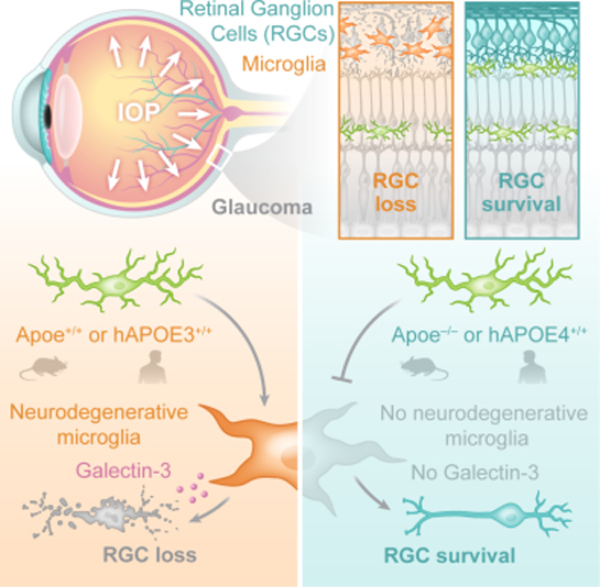
Summary
The apolipoprotein E4 (APOE4) allele is associated with an increased risk of Alzheimer’s disease and a decreased risk of glaucoma, but the underlying mechanisms remain poorly understood. Here, we found that in two mouse glaucoma models, microglia transitioned to a neurodegenerative phenotype characterized by upregulation of Apoe and Lgals3 (Galectin-3), which were also upregulated in human glaucomatous retinas. Mice with targeted deletion of Apoe in microglia or carrying the human APOE4 allele were protected from retinal ganglion cell (RGC) loss despite elevated intraocular pressure (IOP). Similar to Apoe−/− retinal microglia, APOE4-expressing microglia did not upregulate neurodegeneration-associated genes, including Lgals3, following IOP elevation. Genetic and pharmacologic targeting of Galectin-3 ameliorated RGC degeneration, and Galectin-3 expression was attenuated in human APOE4 glaucoma samples. These results demonstrate that impaired activation of APOE4 microglia is protective in glaucoma, and that the APOE-Galectin-3 signaling can be targeted to treat this blinding disease.
Keywords: Microglia, APOE4, neurodegeneration, retina, glaucoma, Alzheimer’s disease, Lgals3, Galectin-3, neuroprotection
eTOC
The apolipoprotein E4 (APOE4) allele is associated with an increased risk of Alzheimer’s disease and a decreased risk of glaucoma, but the underlying mechanisms remain poorly understood. Margeta et al. demonstrate that APOE-Galectin-3 signaling regulates cytotoxic neurodegenerative retinal microglia in glaucoma, and that the APOE4 allele attenuates microglial neurodegenerative phenotype and protects neurons in glaucoma.
Graphical Abstract
Introduction
Glaucoma is a chronic blinding disease caused by progressive loss of retinal ganglion cells (RGCs), currently estimated to affect 80 million people worldwide (Tham et al., 2014). Elevated intraocular pressure (IOP) is the only modifiable risk factor for glaucoma. However, the disease often progresses despite repeated medical and surgical interventions to lower the IOP, and there are no clinically approved therapies that directly promote RGC survival (Almasieh and Levin, 2017). Despite the prevalence and morbidity of glaucoma, the underlying mechanisms that lead to RGC apoptosis in glaucomatous neurodegeneration remain poorly understood. Prior work has implicated the role of oxidative stress and mitochondrial dysfunction, neurotrophic factor deprivation, and astrocyte and microglial reactivity as key converging pathways that result in RGC death in glaucoma (Alqawlaq et al., 2019). Microglia, the resident immune cells of the central nervous system (CNS), have emerged as an important cell type critically involved in many brain neurodegenerations, including Alzheimer’s disease (Butovsky and Weiner, 2018; Hammond et al., 2019). In the healthy adult CNS, microglia play critical roles in maintaining homeostasis by performing constant surveillance, defense, and healthy repair (Colonna and Butovsky, 2017; Li and Barres, 2018). However, microglia can also have harmful effects on the neural tissues during disease by becoming chronically inflammatory (Butovsky and Weiner, 2018; Ising et al., 2019; Marschallinger et al., 2020).
Several prior studies have demonstrated that microglia and recruited myeloid cells play an important role in glaucoma development and progression (Soto and Howell, 2014; Williams et al., 2017; Zeng and Shi, 2018). Activated myeloid cells have been detected in the optic nerves of human glaucomatous eyes (Neufeld, 1999; Yuan and Neufeld, 2001) and early in disease course in the DBA/2J mouse model of glaucoma (Bosco et al., 2015; Bosco et al., 2011; Howell et al., 2012). Mice with reduced levels of myeloid cell activation, either genetically (Bosco et al., 2018; Chidlow et al., 2016) or pharmacologically (Bosco et al., 2008; Cueva Vargas et al., 2016; Liu et al., 2016; Nakazawa et al., 2006; Roh et al., 2012; Wang et al., 2014) were protected from glaucomatous neurodegeneration. However, markers utilized in these studies (Iba1, CD11b, and Cx3cr1) do not differentiate between resident microglia and recruited myeloid cells. Therefore, the individual contributions of these two ontogenetically and functionally distinct cell populations to RGC degeneration in glaucoma remain unknown. Furthermore, the underlying mechanisms by which microglia contribute to RGC death remain poorly understood.
We previously identified a molecular signature of homeostatic microglia that differentiates these cells from recruited myeloid cells and other cell types in the CNS (Butovsky et al., 2014). Microglial genes P2ry12, Tmem119 and Fcrls were found to be specifically expressed in microglia (Butovsky et al., 2014). Furthermore, we and others have found that in mouse models of Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), and multiple sclerosis, microglia switch from a homeostatic to microglial neurodegenerative phenotype (MGnD) (Krasemann et al., 2017), also known as disease-associated microglia (DAM) (Keren-Shaul et al., 2017). This switch is controlled by apolipoprotein E (APOE) and Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) signaling (Krasemann et al., 2017). APOE is the major lipoprotein in the brain while TREM2 is a phosphatidylserine receptor expressed only by myeloid cells; both genes are well-established genetic risk factors for Alzheimer’s disease (Guerreiro et al., 2013; Jonsson et al., 2013). In particular, the APOE4 allele has been identified as the major risk factor for late-onset AD (Saunders et al., 1993; Strittmatter et al., 1993). APOE has also been linked to human glaucoma, with studies by us and others showing that APOE4 is associated with a decreased risk of glaucoma (Lam et al., 2006; Mabuchi et al., 2005; Margeta et al., 2020). This finding is consistent with the literature showing that APOE4 is also associated with a decreased risk of age-related macular degeneration (McKay et al., 2011; Xiying et al., 2017) and decreased photoreceptor degeneration in mice (Levy et al., 2015). However, why the same allele is deleterious in Alzheimer’s disease but protective in eye neurodegenerative diseases remains poorly understood.
In this study, we found that Apoe controls the microglial transition from a homeostatic to a cytotoxic neurodegenerative molecular phenotype in glaucoma, and that targeting this signaling pathway ameliorates glaucomatous RGC degeneration. Similar to Apoe−/− microglia, APOE4 microglia remained homeostatic in glaucoma, which led to decreased RGC degeneration despite elevated IOP. Our findings provide an explanation as to why APOE4 is associated with a decreased risk of glaucoma and show that the APOE signaling pathway is a promising target for neuroprotective treatments for this blinding disease.
Results
Retinal microglia transition to a neurodegenerative MGnD phenotype in the microbead glaucoma model.
To better understand the role of microglia in glaucoma pathogenesis, we investigated the microglial molecular signature in the microbead glaucoma model. To induce elevated IOP, magnetic microbeads were injected in the anterior chamber of wildtype mouse eyes (Chen et al., 2011; Ito et al., 2016; Sappington et al., 2010). Four experimental groups were included: 1) microbead-injected eyes, which exhibited elevated IOP and subsequent optic nerve degeneration (Figure 1A and 1B), 2) contralateral eyes of microbead-injected mice, 3) sham-injected eyes, and 4) naïve eyes of mice that did not undergo any experimental manipulations. One month after microbead injection, retinal microglia were isolated for SmartSeq2 RNAseq using flow cytometry as CD11b+/Ly6C−/Fcrls+ cells (Butovsky et al., 2014) (Figure S1A). We have previously found that sorting with anti-Fcrls antibody effectively differentiates between resident myeloid cells and infiltrating monocytes in chimeric mice transplanted with bone marrow from Cx3cr1GFP/+ donors both in naïve state and after experimental autoimmune encephalomyelitis induction (Butovsky et al., 2014). Furthermore, Fcrls expression in microglia remains stable in neurodegeneration (Krasemann et al., 2017). We further confirmed that the retinal CD11b+/Ly6C−/Fcrls+ myeloid cell population was positive for microglial marker Tmem119 in glaucoma (Figure S1B and S1C). However, it is important to note that peripherally derived monocytes have been shown to upregulate Fcrls and Tmem119 at the RNA level in the brain after microglia depletion (Bennett et al., 2018; Lund et al., 2018b) and in the retina during photoreceptor degeneration (O’Koren et al., 2019). This raises the possibility that the retinal CD11b+/Ly6C−/Fcrls+ myeloid cell population in glaucoma, which we refer to as neurodegenerative microglia (MGnD) in the remainder of the manuscript, may represent a heterogeneous population of resident microglia and monocyte-derived engrafted macrophages. In the microbead-injected eyes, Fcrls+ retinal microglia suppressed homeostatic (M0) microglial genes (e.g. P2ry12, Tmem119, Tgfbr1, Mef2a), and upregulated a number of genes that are also induced in MGnD microglia in the brain, including Apoe, Lgals3, cytokines, and complement (Figure 1C–E; Table S1) (Krasemann et al., 2017). The contralateral eyes of microbead-injected animals also displayed a distinct microglial expression profile (Figure 1C and Table S1), representing cellular signaling, function, movement, and proliferation pathways by Ingenuity Pathway Analysis (IPA). This finding is consistent with published reports showing that induction of glaucoma in one eye can lead to microglial activation in the contralateral eye (Rojas et al., 2014; Sapienza et al., 2016; Tribble et al., 2021).
Figure 1. Retinal microglia transition to a neurodegenerative MGnD phenotype in glaucoma.
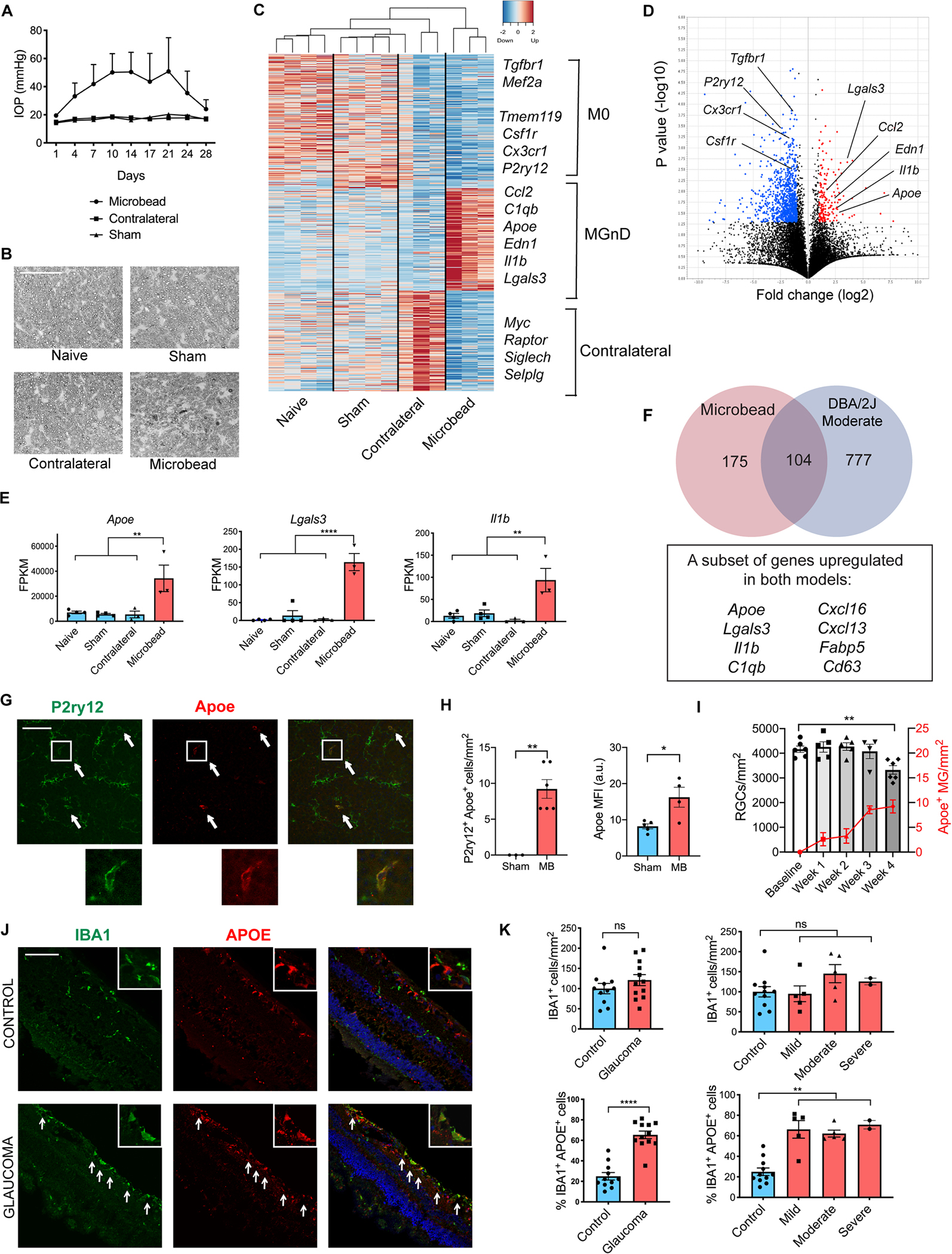
A) Intraocular pressure (IOP) measurements of microbead-injected (MB), contralateral and sham-injected eyes from wildtype C57BL/6J female mice (n=3–4 per group). B) p-phenylenediamine (PPD) staining of optic nerves from MB eyes and controls. Scale bar 50 μm. C) Heatmap of differentially expressed genes between the MB eyes and all three control groups. See also Table S1. D) Volcano plot of differentially expressed genes between MB and sham-injected eyes. Upregulated genes in red; downregulated genes in blue. E) Selected neurodegeneration-associated genes from C. Dot plots showing FPKM values compared using one-way ANOVA. F) Overlap between differentially expressed genes in MB and DBA/2J moderate stage glaucoma eyes. G) Representative images demonstrating colocalization of Apoe and P2ry12 in retinal midperiphery one month after MB injection. Arrows show co-expressing cells. Scale bar 100 μm. H) Quantification of (G). MFI = mean fluorescence intensity of Apoe in P2ry12+ cells. a.u. = arbitrary units. Compared using two-tailed Student’s t-test. I) The time-course of MB-injected wildtype animals showing weekly change in Brn3a+ RGC numbers and P2ry12+ Apoe+ cells per mm2 retina compared using one-way ANOVA (n=4–6 retinas per timepoint). J) Cross-sections of human retinas with and without glaucoma stained with IBA1, APOE, and DAPI. Arrows show co-expressing cells in retinal midperiphery. Scale bar 100 μm. K) Quantification of the number of IBA1+ cells per mm2 of retina and % of IBA1+/APOE+ double-positive cells in control and glaucoma specimens, compared using unpaired t-test with Welch’s correction and one-way ANOVA. Data in A-E and J-K were each obtained from one independent experiment, while data in G-I were pooled from two independent experiments. All results are shown as mean +/− SEM; *P<0.05, **P<0.01, ****P<0.0001, ns = not significant. See also Figures S1 and S2 and Table S1.
Retinal microglia induce the MGnD molecular signature in the DBA/2J glaucoma model.
To further evaluate microglial molecular signature in a different model of glaucoma, we focused on DBA/2J, a genetic model of chronic pigmentary glaucoma. DBA/2J animals have mutations in GpnmbR150X and Tyrp1b genes, and experience IOP elevation and severe loss of RGCs at one year of life with incomplete penetrance (Anderson et al., 2002; Libby et al., 2005). Using the same sorting strategy as shown in Figure S1A, we isolated retinal microglia from 1-year-old DBA/2J animals, which exhibit a range of severity of optic nerve degeneration (Figure S2A) (Libby et al., 2005), as well as DBA/2J Gpnmb+ animals, which serve as isogenic controls (Howell et al., 2007). We also included eyes from DBA/2J animals at 6 months of age, at which point IOP begins to be elevated but no RGC axon degeneration or cell body apoptosis can be detected (Libby et al., 2005). Similar to what we had observed in the microbead glaucoma model, retinal microglia in the DBA/2J model also suppressed homeostatic genes and upregulated Apoe, cytokines, and complement (Figure S2B–D; Table S1). Notably, these changes were absent in the DBA/2J animals at 6 months of age (Figure S2D), demonstrating that it is the presence of neurodegeneration rather than the specific DBA/2J genetic background that is leading to the MGnD microglial phenotype. We next re-analyzed DBA/2J RNAseq data based on the severity of optic nerve degeneration (Figure S2A) and found that MGnD genes were strongly upregulated in retinal microglia from eyes with moderate glaucomatous disease, and less abundantly expressed in eyes with minimal neurodegeneration and severe disease (Figure S2E and S2F). We compared genes upregulated in both the microbead glaucoma model and DBA/2J moderate severity animals and identified a core transcriptional signature shared between the two glaucoma models (Table S1). These genes may play particularly important roles in glaucoma pathogenesis, and include Apoe, Lgals3, Il1b, C1qb, and several cytokines (Figure 1F).
APOE is upregulated in myeloid cells in two murine glaucoma models and in human glaucomatous retinas.
Our prior work has identified APOE as a key regulator of the microglial molecular signature in neurodegeneration (Krasemann et al., 2017). Given that Apoe is highly upregulated in retinal microglia at the RNA level in both glaucoma models (Figure 1F), we confirmed this upregulation at the protein level by demonstrating colocalization of Apoe and Iba1 (a pan-myeloid marker, Figure S1D, S1E and S2G, S2H) and Apoe and P2ry12 (a selective microglial marker, Figure 1G, 1H and S2I, S2J) in microbead-injected and DBA/2J glaucomatous retinas, respectively. We additionally characterized the time-course and spatial distribution of the Apoe+ microglia in the microbead glaucoma model. We found that Apoe+ microglia appear early, one week after microbead injection, before significant RGC degeneration occurs (Figure 1I), and that their appearance inversely correlates with RGC numbers in retinal midperiphery and near periphery (Figure S1F). Next, we investigated whether APOE is upregulated in retinal myeloid cells in human glaucoma. We performed immunohistochemistry using sections from 23 formaldehyde-fixed, paraffin-embedded autopsy eyes: 11 control eyes and 12 eyes with glaucoma that were matched in terms of age, sex, and race (Table S2). Of note, we utilized IBA1+ to quantify myeloid cells in the retina, as we were able to detect a signal with microglia-specific marker TMEM119 in only a small number of samples (Figure S1G and Table S2). While in control eyes we noted few IBA1+ cells expressing APOE, in glaucoma eyes we observed a significant increase in the number of double-positive IBA1+/APOE+ cells, especially in the inner retina (Figure 1J). Quantification demonstrated that while the overall number of IBA1+ cells was not different between control and glaucoma cases, the proportion of IBA1+/APOE+ cells was significantly increased in glaucoma at all stages of disease (Figure 1K). These findings remained significant when we excluded patients with a diagnosis of infection in the autopsy report, demonstrating that the presence of possible systemic inflammation did not influence our results (Figure S1H). Thus, APOE is upregulated in retinal myeloid cells of human glaucoma patients.
Targeting Apoe in long-lived myeloid cells protects from glaucomatous RGC loss.
A previous study has found that animals with a global deletion of Apoe are protected from RGC degeneration in a mouse laser glaucoma model (Omodaka et al., 2014); however, the underlying mechanisms of Apoe-mediated neurodegeneration remained unknown. To investigate the effect of myeloid cell-specific targeting of Apoe on RGC survival in glaucoma, we used Cx3cr1Cre/+:Apoefl/fl mice, in which Apoe is deleted only in myeloid cells (Krasemann et al., 2017). Microbead injection led to a similar degree of IOP elevation in control Cx3cr1WT:Apoefl/fl and Cx3cr1Cre/+:Apoefl/fl mice (Figure S3A). However, while control mice experienced a significant reduction in RGC numbers after IOP elevation, Cx3cr1Cre/+:Apoefl/fl mice were protected from RGC cell body loss (Figure 2A and S3B) and axon loss (Figure 2B and S3C). To confirm that Apoe acts in long-lived myeloid cells (retinal microglia and perivascular macrophages) rather than in peripherally derived myeloid cells to cause RGC loss, we utilized microbead injections in tamoxifen (TAM)-treated Cx3cr1CreERT2/+:Apoefl/fl mice. By deleting Apoe in all myeloid cells using tamoxifen and waiting 30 days for peripheral cells to repopulate, we and others have previously been able to selectively target genes in long-lived myeloid cells in the CNS (Bruttger et al., 2015; Krasemann et al., 2017; Parkhurst et al., 2013). We confirmed that the same TAM regimen efficiently targets Apoe in sorted retinal microglia on RNA (Figure 2C) and protein level (Figure 2D and 2E). Microbeads were injected one month after TAM injection with resulting IOP rise (Figure S3D). IOP elevation led to a significant decrease in RGC numbers in control TAM-treated Cx3cr1WT:Apoefl/fl animals, while TAM-treated Cx3cr1CreERT2/+:Apoefl/fl animals were protected from Brn3a+ RGC cell body loss (Figure 2F and 2G) and axon loss (Figure 2H and 2I). To confirm that targeting of Apoe in long-lived myeloid cells also protects RGC function, we treated a separate cohort of animals with TAM and recorded full-field electroretinograms (ERG) at baseline and one month after microbead injection (Figure S3E). We focused on positive scotopic threshold response (pSTR), as pSTR has previously been demonstrated to be the most sensitive ERG parameter for the detection of functional abnormalities in a rodent model of glaucoma (Bui and Fortune, 2004; Fortune et al., 2004). There were no statistically significant differences in pSTR values at baseline (Figure S3F and 2K). However, one month after microbead injection, Cx3cr1WT:Apoefl/fl animals experienced a significant decrease in pSTR, while Cx3cr1CreERT2/+:Apoefl/fl animals were not different from control littermates (Figure 2J and 2L). Therefore, targeting Apoe in long-lived myeloid cells leads to both structural and functional RGC protection from glaucomatous degeneration.
Figure 2. Targeting Apoe in long-lived myeloid cells protects against RGC loss in microbead-induced glaucoma.
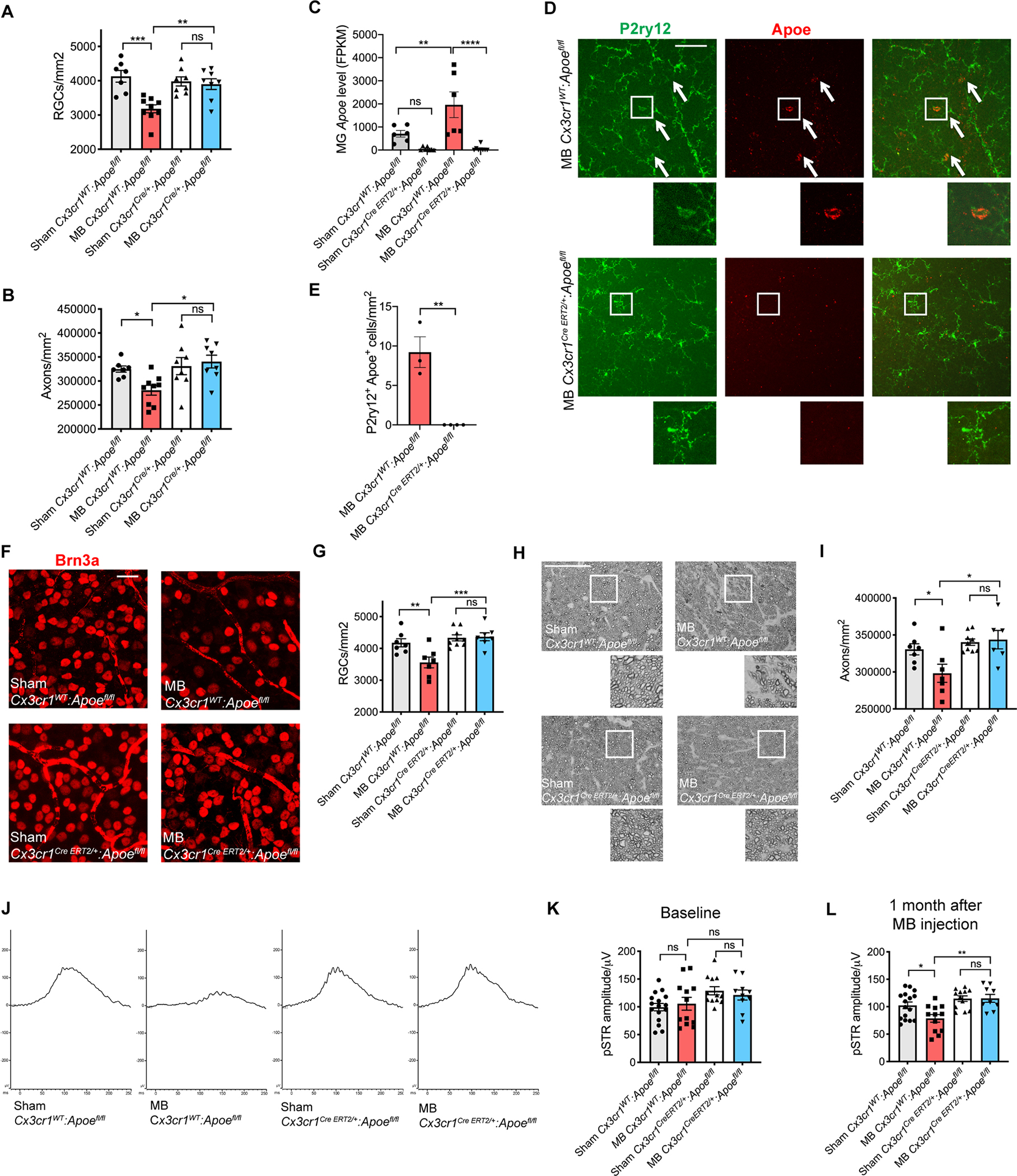
A,B) Quantification of retinal ganglion cell (RGC) cell body numbers (A) and axon counts (B) in microbead-injected (MB) and sham-injected Cx3cr1Cre/+:Apoefl/fl and Cx3cr1WT:Apoefl/fl retinas. Compared using one-way ANOVA (n=7–9 per group). C) Apoe mRNA in sorted retinal microglia (MG) from Cx3cr1CreERT2/+:Apoefl/fl animals and Cx3cr1WT:Apoefl/fl control animals one month after sham or MB injection (n=6–8 per group). Dot plots showing FPKM values compared using one-way ANOVA. D,E) Representative images (D) and quantification (E) showing upregulation of Apoe protein in P2ry12+ cells in MB Cx3cr1WT:Apoefl/fl control animals and lack of upregulation in Cx3cr1CreERT2/+:Apoefl/fl animals, n=3–4 per group. Arrows show co-expressing cells in retinal midperiphery. Scale bar 100 μm. Compared used two-tailed Student’s t-test. F) Representative images of Brn3a+ RGCs in Cx3cr1CreERT2/+:Apoefl/fl and Cx3cr1WT:Apoefl/fl retinas one month after MB or sham injection (n=7–9 per group). Scale bar 20 μm. G) Quantification of RGC cell body numbers compared using one-way ANOVA. H,I) Representative images (H) and quantification (I) of p-phenylenediamine (PPD) staining of optic nerves from animals in (F). Scale bar 50 μm. Compared using one-way ANOVA. J) Representative positive scotopic response (pSTR) electroretinogram traces of MB and sham-injected Cx3cr1CreERT2/+:Apoefl/fl and Cx3cr1WT:Apoefl/fl eyes 1 month after MB injection. K,L) Quantification of pSTR amplitude of animals in (J) at baseline (P>0.55) (K) and one month (L) after MB injection (n=9–16 animals per group). Data in C were obtained from one independent experiment, while the rest of the data were each pooled from 2–3 independent experiments. All results are shown as mean +/− SEM; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, ns = not significant. See also Figure S3.
Apoe−/− retinal microglia remain homeostatic despite elevated IOP in the microbead glaucoma model.
To investigate the mechanism by which microglial Apoe causes RGC degeneration, we performed microbead injections in wildtype and Apoe−/− mice (Figure 3A) and isolated CD11b+/Ly6C−/Fcrls+ retinal microglia for RNAseq analysis. While microglia from wildtype animals upregulated the MGnD core signature including Apoe, Lgals3, proinflammatory cytokines, and complement, Apoe−/− microglia remained homeostatic (Figure 3B–D; Table S3). Importantly, in Apoe−/− mice, the MGnD cluster was strongly suppressed (Figure 3E and 3F). While Lgals3 was one of the most strongly upregulated molecules in glaucoma, its expression was markedly attenuated in Apoe−/− animals (Figure 3C–E). IPA analysis demonstrated that several immune and inflammatory pathways, including neuroinflammation signaling (Figure 3G) and the pan-inflammatory LPS signaling pathway (Figure S4A and S4B) were upregulated in microglia from wildtype glaucomatous retinas, and this activation was suppressed in retinal microglia from Apoe−/− animals. Finally, to confirm these findings on the protein level, we stained glaucomatous wildtype and Apoe−/− retinas for Galectin-3, the protein encoded by the Lgals3 gene. While Galectin-3 was highly upregulated in P2ry12+ microglia in microbead-injected wildtype retinas, this upregulation was abrogated in microbead-injected Apoe−/− animals (Figure 3H and 3I). Furthermore, Galectin-3 and Apoe demonstrated a high degree of colocalization in Iba1+ and P2ry12+ myeloid cells in microbead-injected wildtype retinas (Figure S4C), supporting the idea that these two molecules act in the same signaling pathway.
Figure 3. Apoe−/− retinal microglia remain homeostatic despite elevated IOP in the microbead glaucoma model.
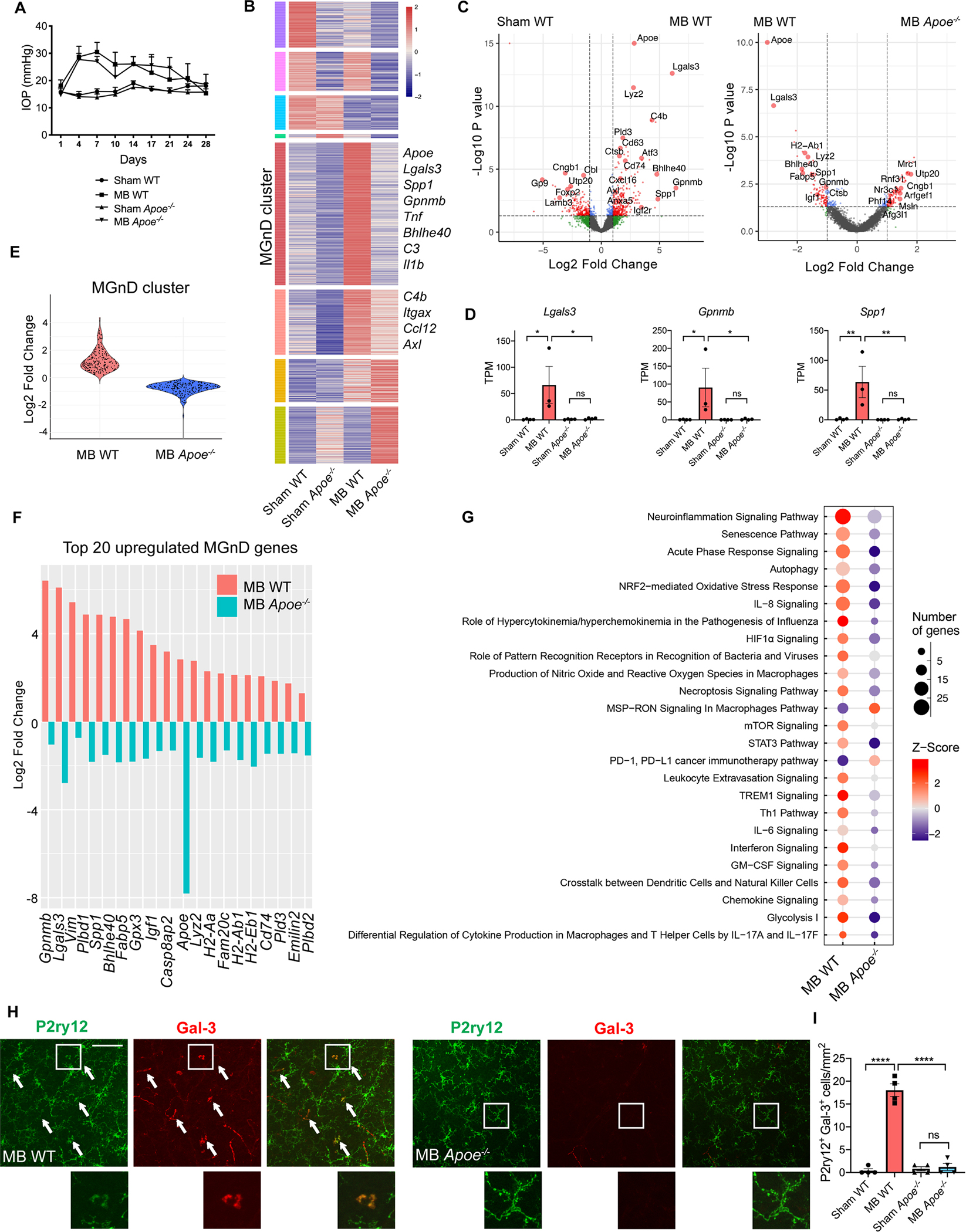
A) Intraocular pressure (IOP) measurements in microbead-injected (MB) and sham-injected wildtype (WT) and Apoe−/− mice (n=3–4 per group). B) Heatmap of differentially expressed microglial genes between MB and sham-injected WT and Apoe−/− mice. MGnD cluster highlighted in red. See also Table S3. C) Volcano plots of differentially expressed microglial genes between MB and sham-injected WT eyes, and MB WT and Apoe−/− eyes. D) Select neurodegeneration-associated genes from (B). Dot plots showing TPM values compared using one-way ANOVA. E) Violin plot showing differences in MGnD cluster gene upregulation between MB WT and Apoe−/− mice. F) The differential expression of the top 20 upregulated MGnD genes between MB WT and Apoe−/− mice. G) Top 25 differentially expressed canonical pathways in retinal microglia from MB WT and Apoe−/− mice as determined by Ingenuity Pathway Analysis. H,I) Representative images (H) and quantification (I) showing upregulation of Galectin-3 protein in P2ry12+ cells in MB WT retinas and lack of upregulation in MB Apoe−/− retinas (n=4 per group). Arrows show co-expressing cells in retinal midperiphery. Scale bar 100 μm. Compared used one-way ANOVA. Data in A-G were obtained from one independent experiment, while data in H-I were obtained from two pooled independent experiments. All results are shown as mean +/− SEM; *P<0.05, **P<0.01, ****P<0.0001, ns = not significant. See also Figure S4 and Table S3.
MGnD microglia directly induce RGC degeneration in vivo.
Our laboratory has previously found that stereotaxically-injected apoptotic neurons induce the MGnD microglial phenotype in the brain (Krasemann et al., 2017). We confirmed that the same is true in the retina by intravitreally injecting UV-irradiated, fluorescently labeled apoptotic neurons into mouse eyes, and 16–24 hours later isolating retinal microglia as phagocytic vs. non-phagocytic (Figure S5A–C). We found that Apoe is upregulated in phagocytic retinal microglia compared to non-phagocytic microglia (Figure S5D) and confirmed this finding on the protein level (Figure S5E). Wildtype phagocytic microglia upregulated MGnD genes including Lgals3, Gpnmb, Spp1, and complement, and this MGnD signature was attenuated in Apoe−/− retinal microglia, similar to what is seen in the microbead glaucoma model (Figure S5F and S5G and Table S4). To test whether MGnD microglia are sufficient to induce RGC degeneration, we next injected fluorescently labeled apoptotic neurons in the brain of YFP-positive Cx3cr1CreERT2/+ animals, isolated phagocytic and non-phagocytic microglia, and injected them intravitreally in the eyes of wild-type recipients (Figure 4A). One month following intravitreal injection, RGC numbers were reduced in eyes injected with phagocytic MGnD microglia compared to those injected with non-phagocytic M0 (homeostatic) microglia or PBS (Figure 4B and 4C). YFP+ donor microglia remained in the vitreous and minimally integrated into the retina (0–2 YFP+ cells per retina), suggesting that the harmful effect of MGnD microglia in this paradigm is mediated by secreted substances rather than direct cellular contact. We confirmed that injected phagocytic MGnD microglia in the vitreous expressed high levels of Apoe protein, while non-phagocytic M0 microglia did not (Figure 4D). We next repeated this experiment with donor microglia from Apoe−/− animals. RGC numbers from eyes injected with phagocytic MGnD Apoe−/− microglia were not statistically different from those injected with non-phagocytic Apoe−/− microglia and PBS, demonstrating that the cytotoxic effect of MGnD microglia is Apoe-dependent (Figure 4E and 4F). Finally, we stained retinas injected with wildtype or Apoe−/− donor microglia with Cleaved caspase 3, a marker of apoptosis (Nagata and Segawa, 2021). We found that Cleaved caspase 3+ cells were present only in retinas injected with wildtype MGnD microglia and restricted to the ganglion cell layer (Figure 4G and 4H), while being minimally present in other retina layers (Figure 4I). These results show that microglia-induced neurodegeneration affects predominantly RGCs in this experimental paradigm.
Figure 4. MGnD microglia induce RGC degeneration in an Apoe-dependent manner.
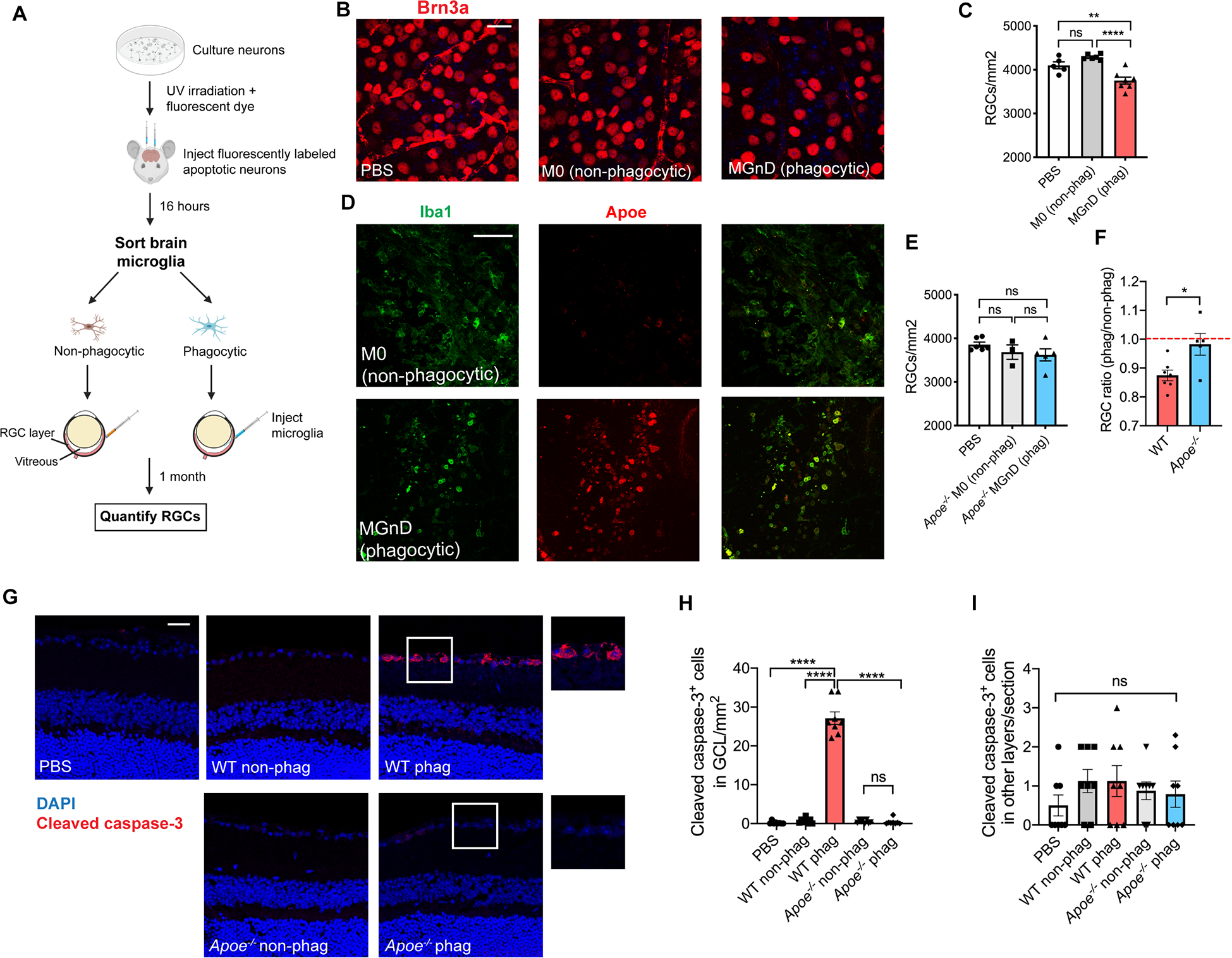
A) Schematic diagram demonstrating the experimental set-up for the apoptotic neuron injection in the brain, followed by microglia isolation and intravitreal injection of 5,000 microglial cells in the eye. B) Representative images of Brn3a+ retinal ganglion cell (RGC) staining of wildtype recipient eyes injected with PBS, M0 (non-phagocytic) and MGnD (phagocytic) microglia from Cx3cr1CreERT2/+ YFP+ donors, n=5–7 eyes per group. Scale bar 20 μm. C) Quantification of Brn3a+ RGC counts compared using one-way ANOVA. D) Donor wildtype phagocytic and non-phagocytic microglia stained for Iba1 and Apoe in the vitreous of wildtype recipients 1 week after intravitreal injection. Scale bar 100 μm. E) Quantification of Brn3a+ RGC counts of wildtype eyes injected with PBS, Apoe−/− non-phagocytic and Apoe−/− phagocytic microglia compared using one-way ANOVA, n=3–6 eyes per group. F) Comparison of RGC counts between experiments in C and E using a two-tailed Student’s t-test. The number of RGCs from eyes injected with phagocytic microglia was normalized to the average number of RGCs from eyes injected with non-phagocytic microglia of the same genotype. G,I) Representative images (G) and quantification (H, I) of Cleaved caspase-3 staining of eyes of wild-type recipients injected with PBS, non-phagocytic and phagocytic microglia from wildtype and Apoe−/− donors (n=8 eyes per group). Scale bar 10 μm. GCL = ganglion cell layer. Compared using one-way ANOVA. All data were obtained from individual independent experiments. All results are shown as mean +/− SEM, *P<0.05. **P<0.01, ****P<0.0001, ns = not significant. See also Figure S5.
Genetic and pharmacologic targeting of Galectin-3 ameliorates RGC degeneration in the microbead glaucoma model.
To further elucidate the mechanism by which microglial Apoe causes RGC degeneration, we sought to identify a cytotoxic downstream effector of Apoe signaling. We focused on Galectin-3 (Lgals3), a secreted carbohydrate-binding lectin that was upregulated in both the microbead and DBA/2J glaucoma models (Figure 1F) and was transcriptionally regulated by Apoe on both the RNA (Figure 3D and S5G) and protein level (Figure 3H). Notably, Lgals3 was one of the genes most strongly affected by loss of Apoe signaling in the microbead glaucoma model (Figure 3C). We performed microbead injections in wildtype and Lgals3−/− animals (Figure 5A) and found that while wildtype animals experienced a significant loss of RGC cell bodies and axons one month after microbead injection, Lgals3−/− animals were protected from neurodegeneration despite elevated IOP (Figure 5B–E). We next pharmacologically targeted Galectin-3 using intravitreal injections of TD139, a selective Galectin-3 inhibitor (Chen et al., 2017; Siew et al., 2019). Wildtype animals were administered intravitreal TD139 or vehicle 14 and 21 days after microbead injection, and RGC survival was assessed one month after microbead injection (Figure 5F). While microbead-injected animals treated with vehicle experienced a loss of RGC cell bodies and axons, animals treated with TD139 were protected from RGC loss (Figure 5G–J), suggesting that pharmacologic inhibition of Galectin-3 is a feasible neuroprotective strategy for the treatment of glaucoma.
Figure 5. Genetic and pharmacologic targeting of Galectin-3 protects against RGC loss in microbead-induced glaucoma.
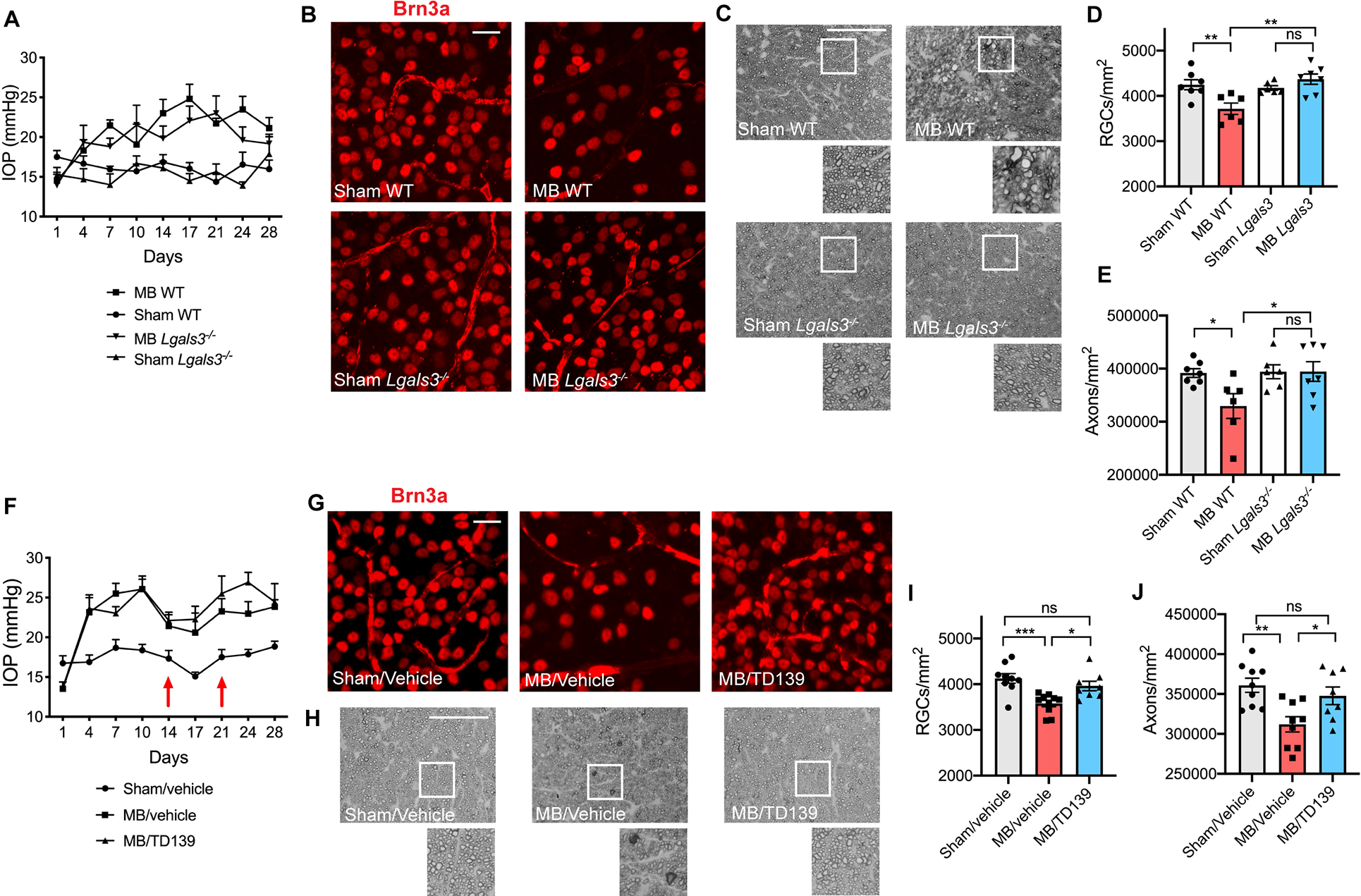
A) Intraocular pressure (IOP) measurements of microbead-injected (MB) and sham-injected wildtype (WT) and Lgals3−/− eyes (n=6–7 per group). B) Representative images of Brn3a+ retinal ganglion cells (RGCs) in MB and sham-injected WT and Lgals3−/− retinas. Scale bar 20 μm. C) Representative images of p-phenylenediamine (PPD) staining of optic nerves from MB and sham-injected WT and Lgals3−/− animals. Scale bar 50 μm. D) Quantification of RGC cell body numbers from (B) compared using one-way ANOVA. E) Quantification of axon counts from (C) compared using one-way ANOVA. F) IOP measurements of MB and sham-injected WT eyes treated with vehicle or Galectin-3 inhibitor TD139 (n=8–10 eyes per group). Intravitreal injections of the inhibitor or vehicle were administered at 2 and 3 weeks after MB injection (red arrows). G) Representative images of Brn3a+ RGCs in WT retinas treated with vehicle or TD139 inhibitor. Scale bar 20 μm. H) Representative images of PPD staining of optic nerves from WT animals treated with vehicle or TD139 inhibitor. Scale bar 50 μm. I-J) Quantification of RGC cell body numbers from (G) and axon counts from (H) compared using one-way ANOVA. Data in A-E and F-J were each pooled from two independent experiments. All results are shown as mean +/− SEM, *P<0.05, **P<0.01, ***P<0.001, ns = not significant.
Humanized APOE4 mice are protected from RGC loss despite IOP elevation.
Human APOE gene has three alleles, APOE2, APOE3 and APOE4. APOE3 is the common allele, while APOE4 is a major risk factor for Alzheimer’s disease (Saunders et al., 1993; Strittmatter et al., 1993). In contrast, APOE4 is associated with a decreased risk of open angle glaucoma (Lam et al., 2006; Mabuchi et al., 2005; Margeta et al., 2020). To investigate whether humanized APOE4 mice are similarly protected from RGC degeneration, we performed microbead injections in APOE2, APOE3 and APOE4 mice (Figure S6A), in which both copies of murine Apoe were replaced with the human isoforms. One month following microbead injection, humanized APOE2 and APOE3 mice showed a significant loss of RGC cell bodies and axons, while humanized APOE4 mice were protected from RGC degeneration (Figure 6A–D). To determine whether APOE3 and APOE4 act cell-autonomously in long-lived myeloid cells, we injected microbeads one month after TAM treatment in APOE3fl/fl and APOE4fl/fl mice with or without the Cx3cr1CreERT2 gene (Figure S6B). Microbead-injected Cx3cr1WT:APOE3fl/fl mice showed loss of RGC cell bodies and axons as compared to sham-treated control group (Figure 6E–H). In contrast, Cx3cr1CreERT2/+:APOE3fl/fl mice, in which APOE3 was targeted in long-lived myeloid cells, were protected from RGC degeneration, while APOE4fl/fl mice were protected from glaucomatous RGC loss regardless of the presence of the Cx3cr1CreERT2 gene (Figure 6E–H). These findings indicate that APOE3 and APOE4 act in long-lived retinal myeloid cells to respectively cause RGC loss or promote RGC survival.
Figure 6. Humanized APOE4 mice are protected from RGC loss in the microbead glaucoma model.
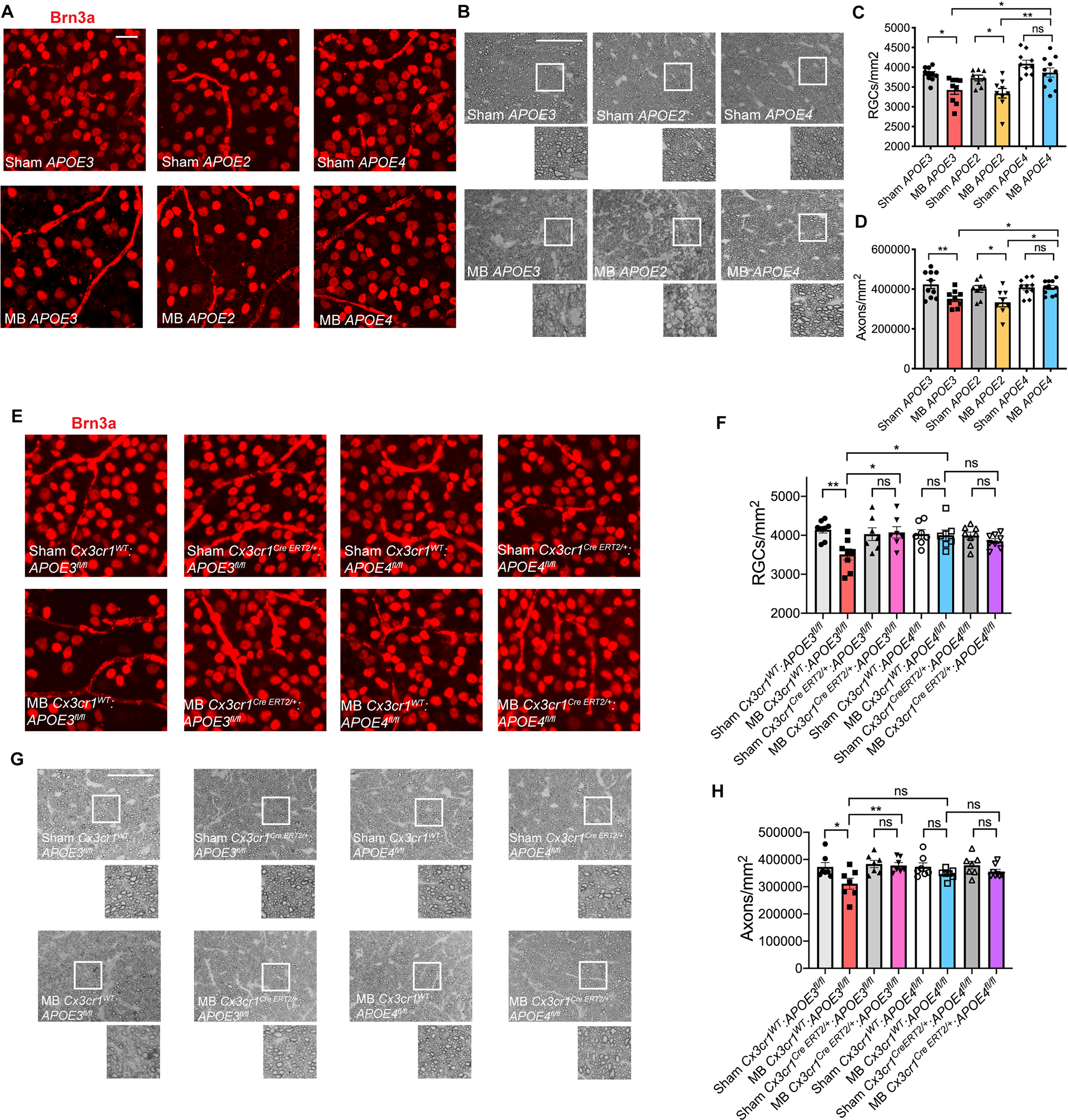
A) Representative images of Brn3a+ retinal ganglion cells (RGCs) in microbead-injected (MB) and sham-injected humanized APOE2, APOE3 and APOE4 retinas (n=8–11 per group). Scale bar 20 μm. B) Representative images of p-phenylenediamine (PPD) staining of optic nerves from MB and sham-injected humanized APOE2, APOE3 and APOE4 animals. Scale bar 50 μm. C) Quantification of RGC cell body numbers in (A) compared using one-way ANOVA. D) Quantification of axon counts in (B) compared using one-way ANOVA. E) Representative images of Brn3a+ RGCs in tamoxifen-treated MB and sham-injected Cx3cr1WT:APOE3fl/fl, Cx3cr1CreERT2/+:APOE3fl/fl, Cx3cr1WT:APOE4fl/fl, Cx3cr1CreERT2/+:APOE4fl/fl retinas (n=7–8 per group). Scale bar 20 μm. F) Quantification of RGC cell body numbers compared using one-way ANOVA. G) Representative images of PPD staining of optic nerves from animals in (E). Scale bar 50 μm. H) Quantification of axon counts compared using one-way ANOVA. Data in A-D and E-H were each pooled from 2–3 independent experiments. All results are shown as mean +/− SEM, *P<0.05, **P<0.01, ns = not significant. See also Figure S6.
APOE4 retinal microglia remain homeostatic in glaucoma.
To investigate the mechanism by which APOE4 promotes RGC survival in glaucoma, we performed microbead injections in humanized APOE3 and APOE4 mice (Figure 7A) and isolated CD11b+/Ly6C−/Fcrls+ retinal microglia for RNAseq analysis. While microglia from APOE3 animals upregulated MGnD molecules (Lgals3, Spp1, Gpnmb, cytokines and complement), APOE4 microglia remained homeostatic, with an attenuated MGnD molecular signature (Figures 7B–E and S6C; Table S5). Of note, Lgals3 was one of the genes whose upregulation was most strongly attenuated in the presence of the APOE4 allele in glaucoma (Figure 7C and 7E). This attenuated MGnD response to elevated IOP is similar to that seen in Apoe−/− animals (Figure 3B–F). Similarly, IPA analysis demonstrated that several immune and inflammatory pathways, including neuroinflammation signaling and pan-inflammatory LPS signaling, were upregulated in microglia from APOE3 glaucomatous retinas, and that this activation was abolished in retinal microglia from APOE4 animals (Figures 7F and S6D). To confirm these findings on the protein level, we stained glaucomatous retinas from humanized APOE3 and APOE4 mice with Galectin-3 antibody. APOE3 mice upregulated Galectin-3 following microbead injection, while in APOE4 microbead-injected retinas, Galectin-3 expression was undetectable (Figure 7G and 7H). We next pharmacologically targeted Galectin-3 in APOE3 and APOE4 animals with intravitreal injections of Galectin-3 inhibitor TD139 using the same experimental paradigm as detailed in Figure 5F. While microbead-injected APOE3 animals treated with TD139 were protected from RGC loss, pharmacologic targeting of Galectin-3 did not confer additional protection in APOE4 animals (Figure S7A–E), consistent with the notion that Galectin-3 is the critical downstream effector of APOE signaling. Finally, we evaluated Galectin-3 expression in human retinal samples. Glaucoma patients with APOE3/APOE3 genotype expressed high levels of Galectin-3 protein in IBA1+ myeloid cells, whereas Galectin-3 expression was attenuated in APOE4 carriers (Figure 7I and 7J and Table S2). Thus, in patients with the APOE4 allele, myeloid cells remain more homeostatic, which may explain the protective effect of this allele in human glaucoma.
Figure 7. APOE4 retinal microglia remain homeostatic in glaucoma.
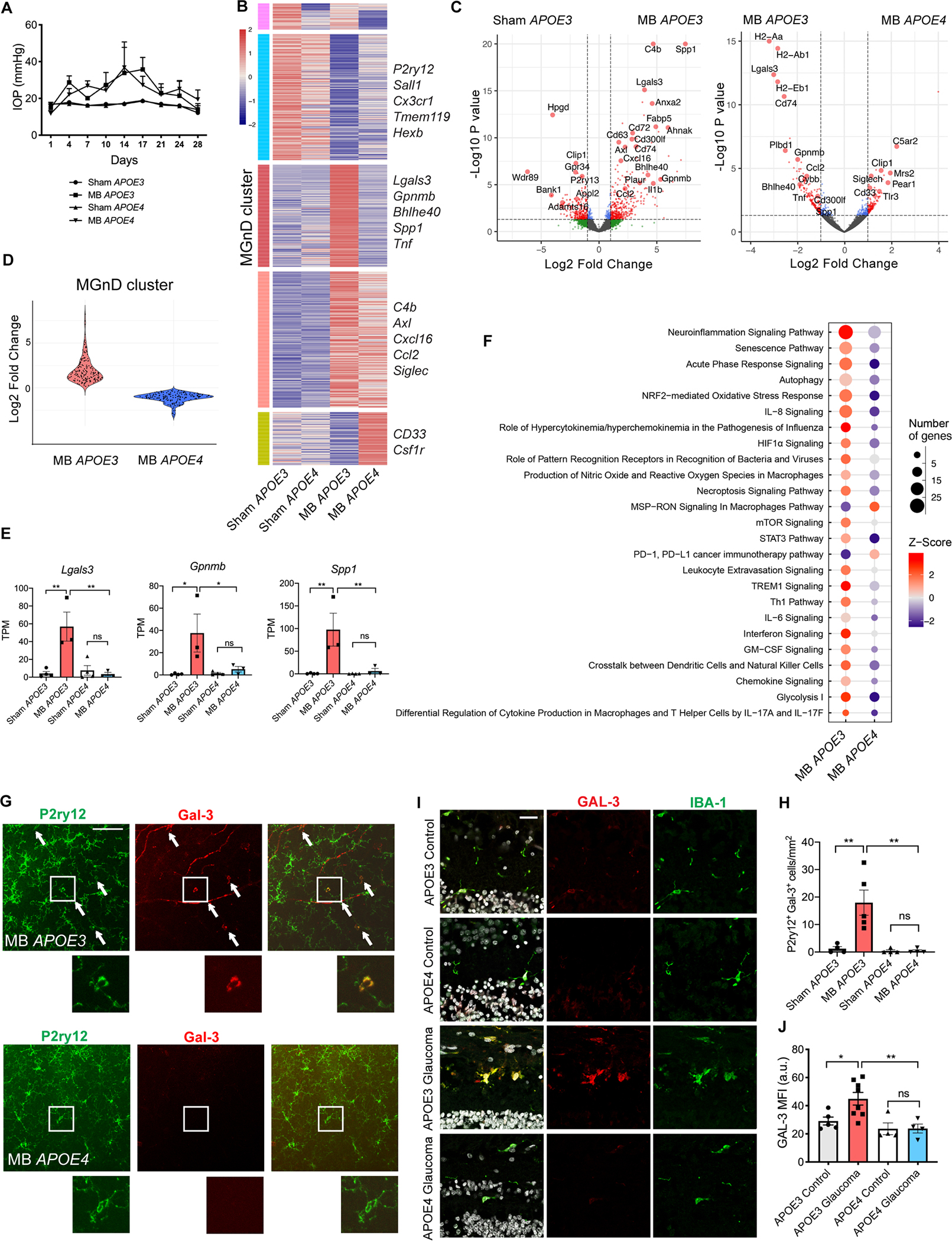
A) Intraocular pressure (IOP) measurements of microbead-injected (MB) and sham-injected humanized APOE3 and APOE4 male mice (n=3–4 per group). B) Heatmap of differentially expressed microglial genes between MB and sham-injected APOE3 and APOE4 mice. MGnD cluster highlighted in red. See also Table S5. C) Volcano plots of differentially expressed microglial genes between MB and sham-injected APOE3 eyes, and MB APOE3 and APOE4 eyes. D) Violin plot showing differences in MGnD cluster gene upregulation between MB APOE3 and APOE4 mice. E) Select neurodegeneration-associated genes from (B). Dot plots showing TPM values compared using one-way ANOVA. F) Top 25 differentially expressed canonical pathways in retinal microglia from MB APOE3 and APOE4 mice as determined by Ingenuity Pathway Analysis. G) Representative images showing upregulation of Galectin-3 protein in P2ry12+ cells in MB APOE3 retinas and lack of upregulation in MB APOE4 retinas, n=4–5 per group. Arrows show co-expressing cells in retinal midperiphery. Scale bar 100 μm. I) Cross-sections of human retinas from APOE3 and APOE4 allele carriers with and without glaucoma stained with IBA1, Galectin-3, and DAPI, n=4–8 per group. Scale bar 25 μm. H) Quantification of (G) shown as the number of P2ry12+ Gal-3+ cells per mm2 retina and compared used one-way ANOVA. J) Quantification of Galectin-3 mean fluorescence intensity (MFI) of human retinal sections in (I) compared used one-way ANOVA. a.u. = arbitrary units. Data in A-F and I-J were each obtained from one independent experiment, while data in G-H were obtained from two pooled independent experiments. All results are shown as mean +/− SEM, *P<0.05, **P<0.01, ns = not significant. See also Figure S6 and Table S5.
Discussion
In this study, we investigated the role of microglia in the pathogenesis of glaucoma by identifying the microglial molecular signature in two murine glaucoma models, the microbead injection model and the DBA/2J model. We find that in both models, microglia transition from a homeostatic to a neurodegenerative transcriptional phenotype characterized by upregulation of Apoe, Lgals3, cytokines, and complement. This transcriptional signature in glaucoma overlaps with the microglial molecular signature in murine models of other neurodegenerative diseases, including Alzheimer’s disease, ALS, and multiple sclerosis (Keren-Shaul et al., 2017; Krasemann et al., 2017). These findings point toward commonalities in the response of microglia to neurodegeneration, regardless of the causative pathogenetic factor or the location of the insult within the CNS.
One of the robustly upregulated genes in microglia in glaucoma was Apoe, the major lipoprotein in the CNS that was previously found to be upregulated in the retina by whole tissue proteomics and in the aqueous humor of glaucoma patients (Inoue et al., 2013; Mirzaei et al., 2017). Here we found that Apoe critically regulates microglial response in a mouse model of glaucoma, corroborating our previous observation that Apoe regulates microglial response to neurodegeneration in brain microglia (Krasemann et al., 2017). Furthermore, targeting Apoe in long-lived myeloid cells (microglia and perivascular macrophages) protected RGCs from degeneration despite elevated IOP. Although several prior investigations suggested that peripheral myeloid cells play an important early role in glaucoma pathogenesis (Harder et al., 2017; Howell et al., 2012; Williams et al., 2019), these studies were largely correlative and conducted exclusively in the DBA/2J glaucoma model, which is known to have underlying immune abnormalities (Anderson et al., 2008; Howell et al., 2013). In contrast, our genetic targeting strategy using the Cx3cr1CreERT2 transgene to target Apoe and human APOE3 and APOE4 alleles clearly demonstrates a critical role for resident myeloid cells in glaucoma pathogenesis. This long-lived Apoe-expressing population is likely heavily enriched in microglia given the sparse numbers of perivascular macrophages in the retina at baseline (Ma et al., 2019).
Our microglial isolation strategy for RNAseq relied on positive selection utilizing the marker Fcrls, which we have previously found to be highly effective in separating microglia from GFP+ infiltrating monocytes in mice transplanted with bone marrow from Cx3cr1GFP/+ donors in both naïve state and during neuroinflammation (Butovsky et al., 2014). Although Fcrls has been found to be expressed at a low level in resident macrophages of the brain in recently published single-cell RNAseq datasets (Jordao et al., 2019), high Fcrls expression is characteristic of microglia (Masuda et al., 2022). Our experimental results using other markers preferentially expressed in microglia (Tmem119, P2ry12) for sorting and immunohistochemistry additionally support significant involvement of retinal microglia in glaucoma. However, recent studies have also demonstrated that monocyte-derived macrophages can upregulate microglial markers upon engraftment in the retina during photoreceptor degeneration (O’Koren et al., 2019), as well as in the brain depleted of microglia in the “empty microglial niche” paradigm (Bennett et al., 2018; Lund et al., 2018b). Future investigations using lineage tracing and single-cell RNA sequencing will be needed to fully map out contributions of different myeloid cell populations to glaucoma pathogenesis (O’Koren et al., 2019; Wieghofer et al., 2021).
One of the key findings of our study is that APOE4, which has been extensively studied as a major risk factor for Alzheimer’s disease (Saunders et al., 1993; Strittmatter et al., 1993), acts similarly to Apoe loss-of-function in terms of microglial activation and effect on RGC survival in glaucoma. Microglia from APOE4 animals remained homeostatic and did not transition to a neurodegenerative phenotype in glaucoma, and RGCs in APOE4 animals did not degenerate despite elevated IOP. These results may explain why APOE4 is associated with a decreased genetic risk of glaucoma (Lam et al., 2006; Mabuchi et al., 2005; Margeta et al., 2020). Furthermore, these findings suggest that there are important differences between glaucoma and Alzheimer’s disease in terms of the underlying disease mechanisms, which may lead to opposing outcomes of MGnD microglial activation. Glaucoma is an optic neuropathy that is characterized by progressive loss of RGCs, and numerous studies have shown that activated myeloid cells producing inflammatory cytokines are detrimental in this disease context (Bosco et al., 2018; Bosco et al., 2008; Chidlow et al., 2016; Cueva Vargas et al., 2016; Liu et al., 2016; Nakazawa et al., 2006; Roh et al., 2012; Wang et al., 2014). In contrast, the role of microglia in Alzheimer’s disease is more complex, as activated microglia are important for both containing and propagating Aβ plaques and hyperphosphorylated tau, toxic deposits that are histopathological hallmarks of this disease (Asai et al., 2015; Gratuze et al., 2021; Griciuc et al., 2019; Huang et al., 2021; Ising et al., 2019; Keren-Shaul et al., 2017; Leyns et al., 2019; McAlpine et al., 2021; Parhizkar et al., 2019; Spangenberg et al., 2019; Wang et al., 2015). While further studies are needed to elucidate the role of APOE4 in microglial activation in Alzheimer’s disease, our work demonstrates that this allele plays a critical role in dampening the response of neurodegenerative microglia in the retina.
The mechanism by which microglia cause RGC degeneration in glaucoma likely involves production of secreted factors, as we showed that MGnD microglia transplanted intravitreally directly caused RGC loss while minimally integrating into the retina, consistent with prior cell transplantation experiments in the eye (Johnson et al., 2010). Indeed, one of the most strongly upregulated genes in glaucoma was Galectin-3, a secreted carbohydrate-binding lectin previously implicated in a number of neurodegenerative diseases, including Alzheimer’s disease (Boza-Serrano et al., 2019), Huntington’s disease (Siew et al., 2019), ALS (Lerman et al., 2012), multiple sclerosis (Jiang et al., 2009) and traumatic optic neuropathy (Abreu et al., 2017). In the majority of the investigated diseases, genetic targeting of Galectin-3 led to improved anatomic and functional outcomes; the only exception was ALS, in which Lgals3−/− animals demonstrated worse neurological symptoms and earlier death (Lerman et al., 2012). Here we show that genetic and pharmacologic targeting of Galectin-3 protects RGCs from degeneration in glaucoma, therefore demonstrating the potential of Galectin-3 inhibitors as a neuroprotective therapy for glaucoma. Furthermore, Galectin-3 expression was upregulated in retinas from APOE3/APOE3 donors and attenuated in APOE4 carriers, and Galectin-3 inhibition did not confer additional protection against RGC loss in humanized APOE4 mice, highlighting the importance of this molecule in mediating the differential effect of APOE allele status on glaucoma pathogenesis.
Galectin-3 may cause neurodegeneration in glaucoma through a variety of mechanisms, including direct cytotoxic effect on RGCs, autocrine/paracrine activation of microglia, and activation of other cell types, including astrocytes. Furthermore, these effects may be mediated by several different transmembrane receptors. For instance, Mer tyrosine kinase (MerTK), a well-characterized Galectin-3 receptor (Caberoy et al., 2012), has been shown to be critical for microglial phagocytosis of stressed neurons (Neher et al., 2013), as well as synapse elimination by astrocytes (Chung et al., 2013). Galectin-3 also binds to microglial receptors Trem2 and Toll-like receptor 4 (TLR4) (Boza-Serrano et al., 2019; Burguillos et al., 2015), and drives paracrine activation of TLR4 in surrounding microglia, thus potentiating their inflammatory response (Burguillos et al., 2015; Liu et al., 2022). Future investigations will be needed to develop a detailed understanding of how Galectin-3-mediated crosstalk between microglia, astrocytes, RGCs, and other retinal cell types contributes to RGC death in glaucoma.
In addition to Galectin-3, neurodegenerative retinal microglia also upregulate cytokines and complement, which have previously been shown to create a proinflammatory cellular milieu and stimulate astrocytes, which in turn can become highly toxic to neurons (Liddelow et al., 2017; Rothhammer et al., 2018). Microglia may also cause RGC death through direct cellular contact, as activated microglia have also been shown to phagocytose living neurons both in vitro during inflammation (Neher et al., 2012; Neher et al., 2011) and during retinal degeneration in vivo (Zhao et al., 2015). Finally, we and others have found that disrupting TGFβ signaling in microglia leads to the homeostatic-to-MGnD phenotypic switch that is sufficient to cause neurodegeneration, paralysis, and death (Butovsky et al., 2014; Lund et al., 2018a; Qin et al., 2018; Utz et al., 2020), as well as retinal gliosis and loss of RGCs and photoreceptors (Ma et al., 2019). Conversely, microglial modulation through viral overexpression of TGFβ1 protected cones in mouse models of retinal degeneration (Wang et al., 2020). These data corroborate our findings presented here that Apoe-mediated microglial activation is sufficient to cause retinal degeneration.
The upstream mechanisms that lead to activation of neurodegenerative microglia in glaucoma remain an open area for investigation. On one hand, we have found that phagocytosis of apoptotic neurons by microglia in the retina and in the brain (Krasemann et al., 2017) is sufficient to cause the switch from homeostatic to MGnD phenotype. On the other hand, we were able to detect Apoe+ microglia early in the microbead glaucoma model, before quantifiable RGC loss took place, and we found that targeting Apoe in long-lived myeloid cells prevented RGC degeneration. These latter findings suggest that microglia act early in glaucoma pathogenesis and can be activated by stimuli other than degenerating neurons, which may include elevated IOP itself (Albalawi et al., 2017; Campagno et al., 2021; Harder et al., 2017). Taken together, our results suggest that Apoe+ microglia play an important role in both initiation and propagation of neurodegeneration in glaucoma.
In summary, here we have demonstrated a critical role for APOE-Galectin-3 signaling in microglia in glaucoma and shown that this signaling cascade can be targeted to develop neuroprotective treatments for this common blinding disorder. In addition, our findings demonstrating that APOE4 impairs the response of neurodegenerative microglia have important mechanistic and therapeutic implications for other CNS neurodegenerations, including Alzheimer’s disease.
Limitations of the study
In this study we relied on bulk RNA sequencing to identify the microglial molecular signature in mouse models of glaucoma. Although powerful, this technique does not provide single cell resolution and therefore cannot determine the full diversity of underlying microglial states and possible contribution of other myeloid cell subpopulations. Future studies will also be needed to elucidate the complete spectrum of microglial transcriptional phenotypes in human glaucomatous retinas, which may be challenging given the limited availability of fresh frozen human retinal tissue. Finally, while we experimentally confirmed that Apoe acts in microglia in a mouse model of glaucoma, for Galectin-3 we relied on global Lgals3 knockout, as Lgals3 floxed mice are not currently available. Future studies will be required to determine if non-microglial cells contribute to the neuroprotective phenotype observed in Lgals3−/− mice, as well as elucidate the mechanism by which Galectin-3 causes RGC death in glaucoma.
STAR*METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Oleg Butovsky (obutovsky@rics.bwh.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Bulk RNA-seq data have been deposited at GEO and will be publicly available as of the date of publication. Accession numbers are listed in the key resources table. All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Brn-3a Antibody, POU-domain protein, clone 5A3.2 | MilliporeSigma | Cat#MAB1585; RRID:AB_94166 |
| Anti Iba1, Rabbit (for Immunocytochemistry) | FUJIFILM Wako Pure Chemical Corporation | Cat#019-19741; RRID:AB_839504 |
| Anti-P2ry12, Rabbit polyclonal | Butovsky Lab, validated in (Butovsky et al., 2015; Butovsky et al., 2014) | N/A |
| Anti-Apolipoprotein E Antibody | Sigma-Aldrich | Cat#AB947; RRID:AB_10770246 |
| Purified Mouse Anti-Human Galectin-3, Clone B2C10 (RUO) | BD Biosciences | Cat#556904; RRID:AB_396531 |
| GFP Polyclonal Antibody | Thermo Fisher Scientific | Cat#A11122; RRID:AB_221569 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat#A-11005; RRID:AB_2534073 |
| Chicken anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21441; RRID:AB_2535859 |
| Rabbit anti-Goat IgG (H+L), Superclonal™ Recombinant Secondary Antibody, Alexa Fluor 555 | Thermo Fisher Scientific | Cat#A27017; RRID:AB_2536081 |
| Rat-monoclonal anti-FCRLS (clone 4G11) | Butovsky lab, validated in (Butovsky et al., 2015; Butovsky et al., 2014) | N/A |
| PerCP/Cyanine5.5 anti-mouse Ly-6C antibody (clone HK1.4) | Biolegend | Cat#128012; RRID:AB_1659241 |
| CD11b Monoclonal Antibody (M1/70), PE-Cyanine7, eBioscience™ | Thermo Fisher Scientific | Cat#25-0112-82; RRID:AB_469588 |
| Cy™3 AffiniPure Donkey Anti-Rabbit IgG (H+L) | Jackson ImmunoResearch Labs | Cat#711-165-152; RRID:AB_2307443 |
| Cleaved Caspase-3 (Asp175) Antibody | Cell Signaling Technology | Cat#9661; RRID:AB_2341188 |
| Anti-IBA1 antibody, Guinea pig | Synaptic Systems | Cat#234 004; RRID:AB_2493179 |
| Anti-TMEM119 antibody, Rabbit | Atlas Antibodies | Cat#HPA051870; RRID:AB_2681645 |
| Galectin-3 Polyclonal antibody, Goat | R&D Systems | Cat#AF1197SP; RRID: AB_2234687 |
| Chicken-anti-rabbit AlexaFluor 647 | Thermo Fisher Scientific | Cat#A-21443; RRID:AB_2535861 |
| Donkey-guineapig AlexaFluor 488 | Jackson ImmunoResearch Labs | Cat#706-545-148; RRID:AB_2340472 |
| Donkey anti-mouse AlexaFluor 488 | Thermo Fisher Scientific | Cat#A-21202; RRID: AB_141607 |
| Biological Samples | ||
| Human eye specimens | Pathology Department, Duke University, See Table S3 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Anhydrous DMSO | Thermo Fisher Scientific | Cat#D12345 |
| Dynabeads™ M-450 Tosylactivated | Thermo Fisher Scientific | Cat#14013 |
| Karnovsky Solution: 16% paraformaldehyde 25% Glutaraldehyde 0.2 M Sodium Cacodylate Buffer CaCl2 (calcium chloride, anhydrous) 1N NaOH |
Electron Microscopy Sciences Electron Microscopy Sciences Electron Microscopy Sciences Thermo Fisher Scientific Electron Microscopy Sciences |
Cat#15710 Cat#16220; CAS#111-30-8 Cat#11653, pH 7.4 Cat#C-76 Cat.#21170-01; CAS# 1310-73-2 |
| Tamoxifen | Sigma-Aldrich | Cat#T5648 |
| TritonX-100 | Roche | Cat#11332481001 |
| Normal Horse Serum | Thermo Fisher Scientific | Cat#NC9909742 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat#A3294 |
| VectaShield with DAPI | Vector Laboratories | Cat#H-1200 |
| Alexa Fluor488 5-SDP Ester | Thermo Fisher Scientific (Molecular Probes) | Cat#A30052 |
| Alexa Flour405 5-NHS Ester | Thermo Fisher Scientific (Molecular Probes) | Cat#A30000 |
| DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) | Thermo Fisher Scientific | Cat#D1306 |
| True Black Lipofuscin Autofluorescence Quencher | Biotium | Cat#23007 |
| Galectin-3 inhibitor (TD139) | Cayman Chemicals | CAS #1450824-22-2 |
| O.C.T. Compound | Tissue-Tek® | Cat#4583 |
| SuperBlock™ (TBS) Blocking Buffer | Thermo Fisher Scientific | Cat#37535 |
| MAXblocks™ Blocking Medium | Active Motif | Cat#15252 |
| Deposited Data | ||
| RNAseq of retinal microglia from microbead-injected wildtype (C57BL/6J) mice | NCBI Gene Expression Omnibus (GEO) | GSE192509 |
| RNAseq of retinal microglia from DBA/2J mice and controls | NCBI Gene Expression Omnibus (GEO) | GSE192505 |
| RNAseq of retinal microglia from microbead-injected Apoe−/− and wildtype mice | NCBI Gene Expression Omnibus (GEO) | GSE192508 |
| RNAseq of phagocytic and nonphagocytic retinal microglia from Apoe−/− and wildtype mice following intravitreal injection of apoptotic neurons | NCBI Gene Expression Omnibus (GEO) | GSE192506 |
| RNAseq of retinal microglia from microbead-injected humanized APOE3 and APOE4 mice | NCBI Gene Expression Omnibus (GEO) | GSE192507 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664 |
| Mouse: Cx3cr1Cre/+ | The Jackson Laboratory | JAX: 025524 |
| Mouse: Cx3cr1CreERT2/+ | The Jackson Laboratory | JAX: 021160 |
| Mouse: Apoe−/− | The Jackson Laboratory | JAX: 002052 |
| Mouse: Lgals3−/− | The Jackson Laboratory | JAX: 006338 |
| Mouse: DBA/2J | The Jackson Laboratory | JAX: 000671 |
| Mouse: DBA/2J Gpnmb+:DBA/2J-Gpnmb+/SjJ | The Jackson Laboratory | JAX: 007048 |
| Mouse: Apoefl/fl | (Wagner et al., 2015) | N/A |
| Mouse: APOE2 | Taconic Biosciences | Taconic: 1547 |
| Mouse: APOE3 | Taconic Biosciences | Taconic: 1548 |
| Mouse: APOE4 | Taconic Biosciences | Taconic: 1549 |
| Mouse: APOE3fl/fl | Cure Alzheimer’s Fund | https://www.alzforum.org/research-models/apoe3-knock-floxed-curealz |
| Mouse: APOE4fl/fl | Cure Alzheimer’s Fund | https://www.alzforum.org/research-models/apoe4-knock-floxed-curealz |
| Software and Algorithms | ||
| FIJI (ImageJ) | NIH | https://imagej.net/Fiji |
| R software (v4.0.3) | N/A | https://cran.r-project.org/ |
| GraphPad Prism 7 | GraphPad Software | https://www.graphpad.com |
| BioRender | BioRender Software | https://biorender.com/ |
| Salmon (v1.4.0) | N/A | https://github.com/COMBINE-lab/salmon |
| Ensembl release 101 | EMBL-EBI | http://aug2020.archive.ensembl.org/index.html |
| DESeq2 (v1.30.1) | N/A | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| pheatmap (v1.0.12) | N/A | https://cran.r-project.org/web/packages/pheatmap/index.html |
| EnhancedVolcano (v1.8) | N/A | https://github.com/kevinblighe/EnhancedVolcano |
| ggplot2 | N/A | https://cran.r-project.org/web/packages/ggplot2/index.html |
| Ingenuity software | Ingenuity Systems | https://www.ingenuity.com |
| Zen Black | Zeiss | https://www.zeiss.com/ |
| Leica application suite software (LAS-AF-lite) | Leica | http://www.leica-microsystems.com |
| Nikon E800 software: DP Controller: 3. 3. 1.292 DP Manager version 3,3,1,222 |
Olympus Life Science Solutions | https://www.olympus-lifescience.com/en/support/downloads/ |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6J, Cx3cr1Cre/+, Cx3cr1CreERT2/+, Apoe−/−, Lgals3−/−, DBA/2J and DBA/2J Gpnmb+ mice were purchased from JAX. Generation of Apoefl/fl mice has been described in (Wagner et al., 2015) and these mice were crossed with Cx3cr1Cre/+ or Cx3cr1CreERT2/+ mice to target Apoe in long-lived myeloid cells. Homozygous APOE2, APOE3, and APOE4 mice, in which endogenous murine Apoe was replaced with the humanized isoforms, were purchased from Taconic Biosciences. APOE3fl/fl and APOE4fl/fl mice were generously provided by the Cure Alzheimer’s Fund and were crossed with Cx3cr1CreERT2/+ mice to target APOE3 and APOE4 in long-lived myeloid cells. If not otherwise stated in the figure legend, mice were a mix of both genders and 6–12 weeks of age at the beginning of the experiments. Mice were matched in terms of gender and age between experimental groups prior to the start of the experiment. Mice were housed under specific pathogen free conditions with food and water ad libitum. Mice did not undergo any procedures prior to their stated use. Mice were euthanized by CO2 inhalation followed by cervical dislocation. The Institutional Animal Care and Use Committee at Harvard Medical School, Brigham and Women’s Hospital, and MEEI Schepens Eye Research Institute approved all experimental procedures involving animals.
Human
Autopsy eyes from 23 patients (11 controls and 12 glaucoma patients) were analyzed (see Table S2 for cohort details). Eye sections and formalin-fixed paraffin-embedded (FFPE) tissue blocks for genotyping were kindly provided by Dr. Alan Proia from the Pathology Department at Duke University. The use of autopsy eyes for research was approved by the Institutional Review Board of Duke University (Pro00083250) and Mass General Brigham (2013P000031) and followed the tenets of the Declaration of Helsinki. As the subjects were deceased and research consent was provided for the autopsy specimens, additional informed consent was not required. To be classified as having glaucoma, patients had to have the diagnosis of glaucoma (as listed in the autopsy report and abstracted from their medical record) and presence of histological changes characteristic of glaucoma. Glaucoma cases and controls were matched in terms of age, sex, and race (as defined in the autopsy report). Eyes with evidence of secondary glaucoma, prior glaucoma surgery, or other significant ocular comorbidities (e.g., moderate or severe diabetic retinopathy, uveitis, advanced age-related macular degeneration) were excluded from the study. In addition, eyes with post-mortem intervals to tissue fixation greater than 72 hours were excluded. Tissues were fixed and embedded in paraffin as described in (Margeta et al., 2018) and paraffin sections were cut at a thickness of 5 μm. Following deparaffination, tissue sections were stained as described in the immunohistochemistry section. For APOE genotyping, DNA extraction from FFPE tissue blocks was conducted at the Genotyping Core Facility at the Harvard T.H. Chan School of Public Health and a TaqMan SNP genotyping assay (rs429358 and rs7412) was conducted at the Partners Personalized Medicine Translational Genomics Core.
METHOD DETAILS
Immunohistochemistry
Mice were euthanized via CO2 inhalation. Cardiac perfusion was performed prior to all Galectin-3 staining to minimize detection of endogenous mouse immunoglobulins. Eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4°C. Following fixation, retinas were dissected from the eyecup and transferred to cold methanol in 1.5 mL Eppendorf tubes for 20 minutes on ice. Retinas were then washed with PBS 0.3% triton, permeabilized by freezing at −80°C for 15 minutes in PBS 0.3% triton and washed again with PBS 0.3% triton. Next, the tissue was blocked for 1 hour at room temperature in blocking buffer (5% NHS, 0.2% BSA, 0.3% Triton-X100 in PBS) and incubated in primary antibody in blocking buffer 1:200 overnight at 4°C. Primary antibody combinations used were rabbit anti-Iba1 (#019–19741, WAKO Chemicals) and goat anti-Apoe (#AB947, Sigma); rabbit anti-P2ry12 [0.4 μg ml−1, Butovsky lab, validated in ref. (Butovsky et al., 2015; Butovsky et al., 2014)] and goat anti-Apoe; rabbit anti-Iba1 and mouse anti-Galectin-3 (#556904, BD Biosciences); rabbit anti-P2ry12 and mouse anti-Galectin-3; rabbit anti-Iba1, mouse anti-Galectin-3, and goat anti-Apoe; rabbit anti-P2ry12, mouse anti-Galectin-3, and goat anti-Apoe; or rabbit anti-GFP (#A-11122, Invitrogen). After primary antibody incubation, retinas were washed 3 X 10 minutes with PBS 0.3% triton and incubated in secondary antibody in blocking buffer 1:400 for 2 hours at room temperature. Secondary antibodies used were goat anti-mouse Alexa 594 (#A-11005, Thermo Fisher) for Brn3a and Galectin-3, chicken anti-rabbit Alexa 488 (#A-21441, Thermo Fisher) for Iba1, P2ry12 and GFP, and rabbit anti-goat Superclonal Alexa 555 (#A-27017, Thermo Fisher) for Apoe. For triple staining of Iba1 or P2ry12, Gal-3, and Apoe, secondary antibodies used were chicken anti-rabbit Alexa 647 (#A-21443, Thermo Fisher) for Iba1 or P2ry12, donkey anti-mouse Alexa 488 (#A-21202, Thermo Fisher) for Galectin-3, and rabbit anti-goat Superclonal Alexa 555 for Apoe. After secondary antibody incubation, retinas were washed again with PBS 0.3% triton, rinsed well with PBS, and mounted vitreous side up using VectaShield with DAPI mounting medium. Of note, for Brn3a staining of retinal ganglion cells, all washes following permeabilization at −80°C were performed using PBS without triton, the primary antibody (#MAB1585, Millipore Sigma) incubation was conducted 1:200 for 4–6 days at 4°C, and the secondary antibody incubation was conducted 1:400 for 48 hours at 4°C. For cleaved caspase staining to assess cell death across retinal layers following adoptive transfer of MGnD microglia, host eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4°C. The fixed eyeballs were transferred to 20% sucrose in PBS at room temperature, embedded in O.C.T. compound (Tissue-Tek; Sakura, Torrance, CA, USA) and cryosectioned at 10 μm. Sections were then mounted on slides, washed with washing buffer (Tween 200 0.05%, PBS) to remove residual O.C.T. compound, blocked with (3% BSA, 1%Triton, PBS) for 1 hour at room temperature, and incubated with rabbit anti-cleaved caspase primary antibody (#9661, Cell Signaling Technology) in blocking buffer (5% NHS, 0.2% BSA, 0.3% Triton-X100 in PBS) 1:100 for 48 hours at 4C. Following primary antibody incubation, sections were washed with PBS and incubated with donkey anti-rabbit Cy3 (#711–165-152, Jackson ImmunoResearch) in blocking buffer (5% NHS, 0.2% BSA, 0.3% Triton-X100 in PBS) 1:1000 for 2 hours at room temperature. Sections were washed again in PBS and mounted using VectaShield with DAPI mounting medium.
For immunohistochemistry of human paraffin-embedded samples, slides were heated for 30 minutes at 60°C, deparaffinized in xylol and rehydrated. After heat-induced antigen retrieval (Citrate buffer pH 6, heated for approx. 30 minutes), slides were permeabilized with 0.2% triton in TBS (TBS-T) for 10 minutes, washed in DI water, and blocked for 1 hour at room temperature (WB blocking buffer, Thermo Fisher). For IBA1 and APOE staining, slides were incubated with rabbit anti-IBA1 primary antibody (#019–19741, WAKO Chemicals) 1:200 in blocking buffer at 4°C overnight, washed with TBS-T, and incubated with chicken anti-rabbit Alexa 488 secondary antibody (#A-21441, Thermo Fisher) 1:400 for 1.5 hours at room temperature and washed again with TBS-T. Slides were then incubated with goat anti-APOE (#AB947, Sigma) 1:200 overnight at 4°C, washed with TBS-T, incubated with rabbit anti-goat Superclonal Alexa 555 (#A-27017, Thermo Fisher) 1:400 for 1.5 hours at room temperature, and thoroughly washed with TBS-T. Finally, slides were embedded using VectaShield with DAPI and imaged using Zeiss Confocal LSM 710. The number and extent of colocalization of IBA1+ and APOE+ cells were quantified using ImageJ in a blinded fashion. For staining with TMEM119 or GAL-3, deparaffinized and antigen retrieved sections were permeabilized with 0.2% triton in PBS (PBS-T) for 10 minutes, washed in DI water, and blocked for 1 hour at room temperature (MAXblock™ Blocking Medium, Active Motif). Slides were incubated with guinea pig anti-IBA1 (#234 004, Synaptic Systems) and rabbit anti-TMEM119 primary antibodies (#HPA051870, Atlas) 1:200 and 1:100, respectively, in staining buffer (MAXbind™ Staining Medium, Active Motif) at 4°C overnight, washed with PBS-T, and incubated with donkey anti-guinea pig 488 (#706–545-148, Jackson ImmunoResearch) and chicken anti-rabbit Alexa 647 (#A-21443, Thermo Fisher) secondary antibodies each 1:200 for 1.5 hours at room temperature and washed again with PBS-T. Slides were then incubated with goat anti-APOE (#AB947, Sigma) 1:200 overnight at 4°C, washed with PBS-T, incubated with rabbit anti-goat Superclonal Alexa 555 secondary antibody (#A-27017, Thermo Fisher), and thoroughly washed with PBS-T. Alternatively, slides were incubated with rabbit anti-IBA1 primary antibody (#019–19741, WAKO Chemicals) 1:500 in staining buffer (MAXbind™ Staining Medium, Active Motif) at 4°C overnight, washed with PBS-T, and incubated with chicken anti-rabbit Alexa 488 secondary antibody (#A-21441, Thermo Fisher) 1:200 for 1.5 hours at room temperature and washed again with PBS-T. Slides were then incubated with goat anti-GAL-3 (#AF1197SP, R&D Systems) 1:200 overnight at 4°C, washed with PBS-T, incubated with rabbit anti-goat Superclonal 555 secondary antibody (#A-27017, Thermo Fisher), and thoroughly washed with PBS-T. Before embedding, sections were treated with True Black (Lipofuscin Autofluorescence Quencher, Biotium) following the manufacturer’s instructions. Finally, slides were embedded using Fluoromount with DAPI and imaged using Leica SP5 Confocal microscope (UMIF; UKE). The number and extent of colocalization of IBA1+ and GAL-3+ cells were quantified in a blinded fashion.
Retinal Ganglion Cell Quantification
Flatmounted retinas were imaged using Zeiss LSM 710 Confocal Microscope. Images were taken at 63x using an oil immersion objective, and 12 images were collected per sample (3 images per quadrant in retinal mid-periphery). Brn3a+ DAPI+ double positive cells were manually counted using ImageJ. RGC count per sample was averaged over the 12 images and converted to cells/mm2.
PPD staining of optic nerves
Optic nerves were dissected and fixed at 4°C with half strength Karnovsky’s fixative (2% paraformaldehyde + 2.5% glutaraldehyde, in 0.1M sodium cacodylate buffer, pH 7.4). Nerves were stored in fixative until tissue processing at the Morphology Core Lab at MEEI Schepens Eye Research Institute. After fixation, samples were rinsed with 0.1M sodium cacodylate buffer, post-fixed with 2% osmium tetroxide in 0.1M sodium cacodylate buffer, then dehydrated with graded ethyl alcohol solutions, transitioned with propylene oxide and resin infiltrated in tEPON-812 epoxy resin (Tousimis, Rockville, Maryland) utilizing an automated EMS Lynx 2 EM tissue processor (Electron Microscopy Sciences, Hatfield, Pennsylvania). Processed tissues were oriented in tEPON-812 epoxy resin and polymerized in silicone molds using an oven set for 60oC for 48 hours. Semi-thin cross-sections were cut at 1-micron with a Histo diamond knife (Diatome, Hatfield, Pennsylvania) on a Leica UC-7 ultramicrotome (Leica Microsystems, Buffalo Grove, IL) and collected on slides then dried on a slide warmer. The slides were stained with 1% paraphenylenediamine (MP Biomedicals LLC, Solon, Ohio) in 50% methanol and 50% isopropanol solution for 1 hour at room temperature, rinsed in three changes of 50% methanol and 50% isopropanol solution for 3 × 1 minutes, then rinsed in 100% ethyl alcohol for 1 minute, air-dried then Permount mountant media (Electron Microscopy Sciences, Hatfield, Pennsylvania) and a glass coverslip was applied over the sections for light microscopic analysis of myelinated axon analysis.
Optic nerve imaging and quantification
Slides were imaged using Nikon E800 Microscope with consistent settings across all images. 6 images were obtained at 60x using an oil immersion objective for quantification such that the entire optic nerve was captured. A representative image was obtained at 100x in the center of the nerve. Quantification was performed using ImageJ software by selecting and cropping a 100 μm × 100 μm square within each 60x image and counting the axons using the automatic Analyze Particles function. Axon count for each sample was obtained by averaging 6 images and converted to axons/mm2.
Conditional deletion of microglial Apoe
To induce Cre-recombinase expression, a dose of tamoxifen (150 mg/kg of body weight) in corn oil (Sigma) was injected intraperitoneally (i.p.) for 2 consecutive days. For genetic depletion of murine Apoe in microglia and peripheral myeloid cells, Apoefl/fl mice were crossed with tamoxifen inducible Cx3cr1CreERT2/+ transgenic mice; for genetic depletion of humanized APOE isoforms from microglia and peripheral myeloid cells, Cx3cr1CreERT2/+:APOE3fl/fl and Cx3cr1CreERT2/+:APOE4fl/fl mice were used. To achieve microglia-specific targeting of Apoe and APOE isoforms at the time of glaucoma induction, 30 days were allotted between tamoxifen administration and magnetic microbead injection to allow for repopulation of peripheral myeloid cells (Bruttger et al., 2015; Krasemann et al., 2017; Parkhurst et al., 2013).
Magnetic Microbead Injection
Microbead injection was performed as previously described (Chen et al., 2011; Ito et al., 2016; Sappington et al., 2010). Briefly, mice were anesthetized by i.p. injection of a mixture of ketamine (100 mg kg−1) and xylazine (10 mg kg−1) and pupils dilated with 1% tropicamide. A small puncture was made in the cornea using a 30-gauge needle. Eyes were injected with 1.5 μL of magnetic microbead solution (2.4 × 106 beads) or PBS for sham injections. All injections were done in the left eye. Beads were attracted to and evenly distributed around the anterior chamber using a small magnet, and eyes were treated with antibiotic eyedrops to reduce risk of infection. Intraocular pressure was monitored as described below.
IOP Measurement
IOP was measured 24 hours after the microbead injection, and then twice a week using a tonometer (TonoLab; Icare, Finland). Mice were anesthetized by isoflurane inhalation (2% to 4% flow). Measurements were conducted at consistent times in the morning and were performed for one month following the microbead injection. The tonometer records six measurements after excluding outlying values and displays an average. The Tonolab-generated average was considered one value, and we recorded five values per eye. The mean of these five values determined the IOP measurement.
Electroretinogram
Adult mice were adapted in a dark room overnight. Mice were anesthetized by i.p. injection of ketamine (100–200 mg/kg) and xylazine (20 mg/kg). Both pupils were dilated with 1% tropicamide, followed by a drop of Genteal to keep the corneas moist. Mice were placed on a heating pad in the Ganzfield bowl to maintain body temperature during the procedure. Two recording electrodes were placed on the corneas, while the reference and ground electrodes were inserted beneath the skin over the cerebellum and tail, respectively. Scotopic threshold response was recorded using flash intensity of 17.65 E-5(P)cd.s/m2. After the ERG recording was complete, the mice were returned to their cages and transferred to a heating pad for recovery until fully sternal. ERG measurements were obtained at baseline and one month after microbead injection.
Mouse microglia isolation and sorting
Microglia isolation was performed according to our previously described protocol (Butovsky et al., 2014; Krasemann et al., 2017). Briefly, mice were euthanized using CO2, eyes removed, and retinas dissected. Single-cell suspensions were prepared and centrifuged over a 37%/70% discontinuous Percoll gradient (GE Healthcare), and mononuclear cells were isolated from the interface. To distinguish resident microglia from recruited myeloid cells, we used a monoclonal antibody that recognizes Fcrls, which is expressed on microglia but not on infiltrating myeloid cells (Butovsky et al., 2014). Isolated cells were stained with anti-Fcrls [clone 4G11, 3 μg ml−1, Butovsky lab, validated in ref. (Butovsky et al., 2015; Butovsky et al., 2014)], CD11b-PeCy7 [clone M1/70, BD Biosciences, 2 μg ml−1] and Ly6C-PerCP/Cy5.5 [clone HK1.4, BioLegend, 2 μg ml−1] antibodies to specifically sort resident microglia as CD11b+, Ly6C−, and Fcrls+ cells. 100 to 600 cells were collected from each retina, normalized to 100 to 300 cells per 5 μl of TCL buffer, and submitted for RNAseq.
Preparation of apoptotic primary neurons
For detailed description of isolation of primary neurons, induction of apoptosis and labeling of neurons, see (Krasemann et al., 2017). Briefly, cerebral hemispheres were removed from embryos at age E18.5, digested, triturated, and seeded on DMEM supplemented with 10% FBS. Media was changed every three days by removing half volume and replacing with 1 × B27 (Invitrogen) in Neurobasal. 7–10 days after culture, neurons were detached from culture plates with PBS washes and apoptosis was induced by UV irradiation with an intensity of 6 × 15 W for 15 min. Neurons were then labeled with fluorescent dye (Alexa488 5-SDP Ester or Alexa405 NHS Ester, Life Technologies/Thermo Fisher Scientific) by incubating for 15 minutes at 37°C and total apoptotic cell number was determined using Trypan Blue staining. Neurons were resuspended at a density of approximately 5×104 cells per 2 μl for stereotactic injection.
Intravitreal injection of apoptotic neurons
To isolate phagocytic and non-phagocytic microglia from the retina following intravitreal injection of apoptotic neurons, neurons were prepared as described above and labeled using AF488 dye. Recipient mice were anesthetized by i.p. injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1) and pupils were dilated with 1% tropicamide. A 30-gauge needle was used to perform the initial puncture just posterior to the limbus, with care being taken to avoid injuring the lens. 2 μl of labeled apoptotic neurons were loaded into a 10 μl Nanofil microsyringe and injected intravitreally into C57BL/6J and Apoe−/− recipients. 16 to 24 hours after the injection, mice were sacrificed by CO2 inhalation. Microglia isolation was performed according to our previously described protocol (Butovsky et al., 2014). Briefly, eyes were removed from mice, retinas dissected and microglia isolated by FACS sorting as described above. 5–6 retinas were pooled per sample according to sex and genotype to increase the yield of phagocytic microglia (100–500 phagocytic cells per sample). Microglia were isolated as CD11b+/Ly6C−/Fcrls+ cells, and phagocytic versus non-phagocytic microglia were further sorted from the CD11b+/Fcrls+ population by detection of Alexa488 or Alexa405 fluorescence.
Intravitreal transfer of MGnD microglia
To determine whether adoptive intravitreal transfer of MGnD microglia is sufficient to induce RGC apoptosis, phagocytic and non-phagocytic microglia were isolated from the brains of Cx3cr1CreERT2/+, C57BL/6J, or Apoe−/− donor mice and transferred to the vitreous of C57BL/6J recipients. For this experiment, apoptotic neurons were cultured and prepared (UV irradiation and fluorescent labeling) as described above using AF405 dye. Donor mice were anesthetized by i.p. injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1) and 2 μl of labeled apoptotic neurons were injected intrahippocampally and intracortically bilaterally (Y: ± 1.5mm; X: −2mm; Z: −2mm and −1mm) using stereotaxic equipment (Harvard Apparatus). After recovery from surgery, animals were returned to their home cages. 16–24 hours post-surgery mice were euthanized by CO2 inhalation, perfused with ice cold HBSS and brains dissected. To isolate phagocytic and non-phagocytic microglia from the brain, approximately 3×4×4 mm of tissue per hemisphere surrounding the apoptotic neuron injection site was dissected and processed in the same way as described above for retinas, following procedures detailed in (Butovsky et al., 2014; Krasemann et al., 2017). Microglia were isolated as CD11b+/Ly6C−/Fcrls+ cells and further sorted from the CD11b+/Fcrls+ population by detection of Alexa405 fluorescence. Once isolated, cells were spun down at 1200 rpm for 7 minutes at 4°C and resuspended in PBS at a concentration of 2500 cells/μl. C57BL/6J recipient mice were anesthetized as described previously and eyes dilated with 1% tropicamide. Mice received 2 μl of either phagocytic microglia, non-phagocytic microglia, or PBS via intravitreal injection performed as described above. One month after injection, mice were sacrificed by CO2 inhalation and eyes collected for immunohistochemistry analysis. This experiment was performed using Cx3cr1CreERT2/+ donor mice (which express YFP under the Cx3cr1 promoter) and C57BL/6J recipients and repeated with C57BL/6J and Apoe−/− donor mice and C57BL/6J recipients, with all experimental parameters kept consistent.
Intravitreal injection of TD139
Galectin-3 inhibitor TD139 (#28400, CAS #1450824–22-2, Cayman Chemicals) stock was prepared by dissolving the solid compound in DMSO to 1000 ng μl−1. Stock was diluted in PBS to a final concentration of 50 ng μl−1 in 5% DMSO/PBS. At two timepoints (2 and 3 weeks after microbead injection), mice were anesthetized as described above and administered 1 μl of Galectin-3 inhibitor or 5% DMSO/PBS vehicle in the microbead-injected eye via intravitreal injection. Sham-injected eyes were treated intravitreally with vehicle. One month after microbead injection, mice were sacrificed by CO2 inhalation and eyes were collected for immunohistochemistry and RGC quantification. Toxicity was assessed prior to the experiment to confirm that retinal ganglion cell density is not affected by TD139 or vehicle treatment under control conditions. This experiment was first performed on C57BL/6J mice and then repeated with matched cohorts of APOE3 and APOE4 humanized mice.
RNA sequencing
Samples were processed according to the Smart-Seq2 protocol and sequenced on Illumina sequencers. Reads in FASTQ were quantified at the transcript level using Salmon (v1.4.0) against the M. musculus. Ensembl gene catalog (August 2020). Differential expression analysis was conducted using R (v4.0.3) and DESeq2 (v1.30.1). Hypothesis testing was performed using either DESeq2’s Wald test on appropriate variable contrasts or with a Likelihood Ratio Test (LRT) for models with greater than two variable conditions being tested. Adjusted P-values were determined using the Benjamini-Hochberg procedure and significance cutoffs were set at 0.05.
Plots
Clustered heatmaps were generated in R using the pheatmap package (v1.0.12). For clustering, the z-scores were calculated using the mean expression of biological replicates per disease stage/condition and then subsequently clustered using K-means. Volcano plots were generated in R using EnhancedVolcano (v1.8). Assorted column, dot, and scatter plots were created with ggplot2 within R.
Ingenuity Pathway Analysis
Data were analyzed using Ingenuity software (Ingenuity Systems, http://www.ingenuity.com). Differentially expressed genes (with corresponding n-fold changes and P-values) were incorporated in canonical pathways and bio-functions and were used for generating biological networks as described previously (Butovsky et al., 2014). Uploaded dataset for analysis was filtered using the following cutoff definitions: fivefold change, P < 0.01.
QUANTIFICATION AND STATISTICAL ANALYSIS
Quantification and statistical analysis
Statistical analysis was done using GraphPad Prism 7 software. Data distribution was assumed to be normal, but this was not formally tested. Data are presented as mean ± SEM and two-tailed Student’s t tests (unpaired) or ANOVA multiple comparison tests were used to assess statistical significance. Mean fluorescence intensity (MFI) analysis of Galectin-3 expression in human and Apoe expression in mouse was performed by quantifying average pixel intensity of Apoe or Galectin-3 within the Iba1+ or P2ry12+ region of interest (ROI) and is reported in arbitrary units (a.u.). Threshold settings for the Iba1+ or P2ry12+ microglial ROI were consistent across all images. Data collection and analysis were blindly performed. Data for each experiment were collected and processed randomly and animals were assigned to various experimental groups randomly as well. All n and P-values and statistical tests are indicated in figure legends. P < 0.05 was considered statistically significant.
Supplementary Material
Table S1: RNAseq data of Fcrls+ retinal microglia from microbead-injected eyes and controls and DBA/2J eyes and controls. Related to Figures 1 and S2.
Table S2: Demographic, clinical, and pathologic characteristics of glaucoma cases and controls. Related to Figures 1 and 7.
Table S3: RNAseq data of Fcrls+ retinal microglia from microbead-injected wildtype and Apoe−/− animals. Related to Figure 3.
Table S4: RNAseq data of Fcrls+ phagocytic and non-phagocytic retinal microglia from wildtype and Apoe−/− animals injected intravitreally with apoptotic neurons. Related to Figure S5.
Table S5: RNAseq data of Fcrls+ retinal microglia from microbead-injected APOE3 and APOE4 animals. Related to Figure 7.
Highlights.
Microglia switch from a homeostatic to a neurodegenerative phenotype in glaucoma
Apoe and APOE3 induce Galectin-3, a key effector of neurodegenerative microglia
Apoe−/− and APOE4 retinal microglia have a decreased response to neurodegeneration
APOE4 allele protects against retinal ganglion cell (RGC) loss in glaucoma
Acknowledgments
This study was supported by the Cure Alzheimer’s Fund (to OB and DMH); BrightFocus Foundation 2020A016806 (OB); NIH/NINDS R01 NS088137 (OB), NIH/NIA R01 AG051812 (OB) and R01 AG054672 (OB); NIH/NEI R01 EY027921 (OB); NIH/NEI K12 EY016335 (MAM), NIH/NEI K08 EY030160 (MAM), American Glaucoma Society Young Clinician Scientist Award (MAM), Research to Prevent Blindness Career Development Award (MAM), Robert M. Sinskey Foundation (MAM). Additional funding: R01 GM132668 (OB); R21 NS104609 (OB), R21 NS101673 (OB); National Multiple Sclerosis Society (5092A1) (OB); National Health and Medical Research Council Australia (RG180378) (OB); Nancy Davis Foundation innovative Award (OB); Amyotrophic Lateral Sclerosis Association Award (OB), NIH/NEI EY025913 (DFC), NIH/NEI EY025259 (DFC), Massachusetts Eye and Ear Summit Fund (DFC), NIH/NEI 5K23EY026988 (EML), Research to Prevent Blindness Ernest & Elizabeth Althouse Special Scholar Award (EML) and Core Grant for Vision Research from NIH/NEI to the Schepens Eye Research Institute (P30EY03790). We thank Lai Ding and the NeuroTechnology Studio at BWH for providing confocal instrument access and consultation on data acquisition and analysis and the UMIF/UKE Hamburg for access to the SP5 confocal microscope.
Footnotes
Declaration of Interests
O.B. and M.A.M. are co-inventors of a patent for the use of Galectin-3 inhibitors for the treatment of glaucoma. O.B.: collaboration with Sanofi, GSK, Regulus Therapeutics; research funding from Sanofi, GSK, miRagen Therapeutics, honoraria for lectures, consultancy: GSK, Camp4. E.M.L and A.D.P.: research funding from Novartis Institutes for BioMedical Research and F. Hoffmann LaRoche.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu CA, De Lima SV, Mendonca HR, Goulart CO, and Martinez AM (2017). Absence of galectin-3 promotes neuroprotection in retinal ganglion cells after optic nerve injury. Histol Histopathol 32, 253–262. [DOI] [PubMed] [Google Scholar]
- Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, and Mitchell CH (2017). The P2X7 Receptor Primes IL-1beta and the NLRP3 Inflammasome in Astrocytes Exposed to Mechanical Strain. Front Cell Neurosci 11, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasieh M, and Levin LA (2017). Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu Rev Vis Sci 3, 91–120. [DOI] [PubMed] [Google Scholar]
- Alqawlaq S, Flanagan JG, and Sivak JM (2019). All roads lead to glaucoma: Induced retinal injury cascades contribute to a common neurodegenerative outcome. Exp Eye Res 183, 88–97. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Nair KS, Amonoo LA, Mehalow A, Trantow CM, Masli S, and John SW (2008). GpnmbR150X allele must be present in bone marrow derived cells to mediate DBA/2J glaucoma. BMC Genet 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, and John SW (2002). Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet 30, 81–85. [DOI] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, and Ikezu T (2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18, 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, Bennett ML, Yaqoob F, Mulinyawe SB, Grant GA, Hayden Gephart M, Plowey ED, and Barres BA (2018). A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 98, 1170–1183 e1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Anderson SR, Breen KT, Romero CO, Steele MR, Chiodo VA, Boye SL, Hauswirth WW, Tomlinson S, and Vetter ML (2018). Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol Ther 26, 2379–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, and Vetter ML (2008). Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci 49, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, and Vetter ML (2015). Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis Model Mech 8, 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco A, Steele MR, and Vetter ML (2011). Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol 519, 599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boza-Serrano A, Ruiz R, Sanchez-Varo R, Garcia-Revilla J, Yang Y, Jimenez-Ferrer I, Paulus A, Wennstrom M, Vilalta A, Allendorf D, et al. (2019). Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol 138, 251–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, et al. (2015). Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 43, 92–106. [DOI] [PubMed] [Google Scholar]
- Bui BV, and Fortune B (2004). Ganglion cell contributions to the rat full-field electroretinogram. J Physiol 555, 153–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguillos MA, Svensson M, Schulte T, Boza-Serrano A, Garcia-Quintanilla A, Kavanagh E, Santiago M, Viceconte N, Oliva-Martin MJ, Osman AM, et al. (2015). Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep 10, 1626–1638. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, Greco DJ, Wu PM, Doykan CE, Kiner O, et al. (2015). Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 77, 75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. (2014). Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, and Weiner HL (2018). Microglial signatures and their role in health and disease. Nat Rev Neurosci 19, 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Alvarado G, Bigcas JL, and Li W (2012). Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol 227, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagno KE, Lu W, Jassim AH, Albalawi F, Cenaj A, Tso HY, Clark SP, Sripinun P, Gomez NM, and Mitchell CH (2021). Rapid morphologic changes to microglial cells and upregulation of mixed microglial activation state markers induced by P2X7 receptor stimulation and increased intraocular pressure. J Neuroinflammation 18, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wei X, Cho KS, Chen G, Sappington R, Calkins DJ, and Chen DF (2011). Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci 52, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Cao Z, Leffler H, Nilsson UJ, and Panjwani N (2017). Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis. Invest Ophthalmol Vis Sci 58, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow G, Ebneter A, Wood JP, and Casson RJ (2016). Evidence Supporting an Association Between Expression of Major Histocompatibility Complex II by Microglia and Optic Nerve Degeneration During Experimental Glaucoma. J Glaucoma 25, 681–691. [DOI] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, and Butovsky O (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva Vargas JL, Belforte N, and Di Polo A (2016). The glial cell modulator ibudilast attenuates neuroinflammation and enhances retinal ganglion cell viability in glaucoma through protein kinase A signaling. Neurobiol Dis 93, 156–171. [DOI] [PubMed] [Google Scholar]
- Fortune B, Bui BV, Morrison JC, Johnson EC, Dong J, Cepurna WO, Jia L, Barber S, and Cioffi GA (2004). Selective ganglion cell functional loss in rats with experimental glaucoma. Invest Ophthalmol Vis Sci 45, 1854–1862. [DOI] [PubMed] [Google Scholar]
- Gratuze M, Chen Y, Parhizkar S, Jain N, Strickland MR, Serrano JR, Colonna M, Ulrich JD, and Holtzman DM (2021). Activated microglia mitigate Abeta-associated tau seeding and spreading. J Exp Med 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griciuc A, Patel S, Federico AN, Choi SH, Innes BJ, Oram MK, Cereghetti G, McGinty D, Anselmo A, Sadreyev RI, et al. (2019). TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron 103, 820–835 e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, et al. (2013). TREM2 variants in Alzheimer’s disease. N Engl J Med 368, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Marsh SE, and Stevens B (2019). Immune Signaling in Neurodegeneration. Immunity 50, 955–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JM, Braine CE, Williams PA, Zhu X, MacNicoll KH, Sousa GL, Buchanan RA, Smith RS, Libby RT, Howell GR, and John SWM (2017). Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A 114, E3839–E3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, and John SW (2007). Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet 8, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Ryan M, Graham LC, Smith RS, and John SW (2013). Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J Neuroinflammation 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, et al. (2012). Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest 122, 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Happonen KE, Burrola PG, O’Connor C, Hah N, Huang L, Nimmerjahn A, and Lemke G (2021). Microglia use TAM receptors to detect and engulf amyloid beta plaques. Nat Immunol 22, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kawaji T, and Tanihara H (2013). Elevated levels of multiple biomarkers of Alzheimer’s disease in the aqueous humor of eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci 54, 5353–5358. [DOI] [PubMed] [Google Scholar]
- Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, Schwartz S, Albasset S, McManus RM, Tejera D, et al. (2019). NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito YA, Belforte N, Cueva Vargas JL, and Di Polo A (2016). A Magnetic Microbead Occlusion Model to Induce Ocular Hypertension-Dependent Glaucoma in Mice. J Vis Exp, e53731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HR, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, Fukada SY, Liu FT, Liew FY, and Lukic ML (2009). Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol 182, 1167–1173. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, and Martin KR (2010). Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci 51, 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, et al. (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368, 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordao MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, et al. (2019). Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. (2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290 e1217. [DOI] [PubMed] [Google Scholar]
- Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, et al. (2017). The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581 e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CY, Fan BJ, Wang DY, Tam PO, Yung Tham CC, Leung DY, Ping Fan DS, Chiu Lam DS, and Pang CP (2006). Association of apolipoprotein E polymorphisms with normal tension glaucoma in a Chinese population. J Glaucoma 15, 218–222. [DOI] [PubMed] [Google Scholar]
- Lerman BJ, Hoffman EP, Sutherland ML, Bouri K, Hsu DK, Liu FT, Rothstein JD, and Knoblach SM (2012). Deletion of galectin-3 exacerbates microglial activation and accelerates disease progression and demise in a SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Brain Behav 2, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O, Calippe B, Lavalette S, Hu SJ, Raoul W, Dominguez E, Housset M, Paques M, Sahel JA, Bemelmans AP, et al. (2015). Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol Med 7, 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns CEG, Gratuze M, Narasimhan S, Jain N, Koscal LJ, Jiang H, Manis M, Colonna M, Lee VMY, Ulrich JD, and Holtzman DM (2019). TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci 22, 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, and Barres BA (2018). Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18, 225–242. [DOI] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, and John SW (2005). Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci 22, 637–648. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang P, Wang J, Yang Z, Huang S, Luo X, Qi J, Shen X, and Zhong Y (2016). The Effect of A2A Receptor Antagonist on Microglial Activation in Experimental Glaucoma. Invest Ophthalmol Vis Sci 57, 776–786. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhao C, Meng J, Li N, Xu Z, Liu X, and Hou S (2022). Galectin-3 regulates microglial activation and promotes inflammation through TLR4/MyD88/NF-kB in experimental autoimmune uveitis. Clin Immunol 236, 108939. [DOI] [PubMed] [Google Scholar]
- Lund H, Pieber M, Parsa R, Grommisch D, Ewing E, Kular L, Han J, Zhu K, Nijssen J, Hedlund E, et al. (2018a). Fatal demyelinating disease is induced by monocyte-derived macrophages in the absence of TGF-beta signaling. Nat Immunol 19, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, Kular L, Needhamsen M, Espinosa A, Nilsson E, et al. (2018b). Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat Commun 9, 4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Silverman SM, Zhao L, Villasmil R, Campos MM, Amaral J, and Wong WT (2019). Absence of TGFbeta signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi F, Tang S, Ando D, Yamakita M, Wang J, Kashiwagi K, Yamagata Z, Iijima H, and Tsukahara S (2005). The apolipoprotein E gene polymorphism is associated with open angle glaucoma in the Japanese population. Mol Vis 11, 609–612. [PubMed] [Google Scholar]
- Margeta MA, Lad EM, and Proia AD (2018). CD163+ macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma. Graefes Arch Clin Exp Ophthalmol 256, 2449–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta MA, Letcher SM, Igo RP Jr., Cooke Bailey JN, Pasquale LR, Haines JL, Butovsky O, Wiggs JL, and consortium N (2020). Association of APOE With Primary Open-Angle Glaucoma Suggests a Protective Effect for APOE epsilon4. Invest Ophthalmol Vis Sci 61, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, et al. (2020). Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci 23, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Amann L, Monaco G, Sankowski R, Staszewski O, Krueger M, Del Gaudio F, He L, Paterson N, Nent E, et al. (2022). Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature. [DOI] [PubMed] [Google Scholar]
- McAlpine CS, Park J, Griciuc A, Kim E, Choi SH, Iwamoto Y, Kiss MG, Christie KA, Vinegoni C, Poller WC, et al. (2021). Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 595, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay GJ, Patterson CC, Chakravarthy U, Dasari S, Klaver CC, Vingerling JR, Ho L, de Jong PT, Fletcher AE, Young IS, et al. (2011). Evidence of association of APOE with age-related macular degeneration: a pooled analysis of 15 studies. Hum Mutat 32, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei M, Gupta VB, Chick JM, Greco TM, Wu Y, Chitranshi N, Wall RV, Hone E, Deng L, Dheer Y, et al. (2017). Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci Rep 7, 12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, and Segawa K (2021). Sensing and clearance of apoptotic cells. Curr Opin Immunol 68, 1–8. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, and Benowitz LI (2006). Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 26, 12633–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Emmrich JV, Fricker M, Mander PK, Thery C, and Brown GC (2013). Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci U S A 110, E4098–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, and Brown GC (2012). Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front Pharmacol 3, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, and Brown GC (2011). Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol 186, 4973–4983. [DOI] [PubMed] [Google Scholar]
- Neufeld AH (1999). Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol 117, 1050–1056. [DOI] [PubMed] [Google Scholar]
- O’Koren EG, Yu C, Klingeborn M, Wong AYW, Prigge CL, Mathew R, Kalnitsky J, Msallam RA, Silvin A, Kay JN, et al. (2019). Microglial Function Is Distinct in Different Anatomical Locations during Retinal Homeostasis and Degeneration. Immunity 50, 723–737 e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omodaka K, Nishiguchi KM, Yasuda M, Tanaka Y, Sato K, Nakamura O, Maruyama K, and Nakazawa T (2014). Neuroprotective effect against axonal damage-induced retinal ganglion cell death in apolipoprotein E-deficient mice through the suppression of kainate receptor signaling. Brain Res 1586, 203–212. [DOI] [PubMed] [Google Scholar]
- Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, Nuscher B, Xiong M, Ghasemigharagoz A, Katzmarski N, et al. (2019). Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 22, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, and Gan WB (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Garrison BS, Ma W, Wang R, Jiang A, Li J, Mistry M, Bronson RT, Santoro D, Franco C, et al. (2018). A Milieu Molecule for TGF-beta Required for Microglia Function in the Nervous System. Cell 174, 156–171 e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, Benowitz LI, and Miller JW (2012). Etanercept, a widely used inhibitor of tumor necrosis factor-alpha (TNF-alpha), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS One 7, e40065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas B, Gallego BI, Ramirez AI, Salazar JJ, de Hoz R, Valiente-Soriano FJ, Aviles-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Trivino A, and Ramirez JM (2014). Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J Neuroinflammation 11, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutierrez-Vazquez C, Hewson P, Staszewski O, et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza A, Raveu AL, Reboussin E, Roubeix C, Boucher C, Degardin J, Godefroy D, Rostene W, Reaux-Le Goazigo A, Baudouin C, and Melik Parsadaniantz S (2016). Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J Neuroinflammation 13, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, and Calkins DJ (2010). The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci 51, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, and et al. (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472. [DOI] [PubMed] [Google Scholar]
- Siew JJ, Chen HM, Chen HY, Chen HL, Chen CM, Soong BW, Wu YR, Chang CP, Chan YC, Lin CH, et al. (2019). Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’s disease. Nat Commun 10, 3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, and Howell GR (2014). The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, Zhang Y, Spevak W, Lin J, Phan NY, et al. (2019). Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat Commun 10, 3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, and Roses AD (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, and Cheng CY (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Tribble JR, Kokkali E, Otmani A, Plastino F, Lardner E, Vohra R, Kolko M, Andre H, Morgan JE, and Williams PA (2021). When Is a Control Not a Control? Reactive Microglia Occur Throughout the Control Contralateral Pathway of Retinal Ganglion Cell Projections in Experimental Glaucoma. Transl Vis Sci Technol 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz SG, See P, Mildenberger W, Thion MS, Silvin A, Lutz M, Ingelfinger F, Rayan NA, Lelios I, Buttgereit A, et al. (2020). Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell. [DOI] [PubMed] [Google Scholar]
- Wagner T, Bartelt A, Schlein C, and Heeren J (2015). Genetic Dissection of Tissue-Specific Apolipoprotein E Function for Hypercholesterolemia and Diet-Induced Obesity. PLoS One 10, e0145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Peng B, and Lin B (2014). Fractalkine receptor regulates microglial neurotoxicity in an experimental mouse glaucoma model. Glia 62, 1943–1954. [DOI] [PubMed] [Google Scholar]
- Wang SK, Xue Y, and Cepko CL (2020). Microglia modulation by TGF-beta1 protects cones in mouse models of retinal degeneration. J Clin Invest 130, 4360–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. (2015). TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieghofer P, Hagemeyer N, Sankowski R, Schlecht A, Staszewski O, Amann L, Gruber M, Koch J, Hausmann A, Zhang P, et al. (2021). Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. EMBO J 40, e105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Braine CE, Kizhatil K, Foxworth NE, Tolman NG, Harder JM, Scott RA, Sousa GL, Panitch A, Howell GR, and John SWM (2019). Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol Neurodegener 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Marsh-Armstrong N, Howell GR, Lasker I.I.o.A., and Glaucomatous Neurodegeneration, P. (2017). Neuroinflammation in glaucoma: A new opportunity. Exp Eye Res 157, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiying M, Wenbo W, Wangyi F, and Qinghuai L (2017). Association of Apolipoprotein E Polymorphisms with Age-related Macular Degeneration Subtypes: An Updated Systematic Review and Meta-analysis. Arch Med Res 48, 370–377. [DOI] [PubMed] [Google Scholar]
- Yuan L, and Neufeld AH (2001). Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res 64, 523–532. [DOI] [PubMed] [Google Scholar]
- Zeng HL, and Shi JM (2018). The role of microglia in the progression of glaucomatous neurodegeneration- a review. Int J Ophthalmol 11, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB, and Wong WT (2015). Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med 7, 1179–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: RNAseq data of Fcrls+ retinal microglia from microbead-injected eyes and controls and DBA/2J eyes and controls. Related to Figures 1 and S2.
Table S2: Demographic, clinical, and pathologic characteristics of glaucoma cases and controls. Related to Figures 1 and 7.
Table S3: RNAseq data of Fcrls+ retinal microglia from microbead-injected wildtype and Apoe−/− animals. Related to Figure 3.
Table S4: RNAseq data of Fcrls+ phagocytic and non-phagocytic retinal microglia from wildtype and Apoe−/− animals injected intravitreally with apoptotic neurons. Related to Figure S5.
Table S5: RNAseq data of Fcrls+ retinal microglia from microbead-injected APOE3 and APOE4 animals. Related to Figure 7.
Data Availability Statement
Bulk RNA-seq data have been deposited at GEO and will be publicly available as of the date of publication. Accession numbers are listed in the key resources table. All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Brn-3a Antibody, POU-domain protein, clone 5A3.2 | MilliporeSigma | Cat#MAB1585; RRID:AB_94166 |
| Anti Iba1, Rabbit (for Immunocytochemistry) | FUJIFILM Wako Pure Chemical Corporation | Cat#019-19741; RRID:AB_839504 |
| Anti-P2ry12, Rabbit polyclonal | Butovsky Lab, validated in (Butovsky et al., 2015; Butovsky et al., 2014) | N/A |
| Anti-Apolipoprotein E Antibody | Sigma-Aldrich | Cat#AB947; RRID:AB_10770246 |
| Purified Mouse Anti-Human Galectin-3, Clone B2C10 (RUO) | BD Biosciences | Cat#556904; RRID:AB_396531 |
| GFP Polyclonal Antibody | Thermo Fisher Scientific | Cat#A11122; RRID:AB_221569 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat#A-11005; RRID:AB_2534073 |
| Chicken anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21441; RRID:AB_2535859 |
| Rabbit anti-Goat IgG (H+L), Superclonal™ Recombinant Secondary Antibody, Alexa Fluor 555 | Thermo Fisher Scientific | Cat#A27017; RRID:AB_2536081 |
| Rat-monoclonal anti-FCRLS (clone 4G11) | Butovsky lab, validated in (Butovsky et al., 2015; Butovsky et al., 2014) | N/A |
| PerCP/Cyanine5.5 anti-mouse Ly-6C antibody (clone HK1.4) | Biolegend | Cat#128012; RRID:AB_1659241 |
| CD11b Monoclonal Antibody (M1/70), PE-Cyanine7, eBioscience™ | Thermo Fisher Scientific | Cat#25-0112-82; RRID:AB_469588 |
| Cy™3 AffiniPure Donkey Anti-Rabbit IgG (H+L) | Jackson ImmunoResearch Labs | Cat#711-165-152; RRID:AB_2307443 |
| Cleaved Caspase-3 (Asp175) Antibody | Cell Signaling Technology | Cat#9661; RRID:AB_2341188 |
| Anti-IBA1 antibody, Guinea pig | Synaptic Systems | Cat#234 004; RRID:AB_2493179 |
| Anti-TMEM119 antibody, Rabbit | Atlas Antibodies | Cat#HPA051870; RRID:AB_2681645 |
| Galectin-3 Polyclonal antibody, Goat | R&D Systems | Cat#AF1197SP; RRID: AB_2234687 |
| Chicken-anti-rabbit AlexaFluor 647 | Thermo Fisher Scientific | Cat#A-21443; RRID:AB_2535861 |
| Donkey-guineapig AlexaFluor 488 | Jackson ImmunoResearch Labs | Cat#706-545-148; RRID:AB_2340472 |
| Donkey anti-mouse AlexaFluor 488 | Thermo Fisher Scientific | Cat#A-21202; RRID: AB_141607 |
| Biological Samples | ||
| Human eye specimens | Pathology Department, Duke University, See Table S3 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Anhydrous DMSO | Thermo Fisher Scientific | Cat#D12345 |
| Dynabeads™ M-450 Tosylactivated | Thermo Fisher Scientific | Cat#14013 |
| Karnovsky Solution: 16% paraformaldehyde 25% Glutaraldehyde 0.2 M Sodium Cacodylate Buffer CaCl2 (calcium chloride, anhydrous) 1N NaOH |
Electron Microscopy Sciences Electron Microscopy Sciences Electron Microscopy Sciences Thermo Fisher Scientific Electron Microscopy Sciences |
Cat#15710 Cat#16220; CAS#111-30-8 Cat#11653, pH 7.4 Cat#C-76 Cat.#21170-01; CAS# 1310-73-2 |
| Tamoxifen | Sigma-Aldrich | Cat#T5648 |
| TritonX-100 | Roche | Cat#11332481001 |
| Normal Horse Serum | Thermo Fisher Scientific | Cat#NC9909742 |
| Bovine Serum Albumin | Sigma-Aldrich | Cat#A3294 |
| VectaShield with DAPI | Vector Laboratories | Cat#H-1200 |
| Alexa Fluor488 5-SDP Ester | Thermo Fisher Scientific (Molecular Probes) | Cat#A30052 |
| Alexa Flour405 5-NHS Ester | Thermo Fisher Scientific (Molecular Probes) | Cat#A30000 |
| DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) | Thermo Fisher Scientific | Cat#D1306 |
| True Black Lipofuscin Autofluorescence Quencher | Biotium | Cat#23007 |
| Galectin-3 inhibitor (TD139) | Cayman Chemicals | CAS #1450824-22-2 |
| O.C.T. Compound | Tissue-Tek® | Cat#4583 |
| SuperBlock™ (TBS) Blocking Buffer | Thermo Fisher Scientific | Cat#37535 |
| MAXblocks™ Blocking Medium | Active Motif | Cat#15252 |
| Deposited Data | ||
| RNAseq of retinal microglia from microbead-injected wildtype (C57BL/6J) mice | NCBI Gene Expression Omnibus (GEO) | GSE192509 |
| RNAseq of retinal microglia from DBA/2J mice and controls | NCBI Gene Expression Omnibus (GEO) | GSE192505 |
| RNAseq of retinal microglia from microbead-injected Apoe−/− and wildtype mice | NCBI Gene Expression Omnibus (GEO) | GSE192508 |
| RNAseq of phagocytic and nonphagocytic retinal microglia from Apoe−/− and wildtype mice following intravitreal injection of apoptotic neurons | NCBI Gene Expression Omnibus (GEO) | GSE192506 |
| RNAseq of retinal microglia from microbead-injected humanized APOE3 and APOE4 mice | NCBI Gene Expression Omnibus (GEO) | GSE192507 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664 |
| Mouse: Cx3cr1Cre/+ | The Jackson Laboratory | JAX: 025524 |
| Mouse: Cx3cr1CreERT2/+ | The Jackson Laboratory | JAX: 021160 |
| Mouse: Apoe−/− | The Jackson Laboratory | JAX: 002052 |
| Mouse: Lgals3−/− | The Jackson Laboratory | JAX: 006338 |
| Mouse: DBA/2J | The Jackson Laboratory | JAX: 000671 |
| Mouse: DBA/2J Gpnmb+:DBA/2J-Gpnmb+/SjJ | The Jackson Laboratory | JAX: 007048 |
| Mouse: Apoefl/fl | (Wagner et al., 2015) | N/A |
| Mouse: APOE2 | Taconic Biosciences | Taconic: 1547 |
| Mouse: APOE3 | Taconic Biosciences | Taconic: 1548 |
| Mouse: APOE4 | Taconic Biosciences | Taconic: 1549 |
| Mouse: APOE3fl/fl | Cure Alzheimer’s Fund | https://www.alzforum.org/research-models/apoe3-knock-floxed-curealz |
| Mouse: APOE4fl/fl | Cure Alzheimer’s Fund | https://www.alzforum.org/research-models/apoe4-knock-floxed-curealz |
| Software and Algorithms | ||
| FIJI (ImageJ) | NIH | https://imagej.net/Fiji |
| R software (v4.0.3) | N/A | https://cran.r-project.org/ |
| GraphPad Prism 7 | GraphPad Software | https://www.graphpad.com |
| BioRender | BioRender Software | https://biorender.com/ |
| Salmon (v1.4.0) | N/A | https://github.com/COMBINE-lab/salmon |
| Ensembl release 101 | EMBL-EBI | http://aug2020.archive.ensembl.org/index.html |
| DESeq2 (v1.30.1) | N/A | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| pheatmap (v1.0.12) | N/A | https://cran.r-project.org/web/packages/pheatmap/index.html |
| EnhancedVolcano (v1.8) | N/A | https://github.com/kevinblighe/EnhancedVolcano |
| ggplot2 | N/A | https://cran.r-project.org/web/packages/ggplot2/index.html |
| Ingenuity software | Ingenuity Systems | https://www.ingenuity.com |
| Zen Black | Zeiss | https://www.zeiss.com/ |
| Leica application suite software (LAS-AF-lite) | Leica | http://www.leica-microsystems.com |
| Nikon E800 software: DP Controller: 3. 3. 1.292 DP Manager version 3,3,1,222 |
Olympus Life Science Solutions | https://www.olympus-lifescience.com/en/support/downloads/ |


