Abstract
Background/Objective
GLP-1R agonists have been shown to reduce fasting and postprandial plasma lipids, both of which are independent risk factors for the development of cardiovascular disease. However, how endogenous GLP-1 – which is rapidly degraded – modulates intestinal and hepatic lipid metabolism is less clear. A vagal gut-brain-axis originating in the portal vein has been proposed as a possible mechanism for GLP-1’s anti-lipemic effects. Here we sought to examine the relationship between vagal GLP-1 signalling and intestinal lipid absorption and lipoprotein production.
Methods
Syrian golden hamsters or C57BL/6 mice received portal vein injections of GLP-1(7-36), and postprandial and fasting plasma TG, TRL TG, or VLDL TG were examined. These experiments were repeated during sympathetic blockade, and under a variety of pharmacological or surgical deafferentation techniques. In addition, hamsters received nodose ganglia injections of a GLP-1R agonist or antagonist to further probe the vagal pathway. Peripheral studies were repeated in a novel GLP-1R KO hamster model and in our diet-induced hamster models of insulin resistance.
Results
GLP-1(7-36) site-specifically reduced postprandial and fasting plasma lipids in both hamsters and mice. These inhibitory effects of GLP-1 were investigated via pharmacological and surgical denervation experiments and found to be dependent on intact afferent vagal signalling cascades and efferent changes in sympathetic tone. Furthermore, GLP-1R agonism in the nodose ganglia resulted in markedly reduced postprandial plasma TG and TRL TG, and fasting VLDL TG and this nodose GLP-1R activity was essential for portal GLP-1s effect. Notably, portal and nodose ganglia GLP-1 effects were lost in GLP-1R KO hamsters and following diet-induced insulin resistance.
Conclusion
Our data demonstrates for the first time that portal GLP-1 modulates postprandial and fasting lipids via a complex vagal gut–brain–liver axis. Importantly, loss or interference with this signalling axis via surgical, pharmacological, or dietary intervention resulted in the loss of portal GLP-1s anti-lipemic effects. This supports emerging evidence that native GLP-1 works primarily through a vagal neuroendocrine mechanism.
Keywords: Glucagon-like peptide-1 (GLP-1), Portal vein, Vagus nerve, Nodose ganglion, Dyslipidemia, Chylomicron
Abbreviations: TG, Triglyceride; TRL, Triglyceride rich lipoprotein; VLDL, Very low-density lipoprotein; GLP-1, Glucagon-like peptide-1; GLP-1R, GLP-1 receptor; IP, intraperitoneal; IV, intravenous; T2D, Type II diabetes; CNS, Central nervous system; SAP, Saponin; Ex-4, Exendin-4
Graphical abstract
1. Introduction
Postprandial lipid metabolism becomes altered in insulin resistant states and type 2 diabetes (T2D), resulting in an overproduction of apolipoprotein B-containing chylomicron (CM) particles, very low-density lipoprotein (VLDL) particles, and elevated plasma triglyceride (TG) levels. This dysregulation in blood lipids, termed dyslipidemia, results in the development of atherogenic fatty remnants, precursors to the development of cardiovascular disease (CVD) [1]. Several hormones have been shown to regulate intestinal and hepatic lipid metabolism, key among them being insulin and gut-derived peptides released upon nutrient consumption. One such peptide, glucagon-like peptide (GLP)-1 has been shown by our laboratory and others to modulate intestinal and hepatic lipoprotein production through a complex gut–brain–liver axis [2,3]. Peripheral administration of GLP-1 receptor (GLP-1R) agonists such as exendin-4 can reduce fasting VLDL accumulation and postprandial chylomicron excursion [2,3]. Activation of GLP-1Rs in the central nervous system (CNS) show a similar result, where intracerebroventricular (ICV) injection of exendin-4 also leads to decreased postprandial lipemia [2]. Importantly, GLP-1R agonists are used extensively in current diabetic care, and new co-agonist compounds are an exciting new avenue for anti-diabetic and anti-obesity pharmaceuticals [4,5].
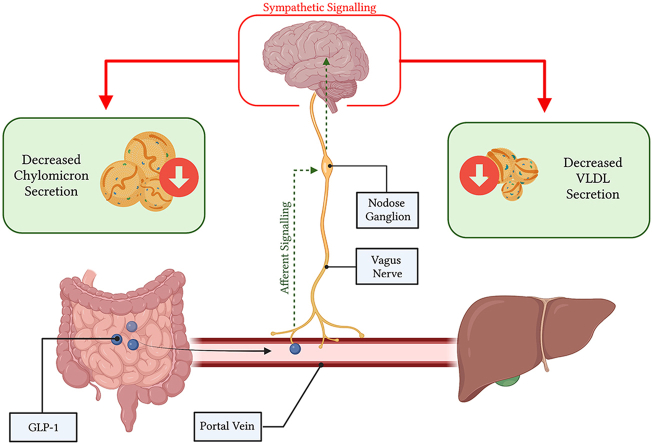
Native GLP-1 produced by enteroendocrine L-cells in the duodenum and distal ileum is rapidly degraded in the circulation, and while pharmacological analogs induce dramatic reductions in lipoprotein secretion, the potential for native GLP-1 to mimic these effects is less evident. Endogenous GLP-1 is secreted into the portal vein which is richly innervated with vagal afferent nerve terminals containing GLP-1Rs, so it is possible that native GLP-1 exerts its effects here via a neuroendocrine mechanism. This notion is supported by recent evidence that GLP-1 is heavily degraded immediately after passing through the portal venous bed by hepatic dipeptidyl peptidase (DPP)-4; limiting its endocrine potential to travel to the pancreatic islet and stimulate insulin release [6]. mRNA for the GLP-1R has been seen in the portal vein [6], and recently has been shown in a Glp1r.tdTomato reporter mouse in both the intra and extra-hepatic portal vein [7]. Indeed, GLP-1 infusion into the portal vein at both periphysiological and pharmacological doses has been shown to significantly stimulate vagal afferent nerve activity [8]. Importantly, portal administration of GLP-1 in rats has been linked closely to insulin release via neuroendocrine signalling; whereas, extra-portal GLP-1 administration has failed to initiate neuron-mediated insulin release [8]. Since insulin has also been shown to be a potent regulator of lipoprotein metabolism this further supports native GLP-1 acting locally within the portal circulation [9].
The GLP-1R-containing vagal afferent nerves overlying the portal bed project towards the caudal brainstem, housing their cell bodies in the nodose ganglia (NG) situated just below the skull. Importantly, the presence of the GLP-1R in nodose ganglia neurons has been demonstrated by RT-PCR, Northern blotting, and in-situ hybridization [10]. Moreover, primary isolated nodose neurons show action potential generation, coupled with increases in intracellular Ca2+ when exposed to GLP-1 [11]. The role of these GLP-1R-containing vagal afferent neurons in regulating peripheral metabolism has been evidenced previously by GLP-1R knockdown in the vagal afferent nerves of rats, which display increased food consumption, accelerated gastric emptying, and post-meal glycemia coupled with depressed insulin secretion [12].
While the effects of GLP-1 on vagal afferent nerves have been studied by others with respect to food consumption, body weight gain, insulin secretion, and glycemia, none have investigated its effects on lipid and lipoprotein metabolism [[12], [13], [14]]. Thus, the aim of the current study was to evaluate the effect native GLP-1 signalling in the portal bed on hepatic and intestinal lipoprotein production. We also sought to determine the relationship between portal and nodose ganglia GLP-1R activation. To do this we employed Syrian golden hamsters and various surgical and pharmacological approaches to elucidate the effect of site-specific activation of GLP-1R on vagal afferent nerves. Together, we demonstrate a novel vagal afferent pathway through which GLP-1 may exert its effects on intestinal and hepatic lipoprotein production.
2. Methods
2.1. Animals & diets
Male Syrian golden hamsters (Mesocricetus auratus) or C57BL/6 mice were purchased from Harlan/Envigo Laboratories (Indianapolis, IN, United States), or bred in-house at the Peter Gilgan Center for Research & Learning. Syrian golden hamsters were chosen as the main experimental model for this study due to the homology of their lipoprotein profiles with human, their innate expression of cholesterol ester transfer protein (CETP; not expressed in rat and mouse), and susceptibility to diet-induced dyslipidemia and hepatic insulin resistance – together making them an ideal model for the study of lipoprotein metabolism. All animals were housed on a 12 h light/dark cycle, with ad libitum access to standard rodent chow and water. Purchased animals were given one week of acclimatization prior to any experimental intervention. For dietary interventions animals were allowed ad-libitum access to high fructose (HF; 60% fructose, 20% casein, D161506) or high-fructose/high-fat/high-cholesterol diet (FFC; 30% fat, 40% fructose, 0.25% cholesterol, D101711) custom made by Dyets Inc (Bethlehem, PA, USA). GLP-1R KO hamsters were developed via crisper cas-9 gene editing in collaboration with Dr. Zhongde Wang’s lab at Utah State University. GLP-1R KO hamsters were bred in a heterozygous x heterozygous breeding scheme to produce KO animals and wildtype (WT) littermates. Age- and sex-matched animals were randomized into experimental groups. All procedures were approved by the Animal Care Committee at the Hospital for Sick Children.
2.2. Pharmacological treatments
Native GLP-1(7-36) (10 μg/kg; Bachem, Torrence, CA, USA) was used for portal vein experiments, and for nodose ganglion GLP-1R agonism experiments Exendin-4 (250ng/ganglia; Bachem, Torrence, CA, USA) was employed. GLP-1 was additionally used for nodose ganglia injections (1μg/ganglia) in GLP-1R KO and FFC-fed hamster studies. The GLP-1R antagonist Exendin 9-39 (Bachem, Torrence, CA, USA) was used as a pre-treatment for both portal (50 nmol/kg; IV) and nodose ganglion studies (500 ng/ganglia). For pharmacological blockade of vagal signalling atropine (40 μg/kg; Sigma Aldrich, Darmstadt, Germany) was administered subcutaneously, and for sympathetic blockade a combination of propranolol (1 mg/kg; ThermoFisher, Waltham, MA, USA) and phentolamine (1 mg/kg; TCI, Portland, OR, USA) was administered intraperitoneally. For denervation experiments capsaicin (25 mg/kg; Millipore, Damstadt, Germany) was administered via three IP injections at 0, 18 and 24 h to denervate small unmylenated primary vagal afferents neurons. Capsaicin was dissolved (25 mg/ml) in a 1:1 solution of dimethyl sulfoxide (DMSO) and 0.9% NaCl in sterile water. For selective deafferentation of vagal GLP-1R-containing neurons Exenatide-Saponin (1 μg/ganglia) or a blank scramble protein Blank-Saponin (1 μg/ganglia) was acquired from Advanced Targeting Systems (Carlsbad, CA, USA). All pharmacological treatments were dissolved in 1x PBS unless otherwise stated.
2.3. Jugular vein cannulation
Hamsters were anesthetized using isoflurane (3% mixed with oxygen) and given meloxicam (2 mg/kg SC). A small paralateral incision was made above the clavicle and the tissue teased away to expose the jugular vein. The vein was isolated, and a small incision made to insert a jugular catheter (filled with heparinized saline). The catheter was tied to the jugular vein using silk suture thread and the catheter is then threaded to back of the neck. The incision over the clavicle was sutured shut and the catheter was tucked under the skin on the back of the neck and sutured in place with silk suture thread.
2.4. Portal vein injection
Animals were anesthetized using isoflurane (3% mixed with oxygen) and meloxicam was given as analgesia (Hamster, 2 mg/kg SC; mouse, 5 mg/kg SC). An incision (1.5–2 cm) was made at the midline starting at the sternum. The portal vein was exposed by externalization of the intestines, which were maintained in sterile gauze soaked in warm saline. The portal vein was then injected with either treatment or vehicle (100 μl volume) using a 30G needle attached to a 1cc syringe. Gauze was applied to staunch bleeding. The intestines were then returned to anatomical position, and the musculo-peritoneum and overlying skin stitched.
2.5. Intraduodenal injection
Hamsters were anesthetized using isoflurane (3% mixed with oxygen) and meloxicam was given as analgesia (2 mg/kg, SC). An incision (1 cm) was made at the midline starting at the sternum and retracted to expose the intestines. The duodenum was exposed by gentle manipulation of the intestine with sterile swabs. Olive oil (200 μl) was then delivered into the duodenum just distal to the pyloric sphincter via a 30G needle attached to a 1cc syringe. The intestines were then returned to anatomical position, and the musculo-peritoneum and overlying skin stitched.
2.6. Vagotomy
Hamsters were anesthetized using isoflurane (3% mixed with oxygen) and meloxicam was given as analgesia (2 mg/kg SC). An incision (1.5–2 cm) was made at the midline starting at the sternum. Using fine forceps, the anterior and posterior vagal trunks were isolated from the esophagus and severed, then peeled back to remove a 2 mm section of the nerve. In sham animals the vagal trunks were isolated but not transected. The intestines were then returned to anatomical position, and the musculo-peritoneum and overlying skin is stitched. Hamsters were given a one-week recovery period prior to portal vein injection experiments.
2.7. Nodose ganglia injection
Hamsters were anesthetized using isoflurane (3% mixed with oxygen) and meloxicam was given as analgesia (2 mg/kg SC). A small incision was made from clavicle to mandible and the platysma was blunt dissected through. The sternomandibular and sternohyodeius muscles were separated by blunt dissection to expose the vagus nerve. The nerve was traced superiorly to the base of the skull to locate the nodose ganglion. The nodose ganglion was then injected just above the superior laryngeal nerve using a custom 34 G needle with a 35° bevel, attached to a 10 μL gastight Hamilton syringe. The incision was then sutured shut. Nodose ganglion injection volume was fixed at 1 μl; for experiments requiring two injections the volume was halved to 0.5 μl per compound.
2.8. Assessment of lipoprotein production
Hamsters were fasted for 16 h and then baseline blood was drawn via a jugular catheter or retro-orbital bleed. Hamsters then underwent surgical injection into the portal vein or nodose ganglia under gas anesthesia. Hamsters were then recovered and left fasted for VLDL experiments or briefly restrained for an oral gavage of olive oil (Sigma–Aldrich, Mississauga, ON; 200 μl) for postprandial experiments. The non-ionic detergents Tyloxapol or Pluronic F-127 (2 g/kg, IV or IP respectively) were administered to halt triglyceride-rich lipoprotein catabolism and triglyceride uptake (via lipoprotein lipase inhibition) and then serial blood draws were performed over a 6 h period. Mice were fasted for 5 h and then baseline blood was collected via the tail vein. Mice then underwent portal vein injection. Following injection mice received pluronic F-127 (2 g/kg, IP) and were fat loaded with a 200 μl gavage of olive oil for postprandial experiments. Serial blood draws were performed over a 2 h period. Experiment-specific details are provided in figure legends. Both portal vein and nodose ganglia test agent injections were performed 15 min prior to olive oil gavage and/or Tyloxapol/Pluronic injection. Stitching of the incision site exceeded no longer than 5 min, then animals were given a minimum of 10 min to recover from the gas anesthesia. Animals begin to display their righting reflex within 3 min and are fully ambulatory and alert after 10 min. All animals were monitored for alertness, normal nesting behaviour, and hunched posture/rapid breathing throughout the timecourse to ensure there was no undue stress from the surgery itself.
2.9. Lipoprotein isolation
Blood samples were collected in heparinized tubes and then centrifuged for 10 min at 4 °C and 6000 rpm (F2402H rotor, Beckman Coulter, ON, Canada). Plasma was isolated and treated with protease inhibitor (SIGMAFAST Protease Inhibitor Cocktail; Sigma–Aldrich). TRL and VLDL fractions were isolated via density ultracentrifugation. Briefly, plasma (150 μl) was overlayed with 4 ml of KBr (1.006 g/ml) and centrifuged for 70 min (for TRL isolation) or 120 min (for VLDL isolation) at 35,000 rpm at 10 °C (SW-55-Ti rotor; Beckman Coulter, ON, Canada). Both fractions were isolated as the upper 300 μl of the tube volume.
2.10. TG, cholesterol, and insulin measurement
TG and cholesterol were determined by enzymatic-based colorimetric assays (Randox, Crumlin, UK) and plasma insulin levels were measured by ELISA (Mercodia, Uppsala, Sweden) according to the manufacturer’s instructions.
2.11. Reverse transcriptase polymerase chain reaction (RT-PCR)
Tissue was flash frozen in liquid nitrogen then crushed with a mortar and pestle. RNA was extracted using TRIzol (Thermo-Fisher Scientific, CA, USA) according to manufacturer’s instructions. Synthesis of cDNA and quantitative RT-PCR were performed as previously described [3].
2.12. Statistical analyses
All results are presented as mean ± SEM. Time course experiments were analyzed via two-way ANOVA with Bonferroni post-hoc analysis. Comparisons between two groups were analyzed via two-tailed unpaired t-tests, and comparisons between 3 or more groups were analyzed via one-way ANOVA. All statistical comparisons were made using GraphPad Prism V8.0 (San Diego, CA, USA). All experiments described here were performed using a between subject paradigm.
3. Results
3.1. Portal vein injection of GLP-1 lowers postprandial and fasting plasma TG and TRL independent of gastric emptying or insulin secretion
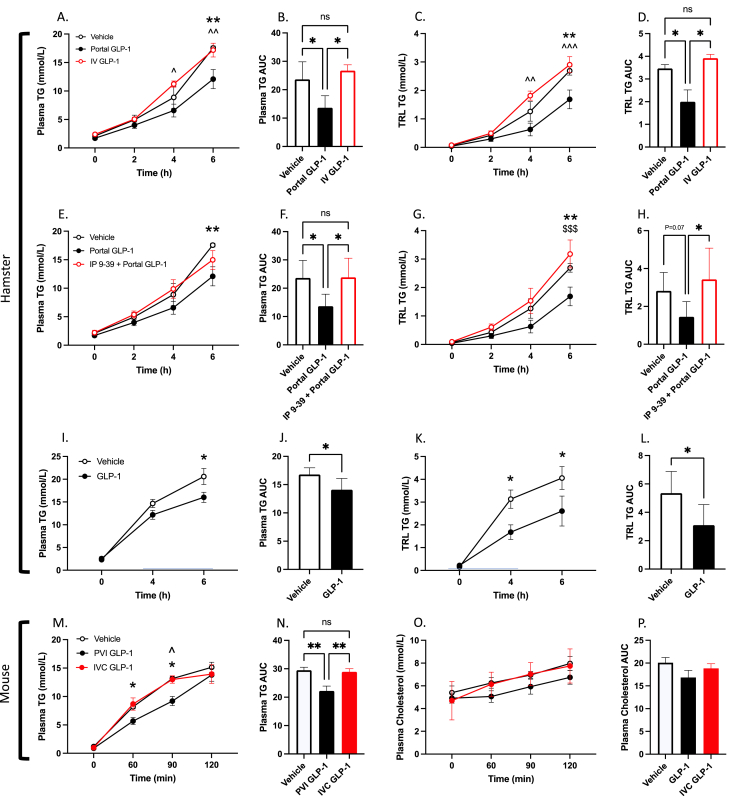
To examine the effect of native GLP-1 in the portal vein on postprandial lipid metabolism, Syrian golden hamsters gavaged with olive oil were surgically injected with an acute bolus of GLP-1 directly into the portal vein. Portal vein injection of GLP-1(7-36) (10 μg/kg) caused a marked decrease in postprandial plasma TG and TRL TG over a 6 h period, and in the plasma and TRL TG AUC (P ≤ 0.05; Figure 1A–D). When the same dose of GLP-1(7-36) was infused into an extra-portal site (IV; jugular vein) this effect was lost, with no divergence from vehicle control (P > 0.05; Figure 1A–D). To confirm this effect was dependent on GLP-1R activation, we pretreated hamsters with an IP injection of the GLP-1R antagonist Exendin 9-39 10 min prior to portal GLP-1(7-36) injection. GLP-1R antagonism was able to completely block the portal GLP-1 effect, and these hamsters exhibited greater plasma and TRL TG accumulation at the 6 h timepoint compared to hamsters treated with portal GLP-1(7-36) alone (≤0.05; Figure 1E–G). Moreover, portal GLP-1 also affected lipid metabolism in the fasted state, as portal injection of GLP-1(7-36) (40 μg/kg) in fasting hamsters was able to elicit significant reductions in fasting plasma lipids and VLDL TG over a 4 h period (P ≤ 0.05; Supp Figure 1A, E).
Figure 1.
Portal vein injection of GLP-1 lowers postprandial plasma TG and TRL TG independent of gastric emptying in both hamster and mouse.A-D: Hamsters were fasted for 16 h then received an injection of GLP-1(7-36) (10 μg/kg) or vehicle (PBS) into the portal vein, or an IV infusion of GLP-1 (10 μg/kg) through the jugular line. Hamsters were then fat-loaded with olive oil via oral gavage (200 μl), received IV tyloxapol (2 g/kg), and blood was drawn over a 6 h period via a jugular catheter. E-H: Alternatively, hamsters were pretreated with IP exendin 9-39 10-minutes prior to portal GLP-1 injection. (A/E) Plasma TG, (B/F) plasma TG AUC, (C/G) TRL TG, (D/H) TRL TG AUC. I-L: 16 h fasted hamsters received portal vein injections of GLP-1(7-36) (10 μg/kg) or vehicle (PBS) and then received an intraduodenal infusion of olive oil (200 μl). Pluronic F-127 was administered (2 g/kg; IP) and blood was drawn via retro-orbital bleed over a 6 h period. (I) Plasma TG, (J) plasma TG AUC, (K) TRL TG, (L) TRL TG AUC. M-P: C57BL/6 mice were fasted for 5 h then received a portal vein injection of GLP-1(7-36) (10 μg/kg) or vehicle (PBS), or an extra-portal injection of GLP-1 (10 μg/kg) into the inferior vena cava (IVC). Mice were then fat loaded with an oral gavage of olive oil (200 μl) and received an IP injection of Pluronic F-127 (2 g/kg). Blood was drawn over a 2 h period via the tail vein. (M) Plasma TG, (N) plasma TG AUC, (O) Plasma cholesterol, (P) plasma cholesterol AUC. Data is presented as means ± SEM (A-H: n=5–6/group; I-L: n=6/group; M-P: n=5–6/group). (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). ∗; Vehicle vs. Portal GLP-1, ˆ; IV GLP-1 vs. Portal GLP-1, $; IP 9–39 + Portal GLP-1 vs. Portal GLP-1.
Since GLP-1 has a well-documented effect on gastric emptying we then sought to identify whether portal GLP-1’s effect on postprandial lipids was due to delayed gastric emptying. To address this, we bypassed the stomach by administering olive oil directly into the duodenum via a surgical injection. GLP-1(7-36) injection into the portal vein of hamsters who received intraduodenal olive oil still manifested significant reductions in postprandial plasma TG and TRL TG over a 6 h period, and in the plasma and TRL TG AUC (P ≤ 0.05; Figure 1I-L). Similarly, GLP-1 is also known to increase insulin secretion – another key modulator of postprandial lipid absorption. Plasma insulin over a 6 h period did not differ between portal GLP-1(7-36) injected and vehicle injected hamsters, or in the insulin AUC (P > 0.05; Supp Figure 2A–B). Lipogenic genes in the jejunum of these hamsters were assayed via RT-PCR, however, we did not see any significant variation in expression despite a significant reduction in jejunal lipid content (P > 0.05; Supp Figure 2C–D).
To confirm our effect was not hamster-specific we repeated portal GLP-1(7-36) injection in mice as well. Experiments in C57BL/6 mice gavaged with olive oil revealed comparable results where portal vein GLP-1(7-36) (10 μg/kg) injection reduced plasma TG at the 60- and 90-minute mark, whereas extra-portal injection (inferior vena cava) did not affect postprandial plasma lipids (P ≤ 0.05; Figure 1M, 1N). As in hamsters, portal GLP-1’s lipid lowering effect in mice was not limited to the postprandial state, as 2 h fasting plasma TG and VLDL TG also showed significant reductions after portal GLP-1(7-36) injection in fasted C56BL/6 mice (P ≤ 0.05; Supp Figure 1I, 1M).
3.2. Loss of vagal afferent signalling abrogates the effects of portal GLP-1 on postprandial plasma TG and TRL TG
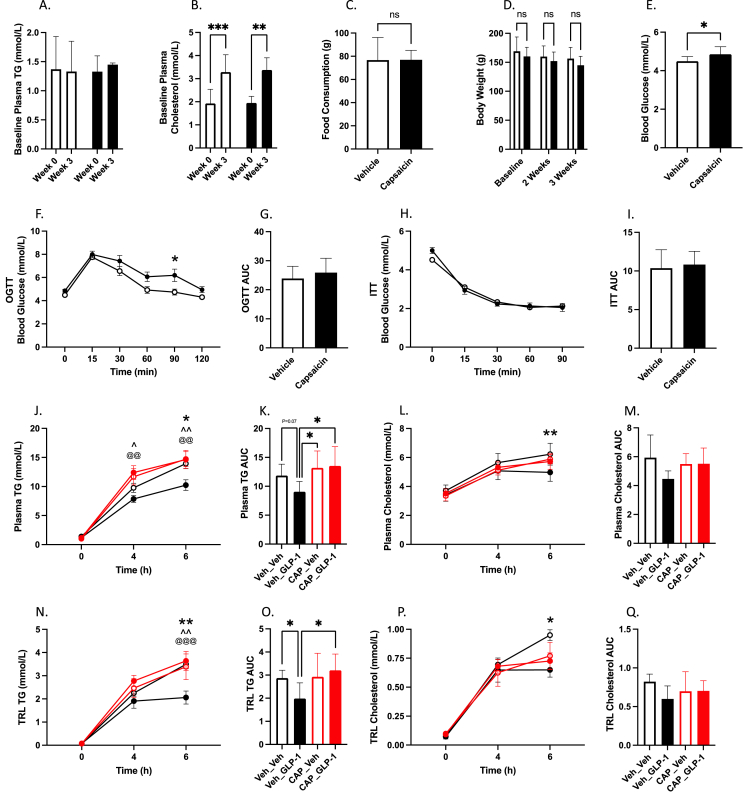
Since GLP-1Rs in the portal bed are located on vagal afferent nerve terminals we next investigated whether vagal afferent signalling is required to elicit reductions in postprandial plasma TG and TRL TG via portal injection of GLP-1. To do this, chow-fed Syrian-golden hamsters were partially deafferented via serial IP injections of capsaicin. Capsaicin treated hamsters showed no change in fasting plasma TG and cholesterol, food consumption, or body weight three weeks after denervation compared to vehicle control (P > 0.05; Figure 2A–E). However, capsaicin-treated hamsters did exhibit elevated fasting blood glucose, and worsened glucose tolerance during OGTT, with no change in insulin sensitivity during ITT (P ≤ 0.05; Figure 2F–I). After olive oil gavage capsaicin-treated hamsters showed no significant difference in plasma or TRL TG and cholesterol compared to vehicle controls, nor were they sensitive to portal vein injection of GLP-1(7-36) (P > 0.05; Figure 2J–Q). Indeed, compared to non-deafferented hamsters injected with portal GLP-1(7-36), both capsaicin treated groups showed significantly higher plasma TG at 4- and 6 h and in the plasma TG AUC, with significantly higher TRL TG at 6 h. Portal GLP-1(7-36) injection in non-deafferented hamsters was the only group to show significant reductions in plasma and TRL TG and cholesterol at the 6 h mark or in the AUC compared to vehicle controls (P ≤ 0.05; Figure 2J–Q).
Figure 2.
Portal GLP-1 is sensitive to capsaicin-deafferentation. Chow-fed Syrian golden hamsters were deafferented via IP capsaicin injection (see methods). Two weeks later hamsters underwent OGTT and ITT (n = 12/group) (F-I), then on the third week hamsters were fasted for 16 h and vehicle-treated (Veh_XX) or capsaicin-treated (CAP_XX) hamsters received portal vein injections of GLP-1(7-36) (10 μg/kg) or vehicle (PBS). Hamsters were then fat loaded via an oral gavage of olive oil (200 μl) and received IP Pluronic F-127 (2 g/kg). Blood was drawn over a 6 h period via retro-orbital bleeds. (A) Baseline plasma TG, (B) baseline plasma cholesterol, (C) food consumption, (D) body weight, (E) 2-week fasting blood glucose, (F) OGTT, (G) OGTT AUC, (H) ITT, (I) ITT AUC, (J) plasma TG, (K) plasma TG AUC, (L) plasma cholesterol, (M) plasma cholesterol AUC (N) TRL TG, (O) TRL TG AUC, (P) TRL cholesterol, (Q) TRL cholesterol AUC. Data is presented as means ± SEM (n=6/group). (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). ∗; Veh_Veh vs. Veh_GLP-1, ˆ; Veh_GLP-1 vs. CAP_Veh, @; Veh_GLP-1 vs. CAP_GLP-1.
To further confirm that vagal afferent signalling was necessary to propagate the effects of portal GLP-1, hamsters underwent subdiaphragmatic truncal vagotomy surgery. Vagotomised hamsters who received portal GLP-1(7-36) (10 μg/kg) no longer exhibited significant reductions in postprandial plasma TG or TRL TG (P > 0.05; Supp Figure 3E–F), however, sham-treated hamsters who received portal GLP-1(7-36) were still able to display significant reductions in postprandial TRL production 6 h post-injection and in the TRL AUC (P ≤ 0.05; Supp Figure 3G,H). Pharmacological inhibition of vagal signalling via atropine pre-treatment showed similar results, where atropine treated animals were no longer sensitive to portal GLP-1(7-36) injection (P > 0.05; Supp Figure 3A–D), whereas vehicle controls still exhibited significant reductions in the plasma and TRL AUCs and in the TRL TG at 6 h (P ≤ 0.05; Supp Figure 3A–D).
3.3. Selective deafferentation of GLP-1R containing vagal neurons in nodose ganglia blocks the portal GLP-1 effect and results in elevated postprandial TG and cholesterol
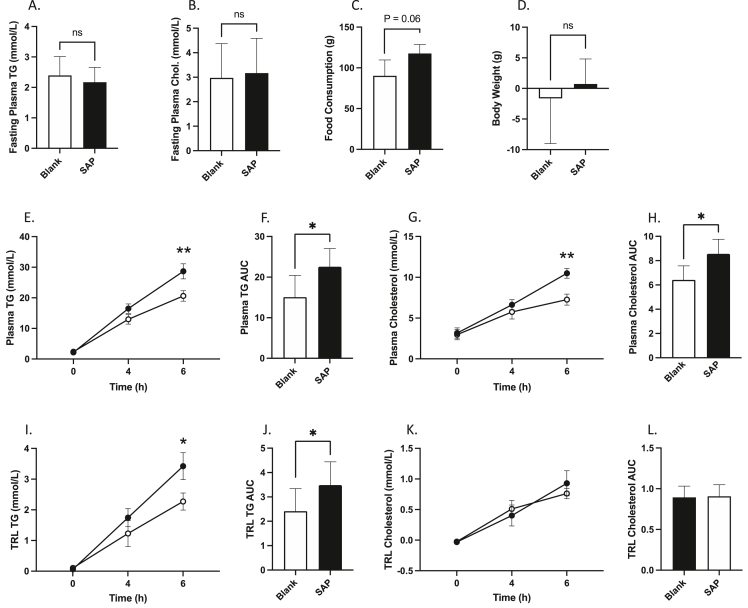
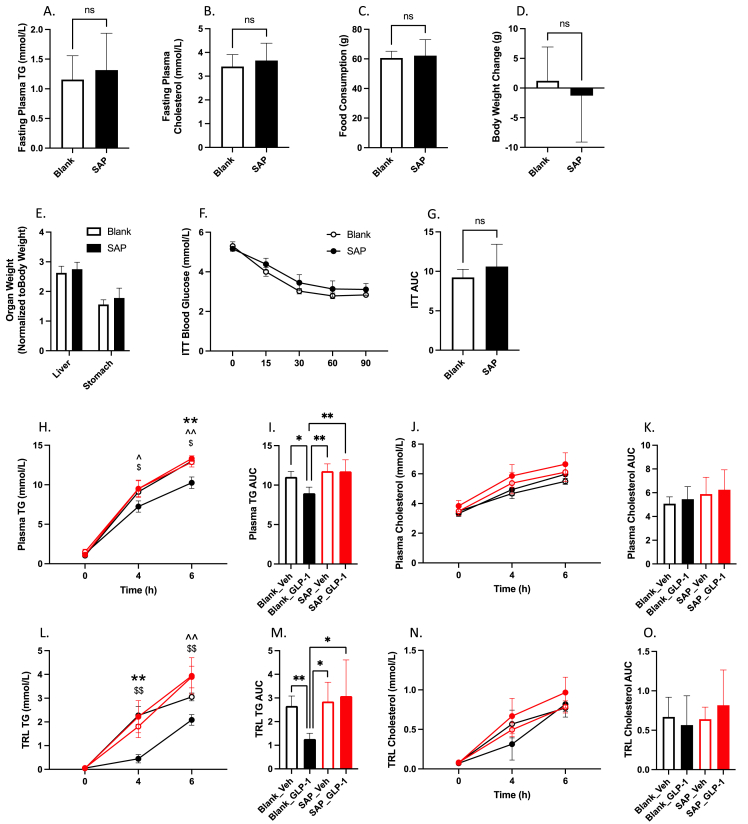
While general interruption of vagal signalling via pharmacological and surgical strategies was able to successfully block the portal GLP-1 effect, we wished to further examine whether the specific loss of GLP-1R-containing vagal neurons was sufficient to alter postprandial lipid homeostasis. To do this we selectively deafferented this subpopulation of vagal afferents via bilateral injections of Saponin-Exenatide into the vagal nodose ganglia. Saponin is a potent neurotoxin which on its own is inert, but upon its endocytosis causes the activation of apoptotic pathways and neuronal death. When linked to a ligand for a G-protein coupled receptor, saponin will be selectively internalized into cells expressing that receptor, thus allowing targeted culling of a subpopulation of neurons. We exploited this pathway to selectively deafferent GLP-1R containing neurons in the nodose ganglion by linking saponin (SAP) to exenatide (a GLP-1R agonist) and injecting it bilaterally. We observed no difference in fasting plasma TG or cholesterol, or in body weight gain with this treatment, however there was a trend toward increased food consumption in the SAP hamsters after two weeks (P > 0.05; Figure 3A–D). However, hamsters who received nodose injections of SAP exhibited elevated postprandial TG and cholesterol accumulation at 6 h post olive oil gavage, which was reflected in the plasma TG and cholesterol AUCs (P ≤ 0.05; Figure 3E–H). This was combined with increased TRL TG at 6 h, and significant rise in the TRL AUC (P ≤ 0.05; Figure 3I–J).
Figure 3.
Selective vagal denervation of GLP-1R-containing neurons results in elevated postprandial blood lipids. Chow-fed Syrian golden hamsters received bilateral nodose ganglia injections of Exenatide-Saponin (SAP) (1ug in 1 μl), or a blank scramble protein linked to Saponin (Blank). Two weeks later hamsters were fasted for 16 h then received an oral gavage of olive oil (200 μl) and were injected with IP Pluronic F-127 (2 g/kg). Blood was collected over a 6 h period via retro-orbital bleeds. (A) 2-week fasting plasma TG, (B) 2-week fasting plasma cholesterol, (C) food consumption, (D) body weight change (E) plasma TG, (F) plasma TG AUC, (G) plasma cholesterol, (H) plasma cholesterol AUC, (I) TRL TG, (J) TRL TG AUC, (K) TRL cholesterol, (L) TRL cholesterol AUC. Data is presented as means ± SEM (n=5/group). (∗P < 0.05, ∗∗P < 0.01).
To confirm that this subpopulation of vagal neurons was necessary for the activity of portal GLP-1 we repeated portal vein injections of GLP-1(7-36) in a new cohort of deafferented animals or vehicle controls. In line with our initial observations deafferentation caused no change in biometrics, fasting plasma TG or cholesterol, or in insulin tolerance (P > 0.05; Figure 4A–G). When deafferented hamsters received portal vein injections of GLP-1(7-36) (10 μg/kg) they showed no reductions in plasma TG or cholesterol in either the plasma or TRL fraction over a 6 h period following olive oil gavage (P > 0.05; Figure 4H–O). Indeed, compared to non-deafferented hamsters treated with portal injections of GLP-1(7-36), both deafferented hamster groups had significantly higher plasma TG at 4- and 6 h post-gavage, and in the plasma TG AUC (P ≤ 0.05; Figure 4H,I). Similar results were seen in the TRL TG and TRL TG AUC, where deafferented hamsters were significantly higher than portal GLP-1(7-36) treated controls (P ≤ 0.05; Figure 3L,M).
Figure 4.
Selective vagal denervation of GLP-1R-containing neurons abrogates the portal GLP-1 effect. Chow-fed Syrian golden hamsters received bilateral nodose ganglia injections of Exenatide-Saponin (SAP) (1ug in 1 μl), or a blank scramble protein linked to Saponin (Blank). Two weeks later hamsters were fasted overnight and then Blank-treated (Blank_XX) or Saponin-treated (SAP_XX) hamsters received a portal vein injection of either GLP-1(7-36) (10 μg/kg) or vehicle (PBS). Hamsters were then fat loaded with an oral gavage of olive oil (200 μl) and given an IP injection of Pluronic F-127 (2 g/kg). Blood was drawn over a 6 h period via retro-orbital bleeds. (A) 2-week fasting plasma TG, (B) 2-week fasting plasma cholesterol, (C) food consumption, (D) body weight change, (E) Organ weight, (F) ITT, (G) ITT AUC, (H) Plasma TG, (I) plasma TG AUC, (J) plasma cholesterol, (K) plasma cholesterol AUC, (L) TRL TG, (M) TRL TG AUC, (N) TRL cholesterol, (O) TRL cholesterol AUC. Data is presented as means ± SEM (A-G: n = 10/group; H-O: n = 5/group). (∗P < 0.05, ∗∗P < 0.01). ∗; Blank_Veh vs. Blank_GLP-1, ˆ; SAP_Veh vs. Blank_GLP-1, $; SAP_GLP-1 vs. Blank_GLP-1.
3.4. Pharmacological blockade of sympathetic signaling abrogates the anti-lipemic effects of portal GLP-1
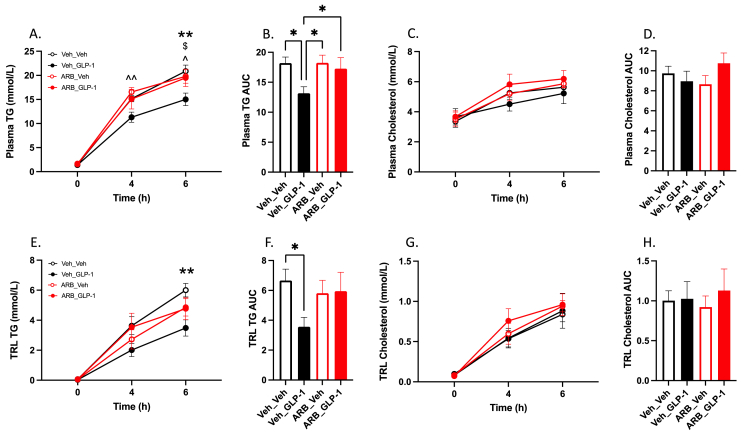
We have previously demonstrated that intracerebroventricular injection of a GLP-1R agonist results in attenuated postprandial plasma TG and TRL TG [2]. This effect was dependent centrally on MCR4 receptor activation and was conducted to the periphery via efferent sympathetic signaling. To connect the disparate components of peripheral and central GLP-1R signaling we hypothesized that portal GLP-1 would rely on changes in efferent sympathetic tone as well. To examine this, we pretreated Syrian golden hamsters gavaged with olive oil with a cocktail of both alpha- and beta-adrenergic receptor antagonists prior to portal GLP-1(7-36) injection. In line with our previous work, sympathetic blockade completely abrogated the portal GLP-1 effect on postprandial plasma and TRL TG at all timepoints (P > 0.05; Figure 5A–B,E–F). Moreover, hamsters treated with adrenergic blockade exhibited significantly higher plasma TG at 4- and 6-h postprandially compared to vehicle controls treated with portal GLP-1(7-36) – this difference was seen in the plasma TG AUC as well (P ≤ 0.05; Figure 5A–B).
Figure 5.
Pharmacological blockade of sympathetic signaling abrogates the anti-lipemic effects of portal GLP-1. Chow-fed Syrian golden hamsters were fasted for 16 h then received and IP injection of alpha- and beta-adrenergic receptor antagonists (ARB_XX) or vehicle (Veh_XX). Ten minutes later hamsters received a portal vein injection of either GLP-1(7-36) (10 μg/kg) or vehicle followed by an oral gavage of olive oil (200 μl) and IP Pluronic F-127 (2 g/kg). Blood was drawn over a 6 h period via retro-orbital bleeds. (A) Plasma TG, (B) plasma TG AUC, (C) plasma cholesterol, (D) plasma cholesterol AUC, (E) TRL TG, (F) TRL TG AUC, (G) TRL cholesterol, (H) TRL cholesterol AUC. Data is presented as means ± SEM (n=5/group). (∗P < 0.05, ∗∗P < 0.01). ∗; Veh_Veh vs. Veh_GLP-1, ˆ; ARB_Veh vs. Veh_GLP-1, $; ARB_GLP-1 vs. Veh_GLP-1.
3.5. Bilateral nodose ganglion injections of Exendin-4 significantly reduce postprandial plasma TG and TRL TG accumulation
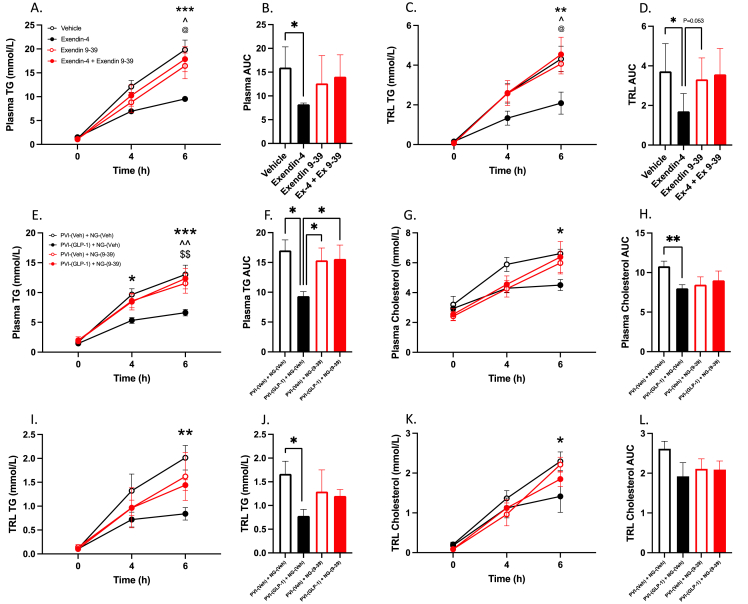
GLP-1R containing neurons in the portal vein project superiorly towards the nodose ganglion, a collection of vagal neuronal cell bodies situated just below the base of the skull. Nodose ganglion neurons have been shown by others to express GLP-1 receptors, and these neuronal bodies project directly to GLP-1-producing neurons in the nucleus tractus solitarius (NTS) [10]. Since we have demonstrated that portal GLP-1Rs require an intact vagal afferent cascade to reduce postprandial and fasting lipoprotein production, we then sought to investigate whether the nodose ganglion was involved in the propagation of the portal GLP-1 signal. To address this, we injected to GLP-1R agonist Exendin-4 into the nodose ganglia to examine the intrinsic effects of GLP-1R activation on lipid metabolism. Hamsters which received bilateral nodose ganglion injections of Exendin-4 (250 ng in 1 μl PBS) displayed significant reductions in postprandial plasma and TRL TG accumulation at 6 h and in the plasma and TRL TG AUC compared to vehicle controls (P ≤ 0.05; Figure 6A–D). Pre-treatment of nodose ganglion neurons with injections of the GLP-1R antagonist Exendin 9-39 (500 ng in 1 μl PBS) blocked the ability of Exendin-4 to illicit reductions in postprandial TG, however injections of Ex 9–39 alone did not significantly alter postprandial plasma and TRL TG (P > 0.05; Figure 6A–D). Similarly, injections of Ex-4 (250 ng in 1 μl PBS) into the nodose ganglia in fasting hamsters was able to significantly lower circulating plasma TG, VLDL TG, and VLDL TG AUC over a 4 h period (P ≤ 0.05; Supp Figure 4A,E,F). Although plasma cholesterol was unaffected, VLDL cholesterol was significantly reduced over a 4 h period, which was also reflected in the VLDL cholesterol AUC (P ≤ 0.05; Supp Figure 4G,H). This corroborates our earlier experiments, and further demonstrates the importance of vagal signalling as an effector of GLP-1’s anti-lipemic properties.
Figure 6.
GLP-1R activation in the nodose ganglia lowers postprandial plasma TG and TRL and is necessary for the anti-lipemic effects of portal GLP-1.A-D: Chow-fed Syrian golden hamsters were fasted for 16 h then received a bilateral nodose ganglion injection of Exendin-4 (250 ng in 1 μl PBS), Exendin 9-39 (500 ng in 1 μl PBS), Exendin 9-39 followed 10 min later by Exendin-4, or vehicle control (1 μl PBS). E-L: Chow-fed Syrian golden hamsters were fasted for 16 h then received bilateral nodose ganglion injections of either Exendin 9-39 (500 ng in 1 μl PBS; PVI-(XX) + NG-(9–39)) or vehicle (1 μl PBS; PVI-(XX) + NG-(Veh). Following this, hamsters received a portal vein injection of either GLP-1(7-36) (10 μg/kg; PVI-(GLP-1) + NG-(XX)) or vehicle (PBS; PVI-(Veh) + NG-(XX)). Following treatment all hamsters were fat loaded with an oral gavage of olive oil (200 μl) and received an IP injection of Pluronic F-127 (2 g/kg). Blood was drawn over a 6 h period via retro-orbital bleed. (A/E) Plasma TG, (B/F) plasma TG AUC, (C/I) TRL TG, (D/J) TRL TG AUC, (G) plasma cholesterol, (H) plasma cholesterol AUC, (K) TRL cholesterol, (L) TRL cholesterol AUC. Data is presented as means ± SEM (A-D: n=4–7/group; E-L: n=5/group). (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). A–D: ∗; Vehicle vs. Exendin-4, ˆ; Exendin 9-39 vs. Exendin 4, @; Exendin 9-39 + Exendin-4 vs. Exendin-4. E–L: ∗; PVI-(Veh) + NG-(Veh) vs. PVI-(GLP-1) + NG-(Veh), ˆ; PVI-(GLP-1) + NG-(9–39) vs. PVI-(GLP-1) + NG-(Veh), $; PVI-(Veh) + NG-(9–39) vs. PVI-(GLP-1) + NG-(Veh).
To elucidate whether portal GLP-1 requires the activity of GLP-1Rs in the nodose to conduct its signal to the NTS in the caudal brainstem, we blocked nodose ganglia GLP-1R with Exendin 9-39 and then did a portal vein injection of GLP-1(7-36) or vehicle. Portal vein injection of GLP-1(7-36) still caused significant reductions in plasma and TRL TG when nodose ganglion GLP-1 receptor signalling was left intact [(PVI-GLP-1 + NG-Veh) vs. (PVI-Veh + NG-Veh)] (P ≤ 0.05; Figure 6E–F,I–J). However, bilateral injection of Ex 9–39 into the nodose ganglion prior to portal vein injection of GLP-1(7-36) abrogated its ability to reduce postprandial TG accumulation [(PVI-GLP-1 + NG-9-39) vs. (PVI-GLP-1 + NG-Veh)] (P > 0.05; Figure 6E–F,I–J). In line with our previous observations, Ex 9–39 injected alone into the nodose ganglion failed to cause alterations in postprandial TG accumulation in either the plasma or TRL TG [(PVI-Veh + NG-9-39) vs. (PVI-Veh + NG-Veh)] (P > 0.05; Figure 6E–F,I–J). Portal injection of GLP-1(7-36) compared to vehicle alone [(PVI-GLP-1 + NG-Veh) vs. (PVI-Veh + NG-Veh)] was able to reduce postprandial plasma and TRL cholesterol over a 6 h period, which was also reflected in the plasma and TRL cholesterol AUC (P ≤ 0.05; Figure 6G–H,K–L).
3.6. The anti-lipemic effects of portal and nodose ganglia GLP-1R activation are abrogated in a novel GLP-1R KO hamster model
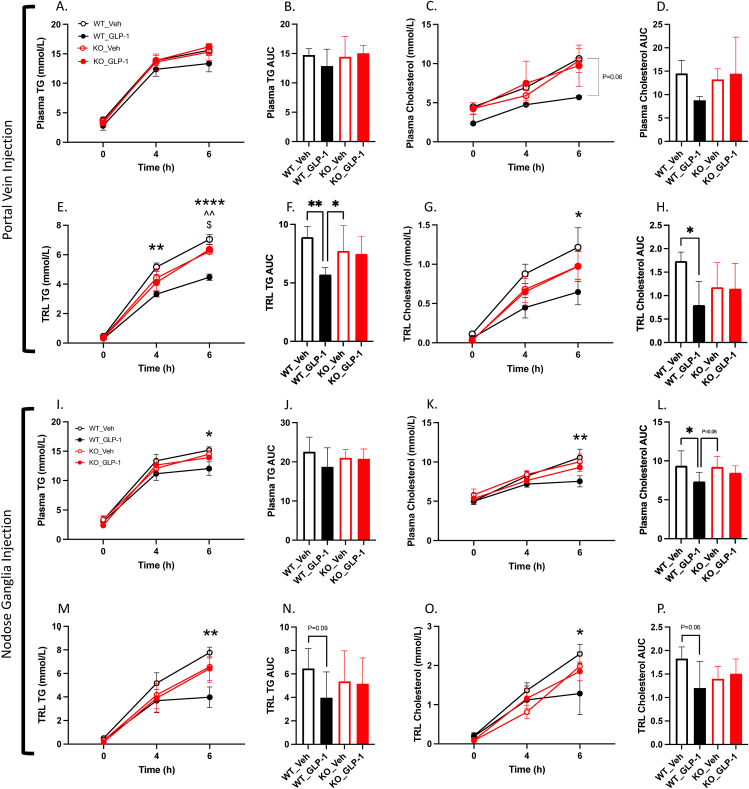
Recently we have developed a novel whole body GLP-1R KO hamster model. To further confirm the observations described above are directly related to GLP-1R signalling we performed portal vein injections of GLP-1(7-36) or vehicle in GLP-1R KO hamsters. As expected, GLP-1R KO hamsters exhibited no reduction in postprandial plasma or TRL TG or cholesterol following portal GLP-1(7-36) injection (P > 0.05; Figure 7A–H). Indeed, both portal GLP-1(7-36) and vehicle treated GLP-1R KO hamsters demonstrated significantly higher TRL TG at the 6 h timepoint compared to wildtype littermates treated with portal GLP-1(7-36). Although we saw no significant changes in plasma TG or cholesterol, wildtype littermates showed significant reductions in TRL TG at 4- and 6 h and in the TRL TG AUC. Similarly, only wildtype littermate controls demonstrated significant reductions in TRL cholesterol at 6 h and in the TRL cholesterol AUC (P ≤ 0.05; Figure 7E–H).
Figure 7.
The anti-lipemic effects of portal and nodose ganglia GLP-1R activation are abrogated in a novel GLP-1R KO hamster model.A-H: GLP-1R KO hamsters or WT littermate controls were fasted for 16 h then received portal vein injections of either GLP-1(7-36) (10 μg/kg) or vehicle (PBS). I-P: Alternatively, GLP-1R KO hamsters or WT littermate controls received bilateral nodose ganglia injections of GLP-1(7-36) (1ug in 1 μl PBS) or Vehicle (1 μl PBS). After treatment all hamsters were fat loaded with an oral gavage of olive oil (200 μl) and then given an IP injection of Pluronic F-127 (2 g/kg). Blood was then drawn over a 6 h period via retro-orbital bleeds. (A/I) Plasma TG, (B/J) plasma TG AUC, (C/K) plasma cholesterol, (D/L) plasma cholesterol AUC, (E/M) TRL TG, (F/N) TRL TG AUC, (G/O) TRL cholesterol, (H/P) TRL cholesterol AUC. Data is presented as means ± SEM (A-H: n=5–6/group; I–P: n=5–7/group). (∗P < 0.05, ∗∗P < 0.01, ∗∗∗∗P < 0.0001). ∗; WT_Veh vs. WT_GLP-1, ˆ; KO_Veh vs. WT_GLP-1, $; KO_GLP-1 vs. WT_GLP-1.
In line with this, when GLP-1(7-36) (500 ng/ganglia) or vehicle control was injected bilaterally into the nodose ganglion of GLP-1R KO hamsters or wildtype littermates, GLP-1(7-36) was able to reduce postprandial plasma TG and cholesterol at 6 h and in the plasma cholesterol AUC in wildtype littermates only (P ≤ 0.05; Figure 7I–L). Correspondingly, only wildtype littermates exhibited reductions in TRL TG and cholesterol at 6 h. At no point did GLP-1R KO hamsters demonstrate any sensitivity to GLP-1(7-36) injection (P > 0.05; Figure 7A–P).
3.7. Vagal afferent GLP-1Rs are sensitive to diet-induced dyslipidemia
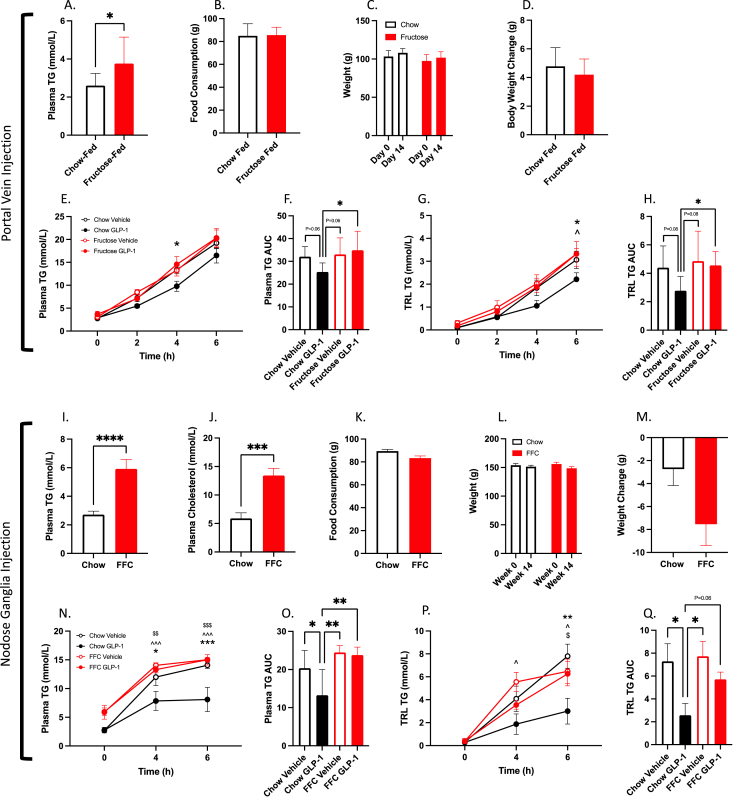
Over the past decade there has been emerging evidence for the concept of GLP-1 resistance. Thus, we sought to investigate whether portal GLP-1R remained sensitive in our fructose-fed hamster model of dyslipidemia and insulin resistance. Hamsters fed a high-fructose diet for 14 days developed significant fasting dyslipidemia (P ≤ 0.05; Figure 8A) despite demonstrating equal feeding activity and weight gain as chow-fed controls (P > 0.05; Figure 8B–D). 14 days of high-fructose feeding completely abrogated the ability of portal vein GLP-1(7-36) (10 μg/kg) to reduce plasma or TRL TG (P > 0.05; Figure 8E–H).
Figure 8.
Vagal afferent GLP-1Rs are sensitive to diet-induced dyslipidemia.A-H: Syrian golden hamsters were fed a high fructose diet or chow control for 2-weeks. Hamsters were then fasted for 16 h before receiving portal vein injections of either GLP-1(7-36) (10 μg/kg) or vehicle (PBS). I-Q: Alternatively, hamsters were fed a fat/fructose/cholesterol enriched diet (FFC) for 4 weeks. Hamsters were then fasted for 16 h before receiving bilateral nodose ganglia injections of GLP-1(7-36) (1ug in 1 μl PBS) or vehicle (1 μl PBS). All hamsters were then fat loaded with an oral gavage of olive oil (200 μl) and then received IV tyloxapol (2 g/kg; A-D) or an IP injection of Pluronic F-127 (2 g/kg; I-L). Blood was then drawn over a 6 h period via jugular catheter (A-D) or retro-orbital bleeds (I-L). (A/I) 2/4-week fasting plasma TG, (B/K) food consumption, (C/L) weight, (D/M) body weight change, (J) 4-week fasting plasma cholesterol, (E/N) plasma TG, (F/O) plasma TG AUC, (G/P) TRL TG, (H/Q) TRL TG AUC. Data is presented as means ± SEM (A-H: n=6–7/group; I-Q: n=6–7/group). (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.0001). ∗; Chow Vehicle vs. Chow GLP-1, ˆ; Chow GLP-1 vs. Fructose/FFC Vehicle, $; Chow GLP-1 vs. Fructose/FFC GLP-1.
Since portal GLP-1R became desensitized to portal GLP-1(7-36) injection, we then investigated whether nodose ganglia GLP-1R showed a similar response. For this study we employed a slightly more aggressive diet enriched with fat, fructose, and cholesterol (FFC) [15]. Hamsters fed an FFC diet for 4 weeks displayed fasting dyslipidemia, with elevations in fasting plasma TG and Cholesterol (P ≤ 0.05; Figure 8I,J). Portal injection of GLP-1(7-36) (10 μg/kg) was no longer able to decrease postprandial plasma or TRL lipids in FFC-fed animals (P > 0.05; Figure 8N–Q), however, chow-fed controls still exhibited significant reductions in both the plasma and TRL TG and cholesterol (P ≤ 0.05; Figure 8N–Q).
4. Discussion
GLP-1R agonists are currently one of the most effective treatments used in T2D to improve glycemic control [16]. Importantly, these agonists also display potent anti-lipemic effects which could reduce the risk of cardiovascular events, the leading cause of death in individuals with T2D [17]. Part of the lipid lowering ability of these GLP-1R agonists is due to the incretin effect, which stimulates insulin release from the pancreas. However, GLP-1R agonists are resistant to degradation by DPP-4 allowing them ample time to circulate and bind to GLP-1Rs around the body. The likelihood of endogenous GLP-1 - which has a circulating half life of approximately 2 min - reaching distant GLP-1Rs in the pancreatic islet is much lower [18]. This becomes even less probable considering only 25% of GLP-1 produced by the gut is secreted to the portal circulation intact, and 40–50% of this secreted product is cleaved in the liver – leaving only 10–15% of active GLP-1 to reach the systemic circulation [19]. Thus, there is mounting evidence for a neuroendocrine signalling mechanism for GLP-1 via afferent vagal nerves in the portal bed where its active concentration is highest [13,[20], [21], [22]]. Indeed, in our experimental model an acute injection of GLP-1(7-36) into the portal vein was sufficient to exert significant reductions in postprandial and fasting plasma TG, VLDL and TRL in both mice and hamsters. This decrease was not seen when an equimolar dose of GLP-1 was administered into the circulation outside of the portal vein (either the jugular vein or IVC), which suggests a site-specific effect. Such specificity supports the notion of a neuroendocrine signalling pathway for GLP-1 originating within the portal venous bed. This is in line with previous reports where infusion of a GLP-1R antagonist into the portal vein but not the jugular vein was able to impair glucose tolerance in rats [13].
In contrast, a recent study by Aulinger et al. found that jugular infusion of GLP-1 in rats was able to produce a greater insulin response compared to infusion into the portal vein [6]. However, their key observations were made under conditions of a continuous infusion of GLP-1; whereas, our experiments were conducted with an acute bolus of GLP-1, perhaps providing less opportunity for jugular GLP-1 to hit peripheral GLP-1Rs in distant tissues. In addition, we pursued a more physiologically relevant approach with an oral fat gavage, activating other classical components of the vagal afferent system such as stretch and chemoreceptors in the stomach and esophagus. Also, in our hamster model we did not see an increase in insulin secretion after portal GLP-1 injection which suggests that portal GLP-1 does not rely on the incretin effect to modulate postprandial lipids. Importantly, milestone experiments by Nishizawa et al. which described this neuro-incretin effect were conducted under constant glucose infusion [20]and the authors determined that the neuronal-mediated insulin component of portal GLP-1 was dependent on ambient glucose levels. Whether the acute portal GLP-1 injection used in our model would induce this neuro-incretin effect under mixed-meal conditions (as opposed to the fat load solely used here) we are less certain. This could be an interesting future direction as GLP-1 has also recently been shown to work synergistically with insulin to stimulate nodose ganglia neuron activity. Iwasaki et al. demonstrated that over 90% of GLP-1 sensitive neurons isolated from mouse nodose ganglia were also responsive to insulin. Co-treatment with GLP-1 and insulin produced greater responses in GLP-1 sensitive neurons and was even able to recruit previously unresponsive neurons to display increases in intracellular [Ca2+] [23].
Along these lines, in addition to stimulating glucose-dependent insulin release, GLP-1 has well-documented effects on gastric emptying, so we wished to confirm whether reductions in postprandial lipids were due to delayed transit of our oral fat gavage - particularly because the effects of GLP-1 on gastric emptying have been shown to be dependent on vagal afferent pathways [21]. To accomplish this, we bypassed the stomach via an intraduodenal infusion of olive oil immediately following portal vein injection of GLP-1. Notably, we still observed a significant decrease in plasma TG and TRL TG, suggesting these effects are at least partially independent of gastric emptying.
Since portal GLP-1 in our model worked in a site-specific manner we then sought to interrogate whether this effect on postprandial and fasting lipids was induced via a vagal signalling axis–as GLP-1R-containing vagal afferent nerve terminals overlay the portal venous bed. To confirm this, we employed a variety of surgical and pharmacological techniques. Deafferentation of small unmyelinated primary vagal afferent neurons was achieved via capsaicin injection. Capsaicin deafferentation has previously been shown to abrogate GLP-1’s effect on gastric emptying [24], and in line with this deafferentation via capsaicin completely blocked the ability of portal GLP-1 to reduce postprandial lipids in our hamster model. Moreover, other non-specific methods such as subdiaphragmatic truncal vagotomy also blocked the anti-lipemic effects of portal GLP-1 injection. Interestingly, vagotomised hamsters showed a lower overall TG profile, however, this may be due to gastroparesis, a common post-operative side effect of vagotomy [[25], [26], [27]]. As above, pharmacological inhibition of vagal signalling with atropine was also able to block the ability of portal GLP-1 to reduce postprandial TG in our hamster model. Finally, to reinforce that it is the loss of GLP-1R-containing vagal neurons specifically that is responsible for the null effect of GLP-1 during deafferentation/denervation, we selectively deafferented this subpopulation of vagal afferent nerves via bilateral nodose ganglia injections of the neurotoxin saponin linked to exenatide. As expected, loss of GLP-1R-containing vagal afferent neurons resulted in a complete loss of the anti-lipemic effects of portal GLP-1 injection. This also decreased the likelihood that portal GLP-1 was stimulating the secretion of bioactive peptides that bound to other vagal afferent nerve populations to modulate lipoprotein metabolism. Thus, we concluded that this subpopulation of vagal afferents was likely essential for the anti-lipemic action portal GLP-1 in our model.
These GLP-1R-containing vagal afferent neurons house their cell bodies in the nodose ganglion, which has also been shown to express the GLP-1R. When we directly stimulated nodose ganglia GLP-1Rs with Exendin-4 we saw marked reductions in postprandial and fasting blood lipids. This occurred independent of stimulation of GLP-1Rs in the portal vein. Moreover, inhibition of GLP-1R in the nodose ganglia with Exendin 9-39 was able to abrogate the ability of portal GLP-1 to reduce postprandial blood lipids, which suggests that these nodose ganglia GLP-1R may be a regulatory component in propagating anti-lipemic signals to the CNS. Whether nodose ganglia GLP-1Rs are being directly activated by circulating GLP-1, or whether their inhibition alters portal GLP-1R sensitivity on vagal afferent nerve terminals is unknown. However, inhibition of nodose GLP-1Rs alone via exendin 9-39 injection did not alter postprandial lipids. In turn, selective deafferentation of GLP-1R-containing nodose neurons via the neurotoxin saporin did cause significant increases in postprandial (but not fasting) plasma TG, plasma cholesterol, and TRL TG following an olive oil gavage. This was paired with a trend towards increased food consumption over a two-week period and elevated liver lipids. Interestingly, nodose ganglia knockdown of GLP-1R containing nerves in rats did not affect food consumption [12], but knockdown in the NTS via an adeno-associated virus resulted in increased food consumption, meal size, and weight gain [28]. It is possible that the subpopulation of GLP-1R containing nerves we deafferented in our model were key in modulating food consumption via other gut peptides such as cholecystokinin (CCK). This is a probability since staining of isolated mouse nodose ganglia revealed that approximately half of CCK1R-expressing neurons in the nodose co-express the GLP-1R, and most GLP-1R-expressing neurons co-expressed CCK1R [29]. We also recognize the potential of portal or nodose ganglia GLP-1Rs to be sensitizing vagal afferents to other circulating factors which regulate lipoprotein metabolism. Certainly, the reverse has been shown where pre-treatment with CCK in food-restricted mice can enhance the satiation effect of GLP-1 via a vagal afferent mechanism [30]. Regardless, experiments in our novel GLP-1R KO hamster model highlighted the importance of the GLP-1R in modulating the vagal response to GLP-1, as KO hamsters did not respond to portal or nodose ganglia injection of its native form.
Previous work in our laboratory has demonstrated that intracerebroventricular (ICV) injection of the GLP-1R agonist Exendin-4 into the third ventricle causes significant reductions in postprandial and fasting plasma lipids [2,3]. Recent work has demonstrated that long-acting agonists such as liraglutide can be directly up taken and deposited into the third ventricle via specialized cells on the blood brain barrier called tanycytes [31]; however, this has yet to be well demonstrated for native GLP-1. Due to native GLP-1s low concentration in the blood and short lifespan we postulate that the endogenous pathways behind central GLP-1R activity may be the product of activated vagal afferents stimulating GLP-1-producing neurons in the NTS; therein, causing the secretion of GLP-1 into hypothalamic nuclei responsible for metabolic control. While not directly addressed here, we were able to provide some evidence to support this hypothesis by investigating the commonalities between how central and vagal GLP-1R activation exert their effects on intestinal lipid metabolism. We have previously demonstrated that the anti-lipemic effects of central GLP-1R signalling were relayed to the periphery via sympathetic signalling, since pharmacological blockade of alpha- and beta-adrenergic receptors blocked the effect of ICV exendin-4 [2]. To align our current model with these findings we repeated portal GLP-1 injection in hamsters who also had their sympathetic signalling impaired via sympathetic blockade. Importantly, sympathetic blockade was able to completely block the ability of portal GLP-1 to reduce postprandial lipids. Together, this presents a potentially unified model of endogenous GLP-1 action; wherein, GLP-1 secreted into the portal vein stimulates GLP-1R on vagal afferent neurons, which project to and stimulate GLP-1 producing neurons in the NTS. This results in GLP-1 secretion into hypothalamic nuclei, in turn depressing hepatic and intestinal lipoprotein production via increased sympathetic tone. This hypothesis is supported by the finding that hamsters treated with sympathomimetic drugs demonstrate depressed fasting plasma TG accumulation over a 2 h period (unpublished data), and peripheral administration of GLP-1R agonists have been shown to increase sympathetic tone [32]. However, to fully probe this hypothesis future experiments are needed to directly link activation of GLP-1R containing vagal afferents to increased hypothalamic GLP-1 levels.
This model for endogenous GLP-1 action also fits well with the relatively recent concept of GLP-1 resistance. While GLP-1R agonists remain effective in individuals with obesity and insulin resistance, this may be due to their ability to activate GLP-1R in a myriad of tissues, such as in the pancreas or brain. Whether vagal sensitivity to endogenous GLP-1, which we here show acts in a narrow site-specific window, would remain unchanged is less certain. This is supported by evidence that children and adults with obesity and T2D have elevated circulating GLP-1 levels compared to lean/non-diabetic counterparts along with higher plasma TG [[33], [34], [35]]. Indeed, in our hamster model, two weeks of feeding a fructose-enriched diet was sufficient to render animals insensitive to portal vein injection of GLP-1. This is consistent with clinical studies where men who consumed a fructose enriched diet for 12 weeks had elevated plasma TG for 2 h after a mixed meal, but no increase in GLP-1 levels [36]. In obese prone rats fed a high fat diet, the ability of exendin-4 to reduce food intake is diminished, which was associated with decreased expression of GLP-1R mRNA in the nodose ganglia [37]. Interestingly, high-fat diet induced inflammation in the nodose ganglion can be seen as early as 24 h; wherein, HFD-fed mice exhibited inflammatory gene expression and microglial infiltration in the nodose ganglion [38]. Consistent with these reports, in our fat/fructose/cholesterol (FFC) dietary hamster model of dyslipidemia, GLP-1 injection into the nodose ganglia was no longer able to elicit reductions in postprandial plasma TG and TRL. This agrees with a recent study where nodose ganglia isolated from high fat diet fed mice exhibited depressed membrane excitability in response to exendin-4 administration [39]. Thus, the development of GLP-1 resistance and dysregulated vagal afferent signalling in insulin resistant states may contribute to the development of dyslipidemia in T2D.
5. Conclusions
Together, the data presented here supports the concept of a complex gut–brain–liver axis for endogenous GLP-1 signalling that is sensitive to dysregulation via diet-induced insulin resistance. Notably, we demonstrate for the first time that native GLP-1 can exert its lipid lowering effects independent of insulin release and gastric emptying via neuroendocrine signalling through the portal venous bed and nodose ganglia. In addition, disruption of vagal afferent GLP-1R signalling via pharmacological inhibition, deafferentation, or poor diet will disrupt this gut–brain axis leading to altered peripheral lipid metabolism. Insights gleaned from these data explain in-part how endogenous GLP-1 signals and becomes dysregulated during states of metabolic dysfunction.
Author contributions
SH designed the study, executed the experiments, analyzed the data, and composed the manuscript. DA executed experiments and edited the manuscript. KA provided the initial conceptual framework for the study, obtained funding, supervised the experiments, and edited the manuscript.
Conflict of Interest
The authors have no conflict of interest to declare.
Acknowledgement
This study was supported by a CIHR (Canadian Institutes of Health Research) foundation grant awarded to KA. SH was supported by provincial OGS (Ontario Graduate Student) scholarships and the Tamarack Award in Diabetes Research awarded by the Banting and Best Diabetes Center. DA was supported by a Restracomp Fellowship awarded by The Hospital for Sick Children, and a CIHR Doctoral Award.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101590.
Contributor Information
Simon Hoffman, Email: simon.hoffman@mail.utoronto.ca.
Danielle Alvares, Email: danielle.alvares@mail.utoronto.ca.
Khosrow Adeli, Email: khosrow.adeli@sickkids.ca.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Alvares D., Hoffman S., Stankovic B., Adeli K. Gut peptide and neuroendocrine regulation of hepatic lipid and lipoprotein metabolism in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2019;1864(3):326–334. doi: 10.1016/j.bbalip.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Farr S., Baker C., Naples M., Taher J., Iqbal J., Hussain M., et al. Central nervous system regulation of intestinal lipoprotein metabolism by glucagon-like peptide-1 via a brain–gut Axis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(5):1092–1100. doi: 10.1161/ATVBAHA.114.304873. [DOI] [PubMed] [Google Scholar]
- 3.Taher J., Baker C.L., Cuizon C., Masoudpour H., Zhang R., Farr S., et al. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Molecular Metabolism. 2014;3(9):823–833. doi: 10.1016/j.molmet.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tschöp M.H., Finan B., Clemmensen C., Gelfanov V., Perez-Tilve D., Müller T.D., et al. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metabolism. 2016;24(1):51–62. doi: 10.1016/j.cmet.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty R.A., Flatt P.R., Irwin N. Is polypharmacy the future for pharmacological management of obesity? Current Opinion in Endocrine and Metabolic Research. 2022;23 doi: 10.1016/j.coemr.2022.100322. [DOI] [Google Scholar]
- 6.Aulinger B.A., Perabo M., Seeley R.J., Parhofer K.G., D'Alessio D.A. Rapid hepatic metabolism blunts the endocrine action of portally infused GLP-1 in male rats. American Journal of Physiology-Endocrinology and Metabolism. 2020;318(2):E189–E197. doi: 10.1152/ajpendo.00298.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen D.B., Grunddal K.V., Pedersen J., Kuhre R.E., Lund M.L., Holst J.J., et al. Using a reporter mouse to map known and novel sites of GLP-1 receptor expression in peripheral tissues of male mice. Endocrinology. 2021;162(3):bqaa246. doi: 10.1210/endocr/bqaa246. [DOI] [PubMed] [Google Scholar]
- 8.Nakabayashi H., Nishizawa M., Nakagawa A., Takeda R., Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. American Journal of Physiology. 1996;271(5 Pt 1):E808–E813. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 9.Pavlic M., Xiao C., Szeto L., Patterson B.W., Lewis G.F. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes. 2010;59(3):580–587. doi: 10.2337/db09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa A., Satake H., Nakabayashi H., Nishizawa M., Furuya K., Nakano S., et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Autonomic Neuroscience. 2004;110(1):36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Kakei M., Yada T., Nakagawa A., Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Autonomic Neuroscience: Basic & Clinical. 2002;102(1–2):39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 12.Krieger J.-P., Arnold M., Pettersen K.G., Lossel P., Langhans W., Lee S.J. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2015;65(1):34–43. doi: 10.2337/db15-0973. [DOI] [PubMed] [Google Scholar]
- 13.Vahl T.P., Tauchi M., Durler T.S., Elfers E.E., Fernandes T.M., Bitner R.D., et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 14.Krieger J.-P., Langhans W., Lee S.J. Vagal mediation of GLP-1’s effects on food intake and glycemia. Physiology & Behavior. 2015;152:372–380. doi: 10.1016/j.physbeh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Nauck M.A., Quast D.R., Wefers J., Meier J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Molecular Metabolism. 2021;46 doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giugliano D., Scappaticcio L., Longo M., Caruso P., Maiorino M.I., Bellastella G., et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovascular Diabetology. 2021;20(1):189. doi: 10.1186/s12933-021-01366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal S., Buring J.E., Rifai N., Mora S., Sacks F.M., Ridker P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298(3):309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 18.Chambers A.P., Sorrell J., Haller A., Roelofs K., Hutch C.R., Kim K.-S., et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metabolism. 2017;25(4):927–934. doi: 10.1016/j.cmet.2017.02.008. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holst J.J. The physiology of glucagon-like peptide 1. Physiological Reviews. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 20.Nishizawa M., Nakabayashi H., Uehara K., Nakagawa A., Uchida K., Koya D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. American Journal of Physiology. Endocrinology and Metabolism. 2013;305(3):E376–E387. doi: 10.1152/ajpendo.00565.2012. [DOI] [PubMed] [Google Scholar]
- 21.Ahrén B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2004;286(2):R269–R272. doi: 10.1152/ajpregu.00423.2003. [DOI] [PubMed] [Google Scholar]
- 22.D'Alessio D. Is GLP-1 a hormone: whether and when? Journal of Diabetes Investigation. 2016;7(Suppl 1):50–55. doi: 10.1111/jdi.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasaki Y., Goswami C., Yada T. Glucagon-like peptide-1 and insulin synergistically activate vagal afferent neurons. Neuropeptides. 2017;65:77–82. doi: 10.1016/j.npep.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 24.I˙meryüz N., Yeğen B.Ç., Bozkurt A., Coşkun T., Villanueva-Peñacarrillo M.L., Ulusoy N.B. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. American Journal of Physiology - Gastrointestinal and Liver Physiology. 1997;273(4):G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 25.Shafi M.A., Pasricha P.J. Post-surgical and obstructive gastroparesis. Current Gastroenterology Reports. 2007;9(4):280–285. doi: 10.1007/s11894-007-0031-2. [DOI] [PubMed] [Google Scholar]
- 26.Anselmi L., Toti L., Bove C., Hampton J., Travagli R.A. A Nigro-Vagal pathway controls gastric motility and is affected in a rat model of Parkinsonism. Gastroenterology. 2017;153(6):1581–1593. doi: 10.1053/j.gastro.2017.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng L.-F., Song J., Fan R.-F., Chen C.-L., Ren Q.-Z., Zhang X.-L., et al. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiologica. 2014;211(2):434–446. doi: 10.1111/apha.12229. [DOI] [PubMed] [Google Scholar]
- 28.Alhadeff A.L., Mergler B.D., Zimmer D.J., Turner C.A., Reiner D.J., Schmidt H.D., et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology. 2017;42(7):1471–1479. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egerod K.L., Petersen N., Timshel P.N., Rekling J.C., Wang Y., Liu Q., et al. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Molecular Metabolism. 2018;12:62–75. doi: 10.1016/j.molmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vana V., Laerke M.K., Rehfeld J.F., Arnold M., Dmytriyeva O., Langhans W., et al. Vagal afferent cholecystokinin receptor activation is required for glucagon-like peptide-1-induced satiation. Diabetes, Obesity and Metabolism. 2022;24(2):268–280. doi: 10.1111/dom.14575. [DOI] [PubMed] [Google Scholar]
- 31.Imbernon M., Saponaro C., Helms H.C.C., Duquenne M., Fernandois D., Deligia E., et al. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metabolism. 2022;34(7):1054–1063. doi: 10.1016/j.cmet.2022.06.002. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto H., Kishi T., Lee C.E., Choi B.J., Fang H., Hollenberg A.N., et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23(7):2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stinson S.E., Jonsson A.E., Lund M.A.V., Frithioff-Bøjsøe C., Aas Holm L., Pedersen O., et al. Fasting plasma GLP-1 is associated with overweight/obesity and cardiometabolic risk factors in children and adolescents. The Journal of Clinical Endocrinology & Metabolism. 2021;106(6):1718–1727. doi: 10.1210/clinem/dgab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L., Sun Y., Wang W., Wang L., Li C. Increased of fasting active glucagon-like peptide-1 is associated with insulin resistance in patients with hypertriglyceridemia. International Journal of Diabetes in Developing Countries. 2022;42(2):211–217. doi: 10.1007/s13410-021-00971-3. [DOI] [Google Scholar]
- 35.Higgins V., Asgari S., Hamilton J.K., Wolska A., Remaley A.T., Hartmann B., et al. Postprandial dyslipidemia, hyperinsulinemia, and impaired gut peptides/bile acids in adolescents with obesity. The Journal of Clinical Endocrinology and Metabolism. 2020;105(4) doi: 10.1210/clinem/dgz261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matikainen N., Söderlund S., Björnson E., Bogl L.H., Pietiläinen K.H., Hakkarainen A., et al. Fructose intervention for 12 weeks does not impair glycemic control or incretin hormone responses during oral glucose or mixed meal tests in obese men. Nutrition, Metabolism, and Cardiovascular Diseases: Nutrition, Metabolism, and Cardiovascular Diseases. 2017;27(6):534–542. doi: 10.1016/j.numecd.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Duca F.A., Sakar Y., Covasa M. Combination of obesity and high-fat feeding diminishes sensitivity to GLP-1R agonist exendin-4. Diabetes. 2013;62(7):2410–2415. doi: 10.2337/db12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waise T.M.Z., Toshinai K., Naznin F., NamKoong C., Md Moin A.S., Sakoda H., et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochemical and Biophysical Research Communications. 2015;464(4):1157–1162. doi: 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- 39.Al Helaili A., Park S.J., Beyak M.J. Chronic high fat diet impairs glucagon like peptide-1 sensitivity in vagal afferents. Biochemical and Biophysical Research Communications. 2020;533(1):110–117. doi: 10.1016/j.bbrc.2020.08.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.