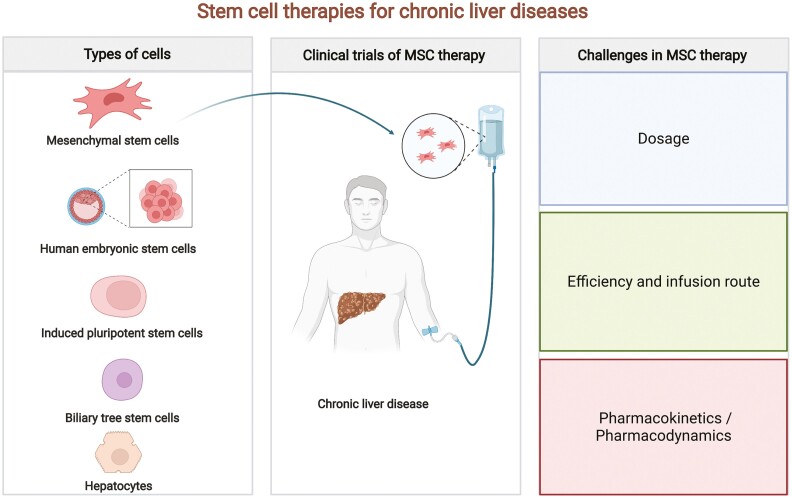
Abstract
Chronic liver diseases have become a significant health issue worldwide and urgently require the development of novel therapeutic approaches, in addition to liver transplantation. Recent clinical and preclinical studies have shown that cell-based therapeutic strategies may contribute to the improvement of chronic liver diseases and offer new therapeutic options to restore liver function through their roles in tissue impairment and immunomodulation. In this review, we summarize the current progress and analyze the challenges for different types of cell therapies used in the treatment of chronic liver diseases currently explored in clinical trials and preclinical studies in animal models. We also discuss some critical issues regarding the use of mesenchymal stem cells (MSCs, the most extensive cell source of stem cells), including therapeutic dosage, transfusion routine, and pharmacokinetics/pharmacodynamics (PK/PD) of transfused MSCs.
Keywords: chronic liver disease, cell therapy, stem cells, mesenchymal stem cell, clinical trial
Graphical Abstract
Graphical Abstract.
Significance Statement.
There is a pressing need for novel therapeutic approaches to the treatment of chronic liver diseases. Stem cell-based therapeutic strategies may contribute to the improvement of chronic liver diseases and offer new therapeutic options to restore liver function. This review provides a detailed account of our current progress and further analyses the challenges of cell therapies for liver diseases. Some critical issues regarding the use of mesenchymal stem cells are also addressed.
Introduction
Liver diseases are a serious threat to human health. It is estimated that up to 800 million people have been affected by chronic liver diseases worldwide, including more than 300 million in China.1-3 Besides viral hepatitis, other common causes of chronic liver disease are obesity, metabolic-associated fatty liver disease, alcoholic liver disease, autoimmune liver disease (primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis), genetics, and other metabolic diseases.4-6 End-stage liver diseases, including decompensated cirrhosis and liver failure, are characterized by portal hypertension and severely impaired liver function, with a series of complications such as ascites, spontaneous peritonitis, coagulation dysfunction, gastrointestinal bleeding, hepatic encephalopathy, and hepatorenal syndrome.7,8 The one-year mortality rate of liver cirrhosis was estimated to be 57%,9 causing 1.32 million deaths worldwide in 2017, accounting for 2.4% of mortalities in the world.4,10 Chronic liver diseases, including decompensated cirrhosis, can develop into acute-on-chronic liver failure, with a further significant increase in mortality (33% at 28 days; 50% at 90 days).11
Current treatments for decompensated cirrhosis or liver failure are still limited, and liver transplantation remains the only available approach to improve survival but is restricted by a shortage of organ resources, rejection after transplantation, and heavy financial costs.12,13 In the past decade, a series of new applications based on cell therapy, including stem cell infusion, hepatocyte transplantation, in vitro artificial liver, and implantation of tissue-engineered organs have been studied as an alternative interventional method for chronic liver diseases. A series of preclinical and clinical studies on cell therapy have shown promising data. However, several gaps remain in the clinical application of MSC treatment for chronic liver diseases. This review focuses on cell therapy for severe liver diseases, summarizes the current progress, and discusses the challenges and unmet issues in this field.
Types of Cells Used for the Treatment of Chronic Liver Diseases
Recently, cell-based therapies, particularly stem cell therapy, are receiving increasing attention. Stem cells and adult liver-originated hepatocytes are often the main cell sources, and they include a type of cell with potential properties of self-renewal and multi-directional differentiation. They can be classified as totipotent, multipotent, and specialized stem cells. They can develop into a complete living organism, various kinds of tissues, and human organs or cells of a certain lineage, under specific conditions. In recent years, with the progress of regenerative medicine and basic research on stem cells, an increasing number of preclinical and clinical studies have been conducted using different types of stem cells,14 as shown in Table 1.
Table 1.
Cell-infusion clinical studies of liver diseases, based on cell-type.
| Cell type | Research phase | Advantages or limitations |
|---|---|---|
| MSCs | Human study (Phase I and II trials) |
• No ethical restriction. • Easy expansion. • Immune regulation, anti-fibrosis, regeneration. • Most clinical research evidence. |
| ESCs | Preclinical study | • Ethical concern. • Risk of tumorigenicityand immune rejection. |
| iPSCs | Preclinical study | • Tumorigenicity • Immunogenicity |
| BTSCs | Human study (Case report) |
• Multipotent stem cells. • Differentiate into hepatocytes and biliary epithelial cells. • Limited source. |
| Hepatocyte | Human study (Small sample size, randomized controlled trial) |
• Limited cell source from liver doner. • Difficult to expand. • Difficult to cryopreserve. • Immune rejection. |
Abbreviations: MSCs, mesenchymal stem cells; ESCs, embryonic stem cells; iPSCs, Induced pluripotent stem cells; BTSCs, biliary tree stem cells; HSCs, hematopoietic stem cells.
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are pluripotent stem cells derived from the mesoderm and can be isolated or prepared from the bone marrow, umbilical cord, fat, pulp, placenta, endometrial tissue, limbus, and amniotic membrane. In the 1960s, Freidenstein et al discovered a group of colony-forming unit-fibroblast cells from bone marrow that can adhere and grow in vitro, with similar morphology to fibroblasts.15,16 Later, these types of cells with the ability of bone and cartilage differentiation were named mesenchymal stem cells, and this name has been widely used17 since then. Properties of MSCs include multi-directional differentiation, immunomodulatory and pro-angiogenic effects, and secretion of various types of growth factors, cytokines, and regulators through paracrine signaling and other pathways, while generally not causing host immune responses due to their low immunogenicity.18,19 Therefore, after being first used in clinical trials for hematological diseases in 1995, approximately a thousand clinical trials have been carried out with MSCs around the world to explore new ways to treat various refractory diseases. At present, MSCs that function as a type of cell-based drug have been approved for the treatment of graft-versus-host disease (GVHD), Crohn’s disease complicated with anal fistula, spinal cord injury, limb ischemia, amyotrophic lateral sclerosis, and other illnesses in the European Union, Canada, South Korea, and Japan.
Human Embryonic Stem Cells (hESCs)
Human embryonic stem cells (hESCs) are pluripotent stem cells from the blastocyst stage of the cell population in the embryo, with unlimited potential for self-proliferation and differentiation into different cell types in vivo.20 hESCs have recently been used in the treatment of many diseases through their induction into a certain spectrum of stem cells in vitro, and hESC-derived cells are commonly used in clinical trials for the treatment of subacute spinal cord injury, age-related macular degeneration, type 1 diabetes, Parkinson’s disease, retinitis pigmentosa, amyotrophic lateral sclerosis, type 1 citrullinemia, and intrauterine adhesions.21,22 For the treatment of liver disease, hESCs have been induced to differentiate into hepatocyte-like cells with the characteristics of mature hepatocytes in vitro.23 The induction of hESCs into hepatocytes and bile duct cells led to the formation of organoids, which shows promise for the construction of liver disease models and the exploration of new therapeutic approaches for liver diseases.24,25 However, due to the ethical and legal issues concerning the source of hESCs, together with the risk of tumorigenicity and immune rejection after cell infusion, there have been no clinical trials involving hESCs for the treatment of chronic liver diseases.
Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (iPSCs) can be derived from various adult somatic cells in vitro through reverse differentiation via a reprogramming technique first reported in 200722,26; they present a pluripotent ability similar to that of hESCs. Since first described, the reprogramming technique has been widely used in disease modeling, drug screening, tissue engineering, and new therapeutics for the treatment of illnesses,27 such as Parkinson’s disease, macular degeneration, retinitis pigmentosa, spinal cord injury, platelet transfusion, GVHD, and cartilage defects.22 iPSCs can be induced into human hepatocytes that resemble normal-functioning hepatocytes. In an animal model of liver injury, iPSCs were reprogrammed into hepatocyte-like cells and the survival rate of mice with acute liver failure.28 iPSCs-derived hepatocyte-like cells have also been used in the development of disease models such as fatty liver disease and ornithine transcarboxylase deficiency.29,30 Bloor et al performed a dose-escalation phase I trial to evaluate the safety and efficacy of iPSC-derived cells by using human peripheral blood monocyte-derived iPSCs for the treatment of steroid-resistant GVHD.31 However, considering their tumorigenicity and immunogenicity, the safety and efficacy of iPSCs need to be thoroughly evaluated before clinical application.32 Thus, there have been no clinical trials on iPSCs for the treatment of chronic liver diseases.
Notably, Taniguchi’s team first developed the human liver bud including endothelial cells,33 later generated human iPSCs-derived liver organoids that were successfully transplanted into infantile piglets through the portal vein with a good safety.34 The preclinical data demonstrated that transplantation of human liver organoids may present a promising therapeutic strategy in the treatment of severe chronic liver diseases; however, the safety and efficacy of transplantation of human liver organoids need to be confirmed in the future clinical trials.
Biliary Tree Stem Cells (BTSCs)
Biliary tree stem cells (BTSCs) are multipotent stem cells located in both extramural peribiliary glands tethered to the exterior surface of bile ducts and intramural peribiliary glands within bile duct walls or in the villi base of the gallbladder. BTSCs express endoderm-specific transcription factors and early surface molecular markers of stem cells.35 BTSCs have the capacity to differentiate into functional liver cells, bile duct, and pancreatic endocrine glands, and play an important role in the development, maturation, and organ regeneration and maintenance of the liver, pancreas, and gallbladder.36 In animal models of drug-induced liver injury, a transfusion of BTSCs was found to promote the repair and regeneration of the injured liver.37 In a clinical trial, Vincenzo et al found that BTSCs could improve the model for end-stage liver disease (MELD) scores, quality of life, and prolong the survival time in patients with decompensated cirrhosis, without significant post-transplant rejection.38 However, there is an ethical concern that limits their clinical application, as the main source of BTSCs is the fetal biliary tree. Thus, they are not extensively used in clinical trials.
Human Hepatocytes
Human hepatocytes from adult donors have been utilized in various attempts to treat liver diseases39 since Mito et al first performed hepatocyte transplantation in a patient with metabolic liver disease in 1992.40 Transplanted hepatocytes were usually prepared from donor livers that were not suitable for transplantation. However, many factors, including inadequate liver supply, varying quality, immunogenicity, the impaired proliferative ability of hepatocytes, inefficient cell migration, and limited space within a severely pathological liver limit the applications of hepatocyte transplant.41 Hepatocytes are usually more suitable for the treatment of inherited metabolic diseases, such as Wilson’s disease, familial cholestasis, and phenylketonuria. Fox et al found that a pre-treatment of irradiating the host liver could improve the engraftment efficiency of transplanted hepatocytes in an animal model, indicating that pre-treatment radiation was safe and could improve the engraftment of transplanted hepatocytes and the long-term survival of patients.41 In addition, trans-differentiation strategy was developed to generate functional hepatocyte-like cells (iHep) from mature cells, which may, in part, solve the limitation of insufficient human primary hepatocytes for the purpose of cell therapy. Two teams reported that transplantation of iHep cells could rescue mice with liver failure respectively in preclinical studies.42,43 Because transplantation of human hepatocytes is with some disadvantages that significantly limit their clinical application, therefore, it is necessary to develop new sources for functional hepatic cell supply or other novel therapeutic approaches in the treatment of severe chronic liver diseases.44
Clinical Trials and Rationale of MSC Therapy for Chronic Liver Diseases
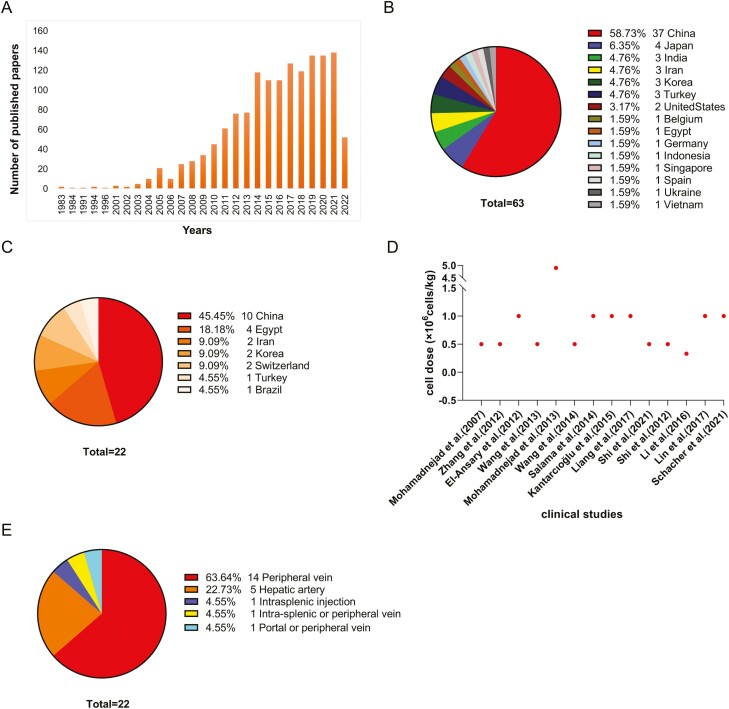
Mesenchymal stem cells (MSCs) are the most commonly used cell source in clinical studies of cell therapy for liver diseases. By searching for “mesenchymal stem cell OR mesenchymal stromal cell AND liver [Title]” on PubMed, 1290 publications were retrieved (year distribution shown in Fig. 1A). Similarly, a search of “mesenchymal stem cell and liver diseases” shows that 63 clinical trials have been registered on ClinicalTrials.gov up to 29 April 2022 (Fig. 1B, 1C).
Figure 1.
A summary of MSCs studies of liver diseases. A. Number of published papers associated with studies on mesenchymal stem cells or mesenchymal stromal cells in liver diseases. These data were obtained on 29 April 2022 (Total = 1290). B. Country and regional distribution of 63 clinical trials registered on ClinicalTrials.gov. C. Country and regional distribution of 22 completed clinical trials shown in Table 2. D. Dosage of MSCs for peripheral intravenous infusion in 14 completed clinical trials shown in Table 2. E. MSC-therapy cell infusion route of 22 completed clinical trials shown in Table 2.
It has been reported that the MELD score, prothrombin time, serum albumin, and total bilirubin were improved in patients with liver cirrhosis or liver failure when they received an MSC infusion.45-51 Suk KT et al conducted a multicenter, open-label, phase II clinical trial to evaluate the treatment of alcoholic liver cirrhosis with autologous bone marrow-derived MSCs.52 A total of 72 patients were randomized into 3 groups, namely, control group, single-infusion group, or double-infusion group. The primary endpoint was the improvement of the fibrosis score, and the secondary endpoints were liver function, Child-Pugh score, and MELD score. Compared to the control group, MSCs significantly improved the fibrosis score and Child-Pugh score at week 24, but there was no significant difference between the single-infusion and double-infusion groups. There was also no significant difference in adverse events among the 3 groups, indicating that MSC infusion is safe and well-tolerated in alcoholic liver cirrhosis patients.52 In another open-label, randomized, controlled trial that enrolled 110 patients with acute-on-chronic liver failure, improvement of liver function, MELD score, control of infection, and fatality were also observed at week 24.53 In recent years, our team has conducted a series of clinical trials using MSCs for treating patients with decompensated cirrhosis, primary biliary cholangitis, acute-on-chronic liver failure, and patients with a post-transplant status. The results revealed that treatment with MSCs could improve the patients’ liver function, increase hepatic functional reserve, reduce post-transplant rejection and complications, and improve quality of life and survival time.54-57 In a 75-month follow-up study of 219 cirrhotic patients who had received an MSC infusion, we found that MSCs could significantly improve patient survival and liver function without increasing tumor incidence and other adverse events.58 However, some studies have also found that MSC infusion did not improve liver function.59-61 The inconsistency in these conclusions may be caused by the varying inclusion and exclusion criteria, endpoints, and sources of MSCs, as well as the small sample size in the majority of trials. In Table 2 and Fig. 1D, we have summarized 22 reported MSC clinical trials for liver cirrhosis and liver failure (11 studies had not been registered on ClinicalTrials.gov).
Table 2.
MSC clinical studies for the treatment of liver cirrhosis and liver failure.
| Country | Author registration number |
Years | Type of study design timing of follow-up visit at endpoint | Liver disease Sample size |
Cell source | Cell dose/each transfusion | Times of infusions | Interval | Infusion route | Endpoints | Major improvements |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iran | Mohamadnejad et al50 / |
2007 | Case series 12 months |
Decompensated liver cirrhosis (n = 4) |
Autologous bone marrow MSC | 3.173 × 107 | 1 | – | Peripheral vein | Safety and feasibility | Creatinine and MELD score |
| Switzerland | Kharaziha et al62 NCT00420134 | 2009 | Single arm 6 months |
Cirrhosis (n = 8) |
Autologous bone marrow MSC | 3-5 × 107 | 1 | – | Portal or peripheral vein | Feasibility, safety and efficacy (LFT and MELD scores) | Creatinine, prothrombin time, and MELD score |
| Egypt | El-Ansary et al63 |
2010 | Case-control 6 months |
Decompensated liver cirrhosis (n = 12) |
Autologous bone marrow MSC | 1 × 107 | 1 | – | Intra-splenic or peripheral vein |
LFT and MELD score improvement | Creatinine, prothrombin time, albumin, bilirubin and MELD score |
| People’s Republic of China | Zhang et al64 NCT0120492 | 2012 | Case-control 24 months |
HBV-related decompensated cirrhosis (n = 45) |
Allogeneic umbilical cord-derived MSC | 0.5 × 106/kg | 3 | Every 4 weeks | Peripheral vein | Safety and efficacy (LFT and MELD scores) | Albumin, bilirubin, MELD score and ascites |
| Egypt | El-Ansary et al47 – |
2012 | Case-control 6 months |
HCV-related decompensated cirrhosis (n = 25) |
Autologous bone marrow MSC | 1 × 106/kg | 1 | – | Peripheral vein | Improvement in LFT and MELD scores | Albumin and MELD score |
| People’s Republic of China | Wang et al56 NCT01662973 | 2013 | Single arm 12 months |
primary biliary cirrhosis (n = 7) |
Allogeneic umbilical cord MSC | 0.5 × 106/kg | 3 | Every 4 weeks | Peripheral vein | Safety and efficacy | Alkaline phosphatase and GGT |
| Iran | Mohamadnejad et al59 – |
2013 | Randomized controlled 12 months |
Decompensated cirrhosis (n = 25) |
Autologous bone marrow MSC | 1.20-2.95 × 108 | 1 | – | Peripheral vein | Absolute change in MELD score | No improvements |
| Egypt | Amin et al46 – |
2013 | Single arm 6 months |
HCV related cirrhosis (n = 20) |
Autologous bone marrow MSC | 1 × 107 | 1 | / | Intrasplenic injection | Safety and efficacy | Albumin, prothrombin time, bilirubin, AST, ALT, and MELD scores |
| People’s Republic of China | Wang et al65 – |
2014 | Single arm 12months |
Primary Biliary Cirrhosis n = 10 |
Allogeneic bone marrowMSC | 3 to 5 × 105/kg | 1 | / | Peripheral vein | Safety and efficacy | Patient quality of life, ALT, AST, GGT and IgM |
| Egypt | Salama et al51 NCT01729221 | 2014 | Randomized controlled 6 months |
HCV-related decompensated cirrhosis (n = 40) |
Autologous bone marrow MSC | 1 × 106/kg | 1 | / | Peripheral vein | Safety and efficacy | Albumin, bilirubin, international normalized ratio, prothrombin, ALT |
| Korea | Jang et al 66NCT01741090 | 2014 | Single arm 6 months |
Alcoholic cirrhosis (n = 11) |
Autologous bone marrow-derived MSC | 5 × 107 | 2 | Every 4 or 8 weeks | Hepatic artery | Improvement of patients’ liver histological features | Child-Pugh score and liver histology |
| People’s Republic of China | Xu et al67 NCT01560845 |
2014 | Randomized controlled 6 months |
HBV related cirrhosis (n = 56) |
Autologous bone marrow-derived MSC | 0.75 ± 0.50 × 106 | 1 | – | Hepatic artery | Absolute change in MELD score and improvement of liver function | Liver function, Treg cells and Th17 cells |
| Turkey | Kantarcıoğlu et al68 NCT01499459 |
2015 | Single arm 12 months |
Liver cirrhosis (n = 25) |
Autologous bone marrow-derived MSC | 1 × 106 | 1 | – | Peripheral vein | Safety and efficacy | Albumin, MELD scores, hepatitis activity index scores |
| Korea | Suk et al69NCT01875081 | 2016 | Randomized controlled 12 months |
Alcoholic cirrhosis (n = 72) |
Autologous bone marrow MSC | 5 × 107 | 1 or 2 | Every 1 month | Hepatic artery | Safety and efficacy | Liver fibrosis and Child-Pugh score |
| People’s Republic of China | Liang et al70 – |
2017 | Single arm 8-70 months |
Cirrhosis associated with autoimmune liver disease (n = 26) | Allogeneic umbilical cord (or cord blood or bone marrow) MSC | 1 × 106/kg | 1 | – | Peripheral vein | Safety and efficacy | ALT, bilirubin, prothrombin time, MELD score |
| Switzerland | Lanthier et al61 |
2017 | Randomized controlled 12 months |
Alcoholic decompensated cirrhosis (n = 58) |
Autologous bone marrow MSC | 0.47 ± 0.15 × 108/kg | 1 | – | Hepatic artery | Safety and efficacy | No improvement |
| People’s Republic of China | Shi et al71NCT01220492 | 2021 | Randomized controlled 75 months |
HBV-related decompensated cirrhosis (n = 219) |
Umbilical cord-derived MSC | 0.5 × 106/kg | 3 | Every 4 weeks | Peripheral vein | Overall survival and liver cancer-free survival | Overall survival, albumin, prothrombin activity, cholinesterase, and total bilirubin |
| People’s Republic of China | Peng et al72 NCT 00956891 | 2011 | Case-control48 months | Chronic hepatitis B liver failure (n = 527) | Autologous bone marrow MSC | 3.4 ± 3.8 × 108 | 1 | – | Hepatic artery | Short-term and long-term efficacy | Albumin, total bilirubin, MELD score, prothrombin time |
| People’s Republic of China | Shi et al55NCT 01218464 | 2012 | Case-control 18 months |
Chronic hepatic failure (n = 43) | Umbilical cord-derived MSC | 0.5 × 106/kg | 3 | Every 4weeks | Peripheral vein | Safety and efficacy | Survival rate, MELD score, globulin, prothrombin activity, direct bilirubin, alanine aminotransferase |
| People’s Republic of China | Li et al73 – |
2016 | Case-control 24 months |
Hepatitis B chronic plus acute liver failure (n = 45) | Umbilical cord stem cell MSC | 0.2 × 108 | 1 | – | Peripheral vein | Safety and efficacy | Albumin, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, prothrombin time (PT), international normalized ratio (INR), Model for End-stage Liver Disease score |
| People’s Republic of China | Lin et al74 – |
2017 | Randomized controlled 6 months | Hepatitis B Chronic acute liver failure n = 110 | Allogeneic bone marrow MSC | 1 to 10 × 105/kg | 4 | Every 1 week | Peripheral vein | Safety and efficacy | 24-week survival rate, MELD score, total bilirubin |
| Brazil | Schacher et al75 – |
2021 | Randomized controlled 3 months |
Chronic hepatic failure (n = 9) | Allogeneic bone marrow MSC | 1 × 106/kg | 5 | Twice in the first and second weeks, and once in the third week. | Peripheral vein | Safety and efficacy | Acute-on-chronic liver failure score |
The rationale for MSC therapy for chronic liver diseases is as follows: (1) owing to the differentiation and regenerative properties of MSCs, they can be stimulated to differentiate into hepatocytes in vitro.76-78 MSCs can also replenish and repair a pathological liver in an animal model79; (2) MSCs exert a range of immunomodulatory effects and regulate innate and adaptive differentiation in vivo including natural killer cells, Kupffer cells, macrophages, dendritic cells, helper T cells, regulatory T cells, and B cells, via direct contact or paracrine signaling to reduce hepatic inflammation and improve host tissue impairment.80-86 (3) MSCs can play a role in improving the hepatic microenvironment and anti-fibrosis. For example, MSCs can secrete interleukin 10 and tumor necrosis factor, which inhibit the activation of hepatic stellate cells (HSCs) and simultaneously induce HSCs apoptosis through the Fas-FasL pathways,87 but they can also induce the regeneration of liver stem cells via hepatocyte growth factor. MSCs can also induce immune cells to produce or directly secrete matrix metalloproteinases for degradation of the extracellular matrix.85,88-90 (4) Ferroptosis is a new form of non-apoptotic cell death that plays a role in the progression of liver diseases. MSCs protect the liver and inhibit the ferroptotic process of hepatocytes through the decrease of intracellular reactive oxygen species (ROS) and Fe2 levels.91 Additionally, Li et al demonstrated that bone marrow MSCs were able to prolong the survival time for fulminant liver failure in a porcine model by blocking the cytokine storm.92 During the COVID-19 pandemic (early 2020), a series of clinical trials were conducted to evaluate the efficiency of MSC therapy for patients with severe COVID-19. Some trials demonstrated that an MSC transfusion could reduce pulmonary inflammation and lesion, improve the convalescence of severe patients, and shorten the length of hospitalization time.93,94 In a multicenter, randomized, double-blind, placebo-controlled trial of 101 patients, we found that MSCs accelerated the restoration of lung lesions and had alleviated pulmonary fibrosis at a one-year follow-up visit.95,96 These findings are consistent with the anti-fibrotic properties of MSCs.
Facing Challenges in MSC Therapy
The treatment of chronic liver diseases with MSCs has yielded some promising findings, but some critical issues in the current protocols remain to be addressed in future studies, including study design, the dosage of transfused MSCs, infusion route of MSCs, and pharmacokinetics and pharmacodynamics (PK/PD) of transfused MSCs in vivo.
Dosage of MSCs
The dosage of MSCs used clinically is a critical issue. Appropriate cell dosage should be carefully determined in the study design based on the source of the cells, patient indication, transfusion time, and infusion route. Phase I clinical studies are often initiated to establish the optimal cell dosages for different indications and infusion routes.31,97-114 Of these, the dose of MSCs administered by peripheral intravenous infusion generally ranges from 5 × 105 to 1 × 106 cells/kg. In a phase I trial of MSC treatment in acute respiratory distress syndrome (ARDS) patients, the low-, medium-, and high-dose groups were 1 × 106 cells/kg, 5 × 106 cells/kg, and 1 × 107 cells/kg, respectively. No infusion-related adverse events were observed in the high-dose group, suggesting that a dose of 1 × 107 cells/kg is safe for ARDS patients.102 As for the treatment of liver diseases, in a dose-escalation study of stem cells, which included a total of 20 patients with decompensated cirrhosis, no adverse events related to cell infusion were observed after 3 rounds of intravenous infusion of umbilical cord stem cells at the highest dose of 2 × 108 cells/time.115 However, the optimal dose for each clinical trial needs to be explored according to the different stages of the disease and administration routine. Figure 2 shows the intravenous infusion dosage used in 14 different clinical studies.
Efficiency and Infusion Route of MSCs
Although unmanipulated, conventional MSCs have been the most widely used in therapeutic studies, extensive efforts have been made to improve the safety and efficiency of MSC transfusions. Some of these strategies, including sorting MSCs to be enriched for stronger functionality, priming MSCs with cytokines, and genetic modification of MSCs, have been developed to enhance the MSCs immunomodulatory potential and/or their homing when they migrate into the target organ with inflammation and loss. MSCs, a heterogeneous population of cells, can be classified into several subgroups. Therefore, pacified and enriched MSCs with selected markers may be more suitable for special conditions than conventional MSCs. For example, MSCs capabilities of chemotaxis, anti-aging, and differentiation could be improved after MSC identification via CD146, CD73, CD271, and CD200.116-118 Furthermore, after coculture with interferon-γ, interleukin-7, and transforming growth factor, the effector cytokines produced by MSCs were increased and their modulation role on immune cells, as well as chemotaxis and proliferative ability, were strengthened.119-121 The gene-editing technique has also been applied to specifically upregulate or silence certain genes (insulin growth factor-like-1, CXCR4, Let7a, etc.) that could result in gene-modified MSCs with stronger anti-fibrotic, immunomodulatory, chemotaxis, anti-apoptotic, differentiated regenerative abilities, and organ-restoration functions.122,123 Although purification methods and gene editing are feasible for MSCs in preclinical studies, there is still a long way to go in terms of cell stability, safety, and compliance with drug-related production specifications. Therefore, the challenge is to balance additional costs and potential logistical/safety concerns.
Different infusion routes may affect the efficacy of MSC treatment. Intravenous infusion is the most common route of MSC administration. Other routes include the hepatic artery, portal vein, and intrahepatic or intra-splenic (Fig. 3) transfusion of MSCs. However, given the differences in the enrolled population, the most suitable source, dosage, and transfusion route of MSC medication have not been confirmed in the reported clinical trials so far, and further randomized, controlled clinical trials with larger sample sizes are needed.
Pharmacokinetics/Pharmacodynamics (PK/PD)
PK/PD measures the distribution of tested drugs and biomarkers in normal or disease models and is further used to analyze the dynamic course of drug absorption, distribution, metabolism, and excretion after drug administration. Therefore, this is an integral part of drug development. PK/PD studies contribute to a better understanding of the relationship between drug exposure, efficacy, and toxicity, and are significant tools to guide the study design for further preclinical and clinical evaluations. Of these, PK/PD-related cell tracking after cell infusion is a key component for evaluating the safety and efficacy of cellular therapy products. Recently, quantitative three-dimensional cryo-imaging, multiple imaging methods, including quantitative magnetic particle imaging, and near-infrared fluorescent semiconductor polymer imaging have been successively used to trace cells after their infusion124-126 to evaluate their distribution across different organs and their changes over time in vivo. These studies, in which MSCs were administered via peripheral intravenous infusion, demonstrated that MSCs were frequently distributed in the liver and lungs of animal models.
Given the characteristics of cell-based products, PK/PD research for application in humans is still in its infancy compared to traditional drugs and may pose uncertain risks to healthy subjects. Therefore, stem cell clinical trials have rarely been conducted in healthy volunteers. In their study, Gholamrezanezhad et al used 125In-oxine-labeled MSCs in decompensated cirrhosis patients and tracked them using MRI.127 MSCs were largely concentrated in the lungs 20 minutes after infusion, and after 2 h, MSCs could be detected in the liver and spleen until 10 days after baseline. These findings are consistent with the conclusions obtained in animal studies and provide a basis for the application of MSCs in the treatment of liver diseases. Accounting for the PK-PD relationship in MSC translational research, combined with better bio-distribution studies, could allow the realization of the potential of a more robust MSC clinical translation.
Perspective
Stem cell therapy, and especially MSC therapy, is generally considered a safe and potentially relevant therapeutic strategy for patients with acute or acute-on-chronic liver failure and decompensated liver cirrhosis. Although these studies provided preliminary evidence on the safety and efficacy of MSC infusions, most clinical trials have been conducted at a single center and with small sample sizes. Further robust, randomized, and controlled clinical studies with a large sample size are required to increase the reliability of MSC therapy and to establish a clinical alternative to treat severe liver diseases. At the same time, owing to the complexity of the clinical process of end-stage liver diseases, the design of the cell-infusion protocol, the time and duration of clinical treatment, and the endpoints at the trials need to be further optimized. The mechanisms of MSC therapy in liver diseases have been studied in vitro; however, cell distribution and related mechanisms in humans have not been fully clarified. We believe that in the near future, several clinical trials will be conducted or completed to generate high-level evidence, which will continuously promote the development of stem cell infusion for the treatment of liver diseases and ultimately benefit the outcome and prognosis of patients with severe liver diseases.
Contributor Information
Tian-Tian Li, Department of Infectious Diseases, Fifth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Infectious Diseases, Beijing, People’s Republic of China; The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, People’s Republic of China.
Ze-Rui Wang, Department of Gastroenterology, First Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China.
Wei-Qi Yao, Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; National Industrial Base for Stem Cell Engineering Products, Tianjin, People’s Republic of China.
En-Qiang Linghu, Department of Gastroenterology, First Medical Center of Chinese PLA General Hospital, Beijing, People’s Republic of China.
Fu-Sheng Wang, Department of Infectious Diseases, Fifth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Infectious Diseases, Beijing, People’s Republic of China.
Lei Shi, Department of Infectious Diseases, Fifth Medical Center of Chinese PLA General Hospital, National Clinical Research Center for Infectious Diseases, Beijing, People’s Republic of China.
Funding
The National Key R&D Program of China (2017YFA0105700), The specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202216), the National Natural Science Foundation of China (82070617), and The Fund of National Clinical Center for Infectious Diseases, PLA General Hospital (NCRC-ID202105,413FZT6).
Conflict of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
F.S.W. proposed initial proposal. F.S.W, L.S. conceived the structure of paper; L.S., T.L., Z.W., and W.Y. drafted the manuscript and drew the figure; L.S., T.L., Z.W., and E.L. collected materials and suggested additional information for the table; F.S.W and L.S. critically revised the manuscript. All authors read and approved the final paper.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. 10.1186/s12916-014-0159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY.. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099-108. 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gines P, Graupera I, Lammert F, et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1(3):256-260. 10.1016/S2468-1253(16)30081-4 [DOI] [PubMed] [Google Scholar]
- 4. Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim D, Li AA, Gadiparthi C, et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154-1163.e3. 10.1053/j.gastro.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh SP, Panigrahi S, Mishra D, Khatua CR.. Alcohol-associated liver disease, not hepatitis B, is the major cause of cirrhosis in Asia. J Hepatol. 2019;70(5):1031-1032. 10.1016/j.jhep.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 7. Arroyo V, Moreau R, Kamath PS, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. 10.1038/nrdp.2016.41 [DOI] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver. European Association for the Study of the L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. [DOI] [PubMed] [Google Scholar]
- 9. Tsochatzis EA, Bosch J, Burroughs AK.. Liver cirrhosis. Lancet. 2014;383(9930):1749-61. 10.1016/S0140-6736(14)60121-5 [DOI] [PubMed] [Google Scholar]
- 10. Allen AM, Kim WR.. Epidemiology and healthcare burden of acute-on-chronic liver failure. Semin Liver Dis. 2016;36(2):123-6. 10.1055/s-0036-1583201 [DOI] [PubMed] [Google Scholar]
- 11. Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144(7): 1426-37, 1437.e1. 10.1053/j.gastro.2013.02.042 [DOI] [PubMed] [Google Scholar]
- 12. European Association for the Study of the Liver. European Association for the Study of the L. Corrigendum to “EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis” [J Hepatol 69 (2018) 406-460]. J Hepatol. 2018;69(5):1207. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Pagan JC, Francoz C, Montagnese S, Senzolo M, Mookerjee RP.. Management of the major complications of cirrhosis: beyond guidelines. J Hepatol. 2021;75(Suppl 1):S135-S146. 10.1016/j.jhep.2021.01.027 [DOI] [PubMed] [Google Scholar]
- 14. Bhatia SN, Underhill GH, Zaret KS, Fox IJ.. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6(245):245sr2. 10.1126/scitranslmed.3005975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83-92. [PubMed] [Google Scholar]
- 16. Till JE, Mc CE.. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213-222. [PubMed] [Google Scholar]
- 17. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-50. 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 18. El Agha E, Kramann R, Schneider RK, et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21(2):166-177. 10.1016/j.stem.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 19. Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ilic D, Ogilvie C.. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35(1):17-25. 10.1002/stem.2450 [DOI] [PubMed] [Google Scholar]
- 21. Trounson A, McDonald C.. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11-22. 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 22. Yamanaka S. Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell. 2020;27(4):523-531. 10.1016/j.stem.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 23. Zaret KS, Grompe M.. Generation and regeneration of cells of the liver and pancreas. Science 2008;322(5907):1490-4. 10.1126/science.1161431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mun SJ, Ryu JS, Lee MO, et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5):970-985. 10.1016/j.jhep.2019.06.030 [DOI] [PubMed] [Google Scholar]
- 25. Ramli MNB, Lim YS, Koe CT, et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology. 2020;159(4):1471-1486.e12. 10.1053/j.gastro.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 26. Zhao T, Fu Y, Zhu J, et al. Single-cell RNA-seq reveals dynamic early embryonic-like programs during chemical reprogramming. Cell Stem Cell. 2018;23(1):31-45.e7. 10.1016/j.stem.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 27. Shi Y, Inoue H, Wu JC, Yamanaka S.. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16(2):115-130. 10.1038/nrd.2016.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagamoto Y, Takayama K, Ohashi K, et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64(5):1068-1075. 10.1016/j.jhep.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 29. Laemmle A, Poms M, Hsu B, et al. Aquaporin 9 induction in human iPSC-derived hepatocytes facilitates modeling of ornithine transcarbamylase deficiency. Hepatology. 2021. 10.1002/hep.32247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Collin de l’Hortet A, Takeishi K, Guzman-Lepe J, et al. Generation of human fatty livers using custom-engineered induced pluripotent stem cells with modifiable SIRT1 metabolism. Cell Metab. 2019;30(2):385-401.e9. 10.1016/j.cmet.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bloor AJC, Patel A, Griffin JE, et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med. 2020;26(11):1720-1725. 10.1038/s41591-020-1050-x [DOI] [PubMed] [Google Scholar]
- 32. Hansel MC, Davila JC, Vosough M, et al. The use of induced pluripotent stem cells for the study and treatment of liver diseases. Curr Protoc Toxicol. 2016;67:14 3 1-143 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481-4. 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- 34. Tsuchida T, Murata S, Hasegawa S, et al. Investigation of clinical safety of human iPS cell-derived liver organoid transplantation to infantile patients in porcine model. Cell Transplant. 2020;29. 10.1177/0963689720964384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54(6):2159-72. 10.1002/hep.24590 [DOI] [PubMed] [Google Scholar]
- 36. Reid LM. Paradoxes in studies of liver regeneration: relevance of the parable of the blind men and the elephant. Hepatology. 2015;62(2):330-3. 10.1002/hep.27917 [DOI] [PubMed] [Google Scholar]
- 37. Kaneko K, Kamimoto K, Miyajima A, Itoh T.. Adaptive remodeling of the biliary architecture underlies liver homeostasis. Hepatology. 2015;61(6):2056-66. 10.1002/hep.27685 [DOI] [PubMed] [Google Scholar]
- 38. Cardinale V, Carpino G, Gentile R, et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014;14:204. 10.1186/s12876-014-0204-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Squires JE, Soltys KA, McKiernan P, et al. Clinical hepatocyte transplantation: what is next? Curr Transplant Rep. 2017;4(4):280-289. 10.1007/s40472-017-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mito M, Kusano M, Kawaura Y.. Hepatocyte transplantation in man. Transplant Proc. 1992;24(6):3052-3. [PubMed] [Google Scholar]
- 41. Soltys KA, Setoyama K, Tafaleng EN, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017;66(5):987-1000. 10.1016/j.jhep.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386-9. 10.1038/nature10116 [DOI] [PubMed] [Google Scholar]
- 43. Guo R, Tang W, Yuan Q, et al. Chemical cocktails enable hepatic reprogramming of mouse fibroblasts with a single transcription factor. Stem Cell Rep. 2017;9(2):499-512. 10.1016/j.stemcr.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A.. Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells 2020;9(2):420. 10.3390/cells9020420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23(10):936-41. 10.1097/MEG.0b013e3283488b00 [DOI] [PubMed] [Google Scholar]
- 46. Amin MA, Sabry D, Rashed LA, et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27(4):607-12. 10.1111/ctr.12179 [DOI] [PubMed] [Google Scholar]
- 47. El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep. 2012;8(3):972-81. 10.1007/s12015-011-9322-y [DOI] [PubMed] [Google Scholar]
- 48. Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34(1):33-41. 10.1111/liv.12218 [DOI] [PubMed] [Google Scholar]
- 49. Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199-205. 10.1097/MEG.0b013e32832a1f6c [DOI] [PubMed] [Google Scholar]
- 50. Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10(4):459-66. doi:07104/AIM.008 [PubMed] [Google Scholar]
- 51. Salama H, Zekri AR, Medhat E, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5(3):70. 10.1186/scrt459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suk KT, Yoon JH, Kim MY, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64(6):2185-2197. 10.1002/hep.28693 [DOI] [PubMed] [Google Scholar]
- 53. Lin BL, Chen JF, Qiu WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017;66(1):209-219. 10.1002/hep.29189 [DOI] [PubMed] [Google Scholar]
- 54. Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112-120. 10.1111/j.1440-1746.2011.07024.x [DOI] [PubMed] [Google Scholar]
- 55. Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10):725-31. 10.5966/sctm.2012-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28(Suppl 1):85-92. 10.1111/jgh.12029 [DOI] [PubMed] [Google Scholar]
- 57. Shi M, Liu Z, Wang Y, et al. A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. 2017;6(12):2053-2061. 10.1002/sctm.17-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shi M, Li YY, Xu RN, et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: a long-term follow-up analysis of the randomized controlled clinical trial. Hepatol Int. 2021;15(6):1431-1441. 10.1007/s12072-021-10199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33(10):1490-6. 10.1111/liv.12228 [DOI] [PubMed] [Google Scholar]
- 60. Yang L, Chang N, Liu X, et al. Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-beta1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J Pathol. 2012;181(1):85-97. 10.1016/j.ajpath.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 61. Lanthier N, Lin-Marq N, Rubbia-Brandt L, et al. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Res Ther. 2017;8(1):88. 10.1186/s13287-017-0541-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kharaziha P, Hellström P, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I–II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199-1205. [DOI] [PubMed] [Google Scholar]
- 63. El-Ansary M, Mogawer S, Abdel-Aziz I, Abdel-Hamid S.. Phase I trial: mesenchymal stem cells transplantation in end stage liver disease. J Am Sci. 2010;6:135-144. [Google Scholar]
- 64. Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112-120. 10.1111/j.1440-1746.2011.07024.x [DOI] [PubMed] [Google Scholar]
- 65. Wang L, Han Q, Chen H, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23(20):2482-2489. 10.1089/scd.2013.0500 [DOI] [PubMed] [Google Scholar]
- 66. Jang Y, Kim Y, Baik S, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34(1):33-41. [DOI] [PubMed] [Google Scholar]
- 67. Xu L, Gong Y, Wang B, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29(8):1620-1628. 10.1111/jgh.12653 [DOI] [PubMed] [Google Scholar]
- 68. Kantarcıoğlu M, Demirci H, Avcu F, et al. Efficacy of autologous mesenchymal stem cell transplantation in patients with liver cirrhosis. Turk J Gastroenterol. 2015;26(3):244-250. [DOI] [PubMed] [Google Scholar]
- 69. Suk K, Yoon J, Kim M, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology (Baltimore, Md). 2016;64(6):2185-2197. [DOI] [PubMed] [Google Scholar]
- 70. Liang J, Zhang H, Zhao C, et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int. J. Rheum. Dis. 2017;20(9):1219-1226. 10.1111/1756-185X.13015 [DOI] [PubMed] [Google Scholar]
- 71. Shi M, Li Y, Xu R, et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: a long-term follow-up analysis of the randomized controlled clinical trial. Hepatol Int. 2021;15(6):1431-1441. 10.1007/s12072-021-10199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Houlihan D, Hopkins L, Suresh S, Armstrong M, Newsome P.. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology (Baltimore, Md). 2011;54(5):1891-2; author reply 2. [DOI] [PubMed] [Google Scholar]
- 73. Li Y, Xu Y, Wu H, et al. Umbilical cord-derived mesenchymal stem cell transplantation in hepatitis B virus related acute-on-chronic liver failure treated with plasma exchange and entecavir: a 24-month prospective study. Stem Cell Rev Rep. 2016;12(6):645-653. 10.1007/s12015-016-9683-3 [DOI] [PubMed] [Google Scholar]
- 74. Lin B, Chen J, Qiu W, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology (Baltimore, Md). 2017;66(1):209-219. [DOI] [PubMed] [Google Scholar]
- 75. Schacher F, Martins Pezzi da Silva A, Silla L, Álvares-da-Silva M.. Bone marrow mesenchymal stem cells in acute-on-chronic liver failure grades 2 and 3: a phase I–II randomized clinical trial. Can J Gastroenterol Hepatol. 2021;2021:3662776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46(1):219-228. 10.1002/hep.21704 [DOI] [PubMed] [Google Scholar]
- 77. Hong SH, Gang EJ, Jeong JA, et al. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330(4):1153-1161. 10.1016/j.bbrc.2005.03.086 [DOI] [PubMed] [Google Scholar]
- 78. Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669-1675. 10.1182/blood-2003-05-1670 [DOI] [PubMed] [Google Scholar]
- 79. Kuo TK, Hung SP, Chuang CH, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008; 134(7): 2111-21, 2121.e1, 21 e1-3. 10.1053/j.gastro.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Prockop DJ, Oh JY.. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14-20. 10.1038/mt.2011.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327-1333. 10.1182/blood-2007-02-074997 [DOI] [PubMed] [Google Scholar]
- 82. Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042-2046. 10.1002/stem.1400 [DOI] [PubMed] [Google Scholar]
- 83. Naji A, Rouas-Freiss N, Durrbach A, et al. Concise review: combining human leukocyte antigen G and mesenchymal stem cells for immunosuppressant biotherapy. Stem Cells. 2013;31(11):2296-2303. 10.1002/stem.1494 [DOI] [PubMed] [Google Scholar]
- 84. Lee KC, Lin HC, Huang YH, Hung SC.. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J Hepatol. 2015;63(6):1405-1412. 10.1016/j.jhep.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 85. Lee CW, Chen YF, Wu HH, Lee OK.. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018;154(1):46-56. 10.1053/j.gastro.2017.09.049 [DOI] [PubMed] [Google Scholar]
- 86. Couwenhoven RI, Luo W, Snead ML.. Co-localization of EGF transcripts and peptides by combined immunohistochemistry and in situ hybridization. J Histochem Cytochem. 1990;38(12):1853-1857. 10.1177/38.12.2254649 [DOI] [PubMed] [Google Scholar]
- 87. Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544-555. 10.1016/j.stem.2012.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Haldar D, Henderson NC, Hirschfield G, Newsome PN.. Mesenchymal stromal cells and liver fibrosis: a complicated relationship. FASEB J. 2016;30(12):3905-3928. 10.1096/fj.201600433R [DOI] [PubMed] [Google Scholar]
- 89. Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45(1):213-222. 10.1002/hep.21477 [DOI] [PubMed] [Google Scholar]
- 90. Meier RP, Mahou R, Morel P, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. 2015;62(3):634-641. 10.1016/j.jhep.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 91. Sun D, Yang L, Zheng W, et al. Protective effects of bone marrow mesenchymal stem Cells (BMMSCS) combined with normothermic machine perfusion on liver grafts donated after circulatory death via reducing the ferroptosis of hepatocytes. Med. Sci. Monit. 2021;27:e930258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shi D, Zhang J, Zhou Q, et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. 2017;66(5):955-964. 10.1136/gutjnl-2015-311146 [DOI] [PubMed] [Google Scholar]
- 93. Shi L, Wang L, Xu R, et al. Mesenchymal stem cell therapy for severe COVID-19. Signal Transduct Target Ther. 2021;6(1):339. 10.1038/s41392-021-00754-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu R, Feng Z, Wang F.. Mesenchymal stem cell treatment for COVID-19. EBioMedicine. 2022;77:103920. 10.1016/j.ebiom.2022.103920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. 10.1038/s41392-021-00488-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shi L, Yuan X, Yao W, et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2021;75:103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277-2286. 10.1016/j.jacc.2009.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164(5):966-972.e6. 10.1016/j.jpeds.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 99. Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254-1266. 10.1002/stem.1634 [DOI] [PubMed] [Google Scholar]
- 100. Perin EC, Borow KM, Silva GV, et al. A Phase II dose–escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117(6):576-584. 10.1161/CIRCRESAHA.115.306332 [DOI] [PubMed] [Google Scholar]
- 101. Skyler JS, Fonseca VA, Segal KR, Rosenstock J, Investigators M-D.. Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose–escalation safety and tolerability pilot study. Diabetes Care. 2015;38(9):1742-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24-32. 10.1016/S2213-2600(14)70291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mordant P, Nakajima D, Kalaf R, et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J Heart Lung Transplant. 2016;35(10):1245-1254. 10.1016/j.healun.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 104. Packham DK, Fraser IR, Kerr PG, Segal KR.. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263-269. 10.1016/j.ebiom.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pers YM, Rackwitz L, Ferreira R, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847-856. 10.5966/sctm.2015-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Alvaro-Gracia JM, Jover JA, Garcia-Vicuna R, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76(1):196-202. 10.1136/annrheumdis-2015-208918 [DOI] [PubMed] [Google Scholar]
- 107. Golpanian S, DiFede DL, Khan A, et al. Allogeneic human mesenchymal stem cell infusions for aging frailty. J Gerontol A Biol Sci Med Sci. 2017;72(11):1505-1512. 10.1093/gerona/glx056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ahn SY, Chang YS, Sung SI, Park WS.. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose–escalation clinical trial. Stem Cells Transl Med. 2018;7(12):847-856. 10.1002/sctm.17-0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Levy ML, Crawford JR, Dib N, et al. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. 2019;50(10):2835-2841. 10.1161/STROKEAHA.119.026318 [DOI] [PubMed] [Google Scholar]
- 110. Schlosser K, Wang JP, Dos Santos C, et al. Effects of mesenchymal stem cell treatment on systemic cytokine levels in a phase 1 dose escalation safety trial of septic shock patients. Crit Care Med. 2019;47(7):918-925. 10.1097/CCM.0000000000003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Singer W, Dietz AB, Zeller AD, et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology. 2019;93(1):e77-e87. 10.1212/WNL.0000000000007720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Abumoawad A, Saad A, Ferguson CM, et al. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020;97(4):793-804. 10.1016/j.kint.2019.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gorman E, Shankar-Hari M, Hopkins P, et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: a phase 1 trial. EClinicalMedicine. 2021;41:101167. 10.1016/j.eclinm.2021.101167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schiess M, Suescun J, Doursout MF, et al. Allogeneic bone marrow-derived mesenchymal stem cell safety in idiopathic parkinson’s disease. Mov Disord. 2021;36(8):1825-1834. 10.1002/mds.28582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fu Q, Jiang S, Wang X, et al. Safety and escalation study of human mesenchymal stem cells for patients with decompensated liver cirrhosis. Chin Hepatol. 2014;019(001):3-7. [Google Scholar]
- 116. Alfaifi M, Eom YW, Newsome PN, Baik SK.. Mesenchymal stromal cell therapy for liver diseases. J Hepatol. 2018;68(6):1272-1285. 10.1016/j.jhep.2018.01.030 [DOI] [PubMed] [Google Scholar]
- 117. Suto EG, Mabuchi Y, Suzuki N, et al. Prospectively isolated mesenchymal stem/stromal cells are enriched in the CD73(+) population and exhibit efficacy after transplantation. Sci Rep. 2017;7(1):4838. 10.1038/s41598-017-05099-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ulrich C, Abruzzese T, Maerz JK, et al. Human placenta-derived CD146-positive mesenchymal stromal cells display a distinct osteogenic differentiation potential. Stem Cells Dev. 2015;24(13):1558-1569. 10.1089/scd.2014.0465 [DOI] [PubMed] [Google Scholar]
- 119. de Witte SF, Franquesa M, Baan CC, Hoogduijn MJ.. Toward development of imesenchymal stem cells for immunomodulatory therapy. Front Immunol. 2015;6:648. 10.3389/fimmu.2015.00648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29(10):1549-1558. 10.1002/stem.698 [DOI] [PubMed] [Google Scholar]
- 121. Pourgholaminejad A, Aghdami N, Baharvand H, Moazzeni SM.. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51-60. [DOI] [PubMed] [Google Scholar]
- 122. Almalki SG, Llamas Valle Y, Agrawal DK.. MMP-2 and MMP-14 silencing inhibits VEGFR2 cleavage and induces the differentiation of porcine adipose-derived mesenchymal stem cells to endothelial cells. Stem Cells Transl Med. 2017;6(5):1385-1398. 10.1002/sctm.16-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lee PH, Tu CT, Hsiao CC, et al. Antifibrotic activity of human placental amnion membrane-derived CD34+ mesenchymal stem/progenitor cell transplantation in mice with thioacetamide-induced liver injury. Stem Cells Transl Med. 2016;5(11):1473-1484. 10.5966/sctm.2015-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schmuck EG, Koch JM, Centanni JM, et al. Biodistribution and clearance of human mesenchymal stem cells by quantitative three-dimensional cryo-imaging after intravenous infusion in a rat lung injury model. Stem Cells Transl Med. 2016;5(12):1668-1675. 10.5966/sctm.2015-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zheng B, von See MP, Yu E, et al. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics. 2016;6(3):291-301. 10.7150/thno.13728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen D, Li Q, Meng Z, et al. Bright polymer dots tracking stem cell engraftment and migration to injured mouse liver. Theranostics. 2017;7(7):1820-1834. 10.7150/thno.18614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gholamrezanezhad A, Mirpour S, Bagheri M, et al. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38(7):961-967. doi: 10.1016/j.nucmedbio.2011.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.