Abstract
Introduction:
Prenatal exposure to organophosphorus pesticides (OPPs) has been associated with neurodevelopmental deficits in children, however evidence linking OPPs with specific cognitive mechanisms, such as executive function (EF), is limited.
Objective:
This study aims to evaluate the association between prenatal exposure to OPPs with multiple measures of EF in preschool-aged children, while considering the role of variant alleles in OPP metabolism genes.
Methods:
We included 262 children with preschool attention-deficit/hyperactivity disorder (ADHD), and 78 typically developing children, from the Preschool ADHD substudy of the Norwegian, Mother, Father, and Child Cohort Study. Participants who gave birth between 2004–2008 were invited to participate in an on-site clinical assessment when the child was approximately 3.5 years; measurements of EF included parent and teacher rating on Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P), and three performance-based assessments. We measured OPP metabolites in maternal urines collected at ~17 weeks’ gestation to calculate total dimethyl- (ΣDMP) and diethyl phosphate (ΣDEP) metabolite concentrations. We estimated multivariable adjusted β’s and 95% confidence intervals (CIs) corresponding to a change in z-score per unit increase in log-ΣDMP/DEP. We further characterized gene-OPP interactions for maternal variants in PON1 (Q192R, M55L), CYP1A2 (1548T>C), CYP1A1 (IntG>A) and CYP2A6 (−47A>C).
Results:
Prenatal OPP metabolite concentrations were associated with worse parent and teacher ratings of emotional control, inhibition, and working memory. A one log-∑DMP increase was associated with poorer teacher ratings of EF on the BRIEF-P (e.g. emotional control domain: β = 0.55, 95% CI: 0.35, 0.74), when weighted to account for sampling procedures. We found less consistent associations with performance-based EF assessments. We found some evidence of modification for PON1 Q192R and CYP2A6 −47A>C. Association with other variants were inconsistent.
Conclusions:
Biomarkers of prenatal OPP exposure were associated with more adverse teacher and parent ratings of EF in preschool-aged children.
Keywords: organophosphorus pesticide, executive function, preschool ADHD, prenatal exposure, MoBa
1. Introduction
Organophosphorus pesticides (OPPs) are a class of insecticides with widespread agricultural use throughout the world (Hertz-Picciotto et al., 2018a). OPPs irreversibly inhibit acetylcholinesterase causing nerve impulses to transmit indefinitely (Fukuto, 1990). OPPs also cause neurologic damage at lower doses (i.e. without overt symptoms) through oxidative stress and effects on proteins involved in fundamental neuronal processes (Slotkin and Seidler, 2007; Terry, 2012). Detoxification pathways include paraoxonase 1 (PON1) and the cytochrome P450 (CYP) superfamily of monooxygenases, which have common genetic variants that may modify expression and/or catalytic efficiency of their respective enzymes (Furlong, 2007; Kaur et al., 2017). Multiple OPPs are approved for use within the European Union/European Economic Community (EU/EEC), including chlorpyrifos (Bjørling-Poulsen et al., 2008). However globally, differences exist in regulations governing the use of OPPs, with some countries banning the use of multiple OPPs, while the same compounds may be permissible in others (Hertz-Picciotto et al., 2018b).
Multiple studies have demonstrated that consumption of conventionally-grown produce is an important route of exposure to OPPs (Oates et al., 2014; Papadopoulou et al., 2019; van den Dries et al., 2019). As such, human exposure may be affected by the import of agricultural products from countries where OPPs are approved for use. For example, a recent investigation of exposure in Norwegian women found that urinary concentrations of OPP metabolites were comparable to those measured in women residing in middle and southern Europe, despite the relatively limited use of OPPs in Norwegian agriculture (Haug et al., 2018), suggesting a role for imported foods as a mechanism of exposure for the Norwegian population (Ye et al., 2009).
Prenatal exposure to OPPs has been associated with a variety of neurodevelopmental deficits in children (Gonzalez-Alzaga et al., 2014), including reduced IQ (Bouchard et al., 2011; Coker et al., 2017; Engel et al., 2016, 2011; Gunier et al., 2017; Rauh et al., 2011, 2006; Rowe et al., 2016; Stein et al., 2018), developmental delay (Liu et al., 2016; Wang et al., 2020, 2017), impaired social responsivity (Furlong et al., 2014), and altered brain morphology (Rauh et al., 2012), microstructure (van den Dries et al., 2020), and activity (Binter et al., 2020). However, several studies have found no link between prenatal exposure and neurodevelopmental endpoints (Cartier et al., 2016; Donauer et al., 2016; Guo et al., 2019; van den Dries et al., 2019) or small and imprecise effects (Jusko et al., 2019; Ntantu Nkinsa et al., 2020). In particular, recent studies in the Generation R cohort in the Netherlands found that prenatal OPP exposure was not associated with children’s nonverbal IQ (Jusko et al., 2019) or traits of attention-deficit hyperactive disorder (ADHD) and autism spectrum disorders (ASD) (van den Dries et al., 2019). Because most studies used non-specific biomarkers of OPP exposure, dimethyl- and diethyl phosphate metabolites, the lack of harmony in these results may arise from differences in the type or extent of OPP parent compound exposure as well as the route of exposure. These differences have implications with regard to the amount of exposure to the parent compound relative to nontoxic preformed OPP metabolites (Lu et al., 2005; Quirós-Alcalá et al., 2012).
While multiple studies have found impacts of prenatal exposure to OPPs on general cognitive abilities (e.g. psychometric intelligence and mental development indices), there is little data examining more specific cognitive mechanisms. Executive function (EF) is an umbrella term for multiple categories of goal-directed, problem-solving behavior that emerge in the preschool period and continue to mature throughout childhood (Anderson, 2002). Research suggests there are three “core EFs”: 1) inhibition, or self-control 2) working memory (WM), the ability to register, maintain and manipulate information, and 3) cognitive flexibility, also called shifting; higher order EFs, such as decision-making, goal setting, and planning are built from upon these core skills (Diamond, 2013). Deficits in EF are common among children diagnosed with neurodevelopmental disorders, such as ASD and ADHD (Margari et al., 2016). However, deficiencies in EF may also be found among individuals without developmental disabilities (Otterman et al., 2019).
A small number of previous studies have included measurements of EF in later childhood or adolescence (Furlong et al., 2017; Sagiv et al., 2021, 2019), and have found relationships between OPP exposure and EF in both positive and negative directions. Studies measuring WM have generally found reduced scores with increasing OPP exposure (Bouchard et al., 2011; Furlong et al., 2017; Rauh et al., 2011; Rowe et al., 2016; Stein et al., 2016). Many of these studies were performed in settings with high community exposure (i.e., communities exposed due to agricultural drift or residential pesticide application), which may not be reflective of contemporary exposure patterns in the general population. In addition, no prior study has assessed the effects OPP exposure on measurements of EF taken during preschool, which are critical for future educational achievement (Diamond, 2016).
The aim of the current study is to evaluate the association of prenatal exposure to OPPs with EF in preschool-aged children, while considering the potentially modifying role of OPP metabolism variants. To address these aims, we leverage a well characterized subset of the Norwegian, Mother, Father, and Child Cohort (MoBa).
2. Materials and methods:
2.1. Study population
MoBa is a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (Magnus et al., 2016, 2006). Pregnant women across Norway were recruited between 1999 and 2008 (Schreuder and Alsaker, 2014). An invitation was sent to women before a routine prenatal ultrasound, which 98% of pregnant women complete before the 20th week of gestation (Backe, 1997). Following enrollment and informed consent for each pregnancy, MoBa included 114,500 children, 95,200 mothers and 75,200 fathers, representing 41% of the invited pregnant women. Participating mothers contributed biospecimens and completed questionnaires throughout pregnancy and provided updates on their child’s health and development longitudinally after birth (Paltiel et al., 2014). The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is now based on regulations related to the Norwegian Health Registry Act.
The study population is nested within a substudy of the MoBa cohort, called the Preschool ADHD Substudy (Overgaard et al., 2014), which oversampled children exhibiting possible ADHD symptoms on the 36-month questionnaire. Selection criteria for the included children has been previously described (Choi et al., 2021). Briefly, children born between 04/2004 and 01/2008 whose mothers reported a high summed scores (>90th percentile) of ADHD-like symptoms (N = 2,798), and a random sample of remaining children (N = 654), were invited to participate in an on-site clinical assessment of preschool ADHD. The enrollment groups were based on summed scores combining six items from the Child Behavior Checklist (Achenbach and Ruffle, 2000) and five items from the DSM-IV-TR criteria for ADHD (American Psychiatric Assocation, 2000). Of those invited, 1,195 children (35%) aged 3 to 4 years (mean = 3.5) took part in a 1-day clinical assessment, of which 870 had an available maternal prenatal urine sample for the measurement of OPP metabolites (sFigure 1). Mothers of children who participated were slightly older, more highly educated, and had fewer children than those who chose not to participate (Skogan et al., 2015).
The analytic sample for this current analysis includes two clinical groups: 1) children with above/subthreshold symptoms of preschool ADHD based on DSM-IV-TR criteria (N = 262) using the Preschool Age Psychiatric Assessment (PAPA) (Egger and Angold, 2004), and 2) children randomly sampled from the eligible population who were subsequently found to have no clinical/subclinical symptoms of ADHD using the PAPA, referred to as the typically developing group (N = 78). Further description of the diagnostic procedures for these children can be found in Kamai et al. (Kamai et al., 2021). Inverse sampling fractions (Richardson et al., 2007) are used to account for the oversampling of children symptomatic for preschool ADHD (further details in statistical analysis below).
2.2. Measurement of Executive Function
Parents and preschool teachers were asked to complete standardized inventories of child behavior before coming to the clinical assessment, which included neuropsychological tests and a semi-structured interview with the child. Methods and results from the preschool clinical assessment of neuropsychological functioning in MoBa have been previously described (Bendiksen et al., 2017; Biele et al., 2022; Overgaard et al., 2021, 2019, 2018b, 2018a, 2016, 2014; Rohrer-Baumgartner et al., 2016, 2016, 2014; Skogan et al., 2016, 2015). From parent and teacher assessments, we selected instruments related to EF (sTable 1). These included parent- and teacher ratings of the Behavior Rating Inventory of Executive Function - Preschool (BRIEF-P), subtests within the Stanford-Binet, 5th edition (SB-5), the A Developmental NEuro-PSYchological Assessment, 2nd edition (NEPSY-II), as well as the cookie delay task (CDT). Additional information on validation of Norwegian translations of the BRIEF-Preschool can be found in Skogan et al. (2016) (Skogan et al., 2016). Briefly, confirmatory factor analyses within the MoBa population supported the original 3-factor solution proposed by the BRIEF-P authors (Gioia et al., 2000). Additional information on neuropsychological testing can be found in Skogan et al. (2014) (Skogan et al., 2014). Briefly, tests were administered by a psychologist, or a trained graduate psychology student with special competence in child neuropsychology and supervised by a child psychologist or psychiatrist. All sessions were videotaped, and data from clinical assessments were reviewed by a child psychologist or psychiatrist and scored according to test algorithms. The SB-5 and NEPSY-II have been translated into Norwegian (Bayliss et al., 2005; Bull et al., 2008).
The BRIEF-P was developed to examine EF within the context of everyday environments in children aged 2–5 (Gioia et al., 1996). The 63-item instrument characterizes five domains of EF: emotional control, inhibition, WM, planning/organization, and shift (ability to move attention freely between tasks). Raters report whether a behavioral descriptor had been a problem for the child on a 3-point scales (never, sometimes, or often) during the past 6 months. The BRIEF-P was filled out by teachers and parents describing behavior in the school and home environment, respectively. For the analysis, we restricted to the emotional control, inhibition, and WM scales, because shift and plan/organize may not have reached a stable functional level of development at this age (Skogan et al., 2016). Raw scores were standardized by age and sex to calculate T scores.
Performance-based assessments were carried out by a psychologist with one parent present, as described previously (Rohrer-Baumgartner et al., 2014). The SB-5 (Roid and Pomplun, 2012) is a test battery used to measure nonverbal and verbal cognitive factors in all ages (2–85 years), including WM. Verbal WM was measured by asking participants to repeat sentences of increasing length. Nonverbal WM is measured with subtests: “delayed response”, where the child was asked to find an object hidden under one of three cups after a few seconds delay, and “block span”, where the child was asked to tap blocks in the same order as the administrator. NEPSY-II (Korkman et al., 1998) is a compendium of tests that examines attention/EF, language, sensorimotor, visuospatial, and memory/learning in children aged 3–12 years. A subtask measuring inhibition called the “Statue task”, where a child is asked to follow instructions to inhibit body movement, eye opening, and vocalization, under a series of increasing distractions. This test is believed to reflect poor inhibitory control and motor persistence (the ability to sustain an action in the absence of reinforcement). The CDT is an experimental task designed to evaluate children’s ability to delay a response to take a piece of cookie until an interval (of varying lengths) is up, signaled by the experimenter clapping their hands. Spearman correlation coefficients between measurements among our study participants are shown in sTable 2.
2.3. Measurement of OPP exposure
We estimated prenatal OPP exposure by measuring 3 dimethyl metabolites: dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), and 3 diethyl metabolites: diethyl phosphate (DEP), diethyl thiophosphate (DETP), and diethyl dithiophosphate (DEDTP), in maternal single spot urine samples collected at ~17 weeks’ gestation (sTable 3), using the ultra-performance liquid chromatography-time-of-flight system (UPLC-TOF) (Cequier et al., 2016). Laboratory quality control (QC) samples spiked at 5 and 50 ng/mL were included in each analytic batch, as well as 4–6 laboratory-blinded pooled QC samples. Average batch-specific coefficients of variation (CVs) for spiked QCs were generally between 5–8%. Average batch-specific CVs for laboratory blinded QCs were higher (9–20%), largely due to the lower mean concentrations among the pooled QC samples (means ranging between 0.4 ng/mL and 2.8 ng/mL, depending on the metabolite). Samples were randomly allocated across analytic batches. Specific gravity (SG) was measured using a pocket refractometer (PAL-10S) from Atago to account for urinary dilution. Metabolites could not be quantified in one participant with above/subthreshold preschool ADHD, who was excluded from the analysis. OPP metabolite concentrations were adjusted for SG (Boeniger et al., 1993) by standardizing all measurements by the geometric mean of the analytic population (OPPSG, i = OPPraw, i * (μSG / (SGi − 1)). Metabolite concentrations below the limit of detection (LOD) were imputed from a log-normal distribution bound by 0 and the LOD (Lubin et al., 2004). SG-adjusted concentrations of dimethyl- and diethyl metabolites were summed by molar weight to calculate total dimethyl- (ΣDMP) and total diethyl phosphate (ΣDEP) concentrations, and were subsequently log (natural) transformed. DEDTP was not included in ΣDEP because nearly 99% of values were below the LOD.
2.4. Measurement of Covariates
We examined factors that could influence prenatal OPP exposure or preschool-aged EF as potential covariates. Maternal characteristics at enrollment were obtained from the baseline questionnaire (completed at ~17 weeks gestation): marital status (married, cohabitating, single/other), education (less than college completed, college completed, more than college, other), parity (nulliparous vs. parous), self-reported depression before pregnancy (i.e. history of depression; yes vs. no), self-reported pre-pregnancy weight and height to calculate pre-pregnancy body mass index (BMI) (kg/m2), smoking and alcohol consumption during the first trimester of pregnancy (any vs. none). Financial problems in the previous 12 months (yes vs. no) was obtained from a questionnaire given at 30 weeks’ gestation. Maternal ADHD score were determined from the ADHD Self-Report Scale in the MoBa questionnaire completed when the child was 3 years old. Characteristics such as maternal age at birth, birth year, and child sex were obtained via data linkage with the Medical Birth Registry of Norway (MBRN) (Irgens, 2000), the national health registry containing information about all births in Norway.
Maternal dietary intake during pregnancy was captured using a food-frequency questionnaire completed by mothers at approximately 22 weeks’ gestation. For the current analysis, we used these dietary data to obtain information on frequency of fruit and raw vegetable consumption (servings/day) and choice of ecologically-grown, or “organic” produce (during pregnancy (seldom/never vs. sometimes/often/usually). In Norway, certification for labeling of organic produce went into effect in 2007 (https://debio.no/english/). Total fish intake (g/day) during pregnancy was also calculated from this questionnaire. To examine potential non-dietary sources of OPP exposure, we obtained information on mother’s self-reported contact with plant care substances (weedkiller, insecticides, and fungicides) in the six months before enrollment from the baseline questionnaire. Paternal use of plant care substances in the six months before wife became pregnant was obtained from the questionnaire administered to the father. We grouped dates of urine collection in June to August, September to November, December to February, and March to May, for seasons. Residence type (living on a farm vs. other) was additionally examined to look at potential agricultural exposure to OPPs and collected in the baseline questionnaire.
2.5. PON1 and CYP genotyping
To evaluate the role of variants in genes critical for OPP metabolism, we characterized single nucleotide polymorphisms (SNPs) in PON1 and CYP1A2, CYP1A1 and CYP2A6. DNA from maternal blood samples collected during pregnancy was extracted using FlexiGene, and candidate SNPs were measured using Sequenom IPLEX. We examined two SNPs in PON1: 1) rs662, “Q192R” and 2) rs854560, “M55L”, and three across CYP1A2, CYP1A1 and CYP2A6: 1) CYP1A2 rs2470890, “1548T>C”, 2) CYP1A1 rs4646421, “IntG>A” and 3) CYP2A6 rs28399433, “−47A>C” (sTable 4). PON1 variants examined are well-characterized (Dardiotis et al., 2019). Q192R is believed to affect substrate specificity, with the QQ genotype (“low-risk” allele) considered to have faster metabolism of toxic OPP forms (Garin et al., 1997) compared with QR/RR (“high-risk allele). 2) M55L is believe to reduce stability and enzyme concentrations (Humbert et al., 1993), although some studies have found conflicting evidence (Dardiotis et al., 2019); several studies have identified LL as the “high-risk” allele compared to MM/ML (“low-risk”). While CYP genes are also involved, less is known about the role of these variants in OPP metabolism; therefore, we examined a selection of SNPs in CYP genes with sufficient frequency of variants alleles in our study participants.
2.6. Statistical analysis
All outcome measures were converted to z-scores and standardized so higher scores correspond to worse (or more adverse) EF. We created a directed acyclic graph (DAG) to identify and select confounders (sFigure 2) (Hernan, 2002). A minimally sufficient set was identified using daggity (http://www.dagitty.net/). We also included a variable for child sex, which is highly associated with EF skills in preschool-aged children, and potential predictors of selection into the study, such as maternal parity, age, and education. For parsimony, we used backwards elimination to remove covariates that did not improve models fit (Weng et al., 2009), when examining the following response variables: 1) teacher and 2) parent ratings of emotional control on the BRIEF-P, and 3) non-verbal WM on the SB-5. Final models were adjusted for fruit consumption (servings/day), raw vegetable consumption (servings/day), age at childbirth, pre-pregnancy BMI, Maternal ADHD score, nulliparity, birth year, season of urine collection, and child sex.
We used multiple imputation with fully conditional specification for missingness in covariates and combined results using Rubin’s rules implemented in PROC MIANALYZE. The imputation model included all covariates, log-ΣDMP/DEP, and the teacher BRIEF-P scales. We used multiple linear regression to estimate β’s and 95% confidence intervals (CI) corresponding to a change in z-score per unit increase in log-ΣDMP/DEP. To account for the sampling procedures, analyses were weighted to the population eligible for the ADHD substudy using sampling fractions estimated separately for enrollment groups (children with high summed scores of ADHD-like symptoms and random sample of remaining children). More details on these procedures and results of a simulation study examining different approaches to weighting can be found in Choi et al. (Choi et al., 2021). To evaluate the potentially modifying role of SNPs in PON1 and CYP genes, we used regression models with interaction terms for the continuous number of variant alleles present (0,1,2) to estimate change in β per allele substitution (Δβ). We determined the presence of modification based on Wald tests (p-value <0.05) for this parameter. For PON1 genes, we used the genotype considered “low-risk” as the referent group, so Δβ’s correspond to change per “high-risk” allele. We performed sensitivity analyses removing weighting from models and stratifying by clinical group. All analyses were conducted using SAS 9.4 (Cary, NC).
3. Results:
Mothers of study participants had an average age at childbirth of 30 years, most were nulliparous (no previous pregnancies) and had a college degree or higher education (Table 1). The proportion of mothers of above/subthreshold preschool ADHD children who were married (44% vs. 54%) and had completed college (35% vs. 24%), were lower compared to mothers of typically developing children. Participating mothers reported a median of ~2.5 servings of fruit and ~0.5 servings of raw vegetables a day during pregnancy, with around one third choosing ecologically-grown fruits (31%) and vegetables (33%) sometimes or more often (compared to seldom/never). Self-reported use of plant care substances (weedkiller, insecticides, and fungicides) in the six months before pregnancy was reported by 4.6% mothers. The proportion of mothers living on a farm (vs. other residence type) was low (2.2%). The median age at clinical assessment for children was 3.5 years. There were slightly more girls than boys in above/subthreshold preschool ADHD children (56%) and typically developing children (54%).
Table 1.
Characteristics of the study population nested in the preschool ADHD study of the Norwegian Mother, Father and Child Cohort Study, 2004–2008
| Maternal characteristicsa | Above/subthreshold Preschool ADHD, n = 262 | Typically developing, n = 78 | Weightedb, n =340 | |||||
|---|---|---|---|---|---|---|---|---|
| Median or N | IQR or (%) | Median or N | IQR or (%) | Median or N | IQR or (%) | |||
| Age at childbirth (years) | 30 | 27–33 | 30 | 28–35 | 30 | 27–34 | ||
| Missing | 1 | |||||||
| Marital status | ||||||||
| Single/other | 15 | (5.7) | 3 | (3.8) | (3.4) | |||
| Cohabitating | 132 | (51) | 33 | (42) | (45) | |||
| Married | 114 | (44) | 42 | (54) | (51) | |||
| Missing | 1 | |||||||
| Education | ||||||||
| Less than college completed | 92 | (35) | 19 | (24) | (25) | |||
| College completed | 110 | (42) | 34 | (44) | (47) | |||
| More than college | 56 | (22) | 22 | (28) | (24) | |||
| Other | 3 | (1.1) | 3 | (3.8) | (3.1) | |||
| Missing | 1 | |||||||
| Financial problems in last 12 months, yes vs. no | 66 | (26) | 20 | (26) | (29) | |||
| Missing | 7 | 1 | ||||||
| Pre-pregnancy BMI (kg/m2) | 23.4 | 21.1–26.0 | 23.5 | 20.8–25.7 | 23.7 | 20.9–26.0 | ||
| Missing | 8 | 1 | ||||||
| Nulliparous, yes vs. no | 157 | (60) | 43 | (56) | (57) | |||
| Missing | 1 | |||||||
| Self-reported depression before pregnancy, yes vs. no | 28 | (11) | 7 | (9.0) | (8.7) | |||
| Maternal ADHD scorec | 1 | 0–3 | 1 | 0–2 | 1 | 0–2 | ||
| Missing | 3 | 1 | ||||||
| Smoking in first trimester, any vs. none | 61 | (23) | 16 | (20) | (25) | |||
| Missing | 1 | 1 | ||||||
| Alcohol consumption in first trimester, any vs. none | 32 | (13) | 8 | (11) | (10) | |||
| ` | Missing | 21 | 5 | |||||
| Fish consumption during pregnancy (g/day) | 24 | 14–36 | 27 | 17–36 | 26 | 17–37 | ||
| Missing | 4 | 3 | ||||||
| Factors associated with OPP exposure | ||||||||
| Contact with plant care substancesd, yes vs. no | 15 | (5.9) | 3 | (3.9) | (4.6) | |||
| Missing | 14 | 2 | ||||||
| Paternal contact with plant care substancese, yes vs. no | 12 | (5.3) | 10 | (14) | (12) | |||
| Missing | 36 | 7 | ||||||
| Living on a farm (vs. other residence type) | 7 | (2.7) | 2 | (2.6) | (2.2) | |||
| Missing | 2 | |||||||
| Fruit consumption during pregnancy (servings/day) | 2.5 | 1.0 –2.5 | 2.5 | 1.0 –2.5 | 2.5 | 1.0 –2.5 | ||
| Missing | 6 | 4 | ||||||
| Raw vegetable consumption during pregnancy (servings/day) | 0.5 | 0.2–0.8 | 0.5 | 0.2–1.0 | 0.5 | 0.2–1.0 | ||
| Missing | 6 | 4 | ||||||
| Use of ecologically-grown fruits during pregnancy (seldom/never vs. sometimes/often/usually)) | 69 | (27) | 25 | (33) | (31) | |||
| Missing | 9 | 3 | ||||||
| Use of ecologically-grown vegetables during pregnancy (seldom/never vs. sometimes/often/usually) | 93 | (37) | 27 | (36) | (33) | |||
| Missing | 10 | 3 | ||||||
| Season of urine collection | ||||||||
| Summer | 68 | (26) | 15 | (19) | (22) | |||
| Fall | 56 | (21) | 12 | (15) | (14) | |||
| Winter | 73 | (28) | 25 | (32) | (32) | |||
| Spring | 65 | (25) | 26 | (33) | (32) | |||
| Child characteristics | ||||||||
| Child age (month) | 41.7 | 40.7–42.4 | 41.6 | 40.6–42.4 | 41.5 | 40.6–42.4 | ||
| Missing | 2 | 1 | ||||||
| Child sex | ||||||||
| Boy | 116 | (44) | 36 | (46) | (43) | |||
| Girls | 146 | (56) | 42 | (54) | (57) | |||
| Child birth year | ||||||||
| 2004 | 26 | (9.9) | 20 | (26) | (22) | |||
| 2005 | 63 | (24) | 30 | (38) | (36) | |||
| 2006 | 90 | (34) | 23 | (29) | (33) | |||
| 2007 | 83 | (32) | 5 | (6.4) | (8.7) | |||
N; number Med; Median; ADHD, attention-deficit/hyperactive disorder; BMI, body mass index; IQR, interquartile range, OPP, organophosphorus pesticide
Maternal characteristics are at the time of enrollment (~15 weeks gestation) unless otherwise stated
To account for the sampling procedures, we performed analysis weighted to the population eligible for the ADHD substudy using sampling fractions. Proportions based on the weighted population.
Maternal ADHD score was from the ADHD Self-Report Scale in the MoBa questionnaire completed when the child was 3 years old.
Mothers were asked about contact weedkiller, insecticides, and fungicides during the six months since enrollment.
Fathers were asked about contact with weedkiller, insecticides, and fungicides in the six months before wife became pregnant.
OPP metabolites were frequently detected in urine samples collected at ~17 weeks’ gestation (sTable 3). DMTP and DEP were most frequently metabolites in both above/subthreshold preschool ADHD children (89% and 41%, respectively) and typically developing children (96% and 51%, respectively). There were no differences in metabolite concentrations between above/subthreshold preschool ADHD and typically developing children per the Wilcoxon ranked-sum test. The distribution of SG-adjusted ∑DMP and ∑DEP (summed by molar weight) is shown in Table 2.
Table 2.
Distribution of total dimethylphosphate and diethylphosphate metabolites in urines collected at ~17 weeks’ gestation
| OPP metabolite level (nmol/L) | N | Geometric Mean | Geometric SD | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ∑DMPa | ||||||||
| Above/subthreshold preschool ADHD | 261 | 5.76 | 1.63 | 3.1 | 25.6 | 56.7 | 113.9 | 1362 |
| Typically developing | 78 | 6.06 | 1.58 | 6.6 | 28.2 | 61.2 | 125.9 | 594 |
| Weightedb | 339 | 6.09 | 1.57 | 3.1 | 27.8 | 64.7 | 136.7 | 1362 |
| ∑DEPa | ||||||||
| Above/subthreshold preschool ADHD | 261 | 3.59 | 1.48 | 2.0 | 10.1 | 18.2 | 34.2 | 241 |
| Typically developing | 78 | 3.81 | 1.43 | 2.9 | 11.1 | 21.7 | 38.0 | 126 |
| Weightedb | 339 | 3.81 | 1.43 | 2.0 | 11.5 | 22.5 | 38.5 | 241 |
OPP, organophosphorous pesticide, DMP, dimethylphosphate, DEP, diethylphosphate; ADHD, attention-deficit/hyperactivity disorder; SD, standard deviation
Dimethyl and diethyl metabolites concentrations were adjusted for specific gravity (SG) by standardizing to the geometric mean. Concentrations below the limit of detection were imputed from a log-normal distribution. Metabolites were summed by molar weight (ΣDEP, ΣDMP). DEDTP was not included in ΣDEP as 99% of values were below the limit of detection. Samples were measured maternal spot urines collected at ~17 weeks’ gestation.
Weighted to the population eligible for the ADHD substudy using sampling fractions.
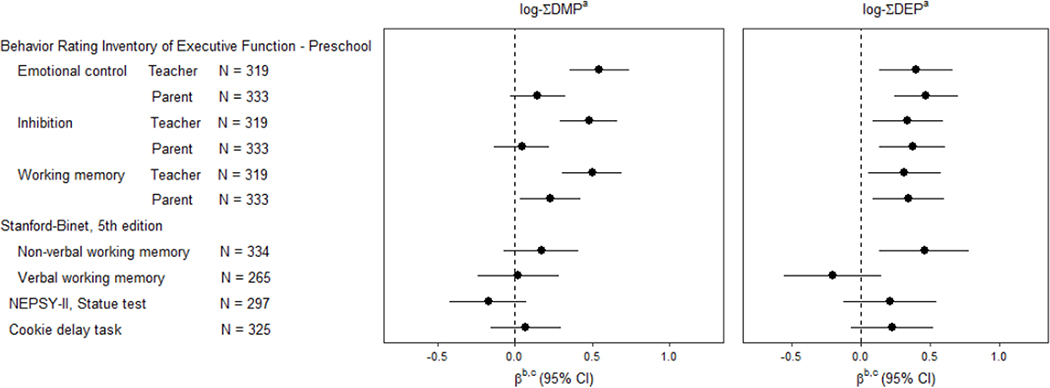
Higher levels of prenatal ∑DMP and ∑DEP was associated with worse (higher z-scores) preschool-aged measurements of EF, particularly parent and teacher ratings on the BRIEF-P, when weighted to account for the study’s sampling procedure (Figure 1; sTable 5). A one unit increase in log-∑DMP was associated with nearly a half z-score increase in teacher ratings of emotional control (β = 0.55, 95% CI: 0.35, 0.74), inhibition (β = 0.48, 95% CI: 0.29, 0.67), and working memory (β = 0.50, 95% CI: 0.30, 0.69). In addition, a one unit increase in log-∑DEP and was associated with worse parent and teacher ratings on the BRIEF-P, with the largest magnitude of association for parent-rated emotional control (β = 0.47, 95% CI: 0.24, 0.70). Associations between OPP metabolite molar sums and changes in BRIEF-P T-scores are shown in sTable 6, with magnitudes of association mostly ranging from 3 to 6 points per log-unit increase in exposure.
Figure 1. Association of log-∑DMP and log-∑DEP with preschool-aged measurements of executive function in regression analysis with weighting by sampling fractions.
CI, confidence interval; DMP, dimethylphosphate, DEP, diethylphosphate, NEPSY, A Developmental NEuro-PSYchological Assessment
a. Dimethyl- and diethyl phosphate metabolites concentrations were adjusted for specific gravity (SG) by standardizing to the geometric mean. Concentrations below the limit of detection were imputed from a log-normal distribution. Metabolites were summed by molar weight (∑DEP, ∑DMP), and log (natural) transformed. DEDTP was not included in ∑DEP as 99% of values were below the limit of detection. Samples were measured maternal spot urines collected at ~17 weeks’ gestation.
b. β for change in z-score per unit increase in log-∑DMP/DEP standardized so higher scores correspond to worse executive function. Weighted to the population eligible for the ADHD substudy using sampling fractions.
c. Adjusted for fruit consumption (servings/day), raw vegetable consumption (servings/day), age at childbirth, pre-pregnancy body mass index (kg/m2), maternal ADHD score, nulliparity, birth year, season of urine collection, and child sex.
Prenatal ∑DMP metabolites were not associated with performance-based assessments of EF (Figure 1, sTable 5), An association between log-∑DEP and poorer non-verbal WM on the SB-5 (β = 0.45, 95% CI: 0.13, 0.78) was observed, however associations with the remaining performance EF assessments were less consistent with this finding.
In sensitivity analyses, we removed weighting by sampling fraction (sTable 7), and stratified models according to clinical group (sTable 8). Associations between OPP metabolites and preschool-aged measurements of EF were attenuated in unweighted analyses, a population that overrepresents children with above/subthreshold preschool ADHD (sTable 7). The association between log-∑DMP and higher z-scores of teacher ratings on the BRIEF-P was reduced in magnitude by around half (e.g. emotion control domain: β = 0.27, 95% CI: 0.05, 0.50). When analyses were stratified by clinical group, some associations between ∑DMP/∑DEP and z-scores of preschool-aged EF measurements were stronger in typically developing children compared to those with above/subthreshold preschool ADHD (sTable 8).
To evaluate the potential modifying role of common variants in PON1, we estimated the change in the association between log-∑DMP and log-∑DEP and teacher ratings on the BRIEF-P, per Q192R and M55L substitution in maternal genotype (sTable 9). For Q192R, the adverse associations between log-∑DMP and multiple teacher ratings of EF among mothers with the QQ genotype was attenuated for those with QR/RR (e.g. Inhibit: Δβ per R substitution = −0.40, 95% CI: −0.75, −0.05), however we did not see patterns of modification of ∑DEP by Q192R. No consistent patterns of association were seen for M55L substitutions in PON1.
We similarly evaluated SNPs in CYP1A2, CYP1A1, and CYP2A6 (sTable 10). The most consistent evidence of effect measure modification was found for the −47A>C allele in CYP2A6. Specifically, the associations of log-∑DEP with poorer teacher ratings of emotional control, inhibition and WM, were attenuated for mothers with C alleles (e.g. emotional control: Δβ per C allele = −0.80, 95% CI: −1.38, −0.22), although there was less consistency for ∑DMP. Among the other CYP genotypes measured, patterns of effect measure modification varied and were not consistently statistically significant.
4. Discussion:
Leveraging a comprehensive, on-site preschool-aged neuropsychological assessment, we examined the relationship between prenatal OPP exposure and preschool-aged EF in a well-characterized subset of the MoBa study. We found that ∑DMP and ∑DEP biomarkers of OPP exposure were associated with higher z-scores of teacher and parent ratings of EF, indicating that elevated prenatal exposure levels are related to poorer EF in the child. For example, a one log-∑DMP increase was associated with poorer teacher ratings of EF on the BRIEF-P, corresponding to ~ 3 to 6 points on the original BRIEF-P scale (T-scores). Increasing ∑DEP was also associated with poorer teacher and parent ratings on the BRIEF-P. No consistent pattern between ∑DMP and performance-based assessments was found, however we observed an association between ∑DEP and worse non-verbal WM on the SB-5. In unweighted analyses, the associations between ∑DMP and ∑DEP and BRIEF-P ratings were attenuated, and when stratifying by clinical group, we found stronger adverse associations in typically developing children compared to children with clinically significant or subthreshold preschool ADHD. We evaluated the potential modifying role of variant alleles and found some evidence of significant modification for Q192R in PON1 and −47A>C in CYP2A6, however patterns of association with other variants were not consistent.
The overall results of this study are in agreement with the majority of the previous OPP literature, which finds deficits in neurodevelopment associated with increasing exposure (Gonzalez-Alzaga et al., 2014). However, there are few papers that have focused specifically on EF, and among them, inconsistent results. A 2017 study by Furlong et al. (Furlong et al., 2017) in an urban birth cohort in New York (Mount Sinai cohort), found that higher concentrations of ∑DMP in third trimester urine samples was associated with an improved “EF factor”, comprised primarily of parent-reported BRIEF domains among children 6–9 years of age. Sagiv et al. (2021) leveraged multiple rater and performance-based assessments of EF in the agricultural CHAMACOS cohort (Sagiv et al., 2021), finding associations between DMP and DEP metabolites and poorer teacher and parent ratings on the BRIEF between 7–12 years of age. In this study, DMP and DEP were most strongly associated with the mother’s BRIEF ratings, compared to maternal reporting on the Connors ADHD/DSM-IV (CADS) and Behavior Assessment System for Children, 2nd ed (BASC-2). Our study results are more closely aligned with those of Sagiv et al. (2021), although our study participants likely received much lower and more indirect exposure to OPPs, primarily via dietary intake of conventionally grown fruits and vegetables (Ye et al., 2009). However, comparisons of DMP and DEP biomarker-based associations across studies is complicated in populations with differing routes of OPP exposure, and thus differing profiles of exposure to parent compounds. It should be noted that DMP and DEP metabolites are non-specific biomarkers of multiple parent compounds whose toxicities vary widely (Sudakin and Stone, 2011). As such, differences in associations across studies may in part relate to differences in the composition of parent compounds to which any specific population is exposed. Direct exposure to pesticides through residential insecticide applications, occupational exposures, or secondary to agricultural drift, is likely to result in a higher magnitude of exposure to the parent compound, and relatively less exposure to preformed DMP and DEP metabolites (Quirós-Alcalá et al., 2012; Sudakin and Stone, 2011). However dietary exposure to OPPs is likely to a mix of parent compound and preformed metabolites (Lu et al., 2005), the latter of which are non-toxic and indistinguishable from parent-compound exposure when measuring diethyl and dimethylphosphate metabolites in urine.
Although we found consistent adverse associations with parent and teacher rated EF domains, we found less consistent evidence of association with performance-based measures. Previous research has shown limited correlation between EF assessments as measured by rater-based and performance measures, indicating that different underlying cognitive constructs are being tapped by these tools (Isquith et al., 2013, 2005; Toplak et al., 2013). We also found limited correlations across performance and rater-based methods (sTable 2). In the preschool period, performance-based assessments of EF may be less precise than in older children, since EFs are not fully developed until adult age. In addition, performance tasks are administered under ideal experimental conditions, in an environment that is highly structured, and with the goals of the assessment clearly defined (Toplak et al., 2013). In contrast, ratings-based assessments ask parents and teachers to reflect on usual behaviors in a typical environment (school or home) that may vary in terms of structure. Therefore, the lack of overlap in our findings is not entirely unexpected.
Metabolism of OPPs is a two-step process where parent compounds are activated to their toxic “oxon” form by CYP genes, then detoxified primarily through hydrolysis by PON1 (Furlong, 2007; Kaur et al., 2017). Variants (i.e. polymorphisms) in these genes may alter their enzymatic activity resulting in exposure to toxic metabolites forms. PON1 has several well characterized variants that affect enzymatic activity and/or serum concentrations, including Q192R and M55L (Dardiotis et al., 2019). Prior studies evaluating modification of OPP associations by Q192R have produced mixed findings. Engel et al. (2011) found that relationships between prenatal DMP and DEP and the mental development index (MDI) of the Bayley Scales of Infant Development-II (BSID-II) measured at 12 months were stronger for mothers with 192QR and RR genotypes, considered slow metabolizers (i.e. “high-risk”), particularly among Black and Hispanic mother-child pairs, but found stronger relationships between prenatal DMP and perceptual reasoning at 6–9 years old for those with the QQ genotype (Engel et al., 2011), who are expected to have faster metabolism and clearance of toxic OPP forms. A pooled analysis of four U.S. birth cohorts additionally found more adverse associations between DEP and the MDI of the BSID at 24 months among mothers with the QQ genotype (Engel et al., 2016). Wang et al. similarly found stronger relationships between DEP and several domains on the Gesell Developmental Scales at 12 months for children of mothers with the QQ genotype in Shandong province, China (Wang et al., 2020). We found some evidence that adverse associations between ∑DMP and teacher ratings of preschool-aged EF were strongest among mothers with the QQ genotype, but no consistent patterns for ∑DEP. Several studies in US cohorts (Eskenazi et al., 2014; Millenson et al., 2017) and a study in Generation R (Jusko et al., 2019) which have assessed neurodevelopment later in childhood (ages 5–9 years) reported no evidence of modification by Q192R. Only one prior study of prenatal OPP exposure has evaluated the M55L, which is believed to reduce stability and enzyme concentrations of PON1; Jusko et al. (2019) found no evidence of modification by M55L for the associations of DMP and DEP with nonverbal IQ measured at 6 years in Generation R (Jusko et al., 2019). Overall, the literature on PON1-mediated modification is inconclusive, potentially due to the lack of specificity in the exposure biomarker, and thus their utility as a marker of susceptibility to OPPs may be limited (Dardiotis et al., 2019).
Despite the role of CYPs in OPP metabolism (Kaur et al., 2017), there has been less attention paid to the potential for common variants to modify the association of prenatal OPP exposure on neurodevelopment. Individual CYPs have different affinities for parent OPP compounds, which change depending on concentration (reviewed in Kaur et al., 2017). While multiple CYPs are involved in OPP metabolism, CYP3A4 has been most consistently demonstrated as a major metabolizer of OPPs (Croom et al., 2010; Kaur et al., 2017). Our study participants lacked sufficient variability in variant frequency to evaluate the IntG>A change in CYP3A4. As such, we evaluated the potential modifying role of SNPs in CYP genes involved in xenobiotic metabolism with sufficient variant frequencies in population. While no patterns were seen for 1548T>C in CYP1A2 and IntG>A in CYP1A1, we found attenuated associations for −47A>C allele changes in CYP2A6, which is believed reduce its enzymatic activity. However, we could find no other published studies that have linked this allele change in CYP2A6 to metabolism of OPPs. Our study extends this literature by examining variants in multiple SNPs in CYP genes for their potential to modify associations to chronic, low dose exposure OPPs during pregnancy and preschool-aged EF.
Strength of this study include the use of a comprehensive battery of EF tests, with concurrent parent and teacher ratings and performance-based assessments, to better characterize the nature of any associations with EF outcomes. Consistency in associations across parent and teacher ratings provides further evidence of the reliability of our findings. This study was nested within the well-characterized MoBa cohort, which collected detailed covariate information on child and maternal characteristics, including dietary patterns during pregnancy. These data allowed us to account for important confounders, like dietary intake of fruits and vegetables, and maternal symptoms of ADHD, which have not been considered in a number of previous investigations. While EFs vary in the general population, children with ADHD tend to have lower scores across a range of EF-related outcomes as compared to typically developing children (Barkley, 1997). Thus, over-sampling children with non-normative EF may have selected for a uniquely susceptible population. Contrary to our expectations, DMP and DEPs were somewhat more strongly associated with EF in typically developing children, which may indicate that other pathways are more prominently related to EF in the setting of ADHD, including heritable pathways. Nonetheless, our design allowed us to explore these questions while accounting for the oversampling of those symptomatic for preschool ADHD. Finally, there has been limited research focused on cognitive effects in the preschool period, however identifying deficits in this period can have important consequences for the child. Previous research has demonstrated the utility of interventions for EF at this age (Sasser et al., 2017; Traverso et al., 2015), with improvements being critical for educational success later in life (Jacobson et al., 2011; Willoughby et al., 2012).
Our study also has several limitations. We were limited to a single spot urine collection at mid-pregnancy to assess prenatal exposure to OPPs. OPPs are rapidly metabolized and a single measurement during pregnancy may not adequately reflect patterns of exposure over the entire period (Spaan et al., 2015). It is possible that study sample, which leveraged an on-site standardized assessment of Preschool ADHD nested within the large and well characterized MoBa cohort, is impacted by self-selection in relation to factors that influenced a mother’s willingness to bring her child in for a long assessment. We attempted to address this by using selection weighing approaches, and included in our multivariable adjusted covariates that may additionally predict participation in the clinical exam, such as maternal education, age, parity and maternal symptoms of ADHD. Finally, while we used a standardized clinical inventory of psychiatric symptoms validated for the preschool period to ascertain ADHD symptoms relative to DSM-IV criteria, this assessment is not equivalent to a diagnostic interview, which would include multiple sources of information and informants for a clinical diagnosis of ADHD. A previous study by Overgaard et al. (2021) in the MoBA, found that these criteria collected at ~3 years identified around half of children with persistent elevation of ADHD symptoms approximately 2 years later (Overgaard et al. 2021).
5. Conclusions
In a study nested in a Norwegian, population-based birth cohort, we found that higher prenatal concentrations of OPP metabolites were associated with worse EF, such as emotional control, inhibition, and working, in preschool-aged children, measured using parent and teacher ratings on the BRIEF-P. OPP usage has reduced in recent decades due to regulations in the Europe, the US, and across the globe (Hertz-Picciotto et al., 2018b). As such, levels of exposure measured among women in this current study may be higher than exposure in pregnant women today. Nonetheless, dietary exposure to OPPs remains widespread even in countries with limited OPP usage, such as Norway, due to importation of agricultural products. Our study adds to the evidence that argues for more aggressive global regulation of OPPs in order to limit exposure during pregnancy.
Supplementary Material
Highlights.
We examined prenatal organophosphate pesticide exposure and child executive functions.
Higher prenatal OPP exposure was associated with worse teacher and parent rated EF.
Associations of OPP with performance assessments of EF were less consistent.
There was some evidence for modification by Q192R in PON1 and −47A>C in CYP2A6.
Acknowledgements:
The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. We are grateful to all the participating families in Norway who take part in this on-going cohort study. We thank the Norwegian Institute of Public Health (NIPH) for generating high-quality genomic data. This research is part of the HARVEST collaboration, supported by the Research Council of Norway (#229624). We also thank the NORMENT Centre for providing genotype data, funded by the Research Council of Norway (#223273), South East Norway Health Authority and KG Jebsen Stiftelsen. We further thank the Center for Diabetes Research, the University of Bergen for providing genotype data and performing quality control and imputation of the data funded by the ERC AdG project SELECTionPREDISPOSED, Stiftelsen Kristian Gerhard Jebsen, Trond Mohn Foundation, the Research Council of Norway, the Novo Nordisk Foundation, the University of Bergen, and the Western Norway health Authorities (Helse Vest).
Funding:
The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. We are grateful to all the participating families in Norway who take part in this on-going cohort study. JT, KR, GC, CM were supported in part by a training grant from the National Institute of Environmental Health Sciences [T32ES007018]. AR was supported by F32ES031832. This research was funded in part by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) R01ES021777, P30 ES010126, and by the Intramural Research Program of the NIH/NIEHS. The Norwegian Mother, Father and Child (MoBa) Controls Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (no. N01-ES-75538), NIH/National Institute of Neurological Disorders and Stroke (NINDS) (no. 1 UO1 NS 047537-01 and no. 2 UO1 NS 047537-06A1).
Footnotes
Ethics:
The current study is based on version 9 of the quality-assured data files released for research in preschool ADHD. The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is currently regulated by the Norwegian Health Registry. The current study was approved by the Regional Committee for Medical Research Ethics in Norway and reviewed and determined to be exempt from further review by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill.
References:
- Achenbach TM, Ruffle TM, 2000. The Child Behavior Checklist and Related Forms for Assessing Behavioral/Emotional Problems and Competencies. Pediatrics in Review 21, 265–271. 10.1542/pir.21-8-265 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Assocation, 2000. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). American Psychiatric Assocation. Washington, DC. [Google Scholar]
- Anderson P, 2002. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8, 71–82. 10.1076/chin.8.2.71.8724 [DOI] [PubMed] [Google Scholar]
- Backe B, 1997. [Routine ultrasonography in obstetric care in Norway, 1994]. Tidsskr Nor Laegeforen 117, 2314–2315. [PubMed] [Google Scholar]
- Barkley RA, 1997. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121, 65–94. 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Bayliss DM, Jarrold C, Baddeley AD, Gunn DM, 2005. The relationship between short-term memory and working memory: complex span made simple? Memory 13, 414–421. 10.1080/09658210344000332 [DOI] [PubMed] [Google Scholar]
- Bendiksen B, Svensson E, Aase H, Reichborn-Kjennerud T, Friis S, Myhre AM, Zeiner P, 2017. Co-Occurrence of ODD and CD in Preschool Children With Symptoms of ADHD. J Atten Disord 21, 741–752. 10.1177/1087054714538655 [DOI] [PubMed] [Google Scholar]
- Biele G, Overgaard KR, Friis S, Zeiner P, Aase H, 2022. Cognitive, emotional, and social functioning of preschoolers with attention deficit hyperactivity problems. BMC Psychiatry 22, 78. 10.1186/s12888-021-03638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binter AC, Bannier E, Saint-Amour D, Simon G, Barillot C, Monfort C, Cordier S, Pelé F, Chevrier C, 2020. Exposure of pregnant women to organophosphate insecticides and child motor inhibition at the age of 10–12 years evaluated by fMRI. Environmental Research 188, 109859. 10.1016/j.envres.2020.109859 [DOI] [PubMed] [Google Scholar]
- Bjørling-Poulsen M, Andersen HR, Grandjean P, 2008. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health 7, 50. 10.1186/1476-069X-7-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J, 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54, 615–627. 10.1080/15298669391355134 [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B, 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119, 1189–1195. 10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R, Espy KA, Wiebe SA, 2008. Short-Term Memory, Working Memory, and Executive Functioning in Preschoolers: Longitudinal Predictors of Mathematical Achievement at Age 7 Years. Developmental Neuropsychology 33, 205–228. 10.1080/87565640801982312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier C, Warembourg C, Le Maner-Idrissi G, Lacroix A, Rouget F, Monfort C, Limon G, Durand G, Saint-Amour D, Cordier S, Chevrier C, 2016. Organophosphate Insecticide Metabolites in Prenatal and Childhood Urine Samples and Intelligence Scores at 6 Years of Age: Results from the Mother–Child PELAGIE Cohort (France). Environmental Health Perspectives 124, 674–680. 10.1289/ehp.1409472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cequier E, Sakhi AK, Haug LS, Thomsen C, 2016. Development of an ion-pair liquid chromatography–high resolution mass spectrometry method for determination of organophosphate pesticide metabolites in large-scale biomonitoring studies. Journal of Chromatography A 1454, 32–41. 10.1016/j.chroma.2016.05.067 [DOI] [PubMed] [Google Scholar]
- Choi G, Villanger GD, Drover SSM, Sakhi AK, Thomsen C, Nethery RC, Zeiner P, Knudsen GP, Reichborn-Kjennerud T, Øvergaard KR, Herring AH, Skogan AH, Biele G, Aase H, Engel SM, 2021. Prenatal phthalate exposures and executive function in preschool children. Environment International 149, 106403. 10.1016/j.envint.2021.106403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E, Gunier R, Bradman A, Harley K, Kogut K, Molitor J, Eskenazi B, 2017. Association between Pesticide Profiles Used on Agricultural Fields near Maternal Residences during Pregnancy and IQ at Age 7 Years. Int J Environ Res Public Health 14. 10.3390/ijerph14050506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croom EL, Wallace AD, Hodgson E, 2010. Human variation in CYP-specific chlorpyrifos metabolism. Toxicology 276, 184–191. 10.1016/j.tox.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Dardiotis E, Aloizou A-M, Siokas V, Tsouris Z, Rikos D, Marogianni C, Aschner M, Kovatsi L, Bogdanos DP, Tsatsakis A, 2019. Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology 411, 24–31. 10.1016/j.tox.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Diamond A, 2016. Why improving and assessing executive functions early in life is critical., in: Griffin JA, McCardle P, Freund LS (Eds.), Executive Function in Preschool-Age Children: Integrating Measurement, Neurodevelopment, and Translational Research. American Psychological Association, Washington, pp. 11–43. [Google Scholar]
- Diamond A, 2013. Executive Functions. Annu. Rev. Psychol. 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donauer S, Altaye M, Xu Y, Sucharew H, Succop P, Calafat AM, Khoury JC, Lanphear B, Yolton K, 2016. An Observational Study to Evaluate Associations Between Low-Level Gestational Exposure to Organophosphate Pesticides and Cognition During Early Childhood. Am J Epidemiol 184, 410–418. 10.1093/aje/kwv447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A, 2004. The Preschool Age Psychiatric Assessment (PAPA): A Structured Parent Interview for Diagnosing Psychiatric Disorders in Preschool Children., in: Handbook of Infant, Toddler, and Preschool Mental Health Assessment. Oxford University Press. New York, NY. Pp. 223–243. [Google Scholar]
- Engel SM, Bradman A, Wolff MS, Rauh VA, Harley KG, Yang JH, Hoepner LA, Barr DB, Yolton K, Vedar MG, Xu Y, Hornung RW, Wetmur JG, Chen J, Holland NT, Perera FP, Whyatt RM, Lanphear BP, Eskenazi B, 2016. Prenatal Organophosphorus Pesticide Exposure and Child Neurodevelopment at 24 Months: An Analysis of Four Birth Cohorts. Environ Health Perspect 124, 822–830. 10.1289/ehp.1409474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS, 2011. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 119, 1182–1188. 10.1289/ehp.1003183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Kogut K, Huen K, Harley KG, Bouchard M, Bradman A, Boyd-Barr D, Johnson C, Holland N, 2014. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environmental Research 134, 149–157. 10.1016/j.envres.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto TR, 1990. Mechanism of Action of Organophosphorus and Carbamate Insecticides. Environmental Health Perspectives 87, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, 2007. Genetic variability in the cytochrome P450–paraoxonase 1 (PON1) pathway for detoxication of organophosphorus compounds. J. Biochem. Mol. Toxicol. 21, 197–205. 10.1002/jbt.20181 [DOI] [PubMed] [Google Scholar]
- Furlong MA, Engel SM, Barr DB, Wolff MS, 2014. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ Int 70, 125–131. 10.1016/j.envint.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong MA, Herring A, Buckley JP, Goldman BD, Daniels JL, Engel LS, Wolff MS, Chen J, Wetmur J, Barr DB, Engel SM, 2017. Prenatal exposure to organophosphorus pesticides and childhood neurodevelopmental phenotypes. Environmental Research 158, 737–747. 10.1016/j.envres.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin MC, James RW, Dussoix P, Blanché H, Passa P, Froguel P, Ruiz J, 1997. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J. Clin. Invest. 99, 62–66. 10.1172/JCI119134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L, 2000. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychology 6, 235–238. 10.1076/chin.6.3.235.3152 [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L, 1996. Behavior rating inventory of executive function:-preschool version (BRIEF-P) Psychological Assessment Resources. Odessa, FL. [Google Scholar]
- Gonzalez-Alzaga B, Lacasana M, Aguilar-Garduno C, Rodriguez-Barranco M, Ballester F, Rebagliato M, Hernandez AF, 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett 230, 104–121. 10.1016/j.toxlet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B, 2017. Prenatal Residential Proximity to Agricultural Pesticide Use and IQ in 7-Year-Old Children. Environ Health Perspect 125, 057002. 10.1289/EHP504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zhang J, Wu C, Lv S, Lu D, Qi X, Jiang S, Feng C, Yu H, Liang W, Chang X, Zhang Y, Xu H, Cao Y, Wang G, Zhou Z, 2019. Associations of prenatal and childhood chlorpyrifos exposure with Neurodevelopment of 3-year-old children. Environmental Pollution 251, 538–546. 10.1016/j.envpol.2019.05.040 [DOI] [PubMed] [Google Scholar]
- Haug LS, Sakhi AK, Cequier E, Casas M, Maitre L, Basagana X, Andrusaityte S, Chalkiadaki G, Chatzi L, Coen M, Bont J. de, Dedele A, Ferrand J, Grazuleviciene R, Gonzalez JR, Gutzkow KB, Keun H, McEachan R, Meltzer HM, Petraviciene I, Robinson O, Saulnier P-J, Slama R, Sunyer J, Urquiza J, Vafeiadi M, Wright J, Vrijheid M, Thomsen C, 2018. In-utero and childhood chemical exposome in six European mother-child cohorts. Environment International 121, 751–763. 10.1016/j.envint.2018.09.056 [DOI] [PubMed] [Google Scholar]
- Hernan MA, 2002. Causal Knowledge as a Prerequisite for Confounding Evaluation: An Application to Birth Defects Epidemiology. American Journal of Epidemiology 155, 176–184. 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Sass JB, Engel S, Bennett DH, Bradman A, Eskenazi B, Lanphear B, Whyatt R, 2018a. Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms. PLoS Med 15, e1002671. 10.1371/journal.pmed.1002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Sass JB, Engel S, Bennett DH, Bradman A, Eskenazi B, Lanphear B, Whyatt R, 2018b. Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms. PLoS Med 15, e1002671. 10.1371/journal.pmed.1002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE, 1993. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet 3, 73–76. 10.1038/ng0193-73 [DOI] [PubMed] [Google Scholar]
- Irgens LM, 2000. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years 5. [PubMed] [Google Scholar]
- Isquith PK, Crawford JS, Espy KA, Gioia GA, 2005. Assessment of executive function in preschool-aged children. Ment. Retard. Dev. Disabil. Res. Rev. 11, 209–215. 10.1002/mrdd.20075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isquith PK, Roth RM, Gioia G, 2013. Contribution of Rating Scales to the Assessment of Executive Functions. Applied Neuropsychology: Child 2, 125–132. 10.1080/21622965.2013.748389 [DOI] [PubMed] [Google Scholar]
- Jacobson LA, Williford AP, Pianta RC, 2011. The role of executive function in children’s competent adjustment to middle school. Child Neuropsychology 17, 255–280. 10.1080/09297049.2010.535654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, van den Dries MA, Pronk A, Shaw PA, Guxens M, Spaan S, Jaddoe VW, Tiemeier H, Longnecker MP, 2019. Organophosphate Pesticide Metabolite Concentrations in Urine during Pregnancy and Offspring Nonverbal IQ at Age 6 Years. Environ Health Perspect 127, 017007. 10.1289/EHP3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai EM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SSM, Hoppin JA, Knudsen GP, Reichborn-Kjennerud T, Zeiner P, Overgaard K, Herring AH, Aase H, Engel SM, 2021. Gestational Phthalate Exposure and Preschool Attention Deficit Hyperactivity Disorder in Norway. Environmental Epidemiology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Jain AK, Singh S, 2017. CYP/PON genetic variations as determinant of organophosphate pesticides toxicity. J Genet 96, 187–201. 10.1007/s12041-017-0741-7 [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S, 1998. NEPSY: A Developmental neuropsychological assessment manual. The Psychological Corporation, San Antonio: TX. [Google Scholar]
- Liu P, Wu C, Chang X, Qi X, Zheng M, Zhou Z, 2016. Adverse Associations of both Prenatal and Postnatal Exposure to Organophosphorous Pesticides with Infant Neurodevelopment in an Agricultural Area of Jiangsu Province, China. Environ Health Perspect 124, 1637–1643. 10.1289/EHP196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Bravo R, Caltabiano LM, Irish RM, Weerasekera G, Barr DB, 2005. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. J Toxicol Environ Health A 68, 209–227. 10.1080/15287390590890554 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environmental Health Perspectives 112, 1691–1696. 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, Handal M, Haugen M, Høiseth G, Knudsen GP, Paltiel L, Schreuder P, Tambs K, Vold L, Stoltenberg C, 2016. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 45, 382–388. 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, 2006. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 35, 1146–1150. 10.1093/ije/dyl170 [DOI] [PubMed] [Google Scholar]
- Margari L, Craig F, Margari F, Legrottaglie A, Palumbi R, De Giambattista C, 2016. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. NDT 1191. 10.2147/NDT.S104620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenson ME, Braun JM, Calafat AM, Barr DB, Huang Y-T, Chen A, Lanphear BP, Yolton K, 2017. Urinary organophosphate insecticide metabolite concentrations during pregnancy and children’s interpersonal, communication, repetitive, and stereotypic behaviors at 8 years of age: The home study. Environ Res 157, 9–16. 10.1016/j.envres.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntantu Nkinsa P, Muckle G, Ayotte P, Lanphear BP, Arbuckle TE, Fraser WD, Bouchard MF, 2020. Organophosphate pesticides exposure during fetal development and IQ scores in 3 and 4-year old Canadian children. Environmental Research 190, 110023. 10.1016/j.envres.2020.110023 [DOI] [PubMed] [Google Scholar]
- Oates L, Cohen M, Braun L, Schembri A, Taskova R, 2014. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environmental Research 132, 105–111. 10.1016/j.envres.2014.03.021 [DOI] [PubMed] [Google Scholar]
- Otterman DL, Koopman-Verhoeff ME, White TJ, Tiemeier H, Bolhuis K, Jansen PW, 2019. Executive functioning and neurodevelopmental disorders in early childhood: a prospective population-based study. Child Adolesc Psychiatry Ment Health 13, 38. 10.1186/s13034-019-0299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard KR, Aase H, Torgersen S, Reichborn-Kjennerud T, Oerbeck B, Myhre A, Zeiner P, 2014. Continuity in features of anxiety and attention deficit/hyperactivity disorder in young preschool children. Eur Child Adolesc Psychiatry 23, 743–752. 10.1007/s00787-014-0538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard KR, Aase H, Torgersen S, Zeiner P, 2016. Co-Occurrence of ADHD and Anxiety in Preschool Children. J Atten Disord 20, 573–580. 10.1177/1087054712463063 [DOI] [PubMed] [Google Scholar]
- Overgaard KR, Oerbeck B, Aase H, Torgersen S, Reichborn-Kjennerud T, Zeiner P, 2018a. Emotional Lability in Preschoolers With Symptoms of ADHD. J Atten Disord 22, 787–795. 10.1177/1087054715576342 [DOI] [PubMed] [Google Scholar]
- Overgaard KR, Oerbeck B, Friis S, Biele G, Pripp AH, Aase H, Zeiner P, 2019. Screening with an ADHD-specific rating scale in preschoolers: A cross-cultural comparison of the Early Childhood Inventory-4. Psychol Assess 31, 985–994. 10.1037/pas0000722 [DOI] [PubMed] [Google Scholar]
- Overgaard KR, Oerbeck B, Friis S, Pripp AH, Aase H, Zeiner P, 2021. Predictive validity of attention-deficit/hyperactivity disorder from ages 3 to 5 Years. Eur Child Adolesc Psychiatry. 10.1007/s00787-021-01750-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard KR, Oerbeck B, Friis S, Pripp AH, Biele G, Aase H, Zeiner P, 2018b. Attention-Deficit/Hyperactivity Disorder in Preschoolers: The Accuracy of a Short Screener. J Am Acad Child Adolesc Psychiatry 57, 428–435. 10.1016/j.jaac.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Paltiel L, Anita H, Skjerden T, Harbak K, Bækken S, Nina Kristin S, Knudsen GP, Magnus P, 2014. The biobank of the Norwegian Mother and Child Cohort Study – present status. Nor J Epidemiol 24. 10.5324/nje.v24i1-2.1755 [DOI] [Google Scholar]
- Papadopoulou E, Haug LS, Sakhi AK, Andrusaityte S, Basagaña X, Brantsaeter AL, Casas M, Fernández-Barrés S, Grazuleviciene R, Knutsen HK, Maitre L, Meltzer HM, McEachan RRC, Roumeliotaki T, Slama R, Vafeiadi M, Wright J, Vrijheid M, Thomsen C, Chatzi L, 2019. Diet as a Source of Exposure to Environmental Contaminants for Pregnant Women and Children from Six European Countries. Environ Health Perspect 127, 107005. 10.1289/EHP5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós-Alcalá L, Bradman A, Smith K, Weerasekera G, Odetokun M, Barr DB, Nishioka M, Castorina R, Hubbard AE, Nicas M, Hammond SK, McKone TE, Eskenazi B, 2012. Organophosphorous pesticide breakdown products in house dust and children’s urine. J Expo Sci Environ Epidemiol 22, 559–568. 10.1038/jes.2012.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R, 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect 119, 1196–1201. 10.1289/ehp.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW, 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118, e1845–1859. 10.1542/peds.2006-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS, 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci U S A 109, 7871–7876. 10.1073/pnas.1203396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, Weiland SK, 2007. Analyses of case-control data for additional outcomes. Epidemiology 18, 441–445. 10.1097/EDE.0b013e318060d25c [DOI] [PubMed] [Google Scholar]
- Rohrer-Baumgartner N, Zeiner P, Eadie P, Egeland J, Gustavson K, Reichborn-Kjennerud T, Aase H, 2016. Language Delay in 3-Year-Old Children With ADHD Symptoms. J Atten Disord 20, 867–878. 10.1177/1087054713497253 [DOI] [PubMed] [Google Scholar]
- Rohrer-Baumgartner N, Zeiner P, Egeland J, Gustavson K, Skogan A, Reichborn-Kjennerud T, Aase H, 2014. Does IQ influence Associations between ADHD Symptoms and other Cognitive Functions in young Preschoolers? Behav Brain Funct 10, 16. 10.1186/1744-9081-10-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH, Pomplun M, 2012. The stanford-binet intelligence scales. The Guilford Press. New York, NY. [Google Scholar]
- Rowe C, Gunier R, Bradman A, Harley KG, Kogut K, Parra K, Eskenazi B, 2016. Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ Res 150, 128–137. 10.1016/j.envres.2016.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Bruno JL, Baker JM, Palzes V, Kogut K, Rauch S, Gunier R, Mora AM, Reiss AL, Eskenazi B, 2019. Prenatal exposure to organophosphate pesticides and functional neuroimaging in adolescents living in proximity to pesticide application. Proc Natl Acad Sci U S A 116, 18347–18356. 10.1073/pnas.1903940116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Harley K, Bradman A, Morga N, Eskenazi B, 2021. Gestational exposure to organophosphate pesticides and longitudinally assessed behaviors related to ADHD and executive function. American Journal of Epidemiology kwab173. 10.1093/aje/kwab173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser TR, Bierman KL, Heinrichs B, Nix RL, 2017. Preschool Intervention Can Promote Sustained Growth in the Executive-Function Skills of Children Exhibiting Early Deficits. Psychol Sci 28, 1719–1730. 10.1177/0956797617711640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder P, Alsaker E, 2014. The Norwegian Mother and Child Cohort Study (MoBa) – MoBa recruitment and logistics. Nor J Epidemiol 24. 10.5324/nje.v24i1-2.1754 [DOI] [Google Scholar]
- Skogan AH, Egeland J, Zeiner P, Øvergaard KR, Oerbeck B, Reichborn-Kjennerud T, Aase H, 2016. Factor structure of the Behavior Rating Inventory of Executive Functions (BRIEF-P) at age three years. Child Neuropsychology 22, 472–492. 10.1080/09297049.2014.992401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogan AH, Zeiner P, Egeland J, Rohrer-Baumgartner N, Urnes A-G, Reichborn-Kjennerud T, Aase H, 2014. Inhibition and working memory in young preschool children with symptoms of ADHD and/or oppositional-defiant disorder. Child Neuropsychology 20, 607–624. 10.1080/09297049.2013.838213 [DOI] [PubMed] [Google Scholar]
- Skogan AH, Zeiner P, Egeland J, Urnes A-G, Reichborn-Kjennerud T, Aase H, 2015. Parent ratings of executive function in young preschool children with symptoms of attention-deficit/-hyperactivity disorder. Behav Brain Funct 11, 16. 10.1186/s12993-015-0060-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, 2007. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull 72, 232–274. 10.1016/j.brainresbull.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan S, Pronk A, Koch HM, Jusko TA, Jaddoe VWV, Shaw PA, Tiemeier HM, Hofman A, Pierik FH, Longnecker MP, 2015. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. J Expo Sci Environ Epidemiol 25, 286–294. 10.1038/jes.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LJ, Gunier RB, Harley K, Kogut K, Bradman A, Eskenazi B, 2018. Early childhood adversity potentiates the adverse association between prenatal organophosphate pesticide exposure and child IQ: the CHAMACOS cohort 19. [DOI] [PMC free article] [PubMed]
- Stein LJ, Gunier RB, Harley K, Kogut K, Bradman A, Eskenazi B, 2016. Early childhood adversity potentiates the adverse association between prenatal organophosphate pesticide exposure and child IQ: The CHAMACOS cohort. Neurotoxicology 56, 180–187. 10.1016/j.neuro.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin DL, Stone DL, 2011. Dialkyl phosphates as biomarkers of organophosphates: The current divide between epidemiology and clinical toxicology. Clinical Toxicology 49, 771–781. 10.3109/15563650.2011.624101 [DOI] [PubMed] [Google Scholar]
- Terry AVJ, 2012. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther 134, 355–365. 10.1016/j.pharmthera.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, West RF, Stanovich KE, 2013. Practitioner Review: Do performance-based measures and ratings of executive function assess the same construct?: Performance-based and rating measures of EF. Journal of Child Psychology and Psychiatry 54, 131–143. 10.1111/jcpp.12001 [DOI] [PubMed] [Google Scholar]
- Traverso L, Viterbori P, Usai MC, 2015. Improving executive function in childhood: evaluation of a training intervention for 5-year-old children. Front. Psychol. 6. 10.3389/fpsyg.2015.00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries MA, Guxens M, Pronk A, Spaan S, El Marroun H, Jusko TA, Longnecker MP, Ferguson KK, Tiemeier H, 2019. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring attention-deficit hyperactivity disorder and autistic traits. Environment International 131, 105002. 10.1016/j.envint.2019.105002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries MA, Lamballais S, El Marroun H, Pronk A, Spaan S, Ferguson KK, Longnecker MP, Tiemeier H, Guxens M, 2020. Prenatal exposure to organophosphate pesticides and brain morphology and white matter microstructure in preadolescents. Environmental Research 191, 110047. 10.1016/j.envres.2020.110047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Ji L, Hu Y, Zhang J, Wang C, Ding G, Chen L, Kamijima M, Ueyama J, Gao Y, Tian Y, 2017. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ Int 108, 119–126. 10.1016/j.envint.2017.08.010 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Ji L, Zhou Y, Shi R, Kamijima M, Ueyama J, Gao Y, Tian Y, 2020. Prenatal exposure to organophosphate pesticides, maternal paraoxonase 1 genotype, and childhood neurodevelopment at 24 months of age in Shandong, China. Environ Sci Pollut Res 27, 1969–1977. 10.1007/s11356-019-06740-4 [DOI] [PubMed] [Google Scholar]
- Weng H-Y, Hsueh Y-H, Messam L.L.McV., Hertz-Picciotto I, 2009. Methods of Covariate Selection: Directed Acyclic Graphs and the Change-in-Estimate Procedure. American Journal of Epidemiology 169, 1182–1190. 10.1093/aje/kwp035 [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Kupersmidt JB, Voegler-Lee ME, 2012. Is preschool executive function causally related to academic achievement? Child Neuropsychology 18, 79–91. 10.1080/09297049.2011.578572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VWV, Tiemeier H, Hoppin JA, Longnecker MP, 2009. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int J Hyg Environ Health 212, 481–491. 10.1016/j.ijheh.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.