Abstract
EULAR highlighted the essential role of digital health in increasing self-management and improving clinical outcomes in patients with arthritis. The objective of this study was to evaluate the efficacy and safety of the digital health application (DHA) in patients with inflammatory arthritis. We assessed demographic parameters, treatment regimen, disease activity, and other patient-reported outcomes at baseline and after 4 weeks of DHA use added to standard care treatment. Of 17 patients, who completed the study, 7 (41.2%) patients were male, ranging from 19 to 63 (40.5 ± 12.2) years. No significant change in antirheumatic treatment was observed during the study. Statistically significant improvements (p < 0.05) were noted for health-related quality of life (increase in Physical Component Summary of Short Form-36 (SF-36) by 23.6%) and disease activity (decrease of Clinical Disease Activity Index and Simple Disease Activity Index by 38.4% and 39.9%, respectively). Clinically significant improvement was demonstrated for SF-36 Total Score (+ 14.4%), disease activity (Rheumatoid Arthritis Disease Activity Index− 5 to 15.9%), and depression (Patient Health Questionnaire− 9 to 13.5%). None of the efficacy parameters showed negative trends. No adverse events were reported throughout the study. The usability level was high i.e., the mean mHealth Application Usability Questionnaire Score of 5.96 (max.: 7.0) demonstrated a high level of application usability. This suggests that using a personalized disease management program based on DHA significantly improves several measures of patient-reported outcomes and disease activity in patients with inflammatory arthritis in a timely manner. These findings highlight the potential of complementary digital therapy in patients with inflammatory arthritis.
Keywords: Rheumatoid arthritis, Spondyloarthritis, mHealth, Mobile applications, Health-related quality of life, Healthy lifestyle
Introduction
Rheumatoid arthritis (RA) and spondyloarthritis (SpA) including psoriatic arthritis (PsA), often subsumed in the SpA spectrum, are the most common chronic arthritides associated with musculoskeletal inflammation, pain, potential reduced health-related quality of life (HRQoL) and physical function [1, 2].
Despite continuous advances in pharmacologic treatment, impairment of HRQoL in arthritis patients has remained substantial compared with the general population [3]. Non-pharmacologic therapy, such as psychological and physical therapy, lifestyle modification, including diet optimization and regular exercise, constitutes a complementary cornerstone of modern treatment. [4, 5].
Recently published recommendations by EULAR [6] highlight the importance of encouraging patient self-management strategies and the crucial role of digital formats [7]. As the large majority of patients with rheumatic and musculoskeletal diseases (RMDs) (91%) regularly uses smartphones [8], digital health application (DHA) represent a promising format to offer patients continuous, on-demand support in achieving disease remission. The COVID-19 pandemic lead to less face-to-face appointments [9], making it challenging for health care professionals (HCPs) to encourage and advise patients, regarding self-management, and an in turn increased usage of DHA [10]. Even though younger RMD patients show higher eHealth literacy [9], rheumatology mHealth options are not restricted to usage by young patients [11, 12].
Increasing the ability to access and share health information, DHA potentially empower patients to take a more active role in self-managing their well-being [13]. Applications addressing chronic conditions have already shown benefits for patients with different chronic conditions. Several DHA have demonstrated the ability to improve outcomes in patients with obesity, depression, and diabetes [7, 14]. Engaging patients with DHA has been proposed in several publications to change patient's health behaviors [15], enhance self-efficacy to manage symptoms [16], decrease health risk behaviors [17], and improve clinical outcomes [18].
Furthermore, DHA can contribute to improved self-efficacy, which in turn is one of the most important psychological factors that reflects patients’ confidence in disease management and was found as a strong predictor of self-management behaviors [19]. Importantly, a high level of self-efficacy also corresponds with lower levels of fatigue, pain, physical and psychological disability in patients with arthritis [20].
Currently, most available apps for RMD patients have not been rigorously evaluated [21, 22] and despite various patient needs [8, 23, 24], only encompass a limited number of functions, mainly symptom tracking using electronic patient-reported outcomes (ePROs). Regular digital tracking of RA symptoms using these tools has been associated with higher adherence, better-managed daily living activities [25] and to an improved patient-provider interaction by discussing information [26]. In contrast to rheumatic patients, rheumatologists show low involvement regarding DHA adoption [10, 27], as for example only a minority of rheumatologists is currently using ePROs and actively reviewing them [28]. Even if ePROs are actively monitored by HCPs, no significant benefit could be shown in a recent randomized controlled trial [29].
The Mida Rheuma App is a CE-certified DHA, to monitor disease burden and to treat patients with RA, SpA, and PsA to improve the patients’ disease activity and HRQoL. The Mida Rheuma App provides a personalized series of treatment action plans in the areas of dietary, mental health, lifestyle factors and physical activity, based on information and preferences that is tracked via a chatbot. The Mida Rheuma App was developed in accordance with recent EULAR guideline recommendations [21, 30, 31] and in close cooperation with patients and doctors. This continuous feedback-loop enables predictive, preventive, personalized and participatory (“P4”) medicine [32].
The objective of this study was to evaluate the efficacy and safety of the Mida Rheuma App added to the conventional treatment in patients with RA, SpA and PsA.
Methods
Study design
This explorative feasibility cross-sectional monocentric study was conducted at the Department of Biomedical Information, Medical Faculty Mannheim of the University Heidelberg between Mar 2021 and Oct 2021. The study was planned to recruit up to 20 subjects to ensure that a minimum of 16 subjects was evaluable for analysis. The sample size was determined according to minimal sample size requirements for pilot studies [33]. Inclusion criteria were the following: male or female participants between the ages of 18 and 65); written informed consent and diagnosis of RA, SpA or PsA, according to American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteria [34] or the Assessment of SpondyloArthritis international Society (ASAS) criteria for axial [35] or peripheral [36] SpA or Classification criteria for Psoriatic Arthritis (CASPAR) criteria [37]. Exclusion criteria were: the presence of conditions that make it impossible or dangerous to operate a display screen for more than 20 min and disorders that significantly complicate the work with their phone. Doses of arthritis-related medications were stable for at least 4 weeks before the screening.
The results of this study were used to inform the study design and sample size of a following randomized clinical trial. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the appropriate institutional review boards (Ethik-Kommission II der Universität Heidelberg Medizinische Fakultät Mannheimersity, protocol #2020-418 M-§23b, dated 26 Dec 2020). Written informed consent was obtained from all patients prior to the study's start.
Intervention
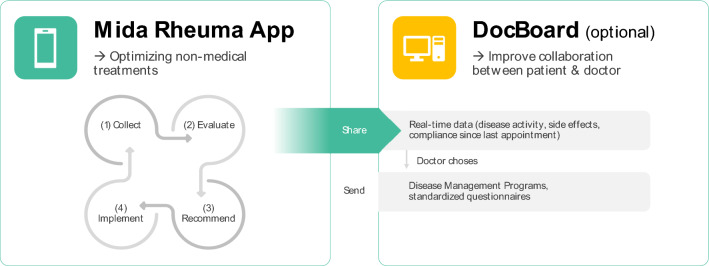
The Midaia Software is a CE-certified digital product, divided into a patient mobile application (Mida Rheuma App) for patients and a respective application (DocBoard Web-App) for the treating physician (see Fig. 1).
Fig. 1.
Midaia Software components
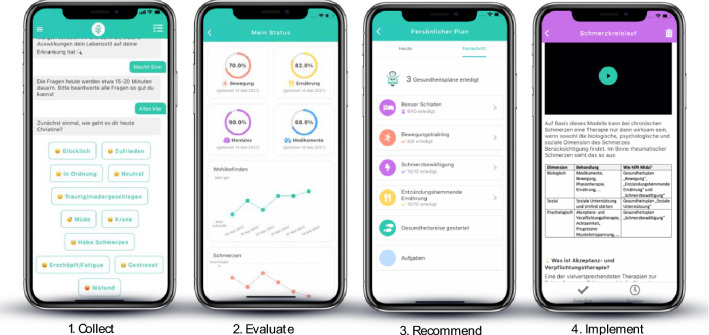
The Mida Rheuma App continuously provides patients with self-management action advice, based on their profile data. Figures 1 and 2 depict this continuous 4-step approach: (1) The conversational health coach Mida collects information on the patient’s disease, well-being, lifestyle factors, mental health, and medication using standardized questionnaires via the conversational health coach Mida.; (2) Based on the collected data, personal behaviors are evaluated, and a personal profile is built around the patient’s disease, well-being, and behavior; (3) This patient profile is cross-checked with recommendations from medical guidelines, medical standards, and state-of-the-art clinical research (Midaia algorithm); to (4) provide patients with personalized disease management action plans, accelerating positive behavior change to adjust the patient’s dietary habits, mental health, lifestyle factors, and physical activity.
Fig. 2.
Mida Rheuma App
Treatment action plans are divided into easy daily tasks, which help patients implement the recommendation into their daily life. Along with these main areas, the Mida Rheuma App also provides patients with programs related to pain and fatigue management, joint protection, motivation, self-efficacy and self-management, smoking cessation, and social areas.
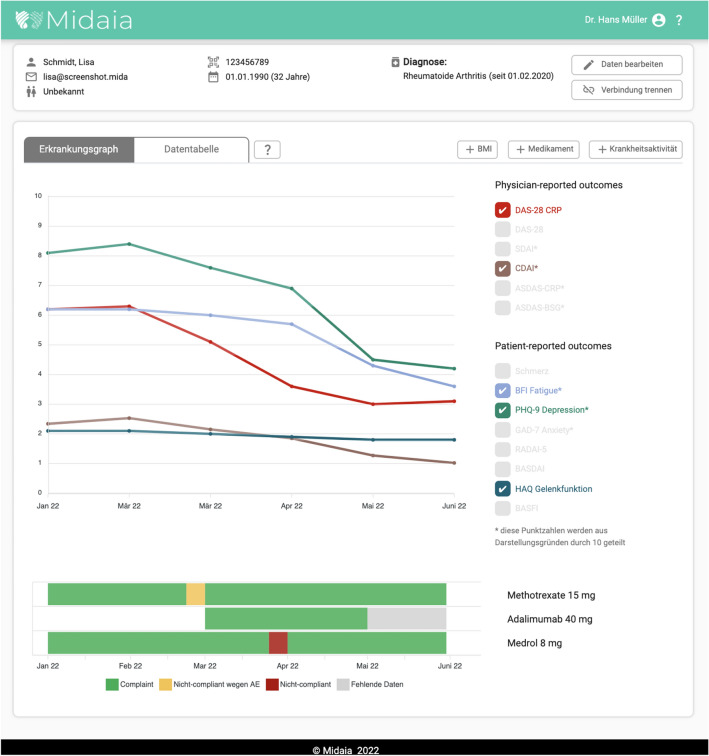
The Mida Rheuma App can be used as standalone software, but also allows data to be shared with via the DocBoard Web-App with treating physicians. The DocBoard Web-App (see Fig. 3) visualizes processed information, including the patients' condition, medication adherence and side effects. In addition, physicians can also add patient data (i.e., joint count, results of laboratory tests) to automatically calculate disease-specific scores and select disease management programs/action plans and standardized questionnaires for their patients in the Mida Rheuma App.
Fig. 3.
DocBoard Web-App
This study excluded the DocBoard Web-App and focused on the Mida Rheuma App.
Patients installed the freely available (App Store and Play Store) Mida Rheuma App using their own mobile phone. Throughout the study, the patient was allowed to complete 3 of 38 currently available action plans. Specific action plans from the list of possible ones were assigned via the Midaia algorithm, considering the patient's current needs at initial assessment. The duration of each action plan varied from 7 to 11 days and patients were reminded daily to fulfill the recommended tasks.
Quality of life assessment
HRQoL was measured by SF-36 Health Survey (SF-36) [38]. The SF-36 Total Score, Physical (PCS) and Mental Component Summary (MCS) scores as well as separate eight scales covering the dimensions of physical functioning, role limitations due to physical function, bodily pain, general health, mental health, role limitations due to emotional health, social functioning, and vitality were evaluated.
Other efficacy assessment
Disease activity was measured by Patient's Global Assessment of Disease Activity (PtGADA, scored from 0 to 100 mm), Patient’s Global Assessment of Pain Intensity (PPAIN, scored from 0 to 100 mm), Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5, scored from 0 to 10 cm, RA patients only) [39], Simple Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) [40]—for RA and PsA patients, and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI, scored from 0 to 10 cm) [41] and Ankylosing Spondylitis Disease Activity Score (ASDAS) [42]– for SpA/PsA patients with axial involvement. Body mass index (BMI), Health Assessment Questionnaire (HAQ) [43], Brief Fatigue Inventory (BFI) [44], and Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al. 2001) were used to evaluate obesity, physical impairment, fatigue, and depression, respectively.
Safety assessment
Clinical safety was addressed by assessing adverse (AEs) and serious adverse events (SAEs). Summaries (number and percentage of subjects) were provided.
Usability assessment
Usability was measured by 18 items MAUQ mHealth App Usability Questionnaire (Zhou et al. 2019) ranged from 1 (strongly agree) to 7 (strongly disagree).
Statistical analysis
IBM SPSS 22.0 software was used for statistical analysis. Demographic and baseline characteristics were summarized using standard descriptive statistics, including sample size, mean, standard deviation (SD), median, minimum, and maximum for continuous variables, and numbers and percentages for categorical variables. The difference between Day 1 and Day 34 was calculated and analyzed to identify if they were statistically significantly different. The data were first checked for a normal distribution using the “D’Agostino and Pearson test for normality”. When a normal distribution was established, the differences in the data sets were examined for their significance using the paired t-test. If no normal distribution of the data has been demonstrated, a nonparametric statistical test (Wilcoxon Test) was used. P-value ≤ 0.05 was considered statistically significant. Clinical significance was determined based on the Man-Son-Hing et al. guideline [45] which considers the relationship between the CI and the minimal clinically important difference (MCID and designated to one of the following: (1) Definite–the MCID is smaller than the lower limit of the CI of the treatment effect, (2) Probable–the MCID is greater than the lower limit of the CI of the treatment effect, but smaller than the treatment effect, (3) Possible–the MCID is less than the upper limit of the CI of the treatment effect, but greater than the treatment effect, and (4) Definitely Not–the MCID is greater than the upper limit of the CI of the treatment effect.
Results
Patients
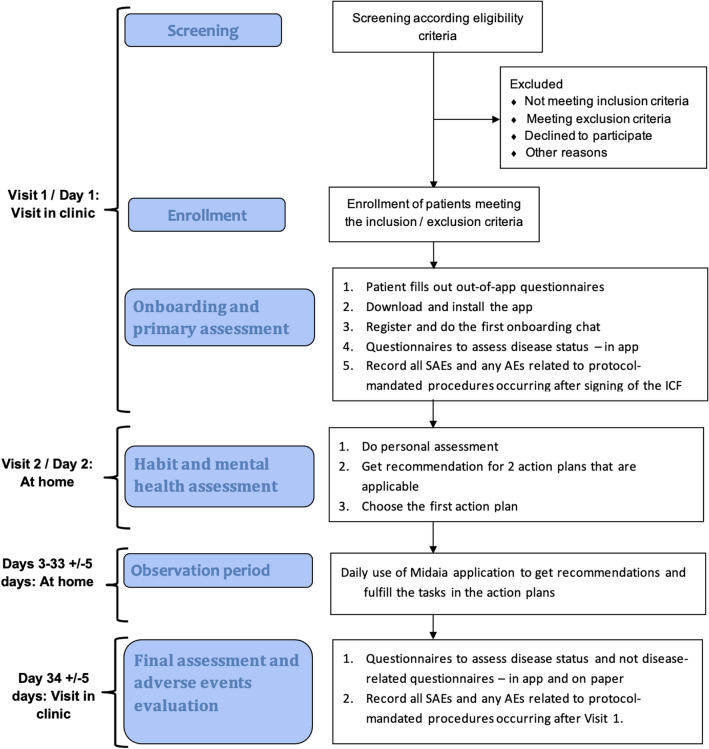
Of 20 patients screened, 19 were enrolled in the study and two patients did not complete the study, (Fig. 4), with a total of 17 patients (12 RA, SpA: 1 axSpA, 4 PsA) completing the study. 7 (41.2%) patients were male, ranging from 19 to 63 (40.5 ± 12.2) years. Patients were treated as follows: 7 non-steroidal anti-inflammatory drugs (41.2%), 2 glucocorticoids (> 5 mg) (11.8%), 3 hydroxychloroquine (17.6%), 10 methotrexate (58.8%), 1 leflunomide (5.9%), 1 sulfasalazine (5.9%), 1 apremilast (5.9%), 3 Janus kinase inhibitors (17.6%), 1 tumor necrosis factor inhibitors (5.9%), 2 IL-6 receptor inhibitors (11.8%), 1 IL-17 inhibitor (5.9%). No substantial intensification of antirheumatic treatment was observed during the study (Suppl. table).
Fig. 4.
Schematic flow chart of protocol
Quality of life
The results showed an improvement in patients’ HRQoL assessed by SF-36 at day 34 (Table 1). Statistically significant improvements were noted by an increase of the sub-scores Physical Component Summary (PCS) of 23.6% (p = 0.024), ‘role limitations due to physical health’–of 76.9% (p = 0.022), and ‘general health’ of 17.1% (p = 0.048). Analyses of clinical significance was based on the definition of 5.0 points as MCID in separate scores and 2.5 in SF-36 Total Score, PCS and MCS scores [46]. According to 95% CIs and MCID relation, PCS, 'role limitations due to physical health,' and 'general health' demonstrated probable clinical significance.
Table 1.
Changes in SF-36 of the patients between the visits *
| Day 1 (n = 17), Mean ± SD | Day 34 (n = 17), Mean ± SD | Difference day1 vs day34 (Mean ± SD, 95% CI) | 95% CI | 90% CI | Improvement, % | P- value day1 vs day34 | |
|---|---|---|---|---|---|---|---|
| SF-36 Total Score | 55.2 ± 20.8 | 63.2 ± 22.7 | − 7.96 ± 15.5 | − 15.9 to 0.006 | − 14.5 to 1.40b | 14.4 | p = 0.050 |
| PCS | 50.6 ± 20.8 | 62.6 ± 23.6 | − 11.9 ± 19.7 | − 22.1 to 1.81b | − 20.3 to 3.60b | 23.6 | p = 0.024 |
| MCS | 59.8 ± 25.0 | 63.8 ± 24.2 | − 4.0 ± 14.9 | − 11.6 to 3.71 | − 10.3 to 2.35 | 6.64 | p > 0.05 |
| Physical functioning | 69.3 ± 21.6 | 70.9 ± 21.3 | − 1.6 ± 11.6 | − 7.57 to 4.39 | − 6.51 to 3.34 | 2.3 | p > 0.05 |
| Role limitations due to physical health | 38.2 ± 41.2 | 67.6 ± 38.1 | − 29.4 ± 47.8 | − 54.0 to 4.84b | − 49.6 to 9.17b | 76.9 | p = 0.022 |
| Role limitations due to emotional problems | 70.6 ± 42.6 | 72.5 ± 40.0 | − 1.9 ± 34.3 | − 19.6 to 15.7 | − 16.5 to 12.6 | 2.78 | p > 0.05 |
| Energy/fatigue | 38.3 ± 21.9 | 44.4 ± 23.1 | − 6.1 ± 14.2 | − 13.4 to 1.24 | − 12.1 to 0.04b | 15.9 | p > 0.05 |
| Emotional well-being | 62.6 ± 20.4 | 66.7 ± 21.9 | − 4.1 ± 8.7 | − 8.61 to 0.30 | − 7.82 to 0.49c | 6.64 | p > 0.05 |
| Social functioning | 67.6 ± 31.5 | 71.3 ± 23.4 | − 3.7 ± 20.6 | − 14.3 to 6.92 | − 12.4 to 5.05 | 5.43 | p > 0.05 |
| Pain | 50.9 ± 23.6 | 60.1 ± 25.4 | − 9.2 ± 25.0 | − 22.1 to 3.60 | − 19.8 to 1.31 | 18.2 | p > 0.05 |
| General health | 44.1 ± 20.1 | 51.7 ± 20.1 | − 7.6 ± 14.5 | − 15.0 to 0.07b | − 13.7 to 1.39b | 17.1 | p = 0.048 |
Bold values indicate clinical and/or statistical significance
adistribution is normal for all data sets
bconfidence intervals exclude 0 and do not cross the MCID (2.5 for SF-36 Total Score, PCS and MCS, 5 for sub-scores, V. Strand, 2012)
cconfidence intervals exclude 0 but cross the MCID
PCS physical component summary, MCS mental component summary
For the SF-36 Total Score, emotional well-being’ and ‘energy/fatigue’ clinical significance was not demonstrated for 95% CI, but it was probable for 90% CI (Table 1).
Other efficacy endpoints
According to SDAI, at baseline, 29.4% of the RA and PsA patients were in remission, 25.2% had low, 29.4% had moderate, and none had high disease activity. The axSpA patient had low disease activity (ASDAS: 2.2). At the end of the study, the number of RA and PsA patients in remission and with low disease activity increased (58.8% and 23.5%, respectively), and the number of patients with moderate disease activity decreased accordingly (11.8%). The axSpA patient had inactive disease (ASDAS: 1.8). According to the p-value (CI 95%), CDAI and SDAI were decreased statistically significantly by 38.4% (p = 0.013) and 39.9% (p = 0.030), respectively.
For other efficacy endpoints, only positive trends without statistical significance were determined. According to other study results, changes of 1.7 points represent an MCID for PHQ-9 [47]; MCID for RADAI-5 was not identified. For PHQ-9 probable clinical importance was demonstrated only for 90% CI (Table 2).
Table 2.
Changes in parameters of the patients between the visits (parameters with normal distribution)
| Day 1, Mean ± SD | Day 34, Mean ± SD | Difference day1 vs day34 (Mean ± SD) | 95% CI | 90% CI | Improvement, % | P- value day1 vs day34 | |
|---|---|---|---|---|---|---|---|
| CDAI (n = 16) | 8.31 ± 7.41 | 5.12 ± 6.05 | 3.19 ± 4.50 | 0.79 to 5.58 | – | 38.4 | p = 0.013 |
| SDAI (n = 16) | 9.06 ± 7.73 | 5.44 ± 6.54 | 3.62 ± 6.05 | 0.40 to 6.85 | – | 39.9 | p = 0.030 |
| PtGADA (n = 17) | 37.8 ± 28.6 | 34.3 ± 25.2 | 3.47 ± 25.0 | − 9.39 to 16.3 | − 7.12 to − 14.1 | 9.18 | p > 0.05 |
| PPAIN (n = 17) | 35.2 ± 27.9 | 33.7 ± 23.1 | 1.5 ± 24.2 | − 10.9 to − 14.0 | − 8.75 to 11.8 | 4.34 | p > 0.05 |
| RADAI-5 (n = 12) | 3.77 ± 1.93 | 3.17 ± 1.98 | 0.60 ± 0.95 | − 0.0034 to 1.20 | 0.11 to 1.09# | 15.9 | P = 0.051 |
| BFI (n = 17) | 3.88 ± 2.69 | 3.86 ± 2.20 | 0.02 ± 1.62 | − 0.81 to 0.85 | − 0.66 to 0.70 | 0.49 | p > 0.05 |
| PHQ-9 (n = 17) | 7.41 ± 3.87 | 6.41 ± 2.61 | 1.00 ± 2.18 | − 0.12 to 2.12 | 0.08 to 1.92a | 13.5 | p > 0.05 |
Bold values indicate clinical and/or statistical significance
aconfidence intervals exclude 0 but cross the MCID (1.7 for PHQ-9, D. Kounali, 2020, not identified for RADAI-5)
SDAI simple disease activity index, CDAI clinical disease activity index, PtGADA patient’s global assessment of disease activity, PPAIN patient’s global assessment of pain intensity, RADAI5 rheumatoid arthritis disease activity index-5, BFI brief fatigue inventory, PHQ9 patient health questionnaire-9
None of the parameters used to assess the effectiveness showed negative trends (Table 1, 2, 3).
Table 3.
Changes in parameters of the patients between the visits (parameters with non-normal distribution)
| Day 1, Median (Min–Max) | Day 34, Median (Min–Max) | P-value Day1 vs Day34 | |
|---|---|---|---|
| BASDAI, n = 3 for both visits | 3.40 (2.40–5.70) | 2.60 (1.60–3.40) | NA |
| HAQ, n = 17 for both visits | 0.50 (0.00–1.50) | 0.62 (0.00–1.38) | p > 0.05 |
| BMI, n = 17 for both visits | 25.9 (21.0–42.8) | 25.6 (21.0–41.3) | p > 0.05 |
BASDAI Bath Ankylosing Spondylitis Disease Activity Index, HAQ Health Assessment Questionnaire, BMI body mass index, NA not applicable
Safety assessment
No adverse events were reported throughout the study.
Usability
The mean value of MAUQ (5.96 ± 0.85) demonstrated a high usability level. The highest ratings were for ‘easy to use’ and ‘easy to learn to use’ the DHA.—6.76 ± 0.44 and 5.82 ± 0.39, respectively. The most problematic rating was that it is not possible to use the DHA with low quality or complete absence of the Internet–4.13 ± 2.50.
Discussion
To our knowledge, this is the first study to show that a mobile application targeting dietary, mental health, lifestyle factors, and physical activity can improve HRQoL when added to usual care in patients with arthritis. After one month of the mobile application use, participants showed clinically and statistically significant improvement across two SF-36 domains (‘role limitations due to physical and 'general health’) and PCS. With a 90 percent confidence interval, SF-36 Total Score and 'emotional well-being' sub-score also demonstrated the clinically meaningful difference between the start and final study time points. Improvement in quality of life in our study is substantial given the insufficient effect of standard drug therapy. According to the German biologics register RABBIT, despite all medication opportunities, impairment of HRQoL (measured by SF-36) in RA patients has remained substantial compared with the general population. Independent of individual medication, only 30% of patients exceeded the PCS’s minimal detectable improvements (MDI), and 20% exceeded the MDI of the MCS. Furthermore, achieving MDI in PCS or MCS is associated with clinical improvement [3]..
A recent RCT reported clinically and statistically significant meaningful improvements in quality of life in patients with systemic lupus erythematosus using a similar app and additional tele-health coaching [48]. Even though study data on the impact of mobile applications on HRQoL are limited for this group of patients with autoimmune arthritis, it was suggested that the use of DHA potentially decreases disease activity and improve functional ability in patients with RA [16] and SpA [49]. It is also possible to improve pain, mood disturbance, and physical function using internet-based self-management strategies, mindfulness-based interventions, and cognitive behavioral therapies using digital products [20]. Potential opportunities for RA self-management mobile applications in reducing disease activity and improving health outcomes are also declared [50]. Among the endpoints we used to assess disease activity, CDAI and SDAI showed statistically significant improvement by 38.4% and 39.9%, respectively, and RADAI-5 demonstrated evidence of clinical importance. There were also clinically significant changes in the level of depression as measured by PHQ-9. This is important because depression is a major mental disorder common in patients with RA or SpA. At the same time, it is not only a psychological problem, since depression level is associated with poor HRQoL, adherence, and treatment response and higher disease activity and functional impairment [51, 52].
An exciting aspect of this study is the demonstrated ability to obtain positive changes with the short-term use of a mobile application which in part may be due to a high level of usability. These early positive results and continuous personalized feedback can help improve app adherence and thus overall performance. Symptoms reported by the patients are translated by the software algorithm to personalized interventions. It is essential that all Mida Rheuma App objectives aimed at helping patients in disease coping are implemented considering the latest EULAR recommendations, and the application development process took place following EULAR points to consider for developing, evaluating, and implementing mobile health applications (Table 4). Almost all recommendations of EULAR guidelines regarding non-drug interventions, including improvement of self-efficacy, were implemented both regarding the content and methods of providing information in the form of a personalized self-management program. Resistance exercises for RA and specific programs for axSpA patients in combination with cardiorespiratory aerobic exercises and a morning stiffness coping program are used in the application. They demonstrated significant improvement in most outcomes in previous studies [53–57]. Depending on the patient's condition, the application also recommends Yoga and Tai Chi, which have a positive impact on symptoms, physical function, disease activity, quality of life, balance, and muscle strength, according to study results [58–61]. Moreover, all exercise programs are divided into difficulty levels and are assigned in accordance with the patient's status. The basis of the effects in the mental component is mindfulness meditation and cognitive behavioral therapy (CBT). It has been proven that regular mindfulness meditation improves chronic pain, depression, and quality of life [62] and CBT could decrease anxiety, depression, and fatigue level in patients with chronic pain and RA [63]. The main aspects of the diet interventions were implementing a diet and weight control aiming for reducing inflammation. In this regard we also recommend action plans to reduce meat consumption and other foods that should be avoided in arthritis, as well as Buchinger fasting. The effectiveness of these recommended treatments has been shown to reduce inflammation, symptoms, and disease activity as well as improve physical functions with a positive effect on comorbidities [64–67].
Table 4.
Implementation of the main non-drug recommendations for patients with inflammatory arthritis in Mida Rheuma App
| Recommendation | Objective of the App | Implementation |
|---|---|---|
| 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases [68] | ||
| Overarching principles (1–5) | To reduce disease activity, improve HRQoL and physical function of patients | Fully implemented. All lifestyle recommendations complement medical treatment and depend on patients’ individual characteristics. Contact with the doctor is enabled |
| Exercise (1–7) | To improve the overall health of patients by optimizing physical activity | Implemented all except 6th (favouring group exercises). Both aerobic and strengthening exercises are recommended with special attention to patients with SpA |
| Diet (1–2) | To reduce inflammation by changing the patient's eating behavior | Fully implemented. A healthy, balanced diet is recommended as a separate program depending on patient needs |
| Alcohol (1–3; 4–NA, gout only) | To reduce inflammation by changing the patient's alcohol behavior | Fully implemented. Recommendations are based on explaining the impact of different doses of alcohol on the course of arthritis and providing advice for reducing the dose in patients who need it based on an assessment of individual alcohol consumption |
| Weight (1–2) | To improve the functional state of patients by normalizing weight for overweight patients | Fully implemented. Patients are involved in the process of achieving and maintaining a healthy weight using a specific program for this purpose, in addition to exercise and dietary advice |
| Smoking (1–2) | To improve disease activity by encouraging patients to stop smoking | Fully implemented. Patients are educated on the effects of smoking on their disease and symptoms. They are supported in stopping to smoke using a specific program for this purpose |
| Work (1) | To improve the overall health of patients by optimizing work activity | Fully implemented. Recommendations are based on the involvement of the patient in the work and ways to optimize it in accordance with the patient's condition |
| 2021 EULAR recommendations for the implementation of self-management strategies in patients with inflammatory arthritis [6] | ||
| Overarching principles (A-B; C–NA, for patient organisations only) | To improve self-efficacy of the patients by changing self-management behaviors | Fully implemented. Evaluation and recommendations related to self-efficacy are implemented as a separate program |
| Recommendations (2–3, 5–6, 8; 1, 4, 7, 9–NA, for healthcare professionals only) | Fully implemented. Evidence-based self-management program includes education, cognitive behavioural therapy, promotion of physical activity, and mental health recommendations | |
| EULAR recommendations for patient education for people with inflammatory arthritis [69] | ||
| Overarching principles (1–2) | To increase the efficiency and safety of patient treatment through additional education | Fully implemented. Education in the application is set up as an interactive learning process. Contact with the doctor is enabled |
| Recommendations (1–6; 7, 8–NA, for healthcare professionals only) | Fully implemented. Patients have access to evidence-based education throughout the course of their disease in a needs-based manner. The effectiveness of education is evaluated. Online interactions are enabled | |
| EULAR points to consider for the development, evaluation and implementation of mobile health applications aiding self-management in people living with rheumatic and musculoskeletal diseases [30] | ||
| Points to consider (1–8, 9–NA,10) | To optimize the process of developing and adapting the application, considering proven effective development approaches | Implemented all except 10th. The information content is up to date, scientifically justifiable, user acceptable and tailored to the individual needs of patients (1, 2). Design development and adaptation was carried out with the participation of patients with RA and SpA and rheumatologists taking into account adaptation for use by patients with impaired hand function (3, 8). All data regarding the developer and funding sources are open (4). Data collection adheres to all applicable regulatory frameworks, such as the European General Data Protection Regulation and the German Federal Data Protection Act (5). The content of the information for patients is aimed at motivating relatively long-term positive behaviour change and improving interaction with the attending physician, the risks and benefits of use are assessed, and actions are taken to minimize them (6, 7). Evaluation of cost–benefit balance (10) is planned |
NA not applicable
The study's main limitations are its pilot design, small sample size, lack of a control group, and short study duration (34 days), leading to bias and influencing the study results. A small study population decreased the ability to clearly define the relationship between results from our study sample and target population. This pilot study's design assumed the possibility of going through the three most important action plans (11 days as maximum for one action plan). Thus, the maximum duration of patient participation in the study was 34 days. The efficacy and safety evaluation period of the planned RCT will be 12 weeks—the standard for this population. Despite the similarity of non-pharmacological treatment in patients with inflammatory arthritis, the effect of the DHA in separate groups of patients with RA, PsA, and SpA requires additional precise evaluation, which will also be conducted in a planned RCT. Given the impact of the Mida Rheuma App on quality of life, a randomized controlled trial with a HRQoL measured using SF-36 as a primary endpoint is planned to confirm efficacy. The evaluation in planned RCT will be based on comparing the effects in the active and control groups.
Conclusion
In summary, usage of the complementary DHA led to significant improvements in quality of life and disease activity.in patients with autoimmune arthritis. Since the pilot design of our study does not allow for final conclusions, a planned randomized controlled trial is needed to confirm these preliminary positive results. Furthermore, rheumatologists and patients need to be educated about the growing number of supportive digital tools available.
Acknowledgements
We thank the patients and their families. We thank Abdullah Khalil for his technical support.
Author Contributions
DF, CP, and FL designed the digital tool, DF and JL developed the study protocol, JL and AB performed the study by recruiting patients, CW contributed to statistical analysis. DF wrote the first draft of the manuscript according to Good Publication Practice guidelines, and all authors commented on previous versions of the manuscript. All authors participated in analyzing and interpreting the data, reviewed and provided important intellectual input and feedback on the draft manuscript, and accepted the last version. All authors, including the statistician, vouch for the completeness and accuracy of the data and analyses. All authors had full access to the data and had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This study is supported by funds from by the Baden-Wuerttemberg Ministry of Science, Research and the Arts through the Center for Innovative Care (ZIV), Grant # 42.04HV.MED(18)/25/1.
Declarations
Conflict of interest
DF, FL, and CP are employees and have an equity interest in Midaia GmbH and have no other disclosures to make. Midaia GmbH developed a mobile application that was used in this study but did not finance this study. JK received financial support from ABATON GmbH, developer of software for rheumatic diseases. SK is founder and managing partner of MED.digital GmbH. He is member of the “Digitalization of health care “ and “Medical Education and University Medicine” committees of the German Medical Association. JL, AB, CW, BK declare that they have no conflict of interest. The investigator (JL) and his respective research team collected all the data. Statistical analyses were conducted by a biostatistician (CW) at Heidelberg University following a prespecified statistical analysis plan.
Ethical approval
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the appropriate institutional review boards (Ethics Committee of Medical Faculty Mannheim, Heidelberg University, protocol #2020-418 M-§23b, 26 Dec 2020). Authors fulfilled the ICMJE authorship criteria.
Informed consent
Written informed consent was obtained from all patients prior to study start.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dmytro Fedkov, Email: fedkovdmytro@gmail.com.
Andrea Berghofen, Email: a-berghofen@web.de.
Christel Weiss, Email: Christel.Weiss@medma.uni-heidelberg.de.
Christine Peine, Email: christine.peine@midaia.com.
Felix Lang, Email: felix.lang@midaia.com.
Johannes Knitza, Email: johannes.knitza@uk-erlangen.de.
Sebastian Kuhn, Email: sebastian.kuhn@uni-bielefeld.de.
Bernhard K. Krämer, Email: bernhard.kraemer@umm.de
Jan Leipe, Email: Jan.Leipe@umm.de.
References
- 1.Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–130. doi: 10.1016/j.semarthrit.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Strand V. Spondyloarthritis is associated with poor function and physical health-related quality of life. J Rheumatol. 2009;36(5):1012–1020. doi: 10.3899/jrheum.081015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhold K, Richter A, Schneider M, et al. Health-related quality of life in patients with long-standing rheumatoid arthritis in the era of biologics: data from the German biologics register RABBIT. Rheumatology (Oxford) 2015;54(10):1858–1866. doi: 10.1093/rheumatology/kev194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham NR, Kashikar-Zuck S. Nonpharmacological treatment of pain in rheumatic diseases and other musculoskeletal pain conditions. Curr Rheumatol Rep. 2013;15(2):306. doi: 10.1007/s11926-012-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary habits and nutrition in rheumatoid arthritis: can diet influence disease development and clinical manifestations. Nutrients. 2020 doi: 10.3390/nu12051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikiphorou E, Santos EJF, Marques A, et al. 2021 EULAR recommendations for the implementation of self-management strategies in patients with inflammatory arthritis. Ann Rheum Dis. 2021;80(10):1278–1285. doi: 10.1136/annrheumdis-2021-220249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Ho AF, Wiener RC, Sambamoorthi U. The association of mobile health applications with self-management behaviors among adults with chronic conditions in the United States. Int J Environ Res Public Health. 2021 doi: 10.3390/ijerph181910351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knitza J, Simon D, Lambrecht A, et al. Mobile health usage, preferences, barriers, and ehealth literacy in rheumatology: patient survey study. JMIR Mhealth Uhealth. 2020;8(8):e19661. doi: 10.2196/19661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejaco C, Alunno A, Bijlsma JW, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis: 2020 doi: 10.1136/annrheumdis-2020-218697. [DOI] [PubMed] [Google Scholar]
- 10.Kernder A, Morf H, Klemm P, et al. Digital rheumatology in the era of COVID-19: results of a national patient and physician survey. RMD Open. 2021 doi: 10.1136/rmdopen-2020-001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambrecht A, Vuillerme N, Raab C, et al. Quality of a supporting mobile app for rheumatic patients: patient-based assessment using the user version of the Mobile Application Scale (uMARS) Front Med (Lausanne) 2021;8:715345. doi: 10.3389/fmed.2021.715345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martini N, Broadbent E, Koo J, et al. Investigating the usability, efficacy and accuracy of a medication entering software system for a healthcare robot. Front Robot AI. 2022;9:814268. doi: 10.3389/frobt.2022.814268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradway M, Årsand E, Grøttland A. Mobile Health: empowering patients and driving change. Trends Endocrinol Metab. 2015;26(3):114–117. doi: 10.1016/j.tem.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Luo D, Wang P, Lu F, Elias J, Sparks JA, Lee YC. Mobile apps for individuals with rheumatoid arthritis: a systematic review. J Clin Rheumatol. 2019;25(3):133–141. doi: 10.1097/RHU.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Freeman B, Li M. Can mobile phone apps influence people's health behavior change? an evidence review. J Med Internet Res. 2016;18(11):e287. doi: 10.2196/jmir.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollard E, Michaud K. A Mobile app with optical imaging for the self-management of hand rheumatoid arthritis: pilot study. JMIR Mhealth Uhealth. 2018;6(10):e12221. doi: 10.2196/12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacoviello BM, Steinerman JR, Klein DB, Silver TL, Berger AG, Luo SX, Schork NJ. Clickotine, a personalized smartphone app for smoking cessation: initial evaluation. JMIR Mhealth Uhealth. 2017;5(4):e56. doi: 10.2196/mhealth.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc. 2017;24(3):619–632. doi: 10.1093/jamia/ocw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do V, Young L, Barnason S, Tran H. Relationships between activation level, knowledge, self-efficacy, and self-management behavior in heart failure patients discharged from rural hospitals. F1000 Res 4. 2015 doi: 10.12688/f1000research.6557.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiRenzo D, Finan P. Self-Efficacy and the Role of Non-Pharmacologic Treatment Strategies to Improve Pain and Affect in Arthritis. Curr Treatm Opt Rheumatol. 2019;5:168–178. doi: 10.1007/s40674-019-00123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knitza J, Tascilar K, Messner E-M, et al. German Mobile Apps in Rheumatology: Review and Analysis Using the Mobile Application Rating Scale (MARS) JMIR Mhealth Uhealth. 2019;7(8):e14991. doi: 10.2196/14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grainger R, Townsley H, White B, Langlotz T, Taylor WJ. Apps for People With Rheumatoid Arthritis to Monitor Their Disease Activity: A Review of Apps for Best Practice and Quality. JMIR Mhealth Uhealth. 2017;5(2):e7. doi: 10.2196/mhealth.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doumen M, Westhovens R, Pazmino S, et al. The ideal mHealth-application for rheumatoid arthritis: qualitative findings from stakeholder focus groups. BMC Musculoskelet Disord. 2021;22(1):746. doi: 10.1186/s12891-021-04624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chahal S, Biln N, Clarke B. Patient Perspectives on a Digital Mobile Health Application for RA. Open Access Rheumatol. 2021;13:63–71. doi: 10.2147/OARRR.S296541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Miedany Y, El Gaafary M, Youssef S, Bahlas S, Almedany S, Ahmed I, Palmer D. Toward electronic health recording: evaluation of electronic patient-reported outcome measures system for remote monitoring of early rheumatoid arthritis. J Rheumatol. 2016;43(12):2106–2112. doi: 10.3899/jrheum.151421. [DOI] [PubMed] [Google Scholar]
- 26.Shaw Y, Courvoisier DS, Scherer A, et al. Impact of assessing patient-reported outcomes with mobile apps on patient-provider interaction. RMD Open. 2021 doi: 10.1136/rmdopen-2021-001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knitza J, Vossen D, Geffken I, et al. Nutzung von Medizin-Apps und Online-Plattformen unter deutschen Rheumatologen : Ergebnisse der rheumadocs-Recherche und DGRh-Kongress-Umfragen von 2016 und 2018. Z Rheumatol. 2019;78(9):839–846. doi: 10.1007/s00393-018-0578-3. [DOI] [PubMed] [Google Scholar]
- 28.Krusche M, Klemm P, Grahammer M, et al. Acceptance, usage, and barriers of electronic patient-reported outcomes among german rheumatologists: survey study. JMIR Mhealth Uhealth. 2020;8(7):e18117. doi: 10.2196/18117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YC, Lu F, Colls J, et al. Outcomes of a mobile app to monitor patient-reported outcomes in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2021;73(8):1421–1429. doi: 10.1002/art.41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najm A, Nikiphorou E, Kostine M, et al. EULAR points to consider for the development, evaluation and implementation of mobile health applications aiding self-management in people living with rheumatic and musculoskeletal diseases. RMD Open. 2019;5(2):e001014. doi: 10.1136/rmdopen-2019-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knitza J, Callhoff J, Chehab G, et al. Positionspapier der Kommission Digitale Rheumatologie der Deutschen Gesellschaft für Rheumatologie e V Aufgaben, Ziele und Perspektiven für eine moderne Rheumatologie. Z Rheumatol. 2020;79(6):562–569. doi: 10.1007/s00393-020-00834-y. [DOI] [PubMed] [Google Scholar]
- 32.Flores M, Glusman G, Brogaard K, Price ND, Hood L. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med. 2013;10(6):565–576. doi: 10.2217/pme.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist. 2005;4(4):287–291. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 34.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 35.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 36.Rudwaleit M, van der Heijde D, Landewé R, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 37.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 38.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leeb BF, Haindl PM, Maktari A, Nothnagl T, Rintelen B. Patient-centered rheumatoid arthritis disease activity assessment by a modified RADAI. J Rheumatol. 2008;35(7):1294–1299. [PubMed] [Google Scholar]
- 40.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S100–S108. [PubMed] [Google Scholar]
- 41.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21(12):2286–2291. [PubMed] [Google Scholar]
- 42.van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(12):1811–1818. doi: 10.1136/ard.2008.100826. [DOI] [PubMed] [Google Scholar]
- 43.Ramey DR, Raynauld JP, Fries JF. The health assessment questionnaire 1992: status and review. Arthritis Care Res. 1992;5(3):119–129. doi: 10.1002/art.1790050303. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 45.Man-Son-Hing M, Laupacis A, O'Rourke K, Molnar FJ, Mahon J, Chan KBY, Wells G. Determination of the clinical importance of study results. J Gen Intern Med. 2002;17(6):469–476. doi: 10.1046/j.1525-1497.2002.11111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strand V, Rentz AM, Cifaldi MA, Chen N, Roy S, Revicki D. Health-related quality of life outcomes of adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol. 2012;39(1):63–72. doi: 10.3899/jrheum.101161. [DOI] [PubMed] [Google Scholar]
- 47.Kounali D, Button KS, Lewis G, et al. How much change is enough? Evidence from a longitudinal study on depression in UK primary care. Psychol Med. 2020 doi: 10.1017/S0033291720003700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan F, Granville N, Malkani R, Chathampally Y. Health-related quality of life improvements in systemic lupus erythematosus derived from a digital therapeutic plus tele-health coaching intervention: randomized controlled pilot trial. J Med Internet Res. 2020;22(10):e23868. doi: 10.2196/23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji X, Wang Y, Ma Y, et al. Improvement of disease management and cost effectiveness in chinese patients with ankylosing spondylitis using a smart-phone management system: a prospective cohort study. Biomed Res Int. 2019;2019:2171475. doi: 10.1155/2019/2171475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollard E, Michaud K. Self-management of rheumatoid arthritis: mobile applications. Curr Rheumatol Rep. 2020;23(1):2. doi: 10.1007/s11926-020-00968-7. [DOI] [PubMed] [Google Scholar]
- 51.Parkinson JT, Foley ÉM, Jadon DR, Khandaker GM. Depression in patients with spondyloarthritis: prevalence, incidence, risk factors, mechanisms and management. Ther Adv Musculoskelet Dis. 2020 doi: 10.1177/1759720X20970028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pankowski D, Wytrychiewicz-Pankowska K, Janowski K, Pisula E. Cognitive impairment in patients with rheumatoid arthritis: a systematic review and meta-analysis. Joint Bone Spine. 2021;89(3):105298. doi: 10.1016/j.jbspin.2021.105298. [DOI] [PubMed] [Google Scholar]
- 53.Baillet A, Vaillant M, Guinot M, Juvin R, Gaudin P. Efficacy of resistance exercises in rheumatoid arthritis: meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2012;51(3):519–527. doi: 10.1093/rheumatology/ker330. [DOI] [PubMed] [Google Scholar]
- 54.Baillet A, Zeboulon N, Gossec L, et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2010;62(7):984–992. doi: 10.1002/acr.20146. [DOI] [PubMed] [Google Scholar]
- 55.Verhoeven F, Guillot X, Prati C, Mougin F, Tordi N, Demougeot C, Wendling D. Aerobic exercise for axial spondyloarthritis—its effects on disease activity and function as compared to standard physiotherapy: A systematic review and meta-analysis. Int J Rheum Dis. 2019;22(2):234–241. doi: 10.1111/1756-185X.13385. [DOI] [PubMed] [Google Scholar]
- 56.Durmus D, Alayli G, Cil E, Canturk F. Effects of a home-based exercise program on quality of life, fatigue, and depression in patients with ankylosing spondylitis. Rheumatol Int. 2009;29(6):673–677. doi: 10.1007/s00296-008-0756-8. [DOI] [PubMed] [Google Scholar]
- 57.García MTF, Carmona L, Almodóvar R, et al. Recomendaciones para la prescripción de ejercicio físico en pacientes con espondiloartritis. Reumatol Clin (Engl Ed) 2019;15(2):77–83. doi: 10.1016/j.reuma.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Mudano AS, Tugwell P, Wells GA, Singh JA. Tai Chi for rheumatoid arthritis. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD004849.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moonaz SH, Bingham CO, Wissow L, Bartlett SJ. Yoga in sedentary adults with arthritis: effects of a randomized controlled pragmatic trial. J Rheumatol. 2015;42(7):1194–1202. doi: 10.3899/jrheum.141129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akyuz G, Kenis-Coskun O. The efficacy of tai chi and yoga in rheumatoid arthritis and Spondyloarthropathies: a narrative biomedical review. Rheumatol Int. 2018;38(3):321–330. doi: 10.1007/s00296-017-3867-2. [DOI] [PubMed] [Google Scholar]
- 61.Ye X, Chen Z, Shen Z, Chen G, Xu X. Yoga for treating rheumatoid arthritis: a systematic review and meta-analysis. Front Med (Lausanne) 2020;7:586665. doi: 10.3389/fmed.2020.586665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199–213. doi: 10.1007/s12160-016-9844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen B, Li Y, Du X, Chen H, Xu Y, Li H, Xu G-Y. Effects of cognitive behavioral therapy for patients with rheumatoid arthritis: a systematic review and meta-analysis. Psychol Health Med. 2020;25(10):1179–1191. doi: 10.1080/13548506.2020.1736312. [DOI] [PubMed] [Google Scholar]
- 64.Khanna S, Jaiswal KS, Gupta B. Managing rheumatoid arthritis with dietary interventions. Front Nutr. 2017;4:52. doi: 10.3389/fnut.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vadell AKE, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am J Clin Nutr. 2020;111(6):1203–1213. doi: 10.1093/ajcn/nqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parvez GMM, Akanda KM (2019) Foods and arthritis: an overview. In: Watson RR, Preedy VR (eds) Bioactive food as dietary interventions for arthritis and related inflammatory diseases (Second Edition). Academic Press, pp i–ii. 10.1016/B978-0-12-813820-5.09993-1
- 67.Deutsche Gesellschaft für Ernährung e.V (DGE) Heilfasten, Basenfasten Intervallfasten. https://www.dge.de/ernaehrungspraxis/diaeten-fasten/heilfasten/?L=0 Accessed 2 Jan. 2020
- 68.Gwinnutt JM, Wieczorek M, Balanescu A, et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2021-222020. [DOI] [PubMed] [Google Scholar]
- 69.Zangi HA, Ndosi M, Adams J, et al. EULAR recommendations for patient education for people with inflammatory arthritis. Ann Rheum Dis. 2015;74(6):954–962. doi: 10.1136/annrheumdis-2014-206807. [DOI] [PubMed] [Google Scholar]