Abstract
Objective
To assess the impact of elevated body mass index (BMI) in the achievement of minimal disease activity (MDA) and several definitions of remission in patients with psoriatic arthritis (PsA) in Switzerland. Secondarily, to assess the overlapping across the study outcomes.
Methods
This observational cohort study in the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) registry included patients with PsA starting their first biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) from 1997 to 30 June 2018. Exposure was BMI category at b/tsDMARD start: overweight, obese, and normal weight (reference). Logistic regression was used to assess the achievement of MDA and remission at ≤12 months, as well as treatment persistence at 1 year, in overweight patients and patients with obesity compared with the normal weight group. Remission was defined by Disease Activity for Psoriatic Arthritis (DAPSA), clinical DAPSA (cDAPSA) and 28-joint Disease Activity Score (DAS28). Additionally, overlapping across study outcomes was investigated.
Results
The study included 306 (39.5%) normal weight patients, 285 (36.8%) overweight patients and 183 (23.6%) patients with obesity. Compared with the normal weight group, patients with obesity had lower odds of achieving MDA at ≤12 months (adjusted OR (ORadj) 0.45, 95% CI 0.24 to 0.82). This was consistent with the observed reduced odds of achieving DAPSA-remission (ORadj 0.42, 95% CI 0.21 to 0.85), cDAPSA-remission (ORadj 0.51, 95% CI 0.27 to 0.96) and DAS28-remission (ORadj 0.51, 95% CI 0.32 to 0.81) in patients with obesity versus normal weight patients. Among the 125 patients achieving MDA, the majority (81.8% normal weight, 80.0% overweight, 78.9% obese) achieved cDAPSA-remission. No differences were observed in the odds to achieving treatment persistence between the BMI strata.
Conclusions
Obesity halved the likelihood of achieving MDA and remission in patients with PsA with b/tsDMARDs compared with those with normal weight, while it did not impact treatment persistence. High overlapping of patients achieving the outcomes MDA and cDAPSA-remission was observed across every BMI group.
Keywords: rheumatology, epidemiology, rheumatology
Strengths and limitations of this study.
The Swiss Clinical Quality Management in Rheumatic Diseases is a nationwide rheumatology registry that represents one of the largest cohorts of patients with rheumatic diseases, including psoriatic arthritis.
The availability of comprehensive patient information—including data on patient characteristics, clinical features and medication—captured the study exposure, outcome and relevant confounders.
Multiple outcomes of clinical success could be evaluated, including minimal disease activity and remission according to Disease Activity for Psoriatic Arthritis (DAPSA), clinical DAPSA and 28-joint Disease Activity Score, thereby increasing the robustness of our results.
Due to the observational nature of the data, missingness was an intrinsic limitation, however, we used multiple imputation to complete baseline variables relevant for the statistical analyses.
The effect on unidimensional outcomes (eg, dactylitis, axial involvement) was not investigated due to the limited number of patients, however, this remains of interest for future studies.
Introduction
Psoriatic arthritis (PsA) is an immune-mediated rheumatic disease,1 with an estimated prevalence of 0.05%–0.42%,2–4 and 5%–41% among patients with psoriasis.3 PsA is a complex and multifactorial disease,5 for which pathological features include musculoskeletal involvement, such as inflammation of the peripheral joints (arthritis), the entheses (enthesitis), the axial skeleton (spondylitis) and the finger and toe digits (dactylitis), as well as extra-articular manifestations involving skin and nails, and potentially other organs.6 Pharmacological treatments include conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs).3 Treatment of PsA aims to maximise health-related quality of life (QoL), through targeting symptoms and structural damage,7 and it is recommended to target low disease activity, minimal disease activity (MDA) or remission.6
One of the most common comorbidities in patients with PsA is obesity,1 8 and higher prevalence of obesity has been reported among patients with PsA (23%–37%) compared with the general population.9–12 Among patients with PsA, obesity has been associated to lower probability of achieving MDA compared with patients with normal weight.10 13 14 Similarly, patients with obesity who have PsA treated with tumour necrosis factor alfa inhibitors (TNFi) showed higher risk of treatment discontinuation compared with patients without obesity,15 as well as lower odds of achieving treatment response compared with patients without obesity15 or normal weight patients.16
The rationale behind the association between obesity and PsA has been previously discussed.5 17 18 In short, obesity has been described as a low-grade inflammatory disease,18 and both obesity and PsA share pathological inflammatory pathways.5 18 19 Further evidence supporting the association between obesity and a worse PsA clinical outcome is the association of weight loss with higher rate of achieving MDA.20 Additionally, obesity is a well-known contributor to the metabolic syndrome (MetS), and MetS was similarly associated with lower likelihood of achieving MDA in patients with PsA.21
Despite the growing evidence on the association between obesity and worse clinical response in patients with PsA, most published observational cohort studies on this topic had relatively small sample size. For example, a systematic review investigating the association between obesity and response in immune-mediated inflammatory diseases identified one randomised clinical trial and eight observational cohort studies in patients with PsA, but six of the included observational cohorts had a sample size ≤330.16 Thus, further investigating this effect, especially in a different and bigger population cohort, remains of interest. Additionally, it is unclear whether the findings would remain consistent across outcome definitions.
Thus, we seek to contribute to the growing body of evidence by performing an observational cohort study aiming to assess the impact of body mass index (BMI) in the achievement of MDA and remission in patients with PsA. Additionally, by including several outcome definitions we aim to investigate the consistency of the findings when considering different aspects of the disease.
Methods
Study design and data source
We performed an observational cohort study in the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) registry from 1 January 1997 to 31 July 2019. The SCQM is a national longitudinal population-based cohort of rheumatic diseases in Switzerland, initiated in 1997.22 SCQM data are recorded during routine clinical practice, and include information on demographics, body height and weight, life-style habits, antirheumatic medication (with start and stop dates), clinical end points, patient-reported outcomes and health standardised surveys.12 22 Diagnosis of PsA is recorded in SCQM following the physician’s criteria.
Study population
Patients with PsA (≥18 years old) starting their first b/tsDMARD in the SCQM registry between 1 June 2020 and 30 June 2018 (inclusive) were included in the study. The first recorded start of b/tsDMARD in the SCQM was defined as the index date. Patients with a b/tsDMARD start date before their first registered visit at SCQM were excluded. Similarly, patients without a baseline record on height and weight were excluded.
Exposure
The exposure of interest was BMI category at the start of the patients’ first b/tsDMARD. Baseline BMI (kg/m2) was calculated using height and weight records (online supplemental equation 1) at index date or as close as possible to this date within a 6-month look-back window. Measures of height and weight are taken in the clinic, during routine visits to the rheumatologist. Patients were classified based on BMI as normal weight (BMI <25 kg/m2), overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥30 kg/m2). The normal weight group was the reference category.
bmjopen-2022-061474supp001.pdf (1.1MB, pdf)
Outcomes
The primary outcome was defined as achievement of MDA within the first year after the index date. MDA was achieved if at least five of the following seven criteria were met: number of tender joint counts (TJC) ≤1; number of swollen joint counts (SJC) ≤1; skin manifestation none or almost none; patient’s joint pain by visual analogue scale (VAS, 0–100) ≤15; patient’s assessment on PsA activity by VAS ≤20; Health Assessment Questionnaire (HAQ) ≤0.5; enthesis points ≤1.23
Secondary outcomes assessed within the first year were: achievement of Disease Activity for Psoriatic Arthritis (DAPSA) remission, defined as DAPSA ≤4; DAPSA-remission or low disease activity (DAPSA-remLDA), defined as DAPSA ≤14; clinical DAPSA (cDAPSA) remission, defined as cDAPSA ≤4 and 28-joint Disease Activity Score (DAS28) remission, defined as DAS28 <2.6. DAPSA, cDAPSA and DAS28 formulas are described in the online supplemental equations 2–5. DAS28-remission was calculated using erythrocyte sedimentation rate (ESR; DAS28-ESR), however, in cases where follow-up data on DAS28-ESR was missing, DAS28 with C reactive protein (CRP; DAS28-CRP) was used instead, if available.
As a tertiary outcome, persistence with the first b/tsDMARD at the end of month 12 was assessed. We allowed for a permissible gap of 1 month between treatment courses of the same b/tsDMARD, as illustrated in the online supplemental figure S1.
Patients with missing information on the study outcomes during the follow-up were categorised as not having achieved the corresponding outcome. In a sensitivity analysis, we re-ran our analyses excluding patients with missing information on outcome during follow-up.
Follow-up
For primary and secondary outcomes, patients were followed from index date until achievement of outcome or a maximum follow-up of 12 months. For the tertiary outcome (treatment persistence), patients were followed until the earliest of the following: treatment stop, start of a new b/tsDMARD or end of observation period (12 months).
In a secondary analysis, all outcomes were assessed with a maximum follow-up of 9 months and 15 months. This was done to investigate if the findings would differ across shorter and longer follow-up times.
Covariates
Baseline variables included demographics, BMI, high education, ever smoking, antirheumatic medication (ie, b/tsDMARD, csDMARD, corticosteroid), inflammatory markers or acute phase reactants (ie, ESR, CRP), physician’s assessment on disease activity and skin, patient-reported disease activity and pain, tender and swollen joint counts (counting 28 joints), composite disease activity scores (ie, DAPSA, cDAPSA, DAS28-ESR), disease-specific manifestations (ie, musculoskeletal manifestations, dactylitis, enthesitis, sacroilitis, spinal involvement, coxitis, peripheral arthritis, nail manifestation), health standardised surveys (ie, HAQ, Short Form-12 (SF-12)) and comorbidities (ie, cardiovascular event/disease, diabetes or other metabolic problems, depression/anxiety). Baseline variables were collected at index date, or as close as possible to that date within a 6-month look-back window, except for composite disease activity scores, disease-specific manifestations and health standardised surveys, which were collected with a 3-month look-back window; information on smoking, cardiovascular event/disease and diabetes, which was included if ever reported prior or at index date and antirheumatic medication, which was collected on the index date.
Additional information on covariates is included in online supplemental text S1.
Data analysis
Patient baseline characteristics were described, and the overweight and obese categories were compared with the normal weight group (reference group) using χ2 test for categorical variables and t-test, analysis of variance or Kruskal-Wallis test for continuous variables. For these tests, missing values did not function as a grouping variable. Statistical significance was defined as p≤0.05.
Subsequently, missingness for key baseline variables was addressed with multiple imputation by chained equation (MICE) using the mice package24 in the R Statistical Software.25 MICE was performed for each study outcome separately, using 50 imputations with 15 interactions for each set. Variables included in the imputations, their original missingness and corresponding applied imputation models are presented in the online supplemental table S1. The 48.32% of the study population had complete information on every variable included in the MICE for the main analysis (online supplemental figure S2). Convergence of imputations was assessed by visual inspection of density plots (online supplemental figure S3).
To investigate the association between BMI categories and the study outcomes, multivariable logistic regression models were conducted (outcome specific) for individual imputed datasets, and the results were pooled to a single estimate according to Rubin’s rules. These models were conducted first, including only sex and age as covariates, and second, adding clinical confounders (full-adjusted). Confounders were chosen based on clinical rational and direct acyclic graphs (online supplemental figure S4), and included: sex (male; female), age, high education (yes/no), ever smoking (yes/no), b/tsDMARD (TNFi; other biologic; tsDMARD), csDMARD at index date (yes/no) and corticosteroid use at index date (yes/no). Additionally, sensitivity analyses were performed whereby we added the respective composite disease activity score or health standardised survey to the fully adjusted models for primary and secondary outcomes to assess their potential mediating impact on the analyses. Another sensitivity analysis addressed the 1-year outcomes after excluding patients with underweight (BMI <18.5 kg/m2)
Lastly, to compare the overlapping across study outcomes, the proportion of patients achieving each outcome (per BMI group) was summarised, and the overlapping of patients achieving individual primary and secondary outcomes during the first year was illustrated with a Venn diagram.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
The study included 774 adult patients with PsA starting their first b/tsDMARD. Online supplemental figure S5 illustrates the cohort selection process. Among included patients, 306 (39.53%) were normal weight, 285 (36.82%) were overweight and 183 (23.64%) were obese. Baseline patient characteristics (prior to imputation) are presented in table 1. Compared with the normal weight group, overweight patients had higher SJC, were less frequently women and had older mean age. Both overweight patients and patients with obesity had lower frequency of high education, and higher patient-reported disease activity and joint pain, while only patients with obesity had higher CRP levels. Compared with the normal weight category, DAPSA and DAS28 were elevated in the overweight group, while cDAPSA was higher in both overweight and obese BMI categories. HAQ and SF-12 with physical components were worse in the patients with obesity, and patients with obesity were more likely to have had a cardiovascular event/disease than the normal weight group.
Table 1.
Patient characteristics at start of first biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD), prior imputation, stratified by body mass index (BMI)
| Normal weight (n=306) | Overweight (n=285) | P value | Obese (n=183) | P value | |
| Sex, women | 172 (56.21) | 126 (44.21) | 0.01 | 101 (55.19) | 0.90 |
| Age, years (mean (SD)) | 47.59 (13.20) | 50.60 (12.52) | 0.01 | 49.50 (11.03) | 0.10 |
| High education (high technical school or university) | 80 (26.14) | 42 (14.74) | 0.00 | 27 (14.75) | 0.01 |
| Missing | 54 (17.65) | 51 (17.89) | 41 (22.4) | ||
| Smoker (ever smoker) | 77 (25.16) | 84 (29.47) | 0.28 | 54 (29.51) | 0.35 |
| Disease duration, years (mean (SD)) | 5.85 (8.07) | 5.54 (6.98) | 0.63 | 4.51 (6.02) | 0.06 |
| Missing | 6 (1.96) | 6 (2.11) | 5 (2.73) | ||
| b/tsDMRAD | 0.87 | 0.35 | |||
| TNFi biologic* | 279 (91.18) | 262 (91.93) | 160 (87.43) | ||
| Other biologic | 9 (2.94) | 9 (3.16) | 6 (3.28) | ||
| tsDMARD‡ | 18 (5.88) | 14 (4.91) | 17 (9.29) | ||
| csDMARD at index | 152 (49.67) | 151 (52.98) | 0.47 | 100 (54.64) | 0.33 |
| Corticosteroid (prednisone) at index | 38 (12.42) | 38 (13.33) | 0.83 | 17 (9.29) | 0.36 |
| HLA-B27+ | 39 (12.75) | 28 (9.82) | 0.30 | 20 (10.93) | 0.88 |
| Missing | 141 (46.08) | 132 (46.32) | 92 (50.27) | ||
| ESR (mm/hour) (median (IQR)) | 10.00 (5.00, 22.00) | 12.00 (6.00, 22.00) | 0.15 | 15.00 (6.00, 23.00) | 0.10 |
| Missing | 38 (12.42) | 43 (15.09) | 24 (13.11) | ||
| CRP (mg/dL) (median (IQR)) | 0.52 (0.20, 0.90) | 0.60 (0.30, 1.10) | 0.18 | 0.80 (0.40, 1.20) | 0.03 |
| Missing | 48 (15.69) | 52 (18.25) | 27 (14.75) | ||
| Swollen joint counts (0–66) (mean (SD)) | 4.70 (5.31) | 5.78 (7.17) | 0.05 | 4.88 (5.34) | 0.73 |
| Missing | 36 (11.76) | 18 (6.32) | 18 (9.84) | ||
| Tender joint counts (0–68) (mean (SD)) | 8.20 (9.23) | 9.18 (10.36) | 0.25 | 8.72 (9.80) | 0.58 |
| Missing | 36 (11.76) | 18 (6.32) | 19 (10.38) | ||
| Physician global disease activity (1–10) (mean (SD)) | 4.42 (2.04) | 4.58 (1.88) | 0.32 | 4.41 (1.85) | 0.96 |
| Missing | 16 (5.23) | 9 (3.16) | 6 (3.28) | ||
| Physician global skin manifestation | 0.11 | 0.07 | |||
| None | 75 (24.51) | 48 (16.84) | 31 (16.94) | ||
| Almost none | 55 (17.97) | 55 (19.3) | 34 (18.58) | ||
| Mild | 56 (18.3) | 66 (23.16) | 36 (19.67) | ||
| Mild to moderate | 35 (11.44) | 30 (10.53) | 18 (9.84) | ||
| Moderate | 27 (8.82) | 35 (12.28) | 33 (18.03) | ||
| Moderate to severe | 19 (6.21) | 28 (9.82) | 13 (7.10) | ||
| Severe | 9 (2.94) | 6 (2.11) | 4 (2.19) | ||
| Missing | 30 (9.80) | 17 (5.96) | 14 (7.65) | ||
| Patient’s assessment on PsA activity (1–10) (mean (SD)) | 5.08 (2.73) | 5.57 (2.50) | 0.05 | 6.05 (2.56) | 0.00 |
| Missing | 82 (26.80) | 57 (20.00) | 46 (25.14) | ||
| Patient’s joint pain (1–10) (mean (SD)) | 4.88 (2.65) | 5.48 (2.39) | 0.01 | 6.18 (2.36) | <0.001 |
| Missing | 76 (24.84) | 54 (18.95) | 44 (24.04) | ||
| Musculoskeletal manifestations | 232 (75.82) | 213 (74.74) | 0.84 | 140 (76.50) | 0.95 |
| Dactylitis | 101 (33.01) | 106 (37.19) | 0.33 | 66 (36.07) | 0.55 |
| Enthesitis | 116 (37.91) | 103 (36.14) | 0.72 | 67 (36.61) | 0.85 |
| Sacroilitis | 72 (23.53) | 64 (22.46) | 0.83 | 27 (14.75) | 0.03 |
| Spinal involvement | 81 (26.47) | 70 (24.56) | 0.66 | 40 (21.86) | 0.30 |
| Coxitis | 13 (4.25) | 8 (2.81) | 0.47 | 15 (8.20) | 0.11 |
| Peripheral arthritis | 141 (46.08) | 138 (48.42) | 0.63 | 94 (51.37) | 0.30 |
| Nail manifestation | 64 (20.92) | 62 (21.75) | 0.88 | 47 (25.68) | 0.27 |
| DAPSA (mean (SD)) | 23.14 (15.73) | 27.94 (18.23) | 0.01 | 26.56 (14.18) | 0.07 |
| Missing | 118 (38.56) | 103 (36.14) | 77 (42.08) | ||
| cDAPSA (mean (SD)) | 22.04 (15.21) | 26.39 (17.57) | 0.01 | 25.60 (13.70) | 0.04 |
| Missing | 107 (34.97) | 80 (28.07) | 71 (38.80) | ||
| DAS28-ESR (mean (SD)) | 3.34 (1.26) | 3.61 (1.33) | 0.02 | 3.44 (1.22) | 0.43 |
| Missing | 51 (16.67) | 49 (17.19) | 34 (18.58) | ||
| SF-12 mcs (mean (SD)) | 45.87 (11.36) | 45.11 (11.66) | 0.49 | 43.85 (11.68) | 0.11 |
| Missing | 77 (25.16) | 78 (27.37) | 51 (27.87) | ||
| SF-12 pcs (mean (SD)) | 38.95 (10.67) | 37.63 (9.71) | 0.18 | 35.79 (9.04) | 0.01 |
| Missing | 77 (25.16) | 78 (27.37) | 51 (27.87) | ||
| HAQ (mean (SD)) | 0.71 (0.66) | 0.79 (0.58) | 0.20 | 0.93 (0.61) | 0.00 |
| Missing | 60 (19.61) | 59 (20.70) | 48 (26.23) | ||
| Cardiovascular event/disease | 26 (8.50) | 39 (13.68) | 0.06 | 31 (16.94) | 0.01 |
| Diabetes or other metabolic problems | 10 (3.27) | 20 (7.02) | 0.06 | 14 (7.65) | 0.05 |
| Depression/Anxiety | 13 (4.25) | 17 (5.96) | 0.45 | 10 (5.46) | 0.69 |
Values are the number and column percentage, unless otherwise specified. Significance tests compare overweight or obese categories with the normal weight group (reference) using χ2 test for categorical variables, and t-test or analysis of variance for continuous variables, but Kruskal-Wallis test for ESR and CRP. For these tests, missing values did not function as a grouping variable. Normal weight (BMI <25 kg/m2); overweight (BMI 25.0–29.9 kg/m2); obese (BMI ≥30 kg/m2).
*Adalimumab, etanercept, infliximab, certolizumab, golimumab.
†Abatacept, secukinumab, tocilizumab, ustekinumab.
‡Apremilast.
CRP, C reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAPSA, Disease Activity Index for Psoriatic Arthritis; DAS28, 28-joint Disease Activity Score; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; HLA-B27+, human leucocyte antigen B27 positive; mcs, mental component summary; n, sample size; pcs, physical component summary; PsA, psoriasis arthritis; SF-12, Short-Form 12 health survey; TNFi, tumor necrosis factor alpha inhibitor.
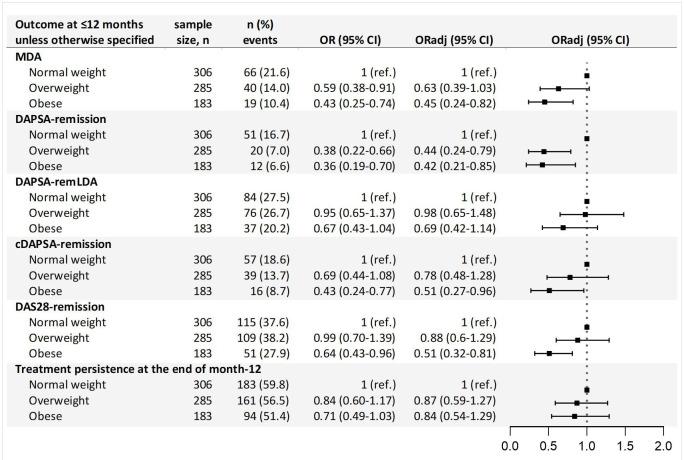
Results from the logistic regression for the primary analysis are presented in figure 1. Compared with the normal weight group, patients with obesity had significantly lower odds of achieving MDA within the first year, with an adjusted OR (ORadj) of 0.45 (95% CI 0.24 to 0.82). Similarly, both overweight patients and patients with obesity had >50% reduced odds of achieving DAPSA-remission (overweight ORadj 0.44 (95% CI 0.24 to 0.79) and obese ORadj 0.42 (95% CI 0.21 to 0.85)), compared with normal weight patients. Additionally, patients with obesity had reduced odds of achieving cDAPSA-remission (ORadj 0.51 (95% CI 0.27 to 0.96)) and DAS28-remission (ORadj 0.51 (95% CI 0.32 to 0.81)) within the first year. No differences were observed across BMI categories on achievement of DAPSA-remLDA or treatment persistence at the end of month 12.
Figure 1.
Results from the multivariable logistic regression investigating the association between body mass index categories and various clinical outcomes. Maximum follow-up 12 months. cDAPSA-remission, clinical Disease Activity for Psoriatic Arthritis (DAPSA) remission; DAPSA-remission, Disease Activity for Psoriatic Arthritis (DAPSA) remission; DAPSA-remLDA, DAPSA remission or low disease activity; DAS28-remission, 28-joint Disease Activity Score remission; MDA, Minimal Disease Activity; n, number; OR, odds ratio adjusting for: sex, age; ORadj, odds ratio adjusting for: sex, age, high educational level, smoker, biologic or targeted synthetic disease-modifying antirheumatic drugs, conventional synthetic disease-modifying antirheumatic drugs, corticosteroid; ref., reference.
The secondary analyses showed that extending the maximum follow-up to 15 months resulted in similar findings to those from the 12 months analyses (table 2). However, in the 9-month analyses, the associations of obesity with DAPSA-remission and with cDAPSA-remission were no longer significant (table 2).
Table 2.
Result from the multivariable logistic regression investigating the association between body mass index (BMI) categories and various clinical outcomes, with maximum follow-up 9 months and 15 months
| sample size, n | Maximum follow-up 9 months | Maximum follow-up 15 months | |||||
| n (%) events | OR (95% CI) | ORadj (95% CI) | N events | OR (95% CI) | ORadj (95% CI) | ||
| MDA | |||||||
| Normal weight | 306 | 45 (14.7) | 1 (ref.) | 1 (ref.) | 86 (28.1) | 1 (ref.) | 1 (ref.) |
| Overweight | 285 | 21 (7.4) | 0.47 (0.27–0.82) | 0.52 (0.28–0.96) | 61 (21.4) | 0.67 (0.45–0.98) | 0.75 (0.48–1.15) |
| Obese | 183 | 12 (6.6) | 0.41 (0.21–0.80) | 0.44 (0.21–0.94) | 30 (16.4) | 0.50 (0.31–0.80) | 0.57 (0.34–0.96) |
| DAPSA-remission | |||||||
| Normal weight | 306 | 31 (10.1) | 1 (ref.) | 1 (ref.) | 67 (21.9) | 1 (ref.) | 1 (ref.) |
| Overweight | 285 | 11 (3.9) | 0.35 (0.17–0.72) | 0.40 (0.18–0.88) | 31 (10.9) | 0.42 (0.26–0.68) | 0.50 (0.30–0.84) |
| Obese | 183 | 8 (4.4) | 0.41 (0.18–0.92) | 0.49 (0.20–1.18) | 17 (9.3) | 0.37 (0.21–0.67) | 0.47 (0.25–0.87) |
| DAPSA-remLDA | |||||||
| Normal weight | 306 | 47 (15.4) | 1 (ref.) | 1 (ref.) | 117 (38.2) | 1 (ref.) | 1 (ref.) |
| Overweight | 285 | 37 (13.0) | 0.81 (0.51–1.30) | 0.88 (0.52–1.50) | 104 (36.5) | 0.91 (0.65–1.27) | 0.90 (0.62–1.31) |
| Obese | 183 | 22 (12.0) | 0.75 (0.43–1.29) | 0.75 (0.40–1.40) | 52 (28.4) | 0.64 (0.43–0.95) | 0.66 (0.42–1.03) |
| cDAPSA-remission | |||||||
| Normal weight | 306 | 36 (11.8) | 1 (ref.) | 1 (ref.) | 77 (25.2) | 1 (ref.) | 1 (ref.) |
| Overweight | 285 | 22 (7.7) | 0.62 (0.35–1.09) | 0.70 (0.38–1.30) | 53 (18.6) | 0.65 (0.43–0.98) | 0.75 (0.48–1.16) |
| Obese | 183 | 12 (6.6) | 0.53 (0.27–1.06) | 0.64 (0.31–1.35) | 23 (12.6) | 0.43 (0.26–0.72) | 0.55 (0.32–0.95) |
| DAS28-remission | |||||||
| Normal weight | 306 | 68 (22.2) | 1 (ref.) | 1 (ref.) | 153 (50) | 1 (ref.) | 1 (ref.) |
| Overweight | 285 | 64 (22.5) | 1.01 (0.68–1.49) | 0.91 (0.58–1.43) | 140 (49.1) | 0.91 (0.65–1.28) | 0.89 (0.61–1.3) |
| Obese | 183 | 29 (15.8) | 0.67 (0.41–1.08) | 0.50 (0.28–0.89) | 70 (38.3) | 0.62 (0.42–0.91) | 0.57 (0.36–0.88) |
| Treatment persistence at the end of follow-up | |||||||
| Normal weight | 306 | 204 (66.7) | 1 (ref.) | 1 (ref.) | 159 (52) | 1 (ref.) | 1 (ref.) |
| Overweight | 285 | 184 (64.6) | 0.86 (0.60–1.21) | 0.91 (0.60–1.36) | 148 (51.9) | 0.96 (0.69–1.34) | 0.97 (0.67–1.42) |
| Obese | 183 | 111 (60.7) | 0.77 (0.52–1.12) | 0.91 (0.57–1.44) | 81 (44.3) | 0.73 (0.51–1.07) | 0.87 (0.57–1.33) |
cDAPSA-remission, clinical Disease Activity for Psoriatic Arthritis (DAPSA) remission; DAPSA-remission, Disease Activity for Psoriatic Arthritis (DAPSA) remission; DAPSA-remLDA, DAPSA remission or low disease activity; DAS28-remission, 28-joint Disease Activity Score remission; MDA, Minimal Disease Activity; n, number; OR, odds ratio adjusting for: sex, age; ORadj, adjusting for: sex, age, high educational level, smoker, b/tsDMARDbiologic or targeted synthetic disease-modifying antirheumatic drug, csDMARDconventional synthetic disease-modifying antirheumatic drugs, corticosteroid.; ref., reference.
In the sensitivity analysis in which the respective composite disease activity score or health standardised survey was included in the model, the previously observed findings in the high BMI groups were attenuated, with the exception of obesity and achievement of MDA (online supplemental table S2). The sensitivity analysis excluding patients with missing information on outcome during the 1-year follow-up yielded stronger reduced odds of achieving MDA and remission among abnormal BMI categories versus the normal weight group (online supplemental table S3). The sensitivity analysis excluding the 12 patients with BMI <18.5 kg/m2 yielded similar results to the main study findings (online supplemental table S4).
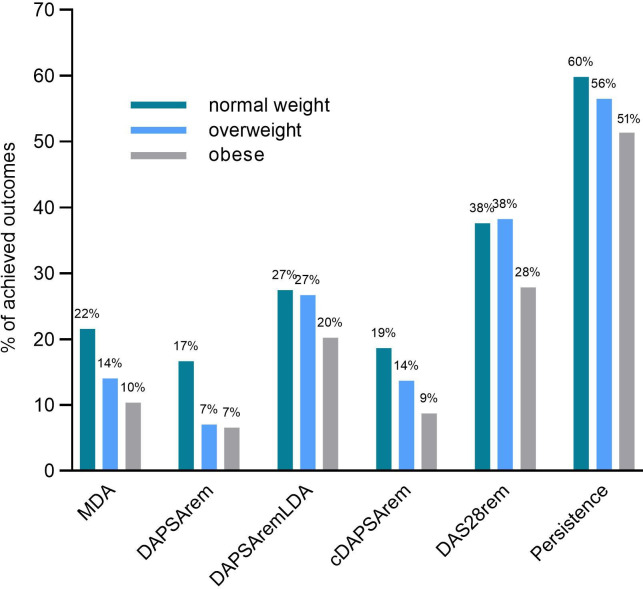
The frequency of achieved outcomes (with 12 months follow-up) per BMI category are presented in figure 2. Overall, 125 patients achieved MDA, 83 DAPSA-remission, 197 DAPSA-remLDA, 112 cDAPSA-remission and 275 DAS28-remission within the first year. Across all outcomes, patients with obesity had a lower prevalence of achieved outcomes. DAS28-remission and treatment persistence had the highest prevalence in all groups, with 37.58% and 59.80% achieved among normal weight patients and 27.87% and 51.37% among patients with obesity, respectively.
Figure 2.
Distribution of patients achieving the study primary and secondary outcomes within the first year, and percentage of patients achieving treatment persistence at the end of month 12, stratified by body mass index category. cDAPSArem, clinical Disease Activity for Psoriatic Arthritis (DAPSA) remission; DAPSArem, DAPSA remission; DAPSA-remLDA, DAPSA remission or low disease activity; DAS28rem, 28-joint Disease Activity Score remission; MDA, Minimal Disease Activity.
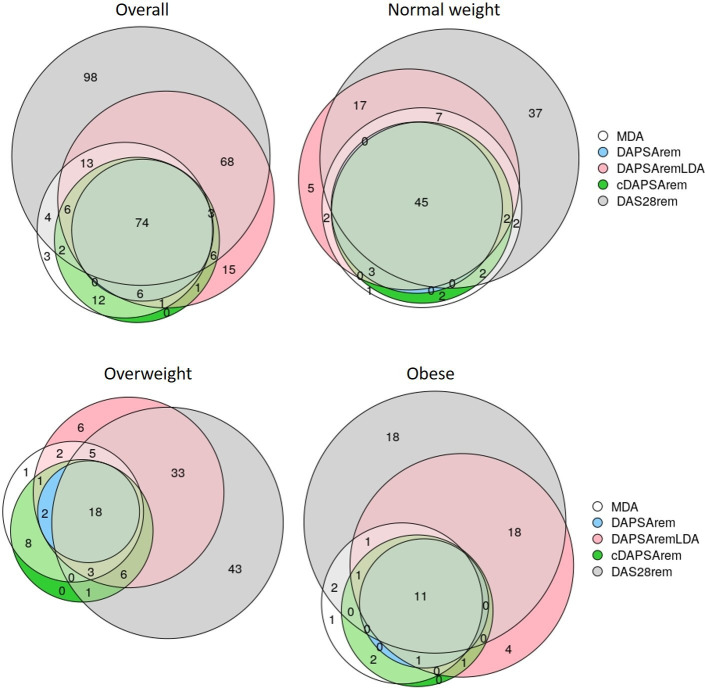
The overlap of patients achieving the outcomes during the first year is illustrated in figure 3, complemented with numerical values in online supplemental table S5. Among the 125 patients achieving MDA (66 normal weight, 40 overweight, 19 with obesity), 80 also achieved DAPSA-remission, of which 48 (72.73%) were normal weight, 20 (50.00%) were overweight and 12 (63.16%) were with obesity. Similarly, among patient with MDA, 54 (81.82%) normal weight, 32 (80.00%) overweight and 15 (78.95%) patients with obesity also achieved cDAPSA-remission. Additionally, MDA overlapped with every remission outcome in 45 (68.18%) normal weight, 18 (45.00%) overweight and 11 (57.89%) patients with obesity.
Figure 3.
Venn diagram depicting the number of patients (counts) achieving the study individual primary and secondary outcomes within the first year, overall and stratifying by body mass index category. cDAPSArem, clinical Disease Activity for Psoriatic Arthritis (DAPSA) remission; DAPSArem, DAPSA remission; DAPSAremLDA, DAPSA remission or low disease activity; DAS28rem, 28-joint Disease Activity Score remission; MDA, Minimal Disease Activity.
Discussion
This observational cohort study found that patients with obesity had a significant 49%–58% reduced odds of achieving MDA, DAPSA-remission, cDAPSA-remission and DAS28-remission within the first year, when compared with normal weight patients. Conversely, being overweight was only associated with a reduced odds of achieving DAPSA remission. In both high BMI categories, the association with achievement of DAPSA-remLDA within the first year and with 1-year treatment persistence, were not statistically significant. Among patients who achieved MDA, the majority also achieved cDAPSA-remission.
Our findings on the association between obesity and lower probability of reaching MDA and remission are consistent with other longitudinal observational studies.10 13 15 In the prospective study by Di Minno et al, obesity was associated with increased risk of not achieving MDA during a 12 months follow-up compared with patients with BMI <30 kg/m2 (HR 4.90, 95% CI 3.04 to 7.87).13 Eder et al reported that, compared with normal weight patients (BMI <25 kg/m2), overweight patients and patients with obesity had 34% and 47% significantly reduced odds of achieving MDA, respectively.10 While we identified a similar OR in the overweight patients and patients with obesity, our results in the overweight group were not statistically significant. In the study by Højgaard et al, obesity was associated with 53% lower odds of achieving European Alliance of Associations for Rheumatology good or moderate (EGOM) response.15 While we did not assess EGOM response, this is a DAS28-driven outcome, and the findings are in agreement with our observed association between obesity and 49% reduced odds for DAS28-remission. Conversely, Iannone et al suggested no significant differences in DAS28-remission rates across BMI categories.26 However, they had a small sample size (135 patients), and their observed lower remission rate in the obese vs normal weight patients was in line with our findings.
Additionally, results from Højgaard et al showed that compared with patients without obesity (BMI <30 kg/m2), patients with obesity were associated with a 60% higher risk of TNFi discontinuation during their study period (median follow-up of 1.5 years).15 While our study did not yield an association between BMI and treatment persistence, these contrasting findings may be explained by the different methodologies. Højgaard et al assessed the time to withdrawal using a survival model, which gives high attention to early outcomes, while we investigated persistence yes/no at a specific timepoint using logistic regression.
In our study, MDA was the main outcome as it covers several aspects from the disease presentation and consequences, and has been associated with patient’s QoL and productivity.27 Additionally, McGagh and Coates suggested that the 66/68 joint counts provide a more realistic picture of joint involvement in PsA, compared with the 28 joint counts, and highlighted the benefits of including patient-reported outcomes.28 Based on this, we identified DAPSA-remission and cDAPSA-remission as optimal secondary outcomes. However, we expect that cDAPSA may be a better fit to study patients with abnormal BMI since obesity was associated with elevated CRP in the general population.29–31 This is further supported by the high overlap of patients achieving MDA and cDAPSA-remission in our study, which was similar across every BMI group.
Regarding the observed higher frequency of achievement of DAS28-remission compared with other remission end points, this may be explained by its narrow focus on peripheral manifestations, potentially underestimating residual disease activity. Nevertheless, the consistency of the observed results on MDA and remission outcomes in the obese group suggests that obesity affects peripheral joints, as well as disease-specific manifestations and the patient’s perspective. However, we note that the different outcome definitions led to contrasting results in the overweight group, suggesting that the effect of overweight on the PsA may not be fully captured by every remission definition. Similarly, the impact of obesity on PsA clinical response was not consistent with the more clinically accessible outcome low disease activity (DAPSA-remLDA).
The reasons for the lower response rates in patients with obesity could be multiple. High body weight can affect the clearance and volume of distribution of b/tsDMARDs.32–34 Adipose tissue has a proinflammatory capacity,35 which could negatively influence drug response. Finally, a relationship between mechanical stress and triggering of musculoskeletal inflammation (deep Köbner phenomenon) in PsA is discussed. Nevertheless, the observed lower odds of achieving MDA or remission in the obese group is of interest, and the consistency across the studied definitions of remission suggests that this effect may be reflected on several factors of the PsA disease.
Finally, as described elsewhere,12 the prevalence of overweight and obesity were higher among patients with PsA in comparison to the general population in Switzerland (Switzerland 2017, people >15 years old, 31% overweight and 11% obese).36 Higher obesity prevalence among patients with PsA in comparison to the reference population was in agreement with prior studies.12
Strengths and limitations
In addition to the large sample size and availability of BMI information (often lacking in real-world data), the key strength of this study is the use of several relevant clinical outcome definitions. While multiple approaches to assess PsA disease activity exist, no single one has been identified as sufficient37 and the choice of the optimal measure remains challenging.28 The consistency of the observed results on MDA and remission outcomes in the obese group reinforces the study findings. However, we did not look at unidimensional outcomes (eg, dactylitis) and this remains of interest for future studies. Additionally, while standard MDA definition includes Psoriasis Activity and Severity Index ≤1 or body surface area ≤3,38 due to data restrictions our MDA definition included a skin manifestation of ‘none’ or ‘almost none’, as reported by the physician.
We did not require a minimum time between treatment start and outcome record. In a post hoc test, we identified that the median time to the record for MDA assessment was between 214 and 245 days, similar across the BMI groups. Additionally, patients could have records of the outcome variable(s) at more than one visit during follow-up. When more than one record was available, all were assessed to identify if successful outcome was achieved.
Intrinsic to real-world data, missingness was a limitation. We addressed missingness at baseline with multiple imputation and missingness during follow-up with sensitivity analyses. Our results were mainly consistent among various sensitivity analyses. For example, the secondary analysis excluding patients who missed information on the outcome during follow-up (instead of treating them as non-achievers of the respective outcome), supported the observed effect of obesity towards MDA and remission, which was even accentuated in this sensitivity analysis. Among secondary analyses varying the duration of follow-up, the 15-month analyses showed consistence with the main findings, and the reduced effect found in the 9-month analyses may be explained by higher missingness of outcome information at shorter follow-up, and therefore lower number of observed events overall.
Limitations to consider when interpreting the results include the potential misclassification of patients in the BMI categories. While overweight and obesity are commonly defined by BMI,39 40 this lacks information on body composition. Thus, although data on waist circumference, skinfold thickness and bioelectrical impedance may provide a better patient classification, this information is extremely limited in real-world data. Additionally, we classified patients with BMI <25 kg/m2 as normal weight, including patients with BMI <18.5 kg/m2, who may be classified as underweight. This was done due to low prevalence of underweight patients with PsA in SCQM12 and is consistent with previous practice in PsA10 26 and other inflammatory rheumatic diseases research in which the majority of studies combine normal and underweight patients.41
It was suggested that patients with obesity may benefit from other non-TNFi b/tsDMARDs, however, the evidence is limited.42 Nevertheless, our results of a lower odds of achieving remission may be largely driven by the high TNFi use in our cohort.
Finally, since weight loss in overweight patients and patients with obesity was identified as a predictor of MDA achievement,20 it remains of interest to perform a similar study to this one but stratifying the overweight patients and patients with obesity by those with and without weight loss.
Conclusion
This study suggests that obesity in patients with PsA is associated with at least a 50% reduction in the likelihood of achieving MDA or remission within the first year after starting b/tsDMARD therapy, when compared with normal weight patients. The consistency of findings across definitions of remission suggests that obesity affects several factors of PsA disease. Conversely, obesity was neither associated with the likelihood of achieving low disease activity nor with treatment persistence. Finally, comparative analyses of b/tsDMARDs within BMI groups is of interest and investigating the benefits of losing weight in this population remains of interest.
Supplementary Material
Acknowledgments
We thank all patients and rheumatologists contributing to the SCQM registry, as well as the entire SCQM staff. A list of rheumatology offices and hospitals which contribute to the SCQM registry can be found at http://www.scqm.ch/institutions. A list of financial supporters of SCQM can be found at http://www.scqm.ch/sponsors. We would like to add a personal thank you to Axel Finckh (University Hospitals of Geneva) for his input regarding the database. AMB acknowledges that her professorship is partly endowed by the Swiss National Pharmacy Association (PharmaSuisse) and the ETH Foundation.
Footnotes
Twitter: @tiozab
Contributors: EV-Y, TB and AMB contributed to the study conceptualisation and methodology; EV-Y performed data curation, formal analysis, visualisation and investigation; EV-Y wrote the original draft manuscript and TB, RM and AMB contributed with revision and editing. All authors read and agreed to the published version of the manuscript. EV-Y is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data belong to the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) and are available only with the approval and permission from the license holder (SCQM).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was reviewed by the ethics commission of the Canton of Zurich (KEK: Req-2020-00045). Pseudonymised data, without access to the code key, was provided by the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) registry to the researchers. Therefore, the commission waived the need for a full ethics authorisation. Prior enrolment at SCQM, signed informed consent was provided by the patients, in accordance with the Declaration of Helsinki. Additionally, withdrawal of participation is possible at any time.
References
- 1.Kumthekar A, Ogdie A. Obesity and psoriatic arthritis: a narrative review. Rheumatol Ther 2020;7:447–56. 10.1007/s40744-020-00215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salaffi F, De Angelis R, Grassi W. MArche pain prevalence, investigation group (MAPPING) study. prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol 2005;23:819–28. [PubMed] [Google Scholar]
- 3.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. 10.1016/j.rdc.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scotti L, Franchi M, Marchesoni A, et al. Prevalence and incidence of psoriatic arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:28–34. 10.1016/j.semarthrit.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 5.Porta S, Otero-Losada M, Kölliker Frers RA, et al. Adipokines, cardiovascular risk, and therapeutic management in obesity and psoriatic arthritis. Front Immunol 2020;11:590749. 10.3389/fimmu.2020.590749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 7.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700.1–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Syrimi Z, Hughes DM, et al. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int 2021;41:275–84. 10.1007/s00296-020-04775-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhole VM, Choi HK, Burns LC, et al. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatology 2012;51:552–6. 10.1093/rheumatology/ker349 [DOI] [PubMed] [Google Scholar]
- 10.Eder L, Thavaneswaran A, Chandran V, et al. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis 2015;74:813–7. 10.1136/annrheumdis-2013-204448 [DOI] [PubMed] [Google Scholar]
- 11.Eder L, Abji F, Rosen CF, et al. The association between obesity and clinical features of psoriatic arthritis: a case-control study. J Rheumatol 2017;44:437–43. 10.3899/jrheum.160532 [DOI] [PubMed] [Google Scholar]
- 12.Vallejo-Yagüe E, Burkard T, Möller B, et al. Comparison of psoriatic arthritis and rheumatoid arthritis patients across body mass index categories in Switzerland. J Clin Med 2021;10:3194. 10.3390/jcm10143194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.di Minno MND, Peluso R, Iervolino S, et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res 2013;65:141–7. 10.1002/acr.21711 [DOI] [PubMed] [Google Scholar]
- 14.Lupoli R, Pizzicato P, Scalera A, et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther 2016;18:297. 10.1186/s13075-016-1194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Højgaard P, Glintborg B, Kristensen LE, et al. The influence of obesity on response to tumour necrosis factor-α inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology 2016;55:2191–9. 10.1093/rheumatology/kew326 [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: a systematic review and meta-analysis. PLoS One 2018;13:e0195123. 10.1371/journal.pone.0195123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Versini M, Jeandel P-Y, Rosenthal E, et al. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13:981–1000. 10.1016/j.autrev.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Russolillo A, Iervolino S, Peluso R, et al. Obesity and psoriatic arthritis: from pathogenesis to clinical outcome and management. Rheumatology 2013;52:62–7. 10.1093/rheumatology/kes242 [DOI] [PubMed] [Google Scholar]
- 19.Neumann E, Hasseli R, Ohl S, et al. Adipokines and autoimmunity in inflammatory arthritis. Cells 2021;10:10020216:216. 10.3390/cells10020216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Minno MND, Peluso R, Iervolino S, et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis 2014;73:1157–62. 10.1136/annrheumdis-2012-202812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa L, Caso F, Ramonda R, et al. Metabolic syndrome and its relationship with the achievement of minimal disease activity state in psoriatic arthritis patients: an observational study. Immunol Res 2015;61:147–53. 10.1007/s12026-014-8595-z [DOI] [PubMed] [Google Scholar]
- 22.Die SCQM Foundation (Swiss clinical quality management in rheumatic diseases). Available: https://www.scqm.ch/en/ueber-uns/ [Accessed 18 May 2021].
- 23.Coates LC, Strand V, Wilson H, et al. Measurement properties of the minimal disease activity criteria for psoriatic arthritis. RMD Open 2019;5:e001002. 10.1136/rmdopen-2019-001002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van BS, Groothuis-Oudshoorn K. mice: multivariate imputation by Chained equations in R. J Stat Software 2011;45:1–67. [Google Scholar]
- 25.R Core Team . R: a language and environmental for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. https://www.r-project.org/ [Google Scholar]
- 26.Iannone F, Fanizzi R, Scioscia C, et al. Body mass does not affect the remission of psoriatic arthritis patients on anti-TNF-α therapy. Scand J Rheumatol 2013;42:41–4. 10.3109/03009742.2012.715186 [DOI] [PubMed] [Google Scholar]
- 27.Coates LC, Orbai A-M, Morita A, et al. Achieving minimal disease activity in psoriatic arthritis predicts meaningful improvements in patients' health-related quality of life and productivity. BMC Rheumatol 2018;2:24. 10.1186/s41927-018-0030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGagh D, Coates LC. Assessment of the many faces of PSA: single and composite measures in PSA clinical trials. Rheumatology 2020;59:i29–36. 10.1093/rheumatology/kez305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yudkin JS, Stehouwer CDA, Emeis JJ, et al. C-Reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction. Arterioscler Thromb Vasc Biol 1999;19:972–8. 10.1161/01.ATV.19.4.972 [DOI] [PubMed] [Google Scholar]
- 30.Hak AE, Stehouwer CD, Bots ML, et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol 1999;19:1986–91. 10.1161/01.ATV.19.8.1986 [DOI] [PubMed] [Google Scholar]
- 31.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131. 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, Eckert D, Hyams JS, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis 2015;21:783–92. 10.1097/MIB.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 33.Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol 2009;65:1211–28. 10.1007/s00228-009-0718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ternant D, Aubourg A, Magdelaine-Beuzelin C, et al. Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit 2008;30:523–9. 10.1097/FTD.0b013e318180e300 [DOI] [PubMed] [Google Scholar]
- 35.Versini M, Jeandel P-Y, Rosenthal E, et al. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13:981–1000. 10.1016/j.autrev.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 36.für SB. Übergewicht und Adipositas - Schweizerische Gesundheitsbefragung 2017 | Publikation. Bundesamt für Statistik, 2020. Available: https://www.bfs.admin.ch/asset/de/14147705 [Accessed 5 Jun 2022].
- 37.Gülfe A, Geborek P, Saxne T. Response criteria for rheumatoid arthritis in clinical practice: how useful are they? Ann Rheum Dis 2005;64:1186–9. 10.1136/ard.2004.027649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. 10.1136/ard.2008.102053 [DOI] [PubMed] [Google Scholar]
- 39.Body mass index - BMI. Available: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi [Accessed 23 Jun 2021].
- 40.Obesity and overweight. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Accessed 8 Jul 2021].
- 41.Lee YX, Kwan YH, Lim KK, et al. A systematic review of the association of obesity with the outcomes of inflammatory rheumatic diseases. Singapore Med J 2019;60:270–80. 10.11622/smedj.2019057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Queiro R. Cardiometabolic comorbidity in the selection of treatment in spondyloarthritis: one step closer to truly personalized medicine? Expert Opin Biol Ther 2021;21:1539–41. 10.1080/14712598.2022.1998448 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061474supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data belong to the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) and are available only with the approval and permission from the license holder (SCQM).