Abstract
Few antibiotics targeting members of the archaeal domain are currently available for genetic studies. Since bacterial antibiotics are frequently directed against competing and related organisms, archaea by analogy might produce effective antiarchaeal antibiotics. Peptide antibiotic (halocin) preparations from euryarchaeal halophilic strains S8a, GN101, and TuA4 were found to be toxic for members of the hyperthermophilic crenarchaeal genus Sulfolobus. No toxicity was evident against representative bacteria or eukarya. Halocin S8 (strain S8a) and halocin R1 (strain GN101) preparations were cytostatic, while halocin A4 (strain TuA4) preparations were cytocidal. Subsequent studies focused on the use of halocin A4 preparations and Sulfolobus solfataricus. Strain TuA4 cell lysates were not toxic for S. solfataricus, and protease (but not nuclease) treatment of the halocin A4 preparation inactivated toxicity, indicating that the A4 toxic factor must be a secreted protein. Potassium chloride supplementation of the Sulfolobus assay medium potentiated toxicity, implicating use of a salt-dependent mechanism. The utility of halocin A4 preparations for genetic manipulation of S. solfataricus was assessed through the isolation of UV-induced resistant mutants. The mutants exhibited stable phenotypes and were placed into distinct classes based on their levels of resistance.
Small subunit (16S) rRNA sequence comparisons have identified a unique lineage or domain of prokaryotic organisms called archaea, which are currently subdivided into the euryarchaea, crenarchaea, and korarchaeota (1, 33, 34). Although prokaryotic in morphology, archaea employ eukaryotic mechanisms for many subcellular processes (32), including replication (2), transcription (12, 14, 15), and translation (5, 13). Cultivated archaea are further divided into the prominent biotypes of methanogens, halophiles (haloarchaea), and hyperthermophiles. Haloarchaea are members of the euryarchaea and thrive under conditions of high salt, while hyperthermophiles can be found in both euryarchaeal and crenarchaeal branches of the archaeal domain. Sulfolobus solfataricus is a hyperthermophilic aerobic crenarchaeote that inhabits acidic terrestrial hot springs. It is capable of both lithoautotrophic growth through sulfur oxidation (27, 35) and heterotrophic growth using a variety of defined carbon and energy sources (6, 8, 9, 23).
Antibiotics are broadly defined as natural, semisynthetic, and wholly synthetic substances that kill or inhibit the growth of microorganisms at low concentrations. One abundant class of bacterial antibiotics is the bacteriocins (3, 7, 11, 25). These secreted proteinaceous compounds are produced by both gram-positive and gram-negative bacteria, range in size from 1 to 100 kDa, and are ribosomally synthesized. They can alter cell membrane integrity, can interfere with transcription, translation, or DNA replication, and frequently are produced during stationary phase. Some archaea do produce proteinaceous antibiotics. For example, Sulfolobus islandicus produces an insoluble cell-associated peptide, termed a sulfolobicin, which is specific only for closely related species (19). Halophilic archaea secrete peptide antibiotics called halocins (or, if small, microhalocins) upon entry into stationary phase and in some cases after entry into stationary phase (20, 22, 26, 28, 29).
Genetic manipulation of archaea continues to lag behind that of bacteria and eukarya. Some of this delay reflects the insensitivity of archaea to conventional antibiotics, limiting the development of selectable genetic markers. However, recent efforts have led to new approaches in this area for methanogens (30) and additional tools for halophiles (17, 18). The frequent cohabitation of archaeal niches by bacteria that compete for the same resources (31) could have fostered the evolution of bacterium-produced antibiotics effective against archaea. One reason why such compounds have remained largely unidentified may be the evolutionary distance between archaea and bacteria whereby antibiotic targets, including components of the translational, transcriptional, and replication systems, evolved divergent structural features. Euryarchaeal halophiles and crenarchaeal hyperthermophiles share key aspects of many subcellular processes which might constitute conserved targets for antibiotic action. This study reports the finding that halocins produced by euryarchaea are effective against crenarchaea and thus act across the main subdivision of the archaeal domain. These compounds represent a new and general class of antiarchaeal toxins.
MATERIALS AND METHODS
Strains and cultivation.
Sulfolobus acidocaldarius DG6, Sulfolobus shibatae B12, and S. solfataricus 98/2 were from laboratory collections and routinely distinguished by 16S rRNA analysis as described previously (24). Saccharomyces cerevisiae, Escherichia coli K-12, Staphylococcus aureus, Bacillus megaterium, and all haloarchaea were also from laboratory collections. Haloarchaea included Halobacterium sp. strain GN101 (produces halocin R1 l1[26]), strain S8a isolated from the Great Salt Lake, Utah (produces halocin S8 [20]), strain TuA4 isolated from Tunisia (produces halocin A4 [21;;1]), and Halobacterium salinarum NRC817 (indicator strain for quantifying halocin activity [20]). The haloarchaeal identity of strain S8 is based on its ability to grow optimally in a medium containing 4.0 M sodium chloride, a unique property of haloarchaea, and the fact that the S8a halocin gene, halS8, has a consensus haloarchaeal promoter and B recognition element sequence (20). The phylogenic identity of strain TuA4 was determined in this study by using domain-specific PCR primers.
For the production of 16 liters of halocin-laden supernatants, haloarchaeal strains S8a, GN101, and TuA4 were grown aerobically at 41°C in eight 4-liter baffled flasks in a New Brunswick G25 incubator at 200 rpm. Culture media for strains S8a and NRC817 were as described elsewhere (20). Culture media for strains TuA4 and GN101 contained 4 M NaCl, 120 mM MgSO4, 100 mM sodium citrate, 30 mM KCl, 10 mM Tris-HCl (pH 7.4), trace elements [50 ng of CuSO4 · 5H2O, 4.55 μg of Fe(NH4)2(SO4)2 · 6H2O, 300 ng of MnSO4 · H2O, and 440 ng of ZnSO4 · 7H2O) per ml], and 1% (wt/vol) Oxoid peptone. Bacteria were cultivated at 37°C in tryptone soy broth to late exponential phase. Overlays were prepared using 0.8% (wt/vol) agar and tryptone soy broth plates solidified with 1.5% (wt/vol) agar. Saccharomyces cerevisiae was cultivated at 26°C in potato-dextrose medium. Overlays were prepared as for bacteria, using potato-dextrose plates solidified with 1.5% agar.
S. solfataricus and S. shibatae were grown at 80°C and S. acidocaldarius was grown at 70°C, as described elsewhere (24). All Sulfolobus species were grown at a pH of 3.0 in screw-cap flasks in a basal salts medium as described previously (10). The basal salts medium was supplemented as indicated with glucose or tryptone to a final concentration of 0.2% (wt/vol) or with yeast extract, casein, and glucose, each to a final concentration of 0.1% (wt/vol). Sodium chloride, lithium chloride, and potassium chloride were added to concentrations of 20 or 200 mM as indicated. Growth was monitored spectrophotometrically at a wavelength of 540 nm. Solid medium was prepared as described elsewhere (10), using 0.6% (wt/vol) Gelrite (Kelco) and basal salts medium containing either 0.2% (wt/vol) tryptone, 0.2% (wt/vol) glucose, or 0.1% (wt/vol) glucose, 0.1% (wt/vol) Casamino Acids, and 0.1% (wt/vol) yeast extract (SR medium), at a pH of 3.0. Magnesium chloride was added at a final concentration of 8.0 mM to solidify the medium. Plates were 100 mm in diameter and contained 35 ml of solidified medium. Gelrite overlays were prepared with a Gelrite concentration of 0.6% (wt/vol) in the corresponding basal salts medium and approximately 109 mid-exponential-phase cells in a total volume of 3 ml. Processed supernatants were spotted in 10-μl volumes on solidified overlays and allowed to dry prior to incubation. Plates were incubated in stacks inside plastic bags at 80°C in plastic containers with sufficient water to prevent desiccation. Growth was monitored daily, and extra water was drained from the plates. Colonies reached a diameter of 2 mm in approximately 6 days for SR medium and in approximately 10 days for minimal glucose medium; overlay lawns appeared in the same time frame.
PCR amplification of 16S rDNA.
PCR was performed using 10 mM potassium chloride, 10 mM ammonium sulfate, 2 mM magnesium chloride, 20 mM Tris-Cl (pH 8.75), 0.1% Triton X-100, 100 μM deoxynucleoside triphosphates, 100 pmol of primers, 2 ng of template DNA, and 2.5 U of recombinant Pfu polymerase (Stratagene). The archaeal primers consisted of Arch21F (5′-TTCCGGTTGATCCC/TGCCGGA-3′) and Arch958R (5′-C/TCCGGCGTTGAC/ATCCAATT-3′); the bacterial primers were Eubact27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and Eubact1492R (5′-GGTTACCTTGTTACGACTT-3′) (4). PCR amplification of the 16S RNA gene from haloarchaeal strain TuA4 was evident only using archaeal domain-specific primers; no amplification product was observed using bacterial domain-specific primers. These results provide further presumptive information that this isolate was archaeal and not bacterial. E. coli K-12 genomic DNA was used as a positive control for the bacterial domain-specific primers, while S. solfataricus genomic DNA was used as a positive control for the archaeal domain-specific primers.
Preparation of processed haloarchaeal supernatants.
Two procedures were used for the preparation of haloarchaeal culture supernatants containing halocin activity. In the first procedure (used in experiments shown in Fig. 1 and Table 1 and for sensitivity determinations using bacteria and eukarya), processed haloarchaeal culture supernatants were prepared by filtering 16 liters of culture through a 0.45-μm-pore-size tangential-flow Pellicon (Millipore) filter to remove the cells. The resulting filtrate was then filtered through a 100-kDa NMWCO tangential-flow filter (Millipore) and then a 30-kDa NMWCO filter (Millipore). The 30-kDa retentate was then heated at 95°C for 2 h to denature contaminating protein, and the flocculant precipitate was removed by filtration using a 0.22-μm-pore-size sterile filter. This material was concentrated further using tangential-flow spin filters (5-kDa NMWCO; Millipore) and desalted by recursive concentration and dilution with 10 mM Tris-HCl (pH 7.4).
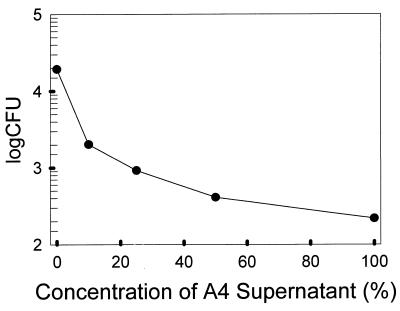
FIG. 1.
Efficiency of plating of S. solfataricus treated with strain TuA4 culture supernatant. Values are averages of replicate samples which varied by less than 10%.
TABLE 1.
Toxicity of processed haloarchaeal culture supernatants toward S. solfataricus
| Haloarchaeon | Zone of inhibitiona (diam [mm]) | Plating efficiencyb | Effect |
|---|---|---|---|
| TuA4 | 12 | 1 | Cytocidal |
| GN101 | 7 | 45 | Cytostatic |
| S8a | 4 | 100 | Cytostatic |
Measured after direct application of 10 μl to the surface of the overlay.
Percentage of colonies resulting from treatment with equal volumes of processed culture supernatants relative to an untreated control. The experiments were repeated four times, and results varied by less than 10%. Values shown are averages for replicate samples which varied by less than 5%.
A more purified supernatant preparation was used for experiments shown in Fig. 2, Table 2, and Table 3 and in experiments involving the enzymatic treatment of haloarchaeal culture supernatants. The 30-kDa retentate was boiled for 2 h, the precipitate was removed by filtration, and the retentate was subjected to acetone precipitation by mixing the supernatant with an equal volume of acetone. The hypersaline layer containing halocin A4 activity was removed, and the remaining acetone was evaporated under a stream of nitrogen gas. This material was concentrated using 5-kDa NMWCO tangential-flow spin filters and subjected to gel filtration column chromatography (2.5- by 110-cm bed volume at 0.04 ml/min) using a P10M matrix (Bio-Rad) buffered with basal salts from the TuA4 growth medium lacking peptone and trace elements. Fractions (0.5 ml) containing activity were pooled and concentrated using 5-kDa NMWCO tangential-flow spin filters and desalted as above. Protein concentrations of these preparations were typically 10 mg/ml. Halocin activity was quantified by serial twofold dilutions to extinction using the sensitive strain H. salinarum NRC817 as described elsewhere (20).
TABLE 2.
Salt potentiation of killing of S. solfataricus by processed strain TuA4 supernatant
| Potassium chloride concn (mM) | Zone of inhibitiona (diam [mm]) | Plating efficiencyb |
|---|---|---|
| 2 | 12 | 1.0 |
| 20 | 13 | 0.8 |
| 200 | 20 | 0.2 |
Measured after incubation for 10 days at 80°C. The experiments were repeated five times, and results varied by less than 10%. Values shown are averages for replicate samples which varied by less than 5%.
Percentage of the value for cells exposed to a sample of processed strain TuA4 supernatant.
TABLE 3.
Resistant mutant phenotypes
| Strain | Zone of inhibitiona (diam [mm])
|
Plating efficiency (%) at 2 mM KClb | |
|---|---|---|---|
| 2 mM KCl | 200 mM KCl | ||
| Class 1 | None | None | 54 |
| Class 2 | None | 4 | 2 |
| Wild type | 10 | 20 | 1 |
Measured after incubation for 10 days at 80°C. The experiments were repeated three times, and the results varied by less than 10%. Values shown are averages for replicate samples which varied by less than 5%.
Mutants were not tested at 200 mM KCl, as they failed to grow under this condition.
Efficiency of plating of S. solfataricus treated with strain TuA4 supernatant.
S. solfataricus cells grown to mid-exponential phase in 0.2% (wt/vol) tryptone were exposed to dilutions of processed TuA4 supernatant prepared in 10 mM Tris-Cl (pH 7.4). Equal volumes of cell culture were mixed with supernatant dilutions. The mixtures were incubated at 80°C for 1 h, after which the cells were pelleted and resuspended in basal salts medium lacking a carbon source and plated on SR medium.
Enzymatic treatment of concentrated strain TuA4 supernatant.
All mesophilic proteases were prepared at a concentration of 10 mg/ml in filter-sterilized stocks. Processed TuA4 supernatant was treated with pronase (Sigma), trypsin (Sigma), or proteinase K (Boehringer Mannheim) at a final concentration of 1.7 mg/ml overnight at 37°C and with thermophilic PreTaq (Life Technologies) at 240 U/ml for 1 h at 75°C. PreTaq was inactivated by the addition of EGTA to a final concentration of 10 mM followed by heating for 15 min at 90°C. Nucleases RNase A (Sigma) and DNase I (Boehringer Mannheim) were prepared at 20 mg/ml. Processed TuA4 supernatant was treated with nucleases at a final concentration of 3 mg/ml for 1 h at 37°C. Enzymatically treated processed TuA4 supernatants were spotted onto S. solfataricus overlays containing 200 mM potassium chloride and 0.2% (wt/vol) glucose as described above. These culture conditions provide the highest level of sensitivity for detecting inhibitory activity. These experiments were repeated three times, and the results varied by less than 10%.
Metabolic labeling.
S. solfataricus cells were grown to mid-exponential phase (λ540 = 0.3) in basal salts medium containing 0.2% (wt/vol) sucrose prior to labeling; 7-ml aliquots of cell culture were removed, and the cells were recovered by centrifugation. The cell pellet was resuspended in basal salts medium lacking a carbon source, and an equal volume of processed TuA4 supernatant was added. The cell supernatant mixture was incubated at 80°C for 1 h, after which the cells were repelleted and resuspended in basal salts with 0.2% (wt/vol) sucrose and 0.5 μCi of Tran35S-label (ICN) was added. The cells were incubated at 80°C for 15 min, after which proteins were precipitated by the addition of cold trichloroacetic acid. Precipitated proteins were recovered by centrifugation, and the amount of radioactivity in each sample was determined by scintillation counting.
Isolation of TuA4 supernatant-resistant mutants of S. solfataricus.
S. solfataricus cells were grown to mid-exponential phase in 0.2% (wt/vol) tryptone medium. Cells were pelleted for 20 min at 5,000 × g, and the supernatant was removed. The pellet was resuspended in 5 ml of basal salts medium lacking a carbon source and irradiated with 260-nm UV light at a distance of 30 cm for 10 min in the dark. The irradiated cells were then grown in 0.2% (wt/vol) tryptone medium in the dark to mid-exponential phase, and a culture sample was removed and mixed with an equal volume of processed TuA4 supernatant. The mixture was incubated at 80°C for 1 h in the dark. Cells were then pelleted and resuspended in basal salts medium lacking a carbon source. Cells were plated in the dark on SR medium and incubated until a colony diameter of approximately 2 mm was obtained. Resulting colonies were purified on SR medium and screened for resistance to desalted TuA4 supernatants. Selected isolates were evaluated further by plating efficiency following exposure to processed TuA4 supernatant to quantify the resistance phenotype.
RESULTS
Growth inhibition of Sulfolobus.
The antimicrobial effect of peptide antibiotics produced by haloarchaea against related organisms has been well documented (29). In an effort to determine whether these peptide antibiotics (halocins) inhibit the growth of distantly related archaea, concentrated processed haloarchaeal culture supernatants were examined for antimicrobial activity against crenarchaeal hyperthermophiles from the genus Sulfolobus. Desalted and size-fractionated culture supernatants from three different haloarchaea were prepared by tangential-flow ultrafiltration through membranes of sequentially smaller pore size. Halocin-laden supernatants were obtained from Halobacterium sp. strain GN101 (halocin R1 [26]), strain S8a, an uncharacterized haloarchaeon from the Great Salt Lake (halocin S8 [20]), and strain TuA4, a newly isolated haloarchaeon from Tunisia (halocin A4 [21]). Two of these haloarchaea, Halobacterium sp. strain GN101 and strain S8a, have previously been shown to produce halocins that are effective in killing or inhibiting the growth of other haloarchaea (20, 26).
An initial determination of growth inhibition was made by spotting processed supernatant on Gelrite overlays containing S. solfataricus cells. Zones of clearing were observed for each processed supernatant tested, but zones differed in size (Table 1) and persistence upon prolonged incubation. The largest zone was observed following treatment with the sample from strain TuA4. Samples from strains GN101 and S8a produced smaller zones of growth inhibition. No growth inhibition was apparent using processed, uninoculated haloarchaeal medium or desalted haloarchaeal cell lysates. These findings indicate that the observed toxicity of processed haloarchaeal culture supernatants against members of the genus Sulfolobus resulted from a secreted factor produced by haloarchaeal cells.
Similar patterns of growth inhibition were observed when the closely related S. shibatae strain B12 and the more distantly related S. acidocaldarius strain DG6 were treated with processed supernatants from the three haloarchaea. Samples from haloarchaeal strain TuA4 were consistently more growth inhibitory than those from strain S8a or GN101. These results indicate the toxicity of haloarchaeal culture supernatants was not limited to S. solfataricus but was genuswide. Other organisms, including the gram-negative bacterium E. coli K-12, the gram-positive bacteria Staphylococcus aureus and B. megaterium, and the eukaryote Saccharomyces cerevisiae, were insensitive to all of the processed haloarchaeal culture supernatants. Together with previous findings demonstrating toxicity against haloarchaea, the present results indicate that haloarchaeal culture supernatants are archaeon specific.
Mechanism of action.
Upon prolonged incubation of S. solfataricus overlay plates treated with the processed supernatants of haloarchaeal strains S8a and GN101, zones of growth inhibition decreased in size and became more turbid by growth of cells within this region. This was not observed for zones of inhibition produced by processed supernatants from haloarchaeal strain TuA4. These results might be explained if processed supernatants of strains S8a and GN101 exerted a cytostatic effect, while that of strain TuA4 exerted a cytocidal effect. To test this possibility, the plating efficiency was determined for S. solfataricus following a 1-h exposure to each of the supernatant preparations (Table 1). Relative to an untreated control, cells exposed to samples from strains S8a and GN101 had plating efficiencies of 100 and 45%, respectively. Similar observations were made following treatment of the other Sulfolobus species. In contrast, samples exposed to processed supernatant of TuA4 resulted in a plating efficiency of only 1%. These results demonstrated that the toxicity of processed supernatants from strains S8a and GN101 required prolonged exposure and are therefore cytostatic in action, while strain TuA4 supernatant required only short-term exposure and is therefore cytocidal in action. Since preparations of haloarchaeal strain TuA4 supernatant acted in a cytocidal fashion, this material was chosen to establish a dose-response relationship for S. solfataricus. The efficiency of plating was determined for S. solfataricus following a 1-h exposure to various concentrations of processed strain TuA4 supernatant (Fig. 1). The smallest amount of supernatant tested was a 1:10 dilution of the processed supernatant sample, which decreased viable counts 10-fold; undiluted supernatant decreased viable counts almost 100-fold.
Antimicrobial agents are known to have numerous targets, including membranes, proton transporters, and various components of translation (3, 7, 11, 25). In an effort to identify the target of the lethal factor secreted by haloarchaeal strain TuA4, S. solfataricus cells were treated at 80°C and examined microscopically. Despite prolonged incubation of more than 6 h, no visible alteration in cell morphology or evidence of cell clumping was observed. Metabolic labeling was undertaken to determine if protein synthesis was altered in cells subjected to halocin exposure. Cells were treated for 1 h at 80°C with processed TuA4 supernatant prior to protein labeling. No net reduction in protein synthesis rates relative to an untreated control was observed.
Composition of TuA4 supernatant.
Processed strain TuA4 supernatant was expected to consist of a range of substances derived primarily from the culture medium (including proteins and small molecules) with minor amounts of substances (e.g., nucleic acids) contributed by cells. Proteases were used to test the importance of proteins on the observed toxicity of the processed culture supernatant. Three mesophilic (ambient temperature) proteases (proteinase K, pronase, and trypsin) were used individually to digest processed supernatant prior to its application to S. solfataricus lawns. No significant variation in the resulting zone of clearing was apparent compared to undigested processed culture supernatant. A fourth protease, thermophilic PreTaq, was also used to treat processed supernatant. Digestion with this protease resulted in the abolition of zone formation on an S. solfataricus lawn, indicating that a protein in the supernatant was responsible for the cytocidal effect. Note that halocin A4 is heat stable: incubation at 75 or 90°C in the absence of PreTaq had no effect on halocin activity. Similar experiments involving treatment of the processed supernatant with DNase I and RNase A failed to diminish the toxicity of processed TuA4 culture supernatant, excluding a role for nucleic acids. Since the processing method eliminates molecules of less than about 5 kDa, small metabolites are also unlikely to be involved in processed TuA4 supernatant toxicity.
Salt-mediated potentiation of killing.
The processed supernatants examined in this study were all desalted prior to use in assays against S. solfataricus. Since some excreted proteins from halophiles are dependent on salt concentration for optimal activity and the cytocidal component of the processed strain TuA4 supernatant appears to be proteinaceous in nature, the consequences of salt addition in the form of monovalent cations was examined (Table 2). Independent addition of sodium chloride and lithium chloride to the medium was tested and found to be lethal to S. solfataricus even at the lowest concentration, precluding their further use. In contrast, potassium chloride addition had no effect on cell viability within the tested concentration range (20 to 200 mM). The basal salts medium for S. solfataricus contains 2 mM potassium; in the presence of this quantity of salt, processed TuA4 supernatant produced a 12-mm zone of clearing and a 1% plating efficiency (Table 1 and Fig. 1). Increasing the potassium concentration 10- and 100-fold increased the zone of clearing and decreased the plating efficiency (Table 2), indicating that potassium chloride can potentiate the lethal effect of the protein present in processed strain TuA4 supernatant.
Isolation of resistant mutants.
S. solfataricus mutants that were resistant to the lethal effect of processed TuA4 supernatant were isolated using UV light mutagenesis. Cells were irradiated with shortwave UV light under conditions that precluded photorepair and resulted in 0.1% survival. Individual isolates were rescreened to confirm the resistant phenotype using the overlay assay. Approximately 20% of the surviving colonies were found to be resistant. These appeared at a frequency of about 10−7 per mutagenized cell. Two distinct classes of resistant mutants were identified, and representative isolates were characterized by the lawn overlay assay (Table 3). Compared to wild type, class 1 mutants were fully resistant to treatment with TuA4 supernatant and produced no zone of clearing. Class 2 mutants exhibited a lower level of resistance and produced a reduced zone of clearing. To quantify the phenotypes of the mutant classes, plating efficiency was determined following a 1-h exposure of processed TuA4 supernatant for a representative of each mutant class (Table 3). At reduced potassium levels (2 mM), the plating efficiency for the most resistant class (class 1) was 54% relative to an untreated control. Class 2 mutants showed a 2% plating efficiency, double that observed for the wild type. Levels of resistance at higher concentrations of potassium chloride could not be determined because the mutants failed to grow under these assay conditions.
DISCUSSION
Processed supernatants from haloarchaeal cultures inhibit the growth of members of the hyperthermophilic crenarchaeal genus Sulfolobus. The inhibition is either cytostatic or cytocidal in nature, depending on the haloarchaeal strain used as a source of the supernatant. Further examination of the cytocidal TuA4 supernatant revealed that the lethal component was a secreted protein, since haloarchaeal cell lysates were not toxic and the toxic activity could be abolished by thermophilic protease treatment. Peptide antibiotics have been identified as excreted products for numerous haloarchaea (29) and have been termed halocins (22) or microhalocins (20). The mechanism of action and target are known only for halocin H6, a Na+/H+ antiporter inhibitor (16). Elucidation of mechanisms for other halocins, including A4, is ongoing.
The addition of salt in the form of potassium chloride was found to potentiate the toxicity of processed haloarchaeal strain TuA4 supernatant for Sulfolobus. Potassium (or sodium, which is not testable in this system) may have the ability to restore proper folding for the protein(s) present in the desalted processed strain TuA4 supernatant. A dependence on monovalent cations like potassium may have arisen as a result of the haloarchaeal use of this cation as a major internal osmoticum to balance elevated external levels of sodium. Alternatively, potassium may increase sensitivity of S. solfataricus to the strain TuA4 toxic factor. Since the natural environment for strain TuA4 approaches saturating sodium chloride concentrations, a requirement for salt stimulation is not unexpected. Whether halocins are salt dependent for action against haloarchaea cannot be determined since these organisms have an obligatory salt requirement.
To evaluate the possibility of using halocins for genetic manipulations, S. solfataricus mutants that were resistant to the toxic effect of processed TuA4 supernatant could be recovered. The infrequent occurrence of resistant mutants suggests that only a limited number of targets can be altered to create a resistant cell. The level of resistance of the mutants, measured as the efficiency of plating, could not be determined at elevated concentrations (200 mM) of potassium chloride due to an inability of the mutants to grow in liquid medium under these conditions. This observation reveals an additional mutant phenotype and may be related to the mechanism of resistance. These findings may lead to improved methods for the genetic manipulation of archaea in general and hyperthermophilic crenarchaea in particular.
Visual examination of S. solfataricus cell morphology following extended exposure to processed strain TuA4 supernatant revealed no cell lysis or other morphological effects. A similar lack of alteration has been observed following treatment of H. salinarum NRC817 (S. Kemper and R. Shand, unpublished data). Such results indicate that the archaeal membrane is not extensively compromised as a consequence of this treatment. Metabolic labeling of S. solfataricus demonstrated there was no alteration in radiolabeled amino acid incorporation following cell treatment, indicating that protein synthesis also is not an A4 target.
The data presented here suggest that there are biological targets for halocin action which are conserved between euryarchaeal halophiles and crenarchaeal hyperthermophiles. Interestingly, preliminary tests with concentrated culture supernatants containing halocins R1, S8, A4, and purified H4 and with the methanoarchaeon Methanosarcina thermophila show that halocin R1 is toxic whereas the other halocins are not (K. Sowers, personal communication). Thus, halocins exhibit broad toxicity toward the primary but distantly related archaeal biotypes. The apparent conservation of halocin toxicity across the archaeal domain suggests that their biological targets must have arisen early during archaeal evolution. The apparent lack of these targets in bacterial prokaryotes and a eukaryote qualifies halocins and their targets as unique components of the archaeal domain.
ACKNOWLEDGMENTS
We thank Melissa Drozda for technical support.
This work was supported by NSF grant MCB-9974453 to P.B. and NIH grant GM59600 to R.F.S.
REFERENCES
- 1.Barns S M, Delwiche C F, Palmer J D, Pace N R. Perspectives on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cann I K, Ishino Y. Archaeal DNA replication: identifying the pieces to solve a puzzle. Genetics. 1999;152:1249–1267. doi: 10.1093/genetics/152.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daw M, Falkiner F. Bacteriocins: nature, function, and structure. Micron. 1996;27:467–479. doi: 10.1016/s0968-4328(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 4.DeLong E F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennis P P. Ancient ciphers: translation in Archaea. Cell. 1997;89:1007–1010. doi: 10.1016/s0092-8674(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 6.Grogan D. Phenotypic characterization of the archebacterial genus Sulfolobus: comparison of five wild-type strains. J Bacteriol. 1989;171:6710–6719. doi: 10.1128/jb.171.12.6710-6719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hancock R, Chapple D. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haseltine C, Rolfsmeier M, Blum P. The glucose effect and regulation of α-amylase synthesis in the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 1996;178:945–950. doi: 10.1128/jb.178.4.945-950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haseltine C, Montalvo-Rodriguez R, Bini E, Carl A, Blum P. Coordinate transcriptional control in the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 1999;181:3920–3927. doi: 10.1128/jb.181.13.3920-3927.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haseltine C, Montalvo-Rodriguez R, Carl A, Bini E, Blum P. Extragenic pleiotropic mutations that repress glycosyl hydrolase expression in the hyperthermophilic archaeon Sulfolobus solfataricus. Genetics. 1999;152:1353–1361. doi: 10.1093/genetics/152.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack R, Tagg J, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klenk H, Palm P, Lottspeich F, Zillig W. Component H of the DNA-dependent RNA polymerase of archaea is homologous to a subunit shared by the three eucaryal nuclear RNA polymerases. Proc Natl Acad Sci USA. 1992;89:407–410. doi: 10.1073/pnas.89.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyrpides N C, Woese C R. Archaeal translation initiation revisited: the initiation factor 2 and eukaryotic initiation factor 2B alpha-beta-delta subunit families. Proc Natl Acad Sci USA. 1998;95:3726–3730. doi: 10.1073/pnas.95.7.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langer D, Hain P, Thuriaux P, Zillig W. Transcription in Archaea: similarity to that in Eucarya. Proc Natl Acad Sci USA. 1995;92:5768–5772. doi: 10.1073/pnas.92.13.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leigh J A. Transcriptional regulation in Archaea. Curr Opin Microbiol. 1999;2:131–134. doi: 10.1016/S1369-5274(99)80023-X. [DOI] [PubMed] [Google Scholar]
- 16.Meseguer I, Torreblanca M, Konishi T. Specific inhibition of the halobacterial Na+/H+ antiporter by halocin H6. J Biol Chem. 1995;270:6450–6455. doi: 10.1074/jbc.270.12.6450. [DOI] [PubMed] [Google Scholar]
- 17.Nuttall S D, Deutschel S E, Irving R A, Serrano-Gomicia J A, Dyall-Smith M L. The ShBle resistance determinant from Streptoalloteichus hindustanus is expressed in Haloferax volcanii and confers resistance to bleomycin. Biochem J. 2000;346:251–254. [PMC free article] [PubMed] [Google Scholar]
- 18.Peck R F, DasSarma S, Krebs M P. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol Microbiol. 2000;35:667–676. doi: 10.1046/j.1365-2958.2000.01739.x. [DOI] [PubMed] [Google Scholar]
- 19.Prangishvili D, Holz I, Stieger E, Nickell S, Kristjansson J, Zillig W. Sulfolobicins, specific proteinaceous toxins produced by strains of the extremely thermophilic archaeal genus Sulfolobus. J Bacteriol. 2000;182:2985–2988. doi: 10.1128/jb.182.10.2985-2988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price L B, Shand R F. Halocin S8: a 36-amino-acid microhalocin from the haloarchaeal strain S8a. J Bacteriol. 2000;182:4951–4958. doi: 10.1128/jb.182.17.4951-4958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rdest S, Sturm M. Bacteriocins from the halobacteria. In: Burgess R, editor. Protein purification: micro to macro. New York, N.Y: Alan R. Liss; 1987. pp. 271–278. [Google Scholar]
- 22.Rodriguez-Valera F, Juez G, Kushner D. Halocins: salt-dependent bacteriocins produced by extremely halophilic rods. Can J Microbiol. 1982;28:151–154. [Google Scholar]
- 23.Rolfsmeier M, Blum P. Purification and characterization of a maltase from the extremely thermophilic crenarchaeote Sulfolobus solfataricus. J Bacteriol. 1995;177:482–485. doi: 10.1128/jb.177.2.482-485.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolfsmeier M, Haseltine C, Bini E, Clark A, Blum P. Molecular characterization of the α-glucosidase gene (malA) from the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 1998;180:1287–1295. doi: 10.1128/jb.180.5.1287-1295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahl H. Gene-encoded antibiotics made in bacteria. Ciba Found Symp. 1994;186:27–42. doi: 10.1002/9780470514658.ch3. [DOI] [PubMed] [Google Scholar]
- 26.Shand R F, Price L B, O'Connor E M. Halocins: protein antibiotics from hypersaline environments. In: Oren A, editor. Microbiology and biogeochemistry of hypersaline environments. Boca Raton, Fla: CRC Press; 1998. pp. 295–306. [Google Scholar]
- 27.Shivvers D, Brock T. Oxidation of elemental sulfur by Sulfolobus acidocaldarius. J Bacteriol. 1973;114:706–710. doi: 10.1128/jb.114.2.706-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torreblanca M, Meseguer I, Rodriguez-Valera F. Halocin H6, a bacteriocin from Haloferax gibbonsii. J Gen Microbiol. 1989;135:2655–2661. [Google Scholar]
- 29.Torreblanca M, Meseguer I, Ventosa A. Production of halocin is a practically universal feature of archaeal halophilic rods. Lett Appl Microbiol. 1994;19:201–205. [Google Scholar]
- 30.Tumbula D L, Whitman W B. Genetics of Methanococcus: possibilities for functional genomics in archaea. Mol Microbiol. 1999;33:1–7. doi: 10.1046/j.1365-2958.1999.01463.x. [DOI] [PubMed] [Google Scholar]
- 31.Ventosa A, Nieto J J, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitman W B, Pfeifer F, Blum P, Klein A. What archaea have to tell biologists. Genetics. 1999;152:1245–1248. doi: 10.1093/genetics/152.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woese C R, Fox G E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woese C R, Kandler O, Wheelis M. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eukarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood A, Kelly D, Norris P. Autotrophic growth of four Sulfolobus strains on tetrathionate and the effect of organic nutrients. Arch Microbiol. 1987;146:382–389. [Google Scholar]