Abstract
Epidemiological data for chronic thromboembolic pulmonary hypertension (CTEPH) are limited and there are conflicting reports regarding its pathogenesis.
A literature review was conducted to identify CTEPH epidemiological data up to June 2014. Data were analysed to provide estimates of the incidence of CTEPH in the USA, Europe and Japan. An epidemiological projection model derived country-specific estimates of future incidence and diagnosis rates of CTEPH.
Overall, 25 publications and 14 databases provided quantitative epidemiological data. In the USA and Europe, the crude annual incidence of diagnosed pulmonary embolism and crude annual full (i.e. diagnosed and undiagnosed) incidence of CTEPH were 66–104 and 3–5 cases per 100 000 population, respectively, while in Japan these rates were lower at 6.7 and 1.9 per 100 000 population, respectively. In 2013, 7–29% of CTEPH cases in Europe and the USA were diagnosed, and the majority of patients were in New York Heart Association functional class III/IV at diagnosis. The projection model indicated that incidence of CTEPH will continue to increase over the next decade.
These data suggest that CTEPH is underdiagnosed and undertreated, and there is an urgent need to increase awareness of CTEPH. High-quality epidemiological studies are required to increase understanding of CTEPH.
Short abstract
Epidemiological data suggest that CTEPH is underdiagnosed and there is an urgent need to improve disease awareness http://ow.ly/J0KC3095U2W
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary hypertension, classified as group 4 in the European Society of Cardiology (ESC)/European Respiratory Society (ERS) 2015 pulmonary hypertension guidelines [1]. CTEPH is a rare chronic condition characterised by pathological changes to the pulmonary arteries and the presence of occlusive organised thromboembolic material within these arteries [2]. Such pulmonary artery obstructions result in an increase in pulmonary vascular resistance, with progressively worsening pulmonary hypertension and subsequent right ventricular failure, which may eventually be fatal [2–4].
The pathogenesis of CTEPH has yet to be fully elucidated and is the focus of ongoing research. The most widely accepted aetiological theory is that CTEPH arises as a complication of acute pulmonary embolism (PE) subsequent to venous thromboembolism (VTE) [2, 5, 6]. It has been proposed that the underlying mechanism giving rise to CTEPH is a misguided vascular remodelling process involving defective angiogenesis and delayed onset of fibrinolysis associated with endothelial dysfunction that prevents complete resolution of the causative PE [7]. However, questions remain regarding this model of CTEPH pathogenesis as a consequence of conflicting epidemiological data for PE and CTEPH. It has been observed that some classical risk factors for VTE do not increase the risk of CTEPH, and that there is distal/microvascular pulmonary artery disease involvement in some patients with CTEPH [4, 8]. Furthermore, reported rates of CTEPH in patients with a history of PE vary widely [9, 10].
Other risk factors for CTEPH include splenectomy, infected ventriculo-atrial shunts, thyroid replacement therapy, lupus anticoagulant/antiphospholipid antibodies, malignancy, infected pacemaker lines and chronic inflammatory conditions, such as osteomyelitis and inflammatory bowel disease [11, 12].
The gold standard treatment for CTEPH is pulmonary endarterectomy (PEA). PEA is potentially curative and is generally indicated in patients with surgically accessible thrombi in the main, lobar or segmental pulmonary arteries [1, 13]. However, PEA is not feasible in all patients, with 24–37% of cases reported as being inoperable due to distal/microvascular thromboembolic disease beyond the potential reach of PEA, the presence of significant comorbidities or refusal of the patient to undergo surgery [10, 12, 14]. Furthermore, despite the high success rate with PEA, it is not always curative. Among patients undergoing PEA for CTEPH, up to 51% of patients have mean pulmonary arterial pressure >25 mmHg post-PEA [15–20].
Thus there is a need for alternative treatments for patients with CTEPH who are either deemed inoperable or have persistent/recurrent pulmonary hypertension after PEA [13, 21–24]. The soluble guanylate cyclase stimulator riociguat has shown efficacy and is the only pharmacotherapy approved for the treatment of inoperable CTEPH and persistent/recurrent CTEPH [1, 25–28]. An emerging interventional technique for the management of inoperable CTEPH is percutaneous balloon pulmonary angioplasty (BPA), which aims to dilate the pulmonary artery branches through the inflation of a small balloon inserted into the artery. While BPA is still in its infancy and can only be performed at specialist centres by physicians experienced in the technique, it may provide a potential treatment option for patients with inoperable CTEPH [29–33].
Determining CTEPH epidemiology is difficult, and there remains an unmet need regarding epidemiological knowledge of the disease. Although CTEPH is a debilitating and life-threatening condition, the early signs and symptoms are often nonspecific, making differential diagnosis difficult. Furthermore, not all patients with CTEPH have a history of previous PE. This can result in uncertainty over the diagnosis and can delay or prevent referral to a specialist. Such confounding factors are highly likely to have resulted in CTEPH being underdiagnosed, and subsequently undertreated [5, 34, 35]. As a result, data quantifying the burden of CTEPH worldwide are limited, both in terms of quantity and quality.
The epidemiological analysis presented here was designed to estimate the incidence and burden of CTEPH in the USA, Europe and Japan, based on a review of the literature, and to apply these findings to a statistical model, enabling country-specific projections of the future burden of CTEPH to be calculated. Such an analysis will advance understanding of CTEPH and identify areas that will require further research.
Methodology
Literature review strategy
The search strategy was initially developed in Medline using search terms selected to identify relevant scientific publications, specifically those containing original epidemiological findings relating to CTEPH. The terms/keywords used for the search were: “chronic thromboembolic pulmonary hypertension” OR “CTEPH” AND “epidemiology”. A Medical Subject Heading subheading was used for the term “epidemiology”, which includes the terms epidemics frequency, surveillance, morbidity, occurrence, outbreaks, prevalence, endemics and incidence. Due to the lack of literature describing CTEPH epidemiology, no specific criteria for study eligibility were used to include or exclude studies. Publications were deemed ineligible and not included if they did not assess one or more of the predefined variables of interest (see the section on screening of articles and data collection) or reported studies with significant bias in the study population, for example studies involving elderly patients only. In addition, older studies that had been superseded by more recent studies were excluded. The keywords given above were used to conduct Medline and PubMed searches. Publications up to June 2014 were included.
Other data sources
In addition, relevant registries and databases were searched for pertinent data: the USA-based Healthcare Cost and Utilization Project Nationwide Inpatient Sample (2010) [36]; the Spanish National Institute of Statistics INEbase Hospital Morbidity Survey (2009–2010) [37]; the French Institute for Public Health Surveillance (2011) [38]; the Japanese Intractable Disease Survey (CTEPH) (2010) [39]; and the UK Hospital Episode Statistics (2010) [40]. Further data included in the analysis were obtained from the Pulmonary Arterial Hypertension (PAH)/CTEPH Treatment Tracking Study, Ipsos Healthcare (2012) [41, 42], unpublished internal audit data (Papworth Hospital (Cambridge, UK) internal audit, 2013), market research and data from the International CTEPH Association.
Screening of articles and data collection
Country-specific data were collected from the screened publications and sources for the following predefined variables: the incidence rate of acute PE in the general population; the survival rate of patients after an acute PE; the proportion of patients with CTEPH following a PE; the proportion of CTEPH cases without a history of PE; the distribution of patients with CTEPH across New York Heart Association (NYHA) functional class (FC) at diagnosis; the proportion of patients with CTEPH referred and assessed for PEA operability; the proportion of patients with CTEPH deemed to be operable; the proportion of patients with CTEPH who underwent PEA; the proportion of patients with CTEPH who survived PEA; and the proportion of patients with CTEPH who developed persistent/recurrent pulmonary hypertension post-PEA.
If more than a single comparable result was collected for one of the variables listed above, the data were aggregated and a weighted average was calculated. These weighted averages were subsequently used in the epidemiological projection model for CTEPH detailed later.
The quality of each dataset was assessed based on sample size, population validity (whether the risk of CTEPH in the sample was likely to be similar to that in the general population) and whether the demographic characteristics of the sample were representative of the general population, together with the likelihood of bias (e.g. restricted age ranges, geographical regions or treatment facilities) in the patient samples. While these are qualitative criteria, datasets were deemed to be of good quality if the publication contained a clear description of the data collection process and included data mostly from large, representative, unbiased sample populations, and few or no additional calculations were needed.
Epidemiological incidence projection methods
Using country-specific population projections taken from the World Bank's health, nutrition and population statistics database [43, 44], an estimate of the future incidence of PE was derived using the age- and sex-specific rates of PE obtained from the literature review. The survival rate following an episode of PE, as estimated from the literature review, was then applied.
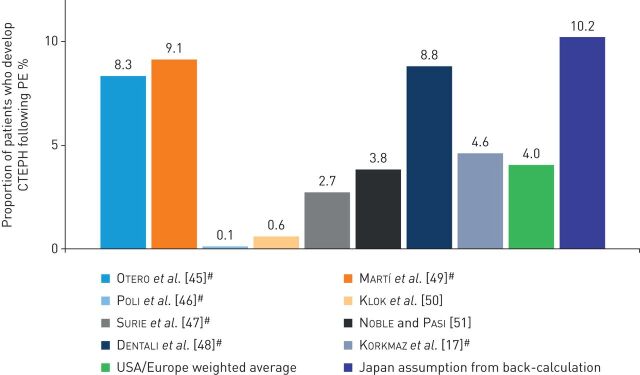
In order to predict the annual full (i.e. diagnosed and undiagnosed) incidence of CTEPH, two approaches were used. For the USA and Europe, the calculation started with the incidence of diagnosed PE (online supplementary figure S1). Taking into account the PE survival rate, the weighted average incidence of CTEPH after an acute PE (4%; figure 1) was applied to the incidence of PE, and then the estimated proportion of patients with CTEPH without a known prior history of PE was applied to give the full annual incidence of CTEPH. Finally, a year- and country-specific diagnosis rate was applied to the full incidence of CTEPH to give the overall number of diagnosed CTEPH cases (online supplementary figure S1). Diagnosis rates for Europe and the USA were back-calculated from the number of PEA surgeries reported in previous years by specialist centres and hospital databases. For Japan, the proportion of patients with PE who subsequently developed CTEPH was back-calculated from the number of patients with CTEPH registered in the Intractable Disease Survey (1590 treated patients were reported in 2011), the incidence of PE [52] and the proportion of CTEPH cases with known PE [53]. Additional assumptions of 20% under-reporting in the Intractable Disease Survey, a diagnosis rate of 20% and a prevalence to incidence ratio of 4.6 were used, with the incidence ratio being determined from a fitted curve of published survival data that showed 50% survival at 5 years and 40% at 10 years post-diagnosis.
FIGURE 1.
Proportion of patients who develop chronic thromboembolic pulmonary hypertension (CTEPH) following pulmonary embolism (PE). #: studies followed-up individual patients after PE.
It was predicted that, through increased disease awareness, the diagnosis rate of CTEPH would increase by +20 percentage points (+10 percentage points in Germany) over the next 20 years, equating to an increase of +1 percentage point per annum in Europe (+0.5 percentage points in Germany) and the USA. For Japan, the diagnosis rate was predicted to increase by +35 percentage points over the next 15 years (2.33 percentage points per annum). The predicted increases were based on the likelihood of increased awareness/education and the availability of medical therapy leading to greater motivation for performing differential diagnostic tests. Finally, diagnosed CTEPH incidence rates were broken down by NYHA FC distribution at diagnosis.
Results
Literature review
The search protocol yielded 1120 potentially relevant publications; 25 were found to provide quantitative epidemiological data relating to the variables of interest that were used in the analysis. In addition, data from 14 other sources (e.g. national databases and market research) were included. Characteristics of the studies are summarised in online supplementary table S1.
Incidence of PE and CTEPH
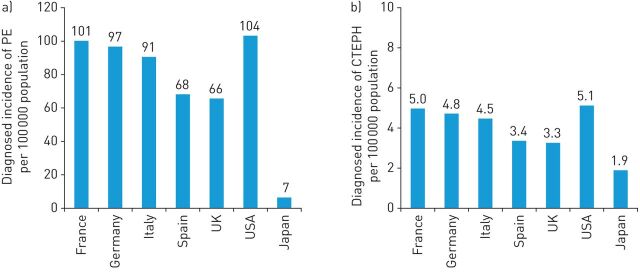
Five data sources gave incidences of PE, including age- and sex-specific rates of PE for the USA and Europe (France, Germany, Spain and the UK) from population-based hospital databases and surveys [36–38, 40, 54]. The crude diagnosed incidence of PE in the USA and Europe ranged from 66 to 104 cases per 100 000 population per year (figure 2). The survival rate at hospital discharge for patients experiencing an acute PE event was reported to be 92% in a large survey of inpatients in the USA [36]. The incidence of PE in Japan was reported in one publication [52] describing a population-based study. The age- and sex-specific rates of PE presented yielded a crude rate of seven cases per 100 000 population per year; much lower than those in the USA and Europe (figure 2).
FIGURE 2.
a) Annual diagnosed incidence of pulmonary embolism (PE), and b) annual full incidence of chronic thromboembolic pulmonary hypertension (CTEPH) per 100 000 population in Europe, the USA and Japan (crude rates for the year 2015).
The calculated crude full incidence (i.e. diagnosed and undiagnosed) of CTEPH in the USA and Europe ranged from 3 to 5 cases per 100 000 population per year, while for Japan the rate was 1.94 per 100 000 population per year (figure 2).
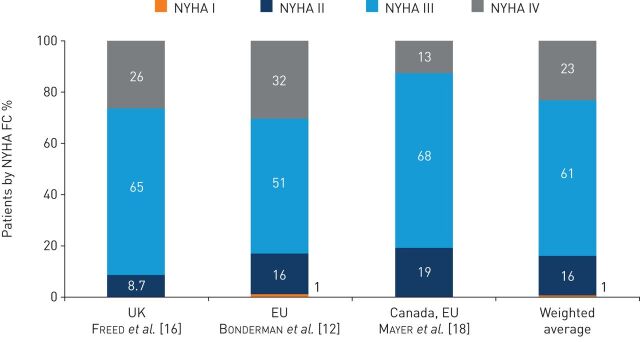
Three studies in European and Canadian populations assessed the NYHA FC at the time of diagnosis among patients with CTEPH [12, 16, 18]. A weighted average of these studies indicated that 1%, 16%, 61% and 23% of patients with CTEPH were in NYHA FC I, II, III and IV, respectively, at diagnosis (figure 3).
FIGURE 3.
Distribution of patients with chronic thromboembolic pulmonary hypertension by New York Heart Association functional class (NYHA FC) at time of diagnosis by region. EU: European Union.
CTEPH incidence after PE
The proportion of patients who develop CTEPH following PE was reported in eight studies conducted in Europe and the USA [17, 45–51]. Japanese data were back-calculated, and further data were obtained from databases/registries. The eight studies in patients from Europe and the USA reported that the incidence of CTEPH in patients following an episode of PE ranged from 0.1% to 9.1%, with a calculated weighted average of 4% (figure 1). The estimate calculated for Japan was higher, with 10% of patients with PE subsequently developing CTEPH.
Data from an international CTEPH registry (covering Canada and countries in Europe) indicated that 25% of patients with CTEPH did not have a documented history of acute PE [10]. In contrast, one Japanese study found that 67% of patients with CTEPH did not have a documented history of acute PE [53].
PEA operability assessment, treatment rate and outcomes
One study showed that suitability for PEA was assessed by a surgeon in 18–47% of patients diagnosed with CTEPH in Europe, the USA and Japan [41].
The proportions of patients with CTEPH who were judged to be suitable candidates for PEA and underwent the procedure were determined in four European studies [10, 12, 14]. PEA was deemed to be feasible in 63–76% of patients in these studies, with a weighted average of 66% (online supplementary figure S2). The proportion of patients with CTEPH judged to be operable who underwent PEA was 71–88%, with a weighted average across the four studies of 85% (online supplementary figure S2). Data from the CTEPH international registry indicated that the proportion of patients with CTEPH judged to be operable or inoperable did not vary significantly according to NYHA FC [10].
PEA survival rates, as assessed in three European studies, were high, with an estimated peri-operative survival rate of ∼95% [15, 18, 55]. A proportion of patients undergoing PEA still experienced pulmonary hypertension after surgery, with the rate of persistent and recurrent pulmonary hypertension following PEA reported in five European studies to be 17–31%, with a weighted average of 30% [5, 10, 16, 17, 19]. However, more recently, Cannon et al. [56] reported results from a UK national cohort of 880 patients who underwent PEA for CTEPH, showing a post-surgical residual pulmonary hypertension rate of 51%.
Results of epidemiological projection
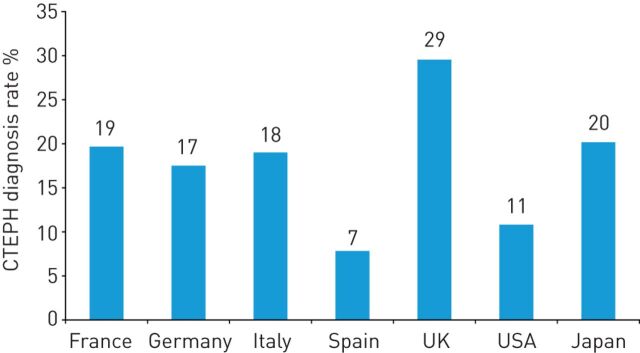
The back-calculated rates of CTEPH diagnosis in Europe and the USA in 2013 ranged from 7% to 29% (figure 4). For 2015, the full annual incidence of CTEPH in France, Germany, Italy, Spain, the UK, the USA and Japan was estimated to be 32 636 cases, or 43 cases per million population (table 1). In addition, it was calculated that only 5334 (16%) of these cases will be diagnosed. Furthermore, an estimated 3860 (72%) diagnosed cases of CTEPH will already be classified as NYHA FC III or IV. Although the cumulative full incidence of CTEPH across the same seven countries is predicted to increase to 37 009 by 2025, it is estimated that a higher proportion will be diagnosed (10 205 cases, 28%) and fewer of these will have reached NYHA FC III or IV at the time of diagnosis (6393 cases, 63%; table 1).
FIGURE 4.
Proportions of patients with chronic thromboembolic pulmonary hypertension (CTEPH) who were diagnosed in Europe, the USA and Japan in 2013.
TABLE 1.
Projected estimates of the annual incidence of full and diagnosed cases of chronic thromboembolic pulmonary hypertension (CTEPH) in Europe, the USA and Japan
| 2015 | 2025 | |||
| Full incidence of CTEPH | Diagnosed incidence of CTEPH | Full incidence of CTEPH | Diagnosed incidence of CTEPH | |
| France | 3310 | 650 | 3744 | 1154 |
| Germany | 3879 | 674 | 4065 | 934 |
| Italy | 2747 | 529 | 2975 | 905 |
| Spain | 1603 | 133 | 1833 | 357 |
| UK | 2087 | 617 | 2347 | 956 |
| USA | 16 566 | 2000 | 19 531 | 4543 |
| Japan | 2444 | 732 | 2513 | 1356 |
| Total | 32 636 | 5334 | 37 009 | 10 205 |
| NYHA FC III/IV | 3860 | 6393 | ||
Data are presented as n. NYHA FC: New York Heart Association functional class.
Discussion
This structured review of the available literature and databases confirms that robust epidemiological data on CTEPH are required. The majority of studies providing these data were undertaken in populations in the USA or Europe. The current epidemiological analysis suggests that the crude full incidence of CTEPH in the USA and Europe ranges from three to five cases per 100 000 population per year. Applying the back-calculated average diagnosis rate of 14.2% for the USA and Europe in 2013, this full incidence equates to a diagnosed incidence of 4–7 cases per million. The full incidence was calculated based on a weighted average of 4% for the proportion of patients who develop CTEPH after PE. Data from the INFORM study, a retrospective US-based claims analysis, suggested the proportion of PE patients developing CTEPH to be 3.8% [57], which is close to the weighted average used in this analysis. A recent review by Kim [58] estimates full incidence of CTEPH in the USA to be 4886 cases per year, based on an incidence of CTEPH after PE of 0.57%, as reported by Klok et al. [50], while the diagnosed incidence of CTEPH in Spain, the UK and Germany is reported to be 0.9, 1.75 and 4 cases per million, respectively [59]. It is clear that when calculating the full incidence of CTEPH, the rate of CTEPH following PE is highly influential on the final projection; however, irrespective of the rate of CTEPH from PE used in estimates, it appears that a large proportion of incident cases of CTEPH are undiagnosed each year. The epidemiological projections made in this study indicate that the full incidence of CTEPH will increase over time from an estimated 32 636 cases (5334 (16%) diagnosed) in 2015 to an estimated 37 009 (10 225 (28%) diagnosed) in 2025 in the seven countries included in the calculation. In addition, the projections indicate that a large proportion of those diagnosed are likely to be in the late stages of the disease.
Nonresolution of acute PE is the most common cause of CTEPH [5] and consequently, past epidemiological research has focused on CTEPH in relation to PE. Early estimates indicated that CTEPH occurs in ∼0.1–0.5% of patients who survive an episode of acute PE [2]; however, this epidemiological analysis shows that more recent data indicate varying and potentially much higher incidences of CTEPH following an acute PE episode, ranging from 0.1% to 9%. Early research reported that there was no documented history of PE or VTE in up to half of all patients with CTEPH, suggesting that PE may not be the only potential cause of CTEPH [4, 8]. Furthermore, recent data from the large international prospective registry of patients with CTEPH indicated that only 25% of patients did not have a history of acute PE or VTE [10]. While it is not clear how many patients experiencing a PE develop CTEPH, the ongoing Follow-Up after Acute Pulmonary Embolism (FOCUS) study is investigating this question. FOCUS is a prospective, multicentre, observational cohort study that will monitor patients with acute PE for 2 years for signs and symptoms of CTEPH, and collect data on the incidence of CTEPH following PE [60].
The rate of PE in Japan appears to be substantially lower than that observed in the USA and Europe, although these data are from a single study. Interestingly, despite Japan having a lower incidence of PE, the rate of CTEPH incidence before intervention is proportionally much higher than those reported in Europe and the USA. Many explanations for the disparities in the incidences of PE and CTEPH between Japan and Europe and the USA have been proposed (e.g. genetics, population demographics and lifestyle) [61–65]. Alternatively, it may be that only severe cases of PE are diagnosed while mild cases are not detected, and consequently not reported.
It is likely that the difficulty in diagnosing CTEPH, due to the lack of an identifiable causative event and/or the lack of early definitive symptoms of CTEPH, will have introduced a certain amount of variability in CTEPH diagnostic rates and impacted these findings. However, such variability may have arisen as a consequence of differences in study design, patient populations or diagnostic methods and criteria [66]. There is a need for physicians to consider that CTEPH may occur without a history of PE [2], and that there may be a proportion of patients with CTEPH in whom asymptomatic PE and VTE occurs without being diagnosed, or a proportion of patients who have CTEPH but are misdiagnosed with other forms of pulmonary hypertension, such as PAH. Furthermore, it should also be considered that some patients who are diagnosed with an acute PE may already have underlying unidentified CTEPH at the time of presentation. This postulation is supported by a prospective multicentre study by Guérin et al. [66] who performed haemodynamic evaluation for CTEPH in 146 patients, with no previous history or other risk factors for pulmonary hypertension at the time of their acute PE diagnosis. Initial echocardiography and multidetector computed tomography data suggested the presence of CTEPH in eight patients, which was confirmed by right heart catheterisation and ventilation/perfusion (V′/Q′) scan in seven of these patients. Age, previous VTE, proximal PE and high levels of systolic pulmonary artery pressure and brain natriuretic peptide were identified as risk factors for CTEPH. These findings suggest that close follow-up and CTEPH screening may be beneficial in patients presenting with acute PE, especially those with high-risk factors for CTEPH. Screening should involve a systematic re-evaluation of systolic pulmonary artery pressure by echocardiography and perfusion defect on V′/Q′ scan, particularly in patients with ongoing dyspnoea 3–6 months after curative anticoagulation of their PE. Use of echocardiography alone is not optimal, as CTEPH may be missed if the echocardiogram shows only mild abnormalities [1, 67].
Geographical variations in CTEPH diagnosis rates identified in this study (for example, the apparently relatively higher diagnosis rate in the UK than in Spain or the USA) may reflect regional variations in diagnostic practices. Gall et al. [41] recently reported the findings of an international physician survey of CTEPH management practice, in which it was found that a relatively higher proportion of patients in Europe than the USA (43% versus 12%) were diagnosed with CTEPH in pulmonary hypertension expert centres. In addition, the use of V′/Q′ scanning in the diagnosis of CTEPH was very low (40% and 45% in pulmonary hypertension centres in Europe and the USA, respectively), contrary to guideline recommendations. The authors suggested that there may be a need for increased education, awareness of guidelines and/or availability of resources to improve CTEPH diagnosis rates worldwide. In addition, data from the INFORM study suggest that V′/Q′ scanning, right heart catheterisation and pulmonary angiography are underused in the USA [57].
This epidemiological study showed that most patients were in NYHA FC III or IV at diagnosis. This, together with the low diagnosis rates derived from the projection model, indicate that CTEPH remains an underrecognised and underdiagnosed disease. This is supported by two recent studies which examined the time delay from onset of symptoms to diagnosis/referral to a pulmonary hypertension expert centre. In one study, Held et al. [68] reported a mean delay from symptom onset to diagnosis of CTEPH of 18±26 months, with most patients presenting with World Health Organization (WHO) FC III or IV. The second study in patients with PAH also identified a significant deterioration in FC associated with a prolonged time between patient-described symptom onset and diagnosis of PAH by right heart catheterisation, which the authors considered could potentially impact on mortality [69], although it should be considered that the natural history of PAH is not the same as that of CTEPH. The consequence of late diagnosis is that treatment options may become limited due to comorbidities and organ damage, and the patient's quality of life may be severely compromised [70]. There appeared to be regional variations in the numbers of patients in NYHA FC III/IV at diagnosis in this study, particularly between Japan and Europe. An international physician survey of CTEPH diagnosis and management practice showed that the majority of patients were in WHO FC III/IV at diagnosis, with some variation in WHO FC between the regions assessed (which included Europe, the USA and Japan) [41]. The reasons for these regional differences are unclear, but may relate to levels of awareness of CTEPH in different regions, which could then impact on likelihood of earlier diagnosis.
PEA is the treatment of choice for CTEPH, as it is potentially curative. However, one study indicated that many patients diagnosed with CTEPH are not assessed by a surgeon for PEA operability [41]. Furthermore, among patients who are assessed and have been deemed to be eligible for PEA, only ∼85% undergo the procedure. Interestingly, it appears that eligibility for PEA in CTEPH is not dependent on disease severity, as there was little variation by NYHA FC [10]. Given the low peri-operative mortality rates when performed in experienced centres, and the improved survival of patients who have undergone PEA compared with those who have not [71], it is important to ensure that all patients with CTEPH are referred for surgical assessment, in line with the 2015 ERS/ESC guidelines for the treatment of pulmonary hypertension [1].
There are a number of limitations to the analyses in this study. While the weighted average incidence of CTEPH after an acute PE of 4% in this study was generally similar to the weighted incidence of 3.8% determined in the INFORM study [57], a subsequent and recent systematic review and meta-analysis of 16 studies has suggested lower incidences of CTEPH after acute PE: 0.56% in “all comers” (n=1186), 3.22% in “survivors” (n=999) and 2.79% in “survivors without major comorbidities” (n=1775) [72], which suggests that the full incidence of CTEPH may be lower. As PEA is performed infrequently in Japan, with physicians favouring BPA, the diagnosis rate for Japan is an assumption, based on the diagnosis rates calculated for the other countries; however, there is a possibility that the real figure is higher.
Nevertheless, the estimates from the epidemiological projection model suggest that CTEPH incidence will continue to increase in the USA, Europe and Japan over the next decade. Moreover, there is a current trend of considerable demographic redistribution towards larger populations of older patients; therefore, the burden of CTEPH is likely to increase further. The increasing incidence of CTEPH predicted here emphasises the need for improved education regarding CTEPH diagnosis and treatment.
In conclusion, more high-quality epidemiological studies, investigating both CTEPH and PE are needed to verify the accuracy of the epidemiological projections in this study. The low diagnosis rates derived from the projection model, and the high proportion of patients who are in NYHA FC III or IV at the time of diagnosis, demonstrate that CTEPH remains an underdiagnosed disease. These factors highlight the need for better recognition of CTEPH and incorporation of current treatment guidelines into daily practice (particularly among physicians outside expert centres) to ensure early diagnosis and appropriate treatment.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table S1, and figures S1 and S2 ERR-0121-2016_Supplementary_data (473.4KB, pdf)
Disclosures
W. Cacheris ERR-0121-2016_Cacheris (1.2MB, pdf)
H. Gall ERR-0121-2016_Gall (1.2MB, pdf)
B. Hinzmann ERR-0121-2016_Hinzmann (1.2MB, pdf)
M.M. Hoeper ERR-0121-2016_Hoeper (1.2MB, pdf)
E. Mayer ERR-0121-2016_Mayer (78.5KB, pdf)
M.J. Richter ERR-0121-2016_Richter (78.6KB, pdf)
Acknowledgements
The literature review and subsequent analyses were performed by Tessellon (St Louis, MO, USA) in cooperation with the commercial insights and analytics department of Bayer AG (Berlin, Germany). Editorial assistance was provided by Adelphi Communications Ltd (Bollington, UK), supported by Bayer AG.
Footnotes
This article has supplementary material available from err.ersjournals.com
Conflict of interest: Disclosures can be found alongside this article at err.ersjournals.com
Provenance: Publication of this peer-reviewed article was sponsored by Bayer AG, Berlin, Germany (principal sponsor, European Respiratory Review issue 143).
References
- 1.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 2.Fedullo P, Kerr KM, Kim NH, et al. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011; 183: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Madani MM, Nakanishi N, et al. Chronic thromboembolic pulmonary hypertension. Lancet Respir Med 2014; 2: 573–582. [DOI] [PubMed] [Google Scholar]
- 4.Peacock A, Simonneau G, Rubin L. Controversies, uncertainties and future research on the treatment of chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc 2006; 3: 608–614. [DOI] [PubMed] [Google Scholar]
- 5.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130: 508–518. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Torbicki A, Dorfmüller P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang IM, Pesavento R, Bonderman D, et al. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013; 41: 462–468. [DOI] [PubMed] [Google Scholar]
- 8.Egermayer P, Peacock AJ. Is pulmonary embolism a common cause of chronic pulmonary hypertension? Limitations of the embolic hypothesis. Eur Respir J 2000; 15: 440–448. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Mayer E, Simonneau G, et al. Chronic thromboembolic pulmonary hypertension. Circulation 2006; 113: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 10.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. [DOI] [PubMed] [Google Scholar]
- 11.Bonderman D, Skoro-Sajer N, Jakowitsch J, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007; 115: 2153–2158. [DOI] [PubMed] [Google Scholar]
- 12.Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009; 33: 325–331. [DOI] [PubMed] [Google Scholar]
- 13.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: Suppl. 25, D92–D99. [DOI] [PubMed] [Google Scholar]
- 14.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 15.Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 16.Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 2011; 141: 383–387. [DOI] [PubMed] [Google Scholar]
- 17.Korkmaz A, Ozlu T, Ozsu S, et al. Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost 2012; 18: 281–288. [DOI] [PubMed] [Google Scholar]
- 18.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. [DOI] [PubMed] [Google Scholar]
- 19.Rahnavardi M, Yan TD, Cao C, et al. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: a systematic review. Ann Thorac Cardiovasc Surg 2011; 17: 435–445. [DOI] [PubMed] [Google Scholar]
- 20.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bresser P, Pepke-Zaba J, Jaïs X, et al. Medical therapies for chronic thromboembolic pulmonary hypertension: an evolving treatment paradigm. Proc Am Thorac Soc 2006; 3: 594–600. [DOI] [PubMed] [Google Scholar]
- 22.Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2004; 23: 637–648. [DOI] [PubMed] [Google Scholar]
- 23.Delcroix M. Chronic post-embolic pulmonary hypertension: a new target for medical therapies? Eur Respir Rev 2013; 22: 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keogh AM, Mayer E, Benza RL, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. J Am Coll Cardiol 2009; 54: Suppl. 1, S67–S77. [DOI] [PubMed] [Google Scholar]
- 25.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 26.Simonneau G, D'Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015; 45: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 27.Simonneau G, D'Armini AM, Ghofrani HA, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. [DOI] [PubMed] [Google Scholar]
- 28.Pepke-Zaba J, Ghofrani HA, Hoeper MM. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiedenroth CB, Liebetrau C, Breithecker A, et al. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2016; 35: 591–596. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. [DOI] [PubMed] [Google Scholar]
- 31.Kim NH. Chronic thromboembolic pulmonary hypertension. Medscape Pulmon Med 2007. www.medscape.com/viewarticle/556058 Date last accessed: March 2, 2017. [Google Scholar]
- 32.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 33.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang IM. Chronic thromboembolic pulmonary hypertension – not so rare after all. N Engl J Med 2004; 350: 2236–2238. [DOI] [PubMed] [Google Scholar]
- 35.Tapson VF, Humbert M. Incidence and prevalence of chronic thromboembolic pulmonary hypertension: from acute to chronic pulmonary embolism. Proc Am Thorac Soc 2006; 3: 564–567. [DOI] [PubMed] [Google Scholar]
- 36.Healthcare Cost and Utilization Project (HCUP). The 2010 Nationwide Inpatient Sample (NIS). www.hcup-us.ahrq.gov/nisoverview.jsp Date last accessed: August 31, 2014. Date last updated: December 14, 2016.
- 37.Instituto Nacional de Estadística (INE). 2009. Base de datos INEbase. Encuesta de morbilidad hospitalaria [INEbase Database. Hospital Morbidity Survey]. www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736176778&menu=ultiDatos&idp=1254735573175 Date last accessed: March 2, 2017. Date last updated: 2015.
- 38.French Institute for Public Health Surveillance (PMSI). 2011. French National Database. www.invs.sante.fr/en Date last accessed: March 2, 2017. Date last updated: 2016.
- 39.Japan Intractable Diseases Research Foundation and Japanese Intractable Diseases Information Center. The Japanese Intractable Disease Survey 2010 (CTEPH). 2010.
- 40.The NHS Information Centre for Health and Social Care. UK Hospital Episode Statistics 2011. http://content.digital.nhs.uk/catalogue/PUB02570 Date last accessed: March 15, 2017. Date last updated: 2014.
- 41.Gall H, Preston IR, Hinzmann B, et al. An international physician survey of chronic thromboembolic pulmonary hypertension management. Pulm Circ 2016; 6: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preston IR, Hinzmann B, Heinz S, et al. An international physician survey of pulmonary arterial hypertension management. Pulm Circ 2016; 6: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Bank. World Bank DataBank HealthStats: Population Estimates and Projections Database. http://data.worldbank.org/data-catalog/population-projection-tables Date last accessed: January 25, 2017. Date last updated: October 4, 2016.
- 44.World Bank. 2017. World Bank DataBank Health Nutrition and Population Statistics. http://databank.worldbank.org/data/reports.aspx?source=health-nutrition-and-population-statistics Date last accessed: January 25, 2017. Date last updated: 2017.
- 45.Otero R, Oribe M, Ballaz A, et al. Echocardiographic assessment of pulmonary arterial pressure in the follow-up of patients with pulmonary embolism. Thromb Res 2011; 127: 303–308. [DOI] [PubMed] [Google Scholar]
- 46.Poli D, Grifoni E, Antonucci E, et al. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis 2010; 30: 294–299. [DOI] [PubMed] [Google Scholar]
- 47.Surie S, Gibson NS, Gerdes VE, et al. Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thromb Res 2010; 125: e202–e205. [DOI] [PubMed] [Google Scholar]
- 48.Dentali F, Donadini M, Gianni M, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 2009; 124: 256–258. [DOI] [PubMed] [Google Scholar]
- 49.Martí D, Gómez V, Escobar C, et al. Incidencia de hipertensión pulmonar tromboembólica crónica sintomática y asintomática. [Incidence of symptomatic and asymptomatic chronic thromboembolic pulmonary hypertension]. Arch Bronconeumol 2010; 46: 628–633. [DOI] [PubMed] [Google Scholar]
- 50.Klok FA, van Kralingen KW, van Dijk AP, et al. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010; 95: 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 2010; 102: Suppl. 1, S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakuma M, Nakamura M, Yamada N, et al. Venous thromboembolism: deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J 2009; 73: 305–309. [DOI] [PubMed] [Google Scholar]
- 53.Tanabe N. Analysis of chronic thromboembolic pulmonary hypertension. Ministry of Health, Wealth and Labor (Japan) Intractable Disease Database, 2008. [Google Scholar]
- 54.Kröger K, Moerchel C, Moysidis T, et al. Incidence rate of pulmonary embolism in Germany: data from the federal statistical office. J Thromb Thrombolysis 2010; 29: 349–353. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins DP, Madani M, Mayer E, et al. Surgical treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J 2013; 41: 735–742. [DOI] [PubMed] [Google Scholar]
- 56.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapson VF, Platt DM, Xia F, et al. Monitoring for pulmonary hypertension following pulmonary embolism: the INFORM study. Am J Med 2016; 129: 978–985. [DOI] [PubMed] [Google Scholar]
- 58.Kim NH. Group 4 pulmonary hypertension: chronic thromboembolic pulmonary hypertension: epidemiology, pathophysiology, and treatment. Cardiol Clin 2016; 34: 435–441. [DOI] [PubMed] [Google Scholar]
- 59.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4: 306–322. [DOI] [PubMed] [Google Scholar]
- 60.Konstantinides S 2016. Follow-Up after Acute Pulmonary Embolism – a Prospective Observational Multicenter Cohort Study – FOCUS. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00005939 (accessed on 27 Aug 2016). [DOI] [PMC free article] [PubMed]
- 61.Kitamukai O, Sakuma M, Takahashi T, et al. Incidence and characteristics of pulmonary thromboembolism in Japan 2000. Intern Med 2003; 42: 1090–1094. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi T, Nakamura M, Sakuma M, et al. Incidence of pulmonary thromboembolism (PTE) and new guidelines for PTE prophylaxis in Japan. Clin Hemorheol Microcirc 2006; 35: 257–259. [PubMed] [Google Scholar]
- 63.Kominami S, Tanabe N, Ota M, et al. HLA-DPB1 and NFKBIL1 may confer the susceptibility to chronic thromboembolic pulmonary hypertension in the absence of deep vein thrombosis. J Hum Genet 2009; 54: 108–114. [DOI] [PubMed] [Google Scholar]
- 64.Kumasaka N, Sakuma M, Shirato K. Incidence of pulmonary thromboembolism in Japan. Jpn Circ J 1999; 63: 439–441. [DOI] [PubMed] [Google Scholar]
- 65.Tanabe N, Kimura A, Amano S, et al. Association of clinical features with HLA in chronic pulmonary thromboembolism. Eur Respir J 2005; 25: 131–138. [DOI] [PubMed] [Google Scholar]
- 66.Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112: 598–605. [DOI] [PubMed] [Google Scholar]
- 67.Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Held M, Grün M, Holl R, et al. Chronisch thromboembolische pulmonale Hypertonie: Latenz bis zur Diagnosesicherung und klinischer Zustand bei Diagnosestellung. [Chronic thromboembolic pulmonary hypertension: time delay from onset of symptoms to diagnosis and clinical condition at diagnosis]. Dtsch Med Wochenschr 2014; 139: 1647–1652. [DOI] [PubMed] [Google Scholar]
- 69.Strange G, Gabbay E, Kermeen F, et al. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ 2013; 3: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathai SC, Ghofrani HA, Mayer E, et al. Quality of life in patients with chronic thromboembolic pulmonary hypertension. Eur Respir J 2016; 48: 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 72.Ende-Verhaar C, Cannegieter S, Vonk-Noordegraaf A, et al. Varying incidences of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the final thread that links them all. Eur Heart J 2016; 37: 406–407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary table S1, and figures S1 and S2 ERR-0121-2016_Supplementary_data (473.4KB, pdf)
W. Cacheris ERR-0121-2016_Cacheris (1.2MB, pdf)
H. Gall ERR-0121-2016_Gall (1.2MB, pdf)
B. Hinzmann ERR-0121-2016_Hinzmann (1.2MB, pdf)
M.M. Hoeper ERR-0121-2016_Hoeper (1.2MB, pdf)
E. Mayer ERR-0121-2016_Mayer (78.5KB, pdf)
M.J. Richter ERR-0121-2016_Richter (78.6KB, pdf)