Abstract
Primary failure of eruption (PFE) is a rare disorder defined as incomplete tooth eruption despite the presence of a clear eruption pathway. PFE is known to be caused by rare variants in the parathyroid hormone 1 receptor gene (PTH1R). Although several PTH1R variants have been reported, the etiology of PFE remains unclear. However, important studies that help elucidate the pathology of PFE have recently been published. The purpose of this review is to summarize current treatment options, clinical symptoms or phenotypes for diagnosis, genetic information including solid evidence in mouse disease models and disease-specific induced pluripotent stem cells, thus approaching the etiology of PFE from the perspective of the latest research.
Keywords: Incomplete tooth eruption, Animal model, Disease-specific induced pluripotent stem cells, Treatment
1. Introduction
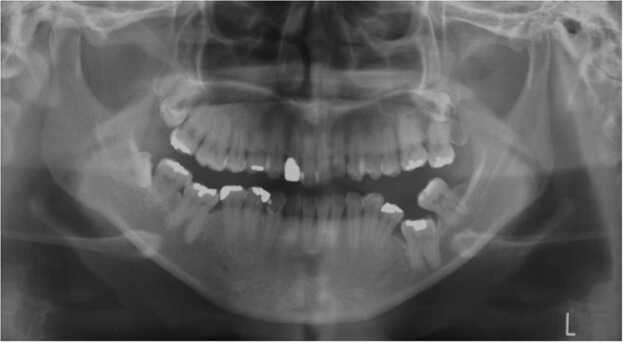
Primary failure of eruption (PFE) is defined as impaired tooth eruption despite the presence of an unobstructed eruption pathway (Fig. 1). PFE involves partial eruption or non-eruption of an initially non-ankylosed tooth due to a disturbed eruption mechanism, resulting in a posterior open bite [1]. The key manifestations were first described in 1981 by Proffit and Vig [2]. Selection of an appropriate treatment strategy depends on the correct diagnosis; however, orthodontic extrusion must be avoided as it can lead to ankylosis [1]. Unfortunately, diagnosis of PFE relies principally on a process of elimination in which all possible causative factors must be considered [3], [4], and many patients have received unsuccessful orthodontic treatment because PFE was not correctly diagnosed. For this reason, a good understanding of the clinical symptoms of PFE is necessary for correct diagnosis and effective treatment. Additionally, genetic information may be useful for diagnostic purposes. The discovery of causative variants of the parathyroid hormone 1 receptor gene (PTH1R) has led to research involving a mouse disease model and disease-specific induced pluripotent stem cells (iPSCs) that has deepened understanding of PFE etiology. Moreover, current studies are expanding the knowledge of PFE pathological conditions. Accordingly, this review summarizes current treatment options, clinical symptoms or phenotype and genetic information, and insights gained from research investigating PFE pathology.
Fig. 1.
a. Oral photographs. The patient was a 29-year-old female with primary failure of tooth eruption. The mandibular first molar was impacted vertically in both sides, and mandibular second pre-molar on the left side was inclined distally, and the mandibular second molar on the right side was inclined mesially. Fig. 1b. Cephalograms. Lateral cephalograms revealed skeletal Class III. The posterior-anterior cephalogram revealed asymmetry with deviation to right side. Fig. 1c. Panoramic radiograph. Panoramic radiograph revealed impaction of the mandibular first molar in both sides. (The permission to reprint of these materials from the Showa Univ J Med Sci 2010;22:151-156.)
2. Management of PFE
In PFE, tooth extrusion cannot be performed because orthodontic force applied to the erupted tooth can lead to ankylosis. Thus, orthodontic treatment is considered insufficient to improve malocclusion caused by PFE [2]. Treatment strategies for PFE vary depending on the clinical conditions. If only a few teeth have moderately abnormal eruption, prosthodontic restoration is recommended to establish satisfactory occlusion. In moderately severe cases, small-segment osteotomy and simultaneous elastic traction of the affected teeth will improve the level of the occlusal plane. Prosthetic treatment for these teeth has been shown to further improve malocclusion [5]. If the use of prosthetic teeth is not sufficient, occlusion may be effectively restored by extracting the affected teeth and replacing them with implants with alveolar ridge augmentation. In more severe cases with many incomplete erupted teeth and a significantly deformed alveolar ridge, prosthetic rehabilitation with a removable prosthetic appliance is effective in improving occlusion at an early stage [6]. In terms of surgical approaches to occlusion, bone grafting can be performed to improve occlusion. Specifically, segmental osteotomy of the alveolar bone affected by PFE can be performed to reposition the segment and subsequently improve the occlusal plane, and a bone graft can be placed between the bone segment and base of the alveolar bone [2], [7]. However, this technique may not be suitable for the maxilla because the thick and poorly elastic palate mucosa resists movement of the segment. This makes it difficult to reposition the segment at an appropriate location. Notably, there is high risk of causing inferior alveolar nerve damage when performing this procedure in the mandible. As such, studies have reported the use of distraction osteogenesis (DOG) of the alveolar bone in patients with severe lateral open bite [8], [9]. However, DOG of the alveolar bone is challenging in terms of controlling the direction of growth because the movement of the distraction divide can only be linear, and the segment tends to be displaced to the palatal side as the bone grows. In these cases, the floating bone concept may be more effective as it allows for 3-dimentional control of the position of the segment by continuously applying pressure to the segment before the bone heals completely [10]. Researchers previously performed DOG of the maxillary alveolar bone in a PFE case with severe lateral open bite, controlling the position of the segment with elastic traction and removing the device before the bone healed completely. This procedure successfully improved the morphology of the dental arch and achieved stable occlusion [11].
Evaluating the severity of clinical symptoms on an individual basis is needed to select the most appropriate treatment option for patients diagnosed with PFE. In other words, familiarity with the clinical symptoms of PFE is extremely important.
3. Clinical symptoms for the diagnosis of PFE
PFE is reported in approximately 0.06% of the population [12]. A prevalence among different populations has not been reported. Hanisch et al. compiled a systematic review of studies on PFE published until 2016 [1]. The review and subsequent articles are summarized in Table 1.
Table 1.
Clinical symptoms of primary failure of tooth eruption (PFE).
| population | Sex distribution | Affected teeth | Primary teeth affected by PFE | Unilateral/bilateral | Family history | Skeletal class | Additional dental anomalies | References |
|---|---|---|---|---|---|---|---|---|
| American, English, German, Saudi Arabian, Indian, French, Japanese, Irish, Danish | male (42.3%), female (57.7%) | Only molars (31.4%); both molars and premolars (68.6%) | Primary teeth affected (24.3%); deciduous teeth not affected (75.7%) | Teeth affected on both sides (64.1%) teeth affected on only one side (35.9%) | Family members affected (84.1%); family members not affected 15.9% | Skeletal classes I (5.9%), II (14.7%), III (79.4%) | Root morphology, impacted teeth, delayed eruption of further teeth, hypodontia, hyperdontia, transposition of teeth, peg-shaped teeth, Mechanical failure of eruption, hyperdontia, hypercementosis | [1] |
| Japanese | 4 females and 2 males | 2 patients with only molars; 2 patients with both molars and premolars; 1 patient with molars, premolars, and incisor; 1 patient with molars and incisor. | 4 patients with teeth affected on both sides; 2 patients with teeth affected on only one side | 3 family members affected; 2 family members not affected | All skeletal class III | Spontaneous reeruption, cervical root resorption | [63] | |
| Brazilian | 3 females and 7 males | Including an affected member with affected deciduous teeth | Affected teeth on both sides; affected teeth on only one side | Three generations of a family with 18 members; 10 members affected by PFE. | [54] | |||
| Caucasian | 2 females and 1 male | Including an affected member with affected deciduous teeth | Affected teeth on both sides; affected teeth on only one side | Two generations of a family with 3 members clinically affected by PFE. | [64] | |||
| Italian | 1 male | Bilateral | [32] | |||||
| Italian | 26 males and 18 females | 14 patients with teeth distal to a PFE-affected molar | 31 patients with affected permanent molars; 20 patients with affected deciduous teeth. | 14 patients with teeth affected bilaterally | [24] |
First and foremost, the easiest way to diagnose PFE is to check for occlusion in parents of patients suspected of having PFE. Almost 85% of patients with PFE had a family member affected by PFE (Table 1) [1]. However, parents may not recognize healthy occlusion since they experienced PFE at a young age; thus, it is strongly recommended to evaluate occlusion in the parents. Indeed, the father or mother may actually have severe malocclusion, even though they declared the absence of any dental problems. The next step in the diagnosis of PFE primarily relies on exclusion, in which all possible causative factors must be considered and eliminated. Table 2 lists the causes of eruption failure, divided into local and systemic causes. In many cases, local factors causing mechanical impairment are responsible for eruption failure. Infraocclusion, immobility, metallic sound on percussion, and radiographic obliteration of the periodontal ligament space are a clue to the diagnosis for mechanical failure of eruption (ankylosis). These characteristics might be differentiated from PFE. However, actuality, mechanical failure of eruption and PFE are often difficult to distinguish [1], [13], [14]. A genetic testing for variants in the PTH1R may be expected to avoid unnecessary treatment intervention [15], [16].
Table 2.
Causes of eruption failure.
| Local causes | Systemic causes |
|---|---|
| Ankylosis | Anemia |
| Ankylosis of decidous teeth | Celiac disease |
| Apical periosontitis of decidous teeth | Cerebral palsy |
| Arch-length deficiency and skeletal pattern | Endocrine disorders: |
| Cysts | Hypothyroidism (cretinism) |
| Ectopic eruption | Hypopituitarism |
| Enamel pearls | Hypoparathyroidism |
| Gingival fibromatosis / gingival hyperplasia | Pseudohypoparathyroidism |
| Impacted primary tooth | Drugs: phenytoin |
| Impaction | Exposure to hypobaria |
| Injuries to primary teeth | Genetic disorders |
| Lack of resorption of decidous tooth | Heavy metal intoxication |
| Mucosal barriers-scar tissue | HIV infection |
| Neoplasms | Ichthyosis |
| Nonodontogenic tumors | Idiopathy |
| Odontogenic tumors | Long-term chemotherapy |
| Oral clefts | Nutrition |
| Premature loss of primary tooth | Oral clefts |
| Primary retention | Prematurity/low birth weight |
| Radiation damage | Radiation damage |
| Regional odontodysplasia | Renal failure |
| Segmental odontomaxillary dysplasia | Vitamin D-resistant rickets |
| Supernumerary teeth | |
| Tongue or lip interpositioning |
4. Genetic testing for PFE
Eruption failure has been found to be a feature in many genetic disorders and syndromes as listed in Table 3. The gene(s) responsible for various disorders and syndromes associated with eruption failures have been identified. Moreover, Decker et al. [15] first reported that a variant in PTH1R was associated with PFE.
Table 3.
Genetic disorders of eruption failure.
| Disease name | Orphanet number | OMIM number | Responsible gene |
|---|---|---|---|
| Aarskog syndrome | 915 | 100050, 305400 | FGD1 |
| Amelogenesis imperfecta | 88661 | 104500, 104510, 104530, 130900, 204650, 204700, 301200, 301201, 612529, 613211, 614832, 615887, 616221, 616270, 617217 | AMELX, ENAM |
| Apert syndrome | 87 | 101200 | FGFR2 |
| Incontinentia pigmenti | 464 | 308300 | IKBKG |
| Carpenter syndrome | 65759 | 201000, 614976 | RAB23, MEGF8 |
| Cherubism | 184 | 118400 | SH3BP2 |
| Cleidocranial dysplasia | 1452 | 119600, 216330 | RUNX2 |
| Down syndrome | 870 | 190685 | trisomy 21 |
| Hypertrichosis lanuginosa congenita | 2222 | 145700 | |
| Costello syndrome | 3071 | 218040 | HRAS |
| Dentin dysplasia | 1653 | 125400, 125420 | DSPP |
| Junctional epidermolysis bullosa | 305 | - | COL17A1, ITGA6, ITGB4, LAMA3, LAMB3, LAMC2, ITGA3 |
| GAPO syndrome | 2067 | 230740 | ANTXR1, TEM8 |
| Gardner syndrome | 79665 | 175100 | APC |
| Gaucher disease | 355 | 230800, 230900, 231000, 231005, 608013, 610539 | GBA, PSAP |
| Hereditary gingival fibromatosis | 2024 | 135300, 605544, 609955, 611010, 617626 | SOS1, REST |
| Gorlin syndrome | 377 | 109400 | PTCH2, PTCH1, SUFU |
| Hallermann-Streiff syndrome | 2108 | 234100 | - |
| Hyperimmunoglobulinemia (Buckley syndrome) Autosomal dominant hyper-IgE syndrome |
2314 | 147060 | STAT3 |
| Hypodontia-dysplasia of nails syndrome (Ectodermal dysplasia 3, Witkop type) | 2228 | 189500 | MSX1 |
| Mucolipidosis type II | 576 | 252500 | GNPTAB |
| Incontinentia pigmenti (Bloch-Sulzberger syndrome) | 464 | 308300 | IKBKG |
| Menkes disease | 565 | 309400 | ATP7A |
| Mucopolysaccharidosis type 6 | 583 | 253200 | ARSB |
| Neurofibromatosis type 1 | 636 | 162200 162210 613675 | NF1 |
| Osteogenesis imperfecta | 666 | 166200 166210 166220 166230 259420 259440 610682 610915 610967 610968 613848 613849 613982 614856 615066 615220 616229 616507 619131 | COL1A1, COL1A2, etc. |
| Osteoglophonic dwarfism | 2645 | 166250 | FGFR1 |
| Osteopetrosis | 53, 2783, 667, etc. | 16600, 607634, 259700, 259710, 611490, 615085, etc. | TCIRG1, ClCN7, LRP5, etc. |
| Otodental dysplasia | 2791 | 166750 | - |
| Parry-Romberg syndrome (progressive hemifacial atrophy) |
1214 | 141300 | - |
| Rapp-Hodgkin syndrome | 3022 | 129400 | TP63 |
| Regional odontodysplasia | 834500 | - | - |
| Rothmund-Thompson syndrome | - | - | - |
| Sclerosteosis | 3152 | 269500, 614305 | SOST, LRP4 |
| SHORT syndrome | 3163 | 269880 | PIK3R1 |
| Singleton-Merten dysplasia | 85191 | 182250, 616298 | IFIH1, DDX58 |
| Infantile spasms syndrome (West Syndrome) | 3451 | 300672, 308350, 613477, 613722, 615006, 616139, 616341, 617065, 617929, 618298 | trisomy 21, the 1p36 deletion or mutations in the ARX or CDKL5 (SPTAN1, PLCB1, ST3GAL3, GRIN2B, SIK1, GUF1, CNPY3, PHACTR1) |
| Neurofibromatosis type 1 | 636 | 162200 162210 613675 | NF1 |
| 22q11 deletion syndrome | 567 | 188400, 192430 | deletion of 22q11.2, TBX1 |
PTH1R variants have also been associated with diseases of bone and cartilage. Table 4 summarizes the PTH1R variants reported so far. A loss-of-function variant in PTH1R can result in Blomstrand osteochondrodysplasia (BOC; Orphanet # 50945; OMIM # 215045), a rare autosomal recessive skeletal dysplasia characterized by advanced endochondral bone maturation and premature ossification of skeletal elements. BOC causes short-limbed dwarfism and perinatal lethality [17]. Similarly, a homozygous PTHIR variant results in Eiken syndrome (Orphanet # 79106; OMIM # 600002), a rare autosomal recessive skeletal disorder characterized by extremely retarded ossification and multiple epiphyseal dysplasia [18]. Patients with Eiken syndrome exhibit abnormal bone modeling in their hands and feet, abnormal pelvic cartilage persistence, and mild growth retardation [18]. In contrast, gain-of-function PTH1R variants have been reported in patients with Jansen’s metaphyseal chondrodysplasia (Orphanet # 33067; OMIM # 156400), an autosomal dominant disorder characterized by pronounced short-limb dwarfism resulting from decelerated chondrocyte differentiation [19].
Table 4.
Potentially pathogenic variants in PTH1R.
| Phenotype | position in chr3 (GRCh37) | HGMD Variant Class | coding DNA (NM_000316.2) | protein (NP_000307.1) | dbSNP | References |
|---|---|---|---|---|---|---|
| Eiken skeletal dysplasia with pseudoepiphyses in the hands and primary failure of tooth eruption | 46935424 | Pathogenic | c 0.103 G>A | p.Glu35Lys | [22] | |
| Primary failure of tooth eruption / Blomstrand chondrodysplasia | 46937356 | Pathogenic | c 0.310 C>T | p.Arg104Ter | rs121434604 | [27], [28] |
| Primary failure of tooth eruption | 46937391 | Likely pathogenic | c.313 + 32 A>G | rs113566258 | [24] | |
| Primary failure of tooth eruption | 46939352 | Pathogenic | c.322delT | p.Cys108ValfsTer82 | [27] | |
| Primary failure of tooth eruption | 46939362 | Pathogenic | c 0.331 G>T | p.Glu111Ter | [27], [30] | |
| Primary failure of tooth eruption | 46939387 | Likely pathogenic | c 0.356 C>T | p.Pro119Leu | rs1364327639 | [25], [27], [30], [31] |
| Primary failure of tooth eruption / Blomstrand chondrodysplasia | 46939426 | Pathogenic | c 0.395 C>T | p.Pro132Leu | rs121434599 | [25], [26], [31], [65] |
| Eiken skeletal dysplasia | 46939432 | Pathogenic | c.401 A>C | p.Tyr134Ser | [18] | |
| Primary failure of tooth eruption | 46939564 | Pathogenic | c 0.425 G>T | p.Gly142Val | [64] | |
| Primary failure of tooth eruption | 46939575 | Pathogenic | c 0.436 C>T | p.Arg146Ter | [27], [30] | |
| Primary failure of tooth eruption | 46939578 | Likely pathogenic | c 0.439 C>T | p.Arg147Cys | rs1323321129 | [25], [27], [31] |
| Ollier disease | 46939587 | Pathogenic | c 0.448 C>T | p.Arg150Cys | rs121434601 | [67] |
| Primary failure of tooth eruption | 46939602 | Pathogenic | c 0.463 G>T | p.Glu155Ter | rs121434605 | [15], [30], [31] |
| Primary failure of tooth eruption | 46939644 | Pathogenic | c 0.505 G>T | p.Glu169Ter | [24], [32] | |
| Primary failure of tooth eruption | 46939683 | Pathogenic | c.543 + 1 G>A | [15], [27], [30], [66] | ||
| Primary failure of tooth eruption | 46939683 | Pathogenic | c.543 + 1 G>T | [27] | ||
| Primary failure of tooth eruption | 46939842 | Pathogenic | c.544–25_544–23delCTG | [31] | ||
| Pseudohypoparathyroidism 1b with neurological involvement | 46939881 | Pathogenic | c 0.557 G>A | p.Arg186His | rs201499146 | [68] |
| Primary failure of tooth eruption | 46939895 | Pathogenic | c.572delA | p.Tyr191SerfsTer14 | [58] | |
| Primary failure of tooth eruption (Hypodontia, skeletal abnormalities of the nasal bridge, clinodactyly, polydactyly, and hallux valgus) | 46939935 | Pathogenic | c 0.611 T > A | p.Val204Glu | [21] | |
| Primary failure of tooth eruption | 46939957 | Pathogenic | c.636dupT | p.Arg213Ter | [27], [30] | |
| Primary failure of tooth eruption | 46940150 | Pathogenic | c.639–2 A>C | [27], [30] | ||
| Primary failure of tooth eruption | 46940150 | Pathogenic | c.639–2 A>G | [27] | ||
| Metaphyseal chondrodysplasia | 46940181 | Pathogenic | c.668 A>G | p.His223Arg | rs121434597 | [19], [69] |
| Ollier disease | 46940277 | Pathogenic | c 0.764 G>A | p.Arg255His | rs1027263198 | [27] |
| Primary failure of tooth eruption | 46940325 | Pathogenic | c.813dupT | p.Ala272CysfsTer127 | [27], [30] | |
| Primary failure of tooth eruption | 46940850 | Pathogenic | c 0.892 T > G | p.Trp298Gly | [31] | |
| Primary failure of tooth eruption | 46940905 | Pathogenic | c 0.947 C>A | p.Ser316Ter | [31] | |
| Primary failure of tooth eruption | 46942515 | Pathogenic | c 0.989 G>T | p.Gly330Val | [31] | |
| Primary failure of tooth eruption | 46942519 | Pathogenic | c.996dupC | p.Ala333ArgfsTer66 | [58] | |
| Primary failure of tooth eruption | 46942542 | Pathogenic | c 0.1016 G>A | p.Trp339Ter | rs760037270 | [27], [30] |
| Primary failure of tooth eruption | 46942559 | Pathogenic | c.1036delC | p.Leu346TrpfsTer9 | [27] | |
| Blomstrand chondrodysplasia | 46942604 | Pathogenic | c.1049 + 29 C>T | [28] | ||
| Primary failure of tooth eruption | 46942901 | Pathogenic | c.1050–3 C>G | [15], [30], [31], [66] | ||
| Primary failure of tooth eruption | 46942936 | Pathogenic | c 0.1082 G>A | p.Trp361Ter | rs1415520107 | [31] |
| Primary failure of tooth eruption | 46942946 | Pathogenic | c.1092delG | p.Val365CysfsTer140 | [70] | |
| Primary failure of tooth eruption / Blomstrand chondrodysplasia | 46942945 | Pathogenic | c.1093delG | p.365CysfsTer141 | rs1304201852 | [27], [29], [30] |
| Primary failure of tooth eruption / Blomstrand chondrodysplasia | 46943287 | Pathogenic | c 0.1148 G>A | p.Arg383Gln | rs398122843 | [17], [25], [27], [31] |
| Metaphyseal chondrodysplasia | 46944032 | Pathogenic | c.1228 A>C | p.Thr410Pro | rs121434598 | [71] |
| Metaphyseal chondrodysplasia | 46944033 | Pathogenic | c 0.1229 C>G | p.Thr410Arg | rs121434602 | [72] |
| Primary failure of tooth eruption | 46944150 | Pathogenic | c.1348_1350delTTC | p.Phe450del | [31] | |
| Primary failure of tooth eruption | 46944238 | Pathogenic | c.1354–1 G>A | [16], [31] | ||
| Metaphyseal chondrodysplasia | 46944258 | Pathogenic | c 0.1373 T > A | p.Ile458Lys | [73] | |
| Metaphyseal chondrodysplasia | 46944258 | Pathogenic | c 0.1373 T > G | p.Ile458Arg | rs121434600 | [69] |
| Eiken skeletal dysplasia | 46944817 | Pathogenic | c 0.1453 C>T | p.Arg485Ter | rs121434603 | [18] |
| Primary failure of tooth eruption | 46944956 | Likely pathogenic | c.1595delC | p.Pro532LeufsTer85 | [24] | |
| Primary failure of tooth eruption | 46945129 | Pathogenic | c 0.1765 T > C | p.Trp589Arg | [24], [74] |
In PTH1R, 48 are registered as pathogenic or likely pathogenic in Human Gene Mutation Database (HGMD) [https://www.hgmd.cf.ac.uk]. In addition, the functional classification of these variants includes 24 missense variants, 9 nonsense variants, 6 frameshift deletions, 3 frameshift insertions, 1 non-frameshift deletion, and 5 intronic variants. Lollipops software [20] was used to confirm the accumulation of genetic regions and functional domains of these variants, and the locations of PTH1R variants associated with PFE and other diseases are shown in Fig. 2. The location of the pathogenic PTH1R variant was observed scattered throughout the gene, with no domain accumulation, and no phenotype-dependent features were observed.
Fig. 2.
Location of variants in the PTH1R, schematized using Lollipops software [20].
One of the reasons for the large variety of phenotypes associated with PTH1R variants is allelic dose. Strong evidence supports that PFE is an autosomal dominant condition associated with heterozygous variants in PTH1R in most cases, whereas homozygous variants are present in extremely rare cases [21], [22], [23], [24]. Although the heterozygous missense PTH1R variant c 0.395 C>T (see Table 4) was reported in Japanese sporadic cases of PFE [25], the homozygous genotype has also been associated with the BOC phenotype [26]. Similarly, the heterozygous PTH1R variants c 0.310 C>T [27], [28], c 0.395 C>T [25], [26], [31], [52], c.1093delG [27], [29], [30], and c 0.1148 G>A [17], [25], [27], [31], have been reported in patients with PFE, while homozygous genotypes have also been associated with the BOC phenotype. Moreover, Jelani et al. [21] reported that the homozygous or biallelic PTH1R variant c 0.611 T > A (see Table 4), which caused a PFE phenotype that appeared to be unique to the family and also caused clinodactyly and nasal bridge deformity, was also identified as a heterozygous genotype associated with the Jansen’s metaphyseal chondrodysplasia phenotype. Moirangthem et al. [22] reported that a patient with autosomal recessive Eiken syndrome (c 0.103 G>A) (see Table 4) also had malposition and impaction in most teeth. In practice, similar to these examples, the disease overlap with PFE may not be a small number of patients. The reason may be due to unconversant oral examinations [15], [31].
As shown in Table 1, almost 15% of patients with PFE had no family members affected by PFE [1]. The patient of PFE without family history, may have spontaneous variant. Therefore, even in sporadic case, a genetic testing brings a valuable advantage for expecting the potential contribution of orthodontic intervention [32].
Although variable phenotypic expression can be observed, almost all PTH1R variants causing PFE are thought to exhibit complete penetrance [31]. However, one study documented a patient with severe PFE that carried the PTH1R variant c 0.505 G>T inherited from an unaffected mother, providing a typical example of incomplete penetrance [32]. This should be considered when interpreting the results of a genetic testing.
It remains unclear whether variants in only PTH1R cause PFE because PTH1R variants are not found in all patients with PFE [1], [27], [30]. The histone methyltransferase 2 C gene (KMT2C) is reportedly strongly associated with PFE [33]. However, affected families in which this gene was identified included family members without all permanent teeth, and the phenotype was somewhat more severe than that in simple PFE. In any case, it is clear that performing a genetic testing for PTH1R, whenever possible, provides useful information to confirm a diagnosis, even in sporadic case [24].
5. PTH1R signaling and tooth eruption
PTH1R encodes parathyroid hormone (PTH) receptor type 1 (PTH1R), a Class B G protein-coupled receptor (GPCR) with 7 transmembrane spanning helixes. PTH1R is activated by two similar ligands with distinct functions, PTH and PTH-related protein (PTHrP), which control the hormonal and local functions of the receptor, respectively. PTH and PTHrP bind to PTH1R in equivalent affinity [34], [35]. Upon ligand binding, PTH1R activates two major second messenger signaling systems, including the adenylyl cyclase/protein kinase A pathway and the phospholipase C/protein kinase C pathway [36]. As a locally acting autocrine and paracrine ligand, PTHrP exerts pleiotropic effects on cell proliferation and differentiation. During development, the PTHrP-PTH1R pathway mediates epithelial-mesenchymal interactions in various organs, such as the skin, hair follicles, mammary glands, pancreas, and developing teeth [37], [38], [39], [40], [41]. This pathway also regulates bone formation in the endochondral pathway [42].
As a result, the PTHrP-PTH1R pathway regulates tooth eruption in multiple aspects. Tooth eruption occurs in three distinct phases: pre-eruptive tooth movement (Phase 1), intra-osseous and supra-osseous eruptive tooth movement (Phase 2), and post-eruptive tooth movement (Phase 3) [43], [44]. Phase 1 (pre-eruptive tooth movement) occurs during the early stages of tooth development, lasting until the commencement of tooth root formation. Enamel and dentin formation at this stage prepares the tooth crown for emergence into the oral cavity. Phase 2 (eruptive tooth movement) occurs during tooth root formation, lasting until the tooth crown reaches the occlusal plane. This phase is divided into two stages: intra-osseous and supra-osseous eruptive tooth movement, and involves the formation of the tooth root, alveolar bone, and periodontal ligament. It also involves the osteoclast-mediated bone resorption of the cortical shell overlaying the tooth crown, which jointly facilitate the emergence of the tooth crown into the oral cavity. Phase 3 (post-eruptive tooth movement) maintains the tooth crown in occlusion as the alveolar bone continues to grow and remodel, which requires continuous maturation of the periodontal attachment apparatus. In this phase, the PTHrP-PTH1R pathway directly regulates the proliferation of dental mesenchymal progenitor cells and their differentiation into cementoblasts, periodontal ligament cells, and alveolar bone osteoblasts. It also indirectly regulates bone-resorbing osteoclasts through the RANKL-RANK axis.
Importantly, tooth eruption requires the formation of the eruption path and the motive force. The sources of the motive force include the dental follicle surrounding the tooth bud, osteogenic activities in the alveolar bone surrounding the tooth root, the formation of the periodontal ligament (PDL) and traction forces generated by the fibers therein, and the development of the tooth root and the cementum in the apical region. Traditionally, tooth eruption has been regarded as a separate and distinct process from tooth root formation, as teeth can emerge into the oral cavity without roots or PDLs [45], [46]. However, recent studies support the emerging theory that these two processes are intertwined [36], [39]. Because the eruption path is cleared in PFE, defective tooth eruption in this condition is likely to be induced by deficiency motive forces, many of which are regulated by the PTHrP-PTH1R pathway.
6. Mouse models of PFE
Various putative loss-of-function mutations in PTH1R are present in patients with PFE [15], [25], [27], [31], [47]. The majority of these PTH1R mutations are heterozygous, while in extremely rare cases, these mutations are also present in homozygous forms [21], [22], [23], [24]. Studies with genetically engineered mice present solid evidence that loss-of-function mutations in Pth1r cause similar skeletal abnormalities as those observed in humans. For example, mice harboring two copies of the Pth1rtm1Hmk allele (Pth1r-/-), which lacks most of the coding region of the Pth1r gene, exhibit perinatal lethality that recapitulates Blomstrand osteochondrodysplasia (BOC) [48]. Interestingly, heterozygous Pth1r mutant mice (Pth1r+/-) do not show overt defects in tooth eruption [49], indicating that Pth1r haploinsufficiency is not sufficient to induce PFE in mice. It is possible that mouse molars are less sensitive to Pth1r gene dosage changes than human molars. Therefore, a better mouse disease model for PFE is needed to understand mechanisms that exclusively affect tooth eruption in molars.
A conditional gene deletion approach based on the Cre-loxP recombination system provides a practical modality to circumvent perinatal lethality of Pth1r-/- mice and test the function of PTH1R in tooth eruption that exclusively occur during postnatal stages. In the Pth1r-floxed allele (Pth1rtm2Hmk), exon 1 of the Pth1r gene, which is essential for ligand binding, is flanked by loxP sites [50]. This allele can be rendered nonfunctional upon Cre-loxP recombination and inactivate PTH1R in a cell type-specific manner. For example, Pth1r can be specifically deleted in dental mesenchymal cells that express osterix (Osx, also known as Sp7) using Osx-cre and Pth1r-floxed alleles. This causes severe failure of tooth eruption in molars associated with truncated roots lacking periodontal ligaments [49], representing an extreme phenotype induced by PTH1R loss-of-function variants. However, these mutant mice do not survive beyond weaning owing to the inability to consume food. A delayed molar eruption phenotype is also observed by Pth1r deletion in Prrx1-expressing progenitor cells using Prrx1-cre [51]. Notably, cells marked by Osx-cre are distributed throughout the alveolar bone and the periodontal tissue surrounding the incisor and molars, while cells marked by Prrx1-cre are predominantly observed in the alveolar bone surrounding incisors and at the base of molars. Delayed eruption, instead of complete failure of eruption, observed in Prrx1-cre-mediated Pth1r mutant mice, is likely due to the restricted distribution of cells marked by Prrx1-cre. Reduced formation of the alveolar bone and PDL at the base of molars, instead of the unresorbed overlaying cortical shell, is likely to explain delayed eruption of Prrx1-cre-mediated Pth1r mutant molars. These findings emphasize the role of the alveolar bone formation in providing the motive force for tooth eruption. The major limitation of these models is that PTH1R is inactivated in the given cell types from embryogenesis. As a result, other bone compartments, including the mandible, skull and long bones, are also severely affected, making it difficult to discern the contribution of each component to the failure to tooth eruption. Therefore, these models lack the phenotypic specificity for tooth eruption in molars and present as conditions that are far more severe than those typically manifested in human PFE.
To inactivate PTH1R postnatally in a tooth root-specific manner, a dental follicle-specific, tamoxifen-inducible PTHrP-creER line can be used to delete PTH1R using PTHrP-creER and Pth1r-floxed allele [52]. By administering tamoxifen at a specific postnatal time point (in this case, postnatal day 3), this inducible cell type-specific approach enables biallelic inactivation of PTH1R in a small number of specific cell types (in this case, PTHrP+ dental follicle cells) at the onset of tooth root formation. This approach can create a cell type-specific “low mosaicism” of PTH1R-deficient cells during tooth root formation, providing substantial mechanical insights into the altered cellular dynamics underlying PFE. Postnatal PTH1R deletion in dental follicle cells exclusively induces failure of tooth eruption in molars, which essentially recapitulate human PFE. The mutant molars exhibit a significant delay in tooth eruption especially first molars, without involving other bone components. Furthermore, the affected molars exhibit dilacerated tooth roots and cementum anomalies, which are often observed in human PFE molars [30]. Interestingly, the mutant mouse PFE phenotype, particularly PFE in first molars, demonstrates aggravated open bite in adult stages [53], which is similar to human cases of PFE reported during a long-term follow-up study [54]. A mouse PFE phenotype is also observed when Pth1r is postnatally inactivated in a much broader type of Osx+ cells using Osx-creER [52], indicating that PTHrP+ dental follicle cells represent an important functional subset of dental mesenchymal cells that orchestrate tooth eruption.
Although these mutant mice do not completely represent the same genetic condition as human patients with PFE, these studies provide important insights into the pathogenesis of PFE (Table 5). PTH1R inactivation drives dental follicle cells to shift from physiological periodontal ligament fibroblasts and cryptal bone alveolar bone osteoblasts to non-physiological precocious cellular cementoblasts. It remains to be determined whether similar mechanisms of cell fate shift also occur in human PFE.
Table 5.
Mouse models of PFE.
| Genotype | Gene symbol: PTH1R | Gene symbol: Cre/CreER drivers | Induction time (tamoxifen) | Tooth eruption phenotype | Reference |
|---|---|---|---|---|---|
| PTH1R-/- | PTH1Rtm1Hmk | N/A | N/A | Perinatal lethal (BOC) | [48] |
| PTH1R+/- | PTH1Rtm1Hmk | N/A | N/A | No phenotype | [49] |
| Osx-Cre; PTH1Rfl/fl | PTH1Rtm2Hmk | Tg(Sp7-tTA,tetO-EGFP/cre)1Amc | N/A | Complete failure of eruption, truncated molar root | [49] |
| Prrx1-Cre; PTH1Rfl/fl | PTH1Rtm2Hmk | Tg(Prrx1-cre)1Cjt | N/A | Delayed eruption | [51] |
| Osx-CreER; PTH1Rfl/fl | PTH1Rtm2Hmk | Tg(Sp7-cre/ERT)1Hmk | P3 | Failure of eruption (especially M1), small mandible | [49] |
| PTHrP-CreER; PTH1Rfl/fl | PTH1Rtm2Hmk | Tg(Pthlh-cre/ERT2)909Nono | P3 | Failure of eruption (especially M1), no bone phenotype | [52] |
7. Etiological study of PFE using the PFE-specific induced pluripotent stem cells (iPSCs)
Yamanaka and his colleagues created mouse and human iPSCs by inducing the reprogramming of somatic cells through the ectopic expression of the four factors (Yamanaka factors), namely, OCT3/4, KLF4, SOX2, and c-Myc [55], [56]. Since iPSCs are free from the ethical problem of using a fertilized egg and the immunological rejection accompanied by embryonic stem cells, human iPSCs are regarded as promising tools for regenerative medicine. Also, disease-specific iPSCs are developing new research tactics in medical sciences. It made it possible to investigate the diseases' pathogenic mechanisms and find therapeutic methods using the disease-specific iPSCs [57].
Several heterozygous variants in the PTH1R have been identified in PFE patients, indicating a close relationship between these genetic variations and the pathogenesis of PFE [25], [31], [47], [58]. Examination of the effects of the introduction of variations in the PTH1R on the cellular functions and observation of the phenotypes of the animals having the modified Pth1r are considered adequate to demonstrate the pathogenic role of PTH1R variation in PFE patients. In addition to these measures, the study using PFE-specific iPSCs is regarded as another potent approach to clarify the pathogenesis of PFE. We established iPSC clones from hematopoietic progenitor cells obtained from a patient with the heterozygous 395 C>T substitution in the PTH1R [59] by introducing the Yamanaka factors using a Sendai virus vector [60]. Using the PFE-specific iPSCs, we tried to explain the role of the genetic variation in PTH1R in the pathogenesis of PFE in in-vitro studies [59].
Alveolar bone resorption is one of the critical events of tooth eruption [61]. Furthermore, it is known that the PTH1R-expressing osteoblasts are responsible for the differentiation and activation of osteoclasts, the bone-resorbing multinucleated giant cells [61]. Hence, we examined the differentiation of the PFE-specific iPSCs and the control iPSCs derived from a healthy volunteer into osteoblast-like cells after cultivation in the medium containing the osteoblast differentiation-inducing factors (β-glycerophosphate, ascorbic acid, and dexamethasone). Differentiation of PFE-specific iPSCs to osteoblast-like cells evaluated by mineralization of the extracellular matrix and the expression of the osteoblast differentiation-related genes (RUNX2, SP7, and BGLAP) was comparable to that of the control iPSCs. Notably, both the osteoblast-like cells derived from PFE-specific iPSCs and those from the control iPSCs expressed equivalent levels of the mRNA and protein of PTH1R [59].
On the other hand, the expression of the mRNA and protein of receptor activator of nuclear factor-κB ligand (RANKL), the essential molecule for osteoclast differentiation and activation [62], in response to PTH was significantly suppressed in the osteoblast-like cells from PFE-specific iPSCs compared to those from the control iPSCs. Active vitamin D3 induced the expression of the mRNA and protein of RANKL comparably in osteoblast-like cells derived from PFE-specific and those from the control iPSCs, indicating that the PTH-PTH1R signaling system leading to the RANKL expression is affected in the osteoblast-like cells derived from PFE-specific iPSCs [59].
The heterozygous 395 C>T variant in the PTH1R is supposed to result in the expression of both standard and P132L amino acid substituted PTH1R in osteoblasts. Our study above indicated that the PTH1R with P132L amino acid substitution in osteoblasts possibly contributed at least in part to the retarded tooth eruption through the incomplete induction of differentiation and activation of osteoclasts in the PFE patient. Detection of gene variants in patients with rare diseases, including PFE, through the techniques such as whole-exome sequencing coupled with studies employing the disease-specific iPSCs, may be a promising approach to clarify the etiology of the diseases.
8. Conclusions
The patient’s clinical symptoms need to be carefully scrutinized. Moreover, genetic testing for PTH1R variants is recommended to avoid unnecessary and ultimately unsuccessful orthodontic intervention. Screening for such variants in a patient with PFE will help in the selection of an appropriate management strategy and allow more realistic treatment options to be offered to the patient. Treatment options must be evaluated on an individual basis, depending on the patient’s age, background, and preferences. Many aspects of PFE pathophysiology remain unclear; however, disease-specific iPSCs present an unprecedented opportunity to replicate both normal and pathological human tissue formation in vitro, thereby enabling the disease to be investigated and an innovative treatment to be developed to be achieved. Use of a mouse disease model is also expected to offer a way to investigate PFE and act as a guide to the discovery of an effective drug.
References
- 1.Hanisch M., Hanisch L., Kleinheinz J., Jung S. Primary failure of eruption (PFE): a systematic review. Head Face Med. 2018;14:5. doi: 10.1186/s13005-018-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proffit W.R., Vig K.W. Primary failure of eruption: a possible cause of posterior open-bite. Am J Orthod. 1981;80:173–190. doi: 10.1016/0002-9416(81)90217-7. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad S., Bister D., Cobourne M.T. The clinical features and aetiological basis of primary eruption failure. Eur J Orthod. 2006;28:535–540. doi: 10.1093/ejo/cjl033. [DOI] [PubMed] [Google Scholar]
- 4.Mubeen S., Seehra J. Failure of eruption of first permanent molar teeth: a diagnostic challenge. J Orthod. 2018;45:129–134. doi: 10.1080/14653125.2018.1462902. [DOI] [PubMed] [Google Scholar]
- 5.Frazier-Bowers S.A., Long S., Tucker M. Primary failure of eruption and other eruption disorders—Considerations for management by the orthodontist and oral surgeon. Semin Orthod. 2016;22:34–44. [Google Scholar]
- 6.Atobe M., Sekiya T., Tamura K., Hamada Y., Nakamura Y. Severe lateral open bite caused by multiple ankylosed teeth: a case report. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2009;107:e14–e20. doi: 10.1016/j.tripleo.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Brady J. Familial primary failure of eruption of permanent teeth. Br J Orthod. 1990;17:109–113. doi: 10.1179/bjo.17.2.109. [DOI] [PubMed] [Google Scholar]
- 8.Kater W.M., Kawa D., Schäfer D., Toll D. Treatment of posterior open bite using distraction osteogenesis. J Clin Orthod. 2004;38:501–504. [PubMed] [Google Scholar]
- 9.Susami T., Matsuzaki M., Ogihara Y., Sakiyama M., Takato T., Sugawara Y., et al. Segmental alveolar distraction for the correction of unilateral open-bite caused by multiple ankylosed teeth: a case report. J Orthod. 2006;33:153–159. doi: 10.1179/146531205225021572. [DOI] [PubMed] [Google Scholar]
- 10.Kunz C., Hammer B., Prein J. Manipulation of callus after linear distraction: a "lifeboat" or an alternative to multivectorial distraction osteogenesis of the mandible? Plast Reconstr Surg. 2000;105:674–679. doi: 10.1097/00006534-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Shirota T., Hishida M., Yamaguchi T., Kurabayashi H., Maki K., Shintani S. Posterior maxillary segmental distraction for the treatment of severe lateral open bite caused by primary failure of tooth eruption: a case report. J Oral Maxillofac Surg Med Pathol. 2013;25:39–42. [Google Scholar]
- 12.Baccetti T. Tooth anomalies associated with failure of eruption of first and second permanent molars. Am J Orthod Dentofac Orthop. 2000;118:608–610. doi: 10.1067/mod.2000.97938. [DOI] [PubMed] [Google Scholar]
- 13.Sharma G., Kneafsey L., Ashley P., Noar J. Failure of eruption of permanent molars: a diagnostic dilemma. Int J Paediatr Dent. 2016;26:91–99. doi: 10.1111/ipd.12163. [DOI] [PubMed] [Google Scholar]
- 14.Raghoebar G.M., Boering G., Jansen H.W., Vissink A. Secondary retention of permanent molars: a histologic study. J Oral Pathol Med. 1989;18:427–431. doi: 10.1111/j.1600-0714.1989.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 15.Decker E., Stellzig-Eisenhauer A., Fiebig B.S., Rau C., Kress W., Saar K., et al. PTHR1 loss-of-function mutations in familial, nonsyndromic primary failure of tooth eruption. Am J Hum Genet. 2008;83:781–786. doi: 10.1016/j.ajhg.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazier-Bowers S.A., Simmons D., Wright J.T., Proffit W.R., Ackerman J.L. Primary failure of eruption and PTH1R: the importance of a genetic diagnosis for orthodontic treatment planning. Am J Orthod Dentofac Orthop. 2010;137:160. doi: 10.1016/j.ajodo.2009.10.019. e1-7. [DOI] [PubMed] [Google Scholar]
- 17.Jobert A.S., Zhang P., Couvineau A., Bonaventure J., Roume J., Le Merrer M., et al. Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Investig. 1998;102:34–40. doi: 10.1172/JCI2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchatelet S., Ostergaard E., Cortes D., Lemainque A., Julier C. Recessive mutations in PTHR1 cause contrasting skeletal dysplasias in Eiken and Blomstrand syndromes. Hum Mol Genet. 2005;14:1–5. doi: 10.1093/hmg/ddi001. [DOI] [PubMed] [Google Scholar]
- 19.Schipani E., Kruse K., Jüppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 20.Jay J.J., Brouwer C. Lollipops in the clinic: information dense mutation plots for precision medicine. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jelani M., Kang C., Mohamoud H.S., Al-Rehaili R., Almramhi M.M., Serafi R., et al. A novel homozygous PTH1R variant identified through whole-exome sequencing further expands the clinical spectrum of primary failure of tooth eruption in a consanguineous Saudi family. Arch Oral Biol. 2016;67:28–33. doi: 10.1016/j.archoralbio.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Moirangthem A., Narayanan D.L., Jacob P., Nishimura G., Mortier G., Girisha K.M. Report of second case and clinical and molecular characterization of Eiken syndrome. Clin Genet. 2018;94:457–460. doi: 10.1111/cge.13413. [DOI] [PubMed] [Google Scholar]
- 23.Jacob P., Soni J.P., Mortier G., Girisha K.M. The third family with Eiken syndrome. Clin Genet. 2019;96:378–379. doi: 10.1111/cge.13601. [DOI] [PubMed] [Google Scholar]
- 24.Grippaudo C., D'Apolito I., Cafiero C., Re A., Chiurazzi P., Frazier-Bowers S.A. Validating clinical characteristic of primary failure of eruption (PFE) associated with PTH1R variants. Prog Orthod. 2021;22:43. doi: 10.1186/s40510-021-00387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi T., Hosomichi K., Narita A., Shirota T., Tomoyasu Y., Maki K., et al. Exome resequencing combined with linkage analysis identifies novel PTH1R variants in primary failure of tooth eruption in Japanese. J Bone Min Res. 2011;26:1655–1661. doi: 10.1002/jbmr.385. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P., Jobert A.S., Couvineau A., Silve C. A homozygous inactivating mutation in the parathyroid hormone/parathyroid hormone-related peptide receptor causing Blomstrand chondrodysplasia. J Clin Endocrinol Metab. 1998;83:3365–3368. doi: 10.1210/jcem.83.9.5245. [DOI] [PubMed] [Google Scholar]
- 27.Roth H., Fritsche L.G., Meier C., Pilz P., Eigenthaler M., Meyer-Marcotty P., et al. Expanding the spectrum of PTH1R mutations in patients with primary failure of tooth eruption. Clin Oral Investig. 2014;18:377–384. doi: 10.1007/s00784-013-1014-3. [DOI] [PubMed] [Google Scholar]
- 28.Hoogendam J., Farih-Sips H., Wÿnaendts L.C., Löwik C.W., Wit J.M., Karperien M. Novel mutations in the parathyroid hormone (PTH)/PTH-related peptide receptor type 1 causing Blomstrand osteochondrodysplasia types I and II. J Clin Endocrinol Metab. 2007;92:1088–1095. doi: 10.1210/jc.2006-0300. [DOI] [PubMed] [Google Scholar]
- 29.Karperien M., van der Harten H.J., van Schooten R., Farih-Sips H., den Hollander N.S., Kneppers S.L., et al. A frame-shift mutation in the type I parathyroid hormone (PTH)/PTH-related peptide receptor causing Blomstrand lethal osteochondrodysplasia. J Clin Endocrinol Metab. 1999;84:3713–3720. doi: 10.1210/jcem.84.10.6033. [DOI] [PubMed] [Google Scholar]
- 30.Pilz P., Meyer-Marcotty P., Eigenthaler M., Roth H., Weber B.H., Stellzig-Eisenhauer A. Differential diagnosis of primary failure of eruption (PFE) with and without evidence of pathogenic mutations in the PTHR1 gene. J Orofac Orthop. 2014;75:226–239. doi: 10.1007/s00056-014-0215-y. [DOI] [PubMed] [Google Scholar]
- 31.Risom L., Christoffersen L., Daugaard-Jensen J., Hove H.D., Andersen H.S., Andresen B.S., et al. Identification of six novel PTH1R mutations in families with a history of primary failure of tooth eruption. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grippaudo C., Cafiero C., D'Apolito I., Re A., Genuardi M., Chiurazzi P., et al. A novel nonsense PTH1R variant shows incomplete penetrance of primary failure of eruption: a case report. BMC Oral Health. 2019;19:249. doi: 10.1186/s12903-019-0944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assiry A.A., Albalawi A.M., Zafar M.S., Khan S.D., Ullah A., Almatrafi A., et al. KMT2C, a histone methyltransferase, is mutated in a family segregating non-syndromic primary failure of tooth eruption. Sci Rep. 2019;9:16469. doi: 10.1038/s41598-019-52935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jüppner H., Abou-Samra A.B., Freeman M., Kong X.F., Schipani E., Richards J., et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 35.Dean T., Vilardaga J.P., Potts J.T., Jr, Gardella T.J. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannstadt M., Jüppner H., Gardella T.J. Receptors for PTH and PTHrP: their biological importance and functional properties. Am J Physiol. 1999;277:F665–F675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 37.Wysolmerski J.J., Broadus A.E., Zhou J., Fuchs E., Milstone L.M., Philbrick W.M. Overexpression of parathyroid hormone-related protein in the skin of transgenic mice interferes with hair follicle development. Proc Natl Acad Sci USA. 1994;91:1133–1137. doi: 10.1073/pnas.91.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wysolmerski J.J., McCaughern-Carucci J.F., Daifotis A.G., Broadus A.E., Philbrick W.M. Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development. 1995;121:3539–3547. doi: 10.1242/dev.121.11.3539. [DOI] [PubMed] [Google Scholar]
- 39.Vasavada R.C., Cavaliere C., D'Ercole A.J., Dann P., Burtis W.J., Madlener A.L., et al. Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem. 1996;271:1200–1208. doi: 10.1074/jbc.271.2.1200. [DOI] [PubMed] [Google Scholar]
- 40.Foley J., Longely B.J., Wysolmerski J.J., Dreyer B.E., Broadus A.E., Philbrick W.M. PTHrP regulates epidermal differentiation in adult mice. J Investig Dermatol. 1998;111:1122–1128. doi: 10.1046/j.1523-1747.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- 41.Philbrick W.M., Dreyer B.E., Nakchbandi I.A., Karaplis A.C. Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci USA. 1998;95:11846–11851. doi: 10.1073/pnas.95.20.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kronenberg H.M. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 43.Richman J.M. Shedding new light on the mysteries of tooth eruption. Proc Natl Acad Sci USA. 2019;116:353–355. doi: 10.1073/pnas.1819412116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagata M., Ono N., Ono W. Mesenchymal progenitor regulation of tooth eruption: a view from PTHrP. J Dent Res. 2020;99:133–142. doi: 10.1177/0022034519882692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cahill D.R., Marks S.C., Jr. Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol. 1980;9:189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang J., Feng J.Q. Signaling pathways critical for tooth root formation. J Dent Res. 2017;96:1221–1228. doi: 10.1177/0022034517717478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian H., Döring F., Kollert S., Rukoyatkina N., Sturm J., Gambaryan S., et al. PTH1R mutants found in patients with primary failure of tooth eruption disrupt g-protein signaling. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanske B., Karaplis A.C., Lee K., Luz A., Vortkamp A., Pirro A., et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 49.Ono W., Sakagami N., Nishimori S., Ono N., Kronenberg H.M. Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat Commun. 2016;7:11277. doi: 10.1038/ncomms11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi T., Chung U.I., Schipani E., Starbuck M., Karsenty G., Katagiri T., et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 51.Cui C., Bi R., Liu W., Guan S., Li P., Song D., et al. Role of PTH1R signaling in Prx1+ mesenchymal progenitors during eruption. J Dent Res. 2020;99:1296–1305. doi: 10.1177/0022034520934732. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi A., Nagata M., Gupta A., Matsushita Y., Yamaguchi T., Mizuhashi K., et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc Natl Acad Sci USA. 2019;116:575–580. doi: 10.1073/pnas.1810200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokavanich N., Gupta A., Nagata M., Takahashi A., Matsushita Y., Yatabe M., et al. A three-dimensional analysis of primary failure of eruption in humans and mice. Oral Dis. 2020;26:391–400. doi: 10.1111/odi.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanno C.M., de Oliveira J.A., Garcia J.F., Roth H., Weber B.H. Twenty-year follow-up of a familial case of PTH1R-associated primary failure of tooth eruption. Am J Orthod Dentofac Orthop. 2017;151:598–606. doi: 10.1016/j.ajodo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Karagiannis P., Takahashi K., Saito M., Yoshida Y., Okita K., Watanabe A., et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol Rev. 2019;99:79–114. doi: 10.1152/physrev.00039.2017. [DOI] [PubMed] [Google Scholar]
- 58.Frazier-Bowers S.A., Hendricks H.M., Wright J.T., Lee J., Long K., Dibble C.F., et al. Novel mutations in PTH1R associated with primary failure of eruption and osteoarthritis. J Dent Res. 2014;93:134–139. doi: 10.1177/0022034513513588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Izumida E., Suzawa T., Miyamoto Y., Yamada A., Otsu M., Saito T., et al. Functional analysis of PTH1R variants found in primary failure of eruption. J Dent Res. 2020;99:429–436. doi: 10.1177/0022034520901731. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura K., Ohtaka M., Takada H., Kurisaki A., Tran N.V.K., Tran Y.T.H., et al. Simple and effective generation of transgene-free induced pluripotent stem cells using an auto-erasable Sendai virus vector responding to microRNA-302. Stem Cell Res. 2017;23:13–19. doi: 10.1016/j.scr.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Wise G.E., King G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi T., Tomoyasu Y., Shirota T., Takahashi M., Nakano H., Kurabayashi H., et al. Craniofacial and dental characteristics of patients with primary failure of tooth eruption in Japanese. Hosp Dent. 2011;23:11–15. [Google Scholar]
- 64.Aziz S., Hermann N.V., Dunø M., Risom L., Daugaard-Jensen J., Kreiborg S. Primary failure of eruption of teeth in two siblings with a novel mutation in the PTH1R gene. Eur Arch Paediatr Dent. 2019;20:295–300. doi: 10.1007/s40368-018-00410-8. [DOI] [PubMed] [Google Scholar]
- 65.Karaplis A.C., He B., Nguyen M.T., Young I.D., Semeraro D., Ozawa H., et al. Inactivating mutation in the human parathyroid hormone receptor type 1 gene in Blomstrand chondrodysplasia. Endocrinology. 1998;139:5255–5258. doi: 10.1210/endo.139.12.6522. [DOI] [PubMed] [Google Scholar]
- 66.Stellzig-Eisenhauer A., Decker E., Meyer-Marcotty P., Rau C., Fiebig B.S., Kress W., et al. Primary failure of eruption (PFE)--clinical and molecular genetics analysis. J Orofac Orthop. 2010;71:6–16. doi: 10.1007/s00056-010-0908-9. [DOI] [PubMed] [Google Scholar]
- 67.Couvineau A., Wouters V., Bertrand G., Rouyer C., Gérard B., Boon L.M., et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. 2008;17:2766–2775. doi: 10.1093/hmg/ddn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerreiro R., Brás J., Batista S., Pires P., Ribeiro M.H., Almeida M.R., et al. Pseudohypoparathyroidism type I-b with neurological involvement is associated with a homozygous PTH1R mutation. Genes Brain Behav. 2016;15:669–677. doi: 10.1111/gbb.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schipani E., Langman C., Hunzelman J., Le Merrer M., Loke K.Y., Dillon M.J., et al. A novel parathyroid hormone (PTH)/PTH-related peptide receptor mutation in Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 1999;84:3052–3057. doi: 10.1210/jcem.84.9.6000. [DOI] [PubMed] [Google Scholar]
- 70.Rhoads S.G., Hendricks H.M., Frazier-Bowers S.A. Establishing the diagnostic criteria for eruption disorders based on genetic and clinical data. Am J Orthod Dentofac Orthop. 2013;144:194–202. doi: 10.1016/j.ajodo.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Schipani E., Langman C.B., Parfitt A.M., Jensen G.S., Kikuchi S., Kooh S.W., et al. Constitutively activated receptors for parathyroid hormone and parathyroid hormone-related peptide in Jansen's metaphyseal chondrodysplasia. N Engl J Med. 1996;335:708–714. doi: 10.1056/NEJM199609053351004. [DOI] [PubMed] [Google Scholar]
- 72.Bastepe M., Raas-Rothschild A., Silver J., Weissman I., Wientroub S., Jüppner H., et al. A form of Jansen's metaphyseal chondrodysplasia with limited metabolic and skeletal abnormalities is caused by a novel activating parathyroid hormone (PTH)/PTH-related peptide receptor mutation. J Clin Endocrinol Metab. 2004;89:3595–3600. doi: 10.1210/jc.2004-0036. [DOI] [PubMed] [Google Scholar]
- 73.Savoldi G., Izzi C., Signorelli M., Bondioni M.P., Romani C., Lanzi G., et al. Prenatal presentation and postnatal evolution of a patient with Jansen metaphyseal dysplasia with a novel missense mutation in PTH1R. Am J Med Genet A. 2013;161A:2614–2619. doi: 10.1002/ajmg.a.36115. [DOI] [PubMed] [Google Scholar]
- 74.Grippaudo C., Cafiero C., D'Apolito I., Ricci B., Frazier-Bowers S.A. Primary failure of eruption: clinical and genetic findings in the mixed dentition. Angle Orthod. 2018;88:275–282. doi: 10.2319/062717-430.1. [DOI] [PMC free article] [PubMed] [Google Scholar]