Abstract
Objective
To quantify the risk of overall and type specific cardiovascular and cerebrovascular diseases as well as venous thromboembolism in women with a history of gestational diabetes mellitus.
Design
Systematic review and meta-analyses.
Data sources
PubMed, Embase, and the Cochrane Library from inception to 1 November 2021 and updated on 26 May 2022.
Review methods
Observational studies reporting the association between gestational diabetes mellitus and incident cardiovascular and cerebrovascular diseases were eligible. Data, pooled by random effects models, are presented as risk ratios (95% confidence intervals). Certainty of evidence was appraised by the Grading of Recommendations, Assessment, Development, and Evaluations.
Results
15 studies rated as moderate or serious risk of bias were included. Of 513 324 women with gestational diabetes mellitus, 9507 had cardiovascular and cerebrovascular disease. Of more than eight million control women without gestational diabetes, 78 895 had cardiovascular and cerebrovascular disease. Compared with women without gestational diabetes mellitus, women with a history of gestational diabetes mellitus showed a 45% increased risk of overall cardiovascular and cerebrovascular diseases (risk ratio 1.45, 95% confidence interval 1.36 to 1.53), 72% for cardiovascular diseases (1.72, 1.40 to 2.11), and 40% for cerebrovascular diseases (1.40, 1.29 to 1.51). Women with gestational diabetes mellitus showed increased risks of incident coronary artery diseases (1.40, 1.18 to 1.65), myocardial infarction (1.74, 1.37 to 2.20), heart failure (1.62, 1.29 to 2.05), angina pectoris (2.27, 1.79 to 2.87), cardiovascular procedures (1.87, 1.34 to 2.62), stroke (1.45, 1.29 to 1.63), and ischaemic stroke (1.49, 1.29 to 1.71). The risk of venous thromboembolism was observed to increase by 28% in women with previous gestational diabetes mellitus (1.28, 1.13 to 1.46). Subgroup analyses of cardiovascular and cerebrovascular disease outcomes stratified by study characteristics and adjustments showed significant differences by region (P=0.078), study design (P=0.02), source of data (P=0.005), and study quality (P=0.04), adjustment for smoking (P=0.03), body mass index (P=0.01), and socioeconomic status (P=0.006), and comorbidities (P=0.05). The risk of cardiovascular and cerebrovascular diseases was, however, attenuated but remained significant when restricted to women who did not develop subsequent overt diabetes (all gestational diabetes mellitus: 1.45, 1.33 to 1.59, gestational diabetes mellitus without subsequent diabetes: 1.09, 1.06 to 1.13). Certainty of evidence was judged as low or very low quality.
Conclusions
Gestational diabetes mellitus is associated with increased risks of overall and type specific cardiovascular and cerebrovascular diseases that cannot be solely attributed to conventional cardiovascular risk factors or subsequent diabetes.
Introduction
The estimated prevalence of gestational diabetes mellitus, defined as glucose intolerance with first onset during pregnancy, ranges from 1% to >30%.1 2 During pregnancy, gestational diabetes mellitus is associated with excess risks of adverse maternal and neonatal outcomes, including pre-eclampsia, preterm birth, stillbirth, large for gestational age, and neonatal hyperinsulinaemia.3 4 5 Although gestational diabetes mellitus usually resolves after birth, a growing number of long term observational studies suggest that the impact persists over time. For example, women with a history of gestational diabetes mellitus were reported to be at increased risks of developing type 2 diabetes, metabolic syndrome, and chronic kidney disease later in life.6 7 8 9
The increased cardiovascular risk in women with gestational diabetes mellitus has been increasingly recognised. A meta-analysis found a nearly twofold higher risk of future overall cardiovascular and cerebrovascular disease in such women, but the analyses on type specific cardiovascular and cerebrovascular diseases as well as on venous thromboembolism were not further evaluated.10 In recent years, the association between gestational diabetes mellitus and type specific cardiovascular and cerebrovascular diseases is gradually being reported.7 10 One of the studies, which included women with gestational diabetes mellitus with and without future development of diabetes mellitus, reported that both groups of women were at increased risks of coronary artery diseases.7 Yet knowledge about specific cardiovascular diseases such as myocardial infraction and heart failure in women with gestational diabetes mellitus is still largely limited. To a large extent, there is a sparsity of evidence on the association of gestational diabetes mellitus with cerebrovascular outcomes and venous thromboembolism. We therefore performed a systematic review and meta-analysis of available evidence from observational studies to quantify the risks of overall and type specific cardiovascular and cerebrovascular diseases in women with gestational diabetes mellitus.
Methods
We developed and followed a protocol for all steps of our systematic review and meta-analysis (see supplementary appendices S1 and S2 for the updated study protocol and protocol deviations). The study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.11
Literature search and inclusion criteria
We searched PubMed, Embase, and the Cochrane Library from inception to 1 November 2021 for potentially relevant studies without language restrictions using the search terms: exposure (gestational diabetes or gestational diabetes mellitus or pregnancy diabetes or pregnancy diabetes mellitus) and outcome (cardiovascular diseases or cerebrovascular disorders or venous thromboembolism or cardiovascular or cerebrovascular or coronary artery disease* or coronary heart disease* or cardiac or ischaemic heart disease* or cardio-cerebrovascular or myocardial infarction or heart failure or angina pectoris or cerebral or stroke or transient ischaemic attack or pulmonary embolism or deep vein thrombosis). The search was updated on 26 May 2022. Supplementary appendix S3 provides full details of the search strategy. In addition, we hand searched reference lists of included articles, and we also searched for relevant studies from the abstracts of conference proceedings of the American Diabetes Association, European Association for the Study of Diabetes, and Annual Meeting of the Society for Maternal-Fetal Medicine (2017-22).
We considered studies eligible for inclusion if they: were observational studies with a retrospective or prospective cohort or case‐control (including nested case‐control) design (cross sectional studies were excluded because the order of occurrence of gestational diabetes mellitus and cardiovascular or cerebrovascular disease is usually hard to determine); reported at least one cardiovascular or cerebrovascular disease or episode of venous thromboembolism in women with a history of gestational diabetes mellitus; included a comparator of women without gestational diabetes mellitus; and presented risk ratios (or odds ratios, hazard ratios, incidence rate ratios) with 95% confidence intervals. Studies were excluded if they lacked an eligible control group or relevant data on outcomes of cardiovascular and cerebrovascular disease. We also excluded publications without original data, such as reviews, editorials, and comments. If studies comprised overlapping cohorts, we chose for analysis the study with the largest cohort or most detailed information. Potential studies in non-English were translated with the aid of translation software or translators if necessary. Study selection was performed in two phases: primary screening of title and abstract, then full text review of potentially eligible articles. Two review authors (WX and YW) independently evaluated eligibility, with discrepancies resolved by a third investigator (ZZ).
Data extraction and outcome assessments
Two authors (WX and YW) independently extracted data from eligible studies using piloted data extraction sheets. Extracted data included first author, publication year, country, setting, duration of follow-up, study design, data source (national or local database), enrolment period, ascertainment of gestational diabetes mellitus and cardiovascular and cerebrovascular diseases, sample size, personal and clinical features of the participants, outcome variables of interest, and adjustment variables.
The primary outcome was the association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases. Secondary outcomes were the association of gestational diabetes mellitus with type specific cardiovascular and cerebrovascular diseases as well as venous thromboembolism (including deep vein thrombosis and pulmonary embolism). Cardiovascular and cerebrovascular diseases was defined as the composite of cardiovascular diseases (including angina pectoris, myocardial infarction, coronary artery diseases, cardiovascular procedures, heart failure, and cardiovascular death) and cerebrovascular diseases (ischaemic stroke, haemorrhagic stroke, and transient ischaemic attack).
Risk of bias and certainty of evidence assessment
Two reviewers (WX and YW) independently assessed the risk of bias of selected studies according to the Risk of Bias in Nonrandomised Studies of Interventions (ROBINS-I) tool.12 This tool consists of seven domains, with bias assessed as due to: confounding, selection of participants, exposure assessment, misclassification during follow-up, missing data, outcome assessment, and selective reporting. Two reviewers (WX and YW) rated the risk of each domain as either low, moderate, serious, critical, or no information. Supplementary appendix S4 provides a detailed description and decision criteria for each domain in ROBINS-I. A senior investigator (ZZ) resolved discrepancies.
Two investigators (WX and YW) independently assessed certainty of evidence for each outcome, with discrepancies resolved by a third reviewer (ZZ). Certainty of evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, which divides evidence into very low, low, moderate, and high levels.13 The quality of evidence from observational studies is initially categorised as low and then upgraded or downgraded based on predefined criteria. Quality can be upgraded for large effect sizes (risk estimates >2 or <0.5 in the absence of plausible confounders), dose-response gradient, or attenuation of the pooled risk estimates by plausible confounders. Conversely, quality can be downgraded for risk of bias (≥25% of the contributing studies were assessed as serious risk of bias), inconsistency (substantial between study heterogeneity, I2 ≥50%), indirectness (presence of factors limiting generalisability of the results), imprecision (95% confidence intervals for risk estimates are wide or cross a minimally important difference of 10% for outcomes (risk ratio 0.9 to 1.1)), and publication bias (evidence of small study effects).
Data synthesis and analysis
Extracted data for meta-analysis were analysed with Stata Statistical Software version 13.0 and R statistical language version R 3.6.0. The P values were two sided, with an alpha level of 0.05 considered significant. Random effect models (DerSimonian and Laird method) were used to calculate pooled risk ratios with 95% confidence intervals for the association between gestational diabetes mellitus and risk of cardiovascular and cerebrovascular events. Hazard ratios and incidence rate ratios were used as good estimators of risk ratios to carry out the statistical estimations. Because the incidence of cardiovascular and cerebrovascular diseases was relatively low in women with gestational diabetes mellitus, odds ratios can be used to approximate risk ratios.14 We selected risk estimates from the multivariate models that were fully adjusted for confounders. For studies that only reported the risk estimates for type specific cardiovascular and cerebrovascular diseases, however, in the absence of overall cardiovascular and cerebrovascular diseases, we summarised type specific risk estimates using either fixed effects or random effects model based on the level of heterogeneity to obtain a combined risk estimates of the study; then we entered the combined risk estimate into the main pooled analysis.15 The heterogeneity across studies was quantified using the I2 statistic (0-25% low heterogeneity, 25-50% moderate heterogeneity, 50-75% substantial heterogeneity, 75-100% high heterogeneity).
To identify the subgroup differences and potential sources of the observed heterogeneity, we carried out subgroup analyses after stratifying for median year of publication (before 2017 v after 2017), study location (North America v Europe v Asia), study design (prospective v retrospective), source of data (nationwide v local database), median duration of follow-up (>10 years v ≤10 years), method for ascertaining gestational diabetes mellitus (diagnostic code v self-report v oral glucose tolerance test), method for ascertaining cardiovascular and cerebrovascular diseases (diagnostic code v others), median sample size (≥100 000 v <100 000), number of cardiovascular and cerebrovascular disease events (≥2500 v <2500), and quality of study (moderate risk of bias v serious risk of bias). To explore whether the association of gestational diabetes mellitus with cardiovascular and cerebrovascular disease was influenced by potential confounders, we performed additional analyses, stratified by several factors, including race, smoking status, body mass index, socioeconomic status, education level, parity, comorbidities, and pregnancy complications. A P value of <0.10 for differences in estimates between these subgroups was considered significant. In addition, to assess the role of subsequent diabetes in the association between gestational diabetes mellitus and cardiovascular and cerebrovascular disease, we further analysed the risk estimate for cardiovascular and cerebrovascular disease in all women with gestational diabetes mellitus and in women with gestational diabetes mellitus who did not develop future overt diabetes. To evaluate the robustness of pooled results, we performed sensitivity analyses by excluding studies one by one, excluding the case-control study, and exclusively including the studies with direct risk estimates for overall cardiovascular and cerebrovascular disease. Potential publication bias was assessed by visualisation of asymmetry in funnel plots (≥10 included studies) in combination with both Egger’s test and Begg’s test.
Patient and public involvement
No patients or members of the public were directly involved in the development or completion of this study owing to time and funding constraints. However, major motivators for research team to complete this study were the first author’s (WX) experience in the management of women with gestational diabetes mellitus during rotation in the endocrinology department, and the strong interest expressed by patients, endocrinologists, and cardiologists in the association between gestational diabetes mellitus and cardiovascular and cerebrovascular outcomes.
Results
Study selection, characteristics, and quality assessment
In our initial and updated search, we identified 32 674 records after the removal of duplicates. Based on screening of titles and abstract reviews, the full text of 100 articles was subsequently reviewed. Fifteen studies were eligible for data extraction and quantitative analysis.7 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Figure 1 shows the flow of records through the review, supplementary appendix S5 includes a list of excluded studies with reasons, and supplementary table S1 summarises the characteristics of the included articles. The included studies were based on 14 datasets and published between 2006 and 2022. Of these studies, four were from Canada,7 20 24 26 three from the United States,16 19 22 two from the United Kingdom,23 29 and one each from Israel,17 Sweden,18 France,21 Iran,25 Korea,27 and Denmark.28 The studies comprised either a retrospective7 16 17 19 20 21 23 24 26 or prospective cohort design,22 25 27 28 29 except for one study, which used a case-control design.18 Apart from one conference abstract,29 the remaining studies were published as full text. All studies reported the case ascertainment of gestational diabetes mellitus, mostly based on diagnostic codes (eg, international classification of diseases)7 19 21 23 24 26 27 28 29 (see supplementary table S1). Of the remaining studies, three used self-report of doctors’ diagnosis16 22 25 and three used the results of an oral glucose tolerance test.17 18 20 In most of the studies, cardiovascular and cerebrovascular disease was ascertained using diagnostic codes from the international classification of diseases.7 17 18 19 20 21 23 24 27 28 29
Fig 1.
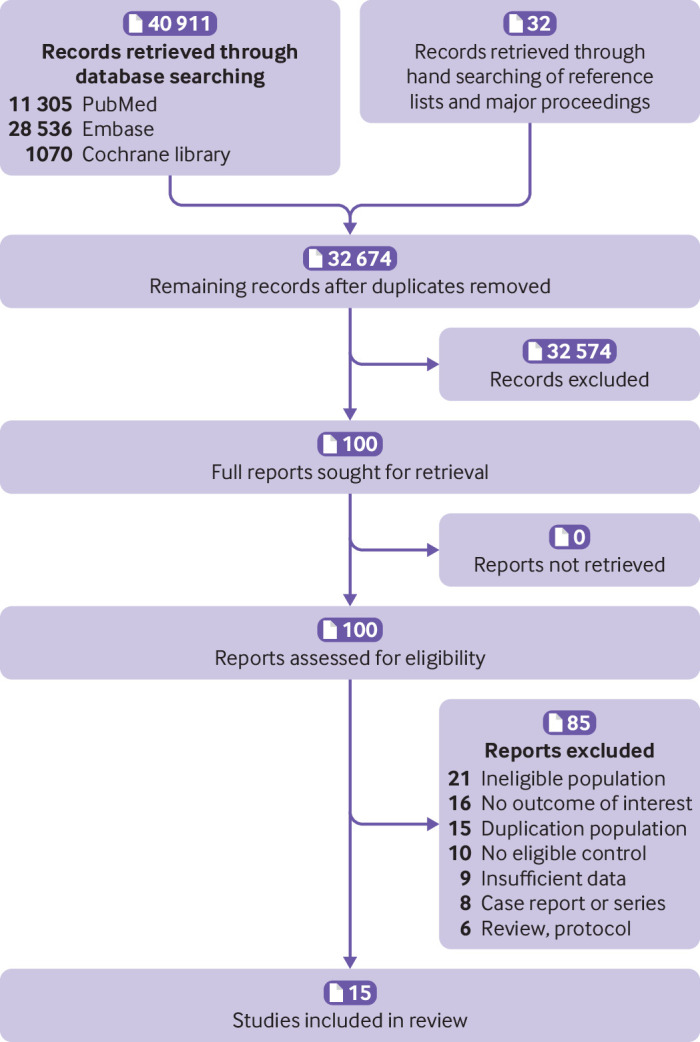
Literature flowchart
Of almost nine million women included in the studies, 102 470 had cardiovascular or cerebrovascular disease. Of 513 324 women with gestational diabetes mellitus, 9507 had cardiovascular and cerebrovascular disease. Of more than eight million control women without gestational diabetes, 78 895 had cardiovascular and cerebrovascular disease. Supplementary table S1 presents the baseline personal and demographic characteristics of the two groups. Table 1 summarises the outcomes of interest, risk estimates, and adjustments across included studies. According to the ROBINS-I tool, seven studies showed a moderate overall risk of bias18 19 22 23 26 28 29 and eight a serious overall risk of bias.7 16 17 20 21 24 25 27 Supplementary table S2 presents the detailed assessment of risk of bias for each domain.
Table 1.
Characteristics of included studies
| Reference | Country | Duration of follow-up (years) | Gestational diabetes mellitus diagnosis | CCVD diagnosis | Study outcome | Sample size | No of CCVD | Adjustments |
|---|---|---|---|---|---|---|---|---|
| Carr 200616 | USA | Not available | Self-report | Self-report | Coronary artery disease, stroke | 982 | 132 | Age, race, menopausal status, and proband status |
| Kessous 201317 | Israel | 10 | Two-step* | International classification of diseases | Admitted to hospital for any cardiovascular reason, complex cardiovascular events, myocardial infarction, simple cardiovascular events, angina pectoris, coronary heart disease, invasive cardiovascular events, percutaneous coronary intervention | 47 909 | 2408 | Age, race |
| Fadl 201418 | Sweden | 9.1 | Oral glucose tolerance test† | International classification of diseases | Ischaemic heart disease, stroke, atherosclerosis or peripheral vascular disease | 15 949 | 2639 | Chronic hypertension, smoking, body mass index, ethnicity, education level, parity |
| Savitz 201419 | USA | 1 | International classification of diseases | International classification of diseases | Coronary heart disease, heart failure, intracranial haemorrhage, stroke, transient ischaemic attack | 849 639 | 660 | Year, age, race, health insurance, gestational hypertension, pre-eclampsia, gestational diabetes, parity, education, prenatal smoking, prenatal care, prepregnancy weight |
| Kaul 201520 | Canada | 5.3 | Oral glucose tolerance test | International classification of diseases | Ischaemic heart disease, cerebrovascular disease | 222 496 | 2319 | Age, pre-eclampsia, parity, smoking status during pregnancy, ethnicity, socioeconomic status, body mass index |
| Goueslard 201621 | France | 7 | International classification of diseases | International classification of diseases | Angina pectoris, myocardial infarction, stroke, heart bypass surgery, coronary angioplasty, carotid endarterectomy, fibrinolysis | 1 515 387 | 3629 | Age |
| Retnakaran 20177 | Canada | 10 | Diagnostic codes | Diagnostic codes | Admitted to hospital for myocardial infarction, acute coronary syndrome, coronary artery bypass surgery, percutaneous coronary intervention, stroke, transient ischaemic attack, carotid endarterectomy | 1 465 682 | 3325 | Age, income, region of residence |
| Tobias 201722 | USA | 25.7 | Self-report | Self-report and medical records | Myocardial infarction, stroke | 1 037 526 | 1140 | Age, menopausal status, current hormone therapy use, race, family history of CCVD, history of pregnancy hypertensive disorders, prepregnancy body mass index, parity, current weight change from prepregnancy, aspirin use, alcohol intake, smoking status, physical activity, Alternative Healthy Eating Index 2010 diet quality score |
| Daly 201823 | UK | 2.9 | Clinical diagnosis and codes | Clinical diagnosis and codes | Coronary artery disease, stroke, transient ischaemic attack | 46 389 | 100 | Age, Townsend fifth, body mass index, smoking, prescribed lipid lowering drug, hypertension |
| McKenzie-Sampson 201824 | Canada | 14.5 | International classification of diseases | International classification of diseases | Ischaemic heart disease, myocardial infarction, angina pectoris, cardiac arrest, heart failure, ischaemic stroke, haemorrhagic stroke | 1 070 667 | 38 268 | Age, parity, time period, socioeconomic deprivation, pre-eclampsia |
| Kabootari 201925 | Iran | 14.1 | Self-report | Electrocardiogram and biomarkers | Coronary heart disease, cardiovascular death, stroke, cerebrovascular death | 4308 | 314 | Age, body mass index, parity, miscarriage, physical activity, hypertension, hypercholesterolaemia |
| Echouffo-Tcheugui 202126 | Canada | 7 | Laboratory test results and international classification of diseases | International classification of diseases | Heart failure | 906 319 | 763‡ | Age, ethnicity, neighbourhood income fifth, rurality, parity, preterm delivery pregestational hypertension, pre-eclampsia, pre-existing CCVD |
| Sun 202127 | Korea | 12.8 | International classification of diseases | International classification of diseases | Admitted to hospital with myocardial infarction, treatment with coronary revascularisation, heart failure, cerebrovascular disease | 1 500 168 | 13 222 | Age, parity, household income, history of pre-eclampsia or hypertension, polycystic ovary syndrome, dyslipidaemia |
| Yu 202128 | Denmark | 16.2 | International classification of diseases | International classification of diseases | Ischaemic heart disease, myocardial infarction, cerebrovascular disease, stroke, heart failure, atrial fibrillation, hypertensive disease, venous thromboembolism, coronary artery bypass surgery or percutaneous coronary intervention, and other types of CCVD | 1 002 486 | 21 220 | Time period of first delivery, parity, age, education, smoking during pregnancy, cohabitation, residence, prepregnancy obesity, country of origin, maternal CCVD history, paternal CCVD history |
| Lee 202229 | UK | 10.3 | International classification of diseases | International classification of diseases | Coronary artery disease, myocardial infarction, ischaemic stroke, peripheral artery disease, heart failure, aortic stenosis, mitral regurgitation, arterial fibrillation/flutter, venous thromboembolism | 219 330 | 13 094 | Age, race, body mass index, smoking, prevalent comorbidities (hypertension, diabetes, dyslipidaemia), drugs |
CCVD=cardiovascular and cerebrovascular diseases.
50 g glucose challenge followed by three hour 100 g glucose challenge.
Fasting capillary whole blood glucose ≥6.1 mmol/L (fasting plasma glucose ≥7.0 mmol/L) and/or two hour blood glucose ≥9.0 mmol/L (plasma glucose ≥10.0 mmol/L).
Number with heart failure.
Primary outcome: overall cardiovascular and cerebrovascular diseases
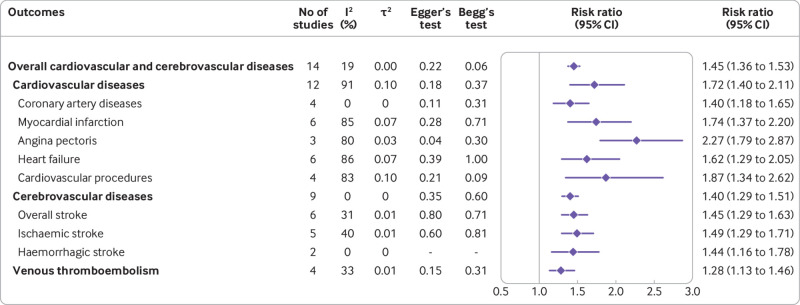
The association between gestational diabetes mellitus and overall cardiovascular and cerebrovascular diseases could be assessed in 14 eligible studies. Compared with women without gestational diabetes mellitus, women with a history of gestational diabetes mellitus showed a 45% increased risk of developing overall cardiovascular and cerebrovascular diseases (risk ratio 1.45, 95% confidence interval 1.36 to 1.53) (fig 2, supplementary figure S1). Heterogeneity of all cardiovascular and cerebrovascular diseases across all studies was very low (I2=19%). Separately, the risks of developing cardiovascular and cerebrovascular diseases were reported in 12 and nine studies, with pooled risk ratios of 1.72 (95% confidence interval 1.40 to 2.11, I2=91%) and 1.40 (1.29 to 1.51, I2=0%), respectively (fig 2, supplementary figures S2 and S3). Further leave-one-out sensitivity analysis or excluding each study one by one, excluding the case-control study18 and excluding the studies without direct information on the risk estimates for total cardiovascular and cerebrovascular diseases17 19 23 24 suggested that the pooled estimate of the primary outcome of gestational diabetes mellitus was robust and not influenced excessively (see supplementary figures S4-S6). We found no evidence of publication bias through visualisation of funnel plots and the results of Egger’s and Begg’s tests (fig 2, supplementary figure S7). Supplementary table S3 summarises the quality of evidence based on the GRADE framework. For the outcomes of cardiovascular and cerebrovascular diseases overall and separately, these findings were considered as either low or very low quality evidence.
Fig 2.
Risk ratios for cardiovascular and cerebrovascular outcomes in women with a history of gestational diabetes mellitus
Secondary outcome: Type specific cardiovascular and cerebrovascular diseases
Women with a history of gestational diabetes mellitus showed substantially increased risks of subsequent coronary artery diseases (risk ratio 1.40, 95% confidence interval 1.18 to 1.65, I2=0%), myocardial infarction (1.74, 1.37 to 2.20, I2=85%), heart failure (1.62, 1.29 to 2.05, I2=86%), angina pectoris (2.27, 1.79 to 2.87, I2=80%), and cardiovascular procedures (1.87, 1.34 to 2.62, I2=83%) compared with women without gestational diabetes mellitus (fig 2, supplementary figures S8-S12). The summary risk ratios for overall stroke, ischaemic stroke, and haemorrhagic stroke were 1.45 (95% confidence interval 1.29 to 1.63, I2=31%), 1.49 (1.29 to 1.71, I2=40%), and 1.44 (1.16 to 1.78, I2=0%), respectively (fig 2, supplementary figures S13-S15). In addition, women with previous gestational diabetes mellitus showed a significantly increased risk of venous thromboembolism compared with their peers (risk ratio 1.28, 95% confidence interval 1.13 to 1.46, I2=0%) (fig 2, supplementary figure S16). Neither Egger’s test nor Begg’s test showed evidence of publication bias for the association of gestational diabetes mellitus with type specific cardiovascular and cerebrovascular diseases (fig 2). According to the GRADE framework, the overall quality of evidence was very low for type specific cardiovascular and cerebrovascular outcomes (supplementary table S3).
Subgroup analyses
Gestational diabetes and cardiovascular and cerebrovascular diseases
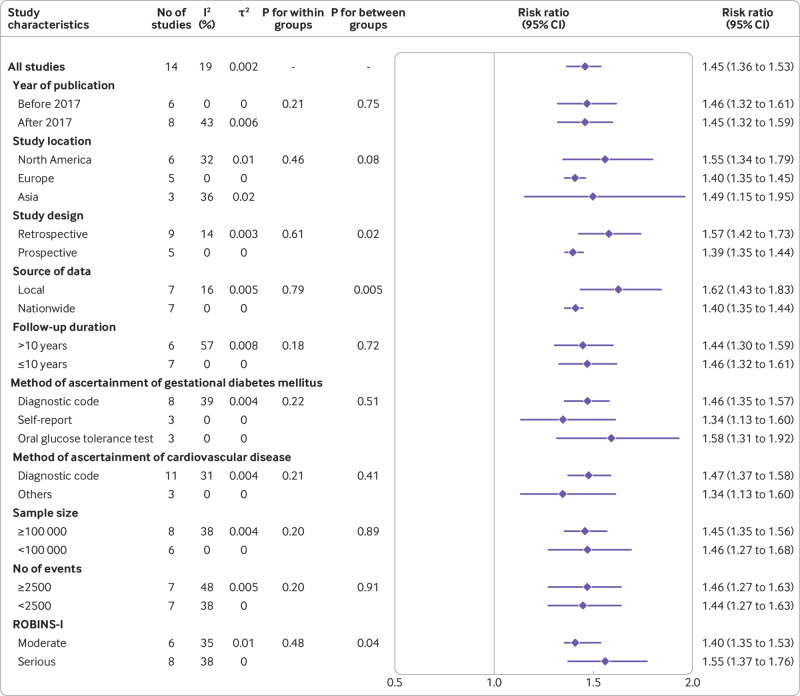
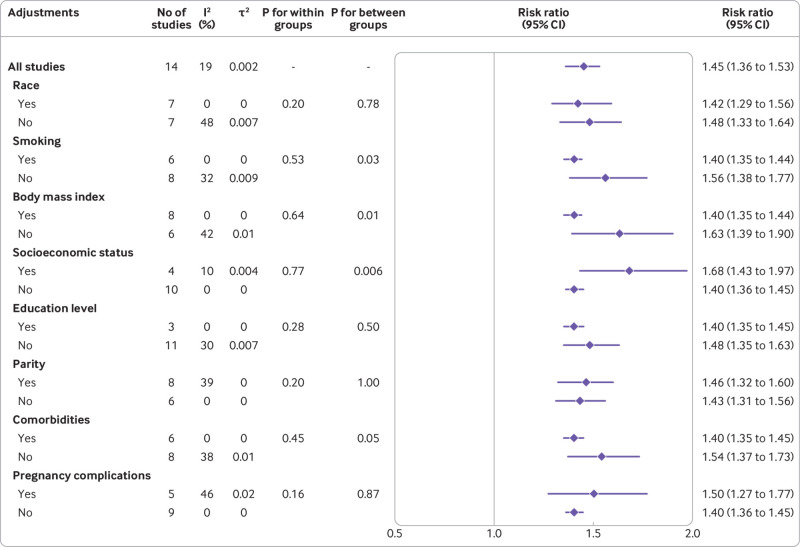
To investigate the presence of subgroup differences for the outcome of cardiovascular and cerebrovascular diseases, we conducted subgroup analyses according to the characteristics of eligible studies, including publication year, region, study design, setting, data source, follow-up period, method for ascertainment of gestational diabetes mellitus or cardiovascular and cerebrovascular diseases, sample size, number of events, and quality of study. Significant differences between subgroups were detected by region (P=0.08), study design (P=0.02), source of data (P=0.005), and quality of study (P=0.04) as well (fig 3, supplementary figures S17-S26). This association was weakened overall in the national, prospective and moderate risk of bias studies compared with local, retrospective and serious risk of bias studies. To establish whether the associations could be influenced by confounding factors, we performed subgroup analyses adjusting for factors such as ethnicity, smoking status, body mass index, socioeconomic status, education level, parity, comorbidities, and pregnancy complications. The test for subgroup differences showed a statistically significant subgroup effect for smoking (P=0.03), body mass index (P=0.01), and socioeconomic status (P=0.006), and for comorbidities (P=0.05), suggesting that these factors might statistically significantly modify the association between gestational diabetes mellitus and cardiovascular and cerebrovascular diseases (fig 4, also see supplementary figures S27-S34). The association was attenuated in the studies that adjusted for smoking, body mass index, and comorbidities but enhanced in the studies that adjusted for socioeconomic status.
Fig 3.
Subgroup analyses of association between gestational diabetes mellitus and overall cardiovascular and cerebrovascular diseases according to study characteristics. ROBINS-I=Risk of Bias in Nonrandomised Studies of Interventions
Fig 4.
Subgroup analyses of association between gestational diabetes mellitus and overall cardiovascular and cerebrovascular diseases according to adjustments
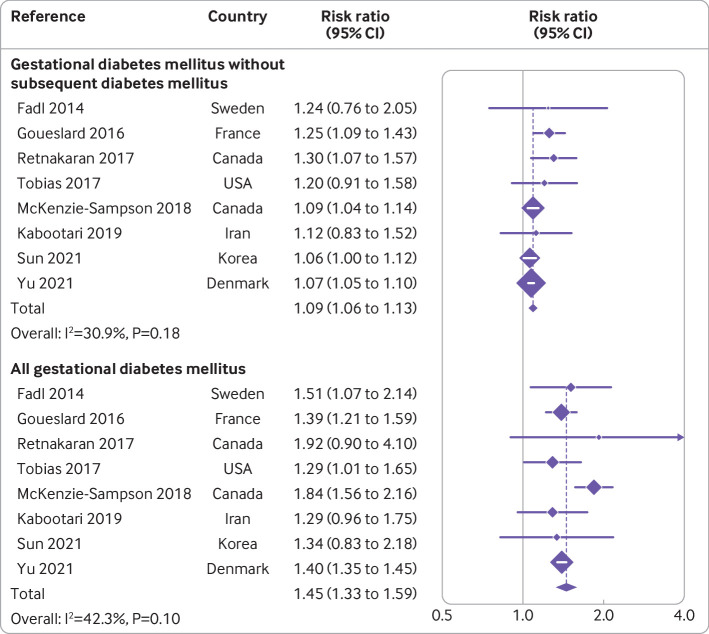
In addition, we investigated the role of future overt diabetes on risk of overall cardiovascular and cerebrovascular diseases in women with previous gestational diabetes mellitus. Eight studies reported the risk of overall cardiovascular and cerebrovascular diseases in both women with gestational diabetes mellitus and women who did not develop future overt diabetes.7 18 21 22 24 25 27 28 Results of the eight studies showed a risk ratio for future incident cardiovascular and cerebrovascular diseases of 1.45 (1.33 to 1.59, I2=42%) in all women with gestational diabetes mellitus. When restricted to the women who did not develop future overt diabetes, the association was attenuated but remained significant (1.09, 1.06 to 1.13, I2=31%) (fig 5).
Fig 5.
Risk ratios for overall cardiovascular and cerebrovascular diseases in all women with gestational diabetes mellitus and in women who did not subsequently develop diabetes
Gestational diabetes and cardiovascular diseases
Subgroup analyses to explore potential sources of significant heterogeneity were inconclusive for cardiovascular diseases, although heterogeneity was reduced in certain specific subgroups, such as studies published before 2017 (I2=0%), retrospective studies (I2=14%), studies with ≤10 years of follow-up (I2=0%), and adjustment for race (I2=0%) (see supplementary tables S4 and S5).
Discussion
Principal findings
The findings of this systematic review and meta-analysis of more than eight million women suggest that those with a history of gestational diabetes mellitus are at significantly increased risks of cardiovascular and cerebrovascular diseases in general and at variable risks for most common types of cardiovascular and cerebrovascular diseases, including myocardial infarction, heart failure, stroke, and venous thromboembolism, even after accounting for ethnicity, sociodemographic characteristics, education level, conventional risk factors for cardiovascular and cerebrovascular diseases, and future incident diabetes. The findings also highlight the need for early intervention in women at high risk of gestational diabetes mellitus, and for continuous monitoring of women with a history of gestational diabetes mellitus after pregnancy.
Comparison with other studies
In recent years, increasing numbers of observational studies have reported the increased risk of adverse cardiovascular outcomes in women with gestational diabetes mellitus compared with their peers without gestational diabetes mellitus. Previous meta-analysis, based on nine studies, concluded that a history of gestational diabetes mellitus was associated with 1.98 times increased risk of future cardiovascular events overall, although the 95% confidence were relatively wide and the level of heterogeneity was high (1.57 to 2.50, I2=98.6%, respectively).10 Moreover, the influence of gestational diabetes mellitus on type specific cardiovascular and cerebrovascular diseases or venous thromboembolism was not analysed at all. in their research. To date, the association of gestational diabetes mellitus with cerebrovascular events and venous thromboembolism is still equivocal, leaving a large knowledge gap on this topic. In our systematic review and meta-analysis, we found that women with a history of gestational diabetes mellitus had a 1.45-fold increased risk of developing incident cardiovascular and cerebrovascular diseases overall, with a 1.72-fold and 1.40-fold increased risk of cardiovascular and cerebrovascular diseases, respectively. In women with gestational diabetes mellitus the risks of common types of cardiovascular and cerebrovascular diseases were increased to variable degrees, including myocardial infarction, heart failure, angina pectoris, cardiovascular procedures, and stroke, as well as venous thromboembolism. In addition, gestational diabetes mellitus remained associated with risk of cardiovascular and cerebrovascular diseases even after accounting for race, sociodemographic features, education level, and conventional risk factors for cardiovascular and cerebrovascular diseases.
This study had several advantages over previous meta-analysis.10 Firstly, we comprehensively and systematically studied the cardiovascular and cerebrovascular profiles of women with gestational diabetes mellitus, to obtain a more complete understanding of the associations. Secondly, we are confident that our results are reliable, because of robustness, lack of publication bias, and overall low heterogeneity. Finally, the strengths of this study include the large sample size and country representativeness, suggesting good generalisability of the results.
Implications from subgroup analyses
In the subgroup analyses, associations between gestational diabetes mellitus and cardiovascular and cerebrovascular disease outcomes differed by geographical region, study design, source of data, quality of study, and adjustment for smoking, body mass index, and socioeconomic status, and for pre-existing comorbidities. This association was attenuated to different degrees, but it remained in the nationwide, prospective and lower risk of bias studies. These high quality studies are more likely to be reliable and provide more precise estimates, whereas the biased studies could overestimate the association.30 As for geographical region, the lowest risk of cardiovascular and cerebrovascular diseases in women with gestational diabetes mellitus was found in Europe compared with Asia and North America. These region specific differences were, however, based on a few reports included in each subgroup, and we also cannot discount spurious results in the presence of multiple subgroup analyses.31 32 Regarding confounders, we found some degree of attenuations in their association after adjustment for smoking status, body mass index, and pre-existing comorbidities. These residual confounding factors are generally thought to artificially inflate the risk estimate for cardio-cerebrovascular events.33 34 35 36 37 38 Conversely, the association was found to be more significant with adjustment for socioeconomic status, but this result needs to be treated with caution. As summarised in recent reviews, the evidence for the association between socioeconomic factors and cardiovascular and cerebrovascular diseases remains uncertain, with causation being speculative.39 40 In summary, the subgroup differences we found highlight the need for high quality studies on the association, specifically the improvement in the design of studies, greater geographical representation, and adequate control for confounding factors to fill the gaps in evidence.
Potential underlying mechanisms
The precise mechanisms of how gestational diabetes mellitus contributed to increased risk of cardiovascular and cerebrovascular diseases remains unknown. The findings from clinical investigations indicated that the increased cardiovascular risk is substantially attributable to the future development of diabetes. In a recent population based cohort study including 10 02 486 women in Demark, around 23% of the increased risks could be explained by the subsequent development of type 2 diabetes.28 In the present study, we also found this increased risk was attenuated in women with gestational diabetes mellitus but no subsequent diabetes (risk ratio 1.09,95% confidence interval 1.06 to 1.13) compared with all women with gestational diabetes (1.45, 1.33 to 1.59) on the basis of eight studies. The trend is consistent with a previous meta-analysis (1.56, 1.04 to 2.32 for women with gestational diabetes mellitus but no subsequent diabetes),10 although the magnitude of risk estimates differed. This trend might be related to several factors: firstly, the present study included three new citations for this outcome,25 27 28 which concerned a relatively large sample size and accounted for a considerable proportion of women when pooling the results. In addition, the different methods applied to pool the results between two studies could have led to the difference. In the present study, the low level of heterogeneity (I2=31%) enabled us to use the fixed effect model with a more precise size of the effect, as well as narrow 95% confidence intervals. But owing to high heterogeneity (I2=98%) in previous meta-analysis, the authors had to use a random effect model with decreased precision and reliability of the results, resulting in the difference in the risk estimates.
Although some pathways may be mediated through subsequent overt diabetes, the significantly increased cardiovascular risk in women with gestational diabetes mellitus and no subsequent diabetes indicated the involvement of other mechanisms. Gestational diabetes mellitus has acute and persistent effects on cardiovascular and cerebrovascular systems. The presence of gestational diabetes mellitus with a brief period of exposure to potentially intense glucose intolerance would contribute to endothelial changes and subsequent endothelial dysfunction, which would be further accelerated in the context of comorbidities such as obesity and dyslipidaemia, even without development of type 2 diabetes, and finally lead to clinically overt cardiovascular and cerebrovascular diseases.41 42 In a recent investigation, women with gestational diabetes mellitus showed a substantial increase in common carotid artery-intima media thickness than controls at 36.2 weeks of gestation (0.81 v 0.55, P<0.001), which may be associated with the increased risk of future cardiovascular and cerebrovascular diseases after pregnancy.43 Moreover, a US multicentre, community based prospective cohort study found a positive correlation between worsening glucose tolerance and substantial increase in risk of coronary artery calcification.44 In addition, researchers also found a strong association between gestational diabetes mellitus and increased left ventricular mass and impaired left ventricular relaxation and systolic function, which were independent of subsequent development of type 2 diabetes.45 In a cross sectional study, women with gestational diabetes mellitus had lower global longitudinal strain of the left ventricle than women with uncomplicated pregnancy.46 Thus, the presence of gestational diabetes mellitus appears to lead to endothelial dysfunction and cardiovascular structural change and dysfunction, which can lead to premature cardiovascular and cerebrovascular diseases in the setting of conventional cardiovascular risk factors.
Limitations of this study
We acknowledge our study has some limitations. Firstly, as with all meta-analyses, the present study was limited by the quality of the included studies. Interpretation of the evidence from observational studies requires caution, as these study types are prone to selection bias, recall bias, and exaggeration of associations. The studies included in our meta-analysis were rated as either moderate or serious risk of bias, largely because of serious bias due to confounding (eg, race and ethnicity, socioeconomic status, maternal education). Confounding bias could affect the validity of our observed associations. Secondly, study designs, data materials, analytical approaches, periods covered, and quality of the studies varied, although the low heterogeneity partially indicated that our results were statistically reliable. Thirdly, no information on repeated measurements of the gestational diabetes mellitus diagnosis during follow-up are available in selected studies. Therefore, we cannot rule out bias due to misclassification during follow-up. Finally, using the GRADE framework we found that the low or very low quality evidence of study outcomes was largely attributed to the nature of the observational study design and potential confounding bias without adjustment for sufficient confounders. We expect quality of evidence to improve with future updates and more high quality studies.
Conclusion
Our systematic review and meta-analysis showed that women with a history gestational diabetes mellitus are at substantially higher risk of future cardiovascular and cerebrovascular diseases overall and of diverse common types of cardiovascular and cerebrovascular diseases. This excess risk cannot be solely attributed to conventional cardiovascular risk factors, which were partially mediated by subsequent diabetes. The findings contribute to a more comprehensive understanding of the adverse cardiovascular and cerebrovascular outcomes associated with gestational diabetes mellitus. Our results highlight the need for early intervention in women at high risk of gestational diabetes mellitus, and for continuous monitoring of women with gestational diabetes mellitus.
What is already known on this topic
The overall increased risk of cardiovascular diseases in women with a history of gestational diabetes mellitus has been increasingly recognised
The impact of gestational diabetes mellitus on type specific cardiovascular and cerebrovascular diseases as well as on venous thromboembolism is, however, largely unclear
What this study adds
In this meta-analysis of 15 unique studies involving more than eight million women, a history of gestational diabetes mellitus was associated with significantly increased risks of overall cardiovascular and cerebrovascular diseases and diverse common cardiovascular and cerebrovascular diseases to varying degrees
The findings cannot be solely attributed to conventional cardiovascular risk factors or subsequent diabetes
The results highlight the need for early intervention in women at high risk of gestational diabetes mellitus, and for continuous monitoring of women with gestational diabetes mellitus
Web extra.
Extra material supplied by authors
Supplementary information: Tables S1-S6, figures S1-S34, and appendices S1-S5
Contributors: ZZ conceived the study, participated in its design and coordination, and critically revised the manuscript. WX had full access to all the data collection, analysis, and interpretation, and drafted the manuscript. YW, SX, LQ, and YY contributed to the process of data collection and data analyses as study investigators. All authors approved the final manuscript. ZZ and WX are the guarantors. All authors had full access to all the data in the study, and the corresponding authors had final responsibility for the decision to submit for publication. The corresponding author (ZZ) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: None.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (ZZ) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The dissemination plan targets a wide audience, including members of the public, patients, patient and public communities, health professionals, and experts in the specialty through various channels: written communication, events and conferences, networks, and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
Study specific summary data are available from the corresponding author (zhuoli.zhang@126.com).
References
- 1. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47. 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 2. Reece EA, Leguizamón G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009;373:1789-97. 10.1016/S0140-6736(09)60515-8. [DOI] [PubMed] [Google Scholar]
- 3. Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2022;377:e067946. 10.1136/bmj-2021-067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu B, Cai J, Xu Y, et al. Early diagnosed gestational diabetes mellitus is associated with adverse pregnancy outcomes: a prospective cohort study. J Clin Endocrinol Metab 2020;105:dgaa633. 10.1210/clinem/dgaa633. [DOI] [PubMed] [Google Scholar]
- 5. Lowe WL, Jr, Scholtens DM, Kuang A, et al. HAPO Follow-up Study Cooperative Research Group . Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019;42:372-80. 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 2020;369:m1361. 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care 2017;40:101-8. 10.2337/dc16-1400. [DOI] [PubMed] [Google Scholar]
- 8. Bomback AS, Rekhtman Y, Whaley-Connell AT, et al. Gestational diabetes mellitus alone in the absence of subsequent diabetes is associated with microalbuminuria: results from the Kidney Early Evaluation Program (KEEP). Diabetes Care 2010;33:2586-91. 10.2337/dc10-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaiser K, Nielsen MF, Kallfa E, Dubietyte G, Lauszus FF. Metabolic syndrome in women with previous gestational diabetes. Sci Rep 2021;11:11558. 10.1038/s41598-021-90832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019;62:905-14. 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xie W, Huang H, Deng X, Gao D, Zhang Z. Modifiable lifestyle and environmental factors associated with onset of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol 2021;84:701-11. 10.1016/j.jaad.2020.08.060. [DOI] [PubMed] [Google Scholar]
- 15. Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol 2011;58:1378-85. 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 16. Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 2006;29:2078-83. 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 17. Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart 2013;99:1118-21. 10.1136/heartjnl-2013-303945. [DOI] [PubMed] [Google Scholar]
- 18. Fadl H, Magnuson A, Östlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG 2014;121:1530-6. 10.1111/1471-0528.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol 2014;180:41-4. 10.1093/aje/kwu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaul P, Savu A, Nerenberg KA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet Med 2015;32:164-73. 10.1111/dme.12635. [DOI] [PubMed] [Google Scholar]
- 21. Goueslard K, Cottenet J, Mariet AS, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol 2016;15:15. 10.1186/s12933-016-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tobias DK, Stuart JJ, Li S, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med 2017;177:1735-42. 10.1001/jamainternmed.2017.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daly B, Toulis KA, Thomas N, et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: a population-based cohort study. PLoS Med 2018;15:e1002488. 10.1371/journal.pmed.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKenzie-Sampson S, Paradis G, Healy-Profitós J, St-Pierre F, Auger N. Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol 2018;55:315-22. 10.1007/s00592-017-1099-2. [DOI] [PubMed] [Google Scholar]
- 25. Kabootari M, Hasheminia M, Guity K, Ramezankhani A, Azizi F, Hadaegh F. Gestational diabetes mellitus in mothers and long term cardiovascular disease in both parents: results of over a decade follow-up of the Iranian population. Atherosclerosis 2019;288:94-100. 10.1016/j.atherosclerosis.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 26. Echouffo-Tcheugui JB, Guan J, Retnakaran R, Shah BR. Gestational diabetes and incident heart failure: a cohort study. Diabetes Care 2021;44:2346-52. 10.2337/dc21-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun J, Kim GR, Lee SJ, Kim HC. Gestational diabetes mellitus and the role of intercurrent type 2 diabetes on long-term risk of cardiovascular events. Sci Rep 2021;11:21140. 10.1038/s41598-021-99993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu Y, Soohoo M, Sørensen HT, Li J, Arah OA. Gestational diabetes mellitus and the risks of overall and type-specific cardiovascular diseases: a population- and sibling-matched cohort study. Diabetes Care 2022;45:151-9. 10.2337/dc21-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SM, Shivakumar M, Park JW, et al. Long-term cardiovascular outcomes of gestational diabetes mellitus: a prospective population-based UK Biobank study. Am J Obstet Gynecol 2022;226(S1):S43-4 10.1016/j.ajog.2021.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ 2005;330:753. 10.1136/bmj.38356.424606.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Best LM, Mughal M, Gurusamy KS. Laparoscopic versus open gastrectomy for gastric cancer. Cochrane Database Syst Rev 2016;3:CD011389. 10.1002/14651858.CD011389.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallach JD, Sullivan PG, Trepanowski JF, Steyerberg EW, Ioannidis JP. Sex based subgroup differences in randomized controlled trials: empirical evidence from Cochrane meta-analyses. BMJ 2016;355:i5826. 10.1136/bmj.i5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Y, Peng N, Chen G, et al. Interaction between smoking and diabetes in relation to subsequent risk of cardiovascular events. Cardiovasc Diabetol 2022;21:14. 10.1186/s12933-022-01447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu G, Hu Y, Zong G, et al. Smoking cessation and weight change in relation to cardiovascular disease incidence and mortality in people with type 2 diabetes: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:125-33. 10.1016/S2213-8587(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aminian A, Wilson R, Zajichek A, et al. Cardiovascular outcomes in patients with type 2 diabetes and obesity: comparison of gastric bypass, sleeve gastrectomy, and usual care. Diabetes Care 2021;44:2552-63. 10.2337/dc20-3023. [DOI] [PubMed] [Google Scholar]
- 36. Iglay K, Hannachi H, Engel SS, et al. Comorbidities in type 2 diabetes patients with and without atherosclerotic cardiovascular disease: a retrospective database analysis. Curr Med Res Opin 2021;37:743-51. 10.1080/03007995.2021.1895736. [DOI] [PubMed] [Google Scholar]
- 37. Wang Y. Stage 1 hypertension and risk of cardiovascular disease mortality in United States adults with or without diabetes. J Hypertens 2022;40:794-803. 10.1097/HJH.0000000000003080. [DOI] [PubMed] [Google Scholar]
- 38. Regensteiner JG, Reusch JEB. Sex differences in cardiovascular consequences of hypertension, obesity, and diabetes: JACC Focus Seminar 4/7. J Am Coll Cardiol 2022;79:1492-505. 10.1016/j.jacc.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ismail SU, Asamane EA, Osei-Kwasi HA, Boateng D. Socioeconomic determinants of cardiovascular diseases, obesity, and diabetes among migrants in the United Kingdom: a systematic review. Int J Environ Res Public Health 2022;19:3070. 10.3390/ijerph19053070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Falkentoft AC, Zareini B, Andersen J, et al. Socioeconomic position and first-time major cardiovascular event in patients with type 2 diabetes: a Danish nationwide cohort study. Eur J Prev Cardiol 2022;28:1819-28. 10.1093/eurjpc/zwab065. [DOI] [PubMed] [Google Scholar]
- 41. Anastasiou E, Lekakis JP, Alevizaki M, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care 1998;21:2111-5. 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 42. Brewster S, Floras J, Zinman B, Retnakaran R. Endothelial function in women with and without a history of glucose intolerance in pregnancy. J Diabetes Res 2013;2013:382670. 10.1155/2013/382670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sonaglioni A, Nicolosi GL, Esposito V, Bianchi S, Lombardo M. Prognostic indicators of persistent carotid intima-media thickness increase in postpartum period in a population of normotensive women with gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2022;269:47-54. 10.1016/j.ejogrb.2021.12.020. [DOI] [PubMed] [Google Scholar]
- 44. Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA Study. Circulation 2021;143:974-87. 10.1161/CIRCULATIONAHA.120.047320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Appiah D, Schreiner PJ, Gunderson EP, et al. Association of gestational diabetes mellitus with left ventricular structure and function: the CARDIA Study. Diabetes Care 2016;39:400-7. 10.2337/dc15-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Company Calabuig AM, Nunez E, Sánchez A, Nicolaides KH, Charakida M, De Paco Matallana C. Three-dimensional echocardiography and cardiac strain imaging in women with gestational diabetes mellitus. Ultrasound Obstet Gynecol 2021;58:278-84. 10.1002/uog.23666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Tables S1-S6, figures S1-S34, and appendices S1-S5
Data Availability Statement
Study specific summary data are available from the corresponding author (zhuoli.zhang@126.com).