Abstract
Anxiety and stress-related disorders are highly prevalent and are characterized by excessive fear to threatening and nonthreatening stimuli. Moreover, there is a large sex bias in vulnerability to anxiety and stress-related disorders—women make up a disproportionately larger number of affected individuals compared with men. Growing evidence suggests that an impaired ability to suppress fear in the presence of safety signals may in part contribute to the development and maintenance of many anxiety and stress-related disorders. However, the sex-dependent impact of stress on conditioned inhibition of fear remains unclear. The present study investigated sex differences in the acquisition and recall of conditioned inhibition in male and female mice with a focus on understanding how stress impacts fear suppression. In these experiments, the training context served as the “fear” cue and an explicit tone served as the “safety” cue. Here, we found a possible sex difference in the training requirements for safety learning, although this effect was not consistent across experiments. Reductions in freezing to the safety cue in female mice were also not due to alternative fear behavior expression such as darting. Next, using footshock as a stressor, we found that males were impaired in conditioned inhibition of freezing when the stress was experienced before, but not after, conditioned inhibition training. Females were unaffected by footshock stress when it was administered at either time. Extended conditioned inhibition training in males eliminated the deficit produced by footshock stress. Finally, exposing male and female mice to swim stress impaired safety learning in male mice only. Thus, we found sex × stress interactions in the learning of conditioned inhibition and sex-dependent effects of stress modality. The present study adds to the growing literature on sex differences in safety learning, which will be critical for developing sex-specific therapies for a variety of fear-related disorders that involve excessive fear and/or impaired fear inhibition.
Most anxiety and trauma-related disorders involve some form of associative learning. Pavlovian fear conditioning, therefore, has been used widely to study the mechanisms of associative learning and memory that form the basis for our understanding of specific aversive conditioning, as well as anxiety and trauma-related neural circuitry (Davis 2000; Maren 2008; Cain et al. 2012; Mahan and Ressler 2012; Steiger et al. 2015; Goode and Maren 2018). In Pavlovian fear conditioning, subjects are presented with a neutral conditional stimulus (CS; e.g., a tone), which is paired with an aversive unconditional stimulus (US; e.g., a footshock). After repeated pairings, the tone predicts a threat and elicits a fear response when presented alone.
In addition to predicting threat in isolation, animals must discriminate between predictors of threat and safety to flexibly respond to varying environmental conditions to make appropriate behavioral responses (e.g., freezing, fighting, and avoidance vs. social interaction, foraging, and reproduction). Most individuals of several species, including humans, rodents, and nonhuman primates, successfully discriminate between predictors of threat and safety in laboratory and nonlaboratory settings (Myers and Davis 2004; Winslow et al. 2008; Kazama et al. 2013). Recent studies suggest that an inability to identify and appropriately respond to predictors of threat and safety contributes to the etiology of several anxiety disorders including generalized anxiety disorder (GAD) and panic disorder (PD), as well as post-traumatic stress disorder (PTSD) (Lissek et al. 2009; Cooper et al. 2018). Similarly, the inability to suppress fear when safety and fear cues are presented simultaneously, a process known as conditioned inhibition, is also implicated in anxiety and trauma-related disorders (Jovanovic et al. 2012; Sijbrandij et al. 2013; Grasser and Jovanovic 2021). Taken together, these clinical findings suggest that the impaired ability to suppress fear is a pathological biomarker of anxiety and trauma-related disorders that could be a behavioral process to target for successful therapeutic intervention.
There are several laboratory methods for investigating conditioned inhibition (Myers and Davis 2004; for review, see Christianson et al. 2012; Krueger and Sangha 2021). However, most, if not all, involve a summation test where the fear cue (CS+) is presented at the same time as a safety cue (CS−). This test assesses the ability of animals to use the CS− to suppress fear in the presence of the CS+. More complicated conditioning designs have been implemented and are required to examine fear suppression in human and nonhuman primates due to configural learning of compound cues (Winslow et al. 2008; Jovanovic et al. 2012; van Rooij and Jovanovic 2019), but have also been implemented successfully in rodents (Myers and Davis 2004) and to examine discrimination among fear, safety, and reward cues (Sangha et al. 2014, 2020; Greiner et al. 2019).
Women are approximately twice as likely as men to be diagnosed with an anxiety or stress-related disorder (Kessler et al. 2005a; McLean et al. 2011). Research on sex differences in conditioned inhibition is quite limited, but recent work has begun to address this important gap in our understanding of critical learning processes involved in anxiety and trauma-related disorders. Findings within the literature, however, have not been consistent across studies (Toufexis et al. 2007; Foilb et al. 2018; Day and Stevenson 2019; Greiner et al. 2019; Day et al. 2020). Some studies have found no sex difference in fear suppression. For instance, one study found that male and female rats exhibit differences in fear discrimination, with female rats displaying better discrimination between CS+ and CS− cues, but there was no apparent sex difference during a summation test, suggesting no differences between males and females in conditioned inhibition (Foilb et al. 2018). Similarly, intact male and female rats were demonstrated to show equivalent conditioned inhibition in an AX+/BX− conditional discrimination procedure (Toufexis et al. 2007). Other studies, however, report sex differences in conditioned inhibition, with female rodents performing worse compared with males. In preweanlings, male rats showed conditioned inhibition in a taste aversion paradigm, whereas females did not suppress aversive responses in the presence of the safety cue (Aranda-Fernandez et al. 2016). Greiner et al. (2019) found that male, but not female, rats successfully suppressed conditioned freezing in the presence of a safety cue in a fear, safety, and reward discrimination task. Having a comprehensive understanding of how males and females learn to inhibit fear in the presence of safety cues is essential for gaining a better understanding of why women have a higher prevalence compared with men for anxiety disorders and PTSD (Kessler et al. 2005b, 2012; McLean et al. 2011). In addition, it is unclear how the experience of stress impacts conditioned inhibition and, if so, whether its effects are sex specific.
Stress × sex interactions have been reported consistently in the literature across several species (for review, see Maeng and Milad 2015). For example, stress facilitates learning of Pavlovian eye blink responses in male rats, but impairs performance in females (Wood and Shors 1998). Witness stress has different effects on some cardiovascular and behavioral responses in male and female rats (Finnell et al. 2017, 2018). In humans, sex × stress interactions have also been observed across several studies involving Pavlovian fear conditioning. Administration of cortisol or exposure to a psychosocial stressor reduces skin conductance responses to a CS+ in men, whereas it enhances responses in women (Merz et al. 2010, 2013). Psychosocial stress also enhances subjective anxiety and salivary cortisol that is associated with enhanced discriminative fear conditioning in men, whereas stress impairs discriminative fear conditioning in females and is not related to salivary cortisol levels (Jackson et al. 2006). Similar to impaired discriminative fear conditioning in women, female rodents generalize contextual fear responses more readily than male rodents, suggesting that stress may impair female rodents’ ability to identify and respond appropriately to fear and nonfear cues (Lynch et al. 2013, 2016; Keiser et al. 2017; Adkins et al. 2019). Overall, these effects highlight a potential role for stress × sex interactions on conditioned inhibition, indicating that females may display impaired fear suppression in the presence of safety cues after stress exposure.
There were two aims for the current study: (1) to identify and evaluate sex differences in conditioned inhibition between male and female mice, and (2) to assess the impact of stress on the acquisition and expression of conditioned inhibition by exposing male and female mice to a stressor before or after conditioning. Because female rodents generalized contextual fear responses more readily than males (Lynch et al. 2013; Keiser et al. 2017), we hypothesized that female mice would display less conditioned inhibition to a safety cue compared with males under the same training conditions. We also hypothesized that stress would have a greater impact on conditioned inhibition in female mice compared with males.
Results
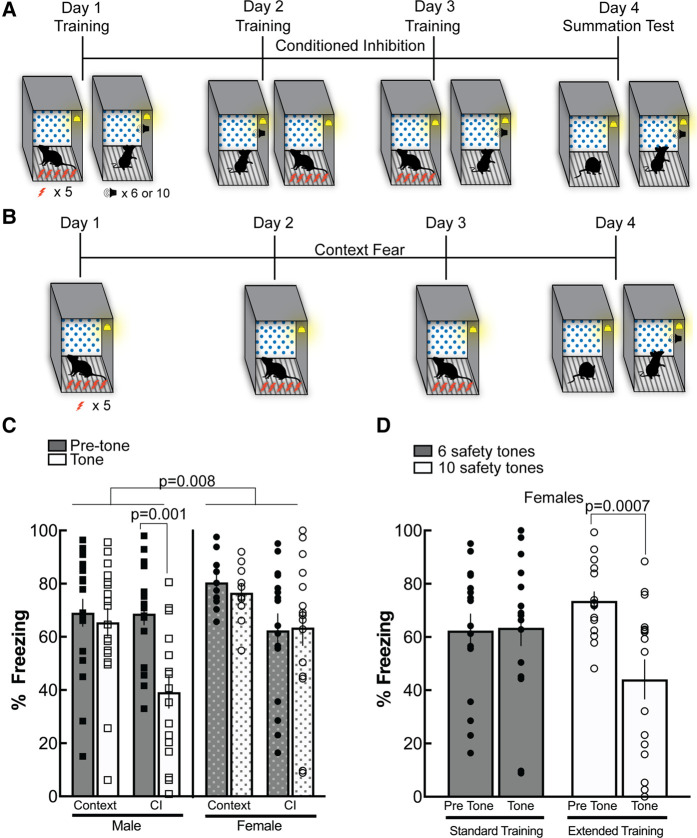
Training conditions for acquisition of conditioned inhibition in male and female mice
A total of 64 male and female B6S1 mice (see “Animals and Housing Conditions” in the Materials and Methods for details) underwent 3 d of training (conditioned inhibition or context alone), during which each day mice were presented with five unsignaled footshocks and six tones as safety cues (Fig. 1A) or they were presented with shocks alone to serve as context fear controls (Fig. 1B). Twenty-four hours after the final day of training, mice were placed back into the training context and the percentage of freezing was measured before and during the tone presentation (Fig. 1A, day 4). There was a significant main effect of training condition (F(1,60) = 9.71, P = 0.0028), a main effect of sex (F(1,60) = 7.34, P = 0.0088), and a main effect of cue presentation (F(1,60) = 4.81, P = 0.0322) (Fig. 1C). There was also a significant sex × cue interaction (F(1,60) = 5.23, P = 0.0257) and a significant training condition × sex × cue three-way interaction (F(1,60) = 5.39, P = 0.0236). Post-hoc analyses showed no significant difference between freezing to the fear cue alone and freezing during the tone in males and females in the context-only groups (P = 0.99 males, P = 0.99 females), suggesting that the tone did not serve as an external inhibitor or innately reduce freezing in this procedure. Males that underwent conditioned inhibition training displayed significantly less freezing during the tone presentation in the summation test compared with pretone freezing, suggesting that the tone served as a conditioned inhibitor (P = 0.001). However, females showed no reduction in freezing to tone during the summation test (P = 0.99) (Fig. 1C). This could suggest that the training conditions used were not sufficient for female mice to acquire conditioned inhibition.
Figure 1.
Training conditions for acquisition of conditioned inhibition in male and female mice. (A) Schematic of conditioned inhibition procedure. Male and female mice underwent three consecutive days of training. Mice received five unsignaled footshocks set to 0.5 mA. Mice were trained in a context that contained a polka dot insert to the rear back wall, stainless steel grid shock floors, dim illumination, and chambers that were cleaned with 70% ethanol. On the first day, following the shock treatment, animals received six tones in the absence of footshocks. The following day, tones were displayed prior to footshock training. On the third day, animals received footshocks with tone presentations after. On day 4, animals were given a summation test to assess their context freezing and inhibition of fear in the presence of the safety tone. (B) Schematic of contextual fear conditioning paradigm. Male and female mice received three consecutive days of context fear conditioning. Five unsignaled footshocks were presented in the context as described above. On the fourth day, animals underwent a summation test where they were presented with the fear-eliciting context, followed by a novel tone. (C) Male mice that received six-tone safety training froze significantly less compared with context-only males (P = 0.001) in the presence of the safety tone. A three-way ANOVA revealed a main effect of sex (P = 0.008) indicating that the six-tone training in males was sufficient to reduce freezing in the presence of the tone, but this training was not sufficient for female conditioned inhibition. (D) Females displayed significant conditioned inhibition (reduced freezing) when they were presented with 10 safety tones during conditioned inhibition training (P = 0.0007). Female mice did not display darting during the tone presentation.
We wondered whether female mice would successfully acquire conditioned inhibition if we used a more extensive training procedure. To test this hypothesis, 32 female mice were used in subsequent experiments. Female mice were presented with five unsignaled footshocks and either six tones (standard training procedure) or 10 tones (extensive training) during the 3-d training procedure. Mice were tested for freezing to context and tone in a summation test as described above. A two-way ANOVA showed there was no main effect of training condition (six vs. 10 tones) (F(1,30) = 0.366; >0.05), but a main effect of tone presentation (F(1,30) = 7.50; P = 0.0103) and a significant training condition × tone presentation interaction (F(1,30) = 8.65; P = 0.0062) (Fig. 1D). Post-hoc comparisons revealed that female mice that underwent the standard conditioned inhibition training (six tones) did not significantly suppress freezing to the tone during the summation test (P = 0.98). Mice that were trained with 10 presentations of the safety cue, however, displayed reduced freezing to the tone during the summation test (P = 0.0007), suggesting they sufficiently learned conditioned inhibition to the safety cue.
Female rats display an alternative behavioral expression of fear in the form of darting (Gruene et al. 2015a,b). Although we observed a significant reduction of freezing to the safety cue tone presentation in female mice that were trained with 10 presentations of the safety cue, it is possible that they used an alternative strategy for behavior expression of fear, such as darting. This could be incorrectly interpreted as fear suppression. To address this possibility, behavior videos from the extended training experiment presented in Figure 1D were extracted and analyzed for darting during the tone presentation using ANY-maze (Stoelting Co.) behavioral tracking software as described previously by Gruene et al. (2015a). Darting behavior was also visually scored based on the same description. We observed no instances of darting behavior as described previously in rats (Gruene et al. 2015a), with average overall velocities in mice during tone presentation equaling 0.1 cm/sec and an average maximum velocity during the tone equaling 1.5 cm/sec, which is far below the 23.5 cm/sec cutoff defined previously (Gruene et al. 2015a) even accounting for differences in rodent size. Therefore, we conclude that under these training and testing conditions for conditioned inhibition, female mice do not display darting as described previously in rats.
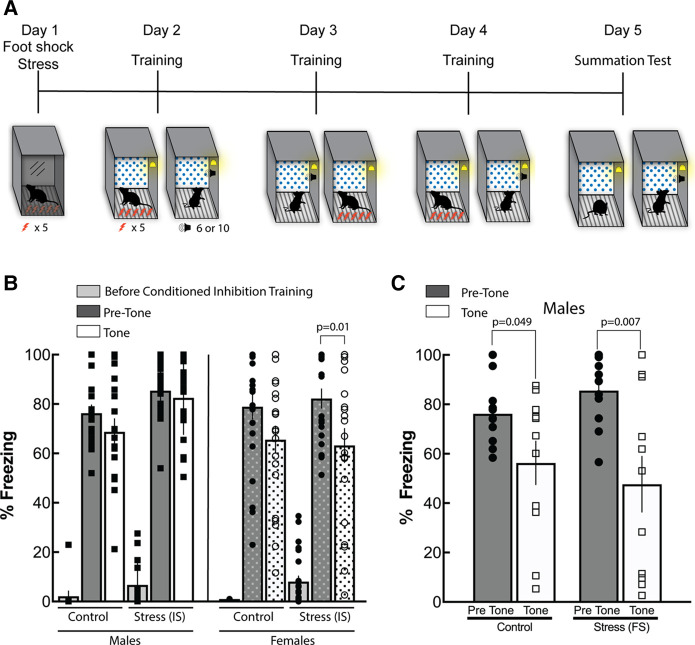
Pretraining stress impairs acquisition of conditioned inhibition in male but not female mice when training is not matched
Next, we wanted to assess the role of stress on the ability of males and females to acquire conditioned inhibition. To do this, 79 males and females were exposed to footshock stress or context exposure (nonstress controls) 24 h before undergoing safety training. Briefly, mice were placed into distinct fear conditioning chambers and were immediately given five 1-mA footshocks within 10 sec (Fig. 2A). Following the last footshock, mice were removed and returned to their home cage. Twenty-four hours later, male and female mice underwent the six- or 10-tone safety training procedure described above, respectively. The different number of safety cue presentations between males and females was conducted so that equivalent conditioned inhibition was obtained in males and females as outlined in our first experiment. Safety training was conducted over three consecutive days, as before. On the final day, mice were placed back into the training context for the summation test (Fig. 2A). A three-way ANOVA revealed a significant main effect of cue presentation (F(1,75) = 16.26, P = 0.0001), but not of sex (F(1,75) = 2.133; P = 0.148) or stress (F(1,75) = 2.293; P = 0.134). These data suggest that regardless of sex or stress condition, mice reduced freezing to the safety tone compared with the fear context alone (Fig. 2B). Results also revealed a significant stress × cue interaction (F(1,75) = 4.233; P = 0.0431). This effect was likely driven by increased freezing in stressed males during the safety tone presentation, although it should be noted that multiple comparisons did not find significant suppression in control males (P = 0.88) or control females (P = 0.15). There was, however, a significant reduction of freezing in stressed females during the summation test (P = 0.01). Because footshock was used as a stressor, we also analyzed the first 100-sec context exposure before delivery of the first footshock during conditioned inhibition training to verify no context fear was expressed by mice that were stressed prior to conditioning. There was a main effect of stress on freezing prior to training (F(1,65) = 8.0; p = 0.006), but no main effect of sex (F(1,65) = 0.001; p = 0.97) and no significant stress × sex interaction (F(1,65) = 0.38; p = 0.54). Although there is a significant difference in freezing between stressed and control mice prior to conditioning, overall freezing for both groups was <8%, which is within baseline freezing levels, suggesting that stressed mice did not develop contextual fear to the conditioning inhibition training context through generalization (Fig. 2B).
Figure 2.
Footshock stress prior to safety training impairs males, but not females, in conditioned inhibition. (A) Schematic of behavioral procedure. Immediate shock or a context pre-exposure was given 1 d prior to conditioned inhibition training. Male and female mice were placed in a distinct context that consisted of no background, IR lights, and stainless steel grid shock floors, and was cleaned with 2% Quatricide. Mice that received immediate shock underwent five unsignaled footshocks set to 1.0 mA within 10 sec. Immediately after the procedure, mice were returned to their home cage. Conditioned inhibition training started the next day as previously described. Male mice received six or 10 safety tone presentation, whereas females received 10 safety tones. (B) Shock stress affected male but not female mice in conditioned inhibition. Female mice that underwent shock stress showed a significant reduction in tone freezing (P = 0.01), indicating a lack of influence from stress on conditioned inhibition. Male mice did not display a significant suppression of freezing in the presence of the safety tone. Shock stress did not induce generalized context fear in either sex, as evidenced by baseline levels of freezing prior to conditioning. (C) When male mice were training with 10 safety tone presentations, they showed significant suppression of freezing to the safety tone during the summation test regardless of stress (P = 0.049 control; P = 0.007 stress).
In the experiment above, fear suppression in males was relatively modest during the summation test. Thus, we next determined whether matching training in males to that of females would reduce the apparent stress-induced impairments on conditioned inhibition. The training procedure was identical to the procedure described, but mice were presented with 10 tones as the safety cue during each training day. A two-way ANOVA found a significant main effect of cue presentation (F(1,20) = 17.61; P = 0.0004), but no main effect of stress (F(1,20) = 0.002, P = 0.96) and no significant stress × cue interaction (F(1,20) = 1.71, P = 0.21) (Fig. 2C). These data suggest matching training in male mice to that of females can overcome the effects of stress on acquisition of conditioned inhibition.
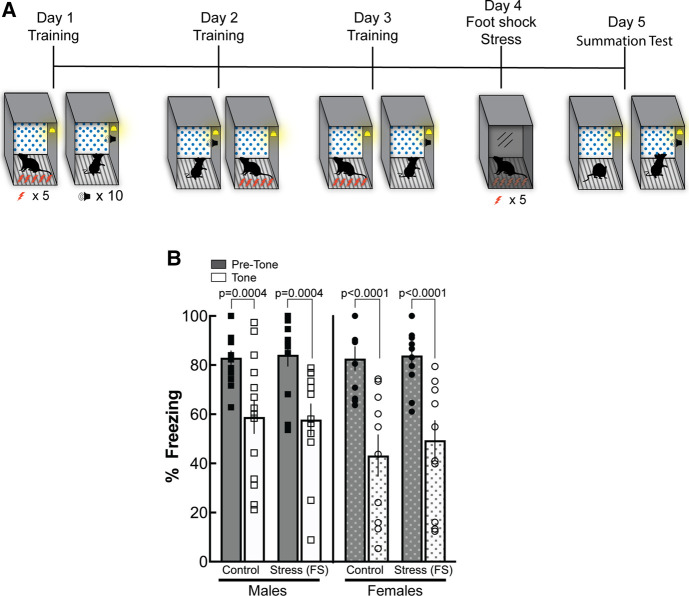
Post-training stress does not impair the recall of conditioned inhibition in male and female mice
To assess the effects of stress on conditioned inhibition if it is experienced after acquisition, 47 male and female mice received footshock stress 24 h after the last day of conditioned inhibition training (Fig. 3A). Given our effects above, we trained males and females using the same conditioned inhibition procedure in which they received five footshocks and 10 presentations of the safety cue. Conditioned inhibition training was conducted over three consecutive days, as before. Twenty-four hours after safety training, mice were placed in distinct fear conditioning chambers (as described above) and were exposed to footshock stress. Following the last footshock, mice were removed and returned to their home cage. Twenty-four hours later, a summation test in the training context was conducted. A three-way ANOVA revealed a significant main effect of cue presentation (F(1,43) = 31.57, P < 0.0001), but not of stress (F(1,43) = 0.1602; P = 0.6910) or sex (F(1,43) = 1.663; P = 0.2041), and no significant interactions. Post-hoc analyses confirmed that nonstressed and stressed control males and females reduced freezing in the presence of the safety cue during the summation test, indicating successful recall of conditioned inhibition regardless of stress exposure (males, P = 0.0004; females, P < 0.0001) (Fig. 3B).
Figure 3.
Footshock stress following safety training does not impair conditioned inhibition in either male or female mice. (A) Schematic of the behavioral procedure. Male and female mice were trained following the conditioned inhibition procedure as previously described. All mice were trained using 10 tones. After three consecutive days of training, mice underwent immediate shock as previously described or received a brief context exposure. The following day, mice underwent a summation test. (B) Following the 10-tone training, male mice that received shock stress following training showed a reduction in freezing in the presence of the safety tone (P = 0.0004), similar to context exposure controls (P = 0.0004). This effect was also seen with female shock stress (P < 0.0001) and context exposure controls (P < 0.0001). Overall, the results suggest that once safety training is acquired, stress does not affect inhibition of fear in the presence of a safety tone.
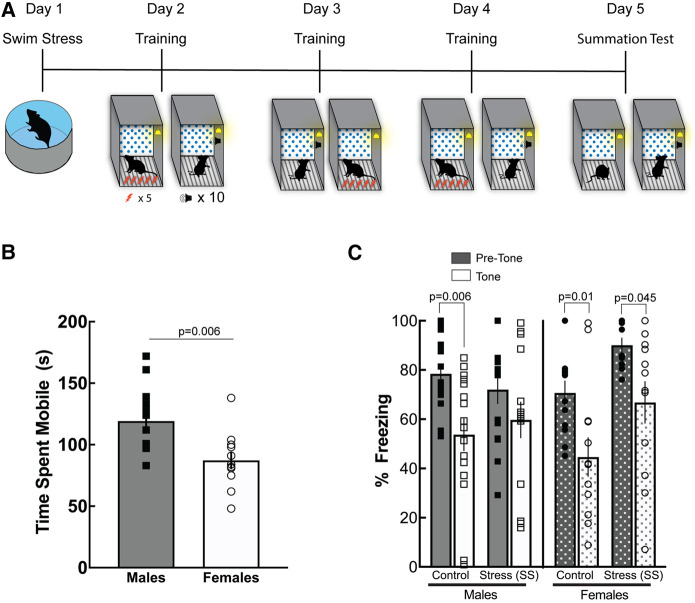
Swim stress impairs acquisition of conditioned inhibition in males but not females
In our previous experiment, we determined that footshock stress prior to training impaired conditioned inhibition in male but not female mice (Fig. 2B). To see whether these impairments generalized to other forms of stress, we used a swim stress procedure for the following experiment. On day 1, half of the mice were exposed to the swim stress procedure, in which they were placed in an 800-mL beaker (10 cm) of ∼25°C water for 5 min (Fig. 4A). Nonstressed control mice remained in their home cage. On days 2–4, male and female mice underwent conditioned inhibition training and then were placed back in the training context for the summation test on day 5. Independent t-tests revealed a significant difference in the time spent mobile between males and females during the swim stress procedure. Females spent significantly less time mobile during the SS (t(22) = 3.04, P = 0.006) (Fig. 4B). Mobility time has been interpreted as a way of active coping during stress (Mitchell et al. 2018). Therefore, these data suggest that female mice spend less time engaging in active coping behaviors during SS compared with males. A three-way ANOVA determined there was a main effect of stress (F(1,52) = 4.026, P = 0.05), a main effect of cue presentation (F(1,52) = 40.40, P < 0.0001), and a stress × sex interaction (F(1,52) = 4.21, P = 0.0452). Post-hoc analyses show that control male and female mice exhibited reduced freezing in the presence of the safety cue (P = 0.006, male; P = 0.011, female), however, only females displayed a significant reduction in freezing during the safety cue compared with males (P = 0.045) after swim stress despite displaying increased overall freezing to the fear and compound fear/safety cue (Fig. 4C). Together, these data suggest that swim stress impaired acquisition of conditioned inhibition in males but not females. Together with the results of the footshock stress experiments, these data suggest that the modality of stress impacts male and female mice differently and should be an important factor to consider when examining sex differences in conditioned inhibition. In the present experiments, females show increased freezing in response to stress overall but can acquire conditioned inhibition regardless of the stress modality. Males, however, show impaired conditioned inhibition when exposed to swim stress before training.
Figure 4.
Swim stress prior to conditioned inhibition impairs males, but not females, in inhibition of freezing. (A) Schematic of behavioral procedures used. Prior to undergoing safety training, male and female mice underwent swim stress for 5 min or remained in their home cage. Twenty-four hours following exposure to swim stress, mice underwent safety training using the 10-tone procedure. (B) Time spent mobile was scored during the 5-min swim stress. Results showed that males spent more time mobile compared with females (P = 0.006). The results show that male mice are impaired in conditioned inhibition if swim stress occurs prior to training, whereas females are not. However, during the swim stress, male mice engage in more active coping mechanisms compared with females. (C) Male mice that did not undergo swim stress showed a significant reduction in freezing during tone presentations (P = 0.006). However, males that were exposed to swim stress prior to safety training did not show a significant reduction in freezing in the presence of the safety tone. Both control (P = 0.01) and swim stress (P = 0.006) female mice showed a significant reduction in freezing during tone presentations.
Discussion
The present experiments demonstrate a sex difference in conditioned inhibition when using context as the “fear” cue and tone as the “safety” cue. In the first experiment, females required more presentations of the safety cue during training compared with males to acquire the same level of conditioned inhibition during the summation test (Fig. 1B). Females, however, did acquire conditioned inhibition when we increased presentations of the safety cue in an extended training procedure. However, this sex difference was not consistent across the experiments, mainly due to males not acquiring sufficient conditioned inhibition to the six-tone training procedure in subsequent experiments. Therefore, it is likely that under nonstressed conditions, male and female mice acquire conditioned inhibition similarly. We found that females were resistant to the effects of footshock stress when the stress was experienced before (Fig. 2B) conditioned inhibition training. In contrast, males were impaired when they were exposed to footshock stress before conditioned inhibition training (Fig. 2B). However, this was likely due to relatively modest fear inhibition exhibited by males during compound presentation of fear and safety cues (see note above) (Fig. 2B). When training conditions were matched to those in females (10 presentations of the safety cue), males were able to acquire conditioned inhibition after the experience of footshock stress. Exposing male and female mice to footshock stress after training had no effect on the ability of either sex to recall conditioned inhibition (Fig. 3B). We next tested whether a different stress modality would reveal a sex difference in the effects of stress on conditioned inhibition. Exposing males and females to swim stress revealed that males, but not females, were impaired in the acquisition of conditioned inhibition. Ultimately, these data suggest that extended safety training can mitigate the impact of stress in both sexes, but that stress modality may interact with sex and the type of learning to differentially impact the ability of mice to suppress fear under appropriate conditions.
Decades of research have established that males and females display separate mechanisms (behaviorally and biologically) for various learning tasks, such as fear learning, fear extinction, and fear generalization (for review, see Frick et al. 2010, 2018; Hussain et al. 2014; Adkins et al. 2019). In addition, several reports demonstrate that males display increased contextual fear learning compared with females (Maren et al. 1994; Markus and Zecevic 1997; Gupta et al. 2001; Gresack et al. 2009; Mizuno and Giese 2010). Others have reported female rats to be less likely to use contextual cues to recall appetitive associative learning and may rely more on other food-associated cues for recall (Anderson and Petrovich 2015, 2018a,b). However, in our context, only control females displayed slightly increased freezing and less variability in responses compared with males (Fig. 1C). The difference in our findings here versus the previous literature could be the result of repeated training sessions, species differences, strain differences, or any combination. Alternatively, females may be more sensitive to repeated context exposures, resulting in enhanced fear responses when returned to the conditioning context. Recent investigations have identified specific neural mechanisms involved in conditioned inhibition and safety learning using male rodents. Of note, the amygdala, medial prefrontal cortex, and ventral hippocampus have all been identified as important loci controlling safety learning in males of multiple species, including humans (Ostroff et al. 2010; Sangha et al. 2013, 2014; McDonald et al. 2018; Meyer et al. 2019). A few studies have directly examined sex differences in safety learning, but the results are inconsistent across groups. For instance, male and female rats exhibit differences in fear discrimination, with female rats displaying better discrimination between CS+ and CS− cues, but no sex difference was observed during summation tests across different types of conditioned inhibition procedures, suggesting no differences between males and females in conditioned inhibition (Toufexis et al. 2007; Foilb et al. 2018; Clark et al. 2019). Other studies, however, report that male rodents display greater fear suppression during summation tests compared with females. For example, in preweanling rats, only males showed suppression of aversive responses in a taste aversion paradigm in the presence of a safety cue (Aranda-Fernandez et al. 2016). In addition, exposure to early life stress in rats produces greater fear responses to certain and uncertain threat cues and increases alcohol consumption in females compared with males (Walker et al. 2018). Greiner et al. (2019), found that male but not female rats successfully suppressed conditioned freezing in the presence of a safety cue in a fear, safety, and reward discrimination task. One explanation for the discrepancies among the studies, the present study included, is that training protocols may have been too extensive to detect differences between males and females. For example, Foilb et al.’s (2018) paradigm consisted of 4 d of training, each consisting of 15 presentations of the safety cue. Here, we found that females successfully acquired conditioned inhibition after 3 d of training and 10 presentations of the safety cue. With the extended training procedure used here, we observed no sex difference in conditioned inhibition. Initially, we attempted to standardize the amount of fear suppression to the compound fear/safety cue during the summation test rather than matching training conditions (Fig. 1). In the first experiment, we found that males were able to learn conditioned inhibition with fewer exposures to the safety cue compared with females. However, in subsequent experiments that integrated stress exposure, we found fear suppression even under control conditions to be more modest than the first experiment. When matching training conditions in males and females, we did not find a sex difference in fear suppression in response to footshock stress but did find a sex difference when mice were exposed to swim stress. Thus, the modality of stress could impact whether a sex difference in safety learning is observed in addition to the different strategies males and females use to learn specific tasks.
Apart from sex differences, several studies have assessed brain regions and neural mechanisms involved in safety learning. This work has primarily been conducted using male rodents. Not surprisingly, the amygdala has emerged as an important structure, which undergoes specific activity and structural changes during safety learning (Ostroff et al. 2010; Sangha et al. 2013). For instance, specific subpopulations of basolateral amygdala neurons change their firing rates in responses to fear + safety cues but not a fear cue alone (Sangha et al. 2013), suggesting that specific neurons in the amygdala might drive fear suppression. Neurotoxic lesions of the ventral hippocampus also impair safety learning, implicating the hippocampus in fear suppression during conditioned inhibition (McDonald et al. 2018). Additionally, ventral hippocampal projections to the prelimbic cortex display increased activity during presentation of a safety cue in human subjects, supporting the role of the hippocampus in fear suppression and establishing a potential neural circuit involved in conditioned inhibition (Meyer et al. 2019). Although these recent studies have established mechanisms regulating conditioned inhibition, much less is known about potential neural mechanisms contributing to the reported sex differences in conditioned inhibition. An extensive c-fos activity study investigating cortical and subcortical regions in male and female rats after fear discrimination identified only the bed nucleus of the stria terminalis (BNST) as sexually dimorphic in its expression of c-fos during fear discrimination. BNST fos expression was greater in male rats during discrimination, and increased fos expression was correlated with reduced freezing to the CS−, but only in females (Foilb et al. 2021). Thus, BNST is a promising target for further investigation of sex differences in conditioned inhibition, and the available data suggest that BNST activity may support fear suppression in females but not males.
In addition to dissecting the specific neural circuits regulating sex differences, it is also important to understand how stress may impact learned safety. Here, we found that footshock stress administered before or after conditioned inhibition had little impact on fear suppression during the summation test in males and females. Previous work using the stress-enhanced fear learning (SEFL) model has generally shown enhanced fear responses, impaired fear extinction, and increased alcohol intake in rodents (Rau and Fanselow 2009; Long and Fanselow 2012; Meyer et al. 2013). In the present study, we did not observe increased freezing to the training context when mice experienced shock before or after undergoing conditioned inhibition training (Figs. 2, 3), but we did observe a relatively small increase in freezing after swim stress in female mice only (Fig. 4C). We only observed an effect of footshock stress in male mice when training conditions were not matched to female mice; when they were matched, there was no deficit in conditioned inhibition observed in either sex. Differences in the results between the present study and those using the SEFL procedure could be due to the different shock protocols. SEFL involves a more intense stress procedure, administering 15 shocks over 90 min, whereas the present experiment administered five shocks in 10 sec and was based on the immediate shock procedure (Fanselow 1990; Rudy et al. 2002). A recent study also used the SEFL protocol prior to safety training and found that it did not impair conditioned inhibition, but did substantially impair fear extinction (Woon et al. 2020). Our data here are largely in accord with those of others demonstrating prior stress does not impair conditioned inhibition in male and female rodents.
Similar to SEFL, acute stress of other modalities enhances Pavlovian fear conditioning in males but tends to impair it in females (e.g., Wood and Shors 1998; Shors et al. 2000; Wolf et al. 2001; Bangasser and Shors 2004, 2007; Waddell et al. 2008). Females’ response to acute stress are influenced by ovarian hormones and estrus cycle stage; ovariectomized females do not show cognitive impairments following acute stress (Wood et al. 2001). Additionally, females in proestrus (when estradiol is high) display less conditioned responding after acute stress exposure compared with females in diestrus (when estradiol is low) (Shors et al. 1998). In the present experiment, we did not track the estrus cycle in female mice, making it difficult to determine whether changing ovarian hormones influence conditioned inhibition. However, there was little adverse effect of stress on conditioned inhibition in female mice observed in any of our experiments, suggesting that ovarian hormones might not influence safety learning in using our procedure. However, exposure to acute stress has been demonstrated to enhance estradiol in females rodents (Shors et al. 1999). Therefore, it is possible that footshock exposure elevated estradiol, which has potent learning-enhancing effects (Frick et al. 2002, 2018; Fernandez et al. 2008; Boulware et al. 2013; Fortress et al. 2013; Frick 2015), thereby enhancing discrimination between the fear cue and safety cue during training despite the prior exposure to stress. The learning-enhancing effects of estradiol, however, are not consistent with the previous literature showing increased estradiol and general impairments in fear conditioning procedures. In addition to footshock stress, we exposed male and female mice to swim stress. The purpose of using swim stress was to use a stressor that is a different modality from the unconditioned stimulus during safety learning (footshock). When mice were exposed to swim stress, we found only male mice were impaired in their ability to acquire conditioned inhibition. This suggests that the modality of the stress may interact with sex to impact fear suppression during conditioned inhibition. Prior studies in rats have shown that females displayed less immobility compared with males in a single-trial forced swim test (Colom-Lapetina et al. 2019), indicating differences in coping strategies across sexes in rats. However, these prior studies investigated swim stress following auditory fear conditioning, which could alter coping strategies to favor more active responses in females compared with males following fear conditioning. Here, we found that females spent significantly less time mobile (more immobility) than males during the swim stress (Fig. 4B), suggesting that females did not engage in active coping equivalent to males, which could be indicative of a greater stress response or different coping mechanisms. We did not examine climbing, head shakes, or diving behavior during the forced swim test, which are also reported to be sex-dependent, with female rats displaying more of these behaviors (Colom-Lapetina et al. 2019). Despite this effect, female mice were not impaired in fear suppression during the summation test after swim stress exposure, suggesting an overall resilience to the effects of stress on safety learning. Regardless, the results of our study show that in some cases and under certain stress conditions, no sex difference in safety learning are apparent, whereas under other stress conditions sex differences emerge.
The present study aimed to understand how male and female mice acquire and retain conditioned inhibition and to assess the impact of stress on learning this task. Our data provide novel and relevant findings suggesting that females might require a more extensive training protocol than males to learn safety cues equivalently. However, we found that males, but not females, were impaired at learning and retaining safety cues following footshock stress and swim stress, but this sex difference is eliminated when the training in males is matched to that of females. Importantly, our data suggest that female mice are not sensitive to different modalities of stress when assessing conditioned inhibition. The present study adds to the growing literature on sex differences in safety learning, which will be critical for developing sex-specific therapies for a variety of fear-related disorders that involve excessive fear and/or impaired fear inhibition.
Materials and Methods
Animals and housing conditions
All experiments used male and female F1 hybrid (B6S1) offspring that were generated from crossing C57BL/6J males and 129S1SvImJ females. Parent C57BL/6J males and 129S1SvImJ females were purchased from Jackson Laboratories, and male and female offspring of these pairs were used for the present experiments. We and others have used B6S1 mice routinely to examine mechanisms of fear learning and memory (Wiltgen and Silva 2007; Wiltgen et al. 2010; Ortiz et al. 2019). All mice were generated from a breeding colony in the Department of Psychological Sciences at Kent State University. Mice were >8 wk before they were used for experiments and were group-housed (two to five mice per cage) with free access to food and water in a room maintained on a 12:12 light:dark cycle. All procedures were conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with the National Institutes of Health guidelines, and with approval by Kent State University Institutional Animal Care and Use Committee guidelines.
Conditioned inhibition
In all experiments, an A+/AB− procedure was used in which the context served as the “fear” cue and an explicit tone served as the “safety” cue (Ostroff et al. 2010) Conditioned inhibition training was performed in four identical conditioning chambers (7 in W × 7 in D × 12 in H) containing two Plexiglas walls, two aluminum side walls, and a stainless steel grid shock floor (Coulbourn Instruments). The training context consisted of the conditioning chamber with a polka dot insert attached to the rear Plexiglas wall, dim illumination, and stainless steel grid floors that were cleaned with 70% ethanol. For day 1 of conditioned inhibition training, mice were placed in the training context and, after 120 sec, were delivered five footshocks (0.5 mA, 1 sec, and 182 ISI). Three minutes (180 sec) after the last footshock, mice were presented with a safety cue (six presentations of 5 kHz, 75-dB tone, 30 sec, and 180 ITI). The tones were explicitly unpaired with the shock to ensure a relationship between the tone and shock was not made. One minute after the last tone, mice were removed from the conditioning chamber. The entire training session lasted 30 min. On day 2 of training, safety tones were presented first with 3 min between the last tone and the first shock presentation. The order of stimulus presentation on day 3 of training was the same as day 1 (Fig. 1). For the first experiment, a separate group of mice was trained in the absence of safety cue exposure to serve as context fear controls (Fig. 1). Standard training to establish conditioned inhibition consisted of three consecutive days of training, with presentations of the footshocks and tones alternating on each day. On day 4, mice were placed back in the training context for a summation test. After 120 sec, mice were presented with two presentations of the safety cue tone (30-sec and 180-sec ITI). Context-only controls were also returned to the conditioning context and received two presentations of a novel tone (same as conditioned inhibition group: 30 sec, 5 kHz, 75 dB, and 180 ITI) 120 sec later to establish that the tone alone did not serve as an external inhibitor and reduce freezing in the absence of conditioned inhibition. Freezing was measured to the context prior to and during the tone using Actimetrics FreezeFrame 5 automated software (Actimetrics). Successful conditioned inhibition occurred if freezing was significantly reduced during the tone presentation (AB−) compared with freezing to the context alone (A+). In a separate and subsequent experiment, we conducted an extensive training procedure consisting of 10 presentations of the safety cue (5 kHz, 75-dB tone, and 30-sec and 100-sec ITI) in the same design as above during the 30-min training sessions. The extended training procedure also consisted of three consecutive days of training, with presentations of the footshocks and tones alternating on each day. On day 4, mice were placed back in the training context for a summation test. After 120 sec, mice were presented with safety cue tones (30 sec and 182 ITI). Freezing was measured as described above. To analyze darting behavior in female mice, FreezFrame 5 videos were extracted, converted to .MOV files, and analyzed for velocity (centimeters per second) during the safety cue tone presentation using ANY-maze (Stoelting Co.) behavioral tracking software, with a 23.5-cm/sec cutoff deemed as darting as described previously (Gruene et al. 2015a). The number of darts was also visually identified and scored by a blind observer as described previously (Gruene et al. 2015a).
Footshock stress
Experiments 2 and 3 used a footshock as a stressor (FS). This procedure was adapted from experiments demonstrating the “immediate shock deficit” in which rodents received footshocks immediately after being placed in the conditioning chamber and were removed immediately after cessation of the shocks. This procedure produces little freezing to the context in which the shocks occurred when rodents are tested for fear memory, suggesting that an association between the training context and aversive stimulus does not occur (Fanselow 1990; Rudy et al. 2002). The advantage of using this procedure is that it induces stress without the potential confound of a competing context fear memory that could impact the conditioned inhibition procedure. Immediate shock was presented in single session 1 d before training or 1 d after training for conditioned inhibition and was conducted in conditioning chambers and behavioral testing room different from those subsequently used for the conditioned inhibition procedure. Immediate shock was performed in two identical conditioning chambers (12 in W × 12 in D × 12 in H) containing two Plexiglas walls, two aluminum side walls, and a stainless steel grid shock floor (Coulbourn Instruments). The context for immediate shock consisted of infrared lights (no visible light), grid floors, and no background, and was cleaned with 2% Quatricide. Mice were placed in the context and were immediately presented with five 1-mA shocks within 10 sec (1-sec ISI). Immediately following the last shock, mice were removed and taken back to their home cage. Twenty-four hours after footshock stress, mice began training or were tested for conditioned inhibition in the summation test.
Swim stress
The swim stress (SS) procedure occurred in a single session 1 d before conditioned inhibition training and followed the protocol used and described by Mitchell et al. (2018). Briefly, mice were transferred from the animal colony to the procedure room at least 1 h prior to testing. Mice were then subjected to a 5-min swim stress. One-thousand-milliliter beakers were filled with 800 mL (10 cm) of ∼25°C water and placed in an open field arena. A digital camera was positioned directly above the acrylic top and used to record swimming behavior for offline scoring. Mobility was defined as active leg movement beyond that required to prevent submersion below the surface and was recorded and analyzed using ANY-maze. After 5 min, mice were thoroughly dried using an absorbent laboratory pad and returned to their home cage. Twenty-four hours later, mice underwent conditioned inhibition training.
Statistical analyses
Freezing behavior was assessed using three-way ANOVA (sex × training condition × cue) with sex and training condition (safety vs. fear; stress vs. no stress) as between-subject variables and cue (pretone vs. tone) as a within-subject variable. In some cases, two-way ANOVA was used. Conditioned inhibition after swim stress was analyzed using two-way ANOVA (sex × stress). For any significant interactions, Tukey's HSD or Sidak's post-hoc tests were used where appropriate.
Acknowledgments
These experiments were funded by National Institutes of Health grant R15MH118705 to A.M.J.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053508.121.
References
- Adkins JM, Jasnow AM, Lynch JF. 2019. Estradiol and sex differences in generalized fear: implications for anxiety disorders. In Estrogens and memory basic research and clinical implications (ed. Frick K), pp. 438–460. Oxford University Press, New York. [Google Scholar]
- Anderson LC, Petrovich GD. 2015. Renewal of conditioned responding to food cues in rats: sex differences and relevance of estradiol. Physiol Behav 151: 338–344. 10.1016/j.physbeh.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LC, Petrovich GD. 2018a. Distinct recruitment of the hippocampal, thalamic, and amygdalar neurons projecting to the prelimbic cortex in male and female rats during context-mediated renewal of responding to food cues. Neurobiol Learn Mem 150: 25–35. 10.1016/j.nlm.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LC, Petrovich GD. 2018b. Ventromedial prefrontal cortex mediates sex differences in persistent cognitive drive for food. Sci Rep 8: 2230. 10.1038/s41598-018-20553-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda-Fernandez PE, Gaztanaga M, Arias C, Chotro MG. 2016. Conditioned inhibition in preweanling rats. Dev Psychobiol 58: 98–106. 10.1002/dev.21359 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. 2004. Acute stress impairs trace eye blink conditioning in females without altering the unconditioned response. Neurobiol Learn Mem 82: 57–60. 10.1016/j.nlm.2004.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. 2007. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci 10: 1401–1403. 10.1038/nn1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci 33: 15184–15194. 10.1523/JNEUROSCI.1716-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Maynard GD, Kehne JH. 2012. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs 21: 1323–1350. 10.1517/13543784.2012.704020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, Sangha S. 2012. Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci 32: 14118–14124. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JW, Drummond SPA, Hoyer D, Jacobson LH. 2019. Sex differences in mouse models of fear inhibition: fear extinction, safety learning, and fear-safety discrimination. Br J Pharmacol 176: 4149–4158. 10.1111/bph.14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom-Lapetina J, Li AJ, Pelegrina-Perez TC, Shansky RM. 2019. Behavioral diversity across classic rodent models is sex-dependent. Front Behav Neurosci 13: 45. 10.3389/fnbeh.2019.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Grillon C, Lissek S. 2018. Impaired discriminative fear conditioning during later training trials differentiates generalized anxiety disorder, but not panic disorder, from healthy control participants. Compr Psychiatry 85: 84–93. 10.1016/j.comppsych.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. 2000. The role of the amygdala in conditioned and unconditioned fear and anxiety. In The amygdala: a functional analysis (ed. Aggleton J), pp. 213–287. Oxford University Press, New York. [Google Scholar]
- Day HLL, Stevenson CW. 2019. The neurobiological basis of sex differences in learned fear and its inhibition. Eur J Neurosci 52: 2466–2486. 10.1111/ejn.14602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HLL, Suwansawang S, Halliday DM, Stevenson CW. 2020. Sex differences in auditory fear discrimination are associated with altered medial prefrontal cortex function. Sci Rep 10: 6300. 10.1038/s41598-020-63405-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. 1990. Factors governing one-trial contextual conditioning. Anim Learn Behav 18: 264–270. 10.3758/BF03205285 [DOI] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28: 8660–8667. 10.1523/JNEUROSCI.1968-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, Wood SK. 2017. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One 12: e0172868. 10.1371/journal.pone.0172868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JE, Muniz BL, Padi AR, Lombard CM, Moffitt CM, Wood CS, Wilson LB, Reagan LP, Wilson MA, Wood SK. 2018. Essential role of ovarian hormones in susceptibility to the consequences of witnessing social defeat in female rats. Biol Psychiatry 84: 372–382. 10.1016/j.biopsych.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Bals J, Sarlitto MC, Christianson JP. 2018. Sex differences in fear discrimination do not manifest as differences in conditioned inhibition. Learn Mem 25: 49–53. 10.1101/lm.045500.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Sansaricq GN, Zona EE, Fernando K, Christianson JP. 2021. Neural correlates of safety learning. Behav Brain Res 396: 112884. 10.1016/j.bbr.2020.112884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem 20: 147–155. 10.1101/lm.026732.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. 2015. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav 74: 4–18. 10.1016/j.yhbeh.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick K, Fernandez S, Bulinski S. 2002. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115: 547–558. 10.1016/S0306-4522(02)00377-9 [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL. 2010. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta 1800: 1045–1055. 10.1016/j.bbagen.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Koss WA. 2018. Estradiol and hippocampal memory in female and male rodents. Curr Opin Behav Sci 23: 65–74. 10.1016/j.cobeha.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S. 2018. Common neurocircuitry mediating drug and fear relapse in preclinical models. Psychopharmacology 236: 415–437. 10.1007/s00213-018-5024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser LR, Jovanovic T. 2021. Safety learning during development: implications for development of psychopathology. Behav Brain Res 408: 113297. 10.1016/j.bbr.2021.113297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner EM, Muller I, Norris MR, Ng KH, Sangha S. 2019. Sex differences in fear regulation and reward-seeking behaviors in a fear-safety-reward discrimination task. Behav Brain Res 368: 111903. 10.1016/j.bbr.2019.111903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, Frick KM. 2009. Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience 159: 451–467. 10.1016/j.neuroscience.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. 2015a. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4: e11352. 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene TM, Roberts E, Thomas V, Ronzio A, Shansky RM. 2015b. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol Psychiatry 78: 186–193. 10.1016/j.biopsych.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. 2001. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats. Brain Res 888: 356–365. 10.1016/S0006-8993(00)03116-4 [DOI] [PubMed] [Google Scholar]
- Hussain D, Shams WM, Brake WG. 2014. Estrogen and memory system bias in females across the lifespan. Transl Neurosci 5: 35–50. 10.2478/s13380-014-0209-7 [DOI] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. 2006. Stress differentially modulates fear conditioning in healthy men and women. Biol Psychiatry 59: 516–522. 10.1016/j.biopsych.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62: 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Schauder KB, McKinnon M, Bachevalier J, Davis M. 2013. A novel AX+/BX− paradigm to assess fear learning and safety-signal processing with repeated-measure designs. J Neurosci Methods 214: 177–183. 10.1016/j.jneumeth.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. 2017. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology 42: 397–407. 10.1038/npp.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Chiu W, Demler O, Merikangas K, Walters E. 2005a. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005b. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. 2012. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21: 169–184. 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JN, Sangha S. 2021. On the basis of sex: differences in safety discrimination vs. conditioned inhibition. Behav Brain Res 400: 113024. 10.1016/j.bbr.2020.113024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. 2009. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther 47: 111–118. 10.1016/j.brat.2008.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long VA, Fanselow MS. 2012. Stress-enhanced fear learning in rats is resistant to the effects of immediate massed extinction. Stress 15: 627–636. 10.3109/10253890.2011.650251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JF III, Cullen PK, Jasnow AM, Riccio DC. 2013. Sex differences in the generalization of fear as a function of retention intervals. Learn Mem 20: 628–632. 10.1101/lm.032011.113 [DOI] [PubMed] [Google Scholar]
- Lynch JF III, Vanderhoof T, Winiecki P, Latsko MS, Riccio DC, Jasnow AM. 2016. Aromatized testosterone attenuates contextual generalization of fear in male rats. Horm Behav 84: 127–135. 10.1016/j.yhbeh.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Maeng LY, Milad MR. 2015. Sex differences in anxiety disorders: interactions between fear, stress, and gonadal hormones. Horm Behav 76: 106–117. 10.1016/j.yhbeh.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. 2012. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35: 24–35. 10.1016/j.tins.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. 2008. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci 28: 1661–1666. 10.1111/j.1460-9568.2008.06485.x [DOI] [PubMed] [Google Scholar]
- Maren S, Deoca B, Fanselow MS. 1994. Sex-differences in hippocampal long-term potentiation (Ltp) and Pavlovian fear conditioning in rats: positive correlation between Ltp and contextual learning. Brain Res 661: 25–34. 10.1016/0006-8993(94)91176-2 [DOI] [PubMed] [Google Scholar]
- Markus EJ, Zecevic M. 1997. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology 25: 246–252. 10.3758/BF03331934 [DOI] [Google Scholar]
- McDonald RJ, Balog RJ, Lee JQ, Stuart EE, Carrels BB, Hong NS. 2018. Rats with ventral hippocampal damage are impaired at various forms of learning including conditioned inhibition, spatial navigation, and discriminative fear conditioning to similar contexts. Behav Brain Res 351: 138–151. 10.1016/j.bbr.2018.06.003 [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG. 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45: 1027–1035. 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. 2010. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35: 33–46. 10.1016/j.psyneuen.2009.07.009 [DOI] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R. 2013. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 38: 2529–2541. 10.1016/j.psyneuen.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. 2013. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res 37: 566–574. 10.1111/acer.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Odriozola P, Cohodes EM, Mandell JD, Li A, Yang R, Hall BS, Haberman JT, Zacharek SJ, Liston C, et al. 2019. Ventral hippocampus interacts with prelimbic cortex during inhibition of threat response via learned safety in both mice and humans. Proc Natl Acad Sci 116: 26970–26979. 10.1073/pnas.1910481116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Gilman TL, Daws LC, Toney GM. 2018. High salt intake enhances swim stress-induced PVN vasopressin cell activation and active stress coping. Psychoneuroendocrinology 93: 29–38. 10.1016/j.psyneuen.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Giese KP. 2010. Towards a molecular understanding of sex differences in memory formation. Trends Neurosci 33: 285–291. 10.1016/j.tins.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. 2004. AX+, BX− discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem 11: 464–475. 10.1101/lm.74704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz S, Latsko MS, Fouty JL, Dutta S, Adkins JM, Jasnow AM. 2019. Anterior cingulate cortex and ventral hippocampal inputs to the basolateral amygdala selectively control generalized fear. J Neurosci 39: 6526–6539. 10.1523/JNEUROSCI.0810-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff LE, Cain CK, Bedont J, Monfils MH, LeDoux JE. 2010. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci 107: 9418–9423. 10.1073/pnas.0913384107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. 2009. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12: 125–133. 10.1080/10253890802137320 [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. 2002. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci 116: 530–538. 10.1037/0735-7044.116.4.530 [DOI] [PubMed] [Google Scholar]
- Sangha S, Chadick JZ, Janak PH. 2013. Safety encoding in the basal amygdala. J Neurosci 33: 3744–3751. 10.1523/JNEUROSCI.3302-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG. 2014. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology 39: 2405–2413. 10.1038/npp.2014.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha S, Diehl MM, Bergstrom HC, Drew MR. 2020. Know safety, no fear. Neurosci Biobehav Rev 108: 218–230. 10.1016/j.neubiorev.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. 1998. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport 9: 419–423. 10.1097/00001756-199802160-00012 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Pickett J, Wood G, Paczynski M. 1999. Acute stress persistently enhances estrogen levels in the female rat. Stress 3: 163–171. 10.3109/10253899909001120 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Beylin AV, Wood GE, Gould E. 2000. The modulation of Pavlovian memory. Behav Brain Res 110: 39–52. 10.1016/S0166-4328(99)00183-7 [DOI] [PubMed] [Google Scholar]
- Sijbrandij M, Engelhard IM, Lommen MJ, Leer A, Baas JM. 2013. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). J Psychiatr Res 47: 1991–1997. 10.1016/j.jpsychires.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Steiger F, Nees F, Wicking M, Lang S, Flor H. 2015. Behavioral and central correlates of contextual fear learning and contextual modulation of cued fear in posttraumatic stress disorder. Int J Psychophysiol 98: 584–593. 10.1016/j.ijpsycho.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. 2007. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor (ER) and ER. J Neurosci 27: 9729–9735. 10.1523/JNEUROSCI.2529-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Jovanovic T. 2019. Impaired inhibition as an intermediate phenotype for PTSD risk and treatment response. Prog Neuropsychopharmacol Biol Psychiatry 89: 435–445. 10.1016/j.pnpbp.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Bangasser DA, Shors TJ. 2008. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci 28: 5290–5294. 10.1523/JNEUROSCI.1129-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RA, Andreansky C, Ray MH, McDannald MA. 2018. Early adolescent adversity inflates threat estimation in females and promotes alcohol use initiation in both sexes. Behav Neurosci 132: 171–182. 10.1037/bne0000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. 2007. Memory for context becomes less specific with time. Learn Mem 14: 313–317. 10.1101/lm.430907 [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, Silva AJ. 2010. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr Biol 20: 1336–1344. 10.1016/j.cub.2010.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. 2008. AX+/BX− discrimination learning in the fear-potentiated startle paradigm in monkeys. Learn Mem 15: 63–66. 10.1101/lm.843308 [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. 2001. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 26: 711–720. 10.1016/S0306-4530(01)00025-7 [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. 1998. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci 95: 4066–4071. 10.1073/pnas.95.7.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. 2001. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning males versus females. Behav Neurosci 115: 175–187. 10.1037/0735-7044.115.1.175 [DOI] [PubMed] [Google Scholar]
- Woon EP, Seibert TA, Urbanczyk PJ, Ng KH, Sangha S. 2020. Differential effects of prior stress on conditioned inhibition of fear and fear extinction. Behav Brain Res 381: 112414. 10.1016/j.bbr.2019.112414 [DOI] [PMC free article] [PubMed] [Google Scholar]