Highlights
-
•
Chronic MDMA users showed elevated FA in corpus callosum and corticospinal tract.
-
•
Negative correlation of FA with MDMA use frequency in isthmus of corpus callosum.
-
•
Neurofilament light chain levels in blood were not elevated in MDMA users.
-
•
MDMA use in humans is not associated with severe white matter lesions.
Keywords: MDMA, Neurotoxicity, Axonal neuropathology, White matter, DTI, Neurofilament light chain
Abbreviations: MDMA, 3,4–methylenedioxymethamphetamine; 5-HT, 5-hydroxytryptamine (serotonin); DTI, diffusion tensor imaging; FA, fractional anisotropy; NfL, neurofilament light chain; CBF, cerebral blood flow
Abstract
3,4–Methylenedioxymethamphetamine (MDMA, “Ecstasy”) is a serotonin- and noradrenaline-releasing substance, currently among the most widely used illicit substances worldwide. In animal studies, repeated exposure to MDMA has been associated with dendritic but also axonal degeneration in the brain. However, translation of the axonal findings, specifically, to humans has been repeatedly questioned and the few existing studies investigating white matter alterations in human chronic MDMA users have yielded conflicting findings. In this study, we combined whole-brain diffusion tensor imaging and neurofilament light chain (NfL) analysis in blood to reveal potential MDMA-induced axonal neuropathology. To this end, we assessed 39 chronic MDMA users and 39 matched MDMA-naïve healthy controls. MDMA users showed increased fractional anisotropy in several white matter tracts, most prominently in the corpus callosum as well as corticospinal tracts, with these findings partly related to MDMA use intensity. However, the NfL levels of MDMA users were not significantly different from those of controls. We conclude that MDMA use is not associated with significant white matter lesions due to the absence of reduced fractional anisotropy and increased NfL levels commonly observed in conditions associated with white matter lesions, including stimulant and ketamine use disorders. Hence, the MDMA-induced axonal degradation demonstrated in animal models was not observed in this human study of chronic MDMA users.
1. Introduction
3,4–Methylenedioxymethamphetamine (MDMA, “Ecstasy”) is a potent serotonin- and noradrenaline releasing drug (Gudelsky and Yamamoto, 2008), inducing euphoria and vigilance in humans and widely consumed in the nightlife scene (Parrott, 2001). After cannabis and cocaine, MDMA is currently one of the most widely used illicit drugs worldwide (UN World Drug Report, 2022). Moreover, the amount of MDMA per tablet, the form in which MDMA is primarily consumed, has more than doubled in the last decade (UN World Drug Report, 2022).
The main targets of MDMA in the brain are monoaminergic transporters, with the highest affinity for serotonin (5-hydroxytryptamine [5-HT]) as well as noradrenaline and, to a much lesser extent, also dopamine transporters (Bershad et al., 2016, Liechti et al., 2000). Psychoactive effects of MDMA are mainly attributed to 5-HT release (Liechti et al., 2000), while noradrenaline release contributes specifically to the stimulant effects (Hysek et al., 2012, Hysek et al., 2011). In line with this, chronic MDMA use in humans has been constantly associated with selective long-term adaptations in the 5–HT system, including lower 5-HT metabolite levels in the cerebrospinal fluid (McCann et al., 1999, McCann et al., 1994), 5-HT depletion (Kish et al., 2000) and reduced 5-HT transporter density in the brain (Müller et al., 2019, Roberts et al., 2016). In addition, chronic MDMA users have consistently been found to display neurocognitive deficits, primarily in the domains of learning and memory, as well as executive functioning (Montgomery and Roberts, 2022, Mustafa et al., 2020, Roberts et al., 2018, Wunderli et al., 2017). These functional impairments have been suggested to result from serotonergic neurotoxicity of MDMA. However, a precise understanding of the underlying mechanisms is still lacking (Montgomery and Roberts, 2022).
Molecular alterations in the human 5-HT system following chronic MDMA exposure are well documented, but how MDMA use affects other brain structures in humans, such as white matter, has not yet been extensively investigated. In rats and non-human primates, several studies have demonstrated that MDMA causes severe axonal degeneration in the brain. This occurs most prominently at serotonergic axons in occipital regions, but has also been observed throughout the cortex (Commins et al., 1987, Hatzidimitriou et al., 1999, O’Hearn et al., 1988, Ricaurte et al., 1985, Ricaurte et al., 1988). The MDMA-induced neuroaxonal damage depends on excessive release of 5-HT, and there is evidence that several mechanisms, such as toxic metabolite formation, increased oxidative stress, hyperthermia, and excitotoxicity are involved (Costa and Gołembiowska, 2022). However, the extent to which these various mechanisms contribute to MDMA-related neurotoxicity remains controversial. Furthermore, Fischer et al. (1995) showed region-specific reinnervation patterns of serotonergic axons after MDMA–induced axonal loss in rats as well as in non-human primates. Specifically, distant targets of 5-HT pathways (e.g., occipital cortex) remained denervated, whereas proximal targets (e.g., amygdala, hypothalamus) exhibited abnormal axonal sprouting resulting in hyper-innervation. Thus, findings suggest long-term reorganization of axonal projections.
In brief, these studies showed widespread axonal degeneration in the brain and demonstrated the neurotoxic potential of MDMA in animals. However, the translation of these results to humans has been repeatedly questioned because i) the doses applied in animal studies may not be comparable to average doses consumed by humans, ii) the route of MDMA administration may be critical since in several animal studies MDMA was injected subcutaneously or intraperitoneally, whereas humans typically ingest MDMA orally, and iii) the species-specific metabolism of MDMA may hamper the transfer of findings in animal models to humans (Curran, 2000, de la Torre and Farré, 2004, Green et al., 2003, Kish, 2002). Therefore, it remains unclear whether the MDMA-induced neurotoxic disruption of axons found in animals also occurs in humans.
So far, the few available studies investigating white matter alterations in regular MDMA users are inconclusive (de Win et al., 2008a, de Win et al., 2008b, Liu et al., 2011, Moeller et al., 2007). These studies applied diffusion tensor imaging (DTI) to compute the degree of water molecular anisotropy (fractional anisotropy, FA), which has been shown to be a reliable measure of white matter alterations. A reduction in FA has been consistently observed in diseases associated with white matter lesions and demyelination (e.g., multiple sclerosis [Welton et al., 2015], stroke [Møller et al., 2007], Alzheimer [Sexton et al., 2011] or adrenoleukodystrophy [Huffnagel et al., 2019]). However, findings in MDMA users regarding alterations in white matter FA are conflicting. Liu et al. (2011) observed increased FA in the internal capsule and decreased FA in the corpus callosum, while de Win et al. (2008a) found decreased FA in the frontoparietal white matter of regular MDMA users. In contrast, Moeller et al., 2007, de Win et al., 2008b observed no white matter alterations indicated by FA. Therefore, no clear conclusion can be drawn from these studies, in contrast to DTI studies in ketamine and stimulant (i.e., cocaine and methamphetamine) users, which have found consistent associations with reduced FA in several white matter tracts (Edward Roberts et al., 2014, Huang et al., 2020, Ma et al., 2017, Michels et al., 2022, Ottino-González et al., 2022, van Son et al., 2016). White matter lesions in ketamine users have been primarily suggested to stem from glutamate-mediated excitotoxicity (Edward Roberts et al., 2014, Strous et al., 2022), while white matter impairments in stimulant users have been linked both to its cerebral vasoconstrictive effects elevating the risk for ischemic lesions (Büttner, 2012) and to the cocaine-adulterant levamisole, which induces leukoencephalopathic and vasculopathic changes (Michels et al., 2022). Furthermore, animal research suggests that enhanced oxidative stress in response to stimulant exposure might affect the expression of myelin-related genes, thereby reducing axonal stability (Albertson et al., 2004, Narayana et al., 2014).
In addition to inconclusive findings, previous studies investigating white matter impairments in MDMA users were limited in several ways by methodology and design. Specifically, appropriate controlling for co-use of other substances was lacking, which is critical since MDMA users tend to display a widespread pattern of polysubstance use (Gouzoulis-Mayfrank and Daumann, 2006). Further, the majority of previous studies used 1.5 Tesla scanners and the only study using a 3 Tesla scanner measured only six gradient directions. These factors are known to drastically reduce the signal-to-noise ratio of diffusion images (Gonen et al., 2001, Jones, 2004). Methodological shortcomings of previous studies applying DTI in substance use contexts were also criticized by a recent review article (Hampton et al., 2019), which suggested several methodological guidelines, that we adhered to for this study.
Complementary to the findings from DTI studies, recent findings revealed elevated neurofilament light chain (NfL) levels in the blood of chronic stimulant and ketamine users (Bavato et al., 2022b, Liu et al., 2021). NfL represents a neuronal cytoskeleton protein present in axons and elevated levels in blood have been established as a sensitive biomarker for neuroaxonal pathology (Khalil et al., 2018). Blood NfL concentration is closely correlated with cerebrospinal fluid NfL concentration (Disanto et al., 2017) and shows positive associations with several measures of structural brain alterations (Alirezaei et al., 2020). A direct association between increased blood NfL levels and a reduction of NfL concentration in specific brain regions has also been demonstrated in patients with neurodegenerative disorders (Ashton et al., 2019). In addition, chronic MDMA exposure has been shown to decrease NfL in the hippocampal tissue of rats (García-Cabrerizo and García-Fuster, 2015), which in turn might lead to increased levels in blood. A recent study further demonstrated that NfL levels were reduced after MDMA treatment in a serotonergic cell line obtained from the rat raphe nucleus (Bavato et al., 2022b), indicating serotonergic neurotoxicity. However, no study has yet examined NfL levels in MDMA users.
In the present study, we used a deep–learning approach to segment white matter into known anatomical tracts and, subsequently, conducted statistical analyses based on FA along these reconstructed fiber bundles. This approach has recently been shown to reliably detect white matter alterations in clinical populations (Wasserthal et al., 2021, Wasserthal et al., 2020, Wasserthal et al., 2018). In contrast to conventional methods such as Tract-Based Spatial Statistics (TBSS; Smith et al., 2006), this approach allows the analysis of DTI metrics in native space and does not suffer from coregistration inaccuracies inherent in TBSS (Zalesky, 2011). Moreover, it allows for precise localization of white matter changes in the brain as the statistical analysis is not restricted to a pseudo-anatomical white matter skeleton. Instead, statistical analysis is performed along anatomically known white matter tracts, facilitating interpretation of effects in terms of functional consequences. Moreover, in addition to the macroscopic DTI approach, we complemented the study with a molecular analysis of NfL concentration in blood, and applied advanced forensic hair toxicology to characterize co–use of other substances, which represent important confounding factors to consider when investigating white matter alterations in substance users. Thus, we aimed to overcome limitations of previous studies and extended the DTI analysis with a sensitive marker of active neuroaxonal pathology. Despite conflicting prior findings, we expected decreased FA and increased NfL levels in chronic MDMA users – based on existing findings in stimulant and ketamine users – and hypothesized alterations are associated with severity of use.
2. Methods
2.1. Participants
Chronic MDMA users (n = 49) as well as MDMA-naïve healthy controls (n = 47) between 18 and 45 years of age were recruited in Zurich, Switzerland. In total, 10 participants from the MDMA user group were excluded due to the absence of MDMA residuals in hair (n = 4), diagnosis of an Axis I DSM-IV psychiatric disorder (n = 1), missing data (n = 1), corrupt data (n = 1), or poor data quality (n = 3; see below). From the control group, a total of n = 8 participants were excluded because either MDMA was detected in the hair analysis (n = 5) or DTI data quality was poor (n = 3). Thus, the final DTI analysis included n = 39 MDMA users and n = 39 control participants. For the analysis of NfL levels, n = 4 participants had to be excluded due to missing blood samples and consequently n = 42 MDMA users and n = 40 controls were analyzed. Both participant groups were matched by sex, age, years of education, verbal intelligence, and nicotine use. General exclusion criteria included acute or previous neurological disorders and severe medical diseases, head injuries, ongoing intake of medication acting on the central nervous system, previous heroin injections, daily cannabis use, and cases of schizophrenia, bipolar disorder, or obsessive–compulsive disorder in first degree relatives. Participants were further excluded if they were screened positively for previous or current DSM-IV Axis I psychiatric disorders, with the exception of substance use disorders in MDMA users. MDMA users were included if they reported a minimum of 25 lifetime occasions of MDMA use, while having consumed the substance at least once during the 4 months prior to study participation (verified by hair analysis). Control participants who reported illegal substance use on more than 15 lifetime occasions, except for cannabis, were excluded. All participants were asked to abstain from illegal substances for at least three days before testing (assessed by urine analysis). Further, they were asked to not drink alcohol for 24 h prior to testing. The Ethics Committee of the Canton Zurich approved the study protocol (BASEC-Nr. 2018–02125) and in accordance with the Declaration of Helsinki, all participants provided written informed consent.
2.2. Clinical and substance use assessment
All participants were screened by trained psychologists for DSM-IV Axis I disorders with the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), with the exception that substance use disorders were assessed using the Structured Clinical Interview for DSM-5 Axis I disorders (First, 2015). The DSM-IV-based MINI was used, as a German version of the DSM-5-based MINI was not available at study start. To further evaluate symptoms of Depression and Attention-Deficit/Hyperactivity Disorder (ADHD), participants filled in the Center for Epidemiologic Studies Depression Scale ([CESD]; Eaton et al., 2004) and the ADHD Self-Rating Scale ([ADHD-SR]; Rösler et al., 2004). By using a standardized German vocabulary test ([Mehrfachwahl-Wortschatz-Intelligenztest]; Lehrl et al., 1995), premorbid verbal intelligence (verbal IQ) was estimated. Moreover, substance use history was assessed with the standardized Interview for Psychotropic Drug Consumption (Quednow et al., 2004) and, additionally, to objectively characterize substance use over the four months prior to testing, a proximal hair sample of 4 cm was taken from the occiput. If the participants' scalp hair was not long enough, body hair was sampled (n = 8). To screen for acute drug exposure, urine samples were collected. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described in detail by Scholz et al. (2021) was used for forensic toxicological hair analysis.
2.3. Magnetic resonance imaging
MRI data were acquired using a 3-Tesla Achieva scanner (Philips Healthcare, Netherlands) equipped with 80 mT/m gradients and a 32–channel receive head coil. Diffusion weighted images were acquired using a single-shot spin-echo echo-planar imaging sequence (repetition time [TR] = 11.8 s, echo time [TE] = 50.1 ms, field of view [FOV] = 220 × 220 mm2, N transversal slices = 60, slice thickness = 2 mm, acquisition matrix = 112 × 110). Diffusion was recorded along 64 directions with a b-value of 1000 s/mm2. Additionally, one non–diffusion–weighted b = 0 s/mm2 scan was performed. To aid with anatomical registration of diffusion weighted images, T1–weighted structural images were acquired with 1 mm isotropic resolution (TR = 8.1 ms, TE = 3.7 ms).
Preprocessing was performed using QSIPrep version 0.12.2 (Cieslak et al., 2021), which is based on Nipype (Gorgolewski et al., 2011). T1–weighted images were corrected for intensity non-uniformity and skull-stripped using ANTs tools (Avants et al., 2011), then used as a structural reference for registration of diffusion images. To correct diffusion images for head motion and eddy current-induced image distortions, FSL eddy was applied (Andersson and Sotiropoulos, 2016). FA maps were computed using tools from TractSeg (Wasserthal et al., 2018).
2.4. Tractometry
Preprocessed and registered diffusion images were fed into TractSeg, a convolutional neuronal network-based model that generates bundle-specific tractograms for anatomically well–known white matter tracts (Wasserthal et al., 2018). In total, 48 white matter bundles were reconstructed separately for each participant (see Supplement for the list of all extracted bundles). All white matter bundles were subdivided into 100 segments by defining 100 equidistant points on the tract's centroid streamline and assigning each point of the tract's streamlines to the nearest segment according to Euclidean distances (Chandio et al., 2020, Yeatman et al., 2012). Subsequently, the mean FA value within each segment was computed per participant, and group differences as well as correlations to NfL levels were then computed per segment of each bundle. An appropriate alpha-level (family-wise error rate (FWE) < 5 %) corrected for multiple comparisons across bundles and segments was obtained using permutation-based non-parametric tests computing the distribution of maximum statistics as described in Nichols & Holmes (2002). Group difference effects were tested using a two-sample t-test and correlation effects using Pearson’s correlation after covariates were regressed out of the data (i.e., a linear regression model was fitted including covariates as predictors and resulting residuals reflected denoised data [the group regressor is included when fitting the model but not in subsequent calculation of residuals]). Covariates included age, gender, handedness (left/right), and co-use of alcohol (amount during six months prior to study based on self-reports), cocaine, amphetamine, cannabis and ketamine (hair residuals reflecting use within four months prior to study). Mean FA values of MDMA users in areas where significant group differences were detected, were correlated with MDMA use intensity (self-reported number of use occasions within six months prior to study) using Pearson correlation and obtained p-values were adjusted for multiple comparisons (Bonferroni correction), as this analysis was repeated for all bundles containing areas of significant group differences. All analyses were performed in Python version 3.8 (Van Rossum and Drake, 2009) applying tools from TractSeg (Wasserthal et al., 2018) and Dipy (Garyfallidis et al., 2014).
2.5. Neurofilament light chain
Blood serum samples were drawn using silica and gel containing tubes (BD Vacutainer). Samples were centrifuged for 15 min at 2000 rpm and 20 °C and subsequently stored at −80 °C. NfL concentration was measured using simple-plex NfL assay (ProteinSimple, CA, USA) on Ella microfluidic system (BioTechne, Minneapolis, USA). To test for group differences regarding NfL concentrations, a linear regression model was applied (alpha level = 5 %). The model included covariates for age, gender, body mass index (BMI) as well as co-use of other substances (i.e., alcohol, cocaine, amphetamine, cannabis, and ketamine). Further, NfL concentrations were tested for correlations with FA values. On the one hand, this correlation was calculated in an exploratory analysis for all segments and each tract to test for a general relationship between NfL and FA. On the other hand, the correlation was calculated only for each segment that showed significant group differences to test whether significant differences in FA were also reflected in the NfL values. Analysis was performed in R version 4.0.3 (R Core Team, 2021).
3. Results
3.1. Demographic and substance use characteristics
MDMA users were well-matched to the control group indicated by the absence of significant group differences regarding demographic variables (Table 1). MDMA was the most frequently used illicit drug in the user group, but cannabis, stimulants, and ketamine were also occasionally taken by some users.
Table 1.
Demographics and substance use of chronic MDMA users and control group.
| Controls (n = 39) | MDMA Users (n = 39) | statistic | p | |
|---|---|---|---|---|
| Age, years | 30.08 (6.74) | 29.92 (6.74) | 0.10 | 0.92 |
| Sex (female / male) | 23/16 | 22/17 | 0.00 | 1.00 |
| Years of school education | 10.03 (1.37) | 10.41 (1.46) | 1.20 | 0.23 |
| Verbal Intelligence | 105.03 (10.62) | 102.87 (10.44) | 0.90 | 0.37 |
| ADHD total score | 10.67 (7.03) | 14.54 (10.51) | 1.91 | 0.060 |
| CESD total score | 10.69 (8.33) | 12.85 (11.58) | 0.94 | 0.35 |
| MDMA | ||||
| Consumed last 6 months (y/n) | 0/39 | 39/0 | 74.05 | <0.001 |
| Dose per week [mg]a | 0.00 (0.00) | 103.05 (137.58) | 4.68 | <0.001 |
| Occasions per week a | 0.00 (0.00) | 0.36 (0.29) | 7.66 | <0.001 |
| Years of use | 0.00 (0.00) | 9.27 (6.14) | 9.44 | <0.001 |
| Cumulative dose lifetime [g] | 0.02 (0.10) | 95.02 (156.42) | 3.79 | <0.001 |
| Cumulative dose lifetime [tablets]b | 0.12 (0.64) | 633.46 (1042.83) | – | – |
| Days since last consumption c | – | 22.56 (18.54), n = 39 | – | – |
| Positive urine testing (y/n) | 0/39 | 0/39 | – | – |
| Hair analysis [pg/mg] d | 0.00 (0.00) | 773.00 (892.53) | 2.32 | 0.023 |
| Nicotine | ||||
| Consumed last 6 months (y/n) | 22/17 | 26/13 | 0.49 | 0.49 |
| Cigarettes per day | 3.09 (5.42) | 4.56 (6.45) | 1.09 | 0.28 |
| Years of use | 7.46 (7.68) | 10.15 (8.83) | 1.44 | 0.15 |
| Alcohol | ||||
| Consumed last 6 months (y/n) | 34/5 | 39/0 | 3.42 | 0.064 |
| Dose per week [g] a | 0.09 (0.13) | 0.15 (0.18) | 1.67 | 0.099 |
| Occasions per week a | 2.12 (1.72) | 2.21 (1.83) | 0.23 | 0.82 |
| Years of use | 12.59 (7.43) | 13.98 (7.13) | 0.84 | 0.40 |
| Cumulative dose lifetime [kg] | 98.20 (140.60) | 149.54 (208.45) | 1.28 | 0.21 |
| Days since last consumption c | 8.03 (24.58), n = 34 | 14.21 (29.73), n = 39 | 0.96 | 0.34 |
| Cannabis | ||||
| Consumed last 6 months (y/n) | 9/30 | 23/16 | 8.96 | 0.003 |
| Dose per week [g] a | 0.05 (0.14) | 0.28 (0.76) | 1.86 | 0.067 |
| Occasions per week a | 0.23 (0.72) | 0.64 (1.37) | 1.66 | 0.10 |
| Years of use | 4.90 (6.79) | 9.26 (7.46) | 2.70 | 0.009 |
| Cumulative dose lifetime [g] | 233.33 (620.40) | 818.42 (1277.29) | 2.57 | 0.012 |
| Days since last consumption c | 34.11 (58.06), n = 9 | 25.00 (30.92), n = 23 | 0.58 | 0.57 |
| Positive urine testing (y/n) | 2/37 | 1/38 | 0.00 | 1.00 |
| Hair analysis [pg/mg] d | 0.00 (0.00) | 0.00 (0.00) | 2.47 | 0.016 |
| Amphetamine | ||||
| Consumed last 6 months (y/n) | 0/39 | 21/18 | 26.07 | <0.001 |
| Dose per week [g] a | 0.00 (0.00) | 0.04 (0.09) | 2.69 | 0.009 |
| Occasions per week a | 0.00 (0.00) | 0.13 (0.26) | 3.08 | 0.003 |
| Years of use | 0.00 (0.00) | 4.84 (7.08) | 4.27 | <0.001 |
| Cumulative dose lifetime [g] | 0.01 (0.04) | 115.27 (361.44) | 1.99 | 0.050 |
| Days since last consumption c | – | 39.14 (45.18), n = 21 | – | – |
| Positive urine testing (y/n) | 0/39 | 0/39 | – | – |
| Hair analysis [pg/mg] d | 0.00 (0.00) | 18.00 (26.69) | 2.53 | 0.013 |
| Cocaine | ||||
| Consumed last 6 months (y/n) | 3/36 | 26/13 | 26.57 | <0.001 |
| Dose per week [g] a | 0.00 (0.00) | 0.10 (0.21) | 2.79 | 0.007 |
| Occasions per week a | 0.00 (0.01) | 0.20 (0.43) | 2.84 | 0.006 |
| Years of use | 0.00 (0.00) | 4.33 (6.28) | 4.31 | <0.001 |
| Cumulative dose lifetime [g] | 0.03 (0.10) | 41.97 (89.61) | 2.92 | 0.005 |
| Days since last consumption c | 56.33 (34.24), n = 3 | 60.65 (61.46), n = 26 | 0.12 | 0.91 |
| Positive urine testing (y/n) | 0/39 | 1/38 | 0.00 | 1.00 |
| Hair analysis [pg/mg] d | 0.00 (0.00) | 167.00 (247.59) | 1.18 | 0.24 |
| Ketamine | ||||
| Consumed last 6 months (y/n) | 0/39 | 14/25 | 14.71 | <0.001 |
| Dose per week [g] a | 0.00 (0.00) | 0.01 (0.03) | 2.22 | 0.029 |
| Occasions per week a | 0.00 (0.00) | 0.05 (0.13) | 2.21 | 0.030 |
| Years of use | 0.00 (0.00) | 0.20 (0.75) | 1.69 | 0.096 |
| Cumulative dose lifetime [g] | 0.00 (0.00) | 1.97 (5.25) | 2.35 | 0.022 |
| Days since last consumption c | – | 60.14 (60.50), n = 14 | – | – |
| Hair analysis [pg/mg] d | 0.00 (0.00) | 0.00 (0.00) | 2.39 | 0.019 |
Values reflect mean and standard deviations, except that for hair residuals the median and median absolute deviation are shown due to a highly right-skewed distribution.
Statistical tests: independent t-tests for quantitative data, chi-squared test for frequency data; significant p-values are shown in bold.
within the past 6 months. In this case, sample size (n) is shown.
During the last 6 months.
In 150 mg tablets.
Days since last consumption is an average including only persons who reported to have used the substance.
Hair residuals reflect sum score of respective substance and its metabolites as quantified via forensic hair toxicology.
3.2. Diffusion tensor imaging
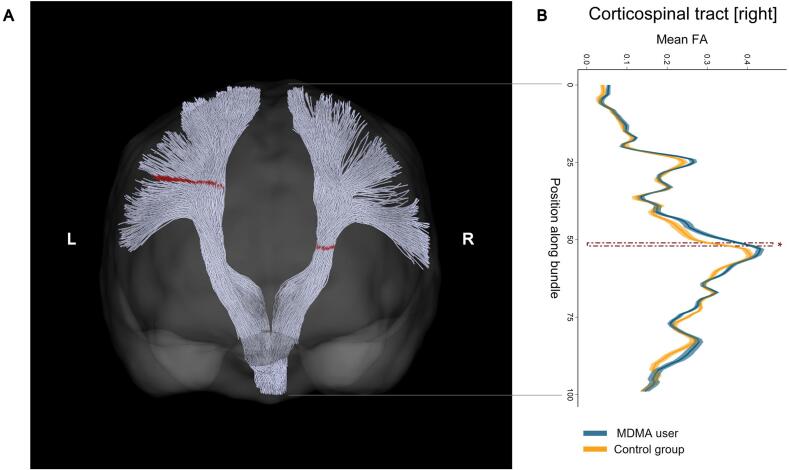
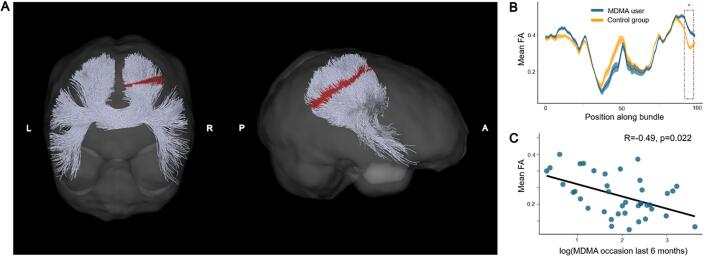
Chronic MDMA users displayed increased FA levels in several reconstructed white matter tracts (see Table 2 for a list of all white matter tracts showing significant group differences). In contrast, no region was detected with decreased FA in MDMA users compared to MDMA-naïve controls. Increased FA levels in MDMA users were found in the left and right corticospinal tract (see Fig. 1). Moreover, other significant areas encompassed several segments of the corpus callosum, specifically along the midline of the genu and anterior midbody, as well as distal areas of the posterior midbody and isthmus (see Fig. 2). The isthmus of the corpus callosum showed the most pronounced group difference and mean FA values of MDMA users within significant segments correlated negatively with MDMA use intensity within the user group, indicated by number of use occasions within six months prior to study (r[37] = -0.49, Bonferroni-corrected p = 0.018, see Fig. 2C). Hence, while MDMA users generally displayed elevated FA levels, this was most pronounced in weak-to-moderate users.
Table 2.
All white matter tracts showing increased FA levels in MDMA users.
| White matter tract | Significant segments1 | FWE-corrected p-value2 |
|---|---|---|
| Corpus callosum [Genu] | 63 [midline] | 0.032 |
| Corpus callosum [anterior midbody] | 43–44 [right of midline] | 0.001 |
| Corpus callosum [posterior midbody] | 13–14, 84 [left and right distal] | 0.030 |
| Corpus callosum [Isthmus] | 92–97 [right distal] | <0.0001 |
| Corticospinal tract [left] | 28 [distal superior] | 0.029 |
| Corticospinal tract [right] | 51 [middle] | 0.032 |
| Inf. occipito-frontal fascicle [left] | 23–25 [distal frontal] | 0.003 |
| Sup. longitudinal fascicle I [left] | 50 [middle] | 0.041 |
| Uncinate fascicle [right] | 62–63 [middle] | 0.038 |
| Thalamo-parietal tract [right] | 54 [middle] | 0.049 |
| Striato-fronto-orbital tract [left] | 45 [middle] | 0.044 |
Numbers reflect segment numbers within tracts, which were all segmented into 100 segments. Thus, number reflects left to right, superior to inferior, or frontal to posterior position along tract depending on their orientation in the brain. In brackets, the relative location of the significant area within the tract is indicated.
In case of multiple significant segments, the minimal p-value is shown.
Fig. 1.
Increased levels of fractional anisotropy (FA) in the left and right corticospinal tract of chronic MDMA users compared to MDMA-naïve control participants. (A) Posterior coronal view of left and right corticospinal tract of an exemplary control participant reconstructed using automatic white matter tract segmentation implemented in TractSeg. Segments in the corticospinal tract depicted in red indicate where MDMA users showed increased FA values after correction for multiple comparison (FWE < 5 %). (B) Mean FA values per segment and respective standard error along the right corticospinal tract are displayed separately for MDMA users (blue) and control participants (yellow). The area marked with a red dashed line corresponds to the significant area also colored in red in (A). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Increased levels of fractional anisotropy (FA) in the isthmus of the corpus callosum of chronic MDMA users compared to MDMA-naïve control participants. (A) Posterior coronal and right sagittal view of the isthmus. The isthmus is located between the body and splenium in the posterior part of the corpus callosum. Segments in the isthmus depicted in red indicate where MDMA users showed increased FA values after correction for multiple comparison (FWE < 5 %). (B) Mean FA values per segment and respective standard error along the isthmus are displayed separately for MDMA users (blue) and control participants (yellow). The area marked with a red dashed line corresponds to the significant area also colored red in (A). (C) Mean FA values of MDMA users in significant areas are negatively correlated with the number of MDMA use occasions during the six months prior to the study (Bonferroni corrected for multiple comparisons), suggesting that although MDMA users generally had elevated FA levels, this effect was most pronounced among weak-to-moderate users. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Neurofilament light chain
Regarding the blood concentrations of NfL, no group difference was observed between MDMA users and the control group (β = -0.56, t(72) = -0.25, p = 0.80; see Fig. 3A for boxplots per group and Supplement for complete regression table). Further, no association between MDMA use frequency and NfL was found (r[40] = -0.18, p = 0.26, see Fig. 3B). In an exploratory analysis correlating NfL with FA in all segments of each tract, no significant correlation survived correction by multiple comparisons (FWE–corrected alpha level: 0.000038; min. p-value: 0.0013; max. Pearson’s correlation coefficient: 0.36). Additionally, to test whether the increased FA levels in significant areas were reflected in NfL levels, mean FA values within these regions were correlated to the latter, but no significant relation was observed (all tests p greater than 0.05, see Supplement Table S2 for detailed results).
Fig. 3.
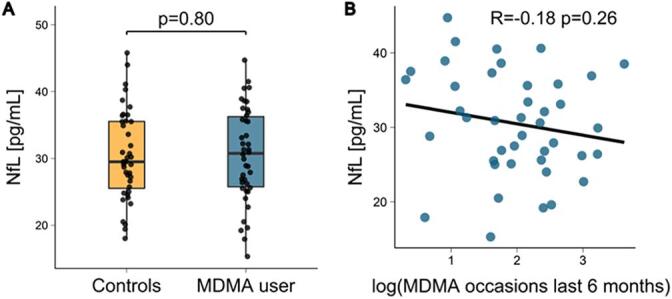
Neurofilament light chain (NfL) levels in chronic MDMA users. (A) No group difference was observed between chronic MDMA users and the control group using a linear regression model including covariates for age, gender, body mass index (BMI) and co-use of other substances. (B) Among MDMA users, NfL concentration was not related to MDMA use intensity indicated by the number of use occasions during the last 6 months prior to study.
4. Discussion
The translation of MDMA-induced axonal degradation findings from animal models to humans has been challenging and previous investigations of white matter impairments in chronic MDMA users were inconclusive (de Win et al., 2008a, de Win et al., 2008b, Liu et al., 2011, Moeller et al., 2007). In this study, we overcame methodological limitations of prior DTI studies in MDMA users by adhering to the guidelines Hampton et al. (2019) proposed. (1) We used a DTI sequence on a 3 T scanner measuring 64 diffusion directions and applied advanced deep–learning based segmentation methods, enabling the detection of group differences along anatomically well-defined tracts. (2) We controlled for co-use of other substances, notably by using results from forensic hair toxicological analysis and, thus, did not have to rely on self-reports only, which are known to be prone to social desirability biases (Latkin et al., 2017). (3) We correlated observed effects to MDMA use intensity. Of note, such an analysis was lacking in previous studies. (4) We complemented the DTI analysis with the measurement of NfL, a molecular marker of active axonal neuropathology, which has not been assessed before in MDMA users.
Our findings are partly in line with the findings of Liu et al. (2011). They observed increased FA in the internal capsule, of which the corticospinal tract constitutes a large part, where we also found increased FA in MDMA users. In contrast, Liu and colleagues found decreased FA in the genu of the corpus callosum where we found increased FA instead. Moreover, our findings are conflicting with those of de Win et al. (2008a) showing reduced FA in frontoparietal white matter and Moeller et al., 2007, de Win et al., 2008b, who did not observe any difference in FA between MDMA users and drug-naïve controls. However, comparability of our study to previous findings is hampered by methodological differences. This is due, first, to the selection of anatomical regions of interest. The analysis of Moeller et al. (2007) was restricted to the corpus callosum and both studies from de Win and colleagues to the frontoparietal white matter. Liu et al. (2011), on the other hand, used a voxel-wise whole brain approach but did not limit their analysis to white matter only. Here, we used a whole-brain approach as no clear a–priori regions of interest could be drawn from the existing literature. Second, because of the anatomical resolution of the analysis, De Win and colleagues computed only one mean FA value over frontoparietal white matter in both hemispheres. Hence, the exact anatomical location of the observed effect remains elusive. Moeller and colleagues computed mean FA in six segments of the corpus callosum, but only included midline parts of the corpus callosum and not its fiber projections. In contrast, in our study we partitioned each white matter tract into 100 segments and hence results obtained allowed for a more fine-grained localization of white matter alterations.
Against our a-priori hypothesis, we did not observe reduced, but rather increased FA in several white matter tracts of chronic MDMA users. Further, this increase was partly negatively associated with use frequency. Although a significant correlation was only observed in the isthmus of the corpus callosum, notably, this area showed the most pronounced group difference effect. The negative correlation indicates that FA decreases with increasing use frequency and thus, might indicate a dose-dependent effect of MDMA use on FA. This finding may be akin to those from a study on alcohol use, which observed an inverted U-association between alcohol use and FA (McEvoy et al., 2018). Nevertheless, for MDMA, animal studies suggested a severe axonal loss (Commins et al., 1987, O’Hearn et al., 1988, Ricaurte et al., 1985, Ricaurte et al., 1988), which was not observed in this human study. Although increased FA in MDMA users might represent potentially abnormal axonal reorganization following axonal loss as observed in animals (Fischer et al., 1995), the results in animals were obtained by selectively staining 5-HT axons in histological slices and it is questionable to which extent we are currently able to detect such fine-grained alterations in DTI since 5-HT axons only constitute a minority of the total axons in the brain (Hornung, 2003, Hornung). As an alternative explanation for the increased FA found in MDMA users, it has been suggested that the white matter changes might be associated with MDMA-induced changes in cerebral blood flow (CBF). Chang et al. (2000) demonstrated that MDMA causes sub-acute reduced CBF in wide-spread cortical regions, observable 2–3 weeks after MDMA administration. However, since findings relating CBF in white matter to FA are currently controversial (Aslan et al., 2011, Giezendanner et al., 2016), and since no long-term changes in CBF were yet observed in MDMA users (see for meta-analysis Müller et al., 2019), it remains questionable whether sub–acute effects on CBF might be related to the increased FA found here in MDMA users.
Inference of biological alterations underlying FA changes is challenging and is particularly complicated by the presence of crossing fibers (Jeurissen et al., 2012, Jones et al., 2013). However, we found the most pronounced FA increase in the corticospinal tract as well as corpus callosum, where crossing fibers are rare (Jeurissen et al., 2012, Oouchi et al., 2007). Therefore, the observed FA increase in these tracts most likely reflects increased fiber density or myelination. This could indicate potentiation of these white matter pathways, as activity–dependent regulation of myelin is a critical factor in neuroplasticity, as has been shown in rodents, in which increased myelination underlying white matter plasticity was reflected in increased FA (Blumenfeld-Katzir et al., 2011, Sampaio-Baptista and Johansen-Berg, 2017). In the present study, a marked increase in FA has been found in the corticospinal tract and the parts of the corpus callosum that bilaterally connect the primary motor and sensory cortex (i.e., anterior and posterior midbody of corpus callosum). Sensorimotor areas are densely innervated by 5–HT axons (Hornung, 2010), and potentiation of associated white matter pathways may reflect enhanced activation of these regions by repeated 5-HT release induced by MDMA. This may be in line with recent findings observing enhanced functional connectivity between raphe nuclei and sensorimotor areas in this sample of human MDMA users (Zimmermann et al., under review). Alternatively, the proposed potentiation of these pathways may be related to altered sensorimotor processing in chronic MDMA users: (1) Potentiation of white matter tracts connected to sensory cortex might be associated with the increased cortical response to sensory stimuli observed in chronic MDMA users (Cowan et al., 2006, Croft et al., 2001). (2) Potentiation of white matter tracts connected to motor cortex might be related to the enhanced motor cortex activation found in MDMA users when performing a motor task (Karageorgiou et al., 2009). The first hypothesis could be tested in a future study on MDMA use examining cortical sensory evoked potentials and their relation to FA in sensory-related white matter tracts, while to examine the second hypothesis, motor cortex activity during a motor task could be correlated with FA in white matter tracts connected to motor cortex. However, ideally the neurobiological mechanism (e.g., extended myelination) underlying increased FA in MDMA users should first be verified in a post–mortem study.
In contrast to studies in cocaine and ketamine users (Bavato et al., 2022a, Liu et al., 2021), we did not observe increased NfL blood levels in MDMA users. However, substance use frequency in these studies were much higher than in our study and both ketamine (N-methyl-d-aspartate receptor antagonist) and cocaine (unselective monoamine transporter inhibitor) have very different mechanisms of action. The absence of elevated NfL levels in MDMA users is in sharp contrast with observed axonal damage in animal studies and, hence, questions the presence of active axonal pathology in MDMA users. On the one hand, the lack of increase of NfL levels could mean that MDMA-induced alterations of neuroaxonal structures are rather limited and/or regional (i.e., in serotonergic axons), and not sufficient to result in a significant increase of NfL levels in blood. On the other hand, NfL levels are coherent with DTI findings and could suggest that brain alterations in chronic MDMA users are due to neuroplastic changes but do not involve axonal damage and neurodegeneration. In this direction, the repeatedly observed memory impairments of MDMA users (Quednow et al., 2006, Wunderli et al., 2017) may stem from molecular alterations in the 5-HT system but not MDMA-induced neurotoxicity (i.e., via loss of neurons and their axons).
4.1. Limitations
A limitation of the present study, and a general challenge for substance use-related research, reflects the higher use of other substances such as cannabis and stimulants in the MDMA user group compared to the control group. Accordingly, the observed effects might be confounded by co-use of other substances than MDMA, although we aimed to rigorously control for co-use in all analyses. In addition, our conclusions are limited by the fact that FA is only an indirect measure of white matter structure and depends on several distinct factors such as myelination, axonal diameter, fiber density as well as membrane permeability (Beaulieu, 2002). Thus, interpretation of FA differences as particular microstructural changes, such as degree of myelination or axon density, without solid biological and theoretical foundations, are challenging (Jones et al., 2013). Interpretation of the underlying mechanism leading to increased FA in this study is further limited given that the analysis was based on FA, while other metrics such as fiber density, radial diffusivity, mean diffusivity and axial diffusivity were not included. However, we used FA as the main outcome variable as all previous studies using the deep learning-based white matter segmentation applied in this study were specifically based on FA.
4.2. Conclusion
In summary, we can nevertheless conclude, with the additional support of complementary NfL results, that – unlike stimulant and ketamine use – chronic MDMA use is not associated with severe white matter lesions but is associated with MDMA-induced changes in white matter diffusion, indicated by increased FA in several white tracts.
CRediT authorship contribution statement
Josua Zimmermann: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Nicole Friedli: Investigation, Formal analysis, Writing – review & editing. Francesco Bavato: Investigation, Writing – review & editing. Philipp Stämpfli: Formal analysis. Rebecca Coray: Investigation, Writing – review & editing. Markus R. Baumgartner: Investigation. Denis Grandgirard: Investigation, Funding acquisition. Stephen L. Leib: Investigation, Funding acquisition. Antje Opitz: Investigation. Erich Seifritz: Funding acquisition. Ann-Kathrin Stock: Writing – review & editing. Christian Beste: Funding acquisition, Writing – review & editing. David M. Cole: Conceptualization, Investigation, Writing – original draft. Boris B. Quednow: Conceptualization, Methodology, Writing – original draft, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to thank Samia Borra, Mariana Carrillo Vázquez, Selina Caviezel, Marco Guglielmo, Xenia Häfeli, Mauro Mey, Anna–Katharina Schmid, and Babette Winter for assistance with recruitment and data acquisition.
Funding
This work was supported by grants of the Swiss National Science Foundation (320030L_179450 to B.B.Q and 189136 to S.L.L.) and by a grant of the Deutsche Forschungsgemeinschaft (BE4045/34-1 to C.B.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103191.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data is only available upon request due to ethical restrictions. The code used for complete analysis can be accessed at https://github.com/jziNeuro/DTI_MDMA_publication.
References
- Albertson D.N., Pruetz B., Schmidt C.J., Kuhn D.M., Kapatos G., Bannon M.J. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J. Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei Z., Pourhanifeh M.H., Borran S., Nejati M., Mirzaei H., Hamblin M.R. Neurofilament Light Chain as a Biomarker, and Correlation with Magnetic Resonance Imaging in Diagnosis of CNS-Related Disorders. Mol. Neurobiol. 2020;57:469–491. doi: 10.1007/s12035-019-01698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N.J., Leuzy A., Lim Y.M., Troakes C., Hortobágyi T., Höglund K., Aarsland D., Lovestone S., Schöll M., Blennow K., Zetterberg H., Hye A. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol. Commun. 2019;7:5. doi: 10.1186/s40478-018-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S., Huang H., Uh J., Mishra V., Xiao G., van Osch M.J.P., Lu H. White matter cerebral blood flow is inversely correlated with structural and functional connectivity in the human brain. NeuroImage. 2011;56:1145–1153. doi: 10.1016/j.neuroimage.2011.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavato, F., Kexel, A.K., Kluwe-Schiavon, B., Maceski, A., Baumgartner, M.R., Seifritz, E., Kuhle, J., Quednow, B.B., 2022a. A longitudinal investigation of blood neurofilament light chain levels in chronic cocaine users. [DOI] [PMC free article] [PubMed]
- Bavato F., Stamatakos S., Ohki C.M.Y., Seifritz E., Romualdi P., Grünblatt E., Quednow B.B. Brain-derived neurotrophic factor protects serotonergic neurons against 3,4-methylenedioxymethamphetamine (“Ecstasy”) induced cytoskeletal damage. J. Neural Transm. 2022;129(5-6):703–711. doi: 10.1007/s00702-022-02502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavato F., Stamatakos S., Ohki C.M.Y., Seifritz E., Romualdi P., Grünblatt E., Quednow B.B. Brain-derived neurotrophic factor protects serotonergic neurons against 3,4-methylenedioxymethamphetamine (“Ecstasy”) induced cytoskeletal damage. J. Neural Transm. 2022 doi: 10.1007/s00702-022-02502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bershad A.K., Miller M.A., Baggott M.J., de Wit H. The effects of MDMA on socio-emotional processing: Does MDMA differ from other stimulants? J. Psychopharmacol. (Oxf.) 2016;30:1248–1258. doi: 10.1177/0269881116663120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T., Pasternak O., Dagan M., Assaf Y., Tang Y.-P. Diffusion MRI of Structural Brain Plasticity Induced by a Learning and Memory Task. PLOS ONE. 2011;6(6):e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner A. Neuropathological Alterations in Cocaine Abuse. Curr. Med. Chem. 2012;19:5597–5600. doi: 10.2174/092986712803988947. [DOI] [PubMed] [Google Scholar]
- Chandio B.Q., Risacher S.L., Pestilli F., Bullock D., Yeh F.-C., Koudoro S., Rokem A., Harezlak J., Garyfallidis E. Bundle analytics, a computational framework for investigating the shapes and profiles of brain pathways across populations. Sci. Rep. 2020;10:17149. doi: 10.1038/s41598-020-74054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Grob C.S., Ernst T., Itti L., Mishkin F.S., Jose-Melchor R., Poland R.E. Effect of ecstasy [3,4-methylenedioxymethamphetamine (MDMA)] on cerebral blood flow: a co-registered SPECT and MRI study. Psychiatry Res. Neuroimaging. 2000;98:15–28. doi: 10.1016/S0925-4927(99)00048-7. [DOI] [PubMed] [Google Scholar]
- Cieslak M., Cook P.A., He X., Yeh F.-C., Dhollander T., Adebimpe A., Aguirre G.K., Bassett D.S., Betzel R.F., Bourque J., Cabral L.M., Davatzikos C., Detre J.A., Earl E., Elliott M.A., Fadnavis S., Fair D.A., Foran W., Fotiadis P., Garyfallidis E., Giesbrecht B., Gur R.C., Gur R.E., Kelz M.B., Keshavan A., Larsen B.S., Luna B., Mackey A.P., Milham M.P., Oathes D.J., Perrone A., Pines A.R., Roalf D.R., Richie-Halford A., Rokem A., Sydnor V.J., Tapera T.M., Tooley U.A., Vettel J.M., Yeatman J.D., Grafton S.T., Satterthwaite T.D. QSIPrep: an integrative platform for preprocessing and reconstructing diffusion MRI data. Nat. Methods. 2021;18:775–778. doi: 10.1038/s41592-021-01185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins D.L., Vosmer G., Virus R.M., Woolverton W.L., Schuster C.R., Seiden L.S. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J. Pharmacol. Exp. Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- Costa G., Gołembiowska K. Neurotoxicity of MDMA: Main effects and mechanisms. Exp. Neurol. 2022;347 doi: 10.1016/j.expneurol.2021.113894. [DOI] [PubMed] [Google Scholar]
- Cowan, R.L., Haga, E., Frederick, B. deB, Dietrich, M.S., Vimal, R.L.P., Lukas, S.E., Renshaw, P.F., 2006. MDMA use is associated with increased spatial BOLD fMRI visual cortex activation in human MDMA users. Pharmacol. Biochem. Behav. 84, 219–228. Doi: 10.1016/j.pbb.2006.04.024. [DOI] [PubMed]
- Croft R.J., Klugman A., Baldeweg T., Gruzelier J.H. Electrophysiological Evidence of Serotonergic Impairment in Long-Term MDMA (“Ecstasy”) Users. Am. J. Psychiatry. 2001;158:1687–1692. doi: 10.1176/appi.ajp.158.10.1687. [DOI] [PubMed] [Google Scholar]
- Curran H.V. Is MDMA ('Ecstasy’) neurotoxic in humans? An overview of evidence and of methodological problems in research. Neuropsychobiology. 2000;42:34–41. doi: 10.1159/000026668. [DOI] [PubMed] [Google Scholar]
- de la Torre R., Farré M. Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol. Sci. 2004;25:505–508. doi: 10.1016/j.tips.2004.08.001. [DOI] [PubMed] [Google Scholar]
- de Win M.M.L., Jager G., Booij J., Reneman L., Schilt T., Lavini C., Olabarriaga S.D., den Heeten G.J., van den Brink W. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain. 2008;131:2936–2945. doi: 10.1093/brain/awn255. [DOI] [PubMed] [Google Scholar]
- de Win M.M.L., Jager G., Booij J., Reneman L., Schilt T., Lavini C., Olabarriaga S.D., Ramsey N.F., den Heeten G.J., van den Brink W. Neurotoxic effects of ecstasy on the thalamus. Br. J. Psychiatry. 2008;193:289–296. doi: 10.1192/bjp.bp.106.035089. [DOI] [PubMed] [Google Scholar]
- Disanto G., Barro C., Benkert P., Naegelin Y., Schädelin S., Giardiello A., Zecca C., Blennow K., Zetterberg H., Leppert D., Kappos L., Gobbi C., Kuhle J. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017;81(6):857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, W.W., Smith, C., Ybarra, M., Muntaner, C., Tien, A., 2004. Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESD-R), in: The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults, Volume 3, 3rd Ed. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, US, pp. 363–377.
- Edward Roberts R., Curran H.V., Friston K.J., Morgan C.J.A. Abnormalities in White Matter Microstructure Associated with Chronic Ketamine Use. Neuropsychopharmacology. 2014;39:329–338. doi: 10.1038/npp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B. The Encyclopedia of Clinical Psychology. John Wiley & Sons Ltd; 2015. Structured Clinical Interview for the DSM (SCID) pp. 1–6. [DOI] [Google Scholar]
- Fischer C., Hatzidimitriou G., Wlos J., Katz J., Ricaurte G. Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/-)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) J. Neurosci. 1995;15:5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabrerizo R., García-Fuster M.J. Chronic MDMA induces neurochemical changes in the hippocampus of adolescent and young adult rats: Down-regulation of apoptotic markers. NeuroToxicology. 2015;49:104–113. doi: 10.1016/j.neuro.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Garyfallidis E., Brett M., Amirbekian B., Rokem A., Van Der Walt S., Descoteaux M., Nimmo-Smith I. Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinformatics. 2014;8 doi: 10.3389/fninf.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giezendanner S., Fisler M.S., Soravia L.M., Andreotti J., Walther S., Wiest R., Dierks T., Federspiel A., Rao H. Microstructure and Cerebral Blood Flow within White Matter of the Human Brain: A TBSS Analysis. PLOS ONE. 2016;11(3):e0150657. doi: 10.1371/journal.pone.0150657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen O., Gruber S., Li B.S.Y., Mlynárik V., Moser E. Multivoxel 3D Proton Spectroscopy in the Brain at 1.5 Versus 3.0 T: Signal-to-Noise Ratio and Resolution Comparison. Am. J. Neuroradiol. 2001;22:1727–1731. [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C., Madison C., Clark D., Halchenko Y., Waskom M., Ghosh S. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front. Neuroinformatics. 2011:5. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E., Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J. Psychopharmacol. (Oxf.) 2006;20:188–193. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- Green A.R., Mechan A.O., Elliott J.M., O’Shea E., Colado M.I. The Pharmacology and Clinical Pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) Pharmacol. Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gudelsky G.A., Yamamoto B.K. ACTIONS OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) ON CEREBRAL DOPAMINERIC, SEROTONERGIC AND CHOLINERGIC NEURONS. Pharmacol. Biochem. Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton W.H., Hanik I.M., Olson I.R. Substance abuse and white matter: Findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288–298. doi: 10.1016/j.drugalcdep.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzidimitriou G., McCann U.D., Ricaurte G.A. Altered Serotonin Innervation Patterns in the Forebrain of Monkeys Treated with (±)3,4-Methylenedioxymethamphetamine Seven Years Previously: Factors Influencing Abnormal Recovery. J. Neurosci. 1999;19:5096–5107. doi: 10.1523/JNEUROSCI.19-12-05096.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung, J.-P., 2010. CHAPTER 1.3 - The Neuronatomy of the Serotonergic System, in: Müller, C.P., Jacobs, B.L. (Eds.), Handbook of Behavioral Neuroscience, Handbook of the Behavioral Neurobiology of Serotonin. Elsevier, pp. 51–64. https://doi.org/10.1016/S1569-7339(10)70071-0.
- Hornung, J.-P., 2003. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat., Special Issue on the Human Brain - The Structural Basis for understanding Human Brain function and dysfunction 26, 331–343. Doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed]
- Huang S., Yang W., Luo J., Yan C., Liu J. White Matter Abnormalities Based on TBSS and Its Correlation With Impulsivity Behavior of Methamphetamine Addicts. Front. Psychiatry. 2020:11. doi: 10.3389/fpsyt.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagel I.C., van Ballegoij W.J.C., Vos J.M.B.W., Kemp S., Caan M.W.A., Engelen M. Longitudinal diffusion MRI as surrogate outcome measure for myelopathy in adrenoleukodystrophy. Neurology. 2019;93:e2133–e2143. doi: 10.1212/WNL.0000000000008572. [DOI] [PubMed] [Google Scholar]
- Hysek C.M., Simmler L.D., Ineichen M., Grouzmann E., Hoener M.C., Brenneisen R., Huwyler J., Liechti M.E. The Norepinephrine Transporter Inhibitor Reboxetine Reduces Stimulant Effects of MDMA (“Ecstasy”) in Humans. Clin. Pharmacol. Ther. 2011;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Hysek C.M., Simmler L.D., Nicola V.G., Vischer N., Donzelli M., Krähenbühl S., Grouzmann E., Huwyler J., Hoener M.C., Liechti M.E., Laks J. Duloxetine Inhibits Effects of MDMA (“Ecstasy“) In Vitro and in Humans in a Randomized Placebo-Controlled Laboratory Study. PLoS ONE. 2012;7(5):e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J., Jones D.K., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2012;34:2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study†. Magn. Reson. Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Karageorgiou J., Dietrich M.S., Charboneau E.J., Woodward N.D., Blackford J.U., Salomon R.M., Cowan R.L. Prior MDMA (Ecstasy) use is associated with increased basal ganglia–thalamocortical circuit activation during motor task performance in humans: An fMRI study. NeuroImage. 2009;46:817–826. doi: 10.1016/j.neuroimage.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M., Teunissen C.E., Otto M., Piehl F., Sormani M.P., Gattringer T., Barro C., Kappos L., Comabella M., Fazekas F., Petzold A., Blennow K., Zetterberg H., Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- Kish S.J. How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol. Biochem. Behav. 2002;71:845–855. doi: 10.1016/s0091-3057(01)00708-0. [DOI] [PubMed] [Google Scholar]
- Kish S.J., Furukawa Y., Ang L., Vorce S.P., Kalasinsky K.S. Striatal serotonin is depleted in brain of a human MDMA (Ecstasy) user. Neurology. 2000;55:294–296. doi: 10.1212/WNL.55.2.294. [DOI] [PubMed] [Google Scholar]
- Latkin C.A., Edwards C., Davey-Rothwell M.A., Tobin K.E. The relationship between social desirability bias and self-reports of health, substance use, and social network factors among urban substance users in Baltimore. Maryland. Addict. Behav. 2017;73:133–136. doi: 10.1016/j.addbeh.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S., Triebig G., Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol. Scand. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Liechti M.E., Baumann C., Gamma A., Vollenweider F.X. Acute Psychological Effects of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) are Attenuated by the Serotonin Uptake Inhibitor Citalopram. Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Liu Y.-L., Bavato F., Chung A.-N., Liu T.-H., Chen Y.-L., Huang M.-C., Quednow B.B. Neurofilament light chain as novel blood biomarker of disturbed neuroaxonal integrity in patients with ketamine dependence. World J. Biol. Psychiatry. 2021;22:713–721. doi: 10.1080/15622975.2021.1907709. [DOI] [PubMed] [Google Scholar]
- Liu H.-S., Chou M.-C., Chung H.-W., Cho N.-Y., Chiang S.-W., Wang C.-Y., Kao H.-W., Huang G.-S., Chen C.-Y. Potential Long-term Effects of MDMA on the Basal Ganglia-Thalamocortical Circuit: A Proton MR Spectroscopy and Diffusion-Tensor Imaging Study. Radiology. 2011;260:531–540. doi: 10.1148/radiol.11101918. [DOI] [PubMed] [Google Scholar]
- Ma L., Steinberg J.L., Wang Q., Schmitz J.M., Boone E.L., Narayana P.A., Moeller F.G. A preliminary longitudinal study of white matter alteration in cocaine use disorder subjects. Drug Alcohol Depend. 2017;173:39–46. doi: 10.1016/j.drugalcdep.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann U.D., Ridenour A., Shaham Y., Ricaurte G.A. Serotonin Neurotoxicity after (±)3,4-Methylenedioxymethamphetamine (MDMA; “Ecstasy”): A Controlled Study in Humans. Neuropsychopharmacology. 1994;10:129–138. doi: 10.1038/npp.1994.15. [DOI] [PubMed] [Google Scholar]
- McCann U.D., Mertl M., Eligulashvili V., Ricaurte G.A. Cognitive performance in (±) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology (Berl.) 1999;143:417–425. doi: 10.1007/s002130050967. [DOI] [PubMed] [Google Scholar]
- McEvoy L.K., Fennema-Notestine C., Elman J.A., Eyler L.T., Franz C.E., Hagler D.J., Hatton S.N., Lyons M.J., Panizzon M.S., Dale A.M., Kremen W.S. Alcohol intake and brain white matter in middle aged men: Microscopic and macroscopic differences. NeuroImage Clin. 2018;18:390–398. doi: 10.1016/j.nicl.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L., Moisa M., Stämpfli P., Hirsiger S., Baumgartner M.R., Surbeck W., Seifritz E., Quednow B.B. The impact of levamisole and alcohol on white matter microstructure in adult chronic cocaine users. Addict. Biol. 2022;27:e13149. doi: 10.1111/adb.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F.G., Steinberg J.L., Lane S.D., Buzby M., Swann A.C., Hasan K.M., Kramer L.A., Narayana P.A. Diffusion Tensor Imaging in MDMA Users and Controls: Association with Decision Making. Am. J. Drug Alcohol Abuse. 2007;33:777–789. doi: 10.1080/00952990701651564. [DOI] [PubMed] [Google Scholar]
- Møller M., Frandsen J., Andersen G., Gjedde A., Vestergaard-Poulsen P., Østergaard L. Dynamic changes in corticospinal tracts after stroke detected by fibretracking. J. Neurol. Neurosurg. Psychiatry. 2007;78:587–592. doi: 10.1136/jnnp.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery C., Roberts C.A. Neurological and cognitive alterations induced by MDMA in humans. Exp. Neurol. 2022;347 doi: 10.1016/j.expneurol.2021.113888. [DOI] [PubMed] [Google Scholar]
- Müller F., Brändle R., Liechti M.E., Borgwardt S. Neuroimaging of chronic MDMA (“ecstasy”) effects: A meta-analysis. Neurosci. Biobehav. Rev. 2019;96:10–20. doi: 10.1016/j.neubiorev.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Mustafa N.S., Bakar N.H.A., Mohamad N., Adnan L.H.M., Fauzi N.F.A.M., Thoarlim A., Omar S.H.S., Hamzah M.S., Yusoff Z., Jufri M., Ahmad R. MDMA and the Brain: A Short Review on the Role of Neurotransmitters in Neurotoxicity. Basic Clin. Neurosci. 2020;11:381–388. doi: 10.32598/bcn.9.10.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana P.A., Herrera J.J., Bockhorst K.H., Esparza-Coss E., Xia Y., Steinberg J.L., Moeller F.G. Chronic cocaine administration causes extensive white matter damage in brain: Diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. Neuroimaging. 2014;221:220–230. doi: 10.1016/j.pscychresns.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hearn E., Battaglia G., Souza E.D., Kuhar M.J., Molliver M.E. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J. Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oouchi H., Yamada K., Sakai K., Kizu O., Kubota T., Ito H., Nishimura T. Diffusion Anisotropy Measurement of Brain White Matter Is Affected by Voxel Size: Underestimation Occurs in Areas with Crossing Fibers. Am. J. Neuroradiol. 2007;28:1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottino-González J., Uhlmann A., Hahn S., Cao Z., Cupertino R.B., Schwab N., Allgaier N., Alia-Klein N., Ekhtiari H., Fouche J.-P., Goldstein R.Z., Li C.-S.-R., Lochner C., London E.D., Luijten M., Masjoodi S., Momenan R., Oghabian M.A., Roos A., Stein D.J., Stein E.A., Veltman D.J., Verdejo-García A., Zhang S., Zhao M., Zhong N., Jahanshad N., Thompson P.M., Conrod P., Mackey S., Garavan H. White matter microstructure differences in individuals with dependence on cocaine, methamphetamine, and nicotine: Findings from the ENIGMA-Addiction working group. Drug Alcohol Depend. 2022;230 doi: 10.1016/j.drugalcdep.2021.109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott A.C. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum. Psychopharmacol. Clin. Exp. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Quednow B.B., Kühn K.-U., Hoenig K., Maier W., Wagner M. Prepulse Inhibition and Habituation of Acoustic Startle Response in Male MDMA (‘Ecstasy’) Users, Cannabis Users, and Healthy Controls. Neuropsychopharmacology. 2004;29:982–990. doi: 10.1038/sj.npp.1300396. [DOI] [PubMed] [Google Scholar]
- Quednow B.B., Jessen F., Kühn K.-U., Maier W., Daum I., Wagner M. Memory deficits in abstinent MDMA (ecstasy) users: neuropsychological evidence of frontal dysfunction. J. Psychopharmacol. (Oxf.) 2006;20:373–384. doi: 10.1177/0269881106061200. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Ricaurte G., Bryan G., Strauss L., Seiden L., Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985;229:986–988. doi: 10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- Ricaurte G.A., Forno L.S., Wilson M.A., DeLanney L.E., Irwin I., Molliver M.E., Langston J.W. (±3,4-Methylenedioxymethamphetamine Selectively Damages Central Serotonergic Neurons in Nonhuman Primates. JAMA. 1988;260:51–55. doi: 10.1001/jama.1988.03410010059035. [DOI] [PubMed] [Google Scholar]
- Roberts C.A., Jones A., Montgomery C. Meta-analysis of molecular imaging of serotonin transporters in ecstasy/polydrug users. Neurosci. Biobehav. Rev. 2016;63:158–167. doi: 10.1016/j.neubiorev.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Roberts C.A., Quednow B.B., Montgomery C., Parrott A.C. MDMA and brain activity during neurocognitive performance: An overview of neuroimaging studies with abstinent ‘Ecstasy’ users. Neurosci. Biobehav. Rev. 2018;84:470–482. doi: 10.1016/j.neubiorev.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Rösler M., Retz W., Retz-Junginger P., Thome J., Supprian T., Nissen T., Stieglitz R.-D., Blocher D., Hengesch G., Trott G.E. Instrumente zur Diagnostik der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS) im Erwachsenenalter. Nervenarzt. 2004;75:888–895. doi: 10.1007/s00115-003-1622-2. [DOI] [PubMed] [Google Scholar]
- Sampaio-Baptista C., Johansen-Berg H. White Matter Plasticity in the Adult Brain. Neuron. 2017;96:1239–1251. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz C., Cabalzar J., Kraemer T., Baumgartner M.R. A Comprehensive Multi-Analyte Method for Hair Analysis: Substance-Specific Quantification Ranges and Tool for Task-Oriented Data Evaluation. J. Anal. Toxicol. 2021;45:701–712. doi: 10.1093/jat/bkaa131. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., Kalu U.G., Filippini N., Mackay C.E., Ebmeier K.P. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging. 2011;32:2322.e5–2322.e18. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Strous, J.F.M., Weeland, C.J., van der Draai, F.A., Daams, J.G., Denys, D., Lok, A., Schoevers, R.A., Figee, M., 2022. Brain Changes Associated With Long-Term Ketamine Abuse, A Systematic Review. Front. Neuroanat. 16. [DOI] [PMC free article] [PubMed]
- United Nations Office on Drugs and Crime, 2022. World Drug Report 2021, 2021st ed. United Nations.
- Van Rossum G., Drake F.L. CreateSpace; Scotts Valley, CA: 2009. Python 3 Reference Manual. [Google Scholar]
- van Son D., Wiers R.W., Catena A., Perez-Garcia M., Verdejo-García A. White matter disruptions in male cocaine polysubstance users: Associations with severity of drug use and duration of abstinence. Drug Alcohol Depend. 2016;168:247–254. doi: 10.1016/j.drugalcdep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Wasserthal J., Neher P., Maier-Hein K.H. TractSeg - Fast and accurate white matter tract segmentation. NeuroImage. 2018;183:239–253. doi: 10.1016/j.neuroimage.2018.07.070. [DOI] [PubMed] [Google Scholar]
- Wasserthal J., Maier-Hein K.H., Neher P.F., Northoff G., Kubera K.M., Fritze S., Harneit A., Geiger L.S., Tost H., Wolf R.C., Hirjak D. Multiparametric mapping of white matter microstructure in catatonia. Neuropsychopharmacology. 2020;45:1750–1757. doi: 10.1038/s41386-020-0691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserthal J., Maier-Hein K.H., Neher P.F., Wolf R.C., Northoff G., Waddington J.L., Kubera K.M., Fritze S., Harneit A., Geiger L.S., Tost H., Hirjak D. White matter microstructure alterations in cortico-striatal networks are associated with parkinsonism in schizophrenia spectrum disorders. Eur. Neuropsychopharmacol. 2021;50:64–74. doi: 10.1016/j.euroneuro.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Welton T., Kent D., Constantinescu C.S., Auer D.P., Dineen R.A. Functionally Relevant White Matter Degradation in Multiple Sclerosis: A Tract-based Spatial Meta-Analysis. Radiology. 2015;275:89–96. doi: 10.1148/radiol.14140925. [DOI] [PubMed] [Google Scholar]
- Wunderli M.D., Vonmoos M., Fürst M., Schädelin K., Kraemer T., Baumgartner M.R., Seifritz E., Quednow B.B. Discrete memory impairments in largely pure chronic users of MDMA. Eur. Neuropsychopharmacol. 2017;27:987–999. doi: 10.1016/j.euroneuro.2017.08.425. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M., Beaulieu C. Tract Profiles of White Matter Properties: Automating Fiber-Tract Quantification. PLOS ONE. 2012;7(11):e49790. doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A. Moderating registration misalignment in voxelwise comparisons of DTI data: a performance evaluation of skeleton projection. Magn. Reson. Imaging. 2011;29:111–125. doi: 10.1016/j.mri.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Zimmermann, J., Friedli, N., Coray, C., Bavato, F., Baumgartner, M.R., Opitz, A., Seifritz, E., Stock, A., Beste, C., Cole, D.M., Quednow, B.B., under review, Short- and long-term neuroplasticity in the human serotonin system assessed via raphe nuclei functional connectivity.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is only available upon request due to ethical restrictions. The code used for complete analysis can be accessed at https://github.com/jziNeuro/DTI_MDMA_publication.