Abstract
Nitrogen-deprived filaments of wild-type or hetC Anabaena sp. produce respectively, at semiregular intervals, heterocysts and weakly fluorescent cells. Unlike heterocysts, the latter cells can divide and elongate, producing a pattern of spaced series of small cells. Because a hetR::gfp fusion is expressed most strongly in the small cells, we propose that these small cells represent a very early stage of heterocyst differentiation. hetC::gfp is expressed most strongly in proheterocysts and heterocysts.
Cyanobacteria are prokaryotes that are capable of oxygen-producing photosynthesis. Some filamentous species, like Anabaena spp., fix dinitrogen under aerobic conditions in specialized cells called heterocysts. These specialized cells differentiate from vegetative cells in response to deprivation of fixed nitrogen. In wild-type Anabaena sp. strain PCC 7120 and many other strains, single heterocysts differentiate at semiregular intervals along vegetative filaments, forming a spacing pattern (23). Heterocysts, in which synthesis of biliprotein-containing antennae stops, are much more weakly autofluorescent than vegetative cells (17). A thick envelope, consisting of an outer, polysaccharide layer and an inner, glycolipid layer, is deposited outside the cell wall of developing heterocysts, forming a barrier to the entry of oxygen (23). En route to becoming a heterocyst, the developing cell stops dividing. However, under certain conditions, developing heterocysts can lose their differentiated character and divide (19, 20).
Genes involved in heterocyst differentiation, principally in Anabaena sp. strain PCC 7120, have been cloned and characterized. However, mechanisms underlying the progression of differentiation are largely unknown. hetR, an autoregulatory gene that encodes a serine-type protease, is required for the initiation of heterocyst differentiation (1, 2, 7, 25). hetC, which encodes a member of the family of ATP-binding cassette exporters and is most similar to such exporters of proteins and peptides, is required for an early step in the differentiation of heterocysts. Expression of hetC, like that of hetR, is under the control of DNA-binding protein NtcA (14). A hetC mutant produces a pattern of non- or weakly fluorescent cells (for simplicity, we shall refer to them as weakly fluorescent) but does not form heterocysts distinguishable by bright-field microscopy (12). Expression of hetR and of other genes, e.g., hepA and patS, involved in heterocyst development and physiology has been localized to proheterocysts and/or heterocysts by use of luxAB, gfp, and lacZ as reporters (1, 10, 13, 16, 22). hepA is involved in synthesis of the polysaccharide layer (11, 21), and patS, which encodes a 17-amino-acid peptide, may regulate the spacing of heterocysts (24). It is not known in which cells hetC is expressed. We present evidence that hetC is expressed most strongly in proheterocysts and heterocysts and is required for the transition to a nondividing state during heterocyst differentiation.
Methods.
Anabaena sp. strain PCC 7120 and its derivatives were grown, selected, and induced to differentiate as described elsewhere (12). Plasmids were introduced into Anabaena sp. strain PCC 7120 and its derivatives by conjugation (8). Measurement or localization of gene expression using luxAB or gfp as reporter employed single-crossover homologous recombination. Products of single and double recombinations were selected as previously described (3, 8). All single recombinations and gene interruptions were verified by Southern blotting. When maintained under selection, single recombinants are very stable, and instability is not evident after several days in the absence of selection (4, 9). Luciferase activity of suspensions was measured as relative luminescence units, i.e., arbitrary ATP photometer units (Turner Designs, Sunnyvale, Calif.) normalized to the concentration of chlorophyll in the sample. Observation of the hetC pattern was performed with a Zeiss Axiophot photomicroscope (9), as was Nomarski microscopy, or with a Leitz Laborlux S microscope fitted with a G filter system (12). Fluorescence and bright-field images for localization of gfp expression were captured with a Hamamatsu Photonics System, model C1966–20 (Photonic Microscopy, Inc., Oak Brook, Ill.), coupled to the Leitz microscope fitted with a Sapphire GFP filter set (Chroma Technology Corp. [Brattleboro, Vt.] Exciter D395/40, dichroic 425DCLP, and emitter D510/40).
hetC is expressed most strongly in proheterocysts and heterocysts.
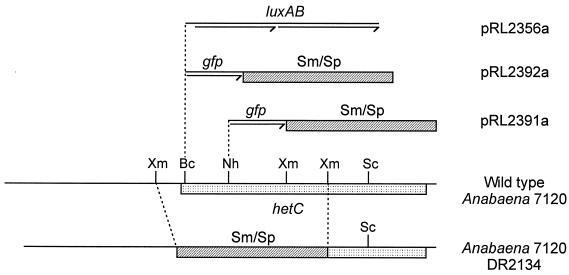
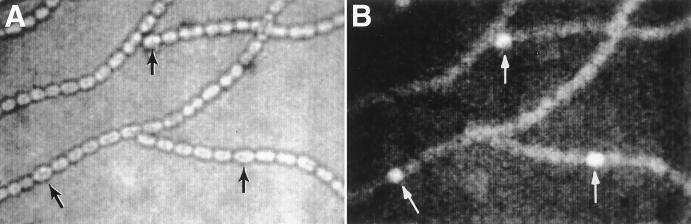
When measured by a transcriptional fusion of luxAB to hetC at its BclI site (pRL2356a in Fig. 1 and Table 1), hetC is seen to be extensively transcriptionally activated by 3.5 h of nitrogen deprivation, comparable to the time of activation of hetR (Table 1) (1) and consistent with qualitative results of primer extension analysis (14). As shown by a hetC::gfp transcriptional fusion of gfp to hetC at its BclI site (in pRL2392a; Fig. 1), hetC is expressed most strongly in proheterocysts and heterocysts (Fig. 2). Over the period of image capture, the initially very low fluorescence by vegetative cells increases markedly. Fluorescence of GFP in excess of the background fluorescence of Anabaena sp. was not visible when gfp was fused to hetC at its NheI site (pRL2391a; Fig. 1 and data not shown), suggesting that transcription of hetC may be attenuated between the BclI and NheI sites.
FIG. 1.
Constructs for study of expression of hetC. In pRL2356a, the hetC promoter is cloned as a 2.2-kb BamHI-BclI fragment from pRL1600 (12) into the BglII site of pRL579 (9). Promoterless gfp was PCR-amplified from pBADgfp (6) with primers CPW135 (5′-TGCCTGCAGGTCGACTCTAGAGGATC-3′) and CPW195 (5′-TCCCGGGTAGCTAGTTAAGAAGGAGATATACATATGG-3′) and cloned into the SmaI site of pUC19 (18); the omega cassette (15), cut with DraI, was cloned into the filled-in EcoRI site; and the 2.7-kb SmaI-PvuII fragment containing the gfp-omega cassette was cloned into the filled-in NheI or BclI site of hetC in pRL1600 and then supplemented with the SphI-cut sacB-oriT-CmrEmr fragment from pRL1075 (1), producing pRL2391a and pRL2392a, respectively. hetC mutant DR2134 was made by double reciprocal recombination with pRL2134, which was constructed by replacing an XmnI fragment of ca. 2.2 kb in pRL1640 (12) with the omega cassette and then transferring a 5.3-kb PstI-SacI fragment between the PstI and SacI sites of pRL271 (1). hetC is transcribed from left to right. Restriction sites: Bc, BclI; Nh, NheI; Sc, ScaI; Xm, XmnI.
TABLE 1.
Luciferase activities of fusions of luxAB to genes involved in the development of heterocysts
| Straina | Luminescence after the following no. of h of nitrogen deprivationb:
|
||||
|---|---|---|---|---|---|
| 0 | 3.5 | 7 | 11 | 22 | |
| WT::pRL2356a (hetC::luxAB) | 2.3 | 59 | 100 | 127 | 104 |
| WT::pRL2215 (hetR::luxAB) | 97 | 269 | 337 | 311 | 272 |
| DR2134::pRL2215 (hetC hetR::luxAB) | 86 | 274 | 284 | 206 | 202 |
| WT::pRL573 (hepA::luxAB) | 5.5 | 7.8 | 57 | 161 | 281 |
| DR2134::pRL573 (hetC hepA::luxAB) | 3.1 | 2.3 | 1.6 | 3.4 | 8.4 |
WT, wild type of Anabaena sp. strain PCC 7120.
Relative luminescence units per microgram of chlorophyll a, calculated from one of three experiments, all of which produced similar results. Standard errors were all less than 30% of the means.
FIG. 2.
Bright-field (A) and fluorescence (B) micrographs of filaments of Anabaena sp. strain PCC 7120::pRL2392a after 24 h of nitrogen deprivation. hetC is expressed most strongly in proheterocysts and heterocysts (arrows).
The semiregularly spaced, weakly fluorescent cells in hetC mutants can divide.
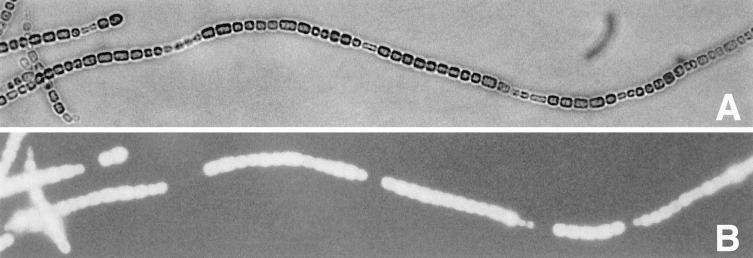
Wild-type Anabaena sp. strain PCC 7120 forms mature heterocysts by 24 h after nitrogen step-down. When excited with light of 350 to 460 nm, the vegetative cells fluoresce brightly, whereas the heterocysts fluoresce weakly. By 48 h (but not 24 h) of nitrogen deprivation, hetC mutants form a pattern of weakly fluorescent cells, often paired, that are indistinguishable from vegetative cells by bright-field microscopy (12). Seeking to determine whether the weakly fluorescent cells would become proheterocysts if nitrogen deprivation was further prolonged, we observed incipient bleaching of many cells after 3 Da at 30 μE m−2 s−1. To prevent bleaching, the light intensity was decreased after 2 Da from 30 to 3 μE m−2 s−1 for 3 Da. The weakly fluorescent cells were found to be elongating and dividing, producing daughter cells of decreased size and forming a pattern of spaced series of diminutive, weakly fluorescent cells (Fig. 3).
FIG. 3.
Nomarski (A) and fluorescence (B) micrographs of a filament of Anabaena sp. strain PCC 7120 hetC mutant DR2134 (Fig. 1) after incubation without fixed nitrogen at 30 μE m−2 s−1 for 2 Da and 3 μE m−2 s−1 for 3 Da, showing a pattern of small, weakly fluorescent cells.
The weakly fluorescent cells represent a very early stage of heterocyst differentiation.
On the basis of the weak fluorescence of the semiregularly spaced cells of a hetC mutant, it was suggested that the cells had initiated heterocyst development but stopped at an early stage (12). However, unlike heterocysts or proheterocysts, these cells elongate and divide. RGSGR is a pentapeptide from the C terminus of PatS that acts as an inhibitor of heterocyst formation (24). Addition of the pentapeptide to 1.3 μM at the time of nitrogen step-down prevented the pattern formation characteristic of hetC mutants (see also reference 24), while its addition at 48 h prevented the formation of diminutive cells from the weakly fluorescent cells (data not shown). Whereas hetC mutant DR1653 (12) showed the phenotype of spaced, weakly fluorescent cells after 48 h of nitrogen deprivation, α41 DR1653, a hetR hetC double mutant, like a hetR mutant (2, 12), showed no pattern formation (data not shown). These observations are consistent with the idea that the weakly fluorescent cells are related to immature heterocysts.
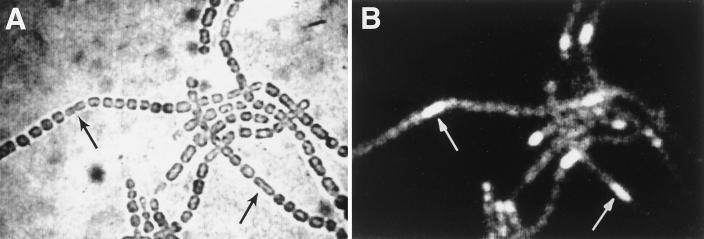
Plasmids pRL2215, derived from pRL881a (1) by deletion of an EcoRV fragment, and pRL573 (22) carry hetR::luxAB and hepA::luxAB transcriptional fusions, respectively. By measuring the luciferase activity of recombinants of pRL2215 and pRL573 with hetC mutant DR2134 and with wild-type Anabaena sp. strain PCC 7120, we found that the hetC mutation has little or no effect on the induction of hetR, as observed by others (14), but nearly completely blocks that of hepA (Table 1). Because hetR is specifically induced in developing heterocysts at a very early stage (1, 2, 5, 10), but a hetC mutation has little effect on the expression of hetR, we used hetR to test whether development is initiated in the weakly fluorescent cells. pRL2380a, bearing hetR::gfp, was constructed by insertion of a 2.7-kb gfp-omega cassette (Fig. 1) into the blunted SacI site of pRL881a (1) and was transferred to hetC mutant DR2134. Fluorescence microscopy with the Sapphire GFP filter showed strongest expression of hetR in the spaced series of diminutive cells (Fig. 4). Using the G filter system, these diminutive cells were found to be only weakly autofluorescent. These observations support the interpretation that the spaced cells in a hetC mutant represent a very early stage of heterocyst differentiation. One effect of the hetC mutation is that unlike proheterocysts in the wild-type strain, these weakly fluorescent cells do not make the transition to a nondividing state, even after 48 h of nitrogen deprivation. One can imagine that HetC is involved in the export of a protein or polypeptide that inhibits the transition to a nondividing state during heterocyst differentiation.
FIG. 4.
Bright-field (A) and fluorescence (B) micrographs of filaments of hetC hetR::gfp strain DR2134::pRL2380a after incubation without fixed nitrogen at 30 μE m−2 s−1 for 2 Da and 3 μE m−2 s−1 for 3 Da, showing strongest expression of a hetR::gfp transcriptional fusion in the dividing and elongating cells (arrows) that are weakly autofluorescent.
Acknowledgments
We thank N. Raikhel for use of the photomicroscope.
This work was supported by the U.S. Department of Energy grant DOE-FG02–91ER20021 and by NSF grant MCB 9723193.
REFERENCES
- 1.Black T A, Cai Y, Wolk C P. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol Microbiol. 1993;9:77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Buikema W J, Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991;5:321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- 3.Cai Y, Wolk C P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990;172:3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Y, Wolk C P. Nitrogen deprivation of Anabaena sp. strain PCC 7120 elicits rapid activation of a gene cluster that is essential for uptake and utilization of nitrate. J Bacteriol. 1997;179:258–266. doi: 10.1128/jb.179.1.258-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y, Wolk C P. Anabaena sp. strain PCC 7120 responds to nitrogen deprivation with a cascade-like sequence of transcriptional activations. J Bacteriol. 1997;179:267–271. doi: 10.1128/jb.179.1.267-271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crameri A, Whitehorn E A, Tate E, Stemmer W P C. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol. 1996;14:315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- 7.Dong U, Huang X, Wu X-Y, Zhao J. Identification of the active site of HetR protease and its requirement for heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 2000;182:1575–1579. doi: 10.1128/jb.182.6.1575-1579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhai J, Wolk C P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- 9.Elhai J, Wolk C P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990;9:3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haselkorn R. Molecular genetics of nitrogen fixation in photosynthetic prokaryotes. In: Tikhonovich I A, Provogov N A, Romanov V I, Newton W E, editors. Nitrogen fixation: fundamentals and applications. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 29–36. [Google Scholar]
- 11.Holland D, Wolk C P. Identification and characterization of hetA, a gene that acts early in the process of mophological differentiation of heterocysts. J Bacteriol. 1990;172:3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khudyakov I, Wolk C P. hetC, a gene coding for a protein similar to bacterial ABC protein exporters, is involved in early regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:6971–6978. doi: 10.1128/jb.179.22.6971-6978.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldener I, Ernst A, Fernández-Piñas F, Wolk C P. Characterization of devA, a gene required for the maturation of proheterocysts in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1994;176:7543–7549. doi: 10.1128/jb.176.24.7543-7549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muro-Pastor A M, Valladares A, Flores E, Herrero A. The hetC gene is a direct target of the NtcA transcriptional regulator in cyanobacterial heterocyst development. J Bacteriol. 1999;181:6664–6669. doi: 10.1128/jb.181.21.6664-6669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prentki P, Binda A, Epstein A. Plasmid vectors for selecting IS1-promoted deletions in cloned DNA: sequence analysis of the omega interposon. Gene. 1991;103:17–23. doi: 10.1016/0378-1119(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 16.Thiel T, Lyons E M, Erker J C, Ernst A. A second nitrogenase in vegetative cells of a heterocyst-forming cyanobacterium. Proc Natl Acad Sci USA. 1995;92:9358–9362. doi: 10.1073/pnas.92.20.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gorkom H J, Donze M. Localization of nitrogen fixation in Anabaena. Nature. 1971;234:231–232. doi: 10.1038/234231b0. [DOI] [PubMed] [Google Scholar]
- 18.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox M, Michison G J, Smith R J. Pattern formation in the blue-green alga. Anabaena. II. Controlled proheterocyst regression. J Cell Sci. 1973;13:637–649. doi: 10.1242/jcs.13.3.637. [DOI] [PubMed] [Google Scholar]
- 20.Wolk C P. Heterocyst germination under defined conditions. Nature. 1965;205:201–202. [Google Scholar]
- 21.Wolk C P. Heterocyst formation in Anabaena. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: ASM Press; 2000. pp. 83–104. [Google Scholar]
- 22.Wolk C P, Elhai J, Kuritz T, Holland D. Amplified expression of a transcriptional pattern formed during development of Anabaena. Mol Microbiol. 1993;7:441–445. doi: 10.1111/j.1365-2958.1993.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolk C P, Ernst A, Elhai J. Heterocyst metabolism and development. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 769–823. [Google Scholar]
- 24.Yoon H-S, Golden J W. Heterocyst pattern formation controlled by a diffusible peptide. Science. 1998;282:935–938. doi: 10.1126/science.282.5390.935. [DOI] [PubMed] [Google Scholar]
- 25.Zhou R, Wei X, Jiang N, Li H, Dong Y, His K L, Zhao J. Evidence that HetR protein is an unusual serine-type protease. Proc Natl Acad Sci USA. 1998;95:4959–4963. doi: 10.1073/pnas.95.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]