ABSTRACT
Although linezolid is effective for multidrug-resistant TB (MDR-TB) tuberculosis treatment, it is associated with cytopenias after 4 weeks of administration. Data on toxicities with long-term use of linezolid and drug pharmacodynamics in MDR-TB treatment are limited, and concerns about toxicity present barriers to wider implementation. This was a secondary analysis of a prospective cohort study of patients treated for MDR-TB in the country of Georgia from 2015 to 2017. Intensive blood sampling 4 to 6 weeks after treatment initiation with linezolid 600 mg daily was performed for pharmacokinetic (PK) analysis, including linezolid trough concentration (Cmin) and area under the curve from 0 to 24 hours (AUC0–24). Linezolid exposure was defined using literature-reported thresholds. Cytopenias were defined using an NIH adverse event (AE) scale. Logistic regression was used to evaluate the relationship between linezolid exposure and cytopenias. Among 76 patients receiving linezolid in their baseline treatment regimen and who had PK data available, cytopenia AEs occurred in 30 (39.5%) for an incidence rate of 46 per 100 person-years. The median duration of linezolid therapy was 526 days. No patients required dose reduction or interruption due to cytopenias. Median linezolid Cmin was 0.235 mg/L (interquartile range [IQR], 0.069 to 0.529), and median AUC0–24 was 89.6 mg·h/L (IQR, 69.2 to 116.2). Cytopenias were associated with linezolid PK parameters (Cmin > 2 mg/L and AUC0–24 > 160 mg·h/L). Cytopenias occurred frequently with long-term use of linezolid 600 mg/day and were associated with PK parameters but did not result in the need for treatment interruption in the management of a cohort of patients with MDR-TB.
KEYWORDS: Mycobacterium tuberculosis, adverse drug effects, cytopenia, linezolid
INTRODUCTION
Tuberculosis (TB) remains a major global health problem, with an estimated 10 million incident cases of TB and 1.5 million deaths due to TB in 2020 and increasing global mortality for the first time in over 20 years (1). Multidrug-resistant TB (MDR-TB) is a major challenge to global TB control efforts and to ending the TB epidemic (1, 2). The development of novel anti-TB drugs and repurposing of existing drugs, such as linezolid, to treat MDR-TB have improved outcomes and shortened treatment regimens from up to 2 years to 6 to 12 months (3–6). Understanding how to best use these new and repurposed drugs is essential for safe and effective treatment of MDR-TB.
Linezolid is an oxazolidinone antibiotic with activity against Mycobacterium tuberculosis that is now included in the WHO DR-TB treatment guidelines as a “group A” drug for MDR-TB treatment to be included in all regimens unless contraindicated (7, 8). However, these guidelines still advise caution with prolonged use of linezolid, as therapy >4 weeks has been associated with adverse effects such as cytopenias and neuropathy (7). Development of cytopenias is related to total linezolid exposure in both a time- and dose-dependent fashion, though the mechanism is not well-defined and the reported duration of linezolid therapy preceding the development of cytopenias is variable and as short as 5 to 14 days (7, 9, 10). While the current recommended treatment duration for MDR-TB is 6 to 18 months, data on long-term use of linezolid and development of toxicities remain limited, particularly in the treatment of TB (11). Recent studies suggest that linezolid-associated adverse events (AEs) occur frequently in patients treated for TB, with 48% of patients in the Nix-TB clinical trial developing cytopenias and 85% requiring linezolid interruption or dose reduction due to an AE (5). Notably, linezolid was dosed at 1,200 mg/day in this trial, while the standard dose per WHO guidelines and used in programmatic settings via national TB programs (NTP) is 600 mg/day (8). To address the safety of this standard dose, we previously conducted a retrospective analysis of patients treated for MDR-TB in the country of Georgia, and it suggested that linezolid dosed at 600 mg/day is tolerable (12). Similar to other countries in the region, Georgia has one of the highest rates of MDR-TB in the world. In 2015, the Georgian NTP implemented new and repurposed drugs, including linezolid, into routine programmatic use for the treatment of MDR-TB (1, 13). Pharmacokinetic/pharmacodynamic data may complement prior clinical data to optimize linezolid use for TB (11).
Pharmacokinetic (PK) parameters that have been proposed as informing linezolid safety include the trough or minimum serum concentration (Cmin) and area under the curve (AUC). While PK parameters for efficacy in TB treatment are not yet defined, a Cmin threshold of >2 mg/L for linezolid efficacy in Gram-positive infections in humans has been proposed based on available clinical data (14, 15). However, values above 2 mg/L have also been associated with mitochondrial protein synthesis toxicity, which is a potential mechanism for cytopenias (16–18). Pharmacologic data for linezolid in the treatment of MDR-TB remain limited. PK modeling utilizing patient data from several settings, including Georgia, suggests that the 600-mg/day dose may maximize efficacy while minimizing toxicity compared to 900-mg or 1,200-mg daily dosing, assuming a low MIC (19). The goal of the present study was to incorporate PK data with clinical outcome data from an existing cohort of patients treated for MDR-TB in Georgia to assess the development of cytopenias with long-term use of linezolid to support safe and effective treatment of MDR-TB.
RESULTS
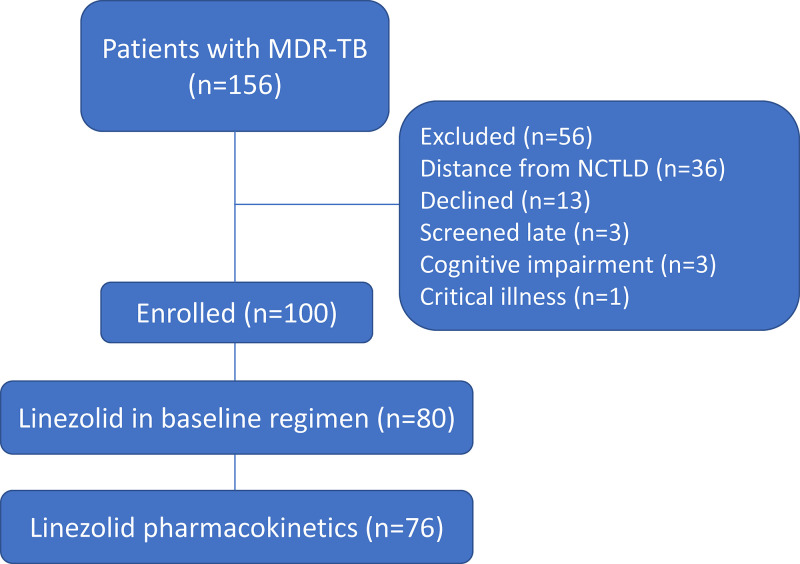
Among a total of 156 patients initiating therapy for MDR-TB, 100 patients provided written informed consent and were enrolled in the study (Fig. 1). Clinical characteristics and outcomes for the parent cohort have been described; treatment outcomes were favorable in 72 (72%), with 91 (91%) achieving sputum culture conversion (20). Linezolid was included in the initial regimen for 80 patients (at a dose of 600 mg daily); 76 had blood drawn for PK evaluation and were included in the primary analysis. The median age of this group was 36.8 years (interquartile range [IQR], 27.7 to 49.9), and most (77%) were male (Table 1). Tobacco use (48.7%) and alcohol use (30.3%) were common. Medical records indicated diabetes mellitus was present in 10 (13.2%) participants, anti-hepatitis C virus (HCV) antibodies in 19 (25.0%), and human immunodeficiency virus (HIV) infection in 2 (2.6%). Clinical outcomes for patients in the primary analysis were favorable overall, with 53/76 (69.7%) cured or completing treatment (Table 1). Patients received linezolid for a median of 526 days (IQR, 305 to 607). The median baseline complete blood count (CBC) values were within normal limits, with white blood cell (WBC) count of 9.4 × 103/μL (IQR, 7.5 to 11.0); hemoglobin, 12.5 g/dL (IQR, 11.3 to 14.0); and platelets, 373 × 103/μL (IQR, 306 to 489) (Table 1). Baseline values were within the AE range for anemia in 21 patients and for thrombocytopenia in 2 patients. The median number of follow-up CBC collections was 8 (IQR, 5 to 12). From baseline to the last recorded CBC value in follow-up, the mean change in hemoglobin was −1.2 g/dL (range, −5.1 to 1.7) and in platelets was 115 × 103/μL (range, −71 to 356). For PK parameters, the median linezolid Cmin was 0.235 mg/L (IQR, 0.069 to 0.529), median maximum serum concentration (Cmax) was 10.24 mg/L (IQR, 7.77 to 12.62), and median AUC from 0 to 24 h (AUC0–24) was 89.6 mg·h/L (IQR, 69.2 to 116.2). Among the 76 patients, 7 (9.2%) had linezolid Cmin above the 2-mg/L efficacy/mitochondrial toxicity threshold, and 0 were above the 7-mg/L thrombocytopenia threshold. For linezolid AUC0–24, 10 (13.2%) had values above the 160-mg·h/L efficacy threshold, and 1 (1.3%) had a value above the 280-mg·h/L toxicity threshold.
FIG 1.
Study population of patients with MDR-TB initiating treatment at the NCTLD from December 2015 to May 2017. NCTLD, National Center for Tuberculosis and Lung Diseases; MDR-TB, multidrug-resistant TB; TB, tuberculosis.
TABLE 1.
Characteristics of patients (n = 76) with MDR-TB who were initiating treatment with linezolid-containing regimensa
| Characteristic | Value |
|---|---|
| Age (median [IQR] [yrs]) | 36.8 (27.7–49.9) |
| Body mass index (median [IQR] [kg/m2]) | 19.9 (18.4–22.4) |
| No. (%) male | 59 (77.6) |
| Any tobacco usee (no. [%]) | 37 (48.7) |
| More than 1 pack per day | 19 (25.0) |
| Any alcohol usee (no. [%]) | 23 (30.3) |
| Heavyb | 8 (10.5) |
| Diabetes mellitus (no. [%]) | 10 (13.2) |
| HIV infection (no. [%]) | 2 (2.6) |
| Hepatitis C antibody positive (no. [%]) | 19 (25.0) |
| Case definitions (no. [%]) | |
| New | 34 (44.7) |
| Relapse | 15 (19.7) |
| After loss to follow-up | 10 (13.2) |
| After failure | 10 (13.2) |
| Other | 7 (9.2) |
| Pulmonary disease only (no. [%]) | 75 (98.7) |
| Cavitary disease (no. [%]) | 47 (61.8) |
| AFB sputum smear positivec (no. [%]) | 54 (71.1) |
| Extensive drug resistance (no. [%]) | 16 (21.1) |
| Baseline laboratory values (median [IQR]) | |
| White blood cells (×103/μL) | 9.4 (7.5–11.0) |
| Hemoglobin (g/dL) | 12.5 (11.3–14.0) |
| Platelets (×103/μL) | 373 (306–488.5) |
| Creatinine (μmol/L) | 71 (61–84) |
| Treatment characteristics (median [IQR]) | |
| SLD treatment duration (days) | 609 (461–622) |
| New drugd treatment duration (days) | 532 (348–608) |
| Linezolid duration (days) | 526 (305–607) |
| Treatment duration prior to linezolid start (days) | 27.5 (0–112) |
| Linezolid stop prior to completion (no. [%]) | |
| Adverse event | 4 (5.3) |
| Poor outcome | 13 (17.1) |
| Clinical outcomes (no. [%]) | |
| Favorable outcome | 53 (69.7) |
| Any acquired drug resistance | 12 (16.2) |
MDR-TB, multidrug-resistant TB; TB, tuberculosis; PK, pharmacokinetic; IQR, interquartile range; HIV, human immunodeficiency virus; SLD, second-line drugs.
“Heavy” represents >15 drinks per week for men and >8 drinks per week for women.
At time of new drug initiation.
New anti-TB drugs include bedaquiline, linezolid, clofazimine, and delamanid.
Reported within 12 weeks of diagnosis.
Incident cytopenias.
Overall, 30 (39.5%) of 76 study participants developed a cytopenia AE (Table 2). An AE for individual CBC parameters occurred in one participant (1.3%) for leukopenia (white blood cells < 2.5 × 103/mm3), in 23 (30.3%) for anemia (hemoglobin < 11.0 g/dL), and in 11 (14.5%) for thrombocytopenia (platelets < 125 × 103/μL). Most of these were grade 1 (23, 76.7%) or grade 2 (11, 36.7%) AEs. There was one grade 3 AE for any cytopenia (thrombocytopenia < 50 × 103/μL) and no grade 4 AE for any parameter. The incidence rate of any cytopenia AE was 46 per 100 person-years, including 35 per 100 person-years for anemia and 16 per 100 person-years for thrombocytopenia. However, only four study participants stopped linezolid due to any AE, and these were not for cytopenias but for neuropathy (n = 2) and hepatotoxicity (n = 2). No participants required dose reduction. Incident cytopenias occurred at a median of 4 months (IQR, 3 to 7) for any cytopenia, 4 months (IQR, 3 to 6) for anemia, and 11 months (IQR, 5 to 13) for thrombocytopenia (see Fig. S1 in the supplemental material). Among the 23 participants with anemia, 22 had recorded resolution at a median of 2 months (range, 1 to 6 months). Among the 11 participants with thrombocytopenia, 9 had recorded resolution at a median of 1 month (range, 1 to 2 months). All cytopenias occurred during the linezolid treatment course.
TABLE 2.
Cytopenia adverse events among patients with MDR-TB initiating treatment with linezolid-containing regimensa
| Adverse event | Data (no. [%]) for |
Incidence rate of any event (per 100 person-yrs) | |||
|---|---|---|---|---|---|
| Any event (n = 76) | Grade 1 | Grade 2 | Grade 3 | ||
| Any adverse event | 30 (39.5) | 46 | |||
| Leukopenia | 1 (1.3) | 1 (1.3) | |||
| Anemia | 23 (30.3) | 14 (18.4) | 9 (11.8) | 35 | |
| Thrombocytopenia | 11 (14.5) | 8 (10.5) | 2 (2.6) | 1 (1.3) | 16 |
Adverse events were defined according to the NIH Division of AIDS (DAIDS) grading table. There were no grade 4 adverse events.
Linezolid exposure and cytopenias.
Logistic regression modeling suggests that linezolid pharmacokinetic exposure as defined by Cmin and AUC0–24 thresholds is associated with development of cytopenias when evaluating the three outcomes of any cytopenia, anemia, and thrombocytopenia (Table 3). Those with Cmin values of >2 mg/L had higher odds of developing any cytopenia than those with Cmin values of ≤2 mg/L (odds ratio [OR], 4.40; 95% confidence interval [CI], 0.79 to 24.4). Those with Cmin values of >2 mg/L also had higher odds of developing anemia than those with Cmin values of ≤2 mg/L (OR, 3.51; 95% CI, 0.72 to 17.2). For both outcomes, these associations are somewhat attenuated in the adjusted model, and similar, though smaller, magnitude associations are shown using linezolid AUC0–24 as the exposure (Table 3). Those study subjects with Cmin values of >2 mg/L were also at higher risk of developing thrombocytopenia than those with Cmin values of ≤2 mg/L (adjusted OR, 5.64; 95% CI, 1.01 to 31.4).
TABLE 3.
Logistic regression analysis of linezolid exposure and cytopenia adverse events among patients with MDR-TB (n = 76)a
| Outcome | No. of patients/total no. of patients (%) | cOR (95% CI) | aORb (95% CI) |
|---|---|---|---|
| Any cytopenia AE | 30 (39.5) | ||
| Cmin > 2 mg/L | 5/7 (71.4) | 4.40 (0.79–24.4) | 3.84 (0.67–22.1) |
| Cmin ≤ 2 mg/L | 25/69 (36.2) | Reference | Reference |
| AUC0–24 > 160 mg·h/L | 6/10 (60.0) | 2.63 (0.67–10.2) | 2.05 (0.49–8.58) |
| AUC0–24 ≤ 160 mg·h/L | 24/66 (36.4) | Reference | Reference |
| Thrombocytopenia AE | 11 (14.5) | ||
| Cmin > 2 mg/L | 3/7 (42.9) | 5.72 (1.08–30.3) | 5.64 (1.01–31.4) |
| Cmin ≤ 2 mg/L | 8/69 (11.6) | Reference | Reference |
| AUC0–24 > 160 mg·h/L | 3/10 (30.0) | 3.11 (0.67–14.5) | 3.24 (0.63–16.7) |
| AUC0–24 ≤ 160 mg·h/L | 8/66 (12.1) | Reference | Reference |
| Anemia AE | 23 (30.3) | ||
| Cmin > 2 mg/L | 4/7 (57.1) | 3.51 (0.72–17.2) | 3.01 (0.58–15.7) |
| Cmin ≤ 2 mg/L | 19/69 (27.5) | Reference | Reference |
| AUC0–24 > 160 mg·h/L | 5/10 (50.0) | 2.67 (0.69–10.3) | 1.88 (0.44–7.98) |
| AUC0–24 ≤ 160 mg·h/L | 18/66 (27.3) | Reference | Reference |
Bold denotes confidence intervals that exclude the null value. NCTLD, National Center for Tuberculosis and Lung Diseases; MDR-TB, multidrug-resistant TB; TB, tuberculosis; AE, adverse event; cOR, crude odds ratio; aOR, adjusted odds ratio; CI, confidence interval.
Adjusted for age and gender.
Linezolid exposure and grade of cytopenias.
Ordinal logistic regression modeling suggests that linezolid pharmacokinetic exposure has an exposure-response relationship with grade of cytopenia AE for thrombocytopenia and anemia. Patients with Cmin values of >2 mg/L had higher odds of higher-grade thrombocytopenia AEs than those with Cmin values of ≤2 mg/L (OR, 5.70; 95% CI, 1.11 to 29.2), with a similar association after adjusting for age and gender (adjusted OR [aOR], 5.95; 95% CI, 1.11 to 32.1) (Table 4). Using tertiles of Cmin as exposure categories, there appears to be an exposure-response relationship, as the corrected OR (cOR) for higher-grade thrombocytopenia among patients with Cmin values of >0.35 mg/L compared to those with Cmin ≤0.12 mg/L was 3.76 (95% CI, 0.68 to 20.8), while among those with Cmin values of >0.12 to 0.35 mg/L, the cOR was 1.55 (95% CI, 0.23 to 10.4). A similar association is seen after adjusting for age and gender. The odds of higher-grade thrombocytopenia and are also greater among those with higher AUC0–24 values than lower AUC0–24 values.
TABLE 4.
Ordinal logistic regression analysis of linezolid exposure and degree of thrombocytopenia among patients with MDR-TB (n = 76)a
| Measure | No. of patients/total no. of patients (%) with AE grade of: |
Data for higher-grade thrombocytopenia (95% CI) |
|||
|---|---|---|---|---|---|
| None | 1 | 2–3 | cOR | aORb | |
| Linezolid Cmin (mg/L) | 65 (85.5) | 8 (10.5) | 3 (3.9) | ||
| >2 | 4/7 (57.1) | 2/7 (28.6) | 1/7 (14.3) | 5.70 (1.11–29.2) | 5.95 (1.11–32.1) |
| ≤2 | 61/69 (88.4) | 6/69 (8.7) | 2/69 (2.9) | Reference | Reference |
| >0.35 | 20/26 (76.9) | 3/26 (11.5) | 3/26 (11.5) | 3.76 (0.68–20.8) | 4.11 (0.71–23.9) |
| >0.12–0.35 | 22/25 (88.0) | 3/25 (12.0) | 1.55 (0.23–10.4) | 1.50 (0.22–10.1) | |
| ≤0.12 | 23/25 (92.0) | 2/25 (8.0) | Reference | Reference | |
| Linezolid AUC0–24 (mg·h/L) | |||||
| >160 | 7/10 (70.0) | 2/10 (20.0) | 1/10 (10.0) | 3.15 (0.69–14.5) | 3.31 (0.65–16.9) |
| ≤160 | 58/66 (87.9) | 6/66 (9.1) | 2/66 (3.0) | Reference | Reference |
| >105 | 20/26 (76.9) | 4/26 (15.4) | 2/26 (7.7) | 3.58 (0.64–19.9) | 3.78 (0.65–22.0) |
| >75.4–105 | 22/25 (88.0) | 2/25 (8.0) | 1/25 (4.0) | 1.62 (0.25–10.7) | 1.61 (0.24–10.7) |
| ≤75.4 | 23/25 (92.0) | 2/25 (8.0) | Reference | Reference | |
Bold denotes confidence intervals which exclude the null value. NCTLD, National Center for Tuberculosis and Lung Diseases; MDR-TB, multidrug-resistant TB; TB, tuberculosis; AE, adverse event; cOR, crude odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Cmin, trough serum concentration; AUC0–24, area under curve.
Adjusted for age and gender.
Study participants with MDR-TB on linezolid who had Cmin values of >2 mg/L had much higher odds of developing higher-grade anemia AE than those with Cmin values of ≤2 mg/L (OR, 13.33; 95% CI, 2.30 to 77.2) (Table 5). There is also an exposure-response relationship, as the OR for higher-grade anemia among patients with Cmin values of >0.35 mg/L compared to those with Cmin values of ≤0.12 mg/L was 2.52 (95% CI, 0.79 to 8.04), while among those with Cmin values of >0.12 to 0.35 mg/L, the OR was 0.74 (0.20 to 2.77). A similar association is seen after adjusting for age and gender. Using AUC0–24 categories of exposure, the odds of higher-grade anemia among patients with AUC0–24 values of >160 mg·h/L were 12.0 times the odds among those with AUC0–24 values of ≤160 mg·h/L (95% CI, 2.31 to 59.8). This association is attenuated by adjustment for age and gender (aOR, 8.74; 95% CI, 1.58 to 48.3). As for Cmin, there appears to be an exposure-response relationship between higher AUC0–24 and higher-grade anemia.
TABLE 5.
Ordinal logistic regression analysis of linezolid exposure and degree of anemia among patients with MDR-TB (n = 76)a
| Measure | No. of patients/total no. of patients (%) with AE grade of: |
Data for higher-grade anemia (95% CI) |
|||
|---|---|---|---|---|---|
| None | 1 | 2–3 | cOR | aORb | |
| Linezolid Cmin (mg/L) | 53 (69.7) | 14 (18.4) | 9 (11.8) | ||
| >2 | 3/7 (42.9) | 4/7 (57.1) | 13.33 (2.30–77.2) c | 19.2 (2.49–149) c | |
| ≤2 | 50/69 (72.5) | 14/69 (20.3) | 5/69 (7.3) | Reference | Reference |
| >0.35 | 15/26 (57.7) | 4/26 (15.4) | 7/26 (26.9) | 2.52 (0.79–8.04) | 1.93 (0.57–6.50) |
| >0.12–0.35 | 20/25 (80.0) | 3/25 (12.0) | 2/25 (8.0) | 0.74 (0.20–2.77) | 0.81 (0.21–3.11) |
| ≤0.12 | 18/25 (72.0) | 7/25 (28.0) | Reference | Reference | |
| Linezolid AUC0–24 (mg·h/L) | |||||
| >160 | 5/10 (50.0) | 5/10 (50.0) | 12.0 (2.41–59.8) c | 8.74 (1.58–48.3) c | |
| ≤160 | 48/66 (72.7) | 14/66 (21.2) | 4/66 (6.1) | Reference | Reference |
| >105 | 14/26 (53.9) | 5/26 (19.2) | 7/26 (26.9) | 2.85 (0.89–9.06) | 2.37 (0.71–7.90) |
| >75.4–105 | 21/25 (84.0) | 2/25 (8.0) | 2/25 (8.0) | 0.58 (0.15–2.29) | 0.63 (0.16–2.54) |
| ≤75.4 | 18/25 (72.0) | 7/25 (28.0) | Reference | Reference | |
Bold denotes confidence intervals which exclude the null value. NCTLD, National Center for Tuberculosis and Lung Diseases; MDR-TB, multidrug-resistant TB; TB, tuberculosis; AE, adverse event; cOR, crude odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Cmin, trough serum concentration; AUC0–24, area under curve.
Adjusted for age & gender.
Polytomous logistic regression for grades 2 to 3 versus none.
DISCUSSION
Among a cohort of patients with MDR-TB who received linezolid at 600 mg once daily as part of their TB treatment regimen, we found that cytopenia adverse events were common, occurring in 40% of patients at an incidence rate of 46 per 100 person-years. However, these AEs were not treatment limiting, as no patients required linezolid interruption or dose reduction due to cytopenias, and most occurred several months into therapy. These findings suggest that long-term use of the 600-mg/day regimen, now standard due to toxicities associated with the 1,200-mg/day regimen, is well tolerated and safe for clinical use. Our findings contrast with those from other TB treatment cohorts using 1,200 mg/day, such as the Nix-TB trial in which most patients (93/109, 85%) required linezolid interruption or dose reduction during a 26-week course due to an AE (5). In a clinical trial using linezolid 600 mg/day, 18% of patients experienced a grade 3 or higher cytopenia AE, while most cytopenia AEs in our study were low grade and unlikely to be clinically significant (21). Additionally, almost all patients with cytopenias experienced count recovery within 2 months. The apparent tolerability of the 600-mg/day dose is consistent with PK modeling data that suggest this dose may minimize toxicity while maintaining efficacy (19, 22). Thus, our study provides much-needed clinical safety data to support use of linezolid at a dose of 600 mg/day for treatment of MDR-TB.
Regression modeling suggests that cytopenia AEs, particularly thrombocytopenia, are associated with linezolid Cmin and AUC0–24 using several different cutoffs, and higher-grade cytopenias are associated with higher PK parameter values. Relatively few (7/76, 9.2%) patients in this cohort had Cmin values above the literature-informed 2-mg/L mitochondrial toxicity threshold compared to at least half of patients receiving linezolid 600 mg or 300 mg daily in studies of drug-resistant TB in South Africa (15, 22). However, the proportion above the toxicity threshold in the South African cohorts was higher than in other studies, and in a separate study, investigators suggested a toxicity trough concentration threshold of 2.5 mg/L (15, 23). This could reflect known heterogeneity between linezolid PK study populations and emphasizes the need for further evaluation of the utility of PK monitoring for linezolid (24). Recent evidence suggests the CYP450 2J2 enzyme accounts for approximately 50% of linezolid hepatic metabolism (25). Differences in CYP2J2 expression or other factors influencing its function, such as diet, could be responsible for variability in linezolid exposures in different populations (26). Contrasting toxicity or AE findings in different populations may also reflect the distribution of mitochondrial rRNA polymorphisms possibly associated with linezolid mitochondrial toxicity (16). Populations in the Caucasus region have low mitochondrial genetic diversity, so including patients from other settings would strengthen or confirm the findings of studies based in Georgia and add to the understanding of PK parameters in global populations (27).
While pharmacokinetic parameters could potentially identify patients at risk of linezolid toxicity, PK analysis for therapeutic drug monitoring (TDM) is not readily available in all centers (28). Although strategies have been proposed to address barriers to implementation, TDM is not currently generalizable to settings where most patients with MDR-TB are treated (29). Alternative linezolid dosing strategies, such as an intensive phase of 1,200 mg daily followed by dosing modification or discontinuation, have been proposed, and preliminary results from the ZeNix trial suggest that a reduced dose or duration of linezolid remains effective while enhancing safety (15, 30, 31). A recent PK modeling study using Nix-TB data supports this conclusion (32). The apparent safety and efficacy of long-term use of linezolid 600 mg/day is reassuring for settings where TDM and frequent laboratory monitoring are challenging.
The evidence from use of linezolid for treatment of Gram-positive bacterial infections indicates cytopenias develop early in therapy, within a few weeks of initiation (10). Among patients with drug-resistant TB treated with linezolid-containing regimens, cytopenias may occur later in the treatment course. In the Nix-TB trial, 52/109 (48%) patients developed cytopenias, with the majority occurring in the first 2 months of therapy, though the proportion was not quantified in study results (5). A study in South Africa of 151 patients reported a 6-month cumulative incidence of anemia of 39%, with a median time to event of 11 weeks (2.75 months) (23). However, in our cohort, the median time to development of cytopenias was 4 months for anemia and 11 months for thrombocytopenia; while this could be due to low linezolid exposure, population differences, or study sampling schedule, pending clinical trial data or further studies with larger sample size may inform whether CBC monitoring can be less frequent while on linezolid. In addition, other oxazolidinones such as sutezolid may offer improved safety profiles for treatment of TB in the future (33).
Our study is subject to certain limitations. Limitations include the single-country cohort, which may limit generalizability to other settings. Few patients had thrombocytopenia at baseline, and missing CBC data remained despite supplemental data collection, which could result in missed cytopenia AE. However, the median number of CBC collections was 11 among those who completed treatment, suggesting that CBC collection was robust for those who remained in care during the study period. Regression modeling produced broad CIs, which often include the null, though the direction of association is generally consistent except in the model evaluating anemia and tertiles of linezolid exposure. The PK data are from a single time point, which may limit the association of PK parameters with incident cytopenias late in therapy and bias the results toward the null. Additionally, PK data for other drugs are not included in this analysis, though the drugs included in treatment regimens for these patients are unlikely to influence the development of cytopenias either directly or via drug-drug interactions and should not impact study findings. Strengths of our study include its population size for investigation of clinical and PK data, well-controlled dosing histories prior to PK sampling, and length of follow-up. Participants received linezolid as long-term therapy for a median of 17.5 months in a programmatic setting; newer linezolid-containing regimens are much shorter at 6 or 9 months (34). These aspects support the use of linezolid for the durations recommended to treat MDR-TB.
In conclusion, among a cohort of patients treated for MDR-TB with regimens containing linezolid 600 mg daily, cytopenias occurred frequently and at a median of 4 months but were of minimal clinical impact, as they did not limit treatment with long-term use. Incident cytopenias may be associated with pharmacokinetic parameters such as Cmin when evaluated early in therapy; this warrants investigation in other settings to inform the utility of TDM. Our findings add to the evidence that this essential anti-TB drug is safe for use in programmatic management of MDR-TB.
MATERIALS AND METHODS
Design and population.
This was a secondary analysis of a National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID)-funded prospective observational cohort of patients with MDR-TB initiating treatment at the National Center for Tuberculosis and Lung Diseases (NCTLD) in Tbilisi, Georgia (20, 35). Eligible patients were those ≥16 years with sputum culture-confirmed TB and multidrug resistance who initiated therapy with bedaquiline, linezolid, clofazimine, and/or delamanid from December 2015 to May 2017. Treatment regimens were individualized based on drug susceptibility testing (DST) results following National TB Program (NTP) and WHO guidelines. Treatment regimens were determined by the NTP Drug Resistance Committee, which meets twice weekly. The standard linezolid dose was 600 mg given once daily, and the standard of care for treatment duration during the study period was 20 to 24 months. Guidelines for linezolid dose reduction included an AE increase in grade to grade 2, which was determined to be related to linezolid, and the decision whether to dose reduce or pause linezolid administration was made by the NCTLD DR-TB Committee. All treatment was administered through directly observed therapy (DOT) at the NCTLD during initial hospitalization. After discharge, DOT was administered 6 days per week at the NCTLD ambulatory department or at 1 of 4 other TB dispensaries in Tbilisi. Medical records and patient interviews were used to collect demographics, medical history, and clinical and laboratory data, including adverse events. Written informed consent was required, and ethics approvals were obtained from the institutional review boards of Emory University and the NCTLD.
Measurements.
Laboratory evaluation included monthly sputum cultures until conversion, defined as 2 consecutive negative sputum cultures ≥28 days apart and at least through 12 months of treatment. Patients were asked to return approximately 6 months posttreatment for follow-up and to provide a sputum sample. Sputum samples underwent acid-fast bacilli (AFB) smear microscopy, and AFB sputum cultures were performed at the National Reference Laboratory using Löwenstein-Jensen–based solid medium and the Bactec mycobacterial growth indicator tube 960 broth culture system. Positive cultures were confirmed to be M. tuberculosis complex using the M. tuberculosis-related antigen test. Phenotypic first- and second-line DST was conducted as previously described, and the MTBDRplus assay was performed on positive culture isolates (36). Neuropathy was assessed only while patients were hospitalized and was not a focus of this study.
CBCs were collected monthly for 12 months and up to 3 later time points at 13, 18, and 20 to 24 months. For pharmacokinetic analyses, 5 to 6 blood samples were collected predose and at 2, 4 to 6, 8 to 10, 12, and 24 h postdose 4 to 6 weeks after treatment initiation. Patients were hospitalized and receiving drugs via DOT for at least 7 days prior to PK sampling. Drug concentrations were measured at the Infectious Diseases Pharmacokinetics Laboratory at the University of Florida (19). Noncompartmental analysis was conducted using Phoenix v7.0 (Certara, Princeton, NJ, USA). Minimum serum concentration (Cmin) and maximum serum concentration (Cmax) were determined based on the time course of drug concentrations (37). Area under the curve (AUC) was estimated using the linear trapezoidal linear interpolation method.
Primary outcome.
Incident cytopenias were evaluated using the NIH Division of AIDS (DAIDS) adverse event (AE) grading scale, which assigns increasing severity of AE from 1 through 4 with decreasing CBC parameters (38). For platelets, a value of <125 × 103/μL is grade 1, <100 × 103/μL is grade 2, <50 × 103/μL is grade 3, and <25 × 103/μL is grade 4 (Table 2). For hemoglobin, a value <11.0 g/dL is grade 1, <10.0 g/dL is grade 2, <9.0 g/dL is grade 3, and <7.0 g/dL is grade 4. For white blood cells, a value of <2.5 × 103/mm3 is grade 1, <2.0 × 103/mm3 is grade 2, <1.5 × 103/mm3 is grade 3, and <1.0 × 103/mm3 is grade 4. An incident cytopenia was defined as either a new graded CBC parameter AE from a previous normal value or an increase in a CBC AE grade if a person had an abnormal baseline CBC parameter. Patients were only counted once when reporting the number of adverse events, and the reported grade was the lowest value in an individual patient.
Primary exposure.
Linezolid exposure as measured by PK parameters was evaluated using values reported in the literature or tertiles of exposure. Previous studies have proposed a toxicity threshold of Cmin of >7 mg/L or AUC0–24 of >280 mg·h/L for thrombocytopenia and an efficacy threshold of Cmin of >2 mg/L or AUC0–24 of >160 mg·h/L (14, 39). A Cmin value of >2 mg/L has also been associated with mitochondrial toxicity. Mouse models of TB have demonstrated an association between hemoglobin and linezolid dose and Cmin, but not AUC (40). Among the study population, the median Cmin was 0.235 mg/L (interquartile range [IQR], 0.069 to 0.529), and the median AUC0–24 was 89.6 mg·h/L (IQR, 69.2 to 116.2). Collected covariates included the baseline characteristics of body mass index, diabetes status, tobacco use, hepatitis B virus infection, cavitary TB disease, and extensive drug resistance, as well as treatment characteristics such as the duration of linezolid therapy, duration of second-line drug therapy, and duration of initial hospitalization.
Statistical analysis.
To describe the incidence of cytopenias with cumulative proportion and incidence rate, person-time was calculated as time to incident cytopenia or assumed to be the 12-month duration of monthly CBC collection. Logistic regression was used to evaluate the relationship between linezolid PK exposure and development of cytopenias. The outcomes were any cytopenia, anemia, or thrombocytopenia anemia as defined by the NIH/DAIDS AE grading table. Exposures were Cmin and AUC0–24 cutoffs from the literature and generated from the receiver operating characteristic (ROC) curve. Ordinal logistic regression was used to describe the dose-response relationship between linezolid PK exposure and severity of cytopenia. The outcomes were grade of anemia or thrombocytopenia as defined by the NIH/DAIDS AE grading table. The three levels of the outcome were none, grade 1, and grade 2 to 3 combined. There were no grade 4 AEs. Exposures were Cmin and AUC0–24 cutoffs from the literature and tertiles of exposure. The proportional odds assumption was assessed using the score test. The proportional odds assumption was not met for the models evaluating anemia AE that used linezolid PK thresholds gathered from the literature (Cmin of 2 mg/L and AUC0–24 of 160 mg·h/L), likely because there were no grade 1 AE in these categories; polytomous logistic regression for grade 2 to 3 anemia versus no anemia was used for these models. Age and gender were included in adjusted models because of the variability in CBC parameters with these characteristics. In univariate analysis, female gender was associated with anemia AE (see Table S1 in the supplemental material), but other potential covariates were not. A second adjusted model, including alcohol use and hepatitis C virus infection, was evaluated because of the potential clinical effect of these factors on CBC parameters via myelosuppression or liver disease. There was no evidence of collinearity between covariates. Models were compared by the likelihood ratio test for nested models.
Data availability.
Data used in this analysis is available via the Emory Dataverse (https://doi.org/10.15139/S3/WL3O44).
ACKNOWLEDGMENTS
We thank the physicians, nurses, and staff at the National Center for Tuberculosis and Lung Diseases (NCTLD) in Tbilisi, Georgia, who provided care for the patients with multidrug-resistant tuberculosis in this study. We also thank Hanna Schurr and Snehaa Krishnan for their contribution to data management.
This work was supported in part by grants from the National Institutes of Health (NIH), including the National Institute of Allergy and Infectious Diseases (K23AI103044 and R21AI122001), the NIH Fogarty International Center (D43TW007124), and the National Center for Advancing Translational Science (to the Georgia Clinical and Translational Science Alliance [Georgia CTSA]; UL1TR002378 and TL1TR002382). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We report no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2021. Global tuberculosis report 2021. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, Kim EK, Lee KM, Lee SS, Park JS, Koh WJ, Lee CH, Kim JY, Shim TS. 2008. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med 178:1075–1082. 10.1164/rccm.200801-132OC. [DOI] [PubMed] [Google Scholar]
- 3.Borisov SE, Dheda K, Enwerem M, Romero Leyet R, D'Ambrosio L, Centis R, Sotgiu G, Tiberi S, Alffenaar JW, Maryandyshev A, Belilovski E, Ganatra S, Skrahina A, Akkerman O, Aleksa A, Amale R, Artsukevich J, Bruchfeld J, Caminero JA, Carpena Martinez I, Codecasa L, Dalcolmo M, Denholm J, Douglas P, Duarte R, Esmail A, Fadul M, Filippov A, Davies Forsman L, Gaga M, Garcia-Fuertes JA, Garcia-Garcia JM, Gualano G, Jonsson J, Kunst H, Lau JS, Lazaro Mastrapa B, Teran Troya JL, Manga S, Manika K, Gonzalez Montaner P, Mullerpattan J, Oelofse S, Ortelli M, Palmero DJ, Palmieri F, Papalia A, Papavasileiou A, Payen MC, Pontali E, et al. 2017. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 49:1700387. 10.1183/13993003.00387-2017. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, Nix TBTT, Nix-TB Trial Team . 2020. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 382:893–902. 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnippel K, Ndjeka N, Maartens G, Meintjes G, Master I, Ismail N, Hughes J, Ferreira H, Padanilam X, Romero R, Te Riele J, Conradie F. 2018. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 6:699–706. 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 7.Vinh DC, Rubinstein E. 2009. Linezolid: a review of safety and tolerability. J Infect 59 (Suppl 1):S59–S74. 10.1016/S0163-4453(09)60009-8. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2019. WHO consolidated guidelines on drug-resistant tuberculosis treatment. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 9.Takahashi Y, Takesue Y, Nakajima K, Ichiki K, Tsuchida T, Tatsumi S, Ishihara M, Ikeuchi H, Uchino M. 2011. Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J Infect Chemother 17:382–387. 10.1007/s10156-010-0182-1. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo D, Orlando G, Cozzi V, Cordier L, Baldelli S, Merli S, Fucile S, Gulisano C, Rizzardini G, Clementi E. 2013. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with gram-positive infections. Int J Antimicrob Agents 41:586–589. 10.1016/j.ijantimicag.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK, Brust JCM, Campbell JR, Chang VWL, Falzon D, Guglielmetti L, Isaakidis P, Kempker RR, Kipiani M, Kuksa L, Lange C, Laniado-Laborin R, Nahid P, Rodrigues D, Singla R, Udwadia ZF, Menzies D, The Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB Treatment 2017 . 2020. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med 8:383–394. 10.1016/S2213-2600(20)30047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikiashvili L, Kipiani M, Schechter MC, Avaliani Z, Kiria N, Kempker RR. 2020. Linezolid use for drug-resistant tuberculosis in Georgia: a retrospective cohort study. Int J Tuber Lung Dis 24:436–443. 10.5588/ijtld.19.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank M, Adamashvili N, Lomtadze N, Kokhreidze E, Avaliani Z, Kempker RR, Blumberg HM. 2019. Long-term follow-up reveals high posttreatment mortality rate among patients with extensively drug-resistant tuberculosis in the country of Georgia. Open Forum Infect Dis 6:ofz152. 10.1093/ofid/ofz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pea F, Viale P, Cojutti P, Del Pin B, Zamparini E, Furlanut M. 2012. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother 67:2034–2042. 10.1093/jac/dks153. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman S, Denti P, Brust JCM, Abdelwahab M, Hlungulu S, Wiesner L, Norman J, Sirgel FA, Warren RM, Esmail A, Dheda K, Gandhi NR, Meintjes G, Maartens G. 2019. Linezolid pharmacokinetics in South African patients with drug-resistant tuberculosis and a high prevalence of HIV coinfection. Antimicrob Agents Chemother 63:e02164-18. 10.1128/AAC.02164-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song T, Lee M, Jeon HS, Park Y, Dodd LE, Dartois V, Follman D, Wang J, Cai Y, Goldfeder LC, Olivier KN, Xie Y, Via LE, Cho SN, Barry CE, III, Chen RY. 2015. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine 2:1627–1633. 10.1016/j.ebiom.2015.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC, Vandecasteele S, Boelaert JR. 2006. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 42:1111–1117. 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein WB, Trotta RF, Rector JT, Tjaden JA, Barile AJ. 2003. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann Pharmacother 37:517–520. 10.1345/aph.1C361. [DOI] [PubMed] [Google Scholar]
- 19.Alghamdi WA, Al-Shaer MH, An G, Alsultan A, Kipiani M, Barbakadze K, Mikiashvili L, Ashkin D, Griffith DE, Cegielski JP, Kempker RR, Peloquin CA. 2020. Population pharmacokinetics of linezolid in tuberculosis patients: dosing regimen simulation and target attainment analysis. Antimicrob Agents Chemother 64:e01174-20. 10.1128/AAC.01174-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempker RR, Mikiashvili L, Zhao Y, Benkeser D, Barbakadze K, Bablishvili N, Avaliani Z, Peloquin CA, Blumberg HM, Kipiani M. 2020. Clinical outcomes among patients with drug-resistant tuberculosis receiving bedaquiline- or delamanid-containing regimens. Clin Infect Dis 71:2336–2344. 10.1093/cid/ciz1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon HS, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park SK, Cho SN, Barry CE, III.. 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 367:1508–1518. 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelwahab MT, Wasserman S, Brust JCM, Dheda K, Wiesner L, Gandhi NR, Warren RM, Sirgel FA, Meintjes G, Maartens G, Denti P. 2021. Linezolid population pharmacokinetics in South African adults with drug-resistant tuberculosis. Antimicrob Agents Chemother 65:e0138121. 10.1128/AAC.01381-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman S, Brust JCM, Abdelwahab MT, Little F, Denti P, Wiesner L, Gandhi NR, Meintjes G, Maartens G. 2022. Linezolid toxicity in patients with drug-resistant tuberculosis: a prospective cohort study. J Antimicrob Chemother 77:1146–1154. 10.1093/jac/dkac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millard J, Pertinez H, Bonnett L, Hodel EM, Dartois V, Johnson JL, Caws M, Tiberi S, Bolhuis M, Alffenaar JC, Davies G, Sloan DJ. 2018. Linezolid pharmacokinetics in MDR-TB: a systematic review, meta-analysis and Monte Carlo simulation. J Antimicrob Chemother 73:1755–1762. 10.1093/jac/dky096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obach RS. 2022. Linezolid metabolism is catalyzed by cytochrome P450 2J2, 4F2, and 1B1. Drug Metab Dispos 50:413–421. 10.1124/dmd.121.000776. [DOI] [PubMed] [Google Scholar]
- 26.Xu M, Ju W, Hao H, Wang G, Li P. 2013. Cytochrome P450 2J2: distribution, function, regulation, genetic polymorphisms and clinical significance. Drug Metab Rev 45:311–352. 10.3109/03602532.2013.806537. [DOI] [PubMed] [Google Scholar]
- 27.Nasidze I, Ling EY, Quinque D, Dupanloup I, Cordaux R, Rychkov S, Naumova O, Zhukova O, Sarraf-Zadegan N, Naderi GA, Asgary S, Sardas S, Farhud DD, Sarkisian T, Asadov C, Kerimov A, Stoneking M. 2004. Mitochondrial DNA and Y-chromosome variation in the caucasus. Ann Hum Genet 68:205–221. 10.1046/j.1529-8817.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 28.Alsultan A, Peloquin CA. 2014. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 74:839–854. 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 29.Vu DH, Bolhuis MS, Koster RA, Greijdanus B, de Lange WC, van Altena R, Brouwers JR, Uges DR, Alffenaar JW. 2012. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 56:5758–5763. 10.1128/AAC.01054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang KC, Yew WW, Cheung SW, Leung CC, Tam CM, Chau CH, Wen PK, Chan RC. 2013. Can intermittent dosing optimize prolonged linezolid treatment of difficult multidrug-resistant tuberculosis? Antimicrob Agents Chemother 57:3445–3449. 10.1128/AAC.00388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conradie F, Everitt D, Olugbosi M, Wills G, Fabiane S, Timm J, Spigelman M. 2021. High rate of successful outcomes treating highly resistant TB in the ZeNix study of pretomanid, bedaquiline and alternative doses and durations of linezolid, p e25755. In 11th IAS Conference on HIV Science. International Aids Society, Berlin, Germany. [Google Scholar]
- 32.Imperial MZ, Nedelman JR, Conradie F, Savic RM. 2022. Proposed linezolid dosing strategies to minimize adverse events for treatment of extensively drug-resistant tuberculosis. Clin Infect Dis 74:1736–1747. 10.1093/cid/ciab699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D, Zhu T, Silvia A, Gobey J, Ellery C, Zhang Y, Eisenach K, Miller P, Diacon AH. 2014. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One 9:e94462. 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 2022. Rapid communication: key changes to the treatment of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 35.Kempker RR. 2022. Data from “clinical outcomes among patients with drug-resistant tuberculosis receiving bedaquiline or delamanid-containing regimens.” 10.15139/S3/WL3O44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tukvadze N, Kempker RR, Kalandadze I, Kurbatova E, Leonard MK, Apsindzelashvili R, Bablishvili N, Kipiani M, Blumberg HM. 2012. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One 7:e31563. 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alghamdi WA, Al-Shaer MH, Kipiani M, Barbakadze K, Mikiashvili L, Kempker RR, Peloquin CA. 2021. Pharmacokinetics of bedaquiline, delamanid and clofazimine in patients with multidrug-resistant tuberculosis. J Antimicrob Chemother 76:1019–1024. 10.1093/jac/dkaa550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Department of Health and Human Services NIoH, National Institute of Allergy and Infectious Diseases, Division of AIDS. 2014. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, version 2.0. https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf. Retrieved 9 March 2022.
- 39.Roger C, Roberts JA, Muller L. 2018. Clinical pharmacokinetics and pharmacodynamics of oxazolidinones. Clin Pharmacokinet 57:559–575. 10.1007/s40262-017-0601-x. [DOI] [PubMed] [Google Scholar]
- 40.Bigelow KM, Deitchman AN, Li SY, Barnes-Boyle K, Tyagi S, Soni H, Dooley KE, Savic R, Nuermberger EL. 2020. Pharmacodynamic correlates of linezolid activity and toxicity in murine models of tuberculosis. J Infect Dis 223:1855–1864. 10.1093/infdis/jiaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and Table S1. Download aac.00408-22-s0001.pdf, PDF file, 0.2 MB (205.2KB, pdf)
Data Availability Statement
Data used in this analysis is available via the Emory Dataverse (https://doi.org/10.15139/S3/WL3O44).