Abstract
To assess the impact of topical agents and dressings on surface wound pH, temperature, and subsequent wound healing. This was a systematic, narrative review of the literature, following the PRISMA (2020) guidelines. The databases searched were Medline PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Library, Embase, Web of Science, and Scopus. Data synthesis and analysis were conducted using a structured narrative synthesis. The quality of the included clinical studies was appraised using the Evidence‐Based Literature (EBL) Critical Appraisal Tool. A total of six clinical studies were assessed as eligible for inclusion, A total of six dressings/topical agents were assessed and the types of wounds included non‐healing chronic wounds. Of the studies, five explored pH and one explored temperature. The EBL validity of the clinical studies was low (mean quality score was 51.3%). The five clinical studies that explored pH investigated different dressings and topical agents reporting an associated reduction in pH and improved wound outcomes. One clinical study investigated the impact of topical sodium nitrite on temperature and found that sodium nitrite increased peri‐wound skin temperature and improved wound outcomes with a reduction in leg ulcer size. Given the low certainty of the evidence, we cannot confidently recommend the use of any particular topical agent or dressing to manipulate pH, or temperature to improve wound outcomes. Thus, there is a need for further research to develop a greater understanding of this topic. Irish Research Council, Enterprise Partnership Scheme.
Keywords: dressings, pH, temperature, topical agents, wound healing
1. INTRODUCTION
Wound healing is a complex process that is influenced by intrinsic and extrinsic factors within the patient and the wound itself. There is evidence to confirm that pH and temperature play an important role in wound healing. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Topical agents and wound dressings form an important part of wound management and their therapeutic availability has increased tenfold during the last decade. 5 , 8 , 9 , 12 However, it is unclear from the literature if topical agents and dressings have any effect on wound pH or wound temperature.
For wounds not progressing or stalled, it is important to achieve a balance between eradicating bacteria and protecting the host cell. 13 , 14 Bacteria produce ammonia, which is liberated from urea by the enzyme ureases; this in turn, results in an alkaline wound environment. The literature reviewed indicated that most bacteria are inhibited in a lower pH environment. 15 , 16 , 17 When an infection is suspected based on a rise in the wound pH, lowering the pH of the wound may be of benefit as it may inhibit the proliferation of the causative organism. 8 , 18 , 19 , 20 , 21 However, it would be incorrect to imply that lowering the pH would eliminate all bacteria as some bacteria can survive in a wider pH range. 5 Further, bacteria in biofilm communities are also able to survive in a wider pH range that would normally be inhibitory to their growth under planktonic conditions. 12 , 22 , 23 , 24 However, a causal relationship between the degree of bacterial contamination and pH value has not yet been established. 10 , 22 The literature has suggested that certain wound dressings and topical agents may alter the wound’s pH and ultimately influence healing, thereby providing a more cost‐effective approach to wound management. 4 , 7 , 16 , 22 , 25 , 26 , 27 , 28 , 29 , 30 These have included, topical polyhexamethylene biguanide (PHMB) and propyl betaine, acetic acid, manuka honey, and silver dressings.
Temperature has an important role to play in the functioning of every system in the body, with all cellular functions affected by it. 9 , 31 , 32 This is also the case in wound healing. 6 , 33 , 34 , 35 At the physiological level, during the inflammatory phase increased temperature in acute wounds increases local dermal blood flow and subcutaneous oxygen tension, resulting in an environment conducive to wound healing. 34 , 36 , 37 Conversely, an increased local temperature of chronic wounds is an indicative sign of wound infection and inflammation and results in delayed healing. 6 , 9 , 26 , 34 , 37 , 38 On the other hand, within acute surgical wounds, healing can also be delayed when the temperature of the wound bed falls below the core body temperature. Previous research conducted by reported that cooler temperatures at the wound site were indicative of wound infection. 39
pH influences biochemical activity in each stage of wound healing and temperature affects all the body’s cellular functions. 6 , 15 , 22 , 31 , 36 , 40 It is postulated that wound dressings and topical agents may lower the pH of acute and non‐healing wounds. This is thought to be a result of the interaction between the wound surface and exudate and the chemical composition and microstructure of the dressing. 41 Many interventions, including, dressings, topical agents, and technologies, are being used to treat infection, and to improve wound healing. In addition, research has demonstrated that antibiotic efficacy is affected by pH. 12 , 42 , 43 Therefore, it would be important to find a dressing or topical agent that has both a therapeutic effect on the healing process and the ability to kill bacteria. 44 There is a potential for altering the wound pH using topical or systemic treatments to enhance wound‐healing outcomes. 15 However, the triggered release of a therapeutic agent may rely on clinical indicators such as pH and temperature. 45 Choosing the right dressing has implications for both patients and the health system as it is recognised that delayed wound healing is costly and can consume resources that could otherwise be used elsewhere. 46 , 47 , 48 , 49 There is currently insufficient evidence on the impact of topical agents/and dressings on biomarkers of wound healing namely pH and temperature. Therefore, this systematic review set out to explore the following;
What is the impact of dressings and topical agents on surface wound pH and/or temperature and subsequent wound healing outcomes?
Does wound pH affect the activity and efficacy of antimicrobial agents?
1.1. Aim
This systematic review was conducted to examine clinical research that explored the impact of topical agents or dressings on surface wound pH and temperature and subsequent wound healing.
2. METHODS
2.1. Outcome measures
The primary outcomes of interest in this review were objective measures of pH, temperature, and wound healing, including measures of wound size, or numbers of completely closed wounds.
2.2. Study selection criteria
All types of quantitative primary research studies published in the English language involving participants, of any age or in any setting, with an acute or chronic open wound, that explored the impact of a topical agent or dressing on pH and/or temperature and wound healing. In vitro and animal studies were excluded. No limits were applied in relation to the year of publication, or geographical location.
2.3. Search strategy
Between May and June 2020, a systematic literature search was undertaken to ensure that all published data relating to the topic were identified. The following databases were searched:
Cochrane Library, Embase, Medline PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science and Scopus.
An initial scoping exercise was performed to identify relevant keywords. Subject headings and mesh terms were then identified and combined using the Boolean tool AND, OR which included;
hydrogen ion concentration, hydrogen ions, pH,
temperature, skin temperature, cutaneous temperature, wound temperature,
wounds and injuries, wounds, leg ulcer, varicose ulcer, stasis ulcer, venous hypertension ulcer, ischaemic ulcer, ischemic wound, ischaemic ulcer, arterial insufficiency wound, arterial insufficiency wound, decubitus ulcer, pressure area, pressure ulcer, bedsore, laceration wound, plantar ulcer, diabetic feet, diabetic foot, feet ulcer, burn wound, burn injury, pilonidal sinus, perianal abscess, surgical wound,
administration cutaneous, topical treatment, topical agents, acetic acid, aloe vera, silver sulfadiazine, silver sulphadiazine, silver compounds, povidone iodine, hydrogen peroxide, hydrogel, alginates, hyaluronic acid, antibacterial agents, antimicrobials, chlorhexidine.
All topical agents were combined with all wound types and derivatives of pH or temperature.
The studies retrieved by the initial search underwent a scanned process by a single review author to exclude irrelevant studies; this was validated by the second review author. Two authors then screened titles and abstracts against the inclusion criteria. Relevant articles in reference lists were also considered. If an article met the inclusion criteria, the full‐text version was retrieved. Full‐text articles were then reviewed independently by one review author and validated by a second review author. In all instances, differences of opinion were resolved by discussion among the authors.
2.4. Data extraction
One review author independently extracted data from eligible studies using a data extraction sheet and table; this was validated by a second author. A data extraction table was used to extract the following information: author; title; date of study; study’s geographical location; funding source; care setting; inclusion/exclusion criteria; participant characteristics; study design details; sample size calculation and sample size; study intervention details; outcome measures; length of follow‐up; loss to follow‐up; results.
2.5. Quality appraisal
The Critical Appraisal Checklist (EBL) devised by Glynn 50 was used to appraise the six clinical studies. Accordingly, the studies were appraised under the headings: population, data collection study design, and results. Applying this tool, the study quality in each category is invalid with a final score of <75. Therefore, the studies that produced results of “Yes” ≥75% or, “No+Unclear” ≤25% were considered good quality. The score from each section was calculated at the end to indicate the validity of the study. The quality of the in vitro studies was not appraised as there was no specific tool available.
2.6. Data synthesis
Meta‐analysis was not feasible due to the heterogeneity of the included studies. A narrative description of the studies was undertaken with studies grouped by the intervention. Following this, a narrative synthesis of the data was undertaken.
3. RESULTS
The search strategy yielded 2083 publications. Following removal of duplicate articles, and screening against the inclusion/exclusion criteria, 21 studies were considered potentially eligible, of which 15 were excluded for reasons. Therefore, six studies were considered eligible for inclusion in this review. Table 1 summarises all the excluded studies and reasons for exclusion, and Figure 1 outlines the flow of studies through this review.
TABLE 1.
Excluded studies with reasons
| Study | Reason for exclusion |
|---|---|
| Trop, Waniek 51 | Wound surface temperature not measured. |
| Coats, Edwards 52 | pH or temperature not measured |
| Andrews, Mowlavi 53 | pH or temperature not measured |
| Cuttle, Kempf 54 | pH or temperature not measured |
| Diggelmann, Zytkovicz 55 | Does not include open wounds. |
| Slone, Linton 56 | This is a review, not a research study. |
| Banerjee, Mishra 57 | Development of a sustained delivery dressing. |
| Prabhu, Prasadi 28 | pH at baseline and study end not included. |
| Percival, McCarty 12 | This is a review. |
| Burke‐Smith, Collier 58 | pH or temperature not measured |
| Finzgar, Melik 59 | pH or temperature not measured |
| Heuer, Hoffmanns 60 | pH or temperature not measured |
| Mehmood, Hariz 61 | pH or temperature not measured |
| Cho and Choi 62 | Does not address wounds. |
| Mohan and Ranganathan 63 | pH or temperature not measured. |
| Koehler, Wallmeyer 64 | Development of a dressing. |
FIGURE 1.
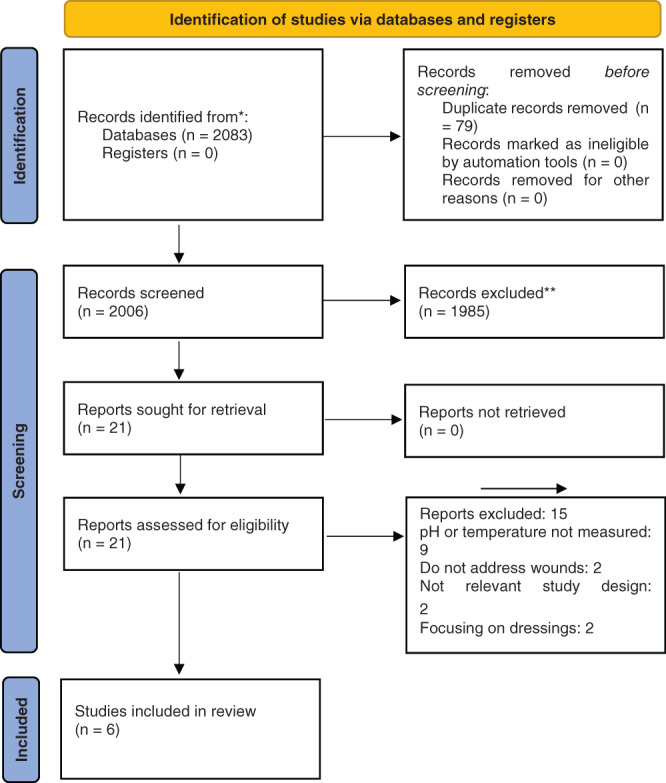
PRISMA 2020 flow diagram for study selection 65
Table 1 displays the excluded studies and the reasons for exclusion.
Figure 1 presents a PRISMA flow diagram mapping out the number of records identified, included, and excluded.
3.1. Description of the included studies
Five of the included studies, explored pH and one study explored temperature. The total sample across the studies was 348 participants, with the sample sizes in the individual clinical studies ranging from 18 to 140 participants. The studies were undertaken across a wide geographical spread among different continents, namely, India (n = 2), United Kingdom (UK) (n = 1), Italy (n = 1), Austria (n = 1), and United States of America (USA) (n = 1). The clinical settings within which the studies took place were acute hospitals, intensive care units, and outpatient clinics. The types of chronic wounds included were leg ulcers, pressure ulcers, burns wounds, trauma wounds, diabetic foot ulcers, and surgical wounds.
3.2. Results of clinical studies
3.2.1. Results: pH: clinical studies
An overview of the results for the pH outcomes is presented in Table 2.
TABLE 2.
Results for pH—clinical studies
| Author, year | Intervention | Comparator | Start of Study | Outcome | Funding |
|---|---|---|---|---|---|
| Romanelli et al., (2010) 4 | Polyhexamethylene biguanide (PHMB) and Betaine and for Wound cleanser | Saline solution in association with standard wound care |
M Median range pH 8. 8.9+/− 0.6 |
M Median range pH 7.0 7.0 +/− 0.3 in (p (P < .05) |
Y Yes Partially financed by B. Braun Medical AG |
| Kumar et al., (2015) 7 | Limited access dressing (LAD) (Negative pressure and moist wound dressing. | Dressed daily with 5% povidone‐iodine soaked gauze |
LAD Day 0: 8.33 ± 0.35 (mean ± SD) Conventional dressing Day 0: 8.31 ± 0.38 |
LAD Day 10: 7.5 ± 0.43 (mean ± SD) Conventional dressing Day 10: 7.9 ± 0.47 (P = .048) |
No |
| Agrawa et al. (2017) 30 | 1% Acetic Acid | No comparator | Start of study pH 9 | End of study pH 7 | No |
| Rafter et al.,(2017) 66 | Manuka Honey | No comparator | Reduction in wound pH at end of study (P < .05) |
Yes Educational grant Advancis Medical. |
|
| Strohal et al., (2018), 67 | Acid oxidising Solution | No comparator |
Day 0: pH (9.25 +/− 0.61). Wound size cm23.06 0.49–32.79 (min/max) |
Day 28: pH (7.68 +/− 0.71) (P = .0001). Wound size cm2.59 0–15.25 (min/max) (P = .001) |
Yes APR Applied Pharma research |
Romanelli et al., 4 conducted a single‐blind, single centre, prospective, controlled explorative comparison trial of 40 participants with chronic leg ulcers. This study evaluated the efficacy and tolerability of a wound cleansing solution containing propyl betaine and PHMB to eliminate or reduce bacterial burden. Propyl betaine and PHMB were used to clean wounds and to moisten absorbent wound dressings. The participants were randomised electronically into two groups; In Group A, 20 participants were treated on alternate days with propyl betaine and PHMB cleansing solution, combined with conventional wound care (polyurethane foam and elastic compression); In group B, 20 participants were treated on alternate days with saline solution combined with conventional wound care (polyurethane foam and elastic compression). Thirty‐eight participants concluded the study, two participants from the control group were lost to follow‐up during treatment. The surface pH was measured using a flat glass electrode connected to a meter (skin pH meter H199181, Hanna Instruments Italy). Wound size was measured with dedicated polarimetry software (Silhouette). Measurements were taken after dressing removal before cleansing. The baseline, median range pH at the wound surface in the group using polyhexamethylene biguanide (PHMB) and betaine was initially 8.9 +/− 0.6. After the 4 weeks, this decreased to 7.0 +/− 0.3. This reduction in pH was statistically significantly lower (P < .05) in the group treated with the active cleanser (PHMB and betaine). The group treated with the PHMB and betaine were reported to have shown significantly better control of the bacterial burden both clinically and by means of instrumental evaluation compared with the control group. However, results for this were not reported in the study.
Kumar and Honnegowda, 7 conducted an RCT on the effect of limited access dressing (LAD) on the surface pH of chronic wounds. LAD combines moist wound healing and negative pressure. One hundred and forty patients were randomised into two groups by simple randomization. Limited access dressings combine moist wound healing with negative pressure utilising an intermittent negative pressure regimen of 30 minutes, followed by a 3.5 hours period of rest. 7 In the LAD group, 64 participants were treated with intermittent negative pressure. In the control group, 76 participants were treated every day with gauze soaked in 5% povidone‐iodine. The wounds were cleaned daily for both groups. The participants were followed up for a period of 10 days. The pH of the wound bed was measured using pH indicator strips MQuant® (Merck). Would size was not measured in this study. Fifty‐six participants were lost to follow up or withdrawn from the study; this included 22 participants in the Limited Access Dressing LAD group and 34 participants in the control group. The findings demonstrated that the LAD treated patients exhibited a reduction in the wound pH as compared with those who received a standard dressing (control). On day 0 the wound surface pH was similar for both groups, in the LAD group the mean and standard deviation (mean ± SD) was 8.33 ± 0.35, whereas it was 8.31 ± 0.38 (mean ± SD) for the control group. On day 10, the mean wound surface pH (± SD) in the LAD group was 7.5 ± 0.43 compared with 7.9 ± 0.47 for the control group (standard dressing). At the end of the study, the mean wound surface pH (± SD) in the LAD group was 0.83 ± 0.52 compared with 0.41 ± 0.26 (P = .048) in the control group. The pH was significantly lower (P = .048) in the LAD group.
Agrawal et al., 30 conducted a prospective analysis study, evaluating the topical use of 1% of acetic acid for the treatment of infected wounds. The pH of 1% of acetic acid was 2.5. One hundred participants with infected wounds of mixed aetiologies, including diabetic, trauma, burns venous ulcers, and graft donor sites were treated with topical application of 1% acetic acid. There was no comparator in this study. Normal saline was used to dilute acetic acid to a concentration of 1%. Following the removal of the old dressing, an immersion bath with 0.1% acetic acid was given for 15 minutes to create an acidic environment. Normal saline was then used to cleanse the wounds. A non‐adhesive sterile Vaseline gauze was placed on the wound, then a gauze soaked in 1% acetic acid solution was placed over this covered with a sterile dressing. Wounds were dressed daily, or on alternate days, for a period of seven to 21 days. During the study period, the patients received no systemic antibiotics. The pH of the wound bed was measured using paper strips. For each wound, a wound swab was collected before commencing acetic acid (1%) and subsequently on day 3, 7, 10, and 14. Wounds were assessed clinically for the amount of discharge, odour, wound size, and quality of granulation tissue. The average pH of infected wounds at the start of the study was alkaline at pH 9.0, the pH decreased with improved granulation tissue to pH 7.0. Whereas, infected wounds were alkaline (pH 9). There was a reported decrease in wound size, inflammation, and induration after treatment with acetic acid, suggestive of wound healing; however, these results were not shown. It is unclear in the study if the pH remained consistently low between dressing changes as measurements are not shown.
Rafter et al., 66 conducted a clinical evaluation study of 100% medical grade Manuka honey for chronic wounds. Twenty‐two participants with a total of 40 chronic wounds were recruited for this study. The type of wounds varied and included pressure ulcers, diabetic foot ulcers, leg ulcers, wounds caused by trauma, and surgical wounds. The Manuka honey dressing was used as a primary dressing with a superabsorbent secondary dressing. The patients were followed over a period of 8 weeks. The participants had their dressing changed on alternate days, the tissue viability nurse consultant performed an assessment of the wound and dressing change once weekly on days 1, 7, 14, 28, 35, 42, 49, and 56. Inpatients had their wound assessed twice a week to ensure concordance with the regimen. When the patients were discharged home, they had their dressings changed every other day by the district nursing teams. A detailed wound assessment was conducted weekly by the tissue viability nurse consultant. A wound swab (n‐108) was taken on days 1, 14, 35, and 49. The pH of the wound bed was measured at every dressing change with a pH indicator strip. The authors were unable to analyse the effectiveness of medical grade Manuka honey on infections as patients were on antibiotics. Results from T‐tests indicated that Manuka honey reduced wound pH significantly (P < .05). pH values for different time points were not shown and wound size was not measured.
Strohal et al., 67 conducted a prospective single arm open‐label clinical case series. This was a pilot study including 30 participants, investigating the use of acid‐oxidising solution (AOS) for chronic leg ulcers. The AOS dressing was applied on each leg ulcer at every dressing change for a period of 35 days. Wounds were dressed daily if critically colonised, and wounds that were not infected were dressed on alternate days. The procedure was as follows; after removal of the old dressing, the ulcers were cleaned with a dry gauze after which, the AOS was sprayed over the entire wound, then after 2 minutes, the ulcers were cleaned again with sterile gauze. The AOS was sprayed again over the entire wound, then a non‐adherent dressing that was soaked in the AOS was placed on the wound and a sterile highly absorbent dressing was then applied on top. In addition, all patients with venous leg ulcers were treated with compression therapy. Patients with non‐venous ulcers did not receive any additional treatment. There was no comparator in this study. Wound size was measured using digital planimetry software and wound pH was measured using a probe and pH meter.
The ulcers showed a highly alkaline pH (9.25 +/− 0.61) at the start of the study. The mean pH decreased significantly (P < .0001), and over time ulcers demonstrated an almost neutral pH value (7.68 +/− 0.71) by visit 5 (day 28 +/−2). At the onset of the study, the median wound size was 3.06cm2 (0.49–32.79 cm2), the size decreased to a median of 0.59cm2 (0–15.25 cm2) at the end of the study. Notably, the researchers reported that the decreased wound size correlated significantly with the reduced pH value of the wound (r = 0.1957, P = .0108). However, this value (r = 0.1957, P = .0108) is substantially below 0.3 or 0.4 and therefore does not indicate a significant correlation between wound size and pH. The researchers reported that there was a statistically significant correlation between the pH change and the successful control of infection was also detected (r = 0.6960; P < .0001).
3.3. Results: temperature: clinical study
One study investigated the impact of a topical agent on wound temperature and reported that topical sodium nitrite cream increased peri‐wound skin temperature as measured using infrared thermography. 68 Baseline wound bed temperature was 32·8°C (SD: ± 1·4) and this increased statistically significantly to 34·9°C (SD: ± 0·7) after the application of the first dose of cream (P < .0001). Following the second dose of cream, a statistically significant increase was also noted; the wound temperature went from 33·0°C (SD: ± 1·1) to 34·8°C (SD: ± 0·7); (P < .0001). There was also an associated increase in temperature reported in the peri‐wound region (r = 0·72, P = .0010).
There was a statistically significant reduction noted in ulcer size; the mean ulcer surface area before commencing treatment was 5.97 cm2 and following treatment, size decreased to 3.26 cm2. In cohort one (0.5% sodium nitrite), there was a 29.9% mean reduction in wound size. In cohort two (1% sodium nitrite), there was a 7.7% mean reduction in wound size, however, wound size increased for one patient). In cohort three (1.5% sodium nitrite), there was a 32.5% mean reduction in wound size. In cohort 3a (1.8% sodium nitrite), there was a mean reduction of 69.7% in wound size and one complete closure). In cohort four (2% sodium nitrite cream), there was an 88.3% mean reduction in wound size and two complete closures. The reductions in wound size correlated with the nitrate dose cohort (r = 0.7, P = .0012).
In summary, six human clinical studies were included in this review. 4 , 7 , 30 , 66 , 67 , 68 Five clinical studies explored pH investigating five different dressings or topical agents including negative pressure dressing, medical grade honey dressing, acid oxidising solution, a solution containing 1% acetic acid, and a solution containing PHMB and betaine. Each of these studies reported a significant reduction in pH and improved wound outcomes at study end, four of the studies reported a reduction in wound size, although in one study this reduction was not statistically significant. 4 One study did not report on wound size, but instead reported time to complete healing, although results for this were not shown. 66 Only, one clinical study investigated the impact of a topical agent on temperature, namely topical sodium nitrite. The study found that sodium nitrite increased peri‐wound skin temperature and improved wound outcomes with an associated reduction in leg ulcer size. 68
3.4. Quality appraisal
Across the clinical studies, the quality appraisal scores ranged from 30% to 64% with an overall mean of 52%. Table 3 illustrates the results of the quality appraisal. The two included RCT's, Kumar and Honnegowda 7 (quality appraisal score: 30%) and Romanelli, Dini 4 (quality appraisal score: 64%) that investigated a Limited Access Dressing and a solution containing propyl betaine and PHMB did not outline the randomization methods, concealment, or blinding. Neither study reported sample size calculation. Romanelli, Dini 4 had a small sample size and thus may not have had enough power to detect differences between groups. The remaining four clinical studies also had low‐quality appraisal scores (mean 50.3%).
TABLE 3.
Quality appraisal
| Overall validity of included studies (%) including the validity of each category | |||||
|---|---|---|---|---|---|
| Authors | Population | Data collection | Study design | Results | Overall Validity |
| Romanelli et al., (2010) 4 | 57% (Not valid) | 80% (Valid) | 60% (Not valid) | 60% (Not valid) | 64% (Not valid) |
| Kumar et al., (2015) 7 | 25% (Not valid) | 50% (Not valid) | 40% (Not valid) | 20% (Not valid) | 30% (Not Valid) |
| Agrawa et al., (2017) 30 | 60% (Not valid) | 33% (Not valid) | 40% (Not valid) | 40% (Not Valid) | 40% (Not valid) |
| Rafter et al.,(2017) 66 | 60% (Not valid) | 50% (Not valid) | 60% (Not valid) | 40% (Not valid) | 52% (Not valid) |
| Strohal et al., (2018), 67 | 66% (Not valid) | 80% (Valid) | 60% (Not valid) | 60% (Not valid) | 66% (Not valid) |
| Minniti et al., 68 | 50% (Not valid) | 40% (Not valid) | 60% (Not valid) | 67% (Not valid) | 58% (Not valid) |
4. DISCUSSION
pH has an important role to play in wound healing and as such it appears that it could possibly be manipulated by topical agents and dressings to improve wound outcomes. However, there is insufficient human studies to support this conclusively. In addition, it is also important to consider that the actual pH of the wound may impact the antimicrobial effectiveness of the topical agent or dressing. 29 , 69 All six interventions will not be discussed, only those important interventions from a clinical practice perspective, including, Manuka honey, Acetic acid (1%), and PHMB and betaine.
Only one study in this review investigated the impact of a topical agent on wound temperature and found that topical sodium nitrite cream increased peri‐wound skin temperature. 68 This was thought to be due to the vasodilating properties of sodium nitrite. However, this was a small dose finding study and therefore the findings should be interpreted with caution.
Manuka honey is an antimicrobial agent with anti‐inflammatory and antioxidant activities. 70 , 71 Manuka honey is comprised of phenolic acids, flavonoids, and other compounds. 71 , 72 The acidity of manuka honey with a pH ranging between 3.2 and 4.5, may assist in the antibacterial action of macrophages. 73 Previous invitro studies by Milne and Connolly 74 and Kiamcoet et al., 75 also found that Manuka honey, medical‐grade honey, and medical‐grade honey combined with ciprofloxacin respectively, lowered pH. However, Kiamco et al., 75 also found that medical‐grade honey alone is not an effective bactericidal. Conversely, research studies 76 , 77 , 78 have shown honey inhibits the growth of most pathogenic bacteria within wounds. Notably, several mechanisms are involved in the antibacterial properties of honey, and these act in synergy. These include osmolarity, the acid pH (3.2 to 4.2), 76 the hydrogen peroxide system, and the presence of phytochemical factors, defensin‐1, and methylglyoxal. 77 , 78 , 79 Moreover, numerous scientific studies 73 , 80 , 81 have shown that honey has, in particular, a positive effect on debridement and a modulating effect on inflammation, thereby promoting the formation of granulation tissue. The acid pH of honey can help to create an acidic wound environment that is considered necessary to maintain optimal conditions for fibroblast activity namely, migration, proliferation, and organisation of collagen. 76 However, despite the plethora of literature available on the use of honey as a potential therapeutic agent for managing chronic wounds, only one clinical study has researched the impact of honey on biomarkers of wound healing such as wound pH. 66 This was a small product evaluation study of 20 participants, which means the findings should be interpreted with caution. No study has researched the impact of honey on wound temperature.
Different acids, including acetic acid (1% and 5%), and an acid oxidising solution have been previously used as treatments for wound healing. 2 , 16 , 82 It is thought that acid solutions contribute to wound healing by creating an acidic environment which helps to control bacterial growth and increase antimicrobial activity. 83 , 84 It has been identified in several studies that lowering the wound pH is effective against different bacterial strains. 2 , 83 , 85 , 86 Historically, acetic acid was used to treat wounds with Pseudomonas infection. 84 Agrawal et al., 28 reported that acetic acid was efficacious against many common isolates including P. aeruginosa, S. aureus, Streptococcus, Proteus mirabilis, Citrobacter spp., C. albicans, Cryptococcus neoformans, Aspergillus niger, and A. fumigatus. Fejfarova et al., 87 in a study on diabetic foot ulcers reported enhanced outcomes of reduced ulcer dimensions using 1% acetic acid, although the results were not statistically significant. Acetic acid (1%) could potentially alter the alkaline pH of infected wounds. 28 Further, whilst 0.1% acetic acid appears to have good antibacterial activity, it is unclear in the included study 30 if the pH remained consistently low between dressing changes. In previous laboratory studies, acetic acid in 1% and 5% concentrations were used to reduce the pH of the wound surface. 2 , 88 However, acetic acid was not considered an effective method of reducing pH as it only reduces the pH for 1 hour, after which the pH resumes its untreated pH value. 2 , 88
One clinical study 4 investigated products that contained PHMB. Propyl Betaine and Polyhexanide (PHMB) is a wound cleanser, which decontaminates and removes exudate, slough, and debris. 4 PHMB belongs to the group of cationic (positively‐charged) biocides known as biguanides. The positively charged groups of molecules can bind quickly to the negatively charged surface of the bacteria, disrupting the bacterial membrane causing damage and the subsequent death of the bacteria. 89 Betaine disrupts the biofilm, PHMB in combination with a betaine creates a low surface tension which results in an increased ability to remove debris, bacteria and biofilm from the wound. 90 , 91 Other invitro studies illustrated that Polyhexadine formulations exhibit increased antimicrobial activity at higher pH values. 73 , 74 Non‐healing wounds have a pH ranging from 7.15 to 8.9. 4 Therefore, products containing PHMB may be useful in reducing the wound pH and thus managing wound infections in chronic wounds or even preparing the wound bed for the wound dressing or topical agent. Interestingly, whilst dressings with iodine and silver are commonly used in clinical practice, there was no clinical research on the effects of these dressings on wound pH.
5. IMPLICATIONS FOR RESEARCH
Although there are many types of topical agents and dressings available, very few studies investigate the effect of dressing/topical agents on wound parameters. There is a lack of robust research on their ability to manipulate the pH or temperature of the wound. Nevertheless, whilst the findings are limited due to the paucity of research and quality of included studies, the findings do indicate that topical agents may potentially manipulate pH to improve wound outcomes. The adoption of biomarkers and technology in the clinical area will incorporate objectivity into wound assessment and improve our understanding of effect dressing and topical agents on these biomarkers. The collection of numerical results could facilitate clear and concise data collection and analysis. 9 Then, there would be great potential to use this knowledge to influence wound healing in the future. This may ultimately lead to the development of a more cost‐effective treatment plan that is tailored to the biomarkers of individual wounds, rather than what is currently a more generic approach. This could potentially result in better clinical outcomes for the patient and more efficient use of resources.
Only one study investigated the effect of a topical agent on wound temperature. Therefore, there is a further need for clinical research in this area.
6. CONCLUSION
The results of this systematic review indicated that that the healing conditions of the wound may be modified by topical agents and dressings applied to the wound. In particular, Manuka honey dressings, Acetic acid PHMB appeared to lower the wound pH resulting in improved wound outcomes. However, significant heterogeneity exists within the studies included in this systematic review and the evidence available is low both in volume and quality. More robust research is needed to determine whether topical agents and dressings have an impact on the biomarkers of wound healing such as pH, temperature and to investigate the effect of pH on the antimicrobial efficacy of topical agents and dressings.
CONFLICT OF INTEREST
No conflict of interest is declared by the authors.
ACKNOWLEDGEMENTS
This research received funding from Irish Research Council, Enterprise Partnership Scheme.
Project ID: EPSPG/2016/159.
Enterprise Partner: Fleming Medical Ltd.
Derwin R, Patton D, Avsar P, Strapp H, Moore Z. The impact of topical agents and dressing on pH and temperature on wound healing: A systematic, narrative review. Int Wound J. 2022;19(6):1397-1408. doi: 10.1111/iwj.13733
DATA AVAILABILITY STATEMENT
It is a systematic review, all data were represented.
REFERENCES
- 1. Gethin G. The significance of surface pH in chronic wounds. Wounds UK. 2007;3:52‐56. [Google Scholar]
- 2. Leveen HH, Falk G, Borek B, et al. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann Surg 1973; 178: 745–753. December 1, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultz G, Mozingo D, Romanelli M, Claxton K. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen. 2005;13:S1‐S11. Review. [DOI] [PubMed] [Google Scholar]
- 4. Romanelli M, Dini V, Barbanera S, et al. Evaluation of the efficacy and tolerability of a solution containing propyl betaine and polihexanide for wound irrigation. Skin Pharmacol Physiol. 2010;23(Suppl):41‐44. doi: 10.1159/000318266 [DOI] [PubMed] [Google Scholar]
- 5. Shukla VK, Shukla D, Tiwary SK, Agrawal S, Rastogi A. Evaluation of pH measurement as a method of wound assessment. J Wound Care. 2007;16:291‐294. [DOI] [PubMed] [Google Scholar]
- 6. Kruse CR, Nuutila K, Lee CCY, et al. The external microenvironment of healing skin wounds. Wound Repair Regen. 2015;23:456‐464. doi: 10.1111/wrr.12303 [DOI] [PubMed] [Google Scholar]
- 7. Kumar P, Honnegowda T. Effect of limited access dressing on surface pH of chronic wounds. Plast Aesthet Res. 2015;2:257‐260. doi: 10.4103/2347-9264.165449 [DOI] [Google Scholar]
- 8. Ono S, Imai R, Ida Y, et al. Increased wound pH as an indicator of local wound infection in second degree burns. Burns. 2015;41:820‐824. doi: 10.1016/j.burns.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 9. Power G, Moore Z, O’Connor T. Measurement of pH, exudate composition and temperature in wound healing: a systematic review. J Wound Care. 2017;26:381‐397. doi: 10.12968/jowc.2017.26.7.381 [DOI] [PubMed] [Google Scholar]
- 10. Gethin G, O’Connor GM, Abedin J, et al. Monitoring of pH and temperature of neuropathic diabetic and nondiabetic foot ulcers for 12 weeks: an observational study. Wound Repair Regen. 2018;26:251‐256. doi: 10.1111/wrr.12628 [DOI] [PubMed] [Google Scholar]
- 11. Gethin G, Ivory JD, Sezgin D, Muller H, O'Connor G, Vellinga A. What is the "normal" wound bed temperature? A scoping review and new hypothesis. Wound Repair Regen. 2021;29:843‐847. doi: 10.1111/wrr.12930 [DOI] [PubMed] [Google Scholar]
- 12. Percival SL, McCarty S, Hunt JA, et al. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014;22:174‐186. doi: 10.1111/wrr.12125 [DOI] [PubMed] [Google Scholar]
- 13. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1‐S28. doi: 10.1046/j.1524-475x.11.s2.1.x [DOI] [PubMed] [Google Scholar]
- 14. Moore Z, Dowsett C, Smith G, et al. TIME CDST: an updated tool to address the current challenges in wound care. J Wound Care. 2019;28:154‐161. doi: 10.12968/jowc.2019.28.3.154 [DOI] [PubMed] [Google Scholar]
- 15. Jones EM, Cochrane CA, Percival SL. The effect of pH on the extracellular matrix and biofilms. Adv Wound Care (New Rochelle). 2015;4:431‐439. doi: 10.1089/wound.2014.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagoba B, Suryawanshi N, Wadher BJ, et al. Acidic environment and wound healing: a review. Wounds. 2015;27:5‐11. [Google Scholar]
- 17. Wiegand C, Abel M, Ruth P, et al. pH influence on antibacterial efficacy of common antiseptic substances. Skin Pharmacol Physiol. 2015;28:147‐158. doi: 10.1159/000367632 [DOI] [PubMed] [Google Scholar]
- 18. Tsukada K, Tokunaga K, Iwama T, et al. He pH changes of pressure ulcers related to the healing process of wounds. Wounds. 1992;4:16‐20. [Google Scholar]
- 19. Dissemond J, Witthoff M, Brauns TC, et al. pH values in chronic wounds: evaluation during modern wound therapy. Hautarzt. 2003;54:959‐965. doi: 10.1007/s00105-003-0554-x [DOI] [PubMed] [Google Scholar]
- 20. Greener B, Hughes AA, Bannister NP, et al. Proteases and pH in chronic wounds. J Wound Care. 2005;14:59‐61. doi: 10.12968/jowc.2005.14.2.26739 [DOI] [PubMed] [Google Scholar]
- 21. Gethin G. The importance of continuous measuring. Wounds UK. 2006;2:60‐68. [Google Scholar]
- 22. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound‐healing: a new perspective for wound‐therapy? Arch Dermatol Res. 2007;298:413‐420. Review. doi: 10.1007/s00403-006-0713-x [DOI] [PubMed] [Google Scholar]
- 23. Wolcott RD, Gontcharova V, Sun Y, Dowd SE. Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag‐encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC Microbiol. 2009;9:226‐226. doi: 10.1186/1471-2180-9-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hostacka A, Ciznar I, Stefkovicova M. Temperature and pH affect the production of bacterial biofilm. Folia Microbiol (Praha). 2010;55:75‐78. doi: 10.1007/s12223-010-0012-y [DOI] [PubMed] [Google Scholar]
- 25. Wilson IA, Henry M, Quill RD, et al. The pH of varicose ulcer surfaces and its relationship to healing. Vasa. 1979;8:339‐342. [PubMed] [Google Scholar]
- 26. Price P, Bale S, Crook H, et al. The effect of a radiant heat dressing on pressure ulcers. J Wound Care. 2000;9:201‐205. doi: 10.12968/jowc.2000.9.4.25972 [DOI] [PubMed] [Google Scholar]
- 27. Daima HK, Selvakannan PR, Kandjani AE, et al. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine‐capped Ag nanoparticles. Nanoscale. 2014;6:758‐765. doi: 10.1039/c3nr03806h [DOI] [PubMed] [Google Scholar]
- 28. Prabhu V, Prasadi S, Pawar V, et al. Does wound pH modulation with 3% citric acid solution dressing help in wound healing: a pilot study. Saudi Surgical Journal. 2014;2:38‐46. Original Article. doi: 10.4103/2320-3846.140690 [DOI] [Google Scholar]
- 29. Percival SL, Finnegan S, Donelli G, et al. Antiseptics for treating infected wounds: efficacy on biofilms and effect of pH. Crit Rev Microbiol. 2016;42:293‐309. doi: 10.3109/1040841X.2014.940495 [DOI] [PubMed] [Google Scholar]
- 30. Agrawal KS, Sarda AV, Shrotriya R, et al. Acetic acid dressings: finding the holy grail for infected wound management. Indian J Plast Surg. 2017;50:273‐280. doi: 10.4103/ijps.IJPS_245_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jain M. Role of tissue conductivity in temperature variation during wound healing process after plastic surgery. Int J Mathem Comput Modell. 2014;18:1114‐1123. [Google Scholar]
- 32. Bryant RA, Nix DP. Acute & Chronic Wounds: Current Management Concepts. 5th ed. St. Louis, Missouri: Elsevier; 2016. [Google Scholar]
- 33. Khan H, Khan MF, Khan BA. Coordination of silver sulfadiazine with glutathione in aqueous medium. Pharm Chem J. 2014. Article in Press;48:269‐272. doi: 10.1007/s11094-014-1091-x [DOI] [Google Scholar]
- 34. Dini V, Salvo P, Janowska A, di Francesco F, Barbini A, Romanelli M. Correlation between wound temperature obtained with an infrared camera and clinical wound bed score in venous leg ulcers. Wounds. 2015;27:274‐278. [PubMed] [Google Scholar]
- 35. Yue JH, Zhang SJ, Sun Q, et al. Local warming therapy for treating chronic wounds: a systematic review. Medicine (Baltimore). 2018;97:e9931. doi: 10.1097/md.0000000000009931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan AA, Banwell PE, Bakker MC, et al. Topical radiant heating in wound healing: an experimental study in a donor site wound model. Int Wound J. 2004;1:233‐240. doi: 10.1111/j.1742-4801.2004.00065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakagami G, Sanada H, Iizaka S, et al. Predicting delayed pressure ulcer healing using thermography: a prospective cohort study. J Wound Care. 2010;19:465‐472. [DOI] [PubMed] [Google Scholar]
- 38. Fierheller M, Sibbald RG. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv Skin Wound Care. 2010;23:369‐379; quiz 380–361. doi: 10.1097/01.Asw.0000383197.28192.98 [DOI] [PubMed] [Google Scholar]
- 39. Siah CR, Childs C, Chia CK, et al. An observational study of temperature and thermal images of surgical wounds for detecting delayed wound healing within four days after surgery. J Clin Nurs. 2019;28:2285‐2295. doi: 10.1111/jocn.14832 [DOI] [PubMed] [Google Scholar]
- 40. Esclamado RM, Damiano GA, Cummings CW. Effect of local hypothermia on early wound repair. Arch Otolaryngol Head Neck Surg. 1990;116:803‐808. [DOI] [PubMed] [Google Scholar]
- 41. Uzun M, Anand SC, Shah T. The effect of wound dressings on the pH stability of fluids. J Wound Care. 2012;21:88‐90, 92–85. doi: 10.12968/jowc.2012.21.2.88 [DOI] [PubMed] [Google Scholar]
- 42. Percival SL, Hill KE, Malic S, et al. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 2011;19:1‐9. doi: 10.1111/j.1524-475X.2010.00651.x [DOI] [PubMed] [Google Scholar]
- 43. Thomas J, Linton S, Corum L, et al. The affect of pH and bacterial phenotypic state on antibiotic efficacy. Int Wound J. 2012;9:428‐435. doi: 10.1111/j.1742-481X.2011.00902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al‐Waili N, Salom K and Al‐Ghamdi AA. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. SciWorldJ 2011; 11: 766–787. April 12, 2011. doi: 10.1100/tsw.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hathaway H, Ajuebor J, Stephens L, et al. Thermally triggered release of the bacteriophage endolysin CHAPK and the bacteriocin lysostaphin for the control of methicillin resistant Staphylococcus aureus (MRSA). Journal of Controlled Release. 2017;245:108‐115. Article. doi: 10.1016/j.jconrel.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763‐771. doi: 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5:1‐8. doi: 10.1136/bmjopen-2015-009283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jarbrink K, Ni G, Sonnergren H, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev 2017; 6: 15. January 26, 2017. doi: 10.1186/s13643-016-0400-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27‐32. doi: 10.1016/j.jval.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 50. Glynn L. A critical appraisal tool for library and information research. Library Hi Tech. 2006;24:387‐399. doi: 10.1108/07378830610692154 [DOI] [Google Scholar]
- 51. Trop M, Waniek G, Zobel G, et al. Procel burn cover used as a total body dressing in burns. Burns. 1995;21:544‐545. Article. doi: 10.1016/0305-4179(95)00028-A [DOI] [PubMed] [Google Scholar]
- 52. Coats TJ, Edwards C, Newton R, et al. The effect of gel burns dressings on skin temperature. Emerg Med J. 2002;19:224‐225. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andrews K, Mowlavi A, Milner SM. The treatment of alkaline burns of the skin by neutralization. Plast Reconstruct Surg. 2003;111:1918‐1921. doi: 10.1097/01.PRS.0000058953.16695.A7 [DOI] [PubMed] [Google Scholar]
- 54. Cuttle L, Kempf M, Kravchuk O, et al. The efficacy of Aloe vera, tea tree oil and saliva as first aid treatment for partial thickness burn injuries. Burns. 2008;34:1176‐1182. doi: 10.1016/j.burns.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 55. Diggelmann KV, Zytkovicz AE, Tuaine JM, et al. Mepilex lite dressings for the management of radiation‐induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83:971‐978. Article. doi: 10.1259/bjr/62011713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Slone W, Linton S, Okel T, Corum L, Thomas JG, Percival SL. The effect of pH on the antimicrobial efficiency of silver alginate on chronic wound isolates. J Am Coll Certif Wound Specialists. 2010;2:86‐90. Review. doi: 10.1016/j.jcws.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Banerjee I, Mishra D, Das T, et al. Caprine (goat) collagen: a potential biomaterial for skin tissue engineering. J Biomater Sci Polym Ed. 2012;23:355‐373. doi: 10.1163/092050610x551943 [DOI] [PubMed] [Google Scholar]
- 58. Burke‐Smith A, Collier J, Jones I. A comparison of non‐invasive imaging modalities: infrared thermography, spectrophotometric intracutaneous analysis and laser Doppler imaging for the assessment of adult burns. Burns. 2015;41:1695‐1707. Article. doi: 10.1016/j.burns.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 59. Finzgar M, Melik Z, Cankar K. Effect of transcutaneous application of gaseous carbon dioxide on cutaneous microcirculation. Clin Hemorheol Microcirc. 2015;60:423‐435. Article. doi: 10.3233/CH-141898 [DOI] [PubMed] [Google Scholar]
- 60. Heuer K, Hoffmanns MA, Demir E, et al. The topical use of non‐thermal dielectric barrier discharge (DBD): nitric oxide related effects on human skin. Nitric Oxide Biol Chem. 2015;44:52‐60. Article. doi: 10.1016/j.niox.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 61. Mehmood N, Hariz A, Templeton S, Voelcker NH. A flexible and low power telemetric sensing and monitoring system for chronic wound diagnostics. Biomed Eng Online. 2015;14:1‐17. doi: 10.1186/s12938-015-0011-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho YS, Choi YH. Comparison of three cooling methods for burn patients: a randomized clinical trial. Burns. 2017;43:502‐508. Article. doi: 10.1016/j.burns.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 63. Mohan P, Ranganathan R. Application of 3D printing in electro induced drug delivery based on wound temperature. Int Res J Pharm. 2017;8:179‐183. Article. doi: 10.7897/2230-8407.0811238 [DOI] [Google Scholar]
- 64. Koehler J, Wallmeyer L, Hedtrich S, et al. pH‐modulating poly(ethylene glycol)/alginate hydrogel dressings for the treatment of chronic wounds. Macromol Biosci 2017;17:1‐11. doi: 10.1002/mabi.201600369 [DOI] [PubMed] [Google Scholar]
- 65. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021; 372: n71. March 31, 2021. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rafter L, Reynolds T, Collier M, et al. A clinical evaluation of Algivon® Plus manuka honey dressings for chronic wounds. Wounds UK. 2017;13:132‐140. [Google Scholar]
- 67. Strohal R, Mittlbock M, Hammerle G. The management of critically colonized and locally infected leg ulcers with an acid‐oxidizing solution: a pilot study. Adv Skin Wound Care. 2018;31:163‐171. doi: 10.1097/01.ASW.0000530687.23867.bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Minniti CP, Gorbach AM, Xu D, et al. Topical sodium nitrite for chronic leg ulcers in patients with sickle cell anaemia: a phase 1 dose‐finding safety and tolerability trial. Lancet Haematol. 2014;1:e95‐e103. Article. doi: 10.1016/S2352-3026(14)00019-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwarzer S, James GA, Goeres D, et al. The efficacy of topical agents used in wounds for managing chronic biofilm infections: a systematic review. J Infect. 2020;80:261‐270. doi: 10.1016/j.jinf.2019.12.017 [DOI] [PubMed] [Google Scholar]
- 70. Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50‐57. doi: 10.1016/j.burns.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 71. Alvarez‐Suarez JM, Gasparrini M, Forbes‐Hernández TY, et al. The composition and biological activity of honey: a focus on Manuka honey. Foods. 2014;3:420‐432. doi: 10.3390/foods3030420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chan CW, Deadman BJ, Manley‐Harris M, Wilkins AL, Alber DG, Harry E. Analysis of the flavonoid component of bioactive New Zealand mānuka (Leptospermum scoparium) honey and the isolation, characterisation and synthesis of an unusual pyrrole. Food Chem. 2013;141:1772‐1781. doi: 10.1016/j.foodchem.2013.04.092 [DOI] [PubMed] [Google Scholar]
- 73. Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27:141‐151. [PubMed] [Google Scholar]
- 74. Milne SD, Connolly P. The influence of different dressings on the pH of the wound environment. J Wound Care. 2014;23:53‐57. [DOI] [PubMed] [Google Scholar]
- 75. Kiamco MM, Atci E, Mohamed A, Call DR, Beyenal H. Hyperosmotic agents and antibiotics affect dissolved oxygen and pH concentration gradients in Staphylococcus aureus biofilms. Appl Environ Microbiol. 2017;83:1‐13. doi: 10.1128/AEM.02783-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lusby PE, Coombes A, Wilkinson JM. Honey: a potent agent for wound healing? J Wound Ostomy Continence Nurs. 2002;29:295‐300. [DOI] [PubMed] [Google Scholar]
- 77. Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1:154‐160. doi: 10.1016/s2221-1691(11)60016-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alam F, Islam MA, Gan SH, et al. Honey: a potential therapeutic agent for managing diabetic wounds. Evid Based Complement Alternat Med. 2014;2014:169130. doi: 10.1155/2014/169130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sood N, Bhardwaj A, Mehta S, Mehta A. Stimuli‐responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23:758‐780. Review. doi: 10.3109/10717544.2014.940091 [DOI] [PubMed] [Google Scholar]
- 80. Vermeulen H, Ubbink DT, Goossens A, et al. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005;92:665‐672. doi: 10.1002/bjs.5055 [DOI] [PubMed] [Google Scholar]
- 81. Cooper RA, Jenkins L, Henriques AF, et al. Absence of bacterial resistance to medical‐grade manuka honey. Eur J Clin Microbiol Infect Dis. 2010;29:1237‐1241. doi: 10.1007/s10096-010-0992-1 [DOI] [PubMed] [Google Scholar]
- 82. Sloss JM, Cumberland N, Milner SM. Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J R Army Med Corps. 1993;139:49‐51. doi: 10.1136/jramc-139-02-04 [DOI] [PubMed] [Google Scholar]
- 83. Schreml S, Szeimies RM, Karrer S, et al. The impact of the pH value on skin integrity and cutaneous wound healing. J Eur Acad Dermatol Venereol. 2010;24:373‐378. doi: 10.1111/j.1468-3083.2009.03413.x [DOI] [PubMed] [Google Scholar]
- 84. Nagoba BS, Selkar SP, Wadher BJ, et al. Acetic acid treatment of pseudomonal wound infections—a review. J Infect Public Health. 2013;6:410‐415. doi: 10.1016/j.jiph.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 85. Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321‐330. doi: 10.1093/jac/34.3.321 [DOI] [PubMed] [Google Scholar]
- 86. Kumaran D, Eswaramoorthy S, Furey W, et al. Structure of staphylococcal enterotoxin C2 at various pH levels. Acta Crystallogr D Biol Crystallogr. 2001;57:1270‐1275. doi: 10.1107/s0907444901011118 [DOI] [PubMed] [Google Scholar]
- 87. Fejfarová V, Tibenská H, Niklová J, et al. Benefits of acidifying agents in local therapy of diabetic foot ulcers infected by pseudomonas sp: a pilot study. Int J Low Extrem Wounds. 2019;18:262‐268. doi: 10.1177/1534734619848573 [DOI] [PubMed] [Google Scholar]
- 88. Kam‐Chi Leung D, Fung‐Ming Mok W, Man‐Wai YD, et al. Use of distilled white vinegar dressing supplement to oral antibiotics in the management of Pseudomonas aeruginosa exit site infection in continuous ambulatory peritoneal dialysis patients. Hong Kong J Nephrol. 2001;3:38‐40. doi: 10.1016/S1561-5413(09)60055-7 [DOI] [Google Scholar]
- 89. Eberlein T, Haemmerle G, Signer M, et al. Comparison of PHMB‐containing dressing and silver dressings in patients with critically colonised or locally infected wounds. J Wound Care. 2012;21:12, 14–16, 18–20. doi: 10.12968/jowc.2012.21.1.12 [DOI] [PubMed] [Google Scholar]
- 90. Andriessen AE, Eberlein T. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds. 2008;20:171‐175. [PubMed] [Google Scholar]
- 91. Bellingeri A, Falciani F, Traspedini P, et al. Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single‐blind RCT. J Wound Care. 2016;25:160, 162–166, 168. doi: 10.12968/jowc.2016.25.3.160 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It is a systematic review, all data were represented.


