Abstract
Objective
To investigate the efficacy of home-based gait training using the wearable Stride Management Assist (SMA) exoskeleton in people with moderately advanced Parkinson's disease.
Methods
This was a single-center, open-label, parallel, randomized controlled trial. We included outpatients with idiopathic Parkinson's disease who were capable of walking independently with or without walk aids and had Hoehn and Yahr stage 2-4 in the ON state. Patients were randomly assigned (1:1 ratio) to receive either SMA gait training (SMA group) or control gait training (control group). All participants underwent gait training for approximately 30 min. These training sessions were conducted 10 times for 3 months. We measured clinical outcomes at baseline and post-intervention. The between-group difference of distance in the three-minute walk test was the primary outcome.
Results
Of the 15 randomly assigned participants, 12 (five in the SMA group) completed this study. The between-group difference was a mean of 13.7 meters (standard error of the mean: 7.8) in the 3-minute walk test (p=0.109). The distance traversed increased from 141.4 m to 154.7 m in the SMA group (p=0.023), whereas there was no marked change in the control group. In addition, although there was a decrease in the physiological cost index from 0.29 to 0.13 in the SMA group (p=0.046), it remained unchanged in the control group.
Conclusion
These findings suggest that home-based SMA gait training may increase the exercise endurance in people with moderately advanced Parkinson's disease.
Keywords: clinical trial, randomized controlled, Parkinson's disease, rehabilitation, wearable robot, gait training
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by various motor and non-motor symptoms (1). As the disease progresses, patients experience disabilities in gait, balance, and posture, which are often not resolved by medication or surgical treatment (1-3). These disabilities create problems with outdoor mobility. In addition, a decrease in physical activity contributes to a worsening of the disease prognosis and deterioration of the quality of life (4).
Exercise and physical therapy compliment medical and surgical therapy by improving balance, gait, and motor coordination (3,4). Resistance training, aerobic exercise, and balance training improve mobility (4). Goal-based and aerobic exercises might induce neuronal plasticity by increasing the blood flow to the brain, enhancing synaptic connectivity, and supporting neural circuits (5). Neurorehabilitation programs, including visual rehabilitation, robot-assisted physiotherapy, tai chi, and dance and music therapy, have been newly developed for the management of PD (3,4,6,7).
The Stride Management Assist exoskeleton (SMA; Honda R&D, Tokyo, Japan) is an automated device worn around the hips that assists individuals during ambulation (8-12). SMA-based gait training was reported to increase step length, walking speed, and spatial symmetry in post-stroke patients (10-12). However, there have been few reports on SMA gait training in people with PD. The device can be easily used in home-based training. In addition, SMA-based rehabilitation would likely enhance the physical and social activities in people with PD.
Our primary aim was to compare the effect of SMA-based gait training with that of control training on the development of physical endurance in patients with moderately advanced PD.
Materials and Methods
Study design
We conducted a single-center, open-label, randomized controlled trial. The participants were randomly assigned (1:1 ratio) to receive either SMA gait training (SMA group) or control gait training (control group). We used the minimization method over two stratification factors of age and disease duration for the random allocation. We set the reference values for the allocation adjustment factor at 75 years old and 8 years' disease duration, according to our previous experience. In addition, we used centralized allocation concealment.
Standard protocol approval, registration, and patient consent
Our study was approved by the ethics committee of the National Hospital Organization, Sagamihara National Hospital. We obtained written informed consent from all participants. In addition, we registered the study with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000040131).
Participants
We recruited people who met the Movement Disorder Society clinically established PD criteria (13) from Kawashima Neurology Clinic. The eligibility criteria were as follows: outpatients with clinically established PD capable of walking independently with/without walking aids; stage 2-4 on the Hoehn and Yahr scale in the ON state; unified Parkinson's disease rating scale (UPDRS) part III score of ≥10 points in the ON state; same medication dose and dose schedule for at least 4 weeks before the enrollment; score ≥26 points for Mini-Mental State Examination (MMSE); and the absence of an unpredictable OFF state.
The exclusion criteria were as follows: patients with clinically established PD who were unable to walk with walking aids; stage 1 or 5 on the Hoehn and Yahr scale in the ON state; UPDRS part III score <10 points in the ON state; score <26 points for MMSE; presence of an unpredictable OFF state; or people with orthopedic and cardiac comorbidities which restrict exercise training.
SMA device
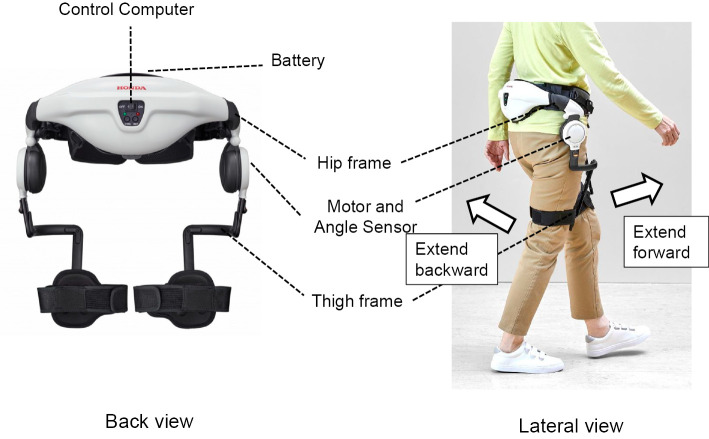
The SMA exoskeleton was certified under ISO 13482 for assistant robots (Fig. 1). The SMA comprises an inverted pendulum model that supports walking, which embodies the theory for bipedal walking of the robot (14). The device can automatically detect the hip joint angle and provide torque to assist in the flexion and extension of the joint (Fig. 1). Its specifications were as follows: width, 430-495 mm; weight (with battery), approximately 2.7 kg; rechargeable lithium-ion battery; single-charge operation time, approximately 60 min; and motor-output, maximum torque 4 N・m. In addition, it has features of both patient-in-charge support (following mode) and device-in-charge support (symmetry mode and step mode) (15).
Figure 1.
Exoskeleton Stride Management Assist (Honda R&D, Tokyo, Japan).
We used both the step and following modes for the gait training. The step mode was preset in the device, and the participants had to repeat three steps using the SMA. While using the following mode, we adjusted the assist torque at the hip joints to an optimum degree by interviewing the participants and having nationally qualified physical or occupational therapists observe the gait.
Intervention
The participants underwent overground gait training for 30 min per session. These training sessions were conducted 10 times for 3 months outdoors near each participant's home on good weather days. We used a home-visit rehabilitation service under the long-term care insurance system of Japan. In the SMA group, the participants performed step training using the step mode for approximately 5 min. In addition, they performed overground walking using the following mode for approximately 25 min. In contrast, the participants in the control group performed similar step training and overground walking without the SMA. We verified the immediate effects of the SMA the first six times on different days in a 10-meter walk test (10MWT) in the SMA group (Fig. 2). All gait training sessions were conducted by nationally qualified physical or occupational therapists.
Figure 2.
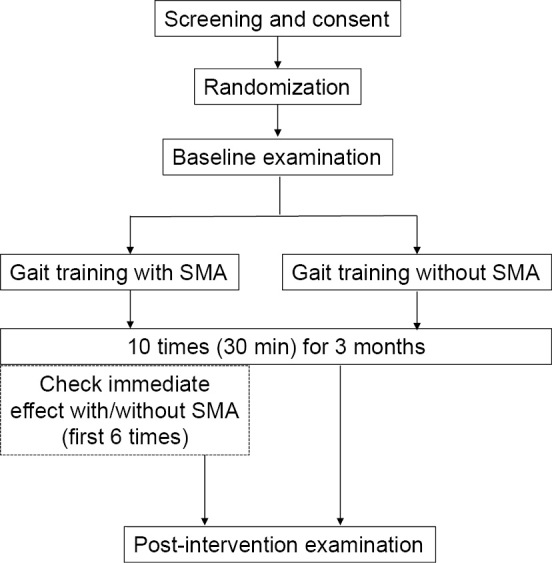
Flow chart of intervention. SMA: Stride Management Assist
Outcome measures
The distance traversed in a 3-minute walk test (3MWT) was the primary outcome. We did not select a 6-minute walk test (6MWT) because some patients are unable to walk for 6 minutes, in our experience. The secondary outcomes comprised the physiological cost index (PCI) in the 3MWT, 10MWT, Berg Balance Scale (BBS), Functional Reach Test (FRT), UPDRS total, part II, and part III in the ON state, Movement Disorders Society-unified Parkinson's disease rating scale (MDS-UPDRS) item 3.11 (freezing of gait), Freezing of Gait Questionnaire (FGQ) (16), Parkinson's Disease Questionnaire-39 (PDQ-39) (17), EuroQOL-5 dimensions-5-level (EQ-5D-5L) (18), and gait and posture analysis using the Mobile Motion Visualizer. A self-selected walking speed was used for all walking tests. The clinical outcomes at baseline and post-intervention were assessed by the same evaluators, who were not blinded. All of the outcomes were measured in the ON state.
Mobile Motion Visualizer
We evaluated participants' gait using the Mobile Motion Visualizer (MMV-001; System Friend, Hiroshima, Japan). This device uses an infrared camera to analyze the gait with ankle joints markers. In addition, it also facilitates analyzing forward and lateral bending of the thoracolumbar spine without markers (19). We defined the angle of the trunk tilt as the angle between the vertical line on the coronal plane and the line connecting the center of the shoulder joints with the center of the hip joints. We evaluated the mean step length, walking speed and average forward and lateral bending angles of thoracolumbar spine while walking for approximately 3 m using the device. The participants completed two gait assessments for each measure, and we calculated the mean of the two assessments.
Statistical analyses
Although there were no preliminary data to predict the changes in either group between baseline and post-intervention in distance traversed in a 3MWT, we suspected that the changes in the SMA and control groups would be 15 m and 2 m with a standard deviation of 10 m for both groups, based on our previous experience. The estimated total sample size for the comparison was 14 patients with a power of 80% using a 2-sided test at a 5% significance level.
We performed a descriptive statistical analysis for all variables. We conducted two-sided unpaired t-tests for the continuous variable and Fisher's exact or chi-squared test for the categorical variables. This facilitated comparing the baseline characteristics between the groups. While the two-sided unpaired t-tests assessed the difference in changes between the two groups, paired t-tests helped determine the differences in changes within the groups. A p value <0.05 was considered statistically significant. We used the SPSS Statistics 25 software program (IBM, Armonk, USA) for statistical analyses.
Results
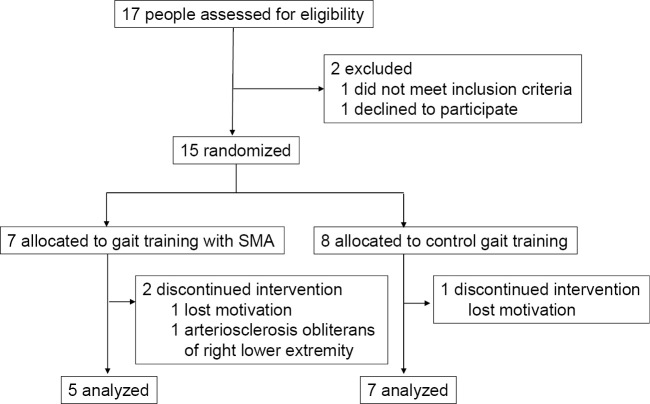
We selected 17 individuals who met our eligibility criteria for the study between October 2017 and March 2020. One person declined to participate, and another failed to meet the inclusion criteria. Ultimately, 15 participants were randomized to the SMA group (n=7) and the control group (n=8). Three of them did not complete the allocated exercise. Thus, we analyzed the outcomes of the remaining 12 participants who completed the intervention (Fig. 3).
Figure 3.
Flow diagram of the participants. SMA: Stride Management Assist
There were no marked differences in the baseline characteristics between the groups (Table 1). Table 2 summarizes the immediate effects of the SMA in the first six trials on different days of five participants in the 10MWT. We observed an increase in the walking speed in the 10MWT from 51.0 m/min to 57.0 m/min (p=0.000). This was concomitant with an increase in the step length from 0.472 m to 0.524 m (p=0.000). In addition, there was a significant improvement in the ranges of flexion and extension mobility and scissor angles of both thighs (Table 2).
Table 1.
Baseline Characteristics of Participants.
| Patients who participated the study | Patients who completed the study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SMA group (n=7) | C group (n=8) | p value | 95% CI | SMA group (n=5) | C group (n=7) | p value | 95% CI | ||
| Age (years) | 77.0 (4.4) | 76.6 (6.3) | 0.90* | -6.5-5.8 | 76.6 (5.3) | 75.4 (5.7) | 0.73* | -8.5-6.1 | |
| Male/Female | 3/4 | 1/7 | 0.28** | 2/3 | 1/6 | 0.53** | |||
| Educational background (years) | 13.6 (3.4) | 14.5 (1.4) | 0.52* | -2.2-4.1 | 13.4 (3.8) | 14.9 (1.1) | 0.45* | -3.3-6.2 | |
| Duration of disease (years) | 9.7 (5.5) | 11.3 (5.4) | 0.60* | -4.6-7.6 | 11.2 (5.8) | 12.4 (4.6) | 0.69* | -5.5-7.9 | |
| Hoehn and Yahr stage | 0.12*** | 0.46*** | |||||||
| 2 | 3 (42.9 %) | 5 (62.5 %) | 3 (60%) | 5 (71.4%) | |||||
| 3 | 4 (57.1 %) | 1 (12.5 %) | 2 (40%) | 1 (14.3%) | |||||
| 4 | 0 | 2 (25.0 %) | 0 | 1 (14.3%) | |||||
| UPDRS total | 35.7 (14.2) | 35.4 (8.9) | 0.96* | -13.3-12.6 | 35.2 (16.8) | 34.0 (8.6) | 0.87* | -17.5-15.1 | |
| UPDRS part II in ON state | 11.0 (8.0) | 10.0 (5.0) | 0.77* | -8.4-6.4 | 11.4 (9.4) | 9.3 (5.0) | 0.62* | -11.4-7.1 | |
| UPDRS part III | 20.0 (5.0) | 19.8 (5.6) | 0.93* | -6.2-5.7 | 19.0 (5.8) | 18.7 (5.2) | 0.93* | -7.4-6.8 | |
| Fall (1 or more/month) | 3 (42.9 %) | 1 (12.5 %) | 0.28** | 2 (40%) | 1 (14.3%) | 0.52** | |||
| Number of comorbidities | 1.3 (1.1) | 1.0 (0.9) | 0.60* | -1.4-0.9 | 0.80 (0.8) | 0.86 (0.9) | 0.91* | -1.1-1.2 | |
| MMSE | 28.3 (1.4) | 28.3 (1.6) | 0.96* | -1.7-1.6 | 28.2 (1.6) | 28.6 (1.4) | 0.68* | -1.6-2.3 | |
| FAB | 14.3 (1.9) | 14.9 (1.5) | 0.51* | -1.3-2.5 | 13.8 (1.8) | 15.0 (1.5) | 0.24* | -0.9-3.3 | |
| Height (cm) | 155.1 (7.3) | 151.0 (9.8) | 0.38* | -13.8-5.7 | 153.7 (6.0) | 151.6 (10.4) | 0.69* | -13.8-9.5 | |
| Weight (kg) | 51.8 (11.9) | 47.8 (6.5) | 0.43* | -14.4-6.5 | 51.2 (11.3) | 48.2 (6.9) | 0.59* | -14.5-8.7 | |
| Body mass index | 21.3 (3.3) | 21.0 (2.5) | 0.84* | -3.5-2.9 | 21.5 (3.4) | 21.0 (2.7) | 0.79* | -4.4-3.5 | |
| LED (mg/day) | 836.5 (296.1) |
761.9 (247.2) |
0.60* | -377.5-228.3 | 758.5 (319.7) |
785.1 (257.5) |
0.88* | -344.0-397.1 | |
| Walking aid | 0.85*** | 0.98*** | |||||||
| Nothing | 2 (28.6 %) | 3 (37.5 %) | 2 (40.0%) | 3 (42.9%) | |||||
| Noldic pole | 1 (14.3 %) | 2 (25.0 %) | 1 (20.0%) | 2 (28.6%) | |||||
| T-cane | 2 (28.6 %) | 1 (12.5 %) | 1 (20.0%) | 1 (14.3%) | |||||
| Walker | 2 (28.6 %) | 2 (25.0 %) | 1 (20.0%) | 1 (14.3%) | |||||
| 3-minute walk test (3MWT, m) | 131.9 (40.4) | 139.5 (44.7) | 0.74* | -40.2-55.4 | 141.4 (32.1) | 142.5 (47.4) | 0.96* | -53.7-55.9 | |
| PCI in 3MWT (beat/m) | 0.46 (0.45) | 0.34 (0.32) | 0.55* | -0.56-0.31 | 0.29 (0.08) | 0.34 (0.13) | 0.78* | 0.14-0.49 | |
Data are mean (SD) or n.
*Unpaired t-test, **Fisher’s exact test, ***Chi-squared test
C group: control group, FAB: frontal assesment battery, LED: levodopa equivalent dose20), MMSE: Mini-Mental State Examination, SMA group: Stride Management Assist group, CI: confidence interval, UPDRS: Unified Parkinson's Disease Rating Scale, PCI: physiological cost index
Table 2.
The Immediate Changes from Device off to on Mode in 10-meter Walk Test (30 Trials).
| Device off | Device on | p | CI | |||||
|---|---|---|---|---|---|---|---|---|
| Walking speed (m/min) | 51.0 (3.0) | 57.0 (3.7) | 0.000 | 3.7-8.4 | ||||
| Walking speed (m/s)* | 0.85 (0.05) | 0.95 (0.06) | ||||||
| Step length (m) | 0.472 (0.023) | 0.524 (0.022) | 0.000 | 3.7-6.8 | ||||
| Cadence (steps/min) | 106.3 (2.4) | 106.6 (2.7) | 0.800 | -2.6-3.3 | ||||
| Range of flexion mobility of left thigh (degrees) | 19.3 (1.3) | 27.8 (1.5) | 0.000 | 6.9-10.0 | ||||
| right thigh (degrees) | 21.5 (1.3) | 29.2 (1.7) | 0.000 | 6.0-9.4 | ||||
| Range of extension mobility of left thigh (degrees) | -5.4 (0.8) | -8.7 (1.0) | 0.000 | -4.8- -2.0 | ||||
| right thigh (degrees) | -5.5 (0.8) | -9.9 (1.1) | 0.000 | -5.9- -3.1 | ||||
| Symmetry of mobility between both thighs | 0.8 (0.03) | 0.9 (0.01) | 0.334 | -0.04-0.10 | ||||
| Range of scissor angle on left thigh (degrees) | 24.8 (1.5) | 37.8 (1.5) | 0.000 | 11.0-14.9 | ||||
| right thigh (degrees) | 26.9 (1.6) | 37.9 (2.0) | 0.000 | 8.8-13.2 | ||||
| Symmetry of scissor angle between both thighs | 0.8 (0.03) | 0.9 (0.02) | 0.109 | -0.01-0.12 |
Data are mean (SEM). *Obtained by calculation. CI: confidence interval
The mean group difference for the walking distance in the 3MWT was 13.7 m [standard error of the mean (SEM), 7.8 m] (p=0.109). There were no significant group differences in the secondary outcomes. However, we observed a reduction in the PCI [mean group difference, 0.18 beats/m (SEM, 0.11 beats/m), p=0.147] and an improvement in the UPDRS total and part III score [mean group difference, 4.9 (SEM, 2.9) and 3.6 (SEM, 2.3), respectively] in the SMA group (Table 3).
Table 3.
Primary and Secondary Outcomes.
| Baseline | 3 months | Within-group change | Between-group difference in change | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMA group | C group | SMA group | C group | SMA group | p value | C group | p value | Mean (SEM) | p value | 95% CI | ||||
| Primary outcome | ||||||||||||||
| 3-minute walk test (3MWT, m) | 141.4 (14.4) | 142.5 (17.9) | 154.7 (15.9) | 142.1 (17.0) | 13.3 (3.7) | 0.023 | -0.4 (6.0) | 0.949 | -13.7 (7.8) | 0.109 | -31.2-3.7 | |||
| Walking speed (m/s)** | 0.79 (0.08) | 0.79 (0.10) | 0.86 (0.09) | 0.79 (0.09) | 0.07 (0.02) | 0.023 | -0.002 (0.033) | 0.949 | -0.08 (0.04) | 0.109 | -0.17-0.02 | |||
| Secondary outcomes | ||||||||||||||
| PCI in 3MWT (beats/min) | 0.29 (0.08) | 0.34 (0.13) | 0.13 (0.05) | 0.35 (0.14) | -0.16 (0.06) | 0.046 | 0.02 (0.09) | 0.850 | 0.18 (0.11) | 0.147 | -0.07-0.43 | |||
| 10-meter walk test (s) | 12.5 (2.5) | 13.7 (2.1) | 11.5 (2.4) | 13.3 (1.7) | -1.0 (0.7) | 0.206 | -0.3 (0.7) | 0.648 | 0.7 (1.0) | 0.488 | -1.5-3.0 | |||
| Walking speed (m/s)** | 0.90 (0.13) | 0.85 (0.14) | 1.01 (0.17) | 0.84 (0.13) | 0.11 (0.06) | 0.165 | -0.01 (0.03) | 0.880 | -0.11 (0.07) | 0.114 | -0.26-0.33 | |||
| Berg Balance Scale | 49 (2.4) |
49.4 (2.3) | 51.0 (2.2) | 49.4 (2.1) | 2.0 (1.3) |
0.200 | 0.0 (0.4) |
1.000 | -2.0 (1.4) | 0.207 | -5.6-1.6 | |||
| Functional Reach Test | 25.3 (2.0) | 21.4 (3.2) | 25.7 (2.5) | 20.1 (3.3) | 0.4 (1.4) | 0.785 | -1.2 (2.4) | 0.626 | -1.6 (3.1) | 0.609 | -8.4-5.2 | |||
| UPDRS total | 35.2 (7.5) | 34.0 (3.3) | 30.6 (5.1) | 34.9 (5.6) | -4.6 (2.8) | 0.172 | 0.3 (1.5) |
0.853 | 4.9 (2.9) |
0.122 | -1.6-11.3 | |||
| UPDRS part II in ON state | 11.4 (4.2) | 9.3 (1.9) |
9.2 (2.9) |
8.9 (2.1) |
-2.2 (2.5) | 0.425 | -0.4 (1.2) | 0.723 | 1.8 (2.5) |
0.490 | -3.7-7.3 | |||
| UPDRS part III | 19.0 (2.6) | 18.7 (1.9) | 15.8 (1.6) | 19.1 (2.6) | -3.2 (2.5) | 0.276 | 0.4 (0.8) |
0.617 | 3.6 (2.3) |
0.148 | -1.5-8.8 | |||
| MDS-UPDRS item 3.11 (freezing of gait) |
1.0 (0.3) |
0.4 (0.2) |
1.0 (0.4) |
0.3 (0.2) |
0.0 (0.3) |
1.000 | -0.1 (0.1) | 0.356 | -0.1 (0.3) | 0.658 | -0.8-0.6 | |||
| Freezing of Gait Questionnaire | 12.2 (1.6) | 8.4 (1.2) |
11.4 (1.7) | 10.6 (1.8) | -0.8 (0.7) | 0.338 | 2.1 (1.6) |
0.239 | 2.9 (2.1) |
0.183 | -1.6-7.5 | |||
| PDQ-39 SI | 28.6 (9.3) | 32.6 (5.9) | 24.9 (6.7) | 31.3 (5.8) | -3.7 (6.0) | 0.566 | -1.3 (2.2) | 0.561 | 2.4 (5.6) |
0.676 | -10.1-14.9 | |||
| EQ-5D-5L utility measure | 0.662 (0.098) | 0.710 (0.060) | 0.701 (0.116) | 0.590 (0.096) | 0.040 (0.080) | 0.649 | -0.120 (0.072) | 0.157 | -0.160 (0.108) | 0.173 | -0.404-0.085 | |||
| Visual analog scale (%) | 59.0 (7.1) | 68.3 (6.0) | 65.0 (10.7) | 66.7 (6.1) | 6.0 (5.8) |
0.358 | -1.7 (3.1) | 0.611 | -7.7 (6.2) | 0.250 | -21.8-6.4 | |||
| Gait and posture analysis using Mobile Motion Visualizer* | ||||||||||||||
| Walking speed (m/min) | 47.6 (2.0) | 37.3 (6.6) | 49.5 (4.3) | 35.3 (4.3) | 1.9 (4.0) |
0.662 | -1.9 (2.5) | 0.485 | -3.8 (4.7) | 0.442 | -14.8-7.1 | |||
| Step length (cm) | 43.9 (1.7) | 33.2 (0.0) | 44.5 (4.3) | 32.3 (0.0) | 0.6 (2.8) |
0.842 | 0.0 (0.0) |
0.529 | 0.0 (0.0) |
0.643 | -0.1-0.1 | |||
| Anterior flexion (degree) | 1.8 (1.3) |
0.8 (3.8) |
2.0 (1.6) |
0.3 (3.5) |
0.2 (0.4) |
0.596 | -0.5 (0.5) | 0.449 | -0.7 (0.7) | 0.340 | -2.3-0.9 | |||
| Lateral flexion (right) (degree) | 5.1 (1.6) |
12.6 (4.9) | 5.2 (1.6) |
12.3 (4.9) | 0.4 (0.4) |
0.405 | 0.3 (3.0) |
0.928 | 0.1 (3.0) |
0.975 | -6.9-7.1 | |||
Data are mean (SEM). *Participants numbers were 5 in SMA group and 5 in C group. **Obtained by calculation
C group: control group, CI: confidence interval, EQ-5D-5L: EuroQOL-5 dimensions-5-level, MDS-UPDRS: Movement Disorders Society-unified Parkinson’s disease rating scale, PCI: physiological cost index, PDQ-39 SI: Parkinson’s Disease Questionnaire-39 Summary Index, UPDRS: unified Parkinson’s disease rating scale, SMA group: Stride Management Assist group, 3MWT: 3-minute walk test
Regarding changes within the groups, we found an increase in the walking distance in the 3MWT from 141.4 m to 154.7 m in the SMA group [95% confidence interval (CI): 2.9 to 23.7, p=0.023], although no marked change was noted in the control group (Table 3). Furthermore, while there was a reduction in the PCI from 0.29 to 0.13 in the SMA group (95% CI: -0.321 to- 0.004, p=0.046), no marked change was seen in the control group. We conducted gait and posture analyses in 10 of the 12 participants (5 per group) using the Mobile Motion Visualizer. However, the visualizer failed to detect improvements in walking speed, step length, or anterior or lateral flexion in either group (Table 3).
We did not change the antiparkinsonian medications during the intervention. Furthermore, no serious adverse events were reported. In addition to the observed considerable acceptability of the intervention, some participants reported walking more easily using the SMA than without it.
Discussion
Our results revealed that gait training using the SMA was able to increase exercise endurance in people with moderately advanced PD. This may be due to the improvement in the walking distance in the 3MWT in the intervention group. The minimal clinically important difference (MCID) of distance in the 6MWT has been reported to range from 14.0 to 30.5 m in various diseases (21). The mean group difference of distance in the 3MWT in our study was 13.7 m, which is over the MCID in the 3MWT. According to Hass et al., the clinically important differences (CIDs) in the walking speed of people with PD were 0.06 m/s, 0.14 m/s, and 0.22 m/s for small, moderate, and large degrees, respectively, according to their distribution-based analysis (22). In addition, the CIDs in the walking speed of people with PD were 0.02 m/s, 0.06 m/s, and 0.10 m/s for small, moderate, and large degrees, respectively, according to anchor-based metrics (22). The immediate change in the walking speed from the OFF to ON mode was 0.10 m/s in our study (Table 2). This in turn corresponded to a moderate CID. Based on the values in Table 3, we can calculate the walking speed in the 3MWT. The between-group difference was 0.08 m/s, whereas the within-group difference for SMA was 0.07 m/s (Table 3). Both belonged to the small CID group in the distribution-based analysis. However, the within-group difference was 0.002 m/s in the control group for the 3MWT. We obtained similar results in the 10MWT. The between-group difference and within-group difference in the SMA group was 0.11 m/s and 0.11 m/s, respectively.
According to Shulman et al., the MCIDs of UPDRS part III score and total score were 2.3-2.7 points and 4.1-4.5 points, respectively (23). The between-group and within-group changes in the SMA group were 3.6 and 3.2 for the UPDRS part III score and 4.9 and 4.6 for the total score, respectively, in our study (Table 3). Thus, our study showed a small improvement in the UPDRS part III score and total score in the SMA group.
The SMA is a light and wearable exoskeleton with a smart appearance. It does not have any special requirement when used for rehabilitation purpose. Furthermore, the torque for each patient can be adjusted by a single physical or occupational therapist. The SMA reduced the energy consumption of healthy young adults during self-selected walking (8). In addition, SMA-based walking exercises not only increased the gait speed but also reduced the glucose metabolism in the hip-associated muscles in elderly women (9). These immediate effects might result in a comfortable walking experience with the aid of SMA. Therefore, the SMA is considered safe and easy to use and an effective device for gait training.
However, most robot-assisted gait training (RAGT) in patients with PD is typically provided by large, static devices that occupy a large space in institutes. Several randomized controlled studies have determined the efficacy of RAGT (24-29). Despite the relatively good results obtained using RAGT compared with control gait training (25,26,28), there were no significant differences in outcomes between the RAGT group and treadmill training (24,27) or balance training groups (29). Krebs et al. defined the success of robotic rehabilitation as therapy that provides more than the “usual” standard of care at a similar or lower cost (30). However, given the large space required and substantial expenditure associated with RAGT, RAGT is far from being the optimal treatment.
Lee Silverman Voice Treatment BIG (LSVT BIG) is an amplitude-oriented training that facilitates reinstating the amplitude and speed of normal movement by recalibrating the participant's perception about movement practice (31,32). Previous studies have reported that LSVT BIG improved the UPDRS-III scores more than home-based exercise, Nordic walking (33), or a shortened protocol (32). LSVT BIG was developed for people with mild to moderate PD. However, it can only be administered by therapists with certification in its performance (31,32) and requires high effort from participants. In contrast, gait training using the SMA requires only a nationally qualified therapist, and participants could fell the effect immediately with SMA. The SMA might automatically reinforce the function of spinal central pattern generators with small effort of participants and could facilitate sensory feedback via proprioceptive and skin afferents (34,35).
Exercise may improve motor symptoms, non-motor symptoms and secondary comorbidities, such as osteoporosis and cardiovascular disease (4). Physical therapy is usually recommended depending on the stage of PD (36). SMA-based rehabilitation can address two of the six core areas for moderately advanced PD (gait and physical capacity and activity) (36). One participant reported being unable to travel long distances using a Nordic pole and was unable to use trains. However, he found he was able to walk longer distances and board trains after undergoing SMA-based training. The effects of Nordic walking have been investigated in people with PD (37), and a positive synergistic effect might be gained in walking while using a Nordic pole and an SMA.
Several limitations associated with the present study warrant mention. First, it was a randomized, controlled, open trial performed at a single facility. Therefore, observer and reporting biases might have influenced the results. Second, our target population included people with PD who were unable to walk outside for a long distance. In addition, those with mild PD might not have needed the SMA. However, the SMA was not available to people with advanced PD who were unable to walk even with a walking aid. Third, our study included a small sample size, and there were more women than men and more Hoehn and Yahr stage 2 patients in the control group than in the SMA group. A larger sample size might have resulted in greater between-group differences and more proportional sex differences and disease severity in both groups. Fourth, a longer-term study to evaluate the extended effect of gait training using this device and a follow-up study might be needed.
In conclusion, SMA-based gait training can increase exercise endurance and walking speed in people with moderately advanced PD. This intervention has the potential to improve both physical and social activities and participation in the International Classification of Functioning, Disability and Health (ICF) model (38,39). The need for home-based care for patients with PD is becoming apparent as society ages (40,41). This method would also facilitate the performance of patient-centered care (40,41). Thus, regional Parkinson's care teams should consider SMA-based gait training as a supporting treatment modality (41). Further studies involving a larger number of participants are warranted.
The authors state that they have no Conflict of Interest (COI).
Acknowledgments
We would like to thank Koji Ohata for his technical suggestions. We would also like to thank the participants of Kawashima Neurology Clinic and their family members.
References
- 1.Kalia LV, Lang AE. Parkinson's disease. Lancet 386: 896-912, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord 23: 790-796, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson's disease: current outlook and future challenges. Parkinsonism Relat Disord 22: S60-S64, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson's disease. Mov Disord 28: 1587-1596, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol 12: 716-726, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med 366: 511-519, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloem BR, Vries NM, Ebersbach G. Nonpharmacological treatments for patients with Parkinson's disease. Mov Disord 30: 1504-1520, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Kitatani R, Ohata K, Takahashi H, Shibuta S, Hashiguchi Y, Yamakami N. Reduction in energy expenditure during walking using an automated stride assistance device in healthy young adults. Arch Phys Med Rehabil 95: 2128-2133, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Shimada H, Hirata T, Kimura Y, et al. Effects of a robotic walking exercise on walking performance in community-dwelling elderly adults. Geriatr Gerontol Int 9: 372-381, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Buesing C, Fish G, O'Donnell M, et al. Effects of a wearable exoskeleton stride management assist system (SMA) on spatiotemporal gait characteristics in individuals after stroke: a randomized controlled trial. J Neuroeng Rehabil 12: 69-82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaraman A, O'Brien MK, Madhavan S, et al. Stride management assist exoskeleton vs functional gait training in stroke: a randomized trial. Neurology 92: e263-e273, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka N, Matsushita S, Sonoda Y, et al. Effect of stride management assist gait training for poststroke hemiplegia: a single center, open-label, randomized controlled trial. J Stroke Cerebrovasc Dis 28: 477-486, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30: 1591-1599, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Takenaka T, Matsumoto T, Yoshiike T. Real-time motion generation and control for biped robot - 1st report: walking gait pattern generation. IEEE/RSJ Int Conf Intell Robot Syst 1084-1091, 2009. [Google Scholar]
- 15.Haarman JA, Reenalda J, Bruurke JH, Kooij H, Rietman JS. The effect of ‘device-in-charge’ versus ‘patient-in charge’ support during robotic gait training on walking ability and balance in chronic stroke survivors: a systemic review. J Rehabil Assist Technol Eng 3: 1-16, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord 6: 165-170, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson C, Fitzpatrik R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing 26: 353-357, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda S, Shiroiwa T, Igarashi A, et al. Developing a Japanese version of the EQ-5D-5L value set [in Japanese]. Hoken Iryo Kagaku (J Natl Inst Public Health) 64: 47-55, 2015. [Google Scholar]
- 19.Tanaka R, Takimoto H, Yamasaki T, Higashi A. Validity of time series kinematical data as measured by markerless motion capture system on a flatland for gait assessment. J Biomech 71: 281-285, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25: 2649-2685, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Rohannon RW, Crouch R. Minimal clinical important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 23: 377-381, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Hass CJ, Bishop M, Moscovich M, et al. Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther 38: 233-238, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol 67: 64-70, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Carda S, Invernizzi M, Baricich A, Comi C, Croquelois A, Cisari C. Robotic gait training is not superior to control treadmill training in Parkinson disease: a single-blind randomized controlled trial. Neurorehabil Neural Repair 26: 1027-1034, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Picelli A, Melotti C, Origrano F, et al. Robot-assisted gait training in patients with Parkinson disease: a randomized controlled trial. Neurorehabil Neural Repair 26: 353-361, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Picelli A, Melotti C, Origano F, Waldner A, Gimigliano R, Smania N. Does robotic gait training improve balance in Parkinson's disease? A randomized controlled trial. Parkinsonism Relat Disord 18: 990-993, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Picelli A, Melotti C, Origano F, Neri R, Waldner A, Smania N. Robot-assisted gait training versus equal intensity treadmill training in patients with mild to moderate Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord 19: 605-610, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Furnari A, Calabró BS, Cola MC, et al. Robotic-assisted gait training in Parkinson's disease: a three-month follow-up randomized clinical trial. Int J Neurosci 127: 996-1004, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Picelli A, Melotti C, Origano F, et al. Robot-assisted gait training is not superior to balance training for improving postural instability in patients with mild to moderate Parkinson's disease: a single-blind randomized controlled trial. Clin Rehabil 29: 339-347, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Krebs HI, Hogan N. Robotic therapy: the tipping point. Am J Phys Med Rehabil 91: S290-S297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox C, Ebersbach G, Raming L, Sapir S. LSVT LOUD and LSVT BIG: behavioral treatment programs for speech and body movement in Parkinson disease. Parkinsons Dis 2012: 391946, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonnell MN, Rischbieth B, Schammer TT, Seaforth C, Shaw AJ, Phillip AC. Lee Silverman Voice Treatment (LSVT)-BIG to improve motor function in people with Parkinson's disease: a systematic review and meta-analysis. Clin Rehabil 32: 607-618, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Ebersbach G, Ebersbach A, Edler D, et al. Comparing in Parkinson's disease - the Berlin LSVTⓇBIG study. Mov Disord 25: 1902-1908, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Bohnen NI, Jahn K. Imaging: what can it tell us about Parkinsonian gait? Mov Disord 28: 1492-1500, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takakusaki K. Neurophysiology of gait: from spinal cord to frontal lobe. Mov Disord 28: 1483-1491, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Keus SHJ, Bloem BR, Hendriks EJM, Bredèro-Cohen AB, Munneke M; the Practice Recommendations Development Group. Evidence-based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Mov Disord 22: 451-460, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Bombieri F, Schena F, Pellegrini B, Barone P, Tinazzi M, Erro R. Walking on four limbs: a systemic review of Nordic walking in Parkinson disease. Parkinsonism Relat Disord 38: 8-12, 2017. [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization. International Classification of Functioning, Disability and Health (ICF) [Internet]. [cited 2001 May 22]. Available from: www.who.int/classifications/icf/en/
- 39.Ohata K. Walking reconstruction: understanding and training gait [in Japanese]. 1st ed. Miwa-Shoten, Tokyo, 2017. [Google Scholar]
- 40.Dorsey ER, Vlaanderen FP, Engelen LJ, et al. Moving Parkinson care to home. Mov Disord 31: 1258-1262, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloem BR, Henderson EJ, Dorsey ER, et al. Integrated and patient-centered management of Parkinson's disease: a network model for reshaping chronic neurological care. Lancet Neurol 19: 623-634, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]