Abstract
Lung cancer is the most lethal cancer type worldwide, with the majority of patients presenting with advanced stage disease. Targeting early stage disease pathogenesis would allow dramatic improvements in lung cancer patient survival. Recently, cell migration has been shown to be an integral process in early lung cancer ontogeny, with preinvasive lung cancer cells shown to migrate across normal epithelium prior to developing into invasive disease. TP53 mutations are the most abundant mutations in human nonsmall cell lung cancers and have been shown to increase cell migration via regulation of Rho-GTPase protein activity. In this review, we explore the possibility of targeting TP53-mediated Rho-GTPase activity in early lung cancer and the opportunities for translating this preclinical research into effective therapies for early stage lung cancer patients.
Short abstract
Preinvasive lung cancer cell migration is a potential novel therapeutic target in early lung cancer http://ow.ly/FJGm305JxMQ
Introduction
Lung cancer is the most lethal cancer type worldwide, with a mortality rate greater than breast, colorectal and prostate cancer combined [1]. Nonsmall cell lung cancers (NSCLCs) account for ∼85% of disease [2] and include squamous cell carcinoma (SqCC), adenocarcinoma (ADC) and large cell carcinoma. Although 5-year post-operative survival is 50% for early-stage (stage I/II) NSCLC, >50% of patients present with stage IV disease that is associated with an abysmal 2% 5-year survival [3]. This is due to our limited understanding of the pathomechanisms driving lung cancer ontogeny, as well as a lack of effective biomarkers and screening tools for diagnosing patients with early stage disease. Thus, in order to identify and treat lung cancers more effectively, an improved understanding of the biochemical, molecular and cellular changes that accompany early lung cancer development is required.
It has been noted through post mortem studies and in patients undergoing longitudinal bronchoscopic surveillance that both preinvasive lesions and invasive SqCCs frequently develop at widely dispersed anatomical locations [4–6]. Indeed, in surveillance studies using autofluorescence bronchoscopy (AFB) and computed tomography, almost 60% of invasive lung cancers were observed in anatomically distinct sites from initially detected preinvasive lesions [7]. More recently, we found that preinvasive SqCC lesions exhibiting TP53 mutations invariably spread throughout the bronchial tree via discontinuous cell migration prior to disease progression [8]. These observations suggest that epithelial cell migration is an integral component of NSCLC development. In this review we focus on the role of cell migration as a potential therapeutic target for early lung cancers.
TP53 mutation, Rho-GTPase signalling and cancer cell migration
TP53 is the most consistently and frequently mutated gene in all NSCLCs, with mutations observed in >80% of SqCCs [9] and >45% of ADCs [10]. Functionally, wild-type TP53 proteins play critical roles in DNA repair, cell cycle regulation, apoptosis and inhibition of cell migration [11]. At a molecular and cellular level, mutations in TP53 promote increased cancer cell migration by altering the cell's internal cytoskeleton via indirect regulation of the Rho-GTPase family of proteins [12].
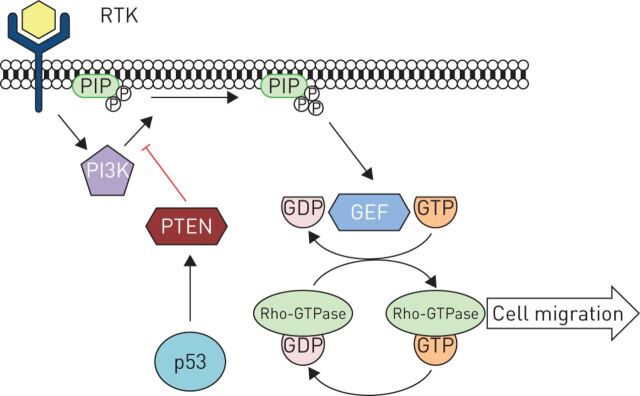
Rho-GTPase proteins coordinate cellular movement by promoting a “grow, grip, pull” system involving cytoskeletal growth at the cell's leading edge, adhesion to the extracellular matrix (ECM) and cytoskeletal contraction to pull the cell forward [13]. Rho-GTPases cycle between an inactive GDP-bound state to the active GTP-bound state upon activation by guanine nucleotide exchange factors (GEFs) [14]. The activation of these GEFs are regulated by upstream phosphoinositide 3-kinase (PI3K) [13]. Thus, PI3K activity, via signalling from membrane-associated receptor tyrosine kinase proteins, regulates Rho-GTPase activation and cell migration (figure 1).
FIGURE 1.
Receptor tyrosine kinase (RTK) responds to external stimuli including extracellular matrix components and chemokines and subsequently activates phosphoinositide 3-kinase (PI3K) to produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3). This, in turn, activates Rho-guanine nucleotide exchange factors (GEF), mediating Rho-GTPase activation and cell migration. Wild-type p53, via phosphatase and tensin homologue (PTEN), negatively regulates PI3K activity and Rho-GTPase-mediated cell migration. Adapted from [13].
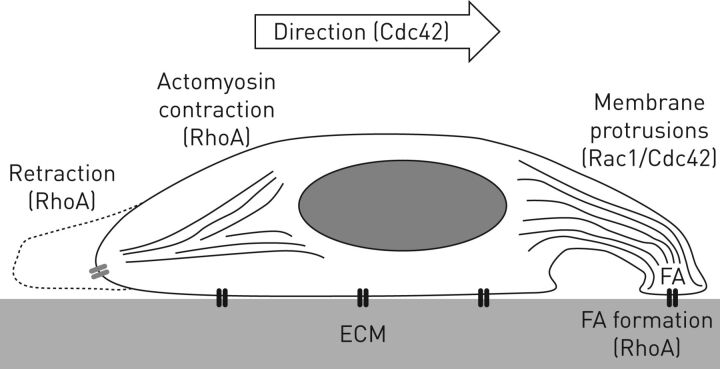
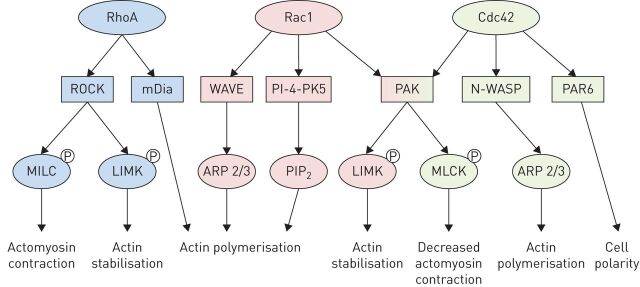
Well-studied Rho-GTPase family members involved in this cell migration system include RhoA, Rac1 and Cdc42, each of which has important roles in the regulation of cell motility. Generally, Cdc42 functions to regulate cell polarity and the formation of actin microspikes and filopodia formation, while Rac1 mediates the formation of larger membrane protrusions (lamellipodia) at the leading edge of cell. Finally, RhoA is involved in focal adhesion formation, actomyosin contraction, stress fibre formation and retraction of the cell's tail [15] (figure 2). Additional components of this system include effector proteins such as Rho-associated kinase (ROCK) that “grow” the actin-based cytoskeleton, integrins that “grip” the ECM and myosin proteins bound to this actin cytoskeleton that provide contractile “pull” forces. Downstream of Rho-GTPases, other effector proteins including mDia, WAVE, N-WASP and PAR6 also coordinate Rac1, RhoA and Cdc42 functions including actin polymerisation, actomyosin contraction and regulation of cell polarity (figure 3) [16, 17].
FIGURE 2.
Cell migration is mediated by Rho-GTPase protein activity. Cell polarity is mediated by Cdc42 activity. Membrane protrusions are formed and are dependent on Rac1 (lamellipodia) and Cdc42 (filopodia) activity. Membrane protrusions grip the extracellular matrix via RhoA-dependent focal adhesion (FA) formation and actomyosin contraction pulls the cell forward via RhoA effector protein Rho-associated kinase (ROCK)-mediated phosphorylation of myosin light chain kinase. Disassembly of focal adhesion and tail retraction is also mediated by RhoA activity. ECM: extracellular matrix.
FIGURE 3.
Rho-GTPases and downstream effector proteins involved in cell motility. RhoA acts via the effector proteins ROCK (RhoA effector protein Rho-associated kinase) and mDia to mediate actomyosin contraction and actin stabilisation. Rac1 acts via WAVE and PI-4-PK5 to mediate actin polymerisation and is involved in membrane protrusions. Cdc42 acts via PAR6 to mediate cell polarity and N-WASP to mediate actin polymerisation.
In normal, noncancerous cells, activation of TP53 following DNA damage or other cellular stress increases phosphatase and tensin homologue (PTEN) activity, leading to inhibition of PI3K, inhibition of downstream GEF activation and reduced Rho-GTPase-dependent cell migration (figure 1). However, in cells exhibiting mutant TP53 this process is disrupted leading to a lack of PTEN-mediated PI3K inhibition, ectopic GEF signalling and enhanced cell migration. In particular, elegant studies using fluorescence lifetime imaging (FLIM)-fluorescence resonance energy transfer (FRET) microscopy in TP53-mutant pancreatic ductal adenocarcinoma cells demonstrate increased motility and invasion in three-dimensional assays in vitro and in vivo [18]. Similarly, fibroblasts derived from TP53-deletion mice showed marked upregulation of GTP-bound RhoA activity and an increased capacity to migrate and invade in comparison with their wild-type counterparts [19]. In addition, human melanocytes with mutated TP53 demonstrated an almost five-fold increase in GTP-bound RhoA activity, coupled with increased migratory capacity [19]. Furthermore, TP53 was found to regulate Cdc42-mediated filopodia formation and cell polarisation in mouse embryonic fibroblasts (MEFs), and TP53-deficient MEFs exhibited constitutive filopodia and an increased ability to migrate [20]. Thus, a clear regulatory interplay exists between TP53 mutations and increased Rho-GTPase activity, contributing to enhanced cancer cell migration.
Interestingly, genes encoding Rho-GTPases themselves are only rarely mutated in human cancers [21, 22], despite their markedly elevated expression and activity [23]. This observation lends further support to the indirect, upstream role of TP53 in regulating activity of these key migratory proteins. Downstream of Rho-GTPases, altered expression of numerous effector proteins including ROCK2, LIMK1 and myosin light-chain (MLC) kinase through altered transcriptional regulation or mutation have been implicated in a variety of metastatic cancers, including both ADC and SqCC NSCLCs [24–26] (figure 3). These findings suggest an important role of downstream Rho-GTPase pathway components in regulating tumour cell migration and subsequent disease progression.
Therapeutic targeting of cancer cell migration
The development of novel therapies to prevent NSCLC progression represents an unmet need in cancer therapy. Reducing preinvasive cancer cell migration speaks to this ideal and the development of pharmacotherapies to reduce tumour cell migration has the potential to improve overall disease mortality. Targeting Rho-GTPase-dependent signalling is attractive as a potential cancer therapy given that the effects of TP53 mutation on cancer cell migration are reversible following RhoA and ROCK inhibition [27]. Towards this end, the small molecule ROCK inhibitor Y27632 has been shown to inhibit migration and invasion in a variety of cancer cell types in vitro, including pancreatic adenocarcinoma [18], melanoma [19] and invasive oesophageal carcinoma [28]. Similarly, ROCK inhibition with the small molecule H-1152 reduced in vitro migration of the murine melanoma cell line B16F10 and limited the cells’ ability to form pulmonary metastases in vivo [29]. Clinically, the potent ROCK inhibitor and vasodilator fasudil is in commercial use in Japan, where it has been shown to be a safe and well-tolerated drug used to prevent cerebral vasospasm post-subarachnoid haemorrhage [30]. Similarly, the direct RhoA inhibitor BA-210, used to treat spinal cord injuries, also has a good safety profile in humans [31]. However, the effect of these drugs on cell migration is as yet unknown.
Upstream of Rho-GTPase signalling, PI3K inhibitors have been explored as potential cancer therapies [32], and several of these are either in use or currently undergoing clinical trials in a variety of human cancers [33]. Notably, the PI3K inhibitor idelalisib is currently approved to treat relapsed chronic lymphocytic leukaemia (CLL), and has recently been approved by the European Medicines Agency for the first-line treatment of CLL patients with TP53 mutations who are not fit for first-line chemotherapy [34]. Targeting PI3K signalling is also of growing interest in the treatment of NSCLC [35], although the effect of PI3K inhibition on lung cancer cell migration remains unknown. Rapamycin, an immunosuppressant and well-known inhibitor of PI3K/mTOR activity, has also demonstrated promising anticancer effects in solid tumours [36, 37]. Interestingly, rapamycin has been shown to inhibit cancer cell migration in vitro via suppression of mTOR-mediated lamellipodia formation, actin reorganisation and focal adhesion formation [38]. These effects were Rho-GTPase dependent, with reduced RhoA, Rac1 and Cdc42 expression in rapamycin-treated cells [39].
In addition to upstream activators and downstream effectors, localisation of Rho-GTPase proteins at the plasma membrane is critical for effective signalling, and is achieved by a series of post-translational modifications including C-terminal cysteine prenylation [40]. Rho-GTPase prenylation is dependent on geranylgeranyltransferase-I, and efficient enzyme activity is dependent on a steady supply of geranylgeranyl pyrophosphate (GGPP) within the cell, the synthesis of which is rate-limited by HMGCoA-reductase. Indeed, HMGCoA-reductase inhibitors (statins) have been shown to reduce Rac1 association with cell membranes and subsequently reduce Rac1 cellular effects, including cell morphology and phagocytosis [41]. Simvastatin has been shown to inhibit migration of human and murine microglial cells at baseline and in response to chemokine stimulation, with associated distorted actin distribution [42]. This effect is reversed by co-incubation with l-mevalonate, indicating an inhibitory dependence on disruption of the mevalonate pathway and HMG-CoA reductase activity [42]. Similarly, treatment of human cultured prostate cancer cells with simvastatin or rosuvastatin reduced colony-forming ability and migration towards the powerful chemoattractant bone marrow stroma, with normal migratory behaviour restored with the addition of mevalonate or GGPP [43]. In addition, epidemiological data demonstrate lower rates of prostate cancer progression in patients taking statins [44–46].
Despite these positive findings, Rho-GTPase inhibition is not without challenges and the use of Rho-GTPase inhibitors for human lung cancers remains unexplored. Rho-GTPase is heavily involved in organ development and repair, making delivery of broad-acting inhibitors potentially precarious. In utero, murine germline ROCK1 deletion causes defective eyelid closure, omphalocoele (nonclosure of the ventral body wall) and widespread epithelial dysfunction [47]. Similarly, 90% of ROCK II knockouts die in utero due to placental dysfunction and intrauterine growth retardation [48]. In normal human lung epithelial cells, Rho-GTPase activity is essential for normal wound repair [49, 50]. However, in bovine epithelial cells, RhoA inhibition via PKC activation was associated with improved wound closure [51]. Thus, the precise interplay of Rho-GTPase activity regulating normal and cancerous lung epithelial cell migration remains unclear. It may therefore be a more sensible approach when designing potential cancer therapeutics to target downstream effector proteins such as ROCK and mDia, rather than Rho-GTPase proteins themselves.
Future directions
Given the disparity in mechanisms underlying cell migration across tissue types, it remains of critical importance to characterise the role of various Rho-GTPase pathway components in normal and cancerous lung epithelial cell migration. Towards this end, methods have recently been developed to isolate and expand primary human bronchial epithelial cells that maintain a normal karyotype and multipotent differentiation capacity [52]. In addition, murine models of both human adeno and squamous NSCLCs, generated via targeted transgenesis or cutaneous application of chemical carcinogens, are available [53–56]. Taken together, these in vitro and in vivo models offer unique opportunities for monitoring the effects of Rho-GTPase activity on lung epithelial cell migration. In addition, human bronchoscopic surveillance using AFB will allow accurate assessment of the migration of clonally distinct preinvasive SqCC lesions. Although the numbers of patients undergoing routine surveillance bronchoscopy remain small, these studies should nonetheless improve our understanding of the earliest stages of disease pathogenesis. Interestingly, the widespread use of statins within these patients may also permit retrospective analysis of the effects of statin-dependent Rho-GTPase inhibition on preinvasive disease progression.
Clinically, targeting the Rho-GTPase signalling pathway to reduce early NSCLC disease progression appears to hold promise. Future in vitro and in vivo studies involving small molecule Rho-GTPase inhibitors will allow precise characterisation of the molecular pathways involved in preinvasive lung cancer cell migration, and propel the use of targets of cell migration towards clinical benefit. Furthermore, the wealth of therapies already available and licenced for human use provides great opportunity for rapid translation of preclinical data into effective therapies for lung cancer patients.
Footnotes
Conflict of interest: None declared.
Provenance: Submitted article, peer reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. . Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 3.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg 2009; 15: 4–9. [PubMed] [Google Scholar]
- 4.Jeanmart M, Lantuejoul S, Fievet F, et al. . Value of immunohistochemical markers in preinvasive bronchial lesions in risk assessment of lung cancer. Clin Cancer Res 2003; 9: 2195–2203. [PubMed] [Google Scholar]
- 5.Jeremy George P, Banerjee AK, Read CA, et al. . Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax 2007; 62: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin WA, Gazdar AF, Haney J, et al. . Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J Clin Invest 1997; 100: 2133–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Boerdonk RA, Smesseim I, Heideman DA, et al. . Close surveillance with long-term follow-up of subjects with preinvasive endobronchial lesions. Am J Respir Crit Care Med 2015; 192: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 8.Pipinikas CP, Kiropoulos TS, Teixeira VH, et al. . Cell migration leads to spatially distinct but clonally related airway cancer precursors. Thorax 2014; 69: 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature 1991; 351: 453–456. [DOI] [PubMed] [Google Scholar]
- 12.Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53? Biol Cell 2006; 98: 141–152. [DOI] [PubMed] [Google Scholar]
- 13.Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal 2013; 25: 1955–1961. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 2002; 16: 1587–1609. [DOI] [PubMed] [Google Scholar]
- 15.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev 2009; 28: 65–76. [DOI] [PubMed] [Google Scholar]
- 16.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev 2009; 28: 5–14. [DOI] [PubMed] [Google Scholar]
- 17.Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol 2015; 36: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timpson P, McGhee EJ, Morton JP, et al. . Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res 2011; 71: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadea G, de Toledo M, Anguille C, et al. . Loss of p53 promotes RhoA–ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol 2007; 178: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadea G, Lapasset L, Gauthier-Rouvière C, et al. . Regulation of Cdc42-mediated morphological effects: a novel function for p53. EMBO J 2002; 21: 2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamoto M, Teramoto H, Matsumoto S, et al. . K-ras and Rho A mutations in malignant pleural effusion. Int J Oncol 2001; 19: 971–976. [DOI] [PubMed] [Google Scholar]
- 22.Rihet S, Vielh P, Camonis J, et al. . Mutation status of genes encoding RhoA, Rac1, and Cdc42 GTPases in a panel of invasive human colorectal and breast tumors. J Cancer Res Clin Oncol 2001; 127: 733–738. [DOI] [PubMed] [Google Scholar]
- 23.Croft DR, Sahai E, Mavria G, et al. . Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res 2004; 64: 8994–9001. [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Morrison C, Wang L, et al. . Identification of somatic mutations in non-small cell lung carcinomas using whole-exome sequencing. Carcinogenesis 2012; 33: 1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan L, Zhang L, Fan K, et al. . MiR-27b targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol Cell Biochem 2014; 390: 85–91. [DOI] [PubMed] [Google Scholar]
- 26.Ramaswamy S, Ross KN, Lander ES, et al. . A molecular signature of metastasis in primary solid tumors. Nat Genet 2003; 33: 49–54. [DOI] [PubMed] [Google Scholar]
- 27.Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep 2012; 13: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikami T, Yoshida K, Sawada H, et al. . Inhibition of Rho-associated kinases disturbs the collective cell migration of stratified TE-10 cells. Biol Res 2015; 48: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teiti I, Florie B, Pich C, et al. . In vivo effects in melanoma of ROCK inhibition-induced FasL overexpression. Front Oncol 2015; 5: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y, Shibuya M, Satoh S, et al. . A postmarketing surveillance study of fasudil treatment after aneurysmal subarachnoid hemorrhage. Surg Neurol 2007; 68: 126–131. [DOI] [PubMed] [Google Scholar]
- 31.Fehlings MG, Theodore N, Harrop J, et al. . A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 2011; 28: 787–796. [DOI] [PubMed] [Google Scholar]
- 32.Crabbe T. Exploring the potential of PI3K inhibitors for inflammation and cancer. Biochem Soc Trans 2007; 35: 253–256. [DOI] [PubMed] [Google Scholar]
- 33.Maira SM, Stauffer F, Schnell C, et al. . PI3K inhibitors for cancer treatment: where do we stand? Biochem Soc Trans 2009; 37: 265–272. [DOI] [PubMed] [Google Scholar]
- 34.Shah A, Mangaonkar A. Idelalisib: a novel PI3Kδ inhibitor for chronic lymphocytic leukemia. Ann Pharmacother 2015; 49: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 35.Fumarola C, Bonelli MA, Petronini PG, et al. . Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 2014; 90: 197–207. [DOI] [PubMed] [Google Scholar]
- 36.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12: 9–22. [DOI] [PubMed] [Google Scholar]
- 37.Fasolo A, Sessa C. mTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs 2008; 17: 1717–1734. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Chen L, Chung J, et al. . Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 2008; 27: 4998–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Luo Y, Chen L, et al. . Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J Biol Chem 2010; 285: 38362–38373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullah N, Mansha M, Casey PJ. Protein geranylgeranyltransferase type 1 as a target in cancer. Curr Cancer Drug Targets 2016; 16: 563–571. [DOI] [PubMed] [Google Scholar]
- 41.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, et al. . Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem 2005; 280: 34202–34209. [DOI] [PubMed] [Google Scholar]
- 42.Kuipers HF, Rappert AA, Mommaas AM, et al. . Simvastatin affects cell motility and actin cytoskeleton distribution of microglia. Glia 2006; 53: 115–123. [DOI] [PubMed] [Google Scholar]
- 43.Brown M, Hart C, Tawadros T, et al. . The differential effects of statins on the metastatic behaviour of prostate cancer. Br J Cancer 2012; 106: 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Platz EA, Leitzmann MF, Visvanathan K, et al. . Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst 2006; 98: 1819–1825. [DOI] [PubMed] [Google Scholar]
- 45.Murtola TJ, Tammela TL, Määttänen L, et al. . Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int J Cancer 2010; 127: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 46.Breau RH, Karnes RJ, Jacobson DJ, et al. . The association between statin use and the diagnosis of prostate cancer in a population based cohort. J Urol 2010; 184: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu Y, Thumkeo D, Keel J, et al. . ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 2005; 168: 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thumkeo D, Keel J, Ishizaki T, et al. . Targeted disruption of the mouse Rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 2003; 23: 5043–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desai LP, Aryal AM, Ceacareanu B, et al. . RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004; 287: L1134–L1144. [DOI] [PubMed] [Google Scholar]
- 50.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010; 298: L715–L731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spurzem JR, Gupta J, Veys T, et al. . Activation of protein kinase A accelerates bovine bronchial epithelial cell migration. Am J Physiol Lung Cell Mol Physiol 2002; 282: L1108–L1116. [DOI] [PubMed] [Google Scholar]
- 52.Butler CR, Hynds RE, Gowers KH, et al. . Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 2016; 194: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu C, Fillmore CM, Koyama S, et al. . Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014; 25: 590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.To MD, Quigley DA, Mao JH, et al. . Progressive genomic instability in the FVB/Kras(LA2) mouse model of lung cancer. Mol Cancer Res 2011; 9: 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudish TM, Opincariu LI, Mozer AB, et al. . N-nitroso-tris-chloroethylurea induces premalignant squamous dysplasia in mice. Cancer Prev Res 2012; 5: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budán F, Varjas T, Nowrasteh G, et al. . Early modification of c-myc, Ha-ras and p53 expressions by N-methyl-N-nitrosourea. In Vivo 2008; 22: 793–797. [PubMed] [Google Scholar]