Abstract
Background:
Periodontal destruction can be the result of different known and yet-to-be-discovered biological pathways. Recent human genetic association studies have implicated interferon-gamma inducible protein 16 (IFI16) and absent in melanoma 2 (AIM2) with high periodontal IL-1β levels and more destructive disease, but mechanistic evidence is lacking. Here we sought to experimentally validate these observational associations and better understand IFI16 and AIM2 roles in periodontitis.
Methods:
Periodontitis was induced in Ifi204−/− (IFI16 murine homolog) and Aim2−/− mice using the ligature model. Chimeric mice were created to identify the main source cells of Ifi204 in the periodontium. IFI16-silenced human endothelial cells were treated with periodontal pathogens in vitro. Periodontal tissues from Ifi204−/− mice were evaluated for alveolar bone (micro-CT), cell inflammatory infiltration (MPO+ staining), Il1b (qRT-PCR), and osteoclast numbers (cathepsin K+ staining).
Results:
Ifi204-deficient mice exhibited >20% higher alveolar bone loss than WT (p<0.05) while no significant difference was found in Aim2−/− mice. Ifi204’s effect on bone loss was primarily mediated by a non-bone marrow source and was independent of Aim2. Ifi204-deficient mice had greater neutrophil/macrophage trafficking into gingival tissues regardless of periodontitis development compared to WT. In human endothelial cells, IFI16 decreased the chemokine response to periodontal pathogens. In murine periodontitis, Ifi204 depletion elevated gingival Il1b and increased osteoclast numbers at diseased sites (p<0.05).
Conclusion:
These findings support IFI16’s role as a novel regulator of inflammatory cell trafficking to the periodontium that protects against bone loss and offers potential targets for the development of new periodontal disease biomarkers and therapeutics.
Keywords: host modulation, inflammasome, inflammatory disease, periodontitis, genetics
One sentence summary:
IFI16 regulates bone destruction in a murine model of experimental periodontitis.
Introduction
Recent findings emerged from genome-wide association studies (GWAS) have expanded our knowledge in genetic susceptibility to periodontal inflammation and disease.1–3 These studies suggest the existence of multiple biological pathways that converge into a similar clinical phenotype characterized by periodontal inflammation and tissue destruction4. Variants that alter periodontal homeostasis and lead to disease may be harbored in genes that affect not only the host immune responses, but also have the potential to modify neuronal signaling pathways, impair the gingival barrier function, or alter the microbial plaque composition.4 While some genes have been recently explored5–8, the biological role of the majority of genes correlated with disease pathogenesis is unknown. Additionally, inflammatory signals other than IL-1β can lead to alveolar bone loss9, 10. The development of future precision oral care requires expanding the knowledge regarding the impact of these novel genes in periodontal health and disease, which would foster the development of targeted therapies and allow for interventions before tissue destruction initiates.11
We have previously reported that individuals with variants in Interferon Gamma-Inducible Protein (IFI)16 and Absent In Melanoma (AIM)2 have increased periodontal disease prevalence and severity.12 These variants also associate with greater than 3-fold higher IL-1β concentrations in gingival fluids compared to individuals without these variants,12 suggesting that these genes likely alter important inflammatory pathways that culminate into periodontitis.13 Additionally, our group previously confirmed periodontal expression of both IFI16 and AIM2 in multiple cells of the human periodontium that included epithelial cells, fibroblasts, endothelial cells, and immune infiltrating cells.12, 14 However, the exact role of these genes in periodontal disease pathogenesis remains unclear. It is known that despite being part of the same AIM2 family of pathogen recognition receptors, both IFI16 and AIM2 sensors have biological functions that vary based on the nature of the harmful encounter, the cellular source, and the type of affected tissue.15–21 In fact, IFI16 regulates the host response by either promoting or impeding inflammation, thereby having both pro-inflammatory and anti-inflammatory functions.15, 20, 22 The objective of the present study was to further our understanding of IFI16 and AIM2 roles in periodontal health and disease using the ligature-induced periodontitis murine model.
Methods
Murine periodontitis model
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina at Chapel Hill (UNC-CH, protocols 16–024.0 and 19–007.0). The study conformed with ARRIVE guidelines.23 Mice were housed at a maximum density of 5 mice/cage under specific pathogen-free (SPF) conditions and maintained in the animal facility during the entire experiments until euthanasia. Eight- to ten-week-old C57Bl/6 WT, Ifi204−/− and Aim2−/− male mice were allocated into healthy or periodontitis groups based on our previous reports utilizing this disease model (maximum of 12 animals/group, based on the 10–20% predicted ligature loss)24. Periodontitis was induced under general anesthesia via the ligature model.24 Briefly, ligature holders and mouse dental beds were printed and assembled prior to experiments (3D printing files protected by UNC-Chapel Hill copyright). Sterile silk sutures SUT-15-1* knotted ~2.5mm apart were placed between the first (M1) and second (M2) maxillary molars. Mice were sacrificed at baseline (health) and 9 days post-periodontitis induction. The 9-day sacrificial timepoint was selected based on our previous study that showed no additional bone loss developed from 9–18 days post-ligature placement.24
Ifi204−/− mice were generated at Yale University, with the approval by the Institutional Animal Care and Use Committee (IACUC) of Yale University (protocol 2019-10538). Ifi204 knockout construct was obtained from KOMP Repository. The Yale Genome Editing Center was used to electroporate and generate ES cells/mouse. The LacZ-Neo reporter was deleted by crossing with FLPe knock-in mice† (016226) to produce IFI204fl. Exons 3–5 were then deleted by crossing with β-actin-cre mice† (019099), followed by 9 generations back-crossing to C57B/6N‡. See Figure S1 in online Journal of Periodontology for detailed information about the Ifi204-depleted mice. Ifi204 cryoprotected sperm is available (048133-UCD, KOMP-UCDavis). Ifi204 knockout genotype was confirmed by forward primer AAGCAGGCTGGCGCCGGAACCGAA and reverse primer AACTCATAAAATCTCAGGTTTG. Aim2 knockout mice were generated at the University of North Carolina at Chapel Hill as previously described25, 26 and genotyped with forward primer GGAACTTCGCTAGACTAGTACGCGTG and reverse primer CAACATTGTACAGATTGAGCAGG, while the wild type animals were confirmed with the forward primer GATGGAGAGTGAGTACCGGGAAATGCTGTT and the reverse primer TCTGCAAGTAGATTGGAGACAGACTCTGGTGA. At sacrifice, mice were weighed, and maxillae and gingival tissues were collected and stored for further analysis.
Real-Time PCR analysis for gene expression
Murine gingival tissues adjacent to the ligature were dissected, placed in 500μL RNALater§ for 24h at 4°C and stored at −80°C until analysis. RNA was isolated using RNAeasy‖ system, and cDNA was generated. cDNA products were amplified and detected with Taqman Gene Expression PCR master mix‖. Taqman probes including Il1b§ (Mm00434228) and Gapdh § (Mm99999915) were used as reference. Taqman probes for human in vitro knockdown experiments included GAPDH§ (Hs02786624) and IFI16§ (Hs00986757). Real-time PCR was quantified by the comparative threshold cycle (delta delta Ct) method.
Micro-computed tomography analysis
At baseline and at 9d-post ligature placement, the maxilla was dissected, fixed in 10% neutral buffered formalin‡‡ for 24h, and transferred to 80% ethanol‡‡ at 4°C. Samples were scanned by micro-computed tomography system (18-μm voxel size). Scanned files were imported into MicroView Standard software§§. The distance between the cementoenamel junction and the alveolar bone crest (CEJ-ABC) at the distal buccal root of the first molar was measured as previously described by our group24. Analysis was done in samples with omitted identification (blinded for WT and Ifi204 samples).
Immunohistochemical analysis
Maxillae were fixed in 10% neutral buffered formalin‡‡ for 24h, decalcified in 10% EDTA‡‡, embedded in paraffin and sectioned at 5μm at the Histology Core, School of Dentistry, University of Michigan (Ann Arbor, MI, USA). Slides were stained for hematoxylin, Ifi204, Aim2, Myeloperoxidase (MPO), and cathepsin K. Immunohistochemistry was performed using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit§§. Briefly, tissues were blocked with a blocking solution‖‖ (cat#017-000-121). Primary antibodies for anti-Ifi204¶¶ (10ug/ml, cat#ab55328), anti-Aim2¶¶ (10ug/ml, cat#ab93015), anti-MPO§§ (5μg/mL, cat# AF3667) and anti-Cathepsin K¶¶ (10ug/ml, cat#ab19027) were incubated at 4°C overnight followed by a biotinylated donkey anti-Rabbit IgG‖‖ (1ug/ml, cat#711-065-152) incubation. Cells that were positive and negative for the MPO staining were counted in the gingival tissues coronally to the alveolar bone crest in the interproximal region of the distal root of the first molar (M1) and the mesial root of the second molar (M2) in each slide as previously described. MPO+ cells are presented as percentage of positive cells for each murine sample.24 Additionally, osteoclasts were identified as cathepsin K+ multinucleated cells adjacent to the bone surface. Cathepsin K + cells were counted as positive cells/slide in health and disease as described by our group.24, 27
Bone marrow-derived macrophages experiment
Murine BMDM were isolated from 8-week-old WT and Ifi204−/− mice as previously described26. Briefly, cells were prepared by flushing bone marrow from the femurs and tibias, followed by lysis of red blood cells with ACK lysis buffer§, and the progenitor cells were differentiated into macrophages by 7-day cultivation in DMEM§ with GM-CSF-containing L929-conditioned media. For inflammasome activation, cells were plated in DMEM with 10% FBS at 24h prior to the experiment. The next day, cells were washed in PBS followed by treatment with ultrapure LPS (200 ng/ml) for 3h to prime the cells in DMEM with 10% FBS§ media. Cells were transfected with poly(dA:dT) (1μg/ml) and interferon stimulatory DNA (ISD) by Lipofectamine 2000 in OptiMem§.
Nigericin was added at 2μM. Supernatants were collected 4h after stimulation and stored for further analysis.
Generation of bone marrow chimeras
Chimeric mice were made to identify the main cell lineages expressing Ifi204 in the periodontium, and determine whether these cells can modulate the bone loss in experimental periodontitis. Mice were matched for sex, age, and weight. Bone marrow transfer experiments were performed by the UNC Animal Core Facility as previously described25, 28. Bone marrow recipient animals, 6 females and 6 males CD45.1† (strain Pep Boy, B6 Cd45.1) were irradiated with 950cGy in a cesium irradiator. Four hours after irradiation, recipient animals received an intravenous injection of 107 bone marrow cells isolated from the femur and tibia of donor mice (CD45.2). A total of 100μl of cells were injected IV into each mouse (107 cells). Mice were kept in individual cages and monitored daily. In accordance with previous studies,25, 28 recipient mice received 2 mg/ml neomycin in drinking water for 14d and bone marrow was allowed to reconstitute an additional 56 days before the mice were used for induction disease. Hematopoietic reconstitution of the peripheral blood of syngeneic bone-marrow transfer mice is complete by day 21 post-transplant, with no significant differences identified in the percentage or absolute counts of lymphocytes, macrophages, neutrophils, and eosinophils.29
Flow cytometric analysis
After bone marrow extraction, erythrocytes and leukocytes were pelleted and resuspended in 5mL of ACK lysis buffer for 5 minutes for erythrocyte lysis. Leukocytes were resuspended in 150μL of PBS. Nonspecific connections were blocked with the purified CD16/CD32 antibody## (1μg/100μL) for 40min at 4°C. Samples were double-labeled with anti-CD45.1-PE## (1:500) and anti-CD45.2-V500## (1:500) antibodies and incubated at 4°C for 60min. The cells were washed and resuspended in 300μL of flow cytometry staining buffer. Cells were analyzed using an LSRII flow cytometer§§§§ and results were obtained using the BDFACSDiva™ software##
Chemokine response of human endothelial cells against periodontal pathogens
Primary human endothelial cells (HUVEC, ATCC# PCS-100-010) were cultured in HuMEC complete medium§ with HUVEC supplement (HuMEC Supplement Kit SKU). Cells were maintained at 37C with CO2. HUVEC were silenced for gene IFI16 through a corresponding lentiviral shRNA vector produced by the Lenti-shRNA Core Facility in UNC at Chapel Hill. Briefly, a SHCLNG-NM_005531 MISSION shRNA Bacterial Glycerol** stock targeting IFI16 was purchased (TRCN0000364688) ‡‡. The IFI16 knockdown stable cell line was generated by infecting the cells under the selection of puromycin. Knockdown efficiency was confirmed at 95% decrease of IFI16 when comparing shIFI16 to scramble control (qRT-PCR). Cells were further assessed in the context of IFI16 silencing upon live periodontal pathogen stimulation (multiplicity of infection of 100 per cell, 4h stimulation). Periodontal pathogens evaluated included P. gingivalis (A7436), A. actinomycetemcomitans (ATCC 33384), and F. nucleatum (ATCC 25586). Supernatant was collected followed by analysis of chemokine (CCL3 and CCL4) and IL-1β responses were evaluated by immunobead multiplex as previously described6.
Statistical analysis
Statistical analysis was based on one-way analyses of variance (ANOVA) followed by Bonferroni-corrected post hoc tests. A p-value less than 0.05 was considered statistically significant. Data are presented as means and standard errors.
Results
Ifi204 reduces periodontal bone loss
Similar to previous findings in humans,12 the current IHC analysis identified expression of Ifi204 (the murine homolog of human IFI16) and Aim2 localized to cells located within the epithelium and connective tissues (Fig. 1A). The expression of Aim2 localized to the inflammatory infiltrate, which increases with development of ligature periodontitis, agrees with our prior finding that Aim2 increases with murine inflammatory bone loss.14 Next, we evaluated whether depletion of Ifi204 (Ifi204−/− mice) affected alveolar bone homeostasis. While no phenotypic bone difference was identified between WT and Ifi204−/− healthy murine tissues, we found Ifi204−/− mice presented ~20% higher bone loss in periodontitis compared to periodontitis development in WT controls (Fig. 1BC, p=0.0117, Figure S2 in online Journal of Periodontology). At 9 days post-ligature placement, WT mice developed 28.8% bone loss compared to 48.3% in Ifi204−/− mice. To address any potential systemic changes that are known to affect bone loss, we evaluated the weight of WT and Ifi204−/− mice. No weight differences were identified between WT and Ifi204−/− mice (WT=22.9mg, Ifi204−/−=22.9mg, p=0.47, data not shown). Next, we evaluated the role of Aim2 in mice with periodontal health and disease, because high AIM2 levels are reported in human periodontitis.30 Additionally, IFI16 has been reported to be a negative regulator of AIM2 inflammasome activation and IL-1β secretion.20 We evaluated Aim2 deficient (Aim2−/−) mice, that we have previously shown to be unable to form the Aim2 inflammasome and secrete IL-1β in response to its ligand, poly dA:dT DNA.25, 26 Our analysis identified no significant effect of Aim2 on the alveolar bone, with Aim2-deficient mice presenting similar bone levels as WT control mice under periodontal health and disease (Fig. 1D). No weight differences were identified between WT and Aim2−/− mice (WT=22.6mg, Aim2−/−=19.9mg, p=0.24, data not shown). Therefore, contrary to reports for an anti-inflammatory action of IFI16 by inhibition of AIM2 inflammasome activation,20, 31 our results indicate that Ifi204 is a negative regulator of periodontal inflammation that is independent of Aim2.
Figure 1: Ifi204 is protective against experimental periodontitis induced bone loss.
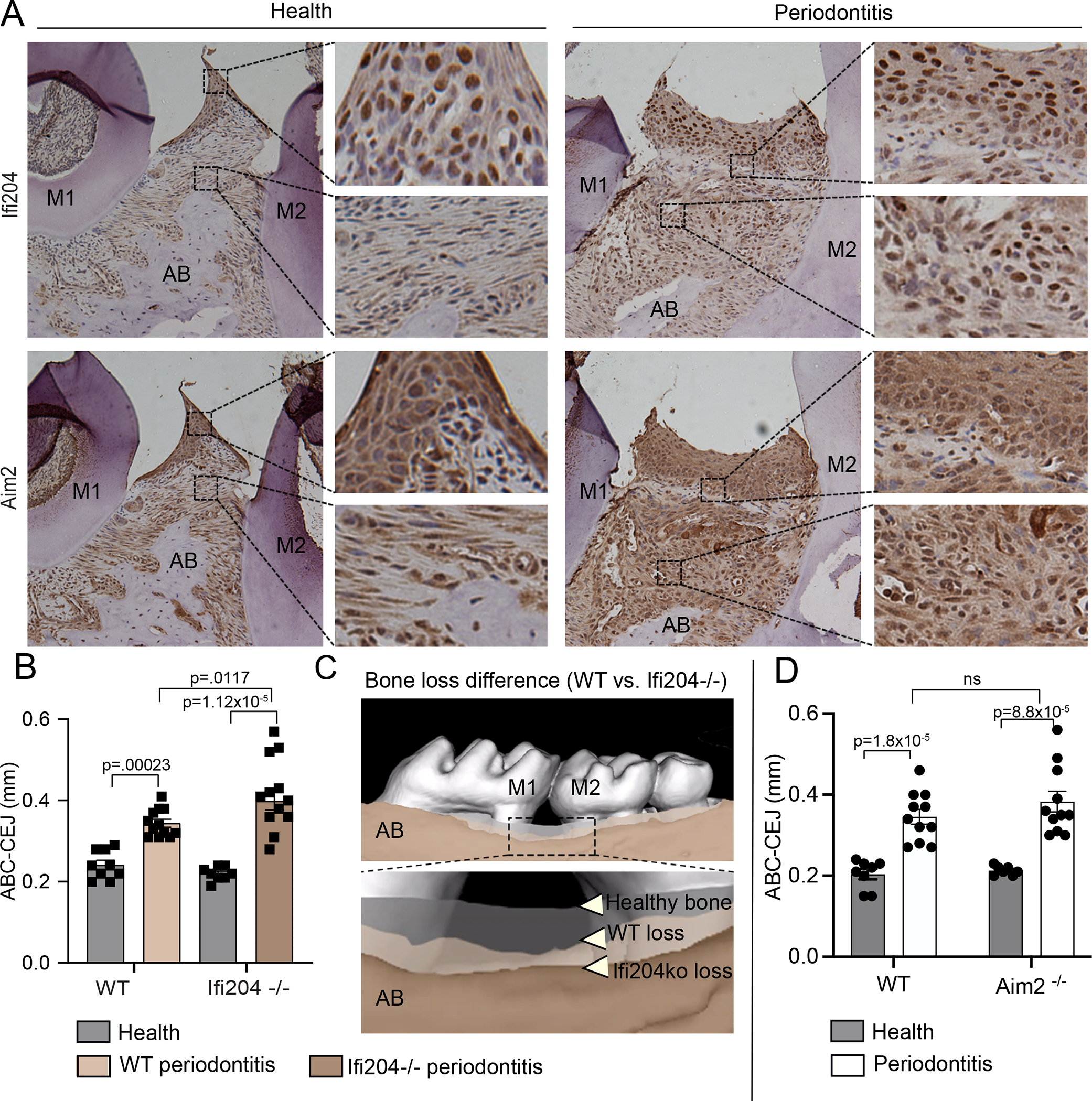
Murine periodontal tissues were evaluated under health and 9-days after ligature placement. A) The expression of Ifi204 and Aim2 was identified in multiple cells of periodontal tissues of WT mice (magnification 20x). B) Alveolar bone levels were measured by micro-CT analysis in Ifi204−/− mice, from the cementoenamel junction to the alveolar bone crest (CEJ-ABC). Ifi204−/− mice showed increased bone loss compared to (healthy) WT. C) Superimposition of the scanned maxilla depicting the bone levels in health and disease (image represents mean loss/group). Healthy bone levels were similar in WT and Ifi204−/− mice (grey color); tan colors illustrate the bone level differences in periodontitis when comparing WT (light) and Ifi204−/−(dark). D) No significant differences in alveolar bone levels identified between WT and Aim2−/− mice. Error bars, s.e. (n=12, dependent on the predicted ligature loss).
A non-bone marrow source of Ifi204 protects against periodontal bone destruction
We next determined the cellular source responsible for the enhanced bone loss in the absence of Ifi204 by generating bone marrow-chimeric mice. To date, the genetic predisposition of patients with the IL-1-genotype has mostly been attributed to an intrinsic exaggerated IL-1β response of immune cells.32 In the present study, WT (CD45.1) mice were exposed to irradiation to deplete the hematopoietic-progenitor source. Irradiated WT (CD45.1) recipient mice were reconstituted with bone-marrow cells derived from either WT (CD45.2) or Ifi204−/− (CD45.2) mice, thereby creating WT or Ifi204 chimeric mice (Fig. 2A). Flow cytometric analysis confirmed successful bone marrow transfer, with no differences identified between CD45.2 reconstitution of chimeric WT and chimeric Ifi204−/− (Fig. 2B). Chimeric mice were then subjected to experimental periodontitis. At 9 days after ligature placement, the alveolar bone loss in both groups of chimeric mice was similar, Ifi204−/− chimera (0.35mm [se=0.02mm]) vs. WT chimera (0.43mm [se=0.04mm]) (Fig. 2C). These results indicate that Ifi204 modulates murine periodontitis via a cellular source that is not derived from the bone marrow. Therefore, the higher bone loss of Ifi204−/− mice does not result from functional changes in infiltrating immune cells and macrophage-derived osteoclasts.
Figure 2: Ifi204 modulation of periodontal bone loss originates from a non-marrow source of cells.
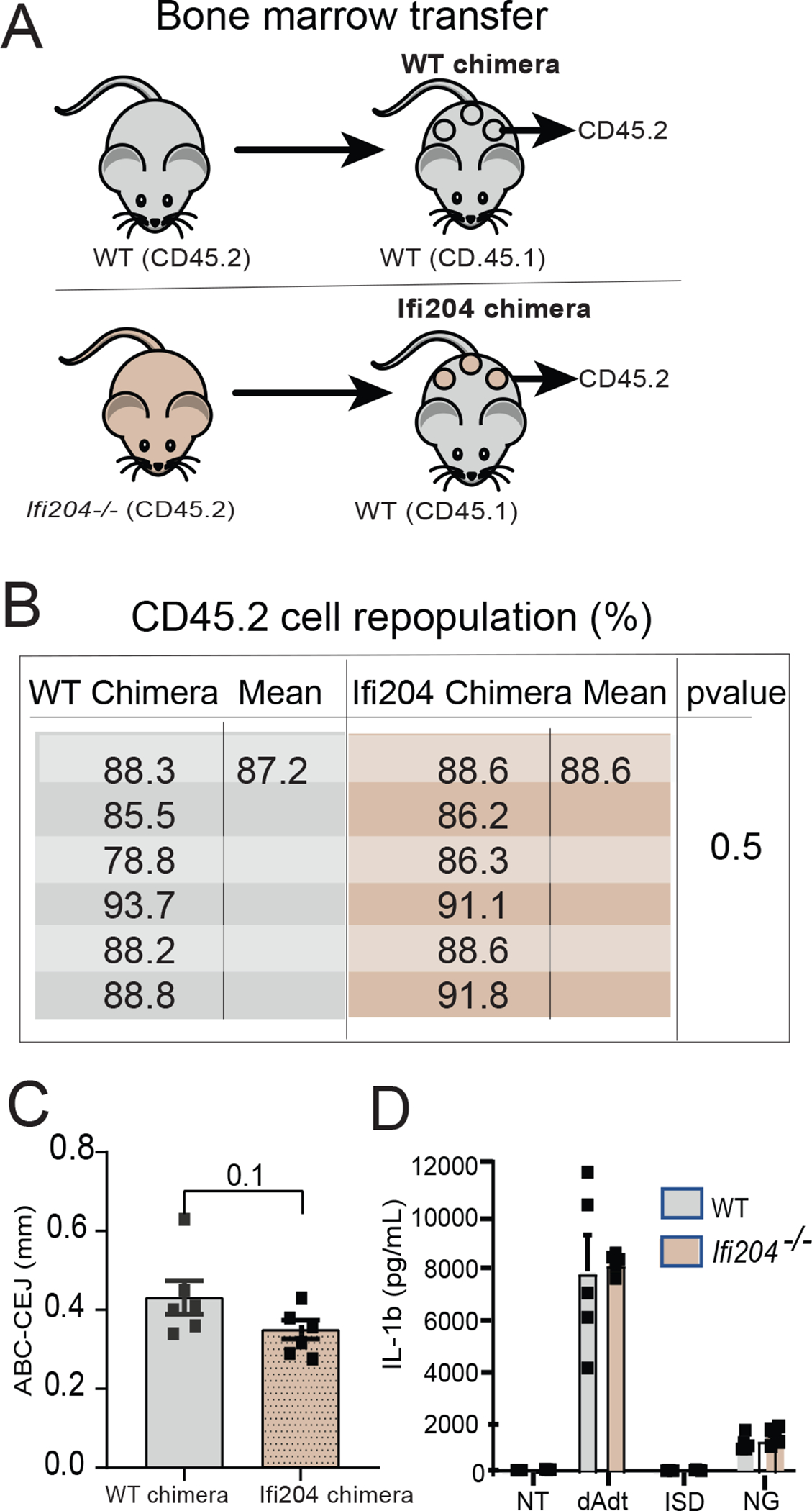
Chimeric mice were created to determine the cellular source responsible for the enhanced bone loss in the absence of Ifi204. A) Schematic representation of the bone marrow transfer (BMT) experiment. WT mice (CD45.1) were exposed to whole-body radiation (8 Gy) to deplete the hematopoietic-progenitor compartment. Irradiated recipient mice were then reconstituted with bone marrow from WT mice or Ifi204−/− (CD45.2). B) Flow cytometry analysis of CD45.2 reconstituted cells in WT chimera and Ifi204 chimera (n=6/group). C) Chimeric mice were evaluated at 9-d post-ligature placement; alveolar bone loss identified of chimeric mice was not different between mice chimeras. Each symbol represents one mouse. Error bars, se; ns, not significant. D) Bone marrow derived macrophages from WT and Ifi204−/− mice were cultured, primed with LPS, and transfected with poly(dA:dT) (Aim2 activation), interferon stimulatory DNA (ISD), or treated with nigericin to activate the Nlrp3 inflammasome. Supernatant was evaluated by ELISA. n=5 mice/group. Each symbol represents one mouse. Error bars, s.e.
Next, we determined macrophage inflammasome activation in vitro by comparing WT and Ifi204−/− cells transfected with poly(dA:dT), a cytosolic double-stranded DNA sequence that triggers formation of the Aim2 inflammasome and subsequent secretion of IL-1β. As controls, BMDM were transfected with interferon stimulatory DNA (ISD) or treated with nigericin, both of which activate the Nlrp3 inflammasome. While all three inflammasome stimulants induced IL-1β from WT BMDM, Ifi204−/− macrophages did not present differences in the IL-1β levels with either Nlrp3 or AIM2 activation (Fig. 2D). These findings indicate that loss of Ifi204 has no effect on IL-1β secretion by bone marrow derived macrophages, suggesting that Ifi204 does not directly modulate the Aim2 inflammasome.
Ifi204 limits inflammatory cell trafficking
Because the chimeric mouse data demonstrated that the Ifi204−/− exaggerated bone phenotype was not related due to functional differences in hematopoietic cells, we further investigated the in vivo contributions of Ifi204 within the periodontal tissues. The presence of neutrophils/macrophages in periodontal tissues of humans and mice is well-documented, and these cells are considered key for host protection against the omnipresent oral microbiota24. Therefore, we evaluated the number of MPO+ cells at the interproximal sites between M1 and M2 before and after ligature placement. Intra-group comparisons revealed that there was an increased inflammatory infiltrate with disease development compared to health regardless of the presence of Ifi204 (Fig. 3AB). We then compared inter-group immune differences between WT and Ifi204−/− mice. Healthy tissues from both groups presented similarly low numbers of infiltrating cells located in the connective tissue, which suggests the absence of active periodontal disease and is in alignment with the similar alveolar bone levels in health of WT and Ifi204−/− mice (Fig. 1) However, ablation of Ifi204 resulted in increased inflammatory cell migration to the epithelial layer of Ifi204−/− healthy gingiva compared to healthy WT controls (Fig. 3AB). The direct contact of oral microorganisms with the gingival epithelia but not the connective tissue supports that Ifi204 alters the host response against the oral bacteria. With induction of ligature-periodontitis, in which bone loss is dependent on the presence of microorganisms24, 33, Ifi204−/− mice presented augmented immune infiltration in both the epithelium and connective tissues at the interproximal diseased site (Fig 3AB). To address the translation of our findings, we evaluated the impact of human IFI16 in the chemotactic response to live classic periodontal pathogens in vitro. Endothelial cells are sources of IFI16/Ifi204 and signal for immune cell trafficking to the periodontium.20, 34 We found that, while the IL-1β response was consistently negligible during bacterial stimulation, IFI16-silenced cells showed a significant increase in levels of the neutrophil/macrophage chemoattractants CCL3 and CCL4 (Fig 3C).
Figure 3: Ifi204 restricts immune infiltration into periodontal tissues.
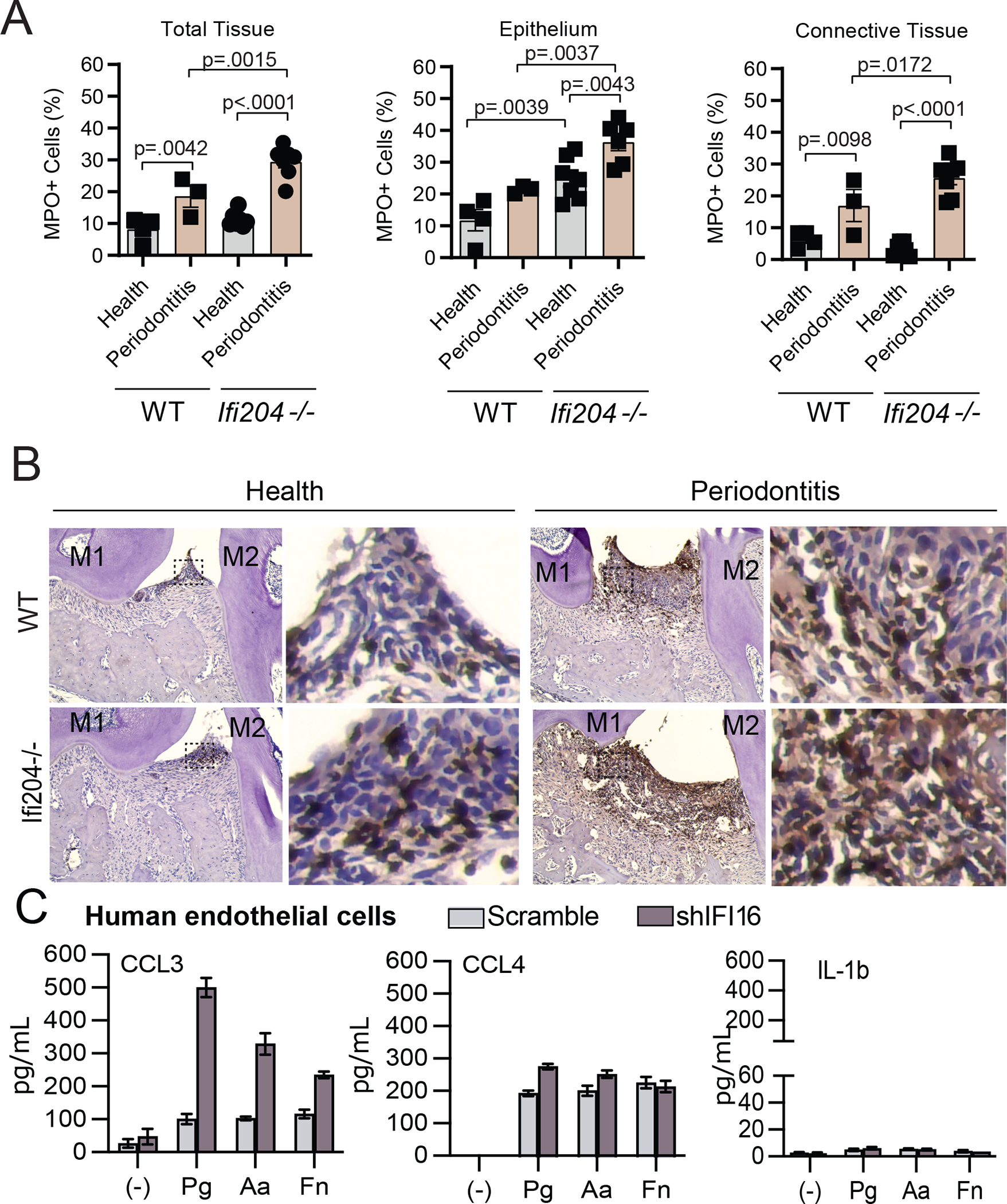
Periodontal MPO staining in WT and Ifi204−/− mice. A) MPO+ cell numbers in the gingival epithelium and connective tissue of healthy and 9d-diseased in WT and Ifi204-depleted mice. B) Representative images of periodontal tissues from WT and Ifi204−/− mice. Researchers were blind to group distribution and genotype when counting MPO+ cells. Results represented as mean, se. M1, M2 = first and second molars. C) Chemokine and IL-1 analysis of IFI16 knockdown human endothelial cells (shIFI16) and scramble controls treated with live periodontal pathogens. Experiments done in triplicates. Error bars, s.e.
Because neutrophils/macrophages are important producers of IL-1β and drivers of osteoclastogenesis24, we evaluated whether the elevated number of immune cells in Ifi204−/− mice would also be reflected in increased IL-1β levels locally. Indeed, periodontitis in Ifi204-depleted mice had dramatic increases in the amount of gingival Il1b comparted to WT (p=0.0009. Fig. 4A) Finally, we evaluated murine periodontal tissues for the presence of bone-resorbing cells. Osteoclasts were identified as cathepsin K+ cells located adjacent to the alveolar bone (Fig 4B). We found the number of osteoclasts (cathepsin K+ cells) located adjacent to the diseased alveolar bone were significantly increased in IFI204−/− mice as compared to WT (p=0.04, Fig. 4 C). Collectively, our studies indicate that Ifi204 derived from non-hematopoietic cells prevents alveolar bone destruction by regulation of immune cell trafficking to periodontal tissues.
Figure 4: IFI16/Ifi204 impacts osteoclast migration to the inflammatory-induced bone lesions.
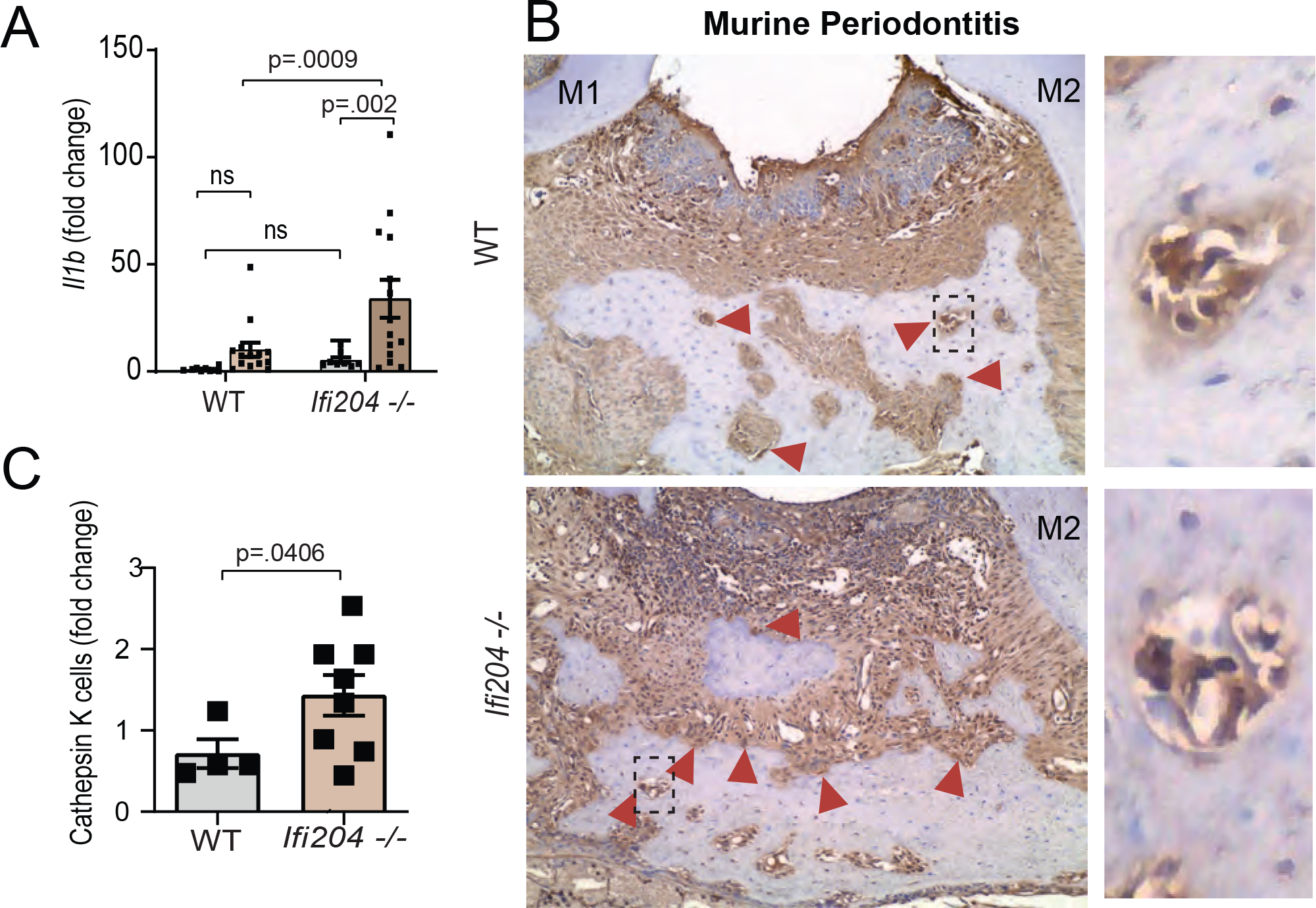
A) Il1b quantification in gingival tissues of WT and Ifi204−/− mice in health and 9d periodontitis. B) Red arrows indicate cathepsin K+ cells. Researchers were blind to group distribution and genotype when counting cathepsin K+ cells. Red arrows= cathepsin K+ cells. C) Cathepsin K histological staining of diseased periodontal tissues displayed a higher number bone-resorbing cells in Ifi204−/− mice (9d post-ligature placement) when compared to WT controls (magnification 20x).
Discussion
Genetic variants of both IFI16 and AIM2 have been associated with increased periodontal IL-1β and more destructive disease in observational human genetic association studies.4, 12 Here, we sought to experimentally assess the role of Ifi204 (the murine homolog of human IFI16) and Aim2 in the regulation of periodontitis development using the ligature model.24 Similar to our previous human findings,12 murine Ifi204 and Aim2 are localized to several cell types present in both healthy and diseased periodontal tissues. We found that Ifi204-deficient mice developed 20% more alveolar bone loss with periodontitis induction, with no bone alterations identified in healthy periodontal tissues. Our findings indicate that Ifi204 attenuates bone destruction independent of Aim2. While Aim2-depletion did not affect alveolar bone levels of mice, neither negatively or positively, the published GWAS data and clinical study support a role for AIM2 in periodontitis.4, 12 The GWAS identified AIM2 SNP as a variant form that may act to precipitate bone-loss in a way that cannot be tested in the current model. Additionally, SNPs in AIM2 were identified as a haplotype block with neighboring gene IFI16.12 Therefore, it is possible that dysfunction of AIM2 impacts periodontal disease development only if associated with IFI16 variants. Despite being an innate immune regulator, Ifi204 prevents alveolar bone loss through a mechanism that is unrelated to functional alterations of hematopoietic cells, which are the key drivers of osteoclastogenesis. Instead, the exaggerated bone damage of Ifi204−/− mice associates with higher trafficking of neutrophils/macrophages to the gingival tissues. In human endothelial cells, IFI16 blunted the chemotactic response against periodontal pathogens. In the diseased murine periodontium, loss of Ifi204 augmented the gingival expression of osteoclast-activating factor Il1b and increased osteoclast numbers present at the ligature site. Taken together, this work implicates IFI16/Ifi204 as having a previously unrecognized role in the regulation of inflammatory cell trafficking that protects from alveolar bone destruction. Because chemotactic signaling precedes immune infiltration and bone resorption, further work is needed to investigate whether a targeted therapeutic approach that regulates IFI16 would be beneficial to prevent disease development in patients with specific “genetic subtypes” of periodontal disease.4, 35
The results from the current study align with the concept of precision oral healthcare.11, 35, 36 Along with several novel genes, the association of IFI16 variants with periodontal disease was previously identified by a GWAS.4 These findings are in support of the existence of biological subtypes of periodontal disease with similar destruction that arise from disturbances on multiple genes with distinct biological functions. However, the roles of most of these newly identified genes have never been explored in the context of periodontal disease. Our study reveals novel information on IFI16 and identifies a biological pathway that increased immune cell transmigration and converged to periodontitis. In support of distinct disease pathways driving bone destruction, it is well documented that patients with rheumatoid arthritis that present an inadequate first-line drug response benefit from therapies that regulate immune cell trafficking, including inhibition of chemokines37, 38 and the IL-6 pathway.22, 39 Importantly, modulation of several chemokines40, 41 including the CCL3 and CCL4 receptors (CCR1 and CCR5)42 reduce immune cell migration and alveolar bone loss in experimental periodontitis. Additional studies will help determine the benefits of modulating the biological pathway led by IFI16 genetic predisposition to prevent disease, including chemokine targeting.
The high GCF-IL-1β levels and increased disease prevalence and severity associated with IFI16 variants suggests that IFI16 is an important modulator of periodontal inflammation.12 Previous studies support a role for IFI16 in mucosal defenses and the development of diseases, including inflammatory bowel disease43, psoriasis15, Behcet’s disease18, and mucosal infections19, 44. Being a multifunctional protein, IFI16 can present “pro-inflammatory” or “anti-inflammatory” roles dependent on the type of encounter, such as inflammasome formation and inhibition.17, 20, 45 Our study’s findings suggest that Ifi204 regulates bone loss by a novel pathway of immune cell trafficking. Although we focus on the periodontitis model, Ifi204 gene also regulates susceptibility to bone destruction in spontaneous murine arthritis.46 Furthermore, a recent transcriptome analysis of Ifi204−/− cells revealed induction of numerous chemoattractant molecules in Ifi204-depleted mice.16 While previous studies support that IFI16 mediates inflammation by regulation of the AIM2 inflammasome, we found that Ifi204 modulates inflammation independent of Aim2.20 As previously discussed, this is likely related to the multi-functional nature of IFI16/Ifi204. In agreement with our human in vitro findings, IFI16 also modulates chemokine production in keratinocytes.15 Furthermore, human IFI16 is implicated in the regulation of NF-kB,47 a major proinflammatory signaling pathway. Given that NF-kB can also promote or resolve inflammation, it will be of great interest to study whether IFI16 local delivery in established periodontal lesions would be beneficial regardless of genetic predisposition.
It is important to acknowledge that the present study has limitations. While our results support that IFI16/Ifi204 alters periodontal immune infiltration, it is possible that the murine bone metabolism is also affected by this gene. Future studies will help determine if IFI16 also affects the osteoblast/osteoclast dynamics, in addition to the influence of dysfunctional IFI16 associated with dysfunctional AIM2. Importantly, human IFI16 and murine homolog Ifi204 are not identical genes, which may impact the translation of our findings. While there is currently no mouse strain that carries the human gene, both proteins have similar innate immune functions16, 17, 21, 45. Most importantly, Ifi204 deletion led to a periodontal phenotype that is similar to what is observed in human studies of IFI16, including increased alveolar bone loss and higher levels of periodontal IL-1β.4, 12, 13 From a clinical perspective, a 20% increased bone loss that we identified in Ifi204-depleted mice may translate into loss of 6–7 teeth in the full dentition in individuals with dysfunctional IFI16. Given that the habit of tooth flossing in older adults associates with the presence 3 additional teeth compared to non-flossers,48 individuals with IFI16 variants may be considered a high-risk group for tooth loss and require close monitoring for periodontal disease development and progression.
Conclusion
Taken together, the data implicate Ifi204 as a novel regulator of periodontal inflammation and provide new insights into the physiologic functions of gene IFI16 in periodontitis.
Supplementary Material
Acknowledgement
We would like to acknowledge Dr. Steven Offenbacher for his invaluable mentorship and support that led to the development of this study. This work was supported in part by grants from the National Institute of Dental and Craniofacial Research (K01DE027087, to J.T.M.), and R01-DK093771. This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (R01-AI153265, to K.V.S.) and the National Center for Advancing Translational Sciences, NIH (KL2TR002490, to K.V.S.) and The Fulbright Program (to T. A.). The authors report no conflicts of interest related to this study.
Footnotes
Thermo Fisher, Waltham, MA.
Qiagen, Germantown, MD.
Applied Biosystems, Waltham, MA.
Sigma-Aldrich, St. Louis, MO.
Jackson Laboratory, Bar Harbor, ME.
BD Biosciences Systems, San Jose, CA.
References
- 1.Divaris K, Monda KL, North KE, et al. Genome-wide association study of periodontal pathogen colonization. J Dent Res 2012;91:21S–28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morelli T, Agler CS, Divaris K. Genomics of periodontal disease and tooth morbidity. Periodontol 2000 2020;82:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divaris K, Monda KL, North KE, et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet 2013;22:2312–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Offenbacher S, Divaris K, Barros SP, et al. Genome-wide association study of biologically informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Hum Mol Genet 2016;25:2113–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing L, Kim S, Sun L, et al. IL-37- and IL-35/IL-37-producing plasma cells in chronic periodontitis. J Dent Res 2019;98:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offenbacher S, Jiao Y, Kim SJ, et al. GWAS for Interleukin-1beta levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation. Nat Commun 2018;9:3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Divaris K, Moss K, et al. The novel ASIC2 locus is associated with severe gingival inflammation. JDR Clin Trans Res 2016;1:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu N, Zhang J, Phillips ST, Offenbacher S, Zhang S. Impaired function of epithelial plakophilin-2 is associated with periodontal disease. J Periodontal Res 2021;56:1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamurthy A, Joshua V, Haj Hensvold A, et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis 2016;75:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J 1994;13:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornman KS, Giannobile WV, Duff GW. Quo vadis: what is the future of periodontics? How will we get there? Periodontol 2000 2017;75:353–371. [DOI] [PubMed] [Google Scholar]
- 12.Marchesan JT, Jiao Y, Moss K, et al. Common polymorphisms in IFI16 and AIM2 genes are associated with periodontal disease. J Periodontol 2017;88:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchesan JT. Inflammasomes as contributors to periodontal disease. J Periodontol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchesan JT, Girnary MS, Moss K, et al. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol 2000 2020;82:93–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao T, Shao S, Li B, et al. Up-regulation of Interferon-inducible protein 16 contributes to psoriasis by modulating chemokine production in keratinocytes. Sci Rep 2016;6:25381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jian J, Wei W, Yin G, Hettinghouse A, Liu C, Shi Y. RNA-Seq analysis of interferon inducible p204-mediated network in anti-tumor immunity. Sci Rep 2018;8:6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerur N, Veettil MV, Sharma-Walia N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 2011;9:363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz-Fernandez L, Garcia-Lozano JR, Montes-Cano MA, et al. Variants of the IFI16 gene affecting the levels of expression of mRNA are associated with susceptibility to Behcet disease. J Rheumatol 2015;42:695–701. [DOI] [PubMed] [Google Scholar]
- 19.Singh VV, Kerur N, Bottero V, et al. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates gamma interferon-inducible protein 16-mediated inflammasomes. J Virol 2013;87:4417–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veeranki S, Duan X, Panchanathan R, Liu H, Choubey D. IFI16 protein mediates the anti-inflammatory actions of the type-I interferons through suppression of activation of caspase-1 by inflammasomes. PLoS One 2011;6:e27040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol 2015;194:3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasonov E, Fatenejad S, Feist E, et al. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis 2021. DOI: 10.1136/annrheumdis-2021-219876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 2020;18:e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchesan J, Girnary MS, Jing L, et al. An experimental murine model to study periodontitis. Nat Protoc 2018;13:2247–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson JE, Petrucelli AS, Chen L, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med 2015;21:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson KV, Junkins RD, Kurkjian CJ, et al. A noncanonical function of cGAMP in inflammasome priming and activation. J Exp Med 2017;214:3611–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchesan JT, Gerow EA, Schaff R, et al. Porphyromonas gingivalis oral infection exacerbates the development and severity of collagen-induced arthritis. Arthritis Res Ther 2013;15:R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Deventer HW, O’Connor W Jr., Brickey WJ, Aris RM, Ting JP, Serody JS. C-C chemokine receptor 5 on stromal cells promotes pulmonary metastasis. Cancer Res 2005;65:3374–3379. [DOI] [PubMed] [Google Scholar]
- 29.Ojielo CI, Cooke K, Mancuso P, et al. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol 2003;171:4416–4424. [DOI] [PubMed] [Google Scholar]
- 30.Xue F, Shu R, Xie Y. The expression of NLRP3, NLRP1 and AIM2 in the gingival tissue of periodontitis patients: RT-PCR study and immunohistochemistry. Arch Oral Biol 2015;60:948–958. [DOI] [PubMed] [Google Scholar]
- 31.Wang PH, Ye ZW, Deng JJ, et al. Inhibition of AIM2 inflammasome activation by a novel transcript isoform of IFI16. EMBO Rep 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornman KS, Crane A, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol 1997;24:72–77. [DOI] [PubMed] [Google Scholar]
- 33.Jiao Y, Darzi Y, Tawaratsumida K, et al. Induction of bone loss by pathobiont-mediated Nod1 signaling in the oral cavity. 2013;13:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montezano AC, Neves KB, Lopes RA, Rios F. Isolation and culture of endothelial cells from large vessels. Methods Mol Biol 2017;1527:345–348. [DOI] [PubMed] [Google Scholar]
- 35.Agler CS, Moss K, Philips KH, et al. Biologically defined or biologically informed traits are more heritable than clinically defined ones: the case of oral and dental phenotypes. Adv Exp Med Biol 2019;1197:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229–2234. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Takeuchi T, Yamanaka H, et al. Efficacy and safety of E6011, an anti-fractalkine monoclonal antibody, in patients with active rheumatoid arthritis with inadequate response to methotrexate: results of a randomized, double-blind, placebo-controlled phase II study. Arthritis Rheumatol 2021;73:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yellin M, Paliienko I, Balanescu A, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 2012;64:1730–1739. [DOI] [PubMed] [Google Scholar]
- 39.Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609–621. [DOI] [PubMed] [Google Scholar]
- 40.Gao L, Faibish D, Fredman G, et al. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J Immunol 2013;190:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glowacki AJ, Yoshizawa S, Jhunjhunwala S, et al. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc Natl Acad Sci U S A 2013;110:18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repeke CE, Ferreira SB Jr., Vieira AE, et al. Dose-response met-RANTES treatment of experimental periodontitis: a narrow edge between the disease severity attenuation and infection control. PLoS One 2011;6:e22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanhove W, Peeters PM, Staelens D, et al. Strong upregulation of AIM2 and IFI16 inflammasomes in the mucosa of patients with active inflammatory bowel disease. Inflamm Bowel Dis 2015;21:2673–2682. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson K, Svensson A, Hait AS, et al. Cutting edge: genetic association between IFI16 single nucleotide polymorphisms and resistance to genital herpes correlates with IFI16 expression levels and HSV-2-induced IFN-beta expression. J Immunol 2017;199:2613–2617. [DOI] [PubMed] [Google Scholar]
- 45.Unterholzner L, Keating SE, Baran M, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 2010;11:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian C, Liu XY, Zhu XD, et al. Ifi204 as the most favored candidate gene that regulates susceptibility to spontaneous arthritis in mice deficient in IL-1ra. Gene Rep 2018;12:21–29. [Google Scholar]
- 47.Diner BA, Li T, Greco TM, et al. The functional interactome of PYHIN immune regulators reveals IFIX is a sensor of viral DNA. Mol Syst Biol 2015;11:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchesan JT, Byrd KM, Moss K, et al. Flossing Is associated with improved oral health in older adults. J Dent Res 2020;99:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


