ABSTRACT
The emergence of artemisinin-resistant parasites in Africa has had a devastating impact, causing most malaria cases and related deaths reported on the continent. In Ethiopia, artemether-lumefantrine (AL) is the first-line drug for the treatment of uncomplicated falciparum malaria. This study is one of the earliest evaluations of artemether-lumefantrine (AL) efficacy in western Ethiopia, 17 years after the introduction of this drug in the study area. This study aimed at assessing PCR- corrected clinical and parasitological responses at 28 days following AL treatment. Sixty uncomplicated falciparum malaria patients were enrolled, treated with standard doses of AL, and monitored for 28 days with clinical and parasitological assessments from September 15 to December 15, 2020. Microscopy was used for patient recruitment and molecular diagnosis of P. falciparum was performed by Var gene acidic terminal sequence (varATS) real-time PCR on dried blood spots collected from each patient from day 0 and on follow-up days 1, 2, 3, 7, 14, 21, and 28. MspI and msp2 genotyping was done to confirm occurrence of recrudescence. Data entry and analysis were done by using the WHO-designed Excel spreadsheet and SPSS version 20 for Windows. A P value of less or equal to 0.05 was considered significant. From a total of 60 patients enrolled in this efficacy study, 10 were lost to follow-up; the results were analyzed for 50 patients. All the patients were fever-free on day 3. The asexual parasite positivity rate on day 3 was zero. However; 60% of the patients were PCR positive on day 3. PCR positivity on day 3 was more common among patients <15 years old as compared with those ≥15 years old (AOR = 6.44, P = 0.027). Only two patients met the case definition of treatment failure. These patients were classified as a late clinical failure as they showed symptoms of malaria and asexual stages of the parasite detected by microscopy on day 14 of their follow-ups. Hence, the Kaplan-Meier analysis of PCR- corrected adequate clinical and parasitological response (ACPR) rate of AL among study participants was 96% (95% CI: 84.9–99). In seven patients, the residual submicroscopic parasitemia persists from day 0 to day 28 of the follow-up. In addition, 16% (8/50) of patients were PCR– and then turned PCR+ after day 7 of the follow-up. AL remains efficacious for the treatment of uncomplicated falciparum malaria in the study area. However, the persistence of PCR-detected residual submicroscopic parasitemia following AL might compromise this treatment and need careful monitoring.
KEYWORDS: therapeutic efficacy study, uncomplicated falciparum malaria, western Ethiopia
INTRODUCTION
Artemisinin-based combination therapies (ACTs) are currently used as a first-line treatment for uncomplicated falciparum malaria in endemic countries (1). Artemisinin resistance with a clinical phenotype manifested by slow parasites clearance was first reported in western Cambodia and has now emerged or spread to other areas of Southeast Asia (2–4).
In Africa, it is predicted that the artemisinin-resistant malaria parasites might spread from Asia or originate de novo. The existence of artemisinin resistance in Africa is concerning as more than 215 million malaria cases and 38,4000 deaths in 2019 were reported in Africa (5). Although ACT is still effective in Africa, there is increasing concern that antimalarial treatment with ACT would be seriously threatened by the emergence of drug-resistant parasites (6).
Some recent studies in Africa have shown reduced P. falciparum susceptibility to ACT and longer parasite clearance time (7–9). In addition, clinically artemisinin-resistant parasites have been reported from Rawada and Uganda (10, 11).
Recent therapeutic efficacy studies (TES) that use quantitative PCR (qPCR) are reporting the persistence of residual submicroscopic parasites after ACT treatment (12–14). Substantial residual submicroscopic parasitemia after microscopically successful artemether-lumefantrine (AL) treatment was reported by qPCR (15).
Recrudescence after ACT treatment might not be the result of inherent parasite resistance (16, 17). Host immunity and pharmacokinetic factors are also determinants of ACT treatment efficacy. Individuals lacking acquired immunity may have higher rates of AL treatment failure (18). In addition, recrudescence was associated with day 7 lumefantrine concentrations in blood (19), concomitant food intake (20), and type of diet taken (21).
Malaria is one of the major health problems in Ethiopia. In 2004, Ethiopia adopted the ACT, artemether-lumefantrine, as the first-line treatment of uncomplicated P. falciparum malaria (22). There are a few TES carried out in Ethiopia with a reported greater than 98% PCR corrected cure rate for AL (23–25). However; there is a paucity of reports on the efficacy of AL in western Ethiopia. Therefore, this study was aimed to assess PCR corrected clinical and parasitological response at 28 days following AL treatment and to give evidence of AL treatment outcomes in the treatment of uncomplicated falciparum malaria in the western part of the country that borders Sudan.
RESULTS
Socio-demographic and baseline clinical characteristics of study participants.
A total of 60 consented, uncomplicated P. falciparum malaria patients were enrolled for the 28 days follow-up. Of these, 16.7% (10/60) patients did not attend the scheduled visits and were found lost to follow-up, and 83.3% of the enrolled patients completed the study (Table 1). No patient was withdrawn from the study.
TABLE 1.
Socio-demographic and baseline clinical characteristics of uncomplicated P. falciparum malaria cases in Sherkole and Horazhab health centers, western Ethiopia, September 15 to December 15, 2021 (n = 50)
| Variables | Frequency | Percent |
|---|---|---|
| Enrolled participants | 60 | |
| Lost to follow-up | 10 | 16.7 |
| Complete the follow-up study | 50 | 83.3 |
| Study health centers | ||
| Sherkole health center | 32 | 64 |
| Horazhab health center | 18 | 36 |
| Sex | ||
| Male | 34 | 68 |
| Female | 16 | 32 |
| Age | ||
| <5 yrs | 5 | 10 |
| 5–15 yrs | 28 | 56 |
| ≥15 yrs | 17 | 34 |
| Ethnicity | ||
| Berta | 36 | 72 |
| Oromo | 8 | 16 |
| Amhara | 5 | 10 |
| Gumz | 1 | 2 |
| Baseline clinical data | ||
| Fever | 40 | 80 |
| Parasitemia: | ||
| <50,000 /μL | 28 | 56 |
| ≥50,000 /μL | 22 | 44 |
Thirty-two of the 50 patients were recruited from Sherkole health center. Most of the study participants were Berta in Ethnicity, and 68% (34/50) of them were males. The mean age of study participants was 15.22 years ± 10.4 SD. The minimum and maximum ages were 2 years and 48 years, respectively. Five of the study participants were under 5 years old, and 56% of the patients were between 5 and 15 years in age (Table 1).
Of patients at enrollment, 40/50 (80%) had fever. The geometric mean of parasitemia at baseline was 21,652 ± 32740 SD parasites/μL. The minimum and maximum parasite densities were 2,349 and 141,793 parasites/μL, respectively. The first, second, and third quartile ranges of parasitemia at baseline were 6,066.75, 37,633.5, and 62,323.25 parasites/μL, respectively (Table 1).
Clinical and parasitological outcomes of AL treatment.
(i) Early and late clinical response. Eight patients had fever on day 1 after they took one dose of AL treatment. On day 2 and day 3, resolution of fever was noted among all patients. For two patients who had fever on day 1, the fever reoccurred on day 7. One patient showed symptoms of malaria (history of fever within 24 h and chills and headache) on day 7. In addition, on day 14 of the follow-up, two patients showed symptoms of malaria (history of fever within 24 h and chills, headache, and back pain).
(ii) Early and late parasitological response. As determined by light microscopy, the prevalence of parasitemia on days 1, 2, and 3 following initiation of AL therapy was 38%, 10%, and 0%, respectively. Parasites were not detected using microscopy, in three patients in which clinical features of malaria were observed on day 7. However, two patients who had the symptoms of malaria on day 14 were microscopically positive during the follow-up period. These two cases had a positive blood slide and repeated full doses of artemether-lumefantrine and were followed up to day 28. Parasites were not seen on the blood smear of the patients on day 21 and day 28 after artemether-lumefantrine retreatment. During the follow-up, gametocytes of P. falciparum were detected among 4 patients on day 1; among 2 patients on day 2; in 1 patient on day 3, and in 1 patient on day 28 (Table 2).
TABLE 2.
Parasite positivity using light microscopy and PCR following AL treatment
| Parasite positivity |
|||
|---|---|---|---|
| Follow-up days | Microscopy | PCR | Gametocytes detected |
| Day 1 | 38% (19/50) | 92% (46/50) | 8% (4/50) |
| Day 2 | 10% (5/50) | 78% (39/50) | 4% (2/50) |
| Day 3 | 0 | 60% (30/50) | 2% (1/50) |
| Day 7 | 0 | 28% (14/50) | 0 |
| Day 14 | 4% (2/50) | 18% (9/50) | 0 |
| Day 21 | 0 | 20% (10/50) | 0 |
| Day 28 | 0 | 20% (10/50) | 2% (1/50) |
The prevalence of parasitemia by PCR on day 1, day 2, and day 3 following initiation of AL therapy was 92%, 78%, and 60%, respectively. Three and two patients in the follow-up who showed clinical features of malaria on day 7 and day14, respectively, were P. falciparum positive using PCR. Following AL treatment, residual submicroscopic parasitemia persisted in some patients after completing the full dose of the treatment. Fourteen of 50 (28%) patients tested were parasite positive on day 7 by PCR. Eight patients who were PCR negative after AL treatment became PCR positive during the follow-up. Of those, 2, 3, and 3 patients became PCR positive on day 14, day 21, and day 28, respectively. In addition, among 7 patients, persistent residual submicroscopic parasitemia was detected from day 0 to day 28 by PCR (Table 2). None of the study participants reported adverse drug reactions to AL.
P. falciparum PCR positivity on day 3 after initiation of AL treatment was not associated with areas of residence, sex, or ethnic group of study participants (P > 0.05), but the PCR positivity on day 3 was more among study participants <15 years old as compared with ≥15 years old (AOR = 6.44, P = 0.027). Fever and parasitemia at baseline were not associated with PCR positivity on day 3 (Table 3).
TABLE 3.
Socio-demographic and baseline clinical characteristics of patients in relation to PCR positivity on day 3 (n = 50)a
| Day 3 parasite positivity |
COR | P value | AOR | P value | ||
|---|---|---|---|---|---|---|
| Characteristics | Positive | Negative | ||||
| Study site | ||||||
| Sherkole | 18 | 14 | 1.556 | 0.472 | 4.82 | 0.06 |
| Kurmuc | 12 | 16 | 1 | 1 | ||
| Ethnicity | ||||||
| Berta | 24 | 12 | 2.67 | 0.129 | 2.21 | 0.375 |
| Others | 6 | 8 | 1 | 1 | ||
| Age | ||||||
| <15 yrs | 24 | 9 | 4.89 | 0.013 | 6.44 | 0.027 |
| ≥15 yrs | 6 | 11 | 1 | 1 | ||
| Baseline clinical data | ||||||
| Fever | ||||||
| Yes | 24 | 16 | 1 | 1 | 1.5 | 0.384 |
| No | 6 | 4 | 1 | 1 | ||
| Parasitemia | ||||||
| <50,000/μL | 15 | 13 | 1.857 | 0.3 | 1.5 | 0.562 |
| ≥50,000/μL | 15 | 7 | 1 | 1 | ||
COR, crude odds ratio; AOR, adjusted odds ratio.
Sex, age, ethnicity, and baseline clinical characteristics of study participants were not associated with PCR positivity after day 7 of the follow-up (P > 0.05), but there was significant association between area of residence and P. falciparum PCR positivity after day 7 of the follow-up (AOR = 0.05, P = 0.002) (Table 4).
TABLE 4.
P. falciparum PCR positivity after day 7 in association with socio-demographic and baseline clinical characteristics of patientsa
| Characteristics | PCR positivity |
COR | P value | AOR | P value | |
|---|---|---|---|---|---|---|
| PCR+ | PCR– | |||||
| Study site | ||||||
| Sherkole | 4 | 28 | 0.091 | 0.001 | 0.05 | 0.002 |
| Kurmuk | 11 | 7 | 1 | 1 | 11 | |
| Sex | ||||||
| Male | 9 | 25 | 0.6 | 0.43 | 0.861 | 0.852 |
| Female | 6 | 10 | 1 | 1 | ||
| Ethnicity | ||||||
| Berta | 11 | 25 | 0.91 | 0.95 | 0.547 | 0.51 |
| Others | 4 | 10 | 1 | 1 | ||
| Age | ||||||
| <15 yrs | 10 | 23 | 0.96 | 0.891 | 0.314 | 0.28 |
| 15 yrs | 5 | 12 | 1 | 1 | ||
| Baseline clinical data | ||||||
| Fever | ||||||
| Yes | 12 | 28 | 1 | 1 | 0.638 | 0.681 |
| No | 3 | 7 | 1 | 1 | ||
| Parasitemia | ||||||
| <50,000/μL | 10 | 18 | 1.9 | 0.323 | 1.12 | 0.83 |
| ≥50,000/μL | 5 | 17 | 1 | 1 | ||
COR, crude odds ratio; AOR, adjusted odds ratio.
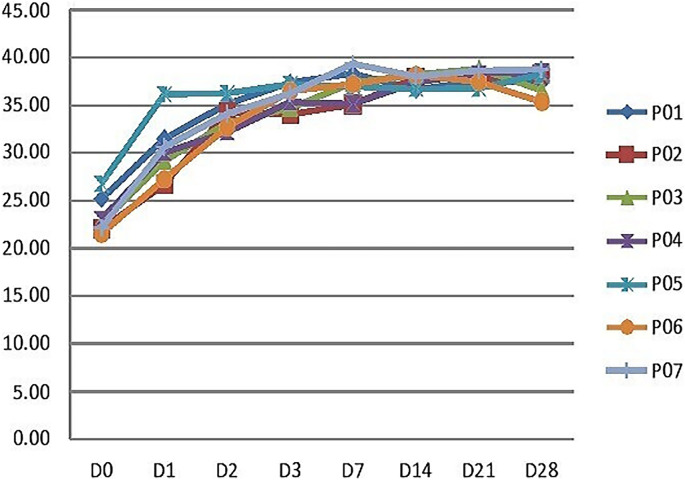
Quantification cycle value analysis of the P. falciparum on day 0 and the follow-up days of the seven patients with persistent PCR positivity indicated the rapid reduction of the parasite density following treatment. However, after day 7 of the treatment the residual submicroscopic parasitemia level remained constant and persisted in patients, as shown in (Fig. 1).
FIG 1.
Quantification cycle (Cq) value of P. falciparum on day 0 and follow-up days of seven patients with persistent PCR positivity as detected by real-time PCR.
(iii) PCR-corrected assessment of AL treatment outcomes. The two patients that showed recurrent malaria on day 14 were confirmed as recrudescence by msp1 and msp2 genotyping of day 0 and day 14 samples (Table 5). Thus, PCR confirmed that AL late clinical failure (LCF) occurred in two patients. Kaplan-Meier estimate of the day 28 efficacy rate of AL among study participants was 96% (95% CI: 84.9–99) with PCR corrected (Fig. S1 in the supplemental material).
TABLE 5.
A measured band lengths of msp1 and msp2 of the two patients with recrudescences on day 0 and day of recurrency
| Patients with treatment failure | Genes | Band length of the Loci in bp |
|
|---|---|---|---|
| Day 0 | Day 14 | ||
| Patient 1 | msp1 | 160 | 159 |
| msp2 | 202, 328, 337 | 202, 328 | |
| Patient 2 | msp1 | 186, 248 | 186, 249 |
| msp2 | 222,342 | 221, 341 | |
DISCUSSION
WHO advocates increased monitoring and surveillance of ACT efficacy against malaria to identify and contain artemisinin resistance (26). Malaria-endemic areas in the Asossa zone are not represented in current surveillance of ACT- resistance malaria in Ethiopia; therapeutic efficacy of AL was not done in this area for more than a decade.
In this study, all patients were fever-free on day 3. Nonetheless, three patients showed clinical features of malaria on day 7. In these patients, the presence of the P. falciparum parasite was confirmed using PCR although this was not detected by microscopy. In addition, on day 14 of the follow-up, two patients showed symptoms of malaria (history of fever within 24 h and chills, headache, and back pain). In these patients, the presence of the parasite was confirmed using both light microscopy and PCR. These findings indicated that AL treatment effectively resolved malaria symptoms on day 3; few patients developed clinical malaria during the follow-up period.
The parasite positivity rate on day 3 by light microscopy was zero. Thus, on day 3 all study patients cleared the parasites based on microscopy. However, 60% of patients were PCR positive on day 3. This PCR positivity after artemether-lumefantrine therapy might be due to residual DNA and/or gametocytes in the absence of viable parasites, indicating that PCR might overestimate parasite prevalence on day 3 after treatment. This posttreatment residual submicroscopic parasite prevalence was comparable with the residual parasitemia on day 3 (68.5%) reported from Faladje, Mali (8). The rate was also higher than 17.7% reported in another study in Mali and Burkina Faso (27).
PCR positivity on day 3 after initiation of the drug was more common in the age group <15 years compared to study participants ≥15 years old (AOR = 6.44, P = 0.027). The association between increasing age and early parasitological clearance has been previously reported (28–30). In high malaria transmission areas, the chance of developing acquired immunity to malaria increases as individuals get older. Acquired immunity is lower in younger individuals, and it has a pivotal role in modulating early parasitological response to treatment with ACTs (29, 31). PCR positivity on day 3 was not associated with baseline parasite density. This finding contrasted with previous findings in Mali and Burkina Faso (27) and Tanzania (32) and could be related to host or parasite factors.
Efficacy data from the current study showed a high cure rate of AL. The PCR-corrected adequate clinical and parasitological response on day 28 was 96%. Two patients (4%) were classified as a late clinical failure as they showed symptoms of malaria and parasite positivity on day 14 detected in microscopy. This study indicated that AL remains highly efficacious for the treatment of uncomplicated P. falciparum infection in the study site. This treatment success was comparable to previous studies done in Ethiopia (23, 33). In neighboring Sudan, a similar high treatment success rate of ACT was reported from a meta-analysis of 20 studies (34).
The persistence of PCR positivity following AL in this study was similar to those in Kenya that reported 37.1% residual parasitemia on day 7 (15), Uganda with 39·9% submicroscopic parasitemia persistence in children on day 17 (14), and Sumatra that reported 39% (35). The finding was also similar to another study that was done in Uganda that reported more than 25% of patients had circulating ring-stage parasites by qRT-PCR at least 14 days postinitiation of ACT or ACT-primaquine (13).
Parasite positivity in PCR after day 7 was more in the Kurmuk district as compared with the Sherkole district (AOR = 0.05, P = 0.002). Persistence of the residual submicroscopic parasitemia in some patients might relate to inadequate artemether-lumefantrine levels in the blood of study participants following treatment. The role of the lumefantrine partner drug is to eliminate the residual parasites and cure the infection (36). Low lumefantrine concentration on day 7 was associated with P. falciparum recrudescence (19). Concomitant food consumption and the type of food taken might affect the absorption of lumefantrine among study participants (20, 21).
Although the treatment is effective in the study area, it is crucial to further assess factors that might relate to PCR positivity following AL treatment. The persistence of positive PCR after curative treatment of AL might be related to several weeks’ persistence of the remaining parasite DNA and gametocytes after treatment without evidence of viable asexual parasites (37, 38). This finding highlights the need of distinguishing active infections from dead pathogens or their debris (39) and submicroscopic gametocytemia (40).
Some patients remained PCR positive during the entire study period and might be reservoir hosts after the 28 days of the follow-up. In these patients, the residual submicroscopic parasitemia persists after AL treatment. This may contribute to the onward transmission of malaria among the surrounding human population. Residual P. falciparum parasitemia after ACT is associated with increased transmission to mosquitoes (41); 1. 88% of mosquitoes became infected after feeding on blood from AL-treated children (42). Asymptomatic recrudescence may be important for the spread of drug-resistant malaria (43, 44).
The persistence of the residual submicroscopic parasitemia in the current study indicated the role of microscopy and PCR in reporting the parasites following treatment with AL. Microscopy alone might not indicate the actual prevalence of parasitemia following initiation of ACT therapy. Thus, therapeutic efficacy studies should consider using ultrasensitive detection of P. falciparum to confirm the presence of low-density parasitemia and/or residual DNA in the absence of viable parasites or gametocytes following treatment of ACTs (45).
Conclusion.
Artemether-lumefantrine remains efficacious for the treatment of uncomplicated P. falciparum in the study area. However, in some patients, there was residual submicroscopic parasitemia after AL treatment. The persistence of PCR positivity and reappearance of PCR detectable parasites following AL treatment has public health implications for continued malaria transmission and may be important for the spread of drug-resistant malaria. There is a need to assess factors that contribute to the PCR positivity of the parasite that might compromise the treatment.
Limitations of the study.
Small sample size, lack of direct observation of the second dose of the drug, and absence of hemoglobin assessment were the limitations of this study.
MATERIALS AND METHODS
Study site and period.
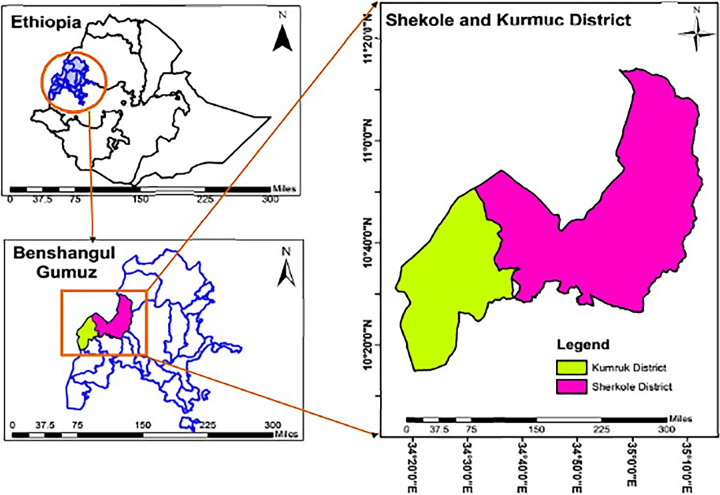
This study was conducted in Sherkole and Horazhab health centers close to the Ethio–Sudan border, located at 10.599 and 34.7803 and 10.56 and 34.2967 latitude and longitude, respectively. The two health centers are found in the Asossa zone in the Benishangul-Gumuz Region of Ethiopia. Sherkole Health Centre is located in the Sherkole district, which is bordered by Sudan in the north (Fig. 2). This district has a refugee camp that houses more than 14,431 Sudanese and South Sudanese (46). The town of Sherkole is about 98 km west of the town of Asossa. Horazhab health center is located in the Kurmuk district, and it is bordered by Sudan in the north and west. It is about 90 km from the town of Asossa (Fig. 2). This study was conducted during the high malaria transmission season from September to December 2020.
FIG 2.
Study area map, Sherkole and Kurmuk districts in the Asossa zone. Benishangul-Gumuz Regional State, western Ethiopia.
Study design.
The study was a prospective study design to assess the clinical and parasitological efficacy of AL for the treatment of uncomplicated falciparum malaria.
Sample size.
The sample size calculation was based on an assumed 97.01% therapeutic efficacy of AL in Ethiopia (33), a 95% confidence level, and 5% precision. Accordingly, the calculated sample size was 45. An additional 20% nonresponse rate was considered, so the sample size became 54.
Study population.
Patients with uncomplicated P. falciparum malaria attending the study health centers who consented to this study were enrolled if they were more than 2 years of age, had a fever (axillary temperature ≥37.5°C), and/or had a history of fever in the last 24 h, mono-infection with P. falciparum, and parasitemia of 2,000 to 200,000 asexual parasites/μL by microscopy. Patients who had received antimalarial drugs 6 days before enrollment were excluded from the study. Out of 50 patients who fulfilled the inclusion criteria and enrolled in the study, 32 and 18 patients were recruited from the Sherkole and Horazhab health centers, respectively. Once eligible patients were enrolled at the two study sites, they were treated with standard doses of AL and monitored for 28 days with clinical and parasitological assessments. Patients' clinical and parasitological assessments were done on day 0 and follow-up days 1, 2, 3, 7, 14, 21, and 28 based on the WHO template protocol for therapeutic efficacy studies (47).
Treatment of patients.
Artemether 20 mg + Lumefantrine 120 mg tablets (Ajanta Pharma Ltd, India) was administered twice daily for 3 days according to the weight of study participants: one tablet for ≥5 kg to <15 kg, two tablets for 15 kg to <25 kg, three tablets for 25 kg to < 35 kg, and four tablets for ≥35 kg. The first daily dose of the drug was directly observed at the study health centers. However, the second daily dose of the drug, taken at home, was not supervised. Then, after each dose, patients were observed for 30 min, and the dose was readministered if vomiting occurred. Adverse events were assessed and recorded during each visit of the patients.
Laboratory diagnosis of P. falciparum.
(i) Microscopy. Microscopy was performed from finger-prick blood collected during days 0, 1, 2, 3, 7, 14, 21, and 28. In addition, dried blood spots (DBS) on filter paper (Whatman No. 1001 320, International Ltd., Maidstone, England) were collected from finger-prick during each subsequent visit of the patients for molecular analysis of the parasite. The DBS was kept in plastic bags with desiccants.
Two blood slides were prepared during each subsequent visit, and both slides were stained with 10% Giemsa for 10–15 min. One of the stained slides was examined at the study health centers to determine the infection status of the patients and to estimate the parasite density. The second stained slide was kept for reading by a second microscopist at the Adama health center. If there was any discrepancy in the parasitemia report between the first and second reader, a third microscopist would read the slides.
Thick and thin Giemsa-stained blood films for parasite counts and species identification were examined at the screening on day 0 to confirm adherence to the inclusion criteria. Thick blood films were examined on days 1, 2, 3, 7, 14, 21, and 28 to quantify malaria parasites. Parasitemia was measured by counting the number of asexual parasites against 200 leucocytes in thick blood films. Parasites per microliter of blood were determined assuming 8,000 white blood cells (WBCs) per microliter of blood.
(ii) Molecular diagnosis. The molecular diagnosis of the parasites was done at Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine. It was performed on DNA extracted from DBS collected from each patient from enrollment (day 0) to days 1, 2, 3, 7, 14, 21, and 28 using the Chelex DNA extraction protocol as earlier described (48).
P. falciparum detection was performed by var gene acidic terminal sequence (varATS) real-time PCR using the Bio-Rad CFX96 real-time PCR machine as previously described (45). This assay had a limit of detection of 0.06 to 0.15 parasites/μL blood and was 10× more sensitive than standard 18S rRNA qPCR (45). Quantification cycle (Cq) values less than 40 were indicative of the varATS amplification in the samples. Discrimination of recrudescence from new infections was determined by comparing merozoite surface protein 1 and 2 alleles (msp1 and msp2) of samples collected at baseline with those collected on the day of recurrent infection observed after day 7.
MspI and msp2 genomic DNA of the parasite was amplified by multiplex primary and nested PCR using allelic specific primers as per published protocol (49). Allelic variants of msp1 (K1, MAD 20, and RO33) and msp2 (FC27 and 3D7) were detected by allelic family-specific nested PCR amplification. Genotypes were distinguished using their fluorescent dye (indicating the allelic family) and by their size, which was determined by Qiaxcel software.
Data analysis.
Data entry and analysis was done by using the WHO-designed Excel spreadsheet and SPSS 20.0 statistical software package (SPSS, Inc., Chicago, USA). AL treatment outcomes were classified based on parasitological and clinical outcomes assessments as recommended by WHO (50). Descriptive statistics were reported. Binary logistic regression analysis was done to determine factors associated with parasite PCR positivity during the follow-up. A P value of less or equal to 0.05 was considered significant.
Ethical consideration.
Ethical approval was obtained from the Ethiopian National Ethic review committee, Addis Ababa, Ethiopia (No. MoSHE 04/246/66) and Aklilu Lemma Institute of Pathobiology, Institutional Review Board, Addis Ababa University (No. ALIPB IRB/19/2012/20). Permission to conduct the study at the health facilities was sought from the relevant regional and district health authorities. Written informed consent was obtained from adult study participants and a parent or guardian of a child. Written informed assent was also taken from children.
Data availability.
The data sets and analyzed result of the study are available on the corresponding author and can be obtained on reasonable request.
ACKNOWLEDGMENTS
We thank all P. falciparum patients for their willingness to participate in the study. We also thank the Medical Research Council Unit the Gambia, at London School of Hygiene and Tropical Medicine, Gambia, for the permission to use the molecular biology laboratory and the PAMGEN H3Africa project for financial support.
Conceptualization: L. Golassa. Supervision of the overall work: L. Golassa, A. Amambua-Ngwa, and S. Dugassa. Methodology: G. Tadele and L. Golassa. Laboratory work: G. Tadele, F. K. Jaiteh, E. Oriero, and M. Oboh. Original draft of the manuscript: G. Tadele. Reviewing and editing the manuscript: L. Golassa, A. Amambua-Ngwa, S. Dugassa, E. Oriero, and M. Oboh. All authors read and approved the final manuscript.
This work was supported by the PAMGEN H3 Africa project (H3A/002/18) and Addis Ababa University, Post Graduate studies.
We declare that we have no competing interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2010. Global report on antimalarial drug efficacy and drug resistance: 2000–2010. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, Lin K, Kyaw MP, Plewes K, Faiz MA, Dhorda M, Cheah PY, Pukrittayakamee S, Ashley EA, Anderson TJC, Nair S, McDew-White M, Flegg JA, Grist EPM, Guerin P, Maude RJ, Smithuis F, Dondorp AM, Day NPJ, Nosten F, White NJ, Woodrow CJ. 2015. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis 15:415–421. 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thuy-Nhien N, Tuyen NK, Tong NT, Vy NT, Thanh NV, Van HT, Huong-Thu P, Quang HH, Boni MF, Dolecek C, Farrar J, Thwaites GE, Miotto O, White NJ, Hien TT. 2017. K13 propeller mutations in Plasmodium falciparum populations in regions of malaria endemicity in Vietnam from 2009 to 2016. Antimicrob Agents Chemother 61:e01578-16. 10.1128/AAC.01578-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. 2020. World malaria report 2020: 20 years of global progress and challenges. WHO, Geneva, Switzerland. [Google Scholar]
- 6.Schallig HD, Tinto H, Sawa P, Kaur H, Duparc S, Ishengoma DS, Magnussen P, Alifrangis M, Sutherland CJ. 2017. Randomized controlled trial of two sequential artemisinin-based combination therapy regimens to treat uncomplicated falciparum malaria in African children: a protocol to investigate the safety, efficacy, and adherence. BMJ Global Health; 2:e000371. 10.1136/bmjgh-2017-000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dama S, Niangaly H, Ouattara A, Sagara I, Sissoko S, Traore OB, Bamadio A, Dara N, Djimde M, Alhousseini ML, Goita S, Maiga H, Dara A, Doumbo OK, Djimde AA. 2017. Reduced ex vivo susceptibility of Plasmodiumfalciparum after oral artemether-lumefantrine treatment in Mali. Malar J 16:59. 10.1186/s12936-017-1700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kone A, Sissoko S, Fofana B, Sangare CO, Dembele D, Haidara AS, Diallo N, Coulibaly A, Traore A, Toure S, Haidara K, Sanogo K, Sagara I, Beshir KB, Gil JP, Doumbo OK, Djimde AA. 2020. Different Plasmodium falciparum clearance times in two Malian villages following artesunate monotherapy. Int J Infectious Diseases 95:399–405. 10.1016/j.ijid.2020.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayogu EE, Ukwe CV, Nna EO. 2015. Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated plasmodium falciparum malaria in Enugu. Trop J Pharm Res 14:1487–1493. 10.4314/tjpr.v14i8.23. [DOI] [Google Scholar]
- 10.Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, Habimana RM, Rucogoza A, Moriarty LF, Sandford R, Piercefield E, Goldman I, Ezema B, Talundzic E, Pacheco MA, Escalante AA, Ngamije D, Mangala J-LN, Kabera M, Munguti K, Murindahabi M, Brieger W, Musanabaganwa C, Mutesa L, Udhayakumar V, Mbituyumuremyi A, Halsey ES, Lucchi NW. 2021. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infectious Diseases 21:1120–1128. 10.1016/S1473-3099(21)00142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Infectious Disease Research and Policy. 2021. Africa Artemisinin-resistant malaria detected in Uganda. https://www.cidrap.umn.edu/news-perspective/2021/09/artemisinin-resistant-malaria-detected-uganda.
- 12.Mwaiswelo R, Ngasala B. 2020. Evaluation of residual submicroscopic Plasmodium falciparum parasites 3 days after initiation of treatment with artemisinin-based combination therapy. Malar J 19:162. 10.1186/s12936-020-03235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H-H, Meibalan E, Zelin J, Daniels R, Eziefula AC, Meyer EC, Tadesse F, Grignard L, Joice RC, Drakeley C, Wirth DF, Volkman SK, Buckee C, Bousema T, Marti M. 2016. Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci Rep 6:26330. 10.1038/srep26330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betson M, Sousa-Figueiredo JC, Atuhaire A, Arinaitwe M, Adriko M, Mwesigwa G, Nabonge J, Kabatereine NB, Sutherland CJ, Stothard JR. 2014. Detection of persistent Plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology 141:1880–1890. 10.1017/S003118201400033X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth JM, Sawa P, Omweri G, Makio N, Osoti V, de Jong MD, Schallig HDFH, Mens PF. 2018. Molecular detection of residual parasitemia after pyronaridine–artesunate or artemether– lumefantrine treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. Am J Trop Med Hyg 99:970–977. 10.4269/ajtmh.18-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ittarat W, Pickard AL, Rattanasinganchan P, Wilairatana P, Looareesuwan S, Emery K, Low J, Udomsangpetch R, Meshnick SR. 2003. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am J Trop Med Hyg 68:147–152. 10.4269/ajtmh.2003.68.147. [DOI] [PubMed] [Google Scholar]
- 17.Akcay SS, Ozyurek S, Inan A, Kuyumcu C, Barkay O, Erol S. 2021. Successful treatment of plasmodium falciparum malaria recrudescence with the same drug despite previous treatment failure. Infectious Diseases and Clinical Microbiology, Haydarpasa Numune Education and Research Hospital, Istanbul, Turkey. https://www.escmid.org/guidelines_publications/escmid_elibrary/material/?mid=16173. Accessed 18 December 2021. [Google Scholar]
- 18.Bourque DL, Chen LH. 2020. Plasmodium falciparum malaria recrudescence after treatment with artemether–lumefantrine. J Travel Medicine 27:taz082. 10.1093/jtm/taz082. [DOI] [PubMed] [Google Scholar]
- 19.WorldWide Antimalarial Resistance Network (WWARN) Lumefantrine PK/PD Study Group. 2015. Artemether-lumefantrine treatment of uncomplicated Plasmodium falciparum malaria: a systematic review and meta-analysis of day 7 lumefantrine concentrations and therapeutic response using individual patient data. BMC Medicine 13:227. 10.1186/s12916-015-0456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borrmann S, Sallas WM, Machevo S, González R, Björkman A, Mårtensson A, Hamel M, Juma E, Peshu J, Ogutu B, Djimdé A, D’Alessandro U, Marrast A-C, Lefèvre G, Kern SE. 2010. The effect of food consumption on lumefantrine bioavailability in African children receiving artemether–lumefantrine crushed or dispersible tablets (Coartem) for acute uncomplicated Plasmodium falciparum malaria. Tropical Medicine and International Health 15:434–441. [DOI] [PubMed] [Google Scholar]
- 21.Premji ZG, Abdulla S, Ogutu B, Ndong A, Falade CO, Sagara I, Mulure N, Nwaiwu O, Kokwaro G. 2008. The content of African diets is adequate to achieve optimal efficacy with fixed-dose artemether-lumefantrine: a review of the evidence. Malar J 7:244. 10.1186/1475-2875-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federal Democratic Republic of Ethiopia Ministry of Health. 2003. Ethiopia: postnatal Care.
- 23.Teklemariam M, Assefa A, Kassa M, Mohammed H, Mamo H. 2017. Therapeutic efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria in a high- transmission area in northwest Ethiopia. PLoS One 12:e0176004. 10.1371/journal.pone.0176004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abamecha A, Yilma D, Addisu W, El-Abid H, Ibenthal A, Noedl H, Yewhalaw D, Moumni M, Abdissa A. 2020. Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Chewaka District, Ethiopia. Malar J 19:240. 10.1186/s12936-020-03307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nega D, Assefa A, Mohamed H, Solomon H, Woyessa A, Assefa Y, Kebede A, Kassa M. 2016. Therapeutic efficacy of artemether-lumefantrine (Coartem) in treating uncomplicated P. falciparum malaria in Metehara, Eastern Ethiopia: a regulatory clinical study. PLoS One 11:e0154618. 10.1371/journal.pone.0154618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. 2014. Artemisinin resistance and artemisinin-based combination therapy efficacy: status report. WHO, Geneva, Switzerland. [Google Scholar]
- 27.Beshir KB, Diallo N, Somé FA, Sombie S, Zongo I, Fofana B, Traore A, Dama S, Bamadio A, Traore OB, Coulibaly SA, Maurice OS, Diarra A, Kaboré JM, Kodio A, Togo AH, Dara N, Coulibaly M, Dao F, Nikiema F, Compaore YD, Kabore NT, Barry N, Soulama I, Sagara I, Sirima SB, Ouédraogo J-B, Djimde A, Sutherland CJ. 2021. Persistent submicroscopic Plasmodium falciparum parasitemia 72 hours after treatment with artemether-lumefantrine predicts 42-day treatment failure in Mali and Burkina Faso. Antimicrob Agents Chemother 65:873. 10.1128/AAC.00873-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorsey G, Gasasira AF, Machekano R, Kamya MR, Staedke SG, Hubbard A. 2004. The impact of age, temperature, and parasite density on treatment outcomes from antimalarial clinical trials in Kampala, Uganda. The American J Tropical Medicine and Hygiene 71:531–536. 10.4269/ajtmh.2004.71.531. [DOI] [PubMed] [Google Scholar]
- 29.Ndour PA, Lopera-Mesa TM, Diakité SAS, Chiang S, Mouri O, Roussel C, Jauréguiberry S, Biligui S, Kendjo E, Claessens A, Ciceron L, Mazier D, Thellier M, Diakité M, Fairhurst RM, Buffet PA. 2015. Plasmodium falciparum clearance is rapid and pitting independent in immune Malian children treated with artesunate for malaria. J Infect Dis 211:290–297. 10.1093/infdis/jiu427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nji AM, Ali IM, Niba PTN, Marie-Solange E, Heumann C, Froeschl G, Mbacham WF. 2021. Discrete survival model analysis of Plasmodium falciparum response to artemisinin-based combination therapies among children in regions of varying malaria transmission in Cameroon. Pathogens 10:1106. 10.3390/pathogens10091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopera-Mesa TM, Doumbia S, Chiang S, Zeituni AE, Konate DS, Doumbouya M, Keita AS, Stepniewska K, Traore K, Diakite SAS, Ndiaye D, Sa JM, Anderson JM, Fay MP, Long CA, Diakite M, Fairhurst RM. 2013. Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity. J Infect Dis 207:1655–1663. 10.1093/infdis/jit082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwaiswelo R, Ngasala B, Jovel I, Xu W, Larsson E, Malmberg M, Gil JP, Premji Z, Mmbando BP, Mårtensson A. 2019. Prevalence of and risk factors associated with polymerase chain reaction-determined Plasmodium falciparum positivity on day 3 after initiation of artemether–lumefantrine treatment for uncomplicated malaria in Bagamoyo District, Tanzania. American Society of Tropical Medicine and Hygiene 100:1179–1186. 10.4269/ajtmh.18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayalew M. 2017. Therapeutic efficacy of artemether lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Ethiopia: a systematic review and meta-analysis. Infectious Dis of Poverty 6:157. 10.1186/s40249-017-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adam S, Ibrahim Y, Gasim GI. 2018. Efficacy and safety of artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Sudan: a systematic review and meta-analysis. Malar J 17:110–118. 10.1186/s12936-018-2265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubis IN, Wijaya H, Lubis M, Lubis CP, Beshir KB, Staedke SG, et al. 2020. Recurrence of Plasmodium malariae and P. falciparum following treatment of uncomplicated malaria in North Sumatera with dihydroartemisinin-piperaquine or artemether-lumefantrine. Open Forum Infec Dis 7:ofaa116. 10.1093/ofid/ofaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO. 2020. Tackling antimalarial drug resistance. WHO, Geneva, Switzerland. https://cdn.who.int/media/docs/default-source/malaria/drug-resistance/who-ucn-gmp-2020-07-eng.pdf?sfvrsn=a2c11533_2&download=true. [Google Scholar]
- 37.Vafa Homann M, Emami SN, Yman V, Stenström C, Sondén K, Ramström H, Karlsson M, Asghar M, Färnert A. 2017. Detection of malaria parasites after treatment in travelers: a 12-months longitudinal study and statistical modelling analysis. EBioMedicine 25:66–72. 10.1016/j.ebiom.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haanshuus CG, Mørch K. 2020. Detection of remaining Plasmodium DNA and gametocytes during follow up after curative malaria treatment among returned travellers in Norway. Malar J 19:296–296. 10.1186/s12936-020-03367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasini EM, Ierssel DV, Vial HJ, Kocken CH. 2013. A novel live-dead staining methodology to study malaria parasite viability. Malar J 12:190. 10.1186/1475-2875-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadesse FG, Lanke K, Nebie I, Schildkraut JA, Gonçalves BP, Tiono AB, Sauerwein R, Drakeley C, Bousema T, Rijpma SR. 2017. Molecular markers for sensitive detection of Plasmodium falciparum asexual stage parasites and their application in a malaria clinical trial. Am J Trop Med Hyg 97:188–198. 10.4269/ajtmh.16-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, Omar SA, Shekalaghe SA, Kaur H, Ndaro A, Chilongola J, Schallig HDFH, Sauerwein RW, Hallett RL, Bousema T. 2013. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin- combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infectious Diseases 208:2017–2024. 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, Manjurano A, Kavishe RA, Beshir KB, Yussuf RU, Omar SA, Hermsen CC, Okell L, Schallig HDFH, Sauerwein RW, Hallett RL, Bousema T. 2013. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infectious Diseases 207:1637–1645. 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- 43.Mumtaz R, Okell LC, Challenger JD. 2020. Asymptomatic recrudescence after artemether–lumefantrine treatment for uncomplicated falciparum malaria: a systematic review and meta-analysis. Malar J 19:453. 10.1186/s12936-020-03520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11:6, 623–639. 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. 2015. Ultra- sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 12:e1001788. 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.UNHCR. 2020. Sherkole camp profile. UNHCR data portal. https://data.unhcr.org/en/documents/details/83700. Accessed 22 October 2021.
- 47.Worldwide Antimalarial Resistance network (WWARN). WHO template protocol for therapeutic efficacy studies. https://www.wwarn.org/tools-resources/who-template-protocol-therapeutic-efficacy-studies.
- 48.Simon N, Shallat J, Wietzikoski CW, Harrington WE. 2020. Optimization of Chelex 100 resin-based extraction of genomic DNA from dried blood spots. Biology Methods and Protocols 5:bpaa009. 10.1093/biomethods/bpaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foley M, Ranford-Cartwright LC, Babiker HA. 1992. Rapid and simple method for isolating malaria DNA from finger prick samples of blood. Molecular and Biochemical Parasitology 53:241–244. 10.1016/0166-6851(92)90026-G. [DOI] [PubMed] [Google Scholar]
- 50.Bloland PB, Ringwald P, Snow RW, Global Partnership to Roll Back Malaria. 2003. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparium malaria. WHO, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/68453. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Download aac.00002-22-s0001.pdf, PDF file, 0.04 MB (43KB, pdf)
Data Availability Statement
The data sets and analyzed result of the study are available on the corresponding author and can be obtained on reasonable request.