Abstract
Objectives:
To compare the impact of two probiotic supplements on fecal microbiota and metabolites, as well as on gut inflammation in human milk-fed preterm infants.
Methods:
In this single-center observational cohort study, we assessed the effects of Bifidobacterium longum subsp. infantis or Lactobacillus reuteri supplementation on the infant gut microbiota by 16S rRNA gene sequencing and fecal metabolome by 1H Nuclear Magnetic Resonance (NMR) spectroscopy. Fecal calprotectin was measured as a marker of enteric inflammation. Aliquots of human or donor milk provided to each infant were also assessed to determine human milk oligosaccharide (HMO) content.
Results:
As expected, each probiotic treatment was associated with increased proportions of the respective bacterial taxon. Fecal HMOs were significantly higher in L. reuteri fed babies despite similar HMO content in the milk consumed. Fecal metabolites associated with bifidobacteria fermentation products were significantly increased in B. infantis supplemented infants. Fecal calprotectin was lower in infants receiving B. infantis relative to L. reuteri (p < 0.01, Wilcoxon rank-sum test) and was negatively associated with the microbial metabolite indole-3-lactate (ILA).
Conclusion:
This study demonstrates that supplementing an HMO-catabolizing Bifidobacterium probiotic results in increased microbial metabolism of milk oligosaccharides and reduced intestinal inflammation relative to a non-catabolizing Lactobacillus probiotic in human milk-fed preterm infants. In this context, Bifidobacterium may provide greater benefit in human milk fed infants via activation of the microbiota-metabolite-immune axis.
Keywords: Bifidobacterium, Lactobacillus, human milk oligosaccharides, premature infant, probiotic, prebiotic, inflammation
Introduction
Early microbial colonization of the intestine is fundamental for neonatal development, as it has the capacity to influence the metabolic and immune function of the host. However, factors including antibiotic exposure (1, 2) and delivery by caesarian section (3) perturb the gut microbiota in preterm infants. Delayed maturation of the microbiota creates collateral complications associated with increased risk of asthma (4), diabetes (5), and obesity (6) in later life. Moreover, necrotizing enterocolitis (NEC), a devastating enteric disease primarily afflicting preterm infants, is linked to disruption in the assembly of the gut microbial community (7). Probiotic administration in preterm infants has been suggested as an effective therapy for reducing risk of NEC and death, presumably by rectifying gut dysbiosis (8–10).
Selection of probiotic species and strain is an important consideration, as its beneficial effects will depend on its ability to compete with other microbial community members while producing bioactive compounds that benefit the host. Human milk oligosaccharides (HMOs) are present at high concentration in human milk, and act as prebiotics to specifically select certain strains of bacteria. Gene clusters involved in HMO utilization have been observed in the genome of some Bifidobacterium species (11), supporting their colonization in breastfed infants (12). However, members of the Lactobacillus genus have a minimal capacity for HMO utilization (13).
We sought to compare the impact of two probiotics, Bifidobacterium longum subsp. infantis EVC001 (B. infantis) and Lactobacillus reuteri DSM 17938 (L. reuteri) in an observational prospective cohort study by examining the efficacy of colonization, and the impact these probiotics have on fecal metabolites and a marker of inflammatory status of preterm infants. To our knowledge this is the first study to explore the differences these organisms exert on preterm infant intestinal microbiota, metabolism, and inflammation in a single center study.
Materials and Methods
Ethical approval and sample collection
This ongoing prospective cohort study of preterm infants was approved by the Institutional Review Board of the University of California Davis in April 2016 and was registered at clinicaltrials.gov (NCT03717584). Parents of infants born at less than 33 weeks gestation were approached for consent either prior to birth or in the first week of life. Collection of specimens began at time of parental consent and included twice weekly stool samples from a soiled diaper, once weekly samples of milk fed to the infant (either mother’s own milk (MOM) or pasteurized donor human milk (PDHM)), and leftover plasma from routine clinical laboratory analyses. Clinical data were collected from the infant’s electronic medical record.
Human study design
Prior to initiation of the cohort study, the UC Davis NICU initiated a standardized feeding protocol that included feeding mother’s colostrum as soon as it was available, a prescribed method of advancement of feeding volume, early fortification with bovine-based human milk fortifier (when the infant was tolerating feeding volumes of 40 mL/kg/d), provision of PDHM when MOM volume was not sufficient (beginning at 48 hours of life), and provision of probiotic microbes in an attempt to decrease risk of death and necrotizing enterocolitis (NEC). When infants reached postmenstrual age of 34 weeks, the probiotic product was stopped, and if the volume of MOM was insufficient, infants received preterm infant formula rather than PDHM.
At the onset of the cohort study, the probiotic product Lactobacillus reuteri DSM 17938 (BioGaia. BioGaia Protectis with Vitamin D, St. Louis, MO) was provided at a daily dose of 5 drops (100 million organisms). This product was continued until our routine prophylactic probiotic product was changed to Bifidobacterium longum subspecies infantis EVC001 (Evivo, Evolve BioSystems, Davis, CA) at a daily dose of 8 billion microbes. Both products’ doses were administered per manufacturer instructions and none of the enrolled infants received both probiotics. The change was based on a clinical consensus among the attending neonatologists established from clinical data from our institution demonstrating effective colonization in preterm infants and prevention of NEC in a preclinical model with a similar strain (B. infantis ATCC 15697) (14, 15). To avoid cross-contamination, enrollment in the cohort study was stopped in Mar 2018 (two months prior to the change in probiotic products) and then restarted in late July 2018 (two months after the change in probiotic).
This prospective cohort study included 45 low birth weight (< 2500 g) premature infants with corrected gestational age (CGA) ranging from 23 to 32 weeks. This study is not a randomized clinical trial and was not powered to look at NEC, sepsis, or death as an outcome. All infants with birth weight < 1500 grams and less than 33 weeks CGA received both human milk and one of the two probiotic products. One infant received L. reuteri during the B. infantis time period at the preference of the attending neonatologist on service. From the 80 infants enrolled in the cohort study from its onset to September 2019, data from all infants treated with a probiotic for whom we had samples of stool (plus or minus milk or plasma) available at 30 and/or 32 weeks postmenstrual age (N=45) were analyzed. We also analyzed stool samples from 5 infants born with birth weight > 1500 grams for whom probiotic supplementation was not clinically indicated (Figure, Supplemental Digital Content 1).
Data collection
A detailed description of sample processing and data collection for 1H NMR Spectroscopy, 16S rRNA gene sequencing and fecal calprotectin are presented in Supplemental Digital Content 2.
Statistical analysis
All statistical analyses and graphics were generated using R (16) (v4.0.2) unless otherwise stated. 16S rRNA gene sequencing data were analyzed with the vegan package (17) (2.5–6) in R. Non-metric multidimensional scaling (NMDS) plots were generated to visualize microbial community structure (vegan::metaMDS, k = 3, distance = “bray”). Compositional differences in fecal microbiota between probiotic groups were assessed for β-diversity through permutational multivariate ANOVA (PERMANOVA) with default parameters of vegan::adonis2 after checking for differences in group dispersion with vegan::betadisper. Differences in proportions of family level taxa were compared using the Wilcoxon-rank sum test.
For metabolite data, normality was assessed using the Shapiro-Wilk test in addition to observing deviations in the residuals of Quantile-Quantile plots. Generalized log transformations (log(y + sqrt(y2 + 1))) were performed to approximate normality for non-normally distributed metabolite data. Spearman’s Rank correlations were used to measure the association in heteroscedastic data for metabolites, plasma, and milk HMOs.
A sigmoidal standard curve was created using a four-point logistic regression model in GraphPad Prism v 8.4.3 for interpolation of fecal calprotectin concentrations. Log concentrations were input into the model and back transformed for visualization. Samples were measured in duplicate and the average OD used. Three data points could not be fit to the standard curve based on either extremely high or low values and were therefore omitted in the analysis. Correlation analysis of fecal calprotectin and fecal indole-3-lactate (independent samples) used partial Spearman’s correlations to adjust for CGA with the ppcor (18) package in R.
All data processing was done by a blinded data analyst. All p-values were corrected for multiple testing by FDR correction with significance assessed as p < 0.05 and statistical trends considered as p < 0.1.
Results
Demographic data from the cohort are summarized in Table 1. Birthweight, gestational age, infant sex, and percentage of infants delivered by cesarean section were similar between cohorts, as were the number of days infants received antibiotics and infant morbidity (including NEC) and mortality. Infants in the B. infantis probiotic treatment group had significantly higher one-minute Apgar scores (p < 0.05); however, at five minutes, the Apgar scores of infants in both groups were similar. The L. reuteri probiotic was started on admission whereas the B. infantis probiotic was started when 3 mL feeding volumes were reached resulting in an earlier Day-of-life (DOL) for the first probiotic dose in infants receiving L. reuteri compared to infants receiving B. infantis (median DOL one and three respectively, p < 0.05). For the duration of the study, all infants consumed either MOM, PDHM, or a combination of both (see Methods for details on feeding protocol) (Table 1). The proportion of infants receiving predominantly or exclusively MOM was similar for the two cohorts (82% and 85% for the L. reuteri and B. infantis groups respectively). Comparison of the milk received by both groups showed no significant difference in HMO composition (Figure, Supplemental Digital Content 3).
Table 1.
Characteristics of infants who received probiotics
| L. reuteri (N=16) | B. infantis (N=29) | p-value | |
|---|---|---|---|
| Birth weight (g), mean (SD) | 1078 (334) | 1165 (334) | 0.41 |
| Gestational age (weeks), mean (SD) | 28 (2.5) | 28.5 (2.1) | 0.38 |
| Male N (%) | 10 (63) | 13 (45) | 0.35 |
| Cesarean N (%) | 9 (56) | 20 (69) | 0.51 |
| One minute Apgar, median (IQR) | 3 (2,4) | 6 (4,7) | 0.023a |
| Five minute Apgar, median (IQR) | 7 (6,8.5) | 7 (6,8) | 0.88 |
| DOL first feeding, median (IQR) | 1 (1,2) | 2 (1,2) | 0.077 |
| DOL full enteral feeding, median (IQR) | 14.5 (10,25) | 13 (9,17) | 0.27 |
| Total days NPO, median (IQR) | 1 (1,3.3) | 2 (1,3) | 0.66 |
| First feeding MOM, N (%) | 12 (75) | 20 (69) | 0.74 |
| Feeding mode; only MOM, N (%) | 7 (44) | 19 (70) | 0.19 |
| Feeding mode; mostly (>50%) MOM, N (%) | 6 (38) | 4 (15) | 0.19 |
| Feeding mode; mostly (>50%) PDHM, N (%) | 3 (19) | 4 (15) | 0.19 |
| Antibiotic days, mean (SD) | 8.6 (13) | 5.1 (9.1) | 0.31 |
| NEC stage 2 or 3, N (%) | 0 (0) | 2 (7) | 0.53 |
| SIP, N (%) | 0 (0) | 2 (7) | 0.53 |
| BPD, N (%) | 7 (44) | 10 (37)b | 0.75 |
| Culture positive sepsis, N (%) | 3 (19) | 3 (10) | 0.65 |
| Death, N (%) | 1 (6) | 1 (4)b | 1.0 |
| Length of hospital stay (days), mean (SD) | 85 (47) | 72 (36) | 0.31 |
| DOL first probiotic dose, median (IQR) | 1 (1,1) | 3 (2,5) | < 0.001a |
| DOL final probiotic dose, median (IQR) | 38 (26,58) | 37 (26,49) | 0.48 |
N, number; SD, standard deviation; IQR, interquartile range; DOL, day of life; MOM, mother’s own milk; NEC, necrotizing enterocolitis; SIP, spontaneous intestinal perforation; BPD, bronchopulmonary dysplasia.
No significant differences between groups except for one minute Apgar score (p < 0.05) and DOL first probiotic dose (p < 0.01).
one set of twins was transferred to another NICU at 33 weeks postmenstrual age and is not included in the calculations for BPD or death.
Taxonomic composition of the fecal microbiota for each infant is displayed in Figure 1A, B. Supplementation with B. infantis significantly increased the proportion of Bifidobacteriaceae compared to infants provided L. reuteri at 30 and 32 weeks CGA (Figure 1C, Wilcoxon rank-sum test, p < 0.01). Conversely, supplementing with L. reuteri resulted in a significantly higher percentage of Lactobacillaceae relative to infants provided B. infantis at both 30 and 32 weeks CGA (Figure 1D, Wilcoxon rank-sum test, p < 0.01). Analysis of β-diversity by PERMANOVA revealed that the fecal microbiota of infants provided B. infantis was distinct from those receiving the L. reuteri by 30 weeks CGA; however, likely due to the small sample size, was not statistically significant (Figure 1E, Adonis, p = 0.37). At 32 weeks CGA, microbial communities separated by probiotic treatment and were statistically different (Figure 1F, Adonis, p = 0.04). Although large proportions of Enterobacteriaceae were present in many preterm infant fecal samples at both timepoints (Figure 1A, B), supplementation with B. infantis revealed a trend toward lower Enterobacteriaceae abundance at 30 weeks CGA; however, no difference was observed at 32 weeks CGA (Figure, Supplemental Digital Content 4).
Figure 1.
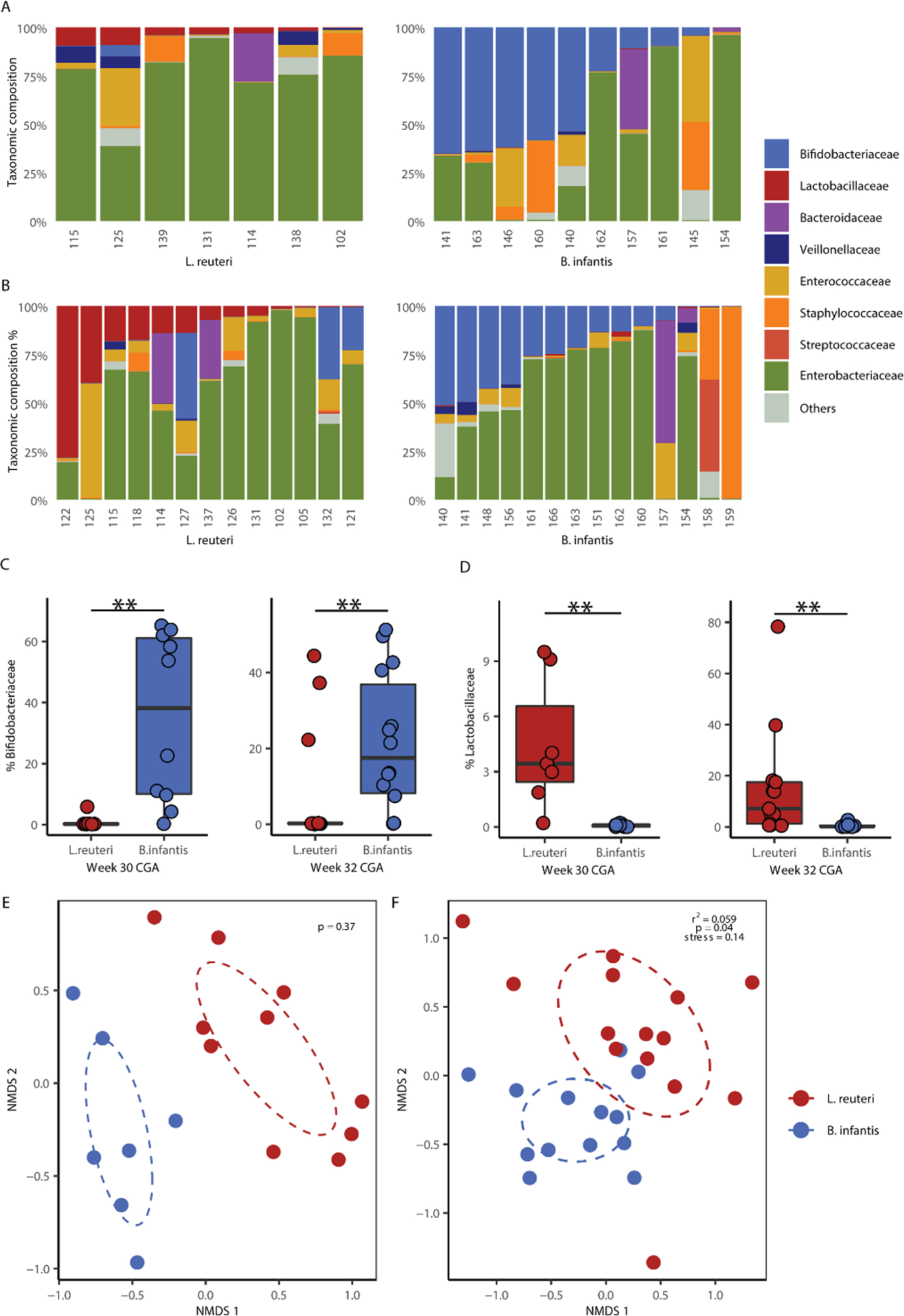
Composition and diversity of infant fecal microbiota communities differ by probiotic supplementation. (A) Taxonomic composition at 30 and (B) 32 weeks CGA. (C) Relative abundance of Bifidobacteriaceae and (D) Lactobacillaceae of fecal microbiota at 30 and 32 weeks CGA. (E) β-diversity for fecal microbiota of infants at 30 and (F) 32 weeks CGA. Taxa classified as unknown or with mean proportions of <1% are grouped in “Others”. Boxplots represent medians and interquartile range (IQR). ** p < 0.01; Wilcoxon rank-sum test with FDR correction for multiple comparisons. Differences in β-diversity were compared using PERMANOVA for Bray-Curtis dissimilarity.
While humans lack the enzymes needed to digest HMOs, certain bacteria harboring HMO-utilization genes have the ability to metabolize these glycans. Residual HMOs in feces have not been digested by intestinal microbes. Univariate analysis of individual fecal HMOs revealed that infants provided L. reuteri had higher 3-fucosyllactose (3-FL), lacto-N-fucopentaose-2 (LNFP II), lacto-N-fucopentaose-3 (LNFP III), 3’-sialyllactose (3’-SL) and 6’-sialyllactose (6’-SL) (p < 0.01) and a trend toward higher 2’- fucosyllactose (2’-FL), lacto-N-fucopentaose-1 (LNFP I), lactodifucotetraose (LDFT) and lacto-N-tetraose (LNT) (p < 0.1) compared to infants provided B. infantis at 30 weeks CGA (Figure 2A, Wilcoxon rank-sum test). At 32 weeks CGA, except for lacto-N-neotetraose (LNnT), all fecal HMOs were significantly elevated in the L. reuteri group compared to the B. infantis group (p < 0.01) suggesting increased utilization of HMOs by B. infantis (Figure 2B, Wilcoxon rank-sum test). Analysis of HMO fermentation products revealed a trend for increased 1,2-propanediol in the B. infantis group (p < 0.1) at 30 weeks CGA (Figure 2C, Wilcoxon rank-sum test), and significantly higher 1,2-propanediol, formate, acetate, pyruvate, and indole-3-lactate (ILA) in the B. infantis group at 32 weeks CGA (acetate, p < 0.05; others, p < 0.01) (Figure 2D, Wilcoxon rank-sum test). Fecal short chain fatty acids (SCFAs), propionate and butyrate, trended higher (p = 0.1) in the group receiving L. reuteri at 30 weeks CGA compared to the group receiving B. infantis; however, by 32 weeks, similar concentrations of propionate and butyrate were observed (Figure, Supplemental Digital Content 5). Total organic acids in feces were also compared to examine the fermentative activity in each probiotic group along with five preterm infants who did not receive a probiotic. The B. infantis group had significantly higher total fecal acids compared to infants that received L. reuteri and those receiving no probiotic (Figure, Supplemental Digital Content 6). Characteristics of infants who did not receive probiotic supplementation are shown in Table, Supplemental Digital Content 7.
Figure 2.
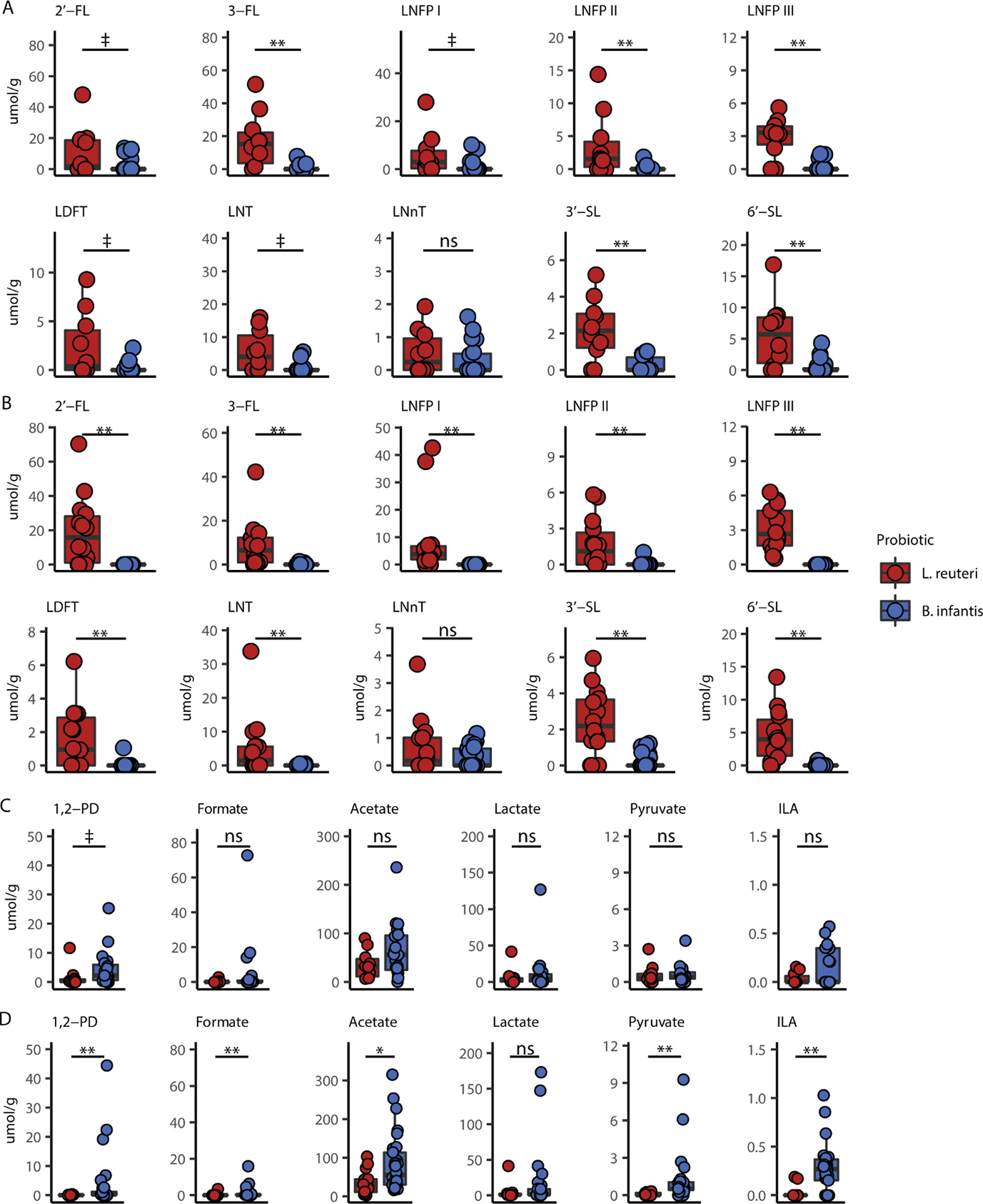
Probiotic supplementation is associated with differences in fecal HMO and metabolite profiles. (A) HMO concentrations in fecal samples from infants at 30 weeks CGA. (B) HMO concentrations in fecal samples from infants at 32 weeks CGA. (C) Fecal metabolites related to bacteria in infants at 30 weeks CGA. (D) Fecal metabolites related to bacteria in infants at 32 weeks CGA. Boxplots represent medians and IQR. ‡ P < 0.10, *P < 0.05, **P < 0.01; Wilcoxon rank-sum test with FDR correction for multiple comparisons. CGA = corrected gestational age; HMO = human milk oligosaccharide; IQR = interquartile range.
Fucosylated HMOs contain one or more fucose residues. A very low abundance of HMOs with an α−1,2-fucosyl linkage is characteristic of mothers who are homozygous for one of the common mutations in the FUT2 gene, and these individuals are commonly referred to as non-secretors. Excluding infants receiving non-secretor milk, correlations were observed between total milk HMO and fecal metabolites, with trends for higher acetate and ILA in infants provided B. infantis at 32 weeks CGA (Figure, Supplemental Digital Content 8)). Plasma acetate and 1,2-propanediol correlated with their respective fecal metabolites for infants in the B. infantis group at 32 weeks CGA suggesting absorption of these compounds into the blood (Figure, Supplemental Digital Content 8). Except for a trend for 1,2-propanediol and total HMO at 30 weeks CGA, no correlations were found to be significant in infants receiving L. reuteri (Figure, Supplemental Digital Content 9), suggesting the associations between fecal acetate or ILA and milk HMOs are stronger in infants receiving B. infantis.
Microbial metabolites including SCFAs and indole derivatives have demonstrated potent anti-inflammatory activity (19, 20). Given that infants supplemented with B. infantis had significantly higher levels of fecal ILA (p < 0.01) (Figure 3A, Wilcoxon rank-sum test), we investigated the effect of probiotic treatment on gut inflammation by examining fecal calprotectin in a subset of infants between 30 and 32 weeks CGA. Infants supplemented with B. infantis had significantly lower fecal calprotectin (p < 0.01) compared to infants provided L. reuteri (Figure 3B, Wilcoxon rank-sum test). After correcting for gestational age at birth, we observed a modest negative correlation for ILA and calprotectin (Figure 3C, partial spearman’s correlation, rho = −0.385, p = 0.02).
Figure 3.
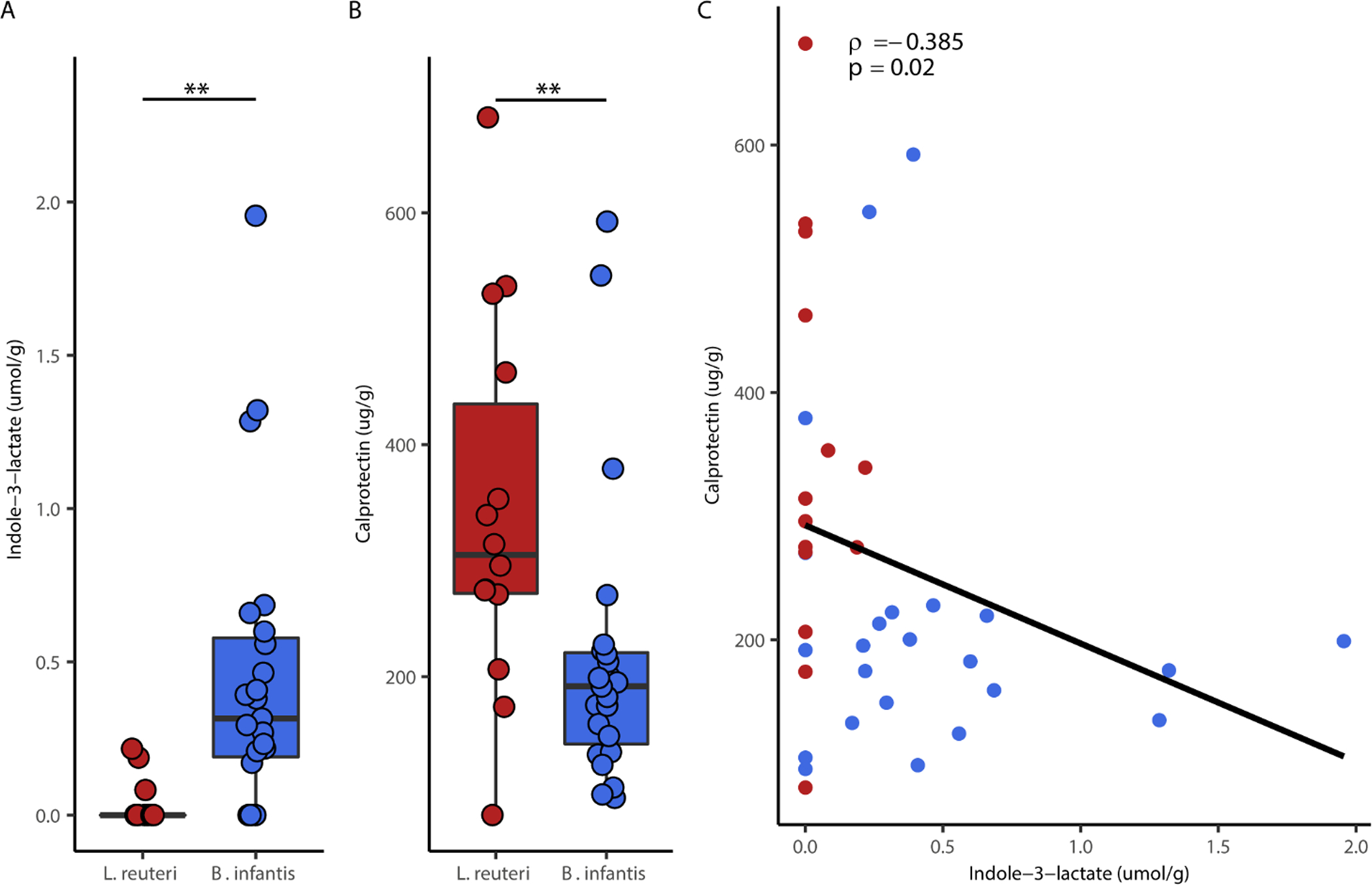
Bifidobacterium treatment is associated with higher ILA and reduced intestinal inflammation. (A) Fecal ILA concentrations for each probiotic group (B) Fecal calprotectin for each probiotic group (C) Partial spearman correlation between fecal ILA and calprotectin adjusted for gestational age at birth. Boxplots represent medians and interquartile range (IQRCorrelation includes independent samples from infants at 30, 31 and 32 weeks CGA. ** p < 0.01; Wilcoxon rank-sum test with FDR correction for multiple comparisons.
Discussion
Routine prophylactic probiotic supplementation of preterm infants remains controversial. Multiple systematic reviews, meta-analyses, and network meta-analyses including results from more than 50 randomized controlled trials involving more than 10,000 preterm infants have demonstrated significant reductions in NEC and death with probiotic supplementation. Interestingly, observational cohort studies including more than 50,000 preterm infants have shown similar reductions in NEC and death during periods of routine probiotic supplementation compared to periods of no supplementation. For these reasons, both the European Society for Pediatric Gastroenterology Hepatology and Nutrition and the American Gastroenterological Association have recently published nuanced recommendations for probiotic administration to this population (21, 22). However, concerns about the purity and numbers of viable organisms in currently available probiotic products, reports of probiotic sepsis and contaminated probiotic products, and lack of evidence as to the optimal probiotic strain and dose have led many neonatologists to avoid probiotic administration, and the Committee on the Fetus and the Newborn of the American Academy of Pediatrics to recommend against routine prophylaxis (23).
Unfortunately, most clinical studies of probiotics in preterm infants compare a probiotic product to placebo or no probiotic, and direct comparisons of differing probiotic strains are rare. Despite more than 20 years of study of probiotics and NEC, many questions remain. This study shows that providing premature infants with B. infantis combined with either MOM or PDHM is associated with greater HMO consumption, greater concentrations of HMO fermentation products, and lower markers of intestinal inflammation compared to infants provided L. reuteri.
Supplementation of B. infantis or L. reuteri increased the relative abundance of Bifidobacteriaceae or Lactobacillaceae in the fecal microbiome of infants receiving these probiotics. Notably, the median relative abundance of Bifidobacteriaceae in the B. infantis supplemented group was higher than Lactobacillaceae in the L. reuteri supplemented group (38.2% vs. 3.4% at 30 weeks CGA and 17.5% vs 7.1% at 32 weeks CGA respectively). This may reflect the adaptation of B. infantis in the infant gut through utilizing HMOs to confer a growth advantage (14). In contrast, L. reuteri strains are unable to consume HMOs (13, 14, 24). In the absence of a privileged nutrient source, competition between L. reuteri and other members of the developing microbial community limit its growth potential. A recent observational study where preterm infants were supplemented with Infloran, a probiotic containing Lactobacillus acidophilus and Bifidobacterium bifidum, reported a greater proportion of Bifidobacterium compared with Lactobacillus in the feces (25).
Metabolites produced by microbes in the gut lumen can interact with epithelial and immune cells by acting as ligands for receptors governing production of inflammatory mediators. Indole-3-lactic acid (ILA), a tryptophan metabolite produced by a number of bacterial genera including Bifidobacterium and Lactobacillus, has been noted for its anti-inflammatory property (26). ILA can bind to the aryl hydrocarbon receptor (AhR) on T cells and colonocytes, affecting expression of anti- and pro-inflammatory cytokines (27). Although both B. infantis and L. reuteri are capable of producing ILA (26, 28), a significantly lower concentration of fecal ILA in infants supplemented with L. reuteri was observed. This may be due to a difference in relative abundance of L. reuteri compared to B. infantis, or to the ability to ferment substrates available in the colon. We have previously shown that B. infantis produced more ILA when grown on HMO vs glucose (19), providing a rationale for increased fecal ILA in infants given B. infantis. Measuring fecal calprotectin, a marker of neutrophil migration to the intestinal mucosa, revealed lower inflammation in infants given B. infantis relative to L. reuteri. Moreover, levels of calprotectin had a modest inverse correlation with fecal ILA supporting the relationship between ILA and immune function.
Strengths of this study include the collection of samples from a single NICU which reduces confounding that may occur from multiple sites. Additionally, we were able to obtain matched samples of milk and feces for most infants at time of sample collection, providing an accurate relationship between microbiota development and milk HMO content. The use of CGAs for sample collection provides an assessment of microbiome development at specific ages. There are limitations and caveats to this study. This is a prospective cohort study and not a randomized control trial. There is no reference group that had not received a probiotic for comparison, as the study was conducted at a single NICU where probiotic treatment is administered as routine standard of care for infants with birth weight < 1500 grams and CGA less than 32 weeks to decrease risk of death and NEC. While the non-parallel design is a strength in that it prevented cross-contamination of the supplemented probiotic strain, it also represents a limitation, i.e., even though there were no differences in the feeding protocol, sample collection, or clinical approaches to infection protection and nutrition between the two time periods, it is possible that unrecognized differences between the two time periods influenced some of the outcomes. Additionally, as recommended by the manufacturers, the B. infantis probiotic was provided at a higher dose (8 × 109 microbes) compared to the L. reuteri probiotic (1 × 108 microbes). The eighty-fold difference in dosages of B. infantis compared to L. reuteri may have increased the abundance of Bifidobacteriaceae measured in the stool relative to the abundance of Lactobacillaceae in infants provided the L. reuteri probiotic.
In summary, we found that probiotic supplementation of premature infants receiving MOM or PDHM with B. infantis resulted in higher proportions of this bacterium and its microbial metabolites in infant feces compared with L. reuteri. We also observed that the bacterial metabolite ILA, which was higher in the feces of B. infantis supplemented infants, was associated with reduced intestinal inflammation. This observational cohort study compares the use of two widely administered probiotics and provides evidence for a potential therapeutic advantage of supplementing MOM or PDHM fed preterm infants with B. infantis to support gut microbial development and metabolism at this critical stage of life.
Supplementary Material
STROBE Statement—Checklist of items that should be included in reports of cohort studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
|
| |||
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 3 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 3 | ||
|
| |||
| Introduction | |||
|
| |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 6 |
|
| |||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 6 |
|
| |||
| Methods | |||
|
| |||
| Study design | 4 | Present key elements of study design early in the paper | 7 |
|
| |||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 7–8 |
|
| |||
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | 7 |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | |||
|
| |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 7–9 |
|
| |||
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 7–9, Supplemental Digital content 2 |
|
| |||
| Bias | 9 | Describe any efforts to address potential sources of bias | 9 |
|
| |||
| Study size | 10 | Explain how the study size was arrived at | 8 |
|
| |||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 7–9, Supplemental Digital Content 1 |
|
| |||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 8–9 |
| (b) Describe any methods used to examine subgroups and interactions | |||
| (c) Explain how missing data were addressed | |||
| (d) If applicable, explain how loss to follow-up was addressed | |||
| (e) Describe any sensitivity analyses | |||
|
| |||
| Results | |||
|
| |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | Supplemental digital content 1, Table 1, pages 8 and 10 |
| (b) Give reasons for non-participation at each stage | |||
| (c) Consider use of a flow diagram | |||
|
| |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | Table 1, page 10 |
| (b) Indicate number of participants with missing data for each variable of interest | |||
| (c) Summarise follow-up time (eg, average and total amount) | |||
|
| |||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | n/a |
|
| |||
|
| |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 10 – 12 |
| (b) Report category boundaries when continuous variables were categorized | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
|
| |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | n/a |
|
| |||
| Discussion | |||
|
| |||
| Key results | 18 | Summarise key results with reference to study objectives | 13 |
|
| |||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 15 |
|
| |||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 15 |
|
| |||
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 15 |
|
| |||
| Other information | |||
|
| |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 1–2 |
Give information separately for exposed and unexposed groups.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
What is Known
Probiotic supplementation in preterm infants is associated with reduced morbidity and mortality.
The efficacy of probiotics is enhanced when combined with prebiotics that support their colonization of the gut.
What is New
This is the first study to directly compare two probiotic strains in preterm infants with respect to the gut microbiota, human milk oligosaccharide metabolism, fecal metabolites, and enteric inflammation.
Acknowledgements
The authors express appreciation to the parents of the enrolled infants, to the nurses for their assistance in collecting specimens, and to the clinical research coordinator, Rosa Pesavento, for data and sample collection and processing.
Conflicts of Interest and Sources of Funding
C.M.S would like to acknowledge funding from the Kinsella Endowed Chair in Food, Nutrition, and Health as well as USDA-NIFA Hatch project 1021411. D.A.M would like to acknowledge funding from NIH (R01 grant: AT008759) and the Peter J. Shields Endowed Chair in Dairy Food Science.
This ongoing prospective cohort study of preterm infants was approved by the Institutional Review Board of the University of California Davis in April 2016 and was registered at clinicaltrials.gov (NCT03717584).
D.A.M. is a co-founder of Evolve Biosystems, a company focused on diet-based manipulation of the gut microbiota, and BCD Biosciences, a company advancing novel bioactive glycans. Neither Evolve Biosystems or BCD Biosciences had a role in the conceptualization, design, data collection, analysis, interpretation of data, preparation and writing of this manuscript, or in the decision to submit the paper for publication.
References
- 1.Yassour M, Vatanen T, Siljander H, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 2016; 8:343ra81–343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006; 118:511–521. [DOI] [PubMed] [Google Scholar]
- 3.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016; 8:343ra82–343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrieta M-C, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015; 7:307ra152–307ra152. [DOI] [PubMed] [Google Scholar]
- 5.Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018; 562:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanislawski MA, Dabelea D, Wagner BD, et al. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a Norwegian birth cohort. mBio 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vongbhavit K, Underwood MA. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin Ther 2016; 38:716–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Sem Pediatric Surg 2018; 27:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B, Shah PS, Afifi J, et al. Probiotics for preterm infants: A national retrospective cohort study. J Perinatol 2019; 39:533–539. [DOI] [PubMed] [Google Scholar]
- 10.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;4:CD005496. [DOI] [PubMed] [Google Scholar]
- 11.Milani C, Turroni F, Duranti S, et al. Genomics of the genus Bifidobacterium reveals species-specific adaptation to the glycan-rich gut environment. Appl Environ Microbiol 2016; 82:980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Parenti M, Grip T, et al. Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: a randomized controlled trial. Sci Rep 2019; 9:11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thongaram T, Hoeflinger JL, Chow J, et al. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J Dairy Sci 2017; 100:7825–7833. [DOI] [PubMed] [Google Scholar]
- 14.Underwood MA, Kalanetra KM, Bokulich NA, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatr 2013; 163:1585–1591.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Underwood MA, Arriola J, Gerber CW, et al. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: Alterations in inflammation, innate immune response, and the microbiota. Pediatr Res 2014; 76:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2020. Available online at https://www.R-project.org/. [Google Scholar]
- 17.Oksanen J, Guillaume Blanchet, Friendly M, et al. vegan: Community ecology package. R package version 2.5–7. R package version 25–7 2020. [Google Scholar]
- 18.Kim S ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods 2015; 22:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrlich AM, Pacheco AR, Henrick BM, et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol 2020; 20:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng N, Gao Y, Zhu W, et al. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS ONE 2020; 15:e0229283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Akker CHP, van Goudoever JB, Shamir R, et al. Probiotics and preterm infants: A position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition committee on nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr 2020; 70:664–680. [DOI] [PubMed] [Google Scholar]
- 22.Su GL, Ko CW, Bercik P, et al. AGA Clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 2020; 159:697–705. [DOI] [PubMed] [Google Scholar]
- 23.Poindexter B Use of probiotics in preterm infants. Pediatrics 2021; 147:e2021051485. [DOI] [PubMed] [Google Scholar]
- 24.Schwab C, Gänzle M. Lactic acid bacteria fermentation of human milk oligosaccharide components, human milk oligosaccharides and galactooligosaccharides: LAB fermentation of HMOs and GOSs. FEMS Microbiol Lett 2011; 315:141–148. [DOI] [PubMed] [Google Scholar]
- 25.Alcon-Giner C, Dalby MJ, Caim S, et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: An observational study. Cell Rep Med 2020; 1:100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng D, Sommella E, Salviati E, et al. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol 2018; 11:1024–1038. [DOI] [PubMed] [Google Scholar]
- 28.Cervantes-Barragan L, Chai JN, Tianero MaD, et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 2017; 357:806. 10.1126/science.aah5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


