Abstract
Background
Long-acting injectable (LAI) antiretroviral therapy (ART) has the potential to improve medication adherence, reduce human immunodeficiency virus (HIV) stigma, and promote equity in care outcomes among people with HIV (PWH). We describe our early experience implementing LAI-cabotegravir/rilpivirine (CAB/RPV) for maintenance HIV-1 treatment.
Methods
We launched a pilot LAI-ART program at a large Ryan White-funded clinic in the Southeast, which accept provider-initiated referrals from April 14, 2021 to December 1, 2021. Our interdisciplinary program team (Clinician-Pharmacy-Nursing) verified clinical eligibility and pursued medication access for eligible patients. We describe (1) demographic and clinical variables of PWH referred and enrolled and (2) early outcomes among those accessing LAI-CAB/RPV.
Results
Among 58 referrals, characteristics were median age 39 (Q1–Q3, 30.25–50) years, 74% male, and 81% Black, and payor source distribution was 26% Private, 21% Medicare, 19% Medicaid, and 34% AIDS Drugs Assistance Program. Forty-five patients (78%) met clinical eligibility for LAI-CAB/RPV; ineligibility concerns included evidence of confirmed or possible RPV resistance (n = 8), HIV nonsuppression (n = 3), possible RPV hypersensitivity (n = 1), and pregnancy (n = 1). Among 45 eligible PWH, 39 (87%) enrolled and 15 (38%) initiated LAI-CAB/RPV after a median of 47 (Q1–Q3, 31–95) days since enrollment.
Conclusions
Implementing LAI-ART at a Southern US Ryan White-funded clinic has been challenged by the following: substantial human resource capital to attain drug, administer injections, and support enrolled patients; delayed therapy initiation due to insurance denials; patient ineligibility primarily due to possible RPV resistance; and inability to provide drug regardless of payor source. These barriers may perpetuate disparities in ART access and outcomes among PWH and should be urgently addressed so that LAI-ART can be offered equitably.
Keywords: cabotegravir/rilpivirine, health equity, implementation science, long-acting injectable antiretroviral therapy, people with HIV
Long-acting injectable antiretroviral therapy may improve adherence, reduce stigma, and promote equity in care outcomes among people with HIV. Implementing a pilot program in the US South was challenged by substantial human resource capital, insurance coverage barriers, and patient ineligibility.
In January 2021, the first ever long-acting injectable (LAI) antiretroviral therapy (ART), cabotegravir/rilpivirine (CAB/RPV), was approved for maintenance human immunodeficiency virus (HIV)-1 treatment in select people with HIV (PWH) with virologic suppression. Although it was initially approved for use on a monthly administration schedule with an oral lead-in (OLI), LAI-CAB/RPV was approved for bimonthly use in February 2022, and the OLI was made optional in March 2022. Long-acting injectable ART serves as a novel alternative to daily oral ART for HIV treatment with the objective of reaching sustained virologic suppression to promote longevity among PWH [1] and effectively eliminate the risk of HIV-1 transmission [2].
The advent of LAI-ART for HIV treatment [3] and prevention [4] is an opportunity to improve the landscape of care for PWH and those at risk, including as a possible tool to help efforts to end the HIV epidemic [5, 6]. Despite remarkable advances in oral ART potency, safety, and tolerability over the past few decades leading to improved life expectancy among PWH [7], only 66% of PWH in the United States achieved viral suppression in 2019 [8]. This gap is magnified by disparities across race, transmission category, and geography, with the South harboring the highest burden of HIV and the worst clinical outcomes [9]. Long-acting injectable ART for HIV-1 treatment has the potential to overcome some of the current management challenges by eliminating pill fatigue, improving medication adherence, reducing HIV stigma [10–12], and ultimately promoting equity in care access and outcomes among PWH [13].
Clinical trial data evaluating the use of LAI-CAB/RPV among treatment-naive and treatment-experienced PWH with ≥5 months of sustained virologic suppression (after induction or maintenance oral ART, respectively) demonstrated remarkable efficacy [14, 15] and high patient satisfaction with use of injectable therapy [16]. However, data on the implementation of LAI-ART use for HIV-1 treatment outside the scope of clinical trials are limited. We report our early experience and some of the principal challenges implementing LAI-CAB/RPV for maintenance HIV-1 treatment in the US South.
METHODS
In April 2021, we launched a pilot program to introduce the use of LAI-CAB/RPV for maintenance HIV-1 treatment at a Ryan White-funded clinic serving >6000 PWH in metropolitan Atlanta, Georgia. The objective of the pilot program was to develop a clinical infrastructure and protocol to initiate LAI-ART among a diverse group of PWH (representing different groups of age, sex, race, and payor source) for maintenance of virologic suppression. Additional goals included gaining familiarity with the landscape of drug procurement (OLI and injectable therapy), training personnel in the administration of 2 gluteal intramuscular (IM)-CAB/RPV injections, and building a system to track and monitor patients for treatment adherence, virologic suppression, tolerability, and acceptability of LAI-CAB/PRV; along with an overarching goal of sustaining and scaling the program so that LAI-ART may be offered equitably to all patients who are interested and clinically eligible.
We describe the process of developing our pilot program and implementing use of LAI-CAB/RPV for HIV-1 treatment, including assembling a multidisciplinary team, soliciting patient referrals (from April 14, 2021 to December 1, 2021), assessing individual clinical eligibility as well as interest and willingness to switch from oral ART, and drug procurement. We also report demographic and clinical variables of PWH referred and enrolled into the pilot program and early outcomes among those accessing LAI-CAB/RPV assessed through March 30, 2022.
Patient Consent Statement
The Emory University Institutional Review Board approved a waiver of informed consent and HIPAA authorization for this study.
RESULTS
Multidisciplinary Team Assembly
We assembled a multidisciplinary team including clinicians, pharmacists, nurses, medication access specialists, and other supporting staff (eg, schedulers, patient navigators) to develop and implement the pilot program. Table 1 describes the specific roles and responsibilities of each team member and an approximated full-time equivalent effort dedicated to the pilot over the initial 12 months of the program. Our team—led by the clinic medical director (physician), a clinician colead of the pilot program (physician), and the pilot program director (clinical pharmacist)—met biweekly beginning in April 2021 through present.
Table 1.
Detailed Roles, Responsibilities, and Effort of Personnel Comprising a Multidisciplinary Team to Develop, Support, and Anticipate Scale-Up of a Pilot Program Implementing Long-Acting Injectable Cabotegravir/Rilpivirine for HIV-1 Maintenance Treatment
| Team Member | Training/Credentials | FTEa Dedicated to the Pilot | Roles and Responsibilities |
|---|---|---|---|
| Medical director of the clinic | Medical doctor | 0.10 |
|
| Clinician co-lead of the pilot program | Medical doctor | 0.10–0.15 |
|
| Program director | Clinical pharmacist (RPh) | 0.25–0.35 |
|
| Pharmacy lead | Clinical pharmacist (PharmD) | 0.10 |
|
| Medication access coordinator | Masters of public health | 0.10–0.15 |
|
| Medical benefits specialist | Health system financial services | 0.05 |
|
| Nursing lead | Registered nurse | <0.05 |
|
| Injection administrator | Certified medical assistant | 0.10 |
|
| Scheduling lead | Patient access supervisor | <0.05 |
|
Abbreviations: ART, antiretroviral therapy; CAB, cabotegravir; FTE, full-time equivalent; HIV, human immunodeficiency virus; LAI, long-acting injectable; RPV, rilpivirine.
The FTE effort provided is approximated and based on an average of the past 12 months, which has included time-intensive periods of program development as well as program maintenance for 15–20 patients currently enrolled and accessing LAI-CAB/RPV; note that FTE effort is funded through the clinic and not designated for effort spent on the pilot program necessarily.
Our initial meetings focused on developing a clinic protocol for implementing LAI-CAB/RPV, including defining patient eligibility criteria, the referral process, workflow design, and clinical decision making. These early concepts were discussed with the clinic community advisory board, and an ongoing National Institutes of Health-funded implementation science R01 simultaneously added patient perspective to the protocol development. We created a clinical database to monitor and track referred and enrolled patients (currently housed on a HIPAA-compliant server in Microsoft Excel). In collaboration with information technology support, we built referral and CAB/RPV prescription capacity and are working to integrate the clinical database into the electronic medical record. Through meetings with ViiV Healthcare, discussions with other clinics and clinicians, and trial and error with each individual insurer, we familiarized ourselves with insurance approval and drug procurement. Nurses and medical assistants were trained on new injection protocols, technique, and documentation (Table 1). We created a script detailing the pilot program for our team to communicate with referred patients deemed clinically eligible to initiate LAI-CAB/RPV, and we assessed their interest and willingness to switch from oral ART as wells as the reason for being interested or not switching; the script also included prompts for confirming preferred contact method and relevant medical history among interested patients (Supplement).
Subsequent meetings focused on reviewing eligibility criteria for referred patients, determining a disposition, and coordinating communication between patients and primary providers. For enrolled patients, we ordered prescriptions, considered how to overcome cases of insurance denials, discussed challenges with drug procurement, reviewed tolerability of the OLI and injectable phases, and evaluated virologic suppression and therapy acceptability at 3 and 6 months post-LAI-ART initiation as applicable (and more frequently if needed).
Protocol Implementation
From April 14, 2021 to December 1, 2021, we accepted provider-initiated referrals of PWH interested in switching from oral ART to LAI-CAB/RPV. Clinician leads verified clinical eligibility based on thorough medical chart review with attention to ART use history, virologic data, available HIV genotype data, concurrent medications, coinfections, and comorbidities. Eligibility was determined according to contemporary on-label use of LAI-CAB/RPV: HIV-1 ribonucleic acid viral load (VL) <200 copies/mL for ≥6 months; no history of virologic failure; no history of resistance or hypersensitivity to either drug; no active hepatitis B virus infection not otherwise treated. For patients in whom prior ART history was not known and/or HIV genotype data was incomplete, our pilot clinician team facilitated a risk-benefit discussion with the patient and primary provider, considering the safety of using LAI-CAB/RPV given available data.
As described in detail in the Supplement, the program leads contacted eligible patients to confirm their interest and willingness to switch using questions as outlined in the script as a guide. If patients declined after learning more about use of LAI-ART and the pilot program, we explored their reasons through semistructured interviews (Supplement). If interest and willingness to switch was confirmed, patients were considered enrolled into the pilot; we asked patients why they were most interested in beginning LAI-ART for HIV treatment and categorized responses based on emergent themes (Supplement). For enrolled patients, a team clinician wrote the prescription for 28-day OLI CAB/RPV, one-time loading dose of IM-CAB/RPV, and monthly maintenance dosing of IM-CAB/RPV (per contemporary approval). A medication access coordinator then pursued drug access by contacting ViiVConnect and/or directly contacting the patients' payor source to determine coverage. As part of the benefits investigation, LAI-CAB/RPV coverage by pharmacy or medical benefits was ascertained, and for the latter, assistance was required from an off-site medical benefits specialist within the main health system. The medication access specialists were instrumental in assisting with prior authorizations and appeals to insurance denials including coordinating communication and documentation among the pilot team, patient's primary care provider, payor source, and ViiV.
Referral Population
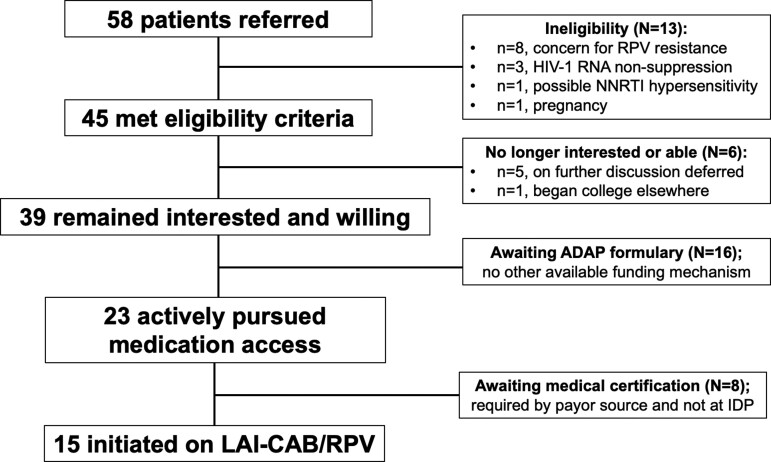
Among 58 referrals, characteristics were median age 39 (Q1–Q3, 30.25–50) years, 74% male, and 81% Black, and payor source distribution was 26% Private, 21% Medicare, 19% Medicaid, and 34% AIDS Drugs Assistance Program (ADAP). Among the 58 patients, there were an average (±standard deviationn) of 1.16 (±1.28) HIV genotypes and 0.36 (±0.91) integrase strand transfer inhibitor (INSTI) genotypes available for review per patient; we did not assess drug resistance by proviral deoxyribonucleic acid (GenoSure) in this population. Forty-five patients (78%) met clinical eligibility for LAI-CAB/RPV; ineligibility concerns included evidence of confirmed or possible rilpivirine resistance (n = 8), HIV nonsuppression (n = 3), possible RPV hypersensitivity (n = 1), and pregnancy (n = 1) (Figure 1).
Figure 1.
Flow of patients from referral to initiation of long-acting injectable (LAI)-cabotegravir/rilpivirine (CAB/RPV) for human immunodeficiency virus (HIV)-1 treatment as part of enrollment into the LAI pilot program. ADAP, AIDS Drug Assistance Program; IDP, Grady Ponce de Leon Center; NNRTI, nonnucleoside reverse-transcriptase inhibitor; RNA, ribonucleic acid.
Of the 8 patients excluded based on nonnucleoside reverse-transcriptase inhibitor (NNRTI) resistance mutations detected on prior genotypes, 4 patients had evidence of mutations contraindicating use of CAB/RPV per data included in the package insert and/or pooled from clinical trials: E138A (n = 1), Y181C (n = 2), and V106I (n = 1) [17, 18]. The other 4 patients had a history of NNRTI use with historic genotype data revealing NNRTI resistance mutations (ie, K103N, G190A, V179E) that could potentially impact rilpivirine susceptibility in the presence of other mutations. In these cases, in which there was concern that additional historical mutations could be present at a low level, the pilot team clinicians engaged in risk-benefit discussions with the patient and primary provider about the safety of using LAI-CAB/RPV; ultimately, the decision was made to not switch before the availability of additional data on drug resistance mutations associated with clinical failure of LAI-CAB/RPV.
Enrolled Population
Among 45 eligible PWH, 39 (87%) enrolled into the pilot program. When contacted for enrollment, 6 patients reported they were no longer interested or willing to switch from daily oral to LAI-ART given the following concerns: “newness” of therapy (n = 2); anticipated side effects of gluteal IM injections (n = 2); and/or inability to secure monthly transportation to clinic (n = 2). For example, one patient asked, “Is this experimental treatment? I don’t want to be a guinea pig”; and despite our team communicating that the medication was approved by the US Food and Drug Administration, the patient elected to wait until more real-world experience was accumulated. A different patient reported nervousness about possible injection site reactions in the buttocks (eg, soreness) given that they work long shifts standing up all day.
Among those who enrolled into the pilot, common themes for choosing to switch included the desire to minimize pill burden or eliminate pill stress (n = 10); participate in a novel therapeutic opportunity lending hope for the future of HIV medicine (n = 3); and/or overcoming pill aversion or intolerance (n = 2). Furthermore, almost all patients stated that LAI-ART would provide them with freedom (1) from their pill as a daily reminder of their HIV diagnosis and also (2) from having to worry about inadvertent HIV disclosure upon others discovering their labeled pill bottles.
Accessing Long-Acting Injectable Cabotegravir/Rilpivirine
Of the 39 patients enrolled, 15 (38%) have successfully initiated LAI-CAB/RPV after a median of 47 (Q1–Q3, 31–95) days from enrollment. Reasons for delays in access or inability to access LAI-CAB/RPV have included the following: lack of drug on the formulary of all payor sources; difficulty deciphering coverage as a pharmacy versus medical benefit; pursuit of prior authorizations and appeals of insurance denials; and delays in accessing the OLI therapy from ViiVConnect (now less relevant in the era of optional OLI therapy). Initially, ViiVConnect was used to help decipher coverage of IM-CAB/RPV as a medical or pharmacy benefit; however, determination would take ≥1 week, and our team streamlined this process by submitting claims directly to the insurer.
Long-acting injectable CAB/RPV remained unavailable for the 16 patients who receive ART through the ADAP given that CAB/RPV was not yet included on the state ADAP formulary. Another 8 patients were awaiting medical certification for CAB/RPV to be covered under medical benefits with 6 recently approved and pending initiation and 2 reconsidering switching due to desire for pregnancy (n = 1) or job requiring travel (n = 1). Among the 15 patients who have accessed LAI-CAB/RPV thus far, payor source distribution was 46% (n = 7) Medicare, 40% (n = 6) Private insurance, and 13% (n = 2) Medicaid. The majority have accessed LAI-CAB/RPV via pharmacy (n = 10) versus medical benefits (n = 5).
Population Who Accessed Long-Acting Injectable Antiretroviral Therapy
Table 2 summarizes demographics, clinical characteristics, and outcomes of the 15 PWH who have successfully accessed LAI-CAB/RPV. One patient was treated as an exception to our eligibility criteria per contemporary label use, and IM-CAB-RPV was administered direct-to-inject (without OLI) given severe malabsorption, limiting their ability to achieve virologic suppression on oral ART. Among the 15 patients, characteristics at the time of enrollment included median (Q1–Q3) or percentage: age 41 (35–53) years, 87% male, 73% non-Hispanic Black, body mass index (BMI) 27.7 (23.2–29.8) kg/m2, blood pressure 125/75 (116–128.5/68–78.75) mmHg, hemoglobin A1c 5.4% (5.1–5.6%), low-density lipoprotein 81 (74.5–109.5) mg/dL, and serum creatinine 1.1 (1–1.2) mg/dL. Average time since HIV diagnosis was 15.0 (±7.9) years, and 67% (n = 10) were men who have sex with men, including 2 whom reported prior injection drug use. Median CD4 count was 530 (Q1–Q3, 269–708) cells/mm3 and median duration of HIV-1 VL <200 copies/mL was 5.9 (Q1–Q3, 3.6–8.5) years. Before switching to CAB/RPV, 87% (n = 13) of patients had been prescribed ≥2 oral ART regimens, 87% (n = 13) were currently prescribed a single tablet regimen, and 93% (n = 14) switched off of an INSTI-containing regimen.
Table 2.
Clinical Characteristics and Early Outcomes of Patients Who Have Accessed Long-Acting Injectable-Cabotegravir/Rilpivirine
| Patient | Age and Sex | Race | Payor Source | Preswitch CD4 Count (Cells/mm3)/CD4% | Time Preswitch HIV-1 RNA <200 cp/mL (Years) | Preswitch ART Regimen | Time From CAB/RPV Script to Initiation (Days) | Oral Lead-in Given? | No. LAI-ART IM Doses Received to Date | Tolerability Concerns | Current Clinical Statusa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 M | NHW | Medicare, part D | 530/32% | 2.23 | TAF/FTC/BIC | 14 | Yes | 9 | None | On LAI-ART, suppressed |
| 2 | 67 F | NHB | Medicare, part D | 777/32% | 8.78 | TAF/FTC/RPV | 42 | Yes | 7 | Injection site pain | On LAI-ART, suppressed |
| 3 | 56 M | NHW | Medicare, part D | 392/25% | 3.54 | TAF/FTC/EVG/c | 20 | Yes | 7 | None | On LAI-ART, suppressed |
| 4 | 48 M | NHB | Medicare, part D | 612/33% | 7.29 | TAF/FTC/EVG/c | 20 | Yes | 6 | Injection site nodules | Discontinued LAI-ARTb |
| 5 | 26 M | NHB | Private | 740/31% | 4.05 | ABC/3TC/DTG | 84 | Yes | 7 | None | On LAI-ART, suppressed |
| 6 | 35 M | NHB | Medicare, part D | 603/42% | 8.25 | TAF/FTC/EVG/c | 61 | Yes | 7 | Injection site pain | On LAI-ART, suppressed |
| 7 | 34 M | NHB | Private | 93/4% | Not suppressedc | TAF/FTC/BIC | 105 | No | 7 | None | On LAI-ART, not suppressed |
| 8 | 38 M | NHB | Private | 263/20% | 1.52 | TAF/FTC/BIC | 63 | Yes | 6 | None | On LAI-ART, suppressed |
| 9 | 42 M | NHB | Medicare, part B | 275/17% | 8.65 | TAF/FTC, DTG | 174 | Yes | 5 | None | Discontinued LAI-ARTd |
| 10 | 38 M | NHW | Private | 254/16% | 4.39 | 3TC/DTG | 154 | Yes | 4 | None | On LAI-ART, suppressed |
| 11 | 60 M | NHB | Private | 479/31% | 3.78 | TAF/FTC, DTG | 167 | Yes | 4 | None | On LAI-ART, suppressed |
| 12 | 30 M | NHB | Medicaid | 748/30% | 7.90 | ABC/3TC/DTG | 47 | Yes | 3 | None | On LAI-ART, suppressed |
| 13 | 55 M | NHW | Private | 603/30% | 10.13 | TAF/FTC/EVG/c | 34 | Yes | 3 | None | On LAI-ART, suppressed |
| 14 | 36 M | NHB | Medicaid | 161/7% | 0.79 | TAF/FTC/BIC | 38 | Yes | 3 | None | On LAI-ART, suppressed |
| 15 | 41 F | NHB | Medicare, part D | 953/48% | 8.54 | 3TC/DTG | 28 | Yes | 3 | Injection site nodules | On LAI-ART, suppressed |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; BIC, bictegravir; DTG, dolutegravir; EVG/c, elvitegravic/cobicistat; HIV, human immunodeficiency virus; IM, intramuscular; LAI, long-acting injectable; NHB, non-Hispanic Black; NHW, non-Hispanic White; RNA, ribonucleic acid; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; VL, viral load.
Suppressed was defined as HIV-1 RNA <200 copies/mL.
Patient elected to discontinue after 6th injection due to persistently painful injection site nodules and concern over medication effectiveness (HIV-1 RNA not detected at time of discontinuation).
Patient with low-level viremia despite reported adherence on oral antiretroviral therapy in the setting of chronic microsporidiosis and vitamin deficiencies.
Patient elected to discontinue after 5th injection given concern for treatment failure in the setting of HIV-1 RNA = 750 copies/mL and HIV genotype revealing nonnucleoside reverse-transcriptase inhibitor mutations (K103N, L100I) not detected on prior available genotypes (HIV-1 RNA <40 copies/mL at time of discontinuation).
Early Outcomes
Outcomes were assessed through March 30, 2022 (twelve months after pilot initiation). Among 15 patients who initiated LAI-CAB/RPV, a median of 6 (3.5–7) injections per patient were administered. Among 15 patients with available VL data obtained 3 months after initiating IM-CAB/RPV, 93% (n = 14) had HIV-1 VL <200 copies/mL. The individual not suppressed was the patient for whom direct-to-inject therapy was pursued; their HIV-1 VL before LAI-CAB/RPV initiation was 767 copies/mL and remained nearly stagnant at 689 copies/mL 6 months later despite ART intensification with fully active oral agents. At the time of LAI-CAB/RPV initiation, the patient's BMI was 20.8 kg/m2 and no injections were missed nor delayed. Excluding this patient, among 10 patients with available VL data obtained 6 months after initiating IM-CAB/RPV, 90% had HIV-1 VL <200 copies/mL. The patient not suppressed at this time point had an HIV-1 VL of 750 copies/mL with a follow-up VL of <40 copies/mL obtained 2 weeks later and associated HIV genotype revealing a K103N and L1001 (these mutations were not detected on prior available genotypes). In this case, the patient's BMI was 27.7 kg/m2 at LAI-CAB/RPV initiation and no injections were missed nor delayed. After a risk-benefit discussion, this patient elected to return to oral ART given the risk of virologic failure associated with LAI-CAB/RPV use in the presence of this combination of drug resistance mutations.
Among patients who have accessed LAI-CAB/RPV thus far, 33% (n = 5) reported injection site reactions including 3 describing discomfort or pain and 2 reporting nodules at the site. Acceptability of LAI-ART assessed at 3 months was 100% (15 of 15), although this was reduced to 80% (8 of 10) among those with 6 months of follow-up. A total of 13 of 15 patients (87%) have continued on LAI-CAB/RPV with one discontinuation described above and another patient electing to discontinue after their 6th injection due to persistently painful injection site nodules and subjective concern over medication effectiveness (their HIV-1 VL was not detected at time of discontinuation). For those continuing on LAI-ART, acceptability has been described as “having a new lease on life,” and overall patients are thrilled to have the experience of long-acting therapy as opposed to having to remember, tolerate, or keep hidden daily pill(s) for HIV treatment.
DISCUSSION
Long-acting injectable ART has potential to revolutionize HIV care delivery and to make progress toward Ending the HIV Epidemic. However, real-world experience using LAI-CAB/RPV has illuminated the value in engaging patients in informed risk-benefits discussion about this therapeutic opportunity and the need for dedicated resources to support the implementation of this novel treatment so that access can be streamlined, supported, and equitable. Substantial and sustained investment in developing infrastructural support, including personnel, is needed to facilitate access and delivery of LAI-ART for a broad range of PWH across a variety of settings as to not widen existing disparities in HIV care [19, 20].
In a pilot program implementing LAI-ART at a Southern US Ryan White-funded clinic, of 58 referrals received and 39 patients enrolled into the program, only 15 (38%) have successfully accessed LAI-CAB/RPV over the course of approximately 12 months since initiating the pilot. An additional 6 patients were recently approved and are pending LAI-ART initiation. Our implementation experience has been challenged by the following: the need for substantial resources (including personnel, time, space); delayed therapy initiation due to insurance denials; patient ineligibility primarily due to concern for potential rilpivirine resistance; and inability to provide drug to patients regardless of payor source. Patients with ADAP prescription coverage represented 34% of pilot referrals (comparable to the clinic at large whereby ∼25% of our patient population is supported by ADAP) and thus far none have been able to access LAI-CAB/RPV. For this group of individuals, while eagerly awaiting formulary approval by Georgia ADAP (anticipated summer 2022), there is no qualifying mechanism via the manufacturer patient assistance programs to support access to LAI-CAB/RPV.
The administrative capacity required to procure and provide LAI-CAB/RPV is significant and can overburden clinics, especially clinics with fewer resources and/or caring for patients with more barriers [21]. Intensive human capital is needed across every implementation phase to (1) consider clinical eligibility thoroughly, access drug, and administer IM-intragluteal injections, (2) support enrolled patients to ensure tracking and retention, and (3) conduct billing and monitor reimbursement. Furthermore, physical infrastructure entails a cold-chain supply and storage of drug kits comprising vials, needles, and syringes and private space for the administration of gluteal IM injections. If there is a 20% uptake of LAI-ART among a clinic population of 3000 PWH, then enrolling 600 patients in the program would translate to 15 (bimonthly) or 30 (monthly) injection visits per day (with drug administration into 2 intragluteal sites per patient). An additional challenge we experienced within our health system was not initially having a clear pathway for accessing drug via medical benefits, thus it was easier for our pilot team to procure the drug for patients with a payor source covering LAI-CAB/RPV via pharmacy benefits; this may be a caveat to our data although an important consideration for other HIV clinics building LAI-ART programs that may not have an existing mechanism to procure the drug via medical benefits.
By adding the option of LAI-CAB/RPV for HIV-1 treatment within the current healthcare system structure, critical resources may be diverted away from PWH who need the most support for adherence and retention to PWH already doing well on therapy. Similarly, the current label use of LAI-CAB/RPV may not match those PWH with the greatest needs, and it is important to consider which patients may stand to benefit the most from this novel therapeutic option [10–12, 22]. We used a centralized approach to implement our pilot program to ensure that accessing LAI-ART was in reach for eligible patients from a variety of backgrounds considering age, sex, race, and payor source; however, careful attention to equitable impact is needed beyond basic demographics upon LAI-ART scale-up and should include dedicated resources to support PWH who have traditionally been at highest risk of poor clinical outcomes. The AIDS Clinical Trial Group (ACTG) protocol 5359, Long-Acting Therapy to Improve Treatment Success in Daily Life (LATITUDE), is actively enrolling PWH with recently documented treatment lapses and thus may provide insights on LAI-ART use among patients with adherence challenges [23]. The study provides focused adherence and retention support, including financial incentives, to encourage participants to meet the viral suppression requirement before LAI-CAB/RPV initiation. Although such an approach may not be feasible to scale in the real-world, this study will provide critical insights into how focused support paired with long-acting and directly observed ART administration may improve treatment adherence and clinical outcomes among PWH with prior adherence challenges.
Furthermore, several referred patients were no longer interested or willing to switch from daily oral to monthly LAI-ART after they were contacted by our program; these patients cited different reasons, for example, concern over therapy newness, transportation needs, desire for pregnancy, etc. Even in our small pilot, individuals changed their mind in as few as 2–3 months between the time of referral and the opportunity for LAI-ART initiation. These data underscore the importance of providers engaging in conversation with potential LAI-ART users about perceived barriers and exploring interest in this novel therapeutic opportunity. Additional qualitative studies may provide useful insights into patient perceptions on accessing LAI-ART and help inform best clinical practices.
Finally, assessing clinical eligibility for LAI-CAB/RPV in the real-world can be challenging. For example, although the CAB/RPV package insert provides guidance on HIV drug resistance mutations that are likely or confirmed to confer resistance to either agent [17], we encountered patients with incomplete HIV genotype data, a particular concern among transient patient populations. Until more real-world data are available, we excluded patients from the pilot who had HIV mutations detected known to compromise rilpivirine susceptibility or that, in the absence of known ART history or genotype timing in relation to ART exposure, suggested prior NNRTI exposure and virologic failure [24]. In pooled data from FLAIR, ATLAS, and ATLAS-2 M, presence of at least 2 of proviral rilpivirine resistance-associated mutations, HIV-1 subtype A6/A1, and/or BMI ≥30 kg/m2 was associated with increased risk of clinical virologic failure through week 48 [18]. Longer term data are needed, especially in the context of real-world use of LAI-CAB/RPV where performing archived genotype testing is not realistic and/or ART history and genotype data may not available.
CONCLUSIONS
In conclusion, our experience in implementing LAI-CAB/RPV for HIV-1 treatment at a Ryan White-funded clinic in the US South has kept us cautiously optimistic about the potential this therapy holds for the patient experience, clinical outcomes, and Ending the HIV Epidemic goals. However, operational and clinical hurdles must be overcome to ensure this therapy has an equitable impact for all populations.
Supplementary Material
Acknowledgments
We thank our patients who enrolled into the pilot program for sharing their experience with us, and we thank the clinic staff and leadership for their time and support of this pilot program.
Author contributions. L. F. C. and J. A. C. designed the study, conducted analyses, provided interpretation of study findings, and drafted the article. D. C.-J., M. A., Z. P. M., K. D., A. C., K. J., M. T.-T., J. S., and B. L. S. conducted the data collection/medical chart abstraction, contributed to interpretation of findings, critically revised the article, and approved the submission. W. S. A. provided guidance on study design and analyses, critically revised the article, and approved the submission.
Financial support. This work was funded by the Center for AIDS Research, Emory University (Grant Number P30-AI-050409). L. F. C. is also funded by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) through the Georgia CTSA (Georgia Clinical & Translational Science Alliance) (Grant Numbers UL1TR002378 and KL2-TL1TR002381) and the Program for Retaining, Supporting, and EleVating Early-career Researchers at Emory (PeRSEVERE) from Emory School of Medicine, a gift from the Doris Duke Charitable Foundation. J. A. C. is also funded by the National Institute of Mental Health of the NIH (Grant Number R01MH123396).
Contributor Information
Lauren F Collins, Department of Medicine, Divison of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA; Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Della Corbin-Johnson, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Meron Asrat, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Zoey P Morton, Department of Medicine, Divison of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA.
Kaylin Dance, Department of Medicine, Divison of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA.
Alton Condra, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Kimberly Jenkins, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Marie Todd-Turner, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Jeri Sumitani, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Bradley L Smith, Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Wendy S Armstrong, Department of Medicine, Divison of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA; Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA.
Jonathan A Colasanti, Department of Medicine, Divison of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, USA; Grady Healthcare System, Infectious Diseases Program, Atlanta, Georgia, USA; Hubert Department of Global Health, Emory University Rollins School of Public Health, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 2. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizzardini G, Overton ET, Orkin C, et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J Acquir Immune Defic Syndr 2020; 85:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 6. Giroir BP. The time is now to end the HIV epidemic. Am J Public Health 2020; 110:22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 2020; 3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC) . Monitoring selected national HIV prevention and care objectives by using HIV surveillance data–United States and 6 dependent areas. 2019. HIV Surveillance Supplemental Report 2021; 26(2).
- 9. Colasanti JA, Armstrong WS. Challenges of reaching 90-90-90 in the southern United States. Curr Opin HIV AIDS 2019; 14:471–80. [DOI] [PubMed] [Google Scholar]
- 10. Carillon S, Gallardo L, Linard F, et al. Perspectives of injectable long acting antiretroviral therapies for HIV treatment or prevention: understanding potential users’ ambivalences. AIDS Care 2020; 32:155–61. [DOI] [PubMed] [Google Scholar]
- 11. Akinwunmi B, Buchenberger D, Scherzer J, et al. Factors associated with interest in a long-acting HIV regimen: perspectives of people living with HIV and healthcare providers in four European countries. Sex Transm Infect 2021; 97:566–73. [DOI] [PubMed] [Google Scholar]
- 12. Philbin MM, Parish C, Bergen S, et al. A qualitative exploration of women's interest in long-acting injectable antiretroviral therapy across six cities in the Women's Interagency HIV study: intersections with current and past injectable medication and substance use. AIDS Patient Care STDS 2021; 35:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hojilla JC, Gandhi M, Satre DD, Johnson MO, Saberi P. Equity in access to long-acting injectables in the USA. Lancet HIV 2022; 9:e145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orkin C, Oka S, Philibert P, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8:e185–96. [DOI] [PubMed] [Google Scholar]
- 15. Swindells S, Lutz T, Van Zyl L, et al. Week 96 extension results of a phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS 2022; 36:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray M, Antela A, Mills A, et al. Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav 2020; 24:3533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Highlights of Prescribing Information- Cabenuva. Research Triangle Park, NC: GlaxoSmithKine; 2021. [Google Scholar]
- 18. Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS 2021; 35:1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. HHS Adults and Adolescents Antiretroviral Guidelines Panel Recommendation for the Long-Acting Injectable Antiretroviral Regimen of Cabotegravir and Rilpivirine. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultARV_GL_ID_2021_CabRpv.pdf. Accessed May 20, 2022.
- 20. Philbin MM, Perez-Brumer A. Promise, perils and cautious optimism: the next frontier in long-acting modalities for the treatment and prevention of HIV. Curr Opin HIV AIDS 2022; 17:72–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. HIV Medicine Association (HIVMA), National Alliance of State and Territorial AIDS Directors, and the American Academy of HIV Medicine . Preparing for Long-Acting Antiretroviral Treatment, 2021. Available at: https://www.hivma.org/globalassets/hivma/long-acting-arvs-_final.pdf. Accessed May 20, 2022. [Google Scholar]
- 22. Philbin M. M. et al. Long-acting injectable ART and PrEP among women in six cities across the United States: a qualitative analysis of who would benefit the most. AIDS Behav 2022; 26:1260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AIDS Clinical Trial Group . A5359: The LATITUDE Study. Available at: https://actgnetwork.org/studies/a5359-the-latitude-study/. Accessed May 20, 2022.
- 24. Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42:1608–18. Available at: https://hivdb.stanford.edu/hivdb/by-patterns/. Accessed May 20, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.