Abstract
Recently, “Technical standards for respiratory oscillometry” was published, which reviewed the physiological basis of oscillometric measures and detailed the technical factors related to equipment and test performance, quality assurance and reporting of results. Here we present a review of the clinical significance and applications of oscillometry. We briefly review the physiological principles of oscillometry and the basics of oscillometry interpretation, and then describe what is currently known about oscillometry in its role as a sensitive measure of airway resistance, bronchodilator responsiveness and bronchial challenge testing, and response to medical therapy, particularly in asthma and COPD. The technique may have unique advantages in situations where spirometry and other lung function tests are not suitable, such as in infants, neuromuscular disease, sleep apnoea and critical care. Other potential applications include detection of bronchiolitis obliterans, vocal cord dysfunction and the effects of environmental exposures. However, despite great promise as a useful clinical tool, we identify a number of areas in which more evidence of clinical utility is needed before oscillometry becomes routinely used for diagnosing or monitoring respiratory disease.
Short abstract
This paper provides a current review of the interpretation, clinical significance and application of oscillometry in respiratory medicine, with special emphasis on limitations of evidence and suggestions for future research. https://bit.ly/3GQPViA
Introduction
The mechanical properties of the lungs are disrupted in many disease states and exposures and contribute to major respiratory symptoms such as dyspnoea. Both the clinical management and our understanding of respiratory disease have benefitted from widely available tests like spirometry and the measurement of absolute lung volumes and gas transfer. However, these measurements require significant patient co-operation and a willingness to undertake maximal respiratory efforts. Oscillometry is a noninvasive method for measuring the mechanical properties of the respiratory system, which can enhance our understanding and management of lung disease [1]. Recently, the European Respiratory Journal published “Technical standards for respiratory oscillometry” [2], which reviewed the physiological basis of oscillometric measures and detailed the technical factors related to standardisation of equipment and test performance, quality assurance and reporting of results. Here we provide a review of the clinical significance and applications of oscillometry. We briefly review the physiological principles of oscillometric measurements and the basics of oscillometry interpretation, highlight potential clinical applications, and identify future work required for oscillometry to become routinely used in clinical practice.
The physiology and performance of oscillometry
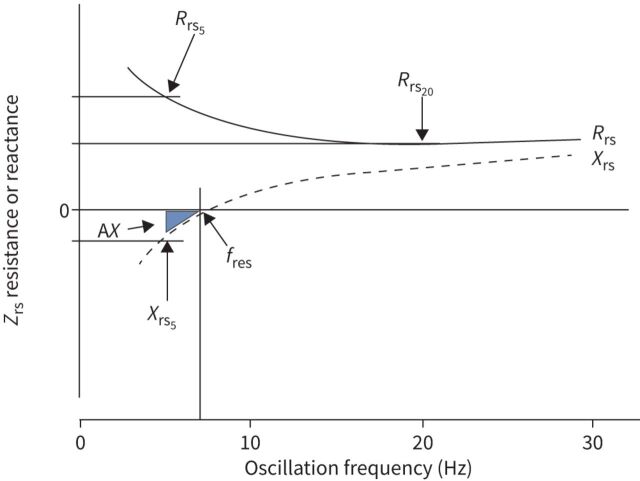
Oscillometry measures the mechanical impedance of the respiratory system (Zrs), representing the resistive and reactive forces that must be overcome to drive an oscillating flow signal into the respiratory system. The forces arise in the respiratory system from 1) the resistance of the airways and tissues to flow (Rrs), 2) the elastance (stiffness) of the lung parenchyma and chest wall in response to changes in volume (encompassed in reactance, Xrs), and 3) the inertance of accelerating gas in the airways (Irs). Zrs has generally been reported from a single frequency or over the frequency range of 5–40 Hz as an average across the whole breathing cycle (i.e. both inspiration and expiration). It has also been reported separately during the inspiratory and expiratory phases. These concepts and how they are measured by oscillometry are described in detail in the technical document. We provide a brief summary of these details in table 1, figure 1 and the supplementary material.
TABLE 1.
Summary of oscillometric parameters with a focus on the medium frequency range likely to be used in clinical practice (5–40 Hz)
| Parameter | Physiological interpretation |
| Z rs | Respiratory system impedance, reflecting the total forces related to resistance, elastance and inertance that must be overcome to drive airflow into and out of the lung. Zrs broadly describes the mechanical properties of the entire respiratory system (airway, parenchyma and chest wall). Zrs is not used in clinical practice per se. Rather, Zrs is represented by its components, respiratory system resistance (Rrs) and reactance (Xrs) as described below. |
| R rs | Resistance of the respiratory system, reflecting frctional losses both in gases as they flow along airways and in tissues of the lung and chest wall as they are stretched and deformed. Rrs at individual frequencies is denoted Rrs5, Rrs8, etc. Changes in Rrs at higher frequencies above ∼5 Hz are reflective of changes in airway resistance, i.e. calibre, and thus sensitive to airway narrowing. As such, Rrs could be increased in clinical situations such as during bronchoconstriction, the presence of excessive mucous or mucous plugging, airway inflammation, and other causes of airway narrowing or obstruction. Tissue resistance becomes progressively more important as frequency decreases below 5 Hz, becoming dominant at normal breathing frequencies (∼0.2 Hz) and lower. |
| Frequency dependence of resistance (e.g. Rrs5–20) | Rrs is largely frequency independent over the medium frequency range among healthy individuals (except in very young children). However, in many respiratory diseases, increased upward inflection of resistance is often evident at low frequencies and therefore frequency dependence is increased. Clinically, the frequency dependence of Rrs is commonly quantified as the difference Rrs5–20. This is thought to primarily be sensitive to heterogeneous narrowing in the peripheral airways, but it may also arise from substantial heterogeneity in narrowing of more central airways, heterogeneity of time constants reflecting airway versus parenchymal disease, and upper airway shunt flow (compliant regions proximal to resistance). |
| X rs | Reactance of the respiratory system, reflecting respiratory system elastance (Ers) due to the combined stiffnesses of the lung and chest wall tissues (below fres; described below), and respiratory system inertance (Irs) due to the mass of gas in the central airways (above the fres). Xrs becomes “more negative” in lung disease, indicating the respiratory system becomes stiffer. Xrs is very dependent on lung volume, with Xrs5 having been demonstrated to be sensitive to airway closure and reflecting communicating lung volume. Intrabreath changes in Xrs5 are useful to detect expiratory flow limitation. To date, Irs is not commonly used as a measure of lung disease clinically, but may be altered in conditions affecting gas flow in the upper and central airways. |
| f res | Resonant frequency, where Ers and Irs make equal and opposite contributions to impedance, (i.e. where Xrs is zero). Ers makes the major contribution to Xrs as frequency decreases below fres, while Irs dominates increasingly above fres. The fres of a healthy adult male is around 8 Hz but is usually higher in lung disease. In children, fres is generally higher than 8 Hz and decreases with age. |
| AX | In contrast to Rrs in health, Xrs is frequency-dependent in the medium frequency range, the magnitude of which is exaggerated in lung disease. The area under the reactance curve (AX) is the area inscribed by the Xrs curve between the lowest measured frequency and fres. AX is thus an integrative measure dominated by the lower frequency components of Xrs, determined predominately by Ers, and affected by the point at which Xrs crosses the frequency axis (fres), which is determined both by Ers and Irs. It has the advantage of having positive rather than negative units and is a measure that evaluates Xrs over a range of frequencies, with units of elastance hPa·L−1. Assessing AX (considering Xrs at all frequencies below fres) in the clinic is potentially more sensitive to changes in the elastic properties of the respiratory system than Xrs at a single frequency. |
FIGURE 1.
Impedance spectrum. Respiratory system impedance (Zrs) is plotted against frequency. Zrs is composed of a real component seen by resistance (Rrs) and an imaginary component expressed as reactance (Xrs). Rrs and Xrs at specific frequencies are noted by the frequency at which they are measured (e.g. Rrs5=Rrs at 5 Hz, Rrs20=Rrs at 20 Hz). The point at which Xrs crosses zero is the resonant frequency (fres). Below fres, Xrs is dominated by elastance, and above fres, Xrs is dominated by inertance. An integrated measure of low frequency Xrs is the area inscribed by Xrs and Zrs=0 starting at the lowest frequency up to fres, known as the area of the Xrs curve, AX. The lowest frequency defining AX is shown at 5 Hz, but it may be estimated starting from any frequency. Note that the Zrs spectrum shown is characteristic for a healthy adult. In healthy young children, the values and frequency dependence of Rrs would be relatively increased, Xrs would be more negative and fres would be shifted markedly to the right (resulting in increased AX).
Much of the evidence for clinical utility of oscillometry, including the studies cited in the current document, have been collected using a wide range of equipment, protocols and reporting formats. Although there is increasing availability of commercial oscillometry devices, comparability across devices remains a challenge, especially with regards to differences in the frequency dependence of resistance and reactance at higher-than-normal impedance [3, 4]. As such, the applicability of existing reference equations (supplementary table S1) remains uncertain for abnormal disease states and across different devices and manufacturers. Until this is resolved, comparisons between healthy and diseased cohorts should ideally be made using the same device, and differences in results based on variability in equipment, protocols and reference values should be considered when interpreting data presented in this review. Efforts are currently underway by the Global Lung Function Initiative to gather data from around the world to develop more universal, robust reference equations that take into account specific equipment and protocols.
Additionally, while oscillometry is a simple test to perform in clinic, its interpretation remains a challenge for many. Further aspects need to be better established before oscillometry gains an evidence-based position in clinical practice, as summarised in table 2. Many of these aspects are highlighted in the following sections, but in particular it is important to note that establishing minimal important clinical differences to assess change in lung function over time is a crucial area for future research.
TABLE 2.
Gaps in knowledge and future needs in defining the clinical applications of oscillometry
| Area of need | What we think we know | Identified gaps/research questions |
| Harmonisation of oscillometry devices [3] | Different metrics between commercial devices as measured impedance is increased. | Established standard testing procedures to compare devices. Encourage manufacturers to revise hardware and firmware/software design. |
| Normative values | Multiple reference populations to determine “normal”, separate for children and adults. | Large, multicentre studies to determine multi-ethnic population normal values across all ages, equivalent to the Global Lung Function Initiative. |
| Minimal clinically important change | Short-term test variability over minutes and days; coefficient of repeatability values in children and adults. Longitudinal data in healthy people to understand variability and changes through life. |
Correlations with clinical progression in disease populations. Minimally clinically important changes for each oscillometry index, which likely vary with disease and treatment. |
| Bronchodilator response | Cut-offs for significant bronchodilator responses, expressed for multiple indices in different ways, from individual studies in healthy people. Good discrimination between health and asthma. Higher sensitivity than spirometry in terms of response and identifying responders. Higher sensitivity than spirometry in identifying individuals with poor asthma control. |
Determine the most useful way to express bronchodilator responses in the clinic. Consistency of such responses within subjects over time. Further studies on clinical correlates of bronchodilator responses, in both healthy and disease populations across all ages. |
| Standardisation of bronchial challenge testing protocol | Good feasibility in younger age groups. Variability across studies in terms of cut-offs. Higher sensitivity than spirometry in terms of response and identifying responders. |
Determine value added to hyperresponsiveness measured by spirometry. Determine standardised cut-offs for the range of challenge agents. Potential feasibility of shorter protocols. |
| Phenotyping in obstructive diseases | Correlations of specific indices with symptoms, imaging and spirometry in terms of baseline measures, changes in response to treatment and prediction of treatment response. | Determine which indices provide the most clinically relevant information by reporting comprehensive, head-to-head comparison across the full range of indices, within disease populations, including patient-centred outcomes. Explore the role of new indices, such as those obtained from within-breath measurements. Sensitivity analyses within disease populations for oscillometry indices. Comprehensive studies correlating oscillometry measures with other phenotyping tools, such as lung imaging and histology. Determine correlations with known and emerging biomarkers of disease, particularly in response to treatment. |
| Grading severity of abnormalities | No data. | Assess degrees of deviation from normal in relation to statistical variation and clinical outcomes. |
| Home monitoring | Potential marker of airway instability in asthma. Sensitive detector of exacerbations in COPD, and utility as guide for intervention in subset of COPD patients (those with previous hospitalisation). |
Role in exacerbation detection and guide for intervention in asthma. Role in prediction of disease progression and responses to treatment in asthma and COPD. Utility in other diseases. |
| Emerging clinical applications | Potential role in identifying lung function deficits in preterm children. Limited utility demonstrated thus far in cystic fibrosis in children, less data in adults. Potential role in detecting early changes in smokers. Potential utility in identifying pathological changes in obesity. Potential in monitoring progression after environmental exposures. Potential in diagnosis of vocal cord dysfunction. Potential role in titrating level of respiratory support in sleep and in COPD patients in the intensive care setting. Potential in identifying clinical progression in lung and bone marrow transplant patients, as well as interstitial lung disease and neuromuscular disease. |
Larger clinical studies beyond proof of concept. Potential for aerosol generation to stratify risk of preventing spread of infection compared to spirometry. |
Oscillometry during infancy
Lung function measurements during infancy have largely been confined to a few specialist centres due to technical complexity, lack of commercially available equipment, and potential risks associated with the measurement (e.g. requirement for sedation). Additionally, the use of a face mask in infants complicates the interpretation of impedance data, since much of the measured Rrs may be attributed to nasal resistance [5]. To date, there is no evidence that impedance measurements in infants contribute to clinical decision-making. However, oscillometric studies have contributed to the pathophysiological understanding of respiratory disease in infants, particularly those with wheezing disorders, as described in the supplementary material.
Preterm birth and bronchopulmonary dysplasia beyond the neonatal intensive care unit
Emerging evidence suggests that oscillometry is a clinically useful measure of lung function in survivors of very preterm birth. Compared to preschoolers delivered at term, those born prematurely have increased Rrs, more negative Xrs and increased area of reactance (AX) and resonant frequency (fres) [6]. Deficits are even greater in premature babies with bronchopulmonary dysplasia (BPD) [7–9]. Xrs measured in the first week of life has been shown to improve prognostication of respiratory outcome in very preterm infants on noninvasive respiratory support. Recently, Evans et al. [10] showed that while all oscillometric outcomes were abnormal in those born very prematurely, Xrs was particularly sensitive at distinguishing term versus preterm preschoolers, suggesting a role for oscillometry in monitoring preterm lung disease. Deficits in oscillometric outcomes persist up to at least adolescence and correlate with respiratory symptoms [11, 12].
To date, only one study has measured oscillometry longitudinally in this population [13]. Interestingly, Xrs and AX deteriorated over time in those with BPD in parallel with declines in spirometry measures, and were found to decrease faster in those children exposed to environmental tobacco smoke and those with respiratory symptoms. Deterioration in these oscillometry outcomes was not associated with structural lung damage on chest computed tomography (CT) scans. The pathophysiology underpinning increased lung stiffness in this population remains unclear, but may reflect failed alveolarisation, structural damage to the parenchyma or inflammatory changes to the lungs.
Asthma
Oscillometric measurements can distinguish adult asthmatics from healthy controls [14–16], asthmatics with different degrees of airway obstruction [17], and between groups of adults with asthma compared to COPD [15, 18–21]. Oscillometry may be especially helpful in diagnosing asthma in patients with preserved spirometry as a more sensitive indicator of abnormal airway physiology [22]. However, while helpful in adults, the delineation between healthy controls and asthmatics using oscillometry is less clear in preschool-aged children, with several studies showing no difference between groups [23–25]. These conflicting findings may be due to differing diagnostic criteria, disease severity or degree of control in the paediatric populations studied who may have smaller differences between health and disease than adults.
Oscillometric outcomes other than resistance and reactance (such as AX and Rrs5–20) may also be useful in supporting a diagnosis of asthma, predicting future loss of asthma control or monitoring clinical treatment changes. In particular, the term “small airway dysfunction” (SAD) has gained traction. The large ATLANTIS cohort revealed that Rrs5–20, AX and Xrs5 were all strong contributors to SAD, with high prevalence in asthma compared to other physiological measures thought to reflect small airways [26]. However, a limitation of the study is that the definition of SAD relied on a statistical, data-driven approach, rather than an independent measure. It also is important to realise that no measures are specific for SAD, including Rrs5–20, which is sensitive to heterogeneouis peripheral airway narrowing, but can also be affected by heterogeneity in the central airways and upper airway shunt (table 1). Furthermore, these relative contributions are likely dependent on disease. We urge caution in the strict interpretation of Rrs5–20 as relating solely to small airways disease, especially when measured using devices that are known to be associated with enhanced frequency dependence of resistance [3, 27–29]. The clinical significance of Rrs5–20 continues to be evaluated in terms of its mechanism [30, 31], prevalence [32] and correlations with measures of severity and control in both children and adults [26, 33, 34].
In addition, oscillometry has provided novel insights into the pathophysiology of asthma via the effects of lung volume on oscillatory mechanics, as well as short- and long-term variations in mechanics over time. These topics are further addressed in the supplementary material. These variations may be a marker of instability, and potentially useful to detect exacerbations or loss of control, particularly in a home telemonitoring setting [35]. Intrabreath changes in oscillometry parameters may also provide additional information beyond conventional parameters: in preschoolers it improved detection of acute obstruction and recurrent wheezers from healthy controls [36], while in adults with severe asthma it distinguished those with poor control from those with good control [37]. While oscillometry measures are generally altered in asthmatics at the population level, oscillometry is arguably of most benefit in clinical practice for the diagnosis of asthma (via bronchodilation or bronchoprovocation) and monitoring response to intervention, as discussed in the following sections.
Bronchodilator response testing
As discussed in the recent technical standards [2], cut-offs indicating a significant bronchodilator response (BDR) using oscillometric indices are reported in healthy adults and children (i.e. 40% decrease in Rrs at 5 Hz, 50% increase in Xrs at 5 Hz and 80% decrease in AX relative to baseline) (supplementary table S2) [2]. Recent work has shown results for BDR in adults [38, 39] that are similar to published data using different instruments [4], suggesting that BDR in oscillometry appears to be robust to different devices and populations. Nevertheless, given the diversity of age ranges, equipment and protocols, further work is still needed to best define a BDR, its significance relative to important patient outcomes and consistency of response over time.
Several studies have shown that a BDR based on oscillometric parameters is better than one based on forced expiratory volume in 1 s (FEV1) at differentiating asthmatic from healthy children [40–42]. However, data relating to BDR in children with “early life” wheeze (i.e. no formal diagnosis of asthma) are less clear, with some studies reporting no differences in BDR between wheezy and non-wheezy preschoolers [43]. In adults, bronchodilation was identified more often using oscillometry than spirometry, and in one study changes in Xrs and AX (but not Rrs) correlated with spirometric responses and identified more subjects with poor asthma control compared to spirometry [44]. Other studies have shown that BDR assessed with oscillometry correlates with poor asthma control [14, 45].
Bronchial challenge testing
Oscillometry can also be used as an alternative to spirometry for conducting bronchial challenges in adults [46–58] and children [59–65]. A summary of studies to date attempting to define airway hyperresponsiveness (AHR) based on oscillometry is shown in supplementary table S3. Results vary widely, ranging from a 20 to 50% increase in Rrs5 and a 20–80% decrease in Xrs5. Provocation studies in children are feasible in patients as young as 3 years old using oscillometry [59, 61]. However, conclusive data indicating which outcome measures and threshold values best reflect a positive bronchial challenge test are currently lacking in this age group. The main advantage of oscillometry for bronchial challenge testing is increased sensitivity of detecting bronchoconstriction, which might shorten the test and reduce the cumulative dose of the agent. However, there may be underestimation of the response occurring in the lungs in children during a challenge due to loss of the oscillatory flow into the upper and large airways, known as upper airway shunt [66]. More information about upper airway shunt is found in the supplementary material.
Deep breaths (i.e. inflation to total lung capacity (TLC)) during spirometry testing, or with some inhalation agents, potentially affect diagnosis, given both the bronchoprotective and bronchodilator effect of deep inhalation in health and in asthma [67–69]. Consequently, oscillometry may be more sensitive than spirometry for detecting AHR in mild asthmatic patients, given their maintained, albeit reduced, response to a deep breath, resulting in a lower provocative dose of methacholine [47, 70] and therefore a shorter testing protocol. However, since avoiding deep breaths may result in AHR even in healthy individuals [71], oscillometry may be less sensitive than spirometry in distinguishing healthy from asthmatic individuals. Of note, patients may prfer oscillometry over spirometry during bronchial challenge testing because they do not have to repeatedly take the deep breaths needed to perform spirometry.
AHR detected by oscillometry is repeatable [47, 49, 61, 72] and correlates with responsiveness based on FEV1 [73], but the correlations of spirometry and oscillometry are inconsistent. There is wide variability across studies, which may be explained by differences in methodology and study populations. However, it appears that oscillometric indices may provide additional information to spirometry. For example, some subjects report symptoms during bronchial challenge without accompanying changes in FEV1, which may be related to the effects of deep inhalation mitigating changes in FEV1; however, concomitant changes in Rrs and AX suggest there is narrowing and/or closure of small airways [74, 75] not detected by spirometry. In summary, oscillometry is a useful tool to detect airway narrowing before spirometry during bronchial challenge testing, but the correlations and thresholds of response are variable, and further studies are needed to determine if oscillometry indices are more clinically relevant than spirometry.
Treatment responses in asthma
In addition to treatment with bronchodilators, differences in Rrs and Xrs in response to different inhaled corticosteroids (ICS) [76, 77], montelukast [78], ICS/long acting β2-agonist (LABA) formulations [79], and ICS versus ICS/LABA [80] have been demonstrated. In general, Rrs, Xrs and AX are more sensitive than spirometry [81]. Recent work has shown that oscillometry and other parameters related to peripheral airway function can be correlated with an improvement in symptoms for patients with poorly controlled asthma receiving ICS/LABA therapy [82]. One study has attempted to determine whether oscillometry could distinguish any benefit of small versus large particle size ICS therapy based on peripheral versus central airway obstruction, respectively; however, this study only looked at the differential treatment effect on symptoms, not oscillometry, and used an arbitrary oscillometric definition of peripheral airway function [83]. Oscillometric indices are also sensitive to improvements in asthma in response to mepolizumab therapy [84]. These findings suggest an important role of oscillometry in identifying and monitoring treatment responses in asthma, complementary to traditional spirometry.
Thus, in asthma, individual oscillometry studies have demonstrated physiological correlations of specific indices with symptoms, imaging, spirometry, changes in response to bronchodilators, bronchial challenges and treatment, and prediction of treatment response. What needs to follow are larger scale trials beyond proof of concept that demonstrate actual clinical utility in terms of improving patient care in asthma, as well as what constitutes a minimal clinically significant difference in oscillometry parameters.
Cystic fibrosis
A number of studies in children with cystic fibrosis (CF) reveal normal oscillometric indices [85–87], with only one study of preschool children with CF reporting abnormal values [88]. Associations between oscillometric indices and other clinical parameters in children with CF are variable [25, 86, 88–92]. There are limited data on the use of oscillometry in adults with CF; however, by one report, resistance and reactance are abnormal in adults with CF and correlate with other measures of lung function [93]. Therefore, the clinical utility of oscillometry in CF remains uncertain, particularly when other clinical tests have shown superior ability to detect early CF lung disease and successfully monitor improvement in lung function with intervention.
COPD
Oscillometry may play an important role in the early detection of the adverse effects of smoking before COPD is diagnosed [94]. Several studies have found a high prevalence of abnormal Zrs in smokers with normal spirometry, mainly in Rrs and Xrs near 5 Hz [95–98], with up to 60% of smokers with normal spirometry (FEV1/forced vital capacity (FVC)>0.70) having some abnormality on oscillometry. Smokers also have greater prevalence of significant BDR compared to nonsmokers [38]. The clinical significance of these findings still needs to be determined by pathologic correlations and prospective clinical studies to establish relevance and utility.
Patients with COPD have significantly higher Rrs and more negative Xrs values than healthy people [99], changes that are proportional to the degree of airway obstruction [100]. Empirical studies demonstrate that Xrs is related to the degree of gas trapping and hyperinflation in the lungs and reflects the amount of communicating lung volume [101]. Xrs and fres relate better to FEV1, and measures of hyperinflation, i.e. inspiratory capacity/TLC and residual volume/TLC ratios, than do resistance measurements [102, 103]. Magnetic resonance imaging-derived measurements of abnormal gas mixing correlate best with Rrs5–19 and AX in COPD patients [104]. Recent work with parametric response mapping by CT has demonstrated strong correlations with oscillometry-derived Rrs5–19 in patients with COPD [105], which is presumed to reflect small airways disease (but may also reflect large airway heterogeneity). A similar finding has been shown using endobronchial optical coherency tomography, which demonstrated that Rrs5–20 correlated with small airways pathology in heavy smokers and patients with COPD [106]. Oscillometric parameters likely related to gas trapping (i.e. Xrs ) in patients with COPD correlated with changes in exercise capacity following completion of pulmonary rehabilitation [107]. Oscillometry may also help in the categorisation of COPD severity [108].
As in asthma, oscillometry parameters are sensitive to treatment in patients with COPD. For example, Xrs near 5 Hz rather than Rrs appears to be more sensitive to bronchodilators [109–113] or ICS/LABA combination treatment [114], or recovery from exacerbations [102, 115–117]. The magnitude of the BDR depends on the disease stage. In the earlier stages of COPD, improvements in oscillometric parameters are greater compared to healthy subjects and are related mainly to an increased bronchodilation of the central airways, improvements in ventilation homogeneity based on the slope of the resistive component of Zrs, and total mechanical load [118].
Examination of intrabreath oscillometry has been especially significant in COPD, where it has been used to demonstrate evidence of tidal expiratory flow limitation (EFLT). Reactance and resistance are higher during expiration compared to inspiration in patients with COPD [119–121], reflecting dynamic airway compression and expiratory airflow limitation [122]. The underlying mechanisms of airway collapse during tidal breathing are uncertain. An empirically defined threshold of the difference between inspiratory and expiratory Xrs has been shown to be a sensitive and specific method for detecting EFLT [123, 124]. The inspiratory–expiratory difference in Xrs and its variability over time is also associated with worse dyspnoea [125]. This index is also associated with more rapid deterioration in exercise capacity over time and an increased likelihood of exacerbations irrespective of the degree of spirometric impairment [126, 127]. EFLT during tidal breathing is an important determinant of dynamic hyperinflation and its identification using oscillometry during noninvasive ventilation allows determining the lowest positive end-expiratory pressure (PEEP) able to abolish it. Recent preliminary studies showed that oscillometry can be incorporated in home noninvasive ventilators for abolishing EFLT by continuously tailoring PEEP to varying lung mechanics [128] leading to reduced hypercapnia and ineffective efforts in stable COPD during nocturnal noninvasive ventilation [129]. Currently, some major limitations to the use of intrabreath monitoring of oscillometry are the lack of normative data and standardisation of analysis and reporting of data.
Oscillometry is often thought to be a better reflection of breathing conditions of everyday life than spirometry, as it directly assesses mechanical impediment to airflow during normal breathing, rather than following deep inhalation; this arguably makes it more useful in COPD, where dyspnoea often occurs even at rest, or where severely obstructed patients can have difficulties with forced manoeuvres. It also makes it amenable to home telemonitoring in COPD, in which feasibility and potential clinical utility has been demonstrated [130] (see supplementary material). Furthermore, the deep inspiration required in spirometry has variable effects on airway calibre in COPD, which may affect clinical correlations [131, 132]. Oscillometry has also revealed greater variations in lung function over time [133, 134] and greater bronchodilator responses in COPD than expected from spirometry [38], again shedding light on disease pathogenesis that goes beyond fixed airway obstruction and reversibility in the larger airways.
In summary, oscillometry may aide in earlier detection of smoking-related effects on the lung and adds insight into the pathophysiology of COPD; however, we still need more data to assess how oscillometry relates to clinical phenotypes of COPD (e.g. predominance of emphysema versus bronchitis) and how it will affect clinical management of patients with COPD.
Bronchiolitis obliterans
Because of its sensitivity to small airway disease, oscillometry has been used to study patients with or at risk of bronchiolitis obliterans. In children, post-infectious bronchiolitis obliterans can be detected by greater changes in Xrs compared to spirometry [135]. In adult lung and bone marrow transplant recipients, bronchiolitis obliterans syndrome may be detected earlier by oscillometry than by conventional spirometry [136–138]. The lack of a need for deep inspiration with oscillometry is also beneficial in patients in the acute postoperative lung transplant period.
Obesity
Impedance measured by oscillometry has been well studied in obesity [139–145]. The effects of obesity are most apparent in Xrs, which suggests that there is an increase in heterogeneous airway narrowing and in airway closure in the lung periphery, although increased stiffness of the chest wall can also contribute [146]. By contrast, there are minimal changes in spirometry and typically no changes in FEV1/FVC ratio in obesity, with the exception of very severe obesity (>40 kg·m−2) [147].
In obesity, Rrs is increased, possibly due to reduced operating lung volume [148–150]. However, this is not the whole reason for this increase [140, 144, 151] as pulmonary circulatory congestion and airway oedema occur in obesity [152, 153] and are correlated with the magnitude of abnormality in both Rrs and Xrs [143]. These changes in airway function make interpretation of impedance in obesity difficult when assessing a patient with potential coexisting airway disease. Obese individuals with clinically and physiologically confirmed asthma have a more negative Xrs than obese, non-asthmatic individuals, probably because of enhanced peripheral airway closure [142, 146]. Changes in Rrs and Xrs with obesity and following bariatric surgery are more pronounced in the supine position with oscillometry being more sensitive to weight loss compared with spirometry [142, 146, 154]. The responses to methacholine bronchial challenge in obese subjects are different compared to non-obese subjects, particularly with regard to bronchoprotection [155] and exaggerated decreases in Xrs [139, 141, 155]. These nuanced differences cannot be easily determined with spirometry. Therefore, obesity is associated with a greater response to bronchoconstriction in terms of changes in the respiratory system elastic properties, but the clinical and pathophysiologic significance of these observations is uncertain.
In children, obesity has been found to be associated with a pattern different from that of obese adults (reduced FEV1/FVC with normal or even increased FEV1 and FVC), called “airway dysanapsis” [156]. Oscillometry may help distinguish the effects of obesity or asthma on the cause of a low FEV1/FVC in children due to dysanapsis or airway narrowing [157]. Increased Rrs5–20 and AX were also found in a cross-sectional study on overweight and obese adolescents [158]. Further research is needed to better define the effects of obesity on oscillometric parameters in overweight and obese children. In both adults and children, it remains unclear how oscillometry data in obesity will contribute to clinical diagnosis and management.
Restrictive diseases
The clinical application of oscillometry in restrictive lung disease is less established in comparison to obstructive disease. Most oscillometric studies of restrictive lung diseases have investigated interstitial lung disease (ILD). Although ILD and obstructive lung diseases are clinically distinct, their corresponding Zrs spectra demonstrate similar patterns of abnormalities, perhaps due to the higher airway resistance found at lower lung volume in ILD. For example, a patient with ILD may demonstrate elevated Rrs, enhanced frequency dependence of Rrs, and more negative Xrs at low frequencies consistent with increased elastance [159–162]. Such findings correlate with the severity of the restriction assessed by either TLC or vital capacity and with the severity of radiographic abnormalities [162–165]. Recent work has shown that while oscillometric parameters in both patients with ILD and COPD correlate well with ventilation unevenness and Rrs, correlations were poor with alterations in static compliance due to emphysema or pulmonary fibrosis [166]. The longitudinal change of Zrs measured by oscillometry in ILD has not been studied.
Oscillometry has also been used to study lung function in pulmonary restriction caused by neuromuscular disease. Conventional physiological studies document diminished vital capacity due to both reduced lung compliance and reduced outward pull of the chest wall in children and adults with neuromuscular disease [167, 168]. While it has been suggested that oscillometry may be useful for the evaluation of neuromuscular disease [169], the data are limited. Technical difficulties in establishing an adequate mouth seal in patients with bulbar weakness may limit applicability in this group. However, oscillometry would appear to have a major advantage over spirometry in detecting lung disease in patients with neuromuscular weakness in general because it does not require muscle force to generate the deep inspiration involved in spirometry.
Vocal cord dysfunction
Paradoxical inspiratory adduction of the vocal cords or reduction in subglottal cross-sectional area induces acute symptoms that is often mistaken for (or associated with) asthma [170]. Detection of inspiratory flow limitation is not widely assessed in clinic and is poorly tolerated, particularly in children. Model analysis has indicated the potential of oscillometry for assessing vocal cord dysfunction (VCD) [171, 172]. It is recommended to carefully examine intratidal changes in Zrs for large positive swings during inspiration [173], especially when tidal inspiratory flow limitation may be suspected [174], and to identify markedly positive differences between inspiratory and expiratory Rrs [175]. Owing to expiratory glottal narrowing, these differences are usually negative in control subjects or stable asthmatics and may be enhanced further in acute bronchoconstriction [175–177]. Large case-control studies are necessary to establish the sensitivity and specificity in VCD.
Sleep apnoea
Obstructive sleep apnoea (OSA) is a very prevalent disease in adults, characterised by recurrent upper airway collapse and obstruction, resulting in nocturnal apnoea and hypopnoea. Given that increase in upper airway resistance is a landmark of OSA, oscillometry is particularly suited for detecting such airway obstruction [178] and hence for application in this disease [179, 180]. Initially, oscillometry was applied to help diagnose OSA in awake patients, since increased susceptibility to airway collapse can be detected by measuring changes in Rrs during continuous negative airway pressure [181]. The effect of posture on oscillometric measures is also enhanced in supine patients with OSA compared to supine patients without OSA, increasing the sensitivity of oscillometry in OSA [182]. Although initial data showed that oscillometry was able to distinguish OSA patients from healthy adults [181], its application for simplified diagnosis has not yet been implemented in routine clinical practice. However, oscillometry is useful for monitoring upper airway collapse during sleep [183–185]. Oscillometry also provides insight into how airway resistance is normalised by application of continuous positive airway pressure (CPAP) [186], and how sleep apnoea and asthma may interact to worsen airway obstruction [187]. Further information on the use of oscillometry in the setting of CPAP is found in the supplementary material.
Environmental and occupational exposures
Oscillometry has been used in monitoring and detection of lung function among people exposed to various environmental or occupational irritants or hazards. Firefighters and those exposed to asbestos have been shown to have abnormalities in oscillometry parameters even with normal spirometric indices [188]. Similar findings are seen in symptomatic people exposed to World Trade Center dust during the 9/11 attacks and suggest the detection of distal airway dysfunction even when spirometry is normal [189, 190]. Exposures to specific inhaled contaminants have been shown to result in changes in Zrs that often but not invariably correlate with changes in spirometry; hence, the two measures may be complementary [191]. Oscillometry has the potential to detect changes years after an environmental exposure. For example, infants exposed to poor air quality from a coal mine fire for a 6-week episode when less than 2 years old had worse AX 3 years after the fire [192]. Furthermore, a study in adolescents showed that exposure to air pollution from local traffic during infancy was associated with abnormal oscillometry parameters suggesting distal airways disease, especially in those with asthma [193]. These studies all suggest that oscillometry may be useful for monitoring lung function in occupational settings and in detecting early changes in lung function in association with environmental exposures.
Of unique concern at the time of this writing is the prevention of spreading contaminated aerosols during lung function testing during the current coronavirus disease 2019 pandemic. Although no data are available, oscillometry is thought to be safer than spirometry in this regard, given that deep inhalation and forced exhalation are not required [194].
Critical care
The measurement of Zrs in critically ill patients receiving ventilatory support poses many unique challenges to the clinician, especially regarding technique and safety [195–200], including managing the interface between the oscillometric generator and the patient's airway [201–203], and the influence of the ventilator circuit on the excitation waveform [204–215]. Further details related to the technical issues of applying oscillometry through the ventilatory circuit are discussed in the supplementary material. Despite technical challenges, the use of oscillometry in ventilated and critically ill patients has yielded tremendous information on the mechanical derangements and pathophysiological processes associated with various respiratory diseases [198, 200, 216–220], such as lung derecruitment [221], parenchymal overdistention [222] and EFLT [124, 223, 224]. In the paediatric patients, oscillometry may be useful for monitoring the effects of positional changes and adjustment of PEEP [220, 225–228] and for improving prediction of respiratory outcomes in extremely preterm newborns receiving invasive ventilation [229]. A recent American Thoracic Society/European Respiratory Society workshop report on the evaluation of respiratory function, including oscillometry in the neonatal and paediatric intensive care units, has recently been published [230]. Oscillometry thus has the potential not only to optimise ventilator settings [226, 231–235], but also to enhance our understanding of the immediate impact of various surgical interventions on lung function [204, 205, 216, 236].
Summary and future research
In summary, oscillometry has shown to be of value for the diagnosing lung disease in our youngest patients and throughout life, and monitoring disease progression, acute exacerbations and treatment effects. While many gaps in our understanding are closing, many still need to be filled in order to ensure a smooth transition of oscillometry from a research tool to a reliable, robust, clinical tool (table 2). In undertaking this review of clinical literature using oscillometry, we have identified several areas where more evidence is required before oscillometry may be used routinely in clinical practice. Looking to the future, we envision many opportunities to develop oscillometry for a wide range of clinical applications [1].
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0208-2021.SUPPLEMENT (267KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
This article has supplementary material available from err.ersjournals.com
Conflict of interest: D.A. Kaminsky reports personal payments made as faculty speaker for Cardiorespiratory Diagnostics Seminar from MGC Diagnostics, Inc. outside the submitted work. Past Chair of ATS Proficiency Standards for Pulmonary Function Laboratories Committee, unpaid.
Conflict of interest: S.J. Simpson has nothing to disclose.
Conflict of interest: K.I. Berger has nothing to disclose.
Conflict of interest: P. Calverley reports receiving consulting fees paid by Phillips Respironics for advisory work on a novel COPD ventilator. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from Phillips Respironics, outside the submitted work.
Conflict of interest: P.L. de Melo reports patent 28727 issued.
Conflict of interest: R.J. Dandurand reports grants or contracts paid to the institution from AstraZeneca, Boehringer-Ingelheim, Covis Pharma, Grifols, MGC Diagnostics, Teva Pharma, Thorasys, and Vyaire, outside the submitted work. Speaking payment from Novartis for L'oscillométrie en clinique: qu'ajoute-t-elle aux évaluations pulmonaires?, 18 September 2019, Boehringer-Ingelheim for L'oscillométrie: vieille physiologie avec un avenir brilliant, 17 November 2020, and Latin American Respiratory Physiology Society for Oscillometry in Asthma and COPD: Interpretation Strategies, 14 November 2020, outside the submitted work. Chairman, Oscillometry Harmonisation Study Group, an international committee academic and industry experts working to standardise oscillometry devices, and Chairman, Respiratory Effectiveness Group Technologies Working Group, Cambridge, U.K. (https://www.regresearchnetwork.org).
Conflict of interest: R.L. Dellacà reports royalties or licenses from Restech, Philips and Vyaire. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from Restech and Philips, outside the submitted work. Support for attending meetings from Philips and Vyaire. Patent issued, owned and licensed from Politecnico di Milano University. Member of the Board of Directors for Restech. Stocks owned for Restech. Free loan of equipment for studies received from Vyaire and Restech.
Conflict of interest: C.S. Farah has nothing to disclose.
Conflict of interest: R. Farré has nothing to disclose.
Conflict of interest: G.L. Hall has nothing to disclose.
Conflict of interest: I. Ioan has nothing to disclose.
Conflict of interest: C.G. Irvin received consulting fees from Medical Graphics Corporation, outside the submitted work.
Conflict of interest: D.W. Kaczka reports support for the present manuscript from University of Iowa. Grants or contracts from Dept of Defence and NIH, outside the submitted work. Consulting fees received from ZOLL Medical, Inc. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from CHEST, ASME, Medical Society of New Zealand, and Johns Hopkins University, outside the submitted work. US patent 10,675,423 B2 (patent on MFOV technique, inventor) and PCT patent pending, patent on MFOV technique pending. Stock or stock options held for OscillaVent, Inc. Loan of ventilator for other projects from ZOLL Medical Inc.
Conflict of interest: G.G. King reports grants or contracts from Restech Italy, NHMRC, Boehringer Ingelheim, CycloPharm, GlaxoSmithKline, Menarini, MundiPharma, Philanthropic individuals and societies, outside the submitted work. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CycloPharm, GlaxoSmithKline, Menarini, MundiPharma and Novartis, outside the submitted work. Leadership or fiduciary role in other board, society, committee or advocacy group for ERS Technical Standards for Respiratory Oscillometry.
Conflict of interest: H. Kurosawa reports receiving a grant from CHEST Co. Ltd. Royalties or licence for CHEST Co. Ltd. Payment or honoraria for lectures received from CHEST Co. Ltd. Nippon, Boehringer Ingelheim, Novartis, and Teijin Pharma, outside the submitted work.
Conflict of interest: E. Lombardi reports grants or contracts from Restech and Sanofi, outside the submitted work. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events received from Angelini, Chiesi, GSK, Novartis and Sanofi, outside the submitted work. Participation on a Data Safety Monitoring Board or Advisory Board for GSK and Novartis.
Conflict of interest: G.N. Maksym reports grants or contracts from National Research Council of Canada, Cyclomedica Inc. Australia, and Lung Association of Nova Scotia, outside the submitted work. Accommodation Expenses received from Thorasys, Thoracic Medical Systems Inc. for attending European Society Meeting 2019. Patents planned, issued or pending for Method and system to acquire oscillometry measurements, owned by Thorasys, Thoracic Medical Systems Inc. Stock or stock options held for Thorasys, Thoracic Medical Systems Inc.
Conflict of interest: F. Marchal has nothing to disclose.
Conflict of interest: E. Oostveen has nothing to disclose.
Conflict of interest: B.W. Oppenheimer has nothing to disclose.
Conflict of interest: P.D. Robinson has nothing to disclose.
Conflict of interest: M. van den Berge reports grants or contracts from GlaxoSmithKline, Novartis, Astra Zeneca, Roche, and Genentech, outside the submitted work.
Conflict of interest: C. Thamrin report grants or contracts from Restech SRL and THORASYS Thoracic. Equipment on loan for research studies from Restech SRL and THORASYS Thoracic, outside the submitted work.
References
- 1.Calverley PMA, Farré R. Oscillometry: old physiology with a bright future. Eur Respir J 2020; 56: 2001815. doi: 10.1183/13993003.01815-2020 [DOI] [PubMed] [Google Scholar]
- 2.King GG, Bates J, Berger KI, et al. . Technical standards for respiratory oscillometry. Eur Respir J 2020; 55: 1900753. doi: 10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 3.Dandurand RJ, Lavoie JP, Lands LC, et al. . Comparison of oscillometry devices using active mechanical test loads. ERJ Open Res 2019; 5: 00160-2019. doi: 10.1183/23120541.00160-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oostveen E, Boda K, van der Grinten CP, et al. . Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J 2013; 42: 1513–1523. doi: 10.1183/09031936.00126212 [DOI] [PubMed] [Google Scholar]
- 5.Hall GL, Hantos Z, Wildhaber JH, et al. . Contribution of nasal pathways to low frequency respiratory impedance in infants. Thorax 2002; 57: 396–399. doi: 10.1136/thorax.57.5.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zannin E, Neumann RP, Dellaca R, et al. . Forced oscillation measurements in the first week of life and pulmonary outcome in very preterm infants on noninvasive respiratory support. Pediatr Res 2019; 86: 382–388. doi: 10.1038/s41390-019-0432-6 [DOI] [PubMed] [Google Scholar]
- 7.Udomittipong K, Sly PD, Patterson HJ, et al. . Forced oscillations in the clinical setting in young children with neonatal lung disease. Eur Respir J 2008; 31: 1292–1299. doi: 10.1183/09031936.00058107 [DOI] [PubMed] [Google Scholar]
- 8.Verheggen M, Wilson AC, Pillow JJ, et al. . Respiratory function and symptoms in young preterm children in the contemporary era. Pediatr Pulmonol 2016; 51: 1347–1355. doi: 10.1002/ppul.23487 [DOI] [PubMed] [Google Scholar]
- 9.Vrijlandt EJ, Boezen HM, Gerritsen J, et al. . Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr 2007; 150: 256–261. doi: 10.1016/j.jpeds.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 10.Evans DJ, Schultz A, Verheggen M, et al. . Identifying pediatric lung disease: a comparison of forced oscillation technique outcomes. Pediatr Pulmonol 2019; 54: 751–758. doi: 10.1002/ppul.24286 [DOI] [PubMed] [Google Scholar]
- 11.Thunqvist P, Gustafsson PM, Schultz ES, et al. . Lung function at 8 and 16 years after moderate-to-late preterm birth: a prospective cohort study. Pediatrics 2016; 137: e20152056. doi: 10.1542/peds.2015-2056 [DOI] [PubMed] [Google Scholar]
- 12.Simpson SJ, Logie KM, O'Dea CA, et al. . Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 2017; 72: 702–711. doi: 10.1136/thoraxjnl-2016-208985 [DOI] [PubMed] [Google Scholar]
- 13.Simpson SJ, Turkovic L, Wilson AC, et al. . Lung function trajectories throughout childhood in survivors of very preterm birth: a longitudinal cohort study. Lancet Child Adolesc Health 2018; 2: 350–359. doi: 10.1016/S2352-4642(18)30064-6 [DOI] [PubMed] [Google Scholar]
- 14.Nair A, Ward J, Lipworth BJ. Comparison of bronchodilator response in patients with asthma and healthy subjects using spirometry and oscillometry. Ann Allergy Asthma Immunol 2011; 107: 317–322. doi: 10.1016/j.anai.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 15.Clement J, Landser FJ, Van de Woestijne KP. Total resistance and reactance in patients with respiratory complaints with and without airways obstruction. Chest 1983; 83: 215–220. doi: 10.1378/chest.83.2.215 [DOI] [PubMed] [Google Scholar]
- 16.Tsuburai T, Suzuki S, Tsurikisawa N, et al. . Use of forced oscillation technique to detect airflow limitations in adult Japanese asthmatics. Arerugi 2012; 61: 184–193. [PubMed] [Google Scholar]
- 17.Cavalcanti JV, Lopes AJ, Jansen JM, et al. . Detection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation technique. Respir Med 2006; 100: 2207–2219. doi: 10.1016/j.rmed.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Shirai T, Mikamo M, et al. . Colored 3-dimensional analyses of respiratory resistance and reactance in COPD and asthma. COPD 2011; 8: 456–463. doi: 10.3109/15412555.2011.626818 [DOI] [PubMed] [Google Scholar]
- 19.Paredi P, Goldman M, Alamen A, et al. . Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax 2010; 65: 263–267. doi: 10.1136/thx.2009.120790 [DOI] [PubMed] [Google Scholar]
- 20.van Noord JA, Clement J, van de Woestijne KP, et al. . Total respiratory resistance and reactance in patients with asthma, chronic bronchitis, and emphysema. Am Rev Respir Dis 1991; 143: 922–927. doi: 10.1164/ajrccm/143.5_Pt_1.922 [DOI] [PubMed] [Google Scholar]
- 21.Yang SC, Lin BY. Comparison of airway hyperreactivity in chronic obstructive pulmonary disease and asthma. Chang Gung Med J 2010; 33: 515–523. [PubMed] [Google Scholar]
- 22.Kim SR, Park KH, Son NH, et al. . Application of impulse oscillometry in adult asthmatic patients with preserved lung function. Allergy Asthma Immunol Res 2020; 12: 832–843. doi: 10.4168/aair.2020.12.5.832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellinckx J, De Boeck K, Bande-Knops J, et al. . Bronchodilator response in 3–6.5 years old healthy and stable asthmatic children. Eur Respir J 1998; 12: 438–443. doi: 10.1183/09031936.98.12020438 [DOI] [PubMed] [Google Scholar]
- 24.Malmberg LP, Pelkonen AS, Haahtela T, et al. . Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax 2003; 58: 494–499. doi: 10.1136/thorax.58.6.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thamrin C, Gangell CL, Udomittipong K, et al. . Assessment of bronchodilator responsiveness in preschool children using forced oscillations. Thorax 2007; 62: 814–819. doi: 10.1136/thx.2006.071290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postma DS, Brightling C, Baldi S, et al. . Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med 2019; 7: 402–416. doi: 10.1016/S2213-2600(19)30049-9 [DOI] [PubMed] [Google Scholar]
- 27.Kuo CR, Jabbal S, Lipworth B. I say IOS you say AOS: comparative bias in respiratory impedance measurements. Lung 2019; 197: 473–481. doi: 10.1007/s00408-019-00247-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares M, Richardson M, Thorpe J, et al. . Comparison of forced and impulse oscillometry measurements: a clinical population and printed airway model study. Sci Rep 2019; 9: 2130. doi: 10.1038/s41598-019-38513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanimura K, Hirai T, Sato S, et al. . Comparison of two devices for respiratory impedance measurement using a forced oscillation technique: basic study using phantom models. J Physiol Sci 2014; 64: 377–382. doi: 10.1007/s12576-014-0329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell AJ, Foy BH, Richardson M, et al. . Functional CT imaging for identification of the spatial determinants of small-airways disease in adults with asthma. J Allergy Clin Immunol 2019; 144: 83–93. doi: 10.1016/j.jaci.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 31.Foy BH, Soares M, Bordas R, et al. . Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med 2019; 200: 982–991. doi: 10.1164/rccm.201812-2322OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson WJ, Zajda E, Lipworth BJ. Are we overlooking persistent small airways dysfunction in community-managed asthma? Ann Allergy Asthma Immunol 2012; 109: 185–189.e2. doi: 10.1016/j.anai.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 33.Manoharan A, Anderson WJ, Lipworth J, et al. . Small airway dysfunction is associated with poorer asthma control. Eur Respir J 2014; 44: 1353–1355. doi: 10.1183/09031936.00082314 [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Aledia AS, Galant SP, et al. . Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. Clin Immunol 2013; 131: 718–723. [DOI] [PubMed] [Google Scholar]
- 35.Gobbi A, Dellacà R, King GG, et al. . Towards predicting individual risk in asthma using daily home monitoring of resistance. Am J Respir Crit Care Med 2017; 195: 265–267. doi: 10.1164/rccm.201603-0537LE [DOI] [PubMed] [Google Scholar]
- 36.Czövek D, Shackleton C, Hantos Z, et al. . Tidal changes in respiratory resistance are sensitive indicators of airway obstruction in children. Thorax 2016; 71: 907–915. doi: 10.1136/thoraxjnl-2015-208182 [DOI] [PubMed] [Google Scholar]
- 37.Chiabai J, Friedrich FO, Fernandes MTC, et al. . Intrabreath oscillometry is a sensitive test for assessing disease control in adults with severe asthma. Ann Allergy Asthma Immunol 2021; 127: 372–377. doi: 10.1016/j.anai.2021.06.005 [DOI] [PubMed] [Google Scholar]
- 38.Jetmalani K, Brown N, Boustany C, et al. . Normal limits for oscillometric bronchodilator responses and relationships with clinical factors. ERJ Open Res 2021; 7: 00439-2021. doi: 10.1183/23120541.00439-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson H, Wollmer P, Sundström J, et al. . Bronchodilator response in FOT parameters in middle-aged adults from SCAPIS: normal values and relationship to asthma and wheezing. Eur Respir J 2021; 58: 2100229. doi: 10.1183/13993003.00229-2021 [DOI] [PubMed] [Google Scholar]
- 40.Marotta A, Klinnert MD, Price MR, et al. . Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol 2003; 112: 317–322. doi: 10.1067/mai.2003.1627 [DOI] [PubMed] [Google Scholar]
- 41.Song TW, Kim KW, Kim ES, et al. . Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol 2008; 19: 763–768. doi: 10.1111/j.1399-3038.2008.00734.x [DOI] [PubMed] [Google Scholar]
- 42.Oostveen E, Dom S, Desager K, et al. . Lung function and bronchodilator response in 4-year-old children with different wheezing phenotypes. Eur Respir J 2010; 35: 865–872. doi: 10.1183/09031936.00023409 [DOI] [PubMed] [Google Scholar]
- 43.Harrison J, Gibson AM, Johnson K, et al. . Lung function in preschool children with a history of wheezing measured by forced oscillation and plethysmographic specific airway resistance. Pediatr Pulmonol 2010; 45: 1049–1056. doi: 10.1002/ppul.21223 [DOI] [PubMed] [Google Scholar]
- 44.Cottee AM, Seccombe LM, Thamrin C, et al. . Bronchodilator response assessed by the forced oscillation technique identifies poor asthma control with greater sensitivity than spirometry. Chest 2020; 157: 1435–1441. doi: 10.1016/j.chest.2019.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo CR, Chan R, Lipworth B. Impulse oscillometry bronchodilator response and asthma control. J Allergy Clin Immunol Pract 2020; 8: 3610–3612. doi: 10.1016/j.jaip.2020.07.031 [DOI] [PubMed] [Google Scholar]
- 46.Bohadana AB, Peslin R, Megherbi SE, et al. . Dose-response slope of forced oscillation and forced expiratory parameters in bronchial challenge testing. Eur Respir J 1999; 13: 295–300. doi: 10.1034/j.1399-3003.1999.13b13.x [DOI] [PubMed] [Google Scholar]
- 47.Broeders ME, Molema J, Hop WC, et al. . Bronchial challenge, assessed with forced expiratory manoeuvres and airway impedance. Respir Med 2005; 99: 1046–1052. doi: 10.1016/j.rmed.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 48.Imahashi Y, Kanazawa H, Ijiri N, et al. . Analysis of the contributing factors to airway hyperresponsiveness by a forced oscillation technique in patients with asthma. Osaka City Med J 2014; 60: 53–62. [PubMed] [Google Scholar]
- 49.McClean MA, Htun C, King GG, et al. . Cut-points for response to mannitol challenges using the forced oscillation technique. Respir Med 2011; 105: 533–540. doi: 10.1016/j.rmed.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 50.Naji N, Keung E, Kane J, et al. . Comparison of changes in lung function measured by plethymography and IOS after bronchoprovocation. Respir Med 2013; 107: 503–510. doi: 10.1016/j.rmed.2012.12.022 [DOI] [PubMed] [Google Scholar]
- 51.Rozen D, Bracamonte M, Sergysels R. Comparison between plethysmographic and forced oscillation techniques in the assessment of airflow obstruction. Respiration 1983; 44: 197–203. doi: 10.1159/000194549 [DOI] [PubMed] [Google Scholar]
- 52.Schmekel B, Smith HJ. The diagnostic capacity of forced oscillation and forced expiration techniques in identifying asthma by isocapnic hyperpnoea of cold air. Eur Respir J 1997; 10: 2243–2249. doi: 10.1183/09031936.97.10102243 [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S, Chonan T, Sasaki H, et al. . Time-course of response in exercise-induced bronchoconstriction. Ann Allergy 1984; 53: 341–346. [PubMed] [Google Scholar]
- 54.van Noord JA, Clement J, van de Woestijne KP, et al. . Total respiratory resistance and reactance as a measurement of response to bronchial challenge with histamine. Am Rev Respir Dis 1989; 139: 921–926. doi: 10.1164/ajrccm/139.4.921 [DOI] [PubMed] [Google Scholar]
- 55.Wesseling GJ, Vanderhoven-Augustin IM, Wouters EF. Forced oscillation technique and spirometry in cold air provocation tests. Thorax 1993; 48: 254–259. doi: 10.1136/thx.48.3.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wouters EF, Polko AH, Schouten HJ, et al. . Contribution of impedance measurement of the respiratory system to bronchial challenge tests. J Asthma 1988; 25: 259–267. doi: 10.3109/02770908809073211 [DOI] [PubMed] [Google Scholar]
- 57.Mansur AH, Manney S, Ayres JG. Methacholine-induced asthma symptoms correlate with impulse oscillometry but not spirometry. Respir Med 2008; 102: 42–49. doi: 10.1016/j.rmed.2007.08.007 [DOI] [PubMed] [Google Scholar]
- 58.Tsurikisawa N, Oshikata C, Tsuburai T, et al. . Physiologic airway responses to inhaled histamine and acetylcholine in patients with mild asthma as analyzed by forced oscillation. Arerugi 2015; 64: 952–970. [DOI] [PubMed] [Google Scholar]
- 59.Alblooshi AS, Simpson SJ, Stick SM, et al. . The safety and feasibility of the inhaled mannitol challenge test in young children. Eur Respir J 2013; 42: 1420–1423. doi: 10.1183/09031936.00041713 [DOI] [PubMed] [Google Scholar]
- 60.Bisgaard H, Klug B. Lung function measurement in awake young children. Eur Respir J 1995; 8: 2067–2075. doi: 10.1183/09031936.95.08122067 [DOI] [PubMed] [Google Scholar]
- 61.Hall GL, Gangell C, Fukushima T, et al. . Application of a shortened inhaled adenosine-5′-monophosphate challenge in young children using the forced oscillation technique. Chest 2009; 136: 184–189. doi: 10.1378/chest.08-2848 [DOI] [PubMed] [Google Scholar]
- 62.Kalliola S, Malmberg LP, Kajosaari M, et al. . Assessing direct and indirect airway hyperresponsiveness in children using impulse oscillometry. Ann Allergy Asthma Immunol 2014; 113: 166–172. doi: 10.1016/j.anai.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 63.Malmberg LP, Makela MJ, Mattila PS, et al. . Exercise-induced changes in respiratory impedance in young wheezy children and nonatopic controls. Pediatr Pulmonol 2008; 43: 538–544. doi: 10.1002/ppul.20805 [DOI] [PubMed] [Google Scholar]
- 64.Nielsen KG, Bisgaard H. The effect of inhaled budesonide on symptoms, lung function and cold air and methacholine responsivenesss in 2- to 5-year-old asthmatic children. Am J Respir Crit Care Med 2000; 162: 1500–1506. doi: 10.1164/ajrccm.162.4.2002019 [DOI] [PubMed] [Google Scholar]
- 65.Schulze J, Smith HJ, Fuchs J, et al. . Methacholine challenge in young children as evaluated by spirometry and impulse oscillometry. Respir Med 2012; 106: 627–634. doi: 10.1016/j.rmed.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 66.Marchal F, Mazurek H, Habib M, et al. . Input respiratory impedance to estimate airway hyperreactivity in children: standard method versus head generator. Eur Respir J 1994; 7: 601–607. doi: 10.1183/09031936.94.07030601 [DOI] [PubMed] [Google Scholar]
- 67.Pellegrino R, Sterk PJ, Sont JK, et al. . Assessing the effect of deep inhalation on airway calibre: a novel approach to lung function in bronchial asthma and COPD. Eur Respir J 1998; 12: 1219–1227. doi: 10.1183/0903.1936.98.12051219 [DOI] [PubMed] [Google Scholar]
- 68.Skloot G, Togias A. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clin Rev Allergy Immunol 2003; 24: 55–72. doi: 10.1385/CRIAI:24:1:55 [DOI] [PubMed] [Google Scholar]
- 69.Lutchen KR, Jensen A, Atileh H, et al. . Airway constriction pattern is a central component of asthma severity. The role of deep inspirations. Am J Respir Crit Care Med 2001; 164: 207–215. doi: 10.1164/ajrccm.164.2.2008119 [DOI] [PubMed] [Google Scholar]
- 70.Weersink EJ, van den Elshout FJ, van Herwaarden CV, et al. . Bronchial responsiveness to histamine and methacholine measured with forced expirations and with the forced oscillation technique. Respir Med 1995; 89: 351–356. doi: 10.1016/0954-6111(95)90007-1 [DOI] [PubMed] [Google Scholar]
- 71.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 1995; 96: 2393–2403. doi: 10.1172/JCI118296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi SH, Sheen YH, Kim MA, et al. . Clinical implications of oscillatory lung function during methacholine bronchoprovocation testing of preschool children. Biomed Res Int 2017; 2017: 9460190. doi: 10.1155/2017/9460190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seccombe LM, Peters MJ, Buddle L, et al. . Exercise-induced bronchoconstriction identified using the forced oscillation technique. Front Physiol 2019; 10: 1411. doi: 10.3389/fphys.2019.01411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger KI, Kalish S, Shao Y, et al. . Isolated small airway reactivity during bronchoprovocation as a mechanism for respiratory symptoms in WTC dust-exposed community members. Am J Ind Med 2016; 59: 767–776. doi: 10.1002/ajim.22639 [DOI] [PubMed] [Google Scholar]
- 75.Segal LN, Goldring RM, Oppenheimer BW, et al. . Disparity between proximal and distal airway reactivity during methacholine challenge. COPD 2011; 8: 145–152. doi: 10.3109/15412555.2011.560127 [DOI] [PubMed] [Google Scholar]
- 76.Hoshino M. Comparison of effectiveness in ciclesonide and fluticasone propionate on small airway function in mild asthma. Allergol Int 2010; 59: 59–66. doi: 10.2332/allergolint.09-OA-0122 [DOI] [PubMed] [Google Scholar]
- 77.Yamaguchi M, Niimi A, Ueda T, et al. . Effect of inhaled corticosteroids on small airways in asthma: investigation using impulse oscillometry. Pulm Pharmacol Ther 2009; 22: 326–332. doi: 10.1016/j.pupt.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 78.Moeller A, Lehmann A, Knauer N, et al. . Effects of montelukast on subjective and objective outcome measures in preschool asthmatic children. Pediatr Pulmonol 2008; 43: 179–186. doi: 10.1002/ppul.20753 [DOI] [PubMed] [Google Scholar]
- 79.Hozawa S, Terada M, Hozawa M. Comparison of budesonide/formoterol Turbuhaler with fluticasone/salmeterol Diskus for treatment effects on small airway impairment and airway inflammation in patients with asthma. Pulm Pharmacol Ther 2011; 24: 571–576. doi: 10.1016/j.pupt.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 80.Houghton CM, Lawson N, Borrill ZL, et al. . Comparison of the effects of salmeterol/fluticasone propionate with fluticasone propionate on airway physiology in adults with mild persistent asthma. Respir Res 2007; 8: 52. doi: 10.1186/1465-9921-8-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larsen G, Morgan W, Heldt G, et al. . Impulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthma. J Allergy Clin Immunol 2009; 123: 861–867. doi: 10.1016/j.jaci.2008.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang FSM, Rutting S, Farrow CE, et al. . Ventilation heterogeneity and oscillometry predict asthma control improvement following step-up inhaled therapy in uncontrolled asthma. Respirology 2020; 25: 827–835. doi: 10.1111/resp.13772 [DOI] [PubMed] [Google Scholar]
- 83.Sugawara H, Saito A, Yokoyama S, et al. . A retrospective analysis of usefulness of impulse oscillometry system in the treatment of asthma. Respir Res 2020; 21: 226. doi: 10.1186/s12931-020-01494-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antonicelli L, Tontini C, Marchionni A, et al. . Forced oscillation technique as method to document and monitor the efficacy of mepolizumab in treating severe eosinophilic asthma. Allergy 2020; 75: 433–436. doi: 10.1111/all.13938 [DOI] [PubMed] [Google Scholar]
- 85.Kerby GS, Rosenfeld M, Ren CL, et al. . Lung function distinguishes preschool children with CF from healthy controls in a multi-center setting. Pediatr Pulmonol 2012; 47: 597–605. doi: 10.1002/ppul.21589 [DOI] [PubMed] [Google Scholar]
- 86.Nielsen KG, Pressler T, Klug B, et al. . Serial lung function and responsiveness in cystic fibrosis during early childhood. Am J Respir Crit Care Med 2004; 169: 1209–1216. doi: 10.1164/rccm.200303-347OC [DOI] [PubMed] [Google Scholar]
- 87.Solymar L, Aronsson P, Sixt R. The forced oscillation technique in children with respiratory disease. Pediatr Pulmonol 1985; 1: 256–261. doi: 10.1002/ppul.1950010507 [DOI] [PubMed] [Google Scholar]
- 88.Gangell CL, Horak F, Patterson HJ, et al. . Respiratory impedance in children with cystic fibrosis using forced oscillations in clinic. Eur Respir J 2007; 30: 892–897. doi: 10.1183/09031936.00003407 [DOI] [PubMed] [Google Scholar]
- 89.Moreau L, Crenesse D, Berthier F, et al. . Relationship between impulse oscillometry and spirometric indices in cystic fibrosis children. Acta Paediatr 2009; 98: 1019–1023. doi: 10.1111/j.1651-2227.2009.01246.x [DOI] [PubMed] [Google Scholar]
- 90.Ramsey KA, Ranganathan SC, Gangell CL, et al. . Impact of lung disease on respiratory impedance in young children with cystic fibrosis. Eur Respir J 2015; 46: 1672–1679. doi: 10.1183/13993003.00156-2015 [DOI] [PubMed] [Google Scholar]
- 91.Ren CL, Rosenfeld M, Mayer OH, et al. . Analysis of the associations between lung function and clinical features in preschool children with cystic fibrosis. Pediatr Pulmonol 2012; 47: 574–581. doi: 10.1002/ppul.21590 [DOI] [PubMed] [Google Scholar]
- 92.Zannin E, Nyilas S, Ramsey KA, et al. . Within-breath changes in respiratory system impedance in children with cystic fibrosis. Pediatr Pulmonol 2019; 54: 737–742. doi: 10.1002/ppul.24281 [DOI] [PubMed] [Google Scholar]
- 93.Lima AN, Faria AC, Lopes AJ, et al. . Forced oscillations and respiratory system modeling in adults with cystic fibrosis. Biomed Eng 2015; 14: 11. doi: 10.1186/s12938-015-0007-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ribeiro CO, Faria ACD, Lopes AJ, et al. . Forced oscillation technique for early detection of the effects of smoking and COPD: contribution of fractional-order modeling. Int J Chron Obstruct Pulmon Dis 2018; 13: 3281–3295. doi: 10.2147/COPD.S173686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faria AC, Costa AA, Lopes AJ, et al. . Forced oscillation technique in the detection of smoking-induced respiratory alterations: diagnostic accuracy and comparison with spirometry. Clinics 2010; 65: 1295–1304. doi: 10.1590/S1807-59322010001200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faria AC, Lopes AJ, Jansen JM, et al. . Evaluating the forced oscillation technique in the detection of early smoking-induced respiratory changes. Biomed Eng 2009; 8: 22. doi: 10.1186/1475-925X-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jetmalani K, Thamrin C, Farah CS, et al. . Peripheral airway dysfunction and relationship with symptoms in smokers with preserved spirometry. Respirology 2017; 23: 512–518. doi: 10.1111/resp.13215 [DOI] [PubMed] [Google Scholar]
- 98.Shinke H, Yamamoto M, Hazeki N, et al. . Visualized changes in respiratory resistance and reactance along a time axis in smokers: a cross-sectional study. Respir Investig 2013; 51: 166–174. doi: 10.1016/j.resinv.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 99.Crim C, Celli B, Edwards LD, et al. . Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med 2011; 105: 1069–1078. doi: 10.1016/j.rmed.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 100.Di Mango AM, Lopes AJ, Jansen JM, et al. . Changes in respiratory mechanics with increasing degrees of airway obstruction in COPD: detection by forced oscillation technique. Respir Med 2006; 100: 399–410. doi: 10.1016/j.rmed.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 101.Milne S, Jetmalani K, Chapman DG, et al. . Respiratory system reactance reflects communicating lung volume in chronic obstructive pulmonary disease. J Appl Physiol 2019; 126: 1223–1231. doi: 10.1152/japplphysiol.00503.2018 [DOI] [PubMed] [Google Scholar]
- 102.Milne S, Hammans C, Watson S, et al. . Bronchodilator responses in respiratory impedance, hyperinflation and gas trapping in COPD. COPD 2018; 15: 341–349. doi: 10.1080/15412555.2018.1458217 [DOI] [PubMed] [Google Scholar]
- 103.Tse HN, Tseng CZ, Wong KY, et al. . Accuracy of forced oscillation technique to assess lung function in geriatric COPD population. Int J Chron Obstruct Pulmon Dis 2016; 11: 1105–1118. doi: 10.2147/COPD.S102222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eddy RL, Westcott A, Maksym GN, et al. . Oscillometry and pulmonary magnetic resonance imaging in asthma and COPD. Physiol Rep 2019; 7: e13955. doi: 10.14814/phy2.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ostridge K, Gove K, Paas KHW, et al. . Using novel computed tomography analysis to describe the contribution and distribution of emphysema and small airways disease in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2019; 16: 990–997. doi: 10.1513/AnnalsATS.201810-669OC [DOI] [PubMed] [Google Scholar]
- 106.Su ZQ, Guan WJ, Li SY, et al. . Significances of spirometry and impulse oscillometry for detecting small airway disorders assessed with endobronchial optical coherence tomography in COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 3031–3044. doi: 10.2147/COPD.S172639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zimmermann SC, Thamrin C, Chan AS, et al. . Relationships between forced oscillatory impedance and 6-minute walk distance after pulmonary rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis 2020; 15: 157–166. doi: 10.2147/COPD.S225543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amaral JL, Lopes AJ, Faria AC, et al. . Machine learning algorithms and forced oscillation measurements to categorise the airway obstruction severity in chronic obstructive pulmonary disease. Comput Methods Programs Biomed 2015; 118: 186–197. doi: 10.1016/j.cmpb.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 109.Borrill ZL, Houghton CM, Tal-Singer R, et al. . The use of plethysmography and oscillometry to compare long-acting bronchodilators in patients with COPD. Br J Clin Pharmacol 2008; 65: 244–252. doi: 10.1111/j.1365-2125.2007.03013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abe T, Setoguchi Y, Kono Y, et al. . Effects of inhaled tiotropium plus transdermal tulobuterol versus tiotropium alone on impulse oscillation system (IOS)-assessed measures of peripheral airway resistance and reactance, lung function and quality of life in patients with COPD: a randomized crossover study. Pulm Pharmacol Ther 2011; 24: 617–624. doi: 10.1016/j.pupt.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 111.Molino A, Simioli F, Stanziola AA, et al. . Effects of combination therapy indacaterol/glycopyrronium versus tiotropium on moderate to severe COPD: evaluation of impulse oscillometry and exacerbation rate. Multidiscip Respir Med 2017; 12: 25. doi: 10.1186/s40248-017-0105-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dellacà R, Pompilio PP, Walker PP, et al. . Effect of bronchodilation on expiratory flow limitation and resting lung mechanics in COPD. Eur Respir J 2009; 33: 1329–1337. doi: 10.1183/09031936.00139608 [DOI] [PubMed] [Google Scholar]
- 113.Diba C, King GG, Berend N, et al. . Improved respiratory system conductance following bronchodilator predicts reduced exertional dyspnoea. Respir Med 2011; 105: 1345–1351. doi: 10.1016/j.rmed.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 114.Timmins SC, Diba C, Schoeffel RE, et al. . Changes in oscillatory impedance and nitrogen washout with combination fluticasone/salmeterol therapy in COPD. Respir Med 2014; 108: 344–350. doi: 10.1016/j.rmed.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 115.Jetmalani K, Timmins S, Brown NJ, et al. . Expiratory flow limitation relates to symptoms during COPD exacerbations requiring hospital admission. Int Obstruct Pulmon Dis 2015; 10: 939–945. doi: 10.2147/COPD.S78332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson MK, Birch M, Carter R, et al. . Measurement of physiological recovery from exacerbation of chronic obstructive pulmonary disease using within-breath forced oscillometry. Thorax 2007; 62: 299–306. doi: 10.1136/thx.2006.061044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stevenson NJ, Walker PP, Costello RW, et al. . Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am Crit Care Med 2005; 172: 1510–1516. doi: 10.1164/rccm.200504-595OC [DOI] [PubMed] [Google Scholar]
- 118.da Costa GM, Faria AC, Di Mango AM, et al. . Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respiration 2014; 88: 101–111. doi: 10.1159/000362691 [DOI] [PubMed] [Google Scholar]
- 119.Ohishi J, Kurosawa H, Ogawa H, et al. . Application of impulse oscillometry for within-breath analysis in patients with chronic obstructive pulmonary disease: pilot study. BMJ Open 2011; 1: e000184. doi: 10.1136/bmjopen-2011-000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silva KK, Faria AC, Lopes AJ, et al. . Within-breath respiratory impedance and airway obstruction in patients with chronic obstructive pulmonary disease. Clinics 2015; 70: 461–469. doi: 10.6061/clinics/2015(07)01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamauchi Y, Kohyama T, Jo T, et al. . Dynamic change in respiratory resistance during inspiratory and expiratory phases of tidal breathing in patients with chronic obstructive pulmonary disease. Int Obstruct Pulmon Dis 2012; 7: 259–269. doi: 10.2147/COPD.S30399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kurosawa H, Kohzuki M. Images in clinical medicine. Dynamic airway narrowing. N Engl J Med 2004; 350: 1036. doi: 10.1056/NEJMicm030626 [DOI] [PubMed] [Google Scholar]
- 123.Dellacà R, Santus P, Aliverti A, et al. . Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J 2004; 23: 232–240. doi: 10.1183/09031936.04.00046804 [DOI] [PubMed] [Google Scholar]
- 124.Dellaca RL, Duffy N, Pompilio PP, et al. . Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. Eur Respir J 2007; 29: 363–374. doi: 10.1183/09031936.00038006 [DOI] [PubMed] [Google Scholar]
- 125.Aarli BB, Calverley PM, Jensen RL, et al. . Variability of within-breath reactance in COPD patients and its association with dyspnoea. Eur Respir J 2015; 45: 625–634. doi: 10.1183/09031936.00051214 [DOI] [PubMed] [Google Scholar]
- 126.Aarli BB, Calverley PM, Jensen RL, et al. . The association of tidal EFL with exercise performance, exacerbations, and death in COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 2179–2188. doi: 10.2147/COPD.S138720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamagami H, Tanaka A, Kishino Y, et al. . Association between respiratory impedance measured by forced oscillation technique and exacerbations in patients with COPD. Int J Chron Obstruct Pulmon Dis 2018; 13: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Milesi I, Porta R, Barbano L, et al. . Automatic tailoring of the lowest PEEP to abolish tidal expiratory flow limitation in seated and supine COPD patients. Respir Med 2019; 155: 13–18. doi: 10.1016/j.rmed.2019.06.022 [DOI] [PubMed] [Google Scholar]
- 129.Zannin E, Milesi I, Porta R, et al. . Effect of nocturnal EPAP titration to abolish tidal expiratory flow limitation in COPD patients with chronic hypercapnia: a randomized, cross-over pilot study. Respir Res 2020; 21: 301. doi: 10.1186/s12931-020-01567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zimmermann SC, Huvanandana J, Nguyen CD, et al. . Day-to-day variability of forced oscillatory mechanics for early detection of acute exacerbations in COPD. Eur Respir J 2020; 56: 1901739. doi: 10.1183/13993003.01739-2019 [DOI] [PubMed] [Google Scholar]
- 131.Cairncross A, Jones R, James A, et al. . Airway wall response to deep inspiration in COPD. Eur Respir J 2016; 48: Suppl. 60, OA3520. doi: 10.1183/13993003.congress-2016.OA3520 [DOI] [Google Scholar]
- 132.Scichilone N, La Sala A, Bellia M, et al. . The airway response to deep inspirations decreases with COPD severity and is associated with airway distensibility assessed by computed tomography. J Appl Physiol 2008; 105: 832–838. doi: 10.1152/japplphysiol.01307.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rutting S, Badal T, Wallis R, et al. . Long-term variability of oscillatory impedance in stable obstructive airways disease. Eur Respir J 2021; 58: 2004318. doi: 10.1183/13993003.04318-2020 [DOI] [PubMed] [Google Scholar]
- 134.Timmins SC, Coatsworth N, Palnitkar G, et al. . Day-to-day variability of oscillatory impedance and spirometry in asthma and COPD. Respir Physiol Neurobiol 2013; 185: 416–424. doi: 10.1016/j.resp.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 135.Lee E, Yoon J, Cho HJ, et al. . Respiratory reactance in children aged three to five years with postinfectious bronchiolitis obliterans is higher than in those with asthma. Acta Paediatr 2017; 106: 81–86. doi: 10.1111/apa.13632 [DOI] [PubMed] [Google Scholar]
- 136.Cho E, Wu JKY, Birriel DC, et al. . Airway oscillometry detects spirometric-silent episodes of acute cellular rejection. Am J Respir Crit Care Med 2020; 201: 1536–1544. doi: 10.1164/rccm.201908-1539OC [DOI] [PubMed] [Google Scholar]
- 137.Hamakawa H, Sakai H, Takahashi A, et al. . Forced oscillation technique as a non-invasive assessment for lung transplant recipients. Adv Exp Med Biol 2010; 662: 293–298. doi: 10.1007/978-1-4419-1241-1_42 [DOI] [PubMed] [Google Scholar]
- 138.Ochman M, Wojarski J, Wiorek A, et al. . Usefulness of the impulse oscillometry system in graft function monitoring in lung transplant recipients. Transplant Proc 2018; 50: 2070–2074. doi: 10.1016/j.transproceed.2017.12.060 [DOI] [PubMed] [Google Scholar]
- 139.Mahadev S, Farah CS, King GG, et al. . Obesity, expiratory flow limitation and asthma symptoms. Pulm Pharmacol Ther 2013; 26: 438–443. doi: 10.1016/j.pupt.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 140.Mahadev S, Salome CM, Berend N, et al. . The effect of low lung volume on airway function in obesity. Respir Physiol Neurobiol 2013; 188: 192–199. doi: 10.1016/j.resp.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 141.Salome CM, Munoz PA, Berend N, et al. . Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes 2008; 32: 502–509. doi: 10.1038/sj.ijo.0803752 [DOI] [PubMed] [Google Scholar]
- 142.Al-Alwan A, Bates JH, Chapman DG, et al. . The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med 2014; 189: 1494–1502. doi: 10.1164/rccm.201401-0178OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oppenheimer BW, Berger KI, Ali S, et al. . Pulmonary vascular congestion: a mechanism for distal lung unit dysfunction in obesity. PLoS ONE 2016; 11: e0152769. doi: 10.1371/journal.pone.0152769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oppenheimer BW, Berger KI, Segal LN, et al. . Airway dysfunction in obesity: response to voluntary restoration of end expiratory lung volume. PLoS ONE 2014; 9: e88015. doi: 10.1371/journal.pone.0088015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oppenheimer BW, Macht R, Goldring RM, et al. . Distal airway dysfunction in obese subjects corrects after bariatric surgery. Surg Obes Relat Dis 2012; 8: 582–589. doi: 10.1016/j.soard.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 146.Peters U, Dechman G, Hernandez P, et al. . Improvement in upright and supine lung mechanics with bariatric surgery affects bronchodilator responsiveness and sleep quality. J Appl Physiol 2018; 125: 1305–1314. doi: 10.1152/japplphysiol.00694.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol 2010; 108: 206–211. doi: 10.1152/japplphysiol.00694.2009 [DOI] [PubMed] [Google Scholar]
- 148.Dattani RS, Swerner CB, Stradling JR, et al. . Exploratory study into the effect of abdominal mass loading on airways resistance and ventilatory failure. BMJ Open Respir Res 2016; 3: e000138. doi: 10.1136/bmjresp-2016-000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yap JC, Watson RA, Gilbey S, et al. . Effects of posture on respiratory mechanics in obesity. J Appl Physiol 1995; 79: 1199–1205. doi: 10.1152/jappl.1995.79.4.1199 [DOI] [PubMed] [Google Scholar]
- 150.Zerah F, Harf A, Perlemuter L, et al. . Effects of obesity on respiratory resistance. Chest 1993; 103: 1470–1476. doi: 10.1378/chest.103.5.1470 [DOI] [PubMed] [Google Scholar]
- 151.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol 2005; 98: 512–517. doi: 10.1152/japplphysiol.00430.2004 [DOI] [PubMed] [Google Scholar]
- 152.Rochester DF, Enson Y. Current concepts in the pathogenesis of the obesity-hypoventilation syndrome. Mechanical and circulatory factors. Am J Med 1974; 57: 402–420. doi: 10.1016/0002-9343(74)90135-1 [DOI] [PubMed] [Google Scholar]
- 153.Kaltman AJ, Goldring RM. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med 1976; 60: 645–653. doi: 10.1016/0002-9343(76)90499-X [DOI] [PubMed] [Google Scholar]
- 154.Peters U, Hernandez P, Dechman G, et al. . Early detection of changes in lung mechanics with oscillometry following bariatric surgery in severe obesity. Appl Physiol Nutr Metab 2016; 41: 538–547. doi: 10.1139/apnm-2015-0473 [DOI] [PubMed] [Google Scholar]
- 155.Skloot G, Schechter C, Desai A, et al. . Impaired response to deep inspiration in obesity. J Appl Physiol 2011; 111: 726–734. doi: 10.1152/japplphysiol.01155.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forno E, Weiner DJ, Mullen J, et al. . Obesity and airway dysanapsis in children with and without asthma. Am J Respir Crit Care Med 2017; 195: 314–323. doi: 10.1164/rccm.201605-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jones MH, Roncada C, Fernandes MTC, et al. . Asthma and obesity in children are independently associated with airway dysanapsis. Front Pediatr 2017; 5: 270. doi: 10.3389/fped.2017.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ekstrom S, Hallberg J, Kull I, et al. . Body mass index status and peripheral airway obstruction in school-age children: a population-based cohort study. Thorax 2018; 73: 538–545. doi: 10.1136/thoraxjnl-2017-210716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Noord JA, Clement J, Cauberghs M, et al. . Total respiratory resistance and reactance in patients with diffuse interstitial lung disease. Eur Respir J 1989; 2: 846–852. [PubMed] [Google Scholar]
- 160.Sokai R, Ito S, Iwano S, et al. . Respiratory mechanics measured by forced oscillation technique in rheumatoid arthritis-related pulmonary abnormalities: frequency-dependence, heterogeneity and effects of smoking. Springerplus 2016; 5: 335. doi: 10.1186/s40064-016-1952-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.de Mesquita Júnior JA, Lopes AJ, Jansen JM, et al. . Using the forced oscillation technique to evaluate respiratory resistance in individuals with silicosis. J Bras Pneumol 2006; 32: 213–220. doi: 10.1590/S1806-37132006000300007 [DOI] [PubMed] [Google Scholar]
- 162.Sugiyama A, Hattori N, Haruta Y, et al. . Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir Med 2013; 107: 875–882. doi: 10.1016/j.rmed.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 163.Aronsson D, Hesselstrand R, Bozovic G, et al. . Airway resistance and reactance are affected in systemic sclerosis. Eur Clin Respir J 2015; 2: 28667. doi: 10.3402/ecrj.v2.28667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fujii M, Shirai T, Mori K, et al. . Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir Physiol Neurobiol 2015; 207: 22–27. doi: 10.1016/j.resp.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 165.Lopes AJ, Mogami R, Camilo GB, et al. . Relationships between the pulmonary densitometry values obtained by CT and the forced oscillation technique parameters in patients with silicosis. Br J Radiol 2015; 88: 20150028. doi: 10.1259/bjr.20150028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takeichi N, Yamazaki H, Fujimoto K. Comparison of impedance measured by the forced oscillation technique and pulmonary functions, including static lung compliance, in obstructive and interstitial lung disease. Int J Chron Obstruct Pulmon Dis 2019; 14: 1109–1118. doi: 10.2147/COPD.S198030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.De Troyer A, Borenstein S, Cordier R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax 1980; 35: 603–610. doi: 10.1136/thx.35.8.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gibson GJ, Pride NB, Davis JN, et al. . Pulmonary mechanics in patients with respiratory muscle weakness. Am Rev Respir Dis 1977; 115: 389–395. [DOI] [PubMed] [Google Scholar]
- 169.Allen J. Pulmonary complications of neuromuscular disease: a respiratory mechanics perspective. Paediatr Respir Rev 2010; 11: 18–23. doi: 10.1016/j.prrv.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 170.Al-Alwan A, Kaminsky D. Vocal cord dysfunction in athletes: clinical presentation and review of the literature. Phys Sportsmed 2012; 40: 22–27. doi: 10.3810/psm.2012.05.1961 [DOI] [PubMed] [Google Scholar]
- 171.Rigau J, Farre R, Trepat X, et al. . Oscillometric assessment of airway obstruction in a mechanical model of vocal cord dysfunction. J Biomech 2004; 37: 37–43. doi: 10.1016/S0021-9290(03)00256-2 [DOI] [PubMed] [Google Scholar]
- 172.Farre R, Navajas D. Forced oscillation: a poorly exploited tool for simply assessing respiratory function in children. Respirology 2016; 21: 982–983. doi: 10.1111/resp.12842 [DOI] [PubMed] [Google Scholar]
- 173.Komarow HD, Young M, Nelson C, et al. . Vocal cord dysfunction as demonstrated by impulse oscillometry. J Allergy Clin Immunol Pract 2013; 1: 387–393. doi: 10.1016/j.jaip.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bey A, Botti S, Coutier-Marie L, et al. . Bronchial or laryngeal obstruction induced by exercise? Front Pediatr 2017; 5: 150. doi: 10.3389/fped.2017.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ioan I, Marchal F, Coffinet L, et al. . Breathing-related changes of respiratory resistance in vocal cord dysfunction. Respirology 2016; 21: 1134–1136. doi: 10.1111/resp.12735 [DOI] [PubMed] [Google Scholar]
- 176.Schweitzer C, Chone C, Marchal F. Influence of data filtering on reliability of respiratory impedance and derived parameters in children. Pediatr Pulmonol 2003; 36: 502–508. doi: 10.1002/ppul.10359 [DOI] [PubMed] [Google Scholar]
- 177.Higenbottam T. Narrowing of glottis opening in humans associated with experimentally induced bronchoconstriction. J Appl Physiol Respir Environ Exerc Physiol 1980; 49: 403–407. [DOI] [PubMed] [Google Scholar]
- 178.Farré R, Peslin R, Rotger M, et al. . Inspiratory dynamic obstruction detected by forced oscillation during CPAP. A model study. Am J Respir Crit Care Med 1997; 155: 952–956. doi: 10.1164/ajrccm.155.3.9117031 [DOI] [PubMed] [Google Scholar]
- 179.Farré R, Montserrat JM, Navajas D. Noninvasive monitoring of respiratory mechanics during sleep. Eur Respir J 2004; 24: 1052–1060. doi: 10.1183/09031936.04.00072304 [DOI] [PubMed] [Google Scholar]
- 180.Farré R, Montserrat JM, Navajas D. Assessment of upper airway mechanics during sleep. Respir Physiol Neurobiol 2008; 163: 74–81. doi: 10.1016/j.resp.2008.06.017 [DOI] [PubMed] [Google Scholar]
- 181.Lorino AM, Lofaso F, Dahan E, et al. . Respiratory impedance response to continuous negative airway pressure in awake controls and OSAS. Eur Respir J 2001; 17: 71–78. doi: 10.1183/09031936.01.17100710 [DOI] [PubMed] [Google Scholar]
- 182.Cao J, Que C, Wang G, et al. . Effect of posture on airway resistance in obstructive sleep apnea-hypopnea syndrome by means of impulse oscillation. Respiration 2009; 77: 38–43. doi: 10.1159/000114146 [DOI] [PubMed] [Google Scholar]
- 183.Badia JR, Farré R, Montserrat JM, et al. . Forced oscillation technique for the evaluation of severe sleep apnoea/hypopnoea syndrome: a pilot study. Eur Respir J 1998; 11: 1128–1134. doi: 10.1183/09031936.98.11051128 [DOI] [PubMed] [Google Scholar]
- 184.Badia JR, Farre R, Rigau J, et al. . Forced oscillation measurements do not affect upper airway muscle tone or sleep in clinical studies. Eur Respir J 2001; 18: 335–339. doi: 10.1183/09031936.01.00085001 [DOI] [PubMed] [Google Scholar]
- 185.Jobin V, Rigau J, Beauregard J, et al. . Evaluation of upper airway patency during Cheyne–Stokes breathing in heart failure patients. Eur Respir J 2012; 40: 1523–1530. doi: 10.1183/09031936.00060311 [DOI] [PubMed] [Google Scholar]
- 186.Navajas D, Farré R, Rotger M, et al. . Assessment of airflow obstruction during CPAP by means of forced oscillation in patients with sleep apnea. Am J Respir Crit Care Med 1998; 157: 1526–1530. doi: 10.1164/ajrccm.157.5.9710026 [DOI] [PubMed] [Google Scholar]
- 187.Cao X, Bradley TD, Bhatawadekar SA, et al. . Effect of simulated obstructive apnea on thoracic fluid volume and airway narrowing in asthma. Am J Respir Crit Care Med 2021; 203: 908–910. doi: 10.1164/rccm.202012-4321LE [DOI] [PubMed] [Google Scholar]
- 188.Schermer T, Malbon W, Newbury W, et al. . Spirometry and impulse oscillometry (IOS) for detection of respiratory abnormalities in metropolitan firefighters. Respirology 2010; 15: 975–985. doi: 10.1111/j.1440-1843.2010.01809.x [DOI] [PubMed] [Google Scholar]
- 189.Friedman SM, Maslow CB, Reibman J, et al. . Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med 2011; 184: 582–589. doi: 10.1164/rccm.201011-1909OC [DOI] [PubMed] [Google Scholar]
- 190.Oppenheimer BW, Goldring RM, Herberg ME, et al. . Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007; 132: 1275–1282. doi: 10.1378/chest.07-0913 [DOI] [PubMed] [Google Scholar]
- 191.Keman S, Willemse B, Wesseling GJ, et al. . A five year follow-up of lung function among chemical workers using flow-volume and impedance measurements. Eur Respir J 1996; 9: 2109–2115. doi: 10.1183/09031936.96.09102109 [DOI] [PubMed] [Google Scholar]
- 192.Shao J, Zosky GR, Hall GL, et al. . Early life exposure to coal mine fire smoke emissions and altered lung function in young children. Respirology 2020; 25: 198–205. doi: 10.1111/resp.13617 [DOI] [PubMed] [Google Scholar]
- 193.Schultz ES, Hallberg J, Gustafsson PM, et al. . Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. J Allergy Clin Immunol 2016; 138: 930–2.e5. doi: 10.1016/j.jaci.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 194.Gupta N, Sachdev A, Gupta D. Oscillometry–A reasonable option to monitor lung functions in the era of COVID-19 pandemic. Pediatr Pulmonol 2021; 56: 14–15. doi: 10.1002/ppul.25121 [DOI] [PubMed] [Google Scholar]
- 195.van de Woestijne KP. The forced oscillation technique in intubated, mechanically-ventilated patients. Pediatr Pulmonol 1993; 6: 767–769. [PubMed] [Google Scholar]
- 196.Farre R. Monitoring respiratory mechanics by forced oscillation in ventilated patients. In: Gullo A, ed. Anaesthesia, Pain, Intensive Care and Emergency Medicine – APICE. Berlin Heidelberg, Springer-Verlag, 2001; pp. 195–204. [Google Scholar]
- 197.Navajas D, Farre R. Forced oscillation assessment of respiratory mechanics in ventilated patients. Crit Care 2001; 5: 3–9. doi: 10.1186/cc972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Beydon L, Malassine P, Lorino AM, et al. . Respiratory resistance by end-inspiratory occlusion and forced oscillations in intubated patients. J Appl Physiol 1996; 80: 1105–1111. doi: 10.1152/jappl.1996.80.4.1105 [DOI] [PubMed] [Google Scholar]
- 199.Peslin R, Felico de Silva J, Chabot F, et al. . Respiratory mechanics studied by multiple linear regression in unsedated ventilated patients. Eur Respir J 1992; 5: 871–878. [PubMed] [Google Scholar]
- 200.Peslin R, Felicio da Silva J, Duvivier C, et al. . Respiratory mechanics studied by forced oscillations during artificial ventilation. Eur Respir J 1993; 6: 772–784. [PubMed] [Google Scholar]
- 201.Farre R, Ferrer M, Rotger M, et al. . Servocontrolled generator to measure respiratory impedance from 0.25 to 26 Hz in ventilated patients at different PEEP levels. Eur Respir J 1995; 8: 1222–1227. doi: 10.1183/09031936.95.08071222 [DOI] [PubMed] [Google Scholar]
- 202.Kaczka DW, Ingenito EP, Lutchen KR. Technique to determine inspiratory impedance during mechanical ventilation: implications for flow-limited patients. Ann Biomed Eng 1999; 27: 340–355. doi: 10.1114/1.146 [DOI] [PubMed] [Google Scholar]
- 203.Navajas D, Farre R, Canet J, et al. . Respiratory input impedance in anesthetized paralyzed patients. J Appl Physiol 1990; 69: 1372–1379. doi: 10.1152/jappl.1990.69.4.1372 [DOI] [PubMed] [Google Scholar]
- 204.Albu G, Babik B, Kesmarky K, et al. . Changes in airway and respiratory tissue mechanics after cardiac surgery. Ann Thorac Surg 2010; 89: 1218–1226. doi: 10.1016/j.athoracsur.2009.12.062 [DOI] [PubMed] [Google Scholar]
- 205.Babik B, Petak F, Asztalos T, et al. . Components of respiratory resistance monitored in mechanically ventilated patients. Eur Respir J 2002; 20: 1538–1544. doi: 10.1183/09031936.02.00000802 [DOI] [PubMed] [Google Scholar]
- 206.Barker SJ, Tremper KK. Pressure losses of endotracheal tubes: preformed versus straight tubes. J Clin Eng 1986; 11: 371–376. doi: 10.1097/00004669-198609000-00011 [DOI] [Google Scholar]
- 207.Hentschel R, Buntzel J, Guttmann J, et al. . Endotracheal tube resistance and inertance in a model of mechanical ventilation of newborns and small infants-the impact of ventilator settings on tracheal pressure swings. Physiol Meas 2011; 32: 1439–1451. doi: 10.1088/0967-3334/32/9/007 [DOI] [PubMed] [Google Scholar]
- 208.Kaczka DW, Hager DN, Hawley ML, et al. . Quantifying mechanical heterogeneity in canine acute lung injury: impact of mean airway pressure. Anesthesiology 2005; 103: 306–317. doi: 10.1097/00000542-200508000-00014 [DOI] [PubMed] [Google Scholar]
- 209.Loring SH, Elliott EA, Drazen JM. Kinetic energy loss and convective acceleration in respiratory resistance measurements. Lung 1979; 156: 33–42. doi: 10.1007/BF02713989 [DOI] [PubMed] [Google Scholar]
- 210.Lorino AM, Beydon L, Mariette C, et al. . A new correction technique for measuring respiratory impedance through an endotrachael tube. Eur Respir J 1996; 9: 1079–1086. doi: 10.1183/09031936.96.09051079 [DOI] [PubMed] [Google Scholar]
- 211.Navajas D, Farre R, Rotger M, et al. . Recording pressure at the distal end of the endotracheal tube to measure respiratory impedance. Eur Respir J 1989; 2: 178–184. [PubMed] [Google Scholar]
- 212.Navalesi P, Hernandez P, Laporta D, et al. . Influence of site of tracheal pressure measurement on in situ estimation of endotracheal tube resistance. J Appl Physiol 1994; 77: 2899–2906. doi: 10.1152/jappl.1994.77.6.2899 [DOI] [PubMed] [Google Scholar]
- 213.Scholz AW, Weiler N, David M, et al. . Respiratory mechanics measured by forced oscillations during mechanical ventilation through a tracheal tube. Physiol Meas 2011; 32: 571–583. doi: 10.1088/0967-3334/32/5/006 [DOI] [PubMed] [Google Scholar]
- 214.Schuessler TF, Bates JHT. A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans Biomed Eng 1995; 42: 860–866. doi: 10.1109/10.412653 [DOI] [PubMed] [Google Scholar]
- 215.Sullivan M, Paliotta J, Saklad M. Endotracheal tube as a factor in measurement of respiratory mechanics. J Appl Physiol 1976; 41: 590–592. doi: 10.1152/jappl.1976.41.4.590 [DOI] [PubMed] [Google Scholar]
- 216.Kaczka DW, Ingenito EP, Body SC, et al. . Inspiratory lung impedance in COPD: effects of PEEP and immediate impact of lung volume reduction surgery. J Appl Physiol 2001; 90: 1833–1841. doi: 10.1152/jappl.2001.90.5.1833 [DOI] [PubMed] [Google Scholar]
- 217.Lorx A, Szabó B, Hercsuth M, et al. . Low-frequency assessment of airway and tissue mechanics in ventilated COPD patients. J Appl Physiol 2009; 107: 1884–1892. doi: 10.1152/japplphysiol.00151.2009 [DOI] [PubMed] [Google Scholar]
- 218.Lorx A, Suki B, Hercsuth M, et al. . Airway and tissue mechanics in ventilated patients with pneumonia. Respir Physiol Neurobiol 2010; 171: 101–109. doi: 10.1016/j.resp.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 219.Farre R, Ferrer M, Rotger M, et al. . Respiratory mechanics in ventilated COPD patients: forced oscillation versus occlusion techniques. Eur Respir J 1998; 12: 170–176. doi: 10.1183/09031936.98.12010170 [DOI] [PubMed] [Google Scholar]
- 220.Gauthier R, Beyaert C, Feillet F, et al. . Respiratory oscillation mechanics in infants with bronchiolitis during mechanical ventilation. Pediatr Pulmonol 1998; 25: 18–31. doi: [DOI] [PubMed] [Google Scholar]
- 221.Dellaca RL, Andersson Olerud M, Zannin E, et al. . Lung recruitment assessed by total respiratory system input reactance. Intensive Care Med 2009; 35: 2164–2172. doi: 10.1007/s00134-009-1673-3 [DOI] [PubMed] [Google Scholar]
- 222.Kaczka DW, Cao K, Christensen GE, et al. . Analysis of regional mechanics in canine lung injury using forced oscillations and 3D image registration. Ann Biomed Eng 2011; 39: 1112–1124. doi: 10.1007/s10439-010-0214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dellacà RL, Rotger M, Aliverti A, et al. . Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J 2006; 27: 983–991. doi: 10.1183/09031936.06.00080005 [DOI] [PubMed] [Google Scholar]
- 224.Suh ES, Pompilio P, Mandal S, et al. . Autotitrating external positive end-expiratory airway pressure to abolish expiratory flow limitation during tidal breathing in patients with severe COPD: a physiological study. Eur Respir J 2020; 56: 1902234. doi: 10.1183/13993003.02234-2019 [DOI] [PubMed] [Google Scholar]
- 225.Dellaca RL, Veneroni C, Vendettuoli V, et al. . Relationship between respiratory impedance and positive end-expiratory pressure in mechanically ventilated neonates. Intensive Care Med 2013; 39: 511–519. doi: 10.1007/s00134-012-2795-6 [DOI] [PubMed] [Google Scholar]
- 226.Raffaeli G, Veneroni C, Ghirardello S, et al. . Role of lung function monitoring by the forced oscillation technique for tailoring ventilation and weaning in neonatal ECMO: new insights from a case report. Front Pediatr 2018; 6: 332. doi: 10.3389/fped.2018.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Vendettuoli V, Veneroni C, Zannin E, et al. . Positional effects on lung mechanics of ventilated preterm infants with acute and chronic lung disease. Pediatr Pulmonol 2015; 50: 798–804. doi: 10.1002/ppul.23049 [DOI] [PubMed] [Google Scholar]
- 228.Wallström L, Veneroni C, Zannin E, et al. . Forced oscillation technique for optimising PEEP in ventilated extremely preterm infants. Eur Respir J 2020; 55: 1901650. doi: 10.1183/13993003.01650-2019 [DOI] [PubMed] [Google Scholar]
- 229.Veneroni C, Wallström L, Sindelar R, et al. . Oscillatory respiratory mechanics on the first day of life improves prediction of respiratory outcomes in extremely preterm newborns. Pediatr Res 2019; 85: 312–317. doi: 10.1038/s41390-018-0133-6 [DOI] [PubMed] [Google Scholar]
- 230.Peterson-Carmichael S, Seddon PC, Cheifetz IM, et al. . An official American Thoracic Society/European Respiratory Society workshop report: evaluation of respiratory mechanics and function in the pediatric and neonatal intensive care units. Ann Am Thorac Soc 2016; 13 S1–11. doi: 10.1513/AnnalsATS.201511-730ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dellacà RL, Zannin E, Kostic P, et al. . Optimisation of positive end-expiratory pressure by forced oscillation technique in a lavage model of acute lung injury. Intensive Care Med 2011; 37: 1021–1030. doi: 10.1007/s00134-011-2211-7 [DOI] [PubMed] [Google Scholar]
- 232.Sellares J, Acerbi I, Loureiro H, et al. . Respiratory impedance during weaning from mechanical ventilation in a mixed population of critically ill patients. Br J Anaesth 2009; 103: 828–832. doi: 10.1093/bja/aep301 [DOI] [PubMed] [Google Scholar]
- 233.Kostic P, Zannin E, Andersson Olerud M, et al. . Positive end-expiratory pressure optimization with forced oscillation technique reduces ventilator induced lung injury: a controlled experimental study in pigs with saline lavage lung injury. Crit Care 2011; 15: R126. doi: 10.1186/cc10236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zannin E, Dellaca RL, Kostic P, et al. . Optimizing positive end-expiratory pressure by oscillatory mechanics minimizes tidal recruitment and distension: an experimental study in a lavage model of lung injury. Crit Care 2012; 16: R217. doi: 10.1186/cc11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zannin E, Ventura ML, Dellaca RL, et al. . Optimal mean airway pressure during high-frequency oscillatory ventilation determined by measurement of respiratory system reactance. Pediatr Res 2014; 75: 493–499. doi: 10.1038/pr.2013.251 [DOI] [PubMed] [Google Scholar]
- 236.Babik B, Asztalos T, Petak F, et al. . Changes in respiratory mechanics during cardiac surgery. Anesth Analg 2003; 96: 1280–1287. doi: 10.1213/01.ANE.0000055363.23715.40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0208-2021.SUPPLEMENT (267KB, pdf)