Abstract
The xps gene cluster is required for the second step of type II protein secretion in Xanthomonas campestris pv. campestris. Deletion of the entire gene cluster caused accumulation of secreted proteins in the periplasm. By analyzing protein abundance in the chromosomal mutant strains, we observed mutual dependence for normal steady-state levels between the XpsL and the XpsM proteins. The XpsL protein was undetectable in total lysate prepared from the xpsM mutant strain, and vice versa. Introduction of the wild-type xpsM gene carried on a plasmid into the xpsM mutant strain was sufficient for reappearance of the XpsL protein, and vice versa. Moreover, both XpsL and XpsM proteins were undetectable in the xpsN mutant strain. They were recovered either by reintroducing the wild-type xpsN gene or by introducing extra copies of wild-type xpsL or xpsM individually. Overproduction of wild-type XpsL and -M proteins simultaneously, but not separately, in the wild-type strain of X. campestris pv. campestris caused inhibition of secretion. Complementation of an xpsL or xpsM mutant strain with a plasmid-borne wild-type gene was inhibited by coexpression of XpsL and XpsM. The presence of the xpsN gene on the plasmid along with the xpsL and the xpsM genes caused more severe inhibition in both cases. Furthermore, complementation of the xpsN mutant strain was also inhibited. In both the wild-type strain and a strain with the xps gene cluster deleted (XC17433), carrying pCPP-LMN, which encodes all three proteins, each protein coprecipitated with the other two upon immunoprecipitation. Expression of pairwise combinations of the three proteins in XC17433 revealed that the XpsL-XpsM and XpsM-XpsN pairs still coprecipitated, whereas the XpsL-XpsN pair no longer coprecipitated.
The type II secretion pathway is used by a wide range of pathogenic gram-negative bacteria for the secretion of extracellular proteins (29, 33, 35). The secreted protein possesses a typical N-terminal signal peptide, which is cleaved by the signal peptidase upon its export across the cytoplasmic membrane through the Sec pathway. A maximum of 14 genes is required for the second step of the type II secretion pathway. Mutation in these genes causes accumulation of the secreted protein in the periplasm. Of the 14 protein components, two were located in the outer membrane, as suggested by sucrose gradient sedimentation analysis (9, 10, 15). The D homologues were demonstrated in various cases to form multimers, by either sucrose gradient sedimentation, gel filtration chromatography, or electron microscopy (3, 8, 18, 22, 24, 25). They were postulated to be the secretion channel. The PulS protein of Klebsiella oxytoca was shown to be copurified with the PulD protein at a 1:1 ratio, presumably as a component of the secretion channel (24). Of the remaining protein components, at least four (G, H, I, and J) have an N-terminal sequence similar to that of the type IV prepilin protein. They were shown to be processed by another protein, designated O except in Pseudomonas aeruginosa, in which it is called XcpA or PilD (2, 11, 16, 30, 40, 41). Disruption of the O protein also caused the secreted protein to accumulate in the periplasm (40). The four pilin-like proteins have been postulated to form pilus-like multimeric structures; thus, they are named pseudopilins. Recently, a pilus-like structure was demonstrated for the PulG protein of K. oxytoca upon electron microscopy by overexpressing the entire pul operon in Escherichia coli (39). The similarity between a fifth pseudopilin, the K protein, and the other pseudopilins is not so obvious (5). The remaining protein components, all of which but one (the C, F, L, M and N proteins) possess at least one putative membrane-anchoring sequence, are cytoplasmic membrane proteins. The last of all, the E protein, is predicted to be a cytoplasmic protein; however, it is associated with the cytoplasmic membrane through the L protein (36).
The cytoplasmic protein EpsE, which exhibited autokinase activity in vitro, was shown to associate with the cytoplasmic membrane via the EpsL protein in Vibrio cholerae (37). Interaction between the OutE and the OutL proteins of Erwinia chrysanthemi was also observed in the yeast two-hybrid system (31). Furthermore, overproduction of a truncated protein composed of the cytoplasmic domain of the OutL protein in the wild-type strain of E. chrysanthemi is inhibitory to normal secretion. Such inhibition was alleviated by overproduction of the wild-type OutE protein, suggesting interaction between the cytoplasmic domain of the OutL and the OutE protein. A nucleotide-binding motif, the Walker A box, with the sequence GXXGXGKT is conserved in all E proteins. Mutation in the nucleotide-binding motif has been shown to eliminate extracellular protein secretion in K. oxytoca, P. aeruginosa, V. cholerae, and E. chrysanthemi (26, 31, 36, 42). Moreover, autokinase activity of the EpsE protein was abolished as a result of mutation in the nucleotide-binding motif (36). In another case, the mutated OutE protein of E. chrysanthemi no longer exhibited OutL-dependent conformational change detected as proteinase K sensitivity (31).
An interactive relationship between the L and the M proteins was suggested primarily by the observation that XcpY (the L homologue) and XcpZ (the M homologue) of the secretion apparatus in P. aeruginosa are mutually required for normal steady-state protein levels (23). Mutual stabilization of the XcpY-XcpZ pair expressed in E. coli was also suggested by the observation that the abundance of each protein was increased by coexpression of the other one (23). Likewise, proteolytic degradation of the EpsL protein of the secretion apparatus of V. cholerae expressed in E. coli was reduced by coexpression of the EpsM protein (37). Although the L and the M proteins in these two microorganisms showed different degrees of instability in the absence of their partners, both observations suggested that the L and the M proteins probably stabilize each other through complex formation. Direct interaction between the EpsL and EpsM proteins of the secretion apparatus in V. cholerae was demonstrated by Sandkvist et al. (37) by coimmunoprecipitation. By exchanging various regions of the EpsL protein of V. cholerae with homologous regions of the ExeL protein of Aeromonas hydrophila, Sandkvist et al. (38) constructed a series of EpsL-ExeL and ExeL-EpsL chimeric proteins. They further demonstrated that the region between amino acid residues 216 and 296 of the EpsL protein is likely to be involved in its complex formation with the EpsM protein. A typical transmembrane sequence predicted within this region is detected at similar positions in all L proteins. It was proposed that complex formation between EpsL and EpsM probably occurs through the transmembrane regions in each protein. A transmembrane sequence is predicted at the N terminus of all M proteins. Coimmunoprecipitation between the PulL and the PulM proteins of K. oxytoca was also observed (28).
The xpsM gene is located upstream of the xpsN gene (19), which is required for extracellular protein secretion in Xanthomonas campestris pv. campestris. It encodes a protein of 213 amino acid residues (accession number M81111). A putative transmembrane segment is predicted at its N terminus (residues 10 to 28). Further upstream is the xpsL gene, which encodes a protein of 373 amino acid residues (GenBank accession number L02630). A putative membrane-anchoring sequence is predicted at residues 215 to 233. To understand the interactive relationship of the XpsM protein with other protein components in X. campestris pv. campestris, we constructed a chromosomal nonpolar mutant strain with a mutation in the xpsM gene. By monitoring the abundance of other Xps proteins with available antibodies, we detected all but XpsL in the mutant strain. Further analysis revealed mutual dependence for normal steady-state levels of the XpsL and -M proteins. Moreover, neither protein was detected in the ΔxpsN mutant strain. Recovery of both was made possible by reintroducing the wild-type xpsN gene or by introducing extra copies of the xpsL or the xpsM gene alone. Coexpression of all three proteins from plasmid-borne genes in the secretion-positive strain caused inhibition. Complementation of the chromosomal mutation in each of the three genes with a plasmid-borne wild-type gene was also inhibited by coexpression with the other two. We further demonstrated that each protein coimmunoprecipitated with the other two when all three were coexpressed. Our interpretation of these observations is discussed below.
MATERIALS AND METHODS
Bacterial strains, plasmids, and antisera.
E. coli BL21(DE3) was a kind gift from F. W. Studier. X. campestris pv. campestris XC1701 was originally isolated as a spontaneous Rifr mutant from a wild isolate, XC17. XC17433 is a secretion mutant strain in which the entire xps gene cluster (xpsEFGHIJKLMND) was deleted (14). XC1709 and XC1712 are chromosomal nonpolar mutant strains with in-frame deletions of xpsN and xpsL, respectively (19; Leu et al., unpublished data). All plasmids used in this study are listed in Table 1. All antisera were prepared from rabbits. Antiserum against XpsN was prepared as described previously (19) and used in immunoblot reactions at 1:250. Antiserum against purified α-amylase was used at 1:500. Antiserum against XpsL (used at 1:200) or against XpsG (used at 1:1,000) was prepared against a nickel-nitrilotriacetic acid column-purified TrxA-His6-XpsLN (N for N-terminal domain) fusion protein or the TrxA-His6-XpsG fusion protein, respectively, that was overexpressed from the T7 promoter in E. coli BL21(DE3) (Leu et al., unpublished data; Chen et al., unpublished data). Antiserum against E. coli OmpA, used in detecting the OprF protein at 1:60,000, was a kind gift from U. Henning.
TABLE 1.
Plasmids
| Plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Plasmids used in raising antibody against XpsM | ||
| pET32a | Ampr; PT7-containing expression vector for expressing N-terminal TrxA-His6 tag fusion | Novagene |
| pET32M | Ampr; a PCR fragment encoding amino acid residues 41 to 185 of XpsM cloned in pET32a at BamHI and SalI sites | This study |
| Plasmids used in constructing chromosomal mutations in xpsM | ||
| pSelect-1 | Tetr; a phagemid vector; ColE1 replicon with an f1(−) ori | Promega |
| pBluescript II SK(−) | Ampr; a phagemid vector; ColE1 replicon with an f1(−) ori | Stratagene |
| pUCD4121 | Camr Sucs; derivative of pTZ18R | C. I. Kado |
| pUC4K | Ampr Kanr; kanr gene cassette can be excised as a restriction fragment | Pharmacia Biotech |
| pKC101 | Tetr; a 6.4-kb EcoRI fragment containing the xpsKLMND genes cloned in pSelect-1 | Wang and Hu (unpublished) data |
| pST102 | Ampr; an EcoRI-SalI fragment of 5.8 kb containing the xpsKLMN genes subcloned in pSelect-1 | This study |
| pST107 | Camr Sucs; an EcoRI-SalI fragment of 5.8 kb containing wild-type xpsKLN and the mutated xpsM gene subcloned at XbaI-SalI sites in pUCD4121 | This study |
| pST108 | Kanr Camr Sucs; a 1.3-kb SalI fragment containing the Kanr gene cassette subcloned from pUC4K at SalI site in pST107 | This study |
| Plasmids used in expressing xpsM in combinations with xpsL and xpsN | ||
| pCPP30 and pCPP33 | Tra− Mob+ Tetr IncP replicon, each with different mutiple cloning sites downstream of Plac | D. W. Bauer |
| pNC2 | SacII-BclI 1.2-kb fragment carrying the xpsN gene subcloned at HindIII-BamHI sites downstream of Plac in pCPP30 | 19 |
| pCPP-30L2 | 2.3-kb NcoI-EcoRI fragment carrying the xpsL gene subcloned at KpnI-EcoRI sites downstream of Plac in pCPP30; xpsM was disrupted by frameshifts; a 0.7-kb fragment in the xpsN region was deleted during cloning | This study |
| pST109 | 1.7-kb MluI-BamHI fragment containing xpsM subcloned at EcoRI-HindIII sites downstream of Plac in pCPP33 | This study |
| pCPP-LMN | 3-kb NcoI-BclI fragment containing the wild-type xpsLMN genes subcloned at KpnI-EcoRI sites downstream of Plac in pCPP30 | This study |
| pCPP-LM | xpsN in pCPP-LMN disrupted by deletion of a 0.6-kb BamHI fragment | This study |
| pCPP-MN | xpsL in pCPP-LMN disrupted by an in-frame deletion of a 0.5-kb StuI fragment | This study |
| pCPP-LN2 | xpsM in pCPP-LMN replaced with the frameshift mutant xpsM gene of pST107 | This study |
Production of antibody against XpsM.
A truncated XpsM protein was overexpressed as a fusion protein with an N-terminal thioredoxin (TrxA) and a His6 tag from the T7 promoter located on the T7 expression vector pET32. The overexpression plasmid pET32M was constructed by cloning a PCR-generated fragment of the xpsM gene encoding amino acids 41 to 185 of XpsM in frame with the trxA gene. The PCR primers used were Mfor (5′-CACTTGGGATCCGACGAATTGCAATCGCTG-3′) and Mrev (5′-CACTTGGTCGACCAGTTCGAAGGCGATGTCCAG-3′). The fusion protein TrxA-His6-XpsM overexpressed in E. coli BL21(DE3) was purified by passing it through a nickel-nitrilotriacetic acid column following the procedures suggested by the supplier. Sera were prepared following the procedures of Harlow and Lane (12). Immunoblot analysis (used at 1:200) indicated that the antiserum could detect in X. campestris pv. campestris XC1701 a protein with an apparent molecular mass of approximately 25 kDa that is absent in XC17433, in which the entire xps gene cluster was deleted (14).
Construction of a chromosomal, nonpolar xpsM mutant.
Following the procedures suggested by the supplier of the Altered Sites II in vitro mutagenesis system (Promega), we introduced two insertions into the xpsM gene located on the plasmid pST101. The primer MAPA, 5′-GCCTTGGGCCCTGTTGCTGCTG-3′, was used for introducing a single C insertion downstream of the 43rd base of the xpsM ORF, which also created an ApaI site. The primer MHD, 5′-GCCAACGAAAGCTTGGCAATGGCCTG-3′, was used for inserting two T's downstream of the 525th base and creating a HindIII site. As a result of two frameshifts, the amino acid sequence between the 15th and the 175th amino acid residues of XpsM was altered without generating a premature stop codon that might exert a polar effect on the downstream gene expression. Subsequently, the mutated xpsM gene was introduced into X. campestris pv. campestris genome following the procedures of Kamoun et al. (17) with slight modifications as described elsewhere (19). Southern blot analysis of chromosomal DNA digested with ApaI and with HindIII in combination with EcoRI confirmed that only the mutated xpsM gene was present in the genome. We designated this strain XC1714.
Triparental conjugation.
A DNA fragment cloned in the broad-host-range vector pCPP30 or pCPP33 (Fig. 1) was introduced into X. campestris pv. campestris via triparental conjugation. E. coli DH5α containing the recombinant plasmid served as the donor. E. coli HB101 containing pRK2013 served as a helper, and X. campestris pv. campestris served as the recipient. Since the conjugation frequency is quite high, we developed a simple cross-streaking method. Picked from fresh colonies, the donor and the helper were streaked onto Luria-Bertani agar plates in parallel, and the recipient was streaked across the donor and the helper in that order. After incubation at 28°C overnight, cells grown near the end of the recipient streak were streaked out for single colonies on the selection plate containing rifampin (100 μg/ml) and kanamycin (50 μg/ml). Streaking for single colonies was repeated to avoid possible E. coli contamination. Plasmid isolated from the putative transconjugant was examined by digestion with restriction enzymes.
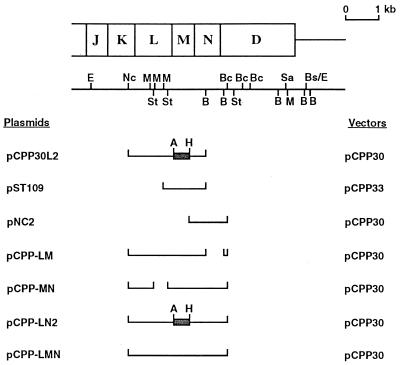
FIG. 1.
Expression of xpsL, -M, and -N genes, separately and in different combinations, from the Plac promoter on broad-host-range vector pCPP30. The locations of the xpsJ, -K, -L, -M, -N, and -D genes are shown on top of the restriction map. The DNA fragment cloned in each plasmid is represented by a single line. The frameshifted xpsM gene is designated by a shaded box flanked by A (ApaI site) and H (HindIII site). Bs/E designates an original BstEII site that was filled in and ligated to an EcoRI linker. The other restriction enzyme sites are abbreviated as follows: Nc, NcoI; M, MluI; St, StuI; B, BamHI; Bc, BclI; Sa, SalI.
Starch plate assay for α-amylase secretion.
The method of Hu et al. (14) for the starch plate assay for α-amylase secretion was used.
Subcellular fractionation.
For subcellular fractionation, the procedures described by Hu et al. (15) were followed with slight modifications. In brief, cells were grown in XOL with 0.4% maltose as the carbon source to an A600 of 1. Supernatant collected upon centrifugation at 11,000 × g for 5 min was saved as the extracellular fraction. Spheroplasts were prepared upon lysozyme (3 mg/ml, wt/vol) treatment of the cells on ice for 1 h in 10 mM HEPES, pH 8.0, containing 20% (wt/vol) sucrose. Supernatant collected from centrifugation of the spheroplasts at 11,000 × g for 30 min was saved as the periplasmic fraction. The spheroplasts were gently rinsed with 10 mM HEPES (pH 8.0) containing 20% sucrose, suspended in 10 mM Tris-HCl (pH 8.0), and saved as the spheroplast fraction. The extracellular and the periplasmic fractions were concentrated upon trichloroacetic acid precipitation as described previously (15). Equivalent amounts of each fraction were loaded on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels for immunoblot analysis of α-amylase distribution.
Sucrose gradient sedimentation analysis.
Total membrane was analyzed on a step sucrose gradient (25 to 61%, wt/wt) following the procedures of Lee et al. (19). Each fraction was analyzed for density, for distribution of the XpsM and OprF (OmpA homologue) proteins, and for succinate dehydrogenase activity as described previously (19).
SDS-PAGE and immunoblot analysis.
For SDS-PAGE, the procedures of Hu et al. (15) were followed, with slight modifications. Separation of total protein on SDS-polyacrylamide gels (10 or 12.5% [wt/vol] acrylamide) was followed by electroblotting onto polyvinylidene difluoride (PVDF) membranes at 100 V for 60 min. At the completion of electrotransfer, the PVDF membrane was soaked in blocking buffer (TBS buffer [10 mM Tris-HCl, pH 7.4; 0.9%, wt/vol, NaCl] plus 5% [wt/vol] nonfat milk powder) for 30 to 60 min before addition of the rabbit antiserum against the targeted protein. Subsequently the mixture was incubated at room temperature for 2 h or at 4°C overnight. Incubation with the goat anti-rabbit immunoglobulin G antibody conjugated with peroxidase for 1 to 2 h was preceded by two washes with TBS buffer (5 to 10 min each time). After repeated washing with TBS buffer, the PVDF membrane was soaked in TBS buffer containing 0.01% (wt/vol) 4-chloro-1-naphthol and 0.2% (wt/vol) H2O2. To minimize the cross-reactive signals on immunoblots, antisera against XpsL and XpsM were preincubated in a final volume of 500 μl with XpsL- or XpsM-negative crude lysate (at 1.5 to 3 mg of protein/ml) at room temperature for 30 min before use. Crude lysates of E. coli BL21(DE3)(pET32a) and XC1712 (xpsL) or XC1714 (xpsM) were prepared by breaking cells through a French press and then collecting supernatant after centrifugation at 785 × g at 4°C for 20 min.
Immunoprecipitation.
The procedures of Lee et al. (19) for immunoprecipitation were followed. Two milligrams of Triton X-100 extract was included in the immunoprecipitation mixture. The percentage of coimmunoprecipitated proteins was estimated by calibrating with immunoprecipitation efficiency of each antibody. Densitometric determination of the protein amounts detected on immunoblot was carried out using TINA 2.09e software. Only signals that fell within a linear range were used in the calculation.
RESULTS
XpsM is a cytoplasmic membrane protein required for extracellular protein secretion.
By analyzing α-amylase secretion on starch plates, we observed that the clear zone produced by the xpsM mutant strain XC1714 was much smaller than that produced by the parental strain (Fig. 2C). In agreement with this observation, immunoblot analysis of subcellular distribution of α-amylase also indicated that the xpsM gene is required for α-amylase secretion across the outer membrane. In contrast to the parental strain XC1701, where α-amylase was mainly present in the extracellular fraction, major portions of α-amylase in the mutant XC1714 were detected in the periplasmic fraction (data not shown). Introduction of the plasmid pST109, which carries a wild-type xpsM gene alone, complemented the secretion defect in XC1714 (Fig. 2C), suggesting that mutation in the chromosomal xpsM gene did not have a polar effect on its downstream gene expression. Sucrose gradient sedimentation analysis of the membrane vesicles prepared from the parental strain XC1701 followed by immunoblot analysis of XpsM indicated that XpsM cofractionated with the cytoplasmic membrane protein succinate dehydrogenase (data not shown), suggesting that it is a cytoplasmic membrane protein.
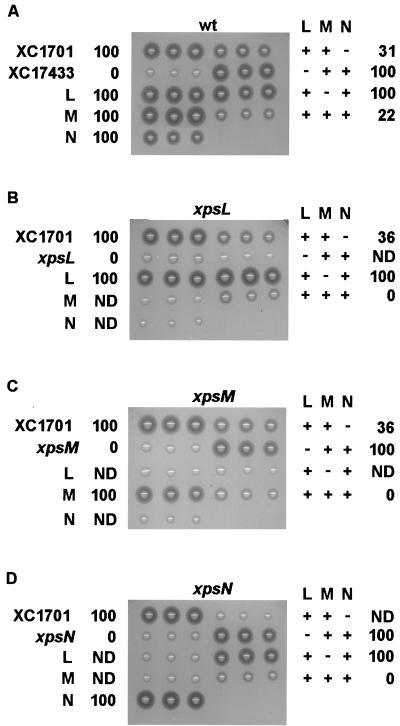
FIG. 2.
Starch plate assay for α-amylase secretion. Freshly grown colonies were transferred with toothpicks in triplicate onto XOL-starch (2%, wt/vol) plates and incubated at 28°C overnight. XC1701 and XC17433 were included as secretion-positive and -negative controls, respectively. Plasmids were introduced into XC1701 (wild-type [wt] parental strain) (A), XC1712 (xpsL mutant) (B), XC1714 (xpsM mutant) (C), and XC1709 (xpsN mutant) (D). L, M, and N represent xpsL, -M, and -N, , respectively. Numbers next to each strain represent the percentage of α-amylase secreted. They were determined by immunoblot analysis of α-amylase in extracellular and cellular fractions. ND, not determined. XC1701(pNC2) tends to form smaller colonies, and so the clear zones on the starch plate appear to be smaller. However, immunoblot analysis detected no α-amylase in the cellular fraction, as indicated by 100% secretion.
Dependence on XpsN for protein abundance of XpsL and -M expressed from chromosomal genes.
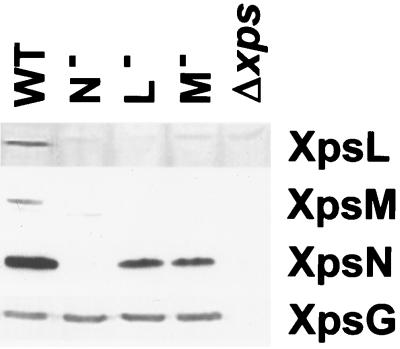
Immunoblot analysis of the xpsM mutant strain XC1714 for other Xps proteins with antibodies available detected all but the XpsL protein. Furthermore, cross-examination of the XpsL, -M, and -N proteins in the chromosomal mutant XC1712 (xpsL), XC1714 (xpsM), and XC1709 (xpsN) revealed complex relationships among them. Both the XpsL and XpsM are undetectable in all strains examined (Fig. 3). The amount of XpsN was clearly reduced in the xpsL and xpsM mutant strains (Fig. 3). All samples were loaded in equal amounts, as judged by immunoblotting with antibody against the pseudopilin XpsG protein and Coomassie blue staining of the gel (data not shown).
FIG. 3.
Abundance of XpsL, XpsM, and XpsN in xpsL, xpsM, and xpsN mutants. X. campestris pv. campestris cells grown in LB medium to an A600 of ∼1 were broken with a sonicator followed by mixing with equal volumes of 2× sample buffer. Ten or fifteen microliters of sample at a cell density of 10 A600 units were loaded on SDS-PAGE gels, followed by immunoblotting with antibody against XpsL, -M, -N, and -G. WT, XC1701; N−, XC1709; L−, XC1712; M−, XC1714; Δxps, XC17433.
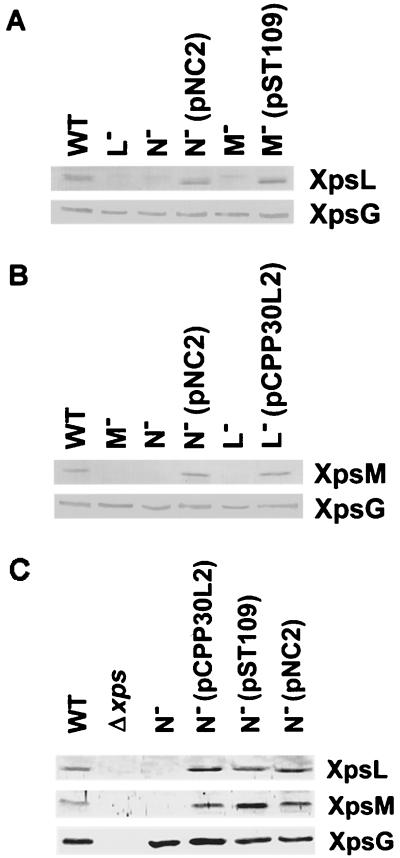
The XpsL protein reappeared in the complemented xpsM mutant strain XC1714(pST109), in which the plasmid carries the wild-type xpsM gene alone, as well as in the complemented xpsN mutant strain XC1709(pNC2), in which the plasmid carries the wild-type xpsN gene alone (Fig. 4A). Similarly, the XpsM protein reappeared in the complemented xpsL mutant strain XC1712(pCPP30L2), in which the plasmid carries the wild-type xpsL gene alone. This protein was also detected in the complemented xpsN mutant strain XC1709(pNC2) (Fig. 4B). On the other hand, in the xpsN mutant strain XC1709, both XpsL and XpsM were recovered by supplementing a plasmid that carries either xpsL or xpsM (Fig. 4C). Slightly larger amounts of XpsM appeared in the strain carrying pST109 (xpsM) than in that carrying pNC2 (xpsN). Presumably, this reflects a higher xpsM gene copy number in the former strain. Interestingly, the increase in the XpsL protein in XC1709(pCPP30L2) was not so obvious, but the reason for this is not clear.
FIG. 4.
Recovery of XpsL (A) and XpsM (B) in complemented strains. (C) Recovery of XpsL and XpsM in an xpsN mutant strain. Samples were prepared as described in the legend to Fig. 3 and are designated as in Fig. 3. The plasmids pNC2, pST109, and pCPP30L2 carry wild-type xpsN, xpsM, and xpsL, respectively.
Overproduction of wild-type XpsL and -M, with or without XpsN, in the wild-type strain inhibited secretion.
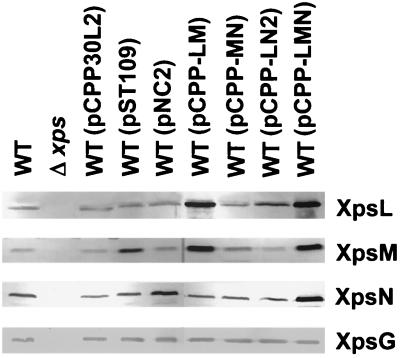
In order to unravel the complex relationships among the XpsL, -M, and -N proteins, we constructed four plasmids, of which one carries all three genes simultaneously (pCPP-LMN) and three encode the three genes in pairs (pCPP-LM, pCPP-MN, and pCPP-LN2). Following introduction of each plasmid into the parental strain XC1701 via triparental conjugation, we examined all transconjugants on starch plates for α-amylase secretion and examined its subcellular distribution by immunoblot analysis. It is clear that none of the singly expressed XpsL, XpsM, and XpsN proteins caused inhibition of α-amylase secretion (Fig. 2A). Only pCPP-LM and pCPP-LMN resulted in inhibition. The latter (22% of total is secreted) caused more severe inhibition than the former (31% of total is secreted). pCPP-MN and pCPP-LN2 did not result in inhibition (Fig. 2A).
Examination of total lysates of these transconjugants on immunoblots for steady-state levels of the XpsL and the XpsM proteins revealed that secretion inhibition correlated with a high steady-state level of XpsL (pCPP30L2) as well as XpsM (pST109) (Fig. 5). These results suggested that overproduction of XpsL and XpsM, which was made possible only by their coexpression from the plasmid, caused secretion inhibition. The results are reminiscent of the observations that the chromosomal xpsL and xpsM genes are required for the detection of each other's protein product (Fig. 3 and 4). The steady-state level of XpsN appeared to be slightly higher when this protein was coexpressed with XpsL and XpsM (pCPP-LMN) than when it was expressed alone (pNC2) (Fig. 5). This agrees with the observation that the normal steady-state level of chromosome-encoded XpsN protein requires the simultaneous presence of XpsL and XpsM (unpublished results).
FIG. 5.
Abundance of XpsL, -M, and -N expressed from different plasmids in XC1701 (wild type [WT]). Sample preparation and immunoblot analysis were conducted as described in the legend to Fig. 3. Δxps designates XC17433.
Coexpression of XpsL, -M, and -N caused negative complementation.
The xpsL (XC1712) and xpsM (XC1714) mutants were complemented in α-amylase secretion by the singly expressed xpsL and xpsM genes, respectively (Fig. 2B and C). Coexpression of each with XpsN did not affect positive complementation. However, when XpsL and XpsM were coexpressed in either mutant strain (xpsL or xpsM), negative complementation was observed (Fig. 2B and C). Coexpression of all three proteins caused a more severe negative effect (0% of total is secreted) than coexpression of XpsL and -M (36% of total is secreted). Negative complementation was also observed in the xpsN mutant strain XC1709 when XpsN was coexpressed with XpsL and XpsM (Fig. 2D). Complementation of the xpsN mutant with the singly expressed xpsN gene was as effective as when XpsN was coexpressed with either XpsL or XpsM (Fig. 2D). These results suggested that negative complementation of all three mutants is probably similar to secretion inhibition in the wild-type strain XC1701 in terms of its correlation with simultaneous overexpression of the XpsL, -M, and -N proteins.
Complex formation detected by coimmunoprecipitation.
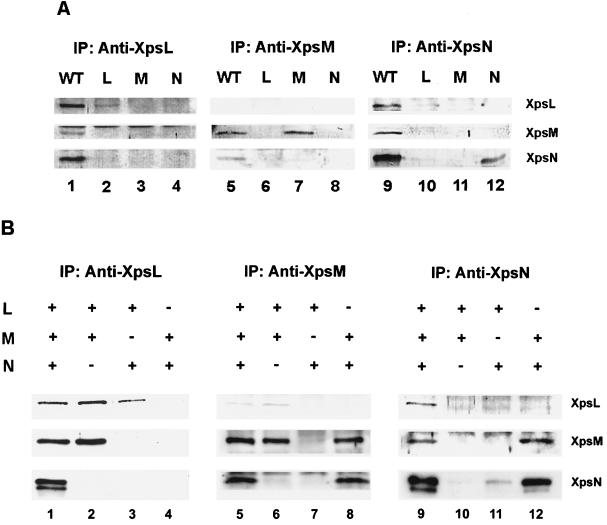
In order to examine the possibility of complex formation among the three proteins, we conducted coimmunoprecipitation experiments on the parental strain XC1701. When antibody against XpsL was included in the immunoprecipitation mixture, all three proteins (XpsL, -M, and -N) were detected on the immunoblot (Fig. 6A). Likewise, all three proteins were precipitated by antibody against XpsN (Fig. 6A). When antibody against XpsM was incubated with the Triton X-100 extract, XpsN was precipitated along with XpsM (Fig. 6A). Immunoprecipitation by antibody against XpsM was approximately three- to fourfold less efficient than that by antibody against the XpsN or XpsL. That could explain why XpsL was not detected in the anti-XpsM antibody precipitate of XC1701.
FIG. 6.
Coimmunoprecipitation. Triton X-100 extracts of membrane proteins prepared from the different strains were immunoprecipitated (IP) with antibody against XpsL, XpsM, or XpsN, followed by immunoblotting with antibody against XpsL (top panels), XpsM (middle panels), or XpsN (bottom panels). “+” and “−” designate presence and absence, respectively, of the gene. (A) XC1701 (lanes 1, 5, and 9), XC17433(pCPP30L2) (lanes 2, 6, and 10), XC17433(pST109) (lanes 3, 7, and 11), and XC17433(pNC2) (lanes 4, 8, and 12); (B) XC17433(pCPP-LMN) (lanes 1, 5, and 9), XC17433(pCPP-LM) (lanes 2, 6, and 10), XC17433 (pCPP-LN2) (lanes 3, 7, and 11), and XC17433(pCPP-MN) (lanes 4, 8, and 12).
In order to determine if each pair of the three proteins coprecipitates in the absence of all other Xps proteins, we performed similar coimmunoprecipitation experiments on XC17433(pCPP-LMN), in which the XpsL, -M, and -N proteins are expressed from plasmid-borne genes without other xps genes. In addition, to determine the relationships within each pair of the three proteins we also conducted coprecipitation experiments on the following strains that express pairwise combinations of the three proteins in the absence of other Xps proteins, XC17433(pCPP-LM), XC17433(pCPP-MN), and XC17433(pCPP-LN2). The results indicated that each protein coprecipitated with the other two in XC17433(pCPP-LMN) (Fig. 6B, lanes 1, 5, and 9). Pairwise combinations revealed that XpsM coprecipitated with either XpsL (Fig. 6B, lanes 2 and 6) or XpsN (Fig. 6B, lanes 8 and 12). In contrast, coprecipitation between XpsL and XpsN was undetectable in the absence of XpsM (Fig. 6B, lanes 3 and 11). A minor band migrating faster than XpsN was detected when XpsN was in high abundance (Fig. 6B, lanes 1, 9, and 12), the reason for which is not clear. We also noted that abundance of the XpsL protein was apparently lower when XpsL was coexpressed with XpsN than in other cases and vice versa (Fig. 6B, lanes 3 and 11). Semiquantitative analysis of the immunoblot from coimmunoprecipitation experiments on XC17433(pCPP-LMN) indicated that approximately 3 to 18% of each protein coprecipitated with one of the other two.
DISCUSSION
By analyzing the XpsL, -M, and -N protein steady-state levels in strains with chromosomal knockouts of each gene, we observed mutual dependence of the XpsL and XpsM for their detection in SDS-polyacrylamide gels. This is similar to the situation in P. aeruginosa (23). Formation of the XpsL-XpsM complex was suggested by their coimmunoprecipitation, as observed in V. cholerae and in K. oxytoca (28, 37). Furthermore, we found that the xpsN gene is required for maintaining both XpsL and -M proteins at normal steady-state levels. In the xpsN knockout strain, both XpsL and -M proteins were undetectable on the immunoblot. Both were recovered when the wild-type xpsN gene alone was reintroduced. Complex formation between XpsN and XpsM was supported by their coimmunoprecipitation, with or without coexpression of XpsL. Coprecipitation between XpsN and the XpsL occurs as long as XpsM is present. In its absence, both XpsN and XpsL levels were reduced. Apparently, XpsM plays a critical role in maintaining both at normal steady-state levels. Decreases in the XpsN and XpsL levels could explain why their coprecipitation was not detected when XpsM was absent. Alternatively, this result could be the consequence of a lack of complex formation due to the absence of XpsM. The number of complexes and their exact composition are not known at the present time.
Introducing extra copies of either xpsL (carried on pCPP30L2) or xpsM (carried on pST109) alone into the xpsN mutant strain XC1709 resulted in the reappearance of both XpsL and XpsM. This observation suggests that detection of XpsL and XpsM in the absence of the xpsN gene is possible if the copy number of one of the genes is raised. We hypothesize that XpsN is involved in maintaining the XpsL-XpsM pair at normal steady-state level by enhancing XpsL-XpsM complex formation or stabilizing the complex. Such a requirement becomes obsolete when the level of either member of the pair is raised, implying a weak association between the XpsL and XpsM. Reliance on XpsN for maintaining their association in normal situations could be bypassed by raising either one of the protein concentrations. Although the XpsN coimmunoprecipitated with either protein, whether XpsN forms a complex with XpsL-XpsM (XpsL-XpsM-XpsN) remains to be determined.
The xpsN mutant strain supplemented with the xpsL or the xpsM gene is unable to secrete (Fig. 2D), suggesting that XpsN plays other roles essential to the secretion process. An association between XpsN and the outer membrane protein XpsD was recently demonstrated with coimmunoprecipitation data (19). Interactive relationships between XpsN and the outer membrane protein on one side and the cytoplasmic membrane XpsL-XpsM complex on the other bear a resemblance to those observed for the TonB protein. TonB is a cytoplasmic membrane protein that serves as the energy transducer for the uptake of iron-siderophore complexes and vitamin B12 in E. coli (7). Sucrose gradient sedimentation analysis indicated that it shuttles between the cytoplasmic membrane and the outer membrane (20). Its interaction with the outer membrane through the receptors for iron-siderophore complexes was suggested by cross-linking experiments (13). In the cytoplasmic membrane, it probably forms a complex with the hexameric ExbB-ExbD complex, also suggested by cross-linking results (13). TonB-dependent activities require proton motive force (PMF) (6, 32). Both PMF and ATP were demonstrated to be required for type II secretion in Aeromonas species (21, 43). Similar to TonB with the ExbB-ExbD complex, the N protein along with the L-M complex probably serves as the energy transducer for the type II secretion apparatus by coupling PMF across the cytoplasmic membrane to drive protein secretion through the channel composed of the D protein in the outer membrane. An alternative (or additional) energy source for type II secretion apparatus could come from the putative “molecular motor” E protein, whose homologues contain an indispensable nucleotide-binding motif. One may speculate that energy generated from E protein could be coupled to protein secretion across the outer membrane through its interaction with L protein (36). Nevertheless, without further experiments we cannot rule out other possible roles for XpsN. For instance, it could serve to signal channel opening, as proposed for the pI protein in the assembly of filamentous phage (34).
The N homologue was not found in E. chrysanthemi or P. aeruginosa. Moreover, PulN in K. oxytoca is not required for the secretion process (28). In these cases, the biological roles played by XpsN in X. campestris pv. campestris could be engaged by another cytoplasmic membrane protein, for instance, the C homologue, whose molecular size and membrane topology are similar to those of the N protein. The abundance of the C homologues in K. oxytoca (27) and P. aeruginosa (4) has been shown to depend on their respective D proteins. Interestingly, C homologues were identified in the type II secretion apparatus of all microorganisms but X. campestris pv. campestris.
Contradictory to our observations, overproduction of the wild-type L protein was demonstrated to be inhibitory in P. aeruginosa and in V. cholerae (1, 23, 38). We have recently been able to raise the XpsL protein level by subcloning the xpsL gene into a broad-host-range vector with a copy number higher than that of pCPP30. By introducing this plasmid into a wild-type strain, we observed very weak inhibition (unpublished results). However, introduction of a second plasmid carrying the xpsM gene alone raised the XpsL protein level (at least 10 times) as well as the inhibitory effect significantly. These observations suggest that it is possible to cause inhibition, albeit very weak, by overproduction of XpsL alone. However, for strong inhibition, simultaneous overproduction of XpsM is necessary. Apparently, the abundance of XpsL relies strongly on XpsM. At the present time we cannot distinguish whether such strong inhibition is caused by overproduced XpsL-XpsM complex or by overproduced XpsL alone.
ACKNOWLEDGMENTS
This work was supported by grants from the National Science Council of the Republic of China, NSC88-2311-B-005-008-B31 and NSC89-2311-B-005-003-B31.
H.-M. Lee and S.-W. Tyan made equal contributions to this work.
REFERENCES
- 1.Ball G, Chapon-Herve V, Bleves S, Michel G, Bally M. Assembly of XcpR in the cytoplasmic membrane is required for extracellular protein secretion in Pseudomonas aeruginosa. J Bacteriol. 1999;181:382–388. doi: 10.1128/jb.181.2.382-388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bally M, Ball G, Badere A, Lazdunski A. Protein secretion in Pseudomonas aeruginosa: the xcpA gene encodes an integral inner membrane protein homologous to Klebsiella pneumoniae secretion function protein PulO. J Bacteriol. 1991;173:479–486. doi: 10.1128/jb.173.2.479-486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 4.Bleves S, Gerard-Vincent M, Lazdunski A, Filloux A. Structure-function analysis of XcpP, a component involved in general secretory pathway-dependent protein secretion in Pseudomonas aeruginosa. J Bacteriol. 1999;181:4012–4019. doi: 10.1128/jb.181.13.4012-4019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleves S, Voulhoux R, Michel G, Lazdunski A, Tommassen J, Filloux A. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family) Mol Microbiol. 1998;27:31–40. doi: 10.1046/j.1365-2958.1998.00653.x. [DOI] [PubMed] [Google Scholar]
- 6.Bradbeer C. The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J Bacteriol. 1993;175:3146–3150. doi: 10.1128/jb.175.10.3146-3150.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen L Y, Chen D Y, Miaw J, Hu N T. XpsD, an outer membrane protein required for protein secretion by Xanthomonas campestris pv. campestris, forms a multimer. J Biol Chem. 1996;271:2703–2708. doi: 10.1074/jbc.271.5.2703. [DOI] [PubMed] [Google Scholar]
- 9.D'Enfert C, Pugsley A P. Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J Bacteriol. 1989;171:3673–3679. doi: 10.1128/jb.171.7.3673-3679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.d'Enfert C, Reyss I, Wandersman C, Pugsley A P. Protein secretion by gram-negative bacteria: characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J Biol Chem. 1989;264:17462–17468. [PubMed] [Google Scholar]
- 11.Dupuy B, Taha M K, Possot O, Marchal C, Pugsley A P. PulO, a component of the pullulanase secretion pathway of Klebsiella oxytoca, correctly and efficiently processes gonococcal type IV prepilin in Escherichia coli. Mol Microbiol. 1992;6:1887–1894. doi: 10.1111/j.1365-2958.1992.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 13.Higgs P I, Myers P S, Postle K. Interactions in the TonB-dependent energy transduction complex: ExbB and ExbD form homomultimers. J Bacteriol. 1998;180:6031–6038. doi: 10.1128/jb.180.22.6031-6038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu N T, Hung M N, Chiou S J, Tang F, Chiang D C, Huang H Y, Wu C Y. Cloning and characterization of a gene required for the secretion of extracellular enzymes across the outer membrane by Xanthomonas campestris pv. campestris. J Bacteriol. 1992;174:2679–2687. doi: 10.1128/jb.174.8.2679-2687.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu N T, Hung M N, Liao C T, Lin M H. Subcellular location of XpsD, a protein required for extracellular protein secretion by Xanthomonas campestris pv. campestris. Microbiology. 1995;141:1395–1406. doi: 10.1099/13500872-141-6-1395. [DOI] [PubMed] [Google Scholar]
- 16.Hu N T, Lee P F, Chen C. The type IV pre-pilin leader peptidase of Xanthomonas campestris pv. campestris is functional without conserved cysteine residues. Mol Microbiol. 1995;18:769–777. doi: 10.1111/j.1365-2958.1995.mmi_18040769.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamoun S, Tola E, Kamdar H, Kado C I. Rapid generation of directed and unmarked deletions in Xanthomonas. Mol Microbiol. 1992;6:809–816. doi: 10.1111/j.1365-2958.1992.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 18.Kazmierczak B I, Mielke D L, Russel M, Model P. pIV, a filamentous phage protein that mediates phage export across the bacterial cell envelope, forms a multimer. J Mol Biol. 1994;238:187–198. doi: 10.1006/jmbi.1994.1280. [DOI] [PubMed] [Google Scholar]
- 19.Lee H M, Wang K C, Liu Y L, Yew H Y, Chen L Y, Leu W M, Chen D C, Hu N T. Association of the cytoplasmic membrane protein XpsN with the outer membrane protein XpsD in the type II protein secretion apparatus of Xanthomonas campestris pv. campestris. J Bacteriol. 2000;182:1549–1557. doi: 10.1128/jb.182.6.1549-1557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letain T E, Postle K. TonB protein appears to transduce energy by shuttling between the cytoplasmic membrane and the outer membrane in Escherichia coli. Mol Microbiol. 1997;24:271–283. doi: 10.1046/j.1365-2958.1997.3331703.x. [DOI] [PubMed] [Google Scholar]
- 21.Letellier L, Howard S P, Buckley J T. Studies on the energetics of proaerolysin secretion across the outer membrane of Aeromonas species: evidence for a requirement for both the protonmotive force and ATP. J Biol Chem. 1997;272:11109–11113. doi: 10.1074/jbc.272.17.11109. [DOI] [PubMed] [Google Scholar]
- 22.Linderoth N A, Simon M N, Russel M. The filamentous phage pIV multimer visualized by scanning transmission electron microscopy. Science. 1997;278:1635–1638. doi: 10.1126/science.278.5343.1635. [DOI] [PubMed] [Google Scholar]
- 23.Michel G, Bleves S, Ball G, Lazdunski A, Filloux A. Mutual stabilization of the XcpZ and XcpY components of the secretory apparatus in Pseudomonas aeruginosa. Microbiology. 1998;144:3379–3386. doi: 10.1099/00221287-144-12-3379. [DOI] [PubMed] [Google Scholar]
- 24.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nouwen N, Stahlberg H, Pugsley A P, Engel A. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J. 2000;19:2229–2236. doi: 10.1093/emboj/19.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Possot O, Pugsley A P. Molecular characterization of PulE, a protein required for pullulanase secretion. Mol Microbiol. 1994;12:287–299. doi: 10.1111/j.1365-2958.1994.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 27.Possot O M, Gerard-Vincent M, Pugsley A P. Membrane association and multimerization of secretion component PulC. J Bacteriol. 1999;181:4004–4011. doi: 10.1128/jb.181.13.4004-4011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Possot O M, Vignon G, Bomchil N, Ebel F, Pugsley A P. Multiple interactions between pullulanase secretion components involved in stabilization and cytoplasmic membrane association of PulE. J Bacteriol. 2000;182:2142–2152. doi: 10.1128/jb.182.8.2142-2152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugsley A P, Dupuy B. An enzyme with type IV prepilin peptidase activity is required to process components of the general extracellular protein secretion pathway of Klebsiella oxytoca. Mol Microbiol. 1992;6:751–760. doi: 10.1111/j.1365-2958.1992.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 31.Py B, Loiseau L, Barras F. Assembly of the type II secretion machinery of Erwinia chrysanthemi: direct interaction and associated conformational change between OutE, the putative ATP-binding component and the membrane protein OutL. J Mol Biol. 1999;289:659–670. doi: 10.1006/jmbi.1999.2803. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds P R, Mottur G P, Bradbeer C. Transport of vitamin B12 in Escherichia coli: some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980;255:4313–4319. [PubMed] [Google Scholar]
- 33.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 34.Russel M. Phage assembly: a paradigm for bacterial virulence factor export? Science. 1994;265:612–614. doi: 10.1126/science.8036510. [DOI] [PubMed] [Google Scholar]
- 35.Salmond G P, Reeves P J. Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 36.Sandkvist M, Bagdasarian M, Howard S P, DiRita V J. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 1995;14:1664–1673. doi: 10.1002/j.1460-2075.1995.tb07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandkvist M, Hough L P, Bagdasarian M M, Bagdasarian M. Direct interaction of the EpsL and EpsM proteins of the general secretion apparatus in Vibrio cholerae. J Bacteriol. 1999;181:3129–3135. doi: 10.1128/jb.181.10.3129-3135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandkvist M, Keith J M, Bagdasarian M, Howard S P. Two regions of EpsL involved in species-specific protein-protein interactions with EpsE and EpsM of the general secretion pathway in Vibrio cholerae. J Bacteriol. 2000;182:742–748. doi: 10.1128/jb.182.3.742-748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauvonnet N, Vignon G, Pugsley A P, Gounon P. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 2000;19:2221–2228. doi: 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strom S M, Nunn D, Lory S. Multiple roles of the pilus biogenesis protein PilD: involvement of PilD in excretion of enzymes from Pseudomonas aeruginosa. J Bacteriol. 1991;173:1175–1180. doi: 10.1128/jb.173.3.1175-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strom S M, Nunn D N, Lory S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc Natl Acad Sci USA. 1993;90:2404–2408. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner L R, Lara J C, Nunn D N, Lory S. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J Bacteriol. 1993;175:4962–4969. doi: 10.1128/jb.175.16.4962-4969.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong K R, Buckley J T. Proton motive force involved in protein transport across the outer membrane of Aeromonas salmonicida. Science. 1989;246:654–656. doi: 10.1126/science.2814486. [DOI] [PubMed] [Google Scholar]