Abstract
Background
Patients with chronic gastrointestinal (GI) disease such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), celiac disease, gastroesophageal reflux disease (GERD), pancreatitis, and chronic liver disease (CLD) often suffer from obesity because of coincidence (IBD, IBS, celiac disease) or related pathophysiology (GERD, pancreatitis and CLD). It is unclear if such patients need a particular diagnostic and treatment that differs from the needs of lean GI patients. The present guideline addresses this question according to current knowledge and evidence.
Objective
The objective of the guideline is to give advice to all professionals working in the field of gastroenterology care including physicians, surgeons, dietitians and others how to handle patients with GI disease and obesity.
Methods
The present guideline was developed according to the standard operating procedure for European Society for Clinical Nutrition and Metabolism guidelines, following the Scottish Intercollegiate Guidelines Network grading system (A, B, 0, and good practice point [GPP]). The procedure included an online voting (Delphi) and a final consensus conference.
Results
In 100 recommendations (3x A, 33x B, 24x 0, 40x GPP, all with a consensus grade of 90% or more) care of GI patients with obesity – including sarcopenic obesity – is addressed in a multidisciplinary way. A particular emphasis is on CLD, especially fatty liver disease, since such diseases are closely related to obesity, whereas liver cirrhosis is rather associated with sarcopenic obesity. A special chapter is dedicated to obesity care in patients undergoing bariatric surgery. The guideline focuses on adults, not on children, for whom data are scarce. Whether some of the recommendations apply to children must be left to the judgment of the experienced pediatrician.
Conclusion
The present guideline offers for the first time evidence‐based advice how to care for patients with chronic GI diseases and concomitant obesity, an increasingly frequent constellation in clinical practice.
Keywords: bariatric surgery, celiac disease, cirrhosis, gastroesophageal reflux disease, inflammatory bowel disease, irritable bowel syndrome, non‐alcoholic fatty liver disease, obesity, pancreatitis, sarcopenic obesity
INTRODUCTION
The guideline focuses on obesity care in patients with obesity and chronic gastrointestinal diseases including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), celiac disease, gastroesophageal reflux disease (GERD), pancreatitis, and chronic liver disease (CLD). A special chapter is dedicated to obesity care in patients undergoing bariatric surgery. A generally accepted goal of obesity therapy also in patients with concomitant gastrointestinal diseases is the reduction of body weight. More precisely, intervention should lead to a selective reduction of fat mass without reducing muscle mass or lean body mass. This ideal cannot be achieved at 100%, neither by non‐surgical nor by surgical means. Therefore, we still use the term body weight reduction instead of body fat reduction, although the work group fully agrees that any obesity therapy needs to aim at preventing of loss of muscle mass as much as possible.
The guideline focuses on adults, not on children, for whom data are scarce. Whether some of the recommendations apply to children must be left to the judgment of the experienced pediatrician.
Prevalence of obesity and sarcopenic obesity in gastrointestinal diseases
The incidence of IBD is rising in parallel with overweight and obesity. Whereas previously, obesity in IBD, in particular Crohn's disease (CD), has been considered unusual (3%) due to higher rates of inflammation and malabsorptive aspects of CD. 1 Recently, a study demonstrated that obesity is a risk factor for the occurrence of CD, but not ulcerative colitis (UC). 2 Cross‐sectional studies show that about 20%–40% of adult patients with IBD are overweight (25 < body mass index (BMI) < 30 kg/m2), and an additional 15%–40% are obese (BMI >30 kg/m2). 3 In a population‐based Scottish study, 18% of the patients were obese (18% of CD; 17.5% of UC patients) compared with 23% of the general population. 4 In an IBD population starting anti‐tumor necrosis factor‐α medication, 4.9% of the patients with obesity and 14.6% of the patients with overweight were sarcopenic, but also 41.5% of the IBD patients with normal weight had sarcopenia. 5 Furthermore, a systematic review reported that 42% of IBD patients were sarcopenic. 6 Thus, obesity and also sarcopenic obesity are quite common in IBD. This highlights the need for nutritional screening in all IBD patients.
A high prevalence of IBS, threefold that in the general population, was reported in obesity centers. 7 Compared to subjects without obesity, patients with obesity were 2.6 times more likely to have IBS (OR 2.6; 95%CI 1.0–6.4) 8 In a cross‐sectional Persian study, IBS was more prevalent among individuals with abdominal obesity compared with normal subjects (23.8% vs. 19%). 9 However, general or abdominal obesity was not associated with odds of IBS. 9 Women have a 1.5 ‐ 3‐fold higher incidence rate of IBS compared to men. 10 In conclusion, these data suggest that there is an association between IBS and obesity, but ‐ different from IBD ‐ sarcopenic obesity seems to play no major role in IBS.
Celiac disease, GERD, and pancreatitis are possible less clearly associated with obesity, compared for example, to CLD such as non‐alcoholic fatty liver disease (NAFLD) and others, but the obesity pandemic finally comprises virtually all types of gastrointestinal diseases. Therefore, the present guideline refers briefly also to celiac disease, GERD, and pancreatitis, some of the more frequent gastrointestinal diseases in clinical practice.
Europe has the highest prevalence of CLD including alcoholic and non‐alcoholic liver disease as well as chronic virus hepatitis. In 2016, the age‐adjusted prevalence of CLD in 35 European countries ranged from 445 (Iceland) to 1,100 (Romania) cases per 100,000 (median 833). 11 The prevalence of sarcopenia in patients with chronic liver cirrhosis is calculated at 48.1% (range, 25%–70%), higher in men (61.6%) compared to women (36%). 12 Alcohol consumption, obesity, and hepatitis B and C virus infections are the main determinants of CLD. 11 In Western European countries, alcohol contributes predominantly to the etiology of cirrhosis and CLD, whereas viral hepatitis is more prevalent in Eastern and Southern European countries. 11 Given the increasing incidence of obesity across most European countries, the incidence of non‐alcoholic liver disease is expected to rise in the future.
Change in body composition in the course of chronic gastrointestinal and liver diseases
An Australian study in 154 IBD patients (70% CD, median age 31 years) showed an increase of BMI in the first 24 months after diagnosis 13 (annual change β = 0.43, 95%CI 0.18, 0.67, p = 0.0006). The proportion of overweight patients increased from 26% at baseline to 31% after 24 months; that for patients with obesity increased from 23% to 31%. These proportions were higher than those of the general Australian population. Over the study period, fat mass index (FMI [kg/height·m2]) increased (β = 0.33 [0.14, 0.53], p = 0.0007), whereas the appendicular skeletal muscle mass index decreased (β = −0.07 [−0.12, −0.01], p = 0.01). Myopenia, defined as appendicular skeletal muscle mass index <1 SD below gender and age‐matched mean, increased from over 19% at baseline to 24% after 24 months (OR = 3.1 [1.2, 7.7]; p = 0.01). The proportion of patients classified as sarcopenic (defined as both appendicular skeletal muscle mass index and grip strengths <1 SD below gender and age‐matched mean) tended to increase but did not reach statistical significance (OR = 2.4 [1.0, 6.0]; p = 0.05). 13 Thus, the risk of obesity as well as the risk of sarcopenic obesity increases in the course of IBD disease.
Although IBS was more prevalent among individuals with abdominal obesity compared with normal‐weight subjects, 9 no data on changes in body composition could be found. In particular, there is no information on the risk of developing sarcopenia in patients with IBS.
In patients with cirrhosis, significant losses in body cell mass and body fat and a redistribution of body water occurred, even in patients with mild disease. In the initial stages, fat loss was more pronounced, followed by an accelerated loss of body cell mass in the advanced stages of liver cirrhosis. 14 Furthermore, sarcopenia was associated with CLD. 15 During an observational study in cirrhosis patients, transitions were observed from normal body composition to sarcopenia and from obesity to sarcopenic obesity. 16 Therefore, there is a need to assess the true extent of malnutrition in these patients.
Obesity‐related risks in patients with chronic gastrointestinal and liver disease
Although in general, obesity is associated with a lower life expectancy, the effect of obesity on IBD‐related health outcomes is unclear. Retrospective studies are inconclusive. On the one hand, patients with obesity with CD were older at diagnosis and obesity was associated with a shorter time to first surgery, 17 a higher rate of perianal disease, and higher hospitalization needs (OR 2.35, 95% CI 1.56–3.52). 18 Moreover, a low BMI tended to be associated with a worse prognosis in UC patients, 19 whereas in another study, obesity was not associated with higher health care utilization and IBD‐related surgeries. 20
In patients with CD, visceral obesity was associated with an increased risk of surgery and penetrating disease; in UC with a higher risk of relapse. 21 However, a meta‐analysis showed that compared to IBD patients without obesity, patients with obesity underwent surgery less frequently (RR 0.82; 95% CI 0.72–0.93). It could be argued that obesity could be a reflection of a less serious IBD since a lower BMI could be the result of inflammatory progression. 22
Obesity might also impair clinical response to IBD treatment. Data from other autoimmune diseases suggest that obesity causes a suboptimal response to therapy, possibly by fast clearance of biologicals causing low trough concentrations. 3 In patients with UC, obesity can negatively affect response to therapy with biologicals. 23 In a longitudinal study in IBD patients, patients with obesity showed higher clinical activity at baseline, but also higher risks of relapse and remaining active disease compared with patients without obesity at 12 months of follow‐up. 24 Besides, obesity poses technical challenges to colorectal surgery possibly increasing the risk of perioperative complications, 3 especially abdominal obesity. 25 Furthermore, compared to IBD‐patients without obesity and with Clostridium difficile, patients with IBD, obesity, and Clostridium difficile had an increased risk of colectomy (adjusted OR 1.60 [1.30–1.96]; p < 0.001), a longer length of hospital stay (∆0.8 days [0.02–1.58]; p = 0.04), higher hospital costs (∆$11,051 [1939–20, 163]; p = 0.02), but no significant difference in mortality risk. 26
Both IBS and obesity have a high impact on the healthcare system and society. However, the association between obesity and IBS is unclear. 26 Subjects with overweight or obesity, and with IBS had greater symptom severity compared with normal‐weight subjects with IBS. 27 Furthermore, a higher body fat percentage predicted a lower quality of life. 10
Sarcopenia is a common feature of advanced cirrhosis. In an observational study of 161 patients with cirrhosis, patients with sarcopenia or sarcopenic obesity had a worse prognosis. 16 The ratio of patients with obesity did not change during this study. However, changes were observed from normal body composition to sarcopenia and from obesity to sarcopenic obesity. The prognosis was worse in patients with sarcopenic obesity, followed by sarcopenia, normal body composition, and visceral obesity, respectively (p = 0.077). 16
Risks related to sarcopenic obesity in patients with chronic gastrointestinal and liver disease
Although sarcopenia did not predict outcomes in a cohort study, a subgroup analysis in overweight IBD patients (BMI ≥25 kg/m2) revealed that sarcopenia was the only significant predictor of the need for surgery (p = 0.002). 5
There is no data on the risk of an adverse event in patients with sarcopenic obesity and IBS compared to non‐sarcopenic patients with IBS, both with and without obesity.
Malnutrition leading to sarcopenia is associated with chronic liver cirrhosis and has an adverse effect on morbidity and mortality. 12 , 16 , 28 A systematic review revealed a mean prevalence rate of sarcopenia of 48.1% (range 25%–70%) 12 Patients with sarcopenia had a 3.23 times higher mortality rate compared to non‐sarcopenic patients (OR 3.23; 95% CI, 2.08–5.01; p < 0.001). An observational study found that the prognosis was worst in sarcopenic obesity, followed by sarcopenia and visceral obesity (p < 0.05). 16
METHODS
General methodology
The present guideline was developed according to the standard operating procedure for European Society for Clinical Nutrition and Metabolism ESPEN guidelines. 29 The guideline was developed by an expert group representing different professions including physicians (SCB, RB, LB, VC, IC, AE, HTK, WK, LL, MLS, JMM, JO, FT, CC), surgeons (MWM, AW, AT) and dietitians (MCK, DVB).
Based on the standard operating procedures for ESPEN guidelines and consensus papers, the first development step of this guideline was the formulation of so‐called PICO questions to address specific patient groups (or problems), interventions, compare different therapies, and be outcome‐related. 29 In total, 45 PICO questions were created and split into eight main chapters entitled “inflammatory bowel disease”, “irritable bowel syndrome”, “celiac disease”, “gastroesophageal reflux disease”, “pancreatitis”, “chronic liver disease”, “management before and after weight loss”, and “Structural requirements”. To answer these PICO questions, a literature search was performed to identify suitable meta‐analyses, systematic reviews, and primary studies (for details see below, “search strategy”). Each PICO question was allocated to subgroups/experts for the different topics and, initially, 98 recommendations answering the PICO questions were formulated. The grading system of the Scottish Intercollegiate Guidelines Network 30 was used to grade the literature. The allocation of studies to the different levels of evidence is shown in Table 1. Supporting the recommendations, the working group added commentaries to explain their basis.
TABLE 1.
Definition of levels of evidence
| 1++ | High‐quality meta‐analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias |
| 1+ | Well‐conducted meta‐analyses, systematic reviews, or RCTs with a low risk of bias |
| 1‐ | Meta‐analyses, systematic reviews, or RCTs with a high risk of bias |
| 2++ | High‐quality systematic reviews of case‐control or cohort studies. High‐quality case‐control or cohort studies with a very low risk of confounding or bias and a high probability that the relationship is causal |
| 2+ | Well‐conducted case‐control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal |
| 2‐ | Case‐control or cohort studies with a high risk of confounding or bias and a significant risk that the relationship is not causal |
| 3 | Non‐analytic studies, for example, case reports, case series |
| 4 | Expert opinion |
Note: According to the Scottish Intercollegiate Guidelines Network (SIGN) grading system. 30 RCT, randomized controlled trial.
The grades of recommendation were decided according to the levels of evidence assigned (Table 2). In some cases, a downgrading from the generated grades of recommendation was necessary based on the levels of evidence according to Table 1 and Table 2, e.g. due to a lack of quality of primary studies included in a meta‐analysis. Such cases are described in the commentaries accompanying the respective recommendations. The wording of the recommendations reflects the grades of recommendations since level A is indicated by the use of the word “shall”, level B by the word “should” and level 0 by the word “can” or “may”. The good practice point|good practice points (GPP) are based on experts' opinions due to the lack of studies, for which the choice of wording was not restricted.
TABLE 2.
Definition of grades of recommendation 29
| A | At least one meta‐analysis, systematic review, or RCT rated as 1++, and directly applicable to the target population; or A body of evidence consisting principally of studies rated as 1+, directly applicable to the target population, and demonstrating overall consistency of results |
| B | A body of evidence including studies rated as 2++, directly applicable to the target population; or A body of evidence including studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results; or and demonstrating overall consistency of results; or Extrapolated evidence from studies rated as 1++ or 1+ |
| 0 | Evidence level 3 or 4; or Extrapolated evidence from studies rated as 2++ or 2+ |
| GPP | Good practice points/expert consensus: Recommended best practice based on the clinical experience of the guideline development group |
Between 10th September and 31st October 2021, an online voting (Delphi round) on the recommendations was performed using the guideline‐services.com platform. All ESPEN, as well as United European Gastroenterology UEG members, were invited to agree or disagree with the recommendations and to provide comments. A first draft of the guideline was also made available to the participants on that occasion. Eighty recommendations reached an agreement >90%, and 16 recommendations reached an agreement of >75–90%. Those recommendations with an agreement higher than 90% (indicating a strong consensus) were directly passed, and all others were revised according to the comments and voted on again during a consensus conference which took online on 25th April 2022. Four recommendations that originally had received more than 90% agreement were also voted on during the consensus conference due to major changes in wording. During the consensus conference, three new recommendations emerging from either the comments from the voters of the online voting or the discussion during the consensus conference were additionally voted on. One recommendation was deleted during the consensus conference. Therefore, the final guideline comprises 100 recommendations. At the consensus conference, all recommendations received an agreement higher than 90% corresponding to “strong consensus” according to Table 3. To support the recommendations and the assigned grades of recommendation, the ESPEN guideline office created evidence tables of relevant meta‐analyses, systematic reviews (randomized) controlled trials, and cohort studies. These evidence tables are available online as supplemental material to this guideline.
TABLE 3.
Classification of the strength of consensus
| Strong consensus | Agreement of >90% of the participants |
| Consensus | Agreement of >75–90% of the participants |
| Majority agreement | Agreement of >50–75% of the participants |
| No consensus | Agreement of <50% of the participants |
Note: According to the AWMF methodology. 31
Search strategy
The literature search was conducted by the working group members between March‐May 2020. The search strategies used are available online as supplemental material to this guideline.
INFLAMMATORY BOWEL DISEASE
Screening & assessment
Which nutrition screening and assessment measures should be performed in patients with IBD and overweight/obesity (BMI >25 kg/m2) to assess nutritional status (obesity, sarcopenic obesity, body composition, micronutrients, etc.) or to optimize treatment?
Recommendation 1
Patients with IBD should be screened for nutritional status at the time of diagnosis and thereafter regularly (at least once a year).
Grade of recommendation GPP – Strong consensus 97% agreement
Commentary
This recommendation is a modified version of recommendation 3A in the ESPEN guideline: Clinical nutrition in IBD. 32 , 33
Adults with IBD are at increased risk of malnutrition, with deficits more common in patients with CD than UC. 34 Patients with obesity may have covert deficits in lean mass which may be unmasked by tools such as skinfold thickness measurement. Patients with active IBD, particularly those whose disease is poorly responsive to medical therapy, are at the highest risk of poor nutrition. In adults, the risk of malnutrition can be assessed with validated screening tools. 35
Malnourished patients with IBD are more likely to be hospitalized following emergency department attendance 36 and are more likely to be admitted to the hospital due to infection. 37 In hospitalized patients, malnutrition is an independent risk factor for venous thromboembolism, 38 non‐elective surgery, 39 longer admission, 34 , 39 and increased mortality. 34
Inflammatory bowel disease patients should be re‐evaluated in case of an acute event such as relapse, if malnutrition or sarcopenia is suspected, or if the patient is at particular risk because of high age that justifies a screening at least twice a year.
Recommendation 2
Nutritional status screening in patients with IBD should comprise anthropometry (body weight, body height) and a validated screening tool (e.g. NRS‐2002* for hospitalized patients, MUST** for other patients).
Grade of recommendation GPP – Strong consensus 94% agreement
*Nutritional Risk Screening.
** Malnutrition Universal Screening Tool.
Commentary
Body weight and body height are two very easy‐to‐determine parameters that are needed for the calculation of the BMI. Body mass index is the basis for both NRS‐2002 and MUST. Both tools are generally recognized and widely recommended. 28 , 40 , 41 , 42 , 43
Recommendation 3
If screening revealed overweight (BMI 25–30 kg/m2), an assessment for waist circumference and liver steatosis should be performed. If screening revealed obesity (BMI >30 kg/m2) or overweight plus increased waist circumference, an assessment for obesity‐related diseases including insulin resistance and low‐grade inflammation should be performed.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
The increasing incidence and prevalence of obesity worldwide cause increments in its prevalence also in IBD patients, reaching approximately one‐third in both CD and UC. 20 , 44 , 45 , 46 Fat gain may also develop over time in patients with IBD, due to multiple causes associated with pathophysiology as well as treatment of the underlying disease. Loss of muscle mass may further develop due to poor dietary intake, increased rates of protein turnover, and loss of nutrients during phases of active disease or from the effect of disease treatments. Corticosteroids used for IBD treatment may cause selective visceral fat deposition 47 as well as an increased net loss of protein in both children and adult patients. 48 , 49 Based on the above observations, the association between obesity and IBD may be further related to the high risk of sarcopenic obesity, that is, the association of excess fat mass and low skeletal muscle mass and function, 50 , 51 , 52 as indeed indicated by a few available reports. 5
In subjects with overweight, an assessment of metabolic risk is recommended, which should include the measurement of waist circumference and liver steatosis by sonography or validated scores. 53 , 54 Additionally, insulin resistance can be estimated by HOMA index, and low‐grade inflammation by C‐reactive protein measurement in serum. In subjects with obesity, the assessment for the presence and impact of obesity‐related diseases (diabetes, hypertension, dyslipidemia; cardiovascular, respiratory, and joint diseases; NAFLD, sleep disorders, etc.) is mandatory. 53 , 54
Recommendation 4
If screening revealed malnutrition or a risk for malnutrition, a more detailed nutritional assessment, for example, according to the GLIM* criteria, should be performed that includes the diagnosis of sarcopenia.
Grade of recommendation GPP ‐ Strong consensus 97% agreement
*Global Leadership Initiative on Malnutrition.
Commentary
The GLIM criteria require for the diagnosis of malnutrition both at least one phenotypic and one etiologic criterion. 55 Phenotypic criteria are defined as weight loss (>5% within the past 6 months or > 10% beyond this time), a low BMI (<20 kg/m2 for Caucasians, < 22 kg/m2 for people over 70 years, 1,5 kg/m2 less for Asians), and a reduced muscle mass (ideally assessed by dual‐energy X‐ray absorptiometry (DXA), alternatively by bioelectrical impedance analysis (BIA)). The etiologic criteria comprise a reduced food intake (plate diagrams, or 7‐day‐food diary) or malassimilation (≤50% of energy requirements for more than 1 week or any reduction for more than 2 weeks or any chronic gastrointestinal condition that harms food assimilation or absorption), or inflammation caused by an acute disease/injury or a chronic disease. 55
In addition to the GLIM criteria, the nutritional assessment can comprise additional anthropometry (waist circumference, forearm or calf circumference, triceps skinfold), functional tests (handgrip strength), and perhaps laboratory tests (albumin, fasting blood glucose, triglycerides).
Since their publication in 2019, the GLIM criteria have been used in three trials to assess malnutrition in presence of gastrointestinal disorders, 56 , 57 one of them in IBD patients. 58 These trials proved the feasibility of the GLIM criteria in gastrointestinal diseases.
Sarcopenia is a particular issue because it is common in patients with overweight and obesity and may predict the need for surgery. 5 Since decreased muscle mass has been reported in 60% of adults with CD compared with healthy subjects, 50 , 59 sarcopenic obesity is another feature of changing phenotype of IBD patients that might impact treatment response and should be assessed accordingly.
Recommendation 5
Patients with IBD and obesity should undergo an appropriate procedure to check for sarcopenia/sarcopenic obesity.
Grade of recommendation GPP ‐ Strong consensus 91% agreement
Commentary
The importance of sarcopenia in obesity and its relevance for prognosis and quality of life is increasingly recognized. Its diagnosis analyzes muscle mass (as part of a body composition analysis) and muscle function (using suitable function tests) obligatory initially and also during the disease. 60 , 61 ESPEN and European Association for the Study of Obesity (EASO) launched an initiative to reach an expert consensus on a definition and diagnostic criteria for sarcopenic obesity. 62 Thereafter, the diagnosis of sarcopenic obesity should be considered in individuals at risk if skeletal muscle function is compromised or skeletal muscle mass is reduced. Screening for sarcopenic obesity is based on the co‐existence of a high BMI or waist circumference with ethnicity‐specific cut‐offs and indicators of sarcopenia such as clinical symptoms, risk factors, or validated questionnaires, for example, “Strength, assistance with walking, rising from a chair, climbing stairs, and falls” (SARC‐F) questionnaire in elderly subjects. Screening should be part of the clinical routine. If positive, muscle function and mass should be evaluated. As functional parameters of the skeletal muscles, we recommend measuring hand muscle strength or knee extensor strength or performing the chair‐stand test as a 5‐time sit‐stand test or 30‐second chair‐stand test. 62 When pathologic functional parameters of skeletal muscle are detected, the diagnostic process continues with the assessment of body composition. Dual‐energy X‐ray absorptiometry and BIA may be recommended as appropriate methods of measuring body composition in patients with overweight or obesity, and other methods such as computed tomography (CT) depending on experience and availability. 62
Sarcopenia is common in the IBD population and can predict the need for surgical intervention. Sarcopenia correlates with major postoperative complications. 6
Recommendation 6
Patients with IBD should be checked for micronutrient deficiencies regularly (at the time of diagnosis and thereafter at least once a year or if clinical signs of deficiencies occur).
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
This recommendation is a modified version of recommendation 6 in the ESPEN guideline: Clinical nutrition in IBD. 32 , 33
Patients with IBD are vulnerable to micronutrient deficits due to gut loss from diarrhea and inadequate dietary intake from anorexia accompanying disease activity. At times when nutrition support is offered then multivitamin and micronutrient supplements should also be offered to ensure an appropriately balanced nutritional intake.
Especially, vitamin B1, B6, B12, A, D, E, K, iron, selenium, and zinc should be looked for (see also ESPEN micronutrient guideline 63 ).
When interpreting blood results of micronutrients and trace elements it is important to consider that many serum values, or markers of status, are positive or negative acute phase reactants. Serum levels rise or fall, as part of the inflammatory response, for example, ferritin, and copper increase but folate, selenium, and zinc decrease in inflammation. 64 In light of this, some authors have examined micronutrient status in patients in clinical disease remission and found deficits in a variety of micronutrients. 65 , 66 Furthermore, deficits may be present even in apparently well‐nourished individuals. 67 These observations highlight the need for routine monitoring (perhaps annually) to screen for deficiency.
A dedicated diet counseling or a daily multivitamin supplement may correct most deficiencies but is no guarantee of adequacy, even over the long term; iron, zinc, and vitamin D are likely to require specific replacement regimens. 68 Poor compliance, particularly in adolescents, is common with multivitamin supplements and patient education about the rationale behind their use is important. 69
Consequences of deranged micronutrient status include anemia, impaired linear growth, and poor bone health. Recent research has focused on vitamin D; it and its receptor may have some immunomodulatory properties, which further highlights the need for specific attention to micronutrient status in patients with IBD.
Which nutrition screening and assessment measures should be performed in patients with IBD and obesity treated or proposed to be treated with biologicals to optimize treatment response and outcome?
Recommendation 7
Patients with obesity and IBD supposed to be treated with biologicals can undergo weight loss therapy in order to optimize the treatment response.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
Patients with IBD and obesity have often inferior responses to biological therapy with biologicals that is related to altered pharmacokinetics and obesity‐mediated chronic low‐grade inflammation. 70 Therefore, nutritional assessment is one of the key points of the management of IBD patients. Overweight and obesity in IBD is recognized as a risk factor associated with increased drug clearance, leading to shorter half‐lives and low drug concentrations. 3 The mechanistic explanation of this situation might be based on impaired absorption of subcutaneously administered active compounds, rapid proteolysis, and a ‘tumor necrosis factor (TNF)‐sink’ phenomenon with inflammatory status caused by obesity. Although not all studies are equivocal, trends toward closer monitoring of body weight, body composition, and weight loss as adjunctive therapy for more successful provision of biologicals are advocated. 70
The usual nutritional screening and assessment techniques are recommended. For details see commentaries to recommendations 1‐3.
Weight loss therapy should consist of fat but not muscle reduction and should follow carefully the general recommendations for obesity therapy.
Recommendation 8
Bone mineral density should be assessed in IBD patients at the time of diagnosis and in patients at risk (chronic active disease, corticosteroid treatment, or previous osteopenia) every one to 2 years.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
Bone mineral density should be assessed in IBD patients using DXA, which allows direct and non‐invasive measurement of bone mass, fat‐free mass, and fat mass. Disturbances in body composition in IBD patients can be accurately measured using the aforementioned gold‐standard method. 13 Reduced bone mineral density described as osteopenia or osteoporosis is one of the most common complications of IBD, encountered in 20%–50% of patients. 13 Low bone mineral density described as osteopenia or osteoporosis is one of the most common complications that correlates with increased fracture risk in IBD patients. 71 The high prevalence of obesity, IBD, and hypovitaminosis D are parallel and overlapping phenomena. Low levels of serum vitamin D are characteristics of both obesity and IBD, as well as sarcopenic obesity. 72 The etiopathogenesis of vitamin D deficiency is multifactorial in IBD patients and develops as a result of malabsorption, inflammation, low dietary intake, low sun exposure, and corticosteroid therapy. 13 , 73 In patients with obesity, vitamin D is being sequestrated in fat tissue, therefore, low serum levels of 25‐OH vitamin D are often measured. 73 Furthermore, obesity is characterized by pro‐inflammatory pathogenic mechanisms and dysbiosis that are also linked to bone alterations in the IBD population. 74
Appropriate screening and prophylaxis of bone alterations in patients with IBD and obesity are therefore even more important in comparison with patients with IBD, but without obesity, and should be done routinely.
Which nutrition screening and assessment measures should be performed in patients with IBD and obesity before and after intestinal surgery?
Recommendation 9
Screening for nutritional status and ‐ if indicated ‐ nutritional assessment shall be performed in patients with IBD and obesity before intestinal surgery to identify the need for perioperative nutritional therapy.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
While agreement exists that patients undergoing surgery should be screened before and after surgery, and should receive nutritional therapy whenever indicated, so far, no evidence is available for the recommendation of a specific screening tool and the measures of assessment in this setting. The ESPEN guideline: Clinical nutrition in IBD states: “Patients with IBD are at risk and therefore should be screened for malnutrition at the time of diagnosis and thereafter on a regular basis. Good practice point|GPP – strong consensus (96% agreement)”. Patients with obesity may have covert deficits in lean mass which may be unmasked by tools such as skinfold thickness measurements. 33
The dietitians' European Crohn's and Colitis Organization (ECCO) working group recommends the Mini Nutritional Assessment for surgical patients, which is in line with a recent study regarding the assessment of patients with IBD in clinical remission. 75 , 76
NRS‐2002 has been well validated for surgical patients in general and is recommended by the ESPEN Guideline: Clinical Nutrition in Surgery. 43 , 77 , 78 MUST is an alternative for the NRS‐2002. Mini Nutritional Assessment may be the most appropriate screening tool for elderly IBD patient. 79 GLIM is recommended for assessment (see also recommendations 2 and 4).
Recommendation 10
Postoperatively, nutritional status should be monitored.
Grade of recommendation GPP ‐ Strong consensus 94% agreement
Commentary
The ESPEN Guideline: Clinical Nutrition in Surgery states: “It is recommended to assess the nutritional status before and after major surgery (GPP)”. 43 Time intervals have to be individualized and related to nutritional therapy after discharge. The GLIM criteria are useful for the assessment of malnutrition and sarcopenia (see also recommendations 4 and 5).
Recommendation 11
In patients before elective surgery, body composition may be performed by validated means such as BIA, DXA, or CT.
Grade of recommendation 0 – Strong consensus 94% agreement
Commentary
Bioelectrical impedance analysis has been shown to detect changes in body composition with an escape to standard nutrition assessment 80 and has been recently recommended as an indicator for the severity of liver disease. 81
When interpreting the results of BIA, which does not assess body composition directly, hydration status should be taken into account. If the hydration status is impaired phase angle may allow defining the nutritional and clinical risk.
Computed tomography derived body composition is well established for the measurement of visceral adipose tissue and skeletal muscle area on the transverse section of L3 in cancer patients and may be used for patients with IBD and obesity as well, especially if performed for other reasons such as IBD staging. 82 , 83
If neither BIA nor CT is available, classical anthropometry (skinfold thickness, arm circumference) or hand grip strength should be performed.
Treatment
Should weight reduction be recommended in patients with IBD and obesity to improve outcomes?
Recommendation 12
Patients with IBD and obesity should be encouraged to lose body weight during the remission phase to improve the course of the disease, reduce obesity‐related comorbidities, and enhance response to therapy with biologicals.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Increased BMI has been associated with treatment failure, risk of hospitalization, and lower endoscopic remission rates in IBD. 84 , 85 , 86 , 87 , 88 Thus, obesity could impact IBD outcomes and further argue for weight reduction (only in the phase of remission) besides the well‐known benefits of weight loss on obesity comorbidities such as diabetes, hypertension and cardiovascular risk, dyslipidemia, and sleep apnea. 54
Current evidence regarding the impact of obesity on the IBD course is controversial. Some authors have speculated that obesity is associated with increased morbidity, disease severity, and more frequent complications such as perianal fistula formation. 18 In contrast, other studies showed that a high BMI might have a favorable effect on IBD prognosis. 19 , 44 However, another study demonstrated no association between BMI and corticosteroid use, hospitalization, and the need for surgery in IBD patients. 20 This discrepancy may be due to the way of assessing obesity, using just BMI or including methods to measure visceral obesity. In the last case, studies using visceral obesity as a measure of obesity have more consistently shown an increased risk of IBD‐related complications and worse surgical outcomes. 89 , 90 , 91
Besides, weight loss after bariatric surgery in IBD patients has proven to be beneficial in the majority of the cases revised in two systematic reviews, with remission or decrease of disease activity and medications. 92 , 93
Patients with IBD and obesity may be less responsive to medical treatment, especially to anti‐TNF drugs, due to high clearance and lower half‐life, if the dosage is not weight adapted. 94 In a cohort study, body composition did not correlate well with BMI, but myopenia was associated with nonresponse to anti‐TNF therapy (sarcopenic obesity). 95 Regarding surgical treatment, patients with CD and obesity have been reported to have an earlier time to first surgery in a retrospective study. 17 However, there is no prospective interventional study investigating the effect of weight loss on IBD course and success of therapy with biologicals. Besides, the adalimumab dose escalation rate increases with higher BMI in CD. 96 In a pooled data analysis, obesity was not associated with treatment failure or active mucosal disease in UC. 97 In another study, no relation between loss of anti‐TNF treatment response and increased BMI in IBD was found. 98 By contrast, obesity may negatively affect weight‐adjusted and fixed‐dose therapies with biologicals. 70
Regarding the details of obesity therapy (reduction of fat mass while preservation of muscle mass) we refer to the current national and international obesity guidelines.
Recommendation 13
Patients with IBD and obesity requiring elective IBD surgery shall be advised to reduce body weight preoperatively.
Grade of recommendation A ‐ Strong consensus 97% agreement
Commentary
In a meta‐analysis, obesity was associated with significantly worse outcomes following IBD‐specific surgery, including longer operative times, greater blood loss, longer length of stay, higher wound infection rates, and higher total postoperative complication rates. 99
Regarding the details of obesity therapy (reduction of fat mass while preservation of muscle mass) we refer to the current national and international obesity guidelines.
Which type of obesity therapy (diet counseling, exercise, multimodal therapy) should be recommended in patients with IBD and overweight/obesity?
Recommendation 14
Obesity therapy for patients with IBD may follow a stepwise approach similar to patients without IBD starting with a diet and lifestyle intervention, but also including anti‐obesity drugs or bariatric surgery if needed.
Grade of recommendation 0 ‐ Strong consensus 97% agreement
Commentary
The downside of obesity in the general population is well known. Concerning gastrointestinal disease, there are additional points to be taken into account.
In IBD patients, obesity might be associated with a more complicated course, a disease less responsive to treatment with biologicals, and a tendency for post‐surgical complications. It could be speculated that treating obesity could result in a better outcome, but this has not been proven. 3 , 100
Treatment. There is a paucity of studies specifically addressing weight loss issues in the groups of patients with gastrointestinal disease.
Life style and dietary interventions. On the whole, lifestyle and dietary interventions carry a low risk of adverse events, especially when carried out under supervised professional guidance. Adherence is usually limited necessitating additional measures. With regards specifically to patients with gastrointestinal diseases, there is a lack of evidence. One study included patients with IBD and prescribed the Mediterranean Diet. There was an improvement in weight, waist circumference, and steatosis. 101 No data exists regarding the effects of overall caloric intake or supervised dietary weight loss on outcomes in IBD patients.
Anti‐obesity drug medication. see recommendation 15.
Bariatric surgery. see recommendation 16.
Which type of obesity therapy (pharmacotherapy) should be recommended in patients with IBD and overweight/obesity?
Recommendation 15
Anti‐obesity drugs can be used in patients with IBD according to their indications, except for orlistat. Orlistat should be avoided in patients with IBD because of the mechanism of action and common side effects.
Grade of recommendation 0 ‐ Strong consensus 91% agreement
Commentary
Therapy with anti‐obesity drugs is currently recommended for patients with a BMI ≥30 kg/m2 or a BMI ≥27 kg/m2 with an obesity‐related disease (e.g. hypertension, type 2 diabetes, sleep apnea). 54 The use of anti‐obesity medications is still limited by reimbursability issues in several countries.
No indication in favor of a specific anti‐obesity drug can be formulated for IBD patients. There are no randomized controlled trials (RCTs) in patients with IBD available for any of the anti‐obesity drugs. Weak recommendations could be formulated only based on the mechanism of action, safety issues, and some uncontrolled small studies.
Orlistat. Gastrointestinal symptoms are the most commonly observed adverse events associated with the use of Orlistat in RCTs and they are primarily a manifestation of the mechanism of action. Commonly observed gastrointestinal symptoms are the following: oily spotting, flatus with discharge, fecal urgency, fatty/oily stool, oily evacuation, increased defecation, and fecal incontinence. Orlistat is contraindicated in patients with chronic malabsorption syndrome. These considerations discourage the use of Orlistat in patients with IBD or IBS.
Liraglutide. Initial experimental data in animals suggest that Glucagon‐like Peptide 1 (GLP‐1) receptor agonists may positively affect homeostasis and immune activity in the gut 102 , 103 and modulate altered visceral sensation in IBS. 104 One case report on the use of liraglutide in a patient with CD has been published. 105 No safety concerns have been raised.
Naltrexone/Bupropion. No data are available for the combination of Naltrexone/Bupropion. Naltrexone alone has been shown to reduce disease activity and improve endoscopic findings in two small uncontrolled studies conducted on 47 adult patients with IBD. 106 Initiation of Naltrexone in IBD patients is followed by reduced dispensing of other drugs considered essential in the treatment of IBD in a population registry. 107 Bupropion alone may have anti‐inflammatory properties and its use is associated with clinical improvements in uncontrolled studies in patients with IBD 108 and case reports. 109
Lorcaserin. No data are available for lorcaserin. No safety concerns have been raised.
Phentermine/Topiramate. No data are available for Phentermine or the combination Phentermine/Topiramate. Initial experimental data suggest that Topiramate may significantly reduce gross pathological signs and microscopic damage in primary affected colon tissue in animal models of IBD. 110 These promising results have been not confirmed in a retrospective cohort study conducted on humans using administrative claims. 111 No safety concerns have been raised.
Should bariatric surgery be recommended for IBD, and if yes which procedure should be preferred?
Recommendation 16
In patients with IBD and BMI >40 kg/m 2 or >, 35 kg/m 2 with obesity‐related comorbidities and previous failed non‐surgical weight‐loss attempts can be offered bariatric surgery, preferably considering non‐malabsorptive procedures not involving the small bowel.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
Although associated with a slightly increased risk of complications, sustained weight loss as induced by bariatric surgery will reduce inflammation and thus improve the severity of IBD in addition to resolving or improving comorbidities. 93 , 112 , 113 , 114 No RCTs or prospective studies were found that compared the different bariatric procedures in patients with IBD (i.e. CD and UC). Patients with CD can have progressive damage, especially to the small intestine with acute flares, and could require intestinal resection. Because of the recommendation of small‐bowel sparing surgeries, 115 it seems safer to perform sleeve gastrectomy (SG) in patients with CD. In patients with UC, it also seems recommended to perform an SG. One of the treatments for UC is total proctocolectomy with ileal pouch‐anal anastomosis. The realization of a Roux‐en‐Y gastric bypass (RYGB) in patients with UC is likely to cause not only technical difficulties for future surgeries (i.e. pouch‐anal anastomosis) but also to increase the bowel frequency due to coloproctectomy.
Although not confirmed in randomized studies, SG is assumed to be superior to RYGB in IBD by only involving the stomach, which might decrease the risk of small intestinal bacterial overgrowth. 116 , 117 , 118 Avoiding anatomical changes in the small intestine might further reduce the risk of complications such as strictures, abscesses, and fistulas and simplify the possible future IBD‐related surgery.
The use of an intragastric balloon in patients with IBD has been evaluated in small series, but the lack of long‐term effects on weight loss as well as reports on complications have limited its use. 119 There are no high‐quality data on the results after other endoscopic procedures for obesity in patients with IBD.
What are energy and protein requirements to be recommended in patients with obesity and IBD without/with altered body composition and low skeletal muscle mass and function?
Recommendation 17
In patients with IBD and overweight/obesity energy requirement can be assessed in absence of indirect calorimetry using validated formula and corrections (based on “adjusted body weight”).
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
In general, the energy requirements of patients with IBD are similar to those of the healthy population. 33 For details see the ESPEN guideline: Clinical nutrition in IBD. 32 , 33 Indirect calorimetry is the preferable means to determine resting energy expenditure. If not available, validated formulas can be used. A well‐established formula to assess energy need is for example, the Harris‐Benedict formula, or the 25 kcal/kg body weight formula (often called “reference body weight”), if indirect calorimetry is not available, which is considered an agreed standard for the individual assessment of energy requirement.
The reference body weight is commonly defined as body weight at a BMI >25 kg/m2. 42 However, calculating energy needs based on reference body weight instead of actual body weight underestimates the needs of individuals with obesity, since adipose tissue utilizes also some energy (4.5 kcal/kg/d), albeit less than muscle tissue (13 kcal/kg/d). 120 The proportion of muscle within the excess weight of an individual with obesity might be roughly 10%. A pragmatic approach is therefore to add one third (33%) of the excess weight (actual body weight – reference body weight) to the reference body weight for all calculations of energy requirements. 42 The resulting body weight is named “adjusted body weight” (adjusted body weight (ABW)) according to the formula ABW = reference body weight + (0.33*(actual body weight – reference body weight)).
Recommendation 18
Protein intake should be increased in IBD patients with active disease and obesity to 1.2–1.5 g/kg ABW/d in adults.
Protein requirements in remission are generally not elevated and provision also in IBD patients with obesity should be similar (0.8–1 g/kg ABW/d in adults) to that recommended for the general population unless sarcopenia or malnutrition is present.
Also in remission, protein intake may be increased (1.2–1.5 g/kg ABW/d) in IBD patients with obesity and sarcopenia or with a high risk of malnutrition after malnutrition screening and assessment are conducted.
Grade of recommendation GPP ‐ Consensus 90% agreement
Commentary
In various studies, overall nutrient provision through oral, enteral, or parenteral routes when appropriate 32 , 121 , 122 , 123 , 124 , 125 is reported to limit protein catabolism in IBD. In the presence of hyper‐catabolism during active IBD flares, high protein recommendations have been proposed with 1.2–1.5 g/kg body weight/d. 32 , 121 , 126 , 127 On the other hand, no strong evidence of enhanced protein requirements has been reported for IBD in remission 32 , 121 and 1 g/kg/d protein has been recommended under these conditions. 32 , 121 In the absence of studies specifically investigating potential differential requirements for patients with overweight or obesity, the above recommendations are proposed to be extended to individuals with IBD and overweight or obesity. Unless accurate measurement of skeletal muscle mass or lean body mass is available using appropriate techniques such as DXA, ABW (see recommendation 17) may represent an acceptable although inevitably approximate reference value to calculate total protein requirements, taking into account metabolically active components of excess body weight. 42 Protein provisions should be probably higher (1.2–1.5 g/kg ABW/d) in the presence of sarcopenia and/or malnutrition. However, the data for this are not conclusive. A meta‐analysis by Hsu et al. 128 showed that nutritional intervention, especially a low‐calorie high protein diet, did not affect muscle mass and grip strength. Finally, weight‐loss programs for individuals with IBD and obesity should be avoided during the active phases of the disease. During remission, weight‐loss programs should include a minimum protein provision of 1 g/kg ABW/d. See also the ESPEN guideline: Clinical nutrition in IBD. 32 , 33
Do we need a particular nutritional intervention in IBD patients with IBD and obesity receiving a (long‐term) therapy with corticosteroids?
Recommendation 19
In patients with IBD and obesity who receive or have received steroid treatment, serum calcium, and 25‐(OH)‐vitamin D should be monitored and supplemented if required to prevent low bone mineral density.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
This recommendation is a modified version of recommendation 11 in the ESPEN guideline: Clinical nutrition in IBD. 33 Corticosteroid is an important agent in IBD treatment with its pros and cons. Osteoporosis is common in IBD with a range from 18% to 42%. 129 , 130 Also, corticosteroid use is a risk factor for osteoporosis in IBD. 131 Vitamin D deficiency contributes to low bone mineral density and is seen as common in IBD patients. 132 , 133 Low serum calcium level stimulates parathormone secretion, which leads to calcium release from bone to serum and ends up with a decreased bone mineral density. 134 IBD patients have lower calcium and phosphate levels when compared to a healthy population. 135 Even though obesity is negatively correlated with osteoporosis in adults, 136 adequate vitamin D and calcium replacement are needed for patients with IBD and obesity receiving corticosteroid therapy.
Weight gain is another side effect of corticosteroid treatment also in patients with obesity. 137 Voluntary weight loss should be preferred in a stable disease course in patients with IBD and obesity. 33 Corticosteroids are mostly used in remission induction in severe disease. Therefore, a strict weight‐reducing diet is not a favorable option in patients with obesity and severe IBD.
Usually, oral supplementation of calcium and vitamin D should be appropriate.
IRRITABLE BOWEL SYNDROME
Screening & assessment
Which nutrition screening and assessment measures should be performed in patients with IBS and overweight/obesity (BMI >25 kg/m 2 ) to assess nutritional status (obesity, sarcopenic obesity, body composition, micronutrients, etc.) to optimize treatment?
Recommendation 20
Patients with IBS should be screened for nutritional status (malnutrition, sarcopenia, overweight, obesity) at the time of diagnosis and thereafter regularly (at least once a year).
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
Nutritional inadequacy is often seen in IBS patients following restrictive diets. People with IBS are likely to follow restrictive diets, like the fermentable oligo‐, di‐, monosaccharides and polyols (FODMAP) diet or a gluten‐free diet, without guidance by a dietitian. In a UK survey in an IBS cohort, 42% of patients believed they had gluten sensitivity and 12% are following a gluten‐free diet 138 A gluten‐free diet might lead to compromised intakes of fiber, calcium, iron, zinc, and folate. 139 Following a low FODMAP diet might lead to inadequate intake of carbohydrates, fiber, iron, B vitamins, and calcium. 140 To guarantee an appropriate nutrient intake, counseling by a dietitian is desirable. 141
Screening should consist at least of documentation of BMI, weight history, appetite, and nutritional intake. In case of suspected malnutrition, validated screening tools such as MUST (see recommendation 2) can be used. Further tools, for example, for assessment of sarcopenia, can be implemented on an individual basis.
Recent ESPEN guidelines 142 state that in clinical practice DXA might be the most accurate instrument to measure body composition in individuals with obesity, but BIA or CT scan can be also used. In a large population study, obesity (high fat mass index) and low muscle mass (low fat‐free mass index) measured by BIA was associated with a longer length of hospital stay compared with a normal fat mass index or fat‐free mass index. 143 Sarcopenia can occur in IBS patients with and without obesity, yet the prevalence is unclear at present.
For further information see commentary to recommendation 2.
Treatment
Should weight reduction be recommended in patients with IBS and overweight/obesity to improve outcomes?
Recommendation 21
Patients with IBS and obesity should be encouraged to lose weight to improve clinical symptoms, primarily by lifestyle modification including dietary regimen and increased physical activity.
Grade of recommendation B ‐ Strong consensus 100% agreement
Overweight patients with IBS can be encouraged to lose weight to improve clinical symptoms, by lifestyle modification including dietary regimen and increased physical activity.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
A higher prevalence of IBS, characterized by abdominal discomfort or pain, associated with altered bowel habits, has been reported in subjects with obesity compared to normal‐weight subjects. 144 In a cross‐sectional study, Lee et al. showed that visceral obesity measured by visceral adipose tissue was associated with IBS. 145 However, due to the scarcity of evidence on this association, it is not yet known whether it is obesity that predisposes to the increased risk of developing IBS or vice versa. The underlying mechanisms could be related to a sedentary lifestyle, dietary pattern, alteration of the levels of anorexigenic hormones, psychological disorders, changes in gut microbiota, and chronic inflammation.
A recent prospective study in subjects with obesity undergoing a 6‐month weight‐loss program with a hypocaloric diet showed that those suffering from IBS experienced a clinically significant improvement in IBS symptoms after the diet, measured by the IBS Severity Scoring System (Irritable Bowel Syndrome Severity Scoring System) and Gastrointestinal Symptom Rating Scale ‐ IBS health scores. 146
A retrospective analysis showed that IBS symptoms in patients with morbid obesity improved after weight reduction surgery by laparoscopic RYGB. 147 However, the evidence is inconsistent and it is too early to recommend bariatric surgery for improvement of symptoms in patients with IBS and obesity.
Which type of obesity therapy (diet counseling, exercise, multimodal therapy) should be recommended in patients with IBS and overweight/obesity?
Recommendation 22
Obesity therapy for patients with IBS may follow a stepwise approach similar to patients without gastrointestinal disease focusing on a diet and lifestyle intervention.
Grade of Recommendation 0 ‐ Strong consensus 100% agreement
Commentary
In a comprehensive review of the literature, the frequency of IBS in adults with obesity is variable and depends on the study population, the prevalence of IBS in subjects with obesity varied from 11.6% to 24%, depending on the study population. 148 , 149
A recent publication describes the success of lifestyle modifications in 88 patients with IBS. 146 Weight loss was recorded in a group of 63 patients with IBS who adhered to the treatment of IBS with the FODMAP diet. 150 Improvement in IBS symptoms was noticed along with weight loss. Which of the two factors – diet composition and/or weight loss is responsible for the improvement is unknown. A question of safety arises whether this weight loss ensues in the development of nutritional deficiencies and unfavorable effect on body composition.
Symptoms similar to those of IBS such as abdominal pain, flatulence, and diarrhea develop frequently post‐bariatric surgery. Irritable bowel syndrome is a common pre‐bariatric surgery symptom with a third of the patients suffering from IBS‐like complaints. 151 In one study 26% of patients, 2 years post‐surgery had IBS‐like symptoms. Irritable bowel syndrome pre‐surgery was found to be among independent preoperative predictors of IBS‐like symptoms at the 2‐year follow‐up visit. Quality of life was lower for patients with IBS‐like symptoms than for patients without IBS‐ like symptoms. 152 When considering a patient with IBS for a bariatric surgery it should be taken into account that IBS symptoms might worsen.
Regarding the details of obesity therapy (reduction of fat mass while preservation of muscle mass) we refer to the current national and international obesity guidelines.
Which type of obesity therapy (pharmacotherapy) should be recommended in patients with IBS and overweight/obesity?
Recommendation 23
Anti‐obesity drugs can be used in patients with IBS according to their indications, however, gastrointestinal side effects and potential interactions with other current treatments should be considered
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
No indication in favor of a specific anti‐obesity drug can be formulated for patients with IBS. There are no RCTs in patients with IBS available for any of the anti‐obesity drugs. Weak recommendations could be formulated only based on the mechanism of action, safety issues, and some uncontrolled small studies. Since some of the side effects of anti‐obesity medications, specifically Orlistat, but also GLP‐1 analogs, are gastrointestinal, it might be speculated that patients with IBS will experience worsening of their symptoms.
Further details: See recommendation 15.
Which type of microbiota therapy should be recommended in patients with IBS and overweight/obesity?
Recommendation 24
Selected probiotics can be recommended for achieving symptom relief in overweight and patients with IBS and obesity.
Grade of recommendation 0 – Strong consensus 93% agreement
Commentary
A large number of studies and several meta‐analyses have investigated the effect of different probiotics and their combinations on IBS symptoms, including pain and discomfort, bloating, flatulence, and global symptoms scores. 153 , 154 , 155 Administered probiotics included Bifidobacterium, Lactobacillus, and Streptococcus strains. 155 Interpretation of study results is hindered by relevant limitations such as large variability in treatment dose, duration, strain combination, and high risk of bias in some studies. 153 , 155 However, selected probiotics have been recommended for patients with IBS at a recommendation grade B, 156 and this recommendation can be extrapolated to IBS patients with obesity at grade 0 because of the extrapolation. For the scope of this guideline, it should be pointed out that no studies have directly addressed microbiota treatment in patients with IBS and overweight or obesity. Some studies have included patients with overweight or obesity with no reported subgroup analyses. 157 , 158 , 159 , 160 There is however no evidence for exclusion of patients with overweight or obesity from reported benefits of selected probiotic treatments.
Prebiotics and synbiotics including inulin, fructan, galactooligosaccharides, and oligosaccharides along with probiotics have been investigated in a smaller number of studies, 153 , 161 making conclusions even more difficult on overall treatment efficacy as well as the superiority of specific combinations. 162 Studies have also investigated the effect of fecal microbiota transplantation on IBS symptoms with published meta‐analyses showing no definitive evidence for efficacy. 163 , 164 , 165
Microbiota treatments should be terminated if no improvement occurs latest within 3 months of treatment. 156
Should bariatric surgery be recommended for IBS, and if yes which procedure should be preferred?
Recommendation 25
Patients with IBS and BMI >40 kg/m 2 or >35 kg/m 2 with obesity‐related comorbidities can be offered bariatric surgery provided that serious attempts to lose weight with non‐surgical methods have been made.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
Irritable bowel syndrome is reported to be more prevalent in obesity. 151 There are sparse and conflicting data in the literature as to the effect on IBS symptoms after bariatric surgery 147 , 152 but efficacy in terms of weight loss and resolution of comorbidities, as well as risks, has not been reported to differ among patients without IBS. On the other hand, it should be considered that bariatric surgery can induce or increase IBS symptoms (see recommendation 22).
Patients with IBS and overweight or obesity should be encouraged to lose weight with conservative measures, as this is always a prerequisite to be considered for bariatric surgery. However, if the goals cannot be reached by this approach, and if obesity is pronounced (grade III) or accompanied by obesity‐related comorbidities (grade II) bariatric surgery can be offered. 147 Because of the limited data available for IBS patients, this recommendation was graded as a GPP.
CELIAC DISEASE
Screening & assessment
Which nutrition screening and assessment measures should be performed in patients with celiac disease and overweight/obesity (BMI >25 kg/m 2 ) to assess nutritional status (obesity, sarcopenic obesity, body composition, micronutrients, etc.) to optimize treatment?
Recommendation 26
Patients with celiac disease should be screened for nutritional status (malnutrition, sarcopenia, micronutrient deficiency, overweight, obesity) at the time of diagnosis and thereafter regularly (at least once a year).
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
Celiac disease is an autoimmune disorder characterized by immune‐mediated mucosal atrophy of the proximal small intestine and subsequent malabsorptive symptoms such as diarrhea and weight loss. 166 Although celiac disease patients have historically been observed as undernourished presenting with low BMI values at the time of diagnosis, nowadays overweight and obesity have increased among celiac patients. 167 , 168 , 169 , 170 A cross‐sectional study showed that up to 32% of patients with celiac disease presented with overweight or obesity. 171 Weight gain, metabolic and nutritional profiles need to be assessed during follow‐up, as some studies show that the metabolic syndrome rate and obesity increase in celiac patients 1 year after starting a gluten‐free diet. In a systematic review published by Valvano, 14 eligible studies were analyzed that showed an increased frequency of NAFLD, weight gain, and alterations of the lipid profile suggesting that profound changes happen in celiac patients on a gluten‐free diet, although the pathophysiology of these derangements is unknown. 169 The features of adult celiac disease ‐ increased gut permeability and small‐intestinal bowel overgrowth, might as well predispose to the occurrence of overweight and obesity, therefore microbiota has to be considered as a possible therapeutic target. 172 Therefore, patients with celiac disease should be screened for nutritional status and might become candidates for weight reduction strategies through lifestyle modification or even bariatric surgery. The impact of weight reduction therapies on celiac disease should be investigated in future clinical trials.
Treatment
Which type of obesity therapy (diet counseling, exercise, multimodal therapy) should be recommended in patients with celiac disease and overweight/obesity?
Recommendation 27
In celiac patients with overweight or obesity, consulting with a registered dietitian should be encouraged to create a healthy eating plan that promotes weight loss and a healthy lifestyle in the course of the disease.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
In contrast to the “classic” celiac presentation of malabsorption and weight loss, overweight and obesity have been respectively described in 40% and 13% of celiac patients, at diagnosis. 173 Furthermore, a gluten‐free diet often results in weight gain due to the improvement in mucosa absorption. Valletta et al. reported that the percentage of overweight subjects almost doubled while on a gluten‐free diet. 174 This may be partially attributed to the hypercaloric content of commercially available gluten‐free foods and bad dietary habits induced by unpalatable, expensive commercial gluten‐free products, replaced by high‐fat commercial gluten‐free foods. 175
In adult celiac disease patients with obesity and metabolic syndrome, what kind of nutritional interventions should be implemented together with a gluten‐free diet?
Recommendation 28
Celiac patients presenting with metabolic syndrome and obesity should comply with a gluten‐free diet, for example, Mediterranean–style gluten‐free diet, with reduced energy content.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
Nutritional profiles of gluten‐free food products have been questioned for the last few decades, and the key inadequacies are low protein and dietary fiber, high calories, fat, sugar, and salt content. 176 Lately, gluten‐free products are often reformulated to become more nutritionally balanced, namely with low simple sugars and high fiber. 175 , 177 A gluten‐free diet may lead to nutritional deficiencies such as fiber, B vitamins, iron, and trace minerals, 175 although the data are conflicting. 178 Mediterranean diet has been proven to be a gold standard for the prevention and therapy of the metabolic syndrome, obesity, and NAFLD, 179 , 180 , 181 and although the data on celiac patients are missing, it would be wise to advise celiac disease patients with obesity‐related problems to adapt their gluten‐free diet to Mediterranean‐style diet.
To comply with a nutritionally balanced gluten‐free diet, patients should be regularly monitored by skilled dietitians or nutritionists and diet therapy should be personalized. 174 The nutritionally balanced gluten‐free diet should be nutrient‐dense, with a high intake of naturally gluten‐free foods (e.g. pseudocereals), with appropriate macronutrient quality and ratios, and rich in micronutrients and phytochemicals. 175
GASTROESOPHAGEAL REFLUX DISEASE
Screening & assessment
Which nutrition screening and assessment measures should be performed in patients with GERD and overweight/obesity (BMI >25 kg/m 2 ) to assess nutritional status (obesity, sarcopenic obesity, body composition, micronutrients, etc.) to optimize treatment?
Recommendation 29
Nutritional status screening should be performed for patients with GERD and overweight or obesity, encompassing basic anthropometric measurements (body weight, body height, BMI, waist circumference)
Grade of recommendation GPP ‐ Strong consensus 96% agreement
Commentary
Obesity has been linked with increased symptoms of GERD 182 and esophageal acid exposure. 183 Epidemiological studies show that obesity is a risk factor for GERD development due to increased intra‐abdominal pressure and gastroesophageal gradient, impaired gastric emptying, and hiatal hernia. 184 Complications connected to longstanding gastroesophageal reflux such as Barrett esophagus erosive esophagitis and esophageal adenocarcinoma are also associated with obesity, especially central obesity. 185 Therapy of GERD patients with obesity implies higher dosages and longer courses of antisecretory drugs, and concomitant use of Ursodeoxycholic acid (UDCA). 184
Therefore, to detect the patients with risk of obesity and especially central obesity, simple procedures such as BMI calculation and waist circumference measurements should be routine screening methods at the time of diagnosis as well as during periodic follow‐up.
Recommendation 30
Sarcopenia and sarcopenic obesity should be assessed, if there are indicators for sarcopenia, using body composition analysis (DXA or BIA) and dynamometry (handgrip strength) in GERD patients with overweight or obesity.
Grade of recommendation GPP ‐ Strong consensus 93% agreement
Commentary
Sarcopenia is associated with GERD, and sarcopenic obesity may be a predictive factor for erosive reflux disease. 186 Therefore, analysis of body composition using dual‐x‐absorptiometry or bioelectric impedance analysis and measurement of handgrip strength should be recommended as useful and simple assessment methods for the diagnosis of sarcopenia and sarcopenic obesity. In parallel, energy intake and protein intake should be assessed.
Indicators for sarcopenia are clinical symptoms suggesting muscle weakness, risk factors, or validated questionnaires, for example, the SARC‐F, in elderly subjects. 62
Treatment
Should weight reduction be recommended in patients with GERD to improve outcomes?
Recommendation 31
Patients with GERD and obesity shall be encouraged to lose body weight and reduce waist circumference.
Grade of recommendation A ‐ Strong consensus 100% agreement
Commentary
Overweight/obesity increases 1.2 – 3‐fold the risk for GERD symptoms. Also, the severity of GERD and its complications are linked to BMI. 187 , 188 Abdominal obesity, which is typically measured in terms of waist circumference, seems to be more important than general obesity, as GERD symptoms or erosive esophagitis were positively associated with abdominal obesity independently of BMI. 189 , 190 Increased abdominal pressure may play a more significant role in subjects with GERD and obesity, meanwhile, the defective esophagogastric barrier is usually found in individuals without obesity. 191
In a large retrospective longitudinal study, weight loss or waist reduction was associated with improvement in GERD symptoms only in subjects with general or abdominal obesity. 192 In a systematic review, even though dietary and lifestyle intervention may improve GERD in patients with obesity; however, the most favorable effect is likely to be found after bariatric surgery, especially after RYGB. 193
Which type of obesity therapy (diet counseling, exercise, multimodal therapy) should be recommended in patients with GERD and overweight/obesity?
Recommendation 32
Patients with overweight or obesity and GERD should undergo weight reduction preferentially through lifestyle modification including dietary regimen and increased physical activity.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Gastroesophageal reflux disease is one of the most common gastrointestinal diseases responsible for many outpatient visits. Obesity is a well‐known risk factor for GERD and patients with GERD and obesity are at increased risk for Barrett's esophagus. 185 The prevalence of GERD in individuals without obesity has been estimated to be 15%–20% while the prevalence is increased to over 60% among the population with obesity.
Several studies have investigated the impact of weight loss on GERD symptoms. In a population‐based cross‐sectional study, intermediate physical activity (once weekly) was associated with a decreased risk of GERD among patients with obesity. 194 It has been shown that controlled weight reduction (at least 10%) by personalized hypocaloric diet and aerobic exercise was associated with improvement of GERD symptoms and reduction of proton pump inhibitor (PPI) use. 195 A retrospective longitudinal study on patients with endoscopic confirmed GERD showed that either weight loss or waist reduction was associated with improvement of GERD symptoms but only in patients with abdominal obesity. 192 The HUNT cohort study from Norway showed a dose‐dependent reduction in heartburn and regurgitation by weight loss. 196 In a prospective trial, weight loss through reduced daily calorie intake, physical activity, and behavioral strategies resulted in a complete resolution of GERD symptoms in a population with overweight/obesity. 197 A systematic review of 16 clinical studies reported that among different lifestyle interventions, weight loss and bed elevation were effective for the resolution of GERD symptoms. 198 Another systematic review in 2016 showed that weight reduction and tobacco smoking cessation were associated with decreased symptoms of GERD. 199
Should bariatric surgery be recommended for GERD, and if yes which procedure should be preferred?
Recommendation 33
In patients with GERD and BMI >40 kg/m 2 or >35 kg/m 2 with obesity‐related comorbidities, bariatric surgery can be considered to achieve weight reduction if non‐surgical interventions failed to achieve the goals. The preferred procedure is RYGB.
Grade of recommendation 0 – Strong consensus 93% agreement
Commentary
Bariatric surgery has been applied as a treatment strategy in patients with GERD and morbid obesity. Most data in this regard derived from small and large series of patients and well‐designed clinical trials are not available. Several surgical approaches have been implemented, however, RYGB is the most effective surgical modality that is associated with weight reduction and improvement of GERD symptoms. It was also associated with decreased esophageal acid exposure and reflux esophagitis. 200 A recent meta‐analysis demonstrated that laparoscopic RYGB was superior to laparoscopic SG for the treatment of GERD symptoms. 201
PANCREATITIS
Screening & assessment
Which nutrition screening and assessment measures should be performed in patients with pancreatitis and overweight/obesity (BMI >25 kg/m 2 ) to assess nutritional status (obesity, sarcopenic obesity, body composition, micronutrients, etc.) or to optimize treatment?
Recommendation 34
In patients with acute pancreatitis and obesity, there is no need for special nutrition care compared to lean patients with acute pancreatitis.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
In all patients with acute pancreatitis, an initial nutrition assessment is recommended. 41 Initial nutritional status characterized by malnutrition as well as obesity are known risk factors for a severe course of acute pancreatitis or complications. 41 Meta‐analyses demonstrated a significantly higher rate of severe pancreatitis [OR = 2.9, 95%CI: 1.8–4.6], local complications (OR = 3.8, 95%CI: 2.4–6.6), systemic complications (OR = 2.3, 95%CI: 1.4–3.8), and death (OR = 2.89, 95%CI: 1.1–7.36) in patients with obesity. 202 The possible pathogenesis of an increased risk for severe pancreatitis in obesity could be unregulated lipolysis of visceral fat enriched in unsaturated triglyceride, thus releasing unsaturated fatty acids which inhibit mitochondrial complexes I and V, cause necrosis, and worsen acute pancreatitis. 203
Although there are some pathophysiological considerations, there is no evidence that patients with acute pancreatitis and obesity need specific nutritional care apart from patients with severe hypertriglyceridemia, which is a distinct entity accounting for 2%–10% of all cases of acute pancreatitis and more frequent in patients with obesity. 204 In these patients, fasting and intravenous hydration are the basis of therapy regardless of the severity of pancreatitis. After the acute episode, the patient should receive detailed instructions on diet therapy. Caloric restriction, decreasing the intake of simple sugars and saturated fat, and increasing the consumption of monounsaturated and poly‐unsaturated fat sources as well as dietary fiber should be recommended. 204
Recommendation 35
Nutritional status screening can be performed for patients with overweight or obesity with chronic pancreatitis, using validated scores for malnutrition and sarcopenia and encompassing basic anthropometric measurements (body weight, body height, BMI, waist circumference).
Grade of recommendation 0 ‐ Strong consensus 97% agreement
Commentary
For chronic pancreatitis, the major risk factor is considered to be alcohol use, with contributions also coming from tobacco use, hypercalcemia, and others. The role of obesity in chronic pancreatitis has been less studied than in other pancreatic diseases (such as acute pancreatitis and pancreatic cancer). Based on systematic review and meta‐analysis, current tobacco use, obesity, and heavy use of alcohol are associated with significant increases in risk for pancreatic diseases. Vegetables and fruit consumption are associated with reduced risk for pancreatic diseases. However, none of the studies included patients with chronic pancreatitis. 205
A recent prospective cohort study on 62 patients with chronic pancreatitis and 66 controls showed that over half of the patients were patients with overweight or obesity and that patients had lower muscle stores, strength, and abnormal vitamin levels. 206
In the setting of metabolic syndrome, chronic hypertriglyceridemia and pancreatic steatosis may be associated with chronic pancreatitis. 207 However, there is insufficient evidence to suggest an association of non‐alcoholic fatty pancreatic disease with the development of chronic inflammation or chronic pancreatitis. 208
In a retrospective study, patients with chronic pancreatitis were more likely to have higher pancreatic fat, but this relationship was not linear with the severity of chronic pancreatitis. In this study, abdominal obesity and pancreatic fat were related with the highest correlation being visceral obesity. 209 In vitro and animal model studies suggest that pancreatic lipomatosis may contribute to β‐cell lipotoxicity and lipoapoptosis, with consequent loss of function. However, data on humans are inconsistent. Unlike the liver, where the triglycerides accumulation is mainly intracellular, pancreatic steatosis is histologically characterized by an increased number of adipocytes and intracellular fat accumulation in both acinar and islet cells, which may precede adipocytes infiltration. It is unknown if intracellular or extracellular triglycerides have a different clinical significance, but adipocytes may influence the function of acinar and islet cells by a paracrine effect, whereas intracellular lipids may lead to lipotoxicity and therefore islet or acinar cells injury. This finding supports the hypothesis that pancreatic fat is exacerbated by visceral fat and has an impact on pancreatic disease, independent of general obesity. In this study, BMI or total body weight was not a significant factor for chronic pancreatitis or type 2 diabetes. 209
In a cross‐sectional study at 26 US Centers, including patients (n = 1,171) with chronic pancreatitis the prevalence of diabetes was (33%) and obesity was associated with an OR 2.38 for type 2 diabetes. 210
Treatment
Is there a specific nutritional treatment for patients with pancreatitis and obesity?
Recommendation 36
Patients with chronic pancreatitis and obesity should be encouraged to lose body weight and reduce waist circumference.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Although the majority of patients with severe chronic pancreatitis present rather with malnutrition than obesity 41 there is also a group of patients with chronic pancreatitis and obesity. The major environmental factors associated with chronic pancreatitis include alcohol abuse (OR, 3.1; 95% CI, 1.87–5.14) as well as smoking (OR, 4.59; 95% CI, 2.91–7.25) 211 – both also major risk factors for cardiovascular and metabolic disease. Adding obesity would increase the risk of cardiovascular disease and metabolic alterations in these patients with chronic pancreatitis.
Therefore, next to the first line of therapy consists of advice to discontinue the use of alcohol and smoking, in patients with chronic pancreatitis and obesity the possibility of weight reduction should be considered if severe malnutrition and sarcopenia have been excluded. Weight loss should be recommended in particular for those individuals with obesity and chronic pancreatitis not related to alcohol or smoking since malnutrition and sarcopenia are less frequent in this subgroup. If a weight loss diet is indicated, the amount of maldigestion and risk for specific malnutrition due to exocrine malfunction should be kept in mind.
Recommendation 37
In patients with severe acute pancreatitis and obesity, an iso‐caloric high protein diet (>1.3 g/kg ABW/d) can be administered in the acute phase. Energy and protein intake should be guided by indirect calorimetry. Apart from the acute phase, patients with acute pancreatitis do not require particular nutritional treatment beyond the recommendations for individuals with obesity in general.
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
In mild or moderate acute pancreatitis usually, no specific diet is necessary regardless of a higher BMI, whereas in severe acute pancreatitis, nutritional support adapted to the metabolic competence has shown to improve clinical outcomes. 41 Due to the changing relationship between fat mass and metabolic active muscle mass with increasing BMI, the measurement of energy expenditure has the best potential to accurately characterize the metabolic situation. If indirect calorimetry is not available, the use of ABW body weight in patients with overweight or obesity is recommended. 42 For definition of ABW see recommendation 17.
Additional metabolic derangements such as decreased glucose tolerance, altered lipid metabolism, lack of micronutrients, and decreased gut motility will need specific attention.
For further details regarding medical nutrition therapy (oral nutritional supplements, enteral and parenteral nutrition) please consult the ESPEN guideline Nutrition in acute and chronic pancreatitis. 41
Recommendation 38
In patients with suspected pancreatic insufficiency, adequate pancreas enzyme replacement therapy may consist of a starting dose of 25,000 units of lipase taken with each meal and increasing the dose as needed up to 75,000 units of lipase per meal.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Pancreatic insufficiency is a state in which there is a failure of pancreatic enzymes to provide adequate digestion. Patients with pancreatic insufficiency can be asymptomatic or symptomatic. Symptomatology can include diarrhea, steatorrhea, flatulence, and abdominal distention. These symptoms can be accompanied by nutritional deficiencies, namely fat‐soluble vitamins. It should be noted that nutritional deficiencies could develop in an asymptomatic patient. These patients are prone to develop deficiencies in fat‐soluble vitamins. Pancreatic insufficiency has been described in various clinical situations such as a result of chronic pancreatitis, in patients with diabetes, in elderly people, and post various surgeries on the gastrointestinal tract. 212 , 213 , 214 40%–80% of patients post gastrectomy and 16% of patients post esophagectomy develop pancreatic insufficiency. 212 The reason for this phenomenon can be a primary pancreatic failure and/or secondary failure due to loss of synchrony between gastric emptying, intestinal motility, and pancreatic biliary secretion.
The most common test for pancreatic activity is fecal elastase whereby a level of <200 μg/g is considered diagnostic for pancreatic insufficiency. The sensitivity of fecal elastase for mild, moderate, and severe exocrine pancreatic insufficiency in patients with chronic pancreatitis is 63%, 100%, and 100%, respectively. 212 The sensitivity of the test results reflects that patients with mild to moderate pancreatic insufficiency could still have normal levels of fecal elastase. This mandates attention to patients who are suspected of suffering from pancreatic insufficiency but have normal levels of fecal elastase since they could be suffering from mild to moderate pancreatic insufficiency and might gain benefit from pancreatic replacement therapy. The majority of replacement therapies consist of enteric‐coated formulas which are activated upon entering the small intestine via a pH‐dependent mechanism. Failure of response to treatment might be caused by too low pH in the small intestine and might be overcome by the addition of PPI or switching to a non‐enteric coated formula. 212 The starting dose should consist of 25,000–50,000 lipase units per meal and 25,000 lipase units per snack. Dose monitoring is important. 212 , 215 The provision of pancreatic enzyme replacement therapy could provide relief of symptoms, but this does not necessarily parallel the normalization of digestion and absorption. The majority of asymptomatic patients with pancreatic insufficiency without replacement pancreatic enzyme therapy and more than half of asymptomatic patients with pancreatic insufficiency and replacement therapy were found to have fat‐soluble vitamin deficiency. 216
Recommendation 39
In cases where pancreatic insufficiency is suspected and a standard enteric‐coated enzyme formulation for pancreas enzyme replacement therapy fails to achieve normalization of fat absorption, PPI treatment and/or an immediate‐release formulation can be tried.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
This recommendation is solely based on clinical practice and therefore grades as a GPP.
CHRONIC LIVER DISEASE
The CLD chapter focuses on NAFLD since this is the type of CLD typically associated with obesity, and advanced stages resulting from NAFLD such as non‐alcoholic steatohepatitis (NASH) and liver cirrhosis. It has been proposed to replace the term NAFLD with metabolic [dysfunction]‐associated fatty liver disease (metabolic [dysfunction]‐associated fatty liver disease (MAFLD)) (metabolic [dysfunction]‐associated fatty liver disease). 217 However, the term MAFLD has not been approved yet by the major international liver societies such as European Association for the Study of the Liver (EASL) or American Association for the Study of the Liver Diseases (AASLD). Moreover, almost all clinical trials have been performed in patients with NAFLD, and not in patients with MAFLD. Since it is not clear yet if NAFLD can be easily replaced by MAFLD, or if definitions for the two terms differ, the guideline working group decided to go for NAFLD presently, which does not exclude that the nomenclature will be changed in the near future.
Screening & assessment
Which screening measures should be performed in patients with CLD and overweight/obesity (BMI >25 kg/m 2 )?
Recommendation 40
Nutritional screening should be performed in all patients with CLD and overweight/obesity at the time of diagnosis and at least once a year during follow‐up.
Grade of Recommendation B ‐ Strong consensus 97% agreement
Commentary
Sarcopenic obesity, sarcopenia and myosteatosis are frequent in patients with cirrhosis. In a study including 678 cirrhotic patients, more than 60% had overweight/obesity, among them more than 30% had sarcopenic obesity. In the whole cohort, 43% had sarcopenia, myosteatosis was more frequent 53%. The presence of these muscle abnormalities was significantly associated with higher long‐term mortality in this study. 218
Recent studies showed that the combination of myosteatosis and sarcopenia was associated with a higher mortality than the presence of each one alone or the absence of both. 219
Sarcopenic obesity and myosteatosis have also a negative impact on liver transplantation and hepatocellular carcinoma management outcomes. EASL and ESPEN in recent guidelines recommend systematic nutritional screening in liver disease and cirrhotic patients. 28 , 220
Recommendation 41
Nutritional screening should be based on specific tools validated for CLD including cirrhosis, for example, the Royal free hospital nutritional prioritizing tool (RFH‐NPT) or the Liver disease undernutrition screening tool (LDUST).
Grade of recommendation B ‐ Strong consensus 93% agreement
Commentary
The RFH‐NPT and the LDUST are the most accurate tools currently available. A recent study compared eight malnutrition screening scores in cirrhosis. Royal free hospital nutritional prioritizing tool and the LDUST were the most accurate with high sensitivity (97.4% and 94.9%, respectively) and negative predictive value (99%, 97.4%, respectively). 221 RFH‐NPT is an independent predictor of cirrhosis complications mortality and the need for liver transplantation. 222 Alternatively, NRS‐2002 or MUST could be used as recommended in ESPEN guidelines. 28 , 40 , 41 , 42 , 43
Recommendation 42
For screening for NAFLD in adults with overweight or obesity, a liver ultrasound should be performed.
Grade of recommendation B ‐ Strong consensus 97% agreement
Commentary
Non‐alcoholic fatty liver disease Assessment and management National Institute for Health and Care Excellenc (NICE) guideline NG49 recommends offering a liver ultrasound to test children and young people for NAFLD if they have type 2 diabetes or metabolic syndrome and do not misuse alcohol. 223 Similarly, European guidelines for the management of NAFLD recommend using ultrasonography as first‐choice imaging in adults at risk for NAFLD. 224 Studies on ultrasound dated from 1983, and with the exception of few ones, study populations were rather small: Paige et al. (2017) n = 61, 225 Dasarathy et al. (2009) n = 73, 226 de Moura Almeida et al. (2008) n = 105, 227 Mottin et al. (2004) n = 1187, 228 Hepburn et al. (2005) n = 122, 229 Jun et al. (2014) n = 3869, 230 Lee et al. 2007 n = 589, 231 Mathiesen et al. 2002 n = 165, 232 Palmentieri et al. (2006) n = 216, 233 Perez et al. (2007) n = 131, 234 Wang et al. (2013) n = 175, 235 Wang et al. (2014) n = 171, 236 Webb et al. (2009) n = 111, 237 and Yajima et al. (1983) n = 45. 238 Most of the studies were performed on subjects undergoing biopsy for suspicion of abnormal liver function or liver disease, hepatitis C, living liver donors, or before bariatric surgery. As noted by eminent authors of the field Castera, Friedrich‐Rust, and Loomba, although, ultrasonography has the limitation that it can only detect steatosis with >2.5%–20% liver fat content and, therefore, a relevant number of patients with steatosis starting at 5% liver fat content can be missed. 239 In a large meta‐analysis overall sensitivity of ultrasound to detect moderate to severe histologically defined fatty liver from the absence of steatosis (n = 34 studies, 2815 participants) was 84.8% (95% CI: 79.5–88.9), specificity was 93.6% (87.2–97.0), the positive likelihood ratio was 13.3 (6.4–27.6), the negative likelihood ratio was 0.16 (0.12–0.22), and the summary area under the ROC curve was 0.93 (0.91–0.95). Ultrasounds have a diagnostic accuracy for the detection of ≥10% of steatosis between 0.91 and 0.93 and specificity between 0.88 ‐ 0.99. 240
Of note, sensitivity, and specificity of ultrasound and fibroscan decreases in those individuals with high BMI/abdominal girth. CT abdomen should be considered in such patients (see also recommendation 45).
Recommendation 43
All NAFLD patients should be screened for non‐communicable diseases such as diabetes, dyslipidemia, cardiovascular disease, chronic kidney disease, polycystic ovarian syndrome, obstructive sleep apnea, osteoporosis, and sarcopenia by anamnesis and in case of a corresponding suspicion by appropriate diagnostic tools.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Non‐alcoholic fatty liver disease subjects with type 2 diabetes/insulin resistance or obesity are at high risk of NASH or fibrotic NAFLD [significant (≥2)/advanced (≥3) fibrosis]. Be aware that NAFLD is strongly associated with metabolic syndrome, and that compared with the general population, NAFLD subjects with type 2 diabetes/insulin resistance or obesity, or fibrotic (F ≥ 2) NAFLD, or NASH patients are at increased risk of cardiovascular and all‐cause mortality.
Staging of NAFLD and the anamnestic screening of the risk of non‐communicable diseases are complementary actions in the management of NAFLD. Type 2 diabetes, atherosclerosis, cardiovascular disease, chronic kidney disease, polycystic ovarian syndrome, obstructive sleep apnea, osteoporosis, and sarcopenia should be taken into account proactively in the management of NAFLD patients. Non‐alcoholic fatty liver disease subjects with type 2 diabetes/insulin resistance or obesity, fibrotic (F ≥ 2) NAFLD or NASH/cirrhotic patients should be promptly screened for cardiovascular disease and related risk factors, chronic kidney disease, obstructive sleep apnea. Screening of colorectal cancer and other extrahepatic malignancies should be proactively implemented according to international guidelines.
Which measures should be performed in patients with CLD and overweight/obesity (BMI >25 kg/m 2 ) to assess nutritional status (obesity, sarcopenic obesity, body composition, micronutrients, etc.) or to optimize treatment?
Recommendation 44
Medium to high‐risk patients according to screening should undergo a detailed nutritional assessment including an assessment of sarcopenia.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
Malnutrition and sarcopenia are risk factors for complications and mortality in cirrhosis and likely other CLD. Malnutrition prevalence is higher in decompensated advanced liver disease compared to compensated CLD. In an Italian prospective study, the prevalence of malnutrition was 23%, 44%, and 57% in the case of cirrhosis Child‐Pugh A, B, and C, respectively. 241 Correction of malnutrition and sarcopenia is an essential part of CLD and especially cirrhosis management. Therefore, a straightforward comprehensive nutritional assessment is mandatory for all patients with CLD.
Overweight/obesity is not a reflection of a better nutritional state. Sarcopenic obesity is a frequent condition associated with advanced CLD in patients with obesity and is related to worse outcomes and mortality. In an analytical study from Canada including 678 cirrhotic patients, the frequency of sarcopenia was 43%, sarcopenic obesity at 20%, and myosteatosis at 52%. Median survival was lower (22–28 months) in patients with muscular abnormalities versus 95 months in patients without muscular abnormalities. 218
Assessment for malnutrition should be systematic in cardiovascular disease/cirrhosis including patients with overweight and obesity. Regular assessment tools such as BIA are challenged by methodological limitations in case of obesity and liver function impairment, as well as in case of fluid retention or insufficient liver metabolism.
EASL guidelines propose the following algorithm to manage cirrhosis/advanced CLD patients according to malnutrition risk (Figure 1). 220 Most of the methods proposed herein are not influenced by obesity besides BMI and other anthropometric measurements.
Nutritional assessment in CLD proposed by EASL is based on the evaluation of muscle mass/sarcopenia using the Skeletal muscle index by measuring the total abdominal muscle area at L3 with magnetic resonance imaging (MRI), or appendicular skeletal muscle mass index by DXA or by measuring lean body mass using BIA. Muscle mass measurement should be completed by a muscle function test, for example, handgrip. Nutritional assessment should also include recording of dietary intake by assessing barriers to eating, and by validated questionnaires or diaries.
FIGURE 1.
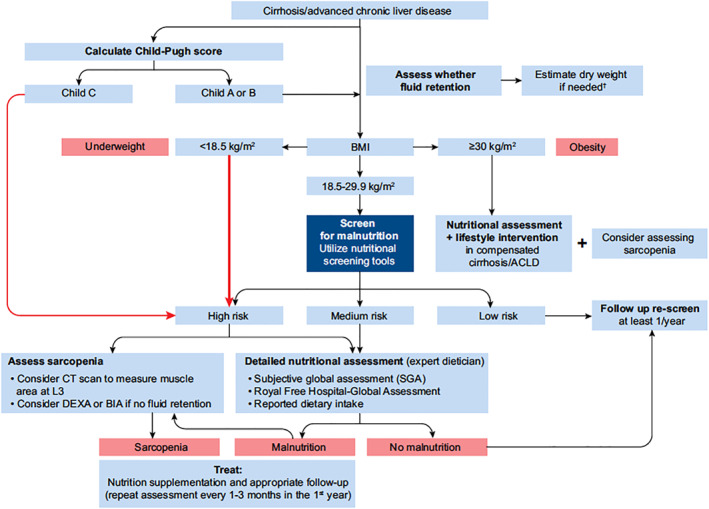
European association for the study of the liver Clinical Practice Guidelines on nutrition in chronic liver disease (CLD) 220
How to assess, preferably through non‐invasive tools, liver steatosis, stage (fibrosis) of CLD, or the presence of primary liver cancers in patients with overweight or obesity to assure adequate diagnosis and treatment?
Recommendation 45
Liver ultrasound should not be used to rule out NAFLD in patients with grade II/III obesity.
Grade of recommendation B ‐ Strong consensus 93% agreement
Commentary
The accuracy of ultrasonography for the diagnosis of liver steatosis is reduced in patients with obesity. 239 Two independent prospective studies enrolled patients with severe obesity (105 with a mean BMI of 43.8 kg/m2 and 187 with a mean BMI of 47.5 kg/m2) undergoing bariatric surgery and intraoperative liver biopsy (histological prevalence of steatosis of 89.5% and 91.4%), the sensitivity and specificity of ultrasound in the diagnosis of hepatic steatosis were: 64.9% and 90.9%, 227 and 49.1% and 75%. 228 Both studies evaluated how BMI affects the performance of ultrasound. Mottin et al. 228 showed that for subjects with BMI between 35 and 40 kg/m2 the prevalence of steatosis in this subgroup was 95.8%, with a sensitivity of 39% and a specificity of 100%, and a positive predictive value of 100%. Alessandro de Moura Almeida et al. 227 the prevalence of steatosis in patients with BMI between 35.0 kg/m2 and 39.9 kg/m2 and in patients with BMI above 40 kg/m2 was 83.3% and 91.3%, respectively. Levels of sensibility, specificity, positive predictive values and negative predictive values in these two BMI categories were, respectively, 65, 75, 92.9, 30, and 64.4, 100, 100, 21.2. As noted by Castera et al. in a recent review, 239 the low sensitivity of the method could be related to the lack of objective criteria for the ultrasound diagnosis of steatosis, and probably, technical problems in performing ultrasound in subjects with grade II and III obesity. Therefore, abdominal ultrasound has not shown to be an accurate method for the diagnosis of hepatic steatosis in patients with morbid obesity. However, since the predictive positive value resulted in variably high the ultrasound remains a pivotal first step in the investigation of suspected NAFLD as confirmed by NICE 223 and EASL guidelines. 224 Recent studies obtained better results using quantitative ultrasound techniques, the ultrasound hepatic‐renal echo‐intensity 235 and the quantitative ultrasound, 225 these may be difficult to apply in clinical practice and have not been tested specifically in patients with morbid obesity.
Instead of ultrasound, a CT abdomen can be used for the diagnosis of NAFLD in patients with grade II/III obesity.
Recommendation 46
Transaminase determination in serum should not be used to rule out NAFLD.
Grade of recommendation B ‐ Strong consensus 97% agreement
Commentary
No papers relevant to the review protocol were identified for alanine aminotransferase (ALT), aspartate aminotransferase (AST), or gamma‐glutamyl transferase (GGT). 242 Liver transaminases should not use to rule out NAFLD, nor to establish the severity of the disease.
Recommendation 47
Selected biomarkers are suitable to assess the presence and the grade of steatosis.
Grade of recommendation 0 ‐ Strong consensus 93% agreement
Commentary
Different tests including biomarkers and/or anthropometric measures and/or clinical data are suitable to assess the presence and the grade of steatosis. SteatoTest, NAFLD liver fat score, Hepatic Steatosis Index, and Fatty Liver Index may be used to diagnose NAFLD in subjects bearing metabolic risk factors/components of the metabolic syndrome in the absence of a history of significant alcohol use or other known liver diseases (for details see Box 1). SteatoTest may be used to diagnose NAFLD in subjects with grade II or III obesity bearing metabolic risk factors/components of metabolic syndrome in the absence of a history of significant alcohol use or another known liver disease. The diagnostic and prognostic performance of hepatic steatosis tests as relevant surrogate biomarkers of solid liver‐related or cardiovascular‐related outcomes needs to be assessed in long term observational or interventional studies.
Box 1 Serum biomarkers and scores to assess liver steatosis.
SteatoTest was developed in subjects bearing several liver risk factors with a median BMI of 25.4 kg/m2 and validated in hepatitis C virus liver disease and alcoholic liver disease subjects. 243 It is a minimally invasive diagnostic test calculated with a formula including alpha2‐macroglobin, apolipoprotein A1, haptoglobin, total bilirubin, AST, ALT, GGT, fasting glucose, total cholesterol, triglycerides, weight and height, adjusted for age and gender. In patients with grade II obesity (BMI >35 kg/m2) underwent to bariatric surgery (n = 288), with an optimal cut‐off of >0.38 SteatoTest predicted the presence of steatosis >5% with sensitivity of 87 and specificity of 50%. 244 The diagnostic for estimating histological moderate/severe (>33%) versus no/mild (0%–33%) steatosis for SteatoTest: AUROC of 0.70 (0.59–0.71). With an optimal cut‐off of >0.69, It predicted moderate/severe steatosis with a sensitivity of 42% and specificity of 79%. 244 In a second study enrolling 112 patients (41% subjects with overweight, 17% subjects with obesity, NAFLD 25% and chronic hepatitis C 36%), the diagnostic for estimating histological moderate/severe (>33%) versus no/mild (0%–33%) steatosis for SteatoTest: AUROC of 0.7 (0.59–0.71). With an optimal cut‐off of >0.94, It predicted moderate/severe steatosis with a sensitivity of 9% and specificity of 42%. 245 As far as the diagnostic for estimating histological moderate/severe (>33%) versus no/mild (0%–33%) steatosis for SteatoTest the quality of evidence of sensitivity and specificity was very low, and the quality of evidence of AUC was very low. Performance of SteatoTest in Patients with grade II/III obesity was also analyzed in a Meta‐Analysis of Individual Patient Data. 243 494 patients with interpretable biopsy and biomarkers using three prospective cohorts of patients with BMI >35 kg/m2 were included. The SteatoTest mean weighted AUROC for advanced steatosis (>33%) was 0.80 (0.79–0.83) significantly greater (Z = 5.2 p = 0.0001) than that of ALT 0.75 (0.73–0.77; p = 0.0001). SteatoTest weighted accuracy was also highly significant in 141 patients with diabetes 0.76 (0.72–0.80; p = 0.0001). Classical AUROC of SteatoTest was 0.71 (0.66–0.75; p = −0.0001). To improve the validation of SteatoTest for steatosis grading a large European consortium analyzed 600 patients with reliable tests and biopsy‐proven NAFLD. 246 This study was one of the first where biopsies were blindly assessed using the new steatosis, activity, and fibrosis score, which provides a reliable and reproducible diagnosis and grading/staging of the three elementary features of NAFLD (steatosis, inflammatory activity) and fibrosis with reduced interobserver variability. The mean non‐binary‐ROC (NonBinAUROC; 95% CI) was 0.822 (0.804–0.840) for SteatoTest and steatosis grades (marked steatosis >33%). Due to the retrospective design and risk of biases the quality of evidence of sensitivity and specificity was very low, quality of evidence of AUC was very low.
Fatty Liver Index, has been conceived as a simple algorithm for the prediction of ultrasound‐detected liver steatosis in the general population (without suspected liver diseases). 247 It includes BMI, waist circumference, triglycerides, and c‐glutamyl‐transferase. More recently the Fatty Liver Index has been assessed in subjects with CLD and BMI ≥28 kg/m2. 248
NAFLD‐liver fat score considered the presence of diabetes, AST/ALT ratio, metabolic syndrome, and insulin to predict ultrasound‐detected fatty liver. Successively this index was investigated in a retrospective study of biopsy‐proven diagnosis in 324 subjects with clinical and/or ultrasonography suspicion of NAFLD. The study population was characterized by a median BMI (kg/m2) of 29 (26–33), median waist circumference (cm) of 101 (92–109), and a high prevalence of type 2 diabetes (41%). The marker displayed an acceptable accuracy in estimating the presence of steatosis of any amount versus no steatosis; AUROCs of 0.80 (0.69–0.88). With an optimal cut‐off of >0.16 NAFLD liver fat score predicted the presence steatosis >5% with sensitivity of 65 and specificity of 87% 248 The diagnostic for estimating histological moderate/severe (>33%) versus no/mild (0%–33%) steatosis decreased to fair; AUROCs of 0.72 (0.66–0.77). With an optimal cut‐off of >0.16 NAFLD liver fat score predicted moderate/severe steatosis with a sensitivity of 78% and specificity of 59%. 248
Hepatic Steatosis Index is calculated according the formula eight x (ALT/AST ratio)+BMI (+2, if female; +2, if diabetes). 249 It showed an acceptable accuracy in estimating the presence of steatosis of any amount versus no steatosis; AUROCs of 0.81 (0.71–0.88). With an optimal cut‐off of >41.6, the Hepatic Steatosis index predicted moderate/severe steatosis with a sensitivity of 61% and specificity of 93%. 249 The diagnostic for estimating histological moderate/severe (>33%) versus no/mild (0%–33%) steatosis decreased to fair; AUROCs of 0.65 (0.66–0.77). An optimal cut‐off of >43.0 Hepatic Steatosis Index predicted moderate/severe steatosis with a sensitivity of 59% and specificity of 68%. 249
How to verify, preferably through non‐invasive tools, liver steatosis, stage (fibrosis) of CLD, or the presence of primary liver cancers in patients with overweight or obesity to assure adequate diagnosis and treatment?
Recommendation 48
The ultrasound‐based controlled attenuation parameter (CAP) and MRI can be used to verify the diagnosis of NAFLD instead of liver biopsy.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
Vibration‐controlled transient elastography has been the pioneer ultrasound‐based technique and is the most widely used worldwide transient elastography and magnetic resonance elastography to provide additional information in patients with NAFLD. The same machine can be used to determine whether steatosis is present: CAP for transient elastography and calculation of the proton‐density fat traction for magnetic resonance elastography. 239 , 250 Regarding CAP the searching strategies identified many papers comprising heterogeneous cohorts of patients affected by different diseases etiologies other than NAFLD. According to a review and meta‐analysis published in 2017 of 3830 patients from 19 studies using the M‐probe (37% hepatitis B, 36% hepatitis C, 20% NAFLD/NASH, 7% other), with a steatosis distribution of 51%/27%/16%/6% for S0/S1/S2/S3, CAP values in dB/m (95% CI) were influenced by several covariates, for example, NAFLD/NASH patients, diabetes and BMI. Optimal cut‐offs were 248 (237–261) and 268 (257–284) for those above S0 and S1 respectively, with, areas under the curves of 0.823 and 0.865, respectively. 250 The NICE guideline considered the level of evidence of CAP for liver steatosis >5% or >30% in previous heterogeneous studies not targeting NAFLD patients from very low to low. 223 Cohorts of patients affected solely by NAFLD or suspected NAFLD have been studied since 2012. These studies were included in the evaluation of the evidence. Thirteen papers have been considered, 11 prospective 251 , 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 and two retrospectives. 255 , 258 Thanks to this explosion of prospective studies conducted in homogenous cohorts the level of evidence increased substantially from early studies on this application to the whole population of liver disease patients.
The company that developed the CAP system does not plan to continue further development of CAP to diagnose NAFLD in patients with obesity because the more the patient is obese the less accurate is CAP for NAFLD monitoring (internal information). Magnetic resonance imaging might be an alternative; however, because of availability and costs, MRI can be performed only on a few selected patients, as stated in the current EASL guideline. 262 A biopsy is usually not recommended for the diagnosis of NAFLD, but NASH and particular differential diagnoses of CLD. 262
Recommendation 49
In case of a negative or unclear ultrasound finding, CAP should be considered to diagnose and stage mild, moderate, and severe hepatic steatosis.
Grade of recommendation B ‐ Strong consensus 96% agreement
Commentary
Very recently even a meta‐analysis 263 appeared including nine of these studies involving 1297 patients with liver biopsy‐proven NAFLD were analyzed. 251 , 253 , 254 , 255 , 258 , 260 , 261 The sensitivity, specificity, diagnostic OR, and area under receiver operating characteristics curves of the pooled data for CAP in diagnosing and staging steatosis in NAFLD patients were assessed. The pooled sensitivity of CAP in detecting mild hepatic steatosis was 87% with a specificity of 91%. The pooled sensitivity of CAP in detecting moderate hepatic steatosis was 85% with a specificity of 74%. For severe steatosis, the pooled sensitivity was 76% with a specificity of 58%. The mean AUROC value for CAP in the diagnosis of mild, moderate, and severe steatosis was 0.96, 0.82, and 0.70, respectively. Subgroup analysis indicated that variation in the geographic regions, cutoffs, age, and BMI could be the potential sources of heterogeneity in the diagnosis of moderate to severe steatosis. As argued by Thomas Carls and colleagues, the ultrasound‐based CAP can be used instead of liver biopsy biopsies for diagnosing fatty liver, taking into account factors such as the underlying disease, BMI, and diabetes, but longitudinal data are needed to demonstrate how CAP relates to clinical outcomes. 250
Recommendation 50
In subjects with grade II/III obesity or suspected NAFLD, an MRI‐PDFF can be performed to confirm the diagnosis of NAFLD.
Grade of recommendation 0 ‐ Strong consensus 93% agreement
Commentary
MRI‐PDFF has an excellent diagnostic value for the assessment of hepatic fat content and classification of histologic steatosis in patients with NAFLD and can be used as a non‐invasive test to validate the diagnosis of NAFLD in individuals with severe obesity or for the longitudinal evaluation of hepatic steatosis in patients under specific NAFLD treatments. The diagnostic accuracy of hepatic proton density fat fraction measured by MRI for the evaluation of liver steatosis with histology as a reference standard was the object of 13 studies. 264 These papers evaluated the diagnostic accuracy of hepatic MRI‐PDFF for the assessment of liver steatosis with histology as a reference standard (scoring system for histological grading of NASH Clinical Research Network (NASH CRN). In eight studies the mean BMI (kg/m2) was 30 or more. All studies except three were prospective. Most of the studies were realized on NAFLD or suspected NAFLD in nine out of 13, two in liver donors, and only one in hepatitis C virus liver disease. 265 , 266 , 267 , 268 , 269 , 270 , 271 , 272 , 273 , 274 , 275 , 276 According to a meta‐analysis of these studies containing 1100 subjects by Qu Y et al., 264 there was a significant threshold effect for liver steatosis ≥ G1. The AUCs for liver steatosis ≥ G1 (NASH CRN), liver steatosis ≥ G2, and liver steatosis = G3 were 0.98 (95% CI 0.76–1.00), 0.91 (95% CI 0.89–0.94), and 0.92 (95% CI 0.89–0.94), respectively. The pooled sensitivities for liver steatosis ≥ G2 and liver steatosis = G3 were 0.83 (95% CI 0.75–0.88) and 0.79 (95% CI 0.63–0.90), respectively; the pooled specificities for liver steatosis ≥ G2 and liver steatosis = G3 were 0.89 (95% CI 0.84–0.92) and 0.89 (95% CI 0.84–0.92), respectively. MRI‐PDFF has high diagnostic accuracy at detecting and grading liver steatosis with histology as a reference standard, suggesting that MRI‐PDFF can provide accurate quantification of liver steatosis in clinical trials and patient care. 264 According another meta‐analysis of six studies 256 , 270 , 271 , 273 , 274 , 275 including 635 subjects by Gu J et al., 277 the summary AUROC values of MRI‐PDFF for classifying steatosis grades 0 versus 1–3, 0–1 versus 2‐3, and 0–2 versus Three were 0.98, 0.91, and 0.90, respectively. Pooled sensitivity and specificity of MRI‐PDFF for classifying steatosis grades 0 versus 1–3, 0–1 versus 2‐3, and 0–2 versus Three were 0.93 and 0.94, 0.74 and 0.90, and 0.74 and 0.87, respectively. This meta‐analysis suggested that MRI‐PDFF has excellent diagnostic value for the assessment of hepatic fat content and classification of histologic steatosis in patients with NAFLD. 239 , 277
How should the progression or regression of liver fibrosis be assessed?
Recommendation 51
Patients with NAFLD and advanced fibrosis or cirrhosis should undergo a surveillance ultrasound of the liver for early detection of hepatocellular carcinoma every 6 months.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Progression or regression of NAFLD includes the disease activity (grading), liver fibrosis (staging) as well as the occurrence of disease‐specific complications such as decompensation of liver cirrhosis or development of hepatocellular carcinoma. Based on prospective trials and meta‐analyses, international clinical guidelines unequivocally recommend hepatocellular carcinoma surveillance performed by experienced personnel in all high‐risk populations using abdominal ultrasound every 6 months. 278 While such high‐level evidence exists on hepatocellular carcinoma surveillance by ultrasound for patients with liver cirrhosis (mostly due to viral hepatitis or alcoholism), patients with NAFLD have a high risk to develop hepatocellular carcinoma, even in non‐cirrhotic livers. 279 Therefore, it appears plausible to include patients with NAFLD at particular risk for hepatocellular carcinoma, that is, patients with advanced (stage F3) fibrosis or cirrhosis, in the same hepatocellular carcinoma surveillance schedule. 278 , 280
Recommendation 52
Fibrosis progression or regression in patients with NAFLD can be monitored after weight loss therapy by non‐invasive procedures or liver biopsy.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
Meta‐analyses from biopsy‐controlled prospective evaluations of patients with NAFLD have convincingly demonstrated that the stage of liver fibrosis is predictive of liver‐related morbidity and mortality. 281 This is the main rationale, why “fibrosis regression” is an accepted endpoint in clinical trials in NASH. 282 Monitoring fibrosis regression would, therefore, be also advisable to monitor disease regression in patients achieving weight loss. Prospective clinical trials evaluating either intense lifestyle modifications, pharmacological interventions (e.g. Glucagon‐like Peptide 1 analogs such as liraglutide or semaglutide), or bariatric surgery have used serial liver biopsies, mostly 1 year after initiating the weight loss intervention, to monitor fibrosis regression. 282 While this is suitable in controlled conditions of a clinical trial, non‐invasive procedures should be preferred in the clinical routine. There is good evidence that several scoring systems (e.g., the Enhanced Liver Fibrosis test), imaging and mechanical procedures (e.g., Magnetic resonance elastography, vibration‐controlled transient elastography (Fibroscan), Acoustic radiation force impulse imaging) have an acceptable degree of accuracy for staging fibrosis. 283 However, the accuracy of non‐invasive tests in monitoring disease regression upon interventions (such as weight loss) is less well defined and awaits further studies. 282 The expert panel acknowledges the need for monitoring fibrosis progression or regression to determine the future risk for liver‐associated complications, but the exact modality (non‐invasive test vs. repeated liver biopsy) is currently based on individual decisions considering the medical condition of the patient, logistic considerations and the potential risks associated with the chosen procedure.
Treatment
Which type of dietary/lifestyle measures for obesity therapy should be recommended in patients with CLD and overweight/obesity?
Recommendation 53
Patients with CLD and overweight or obesity shall undergo weight reduction to improve outcomes.
Grade of recommendation A ‐ Strong consensus 97% agreement
Commentary
Metabolic risk factors seem to be related to severe liver disease in patients with NAFLD according to a recent meta‐analysis of 22 observational studies including 24 million individuals. Type 2 diabetes and obesity were associated with an increased incidence of severe liver disease with HRs 2.5 and 1.2 respectively, and the more metabolic risk factors were present the more the risk of severity increased. Robust data is still lacking to define the impact of metabolic risk factors on liver disease severity and progression. 284
Mortality in cirrhosis is multifactorial, a population‐based study including 52,027 cirrhotic patients in five years showed that the main risk factors related to mortality are portal hypertension‐related complications and decompensations specially hepatorenal syndrome, malignancy, comorbidities (cardiac and renal) and bacterial infections. 285
A recent review and meta‐analysis including 1,495 patients, concluded that liver‐related mortality in NAFLD is exponentially related to an increase in the stage of fibrosis. 286
Weight loss in patients with overweight or obesity and CLD/cirrhosis reduces metabolic risk and liver fibrosis. The first choice of weight loss therapy (namely fat mass reduction) is lifestyle intervention. If the goals cannot be reached by this means, bariatric surgery should be considered. Before starting a weight reduction therapy, severe malnutrition and sarcopenia need to be ruled out.
Intensive lifestyle intervention leading to weight loss ≥10% proved to reduce portal hypertension in a prospective study including 50 patients (patients with overweight or obesity and compensated cirrhotic with portal hypertension). 287
Bariatric surgery improves outcomes in NAFLD including metabolic risk factors (mainly diabetes) and fibrosis (histologically proven). Multiple RCTs and meta‐analyses have been published in this regard. Most specialist societies (EASL, European Association for the Study of Diabetes (EASD), EASO, ESPEN, AASLD, American Association of Clinical Endocrinology (AACE)/The Obesity Society (TOS)/American Society for Metabolic & Bariatric Surgery (ASMBS)/OMA)/American Society of Anesthesiologists (ASA)) recommend weight loss to improve steatosis, liver enzymes, and fibrosis. 28 , 220 , 224 , 288 , 289
Recommendation 54
In patients with obesity and CLD, obesity therapy should start with structured dietary and behavioral lifestyle changes, organized in a multimodality treatment program.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
The guidelines for NAFLD recommend treatment by lifestyle changes including a healthy diet and physical activity. 290 AASLD guidelines recommend for weight loss either a hypocaloric diet alone or in conjunction with increased physical activity (daily reduction of 500–1,000 kcal). 3%–5% weight loss appears to the authors of this guideline to be necessary to improve NASH steatosis, and 7%–10% to improve the majority of histopathologic features, including fibrosis. 288 In a Western cohort of 129 patients with obesity undergoing a 6‐month lifestyle modification program (NAFLD = 58, no NAFLD = 71) patients with NAFLD lost more visceral adipose tissue while weight loss with similar for NAFLD and those without. Non‐alcoholic fatty liver disease was not associated with visceral adipose tissue sarcopenia. 291 There are no specific recommendations according to the type of liver disease.
Recommendation 55
Special attention should be given to sarcopenia during weight‐loss interventions.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
The risk of sarcopenia is high in patients with NAFLD/NASH and it may worsen liver disease progression to fibrosis and overt cirrhosis. 292 , 293 , 294 , 295 , 296 , 297 Given the very high prevalence of overweight and obesity among NAFLD patients, sarcopenic obesity is also common in this setting. The risk of further increases during weight loss; therefore, special attention should be given to sarcopenia. It is related to a poor outcome in cirrhotic patients. 12
Recommendation 56
In chronic liver patients with overweight or obesity, all the advice for the prevention and/or management of non‐communicable preventable diseases (e.g. weight loss, exercise, smoke avoidance, alcohol misuse avoidance) should be always given and proactively promoted and implemented complying with current guidelines for the management of obesity.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Non‐alcoholic fatty liver disease is strongly associated with metabolic syndrome, the components of which include hypertension, hyperglycemia, abdominal obesity, and dyslipidemia. 298 , 299 , 300 , 301 NAFLD has a central role in the complex pathophysiology of metabolic syndrome, type 2 diabetes, and cardiovascular disease. 302 From a clinical and epidemiological point of view, NAFLD should not be considered merely a hepatic manifestation of metabolic syndrome but rather both a consequence as well a predecessor of metabolic syndrome. 299 , 300 , 301 Indeed, it has been recently demonstrated that increased liver fat content in patients with NAFLD is associated with increased rates of metabolic syndrome. There appears to be an association between the quantity of liver fat and the risk for cardiovascular disease in patients with NAFLD. 303 Compared with the general population, NAFLD patients are at increased risk of liver‐related, cardiovascular, and all‐cause mortality. 299 , 300 , 301 Community‐based longitudinal studies determining all‐cause and cause‐specific mortality in patients with NAFLD revealed that patients with NAFLD had higher rates of all‐cause, cardiovascular disease, and liver‐related mortality than the matched general population. 299 , 300 , 301 , 304 , 305 , 306 , 307 , 308 , 309 , 310 , 311 , 312 Finally, a very relevant conclusion in NAFLD biopsied patients was achieved by Ekstedt et al. 313 : the fibrosis stage rather than the presence of NASH predicts the mortality. Liver toxicity of common drugs used in metabolic syndrome outpatients is acceptable and the benefits of drugs given to reduce or prevent outcomes of cardiovascular disease and/or type 2 diabetes include the ones related to the liver disease. 314 , 315 , 316 , 317 , 318 , 319 Advance liver disease determines pharmacokinetic consequences due to the impairment of liver function, and due to frequent multiple therapies and drug interactions. The detrimental effects of adverse drug reactions, as happening in drug‐induced liver injury, are particularly severe in advanced liver disease patients. 314 , 315 , 316 , 317 , 318 , 319 CLD should not be regarded as an absolute limiting factor in the pharmacologic or surgical management of diet‐related non‐communicable diseases (e.g. heart disease, stroke, diabetes/insulin resistance, dyslipidemia, hypertension, gallstones, sarcopenia, osteoporosis) when indicated. Advanced liver disease should be taken into account due to its pharmacokinetic consequences and due to frequent multiple therapies and drug interactions when prescribing drugs. Avoid herbs or integrators at increased risk of drug‐induced liver injury in any case.
Recommendation 57
Non‐alcoholic fatty liver disease/NASH patients with overweight or obesity not undergoing weight‐loss treatment should ingest at least 1 g/kg ABW*/d protein.
Grade of recommendation GPP ‐ Strong consensus 96% agreement
*For definition of ABW see recommendation 17. In CLD patients with ascites, the amount of ascites should be estimated and subtracted from ABW.
Commentary
No studies have compared different protein dietary allowances to identify optimal protein intake to preserve skeletal muscle mass in NAFLD/NASH patients with overweight or obesity. It should however be pointed out that low dietary protein may directly enhance liver fat deposition. 320 On the other hand, in weight‐stable patients not undergoing weight‐loss treatment, recent evidence also suggests a positive impact of higher dietary protein fraction on liver fat and inflammation. 321 , 322 In an RCT in 37 weight‐stable individuals with NAFLD, type 2 diabetes, and an average BMI of 30.2 kg/m2, 30% of dietary macronutrients as animal or plant protein were shown to similarly reduce liver fat by magnetic resonance spectroscopy, circulating levels of hepatic enzymes and markers of inflammation, while insulin sensitivity increased. 321 In a crossover study in 28 individuals with type 2 diabetes, 322 6‐week high‐protein compared to conventional diabetes diet (30 vs. 17% protein content respectively) also was associated with lower hepatic fat content (−2.4 vs. + 0.2%), in addition to lower hemoglobin A1c (HbA1c) and post‐prandial plasma glucose. Given the high emerging prevalence of sarcopenia in individuals with NAFLD/NASH, 292 , 293 , 294 , 295 , 296 , 297 at least 1 g/kg ABW/d of dietary protein is recommended for weight‐stable NAFLD/NASH patients in the absence of malnutrition and sarcopenia, as it equals the recommended allowance for a population with similar risk including geriatric and polymorbid patients. 79 , 323 Unless accurate measurement of skeletal muscle mass or lean body mass is available for example, by DXA, ABW may represent an acceptable although inevitably approximate reference value to calculate total protein requirements, taking into account metabolically active components of excess body weight. 42
Recommendation 58
Non‐alcoholic fatty liver disease/NASH patients with overweight or obesity undergoing a hypocaloric diet to achieve weight loss should ingest 1.2 g/kg ABW/d protein to prevent loss of muscle mass.
Grade of recommendation B ‐ Strong consensus 96% agreement
Commentary
Weight loss in the range of 7%–10% through lifestyle intervention including diet is recommended in NAFLD/NASH patients with overweight or obesity 28 , 324 , 325 , 326 to improve liver steatosis. More pronounced weight loss may be needed to improve liver fibrosis 28 , 101 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 which may require bariatric surgery. Loss of body weight may be accompanied by loss of skeletal muscle mass and lead to sarcopenia which has been reported to be independently associated with fibrosis 297 and may have a detrimental impact on patient morbidity and mortality. 292 , 293 , 294 , 295 , 297 No studies are available on the impact of high protein intake on skeletal muscle mass and function in NAFLD/NASH patients with overweight or obesity undergoing weight‐loss lifestyle programs. Hypocaloric high‐protein diets were however investigated in different patient groups. In a previous meta‐analysis 335 23 studies were included to compare isocaloric high‐versus standard protein intake (1.25 vs. 0.75 g/kg/d) in the context of energy restriction in individuals with obesity. Analyses showed attenuated loss of fat‐free mass despite a more pronounced total body weight loss in high‐protein patient groups. 335 In older adult women with sarcopenia, 1.2 g/desirable body weight/d of protein effectively prevented the reduction of the Muscle Mass index compared to a lower intake of 0.8 g/desirable body weight/d. 336 In another study 337 middle‐aged women receiving 1.2–1.4 g/kg reference body weight/d through a 15 g oral protein supplement for 4 months showed a higher fat‐free mass and muscle strength compared to no change in the control group receiving 0.8–1.0 g/kg reference body weight/d protein. “Desirable body weight in the sense of 336 is equivalent to the reference body weight in 337 and is commonly defined as the body weight at a BMI of 25 kg/m2”. Given the prevalence of sarcopenia in NAFLD/NASH and the potential clinical risk associated with loss of muscle mass and strength, a dietary provision of 1.2 g/kg ABW/d is recommended for NAFLD/NASH individuals with overweight or obesity undergoing weight‐loss programs. For definition of ABW see recommendation 17.
Recommendation 59
Non‐alcoholic fatty liver disease/NASH patients with overweight or obesity and malnutrition or sarcopenia may ingest at least 1.2 g/kg ABW/d protein.
Grade of recommendation GPP ‐ Strong consensus 96% agreement
Commentary
No studies investigating the amount of dietary protein required to improve nutritional status in NAFLD/NASH patients with overweight or obesity and malnutrition or sarcopenia are available, with particular regard to protein requirements to improve skeletal muscle mass or function. High‐protein diets have shown metabolic benefits in non‐malnourished weight stable NAFLD/NASH patients 321 , 322 and higher protein intake favors skeletal muscle protein anabolism and muscle protein accretion in catabolic conditions. At least 1.2 and up to 1.5 g/kg ABW/d dietary protein should be provided to NAFLD/NASH patients with overweight or obesity and malnutrition or sarcopenia.
Recommendation 60
Patients with overweight or obesity and compensated liver cirrhosis should ingest 1.2 g/kg ABW/d protein. Patients with overweight or obesity and compensated liver cirrhosis undergoing weight‐loss programs should ingest 1.2–1.5 g/kg ABW/d protein. Patients with overweight or obesity and compensated liver cirrhosis and malnutrition or sarcopenia should ingest 1.5 g/kg ABW/d protein.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Overweight and obesity are common in patients with compensated liver cirrhosis 338 , 339 and may be associated with a higher risk of decompensation and complications. 338 Obesity prevalence is highest in NAFLD‐associated liver cirrhosis. Liver cirrhosis is a protein‐ and muscle‐catabolic condition due to high total body protein breakdown and decreased protein synthesis. 340 , 341 , 342 , 343 , 344 Elevated protein intake is reported to be well tolerated and effective in liver cirrhosis patients to increase protein anabolism 28 , 345 , 346 also in the presence of malnutrition and sarcopenia. 347 , 348 , 349 , 350 No studies are available specifically investigating these parameters in individuals with overweight or obesity and liver cirrhosis. Recommendations for high dietary protein intakes in the general liver cirrhosis patient population without and with malnutrition and sarcopenia 28 , 345 are therefore extended to the subgroup with overweight and obesity, using ABW to calculate the total requirement taking into account the metabolically active fraction of excess body weight. 42
Different studies also suggested that a 5%–10% weight loss through lifestyle intervention may improve outcomes and reduce disease progression in patients with obesity and compensated liver cirrhosis. 339 , 351 , 352 , 353 Strong evidence is lacking on protein requirements to maintain muscle mass during weight loss programs in patients with obesity and compensated liver cirrhosis. We recommend a higher intake of 1.5 g/kg ABW/d considering the high risk of pre‐existing 292 , 293 , 294 , 295 , 296 , 297 and new‐onset sarcopenia that may occur during weight loss in these patient groups.
Which type of endoscopical procedures for obesity therapy should be recommended in patients with CLD and overweight/obesity?
Recommendation 61
In case of non‐surgical treatment a transient endoscopical gastric balloon can be offered in selected patients with NASH in the absence of portal hypertension.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
Non‐surgical multimodality treatment programs including an endoscopic gastric balloon may achieve significant short‐term weight loss and improvement of comorbidity. 355 The Food and Drug Administration (FDA) approved two liquid‐filled intragastric balloon systems for use in the U.S. (Orbera and ReShape). These systems are partly available also in Europe. Typical risks according to the FDA are hyperinflation with the need for early removal and pancreatitis. 354
Non‐surgical multimodality treatment programs including an endoscopic gastric balloon may achieve significant weight loss and improvement of comorbidity. 355 However, data on patients with CLD is limited. Efficacy and safety of intragastric balloons have been shown for NAFLD in a systematic review and meta‐analysis of nine studies including 442 balloons. 356 Improvement of steatosis was observed in 79.2% of the patients and NAFLD activity score in 83.5%. HOMA‐IR improved in 64.5% of the patients. A reduction in liver volume was observed in 93.3%. In an open‐label prospective study, the effects of intragastric balloon placement in combination with dietary measures and exercise on metabolic and histologic features were investigated in 21 patients with NASH. 357 Six months after intragastric balloon placement weight loss was 11.7 ± 7.7%. Weight loss did not correlate with a reduction in the NAFLD activity score or fibrosis. Significant reductions in HbA1c and waist circumference were observed. The NAFLD activity score improved in 18 of 20 patients with a median decrease of three points (range 1‐4 points). Fibrosis improved by 1.17 stages in 15% of patients and magnetic resonance elastography detected fibrosis by 1.5 stages in 10 of 20 patients. No serious events were observed. In a retrospective analysis of 26 patients with obesity, a significant weight loss was observed six months after intragastric balloon placement, furthermore, blood glucose, HbA1c, FIB‐4, liver stiffness, and CAP were significantly improved. Gastroesophageal reflux symptoms were a side effect, but no severe adverse events occurred. 358
The working group agrees that the gastric balloon should not be used in case of advanced liver cirrhosis with portal hypertension. However, for patients without esophageal varices or other complications of advanced liver cirrhosis, the intragastric balloon can be a supporting intervention that needs an appropriate follow‐up to result in a long‐term solution.
Which type of pharmacotherapy should be recommended in patients with CLD and overweight/obesity?
Recommendation 62
Glucagon‐like Peptide 1 receptor agonists, such as liraglutide or semaglutide, should be recommended as first‐choice anti‐obesity drugs in patients with NASH, provided that the patient does not suffer from decompensated liver disease.
Grade of recommendation B ‐ Strong consensus 96% agreement
Commentary
Weight loss obtained with liraglutide, or orlistat has been associated with the reduction of liver fat content in patients with NAFLD. It seems that the mode of action is weight reduction and not the direct effect of the medication on liver fat content. No evidence is available for the use of anti‐obesity drugs in patients with advanced liver chronic diseases (cirrhosis and liver cancer).
Liraglutide. Several RCTs tested the efficacy of liraglutide in patients with NAFLD and/or NASH, often with a relationship between the amount of weight loss and the degree of histologic improvement of NAFLD. The LEAN trial randomly assigned 52 patients with histologically proved NASH to liraglutide 1.8 or placebo and evaluated the effects with end‐of‐treatment liver biopsy. In this study, 39% of patients receiving liraglutide had resolution of NASH compared with 9% of patients in the placebo group (RR 4.3 [95% CI 1.0–17.7]; p = 0.019). Moreover, 9% of patients in the liraglutide group versus 36% of patients in the placebo group had progression of fibrosis (RR 0.2 [0.1–1.0]; p = 0.04). 359 Several RCTs tested the efficacy of liraglutide 1.8 mg in reducing liver fat content measured with advanced imaging techniques in patients with NAFLD. Some of them demonstrated a better reduction of liver fat content with liraglutide than with placebo, 360 but most did not find significant differences. 361 , 362 , 363 Only liraglutide, along with pioglitazone, showed an improvement in histologic features of NAFLD in a recent systematic review of RCTs evaluating the efficacy and safety of anti‐hyperglycemic drugs in patients with NAFLD with or without diabetes. 364 No data are available for the use of liraglutide in patients with more advanced liver diseases (cirrhosis or liver cancer). No safety concerns have been raised, but the drug is contraindicated in patients with severe liver failure.
Semaglutide. As liragludide, also semaglutide is a GLP‐1 agonist. The advantage of semaglutide is that it requires an s. c. Application once a week only, whereas liraglutide requires a daily injection. Recently, oral semaglutide has also been approved. It needs daily administration. The effectiveness of semaglutide is at least as good as that of liraglutide. 365 , 366 , 367 In many countries, GLP‐1 agonists are reimbursed only for type 2 diabetes and not for obesity, they are expensive There is little evidence that other drug combinations used for the treatment of obesity, such as naltrexone/bupropion or phentermine/topiramate, have a positive benefit in the treatment of NAFLD or that they are safe drugs in advanced CLD (see Box 2).
Box 2 Other pharmacological treatment options in patients with obesity.
Orlistat. The efficacy and safety of orlistat in the treatment of NAFLD and NASH were evaluated in a recent systematic review and meta‐analysis including three RCTs and four single‐arm trials with a total of 330 patients. 368 Improvements were observed in BMI and levels of liver enzymes, but not in liver fibrosis score. 368 In a more recent RCT with quantification of liver fat by MRI, orlistat reduced liver fat content to a greater degree than conventional care. 369 In summary, orlistat may reduce liver fat content and liver enzyme levels in patients with NAFLD. These benefits may be driven primarily by weight loss. 370 No data are available for the use of orlistat in patients with more advanced liver diseases (cirrhosis or liver cancer). No safety concerns have been raised, but the drug is contraindicated in patients with cholestasis.
Dual GLP‐1/glucose‐dependent insulinotropic polypeptide (GIP) agonists. New GLP‐1/GIP agonists such as tirzepatide lack RCTs for NAFLD so far, but retrospective studies suggest a beneficial effect 371 and other trials are ongoing (NCT04166773). Possibly, this new family of drugs will play an important role soon in the treatment of type 2 diabetes, obesity, and their associated comorbidities, including NAFLD.
Naltrexone/Bupropion. No data are available for the use of naltrexone/bupropion in patients with CLD (NAFLD; NASH, cirrhosis, or liver cancer). 370 The combination of naltrexone/bupropion is contraindicated in patients with severe liver failure and it is not recommended in patients with moderate liver dysfunction.
Phentermine/Topiramate. No data are available for the use of phentermine/topiramate in patients with CLD (NAFLD; NASH, cirrhosis, or liver cancer). 370 The combination of phentermine/topiramate is contraindicated in patients with severe liver failure.
Recommendation 63
Prebiotics, probiotics, or synbiotics cannot be recommended to improve NAFLD/NASH in patients with overweight or obesity.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
Randomized clinical trials evaluating prebiotics, probiotics, or synbiotics in the treatment of adult NAFLD have been systematically reviewed and analyzed in several recent meta‐analyses. 372 , 373 , 374 , 375 , 376 , 377 , 378 , 379 , 380 A large majority of analyzed RCTs were based on probiotic administration and they very consistently reported positive effects on liver enzymes. 372 , 373 , 374 , 375 , 376 , 377 , 378 , 379 , 380 In one meta‐analysis, nine studies were included with prebiotic treatment with the highest prevalence of fructooligosaccharides but also including beta‐glucan–supplemented cereals, psyllium husk, xylooligosaccharides, chicory inulin, and fiber extracts; meta‐analyses also found a prebiotics‐induced reduction of plasma ALT and AST. 374 Various meta‐analyses also reported positive effects on ALT and AST of synbiotics with prebiotic components more often represented by fructooligosaccharides. 376 , 377 , 378 In one meta‐analysis, 378 four studies with 235 participants including probiotics and synbiotics demonstrated reduced liver stiffness measured by elastography, an index of inflammation and fibrosis. In the same meta‐analysis, six studies with 384 participants receiving probiotics or synbiotics reported increased odds of improvement in liver fat content in treated patients with moderate‐severe hepatic steatosis graded by ultrasound. 378 Limitations in available evidence include heterogeneity of treatment combinations, their dose, and duration, limited availability of biopsy‐supported NAFLD/NASH diagnosis as well as histologic or MRI evaluation of treatment effects. In a double‐blind RCT in 30 biopsy‐proven NAFLD patients, the 3‐month probiotic treatment caused a significant reduction in ALT, AST, and GGT compared to placebo. 381 Probiotics also reduced intrahepatic triglycerides by magnetic resonance spectroscopy and serum AST in 10 patients. 382 In patients with biopsy‐proven NASH, 24‐week synbiotic treatment with Bifidobacterium longum and fructooligosaccharides and lifestyle modification reduced serum AST and improved NASH histology compared to lifestyle modification alone. 383
Recommendation 64
A Mediterranean diet can be recommended to improve NAFLD/NASH in patients with overweight or obesity.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
In NAFLD subjects with non‐morbid obesity at low risk of having advanced fibrosis according to transient elastography, lifestyle modifications comprising diet and exercise should be offered. Irrespectively of how it is achieved, weight loss reduces hepatic steatosis in patients with overweight or obesity and NAFLD/NASH, 28 , 223 , 325 , 327 while, only substantial weight loss, for example, >9–10% is accompanied by improvement in fibrosis and even full resolution of NASH in paired biopsies. 101 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 A Mediterranean diet has beneficial effects on body weight, insulin sensitivity, and hepatic steatosis and fibrosis, 28 , 223 , 391 , 392 even without weight loss. 393 Moreover, a Mediterranean diet lowers the risk of cardiovascular disease and the development of diabetes, conditions that share common etiological factors with NAFLD, like insulin resistance and obesity. 394 From such data, it has been hypothesized that single food components such as vitamin E could have beneficial effects.
Vitamin E is an antioxidant. Doses of 800 IU of vitamin E improve histologic parameters in non‐diabetic patients (steatosis, inflammation, ballooning, and fibrosis). 395 , 396 Therefore, the recommendation of high doses of vitamin E should be made in non‐diabetic patients with histological lesions proven in liver biopsy, after an open discussion with each patient about the risks and benefits of these doses of vitamin E.
Recommendation 65
Omega‐3‐fatty acids can be used to improve serum triglycerides and liver enzymes in NAFLD/NASH patients with overweight and obesity.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
The effects of omega‐3 fatty acids in NAFLD have been documented in several meta‐analyses. 384 , 385 , 386 , 387
Most of the RCTs included in these meta‐analyses focused on the effects of omega‐3 fatty acids on liver enzymes, omega‐3 fatty acid levels, liver fat content (assessed via MRI/spectroscopy), and steatosis score (assessed via ultrasound) in patients with NAFLD. However, histological measures of disease were unaffected by omega‐3 long‐chain polyunsaturated fatty acid supplementation, 386 , 388 , 389 and histological measures of disease [which were assessed only in patients with NASH] were unaffected by omega‐3 long‐chain polyunsaturated fatty acid supplementation. 386
Recommendation 66
In patients with type 2 diabetes and NAFLD, sodium‐glucose cotransporter‐2 (SGLT‐2) inhibitors can be used to improve glucose control and NAFLD.
Grade of recommendation 0 – Strong consensus 93% agreement
Commentary
SGLT‐2 inhibitors cause weight and fat mass reduction, with improvement of glycemic parameters, insulin resistance, and dyslipidemia as well as long‐term cardiovascular and renal benefits. But they also improve serum levels of liver enzymes, liver fibrosis indices, and liver fat. 390 , 391 However, there are little data on the efficacy of SGLT‐2 on histological parameters of NAFLD. The most common adverse effects of SGLT‐2 inhibitors are genitourinary tract infections. In addition, they may cause diabetic ketoacidosis, dizziness, acute kidney injury, lower limb amputations, and bone fractures. 392
What are the requirements for surgical therapy of obesity in patients with CLD (alcoholic/NAFLD, hepatitis, cholestasis, fibrosis, cirrhosis, or cancer of different origins) and overweight/obesity?
Recommendation 67
Patients with CLD (NAFLD or NASH) with BMI >35 kg/m 2 unresponsive to multimodality treatment should be considered for bariatric surgery.
Grade of recommendation B ‐ Strong consensus 96% agreement
Commentary
In NAFLD there is liver steatosis with hepatocytes infiltrated with fat. Diagnosis is made after other etiologies for fatty liver, such as alcohol consumption, are ruled out. Up to 80% of patients with NAFLD present with obesity. Approximately 10%–25% of patients with silent liver disease develop NASH, and 5%–8% of those will develop liver cirrhosis within 5 years. The degree of fat infiltration is related to BMI and specifically to visceral fat. 393 The resolution of NASH is achieved in 65%–90% of patients achieving ≥7% weight loss. 101 It has been proposed that weight loss of ≥3% is needed to improve steatosis, ≥ 5% to improve inflammation, and ≥10% to improve fibrosis. 394
Patients with fatty liver present frequently with obesity. Weight loss is the first and almost only measure of treatment. In this group of patients, bariatric surgery proved effective. It could even prevent the development of NASH and its complications. In a post‐bariatric‐based population (3,410 patients), compared to a propensity score‐matched group of patients with obesity (46,873 comparison group), bariatric surgery is associated with reduced incidence of NASH and hepatocellular carcinoma. 395 There might be a transient worsening of liver function tests. 396 In patients suffering from NASH, RYGB enabled resolution in 83% of the patients. 397 Histologic improvement was noticed as well. 398 Remission in NASH was found to be durable for 10 years. 399 Bariatric surgery is not associated with increased risk for complications in patients with NAFLD and is highly cost‐effective in patients with NASH compensated cirrhosis and obesity or overweight. 400
In patients with a particular large liver size, a preoperative treatment with either a low‐calory diet or a gastric balloon should be considered.
Recommendation 68
Roux‐en‐Y gastric bypass or laparoscopic SG should be preferred as metabolic surgical procedures in patients with obesity and NAFLD. Both procedures are equally efficacious in ameliorating NAFLD.
Grade of recommendation B ‐ Strong consensus 96% agreement
Commentary
In a secondary outcome analysis of a randomized clinical trial, the influence of SG versus RYGB on liver function in bariatric patients with NAFLD showed no difference between the two procedures. 396 In a comparative study, no difference between RYBG and laparoscopic SG regarding the NAFLD activity score was found. 401 This data was confirmed by other studies. 398 A systematic review and meta‐analysis of RYGB against SG for the amelioration of NAFLD showed that both procedures are equally efficacious. 402 This meta‐analysis included 20 studies, based on four separate criteria: ALT, AST, the NAFLD activity score, and the NAFLD fibrosis score. Another recent meta‐analysis and systematic review included 32 studies and showed that bariatric surgery could lead to a complete resolution of NAFLD after bariatric procedures. 403 However, in some cases, 12% in this meta‐analysis, histologic worsening or de novo NAFLD had appeared after bariatric surgery. 403 Since RYGB was the bariatric procedure with the largest dataset and showed a higher proportion of a complete resolution of NAFLD, the authors were more in favor of RYGB. However, both meta‐analyses have several biases: most of the studies included were retrospective and non‐randomized trials and heterogeneity values were high. In patients with more advanced deterioration of their liver function, SG might have lower mortality. From the American College of Surgeons National Surgical Quality Improvement Program, 3,342 out of 34,169 patients (9.8%) with CLD and Model for End‐Stage Liver Disease (MELD) score >8 were analyzed. An increase in risk for complications with higher MELD score was shown. 30‐day morbidity and mortality were lower after laparoscopic SG compared to laparoscopic RYGB. 404 According to a review of bariatric surgery before, simultaneously or after liver transplantation, 12 studies with a total of 65 patients were analyzed. 405 Complications occurred more often after SG, while mortality was higher after gastric bypass. Sleeve gastrectomy performed after liver transplantation showed the best results.
How should patients with obesity and NASH cirrhosis be managed on the liver transplant waiting list?
Recommendation 69
Metabolic therapies, bariatric endoscopy, and/or bariatric surgery in patients with obesity and NASH cirrhosis managed on the liver transplant waiting list should be currently conducted only within clinical trials or structured programs.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Since morbid obesity is considered a contraindication for liver transplantation, several centers have gained experience in treating patients with obesity and NASH cirrhosis on the waiting list with metabolic medications (e.g. Glucagon‐like Peptide 1 analogs such as liraglutide or semaglutide) or subjected them to bariatric surgery. 406 Laparoscopic SG appears advantageous regarding safety compared to (laparoscopic) RYGB. However, only patients with relatively compensated cirrhosis may be subjected to bariatric surgery, because short‐term complications included bleeding, wound infections, staple line leak, and hepatic encephalopathy, even after SG. 406 An alternative approach to bariatric surgery could be bariatric endoscopic procedures, in which the peri‐procedural risk may be lower. Glucagon‐like Peptide 1 analogs are considered contraindicated in patients with decompensated cirrhosis (i.e. the typical waitlist candidate). The expert panel, therefore, concluded that the above‐listed weight‐loss interventions – pharmacological therapy, bariatric endoscopy, and bariatric surgery – should at present only be conducted within clinical trials or a structured institutional program with ethical approval and a standing data safety monitoring.
Recommendation 70
Nutritional counseling and moderate physical exercise should be offered to patients with obesity and NASH cirrhosis managed on the liver transplant waiting list to support weight loss and improve muscle mass.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Obesity is present in most cases of NASH‐cirrhosis on the waiting list. In patients with compensated cirrhosis, weight reduction by intense lifestyle interventions including nutritional therapy and moderate exercise improved clinical outcomes in several (small) studies. 220 In patients with obesity and decompensated cirrhosis (Child‐Pugh B and C), sarcopenia is a particular concern, supporting the role of physical exercise and sufficient nutritional protein intake to prevent muscle loss. A recent prospective open‐label trial that investigated 16 weeks of personalized hypocaloric normoproteic diet and moderate supervised exercise (60 min/week) in 50 patients with obesity and cirrhosis noted a significant reduction in portal pressure (from 13.9 ± 5.6 mmHg to 12.3 ± 5.2 mmHg; p < 0.0001) without any events of clinical decompensation. 287 These data strongly support nutritional counseling to achieve hypocaloric (−500 to –800 kcal/d) and adequate protein intake (>1.5 g proteins/kg ideal body weight/d), avoid hypomobility, and implement protocols of (supervised) moderate physical activity in NASH patients with obesity on the waiting list.
Recommendation 71
Patients with NASH on the liver transplant waiting list should undergo a thorough multidisciplinary evaluation for cardiovascular and metabolic comorbidities to improve risk stratification for transplant and treatment of comorbidities on the waiting list.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Non‐alcoholic steatohepatitis is projected to become the leading indication for liver transplantation worldwide. While the outcome after liver transplantation is overall similar in patients with NASH cirrhosis compared to other disease etiologies, NASH patients have a higher burden of cardiovascular and metabolic comorbidities and have a substantial risk of disease recurrence after transplantation. The management of patients with obesity and NASH cirrhosis on the liver transplant waiting list should, therefore, aim at improving waitlist survival, optimizing treatment of comorbidities, and reducing the risk of post‐transplant morbidity and mortality. 407
Non‐alcoholic fatty liver disease is a systemic disorder, and comorbidities such as metabolic diseases (type 2 diabetes, dyslipidemia), cardiovascular disease, or renal failure are common and affect transplant risk and long‐term prognosis. A multidisciplinary approach is recommended during waitlist evaluation for capturing these comorbidities, addressing the individual's risk profile, and optimizing pharmacological treatment of the comorbidities. 408 Although no RCTs are substantiating this recommendation, real‐life data from large transplant registries support this approach, because the outcomes of patients transplanted for NASH or cryptogenic cirrhosis were largely similar to those of other etiologies (except for a higher rate of post‐transplant diabetes), despite the higher age of transplant recipients and the higher number of comorbidities. 409 , 410
MANAGEMENT BEFORE AND AFTER WEIGHT LOSS THERAPY/BARIATRIC SURGERY
Before
Which screening and assessment measures should be performed in patients with chronic gastrointestinal diseases (IBD, IBS, CLD) before bariatric surgery?
Recommendation 72
All patients undergoing bariatric surgery, including those with chronic gastrointestinal diseases, should be evaluated for nutritional deficiencies and sarcopenia before intervention.
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
Nutritional deficiencies are more common in individuals with obesity, including protein 411 , iron, 412 and vitamin D. 413 Besides, patients with IBD might be at particular risk for nutritional deficiencies due to decreased nutrient intake, malabsorption, hypermetabolism, pharmacological treatment, or long‐term total parenteral nutrition. 414 , 415 Nutrient screening should minimally include iron status, vitamin B12, folic acid (red blood cell folate, homocysteine, methylmalonic acid optional), and 25‐vitamin D (vitamins A and E optional). 416 More extensive testing should be considered in patients undergoing malabsorptive procedures based on symptoms and risks.
In case of clinical suspicion of sarcopenia, additional evaluations for reduction of muscle mass (e.g. by DXA or BIA) or muscle function (e.g. by handgrip measurement or other functional tests) should be performed.
For further details see ESPEN micronutrient guideline 63 and ESPEN consensus paper on sarcopenic obesity. 62
Recommendation 73
In patients with IBD, gastric endoscopy and colonoscopy should be performed before surgery.
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
This recommendation is solely based on clinical practice and therefore grades as a GPP. In selected cases, for example, clinical suspicion of involvement of the small intestine, magnetic resonance enterography should be performed in addition (see recommendation 74).
Recommendation 74
In patients with CD, a complete gastrointestinal tract assessment should be performed before bariatric surgery.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
Small bowel assessment should be performed before bariatric surgery, especially magnetic resonance enterography. 417 In the case of small bowel involvement, bariatric surgery will be contraindicated. For the same reasons, gastric endoscopy and colonoscopy should be performed. Because in clinically asymptomatic patients, fecal calprotectin can detect a relapse before clinical symptoms occur, the monitoring of fecal calprotectin may be recommended before bariatric surgery. 417 There is no study in the literature evaluating the interest in the fecal calprotectin concentration before bariatric surgery, but ECCO recommendations are in favor of monitoring fecal calprotectin to detect a relapse. 417
For patients with UC, a coloscopy in addition to gastroscopy should be performed before a bariatric procedure. Colonoscopy is mandatory to detect dysplasia or cancer. 418 In the case of dysplasia or cancer, bariatric procedures should be canceled.
Recommendation 75
In patients with CLD, the presence of decompensated cirrhosis should be excluded before bariatric surgery, because of the increased risk following surgery.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
No prospective studies or RCTs were found about assessments needed before bariatric surgery in patients with CLD. In patients with CLD, unknown cirrhosis may be encountered, especially in patients with NASH. In the rare retrospective series or reviews about patients who had undergone bariatric surgery, preoperative assessment is not detailed.
A systematic review of bariatric surgery in patients with cirrhosis included nine studies with a total of 122 patients. 419 In this review, it remains unclear which nutrition screening and preoperative assessment were used.
The working group is convinced that liver cirrhosis is usually a contraindication for bariatric surgery, because of an increased rate of perioperative and long‐term complications, although this position is not substantiated by literature.
According to the German Guideline on bariatric surgery compensated cirrhosis (Child‐Pugh A) is no contraindication for bariatric surgery. 420 Child‐Pugh B or C liver cirrhosis or clinically evident portal hypertension pose serious concerns in indicating bariatric surgery interventions.
Recommendation 76
A psycho‐social evaluation can be performed by a behavioral healthcare specialist before bariatric surgery.
Grade of recommendation 0 ‐ Strong consensus 96% agreement
Commentary
This recommendation is modified from recommendation 30 in Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures. 289 Also eating disorders and other psychopathologies should be assessed and if necessary treated before bariatric surgery.
Bariatric surgery is a treatment option rather than a cosmetic intervention in patients with obesity. Psychologic evaluation before an operation is mandatory for all patients. 289 , 421 There are several controversial results about the relationship between preoperative eating disorders and weight regain after surgery. Problematic eating behaviors, binge eating disorders, and loss of control over eating were not found associated with postoperative weight regain. 422 , 423 , 424 On the other hand, a pilot study showed that preoperative eating disorders can cause postoperative weight regain. 425 Postoperative eating psychopathologies are related to weight regain after surgery, but the relation between preoperative eating psychopathologies and weight regain is still not clear. 426 No doubt, preoperative evaluation for psychologic disorders (eating disorders, substance abuse, mood disorders, etc.) minimizes the risk of postoperative weight control failure according to psychological factors.
After
Do patients with chronic gastrointestinal diseases (IBD, IBS, CLD) and nutritional deficiencies after weight loss need formula diet/multimodal therapy including lifestyle changes?
Recommendation 77
All patients undergoing bariatric surgery, including those with chronic gastrointestinal diseases should be monitored for nutritional deficiencies after bariatric surgery.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
The most common micronutrient deficiencies after bariatric surgery are iron, folic acid, vitamins B1, B12, A, and D. 427 Protein‐and more seldom fat malnutrition is most commonly seen after malabsorptive procedures such as biliopancreatic diversion. 411 Regular nutritional screening in bariatric patients should include vitamin A, B1, B12, D/Calcium, folic acid, and iron. 416
Which nutritional procedures should be performed for which periods in patients with chronic gastrointestinal diseases (IBD, IBS, CLD) after bariatric surgery to reassure adequate treatment?
Recommendation 78
Post‐bariatric surgery patients should ingest adequate amounts of protein to preserve muscle mass and thus prevent sarcopenia.
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
Severe protein deficiency after malabsorptive bariatric surgical procedures is a serious condition that causes the need for hospitalization by 1% per year. 428 There are currently no accepted guidelines on the treatment of protein malnutrition after bariatric surgery. To avoid loss of lean body mass, patients should be given supplementation with 60–90 g protein/d. 429 To achieve this goal, expert diet counseling, as well as protein supplements, can be used.
Recommendation 79
All patients undergoing bariatric surgery, including those with chronic gastrointestinal diseases should be given nutritional supplements to avoid deficiencies after bariatric surgery.
Grade of recommendation B ‐ Strong consensus 96% agreement
Commentary
Post‐bariatric surgery patients are prone to develop multiple nutritional deficiencies mainly protein and micronutrient deficiencies. This ensues in specific deficiencies as well as sarcopenia and osteoporosis. 430 , 431 , 432 , 433 Low intake, as well as malabsorption and/or vomiting, explain these deficiencies. The provision of adequate protein and micronutrients – vitamins and minerals – prevents these deficiencies. Therefore, adequate multivitamin supplementation, ranging from one tablet to two tablets a day according to the surgical procedure, should be recommended to prevent deficiencies. Iron 100 mg/d, vitamin B12 1 mg/d, calcium 500 mg/d, vitamin D 800 U/d, and multivitamin/mineral twice daily should be provided. 434 Additional supplementations may be needed on an individual basis, depending on the type of surgical intervention and selected deficiencies that have been confirmed by laboratory analyses. See also ESPEN micronutrient guideline. 63
Data regarding patients with gastrointestinal disease post‐bariatric surgery and their propensity to develop nutritional deficiencies and/or other metabolic complications is not sufficient to determine specific recommendations for this group of patients.
Recommendation 80
Patients with gastrointestinal disease undergoing bariatric surgery should undergo immediate follow‐up programs specifically designed for post‐bariatric patients along with a follow‐up of their primary disease.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Physical activity aerobic and resistance exercise enables better weight loss along with better physical performance. 435 Patients taking part in a follow‐up program have lower rates of deficiencies. 436 Given this, it is of utmost importance that patients take part in a regular and long‐lasting follow‐up program. Despite the importance of such follow up only about one‐fourth to one‐third of the patients comply with follow‐up 5 years post‐surgery. 437 Failing to take part in such a program is associated with less weight loss and more prominent nutritional deficiencies, though it should be noted that nutritional deficiencies in more than half the patients are found, even in patients taking part in specific post‐bariatric programs. 430
Recommendation 81
Supplementary medical nutritional therapy should be provided to patients with chronic gastrointestinal diseases (IBS, IBD, CLD) if they develop nutritional deficiencies after surgically‐induced weight loss.
Grade of recommendation GPP – Strong consensus 100% agreement
Commentary
If efforts to improve substrate deficiencies, especially protein deficiency, fail by oral supplementation, and enteral nutrition is not tolerated or indicated, parenteral nutrition might be needed. 438 Caution must be taken to avoid the refeeding syndrome by a gradual increase of the provision of calories with an infusion of sufficient amounts of dextrose and prevention/correction of any hypokalemia, hypophosphatemia, and/or hypomagnesemia. 439 Surgical revision might be needed to increase the absorptive surface of the small intestine by lengthening the common channel. 440
Which long‐term care (e.g. dietetic counseling, lifestyle changes) is needed in patients with chronic gastrointestinal diseases (IBD, IBS, CLD) and obesity after initial weight loss/after multimodal therapy/after bariatric surgery?
Recommendation 82
A structured long‐term follow‐up program should be defined and put into place after successful weight loss therapy is achieved by lifestyle intervention or bariatric surgical procedure. The follow‐up program should comprise nutritional screening and assessment, diet recommendations, routine metabolic and nutritional monitoring as well as vitamin, nutrient, and micronutrient supplementation regularly.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
These recommendations have been deducted from recommendation 49ff in AACE/TOS/ASMBS/OMA/ASA 2019 Guidelines. 289
In highly selected patients with well‐controlled invasive blood pressure, bariatric surgery is safe with a low rate of postoperative complications and effective with good weight loss. However, the evidence is limited to small sample sizes and limited periods of follow‐up. 92 , 93 , 112 , 113 , 114 , 116 , 441 , 442 , 443 , 444
Gastrointestinal co‐morbidity is common in patients with obesity and high caloric intake may explain some of the gastrointestinal symptoms. The effect of weight loss surgery on gastrointestinal symptoms is incompletely elucidated. Constipation and satiety increase and food tolerance decreased in the early postoperative period after bariatric surgery. 445 The prevalence of IBS‐like symptoms can increase after RYGB. 152 However, other studies show improvement in gastrointestinal symptoms and therefore quality of life after bariatric surgery. 147 , 446
However, no reliable data for explicit long‐term care in patients with chronic gastrointestinal disease and obesity after a bariatric procedure is available. Therefore, long‐term care in these patients should be performed in analog to patients without chronic gastrointestinal disease and obesity who undergo a bariatric procedure.
Recommendation 83
Patients should perform moderate aerobic physical activity with a minimum of 150 min per week and weight training two to three times a week.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
This recommendation has been deducted from recommendation 51 in AACE/TOS/ASMBS/OMA/ASA 2019 Guidelines. 289
Weight loss, in particular after bariatric procedures, can cause skeletal muscle loss or sarcopenia, associated with a physical disability, poor quality of life, and a higher risk of mortality. 433 Several studies showed a positive correlation between weight loss after bariatric surgery with physical activity. 447 , 448 , 449 Furthermore, physical activity, especially resistance training, after bariatric procedures reduces the risk of sarcopenia and improves a variety of metabolic factors. 450 , 451 , 452 In RCTs, physical activity training twice a week for 6 months after RYGB improved cardiometabolic risk factors and muscle strength, but in the follow‐up, these benefits disappeared compared to controls. 453 , 454 Nevertheless, physical activity induces and maintains the health‐related quality of life improvement for up to 2 years after RYGB. 455
Recommendation 84
Patients should be encouraged to participate in psychotherapeutic interventions or in support groups, self‐monitoring, and/or mobile technologies to improve weight loss and cardiometabolic risks after bariatric procedures.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
This recommendation has been deducted from recommendation 52 in AACE/TOS/ASMBS/OMA/ASA 2019 Guidelines. 289
Patients attending psychotherapeutic interventions, behavioral management, or support groups in combination with bariatric surgery have greater weight loss than patients treated with bariatric surgery only. 456 , 457 , 458 Self‐monitoring leads to improved weight‐loss results. 459 , 460 The incorporation of mobile technologies shows promising results to improve weight loss treatment. 461 , 462 , 463 , 464 , 465 , 466 , 467
Recommendation 85
Weight loss medications may be a useful tool for patients with inadequate weight loss or weight regain after bariatric surgery. Such medications should be prescribed by a specialist only.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
Weight loss medication in conjunction with lifestyle modification may provide weight loss and an improvement in obesity‐related metabolic disorders and complications. 468 , 469 , 470 , 471 Patients who undergo bariatric surgery may incur an inadequate weight loss or weight regain. It has been shown that weight loss medication as an adjunct to bariatric surgery for individuals who have had inadequate weight loss or for individuals who have regained weight after undergoing bariatric surgery may have an additional weight loss benefit. 472 , 473 , 474 , 475 , 476
Is there a special need for the prevention and management of biliary lithiasis and acute pancreatitis in patients with obesity before and during weight loss?
Recommendation 86
Ursodeoxycholic acid shall be prescribed to prevent gallstone formation in patients undergoing weight reduction interventions (lifestyle and diet, endoscopy, and surgery).
Grade of recommendation A ‐ Strong consensus 96% agreement
Commentary
Obesity and rapid weight loss are risk factors for cholelithiasis. Many studies, mainly retrospective, evaluated the incidence of de novo cholecystolithiasis after bariatric surgery, it ranges from 20% to 37%. 477 , 478 The incidence of symptomatic cholecystolithiasis is lower ranging from 3.5% to 8.7% of patients undergoing bariatric surgery. 479 , 480 , 481 Complicated gallstone disease occurs in less than 2% of cases. The average time to develop cholecystolithiasis was 12 months in a retrospective study including 711 cases of gastric sleeve. 479
EASL guidelines 2016 recommend UDCA 500 mg a day until weight stabilization during rapid weight loss, this recommendation was weak. 482 It was based mainly on a meta‐analysis by Stokes et al. including 13 RCTs (two multicentric, dates of publications from 1988 to 2003) with a total number of 1,836 patients, UDCA dose used in studies ranging from 300 to 1,200 mg and duration from six to 18 months, follow up from six to 24 months. Ursodeoxycholic acid was superior to control arms in reducing significantly gallstone formation and cholecystectomy for symptomatic gallstones.
Magouliotis et al conducted a systematic review and meta‐analysis in 2017 including eight studies (six RCTs), different doses of UDCA were used 500–600 mg and 1,000‐1,200 mg, but the conclusion was that UDCA 500–600 mg for six months reduces gallstone formation and cholecystectomies post‐bariatric surgery. 483
The American Associations of Bariatric Surgery, Endocrinology, Obesity, and Anesthesiology published recently guidelines on bariatric surgery perioperative nutrition, metabolic and non‐surgical support, recommending UDCA at the dose of 500 mg once daily for SG and 300 mg twice a day for RYGB or biliopancreatic division with duodenal switch, to prevent gallstone formation. 289
An RCT (UPGRADE trial) is ongoing to better define the effect of UDCA on preventing symptomatic gallstone disease 24 months after bariatric surgery, including 980 patients, using UDCA at 900 mg for six months. It will provide stronger evidence for the use of UDCA for gallstone prevention during rapid weight loss. 484
Ursodeoxycholic acid may not always be required but needs to be considered for selected patients.
Recommendation 87
Cholecystectomy should be proposed for symptomatic patients and those who are asymptomatic undergoing RYGB or biliopancreatic diversion without/with duodenal switch because endoscopic access to the papilla in case of choledocholithiasis is challenging.
If cholecystectomy is indicated it should be performed during bariatric surgery.
Grade of recommendation B ‐ Strong consensus 97% agreement
Commentary
A recent systematic review and meta‐analysis showed that performing cholecystectomy, when it is indicated, concomitantly with bariatric surgery is associated with less postoperative complications and severe complications compared to pre or post‐bariatric surgery but cholecystectomy concomitant to bariatric surgery is related to increase of postoperative complications and mean operative time. 485
The 2019 updated American clinical practice guidelines for the perioperative nutrition, metabolic, and non‐surgical support of patients undergoing bariatric procedures suggest that in asymptomatic patients with known gallstones and a history of RYGB or biliopancreatic diversion without/with duodenal switch, prophylactic cholecystectomy may be considered to avoid choledocholithiasis. Cholecystectomy should be proposed for patients with symptomatic biliary disease. 289
Recommendation 88
Weight loss can be proposed to reduce the recurrence of acute biliary or obesity‐related hypertriglyceridemia pancreatitis.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
Obesity is a risk factor for biliary and hypertriglyceridemia acute pancreatitis. The increase in obesity prevalence is partially responsible for the acute pancreatitis incidence increase. 486 Obesity is a risk factor for the severity of acute pancreatitis. A recent individual patient data meta‐analysis including 1302 patients with acute pancreatitis exploring the relationship between obesity and acute pancreatitis outcomes found that patients with obesity were significantly more at risk to develop organ failure and multiple organ failure than patients without obesity (31% vs. 23% and 20% vs. 12%, p = 0.001 and p < 0.001). Interestingly there was no significant difference between the two groups in terms of mortality or necrosis after adjustment for confounders. 487 Previous meta‐analyses (conventional ones) found a significant impact of obesity on acute pancreatitis severity and mortality. Multiple scoring systems are available to predict the severity of acute pancreatitis: Acute Physiology and Chronic Health Evaluation (APACHE) II, APACHE combined with scoring for obesity (APACHE‐O), the Glasgow scoring system, the Harmless Acute Pancreatitis Score, Prognosis of Acute Pancreatitis 3, the Japanese Severity Score, Pancreatitis Outcome Prediction, and the Bedside Index for Severity in Acute Pancreatitis. 488 International Association of Pancreatology/American Pancreatic Association guidelines advise the use of Systemic Inflammatory Response Syndrome score at admission to predict severity and other clinical, biological, and evolution parameters to predict outcome including BMI. 489 A specific score including obesity has been developed by adapting APACHE II. Acute Physiology and Chronic Health Evaluation combined with scoring for obesity seemed to increase the power of severity prediction. 490 There is no recommended specific acute pancreatitis management algorithm for patients with overweight or obesity. A recent study explored the effect of laparoscopic SG on the recurrence of hypertriglyceridemia acute pancreatitis. In the laparoscopic SG group, there was no recurrence of acute pancreatitis after 12 months of follow‐up compared to 47% in the control group (conventional management of acute pancreatitis). Levels of serum triglycerides normalized in the laparoscopic SG group at 3 months. 491 Future studies are needed to develop specific management of acute pancreatitis in patients with obesity.
Is there a special need for the prevention and management of pancreas insufficiency after bariatric surgery?
Recommendation 89
Post‐bariatric surgery patients who develop a nutritional insufficiency and specifically fat‐soluble vitamin deficiencies despite adequate supplementation should undergo investigation for pancreatic insufficiency.
Grade of recommendation GPP ‐ Strong consensus 97% agreement
Commentary
Pancreas insufficiency is a known complication of bariatric surgery, especially RYGB. The prevalence of pancreatic insufficiency after distal RYGB is 48% and after proximal RYGB is 19%. 492
There are no known means to prevent pancreas insufficiency in post‐bariatric surgery patients.
Recommendation 90
Post‐bariatric surgery patients developing fat‐soluble vitamin deficiencies despite adequate vitamin supplementation should be screened for pancreatic enzyme treatment even if fecal elastase is normal.
Grade of recommendation GPP ‐ Strong consensus 100% agreement
Commentary
For details regarding pancreas enzyme replacement therapy see recommendation 38.
How should hypoglycemia be managed after bariatric surgery?
Recommendation 91
Especially after 1 year of the surgical procedure, characteristic features of post‐bariatric hypoglycemia should be searched for, and differentiated from other types of hypoglycemia.
Grade of recommendation B ‐ Strong consensus 97% agreement
Commentary
Symptomatic hypoglycemia associated with bariatric surgery occurs in some patients more than 1 year after the operative procedure, three to 4 hours after eating a meal with a nonsmall amount of carbohydrates. It is important to distinguish between immediate dumping syndrome after meals (10–60 min), where digestive and vasomotor symptoms predominate, and late dumping syndrome, occurring 60–180 min after meals, with autonomic (adrenergic and cholinergic) and neuroglycopenic symptoms. Tachycardia is a characteristic feature of immediate dumping and low glycemia is of late dumping (glycemia <50 mg/dl). 493
The prevalence of post‐bariatric surgery hypoglycemia depends on the diagnostic cutoff for glycemia and the frequency of glucose measurement after meals. Severe neuroglycopenic hypoglycemia that needs external help from relatives or emergency services may occur in 0.1% of patients who underwent gastric bypass, and in 0.02% with SG. 494 , 495 Mild or moderate hypoglycemia may be identified by a structured questionnaire in 20%–30% of patients 496 and 75% with continuous glucose measurement. 497 Patients with post‐bariatric hypoglycemia after gastric bypass have higher glycemic variability and frequency of glycemia <70 mg/dl, especially at night. These interesting observations point out pathophysiologic mechanisms beyond the prandial changes that have been usually proposed to explain the post‐bariatric hypoglycemia. 498 Patients with post‐bariatric hypoglycemia have postprandial hyperinsulinemia mediated by the combined effects of more rapid nutrient transit from the gastric remnant to the intestine, as well as an enhanced incretin effect. 499 , 500 , 501 , 502 There is no increased GLP‐1 receptor expression in the pancreas or beta‐cell sensitivity to GLP‐1. 503 , 504 However, continuous infusion of GLP‐1 antagonist, exendin 9–39, reduces meal‐induced response after bariatric surgery and prevents hypoglycemia. 505
Younger age, lower BMI, an earlier glucose peak and low glucose levels at 2 hours after an oral glucose tolerance test predicted post‐bariatric hypoglycemia. Prevalence of mild to moderate post‐bariatric hypoglycemia was similar after gastric bypass or SG, with or without previous diabetes. Interestingly, patients with post‐bariatric hypoglycemia experienced smaller weight loss 2 years after bariatric surgery. 506
Recommendation 92
Post‐bariatric hypoglycemia can be diagnosed by glycemia measurement following a provocative mixed meal test.
Grade of recommendation 0 ‐ Strong consensus 97% agreement
Commentary
Assessment of severity and timing of hypoglycemia episodes may be carried out with diaries recording symptoms, type and amount of consumed foods, and physical activity before the symptoms. There are questionnaires designed to screen potential hypoglycemia such as Sigstad Dumping Score, 507 intended more for dumping than for hypoglycemia events, or the Edinburgh Hypoglycemia Scale, Gold and Clarke questionnaires, but they were designed for hypoglycemia in type 1 diabetes and they are not specific or validated for post‐bariatric hypoglycemia. 508 Post‐bariatric hypoglycemia may be more severe after gastric bypass, but SG is also associated with hypoglycemia. 509
Regarding diagnostic tests, the best approach is provocative testing using a mixed meal containing the three macronutrients. 510 However, this test is not standardized neither in the stimulus (the precise composition of the meal, solid or liquid, amount of carbohydrates and proteins, etc.) nor in the diagnostic criteria for hypoglycemia. Continuous glucose monitoring can record glucose variations during the day and their relation to meals, although it may be less accurate in measuring values in the hypoglycemia range. 511 An oral glucose tolerance test is not recommended because the nature of the provocative test is quite different from the usual pattern of meals and it may cause dumping syndrome. Nevertheless, a glucometer is useful to check capillary glucose when symptomatic. 512
Recommendation 93
The treatment of post‐bariatric hypoglycemia should consist primarily of dietary modification, secondarily of medical or endoscopic and surgical therapy.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
- The treatment of post‐bariatric hypoglycemia is based on dietary modification, medical, and surgical therapy. If patients adhere to dietary recommendations, post‐bariatric hypoglycemia can be often solved. However, these patients may have irregular meal patterns that lead them to severe obesity before and hypoglycemia episodes after bariatric surgery. Important pieces of advice for patients with post‐bariatric hypoglycemia are 513 , 514 :
-
•Limit portions of carbohydrates to 30 g per meal, 15 g per snack
-
•Choose low glycemic index carbohydrates and avoid high glycemic index carbohydrates
-
•Give preference to heart‐healthy fats
-
•Consume adequate protein intake
-
•Space meals/snacks three to 4 hours apart
-
•Avoid consuming liquids with meals and chew foods slowly and thoroughly
-
•Avoid alcohol and caffeine
-
•Do not forget post‐bariatric vitamin and mineral intake
-
•
Recommendation 94
If nutrition and drug therapy fail to solve post‐bariatric hypoglycemia, endoscopic and surgical procedures can be performed for the treatment of post‐bariatric hypoglycemia, but partial or total pancreatectomy is not recommended.
Grade of recommendation 0 ‐ Strong consensus 96% agreement
Commentary
If dietary measures are insufficient, drugs can be added. Acarbose inhibits the enzyme α‐glucosidase, which converts polysaccharides into monosaccharides in the intestine. In this way, absorption of glucose is delayed and reduced and as a consequence flattens postprandial glycemic response. The combination of adherence to dietetic changes and regular administration of acarbose may be very effective. However, patients may not complain about both treatments and symptoms persist. Other possible pharmacologic treatments are octreotide, pasireotide, diazoxide, calcium antagonists (nifedipine, verapamil), sitagliptin, and liraglutide. Their efficacy is less well studied than with acarbose. Acarbose reduces hyperglycemia and glycemic variability. In contrast, pasireotide often causes continuous hyperglycemia. 514 , 515 More recent drug treatments for post‐bariatric hypoglycemia are canagliflozin, 516 avexitide, 517 or a Closed‐Loop Glucagon System. 518
Endoscopic techniques may reduce the diameter of the anastomotic mouth and help to maintain weight reduction. They can also be useful in the management of complications related to the surgical procedure, such as gastro‐gastric fistula, marginal bleeding, and ulceration. 519 , 520 Finally, in some cases, surgery can be modified or reverted to correct the post‐bariatric hypoglycemia. 521 , 522 However, partial or total pancreatectomy is not recommended for post‐bariatric hypoglycemia. 523
What is needed to prevent and manage gastrointestinal malignancies in patients who underwent bariatric surgery?
Recommendation 95
Esophagogastroscopy can be performed as a routine diagnostic test before bariatric surgery to rule out Barret esophagus or esophageal and gastric malignancies.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
Obesity is associated with several common cancers such as endometrial, cervix uteri, ovary, breast cancer after menopause, and in men prostate cancer. 54 Weight loss induced by surgery may decrease this increased risk of certain cancers. 524 , 525 However, patients who were treated with bariatric surgery may also develop upper gastrointestinal cancers. As an example, incidental gastrointestinal stromal tumors have been identified during the bariatric surgery procedure. The diligent and careful revision of the left behind gastric chamber in the gastric by‐pass has been associated with a cancer‐free survival of these patients. 526
Due to the anatomical changes introduced by bariatric surgery, these malignancies constitute a diagnostic and therapeutic challenge. Particularly, cancers can develop in the excluded gastric remnant following gastric bypass surgery. Frequently the tumor may be silent and when symptoms appear the disease is in an advanced stage. The diagnostic evaluation may be more difficult and access to the neoplasia for biopsy more complicated than without bariatric surgery. In consequence, the chances of a curative surgery may be decreased. 527
For these reasons, esophagogastroscopy can be considered a routine diagnostic test before bariatric surgery to rule out Barret esophagus or esophageal and gastric malignancies. 528 After bariatric surgery, endoscopy access to lesions may be difficult and a CT scan can be useful to identify them, but the sensitivity is lower than with endoscopy, the lesion has to be larger to be seen, meaning that the tumor already has a volume that may preclude effective surgical treatment. 529
If the digestive tumor is amenable to surgery, the anatomic changes may result in a more difficult procedure, especially regarding the reconstruction of gastrointestinal continuity. In the case of previous SG, the reconstruction options may be a high intrathoracic esophagojejunostomy or a colonic interposition. However, these techniques have a higher risk of anastomotic leakage or vascular complications. 530
Early diagnosis of upper malignancies after bariatric surgery requires a low threshold of suspicion and proceed to rule it out with the most appropriate technique.
STRUCTURAL REQUIREMENTS
Which skills does a clinician need for successful lifestyle intervention in patients with chronic gastrointestinal diseases (IBD, IBS, CLD) to avoid obesity?
Recommendation 96
Clinicians should provide counseling/motivational interviewing/behavioral interventions for lifestyle changes to prevent obesity.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
There is increasing supporting evidence about the importance of well‐structured skills management plans for health professionals to provide a successful follow–up in obesity prevention programs. 531 , 532 , 533 , 534 , 535 , 536
Which methodologies (e.g. shared decision process, guidelines algorithms, mobile apps) does a clinician need for successful lifestyle intervention in patients with chronic gastrointestinal diseases (IBD, IBS, CLD) to avoid obesity?
Recommendation 97
Clinicians should involve patients in a shared decision process about their lifestyle intervention for the prevention of obesity.
Grade of recommendation B ‐ Strong consensus 100% agreement
Commentary
Effective interventions to help people change their behavior require an understanding of their motivations, opportunities, capabilities, and social and physical environment. Planning for lifestyle/behavior change interventions should then be based on knowledge of their specific social context. There is incoming evidence about the effectiveness of such kind of approach. 537 , 538
Recommendation 98
Clinicians may encourage patients to use e‐health tools, ideally under professional supervision, to promote lifestyle changes to prevent/treat obesity.
Grade of recommendation 0 – Strong consensus 100% agreement
Commentary
Although not very strong, there is incoming evidence about the efficacy and usefulness of using a mobile app with health care supervision for lifestyle changes. 539
Recommendation 99
Clinicians may follow guidelines in the prevention of obesity to have a successful outcome through lifestyle intervention.
Grade of recommendation 0 ‐ Strong consensus 100% agreement
Commentary
Current guidelines used in clinical practice can provide a guide to help and an adequate step‐oriented framework for strategic lifestyle interventions. 540
Which settings (e.g. in primary care, in specialized centers) support successful lifestyle intervention in patients with chronic gastrointestinal diseases (IBD, IBS, CLD) to avoid obesity?
Recommendation 100
Primary care should be involved to become a successful setting for lifestyle interventions to prevent obesity.
Grade of recommendation B ‐ Strong consensus 97% agreement
Commentary
There is large evidence of clinical trials showing a modest impact on intervention to deal with obesity prevention and obesity management in clinical practice. As for specific considerations concerning healthy behavioral and screening management attitudes, preventive studies have been mainly done in primary care for both children and the adult population. 541 , 542
DISCLAIMER
These guidelines have been developed with reasonable care and with the best of knowledge available to the authors at the time of preparation. They are intended to assist healthcare professionals and allied healthcare professionals as an educational tool to provide information that may support them in providing care to patients. Patients or other community members using these guidelines shall do so only after consultation with a health professional and shall not mistake these guidelines as professional medical advice. These guidelines must not substitute seeking professional medical and health advice from a health professional.
These guidelines may not apply to all situations and should be interpreted in the light of specific clinical situations and resource availability. It is up to every clinician to adapt these guidelines to local regulations and to each patient's individual circumstances and needs. The information in these guidelines shall not be relied upon as being complete, current or accurate, nor shall it be considered as inclusive of all proper treatments or methods of care or as a legal standard of care.
United European Gastroenterology and ESPEN make no warranty, express or implied, in respect of these guidelines and cannot be held liable for any damages resulting from the application of these guidelines, in particular for any loss or damage (whether direct or indirect) resulting from a treatment based on the guidance given herein.
United European Gastroenterology and ESPEN shall not be held liable to the utmost extent permissible according to the applicable laws for any content available on such external websites, which can be accessed by using the links included herein.
CONFLICT OF INTEREST
The expert members of the working group were accredited by the ESPEN Guidelines Group, the ESPEN Education and Clinical Practice Committee, the ESPEN executive, and the UEG Quality of Care Task Force. All expert members have declared their individual conflicts of interest according to the rules of the International Committee of Medical Journal Editors. If potential conflicts were indicated, they were reviewed by the ESPEN guideline officers and, in cases of doubts, by the ESPEN executive. None of the expert panel had to be excluded from the working group or from co‐authorship because of serious conflicts.
Stephan C. Bischoff reports personal fees from Nestlé, personal fees from Hexal AG, personal fees from Dr. Wild & Co. AG, personal fees from SymbioPharm GmbH, other from Yakult Deutschland GmbH, other from Ardeypharm GmbH, and other from Thieme, outside the submitted work. Luca Busetto reports personal fees from Novo Nordisk, personal fees from Bruno Farmaceutici, personal fees from Rythm, personal fees from Therascience, personal fees from Pronokal, grants from Enzymmanagement, outside the submitted work. Marjo Campmans‐Kuijpers reports personal fees from Janssen, and personal fees from Takeda, outside the submitted work. Laurence Lacaze reports non‐financial support from Nutricia, non‐financial support from Air de Bretagne, and non‐financial support from Fresenius Kabi, outside the submitted work. Miguel Leon‐Sanz reports personal fees from Abbott, grants from Abbott, personal fees from Fresenius Kabi, personal fees from Danone, personal fees from Nestle, personal fees from Persan, personal fees from Takeda, personal fees from Vegenat, outside the submitted work. Johann Ockenga reports personal fees from Dr. Willmar Schwabe GmbH & Co. KG, personal fees from Hexal AG, personal fees from Falk Foundation e.V., outside the submitted work. Frank Tacke reports grants from Allergan, Gilead, BMS, Inventiva, and personal fees from Allergan, Gilead, Galmed, AbbVie, BMS, Boehringer, Galapagos, Intercept, Falk, Inventiva, NovoNordisk outside the submitted work. Darija Vranesic Bender reports personal fees from Abbott, personal fees from Fresenius Kabi, personal fees from Nutricia, personal fees from Nestle, personal fees from Novo Nordisk, outside the submitted work. Arved Weimann reports personal fees from Baxter, grants and personal fees from B. Braun, personal fees from Fresenius Kabi, personal fees from Falk Foundation, grants from Mucos, grants from Seca, outside the submitted work. Cristina Cuerda reports personal fees from FRESENIUS KABI, non‐financial support from PERSAN FARMA, personal fees from NUTRICIA, personal fees from SHIRE, and personal fees from ABBOTT, outside the submitted work. Rocco Barazzoni, Vincenzo Cardinale, Irit Chermesh, Ahad Eshraghian, Haluk Tarik Kani, Wafaa Khannoussi, Juan M. Mendive, Michael W. Müller, and Anders Thorell declare that there are no conflicts of interest.
Supporting information
Supplementary Material 1
Supplementary Material 2
ACKNOWLEDGEMENT
This guideline was financed by ESPEN, the ESPEN, and UEG, the UEG.
Open access funding enabled and organized by Projekt DEAL.
Bischoff SC, Barazzoni R, Busetto L, Campmans‐Kuijpers M, Cardinale V, Chermesh I, et al. European guideline on obesity care in patients with gastrointestinal and liver diseases – Joint European Society for Clinical Nutrition and Metabolism / United European Gastroenterology guideline. United European Gastroenterol J. 2022;10(7):665–722. 10.1002/ueg2.12280
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Landau D‐A, Goldberg A, Levi Z, Levy Y, Niv Y, Bar‐Dayan Y. The prevalence of gastrointestinal diseases in Israeli adolescents and its association with body mass index, gender, and Jewish ethnicity. J Clin Gastroenterol. 2008;42(8):903–9. 10.1097/mcg.0b013e31814685f9 [DOI] [PubMed] [Google Scholar]
- 2. Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, et al. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21(2):361–8. 10.1097/mib.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14(2):110–21. 10.1038/nrgastro.2016.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2(6):370–2. 10.1159/000262276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams DW, Gurwara S, Silver HJ, Horst SN, Beaulieu DB, Schwartz DA, et al. Sarcopenia is common in overweight patients with inflammatory bowel disease and may predict need for surgery. Inflamm Bowel Dis. 2017;23(7):1182–6. 10.1097/mib.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 6. Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2018;25(1):67–73. 10.1093/ibd/izy212 [DOI] [PubMed] [Google Scholar]
- 7. Aasbrenn M, Høgestøl I, Eribe I, Kristinsson J, Lydersen S, Mala T, et al. Prevalence and predictors of irritable bowel syndrome in patients with morbid obesity: a cross‐sectional study. BMC Obes. 2017;4(1):22. 10.1186/s40608-017-0159-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svedberg P, Johansson S, Wallander MA, Hamelin B, Pedersen NL. Extra‐intestinal manifestations associated with irritable bowel syndrome: a twin study. Aliment Pharmacol Ther. 2002;16(5):975–83. 10.1046/j.1365-2036.2002.01254.x [DOI] [PubMed] [Google Scholar]
- 9. Akhondi N, Memar Montazerin S, Soltani S, Saneei P, Hassanzadeh Keshteli A, Esmaillzadeh A, et al. General and abdominal obesity in relation to the prevalence of irritable bowel syndrome. Neuro Gastroenterol Motil. 2019;31(4):e13549. 10.1111/nmo.13549 [DOI] [PubMed] [Google Scholar]
- 10. Sherwin LB, Ozoji OM, Boulineaux CM, Joseph PV, Fourie NH, Abey SK, et al. Gender and weight influence quality of life in irritable bowel syndrome. J Clin Med. 2017;6(11):103. 10.3390/jcm6110103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pimpin L, Cortez‐Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–35. 10.1016/j.jhep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 12. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta‐analysis. PLoS ONE. 2017;12(10):e0186990–e. 10.1371/journal.pone.0186990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, et al. Obesity in inflammatory bowel disease: gains in adiposity despite high prevalence of myopenia and osteopenia. Nutrients. 2018;10(9):1192. 10.3390/nu10091192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Figueiredo FAF, De Mello Perez R, Kondo M. Effect of liver cirrhosis on body composition: evidence of significant depletion even in mild disease. J Gastroenterol Hepatol. 2005;20(2):209–16. 10.1111/j.1440-1746.2004.03544.x [DOI] [PubMed] [Google Scholar]
- 15. Hsu C‐S, Kao J‐H. Sarcopenia and chronic liver diseases. Expet Rev Gastroenterol Hepatol. 2018;12:1229–44. 10.1080/17474124.2018.1534586 [DOI] [PubMed] [Google Scholar]
- 16. Hara N, Iwasa M, Sugimoto R, Mifuji‐Moroka R, Yoshikawa K, Terasaka E, et al. Sarcopenia and sarcopenic obesity are prognostic factors for overall survival in patients with cirrhosis. Intern Med. 2016;55(8):863–70. 10.2169/internalmedicine.55.5676 [DOI] [PubMed] [Google Scholar]
- 17. Hass D, Brensinger C, Lewis J, Lichtenstein G. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:482–8. 10.1016/j.cgh.2005.12.015 [DOI] [PubMed] [Google Scholar]
- 18. Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21(1):51–7. 10.1054/clnu.2001.0503 [DOI] [PubMed] [Google Scholar]
- 19. Stabroth‐Akil D, Leifeld L, Pfützer R, Morgenstern J, Kruis W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis. 2014;30(2):237–42. 10.1007/s00384-014-2051-3 [DOI] [PubMed] [Google Scholar]
- 20. Seminerio JL, Koutroubakis IE, Ramos‐Rivers C, Hashash JG, Dudekula A, Regueiro M, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(12):2857–63. 10.1097/mib.0000000000000560 [DOI] [PubMed] [Google Scholar]
- 21. van der Sloot KW, Bellavance D, Gilpin K, Stewart K, Joshi AD, Garber J, et al. Visceral adiposity, genetic Susceptibility, and risk of complications among individuals with Crohn's disease. Gastroenterology. 2016;150(4):S391. 10.1016/s0016-5085(16)31372-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu Q, Ren J, Li G, Wu X, Li J. The impact of obesity on the clinical course of inflammatory bowel disease: a meta‐analysis. Med Sci Mon Int Med J Exp Clin Res. 2017;23:2599–606. 10.12659/msm.901969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh S, Facciorusso A, Singh AG, Vande Casteele N, Zarrinpar A, Prokop LJ, et al. Obesity and response to anti‐tumor necrosis factor‐α agents in patients with select immune‐mediated inflammatory diseases: a systematic review and meta‐analysis. PLoS ONE. 2018;13(5):e0195123. 10.1371/journal.pone.0195123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jain A, Nguyen NH, Proudfoot JA, Martin CF, Sandborn WJ, Kappelman MD, et al. Impact of obesity on disease activity and patient‐reported outcomes measurement information system (PROMIS) in inflammatory bowel diseases. Am J Gastroenterol. 2019;114(4):630–9. 10.14309/ajg.0000000000000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E. Volumetric fat ratio and not body mass index is predictive of Ileocolectomy outcomes in Crohn's disease patients. Dig Surg. 2014;31(3):219–24. 10.1159/000365359 [DOI] [PubMed] [Google Scholar]
- 26. Shrestha MP, Taleban S. Obesity is associated with increased risk of colectomy in inflammatory bowel disease patients hospitalized with Clostridium difficile infection. Dig Dis Sci. 2018;64(6):1632–9. 10.1007/s10620-018-5423-7 [DOI] [PubMed] [Google Scholar]
- 27. Sadik R, Björnsson E, Simrén M. The relationship between symptoms, body mass index, gastrointestinal transit and stool frequency in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22(1):102–8. 10.1097/meg.0b013e32832ffd9b [DOI] [PubMed] [Google Scholar]
- 28. Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38(2):485–521. 10.1016/j.clnu.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bischoff SC, Singer P, Koller M, Barazzoni R, Cederholm T, van Gossum A. Standard operating procedures for ESPEN guidelines and consensus papers. Clin Nutr. 2015;34(6):1043–51. 10.1016/j.clnu.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 30. Scottish Intercollegiate Guidelines Network (SIGN) . Revised version. In: SIGN 50: a guideline developer's handbook. Edinburgh: SIGN; 2014. [Google Scholar]
- 31. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) . Ständige Kommission leitlinien; 2012. AWMF‐Regelwerk Retrieved from https://www.awmf.org/fileadmin/user_upload/Leitlinien/AWMF‐Regelwerk/AWMF‐Regelwerk.pdf
- 32. Bischoff SC, Escher J, Hebuterne X, Klek S, Krznaric Z, Schneider S, et al. ESPEN practical guideline: clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39(3):632–53. 10.1016/j.clnu.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 33. Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321–47. 10.1016/j.clnu.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein‐calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14(8):1105–11. 10.1002/ibd.20429 [DOI] [PubMed] [Google Scholar]
- 35. Sandhu A, Mosli M, Yan B, Wu T, Gregor J, Chande N, et al. Self‐screening for malnutrition risk in outpatient inflammatory bowel disease patients using the malnutrition universal screening tool (MUST). J Parenter Enteral Nutr. 2015;40(4):507–10. 10.1177/0148607114566656 [DOI] [PubMed] [Google Scholar]
- 36. Gajendran M, Umapathy C, Loganathan P, Hashash JG, Koutroubakis IE, Binion DG. Analysis of hospital‐based emergency department visits for inflammatory bowel disease in the USA. Dig Dis Sci. 2015;61(2):389–99. 10.1007/s10620-015-3895-2 [DOI] [PubMed] [Google Scholar]
- 37. Ananthakrishnan AN, McGinley EL. Infection‐related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7(2):107–12. 10.1016/j.crohns.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 38. Wallaert JB, De Martino RR, Marsicovetere PS, Goodney PP, Finlayson SRG, Murray JJ, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55(11):1138–44. 10.1097/dcr.0b013e3182698f60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A novel risk score to stratify severity of Crohnʼs disease hospitalizations. Am J Gastroenterol. 2010;105(8):1799–807. 10.1038/ajg.2010.105 [DOI] [PubMed] [Google Scholar]
- 40. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. 10.1016/j.clnu.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 41. Arvanitakis M, Ockenga J, Bezmarevic M, Gianotti L, Krznarić Ž, Lobo DN, et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin Nutr. 2020;39(3):612–31. 10.1016/j.clnu.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 42. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. 10.1016/j.clnu.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 43. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623–50. 10.1016/j.clnu.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 44. Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci. 2015;60(8):2436–45. 10.1007/s10620-015-3629-5 [DOI] [PubMed] [Google Scholar]
- 45. Nic Suibhne T, Raftery TC, McMahon O, Walsh C, O'Morain C, O'Sullivan M. High prevalence of overweight and obesity in adults with Crohn's disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7:e241–8. 10.1016/j.crohns.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 46. Pituch‐Zdanowska A, Banaszkiewicz A, Dziekiewicz M, Lazowska‐Przeorek I, Gawronska A, Kowalska‐Duplaga K, et al. Overweight and obesity in children with newly diagnosed inflammatory bowel disease. Adv Med Sci. 2016;61(1):28–31. 10.1016/j.advms.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 47. Barroso T, Conway F, Emel S, McMillan D, Young D, Karteszi H, et al. Patients with inflammatory bowel disease have higher abdominal adiposity and less skeletal mass than healthy controls. Ann Gastroenterol. 2018;31:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Keefe SJ, Ogden J, Rund J, Potter P. Steroids and bowel rest versus elemental diet in the treatment of patients with Crohn's disease: the effects on protein metabolism and immune function. JPEN ‐ J Parenter Enter Nutr. 1989;13(5):455–60. 10.1177/0148607189013005455 [DOI] [PubMed] [Google Scholar]
- 49. Steiner SJ, Noe JD, Denne SC. Corticosteroids increase protein breakdown and loss in newly diagnosed pediatric Crohn disease. Pediatr Res. 2011;70(5):484–8. 10.1203/pdr.0b013e31822f5886 [DOI] [PubMed] [Google Scholar]
- 50. Schneider SM, Al‐Jaouni R, Filippi J, Wiroth JB, Zeanandin G, Arab K, et al. Sarcopenia is prevalent in patients with Crohn's disease in clinical remission. Inflamm Bowel Dis. 2008;14(11):1562–8. 10.1002/ibd.20504 [DOI] [PubMed] [Google Scholar]
- 51. Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 52. Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. 2018;37(6):1787–93. 10.1016/j.clnu.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 53. Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr. 2017;36(4):917–38. 10.1016/j.clnu.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 54. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–24. 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition ‐ a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10(1):207–17. 10.1002/jcsm.12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Skeie E, Tangvik RJ, Nymo LS, Harthug S, Lassen K, Viste A. Weight loss and BMI criteria in GLIM's definition of malnutrition is associated with postoperative complications following abdominal resections – results from a National Quality Registry. Clin Nutr. 2020;39(5):1593–9. 10.1016/j.clnu.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 57. Wojteczek A, Dardzińska JA, Małgorzewicz S, Gruszecka A, Zdrojewski Z. Prevalence of malnutrition in systemic sclerosis patients assessed by different diagnostic tools. Clin Rheumatol. 2019;39(1):227–32. 10.1007/s10067-019-04810-z [DOI] [PubMed] [Google Scholar]
- 58. Marcil V, Levy E, Amre D, Bitton A, Sant'Anna AMGA, Szilagy A, et al. A cross‐sectional study on malnutrition in inflammatory bowel disease: is there a difference based on pediatric or adult age grouping? Inflamm Bowel Dis. 2019;25(8):1428–41. 10.1093/ibd/izy403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Emerenziani S, Biancone L, Guarino MPL, Balestrieri P, Stasi E, Ribolsi M, et al. Nutritional status and bioelectrical phase angle assessment in adult Crohn disease patients receiving anti‐TNFα therapy. Dig Liver Dis. 2017;49(5):495–9. 10.1016/j.dld.2016.12.026 [DOI] [PubMed] [Google Scholar]
- 60. Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018;11(4):294–305. 10.1159/000490361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39(8):2368–88. 10.1016/j.clnu.2019.11.024 [DOI] [PubMed] [Google Scholar]
- 62. Donini Lorenzo M, Busetto L, Bischoff Stephan C, Cederholm T, Ballesteros‐Pomar Maria D, Batsis John A, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Fact. 2022(3):1–15. 10.1159/000521241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berger MM, Shenkin A, Amrein K, Augsburger M, Biesalski H‐K, Bischoff SC, et al. ESPEN Micronutrient guideline. Clin Nutr. 2022. [DOI] [PubMed] [Google Scholar]
- 64. Gerasimidis K, Edwards C, Stefanowicz F, Galloway P, McGrogan P, Duncan A, et al. Micronutrient status in children with IBD. J Pediatr Gastroenterol Nutr. 2013;56(6):e50–e1. 10.1097/mpg.0b013e31828f1e86 [DOI] [PubMed] [Google Scholar]
- 65. Filippi J, Al‐Jaouni R, Wiroth J‐B, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohnʼs disease in remission. Inflamm Bowel Dis. 2006;12(3):185–91. 10.1097/01.mib.0000206541.15963.c3 [DOI] [PubMed] [Google Scholar]
- 66. Geerling BJ, Badart‐Smook A, Stockbrügger RW, Brummer RJ. Comprehensive nutritional status in patients with long‐standing Crohn disease currently in remission. Am J Clin Nutr. 1998;67(5):919–26. 10.1093/ajcn/67.5.919 [DOI] [PubMed] [Google Scholar]
- 67. Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. J Parenter Enteral Nutr. 2007;31(4):311–9. 10.1177/0148607107031004311 [DOI] [PubMed] [Google Scholar]
- 68. Santucci NR, Alkhouri RH, Baker RD, Baker SS. Vitamin and zinc status Pretreatment and Posttreatment in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;59(4):455–7. 10.1097/mpg.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 69. Greenley RN, Stephens KA, Nguyen EU, Kunz JH, Janas L, Goday P, et al. Vitamin and mineral supplement adherence in pediatric inflammatory bowel disease. J Pediatr Psychol. 2013;38(8):883–92. 10.1093/jpepsy/jst037 [DOI] [PubMed] [Google Scholar]
- 70. Singh S, Picardo S, Seow CH. Management of inflammatory bowel diseases in special populations: obese, old, or obstetric. Clin Gastroenterol Hepatol. 2020;18(6):1367–80. 10.1016/j.cgh.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walldorf J, Krummenerl A, Engler K, Busch J, Dollinger MM, Seufferlein T, et al. Health care for osteoporosis in inflammatory bowel disease: unmet needs in care of male patients? J Crohns Colitis. 2013;7(11):901–7. 10.1016/j.crohns.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 72. Szilagyi A. Relationship(s) between obesity and inflammatory bowel diseases: possible intertwined pathogenic mechanisms. Clin J Gastroenterol. 2019;13(2):139–52. 10.1007/s12328-019-01037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Feghaly J, Johnson P, Kalhan A. Vitamin D and obesity in adults: a pathophysiological and clinical update. Br J Hosp Med. 2020;81:1–5. 10.12968/hmed.2019.0291 [DOI] [PubMed] [Google Scholar]
- 74. Sgambato D, Gimigliano F, Musis CD, Moretti A, Toro G, Ferrante E, et al. Bone alterations in inflammatory bowel diseases. World J Clin Cases. 2019;7(15):1908–25. 10.12998/wjcc.v7.i15.1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pieczyńska J, Prescha A, Zabłocka‐Słowińska K, Neubauer K, Smereka A, Grajeta H, et al. Occurrence of dietary risk factors in inflammatory bowel disease: influence on the nutritional status of patients in clinical remission. Adv Clin Exp Med. 2018;28(5):587–92. 10.17219/acem/78590 [DOI] [PubMed] [Google Scholar]
- 76. Sigall‐Boneh R, Levine A, Lomer M, Wierdsma N, Allan P, Fiorino G, et al. Research gaps in diet and nutrition in inflammatory bowel disease. A topical review by D‐ECCO working group [dietitians of ECCO]. J Crohns Colitis. 2017;11(12):1407–19. 10.1093/ecco-jcc/jjx109 [DOI] [PubMed] [Google Scholar]
- 77. Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2009;97(1):92–7. 10.1002/bjs.6805 [DOI] [PubMed] [Google Scholar]
- 78. Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L, Meier R, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27(3):340–9. 10.1016/j.clnu.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 79. Volkert D, Beck AM, Cederholm T, Cruz‐Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. 10.1016/j.clnu.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 80. Valentini L, Schaper L, Buning C, Hengstermann S, Koernicke T, Tillinger W, et al. Malnutrition and impaired muscle strength in patients with Crohn's disease and ulcerative colitis in remission. Nutrition. 2008;24(7‐8):694–702. 10.1016/j.nut.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 81. Pagano AP, Sicchieri JMF, Schiavoni IL, Barbeiro D, Manca CS, da Silva BR, et al. Phase angle as a severity indicator for liver diseases. Nutrition. 2020;70:110607. 10.1016/j.nut.2019.110607 [DOI] [PubMed] [Google Scholar]
- 82. Gomez‐Perez SL, Haus JM, Sheean P, Patel B, Mar W, Chaudhry V, et al. Measuring abdominal circumference and skeletal muscle from a single cross‐sectional computed tomography image: a step‐by‐step guide for clinicians using national institutes of health ImageJ. JPEN ‐ J Parenter Enter Nutr. 2016;40(3):308–18. 10.1177/0148607115604149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martin L, Gioulbasanis I, Senesse P, Baracos VE. Cancer‐associated malnutrition and CT‐defined sarcopenia and myosteatosis are Endemic in overweight and obese patients. J Parenter Enteral Nutr. 2019;44(2):227–38. 10.1002/jpen.1597 [DOI] [PubMed] [Google Scholar]
- 84. Bhalme M, Sharma A, Keld R, Willert R, Campbell S. Does weight‐adjusted anti‐tumour necrosis factor treatment favour obese patients with Crohn’s disease? Eur J Gastroenterol Hepatol. 2013;25(5):543–9. 10.1097/meg.0b013e32835d1f15 [DOI] [PubMed] [Google Scholar]
- 85. Brown P, Clark T, Dowson G, Warren L, Hamlin J, Hull M, et al. Relationship of body mass index to clinical outcomes after infliximab therapy in patients with Crohn’s disease. J Crohns Colitis. 2016;10:1144–50. 10.1093/ecco-jcc/jjw079 [DOI] [PubMed] [Google Scholar]
- 86. Guerbau L, Gerard R, Duveau N, Staumont‐Sallé D, Branche J, Maunoury V, et al. Patients with Crohn's disease with high body mass index present more frequent and rapid loss of response to infliximab. Inflamm Bowel Dis. 2017;23(10):1853–9. 10.1097/mib.0000000000001179 [DOI] [PubMed] [Google Scholar]
- 87. Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(10):2118–24. 10.1097/mib.0b013e31829cf401 [DOI] [PubMed] [Google Scholar]
- 88. Kurnool S, Nguyen NH, Proudfoot J, Dulai PS, Boland BS, Vande Casteele N, et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic‐treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47(11):1472–9. 10.1111/apt.14665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ding Z, Wu XR, Remer EM, Lian L, Stocchi L, Li Y, et al. Association between high visceral fat area and postoperative complications in patients with Crohn's disease following primary surgery. Colorectal Dis. 2016;18(2):163–72. 10.1111/codi.13128 [DOI] [PubMed] [Google Scholar]
- 90. Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn's disease. Clin Gastroenterol Hepatol. 2011;9(8):684–7.e1. 10.1016/j.cgh.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 91. Li Y, Zhu W, Gong J, Zhang W, Gu L, Guo Z, et al. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn's disease. Colorectal Dis. 2015;17(3):225–34. 10.1111/codi.12798 [DOI] [PubMed] [Google Scholar]
- 92. Hudson JL, Barnes EL, Herfarth HH, Isaacs KL, Jain A. Bariatric surgery is a safe and effective option for patients with inflammatory bowel diseases: a case series and systematic review of the literature. Inflamm Intest Dis. 2019;3(4):173–9. 10.1159/000496925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shoar S, Shahabuddin Hoseini S, Naderan M, Mahmoodzadeh H, Ying Man F, Shoar N, et al. Bariatric surgery in morbidly obese patients with inflammatory bowel disease: a systematic review. Surg Obes Relat Dis. 2017;13(4):652–9. 10.1016/j.soard.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 94. Swanson SM, Harper J, Zisman TL. Obesity and inflammatory bowel disease. Curr Opin Gastroenterol. 2018;34(2):112–9. 10.1097/mog.0000000000000422 [DOI] [PubMed] [Google Scholar]
- 95. Ding NS, Malietzis G, Lung PFC, Penez L, Yip WM, Gabe S, et al. The body composition profile is associated with response to anti‐TNF therapy in Crohn's disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther. 2017;46(9):883–91. 10.1111/apt.14293 [DOI] [PubMed] [Google Scholar]
- 96. Bultman E, de Haar C, van Liere‐Baron A, Verhoog H, West RL, Kuipers EJ, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn's disease patients. Aliment Pharmacol Ther. 2012;35(3):335–41. 10.1111/j.1365-2036.2011.04946.x [DOI] [PubMed] [Google Scholar]
- 97. Singh S, Proudfoot J, Xu R, Sandborn WJ. Obesity and response to infliximab in patients with inflammatory bowel diseases: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018;113(6):883–9. 10.1038/s41395-018-0104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Madsen KG, Pottegård A, Hallas J, Kjeldsen J. Treatment failure of TNF‐α inhibitors in obese patients with inflammatory bowel disease—a cohort study. Inflamm Bowel Dis. 2018;24(12):2628–33. 10.1093/ibd/izy178 [DOI] [PubMed] [Google Scholar]
- 99. Hicks G, Abdulaal A, Slesser AAP, Mohsen Y. Outcomes of inflammatory bowel disease surgery in obese versus non‐obese patients: a meta‐analysis. Tech Coloproctol. 2019;23(10):947–55. 10.1007/s10151-019-02080-0 [DOI] [PubMed] [Google Scholar]
- 100. Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22(35):7868–81. 10.3748/wjg.v22.i35.7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, Torres‐Gonzalez A, Gra‐Oramas B, Gonzalez‐Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78. e5; quiz e14‐5. 10.1053/j.gastro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 102. Bang‐Berthelsen CH, Holm TL, Pyke C, Simonsen L, Søkilde R, Pociot F, et al. GLP‐1 induces barrier protective expression in Brunnerʼs glands and regulates colonic inflammation. Inflamm Bowel Dis. 2016;22(9):2078–97. 10.1097/mib.0000000000000847 [DOI] [PubMed] [Google Scholar]
- 103. Duan L, Rao X, Braunstein Z, Toomey AC, Zhong J. Role of incretin Axis in inflammatory bowel disease. Front Immunol. 2017;8:1734. 10.3389/fimmu.2017.01734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nozu T, Miyagishi S, Kumei S, Nozu R, Takakusaki K, Okumura T. Glucagon‐like peptide‐1 analog, liraglutide, improves visceral sensation and gut permeability in rats. J Gastroenterol Hepatol. 2017;33(1):232–9. 10.1111/jgh.13808 [DOI] [PubMed] [Google Scholar]
- 105. Kuwata H, Tsujii S, Fujita N, Okamura S, Iburi T, Mashitani T, et al. Switching from insulin to liraglutide improved glycemic control and the quality of life scores in a case of type 2 diabetes and active Crohn's disease. Intern Med. 2014;53(15):1637–40. 10.2169/internalmedicine.53.2306 [DOI] [PubMed] [Google Scholar]
- 106. Lie MRKL, van der Giessen J, Fuhler GM, de Lima A, Peppelenbosch MP, van der Ent C, et al. Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. J Transl Med. 2018;16(1):55. 10.1186/s12967-018-1427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Raknes G, Simonsen P, Småbrekke L. The effect of low‐dose naltrexone on medication in inflammatory bowel disease: a Quasi experimental before‐and‐after Prescription database study. J Crohns Colitis. 2018;12(6):677–86. 10.1093/ecco-jcc/jjy008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kast RE, Altschuler EL. Remission of Crohn's disease on bupropion. Gastroenterology. 2001;121(5):1260–1. 10.1053/gast.2001.29467 [DOI] [PubMed] [Google Scholar]
- 109. Kane SV, Altschuler EL, Kast RE. Crohn’s disease remission on bupropion. Gastroenterology. 2003;125(4):1290. 10.1016/j.gastro.2003.02.004 [DOI] [PubMed] [Google Scholar]
- 110. Dudley JT, Sirota M, Shenoy M, Pai RK, Roedder S, Chiang AP, et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med. 2011;3(96). 96ra76. 10.1126/scitranslmed.3002648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Crockett SD, Schectman R, Stürmer T, Kappelman MD. Topiramate use does not reduce flares of inflammatory bowel disease. Dig Dis Sci. 2014;59(7):1535–43. 10.1007/s10620-014-3040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aelfers S, Janssen IMC, Aarts EO, Smids C, Groenen MJ, Berends FJ. Inflammatory bowel disease is not a contraindication for bariatric surgery. Obes Surg. 2017;28(6):1681–7. 10.1007/s11695-017-3076-9 [DOI] [PubMed] [Google Scholar]
- 113. Aminian A, Andalib A, Ver MR, Corcelles R, Schauer PR, Brethauer SA. Outcomes of bariatric surgery in patients with inflammatory bowel disease. Obes Surg. 2015;26(6):1186–90. 10.1007/s11695-015-1909-y [DOI] [PubMed] [Google Scholar]
- 114. Colombo F, Rizzi A, Ferrari C, Frontali A, Casiraghi S, Corsi F, et al. Bariatric surgery in patients with inflammatory bowel disease: an accessible path? Report of a case series and review of the literature. J Crohns Colitis. 2014;9(2):185–90. 10.1093/ecco-jcc/jju011 [DOI] [PubMed] [Google Scholar]
- 115. Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, et al. ECCO guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. 2019;14(2):155–68. 10.1093/ecco-jcc/jjz187 [DOI] [PubMed] [Google Scholar]
- 116. Bazerbachi F, Sawas T, Vargas EJ, Haffar S, Deepak P, Kisiel JB, et al. Bariatric surgery is acceptably safe in obese inflammatory bowel disease patients: analysis of the Nationwide inpatient sample. Obes Surg. 2017;28(4):1007–14. 10.1007/s11695-017-2955-4 [DOI] [PubMed] [Google Scholar]
- 117. Gagner M, Hutchinson C, Rosenthal R. Fifth International Consensus Conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(4):750–6. 10.1016/j.soard.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 118. Gero D, Gutschow CA, Bueter M. Does gastric surgery (such as bariatric surgery) impact the risk of intestinal inflammation? Inflamm Intest Dis. 2016;1(3):129–34. 10.1159/000449267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Manguso F, Picascia S, Balzano A. Ulcerative colitis exacerbating after placement of intragastric balloon for the treatment of obesity. Inflamm Bowel Dis. 2008;14(6):872–3. 10.1002/ibd.20373 [DOI] [PubMed] [Google Scholar]
- 120. Wang Z, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB. Resting energy expenditure‐fat‐free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metabol. 2000;279(3):E539–E45. 10.1152/ajpendo.2000.279.3.e539 [DOI] [PubMed] [Google Scholar]
- 121. Forbes A, Escher J, Hebuterne X, Klek S, Krznaric Z, Schneider S, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321–47. 10.1016/j.clnu.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 122. Hannon TS, Dimeglio LA, Pfefferkorn MD, Denne SC. Acute effects of enteral nutrition on protein turnover in adolescents with Crohn disease. Pediatr Res. 2007;61(3):356–60. 10.1203/pdr.0b013e318030d11c [DOI] [PubMed] [Google Scholar]
- 123. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterol. 2012;142(1):46–54. e42; quiz e30. 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 124. Royall D, Jeejeebhoy KN, Baker JP, Allard JP, Habal FM, Cunnane SC, et al. Comparison of amino acid v peptide based enteral diets in active Crohn's disease: clinical and nutritional outcome. Gut. 1994;35(6):783–7. 10.1136/gut.35.6.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Steiner SJ, Pfefferkorn MD, Fitzgerald JF, Denne SC. Protein and energy metabolism response to the initial dose of infliximab in children with Crohn's disease. Inflamm Bowel Dis. 2007;13(6):737–44. 10.1002/ibd.20102 [DOI] [PubMed] [Google Scholar]
- 126. Griffiths RD, Hinds CJ, Little RA. Manipulating the metabolic response to injury. Br Med Bull. 1999;55(1):181–95. 10.1258/0007142991902204 [DOI] [PubMed] [Google Scholar]
- 127. Royall D, Greenberg GR, Allard JP, Baker JP, Jeejeebhoy KN. Total enteral nutrition support improves body composition of patients with active Crohn's disease. JPEN ‐ J Parenter Enter Nutr. 1995;19(2):95–9. 10.1177/014860719501900295 [DOI] [PubMed] [Google Scholar]
- 128. Hsu KJ, Liao CD, Tsai MW, Chen CN. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with sarcopenic obesity: a meta‐analysis. Nutrients. 2019;11(9):2163. 10.3390/nu11092163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):795–841. 10.1053/gast.2003.50106 [DOI] [PubMed] [Google Scholar]
- 130. van Hogezand RA, Hamdy NA. Skeletal morbidity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 2006;41(Suppl 243):59–64. 10.1080/00365520600664276 [DOI] [PubMed] [Google Scholar]
- 131. Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122(7):599–604. 10.1016/j.amjmed.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Blanck S, Aberra F. Vitamin D deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58(6):1698–702. 10.1007/s10620-012-2531-7 [DOI] [PubMed] [Google Scholar]
- 133. Driscoll RH, Jr. , Meredith SC, Sitrin M, Rosenberg IH. Vitamin D deficiency and bone disease in patients with Crohn's disease. Gastroenterol. 1982;83(6):1252–8. 10.1016/s0016-5085(82)80135-2 [DOI] [PubMed] [Google Scholar]
- 134. Sunyecz J. The use of calcium and vitamin D in the management of osteoporosis. Therapeut Clin Risk Manag. 2008;4:827–36. 10.2147/tcrm.s3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Krela‐Kaźmierczak I, Szymczak A, Tomczak M, Łykowska‐Szuber L, Linke K, Eder P. Calcium and phosphate metabolism in patients with inflammatory bowel diseases. Pol Arch Intern Med. 2015;125(7‐8):588–90. 10.20452/pamw.2981 [DOI] [PubMed] [Google Scholar]
- 136. Qiao D, Li Y, Liu X, Zhang X, Qian X, Zhang H, et al. Association of obesity with bone mineral density and osteoporosis in adults: a systematic review and meta‐analysis. Publ Health. 2020;180:22–8. 10.1016/j.puhe.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 137. Savas M, Wester VL, Staufenbiel SM, Koper JW, van den Akker ELT, Visser JA, et al. Systematic evaluation of corticosteroid use in obese and non‐obese individuals: a multi‐cohort study. Int J Med Sci. 2017;14(7):615–21. 10.7150/ijms.19213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aziz I, Branchi F, Pearson K, Priest J, Sanders DS. A study evaluating the Bidirectional relationship between inflammatory bowel disease and self‐reported non‐celiac gluten sensitivity. Inflamm Bowel Dis. 2015;21(4):847–53. 10.1097/mib.0000000000000335 [DOI] [PubMed] [Google Scholar]
- 139. Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten‐free diet in both recently‐diagnosed and long‐term patients with coeliac disease. J Hum Nutr Diet. 2012;26(4):349–58. 10.1111/jhn.12018 [DOI] [PubMed] [Google Scholar]
- 140. Staudacher HM, Lomer MCE, Anderson JL, Barrett JS, Muir JG, Irving PM, et al. Fermentable carbohydrate restriction reduces luminal Bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–8. 10.3945/jn.112.159285 [DOI] [PubMed] [Google Scholar]
- 141. Staudacher HM. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J Gastroenterol Hepatol. 2017;32:16–9. 10.1111/jgh.13688 [DOI] [PubMed] [Google Scholar]
- 142. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. 10.1016/j.clnu.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 143. Kyle UG, Pirlich M, Lochs H, Schuetz T, Pichard C. Increased length of hospital stay in underweight and overweight patients at hospital admission: a controlled population study. Clin Nutr. 2005;24(1):133–42. 10.1016/j.clnu.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 144. Pickett‐Blakely O, Lee L. Irritable Bowel Syndrome . Food and nutrients in disease management. CRC Press; 2009. [Google Scholar]
- 145. Lee CG, Lee JK, Kang Y‐S, Shin S, Kim JH, Lim YJ, et al. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am J Gastroenterol. 2015;110(2):310–9. 10.1038/ajg.2014.422 [DOI] [PubMed] [Google Scholar]
- 146. Aasbrenn M, Lydersen S, Farup PG. A conservative weight loss intervention relieves bowel symptoms in morbidly obese subjects with irritable bowel syndrome: a prospective cohort study. J Obes. 2018;2018:3732753–9. 10.1155/2018/3732753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Clements RH, Gonzalez QH, Foster A, Richards WO, McDowell J, Bondora A, et al. Gastrointestinal symptoms are more intense in morbidly obese patients and are improved with laparoscopic Roux‐en‐Y gastric bypass. Obes Surg. 2003;13:610–4. 10.1381/096089203322190835 [DOI] [PubMed] [Google Scholar]
- 148. Chicco F, Magrì S, Cingolani A, Paduano D, Pesenti M, Zara F, et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm Bowel Dis. 2021;27:1–9. 10.1093/ibd/izaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pickett‐Blakely O. Obesity and irritable bowel syndrome: a comprehensive review. Gastroenterol Hepatol. 2014;10:411–6. [PMC free article] [PubMed] [Google Scholar]
- 150. Frieling T, Heise J, Krummen B, Hundorf C, Kalde S. Tolerability of FODMAP – reduced diet in irritable bowel syndrome – efficacy, adherence, and body weight course. Z Gastroenterol. 2019;57(06):740–4. 10.1055/a-0859-7531 [DOI] [PubMed] [Google Scholar]
- 151. Schneck AS, Anty R, Tran A, Hastier A, Amor IB, Gugenheim J, et al. Increased prevalence of irritable bowel syndrome in a cohort of French morbidly obese patients candidate for bariatric surgery. Obes Surg. 2015;26(7):1525–30. 10.1007/s11695-015-1907-0 [DOI] [PubMed] [Google Scholar]
- 152. Blom‐Høgestøl IK, Aasbrenn M, Chahal‐Kummen M, Brunborg C, Eribe I, Kristinsson J, et al. Irritable bowel syndrome‐like symptoms and health related quality of life two years after Roux‐en‐Y gastric bypass ‐ a prospective cohort study. BMC Gastroenterol. 2019;19(1):204. 10.1186/s12876-019-1103-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta‐analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044–60. 10.1111/apt.15001 [DOI] [PubMed] [Google Scholar]
- 154. Li B, Liang L, Deng H, Guo J, Shu H, Zhang L. Efficacy and safety of probiotics in irritable bowel syndrome: a systematic review and meta‐analysis. Front Pharmacol. 2020;11:332. 10.3389/fphar.2020.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116–27. 10.1016/j.ijsu.2020.01.142 [DOI] [PubMed] [Google Scholar]
- 156. Layer P, Andresen V, Allescher H, Bischoff SC, Claßen M, Elsenbruch S, et al. Update S3‐Leitlinie Reizdarmsyndrom: definition, Pathophysiologie, Diagnostik und Therapie. Gemeinsame Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs‐ und Stoffwechselkrankheiten (DGVS) und der Deutschen Gesellschaft für Neurogastroenterologie und Motilität (DGNM) – Juni 2021 – AWMF‐Registriernummer: 021/016. Z Gastroenterol. 2021;59:1323–415. [DOI] [PubMed] [Google Scholar]
- 157. Enck P, Zimmermann K, Menke G, Muller‐Lissner S, Martens U, Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome‐‐a randomized controlled trial with primary care physicians. Neuro Gastroenterol Motil. 2008;20(10):1103–9. 10.1111/j.1365-2982.2008.01156.x [DOI] [PubMed] [Google Scholar]
- 158. Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6‐month intervention. Aliment Pharmacol Ther. 2005;22(5):387–94. 10.1111/j.1365-2036.2005.02579.x [DOI] [PubMed] [Google Scholar]
- 159. Lyra A, Hillila M, Huttunen T, Mannikko S, Taalikka M, Tennila J, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631–42. 10.3748/wjg.v22.i48.10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic 'functional food' in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13(1):45. 10.1186/1471-230x-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ooi SL, Correa D, Pak SC. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome ‐ what is the current evidence? Compl Ther Med. 2019;43:73–80. 10.1016/j.ctim.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 162. Wilson B, Rossi M, Dimidi E, Whelan K. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2019;109(4):1098–111. 10.1093/ajcn/nqy376 [DOI] [PubMed] [Google Scholar]
- 163. Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta‐analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50(3):240–8. 10.1111/apt.15330 [DOI] [PubMed] [Google Scholar]
- 164. Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, et al. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta‐analysis. Am J Gastroenterol. 2019;114(7):1043–50. 10.14309/ajg.0000000000000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Myneedu K, Deoker A, Schmulson MJ, Bashashati M. Fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta‐analysis. United European Gastroenterol J. 2019;7(8):1033–41. 10.1177/2050640619866990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391(10115):70–81. 10.1016/s0140-6736(17)31796-8 [DOI] [PubMed] [Google Scholar]
- 167. Lojou M, Sahakian N, Dutour A, Vanbiervliet G, Bege T, Gaborit B. Celiac disease and obesity: is bariatric surgery an option? Obes Surg. 2020;30(7):2791–9. 10.1007/s11695-020-04607-z [DOI] [PubMed] [Google Scholar]
- 168. Bascuñán KA, Vespa MC, Araya M. Celiac disease: understanding the gluten‐free diet. Eur J Nutr. 2016;56(2):449–59. 10.1007/s00394-016-1238-5 [DOI] [PubMed] [Google Scholar]
- 169. Valvano M, Longo S, Stefanelli G, Frieri G, Viscido A, Latella G. Celiac disease, gluten‐free diet, and metabolic and liver disorders. Nutrients. 2020;12(4):940. 10.3390/nu12040940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Singh I, Agnihotri A, Sharma A, Verma AK, Das P, Thakur B, et al. Patients with celiac disease may have normal weight or may even be overweight. Indian J Gastroenterol. 2016;35(1):20–4. 10.1007/s12664-016-0620-9 [DOI] [PubMed] [Google Scholar]
- 171. Cheng J, Brar PS, Lee AR, Green PHR. Body mass index in celiac disease. J Clin Gastroenterol. 2010;44(4):267–71. 10.1097/mcg.0b013e3181b7ed58 [DOI] [PubMed] [Google Scholar]
- 172. Farnetti S, Zocco MA, Garcovich M, Gasbarrini A, Capristo E. Functional and metabolic disorders in celiac disease: new implications for nutritional treatment. J Med Food. 2014;17(11):1159–64. 10.1089/jmf.2014.0025 [DOI] [PubMed] [Google Scholar]
- 173. Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten‐free diet. Am J Gastroenterol. 2006;101(10):2356–9. 10.1111/j.1572-0241.2006.00750.x [DOI] [PubMed] [Google Scholar]
- 174. Valletta E, Fornaro M, Cipolli M, Conte S, Bissolo F, Danchielli C. Celiac disease and obesity: need for nutritional follow‐up after diagnosis. Eur J Clin Nutr. 2010;64(11):1371–2. 10.1038/ejcn.2010.161 [DOI] [PubMed] [Google Scholar]
- 175. Theethira TG, Dennis M. Celiac disease and the gluten‐free diet: consequences and recommendations for improvement. Dig Dis. 2015;33(2):175–82. 10.1159/000369504 [DOI] [PubMed] [Google Scholar]
- 176. Dennis M, Lee AR, McCarthy T. Nutritional considerations of the gluten‐free diet. Gastroenterol Clin N Am. 2019;48(1):53–72. 10.1016/j.gtc.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 177. Melini V, Melini F. Gluten‐free diet: gaps and needs for a healthier diet. Nutrients. 2019;11(1):170. 10.3390/nu11010170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Barone M, Della Valle N, Rosania R, Facciorusso A, Trotta A, Cantatore FP, et al. A comparison of the nutritional status between adult celiac patients on a long‐term, strictly gluten‐free diet and healthy subjects. Eur J Clin Nutr. 2015;70(1):23–7. 10.1038/ejcn.2015.114 [DOI] [PubMed] [Google Scholar]
- 179. Kastorini C‐M, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components. J Am Coll Cardiol. 2011;57(11):1299–313. 10.1016/j.jacc.2010.09.073 [DOI] [PubMed] [Google Scholar]
- 180. Godos J, Zappalà G, Bernardini S, Giambini I, Bes‐Rastrollo M, Martinez‐Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: a meta‐analysis of observational studies. Int J Food Sci Nutr. 2016;68(2):138–48. 10.1080/09637486.2016.1221900 [DOI] [PubMed] [Google Scholar]
- 181. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24(19):2083–94. 10.3748/wjg.v24.i19.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sethi S, Richter JE. Diet and gastroesophageal reflux disease. Curr Opin Gastroenterol. 2017;33(2):107–11. 10.1097/mog.0000000000000337 [DOI] [PubMed] [Google Scholar]
- 183. Ayazi S, Hagen JA, Chan LS, DeMeester SR, Lin MW, Ayazi A, et al. Obesity and gastroesophageal reflux: quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J Gastrointest Surg. 2009;13(8):1440–7. 10.1007/s11605-009-0930-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Maev IV, Yurenev GL, Mironova EM, Yureneva‐Thorzhevskaya TV. Phenotype of obesity and gastroesophageal reflux disease in the context of comorbidity in patients with cardiovascular diseases. Ter Arkh. 2019;91(2):126–33. 10.26442/00403660.2019.02.000099 [DOI] [PubMed] [Google Scholar]
- 185. Chang P, Friedenberg F. Obesity and GERD. Gastroenterol Clin N Am. 2014;43(1):161–73. 10.1016/j.gtc.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kim YM, Kim J‐H, Baik SJ, Jung DH, Park JJ, Youn YH, et al. Association between skeletal muscle attenuation and gastroesophageal reflux disease: a health check‐up cohort study. Sci Rep. 2019;9(1):20102. 10.1038/s41598-019-56702-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta‐analysis. Am J Gastroenterol. 2006;101(11):2619–28. 10.1111/j.1572-0241.2006.00849.x [DOI] [PubMed] [Google Scholar]
- 188. Hampel H, Abraham NS, El‐Serag HB. Meta‐analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143(3):199. 10.7326/0003-4819-143-3-200508020-00006 [DOI] [PubMed] [Google Scholar]
- 189. Nam SY, Choi IJ, Ryu KH, Park BJ, Kim HB, Nam BH. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterol. 2010;139(6):1902–11.e2. 10.1053/j.gastro.2010.08.019 [DOI] [PubMed] [Google Scholar]
- 190. Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross‐sectional case‐control study of 7078 Koreans undergoing health check‐ups. Gut. 2008;57(10):1360–5. 10.1136/gut.2007.147090 [DOI] [PubMed] [Google Scholar]
- 191. Nadaleto BF, Herbella FAM, Patti MG. Gastroesophageal reflux disease in the obese: pathophysiology and treatment. Surgery. 2016;159(2):475–86. 10.1016/j.surg.2015.04.034 [DOI] [PubMed] [Google Scholar]
- 192. Park SK, Lee T, Yang HJ, Park JH, Sohn CI, Ryu S, et al. Weight loss and waist reduction is associated with improvement in gastroesophageal disease reflux symptoms: a longitudinal study of 15 295 subjects undergoing health checkups. Neuro Gastroenterol Motil. 2016;29(5):e13009. 10.1111/nmo.13009 [DOI] [PubMed] [Google Scholar]
- 193. De Groot NL, Burgerhart JS, Van De Meeberg PC, De Vries DR, Smout AJPM, Siersema PD. Systematic review: the effects of conservative and surgical treatment for obesity on gastro‐oesophageal reflux disease. Aliment Pharmacol Ther. 2009;30(11‐12):1091–102. 10.1111/j.1365-2036.2009.04146.x [DOI] [PubMed] [Google Scholar]
- 194. Djärv T, Wikman A, Nordenstedt H, Johar A, Lagergren J, Lagergren P. Physical activity, obesity and gastroesophageal reflux disease in the general population. World J Gastroenterol. 2012;18(28):3710–4. 10.3748/wjg.v18.i28.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. de Bortoli N, Guidi G, Martinucci I, Savarino E, Imam H, Bertani L, et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: a comparative study. Dis Esophagus. 2014;29(2):197–204. 10.1111/dote.12319 [DOI] [PubMed] [Google Scholar]
- 196. Ness‐Jensen E, Lindam A, Lagergren J, Hveem K. Weight loss and reduction in gastroesophageal reflux. A prospective population‐based cohort study: the HUNT study. Am J Gastroenterol. 2013;108(3):376–82. 10.1038/ajg.2012.466 [DOI] [PubMed] [Google Scholar]
- 197. Singh M, Lee J, Gupta N, Gaddam S, Smith BK, Wani SB, et al. Weight loss can lead to resolution of gastroesophageal reflux disease symptoms: a prospective intervention trial. Obesity. 2013;21(2):284–90. 10.1002/oby.20279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? Arch Intern Med. 2006;166(9):965. 10.1001/archinte.166.9.965 [DOI] [PubMed] [Google Scholar]
- 199. Ness‐Jensen E, Hveem K, El‐Serag H, Lagergren J. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2016;14(2):175–82.e823. 10.1016/j.cgh.2015.04.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Madalosso CAS, Gurski RR, Callegari‐Jacques SM, Navarini D, Mazzini G, Pereira MS. The impact of gastric bypass on gastroesophageal reflux disease in morbidly obese patients. Ann Surg. 2016;263(1):110–6. 10.1097/sla.0000000000001139 [DOI] [PubMed] [Google Scholar]
- 201. Han Y, Jia Y, Wang H, Cao L, Zhao Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux‐en‐Y gastric bypass: a systematic review and meta‐analysis based on 18 studies. Int J Surg. 2020;76:101–10. 10.1016/j.ijsu.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 202. Dobszai D, Mátrai P, Gyöngyi Z, Csupor D, Bajor J, Erőss B, et al. Body‐mass index correlates with severity and mortality in acute pancreatitis: a meta‐analysis. World J Gastroenterol. 2019;25(6):729–43. 10.3748/wjg.v25.i6.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Khatua B, El‐Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33(5):374–82. 10.1097/mog.0000000000000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Simha V. Management of hypertriglyceridemia. BMJ. 2020:m3109. 10.1136/bmj.m3109 [DOI] [PubMed] [Google Scholar]
- 205. Alsamarrai A, Das SLM, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta‐analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014;12(10):1635–44.e5. 10.1016/j.cgh.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 206. Duggan SN, Smyth ND, O’Sullivan M, Feehan S, Ridgway PF, Conlon KC. The prevalence of malnutrition and fat‐soluble vitamin deficiencies in chronic pancreatitis. Nutr Clin Pract. 2014;29(3):348–54. 10.1177/0884533614528361 [DOI] [PubMed] [Google Scholar]
- 207. Melitas C, Meiselman M. Metabolic pancreatitis: pancreatic steatosis, hypertriglyceridemia, and associated chronic pancreatitis in 3 patients with metabolic syndrome. Case Rep Gastroenterol. 2018;12(2):331–6. 10.1159/000490042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Malli A, Li F, Conwell DL, Cruz‐Monserrate Z, Hussan H, Krishna SG. The burden of systemic adiposity on pancreatic disease: acute pancreatitis, non‐alcoholic fatty pancreas disease, and pancreatic cancer. Jop. 2017;18:365–8. [PMC free article] [PubMed] [Google Scholar]
- 209. Tirkes T, Jeon CY, Li L, Joon AY, Seltman TA, Sankar M, et al. Association of pancreatic steatosis with chronic pancreatitis, obesity, and type 2 diabetes mellitus. Pancreas. 2019;48(3):420–6. 10.1097/mpa.0000000000001252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Bellin MD, Whitcomb DC, Abberbock J, Sherman S, Sandhu BS, Gardner TB, et al. Patient and disease characteristics associated with the presence of diabetes mellitus in adults with chronic pancreatitis in the United States. Am J Gastroenterol. 2017;112(9):1457–65. 10.1038/ajg.2017.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Singh VK, Yadav D, Garg PK. Diagnosis and management of chronic pancreatitis. JAMA. 2019;322(24):2422. 10.1001/jama.2019.19411 [DOI] [PubMed] [Google Scholar]
- 212. Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clin Exp Gastroenterol. 2019;12:129–39. 10.2147/ceg.s168266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Hardt PD, Hauenschild A, Nalop J, Marzeion AM, Jaeger C, Teichmann J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. Pancreatology. 2003;3(5):395–402. 10.1159/000073655 [DOI] [PubMed] [Google Scholar]
- 214. Herzig K‐H, Purhonen A‐K, Räsänen KM, Idziak J, Juvonen P, Phillps R, et al. Fecal pancreatic elastase‐1 levels in older individuals without known gastrointestinal diseases or diabetes mellitus. BMC Geriatr. 2011;11(1):4. 10.1186/1471-2318-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Pezzilli R, Andriulli A, Bassi C, Balzano G, Cantore M, Delle Fave G, et al. Exocrine pancreatic insufficiency in adults: a shared position statement of the Italian Association for the Study of the Pancreas. World J Gastroenterol. 2013;19(44):7930–46. 10.3748/wjg.v19.i44.7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Domínguez‐Muñoz JE, Iglesias‐García J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: is clinical response an appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11:158–62. [PubMed] [Google Scholar]
- 217. Eslam M, Sanyal AJ, George J. MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 218. Montano‐Loza AJ, Angulo P, Meza‐Junco J, Prado CMM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–35. 10.1002/jcsm.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ebadi M, Tsien C, Bhanji RA, Dunichand‐Hoedl AR, Rider E, Motamedrad M, et al. Skeletal muscle pathological fat infiltration (myosteatosis) is associated with higher mortality in patients with cirrhosis. Cells. 2022;11(8):1345. 10.3390/cells11081345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Merli M, Berzigotti A, Zelber‐Sagi S, Dasarathy S, Montagnese S, Genton L, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–93. 10.1016/j.jhep.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Georgiou A, Papatheodoridis GV, Alexopoulou A, Deutsch M, Vlachogiannakos I, Ioannidou P, et al. Evaluation of the effectiveness of eight screening tools in detecting risk of malnutrition in cirrhotic patients: the KIRRHOS study. Br J Nutr. 2019;122(12):1368–76. 10.1017/s0007114519002277 [DOI] [PubMed] [Google Scholar]
- 222. Borhofen SM, Gerner C, Lehmann J, Fimmers R, Görtzen J, Hey B, et al. The royal free hospital‐nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61(6):1735–43. 10.1007/s10620-015-4015-z [DOI] [PubMed] [Google Scholar]
- 223. Glen J, Floros L, Day C, Pryke R. Non‐alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. 2016;354:i4428. 10.1136/bmj.i4428 [DOI] [PubMed] [Google Scholar]
- 224. EASL–EASD–EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388‐402. [DOI] [PubMed] [Google Scholar]
- 225. Paige JS, Bernstein GS, Heba E, Costa EAC, Fereirra M, Wolfson T, et al. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology‐determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2017;208(5):W168–w77. 10.2214/ajr.16.16726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51(6):1061–7. 10.1016/j.jhep.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. de Moura Almeida A, Cotrim HP, Barbosa DB, de Athayde LG, Santos AS, Bitencourt AG, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14(9):1415–8. 10.3748/wjg.14.1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14(5):635–7. 10.1381/096089204323093408 [DOI] [PubMed] [Google Scholar]
- 229. Hepburn MJ, Vos JA, Fillman EP, Lawitz EJ. The accuracy of the report of hepatic steatosis on ultrasonography in patients infected with hepatitis C in a clinical setting: a retrospective observational study. BMC Gastroenterol. 2005;5(1):14. 10.1186/1471-230x-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Jun MJ, Shim JH, Kim SY, Seo N, Kim KM, Lim YS, et al. Clinical implications of preoperative and intraoperative liver biopsies for evaluating donor steatosis in living related liver transplantation. Liver Transpl. 2014;20(4):437–45. 10.1002/lt.23832 [DOI] [PubMed] [Google Scholar]
- 231. Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, et al. Prevalence and risk factors of non‐alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47(2):239–44. 10.1016/j.jhep.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 232. Mathiesen UL, Franzén LE, Aselius H, Resjö M, Jacobsson L, Foberg U, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34(7):516–22. 10.1016/s1590-8658(02)80111-6 [DOI] [PubMed] [Google Scholar]
- 233. Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, et al. The role of bright liver echo pattern on ultrasound B‐mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38(7):485–9. 10.1016/j.dld.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 234. Perez NE, Siddiqui FA, Mutchnick MG, Dhar R, Tobi M, Ullah N, et al. Ultrasound diagnosis of fatty liver in patients with chronic liver disease: a retrospective observational study. J Clin Gastroenterol. 2007;41(6):624–9. 10.1097/01.mcg.0000225680.45088.01 [DOI] [PubMed] [Google Scholar]
- 235. Wang JH, Hung CH, Kuo FY, Eng HL, Chen CH, Lee CM, et al. Ultrasonographic quantification of hepatic‐renal echogenicity difference in hepatic steatosis diagnosis. Dig Dis Sci. 2013;58(10):2993–3000. 10.1007/s10620-013-2769-8 [DOI] [PubMed] [Google Scholar]
- 236. Wang CC, Hsieh TC, Tseng TC, Wang PC, Hsu CS, Lin HH, et al. Factors affecting the diagnostic accuracy of ultrasonography in assessing the severity of hepatic steatosis. J Formos Med Assoc. 2014;113(4):249–54. 10.1016/j.jfma.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 237. Webb M, Yeshua H, Zelber‐Sagi S, Santo E, Brazowski E, Halpern Z, et al. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192(4):909–14. 10.2214/ajr.07.4016 [DOI] [PubMed] [Google Scholar]
- 238. Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver‐kidney contrast. Tohoku J Exp Med. 1983;139(1):43–50. 10.1620/tjem.139.43 [DOI] [PubMed] [Google Scholar]
- 239. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–81.e4. 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta‐analysis. Hepatology. 2011;54(3):1082–90. 10.1002/hep.24452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? Hepatology. 1996;23(5):1041–6. 10.1002/hep.510230516 [DOI] [PubMed] [Google Scholar]
- 242. National Institute for Clinical Excellence . Non‐alcoholic fatty liver disease (NAFLD): assessment and management (NG49). London: National Institute for Health and Clinical Excellence (NICE); 2016. [PubMed] [Google Scholar]
- 243. Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4(1):10. 10.1186/1476-5926-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lassailly G, Caiazzo R, Hollebecque A, Buob D, Leteurtre E, Arnalsteen L, et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol. 2011;23(6):499–506. 10.1097/meg.0b013e3283464111 [DOI] [PubMed] [Google Scholar]
- 245. de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non‐invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32(6):911–8. 10.1111/j.1478-3231.2012.02820.x [DOI] [PubMed] [Google Scholar]
- 246. Munteanu M, Tiniakos D, Anstee Q, Charlotte F, Marchesini G, Bugianesi E, et al. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment Pharmacol Ther. 2016;44(8):877–89. 10.1111/apt.13770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6(1):33. 10.1186/1471-230x-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209–22. 10.1111/apt.12963 [DOI] [PubMed] [Google Scholar]
- 249. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–8. 10.1016/j.dld.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 250. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–30. 10.1016/j.jhep.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 251. Friedrich‐Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, et al. Acoustic radiation force impulse imaging for non‐invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20(4):240–7. 10.1111/j.1365-2893.2012.01646.x [DOI] [PubMed] [Google Scholar]
- 252. Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, et al. Controlled attenuation parameter for non‐invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol. 2013;28(7):1194–201. 10.1111/jgh.12134 [DOI] [PubMed] [Google Scholar]
- 253. Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29(7):1470–6. 10.1111/jgh.12557 [DOI] [PubMed] [Google Scholar]
- 254. Karlas T, Petroff D, Garnov N, Böhm S, Tenckhoff H, Wittekind C, et al. Non‐invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H‐MR spectroscopy. PLoS ONE. 2014;9(3):e91987. 10.1371/journal.pone.0091987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. de Lédinghen V, Wong GL, Vergniol J, Chan HL, Hiriart JB, Chan AW, et al. Controlled attenuation parameter for the diagnosis of steatosis in non‐alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31(4):848–55. 10.1111/jgh.13219 [DOI] [PubMed] [Google Scholar]
- 256. Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–37.e7. 10.1053/j.gastro.2015.11.048 [DOI] [PubMed] [Google Scholar]
- 257. Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, et al. MR spectroscopy‐derived proton density fat fraction is superior to controlled attenuation parameter for detecting and grading hepatic steatosis. Radiology. 2018;286(2):547–56. 10.1148/radiol.2017162931 [DOI] [PubMed] [Google Scholar]
- 258. Chan W‐K, Nik Mustapha NR, Wong GL‐H, Wong VW‐S, Mahadeva S. Controlled attenuation parameter using the FibroScan® XL probe for quantification of hepatic steatosis for non‐alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5(1):76–85. 10.1177/2050640616646528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Naveau S, Voican CS, Lebrun A, Gaillard M, Lamouri K, Njiké‐Nakseu M, et al. Controlled attenuation parameter for diagnosing steatosis in bariatric surgery candidates with suspected nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2017;29(9):1022–30. 10.1097/meg.0000000000000919 [DOI] [PubMed] [Google Scholar]
- 260. Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Marra F, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65(4):1145–55. 10.1002/hep.28843 [DOI] [PubMed] [Google Scholar]
- 261. Lee HW, Park SY, Kim SU, Jang JY, Park H, Kim JK, et al. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PLoS ONE. 2016;11(6):e0157358. 10.1371/journal.pone.0157358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Berzigotti A, Tsochatzis E, Boursier J, Castera L, Cazzagon N, Friedrich‐Rust M, et al. EASL Clinical Practice Guidelines on non‐invasive tests for evaluation of liver disease severity and prognosis ‐ 2021 update. J Hepatol. 2021;75(3):659–89. 10.1016/j.jhep.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 263. Pu K, Wang Y, Bai S, Wei H, Zhou Y, Fan J, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non‐invasive test for steatosis in suspected non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. BMC Gastroenterol. 2019;19(1):51. 10.1186/s12876-019-0961-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Qu Y, Li M, Hamilton G, Zhang YN, Song B. Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: a meta‐analysis. Eur Radiol. 2019;29(10):5180–9. 10.1007/s00330-019-06071-5 [DOI] [PubMed] [Google Scholar]
- 265. Chiang HJ, Lin LH, Li CW, Lin CC, Chiang HW, Huang TL, et al. Magnetic resonance fat quantification in living donor liver transplantation. Transplant Proc. 2014;46(3):666–8. 10.1016/j.transproceed.2013.11.050 [DOI] [PubMed] [Google Scholar]
- 266. Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267(3):767–75. 10.1148/radiol.13121360 [DOI] [PubMed] [Google Scholar]
- 267. Idilman IS, Keskin O, Celik A, Savas B, Elhan AH, Idilman R, et al. A comparison of liver fat content as determined by magnetic resonance imaging‐proton density fat fraction and MRS versus liver histology in non‐alcoholic fatty liver disease. Acta Radiol. 2016;57(3):271–8. 10.1177/0284185115580488 [DOI] [PubMed] [Google Scholar]
- 268. Joe E, Lee JM, Kim KW, Lee KB, Kim SJ, Baek JH, et al. Quantification of hepatic macrosteatosis in living, related liver donors using T1‐independent, T2*‐corrected chemical shift MRI. J Magn Reson Imag. 2012;36(5):1124–30. 10.1002/jmri.23738 [DOI] [PubMed] [Google Scholar]
- 269. Kühn JP, Hernando D, Muñoz del Rio A, Evert M, Kannengiesser S, Völzke H, et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology. 2012;265(1):133–42. 10.1148/radiol.12112520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and Pathologist‐assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153(3):753–61. 10.1053/j.gastro.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Middleton MS, Van Natta ML, Heba ER, Alazraki A, Trout AT, Masand P, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology. 2018;67(3):858–72. 10.1002/hep.29596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61(6):1887–95. 10.1002/hep.27666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, et al. Accuracy of MR imaging‐estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274(2):416–25. 10.1148/radiol.14140754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267(2):422–31. 10.1148/radiol.12120896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Park CC, Hooker C, Hooker JC, Bass E, Haufe W, Schlein A, et al. Assessment of a high‐SNR chemical‐shift‐encoded MRI with complex reconstruction for proton density fat fraction (PDFF) estimation overall and in the low‐fat range. J Magn Reson Imag. 2019;49(1):229–38. 10.1002/jmri.26168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Paparo F, Cenderello G, Revelli M, Bacigalupo L, Rutigliani M, Zefiro D, et al. Diagnostic value of MRI proton density fat fraction for assessing liver steatosis in chronic viral C hepatitis. BioMed Res Int. 2015;2015:758164–11. 10.1155/2015/758164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, et al. Diagnostic value of MRI‐PDFF for hepatic steatosis in patients with non‐alcoholic fatty liver disease: a meta‐analysis. Eur Radiol. 2019;29(7):3564–73. 10.1007/s00330-019-06072-4 [DOI] [PubMed] [Google Scholar]
- 278. Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 279. Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol. 2018;68(2):326–34. 10.1016/j.jhep.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 280. Loomba R, Lim JK, Patton H, El‐Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158(6):1822–30. 10.1053/j.gastro.2019.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Gastroenterology. 2020;158(6):1611–25.e12. 10.1053/j.gastro.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 282. Rinella ME, Tacke F, Sanyal AJ, Anstee QM. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol. 2019;71(4):823–33. 10.1016/j.jhep.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 283. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–64. 10.1053/j.gastro.2020.01.052 [DOI] [PubMed] [Google Scholar]
- 284. Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of population‐based observational studies. PLoS Med. 2020;17(4):e1003100–e. 10.1371/journal.pmed.1003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Charatcharoenwitthaya P, Soonthornworasiri N, Karaketklang K, Poovorawan K, Pan‐Ngum W, Chotiyaputta W, et al. Factors affecting mortality and resource use for hospitalized patients with cirrhosis: a population‐based study. Medicine. 2017;96(32):e7782. 10.1097/md.0000000000007782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology. 2017;65(5):1557–65. 10.1002/hep.29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65(4):1293–305. 10.1002/hep.28992 [DOI] [PubMed] [Google Scholar]
- 288. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis. 2018;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Mechanick JI, Apovian C, Brethauer S, Timothy Garvey W, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures ‐ 2019 update: cosponsored by American association of clinical Endocrinologists/American College of Endocrinology, the obesity society, American society for metabolic and bariatric surgery obesity medicine association, and American society of Anesthesiologists. Obesity. 2020;28:O1–o58. [DOI] [PubMed] [Google Scholar]
- 290. Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non‐alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol. 2018;24(30):3361–73. 10.3748/wjg.v24.i30.3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Rachakonda V, Wills R, DeLany JP, Kershaw EE, Behari J. Differential impact of weight loss on nonalcoholic fatty liver resolution in a North American cohort with obesity. Obesity. 2017;25:1360–8. 10.1002/oby.21890 [DOI] [PubMed] [Google Scholar]
- 292. De Fre CH, De Fre MA, Kwanten WJ, Op de Beeck BJ, Van Gaal LF, Francque SM. Sarcopenia in patients with non‐alcoholic fatty liver disease: is it a clinically significant entity? Obes Rev. 2019;20(2):353–63. 10.1111/obr.12776 [DOI] [PubMed] [Google Scholar]
- 293. Gan D, Wang L, Jia M, Ru Y, Ma Y, Zheng W, et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr. 2020;39(4):1124–30. 10.1016/j.clnu.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 294. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59(5):1772–8. 10.1002/hep.26716 [DOI] [PubMed] [Google Scholar]
- 295. Merli M, Lattanzi B, Aprile F. Sarcopenic obesity in fatty liver. Curr Opin Clin Nutr Metab Care. 2019;22(3):185–90. 10.1097/mco.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 296. Schiavo L, Busetto L, Cesaretti M, Zelber‐Sagi S, Deutsch L, Iannelli A. Nutritional issues in patients with obesity and cirrhosis. World J Gastroenterol. 2018;24(30):3330–46. 10.3748/wjg.v24.i30.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Tovo CV, Fernandes SA, Buss C, de Mattos AA. Sarcopenia and non‐alcoholic fatty liver disease: is there a relationship? A systematic review. World J Hepatol. 2017;9(6):326–32. 10.4254/wjh.v9.i6.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. UK NGC. Extra‐hepatic conditions . Non‐alcoholic fatty liver disease: assessment and management. UK): National Institute for Health and Care Excellence; 2016. [PubMed] [Google Scholar]
- 299. Kumar R, Priyadarshi RN, Anand U. Non‐alcoholic fatty liver disease: growing burden, adverse outcomes and associations. JClin TranslHepatol. 2020;8:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Tariq R, Axley P, Singal AK. Extra‐hepatic manifestations of nonalcoholic fatty liver disease: a review. J Clin Exp Hepatol. 2020;10(1):81–7. 10.1016/j.jceh.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Rosato V, Masarone M, Dallio M, Federico A, Aglitti A, Persico M. NAFLD and extra‐hepatic comorbidities: current evidence on a multi‐organ metabolic syndrome. Int J Environ Res Publ Health. 2019;16(18):3415. 10.3390/ijerph16183415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–44. 10.1038/nrgastro.2013.41 [DOI] [PubMed] [Google Scholar]
- 303. Arulanandan A, Ang B, Bettencourt R, Hooker J, Behling C, Lin GY, et al. Association between quantity of liver fat and cardiovascular risk in patients with nonalcoholic fatty liver disease independent of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2015;13(8):1513–20.e1. 10.1016/j.cgh.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 304. Zhou YY, Zhou XD, Wu SJ, Hu XQ, Tang B, Poucke SV, et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: a meta‐analysis. Eur J Gastroenterol Hepatol. 2018;30:631–6. 10.1097/meg.0000000000001075 [DOI] [PubMed] [Google Scholar]
- 305. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology. 2005;129(1):113–21. 10.1053/j.gastro.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 306. Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma‐glutamyl transpeptidase levels. Hepatology. 2009;50(5):1403–11. 10.1002/hep.23135 [DOI] [PubMed] [Google Scholar]
- 307. Zhou YJ, Li YY, Nie YQ, Huang CM, Cao CY. Natural course of nonalcoholic fatty liver disease in southern China: a prospective cohort study. J Dig Dis. 2012;13(3):153–60. 10.1111/j.1751-2980.2011.00571.x [DOI] [PubMed] [Google Scholar]
- 308. Zeb I, Li D, Budoff MJ, Katz R, Lloyd‐Jones D, Agatston A, et al. Nonalcoholic fatty liver disease and incident cardiac events: the multi‐ethnic study of atherosclerosis. J Am Coll Cardiol. 2016;67(16):1965–6. 10.1016/j.jacc.2016.01.070 [DOI] [PubMed] [Google Scholar]
- 309. Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, Baron JA, et al. Prognosis of patients with a diagnosis of fatty liver‐‐a registry‐based cohort study. Hepato‐Gastroenterology. 2003;50:2101–4. [PubMed] [Google Scholar]
- 310. Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. 10.1002/hep.21327 [DOI] [PubMed] [Google Scholar]
- 311. Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long‐term follow‐up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7(2):234–8. 10.1016/j.cgh.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 312. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357–65. 10.1002/hep.26156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology. 2015;61(5):1547–54. 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 314. EASL Clinical Practice Guidelines . Drug‐induced liver injury. J Hepatol. 2019;70:1222–61. [DOI] [PubMed] [Google Scholar]
- 315. Hoofnagle JH, Björnsson ES. Drug‐induced liver injury ‐ types and phenotypes. N Engl J Med. 2019;381(3):264–73. 10.1056/nejmra1816149 [DOI] [PubMed] [Google Scholar]
- 316. Marrone G, Vaccaro FG, Biolato M, Miele L, Liguori A, Araneo C, et al. Drug‐induced liver injury 2017: the diagnosis is not easy but always to keep in mind. Eur Rev Med Pharmacol Sci. 2017;21:122–34. [PubMed] [Google Scholar]
- 317. Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak‐Ublick GA, Watkins PB, et al. Drug‐induced liver injury. Nat Rev Dis Prim. 2019;5(1):58. 10.1038/s41572-019-0105-0 [DOI] [PubMed] [Google Scholar]
- 318. Teschke R, Schulze J, Eickhoff A, Danan G. Drug induced liver injury: can biomarkers assist RUCAM in causality assessment? Int J Mol Sci. 2017;18(4):803. 10.3390/ijms18040803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, et al. CSH guidelines for the diagnosis and treatment of drug‐induced liver injury. Hepatol Int. 2017;11(3):221–41. 10.1007/s12072-017-9793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Ampong I, Watkins A, Gutierrez‐Merino J, Ikwuobe J, Griffiths HR. Dietary protein insufficiency: an important consideration in fatty liver disease? Br J Nutr. 2020;123(6):601–9. 10.1017/s0007114519003064 [DOI] [PubMed] [Google Scholar]
- 321. Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, et al. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology. 2017;152(3):571–85.e8. 10.1053/j.gastro.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 322. Skytte MJ, Samkani A, Petersen AD, Thomsen MN, Astrup A, Chabanova E, et al. A carbohydrate‐reduced high‐protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62(11):2066–78. 10.1007/s00125-019-4956-4 [DOI] [PubMed] [Google Scholar]
- 323. Gomes F, Schuetz P, Bounoure L, Austin P, Ballesteros‐Pomar M, Cederholm T, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336–53. 10.1016/j.clnu.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 324. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–609. 10.1053/j.gastro.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 325. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 326. Wang RT, Koretz RL, Yee HF, Jr . Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115(7):554–9. 10.1016/s0002-9343(03)00449-2 [DOI] [PubMed] [Google Scholar]
- 327. Barker KB, Palekar NA, Bowers SP, Goldberg JE, Pulcini JP, Harrison SA. Non‐alcoholic steatohepatitis: effect of Roux‐en‐Y gastric bypass surgery. Am J Gastroenterol. 2006;101(2):368–73. 10.1111/j.1572-0241.2006.00419.x [DOI] [PubMed] [Google Scholar]
- 328. Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, et al. Roux‐en‐Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5‐year controlled longitudinal study. Ann Surg. 2014;260(5):893–8. discussion 8‐9. 10.1097/sla.0000000000000945 [DOI] [PubMed] [Google Scholar]
- 329. Dixon JB, Bhathal PS, O'Brien PE. Weight loss and non‐alcoholic fatty liver disease: falls in gamma‐glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;16(10):1278–86. 10.1381/096089206778663805 [DOI] [PubMed] [Google Scholar]
- 330. Harrison SA, Fecht W, Brunt EM, Neuschwander‐Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49(1):80–6. 10.1002/hep.22575 [DOI] [PubMed] [Google Scholar]
- 331. Harrison SA, Fincke C, Helinski D, Torgerson S, Hayashi P. A pilot study of orlistat treatment in obese, non‐alcoholic steatohepatitis patients. Aliment Pharmacol Ther. 2004;20(6):623–8. 10.1111/j.1365-2036.2004.02153.x [DOI] [PubMed] [Google Scholar]
- 332. Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–88. quiz e15‐6. 10.1053/j.gastro.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 333. Stratopoulos C, Papakonstantinou A, Terzis I, Spiliadi C, Dimitriades G, Komesidou V, et al. Changes in liver histology accompanying massive weight loss after gastroplasty for morbid obesity. Obes Surg. 2005;15:1154–60. [DOI] [PubMed] [Google Scholar]
- 334. Tendler D, Lin S, Yancy WS, Jr , Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low‐carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52(2):589–93. 10.1007/s10620-006-9433-5 [DOI] [PubMed] [Google Scholar]
- 335. Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy‐restricted high‐protein, low‐fat compared with standard‐protein, low‐fat diets: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98. 10.3945/ajcn.112.044321 [DOI] [PubMed] [Google Scholar]
- 336. Muscariello E, Nasti G, Siervo M, Di Maro M, Lapi D, D'Addio G, et al. Dietary protein intake in sarcopenic obese older women. Clin Interv Aging. 2016;11:133–40. 10.2147/cia.s96017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Sammarco R, Marra M, Di Guglielmo ML, Naccarato M, Contaldo F, Poggiogalle E, et al. Evaluation of hypocaloric diet with protein supplementation in middle‐aged sarcopenic obese women: a pilot study. Obes Facts. 2017;10(3):160–7. 10.1159/000468153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Berzigotti A, Garcia‐Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54(2):555–61. 10.1002/hep.24418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, et al. Weight‐related effects on disease progression in the hepatitis C antiviral long‐term treatment against cirrhosis trial. Gastroenterology. 2009;137(2):549–57. 10.1053/j.gastro.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85(5):1257–66. 10.1093/ajcn/85.5.1257 [DOI] [PubMed] [Google Scholar]
- 341. McCullough AJ, Mullen KD, Kalhan SC. Defective nonoxidative leucine degradation and endogenous leucine flux in cirrhosis during an amino acid infusion. Hepatology. 1998;28(5):1357–64. 10.1002/hep.510280526 [DOI] [PubMed] [Google Scholar]
- 342. Tessari P, Barazzoni R, Kiwanuka E, Davanzo G, De Pergola G, Orlando R, et al. Impairment of albumin and whole body postprandial protein synthesis in compensated liver cirrhosis. Am J Physiol Endocrinol Metab. 2002;282(2):E304–11. 10.1152/ajpendo.00333.2001 [DOI] [PubMed] [Google Scholar]
- 343. Tessari P, Inchiostro S, Barazzoni R, Zanetti M, Orlando R, Biolo G, et al. Fasting and postprandial phenylalanine and leucine kinetics in liver cirrhosis. Am J Physiol. 1994;267(1):E140–9. 10.1152/ajpendo.1994.267.1.e140 [DOI] [PubMed] [Google Scholar]
- 344. Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, et al. Metabolic and molecular responses to leucine‐enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61(6):2018–29. 10.1002/hep.27717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Nielsen K, Kondrup J, Martinsen L, Dossing H, Larsson B, Stilling B, et al. Long‐term oral refeeding of patients with cirrhosis of the liver. Br J Nutr. 1995;74(4):557–67. 10.1079/bjn19950158 [DOI] [PubMed] [Google Scholar]
- 346. Swart GR, Zillikens MC, van Vuure JK, van den Berg JW. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ. 1989;299(6709):1202–3. 10.1136/bmj.299.6709.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Bories PN, Campillo B. One‐month regular oral nutrition in alcoholic cirrhotic patients. Changes of nutritional status, hepatic function and serum lipid pattern. Br J Nutr. 1994;72(6):937–46. 10.1079/bjn19940097 [DOI] [PubMed] [Google Scholar]
- 348. Manguso F, D'Ambra G, Menchise A, Sollazzo R, D'Agostino L. Effects of an appropriate oral diet on the nutritional status of patients with HCV‐related liver cirrhosis: a prospective study. Clin Nutr. 2005;24(5):751–9. 10.1016/j.clnu.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 349. Norman K, Kirchner H, Freudenreich M, Ockenga J, Lochs H, Pirlich M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non‐neoplastic gastrointestinal disease‐‐a randomized controlled trial. Clin Nutr. 2008;27(1):48–56. 10.1016/j.clnu.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 350. Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12‐month trial. Hepatology. 2008;48(2):557–66. 10.1002/hep.22367 [DOI] [PubMed] [Google Scholar]
- 351. European Association for the Study of the Liver , Berzigotti A, Zelber‐Sagi S, Dasarathy S, Montagnese S, Genton L, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–93. 10.1016/j.jhep.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Macias‐Rodriguez RU, Ilarraza‐Lomeli H, Ruiz‐Margain A, Ponce‐de‐Leon‐Rosales S, Vargas‐Vorackova F, Garcia‐Flores O, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7:e180. 10.1038/ctg.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1920–6.e2. 10.1016/j.cgh.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 354. U.S. Food & Drug Administration . UPDATE: potential risks with liquid‐filled intragastric balloons ‐ letter to health care providers; 2020.
- 355. Weimann A, Fischer M, Oberänder N, Prodehl G, Weber N, Andrä M, et al. Willing to go the extra mile: prospective evaluation of an intensified non‐surgical treatment for patients with morbid obesity. Clin Nutr. 2019;38(4):1773–81. 10.1016/j.clnu.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 356. Chandan S, Mohan BP, Khan SR, Facciorusso A, Ramai D, Kassab LL, et al. Efficacy and safety of intragastric balloon (IGB) in non‐alcoholic fatty liver disease (NAFLD): a comprehensive review and meta‐analysis. Obes Surg. 2021;31(3):1271–9. 10.1007/s11695-020-05084-0 [DOI] [PubMed] [Google Scholar]
- 357. Bazerbachi F, Vargas EJ, Rizk M, Maselli DB, Mounajjed T, Venkatesh SK, et al. Intragastric balloon placement induces significant metabolic and histologic improvement in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2021;19(1):146–54.e4. 10.1016/j.cgh.2020.04.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Salomone F, Currenti W, Magrì G, Boškoski I, Zelber‐Sagi S, Galvano F. Effects of intragastric balloon in patients with nonalcoholic fatty liver disease and advanced fibrosis. Liver Int. 2021;41(9):2112–6. 10.1111/liv.14917 [DOI] [PubMed] [Google Scholar]
- 359. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387(10019):679–90. 10.1016/s0140-6736(15)00803-x [DOI] [PubMed] [Google Scholar]
- 360. Frøssing S, Nylander M, Chabanova E, Frystyk J, Holst JJ, Kistorp C, et al. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: a randomized clinical trial. Diabetes Obes Metabol. 2017;20(1):215–8. 10.1111/dom.13053 [DOI] [PubMed] [Google Scholar]
- 361. Smits MM, Tonneijck L, Muskiet MHA, Kramer MHH, Pouwels PJW, Pieters‐van den Bos IC, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo‐controlled trial. Diabetologia. 2016;59(12):2588–93. 10.1007/s00125-016-4100-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Bizino MB, Jazet IM, de Heer P, van Eyk HJ, Dekkers IA, Rensen PCN, et al. Placebo‐controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre‐specified secondary study on ectopic fat accumulation. Diabetologia. 2020;63(1):65–74. 10.1007/s00125-019-05021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Khoo J, Hsiang JC, Taneja R, Koo SH, Soon GH, Kam CJ, et al. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non‐alcoholic fatty liver disease. Liver Int. 2019;39(5):941–9. 10.1111/liv.14065 [DOI] [PubMed] [Google Scholar]
- 364. Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti‐hyperglycaemic drugs in patients with non‐alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab. 2020;46(6):427–41. 10.1016/j.diabet.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 365. O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet. 2018;392(10148):637–49. 10.1016/s0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- 366. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. 10.1056/nejmoa2032183 [DOI] [PubMed] [Google Scholar]
- 367. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–24. 10.1056/nejmoa2028395 [DOI] [PubMed] [Google Scholar]
- 368. Wang H, Wang L, Cheng Y, Xia Z, Liao Y, Cao J. Efficacy of orlistat in non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Biomed Rep. 2018;9:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Ye J, Wu Y, Li F, Wu T, Shao C, Lin Y, et al. Effect of orlistat on liver fat content in patients with nonalcoholic fatty liver disease with obesity: assessment using magnetic resonance imaging‐derived proton density fat fraction. Therap Adv Gastroenterol. 2019;12:1756284819879047. 10.1177/1756284819879047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Pan CS, Stanley TL. Effect of weight loss medications on hepatic steatosis and steatohepatitis: a systematic review. Front Endocrinol. 2020;11:70. 10.3389/fendo.2020.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS‐3 MRI): a substudy of the randomised, open‐label, parallel‐group, phase 3 SURPASS‐3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406. 10.1016/s2213-8587(22)00070-5 [DOI] [PubMed] [Google Scholar]
- 372. Buss C, Valle‐Tovo C, Miozzo S, Alves de Mattos A. Probiotics and synbiotics may improve liver aminotransferases levels in non‐alcoholic fatty liver disease patients. Ann Hepatol. 2014;13(5):482–8. 10.1016/s1665-2681(19)31246-3 [DOI] [PubMed] [Google Scholar]
- 373. Koutnikova H, Genser B, Monteiro‐Sepulveda M, Faurie JM, Rizkalla S, Schrezenmeir J, et al. Impact of bacterial probiotics on obesity, diabetes and non‐alcoholic fatty liver disease related variables: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open. 2019;9(3):e017995. 10.1136/bmjopen-2017-017995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Loman BR, Hernandez‐Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Nutr Rev. 2018;76(11):822–39. 10.1093/nutrit/nuy031 [DOI] [PubMed] [Google Scholar]
- 375. Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta‐analysis. World J Gastroenterol. 2013;19(40):6911–8. 10.3748/wjg.v19.i40.6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Khan MY, Mihali AB, Rawala MS, Aslam A, Siddiqui WJ. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease ‐ a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol. 2019;31(6):703–15. 10.1097/meg.0000000000001371 [DOI] [PubMed] [Google Scholar]
- 377. Liu L, Li P, Liu Y, Zhang Y. Efficacy of probiotics and synbiotics in patients with nonalcoholic fatty liver disease: a meta‐analysis. Dig Dis Sci. 2019;64:3402–12. 10.1007/s10620-019-05699-z [DOI] [PubMed] [Google Scholar]
- 378. Sharpton SR, Maraj B, Harding‐Theobald E, Vittinghoff E, Terrault NA. Gut microbiome‐targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta‐analysis, and meta‐regression. Am J Clin Nutr. 2019;110(1):139–49. 10.1093/ajcn/nqz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, et al. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Therap Adv Gastroenterol. 2019;12:1756284819878046. 10.1177/1756284819878046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Xiao MW, Lin SX, Shen ZH, Luo WW, Wang XY. Systematic review with meta‐analysis: the effects of probiotics in nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2019;2019:1484598–19. 10.1155/2019/1484598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–5. [PubMed] [Google Scholar]
- 382. Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof‐of‐concept study. Ann Hepatol. 2013;12(2):256–62. 10.1016/s1665-2681(19)31364-x [DOI] [PubMed] [Google Scholar]
- 383. Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo‐oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–53. 10.1007/s10620-011-1887-4 [DOI] [PubMed] [Google Scholar]
- 384. Masterton GS, Plevris JN, Hayes PC. Review article: omega‐3 fatty acids ‐ a promising novel therapy for non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31(7):679–92. 10.1111/j.1365-2036.2009.04230.x [DOI] [PubMed] [Google Scholar]
- 385. Guo X‐f, Yang B, Tang J, Li D. Fatty acid and non‐alcoholic fatty liver disease: meta‐analyses of case‐control and randomized controlled trials. Clin Nutr. 2018;37(1):113–22. 10.1016/j.clnu.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 386. Musa‐Veloso K, Venditti C, Lee HY, Darch M, Floyd S, West S, et al. Systematic review and meta‐analysis of controlled intervention studies on the effectiveness of long‐chain omega‐3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev. 2018;76(8):581–602. 10.1093/nutrit/nuy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Yan J‐H, Guan B‐J, Gao H‐Y, Peng X‐E. Omega‐3 polyunsaturated fatty acid supplementation and non‐alcoholic fatty liver disease: a meta‐analysis of randomized controlled trials. Medicine. 2018;97(37):e12271–e. 10.1097/md.0000000000012271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of Ethyl‐Eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–84.e1. 10.1053/j.gastro.2014.04.046 [DOI] [PubMed] [Google Scholar]
- 389. Scorletti E, Bhatia L, McCormick KG, Clough GF, Nash K, Hodson L, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the WELCOME* study. Hepatology. 2014;60(4):1211–21. 10.1002/hep.27289 [DOI] [PubMed] [Google Scholar]
- 390. Raj H, Durgia H, Palui R, Kamalanathan S, Selvarajan S, Kar SS, et al. SGLT‐2 inhibitors in non‐alcoholic fatty liver disease patients with type 2 diabetes mellitus: a systematic review. World J Diabetes. 2019;10:114–32. 10.4239/wjd.v10.i2.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Chrysavgis L, Papatheodoridi AM, Chatzigeorgiou A, Cholongitas E. The impact of sodium glucose co‐transporter 2 inhibitors on non‐alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36(4):893–909. 10.1111/jgh.15202 [DOI] [PubMed] [Google Scholar]
- 392. Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, et al. Benefits and harms of sodium‐glucose Co‐transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta‐analysis. PLoS ONE. 2016;11:e0166125–e. 10.1371/journal.pone.0166125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Milić S, Lulić D, Štimac D. Non‐alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Hannah WN, Harrison SA. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin Liver Dis. 2016;20(2):339–50. 10.1016/j.cld.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 395. Kwak M, Mehaffey JH, Hawkins RB, Hsu A, Schirmer B, Hallowell PT. Bariatric surgery is associated with reduction in non‐alcoholic steatohepatitis and hepatocellular carcinoma: a propensity matched analysis. Am J Surg. 2020;219(3):504–7. 10.1016/j.amjsurg.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 396. Kalinowski P, Paluszkiewicz R, Ziarkiewicz‐Wróblewska B, Wróblewski T, Remiszewski P, Grodzicki M, et al. Liver function in patients with nonalcoholic fatty liver disease randomized to Roux‐en‐Y gastric bypass versus sleeve gastrectomy. Ann Surg. 2017;266(5):738–45. 10.1097/sla.0000000000002397 [DOI] [PubMed] [Google Scholar]
- 397. Lyo V, Schafer AL, Stewart L. Roux‐en‐Y gastric bypass is a safe and effective option that improves major Co‐Morbidities associated with obesity in an older, veteran population. Am J Surg. 2019;218(4):684–8. 10.1016/j.amjsurg.2019.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. von Schönfels W, Beckmann JH, Ahrens M, Hendricks A, Röcken C, Szymczak S, et al. Histologic improvement of NAFLD in patients with obesity after bariatric surgery based on standardized NAS (NAFLD activity score). Surg Obes Relat Dis. 2018;14(10):1607–16. 10.1016/j.soard.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 399. Tan CH, Al‐Kalifah N, Ser K‐H, Lee Y‐C, Chen J‐C, Lee W‐J. Long‐term effect of bariatric surgery on resolution of nonalcoholic steatohepatitis (NASH): an external validation and application of a clinical NASH score. Surg Obes Relat Dis. 2018;14(10):1600–6. 10.1016/j.soard.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 400. Klebanoff MJ, Corey KE, Samur S, Choi JG, Kaplan LM, Chhatwal J, et al. Cost‐effectiveness analysis of bariatric surgery for patients with nonalcoholic steatohepatitis cirrhosis. JAMA Netw Open. 2019;2:e190047–e. 10.1001/jamanetworkopen.2019.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Froylich D, Corcelles R, Daigle C, Boules M, Brethauer S, Schauer P. Effect of Roux‐en‐Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis. 2016;12(1):127–31. 10.1016/j.soard.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 402. Baldwin D, Chennakesavalu M, Gangemi A. Systematic review and meta‐analysis of Roux‐en‐Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis. 2019;15(12):2123–30. 10.1016/j.soard.2019.09.060 [DOI] [PubMed] [Google Scholar]
- 403. Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2019;17(6):1040–60.e11. 10.1016/j.cgh.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 404. Minhem MA, Sarkis SF, By S, Fares SA, Alami RS. Comparison of early morbidity and mortality between sleeve gastrectomy and gastric bypass in high‐risk patients for liver disease: analysis of American College of surgeons national surgical quality improvement program. Obes Surg. 2018;28(9):2844–51. 10.1007/s11695-018-3259-z [DOI] [PubMed] [Google Scholar]
- 405. Dziodzio T, Biebl M, Öllinger R, Pratschke J, Denecke C. The role of bariatric surgery in abdominal organ transplantation—the next big challenge? Obes Surg. 2017;27(10):2696–706. 10.1007/s11695-017-2854-8 [DOI] [PubMed] [Google Scholar]
- 406. Diwan TS, Lee TC, Nagai S, Benedetti E, Posselt A, Bumgardner G, et al. Obesity, transplantation, and bariatric surgery: an evolving solution for a growing epidemic. Am J Transplant. 2020;20(8):2143–55. 10.1111/ajt.15784 [DOI] [PubMed] [Google Scholar]
- 407. El‐Sherif O, Armstrong MJ. Peculiarities of cirrhosis due to nonalcoholic steatohepatitis (NASH). Semin Liver Dis. 2020;40(01):1–10. 10.1055/s-0039-1697616 [DOI] [PubMed] [Google Scholar]
- 408. Tsochatzis E, Coilly A, Nadalin S, Levistky J, Tokat Y, Ghobrial M, et al. International liver transplantation consensus statement on end‐stage liver disease due to nonalcoholic steatohepatitis and liver transplantation. Transplantation. 2019;103(1):45–56. 10.1097/tp.0000000000002433 [DOI] [PubMed] [Google Scholar]
- 409. Golabi P, Bush H, Stepanova M, Locklear CT, Jacobson IM, Mishra A, et al. Liver transplantation (LT) for cryptogenic cirrhosis (CC) and nonalcoholic steatohepatitis (NASH) cirrhosis: data from the Scientific registry of transplant recipients (SRTR): 1994 to 2016. Medicine (Baltim). 2018;97(31):e11518. 10.1097/md.0000000000011518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2014;12(3):394–402.e1. 10.1016/j.cgh.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 411. Davies DJ, Baxter JM, Baxter JN. Nutritional deficiencies after bariatric surgery. Obes Surg. 2007;17(9):1150–8. 10.1007/s11695-007-9208-x [DOI] [PubMed] [Google Scholar]
- 412. Ammor N, Berthoud L, Gerber A, Giusti V. Nutritional deficiencies in candidates for bariatric surgery. Rev Med Suisse. 2009;5:676–9. [PubMed] [Google Scholar]
- 413. Ducloux R, Nobécourt E, Chevallier J‐M, Ducloux H, Elian N, Altman J‐J. Vitamin D deficiency before bariatric surgery: should supplement intake Be routinely prescribed? Obes Surg. 2011;21(5):556–60. 10.1007/s11695-010-0352-3 [DOI] [PubMed] [Google Scholar]
- 414. Hwang C, Ross V, Mahadevan U. Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis. 2012;18(10):1961–81. 10.1002/ibd.22906 [DOI] [PubMed] [Google Scholar]
- 415. Wędrychowicz A, Zając A, Tomasik P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J Gastroenterol. 2016;22(3):1045–66. 10.3748/wjg.v22.i3.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient‐‐2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21((Suppl 1)):S1–S27. 10.1002/oby.20461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO‐ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2018;13(2):144–64K. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 418. Øresland T, Bemelman WA, Sampietro GM, Spinelli A, Windsor A, Ferrante M, et al. European evidence based consensus on surgery for ulcerative colitis. J Crohns Colitis. 2014;9(1):4–25. 10.1016/j.crohns.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 419. Jan A, Narwaria M, Mahawar KK. A systematic review of bariatric surgery in patients with liver cirrhosis. Obes Surg. 2015;25(8):1518–26. 10.1007/s11695-015-1727-2 [DOI] [PubMed] [Google Scholar]
- 420. Deutsche Gesellschaft für Allgemein‐ und Viszeralchirurgie . S3‐Leitlinie: Chirurgie der Adipositas und metabolischer Erkrankungen; 2018.
- 421. Fried M, Yumuk V, Oppert JM, Scopinaro N, Torres AJ, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Fact. 2013;6(5):449–68. 10.1159/000355480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Conceição EM, Mitchell JE, Pinto‐Bastos A, Arrojado F, Brandão I, Machado PPP. Stability of problematic eating behaviors and weight loss trajectories after bariatric surgery: a longitudinal observational study. Surg Obes Relat Dis. 2017;13(6):1063–70. 10.1016/j.soard.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 423. White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM. Loss of control over eating predicts outcomes in bariatric surgery patients: a prospective, 24‐month follow‐up study. J Clin Psychiatr. 2010;71(02):175–84. 10.4088/jcp.08m04328blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Busetto L, Segato G, De Luca M, De Marchi F, Foletto M, Vianello M, et al. Weight loss and postoperative complications in morbidly obese patients with binge eating disorder treated by laparoscopic adjustable gastric banding. Obes Surg. 2005;15(2):195–201. 10.1381/0960892053268327 [DOI] [PubMed] [Google Scholar]
- 425. Hsu LKG, Sullivan SP, Benotti PN. Eating disturbances and outcome of gastric bypass surgery: a pilot study. Int J Eat Disord. 1997;21(4):385–90. [DOI] [PubMed] [Google Scholar]
- 426. Mauro MFFP, Papelbaum M, Brasil MAA, Carneiro JRI, Coutinho ESF, Coutinho W, et al. Is weight regain after bariatric surgery associated with psychiatric comorbidity? A systematic review and meta‐analysis. Obes Rev. 2019;20(10):1413–25. 10.1111/obr.12907 [DOI] [PubMed] [Google Scholar]
- 427. Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post‐bariatric surgery patient: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2010;95(11):4823–43. 10.1210/jc.2009-2128 [DOI] [PubMed] [Google Scholar]
- 428. Brolin RE. Bariatric surgery and long‐term control of morbid obesity. JAMA. 2002;288(22):2793. 10.1001/jama.288.22.2793 [DOI] [PubMed] [Google Scholar]
- 429. Bock MA. Roux‐en‐Y gastric bypass: the dietitian's and patient's perspectives. Nutr Clin Pract. 2003;18(2):141–4. 10.1177/0115426503018002141 [DOI] [PubMed] [Google Scholar]
- 430. Fox W, Borgert A, Rasmussen C, Kallies K, Klas P, Kothari S. Long‐term micronutrient surveillance after gastric bypass surgery in an integrated healthcare system. Surg Obes Relat Dis. 2019;15(3):389–95. 10.1016/j.soard.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 431. Busetto L, Dicker D, Azran C, Batterham RL, Farpour‐Lambert N, Fried M, et al. Practical recommendations of the obesity management Task force of the European association for the study of obesity for the post‐bariatric surgery medical management. Obes Fact. 2017;10(6):597–632. 10.1159/000481825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Via MA, Mechanick JI. Nutritional and micronutrient care of bariatric surgery patients: current evidence update. Current Obesy Reports. 2017;6(3):286–96. 10.1007/s13679-017-0271-x [DOI] [PubMed] [Google Scholar]
- 433. Voican CS, Lebrun A, Maitre S, Lainas P, Lamouri K, Njike‐Nakseu M, et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS ONE. 2018;13(5):e0197248–e. 10.1371/journal.pone.0197248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Fried M, Yumuk V, Oppert J, Scopinaro N, Torres A, Weiner R, et al. International federation for surgery of obesity and metabolic disorders‐European chapter (IFSO‐EC); European association for the study of obesity (EASO); European association for the study of obesity obesity management Task force (EASO OMTF). Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55. 10.1007/s11695-013-1079-8 [DOI] [PubMed] [Google Scholar]
- 435. Ren Z‐Q, Lu G‐D, Zhang T‐Z, Xu Q. Effect of physical exercise on weight loss and physical function following bariatric surgery: a meta‐analysis of randomised controlled trials. BMJ Open. 2018;8(10):e023208. 10.1136/bmjopen-2018-023208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Boyce SG, Goriparthi R, Clark J, Cameron K, Roslin MS. Can composite nutritional supplement based on the current guidelines prevent vitamin and mineral deficiency after weight loss surgery? Obes Surg. 2015;26(5):966–71. 10.1007/s11695-015-1853-x [DOI] [PubMed] [Google Scholar]
- 437. Thereaux J, Lesuffleur T, Païta M, Czernichow S, Basdevant A, Msika S, et al. Long‐term follow‐up after bariatric surgery in a national cohort. Br J Surg. 2017;104(10):1362–71. 10.1002/bjs.10557 [DOI] [PubMed] [Google Scholar]
- 438. Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez‐Campoy JM, Collazo‐Clavell ML, Guven S, et al. American association of clinical Endocrinologists, the obesity society, and American society for metabolic & bariatric surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Surg Obes Relat Dis. 2008;4(5):S109–S84. 10.1016/j.soard.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 439. Kushner R. Managing the obese patient after bariatric surgery: a case report of severe malnutrition and review of the literature. J Parenter Enteral Nutr. 2000;24(2):126–32. 10.1177/0148607100024002126 [DOI] [PubMed] [Google Scholar]
- 440. Scopinaro N, Adami GF, Marinari GM, Gianetta E, Traverso E, Friedman D, et al. Biliopancreatic diversion. World J Surg. 1998;22(9):936–46. 10.1007/s002689900497 [DOI] [PubMed] [Google Scholar]
- 441. Heshmati K, Lo T, Tavakkoli A, Sheu E. Short‐term outcomes of inflammatory bowel disease after Roux‐en‐Y gastric bypass vs sleeve gastrectomy. J Am Coll Surg. 2019;228(6):893–901.e1. 10.1016/j.jamcollsurg.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 442. Keidar A, Hazan D, Sadot E, Kashtan H, Wasserberg N. The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis. 2015;11(1):132–6. 10.1016/j.soard.2014.06.022 [DOI] [PubMed] [Google Scholar]
- 443. McKenna NP, Habermann EB, Sada A, Kellogg TA, McKenzie TJ. Is bariatric surgery safe and effective in patients with inflammatory bowel disease? Obes Surg. 2019;30(3):882–8. 10.1007/s11695-019-04267-8 [DOI] [PubMed] [Google Scholar]
- 444. Sharma P, McCarty TR, Njei B. Impact of bariatric surgery on outcomes of patients with inflammatory bowel disease: a Nationwide inpatient sample analysis, 2004–2014. Obes Surg. 2017;28(4):1015–24. 10.1007/s11695-017-2959-0 [DOI] [PubMed] [Google Scholar]
- 445. Kvehaugen AS, Farup PG. Changes in gastrointestinal symptoms and food tolerance 6 months following weight loss surgery: associations with dietary changes, weight loss and the surgical procedure. BMC Obes. 2018;5(1):29. 10.1186/s40608-018-0206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446. Rausa E, Kelly ME, Galfrascoli E, Aiolfi A, Cavalcoli F, Turati L, et al. Quality of life and gastrointestinal symptoms following laparoscopic Roux‐en‐Y gastric bypass and laparoscopic sleeve gastrectomy: a systematic review. Obes Surg. 2019;29(4):1397–402. 10.1007/s11695-019-03737-3 [DOI] [PubMed] [Google Scholar]
- 447. Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev. 2010;12(5):366–77. 10.1111/j.1467-789x.2010.00731.x [DOI] [PubMed] [Google Scholar]
- 448. King WC, Chen J‐Y, Bond DS, Belle SH, Courcoulas AP, Patterson EJ, et al. Objective assessment of changes in physical activity and sedentary behavior: pre‐ through 3 years post‐bariatric surgery. Obesity. 2015;23(6):1143–50. 10.1002/oby.21106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Mundi MS, Lorentz PA, Swain J, Grothe K, Collazo‐Clavell M. Moderate physical activity as predictor of weight loss after bariatric surgery. Obes Surg. 2013;23(10):1645–9. 10.1007/s11695-013-0979-y [DOI] [PubMed] [Google Scholar]
- 450. Price PH, Kaizer AM, Inge TH, Eckel RH. Physical activity impacts insulin sensitivity post metabolic bariatric surgery in adolescents with severe obesity. Int J Obes. 2020;44(7):1479–86. 10.1038/s41366-020-0585-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Price PH, Kaizer AM, Daniels SR, Jenkins TM, Inge TH, Eckel RH. Physical activity improves lipid and weight‐loss outcomes after metabolic bariatric surgery in adolescents with severe obesity. Obesity. 2019;27(6):989–96. 10.1002/oby.22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Wefers JF, Woodlief TL, Carnero EA, Helbling NL, Anthony SJ, Dubis GS, et al. Relationship among physical activity, sedentary behaviors, and cardiometabolic risk factors during gastric bypass surgery–induced weight loss. Surg Obes Relat Dis. 2017;13(2):210–9. 10.1016/j.soard.2016.08.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Mundbjerg LH, Stolberg CR, Bladbjerg EM, Funch‐Jensen P, Juhl CB, Gram B. Effects of 6 months supervised physical training on muscle strength and aerobic capacity in patients undergoing Roux‐en‐Y gastric bypass surgery: a randomized controlled trial. Clin Obes. 2018;8(4):227–35. 10.1111/cob.12256 [DOI] [PubMed] [Google Scholar]
- 454. Mundbjerg LH, Stolberg CR, Cecere S, Bladbjerg E‐M, Funch‐Jensen P, Gram B, et al. Supervised physical training improves weight loss after Roux‐en‐Y gastric bypass surgery: a randomized controlled trial. Obesity. 2018;26(5):828–37. 10.1002/oby.22143 [DOI] [PubMed] [Google Scholar]
- 455. Stolberg CR, Mundbjerg LH, Bladbjerg E‐M, Funch‐Jensen P, Gram B, Juhl CB. Physical training following gastric bypass: effects on physical activity and quality of life—a randomized controlled trial. Qual Life Res. 2018;27(12):3113–22. 10.1007/s11136-018-1938-9 [DOI] [PubMed] [Google Scholar]
- 456. Beck NN, Johannsen M, Støving RK, Mehlsen M, Zachariae R. Do postoperative psychotherapeutic interventions and support groups influence weight loss following bariatric surgery? A systematic review and meta‐analysis of randomized and Nonrandomized trials. Obes Surg. 2012;22(11):1790–7. 10.1007/s11695-012-0739-4 [DOI] [PubMed] [Google Scholar]
- 457. Rudolph A, Hilbert A. Post‐operative behavioural management in bariatric surgery: a systematic review and meta‐analysis of randomized controlled trials. Obes Rev. 2013;14(4):292–302. 10.1111/obr.12013 [DOI] [PubMed] [Google Scholar]
- 458. Stewart F, Avenell A. Behavioural interventions for severe obesity before and/or after bariatric surgery: a systematic review and meta‐analysis. Obes Surg. 2015;26(6):1203–14. 10.1007/s11695-015-1873-6 [DOI] [PubMed] [Google Scholar]
- 459. Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel‐Hodge C, Ward DS. The efficacy of a daily self‐weighing weight loss intervention using smart scales and e‐mail. Obesity. 2013;21(9):1789–97. 10.1002/oby.20396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel‐Hodge C, Ward DS. Daily self‐weighing and adverse psychological outcomes: a randomized controlled trial. Am J Prev Med. 2014;46(1):24–9. 10.1016/j.amepre.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Elvin‐Walsh L, Ferguson M, Collins PF. Nutritional monitoring of patients post‐bariatric surgery: implications for smartphone applications. J Hum Nutr Diet. 2017;31(1):141–8. 10.1111/jhn.12492 [DOI] [PubMed] [Google Scholar]
- 462. Sockalingam S, Cassin SE, Wnuk S, Du C, Jackson T, Hawa R, et al. A pilot study on telephone cognitive behavioral therapy for patients six‐months post‐bariatric surgery. Obes Surg. 2017;27(3):670–5. 10.1007/s11695-016-2322-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Spring B, Duncan JM, Janke EA, Kozak AT, McFadden HG, DeMott A, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med. 2013;173(2):105–11. 10.1001/jamainternmed.2013.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Spring B, Pellegrini CA, Pfammatter A, Duncan JM, Pictor A, McFadden HG, et al. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity. 2017;25(7):1191–8. 10.1002/oby.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Svetkey LP, Batch BC, Lin P‐H, Intille SS, Corsino L, Tyson CC, et al. Cell phone intervention for you (CITY): a randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity. 2015;23(11):2133–41. 10.1002/oby.21226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Turner‐McGrievy GM, Wilcox S, Boutté A, Hutto BE, Singletary C, Muth ER, et al. The dietary intervention to enhance tracking with mobile devices (DIET mobile) study: a 6‐month randomized weight loss trial. Obesity. 2017;25(8):1336–42. 10.1002/oby.21889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Zwickert K, Rieger E, Swinbourne J, Manns C, McAulay C, Gibson AA, et al. High or low intensity text‐messaging combined with group treatment equally promote weight loss maintenance in obese adults. Obes Res Clin Pract. 2016;10(6):680–91. 10.1016/j.orcp.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 468. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. 10.3945/ajcn.111.024927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, et al. Executive summary: guidelines (2013) for the management of overweight and obesity in adults. Obesity. 2014;22(S2):S5–S39. 10.1002/oby.20821 [DOI] [PubMed] [Google Scholar]
- 470. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta‐analysis. JAMA. 2016;315(22):2424–34. 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Yanovski SZ, Yanovski JA. Long‐term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(1):74–86. 10.1001/jama.2013.281361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Pajecki D, Halpern A, Cercato C, Mancini M, de Cleva R, Santo MA. Short‐term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir. 2013;40(3):191–5. 10.1590/s0100-69912013000300005 [DOI] [PubMed] [Google Scholar]
- 473. Jester L, Wittgrove AC, Clark GW. Adjunctive use of appetite Suppressant medications for improved weight management in bariatric surgical patients. Obes Surg. 1996;6:412–5. 10.1381/096089296765556476 [DOI] [PubMed] [Google Scholar]
- 474. Schwartz J, Suzo A, Wehr AM, Foreman KS, Mikami DJ, Needleman BJ, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight Recidivism or weight loss plateau post‐bariatric surgery: a retrospective review. Obes Surg. 2015;26(2):452–8. 10.1007/s11695-015-1979-x [DOI] [PubMed] [Google Scholar]
- 475. Stanford FC, Alfaris N, Gomez G, Ricks ET, Shukla AP, Corey KE, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: a multi‐center study. Surg Obes Relat Diseas. 2017;13(3):491–500. 10.1016/j.soard.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Zilberstein B, Pajecki D, Garcia de Brito AC, Gallafrio ST, Eshkenazy R, Andrade CG. Topiramate after adjustable gastric banding in patients with binge eating and difficulty losing weight. Obes Surg. 2004;14(6):802–5. 10.1381/0960892041590926 [DOI] [PubMed] [Google Scholar]
- 477. Guzmán HM, Sepúlveda M, Rosso N, San Martin A, Guzmán F, Guzmán HC. Incidence and risk factors for cholelithiasis after bariatric surgery. Obes Surg. 2019;29(7):2110–4. 10.1007/s11695-019-03760-4 [DOI] [PubMed] [Google Scholar]
- 478. Melmer A, Sturm W, Kuhnert B, Engl‐Prosch J, Ress C, Tschoner A, et al. Incidence of gallstone formation and cholecystectomy 10 Years after bariatric surgery. Obes Surg. 2015;25(7):1171–6. 10.1007/s11695-014-1529-y [DOI] [PubMed] [Google Scholar]
- 479. Alsaif FA, Alabdullatif FS, Aldegaither MK, Alnaeem KA, Alzamil AF, Alabdulkarim NH, et al. Incidence of symptomatic cholelithiasis after laparoscopic sleeve gastrectomy and its association with rapid weight loss. Saudi J Gastroenterol. 2020;26(0):94–8. 10.4103/sjg.sjg_472_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Li VKM, Pulido N, Martinez‐Suartez P, Fajnwaks P, Jin HY, Szomstein S, et al. Symptomatic gallstones after sleeve gastrectomy. Surg Endosc. 2009;23(11):2488–92. 10.1007/s00464-009-0422-6 [DOI] [PubMed] [Google Scholar]
- 481. Sneineh MA, Harel L, Elnasasra A, Razin H, Rotmensh A, Moscovici S, et al. Increased incidence of symptomatic cholelithiasis after bariatric Roux‐en‐Y gastric bypass and previous bariatric surgery: a single center experience. Obes Surg. 2020;30(3):846–50. 10.1007/s11695-019-04366-6 [DOI] [PubMed] [Google Scholar]
- 482. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146‐81. [DOI] [PubMed] [Google Scholar]
- 483. Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Chatedaki C, Sioka E, et al. Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: an updated systematic review and meta‐analysis. Obes Surg. 2017;27(11):3021–30. 10.1007/s11695-017-2924-y [DOI] [PubMed] [Google Scholar]
- 484. Boerlage TC, Haal S, de Brauw LM, Acherman YI, Bruin S, van de Laar AW, et al. Ursodeoxycholic acid for the prevention of symptomatic gallstone disease after bariatric surgery: study protocol for a randomized controlled trial (UPGRADE trial). BMC Gastroenterol. 2017;17:1–8. 10.1186/s12876-017-0674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Tustumi F, Bernardo WM, Santo MA, Cecconello I. Cholecystectomy in patients submitted to bariatric procedure: a systematic review and meta‐analysis. Obes Surg. 2018;28(10):3312–20. 10.1007/s11695-018-3443-1 [DOI] [PubMed] [Google Scholar]
- 486. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61. 10.1053/j.gastro.2013.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Smeets XJ, Knoester I, Grooteman KV, Singh VK, Banks PA, Papachristou GI, et al. The association between obesity and outcomes in acute pancreatitis: an individual patient data meta‐analysis. Eur J Gastroenterol Hepatol. 2019;31(3):316–22. 10.1097/meg.0000000000001300 [DOI] [PubMed] [Google Scholar]
- 488. Forsmark CE, Swaroop Vege S, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–81. 10.1056/nejmra1505202 [DOI] [PubMed] [Google Scholar]
- 489. IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1‐e15. [DOI] [PubMed] [Google Scholar]
- 490. Johnson C, Toh S, Campbell M. Combination of APACHE‐II score and an obesity score (APACHE‐O) for the prediction of severe acute pancreatitis. Pancreatology. 2004;4:1–6. 10.1159/000077021 [DOI] [PubMed] [Google Scholar]
- 491. Song Y, Deng H, Zhou J, Sun J, Zhang X, Ren Y. The effects of laparoscopic sleeve gastrectomy on obesity‐related hypertriglyceridemia‐induced acute pancreatitis. Obes Surg. 2018;28(12):3872–9. 10.1007/s11695-018-3446-y [DOI] [PubMed] [Google Scholar]
- 492. Borbély Y, Plebani A, Kröll D, Ghisla S, Nett PC. Exocrine pancreatic insufficiency after Roux‐en‐Y gastric bypass. Surg Obes Relat Dis. 2016;12:790–4. 10.1016/j.soard.2015.10.084 [DOI] [PubMed] [Google Scholar]
- 493. Kittah NE, Vella A. Management of endocrine disease: pathogenesis and management of hypoglycemia. Eur J Endocrinol. 2017;177(1):R37–47. 10.1530/eje-16-1062 [DOI] [PubMed] [Google Scholar]
- 494. Goldfine AB, Patti ME. How common is hypoglycemia after gastric bypass? Obesity. 2016;24(6):1210–1. 10.1002/oby.21520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post‐gastric bypass hypoglycaemia including 5, 040 patients undergoing surgery for obesity in 1986–2006 in Sweden. Diabetologia. 2010;53:2307–11. 10.1007/s00125-010-1798-5 [DOI] [PubMed] [Google Scholar]
- 496. Lee CJ, Brown TT, Schweitzer M, Magnuson T, Clark JM. The incidence and risk factors associated with developing symptoms of hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2018;14(6):797–802. 10.1016/j.soard.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 497. Kefurt R, Langer FB, Schindler K, Shakeri‐Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux‐En‐Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11(3):564–9. 10.1016/j.soard.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 498. Lee D, Dreyfuss JM, Sheehan A, Puleio A, Mulla CM, Patti ME. Glycemic patterns are distinct in individuals with post‐bariatric hypoglycemia after gastric bypass (PBH‐RYGB). J Clin Endocrinol Metab. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Jacobsen SH, Bojsen‐Møller KN, Dirksen C, Jørgensen NB, Clausen TR, Wulff BS, et al. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose‐tolerant individuals. Diabetologia. 2013;56(10):2250–4. 10.1007/s00125-013-3003-0 [DOI] [PubMed] [Google Scholar]
- 500. Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, et al. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity. 2014;22:2003–9. 10.1002/oby.20791 [DOI] [PubMed] [Google Scholar]
- 501. Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon‐like peptide 1‐stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–14. 10.2337/db11-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Tharakan G, Behary P, Wewer Albrechtsen NJ, Chahal H, Kenkre J, Miras AD, et al. Roles of increased glycaemic variability, GLP‐1 and glucagon in hypoglycaemia after Roux‐en‐Y gastric bypass. Eur J Endocrinol. 2017;177(6):455–64. 10.1530/eje-17-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S, et al. Long‐term effects of bariatric surgery on meal disposal and β‐cell function in diabetic and nondiabetic patients. Diabetes. 2013;62(11):3709–17. 10.2337/db13-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Reubi JC, Perren A, Rehmann R, Waser B, Christ E, Callery M, et al. Glucagon‐like peptide‐1 (GLP‐1) receptors are not overexpressed in pancreatic islets from patients with severe hyperinsulinaemic hypoglycaemia following gastric bypass. Diabetologia. 2010;53(12):2641–5. 10.1007/s00125-010-1901-y [DOI] [PubMed] [Google Scholar]
- 505. Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon‐like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669–80.e2. 10.1053/j.gastro.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Rebelos E, Moriconi D, Scalese M, Denoth F, Molinaro S, Siciliano V, et al. Impact of postprandial hypoglycemia on weight loss after bariatric surgery. Obes Surg. 2020;30(6):2266–73. 10.1007/s11695-020-04465-9 [DOI] [PubMed] [Google Scholar]
- 507. Ahmad A, Kornrich DB, Krasner H, Eckardt S, Ahmad Z, Braslow A, et al. Prevalence of dumping syndrome after laparoscopic sleeve gastrectomy and comparison with laparoscopic Roux‐en‐Y gastric bypass. Obes Surg. 2019;29(5):1506–13. 10.1007/s11695-018-03699-y [DOI] [PubMed] [Google Scholar]
- 508. Geddes J, Wright RJ, Zammitt NN, Deary IJ, Frier BM. An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diabetes Care. 2007;30(11):1868–70. 10.2337/dc07-1432 [DOI] [PubMed] [Google Scholar]
- 509. Capristo E, Panunzi S, De Gaetano A, Spuntarelli V, Bellantone R, Giustacchini P, et al. Incidence of hypoglycemia after gastric bypass vs sleeve gastrectomy: a randomized trial. J Clin Endocrinol Metabol. 2018;103(6):2136–46. 10.1210/jc.2017-01695 [DOI] [PubMed] [Google Scholar]
- 510. Søeby M, Nielsen JB, Pedersen SB, Gribsholt SB, Holst JJ, Richelsen B. Relationship between biochemical and symptomatic hypoglycemia after RYGB. Responses to a mixed meal test: a case‐control study. Surg Obes Relat Dis. 2020;16(9):1179–85. 10.1016/j.soard.2020.04.024 [DOI] [PubMed] [Google Scholar]
- 511. Lupoli R, Lembo E, Ciciola P, Schiavo L, Pilone V, Capaldo B. Continuous glucose monitoring in subjects undergoing bariatric surgery: diurnal and nocturnal glycemic patterns. Nutr Metabol Cardiovasc Dis. 2020;30(11):1954–60. 10.1016/j.numecd.2020.06.029 [DOI] [PubMed] [Google Scholar]
- 512. Emous M, Ubels FL, van Beek AP. Diagnostic tools for post‐gastric bypass hypoglycaemia. Obes Rev. 2015;16(10):843–56. 10.1111/obr.12307 [DOI] [PubMed] [Google Scholar]
- 513. Suhl E, Anderson‐Haynes S‐E, Mulla C, Patti M‐E. Medical nutrition therapy for post‐bariatric hypoglycemia: practical insights. Surg Obes Relat Dis. 2017;13:888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Øhrstrøm CC, Worm D, Højager A, Andersen D, Holst JJ, Kielgast UL, et al. Postprandial hypoglycaemia after Roux‐en‐Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide. Diabetes Obes Metabol. 2019;21(9):2142–51. 10.1111/dom.13796 [DOI] [PubMed] [Google Scholar]
- 515. van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2016;18(1):68–85. 10.1111/obr.12467 [DOI] [PubMed] [Google Scholar]
- 516. Ciudin A, Sánchez M, Hernandez I, Cordero E, Fidilio E, Comas M, et al. Canagliflozin: a new therapeutic option in patients that present postprandial hyperinsulinemic hypoglycemia after Roux‐en‐Y gastric bypass: a pilot study. Obes Fact. 2021;14(3):291–7. 10.1159/000515598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Craig CM, Lawler HM, Lee CJE, Tan M, Davis DB, Tong J, et al. PREVENT: a randomized, placebo‐controlled crossover trial of avexitide for treatment of Postbariatric hypoglycemia. J Clin Endocrinol Metabol. 2021;106(8):e3235–48. 10.1210/clinem/dgab103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Mulla CM, Zavitsanou S, Laguna Sanz AJ, Pober D, Richardson L, Walcott P, et al. A randomized, placebo‐controlled double‐blind trial of a closed‐loop glucagon system for Postbariatric hypoglycemia. J Clin Endocrinol Metabol. 2019;105(4):e1260–e71. 10.1210/clinem/dgz197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Storm AC, Thompson CC. Endoscopic treatments following bariatric surgery. Gastrointest Endosc Clin N Am. 2017;27(2):233–44. 10.1016/j.giec.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clin Obes. 2017;7(2):115–22. 10.1111/cob.12182 [DOI] [PubMed] [Google Scholar]
- 521. Campos GM, Ziemelis M, Paparodis R, Ahmed M, Davis DB. Laparoscopic reversal of Roux‐en‐Y gastric bypass: technique and utility for treatment of endocrine complications. Surg Obes Relat Di. 2014;10(1):36–43. 10.1016/j.soard.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522. Ma P, Ghiassi S, Lloyd A, Haddad A, Boone K, DeMaria E, et al. Reversal of Roux en Y gastric bypass: largest single institution experience. Surg Obes Relat Dis. 2019;15(8):1311–6. 10.1016/j.soard.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 523. Eisenberg D, Azagury DE, Ghiassi S, Grover BT, Kim JJ. ASMBS position statement on postprandial hyperinsulinemic hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2017;13(3):371–8. 10.1016/j.soard.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 524. Sjöström L, Gummesson A, Sjöström CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–62. 10.1016/s1470-2045(09)70159-7 [DOI] [PubMed] [Google Scholar]
- 525. Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, et al. Bariatric surgery and the risk of cancer in a large Multisite cohort. Ann Surg. 2019;269(1):95–101. 10.1097/sla.0000000000002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Mendes JT, Wilson C, Schammel CMG, Scott JD, Schammel DP, Trocha SD. GIST identified during bariatric surgery: to treat or not to treat? Surg Obes Relat Dis. 2020;16(2):282–7. 10.1016/j.soard.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 527. Scozzari G, Trapani R, Toppino M, Morino M. Esophagogastric cancer after bariatric surgery: systematic review of the literature. Surg Obes Relat Dis. 2013;9(1):133–42. 10.1016/j.soard.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 528. Di Lorenzo N, Antoniou SA, Batterham RL, Busetto L, Godoroja D, Iossa A, et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO‐EC, EASO and ESPCOP. Surg Endosc. 2020;34(6):2332–58. 10.1007/s00464-020-07555-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Tse WHW, Kroon HM, van Lanschot JJB. Clinical challenges in upper gastrointestinal malignancies after bariatric surgery. Dig Surg. 2018;35(3):183–6. 10.1159/000477267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Masrur M, Elli E, Gonzalez‐Ciccarelli LF, Giulianotti PC. De novo gastric adenocarcinoma 1 year after sleeve gastrectomy in a transplant patient. Int J Surg Case Rep. 2016;20:10–3. 10.1016/j.ijscr.2015.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Benjamin RM. The Surgeon General's vision for a healthy and fit nation. Publ Health Rep. 2010;125(4):514–5. 10.1177/003335491012500402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Obesity prevention. London: National Institute for Health and Care Excellence (NICE); 2015 Mar. (NICE Clinical Guidelines, No. 43.). 2015. [Google Scholar]
- 533. Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi‐Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease. Circulation. 2004;110(18):2952–67. 10.1161/01.cir.0000145546.97738.1e [DOI] [PubMed] [Google Scholar]
- 534. Association AM. Assessment and management of adult obesity: a primer for physicians. Atlanta; USA2003. [Google Scholar]
- 535. McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;139(11):933–49. 10.7326/0003-4819-139-11-200312020-00013 [DOI] [PubMed] [Google Scholar]
- 536. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults‐‐the evidence report. National institutes of health. Obes Res. 1998;6 (Suppl 2):51s‐209s. [PubMed] [Google Scholar]
- 537. Hardcastle SJ, Taylor AH, Bailey MP, Harley RA, Hagger MS. Effectiveness of a motivational interviewing intervention on weight loss, physical activity and cardiovascular disease risk factors: a randomised controlled trial with a 12‐month post‐intervention follow‐up. Int J Behav Nutr Phys Activ. 2013;10(1):40. 10.1186/1479-5868-10-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Elwyn G, Dehlendorf C, Epstein RM, Marrin K, White J, Frosch DL. Shared decision making and motivational interviewing: achieving patient‐centered care across the spectrum of health care problems. Ann Fam Med. 2014;12(3):270–5. 10.1370/afm.1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Covolo L, Ceretti E, Moneda M, Castaldi S, Gelatti U. Does evidence support the use of mobile phone apps as a driver for promoting healthy lifestyles from a public health perspective? A systematic review of Randomized Control Trials. Patient Educ Counsel. 2017;100(12):2231–43. 10.1016/j.pec.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 540. Lau DCW, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ (Can Med Assoc J). 2007;176(8):S1–S13. 10.1503/cmaj.061409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Brown CL, Perrin EM. Obesity prevention and treatment in primary care. Acad Pediatr. 2018;18(7):736–45. 10.1016/j.acap.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 542. ter Bogt NC, Bemelmans WJ, Beltman FW, Broer J, Smit AJ, van der Meer K. Preventing weight gain by lifestyle intervention in a general practice setting: three‐year results of a randomized controlled trial. Arch Intern Med. 2011;171(4):306–13. 10.1001/archinternmed.2011.22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Supplementary Material 2
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


