Abstract
Objective
To evaluate the association of community-level social vulnerability with COVID-19 vaccine hesitancy and vaccination among pregnant and postpartum individuals.
Methods
Prospective cohort study assessing COVID-19 vaccine hesitancy among pregnant and postpartum individuals. We performed a baseline survey on COVID-19 vaccine hesitancy from 03/22/21 to 04/02/21, and a follow-up survey on COVD-19 vaccination status 3- to 6-months later. The primary exposure was the Centers for Disease Control and Prevention SVI (Social Vulnerability Index), measured in quartiles. Higher SVI quartiles indicated greater community-level social vulnerability with the lowest quartile (quartile 1) as the referent group. The primary outcome was COVID-19 vaccine hesitancy on the baseline survey (uncertainty or refusal of the vaccine), and the secondary outcome was self-report of not being vaccinated (unvaccinated) for COVID-19 on the follow-up survey.
Results
Of 456 assessed individuals, 46% reported COVID-19 vaccine hesitancy on the baseline survey; and of 290 individuals (290/456, 64%) who completed the follow-up survey, 48% (140/290) were unvaccinated. The frequency of baseline vaccine hesitancy ranged from 25% in quartile 1 (low SVI) to 68% in quartile 4 (high SVI), and being unvaccinated at follow-up ranged from 29% in quartile 1 to 77% in quartile 4. As social vulnerability increased, the risk of COVID-19 vaccine hesitancy at baseline increased (quartile 2 aRR (adjusted relative risk): 1.46; 95% CI:0.98 to 2.19; quartile 3 aRR: 1.86; 95% CI:1.28 to 2.71; and quartile 4 aRR: 2.24; 95% CI:1.56 to 3.21), as did the risk of being unvaccinated at follow-up (quartile 2 aRR: 1.00; 95% CI:0.66 to 1.51; quartile 3 aRR: 1.68; 95% CI:1.17 to 2.41; and quartile 4 aRR: 1.82; 95% CI:1.30 to 2.56).
Conclusions
Pregnant and postpartum individuals living in an area with higher community-level social vulnerability were more likely to report COVID-19 vaccine hesitancy and subsequently to be unvaccinated at follow-up.
Keywords: COVID-19, Maternal immunization, Pregnancy, Vaccine uptake, Vaccine hesitancy, Social vulnerability
1. Introduction
Vaccination in pregnancy is a public health priority, as it is an evidence-based strategy to prevent maternal and neonatal morbidity and mortality [1], [2], [3], [4], [5]. Over the past decade, vaccination rates among pregnant and postpartum individuals have remained inadequate with only half of all pregnant individuals being vaccinated for pertussis and influenza annually [6], [7], [8]. During the current COVID-19 pandemic, >50% of pregnant and postpartum individuals have reported vaccine hesitancy [9], [10], [11]. Reasons for vaccine hesitancy in pregnancy are multifactorial and include inconsistent and infrequent provider recommendations, patient perceptions of lack of safety and efficacy data, and barriers to being able to access vaccines [8], [9], [11], [12], [13], [14]. Current guidelines recommend COVID-19 vaccination in pregnancy [15], [16], [17]. COVID-19 infection in pregnancy increases the risk of preeclampsia, fetal growth restriction, and severe maternal morbidity and mortality [18], [19], [20], [21], [22]. While pregnant individuals were initially excluded from COVID-19 vaccine trials, data have since demonstrated the safety and efficacy of COVID-19 vaccines in pregnancy for the mother and fetus [23], [24], [25].
Pregnant individuals who experience a higher burden of adverse social determinants of health are less likely to be vaccinated against influenza and tetanus-diphtheria-acellular pertussis [26], [27], [28], [29], and whether this holds for COVID-19 remains to be studied. Social determinants of health include multiple aspects of the built environment and social setting that impact access and uptake of healthcare services, including access to safe housing, transportation, and neighborhoods; experiences of racism, discrimination, and violence; availability of education, job opportunities, and income; access to nutritious foods and physical activity opportunities; and living in a space with clean air and water [30]. The Social Vulnerability Index (SVI) is a standardized, publicly available, and online index available from the U.S. Centers for Disease Control and Prevention (CDC). It can be used to evaluate community-level social determinants of health based on an individual’s residential location. The SVI provides an overall score as well as four domain scores: socioeconomic status, household composition and disability, minority status and language, and housing type and transportation [31], [32], [33].
The current study evaluated the association between community-level social vulnerability and COVID-19 vaccine hesitancy at baseline and not being vaccinated (unvaccinated) at follow-up among pregnant and postpartum individuals.
2. Methods
2.1. Study setting and patients
We conducted a prospective study of COVID-19 vaccine hesitancy at baseline and subsequent COVID-19 vaccination at follow-up among pregnant and postpartum individuals receiving prenatal and postpartum care at a Midwestern tertiary care academic medical center. Inclusion criteria at enrollment were pregnancy with a confirmed intrauterine gestation or postpartum status < 10 weeks from delivery, English speaking, and receiving prenatal or postpartum care at our center. This study was approved by The Ohio State University Institutional Review Board (ID#: 2021H0023; date: 02/23/21). As the follow-up survey was not planned at the time of the initial assessment, informed consent was obtained at both the baseline and follow-up surveys. We followed Equator (Enhancing the QUAlity and Transparency Of health Research) Network [34] and STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines [35] for the baseline and follow-up surveys, respectively.
As previously described [11], the baseline survey of COVID-19 vaccine hesitancy occurred from March 22, 2021 to April 2, 2021.This was conducted concurrent with state-level guidance making COVID-19 vaccination available for pregnant and postpartum individuals [36], data describing the increased risk of severe COVID-19 infection in pregnancy [18], [19], [20], [21], [22], as well as initial professional guidelines endorsing COVID-19 vaccination in the peripartum period through shared decision making [15], [16]. At the time of the baseline survey, participants may have been recently vaccinated, but this was not assessed. The written baseline survey was completed in person during a scheduled prenatal or postpartum visit. Participants received compensation of five dollars for completing the baseline survey.
The follow-up survey of self-reported COVID-19 vaccination occurred from June 29, 2021 to November 20, 2021, at which time COVID-19 vaccines were widely available and before the Omicron variant was reported [23], [24], [25]. The follow-up survey was conducted following professional guidelines recommending vaccination for pregnant and postpartum individuals, and growing data about the safety and efficacy of COVID-19 vaccination in pregnancy [23]. All previously enrolled participants were contacted three to six months after the baseline survey by either phone or email. If participants did not respond after two phone calls, they were sent an email message. Those who did not respond to the email then received a final phone call. An identical survey by email or telephone was administered in English and was designed to be completed in five minutes.
2.2. Data collection
he baseline survey assessed socio-demographic characteristics and perceptions about vaccination for COVID-19, including willingness, barriers, and facilitators. At follow-up, self-reported COVID-19 vaccination was ascertained (yes/no) since baseline. Participant addresses as well as clinical data were manually abstracted from the electronic health record (EHR). Survey questions were adapted from the CDC adult internet panel survey to assess vaccination in pregnancy [37], the World Health Organization Vaccine Hesitancy Determinants Matrix [38], and the “3 Cs” model (complacency, convenience, and confidence) as outlined by the World Health Organization Vaccine Communications Working Group [39], [40].
The primary exposure was community-level social vulnerability as measured by the CDC 2018 SVI. Participant addresses at baseline were geocoded using ArcGIS and then linked at the census tract level to the SVI [41]. The SVI, which ranged from 0 to 1, was assessed as quartiles. Higher quartile values indicated that the individual resided in an area with greater community-level social vulnerability, compared to the reference quartile 1. We then secondarily assessed each of the four SVI domains (socioeconomic status, household composition and disability, minority status and language, and housing type and transportation) (Table 1 ) [31], [32], [33].
Table 1.
Social vulnerability Index (SVI) domains.
| SVI Domain | Components of SVI domain |
|---|---|
| Overall | |
| Social Vulnerability Index (SVI) | Composite of Domains 1–4 |
| Domains (1–4) | |
| 1: Socioeconomic status | Includes unemployment, education (<high school), and income (poverty) status |
| 2: Household composition and disability | Includes households with single parent, <17 or > 65 years of age, or > 5 years of age with a disability |
| 3: Minority status and language | Includes minority status, and English fluency |
| 4: Housing type and transportation | Includes assessment of multi-unit structures, mobile homes, crowding, no vehicle, and group quarters |
The outcome at baseline was COVID-19 vaccine hesitancy as defined per the World Health Organization Strategic Advisory Group of Experts (SAGE) on Vaccine Hesitancy (i.e. uncertainty or refusal of vaccination despite availability of vaccination services) [40]. The outcome at follow-up was self-report of not being vaccinated for COVID-19 (i.e., unvaccinated). The primary outcome for the follow-up survey was self-report of being unvaccinated because by this time the standard of care was recommended vaccination for pregnant and postpartum individuals, compared to the baseline survey when vaccination had first become available and guidelines were evolving for this population. Vaccination was defined as self-reported receipt of at least one dose of any available COVID-19 vaccine.
Covariates included age, self-reported race and ethnicity, education, current employment status, health insurance, and substance use in pregnancy. These factors were assessed because they have been found to be associated with vaccine hesitancy [38]. Race and ethnicity were self-reported by participants, and was categorized using criteria as outlined by U.S. Vital Statistics [42]. In addition, the survey assessed participant perceptions that were associated with vaccine hesitancy, including: counseling about risks and benefits of vaccination by the obstetrical healthcare provider and the potential benefits of vaccination to the mother and infant [39]. Clinical characteristics inclusive of chronic comorbid conditions that have been associated with increased risk of COVID-19 complications and body mass index (BMI) were manually abstracted from the EHR.
2.3. Statistical analysis
We compared participant characteristics and vaccine perceptions by SVI quartile in the study sample at baseline. We compared categorical variables with a chi-square test and continuous variables with one-way analysis of variance (ANOVA). Modified Poisson regression with robust error variance were used to estimate the associations between SVI quartile score overall and by domain, with baseline COVID-19 vaccine hesitancy and follow-up COVID-19 vaccination. To do so we calculated unadjusted and adjusted risk ratios (RR, aRRs) and 95% confidence intervals (CI). Confounding variables were selected based on prior studies of vaccination in pregnancy and a directed acyclic graph (DAG). The final model for baseline vaccine hesitancy was adjusted for age (<25, 25–30, >30–35, >35 years), race/ethnicity, parity (0, 1, 2 or more), trimester of pregnancy at time of enrollment (1st, 2nd, 3rd, postpartum) and chronic comorbid conditions (0, 1 or more). In addition, the model for vaccination status at follow-up also adjusted for pregnancy status at follow-up (yes/no) and time from baseline to follow-up survey in weeks (continuous). Missing data imputation was not performed as > 99% of participants had complete data at both time points. Because not all participants who completed the baseline survey completed the follow-up survey, we repeated the analysis between SVI and vaccination status at follow-up under a best and worst case scenario, in which all individuals who were lost-to-follow-up were categorized as either vaccinated or unvaccinated, respectively. All statistical analyses were performed using Stata (StataCorp, version 16.1, College Station, TX).
3. Results
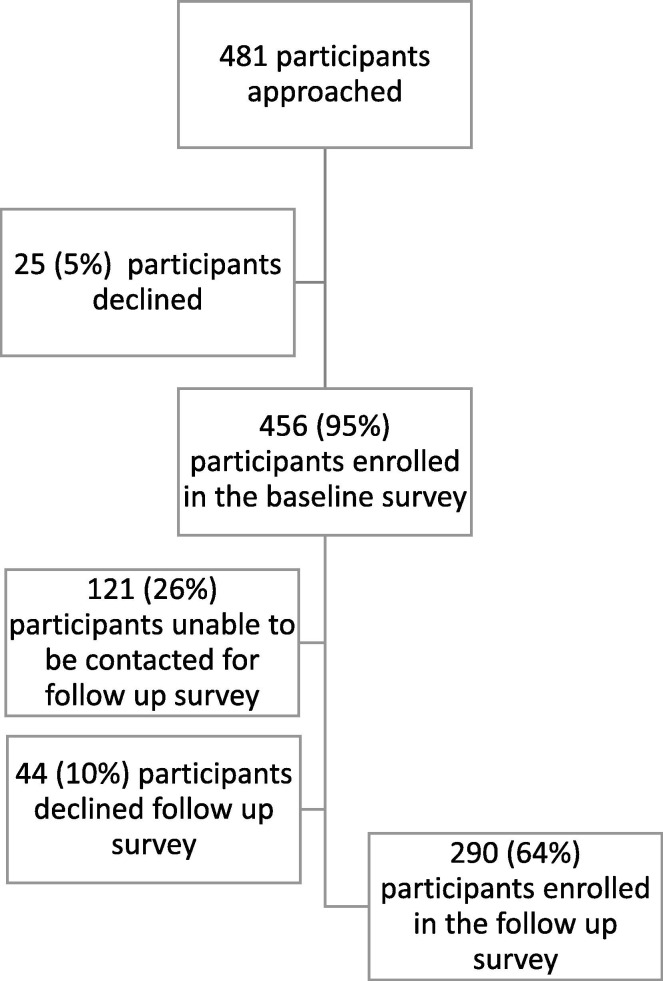
Four-hundred and eighty-one individuals were approached for participation in the baseline survey, of whom 456 individuals (95% pregnant, 5% postpartum) enrolled. Those who declined to enroll (n = 25) had a higher SVI score compared to those who did (mean: 0.63 vs. 0.44; p < 0.01). Of the 456 individuals enrolled, 335 (73%) were able to be contacted for the follow-up survey, of whom 290 (64%) consented to participate (24% pregnant; 76% postpartum) (Appendix Fig. A1 .). Those who did not follow-up (n = 165) had a higher SVI score compared to those who did (mean: 0.51 vs. 0.40; p < 0.001). The median time from enrollment to follow-up was 18 weeks (interquartile range, IQR: 17, 31). The mean age was 29.3 ± 5.4 years, 47% Medicaid beneficiaries, 27% self-identified as non-Hispanic Black, and 52% had a bachelor’s degree or greater (Table 2 ).
Fig. A1.
Flow diagram of participant enrollment in baseline and follow-up survey.
Table 2.
Patient demographic and clinical characteristics by quartile of overall community-level social vulnerability.
| At baseline (n = 456) |
Social Vulnerability Index (SVI), by quartile |
p-value1 | ||||
|---|---|---|---|---|---|---|
|
Overall |
1st quartile (<25%) |
2nd quartile (≥25-49%) |
3rd quartile (≥50-74%) |
4th quartile (≥75-100%) |
||
| N = 456 | n = 114 | n = 111 | n = 116 | n = 115 | ||
| Social Vulnerability Index (SVI) | 0.44 ± 0.30 | 0.07 ± 0.05 | 0.27 ± 0.08 | 0.57 ± 0.08 | 0.83 ± 0.08 | <0.01 |
| Age, years | 29.3 ± 5.4 | 31.2 ± 4.9 | 30.4 ± 5.2 | 20.1 ± 4.9 | 29.5 ± 5.5 | <0.01 |
| Race and ethnicity | ||||||
| Non-Hispanic White Non-Hispanic Black Hispanic None of the above |
271 (59.4) 122 (26.8) 33 (7.2) 30 (6.6) |
80 (70.2) 14 (12.3) 6 (5.3) 14 (12.3) |
78 (70.3) 18 (7.2) 8 (7.2) 7 (6.3) |
75 (64.7) 33 (28.5) 4 (3.5) 4 (3.5) |
38 (33.0) 57 (49.6) 15 (13.0) 5 (4.4) |
<0.01 |
| Education (n = 452) | ||||||
| High school or less Some college Bachelors degree Advanced degree |
130 (28.8) 89 (19.7) 145 (32.1) 88 (19.5) |
10 (8.8) 13 (11.4) 48 (42.1) 43 (37.7) |
17 (15.6) 22 (20.2) 44 (40.4) 26 (23.9) |
47 (40.5) 21 (18.1) 34 (29.3) 14 (12.1) |
56 (49.6) 33 (29.2) 19 (16.8) 5 (4.4) |
<0.01 |
| Parity | ||||||
| 0 1 2 or more |
167 (36.6) 142 (31.1) 147 (32.2) |
49 (43.0) 41 (36.0) 24 (21.1) |
41 (36.9) 33 (29.7) 37 (33.3) |
34 (29.3) 38 (32.8) 44 (37.9) |
43 (37.4) 30 (26.1) 42 (36.5) |
0.08 |
| Employed | 315 (69.1) | 96 (84.2) | 78 (70.3) | 74 (63.8) | 67 (58.3) | <0.01 |
| Health insurance, Medicaid | 215 (47.2) | 28 (24.6) | 39 (35.1) | 68 (58.6) | 80 (69.6) | <0.01 |
| Substance use, current | ||||||
| Tobacco Drug use2 |
42 (9.2) 48 (10.5) |
3 (2.7) 7 (6.1) |
6 (5.4) 9 (8.1) |
18 (15.5) 17 (14.7) |
15 (13.0) 15 (13.0) |
<0.01 0.11 |
| Gestational age, weeks | ||||||
| First trimester Second trimester Third trimester Fourth trimester (postpartum) |
157 (34.4) 231 (50.7) 47 (10.3) 21 (4.6) |
40 (35.1) 58 (50.9) 12 (10.5) 4 (3.5) |
45 (40.5) 53 (47.8) 9 (8.1) 4 (3.6) |
36 (31.0) 57 (49.1) 15 (12.9) 8 (6.9) |
36 (31.3) 63 (54.8) 11 (9.6) 5 (4.4) |
0.76 |
| Body mass index, kg/m2 | 31.6 ± 8.17 | 28.1 ± 6.41 | 31.7 ± 7.97 | 32.7 ± 8.50 | 33.9 ± 8.56 | <0.01 |
| Chronic comorbid conditions | ||||||
| None 1 or more |
136 (29.8) 320 (70.2) |
50 (43.9) 64 (56.1) |
34 (30.6) 77 (69.4) |
26 (22.4) 90 (77.6) |
26 (22.6) 89 (77.4) |
<0.01 |
| Influenza vaccination in the current season | 263 (57.7) | 94 (82.5) | 89 (80.2) | 82 (71.3) | 73 (63.5) | <0.01 |
| Vaccination discussion with provider about COVID-19 (n = 450) | 227 (50.4) | 79 (69.9) | 74 (67.3) | 42 (36.5) | 32 (28.6) | <0.01 |
| Concerned about contracting COVID-19 and impact to self and pregnancy, 1 to 10 | 5.7 ± 3.0 | 6.3 ± 2.9 | 5.8 ± 2.8 | 5.2 ± 3.0 | 5.3 ± 3.1 | 0.01 |
| Benefit of COVID-19 vaccination (n = 415)3 | ||||||
| Mother Baby Both |
93 (22.4) 7 (1.7) 315 (75.9) |
23 (21.3) 3 (2.8) 82 (75.9) |
25 (23.8) 0 (-) 80 (76.2) |
24 (23.3) 3 (2.9) 76 (73.8) |
21 (21.2) 1 (1.0) 77 (77.8) |
0.66 |
Abbreviations: SD: standard deviation; SVI: social vulnerability index.
Data presented as mean ± SD or n (%).
Catagorical variables compared using an overall chi square test and continuous variables compared using one-way analysis of variance (ANOVA).
Reported drug use inclusive of illicit drugs and prescribed opioids.
When asked if the COVID-19 vaccination was for the benefit of the mother, baby, or both.
When the SVI was stratified by quartile, mean SVI score increased from quartile 1 (low SVI) to quartile 4 (high SVI) (quartile 1: 0.12; quartile 2: 0.43; quartile 3: 0.71; and quartile 4: 0.88). And for those with follow-up, mean SVI score at baseline increased from quartile 1 to quartile 4 (1: 0.07; 2: 0.27; 3: 0.57; and 4: 0.83). Individuals in the highest SVI quartile were more likely to identify as non-Hispanic Black, have lower educational attainment, be unemployed, have public insurance, use tobacco, be living with obesity, and have medical comorbidities compared to those in the lowest SVI quartile (overall p < 0.01) (Table 2). Individuals in the highest SVI quartile were less likely to have received the influenza vaccine during the current influenza season, to have discussed any vaccination in pregnancy with their obstetric provider, and to be concerned about contracting COVID-19 in pregnancy (overall p < 0.01).
On the baseline survey, 46% (95% CI: 42% to 51%) reported COVID-19 vaccine hesitancy. The frequency of vaccine hesitancy increased as the SVI increased. In quartile 1 (low SVI), 25% of individuals reported vaccine hesitancy, 38% in quartile 2, 55% in quartile 3, and 68% in quartile 4 (high SVI) (Table 3 ). Similarly, by each SVI domain, the frequency of vaccine hesitancy increased from 24% to 73% from quartile 1 to 4 for socioeconomic status, 28% to 70% for household composition and disability, 33% to 61% for housing type and transportation, but not for minority status and language.
Table 3.
Association between community-level social vulnerability and COVID-19 vaccine hesitancy at baseline SVI Domain.
|
Frequency n (%) |
Unadjusted risk ratio, RR (95% CI) | Adjusted risk ratio, aRR (95% CI)1,2,3,4 | |
|---|---|---|---|
| Overall | 212/456 (46.5) | ||
| Social Vulnerability Index (SVI) | |||
| Quartile 15 Quartile 2 Quartile 3 Quartile 4 |
28/114 (24.6) 42/111 (37.8) 64/116 (55.2) 78/115 (67.8) |
1.00 1.56 (1.03 to 2.34) 2.29 (1.58 to 3.31) 2.83 (1.99 to 4.04) |
1.00 1.46 (0.98 to 2.19) 1.86 (1.28 to 2.71) 2.24 (1.56 to 3.21) |
| Domains (1–4) | |||
| 1: Socioeconomic status | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
28/115 (24.4) 41/113 (36.3) 61/115 (53.0) 82/113 (72.6) |
1.00 1.53 (1.01 to 2.30) 2.22 (1.53 to 3.22) 3.05 (2.15 to 4.32) |
1.00 1.43 (0.95 to 2.14) 1.80 (1.23 to 2.63) 2.39 (1.66 to 3.43) |
| 2: Household composition and disability | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
33/118 (28.0) 39/114 (34.2) 62/113 (54.9) 78/111 (70.3) |
1.00 1.25 (0.84 to 1.84) 1.99 (1.4 to 2.79) 2.56 (1.85 to 3.52) |
1.00 1.22 (0.83 to 1.79) 1.76 (1.26 to 2.47) 2.05 (1.48 to 2.85) |
| 3: Minority status and language | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
59/114 (51.8) 41 (36.0) 45 (39.5) 67 (58.8) |
1.00 0.67 (0.49 to 0.91) 0.76 (0.57 to 1.01) 1.12 (0.89 to 1.42) |
1.00 0.63 (0.48 to 0.85) 0.73 (0.54 to 0.97) 1.00 (0.79 to 1.27) |
| 4: Housing type and transportation | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
38/115 (33.0) 51/113 (45.1) 54/114 (47.4) 69/114 (60.5) |
1.00 1.44 (1.02 to 2.03) 1.51 (1.08 to 2.12) 1.93 (1.41 to 2.64) |
1.00 1.30 (0.94 to 1.80) 1.37 (1.00 to 1.89) 1.65 (1.22 to 2.23) |
Abbreviations: SVI: social vulnerability index, RR: risk ratio, aRR: adjusted risk ratio, CI: confidence interval.
Model adjusted for: age, race and ethnicity, parity, trimester of pregnancy at baseline, and chronic comorbidities.
Unadjusted and adjusted risk ratios calculated using modified Poisson regression with robust error variance.
N = 456 in the unadjusted model and 453 in the adjusted model.
aRR per every one unit increase in SVI quartile.
Mean SVI value (0 to 1) by quartile among individuals with baseline data: <25%, 0.12; ≥25-49%, 0.43; ≥50-74%, 0.71; and ≥ 75–100%, 0.88.
At follow-up, 48% (95% CI: 42% to 53%) reported that they were not vaccinated for COVID-19. The frequency of being unvaccinated for COVID-19 increased as the SVI increased. In quartile 1, 29% of individuals reported being unvaccinated, 32% in quartile 2, 64% in quartile 3, and 77% in quartile 4 (Table 4 ). Similarly, by each SVI domain, the frequency of being unvaccinated increased from 28% to 82% from quartile 1 to 4 for socioeconomic status, 27% to 73% for household composition and disability, 39% to 69% for minority status and language, and 37% to 65% for housing type and transportation.
Table 4.
Association between community-level social vulnerability and being unvaccinated for COVID-19 at follow-up.
| SVI Domain |
Frequency N (%) |
Unadjusted risk ratio, RR (95% CI) | Adjusted risk ratio, aRR (95% CI)1,2,3,4 |
|---|---|---|---|
| Overall | 139/290 (47.9) | ||
| Social Vulnerability Index (SVI) | |||
| Quartile 15 Quartile 2 Quartile 3 Quartile 4 |
26/89 (29.2) 23/73 (31.5) 43/67 (64.2) 47/61 (77.1) |
1.00 1.32 (0.92 to 1.89) 1.16 (0.78 to 1.71) 1.73 (1.23 to 2.43) |
1.00 1.00 (0.66 to 1.51) 1.68 (1.17 to 2.41) 1.82 (1.30 to 2.56) |
| Domains (1–4) | |||
| 1: Socioeconomic status | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
24/87 (27.6) 23/75 (30.7) 39/63 (61.9) 53/65 (81.5) |
1.00 1.11 (0.68 to 1.80) 2.19 (1.47 to 3.26) 2.95 (2.06 to 4.23) |
1.00 1.09 (0.71 to 1.68) 1.73 (1.19 to 2.52) 2.16 (1.53 to 3.04) |
| 2: Household composition and disability | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
24/88 (27.3) 30/78 (38.5) 41/64 (64.1) 44/60 (73.3) |
1.00 1.41 (0.90 to 2.19) 2.30 (1.55 to 3.41) 2.68 (1.84 to 3.90) |
1.00 1.35 (0.92 to 1.99) 1.93 (1.37 to 2.72) 1.83 (1.28 to 2.62) |
| 3: Minority status and language | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
27/69 (39.1) 34/77 (44.2) 31/76 (40.8) 47/68 (69.1) |
1.00 1.13 (0.76 to 1.68) 1.06 (0.71 to 1.60) 1.80 (1.28 to 2.54) |
1.00 0.90 (0.63 to 1.27) 0.93 (0.65 to 1.33) 1.25 (0.92 to 1.69) |
| 4: Housing type and transportation | |||
| Quartile 1 Quartile 2 Quartile 3 Quartile 4 |
31/83 (37.4) 40/80 (50.0) 31/70 (44.3) 37/57 (64.9) |
1.00 1.32 (0.92 to 1.89) 1.16 (0.78 to 1.71) 1.73 (1.23 to 2.43) |
1.00 1.30 (0.96 to 1.78) 1.09 (0.77 to 1.55) 1.42 (1.05 to 1.93) |
Abbreviations: SVI: social vulnerability index, RR: risk ratio, aRR: adjusted risk ratio, CI: confidence interval.
Model adjusted for: age, race and ethnicity, parity, trimester of pregnancy at baseline, chronic comorbidities, time from baseline to follow-up assessment, and pregnant at follow-up.
Unadjusted and adjusted risk ratios calculated using modified Poisson regression with robust error variance.
N = 290 in the unadjusted model and 288 in the adjusted model.
aRR per every one unit increase in SVI quartile.
Mean SVI (0 to 1) by quartile among individuals with follow-up data: <25%, 0.07; ≥25-49%, 0.27; ≥50-74%, 0.57; and ≥ 75–100%, 0.83.
In adjusted analyses, progressing from quartile 2 to quartile 4 (higher SVI) in comparison to quartile 1 (low SVI, reference), the risk of COVID-19 vaccine hesitancy increased (quartile 2 aRR: 1.46; 95% CI: 0.98 to 2.19; quartile 3 aRR: 1.86; 95% CI: 1.28 to 2.71; and quartile 4 aRR: 2.24; 95% CI: 1.56 to 3.21) (Table 3). Similarly, progressing from quartile 2 to quartile 4 in comparison to quartile 1, the risk of being unvaccinated for COVID-19 at follow-up increased (quartile 2 aRR: 1.00; 95% CI 0.66 to 1.51; quartile 3 aRR: 1.68; 95% CI: 1.17 to 2.41; and quartile 4 aRR: 1.82; 95% CI: 1.30 to 2.56) (Table 4).
These results generally held for 3 of 4 SVI domains for vaccine hesitancy at baseline. For the socioeconomic status domain, progressing from quartile 2 to quartile 4, the risk of COVID-19 vaccine hesitancy increased (quartile 2 aRR: 1.43; 95% CI: 0.95 to 2.14; quartile 3 aRR: 1.80; 95% CI: 1.23 to 2.63; and quartile 4 aRR: 2.39; 95% CI: 1.66 to 3.43), as it did for the household composition and disability domain (quartile 2 aRR: 1.22; 95% CI: 0.83 to 1.79; quartile 3 aRR: 1.76; 95% CI: 1.26 to 2.47; and quartile 4 aRR: 2.05; 95% CI: 1.48 to 2.85) and the housing type and transportation domain (quartile 2 aRR: 1.30; 95% CI: 0.94 to 1.80; quartile 3 aRR: 1.37; 95% CI: 1.00 to 1.89; and quartile 4 aRR: 1.65; 95% CI: 1.22 to 2.23), but not the minority status and language domain (Table 3).
Similarly, at follow-up, the risk of being unvaccinated for COVID-19 increased from quartile 2 to quartile 4 for the socioeconomic status domain (quartile 2 aRR: 1.09; 95% CI: 0.71 to 1.68; quartile 3 aRR: 1.73; 95% CI: 1.19 to 2.52; and quartile 4 aRR: 2.16; 95% CI: 1.53 to 3.04), and the household composition and disability domain (quartile 2 aRR: 1.35; 95% CI: 0.92 to 1.99; quartile 3 aRR: 1.93; 95% CI: 1.37 to 2.72; and quartile 4 aRR: 1.83; 95% CI: 1.28 to 2.62) (Table 4). For the domain of housing type and transportation, the risk of being unvaccinated increased from quartile 3 to quartile 4 (quartile 3 aRR: 1.09; 95% CI: 0.77 to 1.55; and quartile 4 aRR: 1.42; 95% CI: 1.05 to 1.93). There was no association between unvaccinated status and the minority status and language domain.
In sensitivity analysis, when the above analyses were repeated under a best and worst case scenario to account for loss-to-follow-up, the association between increasing SVI and the risk of being unvaccinated for COVID-19 held when all participants who did not follow-up were classified as unvaccinated (worst case scenario), as well as when they were classified as vaccinated (best case scenario) (Appendix Table A1 ).
Table A1.
Association between community-level social vulnerability and being unvaccinated for COVID-19 at follow-up.
|
Best case1at follow-up aRR (95% CI)3,4,5,6 |
Worst case2at follow-up aRR (95% CI)3,4,5,6 |
|
|---|---|---|
| Overall | ||
| Social Vulnerability Index (SVI) | ||
| Quartile 1 | 1.00 | 1.00 |
| Quartile 2 | 1.00 (0.66 to 1.51) | 1.00 (0.66 to 1.51) |
| Quartile 3 | 1.68 (1.17 to 2.41) | 1.68 (1.17 to 2.41) |
| Quartile 4 | 1.82 (1.30 to 2.56) | 1.82 (1.30 to 2.56) |
Abbreviations: SVI: social vulnerability index, aRR: adjusted risk ratio, CI: confidence interval.
Best case scenario: individuals who did not follow-up were categorized as vaccinated.
Worst case scenario: individuals who did not follow-up were categorized as unvaccinated.
Model adjusted for: age, parity, race and ethnicity, trimester of pregnancy at baseline, chronic comorbidities; however not adjusted for follow-up covariates, which were not available for those without follow-up (pregnancy status at follow-up, and time to follow-up).
Adjusted risk ratios calculated using modified Poisson regression with robust error variance.
N = 288 in the adjusted model.
aRR per every one unit increase in SVI quartile.
4. Discussion
Pregnant and postpartum individuals living in areas with higher community-level social vulnerability were more likely to report baseline COVID-19 vaccine hesitancy and to be unvaccinated for COVID-19 at follow-up. These findings suggest that experiencing a higher burden of adverse community-level social determinants of health may be associated with persistent COVID-19 vaccine hesitancy in the peripartum period.
In the current study, vaccine hesitancy and follow-up vaccination were assessed at two critical time points: when vaccines first became available for pregnant individuals statewide and then, when vaccines were widely available. Follow-up was at a time when professional guidelines recommended vaccination for all pregnant and postpartum individuals [15], [16], [17], and after publication of population-level data demonstrating the safety of vaccination in pregnancy for both the mother and infant [23], [24], [25]. The findings of the current study are consistent with prior studies that found patient socio-demographic characteristics, such as receipt of public health insurance, lower education attainment, and non-Hispanic Black race, were associated with higher COVID-19 vaccine hesitancy in pregnant [9], [11] and non-pregnant populations [43], [44], [45]. These associations between individual socio-demographic risk factors and vaccine hesitancy have also been previously identified for influenza vaccination in pregnancy [12], [26], [29], [46]. However, the current study focused on a community-level and standardized metric of social determinants of health as opposed to individual socio-demographic risk factors. Outside of pregnancy, higher SVI scores have been associated with an increased risk of being unvaccinated for influenza [47], [48] and COVID-19 [48], [49], [50].
The SVI is an online tool available from the CDC and is readily accessible to healthcare providers across the U.S. Further studies are needed to understand how knowledge of the SVI during the peripartum period could be used to improve vaccine delivery and outcomes in this population. More broadly, whether the SVI could be used as a tool to identify communities in which pregnant and postpartum individuals are at the greatest risk of not receiving recommended vaccinations remains to be studied. Current public health efforts for identifying prevention strategies for COVID-19 are increasingly being implemented based on location and local disease burden. It is possible that interventions to increase vaccination and decrease vaccine hesitancy could be targeted to those pregnant individuals at the greatest risk of not being vaccinated using the SVI as a measure of community-level social determinants of health. However, the efficacy of interventions that address community-level social determinants of health utilizing the SVI to decrease vaccine hesitancy in the peripartum period needs to be studied further. Such programs may include targeting community-based programs to increase vaccination uptake (i.e., mobile clinics, awareness campaigns) to individuals who live in communities with high SVI, and identifying individuals with a high SVI in the EHR for further provider vaccination counseling as part of prenatal care.
There are several limitations to note. Selection bias is possible. Although we had high enrollment (95%) for the initial survey, the follow-up rate was 64%. We performed a sensitivity analysis to examine the robustness of study findings with regards to potential selection bias. While we assessed for prior COVID-19 infection in the household, we did not assess whether the individuals had prior COVID-19 infection. Furthermore, baseline factors associated with vaccine hesitancy, including prior personal exposure to COVID-19 and provider recommendations, were assessed only at enrollment in the current study. These factors may evolve over time given the changing epidemiology of COVID-19 as well as clinical guidelines. Social desirability bias is also possible at follow-up because those who were vaccinated may have been more likely to enroll and or those who did enroll may have been more likely to say they were vaccinated even if they were not. However, self-report of vaccination status has been shown to be accurate outside of pregnancy [44]. Differential follow-up by vaccination status is possible, as those who reported not being vaccinated at 3 months were not reassessed again at 6 months (i.e., at the end of the study period). We did not assess whether documented provider counseling in the EHR varied with patient report of counseling. Furthermore, we were unable to assess whether those in higher SVI categories received differential prenatal care, including inferior provider vaccination counseling. This analysis included individuals from the first through fourth trimesters, and COVID-19 vaccine hesitancy may vary by trimester. However, we adjusted for trimester and pregnancy status in our analysis. Given the relatively small proportion of postpartum participants at baseline and pregnant participants at follow-up, assessing differences in the association between SVI and vaccine hesitancy and vaccination status between pregnant versus postpartum individuals was not possible. We did not assess whether vaccine hesitancy differed by pregnancy status wherein participants would be more or less willing to get vaccinated following the peripartum period in this analysis. Because vaccine hesitancy is likely dynamic, further prospective follow-up of individuals over time is needed. Finally, the current study was a convenience sample at a tertiary care center of only individuals who spoke English. We did not assess participant health literacy which may also impact vaccine hesitancy. These factors may limit the generalizability of our study to other healthcare settings or populations. However, the SVI is a nationally generalizable measure of community level social vulnerability.
In summary, pregnant and postpartum individuals living in an area with higher community-level social vulnerability were more likely to report COVID-19 vaccine hesitancy and to be unvaccinated at follow-up. These results raise the possibility that the SVI could be used as a tool to identify individuals experiencing a higher burden of adverse social determinants of health to decrease vaccine hesitancy and increase vaccination in the peripartum period.
5. Details of ethics approval
This study was approved by The Ohio State University Institutional Review Board (ID#: 2021H0023; date: 02/23/21).
Funding
Dr. Venkatesh was supported by the Care Innovation and Community Improvement Program and the Division of Maternal Fetal Medicine at The Ohio State University Wexner Medical Center.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None
Appendix A.
References
- 1.ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol 2018;131(6):e214–e217. [DOI] [PubMed]
- 2.Mazzilli S., Tavoschi L., Lopalco P.L. Tdap vaccination during pregnancy to protect newborns from pertussis infection. Ann Ig. 2018;30(4):346–363. doi: 10.7416/ai.2018.2226. [DOI] [PubMed] [Google Scholar]
- 3.Becker-Dreps S., Butler A.M., McGrath L.J., et al. Effectiveness of prenatal tetanus, diphtheria, acellular pertussis vaccination in the prevention of infant pertussis in the U.S. Am J Prev Med. 2018;55(2):159–166. doi: 10.1016/j.amepre.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen S.A., Jamieson D.J., Uyeki T.M. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207(3 Suppl):S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 5.Zaman K., Roy E., Arifeen S.E., et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 6.Ding H., Black C.L., Ball S., et al. Influenza Vaccination Coverage Among Pregnant Women — United States, 2016–17 Influenza Season. MMWR Morb Mortal Wkly Rep. 2017;66:1016–1022. doi: 10.15585/mmwr.mm6638a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr S., Van Bennekom C.M., Liang J.L., Mitchell A.A. Tdap Vaccination Coverage During Pregnancy — Selected Sites, United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:1105–1108. doi: 10.15585/mmwr.mm6641a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuen C.Y., Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014;32(36):4602–4613. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Levy A., Singh S., Riley L.E., Prabhu M. Acceptance of COVID-19 vaccination in pregnancy: a survey study. Am J Obstetrics Gynecol Maternal Fetal Med. 2021;3(5) doi: 10.1016/j.ajogmf.2021.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsel C., Moniz M.H., Wagner A.L., Zikmund-Fisher B.J., Hawley S., Jiang L., et al. COVID-19 vaccine hesitancy among reproductive-aged female tier 1A healthcare workers in a United States Medical Center. J Perinatol. 2021;41(10):2549–2551. doi: 10.1038/s41372-021-01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiefer M.K., Mehl R., Costantine M.M., et al. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: a cross-sectional study. BJOG. 2022 doi: 10.1111/1471-0528.17110. [DOI] [PubMed] [Google Scholar]
- 12.Wilson R.J., Paterson P., Jarrett C., Larson H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. 2015;33(47):6420–6429. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Adeyanju G.C., Engel E., Koch L., et al. Determinants of influenza vaccine hesitancy among pregnant women in Europe: a systematic review. Eur J Med Res. 2021;26(1):116. doi: 10.1186/s40001-021-00584-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battarbee A.N., Stockwell M.S., Varner M., et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during august-december 2020. Am J Perinatol. 2022;39(1):75–83. doi: 10.1055/s-0041-1735878. [DOI] [PubMed] [Google Scholar]
- 15.Society for Maternal-Fetal Medicine. Joint Statement Supporting Public Health Measures to combat COVID-19. (https://s3.amazonaws.com/cdn.smfm.org/media/2642/Final_Joint_Statement_on_COVID_19_Health_Measures_12-15-20_LM.pdf). [Accessed 19 January 2022].
- 16.American College of Obstetricians and Gynecologists. Vaccinating Pregnant and Lactating Patients Against COVID-19. (https://www.acog.org/en/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-Pregnant-and-Lactating-Patients-Against-COVID-19). [Accessed 19 January 2022].
- 17.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). (https://www.cdc.gov/vaccines/acip/recommendations.html). [Accessed 19 January 2022].
- 18.Delahoy M., Whitaker M., O’Halloran A., et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-Confirmed COVID-19 — COVID-NET, 13 States, March 1–August 22, 2020. Morbidity Mortality Weekly Rep. 2020;69:1347–1354. doi: 10.15585/mmwr.mm6938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galang R., Chang K., Strid P., Snead M.C., Woodworth K.R., House L.D., et al. Severe coronavirus infections in pregnancy: a systematic review. Obstertrics and Gynecology. 2020;136(2):262–272. doi: 10.1097/AOG.0000000000004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., et al. Maternal death due to COVID-19. Am J Obstetrics Gynecol. 2020;109 doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntley B., Huntley E.S., Di Mascio D., Chen T., Berghella V., Chauhan S.P. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome Coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstertrics Gynecol. 2020;136(2):303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 22.Metz T.D., Clifton R.G., Hughes B.L., et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022 doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. V-safe COVID-19 Vaccine Pregnancy Registry. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html). [Accessed 19 January 2022].
- 24.Male V. Are COVID-19 vaccines safe in pregnancy? Nat Rev Immunol. 2021;3:1–2. doi: 10.1038/s41577-021-00525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimabukuro T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., et al. CDC v-safe COVID-19 pregnancy registry team. preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2272–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding H., Kahn K.E., Black C.L., O'Halloran A., Lu P.J., Williams W.W. Influenza vaccination coverage among pregnant women in the U.S. Am J Prev Med. 2019;56(4):477–486. doi: 10.1016/j.amepre.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Goggins E.R., Williams R., Kim T.G., et al. Assessing influenza vaccination behaviors among medically underserved obstetric patients. J Womens Health (Larchmt) 2021;30(1):52–60. doi: 10.1089/jwh.2020.8582. [DOI] [PubMed] [Google Scholar]
- 28.Qiu X., Bailey H., Thorne C. Barriers and facilitators associated with vaccine acceptance and uptake among pregnant women in high income countries: a mini-review. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.626717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiefer M.K., Mehl R., Costantine M.M., et al. Association between social vulnerability and influenza and tetanus-diphtheria-acellular pertussis vaccination uptake in pregnant and postpartum individuals. Am J Obstet Gynecol MFM. 2022:100603. doi: 10.1016/j.ajogmf.2022.100603. [DOI] [PubMed] [Google Scholar]
- 30.Health.gov. 2022. Social Determinants of Health - Healthy People 2030 | health.gov. [online] Available at: <https://health.gov/healthypeople/objectives-and-data/social-determinants-health> [Accessed 19 January 2022].
- 31.Flanagan B.E., et al. A social vulnerability index for disaster management. J Homeland Security Emergency Manage. 2011;8:1. [Google Scholar]
- 32.Flanagan B.E., Hallisey E.J., Adams E., Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the centers for disease control and prevention's social vulnerability index. J Environ Health. 2018;80(10):34–36. [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention/ Agency for Toxic Substances and Disease Registry/ Geospatial Research A, and Services Program. . CDC/ATSDR Social Vulnerability Index. (https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html). [Accessed 19 January 2022].
- 34.Kelley K., Clark B., Brown V., Sitzia J. Good practice in the conduct and reporting of survey research. Int J Quality Health Care. 2003;15(3):261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 35.Gallo V., Egger M., McCormack V., et al. STrengthening the Reporting of OBservational studies in Epidemiology - Molecular Epidemiology (STROBE-ME): an extension of the STROBE statement. Eur J Clin Invest. 2012;42(1):1–16. doi: 10.1111/j.1365-2362.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 36.Pugh C. Here's everyone who can get the COVID-19 vaccine in Ohio. The Columbus Dispatch. Columbus, OH2021. (https://www.dispatch.com/story/news/2021/03/09/find-out-who-is-eligible-for-a-covid-19-vaccine-in-ohio/4641417001/) [Accessed 19 January 2022].
- 37.Kahn K, Black CL, Ding H, et al. Pregnant women and Tdap vaccination, Internet panel survey, United States, April 2016. . (https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/tdap-report.html#15). [Accessed 19 January 2022].
- 38.Larson H., Jarrett C., Eckersberger E., Smith D.M., Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–2159. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald NSWGoVH. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015;33(34). [DOI] [PubMed]
- 40.Schuster M., Eskola J., Duclos P. SAGE Working Group on Vaccine Hesitancy. Review of vaccine hesitancy: Rationale, remit and methods. Vaccine. 2015;33(34):4157–4160. doi: 10.1016/j.vaccine.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 41.ESRI. ArcGIS Desktop: Release 10. . Redlands, CA: Environmental Systems Research Institute, 2011.
- 42.U.S. Department of health and human services. (2019, June 20). U.S. Census populations with bridged race categories. Centers for Disease Control and Prevention. Retrieved February 19, 2022, from https://www.cdc.gov/nchs/nvss/bridged_race.htm [Accessed 19 January 2022].
- 43.Balasuriya L., Santilli A., Morone J., et al. COVID-19 vaccine acceptance and access among black and latinx communities. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegler A.J., Luisi N., Hall E.W., et al. Trajectory of COVID-19 vaccine hesitancy over time and association of initial vaccine hesitancy with subsequent vaccination. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.26882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul E., Fancourt D. Predictors of uncertainty and unwillingness to receive the COVID-19 booster vaccine: an observational study of 22,139 fully vaccinated adults in the UK. Lancet Reg Health Eur. 2022;14 doi: 10.1016/j.lanepe.2022.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Descamps A., Launay O., Bonnet C., Blondel B. Seasonal influenza vaccine uptake and vaccine refusal among pregnant women in France: results from a national survey. Hum Vaccin Immunother. 2020;16(5):1093–1100. doi: 10.1080/21645515.2019.1688035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strully K.W., Yang T.C. County social vulnerability and influenza vaccine rates: national and local estimates for medicare recipients. Am J Prev Med. 2022;62(1):e1–e9. doi: 10.1016/j.amepre.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Thakore N., Khazanchi R., Orav E.J., Ganguli I. Association of Social Vulnerability, COVID-19 vaccine site density, and vaccination rates in the United States. Healthc (Amst) 2021;9(4) doi: 10.1016/j.hjdsi.2021.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am J Prev Med. 2020;59(3):317–325. doi: 10.1016/j.amepre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oates G.R., Juarez L.D., Horswell R., et al. The association between neighborhood social vulnerability and COVID-19 testing, positivity, and incidence in Alabama and Louisiana. J Community Health. 2021;46(6):1115–1123. doi: 10.1007/s10900-021-00998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]