Abstract
The landscape of asthma has considerably changed after 40 years of inhaled corticosteroid development and nearly 20 years since the first monoclonal antibodies (mAbs) were approved. New members of pharmacological families and more effective drug-delivery devices have been designed but the proportion of uncontrolled patients, unfortunately, remains stable. The most promising treatments now rely on targeted therapies that encourage the improvement of the characterisation of our patients. These clinical (phenotype) or new biological (endotype) tools lead to palpable personalised medicine. This review examines not only the future of mAbs and other new ways of treating asthma but also describes futuristic views based on the paradigm shifts that are ready to occur.
Short abstract
Future treatment for asthma: easier, safer, personalised http://ow.ly/V7b4h
Introduction
Imagining the future of asthma is a valuable exercise. Now is the time to combine the efforts of clinicians, researchers, patients and industry. The therapeutic management of asthma is particularly fascinating when exploring the patient's point of view. “Asthma” as a keyword in any web search engine offers more than 76 million answers and, after official websites, incredibly inventive alternatives appear. Since supply rarely exceeds demand, this illustrates how searchers strongly support the development of alternative ways of management. Moreover, disappointing reports have described quite stable proportions of uncontrolled patients in the general population [1, 2], reminding us of the relative gap between what medicine offers and communicates, and what our expectations are. Scientific peer-reviewed publications on asthma exceed 150 000 articles, with nearly 6% reporting the results of randomised controlled trials. Surprisingly, despite all these therapeutic trials and the availability of the strategy document published by the Global Initiative for Asthma [3], routine options to treat asthma remain limited, and the breadth of the drug panel is quite narrow and strongly dominated by steroids [4].
New paradigms in asthma management have mostly emerged from real-life studies; disease modifiers are seen as the Holy Grail [5–10] and immunotherapies are still forthcoming [11]. Behavioural modifications and their impact, mainly involving the microbiome [12–15], offer a great illustration of gene–environment interactions and suggest attractive opportunities. Environmental control is a challenging concern now embraced by physicians.
Improving what already exists is a valuable option when considering the efficacy and efficiency of currently available options. Improvement of drug delivery, better devices and new members of pharmacological families are subject to continuous and thorough development, but in the near future, treatment will certainly rely on the improvement of patient characterisation. Personalised medicine involves seeking the best ratio between efficacy and feasibility using clinical characteristics (called “phenotypes”) or guided by biomarkers (called “endotypes”) relevant to the underlying pathogenesis of asthma [16–19].
In the future, patients' insights may determine important gaps between research and expectations. Difficulty arises from disease heterogeneity. Addressing issues related to the most serious forms of asthma (e.g. severe asthma, eosinophilic granulomatosis with polyangiitis (EGPA) and allergic bronchopulmonary aspergillosis (ABPA)) is a priority and a dedicated plan similar to that developed for rare diseases may be beneficial. Ultimately, basic science will offer a better understanding of the pivotal mechanisms to achieve the challenges of: 1) controlling severe asthma; and 2) curing mild asthma.
Disease modifiers: changing risk
Behavioural modifications
Avoiding asthma onset is probably the best preventive strategy to develop. To achieve this, identifying people at risk, especially newborns and children, and adopting different behavioural advice are the first steps. A score, the modified Asthma Predictive Index (mAPI), has been developed and thoroughly analysed [20]. In a high-risk cohort, a positive mAPI correlated with the probability of future asthma (table 1).
TABLE 1.
Modified Asthma Predictive Index sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−) and post-test probabilities of asthma in unselected and high-risk populations at years 1, 2 and 3 from ages 6, 8 and 11 years
| Sensitivity % (95% CI) | Specificity % (95% CI) | LR+ (95% CI) | LR− (95% CI) | Unselected# | High risk¶ | |||
| Positive post-test probability % | Negative post-test probability % | Positive post-test probability % | Negative post-test probability % | |||||
| Age 6 years asthma diagnosis | ||||||||
| Year 1 | 11 (4.2–19) | 98 (96–100) | 6.1 (1.8–21) | 0.90 (0.83–0.98) | 43 | 10 | 73 | 28 |
| Year 2 | 12 (4.2–19) | 99 (98–100) | 14 (2.6–80) | 0.89 (0.82–0.97) | 64 | 10 | 86 | 28 |
| Year 3 | 17 (8.4–25) | 99 (98–100) | 21 (4.0–112) | 0.84 (0.75–0.93) | 72 | 9 | 90 | 2 |
| Age 8 years asthma diagnosis | ||||||||
| Year 1 | 8.2 (2.2–14) | 98 (97–100) | 5.3 (1.3–22) | 0.93 (0.87–1.0) | 40 | 10 | 69 | 29 |
| Year 2 | 11 (3.9–18) | 99 (98–100) | 12 (2.1–64) | 0.90 (0.83–0.97) | 59 | 10 | 83 | 28 |
| Year 3 | 19 (8.8–25) | 100 (99–100) | 55 (3.3–913) | 0.83 (0.75–0.92) | 87 | 9 | 96 | 26 |
| Age 11 years asthma diagnosis | ||||||||
| Year 1 | 11 (3.6–18) | 98 (95–100) | 4.9 (1.4–17) | 0.91 (0.83–1.0) | 38 | 10 | 68 | 28 |
| Year 2 | 11 (3.6–19) | 98 (96–100) | 6.8 (1.7–28) | 0.90 (0.83–0.98) | 46 | 10 | 75 | 28 |
| Year 3 | 19 (9.3–28) | 99 (97–100) | 19 (3.6–100) | 0.82 (0.73–0.92) | 70 | 9 | 89 | 26 |
#: 11% theoretical unselected population pre-test probability of asthma; ¶: 30% high-risk Childhood Origins of Asthma population pre-test probability of asthma. Reproduced and modified from [20] with permission from the publisher.
Measuring airway obstruction very early in life may also be appropriate [21], as it suggests inheritance of bronchial airway hyperresponsiveness far earlier than immune system maturation. Bisgaard et al. [21] showed that children developing asthma by age 7 years had a lung function deficit and increased bronchial responsiveness as neonates. Biological understanding of heredity in asthma highlighted impaired priming of the immune system through imbalanced antigen presentation predisposing to a T-helper cell (Th) type 2-oriented response, especially early in life [22, 23]. Subsequently, in line with the Hygiene Hypothesis, it was suggested that a broader diversity of the antigen repertoire would be sufficient for correct maturation of the immune system. Thus, diversity of the microbiome is supposed to be protective and all interventions affecting it, such as diet or antibiotic regimens administered early in life, will increase the likelihood of asthma onset [24, 25]. Of course, some limitations may preclude the success of these methods. Notably, antibiotics can save lives during early childhood, so these data should not be over-interpreted but imply a need for more accurate diagnosis of infection. A growing body of evidence has also highlighted the beneficial impact of interventions sharing similar objectives, such as restricting caesarean delivery to unavoidable cases [26, 27], avoiding maternal and environmental smoke exposure [28–30], and promoting breastfeeding [31, 32]. Moreover, some authors reported advantages of decreasing the “hyper-hygienic” environment and exaggerated exposure restrictions. Regarding the Hygiene Hypothesis, we need to address the question of what is a critical microbial exposure. The A20 protein may be part of the answer to this question. A20 (tumour necrosis factor-α-induced protein 3) is known to regulate interactions between dendritic cells and the airway epithelium. An A20 single-nucleotide polymorphism increases the risk of asthma and allergy in children who grew up on farms, and A20 modulations in different models critically modified asthma signature [33].
Environmental modifications
Politics may play a role in asthma prevention by adoption of the best environmental policies to improve respiratory health by lowering the risks from pollution. Recently, scientific societies have issued strong statements regarding air pollution. Other initiatives are supported by evidence but interventions could not be perfectly analysed in terms of reducing asthma risks. These include issues related to proximity between schools and major traffic areas [34], limiting environmental allergens, assessing and reducing indoor/outdoor air pollution [35], and of course, adapting occupational exposure [36].
The future
The real hopes rely on the ability of any given intervention to significantly affect the natural history of the disease, but demonstrating primary prevention efficacy is rather complex. It largely depends on the long-term incidence of any event related to the disease in a control group. Although specific immunotherapy successfully counteracts the progression of allergy by reducing the extent of sensitisation [37, 38], definitive evidence demonstrating a reduced risk of asthma development is still required. Inhaled corticosteroids (ICS) fail to achieve this goal even when administered very early in life [39]. Prediction of asthma onset is once again critical and strategies based upon genetic profiling may potentially achieved this aim [40, 41]. Nonetheless, limitation of other risk factors should be implemented but their comprehensive analysis is still limited. Vaccines are thought to ideally restore an adapted imbalance between T-cell subsets but to unclear extents [42, 43]. Epigenetic modifications are now regarded as the next step to encompassing evidence of the sustained impaired cross-talk within the airways between structural (epithelium, smooth muscle and fibroblasts) and inflammatory cells. Whether therapeutic interventions may successfully correct critical epigenetic modifications is a fascinating research question [44–48]. Answering it requires an understanding of which epigenetic modifications are responsible for asthma risk, their implications for asthma onset and maintenance, and the associated risks of such treatment. In this setting, microRNA studies, especially the use of antagomirs as potential therapeutic tools, are of great interest [44].
Bronchial thermoplasty was proposed 10 years ago as an interventional technique to reduce airway stiffness and excessive narrowing [49]. Even though the beneficial mechanism of action has not definitely been elucidated, sustained evidence of asthma improvement has been reported in well-designed trials but with a limited number of patients [50–52]. Larger trials are ongoing. Time will tell to what extent this technique affects the natural history of the disease and whether the best responders can be identified.
Therapeutic options
Improving what already exists
New ICS
For 40 years ICS have been used in asthma; today, their dominance is hard to contest and their achievement difficult to challenge from a scientific point of view. Reduced risk of asthma death is not their only achievement [53]: since the global pattern of asthma has changed worldwide, hospital admissions for asthma are becoming rare events [54]. However, ICS' side-effects cannot be ignored since they are now well documented: adrenal insufficiency, diabetes, skin bruising, oral mycosis, reduced bone density and reduced growth in children. Although rare, these events are nonetheless significant, but their incidence rates are insufficient to reverse the very positive benefit/risk balance associated with regular use of ICS in asthma [55, 56]. Notably, patients are more interested in these pitfalls than the benefits of ICS, and poor levels of adherence are potentially related to patients, and sometimes doctors, sharing a mistrust of ICS. Developing new ICS with a better pharmacological profile may potentially address most of these issues. Keeping the good effects and removing the bad requires a perfect understanding of mechanisms of action of ICS. In particular, nongenomic activities and/or selectivity for transactivating genomic properties are often seen as culprits, and some interesting attempts to counter these were proposed recently with modified analogues [57].
Improving drug half-life may also be of interest with a view to improving adherence. Prior experience with long-release intramuscular triamcinolone administration revealed the benefit of addressing adherence issues, but no one should ignore the unacceptable side-effect profile [58].
Other routes of administration have been tested and the concept of unified airways was reinforced by a successful attempt where nasal administration was significantly efficacious in improving airway inflammation [59–62].
New long-acting β-agonists and long-acting muscarinic agonists
New long-acting β-agonists (LABA) have been developed for chronic obstructive pulmonary disease (COPD) and, a priori, these might also reasonably be expected to be effective against asthma. The recently developed once-daily, very long-acting LABA (indacaterol, vilanterol, olodaterol and others [62–66]) potentially offer better bronchodilation patterns relevant to treating severe asthma with persistent airflow obstruction. Whether these drugs will also synergistically improve asthma control and reduce exacerbation rates is unknown at present.
Long-acting muscarinic antagonists initially developed for COPD are now widely used in asthma and tiotropium was recently approved for this indication [67]. Cholinergic pathway inhibition at the epithelial level may partly explain their ability to decrease the exacerbation rate when used as an add-on therapy [68, 69].
As for ICS, improving adherence would rely on improvements in device engineering [70] and pharmacological development to increase release duration and limit side-effects through higher selectivity.
Personalising medicine
The heterogeneity of asthma has been widely reported [18, 71–73]. Until 2000, hypothesis-driven studies, in which each opinion leader promoted their clinical observations and feelings, dominated the literature. Unfortunately, none of these approaches really led to targeted therapies. Atopic status split the asthma world for decades. If gastro-oesophageal reflux disease associated with asthma and premenstrual worsening of asthma were related to different pathophysiological pathways, dedicated therapies should have been beneficial. Later, nocturnal asthma was considered as a specific entity [74] before being integrated as a major item of asthma control. Subsequently, unbiased clustering analysis aimed to identify homogeneous phenotypes of asthma [18]. To date, these attempts have failed to identify the different physiopathology and outcomes underlying each cluster, and at present, there is no ongoing clinical trial stratifying therapeutic strategy according to these clusters.
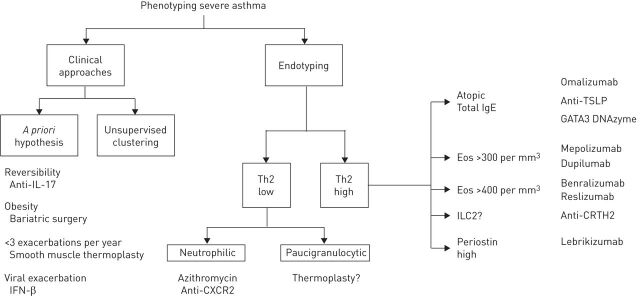
The Gaining Optimum Asthma Control study was pivotal to asthma understanding [75]. This study demonstrated the ability of ICS to gain asthma control in nearly 70% of patients, although total control achievement required the highest therapeutic pressure. Unfortunately, due to the design of the study, there were no data concerning the relevance of a therapeutic “stepping down”. Moreover, the beneficial effect of combination therapy versus fluticasone propionate alone was hardly convincing, as few clinically relevant differences were observed between the two groups. Subsequently, even though clinical heterogeneity is evident through different triggering and precipitating factors, the therapeutic consequences remain homogeneous for most patients, mostly consisting of ICS/LABA. Interestingly, this critical observation raised demands for a rigorous clinical pathway dedicated to distinguishing uncontrolled from severe asthma; in other words, to consider insufficiency of or resistance to ICS as a critical step [76]. Thus, the current strategy promoted by most researchers relies on the biological mechanisms related to the insufficient clinical response to the best standard of care; in practice, high doses of ICS, LABA and a third controller. These biological pathways forming the basis of endotypes of asthma are mainly based on the current Th2/non-Th2 paradigm (figure 1), but identification of the Th2 endotype is not simple. Transcriptomic analysis of sputum samples showed consistency and revealed the feasibility of this identification [77, 78], and some applicability in routine practice [79]. Blood eosinophil count probably represents an excellent compromise between specificity and availability [80]. These results should be considered promising but require confirmation since the number of patients included in the study was very limited. The challenge is now to find the best companion assay identifying the potential responders to any given targeted therapy. Blood eosinophils are easy to measure at low cost with a test that is rapidly available, reproducible throughout the world and shows good consistency in terms of predictive ability. Whether other combined tests will enter routine practice to optimise drug prescription is a matter of great debate.
FIGURE 1.
Severe asthma: from characteristics to phenotypes and to endotypes. IL: interleukin; IFN: interferon; Th: T-helper cell; TSLP: thymic stromal lymphopoietin; GATA3: GATA-binding protein 3; Eos: eosinophils; ILC: innate lymphoid cell; CRTH2: prostaglandin D2 receptor 2.
Important overlaps probably exist between these phenotypes. Because most of the drugs currently being developed target Th2 cytokines, mapping of this expected overlap is required by policy makers and clinicians (figure 1).
Endotype-based strategies: Th2 versus non-Th2
Th2 cytokines and their receptors and Th2 cells have more or less all been targeted (interleukin (IL)-4, IL-5, IL-9, IL-13, IL-23, IL-25, IL-33, IgE and thymic stromal lymphopoietin (TSLP)), and probably, others will be targeted in the future. Targeting of the Th2 subset through Th1 promotion [81–83] or through specific inhibition (GATA3-specific DNAzyme) [84] have been proposed, while the discovery of innate lymphoid cell (ILC)2 allowed their targeting with CRTH2 antagonists, which were rapidly transferred from bench to bedside [85–89]. The ability of each molecule to inhibit the late asthmatic response [57, 84, 90] is now considered an essential avenue of research, even though direct comparisons are difficult.
Th2-orientated therapies
Interleukin-5
The IL-5 story has evolved in two waves and has already been extensively reviewed (more reviews than original contributions are available, as summarised in table 2). Focusing on patients with an eosinophilic inflammation pattern came late in the development of IL-5-targeting agents. The important question at the time of writing is how to understand which threshold should be used for prediction of efficacy and which method should be preferred (blood eosinophils, sputum induction or even exhaled nitric oxide fraction). Potentially, not only will IL-5 targeting be promising in all situations where eosinophils play a debilitating role (EGPA, ABPA and nasal polyposis) but also other nonrespiratory diseases will be considered for such treatment and ongoing clinical trials will be actively surveyed.
TABLE 2.
Anti-interleukin-5 treatment for patients with severe asthma
| Administration | Dose | Patients | Study design | Subjects n | Primary end-point | Secondary end-point | Safety | Ref. | |
| Reslizumab | Intravenously once every 4 weeks over 52 weeks | 3 mg·kg−1 | Hypereosinophilic (induced sputum eosinophils >3%), high-dose ICS, ACQ >1.5, no OCS use | Phase III, double-blind RCT | 106 | Change in ACQ score from baseline to week 15: reslizumab, 0.7; placebo, 0.3 (p=0.054) | Number of exacerbations: OR 0.33 (95% CI 0.10–1.15; p=0.0833) | [91] | |
| Mepolizumab | Intravenously once every 4 weeks over 12 weeks | 250 or 750 mg | ICS <1000 μg per day BDP equivalent | Double-blind RCT | 362 | Change from baseline in morning PEF: no significant differences | Rate of exacerbations: no significant differences | SAEs: No significant differences with placebo | [92] |
| Intravenously once every 4 weeks over 52 weeks | 750 mg | Hypereosinophilic, recurrent severe exacerbations | Double-blind RCT, 2-week course of prednisolone | 61 | Relative risk of severe exacerbations during 50 weeks: 0.57 (95% CI 0.32–0.92; p=0.02) | Change in AQLQ: mean difference between groups +0.35 (95% CI 0.08–0.62; p=0.02) in mepolizumab versus placebo group | [93] | ||
| Intravenously once every 4 weeks over 52 weeks | 75, 250 or 750 mg | Hypereosinophilic, recurrent severe exacerbations | Double-blind RCT | 621 | Reduction of severe exacerbations during 52 weeks: 75 mg, 48% (95% CI 31–61%; p<0.0001); 250 mg, 39% (95% CI 19–54%; p=0.0005); 750 mg, 52% (95% CI 36–64%; p<0.0001) |
Difference from placebo in change in ACQ from baseline: 75 mg, −0.16 (95% CI −0.39–0.07); 250 mg, −0.27 (95% CI −0.51–0·04); 750 mg, −0.20 (95% CI −0.43–0.03) | SAEs: no significant differences with placebo | [94] | |
| Intravenously or subcutaneously once every 4 weeks over 32 weeks | 75 mg i.v. or 100 mg s.c. | Hypereosinophilic, recurrent exacerbations; high-dose ICS for one group | Double-blind, double-dummy RCT | 576 | Reduction of exacerbations during 32 weeks: 75 mg i.v., 47% (95% CI 29–61%; p<0.001); 100 mg s.c., 53% (95% CI 37–65%; p<0.001) | Improvement from baseline in SGRQ: 75 mg i.v., +6.4; 100 mg s.c., 7.0 (p<0.001) Improvement from baseline in ACQ-5: 75 mg i.v., 0.42; 100 mg s.c., 0.44 (p<0.001) | SAEs: no significant differences with placebo | [95] | |
| Subcutaneously once every 4 weeks over 24 weeks | 100 mg | Hypereosinophilic (>300 per mm3), OCS use >6 months | Double-blind RCT; phase of optimisation of OCS regimen | 135 | Reduction in daily OCS dose: OR 2.39 (95% CI 1.25–4.56; p=0.008) | Proportion of patients with >50% reduction of OCS dose: OR 2.26 (95% CI 1.10–4.65; p=0.03) | SAEs: no significant differences with placebo | [96] | |
| Benralizumab | Subcutaneously once every 4 weeks then every 8 weeks over 52 weeks | 2, 20 or 100 mg | Medium/high-dose ICS use, 2–6 exacerbations per year, stratification by eosinophilic status | Phase IIb, double-blind, dose-ranging study; placebo controlled | 324 eosinophilic 285 noneosinophilic | Annual exacerbation rate in eosinophilic individuals: 100 mg, 41% (95% CI 11–60%; p=0.096; 95% CI 80%); no significant differences in 2- or 20-mg group | SAEs: no significant differences; AEs: significantly more nasopharyngitis and injection site reactions | [97] |
ICS: inhaled corticosteroids; ACQ: Asthma Control Questionnaire; OCS: oral corticosteroids; BDP: beclomethasone dipropionate; RCT: randomised controlled trial; PEF: peak expiratory flow; AQLQ: Asthma Quality of Life Questionnaire; SGRQ: St George's Respiratory Questionnaire; SAE: serious adverse event; AE: adverse event.
TABLE 3.
Anti-interleukin-13 treatment for patients with severe asthma
| Drug | Administration | Dose | Patients | Study design | Subjects n | Primary end-point | Secondary end-point | Safety | Ref. |
| Lebrikizumab | Subcutaneously, once-monthly over 6 months | 250 mg | Severe asthma, ICS dose between 200 and 1000 μg fluticasone propionate equivalent | Phase III, double-blind RCT; placebo controlled, dose ranging | 219 | Mean±sem change in FEV1 from baseline at week 12: lebrikizumab +9.8±1.9% versus placebo +4.3±1.5% (p=0.02); relative increase higher in high-periostin subgroup (14±3.1% versus 5.8±2.1%) | No significant results concerning rates of exacerbations and change in ACQ-5 from baseline at week 12 | SAEs: no significant differences; more musculoskeletal events in lebrikizumab group (13.2% versus 5.4%, p=0.045) | [105] |
| Tralokinumab | Subcutaneously, once every 2 weeks over 13 weeks | 150, 300 or 600 mg | Moderate to severe asthma | Phase II, double-blind RCT; placebo controlled | 194 | Mean±sd change in ACQ-6 score from baseline at week 13: tralokinumab −0.76±1.04, placebo −0.61±0.90 (p=0.375) | Mean±sd change from baseline in FEV1 tralokinumab 0.21±0.38 L versus placebo 0.06±0.48 L (p=0.072) Decrease in β2-agonist use (puffs per day): tralokinumab 0.68±1.45 versus placebo 0.10±1.49 (p=0.020) | SAEs: no significant differences with placebo | [102] |
| Lebrikizumab | Subcutaneously once-monthly over 12 weeks | 125, 250 or 500 mg | Stable asthma with no ICS use | Phase II, double-blind RCT; placebo-controlled | 212 | Median (range) relative change in FEV1 from baseline at week 12: 125 mg, +3.5 (−1.1–8.1) (p=0.13); −250 mg, +4.8% (−0.1–9.7) (p=0.05); −500 mg, 2.3% (−2.6–7.3) (p=0.35) | No significant relative change in FEV1 in high-periostin subgroup Time to treatment failure: all groups, HR 0.21 (95% CI 0.09–0.47, p<0.001) No significant change in morning PEF or rescue medication use | SAEs: no significant differences with placebo | [103] |
| GSK67958 | Intravenously once-monthly over 12 weeks | 10 mg·kg−1 | Severe asthma, high-dose ICS | Phase III, double-blind RCT; placebo controlled | 198 | Adjusted least squares mean change in ACQ-7 score from baseline at week 12 (values): GSK67958, −0.31; placebo, −0.17 (p=0.058) | Adjusted least squares mean change from baseline in FEV1: GSK67958 −0.01 versus placebo 0.03 (p=0.276); similar results in patients with increased serum IgE levels or blood eosinophil counts | SAEs: no significant differences with placebo | [104] |
ICS: inhaled corticosteroids; RCT: randomised control trial; FEV1: forced expiratory volume in 1 s; ACQ: Asthma Control Questionnaire; HR: hazard ratio; PEF: peak expiratory flow; SAE: serious adverse event.
Areas of uncertainty will probably be similar to those encountered during the development of omalizumab: the ideal phenotype for introduction, the best dose and frequency of administration, the ideal outcome to follow and the maximal time of exposure in responders, and potential persistent effects. An interesting clustering analysis applied to the randomised trials testing mepolizumab identified body mass index and airway reversibility as significant predictors of efficacy [98].
Interleukin-13
For a decade, IL-13 has been considered a major Th2 cytokine involved in many biological processes relevant to asthma physiopathology and the abundance of data from mouse models is impressive [99–101]. IL-13 receptor is a complex assembly of both IL-4 and IL-13 receptor subunits, allowing for redundancy. Blocking IL-13 signalling is achievable through direct cytokine binding and many monoclonal antibodies (mAbs) have been developed following various clinical strategies [102–105]. Notably, periostin is a downstream IL-13-induced protein that offers a chance to assess the IL-13 signature in the serum. Lebrikizumab has been shown to selectively improve lung function in asthmatics with high levels of circulating periostin [105]. Whether a combination therapy will be further developed and accepted by regulatory agencies is an interesting possibility for the future of asthma. Other IL-13-blocking mAbs failed to achieve significant clinical successes and their development was halted (table 3).
Dupilumab targets both IL-13 and IL-4 [106]: it binds to the α-subunit of the IL-4 receptor and through this blockade, modulates signalling of both the IL-4 and IL-13 pathways. While the targeted population had lower asthmaseverity than those involved in the studies testing the previously described mAbs (patients with moderate asthma were included), this drug allowed a great asthma improvement while tapering the ICS dose until weaning. This trial design may illustrate one possibility for the future treatment of asthma if the cost remains acceptable: subcutaneous administrations of an “as free as possible from side-effects” drug at “as spaced as possible” intervals, liberating people from the constraints of daily treatment.
Other Th2 initiatives
TSLP neutralisation has been proposed as a necessary step to establish definitively the pivotal role of this cytokine in the impaired epithelial–inflammatory crosstalk that is supposed to resume the pathogenesis of Th2-driven asthma. In a recent report, inhibition of the late asthmatic response by this molecule reached 45.9% at day 84 in mild allergic asthmatics [90]. Whether longer-term benefits will be confirmed in this population and in more diverse populations is of great interest.
GATA3 is an important transcription factor for T-cell development. It is involved in Th2 cell activation and signalling [107]. A very original approach opens the possibility of using new specific inhibitors of RNA transcription, DNAzymes, which are short DNA sequences able to cleave specifically recognised RNA molecules. They are very cheap and easy to administer. A German team has presented promising results showing a great reduction in the late asthmatic response, especially in patients with high blood eosinophil levels [84].
Other members of the Th2 family are potentially interesting to target. IL-33 and its receptor ST-2 are receiving particular attention [108]. Whether a companion biomarker can be used to improve identification of the best responders is critical to whether these targets will be used.
Non-Th2-dedicated drugs
Unfortunately, this endotype is far less well understood and very few attempts to treat it are ongoing. The overlap of this endotype with the neutrophilic pattern is usually accepted. Endotype–phenotype translations are not clear at all. Moreover, confusion with ICS and/or oral corticosteroid side-effects is frequently reported and subsequently, targeting neutrophils may potentially be inadequate. CXCR2 neutralisation was nonetheless shown to be safe and potentially relevant in patients with high neutrophil levels in their sputum [109]. CXCR2 is the main IL-8 receptor expressed by neutrophils, so its neutralisation makes them “deaf” to their main activating signal not only at the site of inflammation but also at the bone marrow level.
Low-dose azithromycin regimens have been shown to reduce neutrophilic inflammation in the airways of patients suffering from various chronic diseases, mainly cystic fibrosis (CF) and non-CF bronchiectasis [110]. In an investigator-led trial, it was shown to decrease the exacerbation rate in the neutrophilic subgroup of asthmatic patients [111]. Prospective trials are ongoing.
Phenotype-based strategies
It seems that a certain level of disconnection exists between hypothesised phenotypes and therapeutic trials. Very few biological drugs specifically target clinical characteristics of asthma. Of course, treatment of asthma is not restricted to the development of new drugs and the interesting proposition of specific management has been raised.
Atopic status
Atopy is probably the first phenotypic trait identified in order to personalise asthma treatment. Avoidance and immunotherapy are the two cornerstones of its management [25, 38]. When asthma control remains insufficient even with the help of antiasthma drugs, omalizumab can be used as a rescue add-on therapy. Abundant evidence of its effectiveness is available [112–115]. New outlooks on anti-IgE blockade offer perspectives on improving IgE binding efficacy, decreasing immunisation risks and, potentially, understanding whether atopic status is mandatory [116]. The optimal frequency of administration, duration of treatment (for example, before worsening seasons), etc., of quilizumab are worthwhile issues for both current and future IgE-blocking mAbs [117].
Reversibility: IL-17
High reversibility (over 12% change in forced expiratory volume in 1 s (FEV1) after bronchodilator inhalation) is uncommon and its clinical meaning is controversial. Blocking IL-17 initially appeared to be relevant in neutrophilic asthma but further and more in-depth analysis of animal models revealed an unexpected and direct bronchoconstriction effect [118]. Subgroup analysis of the phase II clinical trial of brodalumab, a mAb directed against IL-17, showed efficacy in patients with a high level of airway reversibility to short-acting β2-agonists [119]. A new trial was subsequently designed within this particular phenotype of asthma but it was terminated early due to a lack of observed efficacy at the pre-specified interim analysis; the decision was not related to safety concerns.
Cough/sputum
Chronic cough and mucus production are disabling symptoms frequently reported by patients. Even though the dedicated tools to describe these symptoms remain poor, some authors have described this phenotypic marker with dedicated targeted therapies for so-called cough-variant asthma [120–122]. Goblet cell hyperplasia is now regarded as a consequence of the remodelling process, mainly through an IL-13-dependent phenomenon [99], but this was not addressed in anti-IL13 trials. Muc5AC knockout mice are more sensitive to viral infections [123], and other pneumoproteins released into the airway lumen also offer interesting defensive activities against inhaled toxicants and microorganisms. Other known triggers involved in goblet cell hyperplasia (NOTCH, SPDEF and others) may represent new therapeutic avenues, but basic science studies are needed. Of note, Grainge et al. [124] completely challenged commonly accepted paradigms. In short, they showed that goblet cell hyperplasia could be induced by a provocation challenge with allergen but also with the nonspecific and noninflammatory methacholine, and could be prevented by inhaled bronchodilators irrespective of the level of airway inflammation.
Chronic airway obstruction
Addressing chronic airflow obstruction in asthma is different than in COPD. The underlying remodelling process is less well understood in asthma; moreover, evidence of it can be seen very early in life. Small airway obstruction is a critical step in COPD progression (in terms of FEV1 decline) but is less clear in asthma. Autopsy studies identified airway remodelling through mucus plugging of the small airways and smooth muscle enlargement as major determinants of chronic airflow obstruction. Smooth muscle hyperplasia is a potential determinant of thermoplasty benefits [52] and the area occupied by the smooth muscle in the bronchial biopsy became an inclusion criterion in an ongoing clinical trial.
Aspirin sensitivity
Interfering with the eicosanoid metabolism appears logical in patients reporting aspirin and other nonsteroidal anti-inflammatory drugs as significant asthma triggers. Leukotriene receptor antagonists were reported as good candidates for this [125, 126]. More recently, the benefit of aspirin tolerance induction on overall asthma control was suggested [127] and dedicated trials are ongoing. Potentially, this phenotype could become a better defined or broader entity.
Obesity
Overweight and obesity are serious conditions by themselves but they become challenging when combined with asthma. The parallel epidemics of asthma and obesity suggest some related pathogenesis but, to date, no common pharmaceutical strategy has emerged. Adipokines may be involved [128, 129]. Steroids are often seen as culprits for weight gain even though this is not always clear. A vicious circle may arise between uncontrolled asthma symptoms, increased steroid use and obesity. Small airway abnormalities have been shown to play an important role in obese people with asthma [130] but the exact nature of the problem is not well understood. Controversial studies have reported benefits from bariatric surgery on asthma outcomes, notably on the level of bronchial hyperresponsiveness [131, 132]; however, bariatric surgery is not always possible. Diagnostic consolidation, a real issue to address in this context, and identification of common biological disturbances are important paths to follow.
Two causes for concern
Allergic bronchopulmonary aspergillosis
ABPA is an incompletely understood phenotype of asthma. Overlapping forms of this disease exist both from the mildest and the most severe sides. It can occur alone or in other contexts such as CF. Depending on the chosen definition, it may appear quite frequent or very rare. Two axes of development can be expected. With regard to antimycotic therapy, nebulised formulations of liposomal amphotericin B can be tried in order to reduce the risks of side-effects including medication interactions [133]. This is quite promising in the field if the risk does not outweigh the benefits. Conversely, targeting IgE and/or eosinophilia is potentially achievable through IgE neutralisation by mAbs [134] or dialysis, or IL-5 pathway blockade. The best time for intervention and a better understanding of how the very particular mucus plugging of the proximal airways leads to irreversible bronchiectasis are unmet needs in ABPA.
Eosinophilic granulomatosis with polyangiitis
Since EGPA has been renamed and its boundaries better described, important efforts have been made to identify coherent, evidence-based therapeutic advice for its treatment. Notably, anti-neutrophil cytoplasmic antibody positivity has allowed for better diagnostic accuracy in many unclear clinical situations. Rituximab was recently shown to be a serious alternative to azathioprine after cyclophosphamide pulses [135]. Omalizumab and mepolizumab were successfully tested as maintenance treatment allowing for tapering of the daily oral steroid dose [136]. A large randomised controlled trial is ongoing in EGPA using monthly subcutaneous administration of mepolizumab [137].
New schemes
Resolution
Severe asthma, whatever the phenotyping definition, is characterised by excessive chronic airway inflammation. The most widely used class of anti-inflammatory and immunosuppressive drugs is the glucocorticoids, which represent the first successful exploitation of an endogenous anti-inflammatory mediator, cortisol.
In response to acute inflammation, a protective host response occurs in order to return to tissue homeostasis, which is called resolution. Lipid mediators, including eicosanoids, prostaglandins and leukotrienes, stimulate blood flow changes, oedema and neutrophil influx to tissues in order to resolve inflammation. Moreover, novel compounds have been identified as active pro-resolving mediators such as lipoxins, protectins, resolvins and maresins (collectively termed specialised pro-resolving mediators (SPMs)).
In severe asthma, which is characterised by asthma instability despite adequate treatment, the generation of lipoxin A4 is reduced [138], leading to the hypothesis that the reduction in pro-resolving activity may be the cause of asthma persistence. Recent findings have shown that the soluble epoxide hydrolase inhibitors would be of interest because they lead to the activation of endogenous pro-resolving mediators such as lipoxin A4 and epoxyeicosatrienoic acids, while at the same time inhibiting pro-inflammatory pathways [139].
Protectin D1 has been documented in condensates of exhaled breath from healthy human subjects [140]. Protectin D1 is the dominant docosadexaenoic acid (DHA)-derived SPM biosynthesised in human eosinophils. In addition, protectin D1 itself negatively regulates the function of eosinophils by inhibiting their chemotaxis and the expression of adhesion molecules. Miyata et al. [141] described an impairment of this negative feedback system mediated by protectin D1 in eosinophils of patients with severe asthma.
Resolvins D1 and E1, which are endogenously generated from the essential fatty acids DHA and eicosapentaenoic acid, promote the resolution of allergic airway inflammation via shared and distinct molecular counter-regulatory pathways. They inhibit aberrant neutrophil trafficking and activation, stimulate efferocytosis of apoptotic neutrophils, and promote antiangiogenic, antifibrotic and anti-infective actions.
Analysis of lipid mediators in murine lungs by metabololipidomics identified increased levels of DHA-derived maresin 1 (MaR1) during the resolution phase of self-limited allergic inflammation. Exogenous MaR1 potently regulated ILC2s and lung inflammation, in part by de novo generation of regulatory T-cells that inhibited ILC2s in a transforming growth factor-β-dependent manner during cell–cell interactions [142]. For these last twp SPMs, no data are available in humans.
Are there potential new therapeutic pathways using these SPMs' action? Benzo-lipoxin A4 is currently under phase I/II study in the treatment of gingivitis. The synthetic resolvin analogue RX-10001 (resolvin E1) is currently in phase II for dry eye syndrome, and RX-10045 is in phase I in asthma, inflammatory bowel disease, rheumatoid arthritis and cardiovascular diseases (both Resolvyx Pharmaceuticals, Cambridge, MA, USA).
Interferon-β: only with symptoms
Another view of asthma management in the future would rely on identification of viral-triggered asthma exacerbations. Rhinoviruses are by far the most common cause of exacerbation. A great deal of research has hypothesised that this increased asthma susceptibility is related to an impaired interferon (IFN) response to infection [143]. Supplementing this IFN defect is an appealing strategy as exacerbations are considered the primary outcome of asthma. These observations required an original trial design where the study began at symptom onset. Intranasal administration of IFN-β starting with symptom onset failed to significantly decrease the severity of exacerbation except in severe asthma patients [144]. Treating asthma “as needed” is another strategy that is currently followed, again with the potential advantage of overcoming the constraints of adherence.
Insight from psychology
“When patients and caregivers are both in trouble, we can reasonably assume that it is the relationship that is difficult” [145]. These words recently written on the subject of difficult-to-treat asthma are relevant because in the age of the internet, a new relationship, possibly a difficult one, is emerging between patients and their physicians. Consultations are sometimes complex and tense, with “expert” patients who are more curious, more anxious, more sceptical, or who self-diagnose. But still, the content of this relationship is clear: a dialogue, just as before, with face-to-face exchanges.
In the recent past, doctors interested in the adherence of their asthma patients placed microchips in medical devices, without their knowledge or consent. Today, caregivers use new technologies to question their patients remotely through an application aiming for early detection of exacerbations. What is concerning here in the relationship could be the desire to control the health behaviour of another, but not so concerning: again, the content and form of this relationship are almost clear to the doctor, the patient and any healthcare provider.
By contrast, what about the use of social networks: patients who are “friends” with their doctor on Facebook or who “follow” them on Twitter? This is an unclear relationship on which the French National Council of the Medical Association clearly ruled in 2011 and warned practitioners against. But what if a virtual closeness, like that in good practice between asthma centres and asthmatic patients, became effective via Facebook? Might this be a way forward? Would asthma be better controlled? And who would control that?
Concluding remarks
Probably not all of the future treatments for asthma described in this article will enter clinical practice; however, they reinforce the essential idea of personalised medicine. Indeed, the challenge is now for physicians not to get lost in the large variety of clinical studies but to succeed in making the connection between one particular patient and the appropriate evidence-based management. After that, great initiatives for improving the natural history of the disease, from diagnosis to prevention, will probably lead to identifying disease modifiers. This challenge requires better understanding in all areas ranging from biology to social sciences.
Footnotes
Previous articles in this series: No. 1: Dombret M-C, Alagha K, Boulet LP, et al. Bronchial thermoplasty: a new therapeutic option for the treatment of severe, uncontrolled asthma in adults. Eur Respir Rev 2014; 23: 510–518. No. 2: Barnig C, Levy BD. Innate immunity is a key factor for the resolution of inflammation in asthma. Eur Respir Rev 2015; 24: 141–153. No. 3: Papaioannou AI, Kostikas K, Zervas E, et al. Control of asthma in real life: still a valuable goal? Eur Respir Rev 2015; 24: 361–369. No. 4: Doberer D, Trejo Bittar HE, Wenzel SE. Should lung biopsies be performed in patients with severe asthma? Eur Respir Rev 2015; 24: 525–539.
Conflict of interest: Disclosures can be found alongside the online version of this article at err.ersjournals.com
Provenance: Submitted article, peer reviewed.
References
- 1.Hankin CS, Bronstone A, Wang Z, et al. Estimated prevalence and economic burden of severe, uncontrolled asthma in the United States. J Allergy Clin Immunol 2013; 131: AB126–AB100. [Google Scholar]
- 2.Slejko JF, Ghushchyan VH, Sucher B, et al. Asthma control in the United States, 2008–2010: indicators of poor asthma control. J Allergy Clin Immunol 2014; 133: 1579–1587. [DOI] [PubMed] [Google Scholar]
- 3.Reddel HK, Humbert M. ERJ September Podcast: Updating the GINA guidelines for asthma control. Eur Respir J 2015; 46: E57. [Google Scholar]
- 4.Carr TF, Kraft M. Update in Asthma 2014. Am J Respir Crit Care Med 2015; 192: 157–163. [DOI] [PubMed] [Google Scholar]
- 5.Tcheurekdjian H, Via M, De Giacomo A, et al. ALOX5AP and LTA4H polymorphisms modify augmentation of bronchodilator responsiveness by leukotriene modifiers in Latinos. J Allergy Clin Immunol 2010; 126: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada M, Obara K, Hirota T, et al. A functional polymorphism in IL-18 is associated with severity of bronchial asthma. Am J Respir Crit Care Med 2009; 180: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 7.Lau MYZ, Dharmage SC, Burgess JA, et al. CD14 polymorphisms, microbial exposure and allergic diseases: a systematic review of gene-environment interactions. Allergy 2014; 69: 1440–1453. [DOI] [PubMed] [Google Scholar]
- 8.Wechsler ME, Kunselman SJ, Chinchilli VM, et al. Effect of β2-adrenergic receptor polymorphism on response to longacting β2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet 2009; 374: 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Nie W, Qian J, et al. Effects of TNF-α polymorphisms on asthma risk: a systematic review and meta-analysis. J Investig Allergol Clin Immunol 2014; 24: 406–417. [PubMed] [Google Scholar]
- 10.Bonini M, Permaul P, Kulkarni T, et al. Loss of salmeterol bronchoprotection against exercise in relation to ADRB2 Arg16Gly polymorphism and exhaled nitric oxide. Am J Respir Crit Care Med 2013; 188: 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol 2015; 136: 556–568. [DOI] [PubMed] [Google Scholar]
- 12.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007; 357: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 13.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet 2013; 381: 861–873. [DOI] [PubMed] [Google Scholar]
- 14.Ege MJ, Mayer M, Normand A-C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011; 364: 701–709. [DOI] [PubMed] [Google Scholar]
- 15.Douwes J, Cheng S, Travier N, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J 2008; 32: 603–611. [DOI] [PubMed] [Google Scholar]
- 16.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol 2015; 135: 299–310. [DOI] [PubMed] [Google Scholar]
- 17.Drazen JM. A step toward personalized asthma treatment. N Engl J Med 2011; 365: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 18.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med 2010; 181: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourdin A, Molinari N, Vachier I, et al. Prognostic value of cluster analysis of severe asthma phenotypes. J Allergy Clin Immunol 2014; 134: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 20.Chang TS, Lemanske RF, Guilbert TW, et al. Evaluation of the modified asthma predictive index in high-risk preschool children. J Allergy Clin Immunol Pract 2013; 1: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med 2012; 185: 1183–1189. [DOI] [PubMed] [Google Scholar]
- 22.Daley D, Park JE, He J-Q, et al. Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes. J Allergy Clin Immunol 2012; 130: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 23.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 2010; 363: 1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell SL, Gold MJ, Hartmann M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012; 13: 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol 2015; 15: 308–322. [DOI] [PubMed] [Google Scholar]
- 26.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy J Br Soc Allergy Clin Immunol 2008; 38: 634–642. [DOI] [PubMed] [Google Scholar]
- 27.van Nimwegen FA, Penders J, Stobberingh EE, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol 2011; 128: 948–955. [DOI] [PubMed] [Google Scholar]
- 28.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2001; 163: 429–436. [DOI] [PubMed] [Google Scholar]
- 29.Simons E, To T, Moineddin R, et al. Maternal second-hand smoke exposure in pregnancy is associated with childhood asthma development. J Allergy Clin Immunol Pract 2014; 2: 201–207. [DOI] [PubMed] [Google Scholar]
- 30.Gergen PJ, Fowler JA, Maurer KR, et al. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics 1998; 101: E8. [DOI] [PubMed] [Google Scholar]
- 31.Dogaru CM, Strippoli M-PF, Spycher BD, et al. Breastfeeding and lung function at school age: does maternal asthma modify the effect? Am J Respir Crit Care Med 2012; 185: 874–880. [DOI] [PubMed] [Google Scholar]
- 32.Silvers KM, Frampton CM, Wickens K, et al. Breastfeeding protects against current asthma up to 6 years of age. J Pediatr 2012; 160: 991–996.e1. [DOI] [PubMed] [Google Scholar]
- 33.Schuijs MJ, Willart MA, Vergote K, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015; 349: 1106–1110. [DOI] [PubMed] [Google Scholar]
- 34.Newman NC, Ryan PH, Huang B, et al. Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. J Pediatr 2014; 164: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet 2014; 383: 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baur X, Sigsgaard T, Aasen TB, et al. Guidelines for the management of work-related asthma. Eur Respir J 2012; 39: 529–545. [DOI] [PubMed] [Google Scholar]
- 37.Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2014; 134: 568–575.e7. [DOI] [PubMed] [Google Scholar]
- 38.Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA 2013; 309: 1278–1288. [DOI] [PubMed] [Google Scholar]
- 39.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006; 354: 1985–1997. [DOI] [PubMed] [Google Scholar]
- 40.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007; 104: 15858–15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koppelman GH, Meerman GJ, Postma DS. Genetic testing for asthma. Eur Respir J 2008; 32: 775–782. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, HayGlass KT, Becker AB, et al. Novel recombinant interleukin-13 peptide-based vaccine reduces airway allergic inflammatory responses in mice. Am J Respir Crit Care Med 2007; 176: 439–445. [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Hayglass KT, Becker AB, et al. Novel cytokine peptide-based vaccines: an interleukin-4 vaccine suppresses airway allergic responses in mice. Allergy 2007; 62: 675–682. [DOI] [PubMed] [Google Scholar]
- 44.Mattes J, Collison A, Plank M, et al. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA 2009; 106: 18704–18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munthe-Kaas MC, Torjussen TM, Gervin K, et al. CD14 polymorphisms and serum CD14 levels through childhood: a role for gene methylation? J Allergy Clin Immunol 2010; 125: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 46.Morales E, Bustamante M, Vilahur N, et al. DNA hypomethylation at ALOX12 is associated with persistent wheezing in childhood. Am J Respir Crit Care Med 2012; 185: 937–943. [DOI] [PubMed] [Google Scholar]
- 47.Perera F, Tang W, Herbstman J, et al. Relation of DNA methylation of 5´-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One 2009; 4: e4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollingsworth JW, Maruoka S, Boon K, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest 2008; 118: 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Dombret M-C, Alagha K, Boulet LP, et al. Bronchial thermoplasty: a new therapeutic option for the treatment of severe, uncontrolled asthma in adults. Eur Respir Rev 2014; 23: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356: 1327–1337. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol 2013; 132: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343: 332–336. [DOI] [PubMed] [Google Scholar]
- 54.Donahue JG, Weiss ST, Livingston JM, et al. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997; 277: 887–891. [PubMed] [Google Scholar]
- 55.Kelly HW, Nelson HS. Potential adverse effects of the inhaled corticosteroids. J Allergy Clin Immunol 2003; 112: 469–478. [PubMed] [Google Scholar]
- 56.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med 2006; 100: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 57.Gauvreau GM, Boulet L-P, Leigh R, et al. A non-steroidal glucocorticoid receptor agonist inhibits allergen-induced late asthmatic responses. Am J Respir Crit Care Med 2015; 191: 161–167. [DOI] [PubMed] [Google Scholar]
- 58.Ogirala RG, Aldrich TK, Prezant DJ, et al. High-dose intramuscular triamcinolone in severe, chronic, life-threatening asthma. N Engl J Med 1991; 324: 585–589. [DOI] [PubMed] [Google Scholar]
- 59.Adams RJ, Fuhlbrigge AL, Finkelstein JA, et al. Intranasal steroids and the risk of emergency department visits for asthma. J Allergy Clin Immunol 2002; 109: 636–642. [DOI] [PubMed] [Google Scholar]
- 60.Nair A, Vaidyanathan S, Clearie K, et al. Steroid sparing effects of intranasal corticosteroids in asthma and allergic rhinitis. Allergy 2010; 65: 359–367. [DOI] [PubMed] [Google Scholar]
- 61.Lohia S, Schlosser RJ, Soler ZM. Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: a meta-analysis. Allergy 2013; 68: 569–579. [DOI] [PubMed] [Google Scholar]
- 62.Woodcock A, Bleecker ER, Lötvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest 2013; 144: 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J 2014; 44: 1548–1556. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis 2014; 9: 629–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapman KR, Rennard SI, Dogra A, et al. Long-term safety and efficacy of indacaterol, a long-acting β₂-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest 2011; 140: 68–75. [DOI] [PubMed] [Google Scholar]
- 66.Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest 2012; 142: 119–127. [DOI] [PubMed] [Google Scholar]
- 67.Kerstjens HAM, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 2012; 367: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 68.Barnes PJ. Neural mechanisms in asthma. Br Med Bull 1992; 48: 149–168. [DOI] [PubMed] [Google Scholar]
- 69.Hayes D, Collins PB, Khosravi M, et al. . Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex. Am J Respir Crit Care Med 2012; 185: 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavorini F, Fontana GA, Usmani OS. New inhaler devices – the good, the bad and the ugly. Respir Int Rev Thorac Dis 2014; 88: 3–15. [DOI] [PubMed] [Google Scholar]
- 71.Moore WC, Fitzpatrick AM, Li X, et al. Clinical heterogeneity in the severe asthma research program. Ann Am Thorac Soc 2013; 10: S118–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siroux V, González JR, Bouzigon E, et al. Genetic heterogeneity of asthma phenotypes identified by a clustering approach. Eur Respir J 2014; 43: 439–452. [DOI] [PubMed] [Google Scholar]
- 73.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010; 11: 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin RJ. Nocturnal asthma: circadian rhythms and therapeutic interventions. Am Rev Respir Dis 1993; 147: S25–S28. [DOI] [PubMed] [Google Scholar]
- 75.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004; 170: 836–844. [DOI] [PubMed] [Google Scholar]
- 76.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 77.Woodruff PG. Subtypes of asthma defined by epithelial cell expression of messenger RNA and microRNA. Ann Am Thorac Soc 2013; 10: Suppl., S186–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters MC, Mekonnen ZK, Yuan S, et al. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol 2014; 133: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katz LE, Gleich GJ, Hartley BF, et al. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014; 11: 531–536. [DOI] [PubMed] [Google Scholar]
- 81.Irifune K, Yokoyama A, Sakai K, et al. Adoptive transfer of T-helper cell type 1 clones attenuates an asthmatic phenotype in mice. Eur Respir J 2005; 25: 653–659. [DOI] [PubMed] [Google Scholar]
- 82.Kline JN, Kitagaki K, Businga TR, et al. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol 2002; 283: L170–L179. [DOI] [PubMed] [Google Scholar]
- 83.Huang TJ, MacAry PA, Eynott P, et al. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol Baltim Md 1950 2001; 166: 207–217. [DOI] [PubMed] [Google Scholar]
- 84.Krug N, Hohlfeld JM, Kirsten A-M, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med 2015; 372: 1987–1995. [DOI] [PubMed] [Google Scholar]
- 85.Stinson SE, Amrani Y, Brightling CE. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J Allergy Clin Immunol 2015; 135: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barnes N, Pavord I, Chuchalin A, et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol 2012; 42: 38–48. [DOI] [PubMed] [Google Scholar]
- 87.Bartemes KR, Kephart GM, Fox SJ, et al. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014; 134: 671–678.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pettipher R, Hunter MG, Perkins CM, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy 2014; 69: 1223–1232. [DOI] [PubMed] [Google Scholar]
- 89.Singh D, Cadden P, Hunter M, et al. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur Respir J 2013; 41: 46–52. [DOI] [PubMed] [Google Scholar]
- 90.Gauvreau GM, O'Byrne PM, Boulet L-P, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370: 2102–2110. [DOI] [PubMed] [Google Scholar]
- 91.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma. Am J Respir Crit Care Med 2001; 184: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 92.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med 2007; 176: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 93.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009; 360: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. [DOI] [PubMed] [Google Scholar]
- 95.Ortega HG, Liu MC, Brusselle GG, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1197. [DOI] [PubMed] [Google Scholar]
- 96.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 97.Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2014; 2: 879–890. [DOI] [PubMed] [Google Scholar]
- 98.Ortega H, Li H, Suruki R, et al. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 2014; 11: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 99.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002; 8: 885–889. [DOI] [PubMed] [Google Scholar]
- 100.Grünig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998; 282: 2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science 1998; 282: 2258–2261. [DOI] [PubMed] [Google Scholar]
- 102.Piper E, Brightling C, Niven R, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J 2013; 41: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noonan M, Korenblat P, Mosesova S, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. J Allergy Clin Immunol 2013; 132: 567–574.e12. [DOI] [PubMed] [Google Scholar]
- 104.De Boever EH, Ashman C, Cahn AP, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol 2014; 133: 989–996. [DOI] [PubMed] [Google Scholar]
- 105.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011; 365: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 106.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368: 2455–2466. [DOI] [PubMed] [Google Scholar]
- 107.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997; 89: 587–596. [DOI] [PubMed] [Google Scholar]
- 108.Lee HY, Rhee CK, Kang JY, et al. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res 2014; 40: 66–76. [DOI] [PubMed] [Google Scholar]
- 109.Nair P, Gaga M, Zervas E, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy J Br Soc Allergy Clin Immunol 2012; 42: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 110.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309: 1251–1259. [DOI] [PubMed] [Google Scholar]
- 111.Brusselle GG, VanderStichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013; 68: 322–329. [DOI] [PubMed] [Google Scholar]
- 112.Deschildre A, Marguet C, Salleron J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J 2013; 42: 1224–1233. [DOI] [PubMed] [Google Scholar]
- 113.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 2011; 139: 28–35. [DOI] [PubMed] [Google Scholar]
- 114.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011; 364: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18: 254–261. [DOI] [PubMed] [Google Scholar]
- 116.Garcia G, Magnan A, Chiron R, et al. A proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthma. Chest 2013; 144: 411–419. [DOI] [PubMed] [Google Scholar]
- 117.Gauvreau GM, Harris JM, Boulet L-P, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci Transl Med 2014; 6: 243ra85. [DOI] [PubMed] [Google Scholar]
- 118.Kudo M, Melton AC, Chen C, et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 2012; 18: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 2013; 188: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 120.Niimi A, Matsumoto H, Minakuchi M, et al. Airway remodelling in cough-variant asthma. Lancet 2000; 356: 564–565. [DOI] [PubMed] [Google Scholar]
- 121.Matsuoka H, Niimi A, Matsumoto H, et al. Inflammatory subtypes in cough-variant asthma: association with maintenance doses of inhaled corticosteroids. Chest 2010; 138: 1418–1425. [DOI] [PubMed] [Google Scholar]
- 122.Rubin BK, Priftis KN, Schmidt HJ, et al. Secretory hyperresponsiveness and pulmonary mucus hypersecretion. Chest 2014; 146: 496–507. [DOI] [PubMed] [Google Scholar]
- 123.Ehre C, Worthington EN, Liesman RM, et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci USA 2012; 109: 16528–16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grainge CL, Lau LCK, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 2011; 364: 2006–2015. [DOI] [PubMed] [Google Scholar]
- 125.Liu MC, Dubé LM, Lancaster J. Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. Zileuton Study Group. J Allergy Clin Immunol 1996; 98: 859–871. [DOI] [PubMed] [Google Scholar]
- 126.Israel E, Cohn J, Dubé L, et al. Effect of treatment with Zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zileuton Clinical Trial Group. JAMA 1996; 275: 931–936. [PubMed] [Google Scholar]
- 127.Świerczyńska-Krępa M, Sanak M, Bochenek G, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J Allergy Clin Immunol 2014; 134: 883–890. [DOI] [PubMed] [Google Scholar]
- 128.Yuksel H, Sogut A, Yilmaz O, et al. Role of adipokines and hormones of obesity in childhood asthma. Allergy Asthma Immunol Res 2012; 4: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kattan M, Kumar R, Bloomberg GR, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol 2010; 125: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marin G, Gamez AS, Molinari N, et al. Distal airway impairment in obese normoreactive women. BioMed Res Int 2013; 2013: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boulet L-P, Turcotte H, Martin J, et al. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med 2012; 106: 651–660. [DOI] [PubMed] [Google Scholar]
- 132.van Huisstede A, Rudolphus A, Castro Cabezas M, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax 2015; 70: 659–667. [DOI] [PubMed] [Google Scholar]
- 133.Poitiers University Hospital. Evaluation of a Therapeutic Strategy Including Nebulised Liposomal Amphotericin B (Ambisome®) in Maintenance Treatment of Allergic Bronchopulmonary Aspergillosis (Cystic Fibrosis Excluded). (NEBULAMB). https://clinicaltrials.gov/ct2/show/NCT02273661?term=nebulamb&rank=1 Date last accessed: September 10, 2015. Date last updated: December 10, 2014.
- 134.van der Ent CK, Hoekstra H, Rijkers GT. Successful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibody. Thorax 2007; 62: 276–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014; 371: 1771–1780. [DOI] [PubMed] [Google Scholar]
- 136.Kim S, Marigowda G, Oren E, et al. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol 2010; 125: 1336–1343. [DOI] [PubMed] [Google Scholar]
- 137.GlaxoSmithKline. A study to investigate mepolizumab in the treatment of eosinophilic granulomatosis with polyangiitis. https://clinicaltrials.gov/ct2/show/NCT02020889 Date last accessed: September 10, 2015. Date last updated: November 19, 2015.
- 138.Bonnans C, Vachier I, Chavis C, et al. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med 2002; 165: 1531–1535. [DOI] [PubMed] [Google Scholar]
- 139.Ono E, Dutile S, Kazani S, et al. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med 2014; 190: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol 2007; 178: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miyata J, Fukunaga K, Iwamoto R, et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immunol 2013; 131: 353–360.e1–e2. [DOI] [PubMed] [Google Scholar]
- 142.Krishnamoorthy N, Burkett PR, Dalli J, et al. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol 2015; 194: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Calışkan M, Bochkov YA, Kreiner-Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med 2013; 368: 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Djukanović R, Harrison T, Johnston SL, et al. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med 2014; 190: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Halimi L, Chanez P, Humbert M. Quand l'asthme est difficile: c'est l'asthmatique qui a des difficultés psychologiques? [When asthma is difficult: is the asthmatic having psychological difficulties?] In: Chanez P, Humbert M, eds. L'Asthme Difficile: Questions Pratiques. [Difficult Asthma: Practical Questions.] Paris, Phase 5, 2011. [Google Scholar]