SUMMARY
The timelines for developing vaccines against infectious diseases are lengthy, and often vaccines that reach the stage of large phase 3 field trials fail to provide the desired level of protective efficacy. The application of controlled human challenge models of infection and disease at the appropriate stages of development could accelerate development of candidate vaccines and, in fact, has done so successfully in some limited cases. Human challenge models could potentially be used to gather critical information on pathogenesis, inform strain selection for vaccines, explore cross-protective immunity, identify immune correlates of protection and mechanisms of protection induced by infection or evoked by candidate vaccines, guide decisions on appropriate trial endpoints, and evaluate vaccine efficacy. We prepared this report to motivate fellow scientists to exploit the potential capacity of controlled human challenge experiments to advance vaccine development. In this review, we considered available challenge models for 17 infectious diseases in the context of the public health importance of each disease, the diversity and pathogenesis of the causative organisms, the vaccine candidates under development, and each model’s capacity to evaluate them and identify correlates of protective immunity. Our broad assessment indicated that human challenge models have not yet reached their full potential to support the development of vaccines against infectious diseases. On the basis of our review, however, we believe that describing an ideal challenge model is possible, as is further developing existing and future challenge models.
KEYWORDS: controlled human infection model, vaccine, human challenge model
INTRODUCTION
The physician-scientist Claude Bernard brought the discipline of experimental medicine to life in the mid-19th century. With his series of groundbreaking discoveries in the field of human physiology, the idea that medicine in the service of human health should be firmly grounded in scientific knowledge gained through experimentation took hold and flourished. The iterative nature of experimental medicine was also a key tenet of Bernard’s teaching. He viewed a scientific theory as the first important step away from the “groping and empiricism” that he eschewed, but he also emphasized that each scientific theory must be further tested and either accepted or discarded as a result of new data (1).
Physicians and scientists early in the 21st century still struggle with the role of experimental medicine in the development of vaccines. The history of vaccine development is largely one of the “groping and empiricism” that Bernard sought to overcome. Edward Jenner developed the smallpox vaccine based on observation (the protected milkmaids), confirmed by experimental medicine in human volunteers (vaccination of children in the community). He had no knowledge of the underlying mechanisms of protective immunity. He did not recommend mass vaccination against smallpox based on a P value from a phase 3 clinical trial but rather on his own careful observations. Nevertheless, the smallpox vaccine that Jenner first developed remains the sole example of the eradication of a disease through vaccination. The polio vaccines developed by Jonas Salk and Albert Sabin and the measles vaccine developed by Maurice Hilleman may also achieve eradications, but these goals have proved more elusive than the world first expected. For many, if not most, of the vaccines in use today, a clear understanding of the mechanism(s) of protection has not been available to guide new vaccine development or improve on vaccine availability.
Today, vaccines are lifesaving tools that underpin improved global health, and yet morbidity and mortality from infectious diseases are still unacceptably high. The reasons for this are many. First, information is incomplete on the fraction of global morbidity and mortality attributable to many of the key pathogens that are potential, or actual, targets of vaccine development, which makes prioritizing overall vaccine development efforts difficult. Second, vaccine development is still largely an empirical process, usually conducted without the guidance of a correlate of protective immunity with which to optimize a vaccine. Vaccines are most often optimized for the strongest measurable immune responses, without foreknowledge of whether a given response or combination of responses will afford protection. The probability of a vaccine development program’s success is therefore difficult to predict prior to the conduct of large, costly, and time-consuming field efficacy trials. Third, even when vaccines exist that can prevent infectious diseases, multiple factors can prevent realization of their full impact, including barriers to access, such as high costs; constraints on supply chain and distribution, such as delivery difficulty to remote areas and limited cold chain capacity; and chronic conditions that diminish immunogenicity, such as immunodeficiency syndromes and environmental enteric dysfunction. Fourth, a legitimate difference of opinion exists on how to make the preclinical and early clinical phases of vaccine development more grounded in scientific data. To date, the scientific community has heavily relied on in vitro and animal model data to guide vaccine development, following a long tradition in the development of new medicines and vaccines. Indeed, Claude Bernard enjoyed the success that he did largely because the aspects of physiology that he studied were sufficiently conserved between animals and humans to permit direct extrapolation. The protective immunity provided by vaccines often seems to be the exception to the rule. Even the best animal models of disease may poorly predict protective immune responses.
“Clinical data trumps all” is a common saying among medical researchers when they become frustrated by the limitations of translating results obtained from animal experiments that are not reproduced when tested in humans. Another phrase heard over and over is that “mice lie and monkeys exaggerate,” hence the continuous stride to learn directly from humans what we need to know for the advancement of medicine or understanding of human physiology. However, direct research in humans is plagued by multiple challenges, including the obvious ethical concerns of subjecting humans to tests that may endanger their health. Additional challenges to address include the enormous genetic, environmental, nutritional, gender, age-related, and other variables among potential research subjects. Some of the mantras in experimental research are the use of a control group and the isolation of the variable studied, so that the results can be interpreted with the least amount of noise. This is usually feasible in animal models but is often impossible to achieve in humans. Indeed, even once robust efficacy data are obtained from a clinical study, results from real-world implementation sometimes fail to match those from carefully controlled trials. To mitigate these risks, the use of a parallel, concurrent control group and the approach to blinding investigators and subjects to avoid bias is mandatory. Challenging humans with pathogenic microorganisms to test new prevention or treatment modalities is a promising approach, as long as the model resembles the disease, the selected participants are as uniform as possible, the control group is appropriate, and the sample size is sufficient to satisfy the hypothesis and other caveats. More often than not, the human challenge field has to be satisfied with generating data progressively to address uncertain findings in the initial attempts to develop the model and expect to accumulate information in a stepwise fashion. With that caveat, human challenge models may indeed be the most promising approach. We acknowledge that while no model is perfect, each can provide unique insights that are otherwise unattainable.
The limitations of animal models for supporting vaccine development have long been recognized. For the last 70 years, human challenge studies with many important pathogens have been conducted to gain more relevant data on pathogenesis, immunity, and the protective efficacy of candidate vaccines. The exact role of human challenge studies in vaccine development, however, is the subject of ongoing debate.
Some of the human challenge models in current use face intrinsic limitations with respect to the selection and availability of challenge strains, their routes of administration, and the capacity to evaluate the full spectrum of clinical disease. Human challenge models may achieve greater impact in their support of vaccine development by increasing focus on models with the fewest intrinsic limitations and a robust candidate vaccine pipeline. Looking across the many challenge models, we recognize common obstacles to be overcome. Coordination of experimental human challenge studies that support vaccine development could accelerate progress, foster collaboration and knowledge sharing, and encourage study consolidation to address problems that are common to broad categories of diseases. Such an approach could also provide broader access to the most advanced technologies that have the potential to accelerate candidate antigen selection, improve the evaluation of immune responses generated by candidate vaccines, and enable the discovery of new mechanisms of resistance to infection.
The goal of this report is to describe the background, context, and present experience with human challenge studies, along with a critical analysis of their role and limitations in support of vaccine development. The framework in which human challenge studies are being conducted is also examined, with the hope of building a comprehensive case that the expanded use of human challenge studies could increase the speed of vaccine development and probability of vaccine success. At the conclusion of this report, we will return to this proposition and provide summary recommendations from the research conducted to date.
APPROACH
We focused our analysis on 17 diseases. The diseases reviewed include the vector-borne diseases malaria and dengue; the enteric diseases cholera, enterotoxigenic Escherichia coli, Shigella, Campylobacter, typhoid fevers (Salmonella), norovirus, Cryptosporidium, rotavirus, and poliovirus; and the respiratory diseases influenza, respiratory syncytial virus, pneumococcus, tuberculosis, pertussis (whooping cough), and severe acute respiratory syndrome coronavirus 2 (the virus responsible for COVID-19). These diseases were selected to cover a range of levels of development of their corresponding human challenge models, from well-developed (malaria, cholera, influenza, etc.) to speculative (COVID-19). The diseases were also selected on the basis of those for which the models have a strong potential for impact on vaccine development.
In most cases, the challenge models involve fully virulent wild-type pathogens. However, in a few cases in which this is neither ethical nor practical, “pseudochallenge” studies with live-attenuated organisms such as vaccine strains have been used as alternatives. These include dengue, rotavirus, poliovirus, and influenza. Challenges with attenuated dengue and influenza viruses are addressed in their respective sections of vector-borne and respiratory diseases, whereas rotavirus and poliovirus are covered together in a dedicated pseudochallenge section under enteric diseases.
The literature searches on each of the diseases were conducted mostly through PubMed (https://pubmed.ncbi.nlm.nih.gov/) at the National Center for Biotechnology Information, U.S. National Library of Medicine, U.S. National Institutes of Health, and through ClinicalTrials.gov. Some of the information available on the websites of the World Health Organization (WHO), the U.S. Centers for Disease Control and Prevention (CDC), and other online sources was also included.
For each disease, the following four topics were researched: (i) epidemiology and public health impact of the disease, (ii) diversity and pathogenesis of the causative organisms, (iii) current vaccine development, and (iv) human challenge models of the disease and their utilization.
The review for each disease area includes figures and tables. Most of the tables in this report represent compilations of available sources. The reference list contains original articles or other sources from which the information that is presented was compiled. Many recent review articles were used for this report, as well as current articles on specific studies or topics that were deemed important to include. Google searches and ClinicalTrials.gov were used to capture unpublished or ongoing studies. It was not feasible in the time available to comprehensively review the literature for any of the diseases included in this report. For each disease area, we include a bibliography of the articles and other sources used to direct the reader to more detailed information for further reading.
This report also includes a section that provides a comprehensive review of the regulatory and ethical considerations related to human challenge studies. The section describes the different types of human challenge studies and compares the regulatory requirements for each type of study in the United States to those in the United Kingdom. The regulatory agencies and advisory groups with jurisdiction over human challenge studies in the United States and the United Kingdom are also described in this section. The ethics section includes considerations for studies conducted with residents of regions of endemicity.
The final section of this report presents our conclusions from the review and analysis we conducted, along with summary recommendations. We also offer specific guidance on the future development and utilization of each of the human challenge models reviewed.
LITERATURE REVIEW ON THE ROLE OF HUMAN CHALLENGE MODELS IN THE DEVELOPMENT OF VACCINES
Vector-Borne Diseases
Malaria
More than 3 billion people live in areas of the world where malaria is endemic. Despite significant advances in vector control and treatment, there were an estimated 229 million malaria cases and 409,000 deaths attributable to malaria in 87 countries where malaria is endemic in 2019 with 95% of the malaria cases concentrated in 29 countries, most of which are in sub-Saharan Africa (2).
The human malaria parasites Plasmodium falciparum and Plasmodium vivax are the most prevalent and clinically significant of the human malaria parasites. Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi cause a smaller fraction of infections worldwide.
(i) Malaria parasite life cycle
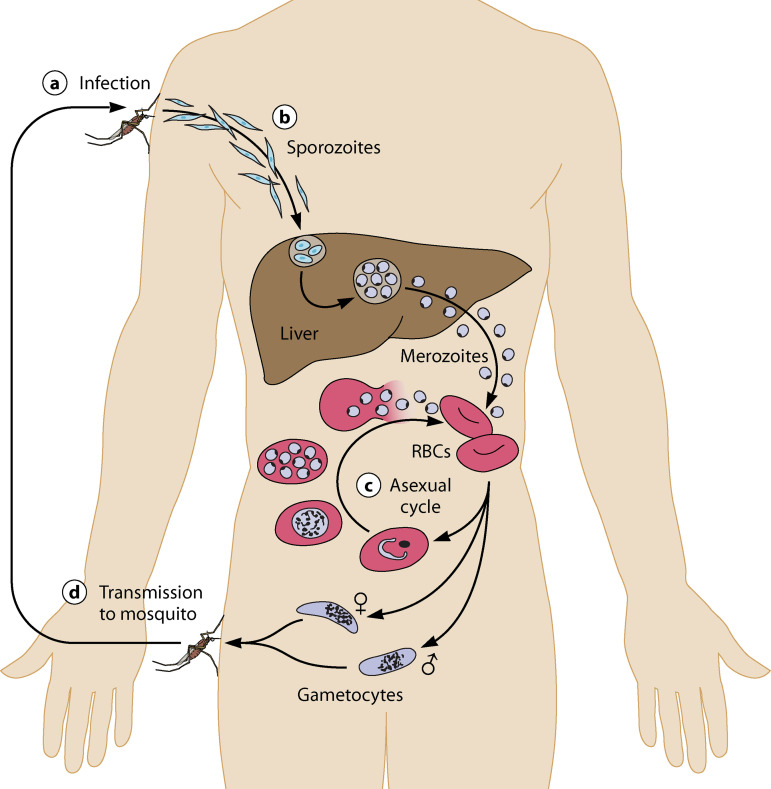
The life cycle of P. falciparum is shown in Fig. 1, which also indicates the parasite life cycle developmental stages at which interventions that include vaccines, small-molecule therapeutics, and biologicals are applied in human challenge models.
FIG 1.
Plasmodium life cycle and malaria challenge platforms.
Briefly, when female malaria-infected Anopheles mosquitoes seeking a blood meal bite the skin of a human, approximately 15 to 100 infectious-stage sporozoites are injected into the skin. The sporozoites migrate to the liver either through the lymphatics or direct blood circulation. Sporozoites traverse the cell membranes of endothelial cells, Kupffer cells, and even several liver hepatocytes before invading into a single hepatocyte to develop into exoerythrocytic-stage parasites. This stage of the life cycle is known as the preerythrocytic stage, the primary target of vaccines that target the sporozoite and developing liver-stage parasite. Sporozoites develop within hepatocytes, where they multiply many 1,000-fold, and then enter blood circulation and invade human erythrocytes. The majority of blood-stage parasites replicate asexually in red blood cells, with cycles of amplification every 48 to 72 h depending on the malaria species. The blood stage of infection is the major cause of malaria symptoms, morbidity, and mortality. A small proportion of blood-stage parasites develop into male and female gametocytes, the sexual stage of the parasite’s life cycle. The gametocytes are the only forms that can reinfect Anopheles mosquitoes when the mosquito bites an infected host to obtain a blood meal. In the mosquito, gametocytes form a zygote, traverse the mosquito’s midgut, and develop into oocyst stages from which infective sporozoites are formed. Sporozoites find their way to the mosquito’s salivary glands, where they can initiate a new infection upon feeding on a susceptible host.
P. vivax differs from P. falciparum in important features. After invasion into hepatocytes, some sporozoites enter a dormant period of quiescence (termed hypnozoite-stage parasites), which may last from several weeks to more than a year. At defined intervals ranging from a few weeks to a year, hypnozoite-stage parasites may resume development within the hepatocyte, multiply, and emerge into the blood stream to initiate repeated cycles of blood-stage infection. In addition, P. vivax gametocytes emerge and develop simultaneously with the asexual-stage parasites and are susceptible to antimalarial drugs used to treat asexual blood-stage parasites.
(ii) Controlled human malaria infection models
Human challenge models have been developed for all three phases of parasite development: the preerythrocytic stage, the blood stage, and the transmission stage. Reviews of challenge models and their citations have recently been published (3–6). The sections that follow describe in detail how these challenge platforms have been developed for P. falciparum, P. vivax, and P. malariae, and we describe how the challenge platforms are used to study the natural history of disease, how they contribute to vaccine development and inform our knowledge of immunologic correlates of protection, and how such challenge models are currently being used in drug development of new antimalarials. Importantly, we comment on the appropriate strengths and limitations of human challenge models in malaria research and development.
The history of challenging humans with malaria parasites is instructive on how society, medicine, and product development have evolved over the last 100 years with respect to the ethical conduct of such trials, the quality management systems of the challenge platforms, the reproducibility of the challenge methodology, the worldwide and country-specific regulatory environment, and the knowledge gained from such studies. Judging the present by the standards of the past should cause one to pause and consider the risk-benefit analysis of utilizing human malaria challenge models to achieve particular outcomes, notably accelerating vaccine and drug development in a safe and reproducible manner.
Human malaria challenge was extensively used in the early 1900s as a mechanism to treat the ravages of neurosyphilis, for which no alternative therapeutic options were available. An early publication described how malaria sporozoites from infected anopheline mosquitoes were used to initiate infection in patients suffering from neurosyphilis (7) and provides the contextual background, despite its limitations in methodology and deficiencies in obtaining informed consent, for the adaptation and development of the safe and reproducible challenge models utilized presently. The knowledge gained from human malaria challenge studies has increased our understanding of the pathogenesis of the disease and the successes and failures related to product development.
(iii) Parasite and mosquito vectors in human malaria challenge models
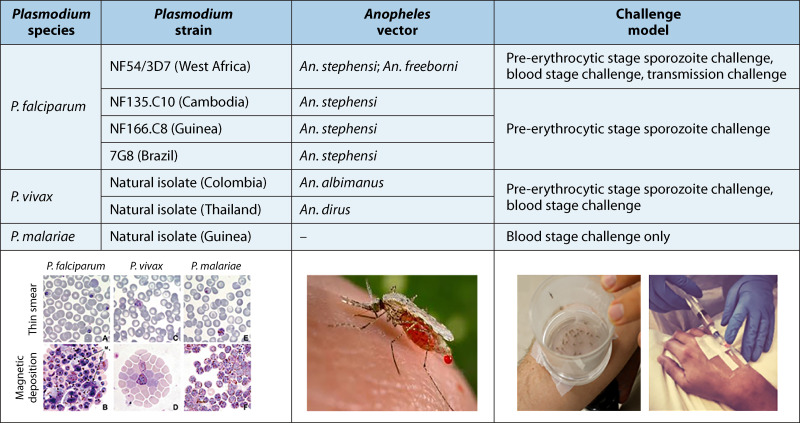
In the modern era, the first demonstration that protection against malaria challenge in a volunteer previously immunized with a live-attenuated parasite took place at the University of Maryland, where human volunteers were challenged with the bite of infected mosquitoes that had previously fed on gametocytemic P. falciparum-infected persons (8). Despite the success of this proof-of-concept trial, it soon became apparent that it was not feasible to rely solely on naturally infected gametocyte-positive human subjects to serve as the source for a mosquito blood meal in order to produce viable infectious sporozoites used to challenge volunteers with the bites of infected Anopheles mosquitoes. The development of a P. falciparum (NF54 strain) asexual- and sexual-stage continuous culture system produced limitless quantities of gametocyte-stage parasites that were placed in membrane feeders where batches of either Anopheles stephensi or Anopheles freeborni mosquitoes could feed on a mixture of P. falciparum and fresh normal blood. After seven to ten days, the mosquito infection rate with oocysts exceeded 90% and salivary gland sporozoites were detected 14 to 17 days postfeeding (9). Volunteers either previously immunized with an experimental vaccine candidate and infectivity controls that did not receive any experimental vaccine were challenged by the bites of five infected mosquitoes, which were permitted to bite and obtain a blood meal for 5 min (9). Volunteers were hospitalized or otherwise congregated together in a hotel and closely monitored daily for the emergence of ring-stage asexual parasites and symptoms. The NF54 strain (and the 3D7 parasite clone of NF54) is sensitive to multiple antimalarials, including chloroquine, atovaquone/proguanil, and artemether/lumefantrine, thus ensuring that infections can reliably be cleared in volunteers. To date, three additional P. falciparum parasite strains have been maintained in continuous culture, producing infectious gametocytes to An. stephensi mosquitoes used in human challenge studies (Fig. 2) (10–12) that differ in genome structure, sequence, and immunogenic potential (13).
FIG 2.
Parasite strains and mosquito vectors commonly used in challenge models.
Compared to P. falciparum parasites, P. vivax parasites cannot be continuously cultured in vitro to produce infectious gametocytes; therefore, the challenge model is limited to using naturally acquired parasites obtained from donor volunteers that present to a health treatment facility with clinical vivax malaria infection (14–17). Small aliquots of blood (~10 mL) obtained from individuals with P. vivax parasitemia must immediately be transferred to membrane feeders where Anopheles dirus or Anopheles albimanus mosquitoes are permitted to feed (Fig. 2).
A third Plasmodium species, P. malariae, obtained from a naturally infected patient, has been used to develop a controlled human malaria challenge model using small aliquots of asexual blood-stage parasites to infect healthy volunteers (18).
A well-developed quality management system is invaluable with regard to the rigorous screening and testing procedures on all aspects of the challenge model. This includes testing for ABO/Rh blood group and screening the infected human donor blood from whom the parasite is isolated for transmissible agents, including HIV, hepatitis B and C viruses, human T-cell leukemia virus, syphilis, and other pathogens, depending on the specific requirements of the regulatory agencies. The insectary and mosquito colony are critical aspects to ensure a safe and reproducible quality management system.
A convening of experts in both preerythrocytic- and blood-stage challenge models was held in 2009 in which general principles were proposed regarding the standardization of the challenge models across centers (19, 20). In addition to procedures to safely protect volunteers from harm, increasingly the application of such challenge models includes both ethical considerations (21, 22) and regulatory oversight (23), which differ depending on the country.
(iv) Preerythrocytic-stage challenge model development
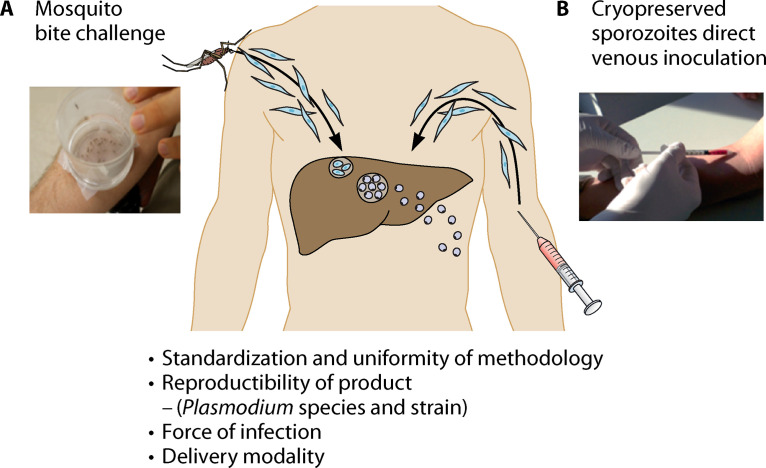
Presently, there are two distinct types of controlled human malaria infection (CHMI) that initiate malaria infection in the host. First, sporozoite infection is initiated by the bites of volunteers by Plasmodium-infected female Anopheles mosquitoes that release sporozoites from the salivary gland while the mosquitoes feed for a blood meal on the skin of the volunteers. We commonly refer to this model as the mosquito bite challenge model. The second method, recently developed, is direct venous inoculation of cryopreserved sporozoites into the host by needle and syringe. Figure 3 illustrates the two challenge methods that use infectious sporozoites to initiate infection.
FIG 3.
Preerythrocytic human challenge models using mosquito bite delivery or direct venous inoculation of infectious sporozoites.
We highlight four factors that impact the operational feasibility and interpretation of the outcomes from each of these two delivery modalities. Any challenge model, independent of pathogen under consideration, should be standardized to ensure the methodology results in uniform infectivity of volunteers that are challenged. The product, defined as the Plasmodium species and/or parasite strain, is generally produced under Good Manufacturing Practice (GMP), since different strains have various susceptibilities to different antimalarial medications to clear blood-stage parasites and differences in potency that affect the prepatent period (time to detection of blood-stage parasitemia). Understanding the “force of infection,” namely, the minimal inoculum required to initiate infection in “control” (no intervention) subjects, is crucial in weighing whether to continue or abandon an intervention such as a vaccine candidate or new antimalarial compound.
Regardless of the particular challenge model, such malaria challenge studies are not statistically powered to detect true differences between those that are protected and those that are not, due to the logistical constraints on the number of subjects that can safely be followed during these phase 2 challenge trials. Typically, the number of human subjects per group has varied from as few as five to ten persons to ~30. These numbers are modest, but can provide a measure of confidence that the outcome of the challenge (presence or absence of blood-stage parasitemia) provides Go/No-Go criteria for further testing in the field under natural transmission. In the sections that follow, we highlight the performance characteristics of each challenge model and provide an exhaustive list of the types of malaria vaccine candidates that have been tested under each model. We also provide a perspective that includes the advantages and limitations of each challenge model.
(v) Mosquito bite challenge model
In 1986, Chulay and colleagues reported on how the bites of five An. stephensi mosquitoes infected with the NF54 strain of P. falciparum could successfully infect adult volunteers using a methodology in which the emergence of parasites into the peripheral blood circulation was closely monitored by detecting ring-stage parasites on thick blood smears (9). This first practical challenge model has been the workhorse over the last 34 years and has been successfully used to rapidly and safely evaluate vaccine efficacy for preerythrocytic vaccine candidates and increasingly to evaluate the new antimalarial therapeutics.
The salient features of the P. falciparum sporozoite challenge model include the continuous use of the model for nearly 30 years to support malaria vaccine and drug development, in which more than 2,000 human volunteers have participated in challenge studies. Human challenge studies have been performed at multiple sites in the United States, Europe, Australia, South America, and Africa. Importantly, World Health Organization consensus guidelines for conducting challenge studies have been established to harmonize across sites (19).
Briefly, frozen blood-stage parasites produced under GMP are thawed and expanded in human erythrocytes in culture. Laboratory-reared An. stephensi mosquitoes are infected by feeding through a thin membrane on cultures containing gametocytes. The presence of sporozoites in the mosquitoes is confirmed two weeks later by dissecting salivary glands, and typically five infected mosquitoes are placed in a mesh-covered carton. Volunteers place their arm over the carton and allow mosquitoes to feed for 5 min. After the feeding opportunity, the researchers verify that the mosquitoes have ingested blood and contain sporozoites.
Volunteer subjects are healthy individuals 18 to 50 years of age selected from the community. The studies are conducted under an institutional review board-approved protocol, and informed consent is obtained. Starting five days after challenge, the volunteers are closely followed for signs and symptoms of malaria and evaluated using thick blood smears. Blood smears permit the counting of parasites under a microscope. During days 9 through 19, when malaria parasites are expected in blood, volunteers are housed at a single hotel with clinical and laboratory staff continuously available. As soon as two or more parasites are detected in thick blood smears, treatment with a standard antimalarial drug is initiated. Blood smears are obtained daily following antimalarial treatment until three consecutive negative smears indicate it is safe to release the volunteer from the study. Volunteers that do not develop malaria by day 28 (well beyond the prepatency period of seven to 25 days for malaria infection) do not receive malaria treatment and are considered “protected.”
A critical component of any challenge model is the force of infection of the inoculum that can achieve a successful infection in the host. Too low of an inoculum will result in too few infected individuals in a “control” group that do not receive a vaccine, making efficacy comparisons to the experimental vaccine group difficult to interpret. Too high an infectious inoculum will overcome any vaccine-induced protection.
It is therefore imperative that the force of infection is sufficiently potent to ensure that every individual in the control group becomes infected after challenge. This requirement poses a risk to the interpretation of efficacy outcomes because of the force of infection (the number of infectious mosquito bites that may overcome vaccine-induced immunity). If the challenge model is insufficiently reproducible (between subjects within a trial or variation between trials), the variability can undermine the interpretation of endpoints, resulting in wasted effort, expense, and premature termination of otherwise promising vaccine candidates. We provide an example that illustrates this point. The authors of a vaccine trial rationalized the use of fewer infectious mosquito bites to try to mimic natural infection (24). The authors noted that “because of the concern that previous experimental challenges conducted with the bites of five infected mosquitoes were unrealistically severe and may have overwhelmed any vaccine-induced immunity, the first four vaccinated volunteers and three nonimmunized control volunteers were challenged with two infected mosquito bites each. The failure of two of three control volunteers to develop patent malaria infections led to a decision to challenge the two remaining groups of volunteers with five infected mosquito bites” (24).
The force of infection that ensures uniformity of infection also depends on the Plasmodium species and strain. The NF54 strain of P. falciparum and a cloned derivative of NF54, 3D7, and produced under GMP, have been used in most mosquito bite challenge models. Graded numbers of NF54-infected mosquito bites (one to five) using aseptically produced infected mosquitoes or different P. falciparum strains have been used in CHMI, but it is not universally accepted that a lower number of infectious mosquito bites (one to three) provides any advantage over the five-bite model. P. vivax challenge models using different numbers of infectious mosquito bites have been developed. Described in a following section in this chapter, the models rely exclusively on natural infection, and thus such natural strains of P. vivax used in challenges are likely to differ with respect to potency and infectiousness. Therefore, whether fewer than five infected bites can consistently result in uniform infection (detection of blood-stage parasitemia) in a subject remains to be confirmed.
Challenging a human volunteer with P. falciparum malaria parasites has intrinsic risks. Malaria can be a life-threatening illness and every challenge has the potential to produce serious adverse events (SAEs). Through the course of more than 1,000 malaria challenges at U.S. Army and U.S. Navy sites, no SAEs or cases of severe malaria have been reported; however, some variation in the conduct of human challenge studies with malaria across challenge centers where such studies are performed does occur. There are no symptoms associated with the liver stage of infection. However, shortly after liver-stage merozoites emerge into the peripheral blood circulation, the accumulation of asexual parasites reaches a threshold whereupon symptoms associated with classical malaria disease, such as fever, myalgias, headaches, nausea, and vomiting, and laboratory abnormalities such as mild thrombocytopenia, emerge (25, 26). As challenge models have been refined over the past two decades with broader international participation, there is not a consensus among the experts on whether or not volunteers participating under informed consent need to be treated immediately upon detection of any parasites in the peripheral circulation in order to protect them from any adverse event. There is a cushion window of several days coincident with a few replication cycles of parasites before treatment is initiated that would allow for scientific inquiry related to whether an intervention would prevent the emergence of clinical symptomatology associated with uncomplicated malaria in addition to the typical endpoints that measure prevention of infection. Untreated subjects will progress to severe disease, and if left untreated, this may result in death. Therefore, it is required that any malaria challenge use parasites (P. falciparum or P. vivax strains) that are susceptible to antimalarial medications. Detecting the presence of asexual blood-stage parasites early in infection is dependent upon highly sensitive and specific diagnostic assays. The preparation and interpretation of thick and thin blood smears by microscopy is the “gold standard” diagnostic assay. Until recently, CHMI studies have relied exclusively on detecting ring-stage parasites on a thick blood smear. The minimal level of detection by microscopy is 10 to 50 parasites per mL of blood, which is significantly lower than antigen rapid detection tests. However, the recent development of the molecular detection of parasites using polymerase chain reaction (PCR) has emerged and has become the go-to standard operating procedure at several CHMI test centers (27–29). The U.S. Food and Drug Administration has qualified one such molecular PCR assay, “Plasmodium falciparum 18S rRNA/rDNA (copies/mL) measured in blood samples by a nucleic acid amplification test assay” for “a monitoring biomarker, that when positive, informs initiation of treatment with an antimalarial drug >6 days following CHMI with P. falciparum sporozoites in healthy subjects (18 to 50 years old) from areas of nonendemicity enrolled in clinical studies for vaccine and/or drug development” (30).
The complexities of the mosquito bite challenge model have limited the number of test centers that can safely conduct CHMI using Anopheles-infected mosquitoes to bite volunteers. The establishment of such challenge centers requires a secure biocontainment insectary with trained entomologists and parasitologists, individuals with training in diagnostics (microscopy and molecular PCR detection), clinicians, and a clinical trial facility. The U.S. military Walter Reed Army Institute of Research (WRAIR) has supported malaria challenge trials using the bites of infectious mosquitoes since the 1980s and has safely conducted dozens of such trials without any study-related SAEs. Using the same exact challenge model standard operating procedures, the CHMI model developed by WRAIR has been transferred to the Center for Infectious Disease Research in Seattle, Washington; the University of Maryland; and the University of Oxford, where the challenge of human volunteers with P. falciparum 3D7 parasites was successfully achieved (31).
(vi) Vaccine efficacy assessed using the mosquito bite challenge model
One of the key applications of the human challenge model for malaria is to assess the potential for vaccine efficacy in small numbers of volunteers. This is particularly relevant for preerythrocytic malaria vaccines that prevent infection. The primary endpoint in CHMI vaccine trials is the presence or absence of blood-stage parasites occurring in a person immunized with an experimental vaccine candidate. Since the 1980s, dozens of malaria vaccine trials have been conducted around the world that use the bites from falciparum-infected anopheline mosquitoes to deliver sporozoites.
The concept that human malaria challenge trials could be exploited to assess vaccine efficacy was first demonstrated by David Clyde and colleagues at the University of Maryland in the early 1970s. Volunteers were immunized with bites from hundreds of irradiated Anopheles mosquitoes infected with P. falciparum and were then challenged with wild-type P. falciparum-infected mosquitoes (8). Subsequently, investigators tested subunit recombinant protein (32) or peptide vaccines (33) based on the circumsporozoite amino acid repeat units and who were challenged. Small numbers of vaccinated subjects did not develop detectable parasitemia and were classified as “protected” compared to those who developed malaria in the control group, which did not receive the vaccine. These first demonstrations initiated the testing of various sporozoite and/or liver-stage antigens either singly or in combination with various expression platforms over the ensuing 30 years. By far, the most extensively investigated malaria vaccine that progressed from CHMI clinical trials to field studies is the RTS,S vaccine (34). The first demonstration that RTS,S could protect a person in a CHMI was in a person immunized with RTS,S formulated with aluminum hydroxide adjuvant. This was followed by the seminal trial that catapulted RTS,S development and subsequent field testing, which demonstrated high-level protective efficacy when RTS,S was formulated with AS01 adjuvants (comprised of monophosphoryl lipid A, QS21, and an oil-in-water formulation). Several RTS,S vaccine CHMI trials followed that improved on the formulation by testing various dosages, number of doses, and schedule. Additional iterations ensued that included testing of a construct that lacked the amino acid repeat units; the combination of additional malaria antigens such as MSP-1 and TRAP with RTS,S (35); and prime-boost combinations using viral vectors expressing malaria genes to prime followed by RTS,S boost (36–39).
In addition to the circumsporozoite protein (CSP) antigen, other P. falciparum antigens expressed as recombinant proteins have been tested in CHMI trials, including PfCS102 (40); LSA1 (41); CelTOS (42, 43); blood-stage vaccine candidates that used mosquito bite challenge, GMZ2 (44) and AMA1 (45); and an epitope virus-like particle vaccine containing a single B-cell and two T-cell epitopes from CSP antigen (46).
Vaccine development platforms, including DNA and viral vectors used singly or in prime-boost configurations, have been tested extensively in CHMI using mosquito bite challenge, and reviews have been excellent. Viral vectors include vaccinia containing seven different malaria antigens from all stages of the life cycle (47), adenovirus 5 (Ad5) vectors (48), and chimpanzee adenovirus 63 (ChAd63) (49). However, most CHMI trials of virus-vectored vaccines were prime-boost combinations that included combinations of DNA/Ad5 (50), DNA/MVA (modified vaccinia virus Ankara) (51), Pox/MVA (52), and ChAd63/MVA (49).
Whole-parasite vaccine constructs have received much attention in recent years and such vaccines are based on the manufacture of aseptic, metabolically active, cryopreserved sporozoite vaccines (53, 54). The evaluation of such whole-parasite vaccines includes genetically modified parasites that prevent development of the parasite in the liver (55) and experimental medicine studies that deliver infectious sporozoites under chemoprophylaxis with different antimalarials given before immunization with the bite of live wild-type infected mosquitoes (56–58).
(vii) Vaccine efficacy assessed by the injection of purified cryopreserved sporozoites
Because mosquito bite challenge is laborious, requiring extensive insectary facilities that are not amenable to transfer across clinical trial sites, a developmental program was initiated more than a decade ago to assess whether sporozoites harvested from the mosquito under GMP could be cryopreserved, thawed, and injected into human volunteers to establish a liver-stage infection with subsequent emergence of blood-stage parasites into the peripheral circulation. It is crucial to note that an infection in a human requires as few as 15 to 100 sporozoites delivered with the bite of a single infected mosquito. Critically, this cannot be achieved with cryopreserved sporozoites, probably because the processes required to harvest, freeze, thaw, and administer sporozoites with a needle and syringe take a toll on parasite viability and infectiousness. Nevertheless, many studies have been conducted to assess whether such cryopreserved sporozoites could be administered in a similar manner to delivery by an infectious mosquito bite (Fig. 3, above). This includes several studies that assessed intradermal (59–61), subcutaneous (62), intramuscular (63, 64), and intravenous administration by a procedure called direct venous inoculation (63, 65). Vaccine efficacy using PfSPZ cryopreserved sporozoites has been studied in CHMI challenge trials, resulting in varying protection related to dosage and number of immunizations and primarily using direct venous inoculation as a method to introduce infectious sporozoites in the challenge model.
There is controversy within the field of malaria vaccinology regarding whether using such cryopreserved sporozoites as a challenge method is a suitable surrogate of natural infection. As stated previously, sporozoites delivered by the bite of infectious mosquitoes are deposited directly into the skin, and traverse through the epithelial, endothelial, and Kupffer cells, the lymphatic system, and venous circulation to arrive in the final destination, a hepatocyte. Vaccines that target sporozoites may elicit antibody-mediated immune responses that neutralize and or kill sporozoites in the skin (and in the peripheral circulation) before entry into hepatocytes. Cryopreserved sporozoites delivered by direct venous inoculation, but not sporozoites delivered by mosquito bite, bypass the skin; thus, this delivery mode would exclude any mechanism of action that relies on activity within the skin. Therefore, one must exert caution when interpreting CHMI vaccine trials that rely on direct venous inoculation. Nevertheless, there are some advantages, including easier implementation, lower cost, no requirement for an insectary, and easier transference of the challenge model to international testing centers.
(viii) Pathogenesis, immunologic, and transcriptional profiling in controlled human malaria infection clinical trials
Several studies have advanced our knowledge of pathogenic processes that take place during malaria infection following CHMI, such as alterations in blood coagulation (66) and the impact of sickle cell trait on time to blood-stage infection after challenge (67).
Significant advances in our understanding of the immunologic mechanisms related to protection obtained from serum and peripheral blood mononuclear cells (PBMCs) from CHMI vaccine trials have been realized and have been nicely reviewed in several publications (68–70). Nevertheless, protection against malaria infection is complex and there are many redundant escape routes the parasite uses to circumvent the immunologic pressures placed on it. This section provides some examples of the variety of methods and tools that have been used to evaluate innate, humoral, and cellular immune responses to various malaria vaccines.
Innate responses following CHMI can be gleaned from interventions that potentiate the innate immune responses, such as prior bacillus Calmette-Guérin vaccination (71). Both humoral (antibody) and cell-mediated immune responses in CHMI trials from semi-immune persons previously exposed to malaria may be different from those observed in persons with no prior malaria exposure or infection (72, 73).
Humoral immune responses to both preerythrocytic- and blood-stage vaccines have increased our knowledge and understanding of how immunization impacts protection against challenge, but no single correlate of protection has been found. One of the more exciting developments coming from CHMI vaccine trials and specifically from individuals protected against malaria challenge is the identification and isolation of monoclonal antibodies derived from plasmablast or memory B-cell populations in immunized persons. These antibodies reveal important insights related to protection against the circumsporozoite antigen on sporozoites and that could form the basis for a new malaria control tool in the form of a prophylaxis against malaria infection (74, 75).
Likewise, multiple immunologic investigations that have examined T-cell responses to virus-vectored malaria vaccines have indicated that antigen-specific CD4+ and CD8+ T cells are induced in subjects protected from malaria infection compared to those not protected; however, no absolute threshold indicative of a correlate of protection has been found (76–79).
Gene expression methodologies using high-resolution transcriptomics have been used to understand the molecular dynamics that occur before, during, and after CHMI (80) in both the parasite (81) and the human host (82–86). Early changes in host transcriptional profiles occur prior to the onset of clinical symptoms in hundreds of genes, uniting pathways from the cell nucleus, intracellular compartments, cell membranes, and extracellular space, providing a glimpse into how parasite infection precipitates a coordinated host response. Of particular interest, in an area that is emerging, is to further understand the transcriptional changes in gene expression that are induced by immunization with whole parasites, RTS,S, or viral vectors and that influence whether a person becomes infected or is protected following CHMI.
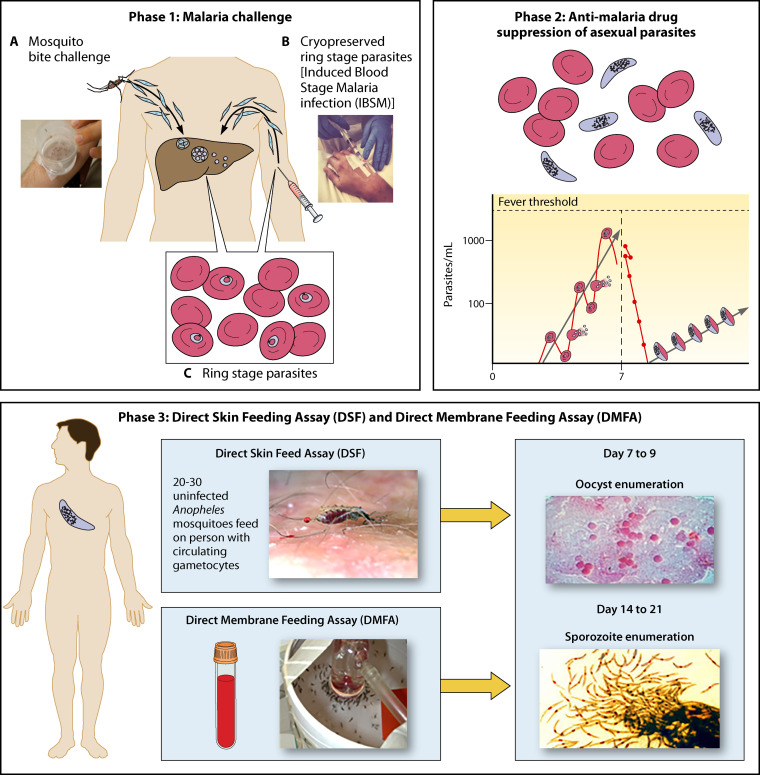
(ix) Blood-stage challenge model
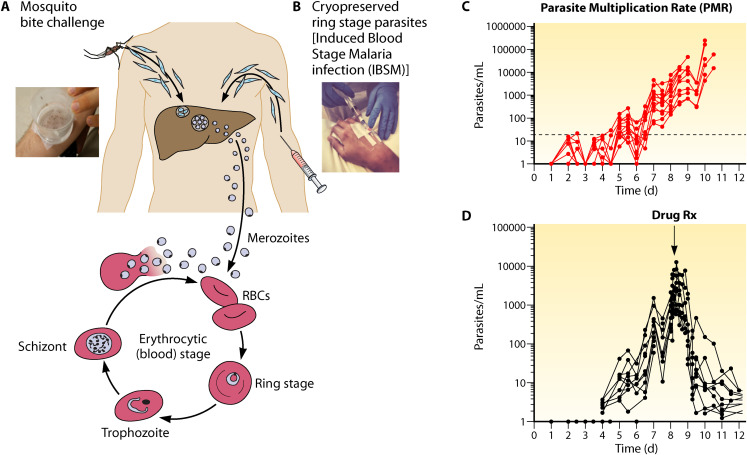
A blood-stage challenge model has been developed for both P. falciparum (87, 88) and P. vivax (89, 90) parasites that can be used to understand specifically the onset of pathogenesis, to evaluate whether new antimalarial drugs can kill parasites, and to evaluate vaccine efficacy that targets postpreerythrocytic stages. This model is illustrated in Fig. 4.
FIG 4.
Blood-stage controlled human malaria infection.
The salient features of this model are similar to the challenge model developed for preerythrocytic malaria interventions. This includes both mosquito bite (Fig. 4A) and the direct venous inoculation (Fig. 4B) of GMP ring-stage parasites into the host, which bypasses the liver stage of infection. Parasite cell banks for intravenous inoculation are prepared according to GMP and cryopreserved in small aliquots that can be readily sent worldwide to clinical trial test centers. Extremely low numbers of ring-stage parasites, ranging from a few hundred to 2,500, can be directly introduced into the host, establishing rounds of blood-stage parasite multiplication every 48 h that are detected by sensitive PCR (Fig. 4C). Both the multiplication and killing of the parasite after administration of experimental or licensed antimalarial drugs can be closely monitored in the host even before the onset of clinical symptoms associated with malaria illness (Fig. 4D). Changes in the host (91) and the parasite (92) occur after blood-stage challenge.
The practical application of the blood-stage challenge model is to evaluate blood-stage vaccines. Both whole organisms and subunit blood-stage vaccines have been evaluated with this challenge model, and immunologic profiling responses, including antibodies that inhibit the growth of the parasite and cell-mediated responses elicited by the vaccine, have been studied (93–95).
(x) Transmission-stage challenge model
Recently, there have been efforts to develop a human challenge model that can measure the transmission of malaria parasite gametocyte stages from an infected human volunteer to anopheline mosquitoes (96–100). The rationale behind the development of such a model is to assess new antimalarial drugs, monoclonal antibody biologics, and vaccines able to interrupt the transmission of the parasite to the mosquito, thereby leading to eventual malaria elimination. The development of such a model is technically challenging since the sexual-stage forms (male and female gametocytes) of the parasite primarily in P. falciparum infection are quite low in density. Briefly, due to factors still not well understood, at some point in the blood-stage life cycle there is a transcriptionally related commitment of a few asexual-stage parasites to become sexual-stage parasites, which results in two forms: male and female gametocytes. Upon blood feeding by a mosquito, both a female and a male gametocyte are taken up, fertilize, and develop into a zygote in the mosquito gut; undergo further differentiation into ookinetes that traverse the midgut epithelium; and develop into oocyst-stage parasites. This initial process of development to the oocyst stage occurs over 7 to 9 days, at which time the oocysts undergo further development into infectious sporozoites (14 to 21 days since the first blood feed), which eventually find their way to the mosquito salivary glands to transmit sporozoites to a new human host. There are several choke points in this sexual-stage part of the life cycle that a challenge model needs to overcome. We illustrate the development of this challenge model in Fig. 5.
FIG 5.
Transmission-stage challenge model.
The first step (step 1) is to establish an infection in the volunteer. This may be accomplished either by the bite of five infectious Anopheles mosquitoes or by the direct venous inoculation of blood-stage parasites into the host. In phase 2 (Fig. 5), asexual-stage blood parasites begin to multiply in the blood. It is critical to not permit the onset of clinical symptomatology in the volunteer, which would necessitate treatment. In step 2, the appearance of sexual-stage parasites is different between P. vivax infections and P. falciparum infections. In P. vivax malaria, sexual-stage parasites develop early in infection, coincident with the appearance of asexual-stage parasites, albeit in extremely low numbers due to their confinement to early reticulocytes and not in mature red blood cells. This early commitment enabled development of a transmission challenge model for P. vivax malaria, which was limited by the parasite-vector compatibility, but proof of concept was demonstrated for the first time. In P. falciparum malaria infection in challenge volunteers, the appearance of gametocytes in the circulation would not normally occur until the parasite density was sufficiently high enough to start the commitment process from the asexual stage to the sexual stage of development, because adverse events such as fever would preclude waiting until gametocytes would appear. Therefore, it is essential to stimulate gametocytogenesis early, before symptoms appear. One strategy adopted is the use of subcurative doses of antimalarials, which results in the appearance of gametocytes in the peripheral circulation. This is illustrated in Fig. 5, phase 2. Critical to this approach is the fine-tuning of antimalarial dosing at concentrations that suppress but do not kill asexual-stage parasites and still induce gametocytogenesis (with drugs such as sulfadoxine-pyremethamine and piperaquine); however, refinement of the approach will require further development.
As the asymptomatic (but infected) volunteer develops gametocytes, there are two methods to determine transmissibility (Fig. 5, phase 3). First, blood is taken and placed into a membrane device in which 20 to 30 uninfected mosquitoes are allowed to feed for 5 to 10 min. This method is referred to as the direct membrane feeding assay. After 7 to 9 days, the mosquitoes are dissected, and the numbers of oocyst-stage parasites are quantified. The second method, the direct skin feed, allows uninfected Anopheles mosquitoes to feed directly on the arms of volunteers infected with gametocytes, and oocyst stages are detected in dissected mosquitoes 7 to 9 days after feeding on the subjects. Proof of concept for both methodologies has been established (99, 100). We are in the early days of the refinement of the transmission-stage model, but it should be amenable to testing many different new drugs and vaccines that conceptually could interrupt transmission.
(xi) Specific considerations for Plasmodium vivax challenge models
The development of a challenge model for P. vivax malaria is unique. The asexual stage of the parasite cannot be cultured in vitro, which precludes the standard methodology for obtaining gametocytes to feed to mosquitoes, which then could produce infectious sporozoites for challenge. Therefore, a predefined strain used in challenge studies cannot be obtained, instead necessitating reliance on naturally occurring vivax strain parasites, which only develop in Duffy-positive reticulocyte blood cells circulating in the population. The parasites that infect the mosquitoes are thus field isolates that vary from experiment to experiment. The requirement that parasites be fully susceptible to antimalarial drugs is more difficult to verify with the field isolates used in the P. vivax model compared to cloned parasites used in the P. falciparum model. The second challenge is the parasite-vector competence, which limits the spectrum of Anopheles mosquito species that can transmit the parasite, such as An. albimanus in South America and An. dirus in Southeast Asia.
The human challenge model for P. vivax also faces an additional safety concern compared to the P. falciparum model. After P. vivax infection, a portion of the parasites lie dormant in the liver as hypnozoites that can cause recurring episodes of malaria if not eliminated. In the current P. vivax challenge model, the antimalarial drug primaquine is prescribed to clear all liver-stage parasites from study volunteers, but long-term follow-up would be required to verify that the clearance of liver stages has occurred. In one such challenge trial, two subjects failed to be cleared with a combination of chloroquine plus primaquine and relapsed multiple times, leading to the discovery that a polymorphism in the cytochrome P450 2D6 allele precludes the metabolism of primaquine to its active component (101).
(xii) Summary
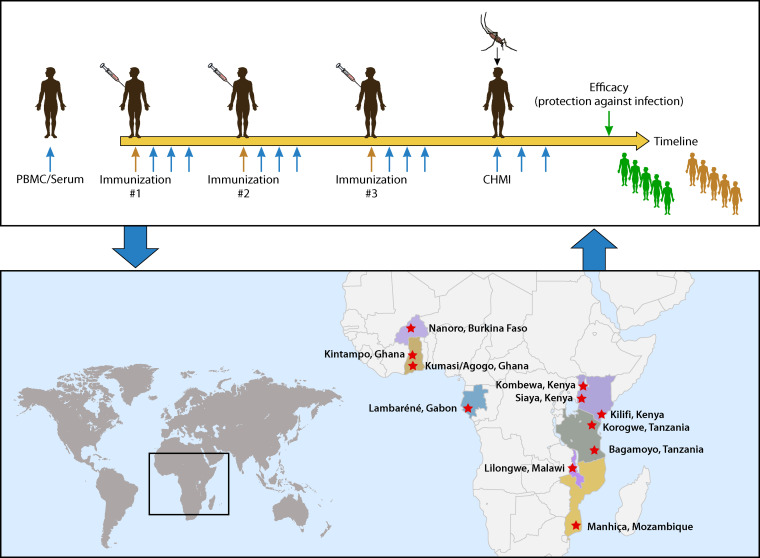
The human challenge models for malaria can be major accelerators for malaria vaccine development. When more of the models become fully developed, they could provide the capacity to evaluate vaccines against P. falciparum and P. vivax malaria, and against the preerythrocytic and blood stages of the parasite life cycle. A wide range of vaccine types targeting different candidate antigens for each of these parasites, and for different stages of infection, could then be systematically compared. Ideally, vaccine development is an iterative process, as illustrated in Fig. 6.
FIG 6.
Malaria vaccine development from controlled human malaria infection to the field and back.
Nevertheless, one would be remiss in relying exclusively on a challenge model without validating a candidate’s vaccine efficacy in the field. As stated previously, the upside is to derisk clinical development in terms of both time and cost. It is equally important to appreciate the limitations of a human challenge model. Such limitations include whether a person has previously been exposed to naturally acquired malaria infection, or whether a person being immunized has concurrent subclinical malaria infection. This cannot be accommodated in challenge models currently used to assess vaccine efficacy. As discussed previously, the force of infection is critical, since only a single mosquito bite is normally sufficient to establish infection. If on the other hand a challenge model requires the bite from five infectious mosquitoes, there is the possibility that one prematurely discards a promising vaccine candidate because insufficient protection is observed after five infected mosquito bites. Other considerations include both host and parasite genetic diversity. Most malaria vaccines using CHMI are tested primarily in volunteers in the United States and Europe. The target for such vaccines is persons residing in sub-Saharan Africa, where the human leukocyte antigen (HLA) class 1 and class 2 alleles differ across populations, rendering interpretation of studies from the “North” to the “South” challenging. In addition, there are potentially vastly different outcomes of challenge studies among previously infected or chronically infected persons. Malaria parasites are extremely genetically diverse, resulting in differences in fitness, invasion potency, and vaccine escape mutations. Relying on a single strain of P. falciparum malaria to adequately predict protection in the field is risky; therefore, quickly transitioning to trials in naturally exposed populations of all ages after having achieved a measure of efficacy after challenge will enable rapid and thoughtful decisions around advancement of promising vaccine candidates.
Dengue
(i) Epidemiology, pathogenesis, diversity, and public health impact of dengue virus
According to the WHO (102), roughly 40% of the world’s population are at risk of infection with dengue virus. The CDC and WHO websites report that approximately 400 million infections with dengue occur annually, with 100 million symptomatic infections, 500,000 cases of dengue hemorrhagic fever, and 22,000 deaths, mostly in children. It is clear that the number of dengue cases has been rapidly expanding (103, 104). No specific drugs exist to treat dengue infection and only a single vaccine, Dengvaxia (chimeric yellow fever dengue-tetravalent dengue vaccine, CYD-TDV, Sanofi Pasteur), has been licensed, although several are in late-stage clinical development.
Dengue is a single-stranded RNA, enveloped flavivirus whose close relatives are the yellow fever, Japanese encephalitis, and West Nile viruses. There are four serotypes of dengue virus (abbreviated DENV): DENV-1, DENV-2, DENV-3, and DENV-4. The E glycoprotein mediates virus attachment and entry into cells and is the target of virus-neutralizing antibodies. Dengue virus has two target cells with different receptors that mediate virus entry. In dendritic cells, DC-SIGN is the receptor (105) and in macrophages the mannose receptor serves this function (106).
Dengue viruses require passage through one of two mosquito species, Aedes aegypti or Aedes albopictus, to complete their life cycle. When these mosquitoes bite a viremic human, the virus is taken up, replicates in the mosquito gut, and spreads to the salivary glands from which the mosquito transmits the virus to humans through bites. In principle, vector control efforts could play a key role in reducing dengue disease burden, but historically these efforts have had mixed results (107–109). Novel approaches such as release of Wolbachia-infected mosquitos hold some promise (110).
While most infections with dengue viruses are asymptomatic, up to 10% of individuals develop dengue fever (DF), characterized by high fever, severe headache, joint pain, rash, and mild bleeding. A small proportion of those with DF progress to dengue hemorrhagic fever and dengue shock syndrome, which can lead to failure of the circulatory system, shock, and death.
The main risk factor for severe dengue disease is infection with a second dengue serotype following primary infection (111). The estimated increase in risk for severe disease with a heterologous secondary infection is 15- to 80-fold.
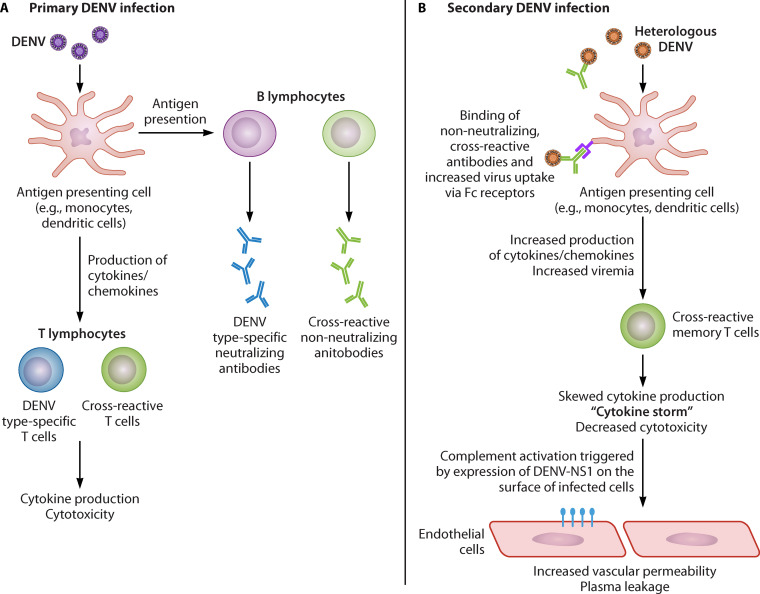
Understanding the underlying mechanisms of disease enhancement upon secondary heterologous infection is critically important for the evaluation and utilization of dengue vaccines and for human challenge models of dengue. In a primary infection, serotype-specific B and T cells successfully curtail the infection (112). However, in a secondary heterologous infection, cross-reactive antibodies not only fail to neutralize the virus but instead help it infect target cells, leading to massive virus production and overproduction of cytokines which increase blood vessel permeability, resulting in dengue hemorrhagic fever and risk of death. These two mechanisms are presented in Fig. 7.
FIG 7.
Potential mechanisms for immune-mediated enhancement of dengue virus infection.
The WHO organized a technical consultation in 2013 among experts on the long-term safety assessment of live-attenuated dengue vaccines to assess the theoretical risks of enhanced disease during clinical trials (113). Long-term follow-up analyses of Dengvaxia (CYD-TDV) phase 3 trials unfortunately indicated that dengue-naive individuals receiving this live-attenuated vaccine were at greater risk of subsequent severe disease (114). In light of these findings, a second WHO guidance on evaluating safety and efficacy of subsequent dengue vaccines, including the importance of long-term follow-up, was released (115).
(ii) Dengue human challenge model
Experimental human infection with dengue viruses has a history of over a century, as described in recent reviews (116, 117). Early work identified durable homotypic protection and short-term heterotypic protection among different dengue serotypes—key results that have both informed and challenged vaccine development against dengue to the present day. In the last few years, a focused effort has come underway to redevelop human dengue infection models that can support vaccine and drug development while meeting current regulatory requirements (118–121).
The first requirement for the redeveloped human dengue infection models is to produce suitably attenuated challenge strains of dengue virus using Good Manufacturing Practice. Researchers at the U.S. Walter Reed Army Institute of Research (WRAIR) took advantage of their previous work on dengue virus attenuation in an effort to develop new challenge strains. Over the years, dengue virus strains have been passaged through a number of cell types in culture (117). Mammen et al. evaluated seven of these attenuated strains by subcutaneous inoculation in flavivirus-naive human volunteers in an inpatient setting. DF was defined as sustained fever for 48 h or more, and by concurrent viremia as defined by virus culture in vitro (122).
Dengue fever has a typical incubation period of seven days or more. Physicians conducted assessments every day for 14 days, and five outpatient visits took place over a period of 6 weeks. Volunteers also underwent chest X-ray, abdominal ultrasound, and punch biopsy of any rash that developed while an inpatient. At study conclusion, volunteers were provided with a medical statement enabling them to notify future medical providers of their potential to develop dengue hemorrhagic fever or dengue shock syndrome upon reexposure to dengue virus (122).
Table 1 details the results of the challenge study. The volunteers challenged with the DENV-2 and DENV-4 strains did not meet the case definition of DF, but those challenged with the DENV-4 strains were infected and had mild perihepatic effusions that were only detectable by ultrasonography. The volunteers challenged with the DENV-1 and DENV-3 strains developed DF, and in some cases, other clinical manifestations of dengue virus infection. The DENV-1 and DENV-3 challenge strains were selected for further use in the challenge model and were manufactured under Good Manufacturing Practice.
TABLE 1.
Clinical manifestations of dengue fever in human volunteers challenged with attenuated strains of four dengue serotypesa
| Strain | Passage history (no.) | Clinical manifestationsb |
|---|---|---|
| DENV-1 45AZ5 | FRhL (20) | Two volunteers were challenged. Both developed DF and one also developed mild effusions. |
| DENV-2 S16803 | Mosquito (1), PGMK (4), PDK (10), FRhL (3) | Three volunteers were challenged with one of these two strains. None developed DF. |
| DENV-2 PR159 | PGMK (6), FRhL (1) | |
| DENV-3 CH53489 cl24/28 | PGMK (4), C6/36 (5), FRhL (1) | Three volunteers were challenged. All developed DF and two developed elevated levels of liver enzymes. |
| DENV-4 341750 | Mosquito (1), PDK (6), FRhL (3) | Two volunteers were challenged. Neither developed DF. |
| DENV-4 341750 | Mosquito (1), PGMK (5), FRhL (4) | Two volunteers were challenged. Neither met the case definition of DF, but both became viremic and developed mild effusions. |
| DENV-4 H-241 | Mosquito (1), C6/36 (2), FRhL (1) |
C6/36, culture-adapted Aedes albopictus larval cells; DENV, dengue virus; DF, dengue fever; FRhL, fetal rhesus macaque lung cells; mosquito, Toxorhynchites amboinensis; PDK, primary dog kidney cells; PGMK, primary green monkey kidney cells.
Effusions are accumulations of fluid that has leaked from small capillaries. Elevated liver enzymes in the blood is evidence of liver damage.
The dengue challenge model described above was also used to study volunteers that had previously received candidate dengue vaccines (116, 123). Ten subjects previously vaccinated with the live-attenuated, tetravalent candidate TDEN under development by WRAIR and GlaxoSmithKline (GSK) were challenged 12 to 24 months after vaccination with the live-attenuated DENV-1 or DENV-3 strains described in Table 2. Four unvaccinated, dengue-naive subjects were challenged as controls, two with each challenge strain.
TABLE 2.
Attenuated dengue virus challenge strainsa
| Strain | Method of production and administration | Stock producer |
|---|---|---|
| DENV-1 45AZ5 | Isolated from a patient infected with dengue in Micronesia in 1974. Challenge doses are reconstituted from freeze-dried stocks in sterile water and administered to healthy adult volunteers by subcutaneous injection. | Walter Reed Army Institute of Research |
| DENV-3 CH53489 cl24/28 | Isolated from a patient infected with dengue in Thailand in 1974. Challenge doses are reconstituted from freeze-dried stocks in sterile water and administered to healthy adult volunteers by subcutaneous injection. | Walter Reed Army Institute of Research |
| rDEN2Δ30 | Recombinant virus derived from DENV-2 Tonga/74, isolated from a patient infected with dengue in the Kingdom of Tonga in 1974, with a 31-nucleotide deletion from the 3′ untranslated region. Challenge doses are thawed from stocks frozen at −80°C and administered to healthy adult volunteers by subcutaneous injection. | U.S. National Institute of Allergy and Infectious Diseases |
DENV, dengue virus.
In this study, many of the volunteers developed DF symptoms without meeting the formal case definition. The main reason was because many were still viremic from their prior vaccination. Four of five vaccinated and one of two control subjects developed elevated liver enzymes, some at high levels. All liver enzymes returned to normal by day 30.
Gunther et al. studied serum levels of soluble cytokine receptors and the levels of gamma interferon (IFN-γ) and other cytokines in PBMCs restimulated with laboratory strains of four dengue serotypes in the 14 subjects from the human challenge study of Sun et al. described above (116). This study suggested dengue virus may suppress cellular immunity during peak viral replication by a mechanism that inhibits production of IFN-γ.
The DENV-1 strain was recently rederived by transfection into FRhL cells and formulated for use in human challenge studies (124). A small phase 1 study successfully demonstrated this strain could infect and cause moderate disease without long-term sequelae.
A second challenge model for DENV serotypes, developed by the U.S. National Institutes of Health, Johns Hopkins University, and the University of Vermont using the genetically attenuated strain rDEN2Δ30 (125), is derived from an isolate from a dengue outbreak in the Kingdom of Tonga in 1974. This strain causes relatively mild disease and is more highly attenuated than the serially passaged strains developed at WRAIR. It was initially developed as a vaccine but found to be insufficiently attenuated for this purpose (118, 126). Administration of 103 PFU as a single subcutaneous dose did not induce fever, but resulted in notable viremia: rDEN2Δ30 was isolated from all ten subjects at a mean peak titer of 2.5 log10 PFU/mL (range, 1.5 to 3.3 log10 PFU/mL). Furthermore, 80% of subjects developed a maculo-papular rash that was graded at moderate severity in 50% of cases. However, these characteristics suggested it could still be useful as a challenge strain. The fact that rDEN2Δ30 is produced by recombinant DNA technology is a distinct advantage with respect to production and characterization of this strain. A further analysis of T-cell responses in subjects challenged with rDEN2Δ30 indicated that responses were generally similar to those induced by natural infection, particularly with respect to nonstructural proteins NS1, NS3, and NS5 (127). These results provided additional support for the suitability of rDEN2Δ30 as a challenge strain in a dengue human infection model.
(iii) Dengue vaccine development
Several candidate dengue vaccines are in early or advanced clinical development and a number of “second generation” candidate vaccines are in the preclinical stage (128–130). Table 3 summarizes the clinical pipeline for candidate dengue vaccines.
TABLE 3.
Dengue vaccines licensed or in clinical developmenta
| Vaccine type | Approach | Development stage | Developer(s) |
|---|---|---|---|
| Live attenuated | |||
| Dengvaxia (CYD-TDV) | Genes encoding prM and E proteins of each of the four dengue serotypes were cloned into the backbone of the yellow fever vaccine YFV 17D. | Licensed | Sanofi Pasteur (licensed from Acambis) |
| TDEN (aka TDV) | Strains of four dengue serotypes attenuated through serial passage in culture were combined for a tetravalent vaccine. The initial vaccine was reformulated after additional passages in serum-free media and was stabilized with carbohydrate rather than serum albumin. | Phase 2 | WRAIR and GSK |
| Butantan-DV (aka Tetra-Vax-DV, TV003) | Attenuation through introduction of a 30-nucleotide deletion (Δ30) in the 3′ untranslated region of the genome of a DENV-4 cDNA clone. The prM and E genes and flanking genome sequences of the three other dengue serotypes were cloned into the DENV-4 backbone. | Phase 3 | U.S. National Institutes of Health, Johns Hopkins University, University of Vermont, and Instituto Butantan |
| TAK-003 (aka DENVax, TDV) | A cDNA clone of the strain DENV-2 PDK-53 attenuated through serial passage in cell culture in Thailand was used as the backbone for insertion of prM and E genes of the other three serotypes. | Phase 3 | CDC and Takeda Vaccines |
| Whole inactivated | |||
| DPIV | Dengue viruses have now been grown to high enough titers in cell culture to permit purification and inactivation. A formalin-inactivated tetravalent vaccine has been produced. | Phase 2 | WRAIR, GSK, and Fiocruz |
| Prime-boost | Priming with live-attenuated TDEN followed by boosting with DPIV is being explored in two trials. | Phase 1 | WRAIR and NMRC |
| Recombinant subunit | |||
| V180 | Stably transformed Drosophila S2 cells produce high levels of a truncated form of the dengue E protein, called 80E. Tetravalent formulations of 80E proteins have been produced with Alhydrogel or Iscomatrix adjuvant. | Phase 1 | Merck |
| TVDV | DNA plasmids expressing prM and E genes of four dengue serotypes have been constructed and combined for a tetravalent vaccine. Various delivery methods, including needle-free injection systems and intradermal electroporation, are being explored. | Phase 1 | NMRC |
aka, also known as; CDC, U.S. Centers for Disease Control and Prevention; DENV, dengue virus; DPIV, dengue purified inactivated vaccine; E, envelope; Fiocruz, Fundação Oswaldo Cruz; GSK, GlaxoSmithKline; NMRC, U.S. Naval Medical Research Center; PDK, primary dog kidney cells; prM, precursor membrane protein; TVDV, tetravalent dengue DNA vaccine; WRAIR, Walter Reed Army Institute of Research.
Dengvaxia, developed by Sanofi Pasteur, is the only one licensed, and the entire development program and deployment experience thus far has been reviewed (131). In a phase 2 trial in Thai school children (132), the overall protection against dengue infection with CYD-TDV was 30.2%. Although some protection was observed with three of the four DENV serotypes, no protection was seen against infection with DENV-2. Phase 3 trials with CYD-TDV were conducted in children in five countries in the Asia-Pacific region (133) and in children in five Latin American countries (134). Phase 3 trials showed 56.5% efficacy and 60.8% efficacy in the Asia-Pacific region and Latin America, respectively. In all three of the trials described above, the level of protection against DENV-3 and DENV-4 was higher than against DENV-1 and DENV-2. These efficacy results were disappointing in light of evidence for induction of neutralizing antibodies, particularly against DENV-2. More concerning was the evidence for increased risk of severe disease in dengue-naive individuals that received Dengvaxia (114), particularly in younger children. These findings have restricted the broad deployment of Dengvaxia and led the WHO to recommend its use only in countries where dengue is endemic with a high burden of disease (at least 70% seropositive) and in individuals with laboratory-confirmed previous exposure to dengue. The WHO has also recommended against use of Dengvaxia in children younger than 9 years old, which includes those most vulnerable to morbidity and mortality caused by dengue. A comparison of B- and T-cell epitopes targeted by Dengvaxia in contrast to two other vaccine candidates (Butantan-DV and TAK-003, described below) suggested lack of targeting appropriate T cell epitopes could account for the limitations observed with Dengvaxia (135).
Another tetravalent live-attenuated dengue vaccine candidate, TDEN (previously known as TDV), is under development by WRAIR and GSK. This vaccine is comprised of strains of four dengue serotypes attenuated through serial passage in dog kidney cells and then in fetal rhesus lung cells, and is in phase 2 (see Table 3 for details). TDEN was initially evaluated in infants, children, and adults in phase 1 and 2 trials (136). Since then, the vaccine has been reformulated with carbohydrate stabilizer rather than serum albumin and lyophilized as a tetravalent product. The reformulated product was taken forward into two phase 2 studies in regions where dengue is endemic. The first was conducted in Thailand and enrolled 120 mostly primed (i.e., dengue seropositive) adults (137). Two doses were administered six months apart. Nearly all vaccinees had a tetravalent response three months following the second dose, as defined by at least a 10-fold increase in reciprocal titers of neutralizing antibodies against all four serotypes. In this study, TDEN was generally safe and well tolerated: there were no vaccine-related serious adverse events or cases of dengue fever and only five subjects receiving TDEN had low-level viremia.
The second phase 2 endemic-setting study with TDEN was conducted in Puerto Rico and enrolled 636 individuals from ages 1 to 50 years (138). Approximately half of these individuals were seropositive at baseline, although this was highly age related: in the youngest age stratum, children under 2 years old, fewer than 10% were seropositive, whereas among adults (21 to 50 years old), 93% were seropositive. As in the Thailand study, subjects received two doses six months apart. Among seronegative and seropositive individuals, the tetravalent response rates were 81 and 99%, respectively, at 1 month after the second dose. The safety profiles between vaccine and placebo groups were similar among both seropositive and seronegative individuals; for example, moderate severity fever was reported by approximately 5% of subjects across all groups. There were no vaccine-related serious adverse events and no confirmed cases of dengue fever. In a follow-on study to examine cell-mediated immune responses, a representative subset of 244 participants was selected and CD4+ T-cell, CD8+ T-cell, and memory B-cell responses were characterized (139). TDEN was found to be poorly to moderately immunogenic by these criteria, regardless of the setting or whether subjects were previously exposed to dengue.
A third live-attenuated tetravalent dengue vaccine candidate (Butantan-DV, previously known as TV003 or Tetra-Vax-DV) is under development by the U.S. National Institutes of Health (NIH), the Instituto Butantan (Brazil), and Johns Hopkins University. This vaccine was attenuated through a 30-nucleotide deletion in the 3′ untranslated region and by additional mutations of a cloned DENV-4 strain. The prM and E genes of the three other serotypes, together with some genome regions flanking the prM and E genes, were then cloned into the attenuated DENV-4 backbone (140). One unique feature of this vaccine is that it is not transmissible to mosquitoes. During development, many different constructs were evaluated alone or in combination (112, 141, 142). Thirteen different phase 1 trials separately evaluated eight different monovalent vaccine constructs. Four monovalent constructs were selected and evaluated in different admixtures in a phase 1 trial. Admixture TV003 produced serum antibody responses against DENV-1 and DENV-4 in 100% of vaccinees, against DENV-3 in 85% of vaccinees, and against DENV-2 in 50% of vaccinees. This vaccine was then advanced to a phase 2 trial in a country, Brazil, where dengue is endemic in collaboration with the Instituto Butantan (143). This study tested a lyophilized formulation of TV003 designated Butantan-DV and found 64% of dengue-naive and 55% of dengue-exposed individuals had a tetravalent neutralizing response, a difference that was not statistically significant. The safety profile for Butantan-DV was acceptable and consistent with phase 1 trials with TV003 conducted in the United States; therefore, Butantan-DV was advanced to a randomized, placebo-controlled phase 3 trial in Brazil, which is ongoing (ClinicalTrials.gov identifier NCT02406729) (144). This trial plans to enroll approximately 17,000 participants from 2 to 59 years of age. Efficacy evaluation of the phase 2 study subjects is also ongoing.
In parallel to the phase 2 trial in Brazil, TV003 was also tested in a dengue controlled human infection model in volunteers in the United States (125). Volunteers received a single dose of either TV003 or placebo and then, 6 months later, were challenged with 103 PFU of rDEN2Δ30. All 21 subjects that received TV003 were completely protected from the challenge and did not experience any viremia or rash. In contrast, all 20 subjects in the placebo group experienced viremia with a mean peak titer of 2.3 ± 0.1 log10 PFU/ml, and 80% experienced a rash. This study demonstrated the potential value of the dengue controlled human infection model in generating proof of concept for a vaccine candidate such as TV003 before investing in a larger, more expensive phase 3 trial. A series of follow-on studies on the immunological responses of participants from this challenge study further demonstrated the utility of the model in conducting detailed analyses that would be more difficult with patients infected in an endemic setting. Nivarthi et al. characterized targets of memory B-cell responses (145), and Graham et al. characterized the quality of vaccine-induced CD4+ and CD8+ T cells specific to the various DENV serotypes (146). In light of the modest efficacy of Dengvaxia, a series of studies was undertaken to compare the immune response of TV003 (147–150). Taken together, these studies suggested that in contrast to Dengvaxia, TV003 induces a robust and balanced immune response against all four DENV serotypes after a single dose, regardless of prior dengue exposure, thus increasing the likelihood that it will confer strong immunity without increasing risk of more severe disease from subsequent infections.
A fourth live-attenuated tetravalent vaccine candidate called TAK-003 (previously known as TDV and DENVax) is under development by the CDC and Takeda Vaccines. It is constructed from a cDNA clone of a DENV-2 strain attenuated through serial passage in dog kidney cells in culture, into which prM and E genes of the other three dengue serotypes were cloned. TAK-003 (and various precursor formulations, admixtures of the four-component attenuated DENV strains, and dose regimens) were tested in at least nine studies in healthy, dengue-naive adult and adolescent volunteers in the United States, Mexico, and Colombia, as well as in both adults and children in countries in Asia and Latin America where dengue is endemic (151–161). Collectively, these studies found that one or two doses induced a tetravalent neutralizing antibody response ranging from 60 to 97% of subjects, depending on previous dengue exposure. The vaccine was well tolerated at multiple dose levels and not associated with serious adverse events. Taken together, these studies supported the safety and efficacy of this vaccine candidate that justified progressing it to a phase 3 study of more than 20,000 children aged 4 to 16 years at 28 study sites in eight countries where dengue is endemic, including five in Latin America and three in Asia (ClinicalTrials.gov identifier NCT02747927) (162). These subjects received two doses subcutaneously of a dose-optimized, lyophilized, and reconstituted formulation or placebo given 3 months apart. It was recently reported that TAK-003 achieved its primary efficacy endpoint in this study of 80.2% overall efficacy in reduction of virologically confirmed dengue (VCD) (163). It was also recently reported that TAK-003 achieved secondary efficacy endpoints, including 76% efficacy in individuals previously exposed to dengue, 66% efficacy in dengue-naive individuals, 90% efficacy against hospitalization due to dengue, and 86% efficacy against dengue hemorrhagic fever (164). Rates of SAEs were similar between the vaccine and placebo groups, and the overall safety profile importantly did not indicate a similar risk as was previously observed with Dengvaxia in dengue-naive vaccinees. In a follow-on report after 2 years, TAK-003 was still 56% efficacious in preventing any VCD and 76% efficacious against hospitalization (165). However, the efficacy in preventing any VCD among 4- and 5-year-olds dropped substantially from 73% in the year 1 to only 25% in year 2. No additional safety risks were noted in year 2. Long-term follow-up of this study to continue to track the safety and efficacy of TAK-003 is still in progress and is expected to be completed at the end of 2021. Ancillary studies have further analyzed the immune response from subjects in some of the studies described above and characterized in detail the humoral and cellular responses associated with immunity induced by TAK-003. In particular, like Butantan-DV (also known as [aka] TV-003) described above, TAK-003 stimulates a relatively balanced profile of neutralizing antibodies (166) and memory B cells (167) against the four DENV serotypes and also stimulates a CD8+ T-cell response against DENV nonstructural proteins NS1, NS3, and NS5, which are associated with a protective immune response (168–170). In contrast, a recent publication highlighted the difference between type-specific neutralizing antibodies against DENV-2 compared to cross-reactive DENV-1, DENV-3, and DENV-4 neutralizing antibodies (171).
An adjuvanted tetravalent dengue purified inactivated vaccine (DPIV) is under development by WRAIR, the Oswaldo Cruz Foundation, and GSK. Three clinical studies comparing various dose levels, adjuvants, and schedules have been completed. These included phase 1 (172) and phase 1/2 (173) studies in dengue-naive adults, and a phase 1 study in Puerto Rican adults (174, 175), most of whom were previously exposed to dengue. In the dengue-naive populations, two doses of DPIV adjuvanted with GSK’s proprietary AS03B adjuvant given intramuscularly one month apart induced a tetravalent neutralizing antibody response in 100% of subjects at 1 month after the second dose. These responses waned somewhat over the following year, but a subset of individuals boosted again at 12 months demonstrated a robust anamnestic response. Among previously dengue-exposed individuals, DPIV stimulated neutralizing antibody titers that generally persisted for at least 3 years. In both populations, DPIV was safe and well tolerated, supporting further development of this candidate. A prime-boost strategy using the live-attenuated TDEN candidate (described above) as a prime followed by boosting with DPIV has been explored in two phase 1 clinical trials conducted by WRAIR and the U.S. Naval Medical Research Center (NMRC) (ClinicalTrials.gov identifiers NCT03141138 and NCT02239614) (176, 177). In the first of these two to report results, it was found that priming with DPIV and boosting with TDEN yielded the higher neutralizing titers and rate of tetravalent seroconversion, as opposed to the opposite sequence (178).
Recombinant subunit and DNA vaccine candidates are also in clinical trials (see Table 3) (128). A tetravalent recombinant subunit vaccine candidate composed of truncations of the E proteins from all four serotypes designated V180 is being developed by Merck. A previous monovalent version composed of only the DENV-1 80E protein was developed by Hawaii Biotech and tested in a phase 1 study (179). V180 contains 10 μg each of DENV-1, DENV-2, and DENV-3 80E and 20 μg of DENV-4 80E. V180 has been tested in a phase 1 trial in healthy, dengue-naive Australian adults comparing various adjuvants and dose levels (180). Tetravalent responses were 71 to 88% after two months but declined to 0 to 43% after 1 year. To investigate responses in the context of prior dengue exposure, V180 was also tested in individuals that had previously received a live-attenuated tetravalent dengue vaccine (TV003 or a related formulation, TV005, described above) and found to be similarly immunogenic (181). NMRC is pursuing a DNA vaccine approach for dengue. A prototype plasmid encoding DENV-1 prM and E delivered by needle-free Biojector intramuscular injection was previously tested in a phase 1 study and found to be safe but only mildly immunogenic (182). A tetravalent dengue DNA vaccine (TVDV) composed of plasmids expressing prM and E proteins from all four serotypes with a lipid adjuvant (Vaxfectin) was then tested in a phase 1 study (183). TVDV was delivered by standard needle-and-syringe intramuscular injection and stimulated minimal neutralizing antibodies, but a notable T-cell IFN-γ response. Alternative delivery approaches for TVDV that may increase immunogenicity, such as intradermal electroporation, have been explored using a nonhuman primate model and may be applied in future clinical studies (184).
An even greater number of candidate dengue vaccines are in preclinical development. Table 4 summarizes the number and types of these vaccine candidates, as reviewed by Schmitz et al. (185) and Redoni et al. (128). The recombinant subunit vaccines are mostly expressed in Escherichia coli or yeast cells. Each of the five virus-vectored vaccines uses a different virus vector. Finally, several candidate vaccines incorporating nonstructural proteins (mainly NS1) are being evaluated in mouse models (186).
TABLE 4.
Candidate dengue vaccines in preclinical developmenta
| Vaccine type | No. of candidates | Developer(s) |
|---|---|---|
| Live attenuated | 3 | Chiang Mai University, Arbovax, Beijing Institute of Microbiology and Epidemiology |
| Inactivated | 1 | NMRC |
| Recombinant subunit | 4 | ICGEB-India, National Health Research Institutes (Taiwan) |
| DNA | 4 | Inovio, Kobe University, CDC, NMRC, Fiocruz |
| Virus-like particles | 4 | Cytos, ICGEB-India, Kobe University, NCGEB-Thailand |
| Virus-vectored | 5 | ICGEB-India, GenPhar/NMRC, University of North Carolina, University of Texas, Themis Bioscience/Institut Pasteur |
CDC, U.S. Centers for Disease Control and Prevention; Fiocruz, Fundação Oswaldo Cruz; ICGEB-India, International Center for Genetic Engineering and Biotechnology; NCGEB-Thailand, National Center for Genetic Engineering and Biotechnology; NMRC, U.S. Naval Medical Research Center.
(iv) Summary
The importance of dengue virus as a threat to international public health has incrementally increased in the last 30 years; yet, large areas of the world (including most of Africa) are at present spared because of the absence of the insect vectors. The intermixing of DENV serotypes and disease enhancement upon heterologous secondary infection could possibly be the engine that has driven the rapid spread and increased morbidity and mortality from dengue virus infection in the last century.
The very nature of the disease makes developing a vaccine against DENV a formidable task (187). The ideal dengue vaccine would provide a similar level of protection against each of the four dengue serotypes. The single live-attenuated tetravalent vaccine licensed to date has generated imbalanced immunity, which has been highlighted as an explanation for its limited efficacy and safety concerns, particularly in dengue-naive children. However, the two live-attenuated candidates that followed into phase 3 trials both induce a more balanced response and seem extremely promising. Alternative approaches, including whole-inactivated and subunit vaccines, are advancing through early clinical development and may augment or complement the live-attenuated approach, for example, as part of a prime-boost strategy.
The development of a human challenge model for dengue has been correspondingly complex (118). A significant limitation, however, is the fact that attenuated challenge strains of DENV-2 and DENV-4 have not been fully characterized in human challenge studies. Furthermore, even the current DENV-1 and DENV-3 challenge strains may be introducing added variability into the model because of their long and often complex passage histories. The newly developed human challenge models for dengue viruses have sparked considerable interest and comment in the community of dengue researchers and vaccine developers (117, 119, 188), along with some valuable suggestions for continued development of the models (189). The newly developed models using attenuated strains of DENV may become important contributors to the overall landscape of dengue vaccine development and introduction.
Enteric Diseases
Cholera
(i) Epidemiology, public health impact, and pathogenesis of Vibrio cholerae
Cholera is a diarrheal disease caused by the bacterium Vibrio cholerae. According to recent estimates, cholera results in 2.8 million cases and nearly 100,000 deaths annually (190). Until the 1970s, the major burden of cholera infection was in South and Southeast Asia, but outbreaks are now frequent in Africa and have also occurred in countries such as Haiti (191) and Yemen (192).
Access to clean water, good sanitation, and proper hygiene will halt the spread of cholera, but these measures are unlikely to be universally available in the near term. Five cholera vaccines are licensed in multiple countries, but most are used in reactive vaccination campaigns in response to outbreaks. To make the best use of vaccine supply, the WHO recommends a cholera vaccine stockpile to contain outbreaks (193).
Long-term vaccination policies may be considered as stakeholders assess the introduction of cholera vaccines into national policies. Bangladesh has taken the lead in this area with several phase 4 trials to assess a cholera vaccine’s feasibility for use and delivery as part of a national vaccine program, and have evaluated parameters such as single-dose regimens, limited-duration storage without refrigeration, and safety in pregnancy (194–197).
More than 200 serogroups of V. cholerae are found in aquatic environments, but only two serogroups (O1 and O139) are associated with cholera outbreaks in humans (198). The O139 serogroup was first identified in India and Bangladesh in 1992 but has since declined. The O1 serogroup has been responsible for the seven global cholera pandemics that have occurred since 1817 (198). The O1 serogroup has two biotypes, called El Tor and classical, which are further divided into serotypes Ogawa and Inaba. Infections with O1 and O139 serogroups are not protective against subsequent infection with the heterologous serogroup (190). Cross-protective immunity between different biotypes and serotypes was evaluated in a human challenge study described below.
V. cholerae are noninvasive but produce an enterotoxin called cholera toxin which binds to ganglioside receptors and causes a massive egress of water and electrolytes and a severe watery diarrhea ensues (198). Treatment consists of oral rehydration and antibiotics which dramatically reduce mortality from approximately 15% to about 1%.
(ii) Cholera vaccine development
Five whole-inactivated oral cholera vaccines are currently available—Dukoral, Shanchol, Euvichol, mORC-Vax, and OraVacs—as well as one live-attenuated vaccine, Vaxchora (see Table 5 for more details). Dukoral, Shanchol, and Euvichol are licensed for international use. Dukoral consists of formalin and heat-inactivated V. cholerae bacteria (O1 serogroup, classical and El Tor biotypes, Inaba and Ogawa serotypes), plus recombinant cholera toxin B subunit (rCTB) which lacks toxigenic activity. Dukoral is administered in three doses, at least 1 week apart, in children younger than 5 years of age. For other age groups, it is given in two doses at least 1 week apart. This vaccine has a requirement for reconstitution in buffer at the time of use in order to protect the rCTB from stomach acid. Dukoral was evaluated in phase 3 efficacy trials in Bangladesh and Peru and in a phase 4 effectiveness trial in Mozambique. In Bangladesh, a double-blind, placebo-controlled trial in 90,000 volunteers showed a cumulative vaccine efficacy over 3 years of 50% in all age groups. The vaccine efficacy was 19% in children less than 5 years of age and 65% in children older than 5 years (199, 200). In a trial of 1,400 adult male recruits from the Peruvian military, vaccine efficacy was 86% after 5 months (201). The Mozambique trial studied the vaccine in 22,000 volunteers (30% of whom were HIV infected) and showed 85% protection after 1 year of follow-up (202). Dukoral has a minimum age requirement of 2 years.
TABLE 5.
Currently available cholera vaccinesa
| Trade name | Producer | Location(s) of licensure | Cost/dose (USD) |
|---|---|---|---|
| Dukoral | Crucell Sweden AB, acquired by Valneva (Sweden) in 2015 | International | $4.70 |
| Shanchol | Shantha Biotechnics, Ltd. (India), acquired by Sanofi Pasteur in 2009 | South and Southeast Asia | $1.84 |
| Euvichol | EuBiologics (South Korea) | International | $1.20 |
| mORC-Vax | Vabiotech (Vietnam) | Vietnam | NA |
| OraVacs | Shanghai United Cell Biotechnology (China) | China and the Philippines | NA |
| Vaxchora | PaxVax, acquired by Emergent BioSolutions (United States) in 2018 | United States | $270 |
NA, information not available.
Shanchol is a vaccine similar to Dukoral, licensed for use in South and Southeast Asia. It contains the same inactivated V. cholerae as Dukoral, but also contains V. cholerae of the O139 serogroup. Shanchol does not contain rCTB. It is administered in two doses at least one week apart. It underwent an efficacy trial in India with 67,000 volunteers, which showed 68% cumulative efficacy over 3 years in all age groups. The vaccine efficacy was 43% over 3 years in children less than 5 years of age (203). Shanchol has a minimum age requirement for administration of 1 year. A version of this vaccine, mORC-Vax, is manufactured in Vietnam for in-country use.
OraVacs is manufactured in China and licensed for use in China and the Philippines. Like Dukoral, this vaccine contains V. cholerae of the O1 serotype, El Tor, and classical biotypes, but it also contains rCTB. OraVacs is administered in enteric-coated capsules in two doses at least one week apart. Efficacy data for this vaccine seem to be unavailable in the literature.
Euvichol, developed by EuBiologics, was first prequalified by the WHO in 2015 (204). Euvichol is an inactivated whole-cell vaccine that has a similar composition as Shanchol: it contains the O1 and O139 serogroups, Inaba and Ogawa serotypes, and the El Tor and classical biotypes. Like Shanchol, it does not contain rCTB. Euvichol was approved by the South Korea Ministry of Food and Drug Safety on the basis of a noninferiority trial in comparison with Shanchol in the Philippines (205). This phase 3 study of 1,263 adults and children found that vibriocidal responses after two doses of each vaccine were very similar: 75 to 80% in adults and roughly 90% in children. A recently developed presentation, Euvichol-Plus, is packaged in plastic tubes rather than glass vials. This presentation reduces product volume and weight, thus facilitating distribution in low-resource settings.
Hillchol (Hilleman Laboratories, India), another killed whole-cell vaccine candidate, is currently progressing through development and represents an important novel approach. Hillchol replaces the four O1 Ogawa/Inaba strains in Dukoral, Shanchol, and Euvichol with a single El Tor Hikojima strain that expresses both Ogawa and Inaba lipopolysaccharide (LPS) (206). In a recent phase 1/2 study in Bangladesh, Hillchol demonstrated a safety and immunogenicity profile that was noninferior to Shanchol (207). Hillchol-B contains the antitoxin component rCTB, which is produced by a novel process that reduces cost relative to previous methods. Hillchol was recently licensed to Bharat Biotech International, which will conduct a phase 3 study to support licensure. Hillchol is intended for use in low- and middle-income countries (LMICs) at an estimated cost of less than U.S. $1 per dose.
A live-attenuated cholera vaccine previously known as CVD 103-HgR, Orochol or Mutachol, is now marketed by Emergent BioSolutions as Vaxchora. This vaccine did not provide significant protection with one dose in a trial involving 67,000 children and adults in Indonesia (208). The vaccine was used effectively in Micronesia to help control a cholera epidemic. In a retrospective analysis, the single-dose vaccine was 79% effective in the target population (209). An early precursor of this vaccine was highly protective in a human challenge model after only a single dose (210). The newer Vaxchora formulation of this vaccine, originally developed by PaxVax and then acquired by Emergent BioSolutions, was recently shown in a phase 3 trial of 197 American adults to be 90% effective against moderate to severe disease caused by experimental infection with V. cholerae 10 days after a single dose, and 80% protective after 3 months (211). PaxVax received U.S. Food and Drug Administration approval for Vaxchora in 2016 for use by adult travelers to countries where cholera is endemic. The cost per dose is approximately $270 (212), which may be acceptable for travelers from high-income countries but is obviously not compatible with introduction in LMICs. Additional studies are needed to determine whether Vaxchora, or an optimized formulation, could play a role in reducing cholera disease burden in LMICs, particularly among children. A recent phase 4 study demonstrated the safety and immunogenicity of Vaxchora among American children and adolescents 2 to 17 years of age is noninferior to adults (213–215).
There has been significant progress in the establishment and deployment of a WHO-coordinated oral cholera vaccine stockpile over the past several years (193). There are now processes in place to receive and evaluate requests for oral cholera vaccine usage in both outbreak and endemic scenarios. As mentioned above, Bangladesh was the first country to actively investigate this option, and other countries, including Mozambique, South Sudan, Cameroon, Uganda, Nigeria, Somalia, and Malawi, have also implemented mass vaccination campaigns (216–222). Further success with this approach will require sustained efforts and commitments from numerous humanitarian organizations, including the WHO, Gavi, the Vaccine Alliance, local ministries of health, and others.
The development of live-attenuated vaccines, which could have potential for greater vaccine efficacy with one dose, has also lagged behind the whole-inactivated vaccine approach. Interest in this area may be renewed with the recent success of Vaxchora described previously. A conjugate vaccine composed of V. cholerae O1 Inaba O-specific polysaccharide and recombinant tetanus toxin heavy chain is approaching phase 1 clinical testing (223–225).
The reluctance to use licensed cholera vaccines for mass vaccination campaigns is based partly on the vaccines’ limited efficacy in clinical trials. When a vaccine of limited efficacy is administered to a substantial fraction of a population, however, the whole population sees the benefit through herd immunity—an effect whereby a critical mass of vaccinated people in a community can break the chain of transmission and actually protect unvaccinated people. Longini et al. have developed a mathematical model to estimate the combined effects of vaccination and herd immunity, and applied their model to oral cholera vaccination in Bangladesh (226). The model suggested 58% vaccine coverage could reduce cases by nearly 20-fold in vaccinated individuals but also by 6-fold in unvaccinated people. These reductions could protect children under 2 years of age, who are ineligible for the most widely licensed cholera vaccines.
(iii) Human challenge studies with Vibrio cholerae
Human challenge studies with V. cholerae have been conducted to study disease pathogenesis, natural history, and immunity, including cross-protection, in addition to the assessment of cholera vaccines (227–232). The challenge strains have been attenuated or wild-type V. cholerae of different serogroups, biotypes, and serotypes grown in the laboratory and administered orally to adult human volunteers. Hospital isolation wards were used to care for and monitor volunteers during these studies and to prevent the spread of cholera in the community. Volunteers were evaluated for the onset, duration, and severity of the watery diarrhea caused by infection with V. cholerae.
One of the earliest human challenge studies with V. cholerae was designed to measure cross-protective immunity across different V. cholerae biotypes and serotypes (227). Infection with either of the classical serotypes provided complete homologous and heterologous protection from disease, and no shedding of the challenge strains by the human volunteers was detected upon rechallenge. Infection with either of the El Tor serotypes provided 90% homologous and 90% heterologous protection from disease, but shedding of the challenge strain did occur in about 30% of rechallenged volunteers. The finding that infection with the El Tor biotype does not fully protect against shedding after reexposure is of critical importance for the epidemiology of cholera infection, since shedding is a major factor in the onward transmission of V. cholerae.
The epidemiology of V. cholerae supports the idea that infections with the El Tor biotype strains are a more serious concern than those with the classical biotype. Strains of the El Tor biotype have largely replaced the classical biotype strains of V. cholerae worldwide (198). Moreover, the circulating strains are actually a hybrid between the classical and El Tor biotypes; they are phenotypically El Tor, but they produce the cholera toxin of the classical biotype (233). It is unclear when this hybrid strain arose, but it is clearly circulating in human populations and causing new outbreaks. The recent protracted cholera epidemic in Haiti is caused by this hybrid strain.
A number of human challenge studies with V. cholerae have been conducted for evaluating candidate vaccines (see Table 6). One whole, killed vaccine and five live-attenuated vaccines have been evaluated for protection against disease in human challenge studies. Nearly 400 human volunteers participated in these studies, in which challenge strains of the O1 and O139 serogroups and of the O1 El Tor Ogawa and Inaba serotypes were used. The overall experience has been that oral challenges with wild-type V. cholerae have been safe in healthy adult volunteers. A three-site cholera human challenge study was conducted with CVD 103-HgR/Vaxchora (211). As noted above, Vaxchora was found to be 80 to 90% protective against moderate-to-severe diarrhea caused by challenge with 1 × 105 CFU of V. cholerae O1 El Tor Inaba N16961. This study was the first use of the cholera human challenge model in a phase 3 study that served as a basis for licensure without additional field studies.
TABLE 6.
Human challenge studies with Vibrio cholerae after administration of candidate vaccines
| Vaccine | No. of volunteers | Challenge strain | Reference |
|---|---|---|---|
| Dukoral with or without cholera toxin B subunit | 35 | O1 El Tor Inaba | Black, 1987 (228) |
| Live-attenuated vaccine CVD 112, based on a serogroup O139 strain of V. cholerae | 23 | O139 | Tacket, 1995 (229) |
| Live-attenuated vaccine CVD 111, based on an O1 El Tor Ogawa strain of V. cholerae | 26 | O1 El Tor Ogawa | Tacket, 1997 (230) |
| Live-attenuated vaccine CVD 103-HgR, based on an O1 classical Inaba strain of V. cholerae | 51 | O1 El Tor Inaba | Tacket, 1999 (210) |
| Peru-15, a live-attenuated vaccine based on an O1 El Tor Inaba strain of V. cholerae | 36 | O1 El Tor Inaba | Cohen, 2002 (231) |
| V. cholerae 638, a live-attenuated vaccine based on an O1 El Tor Ogawa strain | 21 | O1 El Tor Ogawa | Garcia, 2005 (232) |
| Live-attenuated vaccine CVD 103-HgR (Vaxchora formulation) | 197 | O1 El Tor Inaba | Chen, 2016 (211) |
Correlates of protective immunity against cholera have not been conclusively established. In the field, higher vibriocidal antibody titers have been associated with a reduced risk of illness (234) but have not predicted protection in challenge studies (210). Recent field studies have also been used to investigate correlates of protective immunity. These studies have indicated that O1 LPS-specific memory B-cell levels and levels of serum immunoglobulin A against CTB, O1 LPS, and toxin-coregulated pilus A (TcpA) may also be markers for reduced risk of cholera (235, 236). One of these findings was supported by the recent report that CVD 103-HgR (Vaxchora) primed response to TcpA in a human challenge study (237). Gut microbiome characteristics have also been associated with cholera vaccine responses (238).
Human challenge studies have provided some evidence of protective immunity. Vaccinated individuals that are rechallenged show a marked reduction of shedding of the bacteria, which is taken as evidence of antibacterial immunity interfering with survival and growth of vibrios in the intestine. During vaccine development, mucosal sampling is not routinely performed and evaluation has relied on serum vibriocidal antibody titers. A high baseline in individuals residing in areas of cholera endemicity, however, complicates the use of this marker as a surrogate for cholera immunity.
(iv) Summary
Vibrio cholerae is a continuing source of morbidity and mortality in humans living in regions of cholera endemicity of the world. Outbreaks of cholera have continued to occur, and most of the recent outbreaks have been in Africa. The outbreaks in Haiti and Yemen affected 5% of each country’s population. Three internationally licensed cholera vaccines and three other cholera vaccines licensed for a limited geographic region or for use in a single country are currently available and have been used in several mass vaccination campaigns in Haiti, Bangladesh, Iraq, and at least nine countries in sub-Saharan Africa (216, 239). Epidemiological modeling suggests that even modest coverage with vaccines akin to those already available, combined with herd immunity, could lead to major reductions in the numbers of cholera cases in communities where vaccination is provided, including among young children who are ineligible for vaccination.
Human challenge studies have played a significant role in the development of cholera vaccines, especially those studies that demonstrated cross-protection among biotypes and serotypes, and the study of Dukoral with and without CTB. Vaxchora is the first vaccine licensed for travelers solely on the basis of a human challenge study (i.e., in the absence of additional field trials), a significant milestone for the controlled human infection model field. However, as this vaccine is only approved for use by adult travelers, it remains to be seen what impact it will have on reducing burden in LMICs. Human challenge studies could be further applied to evaluate new candidate vaccines for evidence on correlates of protective immunity, especially if safe and well tolerated, and procedures for direct or indirect mucosal sampling could be applied. Challenge studies could also be helpful toward down-selecting candidate live-attenuated vaccines by studying how the immune responses in the field trial of CVD 103-HgR differed from those seen in the challenge model with a precursor of this vaccine.
Enterotoxigenic Escherichia coli
(i) Epidemiology and public health impact of enterotoxigenic Escherichia coli
Enterotoxigenic Escherichia coli (ETEC) remains among the most common bacterial causes of diarrhea-associated morbidity and mortality (240–246). ETEC is often the first bacterial illness that children experience in areas of endemicity, with infants and young children experiencing two to five diarrhea episodes due to ETEC during their first 3 years of life (247–249). Recent studies in sub-Saharan Africa and South Asia conducted under the Global Enteric Multicenter Study (GEMS and GEM-1A) have reaffirmed the continuing importance of ETEC as one of the top four causes of moderate-to-severe diarrhea among children less than 5 years of age in both regions (243, 250). In a prospective community-based assessment of the role of ETEC in morbidity and mortality among infants and young children in low-resource settings, ETEC was also associated with persistent diarrhea, which is consistent with its role in contributing to stunting in these settings (244, 251–256).
It is estimated that ETEC causes about 220 million diarrhea episodes globally, with approximately 75 million episodes in children under 5 years of age and between 19,000 and 42,000 yearly deaths in this age group (240, 256–260). Because ETEC infection is associated with persistent diarrhea, which can lead to stunting early in life, infants and young children are at a higher risk of death due to other infectious disease causes, which may contribute an additional 34,000 deaths annually to the global ETEC mortality burden among infants and young children in low-resource settings. In addition, environmental enteric dysfunction and stunting associated with ETEC infection is thought to contribute to chronic poor health outcomes later in life, such as diabetes, obesity, and hypertension (259, 261), thus further highlighting the importance of developing effective vaccine interventions that induce long-lasting immunity against the most common ETEC strains causing life-threatening illness in infants and young children. ETEC is also the most frequent bacterial cause of diarrhea among travelers to Africa, Asia, and Latin America, including military personnel deployed to these areas. ETEC, in both travel and endemic settings, is becoming increasingly refractory to antibiotic treatment and is now considered a growing antimicrobial resistance threat (262). The Wellcome Trust recently recommended that vaccine development for enteric E. coli-associated illness, including ETEC, be accelerated (262).
ETEC strains have a wide geographic distribution. They are endemic in most of Africa, Asia, and Latin America. Systematic estimates of ETEC prevalence in cases of diarrhea by country or region have been difficult because of complex diagnostics. An improved laboratory assay using real-time quantitative PCR to concurrently detect the presence and relative level of 19 enteropathogens including ETEC has been described (263). Despite improvements in ETEC detection, the true global burden of ETEC-associated morbidity and mortality remains under debate.
(ii) Pathogenesis and diversity of enterotoxigenic Escherichia coli
Enterotoxigenic Escherichia coli is usually acquired by ingesting contaminated food or water. ETEC colonizes the small intestine by binding to epithelial cells using hair-like structures on the surface of ETEC bacteria termed fimbriae, which promote adhesion and the intracellular delivery of enterotoxins. The enterotoxins disrupt enzymes in cellular metabolic pathways that control the transport of electrolytes (such as chloride, sodium, potassium, and calcium) in and out of cells. Toxin delivery results in moderate to massive release of fluid and electrolytes into the lumen of the small bowel, resulting in a life-threatening watery diarrhea.
Two ETEC toxins exist: heat-labile toxin (LT) and heat-stable toxin (ST). LT is a protein that has a similar structure and 80% amino acid homology to cholera toxin. ST is a small peptide consisting of 18 to 20 amino acids that is unique to ETEC. LT and ST interfere with separate metabolic pathways leading to the same effects. Epidemiological studies have shown that strains expressing ST, either alone or in combination with LT, are associated with the most severe episodes of ETEC diarrhea in young children (264). LT-only expressing ETEC strains have tended to be discounted as human pathogens because of their lack of association with moderate-to-severe diarrhea in young children in studies such as GEMS (243, 265). However, it is clear from other epidemiological studies and controlled human infection model (CHIM) trials that subpopulations of LT-only ETEC strains expressing colonization factors, like CS17, can be human pathogens and should be considered in disease burden estimates (248, 249, 266–274). In the community-based MAL-ED study, LT-only expressing ETEC strains were shown to be associated with persistent diarrhea, thus further supporting the concept that at least some LT-only strains can be human pathogens (252). New animal model data as well as in vitro studies also support the pathogenicity of LT-only strains and the potential contribution of LT-producing ETEC strains to not only acute and persistent diarrhea but long-term intestinal sequelae as well, such as environmental enteric dysfunction (EED) and loss of gut barrier function (272, 273). More recent CHIM data also indicates that even asymptomatic ETEC infection can induce significant intestinal inflammation which provided additional evidence that ETEC can contribute to stunting, EED and malnutrition (275, 276).
ETEC encodes proteins termed colonization factor (CF) antigens, by which ETEC are characterized. ETEC strains are also characterized into serogroups using O (lipopolysaccharide) and H (flagella) antigens. Considerable effort has been made to find associations between O, H, CF, and toxins that would identify common constellations of antigens useful for inclusion in vaccines, but when the complexity of this approach was recognized, it was acknowledged that these antigens are “too diverse to be practical unless common epitopes can be identified and exploited” (277).
A subsequent review of the published literature on CF antigen and toxin expression (278) also found that 27% of isolates express LT alone and another 33% of isolates express LT in combination with ST. The most frequently expressed CFs were CFA/I (17%), CFA/II (9%), and CFA/IV (18%). Isidean et al. found marked variation across regions and populations (278). The most widely distributed and commonly found ETEC phenotype was O6:H16 CFA/II LTST, which accounted for 11% of isolates (277).
The CFs were subsequently renamed E. coli surface (CS) antigens in the mid-1990s due to a better understanding of the range of structures the CFs represented (264). In this review, we include reports that were published before and after the CS nomenclature was adopted. Wherever possible, the term “colonization factor” is used to include CF and CS designations.
(iii) Enterotoxigenic Escherichia coli vaccine development
Prevention and treatment options to address diarrheal illness from ETEC exist and are important for reducing the health impact of the high burden of infection. However, they are not always practical to implement and sustain in many low-resource settings due to compliance issues or decreasing effectiveness (i.e., antibiotics). As such, these interventions have limitations with respect to achieving equitable and sustainable coverage. An ETEC vaccine could play a critical and complementary role in the most resource-constrained and highly impacted parts of the world.
The development of an effective ETEC vaccine is an important goal for public health in low- and middle-income countries (LMICs) and would also be of benefit to international travelers to areas of endemicity (279–281). ETEC vaccine development has also been a WHO priority for the last 20 years and a guidance document published in 2006 has helped guide development efforts (282). In addition to potential direct effects on morbidity, mortality, and other ETEC-associated longer-term negative health outcomes, such as stunting, an ETEC vaccine might have indirect effects on decreasing antimicrobial resistance, increasing herd (community) immunity, and protecting from all-cause diarrhea (275). Although several promising oral and parenteral candidate ETEC vaccines have been tested and are in the pipeline at different stages of preclinical and clinical development (see Table 7, below), currently no licensed vaccines against ETEC diarrhea exist. The development of vaccines against these infections has been hampered by technical challenges, insufficient support for coordination of research and development efforts, and a poorly defined market to incentivise investment in product development. In addition, infants and children under 5 years in LMICs (the target age group for an ETEC vaccine) have proven difficult to immunize effectively against enteric pathogens (241, 281, 283). The precise reasons for this are under investigation, but likely relate to poor sanitation and hygiene conditions prevalent in LMICs (see the rotavirus and poliovirus section below for further discussion on this topic), as well as compromised gut health associated with stunting, EED, and malnutrition. To help frame the development of ETEC vaccine preferred product characteristics (PPCs), WHO recently developed a guidance document to help developers target their vaccine development efforts (240). The primary goal of the PPCs was to help guide the development of a safe, effective, and affordable ETEC vaccine that reduces moderate-to-severe diarrheal disease and morbidity in infants and children under 5 years of age in LMICs.
TABLE 7.
Candidate enterotoxigenic Escherichia coli vaccines in developmenta
| Type of vaccine | Candidate | Description | Development stage | Developer(s) |
|---|---|---|---|---|
| Live attenuated | ACE527 | Composed of three attenuated strains of ETEC that collectively express six CF antigens and the B-subunit of ETEC LT | Phase 2b | Acambis and PATH (no longer in clinical development) |
| Whole cell inactivated | ETVAX | An E. coli K-12 strain that overexpresses four CF antigens of ETEC and a hybrid LT/CTB antigen, adjuvanted with dmLT | Phase 2b | Scandinavian Biopharma |
| Live hybrid vectored | Shig-ETEC hybrid | ShigETEC LPS-free cell expressing conserved ETEC and Shigella antigens | Phase 1 | EveliQure |
| Shigella hybrid | Several different live-attenuated candidate Shigella vaccines have been engineered to express ETEC CF antigens | Preclinical | Center for Vaccine Development, University of Maryland | |
| Subunit | ||||
| Antitoxin | Dukoral CTB | There is antigenic similarity between the CTB found in the cholera vaccine Dukoral and LT of ETEC | Licensed for prevention of cholera | Valneva |
| dmLT | dmLT of ETEC | Phase 1 | Tulane University, PATH, and NIH | |
| LT-patch | ETEC LT delivered transcutaneously with a skin patch | Phase 3 | Intercell (no longer in clinical development) | |
| STb | Various mutant toxoids of ETEC ST designed to improve immunogenicity while maintaining safety | Preclinical | University of Bergen | |
| Fimbriae | Fimbrial tip adhesin CfaE | Various constructs designed to block adhesion of ETEC to the intestinal epithelium by inducing antibodies to the tips of fimbriae | Phase 2b | U.S. Naval Medical Research Center |
| Adhesin-toxoid fusion | A CF consensus peptide fused to dmLT-ST mutants | Preclinical | Johns Hopkins University and University of Illinois | |
| Novel proteins | Alternative virulence factorsb | YghJ, a protein secreted by the same pathway as ETEC LT; EatA, a serine protease that degrades mucin and promotes ETEC access to mucosal surfaces; EtpA, a secondary adhesin | Preclinical | Washington University School of Medicine and GlyProVac |
CF, colonization factor; CTB, cholera toxin B-subunit; dmLT, double-mutant heat-labile toxin; E. coli, Escherichia coli; ETEC, enterotoxigenic Escherichia coli; LPS, lipopolysaccharide; LT, heat-labile toxin; NIH, U.S. National Institutes of Health; ST, heat-stable toxin.
ST toxoid and conserved ETEC proteins can be used in combination with other cellular or subunit vaccine options.
The main strategies for the development of ETEC vaccines have been to induce immune responses against CF antigens and against one or both of the ETEC toxins (279). Birth cohort studies in LMICs, phase 2b CHIM studies, and limited phase 2 trials in travelers have indicated that both types of antigens contribute to protective immunity (241, 242, 248, 281–285). Therefore, vaccines have been designed to express some of the most prevalent CF antigens based on the epidemiological data described above. The fact that ETEC strains produce two different toxins also presents a challenge for vaccines. Including the B subunit of LT from ETEC in vaccines is feasible because this subunit has no toxin activity. Mutant forms of the A subunit of ETEC LT that have lost their toxigenic activity are often used in vaccines, but they may not completely mimic the antigenicity of native toxin. Mutant forms of LT, such as double-mutant R192G/L211A (dmLT), can not only serve as safe vaccine antigens but have also been shown to have adjuvant activity for coadministered ETEC antigens and may also make these vaccines more protective (281, 283, 286–288). dmLT has also recently been shown to adjuvant responses to ETEC vaccine antigens when they are given parenterally (by the intradermal or intramuscular routes) (287). The ST of ETEC is homologous to two human proteins and is poorly immunogenic in its native form. It has been proposed that a vaccine that contains five of the CF antigens and LT could provide protection against 80% of global ETEC strains (279). Table 7, compiled from data obtained from Zhang et al., Walker, Bourgeois et al., and Fleckenstein et al., describes the candidate ETEC vaccines in development (242, 279, 281, 289).
A promising live-attenuated ETEC vaccine candidate, ACE527, under development by PATH, was halted for lack of funding. It is comprised of three attenuated strains of ETEC that have been genetically engineered to express multiple CF antigens. Collectively, the three strains express CFA/I, CS1, CS2, CS3, CS5, and CS6. Each of the three strains expresses the B-subunit of the ETEC LT. In a phase 1 trial, ACE527 was found to be safe and well tolerated up to a dose of 1011 CFU. Strong immune responses were elicited to each of the three ETEC strains and to the B subunit of LT (290). A phase 2 trial was then conducted in the ETEC human challenge model (291). The ACE527 vaccine candidate provided substantial protection against the duration of diarrhea and was associated with reduced stool shedding of the challenge strain, an indicator of reduced colonization and reduced stool volume. The vaccine candidate showed significant protection against severe diarrhea (protective efficacy = 41%; P = 0.03). In a subsequent phase 2b immunization and challenge study, the protective efficacy of this vaccine candidate was improved by adding a third dose of vaccine to the immunization regimen and also by adding the dmLT adjuvant to the vaccine formulation (284). The adjuvanted vaccine candidate provided significant protection against severe diarrhea (65.9%; P = 0.01), as well as protection against diarrhea of any severity (58.5%; P = 0.02), when subjects were challenged 6 to 7 months after immunization.
An oral, whole-cell inactivated ETEC vaccine candidate is also under development. An early version of this vaccine candidate combined killed ETEC bacteria expressing five CF antigens with recombinant cholera toxin B (rCTB). It showed short-term efficacy in adult travelers to Mexico and Guatemala (285) but was not efficacious in infants in Egypt or Bangladesh (264). The candidate was reformulated to increase its CF antigen content and to include a hybrid LT/CTB antigen. The reformulated vaccine candidate, called ETVAX, is delivered with dmLT, a mucosal adjuvant. In a phase 1 trial in 129 Swedish adults, the modified candidate was safe and showed improved immunogenicity, especially to CS6, when it was administered with 10 μg of the dmLT adjuvant (288). In more recent clinical studies, the safety and immunogenicity of ETVAX was assessed in infants and young children in Bangladesh (286). This was also the first evaluation of the dmLT adjuvant in a vaccine candidate in this age group. The data showed that infants and children can develop significant intestinal immune responses to this vaccine candidate. Further, the frequency, magnitude, and breadth of these responses could be improved in infants by giving ETVAX with dmLT. The results also suggested that any reduction in immune response due to giving smaller amounts of vaccine in infants could be reversed by including dmLT. In addition, infants given ETVAX with dmLT developed an immune response rapidly after the first dose, while infants given the vaccine candidate alone needed two doses to develop a response. Encouraging ETVAX results were also recently obtained in a phase 2b study conducted in Finnish travelers spending 12 days in Benin as part of a cultural exchange program. The study results confirm the excellent safety profile and positive immunogenicity of ETVAX. While not reaching the study’s protective efficacy goal of 70%, the overall results indicate that ETVAX remains a strong ETEC candidate vaccine that warrants further clinical investigation. ETVAX was 56% efficacious against all severe diarrhea, independent of pathogen (P = 0.025) (292). In addition, ETVAX was 52% protective against more moderate forms of ETEC diarrhea, which can also impact on daily travel-related activities. Among participants with severe diarrhea, significantly fewer participants that received the candidate vaccine received antibiotic or antisecretory drug treatment compared to placebo recipients (P = 0.03), indicating that ETVAX reduced illness severity in the few breakthrough cases that occurred. Given the protective efficacy results against ETEC in Benin and the encouraging results of the descending age study in Bangladeshi infants and young children, ETVAX is moving forward in clinical development with phase 1 and 2b studies in progress in Zambia and The Gambia in infants and young children (Pan-African Clinical Trials Registry trial no. PACTR201905764389804) (293).
Another approach to ETEC vaccine development has been the construction of hybrid vectors expressing ETEC antigens. For example, a polyantigen comprised of ETEC CFs, LT, and ST is being expressed in the live-attenuated strain of Salmonella Typhi ZH9, with the intent to develop a vaccine against both typhoid fever and ETEC. The vaccine has been shown to induce antibodies against the ETEC CF and both of the ETEC toxins in mice (279, 281, 294). Live-attenuated hybrid vaccines based on Shigella flexneri strains expressing ETEC antigens or Shigella mutants designed to unmask conserved antigens shared between ETEC and Shigella are also under development, by the Center for Vaccine Development at the University of Maryland and by EveliQure in Austria, with both candidates moving into phase 1 studies during the 2021 time frame.
Subunit vaccine candidates against ETEC toxins are also under development, with CF antigens and a detoxified form of LT being the primary targets. One approach is to use a conserved protein or peptide component of that protein that is located at the tips of fimbriae. The adhesive protein (adhesin) is involved in the first step of ETEC attachment to mucosal epithelial cells. Antibodies against it might block attachment by a wide spectrum of ETEC strains. Two versions of a vaccine based on this concept are under development by the U.S. Naval Medical Research Center (NMRC) and the University of Illinois working in collaboration with the Johns Hopkins University Bloomberg School of Public Health (281, 287, 295). The Navy program is the most advanced, with phase 1 and 2b trials of two candidate antigens completed. Parenteral delivery of the CfaE adhesin by the intradermal route with mLT induced strong serum and mucosal responses to the adhesin and reduced the severity and incidence of ETEC illness in a CHIM involving challenge with the H10407 strain of ETEC. In addition, intramuscular immunization with CssBA and mLT was also highly immunogenic, inducing both systemic and mucosal immunity as well (296). Funding is currently being sought for a follow-on immunization and challenge study with this antigen-adjuvant combination. A CF consensus peptide fused to dmLT-ST mutants is in preclinical development and has shown encouraging immunogenicity in mouse and rabbit models, as well as showing protective efficacy in the ETEC piglet model (275). Plans are being made to produce pilot lots of this vaccine candidate under current GMP so clinical testing can begin.
Novel virulence factors are also being considered for incorporation into candidate ETEC vaccines. Two of these, described in Table 7, are in preclinical development at the Washington University School of Medicine and in Europe by GlyProVac in Denmark (281, 287, 294).
(iv) Human challenge studies with enterotoxigenic Escherichia coli
The human challenge model for ETEC has been used for more than 40 years. The initial study with the model was reported in 1971. The study’s collaborators at the University of Maryland and the WRAIR established that two ETEC strains isolated from U.S. soldiers in Vietnam with acute, watery diarrhea could cause disease in healthy volunteers. Since that time, at least 32 human challenge studies have been performed using 14 different strains of ETEC and including nearly 600 human volunteers (297–303).
Table 8 describes the ETEC strains that have been used in the human challenge model. Collectively, strains expressing colonization factors CFA/I, CS1, CS2, CS3, CS5, CS6, CS17, CS19, and CS21 have been used. Strains expressing LT, ST, or both have been included in these studies. The challenge strains come from eight different countries. The institutions involved in the development of ETEC challenge strains have included the University of Maryland, Johns Hopkins University, WRAIR, the U.S. Army Medical Research Institute of Infectious Diseases, NMRC, the University of Texas, and the University of Bergen, Norway.
TABLE 8.
Enterotoxigenic Escherichia coli strains used in the human challenge modela
| Challenge strain | Colonization factors and toxins | Country of origin | Strain developer(s) |
|---|---|---|---|
| 214-4 | CF unknown, ST | Mexico | CVD |
| B2C | CS2, CS3, LT/ST | Vietnam | CVD and WRAIR |
| B7A | CS6, LT/ST | Vietnam | CVD, WRAIR, and NMRC |
| DS26-1 | CS19, LT | Saudi Arabia | JHU and NMRC |
| E24377A | CS1, CS3, LT/ST | Egypt | CVD and WRAIR |
| E2528-C1 | CF unknown, LT | Caribbean | CVD |
| H10407 | CFA/I, LT/ST | Bangladesh | University of Texas Medical School, WRAIR, and CVD |
| H1765 | CFAII, LT/ST | Bangladesh | University of Texas Medical School |
| LSN03-016011/A | CS17, LT | Turkey | JHU and NMRC |
| TD255-C4 | CF unknown, LT | Mexico | CVD |
| TW10598 | CS2, CS3, CS21, LT/ST | Guinea-Bissau | University of Bergen and CVD |
| TW10722 | CS5, CS6, ST | Guinea-Bissau | University of Bergen and CVD |
| TW11681 | CFA/I, CS21, ST | Guinea-Bissau | University of Bergen and CVD |
| WS0115A | CS19, LT/ST | Egypt | JHU and NMRC |
CF, colonization factor; CS, coli surface antigen; CVD, Center for Vaccine Development, University of Maryland; JHU, Johns Hopkins University; LT, heat-labile toxin; NMRC, U.S. Naval Medical Research Center; ST, heat-stable toxin; WRAIR, Walter Reed Army Institute of Research.
Table 9 summarizes 30 studies that have been performed with the ETEC human challenge model (300). These studies have been the major sources of information about ETEC pathogenesis and the human immune response to infection with ETEC. Products that have been evaluated in ETEC human challenge studies include probiotic Lactobacillus, bovine colostrum and immunoglobulin G, and bismuth subsalicylate for the prevention of diarrhea; live-attenuated, whole-inactivated, and subunit candidate vaccines; and antibiotics (283, 297–303). Recently, the model has been improved through the development of a severity score that gives another outcome measure when evaluating treatment and preventive interventions (304) and the development of a new ST-only ETEC challenge strain (TW10722) that will help in the development of ST toxoids and ST toxoid-containing vaccines (305, 306). In addition, new applications of the model include microbiota studies and application of advanced systems biology tools to better understand the factors contributing to innate and adaptive immunity parameters triggered by ETEC infection (298, 299, 307–309).
TABLE 9.
Human challenge studies with enterotoxigenic Escherichia colia
| Challenge strain(s) | No. of volunteers | Purpose of study | Institution(s) | Reference |
|---|---|---|---|---|
| B2C, B7A | 24 | Establish ETEC as a cause of diarrhea | CVD and WRAIR | DuPont, 1971 (904) |
| 214-4 | 17 | Establish ST as a virulence factor | CVD | Levine, 1977 (905) |
| H10407 | 13 | Role of CF antigens in pathogenesis and immunity | University of Texas Medical School | Evans, 1978; Satterwhite, 1978 (906, 907) |
| B7A, E2528-C1 | 29 | Capacity of prior infection to protect from homologous or heterologous challenge | CVD | Levine, 1979 (908) |
| H10407, 214-4, TD255-C4 | 13 | Explore whether person-to-person transmission of ETEC can occur | CVD | Levine, 1980 (909) |
| TD255-C4, 214-4, B7A | 48 | Capacity of Lactobacillus to prevent diarrhea in volunteers after challenge with ETEC strains | CVD | Clements, 1981 (910) |
| H10407, B7A | 47 | Compare two antibiotics for treatment of diarrhea in ETEC-challenged volunteers | CVD | Black, 1982 (911) |
| H10407 | 26 | Evaluate a candidate vaccine antigen, somatic pili, for protection from disease in ETEC-challenged volunteers | CVD | Levine, 1982 (912) |
| H10407 | 16 | Evaluate bismuth subsalicylate for protection from diarrhea in ETEC-challenged volunteers | University of Texas Medical School | Graham, 1983 (913) |
| H10407, H1765 | 11 | Evaluate purified CFs as candidate vaccine antigens for protection from disease in ETEC-challenged volunteers | University of Texas Medical School | Evans, 1984 (914) |
| E24377A | 14 | Evaluate CS1 and CS3 colonization factors as antigens for protection from disease in ETEC-challenged volunteers | CVD | Levine, 1984 (915) |
| E24377A | 6 | Evaluate fimbriae antigens as vaccines | CVD | Levine, 1986 (916) |
| H10407 | 14 | Evaluate whole-inactivated ETEC vaccines for protection from challenge | University of Texas Medical School | Evans DG, 1988; Evans DJ, 1988 (917, 918) |
| H10407 | 20 | Protection by milk immunoglobulin concentrate against ETEC challenge | University of Maryland School of Medicine | Tacket, 1988 (919) |
| E24377A | 10 | Vaccination with CF encapsulated in microspheres, followed by challenge with ETEC | University of Maryland School of Medicine | Tacket, 1994 (920) |
| H10407 | 10 | Milk immunoglobulin with activity against CF antigens can protect from challenge | University of Maryland School of Medicine | Freedman, 1998 (921) |
| E24377A | 10 | Milk immunoglobulin does not protect from challenge when administered with a standard meal | University of Maryland School of Medicine | Tacket, 1999 (922) |
| B7A, H10407 | 32 | Pathogenicity of ETEC expressing CFA/I or CS6 in human volunteers, and capacity of ciprofloxacin to resolve symptoms of disease and prevent shedding | U.S. Army Medical Research Institute of Infectious Diseases | Coster, 2007 (923) |
| E24377A | 20 | Protective efficacy of transcutaneous immunization with ETEC LT in a challenge study | JHU and Iomai | McKenzie, 2007 (924) |
| E24377A | 16 | Efficacy of live-attenuated vaccine strain PTL-003 in protection from challenge | JHU and Acambis | McKenzie, 2008 (925) |
| LSN03-016011/A, WS0115A, DS26-1 | 38 | Experimental challenge with ETEC expressing CFs CS17 and CS19 | JHU and NMRC | McKenzie, 2011 (302) |
| LSN0-016011/A | 36 | Protective efficacy of anti-CS17 bovine colostrum passively administered to volunteers | JHU and NMRC | Savarino, 2019 (926) |
| H10407 | 45 | Dose de-escalation study for ETEC challenge strain H10407 | JHU and Gothenburg University | Harro, 2011 (301) |
| H10407 | 30 | Examine lower challenge doses in an effort to refine the model | JHU and PATH | Chakraborty, 2018 (298) |
| H10407 | 36 | Protective efficacy of ACE527 plus dmLT | JHU and PATH | Harro, 2019 (284) |
| TW10598 | 30 | Develop a new ETEC challenge strain for use in vaccine studies | University of Bergen | Skrede, 2014 (303) |
| TW11681 | 27 | Develop a new ST-producing challenge strain for use in vaccine studies | University of Bergen | Sakkestad, 2019 (297) |
| TW10722 | 21 | Develop a new ST-producing challenge strain for use in vaccine studies | University of Bergen | Sakkestad, 2019 (305) |
| B7A | 30 | Protective efficacy of bovine IgG antibodies against CS6 expressing E. coli passively administered to volunteers | JHU and NMRC | Talaat, 2020 (927) |
CF, colonization factor; CS, coli surface antigen; CVD, Center for Vaccine Development, University of Maryland; dmLT, double-mutant heat-labile toxin; E. coli, Escherichia coli; ETEC, enterotoxigenic Escherichia coli; IgG, immunoglobulin G; JHU, Johns Hopkins University; LT, heat-labile toxin; NMRC, U.S. Naval Medical Research Center; ST, heat-stable toxin; WRAIR, Walter Reed Army Institute of Research.
(v) Summary
The status of ETEC as a pathogen of public health importance in much of the world is now firmly established. No vaccine is available against the pathogen, but significant progress is being made with both promising inactivated whole-cell and subunit vaccine candidates moving further into clinical development. A human challenge model, in use for nearly five decades, has been the principal source of our current knowledge of ETEC pathogenesis and has guided antigen selection for the current candidate vaccines in development. The model has undergone a number of improvements in recent years, which has added value to it as an important research tool in the development new preventive interventions for ETEC. The recent successful development of an ST-only expressing challenge strain, in particular, is a significant advance. This advance in the ETEC challenge model portfolio of strains is very timely since efforts to development a safe and immunogenic ST toxoid for inclusion in future ETEC vaccine formulations has accelerated in recent years. Genetic attenuation of the ST toxin by targeted amino acid substitutions has shown promise in yielding safe and immunogenic toxoids in animal models (306), but these encouraging observations need to be confirmed in phase 1 and phase 2b studies. In this regard, we now have a well-characterized human challenge strain that will help support this important vaccine development effort.
The application of the human challenge model to the discovery of correlates of protective immunity has been limited. CHIM study results indicate that both anti-CF and anti-LT toxin immunity can contribute to protection, but more work is needed to better define correlates of protection.
Candidate vaccines against ETEC are in clinical development now that could advance to large field trials based on the results in human challenge studies. These include the inactivated whole-cell ETVAX vaccine, which is poised to begin phase 3 trials in LMICs and in travelers in the 2021/2022 time frame. With continued funding, NMRC’s adhesin-based subunit vaccine approach is also close to moving into late-stage clinical development.
The antigenic diversity of ETEC strains has only limited representation among the most widely used ETEC challenge strains. Nearly 80% of the human challenge studies have used just three strains (H10407, E24377A, and B7A). All three express both LT and ST. The CF antigens collectively expressed by the three strains are CFA/I, CS1, CS3, and CS6. The further development of the LSN03-016011/A strain by Johns Hopkins University and NMRC has provided the field with it first LT-only CS17-positive strain.
As indicated above, a major dilemma facing the field of ETEC vaccine development is whether or not vaccines should induce immunity to ST. Seventy-five percent of ETEC strains express ST, and it is unlikely that the diarrheal disease induced by ETEC can be completely abrogated by vaccines that do not induce immunity to ST. However, ST is poorly immunogenic in humans, which may be due in part to the fact that it closely resembles a self-antigen. A few candidate vaccines in early development appear promising, but they have not moved into human studies. The availability now of a new ST-only ETEC for future CHIM studies will play an important role in evaluating any ST toxoids. In addition, recent proteomic microarray analysis of serum and fecal extracts from subjects experimentally infected with ETEC strains, as well as analysis of samples from adults and children in LMICs, suggests that other protein antigens, such as EtpA, EatA, and YghJ, if added to future vaccine formulations along with CS6, could significantly improve coverage against ETEC strains that produce ST toxin only (289, 295, 310, 311), and the ST-only strain will also play an important role in evaluating these novel vaccine constructs.
Shigella
(i) Epidemiology, diversity, and public health impact of Shigella
Shigella is a major cause of diarrheal disease worldwide. In the Global Enteric Multicenter Study (GEMS) (312–315), Shigella was among the four most common enteric pathogens with attributable risk for moderate to severe diarrhea. Reanalysis of these GEMS data using molecular diagnostics indicates that Shigella remains the most attributable cause of moderate to severe diarrhea in children younger than 5 (313–315). Shigella was the second leading cause of diarrheal mortality in 2016 among all ages, and the leading bacterial cause of diarrhea, accounting for approximately 212,000 deaths. Although Shigella infections occur worldwide, across all age groups, with broad geographical distribution, the greatest burden is among children in low- and middle-income countries (LMICs), where it is estimated to be responsible for between 28,000 (316) and 64,000 deaths (315) among children under 5 years of age. The incidence of Shigella disease is highest among infants and children living in the WHO’s African and Eastern Mediterranean regions (317–326).
Historically, the field has held that dysentery (bloody diarrhea) was the main presentation for Shigella infection, but increasingly sensitive molecular diagnostic tools indicate that Shigella contributes to the burden of nonbloody diarrhea almost as much as it does to bloody diarrhea (319, 320). In addition, growing evidence shows that repeated, nonfatal, moderate to severe Shigella infections contribute to an increasing number of negative health outcomes in children living in LMICs, including stunting, severe malnutrition, and several metabolic disorders developing later in life (314, 318, 321–329), as well as mortality resulting from other infectious diseases (318, 324). In addition, there is growing evidence of increasing antimicrobial-resistant Shigella, and it is accordingly included on WHO and CDC antimicrobial resistance threat lists (262, 330). In light of increasing evidence showing that Shigella can present as nonbloody diarrhea, leading to misdiagnosis and inappropriate administration of antibiotics, this becomes an even greater concern. Not surprisingly, in a recent report highlighting the potential role of vaccines in combating antimicrobial resistance, the Boston Consulting Group and the Wellcome Trust recommended accelerated exploration of multipathogen combined vaccines against high-risk antimicrobial-resistant pathogens like Shigella and Campylobacter (262).
Finally, Shigella is also a major illness among military personnel deployed to LMICs and travelers to areas of endemicity. Research is ongoing to study long-term health issues related to Shigella infections in these populations, and there is already some indication in military populations that Shigella may increase incidence of irritable bowel syndrome (IBS) postinfection (331–335).
Four distinct species of Shigella are recognized, each with wide global distribution. The most prevalent are Shigella flexneri and Shigella sonnei, which together comprise about 90% of moderate to severe diarrhea cases due to Shigella. S. sonnei predominates mostly in high-income countries, whereas S. flexneri tends to predominate in LMICs.
Approximately 50 serotypes of Shigella are recognized, which poses a significant challenge with respect to strain selection for vaccines and CHIM studies. Fortunately, recent multiyear, multicountry studies of the distribution and prevalence of Shigella serotypes have been conducted, as well as a large study focused on mainland China (Table 10) (336). These studies, together with earlier data (337), provide a solid estimate of the most prevalent serotypes worldwide.
TABLE 10.
Recent studies reporting the prevalence of Shigella serotypesa
| Group | Species | Serotypes | Prevalence estimates (%) |
Most prevalent serotypes | ||
|---|---|---|---|---|---|---|
| GEMS study | von Seidlein study | Chang study | ||||
| A | Shigella dysenteriae | 15 | 5.0 | 4.0 | NA | NA |
| B | Shigella flexneri | 15 | 69.5 | 68.0 | 76.2 | 2a, 3a, and 6 |
| C | Shigella boydii | 19 | 5.4 | 6.0 | NA | NA |
| D | Shigella sonnei | 1 | 23.7 | 22.0 | 21.3 | NA |
GEMS, Global Enteric Multicenter Study; NA, information not available.
In association with the GEMS studies in Africa and South Asia, a total of 1,130 Shigella isolates were serotyped (338). S. flexneri accounted for nearly 70% of isolates. Five of the 15 known serotypes of S. flexneri comprised 90% of the identified S. flexneri strains. These serotypes were 2a, 6, 2b, 3a, and 1b, in order of prevalence. S. sonnei comprised 23.7% of isolates, while S. dysenteriae and S. boydii comprised 5 and 5.4%, respectively. These results were in close accord with the data published from 1966 to 1997, as reviewed by Kotloff et al. (337).
In addition, results of the GEMS and an earlier von Seidlein et al. study were also are in close accord (see Table 10). Both studies identified 2a, 3a, and 6 as the most prevalent serotypes of S. flexneri and both studies found that about one in four isolates was S. sonnei.
The recommendation for the future development of Shigella vaccines emerging from this body of work is that a quadrivalent vaccine comprised of S. sonnei and S. flexneri 2a, 3a, and 6 could provide substantial global coverage. Stockpiles of an S. dysenteriae serotype 1 vaccine could be considered as a practical approach to contain future outbreaks (338).
(ii) Pathogenesis of Shigella
Shigella is highly infectious. It is transmitted from person to person or by ingesting contaminated food or water. As few as 100 organisms can cause disease. Infection with Shigella leads to an acute intestinal infection of variable severity (339, 340). At the mild end of the spectrum, a watery diarrhea ensues followed by an inflammatory bacillary dysentery of varying severity, which can include fever, abdominal cramps, and stools containing blood, sheets of white blood cells, and mucus. The histopathology of tissue biopsy specimens from infected individuals shows invasion of the epithelium by inflammatory infiltrates, edema, and most importantly, regions where the colonic epithelium is actually eroded, with the formation of microscopic abscesses. Disease is usually self-limiting, but severe cases require oral rehydration and antibiotic treatment. The more severe sequelae of Shigella infection can include hemolytic-uremic syndrome, IBS, and reactive arthritis (ReA) (339, 340). As indicated above, the high global burden of Shigella infection also makes this enteropathogen a significant contributor to environmental enteric dysfunction and stunting among infants and young children living in areas where Shigella is endemic. It is also suspected that the significant gut inflammation induced by Shigella infection contributes to more long-term sequelae in LMICs, including several metabolic disorders affecting health and productivity later in life, such as diabetes, obesity, and hypertension (318, 322, 324, 326–328).
Shigella species encode three different toxins that are important in pathogenesis. ShET1 is chromosomally encoded and found only in S. flexneri serotypes 2a and 2b. It is a structural homologue of cholera toxin and heat-labile toxin of enterotoxigenic Escherichia coli. ShET2 is encoded on a virulence plasmid and is found in all serotypes. ShET1 and ShET2 cause the watery diarrhea that is the prodrome of disease (341–345).
The most severe form of Shigella disease is caused by infection with S. dysenteriae serotype 1, which is the only Shigella serotype that encodes Shiga toxin. The action of this toxin results in vascular lesions in the colon, kidney, and central nervous system. The resulting hemolytic-uremic syndrome can be life-threatening (337).
Shigella are facultative intracellular pathogens and a constellation of chromosomally encoded and plasmid-encoded genes are involved in the processes, whereby Shigella enter cells, further invade the colonic epithelium, and manipulate and evade host immune responses. The salient features of the invasion process have been reviewed by Schroeder and Hilbi (340).
We have only a limited understanding of how the human immune system contains and eliminates Shigella infection. How the carbohydrate portion of LPS is presented to the immune system in a form that evokes effective cellular and humoral immune responses is unclear (339). The assumption is that humoral and cellular responses to Shigella serotype-specific LPS (and possibly to other antigens including invasion proteins) work together to make subjects immune to shigellosis (332–335, 341–344, 346–351). Field studies and results from recent CHIM studies indicate that higher levels of serum immunoglobulin A or immunoglobulin G antibodies to LPS and invasion proteins are associated with a reduced risk of shigellosis (341–344, 346, 347, 352, 353). In addition, recent immune profiling of antigen-specific antibody responses to conserved invasion proteins done using newly developed proteomic arrays, as well as similar analysis by more traditional enzyme-linked immunosorbent assay methods, indicates that antibodies to IpaB and D may also contribute to protection and that the immune profiles associated with protection against S. flexneri and S. sonnei may differ (344, 354) (see more details below).
(iii) Shigella human challenge model
The Shigella human challenge model was established with the study of Shaughnessy et al. in 1946 using strains FW I through FW V (355). These strains were later classified as S. flexneri 2a. Since that time, at least 18 different Shigella challenge studies have been conducted involving more than 700 human volunteers, using 47 strain-dose combinations (356). The available challenge strains and their utilization are described in Table 11. The majority of the challenge studies have used S. flexneri 2a strains. In the more recent studies with S. flexneri 2a strain 2457T, much lower challenge doses were used than in the initial study with FW I through FW V strains. Nearly 200 volunteers have been challenged with S. sonnei. The challenge studies with strains of S. dysenteriae are few in number and less recent.
TABLE 11.
Shigella human challenge models
| Species | Serotype | Strain(s) | Typical dose range evaluated (CFU) | No. of challenges administered |
|---|---|---|---|---|
| Shigella flexneri | 2a | 2457T | 102 to 104 | 456 |
| FW I to FW V | 108 to 1010 | |||
| Shigella sonnei | NAa | 53G | 5 × 102 | 178 |
| Shigella dysenteriae | 1 | A-1, M-131 | 102 to 104 | 44 |
NA, not applicable, since S. sonnei has a single serotype.
The main difficulty with the Shigella human challenge models has been the lack of an obvious relationship between the challenge dose and attack rates for diarrhea and dysentery (356). The attack rates for doses of S. flexneri strain 2457T have been evaluated in 14 different studies and the attack rates for S. sonnei strain 53G have been evaluated in five studies. Even with similar doses, little consistency in attack rates has been seen. During the course of this work, the delivery of challenge inoculate in buffer has gradually replaced the earlier use of milk or water, and a grading scale for the severity of diarrhea was developed by Black et al. (357). A standard dose has not been adopted to enable comparability among studies.
Variability in attack rates across studies may impact the utility of the human challenge model for discerning differences between Shigella species and serotypes with respect to pathogenesis. It may also make the evaluation of candidate Shigella vaccines more difficult, since many of the candidate vaccines may reduce the severity of disease rather than prevent infection per se. The model has been consistent, however, with respect to rechallenge studies designed to determine whether infection confers protection from disease. Two studies with S. flexneri strain 2457T (358, 359) showed a reduction in disease of about 65% upon rechallenge with the same strain, and prior infection protected 67% of volunteers from disease upon rechallenge with S. sonnei strain 53G (360).
Shigella CHIM studies are usually performed in naive adults from high-income countries, although the major target population is children and adults in LMICs. A dose-escalation human challenge study was conducted in Thailand with S. sonnei strain 53G, the first conducted in an region of endemicity (361). The study was performed in three groups of 12 volunteers each to determine the dose that yielded an attack rate of at least 70% in Thai subjects. An attack rate of 75% was observed with a challenge dose of 1,680 CFU. A reasonable correlation was seen between the shedding of the challenge strain in stool samples and the development of clinical disease in the study volunteers. Further studies in adults in LMICs are being explored, but it remains to be determined what relevance CHIM studies in adults may have for pediatric populations in these settings.
The known postinfection sequelae of Shigella, including ReA and IBS, may need to be specifically addressed in human challenge studies. A meta-analysis of three different epidemiological studies showed a 7-fold increase in risk of IBS following acute gastroenteritis caused by Shigella (362). The extent to which early treatment of shigellosis in the human challenge model may help to reduce the risk of sequelae like IBS and ReA is not clear. Historically, subjects enrolling in Shigella challenge studies have been screened for HLA B27, since this has been shown to be a risk factor for ReA development. The consensus view is that vaccination with Shigella vaccines will not be associated with a higher risk of ReA, even in HLA B27-positive individuals (362). Other ways in which the performance of the Shigella challenge model might be improved include additional standardization with respect to the preparation, administration, and dose range of challenge strains. To this end, an S. sonnei CHIM using a lyophilized lot of strain 53G was established (ClinicalTrials.gov identifier NCT02816346) (354). A dose in the 1,500 to 2,000 CFU range of 53G was selected as the dose for future challenge studies using this product. This model will enable direct comparison of study results between institutions and ensure better consistency over time in the challenge inoculum. Improvements should also include establishing consistent case definitions for diarrhea and dysentery. The case definitions for diarrhea and dysentery could then be systematically aggregated with other clinical and microbiological data to generate a composite score for the severity of disease. Expanding the range of challenge strains to include S. flexneri serotypes 3a and 6 may also be useful. A strong effort to provide standardized guidance for use of the Shigella CHIM was published in a series of papers from a workshop to provide a consensus report on this subject (341–343).
In addition to permitting identification of promising vaccine candidates, CHIM studies enable exploration into immunological markers of vaccine-induced immunity. For example, a core Shigella proteome microarray consisting of more than 2,000 antigen targets common to all Shigella species was used to assess serum samples from volunteers immunized with killed, attenuated, and wild-type S. flexneri 2a (344). These studies identified a series of type III secretion system proteins for which immunoreactivity against was associated with clinical protection.
(iv) Shigella vaccine development
The current vaccine pipeline for Shigella includes both oral (live-attenuated and inactivated whole-cell), as well as subunit candidates for parenteral administration. Live-attenuated vaccines against Shigella were the first to be evaluated clinically. The difficulty with this approach has been to maintain immunogenicity while minimizing reactogenicity. A series of clinical trials with different live-attenuated vaccines developed at the Center for Vaccine Development (CVD) at the University of Maryland has led to selection of strain CVD 1208. This strain, a GuaBA auxotroph in which ShET1 and ShET2 toxins have been deleted, combines low reactogenicity with robust immune responses. Adaptation of this strain to growth on soy media to meet regulatory requirements generated the vaccine candidate CVD 1208S (363). An attenuated vaccine based on S. sonnei with the toxin deletions and a VirG mutation to attenuate through prevention of intercellular spreading was constructed at the U.S. military WRAIR. This strain was selected for the stability of its invasiveness plasmid, a plasmid that is required for expression of O antigen in this serotype. The attenuated S. sonnei vaccine has completed a phase 2b trial (332, 333, 335).
Glycoconjugate vaccines represent another major approach to Shigella vaccine development. Originally developed at the U.S. National Institutes of Health (NIH), in this type of vaccine O polysaccharides from relevant Shigella serotypes are covalently linked to a carrier protein. The NIH prototype vaccine provided 74% protection against S. sonnei outbreaks at Israeli army bases. Unfortunately, the vaccine was not shown to be efficacious in 1- to 4-year-old Israeli children (364). Current work with this approach involves synthetic conjugates, as well as a bioconjugate (332, 335, 346–349). Another novel approach to “O” antigen-based parenteral Shigella vaccines is the Generalized Modules for Membrane Antigens (GMMA) technology from GlaxoSmithKline (333). A prototype bioconjugate vaccine developed by LimmaTech in Switzerland that targeted S. flexneri 2a was recently shown to be highly immunogenic and to induce protection against more severe shigellosis in a phase 2b immunization and challenge study (352). Similar phase 2b immunization and challenge studies to assess the protective potential of the synthetic conjugate and GMMA approaches was also recently completed with results suggesting that the immunogenicity of the vaccine needed to be improved (346, 347, 365). In addition, a promising new subunit vaccine candidate, InvaplexAR-DETOX, recently entered into early-stage clinical development. The vaccine is unique in that it is the only Shigella vaccine in clinical development that attempts to induce both “O” LPS antigen and conserved Ipa protein responses. It also relies on an MsbB mutation in the lipid portion of its LPS antigens to make it less reactogenic for parenteral delivery. Initial safety and immunogenicity results have been very encouraging, and more in-depth immunological studies are still ongoing (351, 366).
In addition to the vaccines described above, a wide range of Shigella vaccine candidates is in the preclinical stage or in phase 1 trials. Some new vaccine candidates seek to exploit conserved antigens, such as proteins involved in the cellular invasion process in addition to or instead of O antigen. Table 12, adapted from recent reviews by Mani et al. and Walker et al. (332, 335), describes these candidate vaccines.
TABLE 12.
Shigella vaccine candidatesa
| Vaccine type | Candidate | Current stage | Developer(s) |
|---|---|---|---|
| Attenuated | CVD 1208S based on S. flexneri 2a | Phase 1 | CVD |
| WRSS1 based on S. sonnei | Phase 2b | WRAIR | |
| ShigETEC LPS-free cell | Phase 1 | EveliQure | |
| Vectored | Ty21a typhoid vaccine expressing O antigens of S. sonnei or a combination of S. sonnei, S. flexneri 2a and 3a, and S. dysenteriae O antigen | Preclinical | Protein Potential |
| Inactivated | Sf2aWC, a formalin-killed S. flexneri 2a whole-cell vaccine | Phase 1 | WRAIR and PATH |
| Shigella truncated mutant (350) | Preclinical | IVI and PATH | |
| Conjugate | Chemical glycoconjugates of S. sonnei and S. flexneri 2a | Phase 3 | NIH Institute for Child Health and Human Development |
| Recombinant bioconjugate | Phase 2b | LimmaTech Biologics and GSK | |
| Synthetic glycoconjugate | Phase 2 | Institut Pasteur | |
| Serotype-dependent mixture | InvaplexAR-DETOX, a chemically defined product consisting of invasion plasmid antigens and O antigens | Phase 1 | WRAIR |
| GMMA consisting of Shigella outer membrane vesicles from an overproducing strain | Phase 2 | GSK | |
| Outer membrane vesicles: naturally secreted outer membrane vesicles of Shigella | Preclinical | University of Navarra, Spain | |
| Serotype-independent proteins | IpaD/IpaB (DB) fusion | Preclinical | PATH |
| PSSP-1 (Pan-Shigella surface protein 1) | Preclinical | IVI | |
| 34-kDa OMP, an outer membrane protein conserved across multiple Shigella species | Preclinical | National Institute of Cholera and Enteric Diseases, India |
CVD, Center for Vaccine Development, University of Maryland; ETEC, enterotoxigenic Escherichia coli; GMMA, Generalized Modules for Membrane Antigens; GSK, GlaxoSmithKline; IVI, International Vaccine Institute, South Korea; LPS, lipopolysaccharide; NIH, U.S. National Institutes of Health; WRAIR, Walter Reed Army Institute of Research. Adapted from references 334 and 337.
Current Shigella vaccine candidates need to provide coverage for up to four serotypes, and the availability of a CHIM for each of these will greatly facilitate their development. Early proof-of-concept work has largely involved S. flexneri 2a and S. sonnei, so CHIMs for these two strains have been established. However, additional work remains to standardize these models to improve consistency and allow comparisons of vaccine candidates across studies. It is expected that as candidates for these strains advance, it will be possible to find field sites permitting phase 3 evaluation of protective efficacy. Licensure studies for other strains may not be feasible in the field, so determination of the effectiveness of a vaccine component against these strains could depend on results from a CHIM trial. These less common strains such as S. flexneri 3a and 6 and possibly S. dysenteriae need to be included among the current challenge strains.
(v) Summary
Diarrheal disease from enteric pathogens is widely acknowledged to be a major worldwide threat to public health. Shigella is one of the most important enteric pathogens for which no safe and effective vaccine is currently available. The development of Shigella vaccines faces several important challenges, including the high diversity of Shigella serotypes and the fact that the protective immunity in communities and in human challenge studies has been serotype specific. Correlates of protective immunity probably include O-antigen-directed antibodies, but the details of the types of responses to be elicited for protective vaccines remain largely undefined beyond that. Recent evidence from Ndungo et al. (344) and Clarkson et al. (354) suggesting that antibody responses to both LPS and conserved protein antigens, like IpaB and IpaC, may contribute to protection needs further investigation.
Human challenge models have played a critical role in defining the pathogenesis and virulence of Shigella species as well as in assessing vaccine potential of various candidates. The challenge models will be even more valuable if the diversity of challenge strains can be expanded, and if studies can be consolidated against standardized and readily available challenge strains.
Campylobacter
(i) Epidemiology and public health impact of Campylobacter jejuni
Campylobacter jejuni is a common enteric bacterial pathogen thought to be responsible for a significant fraction of diarrhea cases worldwide. The Global Enteric Multicenter Study found that Campylobacter infections in India, Bangladesh, and Pakistan were common causes of severe diarrhea in infants and young children (367). Surveillance data are incomplete in many resource-limited countries. One report in 2002 suggested that 40 to 60% of children under the age of 5 in these countries will have experienced at least one episode of campylobacteriosis (368). Postinfection sequelae include Guillain-Barré syndrome (GBS), reactive arthritis (ReA), Crohn’s disease, and irritable bowel syndrome (IBS). More recent burden studies indicate that even asymptomatic Campylobacter infection among infants and young children in low- and middle-income countries (LMICs) can be associated with negative health outcomes, including an increase in intestinal permeability and both intestinal and systemic inflammation, which can contribute to an increased risk of children developing stunting and environmental enteric dysfunction (369). Alarmingly, Campylobacter strains from LMICs have become increasingly antibiotic resistant. In 2017, the U.S. Centers for Disease Control and Prevention, along with the World Health Organization, designated multi-antimicrobial-resistant Campylobacter as a serious threat to public health (262). In addition, a recent Wellcome Trust report urged that better prevention and control measures, like vaccines, be prioritized for accelerated development (262).
(ii) Pathogenesis and diversity of Campylobacter jejuni
In LMIC settings, disease features most commonly reported are watery stool, fever, abdominal pain, vomiting, dehydration, and the presence of fecal leukocytes. Patients are also often underweight and malnourished, which may be secondary to repeated infections or a marker of a poor nutrition state. In addition to these acute disease effects, however, a number of chronic sequelae have been identified.
Among these chronic sequelae, C. jejuni is the most frequent pathogen associated with GBS (370, 371), which is considered to be the leading cause of acute flaccid paralysis worldwide (given the success of polio vaccination campaigns). An estimated 0.1% of Campylobacter-infected individuals develop GBS, which is a serious neurological disorder that can cause a wide range of symptoms, from mild weakness of the extremities to near-complete paralysis and respiratory insufficiency. Molecular mimicry between lipooligosaccharides (LOS) produced by Campylobacter and an autoimmune reaction against human nerve gangliosides is the cause of GBS following campylobacteriosis. Most patients recover in a few months, but the case fatality rate can be as high as 3%. About 20% of GBS patients experience ongoing functional disability, and more than half report severe fatigue for more than 1 year (372). One recent study attributed about 30% of studied cases of GBS to recent infection with C. jejuni (373). In LMICs, Campylobacter-associated GBS may be more common among younger age groups than adults (374). Aside from GBS, other studies in LMICs have identified stunting, microbiome changes, functional bowel disorders, and reactive arthropathies as other chronic consequences of Campylobacter infection and disease (370, 371). As indicated above, these negative health outcomes can also result from chronic asymptomatic infections with Campylobacter, which lead to gut inflammation and a change in gut permeability (369).
ReA associated with Campylobacter infection is also thought to have an immunological basis. The incidence of ReA can be as high as 7% in community outbreaks of campylobacteriosis. Guerry et al. estimate that the postinfective attributable risk of ReA ranges from 1 to 5% (375). Like ReA caused by other pathogens of the gastrointestinal tract, ReA following Campylobacter infection is thought to be associated with HLA B27. Joint symptoms arise about 2 weeks after infection and usually resolve completely, although the symptoms may be chronic or relapsing in 5% of cases (375).
Although diseases like IBS and Crohn’s are sequelae of many cases of campylobacteriosis, data regarding the degree to which Campylobacter is the causative agent are conflicting. Guerry et al. indicate that between 1 and 10% of the risk of IBS is attributable to Campylobacter infection. They cite recent evidence that C. jejuni can breach the intestinal barrier in susceptible individuals, which may set up conditions for chronic inflammatory responses (375).
C. jejuni strains are highly diverse and the basis for this diversity is quite complex. Unlike other bacterial pathogens of the intestinal tract, campylobacteria have a polysaccharide capsule. The capsular polysaccharides (CPS) expressed on the surface of C. jejuni strains are highly variable. More than 40 years ago, Penner and Hennessy developed a serotyping system based on a passive slide agglutination test for heat-stable antigens (376). This system has underpinned our understanding of the role of C. jejuni polysaccharides in epidemiology, pathogenesis, and immunity (375, 377, 378). Forty-seven serotypes, or serotype complexes, of C. jejuni are recognized. The basis for the observed serotypes of Campylobacter is not the typical lipopolysaccharides called “O antigens.” Campylobacteria synthesize an LOS, which is linked to the bacterial cell wall in a different way than O antigens. The Penner typing system is based primarily on CPS, but the LOS can also contribute to serotype specificity (378).
In addition to polysaccharide antigens, several Campylobacter protein targets have been explored as potential antigens, including flagellin (379), flagellin-secreted proteins (380), and the outer membrane protein PorA (381). These may serve as important alternative approaches, given the complexity of Campylobacter polysaccharide expression and variation.
Differences in serotype distribution between LMICs versus high-income countries may also play an important role in differences in long-term sequelae between these settings (375, 378). Such differences may provide important guidance for the selection of Campylobacter strains for inclusion in candidate vaccines and for selecting challenge strains for use in controlled human infection models (CHIMs), as described below.
(iii) Controlled human infection model studies with Campylobacter
Challenge strain selection for use in CHIM studies with Campylobacter has evolved over the years. Experimental human infections with Campylobacter were first reported between 1988 and 1998 (382). The challenge strain used was 81-176, a prototype isolate that was also used for preparing candidate inactivated whole-cell vaccine.
In 2004, Prendergast et al. reported results from the serological characterization of LOS from strain 81-176 (377). The LOS of this strain exhibited ganglioside mimicry. Prendergast et al. then studied human volunteers previously challenged with strain 81-176 and volunteers that had received a whole-inactivated candidate Campylobacter vaccine based on strain 81-176. No detectable, sustained increase in anti-ganglioside antibody responses were seen in the vaccinated or experimentally challenged volunteers, although weak and transient anti-ganglioside antibody responses were observed in some of the volunteers in challenge studies. In six volunteers from a previous challenge study, the Campylobacter strains present in stools were examined and found to have undergone phase variation in their LOS structures.
In 2009, Tribble et al. reported on another study using strain 81-176 (383), which evaluated the relationship of dose to disease severity, as well as the level and duration of protection from reinfection after challenge. The authors observed an illness-dose effect in the study and short-term protection from reinfection. When subjects were challenged 1 year after the initial challenge, less severe illness resulted following rechallenge. Production of IFN-γ by PBMCs after restimulation with Campylobacter antigens was associated with protection from illness.
The Campylobacter human challenge model has been redeveloped since 2009. New challenge strains without the potential for ganglioside mimicry have been selected for use in the Campylobacter challenge model (383, 384). Table 13 describes the criteria used for selecting additional challenge strains. The selection criteria were the avoidance of the molecular mimicry for human antigens, propensity for invasion of epithelial cells, passage history and provenance, and serotype.
TABLE 13.
Selection criteria for Campylobacter strains to be used in human challengea
| Challenge strain | Source | Serotype | Glycolipid mimicry for GBS antigens | Invasiveness for human epithelial cells in vitro |
|---|---|---|---|---|
| 81-176 | NA | HS23/36 | Yes | High |
| RM1221 | NA | NA | No | NA |
| TGH9011 | Laboratory passaged | HS-3 | No | NA |
| BH-01-142 | Primary isolate | HS-3 | No | Low |
| CG8421 | Primary isolate | HS23/36 | No | Low |
GBS, Guillain-Barré syndrome; NA, information not available.
None of the new challenge strains have the potential for the ganglioside mimicry that underlies GBS. Along the way, however, one of the potential challenge strains (RM1221) was found to have LOS that mimic human P blood group antigens. Another potential challenge strain (TGH9011) contains an LOS core that lacks all glycolipid mimicry. However, no information is available on the disease symptoms that TGH9011 has caused and it has been passaged in the laboratory for 25 years or more. The strains ultimately selected for CHIM studies were BH-01-142 and CG8421—both primary isolates with minimal passage in cell culture and low invasiveness for epithelial cells.
Tribble et al. described the initial study with the newly developed challenge model (383). Twenty-three healthy adults that were Campylobacter naive were challenged with 1 × 106 or 1 × 105 CFU of strain CG8421 and followed as inpatients. The attack rates for the two doses were 100 and 93%, and the symptoms of disease were diarrhea, abdominal cramps, nausea, and fever. No major safety concerns arose during the study. However, it should be noted that because of concerns that infection is possibly associated with more chronic functional bowel disorders, postchallenge surveillance for acute and more long-term adverse events has been extended in these more recent challenge studies to 90 and 180 days postchallenge (383–385). Volunteers were treated with strain-sensitive antibiotics no later than 6 days after challenge and were released after antibiotics were started, symptoms were resolved, and two consecutive stool cultures were negative for C. jejuni. After study completion, however, two of the volunteers experienced recrudescent infection, which resolved with subsequent antibiotic treatment. The potential for experimental infection to generate asymptomatic carriers and recrudescence would seem to represent a new obstacle to be overcome (383).
Recent data indicate a lack of homologous protection against C. jejuni rechallenge (385). Of the 15 volunteers administered C. jejuni strain CG8421, 14 developed illness. Eight of the volunteers that developed illness were rechallenged with the same strain three months later. All developed disease with no reduction in severity of illness. Unexpectedly, the immune responses that were seen upon initial infection, which included serum immunoglobulin A (IgA) and immunoglobulin G (IgG), fecal IgA, IgA antibody-secreting cells, and IFN-γ production, were not boosted upon rechallenge. Later, the same investigators examined CD4+ T-cell responses using multiparameter flow cytometry using cryopreserved cells from the previous infection and rechallenge study with strain CG8421 (386). Primary infection elicited proinflammatory cytokines IFN-γ, tumor necrosis factor alpha, interleukin-2 (IL-2), and the chemokine MIP-1β. None of these responses were augmented upon reinfection.
This observation that the immune responses elicited by Campylobacter infection in a CHIM study were not boosted upon rechallenge with the same strain represents a further obstacle in trying to understand how Campylobacter challenge study results should be interpreted and how useful they may be for vaccine development. That being said, natural immunity clearly develops against Campylobacter in field settings; however, protection against illness may be age related or require more than one exposure to be fully protective (387). Another possibility is that the efficient induction of immune memory by Campylobacter may require some level of invasiveness for epithelial cells. Strain CG8421 is poorly invasive for human epithelial cells in vitro (see Table 13). This hypothesis is further supported by earlier controlled human infection model studies with the 81-176 strain of Campylobacter, which is invasive and induces short-term protection against itself (388). The role of invasion in the development of long-term protective immunity needs further investigation, and the development of a new mouse disease model for Campylobacter may help to make these studies more practical (389). In related work, the mucosal adjuvant double-mutant heat-labile toxin (dmLT) was recently coadministered with a dose of the live-attenuated enterotoxigenic Escherichia coli (ETEC) vaccine ACE527 (390). This vaccine was not protective upon rechallenge when given without the adjuvant, but provided strong protection 6 to 7 months after immunization, which included dmLT when challenged with a virulent ETEC strain. These findings could suggest using dmLT with the immunizing challenge with noninvasive strains like CG8421, followed by homologous challenge.
The Campylobacter CHIM has been used to gain insights into the pathogenesis of Campylobacter and how this important bacterial pathogen adapts to the gut environment during the infection and disease process (391), as well as into the mechanism associated with the rapid development of macrolide resistance in vivo (392). The model has also been used to get an early assessment of vaccine efficacy (see the vaccine development section below) and also to evaluate the ability of a new drug, rifaximin, to provide antibiotic prophylaxis against Campylobacter (393). While this study did not demonstrate effective prophylaxis with rifaximin, analysis of gut microbiome from participants indicated potentially significant associations between composition and disease susceptibility (394).
(iv) Campylobacter vaccine development
No licensed vaccine against Campylobacter currently exists and development may need to overcome several challenges (395). The first challenge is identifying those types of vaccines that are most likely to achieve safety, immunogenicity, and strain coverage. Selecting strains for live-attenuated or whole-cell inactivated Campylobacter vaccines is particularly challenging because they must have no potential to elicit anti-ganglioside antibodies in humans. Furthermore, vaccines that can do this and that are limited to a single strain of Campylobacter will somehow need to confer immunity to conserved protein antigens rather than to the highly variable polysaccharide antigens that are immunodominant.
Another potential pitfall in Campylobacter vaccine development is molecular mimicry. Whether or not the full range of human antigens that can be mimicked by Campylobacter is sufficiently understood is unclear. As a case in point, the examination of four potential challenge strains for CHIM studies revealed that one of them, RM1221, unexpectedly mimicked a human blood group antigen (see Table 13) (396). Since Campylobacter has a polysaccharide capsule, conjugate vaccines would seem to be a good option; however, the same proscription against ganglioside and other molecular mimicry would also pertain to the CPS included in the vaccine.
Given the number of globally prevalent serotypes (375, 378) a vaccine based on CPS would need to be at least 8-valent (397). Fortunately, conserved, protective antigens that could be delivered as vectored or subunit vaccines have recently been identified. Two approaches utilizing highly conserved antigens to immunize against Campylobacter are in preclinical development. One is based on the homology between cholera toxin B subunit (CTB) and a 53-kDa major outer membrane protein, PorA, of Campylobacter (381). CTB has been shown to reduce colonization of adult mice challenged with C. jejuni (398). The other approach utilizes a conserved N-glycan heptasaccharide of Campylobacter for immunization. This antigen was displayed on Escherichia coli to immunize chickens and induced up to a 10-log reduction in C. jejuni colonization following challenge (399). This effect was obtained with inactivated whole cells as well as with the live-attenuated strains of the ACE527 ETEC vaccine expressing the heptasaccharide antigen (C. Szymanski, unpublished data). It could be of benefit to compare the anti-Campylobacter potential of the conserved heptasaccharide and CTB antigens in a murine model that has been recently described to demonstrate intestinal disease following oral challenge (389) and determine the possibility for synergy between them. Such studies in this small animal model could pave the way for further clinical studies with these conserved vaccine antigens.
The only Campylobacter vaccine candidate that has recently moved from studies in small animals to the clinic consists of CPS purified from strains 81-176 (HS23/36) and CG8486 (H54complex) conjugated to a recombinant inactivated diphtheria toxin, CRM197. HS23/26 and H54complex are globally prevalent serotypes of Campylobacter (375). The conjugate has been found to be highly immunogenic in mice, and has demonstrated efficacy against diarrheal disease following a high-dose challenge with strain 81-176 in Aotus nancymaae, a New World owl monkey species (400). A phase 1 first-in-human trial was recently completed (ClinicalTrials.gov identifier NCT02067676) (401). The vaccine was well tolerated but was not as immunogenic as preclinical studies had suggested. It is anticipated that an adjuvanted version of the vaccine will go back into phase 1 trials shortly (400).
(v) Summary
Campylobacter infection, together with its postinfection sequelae, constitutes a major public health burden. The Campylobacter CHIM has been modified to eliminate the potential for GBS and is deemed safe for small-scale studies performed under controlled conditions. The current challenge strains do not represent globally prevalent serotypes, and additional strains, should they become available, could improve the relevance of the model. In the Campylobacter challenge model, follow-up of volunteers after study completion is needed to address the potential for recurrent disease and the development of a long-term carrier state. The potential for CHIM studies to cause postinfection sequelae other than GBS is not well understood.
Campylobacter pathogenesis and immunity are incompletely understood. We know little about the relationship of particular serotypes to invasiveness or the potential of different strains to foster postinfection sequelae other than GBS. Also, initial studies with strain CG8421 in the CHIM indicate that infection does not always induce homologous immune protection, or even a measurable memory response (383, 384). Therefore, human challenge with the available strains may provide little information on protective immunity against Campylobacter. The invasiveness for epithelial cells may play a significant role in protective immunity but is incompletely understood. Further unclear is how to limit the potential for invasive strains to damage the intestinal epithelium while studying their potential to induce protective immunity. The availability of a new promising mouse disease model may help investigators gain better insights into the factors contributing to longer-term protective immunity (389).
Vaccine development for Campylobacter faces a number of challenges, some unique to this particular pathogen. Conjugate vaccines that provide coverage for the major C. jejuni serotypes offer an option for further development but face the hurdle of requiring eight serotypes to be included in the vaccine. This may make the vaccine unacceptably complex. Simpler vaccine candidates based on more conserved antigens have been identified and could enter human trials. Campylobacter vaccine development has received relatively little attention compared to other bacterial enteric pathogens, such as ETEC and Shigella, but it, too, is rapidly demonstrating antibiotic resistance (392, 393). Thus, continued refinement of controlled human infection models to accelerate vaccine development for this important pathogen should certainly be pursued.
Salmonella
(i) Epidemiology, diversity, and public health impact of four Salmonella enterica serovars
Salmonellae are pathogenic bacteria that have both an extracellular and an intracellular phase in their life cycle. Salmonella infection is principally the result of ingesting contaminated food or water and disease results from local invasion of the intestinal mucosa or, in the case of typhoid and paratyphoid fevers, local invasion followed by a more generalized systemic spread and dissemination of the bacteria (402, 403). Infected individuals can manifest a carrier state of variable duration that is a source of onward transmission (404).
Human disease following Salmonella infection takes the following main forms that have different degrees of disease severity: enteric fevers, invasive nontyphoidal Salmonella disease (iNTS), and nontyphoidal Salmonella (NTS) gastroenteritis. The enteric fevers, which include typhoid and paratyphoid, carry a 1% case fatality rate worldwide and higher case fatality rates in resource-limited settings and among younger age groups (404). iNTS has a case fatality rate up to 25%, mostly in children younger than 2 years of age and in immunocompromised adults in Africa. NTS gastroenteritis is a more prevalent, usually mild, self-limiting diarrheal disease with an estimated 0.1% case fatality rate (403, 405, 406). The rapid rise in prevalence of antimicrobial-resistant strains among Salmonella enteric pathogens has served to reenergize vaccine development efforts (262, 407–409). Three vaccines against typhoid fever are licensed for global use, while vaccine development efforts for paratyphoid and iNTS are being accelerated (404, 407, 410). This acceleration in vaccine development efforts has been driven by a growing interest among emerging-market manufacturers and global health vaccine developers to fill gaps in development and production capacity.
Salmonella nomenclature is summarized in Table 14. All of the organisms shown in the table belong to Salmonella enterica subspecies 1 and are referred to as serovars. Each serovar of Salmonella contains multiple serotypes.
TABLE 14.
Salmonella enterica serovars that cause severe human diseasea
| Organism | Disease | Host range | Main geographic concentration(s) | Annual global burden of severe disease |
|---|---|---|---|---|
| Salmonella Typhi | Enteric fever | Humans | Africa, Asia, Latin America | 27 million |
| Salmonella Paratyphi A, B, and C | Enteric fever | Humans | South and Southeast Asia | 5 million |
| Salmonella Typhimurium | iNTS | Humans and other warm-blooded animals | Africa, Asia | NA |
| Salmonella Enteritidis | iNTS | Humans and other warm-blooded animals | Africa, Asia | NA |
iNTS, invasive nontyphoidal Salmonella disease; NA, information not available.
The Salmonella enterica serovar Typhimurium (S. Typhimurium) and S. Enteritidis represent an extremely diverse collection, with more than 1,000 serotypes described in humans and a similar number of serotypes in animals (262, 402, 403, 408). S. Typhimurium and S. Enteritidis are a common cause of gastroenteritis in humans. The iNTS bacteria may represent distinct subsets of the S. Typhimurium and S. Enteritidis organisms that are opportunistic pathogens in immune-compromised hosts (262, 403, 407).
The enteric fevers and iNTS have different geographic distributions (405), as summarized in Table 14. Overall, the burden of enteric fever is focused in South and Southeast Asia, whereas highest burden of iNTS is in sub-Saharan Africa. However, the global distribution of the S. enterica serovars that cause severe disease in humans is still fragmented and in continuous flux (410, 411).
The epidemiology of these important S. enterica serovars is incompletely documented at present, especially in Africa (412, 413). For the enteric fevers, this gap is partly due to nonspecific case presentation and lack of a widely applicable, highly sensitive, and specific diagnostic test. Blood culture is still the standard laboratory means of confirming cases of enteric fever, but the very low numbers of bacteria in blood at the onset of symptoms reduces sensitivity. A novel assay for the diagnosis of S. Typhi infection in which very short-term blood culture is combined with PCR for detecting S. Typhi DNA may hold promise in this regard (413, 414).
Overall, global estimates of disease burden are sufficiently precise to establish that both enteric fevers and iNTS are of significant public health impact and warrant use of all available corrective measures. Wain et al. estimate that typhoid and paratyphoid fevers account for about 1% of global mortality from all causes on an annual basis (414). In the context of diarrhea- and enteric fever-related deaths and health impact, the recently completed Global Burden of Disease Study estimated that enteric fevers and NTS, which includes iNTS, accounted for 18% of total enteric disease deaths worldwide and for approximately 19% of the total disability-adjusted life years burden per year for diarrheal disease and enteric fevers (407, 409, 410). The combined typhoid, paratyphoid, and iNTS burden has led to greater interest in developing a combination vaccine that would provide coverage for all four pathotypes of invasive Salmonella (407, 409, 410).
(ii) Pathogenesis and immunity to Salmonella enterica serovars
Natural typhoid infection is usually associated with the detection of serum antibodies and mucosal secretory immunoglobulin A (IgA) intestinal antibody against various S. Typhi antigens; cell-mediated immune responses are also measurable (405, 407, 409, 414–417). In areas where typhoid is endemic, there is an age-related increase in the prevalence and geometric mean titer of anti-virulence antigen (Vi) antibodies (409, 410). Anti-flagellum (H antigen) serum immunoglobulin G (IgG) antibodies following natural infection are long lived (409). Trials of an early prototype Vi conjugate vaccine in Vietnam in the early 1990s demonstrated that the threshold level of IgG antibody to the Vi antigen can be a marker for protection against typhoid fever (418–420).
Cell-mediated immunity also appears to play a part in protection; it has been observed that peripheral blood mononuclear leukocytes of otherwise healthy adults residing in areas where typhoid is endemic that have no history of typhoid, proliferate on exposure to S. Typhi antigens (409, 410). Upon ingestion, these bacteria cross the intestinal barrier and multiply within the underlying macrophages, where they may persist for long periods of time and are shielded from the direct effects of antibodies. They can also take up an extracellular existence, spread from cell to cell, and enter the blood stream and the lymphatic system. Antibodies are the key defense against the extracellular phase and bactericidal and opsonophagocytic antibodies may be the mediator of this defense mechanism (409–411, 415), while T cells are required to clear intracellular bacteria. Although these basic features pertain to both enteric fevers and iNTS, the pathogenesis of the two diseases is quite distinct (406, 407, 414, 415, 418). For this reason, the two diseases may require different approaches for protection with vaccines (413, 414). Pathogenesis and immunity are poorly understood for S. Typhimurium and S. Enteritidis. Human challenge models for iNTS are under consideration but must be approached cautiously because of the high risk of complications in immunocompromised individuals.
(iii) Human challenge models with Salmonella Typhi and Salmonella Paratyphi
For the enteric fevers, human challenge models have been a key source of information about pathogenesis and immunity. Much of our knowledge of the pathogenesis of S. Typhi comes from an early series of experimental human challenge studies conducted at the University of Maryland in the 1960s and 1970s. The use of the early version of the challenge model was suspended in 1974, but a new version was recently developed (421–424).
The initial human challenge studies with Salmonella used the S. Typhi Quailes strain, which was isolated in 1958 from a chronic carrier. This strain expresses a capsular polysaccharide antigen (Vi), the H antigen (flagellin), and the O antigens (lipopolysaccharide [LPS]). The term Vi reflects the fact that the capsular polysaccharide of S. Typhi is a virulence factor. The bacteria were orally administered to 213 volunteers in a dose-escalation study. The study showed higher attack rates and shorter incubation periods with increasing doses from 103 to 109 CFU and provided the first experimentally controlled clinical description of typhoid fever. The typhoid fever seen in experimentally infected volunteers closely resembled that seen in natural infection (411, 421–424).
Subsequent studies at the University of Maryland explored the contributions of Vi and H antigens to strain virulence. Wild-type strains that expressed the Vi and the H antigen were compared to strains that expressed Vi but lacked the H antigen, and to strains that expressed neither of these antigens. These studies established that the attack rate for a given dose was halved in the absence of Vi. At higher doses, however, people infected with strains lacking Vi still developed typhoid.
The early studies with S. Typhi were able to establish a 95% attack rate and a relatively linear dose-response curve. A 2-fold shortening of the incubation period with the higher doses occurred. Importantly, some participants developed bacteremia without fever—a finding postulated to represent early intracellular sequestration of the organism from the immune system.
The clinical features of the typhoid fever in the volunteers that were challenged with S. Typhi were variable. The antibiotic chloramphenicol was administered to participants promptly after the onset of fever, which likely prevented them from developing any of the complications known to occur in natural infection. Intestinal biopsy specimens of some participants showed that enteritis developed during the symptomatic period but resolved without scarring in all participants examined.
The early model development work at the University of Maryland also established a role for human challenge studies in the development of vaccines against S. Typhi. Studies in which human volunteers were administered the live-attenuated oral vaccine Ty21a prior to challenge showed that the vaccine provided up to 87% protective efficacy. In subsequent field trials, this vaccine conferred 96% efficacy over 3 years of follow-up. Ty21a went on to become the world’s only live oral typhoid vaccine and is still in use today.
Even with two widely available licensed typhoid vaccines, a Ty21a live-attenuated vaccine and a Vi capsular polysaccharide vaccine (detailed in the next section), typhoid fever is still a major cause of morbidity and mortality, especially in low-resource countries. According to Waddington et al., “a further paradigm shift in our understanding of disease pathogenesis and host response is required … to advance typhoid control” (411). Efforts to shift the paradigm have resulted in a new human challenge model that differs in several important respects from the earlier version outlined above. The first study using the new model was conducted through a collaboration between the Center for Vaccine Development (CVD) at the University of Maryland and the University of Oxford.
The new model can use challenge strains of either of the agents of enteric fever, S. Typhi or S. Paratyphi. The S. Typhi strain is the same as in the earlier model. Challenge with the newly developed S. Paratyphi A strain is at a target dose of 1 × 103 to 5 × 103 CFU (425). Participants are typhoid-naive volunteers and the studies are conducted on an outpatient basis, unlike in the historical studies. Table 15 further describes the challenge strains and their modes of administration. The purpose of the first published study, conducted at the Centre for Clinical Vaccinology and Tropical Medicine in Oxford, United Kingdom, was to determine the dose of the Quailes strain of S. Typhi required to achieve an attack rate of 60 to 75% in typhoid-naive volunteers (421).
TABLE 15.
Human challenge models for Salmonella Typhi and Salmonella Paratyphi A serovarsa
| Organism | Strain | Method of production and administration |
|---|---|---|
| Salmonella Typhi | Quailes | Isolated from the gallbladder of a chronic carrier in 1958. A challenge dose of 103 to 104 CFU in bicarbonate buffer solution is ingested. |
| Salmonella Paratyphi A | NVGH308 | Ingestion of 1 × 10³ to 5 × 10³ CFU of the challenge strain in bicarbonate buffer solution. |
Both human challenge models have been conducted at the University of Oxford, Oxford, United Kingdom.
The Quailes strain of S. Typhi expresses the Vi antigen and is fully susceptible to the antibiotics used to treat typhoid fever. A fresh working cell bank was manufactured under Good Manufacturing Practice for use in this and subsequent studies. The strain in the cryopreserved cell bank was characterized by full genome sequencing, which confirmed that it contained the expected virulence determinants. The actual doses (determined by bacterial culture of the challenge inoculum) were 1.3 × 103 and 2.0 × 104 CFU.
Forty-one healthy adult volunteers 18 to 60 years of age that had not received typhoid vaccination or been resident for more than 6 months in areas where typhoid is endemic participated in the study. They were carefully screened for a number of general conditions that would make them ineligible, such as the presence of gallstones. S. Typhi forms biofilms on the surface of gallstones, where the organisms are extracellular but impervious to immune clearance. Through the biliary system, the bacteria can be reintroduced into the intestinal tract and are persistently shed (406). The absence of gallstones is thought to reduce, if not eliminate, the potential to establish a carrier state in challenged volunteers (which was doubly ensured by antibiotic treatment before study completion in all challenges volunteers).
Volunteers were followed daily for at least 14 days and then treated with antibiotics, or earlier if indicated. Follow-up visits were at 21, 28, and 60 days after challenge. No volunteers required hospital admission or intravenous antibiotics or fluids. After antibiotic administration, no shedding or carriage of the challenge organisms was detected. Typhoid infection was defined either by fever of 38°C or greater sustained for more than 12 h by day 14 after challenge, or by blood culture for the presence of S. Typhi organisms, or both. Attack rates were 55% with the low-dose challenge and 65% with the high-dose challenge. The clinical profiles of infection varied considerably and represented a range of disease from asymptomatic to clearly symptomatic. Two important observations were that S. Typhi organisms were frequently shed in stools before diagnosis (albeit at low levels) and that infected individuals developed serum antibody responses to H and O antigens, but not to Vi antigen. The Vi antigen is the basis of a licensed typhoid vaccine. According to the human challenge study of Waddington et al., Vi may represent an antigen to which an immune response is not always elicited by infection with S. Typhi (421–424).
The challenge model described above was also used to evaluate a new challenge strain of S. Paratyphi A (see Table 15). This study targeted up to 80 participants and was completed in 2016. Challenge with the NVGH308 strain at an actual median dose of 2.4 × 103 CFU (range, 2.2 × 103 to 2.8 × 103 CFU) was well tolerated and associated with an acceptable safety profile. The resulting spectrum of illness was such that this new model appears suitable for the assessment of vaccine efficacy using endpoints that include bacteremia and other enteric and systemic symptoms (425).
These new challenge models have also been instrumental in gaining new insights into the immunology of immunity to enteric fever (421–424) and in the identification of additional potential immune markers for protection (serum IgA and IgG1) and in supporting policy recommendations by the WHO Strategic Advisory Group of Experts on Immunization for the introduction of Vi conjugate-based anti-typhoid vaccines (410, 421–428).
(iv) Development of vaccines against Salmonella
Table 16 describes the historical and the currently licensed vaccines against S. Typhi. The first vaccines to be developed were whole-inactivated preparations of S. Typhi bacteria administered parenterally. These vaccines consistently showed efficacy against typhoid fever in the 70% range, but they were too reactogenic for general use and their manufacture was discontinued (414). Ty21a is a live-attenuated vaccine developed at the Swiss Serum and Vaccine Institute based on a chemically mutagenized strain of S. Typhi. It is licensed for adults and children over 5 years of age and requires three to four oral doses in capsule form. The vaccine had 87% efficacy in a human challenge model (as earlier described) and showed field efficacy of 95% in Egypt and 67% in Chile. A meta-analysis published by the WHO in 2008 concluded that the vaccine is 51% effective against typhoid fever. Ty21a is licensed for international use (414).
TABLE 16.
Currently licensed Salmonella Typhi vaccines
| Licensed vaccine and trade name | Description | Developer(s) |
|---|---|---|
| Not applicable | Killed whole-cell vaccine | Not currently manufactured due to high rates of reactogenicity |
| Ty21a (Vivotif) | Live-attenuated, oral licensed for adults and children older than 5 years of age | Emergent |
| Vi CPS (various) | Parenteral Vi (capsular polysaccharide antigen) vaccine licensed for adults and children 2 years of age and older | GlaxoSmithKline, Sanofi Pasteur, Bharat Biotech, BioMed, Finlay Institute, and approximately six other manufacturers located in countries of endemicity |
| Vi conjugate (Typbar-TCV, Peda-Typh, Typhibev) | Parenteral Vi conjugated to tetanus toxoid (Typbar-TCV, Peda-Typh) or diphtheria toxoid CRM197 (Typhibev) carrier protein, licensed in India for adults and children 6 months of age and older | Bharat Biotech (Typbar-TCV), Bio-Med (Peda-Typh), Biological E (Typhibev) |
A parenteral vaccine composed of the detergent-purified Vi antigen of S. Typhi is also licensed for international use. The vaccine is 60 to 70% efficacious but only for 2 to 3 years. It is licensed for adults and children older than 2 years of age. Since the vaccine is not conjugated to protein, it does not establish immune memory and cannot be boosted by repeat vaccination. The vaccine is not patent protected and is manufactured by a number of commercial entities under different trade names. The Ty21a and Vi polysaccharide vaccines do afford short-term protection for travelers to regions where typhoid is endemic. Vi conjugate vaccines are also licensed in India for in-country use (404, 407, 410).
The limited efficacy and short-term protection afforded by the Ty21a and unconjugated Vi CPS, as well as the lack of any vaccine against any of the other Salmonella that cause serious disease in humans, has prompted a resurgence of vaccine development against all four of the important Salmonella serovars. Most of the candidate vaccines in development are focused on S. Typhi, but a few candidate vaccines against S. Paratyphi, S. Typhimurium, and S. Enteritidis are under development. Table 17 describes the candidate vaccines in development against all of these organisms. Additional details can be found in recent reviews by Syed et al. (429) and Baliban et al. (430).
TABLE 17.
Candidate vaccines in development against Salmonellaa
| Vaccine type | Vaccine | Development phase | Developer(s) |
|---|---|---|---|
| Salmonella Typhi | |||
| Vi conjugate | Vi-rEPA (recombinant exoprotein A from Pseudomonas aeruginosa) | Phase 3 | U.S. National Institutes of Health, Lanzhou Institute of Biological Products |
| Vi-CRM197 | Phase 3 | EuBiologics, Republic of Korea; PATH | |
| Vi-diphtheria toxoid | Phase 1 or preclinical | International Vaccine Institute/Shantha Biotechnics; SK Chemicals; PT Biofarma; Finlay Institute; Incepta, Bangladesh | |
| Vi-tetanus toxoid | Preclinical | WalVax, China | |
| Vi conjugated to fusion protein PsaA-PdT | Preclinical | Harvard Medical School | |
| O9 conjugate | O9:DT | Preclinical | International Vaccine Institute (Korea) |
| LPS conjugate | O-specific polysaccharide conjugated to diphtheria toxoid | Preclinical | National Institute for Biotechnology and Genetic Engineering (Pakistan) |
| Live-attenuated oral | M01ZH09 | Phase 2 | Prokarium |
| CVD 909 | Phase 2 | University of Maryland | |
| Outer membrane protein | OmpC and OmpF | Phase 1 | Instituto Mexicano del Seguro Social |
| Salmonella Paratyphi | |||
| LPS conjugate | LPS-tetanus toxoid conjugate | Phase 1 | U.S. National Institutes of Health |
| Live attenuated oral | CVD 1902 | Phase 1 | University of Maryland |
| phoPQ mutant | Preclinical | Celldex Therapeutics | |
| Bivalent S. Typhi-S. Paratyphi | |||
| Conjugate | Vi and O:2 antigens of S. Typhi and S. Paratyphi separately conjugated to CRM197 | Preclinical | GSK (licensed to Biological E) |
| Live-attenuated oral bivalent | MO1ZH9 expressing S. Paratyphi LPS (Entervax) | Phase 1 | Prokarium |
| Salmonella Typhimurium | |||
| Live-attenuated oral | CVD 1931 | Preclinical | University of Maryland |
| Regulated delayed attenuation | Preclinical | University of Arizona | |
| WT05 | Phase 1 | Microsciences, Wokingham Berkshire | |
| ruvB mutant | Preclinical | Seoul National University | |
| Hfq deletion mutant | Preclinical | Indian Institute of Science, Bangalore | |
| SA186 lacking ZnuABC transporter | Preclinical | Instituto Superiore de Sanita Roma | |
| MT13 TTSS-2 deficient | Preclinical | Kalinga Institute of Industrial Technology, Odisha University | |
| Adenine methylase mutants | Preclinical | University of California, Santa Barbara | |
| O-protein conjugate | O:4,12-TT | Preclinical | U.S. National Institutes of Health |
| Os-po | Preclinical | National Biotechnology Laboratory, Stockholm | |
| Protein | OmPD outer membrane proteins | Preclinical | University of Birmingham, United Kingdom |
| Salmonella Enteritidis | |||
| Live-attenuated oral | CVD 1944 | Preclinical | University of Maryland |
| O:9 conjugate | O:4,5/O:9-flagellin | Preclinical | University of Maryland |
| S. Typhimurium and S. Enteritidis | |||
| GMMA | Generalized modules for membrane antigens | Preclinical | GSK |
GMMA, Generalized Modules for Membrane Antigens; GSK, GlaxoSmithKline; LPS, lipopolysaccharide; WHO, World Health Organization.
The current vaccine development efforts are focused on three main approaches. The first is conjugating the two important polysaccharide surface antigens (Vi and O) to protein carriers to form the basis for new parenteral vaccines that can be boosted to enhance long-term immunity. This approach recently received a strong endorsement from the WHO, particularly for use in children under 2 years of age (409). Second, several groups are working on developing new live-attenuated vaccines that include different attenuation strategies. The University of Maryland, Prokarium, and others are investigating potential successors to the Ty21a vaccine. Finally, other groups are developing subunit vaccines based on bacterial membrane antigens. Table 17 describes these candidate vaccines (404, 405, 407, 409, 410, 425, 426, 431–435). For S. Typhi, the most clinically advanced candidates are the polysaccharide conjugate vaccines. Two of these vaccines are licensed in India and the Vi conjugate vaccine from Bharat has also achieved WHO prequalification. This vaccine has been shown to be protective in both a controlled human infection model (CHIM), as well as in the field in Nepal. On the strength of this evidence, TCV was deployed in reactive vaccination campaigns against an outbreak of extensively drug-resistant S. Typhi in Pakistan and an outbreak of multidrug-resistant S. Typhi in Zimbabwe (436, 437). In Zimbabwe, within three months of initiating the campaign there was a decrease in number of blood culture-confirmed typhoid cases (438). An evaluation of over 200,000 children in the Pakistan campaign found a vaccine effectiveness of 55 and 97% against extensively drug-resistant S. Typhi (439) and a separate, smaller case-control study in Pakistan found a vaccine effectiveness of 72% (440). Similarly, a cluster-randomized study of over 60,000 children in Bangladesh found an overall vaccine protection of 57% (441). A phase 3 study of over 28,000 children in Malawi compared TCV against meningococcus A vaccine and found an overall efficacy of 81% (442). Two phase 2 studies in Burkina Faso confirmed the safety of TCV and its compatibility with other routine immunizations (443, 444). These results suggest TCV can play a crucial role in mitigating the impact of S. Typhi, including antimicrobial-resistant infections. As indicated above in Table 17, three additional Vi conjugate vaccine candidates are in clinical development.
The LPS-conjugate vaccines (some of which just contain purified O antigen) are in the preclinical stage. Among live-attenuated vaccines, the most advanced are CVD 909 at the University of Maryland and M01ZH09 from Prokarium.
For S. Paratyphi, University of Maryland researchers have developed the live-attenuated vaccine candidate called CVD 1902, which is currently in phase 1. Similarly, Prokarium has a bivalent live-attenuated Typhi-Paratyphi vaccine in phase 1 trials. Celldex Therapeutics has another live-attenuated vaccine candidate in the preclinical stage. A bivalent vaccine in which Vi and O:2 antigens of S. Typhi and S. Paratyphi A are separately conjugated to carrier proteins is in preclinical development at Biological E. The LPS-conjugate vaccine against S. Paratyphi A developed at the U.S. National Institutes of Health failed to show boosting with a second dose given 6 weeks after the first in a phase 1 trial.
For S. Typhimurium, a variety of live-attenuated vaccines are in preclinical stages. The WT05 candidate vaccine, developed by Microsciences in the United Kingdom, has reached phase 1. O antigen conjugate and outer membrane vaccine candidates are also in preclinical stages. Candidate vaccines against S. Enteritidis include live-attenuated CVD 1944 and an O antigen conjugate from University of Maryland, both at the preclinical stage. GSK is applying the Generalized Modules for Membrane Antigens approach for a combined S. Typhimurium/S. Enteritidis vaccine that is in preclinical development.
(v) Summary
Four serovars of S. enterica cause a complex spectrum of disease in humans. Infections with these serovars clearly cause considerable human morbidity and mortality and their geographic spread is unpredictable. The public health threat of these enteropathogens is further amplified by their increasing antibiotic resistance (408, 410). We have only a limited understanding of the pathogenesis and natural immune control of these diseases. Adding to the challenge, two licensed typhoid vaccines are either of moderate efficacy (Ty21a) or of limited durability (Vi polysaccharide antigen vaccine)—factors limiting their widespread use. Neither vaccine works well in the age group at highest risk for illness with these pathogens, infants and children less than 2 years of age. Accordingly, the WHO recently endorsed further development of glycoconjugate vaccines for typhoid fever (426), and positive efficacy data for this approach in both the CHIM and an initial phase 3 efficacy trial in Nepal (431, 434) has driven a major development and testing effort in this area that will likely greatly improve our ability to prevent and control invasive salmonellosis in the future.
Progress can be further accelerated toward better control of Salmonella diseases if several pressing needs are properly addressed. These include improved diagnostic tests that can be widely applied in field settings, improved understanding of the degree to which live-attenuated vaccines can cross-protect against S. Typhi and S. Paratyphi, a clarification of the role of antibodies against the Vi polysaccharide play in protection against S. Typhi and S. Paratyphi, and an understanding of whether it may be feasible to develop vaccines against S. Typhimurium and S. Enteritidis by extrapolation of vaccine concepts that have been moderately successful for S. Typhi and S. Paratyphi bacteria.
For vaccine development against all of the Salmonella diseases, the lack of information on natural immunity and the protective immunity developed after vaccination are critical impediments to progress. Some cross-reaction of the antibody-mediated immunity generated by the licensed vaccine Ty21a and by the live-attenuated vaccine candidate CVD 909 to S. Paratyphi A and B strains does seem to be occurring (422–424). Additional studies will be needed to determine whether these vaccines can provide partial protection against S. Paratyphi. The availability of a new challenge model that includes a strain of S. Paratyphi should be helpful in this regard.
A somewhat confused picture remains with respect to the protection afforded by Vi capsular polysaccharide vaccines since experimental human infection with a strain that expressed this antigen did not elicit detectable anti-Vi antibodies (416). Since so much of the vaccine pipeline depends on Vi antigen conjugate vaccines, some effort toward clarifying such questions might be of benefit to the field. It is interesting to note that comparison of markers for protection induced by the Vi antigens given alone versus those associated with protection induced by Vi conjugates to a protein carrier may differ, further indicating additional studies in this area are needed (432). Similarly, using the new CHIMs to better understand any of the factors may contribute to a better understanding of iNTS pathogenesis and immunity, and would also be a welcome add-on to the field.
The careful work that has supported the development of the new human challenge model for the salmonellae that cause enteric fevers is a fitting tribute to the historical studies that laid much of the groundwork for the licensed vaccines against typhoid fever. The new model has already helped to advance the first Vi conjugate vaccine to WHO prequalification and will be a valuable tool in guiding the development of similar vaccines for paratyphoid and iNTS. Vaccine development for invasive Salmonella disease has a wealth of promising candidates with high probability of success that will lead ultimately to better control of these complex and diverse bacterial pathogens.
Norovirus
(i) Epidemiology, pathogenesis, and antigenic diversity of norovirus
Norovirus, a small, nonenveloped, single-stranded RNA virus, is a leading cause of outbreaks of acute gastroenteritis worldwide. The Global Enteric Multicenter Study identified norovirus as the third most important viral cause of diarrheal disease in children younger than 1 year of age, behind rotavirus and adenovirus 40/41 (445). Similarly, in the most recent Child Health Epidemiology Reference Group analysis of diarrhea-associated mortality under the age of 5, norovirus was the most common viral cause of diarrhea-associated deaths (446). An estimated 677 million infections and 213,000 deaths occur each year across all age groups due to norovirus infection (446). Disease can be more severe in infants and the elderly. The most common modes of norovirus transmission are ingesting contaminated food or water or person-to-person contact. According to some estimates, as few as 30 particles are enough to initiate a new infection.
The mechanisms by which norovirus infection causes acute gastroenteritis are poorly understood (447). Disease onset occurs within 24 to 48 h of infection and includes one or more of the following symptoms: vomiting, watery diarrhea, abdominal cramps, fever, headache, and malaise. Symptoms spontaneously resolve in the great majority of cases within 48 h of onset. Virus particles may be shed in stools long after the resolution of symptoms (448).
The epidemiology of norovirus infection is complex (449–454). The virus occurs in pandemics, in large and small outbreaks, and in sporadic cases of disease. Outbreaks are more common when humans are in confined settings like cruise ships, military installations, prisons, nursing homes, and childcare facilities. The CDC estimates that norovirus may cause 18% of acute gastroenteritis cases worldwide (449). Significant gaps remain regarding the norovirus disease burden in Africa and parts of Latin America, where studies have been infrequent.
Noroviruses are genetically diverse and have been classified based on the amino acid sequences of their capsid protein VP1, which is the target of binding and neutralizing antibodies. Genogroup I contains eight genotypes, including the prototype Norwalk virus that caused an outbreak in school children in Norwalk, Ohio, United States, in 1972 (447). About 10% of human norovirus infections are genogroup I (452). Genogroup II, which causes up to 80% of human infections, contains at least 23 genotypes (452). Genotype GII.4 was associated with seven global outbreaks between 1987 and 2012 (450, 451).
While the propagation of noroviruses in cell culture is not yet routine, producing artificial virus-like particles (VLPs) that faithfully mimic the norovirus capsid structure is possible (450, 455). VLPs representing different genogroups, genotypes, and variants can be produced in laboratories for exploring the impact of genetic diversity on antigenic variation and determining receptor-binding patterns. The receptors for noroviruses are thought to be histo-blood group antigens (HBGA) expressed at mucosal surfaces, including the lumen of the intestinal tract (456–458). Humans that lack a functional FUT2 gene are called nonsecretors because they do not express certain blood group antigens in gastrointestinal secretions VLPs representing different genogroups and genotypes of noroviruses have been used to work out the patterns of binding to different blood group antigens and the capacity of serum antibodies from natural and experimental human infections to block VLP binding to these carbohydrate antigens (451, 456, 459).
Having the capacity to grow the virus in cell culture and to have an animal model in which to conduct preclinical studies of candidate vaccines would be advantageous. Despite considerable effort, the propagation of noroviruses by conventional cell culture techniques is not yet routine; however, efforts to overcome these obstacles are making progress (460–464). Human noroviruses do not infect other normal animal hosts; however, several studies with human norovirus conducted in gnotobiotic (germ-free) piglets suggest that vaccine candidates and probiotics can at least partially suppress clinical symptoms, viral replication, and shedding in these animals (465–468).
(ii) Human challenge models for norovirus
Human challenge studies have been an integral part of norovirus research since the virus was first described (447). In 1972, a rectal swab from a patient linked to an outbreak of diarrhea in school children in Norwalk, Ohio, was used to experimentally inoculate volunteers. The purpose was to reproduce the clinical symptoms of the disease and to identify a small nonenveloped virus, Norwalk virus. Table 18 describes the human challenge models that are currently available and summarizes the collective experience. The challenge studies have been representative of the most important genogroups and genotypes of noroviruses infecting humans. Most of the studies have been conducted with noroviruses of genogroup I, which is thought to be responsible for about 10% of human norovirus infections (452). Two challenge studies have been reported using genogroup II noroviruses, which are thought to be responsible for about 80% of infections in humans. The epidemiologically important genotype II.4 noroviruses are represented by a single challenge study. The challenge pools are purified from the stools of infected individuals and delivered orally. Since secreted HBGA determine human susceptibility to challenge, study volunteers are often prescreened for their blood group and secretor phenotype. Challenge studies are conducted in an inpatient facility. Volunteers are monitored closely following challenge and discharged after symptoms of infection have resolved.
TABLE 18.
Human challenge models for norovirus
| Strain and genotype | Method of production and administration | No. of studies | No. of volunteers |
|---|---|---|---|
| Norwalk virus, GI.1 | A challenge pool was prepared in 2008 from the liquid feces of a single experimentally infected adult by clarification, centrifugation, and serial filtration. The challenge is administered to healthy adults orally. Infection with this challenge pool requires that individuals be secretor positive and lack the blood group B antigen. | 8 | 333 |
| Snow Mountain virus, GII.2 | The virus was purified in 2003 from filtrates of a stool sample and administered to healthy adults orally. Infection with this strain is not dependent on ABO blood type or secretor status. | 1 | 15 |
| Cin-1, GII.4 | Isolated in 2003 from a stool sample from an individual with gastroenteritis. Cin-1 infection is not dependent on ABO blood group, but nonsecretors are resistant to infection. Challenge is delivered to healthy adults orally in sterile water. | 1 | 40 |
Subsequent challenge studies with noroviruses were reported between 1990 and 2003 using clinical isolates of genotype GI.1 viruses similar to Norwalk virus (see Table 18). In 1990, Johnson et al. studied the relationship of preexisting serum antibody titers to protection from disease (469). All 12 human volunteers with serum antibody titers to the challenge virus greater than 1/200 were protected, but only 19 of 30 volunteers with titers less than 1/100 were protected. The first published studies using VLPs for the evaluation of serum antibodies following experimental human challenge were published in 1994 (470, 471). Using this more sensitive assay to study the relationship of serum antibodies to protection from infection in 17 experimentally challenged volunteers, the investigators concluded that preexisting antibodies did not protect from infection. Stored samples from these studies were later used to evaluate virus shedding after experimental challenge by PCR to detect virus genomes and by antigen enzyme-linked immunosorbent assay to detect viral proteins (448). The results indicated that the two assays were well correlated early after infection and that individuals with clinical gastroenteritis after challenge shed ten times more virus than those that did not become ill. The duration of shedding was 4 to 8 weeks or longer in some study subjects.
During this same period, the experimental human challenge study reported by Lindesmith et al. verified the importance of secreted HBGA in norovirus infection (457). These researchers found that individuals that did not express the FUT2 gene (nonsecretors) were fully protected from challenge; and in the same study, they gathered evidence that mucosal IgA responses could protect from infection. Later, this same group studied the reactivity of serum antibodies within and across different genotypes of genogroup I viruses (456). They prepared VLPs from two GI.1 viruses and from GI.2, GI.3, GI.4, and GI.5 viruses. Serum antibodies from volunteers infected with one of the GI.1 strains in their previous challenge study (457) showed cross-reactivity within the GI.1 genotype but not to other GI genotypes.
Between 2010 and 2019, four additional human challenge studies were conducted using genotype I.1 viruses (472–475). One was a challenge study that included 34 human volunteers (474) and showed a correlation between preexisting receptor-blocking antibodies and the clinical outcome (with or without gastroenteritis) after challenge. Binding antibody titers were not correlated with the clinical outcome. In a follow-up study, these investigators used serum antibodies from the volunteers in the previous challenge study to develop a simple hemagglutination inhibition (HAI) assay using human type O erythrocytes (476). These researchers showed that the results from the HAI assay were well correlated with those from the previously used receptor-blocking assay. An HAI titer of greater than 1/40 protected against disease.
Another human challenge study included 57 volunteers and was reported by Atmar et al. in 2014 (475). In this study, individuals with different HBGA and individuals that were either secretors or nonsecretors were studied for their susceptibility to a GI.1 norovirus challenge. All of the volunteers that had blood groups A or O antigens were infected. Individuals with blood group B or AB antigens were not infected, and individuals that were nonsecretors were not infected. For susceptible individuals, the challenge dose required for infection of 50% of volunteers (ID50) was calculated to be 1,320 genomic equivalents, based on a quantitative reverse transcription-PCR (RT-PCR) assay.
Consumption of raw seafood such as oysters carries a risk of infection with enteric pathogens, including norovirus (477). A norovirus human challenge study that enrolled 44 secretor-positive volunteers tested methods of decontaminating raw oysters (472). Volunteers ingested either oysters seeded with 1 × 104 genomic equivalents of norovirus GI.1 strain 8FIIb treated by different high hydrostatic pressure processes, or untreated oysters. Whereas 600 MPa at 6°C for 5 min completely eliminated infectivity from oysters (0 of 10 volunteers infected), oysters treated with only 400 MPa retained infectivity (6 of 19 volunteers infected, 32%). Among volunteers that consumed seeded, untreated oysters, 47% (7 of 15) were infected. A subsequent analysis of gut microbiota from these volunteers identified compositional differences associated with symptomatic and asymptomatic infections (478). A similar study evaluated the persistence of norovirus in water samples stored for various lengths of time (479). Subjects were challenged with a relatively high inoculum of 6.5 × 107 genomic equivalents of GI.1 norovirus 8FIIb, and 10 of 13 were infected and experienced typical clinical symptoms.
A recent norovirus human challenge study with a GI.1 strain was conducted to support future vaccine development (473). Lot 001-09NV was derived from a volunteer from a previous challenge study with strain 8FIIa (457). This new lot was purified and characterized to meet rigorous standards for use in future tests of vaccine candidates. The virulence and clinical symptoms induced by lot 001-09NV were similar to previous studies with related strains. For example, of 16 secretor-positive, blood group A or O individuals challenged with either 3.6 × 105 or 1 × 106 genomic equivalents of norovirus, 9 (56%) met criteria for acute gastroenteritis (diarrhea and/or vomitus), and 11 (69%) were infected, based on shedding norovirus in stool or vomitus by RT-PCR. Furthermore, robust immune responses to GI.1 were observed in infected volunteers: a 30-fold increase in 50% blocking titers (BT50) and 161-fold increase over baseline in IgG titers. Based on these results, lot 001-09NV should be useful for future studies testing vaccine candidates in development (480).
Human challenge studies have also been conducted with three genotypes of the genogroup II norovirus, namely, genotype GII.1, genotype GII.2, and genotype GII.4 noroviruses. In 2005, Lindesmith et al. reported the results of a study of 15 human volunteers challenged with the Snow Mountain strain of genotype II.2 (see Table 18) (481). The investigators found that susceptibility to infection was independent of both the blood type and the secretor phenotype of the volunteers. Nine of fifteen volunteers were infected, and seven developed gastroenteritis. IgG antibodies elicited by infection cross-reacted with VLPs from another GII strain, but not with those from a GI strain. The first human challenge study with a GII.4 norovirus was reported in 2012 (482). Strain Cin-1 (see Table 18) was used to challenge 23 volunteers with the secretor phenotype and 17 nonsecretors. Seventy percent of secretors and six percent of nonsecretors became infected. A small pilot study was conducted with GII.1 Hawaii virus (483). The majority of infected individuals in these three studies developed symptoms of disease.
(iii) Development of norovirus vaccines
The first conventional norovirus vaccine candidate to enter clinical trials was comprised of VLPs of the Norwalk virus administered orally (484, 485). It was well tolerated but poorly immunogenic. The vaccine candidate was then reformulated to include the adjuvant monophosphoryl lipid A (MPL; GlaxoSmithKline) and chitosan (a mucoadherent agent) and evaluated in two additional phase 1 trials using the intranasal route of administration. Results from this study demonstrated that the immunogenicity of the vaccine candidate improved and that safety was maintained (486).
The adjuvanted Norwalk VLP vaccine candidate then advanced to a phase 2 challenge study in human volunteers (487). It contained 100 μg of VLPs produced in a baculovirus expression system, MPL, and chitosan (Archimedes Development). Ninety-eight volunteers were randomized 1:1 to receive vaccine or placebo. The vaccine candidate and placebo were administered intranasally in two doses administered 3 weeks apart. Three weeks after the second dose, 84 of the 90 volunteers that had received two doses of vaccine volunteered for the challenge study and were admitted to an inpatient facility. The next day, they received a homologous strain challenge dose approximately ten times the ID50. Volunteers were cared for and evaluated for at least four days or until the symptoms of disease resolved. The vaccine showed 47% efficacy against the development of disease, and 26% efficacy against infection (487).
A bivalent vaccine candidate similar to the adjuvanted Norwalk VLP vaccine was also evaluated in a human challenge study (488). It contained VLPs of Norwalk virus (genotype GI.1) and VLPs of a consensus sequence GII.4 strain. The bivalent vaccine was administered intramuscularly in MPL adjuvant adsorbed to aluminum hydroxide. The vaccine dose contained 50 μg of each VLP. The dosing schedule was 0 and 4 weeks. At least 1 month after receiving vaccine or placebo, volunteers were admitted to an inpatient challenge facility and challenged with the Cin-1 strain (see Table 18). The vaccine efficacy was low against both infection and disease. According to the protocol, 54% of vaccinees and 63% of controls were infected. The vaccine moderately reduced vomiting and/or diarrhea but did not meet the predefined rates of infection and illness that were the primary study endpoints. Challenge with Norwalk virus was not performed.
The negative results of the initial human challenge study with the bivalent VLP vaccine were somewhat unexpected because the earlier study with the monovalent VLP vaccine seemed to show a moderate level of protection against the development of disease. Although the bivalent vaccine contained VLPs of Norwalk virus, the challenge study was apparently not designed to include a homologous challenge with Norwalk virus, making it difficult to link to the results from the earlier study of the monovalent vaccine. The GII.4 vaccine strain was an artificially derived consensus strain, and it did not exactly match the GII.4 challenge strain. The GII.4 vaccine and challenge strains were mismatched by 19 amino acids in the P2 domain of major capsid protein. Prior to the human challenge study, the GII.4 consensus VLPs were used to immunize rabbits. Hyperimmune sera from immunized rabbits showed variable, but generally good, cross-reactivity among different GII.4 strains (489). The cross-reactivity of serum antibodies from human volunteers vaccinated with the GII.4 consensus VLPs to the challenge strain, however, has not yet been reported. It may also be important that the bivalent VLP vaccine was administered by the intramuscular route, while the monovalent vaccine was delivered intranasally.
The bivalent VLP vaccine candidate TAK-214 was advanced further and recently completed a phase 2b clinical study in the United States (490). Approximately 4,700 adults 18 to 49 years of age were randomized 1:1 to receive either vaccine or placebo and monitored for acute gastroenteritis for 45 days. Because only six cases of GI.1 or GII.4 norovirus were detected, the study had insufficient statistical power to assess the primary endpoint. Therefore, the alpha was amended prior to unblinding in order to provide sufficient power for a secondary endpoint that included all norovirus genogroups detected in the study subjects, most of which were GII.2. In this secondary analysis, the vaccine demonstrated a protective efficacy against moderate or severe disease of 62% (P = 0.0097). In a separate phase 2 study of TAK-214 in roughly 300 adults over 60 years of age, safety and immunogenicity were similar to those seen in younger adults and were unaffected by a second dose after 28 days or by addition of MPL (491). The main sponsor of these studies was the Takeda Pharmaceutical Company, Ltd.
A nonreplicating recombinant adenovirus vector expressing VP1 from a GI.1 strain, designated VXA-G1.1-NN, was recently tested for safety and immunogenicity in a first-in-human phase 1 clinical study of 66 healthy adult volunteers (480). This vaccine candidate was found to be safe, well tolerated, and substantially immunogenic. For example, 78% of vaccinees showed a >2-fold rise in the BT50. The candidate is formulated as an oral tablet that is stable at room temperature to simplify delivery. VXA-G1.1-NN has also been tested along with a GII.4 VP1-expressing adenoviral vector (VXA-G2.4-NS) in a phase 1b study. Both the monovalent and combined bivalent vaccines were safe, well tolerated, and immunogenic, based on IgA antibody-secreting cell response rates: 78% for GI.1 and 93% for GII.4 (492–494). The main sponsor of these studies was Vaxart, Inc.
Eight additional candidate norovirus vaccines based on whole VLPs or smaller protruding domain (P) particles produced from just the protruding portion of VP1 are in preclinical development, along with two adenovirus-vectored norovirus vaccines (450, 455, 466, 495–498). Table 19 summarizes this information and indicates the institutions involved.
TABLE 19.
Candidate norovirus vaccines in development
| Vaccine type | Phase | No. of candidates | Vaccine developer(s) |
|---|---|---|---|
| Bivalent GI.1/GII.4 virus-like particles (TAK-214) | Clinical phase 2b | 1 | Takeda Pharmaceutical Company Limited |
| Nonreplicating recombinant adenovirus vectors (VXA-G1.1-NN, VXA-G2.4-NS) | Clinical phase 1b | 2 | Vaxart, Inc.; University of Maryland |
| Virus-like particles and protruding domain (P) particles | Preclinical | 8 | Arizona State University; University of North Carolina; Virginia-Maryland Regional College of Veterinary Medicine; Cincinnati Children’s Hospital Medical Center; U.S. National Institute of Allergy and Infectious Diseases; UMN Pharma, Inc.; Nanotherapeutics; Kunming University of Science and Technology; Tampere University |
| Recombinant viral vectors (adenovirus) | Preclinical | 2 | Chinese Center for Disease Control; Ohio State University |
(iv) Summary
An eminent group of physicians and virologists published the first human challenge study with norovirus in 1974 (499). Forty years later, human challenge models are a cornerstone of norovirus research and vaccine development. Three different human challenge models are available, representing viruses of the genogroup I and II strains circulating in humans. More than 300 human volunteers have been experimentally challenged in research studies that provide basic information about norovirus pathogenesis, the role of serum antibodies in protection from infection and disease, and the role of HBGA in susceptibility to infection. This information is of fundamental importance for the development of norovirus vaccines. The foundation for norovirus vaccine development has been laid without growing a single norovirus in the laboratory and without the support of an animal model of infection. Human challenge studies have seamlessly woven into the clinical development of the first candidate vaccines against noroviruses.
A careful analysis of strains from norovirus outbreaks in humans over 30 years has provided a clearer understanding of norovirus diversity and the challenge that such diversity poses to vaccine development. Some in the scientific community have proposed that vaccines against the highly variable GII.4 noroviruses may require frequent replacement—a strategy analogous to that used for seasonal influenza vaccines (495). Indeed, the studies discussed here, including the experimental human challenge with GII.4 virus after vaccination, do suggest that protection may be more difficult among the highly variable GII.4 viruses than for the less variable GI noroviruses.
Fortunately, the leading candidate vaccines against norovirus are based on VLPs—a vaccine type that is relatively cost-effective to produce, heat stable in solution, and amenable to long-term storage in a dry powder for intranasal immunization (455). In the future, VLP vaccines against some of the norovirus genogroups and genotypes could be mass produced, stockpiled, and used to contain outbreaks, as well as for mass vaccination campaigns in the most susceptible age groups. The VLP platform may provide the much-needed flexibility for reformulation required for many important diseases caused by highly variable pathogens like noroviruses.
Cryptosporidium
(i) Epidemiology, diversity, pathogenesis, and public health impact of Cryptosporidium
The Global Enteric Multicenter Study identified the apicomplexan parasite Cryptosporidium as the second most frequent cause of childhood diarrhea worldwide (500). In the Institute for Health Metrics and Evaluation’s recent Global Burden of Disease Study, Cryptosporidium was estimated to be responsible for approximately 7% of all diarrhea-associated deaths worldwide and to account for 8% of all diarrhea-associated disability adjusted life years (501, 502). Despite these concerning estimates, these figures still likely underestimate the true disease burden. The development of improved quantitative molecular diagnostic methods may serve to further increase disease estimates and further heighten concerns about the role of Cryptosporidium as a global enteric pathogen (503). Contamination of surface water with Cryptosporidium continues to be the source of outbreaks of disease in high- and low-resource countries alike (504). No vaccine against Cryptosporidium has been developed, and while substantial progress has been made in advancing preclinical drug candidates (505–508), no widely available, highly effective drugs exist to treat the disease. The sole U.S. Food and Drug Administration (FDA)-approved treatment, nitazoxanide, is efficacious in otherwise healthy adults but poorly efficacious in malnourished children and ineffective in severely immunocompromised patients (509), so its uptake in low-resource settings has been limited.
Two species, Cryptosporidium hominis and Cryptosporidium parvum, cause the great majority of human infections. The former is naturally transmitted only in humans, while the latter is common in domesticated animals and can be transmitted to humans zoonotically, as well as person to person. In the general population, rates of seropositivity to Cryptosporidium range from 25 to 60% in different populations and locations (510). While surveillance remains incomplete, C. hominis accounts for roughly 80% of infections worldwide (511).
Cryptosporidium is highly contagious. Infected people and animals typically shed 109 oocysts per g of feces, and ingestion of only a few oocysts from contaminated food or water is sufficient to establish infection. Outbreaks associated with contaminated surface water are common. The largest reported outbreak was in Milwaukee, WI, in 1993, with more than 400,000 infections. Outbreaks in high-income countries remain common (504, 512).
Infection with Cryptosporidium is associated with a wide spectrum of disease (513). Malnourished children in low-resource countries and immunocompromised individuals or those under immune suppressive treatments are the most vulnerable. In most cases, ingestion of oocysts leads to a watery diarrhea that typically lasts 1 to 2 weeks in healthy adults but can persist for weeks beyond that in malnourished and immunocompromised individuals. Microscopically, Cryptosporidium infection is associated with a flattening of the intestinal villi, which may contribute to the malnutrition, environmental enteric dysfunction, and growth retardation seen in infected children. Long-term effects on cognitive and motor development can also be associated with Cryptosporidium infection. The parasite can disseminate to other organs and result in significant mortality in immunocompromised hosts (510, 514). Respiratory infection and symptoms have also been well documented, including in HIV-negative adults and children (515–519); however, their relationship with intestinal infection and symptoms remains unclear.
(ii) Cryptosporidium human challenge model
The initial human challenge studies with Cryptosporidium conducted in the 1990s and early 2000s were designed to determine the number of oocysts of C. parvum required to establish infection and disease and to explore phenotypic diversity of isolates from different animal species. As shown in Table 20, oocysts are either purified from the stools of infected animals (C. parvum) or isolated from humans and propagated in specialized, gnotobiotic pigs (C. hominis). Several methods have been reported for perpetual propagation of C. hominis or C. parvum in cell culture (520–522), but these are not widely available nor used routinely. More recent epidemiology studies highlighting the burden of Cryptosporidium in low- and middle-income countries (LMICs) have rekindled the interest in this model for the purposes of testing drug and vaccine candidates for treatment or prevention of infection with the parasite (523). Studies of the natural history of cryptosporidiosis in LMIC populations demonstrate there are fewer infections among older children (500), suggesting that adaptive immunity induced by a vaccine is achievable. However, comparatively little is understood about the immune response to the parasite; therefore, controlled human infection model (CHIM) studies could play an important role in closing these gaps and supporting development of vaccine candidates.
TABLE 20.
Human challenge model for Cryptosporidium
| Organism | Strain(s) | Method of production and administration | Stock producer |
|---|---|---|---|
| Cryptosporidium parvum | Iowa, Moredun, TAMU, UCP | Oocysts are purified from feces of experimentally infected calves. Adults are orally exposed to measured numbers of oocysts. | Challenge stock prepared at the University of Arizona. Challenges performed at the University of Texas Health Science Center. |
| Cryptosporidium hominis | TU502 | An isolate from a child with cryptosporidiosis has been propagated in gnotobiotic piglets and purified from feces. Adults are orally exposed to measured numbers of oocysts. | Challenge stock prepared at Tufts University School of Medicine. Challenges performed at the University of Texas Health Science Center. |
The initial human challenge study with C. parvum was published by DuPont et al. in 1995 (524). The volunteers for the study were healthy adults that were seronegative for C. parvum. Infection was defined by diarrheal illness and by the detection of oocysts in stools. Follow-up was intensive for the first two weeks and was continued at longer intervals for an additional six weeks.
Doses ranging from 3 × 101 to 1 × 106 oocysts of the Iowa strain of C. parvum were used to challenge 29 volunteers, and 62% of the challenged volunteers became infected. The calculated dose for infection of 50% of volunteers (ID50) was 132 oocysts. Many, but not all, of the subjects that excreted oocysts also had symptoms of infection, including abdominal pain, cramps, and diarrhea. The number of ingested oocysts did not markedly affect the incubation period nor the severity or duration of illness.
These same investigators more closely examined the relationships between patterns of oocyst excretion, challenge dose, and symptoms of disease in infected volunteers. (525). The patterns of oocyst shedding in volunteers with disease symptoms and diarrhea, with disease symptoms without diarrhea, and without disease symptoms altogether were compared. Due to the presence of oocyst shedding in volunteers previously thought not to be shedding, the investigators revised the 50% infectious dose (ID50) for C. parvum to 83 oocysts (526).
In this study, the lack of any obvious relationship between challenge dose and the development of disease symptoms, diarrhea, or the pattern of oocyst shedding was evident. The patterns and duration of shedding were also quite variable. Eight different challenge stocks isolated at different times were used in the study, which may account for some of the variability observed.
The final report in this series appeared in 1998 (527). The characteristic antibody responses that developed during experimental C. parvum infection were studied by immunoblot of oocyst proteins. Serum immunoglobulin G antibodies to proteins with molecular weights of 27, 17, and 15 kDa were those most commonly observed. Some of these antigens were subsequently used in serological screening assays (528).
During the studies with the Iowa strain of C. parvum described above, many of the volunteers underwent upper endoscopy with jejunal biopsy at both pre- and postchallenge time points. These samples were used to study the human mucosal immune response to Cryptosporidium infection and were correlated with the severity of disease symptoms when possible. A series of six articles appeared between 2000 and 2003 reporting the results of these studies, and overall results are summarized in a review published in 2008 (529).
A number of subsequent studies were conducted with the human challenge model for Cryptosporidium between 1999 and 2006. The impact of preexisting immunity on experimental infection with C. parvum was examined in a study by Chappell et al. (526). Human volunteers challenged with four different strains of C. parvum and one strain of C. hominis were examined for disease development and oocyst shedding (530–532). The details and results of these studies are summarized in Table 21.
TABLE 21.
Experimental human challenge studies with Cryptosporidiuma
| Species | Strain | Species of origin | Passage | Volunteers |
Oocyst dose range | Attack rate (%) | ID50 | |
|---|---|---|---|---|---|---|---|---|
| Serostatus | n | |||||||
| Cryptosporidium parvum | Iowa | Bovine | Bovine | Negative | 29 | 30–1,000,00 | 62 | 87 |
| UCP | Bovine | Bovine | Negative | 17 | 500–10,000 | 59 | 1,042 | |
| TAMU | Equine | Human→Bovine | Negative | 14 | 10–500 | 86 | 9 | |
| Moredun | Cervine | Ovine→Bovine | Negative | 16 | 100–3,000 | 69 | 300 | |
| Iowa | Bovine | Bovine | Positive | 17 | 500–50,000 | 62 | 1,880 | |
| Cryptosporidium hominis | TU502 | Human | Porcine | Negative | 21 | 10–500 | 76 | 10 |
ID50, 50% infectious dose. Boldfaced and underlined strains indicate challenge strains that either originated in humans or were passaged through a human, and high attack rates that these strains produced in the human challenge model with low 50% infectious doses (ID50).
In considering the reestablishment of the Cryptosporidium CHIM (523), one element that merits careful consideration is long-term safety risks of volunteers. In the studies conducted in the 1990s and early 2000s, there were no reported safety assessments of adverse events related to challenge beyond 60 days. However, there have been several recent reports of increased incidence of chronic intestinal disorders among populations that experienced naturally occurring outbreaks of cryptosporidiosis. These have included diarrhea, abdominal pain, nausea, or even a formal diagnosis of irritable bowel syndrome according to the Rome III criteria (533–537). These symptoms persisted for several months up to 2 years in approximately 10 to 30% of subjects after the initial acute infection with Cryptosporidium. In case-control studies that also enrolled subjects not infected with Cryptosporidium, the relative risks for these chronic intestinal symptoms typically ranged from 1.5- to 3-fold increased risk. While the precise mechanisms remain to be determined, a full accounting of all associated risk factors is warranted. These observations suggest that future volunteers in a Cryptosporidium CHIM would need to undergo a rigorous informed consent process and be carefully screened for risk factors that might predispose them to long-term sequelae as a result of the challenge. Treating all volunteers with nitazoxanide before discharge may also mitigate longer-term risks.
The C. parvum strains that have been used for human challenge studies differ with respect to their species of origin and their passage history (Table 21). The Iowa and UCP strains have a similar provenance. Both were isolated from calves, in Iowa and Maryland, respectively, and the two strains have been passaged exclusively in calves. A 10-fold difference in ID50 was seen for these strains in the human challenge model. Strain TAMU was accidentally transmitted to a human during the necropsy of a foal and was subsequently propagated in calves. The ID50 for this strain in the human challenge model was 10-fold lower than the ID50 for the Iowa strain and 100-fold lower than the ID50 for the UCP strain. The Moredun strain was isolated from a red deer in Scotland. It was multiply passaged in sheep and then in calves. The ID50 for the Moredun strain was intermediate between that of the Iowa and UCP strains (530, 531).
The Iowa strain of C. parvum has been evaluated in human volunteers with, or without, serologic evidence of prior exposure to C. parvum (525, 538). Table 21 shows that the ID50 increased 20-fold in volunteers with preexisting immunity to C. parvum. One challenge study has been conducted with C. hominis. The challenge strain, called TU502, was isolated from a human infection and passaged in gnotobiotic pigs. In human volunteers without prior exposure to C. hominis, the ID50 was quite low (Table 21). Heterologous cross-protection between species has not been investigated in humans, but studies in gnotobiotic pigs suggest partial cross-protection may occur (539, 540).
Collectively, these studies raised the total number of human volunteers that have been safely experimentally challenged with Cryptosporidium to more than 100. The highest attack rates and lowest ID50 were observed for the two strains that had replicated in humans, TAMU and TU502. These results are highlighted in grey in Table 21. Prior exposure to C. parvum was protective against rechallenge, increasing the ID50 for the Iowa strain by ~20-fold. Clinical experience with C. hominus is limited, but the one strain that has been evaluated had a high attack rate and a low ID50 in seronegative human volunteers.
While no vaccine candidates have been tested against experimental human infection with Cryptosporidium, the challenge model has been used to evaluate bovine hyperimmune colostrum (541). Briefly, pregnant cows were immunized with inactivated C. parvum oocysts and the resulting colostrum was partially purified to yield a concentrate rich in immunoglobulins against Cryptosporidium. Volunteers received 10 g of colostrum or nonfat milk placebo before challenge and then three times daily for 5 days after. There was a nonsignificant trend towards efficacy as measured by a reduction in the incidence of diarrhea; however, the study suffered because of a lower-than-expected attack rate of only 44% infection with the challenge dose of 10,000 oocysts, compared to a 100% attack rate (4 of 4 volunteers infected) in a pilot study with the same dose. Future efforts to standardize the model with a consistent attack rate will be needed to ensure it is suitable for evaluating both therapeutic and vaccine candidates.
(iii) Development of vaccines against Cryptosporidium
The field of vaccine development against Cryptosporidium is at the stage of antigen discovery and exploratory studies in animal models (510, 513, 514, 542). Table 22 summarizes the status of the Cryptosporidium vaccine pipeline and more details can be found in a recent review by Lemieux et al. (543). Two main approaches have been pursued for selecting antigens to be included in vaccines. In one approach, proteins that are exposed on the surface of the sporozoite stage of parasite development are under consideration. These include the apical complex proteins that mediate attachment and invasion (510, 514). The lead target antigens seem to be gp40/15 and cp23. The gp40/15 glycoprotein is cleaved into two subunits that remain covalently attached on the surface of the parasite, and the surface-expressed form of gp40/15 mediates binding to epithelial cells and is associated with the parasitophorous vacuole (544). Cp23, another surface glycoprotein, is also the target of some candidate vaccines. A very large and heavily glycosylated protein, called gp900, is under consideration because it binds to intestinal epithelial cells and inhibits C. parvum attachment in vitro. An antigenic fragment of gp900 (designated SA40), as well as a fragment of another antigenic sporozoite protein, Cpa135 (designated SA35), were determined to have a protective effect as maternal antigens in a BALB/c neonatal mouse model (545).
TABLE 22.
Candidate Cryptosporidium vaccines in preclinical development
| Vaccine type | Phase | No. of candidates | Vaccine developers |
|---|---|---|---|
| DNA vaccines | Preclinical | 6 | Emory University, Chinese Academy of Sciences, Jilin University, Tsinghua University, Shandong Institute of Parasitic Diseases |
| Recombinant bacterial expression vectors | Preclinical | 8 | University of Virginia, Virginia Commonwealth University, Instituto Nacional de Tecnología Agropecuaria, Istituto Superiore di Sanita |
The second approach to Cryptosporidium vaccines is the use of reverse genetics to identify potential target antigens. Using the complete genome sequences of C. parvum and C. hominis, a reverse genetics approach has identified three additional antigens: Cp15, profilin, and apyrase (546). An acidic ribosomal protein that is somehow surface expressed is being investigated in the form of a DNA vaccine (547) or in combination with Cp23 (548). Some of these proteins are being expressed in vectors like Salmonella, recombinant vaccinia, and Lactobacillus (510, 549). Others are being expressed in related protozoan parasites such as Toxoplasma gondii (550) or Tetrahymena thermophila (551). In another study, a reverse genetic approach identified three glycosylphophatidylinositol-anchored proteins: GP60, CpH1, and CpSUB1. These were validated by demonstrating that they bound to sera from Cryptosporidium-infected neonatal calves (552). Furthermore, naturally occurring maternal antibodies against these proteins were found to be present in bovine colostrum and efficiently transferred to neonatal calves.
In the animal health field, cryptosporidiosis is also a serious problem among livestock animals, especially cows. There are no FDA- or U.S. Department of Agriculture-approved vaccines for prevention of cryptosporidiosis in animals; however, there is one preparation marketed as a feed additive, BoviCare-cp (Huvepharma), which may stimulate a protective immune response. BoviCare-cp is composed of digested C. parvum proteins and is recommended to be administered to neonatal calves twice daily for the first seven days of life. Unpublished research suggests that BoviCare-cp reduces diarrhea and oocyst shedding in neonatal calves experimentally challenged with C. parvum (M. Welter, unpublished data). Additional studies are needed to verify these results, and it remains to be determined if this product is efficacious in humans.
(iv) Summary
The intestinal parasite Cryptosporidium came to light as an important intestinal pathogen in the era of HIV/AIDS. For decades, it was generally regarded as an opportunistic pathogen of immune deficiency and the causative agent of occasional outbreaks due to contaminated water and food. The Global Enteric Multicenter Study (500) brought Cryptosporidium into focus as a major cause of morbidity and mortality globally, especially in children younger than 2 years of age.
At this time, the only licensed vaccine against a parasitic disease is the RTS,S vaccine against Plasmodium falciparum malaria. The initial studies with a human challenge model for Cryptosporidium have provided an avenue to better understand pathogenesis and immunity directly in the human host, and to support the development of vaccines against a parasitic disease of newly recognized importance at the earliest possible stage.
Several obstacles must be overcome, however, to develop a human challenge model of broader utility. The isolation and purification of oocysts from the stools of infected animals is not particularly scalable, reproducible, or convenient. Furthermore, no capacity is in place to produce standardized, larger-scale challenge stocks until Cryptosporidium can be adapted to cell culture, as has been done for P. falciparum. Both C. hominus and C. parvum are infectious in neonatal gnotobiotic pigs, both by oral and airborne routes of transmission. Unfortunately, the challenge doses for oral infection that have been used are five orders of magnitude higher than the doses required for infecting 50% of human volunteers with the best available challenge strains (see Table 21). The relevance of airborne transmission to the principal modes of transmission in humans is unclear. The gnotobiotic piglet model is also limited by the fact that specialized facilities are required and neonatal piglets can only be kept in isolation for a short period of time.
Additional studies with C. parvum challenge strains were planned by investigators at the University of Vermont in order to develop a repeated low-dose challenge model to complement the high-dose challenge model developed previously (B. Kirkpatrick, unpublished data). Additional studies using a C. hominis challenge, incorporating rechallenge studies to investigate correlates of protective immunity using highly sophisticated techniques like mass cytometry (to enable high-content immunophenotyping) and sequencing of antibody genes, were planned in collaboration between the University of Maryland and Tufts University (M. Levine and W. Chen, unpublished data). However, because of barriers in obtaining FDA approval related to consistency of challenge inocula, these studies are currently on hold. A key limitation is the lack of a source of Good Manufacturing Practice-certified Cryptosporidium oocysts that would be acceptable to the FDA for administration to CHIM volunteers. Investigators at Radboud University in the Netherlands are also considering establishing a C. parvum challenge model (R. Sauerwein, unpublished data) and may benefit from a more permissive regulatory environment in the European Union.
In light of the seminal GEMS results, young children and infants living in low-resource countries where infection with Cryptosporidium is endemic would most benefit from an effective vaccine. An improved human challenge model for Cryptosporidium would also have great utility in the drug development field, since it would facilitate screening of candidates for clinical effect and early elimination of drug candidates that do not show a treatment benefit in this model. Several drug candidates are advancing in preclinical development and may be ready for testing in a Cryptosporidium CHIM in the near future (505, 506).
Pseudochallenge with live-attenuated pathogens
For many pathogens, challenge with fully virulent wild-type strains is unethical because of safety concerns for participants. In a few notable examples, live-attenuated strains, such as licensed vaccines with well-established safety records, have been used as so-called “pseudochallenge” agents to explore key questions that would otherwise be difficult or impractical to study by either challenge with or natural exposure to virulent wild-type strains. Some examples of this can be found with rotavirus (553), polio (554), influenza (555), and dengue (556). Studies using this approach for rotavirus and polio are summarized in the following sections; studies using live-attenuated dengue vaccine candidates and live-attenuated influenza vaccines as challenge strains are discussed in the corresponding sections.
(i) Rotavirus
(a) Epidemiology and public health impact of rotavirus. Despite recent successes in the development and deployment of vaccines, rotavirus remains the most important pathogen causing diarrheal disease among young children worldwide. As with other enteric diseases, the bulk of the burden falls on impoverished children in low- and middle-income countries (LMICs) with poor access to clean water, sanitation, hygiene, and appropriate nutrition. Rotavirus is responsible for approximately 125,000 deaths among children under 5 years old, although this has decreased from around 500,000 in 2000 (557). Much of this success can be attributed to the approval and introduction of four WHO-prequalified live oral rotavirus vaccines (LORVs): RotaTeq (Merck, USA), Rotarix (GlaxoSmithKline, Belgium), Rotasiil (Serum Institute, India), and Rotavac (Bharat Biotech, India). However, these LORVs have demonstrated only modest efficacy in LMICs (558). The reasons for this observation are still under investigation, and may be multifactorial, but this points to the need for optimization of existing vaccines and/or development of new ones that perform more effectively in LMIC settings. Pseudochallenge studies with LORVs offer one method to accelerating the development of new and improved rotavirus vaccines.
(b) Pathogenesis and diversity of rotavirus. Rotavirus is a nonenveloped, double-stranded RNA virus whose genome contains 11 segments encoding six structural proteins, including VP4 and VP7, which form an outer capsid. The two outer capsid proteins are the target of neutralizing antibodies and determine the rotavirus serotypes. Multiple VP7 serotypes (also called “G” serotypes, since VP7 is glycosylated) have been identified. Similarly, a large number of VP4 (or “P” serotypes for protease-sensitive protein) have been described. Given that most of the rotavirus strains have not been characterized by immunological means, the term “genotype” is frequently used, especially for the initial identification of new viral strains. Some of the predominant genotypes associated with acute disease in children include G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] (559).
A number of host proteins related to histo-blood group antigens have been implicated as rotavirus receptors. Differences in various polysaccharides can modulate virus binding and entry into the host cell, depending on the G and P serotypes (560).
Rotavirus infects via the fecal-oral route and upon infection and replication in the intestinal epithelium triggers acute secretory diarrhea. In addition to the lytic damage to the cells in the tip of the intestinal villi, which limits the absorptive capacity, the virally encoded toxin nonstructural protein 4 stimulates chloride secretion (561). Other mechanisms, including activation of calcium signaling may also contribute to the induction of watery diarrhea (562).
(c) Rotavirus vaccine development. While LORVs have been found to be highly efficacious (90 to 95%) in high-income country settings, their efficacy in low-income countries has been substantially lower (40 to 60%) (558). The precise explanation for this remains to be determined, but various factors have been postulated. One of the most commonly cited is poor immunogenicity because of reduced replication of the LORVs in intestinal epithelial cells of young children with chronic intestinal inflammation, a condition known as environmental enteric dysfunction (563). Other possible explanations are (i) presence of maternal antibodies, given that in low-resource settings women have higher levels of these antibodies in comparison to women living in high-income countries, as antibodies transmitted transplacentally or by breastfeeding interfere with vaccine virus replication (564); and (ii) genetic differences in receptors and coreceptors between residents of high-income countries versus LMICs (565, 566).
One proposed approach to overcome this limitation of LORVs is to develop a parenterally administered rotavirus vaccine that would bypass any deficiencies in mucosal immunity in infants in LMICs (567, 568). There are several use cases for such a vaccine, the simplest being introduction as a stand-alone product. However, recent criticisms about the already large number of vaccines being added to the WHO Expanded Programme on Immunization suggest that such an approach could be difficult to implement. To overcome this criticism, one option would be to combine a parenteral rotavirus vaccine with another existing parenteral vaccine, such as the pentavalent combination (diphtheria-tetanus-pertussis, Haemophilus influenzae type b, and hepatitis B) or other multivalent combination (hexavalent with inactivated polio vaccine, etc.). Another, non-mutually exclusive approach would be to use the parenteral rotavirus vaccine in combination with an LORV in a prime-boost regimen. Experience with other vaccines, including influenza A H5N1 (569, 570) and typhoid (571), suggests that a prime-boost approach could lead to a synergistic response that is superior to either vaccine given individually.
There are several parenteral rotavirus vaccine candidates progressing through development. The most advanced of these is a nonreplicating trivalent subunit rotavirus vaccine consisting of P[4], P[6], and P[8] VP8s conjugated to P2 tetanus toxoid. This candidate recently advanced to a multicountry pivotal phase 3 clinical trial (ClinicalTrials.gov identifier NCT04010448) (572). Another parenteral candidate, earlier in development, is the heat-inactivated rotavirus vaccine CDC-9 strain (573). Several other parenteral rotavirus vaccine candidates are at earlier stages of development (574).
(d) Pseudochallenge studies with a live oral rotavirus vaccine. In a recent phase 1/2 clinical trial of monovalent P2-VP8-P[8] (553), a pseudochallenge model was included as part of the study design as a novel approach to demonstrating vaccine efficacy. Either placebo or the vaccine candidate in three doses of either 30 or 60 μg were given to 162 South African infants at 6, 10, and 14 weeks of age. The primary objectives were to determine vaccine safety/reactogenicity and immunogenicity. As a secondary objective, the pseudochallenge was incorporated as an assessment of vaccine impact on mucosal immunity and viral shedding. The LORV Rotarix was given to vaccinees four weeks after the third P2-VP8-P[8] dose, and then shedding of live Rotarix was measured by enzyme-linked immunosorbent assay at 5, 7, and 9 days postdose. As exploratory objectives, mucosal immune responses before and after Rotarix administration were quantitated. Specifically, serum levels of anti-rotavirus IgA, anti-P2-VP8-P[8] IgA, and IgG geometric mean titers (GMTs) and homotypic neutralizing antibody GMTs were determined (Table 23).
TABLE 23.
Rotarix fecal shedding in infants vaccinated with monovalent P2-VP8-P[8]a
| Parameter | Placebo | P2-VP8-P[8], 30 μg | P2-VP8-P[8], 60 μg | 30 μg and 60 μg, combined |
|---|---|---|---|---|
| No. (%) shedding on day 5, 7, or 9 | 17/44 (39) | 6/45 (13) | 9/46 (20) | 15/91 (17) |
| % reduction compared to placebo (95% CI) | NA | 66 (21–85) | 49 (0–75) | 57 (23–76) |
| P | 0.0087 | 0.0493 | 0.0052 |
Data were reproduced from Groome et al. (553). 95% CI, 95% confidence interval.
In order to determine sample size, it was assumed that 30% of placebo recipients would shed rotavirus. In the final analysis, the most significant result was the number of subjects that shed rotavirus on any day (5, 7, or 9): 39% of placebo recipients compared to 17% of P2-VP8-P[8] recipients (combined 30- and 60-μg groups) shed rotavirus, constituting a 57% reduction (P = 0.0052). Of the 32 shedders, 29 shed Rotarix and 3 shed a G9P[8] strain, presumably the result of natural infection.
This rotavirus pseudochallenge approach was also used in another recent phase 1/2 trial, of trivalent P2-VP8 (575). This trial had a similar design as the one described above: 558 South African infants were enrolled at 6 weeks of age; received three doses of trivalent P2-VP8 or placebo at 6, 10, and 14 weeks of age; and then received Rotarix as a pseudochallenge 4 weeks after the last dose of trivalent P2-VP8. This study tested three dose levels of trivalent P2-VP8: 15, 30, and 90 μg. The reduction in shedding on any of days 5, 7, or 9 after pseudochallenge was not significantly different from placebo for the 15- and 30-μg groups but was significant for the 90-μg group: 45% PCR-confirmed shedding with placebo, versus 27% shedding with 90 μg, a 41% reduction (95% confidence interval [CI] = 0.1 to 65, P = 0.035) (see Table 24).
TABLE 24.
Rotarix fecal shedding in infants vaccinated with trivalent P2-VP8-P[4],[6],[8]a
| Parameter | Placebo | Trivalent, 15 μg | Trivalent, 30 μg | Trivalent, 90 μg |
|---|---|---|---|---|
| No. (%) shedding on day 5, 7, or 9 | 24/53 (45) | 22/52 (42) | 19/56 (34) | 15/56 (27) |
| % reduction compared to placebo (95% CI) | NA | 7 | 25 | 41 (0.1–65) |
| P | NS | NS | 0.035 |
Data were reproduced from Groome et al. (575). NA, not applicable; 95% CI, 95% confidence interval; NS, not significant.
These results served as a proof of concept that reduction in shedding of an orally administered live oral vaccine strain could serve as a valid and informative endpoint in the development of parenterally administered rotavirus vaccines. Importantly, these modest phase 1/2 studies provided strong justification for advancing the vaccine to a much larger phase 3 study now ongoing (ClinicalTrials.gov identifier NCT04010448) (572). The success of this approach also stimulated a pilot study in Zambia that demonstrated reduction viral shedding after a second dose of Rotarix as a live-attenuated challenge (576).
(ii) Polio
(a) Epidemiology and public health impact of polio. Infection with poliovirus is responsible for poliomyelitis (polio), consisting of acute flaccid paralysis that can be fatal as a result of motor neuron damage leading to progressive paralysis. The Global Polio Eradication Initiative (GPEI) has reached the endgame of its campaign, but poliovirus remains endemic in two countries: Afghanistan and Pakistan (577). There are three serotypes of poliovirus (types 1, 2, and 3) and immunization with a single strain does not confer protection against the others. Therefore, a strategy to immunize against all three types is essential for successful eradication. Wild-types 2 and 3 have been eradicated, with the last confirmed cases occurring in 1999 and 2012, respectively. Only type 1 remains circulating in countries of endemicity, with fewer than 180 cases detected annually over the past 5 years (578). This represents substantial progress since the start of the GPEI in 1988, when there were 350,000 confirmed cases worldwide (579).
(b) Current vaccination strategy. Much of the progress in the current eradication campaign was achieved with trivalent oral polio vaccine (tOPV), a live-attenuated vaccine composed of all three strains, also known as the Sabin vaccine. However, one of the risks with use of tOPV is vaccine-associated paralytic polio, which occurs at very low rates of 2 to 4 per 1 million vaccinations. Another risk is reversion of one of the live-attenuated vaccine-derived strains that begins to circulate in communities with insufficient immune coverage, resulting in circulating vaccine-derived poliovirus (cVDPV). Risk of this reversion is particularly notable with type 2 polio because of the genetic instability of the attenuated strains. In order to eliminate these risks, many countries have switched to use of inactivated polio vaccine (IPV), also known as the Salk vaccine, which contains killed virus and therefore lacks the risk of vaccine-associated paralytic polio and generation of cVDPV. IPV generates excellent humoral immunity; however, its key disadvantage is that it is administered systemically and thus does not confer high levels of mucosal immunity in the intestinal tract, the primary site for polio infection. Therefore, subjects receiving IPV only are still at risk of enteric polio infection and may shed virus if exposed to wild polio, thereby contributing to the chain of transmission. Most countries with high levels of vaccine coverage now use IPV to avoid the risks associated with OPV; however, in LMICs with poor sanitation and hygiene and lower vaccination coverage, use of OPV and its associated risks are still prevalent. In 2016, the WHO initiated a phased elimination of OPV, beginning with withdrawal of OPV2 by replacing tOPV with bivalent oral polio vaccine (bOPV, containing only types 1 and 3), and the use of IPV to provide coverage against OPV2. However, during this interim period, subjects that receive IPV and bOPV will still be at risk of shedding type 2 virus in feces if exposed to a type 2 cVDPV. This risk mandates the stockpiling of monovalent oral polio vaccine type 2 (mOPV2) (580) or other short-term measures such as prophylactic or therapeutic antivirals (581) to control an outbreak of cVDPV2. A recent development to counter cVDPV2 outbreaks is the novel OPV2 which is more genetically stable than OPV2 (582, 583).
(c) Pseudochallenge studies with live-attenuated polio vaccines. While challenge studies intentionally infecting subjects with wild-type polioviruses are obviously unethical, the use of challenge with licensed live-attenuated OPV strains with well-established safety profiles has been applied in two approaches as part of the polio eradication endgame and withdrawal of OPVs. The first is challenge studies in the target population of naive infants in order to evaluate the efficacy of IPV and mixed IPV/OPV vaccination regimens. The second is challenge models performed in healthy volunteer adult populations with a history of vaccination with IPV only. These have been used in the development of novel oral polio vaccines and also therapeutics intended for use in outbreak scenarios. In both cases, the primary objective of these challenge studies is typically to evaluate intestinal shedding of the challenge virus as a measure of the extent of mucosal immunity induced by the vaccination regimen.
(d) Challenge studies in infants. A series of phase 2 and phase 3 studies have been conducted with infants in LMICs, in which the subjects received various regimens containing IPV and/or bOPV and then were challenged with either tOPV or mOPV2. Two recently published comprehensive reviews (554, 584) provide detailed overviews of the findings of these studies. In general, they found that one or two doses of IPV in combination with bOPV conferred strong immunity to types 1 and 3, and a moderate level of immunity to type 2. Taken together, these studies provided robust support for the GPEI strategy of switching from tOPV to a mixed IPV/bOPV regimen.
(e) Challenge models in adults. As part of the strategy of replacing tOPV with bOPV, two novel OPV2 vaccine candidates are under development. These live-attenuated strains contain several genetic modifications of the standard OPV2 strain that make them more genetically stable and thus better suited for stockpiling as a means to address cVDPV2 outbreaks and avoid further introduction of cVDPV2. In order to test the safety and efficacy of these candidates, a phase 1 study was conducted in healthy adult volunteers that had a well-documented history of receiving IPV as children (585). This study tested two strains, designated candidate 1 (genotype S2/cre5/S15domV/rec1/hifi3) and candidate 2 (genotype S2/S15domV/CpG40). Both candidates were found to be safe and immunogenic. Fecal shedding was measured by a composite index that accounted for quantity and duration of shedding. This study demonstrated that shed viruses were genetically stable and remained attenuated. The study was conducted in a purpose-built containment facility at the University of Antwerp, Belgium, and dubbed “Poliopolis” in order to ensure strict isolation of subjects shedding virus and prevent accidental release into the environment of any virulent revertants (586). Novel OPV2 candidate 1 was subsequently approved for “Emergency Use Listing” by the WHO (587, 588).
Another approach for mitigating the risk of lack of intestinal immunity with IPV is the coadministration of an adjuvant that enhances the immune response. Previous work with double-mutant heat-labile toxin (dmLT) from ETEC (R192G/L211A) has demonstrated that it is a potent adjuvant when administered orally (589, 590) and that it also has the potential to stimulate mucosal immunity of parenterally coadministered antigens, including IPV (591). This effect has been established in mouse (592) and nonhuman primate preclinical models (B. Norton, unpublished data) and in a recent ETEC subunit vaccine phase 1 clinical study (593).
An ongoing phase 1 study at the University of Antwerp will examine the response of IPV administered with or without dmLT to challenge with bOPV (ClinicalTrials.gov identifier NCT04232943) (594). This study will determine the safety of dmLT when coadministered with IPV, and the efficacy of the adjuvanted vaccine as measured by fecal shedding of bOPV, type-specific fecal IgA, polio neutralizing antibody responses, and other immunological markers.
A similarly designed polio pseudochallenge model study was conducted to support the development of pocapavir, a novel small-molecule antiviral that targets polio and is intended for use in cVDPV outbreaks (595). In this study at the Sahlgrenska University Hospital in Göteborg, Sweden, 144 adults were enrolled, challenged with mOPV1, and then treated with either pocapavir (1,600 mg/day) or placebo. Time to cessation of fecal shedding of mOPV1 was the primary efficacy endpoint. Pocapavir was found to decrease time of shedding by three days compared to placebo (ten versus 13). Unexpectedly, 44% of subjects experienced infection with pocapavir-resistant strains, including several in both the placebo group, as well as in the active treatment group at baseline (i.e., before treatment initiation). The high rate of transmission of resistant strains was attributed to the close quarters of the subjects in the containment facility: for example, sleeping six people to a room and sharing dining and lavatory facilities. When subjects with resistant strains were excluded from the analysis, pocapavir gave a much stronger response and reduced average duration of shedding to only 5.5 days. A follow-up analysis of fecal samples from a subset of volunteers from the placebo group in this study demonstrated that these individuals had neither preexisting intestinal neutralizing antibodies, nor did they mount strongly neutralizing responses after mOPV1 challenge (596), underscoring the vulnerability associated with IPV administration. A recent case study documented the successful use of pocapavir in clearing a vaccine-derived type 3 polio infection in an immunodeficient infant (597), providing additional validation for the role of antivirals in addressing VDPV infections.
(f) Conclusions and future prospects. As the GPEI moves toward global eradication, challenge studies in the target population of infants in LMICs as well as challenge models conducted with adult volunteers will both continue to play crucial roles in developing innovative tools such as novel OPV2 and other interventions that will enable successful completion of this historical global effort. Meanwhile, pseudochallenge models with LORVs have the potential to accelerate development of next-generation parenteral rotavirus vaccines that overcome limitations of the current approaches.
Respiratory Diseases
Influenza
(i) Epidemiology, viral diversity, and public health impact of influenza
Seasonal epidemics of influenza spread rapidly around the world, typically affecting 5 to 15% of the population and causing an estimated 5 million cases of severe infection and 290,000 to 650,000 deaths annually (598). Moreover, four influenza pandemics have occurred in the 20th and early 21st centuries in which a strain emerged that had not previously spread appreciably in humans. These pandemics were associated with high rates of infection, morbidity, and mortality. The 1918 to 1923 “Spanish flu” killed an estimated 50 million people, mostly young adults. Mortality from the subsequent pandemics in 1957 (“Asian flu”), 1968 (“Hong Kong flu”), and 2009 (A[H1N1]) caused 2 million, 1 million, and 0.15 to 0.6 million deaths, respectively (599). While existing vaccines are not fully protective, the impact of their widespread application is notable. For example, during the 2012 to 2013 influenza season in the United States, vaccination was calculated to have prevented 6.6 million influenza virus-associated illnesses, 3.2 million medically attended illnesses, and 79,000 hospitalizations, in spite of the fact that less than 45% of individuals were vaccinated (600).
The influenza virus is diagrammed in Fig. 8. Its genome consists of eight negative, single-stranded RNA segments that are associated with the nucleocapsid protein (NP). The virus has two matrix proteins: M1, which lines the inner surface of the virus envelope and lends rigidity to the structure; and M2, an ion channel-forming peptide that protrudes through the membrane and is partially exposed on the virion surface. The following two proteins coat the outer surface of the virus: neuraminidase (NA), which mediates virus release from infected cells; and hemagglutinin (HA), which binds to the receptor on target cells and mediates virus entry.
FIG 8.
Diagram of influenza virus structural elements.
The HA protein is a major target of influenza vaccines. The HA structure (Fig. 9) is trimeric, with a globular “head” and an elongated “stem” or “stalk.” Each monomer consists of two polypeptide chains (HA1 and HA2) joined by a disulfide bond. HA1 forms the head and is the receptor-binding domain. Broadly neutralizing antibodies against this part of the protein can block receptor engagement. HA2 contains the membrane fusion machinery; a portion of it, called the HA2 endodomain is internal to the virion.
FIG 9.
Structure of influenza hemagglutinin with a globular head domain (red) and elongated stalk domain (green).
Genetic variability is the hallmark of RNA viruses. In influenza, two types of variation occur. “Drift” is when mutations rapidly accumulate in the HA and NA proteins and, to a lesser extent, in structural proteins like the matrix and nucleocapsid. “Shift” is when reassortment of the viral RNA segments occurs. The latter takes place when two viruses infect the same cell and RNA segments of their genomes are randomly packaged into progeny virions. Notably, the highly variable, surface-expressed HA and NA that are key targets of antiviral immunity are encoded on separate RNA segments. Coinfection can generate new HA/NA combinations in a single round of replication.
Influenza viruses can be classified as A or B, based on the amino acid sequences of their HA protein (see Fig. 10). Influenza B is unique to humans, while influenza A is found in humans and a number of animal species, most notably birds and pigs. The influenza A viruses are further classified into groups 1 and 2. Each group is comprised of multiple types that correspond to antigenically distinct proteins (601) and there is further serotype diversity within each subgroup.
FIG 10.
Phylogenetic classification of human influenza A and B viruses according to the hemagglutinin gene.
Influenza A infections in humans include serotypes H1, H2, H3, H5, H7, and H9, which come from both groups of the influenza A viruses. The N1, N2, and N9 serotypes of NA) have been found in viruses spreading in humans. Both strains of influenza B viruses circulate in humans.
Animal reservoirs are a potential source of further diversity of influenza viruses in humans (602). Since the 1918 pandemic, all of the pandemic influenza strains have had two or more genes of animal origin (see Table 25). The 1957 Asian flu strain had HA, NA, and PB1 (an RNA polymerase) from birds. The Hong Kong flu strain had HA, NA, and PB1 genes from birds, but the HA and PB1 were from a different avian source than those in the Asian flu strain. The 2009 influenza A(H1N1) strain replaced all of the genes found in the 1918 Spanish flu strain with those of animal origin. This virus combines genes from two avian and two porcine sources—some shared with previous pandemic strains and others newly introduced from novel avian and porcine sources.
TABLE 25.
Strains of influenza responsible for pandemics spanning a centurya
| Period (yrs) | Pandemic | Strain | Species of origin of HA and NA genes |
|---|---|---|---|
| 1918–1923 | Spanish flu | H1N1 | Human |
| 1957–1958 | Asian flu | H2N2 | Avian |
| 1968–1969 | Hong Kong flu | H3N2 | Avian |
| 2009–2010 | 2009 A(H1N1) | A(H1N1) | Porcine and avian |
HA, hemagglutinin; NA, neuraminidase.
Sporadic human infections with avian, and less frequently, porcine influenza virus continuously occur and are under close surveillance globally. Reassortment with previously human-adapted influenza strains and/or further do novo adaptation of new animal strains to humans are believed necessary to spark global pandemics.
To address the significant genetic variation in influenza viruses, different vaccine approaches have been designed for seasonal and pandemic strains. The unpredictability of pandemic influenza outbreaks has led to influenza vaccine stockpiling against newly identified, novel strains associated with limited outbreaks. For example, the avian influenza strain A(H5N1) none of whose genes match those of previous pandemic strains of avian origin has been newly transmitted to humans. By 2020 the virus (termed “bird flu”) has spread from birds to 861 humans in 17 countries causing 455 deaths (603), a fatality rate exceeding 50%. Presently, influenza A(H5N1) does not easily spread from human to human The United Kingdom stockpiled 17 million doses of a vaccine against this strain, should it begin to spread human to human and spark a new pandemic (604). Another potentially emergent avian strain, influenza A(H7N9), was sporadically observed in humans in mainland China in 2013 and 2014. By 2017, at least 1,564 cases and 612 deaths (a fatality rate of 39.2%) had resulted from this strain (605).
(ii) Influenza vaccines
For decades, influenza vaccines have been made by growing the virus in eggs and inactivating it with formalin or other alkylating agents (Fig. 11). Though highly efficacious, the reactogenicity of unpurified whole-inactivated vaccine led to making “split” preparations, which are better tolerated. More recently, inactivated vaccines are based on the purified HA and NA proteins, which are even less reactogenic (606).
FIG 11.
Inactivated influenza A virus vaccine manufacture.
The great success of live-attenuated vaccines for multiple other viral infections led to sustained efforts for more than two decades to develop live-attenuated influenza vaccines (LAIVs) (607). Aviron (now MedImmune/AstraZeneca, Gaithersburg, MD) introduced the first licensed LAIV in 2003, Fluenz Tetra/FluMist Quadrivalent, based on a cold-adapted A/Ann Arbor/6/1960 strain (608). A similar cold-adapted vaccine based on a Leningrad influenza A strain, termed Ultravac (Microgen, Moscow, Russia), was developed by the Saint Petersburg Institute of Experimental Medicine (IEM) and has been used successfully in Russia since 1987 (609). IEM signed an agreement with the WHO under which they committed to supply pandemic and seasonal candidate vaccine viruses to various developing-country vaccine manufacturers, including China (Changchun BCHT Biotechnology Co., Ltd.), India (Serum Institute of India Pvt., Ltd.), and Thailand (Government Pharmaceutical Organization [GPO]) (610). Serum Institute developed a trivalent LAIV, Nasovac-S, which was proven to be efficacious in studies in children in Bangladesh (611) and was licensed in India and prequalified by the WHO (612). GPO (613) used the Russian strain in the development of a pandemic H5N1 vaccine that was shown to be immunogenic and capable of providing significant priming to a later boost with an H5N1 inactivated vaccine (614). IEM’s findings with an H5N2 LAIV were similar (615). Detailed analysis of the immune responses to LAIVs reveals that while their ability to induce hemagglutination inhibition (HI) and neutralizing antibodies is less than that of inactivated vaccines, LAIVs induce local IgA responses detected in nasal or throat swabs, as well as circulating cytotoxic T-cell responses, which are not commonly observed after inactivated vaccines (616). Despite these advantages, LAIVs are not approved for children under 2 years old, due to increased risk of wheezing. Furthermore, FluMist is not approved for adults over 49 years old because of concerns about decreased efficacy in older individuals.
Genetic drift of the HA and/or the NA genes (driven by the human immune response to natural infection and by vaccination) commonly leads to a need to manufacture new vaccines based on the actual circulating strains. The WHO maintains a worldwide network of laboratories that, together with five central laboratories, characterize up to 30,000 influenza strains yearly. In February of each year, the decision is made on which strains are to be included in the vaccines for the upcoming influenza season in fall and winter. Moreover, in a given year, the seasonal influenza vaccines that are needed may be different for the northern and southern hemispheres (617). In the United States, the FDA requires that seasonal influenza vaccines be relicensed each year (618). Seasonal influenza vaccines are tailored to closely match the circulating strains. Since 2013, they have contained both influenza A and B strains, given their common cocirculation during seasonal outbreaks. Up until a few years ago, seasonal vaccines were trivalent, including an H1, an H3, and a B strain; in the last few years, more than one B strain has circulated, leading to the requirement for quadrivalent vaccines including two A and two B strains. Seasonal vaccines that elicit antibodies to the HA proteins of a hemagglutination titer greater than 1/10 for children and 1/30 for adults are regarded as highly protective against infection and disease.
Currently, more than 25 seasonal influenza vaccines are licensed every year in the United States and 12 are prequalified by the WHO for use in all member countries (619, 620). Most of them are egg derived, but the field is progressively moving toward tissue culture-derived vaccines, which have shown similar profiles of safety and immunogenicity and are less dependent on the growth ability of the virus in eggs and on maintaining egg production and are of more consistent manufacturing and are not contraindicated in individuals allergic to egg proteins. It has been claimed that by circumventing the need for egg culture adaptation, cell-cultured vaccines may result in increased efficacy (621). A cell culture-derived trivalent inactivated influenza virus vaccine produced by Seqirus (headquartered in the United Kingdom) using the Madin-Darby canine kidney cell line was shown to be efficacious in a large efficacy trial with disease endpoints (influenza-like illness) (622). This study was followed by the testing of a quadrivalent version of the vaccine, approved in the European Union (Flucelvax Tetra) and the United States (Flucelvax Quadravalent). The pivotal phase 3 trials of this vaccine in adult and pediatric populations demonstrated the immunogenicity of the quadrivalent cell culture-derived vaccine to be noninferior to that of the trivalent vaccine against shared strains and superior against the influenza B strain absent from the trivalent vaccine (623).
Other approaches to influenza vaccination that have led to licensure include the development of recombinant HA subunit vaccines. Thus, Protein Sciences Corporation (Meriden, CT) developed a trivalent recombinant subunit HA vaccine, Flublok, expressed by a chimeric baculovirus grown in insect cells. The vaccine was well tolerated and immunogenic in adults older than 18 years and even in subjects older than 75 years of age; it demonstrated noninferiority to split vaccines and was shown to be efficacious in a field trial against drifted influenza viruses (624). Flublok was approved by the FDA on a Fast Track Designation. The coverage of this vaccine was recently expanded to include a second influenza B strain to generate Flublok Quadrivalent (625). Of note is the increased immunogenicity of HA subunit vaccines when formulated as nanoparticles, which have potential advantages over inactivated vaccines. Nanoparticles stimulate the innate and adaptive immune systems, exhibit antigen depot effect, extend the period for antigen processing and presentation, and exert an intrinsic adjuvant effect on inducing robust immune responses (626). NanoFlu (Novavax, Inc., Gaithersburg, MD) is a nanoparticle influenza vaccine produced in insect cell lines infected by a chimeric baculovirus carrying the HA genes of the four selected seasonal strains (627). A study of NanoFlu was carried out in 2,652 healthy adults aged 65 and older that received either quadrivalent NanoFlu or Fluzone (Sanofi Pasteur, Inc., Swiftwater, PA). According to a press release, NanoFlu outperformed the Sanofi vaccine on measures of immunogenicity, including significantly higher geometric mean titers and seroconversion rates across all four strains in the vaccines; however, the study results have yet to be published (628).
Meta-analyses across multiple trials of the various seasonal vaccine candidates, including those noted above, reveals little difference with regard to safety or immunogenicity across all the vaccine candidates available. It has been well established that HI titers ≥1:10 are associated with clinical protection, thus demonstrating that a new vaccine induces such titers is frequently sufficient for licensure (629), as long as it is produced in a similar way to vaccines previously licensed on the basis of clinical efficacy. The real challenge for current influenza vaccines is the frequent mismatching between the strains selected for the season and the multiple viral strains that end up circulating, leading to a wide variability in their efficacy. For example, the efficacy of seasonal vaccines ranged from 10% in the 2004 to 2005 season to 60% in the 2010 to 2011 season, with the biggest factor explaining the difference noted as mismatch between the vaccine strains and the circulating strains (630). Between 2000 and 2011, influenza B vaccine strains did not match circulating strains in six influenza seasons. Increased rates of influenza activity were observed in the United States in the fall of 2014, and this was attributed to poor vaccine effectiveness as a result of a mismatch of the H3 component of the current influenza vaccine to circulating strains, leading to an overall effectiveness of 23% (630).
To date, the WHO has prequalified 15 trivalent vaccines, 8 quadrivalent inactivated vaccines, and 1 live-attenuated influenza vaccine. (631), The immunogenicity of seasonal influenza vaccines in children and the elderly, as well as that of vaccines based on pandemic strains, is known to be diminished with respect to that for seasonal vaccines in adults. Several adjuvants have been used to address this limitation, including aluminum hydroxide (alum), MF59, AS03, AF03, virosomes, heat-labile enterotoxin (LT), and more recently the Matrix-M nanoparticle approach by Novavax, Inc. (620). While generally safe and well tolerated, some rare adverse events have discouraged the widespread use of some of these adjuvants; for example, the linkage of LT with Bell’s Palsy.
(iii) Universal influenza vaccines
A large effort is underway to develop vaccines that would not need seasonal reformulation. Immunity to more conserved viral proteins or domains is the target for developing more broadly protective, or even universal, influenza vaccines. The most obvious targets are proteins that are both expressed on the surface of virions (hence accessible to antibodies) and more conserved than the highly variable “head” portion of HA. The HA “stalk,” NA, and the ion channel M2 protein all fall into this category. A vaccine could also target the internal structural proteins of the virus, like NP and M1. In this case, the vaccine-mediated protection would more likely result from the action of cytotoxic T cells. Cytotoxic lymphocytes can specifically recognize and kill virally infected cells when fragments of viral proteins are presented on HLA molecules on the cell surface.
The isolation and characterization of broadly neutralizing antibodies targeting epitopes in the HA stem region is a significant advancement toward identifying targets for universal influenza vaccines. Isolated from the plasmablasts of infected patients or those experimentally vaccinated against the pandemic 2009 influenza A(H1N1) strain (632), these antibodies are extensively affinity matured, suggesting that they originate from memory B cells. Chimeric HA molecules with the head of one strain and the stem of another have been used to separately evaluate the capacity of candidate vaccines to boost antibody responses to the more conserved stem, rather than to the head region (633). A study by Ellebedy et al. found that the best way to boost anti-stem antibody responses was to immunize with a strain to which the volunteers were completely naive. Immunization in a background of preexisting immunity mainly boosted strain-specific responses to the head region (634).
The concept that has emerged from Ellebedy’s research is that vaccines based on influenza strains that have not caused pandemics in humans, but which have sporadically appeared (such as influenza A[H5N1] or A[H7N9] bird flu strains) could establish stronger humoral immune memory to the conserved HA stem. This stronger humoral immune memory to the more conserved portions of HA could, in turn, provide broader protection against pandemic and seasonal influenza strains.
Other conserved antigens that are exposed on the virion surface are being actively investigated as targets of humoral immunity, including the NA (635) and the M2 matrix ion channel protein. A number of subunit vaccines based on the influenza matrix protein are in development (604, 636). Most of them contain only the M2 external domain. This approach is amenable only to influenza A viruses, given that the M2 of influenza B viruses has a different structure and the external portion is too short to be immunogenic.
Other approaches under development include the construction of synthetic peptides that represent mixes or string of B- and/or T-cell epitopes from influenza proteins; epitope-based Multimeric-001 (M-001) vaccine candidate is currently being evaluated in clinical trials. Results from this clinical trial are not yet available (637). A somewhat opposite strategy is the “computationally optimized broadly reactive antigen” approach (638), in which combinations of H1 HA antigens, some naturally derived sequences, and other computer-generated optimal sequences are mixed, with the expectation of inducing responses against diverse H1 influenza strains. Experiments in mice have been promising. A nanoparticle “mosaic” influenza vaccine based on the HA hypervariable receptor-binding domain from sequences of H1N1 influenza strains isolated over a span of more than 90 years is being developed at the National Institutes of Health Vaccine Research Center (639). Another example of epitope-based vaccines is the development of a liposome nanoparticle subunit vaccine by Dhakal et al. (640).
Among the first of the potentially broadly protective candidate vaccines to be evaluated in human challenge studies are those using a vectored approach. The MVA-NP+M1 vaccine expresses NP and M1 from an influenza A(H3N2) strain in the modified vaccinia virus Ankara (MVA) vector. This vector has been extensively attenuated through serial passage in tissue culture. NP and M1 are expressed as a fusion protein. Fifteen volunteers were administered the vaccine, evaluated for cellular immune responses, and challenged 30 days later with an influenza A strain. Three of eleven vaccine recipients and five of eleven controls developed influenza. In vaccine recipients, the symptoms of the disease were blunted and viruses were shed for a shorter period of time (641). A recent study of the MVA-NP+M1 vaccine, newly manufactured on an immortalized cell line, has been reported (642).
The same NP and M1 antigens were expressed in a replication-deficient chimpanzee adenovirus (Ad) vector (643). This vaccine, called ChAdOx1 NP+M1, was evaluated in a phase 1 study, either alone or with an MVA-NP+M1 boost. Although the chimpanzee Ad vaccine produced a similar magnitude of T-cell responses to that seen with MVA-NP+M1, boosting with MVA-NP+M1 did not significantly augment the responses. However, when given in combination with seasonal influenza vaccine, an increase in the antibody response and boosting of T-cell responses were observed (644). A summary of the many novel approaches for influenza vaccine development are diagrammed in Fig. 12 (645). The general expectation is that effective delivery of the wider range of influenza viral antigens that comprise a universal influenza vaccine will likely require novel vaccine types as well (646).
FIG 12.
Range of current and new vaccine approaches against influenza A virus.
Several RNA vaccines for influenza virus are under development. They include synthetic mRNA molecules encoding only the HA, as well as virally derived self-amplifying RNA (sa-RNA) candidates that encode both the antigen of interest and proteins involved in RNA replication. When tested head-to-head, both were protective, but the protection achieved by sa-RNA required only 1.25 μg of sa-RNA compared to 80 μg for the mRNA vaccine. An sa-RNA trivalent vaccine made from H1N1, H3N2 (X31), and B (Massachusetts) protected mice against sequentially administered H1N1 and H3N2 challenges (647).
(iv) Influenza human challenge model
The experience with formal experimental influenza infection to human volunteers is extensive and goes back to 1937, when Smorodintseff et al. (648) infected 72 volunteers with a human influenza virus passaged in ferrets and mice for maintenance. The challenge was safe and well tolerated; only approximately 20% of the subjects had symptoms and they were of mild intensity. Between 1948 and 1988 thousands of quarantined volunteers were infected with respiratory viruses at the UK Medical Research Council Common Cold Unit in Salisbury. Carrat et al. compiled the results of 56 human challenge studies that included 1,280 participants, conducted in the 1990s to early 2000s (649). In most of the studies, volunteers were excluded if they had preexisting antibodies against the challenge strain (usually <1:6). The challenge was mostly given intranasally at doses that ranged from 3 × 1010 to 7.2 × 1010 TCID50 (50% tissue culture infectious dose). Participants were quarantined for seven to nine days and closely monitored for clinical signs and symptoms. Virus shedding was evaluated daily using nasal washes. The review by Carrat et al. provides an overview of important virological and clinical features of influenza infection in individuals with low HI titers to the challenge strain, and over a narrow dose range for the challenge inoculate (649). Overall, 88.2% of challenged volunteers developed influenza infection. Virus shedding was evaluated using nasal washes. Clinical illness was defined by upper and lower respiratory symptoms, ear symptoms, and fever. The clinical and virological features of infection with four different influenza challenge strains are summarized in Table 26.
TABLE 26.
Clinical and virologic features of influenza infection in experimentally challenged human volunteersa
| Challenge strain | No. of volunteers challenged | % infected participants with: |
||
|---|---|---|---|---|
| Virus shedding | Clinical illness | Fever | ||
| Influenza A(H1N1) | 532 | 93.1 | 69.3 | 30.9 |
| Influenza A(H3N2) | 473 | 92.5 | 62.3 | 39.5 |
| Influenza A(H2N2) | 86 | 83.9 | 77.4 | 100 |
| Influenza B | 189 | 81.5 | 62.5 | 7.0 |
Virus shedding was observed in >80% of infected volunteers. Different challenge strains produced somewhat different clinical features. The most variable symptom was fever, which varied from 7 to 100%. Adapted from Carrat et al. (649).
In the early 2000s, influenza human challenge studies came to a halt as a consequence of an adverse outcome in a 21-year-old healthy subject challenged with influenza B in an investigation of peramivir for influenza prophylaxis. The subject presented asymptomatic echocardiogram changes on day 4 after challenge that returned to normal by day 15, but after traveling to Indonesia, where he developed an upper respiratory tract infection, an echocardiogram (taken 51 days after challenge) revealed a reduced ejection fraction, though the subject was symptomatic. Extensive work-up for infectious etiologies revealed nothing, and repeated echocardiograms showed gradual improvement and return to normal. In spite of the lack of direct causality linking myocarditis to the influenza challenge, no further influenza challenge studies were conducted in the United States for nearly a decade (650).
(v) Learning about influenza pathogenesis and immune responses from challenge studies
Important insight into the pathogenesis of influenza viruses has been gained from challenge studies as follows.
(a) On reinfection. Memoli et al. (651) reported the outcome of an intranasal challenge (a rechallenge experiment with influenza strain, A[H1N1]pdm09). Seven subjects challenged with the virus were rechallenged 1 year later. At least three of the seven participants had evidence of repeat infection, and five of the seven showed clinical evidence, demonstrating that sequential reinfection with the same virus may not be a rare phenomenon, which raises questions about the strength of the immune memory induced by infection.
(b) On genetic evolution. Considerable viral evolution occurs during experimental infection with H3N2. In a study by Sobel et al. (652) in which the viral stock contained genetic variants that originated during the passaging process the direct intranasal inoculation resulted in a bottleneck that reduced nonsynonymous genetic diversity in the HA and NP proteins. Intrahost viral evolution continued over the course of infection. A separate study Xiao et al. examined the genetic evolution of the virus in 15 subjects receiving influenza A/California/04/2009(H1N1) and compared it to that of 5 naturally infected subjects, demonstrating that intrahost evolution is different for challenged and naturally infected subjects (653).
(c) Clinical outcomes. Critical insight into what drives influenza-related symptomatology were discerned by inoculation of influenza A/Wisconsin/67/2005 (H3N2) in 17 volunteers. Clinical symptoms were observed in nine of them. Serially drawn blood samples were tested for 25 cytokines, revealed increased levels of IL-6, IL-8, IL-15, monocyte chemotactic protein 1 (MCP-1), and IFN-γ-induced protein 10 (IP-10) as early as 12 to 29 h postinoculation (before symptoms appeared). No such changes were observed in the subjects that remained asymptomatic. Some inflammatory mediators (MCP-1, IP-10, and IL-15) were expressed in circulating cells, while others (IL-6, IL-8, IFN-α, and IFN-γ) were produced at the site of infection (654). A separate transcriptome analysis of peripheral blood was conducted in 41 volunteers that received an intranasal challenge with influenza A (A/Brisbane/59/2007[H1N1] or A/Wisconsin/67/2005[H3N2]) (655). Eighteen (44%) developed symptoms. A gene signature for symptomatic influenza capable of detecting 94% of infected cases was identified from the analysis. The gene signature can be detected as early as 29 h postexposure, about 38 h before peak of clinical symptoms. When used as a tool to investigate patients admitted with clinical influenza, the gene signature discriminated between 2009 influenza A(H1N1)-infected and noninfected individuals with 92% accuracy.
(d) Transmission. The human challenge model for influenza has also been used to study person-to-person transmission of (H1N1)pdm09 providing objective evidence of the effectiveness of such measures as hand-washing and protective masks to prevent influenza transmission (656, 657).
(e) Immune response and correlates of protection. Huang et al. (658) examined the kinetics and magnitude of the B-cell and antibody responses in relation to clinical symptoms and viral shedding in 12 subjects experimentally infected with an influenza A/Brisbane/59/2007(H1N1) strain. The volunteers had little or no detectable HI antibody prior to challenge but developed increasing levels of IgG antibody-secreting B cells within 7 days of exposure, which was taken as evidence for an anamnestic immune response. At the time this response appeared, HI antibodies were still undetectable. An inverse correlation was seen between the magnitude of the response and the viral load and duration of shedding. The quick induction of a recall response suggests that establishing a memory response by vaccination may be feasible (658). Also of interest is the observation that HI antibodies were not well represented in the memory B-cell compartment. Preexisting influenza-specific CD4+ T cells to the nucleoprotein and matrix were correlated with protection from disease in a study conducted by Wilkinson et al. (659). An influenza A(H3N2) strain was used for challenging volunteers previously screened to select those with no detectable antibody to the challenge virus. By day 7 postchallenge, large increases were seen in influenza-specific T cells, indicative of a recall response. Virus shedding and severity of disease were correlated with preexisting CD4+ T cells, but not with CD8+ T cells. The CD4+ T cells showed evidence of cytotoxic activity and responded to peptides from homologous, but also from the heterologous, pandemic strain influenza A(H1N1).
(f) Correlates of protection. In an attempt to discern correlates of protection, Memoli et al. administered wild-type 2009 A(H1N1)pdm influenza A challenge to individuals with HI titers of either >1:40 or <1:40 (660). HI titers of >1:40 were protective against mild and moderate influenza disease. While the baseline HI titer correlated with some reduction in disease severity measures (>1:40 titers), the baseline titers for NA inhibition were better correlated with disease severity. However, in a separate challenge study conducted by Gould et al. using the influenza A/California/2009(H1N1) strain among volunteers preselected for low serum HI titers, no correlation was observed between the titers and outcome of the challenge. On the other hand, a weak, but statistically significant correlation was found between nasal and serum IgA and the duration of nasal shedding, pointing to mucosal IgA as a potential correlate of protection (661).
Park et al. explored the potential correlation between anti-influenza hemagglutinin stalk antibodies and protection from A(H1N1) influenza challenge in 65 volunteers (662). The level of anti-stalk antibodies increased in 64% of the subjects after challenge; however, baseline anti-stalk antibody titers did not correlate with the symptoms induced by the challenge.
Antibody-dependent cellular cytotoxicity (ADCC) has also been proposed as a correlate of protection for influenza and tested in a human challenge study conducted by Jegaskanda et al. (663). Increases in titers of ADCC-mediating antibodies to recombinant HA or to virus-infected cells were observed in adults and children that received LAIV as well as in experimentally infected adults. Preexisting ADCC titers of ≥320 were associated with lower virus replication and a significant reduction in symptoms.
Taking advantage of the recommended use of LAIV in children, Wright et al. used the vaccine as a pseudochallenge agent to examine potential correlates of protection in children (664). After the children were intranasally immunized with LAIV, a second dose of the same vaccine or of an inactivated influenza vaccine was provided. The patterns of immunity induced by the two vaccines were very different; however, the outcome of the second LAIV “challenge” did not correlate with serum or mucosal responses to the vaccine as tested with a battery of assays that included cytokine and chemokine levels. The authors concluded that the mechanism of protective immunity to LAIV could not be defined with traditional methods.
(g) Therapeutics. The influenza challenge model was also employed to assess the therapeutic efficacy of a monoclonal antibody against the influenza hemagglutinin stalk region (MHAA4549A). In a study of 100 subjects challenged with an H3N2 virus (influenza A/Wisconsin/67/2005), the antibody, which was provided to the subjects 24 to 36 h after the challenge, was well tolerated and significantly reduced the viral load and tissue culture infectivity of nasopharyngeal secretions. In addition, it had an impact on influenza symptoms, duration of shedding, and levels of inflammatory markers (665).
(vi) Challenge strains currently under development
Investigators at the U.S. National Institutes of Health and Pennsylvania State University used cloned wild-type A(H1N1) strains manufactured under Good Manufacturing Practice (GMP) to conduct a dose-escalation study in healthy adult volunteers in a quarantine unit (666). Similarly, Watson et al. developed an H1N1 challenge in the United Kingdom (667) using influenza strain A/California/2009(H1N1). The agent was manufactured under current GMP conditions and characterized in accordance with regulatory guidelines. A dose-ascending, open-label clinical study was conducted in 29 healthy young adults seronegative to the challenge strain with three increasing doses of virus. Subjects were challenged intranasally at various doses of the virus and monitored for clinical symptoms, immunological responses, and viral shedding. A dose-dependent increase in clinical signs and symptoms occurred in 75% of subjects receiving the highest dose (3.5 × 106 TCID50), classified as mild (all subjects), moderate (50% of subjects), and severe (16% of subjects); symptoms peaked 4 days after infection. Physician-observed clinical signs were correlated with nasal mucus weight (P < 0.001) and subject-reported symptoms (P < 0.001). Viral shedding peaked at log10 5.16 TCID50 3 days after inoculation and was maintained for approximately 5 days. Symptoms and signs were limited to the upper airways and were of insufficient severity to be of clinical concern.
Using reverse genetics, Memoli et al. developed a GMP-produced wild-type 2009 A(H1N1)pdm virus to be used as an intranasal challenge agent (666). Escalating doses were tested to identify a dose leading to signs of disease in at least 60% of volunteers. A dose of 107 TCID50 caused mild to moderate disease in 69% of subjects. Viral shedding lasted four to five days and significant antibody titer rises were induced by the challenge. Of interest, viral shedding preceded symptoms by 12 to 24 h and terminated 2 to 3 days prior to symptom resolution, indicating that individuals may be infectious before development of symptoms. Most common symptoms were nasal congestion and rhinorrhea, with fever observed in only 10% of the subjects. The Memoli group also developed an influenza A/Bethesda/MM1/H3N2 under GMP (668). Like the H1N1 predecessor, it was used in a dose-escalation challenge trial at doses from 104 to 107 TCID50. Clinical symptoms, viral shedding, and immune responses were evaluated. Only the two higher doses tested (106 and 107) resulted in disease and viral shedding. Of 37 participants challenged, 16 (43%) had viral shedding, and 27 (73%) developed symptoms, with 12 participants (32%) experiencing mild to moderate influenza disease, defined as shedding and symptoms. Similar to the H1N1 challenge, viral shedding was seen one to two days after challenge, preceding the development of clinical symptoms, which peaked on day 3. Only 10 participants (29%) had a ≥4-fold rise in HI antibody titer after challenge. The fact that preexisting HI antibodies were not present in these subjects (by study design) may indicate that preexisting immunity factors other than anti-HA antibody may limit shedding of the two viruses. Nonetheless, this A/Bethesda/MM1/H3N2 challenge virus can be utilized in future studies to further explore pathogenesis and immunity and to evaluate vaccine candidates.
Fullen et al. (669) reported the GMP manufacture of a separate wild-type H3N2, the A/Perth/16/2009(H3N2) strain. Four escalating doses were studied in six volunteers each; infection was observed in approximately 80% of subjects receiving 2.5 × 104 to 4.7 × 106 TCID50 of virus. Symptoms, including runny nose and sneezing, developed more rapidly with the higher doses. Muscle aches and sore throat were only seen with the higher titer, but viral shedding was observed frequently.
As inferred from the studies above, the choice of challenge dose is one of the most critical steps in controlled human infection model studies. Obviously, wild-type virus strains leading to serious infections cannot be used; at the other end, excessively attenuated strains may not be useful to recapitulate even mild cases of infection (670). As an example, a challenge study by Ramos et al. (671), in which a monoclonal antibody was tested, was hard to interpret because the A(H3N2) strain used was overly attenuated or the challenge dose was too low. Only 2 of 24 control (placebo) participants (8%) reported fever, and their nasal secretions averaged only 6 g. In comparison, in a separate challenge study evaluating oseltamivir for efficacy, 12 of 33 patients (36%) receiving placebo had fever after challenge, with influenza A/Texas/36/91(H1N1) virus, and the mean weight of nasal secretions collected was 12 g (672).
As the field moves forward in developing additional influenza challenge models, it is critical to ensure consistency, as much as possible, across the criteria for selection of volunteers; the way in which the inoculum is administered; the collection, treatment, and storage of the samples; clinical scoring of disease severity; laboratory evaluations; etc., in order to properly analyze the data and compare results across studies (673).
(vii) Evaluation of novel vaccines in human challenge studies
Evaluation of vaccine efficacy is the primary goal of developing challenge models, and now that several challenge agents are available, the time is ripe to conduct vaccination challenge studies. A few examples are discussed below.
Lambkin-Williams et al. evaluated the efficacy of aerosolized split inactivated vaccine in a challenge model that uses the A/Panama/2007/1999(H3N2) challenge strain. Subjects immunologically naive to the virus were dosed via nasal spray with proteosome-adjuvanted trivalent influenza vaccine or placebo (674). Vaccine doses of 15 and 30 μg were given either once or twice 14 days apart, and the subjects were challenged with A/Panama/2007/1999(H3N2) 5 weeks later. Immune responses were measured by HI and nasal wash secretory IgA. In all, 57 to 77% of subjects seroconverted to the vaccine, which exhibited 58 to 82% efficacy against any influenza symptoms with seroconversion, 67 to 85% for systemic or lower respiratory illness and seroconversion, and 65 to 100% for febrile illness and seroconversion. The two-dose regimen was found to be superior to the single-dose regimen. The protection observed significantly correlated with prechallenge HI and mucosal secretory IgA titers.
Van Doorn et al. (675) reported the results of a trial of a vaccine candidate termed FLU-v, a peptide vaccine developed by PepTcell (now SEEK, London, United Kingdom) and designed to provide a broadly protective cellular immune response against influenza A and B. Study subjects received one dose of either the vaccine with adjuvant or the adjuvant alone (16 subjects per group) and were challenged 21 days later with A/Wisconsin/67/2005(H3N2) and followed for seven days postchallenge. FLU-v was safe and well tolerated. Subjects in the vaccine group developed FLU-v-specific IFN-δ responses (8.2- to 3.9-fold versus 1.3- to 0.1-fold higher than the control group). The cellular responses observed correlated with reductions in both viral titers and symptoms.
Powell et al. used a challenge model to evaluate the quality of influenza-specific T-cell responses in subjects receiving an MVA vaccine expressing the influenza NP and M1 genes (MVA-NP+M1) (676). The vaccine induced increased expression of granzyme A, perforin, and CD57 on HLA A*02 cells recognizing the M1 58-66 peptide before and after challenge. Similar phenotypic changes have been associated with protection against influenza in other studies. The corresponding efficacy data were published separately (604). Two of eleven vaccinees and five of eleven control subjects developed laboratory-confirmed influenza, including symptoms and virus shedding; the symptoms were less pronounced in the vaccinees.
(viii) Summary
The human challenge models for influenza are safe and provide high rates of infection and disease. They also provide a consistent clinical course and a range of challenge strains with which to evaluate candidate vaccines. Since field trials will only be able to evaluate vaccine-mediated protection against the currently circulating strains, challenge models could be a practical way of comparing the breadth of protection against different influenza serotypes. Efforts to identify target antigens for a universal vaccine are well underway, but the most advanced candidates are still in early stages of development. Universal vaccines will likely include vectored or subunit vaccines, which are unlike the whole-inactivated or live-attenuated seasonal vaccines in current use. The main reason for an approach that differs from seasonal vaccines is because immune responses to the more conserved viral proteins are thought to be subdominant. Eliciting them may require that highly variable proteins (such as the HA1 protein, to which the dominant responses are directed) be removed from some of the vaccines.
Respiratory syncytial virus
(i) Epidemiology, diversity, pathogenesis, and public health impact of respiratory syncytial virus
Respiratory syncytial virus (RSV) is one of the leading causes of mortality in infants and the elderly. A meta-analysis of available data has indicated that, in the absence of effective interventions, 34 million new infections with RSV can be expected each year, leading to 3.4 million hospitalizations and 200,000 deaths (677). Ninety-nine percent of those deaths will occur in low-resource countries around the world. No vaccine or effective drug exists against RSV.
RSV is an enveloped virus with a single-stranded RNA genome. The virus structure is shown in Fig. 13 (678). RSV has three transmembrane glycoproteins that are key targets of antiviral antibodies: G, the attachment protein; F, the fusion protein; and SH, a small hydrophobic protein of unknown function. Inside the virion are the matrix protein M and two regulatory proteins called M2-1 and M2-2. The RNA genome is associated with three proteins: nucleocapsid protein (N), a phosphoprotein (P), and a polymerase (L). The genome also encodes two nonstructural proteins called NS1 and NS2.
FIG 13.
Structural diagram of respiratory syncytial virus.
Two distinct genogroups of RSV are found in humans, termed A and B (679). The genogroup A and B viruses have been analyzed with respect to which of the viral proteins are the most variable between the two genogroups. The G proteins of RSV A and B are the most divergent, with only 53% homology between the two genogroups. The SH and NS1 proteins of RSV A and B strains have 76 and 87% homology, respectively. The F protein has 89% homology between the RSV genogroup A and B viruses (3).
The global epidemiology of RSV infection in infants and young children is incompletely described with respect to the circulation of RSV genogroups and genotypes (680). Overall, about two out of three infections are genogroup A and one out of three are genogroup B. Data from the United States and Europe indicate that RSV epidemics are seasonal and that strain variation often occurs from season to season (681).
The majority of individuals become infected with RSV in early life. By 2 years of age, more than 80% of children around the world will have experienced RSV infection, two-thirds of them under 1 year of age (682). After infection with RSV, protective immunity is incomplete and short lived. The results from experimental human challenge with RSV suggest that previously infected individuals are susceptible to reinfection (683); however, there are limited data from natural infections to confirm this.
RSV infections are associated with a broad spectrum of disease (6). In infants, about two-thirds of infections are limited to the upper respiratory tract, while one-third of infections also involve the lower respiratory tract. In the two-thirds of infants with upper respiratory infection, symptoms of disease are mild, including runny nose and ear infections. The disease is usually self-limiting. Lower respiratory tract infections (LRTIs) with RSV often lead to bronchiolitis, a condition in which inflammatory immune cells infiltrate the air spaces, resulting in mucus production, shedding of damaged epithelial cells, and edema of the wall of the airway. The resulting obstruction of the airway is the major cause of infant mortality from RSV (6). RSV infections are also important causes of morbidity and mortality in the elderly, where repeated episodes of RSV infection may occur in a given individual within a single year (684).
The status of the human immune system is thought to have a major impact on the severity of disease. Our current understanding suggests that differences in the innate immune response to infection in infants under 1 year of age, compared to immune response in older children, underlie the greater mortality of RSV infection in young infants (6). Coinfections with bacterial respiratory pathogens including pneumococcus and Haemophilus are risk factors for severe disease in young children (685–688). The mortality of RSV in older adults is ascribed to weakened immunity, which permits reinfection even in the short period after an episode of RSV, when there is usually a level of protective immunity. Since protective immunity is incomplete and reinfections are common, human infections with RSV and the immune response generated provide few clues to guide the development of RSV vaccines and offer little information about the impact of RSV’s genetic diversity on protective immunity.
(ii) Respiratory syncytial virus vaccine development
RSV vaccine development has proceeded cautiously since an initial serious misstep in the 1960s, when the administration of a formalin-inactivated RSV (FI-RSV) vaccine candidate to infants and children was associated with more severe disease upon subsequent RSV infection. In that trial, 26% of vaccine recipients were hospitalized with severe lower respiratory infections and two deaths were attributed to vaccination (689, 690).
The basic scientific explanation underlying the enhanced disease seen in infants in the FI-RSV vaccine trials continues to be rigorously pursued. Much has been learned in the last 50 years about protective, as well as dysregulated, immunity in RSV infection (691). Protecting against severe RSV disease with antibodies is clearly possible under some circumstances. The RSV-neutralizing monoclonal antibody directed against the fusion (F) glycoprotein, palivizumab, reduces hospitalization for bronchiolitis by 50 to 60% in RSV-infected infants when passively administered in the first few months of life. Neutralizing antibody titers in recently infected adults may reduce the symptoms of subsequent infection, but the protective effect of neutralizing antibodies is short lived (691).
Considerable evidence supports the hypothesis that T-cell immunity is dysregulated in RSV infection (6, 691). The type 1 interferons are key innate defenses against viral infections, and the NS1 and NS2 proteins of RSV suppress production of type 1 IFNs by immune cells. RSV infection of lung epithelial cells in vitro sends inhibitory signals to CD8+ T cells, which would otherwise eliminate virus-infected cells. The immune response to the harmful formalin-inactivated vaccine was biased away from a normally protective Th1 type, cytotoxic T-cell response and toward Th2 and Th17 responses. The catalogue of potential interactions of RSV with the human immune system is long (6) and understanding is still limited.
RSV infection itself does not lead to the enhanced disease upon reinfection that was seen with the FI-RSV in the 1960s. Accordingly, subsequent RSV vaccine development was refocused toward live-attenuated RSV vaccines, which should provoke immune responses more similar to those following natural infection. The collective safety experience with this approach has been acceptable, with no evidence of enhanced disease upon first RSV infection in several hundred study participants that participated in trials of live-attenuated RSV vaccines, as determined through extended follow-up (692).
The first series of live-attenuated vaccines to be developed were cold-passaged, temperature-sensitive (ts) mutants of an RSV genogroup A strain (693). Use of attenuated viruses with a ts phenotype is intended to restrict viral replication to the upper respiratory tract. One vaccine, called cpts-248/404, was considered sufficiently attenuated for more extensive evaluation in infants and young children. The vaccine was infectious in the absence of preexisting immunity to RSV but did not infect RSV-seropositive children. The vaccine did not cause lower respiratory tract illness but did induce a level of nasal congestion in the youngest age group that interfered with feeding.
The second series of live-attenuated RSV vaccines used reverse genetics, whereby attenuating mutations and deletions could be specifically introduced into viral genome (694). Mutations were inserted individually or in combinations stepwise in a series of viruses that were progressively more attenuated. Several of these constructs have entered clinical trials. While the initial constructs yielded a highly attenuated virus that was well tolerated, safe, and moderately immunogenic, evaluation of nasal wash isolates from recipients identified a number of specimens exhibiting loss of the ts phenotype. Constructs to stabilize them were subsequently evaluated separately; for instance, in a study of the strain RSVcps2, 25 of 34 vaccinees (85%) were infected by the vaccine, and 77% shed vaccine virus, but only 59% developed a ≥4-fold rise in RSV serum-neutralizing antibody titers (695). Reversal of the attenuation was not detected.
Additional mutations to stabilize the ts phenotype include deletion of either the M2-2 gene (696) or the NS2 gene (697). These candidates have shown acceptable stability in vitro and have been tested clinically in infants and young children. In the clinical trial, M2-2-deleted virus was shed by 95% of vaccinees; approximately 90% presented a ≥4-fold increase in neutralizing antibodies. NS2 is an interferon antagonist whose deletion diminishes RSV replication, while the increased interferon response to infection may enhance the adaptive immune response. Mutations in the NS2 gene (an IFN antagonist) accompanied by an additional deletion of codon 1313 was restricted in replication, well tolerated, immunogenic, and primed for potent antibody responses after natural exposure to wild-type RSV. At a dose of 105 PFU, the vaccine was overattenuated, but at a 106 PFU dose it was well tolerated, able to replicate in 90% of vaccinees, and immunogenic (80% of the initially seronegative children [1:64]).
A double M2-2/NS2 construct (698) tested one intranasal dose (105 PFU) of the double mutant (n = 21) or placebo (n = 11) in children 6 to 24 months old. All 21 vaccinees were infected with vaccine (based on either shedding or rise in serum RSV antibodies), and 20 shed the virus (based on positivity of nasal wash by both immuno-plaque assay and quantitative PCR). Serum RSV-neutralizing antibodies and anti-RSV F IgG increased ≥4-fold in 95 and 100% of vaccinees, respectively. Mild upper respiratory symptoms and/or fever occurred in 76% of vaccinees and 18% of placebo recipients.
In spite of the several advances made to developed attenuated strains, safety concerns and the limited market existing in industrial countries have deterred the development of any of the candidates discussed above in advanced clinical trials.
Due to successful prophylactic treatment of RSV infection with the F protein-directed antibody palivizumab, several groups have taken a structural-based approach to create nanoparticles, bacterium-like particles, and subunit vaccines using engineered forms of RSV F protein, hoping to elicit a similar protective response against severe disease (699–702). The F protein exists in a metastable prefusion and stable postfusion state, with both epitopes targeted by neutralizing antibodies in natural infection; however, the prefusion form is the major target. In preclinical studies, immunization of mice and rhesus macaques with an F protein containing a stabilized antigenic site Ø (a conformation exposing multiple strong antigenic targets), elicited significant RSV-specific neutralizing activity (702). GlaxoSmithKline (formerly Novartis) is developing both postfusion F protein (703, 704) and an uncleaved monomeric form of F protein that retains the prefusion antigenic targets as subunit vaccine candidates.
Since the disease burden and mortality from RSV is highest in early infancy, RSV vaccines eliciting strong RSV-neutralizing responses are attractive candidates for maternal immunization so that high levels of protective antibodies can be passively transferred to infants before birth. Novavax completed the first maternal immunization efficacy trial of their nanoparticle RSV vaccine in approximately 4,600 pregnant women. The results from this multicountry, randomized, controlled trial showed the vaccine was safe, but it failed to meet its primary efficacy endpoint (705). The observed global vaccine efficacy in reducing medium to severe RSV LRTI was 39.4% (95% CI = 5.3 to 61.2), with 44.4% (95% CI = 19.6 to 61.5) reduction in hospitalizations. While considerably more efficacy was observed in South Africa subcohorts, the study was not sufficiently powered to establish if the higher efficacy reflected variations within the confidence interval. Of note, two additional prespecified endpoints showed vaccine efficacy: 25.3% against all-cause LRTI and 39.1% against all-cause LRTI with significant hypoxemia (<92% oxygen saturation). Other maternal immunogens include vaccine candidates from GlaxoSmithKline (706), Pfizer (707), and the NIAID Vaccine Research Center (708).
A number of vectored and subunit vaccines against RSV are in the preclinical and clinical stages of development. These are reviewed by Loomis and Johnson (709), Morrison and Walsh (710), and Aranda and Polack (711). Some examples of candidate RSV vaccines are shown in Table 27. The current vectored approaches reaching the clinics include adenovirus, bacillus Calmette-Guérin, and Sendai virus vectors expressing RSV proteins, particle vaccines including nanoparticles, and live-attenuated vaccines. These candidate vaccines may enable the evaluation of individual RSV genes for their capacity to elicit and propensity to interfere with cellular and humoral anti-RSV immune responses. They can also contribute to rational antigen selection for a new generation of candidate RSV vaccines.
TABLE 27.
Examples of candidate respiratory syncytial virus vaccines in developmenta
| Vaccine type | Vaccine(s) | Developer(s)b | Phase |
|---|---|---|---|
| Live attenuated | Ts, ΔNS, ΔM2, other | LID/NIAID/NIH; MedImmune, LLC; Sanofi Pasteur | 1 |
| Live chimeric | BCG-RSV | Pontificia Universidad Católica de Chile | 1 |
| Sendai-RSV | St. Jude Children’s Research Hospital, United States | 1 | |
| Particle based | Nanoparticle | Novavax, Inc.* | 3 |
| Subunit protein | F | GSK* | 2 |
| Pfizer, Inc.*; Janssen Vaccines; VRC/NIAID/NIH* | 1 | ||
| G | Beijing Advaccine Biotechnology | 1 | |
| Recombinant vectors | Adeno | Vaxart, Inc. | 1 |
| GSK; Janssen Vaccines | 2 | ||
| Modified vaccinia virus Ankara | Bavarian Nordic A/S | 2 |
BCG, bacillus Calmette-Guérin; GSK, GlaxoSmithKline; LID, Laboratory of Infectious Diseases; NIAID, U.S. National Institute of Allergy and Infectious Diseases; NIH, U.S. National Institutes of Health; RSV, respiratory syncytial virus; VRC, Vaccine Research Center. From the PATH Vaccine Resource Library (928).
*, Development of target protective effects in infants by immunizing pregnant women in the third trimester.
(iii) Respiratory syncytial virus human challenge model
The development of a human challenge model for RSV began in the 1970s (see Table 28). The initial work with the model was facilitated by the availability of a challenge pool of an RSV Genogroup A, genotype 2 virus that had been passaged multiple times in tissue culture (712). From 1981 to 2004, human challenge studies were conducted to investigate the infectivity of RSV by different routes of inoculation (712) and evaluate the relationship of the challenge dose to symptoms of disease, virus shedding, and the development of neutralizing antibodies (713).
TABLE 28.
Respiratory syncytial virus challenge strainsa
| Challenge strain | Method of production and administration | Stock producers |
|---|---|---|
| RSV A2 | An RSV Genogroup A strain was isolated from a pediatric patient in Melbourne, Australia. The virus was expanded by three passages in Vero cells in culture. Healthy adults were challenged via intranasal instillation. | Walter Reed Army Institute of Research and U.S. National Institute of Allergy and Infectious Diseases |
| RSV A Memphis-37 | An RSV genogroup A strain was isolated from a pediatric patient in Tennessee, United States, and produced in Vero cells. Healthy adults were challenged via intranasal instillation. | Meridian Life Sciences, Inc., and Retroscreen Virology, Ltd. |
RSV, respiratory syncytial virus.
Studies with the RSV challenge model waned for nearly a decade, but by 2010 the model had undergone further development with the availability of a new challenge strain called Memphis-37 (714). As described in Table 28, Memphis-37 is a contemporary clinical isolate that was minimally passaged in cell culture.
DeVincenzo et al. (714) used a Memphis-37 challenge strain manufactured under GMP to evaluate the relationship of challenge dose to infection rate, viral load, and severity of disease; 36 healthy adults with exceptionally low neutralizing antibody titers against RSV were quarantined for 13 days following intranasal inoculation of the virus. Over a 500-fold dose range, 77% of volunteers became infected, no significant association was detected between challenge dose and infection rate or peak viral load. Viral load correlated with the severity of disease, which was limited to the upper respiratory tract and the weight of nasal mucus secreted. The production of cytokines after challenge was also evaluated in the study in order to gather evidence pertaining to the prevailing view of RSV immune pathogenesis as an aberrantly expanded Th2 response. Two of the Th2-biased cytokines (IL-4 and IL-10) did not correlate with disease severity, while the levels of several other proinflammatory cytokines and chemokines did track with viral load and disease severity. They suggested that, rather than an immune mechanism, viral load may be the main correlate of the severity of disease. Perhaps important, however, is that the study volunteers may not be representative of the general population with respect to preexisting immunity to RSV.
The newly developed Memphis-37 RSV challenge model has achieved a high attack rate and high degree of consistency in the disease course observed, has established objective and subjective measures of disease severity; and has benefitted from the use of a well-characterized, safe, and appropriate challenge strain manufactured under GMP. However, it is noteworthy, however, that the peak viral load in adults after challenge was ~50-fold lower than that seen in naturally infected infants, which may be explained by the presence of preexisting cellular and humoral immunity that was present even in volunteers with low preexisting serum neutralizing antibodies.
A full genome sequence of Memphis-37 was recently published (715) showing that Memphis-37 is a representative strain within the RSV A genogroup. Of interest, the G protein of RSV A viruses contains a highly conserved amino acid motif that mimics a human chemokine. This mimicry may interfere with leukocyte migration to the lung (716).
The Memphis model has been used for the assessment of anti-RSV drugs, including GS-5806, a viral entry inhibitor that blocks the fusion of virus with the cell membrane (717), as well as JNJ-53718768, another fusion inhibitor. GS-5806 was associated with reduced viral load, mucus weight, and respiratory tract symptoms. A limitation of using the challenge model to assess antivirals is the narrow window for testing the drugs; they have to be given very early to see any effect, while in the case of a natural infection they will likely be given at a point when the disease is already established.
The Memphis-37 model will be of great value to assess the ability of vaccine to block infection as opposed to prevent severe disease. The new wave of vectored and subunit RSV vaccines in preclinical development could benefit from the use of human challenges. Such studies could evaluate the potential of individual RSV proteins, presented to the immune system in different ways, to maintain a high safety profile while overcoming the interference with effective immunity that seems to be a hallmark of RSV infection.
Until recently RSV challenge models had not been applied to vaccine development, in part because of the lack of induction of lower respiratory tract disease; however, a recent study demonstrated the value of the model in vaccine development. Sadoff et al. (718) challenged 53 subjects intranasally with the Memphis 37b RSV strain; 27 of them had received a single intramuscular dose of an Ad26 recombinant virus carrying a prefusion construct of the RSV F protein and had 26 received placebo four weeks earlier. RSV neutralizing antibody titers increased 5.8-fold by 28 days after vaccination. Postchallenge results include a minor drop in RSV infection rates (65.4% among placebos versus 40.7% among vaccinees); however, viral load (VL-AUC: area under the curve of qRT-PCR and quantitative culture) and disease severity were lower among the vaccinated subjects in comparison to the placebo recipients as follows: median VL-AUC qRT-PCR, 0.0 versus 236.0; median VL-AUC quantitative culture, 0.0 versus 109; median RSV AUC clinical severity score 35 versus 167). The study represents a breakthrough that would hopefully lead to other developers using the model.
In addition to its practical use in drug and vaccine development, our understanding of the early immune response to RSV has advanced considerably thanks to the use of the challenge model. An interesting finding is the absence of IgA memory cells after experimental infection, which matches with the known poor and short-lived protection derived from infection (719). Nonetheless, a tight connection exists between IgA and protection from infection, since individuals that do have preexisting mucosal IgA appear to be protected from infection. However, among those that become infected, preexisting IgA levels did not influence the outcome of disease. These findings have been confirmed, with the addition that circulating neutralizing antibodies are also (along with IgA) associated with lower infectivity and higher viral replication. Again, when subjects are infected, no significant protection from disease evolution is observed. When subjects with very low preexisting titers of neutralizing antibody were selected and challenged with RSV, 35 of 42 (77%) were infected. Here again, prechallenge nasal IgA antibodies, as well as neutralizing antibody, were lower among those infected. A trend between the diminished mucosal IgA and disease outcome was suspected but not found. In this study as well, no correlation was found between prechallenge IgA levels and disease outcome.
The relationship between T-cell immunity and RSV infection has been difficult to understand. While it is suspected that depletion of T cells (e.g., immune-compromised subjects) increases the susceptibility to infection, T cells have also been associated with disease enhancement. Jozwick, Chiu, and their group at Imperial College (720) investigated systemic and local RSV-specific CD8+ T cells in 49 RSV-challenged subjects: 26 (53%) developed infection (PCR positive), with viral load peaking at approximately 7 days; 17 suffered symptoms of the common cold (lower respiratory tract); and nine had no symptoms. From 24 of the subjects, they were able to obtain serial samples of bronchial brushings, biopsy specimens, and lavages, which were examined for RSV antigen-specific CD8+ T cells using peptide-specific tetramers. Inflammation was observed in the materials collected and antigen-specific CD8+ T-cell proliferation and activation was present. Circulating antigen-specific activated CD8+ T cells also expanded within ten days of the challenge and viral load measures were inversely correlated with CD8+ proliferation, insinuating a role for CD8+ T cells in detaining viral infection. The subset of resident memory CD8+ T cells was selectively expanded and associated with reduced disease. Rapid recovery was observed in spite of the extensive lower airway inflammation with persistent viral antigen and cellular infiltrates.
The Imperial College team investigated the presence, frequency, and specificity of CD4+ T cells in bronchoalveolar lavage from subjects exposed to the same Memphis-37 challenge strain (721). The frequency of RSV-specific CD4+ T cells strongly correlated with local C-X-C motif chemokine. The team was able to identify 39 epitopes in the virus and quantitate five of them using MHC-II tetramers. The tetramers revealed enrichment of resident memory CD4+ T cells in the lower airway. These cells displayed progressive differentiation, downregulation of costimulatory molecules, and elevated CXCR3 expression.
(iv) Summary
The development of RSV vaccines has been particularly challenging. The obstacles have been many, including the apparent immune dysregulation in RSV infection, the lack of protective immunity in natural infection, and the difficulty involved in extrapolating experimental results from RSV-exposed adults to RSV-naive infants. For live-attenuated vaccines, safety has been maintained, but maintaining a stable attenuated phenotype with some of the vaccine constructs has been difficult. The difficulties in evaluating RSV vaccines in young infants in field trials may very well be offset by the development of robust challenge models; however, the field is in need of additional challenge strains, including some of higher virulence than those currently in use. The potential that vectored and subunit vaccines overcome some of the many obstacles to vaccine development could be realized with the broader application of human challenge studies at the appropriate point in the clinical development process. Meanwhile, the field is effectively using the model to identify potential correlates of protection.
Pneumococcus
(i) Epidemiology, diversity, and pathogenesis of pneumococcus
In the year 2000, before the introduction of the first of the pneumococcal conjugate vaccines (PCVs), the bacterium Streptococcus pneumoniae (also called pneumococcus) caused an estimated 14.5 million episodes of serious disease and 826,000 deaths in children less than 5 years of age (722). This amounted to 11% of all deaths in children under 5, most of them in low- and middle-income countries.
While worldwide introduction of PCVs is far from complete, it was estimated that in 2015, 294,000 pneumococcal deaths occurred among children not infected with HIV (723). Less conservative assumptions result in pneumococcal death estimates as high as 515,000, half of which occurred in India, Nigeria, the Democratic Republic of the Congo, Pakistan, China, and South Sudan. Altogether, they estimated 3.7 million episodes of severe pneumococcus globally in children in 2015. Pneumococcus also causes serious disease in the elderly.
Pneumococcal bacteria exist as chains of varying length surrounded by capsular polysaccharides (CPS) which allow the bacteria to attach to the nasopharyngeal mucosa and establish colonization. Colonizing bacteria can also exist in biofilms in the mucosa (724), in which they exist as aggregates encased in an extracellular matrix. In biofilms, the bacteria are incompletely encapsulated, metabolically less active, and resistant to antimicrobials. Colonization of the nasopharynx with pneumococcus is frequent in infants and young children, and generally declines with age. Rates of colonization may reach 40% in children and 15% in adults (725). Colonization leads to a carrier state in which the bacteria persist without causing serious disease. A fraction of colonized individuals goes on to develop invasive pneumococcal diseases (IPDs), which include bacteremia, meningitis, and bone and joint infections. Pneumococcal pneumonia, a leading cause of severe pneumonia in children, is accompanied by bacteremia and considered invasive in up to 25% of cases (726). Pneumococcus can also develop noninvasive diseases such as otitis media. The factors that govern the transition from an essentially benign carrier state to serious disease are incompletely understood (727). Properties of the bacteria themselves, the effect of coinfection with other bacteria, and the host inflammatory response are thought to be contributing factors. Whether or not bacteria in biofilms are an important source of transmission or progression to invasive disease is an open question.
Close to 100 serotypes of pneumococcus have been identified worldwide on the basis of the binding of serum antibodies to their CPS. The CPS are essential for colonization and are key virulence factors for these bacteria. Immunity mediated by antibodies against the CPS is largely serotype specific.
(ii) Development of pneumococcal vaccines
The first pneumococcal vaccine was licensed in 1983. It included purified CPS of 23 of the most common serotypes, and prevented invasive disease in 57% of vaccinated individuals (726). CPS of many of the most common serotypes, including 6A, 6B, 14, 19A, and 23F, were poorly immunogenic in children less than 2 years of age, however, and the vaccine did not reduce pneumococcal carriage. The duration of protection was also limited.
Conjugating pneumococcal polysaccharides to highly immunogenic carrier proteins largely overcame these problems and launched the era of PCVs. Conjugating polysaccharide antigens to proteins recruits noncognate CD4+ T-cell help for establishing memory responses and promotes class switching and affinity maturation of antibodies. This conversion of the polysaccharide response from T cell independent to T cell dependent is particularly important for children under 2 years old and older adults with declining immune function, since unconjugated polysaccharides are poorly immunogenic in these vulnerable populations (728). Between 2000 and 2010, three different PCVs were licensed (see Table 29).
TABLE 29.
Licensed pneumococcal conjugate vaccines for children less than 5 years of agea
| Vaccine | Manufacturer | Serotypesb | Carrier protein | Indication | Licensure |
|---|---|---|---|---|---|
| PCV7 (Prevnar) | Pfizer, Inc. | 4, 6B, 9V, 14, 18C, 19F, 23F | CRM197, a nontoxic variant of diphtheria toxin | Prevention of IPD and otitis media due to S. pneumoniae | Licensed in 91 countries from 2000 to 2008 |
| PCV13 (Prevnar 13) | Pfizer, Inc. | 4, 6B, 9V, 14, 18C, 19F, 23F, 1, 3, 5, 6A, 7F, 19A | CRM197 | Prevention of IPD and otitis media due to S. pneumoniae | Licensed in 2009/2010 in the United States and Europe |
| PHiD-CV (Synflorix) | GSK | 4, 6B, 9V, 14, 18C, 19F, 23F, 1, 5, 7F | Nontypeable H. influenzae protein D (8 serotypes) and CRM197 (2 serotypes) | Prevention of IPD and otitis media due to S. pneumoniae and H. influenzae | Licensed in more than 60 countries worldwide (not including the United States) in 2009 |
GSK, GlaxoSmithKline; H. influenzae, Haemophilus influenzae; IPD, invasive pneumococcal disease; PCV, pneumococcal conjugate vaccine; S. pneumoniae, Streptococcus pneumoniae.
Serotypes in PCV13 and PHiD-CV that are not included in PCV7 are in boldface type.
The global and regional distribution of pneumococcus serotypes is a key consideration in the utilization of licensed vaccines and for the further development of vaccines against the CPS antigens. Although the first PCV (PCV7) was licensed in 2000, its incorporation into national childhood immunization programs in the most affected countries in the world has lagged, partly due to incomplete information about the match of the seven serotypes in the vaccine to the circulating strains in different countries and regions.
The Pneumococcal Global Serotype Project studied how the fraction of IPD in children less than 5 years of age due to the serotypes included in these vaccines according to geographic region (729). PCV7 showed the most marked regional differences, with better coverage in North America and Europe compared to the rest of the world. These disparities were largely, but not completely, abrogated with the improved strain coverage of the 10-valent and 13-valent vaccines. The latter two vaccines would be expected to cover at least 70% of global IPD episodes in young children (730).
A substantial body of evidence exists to indicate that the licensed PCVs can be highly effective against the serotypes they contain. Over the first 7 years after PCV7 introduction in the United States, a 45% decrease occurred in overall IPD incidence, and a 94% decrease occurred in the incidence of IPD with the serotypes included in the vaccine (731).
Primary colonization with nonvaccine serotypes increased in the post-PCV7 era. In addition, pneumococcus can undergo capsular switching by an exchange of genes among cocolonizing strains, further increasing the incidence of nonvaccine serotypes. Together, these mechanisms can lead to wholesale serotype replacement in which the nonvaccine strains greatly increase in prevalence relative to the vaccine strains. A systematic review of the evidence in 2011 indicated that extensive serotype replacement did occur after PCV7 introduction (732). Disease caused by nonvaccine serotypes in the United States had risen to 88% by 2004, compared to 17% before PCV introduction.
In a recent meta-analysis of 68 studies of IPD in children in countries in which PCV7 had been introduced, serotype 19A was the most predominant cause of childhood IPD, accounting for 21.8% of cases. In countries that introduced higher-valent PCV13, the overall serotype-specific contribution of 19A was lower (14.2%). Overall, non-PCV13 serotypes contributed to 42.2% of cases. PCVs with broader valency, such as PCV15 (729) and PCV20 (733), are now in clinical trials. Initial results from phase 1/2 studies demonstrated two different preparations of PCV15 have safety profiles comparable to that of PCV13 (729). Both PCV15 formulations include serotypes 22F and 33F in addition to those in PCV13, and both induced serotype-specific antibody responses to all 15 serotypes.
A 20-serotype PCV developed by Pfizer that contains 7 new serotypes in addition to the 13 included in PCV13 (734) showed efficacy in a phase 3 trial and has been approved by the U.S. FDA for use in subjects 18 years of age and older (735).
A nonconjugated 23-valent polysaccharide vaccine (Pneumovax 23; Merck) is recommended for adults 65 years or older. It consists of a mixture of purified unconjugated CPS from S. pneumoniae types. The efficacy of CPS vaccines was studied in South Africa in male 16- to 58-year-old gold miners, where it showed efficacies of 76% (for a 6-valent vaccine) and 92% (for a 12-valent vaccine) (736). Similar results have been observed in additional studies of these vaccines in France and elsewhere.
Pneumococcal vaccines have led to a significant and impressive reduction in not only pneumonia, but also sepsis and bacteremia in immunized populations. However, there remain concerns that the current vaccine strategy does not adequately address meningitis, making expansion of the vaccine arsenal an important goal.
(iii) Development of new pneumococcal vaccines
While the current PCVs have been very effective in reducing rates of invasive pneumococcal disease, their effectiveness has been somewhat diminished by the phenomenon of serotype replacement already noted above (737). Given the high number of serotypes currently known, it is cumbersome and inefficient to add additional serotypes to the current vaccines. The alternative is to develop vaccines using antigens different from CPS shared by all S. pneumoniae strains in order to induce a broader response than that of PCVs, stimulating both humoral and cellular immunity. Such vaccines (e.g., whole-cell vaccines, subunit proteins, vectored vaccines, etc.) may be simpler and less expensive to manufacture (738). Work on the development of whole-cell or live-vectored (739) pneumococcal vaccines is also underway. Table 30 below summarizes some of the pneumococcal vaccines in development.
TABLE 30.
Some pneumococcal vaccines in developmenta
| Vaccine type | Institution | Description |
|---|---|---|
| Conjugate | Merck | 15-Valent conjugate vaccine with or without adjuvant (929) |
| Whole-cell | PATH | Whole-cell vaccine formulated in aluminum hydroxide adjuvant (742) |
| Vectored | Genocea | PspA-Lactobacillus lactis vaccine delivery vehicle |
| Multicomponent adenovirus vector | ||
| Particle | Genocea | Lactococcal GEM-based vaccine |
| Subunit | GlaxoSmithKline | Pht proteins |
| Sanofi Pasteur | Recombinant PspA | |
| Genocea | Protein subunit vaccine |
GEM, Gram-positive Enhancer Matrix; Pht, pneumococcal histidine triad; PspA, pneumococcal surface protein A.
Several studies have failed to conclusively identify specific proteins with protective potential. For instance, in studies in which the pneumolysin toxoid and PhtD, two purified S. pneumoniae proteins, were combined with polysaccharide conjugates high prevaccination Ply and PhtD antibody concentrations were observed, but they were not greatly increased by vaccination in 2- to 4-year-olds, and only responses to protein D were observed in the infants (740, 741).
The pathway to licensure for protein-based or whole-cell vaccines is uncertain given that correlates of protection will be difficult to identify and large efficacy superiority trials with disease endpoints (as opposed to carriage endpoints) will be required. Human challenge studies may play a critical role in addressing some of these regulatory issues; at a minimum, they may provide proof of concept that such vaccines are efficacious against one or more of the challenge agents currently in use.
A killed whole-cell vaccine based on a nonencapsulated S. pneumoniae strain developed by PATH was safe and well tolerated when given to adults. The vaccine elicited significant increases (defined arbitrarily as at least a 2-fold rise) in IgG responses to multiple pneumococcal antigens, including PspA and Ply. Functional antibody responses were observed with the highest dose of whole-cell pneumococcal vaccine (0.6 mg). Increases in T-cell cytokine responses, including IL-17A, were also seen among whole-cell pneumococcal vaccines (742). Additional studies have been conducted with this vaccine in toddlers in Kenya; however, further vaccine development is on hold.
(iv) Experimental human carriage model for pneumococcus
Substantial evidence indicates that the highly effective PCVs in current use substantially reduce nasopharyngeal carriage of the vaccine strains, as well as invasive and noninvasive pneumococcal disease in vaccinated individuals (743). Carriage of the vaccine strains is also reduced in unvaccinated individuals (744), presumably the result of herd immunity. Reduction in the rates of nasopharyngeal carriage is a key goal for future pneumococcal vaccines and may be a primary clinical endpoint in future vaccine trials.
Accordingly, a human challenge model has been developed to more broadly evaluate the capacity of candidate pneumococcal vaccines to reduce rates of carriage and to explore immune responses that may be involved in the prevention of the carrier state. The features of the currently used model are detailed in Table 31.
TABLE 31.
Human carriage model for pneumococcus
| Method of production and administration | Challenge strain | Strain provider |
|---|---|---|
| Laboratory cultivation is used to freshly prepare and titrate the challenge inoculum. The inoculum is administered to healthy adult volunteers by intranasal instillation in doses ranging from 10,000 to 140,000 CFU. | P833, a type 23F clinical isolate | Jeffrey Weiser, University of Pennsylvania |
| BHN418, a type 6B clinical isolate | Peter Hermans, University of Nijmegen |
The initial work on this human challenge model was published in 2002 and 2005 by a group at Baylor University (745, 746). Using a serotype 23F clinical isolate, the investigators found that carriage could be established in 6 of 14 healthy, uncolonized volunteers. The duration of colonization was variable. Preexisting serum IgG antibody to the 23F CPS did not correlate with susceptibility to carriage in those subjects inoculated with a 23F strain. A correlation in these volunteers, however, did appear with preexisting serum IgG and secretory IgA responses to PspA. Seven of the eight subjects that did not become colonized had preexisting antibody to PspA, which is one of the antigens included in candidate vaccines in development (see Table 30). Also, the investigators found that six of eight subjects challenged with a serotype 6B clinical isolate became colonized. In a follow-up study, the same group explored responses to a number of other pneumococcal surface proteins, many of which are also under consideration for the development of vaccines. Among the eight proteins evaluated, only the two related choline-binding proteins (PspA and CbpA) generated serum IgG responses in colonized subjects. Unlike PspA, no preexisting serum IgG antibody responses were detected toward CbpA.
After a decade of inactivity, the model underwent further development in Liverpool, United Kingdom, using the same two clinical isolates (23F and 6B) with noncolonized healthy adult volunteers (747). Paradoxically, the first experimental colonization study failed to establish colonization with either strain in all but 1 of the 19 volunteers. By 2013, virtually every aspect of the challenge model, including the important preparation and titration of challenge inoculum, had been refined (748). The more developed study procedure was evaluated in 159 volunteers, and carriage could be established in 22% of volunteers using dose ranges of 11,100 to 313,000 CFU per naris for strain 6B and 9,000 to 84,500 CFU per naris for strain 23F. The most common symptoms after challenge were nonspecific nasal symptoms. None of the volunteers developed symptoms of pneumococcal disease.
The investigators cautioned that reproducibility can be a problem with the inoculum, which seems to stem partly from the propensity of these encapsulated bacteria to aggregate and partly from the loss of viability of the challenge organisms when suspended in the saline used for intranasal administration. The investigators also provided specific guidance on the nasal wash procedure.
Additional studies have been conducted with this model, including an investigation of the role of carriage in protecting against rechallenge with the homologous strain (749). A dose-response curve was first established for the serotype 6B challenge strain, which showed that up to 60% carriage could be established with doses of 40,000 CFU or more. Prechallenge serum IgG titers to the 6B CPS were not correlated with the establishment of carriage. When carriage was established, carriage density was unrelated to challenge dose. After carriage had returned to undetectable levels, ten volunteers were rechallenged with about 40,000 CFU of the same 6B strain, some up to 1 year later. All ten volunteers remained carrier negative. The investigators further studied the capacity of the model to examine antibody responses to several bacterial proteins following the establishment of carriage. They found many bacterial proteins that were immunogenic in humans, which could be potential targets for candidate vaccines.
Several additional publications have reported on using this challenge model (750–752). One stated that pneumococcal carriage does not markedly impact the proportions of other bacteria that colonize the human nasopharynx (753). Other reports suggested that the density and duration of carriage may be influenced by an immunomodulatory response, including the production of transforming growth factor beta 1 and an infiltration of T regulatory cells in the nasopharynx. The investigators suggested that the bacteria themselves may modulate the immune response to facilitate the establishment of the carrier state. Other studies have characterized the colonization “take” and kinetics, as well as the symptoms associated with it (minimal to none).
Likewise, live influenza vaccination (compared to parenteral trivalent influenza vaccine [TIV]) induced fewer symptoms when given before pneumococcus inoculation, with colonization status only affecting the TIV group where more symptoms were reported by colonized participants compared to noncolonized participants following inoculation (n = 12/23 [52.17%] versus n = 13/38 [34.21%], respectively; P < 0.05). When influenza vaccination followed bacterial inoculation, no difference was seen in the symptoms reported between the live-attenuated and trivalent inactivated influenza vaccine groups following inoculation (751).
Taken together, the initial results from the human challenge model show promise for the dissection of immune responses associated with preventing nasopharyngeal carriage of two globally prevalent pneumococcal serotypes, 6B and 23F.
The model has now been used successfully to test the protective effect of PCV13 on colonization by the 6B strain, which was used as the challenge (754). Healthy adults received PCV13 or hepatitis A (control) vaccine. Only minor symptoms were observed after the challenge; colonization rates at any time (the primary endpoint of the study) were 10.4% in the PCV13 group and 47.9% in the control group, a 78% reduction. In addition, the colonization density was reduced by 3-fold. In a subsequent study, the authors examined the role of mucosal IgG to CPS in mediating protection from carriage in the same cohort (755). In the PCV13-vaccinated subjects, IgG levels to the CPS were increased in serum and nasal washes. Nasopharyngeal samples obtained postvaccination heavily agglutinated pneumococcus compared to prevaccination samples among subjects protected, pointing to pneumococcal agglutinating antibodies as the mechanism of protection against carriage acquisition (754). Albeit colonization is not a disease endpoint, it is a surrogate for vaccine-mediated protection and transmission; the impact of PCV on nasopharyngeal colonization in children is well known (756); thus, the model has significant relevance for the future testing of novel vaccines.
(v) Summary
Pneumococcal conjugate vaccines are one of the great success stories in human vaccine development in recent years. The experience since the introduction of these vaccines, however, has been a case study on the selective pressures imposed by vaccines with incomplete strain coverage, and on the interaction of vaccines and drugs. The common denominator for vaccines of all types to prevent childhood morbidity and mortality from pneumococcus may be the prevention of the carrier state. Including the full spectrum of prevalent strains in a single vaccine construct is not possible at the present time. As a result, the strains not in the vaccines grow in prevalence and become the dominant circulating strains and eventually, the vaccines become less effective. Focusing the immune response on more conserved protein antigens may be needed to overcome the limitations of conjugate vaccines. A human carriage model with the potential to evaluate such vaccines was developed and successfully tested against a licensed vaccine. The model generated data suggesting that in noncolonized adult volunteers, establishing carriage may provide short-term protection against the homologous strain. Further, the model clearly demonstrated its usefulness when testing the protective effect of a well-known vaccine, PCV13, which could be used as a positive control in future studies. Vaccination substantially reduced colonization rates and density.
Tuberculosis
(i) Epidemiology, pathogenesis, and public health impact of tuberculosis
Tuberculosis (TB) is an ancient scourge, established in prehistoric human populations before their migration out of Africa (757). In 1882, Robert Koch discovered the intracellular pathogen Mycobacterium tuberculosis and established that it is the causative agent of TB. Today, M. tuberculosis infection causes more human deaths per year than any other pathogen, including HIV and Plasmodium falciparum, the principal agent of human malaria (758). Every year, 10 million people newly develop TB, and 1.5 million die of the disease. The incidence of TB infection in 2018 was 1,310 cases per million of the global population, far exceeding the goal of one case per million set jointly by the WHO and the Stop TB Partnership. Multidrug-resistant, extensively drug-resistant, and even totally drug-resistant strains are also increasing in prevalence. A more effective TB vaccine is urgently needed.
The life cycle of M. tuberculosis in the infected host (Fig. 14) is important to consider for effective vaccine development because it is partly under host immune control. Gengenbacher and Kaufmann (759) describe the life cycle of M. tuberculosis in humans. TB is transmitted by the inhalation of aerosols from the coughs of individuals with active TB. The mycobacteria take up residence in the lung alveoli, where phagocytic cells of the immune system engulf them. Normally, this would lead to a prompt adaptive immune response, but the response is delayed for 2 to 3 weeks in the case of M. tuberculosis infection. During this time, the early innate response to infection recruits macrophages and other immune cells to sites of infection in the lungs, where they become organized as primary granulomas, from which cells come and go. With the development of the adaptive immune response, an infiltration of T cells into the granulomas occurs, which become larger and solid. What happens next is a matter of the balance of immune responses. The granulomas can be static in a standoff with the immune system, but if the response is too inflammatory, the central regions of the granulomas become necrotic and caseous (cheese-like) and exhibit an outgrowth of actively replicating bacteria. In the TB-infected individual, granulomas at all stages can coexist in the lungs. When the liquefying granulomas sufficiently damage the linings of airways, the bacteria escape through coughing to infect new hosts.
FIG 14.
Transmission and pathology of tuberculosis.
In a small fraction of individuals, the infection directly transforms into active TB, and these individuals are an immediate source of new TB cases (Fig. 14). The establishment of latent TB, however, is the normal outcome of infection. Some estimates place the reservoir of individuals latently infected with TB at more than 2.33 billion, or about one-third of the world’s population (756, 758). Some of these individuals experience life-long latency, but the latent infection reactivates in others, at which point the individual once again becomes infectious to others. Reactivation occurs in 2 to 10% of individuals with latent TB each year.
Reinfection of latently infected individuals plays a role in reactivation because a new infection stimulates immune responses that attack and help break down granulomas. In some latently infected individuals, an eventual loss of immune control of the infection occurs. The devastating effects of losing immune control of latent TB in HIV infection are well documented, with continuing active replication and dissemination occurring outside the lungs. The widely used TB vaccine, bacillus Calmette-Guérin (BCG), prevents the dissemination of M. tuberculosis outside of the lungs but does not prevent the establishment of latent infection.
Understanding how many humans are latently infected and what fraction of latently infected individuals will experience reactivation is very important for the control of TB. Vaccines that either prevent the establishment of latency or that prevent reactivation from latency will be of critical importance for TB control. Certain antigens expressed in the M. tuberculosis bacteria are latent but are not expressed in actively replicating M. tuberculosis bacteria. These genes are encoded in part by a 48-gene region of the M. tuberculosis genome called the DosR regulon. A regulon is a group of genes that are turned on or off together, although they may be widely scattered in the genome of an organism (757, 760).
Importantly, DosR regulon-encoded antigens are present in the BCG genome, but they are not expressed. In individuals with prior BCG vaccination, these antigens are recognized poorly, if at all. Correspondingly, vaccination with BCG has little or no capacity to elicit immunity to the latent phase of M. tuberculosis infection.
Immunity to latency antigens is readily and strongly detected in individuals with long-term M. tuberculosis infection that have not experienced a reactivation. Another group of interesting antigens are those involved in the resuscitation of M. tuberculosis after latency, that is, genes that enable the bacteria to return to their metabolically active state. The antigens these genes encode are termed resuscitation promoting, or Rpf, antigens. Immunity to Rpf antigens could curtail the emergence of actively replicating bacteria from a latent infection (757).
Some of the latency and Rpf antigens have been incorporated into candidate TB vaccines. These vaccines are among the first to target multiple phases of the M. tuberculosis life cycle, and they have shown their potential to elicit immune control of latent infection in animal models. Incorporating some of these newly discovered antigens into the BCG genome and engineering BCG to resume expression of them are other potential strategies to take advantage of these new discoveries.
(ii) Tuberculosis vaccines in development
The only currently licensed vaccine against TB is BCG, an attenuated strain of Mycobacterium bovis. This vaccine was developed nearly 100 years ago to prevent serious TB disease in infants, which became common in Europe in the early 20th century (761). It is the most widely used vaccine worldwide. When administered to infants shortly after birth, it can prevent the forms of disease that result from TB dissemination outside the lungs in infants and young children. The protection afforded by BCG vaccination is highly variable (0 to 80% efficacy) and is short lived. Revaccination with BCG later in life does not confer immunity to TB, with or without prior vaccination with BCG.
Table 32 describes some of the candidate vaccines that are in clinical development and was compiled from a number of recent reviews (757, 759–768). The table does not include the more than 20 candidate vaccines in preclinical development.
TABLE 32.
Candidate tuberculosis vaccines in clinical developmenta
| Type of vaccine | Candidate | Description | Stage | Developer(s) |
|---|---|---|---|---|
| BCG replacement vaccine for infants | VPM1002 | Recombinant BCG with deletion of urease C and expression of listeriolysin O to promote class 1 presentation | Phase 3 | Serum Institute of India Pvt., Ltd., and Vakzine Projekt Management GmbH |
| MTBVAC | Live, rationally attenuated M. tuberculosis with stable, engineered deletions in phoP and fadD26 | Phase 2a | University of Zaragoza, Biofabri, and South African Tuberculosis Initiative | |
| Booster vaccine for infants, children, and adolescents with BCG | MVA85A Aeras485 | Ag85A expressed in an MVA vector | Phase 2b | University of Oxford and Aeras |
| H4:IC-31 (Aeras 404) | Ag85B/TB10.4 fusion protein with cationic peptide/TLR-9 adjuvant IC31 | Phase 2 | Aeras, Sanofi Pasteur, and Intercell | |
| Booster vaccine for adults with latent TB | MVA85AAeras 485 | Ag85A expressed in an MVA vector | Phase 2b | University of Oxford and Aeras |
| M72-AS01E | Rv1196/Rv0125 fusion protein with AS01E adjuvant | Phase 2a | GlaxoSmithKline | |
| Ad5 Ag85A | Ag85A expressed in Ad5 vector | Phase 1 | McMaster University, Canada | |
| H56:IC31 | Ag85B/ESAT06/Rv2660 fusion protein with cationic peptide/TLR-9 adjuvant IC31 | Phase 2a | Statens Serum Institute and Intercell | |
| ID93:GLA-SE | Rv2608/Rv3619/Rv3620/Rv1813 fusion protein with stable emulsion/TLR-4 adjuvant | Phase 1 | Infectious Disease Research Institute | |
| SRL172 | Whole-cell inactivated variant of Mycobacterium obuense intended for HIV-infected subjects | Phase 3 | Aeras | |
| DAR-901 | Whole-cell inactivated vaccine derived from SRL172 | Phase 2 | Aeras | |
| Therapeutic vaccine for adults with active TB | Mycobacterium indicus pranii | Killed vaccine against leprosy with observed protection against TB | Phase 3 | Cadila Pharmaceuticals, Ltd., India |
| Mycobacterium vaccae | Atypical Mycobacterium | Phase 2b | Anhui Zhifei Longcom Biopharmaceutical Co., Ltd., China | |
| RUTI | Detoxified liposomal fragments of M. tuberculosis | Phase 2b | Archivel Farma, Spain | |
| Prevention of TB recurrence | VPM1002 | Recombinant BCG with deletion of urease C and expression of listeriolysin O to promote Class 1 presentation | Phase 2/3 | Serum Institute of India Pvt., Ltd. |
BCG, bacillus Calmette-Guérin; MVA, modified vaccinia virus Ankara; TB, tuberculosis; TLR, Toll-like receptor.
Some candidate vaccines are designed to replace BCG for vaccinating infants. VPM1002, originally developed by the Max Plank Institute, Berlin, Germany, and now licensed to Serum Institute of India is a BCG strain that has been redesigned to elicit better CD8+ T-cell responses. It was safe and immunogenic in phase 1 and 2a trials in Germany and South Africa in adults and newborn infants (769, 770). A phase 2b trial in both HIV-exposed and unexposed South African infants has recently been completed; data are awaiting public release (ClinicalTrials.gov identifier NCT02391415) (771), and a multinational phase 3 study is planned (ClinicalTrials.gov identifier NCT04351685) (772). An additional phase 2/3 trial is underway in India to test VPM1002 for the prevention of recurrence in adults previously treated for pulmonary TB (ClinicalTrials.gov identifier NCT03152903) (773).
A live-attenuated, double-deletion mutant of M. tuberculosis, called MTBVAC, originated at University of Zaragoza, Zaragoza, Spain, and has undergone phase 1 and 2a trials (774–777). The vaccine candidate was licensed to Biofabri (Spain) and is now being developed by the South African Tuberculosis Vaccine Initiative. It was recently tested in previously BCG-vaccinated adults, as well as in infants that had not received BCG. MTBVAC was well tolerated and induced long-lasting CD4+ T-cell responses in infants. An efficacy trial of MTBVAC in newborn infants is expected to start in South Africa, Madagascar, and Senegal in 2022 (778).
Another approach to an improved TB vaccine is to design a booster that can be used following BCG vaccination of infants. MVA85A is a candidate vaccine developed by Oxford University and Aeras, in which an antigen from M. tuberculosis is expressed in the modified vaccinia virus Ankara (MVA). The MVA vector is a strain of vaccinia virus that has been stably attenuated by serial passage in cell culture. The MVA85A vaccine was evaluated in a phase 2b trial in more than 4,000 infants in South Africa that were previously vaccinated with BCG but showed little efficacy against infection or disease (779).
A whole new class of candidate vaccines is directed toward the control or eradication of latent TB in adults with prior BCG vaccination. This group is composed of both vectored and subunit vaccines, the latter consisting of fusion proteins delivered with an adjuvant. A phase 2b trial of the MVA85A vectored vaccine in South African and Senegalese adults with HIV has been completed and demonstrated safety and immunogenicity (780). There was no evidence of MVA85A efficacy in preventing TB infection or disease, although with only 650 participants, this study was not well powered for these outcomes. DAR-901, an inactivated whole-cell vaccine based on Mycobacterium obuense (781), is being tested for prevention of infection in an efficacy trial using a three-dose regimen among Tanzanian adolescents (ClinicalTrials.gov identifier NCT02712424) (782). Initial results from this trial suggest DAR-901 was not efficacious at preventing infection; however, the trial has generated data on the predictive value of complete blood count-derived metrics for TB infection risk (783).
A third candidate, ID93, is a subunit TB vaccine candidate comprised of four antigens representing different families of M. tuberculosis proteins (Rv1813, Rv2608 [PPE42], Rv3619 [EsxV], and Rv3620). All four proteins are recognized by M. tuberculosis-exposed individuals (784). ID93 is combined with the Th1-inducing synthetic TLR-4 (Toll-like receptor 4) agonist adjuvant glucopyranosyl lipid adjuvant (GLA). In a recently completed phase 1 study that showed humoral and T-cell responses to all four antigens in the vaccine, GLA had a significant enhancement effect on the polyfunctionality of the induced CD4+ T cells (785).
The H4:IC31 vaccine consists of the fusion of two antigens, Ag85 B and TB10.4, and the IC31 adjuvant, a mixture of KLK, a leucine-rich peptide (KLKL5KLK), and the oligodeoxynucleotide ODN1a, a TLR-9 ligand. After initial phase 1 demonstration of safety and immunogenicity (786), the vaccine was tested in 990 TB-free adolescents that had received BCG at birth and were randomized to receive H4:IC31, BCG revaccination, or placebo (787). Primary outcomes were safety and M. tuberculosis infection, as assessed by QuantiFERON-TB (QFT; Quest Diagnostics) conversion (TB diagnosis is based on immunologic sensitization to M. tuberculosis antigens, as assessed by the tuberculin and/or IFN-γ release assays, typically done with the QFT). Recent infection, as diagnosed by means of the tuberculin skin test or QFT conversion, is associated with a higher risk of disease than is nonconversion or remote conversion (i.e., at least 2 years earlier). An important secondary outcome in the study was sustained QFT conversion to a positive test without reversion to negative status at 3 months and 6 months after conversion. Neither vaccine nor the BCG vaccine prevented initial QFT conversion, but BCG reduced the rate of sustained QFT conversion by 45.4% (P = 0.03), while H4:IC31 reduced it by 30.5% (P = 0.16), a lead finding that may have a potential impact in further development.
The GlaxoSmithKline M72/AS01E vaccine is derived from the M72 recombinant fusion protein from M. tuberculosis antigens (Mtb32A and Mtb39A). In a phase 2b trial conducted in Kenya, South Africa, and Zambia, approximately 3,500 18- to 50-year-old adults with latent M. tuberculosis infection (as determined by IFN-γ release assay) were given either M72/AS01E or placebo twice. Most participants had previously received BCG. Efficacy against progression to bacteriologically confirmed active pulmonary TB disease was evaluated by clinical suspicion of TB confirmed with sputum by means of a PCR test, culture, or both. Ten participants in the M72/AS01E group and 22 in the placebo group presented bacteriologically confirmed active pulmonary TB (primary endpoint) for a vaccine efficacy of 54% (95% CI = 2.9 to 78.2; P = 0.04) (788). The vaccine was not associated with higher rates of serious adverse events. However, unsolicited reports of adverse events in the M72/AS01E group (67.4%) were higher than in the placebo group (45.4%); these were mainly injection site reactions and influenza-like symptoms. Final results of this trial after 3 years of follow-up showed 13 cases in the vaccine arm and 26 cases in the placebo arm giving 50% vaccine efficacy (95% CI = 2 to 74) (789). Additional support for the M72 study for confirmation and expansion of the findings to other populations will be necessary for broad implementation (767).
Aerosol administration of the MVA85A vaccine in BCG-vaccinated adults was recently reported. A priming immunization with the vaccine was well tolerated and highly immunogenic; however, prior intradermal administration of the same vaccine followed by aerosolized MVA85A boosting led to moderate to severe respiratory and systemic adverse events and resulted in modest, significant boosting of the cell-mediated immune response to Ag85. Serum antibodies to Ag85A and MVA were only induced after intradermal vaccination. Aerosolized MVA85A induced significantly higher levels of Ag85A lung mucosal CD4+ and CD8+ T-cell cytokines compared to intradermal vaccination (790).
Therapeutic vaccines are also in development and being designed for use in adults with active TB. Two of these use the strategy of vaccinating with whole, killed preparations of non-TB mycobacteria. A leprosy vaccine (791) and one based on an atypical Mycobacterium from cows (792) are in clinical development in India and China, respectively. A therapeutic vaccine comprised of detoxified liposomal fragments of M. tuberculosis is also under development (793).
(iii) Human challenge model for tuberculosis
A human challenge model that faithfully reproduces human infection with M. tuberculosis is not available now and unlikely to be developed in the near future. The key limitation is that wild-type M. tuberculosis cannot be used for challenge, since infection leads to the establishment of latency. Upon reactivation, latent TB can cause extensive lung damage and can disseminate to other organs. Treatment is available but must be continued for months, and treatment is not a guarantee of cure. Two approaches using BCG as a surrogate for infectious M. tuberculosis using different routes of inoculation have been explored, as described below.
An early attempt to develop a TB human challenge model using BCG focused on intradermal inoculation (794). BCG differs in important respects from M. tuberculosis. BCG is not a human strain of M. tuberculosis; rather, it is an attenuated strain of M. bovis, which infects cows. BCG has large deletions in its genome that arose through the 230 serial passages in culture that Calmette and Guérin used to attenuate BCG in the 1920s. During the further attenuation process that followed the work of Calmette and Guérin, more than 14 substrains of BCG arose, and many of these substrains have been used in the worldwide production of the BCG vaccines (765). Complete genome sequencing of BCG substrains has shown that they contain different subsets of at least 18 “regions of difference” between BCG and M. tuberculosis. A region of difference is a deletion of a block of genes, and the regions of difference between BCG and M. tuberculosis are numbered RD1 through RD18.
Many of the candidate vaccines in development today are based on recombinant BCG strains that have been modified to improve the levels of protection and immune memory, compared to BCG. Different substrains of BCG are being used as the “backbone” into which these modifications are being introduced (765). When a particular substrain of BCG is used to evaluate these candidate vaccines in a human challenge model, the vaccine strain will differ from the challenge strain. Another important difference between M. tuberculosis and BCG is that BCG does not establish latency in humans (757). For this reason, the intradermal BCG challenge model can only evaluate the capacity of vaccines to limit the replication of M. tuberculosis during the initial stage of infection.
The study endpoint for the human TB challenge model using intradermal BCG is to limit mycobacterial growth at the injection site. Parameters for the model were first developed using mice that were inoculated with BCG in the skin of the ear (795). The differences between mouse skin and human skin make extrapolating the experimental results from mice to humans more difficult. The assays developed in mice included bacterial culture from the site of intradermal inoculation and quantitative PCR. In the first human challenge study, healthy adult volunteers (some of whom had undergone prior BCG vaccination) were challenged with BCG by the intradermal route (793). No systemic complications occurred in study volunteers, but all of the BCG-vaccinated and some of the BCG-naive volunteers developed a purulent discharge at the site of inoculation that resolved within four weeks. To gain sufficient material for evaluating the extent of BCG replication after challenge, a punch biopsy of the injection site was performed 1, 2, or 4 weeks after challenge in BCG-naive subjects and 2 weeks after challenge in the BCG-vaccinated subjects. Though unclear in the text of the published study, the figures appear to show that subsets of the group of 28 volunteers that were BGC naive prior to challenge were biopsied at different times. The heterogeneity of the study results at different time points after challenge may stem from the fact that the data came from different volunteers at each time point. Little correlation appeared between the quantification of BCG replication by PCR and by bacterial culture. Overall, the enumeration of bacteria by PCR gave counts that were 10-fold higher than culture.
The human TB challenge model is also designed to permit the evaluation of immune responses after vaccination and challenge. To enable the characterization of local immune responses, the investigators collected cells infiltrating a suction blister generated near the site of inoculation. They also collected blood samples as a source of peripheral blood mononuclear cells (PBMCs) with which to evaluate systemic immune responses. The quantity of cells recovered from blisters was very limited, which precluded an extensive evaluation of immune responses. The investigators measured the fraction of PBMCs secreting IFN-γ after restimulation in culture with the immunodominant M. tuberculosis antigens 85A and TB10.3. The magnitude of the IFN-γ responses prior to challenge did not correlate with the number of live BCG bacterial colonies from skin biopsy specimens after challenge.
In another recent study, five volunteers were challenged with BCG intradermally (796). Swab specimens to quantitate shedding and mycobacterial immunity were collected from the site of vaccination. A comparison of three methods to identify the bacteria—PCR, culture, and time to positivity of mycobacterial growth indicator tubes—was made to evaluate and compare their sensitivity. BCG was detected in swab specimens from all five volunteers by at least one method.
The intradermal BCG challenge model is clearly a significant advance. In its early stages of development, the community of TB researchers received it with enthusiasm, tempered by some of the limitations of the model (797). These include the choice of evaluation method for the primary study endpoint (it is unclear whether this should be PCR, bacterial culture, or both) and difficulty in standardizing the challenge dose (different lots of BCG can vary up to 10-fold with respect to the numbers of infectious bacteria). The model would be improved if invasive procedures such as skin biopsies were not required. Finally, this model will not have the capacity to evaluate the many vaccines in development that include antigens unique to M. tuberculosis, including the antigens uniquely expressed during latency.
Some of these limitations are being addressed. A growth-inhibition assay for M. tuberculosis using cryopreserved PMBCs has been developed, which could replace skin biopsy if it proves to be a valid measure of antimicrobial immunity generated by vaccines. In the initial study, the capacity of PBMCs from BCG-vaccinated individuals to inhibit M. tuberculosis growth was correlated with their IFN-γ enzyme-linked immunospot assay response to a purified protein derivative (PPD) skin test, suggesting that this approach may be useful. Another possible approach would be to develop new readouts, such as luminescence, to measure BCG replication directly at the injection site. A study is underway to evaluate the dose response for the human BCG challenge model, which may permit further standardization of the assay over a meaningful dose range.
Until a well-standardized, broadly applicable human challenge model for TB comes into widespread use, animal models will continue to be used as a principal means of evaluating candidate vaccines. In light of the lack of efficacy in the phase 2b trial in infants of MVA85A (see Table 32), a reassessment of the preclinical animal data that supported the advancement of this candidate vaccine to efficacy trials has occurred (798). The recommendations from McShane et al. include increasing the use of clinical isolates of M. tuberculosis in animal models, powering preclinical studies for the desired level of efficacy in human clinical trials, conducting preclinical studies in the same age groups targeted for human vaccine trials, evaluating protection from infection and from disease, and developing specific animal models for the prevention of latency.
An additional limitation of the animal models for TB is the difficulty of replicating natural infection by the aerosol route in laboratory studies. A study in which guinea pigs were infected, not by intranasal instillation as is done in the laboratory, but by the exhaust air from a hospital TB ward, highlights this difficulty (799). Seventy-five percent of 362 guinea pigs exposed to the exhaust air from the TB ward converted to PPD skin test reactivity, but only 12% developed histopathologic evidence of disease. One-fifth of PPD-positive tests subsequently reverted, but later reappeared—possibly the result of reexposure. These complex dynamics, observed in a setting that more closely resembles natural TB infection, may not be possible to reproduce in animal and human challenge models. In addition, in some of the currently used animal models, M. tuberculosis fails to establish latency (797, 800, 801). In light of the pipeline of candidate vaccines that address latent stages of TB, this is a significant limitation.
An alternative approach using bronchoscopically instilled BCG and M. tuberculosis PPD as challenge agents has recently been reported (802). This study was conducted in 106 healthy South African adult volunteers with a range of prior M. tuberculosis exposure from asymptomatic household contacts to subjects previous treated and recovered from microbiologically confirmed TB disease. Importantly, the challenge was safe: reported adverse events were mild. Using broncheoalveolar lavage fluid collections before and after challenge to examine the immune responses to challenge, the investigators demonstrated several notable changes at the levels of cellular profiles, as well as gene expression and regulation. The bronchioscopic instillation BCG challenge may play an important role in future evaluations of TB vaccine candidates.
A key limitation of the TB human challenge models described above is that neither intradermal inoculation nor bronchioscopic instillation mimic the natural route of aerosol infection. Two clinical studies in healthy BCG-naive adults are ongoing to explore aerosol BCG challenge at University of Oxford. The first will compare aerosol with intradermal BCG administration (ClinicalTrials.gov identifier NCT02709278) (803) and the second will involve bronchioscopic evaluations up to 56 days postchallenge (ClinicalTrials.gov identifier NCT03912207) (804). The objective of these challenge studies is to obtain a body of data indicating aerosol BCG can be safely administered to healthy adult volunteers. In parallel, an attenuated strain of M. tuberculosis is being developed, to be administered by the aerosol route if BCG proves to be safe by this route of administration. Some sort of reporter molecule is envisioned as a readout, since the current attenuated M. tuberculosis is largely, if not completely, replication incompetent. It is not entirely clear how the data would be collected to establish whether the attenuated M. tuberculosis strain could nevertheless establish latency, which may represent an additional concern. These are high hurdles to be overcome, particularly the inclusion of a reporter strain in a challenge study, which has no precedent among regulatory authorities. In any case, these are important efforts, should they prove fruitful, to improve the relevance of the human challenge model for TB.
(iv) Summary
Current drugs and BCG vaccine alone will clearly not be enough to bring TB under control. In the two decades since the WHO declared TB a global emergency, the infusion of resources has made a more complete understanding of the immunology and cell biology of TB infection possible and has led to a robust and diverse pipeline of candidate vaccines. Despite recent disappointments in some TB vaccine clinical trials, reason exists for cautious optimism. On the one hand, the scientific community has a better understanding of why the protection afforded by BCG is so limited and variable. Also, significant discoveries may enable the reengineering of the world’s only TB vaccine for greater efficacy across all age groups. Boosting BCG with subunit vaccines that supply antigens against multiple stages of M. tuberculosis infection has taken hold as a concept and vaccines of this type are in early clinical development, together with vaccines that target only the active replication of M. tuberculosis. Further study of individuals that are latently infected, yet retain immune control of their infection over many years, is clearly warranted. The immune responses in these individuals may hold the key to the development of more effective TB vaccines.
Several human challenge models for evaluating candidate TB vaccines are under development. The challenge strain is BCG, administered by either intradermal inoculation, bronchioscopic instillation, or aerosol in healthy adult volunteers with or without prior vaccination with BCG. To regard the models as tools for downselecting candidate vaccines, accepting the hypothesis that a reduction in the replication of BCG predicts the efficacy of vaccines against M. tuberculosis in humans is necessary. The capacity of animal models to fully support the development of TB vaccines is also limited.
Some experts in the field regard the next 10 to 15 years as a critical time for TB vaccines to demonstrate improved efficacy while maintaining safety (805). They highlight an upcoming bottleneck in the availability of clinical trial sites and capacity. Moreover, unless a strategy to prevent reactivation of disease in the overwhelming numbers of humans that may already be latently infected with TB is established, attaining the goals for TB elimination is likely to be further delayed (756). At the present time, we have no means to identify those people with latent TB that are most likely to progress to active infection, nor do we have a practical way to prevent reactivation short of a new and effective multistage vaccine. One possible roadblock ahead is the potential to cause harm with vaccines that stimulate immune responses against latent TB infection. Part of the damage to the lungs in TB infection may be caused by a vigorous immune attack on granulomas in the lungs, which can damage host cells in the process of attacking the pathogen. Photographs of cavities in the lungs of the TB infected are a vivid reminder of this possibility.
Pertussis
(i) Public health impact of Bordetella pertussis and the evolution of pertussis vaccines
Pertussis, also called whooping cough, is a serious childhood respiratory disease mainly caused by the bacterium Bordetella pertussis. Before the widespread use of whole-cell pertussis vaccines, 115,000 to 270,000 cases of pertussis occurred per year in the United States (806). Whole-cell pertussis vaccines were licensed in the United States in 1914 and came into widespread use after their incorporation into the diphtheria, tetanus toxoid, whole-cell pertussis (DTP) vaccine in 1948. By 1960, DTP vaccines were fully utilized and the rates of pertussis had fallen to 1,200 to 4,000 cases per year in the United States—representing a 99% reduction in the rate of pertussis after vaccine introduction. Pertussis was regarded as a vaccine-preventable disease that was under control; however, the control of pertussis has gradually eroded over the last 30 years in the United States (807) and internationally (808). In 2018, more than 15,000 cases were reported to the CDC. A similar resurgence of pertussis is occurring throughout much of the developed world, in spite of high rates of vaccination with acellular pertussis (aP) vaccines (809–811). The WHO estimated that there were 24.1 million cases of pertussis and 160,700 deaths globally due to pertussis among children under 5 years of age in 2014 (812).
Although pertussis vaccination was accepted as standard practice in the United States from 1960 onward, the initial positive perception of pertussis vaccines had changed by 1990. Whole-cell pertussis vaccines are very reactogenic due to the presence of bacterial endotoxin. Most infants experienced injection site reactions after vaccination, about half developed fever, and a few serious systemic reactions to the vaccine occurred, including isolated cases of panencephalitis that lead to serious concerns (813). A few of the licensed DTP vaccines apparently had manufacturing issues and were found to be associated with poor protection, leading to new outbreaks of pertussis. Concerns about safety and effectiveness, coupled with a number of lawsuits that stemmed from injuries believed to be vaccine associated, caused some manufacturers of DTP vaccines to withdraw their products from the market. With widespread use of the DTP vaccine, generations of parents lacked the experience of witnessing an actual case of pertussis, one more factor influencing public perception about the vaccine. Pertussis vaccination programs in many countries were discontinued and vaccination programs were less accepted in others (805). The basic illness does not provoke an inflammatory immune response and usually occurs without a significant fever. What follows is a severe illness with periods of severe coughing, followed by periods without respiratory symptoms. The cough has a distinctive inspiratory whoop and posttussive emesis. These severe and protracted spasms of coughing up mucus may last for months (814).
The growing reluctance to accept the reactogenic whole-cell pertussis vaccines in the 1970s and 1980s spurred a greater effort to develop acellular vaccines that would not contain endotoxin. Acellular vaccines are based on specific antigens of B. pertussis rather than on the whole bacterium. Multiple candidate antigens were tested in various combinations in the 1980s. The outcome of testing was to select the following antigens for inclusion in acellular vaccines: filamentous hemagglutinin (FHA), pertussis toxin, pertactin, and a combination of fimbriae 2 and 3. By 2002, acellular pertussis vaccines from GlaxoSmithKline and Sanofi Pasteur had been licensed for use in infants in the United States, and the U.S. Advisory Committee on Immunization Practices had recommended that acellular pertussis vaccines completely replace whole-cell pertussis vaccines in U.S. childhood immunization series. Table 33 describes the currently licensed B. pertussis vaccines in the United States.
TABLE 33.
Currently licensed Bordetella pertussis vaccinesa
| Trade name | Valency | Components | Developer | Licensure (yr) |
|---|---|---|---|---|
| Infanrix | DTaP(3) | Pertussis toxin, filamentous hemagglutinin, pertactin, diphtheria toxoid, tetanus toxoid | GlaxoSmithKline | 1997 |
| Daptacel | DTaP(5) | Pertussis toxin, filamentous hemagglutinin, pertactin, fimbrial types 2 and 3, diphtheria toxoid, tetanus toxoid | Sanofi Pasteur | 2002 |
GlaxoSmithKline has licensed additional formulations of the same three pertussis antigens combined with inactivated poliovirus and hepatitis B virus. Sanofi Pasteur has licensed additional formulations of the same five pertussis antigens in different combinations with inactivated poliovirus, Haemophilus influenzae type B, and hepatitis B under the names Tetraxim, Pentaxim, and Hexaxim.
In the decades since their introduction, the acellular vaccines have been safe. They have provided good short-term protection from disease, but the duration of protection is not comparable to that provided by whole-cell pertussis vaccines. The duration of protection has been evaluated in observational studies and during pertussis outbreaks. Both types of studies indicate that the disease-free interval after vaccination has been decreasing over the past two decades in the era of acellular pertussis vaccines.
In 2010/2011, the U.S. state of California experienced the largest outbreak of pertussis in a half-century. The majority of cases were in school-aged children that had only received the acellular vaccines. The outbreak saw 7,200 cases of pertussis and ten infant deaths. The U.S. states of Michigan, Ohio, and Oklahoma, as well as Australia and Ireland, all reported similar outbreaks during the same general time period.
The poor durability of protection with acellular vaccines has prompted the development of new candidate vaccines (815–817); however, difficult it may be to replace a currently used vaccine (815). At the same time, research intended to uncover the basis for the waning efficacy of acellular pertussis vaccines is gaining momentum. Several hypotheses have advanced to explain the poor durability, and possibly lower efficacy, of acellular vaccines compared to whole-cell vaccines. These hypotheses include a different quality of immune response generated by the different vaccines (818), escape of bacterial strains from vaccine-induced immunity (813, 819), and undiagnosed increases in infections with Bordetella parapertussis (a related strain of Bordetella that causes a similar, but milder, respiratory disease in humans) (816, 820). The current pertussis vaccines do not protect against B. parapertussis (819).
A new recombinant acellular pertussis vaccine developed by BioNet-Asia that contains genetically inactivated pertussis toxin and FHA (821) has been tested in adolescents as a monovalent vaccine (aP[PTgen/FHA]) and in combination with tetanus and reduced-dose diphtheria vaccines (TdaP[PTgen/FHA]). Most significant is that 1 year after vaccination, more than 70 subjects still maintained seroconversion, while only 4% of those that had received the TdaP control vaccine had maintained seroconversion (822). Ongoing studies have tested the vaccine in women of child-bearing age and more recently in pregnant women (823).
(ii) Immunity to Bordetella pertussis
Immunity to B. pertussis has been the subject of a comprehensive review (817). The current data on protective immunity come from studies of humans that have received whole-cell and acellular pertussis vaccines, and from a murine respiratory challenge model of pertussis.
Higgs et al. used a combination of human and murine data to provide an informative description of the different mechanisms of immunity that whole-cell and acellular pertussis vaccines may induce (Fig. 15) (817). The killed bacteria in the whole-cell vaccine are engulfed and degraded by phagocytic cells of the immune system which present the bacterial antigens to naive T cells and produce cytokines that drive the differentiation of two types of T cells, Th17 cells which produce an IL-17 that activates neutrophils to take up and degrade intracellular bacteria, and Th1 cells produce IFN-γ that promotes the development of opsonizing and complement-fixing antibodies. These antibodies bind to antigens on the surface of the bacteria and promote their uptake and degradation by activated macrophages and can also directly kill bacteria in the presence of complement. This Th17/Th1-biased immune response is thought to be responsible for the efficacy of whole-cell pertussis vaccines.
FIG 15.
Distinct mechanisms of immunity induced with whole-cell and acellular pertussis vaccines.
In contrast, the cytokines produced in response to acellular vaccines are less driven by pathogen-associated molecular patterns but are influenced by the aluminum hydroxide (alum) adjuvant that is included in the vaccines. Alum drives the production of IL-1, which fosters the development of Th17 cells. Phagocytic cells that take up the acellular vaccine produce IL-4 by an unknown mechanism. IL-4 drives differentiation Th2 cells. Th2 cells produce cytokines that drive antibody production, but the antibodies are of a different subclass that does not opsonize or fix complement. Therefore, the immune responses would not result in the efficient elimination of extracellular bacteria, as occurs with the whole-cell vaccine.
Additional data on the immunity generated by acellular pertussis vaccines have been gathered using a nonhuman primate model of infection with B. pertussis (824). Warfel et al. vaccinated infant baboons with acellular or whole-cell pertussis vaccines and challenged them at 7 months of age with B. pertussis. They measured symptoms of disease and rates of colonization using nasopharyngeal washes after challenge. The acellular pertussis vaccine prevented the most severe symptoms of disease but did not prevent colonization, did not clear colonization any faster than in unvaccinated animals, and did not prevent transmission to unvaccinated contacts. The animals vaccinated with the whole-cell pertussis vaccine had a more rapid clearance of colonization compared to acellular vaccinated animals and unvaccinated controls. Previously infected animals were not colonized upon rechallenge. The T-cell responses to the acellular vaccine differed from the T-cell responses to the whole-cell vaccine and to natural infection. Naturally infected and whole-cell vaccinated animals had B. pertussis-specific Th17 and Th1 memory T cells, whereas animals that received the acellular vaccine had a Th1/Th2 memory response.
A factor that can limit the effectiveness of pertussis vaccines is the increased circulation of bacterial strains that are less susceptible to the vaccine-induced immunity (813, 818).
(iii) Development of pertussis vaccines
Data from a baboon model of pertussis and from human infections indicate that acellular pertussis vaccines provide substantial protection against the most severe symptoms of infection with B. pertussis (823). Therefore, efforts to improve acellular vaccines with respect to the level and durability of protection they afford should proceed in parallel with efforts to develop new vaccines against pertussis. Table 34 describes the B. pertussis vaccines under development. The data were compiled from a number of published articles (816, 825–830).
TABLE 34.
Bordetella pertussis vaccines under developmenta
| Vaccine type | Vaccine details | Development phase | Developer |
|---|---|---|---|
| Live attenuated | B. pertussis strain BPZE1 | Phase 1 | Public Health Agency of Sweden and Institut Pasteur de Lille |
| B. pertussis strain BPZE1f3 expressing serotype 3 fimbria Fim3 | Preclinical | Institut Pasteur de Lille | |
| B. pertussis strain GamLPV | Phase 1/2 | Gamaleya Research Institute, Russia | |
| aroQ mutant B. pertussis (ATCC 9340 strain) | Preclinical | University of Southern Queensland, Australia | |
| Whole cell | DTP low, chemical extraction to reduce lipooligosaccharide from the outer membrane of a whole-cell B. pertussis | Preclinical | Instituto Butantan, Brazil, and Netherlands Vaccine Institute |
| DNA vaccines | DNA plasmid expressing the N-terminal 180-amino-acid fragment (C180) of pertussis toxin S1 subunit | Preclinical | National Institute of Infectious Diseases, Japan |
| DNA plasmid expressing pertussis toxin subunit 1, fragments of pertactin, and filamentous hemagglutinin | Preclinical | Peking Union Medical College and Chinese Academy of Medical Sciences | |
| DNA plasmid encoding a genetically inactivated S1 domain of pertussis toxin | Preclinical | University of Southern Queensland, Australia | |
| Nanoparticle/microparticle | Pertussis toxoid and filamentous hemagglutinin encapsulated in poly-lactide-coglycolide | Preclinical | National University of Ireland |
| Microparticle-based vaccine consisting of pertussis toxoid, polyphosphazene, CpG ODN 10101, and synthetic cationic innate defense regulator peptide 1002 | Preclinical | University of Saskatchewan, Canada | |
| Chitosan-dextran sulfate nanoparticle formulation of pertussis toxoid with IgA adjuvant | Preclinical | Curtin University, Australia | |
| Protein-based | Expression of iron-repressible protein-3 pertussis antigen | Preclinical | Universidad Nacional de La Plata, Argentina |
| Acellular | Outer membrane vesicles expressing lipid adenylate cyclase PagL | Preclinical | Universidad Nacional de La Plata, Argentina |
B. pertussis, Bordetella pertussis; DTP, diphtheria-tetanus-pertussis vaccine; IgA, immunoglobulin A; IgG, immunoglobulin G.
Current acellular pertussis vaccines induce strong antibody and Th2 responses but fail to protect against nasal colonization and transmission of B. pertussis. Furthermore, immunity wanes rapidly after immunization. To improve on their performance several avenues are being pursued, including the introduction of novel adjuvants and intranasal immunization with the same acellular pertussis vaccine (831, 832). In addition, strategies to incorporate additional virulence factors of B. pertussis (e.g., adenylate cyclase toxin or iron regulated protein), and to include adjuvants like Toll-like receptor agonists that drive a stronger Th1 response (815, 816) are being pursued. Another approach under consideration is to present some of the antigens found in current acellular vaccines as DNA-vectored vaccines, or as particle or subunit vaccines. Table 34 describes three DNA vaccines and three particle vaccines that include some of the current acellular vaccine antigens or modified forms of them. An acellular vaccine based on the outer membrane vesicles that Gram-negative bacteria spontaneously secrete is in development (816). All of these candidate vaccines are at the preclinical stage of development.
On the other hand, live-attenuated pertussis vaccines have entered clinical trials. Thus, BPZE1, in which the genes for three toxins have been inactivated or removed, has been shown to protect mice and nonhuman primates. BPZE1 was evaluated in a phase 1 trial and found to be safe, immunogenic, and capable of colonizing the upper respiratory tract in humans (833). Another live-attenuated vaccine, GamLPV, was reported to have completed a phase 1 clinical trial (ClinialTrials.gov identifier NCT03137927) (834) and entered a second phase 1/2 trial (ClinialTrials.gov identifier NCT04036526) (835) at the Gamaleya Research Institute in Russia, but no results have been reported.
(iv) Challenge models for pertussis
Small animal models of B. pertussis infection do not replicate the spectrum of disease seen in humans. Nonhuman primate models of pertussis are under development (823, 836, 837). In their initial study, Warfel et al. compared the spectrum of disease developed after inoculating rhesus macaques or baboons with either of two strains of B. pertussis described in Table 35. These researchers found that seven of seven rhesus macaques were persistently infected with B. pertussis, but only one developed clinical signs of disease. Nine of nine inoculated weanling baboons developed classical symptoms of pertussis. After 6 months, four of the infected baboons and two naive controls were rechallenged with B. pertussis. Both of the controls developed disease, but none of the four convalescent baboons developed disease.
TABLE 35.
Baboon and human challenge models for Bordetella pertussisa
| Host species | Challenge organism | Strains | Method of production and administration | Location |
|---|---|---|---|---|
| Baboon | B. pertussis | Tohama I, obtained from the U.S. Food and Drug Administration, and D425, a clinical isolate provided by the U.S. Centers for Disease Control and Prevention | B. pertussis was grown on Bordet-Gengou agar, resuspended in PBS to a density of 109 to 1010 CFU/ml. Baboons were anesthetized and intubated to deliver 1 mL of the inoculum to the top of the trachea and a catheter was used to deliver 0.5 mL of inoculum to the back of each naris. | U.S. Food and Drug Administration, Center for Biologics Evaluation and Research |
| Human | B. pertussis | B1917, isolated from a Dutch patient and prepared under GMP by Q Biologicals (Belgium) | Frozen vials of B. pertussis were thawed, diluted to the appropriate dose, and directly administered intranasally with 0.5 mL in each naris at 103 to 105 CFU/ml. | National Health Institute for Health Research CRF, Southampton, United Kingdom |
B. pertussis, Bordetella pertussis; CRF, clinical research facility; GMP, Good Manufacturing Practice.
Table 35 provides further details on the route of administration of the challenge strains. The baboons were anesthetized and intubated to deliver part of the inoculum to the top of the trachea, and the remainder of the inoculum was delivered intranasally. As described in the section on immunity to B. pertussis, the baboon model has been used to evaluate immune responses to whole-cell and acellular pertussis vaccines, and to demonstrate that natural infection with B. pertussis provides protection against subsequent colonization and disease. Therefore, the baboon model has demonstrated capacity to evaluate correlates of protection afforded by infection and vaccination. Moreover, a correlation is apparent between the age of the infected baboons and the severity of disease, as observed in humans (835). Airborne transmission of B. pertussis has also been demonstrated in the baboon model (836). Infected baboons could transmit B. pertussis to uninfected cage mates and to uninfected animals housed in cages more than 2 m away.
The baboon model of B. pertussis infection has several limitations. No established, large-scale breeding program exists to ensure an adequate supply of animals for research, and access to infant or juvenile baboons may be even more limited. With respect to evaluating immune responses, reagents that cross-react with baboon antigens are highly limited, particularly those used for separating cells by flow cytometry. Developing such reagents may be a lengthy process.
A human challenge model for pertussis has been discussed for several years (835). One reason to consider this option is that no widely available animal model of pertussis currently exists. In practical terms, the baboon model cannot be expected to provide the capacity needed to support multiple vaccine development programs or to provide the numbers of animals that would be needed to conclusively establish correlates of protective immunity, at least in the near term. A more important limitation for the field of pertussis vaccine development is the lack of adequate field sites with sufficient incidence of pertussis to permit the evaluation of candidate vaccines. Moreover, placebo controls will not be ethically acceptable in field trials, whereas they may be acceptable in the controlled inpatient setting of experimental human infection where prompt treatment is assured. New vaccines will need to demonstrate at least equivalence, if not superiority of the licensed vaccines for preventing disease after infection with B. pertussis, and the required field trials would be large, lengthy, and costly. Smaller comparison trials in a human challenge model could potentially provide some of the data that would support licensure of new pertussis vaccines.
On the other hand, human challenge models for B. pertussis would have significant limitations. Studies would need to be conducted in specialized inpatient facilities with a high level of containment since pertussis is highly contagious by the aerosol route of infection. The delivery of a challenge dose to the lower respiratory tract, as has been done with the baboon model, may not be feasible in humans. Highly effective treatment will be a requirement as soon as infection is established. This limitation is imposed by the fact that antibiotics are effective against B. pertussis early in infection, but there is a “point of no return” beyond which antibiotics are ineffective (813). There is a risk of serious disease, which may not be completely abrogated by early treatment in every case. Since the development of disease will not occur with early treatment, the human challenge model would be limited to the evaluation of vaccines for their capacity to prevent infection rather than to prevent disease.
A protocol for a pertussis human challenge model that addresses some of the issues noted above has been proposed by de Graff et al. (838) and recently implemented (839, 840). The methods of the protocol are summarized in Table 35. Subjects with anti-pertussis toxin IgG concentrations less than 20 IU/mL were inoculated intranasally with wild-type B. pertussis strain B1917 in an inpatient unit. Only minor symptoms were observed. Colonization, assessed by culture and quantitative PCR, was detected in 80% of subjects that received a dose of 105 CFU of the organism. Azithromycin eradicated colonization within 48 h in 88% of colonized individuals. Anti-pertussis toxin IgG seroconversion was observed in 9 of 19 colonized participants and in none of those not colonized. The organism was not detected in environmental samples.
(v) Summary
Acellular pertussis vaccines may need reformulation or replacement if we are to maintain control of B. pertussis in human populations. Given the actual and perceived safety concerns that arose with the use of whole-cell pertussis vaccines, reformulated whole-cell vaccines or live-attenuated vaccines seem unlikely to gain public acceptance. One potentially useful measure would be to remove pertussis vaccines from their current formulations with other vaccines to create a pertussis-only vaccine (815). Such a vaccine could be delivered on its own schedule, with repeated boosting if required. A change in antigen content of the vaccine could be considered (e.g., by adding additional antigens thought to be protective or changing the method for detoxifying pertussis toxin). More substantive changes could include modifying adjuvants or changing the delivery system (e.g., to the intranasal route) (815).
These redesign options for acellular pertussis vaccines must be weighed against the potential to develop new vaccines of clearly superior levels or duration of protection. As shown in the baboon model of B. pertussis infection, preventing lengthy carriage of B. pertussis will be particularly important to prevent transmission to others. To expect that any of the new candidate vaccines would replace the current acellular vaccines in the coming decade would seem to be unrealistic.
The baboon model for pertussis could be significantly expanded, but nature is against us because the establishment of large breeding colonies takes time. Reagents need to be developed for the model to reach its potential for evaluating protective immunity. A recently developed human challenge model may be useful in the evaluation of future vaccines but would present logistical challenges and would be limited to evaluating asymptomatic colonization. The increased severity of disease seen with B. pertussis infection in infants and young children compared to adults is replicated in the baboon model of infection but would not be replicated in a human challenge model with adult volunteers.
Severe acute respiratory syndrome coronavirus 2
A novel coronavirus was identified toward the end of 2019 as the cause of a cluster of pneumonia cases in Wuhan, China. The virus is designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is the causative agent of the disease COVID-19. SARS-CoV-2 spread rapidly around the world, and the outbreak was declared a “Public Health Emergency of International Concern” by the WHO on 30 January 2020; the outbreak of COVID-19 was officially declared a pandemic on 11 March 2020 (841). As of 30 September 2021, more than 236 million cases and more than 4.8 million deaths from COVID-19 had been reported (842). Person-to-person spread of SARS-CoV-2 occurs primarily through respiratory droplets. Infection may also occur by touching an infected surface and then one’s own eyes, nose, or mouth. The detection of viral RNA does not necessarily indicate the presence of infectious virus, and there may be a threshold of viral RNA level below which infectivity is unlikely (843). Several reports have described detection of SARS-CoV-2 RNA and infectious virus in stool specimens (844, 845), but this does not appear to be a significant factor in the spread of infection.
The interval during which an individual with COVID-19 is infectious is uncertain, although SARS-CoV-2 can be transmitted prior to the development of symptoms and throughout the course of illness, with the highest virus levels soon after symptom onset (842, 846–848). The duration of viral shedding is highly variable and may depend on the severity of the illness (842, 844, 849–851). The risk of transmission from an individual with SARS-CoV-2 infection varies by the type and duration of exposure, factors such as the amount of virus in respiratory secretions, and the use of preventive measures.
Due to the immense and urgent need for interventions to stop the pandemic, several clinicians and bioethicists have proposed the development of a SARS-CoV-2 controlled human infection model (CHIM) to accelerate the development of new vaccines and therapeutics despite the unprecedented speed in bringing both conventional (inactivated SARS-CoV-2) and novel (mRNA, viral vector) technologies to the market saving millions of lives. The advancement of a human infection model of SARS-CoV-2 in low-risk volunteer subjects (852–857) provides a unique perspective that is novel and potentially ground-breaking but is not without controversy. The WHO has provided criteria for ethical acceptability of human challenge models using live SARS-CoV-2 challenge virus (858).
First, there needs to be scientific rationale. It is assumed that the application of a SARS-CoV-2 human challenge model would be faster than the conventional clinical trial pathway for testing vaccine candidates and experimental therapeutics. With the COVID-19 pandemic (as of October 2021), well into its second year, considerable progress has been achieved in delivering therapeutics (remdesivir and monoclonal antibodies such as casirivimab and imdevimab, and bamlanivimab and etesevimab) and vaccines (Pfizer, Moderna, Johnson & Johnson, AstraZeneca) without the availability of a standardized and validated human infection challenge model. Nevertheless, a successful human infection model will depend on factors such as (i) availability of Good Manufacturing Practice stocks of virus (859), (ii) advanced knowledge of minimal infectious dose, (iii) secure clinical trial facilities with rigorous infection control procedures for prolonged periods of time, (iv) specific treatment(s) for complications, (v) consensus among policymakers, and (vi) a priori regulatory approval.
Assessment of risk and benefit depends on how comfortable regulatory agencies are with accepting the clinical endpoint in human challenge trials to approve a vaccine without a pivotal trial under natural exposure. If not, then the product sponsor may use challenge studies to either advance or stop further development of that vaccine in order to spare the cost of developing a vaccine that is unlikely to provide an acceptable level of protection. In either case, the risk to any subject in the control group may outweigh any potential benefit, especially when there is no therapeutic intervention that would prevent a serious adverse event related to the challenge, in contrast to challenge with other pathogens for which there are highly effective rescue therapeutics available. Furthermore, the relatively limited understanding of COVID-19 pathogenesis represents an additional risk, in contrast to challenge with pathogens that have been studied for decades and are well characterized. Several authors (851, 852, 857) have argued that because younger persons (i.e., 18 to 49 years) are less at risk for serious complications from COVID-19, this alone justifies consideration of the model. These researchers further argue that if rigorous informed consent is obtained, then it would be ethically justifiable to proceed; however, there is not universal agreement that informed consent alone is ethically justifiable.
The first human challenge model using virus stocks of SARS-CoV-2 has been approved by regulators and ethical committees sponsored by the University of Oxford (NCT04864548). This phase I dose escalation challenge study of up to 64 subjects will be challenged with increasing titers of wild-type SARS-CoV-2 administered intranasally in order to achieve a 50% attack rate as determined by quantitative viral detection and/or qPCR detection in nasopharyngeal secretions at 12-h time points. Rescue treatment monoclonal antibody cocktail (casirivimab and imdevimab) will be administered to subjects after indication of COVID-19 disease beyond mild symptomatology. A Data Safety Monitoring Board will review safety and viral infection data.
Alternatives to intranasal challenge have also been proposed to include oral administration of SARS-CoV-2 that induces a limited infection in the gut without spread to the respiratory tract (860), and attenuated viral challenge strains that induces a self-limiting infection. Finally, coronaviruses different from SARS-CoV-2 and that are known to cause mild upper respiratory symptoms have been considered.
There are several uses cases for SARS-CoV-2 human challenge model development. First, it would accelerate the “Go/No Go” decision at the end of each stage gate in the project both for vaccine and therapeutic development. Second, a SARS-CoV-2 CHIM could be used to define correlates of risk or protection that would then justify regulatory approval. A validated challenge model would also permit us to better understand how long naturally acquired or vaccine-induced immunity lasts, and may also help us separate protection against infection versus protection against ongoing forward transmission especially in asymptomatic infected subjects. Third, a SARS-CoV-2 challenge model could be implemented to test updated vaccines against newly emerging variants during a period when the new variants have been identified but are not yet highly prevalent in the population, thus making traditional clinical trials difficult to conduct or interpret. Fourth, the model could be used to determine reinfection rates by drifted variants in comparison to earlier ones. Finally, it would increase our knowledge and understanding of the early events in viral pathogenesis and immune responses that control replication leading to viral clearance (853).
Notwithstanding the possible advantages offered by a CHIM, a number of limitations, logistical barriers, and critical barriers have been raised (853), including by participants in a recent webinar hosted by the International Alliance for Biological Standardization (861). These limitations include the route of administration, dose-finding studies, the timing vis-à-vis vaccine administration, the generalizability to at-risk vulnerable populations, the requirement for sufficient biocontainment facilities, the GMP compliance of virus stocks from at least two different viral isolates, the need for rescue therapy, and the ethics related to participant selection, premature participant withdrawal necessitating the need for forced quarantine, the risks to study staff, and potential transmission of escaped virus into the general population. Nonetheless, the eagerness to pursue such measures by groups that have never developed, participated in, or led a CHIM trial needs to be balanced by thoughtful considerations of whether classical phase 3 trials should continue to be the gold standard for evaluation of COVID-19 candidate vaccines and therapeutics.
REGULATORY AND ETHICAL CONSIDERATIONS FOR CONDUCTING HUMAN CHALLENGE STUDIES
Three different types of human challenge studies are subject to review by regulatory authorities (see Table 36). These include challenge studies in which a strain of a pathogen is administered to adult volunteers to develop a consistent model and investigate pathogenesis; studies in which a challenge is followed by rechallenge at some later time, with the same or a different challenge strain, to understand the level of protection afforded by prior infection; and studies in which a vaccine is administered prior to challenge to test its protective efficacy. These three types of studies have somewhat different regulatory requirements that depend on the country in which the challenge study is to be conducted.
TABLE 36.
Types of controlled human challenge studies and their regulatory requirementsa
| Study type and description | Examples of study objectives | Regulatory requirements in the United States | Regulatory requirements in the United Kingdom |
|---|---|---|---|
| Challenge study: a challenge strain of a pathogen is administered to healthy adult volunteers. | Establish dose-response curve for infection, time to onset of disease, range of disease symptoms, time to resolution of symptoms if no specific treatment is available, effect of prior natural exposure to the pathogen. Evaluate immune response to infection. Establish or optimize model. | Study must be conducted under an IND application that has been approved by the U.S. Food and Drug Administration. Challenge strains are ideally manufactured in compliance with GMP at a level similar to that required for a product to be studied in a phase 1 clinical trial. Once allowed to proceed, IND applications (or drug master files) for challenge strains may be cross-referenced in subsequent studies. In addition, the study must undergo review and approval by an IRB, or multiple IRBs if the study is to be conducted at multiple sites. Other review agencies may also have jurisdiction under certain circumstances (see Table 37). | Study does not require review by the MHRA if it does not involve a vaccine. The challenge strains do not necessarily need to meet GMP requirements. Review and approval by an IRB is a requirement, and by other agencies in select circumstances (see Table 37). |
| Rechallenge study: a challenge strain of a pathogen is administered to healthy adult volunteers previously challenged with the same strain or a heterologous strain. | Determine whether prior infection protects against reinfection with a homologous or heterologous strain of the pathogen. Identify breadth and/or correlates of protective immunity. | Same as for challenge study. | Same as for challenge study. |
| Vaccine trial using a challenge model: a candidate vaccine is administered to healthy adult volunteers that are later (or in some cases, previously) challenged with one or more strains of the pathogen against which the vaccine (or drugb) is directed. | Evaluate the capacity of a candidate vaccine to prevent infection, colonization/carriage, or transmission of the pathogen; or limit severity of or prevent disease symptoms. Compare different vaccine formulations and/or regimens for the immune responses elicited and level of protection. A candidate vaccine may be directly compared to a licensed vaccine that is suboptimal in some important respect. | Candidate vaccine must be administered under an approved IND application and must undergo ethical review and approval by the relevant IRB(s), in addition to the approvals for the challenge strain and study procedures as described above. | Candidate vaccine must be administered under an MHRA-approved application. The protocol must also undergo ethical review and approval by the relevant IRB(s). |
IND, Investigational New Drug; GMP, Good Manufacturing Practice; IRB, institutional review board; MHRA, Medicines and Healthcare products Regulatory Agency.
The fourth type of challenge study that might be conducted is one in which the challenge agent is used to infect trial subjects that are then treated with investigational drugs for the purposes of drug development. This type of study is not described here, as the focus is on vaccine development.
The requirements from regulatory agencies that have jurisdiction over human challenge studies also vary with the type of study and the country in which the study is conducted (see Table 37). In general, human challenge studies may have a more complex, but perhaps clearer, regulatory requirement in the United States compared to the United Kingdom—the two countries in which the large majority of challenge studies have been conducted. The main differences are that challenge and rechallenge studies that do not include a vaccine are subject to review at the national level in the United States but not in the United Kingdom. Another important difference is that in the United States, the challenge strains themselves usually are required to meet a level of compliance with Good Manufacturing Practice (GMP) similar to that required for vaccines entering phase 1 clinical trials. In the United Kingdom, this condition has not been consistently required.
TABLE 37.
Regulatory agencies and committees with jurisdiction over human challenge studiesa
| United States | Indication for review | United Kingdom | Indication for review |
|---|---|---|---|
| FDA, Office of Vaccines Research and Review | A human challenge study is proposed, with or without a vaccine. | MHRA | A vaccine is included in the human challenge study. |
| IRB | A human challenge study is proposed, with or without a vaccine. | IRB | A human challenge study is proposed, with or without a vaccine. |
| Institutional Biosafety Committee | The challenge strain or vaccine is considered to be a GMO, and the institution where the research is conducted receives U.S. government funding. | Advisory Committee on Releases to the Environment of the Department for Environment, Food and Rural Affairs | The challenge strain or vaccine is considered to be a GMO. |
| U.S. Vaccines and Related Biological Products Advisory Committee to the FDA | The proposed study using a challenge model is a vaccine efficacy trial, particularly if proposed to be a pivotal efficacy trial to support vaccine licensure. |
FDA, U.S. Food and Drug Administration; IRB, institutional review board; GMO, genetically modified organism; MHRA, Medicines and Healthcare products Regulatory Agency.
Human challenge studies may or may not be regulated by a national regulatory authority (NRA); this depends on the country in which the research will take place. In all cases, clinical trials should be reviewed by ethics committees, which are referred to variously depending on the country and location of the clinical study. Terms for these institutions include an institutional review board (IRB), a research ethics committee, an independent ethics committee, or, simply, an ethics committee. For ease of readability in this document, we will use the term IRB to denote the institutional ethics committee responsible for overseeing clinical studies.
In cases in which the vaccine or challenge organism under study is considered to be a genetically modified organism (GMO), additional oversight may include a governmental regulatory agency separate from the NRA or may be performed by the NRA itself. Aspects of the clinical study involving a GMO may also be reviewed at the institute where the research is being conducted, or by an institutional biosafety committee (IBC). For studies receiving funds from the U.S. government review by an IBC is always required (see Table 37).
Below, we address each of the three aforementioned regulatory processes in turn. The regulatory procedures and expectations described reflect actual current practices by regulated industry and clinical trialists conducting studies in which humans are challenged with an infectious organism in the controlled setting of a human challenge study. In some cases, the ideal is described, but as with all regulated clinical studies, a case-by-case basis is considered by regulators and their actual requirements in any given case may not meet the ideal. Instead, their decisions are driven by scientific and ethical considerations, in addition to applicable regulations.
U.S. Procedures for Regulatory Authority Applications
Applications, filings, submissions, or dossiers (the term varies depending on the country) must be submitted to the various regulatory bodies in order to gain approval to conduct a human challenge study. The contents and format of the regulatory filing may also vary depending on to whom it is being submitted. The succeeding subsections will provide summaries on the terminology, contents, and format expected by regulators.
In the United States, human challenge studies are regulated by the NRA (the U.S. Food and Drug Administration [FDA]) and an IRB (or more than one IRB if multiple clinical sites are involved in the clinical study). Furthermore, if the challenge organism or the vaccine is deemed to be a GMO, an IBC will review the trial. In practice, at most U.S. institutions, the IBC will review all studies involving infectious organisms, whether or not they are GMO.
U.S. Food and Drug Administration
The FDA is the NRA that regulates human challenge studies, whether or not they involve a vaccine. The same FDA review office that has jurisdiction for vaccines, the Office of Vaccines Research and Review, is also responsible for challenge organisms. A sponsor of a clinical trial must submit an Investigational New Drug (IND) application to the FDA, which has 30 days to review and approve the proposed clinical trial(s) or place on clinical hold. The FDA prefers submission of separate IND applications for the challenge organism and the vaccine, even if they are to be used in the same clinical trial, at least initially. The rationale for this is that the challenge organism may be used to test other vaccine candidates, particularly if the original vaccine candidate fails (for reasons of safety, immunogenicity, or efficacy). In such a case, the sponsor of the new clinical trial may cross-reference the IND application describing the challenge organism without prejudice due to the failed vaccine candidate. Particularly, if the sponsors are different, this eases the regulatory burden on the new sponsor (and the FDA) to avoid reiterating information the FDA has already reviewed. The IND regulations are promulgated in Title 21 of the U.S. Code of Federal Regulations (CFR) part 312 (862) and subsections. Additional parts of 21 CFR may also apply in regard to product manufacture (863, 864), nonclinical research (865), and human subjects protections (866, 867).
In addition to review by FDA staff, the FDA may seek the advice of its expert advisory committee at its discretion. For vaccines and related products, this would be the Vaccines and Related Biological Products Advisory Committee. Information submitted to the FDA in an IND application is held confidential, protected by their regulations (868). The FDA’s advisory committees are generally held as open, public meetings. Phase 1 clinical trials are not typically the subject of an advisory committee meeting, although they can be. Efficacy trials, particularly pivotal efficacy trials to support product licensure, are generally reviewed by an FDA advisory committee. A human challenge study may fit in this category depending on the claims the sponsor may want to make, though this would be a rare and exceptional situation. The only guidance the FDA provides on human challenge studies specifically and their role in regulatory evaluation is in the 2011 Guidance for Industry: General Principles for the Development of Vaccines to Protect Against Global Infectious Diseases (869).
The contents and format of an IND application are promulgated in 21 CFR 312.23 (870). An alternative format, however, may be used, specifically in the form of a common technical document, an internationally accepted format for regulatory dossiers (871). In either format, the contents expected by the FDA will cover information about the product (in the case of a challenge organism, the manufacture and control of the challenge organism itself), nonclinical research and development, and proposed clinical trial(s). The information about the product or challenge organism includes the microbiology, manufacturing, and controls (referred to as chemistry, manufacturing, and controls), reflecting that the majority of FDA IND reviews are for chemical drugs rather than for vaccines or challenge organisms. The nonclinical information includes pharmacology and toxicology, which for vaccines would be the immunogenicity and proof of concept (e.g., protection from challenge in an animal model), as well as other laboratory-determined characterizations, and the safety studies (in animals and/or in vitro). Finally, clinical information not only includes the protocol for the proposed trial(s) but also several other documents. These include a sample informed consent form, signed and completed FDA forms 1572 and 3674, the investigators’ curriculum vitae and financial disclosures (FDA forms 3454 and 3455), a summary of prior human experience, and an Investigator’s Brochure if the trial will be conducted at multiple sites or by an investigator(s) who was not intimately involved in the product development. The Investigator’s Brochure will be particularly important for human challenge studies conducted at multiple sites, as it will provide necessary information to ensure that all investigators are fully informed about potential risks to the human subjects they will enroll in the study. Another document that may be required is a general investigational plan that describes what the sponsor plans for the overall development of the product, with greater focus on the coming year (872).
Depending on the phase of the clinical trial, the FDA has a sliding scale of expectations for compliance with GMP regulations (863): phase 1 studies do not require full compliance with the GMP regulations, but products used in phase 2 or 3 studies are required to be manufactured and controlled in full compliance with GMP regulations. The FDA recognizes that challenge organisms are not a true product in development, but a tool for vaccine development and for other scientific experiments, such as experimental medicine and human challenge studies. Therefore, even if used in phase 2 or 3 efficacy studies, the FDA will likely have the same expectations for challenge organisms as for phase 1 products. This may not be strictly the case if the challenge organism will be used widely in a relatively large number of phase 2 and 3 human challenge studies or given to larger numbers of individuals (e.g., exceeding 100 to 200 people). But, since the scale of human challenge studies usually involves numbers of subjects more in line with phase 1 vaccine trials, the FDA will not likely expect full compliance with GMP (873, 874). Nonetheless, aspects of GMP will apply to the manufacture and testing of human challenge organisms. For this reason, consulting the FDA’s Guidance for Industry: CGMP for phase 1 Investigational Drugs (872) is recommended for sponsors conducting a human challenge study. Particularly, aspects of production in a multiuse facility, such as stringent changeover procedures and the need for cleaning validation, will be important given the infectious and potentially pathogenic nature of the challenge stock being made. While other processes might not be validated, validation of the cleaning (and disinfecting/sterilizing) procedures will likely be expected.
The FDA has 30 days in which to review the original submission of the IND application. At the end of the 30-calendar-day period, the sponsor may initiate the clinical trial unless they have heard from the FDA that the IND or the protocol has been placed on clinical hold. The grounds for clinical hold are promulgated in 21 CFR 312.42 (875); they primarily focus on the safety of any phase trial or, for phase 2 or 3 studies, the study design must be adequate to meet stated aims. If the IND application is placed on hold, the sponsor must not initiate or must halt the clinical trial (if ongoing already). The FDA has 30 days to provide, in writing, the grounds on which the application was placed on clinical hold and what must be done for the clinical hold to be lifted. When the sponsor provides a complete response to the clinical hold letter (as deemed complete by the FDA), the FDA has another 30 calendar days to review the complete response and provide, in writing, to the sponsor whether the clinical hold has been lifted, and if not, why not, and what further must be done for the hold to be lifted. The FDA may also request modifications to the proposed trial without placing a clinical hold. The sponsor may initiate the clinical trial in that case but should respond to the requested modifications. If a sponsor fails to address the requested modifications, the FDA may not accept the data from the completed clinical trial to support product development. Therefore, making the requested modifications as soon as possible is in the sponsor’s interest, ideally before initiating the trial.
If the investigational product is to be imported into the United States for use in U.S. clinical trial sites, the FDA has jurisdiction to permit the importation once the IND application is in effect (i.e., after the 30-day review and when no clinical hold is in place). U.S. Customs will determine the status of the IND application before allowing the shipment of the investigational product to pass U.S. borders. Shipments of investigational products for an IND application that is on hold or is not yet in effect will be held.
If a U.S. manufacturer wishes to export a product for a clinical trial outside the United States, then the FDA also has jurisdiction if the trial site is in a country that is not on a list of countries with NRAs the United States recognizes as having equivalent responsibility and capability to adequately regulate the product exported from the United States. The import and export regulations are promulgated in 21 CFR 312.110 (876). If the product is being shipped to a country with a competent NRA recognized by the FDA (i.e., in the European Union and European Economic Community, Australia, Canada, Japan, Israel, New Zealand, Switzerland, or South Africa), then the NRA in that country has jurisdiction over the product from the United States that is imported into their country.
Safety reports must also be provided to the FDA for their review. The safety reporting expectations for any clinical trial under an IND application are promulgated in 21 CFR 312.32 (877). Expedited safety reporting is required for serious and unexpected adverse events (within 15 calendar days of the sponsor being informed about the adverse event). Other adverse events must also be reported to the FDA at a frequency determined by the agency, but at least annually. Discussing with the FDA what their reporting expectations would be for expected adverse events from the challenge organism, if a pathogenic one is used, might be appropriate. Primarily, expedited reporting is for serious and unexpected events; however, a clear plan should be described in any protocol submitted for FDA review.
U.S. Institutional Review Boards
Human challenge studies will be held to the same ethical review standards as all research involving human subjects, so the human subject protection standards, including the need to gain approval from an IRB, should apply. This process will be no different than it would for any investigational medical interventional research. Essentially, each clinical trial site and institution participating in the research will require their institution’s IRB approval, unless agreement for review by a central IRB can be obtained. The principal investigator or investigator at the particular trial site will submit to their IRB the proposed clinical protocol, the informed consent documents to be used at that site, the investigators’ and subinvestigators’ curriculum vitae and training records (e.g., Collaborative Institutional Training Initiative training records), and an Investigator’s Brochure if there is one. Also, any recruitment material to be used should be provided for IRB review. The IRB will review and either approve the research, approve the research conditional upon addressing the IRB’s stipulations, or disapprove the research. The clinical trial site must not proceed with a trial until the IRB has approved it and must use the IRB-approved informed consent form and protocol. The IRB will also require updates on the progress of the clinical trial, at least annually. Adverse events occurring during the study must be reported to the IRB for their review and oversight according to the guidelines of the IRB.
U.S. Regulations for Genetically Modified Organisms
If the challenge organism or vaccine is a recombinant organism generated by recombinant DNA technology (i.e., genetic engineering), then it could be considered to be a GMO. GMOs are subject to procedures beyond those required for non-GMOs (i.e., natural organisms or organisms that are the result of manipulated breeding or hybridization). In the United States, the FDA has the authority to review and approve clinical trials conducted with GMOs. The U.S. National Institutes of Health (NIH) also has some oversight responsibilities, if the institution that will perform the research receives U.S. government funding. The NIH Office of Science Policy issued a document explaining the procedures, which are quite complex depending on a number of factors. This document, NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (878), describes the three separate, but overlapping, regulatory procedures of the FDA, IRBs, and IBCs. The Office of Science Policy also provides information governing IBCs. IBCs are responsible for overseeing the research to ensure that the risks to the environment and public health are managed through adequate biological containment, investigator qualifications and training, appropriateness of standard operating procedures (SOPs) and protocols for the research, and compliance (e.g., review of adverse event reports). An IBC may approve or disapprove the research.
United Kingdom Procedures for Regulatory Authority Applications
The European Medicines Agency (EMA) does not approve controlled human infection model (CHIM) study protocols, leaving to the NRAs to decide whether they wish to regulate CHIM studies. Nonetheless, EMA can provide scientific advice to developers on the use of CHIM approaches. The Medicines and Healthcare products Regulatory Agency (MHRA) is the UK’s NRA. The filing to the MHRA should be in the format of the forms for EudraCT or the Integrated Research Application System (879). Similar to the FDA, information on the product and the nonclinical and proposed clinical trials are required for MHRA review. A major difference in the submission is the requirement for a “Qualified Person” with quality assurance responsibilities for the product in the United Kingdom. In the United States, these responsibilities fall to a quality unit, rather than an individual with the specific credentials. Only the Qualified Person may release a clinical trial lot of material for clinical research. Another major difference between the MHRA and the FDA is that the MHRA does not necessarily have an expectation for challenge organisms to meet GMP requirements because the challenge organism is not considered to be a medicinal product (more below). While IRBs have responsibility for reviewing research involving the challenge organism, if there is not a vaccine (or other medical product) used in the study with the challenge, then the MHRA will not review such protocols.
The MHRA does not regulate the development of CHIM agents and has no plans to take up this role. However, if a CHIM study is used to test the efficacy of an intervention (e.g., a vaccine), the study requires approval by MHRA. However, even though there is no formal requirement, MHRA guidance states that GMP standards should be considered to ensure the safety of study subjects and quality of the challenge material. For challenge organisms that are GMOs, the UK Department for Environment, Food and Rural Affairs’ Advisory Committee on Releases to the Environment (ACRE) will assess the risk to the environment and subjects not participating in the study and must give approval before the GMO challenge agent can be administered as part of a CHIM study.
Guidance has been introduced by the European Union Health and Consumers Directorate-General recommending more formal assessment of Non-Investigational Medicinal Products, such as challenge agents, and specifies the appropriate GMP requirements for them (880). Under the new European Union Clinical Trials Regulation, challenge agents fall under a new classification as Auxiliary Medicinal Products (AxMP): a medicinal product used for the needs of a clinical trial as described in the protocol, but not as an investigational medicinal product. Such products shall be manufactured according to GMP or to at least an equivalent standard, in order to ensure appropriate quality. Appropriate GMP requirements foreseen for the safety of the patients should still be applied and the sponsor should ensure that AxMPs are of appropriate quality for the purposes of the trial, taking into account, among other things, the source of the raw materials and any repackaging. It is clearer on the other hand that Good Clinical Practice guidelines must be followed for the use of such AxMP in human trials.
Following Brexit, the United Kingdom withdrew from the European Union on 31 January 2020; however, there was a transition period through 31 December 2020, while the United Kingdom and the European Union negotiated additional arrangements until new rules took effect. During the transition period, the United Kingdom continued to follow the rules of the European Union and MHRA related to clinical research. From 1 January 2021, the MRHA is the UK’s standalone medicines and medical devices regulator.
The World Health Organization’s (WHO) GMP standards are a set of international standards designed to ensure that biological products, including human challenge agents, are manufactured to minimum standards. As a result, the decision of whether to manufacture challenge pathogens to GMP for use in CHIM studies is an important consideration when conducting such a study. While the research community recognizes the need for high quality in the manufacture of human challenge agents, it has been suggested that these standards could be at least “GMP-like,” meaning they would fulfill GMP requirements to as much as is practically possible without being GMP certified. Such a step would enable the manufacture of challenge agents outside of GMP-certified settings, such as in academic laboratories or in countries where GMP facilities might not be as practical. While GMP-like standards would allow this flexibility, it should not be an excuse for adopting low standards of manufacturing, as GMP standards provide reassurance of the safety of agents and the reproducibility of data generated, and GMP-like would need to be justified in each specific case. Consistent with the MHRA position, the requirement for a CHIM agent to be GMP certified, rather than just GMP-like, is the suggestion that the requirement should apply to the cases in which a CHIM study contributes directly to the licensing of a vaccine. In many cases, manufacture of an agent under GMP may not be technically feasible (e.g., helminth challenge agents and Cryptosporidium). In other cases, challenge agents that were developed long ago and found safe in humans were commonly used in trials before GMP guidelines were adopted. Like the United States, however, there is an expectation that IRBs, and potentially IBCs, would review the proposed research. The ethics review procedures in the United Kingdom are similar to those for the U.S. IRB and for this reason will not be reiterated in this section.
United Kingdom Regulations for Genetically Modified Organisms
The Department for Environment, Food, & Rural Affairs and ACRE have responsibility for oversight of GMOs. ACRE’s advice is forwarded to regulators for consideration in evaluating clinical trials (or marketing authorization) for GMOs. If the vaccine or challenge organism could be considered to be a GMO, then the need for consultation with or filing to these bodies should be determined.
Efforts toward Regulatory Convergence on Human Challenge Studies for Vaccine Development
Despite various regulatory procedures in different countries, the WHO has provided a considerations paper to guide regulatory convergence on the subject of human challenge studies: Human Challenge Trials for Vaccine Development: Regulatory Considerations (881). In addition to this guidance, the International Alliance for Biological Standardization has hosted four conferences on the subject of human challenge studies for vaccine development in concert with various cosponsors over the past 6 years: in Strasbourg, France, in 2014 (882); in Rockville, Maryland, United States, in 2017 (883); in Langen, Germany, in 2019 (884); and in Oxford, United Kingdom, in 2020 (885). The meeting reports from these conferences provide recommendations, conclusions, and summaries of the current state-of-the-field in terms of regulatory, ethics, and scientific thinking on this subject.
Regulatory and Ethical Considerations for the Various Applications of Human Challenge Studies to Vaccine Development
Clinical studies in which subjects are intentionally given a virulent or attenuated challenge organism in a controlled manner may be performed for different purposes and thus with differing study designs. These studies may be exploratory in nature. They may also support the development of a challenge model and the establishment of its parameters. Other studies may be similar to conventional clinical trials of candidate vaccines in many respects. Vaccine clinical trials that incorporate a challenge model may be considered phase 1, 2, or 3 and might even be performed as phase 4 studies in certain circumstances.
A developer may conduct human challenge trials to accomplish one or more of a number of aims. The aims of the study determine in what clinical phase the study may be considered to be. Human challenge trials are often a type of efficacy study, but not all would be considered a phase 3 pivotal study. Human challenge trials can have multiple purposes. These purposes could include, but may not be limited to characterization of the challenge stock and model system (titration, symptoms, kinetics, shedding, transmissibility, etc.); gaining a clearer understanding of the pathogenesis of and immunity to the organism in order to guide decisions on what (type and/or quantity of) immune responses a vaccine might need to accomplish to protect against that disease (i.e., to gain insight for vaccine design); and identification of potential immune correlates of protection (which would then require validation in a traditional efficacy study). The use of human challenge models to explore vaccine efficacy can identify an optimal trial design for phase 3 traditional efficacy trial(s) (e.g., case definitions, endpoints, study design aspects), generate appropriate hypotheses to be formally tested in traditional efficacy trials, and establish proof of concept that a particular vaccine candidate might be capable of providing protection. They can be used for derisking or “left-shifting” risk of failure in a vaccine development program, as well as down- or upselecting among various potential lead vaccine candidates to advance only the best to large phase 2b or 3 efficacy trials and to eliminate those that are unworthy of advancement. (In a theoretical vaccine development timeline with early-stage development depicted on the left-hand side and late-stage or advanced development depicted on the right-hand side, shifting the risk of failure earlier [or to the left] in the timeline could result in significant cost [and resource] savings and could minimize opportunity costs by abandoning an unpromising candidate prior to assuming greater expenditures of later-phase clinical trials. Doing so would also minimize the risk to human subjects by avoiding large efficacy studies of nonefficacious vaccines.) In addition, human challenge trials can be used for comparison purposes, as when comparing vaccine performance in endemic settings versus in efficacy trial populations, including evaluating impact of prior immunity or comparing investigational vaccine performance against a licensed vaccine that is suboptimally efficacious (e.g., suboptimal durability, inadequate strain coverage, low efficacy) when a head-to-head trial might be infeasible (e.g., sample size, attack rate, etc.). [Target population(s) in areas of endemicity may differ in important respects to volunteers in human challenge studies, which include prior exposure to the pathogen that might impact the immune response elicited by a vaccine, different nutritional status and gastrointestinal flora, and human genetic markers that could influence vaccine performance. Therefore, the results of a human challenge study may not reproduce results that would otherwise be observed in field trials in regions of endemicity.] Finally, human challenge trials can be used to support emergency use of an investigational vaccine (e.g., in a pandemic), establish a basis for licensure (this purpose would generally be an exception rather than the rule), and to explore postlicensure, whether or not immunity to vaccination wanes and if or when booster doses might be required for durable protection. (Postlicensure exploration might entail a human challenge study in adults to extrapolate when children might need booster doses.)
Not all challenge studies or models would support accomplishing each of the aims above. For example, if the human challenge model system does not adequately mimic the wild-type disease and/or the populations the vaccine would need to protect, then a human challenge trial would likely not be usable as a primary basis for licensure. It might, however, still serve one or more of the other purposes above or could be considered by regulators as supporting evidence for licensure.
In designing any human challenge study, consideration must be given to the study’s aim and its role in vaccine development. Furthermore, the overall regulatory strategy for developing a given vaccine candidate or regimen should reflect the role the model might play in that development. In planning an overall regulatory strategy for development, consideration should be given to whether the time and resources required to develop and validate the human challenge model (if not already available) need to be offset by the degree of left-shifting that could occur in the vaccine’s development timeline. Also, some vaccine companies may be concerned that a human challenge model that is not particularly predictive or relevant from the perspective of testing vaccine efficacy could inaccurately indicate vaccine failure, which would stifle further development of a vaccine candidate that still has promise. Therefore, the task of developing human challenge models generally falls to public interests, since private enterprises often fail to see benefit when comparing to the model’s development costs and resource intensiveness and may balk at the perceived risks to vaccine development. The development of a human challenge model by a public organization that may be willing and able to share the model with multiple industrial vaccine developers could significantly encourage the development of vaccines against diseases for which they currently do not exist or are difficult to develop and license. Of particular note are diseases for which many vaccine candidates have been tried and failed, those considered too difficult to be addressed by vaccination, or those for which development was determined to be unprofitable. Further, in a setting of an emerging disease for which a large number of vaccines are being developed simultaneously, it may be impossible for all candidates to perform large efficacy studies, so downselection to the most promising few through use of a challenge model might be the most efficient and speediest path to vaccine development.
Manufacture, Characterization, and Maintenance of the Challenge Organism
Since the challenge organism will be the subject of regulation through an IND application in the United States, expectations are that the challenge organism will be manufactured and characterized as would any medicinal product for human use (current Good Manufacturing Practice [cGMP] expectations will likely be very similar to those for phase 1 products). Although the challenge organism is not a medicinal product, it will be given to humans and therefore should be relatively safe and pure, of correct identity, and of suitable quality for use in humans. This means that many, though not all, considerations for manufacturing, testing, and characterizing a vaccine product apply to the challenge organism. These considerations entail some aspects of manufacturing according to cGMP and documenting the manufacturing and testing so that a chemistry, manufacturing, and controls section of an IND application may be prepared for regulatory review. While the challenge organism may cause an acceptable level of disease symptoms in the study volunteers, harm resulting from some impurity or unsuitable contents would be unacceptable (risks should be minimized, to be ethically used in humans).
Ideally, a sufficient quantity of the challenge organism stock could be prepared to permit its use in a number of challenge studies. Such studies could be designed to characterize the stock in humans, for the trials for which it was prepared and for use by multiple developers if the stock is to be a standardized challenge for testing multiple developers’ vaccine candidates. In some situations, propagating a human challenge organism in culture or using it directly from a frozen stock may not yet be possible. Some challenge inoculates require fresh propagation immediately preceding their use as a challenge. In these cases, cGMP compliance may be quite difficult. For some bacterial challenges, for example, material scraped freshly from an agar plate might be used. For other challenges, material prepared from infected humans may need to be the source of the challenge material. Whatever the case, regulators should be consulted and agreement gained on the planned manufacture and testing in advance of its use in a human challenge study.
The FDA’s Guidance for Industry: CGMP for Phase 1 Investigational Drugs (872) contains guidance on challenge organism manufacturing. While the guidance permits a phase 1 product to be generated in a laboratory rather than a cGMP-compliant manufacturing facility, the intent of this portion of the guide is really aimed at autologous cell therapies (which are always made in very small batch sizes for a single individual). In some situations, when challenge material is made fresh in a small batch for a given study, discussion should be held with the FDA in advance about whether or not this part of the guidance could apply to a challenge organism. To prepare a sufficient batch size for the purposes of a standardized challenge stock and with suitable containment, an appropriately designed manufacturing facility will most likely be needed. Since establishing a dedicated facility for producing a particular challenge stock is unlikely, considerations in FDA guidance about multiuse facilities should be addressed. In fact, regulatory expectations for control (including cleaning procedures and validation, environmental and personnel monitoring, and changeover procedures) of a multiuse facility are higher than for a dedicated facility where the risk of cross-contamination of the product would be substantially lower. Therefore, although process validation is not expected for phase 1 products, certain validations would be required for a multiuse facility, even if used to produce material for phase 1 investigations (886).
The challenge organism, once developed, will require banking so the challenge used in each trial is homogenous and consistent, just as would be required of a vaccine. Although using a two-tiered banking scheme may be unnecessary, as would be done for cells or vaccine seeds, a “master” seed should be generated at a minimum and banked by storage in appropriate containers and temperature conditions (e.g., frozen in the vapor phase of liquid nitrogen). This master seed may be used as the challenge stock for each human challenge study itself, or it may be used to seed the production of each lot of challenge stock made (if fresh material is required each time). Periodic retitrating of the seed material may be required, preferably in vitro if possible, to ensure the seed is stable under storage conditions. Ideally, a stability program or something akin could be planned for the challenge organism seed stock. Monitoring the viability and titer each time a vial(s) of seed is removed for the purpose of generating a fresh lot of challenge material may be suitable. Trending of these data can aid in monitoring storage stability.
In some cases, the challenge organism may have been genetically altered either by attenuation (growth of a wild-type organism in cell culture, passage through a nonhost species, or some other manner) or by recombinant DNA techniques. In other cases, a wild-type organism might be used. In either case, there may be concern about the organism’s genetic stability. Particularly for genetically altered organisms, assurance that the characteristics determined for that organism are retained when used in a human challenge study requires assessment of genetic stability. Whole-genome sequencing as evidence of genetic stability may be encouraged or required by regulatory agencies. Passaging the organism several times under conditions like those used in production is desirable, as is characterizing the genetic stability of any organisms recovered from humans that have been challenged should they shed the organism. While the latter issue is not strictly one of GMP, genetic stability during production of both the material to be used in the human challenge study and the organism in humans are critical attributes to characterize in order to ensure subject safety and potentially for environmental containment.
Characteristics upon which a manufacturer would release a lot of medicinal product to the clinic (or the market) include safety, purity, potency, identity, and quality. While these characteristics do not translate directly one to one between a vaccine and a challenge organism, some control over attributes that would lead to the use of a challenge organism in the clinic is needed.
While “safety” may seem an inappropriate attribute for a virulent challenge organism, in fact safety and purity are intertwined. An impure material could be unsafe. Particularly, one needs to address whether the challenge organism could be contaminated with other materials that would render it more unsafe or unsafe in unanticipated ways beyond the expected virulence of the challenge organism. Testing should be performed to know whether other microorganisms could be present in the challenge material or if endotoxins are present. Testing for process residuals may be important if the challenge organism is grown in the presence of antibiotics, antibodies, inducers, or other unwanted substances in the final material. This kind of testing is also important if downstream processing steps are used to purify the challenge organism, which could introduce substances undesired in the final material (e.g., cesium from a cesium chloride gradient used to purify a viral preparation).
While the concept of “potency” may not directly apply to the challenge organism, a measure of content or titer of the challenge organism needs to be known for dosing purposes. While the human titer of the challenge organism requires characterizing the material in the human challenge model, an in vitro (or animal) titer should be determined for each lot as a criterion for releasing the material to the clinic. For example, for viruses, a determination of PFU or a TCID50 should be made. For bacteria, the CFU may be a useful measure. In other cases, organisms may be enumerated by microscopic examination, often in conjunction with reagents that increase the sensitivity of detection.
The ability to identify the challenge organism is crucial for confirming the vial (or other container type) of material to be used for a human subject actually contains the challenge organism and not some other product or organism produced in the same facility. For these reasons, an identity test of some type is needed. Quality measures that might be important might include, but not be limited to, appearance, pH, osmolality, or viscosity.
Pathogen Strain Selection and Clinical Assessment following Challenge
Many human challenge models include the development of disease symptoms after challenge. In some cases, no specific treatment for the disease symptoms exists and symptoms resolve naturally due to the human immune response. In many cases, however, treatment for preventing or ameliorating disease symptoms is an integral component of the challenge model. In the latter case, determining in vitro or in animals if and to which antimicrobial drugs the challenge organism is susceptible and/or resistant is an important and current practice. For example, a bacterium used for challenge should be susceptible to well-tolerated and frequently used antibiotics and not require an antibiotic to which allergies are frequent. For challenge with a virus-like influenza, using a strain that is sensitive to oseltamivir or baloxavir could be important. Susceptibility to the antimicrobial should be normal and not require higher than normal doses or longer treatment times to cure the subject of the challenge organism.
Clinical case ascertainment may be important to demonstrate that symptoms of illness are due to the challenge organism and not some concomitant infection. Trial subjects could have been exposed to either the same or a different disease organism before enrolling in the trial or during the trial itself if not confined to inpatient settings. Specimens should be taken not only to define kinetics or assess shedding and cure but also to determine whether the challenge organism and no other organism is present in the relevant anatomical compartment. For example, if a subject has diarrhea, then stool specimens should be collected and analyzed. For respiratory symptoms, collecting and analyzing sputum samples or nasal washes may be necessary.
A severity scale for the disease endpoints that will be key to defining the impact of vaccination to ameliorate disease in the human challenge model should be defined. The range of severity seen without intervention in the initial characterization of the challenge model may need to be compared to the range of severity seen following vaccination for those vaccines that do not prevent all disease symptoms. Of course, a balance between the severity of the disease caused by the challenge and the safety of the human volunteers enrolled must be met while also maintaining scientific integrity of the study. In human challenge studies, the development of disease symptoms is often limited to the mild end of the spectrum of disease that the pathogen causes in natural infection and should generally avoid severe disease symptoms. For these reasons, in many cases human challenge studies of candidate vaccines will be limited to an assessment of the vaccine to ameliorate mild symptoms, with the hope that limiting mild disease will extrapolate to more severe disease in natural infection. Ideally, human subjects should be treated to cure the disease as soon as they express reliable disease symptoms or markers on which to assess impact of vaccination. Again, the ethical precept of minimizing risk applies.
Clinical Site Health and Safety Requirements
In addition to manufacturing under an appropriate biosafety level (BSL), the clinical trial may need to be conducted with biosafety in mind. If the challenge organism or the vaccine is a recombinant organism and if the challenge organism is virulent or not endemic in the clinical trial location, subjects may require housing in a quarantine unit during the trial and for the duration of shedding, if any. Waste generated in the quarantine unit, including excretions that might normally flow into the sewer system, will likely need to be decontaminated or incinerated to prevent the spread of a virulent challenge organism or a recombinant organism. Using a quarantine unit with negative air pressure to contain respiratory or airborne pathogens may be necessary.
Defined laboratory BSLs range from 1 to 4, with 1 being rudimentary and standard laboratory practices and 4 being full containment, including wearing personal protective gear and respirators that prevent contact between the laboratory scientist and the disease organism. For details on these BSL procedures, consult the WHO Laboratory Biosafety Manual (887). Most organisms that might be used for a human challenge study would likely be BSL2, which entails but is not limited to restricted access with posting of biohazard signage; standard laboratory personal protective gear such as a laboratory coat, safety glasses, and gloves; the availability and use of biosafety cabinets; and access to an autoclave for decontamination purposes.
Laboratory BSL guidelines do not strictly translate to a clinical trial setting. Instead, consideration must be given to whether the trial should be conducted as an outpatient trial with no additional precautions, an outpatient trial with additional precautions (e.g., bandaging to cover a site of inoculation from which an organism might shed), or as an inpatient trial in a quarantine unit. Most vaccine trials are conducted on an outpatient basis with no specific additional precautions beyond informing the trial subject to possibly avoid contact (for live vaccines) with children, pregnant women, or immunosuppressed people. In some cases (such as when investigating a recombinant poxvirus), the site of inoculation or scarification might be bandaged, and careful instructions given for changing the bandaging and returning the bandaging materials to the trial site for decontamination and destruction. However, human challenge studies may require more stringent biosafety controls.
Many human challenge studies are performed in quarantine to ensure that volunteers are closely monitored, clinical data are accurately collected, and care and treatment is administered during the course of the study. Quarantine also reduces (and hopefully eliminates) the possibility of spreading the virulent or recombinant organism, which could be excreted, respired, or secreted by trial subjects. However, not all human challenge studies require such containment. Closely monitoring the trial subjects on an outpatient basis and providing them with clear instructions and a means of rapid communication (e.g., mobile phone) could be possible. In particular, if the challenge organism is a vaccine (such as bacillus Calmette-Guérin for tuberculosis) or a vaccine candidate that was perhaps underattenuated and the organism itself is endemic or substantially similar to an endemic organism, then outpatient trials may be performed. Also, if the challenge organism is endemic, an outpatient trial may be considered, even if the organism is virulent. If the challenge organism cannot be spread person to person readily, consideration may also be given to an outpatient setting with precautions even if the disease is not endemic. Inpatient quarantine would be expected if the challenge organism is not endemic, is substantially different from endemic strains, causes substantial symptoms of illness that require round-the-clock monitoring, and/or is a recombinant organism that would be considered a GMO that could be released into the environment inappropriately. Challenged individuals might be required to remain at the clinical trial site following challenge, not necessarily for containment but for frequent trial endpoint monitoring and specimen collection.
Furthermore, conducting the human challenge study in a facility that uses negative air pressure might be necessary so that air flows into the unit from outside, containing pathogenic respiratory or airborne challenge organisms inside the quarantine unit and preventing their escape into the outside environment. Consideration must be given to the ability of the challenge organism to be excreted or secreted from the trial subject into their environment, including onto or into other people. If the challenge organism might be spread person to person by close or casual contact, the potential for transmissibility might be high. In characterizing the human challenge model, this possibility should be explored, as some individuals in an inpatient trial may not get challenged but may become infected from interacting with challenged individuals within the inpatient setting. Although this scenario does not take place in every human challenge study, the possibility of it occurring makes it worth addressing. Overall, transmissibility must be presumed and accounted for in the study design whenever a live organism is used. A clear exception is if the disease organism cannot be spread person to person. If the vector for spread (e.g., mosquitos) is present in the locale of the trial site (i.e., the catchment area from where the trial subjects would be selected), then transmissibility should still be presumed.
In the event that materials used in both inpatient and outpatient studies become contaminated with the challenge organism, such biohazardous waste must be handled with precautions and decontaminated before disposal into municipal waste. An organism that may be spread through urine or feces into the municipal sewer system must be collected and decontaminated before sewer system or standard waste disposal if the organism is not endemic. Challenge organisms such as enteric pathogens that can cause diarrhea must be contained by ensuring that all feces and diarrheal fluids are collected in some type of container rather than allowed to flow into the sewer. These excretions must be decontaminated prior to disposal. Such precautions are in current practice.
Clinical trial staff and anyone working at the clinical trial site (especially a quarantine unit) could be at risk of exposure to infection with the challenge organism. Consideration must be given to staff health and safety regardless of the staff member’s role (e.g., a clinical trialist, a janitor responsible for cleaning and removal of waste materials, or someone bringing meals to subjects contained in-house). Appropriate precautions should be taken to prevent spreading the challenge organism to anyone susceptible to infection with it. Such precautions may include, but not be limited to, wearing personal protective gear, regular health screenings, and vaccination if a licensed vaccine is available.
Risk Assessments and Standard Operating Procedures for Human Challenge Studies
Informal or formal risk assessments that identify potential hazards, characterize risks, and plan for and develop risk management strategies should be considered for both manufacturing and clinical studies. The International Organization for Standardization or the International Council for Harmonization describes useful tools for conducting formal risk assessments. Commonly used risk assessment tools for manufacturing include Failure Mode Effects Analysis and Hazard Analysis and Critical Control Points, which could lend themselves to either quantitative analysis or semiquantitative analysis (i.e., giving relative weights to ranks such as high, medium, and low). One such semiquantitative tool gives ranks of 1, 2, and 3 and multiplies scores assigned to the probability of a given event’s occurrence, the probability of promptly and actionably detecting the event if it occurs, and the severity of the event’s impact. Higher scores require more attention in the risk management plan. Whatever tool is used, the purpose of the risk assessment is to consider various scenarios in which a hazard may occur and to make decisions about how best to mitigate or manage such risks. Planning overlapping fail-safes whenever feasible is prudent.
With any regulated environment, operating according to standardized and documented procedures that have been approved by an oversight group (e.g., a quality assurance unit) is imperative. SOPs are expected not only for manufacturing the challenge organism (essential components of GMP) but also for clinical practices, including, but not limited to, the challenge procedure itself. To ensure reproducible effects in challenges, a documented standardized procedure should be put in place as part of the development of the human challenge model. Personnel involved in such procedures must be trained on the SOPs and the training documented for regulatory review and oversight. A human challenge model may involve dozens of SOPs to cover all facets of the model. These SOPs need to be prospectively written and approved, complied with, and documented to prove that either no SOP deviations occurred or that deviations that did occur were held subject to investigation and corrective/preventive action. Once the human challenge model is established, future challenges will be governed by the SOPs written during the development process.
Ethical Considerations
By their very nature, human challenge studies have unique ethical considerations and confront our ethical precepts about trial participation. In general, human challenge studies are undertaken for generalizable knowledge rather than for direct benefit to the participants. If the challenge study includes a vaccine, the knowledge gained may directly increase the probability that an efficacious vaccine will be developed; yet, this benefit is not likely to be directly linked to participation in a human challenge study, since many diseases for which human challenge models have been developed are not endemic in the countries where challenge studies are typically performed or are not relevant to the populations participating in the studies (e.g., pediatric infections). Due to the ethical considerations associated with human challenge models, the risk to participating volunteers must be kept to the absolute minimum required for such models to have any utility in advancing vaccine development. Since the risks of participating in human challenge models are highly pathogen specific, any assessment of how to control these risks must be specific to the challenge model in question.
One of the most important considerations is whether the utility of the challenge model depends on the development of disease symptoms. This is generally the case for the human challenge models in current use, with some notable exceptions, such as the sporozoite challenge model for malaria. A human challenge model should not be acceptable if it uses a challenge strain of a pathogen that causes serious symptoms or conditions that do not resolve naturally and cannot be cured with a therapeutic intervention. Human challenge studies cannot use an organism that has significant potential to lead to chronic symptoms or other long-term sequelae, nor can they use subjects considered to be vulnerable (such as children; pregnant or lactating women; immunosuppressed or autoimmune individuals; individuals with diminished capacity to provide informed consent; or other such persons with abnormal risks, such as asthmatics and transplant recipients). Therefore, the current practice is that only healthy adults fully capable of giving informed consent can be considered for enrollment in a human challenge study and that practice should continue. A notable exception is pediatric studies using licensed live-attenuated vaccines with well-established safety profiles (e.g., rotavirus or poliovirus) as challenge agents.
Worth acknowledging is that different individuals are willing to accept risk to differing degrees. Some people are risk averse, and others are risk-takers. While a human challenge study should not expose subjects to undue risks, the level of risk is higher than in a typical vaccine trial. Individuals such as firefighters, military personnel, extreme sports enthusiasts, skydivers, and police officers may accept more risk in life than others—sometimes for the greater good of humanity or sometimes for their own personal gain or enjoyment. Given that a human challenge study should be designed with the goal of obtaining knowledge that will aid the greater good, many individuals would likely accept the risks of such a trial. Therefore, stating a priori what level of risk is acceptable in a human challenge study can be difficult.
Nonetheless, the ethical precept of beneficence requires that researchers minimize risks and maximize benefits. While the risks required in a human challenge study are generally anticipated, the study should be developed to minimize those risks as much as possible while maintaining scientific integrity. Consideration must be given to potential individual benefits and risks, as well as to potential societal benefits and risks (such as release into the environment of a pathogen that might not otherwise be present). In a clinical trial, ethics provisions are made for situations in which there may be greater than minimal risk but little (or no) potential for individual benefit. In these cases, knowledge may be gained that will be of benefit to the larger societal population with whom the potential trial participant shares significant characteristics. Asking trial participants to accept the risk from a challenge can be compared to the justifications that support inclusion of placebos in controlled clinical trials.
While all clinical trials require thorough informed consent procedures, human challenge studies require an informed consent process that ensures that individuals enrolling in the trial understand they will be intentionally infected with an infectious disease-causing organism and that they are likely to get ill and suffer a predictable level of disease symptoms. While all trials must minimize risk and maximize benefits, these studies may require individuals to experience higher risks than other trials. Participation in human challenge studies cannot be considered an everyday risk even though the infectious diseases the investigators are attempting to model are circulating in the human population. Despite the highly controlled environment and high standard of care provided for volunteers, intentional inoculation with a pathogen carries a potentially greater risk to a participant than that expected from natural pathogen exposure. Participants must understand this. The recommendation is that some means of assessing participant understanding of the information conveyed during the informed consent process be utilized in a human challenge study (e.g., a prestudy quiz that must be passed with a very high percentage of correct answers).
It is important that the study sponsor ensures trial participants that experience study-related injuries receive the best possible medical care. Although every precaution should be taken in a human challenge study to ensure that individuals suffer no more harm than is scientifically necessary to gain a credible result from the study, eventualities may occur in which a trial participant experiences more harm than foreseen and that study procedures could not prevent. While this should be a rare exception, the possibility nonetheless exists and necessitates insurance. This would be true whether or not the trial uses an investigational vaccine candidate. The vaccine itself would put trial participants at risk of unforeseen adverse events. In a human challenge study that uses an investigational vaccine candidate, the separate risks inherent in the administration of the candidate vaccine and in the challenge with the pathogen should be separately communicated and distinguished in the information provided to study volunteers.
In most cases, a human challenge study should involve treatment to either cure or, at a minimum, ameliorate disease symptoms. Bacterial diseases can generally be treated with antibiotics. For some parasites and viruses, anti-infectives and antiviral drugs, respectively, are available. For diseases that have no available treatment, palliative therapy to minimize severity and relieve symptoms should be available to trial participants as part of the study. For example, with diarrheal diseases, fluid and electrolyte replacement must be available for use as needed. For febrile illnesses, antipyretics should be available for use. This is consistent with current practices.
Practical and Ethical Considerations of Controlled Human Infection Model Studies in Regions of Endemicity
While the vast majority of controlled human infection model (CHIM) studies have been conducted in high-income countries (HICs), in recent years there has been increasing interest in conducting such studies in regions of endemicity, including low- and middle-income countries (LMICs). While such studies heighten sensitivity to ethical concerns, there are also important benefits to be obtained, and with proper oversight and precautions, it is possible to conduct them ethically. One benefit is that in the context of vaccine development, residents of regions of endemicity may have different immunological responses to vaccines compared to residents of HICs, who are typically immunologically naive (888). This could occur because of either increased exposure to the corresponding pathogens due to living in a region of endemicity or, alternatively, because of genetic differences. Another emerging factor is that the microbiota of endemic-region residents differs substantially from residents of HICs (889), and these differences may result in differential susceptibility to disease as well as differential vaccine responses (890, 891). Already, CHIM studies have been conducted in regions of endemicity, including with malaria (892–895), cholera (896, 897), and shigellosis (898, 899). Additional studies are in progress or will be initiated within the next few years (900).
One of the major concerns about conducting challenges in LMICs is the needed availability of state-of-the art expertise, equipment, and facilities to diagnose and be able to treat any unexpected safety outcomes to the highest possible standards. This has been partially addressed by the fact that currently used challenge agents have been tested before in HICs, where all potential unexpected outcomes and complications can be rapidly addressed.
Current experience has indicated that early and robust engagement with local communities, public health officials, and regulatory authorities is crucial for acceptance and success (901). Ideally, the principal investigator for such a study should be a respected local investigator, rather than someone from outside the community. Ideally, these studies should also be approved by ethical review committees from both the local region as well as the sponsor’s or developer’s home country (assuming this is an HIC). In some cases, informed consent may involve not only individual volunteers for the study but also local authorities or the members of the local community (902). Frequent communications about risks and benefits of the study should be delivered to local community members through multiple channels to ensure the utmost transparency. Financial compensation and the potential for inducement must be considered in LMIC settings even more carefully than in HICs (903). CHIM studies in endemic settings have the potential to accelerate the development of efficacious vaccines and drugs that benefit these communities, but they must be approached cautiously and with a high sensitivity to local conditions.
Summary
The regulatory and ethical considerations for human challenge studies are numerous. While some are common to any clinical study of a candidate vaccine against an infectious disease, some are unique due to the specific nature of human challenge studies. International regulatory authorities lack harmonization as to whether human challenge studies that do not involve an investigational medicinal product (such as a vaccine candidate or new drug) require submitting a request for clinical trial authorization from an NRA. The United States has a requirement to file an IND application, which brings with it many considerations, such as the appropriate application of some level of compliance with GMP to the manufacture and control of the human challenge stock. In addition, scientific considerations for the validity of the human challenge model and the reliability and credibility of the data to be derived during its use invoke additional regulatory expectations. Standardized procedures are imperative for this purpose.
Special facilities and potential requirements of containment (e.g., inpatient quarantine units) may be necessary. A risk assessment may aid in considering what potential risks a human challenge study might pose so that researchers can strive to minimize risks. Ethical considerations are particularly key in a human challenge study, and only fully informed and consenting healthy adults should be considered for enrollment. These expectations are reflected in current human challenge study practices and should continue. Ultimately, the scientific integrity and design of the human challenge study is what drives decisions, but this must be balanced with the need to minimize risks and maximize benefits to study participants. Only data derived with credibility and reliability will be acceptable for regulatory decision-making. Studies performed with scientific rigor, while reflecting the pragmatic needs of studies performed in humans, should provide the necessary credibility and reliability.
CONCLUSION AND RECOMMENDATIONS
The fundamental processes by which vaccines are being developed have come under scrutiny. With the exception of the extraordinary success achieved by COVID-19 vaccines, over the years candidate vaccines entering advanced clinical development offer a low probability of success. In contrast, the success of COVID-19 vaccines was driven by the urgency of a global pandemic, unprecedented resources allocated to their development, unprecedented sharing of data between scientists prior to publication, and the decades-long foundation of basic research understanding mRNA and other vaccine platforms (adenovirus, protein subunits, inactivated viruses) and coronaviruses on which the clinical development was built. Further, taking vaccines through several development steps is a lengthy process even if some of the studies can take place in tandem. We have considered in this report the possibility that the expanded development and use of human challenge models could provide, in a shorter time than required for efficacy trials, data that are more predictive of the efficacy of candidate vaccines in large field trials. Our overall conclusion is that human challenge studies can clearly play a more pivotal role in vaccine development than they do today. In the following sections, the recommended steps that may be taken to realize the full potential of human challenge studies to support and advance vaccine development are described.
Overarching Needs for the Field of Human Challenge Studies
Human challenge models can be improved in the following ways virtually across the board. These measures could be implemented individually for each different challenge model. Whenever it is possible to identify groups of challenge models with similar needs, efficiencies will be gained by involving a range of experts across different diseases to maximize opportunities for shared learning.
These improvements include the increased standardization and validation of study procedures, data collection, and data analysis, as well as the enhanced availability of more numerous, updated, and relevant challenge strains. Clinical trial registries such as ClinicalTrials.gov could play an important role in standardizing procedures for data collection and reporting. Human challenge models would benefit from better defined clinical endpoints for vaccine studies and increased use of endpoints that can be directly applied in subsequent field trials. Standardized endpoints are critical for challenge studies with naive volunteers, as they can facilitate translation to real-world applications in vaccine recipients with some immunity from prior natural infection. More focused and comprehensive studies to identify correlates of protective immunity using conventional immune assays and new technologies will improve the development of vaccines overall. It is critical to plan for longer-term follow-up of volunteers for challenge studies in which there is any known potential for postinfection sequelae or precedence for failure to clear the challenge strain from volunteers at study conclusion. Better integration of vaccine studies conducted with human challenge models into the overall development plans for candidate vaccines, including criteria to specify which results will curtail further development, will result in significant savings in time and development costs. One pitfall is challenging with a dose of pathogen that is higher than physiologically relevant and thus setting an unreasonably high bar, resulting in termination of a vaccine that would provide protection against natural exposure. Finally, it is necessary to develop systematic approaches to efficiently address the anticipated regulatory and ethical concerns that may accompany the proliferation of human challenge studies.
Critical Evaluation and Recommendations To Increase the Capacity of Human Challenge Models To Support Vaccine Development
Each of the human challenge models described in this report has a unique set of characteristics that must be considered to determine what role the model can play in vaccine development. These characteristics include the depth of experience with the model, the availability of appropriate challenge strains, their ability to mimic natural infection, the capacity to adequately manage the risk to volunteers, and the potential to gather data with the model that can clearly advance vaccine development for the disease in question. Some models clearly have inherent obstacles to further development, and in these instances, it may not be technically feasible to substantially improve the model. Such models have limited relevance to natural infection and disease and consequently may have a smaller role to play in the development of vaccines, compared to conventional clinical trials. The requirements for a challenge model may vary according to the stage of vaccine development to which it will be applied. A lot depends on the number of volunteers that will participate in challenge studies with the model and on the quality of data the model will need to provide in order to fulfill its purpose for a given study. A case-by-case assessment is offered for each challenge model, along with concerns and recommendations for further development, if any, that have arisen from document review and expert consultations conducted for this report. The basis for the summary recommendations provided in Table 38 is further described below.
TABLE 38.
Summary recommendations for the continued development and utilization of human challenge models for 17 infectious diseasesa
| Disease | Challenge agent(s) | Recommendation from the literature review |
|---|---|---|
| Malaria | Plasmodium falciparum | Proceed. Few concerns identified, if any. |
| Cholera | Vibrio cholerae | |
| Pneumococcus | Streptococcus pneumoniae | |
| Rotavirus | Live oral rotavirus vaccine | |
| Poliomyelitis | Oral poliovirus vaccine | |
| Influenza | Influenza strains pertinent to the development of universal influenza vaccines | |
| Typhoid/Paratyphoid | Salmonella Typhi and Salmonella Paratyphi serovars | |
| ETEC | ETEC strains expressing selected toxins and colonization factors | Proceed with caution. Minor concerns identified. |
| Shigellosis | Shigella flexneri serotypes and Shigella sonnei | |
| Norovirus | Norovirus genogroups and genotypes | |
| RSV | RSV strains of genogroups A and B | |
| Dengue | Attenuated dengue virus serotypes 1 through 4 | |
| Malaria | Plasmodium vivax | Address major concerns before proceeding. |
| Campylobacteriosis | Campylobacter jejuni serotypes | |
| Tuberculosis | Bacillus Calmette-Guérin | |
| Pertussis | Bordetella pertussis | |
| Cryptosporidiosis | Cryptosporidium parvum and Cryptosporidium hominis | |
| COVID-19 | SARS-CoV-2 |
COVID-19, Coronavirus disease 2019; ETEC, enterotoxigenic Escherichia coli; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
We identified no significant concerns with the malaria challenge model using a Plasmodium falciparum challenge. Our findings recommend the continued use of the sporozoite model, increased utilization of the blood-stage model, and development of a transmission-blocking model to enable development of new malaria vaccines against multiple stages of the parasite life cycle. However, major concerns with the malaria challenge model using Plasmodium vivax need to be addressed before proceeding. A recommendation for this model is to decrease risk for challenge participants by replacing field strains with a cloned challenge strain of known susceptibility to antimalarial drugs, even though doing so could be more difficult than what has been done for P. falciparum due to the lack of a cell culture system for growth of the parasite. Additional steps are also recommended to minimize or eliminate the potential for recrudescence of liver-stage P. vivax in study volunteers, either by genetically modifying a cloned challenge strain to eliminate potential for dormant liver-stage parasites or by verifying the capacity of drug regimens to clear liver-stage parasites at study completion, or both.
We identified minor concerns with the challenge models for dengue that should be monitored as these studies move forward. A recommendation is to limit the use of challenge strains attenuated through serial passage in tissue culture until a fuller understanding is gained of their potential to cause severe disease in adult volunteers without preexisting immunity to the pathogen. The genetically attenuated challenge strains do not raise significant concerns for severe disease, and their continued use is recommended. The potential for severe disease in natural infection with a heterologous serotype following primary infection is well documented. Rechallenge studies or simultaneous challenge with heterologous serotypes of dengue viruses should be avoided with both genetically and tissue culture-attenuated strains until additional data indicate that this has no potential to induce serious disease outcomes in study participants.
We identified no significant concerns with the challenge model for cholera. Cholera bacteria are noninvasive for the human gastrointestinal tract and supportive care in the context of a challenge study and antibiotic treatment can effectively minimize any significant risk of severe disease. The cholera challenge model should continue to be fully utilized and continue to focus on the serogroup O1 El Tor biotype, since it is responsible for the majority of cholera outbreaks worldwide (including the recent severe and protracted outbreaks in Haiti and Yemen).
There are only minor elements of concern with the human challenge model for ETEC, which relate to the need for additional reagents and the types of vaccines that may be evaluated, rather than to the challenge model itself. Like cholera, ETEC strains are noninvasive for the human gastrointestinal tract. There is need for further characterization of recently developed challenge strains expressing heat-stable toxin (ST), but not heat-labile toxin, to support the development of vaccines that target ST. ST is not immunogenic in humans because it mimics two human peptide hormones. Ensuring that candidate vaccines that seek to render ST more immunogenic have no potential to invoke an autoimmune response in human volunteers is important.
There is one element of concern with the Shigella human challenge model that warrants two recommendations for improvement. Shigella is invasive for the human gastrointestinal tract, which raises the level of vigilance required to maintain the safety of volunteers. The concern relates to the potential for serious postinfection sequelae, which can occur at low frequency in natural infection. These sequelae include hemolytic-uremic syndrome, irritable bowel syndrome, and reactive arthritis. Human challenge studies use antibiotic treatment early in the course of disease development to mitigate this risk, but the residual potential for postinfection sequelae in human challenge studies is not known. Longer-term follow-up of volunteers should be considered and the data should be reviewed by experts not directly involved in the studies. The Shigella human challenge model could be improved by (i) gaining a fuller understanding of the relationship between challenge dose and attack rate for disease development and (ii) developing new challenge strains that represent more of the prevalent Shigella flexneri serotypes.
We identified several major concerns with the human challenge model for Campylobacter. The most important is the clear, but incompletely defined, potential for serious postinfection sequelae in adult volunteers. These include Guillain-Barré syndrome, irritable bowel syndrome, and reactive arthritis. Antibiotic treatment of study volunteers undoubtedly mitigates this risk to some extent, but the observation that some volunteers experienced recurrent disease episodes after study completion despite treatment raises concerns. The selection and evaluation of Campylobacter challenge strains is another area of concern. The potential for Guillain-Barré syndrome arises from ganglioside mimicry, in which the bacteria produce a carbohydrate capsule that invokes an immune response that cross-reacts with human nerve gangliosides. Strains with this potential have been disqualified as challenge strains, but these bacteria may still have the potential to mimic other human carbohydrates. One strain of Campylobacter that mimics human blood group antigens has been described. Phase variation is another challenge facing the human challenge model for Campylobacter. The range of carbohydrates expressed in the Campylobacter capsule depends on growth conditions. That additional carbohydrates that mimic human antigens are expressed upon infection of humans, but not under bacterial culture conditions, is possible. Therefore, in vitro screening assays applied to the carbohydrates that Campylobacter produces cannot be sufficiently comprehensive to fully rule out the possibility of mimicry for the full range of human carbohydrate antigens. The current challenge strains have apparently been safe, but they do not represent the globally prevalent strains. If additional human challenge studies with Campylobacter are performed, continuing the long-term follow-up of volunteers will be essential.
Salmonella Typhi and Salmonella Paratyphi serovars are invasive for the human gastrointestinal tract, and they have the potential to establish a long-term carrier state. In human challenge studies with these organisms, prompt antibiotic treatment has been successful in preventing both severe gastroenteritis as well as establishment of a carrier state in volunteers; therefore, we have no significant concerns about this model. Severe disease and carriage should continue to be closely monitored in future studies with the S. Typhi and S. Paratyphi challenge models. The potential for Salmonella Typhimurium and Salmonella Enteritidis serovars, the causative agents of invasive nontyphoidal Salmonella disease in infants and immunocompromised individuals in Africa, to cause severe disease in immunocompetent adults is incompletely understood. This lack of information regarding disease pathogenesis precludes the establishment of challenge models for these organisms at the present time. Improvements are needed with respect to the S. Typhi challenge strain, which is decades old and should be replaced with a contemporary isolate. Experience with the newly developed S. Paratyphi challenge strain is limited and use of the human challenge model with this strain should be expanded cautiously.
We identified minor concerns with the human challenge model for norovirus, related to challenge pools that are purified directly from infected humans. Recent successes with growing norovirus in tissue culture should permit derivation of cloned viruses that can be manufactured under Good Manufacturing Practice (GMP). The focus on the GII.4 genotype strains is appropriate and should be expanded, since this serotype is responsible for the majority of infections. The use of the GI.1 serotype strains should also continue.
We identified major concerns with the human challenge model for Cryptosporidium. The most significant is the recent decision from the U.S. Food and Drug Administration that Cryptosporidium oocysts for use in a controlled human infection model study must be produced in compliance with GMP. No such source currently exists and establishment of this manufacturing capacity will require substantial effort and resources. Another key factor is the importance of gaining a better understanding of the potential for pulmonary infection by airborne transmission of these organisms. If so, knowing under what circumstances this can occur is important because it would impose new requirements on human challenge studies with respect to quarantine and physical containment. A variable duration of oocyst shedding following challenge occurs, and the time period during which this takes place may need further definition. However, no transmission to household contacts was documented in the studies conducted to date. Efforts to grow Cryptosporidium in routine, large-scale tissue culture should be redoubled to avoid the need for deriving challenge organisms directly from infected humans and animals. The experience with the Cryptosporidium hominis human challenge model is limited and should be expanded cautiously.
We identified no significant concerns with the challenge models for rotavirus and poliovirus. Both use licensed live oral vaccines that have been administered in multimillions of doses. Challenge studies for both have been conducted in the target population of infants without any serious safety concerns. For both diseases, these challenge studies have the potential to advance important next-generation vaccines that could significantly impact disease burden: subunit or inactivated vaccines for rotavirus and genetically stabilized novel oral poliovirus vaccines in the case of polio.
There are few, if any, concerns with the human challenge model for influenza virus. The strains used in this model are seasonal, low-pathogenicity strains that are distinct from the highly pathogenic avian influenza strains that have a high case fatality rate. The development of universal influenza vaccines would be better supported if additional challenge strains were manufactured under GMP to permit a more widely distributed and concerted effort in the United States and the United Kingdom.
Only minor concerns were identified with the human challenge model for respiratory syncytial virus (RSV). It would be optimal to redevelop the RSV genogroup A Memphis-37 challenge strain to gain approval by the U.S. Food and Drug Administration and to develop an RSV genogroup B challenge strain (genogroup B strains cause up to one-third of RSV infections worldwide). The human challenge model for RSV may have limited capacity to be instructive regarding correlates of protective immunity because (i) infection with RSV does not protect against subsequent challenge with the same strain and (ii) protection against the mild, upper respiratory disease that develops in adult volunteers may be poorly predictive of protection against bronchiolitis in infants.
Significant advances have been realized in the development of the human challenge model for pneumococcus from studies conducted in the United Kingdom. The manufacturing of challenge strains representing additional prevalent serotypes would increase the capacity of the model to evaluate candidate vaccines in development, especially if these strains were to be manufactured under GMP for utilization in the United States and the United Kingdom. The clinical endpoint for the challenge model, prevention of pneumococcal carriage in the nasopharynx of adult volunteers, may not have predictive value for evaluating vaccine success in infants and young children that are most affected by severe disease. The degree to which positive results with adults in the human challenge model will extrapolate to preventing development of severe disease in infants is incompletely understood.
The development of a tuberculosis (TB) human challenge model that is relevant to human infection with Mycobacterium tuberculosis faces many hurdles. The newly developed models using intradermal or bronchioscopic administration of bacillus Calmette-Guérin (BCG) as a challenge strain do not represent the causative organism, route of infection, or disease development as they occur in natural infection. Whether a model of improved relevance can be developed while maintaining volunteer safety is unclear at present. Meanwhile, intradermal or bronchioscopic challenge with BCG may support the development of TB vaccines designed to improve upon the licensed BCG vaccine. The development of TB vaccines that reduce the establishment of latent TB, however, is not supported by the current models, in which latency antigens are not expressed.
An improved vaccine against Bordetella pertussis, the bacterium that causes whooping cough, is needed because the acellular pertussis vaccine in current use does not provide durable immunity. Infant baboons can be infected with B. pertussis and develop a disease that closely mimics whooping cough in human infants. The baboon challenge model can be used to evaluate candidate vaccines but may be limited in its capacity to identify correlates of protective immunity because of the difficulty in extrapolating immune responses from animals to humans. A human challenge model for pertussis has recently been implemented and has the potential to complement the baboon model. The human challenge model requires that volunteers receive prompt antibiotic treatment soon after infection is established and may therefore be limited to the evaluation of the ability of vaccine candidates to prevent B. pertussis colonization, rather than disease.
The global pandemic of severe acute respiratory syndrome coronavirus 2 has stimulated an important discussion about the potential for a COVID-19 human challenge model. Such a model could play a role in accelerating the development of vaccines to prevent COVID-19 infection and disease. The model could additionally answer key questions about the transmissibility and correlates of protection that would be more difficult to address in a traditional field study. The lack of a reliable rescue treatment has led many to conclude it would be unethical to conduct such a study at the present time. However, a vocal, growing minority has argued that the massive, unprecedented scope of the COVID-19 pandemic shifts the risk-benefit calculation and justifies conducting human challenge studies.
Forging a New Community of Experts in Human Challenge Studies under the Aegis of a Global Health Experimental Medicine Network
The level of enthusiasm for the use of the controlled human infection concept in the scientific community is substantially increasing. To forge support for the concept, it may be necessary to demonstrate that the risks can be mitigated and the expected benefits will accrue. This was recently achieved for some vaccines, leading to better understanding of how they work and acceleration of their development.
The expansion of human challenge studies should be approached with an abundance of caution and with clear distinctions made among the available human challenge models according to their strengths and limitations. The expanded use of a given model is best initiated in experienced hands, and dissemination of shared learning and leadership can be provided by seasoned veterans in the field.
Considering a phased development plan in which the initial studies are conducted in a loosely constituted network may be useful before establishing an elaborate (and often bureaucratic) structure. In addition, projects in which the benefits of human challenge studies are most likely to be realized in the near term should be targeted. Twelve human challenge models are available now for which we identified no major concerns (see Table 38). From these, several groups actively working with them have joined forces to establish experimental medicine networks to capitalize on the advances made.
The vision of a new approach to vaccine development against many of the diseases of global public health importance is bold and inspiring. Momentum could build quickly as additional vaccines are tested and proven efficacious in the most affected populations, with the help, in large part, of the results of human challenge models. This approach, however, is neither suitable nor feasible for certain important diseases. For these, the traditional approaches to vaccine development must continue to be supported and applied. Where essential scientific knowledge can be gained and the safety of volunteers maintained, human challenge studies can play a greater role in vaccine development. Human immune responses to infection hold the key to vaccine development, and the controlled setting of a human challenge study can provide the means to evaluate these immune responses with a depth and sophistication never dreamed of by the developers of most of the vaccines we use today. Putting the best science behind the quest for new vaccines would be something the founder of experimental medicine, Claude Bernard, would heartily endorse.
ACKNOWLEDGMENTS
We thank Francine E. McCutchan for important contributions in the initial stages of this project, Mariana Zarate for bibliography assistance, and Stephanie M. Cascio for editorial assistance.
This study was funded by a grant to PATH from the Bill & Melinda Gates Foundation (OPP1140594). The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The authors declare they have no financial conflicts of interest in any of the products described here.
Conceptualization—Jorge Flores; funding acquisition—Jorge Flores; writing (original draft)—Robert K. M. Choy, A. Louis Bourgeois, Christian F. Ockenhouse, Richard I. Walker, Rebecca L. Sheets, and Jorge Flores; writing (review and editing)—Robert K. M. Choy, A. Louis Bourgeois, Christian F. Ockenhouse, Richard I. Walker, Rebecca L. Sheets, and Jorge Flores.
Biographies

Robert K. M. Choy is Director, Research and Development, in PATH’s Center for Vaccine Innovation and Access. He heads PATH’s efforts to use controlled human infection models to accelerate vaccine development for pathogens of global health importance, including Shigella, Salmonella, enterotoxigenic Escherichia coli, polio, and Plasmodium falciparum. He also currently leads projects to develop vaccines, therapeutics, and diagnostic tools for enteric and diarrheal diseases such as rotavirus, cholera, cryptosporidiosis, and environmental enteric dysfunction. Before joining PATH, he worked at Exelixis, Inc., using model organism genomics and cell-based high-throughput screening to identify and characterize novel drug targets across a range of therapeutic areas, from regenerative medicine to oncology, neuroscience, and metabolic disease. Dr. Choy received his Ph.D. in molecular and cellular biology from the University of Washington.

A. Louis Bourgeois is a Science Officer in the Enteric and Diarrheal Diseases program in PATH’s Center for Vaccine Innovation and Access. For the past 35 years, he has worked extensively in ETEC, Shigella, and Campylobacter vaccine development. Dr. Bourgeois began his career as a member of the U.S. Navy’s Enteric Disease Research Program and continued as a faculty member in the Center for Immunization Research, Johns Hopkins Bloomberg School of Public Health (JHBSPH) in Baltimore, MD, and more recently at PATH. While working on bacterial enteric vaccines, he has also helped refine controlled human infection models for ETEC, Shigella, and Campylobacter and helped develop and test a new mucosal adjuvant, dmLT, which has shown immune-enhancing activity for both oral and parenterally administered vaccines impacting on both protein and polysaccharide antigens. Dr. Bourgeois holds a Ph.D. in microbiology from Georgetown University and a Master of Public Health from JHBSPH.

Christian F. Ockenhouse is Senior Medical Officer and Director, Medical and Clinical Affairs, in the Malaria Disease Area of the Center for Vaccine Innovation and Access. He leads the clinical development of preerythrocytic, blood-stage, and transmission-blocking malaria vaccines at PATH and has more than 30 years of experience in developing human challenge models against all stages of the malaria life cycle. Before joining PATH, he led the first Joint U.S. Military Malaria Vaccine Program between the U.S. Army and U.S. Navy at the Walter Reed Army Institute of Research. He received his Ph.D. in immunology and parasitology from New York University and his M.D. from the Medical College of Pennsylvania (Drexel University).

Richard I. Walker was Director of the Enteric Vaccine Initiative at PATH, where he currently serves as Director for Special Products in the Enteric and Diarrheal Diseases program in PATH’s Center for Vaccine Innovation and Access. He was a microbiologist in the U.S. Navy for 23 years, during which he studied opportunistic infections, mucosal immunization, and enteric infections. His military assignments included the Radiological Defense Laboratory (San Francisco, CA), the Naval Medical Research Unit No. 2 (Taipei, Taiwan), and the Armed Forces Radiobiology Research Institute and Naval Medical Research Institute (both in Bethesda, MD). Upon retirement from the U.S. Navy, Dr. Walker worked at the National Vaccine Program Office, Antex Biologics, and at the U.S. Food and Drug Administration, where he was Director of the Division of Bacterial Parasitic and Allergenic Products. At PATH, he has focused on vaccine development for ETEC and Shigella and promoted the development of dmLT as a mucosal adjuvant.

Rebecca L. Sheets is the Principal Consultant of Grimalkin Partners, an Adjunct Professor at the Catholic University of America, and Vice President of the North American affiliate of the International Alliance for Biological Standardization. For 10 years, she served as a Scientific Reviewer for the U.S. Food and Drug Administration. In 2013, she retired from the U.S. Public Health Service at the rank of Captain (O-6), having served 11 years as a Vaccine Scientific and Regulatory Specialist in the Division of AIDS and Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health. She is the author of Fundamentals of Biologicals Regulation: Vaccines and Biotechnology Medicines, published by Imprint Press in 2017, and has authored more than 40 peer-reviewed journal articles. She is a frequent lecturer, moderator, and discussant at international conferences. Dr. Sheets received her Ph.D. in pathology from the University of Southern California School of Medicine.

Jorge Flores’ trajectory in medical research spans more than 50 years. His early research focused on the mechanism of action of cholera toxin (Massachusetts General Hospital/Harvard Medical School, 1970–1975) and the etiology of infantile diarrhea (Central University of Venezuela, 1975–1979), which led to his pivotal involvement in the development of rotavirus vaccines at the NIAID, National Institutes of Health. In 1994, Dr. Flores moved to the NIAID Division of AIDS (1994–2010), where he led the Clinical Branch in charge of HIV vaccine development, including the planning, conduct, and oversight of more than 60 clinical trials of 40+ HIV vaccine candidates. He joined PATH in 2010 to lead a vaccine clinical team that over the last 11 years completed more than 70 phase 1 to 3 trials of pediatric and adult vaccines, including four new rotavirus vaccines, as well as vaccines for pneumococcus, meningococcus, influenza, malaria, and COVID-19.
REFERENCES
- 1.Geenen V. 2020. Claude Bernard (1813–1878), the father of modern physiology and experimental medicine. http://orbi.ulg.ac.be/bitstream/2268/79049/1/C.BERNARD%20Text.pdf. Accessed 28 May 2022.
- 2.World Health Organization. 2020. World malaria report 2020. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240015791. [Google Scholar]
- 3.Spring M, Polhemus M, Ockenhouse C. 2014. Controlled human malaria infection. J Infect Dis 209(Suppl 2):S40–S45. doi: 10.1093/infdis/jiu063. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MM, Loiseau C, McCarthy JS, Doolan DL. 2019. Human challenge models: tools to accelerate the development of malaria vaccines. Expert Rev Vaccines 18:241–251. doi: 10.1080/14760584.2019.1580577. [DOI] [PubMed] [Google Scholar]
- 5.Roestenberg M, Hoogerwerf MA, Ferreira DM, Mordmüller B, Yazdanbakhsh M. 2018. Experimental infection of human volunteers. Lancet Infect Dis 18:e312–e322. doi: 10.1016/S1473-3099(18)30177-4. [DOI] [PubMed] [Google Scholar]
- 6.Stanisic DI, McCarthy JS, Good MF. 2017. Controlled human malaria infection: applications, advances, and challenges. Infect Immun 86:e00479-17. doi: 10.1128/IAI.00479-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayne B. 1933. The injection of mosquito sporozoites in malaria therapy. Public Health Rep 48:909–916. doi: 10.2307/4580870. [DOI] [Google Scholar]
- 8.Clyde DF, Most H, McCarthy VC, Vanderberg JP. 1973. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, Quakyi IA, Carter R, Trosper JH, Hockmeyer WT. 1986. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg 35:66–68. doi: 10.4269/ajtmh.1986.35.66. [DOI] [PubMed] [Google Scholar]
- 10.Langenberg MCC, Wammes LJ, McCall MBB, Bijker EM, van Gemert GJ, Graumans W, van de Vegte-Bolmer MG, Teelen K, Hermsen CC, Koelewijn R, van Hellemond JJ, van Genderen PJJ, Sauerwein RW. 2018. Controlled human malaria infection with graded numbers of Plasmodium falciparum NF135.C10- or NF166.C8-infected mosquitoes. Am J Trop Med Hyg 99:709–712. doi: 10.4269/ajtmh.18-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, Gordon DM, Stoute JA, Church LW, Sedegah M, Heppner DG, Ballou WR, Richie TL. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 12.Laurens MB, Berry AA, Travassos MA, Strauss K, Adams M, Shrestha B, Li T, Eappen A, Manoj A, Abebe Y, Murshedkar T, Gunasekera A, Richie TL, Lyke KE, Plowe CV, Kennedy JK, Potter GE, Deye GA, Sim BKL, Hoffman SL. 2019. Dose-dependent infectivity of aseptic, purified, cryopreserved Plasmodium falciparum 7G8 sporozoites in malaria-naive adults. J Infect Dis 220:1962–1966. doi: 10.1093/infdis/jiz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moser KA, Drábek EF, Dwivedi A, Stucke EM, Crabtree J, Dara A, Shah Z, Adams M, Li T, Rodrigues PT, Koren S, Phillippy AM, Munro JB, Ouattara A, Sparklin BC, Dunning Hotopp JC, Lyke KE, Sadzewicz L, Tallon LJ, Spring MD, Jongsakul K, Lon C, Saunders DL, Ferreira MU, Nyunt MM, Laufer MK, Travassos MA, Sauerwein RW, Takala-Harrison S, Fraser CM, Sim BKL, Hoffman SL, Plowe CV, Silva JC. 2020. Strains used in whole organism Plasmodium falciparum vaccine trials differ in genome structure, sequence, and immunogenic potential. Genome Med 12:6. doi: 10.1186/s13073-019-0708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne RO, Griffin PM, McCarthy JS, Draper SJ. 2017. Plasmodium vivax controlled human malaria infection: progress and prospects. Trends Parasitol 33:141–150. doi: 10.1016/j.pt.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera S, Fernandez O, Manzano MR, Murrain B, Vergara J, Blanco P, Palacios R, Vélez JD, Epstein JE, Chen-Mok M, Reed ZH, Arévalo-Herrera M. 2009. Case report: successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg 81:740–746. doi: 10.4269/ajtmh.2009.09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera S, Solarte Y, Jordán-Villegas A, Echavarría JF, Rocha L, Palacios R, Ramírez O, Vélez JD, Epstein JE, Richie TL, Arévalo-Herrera M. 2011. Consistent safety and infectivity in sporozoite challenge model of Plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hyg 84:4–11. doi: 10.4269/ajtmh.2011.09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov. 2020. NCT00935623: development of a safe and reproducible human sporozoite challenge model for Plasmodium vivax in healthy adults in the United States. https://clinicaltrials.gov/ct2/show/NCT00935623.
- 18.Woodford J, Collins KA, Odedra A, Wang C, Kyung JI, Domingo GJ, Watts R, Marquart L, Berriman M, Otto TD, McCarthy JS. 2020. An experimental human blood stage model for studying Plasmodium malariae infection. J Infect Dis 221:948–955. doi: 10.1093/infdis/jiz102. [DOI] [PubMed] [Google Scholar]
- 19.Laurens MB, Duncan CJ, Epstein JE, Hill AV, Komisar JL, Lyke KE, Ockenhouse CF, Richie TL, Roestenberg M, Sauerwein RW, Spring MD, Talley AK, Moorthy VS, Consensus Group on Design of Clinical Trials of Controlled Human Malaria Infection . 2012. A consultation on the optimization of controlled human malaria infection by mosquito bite for evaluation of candidate malaria vaccines. Vaccine 30:5302–5304. doi: 10.1016/j.vaccine.2012.04.088. [DOI] [PubMed] [Google Scholar]
- 20.Moorthy VS, Diggs C, Ferro S, Good MF, Herrera S, Hill AV, Imoukhuede EB, Kumar S, Loucq C, Marsh K, Ockenhouse CF, Richie TL, Sauerwein RW. 2009. Report of a consultation on the optimization of clinical challenge trials for evaluation of candidate blood stage malaria vaccines, 18–19 March 2009, Bethesda, MD, USA. Vaccine 27:5719–5725. doi: 10.1016/j.vaccine.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Kraft SA, Duenas DM, Kublin JG, Shipman KJ, Murphy SC, Shah SK. 2019. Exploring ethical concerns about human challenge studies: a qualitative study of controlled human malaria infection study participants’ motivations and attitudes. J Empir Res Hum Res Ethics 14:49–60. doi: 10.1177/1556264618820219. [DOI] [PubMed] [Google Scholar]
- 22.Njue M, Njuguna P, Kapulu MC, Sanga G, Bejon P, Marsh V, Molyneux S, Kamuya D. 2018. Ethical considerations in controlled human malaria infection studies in low resource settings: experiences and perceptions of study participants in a malaria challenge study in Kenya. Wellcome Open Res 3:39. doi: 10.12688/wellcomeopenres.14439.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chattopadhyay R, Pratt D. 2017. Role of controlled human malaria infection (CHMI) in malaria vaccine development: a U.S. Food and Drug Administration (FDA) perspective. Vaccine 35:2767–2769. doi: 10.1016/j.vaccine.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Fries LF, Gordon DM, Schneider I, Beier JC, Long GW, Gross M, Que JU, Cryz SJ, Sadoff JC. 1992. Safety, immunogenicity, and efficacy of a Plasmodium falciparum vaccine comprising a circumsporozoite protein repeat region peptide conjugated to Pseudomonas aeruginosa toxin A. Infect Immun 60:1834–1839. doi: 10.1128/iai.60.5.1834-1839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Church LW, Le TP, Bryan JP, Gordon DM, Edelman R, Fries L, Davis JR, Herrington DA, Clyde DF, Shmuklarsky MJ, Schneider I, McGovern TW, Chulay JD, Ballou WR, Hoffman SL. 1997. Clinical manifestations of Plasmodium falciparum malaria experimentally induced by mosquito challenge. J Infect Dis 175:915–920. doi: 10.1086/513990. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JE, Rao S, Williams F, Freilich D, Luke T, Sedegah M, de la Vega P, Sacci J, Richie TL, Hoffman SL. 2007. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis 196:145–154. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 27.Bejon P, Andrews L, Andersen RF, Dunachie S, Webster D, Walther M, Gilbert SC, Peto T, Hill AV. 2005. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Dis 191:619–626. doi: 10.1086/427243. [DOI] [PubMed] [Google Scholar]
- 28.Kamau E, Alemayehu S, Feghali KC, Komisar J, Regules J, Cowden J, Ockenhouse CF. 2014. Measurement of parasitological data by quantitative real-time PCR from controlled human malaria infection trials at the Walter Reed Army Institute of Research. Malar J 13:288. doi: 10.1186/1475-2875-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy SC, Prentice JL, Williamson K, Wallis CK, Fang FC, Fried M, Pinzon C, Wang R, Talley AK, Kappe SH, Duffy PE, Cookson BT. 2012. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg 86:383–394. doi: 10.4269/ajtmh.2012.10-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Food and Drug Administration, Office for Drug Evaluation and Research. 2021. Qualification decision and executive summary. US FDA, Bethesda, MD. https://www.fda.gov/media/119436/download. [Google Scholar]
- 31.Talley AK, Healy SA, Finney OC, Murphy SC, Kublin J, Salas CJ, Lundebjerg S, Gilbert P, Van Voorhis WC, Whisler J, Wang R, Ockenhouse CF, Heppner DG, Kappe SH, Duffy PE. 2014. Safety and comparability of controlled human Plasmodium falciparum infection by mosquito bite in malaria-naive subjects at a new facility for sporozoite challenge. PLoS One 9:e109654. doi: 10.1371/journal.pone.0109654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballou WR, Hoffman SL, Sherwood JA, Hollingdale MR, Neva FA, Hockmeyer WT, Gordon DM, Schneider I, Wirtz RA, Young JF. 1987. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet 1:1277–1281. doi: 10.1016/S0140-6736(87)90540-X. [DOI] [PubMed] [Google Scholar]
- 33.Herrington DA, Clyde DF, Losonsky G, Cortesia M, Murphy JR, Davis J, Baqar S, Felix AM, Heimer EP, Gillessen D, Nardin E, Nussenzweig RS, Nussenzweig V, Hollingdale MR, Levine MM. 1987. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328:257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 34.Regules JA, Cummings JF, Ockenhouse CF. 2011. The RTS,S vaccine candidate for malaria. Expert Rev Vaccines 10:589–599. doi: 10.1586/erv.11.57. [DOI] [PubMed] [Google Scholar]
- 35.Kester KE, Gray Heppner D, Jr, Moris P, Ofori-Anyinam O, Krzych U, Tornieporth N, McKinney D, Delchambre M, Ockenhouse CF, Voss G, Holland C, Beckey JP, Ballou WR, Cohen J, RTS,S/TRAP Group . 2014. Sequential phase 1 and phase 2 randomized, controlled trials of the safety, immunogenicity and efficacy of combined pre-erythrocytic vaccine antigens RTS,S and TRAP formulated with AS02 adjuvant system in healthy, malaria naive adults. Vaccine 32:6683–6691. doi: 10.1016/j.vaccine.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 36.Ockenhouse CF, Regules J, Tosh D, Cowden J, Kathcart A, Cummings J, Paolino K, Moon J, Komisar J, Kamau E, Oliver T, Chhoeu A, Murphy J, Lyke K, Laurens M, Birkett A, Lee C, Weltzin R, Wille-Reece U, Sedegah M, Hendriks J, Versteege I, Pau MG, Sadoff J, Vanloubbeeck Y, Lievens M, Heerwegh D, Moris P, Guerra Mendoza Y, Jongert E, Cohen J, Voss G, Ballou WR, Vekemans J. 2015. Ad35.CS.01-RTS,S/AS01 heterologous prime boost vaccine efficacy against sporozoite challenge in healthy malaria-naive adults. PLoS One 10:e0131571. doi: 10.1371/journal.pone.0131571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, Andrews L, Bejon P, Poulton I, Butcher G, Watkins K, Sinden RE, Leach A, Moris P, Tornieporth N, Schneider J, Dubovsky F, Tierney E, Williams J, Heppner DG, Jr, Gilbert SC, Cohen J, Hill AV. 2006. A clinical trial of prime-boost immunization with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine 24:2850–2859. doi: 10.1016/j.vaccine.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 38.Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, Payne RO, Venkatraman N, de Barra E, Snudden CM, Poulton ID, de Graaf H, Sukhtankar P, Roberts R, Ivinson K, Weltzin R, Rajkumar BY, Wille-Reece U, Lee CK, Ockenhouse CF, Sinden RE, Gerry S, Lawrie AM, Vekemans J, Morelle D, Lievens M, Ballou RW, Cooke GS, Faust SN, Gilbert S, Hill AV. 2016. Safety and high level efficacy of the combination malaria vaccine regimen of RTS,S/AS01B with chimpanzee adenovirus 63 and modified vaccinia Ankara vectored vaccines expressing ME-TRAP. J Infect Dis 214:772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, Collins KA, Edwards NJ, Douglas AD, Anagnostou NA, Ewer KJ, Havelock T, Mahungu T, Bliss CM, Miura K, Poulton ID, Lillie PJ, Rampling T, Ewer KJ, Bowyer G, Edwards NJ, Wright D, Sridhar S, Payne R, Powlson J, Bliss C, Venkatraman N, Poulton ID, de Graaf H, Gbesemete D, Grobbelaar A, Davies H, et al. 2018. Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP. NPJ Vaccines 3:49. doi: 10.1038/s41541-018-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genton B, D’Acremont V, Lurati-Ruiz F, Verhage D, Audran R, Hermsen C, Wolters L, Reymond C, Spertini F, Sauerwein R. 2010. Randomized double-blind controlled phase I/IIa trial to assess the efficacy of malaria vaccine PfCS102 to protect against challenge with Plasmodium falciparum. Vaccine 28:6573–6580. doi: 10.1016/j.vaccine.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 41.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, Walsh DS, Yoon IK, Prosperi C, Juompan LY, Lanar DE, Krzych U, Hall BT, Ware LA, Stewart VA, Williams J, Dowler M, Nielsen RK, Hillier CJ, Giersing BK, Dubovsky F, Malkin E, Tucker K, Dubois MC, Cohen JD, Ballou WR, Heppner DG, Jr.. 2010. Recombinant liver stage antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-γ/IL-2 CD4 T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine 28:5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 42.ClinicalTrials.gov. 2019. NCT01540474: phase 1 study with the vaccine candidate Plasmodium falciparum malaria protein (FMP012), an E. coli-expressed cell-traversal protein, administered intramuscularly in healthy malaria-naive adults. https://clinicaltrials.gov/ct2/show/NCT01540474.
- 43.ClinicalTrials.gov. 2019. NCT02174978: phase 1 clinical trial with controlled human malaria infection (CHMI) open-label dose safety, reactogenicity, immunogenicity, and efficacy of the vaccine candidate Plasmodium falciparum malaria protein (FMP012), administered intramuscularly with AS01B adjuvant system in healthy malaria-naive adults. https://clinicaltrials.gov/ct2/show/NCT02174978.
- 44.Dejon-Agobe JC, Ateba-Ngoa U, Lalremruata A, Homoet A, Engelhorn J, Nouatin OP, Edoa JR, Fernandes JF, Esen M, Mouwenda YD, Betouke Ongwe EM, Massinga- Loembe M, Hoffman SL, Sim BKL, Theisen M, Kremsner PG, Adegnika AA, Lell B, Mordmüller B. 2019. Controlled human malaria infection of healthy adults with lifelong malaria exposure to assess safety, immunogenicity, and efficacy of the asexual blood-stage malaria vaccine candidate GMZ2. Clin Infect Dis 69:1377–1384. doi: 10.1093/cid/ciy1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, Bergmann-Leitner E, Stewart VA, Bittner S, Juompan L, Kortepeter MG, Nielsen R, Krzych U, Tierney E, Ware LA, Dowler M, Hermsen CC, Sauerwein RW, de Vlas SJ, Ofori-Anyinam O, Lanar DE, Williams JL, Kester KE, Tucker K, Shi M, Malkin E, Long C, Diggs CL, Soisson L, Dubois MC, Ballou WR, Cohen J, Heppner DG, Jr.. 2009. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS One 4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, Maier C, Andrews L, Andersen RF, Gilbert S, Poulton I, Webster D, Dubovsky F, Tierney E, Sarpotdar P, Correa S, Huntcooke A, Butcher G, Williams J, Sinden RE, Thornton GB, Hill AV. 2005. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine 23:857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Ockenhouse CF, Sun PF, Lanar DE, Wellde BT, Hall BT, Kester K, Stoute JA, Magill A, Krzych U, Farley L, Wirtz RA, Sadoff JC, Kaslow DC, Kumar S, Church LW, Crutcher JM, Wizel B, Hoffman S, Lalvani A, Hill AV, Tine JA, Guito KP, de Taisne C, Anders R, Ballou WR. 1998. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J Infect Dis 177:1664–1673. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- 48.Tamminga C, Sedegah M, Regis D, Chuang I, Epstein JE, Spring M, Mendoza-Silveiras J, McGrath S, Maiolatesi S, Reyes S, Steinbeiss V, Fedders C, Smith K, House B, Ganeshan H, Lejano J, Abot E, Banania GJ, Sayo R, Farooq F, Belmonte M, Murphy J, Komisar J, Williams J, Shi M, Brambilla D, Manohar N, Richie NO, Wood C, Limbach K, Patterson NB, Bruder JT, Doolan DL, King CR, Diggs C, Soisson L, Carucci D, Levine G, Dutta S, Hollingdale MR, Ockenhouse CF, Richie TL. 2011. Adenovirus-5-vectored P falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS One 6:e25868. doi: 10.1371/journal.pone.0025868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Longley R, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AV. 2015. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis 211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, Ganeshan H, Belmonte M, Farooq F, Abot E, Banania JG, Huang J, Newcomer R, Rein L, Litilit D, Richie NO, Wood C, Murphy J, Sauerwein R, Hermsen CC, McCoy AJ, Kamau E, Cummings J, Komisar J, Sutamihardja A, Shi M, Epstein JE, Maiolatesi S, Tosh D, Limbach K, Angov E, Bergmann-Leitner E, Bruder JT, Doolan DL, King CR, Carucci D, Dutta S, Soisson L, Diggs C, Hollingdale MR, Ockenhouse CF, Richie TL. 2013. DNA prime/adenovirus boost malaria vaccine encoding P falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One 8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, Andersen RF, Bejon P, Goonetilleke N, Poulton I, Webster DP, Butcher G, Watkins K, Sinden RE, Levine GL, Richie TL, Schneider J, Kaslow D, Gilbert SC, Carucci DJ, Hill AV. 2006. A DNA prime-modified vaccinia virus Ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun 74:5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, McConkey SJ, Poulton I, Andrews L, Andersen RF, Bejon P, Butcher G, Sinden R, Skinner MA, Gilbert SC, Hill AV. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci USA 102:4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, Lyke KE, Mordmüller B, Alonso P, Duffy PE, Doumbo OK, Sauerwein RW, Tanner M, Abdulla S, Kremsner PG, Seder RA, Hoffman SL. 2015. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33:7452–7461. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollingdale MR, Sedegah M. 2017. Development of whole sporozoite malaria vaccines. Expert Rev Vaccines 16:45–54. doi: 10.1080/14760584.2016.1203784. [DOI] [PubMed] [Google Scholar]
- 55.Spring M, Murphy J, Nielsen R, Dowler M, Bennett JW, Zarling S, Williams J, de la Vega P, Ware L, Komisar J, Polhemus M, Richie TL, Epstein J, Tamminga C, Chuang I, Richie N, O’Neil M, Heppner DG, Healer J, O’Neill M, Smithers H, Finney OC, Mikolajczak SA, Wang R, Cowman A, Ockenhouse C, Krzych U, Kappe SHI. 2013. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 31:4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Rénia L, van der Ven A, Hermsen CC, Sauerwein R. 2009. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 57.Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, Gmeiner M, Campo JJ, Esen M, Ruben AJ, Held J, Calle CL, Mengue JB, Gebru T, Ibáñez J, Sulyok M, James ER, Billingsley PF, Natasha KC, Manoj A, Murshedkar T, Gunasekera A, Eappen AG, Li T, Stafford RE, Li M, Felgner PL, Seder RA, Richie TL, Sim BK, Hoffman SL, Kremsner PG. 2017. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542:445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Healy SA, Murphy SC, Hume JCC, Shelton L, Kuntz S, Van Voorhis WC, Moodie Z, Metch B, Wang R, Silver-Brace T, Fishbaugher M, Kennedy M, Finney OC, Chaturvedi R, Hobbs CV, Warner-Lubin M, Talley AK, Wong-Madden S, Stuart K, Wald A, Kappe SH, Kublin JG, Duffy PE. 2020. Chemoprophylaxis vaccination: phase 1 study to explore stage-specific immunity to Plasmodium falciparum in U.S. adults. Clin Infect Dis 71:1481–1490. doi: 10.1093/cid/ciz1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyke KE, Laurens MB, Strauss K, Adams M, Billingsley PF, James E, Manoj A, Chakravarty S, Plowe CV, Li ML, Ruben A, Edelman R, Green M, Dube TJ, Sim BKL, Hoffman SL. 2015. Optimizing intradermal administration of cryopreserved Plasmodium falciparum sporozoites in controlled human malaria infection. Am J Trop Med Hyg 93:1274–1284. doi: 10.4269/ajtmh.15-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, Mpina M, Ali Juma O, Schindler T, Huber E, Gunasekera A, Manoj A, Simon B, Saverino E, Church LWP, Hermsen CC, Sauerwein RW, Plowe C, Venkatesan M, Sasi P, Lweno O, Mutani P, Hamad A, Mohammed A, Urassa A, Mzee T, Padilla D, Ruben A, Sim BKL, Tanner M, Abdulla S, Hoffman SL. 2014. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 91:471–480. doi: 10.4269/ajtmh.14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheehy SH, Spencer AJ, Douglas AD, Sim BK, Longley RJ, Edwards NJ, Poulton ID, Kimani D, Williams AR, Anagnostou NA, Roberts R, Kerridge S, Voysey M, James ER, Billingsley PF, Gunasekera A, Lawrie AM, Hoffman SL, Hill AV. 2013. Optimising controlled human malaria infection studies using cryopreserved P falciparum parasites administered by needle and syringe. PLoS One 8:e65960. doi: 10.1371/journal.pone.0065960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. 2011. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334:475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 63.Gómez-Pérez GP, Legarda A, Muñoz J, Sim BK, Ballester MR, Dobaño C, Moncunill G, Campo JJ, Cisteró P, Jimenez A, Barrios D, Mordmüller B, Pardos J, Navarro M, Zita CJ, Nhamuave CA, García-Basteiro AL, Sanz A, Aldea M, Manoj A, Gunasekera A, Billingsley PF, Aponte JJ, James ER, Guinovart C, Antonijoan RM, Kremsner PG, Hoffman SL, Alonso PL. 2015. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naive volunteers: effect of injection volume and dose on infectivity rates. Malar J 14:306. doi: 10.1186/s12936-015-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodgson SH, Juma E, Salim A, Magiri C, Kimani D, Njenga D, Muia A, Cole AO, Ogwang C, Awuondo K, Lowe B, Munene M, Billingsley PF, James ER, Gunasekera A, Sim BK, Njuguna P, Rampling TW, Richman A, Abebe Y, Kamuyu G, Muthui M, Elias SC, Molyneux S, Gerry S, Macharia A, Williams TN, Bull PC, Hill AV, Osier FH, Draper SJ, Bejon P, Hoffman SL, Ogutu B, Marsh K. 2014. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol 5:686. doi: 10.3389/fmicb.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, Kamate B, Samake Y, Guindo MA, Dolo A, Niangaly A, Niaré K, Zeguime A, Sissoko K, Diallo H, Thera I, Ding K, Fay MP, O’Connell EM, Nutman TB, Wong-Madden S, Murshedkar T, Ruben AJ, Li M, Abebe Y, Manoj A, Gunasekera A, Chakravarty S, Sim BKL, Billingsley PF, James ER, Walther M, Richie TL, Hoffman SL, Doumbo O, Duffy PE. 2017. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 17:498–509. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riedl J, Mordmüller B, Koder S, Pabinger I, Kremsner PG, Hoffman SL, Ramharter M, Ay C. 2016. Alterations of blood coagulation in controlled human malaria infection. Malar J 15:15. doi: 10.1186/s12936-015-1079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lell B, Mordmüller B, Dejon Agobe JC, Honkpehedji J, Zinsou J, Mengue JB, Loembe MM, Adegnika AA, Held J, Lalremruata A, Nguyen TT, Esen M, Kc N, Ruben AJ, Chakravarty S, Lee Sim BK, Billingsley PF, James ER, Richie TL, Hoffman SL, Kremsner PG. 2018. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after controlled human malaria infection in adults in Gabon. Am J Trop Med Hyg 98:508–515. doi: 10.4269/ajtmh.17-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCall MBB, Kremsner PG, Mordmüller B. 2018. Correlating efficacy and immunogenicity in malaria vaccine trials. Semin Immunol 39:52–64. doi: 10.1016/j.smim.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Mo AX, Pesce J, Hall BF. 2015. Exploring immunological mechanisms of the whole sporozoite vaccination against Plasmodium falciparum malaria. Vaccine 33:2851–2857. doi: 10.1016/j.vaccine.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 70.Scholzen A, Sauerwein RW. 2016. Immune activation and induction of memory: lessons learned from controlled human malaria infection with Plasmodium falciparum. Parasitology 143:224–235. doi: 10.1017/S0031182015000761. [DOI] [PubMed] [Google Scholar]
- 71.Walk J, de Bree LCJ, Graumans W, Stoter R, van Gemert GJ, van de Vegte-Bolmer M, Teelen K, Hermsen CC, Arts RJW, Behet MC, Keramati F, Moorlag S, Yang ASP, van Crevel R, Aaby P, de Mast Q, van der Ven AJAM, Stabell Benn C, Netea MG, Sauerwein RW. 2019. Outcomes of controlled human malaria infection after BCG vaccination. Nat Commun 10:874. doi: 10.1038/s41467-019-08659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdi AI, Hodgson SH, Muthui MK, Kivisi CA, Kamuyu G, Kimani D, Hoffman SL, Juma E, Ogutu B, Draper SJ, Osier F, Bejon P, Marsh K, Bull PC. 2017. Plasmodium falciparum malaria parasite var gene expression is modified by host antibodies: longitudinal evidence from controlled infections of Kenyan adults with varying natural exposure. BMC Infect Dis 17:585. doi: 10.1186/s12879-017-2686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obiero JM, Shekalaghe S, Hermsen CC, Mpina M, Bijker EM, Roestenberg M, Teelen K, Billingsley PF, Sim BK, James ER, Daubenberger CA, Hoffman SL, Abdulla S, Sauerwein RW, Scholzen A. 2015. Impact of malaria preexposure on antiparasite cellular and humoral immune responses after controlled human malaria infection. Infect Immun 83:2185–2196. doi: 10.1128/IAI.03069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kisalu NK, Idris AH, Weidle C, Flores-Garcia Y, Flynn BJ, Sack BK, Murphy S, Schön A, Freire E, Francica JR, Miller AB, Gregory J, March S, Liao HX, Haynes BF, Wiehe K, Trama AM, Saunders KO, Gladden MA, Monroe A, Bonsignori M, Kanekiyo M, Wheatley AK, McDermott AB, Farney SK, Chuang GY, Zhang B, Kc N, Chakravarty S, Kwong PD, Sinnis P, Bhatia SN, Kappe SHI, Sim BKL, Hoffman SL, Zavala F, Pancera M, Seder RA. 2018. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 24:408–416. doi: 10.1038/nm.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oyen D, Torres JL, Wille-Reece U, Ockenhouse CF, Emerling D, Glanville J, Volkmuth W, Flores-Garcia Y, Zavala F, Ward AB, King CR, Wilson IA. 2017. Structural basis for antibody recognition of the NANP repeats in Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci USA 114:E10438–E10445. doi: 10.1073/pnas.1715812114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun P, Schwenk R, White K, Stoute JA, Cohen J, Ballou WR, Voss G, Kester KE, Heppner DG, Krzych U. 2003. Protective immunity induced with malaria vaccine, RTS,S, is linked to Plasmodium falciparum circumsporozoite protein-specific CD4 and CD8 T cells producing IFN-γ. J Immunol 171:6961–6967. doi: 10.4049/jimmunol.171.12.6961. [DOI] [PubMed] [Google Scholar]
- 77.Moris P, Jongert E, van der Most RG. 2018. Characterization of T-cell immune responses in clinical trials of the candidate RTS,S malaria vaccine. Hum Vaccin Immunother 14:17–27. doi: 10.1080/21645515.2017.1381809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwenk R, Lumsden JM, Rein LE, Juompan L, Kester KE, Heppner DG, Krzych U. 2011. Immunization with the RTS,S/AS malaria vaccine induces IFN-γ+ CD4 T cells that recognize only discrete regions of the circumsporozoite protein and these specificities are maintained following booster immunizations and challenge. Vaccine 29:8847–8854. doi: 10.1016/j.vaccine.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 79.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AV. 2005. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol 174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 80.Barton AJ, Hill J, Pollard AJ, Blohmke CJ. 2017. Transcriptomics in human challenge models. Front Immunol 8:1839. doi: 10.3389/fimmu.2017.01839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoo R, Bruske E, Dimonte S, Zhu L, Mordmüller B, Sim BKL, Kremsner PG, Hoffman SL, Bozdech Z, Frank M, Preiser PR. 2019. Transcriptome profiling reveals functional variation in Plasmodium falciparum parasites from controlled human malaria infection studies. EBioMedicine 48:442–452. doi: 10.1016/j.ebiom.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ockenhouse CF, Hu WC, Kester KE, Cummings JF, Stewart A, Heppner DG, Jedlicka AE, Scott AL, Wolfe ND, Vahey M, Burke DS. 2006. Common and divergent immune response signaling pathways discovered in peripheral blood mononuclear cell gene expression patterns in presymptomatic and clinically apparent malaria. Infect Immun 74:5561–5573. doi: 10.1128/IAI.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran TM, Bijker EM, Haks MC, Ottenhoff THM, Visser L, Schats R, Venepally P, Lorenzi H, Crompton PD, Sauerwein RW. 2019. Whole-blood transcriptomic signatures induced during immunization by chloroquine prophylaxis and Plasmodium falciparum sporozoites. Sci Rep 9:8386. doi: 10.1038/s41598-019-44924-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van den Berg RA, Coccia M, Ballou WR, Kester KE, Ockenhouse CF, Vekemans J, Jongert E, Didierlaurent AM, van der Most RG. 2017. Predicting RTS,S vaccine-mediated protection from transcriptomes in a malaria-challenge clinical trial. Front Immunol 8:557. doi: 10.3389/fimmu.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, Ballou WR, Jongert E, Wille-Reece U, Ockenhouse C, Aderem A, Zak DE, Sadoff J, Hendriks J, Wrammert J, Ahmed R, Pulendran B. 2017. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci USA 114:2425–2430. doi: 10.1073/pnas.1621489114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dunachie S, Berthoud T, Hill AV, Fletcher HA. 2015. Transcriptional changes induced by candidate malaria vaccines and correlation with protection against malaria in a human challenge model. Vaccine 33:5321–5331. doi: 10.1016/j.vaccine.2015.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCarthy JS, Sekuloski S, Griffin PM, Elliott S, Douglas N, Peatey C, Rockett R, O’Rourke P, Marquart L, Hermsen C, Duparc S, Möhrle J, Trenholme KR, Humberstone AJ. 2011. A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One 6:e21914. doi: 10.1371/journal.pone.0021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engwerda CR, Minigo G, Amante FH, McCarthy JS. 2012. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol 28:515–521. doi: 10.1016/j.pt.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, Elliott S, Whiley D, Sloots T, Winzeler EA, Trenholme KR. 2013. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis 208:1688–1694. doi: 10.1093/infdis/jit394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffin P, Pasay C, Elliott S, Sekuloski S, Sikulu M, Hugo L, Khoury D, Cromer D, Davenport M, Sattabongkot J, Ivinson K, Ockenhouse C, McCarthy J. 2016. Safety and reproducibility of a clinical trial system using induced blood stage Plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLoS Negl Trop Dis 10:e0005139. doi: 10.1371/journal.pntd.0005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woodberry T, Minigo G, Piera KA, Amante FH, Pinzon-Charry A, Good MF, Lopez JA, Engwerda CR, McCarthy JS, Anstey NM. 2012. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J Infect Dis 206:333–340. doi: 10.1093/infdis/jis366. [DOI] [PubMed] [Google Scholar]
- 92.Stanisic DI, Gerrard J, Fink J, Griffin PM, Liu XQ, Sundac L, Sekuloski S, Rodriguez IB, Pingnet J, Yang Y, Zhou Y, Trenholme KR, Wang CY, Hackett H, Chan JA, Langer C, Hanssen E, Hoffman SL, Beeson JG, McCarthy JS, Good MF. 2016. Infectivity of Plasmodium falciparum in malaria-naive individuals is related to knob expression and cytoadherence of the parasite. Infect Immun 84:2689–2696. doi: 10.1128/IAI.00414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Payne RO, Milne KH, Elias SC, Edwards NJ, Douglas AD, Brown RE, Silk SE, Biswas S, Miura K, Roberts R, Rampling TW, Venkatraman N, Hodgson SH, Labbé GM, Halstead FD, Poulton ID, Nugent FL, de Graaf H, Sukhtankar P, Williams NC, Ockenhouse CF, Kathcart AK, Qabar AN, Waters NC, Soisson LA, Birkett AJ, Cooke GS, Faust SN, Woods C, Ivinson K, McCarthy JS, Diggs CL, Vekemans J, Long CA, Hill AV, Lawrie AM, Dutta S, Draper SJ. 2016. Demonstration of the blood-stage Plasmodium falciparum controlled human malaria infection model to assess efficacy of the P falciparum apical membrane antigen 1 vaccine, FMP2.1/AS01. J Infect Dis 213:1743–1751. doi: 10.1093/infdis/jiw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loughland JR, Minigo G, Sarovich DS, Field M, Tipping PE, Montes de Oca M, Piera KA, Amante FH, Barber BE, Grigg MJ, William T, Good MF, Doolan DL, Engwerda CR, Anstey NM, McCarthy JS, Woodberry T. 2017. Plasmacytoid dendritic cells appear inactive during sub-microscopic Plasmodium falciparum blood-stage infection, yet retain their ability to respond to TLR stimulation. Sci Rep 7:2596. doi: 10.1038/s41598-017-02096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loughland JR, Minigo G, Burel J, Tipping PE, Piera KA, Amante FH, Engwerda CR, Good MF, Doolan DL, Anstey NM, McCarthy JS, Woodberry T. 2016. Profoundly reduced CD1c myeloid dendritic cell HLA-DR and CD86 expression and increased tumor necrosis factor production in experimental human blood-stage malaria infection. Infect Immun 84:1403–1412. doi: 10.1128/IAI.01522-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farid R, Dixon MW, Tilley L, McCarthy JS. 2017. Initiation of gametocytogenesis at very low parasite density in Plasmodium falciparum infection. J Infect Dis 215:1167–1174. doi: 10.1093/infdis/jix035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao P, Collins KA, Zaloumis S, Wattanakul T, Tarning J, Simpson JA, McCarthy J, McCaw JM. 2019. Modeling the dynamics of Plasmodium falciparum gametocytes in humans during malaria infection. Elife 8:e49058. doi: 10.7554/eLife.49058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collins KA, Wang CY, Adams M, Mitchell H, Rampton M, Elliott S, Reuling IJ, Bousema T, Sauerwein R, Chalon S, Möhrle JJ, McCarthy JS. 2018. A controlled human malaria infection model enabling evaluation of transmission-blocking interventions. J Clin Invest 128:1551–1562. doi: 10.1172/JCI98012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reuling IJ, van de Schans LA, Coffeng LE, Lanke K, Meerstein-Kessel L, Graumans W, van Gemert GJ, Teelen K, Siebelink-Stoter R, van de Vegte-Bolmer M, de Mast Q, van der Ven AJ, Ivinson K, Hermsen CC, de Vlas S, Bradley J, Collins KA, Ockenhouse CF, McCarthy J, Sauerwein RW, Bousema T. 2018. A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. Elife 7:e31549. doi: 10.7554/eLife.31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alkema M, Reuling IJ, de Jong GM, Lanke K, Coffeng LE, van Gemert GJ, van de Vegte-Bolmer M, de Mast Q, van Crevel R, Ivinson K, Ockenhouse CF, McCarthy JS, Sauerwein R, Collins KA, Bousema T. 2021. A randomized clinical trial to compare P falciparum gametocytaemia and infectivity following blood-stage or mosquito bite induced controlled malaria infection. J Infect Dis 224:1257–1265. doi: 10.1093/infdis/jiaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. 2013. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 102.World Health Organization. 2020. Dengue and severe dengue: key facts. WHO, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. [Google Scholar]
- 103.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, Pigott DM, Shearer FM, Johnson K, Earl L, Marczak LB, Shirude S, Davis Weaver N, Gilbert M, Velayudhan R, Jones P, Jaenisch T, Scott TW, Reiner RC, Jr, Hay SI. 2019. The current and future global distribution and population at risk of dengue. Nat Microbiol 4:1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller JL, de Wet BJM, deWet BJM, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. 2008. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog 4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.World Health Organization. 2017. Global vector control response 2017–2030. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/9789241512978. [Google Scholar]
- 108.Sim S, Ng LC, Lindsay SW, Wilson AL. 2020. A greener vision for vector control: the example of the Singapore dengue control programme. PLoS Negl Trop Dis 14:e0008428. doi: 10.1371/journal.pntd.0008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dalpadado R, Gunathilaka N, Amarasinghe D, Udayanaga L. 2021. A challenge for a unique dengue vector control programme: assessment of the spatial variation of insecticide resistance status amongst Aedes aegypti and Aedes albopictus populations in Gampaha District, Sri Lanka. Biomed Res Int 2021:6619175. doi: 10.1155/2021/6619175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gesto JSM, Pinto SB, Dias FBS, Peixoto J, Costa G, Kutcher S, Montgomery J, Green BR, Anders KL, Ryan PA, Simmons CP, O’Neill SL, Moreira LA. 2021. Large-scale deployment and establishment of Wolbachia into the Aedes aegypti population in Rio de Janeiro, Brazil. Front Microbiol 12:711107. doi: 10.3389/fmicb.2021.711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP, Jr, Srikiatkhachorn A. 2007. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 77:910–913. doi: 10.4269/ajtmh.2007.77.910. [DOI] [PubMed] [Google Scholar]
- 112.McArthur MA, Sztein MB, Edelman R. 2013. Dengue vaccines: recent developments, ongoing challenges and current candidates. Expert Rev Vaccines 12:933–953. doi: 10.1586/14760584.2013.815412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bentsi-Enchill AD, Schmitz J, Edelman R, Durbin A, Roehrig JT, Smith PG, Hombach J, Farrar J, Live Dengue Vaccines Technical Consultation Reporting Group . 2013. Long-term safety assessment of live attenuated tetravalent dengue vaccines: deliberations from a WHO technical consultation. Vaccine 31:2603–2609. doi: 10.1016/j.vaccine.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, Moodie Z, Westling T, Mascareñas C, Frago C, Cortés M, Chansinghakul D, Noriega F, Bouckenooghe A, Chen J, Ng SP, Gilbert PB, Gurunathan S, DiazGranados CA. 2018. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 379:327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- 115.Vannice KS, Wilder-Smith A, Barrett ADT, Carrijo K, Cavaleri M, de Silva A, Durbin AP, Endy T, Harris E, Innis BL, Katzelnick LC, Smith PG, Sun W, Thomas SJ, Hombach J. 2018. Clinical development and regulatory points for consideration for second-generation live attenuated dengue vaccines. Vaccine 36:3411–3417. doi: 10.1016/j.vaccine.2018.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gunther VJ, Putnak R, Eckels KH, Mammen MP, Scherer JM, Lyons A, Sztein MB, Sun W. 2011. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine 29:3895–3904. doi: 10.1016/j.vaccine.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 117.Lyons AG. 2014. The human dengue challenge experience at the Walter Reed Army Institute of Research. J Infect Dis 209(Suppl 2):S49–S55. doi: 10.1093/infdis/jiu174. [DOI] [PubMed] [Google Scholar]
- 118.Larsen CP, Whitehead SS, Durbin AP. 2015. Dengue human infection models to advance dengue vaccine development. Vaccine 33:7075–7082. doi: 10.1016/j.vaccine.2015.09.052. [DOI] [PubMed] [Google Scholar]
- 119.Thomas SJ. 2013. Dengue human infection model: reestablishing a tool for understanding dengue immunology and advancing vaccine development. Hum Vaccin Immunother 9:1587–1590. doi: 10.4161/hv.24188. [DOI] [PubMed] [Google Scholar]
- 120.Whitehorn J, Van VC, Simmons CP. 2014. Dengue human infection models supporting drug development. J Infect Dis 209(Suppl 2):S66–S70. doi: 10.1093/infdis/jiu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eckels KH, Dubois DR, Putnak R, Vaughn DW, Innis BL, Henchal EA, Hoke CH, Jr.. 2003. Modification of dengue virus strains by passage in primary dog kidney cells: preparation of candidate vaccines and immunization of monkeys. Am J Trop Med Hyg 69:12–16. doi: 10.4269/ajtmh.2003.69.12. [DOI] [PubMed] [Google Scholar]
- 122.Mammen MP, Lyons A, Innis BL, Sun W, McKinney D, Chung RCY, Eckels KH, Putnak R, Kanesa-Thasan N, Scherer JM, Statler J, Asher LV, Thomas SJ, Vaughn DW. 2014. Evaluation of dengue virus strains for human challenge studies. Vaccine 32:1488–1494. doi: 10.1016/j.vaccine.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 123.Sun W, Eckels KH, Putnak JR, Lyons AG, Thomas SJ, Vaughn DW, Gibbons RV, Fernandez S, Gunther VJ, Mammen MP, Jr, Statler JD, Innis BL. 2013. Experimental dengue virus challenge of human subjects previously vaccinated with live attenuated tetravalent dengue vaccines. J Infect Dis 207:700–708. doi: 10.1093/infdis/jis744. [DOI] [PubMed] [Google Scholar]
- 124.Endy TP, Wang D, Polhemus ME, Jarman RG, Jasper LE, Gromowski G, Lin L, De La Barra RA, Friberg H, Currier JR, Abbott M, Ware L, Klick M, Paolino KM, Blair DC, Eckels K, Rutvisuttinunt W, Thomas SJ. 2021. A phase 1, open-label assessment of a dengue virus-1 live virus human challenge strain. J Infect Dis 223:258–267. doi: 10.1093/infdis/jiaa351. [DOI] [PubMed] [Google Scholar]
- 125.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, Carmolli MP, Luke CJ, Diehl SA, Durbin AP. 2016. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci Transl Med doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 126.ClinicalTrials.gov. 2021. NCT01931176: a phase 1 evaluation of the safety and immunogenicity of rDEN2Δ30-7169, a live attenuated monovalent dengue virus vaccine. https://clinicaltrials.gov/ct2/show/study/NCT01931176.
- 127.Grifoni A, Angelo M, Sidney J, Paul S, Peters B, de Silva AD, Phillips E, Mallal S, Diehl SA, Botten J, Boyson J, Kirkpatrick BD, Whitehead SS, Durbin AP, Sette A, Weiskopf D. 2017. Patterns of cellular immunity associated with experimental infection with rDEN2Δ30 (Tonga/74) support its suitability as a human dengue virus challenge strain. J Virol 91:e02133-16. doi: 10.1128/JVI.02133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Redoni M, Yacoub S, Rivino L, Giacobbe DR, Luzzati R, Di Bella S. 2020. Dengue: status of current and under-development vaccines. Rev Med Virol 30:e2101. doi: 10.1002/rmv.2101. [DOI] [PubMed] [Google Scholar]
- 129.Wilder-Smith A. 2020. Dengue vaccine development: status and future. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 63:40–44. doi: 10.1007/s00103-019-03060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deng SQ, Yang X, Wei Y, Chen JT, Wang XJ, Peng HJ. 2020. A review on dengue vaccine development. Vaccines (Basel) 8:63. doi: 10.3390/vaccines8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas SJ, Yoon IK. 2019. A review of Dengvaxia: development to deployment. Hum Vaccin Immunother 15:2295–2314. doi: 10.1080/21645515.2019.1658503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 133.Capeding MR, Tran NH, Hadinegoro SR, Ismail H, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon I-K, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A, The CYD14 Study Group . 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 134.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Miranda Montoya MC, Cortés Supelano M, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F. 2015. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 135.Pinheiro JR, Camilo Dos Reis E, Souza RDSO, Rocha ALS, Suesdek L, Azevedo V, Tiwari S, Rocha BGS, Birbrair A, Méndez EC, Luiz WB, Amorim JH. 2021. Comparison of neutralizing dengue virus B cell epitopes and protective T cell epitopes with those in three main dengue virus vaccines. Front Immunol 12:715136. doi: 10.3389/fimmu.2021.715136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun W, Cunningham D, Wasserman SS, Perry J, Putnak JR, Eckels KH, Vaughn DW, Thomas SJ, Kanesa-Thasan N, Innis BL, Edelman R. 2009. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naive adults. Hum Vaccin 5:33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 137.Watanaveeradej V, Gibbons RV, Simasathien S, Nisalak A, Jarman RG, Kerdpanich A, Tournay E, De La Barrerra R, Dessy F, Toussaint JF, Eckels KH, Thomas SJ, Innis BL. 2014. Safety and immunogenicity of a rederived, live-attenuated dengue virus vaccine in healthy adults living in Thailand: a randomized trial. Am J Trop Med Hyg 91:119–128. doi: 10.4269/ajtmh.13-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bauer K, Esquilin IO, Cornier AS, Thomas SJ, Quintero Del Rio AI, Bertran-Pasarell J, Morales Ramirez JO, Diaz C, Carlo S, Eckels KH, Tournay E, Toussaint JF, De La Barrera R, Fernandez S, Lyons A, Sun W, Innis BL. 2015. A phase II, randomized, safety and immunogenicity trial of a re-derived, live-attenuated dengue virus vaccine in healthy children and adults living in Puerto Rico. Am J Trop Med Hyg 93:441–453. doi: 10.4269/ajtmh.14-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moris P, Bauer KM, Currier JR, Friberg H, Eckels KH, Esquilin IO, Gibbons RV, Innis BL, Jarman RG, Simasathien S, Sun P, Thomas SJ, Watanaveeradej V. 2019. Cell-mediated immune responses to different formulations of a live-attenuated tetravalent dengue vaccine candidate in subjects living in dengue endemic and non-endemic regions. Hum Vaccin Immunother 15:2090–2105. doi: 10.1080/21645515.2019.1581536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. 2011. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 29:7242–7250. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murrell S, Wu SC, Butler M. 2011. Review of dengue virus and the development of a vaccine. Biotechnol Adv 29:239–247. doi: 10.1016/j.biotechadv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 142.Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, Tibery C, Sabundayo BP, Shaffer D, Talaat KR, Hynes NA, Wanionek K, Carmolli MP, Luke CJ, Murphy BR, Subbarao K, Whitehead SS. 2013. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 207:957–965. doi: 10.1093/infdis/jis936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kallas EG, Precioso AR, Palacios R, Thomé B, Braga PE, Vanni T, Campos LMA, Ferrari L, Mondini G, da Graça Salomão M, da Silva A, Espinola HM, do Prado Santos J, Santos CLS, Timenetsky M, Miraglia JL, Gallina NMF, Weiskopf D, Sette A, Goulart R, Salles RT, Maestri A, Sallum AME, Farhat SCL, Sakita NK, Ferreira JCOA, Silveira CGT, Costa PR, Raw I, Whitehead SS, Durbin AP, Kalil J. 2020. Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine Butantan-DV in adults in Brazil: a two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect Dis 20:839–850. doi: 10.1016/S1473-3099(20)30023-2. [DOI] [PubMed] [Google Scholar]
- 144.ClinicalTrials.gov. 2020. NCT02406729: phase III trial to evaluate efficacy and safety of a dengue 1,2,3,4 (attenuated) vaccine. https://clinicaltrials.gov/ct2/show/NCT02406729.
- 145.Nivarthi UK, Tu HA, Delacruz MJ, Swanstrom J, Patel B, Durbin AP, Whitehead SS, Pierce KK, Kirkpatrick BD, Baric RS, Nguyen N, Emerling DE, de Silva AM, Diehl SA. 2019. Longitudinal analysis of acute and convalescent B cell responses in a human primary dengue serotype 2 infection model. EbioMed 41:465–478. doi: 10.1016/j.ebiom.2019.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Graham N, Eisenhauer P, Diehl SA, Pierce KK, Whitehead SS, Durbin AP, Kirkpatrick BD, Sette A, Weiskopf D, Boyson JE, Botten JW. 2020. Rapid induction and maintenance of virus-specific CD8 TEMRA and CD4 TEM cells following protective vaccination against dengue virus challenge in humans. Front Immunol 11:479. doi: 10.3389/fimmu.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kirkpatrick BD, Durbin AP, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Diehl SA, Elwood D, Jarvis AP, Sabundayo BP, Lyon CE, Larsson CJ, Jo M, Lovchik JM, Luke CJ, Walsh MC, Fraser EA, Subbarao K, Whitehead SS. 2015. Robust and balanced immune responses to all 4 dengue virus serotypes following administration of a single dose of a live attenuated tetravalent dengue vaccine to healthy, flavivirus-naive adults. J Infect Dis 212:702–710. doi: 10.1093/infdis/jiv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Durbin AP, Kirkpatrick BD, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Opert K, Jarvis AP, Sabundayo BP, McElvany BD, Sendra EA, Larsson CJ, Jo M, Lovchik JM, Luke CJ, Walsh MC, Fraser EA, Subbarao K, Whitehead SS. 2016. A 12-month-interval dosing study in adults indicates that a single dose of the National Institute of Allergy and Infectious Diseases tetravalent dengue vaccine induces a robust neutralizing antibody response. J Infect Dis 14:832–835. doi: 10.1093/infdis/jiw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Whitehead SS, Durbin AP, Pierce KK, Elwood D, McElvany BD, Fraser EA, Carmolli MP, Tibery CM, Hynes NA, Jo M, Lovchik JM, Larsson CJ, Doty EA, Dickson DM, Luke CJ, Subbarao K, Diehl SA, Kirkpatrick BD. 2017. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl Trop Dis 11:e0005584. doi: 10.1371/journal.pntd.0005584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whitehead SS. 2016. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD™ vaccine? Expert Rev Vaccines 15:509–517. doi: 10.1586/14760584.2016.1115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Osorio JE, Velez ID, Thomson C, Lopez L, Jimenez A, Haller AA, Silengo S, Scott J, Boroughs KL, Stovall JL, Luy BE, Arguello J, Beatty ME, Santangelo J, Gordon GS, Huang CY, Stinchcomb DT. 2014. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 14:830–838. doi: 10.1016/S1473-3099(14)70811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.George SL, Wong MA, Dube TJ, Boroughs KL, Stovall JL, Luy BE, Haller AA, Osorio JE, Eggemeyer LM, Irby-Moore S, Frey SE, Huang CY, Stinchcomb DT. 2015. Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis 212:1032–1041. doi: 10.1093/infdis/jiv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rupp R, Luckasen GJ, Kirstein JL, Osorio JE, Santangelo JD, Raanan M, Smith MK, Wallace D, Gordon GS, Stinchcomb DT. 2015. Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a phase 1b randomized study. Vaccine 33:6351–6359. doi: 10.1016/j.vaccine.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 154.Jackson LA, Rupp R, Papadimitriou A, Wallace D, Raanan M, Moss KJ. 2018. A phase 1 study of safety and immunogenicity following intradermal administration of a tetravalent dengue vaccine candidate. Vaccine 36:3976–3983. doi: 10.1016/j.vaccine.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 155.Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, Oh HM, Raanan M, Sariol CA, Shek LP, Simasathien S, Smith MK, Velez ID, Wallace D, Gordon GS, Stinchcomb DT. 2016. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis 213:1562–1572. doi: 10.1093/infdis/jiv762. [DOI] [PubMed] [Google Scholar]
- 156.Sáez-Llorens X, Tricou V, Yu D, Rivera L, Tuboi S, Garbes P, Borkowski A, Wallace D. 2017. Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 17:615–625. doi: 10.1016/S1473-3099(17)30166-4. [DOI] [PubMed] [Google Scholar]
- 157.Sáez-Llorens X, Tricou V, Yu D, Rivera L, Jimeno J, Villarreal AC, Dato E, Mazara S, Vargas M, Brose M, Rauscher M, Tuboi S, Borkowski A, Wallace D. 2018. Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2–17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 18:162–170. doi: 10.1016/S1473-3099(17)30632-1. [DOI] [PubMed] [Google Scholar]
- 158.Tricou V, Sáez-Llorens X, Yu D, Rivera L, Jimeno J, Villarreal AC, Dato E, Saldaña de Suman O, Montenegro N, DeAntonio R, Mazara S, Vargas M, Mendoza D, Rauscher M, Brose M, Lefevre I, Tuboi S, Borkowski A, Wallace D. 2020. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2–17 years: a randomised, placebo-controlled, phase 2 trial. Lancet 395:1434–1443. doi: 10.1016/S0140-6736(20)30556-0. [DOI] [PubMed] [Google Scholar]
- 159.Tricou V, Low JG, Oh HM, Leo YS, Kalimuddin S, Wijaya L, Pang J, Ling LM, Lee TH, Brose M, Hutagalung Y, Rauscher M, Borkowski A, Wallace D. 2020. Safety and immunogenicity of a single dose of a tetravalent dengue vaccine with two different serotype-2 potencies in adults in Singapore: a phase 2, double-blind, randomised, controlled trial. Vaccine 38:1513–1519. doi: 10.1016/j.vaccine.2019.11.061. [DOI] [PubMed] [Google Scholar]
- 160.Turner M, Papadimitriou A, Winkle P, Segall N, Levin M, Doust M, Johnson C, Lucksinger G, Fierro C, Pickrell P, Raanan M, Tricou V, Borkowski A, Wallace D. 2020. Immunogenicity and safety of lyophilized and liquid dengue tetravalent vaccine candidate formulations in healthy adults: a randomized, phase 2 clinical trial. Hum Vaccin Immunother 16:2456–2464. doi: 10.1080/21645515.2020.1727697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Biswal S, Mendez Galvan JF, Macias Parra M, Galan-Herrera JF, Carrascal Rodriguez MB, Rodriguez Bueno EP, Brose M, Rauscher M, LeFevre I, Wallace D, Borkowski A. 2021. Immunogenicity and safety of a tetravalent dengue vaccine in dengue-naive adolescents in Mexico City. Rev Panam Salud Publica 45:e67. doi: 10.26633/RPSP.2021.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.ClinicalTrials.gov. 2021. NCT02747927: a phase III, double-blind, randomized, placebo-controlled trial to investigate the efficacy, safety and immunogenicity of a tetravalent dengue vaccine (TDV) administered subcutaneously in healthy children aged 4 to 16 years old. https://clinicaltrials.gov/ct2/show/NCT02747927.
- 163.Biswal S, Reynales H, Saez-Llorens X, Lopez P, Borja-Tabora C, Kosalaraksa P, Sirivichayakul C, Watanaveeradej V, Rivera L, Espinoza F, Fernando L, Dietze R, Luz K, Venâncio da Cunha R, Jimeno J, López-Medina E, Borkowski A, Brose M, Rauscher M, LeFevre I, Bizjajeva S, Bravo L, Wallace D, for the TIDES Study Group . 2019. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med 381:2009–2019. doi: 10.1056/NEJMoa1903869. [DOI] [PubMed] [Google Scholar]
- 164.Biswal S, Borja-Tabora C, Martinez Vargas L, Velásquez H, Theresa Alera M, Sierra V, Rodriguez-Arenales EJ, Yu D, Wickramasinghe VP, Duarte Moreira E, Jr, Fernando AD, Gunasekera D, Kosalaraksa P, Espinoza F, López-Medina E, Bravo L, Tuboi S, Hutagalung Y, Garbes P, Escudero I, Rauscher M, Bizjajeva S, LeFevre I, Borkowski A, Saez-Llorens X, Wallace D, The TIDES Study Group . 2020. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: a randomised, placebo-controlled, phase 3 trial. Lancet 395:1423–1433. doi: 10.1016/S0140-6736(20)30414-1. [DOI] [PubMed] [Google Scholar]
- 165.López-Medina E, Biswal S, Saez-Llorens X, Borja-Tabora C, Bravo L, Sirivichayakul C, Vargas LM, Alera MT, Velásquez H, Reynales H, Rivera L, Watanaveeradej V, Rodriguez-Arenales EJ, Yu D, Espinoza F, Dietze R, Fernando L, Wickramasinghe P, Duarte Moreira E, Jr, Fernando AD, Gunasekera D, Luz K, da Cunha RV, Tricou V, Rauscher M, Liu M, LeFevre I, Wallace D, Kosalaraksa P, Borkowski A, The TIDES Study Group . 2020. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents two years after vaccination. J Infect Dis 15:jiaa761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tricou V, Low JG, Oh HM, Leo YS, Kalimuddin S, Wijaya L, Pang J, Ling LM, Lee TH, Brose M, Hutagalung Y, Rauscher M, Borkowski A, Wallace D. 2020. Safety and immunogenicity of a single dose of a tetravalent dengue vaccine with two different serotype-2 potencies in adults in Singapore: a phase 2, double-blind, randomised, controlled trial. Vaccine 38:1513–1519. doi: 10.1016/j.vaccine.2019.11.061. [DOI] [PubMed] [Google Scholar]
- 167.Michlmayr D, Andrade P, Nascimento EJM, Parker A, Narvekar P, Dean HJ, Harris E. 2021. Characterization of the type-specific and cross-reactive B-cell responses elicited by a live-attenuated tetravalent dengue vaccine. J Infect Dis 223:247–257. doi: 10.1093/infdis/jiaa346. [DOI] [PubMed] [Google Scholar]
- 168.Chu H, George SL, Stinchcomb DT, Osorio JE, Partidos CD. 2015. CD8 T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 212:1618–1628. doi: 10.1093/infdis/jiv258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waickman AT, Friberg H, Gargulak M, Kong A, Polhemus M, Endy T, Thomas SJ, Jarman RG, Currier JR. 2019. Assessing the diversity and stability of cellular immunity generated in response to the candidate live-attenuated dengue virus vaccine TAK-003. Front Immunol 10:1778. doi: 10.3389/fimmu.2019.01778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sharma M, Glasner DR, Watkins H, Puerta-Guardo H, Kassa Y, Egan MA, Dean H, Harris E. 2020. Magnitude and functionality of the NS1-specific antibody response elicited by a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 221:867–877. doi: 10.1093/infdis/jiz081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.White LJ, Young EF, Stoops MJ, Henein SR, Adams EC, Baric RS, de Silva AM. 2021. Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003). PLoS Negl Trop Dis 15:e0009258. doi: 10.1371/journal.pntd.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schmidt AC, Lin L, Martinez LJ, Ruck RC, Eckels KH, Collard A, De La Barrera R, Paolino KM, Toussaint JF, Lepine E, Innis BL, Jarman RG, Thomas SJ. 2017. Phase 1 randomized study of a tetravalent dengue purified inactivated vaccine in healthy adults in the United States. Am J Trop Med Hyg 96:1325–1337. doi: 10.4269/ajtmh.16-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lin L, Lyke KE, Koren M, Jarman RG, Eckels KH, Lepine E, McArthur MA, Currier JR, Friberg H, Moris P, Keiser PB, De La Barrera R, Vaughn DW, Paris RM, Thomas SJ, Schmidt AC. 2020. Safety and immunogenicity of an AS03B-adjuvanted inactivated tetravalent dengue virus vaccine administered on varying schedules to healthy U.S. adults: a phase 1/2 randomized study. Am J Trop Med Hyg doi: 10.4269/ajtmh.19-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Diaz C, Lin L, Martinez LJ, Eckels KH, Campos M, Jarman RG, De La Barrera R, Lepine E, Toussaint JF, Febo I, Innis BL, Thomas SJ, Schmidt AC. 2018. Phase I randomized study of a tetravalent dengue purified inactivated vaccine in healthy adults from Puerto Rico. Am J Trop Med Hyg 98:1435–1443. doi: 10.4269/ajtmh.17-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Diaz C, Koren M, Lin L, Martinez LJ, Eckels KH, Campos M, Jarman RG, De La Barrera R, Lepine E, Febo I, Vaughn DW, Wilson TM, Paris RM, Schmidt AC, Thomas SJ. 2020. Safety and immunogenicity of different formulations of a tetravalent dengue purified inactivated vaccine in healthy adults from Puerto Rico: final results after 3 years of follow-up from a randomized, placebo-controlled phase I study. Am J Trop Med Hyg 102:951–954. doi: 10.4269/ajtmh.19-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.ClinicalTrials.gov. 2020. NCT03141138: a phase 1, randomized, open-label, comparison of heterologous prime-boost vaccination schedules of tetravalent dengue virus purified inactivated vaccine (PIV) and tetravalent dengue virus live attenuated vaccine (LAV) in healthy adults. https://clinicaltrials.gov/ct2/show/NCT03141138.
- 177.ClinicalTrials.gov. 2021. NCT02239614: a phase 1, randomized, open-label, single-center, study of TDENV-PIV and LAV dengue vaccine platforms as part of a heterologous prime-boost strategy in healthy adults in a nonendemic region. https://clinicaltrials.gov/ct2/show/NCT02239614.
- 178.Lin L, Koren MA, Paolino KM, Eckels KH, De La Barrera R, Friberg H, Currier JR, Gromowski GD, Aronson NE, Keiser PB, Sklar MJ, Sondergaard EL, Jasper LE, Endy TP, Jarman RG, Thomas SJ. 2021. Immunogenicity of a live-attenuated dengue vaccine using a heterologous prime-boost strategy in a phase 1 randomized clinical trial. J Infect Dis 223:1707–1716. doi: 10.1093/infdis/jiaa603. [DOI] [PubMed] [Google Scholar]
- 179.Manoff SB, George SL, Bett AJ, Yelmene ML, Dhanasekaran G, Eggemeyer L, Sausser ML, Dubey SA, Casimiro DR, Clements DE, Martyak T, Pai V, Parks DE, Coller BA. 2015. Preclinical and clinical development of a dengue recombinant subunit vaccine. Vaccine 33:7126–7134. doi: 10.1016/j.vaccine.2015.09.101. [DOI] [PubMed] [Google Scholar]
- 180.Manoff SB, Sausser M, Falk Russell A, Martin J, Radley D, Hyatt D, Roberts CC, Lickliter J, Krishnarajah J, Bett A, Dubey S, Finn T, Coller BA. 2019. Immunogenicity and safety of an investigational tetravalent recombinant subunit vaccine for dengue: results of a phase I randomized clinical trial in flavivirus-naive adults. Hum Vaccin Immunother 15:2195–2204. doi: 10.1080/21645515.2018.1546523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Durbin AP, Pierce KK, Kirkpatrick BD, Grier P, Sabundayo BP, He H, Sausser M, Russell AF, Martin J, Hyatt D, Cook M, Sachs JR, Wen-Tseng Lee A, Wang L, Coller BA, Whitehead SS. 2020. Immunogenicity and safety of a tetravalent recombinant subunit dengue vaccine in adults previously vaccinated with a live attenuated tetravalent dengue vaccine: results of a phase-I randomized clinical trial. Am J Trop Med Hyg doi: 10.4269/ajtmh.20-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, Wu SJ, Sun P, Kochel T, Raviprakash K, Hayes CG, Porter KR. 2011. Evaluation of a prototype dengue-1 DNA vaccine in a phase 1 clinical trial. Vaccine 29:960–968. doi: 10.1016/j.vaccine.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 183.Danko JR, Kochel T, Teneza-Mora N, Luke TC, Raviprakash K, Sun P, Simmons M, Moon JE, De La Barrera R, Martinez LJ, Thomas SJ, Kenney RT, Smith L, Porter KR. 2018. Safety and immunogenicity of a tetravalent dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am J Trop Med Hyg 98:849–856. doi: 10.4269/ajtmh.17-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Williams M, Ewing D, Blevins M, Sun P, Sundaram AK, Raviprakash KS, Porter KR, Sanders JW. 2019. Enhanced immunogenicity and protective efficacy of a tetravalent dengue DNA vaccine using electroporation and intradermal delivery. Vaccine 37:4444–4453. doi: 10.1016/j.vaccine.2019.06.083. [DOI] [PubMed] [Google Scholar]
- 185.Schmitz J, Roehrig J, Barrett A, Hombach J. 2011. Next generation dengue vaccines: a review of candidates in preclinical development. Vaccine 29:7276–7284. doi: 10.1016/j.vaccine.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 186.Wan SW, Lin CF, Wang S, Chen YH, Yeh TM, Liu HS, Anderson R, Lin YS. 2013. Current progress in dengue vaccines. J Biomed Sci 20:37. doi: 10.1186/1423-0127-20-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Halstead SB, Dans LF. 2019. Dengue infection and advances in dengue vaccines for children. Lancet Child Adolesc Health 3:734–741. doi: 10.1016/S2352-4642(19)30205-6. [DOI] [PubMed] [Google Scholar]
- 188.Durbin AP, Whitehead SS. 2013. The dengue human challenge model: has the time come to accept this challenge? J Infect Dis 207:697–699. doi: 10.1093/infdis/jis749. [DOI] [PubMed] [Google Scholar]
- 189.Endy TP. 2014. Dengue human infection model performance parameters. J Infect Dis 209(Suppl 2):S56–S60. doi: 10.1093/infdis/jiu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis 204:912–918. doi: 10.1093/infdis/jir416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pan American Health Organization, World Health Organization. 2018. Epidemiological update: cholera. PAHO/WHO, Washington, DC. https://www.paho.org/hq/index.php?option=com_docman&view=download&category_slug=cholera-2219&alias=46635-11-october-2018-cholera-epidemiological-update&Itemid=270&lang=en. [Google Scholar]
- 192.World Health Organization Regional Office for the Eastern Mediterranean. 2020. Cholera situation in Yemen, January 2020. WHO EMRO, Cairo, Egypt. https://reliefweb.int/sites/reliefweb.int/files/resources/EMCSR252E.pdf. [Google Scholar]
- 193.Pezzoli L, on behalf of the Oral Cholera Vaccine Working Group of the Global Task Force on Cholera Control . 2020. Global cholera vaccine use, 2013–2018. Vaccine 38(Suppl 1):A132–A140. doi: 10.1016/j.vaccine.2019.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qadri F, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Begum YA, Bhuiyan TR, Chowdhury MI, Uddin MJ, Khan JAM, Chowdhury AI, Rahman A, Siddique SA, Asaduzzaman M, Akter A, Khan A, You YA, Siddik AU, Saha NC, Kabir A, Riaz BK, Biswas SK, Begum F, Unicomb L, Luby SP, Cravioto A, Clemens JD. 2015. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet 386:1362–1371. doi: 10.1016/S0140-6736(15)61140-0. [DOI] [PubMed] [Google Scholar]
- 195.Saha A, Khan A, Salma U, Jahan N, Bhuiyan TR, Chowdhury F, Khan AI, Khanam F, Muruganandham S, Reddy S, Dhingra KMS, Clemens JD, Cravioto A, Qadri F. 2016. The oral cholera vaccine Shanchol™ when stored at elevated temperatures maintains the safety and immunogenicity profile in Bangladeshi participants. Vaccine 34:1551–1558. doi: 10.1016/j.vaccine.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 196.Khan AI, Ali M, Chowdhury F, Saha A, Khan IA, Khan A, Akter A, Asaduzzaman M, Islam MT, Kabir A, You YA, Saha NC, Cravioto A, Clemens JD, Qadri F. 2017. Safety of the oral cholera vaccine in pregnancy: retrospective findings from a subgroup following mass vaccination campaign in Dhaka, Bangladesh. Vaccine 35:1538–1543. doi: 10.1016/j.vaccine.2017.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Qadri F, Ali M, Lynch J, Chowdhury F, Khan AI, Wierzba TF, Excler J-L, Saha A, Excler J-L, Islam MT, Begum YA, Bhuiyan TR, Khanam F, Chowdhury MI, Khan IA, Kabir A, Riaz BK, Akter A, Khan A, Asaduzzaman M, Kim DR, Siddik AU, Saha NC, Cravioto A, Singh AP, Clemens JD. 2018. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis 18:666–674. doi: 10.1016/S1473-3099(18)30108-7. [DOI] [PubMed] [Google Scholar]
- 198.Lopez AL, Gonzales ML, Aldaba JG, Nair GB. 2014. Killed oral cholera vaccines: history, development and implementation challenges. Ther Adv Vaccines 2:123–136. doi: 10.1177/2051013614537819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Clemens JD, Harris JR, Khan MR, Kay BA, Yunus M, Svennerhold A-M, Sack DA, Chakraborty J, Stanton BF, Khan MU, Atkinson W, Holmgren J. 1986. Field trial of oral cholera vaccines in Bangladesh. Lancet 2:124–127. doi: 10.1016/S0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- 200.Clemens JD, Sack DA, Harris JR, van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, Stanton BF, Kay BA, Eeckels R, Walter S, Svennerholm A-M, Holmgren J. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273. doi: 10.1016/0140-6736(90)90080-O. [DOI] [PubMed] [Google Scholar]
- 201.Sanchez JL, Vasquez B, Begue RE, Meza R, Castellares G, Cabezas C, Watts DM, Svennerholm A-M, Sadoff JC, Taylor DN. 1994. Protective efficacy of oral whole-cell/recombinant-B-subunit cholera vaccine in Peruvian military recruits. Lancet 344:1273–1276. doi: 10.1016/s0140-6736(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 202.Lucas ME, Deen JL, von Seidlein L, Wang X-Y, Ampuero J, Puri M, Ali M, Ansaruzzaman M, Amos J, Macuamule A, Cavailler P, Guerin PJ, Mahoudeau C, Kahozi-Sangwa P, Chaignat C-L, Barreto A, Songane FF, Clemens JD. 2005. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 352:757–767. doi: 10.1056/NEJMoa043323. [DOI] [PubMed] [Google Scholar]
- 203.Kanungo S, Sen B, Ramamurthy T, Sur D, Manna B, Pazhani GP, Chowdhury G, Jhunjhunwala P, Nandy RK, Koley H, Bhattacharya MK, Gupta S, Goel G, Dey B, Thungapathra M, Nair GB, Ghosh A, Mahalanabis D. 2014. Safety and immunogenicity of a live oral recombinant cholera vaccine VA1.4: a randomized, placebo controlled trial in healthy adults in a cholera endemic area in Kolkata, India. 9:e99381. doi: 10.1371/journal.pone.0099381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Odevall L, Hong D, Digilio L, Sahastrabuddhe S, Mogasale V, Baik Y, Choi S, Kim JH, Lynch J. 2018. The Euvichol story: development and licensure of a safe, effective, and affordable oral cholera vaccine through global public private partnerships. Vaccine 36:6606–6614. doi: 10.1016/j.vaccine.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Baik YO, Choi SK, Olveda RM, Espos RA, Ligsay AD, Montellano MB, Yeam JS, Yang JS, Park JY, Kim DR, Desai SN, Singh AP, Kim IY, Kim CW, Park S-N. 2015. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol versus Shanchol) in the Philippines. Vaccine 33:6360–6365. doi: 10.1016/j.vaccine.2015.08.075. [DOI] [PubMed] [Google Scholar]
- 206.Karlsson SL, Ax E, Nygren E, Källgård S, Blomquist M, Ekman A, Benktander J, Holmgren J, Lebens M. 2014. Development of stable Vibrio cholerae O1 Hikojima type vaccine strains coexpressing the Inaba and Ogawa lipopolysaccharide antigens. PLoS One 9:e108521. doi: 10.1371/journal.pone.0108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chowdhury F, Ali Syed K, Akter A, Rahman Bhuiyan T, Tauheed I, Khaton F, Biswas R, Ferdous J, Al Banna H, Ross AG, McMillan N, Sharma T, Kanchan V, Pal Singh A, Gill D, Lebens M, Nordqvist S, Holmgren J, Clemens JD, Qadri F. 2021. A phase I/II study to evaluate safety, tolerability and immunogenicity of Hillchol, an inactivated single Hikojima strain based oral cholera vaccine, in a sequentially age descending population in Bangladesh. Vaccine 39:4450–4457. doi: 10.1016/j.vaccine.2021.06.069. [DOI] [PubMed] [Google Scholar]
- 208.Holmgren J, Svennerholm AM. 2012. Vaccines against mucosal infections. Curr Opin Immunol 24:343–353. doi: 10.1016/j.coi.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 209.Calain P, Chaine J-P, Johnson E, Hawley M-L, O’Leary MJ, Oshitani H, Chaignat C-L. 2004. Can oral cholera vaccination play a role in controlling a cholera outbreak? Vaccine 22:2444–2451. doi: 10.1016/j.vaccine.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 210.Tacket CO, Cohen MB, Wasserman SS, Losonsky G, Livio S, Kotloff K, Edelman R, Kaper JB, Cryz SJ, Giannella RA, Schiff G, Levine MM. 1999. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect Immun 67:6341–6345. doi: 10.1128/IAI.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen WH, Cohen MB, Kirkpatrick BD, Bready RC, Galloway D, Gurwith M, Hall RH, Kessler RA, Lock M, Haney D, Lyon CE, Pasetti MF, Simon JK, Szabo F, Tennant S, Levine MM. 2016. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis 62:1329–1335. doi: 10.1093/cid/ciw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mosley JF, II, Smith LL, Brantley P, Locke D, Como M. 2017. Vaxchora: the first FDA-approved cholera vaccination in the United States. P T 42:638–640. [PMC free article] [PubMed] [Google Scholar]
- 213.McCarty JM, Gierman EC, Bedell L, Lock MD, Bennett S. 2020. Safety and immunogenicity of live oral cholera vaccine CVD 103-HgR in children and adolescents aged 6–17 years. Am J Trop Med Hyg 102:48–57. doi: 10.4269/ajtmh.19-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McCarty JM, Cassie D, Bedell L, Lock MD, Bennett S. 2020. Safety and immunogenicity of live oral cholera vaccine CVD 103-HgR in children aged 2–5 years in the United States. Am J Trop Med Hyg 104:861–865. doi: 10.4269/ajtmh.20-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McCarty JM, Cassie D, Bedell L, Lock MD, Bennett S. 2021. Long-term immunogenicity of live oral cholera vaccine CVD 103-HgR in adolescents aged 12–17 years in the United States. Am J Trop Med Hyg 104:1758–1760. doi: 10.4269/ajtmh.20-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Semá Baltazar C, Rafael F, Langa JPM, Chicumbe S, Cavailler P, Gessner BD, Pezzoli L, Barata A, Zaina D, Inguane DL, Mengel MA, Munier A. 2018. Oral cholera vaccine coverage during a preventive door-to-door mass vaccination campaign in Nampula, Mozambique. 13:e0198592. doi: 10.1371/journal.pone.0198592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Abubakar A, Azman AS, Rumunu J, Ciglenecki I, Helderman T, West H, Lessler J, Sack DA, Martin S, Perea W, Legros D, Luquero FJ. 2015. The first use of the global oral cholera vaccine emergency stockpile: lessons from South Sudan. PLoS Med 12:e1001901. doi: 10.1371/journal.pmed.1001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Msyamboza KP, M’bang’ombe M, Hausi H, Chijuwa A, Nkukumila V, Kubwalo HW, Desai S, Pezzoli L, Legros D. 2016. Feasibility and acceptability of oral cholera vaccine mass vaccination campaign in response to an outbreak and floods in Malawi. Pan Afr Med J 23:203. doi: 10.11604/pamj.2016.23.203.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Amani A, Tatang CA, Bayiha CN, Woung M, Ngo Bama S, Nangmo A, Mbang MA, Epee Douba E. 2021. A reactive vaccination campaign with single dose oral cholera vaccine (OCV) during a cholera outbreak in Cameroon. Vaccine 39:1290–1296. doi: 10.1016/j.vaccine.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 220.Bwire G, Roskosky M, Ballard A, Brooks WA, Okello A, Rafael F, Ampeire I, Orach CG, Sack DA. 2020. Use of surveys to evaluate an integrated oral cholera vaccine campaign in response to a cholera outbreak in Hoima District, Uganda. BMJ Open 10:e038464. doi: 10.1136/bmjopen-2020-038464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ngwa MC, Alemu W, Okudo I, Owili C, Ugochukwu U, Clement P, Devaux I, Pezzoli L, Oche JA, Ihekweazu C, Sack DA. 2020. The reactive vaccination campaign against cholera emergency in camps for internally displaced persons, Borno, Nigeria, 2017: a two-stage cluster survey. BMJ Glob Health 5:e002431. doi: 10.1136/bmjgh-2020-002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lubogo M, Mohamed AM, Ali AH, Ali AH, Popal GR, Kiongo D, Bile KM, Malik M, Abubakar A. 2020. Oral cholera vaccination coverage in an acute emergency setting in Somalia, 2017. Vaccine 38:A141–A147. doi: 10.1016/j.vaccine.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 223.Global Task Force on Cholera Control. 2021. Preclinical toxicology testing and IND application for a novel cholera conjugate vaccine. Global Task Force on Cholera Control/WHO, Geneva, Switzerland. https://www.gtfcc.org/research/preclinical-toxicology-testing-and-ind-application-for-a-novel-cholera-conjugate-vaccine/. [Google Scholar]
- 224.Sayeed MA, Bufano MK, Xu P, Eckhoff G, Charles RC, Alam MM, Sultana T, Rashu MR, Berger A, Gonzalez-Escobedo G, Mandlik A, Bhuiyan TR, Leung DT, LaRocque RC, Harris JB, Calderwood SB, Qadri F, Vann WF, Kováč P, Ryan ET. 2015. A cholera conjugate vaccine containing O-specific polysaccharide (OSP) of V. cholerae O1 Inaba and recombinant fragment of tetanus toxin heavy chain (OSP:rTTHc) induces serum, memory and lamina proprial responses against OSP and is protective in mice. PLoS Negl Trop Dis 9:e0003881. doi: 10.1371/journal.pntd.0003881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Akter A, Kelly M, Charles RC, Harris JB, Calderwood SB, Bhuiyan TR, Biswas R, Xu P, Kováč P, Qadri F, Ryan ET. 2021. Parenteral vaccination with a cholera conjugate vaccine boosts vibriocidal and anti-OSP responses in mice previously immunized with an oral cholera vaccine. Am J Trop Med Hyg 104:2024–2030. doi: 10.4269/ajtmh.20-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Longini IM, Jr, Nizam A, Ali M, Yunus M, Shenvi N, Clemens JD. 2007. Controlling endemic cholera with oral vaccines. PLoS Med 4:e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Levine MM, Kaper JB, Black RE, Clements ML. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev 47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Black RE, Levine MM, Clements ML, Young CR, Svennerholm AM, Holmgren J. 1987. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun 55:1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tacket CO, Losonsky G, Nataro JP, Comstock L, Michalski J, Edelman R, Kaper JB, Levine MM. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis 172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 230.Tacket CO, Kotloff KL, Losonsky G, Nataro JP, Michalski J, Kaper JB, Edelman R, Levine MM. 1997. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD 111. Am J Trop Med Hyg 56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 231.Cohen MB, Giannella RA, Bean J, Taylor DN, Parker S, Hoeper A, Wowk S, Hawkins J, Kochi SK, Schiff G, Killeen KP. 2002. Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect Immun 70:1965–1970. doi: 10.1128/IAI.70.4.1965-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Garcia L, Jidy MD, Garcia H, Rodríguez BL, Fernández R, Año G, Cedré B, Valmaseda T, Suzarte E, Ramírez M, Pino Y, Campos J, Menéndez J, Valera R, González D, González I, Pérez O, Serrano T, Lastre M, Miralles F, Del Campo J, Maestre JL, Pérez JL, Talavera A, Pérez A, Marrero K, Ledón T, Fando R. 2005. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect Immun 73:3018–3024. doi: 10.1128/IAI.73.5.3018-3024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ansaruzzaman M, Bhuiyan NA, Safa A, Sultana M, McUamule A, Mondlane C, Wang X-Y, Deen JL, von Seidlein L, Clemens JD, Lucas M, Sack DA, Nair GB. 2007. Genetic diversity of El Tor strains of Vibrio cholerae O1 with hybrid traits isolated from Bangladesh and Mozambique. Int J Med Microbiol 297:443–449. doi: 10.1016/j.ijmm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 234.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis 151:236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 235.Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, Nazim M, LaRocque RC, Ryan ET, Calderwood SB, Qadri F, Harris JB. 2012. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol 19:842–848. doi: 10.1128/CVI.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque ASG, Ryan ET, Qadri F, Calderwood SB. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mayo-Smith LM, Simon JK, Chen WH, Haney D, Lock M, Lyon CE, Calderwood SB, Kirkpatrick BD, Cohen M, Levine MM, Gurwith M, Harris JB. 2017. The live attenuated cholera vaccine CVD 103-HgR primes responses to the toxin-coregulated pilus antigen TcpA in subjects challenged with wild-type Vibrio cholerae. Clin Vaccine Immunol 24:e00470-16. doi: 10.1128/CVI.00470-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chac D, Bhuiyan TR, Saha A, Alam MM, Salma U, Jahan N, Chowdhury F, Khan AI, Ryan ET, LaRocque R, Harris JB, Qadri F, Weil AA. 2021. Gut microbiota and development of Vibrio cholerae-specific long-term memory B cells in adults after whole-cell killed oral cholera vaccine. Infect Immun 89:e0021721. doi: 10.1128/IAI.00217-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Khan AI, Khan IA, Siddique SA, Rahman A, Islam MT, Bhuiya MAI, Saha NC, Biswas PK, Saha A, Chowdhury F, Qadri F. 2019. Feasibility, coverage and cost of oral cholera vaccination conducted by icddr,b using the existing National Immunization Service delivery mechanism in rural setting Keraniganj, Bangladesh. Hum Vaccin Immunother 15:1302–1309. doi: 10.1080/21645515.2018.1528833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Giersing B, Khalil I. 2020. WHO preferred product characteristics for vaccine against enterotoxigenic Escherichia coli. World Health Organization, Department of Immunizations, Vaccines and Biologicals, Geneva, Switzerland. https://www.who.int/publications/i/item/who-preferred-product-characteristics-for-vaccines-against-enterotoxigenic-escherichia-coli. [Google Scholar]
- 241.Svennerholm AM, Tobias J. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev Vaccines 7:795–804. doi: 10.1586/14760584.7.6.795. [DOI] [PubMed] [Google Scholar]
- 242.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev Vaccines 11:677–694. doi: 10.1586/erv.12.37. [DOI] [PubMed] [Google Scholar]
- 243.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 244.Lamberti LM, Bourgeois AL, Fischer Walker CL, Black RE, Sack D. 2014. Estimating diarrheal illness and deaths attributable to shigellae and enterotoxigenic Escherichia coli among older children, adolescents, and adults in South Asia and Africa. PLoS Negl Trop Dis 8:e2705. doi: 10.1371/journal.pntd.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 246.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, et al. 2012. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rao M, Abu-Elyazeed R, Savarino SJ, Naficy AB, Wierzba TF, Abdel-Messih I, Shaheen H, Frenck RW, Svennerholm A-M, Clemens JD. 2003. High disease burden of diarrhea due to enterotoxigenic Escherichia coli among rural Egyptian infants and young children. J Clin Microbiol 41:4862–4864. doi: 10.1128/JCM.41.10.4862-4864.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm A-M. 2007. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun 75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mansour A, Shaheen H, Amine M, Hassan K, Sanders JW, Riddle MS, Armstrong AW, Svennerholm AM, Sebeny PJ, Klena JD, Young SYN, Frenck RW. 2014. Diarrhea burden due to natural infection with enterotoxigenic Escherichia coli in a birth cohort in a rural Egyptian community. J Clin Microbiol 52:2595–2603. doi: 10.1128/JCM.00215-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kotloff KL, Nasrin D, Blackwelder WC, Wu Y, Farag T, Panchalingham S, Sow SO, Sur D, Zaidi AKM, Faruque ASG, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ahmed S, Qureshi S, Quadri F, Hossain A, Das SK, Antonio M, Hossain MJ, Mandomando I, Acácio S, Biswas K, Tennant SM, Verweij JJ, Sommerfelt H, Nataro JP, Robins-Browne RM, Levine MM. 2019. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS. ). Lancet Glob Health 7:e568–e584. doi: 10.1016/S2214-109X(19)30076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, MAL-ED Network Investigators . 2018. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from MAL-ED cohort study. Lancet Glob Health 6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e574. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lee G, Paredes Olortegui M, Yori PP, Black RE, Caulfield L, Banda Chavez C, Hall E, Pan WK, Meza R, Kosek M. 2014. Effects of Shigella-, Campylobacter-, and ETEC-associated diarrhoea on childhood growth. Pediatr Infect Dis J 33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 254.Platts-Mills JA, Taniuchi M, Uddin MJ, Sobuz SU, Mahfuz M, Gaffar SA, Mondal D, Hossain MI, Islam MM, Ahmed AS, Petri WA, Haque R, Houpt ER, Ahmed T. 2017. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr 105:1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Anderson JD, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, Rheingans R. 2019. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhea infections in 79 low-income and lower middle-income countries: a modeling analysis. Lancet Glob Health 7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bagamian KH, Anderson JD, Muhib F, Cumming O, Laytner LA, Wierzba TF, Rheingans R. 2020. Heterogeneity in enterotoxigenic Escherichia coli and Shigella infections in children under 5 years of age from 11 African countries: a subnational approach quantifying risk, mortality, morbidity and stunting. Lancet Glob Health 8:e101–e112. doi: 10.1016/S2214-109X(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.GBD 2016 Diarrhoeal Disease Collaborators. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, Kotloff KL, Levine MM, Luby SP, MacLennan CA, Pan WK, Pavlinac PB, Platts-Mills JA, Qadri F, Riddle MS, Ryan ET, Shoultz DA, Steele AD, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC, Jr.. 2018. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Troeger C, Colombara DV, Rao PC, Khalil IA, Brown A, Brewer TG, Guerrant RL, Houpt ER, Kotloff KL, Misra K, Petri WA, Jr, Platts-Mills J, Riddle MS, Swartz SJ, Forouzanfar MH, Reiner RC, Jr, Hay SI, Mokdad AH. 2018. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health 6:e255–e69. doi: 10.1016/S2214-109X(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Butkeviciute E, Prudden HJ, Jit M, Smith PG, Kang G, Riddle MS, Lopman BA, Pitzer VE, Lanata CF, Platts-Mills JA, Breiman RF, Giersing BK, Hasso-Agopsowicz M. 2021. Global diarrhoea-associated mortality estimates and models in children: recommendations for dataset and study selection. Vaccine 39:4391–4398. doi: 10.1016/j.vaccine.2021.05.086. [DOI] [PubMed] [Google Scholar]
- 261.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. 2013. The impoverished gut: a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.The Boston Consulting Group. 2018. Vaccines to tackle drug resistant infections: an evaluation of R&D opportunities. BCG, Boston, MA. https://vaccinesforamr.org/wp-content/uploads/2018/09/Vaccines_for_AMR.pdf. [Google Scholar]
- 263.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Qadri F, Svennerholm AM, Faruque AS, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque AS, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Clemens J, Savarino S, Abu-Elyazeed R, Safwat M, Rao M, Wierzba T, Svennerholm AM, Holmgren J, Frenck R, Park E, Naficy A. 2004. Development of pathogenicity driven definitions of outcomes for a field trial of a killed oral vaccine against enterotoxigenic Escherichia coli in Egypt: application of an evidence-based method. J Infect Dis 189:2299–2307. doi: 10.1086/386288. [DOI] [PubMed] [Google Scholar]
- 267.McConnell M, Hibberd M, Penny M, Scotland S, Cheasty T, Rowe B. 1991. Surveys of human enterotoxigenic Escherichia coli from three different geographical areas for possible colonization factors. Epidemiol Infect 106:477–484. doi: 10.1017/s0950268800067522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shaheen HI, Khalil SB, Rao MR, Abu Elyazeed R, Wierzba TF, Peruski LF, Jr, Putnam S, Navarro A, Morsy BZ, Cravioto A, Clemens JD, Svennerholm AM, Savarino SJ. 2004. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J Clin Microbiol 42:5588–5595. doi: 10.1128/JCM.42.12.5588-5595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Viboud GI, Jouve MJ, Binsztein N, Vergara M, Rivas M, Quiroga M, Svennerholm AM. 1999. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J Clin Microbiol 37:2829–2833. doi: 10.1128/JCM.37.9.2829-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Steinsland H, Valentiner-Branth P, Perch M, Dias F, Fischer TK, Aaby P, Mølbak K, Sommerfelt H. 2002. Enterotoxigenic Escherichia coli infections and diarrhea in a cohort of young children in Guinea-Bissau. J Infect Dis 186:1740–1747. doi: 10.1086/345817. [DOI] [PubMed] [Google Scholar]
- 271.McKenzie R, Porter CK, Cantrell JA, Denearing B, O’Dowd A, Grahek SL, Sincock SA, Woods C, Sebeny P, Sack DA, Tribble DR, Bourgeois AL, Savarino SJ. 2011. Volunteer challenge with enterotoxigenic Escherichia coli that express intestinal colonization fimbriae CS17 and CS19. J Infect Dis 204:60–64. doi: 10.1093/infdis/jir220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bolick D, Medeiros PHQS, Ledwaba SE, Lima AAM, Nataro JP, Barry EM, Guerrant RL. 2018. Critical role of zinc in a new murine model of enterotoxigenic Escherichia coli diarrhea. Infect Immun 86:e001183-18. doi: 10.1128/IAI.00183-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kreisberg RB, Harper J, Strauman MC, Marohn M, Clements JD, Nataro JP. 2011. Induction of increased permeability of polarized enterocyte monolayers by enterotoxigenic Escherichia coli heat-labile enterotoxin. Am J Trop Med Hyg 84:451–455. doi: 10.4269/ajtmh.2011.10-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Crofts AA, Giovanetti SM, Rubin EJ, Poly FM, Gutiérrez RL, Talaat KR, Porter CK, Riddle MS, DeNearing B, Brubaker J, Maciel M, Alcala AN, Chakraborty S, Prouty MG, Savarino SJ, Davies BW, Trent MS. 2018. Enterotoxigenic Escherichia coli virulence gene regulation in human infections. Proc Natl Acad Sci USA 115:E8968–E8976. doi: 10.1073/pnas.1808982115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Khalil I, Walker R, Porter CK, Muhib F, Chilengi R, Cravioto A, Guerrant R, Svennerholm AM, Qadri F, Baqar S, Kosek M, Kang G, Lanata C, Armah G, Wierzba T, Hasso-Agopsowicz M, Giersing B, Louis Bourgeois A. 2021. Enterotoxigenic Escherichia coli (ETEC) vaccines: priority activities to enable product development, licensure, and global access. Vaccine 39:4266–4277. doi: 10.1016/j.vaccine.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Brubaker J, Zhang X, Bourgeois AL, Harro C, Sack DA, Chakraborty S. 2021. Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model. Gut Microbes 13:1–13. doi: 10.1080/19490976.2021.1891852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wolf MK. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev 10:569–584. doi: 10.1128/CMR.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 279.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 280.Levine MM, Barry EM, Chen WH. 2019. A roadmap for enterotoxigenic Escherichia coli vaccine development based on volunteer challenge studies. Hum Vaccin Immunother 15:1357–1378. doi: 10.1080/21645515.2019.1578922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bourgeois AL, Wierzba TF, Walker RI. 2016. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 282.World Health Organization. 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec 81:97–104. [PubMed] [Google Scholar]
- 283.Qadri F, Ali M, Lynch J, Chowdhury F, Khan AI, Wierzba TF, Excler J-L, Saha A, Islam MT, Begum YA, Bhuiyan TR, Khanam F, Chowdhury MI, Khan IA, Kabir A, Riaz BK, Akter A, Khan A, Asaduzzaman M, Kim DR, Siddik AU, Saha NC, Cravioto A, Singh AP, Clemens JD. 2018. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis 18:666–674. doi: 10.1016/S1473-3099(18)30108-7. [DOI] [PubMed] [Google Scholar]
- 284.Harro C, Louis Bourgeois A, Sack D, Walker R, DeNearing B, Brubaker J, Maier N, Fix A, Dally L, Chakraborty S, Clements JD, Saunders I, Darsley MJ. 2019. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 37:1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sack DA, Shimko J, Torres O, Bourgeois AL, Francia DS, Gustafsson B, Kärnell A, Nyquist I, Svennerholm AM. 2007. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 25:4392–4400. doi: 10.1016/j.vaccine.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 286.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, Wiklund G, Kaim J, Löfstrand M, Carlin N, Bourgeois AL, Maier N, Fix A, Wierzba T, Walker RI, Svennerholm A-M. 2020. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis 20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Maciel M, Trop S, Kim A, Ward E, Villar Z, Lee TK, Jaep K, Porter C, Poole S, Prouty MG. 2019. Serological and α4β7 antibody-secreting cell responses after intramuscular immunization with CssBA, a CS6-subunit based enterotoxigenic E. coli vaccine candidate, and LT(R192G/L211A) as adjuvant. 10th International Conference on Vaccines for Enteric Diseases, 16–18 October 2019. 10th International Conference on Vaccines for Enteric Diseases, Lausanne, Switzerland. [Google Scholar]
- 288.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm AM. 2014. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine 32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 289.Fleckenstein J, Sheikh A, Qadri F. 2014. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert Rev Vaccines 13:631–639. doi: 10.1586/14760584.2014.905745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Turner AK, Stephens JC, Beavis JC, Greenwood J, Gewert C, Randall R, Freeman D, Darsley MJ. 2011. Generation and characterization of a live attenuated enterotoxigenic Escherichia coli combination vaccine expressing six colonization factors and heat-labile toxin subunit B. Clin Vaccine Immunol 18:2128–2135. doi: 10.1128/CVI.05345-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. 2012. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 19:1921–1931. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Scandinavian Biopharma. 2020. Promising preliminary findings from a phase 2b study of the oral vaccine ETVAX against travelers’ diarrhea in Finnish travelers to Benin, West Africa. Scandinavian Biopharma, Solna, Sweden. (Press release.) http://www.mynewsdesk.com/se/scandinavian-biopharma/pressreleases/promising-preliminary-findings-from-a-phase-2b-study-of-the-oral-vaccine-etvax-r-against-travelers-diarrhea-in-finnish-travelers-to-benin-west-africa-3002135. [Google Scholar]
- 293.Pan-African Clinical Trials Registry Trial. 2020. PACTR201905764389804: a phase 1 age descending placebo controlled clinical trial to examine the safety, tolerability, and immunogenicity of an oral inactivated ETEC vaccine (ETVAX) in healthy adults and children in Zambia. https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=7094.
- 294.Barry E, Cassels F, Riddle M, Walker R, Wierzba T. 2019. Vaccines against Shigella and enterotoxigenic Escherichia coli: a summary of the 2018 VASE conference. Vaccine 7:4768–4774. doi: 10.1016/j.vaccine.2019.02.070. [DOI] [PubMed] [Google Scholar]
- 295.Chakraborty S, Randall A, Vickers TJ, Molina D, Harro CD, DeNearing B, Brubaker J, Sack DA, Bourgeois AL, Felgner PL, Liang X, Mani S, Wenzel H, Townsend RR, Gilmore PE, Darsley MJ, Rasko DA, Fleckenstein JM. 2018. Human experimental challenge with enterotoxigenic Escherichia coli elicits immune responses to canonical and novel antigens relevant to vaccine development. J Infect Dis 218:1436–1446. doi: 10.1093/infdis/jiy312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lee T, Gutiérrez RL, Maciel M, Poole S, Testa KJ, Trop S, Duplessis C, Lane A, Riddle MS, Hamer M, Alcala A, Prouty M, Maier N, Erdem R, Louis Bourgeois A, Porter CK. 2021. Safety and immunogenicity of intramuscularly administered CS6 subunit vaccine with a modified heat-labile enterotoxin from enterotoxigenic Escherichia coli. Vaccine 39:5548–5556. doi: 10.1016/j.vaccine.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sakkestad ST, Steinsland H, Skrede S, Kleppa E, Lillebø K, Sævik M, Søyland H, Rykkje Heien A, Gjerde Tellevik M, Barry EM, Sommerfelt H, Hanevik K. 2019. Experimental infection of human volunteers with a heat-stable enterotoxin-producing enterotoxigenic Escherichia coli strain TW11681. Pathogens 22:84. doi: 10.3390/pathogens8020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chakraborty S, Harro C, DeNearing B, Brubaker J, Connor S, Maier N, Dally L, Flores J, Bourgeois AL, Walker R, Sack DA. 2018. Impact of lower challenge doses of enterotoxigenic Escherichia coli on clinical outcome, intestinal colonization and immune responses in adult volunteers. PLoS Negl Trop Dis 12:e0006442. doi: 10.1371/journal.pntd.0006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.McArthur MA, Chen WH, Magder L, Levine MM, Sztein MB. 2017. Impact of CD4 T cell responses on clinical outcome following oral administration of wild-type enterotoxigenic Escherichia coli in humans. PLoS Negl Trop Dis 11:e0005291. doi: 10.1371/journal.pntd.0005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Porter CK, Riddle MS, Tribble DR, Louis Bougeois A, McKenzie R, Isidean SD, Sebeny P, Savarino SJ. 2011. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29:5869–5885. doi: 10.1016/j.vaccine.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 301.Harro C, Chakraborty S, Feller A, DeNearing B, Cage A, Ram M, Lundgren A, Svennerholm AM, Bourgeois AL, Walker RI, Sack DA. 2011. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin Vaccine Immunol 18:1719–1727. doi: 10.1128/CVI.05194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.McKenzie R, Porter CK, Cantrell JA, Denearing B, O’Dowd A, Grahek SL, Sincock SA, Woods C, Sebeny P, Sack DA, Tribble DR, Bourgeois AL, Savarino SJ. 2011. Volunteer challenge with enterotoxigenic Escherichia coli that express intestinal colonization factor fimbriae CS17 and CS19. J Infect Dis 204:60–64. doi: 10.1093/infdis/jir220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Skrede S, Steinsland H, Sommerfelt H, Aase A, Brandtzaeg P, Langeland N, Cox RJ, Saevik M, Wallevik M, Skutlaberg DH, Tellevik MG, Sack DA, Nataro JP, Guttormsen AB. 2014. Experimental infection of healthy volunteers with enterotoxigenic Escherichia coli wild-type strain TW10598 in a hospital ward. BMC Infect Dis 14:482. doi: 10.1186/1471-2334-14-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Porter CK, Riddle MS, Alcala AN, Sack DA, Harro C, Chakraborty S, Gutierrez RL, Savarino SJ, Darsley M, McKenzie R, DeNearing B, Steinsland H, Tribble DR, Bourgeois AL. 2016. An evidenced-based scale of disease severity following human challenge with enterotoxigenic Escherichia coli. PLoS One 11:e149358. doi: 10.1371/journal.pone.0149358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sakkestad ST, Steinsland H, Skrede S, Lillebø K, Skutlaberg DH, Guttormsen AB, Zavialov A, Paavilainen S, Søyland H, Sævik M, Heien AR, Tellevik MG, Barry E, Langeland N, Sommerfelt H, Hanevik K. 2019. A new human challenge model for testing heat-stable toxin-based vaccine candidates for enterotoxigenic Escherichia coli diarrhea-dose optimization, clinical outcomes and CD4 T cells. PLoS Negl Trop Dis 13:e0007823. doi: 10.1371/journal.pntd.0007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Govasli ML, Diaz Y, Zegeye ED, Darbakk C, Taxt AM, Puntervoll P. 2018. Purification and characterization of native and vaccine candidate mutant enterotoxigenic Escherichia coli heat-stable toxins. Toxins (Basel) 10:274. doi: 10.3390/toxins10070274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang WE, Suchindran S, Nicholson BP, McClain MT, Burke T, Ginsburg GS, Harro CD, Chakraborty S, Sack DA, Woods CW, Tsalik EL. 2016. Transcriptomic analysis of the host response and innate resilience to enterotoxigenic Escherichia coli infection in humans. J Infect Dis 213:1495–1504. doi: 10.1093/infdis/jiv593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Pop M, Paulson JN, Chakraborty S, Astrovskaya I, Lindsay BR, Li S, Bravo HC, Harro C, Parkhill J, Walker AW, Walker RI, Sack DA, Stine OC. 2016. Individual-specific changes in the human gut microbiota after challenge with enterotoxigenic Escherichia coli and subsequent ciprofloxacin treatment. BMC Genomics 17:440. doi: 10.1186/s12864-016-2777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lindsay BR, Chakraborty S, Harro C, Li S, Nataro JP, Sommerfelt H, Sack DA, Colin Stine O. 2014. Quantitative PCR and culture evaluation for enterotoxigenic Escherichia coli (ETEC) associated diarrhea in volunteers. FEMS Microbiol Lett 352:25–31. doi: 10.1111/1574-6968.12362. [DOI] [PubMed] [Google Scholar]
- 310.Kuhlmann FM, Martin J, Hazen TH, Vickers TJ, Pashos M, Okhuysen PC, Gómez-Duarte OG, Cebelinski E, Boxrud D, Del Canto F, Vidal R, Qadri F, Mitreva M, Rasko DA, Fleckenstein JM. 2019. Conservation and global distribution of non-canonical antigens in enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 13:e0007825. doi: 10.1371/journal.pntd.0007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chakraborty S, Randall A, Vickers TJ, Molina D, Harro CD, DeNearing B, Brubaker J, Sack DA, Bourgeois AL, Felgner PL, Liang X, Mani S, Wenzel H, Townsend RR, Gilmore PE, Darsley MJ, Rasko DA, Fleckenstein JM. 2019. Interrogation of a live-attenuated enterotoxigenic Escherichia coli vaccine highlights features unique to wild-type infections. NPJ Vaccines 4:37. doi: 10.1038/s41541-019-0131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne M, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 313.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque ASG, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhea in children: a reanalysis of the GEMS case control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. 2017. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 35:6783–6789. doi: 10.1016/j.vaccine.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 315.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, Kotloff KL, Levine MM, Luby SP, MacLennan CA, Pan WK, Pavlinac PB, Platts-Mills JA, Qadri F, Riddle MS, Ryan ET, Shoultz DA, Steele AD, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC, Jr.. 2018. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE; Child Health Epidemiology Reference Group of the World Health Organization and UNICEF . 2013. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lamberti LM, Bourgeois AL, Fischer Walker CL, Black RE, Sack D. 2014. Estimating diarrheal illness and deaths attributable to Shigellae and enterotoxigenic Escherichia coli among older children, adolescents, and adults in South Asia and Africa. PLoS Negl Trop Dis 8:e2705. doi: 10.1371/journal.pntd.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Anderson JD, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, Rheingans R. 2019. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health 7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Quresh S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhea in developing countries: a multisite birth cohort study (MAL-ED. ). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tickell KD, Brander RL, Atlas HE, Pernica JM, Walson JL, Pavlinac PB. 2017. Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis. Lancet Glob Health 5:e1235–e1248. doi: 10.1016/S2214-109X(17)30392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Hosangadi D, Smith PG, Giersing BK. 2019. Considerations for using ETEC and Shigella disease burden estimates to guide vaccine development strategy. Vaccine 37:7372–7380. doi: 10.1016/j.vaccine.2017.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. 2013. The impoverished gut: a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lindsay B, Saha D, Sanogo D, Das SK, Omore R, Farag TH, Nasrin D, Li S, Panchalingam S, Levine MM, Kotloff K, Nataro JP, Magder L, Hungerford L, Faruque ASG, Oundo J, Hossain MA, Adeyemi M, Stine OC. 2015. Association between Shigella infection and diarrhea varies based on location and age of children. Am J Trop Med Hyg 93:918–924. doi: 10.4269/ajtmh.14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Anderson JD, Bagamian KH, Muhib F, Baral R, Laytner LA, Amaya M, Wierzba T, Rheingans R. 2019. Potential impact and cost-effectiveness of future ETEC and Shigella vaccines in 79 low- and lower middle-income countries. Vaccine X 1:100024. doi: 10.1016/j.jvacx.2019.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R, the Maternal and Child Nutrition Study Group . 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 326.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, MAL-ED Network Investigators . 2018. Use of quantitative molecular diagnostics methods to investigate the effect of enteropathogens infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lee G, Paredes Olortegui M, Yori PP, Black RE, Caulfield L, Chavez CB, Hall E, Pan WK, Meza R, Kosek M. 2014. Effects of Shigella-, Campylobacter-, and ETEC-associated diarrhoea on childhood growth. Pediatr Infect Dis J 33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 328.Platts-Mills JA, Taniuchi M, Uddin MJ, Sobuz SU, Mahfuz M, Gaffar SA, Mondal D, Hossain MI, Islam MM, Ahmed AS, Petri WA, Haque R, Houpt ER, Ahmed T. 2017. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr 105:1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhea in developing countries: a multisite birth cohort study (MAL-ED. ). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.World Health Organization. 2017. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12. [Google Scholar]
- 331.Porter CK, Kowalcyk B, Riddle MS. 2016. Chronic health consequences of acute enteric infections in the developed world. Am J Gastroenterol Suppl 3:12–23. doi: 10.1038/ajgsup.2016.10. [DOI] [Google Scholar]
- 332.Mani S, Wierzba T, Walker RI. 2016. Status of vaccine research and development for Shigella. Vaccine 34:2887–2894. doi: 10.1016/j.vaccine.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 333.Riddle MS, Chen WH, Kirkwood CD, MacLennan CA. 2018. Update on vaccines for enteric pathogens. Clin Microbiol Infect 24:1039–1045. doi: 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 334.Nataro JP, Guerrant RL. 2017. Chronic consequences on human health induced by microbial pathogens: growth faltering among children in developing countries. Vaccine 35:6807–6812. doi: 10.1016/j.vaccine.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 335.Walker RI, Wierzba TF, Mani S, Bourgeois AL. 2017. Vaccines against Shigella and enterotoxigenic Escherichia coli: a summary of the 2016 VASE Conference. Vaccine 35:6775–6782. doi: 10.1016/j.vaccine.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 336.Chang Z, Lu S, Chen L, Jin Q, Yang J. 2012. Causative species and serotypes of shigellosis in mainland China: systematic review and meta-analysis. 7:e52515. doi: 10.1371/journal.pone.0052515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 338.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, Qureshi S, Ramamurthy T, Tamboura B, Adegbola RA, Hossain MJ, Saha D, Sen S, Faruque ASG, Alonso PL, Breiman RF, Zaidi AKM, Sur D, Sow SO, Berkeley LY, O’Reilly CE, Mintz ED, Biswas K, Cohen D, Farag TH, Nasrin D, Wu Y, Blackwelder WC, Kotloff KL, Nataro JP, Levine MM. 2014. Shigella isolates from the Global Enteric Multicenter Study inform vaccine development. Clin Infect Dis 59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Philpott DJ, Edgeworth JD, Sansonetti PJ. 2000. The pathogenesis of Shigella flexneri infection: lessons from in vitro and in vivo studies. Philos Trans R Soc Lond B Biol Sci 355:575–586. doi: 10.1098/rstb.2000.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Schroeder GN, Hilbi H. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev 21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.MacLennan CA, Aguilar AO, Steele AD. 2019. Consensus report on Shigella controlled human infection model: introduction and overview. Clin Infect Dis 69:S577–S579. doi: 10.1093/cid/ciz886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Talaat KR, Bourgeois AL, Frenck RW, Chen WH, MacLennan CA, Riddle MS, Suvarnapunya AE, Brubaker JL, Kotloff KL, Porter CK. 2019. Consensus report on Shigella controlled human infection model: conduct of studies. Clin Infect Dis 69:S580–S590. doi: 10.1093/cid/ciz892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kaminski RW, Pasetti MF, Aguilar AO, Clarkson KA, Rijpkema S, Bourgeois AL, Cohen D, Feavers I, MacLennan CA. 2019. Consensus report on Shigella controlled human infection model: immunological assays. Clin Infect Dis 69:S596–S601. doi: 10.1093/cid/ciz909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ndungo E, Randall A, Hazen TH, Kania DA, Trappl-Kimmons K, Liang X, Barry EM, Kotloff KL, Chakraborty S, Mani S, Rasko DA, Pasetti MF. 2018. A novel Shigella proteome microarray discriminates targets of human antibody reactivity following oral vaccination and experimental challenge. mSphere 3:e00260-18. doi: 10.1128/mSphere.00260-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Venkatesan MM, Ranallo RT. 2006. Live-attenuated Shigella vaccines. Expert Rev Vaccines 5:669–686. doi: 10.1586/14760584.5.5.669. [DOI] [PubMed] [Google Scholar]
- 346.Alaimo C. 2019. Development of Shigella multivalent bioconjugate vaccine: toward a phase 1/2 study in Kenyan infants. Presentation at the 10th International Conference on Vaccines for Enteric Diseases, 16–18 October 2019. 10th International Conference on Vaccines for Enteric Diseases, Lausanne, Switzerland. [Google Scholar]
- 347.Martin LB, Ndiaye AGW, Podda A, Saul A. 2019. Shigella vaccine based on GMMA-technology: inducing high level persistent and boostable antibody responses in human populations. Presentation at the 10th International Conference on Vaccines for Enteric Diseases, 16–18 October 2019. 10th International Conference on Vaccines for Enteric Diseases, Lausanne, Switzerland. [Google Scholar]
- 348.Ravenscroft N, Braun M, Schneider J, Dreyer AM, Wetter M, Haeuptle MA, Kemmler S, Steffen M, Sirena D, Herwig S, Carranza P, Jones C, Pollard AJ, Wacker M, Kowarik M. 2019. Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 29:669–680. doi: 10.1093/glycob/cwz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Barel LA, Mulard LA. 2019. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: from concept to efficacy in human. Hum Vaccin Immunother 15:1338–1356. doi: 10.1080/21645515.2019.1606972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kim MJ, Moon YH, Kim H, Rho S, Shin YK, Song M, Walker R, Czerkinsky C, Kim DW, Kim JO. 2018. Cross-protective Shigella whole-cell vaccine with a truncated O-polysaccharide chain. Front Microbiol 9:2609. doi: 10.3389/fmicb.2018.02609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Clarkson KA, Gutierrez R, Turbyfill KR, Detizio KR, Vortherms A. 2019. GMP manufacture, characterization and clinical evaluation of Shigella flexneri 2a detoxified artificial Invaplex. Presentation at the 10th International Conference on Vaccines for Enteric Diseases, 16–18 October 2019. 10th International Conference on Vaccines for Enteric Diseases, Lausanne, Switzerland. [Google Scholar]
- 352.Clarkson KA, Talaat KR, Alaimo C, Martin P, Bourgeois AL, Dreyer A, Porter CK, Chakraborty S, Brubaker J, Elwood D, Frölich R, DeNearing B, Weerts HP, Feijoo B, Halpern J, Sack D, Riddle MS, Fonck VG, Kaminski RW. 2021. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine 66:103308. doi: 10.1016/j.ebiom.2021.103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cohen D, Green MS, Block C, Slepon R, Ofek I. 1991. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol 29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Clarkson KA, Frenck RW, Jr, Dickey M, Suvarnapunya AE, Chandrasekaran L, Weerts HP, Heaney CD, McNeal M, Detizio K, Parker S, Hoeper A, Bourgeois AL, Porter CK, Venkatesan MM, Kaminski RW. 2020. Immune response characterization after controlled infection with lyophilized Shigella sonnei 53G. mSphere 5:e00988-19. doi: 10.1128/mSphere.00988-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Shaughnessy HJ, Olsson RC. 1946. Experimental human bacillary dysentery; polyvalent dysentery vaccine in its prevention. JAMA 132:362–368. doi: 10.1001/jama.1946.02870420002002. [DOI] [PubMed] [Google Scholar]
- 356.Porter CK, Thura N, Ranallo RT, Riddle MS. 2013. The Shigella human challenge model. Epidemiol Infect 141:223–232. doi: 10.1017/S0950268812001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Black RE, Levine MM, Clements ML, Young CR, Svennerholm AM, Holmgren J. 1987. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun 55:1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kotloff KL, Nataro JP, Losonsky GA, Wasserman SS, Hale TL, Taylor DN, Sadoff JC, Levine MM. 1995. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine 13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 359.DuPont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, Gangarosa EJ. 1972. Immunity in shigellosis II protection induced by oral live vaccine or primary infection. J Infect Dis 125:12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- 360.Herrington DA, Van de Verg L, Formal SB, Hale TL, Tall BD, Cryz SJ, Tramont EC, Levine MM. 1990. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella Typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 8:353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- 361.Bodhidatta L, Pitisuttithum P, Chamnanchanant S, Chang KT, Islam D, Bussaratid V, Venkatesan MM, Hale TL, Mason CJ. 2012. Establishment of a Shigella sonnei human challenge model in Thailand. Vaccine 30:7040–7045. doi: 10.1016/j.vaccine.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gaston JS, Inman RD, Ryan ET, Venkatesan MM, Barry EM, Hale TL, Bourgeois AL, Walker RI. 2009. Vaccination of children in low-resource countries against Shigella is unlikely to present an undue risk of reactive arthritis. Vaccine 27:5432–5434. doi: 10.1016/j.vaccine.2009.06.107. [DOI] [PubMed] [Google Scholar]
- 363.Kotloff KL, Simon JK, Pasetti MF, Sztein MB, Wooden SL, Livio S, Nataro JP, Blackwelder WC, Barry EM, Picking W, Levine MM. 2007. Safety and immunogenicity of CVD 1208S, a live, oral DeltaguaBA deltasen deltaset Shigella flexneri 2a vaccine grown on animal-free media. Hum Vaccin 3:268–275. doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- 364.Passwell JH, Ashkenazi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, Robbins JB, Schneerson R, The Israeli Shigella Study Group . 2010. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine 28:2231–2235. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Frenck RW, Jr, Conti V, Ferruzzi P, Ndiaye AGW, Parker S, McNeal MM, Dickey M, Granada JP, Cilio GL, De Ryck I, Necchi F, Suvarnapunya AE, Rossi O, Acquaviva A, Chandrasekaran L, Clarkson KA, Auerbach J, Marchetti E, Kaminski RW, Micoli F, Rappuoli R, Saul A, Martin LB, Podda A. 2021. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine 39:101076. doi: 10.1016/j.eclinm.2021.101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Turbyfill KR, Clarkson KA, Vortherms AR, Oaks EV, Kaminski RW. 2018. Assembly, biochemical characterization, immunogenicity, adjuvanticity, and efficacy of Shigella artificial Invaplex. mSphere 3:e00583-17. doi: 10.1128/mSphere.00583-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 368.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. 2002. Human campylobacteriosis in developing countries. Emerg Infect Dis 8:237–244. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, Seidman JC, McCormick BJ, Shrestha S, Samie A, Mahfuz M, Qureshi S, Hotwani A, Babji S, Trigoso DR, Lima AA, Bodhidatta L, Bessong P, Ahmed T, Shakoor S, Kang G, Kosek M, Guerrant RL, Lang D, Gottlieb M, Houpt ER, Platts-Mills JA, Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) Network Investigators . 2016. Epidemiology and impact of Campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis 63:1171–1179. doi: 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lee G, Pan W, Yori PP, Olortegui MP, Tilley D, Gregory M, Oberhelman R, Burga R, Chavez CB, Kosek M. 2013. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis 7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Riddle MS, Gutierrez RL, Verdu EF, Porter CK. 2012. The chronic gastrointestinal consequences associated with Campylobacter. Curr Gastroenterol Rep 14:395–405. doi: 10.1007/s11894-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 372.Nyati KK, Nyati R. 2013. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: an update. Biomed Res Int 2013:852195. doi: 10.1155/2013/852195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Poropatich KO, Walker CL, Black RE. 2010. Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: a systematic review. J Health Popul Nutr 28:545–552. doi: 10.3329/jhpn.v28i6.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wierzba TF, Abdel-Messih IA, Gharib B, Baqar S, Hendaui A, Khalil I, Omar TA, Khayat HE, Putnam SD, Sanders JW, Ng L-K, Price LJ, Scott DA, Frenck RR. 2008. Campylobacter infection as a trigger for Guillain-Barré syndrome in Egypt. 3:e3674. doi: 10.1371/journal.pone.0003674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. 2012. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol 2:7. doi: 10.3389/fcimb.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Penner JL, Hennessy JN. 1980. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol 12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Prendergast MM, Tribble DR, Baqar S, Scott DA, Ferris JA, Walker RI, Moran AP. 2004. In vivo phase variation and serologic response to lipooligosaccharide of Campylobacter jejuni in experimental human infection. Infect Immun 72:916–922. doi: 10.1128/IAI.72.2.916-922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Pike BL, Guerry P, Poly F. 2013. Global distribution of Penner serotypes: a systematic review. 8:e67375. doi: 10.1371/journal.pone.0067375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lee LH, Burg E, III, Baqar S, Bourgeois AL, Burr DH, Ewing CP, Trust TJ, Guerry P. 1999. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect Immun 67:5799–5805. doi: 10.1128/IAI.67.11.5799-5805.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Baqar S, Applebee LA, Gilliland TC, Jr, Lee LH, Porter CK, Guerry P. 2008. Immunogenicity and protective efficacy of recombinant Campylobacter jejuni flagellum-secreted proteins in mice. Infect Immun 76:3170–3175. doi: 10.1128/IAI.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Albert MJ, Haridas S, Steer D, Dhaunsi GS, Smith AI, Adler B. 2007. Identification of a Campylobacter jejuni protein that cross-reacts with cholera toxin. Infect Immun 75:3070–3073. doi: 10.1128/IAI.00139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J Infect Dis 157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 383.Tribble DR, Baqar S, Carmolli MP, Porter C, Pierce KK, Sadigh K, Guerry P, Larsson CJ, Rockabrand D, Ventone CH, Poly F, Lyon CE, Dakdouk S, Fingar A, Gilliland T, Jr, Daunais P, Jones E, Rymarchyk S, Huston C, Darsley M, Kirkpatrick BD. 2009. Campylobacter jejuni strain CG8421: a refined model for the study of campylobacteriosis and evaluation of Campylobacter vaccines in human subjects. Clin Infect Dis 49:1512–1519. doi: 10.1086/644622. [DOI] [PubMed] [Google Scholar]
- 384.Poly F, Read TD, Chen YH, Monteiro MA, Serichantalergs O, Pootong P, Bodhidatta L, Mason CJ, Rockabrand D, Baqar S, Porter CK, Tribble D, Darsley M, Guerry P. 2008. Characterization of two Campylobacter jejuni strains for use in volunteer experimental-infection studies. Infect Immun 76:5655–5667. doi: 10.1128/IAI.00780-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kirkpatrick BD, Lyon CE, Porter CK, Maue AC, Guerry P, Pierce KK, Carmolli MP, Riddle MS, Larsson CJ, Hawk D, Dill EA, Fingar A, Poly F, Fimlaid KA, Hoq F, Tribble DR. 2013. Lack of homologous protection against Campylobacter jejuni CG8421 in a human challenge model. Clin Infect Dis 57:1106–1113. doi: 10.1093/cid/cit454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Fimlaid KA, Lindow JC, Tribble DR, Bunn JY, Maue AC, Kirkpatrick BD. 2014. Peripheral CD4 T cell cytokine responses following human challenge and re-challenge with Campylobacter jejuni. 9:e112513. doi: 10.1371/journal.pone.0112513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Rao MR, Naficy AB, Savarino SJ, Abu-Elyazeed R, Wierzba TF, Peruski LF, Abdel-Messih I, Frenck R, Clemens JD. 2001. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol 154:166–173. doi: 10.1093/aje/154.2.166. [DOI] [PubMed] [Google Scholar]
- 388.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P, Burg EII, Moran AP, Applebee L, Bourgeois AL. 2010. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun 78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Giallourou N, Medlock GL, Bolick DT, Medeiros PH, Ledwaba SE, Kolling GL, Tung K, Guerry P, Swann JR, Guerrant RL. 2018. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog 3:e1007083. doi: 10.1371/journal.ppat.1007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Harro C, Bourgeois AL, Sack D, Walker R, DeNearing B, Brubaker J, Maier N, Fix A, Dally L, Chakraborty S, Clements JD, Saunders I, Darsley MJ. 2019. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 37:1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Crofts AA, Poly FM, Ewing CP, Kuroiwa JM, Rimmer JE, Harro C, Sack D, Talaat KR, Porter CK, Gutierrez RL, DeNearing B, Brubaker J, Laird RM, Maue AC, Jaep K, Alcala A, Tribble DR, Riddle MS, Ramakrishnan A, McCoy AJ, Davies BW, Guerry P, Trent MS. 2018. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol 3:494–502. doi: 10.1038/s41564-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Lindow JC, Poly F, Tribble DR, Guerry P, Carmolli MP, Baqar S, Porter CK, Pierce KK, Darsley MJ, Sadigh KS, Dill A, Kirkpatrick BD, the Campylobacter Study Team . 2010. Caught in the act: in vivo development of macrolide resistance to Campylobacter jejuni infection. J Clin Microbiol 48:3012–3015. doi: 10.1128/JCM.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Rimmer JE, Harro C, Sack DA, Talaat KR, Gutierrez RL, DeNearing B, Brubaker J, Laird RM, Poly F, Maue AC, Jaep K, Alcala A, Mochalova Y, Gariepy CL, Chakraborty S, Guerry P, Tribble DR, Porter CK, Riddle MS. 2018. Rifaximin fails to prevent campylobacteriosis in the human challenge model: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 66:1435–1441. doi: 10.1093/cid/cix1014. [DOI] [PubMed] [Google Scholar]
- 394.Stamps BW, Kuroiwa J, Isidean SD, Schilling MA, Harro C, Talaat KR, Sack DA, Tribble DR, Maue AC, Rimmer JE, Laird RM, Porter CK, Goodson MS, Poly F. 2021. Exploring changes in the host gut microbiota during a controlled human infection model for Campylobacter jejuni. Front Cell Infect Microbiol 11:702047. doi: 10.3389/fcimb.2021.702047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Poly F, Noll AJ, Riddle MS, Porter CK. 2019. Update on Campylobacter vaccine development. Hum Vaccin Immunother 15:1389–1400. doi: 10.1080/21645515.2018.1528410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Houliston RS, Vinogradov E, Dzieciatkowska M, Li J, St Michael F, Karwaski MF, Brochu D, Jarrell HC, Parker CT, Yuki N, Mandrell RE, Gilbert M. 2011. Lipooligosaccharide of Campylobacter jejuni: similarity with multiple types of mammalian glycans beyond gangliosides. J Biol Chem 286:12361–12370. doi: 10.1074/jbc.M110.181750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Clarke TN, Schilling MA, Melendez LA, Isidean SD, Porter CK, Poly FM. 2021. A systematic review and meta-analysis of Penner serotype prevalence of Campylobacter jejuni in low- and middle-income countries. PLoS One 16:e0251039. doi: 10.1371/journal.pone.0251039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Albert MJ, Mustafa AS, Islam A, Haridas S. 2013. Oral immunization with cholera toxin provides protection against Campylobacter jejuni in an adult mouse intestinal colonization model. mBio 4:e00246-13. doi: 10.1128/mBio.00246-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Nothaft H, Davis B, Lock YY, Perez-Munoz ME, Vinogradov E, Walter J, Coros C, Szymanski CM. 2016. Engineering the Campylobacter jejuni N-glycan to create an effective chicken vaccine. Sci Rep 6:26511. doi: 10.1038/srep26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Laird R, Ramakrishnan A, Schumack NM, Nunez G, Espinoza N, Nieto M, Castillo R, Rojas J, McCoy AJ, Beck Z, Matyas GR, Alving CR, Guerry P, Poly F. 2019. Enhanced immunogenicity and protective efficacy of a Campylobacter jejuni conjugate vaccine coadministered with liposomes containing monophosphoryl lipid A and QS-21 in Aotus nancymaae non-human. Presentation at the 10th International Conference on Vaccines for Enteric Diseases, 16–18 October 2019. 10th International Conference on Vaccines for Enteric Diseases, Lausanne, Switzerland. [Google Scholar]
- 401.ClinicalTrials.gov. 2021. NCT02067676: safety and immunogenicity evaluation of an intramuscular capsule-conjugate Campylobacter vaccine (CJCV1). https://clinicaltrials.gov/ct2/show/study/NCT02067676.
- 402.Brenner FW, Villar RG, Angulo FJ, Tauxe R, Swaminathan B. 2000. Salmonella nomenclature. J Clin Microbiol 38:2465–2467. doi: 10.1128/JCM.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Gal-Mor O, Boyle EC, Grassl GA. 2014. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Waddington CS, Darton TC, Pollard AJ. 2014. The challenge of enteric fever. J Infect Dis 68(Suppl 1):S38–S50. doi: 10.1016/j.jinf.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 405.MacLennan CA. 2014. Antibodies and protection against invasive Salmonella disease. Front Immunol 5:635. doi: 10.3389/fimmu.2014.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. 2012. Host-pathogen interaction in invasive salmonellosis. PLoS Pathog 8:e1002933. doi: 10.1371/journal.ppat.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.MacLennan C, Steel D. 2019. Invasive non-typhoid Salmonella vaccines. Presentation at the World Health Organization Product Development for Vaccines Advisory Committee meeting, 26–28 June 2019. World Health Organization, Geneva, Switzerland. https://cdn.who.int/media/docs/default-source/immunization/pdvac/pdvac-2019/day-2-2019-pdvac-presentation-(1).pdf. [Google Scholar]
- 408.World Health Organization. 2015. Global action plan on antimicrobial resistance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 409.World Health Organization. 2020. Recommendations to assure the quality, safety and efficacy of typhoid conjugate vaccines. WHO Technical Report Series, no. 987, annex 3. WHO, Geneva, Switzerland. [Google Scholar]
- 410.MacLennan CA, Martin LB, Micoli F. 2014. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother 10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Waddington CS, Darton TC, Woodward WE, Angus B, Levine MM, Pollard AJ. 2014. Advancing the management and control of typhoid fever: a review of the historical role of human challenge studies. J Infect 68:405–418. doi: 10.1016/j.jinf.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 412.Darton TC, Blohmke CJ, Pollard AJ. 2014. Typhoid epidemiology, diagnostics, and the human challenge model. Curr Opin Gastroenterol 30:7–17. doi: 10.1097/MOG.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 413.Zhou L, Pollard AJ. 2010. A fast and highly sensitive blood culture PCR method for clinical detection of Salmonella enterica serovar Typhi. Ann Clin Microbiol Antimicrob 9:14. doi: 10.1186/1476-0711-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. 2015. Typhoid fever. Lancet 385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PubMed] [Google Scholar]
- 415.Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G, Cross AS, Galen JE, Pasetti MF, Levine MM. 2014. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin Vaccine Immunol 21:712–721. doi: 10.1128/CVI.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Bajracharya D, Khan MI, Pach A, III, Shrestha P, Joshi N, Upreti SR, Wierzba T, Puri M, Sahastrabuddhe S, Ochiai RL. 2014. 25 years after Vi typhoid vaccine efficacy study, typhoid affects significant number of population in Nepal. PLoS One 9:e77974. doi: 10.1371/journal.pone.0077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacLennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 44:1215–1221. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Achtman M, Wain J, Weill FX, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A, Dougan G, Harrison LH, Brisse S, S. Enterica MLST Study Group . 2012. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog 8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 420.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, Thompson BAV, Kerridge SA, Kingsley RA, Zhou L, Holt KE, Yu L-M, Lockhart S, Farrar JJ, Sztein MB, Dougan G, Angus B, Levine MM, Pollard AJ. 2014. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 58:1230–1240. doi: 10.1093/cid/ciu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Sztein MB, Salerno-Goncalves R, McArthur MA. 2014. Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward. Front Immunol 5:516. doi: 10.3389/fimmu.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Pakkanen SH, Kantele JM, Herzog C, Kantele A. 2014. Cross-reactive immune response elicited by parenteral Vi polysaccharide typhoid vaccine against non-typhoid Salmonellae. Vaccine 32:544–551. doi: 10.1016/j.vaccine.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 424.Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. 2014. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol 21:427–434. doi: 10.1128/CVI.00786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Dobinson HC, Gibani MM, Jones C, Thomaides-Brears HB, Voysey M, Darton TC, Waddington CS, Campbell D, Milligan I, Zhou L, Shrestha S, Kerridge SA, Peters A, Stevens Z, Podda A, Martin LB, D’Alessio F, Thanh DP, Basnyat B, Baker S, Angus B, Levine MM, Blohmke CJ, Pollard AJ. 2017. Evaluation of the clinical and microbiological response to Salmonella Paratyphi A infection in the first paratyphoid human challenge model. Clin Infect Dis 64:1066–1075. doi: 10.1093/cid/cix042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.World Health Organization. 2018. Typhoid vaccines: WHO position paper–March 2018. Wkly Epidemiol Rec 93:153–172. doi: 10.1016/j.vaccine.2018.04.022. [DOI] [Google Scholar]
- 427.Szu SC, Hunt S, Xie G, Robbins JB, Schneerson R, Gupta R, Zhao Z, Tan X. 2013. A human IgG anti-Vi reference for Salmonella Typhi with weight-based antibody units assigned. Vaccine 31:1970–1974. doi: 10.1016/j.vaccine.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Rijpkema S, Hockley J, Logan A, Rigsby P, Atkinson E, Jin C, Goldblatt D, Liang H, Bachtiar NS, Yang JS, Goel A, Ramasamy V, Pasetti MF, Pollard AJ, anti-Vi IgG working group . 2018. Establishment of the first international standard for human anti-typhoid capsular Vi polysaccharide IgG. Biologicals 56:29–38. doi: 10.1016/j.biologicals.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Syed KA, Saluja T, Cho H, Hsiao A, Shaikh H, Wartel TA, Mogasale V, Lynch J, Kim JH, Excler JL, Sahastrabuddhe S. 2020. Review on the recent advances on typhoid vaccine development and challenges ahead. Clin Infect Dis 71(Suppl 2):S141–S150. doi: 10.1093/cid/ciaa504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Baliban SM, Lu YJ, Malley R. 2020. Overview of the nontyphoidal and paratyphoidal Salmonella vaccine pipeline: current status and future prospects. Clin Infect Dis 71:S151–S154. doi: 10.1093/cid/ciaa514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, Harris V, Gardner J, Nebykova A, Kerridge SA, Hill J, Thomaides-Brears H, Blohmke CJ, Yu LM, Angus B, Pollard AJ. 2017. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomized controlled, phase 2b trial. Lancet 390:2472–2480. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Dahora LC, Jin C, Spreng RL, Feely F, Mathura R, Seaton KE, Zhang L, Hill J, Jones E, Alam SM, Dennison SM, Pollard AJ, Tomaras GD. 2019. IgA and IgG1 specific to Vi polysaccharide of Salmonella Typhi correlate with protection status in a typhoid fever controlled human infection model. Front Immunol 10:2582. doi: 10.3389/fimmu.2019.02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Lee EY, Park JY, Kim DR, Song M, Sahastrabuddhe S, Kim H, Chon Y, Yang JS. 2020. Comparison of anti-Vi IgG responses between two clinical studies of typhoid Vi conjugate vaccines (Vi-TT versus Vi-DT). PLoS Negl Trop Dis 14:e0008171. doi: 10.1371/journal.pntd.0008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, Liu X, Tonks S, Mazur O, Farooq YG, Clarke J, Hill J, Adhikari A, Dongol S, Karkey A, Bajracharya B, Kelly S, Gurung M, Baker S, Neuzil KM, Shrestha S, Basnyat B, Pollard AJ, TyVAC Nepal Study Team . 2019. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med 381:2209–2218. doi: 10.1056/NEJMoa1905047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Choi SK, Baik YO, Kim CW, Kim SK, Oh IN, Yoon H, Yu D, Lee C. 2021. An open-label, comparative, single dose, clinical phase I study to assess the safety and immunogenicity of typhoid conjugate vaccine (Vi-CRM197) in healthy Filipino adults. Vaccine 39:2620–2627. doi: 10.1016/j.vaccine.2021.03.089. [DOI] [PubMed] [Google Scholar]
- 436.Yousafzai MT, Qamar FN, Shakoor S, Saleem K, Lohana H, Karim S, Hotwani A, Qureshi S, Masood N, Rauf M, Khanzada JA, Kazi M, Hasan R. 2019. Ceftriaxone-resistant Salmonella Typhi outbreak in Hyderabad City of Sindh, Pakistan: high time for the introduction of typhoid conjugate vaccine. Clin Infect Dis 68(Suppl 1):S16–S21. doi: 10.1093/cid/ciy877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Appiah GD, Chung A, Bentsi-Enchill AD, Kim S, Crump JA, Mogasale V, Pellegrino R, Slayton RB, Mintz ED. 2020. Typhoid outbreaks, 1989–2018: implications for prevention and control. Am J Trop Med Hyg 102:1296–1305. doi: 10.4269/ajtmh.19-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Olaru ID, Mtapuri-Zinyowera S, Feasey N, Ferrand RA, Kranzer K. 2019. Typhoid Vi-conjugate vaccine for outbreak control in Zimbabwe. Lancet Infect Dis 19:930. doi: 10.1016/S1473-3099(19)30425-6. [DOI] [PubMed] [Google Scholar]
- 439.Yousafzai MT, Karim S, Qureshi S, Kazi M, Memon H, Junejo A, Khawaja Z, Ur Rehman N, Ansari MS, Ali R, Ujjan IU, Lohana HM, Memon NM, Hussain M, Nigar R, Bar-Zeev N, Qamar FN. 2021. Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study. Lancet Glob Health 9:e1154–e1162. doi: 10.1016/S2214-109X(21)00255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Batool R, Tahir Yousafzai M, Qureshi S, Ali M, Sadaf T, Mehmood J, Ashorn P, Naz Qamar F. 2021. Effectiveness of typhoid conjugate vaccine against culture-confirmed typhoid in a peri-urban setting in Karachi: a case-control study. Vaccine 39:5858–5865. doi: 10.1016/j.vaccine.2021.08.051. [DOI] [PubMed] [Google Scholar]
- 441.Qadri F, Khanam F, Liu X, Theiss-Nyland K, Biswas PK, Bhuiyan AI, Ahmmed F, Colin-Jones R, Smith N, Tonks S, Voysey M, Mujadidi YF, Mazur O, Rajib NH, Hossen MI, Ahmed SU, Khan A, Rahman N, Babu G, Greenland M, Kelly S, Ireen M, Islam K, O’Reilly P, Scherrer KS, Pitzer VE, Neuzil KM, Zaman K, Pollard AJ, Clemens JD. 2021. Protection by vaccination of children against typhoid fever with a Vi-tetanus toxoid conjugate vaccine in urban Bangladesh: a cluster-randomised trial. Lancet 398:675–684. doi: 10.1016/S0140-6736(21)01124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Patel PD, Patel P, Liang Y, Meiring JE, Misiri T, Mwakiseghile F, Tracy JK, Masesa C, Msuku H, Banda D, Mbewe M, Henrion M, Adetunji F, Simiyu K, Rotrosen E, Birkhold M, Nampota N, Nyirenda OM, Kotloff K, Gmeiner M, Dube Q, Kawalazira G, Laurens MB, Heyderman RS, Gordon MA, Neuzil KM, TyVAC Malawi Team . 2021. Safety and efficacy of a typhoid conjugate vaccine in Malawian children. N Engl J Med 385:1104–1115. doi: 10.1056/NEJMoa2035916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Sirima SB, Ouedraogo A, Barry N, Siribie M, Tiono A, Nébié I, Konaté A, Berges GD, Diarra A, Ouedraogo M, Bougouma EC, Soulama I, Hema A, Datta S, Liang Y, Rotrosen ET, Tracy JK, Jamka LP, Oshinsky JJ, Pasetti MF, Neuzil KM, Laurens MB. 2021. Safety and immunogenicity of Vi-typhoid conjugate vaccine co-administration with routine 9-month vaccination in Burkina Faso: a randomized controlled phase 2 trial. Int J Infect Dis 108:465–472. doi: 10.1016/j.ijid.2021.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Sirima SB, Ouedraogo A, Barry N, Siribie M, Tiono AB, Nébié I, Konaté AT, Berges GD, Diarra A, Ouedraogo M, Soulama I, Hema A, Datta S, Liang Y, Rotrosen ET, Tracy JK, Jamka LP, Neuzil KM, Laurens MB. 2021. Safety and immunogenicity of co-administration of meningococcal type A and measles-rubella vaccines with typhoid conjugate vaccine in children aged 15–23 months in Burkina Faso. Int J Infect Dis 102:517–523. doi: 10.1016/j.ijid.2020.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque SG, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, Duarte ASR, Black RE, Angulo FJ. 2015. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS One 10:e0142927. doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Karst SM, Zhu S, Goodfellow IG. 2015. The molecular pathology of noroviruses. J Pathol 235:206–216. doi: 10.1002/path.4463. [DOI] [PubMed] [Google Scholar]
- 448.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. 2008. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. 2014. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 14:725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Ramani S, Atmar RL, Estes MK. 2014. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol 30:25–33. doi: 10.1097/MOG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Cannon JL, Lindesmith LC, Donaldson EF, Saxe L, Baric RS, Vinje J. 2009. Herd immunity to GII.4 noroviruses is supported by outbreak patient sera. J Virol 83:5363–5374. doi: 10.1128/JVI.02518-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, Rocks JJ, Kiel J, Montes JS, Moe CL, Eisenberg JNS, Leon JS. 2012. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect 140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Sunil V, Holt AM, Dixon R, Dingman D, Noseworthy AL. 2013. Norovirus-like virus outbreak at a correctional facility in Haliburton Kawartha Pine Ridge District Health Unit, March to April 2008. J Correct Health Care 19:269–277. doi: 10.1177/1078345813499311. [DOI] [PubMed] [Google Scholar]
- 454.Greig JD, Lee MB, Harris JE. 2011. Review of enteric outbreaks in prisons: effective infection control interventions. Public Health 125:222–228. doi: 10.1016/j.puhe.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 455.Richardson C, Bargatze RF, Goodwin R, Mendelman PM. 2013. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev Vaccines 12:155–167. doi: 10.1586/erv.12.145. [DOI] [PubMed] [Google Scholar]
- 456.Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. 2010. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat Med 9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 458.Shirato H. 2011. Norovirus and histo-blood group antigens. Jpn J Infect Dis 64:95–103. doi: 10.7883/yoken.64.95. [DOI] [PubMed] [Google Scholar]
- 459.Swanstrom J, Lindesmith LC, Donaldson EF, Yount B, Baric RS. 2014. Characterization of blockade antibody responses in GII.2.1976 Snow Mountain virus-infected subjects. J Virol 88:829–837. doi: 10.1128/JVI.02793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Bhar S, Jones MK. 2019. In vitro replication of human norovirus. Viruses 11:547. doi: 10.3390/v11060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Jones MK, Grau KR, Costantini V, Kolawole AO, de Graaf M, Freiden P, Graves CL, Koopmans M, Wallet SM, Tibbetts SA, Schultz-Cherry S, Wobus CE, Vinjé J, Karst SM. 2015. Human norovirus culture in B cells. Nat Protoc 10:1939–1947. doi: 10.1038/nprot.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK. 2016. Replication of human noroviruses in stem cell-derived human enteroids. Science 353:1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Sato S, Hisaie K, Kurokawa S, Suzuki A, Sakon N, Uchida Y, Yuki Y, Kiyono H. 2019. Human norovirus propagation in human induced pluripotent stem cell-derived intestinal epithelial cells. Cell Mol Gastroenterol Hepatol 7:686–688. doi: 10.1016/j.jcmgh.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Craig K, Dai X, Li A, Lu M, Xue M, Rosas L, Gao TZ, Niehaus A, Jennings R, Li J. 2019. A lactic acid bacteria (LAB)-based vaccine candidate for human norovirus. Viruses 11:213. doi: 10.3390/v11030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Kocher J, Bui T, Giri-Rachman E, Wen K, Li G, Yang X, Liu F, Tan M, Xia M, Zhong W, Jiang X, Yuan L. 2014. Intranasal P particle vaccine provided partial cross-variant protection against human GII.4 norovirus diarrhea in gnotobiotic pigs. J Virol 88:9728–9743. doi: 10.1128/JVI.01249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Lei S, Ramesh A, Twitchell E, Wen K, Bui T, Weiss M, Yang X, Kocher J, Li G, Giri-Rachman E, Trang NV, Jiang X, Ryan EP, Yuan L. 2016. High protective efficacy of probiotics and rice bran against human norovirus infection and diarrhea in gnotobiotic pigs. Front Microbiol 7:1699. doi: 10.3389/fmicb.2016.01699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Lei S, Twitchell EL, Ramesh AK, Bui T, Majette E, Tin CM, Avery R, Arango-Argoty G, Zhang L, Becker-Dreps S, Azcarate-Peril MA, Jiang X, Yuan L. 2019. Enhanced GII.4 human norovirus infection in gnotobiotic pigs transplanted with a human gut microbiota. J Gen Virol 100:1530–1540. doi: 10.1099/jgv.0.001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis 161:18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- 470.Graham DY, Jiang X, Tanaka T, Opekun AR, Madore HP, Estes MK. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis 170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 471.Gray JJ, Cunliffe C, Ball J, Graham DY, Desselberger U, Estes MK. 1994. Detection of immunoglobulin M (IgM), IgA, and IgG Norwalk virus-specific antibodies by indirect enzyme-linked immunosorbent assay with baculovirus-expressed Norwalk virus capsid antigen in adult volunteers challenged with Norwalk virus. J Clin Microbiol 32:3059–3063. doi: 10.1128/jcm.32.12.3059-3063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Leon JS, Kingsley DH, Montes JS, Richards GP, Lyon GM, Abdulhafid GM, Seitz SR, Fernandez ML, Teunis PF, Flick GJ, Moe CL. 2011. Randomized, double-blinded clinical trial for human norovirus inactivation in oysters by high hydrostatic pressure processing. Appl Environ Microbiol 77:5476–5482. doi: 10.1128/AEM.02801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Mateo R, Lindesmith LC, Garg SJ, Gottlieb K, Lin K, Said S, Leon JS, Sims AC, Weber DJ, Baric RS, Tucker SN, Taylor DN. 2020. Production and clinical evaluation of Norwalk GI.1 virus Lot 001-09NV in norovirus vaccine development. J Infec Dis 221:919–926. doi: 10.1093/infdis/jiz540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. 202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. 2014. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Czako R, Atmar RL, Opekun AR, Gilger MA, Graham DY, Estes MK. 2012. Serum hemagglutination inhibition activity correlates with protection from gastroenteritis in persons infected with Norwalk virus. Clin Vaccine Immunol 19:284–287. doi: 10.1128/CVI.05592-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Le Guyader FS, Bon F, DeMedici D, Parnaudeau S, Bertone A, Crudeli S, Doyle A, Zidane M, Suffredini E, Kohli E, Maddalo F, Monini M, Gallay A, Pommepuy M, Pothier P, Ruggeri FM. 2006. Detection of multiple noroviruses associated with an international gastroenteritis outbreak linked to oyster consumption. J Clin Microbiol 44:3878–3882. doi: 10.1128/JCM.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Patin NV, Peña-Gonzalez A, Hatt JK, Moe C, Kirby A, Konstantinidis KT. 2020. The role of the gut microbiome in resisting norovirus infection as revealed by a human challenge study. mBio 11:e02634-20. doi: 10.1128/mBio.02634-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Seitz SR, Leon JS, Schwab KJ, Lyon GM, Dowd M, McDaniels M, Abdulhafid G, Fernandez ML, Lindesmith LC, Baric RS, Moe CL. 2011. Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol 77:6884–6888. doi: 10.1128/AEM.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Kim L, Liebowitz D, Lin K, Kasparek K, Pasetti MS, Garg SJ, Gottlieb K, Trager G, Tucker SN. 2018. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight 3:e121077. doi: 10.1172/jci.insight.121077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Frenck R, Bernstein DI, Xia M, Huang P, Zhong W, Parker S, Dickey M, McNeal M, Jiang X. 2012. Predicting susceptibility to norovirus GII.4 by use of a challenge model involving humans. J Infect Dis 206:1386–1393. doi: 10.1093/infdis/jis514. [DOI] [PubMed] [Google Scholar]
- 483.Kirby AE, Streby A, Moe CL. 2016. Vomiting as a symptom and transmission risk in norovirus illness: evidence from human challenge studies. PLoS One 11:e0143759. doi: 10.1371/journal.pone.0143759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. 2003. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol 108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 485.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterol 117:40–48. doi: 10.1016/S0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 486.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, Bargatze RF, Sztein MB, Tacket CO. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis 202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. 2011. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinjé J, Gregoricus N, Frenck RW, Jr, Moe CL, Al-Ibrahim MS, Barrett J, Ferreira J, Estes MK, Graham DY, Goodwin R, Borkowski A, Clemens R, Mendelman PM. 2014. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis 11:870–888. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. 2012. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 30:3580–3586. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Sherwood J, Mendelman PM, Lloyd E, Liu M, Boslego J, Borkowski A, Jackson A, Faix D, US Navy Study Team . 2020. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine 38:6442–6449. doi: 10.1016/j.vaccine.2020.07.069. [DOI] [PubMed] [Google Scholar]
- 491.Treanor J, Sherwood J, Cramer JP, Le Cam Bouveret N, Lin S, Baehner F, Borkowski A, NOR-204 Investigators . 2020. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine 38:5842–5850. doi: 10.1016/j.vaccine.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 492.ClinicalTrials.gov. 2020. NCT03897309: a phase 1b, randomized, double-blind, placebo-controlled, multi-center safety and immunogenicity study of adenoviral-vector based oral norovirus vaccines expressing GI.1 or GII.4 VP1 with monovalent or bivalent dosing. https://clinicaltrials.gov/ct2/show/NCT03897309.
- 493.Vaxart, Inc. 2019. Vaxart’s tableted oral bivalent norovirus vaccine meets primary and secondary endpoints in phase 1b study. Vaxart, South San Francisco, CA. (Press release.) https://www.globenewswire.com/news-release/2019/09/25/1920587/0/en/Vaxart-s-Tableted-Oral-Bivalent-Norovirus-Vaccine-Meets-Primary-and-Secondary-Endpoints-in-Phase-1b-Study.html. [Google Scholar]
- 494.Taylor D. 2019. Progress on the development of an oral, bivalent norovirus vaccine. Presentation at the 10th International Conference on Vaccines for Enteric Diseases, 17 October 2019. 10th International Conference on Vaccines for Enteric Diseases, Lausanne, Switzerland. [Google Scholar]
- 495.Debbink K, Lindesmith LC, Donaldson EF, Swanstrom J, Baric RS. 2014. Chimeric GII.4 norovirus virus-like-particle-based vaccines induce broadly blocking immune responses. J Virol 88:7256–7266. doi: 10.1128/JVI.00785-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Esposito S, Principi N. 2020. Norovirus vaccine: priorities for future research and development. Front Immunol 11:1383. doi: 10.3389/fimmu.2020.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Chen W, Kang T, Yuan R, Shao C, Jing S. 2020. Immunogenicity and protective potency of norovirus GII.17 virus-like particle-based vaccine. Biotechnol Lett 42:1211–1218. doi: 10.1007/s10529-020-02837-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Lampinen V, Heinimäki S, Laitinen OH, Pesu M, Hankaniemi MM, Blazevic V, Hytönen VP. 2021. Modular vaccine platform based on the norovirus-like particle. J Nanobiotechnol 19:25. doi: 10.1186/s12951-021-00772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, Kapikian AZ, Chanock RM. 1974. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis 129:709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]
- 500.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 501.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, et al. 2012. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 502.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Widerström M, Schönning C, Lilja M, Lebbad M, Ljung T, Allestam G, Ferm M, Björkholm B, Hansen A, Hiltula J, Långmark J, Löfdahl M, Omberg M, Reuterwall C, Samuelsson E, Widgren K, Wallensten A, Lindh J. 2014. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg Infect Dis 20:581–589. doi: 10.3201/eid2004.121415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Manjunatha UH, Vinayak S, Zambriski JA, Chao AT, Sy T, Noble CG, Bonamy GMC, Kondreddi RR, Zou B, Gedeck P, Brooks CF, Herbert GT, Sateriale A, Tandel J, Noh S, Lakshminarayana SB, Lim SH, Goodman LB, Bodenreider C, Feng G, Zhang L, Blasco F, Wagner J, Leong FJ, Striepen B, Diagana TT. 2017. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 546:376–380. doi: 10.1038/nature22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Lunde CS, Stebbins EE, Jumani RS, Hasan MM, Miller P, Barlow J, Freund YR, Berry P, Stefanakis R, Gut J, Rosenthal PJ, Love MS, McNamara CW, Easom E, Plattner JJ, Jacobs RT, Huston CD. 2019. Identification of a potent benzoxaborole drug candidate for treating cryptosporidiosis. Nat Commun 10:2816. doi: 10.1038/s41467-019-10687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Baragaña B, Forte B, Choi R, Nakazawa Hewitt S, Bueren-Calabuig JA, Pisco JP, Peet C, Dranow DM, Robinson DA, Jansen C, Norcross NR, Vinayak S, Anderson M, Brooks CF, Cooper CA, Damerow S, Delves M, Dowers K, Duffy J, Edwards TE, Hallyburton I, Horst BG, Hulverson MA, Ferguson L, Jiménez-Díaz MB, Jumani RS, Lorimer DD, Love MS, Maher S, Matthews H, McNamara CW, Miller P, O’Neill S, Ojo KK, Osuna-Cabello M, Pinto E, Post J, Riley J, Rottmann M, Sanz LM, Scullion P, Sharma A, Shepherd SM, Shishikura Y, Simeons FRC, Stebbins EE, Stojanovski L, Straschil U, Tamaki FK, Tamjar J, et al. 2019. Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proc Natl Acad Sci USA 116:7015–7020. doi: 10.1073/pnas.1814685116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Huang W, Hulverson MA, Choi R, Arnold SLM, Zhang Z, McCloskey MC, Whitman GR, Hackman RC, Rivas KL, Barrett LK, Ojo KK, Van Voorhis WC, Fan E. 2019. Development of 5-aminopyrazole-4-carboxamide-based bumped-kinase inhibitors for cryptosporidiosis therapy. J Med Chem 62:3135–3146. doi: 10.1021/acs.jmedchem.9b00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. 2007. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol 63:387–393. doi: 10.1111/j.1365-2125.2007.02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Mead JR. 2014. Prospects for immunotherapy and vaccines against Cryptosporidium. Hum Vaccin Immunother 10:1505–1513. doi: 10.4161/hv.28485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, Panchalingam S, Sur D, Zaidi AKM, Faruque ASG, Saha D, Adegbola R, Alonso PL, Breiman RF, Bassat Q, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ahmed S, Qureshi S, Quadri F, Hossain A, Das SK, Antonio M, Hossain MJ, Mandomando I, Nhampossa T, Acácio S, Omore R, Oundo JO, Ochieng JB, Mintz ED, O’Reilly CE, Berkeley LY, Livio S, Tennant SM, Sommerfelt H, Nataro JP, Ziv-Baran T, Robins-Browne RM, Mishcherkin V, Zhang J, Liu J, Houpt ER, Kotloff KL, Levine MM. 2016. The Burden of CRYPTOSPORIDIUM diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 10:e0004729. doi: 10.1371/journal.pntd.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Gharpure R, Perez A, Miller AD, Wikswo ME, Silver R, Hlavsa MC. 2019. Cryptosporidiosis outbreaks—United States, 2009–2017. MMWR Morb Mortal Wkly Rep 68:568–572. doi: 10.15585/mmwr.mm6825a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Checkley W, White AC, Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA, Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Borad A, Ward H. 2010. Human immune responses in cryptosporidiosis. Future Microbiol 5:507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Iroh Tam PY, Chisala M, Nyangulu W, Thole H, Nyirenda J. 2021. Respiratory cryptosporidiosis in Malawian children with diarrheal disease. PLoS Negl Trop Dis 15:e0009643. doi: 10.1371/journal.pntd.0009643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Sponseller JK, Griffiths JK, Tzipori S. 2014. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clin Microbiol Rev 27:575–586. doi: 10.1128/CMR.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, Griffiths JK. 2010. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: potential for respiratory transmission. Clin Infect Dis 50:1366–1372. doi: 10.1086/652140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Mor SM, Ascolillo LR, Nakato R, Ndeezi G, Tumwine JK, Okwera A, Sponseller JK, Tzipori S, Griffiths JK. 2018. Expectoration of Cryptosporidium parasites in sputum of human immunodeficiency virus-positive and -negative adults. Am J Trop Med Hyg 98:1086–1090. doi: 10.4269/ajtmh.17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Nyangulu W, Van Voorhis W, Iroh TP. 2019. Evaluating respiratory cryptosporidiosis in pediatric diarrheal disease: protocol for a prospective, observational study in Malawi. BMC Infect Dis 19:728. doi: 10.1186/s12879-019-4380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Morada M, Lee S, Gunther-Cummins L, Weiss LM, Widmer G, Tzipori S, Yarlett N. 2016. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 46:21–29. doi: 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 521.Wilke G, Funkhouser-Jones LJ, Wang Y, Ravindran S, Wang Q, Beatty WL, Baldridge MT, VanDussen KL, Shen B, Kuhlenschmidt MS, Kuhlenschmidt TB, Witola WH, Stappenbeck TS, Sibley LD. 2019. A stem-cell-derived platform enables complete Cryptosporidium development in vitro and genetic tractability. Cell Host Microbe 26:123–134. doi: 10.1016/j.chom.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Miller CN, Jossé L, Brown I, Blakeman B, Povey J, Yiangou L, Price M, Cinatl J, Jr, Xue WF, Michaelis M, Tsaousis AD. 2018. A cell culture platform for Cryptosporidium that enables long-term cultivation and new tools for the systematic investigation of its biology. Int J Parasitol 48:197–201. doi: 10.1016/j.ijpara.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Jumani RS, Blais J, Tillmann HC, Segal F, Wetty D, Ostermeier C, Nuber N, Lakshman J, Aziz N, Chandra R, Chen WH, Chappell CL, Diagana TT, Manjunatha UH. 2021. Opportunities and challenges in developing a Cryptosporidium controlled human infection model for testing antiparasitic agents. ACS Infect Dis 7:959–968. doi: 10.1021/acsinfecdis.1c00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med 332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 525.Chappell CL, Okhuysen PC, Sterling CR, DuPont HL. 1996. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J Infect Dis 173:232–236. doi: 10.1093/infdis/173.1.232. [DOI] [PubMed] [Google Scholar]
- 526.Chappell CL, Okhuysen PC, Sterling CR, Wang C, Jakubowski W, Dupont HL. 1999. Infectivity of Cryptosporidium parvum in healthy adults with preexisting anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg 60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 527.Moss DM, Chappell CL, Okhuysen PC, DuPont HL, Arrowood MJ, Hightower AW, Lammie PJ. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J Infect Dis 178:827–833. doi: 10.1086/515377. [DOI] [PubMed] [Google Scholar]
- 528.Priest JW, Moss DM. 2020. Measuring Cryptosporidium serologic responses by multiplex bead assay. Methods Mol Biol 2052:61–85. doi: 10.1007/978-1-4939-9748-0_5. [DOI] [PubMed] [Google Scholar]
- 529.Pantenburg B, Dann SM, Wang HC, Robinson P, Castellanos-Gonzalez A, Lewis DE, White AC, Jr.. 2008. Intestinal immune response to human Cryptosporidium sp. infection. Infect Immun 76:23–29. doi: 10.1128/IAI.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis 180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 531.Okhuysen PC, Rich SM, Chappell CL, Grimes KA, Widmer G, Feng X, Tzipori S. 2002. Infectivity of a Cryptosporidium parvum isolate of cervine origin for healthy adults and interferon-gamma knockout mice. J Infect Dis 185:1320–1325. doi: 10.1086/340132. [DOI] [PubMed] [Google Scholar]
- 532.Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, Tzipori S. 2006. Cryptosporidium hominis: experimental challenge of healthy adults. Am J Trop Med Hyg 75:851–857. doi: 10.4269/ajtmh.2006.75.851. [DOI] [PubMed] [Google Scholar]
- 533.Rehn M, Wallensten A, Widerström M, Lilja M, Grunewald M, Stenmark S, Kark M, Lindh J. 2015. Post-infection symptoms following two large waterborne outbreaks of Cryptosporidium hominis in northern Sweden, 2010–2011. BMC Public Health 15:529. doi: 10.1186/s12889-015-1871-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Lilja M, Widerström M, Lindh J. 2018. Persisting post-infection symptoms 2 years after a large waterborne outbreak of Cryptosporidium hominis in northern Sweden. BMC Res Notes 11:625. doi: 10.1186/s13104-018-3721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Iglói Z, Mughini-Gras L, Lochlainn LN, Barrasa A, Sane J, Mooij S, Schimmer B, Roelfsema J, van Pelt W, Kortbeek T. 2018. Long-term sequelae of sporadic cryptosporidiosis: a follow-up study. Eur J Clin Microbiol Infect Dis 37:1377–1384. doi: 10.1007/s10096-018-3268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Stiff RE, Davies AP, Mason BW, Hutchings HA, Chalmers RM. 2017. Long-term health effects after resolution of acute Cryptosporidium parvum infection: a 1-year follow-up of outbreak-associated cases. J Med Microbiol 66:1607–1611. doi: 10.1099/jmm.0.000609. [DOI] [PubMed] [Google Scholar]
- 537.Carter BL, Stiff RE, Elwin K, Hutchings HA, Mason BW, Davies AP, Chalmers RM. 2019. Health sequelae of human cryptosporidiosis: a 12-month prospective follow-up study. Eur J Clin Microbiol Infect Dis 38:1709–1717. doi: 10.1007/s10096-019-03603-1. [DOI] [PubMed] [Google Scholar]
- 538.Chappell CL, Okhuysen PC, Sterling CR, Wang C, Jakubowski W, Dupont HL. 1999. Infectivity of Cryptosporidium parvum in healthy adults with preexisting anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg 60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 539.Sheoran AS, Pina-Mimbela R, Keleher A, Girouard D, Tzipori S. 2018. Infection with anthroponotic Cryptosporidium parvum does not fully protect the host against a subsequent challenge with C. hominis. Microbes Infect 20:267–270. doi: 10.1016/j.micinf.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 540.Sheoran A, Wiffin A, Widmer G, Singh P, Tzipori S. 2012. Infection with Cryptosporidium hominis provides incomplete protection of the host against Cryptosporidium parvum. J Infect Dis 205:1019–1023. doi: 10.1093/infdis/jir874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Okhuysen PC, Chappell CL, Crabb J, Valdez LM, Douglass ET, DuPont HL. 1998. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis 26:1324–1329. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 542.Borad A, Ward H. 2010. Human immune responses in cryptosporidiosis. Future Microbiol 5:507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Lemieux MW, Sonzogni-Desautels K, Ndao M. 2017. Lessons learned from protective immune responses to optimize vaccines against cryptosporidiosis. Pathogens 7:2. doi: 10.3390/pathogens7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Cui Z, Wang L, Wang Y, Li J, Wang R, Sun M, Zhang L. 2020. Cryptosporidium parvum gp40/15 is associated with the parasitophorous vacuole membrane and is a potential vaccine target. Microorganisms 8:363. doi: 10.3390/microorganisms8030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Tosini F, Ludovisi A, Tonanzi D, Amati M, Cherchi S, Pozio E, Gómez-Morales MA. 2019. Delivery of SA35 and SA40 peptides in mice enhances humoral and cellular immune responses and confers protection against Cryptosporidium parvum infection. Parasit Vectors 12:233. doi: 10.1186/s13071-019-3486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Manque PA, Tenjo F, Woehlbier U, Lara AM, Serrano MG, Xu P, Alves JM, Smeltz RB, Conrad DH, Buck GA. 2011. Identification and immunological characterization of three potential vaccinogens against Cryptosporidium species. Clin Vaccine Immunol 18:1796–1802. doi: 10.1128/CVI.05197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Benitez A, Priest JW, Ehigiator HN, McNair N, Mead JR. 2011. Evaluation of DNA encoding acidic ribosomal protein P2 of Cryptosporidium parvum as a potential vaccine candidate for cryptosporidiosis. Vaccine 29:9239–9245. doi: 10.1016/j.vaccine.2011.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Liu K, Zai D, Zhang D, Wei Q, Han G, Gao H, Huang B. 2010. Divalent Cp15-23 vaccine enhances immune responses and protection against Cryptosporidium parvum infection. Parasite Immunol 32:335–344. doi: 10.1111/j.1365-3024.2009.01191.x. [DOI] [PubMed] [Google Scholar]
- 549.Roche JK, Rojo AL, Costa LB, Smeltz R, Manque P, Woehlbier U, Bartelt L, Galen J, Buck G, Guerrant RL. 2013. Intranasal vaccination in mice with an attenuated Salmonella enterica serovar 908htr A expressing Cp15 of Cryptosporidium: impact of malnutrition with preservation of cytokine secretion. Vaccine 31:912–918. doi: 10.1016/j.vaccine.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Driskell I, O’Connor RM. 2020. Production and purification of functional Cryptosporidium glycoproteins by heterologous expression in Toxoplasma gondii. Methods Mol Biol 2052:87–102. doi: 10.1007/978-1-4939-9748-0_6. [DOI] [PubMed] [Google Scholar]
- 551.Elguero ME, Tomazic ML, Montes MG, Florin-Christensen M, Schnittger L, Nusblat AD. 2019. The Cryptosporidium parvum gp60 glycoprotein expressed in the ciliate Tetrahymena thermophila is immunoreactive with sera of calves infected with Cryptosporidium oocysts. Vet Parasitol 271:45–50. doi: 10.1016/j.vetpar.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 552.Tomazic ML, Rodriguez AE, Lombardelli J, Poklepovich T, Garro C, Galarza R, Tiranti K, Florin-Christensen M, Schnittger L. 2018. Identification of novel vaccine candidates against cryptosporidiosis of neonatal bovines by reverse vaccinology. Vet Parasitol 264:74–78. doi: 10.1016/j.vetpar.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 553.Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, Flores J, Cryz S. 2017. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 17:843–853. doi: 10.1016/S1473-3099(17)30242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Bandyopadhyay AS, Modlin JF, Wenger J, Gast C. 2018. Immunogenicity of new primary immunization schedules with inactivated poliovirus vaccine and bivalent oral polio vaccine for the polio endgame: a review. Clin Infect Dis 67:S35–S41. doi: 10.1093/cid/ciy633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Wright PF, Hoen AG, Ilyushina NA, Brown EP, Ackerman ME, Wieland-Alter W, Connor RI, Jegaskanda S, Rosenberg-Hasson Y, Haynes BC, Luke CJ, Subbarao K, Treanor JJ. 2016. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 3:ofw108. doi: 10.1093/ofid/ofw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Larsen CP, Whitehead SS, Durbin AP. 2015. Dengue human infection models to advance dengue vaccine development. Vaccine 33:7075–7082. doi: 10.1016/j.vaccine.2015.09.052. [DOI] [PubMed] [Google Scholar]
- 557.Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Petri WA, Jr, Riddle MS, Steele AD, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC, Jr.. 2018. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 172:958–965. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Steele AD, Victor JC, Carey ME, Tate JE, Atherly DE, Pecenka C, Diaz Z, Parashar UD, Kirkwood CD. 2019. Experiences with rotavirus vaccines: can we improve rotavirus vaccine impact in developing countries? Hum Vaccin Immunother 15:1215–1227. doi: 10.1080/21645515.2018.1553593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Sadiq A, Bostan N, Yinda KC, Naseem S, Sattar S. 2018. Rotavirus: genetics, pathogenesis and vaccine advances. Rev Med Virol 28:e2003. doi: 10.1002/rmv.2003. [DOI] [PubMed] [Google Scholar]
- 560.Sharma S, Hagbom M, Svensson L, Nordgren J. 2020. The impact of human genetic polymorphisms on rotavirus susceptibility, epidemiology, and vaccine take. Viruses 12:324. doi: 10.3390/v12030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Hyser JM, Estes MK. 2009. Rotavirus vaccines and pathogenesis: 2008. Curr Opin Gastroenterol 25:36–43. doi: 10.1097/MOG.0b013e328317c897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Crawford SE, Hyser JM, Utama B, Estes MK. 2012. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc Natl Acad Sci USA 109:E3405–E3413. doi: 10.1073/pnas.1216539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Mwape I, Bosomprah S, Mwaba J, Mwila-Kazimbaya K, Laban NM, Chisenga CC, Sijumbila G, Simuyandi M, Chilengi R. 2017. Immunogenicity of rotavirus vaccine (Rotarix™) in infants with environmental enteric dysfunction. PLoS One 12:e0187761. doi: 10.1371/journal.pone.0187761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Mwila K, Chilengi R, Simuyandi M, Permar SR, Becker-Dreps S. 2017. Contribution of maternal immunity to decreased rotavirus vaccine performance in low- and middle-income countries. Clin Vaccine Immunol 24:e00405-16. doi: 10.1128/CVI.00405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Williams FB, Kader A, Colgate ER, Dickson DM, Carmolli M, Uddin MI, Sharmin S, Islam S, Bhuiyan TR, Alam M, Nayak U, Mychaleckyj JC, Petri WA, Haque R, Qadri F, Kirkpatrick BD, Lee B. 2021. Maternal secretor status affects oral rotavirus vaccine response in breastfed infants in Bangladesh. J Infect Dis 224:1147–1151. doi: 10.1093/infdis/jiaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Ahmed T. 2020. 15th Asian Conference on Diarrhoeal Disease and Nutrition, abstr PP-018. 15th Asian Conference on Diarrhoeal Disease and Nutrition, Dhaka, Bangaladesh. [Google Scholar]
- 567.Jiang B, Gentsch JR, Glass RI. 2008. Inactivated rotavirus vaccines: a priority for accelerated vaccine development. Vaccine 26:6754–5758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 568.Fix AD, Harro C, McNeal M, Dally L, Flores J, Robertson G, Boslego JW, Cryz S. 2015. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine 33:3766–3772. doi: 10.1016/j.vaccine.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 569.Talaat KR, Luke CJ, Khurana S, Manischewitz J, King LR, McMahon BA, Karron RA, Lewis KD, Qin J, Follmann DA, Golding H, Neuzil KM, Subbarao K. 2014. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis 209:1860–1869. doi: 10.1093/infdis/jiu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Rudenko L, Naykhin A, Donina S, Korenkov D, Petukhova G, Isakova-Sivak I, Losev I, Stukova M, Erofeeva M, Nikiforova A, Power M, Flores J. 2015. Assessment of immune responses to H5N1 inactivated influenza vaccine among individuals previously primed with H5N2 live attenuated influenza vaccine. Hum Vaccin Immunother 11:2839–2848. doi: 10.1080/21645515.2015.1069931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Wahid R, Pasetti MF, Maciel M, Jr, Simon JK, Tacket CO, Levine MM, Sztein MB. 2011. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans. Clin Immunol 138:187–200. doi: 10.1016/j.clim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.ClinicalTrials.gov. 2021. NCT04010448: a phase 3 double-blind, randomized, active comparator-controlled, group-sequential, multinational trial to assess the safety, immunogenicity and efficacy of a trivalent rotavirus P2-VP8 subunit vaccine in prevention of severe rotavirus gastroenteritis in healthy infants. https://clinicaltrials.gov/ct2/show/NCT04010448.
- 573.Esona MD, Foytich K, Wang Y, Shin G, Wei G, Gentsch JR, Glass RI, Jiang B. 2010. Molecular characterization of human rotavirus vaccine strain CDC-9 during sequential passages in Vero cells. Hum Vaccin 6:10409. doi: 10.4161/hv.6.3.10409. [DOI] [PubMed] [Google Scholar]
- 574.Fix A, Kirkwood CD, Steele D, Flores J. 2020. Next-generation rotavirus vaccine developers meeting: summary of a meeting sponsored by PATH and the Bill and Melinda Gates Foundation (19–20 June 2019, Geneva). Vaccine 38:8247–8254. doi: 10.1016/j.vaccine.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 575.Groome MJ, Fairlie L, Morrison J, Fix A, Koen A, Masenya M, Jose L, Madhi SA, Page N, McNeal M, Dally L, Cho I, Power M, Flores J, Cryz S. 2020. Safety and immunogenicity of a parenteral trivalent P2-VP8 subunit rotavirus vaccine: a multisite, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 20:851–863. doi: 10.1016/S1473-3099(20)30001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Chilengi R, Simuyandi M, Chibuye M, Chirwa M, Sukwa N, Laban N, Chisenga C, Silwamba S, Grassly N, Bosomprah S. 2020. A pilot study on use of live attenuated rotavirus vaccine (Rotarix™) as an infection challenge model. Vaccine 38:7357–7362. doi: 10.1016/j.vaccine.2020.09.023. [DOI] [PubMed] [Google Scholar]
- 577.Global Polio Eradication Initiative. 2020. Endemic countries. Global Polio Eradication Initiative, Geneva, Switzerland. https://polioeradication.org/where-we-work/polio-endemic-countries/. [Google Scholar]
- 578.World Health Organization. 2021. Global wild poliovirus 2016–2021. WHO, Geneva, Switzerland. https://polioeradication.org/wp-content/uploads/2021/10/weekly-polio-analyses-WPV-20211012.pdf. [Google Scholar]
- 579.World Health Organization. 2019. Poliomyelitis: key facts. WHO, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/poliomyelitis. [Google Scholar]
- 580.Garon J, Seib K, Orenstein WA, Ramirez Gonzalez A, Chang Blanc D, Zaffran M, Patel M. 2016. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines 15:693–708. doi: 10.1586/14760584.2016.1140041. [DOI] [PubMed] [Google Scholar]
- 581.Collett MS, Neyts J, Modlin JF. 2008. A case for developing antiviral drugs against polio. Antiviral Res 79:179–187. doi: 10.1016/j.antiviral.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 582.De Coster I, Leroux-Roels I, Bandyopadhyay AS, Gast C, Withanage K, Steenackers K, De Smedt P, Aerssens A, Leroux-Roels G, Oberste MS, Konopka-Anstadt JL, Weldon WC, Fix A, Konz J, Wahid R, Modlin J, Clemens R, Costa Clemens SA, Bachtiar NS, Van Damme P. 2021. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials. Lancet 397:39–50. doi: 10.1016/S0140-6736(20)32541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Sáez-Llorens X, Bandyopadhyay AS, Gast C, Leon T, DeAntonio R, Jimeno J, Caballero MI, Aguirre G, Oberste MS, Weldon WC, Konopka-Anstadt JL, Modlin J, Bachtiar NS, Fix A, Konz J, Clemens R, Costa Clemens SA, Rüttimann R. 2021. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials. Lancet 397:27–38. doi: 10.1016/S0140-6736(20)32540-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Ciapponi A, Bardach A, Rey Ares L, Glujovsky D, Cafferata ML, Cesaroni S, Bhatti A. 2019. Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis. Cochrane Database Syst Rev 12:CD011260. doi: 10.1002/14651858.CD011260.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Van Damme P, De Coster I, Bandyopadhyay AS, Revets H, Withanage K, De Smedt P, Suykens L, Oberste MS, Weldon WC, Costa-Clemens SA, Clemens R, Modlin J, Weiner AJ, Macadam AJ, Andino R, Kew OM, Konopka-Anstadt JL, Burns CC, Konz J, Wahid R, Gast C. 2019. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 394:148–158. doi: 10.1016/S0140-6736(19)31279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Van Damme P, Coster I, Bandyopadhyay AS, Suykens L, Rudelsheim P, Neels P, Oberste MS, Weldon WC, Clemens R, Revets H. 2019. Poliopolis: pushing boundaries of scientific innovations for disease eradication. Future Microbiol 14:1321–1330. doi: 10.2217/fmb-2019-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.World Health Organization. 2020. First ever vaccine listed under WHO emergency use. WHO, Geneva, Switzerland. https://www.who.int/news/item/13-11-2020-first-ever-vaccine-listed-under-who-emergency-use. [Google Scholar]
- 588.Global Polio Eradication Initiative. 2020. Novel oral polio vaccine type 2 (nOPV2) granted EUL recommendation. GPEI, Geneva, Switzerland. https://polioeradication.org/news-post/novel-oral-polio-vaccine-type-2-nopv2-granted-interim-emergency-use-listing-recommendation/. [Google Scholar]
- 589.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, Khan A, Rahman SIA, Khanam F, Lundgren A, Wiklund G, Kaim J, Löfstrand M, Carlin N, Bourgeois AL, Maier N, Fix A, Wierzba T, Walker RI, Svennerholm A-M. 2020. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis 20:208–229. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Harro C, Louis Bourgeois A, Sack D, Walker R, DeNearing B, Brubaker J, Maier N, Fix A, Dally L, Chakraborty S, Clements JD, Saunders I, Darsley MJ. 2019. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 37:1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Clements JD, Norton EB. 2018. The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere 3:e00215-18. doi: 10.1128/mSphere.00215-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Norton EB, Bauer DL, Weldon WC, Oberste MS, Lawson LB, Clements JD. 2015. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine 33:1909–1915. doi: 10.1016/j.vaccine.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 593.Lee T, Gutiérrez RL, Maciel M, Poole S, Testa KJ, Trop S, Duplessis C, Lane A, Riddle MS, Hamer M, Alcala A, Prouty M, Maier N, Erdem R, Louis Bourgeois A, Porter CK. 2021. Safety and immunogenicity of intramuscularly administered CS6 subunit vaccine with a modified heat-labile enterotoxin from enterotoxigenic Escherichia coli. Vaccine 39:5548–5556. doi: 10.1016/j.vaccine.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.ClinicalTrials.gov. 2021. NCT04232943: a phase 1 randomized study to examine the safety, tolerability, and immunogenicity of inactivated poliovirus vaccine (IPV) with or without E. coli double mutant heat labile toxin (dmLT) and impact on poliovirus shedding post-bOPV challenge in healthy IPV-primed adult subjects. https://clinicaltrials.gov/ct2/show/NCT04232943.
- 595.Collett MS, Hincks JR, Benschop K, Duizer E, van der Avoort H, Rhoden E, Liu H, Oberste MS, McKinlay MA, Hartford M. 2017. Antiviral activity of pocapavir in a randomized, blinded, placebo-controlled human oral poliovirus vaccine challenge model. J Infect Dis 215:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Brickley EB, Connor RI, Wieland-Alter WF, Collett MS, Hartford M, Van Der Avoort H, Boesch AW, Weiner JA, Ackerman ME, McKinlay MA, Arita M, Bandyopadhyay AS, Modlin JF, Wright PF. 2019. Intestinal antibody responses to a live oral poliovirus vaccine challenge among adults previously immunized with inactivated polio vaccine in Sweden. BMJ Glob Health 4:e001613. doi: 10.1136/bmjgh-2019-001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Copelyn J, Hincks JR, Wilmshurst JM, Petersen W, Howard W, Jallow S, Moonsamy S, Seakamela L, Suchard M, Collett MS, Eley B. 2020. Clearance of immunodeficiency-associated vaccine-derived poliovirus infection with pocapavir. Pediatr Infect Dis J 39:435–437. doi: 10.1097/INF.0000000000002584. [DOI] [PubMed] [Google Scholar]
- 598.World Health Organization. 2017. Up to 650 000 people die of respiratory diseases linked to seasonal flu each year. WHO, Geneva, Switzerland. https://www.who.int/news-room/detail/14-12-2017-up-to-650-000-people-die-of-respiratory-diseases-linked-to-seasonal-flu-each-year. [Google Scholar]
- 599.Centers for Disease Control and Prevention. 2019. Past pandemics (history and resources). CDC, Atlanta, GA. https://www.cdc.gov/flu/pandemic-resources/1918-pandemic-h1n1.html. [Google Scholar]
- 600.Centers for Disease Control and Prevention. 2013. Estimated influenza illnesses and hospitalizations averted by influenza vaccination: United States, 2012–13 influenza season. Morb Mortal Wkly Rep 62:997–1000. [PMC free article] [PubMed] [Google Scholar]
- 601.Pica N, Palese P. 2013. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med 64:189–202. doi: 10.1146/annurev-med-120611-145115. [DOI] [PubMed] [Google Scholar]
- 602.Taubenberger JK, Kash JC. 2010. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 7:440–451. doi: 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.World Health Organization. 2020. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2022. WHO, Geneva, Switzerland. https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2022-13-may-2022. [Google Scholar]
- 604.Oxford JS. 2013. Towards a universal influenza vaccine: volunteer virus challenge studies in quarantine to speed the development and subsequent licensing. Br J Clin Pharmacol 76:210–216. doi: 10.1111/bcp.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Quan C, Shi W, Yang Y, Yang Y, Liu X, Xu W, Li H, Li J, Wang Q, Tong Z, Wong G, Zhang C, Ma S, Ma Z, Fu G, Zhang Z, Huang Y, Song H, Yang L, Liu WJ, Liu Y, Liu W, Gao GF, Bi Y. 2020. New threats from H7N9 influenza virus: spread and evolution of high- and low-pathogenicity variants with high genomic diversity in wave five. J Virol 92:e00301-18. doi: 10.1128/JVI.00301-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Fiore AE, Bridges CB, Katz JM, Cox NJ. 2012. Inactivated influenza vaccines, p 257–293. In Plotkin SA, Orenstein W, Offit P (ed), Vaccines, 6th ed. Elsevier Saunders, Philadelphia, PA. [Google Scholar]
- 607.Chanock RM, Murphy BR, Collins PL, Coelingh KV, Olmsted RA, Snyder MH, Spriggs MK, Prince GA, Moss B, Flores J, Gorziglia M, Kapikian AZ. 1988. Live viral vaccines for respiratory and enteric tract diseases. Vaccine 6:129–133. doi: 10.1016/S0264-410X(88)80014-8. [DOI] [PubMed] [Google Scholar]
- 608.. Carter NJ, Curran MP. 2011. Live attenuated influenza vaccine (FluMist; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 71:1591–1622. doi: 10.2165/11206860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 609.Rudenko LG, Slepushkin AN, Monto AS, Kendal AP, Grigorieva EP, Burtseva EP, Rekstin AR, Beljaev AL, Bragina VE, Cox N. 1993. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. J Infect Dis 168:881–887. doi: 10.1093/infdis/168.4.881. [DOI] [PubMed] [Google Scholar]
- 610.Rudenko L, Yeolekar L, Kiseleva I, Isakova-Sivak I. 2016. Development and approval of live attenuated influenza vaccines based on Russian master donor viruses: process challenges and success stories. Vaccine 34:5436–5441. doi: 10.1016/j.vaccine.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Lewis KDC, Ortiz JR, Rahman MZ, Levine MZ, Rudenko L, Wright PF, Katz JM, Dally L, Rahman M, Isakova-Sivak I, Ilyushina NA, Matyushenko V, Fry AM, Lindstrom SE, Bresee JS, Brooks WA, Neuzil KM. 2019. Immunogenicity and viral shedding of Russian-backbone, seasonal, trivalent, live, attenuated influenza vaccine in a phase II, randomized, placebo-controlled trial among preschool-aged children in urban Bangladesh. Clin Infect Dis 69:777–785. doi: 10.1093/cid/ciy1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Kulkarni PS, Agarkhedkar S, Lalwani S, Bavdekar AR, Jog S, Raut SK, Parulekar V, Agarkhedkar SS, Palkar S, Mangrule S. 2014. Effectiveness of an Indian-made attenuated influenza A(H1N1)pdm 2009 vaccine: a case control study. Hum Vaccin Immunother 10:566–571. doi: 10.4161/hv.27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Surichan S, Wirachwong P, Supachaturas W, Utid K, Theerasurakarn S, Langsanam P, Lakornrach P, Nitisaporn L, Chansikkakorn C, Vangkanonta W, Kaweepornpoj R, Poopipatpol K, Thirapakpoomanunt S, Srichainak S, Artavatkun W, Chokevivat V, Wibulpolprasert S. 2011. Development of influenza vaccine production capacity by the Government Pharmaceutical Organization of Thailand: addressing the threat of an influenza pandemic. Vaccine 29(Suppl 1):A29–A33. doi: 10.1016/j.vaccine.2011.04.120. [DOI] [PubMed] [Google Scholar]
- 614.Pitisuttithum P, Boonnak K, Chamnanchanunt S, Puthavathana P, Luvira V, Lerdsamran H, Kaewkungwal J, Lawpoolsri S, Thanachartwet V, Silachamroon U, Masamae W, Schuetz A, Wirachwong P, Thirapakpoomanunt S, Rudenko L, Sparrow E, Friede M, Kieny MP. 2017. Safety and immunogenicity of a live attenuated influenza H5 candidate vaccine strain A/17/Turkey/Turkey/05/133 H5N2 and its priming effects for potential pre-pandemic use: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 17:833–842. doi: 10.1016/S1473-3099(17)30240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Rudenko L, Naykhin A, Donina S, Korenkov D, Petukhova G, Isakova-Sivak I, Losev I, Stukova M, Erofeeva M, Nikiforova A, Power M, Flores J. 2015. Assessment of immune responses to H5N1 inactivated influenza vaccine among individuals previously primed with H5N2 live attenuated influenza vaccine. Hum Vaccin Immunother 11:2839–2848. doi: 10.1080/21645515.2015.1069931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Isakova-Sivak I, Grigorieva E, Rudenko L. 2020. Insights into current clinical research on the immunogenicity of live attenuated influenza vaccines. Expert Rev Vaccines 19:43–55. doi: 10.1080/14760584.2020.1711056. [DOI] [PubMed] [Google Scholar]
- 617.Stohr K, Bucher D, Colgate T, Wood J. 2012. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol Biol 865:147–162. doi: 10.1007/978-1-61779-621-0_9. [DOI] [PubMed] [Google Scholar]
- 618.Weir JP, Gruber MF. 2016. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses 10:354–360. doi: 10.1111/irv.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.World Health Organization. 2020. WHO prequalified vaccines. World Health Organization, Geneva, Switzerland. https://extranet.who.int/gavi/PQ_Web/Default.aspx?nav=2WHO%20link%20[23]%20WHO%20link. [Google Scholar]
- 620.Tregoning JS, Russell RF, Kinnear E. 2018. Adjuvanted influenza vaccines. Hum Vaccin Immunother 14:550–564. doi: 10.1080/21645515.2017.1415684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Pérez Rubio A, Eiros JM. 2018. Cell culture-derived flu vaccine: present and future. Hum Vaccin Immunother 14:1874–1882. doi: 10.1080/21645515.2018.1460297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, Holmes S. 2010. Clinical efficacy of cell culture-derived and egg‐derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis 51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 623.Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, MaCurdy T, Forshee R. 2019. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis 220:1255–1264. doi: 10.1093/infdis/jiy716. [DOI] [PubMed] [Google Scholar]
- 624.Cox MM, Patriarca PA, Treanor J. 2008. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir Viruses 2:211–219. doi: 10.1111/j.1750-2659.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Dunkle LM, Izikson R, Patriarca P, Goldenthal KL, Muse D, Callahan J, Cox MMJ, PSC12 Study Team . 2017. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 376:2427–2436. doi: 10.1056/NEJMoa1608862. [DOI] [PubMed] [Google Scholar]
- 626.Wang Y, Deng L, Kang SM, Wang BZ. 2018. Universal influenza vaccines: from viruses to nanoparticles. Expert Rev Vaccines 17:967–976. doi: 10.1080/14760584.2018.1541408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Shinde V, Fries L, Wu Y, Agrawal S, Cho I, Thomas DN, Spindler M, Lindner E, Hahn T, Plested J, Flyer D, Massare MJ, Zhou B, Fix A, Smith G, Glenn GM. 2018. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 378:2346–2348. doi: 10.1056/NEJMc1803554. [DOI] [PubMed] [Google Scholar]
- 628.Novavax. 2020. Novavax’ NanoFlu achieves all primary endpoints in phase 3 clinical trial. https://ir.novavax.com/2020-03-24-Novavax-NanoFlu-Achieves-All-Primary-Endpoints-In-Phase-3-Clinical-Trial.
- 629.US Food and Drug Administration (FDA), Center for Biologics Evaluation and Research. 2007. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. FDA, Rockville, MD. https://www.fda.gov/media/73706/download. [Google Scholar]
- 630.Lamb YN. 2019. Cell-based quadrivalent inactivated influenza virus vaccine (Flucelvax Tetra/Flucelvax Quadrivalent): a review in the prevention of influenza. Drugs 79:1337–1348. doi: 10.1007/s40265-019-01176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.World Health Organization. 2020. WHO prequalified vaccines. World Health Organization, Geneva, Switzerland. https://extranet.who.int/gavi/PQ_Web/Default.aspx?nav=2. [Google Scholar]
- 632.Wang W, Vassell R, Song HS, Chen Q, Keller PW, Verma S, Alvarado-Facundo E, Wan H, Schmeisser F, Meseda CA, Weir JP, Weiss CD. 2019. Generation of a protective murine monoclonal antibody against the stem of influenza hemagglutinins from group 1 viruses and identification of resistance mutations against it. PLoS One 14:e0222436. doi: 10.1371/journal.pone.0222436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Krammer F, Palese P. 2019. Universal influenza virus vaccines that target the conserved hemagglutinin stalk and conserved sites in the head domain. J Infect Dis 219:S62–S67. doi: 10.1093/infdis/jiy711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, Edupuganti S, Spearman P, Andrews SF, Wilson PC, García-Sastre A, Mulligan MJ, Mehta AK, Palese P, Ahmed R. 2014. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci USA 111:13133–13138. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Jagadesh A, Salam AA, Mudgal PP, Arunkumar G. 2016. Influenza virus neuraminidase (NA): a target for antivirals and vaccines. Arch Virol 161:2087–2094. doi: 10.1007/s00705-016-2907-7. [DOI] [PubMed] [Google Scholar]
- 636.Mezhenskaya D, Isakova-Sivak I, Rudenko L. 2019. M2e-based universal influenza vaccines: a historical overview and new approaches to development. J Biomed Sci 26:76. doi: 10.1186/s12929-019-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.van Doorn E, Liu H, Ben-Yedidia T, Hassin S, Visontai I, Norley S, Frijlink HW, Hak E. 2017. Evaluating the immunogenicity and safety of a BiondVax-developed universal influenza vaccine (Multimeric-001) either as a standalone vaccine or as a primer to H5N1 influenza vaccine: phase IIb study protocol. Medicine (Baltimore, MD) 96:e6339. doi: 10.1097/MD.0000000000006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Darricarrère N, Pougatcheva S, Duan X, Rudicell RS, Chou TH, DiNapoli J, Ross TM, Alefantis T, Vogel TU, Kleanthous H, Wei CJ, Nabel GJ. 2018. Development of a pan-H1 influenza vaccine. J Virol 92:e01349-18. doi: 10.1128/JVI.01349-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong WP, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS. 2019. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol 20:362–372. doi: 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Dhakal S, Cheng X, Salcido J, Renu S, Bondra K, Lakshmanappa YS, Misch C, Ghimire S, Feliciano-Ruiz N, Hogshead B, Krakowka S, Carson K, McDonough J, Lee CW, Renukaradhya GJ. 2018. Liposomal nanoparticle-based conserved peptide influenza vaccine and monosodium urate crystal adjuvant elicit protective immune response in pigs. Int J Nanomedicine (Lond) 13:6699–6715. doi: 10.2147/IJN.S178809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, Hamill M, Peng Y, Blais ME, Duncan CJ, Sheehy SH, Havelock T, Faust SN, Williams RL, Gilbert A, Oxford J, Dong T, Hill AV, Gilbert SC. 2012. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Folegatti PM, Bellamy D, Flaxman A, Mair C, Ellis C, Ramon RL, Ramos Lopez F, Mitton C, Baker M, Poulton I, Lawrie A, Roberts R, Minassian A, Ewer KJ, Evans TG, Hill AVS, Gilbert SC. 2019. Safety and immunogenicity of the heterosubtypic influenza A vaccine MVA-NP+M1 manufactured on the AGE1.CR.pIX avian cell line. Vaccines 7:33. doi: 10.3390/vaccines7010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, Lambe T, Gilbert SC. 2014. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol Ther 22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Antrobus RD, Berthoud TK, Mullarkey CE, Hoschler K, Coughlan L, Zambon M, Hill AV, Gilbert SC. 2014. Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol Ther 22:233–238. doi: 10.1038/mt.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Reperant LA, Rimmelzwaan GF, Osterhaus AD. 2014. Advances in influenza vaccination. F1000Prime Rep 6:47. doi: 10.12703/P6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Tripp RA, Tompkins SM. 2014. Virus-vectored influenza virus vaccines. Viruses 6:3055–3079. doi: 10.3390/v6083055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H, Reece ST, Sahin U, Tregoning JS. 2018. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther 26:446–455. doi: 10.1016/j.ymthe.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Smorodintseff AA, Tushinsky MD, Drobyshevskaya AI, Korovin AA, Osetroff AI. 1937. Investigation on volunteers infected with the influenza virus. Am J Med Sci 194:159–170. doi: 10.1097/00000441-193708000-00002. [DOI] [Google Scholar]
- 649.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, Valleron AJ. 2008. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 167:775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 650.Sherman AC, Mehta A, Dickert NW, Anderson EJ, Rouphael N. 2019. The future of flu: a review of the human challenge model and systems biology for advancement of influenza vaccinology. Front Cell Infect Microbiol 9:107. doi: 10.3389/fcimb.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Memoli MJ, Han A, Walters KA, Czajkowski L, Reed S, Athota R, Angela Rosas L, Cervantes-Medina A, Park JK, Morens DM, Kash JC, Taubenberger JK. 2020. Influenza A reinfection in sequential human challenge: implications for protective immunity and “universal” vaccine development. Clin Infect Dis 70:748–753. doi: 10.1093/cid/ciz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Sobel Leonard A, McClain MT, Smith GJ, Wentworth DE, Halpin RA, Lin X, Ransier A, Stockwell TB, Das SR, Gilbert AS, Lambkin-Williams R, Ginsburg GS, Woods CW, Koelle K. 2016. Deep sequencing of influenza A virus from a human challenge study reveals a selective bottleneck and only limited intrahost genetic diversification. J Virol 90:11247–11258. doi: 10.1128/JVI.01657-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Xiao Y, Park JK, Williams S, Ramuta M, Cervantes-Medina A, Bristol T, Smith S, Czajkowski L, Han A, Kash JC, Memoli MJ, Taubenberger JK. 2019. Deep sequencing of 2009 influenza A/H1N1 virus isolated from volunteer human challenge study participants and natural infections. Virology 534:96–107. doi: 10.1016/j.virol.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.McClain MT, Henao R, Williams J, Nicholson B, Veldman T, Hudson L, Tsalik EL, Lambkin-Williams R, Gilbert A, Mann A, Ginsburg GS, Woods CW. 2016. Differential evolution of peripheral cytokine levels in symptomatic and asymptomatic responses to experimental influenza virus challenge. Clin Exp Immunol 183:441–451. doi: 10.1111/cei.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Woods CW, McClain MT, Chen M, Zaas AK, Nicholson BP, Varkey J, Veldman T, Kingsmore SF, Huang Y, Lambkin-Williams R, Gilbert AG, Hero AO, 3rd, Ramsburg E, Glickman S, Lucas JE, Carin L, Ginsburg GS. 2013. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS One 8:e52198. doi: 10.1371/journal.pone.0052198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Killingley B, Enstone J, Booy R, Hayward A, Oxford J, Ferguson N, Nguyen Van-Tam J, Influenza Transmission Strategy Development Group . 2011. Potential role of human challenge studies for investigation of influenza transmission. Lancet Infect Dis 11:879–886. doi: 10.1016/S1473-3099(11)70142-6. [DOI] [PubMed] [Google Scholar]
- 657.Killingley B, Enstone JE, Greatorex J, Gilbert AS, Lambkin-Williams R, Cauchemez S, Katz JM, Booy R, Hayward A, Oxford J, Bridges CB, Ferguson NM, Nguyen Van-Tam JS. 2012. Use of a human influenza challenge model to assess person-to-person transmission: proof-of-concept study. J Infect Dis 205:35–43. doi: 10.1093/infdis/jir701. [DOI] [PubMed] [Google Scholar]
- 658.Huang KY, Li CK, Clutterbuck E, Chui C, Wilkinson T, Gilbert A, Oxford J, Lambkin-Williams R, Lin TY, McMichael AJ, Xu XN. 2014. Virus-specific antibody secreting cell, memory B-cell, and sero-antibody responses in the human influenza challenge model. J Infect Dis 209:1354–1361. doi: 10.1093/infdis/jit650. [DOI] [PubMed] [Google Scholar]
- 659.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. 2012. Preexisting influenza-specific CD4 T cells correlate with disease protection against influenza challenge in humans. Nat Med 18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 660.Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT, Jr, Taubenberger JK. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7:e00417-16. doi: 10.1128/mBio.00417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Gould VMW, Francis JN, Anderson KJ, Georges B, Cope AV, Tregoning JS. 2017. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front Microbiol 8:900. doi: 10.3389/fmicb.2017.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Park JK, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Rosas LA, Cervantes-Medina A, Taubenberger JK, Memoli MJ. 2018. Evaluation of preexisting anti-hemagglutinin stalk antibody as a correlate of protection in a healthy volunteer challenge with influenza A/H1N1pdm virus. mBio 9:e02284-17. doi: 10.1128/mBio.02284-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Jegaskanda S, Luke C, Hickman HD, Sangster MY, Wieland-Alter WF, McBride JM, Yewdell JW, Wright PF, Treanor J, Rosenberger CM, Subbarao K. 2016. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J Infect Dis 214:945–952. doi: 10.1093/infdis/jiw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Wright PF, Hoen AG, Ilyushina NA, Brown EP, Ackerman ME, Wieland-Alter W, Connor RI, Jegaskanda S, Rosenberg-Hasson Y, Haynes BC, Luke CJ, Subbarao K, Treanor JJ. 2016. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 3:ofw108. doi: 10.1093/ofid/ofw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.McBride JM, Lim JJ, Burgess T, Deng R, Derby MA, Maia M, Horn P, Siddiqui O, Sheinson D, Chen-Harris H, Newton EM, Fillos D, Nazzal D, Rosenberger CM, Ohlson MB, Lambkin-Williams R, Fathi H, Harris JM, Tavel JA. 2017. Phase 2 randomized trial of the safety and efficacy of MHAA4549A, a broadly neutralizing monoclonal antibody, in a human influenza A virus challenge model. Antimicrob Agents Chemother 61:e01154-17. doi: 10.1128/AAC.01154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Memoli MJ, Czajkowski L, Reed S, Athota R, Bristol T, Proudfoot K, Fargis S, Stein M, Dunfee RL, Shaw PA, Davey RT, Taubenberger JK. 2015. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin Infect Dis 60:693–702. doi: 10.1093/cid/ciu924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Watson JM, Francis JN, Mesens S, Faiman GA, Makin J, Patriarca P, Treanor JJ, Georges B, Bunce CJ. 2015. Characterization of a wild-type influenza (A/H1N1) virus strain as an experimental challenge agent in humans. Virol J 12:13. doi: 10.1186/s12985-015-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Han A, Czajkowski LM, Donaldson A, Baus HA, Reed SM, Athota RS, Bristol T, Rosas LA, Cervantes-Medina A, Taubenberger JK, Memoli MJ. 2019. A dose-finding study of a wild-type influenza A(H3N2) virus in a healthy volunteer human challenge model. Clin Infect Dis 69:2082–2090. doi: 10.1093/cid/ciz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Fullen DJ, Noulin N, Catchpole A, Fathi H, Murray EJ, Mann A, Eze K, Balaratnam G, Borley DW, Gilbert A, Lambkin-Williams R. 2016. Accelerating influenza research: vaccines, antivirals, immunomodulators and monoclonal antibodies. The manufacture of a new wild-type H3N2 virus for the human viral challenge model. PLoS One 11:e0145902. doi: 10.1371/journal.pone.0145902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Fry AM, Zhong W, Gubareva LV. 2015. Advancing treatment options for influenza: challenges with the human influenza challenge. J Infect Dis 211:1033–1035. doi: 10.1093/infdis/jiu543. [DOI] [PubMed] [Google Scholar]
- 671.Ramos EL, Mitcham JL, Koller TD, Bonavia A, Usner DW, Balaratnam G, Fredlund P, Swiderek KM. 2015. Efficacy and safety of treatment with an anti-M2e monoclonal antibody in experimental human influenza. J Infect Dis 211:1038–1044. doi: 10.1093/infdis/jiu539. [DOI] [PubMed] [Google Scholar]
- 672.Hayden FG, Treanor JJ, Betts RF, Lobo M, Esinhart JD, Hussey EK. 1996. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA 275:295–299. doi: 10.1001/jama.1996.03530280047035. [DOI] [PubMed] [Google Scholar]
- 673.Lambkin-Williams R, Noulin N, Mann A, Catchpole A, Gilbert AS. 2018. The human viral challenge model: accelerating the evaluation of respiratory antivirals, vaccines and novel diagnostics. Respir Res 19:123. doi: 10.1186/s12931-018-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Lambkin-Williams R, Gelder C, Broughton R, Mallett CP, Gilbert AS, Mann A, He D, Oxford JS, Burt D. 2016. An intranasal proteosome-adjuvanted trivalent influenza vaccine is safe, immunogenic and efficacious in the human viral influenza challenge model. Serum IgG and mucosal IgA are important correlates of protection against illness associated with infection. PLoS One 11:e0163089. doi: 10.1371/journal.pone.0163089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.van Doorn E, Pleguezuelos O, Liu H, Fernandez A, Bannister R, Stoloff G, Oftung F, Norley S, Huckriede A, Frijlink HW, Hak E. 2017. Evaluation of the immunogenicity and safety of different doses and formulations of a broad spectrum influenza vaccine (FLU-v) developed by SEEK: study protocol for a single-center, randomized, double-blind and placebo-controlled clinical phase IIb trial. BMC Infect Dis 17:241. doi: 10.1186/s12879-017-2341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Powell TJ, Peng Y, Berthoud TK, Blais ME, Lillie PJ, Hill AV, Rowland-Jones SL, McMichael AJ, Gilbert SC, Dong T. 2013. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP+M1 vaccine. PLoS One 8:e62778. doi: 10.1371/journal.pone.0062778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Jung HE, Kim TH, Lee HK. 2020. Contribution of dendritic cells in protective immunity against respiratory syncytial virus infection. Viruses 12:102. doi: 10.3390/v12010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Sullender WM. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin Microbiol Rev 13:1–15. doi: 10.1128/CMR.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Iwane MK, Farnon EC, Gerber SI. 2013. Importance of global surveillance for respiratory syncytial virus. J Infect Dis 208(Suppl 3):S165–S166. doi: 10.1093/infdis/jit484. [DOI] [PubMed] [Google Scholar]
- 681.Tan L, Lemey P, Houspie L, Viveen MC, Jansen NJ, van Loon AM, Wiertz E, van Bleek GM, Martin DP, Coenjaerts FE. 2012. Genetic variability among complete human respiratory syncytial virus subgroup A genomes: bridging molecular evolutionary dynamics and epidemiology. PLoS One 7:e51439. doi: 10.1371/journal.pone.0051439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. 2014. Immunity to RSV in early-life. Front Immunol 5:466. doi: 10.3389/fimmu.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Hall CB, Walsh EE, Long CE, Schnabel KC. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 684.Falsey AR, Walsh EE. 2000. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 13:371–384. doi: 10.1128/CMR.13.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Brealey JC, Chappell KJ, Galbraith S, Fantino E, Gaydon J, Tozer S, Young PR, Holt PG, Sly PD. 2018. Streptococcus pneumoniae colonization of the nasopharynx is associated with increased severity during respiratory syncytial virus infection in young children. Respirology 23:220–227. doi: 10.1111/resp.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Valley-Omar Z, Tempia S, Hellferscee O, Walaza S, Variava E, Dawood H, Kahn K, McMorrow M, Pretorius M, Mtshali S, Mamorobela E, Wolter N, Venter M, von Gottberg A, Cohen C, Treurnicht FK. 2022. Human respiratory syncytial virus diversity and epidemiology among patients hospitalized with severe respiratory illness in South Africa, 2012–2015. Influenza Other Respir Viruses 16:222–235. doi: 10.1111/irv.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Caballero MT, Bianchi AM, Grigaites SD, De la Iglesia Niveyro PX, Nuño A, Valle S, Afarian G, Esperante SA, Ferretti AJP, Jares Baglivo S, De Luca J, Alvarez-Paggi D, Diamanti A, Bassat Q, Polack FP, RSV Mortality Network . 2021. Community mortality due to respiratory syncytial virus in Argentina: population-based surveillance study. Clin Infect Dis 73:S210–S217. doi: 10.1093/cid/ciab497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Pacheco GA, Gálvez NMS, Soto JA, Andrade CA, Kalergis AM. 2021. Bacterial and viral coinfections with the human respiratory syncytial virus. Microorganisms 9:1293. doi: 10.3390/microorganisms9061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 690.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 691.Openshaw PJ, Chiu C. 2013. Protective and dysregulated T cell immunity in RSV infection. Curr Opin Virol 3:468–474. doi: 10.1016/j.coviro.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O’Shea AF, Gruber WC, Murphy BR. 2007. The absence of enhanced disease with wild-type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, Wittek AE, Casey R, Eichelberger M, Thumar B, Randolph VB, Udem SA, Chanock RM, Murphy BR. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 694.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, Gruber W, Murphy BR, Collins PL. 2005. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis 191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 695.Buchholz UJ, Cunningham CK, Muresan P, Gnanashanmugam D, Sato P, Siberry GK, Rexroad V, Valentine M, Perlowski C, Schappell E, Thumar B, Luongo C, Barr E, Aziz M, Yogev R, Spector SA, Collins PL, McFarland EJ, Karron RA, International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1114 Study Team . 2018. Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis 217:1338–1346. doi: 10.1093/infdis/jiy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.McFarland EJ, Karron RA, Muresan P, Cunningham CK, Valentine ME, Perlowski C, Thumar B, Gnanashanmugam D, Siberry GK, Schappell E, Barr E, Rexroad V, Yogev R, Spector SA, Aziz M, Patel N, Cielo M, Luongo C, Collins PL, Buchholz UJ, International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 2000 Study Team . 2018. Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis 217:1347–1355. doi: 10.1093/infdis/jiy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Karron RA, Luongo C, Mateo JS, Wanionek K, Collins PL, Buchholz UJ. 2020. Safety and immunogenicity of the respiratory syncytial virus vaccine RSV/ΔNS2/Δ1313/I1314L in RSV-seronegative children. J Infect Dis 222:82–91. doi: 10.1093/infdis/jiz408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.McFarland EJ, Karron RA, Muresan P, Cunningham CK, Perlowski C, Libous J, Oliva J, Jean-Philippe P, Moye J, Schappell E, Barr E, Rexroad V, Fearn L, Cielo M, Wiznia A, Deville JG, Yang L, Luongo C, Collins PL, Buchholz UJ. 2020. Live-attenuated respiratory syncytial virus vaccine with M2-2 deletion and with small hydrophobic noncoding region is highly immunogenic in children. J Infect Dis 221:2050–2059. doi: 10.1093/infdis/jiaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Rigter A, Widjaja I, Versantvoort H, Coenjaerts FE, van Roosmalen M, Leenhouts K, Rottier PJ, Haijema BJ, de Haan CA. 2013. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS One 8:e71072. doi: 10.1371/journal.pone.0071072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Swanson KA, Balabanis K, Xie Y, Aggarwal Y, Palomo C, Mas V, Metrick C, Yang H, Shaw CA, Melero JA, Dormitzer PR, Carfi A. 2014. A monomeric uncleaved respiratory syncytial virus F antigen retains prefusion-specific neutralizing epitopes. J Virol 88:11802–11810. doi: 10.1128/JVI.01225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, Zhou B, Lu H, Boddapati S, Li J, Flyer D, Glenn G. 2012. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One 7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA 108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Leroux-Roels G, De Boever F, Maes C, Nguyen TL, Baker S, Gonzalez Lopez A. 2019. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 37:2694–2703. doi: 10.1016/j.vaccine.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 705.Jares Baglivo S, Polack FP. 2019. The long road to protect infants against severe RSV lower respiratory tract illness. F1000Res 8:F1000. doi: 10.12688/f1000research.18749.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Schwarz TF, McPhee RA, Launay O, Leroux-Roels G, Talli J, Picciolato M, Gao F, Cai R, Nguyen TL, Dieussaert I, Miller JM, Schmidt AC. 2019. Immunogenicity and safety of 3 formulations of a respiratory syncytial virus candidate vaccine in nonpregnant women: a phase 2, randomized trial. J Infect Dis 220:1816–1825. doi: 10.1093/infdis/jiz395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Pfizer, Inc. 2018. Pfizer begins a phase 1/2 study to evaluate respiratory syncytial virus (RSV) vaccine. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_begins_a_phase_1_2_study_to_evaluate_respiratory_syncytial_virus_rsv_vaccine-0.
- 708.Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, Holman LA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, Gaudinski MR, Kumar A, Chang LA, Moin SM, Hill JP, DiPiazza AT, Schwartz RM, Kueltzo L, Cooper JW, Chen P, Stein JA, Carlton K, Gall JG, Nason MC, Kwong PD, Chen GL, Mascola JR, McLellan JS, Ledgerwood JE, Graham BS, VRC 317 Study Team . 2019. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 365:505–509. doi: 10.1126/science.aav9033. [DOI] [PubMed] [Google Scholar]
- 709.Loomis RJ, Johnson PR. 2013. Gene-based vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol 372:307–324. doi: 10.1007/978-3-642-38919-1_15. [DOI] [PubMed] [Google Scholar]
- 710.Morrison TG, Walsh EE. 2013. Subunit and virus-like particle vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol 372:285–306. doi: 10.1007/978-3-642-38919-1_14. [DOI] [PubMed] [Google Scholar]
- 711.Aranda SS, Polack FP. 2019. Prevention of pediatric respiratory syncytial virus lower respiratory tract illness: perspectives for the next decade. Front Immunol 10:1006. doi: 10.3389/fimmu.2019.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Hall CB, Douglas RG, Jr, Schnabel KC, Geiman JM. 1981. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun 33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. 2004. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res 63:191–196. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 714.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, Oxford J, Pareek R, Moore R, Walsh E, Studholme R, Dorsett P, Alvarez R, Lambkin-Williams R. 2010. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Kim YI, DeVincenzo JP, Jones BG, Rudraraju R, Harrison L, Meyers R, Cehelsky J, Alvarez R, Hurwitz JL. 2014. Respiratory syncytial virus human experimental infection model: provenance, production, and sequence of low-passaged Memphis-37 challenge virus. PLoS One 9:e113100. doi: 10.1371/journal.pone.0113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. 2006. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1 T cell responses. J Immunol 176:1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- 717.DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, McBride S, Lambkin-Williams R, Jordan R, Xin Y, Ramanathan S, O’Riordan T, Lewis SA, Li X, Toback SL, Lin S-L, Chien JW. 2014. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 718.Sadoff J, De Paepe E, DeVincenzo J, Gymnopoulou E, Menten J, Murray B, Bastian AR, Vandebosch A, Haazen W, Noulin N, Comeaux C, Heijnen E, Eze K, Gilbert A, Lambkin-Williams R, Schuitemaker H, Callendret B. 2021. Prevention of respiratory syncytial virus infection in healthy adults by a single immunization of Ad26.RSV.preF in a human challenge study. J Infect Dis doi: 10.1093/infdis/jiab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, de Haan CA, Wrammert J, Openshaw PJ, Chiu C, Mechanisms of Severe Acute Influenza Consortium Investigators . 2015. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med 191:1040–1049. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EHC, Sykes A, Maybeno M, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C. 2015. RSV-specific airway resident memory CD8 T cells and differential disease severity after experimental human infection. Nat Commun 6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Guvenel A, Jozwik A, Ascough S, Ung SK, Paterson S, Kalyan M, Gardener Z, Bergstrom E, Kar S, Habibi MS, Paras A, Zhu J, Park M, Dhariwal J, Almond M, Wong EH, Sykes A, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C. 2020. Epitope-specific airway-resident CD4 T cell dynamics during experimental human RSV infection. J Clin Invest 130:523–538. doi: 10.1172/JCI131696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team . 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 723.Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H, Rudan I, Black R, Knoll MD. 2018. Burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Gilley RP, Orihuela CJ. 2014. Pneumococci in biofilms are noninvasive: implications on nasopharyngeal colonization. Front Cell Infect Microbiol 4:163. doi: 10.3389/fcimb.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. 2009. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124:e1–e11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Tan TQ. 2012. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev 25:409–419. doi: 10.1128/CMR.00018-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Vernatter J, Pirofski LA. 2013. Current concepts in host-microbe interaction leading to pneumococcal pneumonia. Curr Opin Infect Dis 26:277–283. doi: 10.1097/QCO.0b013e3283608419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Jha V, Janoff EN. 2019. Complementary role of CD4 T cells in response to pneumococcal polysaccharide vaccines in humans. Vaccines (Basel) 7:18. doi: 10.3390/vaccines7010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Rupp R, Hurley D, Grayson S, Li J, Nolan K, McFetridge RD, Hartzel J, Abeygunawardana C, Winters M, Pujar H, Benner P, Musey L. 2019. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother 15:549–559. doi: 10.1080/21645515.2019.1568159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O’Brien KL. 2010. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the Pneumococcal Global Serotype Project. PLoS Med 7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 732.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Thompson A, Lamberth E, Severs J, Scully I, Tarabar S, Ginis J, Jansen KU, Gruber WC, Scott DA, Watson W. 2019. Phase 1 trial of a 20-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 37:6201–6207. doi: 10.1016/j.vaccine.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 734.Gallagher GM. 2020. 20vPnC pneumococcal conjugate vaccine candidate shows promise in phase 3 study. https://www.contagionlive.com/news/20vpnc-pneumococcal-conjugate-vaccine-candidate-shows-promise-in-phase-3-study.
- 735.US Food and Drug Administration. 2021. Prevnar 20 product information. Food and Drug Administration, Bethesda, MD. https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20. [Google Scholar]
- 736.Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. 1977. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA 238:2613–2616. doi: 10.1001/jama.1977.03280250039019. [DOI] [PubMed] [Google Scholar]
- 737.Briles DE, Paton JC, Mukerji R, Swiatlo E, Crain MJ. 2019. Pneumococcal vaccines. Microbiol Spectr https://www.asmscience.org/content/journal/microbiolspec/10.1128/microbiolspec.GPP3-0028-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Principi N, Esposito S. 2018. Development of pneumococcal vaccines over the last 10 years. Expert Opin Biol Ther 18:7–17. doi: 10.1080/14712598.2018.1384462. [DOI] [PubMed] [Google Scholar]
- 739.Wang S, Curtiss R, III.. 2014. Development of Streptococcus pneumoniae vaccines using live vectors. Vaccines (Basel) 2:49–88. doi: 10.3390/vaccines2010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Odutola A, Ota MO, Ogundare EO, Antonio M, Owiafe P, Worwui A, Greenwood B, Alderson M, Traskine M, Verlant V, Dobbelaere K, Borys D. 2016. Reactogenicity, safety and immunogenicity of a protein-based pneumococcal vaccine in Gambian children aged 2–4 years: a phase II randomized study. Hum Vaccin Immunother 12:393–402. doi: 10.1080/21645515.2015.1111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Odutola A, Ota MOC, Antonio M, Ogundare EO, Saidu Y, Owiafe PK, Worwui A, Idoko OT, Owolabi O, Kampmann B, Greenwood BM, Alderson M, Traskine M, Swinnen K, Verlant V, Dobbelaere K, Borys D. 2019. Immunogenicity of pneumococcal conjugate vaccine formulations containing pneumococcal proteins, and immunogenicity and reactogenicity of co-administered routine vaccines: a phase II, randomised, observer-blind study in Gambian infants. Vaccine 37:2586–2599. doi: 10.1016/j.vaccine.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 742.Keech CA, Morrison R, Anderson P, Tate A, Flores J, Goldblatt D, Briles D, Hural J, Malley R, Alderson MR. 2020. A phase 1 randomized, placebo-controlled, observer-blinded trial to evaluate the safety and immunogenicity of inactivated Streptococcus pneumoniae whole-cell vaccine in adults. Pediatr Infect Dis J 39:345–351. doi: 10.1097/INF.0000000000002567. [DOI] [PubMed] [Google Scholar]
- 743.Fleming-Dutra KE, Conklin L, Loo JD, Knoll MD, Park DE, Kirk J, Goldblatt D, Whitney CG, O’Brien KL. 2014. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J 33(Suppl 2):S152–S160. doi: 10.1097/INF.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. 2013. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 32:133–145. doi: 10.1016/j.vaccine.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 745.McCool TL, Cate TR, Moy G, Weiser JN. 2002. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med 195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.McCool TL, Cate TR, Tuomanen EI, Adrian P, Mitchell TJ, Weiser JN. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect Immun 71:5724–5732. doi: 10.1128/IAI.71.10.5724-5732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Wright AK, Ferreira DM, Gritzfeld JF, Wright AD, Armitage K, Jambo KC, Bate E, El Batrawy S, Collins A, Gordon SB. 2012. Human nasal challenge with Streptococcus pneumoniae is immunising in the absence of carriage. PLoS Pathog 8:e1002622. doi: 10.1371/journal.ppat.1002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Gritzfeld JF, Wright AD, Collins AM, Pennington SH, Wright AK, Kadioglu A, Ferreira DM, Gordon SB. 2013. Experimental human pneumococcal carriage. J Vis Exp 72:50115. doi: 10.3791/50115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Ferreira DM, Neill DR, Bangert M, Gritzfeld JF, Green N, Wright AKA, Pennington SH, Bricio-Moreno L, Bricio Moreno L, Moreno AT, Miyaji EN, Wright AD, Collins AM, Goldblatt D, Kadioglu A, Gordon SB. 2013. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med 187:855–864. doi: 10.1164/rccm.201212-2277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Morton B, Burr S, Jambo K, Rylance J, Henrion MYR, Banda NP, Nsomba E, Kapumba B, Manda-Taylor L, Masesa C, Ferrreira D, Gordon SB, MARVELS Consortium . 2020. A pneumococcal controlled human infection model in Malawi: transfer of an established pneumococcal carriage model from Liverpool, UK to Blantyre, Malawi: a feasibility study. Wellcome Open Res 5:25. doi: 10.12688/wellcomeopenres.15689.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Nikolaou E, Jochems SP, Mitsi E, Pojar S, Blizard A, Reiné J, Solórzano C, Negera E, Carniel B, Soares-Schanoski A, Connor V, Adler H, Zaidi SR, Hales C, Hill H, Hyder-Wright A, Gordon SB, Rylance J, Ferreira DM. 2021. Experimental human challenge defines distinct pneumococcal kinetic profiles and mucosal responses between colonized and non-colonized adults. mBio 12:e02020-20. doi: 10.1128/mBio.02020-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Hales C, Jochems SP, Robinson R, Solórzano C, Carniel B, Pojar S, Reiné J, German EL, Nikolaou E, Mitsi E, Hyder-Wright AD, Hill H, Adler H, Connor V, Zaidi S, Lowe C, Fan X, Wang D, Gordon SB, Rylance J, Ferreira DM. 2020. Symptoms associated with influenza vaccination and experimental human pneumococcal colonization of the nasopharynx. Vaccine 38:2298–2306. doi: 10.1016/j.vaccine.2020.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Shak JR, Cremers AJ, Gritzfeld JF, de Jonge MI, Hermans PW, Vidal JE, Klugman KP, Gordon SB. 2014. Impact of experimental human pneumococcal carriage on nasopharyngeal bacterial densities in healthy adults. PLoS One 9:e98829. doi: 10.1371/journal.pone.0098829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Collins AM, Wright AD, Mitsi E, Gritzfeld JF, Hancock CA, Pennington SH, Wang D, Morton B, Ferreira DM, Gordon SB. 2015. First human challenge testing of a pneumococcal vaccine: a double-blind randomized controlled trial. Am J Respir Crit Care Med 192:853–858. doi: 10.1164/rccm.201503-0542OC. [DOI] [PubMed] [Google Scholar]
- 755.Mitsi E, Roche AM, Reiné J, Zangari T, Owugha JT, Pennington SH, Gritzfeld JF, Wright AD, Collins AM, van Selm S, de Jonge MI, Gordon SB, Weiser JN, Ferreira DM. 2017. Agglutination by anti-capsular polysaccharide antibody is associated with protection against experimental human pneumococcal carriage. Mucosal Immunol 10:385–394. doi: 10.1038/mi.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756.Esposito S, Principi N. 2015. Pneumococcal vaccines and the prevention of community-acquired pneumonia. Pulm Pharmacol Ther 32:124–129. doi: 10.1016/j.pupt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 757.Esmail H, Barry CE, III, Young DB, Wilkinson RJ. 2014. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci 369:20130437. doi: 10.1098/rstb.2013.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Ottenhoff TH, Kaufmann SH. 2012. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog 8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Gengenbacher M, Kaufmann SH. 2012. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev 36:514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Geluk A, van Meijgaarden KE, Joosten SA, Commandeur S, Ottenhoff TH. 2014. Innovative strategies to identify Mycobacterium tuberculosis antigens and epitopes using genome-wide analyses. Front Immunol 5:256. doi: 10.3389/fimmu.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Weiner J, III, Kaufmann SH. 2014. Recent advances towards tuberculosis control: vaccines and biomarkers. J Intern Med 275:467–480. doi: 10.1111/joim.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Rowland R, McShane H. 2011. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines 10:645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Hokey DA, Ginsberg A. 2013. The current state of tuberculosis vaccines. Hum Vaccin Immunother 9:2142–2146. doi: 10.4161/hv.25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Junqueira-Kipnis AP, Marques Neto LM, Kipnis A. 2014. Role of fused Mycobacterium tuberculosis immunogens and adjuvants in modern tuberculosis vaccines. Front Immunol 5:188. doi: 10.3389/fimmu.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Montagnani C, Chiappini E, Galli L, de Martino M. 2014. Vaccine against tuberculosis: what’s new? BMC Infect Dis 14:S2. doi: 10.1186/1471-2334-14-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.da Costa AC, Nogueira SV, Kipnis A, Junqueira-Kipnis AP. 2014. Recombinant BCG: innovations on an old vaccine. Scope of BCG strains and strategies to improve long-lasting memory. Front Immunol 5:152. doi: 10.3389/fimmu.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Brazier B, McShane H. 2020. Towards new TB vaccines. Semin Immunopathol 42:315–331. doi: 10.1007/s00281-020-00794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Hatherill M, White RG, Hawn TR. 2019. Clinical development of new TB vaccines: recent advances and next steps. Front Microbiol 10:3154. doi: 10.3389/fmicb.2019.03154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Kaufmann SH, Cotton MF, Eisele B, Gengenbacher M, Grode L, Hesseling AC, Walzl G. 2014. The BCG replacement vaccine VPM1002: from drawing board to clinical trial. Expert Rev Vaccines 13:619–630. doi: 10.1586/14760584.2014.905746. [DOI] [PubMed] [Google Scholar]
- 770.Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B, Johnstone H, van der Spuy G, Maertzdorf J, Kaufmann SHE, Hesseling AC, Walzl G, Cotton MF. 2017. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin Vaccine Immunol 24:e00439-16. doi: 10.1128/CVI.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.ClinicalTrials.gov. 2020. NCT02391415: phase II double-blind, randomized, controlled study to evaluate safety and immunogenicity of VPM1002 compared with BCG in HIV-exposed and HIV-unexposed, BCG-naive newborn infants. https://clinicaltrials.gov/ct2/show/NCT02391415.
- 772.ClinicalTrials.gov. 2020. NCT04351685: a multicenter, phase III, double-blind, randomized, active-controlled study to evaluate the efficacy and safety of VPM1002 in comparison to BCG in prevention of Mycobacterium tuberculosis infection in newborn infants. https://clinicaltrials.gov/ct2/show/NCT04351685.
- 773.ClinicalTrials.gov. 2021. NCT03152903: a multicenter phase II/III double-blind, randomized, placebo controlled study to evaluate the efficacy and safety of VPM1002 in the prevention of tuberculosis (TB) recurrence in pulmonary TB patients after successful TB treatment. https://clinicaltrials.gov/ct2/show/NCT03152903.
- 774.Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, Fernandez C, Parra A, Cardona PJ, Vilaplana C, Ausina V, Williams A, Clark S, Malaga W, Guilhot C, Gicquel B, Martin C. 2013. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated Mycobacterium tuberculosis-based vaccine to enter clinical trials. Vaccine 31:4867–4873. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 775.Solans L, Uranga S, Aguilo N, Arnal C, Gomez AB, Monzon M, Badiola JJ, Gicquel B, Martin C. 2014. Hyper-attenuated MTBVAC erp mutant protects against tuberculosis in mice. Vaccine 32:5192–5197. doi: 10.1016/j.vaccine.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 776.Spertini F, Audran R, Chakour R, Karoui O, Steiner-Monard V, Thierry AC, Mayor CE, Rettby N, Jaton K, Vallotton L, Lazor-Blanchet C, Doce J, Puentes E, Marinova D, Aguilo N, Martin C. 2015. Safety of human immunization with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med 3:953–962. doi: 10.1016/S2213-2600(15)00435-X. [DOI] [PubMed] [Google Scholar]
- 777.Tameris M, Mearns H, Penn-Nicholson A, Gregg Y, Bilek N, Mabwe S, Geldenhuys H, Shenje J, Luabeya AKK, Murillo I, Doce J, Aguilo N, Marinova D, Puentes E, Rodríguez E, Gonzalo-Asensio J, Fritzell B, Thole J, Martin C, Scriba TJ, Hatherill M, MTBVAC Clinical Trial Team . 2019. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: a randomised controlled, double-blind dose-escalation trial. Lancet Respir Med 7:757–770. doi: 10.1016/S2213-2600(19)30251-6. [DOI] [PubMed] [Google Scholar]
- 778.Martín C, Marinova D, Aguiló N, Gonzalo-Asensio J. 2021. MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG. Vaccine 39:7277–7285. doi: 10.1016/j.vaccine.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 779.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, MVA85A 020 Trial Study Team . 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dièye S, Dieye TN, Esmail H, Goliath R, Huygen K, January V, Ndiaye I, Oni T, Raine M, Romano M, Satti I, Sutton S, Thiam A, Wilkinson KA, Mboup S, Wilkinson RJ, McShane H, MVA85A 030 Trial Investigators . 2015. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 3:190–200. doi: 10.1016/S2213-2600(15)00037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.von Reyn CF, Lahey T, Arbeit RD, Landry B, Kailani L, Adams LV, Haynes BC, Mackenzie T, Wieland-Alter W, Connor RI, Tvaroha S, Hokey DA, Ginsberg AM, Waddell R. 2017. Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: a randomized, controlled trial of DAR-901. PLoS One 12:e0175215. doi: 10.1371/journal.pone.0175215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.ClinicalTrials.gov. 2020. NCT02712424: a phase 2, randomized, placebo-controlled, double-blind, study of the prevention of infection with Mycobacterium tuberculosis among adolescents who have previously received BCG. https://clinicaltrials.gov/ct2/show/study/NCT02712424.
- 783.Rees CA, Pineros DB, Amour M, Munseri P, Said J, Magohe A, Matee M, Pallangyo K, von Reyn CF. 2020. The potential of CBC-derived ratios (monocyte-to-lymphocyte, neutrophil-to-lymphocyte, and platelet-to-lymphocyte) to predict or diagnose incident TB infection in Tanzanian adolescents. BMC Infect Dis 20:609. doi: 10.1186/s12879-020-05331-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, Vergara J, Mabwe S, Bilek N, Geldenhuys H, Luabeya AK, Ellis R, Ginsberg AM, Hanekom WA, Reed SG, Coler RN, Scriba TJ, Hatherill M, The TBVPX-114 Study Team . 2018. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med 6:287–298. doi: 10.1016/S2213-2600(18)30077-8. [DOI] [PubMed] [Google Scholar]
- 785.Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, Rolf T, Lu L, Alter G, Hokey D, Jayashankar L, Walker R, Snowden MA, Evans T, Ginsberg A, Reed SG, The TBVPX-113 Study Team . 2018. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines 3:34. doi: 10.1038/s41541-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Bekker LG, Dintwe O, Fiore-Gartland A, Middelkoop K, Hutter J, Williams A, Randhawa AK, Ruhwald M, Kromann I, Andersen PL, DiazGranados CA, Rutkowski KT, Tait D, Miner MD, Andersen-Nissen E, De Rosa SC, Seaton KE, Tomaras GD, McElrath MJ, Ginsberg A, Kublin JG, HVTN 602/Aeras A-042 Protocol Team . 2020. A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClinicalMedicine 21:100313. doi: 10.1016/j.eclinm.2020.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, Mabwe S, Makhethe L, Erasmus M, Toefy A, Mulenga H, Hanekom WA, Self SG, Bekker LG, Ryall R, Gurunathan S, DiazGranados CA, Andersen P, Kromann I, Evans T, Ellis RD, Landry B, Hokey DA, Hopkins R, Ginsberg AM, Scriba TJ, Hatherill M, C-040–404 Study Team . 2018. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, Ayles HM, Henostroza G, Thienemann F, Scriba TJ, Diacon A, Blatner GL, Demoitié MA, Tameris M, Malahleha M, Innes JC, Hellström E, Martinson N, Singh T, Akite EJ, Khatoon Azam A, Bollaerts A, Ginsberg AM, Evans TG, Gillard P, Tait DR. 2018. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 379:1621–1634. doi: 10.1056/NEJMoa1803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, Scriba TJ, Akite EJ, Ayles HM, Bollaerts A, Demoitié MA, Diacon A, Evans TG, Gillard P, Hellström E, Innes JC, Lempicki M, Malahleha M, Martinson N, Mesia Vela D, Muyoyeta M, Nduba V, Pascal TG, Tameris M, Thienemann F, Wilkinson RJ, Roman F. 2019. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 381:2429–2439. doi: 10.1056/NEJMoa1909953. [DOI] [PubMed] [Google Scholar]
- 790.Manjaly Thomas ZR, Satti I, Marshall JL, Harris SA, Lopez Ramon R, Hamidi A, Minhinnick A, Riste M, Stockdale L, Lawrie AM, Vermaak S, Wilkie M, Bettinson H, McShane H. 2019. Alternate aerosol and systemic immunization with a recombinant viral vector for tuberculosis, MVA85A: a phase I randomised controlled trial. PLoS Med 16:e1002790. doi: 10.1371/journal.pmed.1002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Sharma SK, Katoch K, Sarin R, Balambal R, Kumar Jain N, Patel N, Murthy KJR, Singla N, Saha PK, Khanna A, Singh U, Kumar S, Sengupta A, Banavaliker JN, Chauhan DS, Sachan S, Wasim M, Tripathi S, Dutt N, Jain N, Joshi N, Penmesta SRR, Gaddam S, Gupta S, Khamar B, Dey B, Mitra DK, Arora SK, Bhaskar S, Rani R. 2017. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in category II pulmonary tuberculosis in a randomized trial. Sci Rep 7:3354. doi: 10.1038/s41598-017-03514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Weng H, Huang JY, Meng XY, Li S, Zhang GQ. 2016. Adjunctive therapy of Mycobacterium vaccae vaccine in the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Biomed Rep 4:595–600. doi: 10.3892/br.2016.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Nell AS, D’lom E, Bouic P, Sabaté M, Bosser R, Picas J, Amat M, Churchyard G, Cardona P-J. 2014. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS One 9:e89612. doi: 10.1371/journal.pone.0089612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Minassian AM, Satti I, Poulton ID, Meyer J, Hill AV, McShane H. 2012. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis Bacille Calmette-Guérin. J Infect Dis 205:1035–1042. doi: 10.1093/infdis/jis012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Minassian AM, Ronan EO, Poyntz H, Hill AV, McShane H. 2011. Preclinical development of an in vivo BCG challenge model for testing candidate TB vaccine efficacy. PLoS One 6:e19840. doi: 10.1371/journal.pone.0019840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Blazevic A, Xia M, Turan A, Tennant J, Hoft DF. 2017. Pilot studies of a human BCG challenge model. Tuberculosis (Edinb) 105:108–112. doi: 10.1016/j.tube.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 797.Hokey DA. 2014. TB vaccines: the (human) challenge ahead. Mycobact Dis 4:e128. doi: 10.4172/2161-1068.1000e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.McShane H, Williams A. 2014. A review of preclinical animal models utilized for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb) 94:105–110. doi: 10.1016/j.tube.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, Orme IM, Nardell EA. 2011. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 91:329–338. doi: 10.1016/j.tube.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. 2010. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol 17:1170–1182. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Skerry C, Pokkali S, Pinn M, Be NA, Harper J, Karakousis PC, Jain SK. 2013. Vaccination with recombinant Mycobacterium tuberculosis PknD attenuates bacterial dissemination to the brain in guinea pigs. PLoS One 8:e66310. doi: 10.1371/journal.pone.0066310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Davids M, Pooran A, Hermann C, Mottay L, Thompson F, Cardenas J, Gu J, Koeuth T, Meldau R, Limberis J, Gina P, Srivastava S, Calder B, Esmail A, Tomasicchio M, Blackburn J, Gumbo T, Dheda K. 2020. A human lung challenge model to evaluate the safety and immunogenicity of PPD and live bacillus Calmette-Guérin. Am J Respir Crit Care Med 201:1277–1291. doi: 10.1164/rccm.201908-1580OC. [DOI] [PubMed] [Google Scholar]
- 803.ClinicalTrials.gov. 2020. NCT02709278: a clinical challenge trial to evaluate controlled human infection with BCG administered by the aerosol inhaled route compared with the intradermal route in healthy, BCG-naive, UK adult volunteers. https://clinicaltrials.gov/ct2/show/NCT02709278.
- 804.ClinicalTrials.gov. 2020. NCT03912207: a human challenge study to evaluate innate and adaptive immune responses to a controlled human infection with BCG administered by the aerosol inhaled route in healthy, BCG-naive, UK adult volunteers. https://clinicaltrials.gov/ct2/show/NCT03912207.
- 805.Andersen P, Scriba TJ. 2019. Moving tuberculosis vaccines from theory to practice. Nat Rev Immunol 19:550–562. doi: 10.1038/s41577-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 806.Klein NP. 2014. Licensed pertussis vaccines in the United States. Hum Vaccin Immunother 10:2684–2690. doi: 10.4161/hv.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, Schley AW, Anderson WJ, Grigoryan A, Aranas AE, Wodajo MS, Abellera JP, Centers for Disease Control and Prevention . 2014. Summary of notifiable diseases—United States, 2012. MMWR Morb Mortal Wkly Rep 61:1–121. [PubMed] [Google Scholar]
- 808.World Health Organization. 2019. Pertussis reported cases. World Health Organization, Geneva, Switzerland. https://immunizationdata.who.int/pages/incidence/pertussis.html. [Google Scholar]
- 809.Hozbor D, Mooi F, Flores D, Weltman G, Bottero D, Fossati S, Lara C, Gaillard ME, Pianciola L, Zurita E, Fioriti A, Archuby D, Galas M, Binsztein N, Regueira M, Castuma C, Fingermann M, Graieb A. 2009. Pertussis epidemiology in Argentina: trends over 2004–2007. J Infect 59:225–231. doi: 10.1016/j.jinf.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 810.Quinn HE, McIntyre PB. 2007. Pertussis epidemiology in Australia over the decade 1995–2005: trends by region and age group. Commun Dis Intell Q Rep 31:205–215. [PubMed] [Google Scholar]
- 811.Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE, EUVAC-NET Group . 2005. Resurgence of pertussis in Europe. Pediatr Infect Dis J 24:761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 812.Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. 2017. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 17:974–980. doi: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- 813.Tam J, Tran D, Bettinger JA, Moore D, Sauvé L, Jadavji T, Tan B, Vaudry W, Halperin SA, Top KA, Canadian Immunization Monitoring Program Active Investigators . 2020. Review of pediatric encephalitis and encephalopathy cases following immunization reported to the Canadian Immunization Monitoring Program Active (IMPACT) from 1992 to 2012. Vaccine 38:4457–4463. doi: 10.1016/j.vaccine.2020.04.035. [DOI] [PubMed] [Google Scholar]
- 814.Cherry JD. 2013. Pertussis: challenges today and for the future. PLoS Pathog 9:e1003418. doi: 10.1371/journal.ppat.1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Burns DL, Meade BD, Messionnier NE. 2014. Pertussis resurgence: perspectives from the working group meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J Infect Dis 209(Suppl 1):S32–S35. doi: 10.1093/infdis/jit491. [DOI] [PubMed] [Google Scholar]
- 816.Meade BD, Plotkin SA, Locht C. 2014. Possible options for new pertussis vaccines. J Infect Dis 209(Suppl 1):S24–S27. doi: 10.1093/infdis/jit531. [DOI] [PubMed] [Google Scholar]
- 817.Rumbo M, Hozbor D. 2014. Development of improved pertussis vaccine. Hum Vaccin Immunother 10:2450–2453. doi: 10.4161/hv.29253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Higgs R, Higgins SC, Ross PJ, Mills KH. 2012. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol 5:485–500. doi: 10.1038/mi.2012.54. [DOI] [PubMed] [Google Scholar]
- 819.Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, Cassiday PK, Chiang CS, Dalby T, Fry NK, Gaillard ME, van Gent M, Guiso N, Hallander HO, Harvill ET, He Q, van der Heide HG, Heuvelman K, Hozbor DF, Kamachi K, Karataev GI, Lan R, Lutyńska A, Maharjan RP, Mertsola J, Miyamura T, Octavia S, Preston A, Quail MA, Sintchenko V, Stefanelli P, Tondella ML, Tsang RS, Xu Y, Yao SM, Zhang S, Parkhill J, Mooi FR. 2014. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. mBio 5:e01074. doi: 10.1128/mBio.01074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Bottero D, Gaillard ME, Errea A, Moreno G, Zurita E, Pianciola L, Rumbo M, Hozbor D. 2013. Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine 31:5262–5268. doi: 10.1016/j.vaccine.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 821.Sirivichayakul C, Chanthavanich P, Limkittikul K, Siegrist CA, Wijagkanalan W, Chinwangso P, Petre J, Hong Thai P, Chauhan M, Viviani S. 2017. Safety and immunogenicity of a combined tetanus, diphtheria, recombinant acellular pertussis vaccine (TdaP) in healthy Thai adults. Hum Vaccin Immunother 13:136–143. doi: 10.1080/21645515.2016.1234555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Pitisuttithum P, Chokephaibulkit K, Sirivichayakul C, Sricharoenchai S, Dhitavat J, Pitisuthitham A, Phongsamart W, Boonnak K, Lapphra K, Sabmee Y, Wittawatmongkol O, Chauhan M, Wijagkanalan W, Hommalai G, Fortuna L, Chinwangso P, Poredi IK, van den Biggelaar AHJ, Pham HT, Viviani S. 2018. Antibody persistence after vaccination of adolescents with monovalent and combined acellular pertussis vaccines containing genetically inactivated pertussis toxin: a phase 2/3 randomised, controlled, non-inferiority trial. Lancet Infect Dis 18:1260–1268. doi: 10.1016/S1473-3099(18)30375-X. [DOI] [PubMed] [Google Scholar]
- 823.Thai Clinical Trials Registry. 2020. Study ID TCTR20180725004: immunogenicity and safety of various recombinant acellular pertussis-containing vaccines in healthy pregnant women. http://www.thaiclinicaltrials.org/show/TCTR20180725004.
- 824.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Locht C, Mielcarek N. 2014. Live attenuated vaccines against pertussis. Expert Rev Vaccines 13:1147–1158. doi: 10.1586/14760584.2014.942222. [DOI] [PubMed] [Google Scholar]
- 826.Marzouqi I, Richmond P, Fry S, Wetherall J, Mukkur T. 2010. Development of improved vaccines against whooping cough: current status. Hum Vaccin 6:543–553. doi: 10.4161/hv.6.7.11413. [DOI] [PubMed] [Google Scholar]
- 827.Garlapati S, Eng NF, Kiros TG, Kindrachuk J, Mutwiri GK, Hancock RE, Halperin SA, Potter AA, Babiuk LA, Gerdts V. 2011. Immunization with PCEP microparticles containing pertussis toxoid, CpG ODN and a synthetic innate defense regulator peptide induces protective immunity against pertussis. Vaccine 29:6540–6548. doi: 10.1016/j.vaccine.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 828.Sharma S, Mukkur TK, Benson HA, Chen Y. 2012. Enhanced immune response against pertussis toxoid by IgA-loaded chitosan-dextran sulfate nanoparticles. J Pharm Sci 101:233–244. doi: 10.1002/jps.22763. [DOI] [PubMed] [Google Scholar]
- 829.Alvarez Hayes J, Erben E, Lamberti Y, Principi G, Maschi F, Ayala M, Rodriguez ME. 2013. Bordetella pertussis iron regulated proteins as potential vaccine components. Vaccine 31:3543–3548. doi: 10.1016/j.vaccine.2013.05.072. [DOI] [PubMed] [Google Scholar]
- 830.Dewan KK, Linz B, DeRocco SE, Harvill ET. 2020. Acellular pertussis vaccine components: today and tomorrow. Vaccines 8:217. doi: 10.3390/vaccines8020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Allen AC, Wilk MM, Misiak A, Borkner L, Murphy D, Mills KHG. 2018. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol 11:1763–1776. doi: 10.1038/s41385-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 832.Shi W, Kou Y, Jiang H, Gao F, Kong W, Su W, Xu F, Jiang C. 2018. Novel intranasal pertussis vaccine based on bacterium-like particles as a mucosal adjuvant. Immunol Lett 198:26–32. doi: 10.1016/j.imlet.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 833.Cauchi S, Locht C. 2018. Non-specific effects of live attenuated pertussis vaccine against heterologous infectious and inflammatory diseases. Front Immunol 9:2872. doi: 10.3389/fimmu.2018.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.ClinialTrials.gov. 2020. NCT03137927: a phase I clinical study of a GamLPV, a live intranasal Bordetella pertussis vaccine; a randomized placebo-controlled dose-escalating study of single-use GamLPV safety and tolerability in healthy human volunteers. https://clinicaltrials.gov/ct2/show/NCT03137927.
- 835.ClinialTrials.gov. 2020. NCT04036526: a randomized double-blind placebo-controlled comparative research of potency and safety of a GamLPV, a live intranasal Bordetella pertussis vaccine, using two dosing schedules and methods of application in healthy human volunteers. https://clinicaltrials.gov/ct2/show/NCT04036526.
- 836.Merkel TJ, Halperin SA. 2014. Nonhuman primate and human challenge models of pertussis. J Infect Dis 209(Suppl 1):S20–S23. doi: 10.1093/infdis/jit493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. 2012. Nonhuman primate model of pertussis. Infect Immun 80:1530–1536. doi: 10.1128/IAI.06310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.de Graaf H, Gbesemete D, Gorringe AR, Diavatopoulos DA, Kester KE, Faust SN, Read RC. 2017. Investigating Bordetella pertussis colonization and immunity: protocol for an inpatient controlled human infection model. BMJ Open 7:e018594. doi: 10.1136/bmjopen-2017-018594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.de Graaf H, Ibrahim M, Hill AR, Gbesemete D, Vaughan AT, Gorringe A, Preston A, Buisman AM, Faust SN, Kester KE, Berbers GAM, Diavatopoulos DA, Read RC. 2020. Controlled human infection with Bordetella pertussis induces asymptomatic, immunizing colonization. Clin Infect Dis 71:403–411. doi: 10.1093/cid/ciz840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Merkel TJ. 2020. Toward a controlled human infection model of pertussis. Clin Infect Dis 71:412–414. doi: 10.1093/cid/ciz842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.World Health Organization. 2020. Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization, Geneva, Switzerland. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. [Google Scholar]
- 842.Worldometer. 2021. Coronavirus cases. https://www.worldometers.info/coronavirus/.
- 843.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 844.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. 2020. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. 2020. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, January-March 2020: retrospective cohort study. BMJ 369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. 2020. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, Yip CC, Cai JP, Chan JM, Chik TS, Lau DP, Choi CY, Chen LL, Chan WM, Chan KH, Ip JD, Ng AC, Poon RW, Luo CT, Cheng VC, Chan JF, Hung IF, Chen Z, Chen H, Yuen KY. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. 2020. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 849.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. 2020. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, Li Y, Ni Q, Zou R, Li X, Xu M, Zhang Y, Zhao H, Zhang X, Yu L, Su J, Lang G, Liu J, Wu X, Guo Y, Tao J, Shi D, Yu L, Cao Q, Ruan B, Liu L, Wang Z, Xu Y, Liu Y, Sheng J, Li L. 2020. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 71:799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Eyal N, Lipsitch M, Smith PG. 2020. Human challenge studies to accelerate coronavirus vaccine licensure. J Infect Dis 221:1752–1756. doi: 10.1093/infdis/jiaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Plotkin SA, Caplan A. 2020. Extraordinary diseases require extraordinary solutions. Vaccine 38:3987–3988. doi: 10.1016/j.vaccine.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Nguyen LC, Bakerlee CW, McKelvey TG, Rose SM, Norman AJ, Joseph N, Manheim D, McLaren MR, Jiang S, Barnes CF, Kinniment M, Foster D, Darton TC, Morrison J, for the 1Day Sooner Research Team . 2020. Evaluating use cases for human challenge trials in accelerating SARS-CoV-2 vaccine development. Clin Infect Dis doi: 10.1093/cid/ciaa935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Baay M, Neels P. 2021. Controlled human infection to speed up SARS-CoV-2 vaccine development. Front Immunol 12:658783. doi: 10.3389/fimmu.2021.658783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Rapeport G, Smith E, Gilbert A, Catchpole A, McShane H, Chiu C. 2021. SARS-CoV-2 human challenge studies: establishing the model during an evolving pandemic. N Engl J Med 385:961–964. doi: 10.1056/NEJMp2106970. [DOI] [PubMed] [Google Scholar]
- 857.Deming ME, Michael NL, Robb M, Cohen MS, Neuzil KM. 2020. Accelerating development of SARS-CoV-2 vaccines: the role for controlled human infection models. N Engl J Med 383:e63. doi: 10.1056/NEJMp2020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.World Health Organization. 2020. Key criteria for the ethical acceptability of COVID-19 human challenge studies. WHO, Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/331976/WHO-2019-nCoV-Ethics_criteria-2020.1-eng.pdf?ua=1. [Google Scholar]
- 859.Johnson CY. 2020. U.S. will prepare coronavirus strain for potential human challenge trials. Washington Post, Washington, DC. https://www.washingtonpost.com/health/2020/08/14/us-will-prepare-coronavirus-strain-potential-human-challenge-trials/. [Google Scholar]
- 860.Hausdorff WP, Flores J. 2021. Low-dose and oral exposure to SARS-CoV-2 may help us understand and prevent severe COVID-19. Int J Infect Dis 103:37–41. doi: 10.1016/j.ijid.2020.11.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Baay M, Neels P. 2020. SARS-CoV-2 controlled human infection models: ethics, challenge agent production and regulatory issues. Biologicals 15:69–74. doi: 10.1016/j.biologicals.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 312. CFR part 312, vol 5. FDA, Rockville, MD. [Google Scholar]
- 863.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 600. CFR part 600, vol 7. FDA, Rockville, MD. [Google Scholar]
- 864.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, parts 210 and 211. CFR Parts 210 and 211, vol 4. FDA, Rockville, MD. [Google Scholar]
- 865.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 58. CFR Part 58, vol 1. FDA, Rockville, MD. [Google Scholar]
- 866.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 50. CFR Part 50, vol 1. FDA, Rockville, MD. [Google Scholar]
- 867.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 56. CFR Part 56, vol 1. FDA, Rockville, MD. [Google Scholar]
- 868.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 312.130. CFR Part 312.130, vol 5. FDA, Rockville, MD. [Google Scholar]
- 869.US Food and Drug Administration, Center for Biologics Evaluation and Research. 2011. Guidance for industry: general principles for the development of vaccines to protect against global infectious diseases. FDA, Rockville, MD. https://www.fda.gov/media/82306/download. [Google Scholar]
- 870.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 312.23, 5. CFR Part 312.23. FDA, Rockville, MD. [Google Scholar]
- 871.International Conference on Harmonization. 2000. Topic M4: the common technical document. ICH, Geneva, Switzerland. https://www.ich.org/page/ctd. [Google Scholar]
- 872.US Department of Health and Human Services, Food and Drug Administration. 2021. Statement of Investigator (Title 21, Code of Federal Regulations (CFR) part 312). FDA, Rockville, MD. http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM074728.pdf. [Google Scholar]
- 873.US Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Office of Regulatory Affairs. 2008. Guidance for industry: CGMP for phase 1 investigational drugs. FDA, Rockville, MD. https://www.fda.gov/media/70975/download. [Google Scholar]
- 874.Ramanathan R, Stibitz S, Pratt D, Roberts J. 2019. Use of controlled human infection models (CHIMs) to support vaccine development: US regulatory considerations. Vaccine 37:4256–4261. doi: 10.1016/j.vaccine.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 875.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 312.42. CFR Part 312.42, vol 5. FDA, Rockville, MD. [Google Scholar]
- 876.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 312.110. CFR Part 312.110, vol 5. FDA, Rockville, MD. [Google Scholar]
- 877.US Food and Drug Administration. 2014. Code of Federal Regulations Title 21, part 312.32. CFR Part 312.32, vol 5. FDA, Rockville, MD. [Google Scholar]
- 878.US National Institutes of Health. 2019. NIH guidelines for research involving recombinant or synthetic nucleic acid molecules. NIH, Bethesda, MD. https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf. [Google Scholar]
- 879.US National Institute of Allergy and Infectious Diseases. 2020. ClinRegs: United Kingdom. NIH, Bethesda, MD. https://clinregs.niaid.nih.gov/country/united-kingdom/united-states#_top. [Google Scholar]
- 880.European Commission, Health and Consumers Directorate-General. 2011. The rules governing medicinal products in the European Union—volume 10: guidance documents applying to clinical trials guidance on Investigational Medicinal Products (IMPs) and ‘Non Investigational Medicinal Products’ (NIMPs), rev. 1. European Commission, Brussels, Belgium. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/imp_03-2011.pdf. [Google Scholar]
- 881.Sheets R, Knezevic I. 2017. Annex 10: human challenge trials for vaccine development: regulatory considerations, p 575–587. In WHO Expert Committee on Biological Standardization, sixty-seventh report. WHO Technical Report Series 1004. WHO, Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/255657/9789241210133-eng.pdf;jsessionid=625E4C06254910A2F4E136C6C10EF9D5?sequence=1. [Google Scholar]
- 882.Sheets RL, Fritzell B, Aguado de Ros MT. 2016. Human challenge trials in vaccine development: Strasbourg, September 29–October 1, 2014. Biologicals 44:37–50. doi: 10.1016/j.biologicals.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 883.Baay MFD, Richie TL, Neels P, Session chairs at the second Human Challenge Trials meeting . 2019. Human challenge trials in vaccine development, Rockville, MD, USA, September 28–30, 2017. Biologicals 61:85–94. doi: 10.1016/j.biologicals.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 884.Bekeredjian-Ding I, Van Molle W, Baay M, Neels P, PEI Speakers and Session Chairs . 2020. Human challenge trial workshop: focus on quality requirements for challenge agents, Langen, Germany, October 22, 2019. Biologicals 66:53–61. doi: 10.1016/j.biologicals.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 885.Pollard AJ, Sauerwein R, Baay M, Neels P. 2020. Third human challenge trial conference, Oxford, United Kingdom, February 6–7, 2020, a meeting report. Biologicals. doi: 10.1016/j.biologicals.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 886.Sheets RL, Rangavajhula V, Pullen JK, Butler C, Mehra V, Shapiro S, Pensiero M. 2015. Now that you want to take your HIV/AIDS vaccine/biological product research concept into the clinic: what are the “cGMP”? Vaccine 33:1757–1766. doi: 10.1016/j.vaccine.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.World Health Organization. 2004. Laboratory biosafety manual, 3rd ed. WHO, Geneva, Switzerland. https://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/. [Google Scholar]
- 888.Nouatin O, Ateba Ngoa U, Ibáñez J, Dejon-Agobe JC, Mordmüller B, Edoa JR, Mougeni F, Brückner S, Bouyoukou Hounkpatin A, Esen M, Theisen M, Moutairou K, Hoffman SL, Issifou S, Luty AJF, Loembe MM, Agnandji ST, Lell B, Kremsner PG, Adegnika AA. 2020. Effect of immune regulatory pathways after immunization with GMZ2 malaria vaccine candidate in healthy lifelong malaria-exposed adults. Vaccine 38:4263–4272. doi: 10.1016/j.vaccine.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, Lucas SK, Beura LK, Thompson EA, Till LM, Batres R, Paw B, Pergament SL, Saenyakul P, Xiong M, Kim AD, Kim G, Masopust D, Martens EC, Angkurawaranon C, McGready R, Kashyap PC, Culhane-Pera KA, Knights D. 2018. US immigration westernizes the human gut microbiome. Cell 175:962–972. doi: 10.1016/j.cell.2018.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, Tate J, de Weerth C, Giaquinto C, Wiersinga WJ, Lewis KD, de Vos WM. 2017. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infect Dis 215:34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.Harris V, Ali A, Fuentes S, Korpela K, Kazi M, Tate J, Parashar U, Wiersinga WJ, Giaquinto C, de Weerth C, de Vos WM. 2018. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 9:93–101. doi: 10.1080/19490976.2017.1376162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, Mpina M, Ali Juma O, Schindler T, Huber E, Gunasekera A, Manoj A, Simon B, Saverino E, Church LWP, Hermsen CC, Sauerwein RW, Plowe C, Venkatesan M, Sasi P, Lweno O, Mutani P, Hamad A, Mohammed A, Urassa A, Mzee T, Padilla D, Ruben A, Sim BKL, Tanner M, Abdulla S, Hoffman SL. 2014. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 91:471–480. doi: 10.4269/ajtmh.14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Hodgson SH, Juma E, Salim A, Magiri C, Kimani D, Njenga D, Muia A, Cole AO, Ogwang C, Awuondo K, Lowe B, Munene M, Billingsley PF, James ER, Gunasekera A, Sim BK, Njuguna P, Rampling TW, Richman A, Abebe Y, Kamuyu G, Muthui M, Elias SC, Molyneux S, Gerry S, Macharia A, Williams TN, Bull PC, Hill AV, Osier FH, Draper SJ, Bejon P, Hoffman SL, Ogutu B, Marsh K. 2014. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol 5:686. doi: 10.3389/fmicb.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Lell B, Mordmüller B, Dejon Agobe JC, Honkpehedji J, Zinsou J, Mengue JB, Loembe MM, Adegnika AA, Held J, Lalremruata A, Nguyen TT, Esen M, Kc N, Ruben AJ, Chakravarty S, Lee Sim BK, Billingsley PF, James ER, Richie TL, Hoffman SL, Kremsner PG. 2018. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after controlled human malaria infection in adults in Gabon. Am J Trop Med Hyg 98:508–515. doi: 10.4269/ajtmh.17-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Dejon-Agobe JC, Ateba-Ngoa U, Lalremruata A, Homoet A, Engelhorn J, Nouatin OP, Edoa JR, Fernandes JF, Esen M, Mouwenda YD, Betouke Ongwe EM, Massinga-Loembe M, Hoffman SL, Sim BKL, Theisen M, Kremsner PG, Adegnika AA, Lell B, Mordmüller B. 2019. Controlled human malaria infection of healthy adults with lifelong malaria exposure to assess safety, immunogenicity, and efficacy of the asexual blood-stage malaria vaccine candidate GMZ2. Clin Infect Dis 69:1377–1384. doi: 10.1093/cid/ciy1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Suntharasamai P, Migasena S, Vongsthongsri U, Supanaranond W, Pitisuttitham P, Supeeranan L, Chantra A, Naksrisook S. 1992. Clinical and bacteriological studies of El Tor cholera after ingestion of known inocula in Thai volunteers. Vaccine 10:502–505. doi: 10.1016/0264-410x(92)90347-m. [DOI] [PubMed] [Google Scholar]
- 897.Pitisuttithum P, Cohen MB, Phonrat B, Suthisarnsuntorn U, Bussaratid V, Desakorn V, Phumratanaprapin W, Singhasivanon P, Looareesuwan S, Schiff GM, Ivanoff B, Lang D. 2001. A human volunteer challenge model using frozen bacteria of the new epidemic serotype, V. cholerae O139 in Thai volunteers. Vaccine 20:920–925. doi: 10.1016/s0264-410x(01)00381-4. [DOI] [PubMed] [Google Scholar]
- 898.Bodhidatta L, Pitisuttithum P, Chamnanchanant S, Chang KT, Islam D, Bussaratid V, Venkatesa MM, Hale TL, Mason CJ. 2012. Establishment of a Shigella sonnei human challenge model in Thailand. Vaccine 30:7040–7045. doi: 10.1016/j.vaccine.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899.Pitisuttithum P, Islam D, Chamnanchanunt S, Ruamsap N, Khantapura P, Kaewkungwal J, Kittitrakul C, Luvira V, Dhitavat J, Venkatesan MM, Mason CJ, Bodhidatta L. 2016. Clinical trial of an oral live Shigella sonnei vaccine candidate, WRSS1, in Thai adults. Clin Vaccine Immunol 23:564–575. doi: 10.1128/CVI.00665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Kapulu MC, Njuguna P, Hamaluba MM, CHMI-SIKA Study Team . 2018. Controlled human malaria infection in semi-immune Kenyan adults (CHMI-SIKA): a study protocol to investigate in vivo Plasmodium falciparum malaria parasite growth in the context of preexisting immunity. Wellcome Open Res 3:155. doi: 10.12688/wellcomeopenres.14909.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Njue M, Njuguna P, Kapulu MC, Sanga G, Bejon P, Marsh V, Molyneux S, Kamuya D. 2018. Ethical considerations in controlled human malaria infection studies in low resource settings: experiences and perceptions of study participants in a malaria challenge study in Kenya. Wellcome Open Res 3:39. doi: 10.12688/wellcomeopenres.14439.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902.Elliott AM, Roestenberg M, Wajja A, Opio C, Angumya F, Adriko M, Egesa M, Gitome S, Mfutso-Bengo J, Bejon P, Kapulu M, Seager Z, Lutalo T, Nazziwa WB, Muwumuza A, Yazdanbakhsh M, Kaleebu P, Kabatereine N, Tukahebwa E. 2018. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: report of a stakeholders’ meeting held in Entebbe, Uganda. AAS Open Res 1:2. doi: 10.12688/aasopenres.12841.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Njue M, Kombe F, Mwalukore S, Molyneux S, Marsh V. 2014. What are fair study benefits in international health research? Consulting community members in Kenya. PLoS One 9:e113112. doi: 10.1371/journal.pone.0113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.DuPont HL, Formal SB, Hornick RB, Snyder MJ, Libonati JP, Sheahan DG, LaBrec EH, Kalas JP. 1971. Pathogenesis of Escherichia coli diarrhea. N Engl J Med 285:1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- 905.Levine MM, Caplan ES, Waterman D, Cash RA, Hornick RB, Snyder MJ. 1977. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect Immun 17:78–82. doi: 10.1128/iai.17.1.78-82.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Evans DG, Satterwhite TK, Evans DJ, Jr, DuPont HL. 1978. Differences in serological responses and excretion patterns of volunteers challenged with enterotoxigenic Escherichia coli with and without the colonization factor antigen. Infect Immun 19:883–888. doi: 10.1128/iai.19.3.883-888.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Satterwhite TK, Evans DG, DuPont HL, Evans DJ, Jr.. 1978. Role of Escherichia coli colonization factor antigen in acute diarrhoea. Lancet 2:181–184. doi: 10.1016/s0140-6736(78)91921-9. [DOI] [PubMed] [Google Scholar]
- 908.Levine MM, Nalin DR, Hoover DL, Bergquist EJ, Hornick RB, Young CR. 1979. Immunity to enterotoxigenic Escherichia coli. Infect Immun 23:729–736. doi: 10.1128/iai.23.3.729-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Levine MM, Rennels MB, Cisneros L, Hughes TP, Nalin DR, Young CR. 1980. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am J Epidemiol 111:347–355. doi: 10.1093/oxfordjournals.aje.a112906. [DOI] [PubMed] [Google Scholar]
- 910.Clements ML, Levine MM, Black RE, Robins-Browne RM, Cisneros LA, Drusano GL, Lanata CF, Saah AJ. 1981. Lactobacillus prophylaxis for diarrhea due to enterotoxigenic Escherichia coli. Antimicrob Agents Chemother 20:104–108. doi: 10.1128/AAC.20.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Black RE, Levine MM, Clements ML, Cisneros L, Daya V. 1982. Treatment of experimentally induced enterotoxigenic Escherichia coli diarrhea with trimethoprim, trimethoprim-sulfamethoxazole, or placebo. Rev Infect Dis 4:540–545. doi: 10.1093/clinids/4.2.540. [DOI] [PubMed] [Google Scholar]
- 912.Levine MM, Black RE, Brinton CC, Clements ML, Fusco P, Hughes TP, O’Donnell S, Robins-Browne R, Wood S, Young CR. 1982. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis Suppl 33:83–95. [PubMed] [Google Scholar]
- 913.Graham DY, Estes MK, Gentry LO. 1983. Double-blind comparison of bismuth subsalicylate and placebo in the prevention and treatment of enterotoxigenic Escherichia coli-induced diarrhea in volunteers. Gastroenterol 85:1017–1022. doi: 10.1016/S0016-5085(83)80066-3. [DOI] [PubMed] [Google Scholar]
- 914.Evans DG, Graham DY, Evans DJ, Jr.. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers: response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87:934–940. doi: 10.1016/0016-5085(84)90091-X. [DOI] [PubMed] [Google Scholar]
- 915.Levine MM, Ristaino P, Marley G, Smyth C, Knutton S, Boedeker E, Black R, Young C, Clements ML, Cheney C. 1984. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun 44:409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Levine M, Morris JG, Losonsky G, Boedeker EC, Rowe B. 1986. Fimbriae (pili) adhesins as vaccines, p 143–145. In Lark DL, Mormakr S, Uhlin B-E, Wolf-Wath H (ed), Protein-carbohydrate interactions in biologic systems: the molecular biology of microbial pathogenicity. Academic Press, London, United Kingdom. [Google Scholar]
- 917.Evans DG, Evans DJ, Jr, Opekun AR, Graham DY. 1988. Non-replicating oral whole cell vaccine protective against enterotoxigenic Escherichia coli (ETEC) diarrhea: stimulation of anti-CFA (CFA/I) and anti-enterotoxin (anti-LT) intestinal IgA and protection against challenge with ETEC belonging to heterologous serotypes. FEMS Microbiol Immunol 1:117–125. doi: 10.1111/j.1574-6968.1988.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 918.Evans DJ, Jr, Evans DG, Opekun AR, Graham DY. 1988. Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiol Immunol 1:9–18. doi: 10.1111/j.1574-6968.1988.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 919.Tacket CO, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Levine MM. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med 318:1240–1243. doi: 10.1056/NEJM198805123181904. [DOI] [PubMed] [Google Scholar]
- 920.Tacket CO, Reid RH, Boedeker EC, Losonsky G, Nataro JP, Bhagat H, Edelman R. 1994. Enteral immunization and challenge of volunteers given enterotoxigenic Escherichia coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12:1270–1274. doi: 10.1016/s0264-410x(94)80038-2. [DOI] [PubMed] [Google Scholar]
- 921.Freedman DJ, Tacket CO, Delehanty A, Maneval DR, Nataro J, Crabb JH. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis 177:662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- 922.Tacket CO, Losonsky G, Livio S, Edelman R, Crabb J, Freedman D. 1999. Lack of prophylactic efficacy of an enteric-coated bovine hyperimmune milk product against enterotoxigenic Escherichia coli challenge administered during a standard meal. J Infect Dis 180:2056–2059. doi: 10.1086/315157. [DOI] [PubMed] [Google Scholar]
- 923.Coster TS, Wolf MK, Hall ER, Cassels FJ, Taylor DN, Liu CT, Trespalacios FC, DeLorimier A, Angleberger DR, McQueen CE. 2007. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect Immun 75:252–259. doi: 10.1128/IAI.01131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.McKenzie R, Bourgeois AL, Frech SA, Flyer DC, Bloom A, Kazempour K, Glenn GM. 2007. Transcutaneous immunization with the heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double-blind, placebo-controlled challenge study. Vaccine 25:3684–3691. doi: 10.1016/j.vaccine.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 925.McKenzie R, Darsley M, Thomas N, Randall R, Carpenter C, Forbes E, Finucane M, Sack RB, Hall E, Bourgeois AL. 2008. A double-blind, placebo-controlled trial to evaluate the efficacy of PTL-003, an attenuated enterotoxigenic Escherichia coli (ETEC) vaccine strain, in protecting against challenge with virulent ETEC. Vaccine 26:4731–4739. doi: 10.1016/j.vaccine.2008.06.064. [DOI] [PubMed] [Google Scholar]
- 926.Savarino SJ, McKenzie R, Tribble DR, Porter CK, O’Dowd A, Sincock SA, Poole ST, DeNearing B, Woods CM, Kim H, Grahek SL, Brinkley C, Crabb JH, Bourgeois AL. 2019. Hyperimmune bovine colostral anti-CS17 antibodies protect against enterotoxigenic Escherichia coli diarrhea in a randomized, doubled-blind, placebo-controlled human infection model. J Infect Dis 220:505–513. doi: 10.1093/infdis/jiz135. [DOI] [PubMed] [Google Scholar]
- 927.Talaat KR, Porter CK, Bourgeois AL, Lee TK, Duplessis CA, Maciel M, Jr, Gutierrez RL, DeNearing B, Adjoodani B, Adkinson R, Testa KJ, Feijoo B, Alcala AN, Brubaker J, Beselman A, Chakraborty S, Sack D, Halpern J, Trop S, Wu H, Jiao J, Sullivan E, Riddle MS, Joseph SS, Poole ST, Prouty MG. 2020. Oral delivery of hyperimmune bovine serum antibodies against CS6-expressing enterotoxigenic Escherichia coli as a prophylactic against diarrhea. Gut Microbes doi: 10.1080/19490976.2020.1732852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.PATH Vaccine Resource Library. 2020. RSV and mAb trial tracker. https://www.path.org/resources/rsv-and-mab-trial-tracker/.
- 929.Greenberg D, Hoover PA, Vesikari T, Peltier C, Hurley DC, McFetridge RD, Dallas M, Hartzel J, Marchese RD, Coller BG, Stek JE, Abeygunawardana C, Winters MA, MacNair JE, Pujar NS, Musey L. 2018. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine 36:6883–6891. doi: 10.1016/j.vaccine.2018.02.113. [DOI] [PubMed] [Google Scholar]