Abstract
Background and Objectives
Lifestyle activities, such as physical activity and cognitive stimulation, may mitigate age-associated cognitive decline, delay dementia onset, and increase cognitive reserve. Whether the association between lifestyle activities and cognitive reserve differs by sex and APOE4 status is an understudied yet critical component for informing targeted prevention strategies. The current study examined interactions between sex and physical or cognitive activities on cognitive reserve for speed and memory in older adults.
Methods
Research participants with unimpaired cognition, mild cognitive impairment, or dementia from the Washington Heights-Inwood Columbia Aging Cohort were included in this study. Cognitive reserve scores for speed and memory were calculated by regressing out hippocampal volume, total gray matter volume, and white matter hyperintensity volume from composite cognitive scores for speed and memory, respectively. Self-reported physical activity was assessed using the Godin Leisure Time Exercise Questionnaire, converted to metabolic equivalents (METS). Self-reported cognitive activity (COGACT) was calculated as the sum of 3 yes/no questions. Sex by activity interactions and sex-stratified analyses were conducted using multivariable linear regression models, including a secondary analysis with APOE4 as a moderating factor.
Results
Seven hundred fifty-eight participants (mean age = 76.11 ± 6.31 years, 62% women) were included in this study. Higher METS was associated with greater speed reserve in women (β = 0.04, CI 0.0–08) but not in men (β = 0.004, CI −0.04 to 0.05). METS was not associated with memory reserve in women or men. More COGACT was associated with greater speed reserve in the cohort (β = 0.13, CI 0.05–0.21). More COGACT had a trend for greater memory reserve in women (β = 0.06, CI −0.02 to 0.14) but not in men (β = −0.04, CI −0.16 to 0.08). Only among women, APOE4 carrier status attenuated relationships between METS and speed reserve (β = −0.09, CI −0.22 to 0.04) and between COGACT and both speed (β = −0.26, CI −0.63 to 0.11) and memory reserves (β = −0.20, CI −0.50.0 to 093).
Discussion
The associations of self-reported physical and cognitive activities with cognitive reserve are more pronounced in women, although APOE4 attenuates these associations. Future studies are needed to understand the causal relationship among sex, lifestyle activities, and genetic factors on cognitive reserve in older adults to best understand which lifestyle activities may be most beneficial and for whom.
With a scarcity of disease-modifying treatments for Alzheimer disease (AD), prevention is critical. Enhancing cognitive reserve, operationalized as preserved cognitive health despite the presence of brain pathology,1,2 is a promising path toward dementia prevention. Lifestyle factors such as physical activity and cognitive stimulation are 2 modifiable behaviors that may enhance cognitive reserve and reduce dementia risk,3–8 yet it is unclear who benefits most from these activities. Sex differences associated with cognitive benefits of physical activity are evident but mixed. Although meta-analytic reviews aggregating retrospective data suggest a stronger relationship between exercise and cognition in women than men,9,10 recent prospective studies, including an exercise randomized controlled trial, show larger cognitive gains following exercise in men than in women.11 Even less is known about sex differences on the benefit of cognitive stimulation on cognitive reserve.5 Factors such as sex, APOE4, the major genetic risk factor for late-onset AD, and their interaction importantly affect cognitive trajectories, yet little is known on whether they moderate the beneficial effects of modifiable lifestyle activities on cognitive reserve. The current study investigated whether associations of physical and cognitive activities with cognitive reserve are modified by sex and APOE4 carrier status in multiethnic, community-dwelling older adults. This approach will enable a better understanding of which modifiable factors may influence cognitive reserve and in whom.
Methods
Participants
Study participants were selected from the Washington Heights/Hamilton Heights-Inwood Columbia Aging Project (WHICAP). WHICAP recruitment was conducted in 3 waves, starting in 1992, 1999, and 2009. Participants were English or Spanish speaking, Medicare eligible, racially and ethnically diverse residents of Northern Manhattan.12 The WHICAP inclusion criteria included individuals aged 65 years and older and language competency in English or Spanish. Exclusion criteria included dementia diagnosis for the 1999 and 2009 waves only.12,13 No other health-related exclusions were included to maximize population representativeness. Participants completed assessments for health status, functional ability, neurologic status, and neuropsychological test performance.14 A subset of participants received high-resolution structural T1 MRI brain scans at 1.5T or 3T MRI field strength.15 Their first imaging visit was selected for the analysis. Participants met eligibility for inclusion in this study if they had the following available data no more than 12 months apart (when applicable): (1) MRI data, (2) lifestyle activities data, (3) neuropsychological data, (4) APOE genotype, and (5) demographic information including age at the time of scan, sex, education, and diagnosis. Because of differing sex physiologies and systematic social norms that cannot be differentiated in our current study design, the terms women and men used in the present study refer to sex differences that may have biological, physiologic, or social etiologies.
Diagnoses were assigned through diagnostic consensus conferences attended by a panel of neurologists, psychiatrists, and neuropsychologists using a combination of neuropsychological, functional, and neurologic assessments.13,16 Dementia diagnoses also included use of the DSM-III criteria for dementia. The flowchart shows the final sample size of 758 participants (eFigure 1, links.lww.com/WNL/C103).
Standard Protocol Approvals, Registrations, and Patient Consents
The project design and protocol were approved by the Institutional Review Boards of the Columbia Presbyterian Medical Center and the New York State Psychiatric Institute. Participants provided written informed consent at the enrollment.
MRI
1.5T and 3T MRI scans were collected at Columbia University Medical Center with the following parameters: 1.5T Phillips Intera MRI scanner (T1-weighted structural MRI: repetition time [TR] 20 ms, echo time [TE] 2.1 ms, FOV 240 × 256 × 160 matrix, and 1.3 mm slice thickness; T2-FLAIR: TR 11,000 ms, TE 144 ms, inversion time = 2,800, FOV 25 cm × 256 × 192 matrix, and 3 mm slice thickness; proton density: TR 2675 ms, TE 12 ms, FOV 220 × 165×140, and 4 mm slice thickness) and 3T Philips MRI scanner (T1-weighted structural MRI: TR 6.6 ms, TE 3 ms, FOV 256 × 256 × 165, and 1 mm slice thickness; T2-FLAIR: TR 8.0 seconds, TE 332 ms, FOV 240 × 240 × 180, and 0.43 mm slice thickness). Hippocampal volumes, total gray matter volumes, and white matter hyperintensity (WMH) volumes were calculated using FreeSurfer and in-house processing pipeline as previously described.15,17 Hippocampal volumes and total gray matter volume were regressed against total intracranial volume. WMHs were log transformed to normalize their distribution. Scanner field strength was covaried for in the analysis using the recruitment cohort categorical variable, as described below.
APOE Genotyping
APOE genotype was determined using restriction isotyping.18 Participants with at least one E4 allele were classified as APOE4 carriers. All other participants were classified as APOE4 noncarriers. Analyses were repeated excluding APOE E2/E2 (n = 7) and E2/E4 genotype (n = 20).
Physical and Cognitive Activity Measures
Physical activity measures of duration, intensity, and frequency were collected using the Godin leisure time exercise questionnaire.19 Participants' typical weekly physical activity was evaluated in terms of metabolic equivalent of task (METS) and was constructed as number of minutes × number of times × coefficient (9 for vigorous, 5 for moderate, and 3 for light activities corresponding to the metabolic equivalent) based on previously published work.20–22 A natural logarithm of physical activity was taken, and participants with no physical activity were bottom coded and assigned the lower limit of the score distribution. Cognitive activities (COGACT) were defined as an aggregate score based on self-reported participation (yes/no) in the following activities in the preceding 13 months: reading magazines, newspapers, or books; going to classes; and playing cards, games, or bingo.23 These measures were collected within 12 months of the MRI scan date.
Domain-Specific Cognitive Reserve Measures
In previously published work, confirmatory factor analysis identified the Selective Reminding Test (total recall, immediate recall, and delayed recognition) and Color Trails Test A and B as domain-specific scores for memory and speed domains, respectively.24 We used a residual approach to quantify domain-specific cognitive reserve.1,25 Memory and speed factor scores were regressed on hippocampal volumes, total gray matter volume, and WMH volume. Sex was not included as a factor in the regression models. The resulting residuals from these regressions were used as proxies to represent their respective domain-specific cognitive reserve according to prior approaches to quantify cognitive reserve.1,25 Higher residual values indicate better speed or memory function.
Statistical Analyses
Analyses were conducted in SPSS Statistics 25 (IBM Corp., Released 2017; IBM SPSS Statistics for Windows, Version 25.0; Armonk, NY: IBM Corp.) and R.26 Sex differences of participants' demographic variables were compared using t tests for continuous variables and χ2 tests for categorical variables. Analysis of variance tests with interaction terms for physical or cognitive activities by sex were conducted to assess group differences in speed and memory reserves independently. Main effects were considered significant if type I error rate (α) was less than or equal to 0.05. Power analysis for one-way analysis of variance F tests was used to determine significance levels for interactions. Interactions with p < 0.20 significance were subsequently probed with sex-stratified analyses (power = 0.6).27 Further analyses explored the moderating role of APOE4 in activity-reserve relationships by testing activity by sex by APOE4 3-way interactions. Activities by APOE4 interactions with a corresponding p < 0.05 were probed with APOE4-stratified analyses. Covariates for the analyses included age, sex (when applicable), race/ethnicity, education, recruitment cohort, diagnosis (cognitively unimpaired, MCI, or dementia), and APOE4 status (when applicable). APOE4 noncarriers, men, non-Hispanic White participants, and dementia diagnosis were used as the referent groups in the analyses. Partial R2-s were used to calculate the coefficient of determination of METS and COGACT.
Sensitivity analyses assessed whether imaging subsamples from wave 2 and wave 3 should be combined or considered separately by adjusting the reserve measures to reflect differences in association patterns and conducting the Student t test. In addition, participants were grouped by cognitive impairment status (cognitively normal [CN] vs cognitively impaired [MCI/AD]), and the modifying effect of the group on activity—domain reserve relationship was tested to assess whether the relationships were driven by group. The modifying effect of race/ethnicity was also evaluated in the sensitivity analyses.
Data Availability
Data can be requested and approved from the WHICAP Publication Committee (reference): cumc.co1.qualtrics.com/jfe/form/SV_6x5rRy14B6vpoqN.
Results
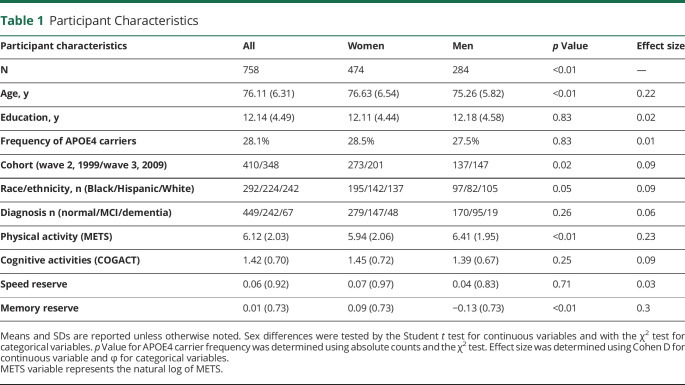
Demographic variables, physical and COGACT measures, and domain reserve scores in the cohort are summarized in Table 1. Men and women did not differ significantly on education, APOE4 carrier status, race/ethnicity, diagnosis, self-reported cognitive activities, and speed reserve. Women participants were older, reported less physical activity, and had larger memory reserve. For cognitive activities, women and men had similar reading and card-playing habits, but more women than men attended classes.
Table 1.
Participant Characteristics
Physical Activity (METS) vs Speed and Memory Reserves
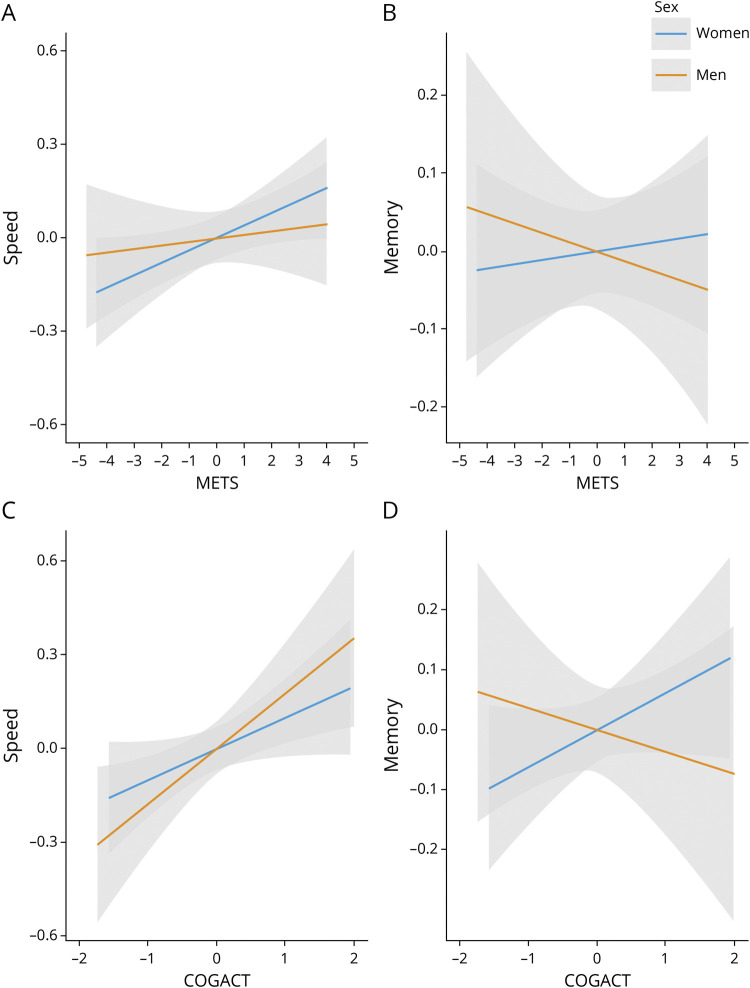
METS was associated with speed reserve among all participants (β = 0.05, 95% Confidence Interval [CI] 0.01–0.08, p = 0.01, model R2 = 0.33, METS R2 = 0.05); however, a METS by sex interaction (β = −0.05, CI −0.1 to 0.08, p = 0.09, interaction R2 = 0.04) indicated the relationship differed by sex. Sex-stratified analyses revealed a significant relationship among women (β = 0.04, CI 0–0.08, p = 0.05, model R2 = 0.36, METS R2 = 0.08) that was not observed in men (β = 0.004, CI −0.04 to 0.05, p = 0.85, model R2 = 0.26, METS R2 < 0.01) (see Figure 1A, eTable 1, links.lww.com/WNL/C103, for model estimates).
Figure 1. Association Between Physical (METS) and Cognitive (COGACT) Activities and Speed and Memory Reserves.
The (added variable) plots demonstrate the association between METS and speed and memory reserves (A and B) and between COGACT and speed and memory reserves (C and D) stratified by sex by regressing out the covariates from both the dependent and independent variables in each panel.
METS was not associated with memory reserve (β = 0.004, CI −0.04 to 0.05, p = 0.82, model R2 = 0.32, METS R2 = 0.02), and a METS by sex interaction was not observed (β = −0.01, CI −0.06 to 0.03, p = 0.60, interaction R2 < 0.01) (Figure 1B, eTable 1, links.lww.com/WNL/C103).
COGACT vs Speed and Memory Reserves
COGACT was associated with speed reserve among all participants (β = 0.13, CI 0.05–0.21, p = 0.003, model R2 = 0.33, COGACT R2 < 0.01), and a COGACT by sex interaction was not observed (β = −0.01, CI −0.17 to 0.16, p = 0.93, interaction R2 < 0.01) (Figure 1C, eTable 2, links.lww.com/WNL/C103).
COGACT was not associated with memory reserve among all participants (β = 0.04, CI −0.03 to 0.11), p = 0.26, model R2 = 0.33, COGACT R2 = 0.07); however, a COGACT by sex interaction was observed (β = −0.11, CI −0.24 to 0.02, p = 0.11, interaction R2 = 0.01), revealing a trend among women (β = 0.06, CI −0.02 to 0.14, p = 0.15, model R2 = 0.37, COGACT R2 = 0.07) and no association among men (β = −0.04, CI −0.16 to 0.08, p = 0.52, model R2 = 0.28, interaction R2 = 0.02) (Figure 1D, eTable 2, links.lww.com/WNL/C103).
APOE4 Attenuates the Relationship Between Lifestyle Activity and Cognitive Reserve in Women
Planned secondary analyses assessed the association of activity (METS and COGACT), sex, and APOE4 status on reserve (speed and memory). Among women, the recruitment cohort, diagnoses, and age differed between APOE4 carriers and noncarriers (p < 0.01), such that the frequency of APOE4 carriers was lower in wave 2, and APOE4 carriers were more likely to be CN and younger. There was a difference in education between APOE4 carriers and noncarriers among men (p = 0.04).
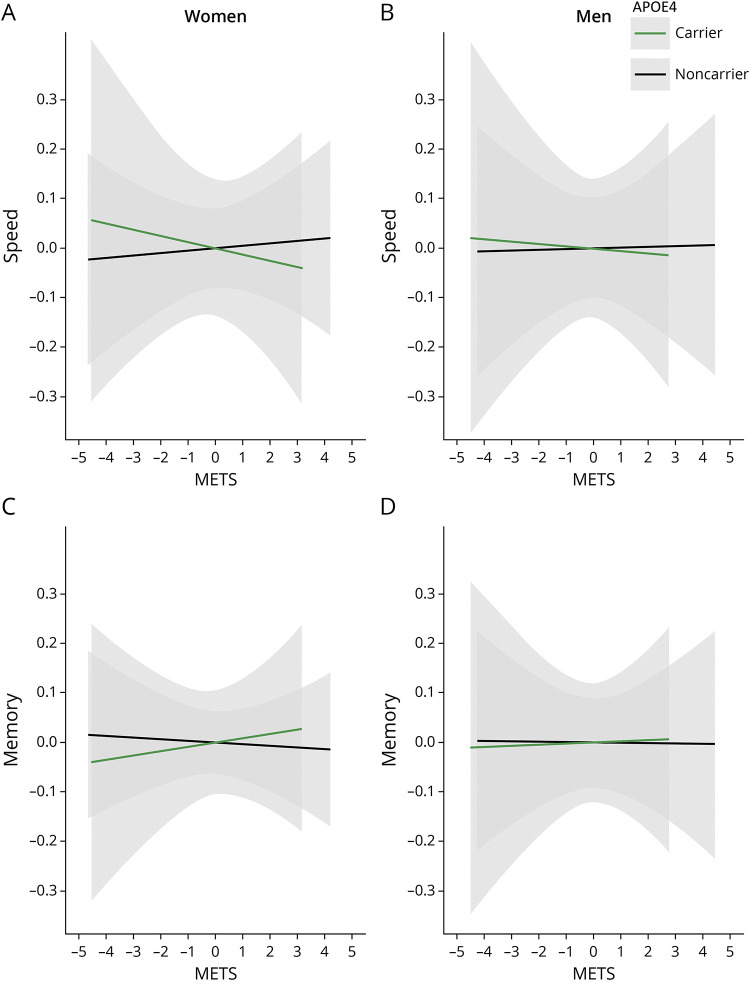
There was a significant METS by sex by APOE4 interaction on speed reserve (β = −0.09, CI −0.22 to 0.04, p = 0.17, model R2 = 0.33, interaction R2 = 0.02). Sex-stratified analyses indicated that the presence of APOE4 allele moderated the relationships between METS and speed reserve among women (β = 0.08, CI 0–0.15, p = 0.05, model R2 = 0.37, interaction R2 = 0.08), such that APOE4 noncarrier women had a stronger association between METS and speed reserve (β = 0.06, CI 0.02–0.11, p < 0.01, model R2 = 0.42, METS R2 = 0.03) (Figure 2A) compared with APOE4 carrier women (β = −0.03, CI −0.10 to 0.05, p = 0.52, model R2 = 0.36, METS R2 = 0.02). No significant METS effect (β = 0.02, CI −0.08 to 0.11, p = 0.71, model R2 = 0.27, METS R2 < 0.01) or APOE4 allele moderation was observed among men (β = −0.02, CI −0.12 to 0.09, p = 0.74, interaction R2 < 0.01) (Figure 2B). The METS by sex by APOE4 interaction for memory reserve was not significant (β = −0.04, CI −0.14 to 0.07, p = 0.48, model R2 = 0.32, interaction R2 < 0.01) (Figure 2, C and D).
Figure 2. Sex-Stratified Associations for Physical Activity (METS) and Speed and Memory Reserves by APOE4 Carrier Status.
The plots demonstrate the association between METS and speed and memory reserves among women (A and C) and men (B and D) stratified by APOE4 carrier status by regressing out the covariates from both the dependent and independent variables in each panel.
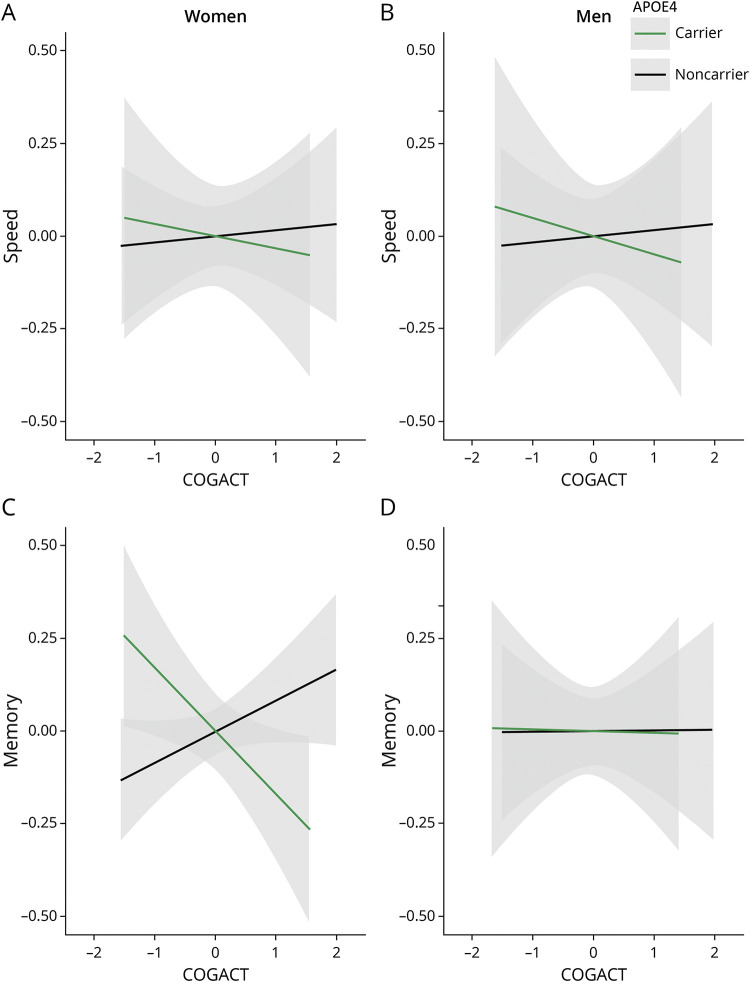
The COGACT by sex by APOE4 interaction effect on speed reserve was significant (β = −0.26, CI −0.63 to 0.11, p = 0.16, model R2 = 0.34, interaction R2 = 0.03). Sex-stratified analyses found that APOE4 allele moderated the COGACT-speed reserve relationship among women (β = 0.24, CI 0.03–0.44, p = 0.02, model R2 = 0.37, interaction R2 = 0.01), such that increased engagement in COGACT was associated with higher speed reserve for APOE4 noncarrier women (β = 0.20, CI 0.07–0.32, p < 0.01, model R2 = 0.37, COGACT R2 = 0.03). This association was not observed among women APOE4 carriers (β = −0.11, CI −0.31 to 0.09, p = 0.27, model R2 = 0.43, COGACT R2 = 0.01) (Figure 3A). Among men, no significant APOE4 moderation was found for COGACT-speed relationships (β = −0.04, CI −0.34 to 0.25, p = 0.77, model R2 = 0.28, interaction R2 < 0.01) (Figure 3B).
Figure 3. Sex-Stratified Associations for Cognitive Activities (COGACT) and Speed and Memory Reserves by APOE4 Carrier Status.
The plots demonstrate the association between COGACT and speed and memory reserves among women (A and C) and men (B and D) stratified by APOE4 carrier status by regressing out the covariates from both the dependent and independent variables in each panel.
COGACT by sex by APOE4 interaction on memory reserve was significant (β = −0.20, CI −0.50 to 0.093, p = 0.18, model R2 = 0.33, interaction R2 = 0.02). Sex-stratified analyses found an APOE4 moderation among women (β = 0.24, CI 0.08–0.40, p < 0.01, model R2 = 0.34, COGACT R2 = 0.02) (Figure 3C). There was a significant association among APOE4 noncarrier women (β = 0.15, CI 0.05–0.25, p < 0.01, model R2 = 0.34, COGACT R2 = 0.03), whereas a nonsignificant COGACT-memory reserve association was observed among APOE4 carrier women (β = −0.10, CI −0.25 to 0.05, p = 0.19, model R2 = 0.36, COGACT R2 = 0.01) (Figure 3C). APOE4 moderation of COGACT-memory reserve was not significant among men (β = 0.05, CI −0.21 to 0.31, p = 0.70, model R2 = 0.28, interaction R2 < 0.01) (Figure 3D). Analyses were repeated excluding those with an APOE E2/E2 or E2/E4 genotype, and the findings did not change.
Sensitivity Analyses by Group (CN vs MCI/AD and Race/Ethnicity)
Demographic variables, physical and COGACT measures, and domain reserve scores stratified by recruitment cohorts to account for different scanners and different MRI sequences are summarized in eTable 3, links.lww.com/WNL/C103. After adjusting for key demographic variables (i.e., age, sex, APOE4, education, and diagnosis), there were no significant differences in the memory reserve between the cohorts (p = 0.44) and in the speed reserve between the cohorts (p = 0.39). Because there were no significant differences in the reserve measurements based on the MRI scanner, no further cohort-stratified analyses were conducted.
The results did not change for sensitivity analyses stratified by impairment group. Stratified analyses revealed that the relationship between METS and speed reserve was primarily driven by CN participants (eTable 4, links.lww.com/WNL/C103). All reported interactions were observed for CN participants. Although there was a significant interaction with impairment, the relationship between METS and memory was not significant in either subgroup (eTable 4, links.lww.com/WNL/C103).
Impairment status significantly moderated COGACT-speed reserve relationship (β = 0.17, CI 0.01–0.33, p = 0.04, model R2 = 0.32, interaction R2 < 0.01, eTable 5, links.lww.com/WNL/C103). The relationship between COGACT and speed reserve was primarily driven by participants with MCI and dementia. Impairment was not a modifier in COGACT-memory reserve relationship (β = 0.05, CI −0.08 to 0.18, p = 0.45, model R2 = 0.31, COGACT R2 < 0.01, eTable 5, links.lww.com/WNL/C103). Impairment was not a significant moderator in reserve—activity by sex by APOE4 relationships. Race/ethnicity was not a significant moderator of any of the relationships of interest (eTables 6–7, links.lww.com/WNL/C103).
Discussion
Understanding how the relationship between lifestyle activities and cognitive reserve is modified by sex and APOE4 status is needed to inform strategies for Alzheimer prevention and clinical trials. In the present study, physical activity was associated with speed reserve in women but not men. Based on the effect sizes observed for physical activity (METS) and age, a 2-fold increase in physical activity would be equivalent to an estimated 2.75 fewer years of processing speed aging in women. Physical activity was not associated with memory reserve in women or men. While cognitive activities were positively associated with speed reserve in both women and men; they were positively associated with memory reserve in women only. Each additional COGACT corresponded to 13 fewer years of processing speed aging (10 years among women and 17 years among men). Furthermore, the associations observed for women were attenuated by APOE4 carrier status, such that APOE4 had a negative effect on the association between lifestyle activities and cognitive reserve in women only. Overall, our findings suggest that sex and APOE4 carrier status are important factors to consider in the association between the beneficial effects of lifestyle activities on cognitive reserve.
It is widely believed that physical activity confers brain benefit by maintaining or enhancing brain integrity through neuronal growth, synaptic plasticity, or dendritic spine growth based on animal studies.28–30 In the current study, physical activity mapped onto speed reserve, despite controlling for hippocampal volume, total gray matter volume, and white matter hyperintensities. These observations suggest that in addition to physical activity preserving brain volume, physical activity may also maintain other aspects of brain health not captured by structural markers, such as functional brain networks and brain perfusion, important for processing speed.31,32 This relationship is consistent with the literature that reports that aerobic exercise more often affects frontally mediated processes, such as executive function and processing speed, than hippocampal-mediated processes, such as episodic memory.33–35 However, the effects of aerobic exercise on cognitive domains are not always consistent likely due to differences in population studied, type, duration, and intensity.36
Contrary to physical activity, which was only associated with speed reserve, cognitively stimulating activities such as reading or group classes were associated with both speed and memory reserve. Cognitive activities have been postulated to help maintain the integrity of the brain4 in a way that reflects the cognitive demands of the specific activity. For example, if playing a card game uses executive skills, playing card games more frequently would confer longer-term benefits in the cognitive domain of executive functioning.37 Based on the nature of the cognitive activities reported in the present study, such as card games and reading, these activities could conceivably involve both processing speed and memory functions. However, reverse causality cannot be ruled out, such that those with greater speed or memory reserve are more likely to engage in these types of cognitively stimulating activities, and these relationships may differ in those with normal cognition compared with those with impaired cognition.
The sex-specific associations of physical activity–speed (observed in both) and COGACT-memory (observed only in women) may be related to the types of activities women vs men engaged in. Although no differences were observed for card-playing and reading behaviors by sex, women did report higher levels of group-based classes than men. Contrary to card play and reading activities, group-based classes inherently encompass a social component that may differentially engage cognitive abilities.
These sex effects may be further modified38 by interactions with APOE4, the major genetic risk for late-onset AD.39 Compared with noncarrier women or APOE4 carrier men, women with an APOE4 allele have increased lifetime risk for AD,40,41 smaller adjusted hippocampal volumes,42–44 more pathologic levels of CSF abeta and tau,45,46 more senile plaques and neurofibrillary tangles postmortem,47 and poorer cognition.42,43 More recently, studies indicate that APOE4 carrier women also have greater tau burden based on CSF and tau PET imaging48,49 and have steeper rates of cognitive decline50 than women without an APOE4 allele. Findings from these previous studies are consistent with the unfolding story that APOE4 may dampen the beneficial relationships between lifestyle activities and cognitive reserve, with a unique effect in women, as found in the present study. Several mechanistic underpinnings may explain this unique decrement in APOE4 carrier women. For example, women experience dramatic reductions in estrogen production after menopause, which can lead to metabolic deficiencies and ultimately cognitive decline. The double-hit of APOE4 carriage exacerbates these negative effects by further reducing the availability of bioenergetic fuel.51
There are several notable limitations. The current study is focused on a cohort living in Northern Manhattan communities and thus excludes the activity patterns of suburban and rural community dwellers. The activity scores were derived from self-reported questionnaire answers, which may include some level of recall bias in reporting by sex or in those individuals with cognitive impairment. Notably, however, the observed associations between lifestyle activities and cognitive reserve were also detected in the impaired group. Structural and societal factors, which are reflected in part, by educational opportunities and attainment, are major determinants of cognitive reserve and were not directly measured or assessed in this study. Future studies with controlled activity interventions or the utilization of objective activity levels (e.g., accelerometers) to measure physical activity would help determine the validity of our findings. The present study is limited to participants who volunteered for MRI studies. Those who volunteer for MRI studies generally tend to self-report good or excellent health, thereby possibly restricting our findings to a healthier cohort. Finally, the present study uses an observational cross-sectional design limiting conclusions on causality.
Overall, the findings from the present study can be used to develop more precise lifestyle recommendations based on sex and APOE4 status. Future studies are needed to test the causal relationship between lifestyle activities and cognitive reserve and how causality is modified by sex and APOE4. Observing these sex- and APOE4-specific associations in a community-dwelling and racially-diverse cohort is a strength. Despite the large literature on modifiable lifestyle risk factors for AD, more studies are needed to establish causality to better inform dementia prevention approaches. It is indeed possible that a combination of modifiable lifestyle factors will need to be engaged for greater effect and that this may differ between women and men and by those with APOE4.
Glossary
- AD
Alzheimer disease
- CN
cognitively normal
- COGACT
cognitive activities
- DSM-III
Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition
- MCI
mild cognitive impaired
- METS
metabolic equivalent of task
- TE
echo time
- TR
repetition time
- WHICAP
Washington Heights/Hamilton Heights-Inwood Columbia Aging Project
- WMH
white matter hyperintensity
Appendix. Authors
Footnotes
CME Course NPub.org/cmelist
Study Funding
WHICAP: Data collection and sharing for this project was supported by the Washington Heights-Inwood Columbia Aging Project (WHICAP, PO1AG07232, R01AG037212, RF1AG054023, and RF1 AG066107) funded by the National Institute on Aging (NIA) and by the National Center for Advancing Translational Sciences, NIH, through Grant Number UL1TR001873. This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study. This project, developed out of the NIH-funded 2018 Advanced Psychometrics Workshop on Cognitive Aging conference, was approved by the WHICAP executive committee. Data were received on May 29, 2019. The authors were supported by the following NIH grants: J.P. and V.A.: AG054617; K.B.C.: AG061253; L.B.Z.: AG047963 and AG054520; S.E.T.: AG050723; and Y.G.: AG061008 and AG059013). N.M.A. was funded by the Intramural Research Program, National Institute on Aging, NIH.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Reed BR, Mungas D, Farias ST, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(pt 8):2196-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516. [DOI] [PubMed] [Google Scholar]
- 4.Cheng ST. Cognitive reserve and the prevention of dementia: the role of physical and cognitive activities. Curr Psychiatry Rep. 2016;18(9):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casaletto KB, Rentería MA, Pa J, et al. Late-life physical and cognitive activities independently contribute to brain and cognitive resilience. J Alzheimers Dis. 2020;74(1):363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191-198. [DOI] [PubMed] [Google Scholar]
- 7.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol Psychiatry. 2013;18:864-874. [DOI] [PubMed] [Google Scholar]
- 8.Voss MW, Vivar C, Kramer AF, Van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17(10):525-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barha CK, Liu‐Ambrose T. Sex differences in exercise efficacy: is midlife a critical window for promoting healthy cognitive aging? FASEB J. 2020;34:11329-11336. [DOI] [PubMed] [Google Scholar]
- 10.Barha CK, Hsiung GR, Best JR, et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. J Alzheimers Dis. 2017;60(4):1397-1410. [DOI] [PubMed] [Google Scholar]
- 11.Stern Y, Lee S, Predovan D, Sloan RP. Sex moderates the effect of aerobic exercise on some aspects of cognition in cognitively intact younger and middle-age adults. J Clin Med. 2019;8(6):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang MX, Cross P, Andrews H, et al. Incidence of AD in african-Americans, caribbean hispanics, and caucasians in northern manhattan. Neurology. 2001;56(1):49-56. [DOI] [PubMed] [Google Scholar]
- 13.Manly JJ, Bell-Mcginty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62(11):1739-1746. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y. Diagnosis of dementia in a heterogeneous population. Arch Neurol. 1992;49:453. [DOI] [PubMed] [Google Scholar]
- 15.Brickman AM, Tosto G, Gutierrez J, et al. An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology. 2018;91(15):e1402–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayeux R, Reitz C, Brickman AM, et al. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment-part 1. Alzheimers Dement. 2011;7:15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brickman AM, Zahodne LB, Guzman VA, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging. 2015;36(1):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545-548. [PubMed] [Google Scholar]
- 19.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141-146. [PubMed] [Google Scholar]
- 20.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y, Beato JM, Amarante E, et al. Assessment of leisure time physical activity and brain health in a multiethnic cohort of older adults. JAMA Netw Open. 2020;3(11):e2026506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino E, Manly JJ, Schupf N, Mayeux R, Gu Y. Current and past leisure time physical activity in relation to risk of Alzheimer's disease in older adults. Alzheimers Dement. 2019;15(12):1603-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helzner EP, Scarmeas N, Cosentino S, Portet F, Stern Y. Leisure activity and cognitive decline in incident Alzheimer disease. Arch Neurol. 2007;64(12):1749-1754. [DOI] [PubMed] [Google Scholar]
- 24.Scarmeas N. Education and rates of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;77(3):308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahodne LB, Manly JJ, Brickman AM, Siedlecki KL, Decarli C, Stern Y. Quantifying cognitive reserve in older adults by decomposing episodic memory variance: replication and extension. J Int Neuropsychol Soc. 2013;19(8):854-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. Available at: R-project.org/. [Google Scholar]
- 27.Buckley JP, Doherty BT, Keil AP, Engel SM. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect. 2017;125(6):067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creer DJ, Romberg C, Saksida LM, Van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague–Dawley rats in vivo. Neuroscience. 2004;124:71-79. [DOI] [PubMed] [Google Scholar]
- 31.Boraxbekk C-J, Salami A, Wåhlin A, Nyberg L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network: a multimodal approach. NeuroImage. 2016;131:133-141. [DOI] [PubMed] [Google Scholar]
- 32.Hsu CL, Best JR, Wang S, et al. . The impact of aerobic exercise on fronto-parietal network connectivity and its relation to mobility: an exploratory analysis of a 6-month randomized controlled trial. Front Hum Neurosci. 2017:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418-419. [DOI] [PubMed] [Google Scholar]
- 34.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52(1):119-130. [DOI] [PubMed] [Google Scholar]
- 35.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults. Psychol Sci. 2003;14:125-130. [DOI] [PubMed] [Google Scholar]
- 36.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane RL, Butler M, Fink HA, et al. . Interventions to Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer's-type Dementia [Internet]. Agency for Healthcare Research and Quality (US)Cognitive Training; 2017. Comparative Effectiveness Reviews, No. 188. 4A, Results: Cognitive Training. [PubMed] [Google Scholar]
- 38.Barha CK, Liu-Ambrose T. Exercise and the aging brain: considerations for sex differences. Brain Plast. 2018;4(1):53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921-923. [DOI] [PubMed] [Google Scholar]
- 40.Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA. 1997;278:1349. [PubMed] [Google Scholar]
- 41.Neu SC, Pa J, Kukull W, et al. . Apolipoprotein E genotype and sex risk factors for Alzheimer disease. JAMA Neurol. 2017;74:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobel Z, Isenberg AL, Raghupathy D, Mack W, Pa J. APOE ɛ4 gene dose and sex effects on Alzheimer's disease MRI biomarkers in older adults with mild cognitive impairment. J Alzheimer's Dis. 2019;71:647-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleisher A. Sex, apolipoprotein E ε4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953. [DOI] [PubMed] [Google Scholar]
- 44.Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11(1):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damoiseaux JS, Seeley WW, Zhou J, et al. . Gender modulates the APOE4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32:8254-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24-28. [DOI] [PubMed] [Google Scholar]
- 48.Buckley RF, Scott MR, Jacobs HIL, et al. Sex mediates relationships between regional tau pathology and cognitive decline. Ann Neurol. 2020;88(5):921-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer's disease: findings from three well-characterized cohorts. Alzheimers Dement. 2018;14:1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). 2015;1:103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be requested and approved from the WHICAP Publication Committee (reference): cumc.co1.qualtrics.com/jfe/form/SV_6x5rRy14B6vpoqN.