ABSTRACT
Prothionamide, a second-line drug for multidrug-resistant tuberculosis (MDR-TB), has been in use for a few decades. However, its pharmacokinetic (PK) profile remains unclear. This study aimed to develop a population PK model for prothionamide and then apply the model to determine the optimal dosing regimen for MDR-TB patients. Multiple plasma samples were collected from 27 MDR-TB patients who had been treated with prothionamide at 2 different study hospitals. Prothionamide was administered according to the weight-band dose regimen (500 mg/day for weight <50 kg and 750 mg/day for weight >50 kg) recommended by the World Health Organization. The population PK model was developed using nonlinear mixed-effects modeling. The probability of target attainment, based on systemic exposure and MIC, was used as a response target. Fixed-dose regimens (500 or 750 mg/day) were simulated to compare the efficacies of various dosing regimens. PK profiles adequately described the two-compartment model with first-order elimination and the transit absorption compartment model with allometric scaling on clearance. All dosing regimens had effectiveness >90% for MIC values <0.4 μg/mL in 1.0-log kill target. However, a fixed dose of 750 mg/day was the only regimen that achieved the target resistance suppression of ≥90% for MIC values of <0.2 μg/mL. In conclusion, fixed-dose prothionamide (750 mg/day), regardless of weight-band, was appropriate for adult MDR-TB patients with weights of 40 to 67 kg.
KEYWORDS: prothionamide, multidrug-resistant tuberculosis, population pharmacokinetics
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the most common infectious causes of mortality worldwide. In particular, drug-resistant TB (DR-TB) is a global public health threat. Despite improvements in the diagnosis and treatment of DR-TB, treatment is successful in only 57% of affected patients. Multidrug-resistant TB (MDR-TB), a subtype of DR-TB, is resistant to both isoniazid and rifampin (1). The goal of treatment for TB, including MDR-TB, is to cure infection and minimize transmission. However, treatment for MDR-TB is complicated because the available drugs are toxic; moreover, few drugs are effective against MDR-TB. According to recent guidelines by the World Health Organization (WHO), American Thoracic Society, Centers for Disease Control and Prevention, European Respiratory Society, and Infectious Disease Society of America, a combination of at least five susceptible drugs from groups A, B, or C of MDR-TB regimens is recommended during the intensive phase of treatment (5 to 7 months after sputum culture conversion); a combination of four drugs is recommended during the continuation phase of treatment (15 to 21 months after culture conversion) (2, 3).
Prothionamide (PTO), developed in the late 1950s (4), was commonly used as a second-line drug to treat MDR-TB patients in previous decades. PTO is currently classified in group C of MDR-TB regimens, which consists of drugs that are recommended for use when five more effective drugs cannot be used (2, 3). The WHO guidelines recommend daily PTO doses at 15 to 20 mg/kg or using a weight-band dose regimen for MDR-TB patients (3). Despite long-term experience with PTO, its pharmacokinetics (PK) with weight-based or weight-band doses in MDR-TB patients are unclear (5, 6). In addition, no previous studies have analyzed the PK characteristics of PTO using a population-based approach.
In this study, we aimed to develop a population PK model, using PTO plasma concentrations collected in MDR-TB patients, and then evaluate the weight-based dosing regimen, using a simulation based on MICs effective against drug-resistant M. tuberculosis.
RESULTS
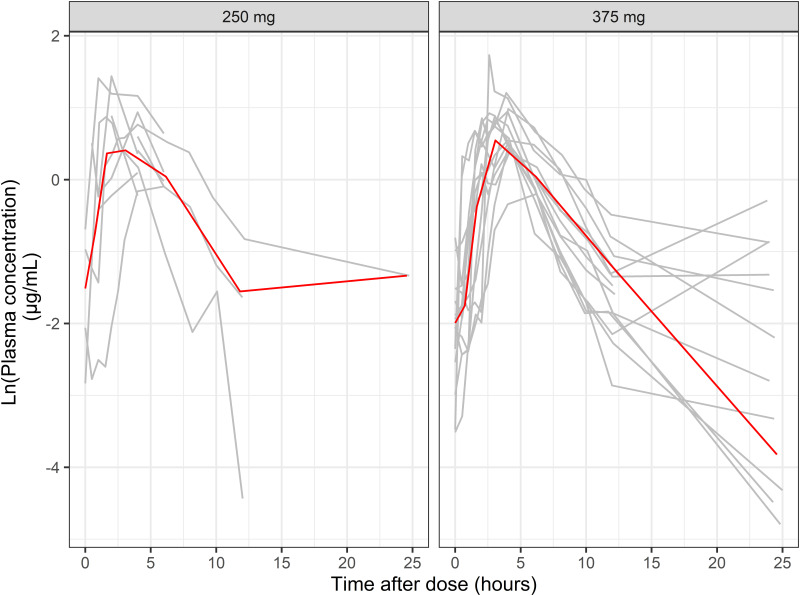
We included 27 patients in this study, including 10 (group A) and 17 (group B) from Chang et al. (7) and Lee et al. studies (6), respectively. The demographics of MDR-TB patients are summarized in Table 1. We included 247 PTO samples in this study. Figure 1 depicts the plasma concentration-time profiles according to PTO doses. We excluded 44 samples with concentrations below the limit of quantitation from a total of 291 samples during model development.
TABLE 1.
Baseline characteristics of study participants
| Characteristic | Value |
|---|---|
| Total no. of patients | 27 |
| No. of male patients/no. of female patients | 22/5 |
| Age (mean [range] [yrs]) | 45 (23–89) |
| Wt (mean [range] [kg]) | 58 (40–67) |
| BMI (mean [range] [kg/m2]) | 20 (14–23) |
| Sampling time after dose administration (h) | Group A, 0, 0.5, 1, 2, 4, 6, and 12; group B, 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 |
| Coadministration of anti-TB drug (no. of patients)a | |
| CS | 27 |
| PAS | 25 |
| KAN | 25 |
| STR | 19 |
| OFX | 17 |
| MXF | 7 |
| PZA | 6 |
| EMB | 2 |
| LVX | 1 |
| LZD | 1 |
CS, cycloserine; PAS, p-aminosalicylic acid; KAN, kanamycin; STR, streptomycin; OFX, ofloxacin; MXF, moxifloxacin; PZA, pyrazinamide; EMB, ethambutol; LVX, levofloxacin; LZD, linezolid.
FIG 1.
Plasma concentration-time profile of prothionamide (PTO) after dose administration. Gray and red lines represent individual profile and median, respectively. One patient who was administered 500 mg of PTO was excluded.
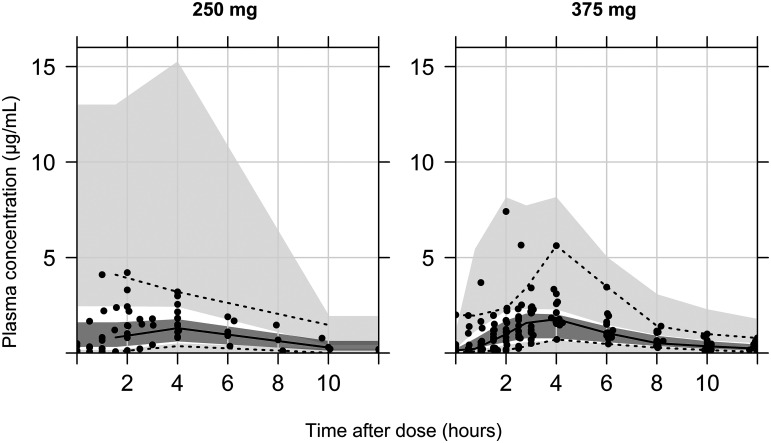
PTO profiles explained the two-compartment model with first-order elimination and the transit absorption compartment model (see Fig. S1 in the supplemental material). The one- and three-compartment model with an additional flip-flop kinetics model for the absorption-elimination phase and a lag time model for the absorption phase was conducted as well, and yet there was no significant improvement of the model on the diagnostic criteria. Allometric scaling was applied to scale weight-related changes in systemic clearance (CL), using a fixed coefficient (0.75). However, no other covariates were found significant by stepwise covariate modeling (SCM). Table 2 summarizes the estimates and bootstrap results for the included parameters. Figure 2 and Fig. S2 display the goodness-of-fit and visual predictive check plots for the final model.
TABLE 2.
Estimates of parameters for prothionamide population pharmacokinetic modelb
| Parameter | Population estimate |
Bootstrap estimate |
||
|---|---|---|---|---|
| Value | RSE (%) | Median | 5th–95th percentile | |
| Ka (hr−1) | 0.884 | 5.9 | 0.868 | 0.620–1.46 |
| V (L) | 26.7 | 6.3 | 23.1 | 9.90–37.6 |
| CL (L/h) | 11.4 | 19.3 | 9.09 | 4.11–14.0 |
| Q (L/h) | 6.58 | 27.4 | 5.59 | 2.81–11.2 |
| Vp (L) | 354 | 28.5 | 327 | 163–705 |
| F (%) | 43.6 | 18.4 | 36.9 | 20.3–56.1 |
| NN | 0.237 | 5.6 | 0.245 | 0.219–0.275 |
| MTT | 3.12 | 5.2 | 3.06 | 2.38–3.25 |
| Power coefficient of CL-wta | 0.75 | |||
| IIV %CV | ||||
| V | 91.6 | 17.0 | 86.9 | 47.9–207 |
| CL | 77.9 | 18.8 | 81.9 | 53.4–130 |
| Ka | 75.9 | 36.0 | 71.6 | 22.4–293 |
| Q | 114.8 | 17.8 | 98.4 | 51.1–157 |
| RV | ||||
| Proportional error (%) | 21.0 | 5.9 | 20.4 | 16.1–25.0 |
Value was fixed. Power coefficient equation is .
RSE, relative standard error; MTT, mean time of transit; V, volume of distribution; IIV, interindividual variability; RV, residual variability; CL, clearance; CV, coefficient of variation; NN, number of transit compartments; Vp, peripheral volume of distribution; F, oral bioavailability.
FIG 2.
Visual predictive check (VPC) plot of final population pharmacokinetics model for prothionamide (PTO), stratified on dosing regimen (250 mg and 375 mg). Solid line, median for observation; top and bottom dashed lines, 95th and 5th percentiles for observation, respectively; gray blocks, median prediction with 95% confidence interval (CI); top and bottom light gray blocks, 95th and 5th predictions with 95% CI, respectively.
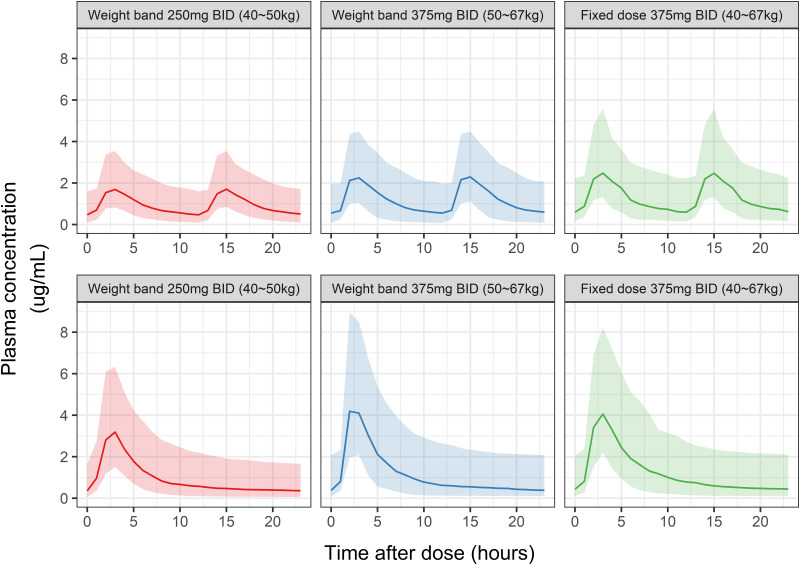
The efficacies of weight-band (50-kg bands) and fixed-dose (strength, 500 or 750 mg/day; frequency, once [QD] or twice [BID] per day) PTO regimens were compared using eight simulated scenarios. Figure 3 displays the simulated PK profiles for each regime except for fixed 250-mg BID or 500-mg QD doses. There were no significant differences between the simulated PK profiles of weight-band and fixed-dose (750-mg QD or 375-mg BID) PTO regimens. In addition, the simulation result of the steady-state PK profile suggested significantly lower systemic exposures for the 250-mg BID/500-mg QD weight-band regimen than the 375-mg BID/750-mg QD weight-band and fixed-dose regimens (Fig. 3). The overall differences among these regimens were >15% for concentrations at 2 (C2) and 6 (C6) h after the dose, peak concentration (Cmax), and AUC for the dosing interval (AUCtau) at steady state (Table S1).
FIG 3.
Simulation pharmacokinetic profiles for prothionamide (PTO) at steady state after twice-daily (BID, top) and once-daily (QD, bottom) doses. The two panels on the left and middle display profiles for weight-band dose regimens, and the right panel displays the profile for fixed-dose regimen. Line, median; shaded area, 10th to 90th percentiles.
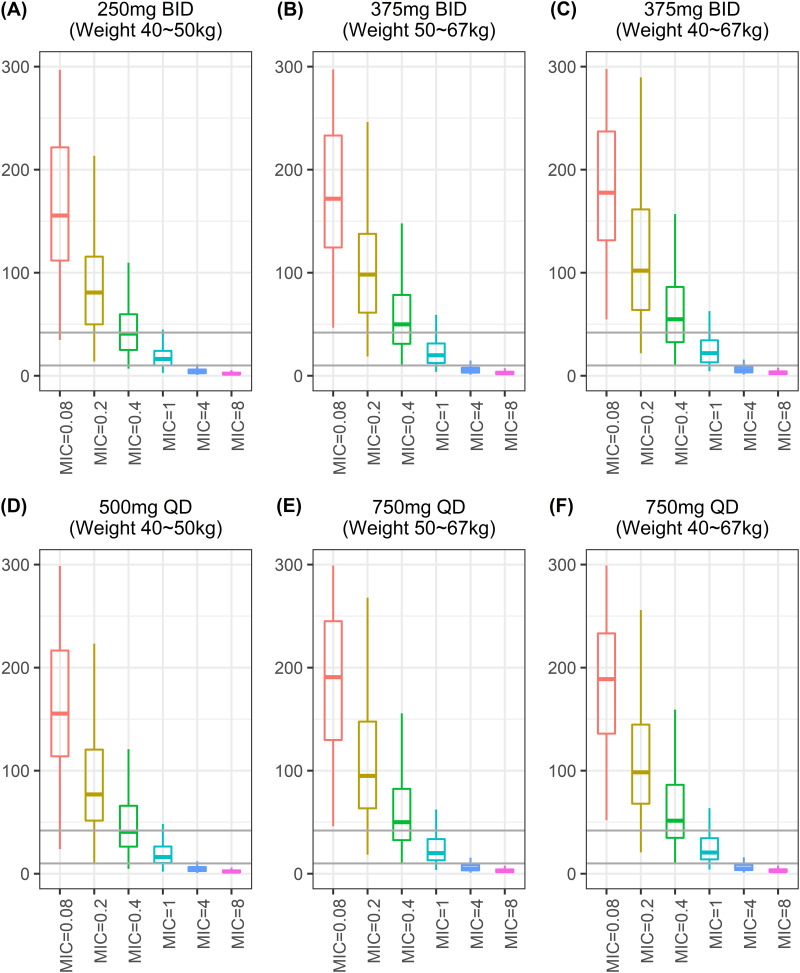
The free fraction of area under the concentration curve at steady state (fAUCss) and MIC value were used to calculate fAUCss/MIC to determine the PK/pharmacodynamic (PD) target (fAUCss/MIC ≥ 10 or fAUCss/MIC ≥ 42) (Table 3 and Fig. 4). Figure 5 displays the probability target attainment (PTA [%]) for the dosing regimens. Although all dosing regimens had effectiveness of >90% for MIC values <0.4 μg/mL using 1.0-log-kill (fAUCss/MIC ≥ 10), a fixed-dose regimen (750 mg QD or 375 mg BID) was the only regimen that achieved resistance suppression criteria (fAUCss/MIC ≥ 42) of ≥90% for MIC values <0.2 μg/mL.
TABLE 3.
Probability of target attainment according to prothionamide dosing regimensa
| MIC (μg/mL) | BID schedule |
QD schedule |
||||||
|---|---|---|---|---|---|---|---|---|
| WT band dose |
Fixed dose |
WT band dose |
Fixed dose |
|||||
| 250 mg (40–50 kg) | 375 mg (50–67 kg) | 250 mg (40–67 kg) | 375 mg (40–67 kg) | 500 mg (40–50 kg) | 750 mg (50–67 kg) | 500 mg (40–67 kg) | 750 mg (40–67 kg) | |
| Target fAUC/MIC ratio ≥ 10 | ||||||||
| 0.08 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.2 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.4 | 98 | 100 | 97 | 100 | 98 | 99 | 97 | 100 |
| 1 | 75 | 84 | 66 | 90 | 78 | 86 | 76 | 87 |
| 4 | 7 | 15 | 8 | 18 | 10 | 17 | 7 | 20 |
| 8 | 2 | 1 | 0 | 2 | 1 | 3 | 1 | 2 |
| Target fAUC/MIC ratio ≥ 42 | ||||||||
| 0.08 | 99 | 100 | 98 | 100 | 99 | 100 | 99 | 100 |
| 0.2 | 84 | 89 | 74 | 94 | 85 | 91 | 83 | 92 |
| 0.4 | 48 | 62 | 42 | 61 | 48 | 62 | 45 | 63 |
| 1 | 7 | 13 | 7 | 17 | 10 | 14 | 6 | 19 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
BID, twice daily; QD, once daily; fAUCss, free fraction of area under the concentration curve at steady state; MIC; minimum inhibitory concentration.
FIG 4.
Simulated target attainment (fAUCss/MIC ≥10 for 1.0 log-kill target or ≥42 for resistance suppression target) for weight-band and fixed-dose regimens. (A) Weight-band (weight range, 40 to 50 kg) 250-mg twice-daily (BID) dose; (B) weight-band (weight range, 50 to 67 kg) 375-mg BID dose; (C) fixed-dose 375-mg BID dose; (D) weight-band (weight range, 40 to 50 kg) 500-mg once-daily (QD) dose; (E) weight-band (weight range, 50 to 67 kg) 750-mg QD dose; (F) fixed-dose 750-mg QD dose. Bottom and top solid lines in each figure represent fAUCss/MIC values ≥10 and ≥42, respectively.
FIG 5.
Probability target attainment (PTA [%]) and MIC of prothionamide (PTO) for M. tuberculosis. Solid and broken lines in each panel represent 90% and 80% values, respectively.
DISCUSSION
In this study, we developed a population model to explore the PK and effectiveness of PTO for MDR-TB patients. According to the final model, the estimated CL and estimated volume of distribution (V) for MDR-TB patients were 11.4 L/h and 26.7 L/h, respectively; these did not significantly differ from healthy individuals (8). In addition, considering the range of time to reach peak plasma concentration (Tmax, 1.5 to 6.0 h) reported in studies of healthy individuals, we did not expect to find any differences in absorption rates for PTO between healthy individuals and MDR-TB patients (5, 8, 9).
Our results suggest that a fixed-dose regimen is superior to a weight-band dosing regimen for adults by means of convenience and efficacy. The WHO and Korean guidelines recommend weight-band PTO dosing (500 mg/day for weight of 30 to 50 kg and 750 mg/day for weight >50 kg). However, the steady-state PK profile simulation result suggested significantly lower systemic exposures for the 250-mg BID/500-mg QD weight-band regimen than for the 375-mg BID/750-mg QD weight-band and fixed-dosing regimens (Fig. 3). The differences in PK cast doubt on the weight-band PTO dosing regimen. Simulation results and PK patterns for the fixed-dose 375-mg BID/750-mg QD and weight-band 375-mg BID/750-mg QD dosing regimens also add to the doubt (see Table S1 in the supplemental material). In addition, the superiority of a fixed-dose 375-mg BID/750-mg QD regimen was suggested by the lack of differences in the objective function values (OFVs) of models, with or without scaling for the effects of weight on CL (ΔOFV between models = 1.138).
Because higher systemic exposure may improve drug efficacy against infectious diseases, weight-band dosing with a higher dose (375 mg BID/750 mg QD) was expected to be superior to weight-band dosing with a lower dose (250 mg BID/500 mg QD). The simulated PK/PD target results emphasize the usefulness of a fixed-dose 375-mg BID/750-mg QD regimen, which was the only regimen with PTA >90% for MIC values of <1 μg/mL and <0.2 μg/mL in fAUCss/MIC of ≥10 and ≥42, respectively (Fig. 5; Table 3). However, the PTA for the weight-band 250 mg BID/500 mg QD dosing regimen rapidly decreased to 75% for fAUCss/MIC of ≥10 and 42% for fAUCss/MIC of ≥42 at MIC values of 1 μg/mL and 0.2 μg/mL, respectively (Fig. 5; Table 3); these results suggested inadequate systemic drug exposure. Tan et al. reported an MIC of almost 0.4 μg/mL PTO among 248 samples of M. tuberculosis (10). Although none of the regimens had a PTA of 80% at this MIC value in fAUCss/MIC of ≥42 for resistance suppression, the fixed-dose 375-mg BID/750-mg QD regimen had 30% higher levels than the weight-band 250-mg BID/500-mg QD dosing regimens. Adverse events were not expected to increase with the increased dose in the fixed-dose regimen for patients with weight <50 kg because PTO has fewer side effects than ethionamide (11). The dose of 750 mg/day for patients with weight <50 kg falls within the range of 15 to 20 mg/kg recommended by WHO as well (3).
In the present study, the interindividual variability (IIV) for V, CL, absorption rate constant (Ka), and inter-compartmental clearance (Q) were 91.6%, 77.9%, 75.9%, and 114.8%, respectively. These high IIVs may advocate for the practice of therapeutic drug monitoring (TDM). Especially for MDR-TB patients with few effective drugs for their regimen, the use of TDM may be beneficial to optimize the treatment effect of available drugs.
To date, only noncompartmental analyses were conducted for PTO, and this study was the first to develop a population PK model of the drug. Meanwhile, population PK models for ETO have been developed from clinical trials of patients with tuberculosis (12–14). According to previous research, the oral bioavailability of PTO and ETO were 0.9 and 1.1, respectively (5, 15). The estimated bioavailability of PTO in MDR-TB patients in this study was 43.6% in this study. This difference may suggest that PK of PTO may differ in MDR-TB patients compared to healthy subjects. In the case of ETO, it has been reported to result in a lower AUC for TB patients, perhaps due to decreased bioavailability (13). Considering that ETO and PTO have similar PK properties (15) and that insufficient research was conducted until now to make a precise comparison of the PK of PTO between MDR-TB patients and healthy individuals, we may suggest a decrease in bioavailability for PTO as well.
There were several limitations to our study, including its small sample size. First, our results are only applicable to Korean MDR-TB patients because our model was developed based on a data set of Korean patients. Second, the weight range used for simulation of the fixed and weight-band dosing regimens was 40 to 67 kg; caution should be exercised when applying the results to patients with weights outside this range. Finally, we did not evaluate the adverse effects of PTO; therefore, unexpected adverse effects may occur with fixed-dosing regimens.
In conclusion, we successfully developed a population PK model for PTO in MDR-TB patients and then compared the systemic exposure and PK/PD targets between weight-band and fixed-dosing regimens. A fixed-dose 350-mg BID/750-mg QD PTO regimen provides the most optimized doses for MDR-TB patients with weights of 40 to 67 kg. To our knowledge, this is the first study to provide evidence for the superiority of a fixed-dose PTO regimen for adult MDR-TB patients. The use of a fixed-dose regimen is expected to improve MDR-TB treatment by simplifying the dosing schedule.
MATERIALS AND METHODS
Study population and data collection.
MDR-TB patients from two separate clinical trials (Chang et al. [7] and Lee et al. [6]) were included in this study. All patients were adults with stable pulmonary MDR-TB and had received second-line anti-TB drugs for more than 2 weeks. M. tuberculosis was identified in the sputum or bronchial washing fluid, and resistance to isoniazid and rifampicin was evaluated using drug susceptibility testing on solid media. In accordance with current guidelines, all patients received at least 3 second-line anti-TB drugs (levofloxacin, moxifloxacin, ofloxacin, cycloserine, p-aminosalicylic acid, intramuscular kanamycin, streptomycin, linezolid, ethambutol, or pyrazinamide) with PTO.
PTO was administered orally, and the dose was decided based on each patient’s clinical status and corresponding weight-band dosing regimen (500 mg/day for weight of 30 to 50 kg and 750 mg/day for weight >50 kg), in accordance with the Korean guidelines for tuberculosis (4th edition), modified from the WHO recommendations (3, 16). Plasma PTO levels were measured in blood samples collected at least 6 times from each patient (group A, 0.5, 1, 2, 4, 6, and 12 h after the dose; group B, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, and 24 h after the dose). PTO levels were measured using validated ultraperformance liquid chromatography-tandem mass spectrometry for patients in group A and high-performance liquid chromatography using UV detection for patients in group B, in accordance with previously described methods (17, 18). The lower limits of quantification were 0.5 mL μg/and 0.008 μg/mL for groups A and B, respectively.
Each study was approved by the institutional review board where it was operated by each institute and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Population pharmacokinetic analysis and model evaluation.
The population PK model for PTO was developed using nonlinear mixed-effects modeling software (NONMEM; version 7.4; Icon Plc, Dublin, Ireland) and Perl-speaks-NONMEM (PsN; version 4.9.0; Icon Plc) (19). All parameters were estimated using first-order conditional estimation with interaction.
Various structural models were tested, including one-, two-, and three-compartment models. In addition, a transit compartment model for the absorption phase, a flip-flop kinetics model for the absorption-elimination phase, a lag time model for the absorption phase, and allometric scaling with body weight as a structural parameter were also evaluated (20, 21). The interindividual variability (IIV) of structural PK parameters was implemented as an exponential relationship; residual variability was chosen from the additive, proportional, and combined models. The appropriate model was selected using numerical (e.g., OFV, shrinkage of IIV and residual variability, and precision of estimation values) and visual (e.g., goodness-of-fit and visual predictive check) criteria.
SCM in PsN was used to explore parameter-covariate relationships. The level of significance for covariate screening was set at P values of <0.05 for forward selection and P values of <0.01 for backward elimination. The covariates included sex, age, weight, body mass index, and coadministration of drugs. A nonparametric bootstrap method (n = 500) was used to obtain the precision values for parameters to validate the internal model.
Simulation for probability target attainment.
To calculate the PTA, Monte Carlo simulation (n = 1,000) was performed with the population PK model to optimize the PTO dose. The PTO fAUCss was calculated using noncompartmental analysis of the simulation results by the ncappc package of R software (R Foundation for Statistical Computing, Vienna, Austria) (22). The free fraction was assumed to be 70% (12, 23). The range of MIC values for M. tuberculosis was set at 0.8 to 8 μg/mL, as reported by Tan et al. (10).
For the PK/PD target, fAUCss/MIC values of 10 for 1.0-log kill and 42 for resistance suppression were used, based on the values for ethionamide (12, 24). Ethionamide values were used because ethionamide and PTO exhibit similar effects and can be used interchangeably (25). The PTA for each regimen and the MIC were evaluated using fAUCss/MIC values of ≥10 or ≥42. Simulation scenarios were developed in accordance with the WHO recommendations for PTO dosing and selected covariates. The weight-band (500 mg/day for weight of <50 kg or 750 mg/day for weight ≥50) and fixed-dose (500- or 750-mg/day) PTO regimen (frequency, once [QD]- or twice [BID]-per-day) scenarios were simulated.
ACKNOWLEDGMENTS
This study was funded by Chungnam National University, as well as a grant by the Institute for Information and Communications Technology Planning and Evaluation, funded by the government of the Republic of Korea (MSIT; no. 2020-0-01441; Artificial Intelligence Convergence Research Center, Chungnam National University, South Korea). No. RS-2022- 00155857, Artificial Intelligence Convergence Innovation Human Resources Development (Chungnam National University) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2022R1A2C1010929).
Footnotes
Supplemental material is available online only.
Contributor Information
Young-Ran Yoon, Email: yry@knu.ac.kr.
Radojka M. Savic, Email: rada.savic@ucsf.edu.
REFERENCES
- 1.World Health Organization. 2020. Global tuberculosis report 2020. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, Cattamanchi A, Peter Cegielski J, Chen L, Daley CL, Dalton TL, Duarte R, Fregonese F, Robert Horsburgh C, Khan FA, Kheir F, Lan Z, Lardizabal A, Lauzardo M, Mangan JM, Marks SM, McKenna L, Menzies D, Mitnick CD, Nilsen DM, Parvez F, Peloquin CA, Raftery A, Simon Schaaf H, Shah NS, Starke JR, Wilson JW, Wortham JM, Chorba T, Seaworth B, Lardizabal A, Raftery RN. 2019. Treatment of drug-resistant tuberculosis an official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 200:e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2019. Consolidated guidelines on tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Willand N, Flipo M, Villemagne B, Baulard A, Deprez B. 2019. Recent advances in the design of inhibitors of mycobacterial transcriptional regulators to boost thioamides anti-tubercular activity and circumvent acquired-resistance. Annu Rep Med Chem 52:131–152. doi: 10.1016/bs.armc.2019.06.003. [DOI] [Google Scholar]
- 5.Jenner PJ, Smith SE. 1987. Plasma levels of ethionamide and prothionamide in a volunteer following intravenous and oral dosages. Lepr Rev 58:31–37. doi: 10.5935/0305-7518.19870004. [DOI] [PubMed] [Google Scholar]
- 6.Lee HW, Kim DW, Park JH, Kim SD, Lim MS, Phapale PB, Kim EH, Park SK, Yoon YR. 2009. Pharmacokinetics of prothionamide in patients with multidrug-resistant tuberculosis. Int J Tuber Lung Dis 13:1161–1166. [PubMed] [Google Scholar]
- 7.Chang MJ, Jin B, Chae J, Yun H, Kim ES, Lee YJ, Cho YJ, Yoon H, Lee CT, Park KU, Song J, Lee JH, Park JS. 2017. Population pharmacokinetics of moxifloxacin, cycloserine, p-aminosalicylic acid and kanamycin for the treatment of multi-drug-resistant tuberculosis. Int J Antimicrob Agents 49:677–687. doi: 10.1016/j.ijantimicag.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Park SI, Oh J, Jang K, Yoon J, Moon SJ, Park JS, Lee JH, Song J, Jang IJ, Yu KS, Chung JY. 2015. Pharmacokinetics of second-line antituberculosis drugs after multiple administrations in healthy volunteers. Antimicrob Agents Chemother 59:4429–4435. doi: 10.1128/AAC.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenner PJ, Ellard GA, Gruer PJ, Aber VR. 1984. A comparison of the blood levels and urinary excretion of ethionamide and prothionamide in man. J Antimicrob Chemother 13:267–277. doi: 10.1093/jac/13.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y, Su B, Zheng H, Wang Y, Pang Y. 2017. Prothionamide susceptibility testing of Mycobacterium tuberculosis using the resazurin microtitre assay and the BACTECMGIT 960 system. Eur J Clin Microbiol Infect Dis 36:779–782. doi: 10.1007/s10096-016-2859-6. [DOI] [PubMed] [Google Scholar]
- 11.Scardigli A, Caminero JA, Sotgiu G, Centis R, D'Ambrosio L, Migliori GB. 2016. Efficacy and tolerability of ethionamide versus prothionamide: a systematic review. Eur Respir J 48:946–952. doi: 10.1183/13993003.00438-2016. [DOI] [PubMed] [Google Scholar]
- 12.Al-Shaer MH, Märtson AG, Alghamdi WA, Alsultan A, An G, Ahmed S, Alkabab Y, Banu S, Houpt ER, Ashkin D, Griffith DE, Cegielski JP, Heysell SK, Peloquin CA. 2020. Ethionamide population pharmacokinetic model and target attainment in multidrug-resistant tuberculosis. Antimicrob Agents Chemother 64:e00713-20. doi: 10.1128/AAC.00713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M, Namdar R, Stambaugh JJ, Starke JR, Bulpitt AE, Berning SE, Peloquin CA. 2002. Population pharmacokinetics of ethionamide in patients with tuberculosis. Tuberculosis (Edinb) 82:91–96. doi: 10.1054/tube.2002.0330. [DOI] [PubMed] [Google Scholar]
- 14.Bjugård Nyberg H, Draper HR, Garcia-Prats AJ, Thee S, Bekker A, Zar HJ, Hooker AC, Schaaf HS, McIlleron H, Hesseling AC, Denti P. 2020. Population pharmacokinetics and dosing of ethionamide in children with tuberculosis. Antimicrob Agents Chemother 64:e01984-19. doi: 10.1128/AAC.01984-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatesan K. 1989. Clinical pharmacokinetic considerations in the treatment of patients with leprosy. Clin Pharmacokinet 16:365–386. doi: 10.2165/00003088-198916060-00003. [DOI] [PubMed] [Google Scholar]
- 16.Joint Committee for the Revision of Korean Guideline for Tuberculosis. 2020. Korean guidelines for tuberculosis, 4th ed. Joint Committee for the Revision of Korean Guideline for Tuberculosis. [Google Scholar]
- 17.Han M, Jun SH, Lee JH, Park KU, Song J, Song SH. 2013. Method for simultaneous analysis of nine second-line anti-tuberculosis drugs using UPLC-MS/MS. J Antimicrob Chemother 68:2066–2073. doi: 10.1093/jac/dkt154. [DOI] [PubMed] [Google Scholar]
- 18.Bartels H, Bartels R. 1998. Simple, rapid and sensitive determination of protionamide in human serum by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 707:338–341. doi: 10.1016/s0378-4347(97)00584-7. [DOI] [PubMed] [Google Scholar]
- 19.Lindbom L, Pihlgren P, Jonsson EN, Jonsson N. 2005. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 21.Back HM, Lee JB, Han N, Goo S, Jung E, Kim J, Song B, An SH, Kim JT, Rhie SJ, Ree YS, Chae JW, Kim J, Yun HY. 2019. Application of size and maturation functions to population pharmacokinetic modeling of pediatric patients. Pharmaceutics 11:259. doi: 10.3390/pharmaceutics11060259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acharya C, Hooker AC, Türkyılmaz GY, Jönsson S, Karlsson MO. 2016. A diagnostic tool for population models using non-compartmental analysis: the ncappc package for R. Comput Methods Programs Biomed 127:83–93. doi: 10.1016/j.cmpb.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. 2018. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande D, Pasipanodya JG, Mpagama SG, Srivastava S, Bendet P, Koeuth T, Lee PS, Heysell SK, Gumbo T. 2018. Ethionamide pharmacokinetics/pharmacodynamics-derived dose, the role of MICs in clinical outcome, and the resistance arrow of time in multidrug-resistant tuberculosis. Clin Infect Dis 67:S317–S326. doi: 10.1093/cid/ciy609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. 2018. Technical report on the pharmacokinetics and pharmacodynamics (PK/PD) of medicines used in the treatment of drug-resistant tuberculosis, p 63. World Health Organization, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2 and Table S1. Download aac.01893-21-s0001.pdf, PDF file, 0.1 MB (130.4KB, pdf)