inhibition of SAA metabolism by downregulating HNF4α enhanced EMT, and exacerbated sorafenib resistance, with an unknown mechanism.296 Since methionine is an essential amino acid and its bioavailability relies on diet, regulating methionine intake may be beneficial for tumor treatment and reversal of drug resistance.297
The metabolites also have non-metabolic modifying functions. Fumarate accumulates in cells lacking fumarate hydratase (FH) and directly reacts with PTEN at cysteine-211 (C211) to form S-(2-succino)-cysteine. Succinated C211 blocks the binding of PTEN to the cell membrane, thereby reducing its inhibitory effect on the PI3K/AKT pathway, leading to sustained activation of the pathway and further sunitinib resistance of type 2 papillary renal cell carcinoma.298 Simultaneous use of AKT inhibitors or enhancing FH expression may improve drug resistance.
Cancer cells gradually develop metabolic adaptations and resistance during TKI treatment, suggesting that tumor metabolic reprogramming is an effective mechanism for tumor cells to resist TKI. Changes in metabolite levels in the TME offer new insights into TKI resistance. There are studies supporting the use of glycolysis inhibitors to ameliorate sunitinib resistance.299 Thus, targeting tumor metabolic adaptation and altering metabolites levels and composition of TME may improve cancer treatment.300 In addition, metabolic changes help diagnose tumors and detect TKI resistance on a macroscopic scale. In other words, identifying specific metabolites or combinations of several metabolites may be helpful to characterize an individual tumor, in which metabolomics has great potential.301 Metabolomics was used to characterize the metabolic profile of cancer cachexia. A diagnostic model using serum myostatin, leucine, and phenyl acetate levels can reach a diagnostic accuracy of 94.64%, showing potential for practical application.302 It is worth exploring its application in TKIs resistance to tumors.
Cell death inhibition
Cell death resistance is a hallmark of cancer. Cell death pathways include apoptosis and non-apoptotic forms, such as ferroptosis, autophagy, necroptosis, and pyroptosis. Tumor cells that develop death resistance can survive and proliferate continuously, resulting in tumor survival, recurrence, and treatment resistance.
Apoptosis inhibition
In cancer, dysregulated apoptotic signaling, particularly the activation of the anti-apoptotic pathways, allows cancer cells to escape apoptosis, leading to uncontrolled proliferation and TKI resistance.
The Bcl-2 family plays a vital role in regulating apoptosis and controls the intrinsic mitochondrial apoptosis pathway. The family can be divided into two major groups: one group exerts anti-apoptotic effects, such as bcl2 apoptosis regulator (BCL-2), BCL-X, BCL-W, and MCL1 apoptosis regulator (MCL-1), and the other one plays a pro-apoptotic role, such as BIM, PUMA, and NADPH oxidase activator (NOXA).303 Family members antagonize each other and regulate cell death. Overexpression of anti-apoptotic members or low expression of pro-apoptotic members can lead to survival and drug resistance.115
Studies have shown that BIM knockdown can significantly attenuate or eliminate the therapeutic effects of third-generation EGFR-TKIs, indicating that low BIM expression is a major cause of TKI resistance. BIM downregulation occurs during the overactivity of PI3K/AKT and RAS/MAPK. These pathways phosphorylate the transcription factor FOXO and downregulate BIM expression.304 For example, hypoxia upregulates FGFR to activate the RAS/MAPK pathway, thereby downregulating BIM expression and inducing osimertinib resistance.100 EMT-TFS can also downregulate BIM. In addition, epigenetic modification can also change BIM expression. BIM deletion polymorphisms are one of the mechanisms of primary resistance to EGFR-TKIs in approximately 21% of the East Asian population.305 They express BIM molecules without pro-apoptotic activity, resulting in impaired apoptosis. NSCLC with BIM deletion polymorphism was resistant to all three generations of EGFR-TKIs. But it is still controversial whether BIM deletion polymorphism affects the survival of patients, and further studies are needed.35
Apart from BIM, some cell lines also overexpress anti-apoptotic proteins such as BCL-2. Similar to BIM, high BCL-2 expression occurs because of PI3K/AKT and RAS/MAPK pathways overactivity.306 Epigenetic modifications can also regulate BCL-2 expression. Elongator (ELP) complexes can promote MCL-1 expression through tRNA modification, resulting in triple-negative breast cancer resistance to erlotinib.307 Other mechanisms, such as gene hypomethylation, gene rearrangements, and silencing mutations in noncoding RNAs that regulate BCL-2, can also upregulate BCL-2.303 However, some of these factors have not yet been demonstrated to be associated with TKI resistance. BCL-2 leads to TKI resistance partly by downregulating BIM through binding to contemporaneous low expression of BIM.
The induction of apoptosis is a potential solution for drug resistance. Upregulation of pro-apoptotic factors or downregulation of anti-apoptotic factors is effective. The combination of vorinostat, an inducer of BIM expression, and metformin, an inhibitor of anti-apoptotic proteins, enhanced the sensitivity of drug-resistant NSCLC to gefitinib.308 Inhibition of Mcl-1 and activation of Bax improve acquired resistance to osimertinib and other third-generation EGFR-TKIs in NSCLC.309 Similarly, inducing the expression of NOXA and bound Mcl-1 by ixazomib, a proteasome inhibitor, attenuated resistance to the ALK-TKI alectinib in ALK-rearranged NSCLC cell lines.310 The MCL-1 inhibitor, S63845, reversed drug resistance by enhancing apoptosis in regorafenib-resistant CRC cells.311 In addition, inhibition of DNA damage-induced apoptosis suppressor (DDIAS), an anti-apoptotic protein that binds to and promotes STAT3 signaling to inhibit apoptosis, led to apoptosis in gefitinib-resistant cells.312 In cancer cells with high expression of anti-apoptotic molecules, simply elevating the expression of pro-apoptotic molecules may not be sufficient to induce apoptosis. Hence, it may be more reasonable to directly target anti-apoptotic proteins instead of upregulating pro-apoptotic proteins.310 Mitochondria are the junction of apoptosis, and interference with mitochondrial function can induce apoptosis. Piperlongumine, the piper longum extract, can strongly induce ROS production and mitochondrial dysfunction and activate the AMP-activated protein kinase (AMPK) pathway, inhibiting cell growth and survival.313 Blocking the cell cycle under certain conditions can also induce apoptosis. Cyclin-dependent kinase (CDK) is the key kinase in cell cycle regulation, and CDK inhibitors can attenuate TKI resistance. TKI in combination with CDK7/12 inhibitor THZ1, CDK9 inhibitors alvocidib, and dinaciclib had a greater impact on apoptosis in drug-resistant cells.314–316
Non-apoptotic cell death inhibition
In addition to apoptosis, there are several forms of programmed cell death, such as ferroptosis, autophagy, necroptosis, and pyroptosis. These non-apoptotic forms of cell death are also closely related to TKI resistance.
Ferroptosis
Ferroptosis is a form of programmed cell death caused by iron-dependent peroxidation of polyunsaturated phospholipids in the cell membrane, and it is activated by two main pathways.317 The extrinsic pathway refers to the inhibition of cell membranes transport proteins such as cysteine/glutamate transport proteins, also known as the xc- system, or the activation of ferroportin. The former reduces GSH levels by decreasing GSH precursor uptake and increasing peroxides accumulation, while the latter increases intracellular iron and causes PUFA peroxidation by Fenton’s reaction with intracellular ROS. The intrinsic pathway refers to the direct inhibition of intracellular antioxidant enzymes such as glutathione peroxidase 4 (GPX4), leading to peroxides accumulation.318 The accumulation of oxidants in both pathways finally leads to ferroptosis. Tumor cells develop several mechanisms to escape ferroptosis-induced cell death, causing drug resistance. Inhibition of lipid peroxidation prevents ferroptosis and enhances cell survival and drug resistance.
Nuclear factor erythroid 2-related factor 2 (NRF2) pathway activation prevents ferroptosis and increases TKI resistance. Sorafenib induces p62 expression to block NRF2 ubiquitinated degradation and to enhance its nuclear translocation by inactivating Kelch-like ECH-associated protein 1 (KEAP1).319 Sirtuin 6 (SIRT6) and inhibitor of apoptosis-stimulating protein of p53 (iASPP) also bind to Keap1 to strengthen NRF2-mediated antioxidant defense. Overexpression of SIRT6 is associated with sorafenib resistance in gastric cancer cell lines, but iASPP need further exploration.320,321 In the nuclear, NRF2 binds to the cis-transcriptional regulatory element antioxidant response element (ARE). It interacts with transcriptional coactivators such as MAF-BZIP transcription factor G (MAFG), activating the transcription of NAD(P)H quinone dehydrogenase 1 (NQO1), heme oxygenase 1 (HMOX1), and ferritin heavy chain 1 (FTH1) to increase the expression of antioxidants and inhibit ferroptosis. Metallothionein-1G (MT-1G), ABCC5, GPX4, and superoxide dismutase (SOD) are also transcriptional targets of NRF2. MT-1G is a ROS scavenger that negatively regulates ferroptosis.320,322–324 ABCC5 stabilizes SCL7A11 to increase GSH precursor uptake and simultaneously enhance sorafenib efflux, leading to TKI resistance.325 Apart from NRF2 pathways, the Hippo pathway downstream transcriptional complex YAP/TAZ (Tafazzin)-TEAD (TEA domain transcription factor) is a key factor for sorafenib resistance in HCC. As a transcription factor, YAP/TAZ directly induces xc-system subunit SLC7A11 expression and synergistically induces its expression by increasing activating transcription factor 4 (ATF4) stability. High SLC7A11 expression increases intracellular GSH synthesis, attenuates lipid peroxidation, and inhibits ferroptosis.326 Additionally, Tumor cells-derived stearoyl-CoA desaturase-1 (SCD1), tumor endothelial cells (TECs)- and adipocytes-derived fatty acid-binding protein 4 (FABP4) induce ferroptosis resistance in breast cancer cells treated with sunitinib or sorafenib.327 Mechanistically, SCD1 desaturates fatty acids to generate MUFAs, while FABP4 maintains lipid droplet (LD) formation in tumor cells to reduce LD destruction-induced ROS production and inhibit the ferroptosis.328 Moreover, Sorafenib initially induces ferroptosis inhibiting cysteine uptake, but intracellular cysteine deficiency may lead to mitochondrial membrane potential hyperpolarization and mitochondrial dysfunction,329 further activating PI3K-RAC1(RAC family small GTPase1)-PAK1 signaling and enhancing micropinocytosis to replenish intracellular depleted cysteine and resist ferroptosis.330
GPX4 overexpression is observed in the hyper mesenchymal state caused by EMT and is associated with erlotinib resistance.331 GPX4 can scavenge peroxides, allowing cells to resist ferroptosis, but the relationship between EMT and GPX4 is not clear, and EMT can also lead to drug resistance through non-ferroptosis mechanisms. Consequently, the relationship between EMT and ferroptosis deserves further studies. The current study shows that GPX4 inhibition strongly induces ferroptosis of mesenchymal-like cancer cells compared with its negligible effect on epithelioid cancer cells, leading to the speculation that highly mesenchymal-like cells are more susceptible to ferroptosis, which may be related to high baseline transcription of ZEB1 in mesenchymal-like cells. ZEB1 induces the upregulation of PPARγ, the primary regulator of hepatic lipid metabolism, to promote lipid oxidative metabolism.332 In addition, ZEB1 induces plasma membrane remodeling via EMT, which is the site of lipoxygenase-induced PUFA oxidation. Therefore, it may affect peroxide accumulation, but more specific mechanisms remain to be investigated.
Inducing ferroptosis can significantly decrease drug-resistant tumor cell viability.317 We have reviewed that TKI resistance is linked to ferroptosis inhibition. Therefore, re-activating ferroptosis may reduce the TKI resistance. Inhibition of GPX4 is an effective method to target the intrinsic pathway.333 Inhibition of GPX4 could overcome the EGFR-TKI resistance by inducing ferroptosis in NSCLC and LUAD cell lines.331,334 Reducing GSH precursor uptake, increasing intracellular ferric ion concentration, and increasing ROS levels are helpful for extrinsic pathways. Amiloride inhibited the effect of macropinocytosis, and the combination with sorafenib decreased HCC cells’ drug resistance.330 The combination of the lysosomal disruptors, Siramesine, and lapatinib upregulated transferrin to increase ferric ion transport into the cancer cells and downregulated ferroportin-1 to decrease ferric ion transport out of the cell, thereby inducing intracellular ferric ion accumulation, ROS production, and ferroptosis.335 Moreover, disulfiram-copper (DSF/Cu) significantly damaged mitochondria, accelerated ROS accumulation, induced lipid peroxidation, and potentiated the cytotoxic effect of sorafenib in vitro and vivo by simultaneously inhibiting the signal pathway of NRF2.336 Additionally, cryptotanshinone (CTS) may inhibit the expression of the antioxidants such as catalase (CAT), HMOX1, and SCD and alleviate gefitinib resistance in NSCLC cells.294 Since lipid peroxidation happens throughout ferroptosis, targeting lipid metabolism to induce ferroptosis or, even more broadly, targeting the tumor metabolic reprogramming may improve drug resistance. For instance, there are certain crosstalks between mannose metabolism and lipid metabolism. Inhibition of high mannose phosphonic isomerase (MPI) can decrease TLT3-TKI resistance in AML with FLT-ITD. Mechanistically, MPI knockdown triggers defective protein glycosylation, which can induce the unfolded protein response (UPR) and drive ATF6 transcriptionally inhibits PPARα, increasing PUFA levels and inducing ferroptosis.337
In summary, some TKIs can induce ferroptosis, allowing tumor cells to develop drug resistance. During TKI administration, tumor cells can resist lipid peroxidation-induced ferroptosis by activating antioxidant pathways, enhancing GSH biosynthesis, and upregulating GPX4, leading to drug resistance. In addition, lipid metabolism reprogramming is closely related to ferroptosis, and they can potentiate each other, increasing tumor cells survival. Moreover, numerous factors can influence ferroptosis, such as P53 mutations and hypoxia, and ferroptosis may cause inflammation-related immunosuppression in TME, enhancing tumor growth.318 Therefore, targeting ferroptosis is rational to reverse TKI resistance.
Autophagy
Autophagy is both a tumor suppressor pathway and a survival mechanism for tumor cells to withstand metabolic stress and resist treatment.337 mTOR is a critical regulator of autophagy. TKIs can induce autophagy by reducing mTOR338 phosphorylation through several pathways such as PI3K/AKT, MAPK, and JAK/STAT.339 There are studies discussing the detrimental role of TKIs-induced autophagy.340–342 However, given the paradoxical role of autophagy, the outcome of TKI-induced autophagy is not definitively determined yet. Here, autophagy-mediated TKI resistance was reviewed.
In FLT-TKIs resistant AML cells with FLT3-ITD, FLT3-ITD-mediated aberrant activation of FLT3 induced autophagy by upregulating the activity of ATF4. High-level autophagy results in cancer cell survival and resistance to FLT-TKIs.343 In erlotinib-resistant NSCLC, high GATA binding protein 6 (GATA6) and a threefold increase in autophagic activity were observed. In contrast, GATA6 knockdown reduced autophagy and restore drug sensitivity, suggesting the interaction among GATA6, autophagy, and TKI resistance.344 Furthermore, abnormal AXL activation also increased autophagic flux in NSCLC cell lines. It prevented caspase-mediated apoptosis, leading to resistance to the first- and third-generation EGFR-TKIs erlotinib and rociletinib. The cytoprotective effect leading to drug resistance was independent of AXL-induced EMT.345 Apart from mTOR-induced autophagy, AMPK could induce autophagy in response to intracellular ATP deficiency caused by nutrient deprivation or mitochondrial function inhibition. It was shown that BCR-ABL TKI nilotinib induces AMPK activation and phosphorylates Unc-51-like autophagy activating kinase 1 (ULK1). ULK1 activates downstream targets such as autophagy-related 13 (ATG13), thereby increasing autophagic flux to maintain CML cell survival. This shows that TKI may alter tumor energy or metabolic status and adaptively activate autophagy to maintain cell survival.346 Mitochondrial autophagy is a specific type that can be activated by PTEN-induced kinase 1 (PINK1)-Parkin signaling. Hypoxia/HIF-1α can upregulate miR-210-5p in HCC, downregulating ATPase family AAA domain-containing 3 A (ATAD3A) expression and triggers mitochondrial autophagy via the PINK/Parkin pathway and leads to sorafenib resistance. The mechanism may be overactivated mitochondrial autophagy and reduced ROS production, leading to death resistance.347
In summary, autophagy can support tumor cell proliferation and contribute to drug resistance in some cases because of its role in energy and nutrient metabolism. However, the detailed mechanism of autophagy-dependent proliferation and apoptosis resistance needs further investigation. In autophagy-induced drug resistance, inhibition of autophagy can restore TKI sensitivity. Afatinib combined with the autophagy inhibitor, CQ, has greater anti-cancer efficacy.341 Azithromycin combined with gefitinib enhances pancreatic cancer cell death by inhibiting autophagy.348 Furthermore, a phase II randomized clinical trial (CHOICES) demonstrated that imatinib combined with HCQ for chronic phase CML achieved a higher (20.8%, P = 0.059) cytogenetic remission rate at 24 months compared with imatinib alone.349
Paradoxically, some studies have shown that combining TKIs and autophagy inhibitors such as Beclin-1 tyrosine phosphomimetic mutant or BCL-2 enhances tumor growth, dedifferentiation, and TKI resistance.350 These observations suggest that autophagy inhibition can lead to TKI resistance, but the exact mechanism is unclear. Beclin1-induced autophagy requires Beclin autophosphorylation to be released from BCL2/BCL-XL proteins, showing the link between apoptosis and autophagy.351 The relationship between apoptosis and autophagy may contribute to the development of drug resistance. Moreover, hypercholesterolemia is associated with sorafenib resistance. High SCAP induced by disordered lipid metabolism can inhibit autophagy by downregulating AMPK activity.351 However, the effect of decreased autophagic flux on drug resistance is not clear.337
In cases where autophagy inhibition is harmful, the induction of autophagy is beneficial for TKI therapy.352 Serine and arginine-rich splicing factor 1 (SRSF1) is an autophagy suppressor, and its knockdown may activate autophagy and inhibit the proliferation of gefitinib-resistant cancer cells.353 Cannabidiol promotes mitochondrial autophagy in CML cells by activating transient receptor potential vanilloid type 2 (TRPV2). Cannabidiol exerts a synergistic effect with imatinib in imatinib-resistant CML cells.354 In AML carrying FLT3-ITD, FLT-3-ITD molecules were detected in the autophagosome after induction of autophagy with the proteasome inhibitor bortezomib. It suggests that autophagy is involved in the posttranslational degradation of aberrant PTKs, and induction of autophagy is undoubtedly beneficial in this case. Bortezomib can sensitize cancer cells to FLT-3 TKI quizartinib in combination therapy.355
More interestingly, lapatinib, a dual inhibitor of EGFR and ERBB2, promotes autophagy in cells expressing only EGFR but inhibits autophagy in cells expressing ERBB2.356 These findings suggest that TKIs may act differently in tumors with different mutation profiles. The contradictory results regarding the role of autophagy in TKI resistance reflect the uncertain role of autophagy in cancer treatment.357 Therefore, some concerns require further exploration: (I) the relationship between abnormal PTK activation and autophagy; (II) whether all TKIs induce autophagy and how to define the protective and toxic effects of TKI-induced autophagy on cancer cells. Since autophagy is associated with both apoptotic cell death and some types of non-apoptotic cell death, exploring the intrinsic link between different types of cell death is pivotal for understanding TKI resistance.
Other nonapoptotic cell death inhibition
Necroptosis and pyroptosis are also emerging forms of nonapoptotic cell death, and recent findings suggest their involvement in TKI resistance.
Necroptosis, also called programmed necrosis, is a necrotic cell death mediated by RIPK1 and RIPK3 kinase. Under the stimulation of TNFα, lipopolysaccharide/Toll-like receptors, interferon (IFN), T-cell receptors, etc.,358 cells form a necrosome complex (RIPK1, RIPK3, MLKL), oligomerizing and translocating mixed lineage kinase domain-like pseudokinase (MLKL) to the cell membrane to initiate necroptosis. It causes the release of damage-associated molecular patterns (DAMPs) and inflammation.359 Some TKIs induce necroptosis, so it is reasonable to consider the role of necroptosis inhibition in TKI resistance.
Hypoxia is also important. A TKI with anti-angiogenesis effects exacerbates hypoxia. HIF induces HSP90α expression, which binds the necrosome complex, induces MLKL degradation by autophagic lysosomes, blocks necroptosis, and induces HCC resistance to sorafenib.360 Epigenetics contributes to necroptosis resistance. It was shown that overexpression of miR-92a-1-5p in Ph+CML cells inhibits MLKL expression and correlates with BCR-ABL-TKI resistance.361 ROS also induces necroptosis. It can promote RIP3 autophosphorylation and necrosome complex assembly.362 These findings raise the question of whether reduced ROS production prevents necroptosis during treatment with TKIs.
Interestingly, sorafenib was shown to directly target RIPK1 and RIPK3 to block the second mitochondria-derived activator of caspases (Smac)-induced necroptosis in CML cell lines. It is consistent with clinical reports that low micromolar concentrations of sorafenib could inhibit necroptosis.363 An animal study explained that sorafenib acts differently depending on the concentration.364 Lower concentrations inhibit necroptosis without affecting NF-κB activation, while higher concentrations induce non-necroptotic forms of cell death. In addition to sorafenib, ponatinib and pazopanib have also been identified as inhibitors of necroptosis.365 In other words, some TKIs initially inhibit necroptosis, but the pros and cons of this effect are unknown, and further studies regarding the relationship between TKI and necroptosis are needed.
Targeting necroptosis-associated molecules is a promising approach to improving drug resistance. TNF-α is a trigger molecule for necroptosis, but upregulation of its expression is ineffective in inducing necroptosis, and TNF-α levels exactly positively correlate with sorafenib resistance, for high TNF-α will lead to EMT and it can also induce activation of the NF-κB pathway to avoid cell death.36617-demethoxygeldanamycin (17-AAG) is a targeted inhibitor of HSP90α. Animal studies have shown that 17-AAG reversed sorafenib resistance in HCC.360 Moreover, shikonin is a small molecule from traditional Chinese medicine that can induce necroptosis. It activated the necrosome complex and overcame the resistance of Ph+ CML to BCR-ABL TKI and RCC cells to sunitinib.361,367 In addition, shikonin downregulated cell cycle-activating proteins and inhibited the proliferation of drug-resistant cells through cycle arrest. It also directly inhibited the AKT/mTOR pathway and enhanced TKI function.367 Apurinic/pyrimidinic endonuclease 1 (APE1) is responsible for DNA damage repair. APE1 inhibitors induced DNA damage in NSCLC cell lines, thereby inducing apoptosis, pyroptosis, and necroptosis and overcoming erlotinib resistance, suggesting that inducing cell death by targeting DNA repair can improve cancer treatment.368 Nuclear protein 1 (NUPR1) is not expressed in normal tissues but is highly expressed in precancerous lesions of the liver. Inhibition of its expression by ZZW-115 resulted in a substantial decrease in intracellular ATP concentrations and induced apoptosis and necroptosis, suggesting that NURP1 is an effective drug target.369
Pyroptosis is an intensely inflammatory lytic programmed cell death. The typical pyroptosis pathway is initiated by assembling large supramolecular complexes called inflammasomes in response to signaling stimuli.370 Inflammasomes can activate specific caspases, which cleave and activate the effector molecule gasdermin (GSDM). It causes pore formation on the cell membrane, leading to cancer cells swelling and rupture,371 resulting in the release of proinflammatory molecules and inflammatory cells infiltration.
The relationship between pyroptosis and cancer is now quite unclear, the pyroptosis-mediated inflammatory response can induce an immune response that contributes to tumor regression, but pyroptosis may sometimes induce a supportive TME.372 Theoretically, pyroptosis inhibition may help TKI resistance if the TKI acts through pyroptosis. However, only a few TKIs have been shown to kill tumor cells through pyroptosis. Studies have shown that sorafenib can induce pyroptosis in TAMs and release inflammatory mediators to induce NK cells-mediated cytotoxicity.373 Dasatinib can directly induce pyroptosis in tumor cells.374 Pyroptosis inhibition may help overcome drug resistance, but it has been poorly explored. In general, a few studies report necroptosis and pyroptosis with TKI resistance, and in-depth studies of both can help find new therapeutic targets.
Death resistance is the characteristic of cancer cells. The adaptive changes that occur in the TME during TKI treatment can exacerbate death resistance and lead to the development of drug resistance. However, programmed cell death is a double-edged sword, and inducing cancer cell death may not be totally beneficial for cancer treatment.375 Selective manipulation of cell death is a potential approach for tumor eradication and reversal of drug resistance (Fig. 5).
Fig. 5. Cell death inhibition in TKI resistance.
Under TKI pressure, tumor cells can resist apoptosis by downregulating proapoptotic molecules and/or upregulating apoptosis-inhibiting molecules. They can also resist ferroptosis by reducing lipid peroxidation through adaptive activation of antioxidant pathways and enhanced production of antioxidant substances such as GSH. Autophagy can also affect TKI resistance, but the relationship between autophagy levels and TKI resistance varies between contexts. Necroptosis is initiated by the necrosome complex, and factors such as ncRNA and hypoxia can inhibit necroptosis by suppressing the assembly of the necrosome complex
Epigenetic modification
Epigenetics bridges the gap between genotype and phenotype. It regulates chromatin structure and gene expression by covalent modification of histones and nucleic acids through multiple modalities without altering nucleotide sequences.376 Epigenetics is closely associated with carcinogenesis and drug resistance and is involved in drug-tolerant persisters (DTPs).377–379
Noncoding RNA (ncRNA)
ncRNAs do not encode proteins but are involved in posttranscriptional modifications. ncRNAs mainly include microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs). ncRNAs regulate gene expression without altering gene sequence and are deeply involved in epigenetic modification. Their expression levels are often abnormal in tumors, which is associated with tumor progression and TKI resistance (Table 3).
Table 3.
ncRNAs related to TKI resistance
| ncRNAs | Upstream molecules or pathways | Target molecules or pathways | Effect |
|---|---|---|---|
| miRNA | |||
| miR-212455(↓) | — | ABCG2 | Increased drug efflux |
| miR-452-5p135(↑) | — | DUSP6 | Activating the RAS/MAPK pathway |
| miR-210-5p347(↑) | Hypoxia/HIF | ATAD3A/ PINK1/PARKIN | Activation of mitochondrial autophagy |
| miR-92a-1-5p361(↑) | — | MLKL | Necroptosis inhibition |
| miR-200247 | — | ZEB1/2 | EMT |
| miR-186247 | — | TWIST | EMT |
| miR-34a247 | — | SNAIL | EMT |
| miRNA-125-5p382(↑) | — | ataxin-1 | EMT |
| miR-424383(↑) | — | CBX4 | nuclear translocation of YAP1 cancer stem cell phenotype |
| miR-25-3p384(↑) | — | PTEN | Activating the PI3K / Akt pathway to induce M2 polarization of macrophages |
| miR-130b-3p384(↑) | — | ||
| miR-125d-5p385(↑) | c-Jun/PUMA | Apoptosis resistance | |
| miR-494386(↑) | — | PTEN, P27, PUMA | Activating the PI3K / Akt pathway Apoptosis resistance |
| miR-30a-5p387(↓) | — | CLCF1 | Activating the PI3K / Akt pathway Promoting glycolysis |
| miR-30b/c388(↑) | MET | BIM | Apoptosis resistance |
| miR-335389(↑) | — | AXL | Activating the PI3K / Akt pathway |
| miR-196a390(↑) | NRF2 | GLTP | Cell proliferation |
| miR-221392(↑) | NF-κB pathway | p27kip1 | Apoptosis resistance |
| lncRNA | |||
| lncRNA-RP11-390F4.3259(↑) | hypoxia/HIF-1α | Snail,Twist1, ZEB1, ZEB2 | EMT |
| lncRNA-ECVSR394(↑) | — | ERβ/HIF2-α | cancer stem cell phenotype increasing vascular mimicry |
| lncRNA-CASC9395(↑) | — | EZH2/DUSP1 | Activating the RAS/MAPK pathway |
| LncRNA-PCAT-1396(↑) | — | PI3K/Akt pathway | Activating the PI3K / Akt pathway |
| LncRNA-HIF1A-AS2397(↑) | HIF-1α | miR-146b-5p/IL-6/STAT3 | Activating the IL-6/STAT3 pathway to promote proliferation |
| lncRNA-RP11616M22.7398(↑) | — | RASSF | Regulation of hippo pathway |
| circRNA | |||
| circFN1399(↑) | — | miR-1205/E2F1 | Cell proliferation and apoptosis resistance |
| circFOXM1400(↑) | — | miR-1324/MECP2 | Cell proliferation and apoptosis resistance |
| circRNA hsa_circ_0005576401(↑) | — | miR-512-5p/IGF-1R | Alternative activation of PTK |
| circRNA-SORE402(↑) | — | YBX1 | apoptosis resistance |
| circ-E-Cad405,406(↑) | C-E-Cad/EGFR | Alternative activation of PTK | |
| CircRNA-ASK1426(↓) | YTHDF2 | ASK1-272a.a/PI3K/AKT | Activating the RAS/MAPK pathway |
ABCG2 ATP Binding Cassette Subfamily G Member 2, AKT AKT serine/threonine kinase, ATAD3A ATPase family AAA domain containing 3a, AXL AXL receptor tyrosine kinase, BIM bcl-2 interacting mediator of cell death, CBX4 chromobox 4, CLCF1 cardiotrophin-like cytokine factor 1, DUSP dual specificity phosphatase, E2F1 E2F transcription factor 1, EGFR epidermal growth factor receptor, EMT epithelial-mesenchymal transition, ERβ estrogen receptor β, EZH2 enhancer of zeste homolog 2, GLTP glycolipid transfer protein, HIF-1α hypoxia inducible factor 1 subunit alpha, IGF insulin-like growth factor, JNK c-Jun N-terminal kinases, MAPK1 mitogen-activated protein kinase 1, MECP2 methyl-CPG binding protein 2, MET MET proto-oncogene, MLKL mixed lineage kinase domain like pseudokinase, NRF2 nuclear factor erythroid 2-related factor 2, p27kip1 cyclin-dependent kinase inhibitor 1b, PI3K phosphatidylinositol-4,5-bisphosphate 3-kinase, PINK1 PTEN induced kinase 1, PRKN Parkin, PTEN phosphatase and tensin homolog, PUMA P53 up-regulated modulator of apoptosis; RASSF1 ras association domain family member 1, SNAIL snail family transcriptional repressor, STAT signal transducer and activator of transcription, TWIST twist family BHLH transcription factor, YBX1 Y-Box binding protein1, YTHDF2 YTH N6-methyladenosine RNA binding protein 2, ZEB zinc finger e-box binding homeobox.
The relationship between miRNAs dysregulation and TKI resistance is continuously being revealed. miRNAs participate in various pathological processes and are potential markers for prognosis and systemic treatment response.6,380miRNAs are associated with EMT. ZEB1/2 are repressors of miR-200. miR-200 targets ZEB family members by establishing double negative regulatory feedback loops to confine ZEB expression. A similar scenario exists between TWIST and miR-186, SNAIL and miR-34a. On the contrary, three EMT-TF/miRNA groups form a self-enforcing regulatory network.247 SH3 domain-containing kinase binding protein 1 (SHKBP1) is a direct target of miR-499a that ZEB1 inhibits. SHKBP1 regulates EGFR activity. TGF-β upregulates ZEB1 in osteosarcoma, which leads to higher expression of SHKBP1 through miR-499a. SHKBP1 also increases EGFR activity, which occurs concomitantly with a TGF-β-induced EMT-associated kinase switch to an AKT-activated with EGFR-independent state. Meanwhile, AKT upregulates SHKBP1 expression, enhancing EGFR activity.381 In HCC, upregulation of miRNA-125-5p suppressed ataxin-1 expression and induced Snail-mediated EMT, enhancing cancer cell stemness and leading to sorafenib resistance.382 In addition, miR-424 could target chromo box 4 (CBX4) to induce YAP nuclear translocation, which enhances tumor stemness and sorafenib resistance.383 Additionally, tumor cells can release miRNAs from exosome to TME and convey resistance to other cells. For example, cancer cells-released miR-25-3p and miR-130b-3p can promote TAM-M2 polarization, enhance VEGF expression, and induce EMT, thus leading to multidrug resistance in colon cancer.384
miRNA could regulate cell death. miR-125d-5p, miR-494, and miR-30a-5p overexpression in HCC cell lines are associated with sorafenib resistance. Among them, miR-125d-5p inhibits c-Jun-mediated PUMA transcriptional induction. Low levels of PUMA increase mitochondrial membrane potential and decrease ROS levels, leading to apoptosis resistance.385 miR-494 inhibits PTEN expression, thus activating PI3K/Akt/mTOR pathway, and downregulates PUMA to resist apoptosis.386 miR-30a-5p downregulation leads to high CLCF1 expression, which activates GLUT3 and HK2 expression through the PI3K/AKT pathway to enhance glycolysis-induced drug resistance.387 Besides, miR-30c is highly expressed in NSCLC with MET mutations, and it directly targets the 3’-untranslated region of BIM to inhibit its transcription, leading to apoptosis resistance.388 Moreover, miR-335 regulates AXL expression, which leads to EGFR-TKI resistance in NSCLC.389 In breast cancer cell lines, lapatinib induces Src to activate the NF-κB pathway to upregulate miR-221. High miR-221 leads to death resistance by inhibiting cyclin-dependent kinase inhibitor 1B (p27kip1).390 Additionally, studies have shown that activation of the NRF2 pathway, which is related to ferroptosis, increased miR-196a expression. Glycolipid transfer protein (GLTP) was a direct target of miR-196a, whose downregulation led to NSCLC resistance to gefitinib.391 The detail of GLTP in TKI resistance hasn’t been clarified, but it is involved in several processes (autophagy, inflammation, cell death, etc.) and may be a drug target.392
Apart from miRNAs, other ncRNAs like lncRNAs and circRNAs are associated with TKI-related drug resistance.393 In sunitinib-treated RCC, lncRNA-ECVSR can enhance the stability of estrogen receptor beta (Erβ) mRNA to upregulate HIF-2α transcription, promote vascular mimicry, cancer stemness, and RCC progression.394 LncRNA-CASC9 suppresses DUSP1 expression by recruiting histone methyltransferase enhancer of zeste homolog 2 (EZH2). Decreased DUSP1 expression results in continuous RAS/MAPK activation and causes gefitinib resistance in NSCLC.395 Moreover, lncRNA-PCAT-1 may increase the phosphorylation of AKT and GSK3, resulting in sustained activation of PI3K/AKT and gefitinib resistance.396 LncRNA-HIF1A-AS2 induces IL-6 expression by binding to miR-146b-4p and activating the IL-6/STAT pathway. IL-6 overexpression results in LUAD resistance.397 High lncRNA-RP11616M22.7 are associated with imatinib resistance in GIST, which may lie in the regulation of the hippo pathway by Ras association domain family member 1 (RASSF1).398 As for circRNA, sorafenib induces circFN1 and circFOXM1 expression in HCC, thereby promoting E2F transcription factor 1 (E2F1) and methyl-CpG binding protein 2 (MECP2) expression through circFN1/miR-1205/E2F1 and circFOXM1/miR-1324/MECP2 pathways. These molecular interactions were shown to promote cell proliferation, metastasis, and apoptosis resistance.399,400 CircRNA hsa_circ_0005576 directly interacts with miR-512-5p in LUAD cell lines and upregulates miR-512-5p/IGF-1R pathway, leading to osimertinib resistance.401 In addition, circRNA-SORE binds to Y-box binding protein 1 (YBX1) to block the interaction between YBX1 and the pre-mRNA-processing factor 19 (PRP19) (an E3 ubiquitin ligase).402 Consequently, PRP19-mediated YBX degradation is inhibited, and high YBX is related to the apoptosis resistance.403 Moreover, circRNA-SORE has also been shown to convey sorafenib resistance to other HCC cells via exosomes.402 In addition to its posttranscriptional regulatory function, circRNA can directly encode proteins.404 The secreted E-calmodulin variant protein (C-E-Cad) encoded by circular E-cadherin (circ-E-Cad) RNA was found to bind to the EGFR CR2 structural domain to activate EGFR without EGF, provoking GBM resistance to EGFR therapy.405,406
By sharing common signaling molecules, different ncRNA regulatory axes form an extensive ncRNA regulatory network that regulates TKI resistance. Preliminary circRNA/lncRNA-miRNA–mRNA networks have been constructed to shed light on the role of ncRNAs in regulating signaling pathways and drug resistance.407
Given the complicated and delicate regulatory mechanisms of ncRNAs and their involvement in regulating multiple pathways, ncRNAs are promising therapeutic targets. Some ncRNA drugs have been used in clinical trials but not for treating TKI-resistant tumors. Preclinical studies have shown the therapeutic potential of ncRNAs. For example, increased miR-424 levels induced CBX4 depolymerization to reduce YAP1 nuclear translocation, and improved sorafenib resistance.383 MiR-210-5p antagomir in combination with sorafenib alleviated sorafenib resistance.347 In addition, paeonol inhibits proliferation and promotes apoptosis of apatinib-resistant gastric cancer cells via lncRNA-00665/miR-665/MAPK1 axis.408 CircKCNN2 upregulates methyl-CpG binding domain protein 2 (MBD2) via miR-520c-3p. miR-520c-3p mediates epigenetic transcriptional silencing of FGFR4, which increases the efficacy of lenvatinib in HCC.409 In addition, ncRNAs have a prognostic value. Studies have shown that elevated levels of circulating miR-125d-5p often predict a low response to sorafenib in HCC, and antagonizing miR-125d-5p may attenuate drug resistance.385 In short, modulating ncRNA expression by ncRNA antagonists, analogs, or by targeting ncRNA upstream regulators can help reverse drug resistance.
Other epigenetic modifications
Other types of epigenetic modifications such as DNA methylation, histone modification, RNA methylation, and chromatin remodeling are also involved in TKI resistance.
DNA methylation is one of the first epigenetic regulatory mechanisms affecting gene transcriptional activity. Studies have shown that compared with other NSCLC cell lines with the methylated EGFR promoter region, NSCLC cell lines with the unmethylated EGFR promoter region are more sensitive to gefitinib, indicating that EGFR promoter methylation is a potential mechanism of acquired resistance.410 Aberrant methylation of gamma-aminobutyric acid type B receptor subunit 2 (GABBR2) can be seen in erlotinib-treated NSCLC cell lines, which leads to its overexpression. High GABBR2 activates ERK phosphorylation, significantly preventing erlotinib-induced apoptosis.411 Moreover, in HCC, hypermethylation of the CPG island at the ADAMTS-like 5 (ADAMTSL5) locus results in its overexpression, but reduced expression and/or phosphorylation of several RTKs (MET, EGFR, PDGFRβ, IGF1Rβ, and FGFR4) and increased sensitivity to sorafenib, lenvatinib, and regorafenib were witnessed when low the ADAMTSL5 level,412 suggesting that ADAMTSL5 may contribute to TKI resistance through regulating several RTKs activity.
Histone modifications regulate gene transcription by altering chromatin structure. Abnormal histone modifications are associated with TKI resistance. Histone methylation mediated by histone methyltransferase (HMT) may contribute to TKI resistance. Set domain containing 2 (SETD2), an HMT responsible for trimethylation modification of lysine at position 20 of histone H3 (H3K20me3), leads to transcriptional repression. Low SETD2 promotes the MYCN (n-myc) transcription in CML cells. MYCN can promote proliferation, immortalization, stemness, and carcinogenesis and aggravate imatinib resistance.413 However, the effect of MYCN upregulation on CML resistance has not been fully elucidated. Furthermore, the EZH2 is an HMT responsible for trimethylation modification of lysine at position 27 of histone H3 (H3K27me3). In HCC, HOXB cluster antisense RNA 3 (HOXB-AS3) interacts with EZH2 to recruit EZH2 to the Dicer’s promoter to add H3K27me3 modification, leading to Dicer transcriptional repression, resulting in the acquisition of stem cell-like properties and sorafenib resistance.414 Dicer is an RNase III nucleic acid endonuclease in the cytoplasm that is essential for mRNA maturation. However, there are still gaps in the relationship between Dicer downregulation and TKI resistance. Additionally, deletion of lysine methyltransferase 5 C (KMT5C), an HMT responsible for histone H4 lysine 20 trimethylation (H4K20me3), upregulates lncRNA-LINC01510 and promotes MET transcription, leading to EGFR-TKI resistance in NSCLC through activation of the bypass pathway.415 SET and MYND domain containing 2 (SMYD2) are highly expressed in ccRCC, and associated with multidrug resistance, including sunitinib in RCC. Mechanistically, SMYD2 binds to the promoter of miR-125b, inhibiting the expression of Dickkopf WNT signaling pathway inhibitor 3 (DKK3) and promoting proliferation and migration.416
Notably, a vitro experiment revealed changes in epigenetic modifications of GB with ALK kinase mutations and MYCN amplification from drug sensitivity to drug resistance under intervention with an ALK inhibitor. MYCN expression was initially reduced due to H3K27me3 of its locus, and gradually, the promoter of the brother of the regulator of the imprinted site (BORIS) was hypermethylated and further promoted BORIS expression. BORIS could bind to chromatin loop anchors of the DNA regulatory region containing super-enhancers and promote the expression of genes in this region, resulting in full drug resistance.417,418 This study showed progressive epigenetic modifications in tumors from drug sensitivity to complete drug resistance, supporting the role of epigenetic modifications in reversible drug resistance.
Abnormal histone acetylation modifications also lead to TKI resistance. Studies have shown that high CD13 is associated with sorafenib resistance in HCC. Mechanistically, CD13 stabilizes histone deacetylase 5 (HDAC5), which is critical in regulating LSD1 protein stability through post-translational modification. LSD demethylates the NF-κB catalytic unit p65 to activate the NF-κB pathway in an HDAC5-dependent manner, resulting in cell survival.419 In addition, upregulated lysine demethylase 6 A (KDM6A) in CML can be recruited to the NTRK1 promoter by the transcription factor YY1. KDM6A enhances NTRK1 function and induces imatinib resistance by bypassing pathways.420
RNA modification is a vital process that regulates posttranscriptional gene expression. mRNA N6-methyladenosine (m6A) modification can affect gene transcription by influencing mRNA stability, and m6A methyltransferase (METTL) plays an important role in TKI resistance. METTL3 is overexpressed in NSCLC, and it positively regulates autophagy by strengthening the stability of autophagy-related mRNAs such as ATG5 and ATG7, resulting in gefitinib resistance.421 METTL3 also changes MET levels to modulate PI3K/AKT pathway, leading to LUAD resistance to gefitinib.422 In addition, METTL3 upregulates AXL expression and induces EMT in ovarian cancer.423 Similarly, METTL7B can increase the expression of GPX4, HMOX1, and SOD1 by m6A modification, leading to ferroptosis resistance and TKI resistance in LUAD (see Ferroptosis).424 Moreover, METTL-KIAA1429 can enhance homeobox A1 (HOXA1) mRNA stability and lead to gefitinib resistance in NSCLC through HOXA1 expression,425 but HOXA1 is considered an oncogene whose function is currently unknown. The m6A modification mediated by YTH N6-methyladenosine RNA binding protein 2 (YTHDF2) can increase circRNA-ASK1 degradation, thereby attenuating the inhibitory effect of the circRNA-ASK1-encoded product ASK1-272a.a on AKT pathway and increasing LUAD resistance to gefitinib.426 YTHDF also mediates m6A modification of lncRNA-TUSC7 and abolishes the inhibitory effect of miRNA-146 on the Notch pathway, whose activation is related to EMT and LUAD resistance to erlotinib.427
FTO alpha-ketoglutarate-dependent dioxygenase (FTO) is an m6A demethylase, and it can be induced to express by TKIs in leukemia. FTO increases the stability of cell survival- and proliferation-related mRNAs through m6A demethylation, allowing cells to rapidly upregulate the expression of anti-apoptotic molecules such as BCL-2 and proliferative genes such as MERTK. This enables cells to withstand the initial TKI attack. Moreover, anti-apoptotic gene expression can be maintained, developing a reversible drug-resistant phenotype. An FTO inhibitor, rehein, can restore TKI sensitivity.428 Moreover, FTO can enhance FLT3-ITD expression to promote acute promyelocytic leukemia (APL) cell survival and proliferation. FTO-mediated post-transcriptional repression of retinoic acid receptor alpha (RARA), ankyrin repeat and SOCS box containing 2 (ASB2) are involved in the upregulation of FLT3-ITD.429 As an m6A reader, insulin-like growth factor binding protein 2 (IGFBP2) enhances ERBB2 mRNA translation, induces HER2 expression, and leads to selumetinib resistance. Lapatinib targets both IGFBP2 and BRBB2 to reverse selumetinib resistance.430
RNA binding protein (RBP) is also a kind of RNA modification. RBP MUSASHI-2 (MSI2) binds to the mRNA of Nanog (Nanog homeobox), a core stem cell factor. High Nanog is associated with cancer stem cell-like properties and leads to death resistance, resulting in osimertinib resistance of LUAD, and drug sensitivity restored after MSI knockdown.431
Chromatin remodeling in GB may be associated with reversible chronic tumor resistance to TKI. Under the TKI therapy, some GB cells gradually transform into reversibly resistant cells with upregulation of histone demethylase (KDM), extensive redistribution of H3K27me3, and overactivation of the Notch pathway. KDM and H3K27me3 increase target gene expression. The notch pathway may be associated with stem cell properties acquirement.432 The link between these alterations and drug resistance needs further investigation.
So far, some epigenetic drugs have been approved for cancer treatment, and some preclinical studies showed the promising efficacy of combination therapy with TKIs and epigenetic drugs. Aberrant DNA hypermethylation is associated with lower TKI sensitivity, and imatinib combined with the DNA methyltransferase inhibitor (DNMTi) decitabine enhanced TKI-induced cell apoptosis and induced oncogene expression, exhibiting promising antitumor effects.433 Moreover, DI-1 enhanced the cabozantinib sensitivity in osteosarcoma by inhibiting DNMT-1.434 Intervention with histone modifications is also a potential option. EZH2 overexpression is associated with poor prognosis, providing a reason for EZH2 inhibition. In sorafenib-resistant TC cell lines, sorafenib sensitivity was restored when combined with EZH2 inhibitor.435 Furthermore, sorafenib has been shown to induce apoptosis in HCC cells by accelerating the EZH2 degradation, suggesting that EZH2 inhibitors in combination with TKI reverses drug resistance and potentially enhances the drug efficacy before resistance.436 2,4-pyrimidinediamine derivative 10 f is a dual ALK and HDAC inhibitor. It can treat ceritinib- or crizotinib-resistant NSCLC cell lines.437 Tazemetostat is the first FDA-approved EZH2 inhibitor recently,438 whose combination with TKIs deserves further study.
Histone deacetylase inhibitors (HDACi) are suitable candidates for combination therapy. HDACis combined with EGFR-TKIs effectively overcomes TKIs resistance.439–441 For example, the combination of vorinostat and osimertinib attenuates apoptosis resistance in NSCLC cell lines with BIM deletion polymorphisms.442 The combination of vorinostat and brigatinib has been effective in LUAD cell lines with third-generation EGFR-TKIs resistance mutations.443 Epigenetic regulatory interventions are diverse, but it is clear that epigenetic manipulation is an effective strategy to restore TKI sensitivity.444,445
Overall, Tumor cells can acquire drug resistance through epigenetic reprogramming. However, the presence of various epigenetic modification methods makes research challenging. More importantly, epigenetic modification does not change the gene sequence. In other words, epigenetic modification is reversible, so proper epigenetic intervention may be the key to reversing drug resistance (Table 4).
Table 4.
Preclinical studies of TKI in combination with other drugs to reverse drug resistance
| TKI | Cancer | Target | Anticancer therapies | Type | anti-resistance mechanism | Ref. |
|---|---|---|---|---|---|---|
| Reversing EMT | ||||||
| Erlotinib | NSCLC | TGF-βR | LY364947 | TGF-β inhibitor | Inhibiting TGF-β and reversing EMT | 268 |
| Selumetinib | NSCLC | AXL | bemcentinib | AXL TKI | Reducing TGF-β and blockade | 271 |
| EGFR-TKI | NSCLC | AuroraA | alisertib | AuroraA kinase inhibitor | reversing EMT | 272 |
| Osimertinib | NSCLC | AuroraB |
GSK1070916 ENMD-2076 PF-003814735 |
AuroraB kinase inhibitor | reversing EMT | 253 |
|
Crizotinib Alectinib Ceritinib |
NSCLC | AXL |
Ganetespib R428 |
HSP90α inhibitor AXL-TKI |
Inhibiting AXL and reversing EMT | 266 |
| Sorafenib | HCC | AXL | R428 | AXL-TKI | Inhibiting AXL and reversing EMT | 267 |
| Erlotinib | HNSCC | TrkA | siTrkA1, siTrkA3 | TrkA siRNA | Reversing EMT through blockading NGF/TrkA/STAT3 pathway | 170 |
| sunitinib | RCC | Plk1 |
ab19777 NB100-122 |
Plk1 antibody HIF-2α antibody |
Reversing EMT through blockading HIF-2α/ Plk1 | 257 |
| Metabolism intervention | ||||||
| regorafenib | HCC | HK1 | HK1 shRNA | HK1 shRNA | Intervening metabolism to reverse drug resistance | 277 |
| Imatinib | CML | PKM2 | PKM2 siRNA | PKM2 siRNA | Reducing glycolysis and PI3K / AKT pathway activity and inducing apoptosis | 279 |
| Gefitinib | NSCLC | lactate | NHI-Glc-2 | LDH inhibitor | Reducing the secretion of HGF of CAFs by reducing the stimulation of lactate | 275 |
|
Gefitinib Erlotinib |
NSCLC | OXPHOS complex I | Phenformin | OXPHOS inhibitor | Inhibiting proliferation by inhibiting mitochondrial OXPHOS | 284 |
| Gefitinib | NSCLC | SREBP | Anti-SREBP1 | SREBP1antibody | Intervening lipid metabolism to induce ROS production and induce apoptosis | 289 |
| Gefitinib | NSCLC | PPARα | fenofibrate | lipid-lowering drug | Intervening cholesterol metabolism and induces apoptosis | 291 |
| Blocking aberrant activation of downstream pathways | ||||||
| Imatinib | CML | mTOR | everolimus | mTOR inhibitor | Inducing apoptosis and cell cycle arrest by blockading mTOR | 106 |
| Sunitinib | RCC | mTOR | Rapalink-1 | mTOR inhibitor | Inducing apoptosis by blockading mTOR, MAPK signaling pathway | 109 |
|
Gefitinib Osimertinib |
NSCLC | FGFR, AKT |
GSK2141795 |
FGFR TKI AKT inhibitor |
Blockading PI3K/AKT signaling pathway through dual inhibition of FGFR and AKT | 110 |
| Sorafenib | HCC | MET, MET |
MK2206 capmatinib |
AKT inhibitor MET TKI |
Blocking bypass activation of downstream signaling pathway | 111 |
| BCR-ABL TKI | AML | RAF | asciminib | RAF inhibitor | Blockading RAS/MAPK signaling pathway through inhibition of RAF | 113 |
| Regorafenib | gastric cancerr | MEK |
Erdafitinib Trametinib |
FGFR TKI MEK inhibitor |
Blockading RAS/MAPK signaling pathway through dual inhibition of FGFR and MEK | 116 |
| erlotinib | ATC | MEK | Trametinib | MEK inhibitor | Blockading RAS/MAPK signaling pathway through dual inhibition of EGFR and MEK | 117 |
| entrectinib | PDAC | MEK | cobimetinib | MEK inhibitor | Blockading RAS/MAPK signaling pathway through dual inhibition of NTRK1 and MEK | 118 |
| Dasatinib | EC | MEK | Trametinib | MEK inhibitor | Blockading RAS/MAPK signaling pathway through dual inhibition of EphA2 and MEK | 119 |
| Dasatinib | NB | ERK | ulixertinib | ERK inhibitor | Blockading MEK/ERK signaling pathway which activated by EPO/NGF through inhibition of ERK | 114 |
| Lenvatinib | ATC | PAK | KPT-9274 | PAK inhibitor | Blockading RAS signaling pathway and promote apoptosis | 122 |
| EGFR-TKI | NSCLC | MEK, PI3K |
MEK162 BKM120 |
MEK inhibitor PI3K inhibitor |
Dual inhibition of PI3K/AKT and RAS/MAPK pathways | 123 |
| Lapatinib | GIST | MEK, PI3K |
Refametinib copanlisib |
MEK inhibitor PI3K inhibitor |
Dual inhibition of PI3K/AKT and RAS/MAPK pathways | 124 |
| ALK-TKI | ALCL | SHP3 | SHP3 inhibitor | SHP3 inhibitor | Inhibiting the RAS pathway overactivation | 136 |
| ALK-TKI | NB | PIM | AZD1208 | PIM inhibitor | Blockading JAK/STAT3 signaling pathway | 171 |
| Gefitinib | GB | IGFIRβ | Anti-IGFIRβ | IGFIRβ antibody | Enhancing the inhibition of AKT and MEK/ERK pathway, inducing apoptosis | 183 |
| Lenvatinib | HCC | EGFR | Gefitinib | EGFR-TKI | Blocking the bypass activation of ERK pathway by inhibiting the EGFR | 186 |
| gefitinib | FAK | defactinib | FAK-TKI | Enhancing the inhibition of AKT and MEK/ERK pathway, inducing apoptosis | ||
| regorafenib | CRC | IGFR | aspirin | IGF inhibitor | Enhancing the inhibition of AKT and MEK/ERK pathway, inducing apoptosis | 189 |
| Osimertinib | NSCLC | MET | berberine | MET inhibitor | Blockading the bypass activation caused by MET-amplification | 190 |
| imatinib | GIST | HSP90 | TAS-116 | HSP90 inhibitor | Blockading the bypass activation caused by aberrant activation of KIT overexpression | 191 |
| Lapatinib | Brest cancer | HSP90 | 17-DMAG | HSP90 inhibitor | Not clear, inhibiting the cell proliferation and protein expression | 192 |
| Alectinib | NSCLC | GAB | Phenformin | GAB inhibitor | Blockading the bypass activation caused by aberrant activation of HGF/MET | 193 |
| Induction of cell death | ||||||
| EGFR-TKI | NSCLC |
BIM BCL-2 |
Vorinostat Metformin |
Bim inducer Bcl-2 inhibitor |
Inducing apoptosis | 308 |
| alectinib | NSCLC | NOXA | ixazomib | proteasome inhibitor | Inducing apoptosis by increasing the expression of NOXA | 310 |
| regofenib | CRC | MCL-1 | S63845 | MCL-1 inhibitor | Inducing apoptosis by decreasing the expression of MCL-1 | 311 |
| Sorafenib | HCC | Mitochondria | piperlongumine | – | Inducing ROS generation, mitochondrial dysfunction and cell cycle arrest, activating the AMPK signaling pathway. | 313 |
| crizotinib | LUAD | CDK |
THZ1 Alvocidib dinaciclib |
CDK7/12 inhibitor CDK9 inhibitor |
Inducing apoptosis by cell cycle arrest | 314 |
| lapatinib | Breast cancer | CDK | THZ1 | CDK7 inhibitor | Inducing apoptosis by cell cycle arrest | 315 |
| lapatinib | Breast cancer | CDK | dinaciclib | CDK12 inhibitor | Enhancing inhibition of PI3K/AKT pathway and inducing apoptosis | 316 |
| Gefitinib | NSCLC | GPX4 | fluvastatin | GPX4 inhibitor | Inducing ferroptosis | 331 |
| lapatinib | Breast cancer |
Transferrin ferroportin-1 |
Siramesine | lysosome disrupting agent | Increasing the intake of Fe and inducing ferroptosis | 335 |
| sorafenib | HCC |
Mitochondria ML385 |
DSF/Cu |
Mitochondrial functional interference agents NRF inhibitor |
Increasing ROS generation, mitochondrial dysfunction and inhibiting antioxidant effect of NRF pathway to induce ferroptosis | 336 |
| Gefitinib | NSCLC | Muti-target | CTS | – | Decreasing the expression of CAT, HMOX1 and SCD to enhance vulnerability to oxidative stress | 294 |
| Sorafenib | HCC | – | amiloride | Micropinocytosis inhibitor | Inhibiting micropinocytosis to reducing the intracellular cysteine intake and inducing ferroptosis | 330 |
| afatinib | LUAD | – | CQ | Autophagy inhibitor | Inhibiting autophagy to induce cell death | 341 |
| gefitinib | Pancreatic cancer | – | azithromycin | Autophagy inhibitor | Inhibiting autophagy to induce cell death | 348 |
| imatinib | CML | cannabidiol | Autophagy inducer | Inducing autophagy to induce cell death | 354 | |
| quizartinib | CML | – | bortezomib | proteasome inhibitor | Inducing autophagy to enhance degradation of FLT3-ITD | 355 |
| sorafenib | HCC | HSP90α | 17-AAG | HSP90α inhibitor | Inducing necroptosis | |
| Sunitinib | RCC | – | Shikonin | -– | Inducing necroptosis and inhibiting mTOR | 367 |
| Erlotinib | NSCLC | APE | NO.0449-0145 | APE inhibitor | Inducing necroptosis, apoptosis, pyroptosis | 368 |
| Intervention of epigenetic modifications | ||||||
| Imatinib | CML | DNMT | decitabine | DNMTis | Inducing apoptosis and expression of tumor suppressor gene | 433 |
| cabozatinib | OS | DNMT-1 | DI-1 | DNMTis | Enhancing sensitivity to TKI | 434 |
| Sorafenib | TC | EZH2 | EPZ-6438 | EZH2 inhibitor | decreasing H3K27me3 and increasing H3K27ac levels | 435 |
| Osimertinib | NSCLC | HDAC | SAHA | HDACi | Inhibiting histone deacetylase and autophagy | 439 |
| Gefitinib | NSCLC | HDAC | GCJ-490A | HDACi | Increasing histone acetylation at the IKKα promoter and enhancing IKKα transcription, through which MET level decreased | 440 |
| Osimertinib | LUAD | HDAC3 | vorinostat | HDACi | Increasing BIM expression and inducing apoptosis | 443 |
| EGFR-TKI | LUAD |
HDAC ALK |
Vorinostat brigatinib |
HDACi ALK-TKI |
Inhibiting PI3K/AKT pathway and inducing apoptosis | 443 |
17-AAG 17-demethoxygeldanamycin, AKT AKT serine/threonine kinase, ALCL anaplastic large cell lymphoma, ALK anaplastic lymphoma receptor tyrosine kinase, APE1 apurinic/apyrimidinic endonuclease 1, ATC anaplastic thyroid carcinoma, AXL AXL receptor tyrosine kinase, BIM bcl-2 interacting mediator of cell death, CAF cancer-associated fibroblasts, CAT catalase, CDK cyclin-dependent kinase, CML chronic myelocytic leukemia, CQ chloroquine, CRC colorectal cancer, DNMTi DNA methyltransferase inhibitors, EC endometrial cancer, EMT epithelial-mesenchymal transition, EPO erythropoietin, EZH2 enhancer of zeste homolog 2, FAK focal adhesion kinas, FGFR fibroblast growth factor receptor, FGFR fibroblast growth factor receptor, GAB1 GEB2 associated binding protein 1, GBM glioblastoma, GPX4 glutathione peroxidase 4, H3K27me3 trimethylation of lys-27 in histone 3, HCC hepatocellular carcinoma, HDACi histone deacetylase inhibitors, HGF hepatocyte growth factor, HK1 hexokinase 1, HMOX1 heme oxygenase1, HSP70 heat shock protein 70, IGFR insulin-like gOriginal rowth factor receptor, KIT KIT proto-oncogene; LDH lactate dehydrogenase, LUAD lung adenocarcinoma, MCL-1 mcl1 apoptosis regulator, MEK MAP kinase kinase, MET MET proto-oncogene, mTOR mechanistic target of rapamycin kinase, NB neuroblastoma, NGF nerve growth factor, NOXA NADPH oxidase activator, NSCLC non-small cell lung cancer, NTRK:neurotrophic receptor tyrosine kinase 1, OS osteosarcoma, OXPHOS mitochondrial oxidative phosphorylation system, PAK1 P21 (RAC1) activated kinase 1,PDAC pancreatic ductal adenocarcinoma, PIM Pim-1 proto-oncogene, PKM2 pyruvate kinase m2, Plk1 polo-like kinase 1, PPARα peroxisome proliferator activated receptor alpha, RCC renal cell carcinoma, ROS reactive oxygen species, SCD1 stearoyl-coA desaturase, SREBP1 sterol regulatory element binding protein 1, TC thyroid cancer, TGF-β transforming growth factor beta, TrkA tropomyosin receptor kinase A.
Abnormal metabolism of TKIs
Drug delivery
Absorption, distribution, metabolism, and excretion (ADME) of TKIs require trans-membrane transport through transporter proteins, and transporter proteins can also exacerbate drug resistance by impeding trans-membrane transport of drugs. Drug transporters located in enterocytes, hepatocytes, renal proximal tubular cells, and physiological barriers such as the blood-brain barrier are directly involved in drug absorption, distribution, and elimination. They also regulate the intracellular concentration of metabolic enzymes, thereby indirectly affecting drug metabolism.8 The altered activity of transporter proteins can decrease the absorption of TKIs or increase their excretion. Thus, TKI concentrations in circulation and targeted regions are inadequate to inhibit abnormal PTKs completely. Furthermore, drug transporters are essential for TKI uptake or excretion by cancer cells. Reduced influx or increased efflux of TKIs mediated by drug transporters in cancer cell membranes can lead to drug resistance. Various mechanisms, such as genetic variations, epigenetic factors, and transporter-mediated drug interactions, can affect the expression and function of drug transporters in cancer cells, resulting in different clinical outcomes.8
Theoretically, reducing the activity or expression of transporter proteins can decrease intracellular drug accumulation.443 Several studies have shown that the solute carrier (SLC) transporter proteins are responsible for TKI uptake by cancer cells,8 and weak clinical response of CML to imatinib was associated with reduced SLC22A1 expression and activity.446 However, most TKIs cannot be considered the substrates of SLC, because TKIs concentration decreased in most cells transfected with SLC.8 Therefore, further studies are needed to clarify the cellular mechanisms of TKI uptake and to measure the effect of transporter proteins on TKI resistance.
Compared with drug influx, drug efflux is considered a more prevalent sector of drug resistance. Increased drug efflux can reduce its intracellular concentrations below the therapeutic levels, resulting in drug resistance. The efflux of TKIs mainly relies on the ATP binding cassette (ABC) family proteins, such as ATP binding cassette subfamily B member 1 (ABCB1, p-gp) and breast cancer resistance protein (BCRP).447 Single nucleotide polymorphisms (SNPs) of ABC may affect TKI efflux. For instance, 1199 G > A SNP in ABCB1 increased imatinib, nilotinib, and dasatinib efflux compared with the wild genotype.448 However, 421 G > A SNP in ABC subfamily G member 2 (ABCG2) reduced the affinity of TKIs to ABCG2 and prevented drug resistance compared with wild genotype.449 It may be because SNP in the ATPase structural domain of ABCG2 can affect ABCG activity to reduce drug efflux.450 In addition, increased expression or enhanced function of p-gp can also facilitate drug efflux and drug resistance.441,442 Gene rearrangements can lead to the insertion of a more active promoter in MDR-1 (p-gp) mRNA,451,452 which can still translate biologically active abnormal p-gp to increase drug efflux.453 It may be a reason for ceritinib resistance in NSCLC cell lines with ALK rearrangement.454 Epigenetic modifications also regulate ABC expression. MiR-212 is downregulated in imatinib-treated CML, which could upregulate ABCG2 and lead to resistance.455 Many factors affect the function of drug transporters, but the relationship between some of them and TKI resistance needs further research. Interestingly, research in sunitinib-resistant metastatic RCC cell lines indicated a novel way of TKIs efflux. Sunitinib promotes TFE3 (transcription factor binding to IGHM enhancer 3) translocation into the nucleus, increasing the expression of extended-synaptotagmin-1 (ESYT1) that is an endoplasmic reticulum protein. ESTY1 formed a dimer with synaptotagmin-7 (SYT7) on the lysosome and induced endoplasmic reticulum breakage, Ca2+ release, and lysosomal exocytosis. Lysosomal exocytosis can increase sunitinib efflux and simultaneously release histone B out of the cell to degrade the ECM and facilitate tumor metastasis, and Ca2+ induces lysosomal biogenesis and forms a positive feedback.456
Pregnane X receptor (PXR), a transcriptional activator, can sense and remove foreign substances by regulating the expression of drug-metabolizing enzymes and drug transporters.457 In prostate cancer, PXR leads to dasatinib resistance but increases erlotinib, dabrafenib, and afatinib sensitivity. PXR-induced upregulation of cytochrome p450 family three subfamilies A member 4 (CYP3A4) may lead to dasatinib resistance. CYP3A4 metabolizes dasatinib to its inactive form, and PXR increases dasatinib efflux. In contrast, afatinib sensitivity may be because of PXR-induced upregulation of SLC16A1, which increases the intracellular concentration of afatinib. PXR also increases the active form of afatinib in a CYP-dependent manner.458 It supports the concept that drugs can enter tumor cells differently, and the pharmacokinetic parameters of different TKIs can affect their clinical efficacy.
Despite the role of ABC inhibition in reversing drug resistance, ABC inhibitors almost failed in clinical trials because of their serious adverse effects and drug interactions.459 ABC transporters are not specifically expressed on tumor cells, but they are also widely distributed in other cells such as hepatic and intestinal cells, which are related to drug uptake and efflux. TKI delivery by nanocarriers may improve the situation. Nanocarriers enter the cells by endocytosis, which simultaneously maintains targeting and attenuates the influence of transporter proteins.460 In addition, some TKIs rely on ABC to out the cell but inhibit ABC at the same time. Studies have shown that nilotinib is a potent inhibitor of P-gp and BCRP, and concurrent administration of nilotinib with afatinib in animal models reduced the clearance of afatinib by 65.3%.461 The possible reason lies in the homology of the ATP binding sites of ABC and TKI-targeted PTKs, implicating that combinations or higher doses of TKIs may be effective.462 However, most of these studies were conducted in vitro and could not measure the impact of ABCs on drug uptake and efflux in different organs. Therefore, in vivo studies are needed to clarify the relationship between TKI and ABC.
Lysosomal sequestration
Lysosomes are acidic digestive vesicles composed of lipoprotein membranes and over 50 hydrolytic enzymes. Hydrophobic, weakly basic TKIs can enter the lysosomes by pH gradient-driven free diffusion or ABC transporters. Thereafter, protonated TKIs lose their ability to penetrate the membrane, being isolated in vesicles far from their target, named lysosomal sequestration. Several TKIs such as sunitinib, erlotinib, and sorafenib undergo lysosomal sequestration.463 In addition, protonated TKIs in the lysosomes can prevent the mTOR complex (mTORc)-mediated inhibition of transcription factor EB (TFEB). TFEB is responsible for lysosomal biogenesis. It can also increase lysosomal membrane permeability, leading to calcium expelling to activate calcineurin (CaN). Activated CaN dephosphorylates TFEB.464 Dephosphorylated TFEB enters the nucleus and stimulates lysosomal biogenesis, sequesters more TKIs, and exacerbates drug resistance. But such resistance is reversible. Drug-free cultures of sunitinib-resistant tumor cells can normalize the lysosomal capacity and restore drug sensitivity upon redosing.463
Inhibition of lysosomal sequestration is an effective strategy to attenuate drug resistance. The combination of nintedanib and inhibitors of lysosomal acidification, such as bafilomycin A1 or chloroquine (CQ), significantly inhibited tumor growth in nintedanib-resistant tumors in mice.465 Bafilomycin A1 is an inhibitor of H+-ATPase in the lysosomal membrane and attenuates the acidic condition of the lysosome. Although bafilomycin A1 cannot be used for clinical purposes due to its toxicity, hydroxychloroquine (HCQ) and its derivatives have shown promising results in clinical trials.349 The mechanism responsible for HCQ function is unclear, but it is assumed that HCQ prevents lysosomal accumulation of the drug.466 Moreover, drugs targeting other components of the lysosome, such as V-ATPase, acid sphingomyelinase, cathepsin, and HSP70, markedly impair lysosomal degradation.466 Future studies are needed to uncover the effects of these drugs on TKI resistance. Moreover, it has been shown that different TKIs accumulate in different cellular components,467 and lysosomal accumulation seems to be related to the TKI’s base dissociation constant (pkb). The lysosomal accumulation of the drug increases with pkb.462 Therefore, assessing the physicochemical properties of TKI and modifying it may prevent lysosomal sequestration of the drug (Fig. 6).462
Fig. 6. Abnormal TKI drug metabolism.
After entering the cell, TKI needs to compete with ATP to bind to the ATP site of abnormal PTK to block the downstream pathway. When the TKI binding site is mutated, it will result in the inability of the TKI to bind and drug resistance will develop. In addition, after TKI enters the cell, the weakly basic TKI will be driven into the acidic lysosomes and protonated and lose the ability to cross the membrane, which leads to a decrease in intracellular TKI on the one hand and stimulates lysosome production on the other, forming a vicious circle. In addition, tumor cells can also promote TKI efflux from cells by high expression of drug efflux proteins, such as ABC, further reducing TKI concentration
Drug-drug interaction (DDI)
Generally, oncology patients require several drugs to achieve a better outcome. Drugs compete with each other for protein binding, and it’s where DDI limits or enhances drug efficacy. DDI can be divided into PK-DDI and pharmacodynamics (PD) DDI.468 PK-DDI involves the entire process of drug ADME. PD-DDI is associated with synergistic, antagonistic, or additive reactions between drugs.469 DDI-mediated reduction in drug efficacy is multilayered, and some patients may not achieve optimal efficacy as a result.470
TKIs are mostly administered orally, and their absorption is inevitably affected by gastric acid PH, drug transporters, and intestinal enzymes.469 Suffering from gastrointestinal adverse effects such as gastroesophageal reflux, many patients take acid-reducing agents (ARAs) during cancer treatment. But ARA-mediated gastric acid pH increase can reduce the solubility of the weakly basic TKI and affect drug absorption and efficacy.471 An early study examined the effect of the omeprazole, a proton pump inhibitor (PPI), on the PK of 100 mg dasatinib in 14 healthy subjects, in which dasatinib was administered four days after the omeprazole application. A 40% decrease in the bioavailability of dasatinib was reported, and the area under the curve from zero to infinity (AUCinf) and peak concentration (Cmax) were reduced by 43% and 42%, respectively.472 Similarly, another clinical trial showed that erlotinib administered concomitantly with PPIs increased the risk of death in lung cancer patients by 21% compared with TKI alone, and discontinuation of TKI did not affect the reduced survival in this group of patients.470,473 That was to say, in the presence of PPI, the survival rate of lung cancer patients did not change with and without TKI use. PPI use and decreased TKI absorption may be the reason behind TKIs ineffectiveness. Moreover, the PK of erlotinib is significantly lower when administered concomitantly with PPIs and histamine H2-receptor antagonists. Therefore, concomitant use of TKIs and ARAs is not recommended in clinical.472 Unfortunately, there is no systematic recommendation for PPI usage during TKIs therapy, and there is no convincing prospective study measuring the interaction between PPIs and TKIs.473 The impact of ARAs on the efficacy of TKIs may be underestimated. Therefore, future studies focusing on the interaction between TKIs and ARAs are essential to improve the efficacy of both TKIs and ARAs.
Drug metabolizing enzymes and drug transporters are also involved in DDIs. CYP450 is the main enzyme that metabolizes TKIs. The plasma concentrations of TKIs decrease when TKIs are concomitantly administered with hepatic enzyme inducers such as rifampin, phenytoin sodium, and carbamazepine. For instance, rifampin reduces the total area under the curve (AUC) and Cmax of bosutinib by approximately 90%.474 In addition, co-administration of rifampin with nintedanib reduced the AUC of nintedanib by 50% and the Cmax by 60%, but the nintedanib degradation was lightly affected by CYP, and the phenomenon attributed to rifampicin-mediated elevated p-gp expression.475
In conclusion, DDI is a double-edged sword. It can enhance TKIs efficacy on the one hand, but it may reduce efficacy and enhance side effects sometimes, which lead to patients’ low compliance and treatment failure. Therefore, it is crucial to understand the pharmacokinetics of each TKI and rationally prescribe the drug in combination with other drugs.
Outlook
TKIs are now widely used for several types of cancer. Meanwhile, TKI resistance is a major challenge in treating cancer and markedly reduces patients’ compliance, survival, and quality of life. Therefore, exploring the mechanisms behind TKI resistance is essential. Therefore, we summarize the mechanism and development of tumor with TKI resistance in this review. First, the main reason is the abnormal activation of PTK-related signaling pathways involved in gene mutations. In addition to mutations, TME is deeply involved in tumor development and TKI resistance. TME is pivotal for tumor evolution, contributes to EMT and metabolic reprogramming, and plays an important role in TKI resistance. Cell death resistance, tumor metabolism, and immune reprogramming are involved in TKI resistance and can be novel targets for drug development.476 Epigenetic modification is a reversible mechanism of TKI resistance that can also be a therapeutic target. Several RTKs, such as AXL and NRTKs, are primed to play an essential role in TKI resistance and are promising targets for reversing drug resistance.477 However, future studies are needed to provide a deeper insight into these mechanisms and their crosstalk. Besides, Appropriate therapeutic strategies can improve drug resistance. Advanced knowledge about DDI and TKIs pharmacokinetics is necessary to achieve optimal treatment efficacy. Combined therapy can also attenuate several resistance mechanisms. Herein, clinical trials using TKIs combined with other drugs or using two TKIs are ongoing. The use of TKIs with concomitant chemotherapy and immunotherapy is also of interest. However, DDI and adverse effects caused by multidrug combinations must be considered. Understanding the molecular mechanisms of the disease and relevant pharmacokinetic information is pivotal for choosing the best therapeutic approach.
Due to the heterogeneity of TKI resistance mechanisms in different types of tumors and each patient, a single therapeutic strategy cannot overcome drug resistance in all patients.199 Because of the individualized and dynamic nature of TKI resistance mechanisms in tumor cells and the existence of several resistance mechanisms in a single tumor cell, TKI resistance cannot be easily reversed. Precision medicine for individualized biological profiles may be helpful in the coming years. Recently, the breakthrough in biotechnology, liquid biopsies, and next-generation sequencing technologies improved the detection of TKI resistance mutations.478 Tumor and cell-free DNA analysis, immunolabeling, proteomics, metabolomics, and RNA analysis can identify the biological characteristics of TKI resistant tumors.479 They improve the identification of TKI resistance mechanisms and optimize the therapeutic approach. Based on new mechanisms of TKI resistance, better detection methods and treatment modalities are under consideration. Hence, a deeper insight into the mechanisms of TKI resistance is crucial.
Acknowledgements
Figures were created with Adobe illustrator 26.0.3(64-bit). No funding was secured specifically for this article.
Author contributions
Y.Y. and Q.L. conceptualized the idea, S.L. and Y.W. researched data and collected the references, Y.Y. and S.L. wrote the manuscript, S.L. and Y.Z. made the diagrams. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yang Yang, Shuo Li, Yujiao Wang
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao, Q. et al. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer. 17, 36 (2018). [DOI] [PMC free article] [PubMed]
- 4.Serrano C, George S. Gastrointestinal stromal tumor: challenges and opportunities for a new decade. Clin. Cancer Res. 2020;26:5078–5085. doi: 10.1158/1078-0432.CCR-20-1706. [DOI] [PubMed] [Google Scholar]
- 5.Ngo MT, et al. The role of IGF/IGF-1R signaling in hepatocellular carcinomas: stemness-related properties and drug resistance. Int. J. Mol. Sci. 2021;22:1931. doi: 10.3390/ijms22041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonetti A, et al. MicroRNAs as a drug resistance mechanism to targeted therapies in EGFR-mutated NSCLC: Current implications and future directions. Drug Resist. Updat. 2019;42:1–11. doi: 10.1016/j.drup.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Ng KP, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat. Med. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 8.Neul C, et al. Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol. Sci. 2016;37:904–932. doi: 10.1016/j.tips.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Solouki S, August A, Huang W. Non-receptor tyrosine kinase signaling in autoimmunity and therapeutic implications. Pharmacol. Ther. 2019;201:39–50. doi: 10.1016/j.pharmthera.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: characterisation, mechanism of action and therapeutic interests for bone cancers. J. Bone Oncol. 2015;4:1–12. doi: 10.1016/j.jbo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler, D. L. & Yarden, Y. Receptor Tyrosine Kinases: Family and Subfamilies (Springer International Publishing, Cham, 2015).
- 12.Siveen KS, et al. Role of non receptor tyrosine kinases in hematological malignances and its targeting by natural products. Mol. Cancer. 2018;17:31. doi: 10.1186/s12943-018-0788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer. 2018;17:58. doi: 10.1186/s12943-018-0782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouwer Sde, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification. Clin. Cancer Res. 2010;16:4353–4362. doi: 10.1158/1078-0432.CCR-09-2660. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan A, Ahsan A. Mechanisms of resistance to EGFR tyrosine kinase inhibitors and therapeutic approaches: an update. Adv. Exp. Med. Biol. 2016;893:137–153. doi: 10.1007/978-3-319-24223-1_7. [DOI] [PubMed] [Google Scholar]
- 16.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Antona C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr. Relat. Cancer. 2010;17:7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 18.Drilon A, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann. Oncol. 2016;27:920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 20.Paul MK, Mukhopadhyay AK. Tyrosine kinase - Role and significance in Cancer. Int. J. Med. Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay LM, et al. Genomic profiling of 114,200 advanced cancers identifies recurrent kinase domain duplications (KDD) and oncogenic rearrangements (RE) across diverse tumor types. Ann. Oncol. 2017;28:v595. doi: 10.1093/annonc/mdx391. [DOI] [Google Scholar]
- 22.Bhullar KS, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Cancer. 2018;17:48. doi: 10.1186/s12943-018-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roskoski R. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol. Res. 2016;103:26–48. doi: 10.1016/j.phrs.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Sutanto F, Konstantinidou M, Dömling A. Covalent inhibitors: a rational approach to drug discovery. Rsc. Med. Chem. 2020;11:876–884. doi: 10.1039/D0MD00154F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks ED. Asciminib: First Approval. Drugs. 2022;82:219–226. doi: 10.1007/s40265-021-01662-3. [DOI] [PubMed] [Google Scholar]
- 26.Bellora F, et al. Imatinib and nilotinib off-target effects on human NK Cells, monocytes, and M2 macrophages. J. Immunol. 2017;199:1516–1525. doi: 10.4049/jimmunol.1601695. [DOI] [PubMed] [Google Scholar]
- 27.Leonard JT, et al. Concomitant use of a dual Src/ABL kinase inhibitor eliminates the in vitro efficacy of blinatumomab against Ph+ ALL. Blood. 2021;137:939–944. doi: 10.1182/blood.2020005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derosa L, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 2020;78:195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Tang CP, et al. GCN2 kinase activation by ATP-competitive kinase inhibitors. Nat. Chem. Biol. 2022;18:207–215. doi: 10.1038/s41589-021-00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 31.Abdelgalil AA, Alkahtani HM. & Al-Jenoobi, F. I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 2019;44:239–266. doi: 10.1016/bs.podrm.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Cohen P, Cross D, Janne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat. Rev. Drug Discov. 2021;20:551–569. doi: 10.1038/s41573-021-00195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 34.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He, J., Huang, Z., Han, L., Gong, Y. & Xie, C. Mechanisms and management of 3rdgeneration EGFRTKI resistance in advanced nonsmall cell lung cancer (Review). Int. J. Oncol. 59, 90 (2021). [DOI] [PMC free article] [PubMed]
- 36.Tilio M, et al. Irreversible inhibition of Δ16HER2 is necessary to suppress Δ16HER2-positive breast carcinomas resistant to Lapatinib. Cancer Lett. 2016;381:76–84. doi: 10.1016/j.canlet.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 37.Gramza AW, Corless CL, Heinrich MC. Resistance to Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2009;15:7510–7518. doi: 10.1158/1078-0432.CCR-09-0190. [DOI] [PubMed] [Google Scholar]
- 38.Sharma GG, et al. A Compound L1196M/G1202R ALK Mutation in a Patient with ALK-Positive Lung Cancer with Acquired Resistance to Brigatinib Also Confers Primary Resistance to Lorlatinib. J. Thorac. Oncol. 2019;14:e257–e259. doi: 10.1016/j.jtho.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, et al. PDGFRB mutation and tyrosine kinase inhibitor resistance in Ph-like acute lymphoblastic leukemia. Blood. 2018;131:2256–2261. doi: 10.1182/blood-2017-11-817510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondal J, Tiwary P, Berne BJ. How a kinase inhibitor withstands gatekeeper residue mutations. J. Am. Chem. Soc. 2016;138:4608–4615. doi: 10.1021/jacs.6b01232. [DOI] [PubMed] [Google Scholar]
- 41.Mullard A. Cancer drug developers counteract kinase gatekeeper mutations. Nat. Rev. Drug Discov. 2015;14:667–668. doi: 10.1038/nrd4742. [DOI] [PubMed] [Google Scholar]
- 42.Yun C-H, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc. Natl Acad. Sci. U.S.A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018;29:i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serrano C, et al. KRAS and KIT Gatekeeper Mutations Confer Polyclonal Primary Imatinib Resistance in GI Stromal Tumors: Relevance of Concomitant Phosphatidylinositol 3-Kinase/AKT Dysregulation. J. Clin. Oncol. 2015;33:e93–6. doi: 10.1200/JCO.2013.48.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subbiah V, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terzyan SS, et al. Structural basis of resistance of mutant RET protein-tyrosine kinase to its inhibitors nintedanib and vandetanib. J. Biol. Chem. 2019;294:10428–10437. doi: 10.1074/jbc.RA119.007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon BJ, et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J. Thorac. Oncol. 2020;15:541–549. doi: 10.1016/j.jtho.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen T, et al. The L730V/I RET roof mutations display different activities toward pralsetinib and selpercatinib. NPJ Precis. Oncol. 2021;5:48. doi: 10.1038/s41698-021-00188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ou S-HI, et al. Emergence of novel and dominant acquired EGFR solvent-front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M) NSCLC patient who progressed on osimertinib. Lung cancer. 2017;108:228–231. doi: 10.1016/j.lungcan.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Drilon A, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 51.Facchinetti F, et al. Facts and New Hopes on Selective FGFR Inhibitors in Solid Tumors. Clin. Cancer Res. 2020;26:764–774. doi: 10.1158/1078-0432.CCR-19-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi Y, et al. Characterization of EGFR T790M, L792F, and C797S Mutations as Mechanisms of Acquired Resistance to Afatinib in Lung Cancer. Mol. Cancer Ther. 2017;16:357–364. doi: 10.1158/1535-7163.MCT-16-0407. [DOI] [PubMed] [Google Scholar]
- 53.Yanagitani N, et al. Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci. 2020;111:932–939. doi: 10.1111/cas.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang J, et al. Clinicopathological features and resistance mechanisms in HIP1-ALK-rearranged lung cancer: A multicenter study. GENE CHROMOSOME CANC. 2022;61:177–186. doi: 10.1002/gcc.23005. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y, et al. A novel ROS1 G2032 K missense mutation mediates lorlatinib resistance in a patient with ROS1-rearranged lung adenocarcinoma but responds to nab-paclitaxel plus pembrolizumab. Lung Cancer. 2020;143:55–59. doi: 10.1016/j.lungcan.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Lin JJ, et al. Spectrum of Mechanisms of Resistance to Crizotinib and Lorlatinib in ROS1 Fusion-Positive Lung Cancer. Clin. Cancer Res. 2021;27:2899–2909. doi: 10.1158/1078-0432.CCR-21-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, et al. Disease progression patterns and molecular resistance mechanisms to crizotinib of lung adenocarcinoma harboring ROS1 rearrangements. NPJ Precis. Oncol. 2022;6:20. doi: 10.1038/s41698-022-00264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keddy C, et al. Resistance Profile and Structural Modeling of Next-Generation ROS1 Tyrosine Kinase Inhibitors. Mol. Cancer Ther. 2022;21:336–346. doi: 10.1158/1535-7163.MCT-21-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Shen T, Mooers BHM, Hilberg F, Wu J. Drug resistance profiles of mutations in the RET kinase domain. Br. J. Pharmacol. 2018;175:3504–3515. doi: 10.1111/bph.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Liu Xu,X, Wen J, Zhang C, Fan M. Identification of a EML4-ALK exon 19 fusion variant in lung adenocarcinoma and alectinib resistance. Lung Cancer. 2021;160:32–35. doi: 10.1016/j.lungcan.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Zhang JQ, et al. The V654A second-site KIT mutation increases tumor oncogenesis and STAT activation in a mouse model of gastrointestinal stromal tumor. Oncogene. 2020;39:7153–7165. doi: 10.1038/s41388-020-01489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng C, et al. Spectrum of activity of dasatinib against mutant KIT kinases associated with drug-sensitive and drug-resistant gastrointestinal stromal tumors. Gastric Cancer. 2020;23:837–847. doi: 10.1007/s10120-020-01069-1. [DOI] [PubMed] [Google Scholar]
- 63.Florou V, et al. Clinical Activity of Selitrectinib in a Patient With Mammary Analogue Secretory Carcinoma of the Parotid Gland With Secondary Resistance to Entrectinib. J. Natl Compr. Canc. Netw. 2021;19:478–482. doi: 10.6004/jnccn.2021.7022. [DOI] [PubMed] [Google Scholar]
- 64.Estupiñan Velasquez HY, et al. Combination of Gatekeeper Mutations and Cysteine 481 Replacement Causes Super Resistance to the Irreversible BTK Inhibitors Ibrutinib, Acalabrutinib and Zanubrutinib. Blood. 2019;134:5759. doi: 10.1182/blood-2019-123398. [DOI] [Google Scholar]
- 65.Nakaoku T, et al. A secondary RET mutation in the activation loop conferring resistance to vandetanib. Nat. Commun. 2018;9:625. doi: 10.1038/s41467-018-02994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byron SA, et al. The N550K/H mutations in FGFR2 confer differential resistance to PD173074, dovitinib, and ponatinib ATP-competitive inhibitors. Neoplasia. 2013;15:975–988. doi: 10.1593/neo.121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, et al. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol. Cell. 2007;27:717–730. doi: 10.1016/j.molcel.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoemberger M, Pitsawong W, Kern D. Cumulative mechanism of several major imatinib-resistant mutations in Abl kinase. Proc. Natl Acad. Sci. U.S.A. 2020;117:19221–19227. doi: 10.1073/pnas.1919221117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu T, et al. Repurposing cabozantinib to GISTs: Overcoming multiple imatinib-resistant cKIT mutations including gatekeeper and activation loop mutants in GISTs preclinical models. Cancer Lett. 2019;447:105–114. doi: 10.1016/j.canlet.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 70.Jones RL, et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: Long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur. J. Cancer. 2021;145:132–142. doi: 10.1016/j.ejca.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia Z-J, et al. SAF-189s, a potent new-generation ROS1 inhibitor, is active against crizotinib-resistant ROS1 mutant-driven tumors. Acta Pharmacol. Sin. 2021;42:998–1004. doi: 10.1038/s41401-020-00513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Y, et al. A new ALK inhibitor overcomes resistance to first- and second-generation inhibitors in NSCLC. EMBO Mol. Med. 2022;14:e14296. doi: 10.15252/emmm.202114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith BD, et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell. 2019;35:738–751.e9. doi: 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Wu D, et al. LY2874455 potently inhibits FGFR gatekeeper mutants and overcomes mutation-based resistance. Chem. Commun. (Camb.) 2018;54:12089–12092. doi: 10.1039/C8CC07546H. [DOI] [PubMed] [Google Scholar]
- 75.To C, et al. Single and Dual Targeting of Mutant EGFR with an Allosteric Inhibitor. Cancer Discov. 2019;9:926–943. doi: 10.1158/2159-8290.CD-18-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang J, et al. Mutant-Selective Allosteric EGFR Degraders are Effective Against a Broad Range of Drug-Resistant Mutations. Angew. Chem. Int. Ed. Engl. 2020;59:14481–14489. doi: 10.1002/anie.202003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen, H. et al. Conformational Constrained 4-(1-Sulfonyl-3-indol)yl-2-phenylaminopyrimidine Derivatives as New Fourth-Generation Epidermal Growth Factor Receptor Inhibitors Targeting T790M/C797S Mutations. J. Med. Chem. (2022). [DOI] [PubMed]
- 78.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Larotrectinib (Bethesda (MD), 2012). [PubMed]
- 79.Laetsch TW, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: a multicentre, open-label, phase 1 study. Lancet Oncol. 2018;19:705–714. doi: 10.1016/S1470-2045(18)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drilon A, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong DS, et al. Larotrectinib in adult patients with solid tumours: a multi-centre, open-label, phase I dose-escalation study. Ann. Oncol. 2019;30:325–331. doi: 10.1093/annonc/mdy539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan S, et al. Structural Optimization and Structure-Activity Relationship Studies of 6,6-Dimethyl-4-(phenylamino)-6H-pyrimido5,4-b1,4oxazin-7(8H)-one Derivatives as A New Class of Potent Inhibitors of Pan-Trk and Their Drug-Resistant Mutants. J. Med. Chem. 2022;65:2035–2058. doi: 10.1021/acs.jmedchem.1c01597. [DOI] [PubMed] [Google Scholar]
- 83.Saleem H, et al. The TICking clock of EGFR therapy resistance in glioblastoma: Target Independence or target Compensation. Drug Resist. Updat. 2019;43:29–37. doi: 10.1016/j.drup.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Liu, Z. et al. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Sci. Immunol. 4, eaav6473 (2019). [DOI] [PubMed]
- 85.Reguera-Nuñez E, Man S, Xu P, Kerbel RS. Preclinical impact of high dose intermittent antiangiogenic tyrosine kinase inhibitor pazopanib in intrinsically resistant tumor models. Angiogenesis. 2018;21:793–804. doi: 10.1007/s10456-018-9623-8. [DOI] [PubMed] [Google Scholar]
- 86.Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat. Rev. 2018;65:1–10. doi: 10.1016/j.ctrv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 87.Vendrell JA, et al. EGFR-dependent mechanisms of resistance to osimertinib determined by ctDNA NGS analysis identify patients with better outcome. Transl. Lung Cancer Res. 2021;10:4084–4094. doi: 10.21037/tlcr-21-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi Y-R, et al. Early On-Treatment Prediction of the Mechanisms of Acquired Resistance to EGFR Tyrosine Kinase Inhibitors. Cancers. 2022;14:1512. doi: 10.3390/cancers14061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ali Z, et al. Zebrafish patient-derived xenograft models predict lymph node involvement and treatment outcome in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022;41:58. doi: 10.1186/s13046-022-02280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart EL, et al. Clinical Utility of Patient-Derived Xenografts to Determine Biomarkers of Prognosis and Map Resistance Pathways in EGFR-Mutant Lung Adenocarcinoma. J. Clin. Oncol. 2015;33:2472–2480. doi: 10.1200/JCO.2014.60.1492. [DOI] [PubMed] [Google Scholar]
- 91.Robichaux JP, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature. 2021;597:732–737. doi: 10.1038/s41586-021-03898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papadimitrakopoulou VA, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann. Oncol. 2018;29:viii741. doi: 10.1093/annonc/mdy424.064. [DOI] [Google Scholar]
- 93.Kehagias P, et al. Abstract 1082: Regorafenib resistance is associated with senescence-like phenotype and EMT in colorectal cancer (CRC) Cancer Res. 2021;81:1082. doi: 10.1158/1538-7445.AM2021-1082. [DOI] [Google Scholar]
- 94.Li B, et al. Conjoined hyperactivation of the RAS and PI3K pathways in advanced GIST. J. Clin. Oncol. 2016;34:e22520–e22520. doi: 10.1200/JCO.2016.34.15_suppl.e22520. [DOI] [Google Scholar]
- 95.Veeraraghavan J, et al. Abstract 1077: Acquired neratinib resistance is associated with acquisition of HER2 and PIK3CA mutations and can be overcome using potent drug combinations in HER2-positive breast cancer models. Cancer Res. 2021;81:1077. doi: 10.1158/1538-7445.AM2021-1077. [DOI] [Google Scholar]
- 96.Quentmeier H, Eberth S, Romani J, Zaborski M, Drexler HG. BCR-ABL1-independent PI3Kinase activation causing imatinib-resistance. J. Hematol. Oncol. 2011;4:6. doi: 10.1186/1756-8722-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim S, et al. Acquired Resistance of MET-Amplified Non-small Cell Lung Cancer Cells to the MET Inhibitor Capmatinib. Cancer Res. Treat. 2019;51:951–962. doi: 10.4143/crt.2018.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park H-R, et al. Acquired Resistance to Third-Generation EGFR Tyrosine Kinase Inhibitors in Patients With De Novo EGFRT790M-Mutant NSCLC. J. Thorac. Oncol. 2021;16:1859–1871. doi: 10.1016/j.jtho.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Yang L, et al. Synergistic therapeutic effect of combined PDGFR and SGK1 inhibition in metastasis-initiating cells of breast cancer. Cell Death Differ. 2020;27:2066–2080. doi: 10.1038/s41418-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu Y, et al. Hypoxia Induces Resistance to EGFR Inhibitors in Lung Cancer Cells via Upregulation of FGFR1 and the MAPK Pathway. Cancer Res. 2020;80:4655–4667. doi: 10.1158/0008-5472.CAN-20-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato H, et al. MAPK Pathway Alterations Correlate with Poor Survival and Drive Resistance to Therapy in Patients with Lung Cancers Driven by ROS1 Fusions. Clin. Cancer Res. 2020;26:2932–2945. doi: 10.1158/1078-0432.CCR-19-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mian AA, Zafar U, Ahmed SMA, Ottmann OG, Lalani E-NMA. Oncogene-independent resistance in Philadelphia chromosome - positive (Ph+) acute lymphoblastic leukemia (ALL) is mediated by activation of AKT/mTOR pathway. Neoplasia. 2021;23:1016–1027. doi: 10.1016/j.neo.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Motzer RJ, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 104.Hutson TE, et al. A Single-arm, Multicenter, Phase 2 Study of Lenvatinib Plus Everolimus in Patients with Advanced Non-Clear Cell Renal Cell Carcinoma. Eur. Urol. 2021;80:162–170. doi: 10.1016/j.eururo.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 105.Motzer R, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 106.Alves R, et al. Everolimus in combination with Imatinib overcomes resistance in Chronic myeloid leukaemia. Med Oncol. 2019;36:30. doi: 10.1007/s12032-019-1253-5. [DOI] [PubMed] [Google Scholar]
- 107.Fang W, et al. PI3K-AKT-mTOR pathway alterations in advanced NSCLC patients after progression on EGFR-TKI and clinical response to EGFR-TKI plus everolimus combination therapy. Transl. Lung Cancer Res. 2020;9:1258–1267. doi: 10.21037/tlcr-20-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Massarelli E, et al. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2015;26:1476–1480. doi: 10.1093/annonc/mdv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuroshima K, et al. Potential new therapy of Rapalink-1, a new generation mammalian target of rapamycin inhibitor, against sunitinib-resistant renal cell carcinoma. Cancer Sci. 2020;111:1607–1618. doi: 10.1111/cas.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terp MG, et al. Combined FGFR and Akt pathway inhibition abrogates growth of FGFR1 overexpressing EGFR-TKI-resistant NSCLC cells. NPJ Precis. Oncol. 2021;5:65. doi: 10.1038/s41698-021-00208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han P, et al. Dual inhibition of Akt and c-Met as a second-line therapy following acquired resistance to sorafenib in hepatocellular carcinoma cells. Mol. Oncol. 2017;11:320–334. doi: 10.1002/1878-0261.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reck M, Carbone DP, Garassino M, Barlesi F. Targeting KRAS in non-small-cell lung cancer: recent progress and new approaches. Ann. Oncol. 2021;32:1101–1110. doi: 10.1016/j.annonc.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Rouhimoghadam M, et al. Exploiting LY3009120 and Asciminib Combination to Target TKI-Resistant CML. Blood. 2021;138:3600. doi: 10.1182/blood-2021-153353. [DOI] [Google Scholar]
- 114.Lebedev T, et al. Growth factor signaling predicts therapy resistance mechanisms and defines neuroblastoma subtypes. Oncogene. 2021;40:6258–6272. doi: 10.1038/s41388-021-02018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi P, et al. Overcoming Acquired Resistance to AZD9291, A Third-Generation EGFR Inhibitor, through Modulation of MEK/ERK-Dependent Bim and Mcl-1 Degradation. Clin. Cancer Res. 2017;23:6567–6579. doi: 10.1158/1078-0432.CCR-17-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lau DK, et al. Rapid Resistance of FGFR-driven Gastric Cancers to Regorafenib and Targeted FGFR Inhibitors can be Overcome by Parallel Inhibition of MEK. Mol. Cancer Ther. 2021;20:704–715. doi: 10.1158/1535-7163.MCT-20-0836. [DOI] [PubMed] [Google Scholar]
- 117.Choi Y-S, et al. Effects of a dabrafenib and erlotinib combination treatment on anaplastic thyroid carcinoma. Endocr. Relat. Cancer. 2022;29:307–319. doi: 10.1530/ERC-22-0022. [DOI] [PubMed] [Google Scholar]
- 118.Vaishnavi A, et al. Inhibition of MEK1/2 Forestalls the Onset of Acquired Resistance to Entrectinib in Multiple Models of NTRK1-Driven Cancer. Cell Rep. 2020;32:107994. doi: 10.1016/j.celrep.2020.107994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu Y, et al. MEK inhibition overcomes resistance to EphA2-targeted therapy in uterine cancer. Gynecol. Oncol. 2021;163:181–190. doi: 10.1016/j.ygyno.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim R, et al. A Phase I Trial of Trametinib in Combination with Sorafenib in Patients with Advanced Hepatocellular Cancer. Oncologist. 2020;25:e1893–e1899. doi: 10.1634/theoncologist.2020-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morales ML, et al. MEK inhibition enhances the response to tyrosine kinase inhibitors in acute myeloid leukemia. Sci. Rep. 2019;9:18630. doi: 10.1038/s41598-019-54901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khan HY, et al. Targeting XPO1 and PAK4 in 8505 C Anaplastic Thyroid Cancer Cells: Putative Implications for Overcoming Lenvatinib Therapy Resistance. Int. J. Mol. Sci. 2019;21:237. doi: 10.3390/ijms21010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qu G-P, et al. Dual targeting of MEK and PI3K effectively controls the proliferation of human EGFR-TKI resistant non-small cell lung carcinoma cell lines with different genetic backgrounds. BMC Pulm. Med. 2021;21:208. doi: 10.1186/s12890-021-01571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mezynski MJ, et al. Targeting the PI3K and MAPK pathways to improve response to HER2-targeted therapies in HER2-positive gastric cancer. J. Transl. Med. 2021;19:184. doi: 10.1186/s12967-021-02842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ning G, et al. A novel treatment strategy for lapatinib resistance in a subset of HER2-amplified gastric cancer. BMC Cancer. 2021;21:923. doi: 10.1186/s12885-021-08283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sekino Y, et al. PTEN Is Involved in Sunitinib and Sorafenib Resistance in Renal Cell Carcinoma. Anticancer Res. 2020;40:1943–1951. doi: 10.21873/anticanres.14149. [DOI] [PubMed] [Google Scholar]
- 127.Zhong J, et al. Potential Resistance Mechanisms Revealed by Targeted Sequencing from Lung Adenocarcinoma Patients with Primary Resistance to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) J. Thorac. Oncol. 2017;12:1766–1778. doi: 10.1016/j.jtho.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 128.Malchers F, et al. Mechanisms of Primary Drug Resistance in FGFR1-Amplified Lung Cancer. Clin. Cancer Res. 2017;23:5527–5536. doi: 10.1158/1078-0432.CCR-17-0478. [DOI] [PubMed] [Google Scholar]
- 129.Cortot AB, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73:834–843. doi: 10.1158/0008-5472.CAN-12-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang W, et al. Reduced PHLPP Expression Leads to EGFR-TKI Resistance in Lung Cancer by Activating PI3K-AKT and MAPK-ERK Dual Signaling. Front. Oncol. 2021;11:665045. doi: 10.3389/fonc.2021.665045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu W, et al. OTUD1 stabilizes PTEN to inhibit the PI3K/AKT and TNF-alpha/NF-kappaB signaling pathways and sensitize ccRCC to TKIs. Int. J. Biol. Sci. 2022;18:1401–1414. doi: 10.7150/ijbs.68980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi J, et al. Transglutaminase 2 induces intrinsic EGFR-TKI resistance in NSCLC harboring EGFR sensitive mutations. Am. J. Cancer Res. 2019;9:1708–1721. [PMC free article] [PubMed] [Google Scholar]
- 133.Yin X, et al. Histone demethylase RBP2 mediates the blast crisis of chronic myeloid leukemia through an RBP2/PTEN/BCR-ABL cascade. Cell. Signal. 2019;63:109360. doi: 10.1016/j.cellsig.2019.109360. [DOI] [PubMed] [Google Scholar]
- 134.Kim I-G, et al. Targeting therapy-resistant lung cancer stem cells via disruption of the AKT/TSPYL5/PTEN positive-feedback loop. Commun. Biol. 2021;4:778. doi: 10.1038/s42003-021-02303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin X, et al. MiR-452-5p promotes colorectal cancer progression by regulating an ERK/MAPK positive feedback loop. Aging. 2021;13:7608–7626. doi: 10.18632/aging.202657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karaca Atabay E, et al. Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK + anaplastic large cell lymphoma. Blood. 2022;139:717–731. doi: 10.1182/blood.2020008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dong H, et al. Tyrosine Phosphatase PTPRO Deficiency in ERBB2-Positive Breast Cancer Contributes to Poor Prognosis and Lapatinib Resistance. Front. Pharmacol. 2022;13:838171. doi: 10.3389/fphar.2022.838171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mäkelä E, et al. Discovery of a Novel CIP2A Variant (NOCIVA) with Clinical Relevance in Predicting TKI Resistance in Myeloid Leukemias. Clin. Cancer Res. 2021;27:2848–2860. doi: 10.1158/1078-0432.CCR-20-3679. [DOI] [PubMed] [Google Scholar]
- 139.Fang L, et al. HP-1 inhibits the progression of ccRCC and enhances sunitinib therapeutic effects by suppressing EMT. Carbohydr. Polym. 2019;223:115109. doi: 10.1016/j.carbpol.2019.115109. [DOI] [PubMed] [Google Scholar]
- 140.Engelman JA, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 141.Vaquero J, et al. The IGF2/IR/IGF1R Pathway in Tumor Cells and Myofibroblasts Mediates Resistance to EGFR Inhibition in Cholangiocarcinoma. Clin. Cancer Res. 2018;24:4282–4296. doi: 10.1158/1078-0432.CCR-17-3725. [DOI] [PubMed] [Google Scholar]
- 142.Hayakawa D, et al. Activation of insulin-like growth factor-1 receptor confers acquired resistance to osimertinib in non-small cell lung cancer with EGFR T790M mutation. Thorac. Cancer. 2020;11:140–149. doi: 10.1111/1759-7714.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Okura N, et al. ONO-7475, a Novel AXL Inhibitor, Suppresses the Adaptive Resistance to Initial EGFR-TKI Treatment in EGFR-Mutated Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020;26:2244–2256. doi: 10.1158/1078-0432.CCR-19-2321. [DOI] [PubMed] [Google Scholar]
- 144.Shaw AT, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Piotrowska Z, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov. 2018;8:1529–1539. doi: 10.1158/2159-8290.CD-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lovly CM, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat. Med. 2014;20:1027–1034. doi: 10.1038/nm.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liefers-Visser JAL, Meijering RAM, Reyners AKL, van der Zee AGJ, Jong Sde. IGF system targeted therapy: Therapeutic opportunities for ovarian cancer. Cancer Treat. Rev. 2017;60:90–99. doi: 10.1016/j.ctrv.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Cascone T, et al. The HGF/c-MET Pathway Is a Driver and Biomarker of VEGFR-inhibitor Resistance and Vascular Remodeling in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017;23:5489–5501. doi: 10.1158/1078-0432.CCR-16-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin Y-T, et al. Resistance profiles of anaplastic lymphoma kinase tyrosine kinase inhibitors in advanced non-small-cell lung cancer: a multicenter study using targeted next-generation sequencing. Eur. J. Cancer. 2021;156:1–11. doi: 10.1016/j.ejca.2021.06.043. [DOI] [PubMed] [Google Scholar]
- 150.Dagogo-Jack I, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res. 2020;26:2535–2545. doi: 10.1158/1078-0432.CCR-19-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dumas P-Y, et al. Hematopoietic niche drives FLT3-ITD acute myeloid leukemia resistance to quizartinib via STAT5-and hypoxia-dependent upregulation of AXL. Haematologica. 2019;104:2017–2027. doi: 10.3324/haematol.2018.205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Banerjee S, et al. KITlow Cells Mediate Imatinib Resistance in Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2021;20:2035–2048. doi: 10.1158/1535-7163.MCT-20-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Su B, et al. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021;24:352–367. doi: 10.1007/s10120-020-01126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruan H, Li S, Bao L, Zhang X. Enhanced YB1/EphA2 axis signaling promotes acquired resistance to sunitinib and metastatic potential in renal cell carcinoma. Oncogene. 2020;39:6113–6128. doi: 10.1038/s41388-020-01409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding J, et al. Targeting the EphB4 receptor tyrosine kinase sensitizes HER2-positive breast cancer cells to Lapatinib. Cancer Lett. 2020;475:53–64. doi: 10.1016/j.canlet.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 156.Leung HW, et al. EPHB2 Activates β-Catenin to Enhance Cancer Stem Cell Properties and Drive Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res. 2021;81:3229–3240. doi: 10.1158/0008-5472.CAN-21-0184. [DOI] [PubMed] [Google Scholar]
- 157.Furlan A, Kherrouche Z, Montagne R, Copin MC, Tulasne D. Thirty years of research on met receptor to move a biomarker from bench to bedside. Cancer Res. 2014;74:6737–6744. doi: 10.1158/0008-5472.CAN-14-1932. [DOI] [PubMed] [Google Scholar]
- 158.Liu X, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin. Cancer Res. 2011;17:7127–7138. doi: 10.1158/1078-0432.CCR-11-1157. [DOI] [PubMed] [Google Scholar]
- 159.Feng H, et al. FOXK2 transcriptionally activating VEGFA induces apatinib resistance in anaplastic thyroid cancer through VEGFA/VEGFR1 pathway. Oncogene. 2021;40:6115–6129. doi: 10.1038/s41388-021-01830-5. [DOI] [PubMed] [Google Scholar]
- 160.Ma X-L, et al. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J. Hematol. Oncol. 2020;13:11. doi: 10.1186/s13045-020-0845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koike, N. The Role of Stem Cells in the Hepatobiliary System and in Cancer Development: a Surgeon’s Perspective. In Stem Cells and Cancer in Hepatology (ed. Zheng, Y.) 211–253 (Academic Press, 2018).
- 162.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 163.Fu Y, et al. Abnormally activated OPN/integrin alphaVbeta3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J. Hematol. Oncol. 2020;13:169. doi: 10.1186/s13045-020-01009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar R, et al. Specific, targetable interactions with the microenvironment influence imatinib-resistant chronic myeloid leukemia. Leukemia. 2020;34:2087–2101. doi: 10.1038/s41375-020-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li L, et al. Ibrutinib reverses IL-6-induced osimertinib resistance through inhibition of Laminin α5/FAK signaling. Commun. Biol. 2022;5:155. doi: 10.1038/s42003-022-03111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo G, et al. A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat. Neurosci. 2017;20:1074–1084. doi: 10.1038/nn.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ou B, et al. A positive feedback loop of β-catenin/CCR2 axis promotes regorafenib resistance in colorectal cancer. Cell Death Dis. 2019;10:643. doi: 10.1038/s41419-019-1906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiong W, et al. RRM2 Regulates Sensitivity to Sunitinib and PD-1 Blockade in Renal Cancer by Stabilizing ANXA1 and Activating the AKT Pathway. Adv. Sci. (Weinh.) 2021;8:e2100881. doi: 10.1002/advs.202100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yoshida R, et al. Activation of Src signaling mediates acquired resistance to ALK inhibition in lung cancer. Int. J. Oncol. 2017;51:1533–1540. doi: 10.3892/ijo.2017.4140. [DOI] [PubMed] [Google Scholar]
- 170.Lin C, et al. Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer Lett. 2020;472:81–96. doi: 10.1016/j.canlet.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 171.Trigg RM, et al. The targetable kinase PIM1 drives ALK inhibitor resistance in high-risk neuroblastoma independent of MYCN status. Nat. Commun. 2019;10:5428. doi: 10.1038/s41467-019-13315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Z, Ma L, Sun Y, Yu W, Wang X. Targeting STAT3 signaling overcomes gefitinib resistance in non-small cell lung cancer. Cell Death Dis. 2021;12:561. doi: 10.1038/s41419-021-03844-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zheng Q, et al. A novel STAT3 inhibitor W2014-S regresses human non-small cell lung cancer xenografts and sensitizes EGFR-TKI acquired resistance. Theranostics. 2021;11:824–840. doi: 10.7150/thno.49600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen J, et al. Single-cell DNA-seq depicts clonal evolution of multiple driver alterations in osimertinib-resistant patients. Ann. Oncol. 2022;33:434–444. doi: 10.1016/j.annonc.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 175.Abou-Alfa GK, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schöffski P, et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 ‘CaboGIST’. Eur. J. Cancer. 2020;134:62–74. doi: 10.1016/j.ejca.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 177.Yun MR, et al. Repotrectinib Exhibits Potent Antitumor Activity in Treatment-Naïve and Solvent-Front-Mutant ROS1-Rearranged Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020;26:3287–3295. doi: 10.1158/1078-0432.CCR-19-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shi T, et al. Evaluating the Effect of Lenvatinib on Sorafenib-Resistant Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2021;22:13071. doi: 10.3390/ijms222313071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mizuta H, et al. Gilteritinib overcomes lorlatinib resistance in ALK-rearranged cancer. Nat. Commun. 2021;12:1261. doi: 10.1038/s41467-021-21396-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dumas P-Y, et al. Dual Inhibition of FLT3 and AXL by Gilteritinib Overcomes Hematopoietic Niche-Driven Resistance Mechanisms in FLT3-ITD Acute Myeloid Leukemia. Clin. Cancer Res. 2021;27:6012–6025. doi: 10.1158/1078-0432.CCR-20-3114. [DOI] [PubMed] [Google Scholar]
- 181.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Entrectinib (Bethesda MD, 2012). [PubMed]
- 182.Li F, Zhu T, Cao B, Wang J, Liang L. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur. J. Cancer. 2017;84:184–192. doi: 10.1016/j.ejca.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 183.Ma Y, et al. InsR/IGF1R Pathway Mediates Resistance to EGFR Inhibitors in Glioblastoma. Clin. Cancer Res. 2016;22:1767–1776. doi: 10.1158/1078-0432.CCR-15-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sequist LV, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. [DOI] [PubMed] [Google Scholar]
- 185.Wu Y-L, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir. Med. 2020;8:1132–1143. doi: 10.1016/S2213-2600(20)30154-5. [DOI] [PubMed] [Google Scholar]
- 186.Jin H, et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature. 2021;595:730–734. doi: 10.1038/s41586-021-03741-7. [DOI] [PubMed] [Google Scholar]
- 187.Dawson JC, Serrels A, Stupack DG, Schlaepfer DD, Frame MC. Targeting FAK in anticancer combination therapies. Nat. Rev. Cancer. 2021;21:313–324. doi: 10.1038/s41568-021-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suzuki C, et al. Inhibition of EGFR and MEK surmounts entrectinib resistance in a brain metastasis model of NTRK1-rearranged tumor cells. Cancer Sci. 2022;113:2323. doi: 10.1111/cas.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo Y, Mehrabi Nasab E, Hassanpour F, Athari SS. Linsitinib and aspirin as the IGF1-R antagonists, inhibit regorafenib-resistant chemotherapy in colon cancer. Saudi J. Biol. Sci. 2022;29:872–877. doi: 10.1016/j.sjbs.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen Z, et al. The natural product berberine synergizes with osimertinib preferentially against MET-amplified osimertinib-resistant lung cancer via direct MET inhibition. Pharmacol. Res. 2022;175:105998. doi: 10.1016/j.phrs.2021.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saito Y, et al. TAS-116 inhibits oncogenic KIT signalling on the Golgi in both imatinib-naïve and imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer. 2020;122:658–667. doi: 10.1038/s41416-019-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee HJ, et al. HSP90 Inhibitor, 17-DMAG, Alone and in Combination with Lapatinib Attenuates Acquired Lapatinib-Resistance in ER-positive, HER2-Overexpressing Breast Cancer Cell Line. Cancers. 2020;12:2630. doi: 10.3390/cancers12092630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen H, et al. Metformin reduces HGF-induced resistance to alectinib via the inhibition of Gab1. Cell Death Dis. 2020;11:111. doi: 10.1038/s41419-020-2307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Arosio G, et al. Synergistic Drug Combinations Prevent Resistance in ALK + Anaplastic Large Cell Lymphoma. Cancers. 2021;13:4422. doi: 10.3390/cancers13174422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schwab, M. Encyclopedia of Cancer (Springer Berlin Heidelberg, 2011).
- 196.Rong X, et al. Molecular Mechanisms of Tyrosine Kinase Inhibitor Resistance Induced by Membranous/Cytoplasmic/Nuclear Translocation of Epidermal Growth Factor Receptor. J. Thorac. Oncol. 2019;14:1766–1783. doi: 10.1016/j.jtho.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 197.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat. Rev. Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 198.Shuai S, et al. TIP30 overcomes gefitinib resistance by regulating cytoplasmic and nuclear EGFR signaling in non-small-cell lung cancer. Cancer Sci. 2021;112:4139–4150. doi: 10.1111/cas.15000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lee P-C, et al. Targeting PKCδ as a Therapeutic Strategy against Heterogeneous Mechanisms of EGFR Inhibitor Resistance in EGFR-Mutant Lung Cancer. Cancer Cell. 2018;34:954–969.e4. doi: 10.1016/j.ccell.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Basu A, Pal D. Two faces of protein kinase Cδ: the contrasting roles of PKCδ in cell survival and cell death. SCIENTIFIC WORLD J. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen M-K, Hung M-C. Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases. FEBS J. 2015;282:3693–3721. doi: 10.1111/febs.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu J, et al. Cancer-Associated Fibroblasts Provide a Stromal Niche for Liver Cancer Organoids That Confers Trophic Effects and Therapy Resistance. Cell. Mol. Gastroenterol. Hepatol. 2021;11:407–431. doi: 10.1016/j.jcmgh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Straussman R, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huang W-C, et al. The MEK/ERK/miR-21 Signaling Is Critical in Osimertinib Resistance in EGFR-Mutant Non-Small Cell Lung Cancer Cells. Cancers. 2021;13:6005. doi: 10.3390/cancers13236005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sepe P, et al. Prospective Translational Study Investigating Molecular PrEdictors of Resistance to First-Line PazopanIb in Metastatic reNal CEll Carcinoma (PIPELINE Study) Am. J. Clin. Oncol. 2020;43:621–627. doi: 10.1097/COC.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 206.Shintani Y, et al. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J. Thorac. Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 207.Yamada D, et al. Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Eur. J. Cancer. 2013;49:1725–1740. doi: 10.1016/j.ejca.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 208.Zhang R, et al. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci. 2020;111:406–417. doi: 10.1111/cas.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gao L, et al. The BAFF/NFκB axis is crucial to interactions between sorafenib-resistant HCC cells and cancer-associated fibroblasts. Cancer Sci. 2021;112:3545–3554. doi: 10.1111/cas.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yi Y, et al. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 211.Oshi M, et al. Annexin A1 Expression Is Associated with Epithelial-Mesenchymal Transition (EMT), Cell Proliferation, Prognosis, and Drug Response in Pancreatic Cancer. Cells. 2021;10:653. doi: 10.3390/cells10030653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Song M, et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology. 2021;73:1717–1735. doi: 10.1002/hep.31792. [DOI] [PubMed] [Google Scholar]
- 213.Watson SS, et al. Microenvironment-Mediated Mechanisms of Resistance to HER2 Inhibitors Differ between HER2 + Breast Cancer Subtypes. Cell Syst. 2018;6:329–342.e6. doi: 10.1016/j.cels.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Joosten SC, et al. Resistance to sunitinib in renal cell carcinoma: From molecular mechanisms to predictive markers and future perspectives. Biochim. Biophys. Acta. 2015;1855:1–16. doi: 10.1016/j.bbcan.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 215.Makhov P, et al. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018;17:1355–1364. doi: 10.1158/1535-7163.MCT-17-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yin X, et al. Metformin enhances gefitinib efficacy by interfering with interactions between tumor-associated macrophages and head and neck squamous cell carcinoma cells. Cell. Oncol. (Dordr.) 2019;42:459–475. doi: 10.1007/s13402-019-00446-y. [DOI] [PubMed] [Google Scholar]
- 217.Ma S, et al. Epiregulin confers EGFR-TKI resistance via EGFR/ErbB2 heterodimer in non-small cell lung cancer. Oncogene. 2021;40:2596–2609. doi: 10.1038/s41388-021-01734-4. [DOI] [PubMed] [Google Scholar]
- 218.Massip-Copiz M, Clauzure M, Valdivieso ÁG, Santa-Coloma TA. Epiregulin (EREG) is upregulated through an IL-1β autocrine loop in Caco-2 epithelial cells with reduced CFTR function. J. Cell. Biochem. 2018;119:2911–2922. doi: 10.1002/jcb.26483. [DOI] [PubMed] [Google Scholar]
- 219.Liu S, et al. EREG-driven oncogenesis of Head and Neck Squamous Cell Carcinoma exhibits higher sensitivity to Erlotinib therapy. Theranostics. 2020;10:10589–10605. doi: 10.7150/thno.47176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dong N, et al. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br. J. Cancer. 2019;121:22–33. doi: 10.1038/s41416-019-0482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang, Z. et al. ‘γδTcells-IL-17A-Neutrophils’ Axis Drive Immunosuppression and Confer VEGFR2-TKI Refractoriness to Breast Cancer Therapy. SSRN. 10.2139/ssrn.3548733 (2020).
- 222.Zhou S-L, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 223.Gao Y, et al. CCL22 signaling contributes to sorafenib resistance in hepatitis B virus-associated hepatocellular carcinoma. Pharmacol. Res. 2020;157:104800. doi: 10.1016/j.phrs.2020.104800. [DOI] [PubMed] [Google Scholar]
- 224.Yang P, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gao Y, et al. Intratumoral stem-like CCR4 + regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J. Hepatol. 2022;76:148–159. doi: 10.1016/j.jhep.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 226.Lei X, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 227.Ko JS, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xin H, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Feng PH, et al. S100A9(+) MDSC and TAM-mediated EGFR-TKI resistance in lung adenocarcinoma: the role of RELB. Oncotarget. 2018;9:7631–7643. doi: 10.18632/oncotarget.24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Eluard B, et al. The alternative RelB NF-κB subunit is a novel critical player in diffuse large B-cell lymphoma. Blood. 2022;139:384–398. doi: 10.1182/blood.2020010039. [DOI] [PubMed] [Google Scholar]
- 231.Ge Q-L, et al. RelB/NF-κB links cell cycle transition and apoptosis to endometrioid adenocarcinoma tumorigenesis. Cell Death Dis. 2016;7:e2402. doi: 10.1038/cddis.2016.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Deng X, et al. Myeloid-derived suppressor cells promote tumor growth and sorafenib resistance by inducing FGF1 upregulation and fibrosis. Neoplasia. 2022;28:100788. doi: 10.1016/j.neo.2022.100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen R, et al. Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: from modulation to combination therapy targeting the microenvironment. Cancer Cell Int. 2022;22:73. doi: 10.1186/s12935-021-02435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rini BI, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 235.Makker V, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2020;38:2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Makker V, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lee C-H, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946–958. doi: 10.1016/S1470-2045(21)00241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Finn RS, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gettinger S, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J. Thorac. Oncol. 2018;13:1363–1372. doi: 10.1016/j.jtho.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 241.Cui Q, et al. Osimertinib Rechallenge With Bevacizumab vs. Chemotherapy Plus Bevacizumab in EGFR-Mutant NSCLC Patients With Osimertinib Resistance. Front. Pharmacol. 2021;12:746707. doi: 10.3389/fphar.2021.746707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhao S, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol. Res. 2019;7:630–643. doi: 10.1158/2326-6066.CIR-17-0640. [DOI] [PubMed] [Google Scholar]
- 243.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4:1112–1115. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bertol BC, et al. Lenvatinib Plus Anti-PD-1 Combination Therapy for Advanced Cancers: Defining Mechanisms of Resistance in an Inducible Transgenic Model of Thyroid Cancer. Thyroid. 2022;32:153–163. doi: 10.1089/thy.2021.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 246.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tulchinsky E, Demidov O, Kriajevska M, Barlev NA, Imyanitov E. EMT: A mechanism for escape from EGFR-targeted therapy in lung cancer. Biochim. Biophys. Acta Rev. Cancer. 2019;1871:29–39. doi: 10.1016/j.bbcan.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 248.Yoshida T, et al. ZEB1 Mediates Acquired Resistance to the Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PLoS One. 2016;11:e0147344. doi: 10.1371/journal.pone.0147344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Weng CH, et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene. 2019;38:455–468. doi: 10.1038/s41388-018-0454-2. [DOI] [PubMed] [Google Scholar]
- 250.Hwang HS, et al. Epithelial-mesenchymal transition as a mechanism of resistance to tyrosine kinase inhibitors in clear cell renal cell carcinoma. Lab. Invest. 2019;99:659–670. doi: 10.1038/s41374-019-0188-y. [DOI] [PubMed] [Google Scholar]
- 251.Hänze J, Henrici M, Hegele A, Hofmann R, Olbert PJ. Epithelial mesenchymal transition status is associated with anti-cancer responses towards receptor tyrosine-kinase inhibition by dovitinib in human bladder cancer cells. BMC Cancer. 2013;13:589. doi: 10.1186/1471-2407-13-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yochum ZA, et al. Targeting the EMT transcription factor TWIST1 overcomes resistance to EGFR inhibitors in EGFR-mutant non-small-cell lung cancer. Oncogene. 2019;38:656–670. doi: 10.1038/s41388-018-0482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tanaka K, et al. Targeting Aurora B kinase prevents and overcomes resistance to EGFR inhibitors in lung cancer by enhancing BIM- and PUMA-mediated apoptosis. Cancer Cell. 2021;39:1245–1261.e6. doi: 10.1016/j.ccell.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Soucheray M, et al. Intratumoral Heterogeneity in EGFR-Mutant NSCLC Results in Divergent Resistance Mechanisms in Response to EGFR Tyrosine Kinase Inhibition. Cancer Res. 2015;75:4372–4383. doi: 10.1158/0008-5472.CAN-15-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dopeso H, et al. PHD3 Controls Lung Cancer Metastasis and Resistance to EGFR Inhibitors through TGFα. Cancer Res. 2018;78:1805–1819. doi: 10.1158/0008-5472.CAN-17-1346. [DOI] [PubMed] [Google Scholar]
- 256.Oh H, et al. Therapy-Induced Transdifferentiation Promotes Glioma Growth Independent of EGFR Signaling. Cancer Res. 2021;81:1528–1539. doi: 10.1158/0008-5472.CAN-20-1810. [DOI] [PubMed] [Google Scholar]
- 257.Dufies M, et al. Plk1, upregulated by HIF-2, mediates metastasis and drug resistance of clear cell renal cell carcinoma. Commun. Biol. 2021;4:166. doi: 10.1038/s42003-021-01653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yang M-H, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 259.Peng P-H, Chieh-Yu Lai J, Hsu K-W, Wu K-J. Hypoxia-induced lncRNA RP11-390F4.3 promotes epithelial-mesenchymal transition (EMT) and metastasis through upregulating EMT regulators. Cancer Lett. 2020;483:35–45. doi: 10.1016/j.canlet.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 260.Tam SY, Wu VWC, Law HKW. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020;10:486. doi: 10.3389/fonc.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol. Cancer. 2019;18:153. doi: 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhu X, Chen L, Liu L, Niu X. EMT-Mediated Acquired EGFR-TKI Resistance in NSCLC: Mechanisms and Strategies. Front. Oncol. 2019;9:1044. doi: 10.3389/fonc.2019.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Clement MS, Gammelgaard KR, Nielsen AL, Sorensen BS. Epithelial-to-mesenchymal transition is a resistance mechanism to sequential MET-TKI treatment of MET-amplified EGFR-TKI resistant non-small cell lung cancer cells. Transl. Lung Cancer Res. 2020;9:1904–1914. doi: 10.21037/tlcr-20-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee YS, et al. Anti-cancer Effects of HNHA and Lenvatinib by the Suppression of EMT-Mediated Drug Resistance in Cancer Stem Cells. Neoplasia. 2018;20:197–206. doi: 10.1016/j.neo.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhang Z, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nakamichi S, et al. Overcoming drug-tolerant cancer cell subpopulations showing AXL activation and epithelial-mesenchymal transition is critical in conquering ALK-positive lung cancer. Oncotarget. 2018;9:27242–27255. doi: 10.18632/oncotarget.25531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pinato DJ, et al. Integrated analysis of multiple receptor tyrosine kinases identifies Axl as a therapeutic target and mediator of resistance to sorafenib in hepatocellular carcinoma. Br. J. Cancer. 2019;120:512–521. doi: 10.1038/s41416-018-0373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Serizawa M, Takahashi T, Yamamoto N, Koh Y. Combined treatment with erlotinib and a transforming growth factor-β type I receptor inhibitor effectively suppresses the enhanced motility of erlotinib-resistant non-small-cell lung cancer cells. J. Thorac. Oncol. 2013;8:259–269. doi: 10.1097/JTO.0b013e318279e942. [DOI] [PubMed] [Google Scholar]
- 269.Goyette M-A, et al. The Receptor Tyrosine Kinase AXL Is Required at Multiple Steps of the Metastatic Cascade during HER2-Positive Breast Cancer Progression. Cell Rep. 2018;23:1476–1490. doi: 10.1016/j.celrep.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 270.Goyette M-A, et al. Targeting Axl favors an antitumorigenic microenvironment that enhances immunotherapy responses by decreasing Hif-1α levels. Proc. Natl Acad. Sci. USA. 2021;118:e2023868118. doi: 10.1073/pnas.2023868118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Konen JM, et al. Dual Inhibition of MEK and AXL Targets Tumor Cell Heterogeneity and Prevents Resistant Outgrowth Mediated by the Epithelial-to-Mesenchymal Transition in NSCLC. Cancer Res. 2021;81:1398–1412. doi: 10.1158/0008-5472.CAN-20-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang CY, et al. Alisertib inhibits migration and invasion of EGFR-TKI resistant cells by partially reversing the epithelial-mesenchymal transition. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:119016. doi: 10.1016/j.bbamcr.2021.119016. [DOI] [PubMed] [Google Scholar]
- 273.Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat. Rev. Cancer. 2021;21:669–680. doi: 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- 274.Noel BM, et al. Multiomic Profiling of Tyrosine Kinase Inhibitor-Resistant K562 Cells Suggests Metabolic Reprogramming To Promote Cell Survival. J. Proteome Res. 2019;18:1842–1856. doi: 10.1021/acs.jproteome.9b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Apicella M, et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018;28:848–865.e6. doi: 10.1016/j.cmet.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 276.Ishibashi K, et al. MP72-05 IL-6-Triggered positive feedback loop for nfκb signaling induces tki resistance through metabolic alterations on renal cell carcinoma cells. J. Urol. 2018;199:e953–e954. doi: 10.1016/j.juro.2018.02.2289. [DOI] [Google Scholar]
- 277.Sofer S, et al. A genome-wide CRISPR activation screen reveals Hexokinase 1 as a critical factor in promoting resistance to multi-kinase inhibitors in hepatocellular carcinoma cells. FASEB J. 2022;36:e22191. doi: 10.1096/fj.202101507RR. [DOI] [PubMed] [Google Scholar]
- 278.Gao R, et al. USP29-mediated HIF1α stabilization is associated with Sorafenib resistance of hepatocellular carcinoma cells by upregulating glycolysis. Oncogenesis. 2021;10:52. doi: 10.1038/s41389-021-00338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tong L, et al. PKM2 Mediates Chronic Myeloid Leukemia Imatinib Resistance By Regulating Glycolysis Energy Metabolism. Blood. 2018;132:1724. doi: 10.1182/blood-2018-99-113960. [DOI] [Google Scholar]
- 280.Li Q, et al. Nuclear PKM2 contributes to gefitinib resistance via upregulation of STAT3 activation in colorectal cancer. Sci. Rep. 2015;5:16082. doi: 10.1038/srep16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lynch CJ, et al. Role of hepatic carbonic anhydrase in de novo lipogenesis. Biochem. J. 1995;310:197–202. doi: 10.1042/bj3100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rostovtseva TK, et al. Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease. J. Biol. Chem. 2015;290:18467–18477. doi: 10.1074/jbc.M115.641746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sharma N, Bhushan A, He J, Kaushal G, Bhardwaj V. Metabolic plasticity imparts erlotinib-resistance in pancreatic cancer by upregulating glucose-6-phosphate dehydrogenase. Cancer Metab. 2020;8:19. doi: 10.1186/s40170-020-00226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim S, et al. Enhanced Sensitivity of Nonsmall Cell Lung Cancer with Acquired Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors to Phenformin: The Roles of a Metabolic Shift to Oxidative Phosphorylation and Redox Balance. Oxid. Med. Cell. Longev. 2021;2021:5428364. doi: 10.1155/2021/5428364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sato T, et al. Metabolomic Analysis to Elucidate Mechanisms of Sunitinib Resistance in Renal Cell Carcinoma. Metabolites. 2020;11:1. doi: 10.3390/metabo11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iyer SR, et al. MYC promotes tyrosine kinase inhibitor resistance in ROS1 fusion-positive lung cancer. Mol. Cancer Res. 2022;20:722–734. doi: 10.1158/1541-7786.MCR-22-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Leone RD, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rysman E, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 289.Xu C, et al. Lipidomics reveals that sustained SREBP-1-dependent lipogenesis is a key mediator of gefitinib-acquired resistance in EGFR-mutant lung cancer. Cell Death Discov. 2021;7:353. doi: 10.1038/s41420-021-00744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Feng WW, et al. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 2019;29:3405–3420.e5. doi: 10.1016/j.celrep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wang M-S, et al. PPARα agonist fenofibrate relieves acquired resistance to gefitinib in non-small cell lung cancer by promoting apoptosis via PPARα/AMPK/AKT/FoxO1 pathway. Acta Pharmacol. Sin. 2022;43:167–176. doi: 10.1038/s41401-021-00638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pan Z, et al. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Mol. Cancer. 2022;21:77. doi: 10.1186/s12943-022-01547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Swinnen JV, Dehairs J, Talebi A. Membrane Lipid Remodeling Takes Center Stage in Growth Factor Receptor-Driven Cancer Development. Cell Metab. 2019;30:407–408. doi: 10.1016/j.cmet.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 294.Cai P, et al. Comparative Proteomics Analysis Reveals the Reversal Effect of Cryptotanshinone on Gefitinib-Resistant Cells in Epidermal Growth Factor Receptor-Mutant Lung Cancer. Front. Pharmacol. 2022;13:837055. doi: 10.3389/fphar.2022.837055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wei L, et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat. Commun. 2019;10:4681. doi: 10.1038/s41467-019-12606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Xu Q, et al. HNF4α regulates sulfur amino acid metabolism and confers sensitivity to methionine restriction in liver cancer. Nat. Commun. 2020;11:3978. doi: 10.1038/s41467-020-17818-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gao X, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397–401. doi: 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ge X, et al. Fumarate inhibits PTEN to promote tumorigenesis and therapeutic resistance of type2 papillary renal cell carcinoma. Mol. Cell. 2022;82:1249–1260. doi: 10.1016/j.molcel.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 299.Pisarsky L, et al. Targeting Metabolic Symbiosis to Overcome Resistance to Anti-angiogenic Therapy. Cell Rep. 2016;15:1161–1174. doi: 10.1016/j.celrep.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. 2017;14:11–31. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- 301.Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019;20:353–367. doi: 10.1038/s41580-019-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yang Q-J, et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle. 2018;9:71–85. doi: 10.1002/jcsm.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Adams CM, Clark-Garvey S, Porcu P, Eischen CM. Targeting the Bcl-2 Family in B Cell Lymphoma. Front. Oncol. 2018;8:636. doi: 10.3389/fonc.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sionov RV, Vlahopoulos SA, Granot Z. Regulation of Bim in Health and Disease. Oncotarget. 2015;6:23058–23134. doi: 10.18632/oncotarget.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li X, et al. BIM Deletion Polymorphism Confers Resistance to Osimertinib in EGFR T790M Lung Cancer: a Case Report and Literature Review. Target. Oncol. 2018;13:517–523. doi: 10.1007/s11523-018-0573-2. [DOI] [PubMed] [Google Scholar]
- 306.Su Y, et al. Targeting PI3K, mTOR, ERK, and Bcl-2 signaling network shows superior antileukemic activity against AML ex vivo. Biochem. Pharmacol. 2018;148:13–26. doi: 10.1016/j.bcp.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cruz-Gordillo P, Honeywell ME, Harper NW, Leete T, Lee MJ. ELP-dependent expression of MCL1 promotes resistance to EGFR inhibition in triple-negative breast cancer cells. Sci. Signal. 2020;13:eabb9820. doi: 10.1126/scisignal.abb9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chen H, et al. Vorinostat and metformin sensitize EGFR-TKI resistant NSCLC cells via BIM-dependent apoptosis induction. Oncotarget. 2017;8:93825–93838. doi: 10.18632/oncotarget.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ma G, et al. Overcoming acquired resistance to third-generation EGFR inhibitors by targeting activation of intrinsic apoptotic pathway through Mcl-1 inhibition, Bax activation, or both. Oncogene. 2022;41:1691–1700. doi: 10.1038/s41388-022-02200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tanimoto A, et al. Proteasome Inhibition Overcomes ALK-TKI Resistance in ALK-Rearranged/TP53-Mutant NSCLC via Noxa Expression. Clin. Cancer Res. 2021;27:1410–1420. doi: 10.1158/1078-0432.CCR-20-2853. [DOI] [PubMed] [Google Scholar]
- 311.Song X, et al. Mcl-1 inhibition overcomes intrinsic and acquired regorafenib resistance in colorectal cancer. Theranostics. 2020;10:8098–8110. doi: 10.7150/thno.45363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Im J-Y, et al. DGG-100629 inhibits lung cancer growth by suppressing the NFATc1/DDIAS/STAT3 pathway. Exp. Mol. Med. 2021;53:643–653. doi: 10.1038/s12276-021-00601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zheng L, et al. Piperlongumine synergistically enhances the antitumour activity of sorafenib by mediating ROS-AMPK activation and targeting CPSF7 in liver cancer. Pharmacol. Res. 2022;177:106140. doi: 10.1016/j.phrs.2022.106140. [DOI] [PubMed] [Google Scholar]
- 314.Paliouras AR, et al. Vulnerability of drug-resistant EML4-ALK rearranged lung cancer to transcriptional inhibition. EMBO Mol. Med. 2020;12:e11099. doi: 10.15252/emmm.201911099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sun B, et al. Inhibition of the transcriptional kinase CDK7 overcomes therapeutic resistance in HER2-positive breast cancers. Oncogene. 2020;39:50–63. doi: 10.1038/s41388-019-0953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Li H, et al. CDK12 inhibition enhances sensitivity of HER2 + breast cancers to HER2-tyrosine kinase inhibitor via suppressing PI3K/AKT. Eur. J. Cancer. 2021;145:92–108. doi: 10.1016/j.ejca.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 317.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer. 2019;19:405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 318.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 319.Sun X, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Cai S, et al. SIRT6 silencing overcomes resistance to sorafenib by promoting ferroptosis in gastric cancer. Biochem. Biophys. Res. Commun. 2021;577:158–164. doi: 10.1016/j.bbrc.2021.08.080. [DOI] [PubMed] [Google Scholar]
- 321.Ge W, et al. iASPP Is an Antioxidative Factor and Drives Cancer Growth and Drug Resistance by Competing with Nrf2 for Keap1 Binding. Cancer Cell. 2017;32:561–573.e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 322.Sun X, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500. doi: 10.1002/hep.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Houessinon A, et al. Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol. Cancer. 2016;15:38. doi: 10.1186/s12943-016-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ma C-S, et al. NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharmacol. Sin. 2021;42:613–623. doi: 10.1038/s41401-020-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Huang W, et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia. 2021;23:1227–1239. doi: 10.1016/j.neo.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gao R, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol. Med. 2021;13:e14351. doi: 10.15252/emmm.202114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Luis G, et al. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43:102006. doi: 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs—mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015;11:592–605. doi: 10.1038/nrendo.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Gao M, et al. Role of Mitochondria in Ferroptosis. Mol. Cell. 2019;73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Byun J-K, et al. Macropinocytosis is an alternative pathway of cysteine acquisition and mitigates sorafenib-induced ferroptosis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022;41:98. doi: 10.1186/s13046-022-02296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Viswanathan VS, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Gubelmann C, et al. Identification of the transcription factor ZEB1 as a central component of the adipogenic gene regulatory network. eLife. 2014;3:e03346. doi: 10.7554/eLife.03346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hangauer MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zhang C, et al. Identification of GPX4 as a therapeutic target for lung adenocarcinoma after EGFR-TKI resistance. Transl. Lung Cancer Res. 2022;11:786–801. doi: 10.21037/tlcr-22-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ren X, et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol. 2021;46:102122. doi: 10.1016/j.redox.2021.102122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Woodley K, et al. Mannose Metabolism Is a Metabolic Vulnerability Unveiled By Standard and Novel Therapies in Acute Myeloid Leukemia. Blood. 2021;138:508. doi: 10.1182/blood-2021-148489. [DOI] [Google Scholar]
- 338.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 339.Li YJ, et al. Autophagy and multidrug resistance in cancer. Chin. J. Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Pagotto A, et al. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 2017;8:e2943. doi: 10.1038/cddis.2017.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hu X, et al. Blocking autophagy improves the anti-tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Sci. Rep. 2017;7:4559. doi: 10.1038/s41598-017-04258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Guti E, et al. The multitargeted receptor tyrosine kinase inhibitor sunitinib induces resistance of HER2 positive breast cancer cells to trastuzumab-mediated ADCC. Cancer Immunol. Immunother. 2022;71:2151–2168. doi: 10.1007/s00262-022-03146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Heydt Q, et al. Oncogenic FLT3-ITD supports autophagy via ATF4 in acute myeloid leukemia. Oncogene. 2018;37:787–797. doi: 10.1038/onc.2017.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ma R, et al. GATA6-upregulating autophagy promotes TKI resistance in nonsmall cell lung cancer. Cancer Biol. Ther. 2019;20:1206–1212. doi: 10.1080/15384047.2019.1599665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Lotsberg ML, et al. AXL Targeting Abrogates Autophagic Flux and Induces Immunogenic Cell Death in Drug-Resistant Cancer Cells. J. Thorac. Oncol. 2020;15:973–999. doi: 10.1016/j.jtho.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ianniciello A, et al. ULK1 inhibition promotes oxidative stress-induced differentiation and sensitizes leukemic stem cells to targeted therapy. Sci. Transl. Med. 2021;13:eabd5016. doi: 10.1126/scitranslmed.abd5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wu H, et al. Mitophagy promotes sorafenib resistance through hypoxia-inducible ATAD3A dependent Axis. J. Exp. Clin. Cancer Res. 2020;39:274. doi: 10.1186/s13046-020-01768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mukai S, et al. Macrolides sensitize EGFR-TKI-induced non-apoptotic cell death via blocking autophagy flux in pancreatic cancer cell lines. Int. J. Oncol. 2016;48:45–54. doi: 10.3892/ijo.2015.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Horne GA, et al. A randomised phase II trial of hydroxychloroquine and imatinib versus imatinib alone for patients with chronic myeloid leukaemia in major cytogenetic response with residual disease. Leukemia. 2020;34:1775–1786. doi: 10.1038/s41375-019-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wei Y, et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Menon MB, Dhamija S. Beclin 1 Phosphorylation - at the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018;6:137. doi: 10.3389/fcell.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Li D, et al. Cholesterol sensor SCAP contributes to sorafenib resistance by regulating autophagy in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022;41:116. doi: 10.1186/s13046-022-02306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lv Y, et al. SRSF1 inhibits autophagy through regulating Bcl-x splicing and interacting with PIK3C3 in lung cancer. Signal Transduct. Target. Ther. 2021;6:108. doi: 10.1038/s41392-021-00495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Maggi F, et al. The effects of cannabidiol via TRPV2 channel in chronic myeloid leukemia cells and its combination with imatinib. Cancer Sci. 2022;113:1235–1249. doi: 10.1111/cas.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Larrue C, et al. Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells. Blood. 2016;127:882–892. doi: 10.1182/blood-2015-05-646497. [DOI] [PubMed] [Google Scholar]
- 356.Chen Y, et al. Erb-b2 Receptor Tyrosine Kinase 2 (ERBB2) Promotes ATG12-Dependent Autophagy Contributing to Treatment Resistance of Breast Cancer Cells. Cancers. 2021;13:1038. doi: 10.3390/cancers13051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Huang X, et al. Bypassing drug resistance by triggering necroptosis: recent advances in mechanisms and its therapeutic exploitation in leukemia. J. Exp. Clin. Cancer Res. 2018;37:310. doi: 10.1186/s13046-018-0976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Gong Y, et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer. 2019;18:100. doi: 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Liao Y, et al. HSP90α mediates sorafenib resistance in human hepatocellular carcinoma by necroptosis inhibition under hypoxia. Cancers. 2021;13:243. doi: 10.3390/cancers13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Huang X, Chen Z, Ni F, Ye X, Qian W. Shikonin overcomes drug resistance and induces necroptosis by regulating the miR-92a-1-5p/MLKL axis in chronic myeloid leukemia. Aging. 2020;12:17662–17680. doi: 10.18632/aging.103844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang Y, et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Feldmann F, Schenk B, Martens S, Vandenabeele P, Fulda S. Sorafenib inhibits therapeutic induction of necroptosis in acute leukemia cells. Oncotarget. 2017;8:68208–68220. doi: 10.18632/oncotarget.19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Martens S, et al. Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury. Cell Death Dis. 2017;8:e2904. doi: 10.1038/cddis.2017.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Fauster A, et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Tan W, et al. TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2019;40:446–456. doi: 10.1016/j.ebiom.2018.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Markowitsch SD, et al. Shikonin Inhibits Cell Growth of Sunitinib-Resistant Renal Cell Carcinoma by Activating the Necrosome Complex and Inhibiting the AKT/mTOR Signaling Pathway. Cancers. 2022;14:1114. doi: 10.3390/cancers14051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Long K, et al. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death Dis. 2021;12:503. doi: 10.1038/s41419-021-03804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lan W, et al. Targeting NUPR1 with the small compound ZZW-115 is an efficient strategy to treat hepatocellular carcinoma. Cancer Lett. 2020;486:8–17. doi: 10.1016/j.canlet.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 370.Nirmala JG, Lopus M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020;36:145–164. doi: 10.1007/s10565-019-09496-2. [DOI] [PubMed] [Google Scholar]
- 371.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 372.Karki R, Kanneganti T-D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer. 2019;19:197–214. doi: 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hage C, et al. Sorafenib Induces Pyroptosis in Macrophages and Triggers Natural Killer Cell-Mediated Cytotoxicity Against Hepatocellular Carcinoma. Hepatology. 2019;70:1280–1297. doi: 10.1002/hep.30666. [DOI] [PubMed] [Google Scholar]
- 374.Zhang J, Chen Y, He Q. Distinct characteristics of dasatinib-induced pyroptosis in gasdermin E-expressing human lung cancer A549 cells and neuroblastoma SH-SY5Y cells. Oncol. Lett. 2020;20:145–154. doi: 10.3892/ol.2020.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Koren E, Fuchs Y. Modes of regulated cell death in cancer. Cancer Discov. 2021;11:245–265. doi: 10.1158/2159-8290.CD-20-0789. [DOI] [PubMed] [Google Scholar]
- 376.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 377.Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 2020;19:776–800. doi: 10.1038/s41573-020-0077-5. [DOI] [PubMed] [Google Scholar]
- 378.Alves R, et al. Resistance to tyrosine kinase inhibitors in chronic myeloid leukemia-from molecular mechanisms to clinical relevance. Cancers. 2021;13:4820. doi: 10.3390/cancers13194820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Sharma SV, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Baumgartner U, et al. miR-19b enhances proliferation and apoptosis resistance via the EGFR signaling pathway by targeting PP2A and BIM in non-small cell lung cancer. Mol. Cancer. 2018;17:44. doi: 10.1186/s12943-018-0781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wang T, et al. The TGFbeta-miR-499a-SHKBP1 pathway induces resistance to EGFR inhibitors in osteosarcoma cancer stem cell-like cells. J. Exp. Clin. Cancer Res. 2019;38:226. doi: 10.1186/s13046-019-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Hirao A, et al. MiR-125b-5p Is Involved in Sorafenib Resistance through Ataxin-1-Mediated Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Cancers. 2021;13:4917. doi: 10.3390/cancers13194917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhao W, et al. Inhibiting CBX4 efficiently protects hepatocellular carcinoma cells against sorafenib resistance. Br. J. Cancer. 2021;124:1237–1248. doi: 10.1038/s41416-020-01240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Wang D, et al. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36–52. doi: 10.1016/j.canlet.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 385.Fernández-Tussy P, et al. Anti-miR-518d-5p overcomes liver tumor cell death resistance through mitochondrial activity. Cell Death Dis. 2021;12:555. doi: 10.1038/s41419-021-03827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pollutri D, et al. The epigenetically regulated miR-494 associates with stem-cell phenotype and induces sorafenib resistance in hepatocellular carcinoma. Cell Death Dis. 2018;9:4. doi: 10.1038/s41419-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Zhang Z, et al. The miR-30a-5p/CLCF1 axis regulates sorafenib resistance and aerobic glycolysis in hepatocellular carcinoma. Cell Death Dis. 2020;11:902. doi: 10.1038/s41419-020-03123-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 388.Garofalo M, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat. Med. 2011;18:74–82. doi: 10.1038/nm.2577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 389.Safaric Tepes P, et al. An epigenetic switch regulates the ontogeny of AXL-positive/EGFR-TKi-resistant cells by modulating miR-335 expression. Elife. 2021;10:e66109. doi: 10.7554/eLife.66109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Liu B-J, et al. miR-196a Upregulation Contributes to Gefitinib Resistance through Inhibiting GLTP Expression. Int. J. Mol. Sci. 2022;23:1785. doi: 10.3390/ijms23031785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Mishra SK, et al. Emerging roles for human glycolipid transfer protein superfamily members in the regulation of autophagy, inflammation, and cell death. Prog. Lipid Res. 2020;78:101031. doi: 10.1016/j.plipres.2020.101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Huynh TK, et al. miR-221 confers lapatinib resistance by negatively regulating p27kip1 in HER2-positive breast cancer. Cancer Sci. 2021;112:4234–4245. doi: 10.1111/cas.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wen C, et al. Screening Circular RNAs Related to Acquired Gefitinib Resistance in Non-small Cell Lung Cancer Cell Lines. J. Cancer. 2020;11:3816–3826. doi: 10.7150/jca.39783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.He M, et al. Sunitinib increases the cancer stem cells and vasculogenic mimicry formation via modulating the lncRNA-ECVSR/ERβ/Hif2-α signaling. Cancer Lett. 2022;524:15–28. doi: 10.1016/j.canlet.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 395.Chen Z, et al. Long non-coding RNA CASC9 promotes gefitinib resistance in NSCLC by epigenetic repression of DUSP1. Cell Death Dis. 2020;11:858. doi: 10.1038/s41419-020-03047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wang S, et al. Relationship between long non-coding RNA PCAT-1 expression and gefitinib resistance in non-small-cell lung cancer cells. Respir. Res. 2021;22:146. doi: 10.1186/s12931-021-01719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Si J, et al. HIF1A-AS2 induces osimertinib resistance in lung adenocarcinoma patients by regulating the miR-146b-5p/IL-6/STAT3 axis. Mol. Ther. Nucleic Acids. 2021;26:613–624. doi: 10.1016/j.omtn.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Shao Y, et al. RP11-616M22.7 recapitulates imatinib resistance in gastrointestinal stromal tumor. Mol. Ther. Nucleic Acids. 2021;25:264–276. doi: 10.1016/j.omtn.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Yang C, et al. circFN1 Mediates Sorafenib Resistance of Hepatocellular Carcinoma Cells by Sponging miR-1205 and Regulating E2F1 Expression. Mol. Ther. Nucleic Acids. 2020;22:421–433. doi: 10.1016/j.omtn.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Weng H, et al. circFOXM1 contributes to sorafenib resistance of hepatocellular carcinoma cells by regulating MECP2 via miR-1324. Mol. Ther. Nucleic Acids. 2021;23:811–820. doi: 10.1016/j.omtn.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Liu S, et al. Hsa_circ_0005576 promotes osimertinib resistance through the miR-512-5p/IGF1R axis in lung adenocarcinoma cells. Cancer Sci. 2022;113:79–90. doi: 10.1111/cas.15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Xu J, et al. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct. Target. Ther. 2020;5:298. doi: 10.1038/s41392-020-00375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Feng M, et al. YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood. 2021;138:71–85. doi: 10.1182/blood.2020009676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 405.Gao X, et al. Circular RNA-encoded oncogenic E-cadherin variant promotes glioblastoma tumorigenicity through activation of EGFR-STAT3 signalling. Nat. Cell Biol. 2021;23:278–291. doi: 10.1038/s41556-021-00639-4. [DOI] [PubMed] [Google Scholar]
- 406.Harjes U. EGFR is going circular. Nat. Rev. Cancer. 2021;21:280. doi: 10.1038/s41568-021-00350-4. [DOI] [PubMed] [Google Scholar]
- 407.Dai C, et al. Construction of a circRNA-miRNA-mRNA Regulated Pathway Involved in EGFR-TKI Lung Adenocarcinoma Resistance. Technol. Cancer Res. Treat. 2021;20:15330338211056809. doi: 10.1177/15330338211056809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Li M, Cai O, Yu Y, Tan S. Paeonol inhibits the malignancy of Apatinib-resistant gastric cancer cells via LINC00665/miR-665/MAPK1 axis. Phytomedicine. 2022;96:153903. doi: 10.1016/j.phymed.2021.153903. [DOI] [PubMed] [Google Scholar]
- 409.Liu D, et al. circKCNN2 suppresses the recurrence of hepatocellular carcinoma at least partially via regulating miR-520c-3p/methyl-DNA-binding domain protein 2 axis. Clin. Transl. Med. 2022;12:e662. doi: 10.1002/ctm2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wang Q, Li M, Hu C. Relationship between EGFR Promoter Region Methylation and Secondary Resistance Which may be Induced by Gefitinib. Zhongguo Fei Ai Za Zhi. 2015;18:193–198. doi: 10.3779/j.issn.1009-3419.2015.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Niu X, et al. Genome-wide DNA Methylation Analysis Reveals GABBR2 as a Novel Epigenetic Target for EGFR 19 Deletion Lung Adenocarcinoma with Induction Erlotinib Treatment. Clin. Cancer Res. 2017;23:5003–5014. doi: 10.1158/1078-0432.CCR-16-2688. [DOI] [PubMed] [Google Scholar]
- 412.Arechederra M, et al. ADAMTSL5 is an epigenetically activated gene underlying tumorigenesis and drug resistance in hepatocellular carcinoma. J. Hepatol. 2021;74:893–906. doi: 10.1016/j.jhep.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 413.Sheng Y, et al. Downregulation of the histone methyltransferase SETD2 promotes imatinib resistance in chronic myeloid leukaemia cells. Cell Prolif. 2019;52:e12611. doi: 10.1111/cpr.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Tseng C-F, Chen L-T, Wang H-D, Liu Y-H, Shiah S-G. Transcriptional suppression of Dicer by HOXB-AS3/EZH2 complex dictates sorafenib resistance and cancer stemness. Cancer Sci. 2022;113:1601–1612. doi: 10.1111/cas.15319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Pal AS, et al. Loss of KMT5C Promotes EGFR Inhibitor Resistance in NSCLC via LINC01510-Mediated Upregulation of MET. Cancer Res. 2022;82:1534–1547. doi: 10.1158/0008-5472.CAN-20-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Yan L, et al. Inhibition of SMYD2 suppresses tumor progression by down-regulating microRNA-125b and attenuates multi-drug resistance in renal cell carcinoma. Theranostics. 2019;9:8377–8391. doi: 10.7150/thno.37628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Berko ER, Mossé YP. Thrown for a Loop: Awakening BORIS to Evade ALK Inhibition Therapy. Cancer Cell. 2019;36:345–347. doi: 10.1016/j.ccell.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Debruyne DN, et al. BORIS promotes chromatin regulatory interactions in treatment-resistant cancer cells. Nature. 2019;572:676–680. doi: 10.1038/s41586-019-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Hu B, et al. CD13 promotes hepatocellular carcinogenesis and sorafenib resistance by activating HDAC5-LSD1-NF-κB oncogenic signaling. Clin. Transl. Med. 2020;10:e233. doi: 10.1002/ctm2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhang C, et al. KDM6A promotes imatinib resistance through YY1-mediated transcriptional upregulation of TRKA independently of its demethylase activity in chronic myelogenous leukemia. Theranostics. 2021;11:2691–2705. doi: 10.7150/thno.50571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Liu S, et al. The mechanism of m(6)A methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by beta-elemene. Cell Death Dis. 2020;11:969. doi: 10.1038/s41419-020-03148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Gao F, et al. RNA methyltransferase METTL3 induces intrinsic resistance to gefitinib by combining with MET to regulate PI3K/AKT pathway in lung adenocarcinoma. J. Cell. Mol. Med. 2021;25:2418–2425. doi: 10.1111/jcmm.16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Hua W, et al. METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecol. Oncol. 2018;151:356–365. doi: 10.1016/j.ygyno.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 424.Song H, et al. Methyltransferase like 7B is a potential therapeutic target for reversing EGFR-TKIs resistance in lung adenocarcinoma. Mol. Cancer. 2022;21:43. doi: 10.1186/s12943-022-01519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Tang J, Han T, Tong W, Zhao J, Wang W. N6-methyladenosine (m6A) methyltransferase KIAA1429 accelerates the gefitinib resistance of non-small-cell lung cancer. Cell Death Discov. 2021;7:108. doi: 10.1038/s41420-021-00488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wang T, et al. A novel protein encoded by circASK1 ameliorates gefitinib resistance in lung adenocarcinoma by competitively activating ASK1-dependent apoptosis. Cancer Lett. 2021;520:321–331. doi: 10.1016/j.canlet.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 427.Li K, et al. M6A associated TSUC7 inhibition contributed to Erlotinib resistance in lung adenocarcinoma through a notch signaling activation dependent way. J. Exp. Clin. Cancer Res. 2021;40:325. doi: 10.1186/s13046-021-02137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Yan F, et al. A dynamic N6-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res. 2018;28:1062–1076. doi: 10.1038/s41422-018-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Li Z, et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Sa R, et al. IGF2BP2-dependent activation of ERBB2 signaling contributes to acquired resistance to tyrosine kinase inhibitor in differentiation therapy of radioiodine-refractory papillary thyroid cancer. Cancer Lett. 2022;527:10–23. doi: 10.1016/j.canlet.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 431.Yiming R, et al. MUSASHI-2 confers resistance to third-generation EGFR-tyrosine kinase inhibitor osimertinib in lung adenocarcinoma. Cancer Sci. 2021;112:3810–3821. doi: 10.1111/cas.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Liau BB, et al. Adaptive Chromatin Remodeling Drives Glioblastoma Stem Cell Plasticity and Drug Tolerance. Cell Stem Cell. 2017;20:233–246 e7. doi: 10.1016/j.stem.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Kamachi K, et al. Targeting DNMT1 by demethylating agent OR-2100 increases tyrosine kinase inhibitors-sensitivity and depletes leukemic stem cells in chronic myeloid leukemia. Cancer Lett. 2022;526:273–283. doi: 10.1016/j.canlet.2021.11.032. [DOI] [PubMed] [Google Scholar]
- 434.Wang J-H, Zeng Z, Sun J, Chen Y, Gao X. A novel small-molecule antagonist enhances the sensitivity of osteosarcoma to cabozantinib in vitro and in vivo by targeting DNMT-1 correlated with disease severity in human patients. Pharmacol. Res. 2021;173:105869. doi: 10.1016/j.phrs.2021.105869. [DOI] [PubMed] [Google Scholar]
- 435.Wang Z, Dai J, Yan J, Zhang Y, Yin Z. Targeting EZH2 as a novel therapeutic strategy for sorafenib-resistant thyroid carcinoma. J. Cell. Mol. Med. 2019;23:4770–4778. doi: 10.1111/jcmm.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Wang S, et al. Sorafenib suppresses growth and survival of hepatoma cells by accelerating degradation of enhancer of zeste homolog 2. Cancer Sci. 2013;104:750–759. doi: 10.1111/cas.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Pan T, et al. Discovery of 2,4-pyrimidinediamine derivatives as potent dual inhibitors of ALK and HDAC. Eur. J. Med. Chem. 2021;224:113672. doi: 10.1016/j.ejmech.2021.113672. [DOI] [PubMed] [Google Scholar]
- 438.Hoy SM. Tazemetostat: first approval. Drugs. 2020;80:513–521. doi: 10.1007/s40265-020-01288-x. [DOI] [PubMed] [Google Scholar]
- 439.Cao P, et al. Combining EGFR-TKI With SAHA Overcomes EGFR-TKI-Acquired Resistance by Reducing the Protective Autophagy in Non-Small Cell Lung Cancer. Front. Chem. 2022;10:837987. doi: 10.3389/fchem.2022.837987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.He, T. et al. The HDAC inhibitor GCJ-490A suppresses c-Met expression through IKKα and overcomes gefitinib resistance in non-small cell lung cancer. Cancer Biol. Med. 10.20892/j.issn.2095-3941.2021.0130 (2022). [DOI] [PMC free article] [PubMed]
- 441.Peralta-Arrieta I, et al. Failure to EGFR-TKI-based therapy and tumoural progression are promoted by MEOX2/GLI1-mediated epigenetic regulation of EGFR in the human lung cancer. Eur. J. Cancer. 2022;160:189–205. doi: 10.1016/j.ejca.2021.10.032. [DOI] [PubMed] [Google Scholar]
- 442.Tanimoto A, et al. Histone Deacetylase 3 Inhibition Overcomes BIM Deletion Polymorphism-Mediated Osimertinib Resistance in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2017;23:3139–3149. doi: 10.1158/1078-0432.CCR-16-2271. [DOI] [PubMed] [Google Scholar]
- 443.Lin C-Y, et al. Vorinostat combined with brigatinib overcomes acquired resistance in EGFR-C797S-mutated lung cancer. Cancer Lett. 2021;508:76–91. doi: 10.1016/j.canlet.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 444.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv. Pharm. Bull. 2017;7:339–348. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zimmerman EI, et al. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin. Cancer Res. 2013;19:1458–1466. doi: 10.1158/1078-0432.CCR-12-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.White DL, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110:4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 447.Hegedus C, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br. J. Pharmacol. 2009;158:1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Dessilly G, et al. ABCB1 1199GA polymorphism (rs2229109) affects the transport of imatinib, nilotinib and dasatinib. Pharmacogenomics. 2016;17:883–890. doi: 10.2217/pgs-2016-0012. [DOI] [PubMed] [Google Scholar]
- 449.Skoglund K, et al. Single-nucleotide polymorphisms of ABCG2 increase the efficacy of tyrosine kinase inhibitors in the K562 chronic myeloid leukemia cell line. Pharmacogenet. Genomics. 2014;24:52–61. doi: 10.1097/FPC.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 450.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int. J. Cancer. 2004;109:238–246. doi: 10.1002/ijc.11669. [DOI] [PubMed] [Google Scholar]
- 451.Luedtke D, Marzin K, Jungnik A, Wangenheim Uvon, Dallinger C. Effects of Ketoconazole and Rifampicin on the Pharmacokinetics of Nintedanib in Healthy Subjects. Eur. J. Drug Metab. Pharmacokinet. 2018;43:533–541. doi: 10.1007/s13318-018-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Eadie LN, Hughes TP, White DL. ABCB1 Overexpression Is a Key Initiator of Resistance to Tyrosine Kinase Inhibitors in CML Cell Lines. PloS one. 2016;11:e0161470. doi: 10.1371/journal.pone.0161470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Huff LM, Wang Z, Iglesias A, Fojo T, Lee J-S. Aberrant transcription from an unrelated promoter can result in MDR-1 expression following drug selection in vitro and in relapsed lymphoma samples. Cancer Res. 2005;65:11694–11703. doi: 10.1158/0008-5472.CAN-04-1349. [DOI] [PubMed] [Google Scholar]
- 454.Katayama R, et al. P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine. 2016;3:54–66. doi: 10.1016/j.ebiom.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Turrini E, et al. MicroRNA profiling in K-562 cells under imatinib treatment: influence of miR-212 and miR-328 on ABCG2 expression. Pharmacogenet. Genomics. 2012;22:198–205. doi: 10.1097/FPC.0b013e328350012b. [DOI] [PubMed] [Google Scholar]
- 456.Li L, et al. Sunitinib treatment promotes metastasis of drug-resistant renal cell carcinoma via TFE3 signaling pathway. Cell Death Dis. 2021;12:220. doi: 10.1038/s41419-021-03511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Xing Y, Yan J, Niu Y. PXR: a center of transcriptional regulation in cancer. Acta Pharm. Sin. B. 2020;10:197–206. doi: 10.1016/j.apsb.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Matheux A, et al. PXR modulates the prostate cancer cell response to afatinib by regulating the expression of the monocarboxylate transporter SLC16A1. Cancers. 2021;13:3635. doi: 10.3390/cancers13143635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Robey RW, et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Da Silva CG, Peters GJ, Ossendorp F, Cruz LJ. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother. Pharmacol. 2017;80:881–894. doi: 10.1007/s00280-017-3427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.D’Cunha RR, Murry DJ, An G. Nilotinib alters the efflux transporter-mediated pharmacokinetics of afatinib in mice. J. Pharm. Sci. 2019;108:3434–3442. doi: 10.1016/j.xphs.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 462.Klerk DJ, de, Honeywell RJ, Jansen G, Peters GJ. Transporter and lysosomal mediated (multi)drug resistance to tyrosine kinase inhibitors and potential strategies to overcome resistance. Cancers. 2018;10:503. doi: 10.3390/cancers10120503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Gotink KJ, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin. Cancer Res. 2011;17:7337–7346. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Yue S, et al. FGFR-TKI resistance in cancer: current status and perspectives. J. Hematol. Oncol. 2021;14:23. doi: 10.1186/s13045-021-01040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Englinger B, et al. Intrinsic fluorescence of the clinically approved multikinase inhibitor nintedanib reveals lysosomal sequestration as resistance mechanism in FGFR-driven lung cancer. J. Exp. Clin. Cancer Res. 2017;36:122. doi: 10.1186/s13046-017-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Piao S, Amaravadi RK. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016;1371:45–54. doi: 10.1111/nyas.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Honeywell R, Hitzerd S, Kathmann I, Peters G. Subcellular localization of several structurally different tyrosine kinase inhibitors. ADMET DMPK. 2018;6:258–266. doi: 10.5599/admet.514. [DOI] [Google Scholar]
- 468.Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat. Rev. Cancer. 2006;6:546–558. doi: 10.1038/nrc1887. [DOI] [PubMed] [Google Scholar]
- 469.Assaraf YG, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updat. 2019;46:100645. doi: 10.1016/j.drup.2019.100645. [DOI] [PubMed] [Google Scholar]
- 470.van Leeuwen RWF, et al. Tyrosine kinase inhibitors and proton pump inhibitors: an evaluation of treatment options. Clin. Pharmacokinet. 2017;56:683–688. doi: 10.1007/s40262-016-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Herbrink M, Nuijen B, Schellens JH, Beijnen JH. Variability in bioavailability of small molecular tyrosine kinase inhibitors. Cancer Treat. Rev. 2015;41:412–422. doi: 10.1016/j.ctrv.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 472.Budha NR, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin. Pharmacol. Ther. 2012;92:203–213. doi: 10.1038/clpt.2012.73. [DOI] [PubMed] [Google Scholar]
- 473.Uchiyama AAT, et al. Proton pump. Curr. Oncol. 2021;28:783–799. doi: 10.3390/curroncol28010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Abbas R, Boni J, Sonnichsen D. Effect of rifampin on the pharmacokinetics of bosutinib, a dual Src/Abl tyrosine kinase inhibitor, when administered concomitantly to healthy subjects. Drug Metab. Pers. Ther. 2015;30:57–63. doi: 10.1515/dmdi-2014-0026. [DOI] [PubMed] [Google Scholar]
- 475.Wind S, et al. Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin. Pharmacokinet. 2019;58:1131–1147. doi: 10.1007/s40262-019-00766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Nezhadi S, Saadat E, Handali S, Dorkoosh F. Nanomedicine and chemotherapeutics drug delivery: challenges and opportunities. J. Drug. Target. 2021;29:185–198. doi: 10.1080/1061186X.2020.1808000. [DOI] [PubMed] [Google Scholar]
- 477.Fuse MJ, et al. Mechanisms of Resistance to NTRK Inhibitors and Therapeutic Strategies in NTRK1-Rearranged Cancers. Mol. Cancer Ther. 2017;16:2130–2143. doi: 10.1158/1535-7163.MCT-16-0909. [DOI] [PubMed] [Google Scholar]
- 478.Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum. Genomics. 2019;13:34. doi: 10.1186/s40246-019-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat. Rev. 2020;86:102019. doi: 10.1016/j.ctrv.2020.102019. [DOI] [PMC free article] [PubMed] [Google Scholar]