Abstract
Epithelial mesenchymal transition (EMT) is a process comprising cellular and molecular events which result in cells shifting from an epithelial to a mesenchymal phenotype. Periodontitis is a destructive chronic disease of the periodontium initiated in response to a dysbiotic microbiome, and dominated by Gram-negative bacteria in the subgingival niches accompanied by an aberrant immune response in susceptible subjects. Both EMT and periodontitis share common risk factors and drivers, including Gram-negative bacteria, excess inflammatory cytokine production, smoking, oxidative stress and diabetes mellitus. In addition, periodontitis is characterized by down-regulation of key epithelial markers such as E-cadherin together with up-regulation of transcriptional factors and mesenchymal proteins, including Snail1, vimentin and N-cadherin, which also occur in the EMT program. Clinically, these phenotypic changes may be reflected by increases in microulceration of the pocket epithelial lining, granulation tissue formation, and fibrosis. Both in vitro and in vivo data now support the potential involvement of EMT as a pathogenic mechanism in periodontal diseases which may facilitate bacterial invasion into the underlying gingival tissues and propagation of inflammation. This review surveys the available literature and provides evidence linking EMT to periodontitis pathogenesis.
Keywords: Epithelial mesenchymal transition, Periodontitis, Transcription factor, Cell adhesion, Growth factor, Periodontal pathogen
1. Introduction
Periodontitis is a chronic inflammatory disease affecting the tooth’s supporting structures. The disease is initiated and propagated by a dysbiotic dental biofilm, predominated by Gram-negative anaerobes, and in the presence of an aberrant immune response in a genetically susceptible host [1]. If periodontitis-associated tissue destruction is not diagnosed and appropriately managed it can lead to hard and soft tissue breakdown, and ultimately tooth loss [2]. According to the World Health Organization, between 35% and 50% of the global population are affected by periodontitis with different levels of severities [3]. In 2015, the Global Burden of Disease reported that the prevalence of severe periodontitis worldwide was 7.4%, while the prevalence of milder forms of periodontitis may be as high as 50% [4]. Worldwide, data demonstrate that periodontitis seriously affects an individuals’ quality of life and has profound economic and health burdens [5], [6], [7], [8]. Furthermore, periodontal disease-associated inflammatory events are not isolated from other organs and bodily systems. The local dysbiotic subgingival microbiota can upregulate systemic levels of cytokines either directly by entry of periodontal pathogens into the blood stream or indirectly due to the inflammatory microenvironment of periodontal pockets acting as a focus for proinflammatory cytokine production. These findings are supported by a relatively large number of studies that highlight the association between periodontitis and several systemic diseases and conditions, including diabetes mellitus, cardiovascular disease, adverse pregnancy outcomes (APO), gastrointestinal disease, osteoporosis and cancers [9], [10], [11], [12], [13].
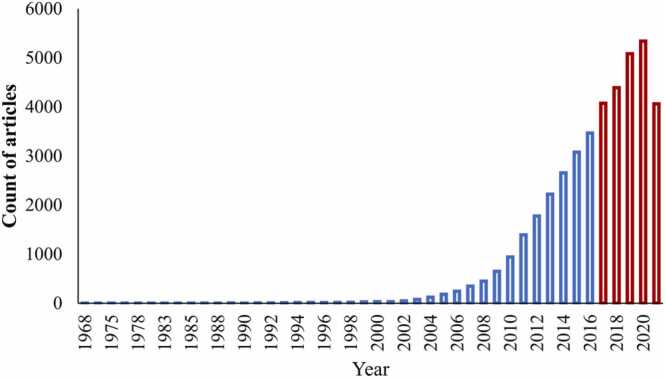
Epithelial-mesenchymal transition (EMT) is a process by which epithelial cells acquire a mesenchymal phenotype following complete or partial loss of their original phenotype [14], [15], [16]. The EMT process requires a series of orchestrated cellular and molecular events including an increased resistance to apoptosis and anoikis, loss of apico-basal polarity, dissociation of adhesion proteins, and cytoskeleton architectural reorganization. These events are associated with the simultaneous up-regulation of mesenchymal phenotypic markers and down-regulation of epithelial phenotypic markers [14], [15], [16], [17]. Transitioned cells consequently exhibit increased mobility together with increased enzymatic activity which results in remodeling and degradation of the underlying basement membrane (BM) [18], [19]. The outcome of these events can be a compromise in the integrity of epithelial-barrier function. Notably, these tissue changes are a hallmark of periodontal pocketing and enable the invasion of periodontal pathogens to the underlying mucosal tissues [20]. Over recent decades the interest in the role of EMT in developmental and disease biology has grown exponentially with the majority of the research on EMT being published in the past 5 years (Fig. 1). Notably, over half of these studies relate to cancer metastasis.
Fig. 1.
Growth of the EMT-related literature. The first experimental analysis of epithelial–mesenchymal transition (EMT) in development was published in 1968. The relationship of EMT to growth factors was identified in 1989 and the transcriptional regulation of EMT was identified in 1994. More recent studies link EMT to metastasis, organ fibrosis and stem cells.
(retrieved from PubMed at https://pubmed.ncbi.nlm.nih.gov/ using the search terms: epithelial-mesenchymal transition, epithelial-mesenchymal transformation in April 2022)
Interestingly, there are several risk factors and promoters common to both periodontitis progression and EMT. EMT-inducers include Gram-negative bacteria and their virulence factors, exposure to chronically elevated levels of inflammatory cytokines, tobacco smoke exposure and hypoxia [21], [22], [23], [24]. Furthermore, systemic diseases, such as diabetes mellitus, have been shown to be associated with EMT-induction [25], [26]. The association of these pathogenic and environmental risk factors with periodontitis is well-established and this highlights the potential involvement of EMT as a pathogenic mechanism in periodontitis. Consequently, this review surveys the current literature to identify evidence for the potential involvement of EMT in periodontitis pathogenesis.
2. Types of EMT
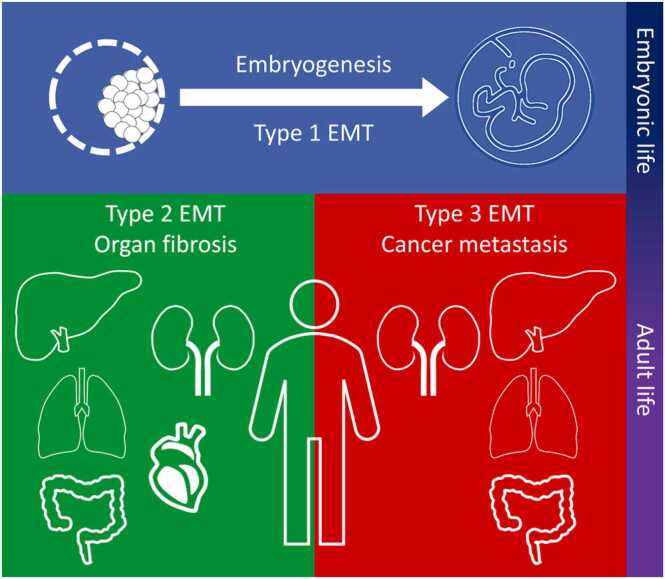
Developmental biologists have defined EMT “as a phenotypic transformation linked with metastasis and a morphological conversion taking place at particular sites within embryonic epithelia to produce individual migratory cells” [27]. Comparable observations are also reported by cancer researchers [28], [29], [30]. Consequently, based on the biological context, EMT has now been classified into three types (Fig. 2) [31], [32].
Fig. 2.
Types of epithelial-mesenchymal transition (EMT). Type 1 EMT is evident during embryonic life and considered as an integral mechanism for the gastrulation and development of the organs. During adult life, type 2 and 3 EMT are classified as pathological processes responsible for the fibrosis of organs and cancer metastasis, respectively.
Type 1 EMT was first highlighted in the 1960 s by the pioneering work of Elizabeth Hay using chick embryos [33]. During embryonic life, EMT and its reverse process, mesenchymal-epithelial transition, are physiological processes necessary for implantation of the embryo and its subsequent transition into a three-layered structure during gastrulation [34]. EMT is an integral mechanism in organ and tissue formation, including neural crest tissue development [35], [36], heart valve development [36], and Müller duct regression [37]. Data derived from mouse developmental models have identified that EMT is regulated by several key signaling pathways. Studies of aberrant Wnt expression during gastrulation have shown abnormal embryonic morphology due to unsuccessful EMT induction [38]. Further studies using chick embryos have demonstrated that blocking Wnt8 signaling results in failure of primitive streak formation [39], and similar outcomes have also been obtained in studies of Wnt3 signaling in developing mice [40]. Further analysis has demonstrated that Wnt signaling is mediated by members of the transforming growth factor (TGF)-β superfamily, including Nodal and vegetal-1, and their deficiency can also lead to EMT failure with morphological consequences, such as embryonic asymmetry [41], [42].
During adult life, EMT is regarded as a pathological process either involved in organ fibrosis (Type 2) or cancer metastasis (Type 3). Chronic inflammation is reported to be a potent inducer of Type 2 EMT and is responsible for compromising epithelial barrier integrity and organ dysfunction [28]. Consequently, chronic and persistent inflammation leading to increased production of inflammatory cytokines and chemokines, such as TGF-β1 and interleukins (IL), is considered a major factor in the development of EMT type 2 [43]. Although inflammation is regarded as a normal tissue defense mechanism, an aberrant and prolonged inflammatory response has been reported to be involved in EMT induction [44], [45], [46]. EMT has therefore been shown to arise and contribute to various types of inflammation-associated fibrosis including ones involved in lung, liver, and kidney tissues [47]. Notably, Type 2 EMT is also reported as being essential for scar tissue formation and tissue repair [48], [49]. Indeed, data indicate that overexpression of key transcriptional regulators of EMT in human keratinocytes in vitro are responsible for increasing wound healing processes involving cell proliferation and desmosome function [50].
In Type 3 EMT, reports from studies utilizing mouse and in vitro cell models have shown that epithelial cancer cells are characterized by increased expression of mesenchymal markers, including vimentin, fibroblast specific protein (FSP)− 1, and α-smooth muscle actin (SMA), and down-regulated expression of epithelial markers, including E-cadherin and β-catenin [51]. Current data indicates that neoplastic cells in the early stages of carcinoma exist in an epithelial-like state, but as the tumor progresses they gradually acquire mesenchymal characteristics and the resulting quasi-mesenchymal cells then become resistant to various treatment regimens [52]. In addition to elevated therapeutic resistance, activation of an EMT program in mammary carcinoma cells has led to a tumor-initiating state, also termed the cancer stem cell state [53], [54]. This EMT-induced acquisition of stemness can also occur in several other types of carcinomas. Data from many research groups have suggested that the EMT program is an integral metastatic mechanism found in all types of carcinomas [55], [56], [57], [58].
3. Biomarkers of EMT
During EMT, expression of certain biomarkers is markedly changed (Table 1) and these are considered as the hallmarks of the EMT process [59], [60]. Nevertheless, definition of EMT is not limited to molecular changes and includes several other cellular and behavioral criteria. Recently the case definition of EMT was extensively reviewed by Yang and coauthors [16] and they outlined a set of criteria to enable diagnosis of cells undergoing EMT. Several of these changes are also described in more detail below.
Table 1.
Molecular markers, and their expression level changes, which occur during EMT.
| Category/Function | Biomarker | EMT associated changes | Publications |
|---|---|---|---|
| Cell-cell attachment molecules | E-cadherin | ↓ | [28], [78] |
| ZO-1, Occludins, Claudins | ↓ | [79], [80] | |
| Desmoplakin, plakoglobin | ↓ | [81] | |
| N-cadherin | ↑ | [82] | |
| Cytoskeletal molecules | β-catenin | ↓ | [83], [84] |
| Cytokeratin | ↓ | [85], [86] | |
| α-SMA | ↑ | [87] | |
| Vimentin | ↑ | [88] | |
| FSP-1 | ↑ | [89] | |
| Transcriptional factors | Snail¸ Slug (Snail-2) | ↑ | [14], [83] |
| Twist | ↑ | [90] | |
| LEF-1 | ↑ | [91] | |
| ZEB-1 | ↑ | [92] | |
| NF-κB | ↑ | [2], [93] | |
| SOX 10 | ↑ | [94], [95] | |
| FOXC2 | ↑ | [96], [97] | |
| ECM proteins | Collagen I, III | ↑ | [98] |
| Collagen IV | ↓ | [99] | |
| Fibronectin | ↑ | [45] | |
| Laminin | ↓ | [100] | |
| Cell-BM attachment proteins | Integrin α6β4 | ↓ | [14] |
| Integrin α5β1, αVβ6 | ↑ | [101], [102] | |
| Proteolytic enzymes | MMP-9, − 2, − 3 | ↑ | [45], [103] |
Abbreviations: ZO-1: Zonula occludens-1, α-SMA: α-smooth muscle actin, FSP-1: Fibroblast-specific protein 1, LEF-1: Lymphoid enhancer-binding factor 1, ZEB-1: zinc finger and homeodomain transcription factor 1, NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, MMP: Matrix metallopeptidase.
E-cadherin is a calcium ion-dependent transmembrane protein, it is the basic structural unit forming adherens junctions which unite the epithelial cells into a coherent tissue structure [61]. During morphogenesis, cadherins regulate cell arrangement, their localisation, and movement in response to extracellular signaling [62]. Snail1 is a zinc finger protein that, in humans is encoded by the SNAI1 gene [63], and belongs to a family of transcription factors (TFs) that promote the repression of E-cadherin [64]. Snail1 activity is consider a major EMT-inducer as it is highly expressed during the EMT process at a relatively early stage. In addition, Snail1 is responsible for inducing and modulating other EMT-TFs, including ZEB1/2 and Slug [64], which collectively activate the EMT programs during development, fibrosis, and cancer progression [65]. Snail1 activity suppresses E-cadherin levels by combining the E-box sequence in the proximal promoter region of the E-cadherin gene [66], [67], this thereby increases the likelihood of cells acquiring the EMT-phenotype.
β-catenin is a bi-functional protein involved in the regulation and coordination of cell adhesion and gene transcription. β-catenin is responsible for the cytoplasmic anchoring of cadherins [68] and acts as an intracellular signal transducer for the Wnt signaling pathway [69], [70]. The cadherin-catenin complex is integral for the formation and maintenance of coherent epithelial layers. In addition, as part of the complex, β-catenin regulates cell growth, and the transmission of contact inhibition signals which are essential to halt mitosis once the epithelial sheet is formed [71].
Vimentin is a type III intermediate filament cytoskeletal protein expressed in mesenchymal cells [72] and is the main cytosolic component of these cells. Consequently, vimentin is often used as a biomarker of mesenchymal cells or to detect cells undergoing EMT [73]. Vimentin plays several roles, including cell regulation, interactions with signaling proteins and adhesion molecules [74], [75]. The dynamic nature of vimentin is important for cell flexibility [76] and plasticity to support essential cellular functions and cell reorganization during mitosis [77].
4. Features of EMT
4.1. EMT-associated cellular junctions and cytoskeletal changes
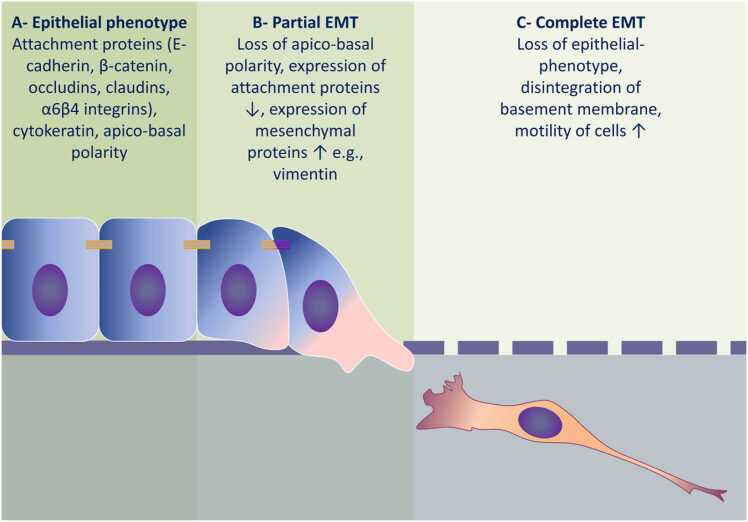
Fig. 3 illustrates the progression of EMT-associated molecular and cellular events. Initially there is disruption of polarity and dissociation of the cellular attachment proteins which occurs in target cells in response to extracellular sensing. The TGF-β pathway plays a central role in EMT-induction in different tissues [104], [105] and the TGF-β superfamily members bind to specific cell surface receptors. This binding subsequently results in the intracellular phosphorylation of SMAD2 and SMAD3 which then complex with SMAD4. This complex then translocates to the nucleus where it binds to specific DNA-motifs resulting in regulation of the expression of a relatively large subset of genes which drive the acquisition of the mesenchymal phenotype [106]. This transcriptional activity involves the DNA-binding TFs described above including Snail, Twist, and other basic helix-loop-helix TFs [107], [108]. These EMT-TFs further upregulate the expression of TGF-β ligands, instigating the establishment of autocrine signaling that drives the EMT process [109], [110]. This canonical signaling collaborates with several other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK) and Rho- GTPase pathways, which also contribute to the activation of the EMT program [106]. The combined effect of these TFs is the suppression of expression of epithelial markers responsible for cell attachment and polarity [111], [112].
Fig. 3.
Progression of molecular and cellular events in EMT. A) Under homeostatic conditions, epithelial cells form coherent epithelial sheets and express molecules uniquely associated with the epithelial-phenotype, such as E-cadherin, β-catenin, occludins, and α6β4 integrins. B) Exposure of epithelial cells to EMT-inducer(s), e.g., cytokines or Gram-negative bacterial components, triggers the EMT process. Early events include loss of apico-basal polarity followed by downregulation of attachment proteins concomitant with up-regulation of mesenchymal proteins, such as vimentin. At this stage, both epithelial and mesenchymal biomarkers are co-expressed in the same cell and this is defined as partial EMT. C) Prolonged exposure to EMT-inducers results in a complete loss of the epithelial phenotype and acquisition of a mesenchymal phenotype. Transitioned cells exhibit increased motility together with increased expression of matrix metalloproteinases that degrade the basement membrane, thereby, facilitating migration of these mesenchymal cells to the underlying connective tissue.
Epithelial cells display apical–basal polarity which is organized by the complexes that are physically and functionally integrated within the cell junction architecture. In vertebrate cells, the localization of these polarity complexes defines the cellular compartments. For instance, partitioning-defective proteins and their complexes, and the Crumbs complexes are located apically in association with tight junctions (TJs) and define the apical compartment, while the location of Scribble (SCRIB) defines the basolateral compartment [113]. Consequently, the removal of the epithelial junctions during EMT confers a loss of apical–basal polarity. The association between junctional and polarity proteins is observed when the expression of E-cadherin is down-regulated and this is a key feature of cells undergoing EMT. Subsequently, SCRIB is prevented from interacting with the lateral plasma membrane [114], and the decreasing SCRIB and/or E-cadherin levels reduce adhesion and increase cell motility [115].
During EMT, alongside the down-regulation of the expression of E-cadherin there is structural reorganization of other epithelial markers, in particular cytoskeletal cytokeratin proteins. Conversely, the expression of markers associated with the mesenchymal-phenotype are activated. Subsequently, the well characterized marker of EMT induction, N-cadherin, is involved in the process termed ‘cadherin switching’, whereby epithelial E-cadherin is replaced by its mesenchymal counterpart, N-cadherin [116], [117]. Other epithelial junctions are then removed and the junctional proteins become translocated and/or degraded. For instance, the dissolution of TJs is accompanied by decrease in claudin and occludins expression and diffusion of zonula occludens (ZO)− 1, which is responsible for the apical seal between the cells [118]. During the destabilization of adherens junctions, E-cadherin is cleaved at the plasma membrane and subsequently degraded [119]. Consequently, β-catenin is released and translocates to the nucleus where it collaborates with T-cell factor and lymphoid enhancer factor to activate Wnt gene expression [120]. Notably, and as has been highlighted above, the blocking of Wnt/β-catenin signaling results in inhibition of EMT and is reported to prevent renal fibrosis [121]. The p120-catenin (catenin-δ1) also accumulates in the nucleus and is involved in transcription regulation after decreasing E-cadherin levels [122]. As EMT progresses, expression of junctional proteins is transcriptionally repressed and this is also implicated in the loss of epithelial junctions and adhesions [112], [123], [124].
4.2. Loss of cell-extracellular matrix adhesion and motility
Cells undergoing EMT rearrange their cortical actin to allow for dynamic cell elongation and directional movement [34], [119], [125]. The transitioned cells are characterized by increased contractility and the formation of actin stress fibers [126], however, the molecular mechanisms that control actin dynamics during EMT remain to be entirely elucidated. Notably, some Rho-GTPases have been shown to regulate the dynamics and rearrangement of actin during EMT, e.g., RhoA promotes actin stress fiber formation, while Ras-related C3 botulinum toxin substrate 1 and cell division control protein 42 homolog mainly promote the formation of motility-associated cellular structures, such as lamellipodia and filopodia [127], [128]. Additionally, the migratory phenotype is associated with the up-regulation of vimentin which is essential for the process of cellular protrusion formation and the maturation of actin networks [129].
The EMT-phenotype is further characterised by the release of matrix metalloproteinases (MMP) which facilitate cell invasion and migration by their degradation of the underlying basement membrane (BM). The association between EMT and MMP has been particularly well demonstrated in mouse xenograft models of human gastric cancer which have shown that increased MMP-9 expression, together with the EMT-phenotype, increased the frequency of lung metastases [130]. Changes in the levels of MMP-mediated EMT are also associated with the substitution of epithelial integrins with mesenchymal-associated molecules. During EMT, epithelial integrin α6β4 is down-regulated while the mesenchymal integrin α5β1 is concomitantly up-regulated, and this increases the tendency of cells to adhere to fibronectin in the extracellular matrix (ECM). Additionally, alteration of integrin expression itself triggers EMT via activating signaling, including the TGF-β/Smad pathway, and integrin-linked kinases [131].
5. Role of bacteria in promoting EMT
The role of bacteria, especially Gram-negative anaerobes, in the activation of EMT has now been investigated in many studies. Helicobacter pylori, is a microaerophilic Gram-negative bacteria found primarily in the stomach and is associated with loss of epithelial integrity in the mucosa leading to gastric ulceration. The injection of H. pylori proteins into gastric epithelial cells has been shown to result in the disruption of epithelial barrier function [132]. The effects of this bacterium reportedly occur due to its ability to down-regulate ZO1-mediated TJs causing dissociation of cell-cell attachment and loss of the apical seal, thereby facilitating cellular invasion into underlying tissues. Persistent exposure of gastric epithelium to H. pylori has been shown to result in morphological changes indicative of mesenchymal-like cells [132]. In addition, specific toxins and virulence factors from this bacterium, e.g., CagA, are can induce morphological changes and increase cellular migratory ability [133], [134], [135]. These data support the role of H. pylori as a major risk factor for gastric and duodenal ulceration and gastric carcinoma which potentially act via EMT-induction [136], [137].
In vitro and in vivo studies have demonstrated that EMT-induction can occur in response to bacterial lipopolysaccharide (LPS)-toll-like receptor (TLR)− 4 mediated nuclear factor kappa B (NF-κB) signaling [138]. Intranasal inoculation of mice with LPS results in increased Snail activity and subsequent down-regulation of claudins, the subunits of TJ, breaking the apical epithelial seal via activation of TLR-4 signaling in vivo [139]. Additionally, colonization of the nasal epithelium with Streptococcus pneumoniae and Haemophillus influenza has been shown to cause down-regulation of claudins, also mediated by TLR-dependent mechanisms. The subsequent loss of epithelial coherence enabled invasion of these bacteria to the underlying tissues. Furthermore, findings obtained using an in vitro model of lung disease which used primary human bronchial epithelial cells, demonstrated a similar pattern of claudin down-regulation in response to bacterial exposure following increased expression of Snail, which required activation of the p38 MAPK/TGF-β signaling [139], [140].
6. Pathogenesis of periodontitis
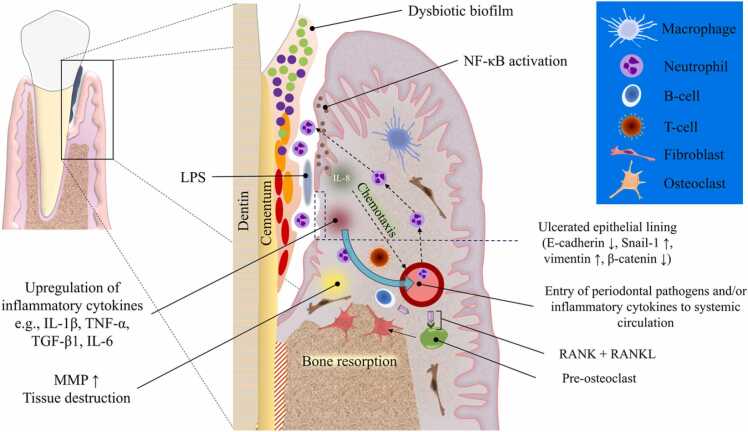
Initiation and progression of periodontitis is attributed to key bacteria in the dental biofilm which stimulate the inflammatory immune response, leading to tissue damage in susceptible individuals (Fig. 4). Indeed, periodontal disease is known to be the result of complex interactions between the pathogenic subgingival biofilm and the host’s immune-inflammatory responses. The subgingival microbiota has been reported to contain more than 700 bacteria species [141]. However, only a relatively small number of bacteria are reportedly to be closely associated with periodontitis progression, and includes the key periopathogens Fusobacterium nucleatum, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans [142].
Fig. 4.
Pathogenesis of periodontitis. Dysbiosis of subgingival microbiome results in an increased populations of red and orange bacteria such as Porphyromonas gingivalis and Fusobacterium nucleatum. The virulence factors described in the main text body trigger an inflammatory response mainly via Toll-like receptor signaling which activates intracellular NF-κB which in turn increases interleukin (IL) production. IL-8, in particular, acts as a chemotactic agent for inflammatory cells such as neutrophils and macrophages, recruiting them from nearby blood vessels. Cellular (T-cells) and humoral (B-cells) immune cells also increase in numbers due to the chemotactic gradients generated in association with chronic inflammation. These inflammatory events lead to further escalation of inflammatory mediator production which can compromise the integrity of the epithelial pocket lining and drive the EMT process. Additionally, tissue destruction and bone resorption increase due to overexpression of matrix metalloproteinases and RANKL, respectively. Subsequent exposure of the underlying connective tissue and increased vascular permeability facilitate the entry of periodontal pathogens and/or inflammatory cytokines to the circulation thereby contributing to the impact of periodontitis on systemic diseases.
The first physical barrier between the bacteria of the dental biofilm and the underlying mucosal connective tissue (CT) is the sulcular and junctional epithelium (JE). Periodontitis development not only relies on the change to a dysbiotic biofilm, but also the presence of host risk factors. For instance, an aberrant immune response, such as defective chemotaxis or hyperresponsive reactive oxygen species production by polymorphonuclear leukocytes (PMN), in the presence of the dysbiotic biofilm, leads to a chain of tissue destructive events in the periodontium [143]. Furthermore, as the biofilm advances apically, the body responds by the apical migration of the JE resulting in periodontal pocket formation. With the increasing periodontal pocket depth, the complexity and diversity of the subgingival microbiome is increased and is dominated by Gram-negative anaerobes. Key periodontal pathogens also produce several potent virulence factors, e.g. fimbriae and lectin-type adhesins, a polysaccharide capsule, LPS, hemagglutinins, potent proteinases, toxic products of metabolism, outer membrane vesicles, and numerous enzymes, which trigger and maintain an intense immune response which involves subsequent inflammatory mediator release from the pocket epithelium [144]. Increased expression of MMPs and prostaglandin E2 by the host in addition to receptor activator of nuclear factor-kappa B ligand (RANKL) stimulation participate in increasing the pocket depth and drive alveolar bone resorption [145], [146]. Under normal physiologic conditions, bone remodeling is orchestrated by RANKL and its antagonist osteoprotegerin (OPG) [147]. The RANKL/OPG system is disrupted during periodontitis as the levels of RANKL are markedly upregulated; thereby, overwhelming the decoy action of OPG and tipping the balance towards increased osteolytic activity [148]. Interestingly, RANKL can induce EMT via activating NF-κB signaling pathway [149], [150] and increasing nuclear translocation of EMT-associated transcription factors, such as Snail and Twist [149]. In the healthy periodontium, neutrophils are resident in relatively small numbers, however, once the bacterial infection induces the host pro-inflammatory response; neutrophils are recruited in relatively large numbers and release enzymes and oxygen radicals which aim to reduce the bacterial load. There is reportedly an exaggerated neutrophilic response during periodontal disease and the molecules which aim to kill bacteria also result in local tissue damage, further exacerbating the immune response resulting in a non-resolving chronic inflammatory lesion [151].
Within the periodontal pocket, the early and initial response of the host to the dysbiotic subgingival biofilm is characterised by the recruitment of inflammatory cells [152]. At later stages of disease, the growing populations of immune cells also contribute to the inflammatory cytokine milieu which is increasingly rich in cytokines, such as TNF-α, IL-1, IL-4, IL-10, interferon-γ, and TGF-β [153]. In addition, CD4 + T-cells produce RANKL promoting bone breakdown and absorption [154].
The loss of epithelial integrity consequently provides a portal for entry of the periodontal bacteria into the underlying CT where key fibroblast populations participate in the defense mechanism also by secreting increased levels of inflammatory cytokines. In addition, they secrete a fibrous ECM that is aimed at walling off the inflammatory site to limit the invasion of the periodontal bacteria into deeper tissues [155], [156]. Gingival fibroblasts under disease conditions can also increase their secretion of proteolytic enzymes and pro-inflammatory proteins, e.g., MMP-2 and prostaglandin E2 [157]; which contribute to soft tissue destruction and bone resorption. This inflammatory environment therefore contains conditions which are potent for the local driving of EMT.
7. In vitro, animal model and clinical evidence supporting a role for EMT in periodontal disease
It is well known that the inflammation aims to provide protection which limits the bacterial invasion. However, when the inflammatory response does not resolve and becomes chronic, there is a disruption of the epithelial barrier exposing the CT to periodontal pathogens which further contribute to the local inflammatory response [158]. Notably, the increased amount of the granulation tissue in the periodontal pockets indicates fibrosis which, together with loss of epithelial integrity, are hallmarks of Type 2 EMT [18]. Implication of EMT as a pathogenic mechanism of gingival fibrosis associated with disruptions of the basement membrane was previously indicated by several studies [45], [100], [159]. Gingival biopsies from periodontitis patients suggested that remnant embryonic cells contribute to the development of the reactive lining epithelium of periodontal pockets [160]. Immunohistochemical and real-time PCR analyses showed that a Hertwig’s epithelial root sheath (HERS) cell line co-expresses mesenchymal markers (vimentin and N-cadherin) and epithelial proteins (cytokeratin14, E-cadherin, and p63) when stimulated with inflammatory cytokines [161]. Consistent results were reported by Itaya et al. (2017) who demonstrated that fragmented HERS in periodontal ligament (PDL) underwent EMT when stimulated with TGF-β1, where the expression of E-cadherin was downregulated with associated upregulation of N-cadherin and extracellular matrix proteins [162]. Additionally, multi-lineage potential was observed in stem cells present in the epithelial cell rests of Malassez of the PDL via EMT leading to co-expression of both epithelial and mesenchymal markers in these cells [163]. Granulation tissues isolated from periodontal pockets, infected with red complex bacteria, exhibited strong expression of Collagen type I and mesenchymal/fibroblastic cells of embryonic origin [164]. This could indicate that EMT is a major contributor to increasing resident fibroblast populations resulting in fibrosis of periodontal pockets and non-resolving chronic inflammatory lesion.
Indeed, evidence indicate that periodontal pathogens invade relatively deeply into tissues penetrating through the inflamed and damaged periodontal pocket epithelium [156], [165], [166]. Bleeding on probing (BOP) is evident in active periodontal pockets and this clearly indicates the presence of microulceration of the pocket epithelium [144], [167]. The cellular and tissue changes present in disease combined with the presence of key cytokines and other molecular mediators implicate the potential involvement of EMT as a mechanism in periodontal disease progression. Indeed, several recent studies have now shown EMT-associated changes in periodontally diseased tissues. For example, gingival samples from patients with periodontal disease demonstrate increased expression of fibronectin and integrin αvβ6 in the CT, and epithelial cell cultures derived from the same patient showed an increased expression of fibronectin, Slug, MMP-9, MMP-13, and MMP-2 [168], [169]. Consistently, the expression of the epithelial αvβ6 Integrin was downregulated in gingival epithelial cell cultures exposed to multiple virulence factors derived from periodontal bacteria [170]. Additionally, expression of proteins involved in Wnt/β-catenin signaling, one of the main EMT-inducing pathways, markedly increased in periodontitis as compared with periodontal health [171], [172].
As highlighted above, several bacterial species are regarded as being key to the pathogenesis of periodontitis. One of the most important bacteria is P. gingivalis, which is a Gram-negative anaerobe, member of the red complex and is considered a keystone periodontal pathogens that mediates the transition from a symbiotic to dysbiotic dental biofilm [173]. Studies have shown that P. gingivalis-infected gingival epithelial cells (GEC) demonstrate activation of anti-apoptotic pathways, e.g., JAK/STAT and PI3K/Akt,signalingwhich are also involved in the inflammatory and EMT processes [174]. The JAK/STAT pathway activates NF-κB signaling leading to increased cytokine production [175] and the PI3K/Akt pathway, is involved in upregulating TLR-4 mRNA expression in response to bacterial LPS [176]. Phosphorylation of Akt, and its consequent activation, also induces NF-κB signaling which increases the transcription of several anti-apoptotic genes in GECs [177]. Several other periodontal bacteria can also induce host cell NF-κB-mediated responses, promote cell survival, increase cell migration and invasion, and increase the expression of EMT promoting cytokines [178], [179]. In addition, P. gingivalis exposure increases phosphorylation of the GSK3β enzyme and overexpression of the transcription factors, Slug and Snail, in GECs which increases ZEB1 levels that in turn can inhibit expression of E-cadherin and upregulate vimentin, β-catenin and MMP-2, − 7 and − 9. These data are derived from animal and in vitro models of GECs [180]. These molecular changes affect the P. gingivalis-infected GECs, resulting in them demonstrating a mesenchymal phenotype [181]. Furthermore, gingival samples obtained from patients with periodontitis have indicated that P. gingivalis infection compromises epithelial connections by exploiting internal cellular mechanisms to down-regulate E-cadherin and increase expression of IL-1β. These findings were also supported by results from an experimental animal model following injection P. gingivalis-LPS into the rat gingival sulcus [182] and exposing human gingival epithelial cells to P. gingivalis-LPS [183]. The same outcomes were observed in both of these studies with E-cadherin being down-regulated [182], [183] and IL-1β being up-regulated [182]. Interestingly, treatment with antioxidants, such as vitamin E and ascorbic acid, which are also known anti-EMT agents, was able to restore the impaired epithelial integrity [183]. The potential of P. gingivalis to induce EMT is not only limited to the periodontal pocket niche; results from an experimental animal model showed that P. gingivalis-LPS injection exacerbated atherosclerosis via TNF-α-induced EMT of endothelial cells [184].
F. nucleatum is an opportunistic Gram-negative anaerobe belonging to the orange complex group and is abundantly detected in periodontal disease [11]. It is responsible for altering the microenvironment of the subgingival microbiome and enables colonization with red complex bacteria, thereby providing the bridging between the primary colonizers and the more pathogenic bacteria. The role of F. nucleatum in disease is however not only limited to periodontitis but extends to other systemic conditions including APO, gastrointestinal disease, atherosclerosis, and carcinomas [9], [11], [13], [185]. Interestingly, F. nucleatum has recently been reported to be involved in driving EMT [186]. Data demonstrate that F. nucleatum exposure reduces levels of epithelial markers, including E-cadherin, and increases levels of EMT-associated transcription factors, including Snail and Slug, in the epithelium of progressive colorectal cancer [187] as well as periodontitis [20]. Moreover, ZEB1 levels have been shown to be up-regulated in oral cancer cells when stimulated by F. nucleatum [188]. Indeed, both P. gingivalis and F. nucleatum have been linked to the increased invasiveness of oral squamous cell carcinoma (OSCC) [189], [190], [191]. Results from a recent study have shown that F. nucleatum can induce EMT via downstream activation of lncRNA MIR4435–2HG/miR‐296–5p/Akt2/SNAI1 signaling in OSCC and noncancerous human immortalized oral epithelial cells [192]. Consequently, F. nucleatum, potentially promotes increased metastatic ability in OSCC [188]. Similarly, results from a systematic review have suggested that P. gingivalis is involved in different stage of OSCC including EMT of malignant cells [193]. Notably, periodontal pathogens a reportedly represent a potential a risk factor for development of oral cancer [194]; therefore, monitoring patients with severe periodontitis and maintaining their oral hygiene could significantly reduce the risk of developing OSCC [194].
Other studies using an in vitro model of periodontitis, have shown that the exposure of primary oral keratinocytes to F. nucleatum and P. gingivalis, results in hallmark EMT changes, including up-regulation of vimentin, N-cadherin, MMP-2, and Snail1, along with down-regulation of E-cadherin and an increased migratory ability [20]. These events were also associated with loss of epithelial coherence as indicated by lowering resistance of the epithelial monolayer to the passage of an electric current [20]. Other studies have shown that exposure of oral keratinocytes to F. nucleatum, P. gingivalis or their components leads to changes in cytokeratin expression and increased transcription of EMT-promoting cytokines, such as TNF-α and IL-6 as well as promoting the migratory ability of epithelial cells [195]. A key EMT risk factor is the chronic exposure to cytokines, of which TGF-β1 is known to be one of the main drivers [105], [196]. Notably, periodontitis progression is associated with up-regulation of TGF-β1, as studies of periodontal tissue samples derived from patients with advanced periodontitis showed significantly higher TGF-β1 levels compared with healthy controls [197].
The potential of periodontal pathogens to induce EMT has been more directly demonstrated in a previous analysis of gingival tissue samples obtained from individuals with advanced periodontitis. Data demonstrated down-regulation of cell adhesion proteins such as E-cadherin and connexins-26 and − 43 in the lining epithelium of periodontal pockets [198]. Notably, a direct correlation between the severity of periodontitis and the expression of the EMT biomarkers was demonstrated [199]. Analysis of tissue samples from patients with periodontitis has also demonstrated down-regulation of E-cadherin which is a major indicator of EMT [200]. Additionally, tissue samples from periodontal pockets and subgingival plaque samples demonstrates activation of RANKL transcription, which is also proposed to be an EMT-inducing factor, and this was associated with a significant increase in the levels and presence of P. gingivalis at disease sites [201].
8. Summary
EMT was initially described as an integral physiologic mechanism responsible for plasticity of cells during embryogenesis. More recently, it has been recognized as a pathologic process, in particular during cancer metastasis, inflammatory diseases and organ fibrosis. Cumulative evidence now indicates the potential involvement of EMT in the pathogenesis of periodontitis. This linkage is based on several factors which include both processes sharing common risk factors and drivers, including Gram-negative anaerobes, persistent exposure to cytokines, cigarette smoking, diabetes mellitus, hypoxia, and increased local oxidative stress. It is also notable that exposure of epithelial cells to titanium can also drive EMT and this therefore may have implications for the related gum disease of peri-implantitis [202].
While inflammation is aimed at providing a protective mechanism; in susceptible individuals, the exaggerated and chronic immune reaction, in response to the dysbiotic subgingival microbiome, is responsible for the initiation and progression of periodontitis. Current evidence now suggests that periodontal pocket epithelial cells may manifest aspects of EMT due to this prolonged exposure to cytokines which are locally released in response to exposure to Gram-negative bacteria, their components and virulence factors, together with other environmental risk factors. Consequently, the loss of epithelial coherence allows the spread of periodontal pathogens and their virulence factors deeper into the oral tissues, propagating the inflammation, frustrating the healing process and increasing the severity of periodontal tissue destruction. Furthermore, the penetration of the bacteria and cytokines may contribute to the systemic impact and health burden of periodontitis.
Currently, the available level of evidence about EMT-induction during periodontitis in human is scarce which necessities conducting further pre-clinical and clinical studies to better characterise the role of EMT in the development and progression of periodontitis. Available studies indirectly and separately investigate EMT-associated molecular, cellular, and functional features in periodontitis. Studying these events as one mechanism will better clarify their role in the pathogenesis of periodontitis. Targeted studies could identify novel therapeutic opportunities, including the potential application of repurposed drugs currently used in other indications, now aimed at halting EMT in periodontitis. Such an approach could enable restoration of a homeostatic and cohesive tissue architecture, whilst enabling a controlled and resolving dynamic inflammatory response which would be beneficial for the host.
Acknowledgment
This study was self-funded. No sponsor was involved in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Conflicts of interest
Authors confirm that there are no known conflicts of interest associated with this publication.
Footnotes
Scientific field of dental Science: Periodontology, molecular/cellular biology, microbiology, immunology.
Contributor Information
Saif S. Saliem, Email: Drsaifjuma@codental.uobaghdad.edu.iq.
Ali A. Abdulkareem, Email: ali.abbas@codental.uobaghdad.edu.iq.
References
- 1.Wolff L., Dahlén G., Aeppli D. Bacteria as risk markers for periodontitis. J Periodo. 1994;65:498–510. doi: 10.1902/jop.1994.65.5s.498. [DOI] [PubMed] [Google Scholar]
- 2.Martin J.A., Page R.C., Loeb C.F., Levi P.A., Jr. Tooth loss in 776 treated periodontal patients. J Periodo. 2010;81:244–250. doi: 10.1902/jop.2009.090184. [DOI] [PubMed] [Google Scholar]
- 3.Petersen P.E., Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60:15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 4.Billings M., Holtfreter B., Papapanou P.N., Mitnik G.L., Kocher T., Dye B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Clin Periodo. 2018;45(Suppl 20):S130–s48. doi: 10.1111/jcpe.12944. [DOI] [PubMed] [Google Scholar]
- 5.Botelho J., Machado V., Leira Y., Proença L., Chambrone L., Mendes J.J. Economic burden of periodontitis in the United States and Europe: an updated estimation. J Periodo. 2022;93:373–379. doi: 10.1002/JPER.21-0111. [DOI] [PubMed] [Google Scholar]
- 6.Durham J., Fraser H.M., McCracken G.I., Stone K.M., John M.T., Preshaw P.M. Impact of periodontitis on oral health-related quality of life. J Dent. 2013;41:370–376. doi: 10.1016/j.jdent.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Fischer R.G., Lira Junior R., Retamal-Valdes B., Figueiredo L.C., Malheiros Z., Stewart B., et al. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz Oral Res. 2020;34 doi: 10.1590/1807-3107bor-2020.vol34.0026. [DOI] [PubMed] [Google Scholar]
- 8.Bishop C. Time to take gum disease seriously: the societal and economic impact of periodontitis. Econ Intell Unit. 2021;2022:1–46. [Google Scholar]
- 9.Vander Haar E.L., So J., Gyamfi-Bannerman C., Han Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: epidemiological and mechanistic evidence. Anaerobe. 2018;50:55–59. doi: 10.1016/j.anaerobe.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zardawi F., Gul S., Abdulkareem A., Sha A., Yates J. Association between periodontal disease and atherosclerotic cardiovascular diseases: revisited. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.625579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han Y.W. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Rawi N.H., Imran N.K., Abdulkareem A.A., Abdulsattar A.M., Uthman A.T. Association between maternal periodontitis, acute-phase reactants and preterm birth. Oral Dis. 2021 doi: 10.1111/odi.13851. [DOI] [PubMed] [Google Scholar]
- 13.Wu J., Li Q., Fu X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl Oncol. 2019;12:846–851. doi: 10.1016/j.tranon.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das V., Bhattacharya S., Chikkaputtaiah C., Hazra S., Pal M. The basics of epithelial-mesenchymal transition (EMT): a study from a structure, dynamics, and functional perspective. J Cell Physiol. 2019 doi: 10.1002/jcp.28160. [DOI] [PubMed] [Google Scholar]
- 16.Yang J., Antin P., Berx G., Blanpain C., Brabletz T., Bronner M., et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–352. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismael M.K. The prognostic value of some epithelial-mesenchymal transition markers and metastasis-related markers in human transitional cell carcinoma of the bladder. Baghdad Sci J. 2018;15:244–252. [Google Scholar]
- 18.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radisky D.C. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 20.Abdulkareem A.A., Shelton R.M., Landini G., Cooper P.R., Milward M.R. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. J Periodontal Res. 2018;53:565–574. doi: 10.1111/jre.12546. [DOI] [PubMed] [Google Scholar]
- 21.Hofman P., Vouret-Craviari V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes. 2012;3:176–185. doi: 10.4161/gmic.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 23.Vu T., Jin L., Datta P.K. Effect of cigarette smoking on epithelial to mesenchymal transition (EMT) in lung cancer. J Clin Med. 2016;5:44. doi: 10.3390/jcm5040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou W., Hu S., Li C., Ma H., Wang Q., Meng G., et al. Cigarette smoke induced lung barrier dysfunction, emt, and tissue remodeling: a possible Link between COPD and lung cancer. Biomed Res Int. 2019;2019:2025636. doi: 10.1155/2019/2025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C.M., Juan S.H., Pai M.H., Chou H.C. Hyperglycemia induces epithelial-mesenchymal transition in the lungs of experimental diabetes mellitus. Acta Histochem. 2018;120:525–533. doi: 10.1016/j.acthis.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P., Lu B., Zhu R., Yang D., Liu W., Wang Q., et al. Hyperglycemia accelerates inflammaging in the gingival epithelium through inflammasomes activation. J Periodontal Res. 2021;56:667–678. doi: 10.1111/jre.12863. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.H., Xing T., Yang Z., Dudek R., Lu Q., Chen Y.H. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017:7. doi: 10.3390/jcm7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 30.Agnoletto C., Caruso C., Garofalo C. Heterogeneous circulating tumor cells in sarcoma: implication for clinical practice. Cancers. 2021:13. doi: 10.3390/cancers13092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blick T., Widodo E., Hugo H., Waltham M., Lenburg M.E., Neve R.M., et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastas-- 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 32.Trimboli A.J., Fukino K., de Bruin A., Wei G., Shen L., Tanner S.M., et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 33.Hay ED, editor Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. Epithelial-Mesenchymal Interactions; 18th Hahnemann Symposium, 1968; 1968: Williams & Wilkins.
- 34.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 35.Newgreen D.F., Ritterman M., Peters E.A. Morphology and behaviour of neural crest cells of chick embryo in vitro. Cell Tissue Res. 1979;203:115–140. doi: 10.1007/BF00234333. [DOI] [PubMed] [Google Scholar]
- 36.Thiery J.P., Duband J.L., Rutishauser U., Edelman G.M. Cell adhesion molecules in early chicken embryogenesis. Proc Natl Acad Sci USA. 1982;79:6737–6741. doi: 10.1073/pnas.79.21.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trelstad R.L., Hayashi A., Hayashi K., Donahoe P.K. The epithelial-mesenchymal interface of the male rate Mullerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- 38.Pöpperl H., Schmidt C., Wilson V., Hume C.R., Dodd J., Krumlauf R., et al. Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005. doi: 10.1242/dev.124.15.2997. [DOI] [PubMed] [Google Scholar]
- 39.Skromne I., Stern C.D. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915–2927. doi: 10.1242/dev.128.15.2915. [DOI] [PubMed] [Google Scholar]
- 40.Liu P., Wakamiya M., Shea M.J., Albrecht U., Behringer R.R., Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 41.Skromne I., Stern C.D. A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech Dev. 2002;114:115–118. doi: 10.1016/s0925-4773(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 42.Chea H.K., Wright C.V., Swalla B.J. Nodal signaling and the evolution of deuterostome gastrulation. Dev Dyn. 2005;234:269–278. doi: 10.1002/dvdy.20549. [DOI] [PubMed] [Google Scholar]
- 43.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López-Novoa J.M., Nieto M.A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sume S.S., Kantarci A., Lee A., Hasturk H., Trackman P.C. Epithelial to mesenchymal transition in gingival overgrowth. Am J Pathol. 2010;177:208–218. doi: 10.2353/ajpath.2010.090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou B., von Gise A., Ma Q., Hu Y.W., Pu W.T. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev Biol. 2010;338:251–261. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins R.G., Moore B.B., Chambers R.C., Eickelberg O., Königshoff M., Kolb M., et al. An official American thoracic society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol. 2017;56:667–679. doi: 10.1165/rcmb.2017-0096ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savagner P., Arnoux V. Epithelio-mesenchymal transition and cutaneous wound healing. Bull Acad Natl Med. 2009;193:1981–1991. [PubMed] [Google Scholar]
- 49.Stone R.C., Pastar I., Ojeh N., Chen V., Liu S., Garzon K.I., et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365:495–506. doi: 10.1007/s00441-016-2464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savagner P., Kusewitt D.F., Carver E.A., Magnino F., Choi C., Gridley T., et al. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- 51.Yang J., Weinberg R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Shibue T., Weinberg R.A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morel A.-P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002888. e2888-e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moody S.E., Perez D., Pan T.C., Sarkisian C.J., Portocarrero C.P., Sterner C.J., et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Baumgart E., Cohen M.S., Silva Neto B., Jacobs M.A., Wotkowicz C., Rieger-Christ K.M., et al. Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res. 2007;13:1685–1694. doi: 10.1158/1078-0432.CCR-06-2330. [DOI] [PubMed] [Google Scholar]
- 57.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A., Bailey J.M., McAllister F., et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye X., Tam W.L., Shibue T., Kaygusuz Y., Reinhardt F., Ng Eaton E., et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeisberg M., Neilson E.G. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scanlon C.S., Van Tubergen E.A., Inglehart R.C., D’Silva N.J. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2012;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alimperti S., Andreadis S.T. CDH2 and CDH11 act as regulators of stem cell fate decisions. Stem Cell Res. 2015;14:270–282. doi: 10.1016/j.scr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gumbiner B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 63.Paznekas W.A., Okajima K., Schertzer M., Wood S., Jabs E.W. Genomic organization, expression, and chromosome location of the human SNAIL gene (SNAI1) and a related processed pseudogene (SNAI1P) Genomics. 1999;62:42–49. doi: 10.1006/geno.1999.6010. [DOI] [PubMed] [Google Scholar]
- 64.Stemmler M.P., Eccles R.L., Brabletz S., Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21:102–112. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- 65.Barrallo-Gimeno A., Nieto M.A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 66.Batlle E., Sancho E., Francí C., Domínguez D., Monfar M., Baulida J., et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 67.Dong C., Wu Y., Wang Y., Wang C., Kang T., Rychahou P.G., et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene. 2013;32:1351–1362. doi: 10.1038/onc.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCrea P.D., Turck C.W., Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 69.Peifer M., Rauskolb C., Williams M., Riggleman B., Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development. 1991;111:1029–1043. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- 70.Noordermeer J., Klingensmith J., Perrimon N., Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- 71.Nelson W.J. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cabeen M.T., Jacobs-Wagner C. The bacterial cytoskeleton. Annu Rev Genet. 2010;44:365–392. doi: 10.1146/annurev-genet-102108-134845. [DOI] [PubMed] [Google Scholar]
- 73.Windoffer R., Beil M., Magin T.M., Leube R.E. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol. 2011;194:669–678. doi: 10.1083/jcb.201008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhattacharya R., Gonzalez A.M., Debiase P.J., Trejo H.E., Goldman R.D., Flitney F.W., et al. Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J Cell Sci. 2009;122:1390–1400. doi: 10.1242/jcs.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J., Yang C., Kim E.J., Jang J., Kim S.J., Kang S.M., et al. Vimentin filaments regulate integrin-ligand interactions by binding to the cytoplasmic tail of integrin β3. J Cell Sci. 2016;129:2030–2042. doi: 10.1242/jcs.180315. [DOI] [PubMed] [Google Scholar]
- 76.Goldman R.D., Khuon S., Chou Y.H., Opal P., Steinert P.M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971–983. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duarte S., Viedma-Poyatos Á., Navarro-Carrasco E., Martínez A.E., Pajares M.A., Pérez-Sala D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat Commun. 2019;10:4200. doi: 10.1038/s41467-019-12029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peinado H., Ballestar E., Esteller M., Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikenouchi J., Matsuda M., Furuse M., Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 80.Polette M., Mestdagt M., Bindels S., Nawrocki-Raby B., Hunziker W., Foidart J.M., et al. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- 81.Savagner P., Yamada K.M., Thiery J.P. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang S.-B., He X.-J., Xia Y.-J., Hu W.-J., Luo J.-G., Zhang J., et al. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2305–2315. doi: 10.2147/OTT.S101853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Medici D., Hay E.D., Olsen B.R. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan D., Avtanski D., Saxena N.K., Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287 doi: 10.1074/jbc.M111.322800. 8598-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–vii92. doi: 10.1093/annonc/mdq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Serrano M.J., Ortega F.G., Alvarez-Cubero M.J., Nadal R., Sanchez-Rovira P., Salido M., et al. EMT and EGFR in CTCs cytokeratin negative non-metastatic breast cancer. Oncotarget. 2014;5:7486–7497. doi: 10.18632/oncotarget.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ding L., Zhang Z., Shang D., Cheng J., Yuan H., Wu Y., et al. α-Smooth muscle actin-positive myofibroblasts, in association with epithelial-mesenchymal transition and lymphogenesis, is a critical prognostic parameter in patients with oral tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43:335–343. doi: 10.1111/jop.12143. [DOI] [PubMed] [Google Scholar]
- 88.Mendez M.G., Kojima S., Goldman R.D. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. Faseb J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okada H., Danoff T.M., Kalluri R., Neilson E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 90.Eckert M.A., Lwin T.M., Chang A.T., Kim J., Danis E., Ohno-Machado L., et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim K., Lu Z., Hay E.D. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 92.Xu J., Lamouille S., Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huber M.A., Azoitei N., Baumann B., Grünert S., Sommer A., Pehamberger H., et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He P., Jin X. SOX10 induces epithelial-mesenchymal transition and contributes to nasopharyngeal carcinoma progression. Biochem Cell Biol. 2018;96:326–331. doi: 10.1139/bcb-2017-0160. [DOI] [PubMed] [Google Scholar]
- 95.Dravis C., Spike B.T., Harrell J.C., Johns C., Trejo C.L., Southard-Smith E.M., et al. Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep. 2015;12:2035–2048. doi: 10.1016/j.celrep.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Børretzen A., Gravdal K., Haukaas S.A., Beisland C., Akslen L.A., Halvorsen O.J. FOXC2 expression and epithelial-mesenchymal phenotypes are associated with castration resistance, metastasis and survival in prostate cancer. J Pathol Clin Res. 2019;5:272–286. doi: 10.1002/cjp2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hollier B.G., Tinnirello A.A., Werden S.J., Evans K.W., Taube J.H., Sarkar T.R., et al. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981–1992. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shintani Y., Maeda M., Chaika N., Johnson K.R., Wheelock M.J. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008;38:95–104. doi: 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song W., Jackson K., McGuire P.G. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev Biol. 2000;227:606–617. doi: 10.1006/dbio.2000.9919. [DOI] [PubMed] [Google Scholar]
- 100.Kantarci A., Nseir Z., Kim Y.S., Sume S.S., Trackman P.C. Loss of basement membrane integrity in human gingival overgrowth. J Dent Res. 2011;90:887–893. doi: 10.1177/0022034511404703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y., Yang J., Dai C., Wu C., Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maschler S., Wirl G., Spring H., Bredow D.V., Sordat I., Beug H., et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 103.Cheng S., Lovett D.H. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Clin Pathol. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bierie B., Moses H.L. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 105.Xu J., Lamouille S., Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Derynck R., Muthusamy B.P., Saeteurn K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 108.Massagué J. TGFbeta in. Cancer Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dhasarathy A., Phadke D., Mav D., Shah R.R., Wade P.A. The transcription factors Snail and Slug activate the transforming growth factor-beta signaling pathway in breast cancer. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grande M.T., Sánchez-Laorden B., López-Blau C., De Frutos C.A., Boutet A., Arévalo M., et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–997. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 111.Thiery J.P., Huang R. Linking epithelial-mesenchymal transition to the well-known polarity protein Par6. Dev Cell. 2005;8:456–458. doi: 10.1016/j.devcel.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 113.Johnston D., Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. (St) [DOI] [PubMed] [Google Scholar]
- 114.Navarro C., Nola S., Audebert S., Santoni M.J., Arsanto J.P., Ginestier C., et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- 115.Qin Y., Capaldo C., Gumbiner B.M., Macara I.G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hazan R.B., Qiao R., Keren R., Badano I., Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 117.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 118.Huang R.Y., Guilford P., Thiery J.P. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125:4417–4422. doi: 10.1242/jcs.099697. [DOI] [PubMed] [Google Scholar]
- 119.Yilmaz M., Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastas-- Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 120.Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 121.Feiteng C., Lei C., Deng L., Chaoliang X., Zijie X., Yi S., et al. Relaxin inhibits renal fibrosis and the epithelial-to-mesenchymal transition via the Wnt/β-catenin signaling pathway. Ren Fail. 2022;44 doi: 10.1080/0886022X.2022.2044351. 513-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kourtidis A., Ngok S.P., Anastasiadis P.Z. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bax N.A.M., Pijnappels D.A., van Oorschot A.A.M., Winter E.M., de Vries A.A.F., van Tuyn J., et al. Epithelial-to-mesenchymal transformation alters electrical conductivity of human epicardial cells. J Cell Mol Med. 2011;15:2675–2683. doi: 10.1111/j.1582-4934.2011.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Craene B., Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 125.Yilmaz M., Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 126.Haynes J., Srivastava J., Madson N., Wittmann T., Barber D.L. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol Biol Cell. 2011;22:4750–4764. doi: 10.1091/mbc.E11-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nelson W.J. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Godde N.J., Galea R.C., Elsum I.A., Humbert P.O. Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions. J Mammary Gland Biol Neoplasia. 2010;15:149–168. doi: 10.1007/s10911-010-9180-2. [DOI] [PubMed] [Google Scholar]
- 129.Schoumacher M., Goldman R.D., Louvard D., Vignjevic D.M. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoo Y.A., Kang M.H., Lee H.J., Kim B.H., Park J.K., Kim H.K., et al. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]
- 131.Kim Y., Kugler M.C., Wei Y., Kim K.K., Li X., Brumwell A.N., et al. Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Blaser M.J. Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep. 2006;7:956–960. doi: 10.1038/sj.embor.7400812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Segal E.D., Cha J., Lo J., Falkow S., Tompkins L.S. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Odenbreit S., Püls J., Sedlmaier B., Gerland E., Fischer W., Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 135.Bakir W.A., Hussein S.M., Mahmood N.A. Determination of enzymatic antioxidant in iraqi patients with chronic gastritis. Iraqi J Pharm Sci. 2008;17:26–31. [Google Scholar]
- 136.Wroblewski L.E., Peek R.M., Jr., Wilson K.T. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baj J., Korona-Głowniak I., Forma A., Maani A., Sitarz E., Rahnama-Hezavah M., et al. Mechanisms of the epithelial-mesenchymal transition and tumor microenvironment in Helicobacter pylori-Induced Gastric. Cancer Cells. 2020;9:1055. doi: 10.3390/cells9041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yin Y., Li F., Li S., Cai J., Shi J., Jiang Y. TLR4 influences hepatitis B virus related hepatocellular carcinoma by regulating the Wnt/β-catenin pathway. Cell Physiol Biochem. 2017;42:469–479. doi: 10.1159/000477594. [DOI] [PubMed] [Google Scholar]
- 139.Clarke T.B., Francella N., Huegel A., Weiser J.N. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe. 2011;9:404–414. doi: 10.1016/j.chom.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beisswenger C., Lysenko E.S., Weiser J.N. Early bacterial colonization induces toll-like receptor-dependent transforming growth factor beta signaling in the epithelium. Infect Immun. 2009;77:2212–2220. doi: 10.1128/IAI.01224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar P.S., Griffen A.L., Moeschberger M.L., Leys E.J. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lovegrove J.M. Dental plaque revisited: bacteria associated with periodontal disease. J N Z Soc Periodo. 2004:7–21. [PubMed] [Google Scholar]
- 143.Scott D.A., Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83. doi: 10.1159/000329672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tribble G.D., Lamont R.J. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000. 2010;52:68–83. doi: 10.1111/j.1600-0757.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kornman K.S., Page R.C., Tonetti M.S. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 146.Hannas A.R., Pereira J.C., Granjeiro J.M., Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 147.Boyce B.F., Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 148.Belibasakis G.N., Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodo. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 149.Tsubaki M., Komai M., Fujimoto S., Itoh T., Imano M., Sakamoto K., et al. Activation of NF-κB by the RANKL/RANK system up-regulates snail and twist expressions and induces epithelial-to-mesenchymal transition in mammary tumor cell lines. J Exp Clin Cancer Res. 2013;32:62. doi: 10.1186/1756-9966-32-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Palafox M., Ferrer I., Pellegrini P., Vila S., Hernandez-Ortega S., Urruticoechea A., et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72:2879–2888. doi: 10.1158/0008-5472.CAN-12-0044. [DOI] [PubMed] [Google Scholar]
- 151.Chapple I.L.C., Gilbert A.D., Wilson N.H.F. Understanding Periodontal Diseases: Assessment and Diagnostic Procedures in Practice. Quintessence Publishing Co; London, England: 2002. [Google Scholar]
- 152.Benakanakere M., Kinane D.F. Innate cellular responses to the periodontal biofilm. Front Oral Biol. 2011;15:41–55. doi: 10.1159/000329670. [DOI] [PubMed] [Google Scholar]
- 153.Graves D. Cytokines that promote periodontal tissue destruction. J Periodo. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 154.Vernal R., Dutzan N., Hernández M., Chandía S., Puente J., León R., et al. High expression levels of receptor activator of nuclear factor-kappa B ligand associated with human chronic periodontitis are mainly secreted by CD4+ T lymphocytes. J Periodo. 2006;77:1772–1780. doi: 10.1902/jop.2006.050376. [DOI] [PubMed] [Google Scholar]
- 155.Ahn S.H., Chun S., Park C., Lee J.H., Lee S.W., Lee T.H. Transcriptome profiling analysis of senescent gingival fibroblasts in response to Fusobacterium nucleatum infection. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palm E., Khalaf H., Bengtsson T. Porphyromonas gingivalis downregulates the immune response of fibroblasts. BMC Microbiol. 2013;13:155. doi: 10.1186/1471-2180-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Page R.C., Kornman K.S. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 158.Cekici A., Kantarci A., Hasturk H., Van, Dyke T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alshargabi R., Sano T., Yamashita A., Takano A., Sanada T., Iwashita M., et al. SPOCK1 is a novel inducer of epithelial to mesenchymal transition in drug-induced gingival overgrowth. Sci Rep. 2020;10:9785. doi: 10.1038/s41598-020-66660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hunter N., Nicholls B., Srivastava M., Chapple C.C., Zoellner H.F., Gibbins J.R. Reactive pocket epithelium in untreated chronic periodontal disease: possible derivation from developmental remnants of the enamel organ and root sheath. J Oral Pathol Med. 2001;30:178–186. doi: 10.1034/j.1600-0714.2001.300308.x. [DOI] [PubMed] [Google Scholar]
- 161.Akimoto T., Fujiwara N., Kagiya T., Otsu K., Ishizeki K., Harada H. Establishment of Hertwig's epithelial root sheath cell line from cells involved in epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2011;404:308–312. doi: 10.1016/j.bbrc.2010.11.112. [DOI] [PubMed] [Google Scholar]
- 162.Itaya S., Oka K., Ogata K., Tamura S., Kira-Tatsuoka M., Fujiwara N., et al. Hertwig's epithelial root sheath cells contribute to formation of periodontal ligament through epithelial-mesenchymal transition by TGF-β. Biomed Res. 2017;38:61–69. doi: 10.2220/biomedres.38.61. [DOI] [PubMed] [Google Scholar]
- 163.Xiong J., Mrozik K., Gronthos S., Bartold P.M. Epithelial cell rests of Malassez contain unique stem cell populations capable of undergoing epithelial-mesenchymal transition. Stem Cells Dev. 2012;21:2012–2025. doi: 10.1089/scd.2011.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ronay V., Belibasakis G.N., Schmidlin P.R., Bostanci N. Infected periodontal granulation tissue contains cells expressing embryonic stem cell markers. A pilot study. Schweiz Mon Zahnmed. 2013;123:12–16. [PubMed] [Google Scholar]
- 165.Dabija-Wolter G., Cimpan M.R., Costea D.E., Johannessen A.C., Sørnes S., Neppelberg E., et al. Fusobacterium nucleatum enters normal human oral fibroblasts in vitro. J Periodo. 2009;80:1174–1183. doi: 10.1902/jop.2009.090051. [DOI] [PubMed] [Google Scholar]
- 166.Amornchat C., Rassameemasmaung S., Sripairojthikoon W., Swasdison S. Invasion of porphyromonas gingivalis into human gingival fibroblasts in vitro. J Int Acad Periodo. 2003;5:98–105. [PubMed] [Google Scholar]
- 167.Costerton J.W., Lewandowski Z., DeBeer D., Caldwell D., Korber D., James G. Biofilms, the customized microniche. J Bacteriol. 1994;176:2137–2142. doi: 10.1128/jb.176.8.2137-2142.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sume S., Kantarci A., Lee A., Hasturk H., Trackman P. Epithelial to MESENCHYMAL TRANSITION IN GINGIVAL OVErgrowth. Am J Clin Pathol. 2010;177:208–218. doi: 10.2353/ajpath.2010.090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matuszczak E., Cwalina I., Tylicka M., Wawrzyn K., Nowosielska M., Sankiewicz A., et al. Levels of selected matrix metalloproteinases-MMP-1, MMP-2 and fibronectin in the saliva of patients planned for endodontic treatment or surgical extraction. J Clin Med. 2020:9. doi: 10.3390/jcm9123971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bi J., Koivisto L., Pang A., Li M., Jiang G., Aurora S., et al. Suppression of αvβ6 Integrin Expression by Polymicrobial Oral Biofilms in Gingival Epithelial Cells. Sci Rep. 2017;7:4411. doi: 10.1038/s41598-017-03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chatzopoulos G.S., Koidou V.P., Wolff L.F. Expression of Wnt signaling agonists and antagonists in periodontitis and healthy subjects, before and after non-surgical periodontal treatment: a systematic review. J Periodontal Res. 2022;57:698–710. doi: 10.1111/jre.13029. [DOI] [PubMed] [Google Scholar]
- 172.Liu X., Zhang Z., Pan S., Shang S., Li C. Interaction between the Wnt/β-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res. 2018;53:842–852. doi: 10.1111/jre.12574. [DOI] [PubMed] [Google Scholar]
- 173.Hajishengallis G., Darveau R.P., Curtis M.A. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shouda T., Yoshida T., Hanada T., Wakioka T., Oishi M., Miyoshi K., et al. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001;108:1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ahmad S.F., Ansari M.A., Zoheir K.M., Bakheet S.A., Korashy H.M., Nadeem A., et al. Regulation of TNF-α and NF-κB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology. 2015;220:889–898. doi: 10.1016/j.imbio.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 176.Jiang D., Li D., Cao L., Wang L., Zhu S., Xu T., et al. Positive feedback regulation of proliferation in vascular smooth muscle cells stimulated by lipopolysaccharide is mediated through the TLR 4/Rac1/Akt pathway. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lieberthal W., Levine J.S. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 178.Hoare A., Soto C., Rojas-Celis V., Bravo D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat Inflamm. 2019;2019:1029857. doi: 10.1155/2019/1029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee J., Roberts J.S., Atanasova K.R., Chowdhury N., Han K., Yilmaz Ö. Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. doi: 10.3389/fcimb.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yost S., Stashenko P., Choi Y., Kukuruzinska M., Genco C.A., Salama A., et al. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int J Oral Sci. 2018;10:32. doi: 10.1038/s41368-018-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sztukowska M.N., Ojo A., Ahmed S., Carenbauer A.L., Wang Q., Shumway B., et al. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell Microbiol. 2016;18:844–858. doi: 10.1111/cmi.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li Y., Li B., Liu Y., Wang H., He M., Liu Y., et al. Porphyromonas gingivalis lipopolysaccharide affects oral epithelial connections via pyroptosis. J Dent Sci. 2021;16:1255–1263. doi: 10.1016/j.jds.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Abe-Yutori M., Chikazawa T., Shibasaki K., Murakami S. Decreased expression of E-cadherin by Porphyromonas gingivalis-lipopolysaccharide attenuates epithelial barrier function. J Periodontal Res. 2017;52:42–50. doi: 10.1111/jre.12367. [DOI] [PubMed] [Google Scholar]
- 184.Suh J.S., Kim S., Boström K.I., Wang C.-Y., Kim R.H., Park N.-H. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial–mesenchymal transition in mice. Int J Oral Sci. 2019;11:21. doi: 10.1038/s41368-019-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Figuero E., Sánchez-Beltrán M., Cuesta-Frechoso S., Tejerina J.M., del Castro J.A., Gutiérrez J.M., et al. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodo. 2011;82:1469–1477. doi: 10.1902/jop.2011.100719. [DOI] [PubMed] [Google Scholar]
- 186.Harrandah A.M., Chukkapalli S.S., Bhattacharyya I., Progulske-Fox A., Chan E.K.L. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J Oral Microbiol. 2020;13:1849493. doi: 10.1080/20002297.2020.1849493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yu M.R., Kim H.J., Park H.R. Fusobacterium nucleatum accelerates the progression of colitis-associated colorectal cancer by promoting EMT. Cancers. 2020:12. doi: 10.3390/cancers12102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shao W., Fujiwara N., Mouri Y., Kisoda S., Yoshida K., Yoshida K., et al. Conversion from epithelial to partial-EMT phenotype by Fusobacterium nucleatum infection promotes invasion of oral cancer cells. Sci Rep. 2021;11:14943. doi: 10.1038/s41598-021-94384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abdulkareem A.A., Shelton R.M., Landini G., Cooper P.R., Milward M.R. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adh Migr. 2018;12:127–137. doi: 10.1080/19336918.2017.1322253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lamont R.J., Fitzsimonds Z.R., Wang H., Gao S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontol. 2022;2000 doi: 10.1111/prd.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Harrandah A.M., Chukkapalli S.S., Bhattacharyya I., Progulske-Fox A., Chan E.K.L. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J Oral Microbiol. 2020;13:1849493. doi: 10.1080/20002297.2020.1849493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang S., Li C., Liu J., Geng F., Shi X., Li Q., et al. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020;287:4032–4047. doi: 10.1111/febs.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lafuente Ibáñez de Mendoza I., Maritxalar Mendia X., García de la Fuente A.M., Quindós Andrés G., Aguirre Urizar J.M. Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: a systematic review. J Periodontal Res. 2020;55:13–22. doi: 10.1111/jre.12691. [DOI] [PubMed] [Google Scholar]
- 194.Komlós G., Csurgay K., Horváth F., Pelyhe L., Németh Z. Periodontitis as a risk for oral cancer: a case–control study. BMC Oral Health. 2021;21:640. doi: 10.1186/s12903-021-01998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Palena C., Hamilton D.H., Fernando R.I. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. 2012;8:713–722. doi: 10.2217/fon.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim B.N., Ahn D.H., Kang N., Yeo C.D., Kim Y.K., Lee K.Y., et al. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci Rep. 2020;10:10597. doi: 10.1038/s41598-020-67325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mize T.W., Sundararaj K.P., Leite R.S., Huang Y. Increased and correlated expression of connective tissue growth factor and transforming growth factor beta 1 in surgically removed periodontal tissues with chronic periodontitis. J Periodontal Res. 2015;50:315–319. doi: 10.1111/jre.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ye P., Chapple C.C., Kumar R.K., Hunter N. Expression patterns of E-cadherin, involucrin, and connexin gap junction proteins in the lining epithelia of inflamed gingiva. J Pathol. 2000;192:58–66. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH673>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 199.Wadie K.W., Bashir M.H., Abbass M.M.S. Epithelial-mesenchymal transition in gingival tissues from chronic periodontitis patients: a case-control study. Dent Med Probl. 2021;58:311–319. doi: 10.17219/dmp/133514. [DOI] [PubMed] [Google Scholar]
- 200.Nagarakanti S., Ramya S., Babu P., Arun K.V., Sudarsan S. Differential expression of E-cadherin and cytokeratin 19 and net proliferative rate of gingival keratinocytes in oral epithelium in periodontal health and disease. J Periodo. 2007;78:2197–2202. doi: 10.1902/jop.2007.070070. [DOI] [PubMed] [Google Scholar]
- 201.Wara-aswapati N., Surarit R., Chayasadom A., Boch J.A., Pitiphat W. RANKL upregulation associated with periodontitis and Porphyromonas gingivalis. J Periodo. 2007;78:1062–1069. doi: 10.1902/jop.2007.060398. [DOI] [PubMed] [Google Scholar]
- 202.Setyawati M.I., Sevencan C., Bay B.H., Xie J., Zhang Y., Demokritou P., et al. Nano-TiO(2) drives epithelial-mesenchymal transition in intestinal epithelial cancer cells. Small. 2018;14 doi: 10.1002/smll.201800922. [DOI] [PubMed] [Google Scholar]