Abstract
Intracellular vesicle trafficking is the fundamental process to maintain the homeostasis of membrane-enclosed organelles in eukaryotic cells. These organelles transport cargo from the donor membrane to the target membrane through the cargo containing vesicles. Vesicle trafficking pathway includes vesicle formation from the donor membrane, vesicle transport, and vesicle fusion with the target membrane. Coat protein mediated vesicle formation is a delicate membrane budding process for cargo molecules selection and package into vesicle carriers. Vesicle transport is a dynamic and specific process for the cargo containing vesicles translocation from the donor membrane to the target membrane. This process requires a group of conserved proteins such as Rab GTPases, motor adaptors, and motor proteins to ensure vesicle transport along cytoskeletal track. Soluble N-ethyl-maleimide-sensitive factor (NSF) attachment protein receptors (SNARE)-mediated vesicle fusion is the final process for vesicle unloading the cargo molecules at the target membrane. To ensure vesicle fusion occurring at a defined position and time pattern in eukaryotic cell, multiple fusogenic proteins, such as synaptotagmin (Syt), complexin (Cpx), Munc13, Munc18 and other tethering factors, cooperate together to precisely regulate the process of vesicle fusion. Dysfunctions of the fusogenic proteins in SNARE-mediated vesicle fusion are closely related to many diseases. Recent studies have suggested that stimulated membrane fusion can be manipulated pharmacologically via disruption the interface between the SNARE complex and Ca2+ sensor protein. Here, we summarize recent insights into the molecular mechanisms of vesicle trafficking, and implications for the development of new therapeutics based on the manipulation of vesicle fusion.
Keywords: Vesicle formation, Vesicle transport, Vesicle fusion, Fusogenic proteins, Disease therapy
Introduction
In eukaryotic cells, vesicle trafficking dependent signal transduction occurs via exchange of the content between membrane-enclosed organelles. Endoplasmic reticulum (ER), Golgi apparatus, endosomes, multivesicular body, lysosome, and plasma membrane are well characterized vesicle trafficking organelles [1]. Cargo containing-vesicle trafficking is highly specific and dynamic, which is regulated by multiple proteins that are conserved throughout eukaryotic evolution [2, 3]. Vesicle trafficking initiates from vesicle formation via membrane budding, followed by vesicle transport among intracellular organelles, and is accomplished by vesicle fusion with the target membrane [4]. Up to one third of all proteins in eukaryotic cells involve in these vesicle trafficking pathways. These events are orchestrated by mutiple proteins and protein complexes, including coat proteins (such as coat protein complex II (COPII), COPI, and clathrin), small GTP-binding proteins, tethering proteins, and fusogenic proteins [3, 5–11].
Dysfunctional vesicle trafficking could lead to the development of a wide range of diseases, for example in the nervous system, dysregulation of synaptic vesicle fusion in presynapse leads to neurodegenerative disease; in the respiratory system, abnormal secretory activity of airway epithelial cells results in several respiratory disorders, such as asthma and cystic fibrosis. Here, we present a general working model for how vesicles are transported between membrane organelles, and propose a potential new therapeutic strategy for diseases that are related to vesicle fusion.
Vesicle formation
In mammalian cells, vesicle formation is a complex and delicate process achieved by trapping cargo molecules into carriers on the intracellular membrane compartments. Several intracellular organelles are involved in this process, such as ER, Golgi, and plasma membrane. Different coat proteins mediate different budding events, by shaping of transport vesicles and selecting desired cargo molecules through direct or indirect interactions. COPII-, COPI- and clathrin-coated vesicles (CCVs) make up most of intracellular trafficking vesicles. They share conserved molecular rationale for vesicle formation, although the involved molecules are different from each other. In this review, we summarize the studies for the three well defined pathways of vesicle formation that are mediated by COPII, COPI and clathrin (Fig. 1, Table 1).
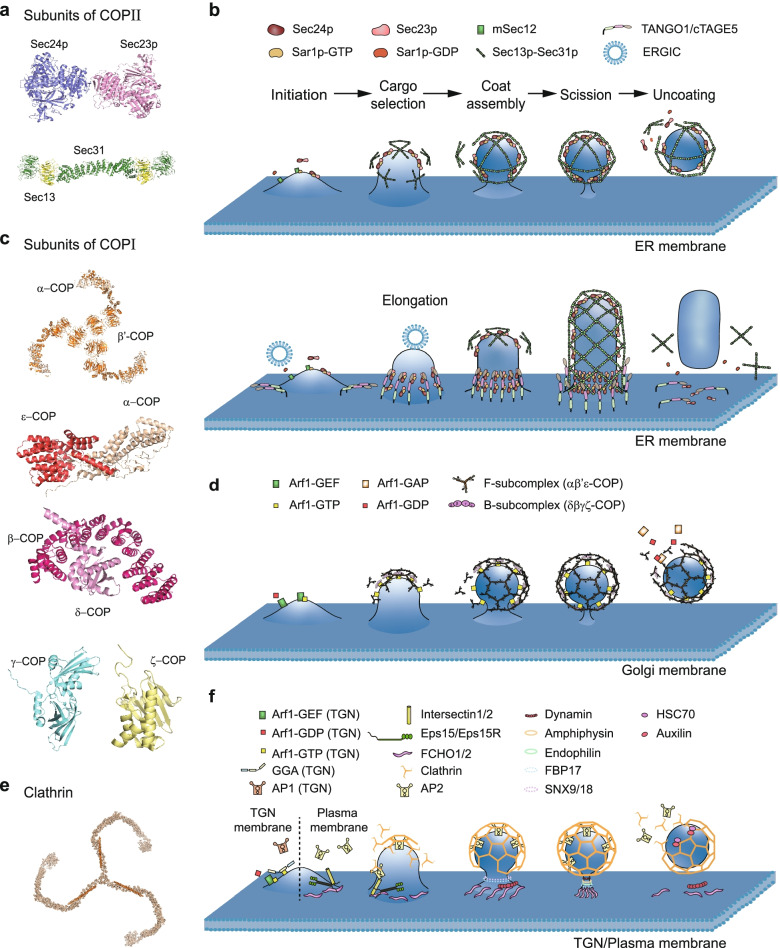
Fig. 1.
The coat protein mediated vesicle formation. a Crystal structure of the Sec23p-Sec24p subcomplex in Homo sapiens (PDB ID: 3EG9) [12] and cryo-EM structure of the Sec13-Sec31 subcomplex in Saccharomyces cerevisiae (PDB ID: 4BZK) [13]. b COPII-coated vesicles formation. Upper panel, COPII-coated vesicles derived from the ER exit site (ERES). Lower panel, formation of a mega-COPII-coated carrier at the ER. c Crystal structure of αβ’-COP subcomplex in Saccharomyces cerevisiae (PDB ID: 3MKQ) [14], αε-COP subcomplex in Bos taurus (PDB ID: 3MKR) [14], βδ-COP subcomplex in Chaetomium thermophilum (PDB ID: 5MU7) [15], the γ-COP subunit in Homo sapiens (PDB ID: 1R4X) [16] and NMR structure of the ζ-COP subunit in Homo sapiens (PDB ID: 2HF6) [17] of the COPI complex. d COPI-coated vesicles formation. COPI-coated vesicles appear in retrograde transport from the Golgi apparatus to the ER and between the Golgi cisternae. e Cryo-EM structure of clathrin triskelia in Bos taurus (PDB ID: 3IYV) [18]. f CCVs formation from TGN (on the left of the dotted line) and plasma membrane (on the right of the dotted line). The copyright permission of panels b, d, and f are from [7]
Table 1.
Classic proteins involved in COPII-, COPI-, and clathrin-mediated vesicle formation
| COPII | COPI | Clathrin | ||
|---|---|---|---|---|
| Location | ER | cis-Golgi | TGN | Plasma membrane |
| Initiation protein |
mSec12 Sar1p-GTP |
Arf1-GTP Arf1-GEF (GBF1) |
Arf1-GTP Arf1-GEF |
FCHO1/2 intersectin1/2 eps15/eps15R |
| Adaptor protein |
Sec24p Sec23p p24 TANGO1/cTAGE5 |
Arf1 Arf1-GEF |
AP1 GGAs |
AP2 |
| Cargo molecule |
Collagens chylomicron Proteins containing diphenylalanine motif |
Proteins containing di-lysine motifs (K(X)KXX), or RXR motifs | Proteins containing YXXΦ, LL motifs, or (D/E)XXXL(L/I) motif | Proteins containing YXXΦ, LL motifs, or (D/E)XXXL(L/I) motif |
| Coat protein |
Sar1p Sec23p Sec24p Sec13p Sec31p |
α-COP β′-COP β-COP δ-COP γ-COP ζ-COP |
Clathrin | Clathrin |
| Membrane scission protein |
Sec23p Sar1p-GTP |
Arf-GAPs PLD2 BARS |
Dynamin Amphiphysin |
Dynamin Endophilin Amphiphysin FBP17 SNX9/18 |
| Uncoating protein |
Sec23p Sar1p-GTP |
Arf1-GTP Arf1-GAPs |
Auxilin HSC70 AP1 |
Auxilin HSC70 AP2 |
COPII-coated vesicle formation
COPII-coated vesicles transport proteins and membranes from the ER to the Golgi apparatus. The crystal structure of COPII complex has revealed the five subunits: the small GTPase Sar1p and two heterodimeric complexes, Sec23p-Sec24p [12] and Sec13p-Sec31p [13] (Fig. 1a). These proteins support vesicle budding, cargo selection, coat assembly, and uncoating of ER derived COPII-coated vesicle (Fig. 1b).
The small GTPase Sar1p acts as a “seed” in the stage of initiation of COPII-coated vesicle formation. In a microsome-based vesicle formation assay, it was found that Sar1p buried an amphipathic helix into the ER membrane [19], when it was transformed to Sar1p-GTP by mSec12, which is an ER-linked membrane protein that functions as a guanine exchange factor (GEF) for Sar1p [20]. Afterwards, Sar1p-GTP assembles the vesicle coat by sequentially recruiting the heterodimeric Sec23p-Sec24p subcomplex of COPII. Sec24p is not only involved in the assembly of the COPII coat, but also serves as a platform for cargo selection by recognizing cargo molecules with the specific regions such as diphenylalanine motif. A recent structural study of COPII suggested that Sec24p might have multiple cargo binding sites that are capable of capturing multiple cargoes, although further evidences are still needed [21]. Moreover, the membrane associated proteins of p24 family are recruited to COPII-coated vesicles by binding to Sec23p via a cytosolic diphenylalanine motif [22]. These membrane proteins, acting as cargo adaptors, are required for efficient ER-to-Golgi transport of cargo proteins [23–25]. At the same time as cargo selection, Sec23p also recruits the rod-shaped heterotetramer of Sec13p-Sec31p complex through a flexible proline-rich domain (PRD) in the C-terminal half of Sec31p [26]. Structural studies reveal that the heterotetramer of Sec13p-Sec31p complex consists of a β-propeller domain and an α-solenoid domain at N-terminal half of Sec31p, and these domains are separated by a blade insertion motif, which binds to the Sec13p β-propeller domain to form the heterotetrameric complex (Fig. 1a) [13, 26, 27]. Thereafter, with the assembly of this larger coating complex, it induces the formation of coated carriers by bending the membrane in the stage of coat assembly [13, 28–30]. Then the COPII coat complex gradually coalesces into an icosidodecahedral polymer shell, and segregates vesicle from the donor membrane [24, 31, 32]. In the stage of membrane scission, Sec23p acts as a GTPase-activating protein (GAP) to activate Sar1p-GTP hydrolysis, leading to the cleavage of vesicle membrane [33, 34]. Sar1p-GDP is thought to be released after GTP hydrolysis, resulting in vesicle uncoating before the vesicle fuses with the target membrane (Fig. 1b).
For the transport of large and irregular cargoes from the ER, such as procollagen and chylomicron, it requires the formation of COPII-coated mega-vesicles with a diameter larger than 300 nm [35]. The formation of COPII-coated mega-vesicles is similar as regular COPII-coated vesicles (Fig. 1b), except the involvement of three TANGO1 family proteins: cTAGE5, TANGO1 and short TANGO1. Structurally, the N-terminus of TANGO1 contains a SH3-like domain, which binds to collagens via HSP47, while the cytoplasmic domain of cTAGE5 contains two coiled-coil (CC) domains and a PRD that is similar to TANGO1 [36]. The immunofluorescence and co-immunoprecipitation (Co-IP) experiments revealed that the CC1 domain was essential for the recruitment of mSec12 to the ERES [37]. Moreover, TANGO1-family proteins form a ring-like structure and such a ring filament would resist to the elastic stresses and prevent membrane from further shrinking [36]. Additionally, this ring will exert a tight control over the timing and the activity of Sar1p-GTP hydrolysis at the base of a COPII bud [36]. Furthermore, TANGO1 recruits ER-Golgi intermediate compartment (ERGIC) membranes via the CC1 domain. The ERGIC membrane can fuse with cargo-enriched domain at ERES by the SNARE proteins, leading to the growth of the cargo-enriched domain into a tube-like structure. Once the tubule is large enough to accommodate these large and long cargos, the SH3-like domain and PRD of TANGO1 dissociate from cargo molecules, and the Sec23p-Sec24p subcomplex, respectively. Afterwards, the Sec13p-Sec31p subcomplex is recruited to the neck of the tubule, leading to the membrane cleavage and generation of COPII-coated mega-vesicles from the ER [35].
COPI-coated vesicle formation
COPI-coated vesicles transport cargo proteins and lipid molecules from the Golgi membrane to the ER membrane, or between Golgi membrane compartments. The cryo-electron microscopy (cryo-EM) structure of COPI [38] revealed that the coatomer, multimeric complexes are composed of seven core subunits in the cytosol including α-COP, β’-COP, ε-COP, β-COP, δ-COP, γ-COP, and ζ-COP (Fig. 1 c), which are divided into cage-like subcomplex (B-subcomplex) and linker-like subcomplex (F-subcomplex). The B-subcomplex is a trimeric αβ’ε-COP, consisting of α-, β’-, and ε-subunits, while the F-subcomplex is a tetrameric βδγζ-COP, consisting of β-, γ-, δ-, and ζ-subunits [14–17, 39–42]. The B-subcomplex binds membrane-anchored dilysine cargo via the N-terminal WD-repeat domains of α-COP and β’-COP [43, 44], while the F-subcomplex is recruited by ADP-ribosylation factor 1 (Arf1)-GTP binding to γ-COP and β-COP subunits [45]. Coat assembly starts when the B-subcomplex, the F-subcomplex and intercalated cargoes are highly concentrated on the membrane through multivalent interactions (Fig. 1d).
The small GTPase Arf1 is activated to initiate the formation of COPI-coated vesicles by interacting with the homology downstream of Sec7d-1 (HDS1) and HDS2 domains of GBF1 (a guanine nucleotide exchange factor for Arf1) [46]. After initiation, the cis-Golgi localized GBF1 recruits the coatomer to the membrane as intact complexes via binding to the appendage domain of γ-COP [47]. The subunits of yeast β’-COP and part of α-COP were crystallized to form a triskelion-like structure which had been proposed to form a polygonal cage as shown in Fig. 1c [14]. In the crystal structure of the B-subcomplex, ε-COP binds to the C-terminal domain of α-COP, while β’-COP binds to a part of α-COP (Fig. 1c) [14]. Moreover, at the stage of cargo sorting, cargo molecules are selectively packaged into COPI-coated vesicles for transport. The mechanism of cargo sorting involves the interaction among the Golgi membrane, specific motif sequences in the cargo molecules, and different subunits of COPI. For example, after the recruitment of Arf1-GTP and COPI onto the cis-Golgi membrane, the α-solenoid domain of β’- and α-COP form an arch above the βδγζ-COP subcomplex by orienting their N-terminal β-propeller domains, such that the dilysine motif (K(X)KXX, K, Lys; X, any amino acid), or motif like RKR (R, Arg; K, Lys) of cargo binding site locates on the Golgi membrane [38, 48], and recruits molecules such as escaped ER-resident protein cargoes and soluble ER protein cargoes, which are then retro-transferred from the Golgi apparatus back to the ER [39]. When the COPI complex further concentrates and binds to cargo molecules, the B-subcomplex and the F-subcomplex intertwine to form a triply folded structure, which are connected by flexibly attached domains. One set of interactions is formed by the μ-homology domain of δ-COP and another by ε-COP and the C-terminal domain of α-COP [38]. At the stage of coat assembly, this triply folded structure deforms the planar Golgi membrane, leading to positive curvature and budding of the vesicles. In the late stage of the COPI-coated vesicle formation, the Golgi membrane is dominated by negative curvature. In morphometrical and biochemical assays, it was found that BrefeldinA-ADP-ribosylated substrate (BARS), a peripheral Golgi membrane protein, are essential for COPI-coated vesicle membrane scission [49]. In the stage of membrane scission, BARS together with COPI-associated proteins (Arf1, Arf-GAPs, and coatomer) drive the COPI complex gradually shrink to a narrow neck, and a spherical cage-like vesicles are formed via splitting the neck by phosphatidic acid that is produced by phospholipase D2 (PLD2) [50]. After the COPI-coated vesicles detaching from the Golgi membrane, Arf1-GTP is hydrolyzed under the action of Arf1-GAPs, in which Arf1-GAPs form a myristoyl-binding pocket, allowing to initiate the disassembly of the COPI complex and the uncoating of the vesicles. Last, a synthetic peptide of FFXXRRXX (F, Phe; R, Arg; X, any amino acid) with the sorting signal at the C-terminus of the p24 protein hp24a, was reported to significantly inhibit Arf1-GTP hydrolysis, while other peptides with the same sorting signal have no effect. It indicates that different cargoes may have different effects on the rate of vesicle uncoating [51], although the detailed molecule mechanism of Arf1-GTP hydrolysis and uncoating of COPI-coated vesicles is not fully understood.
Clathrin-coated vesicle formation
CCVs transport cargo molecules from the trans-Golgi network (TGN) membrane to the endosome membrane, and from the plasma membrane to the endosome membrane (so called endocytosis). As observed from crystal structure (Fig. 1e), clathrin resembles a spider-like molecule with three legs radiating from a central hub [18, 52] Antiparallel interactions of the legs of triskelions centered on adjacent vertices of the lattice allows the cage to be built [53, 54]. The CCVs formation from plasma membrane and Golgi membrane shares a conserved molecule mechanism except the recruitment of different adaptor proteins and cargo molecules, in which the adaptor protein 2 (AP2) and cargos containing YXXΦ (X, any residue; Φ, a residue with a bulky hydrophobic side chain) motif, (D/E)XXXL(L/I) motif (D, Asp; E, Glu; L, Leu; I, Ile), and LL motif ((-)(2–4)XLL, (-), negatively charged residue; X, polar residue; L, Leu) are recruited to the plasma membrane by Fer/CIP4 homology domain only protein 1/2 (FCHO1/2), eps15, eps15R (eps15 related), intersectin1/2 and phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) [55–59], while AP1, Golgi-localized, gamma-adaptin ear homology, Arf-binding protein protein (GGA protein) and cargo containing (D/E)XXXL(L/I) motif and LL motif are recruited to TGN by Arf1-GTP and phosphatidylinositol-4-monophosphate (PI4P) [60, 61].
Here we take the endocytic pathway originating from the plasma membrane as an example to discuss the molecule mechanism of the CCV formation (Fig. 1f). In mammalian cells, the endocytic proteins consists at least of the adaptor proteins FCHO1/2, AP2, and the scaffold proteins eps15, eps15R, and intersectin1/2 in the initiation [54, 57, 62, 63]. The CCV formation is initiated by the increase of the endocytic proteins at the plasma membrane. FCHO1/2 locates to plasma membrane via PI(4,5)P2, and recruits eps15, eps15R, intersectin1/2 via the C-terminal AP2-μ homology domain (μHD), increasing the likelihood of initiating endocytic events [57]. Afterwards, AP2 is recruited by the μHD of FCHO1/2 and Asp-Pro-Phe triplet-based motif of eps15/eps15R [64], while clathrin is recruited by AP2 when it binds to PI(4,5)P2 and YXXΦ- and LL-containing cargoes [58, 59, 65], followed by the clathrin-coated pit (CCP) formation and the coat expansion. In the stage of cargo selection, clathrin recruits various membrane cargo proteins to the clathrin-coated site, via the adaptor proteins such as AP2, and the accessory proteins such as AP180 and epsin [7]. In the stage of coat assembly, clathrin bends the membrane during vesicle formation in the assistance of its adaptors. For example, crystal structure revealed that the amphipathic helix of the epsin N-terminal homology (ENTH) domain of epsin has createed local membrane defects and facilitates membrane curvature formation [66]. As the clathrin lattice rapidly reorganizes and propagates, membrane deformation is driven to provide the lattice flexibility to accommodate the changes in membrane curvature [67]. In the step of membrane scission, detachment of the coated vesicles from the plasma membrane is mediated by GTPase protein dynamin, which mechanically pushes out their junctions via proteolysis of GTP [68]. Other membrane binding proteins also play important roles in membrane scission, including endophilin, amphiphysin, formin-binding protein 17 (FBP17), SNX9/18, and so on [7, 67, 69–72]. Before vesicle fusion, CCVs need disassembly of the clathrin lattice during uncoating stage. Auxilin is a member of the DnaJ class of proteins that contains a conserved J domain [73] and activates the ATPase HSC70 [74]. Auxilin locates to CCVs by its clathrin binding domain, and recruits the uncoating protein HSC70 [75]. In the response of the hydrolysis ATP, HSC70 disrupts clathrin–clathrin interactions, leading to the disassembly of the clathrin coat. And then HSC70 is released and reused for the next round of vesicle formation [67].
Intracellular vesicle transport
Intracellular vesicle transport includes the ER-to-Golgi transport, the retrograde Golgi-to-ER transport, the TGN transport, the endocytic vesicle transport, and the membrane trafficking in autophagy (Fig. 2a). The intracellular vesicle transport relies on a cytoskeletal track, which involves Rab GTPases, motor adaptors, as well as motor proteins such as kinesin, dynein, myosin (Fig. 2b) [76–80]. Rab GTPases belong to the Ras superfamily of small GTPases [81]. GTP-bound Rab regulates intracellular vesicle transport by binding to the vesicle membrane and recruiting the effector molecules, such as motor proteins, motor adaptors, while GDP-bound Rab is inactive and distributed to the cytosol [81]. Each Rab protein localizes in a specific cellular compartment and recruits a different set of effector molecules, realizing precise spatial regulation of vesicle transport [81, 82]. Different proteins regulate different vesicle transport pathways by untilzing a conserved molecular mechanism in vesicle translocation, vesicle tethering and vesicle fusion with target membrane. We summarize the corresponding factors involved in the vesicle transport pathway in Table 2.
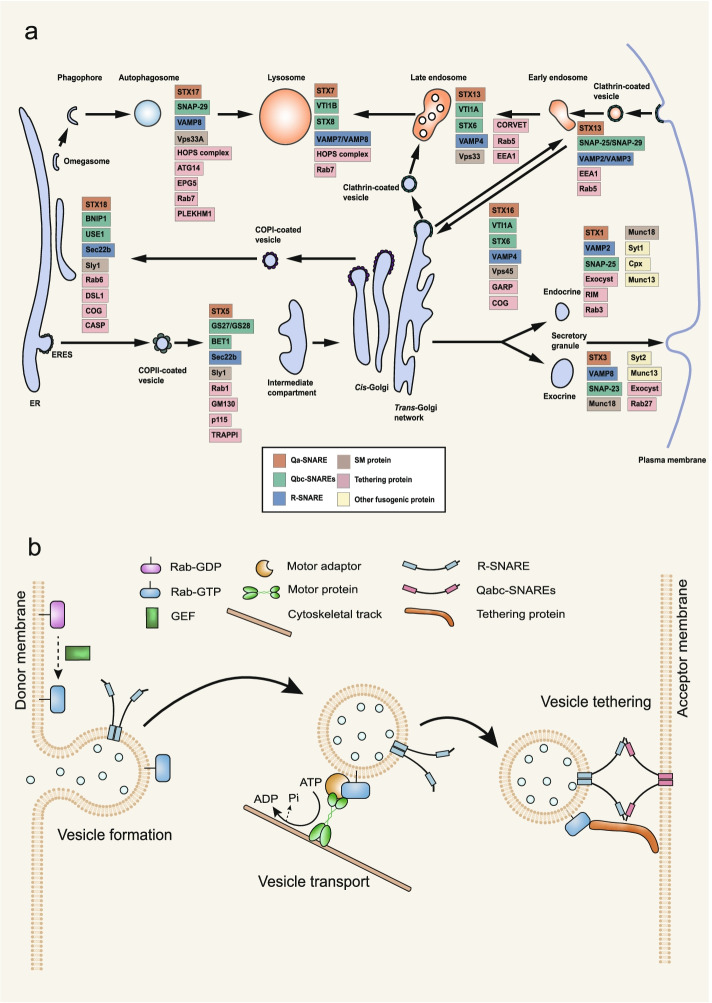
Fig. 2.
Intracellular vesicle trafficking pathways. a The SNAREs, SM proteins, tethering proteins and other fusogenic proteins in intracellular vesicle trafficking pathways. In mammalian cells, intracellular vesicle trafficking includes the ER-to-Golgi transport, the retrograde Golgi-to-ER transport, the TGN transport, the endocytic pathway, the membrane trafficking in autophagy and others. Different SNAREs and fusogenic proteins are assigned to different vesicle trafficking pathways. (Red, Qa-SNARE family; Light green, Qbc-SNARE family; Blue, R-SNARE family; Brown, SM protein; Pink, Tethering protein; Yellow, other fusogenic protein). Vps45, Vacuolar protein sorting 45 homolog; GARP, Golgi-associated retrograde protein. b The working model of vesicle transport and tethering. Rab GTPases mediates vesicle transport along the cytoskeleton tracks (actin filaments or microtubules) by indirectly binding via motor adaptors or directly binding to motor proteins. After cargo containing vesicles being loaded, motor proteins drive vesicles moving along cytoskeleton tracks to the destination by hydrolysis of ATP. Afterwards, GTP-bound Rab recruits tethering proteins to dock vesicles with the acceptor membrane. The copyright permission of panels a and b are from [3, 78]
Table 2.
Classic proteins involve in different vesicle transport pathways
| Transport pathways | Rab GTPases | Motor adaptor | Motor protein | Cytoskeleton | Tethering protein |
|---|---|---|---|---|---|
| ER to Golgi transport | Rab1 | Sec23 | Dynein-dynactin complex, KIF5B | Microtubule | TRAPPI, p115, GM130 |
| Retrograde transport from the Golgi to the ER | Rab6A | BicaudalD |
COPI-dependent: Rabkinesin-6, KIF1C COPI-independent: Dynein-dynactin complex |
COPI-dependent: Microtubule COPI-independent: Microtubule |
COPI-dependent: Dsl1, COG, CASP |
| Transport from TGN to plasma membrane |
Endocrine secretion: Rab3 Exocrine secretion: Rab27, Rab3D |
Endocrine secretion: DENN/MADD Exocrine secretion: MyRIP |
Endocrine secretion: KIF1A Exocrine secretion: Myosin V |
Endocrine secretion: Microtubule Exocrine secretion: Actin |
Endocrine secretion: Exocyst, RIM Exocrine secretion: Exocyst |
| Transport from TGN to endosome | Rab14 | / | KIF16B | Microtubule | CORVET |
| Transport from endosomes to TGN | Rab6 | SNX6 | Dynein-dynactin complex | Microtubule | GARP, COG |
| Transport from plasma membrane to early endosome | Rab5 | Dab2 | Myosin VI | Actin | EEA1 |
| Transport from recycling endosome to plasma membrane | Rab11 | FIP2 | Myosin Vb | Actin | Exocyst |
| Transport from endosome to late endosome | Rab5 | HOOK | Dynein-dynactin complex | Microtubule | / |
| Transport from endosome to lysosome | Rab7 | RILP | Dynein-dynactin complex | Microtubule | HOPS |
| Transport from autophagosome to lysosome | Rab7 |
FYCO1 LC3 |
Kinesin-1 | Microtubule | EPG5, HOPS, ATG14, PLEKHM1 |
ER-to-Golgi transport
Most membrane proteins, secretory proteins and lipids are synthesized in the ER, and then transported to different membrane organelles, which is critical for protein secretion and sorting [83, 84]. Newly synthesized proteins are transported to the Golgi apparatus via the ER-derived COPII-coated vesicles (Fig. 2a) [84]. As discussed in the section of vesicle formation, COPII-coated vesicles can cluster into larger vesicular-tubular structure by homotypic fusion [85]. This vesicular-tubular structure forms the ERGIC [86], an organelle before the cargos being transported to the Golgi apparatus.
Vesicle transport from the ER to Golgi is dependent on the dynein-dynactin complex, which crosslinks COPII-coated vesicle and microtubule [87, 88]. It was found that the Sec23p subunit of the COPII directly bound to the C-terminal cargo-binding domain of the p150Glued subunit of the dynein–dynactin complex by two-hybrid screening experiments [89]. In addition, the N-terminal region of p150Glued contains the cytoskeleton-associated protein glycine-rich domain, the basic domain, and the serine/proline-rich domain [90]. And it was found that the p150Glued interacted with the dynein and microtubules via its microtubule binding domain in the magic angle spinning NMR spectroscopy [91]. After cargo containing COPII-coated vesicles loaded, the dynein–dynactin complex drives COPII-coated vesicle moving along microtubule track to the Golgi apparatus by hydrolysis of ATP via the AAA (ATPases associated with diverse cellular activities) subunit of dynein [89, 92].
After COPII-coated vesicles are transported to the cis-Golgi network (CGN), the transport protein particle I (TRAPPI) is required for COPII-coated vesicle tethering [93]. It was found that the Bet3 subunit of TRAPPI could bind to the Sec23p subunit of COPII by the Pull-down experiments [94]. In addition, TRAPPI also acts as GEFs that specifically activate Rab1 by catalyzing GDP/GTP nucleotide exchange [94]. Rab1 then recruits its effector proteins golgins such as p115, Golgi matrix protein 130 (GM130), to tether COPII-coated vesicles to the CGN [95, 96]. Golgins are anchored to the Golgi membrane through their C-terminus. In AFM experiments, it was found that golgins could extend a considerable distance into the surrounding cytoplasm due to their coiled-coil structure property, which ensures the capture of vesicles at a long range and increases the efficiency of vesicle trafficking [97, 98].
Membrane fusion between COPII-coated vesicle and the CGN relies on a conserved SNARE complex consisting of syntaxin-5 (STX5), Golgi SNAREs of 27 kDa (GS27)/28 kDa (GS28), blocked early in transport 1 (BET1), and Sec22b [3]. Each of the SNARE proteins contributes one SNARE motif, and the formation of the four-helical bundle of SNARE complex drives membrane fusion between the COPII-coated vesicle and the Golgi membrane. The Sec1/Munc18 (SM) protein Sly1 binds to the N-terminal peptides of STX5 via its N-terminal domain, loosening the closed conformation of STX5 to facilitate the SNARE complex formation [99–101]. The detailed molecular mechanisms of SNARE and SM protein mediated vesicle fusion are discussed in section of molecular mechanism of vesicle fusion. Dysfunctions of these fusogenic proteins could cause various physiological deficits in cells. For example, it was found that the misfolded Rab1 led to the dysfunction of the ER to Golgi transport, genetically and pathologically causing neurodegeneration in amyotrophic lateral sclerosis (ALS) in mice [102]. STX5, as an integral component of the ER-derived COPII-coated vesicles, plays a role in maintaining the morphology of the Golgi apparatus [103]. GS27 and GS28 are important membrane trafficking proteins between the ER and the Golgi apparatus and between the Golgi subcompartments [104]. In the functional assays for the assessment of the pathogenicity, it was found that mutation of GS27 resulted in progressive myoclonus epilepsies [105, 106]. BET1 mainly exists at the ER and the cis-Golgi membrane. Downregulation of BET1 by siRNAs impairs the ER-to-Golgi transport [107]. Furthermore, the ER protein Sec22b promotes efficient membrane fusion in both anterograde and retrograde transport [108], and mutation of Sec22b has been implicated in atherosclerosis and Alzheimer's disease (AD) [109–111].
Retrograde Golgi-to-ER transport
The retrograde Golgi-to-ER trafficking is responsible for the recovery and transport of escaped proteins or recycled membrane back to the ER [112]. This retrieval is critical for maintaining the homeostasis of the ER and the Golgi apparatus in mammalian cell [113]. The COPI-coated vesicles mediate the retrograde transport from the Golgi apparatus to the ER (Fig. 2a) [114–116].
The transport of COPI-coated vesicles from the Golgi apparatus to the ER is dependent on GTPases Rab6A and motor kinesin family proteins. Golgi located Rab6A is one of Rab6 isoforms that are ubiquitously expressed in mammalian cells. It was found that GTP-bound Rab6A was active and bound to COPI-coated vesicles via hydrophobic geranylgeranyl modified cysteine residues at C-terminus, meanwhile Rab6A also directly bound to the C-terminal domain of the motor protein Rabkinesin-6 on microtubule in two-hybrid assay and Co-IP experiments [117]. In addition, kinesin family member 1C (KIF1C), as a member of the Unc104 subfamily of kinesin-like proteins, also involved in vesicle transport from the Golgi apparatus to the ER [118]. It was reported that the C-terminus of KIF1C directly bound to Rab6A, while its N-terminal domain moved along microtubules [119]. Note that, some reports also suggested the existence of a COPI-independent Golgi-to-ER transport which is regulated by the dynein-dynactin motor complex and its adaptor BicaudalD [120]. And this COPI-independent Golgi-to-ER transport may be responsible for the retrograde transport of glycosylation enzymes and Shiga-like toxin through tubular carriers rather than vesicles [121–123].
The tethering of COPI-coated vesicles to the ER is mediated by Dsl1 [124]. The Dsl1 complex (also called STX18 complex) consists of three subunits (Dsl1, Sec39 and Tip20) [124]. The Dsl1 complex is a tethering complex that located in the ER and recognizes the Golgi-derived COPI-coated vesicles [125]. In the Pull-down experiments, it was found that COPI-coated vesicles were tethered to ER membrane by the central acidic domain of the Dsl1 interacting with the COPI subunits (α-, ɛ-, and δ-COP) [124, 126]. In addition, the conserved oligomeric Golgi (COG) complex, and Caspase (CASP) (the alternatively spliced product of the gene encoding the CCAAT-displacement protein transcription factor) also act as tethering proteins to associate COPI-coated vesicles with ER membrane [127–129].
Once vesicle tethered, membrane fusion between the COPI-coated vesicle and the ER will occur by the formation of SNARE complex consisting of STX18, B-cell lymphoma-2 interacting protein 1 (BNIP1), unconventional SNARE (primary sequence is less conserved in SNARE motif) in the ER1 (USE1), Sec22b [3]. The ER membrane-localized STX18, BNIP1, and USE1 form a ternary SNARE complex with Sec22b on vesicle to promote membrane fusion, thereby transporting the ER proteins from the ERGIC and CGN back to the ER [130]. The SM protein Sly1 also involved in mediating the membrane fusion between the COPI-coated vesicle and the ER by interacting with STX18 via its conserved N-terminal motif [99, 131]. The consequence of the membrane fusion driven by these fusogenic proteins is to maintain various normal physiological functions in cell. For example, knockdown of STX18 induces segregation of the smooth and rough ER, suggesting a role of STX18 in the organization of the ER membrane [132], while deletion of BNIP1 disrupts the three-way junctions of the ER network [133]. Mammalian USE1 is also known as MAPK-activating protein PM26 or p31, and deletion of USE1 causes death of mice due to the disruption of ER structures [134, 135].
Trans-Golgi network transport
The TGN, as a cargo sorting station, generates distinct transport carriers for various destinations [136]. Membrane trafficking from the TGN is crucial for both the endocrine and exocrine cells. Secretory vesicles are formed at specific membrane region of the TGN via envelopment of the dense-core aggregates of secretory proteins by clathrin [137–139]. TGN transport includes the vesicle transport from the TGN to plasma membrane, the vesicle transport from the TGN to endosome, and the vesicle transport from endosome to the TGN (Fig. 2a). Here, we only focus on the molecular mechanism of vesicle transport from the TGN to plasma membrane which is well established and shares a conserved molecular mechanism with other TGN transport pathways (Table 2) [140–146].
In endocrine secretion, the vesicle transport from the TGN to plasma membrane involves Rab3, the adaptor protein DENN/MADD and the motor protein KIF1A. In presynapse, GTP-bound Rab3 locates on synaptic vesicles via geranylgeranyl modified cysteine residues at C-terminus and recruits its effector protein DENN/MADD, which acts as an adaptor protein to crosslink the synaptic vesicle and the CC3 domain of KIF1A [147]. The KIF1A consists of a motor domain at the N-terminal side, three CC domains and a pleckstrin homology (PH) domain at the C-terminal side. In details, the motor domain associates with microtubule via the microtubule-binding domains, while CC1 domain regulates the motor activity by inhibition of the motor domain. Moreover, both the CC1 and CC2 domain regulate motor dimerization [148, 149], and the PH domain mediates the recruitment of cargo containing vesicle via binding to PI(4,5)P2 [150]. By overexpression or knockdown of KIF1A, it was found that the overactivation of KIF1A increased the number of synaptic vesicles at active zone of the presynapse, while the depletion of KIF1A resulted in a decrease in the number of synaptic vesicles [151], suggesting that the KIF1A is important for the axonal transport of synaptic vesicles to plasma membrane [150].
The exocyst complex is required for vesicle tethering to the plasma membrane [152]. The vesicle located Rab GTPase Sec4 (the yeast homolog of Rab8), recruits the exocyst complex to secretory vesicles through interacting with the subunit of Sec15 [153]. The exocyst consists of the eight subunits EXOC1-8 in mammalian cell, corresponding to Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo8 in yeast [152]. In the co-sedimentation experiments, it was found that EXOC1 bound with PI(4,5)P2 on plasma membrane via its PH domain-like region at its N-terminus [154], while EXOC7 bound with PI(4,5)P2 via a patch of basic residues at its C-terminus [155]. In mammalian cell, Rab-interacting molecule (RIM), as the effector protein of Rab3, is also required for vesicle tethering to the plasma membrane. In liposome binding experiments, it was found that RIM could interact with vesicle located GTP-bound Rab3 via its two α-helical regions at N-terminus, and could bind to PI(4,5)P2-containing membrane via its C2B domain [156]. In addition, it was repored that RIM protein also involved in tethering N- and P/Q-type Ca2+ channels to presynaptic active zone via a direct PDZ-domain interaction in RIM conditional knockout mice [157]. Moreover, the RIM’s Zinc finger (ZF) domain could interact with C2A domain of Munc13 to facilitate vesicle tethering [158].
CCVs transport from the Golgi to endosomes, lysosomes, or plasma membrane, also relies on SNARE proteins [3]. For example, neurotransmitter release is mediated by a ternary SNARE complex consisting of STX1A, synaptosomal-associated protein 25 kDa A (SNAP-25A), and vesicle associated membrane protein 2 (VAMP2) [159]. A ternary SNARE complex consisting of STX7, vesicle transport through interaction with t-SNAREs 1B (VTI1B), STX8 and VAMP8 drives homotypic fusion of late endosomes [160, 161], while VAMP7 determines heterotypic fusion of late endosomes [162]. In addition, cargo molecules from endosomes can also be transported back to the TGN. The ternary SNARE complex involved in the endosome-to-TGN trafficking is STX6, STX16, vesicle transport through interaction with t-SNAREs homolog 1A (VTI1A), VAMP4 [145, 163–165]. The detailed molecular mechanisms of SNARE mediated vesicle fusion are discussed in section of molecular mechanism of vesicle fusion.
Endocytic pathway
The endocytic transport is critical for the uptake of extracellular components, receptor internalization and the regulation of cell signaling [166]. In the endocytic pathway, CCVs with internalized molecules are transported to early endosomes for cargo sorting. Some molecules such as recycling receptors and lipid membrane are transported back to plasma membrane via recycling endosomes, while others including ubiquitylated proteins and downregulated receptors are transported to late endosomes and lysosomes for degradation [167]. Endocytic vesicle transport among several intracellular organelles of early endosomes, late endosomes and lysosomes also shares a similar molecular mechanism, although the regulatory proteins are different (Fig. 2a) (Table 2) [168–174]. Here, we take the endocytic transport of the clathrin-coated endocytic vesicle from plasma membrane to endosome for example to elucidate the molecular mechanism of the key regulators involved in the pathway.
The transport of the clathrin-coated endocytic vesicle from plasma membrane to early endosomes involves AP-2, Disabled 2 (Dab2) and myosin VI [175]. The CCVs located AP-2 binds to the DPF motifs of adaptor protein Dab2 at the central region [176]. Moreover, the C-terminal region of Dab2 binds to the divergent tail domain of myosin VI, and induces the dimerization of myosin VI, which is essential for its motion activity [176]. The highly conserved motor domain of myosin VI is responsible for binding to F-actin and transporting vesicle along actin filament by converting energy from ATP hydrolysis into directional motion [176]. For more details about the endocytic transport of CCVs, we refer to the other reviews [7, 67, 166, 177].
The tethering protein early endosomal antigen 1 (EEA1) is required for the clathrin-coated endocytic vesicle tethering with early endosomes [178, 179]. EEA1 is predominantly localized to the early endosomes as the specific biomarker. The crystal structure of EEA1 revealed that EEA1 could form into a ~ 200 nm long coiled-coil homodimer, composed of an N-terminal C2H2 ZF domain and a C-terminal FYVE (Fab 1, YOTB, Vac 1, and EEA1) domain, in which the FYVE domain interacts with phosphatidylinositol-3-phosphate (PI3P), which is essential for early endosomal membrane to recruit EEA1, while the C2H2 ZF domain binds to GTP-bound Rab5 on the clathrin-coated endocytic vesicles [180]. Moreover, the tethering protein class C core vacuole/endosome tethering (CORVET) is involved in the homotypic fusion of early endosomes. Early endosomes located CORVET, consists of Vps3, Vps8, Vps11, Vps16, Vps18, and Vps33 subunit [181], in which the subcomplex of Vps3 and Vps8 physically and genetically interact with the Rab5 for early endosomes tethering [182].
A ternary SNARE complex consisting of STX13, SNAP-25/SNAP-29 and VAMP2/VAMP3 drives the heteromorphic fusion of the clathrin-coated endocytic vesicle and early endosome [10]. STX13 and SNAP-25/SNAP-29 contributes Qa- and Qbc-SNARE motifs, respectively, while VAMP2/VAMP3 contributes the other arginine-containing SNAREs (R-SNARE) motif, thereby assembling into the four-helical bundle of SNARE complex, and mediating the membrane fusion [10]. On the other hand, homotypic membrane fusion of early endosomes is essential for the formation of sorting endosomes. Homotypic membrane fusion of early endosomes relies on a conserved SNARE complex consisting of STX13, VTI1A, STX6, and VAMP4, although such SNARE pairing does not suffice to determine the specificity of early endosome fusion [183]. Besides, the SM proteins Vps33, one subunit of the CORVET/ homotypic fusion and protein sorting (HOPS), is also critical for the homotypic membrane fusion of early endosomes. In reconstituted liposome fusion assay, it was found that Vps33 promoted fusion pore opening by enhancing the formation of SNARE complex [184].
Membrane trafficking in autophagy
Autophagy is a highly regulated catabolic process in eukaryotic cells, which uses lysosomes to degrade large protein aggregates, damaged organelles and other components to cope with internal and external stress and to maintain cell homeostasis [185]. The process of macroautophagy includes five stages: autophagy induction, nucleation process, extension of autophagosome, fusion of autophagosome and lysosome, and degradation within autophagosome [186].
The retrograde transport of autophagosomes to lysosomes involves Rab7, LC3, FYVE and coiled-coil domain-containing protein 1 (FYCO1), kinesin-1. Rab7 recruits its effector protein FYCO1, which binds to microtubule-associated proteins 1A/1B light chain 3B (LC3B) and PI3P on autophagosomes via FYVE domain [187, 188]. In immunoprecipitations and immunoblots experiments, it was found that the phosphorylation of LC3B would reduce the binding affinity with FYCO1 [189]. Moreover, bead capture based Pull-down assays indicated that the middle part of FYCO1 (residues 585–1233) bound directly to the kinesin light chain 2 of kinesin-1 [190], and protrudin, an ER protein, promotes such interaction in human and rat cell lines, which is required for the retrograde transport of autophagosomes to lysosomes along kinesin-1 associated microtubule [187, 189, 190].
Ectopic P granules protein 5 homolog (EPG5), as an effector protein of Rab7, serves as a tethering factor to ensure the specificity of autophagosome-lysosome/late endosome fusion. EPG5 is recruited to the lysosome or late endosome by interacting with ATG8 homolog human LC3B/LC3C on autophagosome via its two LC3-interacting regions with a conserved sequence (W/F/Y-X1-X2-I/L/V) [191]. Pleckstrin homology domain-containing family member 1 (PLEKHM1), as another Rab7 effector protein, is localized on late endosomes and lysosomes. It was found that PLEKHM1 bound to ATG8 family proteins, preferentially to GABARAPs, to capture autophagosome by a yeast two-hybrid system [192, 193]. ATG14, an essential autophagy-specific regulator of the class III phosphatidylinositol 3-kinase complex, promotes membrane tethering of autophagosome. In a reconstituted liposome fusion assay, it was found that ATG14 homo-oligomerization enhanced their ability to promote membrane tethering [194]. Moreover, ATG14 interacts with the SNARE core domain of STX17 through its CC domain and stabilizes the STX17-SNAP-29 complex to promote membrane fusion of autophagosomes and lysosomes [194]. The HOPS complex also plays a tethering role in autophagy. It was found that HOPS was recruited to the autophagosome membrane by binding to Rab7 and phospholipids such as PI3P in protein-lipid overlay assays [195]. In summary, multiple tethering factors may act coordinately to ensure the efficiency and specificity of vesicle tethering in autophagy.
SNAREs (STX17, SNAP-29, and VAMP8) are essential for autophagosome formation and degradation [194, 196]. STX17 is widely expressed in a variety of tissues and mainly located on the ER membrane, mitochondria and cytoplasm [197]. When autophagy is activated, STX17 and SNAP-29 are recruited to the autophagosome membrane, and interact with VAMP8 on the lysosome to form the ternary SNARE complex, which drives the membrane fusion of autophagosome and lysosome, leading to the completion of autophagy [194, 198]. Besides of SNARE protein, the SM protein Vps33A [199] also binds to the SNARE complex at the groove of the four-α-helical bundle, promoting the vesicle fusion by stabilizing the STX17-SNAP-29-VAMP8 complex [200].
Molecular mechanism of vesicle fusion
Vesicle fusion with a target membrane is the end of a particular vesicle trafficking pathway. Vesicle fusion involves several steps, such as vesicle tethering or docking, vesicle priming, hemifusion, fusion pore opening and the SNARE complex disassembly [3, 201–203]. To achieve this, the repulsive force generated by the negatively charged lipid bilayers and the dehydration of the water layers at the lipid headgroups have to be overcome. The SNARE complex formation can produce the energy to drive two membranes fusion via the formation of four-helix bundles [204]. Without regulators, membrane fusion occurs spontaneously by a thermodynamically-driven process of the SNARE complex formation [205]. To precisely regulate the vesicle fusion, other fusogenic proteins, such as the tethering factor Rab and RIM, Ca2+ sensor protein Syts, Cpx, Munc18, Munc13, NSF and α-SNAP cooperate to ensure vesicle fusion event occurs at defined fusion site and under precise control [159, 202, 206, 207]. Intensive research that began over 40 years has resulted in an understanding of the molecular mechanism of SNARE-mediated vesicle fusion.
SNARE proteins
SNARE proteins involve in most of the intracellular vesicle trafficking pathway except the homotypic fusion between the mitochondria and the ER membrane [208]. The nomenclature of SNARE proteins stems to their discovery as membrane receptors for soluble NSF attachment protein (SNAP) [209–215]. SNARE proteins have a superfamily of 36 homologs in human, here we mainly focus on the best characterized neuronal SNAREs. SNARE proteins are characterized by the SNARE motif in the membrane proximal region, which is evolutionary conserved domain of 60 ~ 70 residues with heptad repeats (Fig. 3a). The SNARE hypothesis was proposed in 1993, in which it postulated that a distinct vesicle-SNARE (v-SNARE) pairs with the target membrane-SNARE (t-SNARE), and such specific interaction drives two membranes to fuse [216].
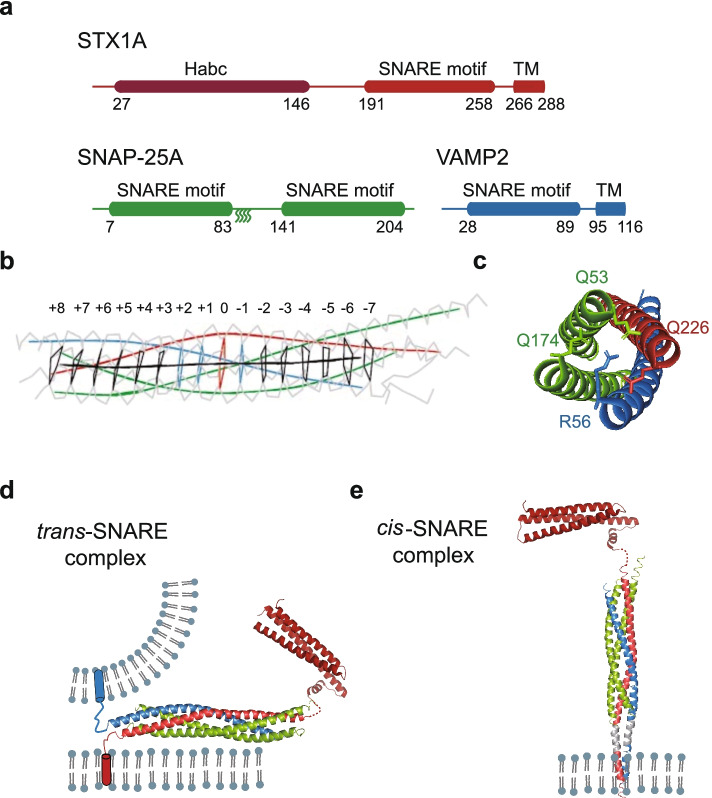
Fig. 3.
SNARE proteins and SNARE complex. a Domain diagrams of the STX1A (red) with Habc domain (dark red), SNAP-25A (green) and VAMP2 (blue). For SNAP-25A, the palmitoylation sites within the cysteine cluster (C85, C88, C90, C92) of the linker region are presented as curve lines. The numbers of residues are indicated below each diagram. The color code for each SNARE protein is the same in other figures. TM, transmembrane. b 16 stacked layers (Red: 0; Blue: -1, + 1 and + 2, that are closest to the ideal leucine-zipper geometry; Black: others) of the SNARE complex are indicated in Cα traces (gray), the superhelical axis (black), the helical axes of STX1A, SNAP-25A and VAMP2 are shown as red, green, and blue respectively. c The side chains involved in the ionic “0” layer of SNARE complex are shown as sticks. d The ribbon diagram shows the structure of the trans-SNARE complex in Rattus norvegicus (PDB ID: 1SFC) [217] with STX1A Habc domain (PDB ID: 3C98) [218] on two opposing membranes. e The ribbon diagram shows the structure of the cis-SNARE complex in Rattus norvegicus (PDB ID: 3IPD) [219] on plasma membrane after a full fusion event. The copyright permission of panels b and c are from [217]
The first identified SNAREs in mammalian cells were synaptic STX1A [209], SNAP-25A [213] and VAMP [214], while the yeast SNAREs were independently discovered via genetic screening [212]. STX1A and SNAP-25A are generally located on the plasma membrane, while VAMP2 is primarily located on the synaptic vesicles. Both STX and VAMP are anchored to membrane via a single helical transmembrane domain at the C-terminus, whereas SNAP-25A resides on the plasma membrane via the palmitoylation modification of the four cysteine residues on the linker region (Fig. 3a). In the ternary neuronal SNARE complex, the four SNARE motifs form a parallel four-helix bundle, of which SNAP-25A contributes two SNARE motifs, STX1A and VAMP2 each contribute one SNARE motif, respectively [217].
The SNARE complex of four-helix bundle consists of 16 stacked layers of interacting side chains (Fig. 3b) [220]. The “-1”, “ + 1” and “ + 2” layers of the complex are at the center position, which are closest to the ideal leucine-zipper geometry and amino acid composition (Fig. 3b). In the middle of the SNARE motif, it is a highly conserved “0” layer (Fig. 3b), composed of one arginine residue from VAMP2, one glutamine residue from STX1A, and two glutamine residues from SNAP-25A (Fig. 3c). According to this remarkable feature, SNARE proteins are also classified as glutamine-containing SNAREs (Q-SNARE) and R-SNARE, respectively. The QabcR-complex groups in “0” layer of the SNARE complex are highly conserved throughout all species [220].
SNARE proteins that are located in opposing membranes form the trans-SNARE conformation (Fig. 3d), and drive membrane fusion by releasing free energy (~ 36 kBT) [204, 221] during the zippering of the SNARE complex, which ends in the cis-SNARE conformation (Fig. 3e) [219]. The fusion pore is a vital transient state in the final step of each trafficking pathway. The process of fusion pore opening and dilation is highly dynamic, which might determine the fate of trafficking vesicles, either fusing with the plasma membrane or via a “kiss-and-run” route (fusion pore closes rapidly without full dilation) [222]. The copy number and dynamics of trans-SNARE complex are critical for fusion pore formation [223]. To induce lipid stalk formation, the energy required to overcome the hydration-force barrier is around 40 ~ 90 kBT [224]. Considering the additional energy needed for the pore formation and dilation, more energy would be required to drive a full fusion event. Thus, one SNARE complex may be sufficient for lipid exchange between two membranes, i.e. a hemifusion intermediate state [225], but more SNARE complexes are required for a full fusion event [226–228].
Post-translational modifications (PTM) of SNARE proteins, for example phosphorylation, palmitoylation, acetylation, and O-GlcNAcylation, play an important role in the regulation of membrane trafficking [229]. In the vesicle trafficking pathway of autophagy, deacetylation of STX17 enhances the binding to SNAP-29 and facilitates the formation of the STX17-SNAP-29-VAMP8 complex, thereby further promoting autophagosome-lysosome fusion [198]. Additionally, both O-GlcNAcylation of SNAP-29 and phosphorylation of VAMP8 hinder the SNARE complex assembly [230–233]. Moreover, PTM of SNARE proteins are also involved in other vesicle trafficking pathways. Phosphorylation of the residues within the SNARE motif of VAMP8 inhibits mast cell secretion [232], which might be important for preventing overreach reaction such as anaphylactic shock. Palmitoylation of SNAP-25A is essential for exocytosis in neuroendocrine cells [234], while ubiquitination of STX5 inhibits the SNARE complex assembly and disrupts Golgi membrane fusion during early mitosis [235]. Taken together, PTM of SNAREs is crucial to their localization and the SNARE complex formation in membrane trafficking pathway.
Synaptotagmin
The Syts, as the Ca2+ sensor protein, play a key role in regulating Ca2+-triggered membrane fusion [236, 237]. Syts are an evolutionary conserved family of proteins that consist of N-terminal single transmembrane domain, an unstructured linker region, and two cytoplasmic protein Kinase C-like C2 domains, termed C2A and C2B, respectively (Fig. 4a) [236, 238, 239].
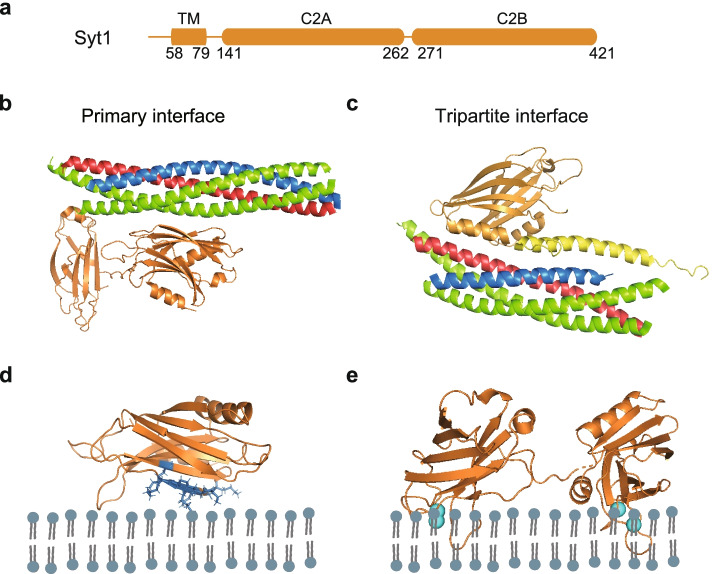
Fig. 4.
Syt1 and its interactions with SNARE complex and membrane. a Domain diagram of Syt1. Residue numbers are indicated below the diagram. b Crystal structure of the primary interface of SNARE-Syt1 complex in Rattus norvegicus (PDB ID: 5CCI) [240]. c Crystal structure of the tripartite interface of SNARE-Syt1-Cpx1 complex in Rattus norvegicus (PDB ID: 5W5D) [241]. d Model of polybasic region of C2B domain interacting with the anionic membrane (PDB ID: 1K5W) [242]. The side chains of the residues in the polybasic region (K313, K321, R322, K324, K326, K325 and K327) are shown in blue sticks. e Crystal structure of the tandem Syt1 C2AB (orange) domains in Rattus norvegicus (PDB ID: 5KJ7) [239] in the presence of Ca2+ (cyan sphere)
There are 16 isoforms of mammalian Syts [243]. Among these isoforms, Syt1, Syt2 and Syt9 are the Ca2+ sensor for evoked synchronous synaptic vesicle fusion or secretory granule secretion. In addition, Syt7 acts as a redundant Ca2+ sensor for Ca2+-dependent asynchronized release [244–246]. Here, we only focus on Syt1 isoform that is located on the synaptic vesicle and is critical for synchronous Ca2+-triggered synaptic vesicle fusion.
In absence of Ca2+, Syt1 binds to both the anionic membranes via the polybasic region and possibly arginine apex, as well as the SNARE complex via the primary interface and tripartite interfaces (Fig. 4b-d) [240–242, 247], which is vital for vesicle docking and priming [240, 248–259]. Note that, the interaction between the SNARE complex and the polybasic region of Syt1 was also observed in the absence of membrane environment by NMR experiments [249]. In vitro reconstitution fusion assay and cryo-EM of cultured neuron experiments demonstrated that Syt1 bound to the heterodimer of STX1A-SNAP-25A subcomplex to mediate vesicle docking [260, 261]. It was found that Syt1 clamped the frequency of miniature spontaneous fusion events in the absence of Ca2+ by in vitro reconstitution assay and neuronal culture experiments [262–265]. In addition, ring-like oligomers of Syt1 was observed on synthetic membrane in cryo-EM experiments, and destruction of the oligomer ring will lead to the increase of spontaneous fusion in the absence of Ca2+ [263, 264, 266]. However, such ring-like structure have not yet been found in cryo-ET studies of synaptosomes [267]. In another model, Syt1 and Cpx1 can lock two membranes in the pre-fusion state by interacting with the SNARE complex via a tripartite interface and a primary interface (Fig. 4b-c), which is thought to be critical in promoting Ca2+-triggered neurotransmitter release [241]. A different model has been recently proposed that Syt1 may dissociate from the primary complex upon Ca2+ binding to PI(4,5)P2 containing membrane environment, and subsequent followed by conformational change of Cpx [268]. Note that, the primary interface has been confirmed in several studies in solution as well as in neurons, while further studies are required to fully corroborate the physiological importance of the tripartite interface.
When Syt1 binds to Ca2+ via the region located on the bottom of the each C2 domain (Fig. 4e), synchronized neurotransmitter release occurs [269–273]. In Syt1-deficient mammalian neurons, Syt1 mutants that disrupt the Ca2+ binding sites of the Syt1 C2A domain can partially rescue the phenotype in the Ca2+-evoked neurotransmitter release, while mutants that disrupt the Ca2+ binding sites of the Syt1 C2B domain cannot, suggesting a more vital role of C2B domain than C2A domain [265, 274, 275]. Reducing the Ca2+ binding affinity of Syt1 in mice causes a correspondingly reduction in Ca2+ sensitivity of fusion [253, 276], proving that Syt1 is the Ca2+ sensor in synaptic vesicle fusion. One of established working model for Ca2+-triggered synaptic vesicle fusion is a synergistic interaction among Syt1, the SNARE complex, and anionic membrane. In this model, the primary interface between Syt1 and the SNARE complex [240] and the interaction between the polybasic region of Syt1 and anionic lipids [249, 268, 277] may serve as a scaffold, while the fusion loop of C2 domain inserts into anionic membrane upon Ca2+ binding, and produces the local positive membrane curvature on the plasma membrane, which substantially increases the fusion probability by lowering the energy barrier of hydration force [268, 278–281]. It has also been shown that Syt1-Ca2+ substantially promotes the fusion pores opening and expansion by cooperating with the SNARE proteins [262, 282] or interacting with membrane by two conserved arginine residues in Syt1 C2B domain [247].
Although the interaction between the polybasic region of Syt1 and the SNARE complex was observed in numerous biochemical studies and NMR experiments [249, 268], in vitro reconstitution experiments suggested that Syt1 promoted Ca2+-triggered vesicle fusion by binding to PI(4,5)P2-containing membrane rather than SNARE at physiological ion concentration [283]. Such controversial results under different experimental conditions suggest a rather dynamic interaction between Syt1 and the SNARE complex.
Besides, the cryo-ET analysis of mouse hippocampal synapses revealed that Syt1 loosely docked synaptic vesicles to plasma membrane within 2 ~ 12 nm by interacting with PI(4,5)P2, while the SNARE complex brought synaptic vesicles closer within 0 ~ 2 nm [284, 285]. Additionally, by functional reconstitution in liposome fusion, it suggested C2 domains were capable of decreasing the gap between synaptic vesicle and target membrane by crosslinking opposing membranes [286, 287]. Using reconstituted proteoliposome, it was found that under low ionic, Syt1 functions as a distance regulator that tethers the liposomes close enough for membrane fusion in the presence of Ca2+ [287]. Moreover, both in vitro and in vivo studies showed that the linker between the C2 domains, and the juxta membrane linker between the transmembrane domain and the C2A domain are also important for Syt1 function in Ca2+-triggered vesicle fusion, possibly by regulating the distance between two membranes [288–291].
Complexin
Cpx, also known as synaphin, is a small cytoplasmic protein, which is largely unstructured in solution [292]. There are four homologs in the mammalian Cpx family. Cpx1 and Cpx2 mainly exist in synapse, while Cpx3 and Cpx4 are found in the optic nerve [293, 294]. The difference in subtype distribution implies the functional difference among the isomers of Cpx. Here, we focus on the homolog Cpx1 whose primary sequence is highly conserved in different species [293, 295, 296].
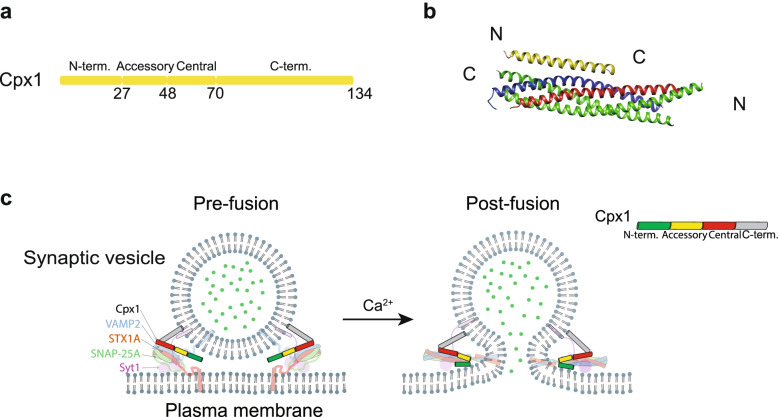
Cpx1 consists of a N-terminal domain, an accessory α-helix domain, a central α-helix domain and a flexible C-terminal domain (Fig. 5a). The N-terminal domain of Cpx1 plays a key role in activation of fast synchronous release in mammalian cell [297, 298], probably via interaction with the plasma membrane and the C-terminal end of the SNARE complex [299, 300]. The accessory domain plays a role in reducing spontaneous release in cultured neuron based electrophysiology experiments and in vitro reconstituted systems, although its molecular mechanism is still controversial [301–303]. Cpx1 binds to the SNARE complex via its central α-helix domain, which inserts into a groove formed by VAMP2 and STX1A in an anti-parallel orientation (Fig. 5b) [302, 304, 305] Moreover, the C-terminus of Cpx1 is attached to synaptic vesicle via sensing the membrane curvature of synaptic vesicle, and such localization on synaptic vesicle is critical for inhibiting spontaneous release [299, 306]. Furthermore, the C-terminal domain has an effect on synaptic vesicle priming in neurons [299, 307, 308]. The C-terminal deletion of Cpx1 cannot inhibit spontaneous release in neuron, but it still stimulates Ca2+-triggered release in both cultured neuron and reconstitution system [299, 307–309].
Fig. 5.
Cpx1 and its regulatory function. a Domain diagram of Cpx1. Residue numbers are indicated below the diagram. b Crystal structure of the SNARE-Cpx1 complex in Rattus norvegicus (PDB ID: 1KIL) [305]. c Models of how Cpx1 regulates synaptic vesicle fusion. In absence of Ca2+, the core fusion machinery is locked to a pre-fusion stage via several interactions from Cpx1: the central α-helical domain interacts with SNARE complex; the accessory α-helical domain involves in a tripartite interface with Syt1 and SNARE complex; C-terminal domain is associated with synaptic vesicle (left). When Ca2+ influx, Cpx1 inhibition could be released by a conformational change of the tripartite interface upon Ca2+ binding to Syt1, meanwhile Cpx1 synchronizes synaptic vesicle fusion via its N-terminal domain binds to C-terminus of trans-SNARE complex and plasma membrane (right)
Cpx1 acts both as an activator and as an inhibitor for neurotransmitter release, giving rise to a major controversy (Fig. 5c) [295]. In the presence of Ca2+, Cpx1 activates synchronous neurotransmitter release via its N-terminal region and the central α-helix domain binding to the SNARE complex (Fig. 5c) [292, 295, 296, 298, 303, 304, 308–313], while in the absence of Ca2+, Cpx1 has an inhibitory function on spontaneous release via the accessory α-helix domain and the C-terminal domain, although this function is less conserved among species (Fig. 5c) [299, 304, 308, 309, 314, 315]. Site-directed mutagenesis in Drosophila showed that the Cpx clamping function was predominantly maintained by its accessory helix, and molecular modeling results suggested that the Cpx accessory domain interacted with the truncated C-terminus of VAMP2 and the lipid bilayer of synaptic vesicle to prevent the SNARE complex fully assembly [316]. Energetic measurements by surface forces apparatus revealed that Cpx1 facilitated SNAREpin assembly sequentially by doubling the distance of intermembranes at which the SNAREs alone that can engage, and then clamped trans-SNAREpin formation by binding to the C-terminus of SNARE complex, into a half-zippered intermediate state [317, 318]. Besides, the crystal structure of SNARE-Cpx1 complex revealed that Cpx1 could organize SNAREs into zig-zag topology to prevent spontaneous fusion, although a C-terminal SNARE motif truncated VAMP2 was used to mimic trans-SNARE state [319]. However, the follow-up studies suggested that Cpx mutants in Drosophila which disrupted the zig-zag topology of SNARE-Cpx1 complex, could still rescue Ca2+-evoked neurotransmitter release, indicating that crosslinked the trans–SNARE complex by Cpx is not a prerequisite for synchronized neurotransmitter release [320]. Last, the crystal structure of the primed pre-fusion SNARE-Cpx-Syt1 complex revealed an unexpected tripartite interface and the mutations of the interface severely impaired Ca2+-evoked synchronous release in neurons, suggesting that interface is essential for the primed pre-fusion state [241].
Munc13 and Munc18
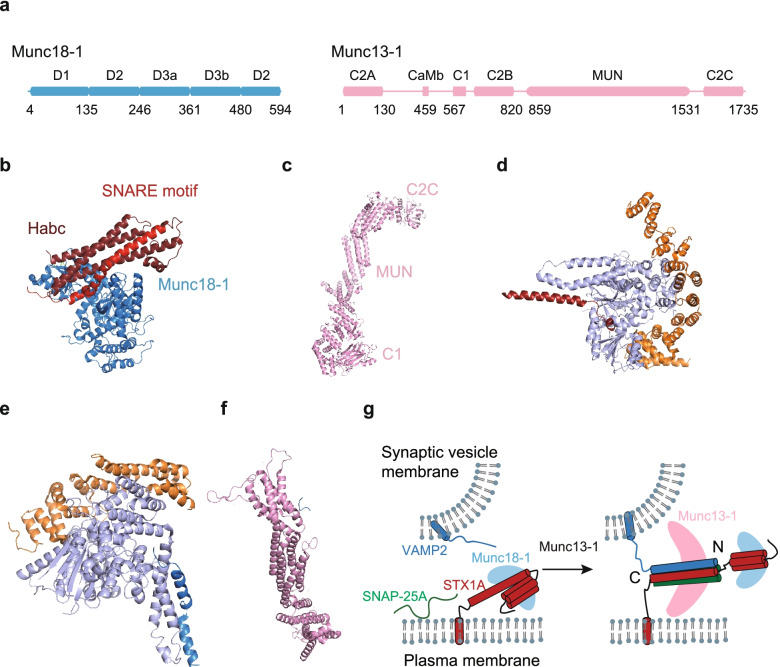
Munc13 and Munc18 regulate synaptic vesicle priming via orchestrating the SNARE complex properly assembly [321–325]. Here we focus on Munc13-1 and Munc18-1 that are well studied in synapse. The structure of Munc18-1 protein includes five domains (Fig. 6a) [326, 327]. Structural studies by crystallography and electron paramagnetic resonance spectroscopy revealed that Munc18-1 captured STX1A, locking it into a heterodimeric complex in a closed conformation [218, 328–330]. This state kinetically prevents the formation of ternary SNARE complex [218, 330, 331] until the presence of Munc13-1. The crystal structure of the Munc18-1-STX1A complex revealed the interaction of STX1A with the pocket formed by domains 1 and 3 of Munc18-1 (Fig. 6b) [329, 330] explaining the inability of the SNARE motif of STX1A to interact with SNAP-25A and VAMP2. Although such closed conformation is not valuable for vesicle fusion due to its hindrance to the SNARE complex formation, it may be required for the recruitment of Munc18-1 to fusion sites of active zone [332], and it may serve as template for the SNARE complex assembly [323, 333].
Fig. 6.
Munc18-1 and Munc13-1. a Domain diagrams of Munc18-1 (blue) and Munc13-1 (pink). Residue numbers are indicated below the diagrams. b Crystal structure of the STX1A-Munc18-1 complex in Rattus norvegicus (PDB ID: 3C98) [218]. The Habc domain (dark red) of STX1A locks the SNARE motifs (red) in the closed conformation by the interaction of Munc18-1. c Cryo-EM crystal structure of the C1C2BMUNC2C fragment of Munc13-1 in Rattus norvegicus (PDB ID: 7T7V) [334]. d Crystal structure of the Qa-SNARE Vam3 (red) bound to the SM protein Vps33 (light blue) in Chaetomium thermophilum (PDB ID: 5BUZ) [321]. e Crystal structure of the R-SNARE Nyv1 (marine) bound to the SM protein Vps33 in Chaetomium thermophilum (PDB ID: 5BV0) [321]. f Crystal structure of the VAMP2 (marine, residues 87–92)-MUN (pink) complex in Rattus norvegicus. (PDB ID: 6A30) [333]. g Model of Munc13-1 (pink) catalyzing the transfer of STX1A from the Munc18-1 (light blue)-STX1A complex to the properly assembled ternary trans-SNARE complex. The color codes for STX1A, SNAP-25A and VAMP2, are red, green, and blue, respectively
Munc13 enables STX1A to transit into open conformation and it acts as a chaperons to promote ternary neuronal SNARE complex formation [335–337]. Munc13-1 consists of a C1 domain, a calmodulin-binding domain, two C2 domains (C2A, C2B), a MUN domain, and another C2 domain (C2C) (Fig. 6a). The crystal structure of the C1C2BMUNC2C fragment of Munc13-1 revealed intramolecular interactions among the C1, C2B, and MUN domains (Fig. 6c) [334, 338] The C1 and C2B domain of Munc13-1 binds to diacyglycerol and PI(4,5)P2 on the plasma membrane, respectively, and regulates the priming of synaptic vesicles, probably via increasing the local concentration of MUN domain [323, 339]. The C2A domain of Munc13-1 interacts with RIM [340], which might be essential for the vesicle tethering and priming [341]. The MUN domain of Munc13 performs two functions: (1) catalyzes the transfer of STX1A from the STX1A-Munc18 complex to the ternary trans-SNARE complex [333, 335, 342], and (2) promotes the parallel configuration assembly of the SNARE complex with Munc18 [323, 343, 344]. Last, the C2C domain of Munc13-1 activates Ca2+-evoked release in chromaffin granule secretion and neurotransmission in C. elegans, likely via bridging two opposing membranes [345–347].
It was found a novel autoinhibitory role for the C2B domain of Munc13 by deletion of C2B of C. elegans Munc13 ortholog UNC-13 in electrophysiology experiments and the autoinhibition by C2B was relieved by Ca2+ binding to C2B and stabilized by the neighboring C1 domain [348, 349]. In addition, NMR experiments revealed the interaction between Munc18-1 and VAMP2, and this interaction was inhibited by a L348R mutation in Munc18-1 but stimulated by the D326K, which might contribute to the autoinhibition of VAMP2 [350]. Moreover, the mutation of Munc18-1 Q301D inhibited lipid mixing in a reconstituted fusion assay and its expression in Munc18-1 deficient neurons severely reduced synaptic transmission [351]. The mutations of VAMP2 that impair the function of Munc18-1 to promote trans-SNARE zippering, strongly inhibit spontaneous and synchronized neurotransmitter release in cultured neurons [352]. Furthermore, the crystal structures of Vps33 with Qa-SNARE Vam3 or R-SNARE Nyv1 and Munc13 with short VAMP2 fragment suggested Munc18 and Munc13 could bind to individual SNARE and align them into a correct orientation (Fig. 6d-f) [321, 323, 333]. Last, the recent functional study suggested that Munc13 promoted the proper SNARE complex assembly together with Munc18, which was critical for the physiological functions of Munc13 in priming and short term presynaptic plasticity [323]. Taken together, a working model has been proposed for Munc13 and Munc18, in which Munc13 and Munc18 may act as a template for the SNARE complex properly assembly to ensure synaptic vesicle priming (Fig. 6g).
Cooperation of the fusogenic proteins
Intensive studies of the molecular mechanism of SNARE-mediated vesicle fusion has resulted in a possible working model. 1) As a starting complex, Munc18 locks STX into a closed conformation on the plasma membrane [353]. Secretory vesicles are recruited to a delicate fusion site by the interaction with tethering factors such as Rab, RIM and Munc13 [354–357]. 2) In the step of vesicle priming, also known as the step of ready for fusion, or readily releasable pool, Munc13 catalyzes the transit of STX from the closed conformation of Munc18-STX complex into the proper trans-SNARE complex [323, 342, 353, 358]. Meanwhile, via interacting with the trans-SNARE complex, vesicle membrane and plasma membrane, Cpx1 and Syt1 lock the fusion complex into a prefusion state [206]. 3) In the step of Ca2+-triggered vesicle fusion, the fusion loop of Syt binds to Ca2+ and inserts into the anionic membrane [252, 254, 359, 360]. Meanwhile Syt1 interacts with the anionic lipid via the polybasic region, and with the SNARE complex via the primary interface, thereby inducing fusion pore opening via the cis-SNARE complex formation [240, 268, 336, 359, 361]. 4) After the cargo is released through the fusion pore, the cis-SNARE complex is disassembled by NSF and α-SNAP in response to ATP hydrolysis [362–367]. The disassociated SNARE proteins are then available for a new round of vesicle formation and fusion. In summary, SNARE-mediated vesicle fusion is highly dynamical and precisely controlled by Syt, Cpx, Munc13, Munc18 and others, including probably unidentified regulators, and dysfunction of any fusogenic protein in each substep of vesicle fusion could result in the development of a wide-range of membrane fusion related diseases.
Dysfunctions of vesicle trafficking-related proteins and diseases
Vesicle trafficking and fusion between organelles, as an important cell signaling pathway, maintain intracellular material exchange and intercellular signal transmission. Vesicle trafficking disorder can lead to a series of diseases, such as nervous system diseases, respiratory system diseases, immune system diseases, diabetes and so on [368, 369]. Pathological changes include the obstruction of vesicle trafficking and over secretion of vesicle cargo, so that reverse manipulation of vesicle trafficking could be the potential therapy for vesicle trafficking disorder related diseases. Since numerous proteins are involved in vesicle trafficking, and it is not realistic to discuss all the dysfunctions of these proteins related diseases. In this section, we only focus on the dysfunction of fusogenic proteins related diseases, because the molecular mechanism of membrane fusion between vesicle and cell membrane has been well elucidated, and manipulating these proteins are less likely to have an impact on the early steps of vesicle trafficking. Moreover, recent study shows that Ca2+-triggered membrane fusion can be manipulated to prevent the airway mucus occlusion in mice with asthma [370]. It suggests that the membrane fusion process can be controlled pharmacologically and targeting to the vesicle trafficking-related protein could be a novel therapeutics strategy for the drug development across a wide range of therapeutic areas.
Respiratory airway diseases
In the surface epithelium of intrapulmonary airway system, the secretory cells express and secrete mucins to extracellular airway lumen [371]. In the healthy state, mucin secretion is at low baseline level which is critical for the defense and clearance of inhaled particle and pathogens. When allergic mucus metaplasia occurs, the mucus is greatly increased in production and accumulated in the airway lumen. Mucus hypersecretion is a major cause of airway obstruction in the pathophysiology of airway related diseases, such as asthma and cystic fibrosis.
Up to now, most drugs are developed to reduce inflammation or expand the airways [371, 372], but the most serious issue is airway obstruction caused by mucus accumulation. The drugs approved to deal with mucus are targeted on mucin production, mucus hydration or mucus digestion [371, 373, 374]. Although some of available drugs can improve lung health in clinical trials [375], the limitations on their applications are also obvious. For example, treatments that inhibit mucin over production failed to reduce the mucin storage in airway epithelial cells in a clinic trial [376]. Moreover, treatments that reduce mucus accumulation via digesting DNA polymers in the mucus layer are only suitable for cystic fibrosis but not for other obstructive lung disease [377]. Finally, cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy is restricted to the patients with certain genetic mutations [378].
A novel strategy for reducing mucus accumulation is to inhibit mucus hypersecretion (Fig. 7a-b). Mucus hypersecretion is mediated by the SNARE proteins and the Ca2+ sensor Syt2. In stimulated mucin secretion, when agonists like ATP bind to purinergic receptors coupled to G proteins in the plasma membrane, inositol triphosphate (IP3) is generated by phospholipase C. Then, Ca2+ is released from ER via the activated IP3 receptor. In turn, Ca2+ binds to Syt2 and accelerates mucin secretion [371].
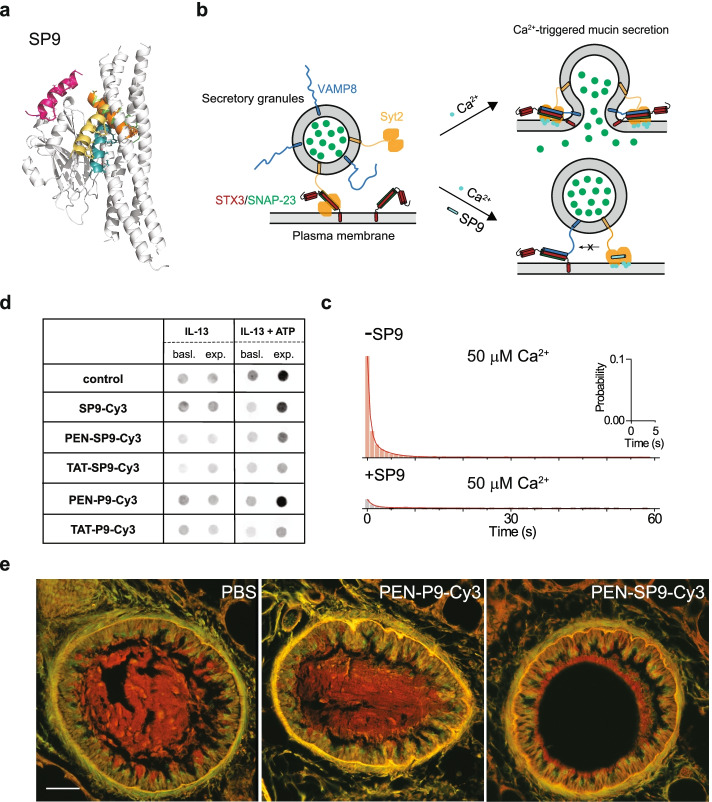
Fig. 7.
Inhibition of mucus hypersecretion by a stapled peptide. a Molecular dynamics simulations of SP9-Syt1-C2B complex superimposed on the structure of the primary interface (gray). b The therapeutic strategy to inhibit mucus hypersecretion. Binding of SP9 to Syt2 blocks the interaction between Syt2 and SNARE complex, thereby disrupts the Ca2+-triggered mucus hypersecretion. c SP9 inhibits Ca2+-triggered fusion probability in a reconstituted system. d SP9 inhibits mucin secretion from airway epithelial cells. Image represents western blots for MUC5AC on apical surface of untreated HAECs, or HAECs treated with different peptides. e SP9 inhibits mucin secretion and airway occlusion in mice. Images represents transverse sections of bronchial airways of mice with asthma pre-treated with PBS or peptides. Panels a, c-e, are adapted from [370]
Previous studies demonstrated that the primary SNARE-Syt interface is a genuine and specific interface and conserved in all species [240]. Disruption of the primary interface by mutations abolished fast synchronous release in cultured neurons and greatly reduces the Ca2+-triggered fusion probability in single-vesicle fusion experiments. Based on the structure of the SNARE-Syt complex, an engineered stapled peptide SP9 derived from the SNAP-25A fragment was designed to compete with the SNARE-Syt interaction (Fig. 7b). In a reconstituted fusion system, SP9 substantially reduced the Ca2+-triggered membrane fusion but has only mild effect on Ca2+-independent membrane fusion (Fig. 7c). Moreover, after being delivered into airway epithelial cells via conjugating to a cell penetrating peptide, SP9 strongly suppressed stimulated mucin secretion, but not incurred toxicity because of the mild effect on basal mucin secretion (Fig. 7d). Finally, short term treatment of mice with aerosolized SP9 resulted in substantial peptide uptake into airway epithelial cells, and markedly reduced methacholine-stimulated, Ca2+-triggered mucin secretion and airway mucus occlusion, but not baseline mucin secretion (Fig. 7e). Thus, disruption of the SNARE-Syt2 mediated mucus hypersecretion could be a new therapeutic strategy for obstructive lung disease.
Many approved therapeutic peptides have shown a low rate of immunogenicity in clinical trials [379–381], so SP9, as a therapeutic compound, is unlikely to cause any immune response. However, considerable optimization and pharmacokinetic studies are required before moving to clinical trials, including maximizing cellular uptake, improving intracellular stability, and increasing potency. In summary, stimulated membrane fusion processes, such as neurotransmitter release or mucin secretion, can be manipulated pharmacologically by compounds that disrupt the interaction between the fusogenic proteins and Ca2+ sensors. From a broader perspective, it suggests that targeting to fusogenic proteins such as Syt, have therapeutic value in the pathophysiology of common membrane fusion disorder related diseases.
Neurological diseases
Neurodegenerative diseases
In nervous system, almost all the neurodegenerative diseases are associated with dysregulation of synaptic vesicle trafficking. Abnormal expression or dysfunction of fusogenic proteins results in series of diseases in neuronal system. Accumulation of Aβ in neurons is one of the hallmarks for AD patients. Besides, in AD patients and mice, oligomerized Aβ inhibited the SNARE complex formation via competitively binding to STX1A with VAMP2, leading to the defects in neurotransmission and cognitive function [382, 383]. In addition, in AD brain, it was found that expression level of Syt1 was significantly reduced in the regions related to cognitive and memory functions by the experiments of mass spectroscopy and western blot [384, 385]. And one microRNA miR-34c in AD mice negatively regulated Syt1 expression, while miR-34c antagomir markedly increased the brain levels of Syt1, rescuing synaptic and memory deficits [386]. Moreover, in AD mice, deletion of Munc18-1 displayed dysregulation of tau phosphorylation, neurofibrillary tangle accumulation and alterations of the ubiquitination state which are also the hallmarks of AD [387].
Parkinson’s disease (PD) is characterized by the accumulation of misfolded and fibrillary forms of α-synuclein (α-syn) in neurons. α-syn plays a role in the formation of SNARE complex as a chaperon via binding to N-terminal of VAMP2, and regulates vesicle trafficking and synaptic transmission [388]. Whereas pathotype mutations of α-syn from rare inherited PD inhibit vesicle fusion and neurotransmitter release via binding to anionic membrane [389–391].
Moreover, it was reported that modification of VAMP2 with a “non-cleavable” N-terminal ubiquitin substrate could lead to progressive impairment of synaptic transmission at the neuromuscular junction followed by the degeneration of motor nerve terminals (i.e. ALS) [392].
Neuropsychiatric diseases
For schizophrenia disease, expression levels of SNAP-25A and Cpx1 are reduced [393–395], while the interaction between Cpx1 and SNAREs increases in schizophrenia patients [396]. Moreover, it is observed that Ser14 phosphorylated STX1 is decreased in post mortem prefrontal cortex of schizophrenia patients [397], although the function of phosphorylation of STX1 was still unclear. Some studies suggested that phosphorylation of STX1 was required for the SNARE complex formation because it could enhance the binding to SNAP-25 and Munc18-1 [398]. But in other studies, phosphorylation of STX1 inhibited the probability of vesicle exocytosis in neuronal cells, because of its function in regulating the N-terminal interaction with Munc18-1, which promotes vesicle docking at the plasma membrane [399]. Last, one study revealed that phosphorylation of STX1 reduced vesicle exocytosis from slowly releasable pool without effect on the readily releasable pool [400].
In the plasma samples of bipolar disorder (BD) patients, the Syt7 mRNA level was significantly reduced, and in Syt7 knockout mice, mood cycling symptoms of BD was observed [401, 402]. As a result of Syt7 defects, the activity of GluN2B-NMDARs was attenuated by disruption of spontaneous glutamate release, which induced mania-like behavioral abnormalities [403]. After being treated with clinical BD drugs such as olanzapine, which could induce a significant extracellular release of glutamate in mice by inhibiting the activity of the catabolic enzyme D-aspartate oxidase, it could efficiently prevent the behavioral abnormalities of Syt7 knockout mice [404].
Other neurological diseases
In other cases, hypersecretion of neurotransmitter and sensory neurochemicals, such as substance P, calcitonin gene‐related peptide, glutamate and so on, could cause neuropathic by conveying pain sensation from the peripheral to the central nervous system including the spinal cord [405]. Botulinum neurotoxin type A (BoNTA) is known to cleave SNAP‐25, and prevent synaptic vesicle fusion. In a double‐blind placebo‐controlled trials, the efficacy of BoNTA was confirmed for the prevention of headaches in chronic migraine patients [406]. Although BoNTA is a potential drug candidate, its therapeutic use is limited because of its high toxicity. It was designed a small peptide drug candidate DD04107 (Palmitoyl-EEMQRR-NH2) that to substituted BoNTA by destabilizing the SNARE complex [407, 408]. This peptide was also found to interact with Syt1-C2B domain at the primary interface selectively, suggesting that Syt1 is a potential new analgesic target [407]. Similarly, suppressing Syt1 expression by stimulating acupoints of rats with electroacupuncture could attenuate neuropathic pain, indicating Syt1 involves in the development of neuropathic pain [409].
Numerous mutations of SNAREs and Syt1 were found in patients with neurodevelopmental disorders, including developmental and epileptic encephalopathies (DEEs), and it was likely due to the mutants caused abnormality in neurotransmitter release [410–413]. For example, most of the mutations on SNARE proteins occurred at the SNARE motif, which disrupt the SNARE complex formation [410, 412, 413]. In addition, mutations of SNAP-25 (K40E, V48F and D166Y) on the residues in the primary interface, impaired SNARE-Syt1 interaction [413]. Moreover, mutations of STX1 on N-terminal peptide and Habc domains, interfered the interaction with Munc18-1, while mutations of STX1 on the C-terminal transmembrane domain, impaired its location at plasma membrane [410]. Finally, mutations (D304G, D366E, I368T, N371K) of Syt1 occured in Ca2+ binding site and fusion loop, could severely interfere Ca2+-evoked neurotransmitter release [411].
Endocrine system disease
The main cause of type 2 diabetes mellitus (T2DM) is dysfunction of insulin secretion in pancreatic β-cells and insulin resistance in skeletal muscle, liver and fatty tissue [414]. The glucose homeostasis is regulated by insulin, which raises glucose uptake into skeletal and fat cells from blood. In response to insulin, glucose transporter 4 (GLUT4) is translocated to the plasma membrane by fusion with GLUT4 storage vesicles (GSVs), and the failure of this process is an early step in the development of insulin resistance and T2DM [415]. GSV fusion with the plasma membrane is regulated by SNAREs (STX4, SNAP-23 and VAMP2) [416]. The insulin receptor catalyzes phosphorylation of Munc18-3 at Tyr219 and Tyr521 and resulted in Munc18-3 switching the binding from STX4 to a double C2-like domain-containing protein-β (DOC2β) [417, 418]. Inhibition of GSV fusion induced the insulin resistance in muscle cell, which in turn might interfere insulin secretion in pancreatic β-cells [419]. Furthermore, the insulin secretion is also regulated by SNARE and other fusogenic proteins. Based on the resources of secretory granules that are pre-docked or not, insulin secretion exhibits a biphasic pattern [420]. For the secretion of pre-docked granules, it is a transient but rapid process that regulated by SNAREs (STX1A, SNAP-25 and VAMP2), Munc18-1, Munc13-1 and Syt7, and triggered by Ca2+, while for the secretion of non-docked granules, it is a 5 ~ 10 min persisting release process that regulated by SNAREs (STX3, SNAP-25 and VAMP8), and Munc18-2 [421, 422]. In T2DM mice, treating with fibroblast growth factor 21 could promote the expression of SNAREs to elevate insulin secretion, thereby maintaining insulin homeostasis and pancreatic β‐cell function [423]. Therefore, manipulation of GLUT4 translocation and insulin secretion could be considered as a potential treatment of T2DM.
Immune system diseases
Familial hemophagocytic lymphohistiocytosis (FHL) is a genetically heterogeneous disease with defective cytotoxicity caused by dysfunction of cytolytic granules secretion at the immunological synapse. The lytic granules secretion is mediated by STX11, SNAP-23, VAMP7, Munc18-2, and Munc13-4, and mutations of these proteins cause defective immune function [424–428]. In FHL3 patients, various mutations of Munc13-4 were found, such as various truncations of Munc13-4 caused by different splice sites, nonsense mutations, and frameshift. All the mutations of Munc13-4 were found to interfere the priming step of cytolytic granules secretion [428]. In addition, mutations of STX11 and Munc18-2 were associated with FHL4 and FHL5, respectively [424, 429]. It was found that in FHL4 the defect in the interaction between STX11 and Munc18-2 was caused by mutations of STX11 (R4A, L58P), while mutations of Munc18-2 (E132A, P477L) in FHL5, disrupted cytolytic vesicle fusion [430, 431]. Moreover, in FHL4 patients, the nonsense mutations of STX11 (W382X, Q268X) led to the lack of the C-terminal cysteine-rich motif, resulting in its failure of localization on plasma membrane [432]. While in FHL5 patients, lack of Munc18‐2 also resulted in the failure of localization of STX11 to the plasma membrane of cytotoxic T-lymphocytes, suggesting the localization of STX11 onto plasma membrane depends on Munc18‐2 [433].
Histamine, as a key factor in allergic disease, is stored in histamine granules in mast cells and degranulated after allergen-mediated cross-linking of immunoglobulin E on mast cells. Histamine secretion from mast cell is mediated by SNAREs (STX3, SNAP-23, VAMP8), Munc18-2, and Munc13-4 [434–436]. Atopic dermatitis is a chronic inflammatory dermal disease caused by histamine with symptoms including inflammation, itching, and dry skin. 1-Iodohexadecane treatment significantly decreased VAMP8 expression level, therefore reduced histamine release in mast cells and alleviates the symptoms for the mice with atopic dermatitis [437]. In another study, peptides derived from various SNARE motifs, such as a N-terminal-mimicking peptides derived from VAMP8, was investigated for their potential inhibitory effects against the formation of SNARE complex and mast cell degranulation [438]. Thus, inhibiting the mast cell degranulation could be a potential strategy to prevent histamine secretion and to alleviate atopic dermatitis symptom.
Conclusions and perspectives
Intracellular vesicle trafficking builds the communication network inside cells, which is important for maintaining the homeostasis of intracellular organelles. Although the function and mechanism of vesicle trafficking-related protein has been intensively investigated, the questions that how these supramolecule of the protein complex works together remain to be answered. For example, how specific phosphoinositide levels are controlled on CCPs during vesicle formation? Second, how do Syt1, Cpx1 and SNAREs cooperate to inhibit vesicle fusion in the absence of Ca2+? Third, how do Munc13, Munc18 and SNAREs cooperate to ensure the proper SNARE complex formation? Last but not least, what is the arrangement of supramolecule of the fusogenic proteins complex around the fusion pore in response to Ca2+? Understanding how these key proteins correctly coordinate during vesicle trafficking can help us to interpret how pathogenic factors disrupt the precisely regulated pathway. For example, in airway system, different Munc18 isoforms mediate baseline and stimulated airway mucin secretion, but the molecular mechanism underneath is still unclear. Further research into the pathology and mechanism of how Munc18 and Syt2 cooperate to induce stimulated mucin secretion, would aid in the development of new therapeutic approaches to selectively manipulate stimulated mucin secretion for obstructive pulmonary disease.
Dysfunctions of membrane fusion are closely related to the occurrence of various diseases, although the underlying molecular mechanism are not well understood. Current data suggests that the fusogenic proteins could serve as a starting-point of therapeutic target for vesicle fusion disorder related diseases, and manipulating vesicle fusion could be an entirely new therapeutic strategy for drugs development. Up to now, several potential drugs show great efficacy to treat corresponding diseases in experiment model, such as SP9, and small VAMP8 peptide, and DD04107 that could inhibit neurotransmitter release by destabilizing the SNARE complex, is currently active in phase II clinical trials for neuropathic pain. Considering the mechanism of SNARE mediated vesicle fusion is conserved and universal in different organelles and tissues, inhibition or manipulation of membrane fusion processes would have wide-ranging applications for the therapy of membrane fusion disorder related diseases, including obstructive lung disease, viral-host membrane entry and virus exocytosis. However, it is worth noting that due to the highly conservative mechanism, special attention should be paid to whether the related drugs may have extensive toxicity in multiple organs and tissues. In the follow-up drug research, more efforts might be needed to improve the organ selectivity and targeting of drugs.
Acknowledgements
We thank Axel T. Brunger and Jiajie Diao for critical reading of this review.
Abbreviations
- AAA
ATPases associated with diverse cellular activities
- AD
Alzheimer's disease
- ALS
Amyotrophic lateral sclerosis
- AP1
Adaptor protein 1
- AP2
Adaptor protein 2
- Arf1
ADP-ribosylation factor 1
- BARS
BrefeldinA-ADP-ribosylated substrate
- BD
Bipolar disorder
- BET1
Blocked early in transport 1
- BNIP1
B-cell lymphoma-2 interacting protein 1
- BoNTA
Botulinum neurotoxin type A
- CAPS
Caspase
- CC
Coiled-coil
- CCP
Clathrin-coated pit
- CCV
Clathrin-coated vesicle
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CGN
Cis-Golgi network
- COG
Conserved oligomeric Golgi
- Co-IP
Co-immunoprecipitation
- COPII
Coat protein complex II
- COPI
Coat protein complex I
- CORVET
Class C core vacuole/endosome tethering
- cryo-EM
Cryo-electron microscopy
- Dab2
Disabled 2
- DEEs
Developmental and epileptic encephalopathies
- DOC2β
Double C2-like domain-containing protein-β
- EEA1
Early endosomal antigen 1
- ENTH
Epsin N-terminal homology
- EPG5
Ectopic P granules protein 5 homolog
- eps15R
Eps15 related
- ER
Endoplasmic reticulum
- ERES
ER exit site
- ERGIC
ER-Golgi intermediate compartment
- FBP17
Formin-binding protein 17
- FCHO1/2
Fer/CIP4 homology domain only protein 1/2
- FHL
Familial hemophagocytic lymphohistiocytosis
- FYCO1
FYVE and coiled-coil domain-containing protein 1
- FYVE
Fab 1, YOTB, Vac 1, and EEA1
- GAP
GTPase-activating protein
- GARP
Golgi-associated retrograde protein
- GBF1
Guanine nucleotide exchange factor for Arf1
- GEF
Guanine exchange factor
- GGA
Golgi-localized, gamma-adaptin ear homology, Arf-binding protein
- GS27
Golgi SNAREs of 27 kDa
- GS28
Golgi SNAREs of 28 kDa
- GSVs
GLUT4 storage vesicles
- HDS1
Homology downstream of Sec7d-1
- HOPS
Homotypic fusion and protein sorting
- IP3
Inositol triphosphate
- KIF1C
Kinesin family member 1C
- LC3B
Microtubule-associated proteins 1A/1B light chain 3B
- PD
Parkinson’s disease
- PH
Pleckstrin homology
- PI(4,5)P2
Phosphatidylinositol-4,5-bisphosphate
- PI3P
Phosphatidylinositol-3-phosphate
- PI4P
Phosphatidylinositol-4-monophosphate
- PLD2
Produced by phospholipase D2
- PLEKHM1
Pleckstrin homology domain-containing family member 1
- PRD
Proline-rich domain
- PTM
Post-translational modifications
- Q-SNARE
Glutamine-containing SNAREs
- RIM
Rab-interacting molecule
- R-SNARE
Arginine-containing SNAREs
- SM
Sec1/Munc18
- SNAP
Soluble NSF attachment protein
- SNAP-25A
Synaptosomal-associated protein 25 kDa A
- STX5
Syntaxin-5
- T2DM
Type 2 diabetes mellitus
- TGN
Trans-Golgi network
- TRAPPI
Transport protein particle I
- t-SNARE
Target membrane-SNARE
- USE1
Unconventional SNARE in the ER1
- VAMP2
Vesicle associated membrane protein 2
- Vps45
Vacuolar protein sorting homolog
- v-SNARE
Vesicle-SNARE
- VTI1A
Vesicle transport through interaction with t-SNAREs homolog 1A
- VTI1B
Vesicle transport through interaction with t-SNAREs 1B
- ZF
Zinc finger
- α-syn
α-Synuclein
- μHD
μ Homology domain
Authors’ contributions
Lele Cui, Hao Li, Yufeng Xi, Qianli Hu, Jiaqi Fan and Ying Lai jointly contributed to the first draft of the article and the figures. Qianli Hu, Huimin Liu, Jiaqi Fan, Yijuan Xiang, Xing Zhang, and Weiwei Shui revised the manuscript. Ying Lai conceived the presented idea, revised the manuscript again and approved the final version. All authors approved this manuscript for publication.
Funding
This work was supported by the National Natural Science Foundation of China (31900688, 32170686 and 00402054A1337) and the Sichuan University (20822041D4058).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors concur with this publication.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Lele Cui, Hao Li, Yufeng Xi and Qianli Hu contributed equally to this work.
References
- 1.Cohen S, Valm AM, Lippincott-Schwartz J. Interacting organelles. Curr Opin Cell Biol. 2018;53:84–91. doi: 10.1016/j.ceb.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/S0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/NRM2002. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Béthune J, Wieland FT. Assembly of COPI and COPII vesicular coat proteins on membranes. Annu Rev Biophys. 2018;47:63–83. doi: 10.1146/annurev-biophys-070317-033259. [DOI] [PubMed] [Google Scholar]
- 6.Langemeyer L, Fröhlich F, Ungermann C. Rab GTPase function in endosome and lysosome biogenesis. Trends Cell Biol. 2018;28:957–970. doi: 10.1016/j.tcb.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 7.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DG, Pimpl P. Clathrin and post-Golgi trafficking: a very complicated issue. Trends Plant Sci. 2014;19:134–139. doi: 10.1016/j.tplants.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Sun Z, Brodsky JL. Protein quality control in the secretory pathway. J Cell Biol. 2019;218:3171–3187. doi: 10.1083/jcb.201906047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Li L, Hong W. SNARE proteins in membrane trafficking. Traffic. 2017;18:767–775. doi: 10.1111/tra.12524. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Hughson FM. Chaperoning SNARE folding and assembly. Annu Rev Biochem. 2021;90:581–603. doi: 10.1146/annurev-biochem-081820-103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27:2918–2928. doi: 10.1038/EMBOJ.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanetti G, Prinz S, Daum S, Meister A, Schekman R, Bacia K, et al. The structure of the COPII transport-vesicle coat assembled on membranes. Elife. 2013;2:e00951. doi: 10.7554/ELIFE.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C, Goldberg J. Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats. Cell. 2010;142:123–132. doi: 10.1016/j.cell.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodonova SO, Aderhold P, Kopp J, Ganeva I, Röhling S, Hagen WJH, et al. 9Å structure of the COPI coat reveals that the Arf1 GTPase occupies two contrasting molecular environments. Elife. 2017;6:e26691. doi: 10.7554/ELIFE.26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson PJ, Frigerio G, Collins BM, Duden R, Owen DJ. γ-COP appendage domain – structure and function. Traffic. 2004;5:79–88. doi: 10.1111/J.1600-0854.2004.00158.X. [DOI] [PubMed] [Google Scholar]
- 17.Yu W, Lin J, Jin C, Xia B. Solution structure of human ζ-COP: direct evidences for structural similarity between COP I and clathrin-adaptor coats. J Mol Biol. 2009;386:903–912. doi: 10.1016/J.JMB.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 18.Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432:573–579. doi: 10.1038/nature03079. [DOI] [PubMed] [Google Scholar]
- 19.Drin G, Antonny B. Helices sculpt membrane. Nature. 2005;437:1247–1248. doi: 10.1038/4371247a. [DOI] [PubMed] [Google Scholar]
- 20.Weissman JT, Plutner H, Balch WE. The mammalian guanine nucleotide exchange factor mSec12 is essential for activation of the Sar1 GTPase directing endoplasmic reticulum export. Traffic. 2001;2:465–475. doi: 10.1034/J.1600-0854.2001.20704.X. [DOI] [PubMed] [Google Scholar]
- 21.Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, et al. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/S0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez M, Dejgaard K, Füllekrug J, Dahan S, Fazel A, Paccaud JP, et al. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/JCB.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schimmöller F, Singer-Krüger B, Schröder S, Krüger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser C. Thinking about p24 proteins and how transport vesicles select their cargo. Proc Natl Acad Sci U S A. 2000;97:3783–3785. doi: 10.1073/pnas.97.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney GE, Bowen NJ. p24 proteins, intracellular trafficking, and behavior: drosophila melanogaster provides insights and opportunities. Biol Cell. 2004;96:271–278. doi: 10.1016/j.biolcel.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Hutchings J, Stancheva VG, Brown NR, Cheung ACM, Miller EA, Zanetti G. Structure of the complete, membrane-assembled COPII coat reveals a complex interaction network. Nat Commun. 2021;12:1–13. doi: 10.1038/s41467-021-22110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fath S, Mancias JD, Bi X, Goldberg J. Structure and organization of coat proteins in the COPII cage. Cell. 2007;129:1325–1336. doi: 10.1016/J.CELL.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 28.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 29.Stagg SM, Gürkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, et al. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 30.Hutchings J, Stancheva V, Miller EA, Zanetti G. Subtomogram averaging of COPII assemblies reveals how coat organization dictates membrane shape. Nat Commun. 2018;9:1–8. doi: 10.1038/s41467-018-06577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, et al. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 32.Stagg SM, LaPointe P, Razvi A, Gürkan C, Potter CS, Carragher B, et al. Structural basis for cargo regulation of COPII coat assembly. Cell. 2008;134:474–484. doi: 10.1016/j.cell.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23•Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635. doi: 10.1016/J.DEVCEL.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24–Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 35.Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/J.CELL.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Raote I, Saxena S, Campelo F, Malhotra V. TANGO1 marshals the early secretory pathway for cargo export. BBA-Biomenbranes. 2021;1863:183700. doi: 10.1016/J.BBAMEM.2021.183700. [DOI] [PubMed] [Google Scholar]
- 37.Saito K, Yamashiro K, Shimazu N, Tanabe T, Kontani K, Katada T. Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export. J Cell Biol. 2014;206:751–762. doi: 10.1083/JCB.201312062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dodonova SO, Diestelkoetter-Bachert P, Von Appen A, Hagen WJH, Beck R, Beck M, et al. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 2015;349:195–198. doi: 10.1126/SCIENCE.AAB1121. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Navarro N, Miller E. Protein sorting at the ER-Golgi interface. J Cell Biol. 2016;215:769–778. doi: 10.1083/jcb.201610031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters MG, Serafini T, Rothman JE. “Coatomer”: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- 41.Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–3917. doi: 10.1093/EMBOJ/19.15.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowe M, Kreis TE. In Vitro assembly and disassembly of coatomer. J Biol Chem. 1995;270:31364–31371. doi: 10.1074/JBC.270.52.31364. [DOI] [PubMed] [Google Scholar]
- 43.Jackson LP, Lewis M, Kent HM, Edeling MA, Evans PR, Duden R, et al. Molecular Basis for Recognition of Dilysine Trafficking Motifs by COPI. Dev Cell. 2012;23:1255–1262. doi: 10.1016/J.DEVCEL.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma W, Goldberg J. Rules for the recognition of dilysine retrieval motifs by coatomer. EMBO J. 2013;32:926–937. doi: 10.1038/EMBOJ.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X, Breitman M, Goldberg J. A structure-based mechanism for Arf1-dependent recruitment of coatomer to membranes. Cell. 2012;148:530–542. doi: 10.1016/J.CELL.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quilty D, Chan CJ, Yurkiw K, Bain A, Babolmorad G, Melançon P. The Arf-GDP-regulated recruitment of GBF1 to Golgi membranes requires domains HDS1 and HDS2 and a Golgi-localized protein receptor. J. Cell Sci. 2019;132:jcs208199. doi: 10.1242/JCS.208199/258340/AM/THE-ARF-GDP-REGULATED-RECRUITMENT-OF-GBF1-TO-GOLGI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng Y, Golinelli-Cohen MP, Smirnova E, Jackson CL. A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor. EMBO Rep. 2009;10:58–64. doi: 10.1038/EMBOR.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zerangue N, Schwappach B, Yuh NJ, Lily YJ. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/S0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 49.Welgert R, Silletta MG, Spanò S, Turacchie G, Cericola C, Colanzi A, et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 50.Yang J-S, Gad H, Lee SY, Mironov A, Zhang L, Beznoussenko GV, et al. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat Cell Biol. 2008;10:1146–1153. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- 52.Ter Haar E, Musacchio A, Harrison SC, Kirchhausen T. Atomic structure of clathrin: A β propeller terminal domain joins an α zigzag linker. Cell. 1998;95:563–573. doi: 10.1016/S0092-8674(00)81623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungewickell E, Branton D. Assembly units of clathrin coats. Nature. 1981;289:420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- 54.Pearse BMF. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976;73:1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milosevic I. Endocytic machinery at the neuronal synapse. In: Mochida S, editor. Presynaptic Terminals. Tokyo: Springer Japan; 2015. pp. 223–256. [Google Scholar]
- 56.Wood KM, Smith CJ. Clathrin: the molecular shape shifter. Biochem J. 2021;478:3099–3123. doi: 10.1042/BCJ20200740. [DOI] [PubMed] [Google Scholar]
- 57.Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, et al. FCHo proteins are nucleators of Clathrin-Mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/SCIENCE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly BT, McCoy AJ, Späte K, Miller SE, Evans PR, Höning S, et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kozik P, Francis RW, Seaman MNJ, Robinson MS. A Screen for Endocytic Motifs Traffic. 2010;11:843–855. doi: 10.1111/J.1600-0854.2010.01056.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem. 2011;286:2022–2030. doi: 10.1074/JBC.M110.197178/ATTACHMENT/8F6CAB17-4C39-4D10-9E9A-C207857C150F/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, et al. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 γ–σ1 and AP-3 δ–σ3 hemicomplexes. J Cell Biol. 2003;163:1281–1290. doi: 10.1083/JCB.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLOS Biol. 2011;9:e1000604. doi: 10.1371/JOURNAL.PBIO.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umasankar PK, Sanker S, Thieman JR, Chakraborty S, Wendland B, Tsang M, et al. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning. Nat Cell Biol. 2012;14:488–501. doi: 10.1038/ncb2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L, Umasankar PK, Wrobel AG, Lymar A, McCoy AJ, Holkar SS, et al. Transient Fcho1/2⋅Eps15/R⋅AP-2 nanoclusters prime the AP-2 clathrin adaptor for cargo binding. Dev Cell. 2016;37:428–443. doi: 10.1016/J.DEVCEL.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly BT, Graham SC, Liska N, Dannhauser PN, Höning S, Ungewickell EJ, et al. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 2014;345:459–463. doi: 10.1126/SCIENCE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ford MGJ, Mills IG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 67.Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 68.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 69.Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol. 2011;22:18–26. doi: 10.1016/j.semcdb.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gillon AD, Latham CF, Miller EA. Vesicle-mediated ER export of proteins and lipids. Biochim Biophys Acta. 2012;1821:1040–1049. doi: 10.1016/j.bbalip.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundmark R, Carlsson SR. SNX9 – a prelude to vesicle release. J Cell Sci. 2009;122:5–11. doi: 10.1242/JCS.037135. [DOI] [PubMed] [Google Scholar]
- 72.Håberg K, Lundmark R, Carlsson SR. SNX18 is an SNX9 paralog that acts as a membrane tubulator in AP-1-positive endosomal trafficking. J Cell Sci. 2008;121:1495–1505. doi: 10.1242/jcs.028530. [DOI] [PubMed] [Google Scholar]
- 73.Ungewickell E, Ungewickell H, Holstein SEH, Lindner R, Prasad K, Barouch W, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- 74.Holstein SEH, Ungewickeu H, Ungewickeu E, Ungewickell E, Ungewickell H, Holstein SE, et al. Mechanism of clathrin basket dissociation: separate functions of protein domains of the DnaJ homologue auxilin. J Cell Biol. 1996;135:925–937. doi: 10.1083/JCB.135.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greener T, Zhao X, Nojima H, Eisenberg E, Greene LE. Role of Cyclin G-associated Kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J Biol Chem. 2000;275:1365–1370. doi: 10.1074/JBC.275.2.1365. [DOI] [PubMed] [Google Scholar]
- 76.Barlan K, Gelfand VI. Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harb Perspect Biol. 2017;9:a025817. doi: 10.1101/cshperspect.a025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Titus MA. Myosin-driven intracellular transport. Cold Spring Harb Perspect Biol. 2018;10:a021972. doi: 10.1101/cshperspect.a021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 79.Hong W, Lev S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 2014;24:35–43. doi: 10.1016/j.tcb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 80.Lu L, Hong W. From endosomes to the trans-Golgi network. Semin Cell Dev Biol. 2014;31:30–39. doi: 10.1016/j.semcdb.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 81.Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res. 2014;328:1–19. doi: 10.1016/j.yexcr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 82.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 83.McCaughey J, Stephens DJ. ER-to-Golgi transport: a sizeable problem. Trends Cell Biol. 2019;29:940–953. doi: 10.1016/j.tcb.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2011;14:20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 85.Bentley M, Liang Y, Mullen K, Xu D, Sztul E, Hay JC. SNARE status regulates tether recruitment and function in homotypic COPII vesicle fusion. J Biol Chem. 2006;281:38825–38833. doi: 10.1074/jbc.M606044200. [DOI] [PubMed] [Google Scholar]
- 86.Saraste J, Marie M. Intermediate compartment (IC): from pre-Golgi vacuoles to a semi-autonomous membrane system. Histochem Cell Biol. 2018;150:407–430. doi: 10.1007/s00418-018-1717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 88.Gupta V, Palmer KJ, Spence P, Hudson A, Stephens DJ. Kinesin-1 (uKHC/KIF5B) is required for bidirectional motility of ER exit sites and efficient ER-to-Golgi transport. Traffic. 2008;9:1850–1866. doi: 10.1111/j.1600-0854.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- 89.Watson P, Forster R, Palmer KJ, Pepperkok R, Stephens DJ. Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol. 2005;7:48–55. doi: 10.1038/ncb1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urnavicius L, Zhang K, Diamant AG, Motz C, Schlager MA, Yu M, et al. The structure of the dynactin complex and its interaction with dynein. Science. 2015;347:1441–1446. doi: 10.1126/science.aaa4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo C, Williams JC, Polenova T. Conformational flexibility of p150(Glued)(1–191) subunit of dynactin assembled with microtubules. Biophys J. 2019;117:938–949. doi: 10.1016/j.bpj.2019.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeWitt MA, Cypranowska CA, Cleary FB, Belyy V, Yildiz A. The AAA3 domain of cytoplasmic dynein acts as a switch to facilitate microtubule release. Nat Struct Mol Biol. 2015;22:73–80. doi: 10.1038/nsmb.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–763. doi: 10.1038/nrm2999. [DOI] [PubMed] [Google Scholar]
- 94.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, et al. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 95.Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis–Golgi tethering. Traffic. 2001;2:268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 96.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 97.Witkos TM, Lowe M. Recognition and tethering of transport vesicles at the Golgi apparatus. Curr Opin Cell Biol. 2017;47:16–23. doi: 10.1016/j.ceb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Cheung PP, Limouse C, Mabuchi H, Pfeffer SR. Protein flexibility is required for vesicle tethering at the Golgi. Elife. 2015;4:e12790. doi: 10.7554/eLife.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamaguchi T, Dulubova I, Min S-W, Chen X, Rizo J, Südhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 100.Dascher C, Balch WE. Mammalian Sly1 regulates syntaxin 5 function in endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:15866–15869. doi: 10.1074/jbc.271.27.15866. [DOI] [PubMed] [Google Scholar]
- 101.Demircioglu FE, Burkhardt P, Fasshauer D. The SM protein Sly1 accelerates assembly of the ER-Golgi SNARE complex. Proc Natl Acad Sci U S A. 2014;111:13828–13833. doi: 10.1073/pnas.1408254111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soo KY, Halloran M, Sundaramoorthy V, Parakh S, Toth RP, Southam KA, et al. Rab1-dependent ER-Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS. Acta Neuropathol. 2015;130:679–697. doi: 10.1007/s00401-015-1468-2. [DOI] [PubMed] [Google Scholar]
- 103.Miyazaki K, Wakana Y, Noda C, Arasaki K, Furuno A, Tagaya M. Contribution of the long form of syntaxin 5 to the organization of the endoplasmic reticulum. J Cell Sci. 2012;125:5658–5666. doi: 10.1242/jcs.105304. [DOI] [PubMed] [Google Scholar]
- 104.Fusella A, Micaroni M, Di Giandomenico D, Mironov AA, Beznoussenko GV. Segregation of the Qb-SNAREs GS27 and GS28 into Golgi vesicles regulates intra-Golgi transport. Traffic. 2013;14:568–584. doi: 10.1111/tra.12055. [DOI] [PubMed] [Google Scholar]
- 105.Praschberger R, Lowe SA, Malintan NT, Giachello CNG, Patel N, Houlden H, et al. Mutations in Membrin/GOSR2 reveal stringent secretory pathway demands of dendritic growth and synaptic integrity. Cell Rep. 2017;21:97–109. doi: 10.1016/j.celrep.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Völker JM, Dergai M, Abriata LA, Mingard Y, Ysselstein D, Krainc D, et al. Functional assays for the assessment of the pathogenicity of variants of GOSR2, an ER-to-Golgi SNARE involved in progressive myoclonus epilepsies. Dis Model Mech. 2017;10:1391–1398. doi: 10.1242/dmm.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Donkervoort S, Krause N, Dergai M, Yun P, Koliwer J, Gorokhova S, et al. BET1 variants establish impaired vesicular transport as a cause for muscular dystrophy with epilepsy. EMBO Mol Med. 2021;13:e13787. doi: 10.15252/emmm.202013787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siddiqi S, Mani AM, Siddiqi SA. The identification of the SNARE complex required for the fusion of VLDL-transport vesicle with hepatic cis-Golgi. Biochem J. 2010;429:391–401. doi: 10.1042/BJ20100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reus LM, Pasaniuc B, Posthuma D, Boltz T, Pijnenburg YAL, Ophoff RA. Gene expression imputation across multiple tissue types provides insight into the genetic architecture of frontotemporal dementia and its clinical subtypes. Biol Psychiatry. 2021;89:825–835. doi: 10.1016/j.biopsych.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berchtold NC, Coleman PD, Cribbs DH, Rogers J, Gillen DL, Cotman CW. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and alzheimer’s disease. Neurobiol Aging. 2013;34:1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Y, Tan W, Sheng W, Li X. Identification of Biomarkers associated with alzheimer’s disease by bioinformatics analysis. Am J Alzheimers Dis Other Demen. 2016;31:163–168. doi: 10.1177/1533317515588181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 113.Spang A. Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5:a013391. doi: 10.1101/cshperspect.a013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson LP. Structure and mechanism of COPI vesicle biogenesis. Curr Opin Cell Biol. 2014;29:67–73. doi: 10.1016/j.ceb.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 115.Letourneur F, Gaynor EC, Hennecke S, Démollière C, Duden R, Emr SD, et al. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 116.Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 117.Echard A, Jollivet F, Martinez O, Lacapère JJ, Rousselet A, Janoueix-Lerosey I, et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- 118.Dorner C, Ciossek T, Müller S, Møller PH, Ullrich A, Lammers R. Characterization of KIF1C, a new kinesin-like protein involved in vesicle transport from the Golgi apparatus to the endoplasmic reticulum. J Biol Chem. 1998;273:20267–20275. doi: 10.1074/jbc.273.32.20267. [DOI] [PubMed] [Google Scholar]
- 119.Lee PL, Ohlson MB, Pfeffer SR. Rab6 regulation of the kinesin family KIF1C motor domain contributes to Golgi tethering. Elife. 2015;4:e06029. doi: 10.7554/eLife.06029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- 121.Heffernan LF, Simpson JC. The trials and tubule-ations of Rab6 involvement in Golgi-to-ER retrograde transport. Biochem Soc Trans. 2014;42:1453–1459. doi: 10.1042/BST20140178. [DOI] [PubMed] [Google Scholar]
- 122.Girod A, Storrie B, Simpson JC, Johannes L, Goud B, Roberts LM, et al. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- 123.White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spang A. The DSL1 complex: the smallest but not the least CATCHR. Traffic. 2012;13:908–913. doi: 10.1111/j.1600-0854.2012.01362.x. [DOI] [PubMed] [Google Scholar]
- 125.Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, et al. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139:1119–1129. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andag U, Schmitt HD. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278:51722–51734. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]
- 127.Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. [DOI] [PubMed] [Google Scholar]
- 129.Sohda M, Misumi Y, Yamamoto A, Nakamura N, Ogata S, Sakisaka S, et al. Interaction of Golgin-84 with the COG complex mediates the intra-Golgi retrograde transport. Traffic. 2010;11:1552–1566. doi: 10.1111/j.1600-0854.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 130.Hatsuzawa K, Hirose H, Tani K, Yamamoto A, Scheller RH, Tagaya M. Syntaxin 18, a SNAP receptor that functions in the endoplasmic reticulum, intermediate compartment, and cis-Golgi vesicle trafficking. J Biol Chem. 2000;275:13713–13720. doi: 10.1074/jbc.275.18.13713. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Gallwitz D, Peng R. Structure-based functional analysis reveals a role for the SM protein Sly1p in retrograde transport to the endoplasmic reticulum. Mol Biol Cell. 2005;16:3951–3962. doi: 10.1091/mbc.e05-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iinuma T, Aoki T, Arasaki K, Hirose H, Yamamoto A, Samata R, et al. Role of syntaxin 18 in the organization of endoplasmic reticulum subdomains. J Cell Sci. 2009;122:1680–1690. doi: 10.1242/jcs.036103. [DOI] [PubMed] [Google Scholar]
- 133.Nakajima K, Hirose H, Taniguchi M, Kurashina H, Arasaki K, Nagahama M, et al. Involvement of BNIP1 in apoptosis and endoplasmic reticulum membrane fusion. EMBO J. 2004;23:3216–3226. doi: 10.1038/sj.emboj.7600333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nieradka A, Ufer C, Thiadens K, Grech G, Horos R, van Coevorden-Hameete M, et al. Grsf1-induced translation of the SNARE protein Use1 is required for expansion of the erythroid compartment. PLoS ONE. 2014;9:e104631. doi: 10.1371/journal.pone.0104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uemura T, Sato T, Aoki T, Yamamoto A, Okada T, Hirai R, et al. p31 deficiency influences endoplasmic reticulum tubular morphology and cell survival. Mol Cell Biol. 2009;29:1869–1881. doi: 10.1128/mcb.01089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo Y, Sirkis DW, Schekman R. Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol. 2014;30:169–206. doi: 10.1146/annurev-cellbio-100913-013012. [DOI] [PubMed] [Google Scholar]
- 137.McNiven MA, Thompson HM. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/SCIENCE.1118133/SUPPL_FILE/MCNIVEN.SOM.PDF. [DOI] [PubMed] [Google Scholar]
- 138.Dell’Angelica EC, Bonifacino JS. Coatopathies: genetic disorders of protein coats. Annu Rev Cell Dev Biol. 2019;35:131–168. doi: 10.1146/annurev-cellbio-100818-125234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 140.Jacobs DT, Weigert R, Grode KD, Donaldson JG, Cheney RE. Myosin Vc is a molecular motor that functions in secretory granule trafficking. Mol Biol Cell. 2009;20:4471–4488. doi: 10.1091/mbc.e08-08-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Desnos C, Schonn J-S, Huet S, Tran VS, El-Amraoui A, Raposo G, et al. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J Cell Biol. 2003;163:559–570. doi: 10.1083/jcb.200302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marchelletta RR, Jacobs DT, Schechter JE, Cheney RE, Hamm-Alvarez SF. The class V myosin motor, myosin 5c, localizes to mature secretory vesicles and facilitates exocytosis in lacrimal acini. Am J Physiol Cell Physiol. 2008;295:C13–28. doi: 10.1152/ajpcell.00330.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ueno H, Huang X, Tanaka Y, Hirokawa N. KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev Cell. 2011;20:60–71. doi: 10.1016/j.devcel.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 144.Hong Z, Yang Y, Zhang C, Niu Y, Li K, Zhao X, et al. The retromer component SNX6 interacts with dynactin p150(Glued) and mediates endosome-to-TGN transport. Cell Res. 2009;19:1334–1349. doi: 10.1038/cr.2009.130. [DOI] [PubMed] [Google Scholar]
- 145.Laufman O, Hong WJ, Lev S. The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J Cell Biol. 2011;194:459–472. doi: 10.1083/JCB.201102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hirata T, Fujita M, Nakamura S, Gotoh K, Motooka D, Murakami Y, et al. Post-Golgi anterograde transport requires GARP-dependent endosome-to-TGN retrograde transport. Mol Biol Cell. 2015;26:3071–3084. doi: 10.1091/mbc.E14-11-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]
- 148.Huo L, Yue Y, Ren J, Yu J, Liu J, Yu Y, et al. The CC1-FHA tandem as a central hub for controlling the dimerization and activation of kinesin-3 KIF1A. Structure. 2012;20:1550–1561. doi: 10.1016/j.str.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 149.Ren J, Wang S, Chen H, Wang W, Huo L, Feng W. Coiled-coil 1-mediated fastening of the neck and motor domains for kinesin-3 autoinhibition. Proc Natl Acad Sci U S A. 2018;115:E11933–E11942. doi: 10.1073/pnas.1811209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hummel JJA, Hoogenraad CC. Specific KIF1A-adaptor interactions control selective cargo recognition. J Cell Biol. 2021;220:e202105011. doi: 10.1083/jcb.202105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiba K, Takahashi H, Chen M, Obinata H, Arai S, Hashimoto K, et al. Disease-associated mutations hyperactivate KIF1A motility and anterograde axonal transport of synaptic vesicle precursors. Proc Natl Acad Sci U S A. 2019;116:18429–18434. doi: 10.1073/pnas.1905690116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mei K, Guo W. The exocyst complex. Curr Biol. 2018;28:R922–R925. doi: 10.1016/j.cub.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 153.Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. Embo J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baek K, Knödler A, Lee SH, Zhang X, Orlando K, Zhang J, et al. Structure-function study of the N-terminal domain of exocyst subunit Sec3. J Biol Chem. 2010;285:10424–10433. doi: 10.1074/jbc.M109.096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell. 2007;18:4483–4492. doi: 10.1091/mbc.e07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Jong APH, Roggero CM, Ho M-R, Wong MY, Brautigam CA, Rizo J, et al. RIM C(2)B domains target presynaptic active zone functions to PIP(2)-containing membranes. Neuron. 2018;98:335–349.e7. doi: 10.1016/j.neuron.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Camacho M, Basu J, Trimbuch T, Chang S, Pulido-Lozano C, Chang S-S, et al. Heterodimerization of Munc13 C(2) A domain with RIM regulates synaptic vesicle docking and priming. Nat Commun. 2017;8:15293. doi: 10.1038/ncomms15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/SCIENCE.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Atlashkin V, Kreykenbohm V, Eskelinen EL, Wenzel D, Fayyazi A, Fischer von Mollard G. Deletion of the SNARE vti1b in mice results in the loss of a single SNARE partner, syntaxin 8. Mol Cell Biol. 2003;23:5198–5207. doi: 10.1128/MCB.23.15.5198-5207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miller SE, Collins BM, McCoy AJ, Robinson MS, Owen DJ. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature. 2007;450:570–574. doi: 10.1038/nature06353. [DOI] [PubMed] [Google Scholar]
- 162.Pryor PR, Mullock BM, Bright NA, Lindsay MR, Gray SR, Richardson SC, et al. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5:590–595. doi: 10.1038/sj.embor.7400150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–664. doi: 10.1083/JCB.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tran TH, Zeng Q, Hong W. VAMP4 cycles from the cell surface to the trans-Golgi network via sorting and recycling endosomes. J Cell Sci. 2007;120:1028–1041. doi: 10.1242/jcs.03387. [DOI] [PubMed] [Google Scholar]
- 165.Wang Y, Tai G, Lu L, Johannes L, Hong W, Tang BL. Trans-Golgi network syntaxin 10 functions distinctly from syntaxins 6 and 16. Mol Membr Biol. 2005;22:313–325. doi: 10.1080/09687860500143829. [DOI] [PubMed] [Google Scholar]
- 166.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 167.Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- 168.Wieffer M, Maritzen T, Haucke V. SnapShot: endocytic trafficking. Cell. 2009;137(382):e1–3. doi: 10.1016/j.cell.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 169.Takahashi S, Kubo K, Waguri S, Yabashi A, Shin H-W, Katoh Y, et al. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J Cell Sci. 2012;125:4049–4057. doi: 10.1242/jcs.102913. [DOI] [PubMed] [Google Scholar]
- 170.Hammer JA, 3rd, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol. 2011;13:13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- 171.Xiang X, Qiu R, Yao X, Arst HNJ, Peñalva MA, Zhang J. Cytoplasmic dynein and early endosome transport. Cell Mol Life Sci. 2015;72:3267–3280. doi: 10.1007/s00018-015-1926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 175.Hasson T. Myosin VI: two distinct roles in endocytosis. J Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- 176.Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, et al. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- 177.Schafer DA. Coupling actin dynamics and membrane dynamics during endocytosis. Curr Opin Cell Biol. 2002;14:76–81. doi: 10.1016/s0955-0674(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 178.Rubino M, Miaczynska M, Lippé R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol Chem. 2000;275:3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- 179.Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mishra A, Eathiraj S, Corvera S, Lambright DG. Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of Early Endosomal Autoantigen 1 (EEA1) Proc Natl Acad Sci U S A. 2010;107:10866–10871. doi: 10.1073/pnas.1000843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Solinger JA, Spang A. Tethering complexes in the endocytic pathway: CORVET and HOPS. FEBS J. 2013;280:2743–2757. doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]
- 182.Plemel RL, Lobingier BT, Brett CL, Angers CG, Nickerson DP, Paulsel A, et al. Subunit organization and Rab interactions of Vps-C protein complexes that control endolysosomal membrane traffic. Mol Biol Cell. 2011;22:1353–1363. doi: 10.1091/mbc.E10-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc Natl Acad Sci U S A. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pieren M, Schmidt A, Mayer A. The SM protein Vps33 and the t-SNARE H(abc) domain promote fusion pore opening. Nat Struct Mol Biol. 2010;17:710–717. doi: 10.1038/nsmb.1809. [DOI] [PubMed] [Google Scholar]
- 185.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pankiv S, Alemu EA, Brech A, Bruun J-A, Lamark T, Overvatn A, et al. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pankiv S, Johansen T. FYCO1: linking autophagosomes to microtubule plus end-directing molecular motors. Autophagy. 2010;6:550–552. doi: 10.4161/auto.6.4.11670. [DOI] [PubMed] [Google Scholar]
- 189.Nieto-Torres JL, Shanahan S-L, Chassefeyre R, Chaiamarit T, Zaretski S, Landeras-Bueno S, et al. LC3B phosphorylation regulates FYCO1 binding and directional transport of autophagosomes. Curr Biol. 2021;31:3440–3449.e7. doi: 10.1016/j.cub.2021.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- 191.Wang Z, Miao G, Xue X, Guo X, Yuan C, Wang Z, et al. The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol Cell. 2016;63:781–795. doi: 10.1016/j.molcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 192.Tabata K, Matsunaga K, Sakane A, Sasaki T, Noda T, Yoshimori T. Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol Biol Cell. 2010;21:4162–4172. doi: 10.1091/mbc.E10-06-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McEwan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, et al. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol Cell. 2015;57:39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 194.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y, et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature. 2015;520:563–566. doi: 10.1038/nature14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tian X, Teng J, Chen J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy. 2021;17:2680–2688. doi: 10.1080/15548627.2020.1823124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li Y, Cheng X, Li M, Wang Y, Fu T, Zhou Z, et al. Decoding three distinct states of the Syntaxin17 SNARE motif in mediating autophagosome-lysosome fusion. Proc Natl Acad Sci U S A. 2020;117:21391–21402. doi: 10.1073/pnas.2006997117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shen Q, Shi Y, Liu J, Su H, Huang J, Zhang Y, et al. Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy. 2021;17:1157–1169. doi: 10.1080/15548627.2020.1752471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lobingier BT, Merz AJ. Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Mol Biol Cell. 2012;23:4611–4622. doi: 10.1091/mbc.E12-05-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Saleeb RS, Kavanagh DM, Dun AR, Dalgarno PA, Duncan RR. A VPS33A-binding motif on syntaxin 17 controls autophagy completion in mammalian cells. J Biol Chem. 2019;294:4188–4201. doi: 10.1074/jbc.RA118.005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/S0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 202.Brunger AT, Choi UB, Lai Y, Leitz J, Zhou Q. Molecular mechanisms of fast neurotransmitter release. Annu Rev Biophys. 2018;47:469–497. doi: 10.1146/ANNUREV-BIOPHYS-070816-034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu Y, Zhang F, Su Z, McNew JA, Shin YK. Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol. 2005;12:417–422. doi: 10.1038/NSMB921. [DOI] [PubMed] [Google Scholar]
- 204.Gao Y, Zorman S, Gundersen G, Xi Z, Ma L, Sirinakis G, et al. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science. 2012;337:1340–1343. doi: 10.1126/SCIENCE.1224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 206.Brunger AT, Choi UB, Lai Y, Leitz J, White KI, Zhou Q. The pre-synaptic fusion machinery. Curr Opin Struct Biol. 2019;54:179–188. doi: 10.1016/j.sbi.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brunger AT, Leitz J, Zhou Q, Choi UB, Lai Y. Ca2+-triggered synaptic vesicle fusion initiated by release of inhibition. Trends Cell Biol. 2018;28:631–645. doi: 10.1016/J.TCB.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gao S, Hu J. Mitochondrial fusion: the machineries in and out. Trends Cell Biol. 2021;31:62–74. doi: 10.1016/J.TCB.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 209.Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 210.Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-T. [DOI] [PubMed] [Google Scholar]
- 211.Malhotra V, Orci L, Glick BS, Block MR, Rothman JE. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988;54:221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- 212.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 213.Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, et al. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/JCB.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Trimble WS, Cowan DM, Scheller RH. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988;85:4538–4542. doi: 10.1073/PNAS.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wilson DW, Wilcox CA, Flynn GC, Chen E, Kuang WJ, Henzel WJ, et al. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989;339:355–359. doi: 10.1038/339355A0. [DOI] [PubMed] [Google Scholar]
- 216.Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 217.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 a resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 218.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/EMBOJ.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stein A, Weber G, Wahl MC, Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–528. doi: 10.1038/NATURE08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fasshauer D, Bryan Sutton R, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci U S A. 1998;95:15781–15786. doi: 10.1073/PNAS.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li F, Pincet F, Perez E, Eng WS, Melia TJ, Rothman JE, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14:890–896. doi: 10.1038/NSMB1310. [DOI] [PubMed] [Google Scholar]
- 222.He L, Wu LG. The debate on the kiss-and-run fusion at synapses. Trends Neurosci. 2007;30:447–455. doi: 10.1016/J.TINS.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 223.Bao H, Das D, Courtney NA, Jiang Y, Briguglio JS, Lou X, et al. Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature. 2018;554:260–263. doi: 10.1038/nature25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Aeffner S, Reusch T, Weinhausen B, Salditt T. Energetics of stalk intermediates in membrane fusion are controlled by lipid composition. Proc Natl Acad Sci U S A. 2012;109:E1609–E1618. doi: 10.1073/PNAS.1119442109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Van Den Bogaart G, Holt MG, Bunt G, Riedel D, Wouters FS, Jahn R. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol. 2010;17:358–364. doi: 10.1038/NSMB.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hernandez JM, Stein A, Behrmann E, Riedel D, Cypionka A, Farsi Z, et al. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science. 2012;336:1581–1584. doi: 10.1126/SCIENCE.1221976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, et al. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sinha R, Ahmed S, Jahn R, Klingauf J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc Natl Acad Sci U S A. 2011;108:14318–14323. doi: 10.1073/PNAS.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Czuba LC, Hillgren KM, Swaan PW. Post-translational modifications of transporters. Pharmacol Ther. 2018;192:88–99. doi: 10.1016/j.pharmthera.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Guo B, Liang Q, Li L, Hu Z, Wu F, Zhang P, et al. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat Cell Biol. 2014;16:1215–1226. doi: 10.1038/ncb3066. [DOI] [PubMed] [Google Scholar]
- 231.Chen Q, Hao M, Wang L, Li L, Chen Y, Shao X, et al. Prefused lysosomes cluster on autophagosomes regulated by VAMP8. Cell Death Dis. 2021;12:939. doi: 10.1038/s41419-021-04243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Malmersjö S, Di Palma S, Diao J, Lai Y, Pfuetzner RA, Wang AL, et al. Phosphorylation of residues inside the SNARE complex suppresses secretory vesicle fusion. Embo J. 2016;35:1810–1821. doi: 10.15252/embj.201694071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huang H, Ouyang Q, Zhu M, Yu H, Mei K, Liu R. mTOR-mediated phosphorylation of VAMP8 and SCFD1 regulates autophagosome maturation. Nat Commun. 2021;12:6622. doi: 10.1038/s41467-021-26824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Greaves J, Prescott GR, Gorleku OA, Chamberlain LH. Regulation of SNAP-25 trafficking and function by palmitoylation. Biochem Soc Trans. 2010;38:163–166. doi: 10.1042/BST0380163. [DOI] [PubMed] [Google Scholar]
- 235.Huang S, Tang D, Wang Y. Monoubiquitination of Syntaxin 5 regulates Golgi membrane dynamics during the cell cycle. Dev Cell. 2016;38:73–85. doi: 10.1016/j.devcel.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 237.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 238.Mittelsteadt T, Seifert G, Alvárez-Barón E, Steinhäuser C, Becker AJ, Schoch S. Differential mRNA expression patterns of the synaptotagmin gene family in the rodent brain. J Comp Neurol. 2009;512:514–528. doi: 10.1002/CNE.21908. [DOI] [PubMed] [Google Scholar]
- 239.Lyubimov AY, Uervirojnangkoorn M, Zeldin OB, Zhou Q, Zhao M, Brewster AS, et al. Advances in X-ray free electron laser (XFEL) diffraction data processing applied to the crystal structure of the synaptotagmin-1 / SNARE complex. Elife. 2016;5:e18740. doi: 10.7554/eLife.18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhou Q, Lai Y, Bacaj T, Zhao M, Lyubimov AY, Uervirojnangkoorn M, et al. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature. 2015;525:62–67. doi: 10.1038/NATURE14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhou Q, Zhou P, Wang AL, Wu D, Zhao M, Südhof TC, et al. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 2017;548:420–425. doi: 10.1038/nature23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fernandez I, Araç D, Ubach J, Gerber SH, Shin O, Gao Y, et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 243.Wolfes AC, Dean C. The diversity of synaptotagmin isoforms. Curr Opin Neurobiol. 2020;63:198–209. doi: 10.1016/J.CONB.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 244.Sugita S, Han W, Butz S, Liu X, Fernández-Chacón R, Lao Y, et al. Synaptotagmin VII as a plasma membrane Ca(2+) sensor in exocytosis. Neuron. 2001;30:459–473. doi: 10.1016/S0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 245.Schonn JS, Maximov A, Lao Y, Südhof TC, Sørensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 2008;105:3998–4003. doi: 10.1073/PNAS.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147:666–677. doi: 10.1016/J.CELL.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nyenhuis SB, Karandikar N, Kiessling V, Kreutzberger AJB, Thapa A, Liang B, et al. Conserved arginine residues in synaptotagmin 1 regulate fusion pore expansion through membrane contact. Nat Commun. 2021;12:761. doi: 10.1038/s41467-021-21090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brose N, Petrenko AG, Sudhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/SCIENCE.1589771. [DOI] [PubMed] [Google Scholar]
- 249.Brewer KD, Bacaj T, Cavalli A, Camilloni C, Swarbrick JD, Liu J, et al. Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution. Nat Struct Mol Biol. 2015;22:555–564. doi: 10.1038/NSMB.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pérez-Lara Á, Thapa A, Nyenhuis SB, Nyenhuis DA, Halder P, Tietzel M, et al. PtdInsP 2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. Elife. 2016;5:e15886. doi: 10.7554/ELIFE.15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang S, Li Y, Ma C. Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion. Elife. 2016;5:e14211. doi: 10.7554/ELIFE.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J Biol Chem. 1998;273:13995–14001. doi: 10.1074/JBC.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 253.Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 254.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11:36–44. doi: 10.1038/NSMB709. [DOI] [PubMed] [Google Scholar]
- 255.Kuo W, Herrick DZ, Ellena JF, Cafiso DS. The calcium-dependent and calcium-independent membrane binding of synaptotagmin 1: two modes of C2B binding. J Mol Biol. 2009;387:284–294. doi: 10.1016/J.JMB.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Choi UB, Strop P, Vrljic M, Chu S, Brunger AT, Weninger KR. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat Struct Mol Biol. 2010;17:318–324. doi: 10.1038/NSMB.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vrljic M, Strop P, Hill RC, Hansen KC, Chu S, Brunger AT. Post-translational modifications and lipid binding profile of insect cell-expressed full-length mammalian synaptotagmin 1. Biochemistry. 2011;50:9998–10012. doi: 10.1021/BI200998Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kochubey O, Schneggenburger R. Synaptotagmin increases the dynamic range of synapses by driving Ca2+-evoked release and by clamping a near-linear remaining Ca2+ sensor. Neuron. 2011;69:736–748. doi: 10.1016/J.NEURON.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 259.Davletov BA, Südhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993;268:26386–26390. doi: 10.1016/s0021-9258(19)74326-9. [DOI] [PubMed] [Google Scholar]
- 260.de Wit H, Walter AM, Milosevic I, Gulyás-Kovács A, Riedel D, Sørensen JB, et al. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138:935–946. doi: 10.1016/j.cell.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 261.Kim JY, Choi BK, Choi MG, Kim SA, Lai Y, Shin YK, et al. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 2012;31:2144–2155. doi: 10.1038/EMBOJ.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Das D, Bao H, Courtney KC, Wu L, Chapman ER. Resolving kinetic intermediates during the regulated assembly and disassembly of fusion pores. Nat Commun. 2020;11:231. doi: 10.1038/S41467-019-14072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Tagliatti E, Bello OD, Mendonça PRF, Kotzadimitriou D, Nicholson E, Coleman J, et al. Synaptotagmin 1 oligomers clamp and regulate different modes of neurotransmitter release. Proc Natl Acad Sci U S A. 2020;117:3819–3827. doi: 10.1073/PNAS.1920403117/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ramakrishnan S, Bera M, Coleman J, Krishnakumar SS, Pincet F, Rothman JE. Synaptotagmin oligomers are necessary and can be sufficient to form a Ca 2+ -sensitive fusion clamp. FEBS Lett. 2019;593:154–162. doi: 10.1002/1873-3468.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Xu J, Pang ZP, Shin OH, Südhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/NN.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang J, Li F, Bello OD, Sindelar CV, Pincet F, Krishnakumar SS, et al. Circular oligomerization is an intrinsic property of synaptotagmin. Elife. 2017;6:e27441. doi: 10.7554/eLife.27441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.C Papantoniou U Laugks J Betzin C Capitanio S Schoch N Brose et al 2022 Synaptic vesicle-bound molecular bridges organize sequential vesicle states along parallel pathways BioRxiv10.1101/2022.04.10.487799
- 268.Voleti R, Jaczynska K, Rizo J. Ca(2+)-dependent release of synaptotagmin-1 from the SNARE complex on phosphatidylinositol 4,5-bisphosphate-containing membranes. Elife. 2020;9:e57154. doi: 10.7554/eLife.57154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ubach J, Zhang X, Shao X, Südhof TC, Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998;17:3921–3930. doi: 10.1093/EMBOJ/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. J Biol Chem. 1998;273:1596–1604. doi: 10.1074/JBC.273.3.1596. [DOI] [PubMed] [Google Scholar]
- 271.Grobler JA, Essen LO, Williams RL, Hurley JH. C2 domain conformational changes in phospholipase C-delta 1. Nat Struct Biol. 1996;3:788–795. doi: 10.1038/NSB0996-788. [DOI] [PubMed] [Google Scholar]
- 272.Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 273.Sutton RB, Sprang SR. Structure of the protein kinase Cbeta phospholipid-binding C2 domain complexed with Ca2+ Structure. 1998;6:1395–1405. doi: 10.1016/S0969-2126(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 274.Nishiki TI, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Shin OH, Xu J, Rizo J, Südhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci U S A. 2009;106:16469–16474. doi: 10.1073/PNAS.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Pang ZP, Shin OH, Meyer AC, Rosenmund C, Südhof TC. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, et al. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat Struct Mol Biol. 2013;20:679–686. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Davis AF, Bai J, Fasshauer D, Wolowick MJ, Lewis JL, Chapman ER. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–376. doi: 10.1016/S0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 279.Lai AL, Tamm LK, Ellena JF, Cafiso DS. Synaptotagmin 1 modulates lipid acyl chain order in lipid bilayers by demixing phosphatidylserine. J Biol Chem. 2011;286:25291–25300. doi: 10.1074/JBC.M111.258848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 281.Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wu Z, Dharan N, McDargh ZA, Thiyagarajan S, O’shaughnessy B, Karatekin E. The neuronal calcium sensor Synaptotagmin-1 and SNARE proteins cooperate to dilate fusion pores. Elife. 2021;10:e68215. doi: 10.7554/ELIFE.68215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Park Y, Seo JB, Fraind A, Pérez-Lara A, Yavuz H, Han K, et al. Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat Struct Mol Biol. 2015;22:815–823. doi: 10.1038/nsmb.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chen Y, Wang YH, Zheng Y, Li M, Wang B, Wang QW, et al. Synaptotagmin-1 interacts with PI(4,5)P2 to initiate synaptic vesicle docking in hippocampal neurons. Cell Rep. 2021;34:108842. doi: 10.1016/j.celrep.2021.108842. [DOI] [PubMed] [Google Scholar]
- 285.Grushin K, Wang J, Coleman J, Rothman JE, Sindelar CV, Krishnakumar SS. Structural basis for the clamping and Ca(2+) activation of SNARE-mediated fusion by synaptotagmin. Nat Commun. 2019;10:2413. doi: 10.1038/s41467-019-10391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lin C-C, Seikowski J, Pérez-Lara A, Jahn R, Höbartner C, Walla PJ. Control of membrane gaps by synaptotagmin-Ca2+ measured with a novel membrane distance ruler. Nat Commun. 2014;5:5859. doi: 10.1038/ncomms6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.van den Bogaart G, Thutupalli S, Risselada JH, Meyenberg K, Holt M, Riedel D, et al. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat Struct Mol Biol. 2011;18:805–812. doi: 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Liu H, Bai H, Xue R, Takahashi H, Edwardson JM, Chapman ER. Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission. Nat Neurosci. 2014;17:670–677. doi: 10.1038/NN.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bai H, Xue R, Bao H, Zhang L, Yethiraj A, Cui Q, et al. Different states of synaptotagmin regulate evoked versus spontaneous release. Nat Commun. 2016;7:10971. doi: 10.1038/NCOMMS10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lai Y, Lou X, Jho Y, Yoon TY, Shin YK. The synaptotagmin 1 linker may function as an electrostatic zipper that opens for docking but closes for fusion pore opening. Biochem J. 2013;456:25–33. doi: 10.1042/BJ20130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lee J, Littleton JT. Transmembrane tethering of synaptotagmin to synaptic vesicles controls multiple modes of neurotransmitter release. Proc Natl Acad Sci U S A. 2015;112:3793–3798. doi: 10.1073/PNAS.1420312112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.McMahon HT, Missler M, Li C, Südhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 293.Cho RW, Song Y, Littleton JT. Comparative analysis of Drosophila and mammalian complexins as fusion clamps and facilitators of neurotransmitter release. Mol Cell Neurosci. 2010;45:389–397. doi: 10.1016/J.MCN.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Reim K, Mansour M, Varoqueaux F, McMahon HT, Südhof TC, Brose N, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/S0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 295.Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr Biol. 2011;21:97–105. doi: 10.1016/J.CUB.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yang X, Pei J, Kaeser-Woo YJ, Bacaj T, Grishin NV, Südhof TC. Evolutionary conservation of complexins: from choanoflagellates to mice. EMBO Rep. 2015;16:1308–1317. doi: 10.15252/EMBR.201540305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Xue M, Reim K, Chen X, Chao HT, Deng H, Rizo J, et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat Struct Mol Biol. 2007;14:949–958. doi: 10.1038/NSMB1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lai Y, Choi UB, Zhang Y, Zhao M, Pfuetzner RA, Wang AL, et al. N-terminal domain of complexin independently activates calcium-triggered fusion. Proc Natl Acad Sci U S A. 2016;113:E4698–E4707. doi: 10.1073/PNAS.1604348113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gong J, Lai Y, Li X, Wang M, Leitz J, Hu Y, et al. C-terminal domain of mammalian complexin-1 localizes to highly curved membranes. Proc Natl Acad Sci U S A. 2016;113:E7590–E7599. doi: 10.1073/PNAS.1609917113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Xue M, Craig TK, Xu J, Chao HT, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol. 2010;17:568–575. doi: 10.1038/NSMB.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Radoff DT, Dong Y, Snead D, Bai J, Eliezer D, Dittman JS. The accessory helix of complexin functions by stabilizing central helix secondary structure. Elife. 2014;3:1–17. doi: 10.7554/ELIFE.04553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, et al. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/SCIENCE.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/NN1980. [DOI] [PubMed] [Google Scholar]
- 304.Xue M, Lin YQ, Pan H, Reim K, Deng H, Bellen HJ, et al. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Chen X, Tomchick DR, Kovrigin E, Araç D, Machius M, Südhof TC, et al. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/S0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 306.Wragg RT, Snead D, Dong Y, Ramlall TF, Menon I, Bai J, et al. Synaptic vesicles position complexin to block spontaneous fusion. Neuron. 2013;77:323–334. doi: 10.1016/j.neuron.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Dhara M, Yarzagaray A, Schwarz Y, Dutta S, Grabner C, Moghadam PK, et al. Complexin synchronizes primed vesicle exocytosis and regulates fusion pore dynamics. J Cell Biol. 2014;204:1123–1140. doi: 10.1083/JCB.201311085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Yang X, Cao P, Südhof TC. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proc Natl Acad Sci U S A. 2013;110:20777–20782. doi: 10.1073/PNAS.1321367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kaeser-Woo YJ, Yang X, Südhof TC. C-terminal complexin sequence is selectively required for clamping and priming but not for Ca2+ triggering of synaptic exocytosis. J Neurosci. 2012;32:2877–2885. doi: 10.1523/JNEUROSCI.3360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hobson RJ, Liu Q, Watanabe S, Jorgensen EM. Complexin maintains vesicles in the primed state in C. elegans. Curr Biol. 2011;21:106–113. doi: 10.1016/J.CUB.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cao P, Yang X, Südhof TC. Complexin activates exocytosis of distinct secretory vesicles controlled by different synaptotagmins. J Neurosci. 2013;33:1714–1727. doi: 10.1523/JNEUROSCI.4087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rendón WO, Martínez-Alonso E, Tomás M, Martínez-Martínez N, Martínez-Menárguez JA. Golgi fragmentation is Rab and SNARE dependent in cellular models of Parkinson’s disease. Histochem Cell Biol. 2013;139:671–684. doi: 10.1007/S00418-012-1059-4. [DOI] [PubMed] [Google Scholar]
- 313.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/SCIENCE.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Troy LJ. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J Neurosci. 2012;32:18234–18245. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Trimbuch T, Xu J, Flaherty D, Tomchick DR, Rizo J, Rosenmund C. Re-examining how complexin inhibits neurotransmitter release. Elife. 2014;3:e02391. doi: 10.7554/ELIFE.02391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Brady J, Vasin A, Bykhovskaia M. the accessory helix of complexin stabilizes a partially unzippered state of the SNARE complex and mediates the complexin clamping function in vivo. eNeuro. 2021;8(2):ENEURO.0526-20.2021. doi: 10.1523/ENEURO.0526-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Li F, Pincet F, Perez E, Giraudo CG, Tareste D, Rothman JE. Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state. Nat Struct Mol Biol. 2011;18:941–946. doi: 10.1038/nsmb.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bykhovskaia M, Jagota A, Gonzalez A, Vasin A, Littleton JT. Interaction of the complexin accessory helix with the C-terminus of the SNARE complex: molecular-dynamics model of the fusion clamp. Biophys J. 2013;105:679–690. doi: 10.1016/j.bpj.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kümmel D, Krishnakumar SS, Radoff DT, Li F, Giraudo CG, Pincet F, et al. Complexin cross-links prefusion SNAREs into a zigzag array. Nat Struct Mol Biol. 2011;18:927–933. doi: 10.1038/nsmb.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Cho RW, Kümmel D, Li F, Baguley SW, Coleman J, Rothman JE, et al. Genetic analysis of the Complexin trans-clamping model for cross-linking SNARE complexes in vivo. Proc Natl Acad Sci U S A. 2014;111:10317–10322. doi: 10.1073/pnas.1409311111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Baker RW, Jeffrey PD, Zick M, Phillips BP, Wickner WT, Hughson FM. A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science. 2015;349:1111–1114. doi: 10.1126/science.aac7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Junge HJ, Rhee J-S, Jahn O, Varoqueaux F, Spiess J, Waxham MN, et al. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 323.Lai Y, Choi UB, Leitz J, Rhee HJ, Lee C, Altas B, et al. Molecular mechanisms of synaptic vesicle priming by Munc13 and Munc18. Neuron. 2017;95:591–607.e10. doi: 10.1016/J.NEURON.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 325.Imig C, Min S-W, Krinner S, Arancillo M, Rosenmund C, Südhof TC, et al. The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron. 2014;84:416–431. doi: 10.1016/j.neuron.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 326.Gotoh K, Yokota H, Kikuya E, Watanabe T, Oishi M. Genomic structure of MUNC18-1 protein, which is involved in docking and fusion of synaptic vesicles in brain. J Biol Chem. 1998;273:21642–21647. doi: 10.1074/JBC.273.34.21642. [DOI] [PubMed] [Google Scholar]
- 327.Graham ME, Prescott GR, Johnson JR, Jones M, Walmesley A, Haynes LP, et al. Structure-function study of mammalian Munc18–1 and C. elegans UNC-18 implicates domain 3b in the regulation of exocytosis. PLoS One. 2011;6:e17999. doi: 10.1371/JOURNAL.PONE.0017999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dawidowski D, Cafiso DS. Munc18-1 and the Syntaxin-1 N Terminus regulate open-closed states in a t-SNARE complex. Structure. 2016;24:392–400. doi: 10.1016/J.STR.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Colbert KN, Hattendorf DA, Weiss TM, Burkhardt P, Fasshauer D, Weis WI. Syntaxin1a variants lacking an N-peptide or bearing the LE mutation bind to Munc18a in a closed conformation. Proc Natl Acad Sci U S A. 2013;110:12637–12642. doi: 10.1073/PNAS.1303753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Misura KMS, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 331.Chen X, Lu J, Dulubova I, Rizo J. NMR analysis of the closed conformation of syntaxin-1. J Biomol NMR. 2008;41:43–54. doi: 10.1007/S10858-008-9239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Shen J, Rathore SS, Khandan L, Rothman JE. SNARE bundle and syntaxin N-peptide constitute a minimal complement for Munc18-1 activation of membrane fusion. J Cell Biol. 2010;190:55–63. doi: 10.1083/JCB.201003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wang S, Li Y, Gong J, Ye S, Yang X, Zhang R, et al. Munc18 and Munc13 serve as a functional template to orchestrate neuronal SNARE complex assembly. Nat Commun. 2019;10:69. doi: 10.1038/s41467-018-08028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Grushin K, Kalyana Sundaram RV, Sindelar CV, Rothman JE. Munc13 structural transitions and oligomers that may choreograph successive stages in vesicle priming for neurotransmitter release. Proc Natl Acad Sci U S A. 2022;119:e2121259119. doi: 10.1073/pnas.2121259119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542–549. doi: 10.1038/NSMB.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/NATURE11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang S, Choi UB, Gong J, Yang X, Li Y, Wang AL, et al. Conformational change of syntaxin linker region induced by Munc13s initiates SNARE complex formation in synaptic exocytosis. Embo J. 2017;36:816–829. doi: 10.15252/EMBJ.201695775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Xu J, Camacho M, Xu Y, Esser V, Liu X, Trimbuch T, et al. Mechanistic insights into neurotransmitter release and presynaptic plasticity from the crystal structure of Munc13-1 C1C2BMUN. Elife. 2017;6:e22567. doi: 10.7554/ELIFE.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, et al. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/S0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 340.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, et al. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/SJ.EMBOJ.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kaeser P. Pushing synaptic vesicles over the RIM. Cell Logist. 2011;1:106–110. doi: 10.4161/CL.1.3.16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yang X, Wang S, Sheng Y, Zhang M, Zou W, Wu L, et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat Struct Mol Biol. 2015;22:547–554. doi: 10.1038/NSMB.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wang X, Gong J, Zhu L, Wang S, Yang X, Xu Y, et al. Munc13 activates the Munc18-1/syntaxin-1 complex and enables Munc18-1 to prime SNARE assembly. Embo J. 2020;39:e103631. doi: 10.15252/embj.2019103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Shu T, Jin H, Rothman JE, Zhang Y. Munc13-1 MUN domain and Munc18-1 cooperatively chaperone SNARE assembly through a tetrameric complex. Proc Natl Acad Sci U S A. 2020;117:1036–1041. doi: 10.1073/PNAS.1914361117/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Madison JM, Nurrish S, Kaplan JM. UNC-13 interaction with syntaxin is required for synaptic transmission. Curr Biol. 2005;15:2236–2242. doi: 10.1016/J.CUB.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 346.Stevens DR, Wu ZX, Matti U, Junge HJ, Schirra C, Becherer U, et al. Identification of the minimal protein domain required for priming activity of Munc13-1. Curr Biol. 2005;15:2243–2248. doi: 10.1016/J.CUB.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 347.Liu X, Seven AB, Camacho M, Esser V, Xu J, Trimbuch T, et al. Functional synergy between the Munc13 C-terminal C1 and C2 domains. Elife. 2016;5:e13696. doi: 10.7554/ELIFE.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Michelassi F, Liu H, Hu Z, Dittman JS. A C1–C2 module in Munc13 inhibits calcium-dependent neurotransmitter release. Neuron. 2017;95:577–590.e5. doi: 10.1016/j.neuron.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Palfreyman MT, Jorgensen EM. Unc13 Aligns SNAREs and superprimes synaptic vesicles. Neuron. 2017;95:473–475. doi: 10.1016/j.neuron.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 350.Sitarska E, Xu J, Park S, Liu X, Quade B, Stepien K, et al. Autoinhibition of Munc18-1 modulates synaptobrevin binding and helps to enable Munc13-dependent regulation of membrane fusion. Elife. 2017;6:e24278. doi: 10.7554/eLife.24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.André T, Classen J, Brenner P, Betts MJ, Dörr B, Kreye S, et al. The interaction of Munc18–1 Helix 11 and 12 with the central region of the VAMP2 SNARE motif is essential for SNARE templating and synaptic transmission. eNeuro. 2020;7:ENEURO.0278-20.2020. doi: 10.1523/ENEURO.0278-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shen C, Rathore SS, Yu H, Gulbranson DR, Hua R, Zhang C, et al. The trans-SNARE-regulating function of Munc18-1 is essential to synaptic exocytosis. Nat Commun. 2015;6:8852. doi: 10.1038/ncomms9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339:421–425. doi: 10.1126/SCIENCE.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Coppola T, Perret-Menoud V, Lüthi S, Farnsworth CC, Glomset JA, Regazzi R. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. Embo J. 1999;18:5885–5891. doi: 10.1093/EMBOJ/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Wu X, Cai Q, Shen Z, Chen X, Zeng M, Du S, et al. RIM and RIM-BP form presynaptic active-zone-like condensates via phase separation. Mol Cell. 2019;73:971–984.e5. doi: 10.1016/J.MOLCEL.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 356.Bello OD, Zanetti MN, Mayorga LS, Michaut MA. RIM, Munc13, and Rab3A interplay in acrosomal exocytosis. Exp Cell Res. 2012;318:478–488. doi: 10.1016/J.YEXCR.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.James DJ, Martin TFJ. CAPS and Munc13: CATCHRs that SNARE vesicles. Front. Endocrinol. (Lausanne) 2013;4:187. doi: 10.3389/FENDO.2013.00187/PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, et al. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/NSMB1001. [DOI] [PubMed] [Google Scholar]
- 359.Paddock BE, Striegel AR, Hui E, Chapman ER, Reist NE. Ca2+-dependent, phospholipid-binding residues of synaptotagmin are critical for excitation-secretion coupling in vivo. J Neurosci. 2008;28:7458–7466. doi: 10.1523/JNEUROSCI.0197-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Lee HK, Yang Y, Su Z, Hyeon C, Lee TS, Lee HW, et al. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science. 2010;328:760–763. doi: 10.1126/SCIENCE.1187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lai Y, Diao J, Liu Y, Ishitsuka Y, Su Z, Schulten K, et al. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin. Proc Natl Acad Sci U S A. 2013;110:1333–1338. doi: 10.1073/PNAS.1218818110/-/DCSUPPLEMENTAL/PNAS.201218818SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Choi UB, Zhao M, White KI, Pfuetzner RA, Esquivies L, Zhou Q, et al. NSF-mediated disassembly of on- and off-pathway SNARE complexes and inhibition by complexin. Elife. 2018;7:e36497. doi: 10.7554/ELIFE.36497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Lenzen CU, Steinmann D, Whiteheart SW, Weis WI. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell. 1998;94:525–536. doi: 10.1016/S0092-8674(00)81593-7. [DOI] [PubMed] [Google Scholar]
- 364.Ryu JK, Min D, Rah SH, Kim SJ, Park Y, Kim H, et al. Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover. Science. 2015;347:1485–1489. doi: 10.1126/SCIENCE.AAA5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ungermann C, Nichols BJ, Pelham HRB, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998;140:61–69. doi: 10.1083/JCB.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhao C, Slevin JT, Whiteheart SW. Cellular functions of NSF: not just SNAPs and SNAREs. FEBS Lett. 2007;581:2140–2149. doi: 10.1016/J.FEBSLET.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Zhao M, Wu S, Zhou Q, Vivona S, Cipriano DJ, Cheng Y, et al. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015;518:61–67. doi: 10.1038/nature14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ammendolia DA, Bement WM, Brumell JH. Plasma membrane integrity: implications for health and disease. BMC Biol. 2021;19:71. doi: 10.1186/S12915-021-00972-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Yarwood R, Hellicar J, Woodman PG, Lowe M. Membrane trafficking in health and disease. DMM Dis. Model. Mech. 2020;13:dmm043448. doi: 10.1242/DMM.043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lai Y, Fois G, Flores JR, Tuvim MJ, Zhou Q, Yang K, et al. Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides. Nature. 2022;603:949–956. doi: 10.1038/s41586-022-04543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMRA0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA. 2019;321:745–746. doi: 10.1001/JAMA.2019.0131. [DOI] [PubMed] [Google Scholar]
- 373.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015;7:276ra27. doi: 10.1126/SCITRANSLMED.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Boucher RC. Muco-obstructive lung diseases. N Engl J Med. 2019;380:1941–1953. doi: 10.1056/NEJMRA1813799. [DOI] [PubMed] [Google Scholar]
- 375.Yang C, Montgomery M. Dornase alfa for cystic fibrosis. Cochrane database Syst. Rev. 2021;3:CD001127. doi: 10.1002/14651858.CD001127.PUB5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Woodruff PG, Wolff M, Hohlfeld JM, Krug N, Dransfield MT, Sutherland ER, et al. Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:438–445. doi: 10.1164/RCCM.200909-1415OC. [DOI] [PubMed] [Google Scholar]
- 377.Fahya BJV, Kimb KW, Liub J, Bousheya BHA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–852. doi: 10.1016/S0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 378.Bartoszewski R, Matalon S, Collawn JF. Ion channels of the lung and their role in disease pathogenesis. Am J Physiol Lung Cell Mol Physiol. 2017;313:L859–L872. doi: 10.1152/AJPLUNG.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ali AM, Atmaj J, Van Oosterwijk N, Groves MR, Dömling A. stapled peptides inhibitors: a new window for target drug discovery. Comput Struct Biotechnol J. 2019;17:263–281. doi: 10.1016/J.CSBJ.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lau JL, Dunn MK. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg Med Chem. 2018;26:2700–2707. doi: 10.1016/J.BMC.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 381.Moiola M, Memeo MG, Quadrelli P. Stapled peptides-a useful improvement for peptide-based drugs. Molecules. 2019;24:3654. doi: 10.3390/MOLECULES24203654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, et al. Progressive accumulation of amyloid-β oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yang Y, Kim J, Kim HY, Ryoo N, Lee S, Kim YS, et al. Amyloid-β Oligomers may impair SNARE-mediated exocytosis by direct binding to syntaxin 1a. Cell Rep. 2015;12:1244–1251. doi: 10.1016/j.celrep.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sze CI, Bi H, Kleinschmidt-Demasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. J Neurol Sci. 2000;175:81–90. doi: 10.1016/S0022-510X(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 385.Yoo BC, Cairns N, Fountoulakis M, Lubec G. Synaptosomal proteins, beta-soluble N-ethylmaleimide-sensitive factor attachment protein (Beta-SNAP), gamma-SNAP and synaptotagmin I in brain of patients with Down syndrome and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12:219–225. doi: 10.1159/000051261. [DOI] [PubMed] [Google Scholar]
- 386.Shi Z, Zhang K, Zhou H, Jiang L, Xie B, Wang R, et al. Increased miR-34c mediates synaptic deficits by targeting synaptotagmin 1 through ROS-JNK-p53 pathway in Alzheimer’s disease. Aging Cell. 2020;19:e13125. doi: 10.1111/acel.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Law C, Profes MS, Levesque M, Kaltschmidt JA, Verhage M, Kania A. Normal molecular specification and neurodegenerative disease-like death of spinal neurons lacking the SNARE-associated synaptic protein Munc18-1. J Neurosci. 2016;36:561–576. doi: 10.1523/JNEUROSCI.1964-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/SCIENCE.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lai Y, Kim S, Varkey J, Lou X, Song JK, Diao J, et al. Nonaggregated α-synuclein influences SNARE-dependent vesicle docking via membrane binding. Biochemistry. 2014;53:3889–3896. doi: 10.1021/BI5002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Choi BK, Choi MG, Kim JY, Yang Y, Lai Y, Kweon DH, et al. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci U S A. 2013;110:4087–4092. doi: 10.1073/PNAS.1218424110/-/DCSUPPLEMENTAL/PNAS.201218424SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, et al. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. doi: 10.7554/ELIFE.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Liu Y, Li H, Sugiura Y, Han W, Gallardo G, Khvotchev M, et al. Ubiquitin-synaptobrevin fusion protein causes degeneration of presynaptic motor terminals in Mice. J Neurosci. 2015;35:11514–11531. doi: 10.1523/JNEUROSCI.5288-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Thompson PM, Sower AC, Perrone-Bizzozero NI. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry. 1998;43:239–243. doi: 10.1016/S0006-3223(97)00204-7. [DOI] [PubMed] [Google Scholar]
- 394.Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, et al. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/SJ.MP.4001319. [DOI] [PubMed] [Google Scholar]
- 395.Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V, et al. Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry. 2002;7:484–492. doi: 10.1038/SJ.MP.4000978. [DOI] [PubMed] [Google Scholar]
- 396.Ramos-Miguel A, Beasley CL, Dwork AJ, Mann JJ, Rosoklija G, Barr AM, et al. Increased SNARE protein-protein interactions in orbitofrontal and anterior cingulate cortices in schizophrenia. Biol Psychiatry. 2015;78:361–373. doi: 10.1016/J.BIOPSYCH.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Castillo MA, Ghose S, Tamminga CA, Ulery-Reynolds PG. Deficits in syntaxin 1 phosphorylation in schizophrenia prefrontal cortex. Biol Psychiatry. 2010;67:208–216. doi: 10.1016/J.BIOPSYCH.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 398.Barak-Broner N, Singer-Lahat D, Chikvashvili D, Lotan I. CK2 Phosphorylation is required for regulation of Syntaxin 1A activity in Ca(2+)-triggered release in neuroendocrine cells. Int J Mol Sci. 2021;22:13556. doi: 10.3390/ijms222413556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Rickman C, Duncan RR. Munc18/Syntaxin interaction kinetics control secretory vesicle dynamics. J Biol Chem. 2010;285:3965–3972. doi: 10.1074/jbc.M109.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Shi VH, Craig TJ, Bishop P, Nakamura Y, Rocca D, Wilkinson KA, et al. Phosphorylation of Syntaxin-1a by casein kinase 2α regulates pre-synaptic vesicle exocytosis from the reserve pool. J Neurochem. 2021;156:614–623. doi: 10.1111/jnc.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Shen W, Wang QW, Liu YN, Marchetto MC, Linker S, Lu SY, et al. Synaptotagmin-7 is a key factor for bipolar-like behavioral abnormalities in mice. Proc Natl Acad Sci U S A. 2020;117:4392–4399. doi: 10.1073/PNAS.1918165117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wang QW, Lu SY, Liu YN, Chen Y, Wei H, Shen W, et al. Synaptotagmin-7 deficiency induces mania-like behavioral abnormalities through attenuating GluN2B activity. Proc Natl Acad Sci U S A. 2020;117:31438–31447. doi: 10.1073/pnas.2016416117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wang QW, Wang YH, Wang B, Chen Y, Lu SY, Yao J. Synaptotagmin-7-mediated activation of spontaneous NMDAR currents is disrupted in bipolar disorder susceptibility variants. PLoS Biol. 2021;19:e3001323. doi: 10.1371/JOURNAL.PBIO.3001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Sacchi S, De NV, Paolone G, Nuzzo T, Iannotta M, Belardo C, et al. Olanzapine, but not clozapine, increases glutamate release in the prefrontal cortex of freely moving mice by inhibiting D-aspartate oxidase activity. Sci Rep. 2017;7:46288. doi: 10.1038/srep46288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 406.Dodick DW, Turkel CC, Degryse RE, Diener HC, Lipton RB, Aurora SK, et al. Assessing clinically meaningful treatment effects in controlled trials: Chronic migraine as an example. J Pain. 2015;16:164–175. doi: 10.1016/j.jpain.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 407.Butrón D, Zamora-Carreras H, Devesa I, Treviño MA, Abian O, Velázquez-Campoy A, et al. DD04107-Derived neuronal exocytosis inhibitor peptides: evidences for synaptotagmin-1 as a putative target. Bioorg Chem. 2021;115:105231. doi: 10.1016/J.BIOORG.2021.105231. [DOI] [PubMed] [Google Scholar]
- 408.Blanes-Mira C, Merino JM, Valera E, Fernández-Ballester G, Gutiérrez LM, Viniegra S, et al. Small peptides patterned after the N-terminus domain of SNAP25 inhibit SNARE complex assembly and regulated exocytosis. J Neurochem. 2004;88:124–135. doi: 10.1046/j.1471-4159.2003.02133.x. [DOI] [PubMed] [Google Scholar]
- 409.Wan J, Nan S, Liu J, Ding M, Zhu H, Suo C, et al. Synaptotagmin 1 is involved in neuropathic pain and electroacupuncture-mediated analgesic effect. Int J Mol Sci. 2020;21:968. doi: 10.3390/IJMS21030968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wolking S, May P, Mei D, Møller RS, Balestrini S, Helbig KL, et al. Clinical spectrum of STX1B-related epileptic disorders. Neurology. 2019;92:E1238–E1249. doi: 10.1212/WNL.0000000000007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Baker K, Gordon SL, Melland H, Bumbak F, Scott DJ, Jiang TJ, et al. SYT1-associated neurodevelopmental disorder: a case series. Brain. 2018;141:2576–2591. doi: 10.1093/BRAIN/AWY209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Salpietro V, Malintan NT, Llano-Rivas I, Spaeth CG, Efthymiou S, Striano P, et al. Mutations in the neuronal vesicular SNARE VAMP2 affect synaptic membrane fusion and impair human neurodevelopment. Am J Hum Genet. 2019;104:721–730. doi: 10.1016/J.AJHG.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Alten B, Zhou Q, Shin OH, Esquivies L, Lin PY, White KI, et al. Role of aberrant spontaneous neurotransmission in SNAP25-associated encephalopathies. Neuron. 2021;109:59–72.e5. doi: 10.1016/J.NEURON.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Prim. 2015;1:1–22. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 415.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799A. [DOI] [PubMed] [Google Scholar]
- 416.Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci U S A. 1996;93:15169–15173. doi: 10.1073/PNAS.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Jewell JL, Oh E, Bennett SM, Meroueh SO, Thurmond DC. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J Biol Chem. 2008;283:21734–21746. doi: 10.1074/JBC.M710445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Jewell JL, Oh E, Ramalingam L, Kalwat MA, Tagliabracci VS, Tackett L, et al. Munc18c phosphorylation by the insulin receptor links cell signaling directly to SNARE exocytosis. J Cell Biol. 2011;193:185–199. doi: 10.1083/JCB.201007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Defronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773. doi: 10.2337/DB09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Aoyagi K, Ohara-Imaizumi M, Nagamatsu S. Regulation of resident and newcomer insulin granules by calcium and SNARE proteins. Front Biosci. 2011;16:1197–1210. doi: 10.2741/3784. [DOI] [PubMed] [Google Scholar]
- 421.Gaisano HY. Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis. Diabetes, Obes Metab. 2017;19:115–123. doi: 10.1111/DOM.13001. [DOI] [PubMed] [Google Scholar]
- 422.Thurmond DC, Gaisano HY. Recent insights into beta-cell exocytosis in type 2 diabetes. J Mol Biol. 2020;432:1310–1325. doi: 10.1016/j.jmb.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Pan Y, Wang B, Zheng J, Xiong R, Fan Z, Ye Y, et al. Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner. J Cell Mol Med. 2019;23:1059–1071. doi: 10.1111/jcmm.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.D’Orlando O, Zhao F, Kasper B, Orinska Z, Müller J, Hermans-Borgmeyer I, et al. Syntaxin 11 is required for NK and CD8+ T-cell cytotoxicity and neutrophil degranulation. Eur J Immunol. 2013;43:194–208. doi: 10.1002/EJI.201142343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Hellewell AL, Foresti O, Gover N, Porter MY, Hewitt EW. Analysis of familial hemophagocytic lymphohistiocytosis type 4 (FHL-4) mutant proteins reveals that S-acylation is required for the function of syntaxin 11 in natural killer cells. PLoS ONE. 2014;9:e98900. doi: 10.1371/JOURNAL.PONE.0098900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Krzewski K, Gil-Krzewska A, Watts J, Stern JNH, Strominger JL. VAMP4- and VAMP7-expressing vesicles are both required for cytotoxic granule exocytosis in NK cells. Eur J Immunol. 2011;41:3323–3329. doi: 10.1002/EJI.201141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Pagel J, Beutel K, Lehmberg K, Koch F, Maul-Pavicic A, Rohlfs AK, et al. Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5) Blood. 2012;119:6016–6024. doi: 10.1182/BLOOD-2011-12-398958. [DOI] [PubMed] [Google Scholar]
- 428.Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/S0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 429.Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Müller M-L, Chiang SCC, Meeths M, Tesi B, Entesarian M, Nilsson D, et al. An N-Terminal Missense Mutation in STX11 Causative of FHL4 Abrogates Syntaxin-11 Binding to Munc18-2. Front Immunol. 2014;4:515. doi: 10.3389/fimmu.2013.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Hackmann Y, Graham SC, Ehl S, Höning S, Lehmberg K, Aricò M, et al. Syntaxin binding mechanism and disease-causing mutations in Munc18-2. Proc Natl Acad Sci U S A. 2013;110:E4482–E4491. doi: 10.1073/pnas.1313474110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Dieckmann NMG, Hackmann Y, Aricò M, Griffiths GM. Munc18-2 is required for Syntaxin 11 localization on the plasma membrane in cytotoxic T-lymphocytes. Traffic. 2015;16:1330–1341. doi: 10.1111/tra.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Sanchez E, Gonzalez EA, Moreno DS, Cardenas RA, Ramos MA, Davalos AJ, et al. Syntaxin 3, but not syntaxin 4, is required for mast cell–regulated exocytosis, where it plays a primary role mediating compound exocytosis. J Biol Chem. 2019;294:3012. doi: 10.1074/JBC.RA118.005532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Brochetta C, Suzuki R, Vita F, Soranzo MR, Claver J, Madjene LC, et al. Munc18-2 and syntaxin 3 control distinct essential steps in mast cell degranulation. J Immunol. 2014;192:41–51. doi: 10.4049/JIMMUNOL.1301277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Rodarte EM, Ramos MA, Davalos AJ, Moreira DC, Moreno DS, Cardenas EI, et al. Munc13 proteins control regulated exocytosis in mast cells. J Biol Chem. 2018;293:345–358. doi: 10.1074/JBC.M117.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Kim DY, Won K-J, Hwang DIl, Kim NY, Kim B, Lee HM. 1-Iodohexadecane Alleviates 2,4-Dinitrochlorobenzene-induced atopic dermatitis in Mice: possible involvements of the skin barrier and mast cell SNARE proteins. Molecules. 2022;27:1560. doi: 10.3390/MOLECULES27051560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Yang Y, Kong B, Jung Y, Park JB, Oh JM, Hwang J, et al. Soluble N-ethylmaleimide-sensitive factor attachment protein receptor-derived peptides for regulation of mast cell degranulation. Front Immunol. 2018;9:725. doi: 10.3389/FIMMU.2018.00725/PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.