Summary
Background
Women represent a meaningful proportion of new HIV diagnoses, with Black women comprising 58% of new diagnoses among women. As HIV infection also increases risk of chronic kidney disease (CKD), understanding CKD risk among women with HIV (WWH), particularly Black women, is critical.
Methods
In this longitudinal cohort study of people with HIV (PWH) enrolled in CFAR Network of Integrated Clinical Systems (CNICS), a multicentre study comprised of eight academic medical centres across the United States from Jan 01, 1996 and Nov 01, 2019, adult PWH were excluded if they had ≤2 serum creatinine measurements, developed CKD prior to enrollment, or identified as intersex or transgendered, leaving a final cohort of 33,998 PWH. The outcome was CKD development, defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1·73 m2 calculated using the CKD-EPI equation, for ≥90 days with no intervening higher values.
Findings
Adjusting for demographic and clinical characteristics, WWH were 61% more likely to develop CKD than men (adjusted hazard ratio [aHR]: 1·61, 95% CI: 1·46-1·78, p<0·001). This difference persisted after further adjustment for APOL1 risk variants (aHR female sex: 1·92, 95% CI: 1·63-2·26, p<0·001) and substance abuse (aHR female sex: 1·70, 95% CI: 1·54-1·87, p<0·001).
Interpretation
WWH experienced increased risk of CKD. Given disparities in care among patients with end-stage kidney disease, efforts to engage WWH in nephrology care to improve chronic disease management are critical.
Funding
US National Institutes of Health.
Keywords: HIV, Kidney disease, Health disparities
Research in context.
Evidence before this study
We searched PubMed for research articles published up to September 1, 2021 using combinations, abbreviations, and variations of the terms “HIV”, “women”, “kidney disease”, “kidney failure”, “chronic kidney disease”, and “end-stage kidney disease.” We identified multiple cohort studies from the United States, Europe, and Brazil through this search. Consistently these studies demonstrated female sex was a risk factor for either chronic kidney disease or end-stage kidney disease among people with HIV. These studies, however, were limited in their approach or by the data available to them.
Added value of this study
To our knowledge, this is the first study to examine the association of female sex with chronic kidney disease after accounting for sex-specific differences in serum creatinine levels and known genetic risk factors for kidney disease. We used the CFAR Network of Integrated Clinical Systems (CNICS) to examine the association of female sex with incident chronic kidney disease development. Even after accounting for known genetic risk factors and African ancestry, female sex was associated with increased risk of chronic kidney disease as compared to men. Moreover, accounting for patient-reported outcomes such as substance use and for sex-based differences in serum creatinine level failed to mitigate this heightened risk of chronic kidney disease development.
Implications of all the available evidence
Women with HIV in the United States appear to have increased risk of chronic kidney disease. While the majority of women with HIV are African American or Black, accounting for African ancestry or APOL1 renal risk variants does not completely mitigate this risk. Thus, early detection of kidney dysfunction and linkage to nephrology care are crucial for women with HIV.
Alt-text: Unlabelled box
Introduction
Kidney disease has emerged as a leading comorbidity among people with HIV (PWH) with estimated chronic kidney disease (CKD) prevalence between 2·4% and 17·0%.1, 2, 3 Beyond established CKD risk factors such as aging, obesity, diabetes, and hypertension, PWH also accrue risk attributable to HIV-specific factors such as HIV viraemia, low CD4 counts, and the nephrotoxic effects of antiretroviral therapy (ART), which can approximate the level of risk associated with traditional risk factors.4, 5, 6, 7, 8, 9, 10 While effective ART may slow the decline of estimated glomerular filtration rate (eGFR), it does not guarantee this deterioration will be completely halted.11 Thus, without efforts targeted to halt this process, some PWH will progress to CKD and, ultimately, to end-stage kidney disease (ESKD) with some studies demonstrating 2·1-fold higher odds of kidney impairment among PWH as compared to those without HIV.6,12,13 Moreover, once PWH develop CKD, they are two-fold more likely to die than individuals without HIV, necessitating efforts to further understand development and progression of CKD in this population.14
Consistent with the studies conducted among those without HIV, disparities in CKD/ESKD among PWH are well-established, including sex-specific disparities.15 In a cross-sectional study from Brazil, women with HIV (WWH) experienced 1·2-fold higher odds of mild kidney impairment compared to male counterparts.16 A multicentre study of PWH in the United States corroborated this finding, demonstrating both Black women and non-Black women experienced 1·5-fold higher incidence rates of CKD as compared to men.7 Critically, however, neither study accounted for sex-based differences in baseline serum creatinine levels. Existing eGFR equations include a correction for female sex, as women typically have lower serum creatinine levels than men attributable to lesser muscle mass or hormonal differences between the sexes,17, 18, 19 that results in the assignment of a lower eGFR to women as compared to men with the same serum creatinine.17,20 Thus, accounting for sex-based differences in serum creatinine when examining risk of CKD is crucial.
The burden of CKD risk is also not equitably distributed across racial groups, as Black individuals comprise the majority of CKD/ESKD cases among PWH.5 Evidence suggests that perhaps genetic risk factors, specifically apolipoprotein-L1 (APOL1), may contribute to this enhanced level of risk among Black PWH.21, 22, 23 Additionally, APOL1 is highly correlated with genetic African ancestry, which has been shown to significantly increase serum creatinine, resulting in lower eGFRs.24 Self-reported Black race, however, is not a good surrogate for APOL1 or for genetic African ancestry and, instead, serves as a surrogate for societal factors such as culture, diet, and discrimination.25 Beyond self-reported race and genetic markers, other factors such as socioeconomic status, HIV-related stigma, and systemic racism may also contribute to the observed racial disparity in CKD/ESKD, as these factors influence retention in care and ART adherence through increased depression, lower self-efficacy, and avoidant coping.26, 27, 28, 29, 30 While Black individuals are disproportionately represented among new HIV diagnoses, this inequity is starker among WWH of whom 58% of new diagnoses occur among Black women.31 Thus, as the majority of WWH are Black,32 inability to control for genetic risk factors such as APOL1 or for sex-based differences in serum creatinine levels may have confounded previous estimates of CKD risk. As such, this study was designed to determine whether WWH are at increased risk of CKD after adjusting for both sex-based differences in baseline serum creatinine levels and known genetic CKD risk variants. We hypothesized female sex was associated with increased risk of CKD development.
Methods
Study design and population
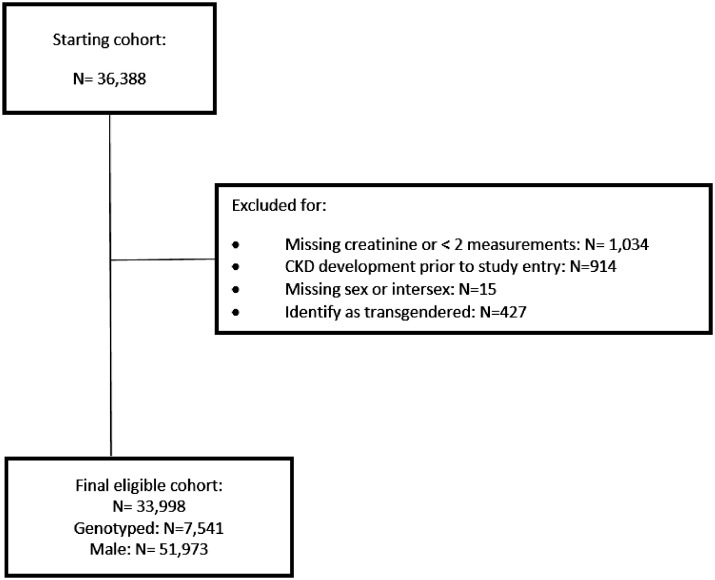
The CNICS cohort is a prospective, longitudinal, multicentre cohort of adult PWH. Participants enrolled at eight academic medical centres across the US (Case Western Reserve University, University of Alabama at Birmingham, University of California San Francisco, University of Washington, University of California San Diego, Fenway Health/Harvard University, University of North Carolina Chapel Hill, and Johns Hopkins University). CNICS collects detailed clinical, demographic and laboratory data including participant age, sex, self-reported race/ethnicity, comorbidities, medications, lab results, and vital status.33 Patients must provide written informed consent to participate in CNICS. Of the >37,000 current adult participants, more than 8,000 have genetic data available for analysis to date with ongoing expansion. PWH were excluded if they had ≤2 creatinine measurements during the study period (01/01/1996-11/01/2019), developed CKD prior to study entry (defined as an eGFR <60 mL/min/1·73m2 for ≥90 days without an intervening higher value), or identified as intersex or transgendered, leaving a final cohort of 33,998 PWH (Figure 1). The Institutional Review Board at the University of Alabama Birmingham approved this study (protocol: 30001300). This study adhered to the items outlined in the STROBE reporting tool.
Figure 1.
Cohort construction diagram.
There were 36,388 PWH enrolled in CNICS. 1,034 PWH were excluded for either no serum creatinine measurements or fewer than 2 measurements during the study period. 914 PWH developed CKD prior to study enrollment, 15 were either intersex or had no birth sex recorded, and 427 PWH were transgendered. Consequently, a final cohort of 33,998 PWH was derived of whom 6,105 were female and 27,893 were male.
Genotyping
DNA was extracted from peripheral blood mononuclear cells or buffy coats using the FlexiGene DNA kit (Qiagen Inc, Chatsworth, CA). Genotyping was performed using the expanded Illumina Multi-Ethnic Genotyping Array as described elsewhere.34 Single nucleotide polymorphisms (SNPs) with call rates < 95%, minor allele frequency < 5%, deviation from Hardy-Weinberg equilibrium (p-value < 1 × 10−29), and regions of high linkage disequilibrium (LD),35 as well as samples with call rates < 95%, sex discrepancies between genotype data and self-report, and pairwise identity- by- descent (‘pi-hat’) > 0·9 were excluded as implemented in Plink v1·9.36 For linkage disequilibrium pruning, linked SNPs were removed using r2 ≥ 0·1. The ADMIXTURE algorithm was used to determine the global proportions of genetic African ancestry,37,38 and APOL1 was assessed using the recessive model (0/1 vs. 2).39
Patient-reported outcomes
PWH were surveyed approximately every 6 months as part of routine clinical care using validated instruments for substance abuse (drug, alcohol, and tobacco use). These surveys were administered via a web-based survey software application that may reduce social desirability bias. Data from the Alcohol Use Disorders Identification Test and the National Institute of Drug Abuse-modified Alcohol, Smoking, and Substance Involvement Test were utilized to examine the association of past or current substance abuse with CKD development.40, 41, 42, 43 Each substance (marijuana, methamphetamine, illicit opioid, intravenous drug use, cocaine/crack, and high risk alcohol use) was examined independently given the distinctly different mechanisms by which they may impact kidney function.
Statistical analyses
Outcome of interest was CKD development, defined as an eGFR of <60 mL/min/1·73m2 for ≥90 days with no intervening higher values.44 eGFR was calculated using the 2009 CKD-EPI equation.17 Time-at-risk was defined as time from study enrollment to CKD development, death, or loss to follow-up (defined as one year after the last creatinine measurement). In Cox proportional hazards models, PWH were censored if time-at-risk ended in death or loss to follow-up. Women are known to have a longer life expectancy than men in developed countries and as such, there was the potential for this differential death rate to bias our results. Accordingly, Fine and Gray competing risks models were performed in which death was treated as a competing risk for CKD development.
Covariates
The primary exposure was self-reported sex at birth. In models among genotyped PWH, the primary exposure was biological sex. Variables considered for both Cox and Fine and Gray model inclusion included the following: age at study entry, self-reported race, baseline serum creatinine, body mass index, hypertension, diabetes, hepatitis C infection, hepatitis B infection, CD4 count, log-transformed HIV viral load, baseline tenofovir use, baseline protease inhibitor use, baseline indinavir use, baseline atazanavir use, baseline lopinavir use, baseline integrase inhibitor use, total antiretroviral therapy use, and study enrollment year. Functional form for continuous variables was assessed using Martingale residuals to ensure the linear form of continuous variables was most appropriate. Variables were retained for model inclusion if significant at p<0·10. Missingness of variables was compared across sexes to assess for potential bias. All analyses were presented are complete cases analyses. The proportional hazards assumption was tested by interacting all predictor variables with the log-function of survival time.
Sub-group and sensitivity analyses
Genetic data and patient-reported outcomes were only available on select PWH based on availability of samples genotyped to date and introduction of the surveys within each site respectively. Given the potential for unintended bias due to estimation of risk in select sub-groups, models were built within each sub-group to permit inclusion of their unique data elements and confirm our inferences with respect to female sex. Models were stratified by era of study enrollment, defined as five calendar year periods, to assess for potential confounding by secular trends, specifically improvement in HIV-specific therapeutics. Inferences were confirmed in each of our sub-cohorts. The inclusion of race in existing eGFR estimating equations has recently been debated,45,46 with many centres dropping the race coefficient from these calculations. To increase generalizability, eGFR was recalculated excluding the race coefficient from the CKD-EPI equation, and additional analyses were performed. As both body mass index (BMI) and eGFR at study entry could confound the relationship between female sex and CKD development, sensitivity analyses in which both factors were controlled for were conducted. Inferences, presented in the Supplemental Materials, were consistent with those presented in the body of this manuscript demonstrating female sex's association with increased risk of CKD.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to the dataset used in this study. JEL had final responsibility for the decision to submit this manuscript for publication.
Results
The CNICS cohort was examined using three distinct cohorts (overall, genotyped, and PRO) as there were notable differences in patient demographics and era of study entry. There was a lower proportion of Hispanic PWH in the genotyped cohort as compared to the overall and PRO cohorts (9·0% vs. 12·0% vs. 13·7%), and a higher proportion of Black PWH (43·4% vs. 38·5% vs. 38·6%). Diabetes was more common (19·0% vs. 11·7% vs. 15·5%) and CD4 count lower in the genotyped cohort as compared to the overall and PRO cohorts (Table 1).
Table 2.
Baseline characteristics by birth sex.
| Female | Male | P-value | |
|---|---|---|---|
| N=6,105 | N=27,893 | ||
| Demographics | |||
| Age at study entry, median (IQR) | 39 (31-46) | 39 (31-46) | 0·17 |
| Hispanic, n (%) | 484 (7·8) | 3,708 (13·0) | <0·0001 |
| Black race (ref: White), n (%) | 3,964 (63·9) | 9,474 (33·2) | <0·0001 |
| HIV Characteristics | |||
| ARV at baseline, n (%) | 3,152 (50·8) | 14,966 (52·4) | 0·02 |
| CD4 count, median (IQR) | 362 (167-587) | 352 (159-557) | <0·0001 |
| Log viral copy-years, median (IQR) | 4·8 (3·0-6·0) | 4·2 (2·5-4·9) | 0·01 |
| ART duration, yrs | 0 (0-245) | 0 (0-540) | <0.0001 |
| PI duration, days | 379 (91-1,283) | 697 (195-1,623) | 0.05 |
| INSTI duration, days | 256 (58-796) | 363 (66-1,190) | 0.24 |
| Atazanavir duration, days | 351 (73-1,076) | 548 (126, 1,676) | 0.61 |
| Indinavir duration, days | 365 (103-763) | 580 (204-1,190) | <0.0001 |
| Comorbid Conditions | |||
| HCV infection, n (%) | 1,587 (25·6) | 5,371 (18·8) | <0·0001 |
| HBV infection, n (%) | 224 (3·6) | 1,797 (6·3) | <0·0001 |
| Diabetes, n (%) | 1,010 (16·3) | 2,942 (10·3) | <0·0001 |
| Hypertension, n (%) | 1,027 (16·6) | 2,803 (9·8) | <0·0001 |
| 2 APOL1 risk variantsa, n (%) | 152 (10·4) | 353 (5·8) | <0·0001 |
| Baseline creatinine, median (IQR) | 0·8 (0·7-1·0) | 1·0 (0·9-1·2) | <0·0001 |
| Baseline eGFR, median (IQR) | 93 (71-112) | 94 (77-109) | 0·04 |
| Patient Reported Outcomes | |||
| High-risk alcohol abuse, n (%) | 1,750 (28·7) | 8,218 (29·5) | <0·0001 |
| IDU, n (%)b | 161 (16·8) | 1,110 (21·6) | 0·001 |
| Amphetamine use, n (%)b | 334 (16·9) | 4,166 (42·1) | <0·0001 |
| Marijuana use, n (%)b | 982 (49·8) | 6,905 (70·4) | <0·0001 |
| Opiate use, n (%)b | 394 (21·3) | 2,122 (22·1) | 0·49 |
| Crack/cocaine use, n (%)b | 764 (38·6) | 5,009 (50·4) | <0·0001 |
| Study Entry Era | |||
| 1995–1999 | 1,112 (18.1) | 4,157 (14.8) | <0.0001 |
| 2000–2004 | 1,700 (27.7) | 6,825 (24.3) | |
| 2005–2009 | 1,350 (22.0) | 6,871 (24.4) | |
| 2010–2014 | 1,376 (22.4) | 7,032 (25.0) | |
| 2015–2020 | 607 (9.9) | 3,240 (11.5) |
Among genotyped only.
Among PRO cohort only.
ARV: antiretroviral use, HCV: hepatitis C virus, HBV: hepatitis B virus, APOL1: apolipoprotein 1, eGFR: estimated glomerular filtration rate, CKD: chronic kidney disease, IDU: intravenous drug use.
Table 1.
Baseline characteristics by cohort among people living with HIV engaged in HIV care.
| Overall |
Genotyped |
PRO cohort |
||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| N=6,105 | N=27,893 | N=1,445 | N=6,017 | N=2,844 | N=12,827 | |
| Demographics | ||||||
| Age at study entry, median (IQR) | 39 (31-46) | 39 (31-46) | 39 (32-46) | 40 (33-46) | 39 (32-47) | 39 (31-47) |
| Hispanic, n (%) | 479 (7·9) | 3,629 (13·0) | 67 (4 6) | 603 (10 0) | 252 (8 9) | 1,895 (14 8) |
| Black race, n (%) | 3,909 (64 0) | 9,301 (33·4) | 1,006 (69 6) | 2,248 (37 4) | ||
| HIV Characteristics | ||||||
| ARV at baseline, n (%) | 3,152 (50·8) | 14,966 (52·4) | 794 (55·0) | 3,016 (50 1) | 1,391 (48 9) | 5,907 (46 1) |
| CD4 count, median (IQR) | 363 (168-588) | 351 (158-557) | 352 (154-572) | 319 (129-521) | 381 (179-608) | 362 (172-566) |
| Log viral copy-years, median (IQR) | 9·8 (7·8-11 3) | 9 4 (7 5-11 0) | 10 6 (9 0-11 8) | 10 2 (8 4-11 5) | 9 8 (7 8-11 4) | 9 3 (7 4-11 1) |
| Comorbid Conditions | ||||||
| HCV infection, n (%) | 1,574 (25·8) | 5,313 (19.1) | 397 (27 5) | 1,365 (22 7) | 655 (23 0) | 2,344 (18 3) |
| HBV infection, n (%) | 222 (3·6) | 1,781 (6·4) | 66 (4 6) | 471 (7 8) | 100 (3 5) | 809 (6 3) |
| Diabetes, n (%) | 1,007 (16·6) | 2,930 (10·6) | 343 (23 8) | 1,076 (17 9) | 619 (21 9) | 1,803 (14 1) |
| Hypertension, n (%) | 1,023 (16·9) | 2,781 (10.1) | 601 (21 2) | 1,529 (12 0) | ||
| 2 APOL1 risk variantsa, n (%) | – | 148 (10·2) | 350 (5·8) | |||
| ≥50% African ancestry | – | 1,003 (69 4) | 2,224 (37 0) | |||
| Baseline creatinine, median (IQR) | 0·8 (0·7-1·0) | 1·0 (0·9-1·2) | 0 9 (0·7-1·1) | 1 1 (0 9-1 2) | 0 9 (0 7-1 0) | 1 0 (0 9-1 2) |
| Baseline eGFR, median (IQR) | 93 (71-112) | 94 (77-109) | 85 (63-103) | 87 (70-103) | 88 (69-106) | 89 (74-104) |
| BMI, kg/m2 | ||||||
| <19 | 333 (6 0) | 1,025 (4 0) | 62 (4 5) | 221 (3 8) | 136 (4 8) | 470 (3 7) |
| 19-24 | 1,894 (34 2) | 12,141 (47 8) | 456 (33 1) | 2,724 (46 4) | 891 (31 7) | 5,928 (46 8) |
| 25-29 | 1,506 (27 2) | 8,778 (34 6) | 405 (29 4) | 2,108 (35 9) | 789 (28 0) | 4,429 (35 0) |
| ≥30 | 1,801 (32 5) | 3,456 (13 6) | 453 (32 9) | 820 (14 0) | 999 (35 5) | 1,841 (14 5) |
| Patient Reported Outcomes | ||||||
| High risk alcohol abuse | – | – | 397 (14 0) | 2,183 (17 0) | ||
| IDU | – | – | 189 (14.2) | 1,213 (18 8) | ||
| Methamphetamine use | – | – | 395 (14 6) | 4,660 (37 3) | ||
| Marijuana use | – | – | 1,327 (48 9) | 8,602 (69 0) | ||
| Illicit opioid use | – | – | 473 (18 3) | 2,418 (19 9) | ||
| Cocaine/crack use | 985 (36 2) | 5,928 (4761) | ||||
| Study Entry Era | ||||||
| 1995-1999 | 1,088 (17 8) | 4,062 (14 6) | 352 (24 4) | 1,188 (19 7) | 335 (11 8) | 1,134 (8 8) |
| 2000-2004 | 1,696 (27.8) | 6,791 (24 4) | 484 (33 5) | 1,584 (26 3) | 594 (20 9) | 2,159 (16 8) |
| 2005-2009 | 1,346 (22 1) | 6,834 (24 5) | 423 (29 3) | 2,063 (34 3) | 742 (26 1) | 3,414 (26 6) |
| 2010-2014 | 1,371 (22 5) | 6,993 (25 1) | 184 (12 7) | 1,153 (19 2) | 883 (31 1) | 4,381 (34 2) |
| 2015-2020 | 604 (9.9) | 3,213 (11 5) | 2 (0 1) | 29 (0 5) | 290 (10 2) | 1,739 (13 6) |
Among genotyped only.
ARV: antiretroviral use, HCV: hepatitis C virus, HBV: hepatitis B virus, APOL1: apolipoprotein 1, eGFR: estimated glomerular filtration rate, CKD: chronic kidney disease, IDU: intravenous drug use.
Overall cohort
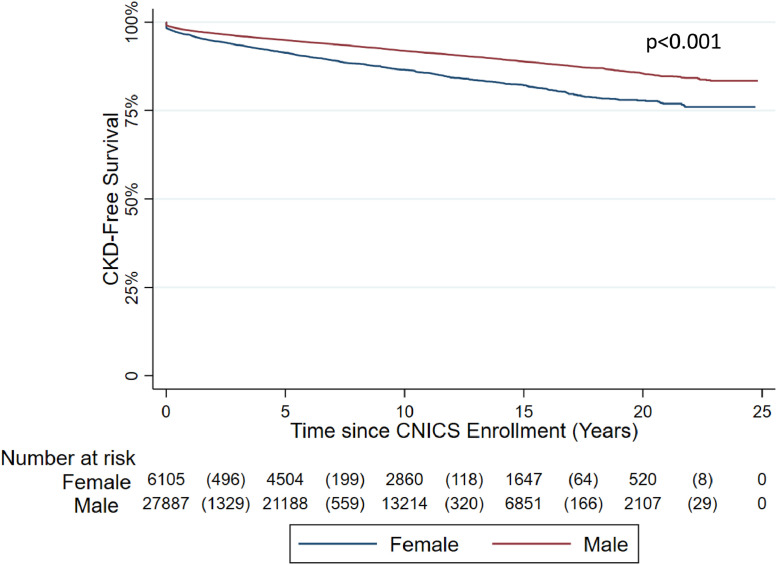
There were 33,998 PWH in the overall CNICS cohort. WWH were more commonly Black (64.0% vs. 33.4%), had higher prevalence of HCV co-infection (25.8% vs. 19.1%), higher prevalence of diabetes (16.6% vs. 10.6%), and higher prevalence of BMI ≥30 (32.5% vs. 13.6%). Median baseline serum creatinine was lower among WWH as compared to men (0.8, IQR: 0.7-1.0, vs. 1.0, IQR: 0.9-1.2) (Table 1).The cumulative incidence of CKD during the study period was 24% among women and 19% among men. Percent of WWH free of CKD at one, three, and five years were 94.2%, 90.4%, and 87.9% as compared to 95.3%, 92.7%, and 90.6% of men with HIV (p<0.0001) (Figure 2). In unadjusted analyses, female sex was associated with a 67% increased risk of CKD (HR: 1·67, 95% 1·39-2·01, p<0·001) (Supplemental Tables 1 and 3). While the absolute risk difference in CKD development was 5%, after adjustment for sex-based differences in baseline serum creatinine and known risk factors, female sex was associated with a 61% increased risk of CKD as compared to men (adjusted hazard ratio [aHR]: 1·61, 95% CI: 1·46-1·78, p<0·001) (Table 3) and a 63% increased risk of CKD after treating death as a competing risk (adjusted subdistribution hazard ratio [asdHR]: 1·63, 95% CI: 1·50-1·78, p<0·001) (Table 4) The calculated E-value, defined as the strength of association with CKD an unknown confounder would need to impact the observed association between female sex and CKD, was 2.60 (lower limit: 2.28) and 2.64 (lower limit: 2.37) for the Cox proportional hazards and Fine and Gray competing risk regressions respectively. After controlling for both BMI and baseline eGFR, female sex was associated with 36% and 29% increased risk of CKD development in both Cox proportional hazards models and Fine and Gray competing risk regressions respectively (aHR: 1.36, 95% CI: 1.22-1.52, p<0.0001; asdHR: 1.29, 1.18-1.42, p<0.0001) (Supplemental Tables 3 and 4).
Figure 2.
Survival curve for CKD development among PWH by birth sex.
PWH were followed from date of study enrollment to the earliest of CKD development, loss to follow-up, or death. Women with HIV, shown in red, had lower CKD-free survival as compared to men with HIV, shown in blue (p<0.001).
Table 3.
Adjusted risk of chronic kidney disease development among people living with HIV using Cox proportional hazards regression.
| Overall | Genotyped | Patient Reported Outcomes Cohort | Genotyped and Patient Reported Outcomes | |
|---|---|---|---|---|
| N=33,998 | N=7,462 | N=15,671 | N=5,344 | |
| aHR (95% CI)a | aHR (95% CI)a | aHR (95% CI)a | aHR (95% CI)a | |
| Demographics | ||||
| Female sex | 1·61 (1·46-1·78) | 1·92 (1·64-2·26) | 1·70 (1·54-1·87) | 1·76 (1·46-2·12) |
| Age at study entry | 1·08 (1·08-1·09) | 1·08 (1·07-1·09) | 1·09 (1·086-1·10) | 1·08 (1·07-1·09) |
| Black race | 0·71 (0·65-0·78) | 0·52 (0·34-0·79) | 0·65 (0·59-0·71) | 0·63 (0·39-1·02) |
| Baseline creatinine | 1·07 (1·05-1·09) | 1·46 (1·39-1·55) | 1·45 (1·43-1·48) | 1·41 (1·31-1·52) |
| APOL1 risk | – | 1·35 (1·03-1·81) | – | 1·65 (1·22-2·24) |
| African ancestry | – | 1·10 (0·67-1·80) | – | 0·85 (0·49-1·49) |
| Comorbid Conditions | ||||
| HCV infection | 1·25 (1·14-1·37) | 1·21 (1·02-1·45) | 1·25 (1·14-1·36) | – |
| HBV infection | 1·43 (1·24-1·66) | – | 1·26 (1·09-1·46) | – |
| Diabetes | 1·65 (1·50-1·82) | 1·42 (1·21-1·67) | 1·39 (1·28-1·52) | 1·34 (1·14-1·58) |
| Hypertension | 1·77 (1·59-1·97) | 1·51 (1·25-1·82) | 1·42 (1·29-1·57) | 1·48 (1·22-1·80) |
| ART Naïve | 0·81 (0·74-0·89) | – | 0·80 (0·73-0·88) | 0·42 (0·17-1·05) |
| Log Viral Copy-years | 1·02 (1·00-1·04) | 1·01 (1·00-1·05) | 1·03 (1·00-1·06) | – |
| ART duration, yrs. | 0·97 (0·95-0·99) | – | 0·93 (0·92-0·95) | 0·96 (0·93-0·99) |
| PI duration, yrs. | 1·03 (1·00-1·05) | – | 1·03 (1·01-1·06) | – |
| INSTI duration, yrs. | 1·10 (1·06-1·14) | 1·14 (1·06-1·23) | 1·11 (1·08-1·14) | 1·15 (1·06-1·24) |
| Atazanavir duration, yrs. | – | – | 1·07 (1·04-1·11) | – |
| Indinavir duration, yrs. | – | – | 0·88 (0·83-0·94) | – |
| CD4 < 200 cells/mL | 1·38 (1·27-1·51) | 1·20 (1·02-1·40) | 1·20 (1·11-1·31) | 1·24 (1·06-1·46) |
| High-risk alcohol use | – | – | 0·68 (0·59-0·78) | 0·64 (0·50-0·83) |
| Methamphetamine use | – | – | 0·85 (0·70-1·02) | 0·85 (0·70-1·02) |
| Illicit opioid use | – | – | – | 1·24 (1·02-1·52) |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCV, Hepatitis C virus; HBV, Hepatitis B virus; ART: antiretroviral therapy; VCY: viral copy-years.
Bold indicates significance at p<0.05.
Table 4.
Adjusted risk of chronic kidney disease development among people living with HIV using Fine and Gray competing risks regression.
| Overall | Genotyped | Patient Reported Outcomes Cohort | Genotyped and Patient Reported Outcomes | |
|---|---|---|---|---|
| N=33,998 | N=7,462 | N=15,671 | N=5,344 | |
| aHR (95% CI)a | aHR (95% CI)a | aHR (95% CI)a | aHR (95% CI)a | |
| Demographics | ||||
| Female sex | 1·63 (1·50-1·78) | 1·79 (1·47-2·18) | 1·60 (1·26-2·04) | 1·60 (1·33-1·92) |
| Age at study entry | 1·07 (1·06-1·07) | 1·07 (1·06-1·08) | 1·07 (1·06-1·08) | 1·07 (1·06-1·08) |
| Black race | 0·79 (0·73-0·86) | 0·73 (0·54-0·99) | 0·58 (0·49-0·69) | 0·64 (0·41-1·00) |
| Baseline creatinine | 1·07 (1·05-1·08) | 1·39 (1·34-1·44) | 1·44 (1·37-1·52) | 1·41 (1·31-1·51) |
| APOL1 risk | – | 1·56 (1·15-2·14) | – | 1·64 (1·18-2·28) |
| African ancestry | – | 0·76 (0·48-1·22) | – | 0·83 (0·48-1·41) |
| Comorbid Conditions | ||||
| HCV infection | 1·11 (1·02-1·21) | – | – | – |
| HBV infection | 1·43 (1·25-1·63) | – | – | – |
| Diabetes | 1·98 (1·81-2·16) | 1·50 (1·25-1·79) | 1·37 (1·16-1·63) | 1·36 (1·16-1·59) |
| Hypertension | 1·93 (1·75-2·12) | 1·65 (1·49-1·79) | 1·60 (1·44-1·78) | 1·58 (1·32-1·90) |
| ART Naïve | 0·86 (0·79-1·07) | – | – | – |
| Log Viral Copy-years | 1·06 (1·04-1·07) | 1·05 (1·02-1·07) | 1·06 (1·03-1·08) | 1·07 (1·03-1·10) |
| ART duration, yrs. | 0·98 (0·96-0·99) | – | – | – |
| PI duration, yrs. | 1·02 (0·999-1·05) | – | – | – |
| Tenofovir duration, yrs. | – | 1·05 (0·998-1·11) | – | – |
| INSTI duration, yrs. | 1·10 (1·06-1·13) | 1·11 (1·05-1·17) | 1·12 (1·08-1·15) | 1·11 (1·03-1·20) |
| Indinavir duration, yrs. | – | 0·93 (0·86-1·00) | 0·86 (0·80-0·93) | – |
| CD4 < 200 cells/mL | 1·33 (1·23-1·44) | 1·14 (1·04-1·25) | 1·14 (1·03-1·27) | 1·18 (1·02-1·37) |
| High-risk alcohol use | – | – | 0·60 (0·46-0·79) | 0·64 (0·50-0·81) |
| Methamphetamine use | – | – | – | 0·87 (0·73-1·04) |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCV, Hepatitis C virus; HBV, Hepatitis B virus; ART: antiretroviral therapy; VCY: viral copy-years.
Bold indicates significance at p<0.05.
Genotyped cohort
There were 7,435 PWH in the CNICS cohort with serum creatinine values who were also genotyped at the APOL1 locus with accompanying genetic African ancestry data available for analysis. WWH were more commonly Black (69.6% vs. 37.4%), more commonly had 2 APOL1 renal risk variants (10.2% vs. 5.8%), had higher prevalence of diabetes (23.8% vs. 17.9%), and higher prevalence of BMI ≥30 (32.9% vs. 14.0%) as compared to men (Table 1). There was substantial variation in the proportion of genetic African ancestry with many PWH having admixed ancestry (Supplemental Figure 1). In unadjusted analyses, female sex was associated with 55% increased risk of CKD as compared to men (HR: 1·55, 95% CI: 1·26-1·90, p<0·001) (Supplemental Tables 1 and 3). After adjustment for demographic characteristics, comorbid conditions, HIV characteristics, APOL1 CKD risk variants, and genetic African ancestry, female sex was associated with 92% increased risk of CKD as compared to men (aHR: 1·92, 95% CI: 1·63-2·26, p<0·001) (Table 3). Genetic African ancestry was not significantly associated with CKD, but presence of two APOL1 risk variants was associated with 35% increased risk (aHR: 1·35, 95% 1·03-1·81, p<0·001) (Table 3). After accounting for death as a competing risk, female sex was associated with a 79% increased risk of CKD and 2 APOL1 risk variants with 56% increased risk (asdHR: 1·79, 95% CI: 1·47-2·18, p<0·001; asdHR: 1·56, 95% CI: 1·15-2·14, p<0·001) (Table 4). The calculated E-value was 3.25 (lower limit: 2.64) and 2.98 (lower limit: 2.28) for the Cox proportional hazards and Fine and Gray competing risk regressions respectively. Lastly, after controlling for both BMI and baseline eGFR in addition to APOL1 renal risk factors and African ancestry, female sex was associated with 36% and 29% increased risk of CKD development in both Cox proportional hazards models and Fine and Gray competing risk regressions respectively (aHR: 1.36, 95% CI: 1.16-1.60, p<0.0001; asdHR: 1.29, 95% CI: 1.06-1.57, p=0.01) (Supplemental Tables 3 and 4).
Patient reported outcomes cohort
There were 15,671 PWH with history of substance use data and with creatinine data. WWH were less commonly Hispanic (8.9% vs. 14.8%), more commonly coinfected with HCV (23.0% vs. 18.3%), and more commonly had diabetes (21.9% vs. 14.1%). BMI of 30 or greater was more common among WWH than men (35.5% vs. 14.5%). Women less commonly reported methamphetamine use (14.6% vs. 37.3%), marijuana use (48.9% vs. 69.0%), and cocaine/crack use (36.2% vs. 47.6%). (Table 1). In unadjusted analyses, female sex was associated with 47% increased risk of CKD as compared to men (aHR: 1·47, 95% CI: 1·24-1·74, p<0·001) (Supplemental Tables 1 and 2). After adjusting for demographics, comorbid conditions, HIV-specific risk factors, and high-risk alcohol use and methamphetamine use, female sex was associated with 70% increased risk of CKD development as compared to men (aHR: 1·70, 95% CI: 1·54-1·87, p<0·001) (Table 3). Female sex was associated with a 60% increased risk of CKD, after accounting for death as a competing risk (asdHR: 1·60, 95% CI: 1·26-2·04, p<0·001) (Table 4). Female sex was associated with 41% and 32% increased risk of CKD development in both Cox proportional hazards models and Fine and Gray competing risk regressions respectively, even after accounting for the potential confounding effects of BMI, baseline eGFR, and high-risk alcohol use (aHR: 1.41, 95% CI: 1.17-1.70, p<0.0001; asdHR: 1.32, 95% CI: 1.14-1.52, p<0.0001) Supplemental Tables 3 and 4). Inclusion of APOL1 and African ancestry with substance use data yielded similar inferences (aHR: 1·82, 95% CI: 1·40-2·38, p<0·001; asdHR: 1·60, 95% CI: 1·33-1·92, p<0·001) (Tables 3 and 4). Addition of genetic factors to models containing BMI and baseline eGFR suggested female sex was associated with 35% and 22% increased risk of CKD development in both Cox proportional hazards models and Fine and Gray competing risk regressions respectively (aHR: 1.35, 95% CI: 1.02-1.78, p=0.04; asdHR: 1.22, 95% CI: 1.02-1.45, p=0.03) (Supplemental Tables 3 and 4). The calculated E-value was 2.79 (lower limit: 2.45) and 3.04 (lower limit: 2.58) for the Cox proportional hazards and Fine and Gray competing risk regressions respectively.
Discussion
This comprehensive study of kidney function in PWH is the first to account for sex-based differences in serum creatinine and genetic risk factors for CKD. We found female sex was consistently associated with increased risk of CKD independent of baseline serum creatinine. Moreover, the disparity in CKD was not mitigated after adjustment for APOL1 and degree of African ancestry. Inclusion of history of substance abuse similarly failed to mitigate the increased risk of CKD found among WWH compared to men.
Several studies have demonstrated an increased risk of CKD in women regardless of HIV status with multiple proposed biological mechanisms. Specifically, the increased risk of CKD among WWH observed in this study may in part be due to biologic factors such as sex-specific differences in antiretroviral pharmacokinetic parameters, pregnancy, or genetic variants located on sex chromosomes. Multiple studies have suggested women have higher plasma drug concentrations than men at the same dosing level.47,48 While tenofovir alafenamide has similar tolerability to tenofovir disoproxil fumarate (TDF) and lower risk of adverse events, including those specific to kidney injury,49 TDF was the most common formulation of tenofovir prescribed in this retrospective cohort, reflecting contemporary practice patterns. Women have been reported to have approximately 19% higher concentrations of TDF than men,50 which may explain some of the increased risk of CKD development among women observed in this study as TDF plasma concentrations are associated with increased risk of kidney tubular dysfunction.51 Additionally, numerous studies suggest, when engaged in HIV care, WWH have lower HIV viral loads than men and higher levels of inflammatory markers.52, 53, 54, 55, 56, 57, 58 Even with effective ART therapy, this increased inflammation may persist and mimic the natural inflammation process associated with aging in the general population thereby increasing the baseline risk of CKD development among PWH.59,60 As an experience unique to women, the effect of pregnancy on renal function must also be considered. It is known women with low baseline eGFR experience heightened risk of CKD progression in pregnancy and, as we were unable to account for pregnancy history, may contribute to some of the unexplained, increased risk observed in this study.61 Relatedly, sex hormones have been implicated in CKD risk and progression, with estrogen protective against kidney disease and testosterone increasing risk of kidney disease.62,63 Last, genetic analyses of sex chromosomes are infrequent, with many of those published performed incorrectly.64 Thus, it is relatively unknown whether there are risk variants for CKD on the X chromosome or perhaps a protective variant present on the Y chromosome. Further work exploring the potential for sex-specific genetic variants is needed. Cumulatively, these biologic factors may explain some proportion of the observed disparity in CKD development.
Beyond biologic factors, social factors including access to and compliance with care are known to differ between men and WWH and may contribute to the increased CKD risk observed among WWH in this study.65,66 WWH have been found to disengage from care more frequently than men due to a number of potential reasons including increased depression, greater stigma associated with HIV infection, and lower socioeconomic status.67,68 Recent work found HIV stigma was highest among Black, Hispanic/Latinx, and white women as compared to white men with potentially important implications for clinical care. Stigma was associated with lower ART use and adherence, greater numbers of missed HIV care visits, and increased prevalence of symptoms associated with depression or anxiety.66,67 Moreover, WWH face multiple hurdles to clinic attendance including insurance issues and presence of childcare. Recent work among WWH highlighted private insurance was associated with three-fold higher odds of retention in care as compared to lack of insurance with no AIDS Drug Assistance Program coverage.69 In a mixed methods study of WWH in the Southeast, women reported childcare as disruptive to their schedules, leading them to subsequently forget to take their ART,70, 71, 72 though other research has reported children act as a meaningful facilitator of retention in care as WWH recognize the need to care for themselves in order to subsequently care for their children.73 Additionally, substance use among WWH has been consistently associated with failure to achieve viral suppression.74, 75, 76 As active viraemia is a known risk factor for kidney disease progression,77 these may represent meaningful points of intervention as actions taken to reduce HIV stigma and eliminate barriers to clinical attendance may improve retention in care, ART adherence, and chronic disease management. Consequently, interpersonal and societal factors may compound upon and interact with each other to increase risk factors for CKD and perhaps for subsequent CKD risk.
The finding of increased CKD risk among WWH has been described in previous cohort studies but is incongruent with data highlighting the significantly lower rates of CKD progression and ESKD development among women in the general population. Correspondingly, existing risk calculators for ESKD confer protective status to women.78 Among PWH, this discrepancy between risk of CKD and ESKD also persists. In adjusted analyses of a national cohort of PWH, WWH experienced no significant increased risk of ESKD as compared to men.5 The reasons for this apparent contradiction are unclear. Age has been shown to act as a significant effect modifier of risk such that ESKD risk is lower among older women as compared to younger men, but higher among younger women as compared to younger men, potentially motivating further study of the role of sex hormones in CKD progression and ESKD development.79 Additionally, the CKD-EPI equation itself may contribute to some of this perceived increased risk of CKD as, at any given serum creatinine level, it assigns women a lower eGFR than it does men and thereby also assigns them a more advanced CKD stage.17 Nevertheless, understanding CKD risk among WWH and measures that may mitigate such risk is of paramount importance given the known disparities in ESKD care.
As with all observational studies, there are limitations to these analyses. While this is a diverse cohort of PWH, our analysis is restricted to those who enroll in CNICS at eight academic medical centres across the United States and may not reflect all PWH in the United States including those who are not yet diagnosed or not in care. As patients must consent to participate in CNICS, selection bias is possible in which patients who are more engaged in care are also more likely to participate in CNICS. However, CNICS consent rates are high (>90%) so the impact of this limitation is likely minimal. While CNICS has standardized protocols at enrollment to capture prior ART regimens and other HIV history, there may be missing data for HIV care occurring prior to CNICS enrollment. As the majority of WWH are Black and Black individuals are known to present later to care, and while we control for CD4 count and HIV viral load,80,81 we cannot fully account for this disparity in access to care that may confound our findings. A new race-less eGFR equation was recently published.82 Given time lags in adoption, these data reflect practice during the study period. Moreover, creatinine-based estimates of kidney function are more imprecise than cystatin C-based estimates but, as cystatin C values are not routinely captured in clinical practice, we were reliant upon creatinine-based estimating equations. Similarly, we were unable to assess other measures of kidney disease among PWH, such as proteinuria, as these data were not captured within this dataset. As such, our inferences are limited to kidney function reflected in creatinine-based estimating equations. Among PWH, there are numerous factors that may influence serum creatinine levels and subsequently affect ascertainment of CKD, of which the most pertinent may be ART. Ultimately, this interrelationship between ART, serum creatinine levels, and ascertainment of CKD may illustrate the importance of a holistic approach to assessing kidney function that considers serum creatinine levels in the context of patient drug regimens or that even prioritizes collection of additional labs such as cystatin C or iothalamate GFRs among patients whose kidney function appears particularly impaired. Importantly, models were built to best estimate the measure of association for sex rather than other characteristics such as race, ART regimens, and history of substance use. As such, those covariates are subject to unmeasured confounding, bias, and effect modification and must subsequently be interpreted with caution.83 Last, there are other sex-specific differences including hormones, physiology, and pregnancy history for which we could not adjust that may confound our results. Despite these limitations, this study has a large sample size with racial and geographic diversity that enhance the generalizability of our findings.
This study is the first to clearly associate female sex with increased risk of CKD development by accounting for sex-based differences in serum creatinine, genetic factors, and other risk factors including substance use. Importantly, these data highlight this increased risk persisted even after adjustment for covariates known to contribute to diminished kidney function. As life expectancy for this population increases, managing CKD risk among WWH is critical, particularly given the epidemiology of HIV infections, known disparities in access to kidney transplantation, and risk of subsequent mortality. Further work examining the mechanisms by which WWH are at increased CKD risk is warranted.
Contributors
DS, HMC, RDM, KC, JMJ, JJE, MS, IP, and JEL contributed to data curation, funding acquisition, manuscript review and editing. BAS and PM accessed and verified the underlying data, and contributed to data analysis and manuscript drafting. WL contributed to data curation, manuscript review, and editing. GN, HF, SM, PP, BJ, and EM contributed to manuscript review and editing.
Data sharing statement
The data supporting these findings are publicly available upon request through the CFAR Network of Integrated Clinical Systems (CNICS).
Declaration of interests
JEL reports grands and funding from Hansa, United Therapeutics, honoraria from Sanofi, and non-monetary support from the FDA and Davita, and reports support from the NIH for the present study. KC reports an investigator-initiated grant from Gilead Sciences and serves as a medical advisory board member for Gilead Sciences. GN reports R01-DK127139, R01-HL155915, R56-DK126930, and funding from Renalytix. GN also receives consulting fees from Renalytx (as well as royalties), Variant Bio, Qiming Capital, Cambridge Healthcare, Daiichi Sankyo (as well as honoraria). GN also has patent with Renalytx and participates in advisory boards for Renalytix and Pensieve Health. Finally, GN has stock/stock options in Renaltyx, Pensieve Health, Vierici Dx, Nexus I Connect, and Data2Wisdom LLC. SM reports honoraria from CareDx and serves on their advisory board, and reports NIH grants/contracts for use of CCR5 in HIV-positive transplant recipients and the prospective HOPE In-Action trial. DS has entered into a consulting agreement with IMS Consulting and Expert Services, has been elected as Councilor at Large to the American Society of Transplantation Board of Directors, and reports funding from the NIH for the present study. HC reports funding from the NIH (for the present study), AHRQ, Viiv, and serves on the Office of AIDS Advisory Council for the NIH. MS serves on the advisory or DSMB board for I-SPY, an NIH-funded study, and in a leadership role for IAS-USA. JJE reports financial support for the present study from the NIH; grants (outside the present study) from ViiV Healthcare, Gilead Science and Janssen; and consulting fees (outside the present study) from Merck, ViiV Healthcare, Gilead Sciences, and Jansssen. BJ and BAS report funding from the NIH. RM and IP report financial support from the NIH for the present study. All other authors declare no competing interests.
Acknowledgements
This study was funded by the NIH R01DK117675 (PI: Locke) and R01DA047045 (PI: Crane). Additional funding for CNICS was from the National Institute of Allergy and Infectious Diseases (NIAID) [CNICS R24 AI067039; UW CFAR NIAID Grant P30 AI027757; UAB CFAR grant P30 AI027767; UNC CFAR grant P30 AI50410; and JHU CFAR grant P30 AI094189]; the National Institute of Alcohol Abuse and Alcoholism (NIAAA) [U24AA020801, U01AA020793 and U01AA020802], National Human Genome Research Institute (NHGRI) [RO1HG010649], and the National Institute on Drug Abuse (NIDA) [R01DA047045].
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101653.
Appendix. Supplementary materials
References
- 1.Ryom L, Kirk O, Lundgren JD, et al. Advanced chronic kidney disease, end-stage renal disease and renal death among HIV-positive individuals in Europe. HIV Med. 2013;14:503–508. doi: 10.1111/hiv.12038. [DOI] [PubMed] [Google Scholar]
- 2.Campbell LJ, Ibrahim F, Fisher M, Holt SG, Hendry BM, Post FA. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 3.Szczech LA, Gange SJ, van der Horst C, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 4.Scherzer R, Gandhi M, Estrella MM, et al. A chronic kidney disease risk score to determine tenofovir safety in a prospective cohort of HIV-positive male veterans. AIDS. 2014;28:1289–1295. doi: 10.1097/QAD.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60:941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas GM, Mehta SH, Atta MG, et al. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21:2435–2443. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- 7.Wong C, Gange SJ, Buchacz K, et al. First occurrence of diabetes, chronic kidney disease, and hypertension among North American HIV-infected adults, 2000-2013. Clin Infect Dis. 2017;64:459–467. doi: 10.1093/cid/ciw804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D Studya. J Infect Dis. 2013;207:1359–1369. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flandre P, Pugliese P, Cuzin L, et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol. 2011;6:1700–1707. doi: 10.2215/CJN.09191010. [DOI] [PubMed] [Google Scholar]
- 10.Campbell L, Ibrahim F, Fisher M, Holt S, Hendry B, Post F. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi AI, Shlipak MG, Hunt PW, Martin JN, Deeks SG. HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. AIDS. 2009;23:2143–2149. doi: 10.1097/QAD.0b013e3283313c91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Althoff KN, McGinnis KA, Wyatt CM, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2014;60:627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kooij KW, Vogt L, Wit FWNM, et al. Higher prevalence and faster progression of chronic kidney disease in human immunodeficiency virus-infected middle-aged individuals compared with human immunodeficiency virus-uninfected controls. J Infect Dis. 2017;216:622–631. doi: 10.1093/infdis/jix202. [DOI] [PubMed] [Google Scholar]
- 14.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O'Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007;72:1380–1387. doi: 10.1038/sj.ki.5002541. [DOI] [PubMed] [Google Scholar]
- 15.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151–164. doi: 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
- 16.Cristelli MP, Trullàs JC, Cofán F, et al. Prevalence and risk factors of mild chronic renal failure in HIV-infected patients: influence of female gender and antiretroviral therapy. Braz J Infect Dis. 2018;22:193–201. doi: 10.1016/j.bjid.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hödlmoser S, Winkelmayer WC, Zee J, et al. Sex differences in chronic kidney disease awareness among US adults, 1999 to 2018. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243431. e0243431-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gault MH, Chafe L, Prabhakaran V. Mid-menstrual cycle decline in creatinine and urea clearances. Nephron. 1994;67:158–166. doi: 10.1159/000187919. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27:2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasembeli AN, Duarte R, Ramsay M, et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. 2015;26:2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman BI, Limou S, Ma L, Kopp JB. APOL1-associated nephropathy: a key contributor to racial disparities in CKD. Am J Kidney Dis. 2018;72:S8–s16. doi: 10.1053/j.ajkd.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udler MS, Nadkarni GN, Belbin G, et al. Effect of genetic African ancestry on eGFR and kidney disease. J Am Soc Nephrol. 2015;26:1682–1692. doi: 10.1681/ASN.2014050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norton JM, Moxey-Mims MM, Eggers PW, et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27:2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson CA, MO Johnson, Neilands TB, et al. Internalized HIV stigma predicts suboptimal retention in care among people living with HIV in the United States. AIDS Patient Care STDS. 2021;35:188–193. doi: 10.1089/apc.2020.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinn K, Dickson-Gomez J, Broaddus M, Kelly JA. "It's almost like a crab-in-a-barrel situation": stigma, social support, and engagement in care among black men living with HIV. AIDS Educ Prevent. 2018;30:120–136. doi: 10.1521/aeap.2018.30.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweeney SM, Vanable PA. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav. 2016;20:29–50. doi: 10.1007/s10461-015-1164-1. [DOI] [PubMed] [Google Scholar]
- 29.Blake Helms C, Turan JM, Atkins G, et al. Interpersonal mechanisms contributing to the association between HIV-related internalized stigma and medication adherence. AIDS Behav. 2017;21:238–247. doi: 10.1007/s10461-016-1320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earnshaw VA, Bogart LM, Laurenceau JP, et al. Internalized HIV stigma, ART initiation and HIV-1 RNA suppression in South Africa: exploring avoidant coping as a longitudinal mediator. J Int AIDS Soc. 2018;21:e25198. doi: 10.1002/jia2.25198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan PS, Satcher Johnson A, Pembleton ES, et al. Epidemiology of HIV in the USA: epidemic burden, inequities, contexts, and responses. Lancet. 2021;397:1095–1106. doi: 10.1016/S0140-6736(21)00395-0. [DOI] [PubMed] [Google Scholar]
- 32.Desir FA, Lesko CR, Moore RD, et al. One size fits (n)one: the influence of sex, age, and sexual Human Immunodeficiency Virus (HIV) acquisition risk on racial/ethnic disparities in the HIV care continuum in the United States. Clin Infect Dis. 2019;68:795–802. doi: 10.1093/cid/ciy556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS research network of integrated clinical systems. Int J Epidemiol. 2008;37:948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H, Sewda A, Marquez-Luna C, et al. Genetic architecture of cardiometabolic risks in people living with HIV. BMC Med. 2020;18:288. doi: 10.1186/s12916-020-01762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price AL, Weale ME, Patterson N, et al. Long-range LD can confound genome scans in admixed populations. Am J Hum Genet. 2008;83:132–139. doi: 10.1016/j.ajhg.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou H, Alexander D, Lange K. A quasi-Newton acceleration for high-dimensional optimization algorithms. Stat Comput. 2011;21:261–273. doi: 10.1007/s11222-009-9166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 41.Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 42.Crane HM, McCaul ME, Chander G, et al. Prevalence and factors associated with hazardous alcohol use among persons living with HIV across the US in the current era of antiretroviral treatment. AIDS Behav. 2017;21:1914–1925. doi: 10.1007/s10461-017-1740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nance RM, Trejo MEP, Whitney BM, et al. Impact of abstinence and of reducing illicit drug use without abstinence on human immunodeficiency virus viral load. Clin Infect Dis. 2020;70:867–874. doi: 10.1093/cid/ciz299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M, Sequist TD. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129 doi: 10.1016/j.amjmed.2015.08.025. 153-62.e7. [DOI] [PubMed] [Google Scholar]
- 45.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322:113–114. doi: 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 46.Yudell M, Roberts D, DeSalle R, Tishkoff S. Taking race out of human genetics. Science. 2016;351:564–565. doi: 10.1126/science.aac4951. [DOI] [PubMed] [Google Scholar]
- 47.Venuto CS, Mollan K, Ma Q, et al. Sex differences in atazanavir pharmacokinetics and associations with time to clinical events: AIDS Clinical Trials Group Study A5202. J Antimicrob Chemother. 2014;69:3300–3310. doi: 10.1093/jac/dku303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark R. Sex differences in antiretroviral therapy-associated intolerance and adverse events. Drug Saf. 2005;28:1075–1083. doi: 10.2165/00002018-200528120-00003. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: meta-analysis. Medicine. 2016;95 doi: 10.1097/MD.0000000000005146. e5146-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2018;62:e01710–e01717. doi: 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andany N, Walmsley SL. What's new for antiretroviral treatment in women with HIV. J Virus Eradic. 2016;2:67–77. doi: 10.1016/S2055-6640(20)30472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 53.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis. 2014;209(suppl 3):S86–S92. doi: 10.1093/infdis/jiu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–322. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 55.Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep. 2018;15:136–146. doi: 10.1007/s11904-018-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziegler S, Altfeld M. Sex differences in HIV-1-mediated immunopathology. Curr Opin HIV AIDS. 2016;11:209–215. doi: 10.1097/COH.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathad JS, Gupte N, Balagopal A, et al. Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr. 2016;73:123–129. doi: 10.1097/QAI.0000000000001095. (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meier A, Chang JJ, Chan ES, et al. Sex differences in the toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3. In: Alt FW, ed. Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune activation in HIV-infected aging women on antiretrovirals–implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063804. e63804-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hladunewich MA. Chronic kidney disease and pregnancy. Semin Nephrol. 2017;37:337–346. doi: 10.1016/j.semnephrol.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Neugarten J, Golestaneh L. Influence of sex on the progression of chronic kidney disease. Mayo Clin Proc. 2019;94:1339–1356. doi: 10.1016/j.mayocp.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Lima-Posada I, Bobadilla NA. Understanding the opposite effects of sex hormones in mediating renal injury. Nephrology. 2021;26:217–226. doi: 10.1111/nep.13806. [DOI] [PubMed] [Google Scholar]
- 64.Wise AL, Gyi L, Manolio TA. eXclusion: toward integrating the X chromosome in genome-wide association analyses. Am J Hum Genet. 2013;92:643–647. doi: 10.1016/j.ajhg.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matson TE, McGinnis KA, Rubinsky AD, et al. Gender and alcohol use: influences on HIV care continuum in a national cohort of patients with HIV. AIDS. 2018;32:2247–2253. doi: 10.1097/QAD.0000000000001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beer L, Tie Y, McCree DH, et al. HIV stigma among a national probability sample of adults with diagnosed HIV-United States, 2018-2019. AIDS Behav. 2021;26(suppl 1):39–50. doi: 10.1007/s10461-021-03414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turan B, Rice WS, Crockett KB, et al. Longitudinal association between internalized HIV stigma and antiretroviral therapy adherence for women living with HIV: the mediating role of depression. AIDS. 2019;33:571–576. doi: 10.1097/QAD.0000000000002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denning P, DiNenno E. XVIII International AIDS Conference. 2010. Communities in crisis: is there a generalized HIV epidemic in impoverished urban areas of the United States. [Google Scholar]
- 69.Kay ES, Edmonds A, Ludema C, et al. Health insurance and AIDS Drug Assistance Program (ADAP) increases retention in care among women living with HIV in the United States. AIDS Care. 2021;33:1044–1051. doi: 10.1080/09540121.2020.1849529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boehme AK, Davies SL, Moneyham L, Shrestha S, Schumacher J, Kempf M-C. A qualitative study on factors impacting HIV care adherence among postpartum HIV-infected women in the rural southeastern USA. AIDS Care. 2014;26:574–581. doi: 10.1080/09540121.2013.844759. [DOI] [PubMed] [Google Scholar]
- 71.Blank AE, Fletcher J, Verdecias N, Garcia I, Blackstock O, Cunningham C. Factors associated with retention and viral suppression among a cohort of HIV+ women of color. AIDS Patient Care STDS. 2015;29(suppl 1):S27–S35. doi: 10.1089/apc.2014.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merenstein D, Schneider MF, Cox C, et al. Association of child care burden and household composition with adherence to highly active antiretroviral therapy in the Women's Interagency HIV Study. AIDS Patient Care STDS. 2009;23:289–296. doi: 10.1089/apc.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buchberg MK, Fletcher FE, Vidrine DJ, et al. A mixed-methods approach to understanding barriers to postpartum retention in care among low-income, HIV-infected women. AIDS Patient Care STDS. 2015;29:126–132. doi: 10.1089/apc.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Labisi TO, Podany AT, Fadul NA, Coleman JD, King KM. Factors associated with viral suppression among cisgender women living with human immunodeficiency virus in the United States: an integrative review. Womens Health. 2022;18 doi: 10.1177/17455057221092267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reid R, Dale SK. Moderating effects of social support on the relationship between substance use disorders and HIV viral load and medication adherence among black women living with HIV in the United States. AIDS Care. 2021;16:1–10. doi: 10.1080/09540121.2021.2001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leddy AM, Zakaras JM, Shieh J, et al. Intersections of food insecurity, violence, poor mental health and substance use among US women living with and at risk for HIV: evidence of a syndemic in need of attention. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG. HIV viremia and changes in kidney function. AIDS. 2009;23:1089–1096. doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315:164–174. doi: 10.1001/jama.2015.18202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricardo AC, Yang W, Sha D, et al. Sex-related disparities in CKD progression. J Am Soc Nephrol. 2019;30:137–146. doi: 10.1681/ASN.2018030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demeke HB, Johnson AS, Zhu H, Gant Z, Duffus WA, Dean HD. HIV infection-related care outcomes among U.S.-born and non-U.S.-born blacks with diagnosed HIV in 40 U.S. areas: The National HIV Surveillance System, 2016. Int J Environ Res Public Health. 2018;15:2404. doi: 10.3390/ijerph15112404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ojikutu BO, Mazzola E, Fullem A, et al. HIV testing among black and hispanic immigrants in the United States. AIDS Patient Care STDS. 2016;30:307–314. doi: 10.1089/apc.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.