Abstract
Probiotics are live microorganisms that confer health benefits to host organisms when consumed in adequate amounts and are often incorporated into foods for human consumption. However, this has negative implications on their viability as large numbers of these beneficial bacteria are deactivated when subjected to harsh conditions during processing, storage, and passage through the gastrointestinal tract. To address these issues, numerous studies on encapsulation techniques to protect probiotics have been conducted. This review focuses on emulsion technology for probiotic encapsulation, with a special focus on Pickering emulsions. Pickering emulsions are stabilized by solid particles, which adsorb strongly onto the liquid-liquid interfaces to prevent aggregation. Pickering emulsions have demonstrated enhanced stability, high encapsulation efficiency, and cost-effectiveness compared to other encapsulation techniques. Additionally, Pickering emulsions are regarded as safe and biocompatible and utilize natural materials, such as cellulose and chitosan derived from plants, shellfish, and fungi, which may also be viewed as more acceptable in food systems than common synthetic and natural molecular surfactants. This article reviews the current status of Pickering emulsion use for probiotic delivery and explores the potential of this technique for application in other fields, such as livestock farming, pet food, and aquaculture.
Keywords: Encapsulation, Probiotics, Viability, Emulsions, Pickering emulsions
Graphical abstract
Highlights
-
•
Probiotics play an important role in maintaining the health of humans and animals.
-
•
Encapsulation improves probiotic viability in harsh environments.
-
•
Probiotics can be encapsulated by many techniques such as emulsification.
-
•
Pickering emulsions use particles instead of molecules to stabilize emulsions.
-
•
Natural particles are more acceptable to some consumers than synthetic emulsifiers.
1. Introduction
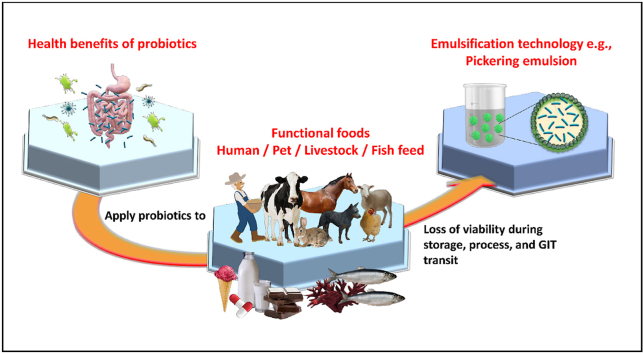
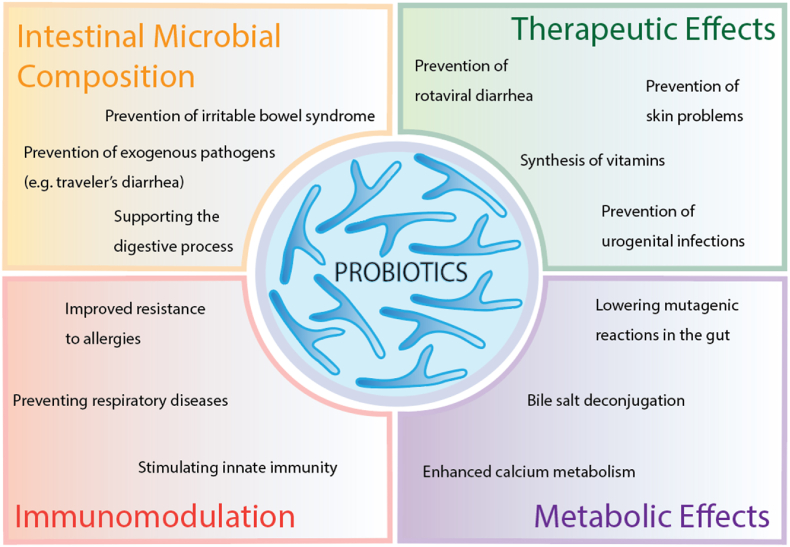
The term “probiotic” is derived from the Latin word pro meaning “for,” and the Greek word βιωτικός, meaning “of or pertaining to life” (Liddell and Robert, 1889) and can be defined as “live microorganisms (bacteria and yeast) which, when administered in adequate amounts, confer a health benefit to the host” (FAO/WHO, 2001). Probiotics were first associated with maintaining health over a century ago when Metchnikoff suggested that the consumption of fermented milk products was responsible for the long life of Bulgarian peasants (Metchnikoff, 1908). Over the decades, evidence pointing to the beneficial effects of probiotics on human health has continued to expand. The increased use of antibiotics, radiation, and immunosuppressive treatments has been linked to changes in the composition of gut flora (Gupta and Garg, 2009). The introduction of beneficial bacterial species into the gastrointestinal tract represents a strategy to revive the microbial equilibrium and prevent disease (Gupta and Garg, 2009). In addition to modulating gut functionality, probiotics have been associated with boosting immunity and brain function, reducing serum cholesterol, suppressing endogenous and exogenous pathogens, reducing symptoms of lactose intolerance, decreasing the prevalence of allergies, and reducing the risks of certain cancers (Parvez et al., 2006; Kechagia et al., 2013; Rokka and Rantamäki, 2010). Fig. 1 summarizes several health benefits of probiotic consumption. The attractive properties of probiotics have led to an increased interest in the development of probiotic formulations, including novel functional foods, which can confer significant health benefits to consumers. The most commonly used probiotics include strains of the Bifidobacterium and Lactobacillus genera (Vlasova et al., 2016). Some fungal strains belonging to Saccharomyces have also been used in probiotic formulations (Ansari et al., 2021). Probiotics are traditionally added to dairy products, such as cheeses (Sharifi et al., 2021), yogurts (Afzaal et al., 2019), and milk (Abesinghe et al., 2020), but recently non-dairy products, such as meats, bread, juices, and chocolates have also been explored as probiotic delivery matrices (Aspri et al., 2020). However, despite the growing market for probiotic formulations, maintaining the viability of these products through processing, storage, and passage through the gastrointestinal tract remains a challenge (Rajam and Subramanian, 2022).
Fig. 1.
Beneficial health effects of probiotic bacteria on human health. Data from (Markowiak and Ślizewska, 2017).
Various microencapsulation techniques have been explored in the food industry as methods to protect probiotics from harsh conditions. In particular, Pickering emulsions have emerged as an attractive strategy for encapsulation, with various reviews published in recent years. Pickering emulsion stabilization by hybrid nanoparticles (Tavasoli et al., 2022) and protein nanoparticles (Zhang et al., 2021) has been reviewed, as well as the potential of Pickering multiple emulsions (Klojdová and Stathopoulos, 2022) and high internal-phase Pickering emulsions (Abdullah et al., 2020) in food applications. However, to the best of our knowledge, no review article focusing on Pickering emulsions for probiotic encapsulation has been reported. Furthermore, probiotic encapsulation by emulsification for animal and fish feeds has not been extensively explored. In this review, probiotic encapsulation by emulsification techniques is discussed in detail, with an emphasis on Pickering emulsions and applications for animals.
2. Challenges faced in probiotic delivery
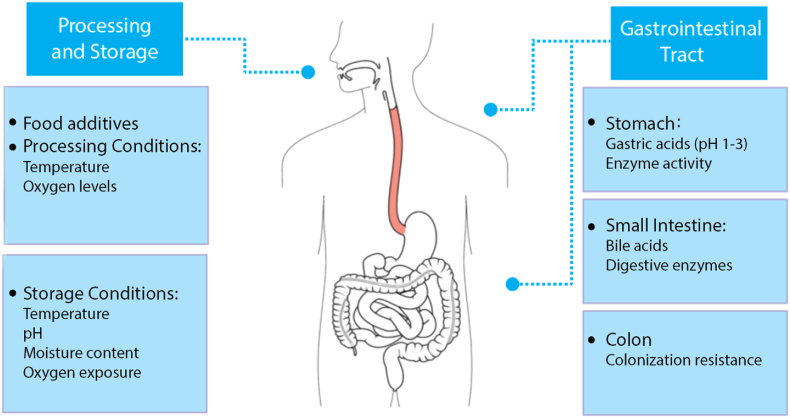
Bacterial viability refers to the ability of a cell to grow and be able to generate a colony of cells under the required environmental conditions (Wilkinson, 2018). Sufficiently large amounts of probiotic bacteria must be able to pass through the gastrointestinal tract (GIT) and colonize the colon in order to confer health benefits to host organisms. To ensure this, it is commonly accepted that probiotic products should contain a minimum of 106–107 CFU/mL or CFU/g of viable cells to generate beneficial health effects (Liu et al., 2018). Often however, the addition of probiotic cells directly to foods significantly reduces the cell viability, causing it to drop below the recommended levels and reduces their efficacy. The composition and distinct physicochemical characteristics of food matrices, such as osmotic pressure, oxygen levels, pH and food preservatives or additives can adversely affect bacterial cell growth and metabolism, thus reducing the stability and functionality of the incorporated probiotics till the food is consumed (Saarela et al., 2006). Probiotic viability is also impacted during processing, storage, and GIT transit as summarized in Fig. 2. Although microencapsulation of probiotics aims to address viability issues, it is important to consider all factors affecting probiotic survival while developing probiotic formulations so that any harmful agents or conditions can be avoided.
Fig. 2.
Factors affecting the viability of probiotics. Adapted from (Terpou et al., 2019).
2.1. Food processing and storage
Probiotics are exposed to various environmental stresses during food production, storage, and transport, causing a loss of viable cells before they are even ingested. High levels of oxygen exposure and relative humidity are detrimental to many different probiotic strains (Tripathi and Giri, 2014). These microorganisms are primarily anaerobic or microaerophilic, and high oxygen levels during the fermentation process or storage period can severely compromise their viability (Donaldson et al., 2015). Oxygen is directly toxic to some strains and may also oxidize other components such as fats, producing free radicals, which are toxic to probiotic cells (Korbekandi et al., 2011). Certain cultures also produce peroxides such as hydrogen peroxide when exposed to oxygen, which are toxic and cause cell death when accumulated in large amounts (Talwalkar et al., 2004). The moisture content of probiotic products can also influence the shelf-life of live bacteria. Exposure to moisture activates the bacteria and triggers the early onset of degradation since activation is intended to occur after ingestion by the consumer (Zhao et al., 2018). Studies have shown that increasing the relative humidity of the storage environment can increase the rate where viability is lost (Ying et al., 2010).
Other factors influencing the viability of probiotics include temperature, pH and the presence of food additives. Bacteria present in the human gut have evolved to survive at human body temperature, and therefore, are sensitive to elevated temperatures encountered during food processing steps, such as pasteurization, sterilization, and cooking (Anal and Singh, 2007). Furthermore, the viability during storage is reduced at elevated temperatures (Gardiner et al., 2000). Optimal storage temperatures tend to depend on the strain as well as the presence of food additives. For Bifidobacteria, Bruno and Shah (2003) found that viability was maximized when stored at −18 °C, and storage at 20 °C led to a significant loss of viable cells (Bruno and Shah, 2003). The survival of probiotics during storage is also impacted by pH (Perricone et al., 2015), a particular concern when added to beverages such as fruit juices. Compatibility with other food ingredients also plays a role in probiotic survival. Commonly used additives such as sugars and aromatic compounds can have drastic effects on the growth and viability of some probiotic strains (Vinderola et al., 2002).
2.2. Harsh conditions in the GIT
After ingestion, probiotics are exposed to various harsh environmental conditions in the upper GIT, particularly in the stomach and small intestine, which further impact their viability. Commonly used strains such as Lactobacillus and Bifidobacterium have been found to be particularly sensitive to harsh conditions encountered in the human gut, such as gastric acids and bile salts, which limit their capacity to improve human health (Cook et al., 2012). A previous study assessing the viability of commercial probiotics during GIT transit found that formulations saw a 106-fold reduction in viable bacterial cells after 5 min of incubation in gastric fluids (Dodoo et al., 2017). As a result, by the time they reach the colon, there are insufficient viable cells remaining to colonize the mucosal surfaces.
Probiotics are generally optimized to survive at pH conditions within the colon, which is typically around 6 to 7. However, before reaching the colon, they pass through the stomach, where they are exposed to acidic gastric fluid, which has a pH ranging from 1 to 3. This environment can be extremely lethal to probiotics, causing a reduction of the cytoplasmic pH. The high levels of H+ ions causes a decrease in the activity of glycolytic enzymes and reduces the activity of the F1F0-ATPase proton pumps, which are responsible for their survival in acidic environments (Cotter and Hill, 2003). Additionally, enzyme activity, mechanical churning, and high ionic strength in the stomach can have adverse effects on viability (Sarao and Arora, 2017). After passing through the stomach, probiotic bacteria enter the small intestine, where they encounter bile acids and digestive enzymes, including lipases, proteases, and amylases (Han et al., 2021). These conditions can disrupt cell membranes and cause DNA damage, further reducing probiotic viability (Hamner et al., 2013). A previous study has shown that the viability of Lactobacillus was significantly reduced in simulated intestinal fluid (Yao et al., 2017). Furthermore, once probiotics reach the colon, they must compete with the bacteria in the colon for nutrients and adhesion sites (Zmora et al., 2018). Therefore, it is essential that there are enough viable probiotic cells, as a large number is excreted due to the colonization resistance.
3. Current strategies for probiotic encapsulation and delivery
Improving probiotic stability and colon-adhesion continues to be a challenge for commercial probiotic supplements since their viability may be lowered below the desired levels during manufacturing, storage, and digestion. Therefore, probiotics must be encapsulated in protective systems that shield the probiotic cells from external conditions and enhances bacterial vitality (Reque and Brandelli, 2021). Encapsulation may also increase the efficacy of probiotics by controlling their release at the site of action in the intestine.
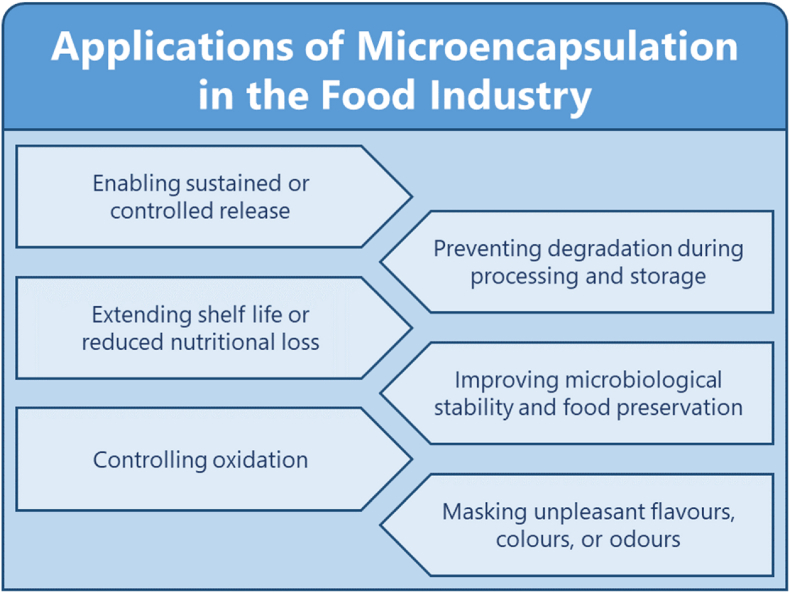
Encapsulation is a physicochemical or mechanical technique to protect one substance within one or more coating materials, with the produced particles ranging from a few nanometers (nm) to a few millimeters (mm) in size (Khosravi Zanjani et al., 2014). Encapsulation techniques have been designed to protect encapsulated materials from environmental changes, such as pH, oxygen, heat, or UV radiation. Encapsulation systems can also be functionalized to mask unpleasant flavours or odours; impart stimuli-responsiveness; and control targeted, sustained, and burst release. The encapsulation of probiotics, vitamin C, insulin, and other active ingredients is an established procedure in the food industry (Iravani et al., 2015) (Fig. 3).
Fig. 3.
Important applications of microencapsulation. Reproduced from (Iravani et al., 2015; Gómez et al., 2018).
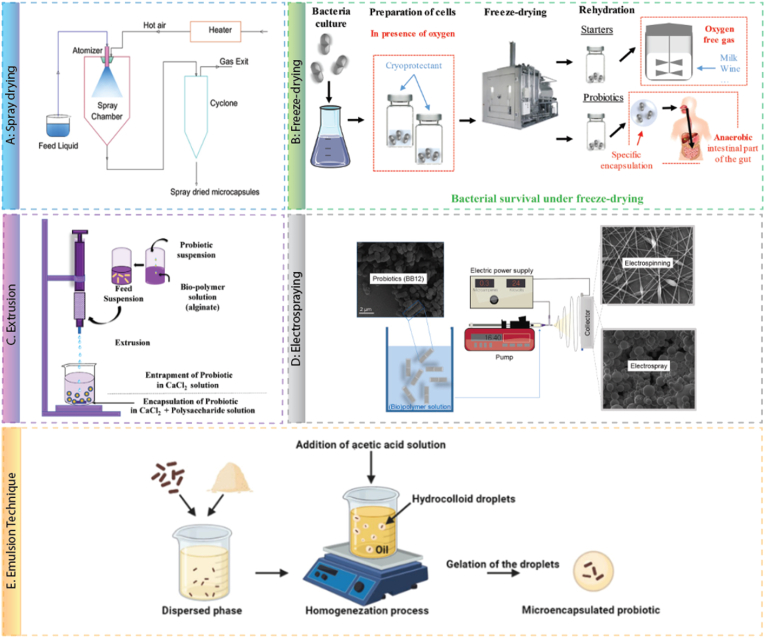
Various encapsulation techniques, such as spray drying, emulsification, extrusion, electrospraying, and freeze-drying, are currently used to produce probiotic formulations (Rokka and Rantamäki, 2010). The advantages and disadvantages of each of these techniques are presented in Table 1. Prior to implementation, it is necessary to select the appropriate encapsulation strategy based on the probiotic species, as different probiotic species have different levels of sensitivity to environmental conditions (Šipailienė and Petraitytė, 2018). However, most reports tend to favour gentle and non-aggressive operations as well as non-toxic solvents to maintain viability during the encapsulation process.
Table 1.
Pros and cons of the different encapsulation technologies for probiotics. Adapted from (Mendes and Chronakis, 2021).
| Spray-drying | Freeze-drying | Extrusion | Electrospraying | Emulsification | |
|---|---|---|---|---|---|
| Encapsulation Efficiency | * | - | ** | *** | * |
| Cell viability | ** | ** | * | ** | ** |
| Temperature regularity | - | - | - | *** | ** |
| Cost | ** | - | * | * | * |
| Material flexibility | * | *** | - | *** | - |
| Scalability | *** | *** | *** | * | *** |
***: Best; **: Good; *: Fair; -: Worst.
3.1. Spray drying
Among the various encapsulation processes, spray-drying (Fig. 4A) is one of the most common methods of microencapsulation used in the food, chemical, pharmaceutical, and cosmetic industries. This method is suitable for continuous mass production and for obtaining a uniform particle size distribution (Pupa et al., 2021). The principle of spray drying technology is that a polymer solution containing an active material, such as a suspension, solution, or emulsion is sprayed into a drying chamber, and the solvent is rapidly evaporated by the flow of heated gas and solidified into nano- or micro-sized particles (Piñón-Balderrama et al., 2020). Tuning the operating conditions and the design of the equipment are critical to producing a powder with the desired characteristics and good probiotic viability. Important parameters include flow pattern, inlet temperature, flow rates, and carrier agent characteristics. To minimize reduction in probiotic viability, a co-current flow of sprayed material and heated carrier is preferred as it ensures the dried particles are exposed to the least amount of heat (Sehrawat et al., 2022). Despite the benefits of spray drying, the high temperatures required to facilitate water removal diminish the viability and activity of probiotics in the final product, which also impacts the storage stability (Gbassi and Vandamme, 2012).
Fig. 4.
Schematic representations of probiotic encapsulation technologies. A) Spray drying. Reproduced from (Rajam and Subramanian, 2022), B) Freeze-drying. Reproduced from (Bodzen et al., 2020), C) Extrusion. Reproduced from (Gurram et al., 2021), D) Electrospraying. Reproduced from (Mendes and Chronakis, 2021), and E) Emulsification. Reproduced from (Camelo-Silva et al., 2022).
3.2. Freeze-drying
Freeze drying (Fig. 4B) is ideal for dehydrating heat-sensitive substances since it removes moisture from a frozen solution by sublimation at reduced pressure. This process is suitable for the long-term preservation of probiotics, which is convenient for the distribution and storage of probiotic formulations. There are three steps in the freeze-drying process. (1) Very low temperature (between −70 °C and −80 °C) is used to freeze the solution. (2) Primary drying is used to partially dry the solution by reducing pressure, followed by (3) secondary drying, which removes the remaining water (Yadegari et al., 2017). During these three steps, the probiotics are exposed to a variety of stress factors, especially dehydration, which negatively impacts the viability of the probiotics (Santivarangkna et al., 2011). To address this, cryoprotectants need to be used to prevent cold damage and increase the probiotics' survivability. Sugars, polymers, and proteins are commonly utilized to shield against freeze-drying and storage conditions (Cui et al., 2018; Shu et al., 2018; Romyasamit et al., 2021). Additionally, this procedure is time-consuming and expensive; compared to spray drying, and it generally costs about six times more to remove 1 kg of water (Barbosa et al., 2015).
3.3. Extrusion
The extrusion method is known to display excellent probiotic survival rates of 85–90% in gastric acid and bile conditions compared to other methods (Krasaekoopt et al., 2003). The technique avoids high temperatures during processing, allowing a higher survival rate of probiotics. In the extrusion method, a hydrocolloid material such as alginate, carrageenan, gelatin, or chitosan is typically used as a coating material. In short, the hydrocolloid solution is mixed with probiotics, passed through a syringe (lab scale) or extruder (pilot scale), and discharged into a gelling solution containing a multivalent cation (e.g., CaCl2) to prepare a hard capsule, as depicted in Fig. 4C. This method is simple, low-cost, and promotes high levels of cell retention. However, it has been pointed out that the size of the capsules is limited by the diameter of the nozzle and it is challenging to scale up the process. Electrospraying, jet cutting, vibrating-nozzle techniques, and spinning disc atomization are some methods that may be integrated to improve the scalability and particle size limitations of extruded microcapsules (Sultana et al., 2022). The resulting gels were also shown to be sensitive to extreme pH conditions, though multilayer coatings have been explored to address this issue (Khosravi Zanjani et al., 2014).
3.4. Electrospraying
Electrospraying (Fig. 4D) is a technique used to produce nano- or microparticles that involves injecting a sufficiently electrically conductive and viscous polymer solution into a capillary, then applying an electrostatic force to the solution at the tip of the needle. The electrically charged droplets are drawn towards a metallic collector via electrstatic attraction (Phuong Ta et al., 2021). Because of the simple structure of the device, electrospraying is straightforward to operate under moderate conditions and can generate particles with a monodisperse distribution (Premjit and Mitra, 2021). Research is now being conducted to produce drug and probiotic-containing particles using electrospraying since it does not require high temperatures, pressures, or harsh chemical conditions (Zaeim et al., 2018). In a recent study, Lactobacillus Plantarum was encapsulated within alginate particles and coated with chitosan via electrospraying. The fabricated particles demonstrated good mucosal retention, which allowed the probiotics to remain longer in the digestive tract and interact with the microbiome (Phuong Ta et al., 2021). However, electrospraying may produce small quantities of particles, and occasionally cross-linking chemicals are required. Furthermore, the solvent in which the polymer is dissolved is not entirely removed, which may result in improper particle formation (Tapia-Hernández et al., 2015).
3.5. Emulsification
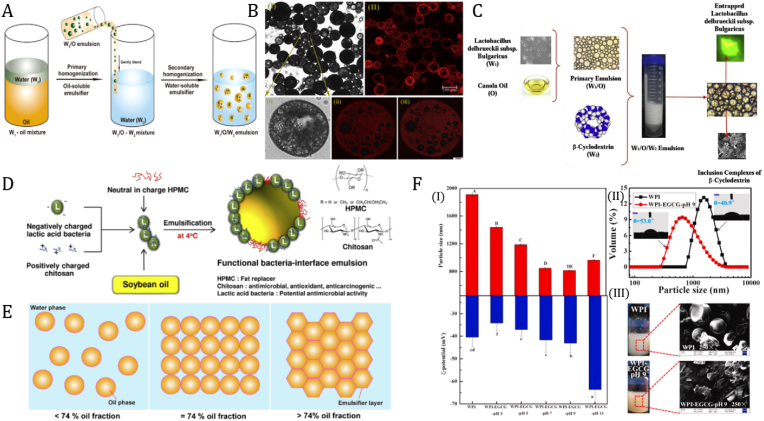
An emulsion is an example of a colloidal system with at least two immiscible liquids, such as oil and water, with one dispersing in the other (Chappat, 1994). Emulsions can be utilized to encapsulate probiotic cells, as shown in Fig. 4E. The two types of simple emulsions commonly used are (a) oil-in-water (O/W) and (b) water-in-oil (W/O) (Bai et al., 2017). Emulsions can be produced through physical (mechanical) and chemical processes (interfacial chemistry) (Karbstein and Schubert, 1995; Zafeiri et al., 2017). Chemical methods are dependent on the hydrophilic-hydrophobic balance (HLB) value of the emulsifier and the concentration of the emulsifier (Dominguez and Abreu, 2016). Physical methods utilize an emulsifying device with high shear or high-pressure capabilities. A homogenizing mixer, such as a high-speed or ultrasonic homogenizer, is used to prepare small particles, while a microfluidizer can be used to produce nano- and microemulsions. Often, a combination of physical and chemical methods are used to prepare these emulsions.
When a W/O emulsion is emulsified in water, a W/O/W emulsion can be produced (Fig. 6A). The term “multiple emulsion” or “double emulsion” is widely used to refer to this type of emulsion. A W/O/W emulsion can typically be produced in two steps. First, a stable W/O emulsion is prepared under high shear, which is then re-dispersed in an aqueous solution containing a hydrophilic emulsifier under lower shear conditions. When a high shear is applied in the second step, coalescence can occur due to the collision of water droplets and the rupture of the oil phase (Dominguez and Abreu, 2016).
Fig. 6.
A) Schematic diagram showing the production of multiple emulsions (W1/O/W2) using the two-step emulsification procedure. Reproduced from (Bai et al., 2021). B) Confocal laser scanning micrographs of LA encapsulated in PDE after 14 days' storage under bright and fluorescence fields (I-II); Images collected under high-magnification marked with an arrow (i-iii), the images showed the oil phase in red (stained with Nile red and excited at 549 nm) and LA in blue (stained with STYO-9 and excited at 483 nm). Reproduced from (Wang et al., 2020). C) Schematic showing the encapsulation of L. dellbrueckii in multiple Pickering emulsions stabilized by β-cyclodextrin inclusion complexes. Reproduced from (Eslami et al., 2017). D) Preparation scheme of CL- and CL/HPMC-stabilized emulsion at different concentrations of HPMC. Reproduced from (Rattanaburi et al., 2019). E) Schematic showing (not to scale) the oil phase structure of HIPEs at different internal phase volume fractions. Reproduced from (Bai et al., 2021). F) (I) Particle sizes and ζ-potentials of WPI and WPI-EGCG covalent conjugates. (II) Particle size distributions, three-phase contact angles (θ), and (III) SEM images of WPI and WPI-EGCG-pH 9 covalent conjugate freeze-dried samples. Reproduced from (Qin et al., 2021).
The types of emulsion systems used in probiotic encapsulation can be broadly divided into two categories based on the stabilizing surfactants used. There are conventional emulsions stabilized with molecular (or conventional) surfactants and particle-stabilized Pickering emulsions, which will be reviewed in Section 3.4. Various conventional surfactants have been used to encapsulate probiotics for oral delivery in combination with other novel approaches such as nanogels and structured emulsion gels. For instance, Ding et al. (2022) generated W/O/W emulsions with the inner aqueous phase loaded with probiotic cells and various protectant compounds. The oil phase consisted of soybean oil and polyglycerol polyricinoleate (PGPR), a common food-safe molecular surfactant, stabilizing both the W1/O and O/W2 interfaces (Ding et al., 2022; Wu et al., 2021a). The outer aqueous phase was additionally stabilized by a nanogel matrix of carboxymethyl konjac glucomannan-chitosan (CMKGM-CS), allowing for reduced droplet diameter and higher emulsified phase volume. The nanogel W/O/W emulsions were additionally encapsulated in alginate hydrogel beads, further improving protection, and providing a controlled release mechanism at the target site through pH-responsive behavior. In another set of studies, monoglyceride emulsion gels were investigated in the stabilization of O/W emulsions for food systems where these types of emulsions occur (Melchior et al., 2021, 2022; Marino et al., 2017). Contrary to W/O and W/O/W emulsions that are widely reported in the literature, the probiotic cells were present in the continuous phase, where they are more vulnerable to processing and digestive stresses. Despite this, gel complexes formed by oil emulsion droplets, crystalline monoglyceride lamellae, and milk proteins formed a dense structure protecting the probiotic cells under processing, storage, and gastric conditions.
A review of conventional emulsification techniques for probiotic encapsulation in papers published prior to January 2021 by Camelo-Silva et al. describes the state of the field and the established emulsification methods commonly used (Camelo-Silva et al., 2022). It also highlights two emerging technologies for future consideration; membrane and microfluidic emulsification. Membrane emulsification involves the introduction of the dispersed phase of the target emulsion into the continuous phase through a porous membrane. Each pore can produce separate micro- or nanosized droplets, resulting in highly monodisperse emulsions through a relatively high throughput continuous process when the membrane and process conditions can be precisely controlled (Camelo-Silva et al., 2022). As of June 2022, no additional papers using the membrane emulsification process for probiotic emulsification have been published.
Microfluidic emulsification uses the intersection of microchannels in various configurations to generate droplets of the dispersed phase in a continuous phase. Common configurations include the T-junction, flow-focusing, and co-flow geometries (Shembekar et al., 2016). Recently, Quintana et al., 2021a, Quintana et al., 2021b investigated the application of flow-focusing microfluidic chips in the generation of lecithin stabilized W/O emulsions for the encapsulation of the probiotic Lactobacillus plantarum CIDCA 83114 in okara oil, a soy by-product (Quintana et al., 2021; Quintana et al., 2021). Okara oil, a soy byproduct, was used as the continuous phase and a block copolymer containing poly(acrylic acid), poly(ethylene oxide), and poly(propylene oxide) (PPP12) with a demonstrated resistance to gastrointestinal decomposition was used in the dispersed phase. They found that the microfluidic method could be used to reliably generate a steady flow of uniform spherical emulsion droplets, and that larger diameter microchannels were advantageous due to fewer issues with flow obstruction and greater flow rates.
3.6. Pickering emulsions
Pickering emulsions have recently emerged as an excellent strategy to stabilize emulsion systems. Unlike conventional emulsions, Pickering emulsions are attractive in food research since they are free of low molecular weight surfactants and the irreversible adsorption of solid particles at the oil-water interface can produce safe, stable, and environmentally friendly systems (Baek et al., 2019). Compared to conventional surfactant-stabilized emulsions, Pickering emulsions exhibit enhanced stability and lower toxicity (when biocompatible, food-grade particles are used) (Anjali and Basavaraj, 2018).
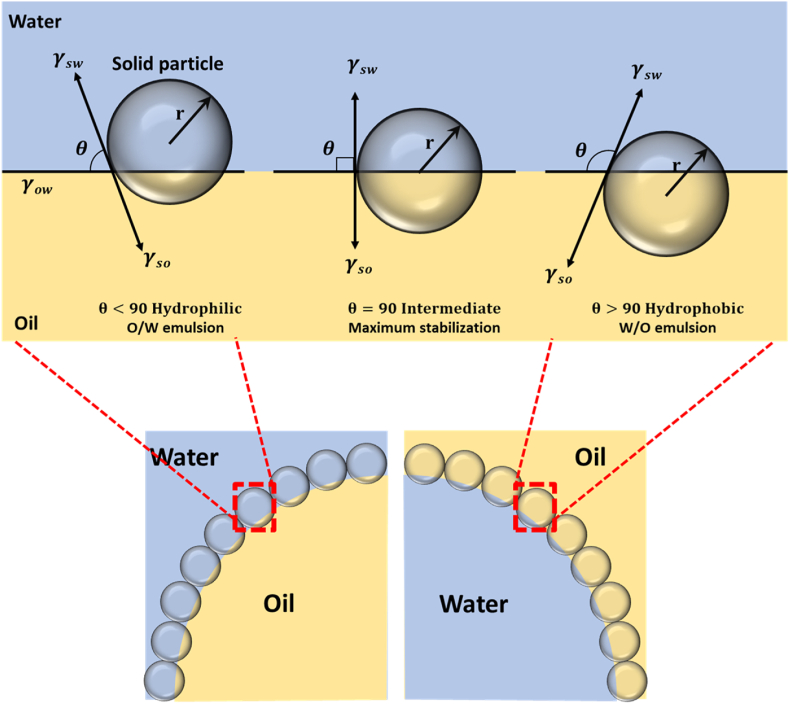
Pickering emulsions are stabilized by adsorbing nano- or micro-sized solid particles at the oil-water interface to prevent coalescence of the emulsion droplets. The large interfacial adsorption energies of Pickering emulsifiers allow for irreversible adsorption, unlike conventional emulsifiers (Fujisawa et al., 2017). The particles must have a contact angle which is measured at a three-phase boundary (θ) that has sufficient interface adsorption capacities (Chen et al., 2020) and are selected by emulsion types (Gonzalez Ortiz et al., 2020) (Fig. 5). Hydrophilic particles have a contact angle, measured at the water side of the interface, smaller than 90° and a large part of the particle surface is present on the aqueous phase. On the other hand, the lipophilic particles have a contact angle greater than 90°, and a large part of the particle surface is present on the oil phase (Rayner et al., 2014). Therefore, hydrophilic particles tend to form an O/W emulsion, and lipophilic particles tend to form a W/O emulsion. A solid particle with a contact angle close to 90° forms the most stable emulsion with the highest irreversible adsorption at the interface between water and oil.
Fig. 5.
Solid particle wetting and contact angle leading to the formation of O/W or W/O emulsion. Adapted from (Saffarionpour, 2020).
When spherical particles are adsorbed at the interface of two fluids (e.g., oil ( and water () the attachment energy can be described by the equations below.
| (1) |
| (2) |
The energy of adsorption E represents the energy required to remove a particle of radius from an oil and water interface with an interfacial tension γow. Equations (1), (2) describe the adsorption energy to the interface when the particles exist in the water or oil phase in the initial state, respectively. The wettability of particles at the fluid interface can be characterized by the three-phase contact angle (θc) at the three-phase (particle-oil-water) interface. For example, when a neutrally wetting (θc = 90°) particle with a radius of 1 mm is adsorbed at the oil-water interface, its energy is approximately 108 kBT, which corresponds to a very large energy barrier. Here, kBT is the thermal energy of one particle, kB is the Boltzmann's constant, and T is the temperature. The adsorption energy indicates that the particles adsorbed to the interface are irreversible, and the particles cannot be spontaneously desorbed from the interface unless a significant amount of energy is supplied (Binks and Fletcher, 2001; Binks, 2002).
The Pickering emulsion system can be used to encapsulate probiotics to enhance bacterial survivability in liquid form. For instance, Wang et al. (2020) developed a Pickering double emulsion (PDE) of the W1/O/W2 type for the purpose of encapsulating Lactobacillus acidophilus for targeted delivery to the colon (Wang et al., 2020). The unique structure of these emulsions makes them attractive for food applications as they can be tuned to possess triggered release as well as flavour masking. The author improved on the previous approach of a double emulsion stabilized with a pair of low-molecular surfactants, which is not stable against droplet coalescence or aggregation, to demonstrate outstanding stability with the exterior interface layer comprising bacteria cellulose (BC) nanoparticles. The double emulsion was composed of sodium alginate with CaCl2 (W1), soybean oil containing polyglycerol polyricinoleate (O), and bacteria cellulose solution (W2). Confocal laser scanning microscopy was used to investigate the microstructure of the PDE that had the greatest stability after 14 days of storage at 4 °C (Fig. 6B). Under simulated gastrointestinal conditions, around 84.24% of encapsulated cells remained alive while free cells exhibited greater loss of viability, owing to their poor tolerance to low pH conditions. In addition, they found that the colon-adhesion efficacy of probiotics-loaded Pickering emulsions after GIT transit was 43.27%, which was three times higher than unencapsulated probiotic cells. This is most likely the result of the presence of micelles and sodium alginate, which improve the rate of adherence of probiotics in the colon. In another study, Eslami et al. (2017) conducted a comprehensive investigation on multiple emulsion formation and stabilization using β-cyclodextrin (β-CD) inclusion complexes for Lactobacillus dellbrueckii (Eslami et al., 2017). The multiple emulsion (W1/O/W2) is composed of the primary emulsion (W1/O), which has a probiotic containing an aqueous phase and oil with span-80, which is introduced to the outer aqeous β-CD or Tween-80 solution (Fig. 6C). The authors discovered that β-CD was more effective than Tween 80 surfactant at stabilizing microorganisms. Even though Tween-80 stabilized double emulsions with long-term stability, the high survival of cells using β-CD demonstrated that solid particles adsorbed at the oil-water interface were a good option for probiotic encapsulation. Rattanaburi et al. (2019) prepared Pickering emulsions stabilized by hydroxypropyl methylcellulose (HPMC) as a typical anionic polymer with chitosan and Lactococcus lactis IO-1 (L. lactis IO-1), as shown in Fig. 6D. (Rattanaburi et al., 2019). The Pickering emulsions possessed the health benefits of chitosan with the antibacterial activity of bacteriocin, which was generated by L. lactis. The self-association of positively charged chitosan with negatively charged cells of the L. lactis altered the surface properties of the bacteria, allowing it to be used as a soft hydrophobic material suited for Pickering emulsification. The network of lactic acid and chitosan did not reduce the interfacial tension but was adsorbed at the oil-water interface. Hydroxypropyl cellulose was able to aid in the stability of the emulsion by filling the gaps between the chitosan and lactic acid bacteria network present at the oil interface, and acted as an effective steric barrier at the oil-water interface (Rattanaburi et al., 2019). Recent research has demonstrated that Lactobacillus plantarum was successfully encapsulated using whey protein isolate (WPI)/(−)-epigallocatechin-3-gallate (EGCG) covalent conjugate nanoparticles as an emulsifier within the Pickering high internal phase emulsions to enhance its durability at storage and gastrointestinal conditions. The term “high internal phase emulsions,” or “HIPEs,” refers to emulsions that have a highly viscous or gel-like network, and are typically formed by a minimal oil fraction (φ) of 0.74 (Fig. 6E). These emulsions have been utilized for purposes including the protection of bioactive substances, the delivery of drugs, and the creation of porous polymer materials. In the study, WPI-EGCG covalent conjugates were formed into nanoparticles with amphiphilic wettability (53.0°) using a free-radical induction procedure at a pH of 9 (Fig. 6F). These nanoparticles had an average size of 814 nm and could effectively stabilize Pickering emulsions. Additionally, they had an inhibitory influence on digestive enzymes and improved their antioxidant properties, which resulted in a slight reduction of probiotic activity compared to free probiotics in the stomach and colon (Qin et al., 2021). A summary of recent work using Pickering emulsions for the encapsulation of probiotics in food applications is summarized in Table 2.
Table 2.
Summary of probiotic microencapsulation using Pickering emulsions and the Pickering emulsifiers used.
| Emulsion Type | Probiotic Identity | Pickering Emulsifier | Reference |
|---|---|---|---|
| O/W | None tested | WPI-phytosterol nanoparticles | Zhou et al. (2022) |
| Lactobacillus casei K17 | Phytoglycogen nanoparticles | Wang & Chen (2021) | |
| Lactobacillus lactis IO-1 | HPMC-chitosan-L. lactis conjugate | Rattanaburi et al. (2019) | |
| O/W HIPE | Lactobacillus plantarum | WPI-EGCG | Qin et al. (2021) |
| Lactobacillus rhamnosus GG | β-lactoglobulin-propylene glycol alginate composite nanoparticles | Su et al. (2021) | |
| W/O | Saccharomyces cerevisiae | Amidine latex particles | Hamad (2012) |
| W/Oa | None tested | Butyl methacrylate derivatives | Sabatini et al. (2021) |
| Foam | Lactobacillus acidophilus | Chitosan coated L. acidophilus | (Yucel Falco et al., 2017) |
| W/O/W | Lactobacillus acidophilus | BC Nanoparticle | Wang et al. (2020) |
| Lactobacillus delbrueckii | β-cyclodextrin | Eslami et al. (2017) | |
| Lactobacillus salivarius NRRL B-30514 | Sugar beet pectin | Zhang et al. (2016) |
W/O Pickering emulsion used as an intermediate synthesis state for the production of polymer microcapsules.
An important factor to consider when discussing the benefits of utilizing Pickering emulsions for probiotic encapsulation is the improved oxygen resistance. For the avoidance of oxygen (O2) diffusion and migration, Pickering emulsion particle-based film creation for food has been advanced in recent years. Typically, the majority of food oxidation events including lipid oxidation are caused by the presence of O2 and are associated with unpleasant tastes, nutrient loss, rancidity, and deterioration (Trinh et al., 2022). Edible coatings are intended to have limited moisture and oxygen permeability, preserve or improve visual quality, and give mechanical rigidity for food applications. For instance, the incorporation of polylactic acid (PLA) (Zhu et al., 2018); ZnO nanoparticle (Wu et al., 2021b); sodium starch octenyl succinate (SSOS) (Sun et al., 2020); zein/chitosan colloidal particle (ZCCPEs) (Shi et al., 2016); and konjac glucomannan (KGM) (Liu et al., 2021) based Pickering emulsion bi- or multi-layer films have been developed and studied. A dense and rigid solid particles-based network was observed to partition to the interface of the droplet in the Pickering emulsion. The distinctive interfacial structure may perform as a natural barrier to O2, hence decreasing oxygen permeability.
Additionally, there is strong evidence that the liquid-liquid interfacial zone plays a very important role in oxidative stability of multiphase systems (Berton-Carabin et al., 2014). In their review of the stabilization of lipids in O/W emulsions by emulsifiers, Berton-Carabin et al. (2014) suggested that more controlled studies are necessary to derive the necessary conclusions due to the complexity of the process. However, studies suggested that both conventional and Pickering emulsifiers played a role in the inhibition of lipid oxidation. Antioxidants are another effective tool for reducing oxidation in emulsions and Laguerre et al. (2015) indicated that the location of the antioxidants in the multiphase system played an important role in their efficacy. Especially, antioxidant activity appeared to increase when located at the interfaces, hence the adsorption of antioxidant-modified Pickering emulsifiers to interfaces could be an effective solution (Laguerre et al., 2015).
4. Encapsulated probiotics for various applications
Previous sections focus on the encapsulation of probiotics for human applications. However, the beneficial effects of probiotics are not restricted to humans. Probiotic bacteria have also been shown to enhance the health of animal pets, livestock, and farmed fish, prompting research on novel encapsulation techniques for application in these fields. This section reviews the current status of encapsulated probiotics for farmed animals, pets, and fish, highlighting recent publications which have focused on emulsification technology.
4.1. Farmed animals and pets
Probiotics play an important role in the health of domesticated animals, and have found their way into livestock, poultry and pet feed. The use of probiotics in poultry and livestock has had positive impacts on growth performance, feed utilization, disease resistance, and immunostimulation (Gopal and Dhanasekaran, 2021). Similarly, for pets such as cats and dogs, probiotics have been associated with reduced stress, immune system modulation, and protection against infections caused by intestinal pathogens (Lee et al., 2022). However, issues pertaining to loss of viability due to storage conditions and GIT transit apply to animal probiotic formulations too, prompting researchers to investigate the potential of microencapsulation techniques in these fields as well. Researchers have explored the use of spray drying (Pupa et al., 2021) and extrusion techniques (Atia et al., 2018) to encapsulate probiotics for application in poultry and livestock feeds. Pupa et al. (2021) developed a double-encapsulated coating using 1.5% alginate and 0.5% chitosan by extrusion, emulsion, and spray-drying methods for the encapsulation of Lactobacillus plantarum strains 31F, 25F, 22F, Pediococcus pentosaceus 77F, and P. acidilactici 72N (Pupa et al., 2021). They found that all three methods resulted in high encapsulation efficiencies, and spray dried particles had the smallest diameter, which enabled easy mixing with livestock feed. The encapsulated probiotics were stable after 6 months storage at room temperature and retained their acid-bile tolerance and antibacterial capabilities. Atia et al. (2018) developed a probiotic formula consisting of alginate and inulin beads for potential application as growth promoters for swine (Atia et al., 2018). Pediococcus acidilactici UL5, Lactobacillus reuteri, and Lactobacillus salivarius were encapsulated to study the release and behaviour through the GIT using in vitro models. Scanning electron and fluorescence microscopic results showed that beads containing 5% inulin were the most stable in the stomach and small intestine. The beads were degraded in 3 h when incubated in fermented media designed to mimic the colon. Therefore, this study showed how alginate-inulin beads protected the probiotics in the upper GIT and exhibited controlled release in the colon.
Although, there is limited literature available on applications in pet feed, an interesting study by Baroncello et al. (2020) demonstrated the potential for encapsulated probiotics in this field. Saccharomyces boulardii was encapsulated in sodium alginate microcapsules coated with chitosan using an extrusion technique (Baroncello et al., 2020). The microcapsules were incorporated into a meat snack for cats and dogs. Results of the study showed that the snack had a 120-day shelf life and an acceptability index of 77.8%, with beneficial effects when added to pet snacks with high levels of protein.
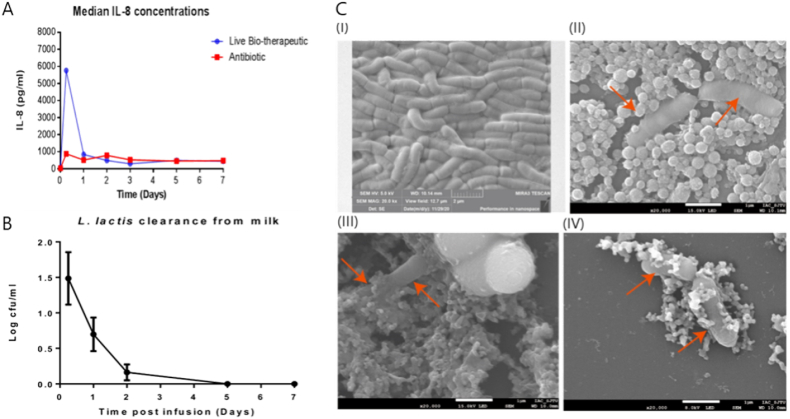
In recent years, there have also been publications utilizing emulsions and Pickering emulsions for these applications. Kitching et al. (2019) developed paraffin-based emulsions to encapsulate Lactococcus lactis DPC3147 to treat bovine mastitis in cows (Kitching et al., 2019). Results showed that the encapsulated probiotics had similar efficacy to commercial antibiotics, with a robust immune response against bovine mastitis pathogens being triggered by the administration of L. lactis DPC3147 (Fig. 7A). Additionally, L. lactis cells were cleared from treated cows’ milk within 5 days (Fig. 7B), reducing the amount of milk withheld from the market due to treatment. Wang and Chen (2021) investigated the effects of adding 10% Pickering emulsions to novel nano-protectants for Lactobacillus casei K17 (Wang and Chen, 2021). The morphologies of the different protectants were compared and shown in Fig. 7C, where it is evident that protectants containing Pickering emulsion (IV) produced a denser covering layer. The formulation containing Pickering emulsions was most effective at protecting probiotics during feed storage, freeze-drying, and in simulated GIT environments.
Fig. 7.
A) Quantification of IL-8 concentrations in milk samples from cows with bovine mastitis over time after treatment with L. lactis encapsulated in paraffin-based emulsion and commercial antibiotic, IL-8 is used as a biomarker for immune response. Reproduced from (Kitching et al., 2019). B) Clearance of L. lactis from milk samples occurs quickly, with no culturable cells remaining after 5 days from treatment. Reproduced from (Kitching et al., 2019). C) Scanning electron micrographs of L. casei, (I) without protectant, (II),(III) with protectant not containing Pickering emulsions, and (IV) protectant containing 10% Pickering emulsions. Reproduced from (Wang and Chen, 2021).
4.2. Aquatic animals
The declining productivity of the fish farming industry coupled with increasing environmental damage caused by aquaculture practices has prompted the use of probiotics in aquatic organisms. Probiotics have promoted sustainable aquaculture by acting as growth promoters, increasing resistance to certain diseases, enhancing immune response in fish, and improving water quality (Mondal et al., 2022). Furthermore, studies have found that probiotics can improve the digestion of other nutrients, increase stress tolerance, and encourage reproduction in aquatic animals (Martínez Cruz et al., 2012).
However, simple incorporation of probiotics in fish feeds leads to high losses in viability prior to reaching their target, thus reducing the benefits to fish health. Various encapsulation techniques have been explored to protect probiotics from harsh conditions and maintain their viability. One of the first studies assessing the usefulness of encapsulation techniques in fish feed was conducted by Rosas-Ledesma et al. (2012). The authors stressed the importance of probiotic survival under stressful conditions, such as low water content, which is frequently the case with animal feeds (Rosas-Ledesma et al., 2012). Calcium alginate beads were prepared for the encapsulation of Shewanella putrefaciens Pdp11 using various concentrations of alginate and calcium chloride. The results from this study showed that the percentage of encapsulated cells was above 80%, with the lowest efficiency rates corresponding to higher calcium availability. However, the capsules had to be stored at 4 °C in order to achieve 90% survival over a month. At 22 °C, a 40% loss in viability was observed over 30 days. The study further demonstrated that the encapsulated probiotics could survive through the GIT of sole fish (Rosas-Ledesma et al., 2012).
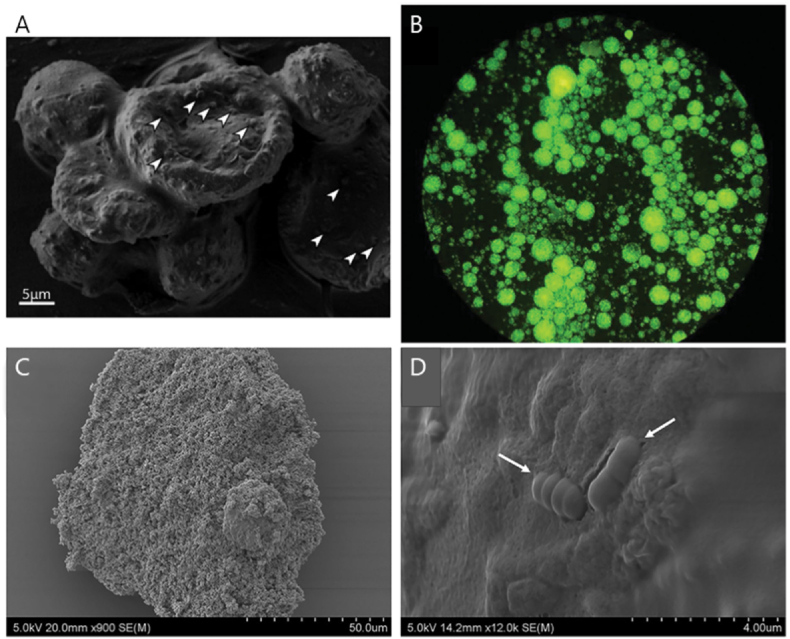
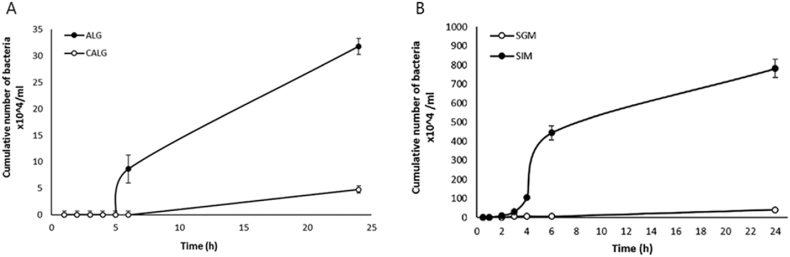
Additionally, there have been a few studies on emulsification as an encapsulation technique for aquaculture applications. Ghosh et al. (2016) prepared water-in-oil emulsions consisting of alginate, calcium chloride, and canola oil to encapsulate Enterobacter sp. (Fig. 8A) and tested them with rainbow trout. Results showed that the cumulative mortality was lower for fish fed with encapsulated probiotics compared to free probiotics (Ghosh et al., 2016). Recently, a study conducted by Vega-Carranza et al. (2021) utilized emulsion technology to encapsulate Bacillus licheniformis, a marine probiotic, in alginate microparticles and evaluated its controlled and targeted release within a simulated shrimp digestive tract (Vega-Carranza et al., 2021). The synthesized alginate microparticles were capable of carrying, protecting, and delivering a viable number of probiotics (51.29%) to the shrimp's intestine. In addition to protecting probiotics from harsh conditions, encapsulation promotes controlled release of the bacteria at its target. Masoomi Dezfooli et al. (2021) demonstrated the ability of chitosan-coated alginate microparticles (Fig. 8B–D, prepared by emulsification) to protect Exiguobacterium from sea water (Fig. 9A) and simulated gastric media (Fig. 9B), and release them in simulated intestinal fluids of black-footed abalone fish. The bacteria were released slowly over 24 h, and the cumulative release was always higher in intestinal fluid, suggesting that these microparticles could be used as a controlled release system for delivering viable probiotic bacteria to the GIT of abalone (Masoomi Dezfooli et al., 2021).
Fig. 8.
A) SEM image of Enterobacter-loaded alginate beads, inclusions of Enterobacter specimens on the surface are indicated. Adapted from (Ghosh et al., 2016). B) Fluorescence micrograph of stained Exiguobacterium loaded chitosan-coated alginate microcapsules under 100x magnification. Adapted from (Masoomi Dezfooli et al., 2021). C, D) SEM images of Exiguobacterium loaded chitosan-coated alginate microcapsules, Exiguobaterium inclusions indicated in D. Adapted from (Masoomi Dezfooli et al., 2021).
Fig. 9.
A) Release of B. licheniformis from alginate (ALG) and chitosan-alginate (CALG) microparticles in seawater (n = 3). Reproduced from (Masoomi Dezfooli et al., 2021). B) Release of encapsulated B. licheniformis over time in simulated gastric media (SGM) and simulated intestinal media (SIM) of abalone (n = 3). Reproduced from (Masoomi Dezfooli et al., 2021).
5. Conclusions and future perspective
The encapsulation of probiotics using Pickering emulsions is an attractive approach to enhance the viability of living organisms. Pickering emulsions offer a safer and environmentally friendly alternative to current encapsulation techniques, with comparable or improved success in maintaining probiotic viability during delivery. Some important advances in emulsification techniques, including the development of multiple emulsions and high internal phase emulsions, were highlighted, which can also be stabilized by solid particles. These technologies can play an important role in the development of novel functional foods. However, it remains to be seen whether these lab-tested technologies can be translated to large-scale commercial production. Additionally, further in vivo study is required to provide information on safety and efficacy.
While there has been significant advancement in the field of functional foods, there is very limited literature available on the use of Pickering emulsions for probiotic encapsulation in aquaculture and farming. Pickering emulsions have been previously applied to encapsulate essential oils as well as oral vaccines for delivery to fish. Applying this technique for probiotic encapsulation could further enhance the health of farmed fish as well as livestock. Pickering emulsions offer a greener alternative to current technologies along with higher protection of bacterial cells, thus promoting more productive and sustainable farming practices. The research in this field thus far shows that it is a promising technique worth further exploration.
CRediT authorship contribution statement
Fatemah Haji: Conceptualization, Formal analysis, Writing – original draft. James Cheon: Conceptualization, Formal analysis, Writing – original draft. Jiyoo Baek: Conceptualization, Formal analysis, Writing – original draft. Qi Wang: Supervision, Writing – review & editing. Kam Chiu Tam: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
K. C. Tam wishes to acknowledge the funding from CFI and NSERC.
Editor name: Dr. Yeonhwa Park
Data availability
No data was used for the research described in the article.
References
- Abdullah, Weiss J., Ahmad T., Zhang C., Zhang H. A review of recent progress on high internal-phase Pickering emulsions in food science. Trends Food Sci. Technol. 2020;106:91–103. doi: 10.1016/j.tifs.2020.10.016. [DOI] [Google Scholar]
- Abesinghe A.M.N.L., Priyashantha H., Prasanna P.H.P., Kurukulasuriya M.S., Ranadheera C.S., Vidanarachchi J.K. Inclusion of probiotics into fermented buffalo (Bubalus bubalis) milk: an overview of challenges and opportunities. Fermentation. 2020;6(4):1–24. doi: 10.3390/fermentation6040121. [DOI] [Google Scholar]
- Afzaal M., Khan A.U., Saeed F., Ahmed A., Ahmad M.H., Maan A.A., et al. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 2019;7(12):3931–3940. doi: 10.1002/fsn3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anal A.K., Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007;18(5):240–251. doi: 10.1016/j.tifs.2007.01.004. [DOI] [Google Scholar]
- Anjali T.G., Basavaraj M.G. Influence of pH and salt concentration on pickering emulsions stabilized by colloidal peanuts. Langmuir. 2018;34(44):13312–13321. doi: 10.1021/acs.langmuir.8b02913. [DOI] [PubMed] [Google Scholar]
- Ansari F., Alian Samakkhah S., Bahadori A., Jafari S.M., Ziaee M., Khodayari M.T., Pourjafar H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2021:1–29. doi: 10.1080/10408398.2021.1949577. 0(0) [DOI] [PubMed] [Google Scholar]
- Aspri M., Papademas P., Tsaltas D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation. 2020;6(1):1–20. doi: 10.3390/fermentation6010030. [DOI] [Google Scholar]
- Atia A., Gomaa A., Fernandez B., Subirade M., Fliss I. Study and understanding behavior of alginate-inulin synbiotics beads for protection and delivery of antimicrobial-producing probiotics in colonic simulated conditions. Probiotic Antimicrobiol. Protein. 2018;10(2):157–167. doi: 10.1007/s12602-017-9355-x. [DOI] [PubMed] [Google Scholar]
- Baek J., Wahid-Pedro F., Kim K., Kim K., Tam K.C. Phosphorylated-CNC/modified-chitosan nanocomplexes for the stabilization of Pickering emulsions. Carbohydr. Polym. 2019;206:520–527. doi: 10.1016/j.carbpol.2018.11.006. July 2018. [DOI] [PubMed] [Google Scholar]
- Bai L., Huan S., Li Z., McClements D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: gum Arabic, beet pectin, and corn fiber gum. Food Hydrocolloids. 2017;66:144–153. doi: 10.1016/j.foodhyd.2016.12.019. [DOI] [Google Scholar]
- Bai L., Huan S., Rojas O.J., McClements D.J. Recent innovations in emulsion science and technology for food applications. J. Agric. Food Chem. 2021;69(32):8944–8963. doi: 10.1021/acs.jafc.1c01877. [DOI] [PubMed] [Google Scholar]
- Barbosa J., Borges S., Amorim M., Pereira M.J., Oliveira A., Pintado M.E., Teixeira P. Comparison of spray drying, freeze drying and convective hot air drying for the production of a probiotic orange powder. J. Funct.Foods. 2015;17:340–351. doi: 10.1016/j.jff.2015.06.001. [DOI] [Google Scholar]
- Baroncello S., Candiago N.T., Gelinski J.M.L.N., Caliari V., Baratto C.M. Meat pet snacks by containing encapsulated Saccharomyces boulardii. Euro J. Agri. Food Sci. 2020;2(4) [Google Scholar]
- Berton‐Carabin C.C., Ropers M., Genot C. Lipid oxidation in oil‐in‐water emulsions: involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014;13(5):945–977. [Google Scholar]
- Binks B.P., Fletcher P.D.I. Particles adsorbed at the Oil−Water interface: a theoretical comparison between spheres of uniform wettability and “janus” particles. Langmuir. 2001;17(16):4708–4710. doi: 10.1021/la0103315. [DOI] [Google Scholar]
- Binks Bernard P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002;7(1):21–41. doi: 10.1016/S1359-0294(02)00008-0. [DOI] [Google Scholar]
- Bodzen A., Iaconelli C., Charriau A., Dupont S., Beney L., Gervais P. Specific gaseous conditions significantly improve lactobacillus casei and Escherichia coli survival to freeze drying and rehydration. Appl. Food Biotechnol. 2020;7(1):1–9. doi: 10.22037/afb.v7i1.26343. [DOI] [Google Scholar]
- Bruno F.A., Shah N.P. Viability of two freeze-dried strains of Bifidobacterium and of commercial preparations at various temperatures during prolonged storage. J. Food Sci. 2003;68(7):2336–2339. doi: 10.1111/j.1365-2621.2003.tb05769.x. [DOI] [Google Scholar]
- Camelo-Silva C., Silvani S., Alan V., Marco A., Luccio D. Innovation and trends in probiotic microencapsulation by emulsification techniques. Food Eng. Rev. 2022 doi: 10.1007/s12393-022-09315-1. [DOI] [Google Scholar]
- Chappat M. Some applications of emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1994;91:57–77. doi: 10.1016/0927-7757(94)02976-8. [DOI] [Google Scholar]
- Chen L., Ao F., Ge X., Shen W. Food-grade pickering emulsions: preparation, stabilization and applications. Molecules. 2020;25 doi: 10.3390/molecules25143202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M.T., Tzortzis G., Charalampopoulos D., Khutoryanskiy V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Contr. Release. 2012;162(1):56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Cotter P.D., Hill C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003;67(3):429–453. doi: 10.1128/mmbr.67.3.429-453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Hang F., Liu X., Xu Z., Liu Z., Zhao J., Chen W. Effect of acids produced from carbohydrate metabolism in cryoprotectants on the viability of freeze-dried Lactobacillus and prediction of optimal initial cell concentration. J. Biosci. Bioeng. 2018;125(5):513–518. doi: 10.1016/j.jbiosc.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Ding X., Xu Y., Wang Y., Xie L., Liang S., Li D., Zhan X. Carboxymethyl konjac glucomannan-chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics. Carbohydr. Polym. 2022;289 doi: 10.1016/j.carbpol.2022.119438. [DOI] [PubMed] [Google Scholar]
- Dodoo C.C., Wang J., Basit A.W., Stapleton P., Gaisford S. Targeted delivery of probiotics to enhance gastrointestinal stability and intestinal colonisation. Int. J. Pharm. 2017;530(1):224–229. doi: 10.1016/j.ijpharm.2017.07.068. [DOI] [PubMed] [Google Scholar]
- Dominguez M.S., Abreu C.R. Elsevier; 2016. Nanocolloids: A Meeting Point for Scientists and Technologists. [Google Scholar]
- Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2015;14(1):20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami P., Davarpanah L., Vahabzadeh F. Encapsulating role of β-cyclodextrin in formation of pickering water-in-oil-in-water (W1/O/W2) double emulsions containing Lactobacillus dellbrueckii. Food Hydrocolloids. 2017;64:133–148. doi: 10.1016/j.foodhyd.2016.10.035. [DOI] [Google Scholar]
- FAO/WHO . third ed. 2001. Probiotics in Food; pp. 413–426. Chemical and Functional Properties of Food Components. [DOI] [Google Scholar]
- Fujisawa S., Togawa E., Kuroda K. Facile route to transparent, strong, and thermally stable nanocellulose/polymer nanocomposites from an aqueous pickering emulsion. Biomacromolecules. 2017;18(1):266–271. doi: 10.1021/acs.biomac.6b01615. [DOI] [PubMed] [Google Scholar]
- Gardiner G.E., O'Sullivan E., Kelly J., Auty M.A.E., Fitzgerald G.F., Collins J.K., Stanton C. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl. Environ. Microbiol. 2000;66(6):2605–2612. doi: 10.1128/AEM.66.6.2605-2612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbassi G.K., Vandamme T. Probiotic encapsulation technology: from microencapsulation to release into the gut. Pharmaceutics. 2012;4 doi: 10.3390/pharmaceutics4010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B., Cain K.D., Nowak B.F., Bridle A.R. Microencapsulation of a putative probiotic Enterobacter species, C6-6, to protect rainbow trout, Oncorhynchus mykiss (Walbaum), against bacterial coldwater disease. J. Fish. Dis. 2016;39(1):1–11. doi: 10.1111/jfd.12311. [DOI] [PubMed] [Google Scholar]
- Gómez B., Barba F.J., Domínguez R., Putnik P., Bursać Kovačević D., Pateiro M., Lorenzo J.M. Microencapsulation of antioxidant compounds through innovative technologies and its specific application in meat processing. Trends Food Sci. Technol. 2018;82:135–147. doi: 10.1016/j.tifs.2018.10.006. [DOI] [Google Scholar]
- Gonzalez Ortiz D., Pochat-Bohatier C., Cambedouzou J., Bechelany M., Miele P. Current trends in pickering emulsions: particle morphology and applications. Engineering. 2020;6(4):468–482. doi: 10.1016/j.eng.2019.08.017. [DOI] [Google Scholar]
- Gopal V., Dhanasekaran D. In: Chapter 22 - Probiotics as a Growth Promotant for Livestock and Poultry Production (D. Dhanasekaran & A. B. T.-A. Sankaranarayanan P., editor. 2021. [DOI] [Google Scholar]
- Gupta V., Garg R. Probiotics. Indian J. Med. Microbiol. 2009;27(3):202–209. doi: 10.4103/0255-0857.53201. [DOI] [PubMed] [Google Scholar]
- Gurram S., Jha D.K., Shah D.S., Kshirsagar M.M., Amin P.D. Insights on the critical parameters affecting the probiotic viability during stabilization process and formulation development. AAPS PharmSciTech. 2021;22(5) doi: 10.1208/s12249-021-02024-8. [DOI] [PubMed] [Google Scholar]
- Hamad S.A. 2012. Novel Techniques for Microencapsulatıon of Probiotic Bacteria. [Google Scholar]
- Hamner S., McInnerney K., Williamson K., Franklin M.J., Ford T.E. Bile salts affect expression of Escherichia coli O157:H7 genes for virulence and iron acquisition, and promote growth under iron limiting conditions. PLoS One. 2013;8(9):1–14. doi: 10.1371/journal.pone.0074647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Lu Y., Xie J., Fei Y., Zheng G., Wang Z., Li L. Probiotic gastrointestinal transit and colonization after oral administration: a long journey. Front. Cell. Infect. Microbiol. 2021;11:1–12. doi: 10.3389/fcimb.2021.609722. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S., Korbekandi H., Mirmohammadi S.V. Technology and potential applications of probiotic encapsulation in fermented milk products. J. Food Sci. Technol. 2015;52(8):4679–4696. doi: 10.1007/s13197-014-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbstein H., Schubert H. Developments in the continuous mechanical production of oil-in-water macro-emulsions. Chem. Eng. Process: Process Intensif. 1995;34(3):205–211. doi: 10.1016/0255-2701(94)04005-2. [DOI] [Google Scholar]
- Kechagia M., Basoulis D., Konstantopoulou S., Dimitriadi D., Gyftopoulou K., Skarmoutsou N., Fakiri E.M. Hindawi Publishing Corporation; 2013. Health Benefit of Probiotic: A Review; pp. 1–7. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi Zanjani M.A., Ghiassi Tarzi B., Sharifan A., Mohammadi N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. (IJPR) : Int. J. Phys. Res. 2014;13(3):843–852. https://pubmed.ncbi.nlm.nih.gov/25276184 [PMC free article] [PubMed] [Google Scholar]
- Kitching M., Mathur H., Flynn J., Byrne N., Dillon P., Sayers R., Ross R.P. A live bio-therapeutic for mastitis, containing lactococcus lactis DPC3147 with comparable efficacy to antibiotic treatment. Front. Microbiol. 2019;10:1–11. doi: 10.3389/fmicb.2019.02220. SEP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klojdová I., Stathopoulos C. The potential application of pickering multiple emulsions in food. Foods. 2022;11 doi: 10.3390/foods11111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbekandi H., Mortazavian A., Iravani S. 2011. Technology and Stability of Probiotic in Fermented Milks Containing Probiotics and Prebiotics. [Google Scholar]
- Krasaekoopt W., Bhandari B., Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003;13(1):3–13. doi: 10.1016/S0958-6946(02)00155-3. [DOI] [Google Scholar]
- Laguerre M., Bayrasy C., Panya A., Weiss J., McClements D.J., Lecomte J., Villeneuve P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015;55(2):183–201. doi: 10.1080/10408398.2011.650335. [DOI] [PubMed] [Google Scholar]
- Lee D., Goh T.W., Kang M.G., Choi H.J., Yeo S.Y., Yang J., et al. Perspectives and advances in probiotics and the gut microbiome in companion animals. J. Anim. Sci. Technol. 2022;64(2):197–217. doi: 10.5187/jast.2022.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell Scott, Robert H.G. Clarendon Press; Oxford: 1889. An Intermediate Greek-English Lexicon. [Google Scholar]
- Liu H., Gong J., Chabot D., Miller S.S., Cui S.W., Zhong F., Wang Q. Improved survival of Lactobacillus zeae LB1 in a spray dried alginate-protein matrix. Food Hydrocolloids. 2018;78:100–108. doi: 10.1016/j.foodhyd.2017.07.004. [DOI] [Google Scholar]
- Liu Z., Shen R., Yang X., Lin D. Characterization of a novel konjac glucomannan film incorporated with Pickering emulsions: effect of the emulsion particle sizes. Int. J. Biol. Macromol. 2021;179:377–387. doi: 10.1016/j.ijbiomac.2021.02.188. [DOI] [PubMed] [Google Scholar]
- Marino M., Innocente N., Calligaris S., Maifreni M., Marangone A., Nicoli M.C. Viability of probiotic Lactobacillus rhamnosus in structured emulsions containing saturated monoglycerides. J. Funct.Foods. 2017;35:51–59. doi: 10.1016/j.jff.2017.05.012. [DOI] [Google Scholar]
- Markowiak P., Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9) doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Cruz P., Ibáñez A.L., Monroy Hermosillo O.A., Ramírez Saad H.C. Use of probiotics in aquaculture. ISRN Microbiology. 2012:1–13. doi: 10.5402/2012/916845. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoomi Dezfooli S., Gutierrez-Maddox N., Alfaro A.C., Seyfoddin A. Development of a microencapsulated probiotic delivery system for New Zealand black-footed abalone (Haliotis iris) Pharmaceut. Dev. Technol. 2021;26(4):390–402. doi: 10.1080/10837450.2021.1876090. [DOI] [PubMed] [Google Scholar]
- Melchior S., Calligaris S., Marino M., D'Este F., Honsell G., Nicoli M.C., Innocente N. Digestive protection of probiotic Lacticaseibacillus rhamnosus in Ricotta cheese by monoglyceride structured emulsions. Int. J. Food Sci. Technol. 2022;57(5):3106–3115. doi: 10.1111/ijfs.15641. [DOI] [Google Scholar]
- Melchior S., Marino M., D'Este F., Innocente N., Nicoli M.C., Calligaris S. Effect of the formulation and structure of monoglyceride-based gels on the viability of probiotic: lactobacillus rhamnosus upon in vitro digestion. Food Funct. 2021;12(1):351–361. doi: 10.1039/d0fo01788d. [DOI] [PubMed] [Google Scholar]
- Mendes A.C., Chronakis I.S. Electrohydrodynamic encapsulation of probiotics: a review. Food Hydrocolloids. 2021;117 doi: 10.1016/j.foodhyd.2021.106688. [DOI] [Google Scholar]
- Metchnikoff E. The prolongation of life; optimistic studies : Metchnikoff. Elie. 1908;44:1864–1945. https://archive.org/details/prolongationofli00metciala/page/n6 1845-1916. 0. [Google Scholar]
- Mondal S., Mondal D., Mondal T., Malik J. In: Chapter 17 - Application of Probiotic Bacteria for the Management of Fish Health in Aquaculture. Dar G.H., Bhat R.A., Qadri H., Al-Ghamdy K.M., Hakeem K.R.B.T.-B.F.D., editors. 2022. [DOI] [Google Scholar]
- Parvez S., Malik K.A., Ah Kang S., Kim H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006;100(6):1171–1185. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- Perricone M., Bevilacqua A., Altieri C., Sinigaglia M., Corbo M.R. Challenges for the production of probiotic fruit juices. Beverages. 2015;1(2):95–103. doi: 10.3390/beverages1020095. [DOI] [Google Scholar]
- Phuong Ta L., Bujna E., Kun S., Charalampopoulos D., Khutoryanskiy V.V. Electrosprayed mucoadhesive alginate-chitosan microcapsules for gastrointestinal delivery of probiotics. Int. J. Pharm. 2021;597 doi: 10.1016/j.ijpharm.2021.120342. [DOI] [PubMed] [Google Scholar]
- Piñón-Balderrama C.I., Leyva-Porras C., Terán-Figueroa Y., Espinosa-Solís V., Álvarez-Salas C., Saavedra-Leos M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes. 2020;8 doi: 10.3390/pr8080889. [DOI] [Google Scholar]
- Premjit Y., Mitra J. Optimization of electrospray-assisted microencapsulation of probiotics (leuconostoc lactis) in soy protein isolate-oil particles using box-behnken experimental design. Food Bioprocess Technol. 2021;14(9):1712–1729. doi: 10.1007/s11947-021-02670-7. [DOI] [Google Scholar]
- Pupa P., Apiwatsiri P., Sirichokchatchawan W., Pirarat N., Muangsin N., Shah A.A., Prapasarakul N. The efficacy of three double-microencapsulation methods for preservation of probiotic bacteria. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-93263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X.-S., Gao Q.-Y., Luo Z.-G. Enhancing the storage and gastrointestinal passage viability of probiotic powder (Lactobacillus Plantarum) through encapsulation with pickering high internal phase emulsions stabilized with WPI-EGCG covalent conjugate nanoparticles. Food Hydrocolloids. 2021;116 doi: 10.1016/j.foodhyd.2021.106658. [DOI] [Google Scholar]
- Quintana G., Gerbino E., Alves P., Simões P.N., Rúa M.L., Fuciños C., Gomez-Zavaglia A. Microencapsulation of Lactobacillus plantarum in W/O emulsions of okara oil and block-copolymers of poly(acrylic acid) and pluronic using microfluidic devices. Food Res. Int. 2021;140 doi: 10.1016/j.foodres.2020.110053. [DOI] [PubMed] [Google Scholar]
- Quintana G., Gerbino E., Gómez-Zavaglia A. In: Microfluidic Glass Capillary Devices: an Innovative Tool to Encapsulate Lactiplantibacillus Plantarum BT - Basic Protocols in Encapsulation of Food Ingredients. Gomez-Zavaglia A., editor. 2021. [DOI] [Google Scholar]
- Rajam R., Subramanian P. Encapsulation of probiotics: past, present and future. Beni-suef Univ. J. Basic. Appl. Sci. 2022;11(1) doi: 10.1186/s43088-022-00228-w. [DOI] [Google Scholar]
- Rattanaburi P., Charoenrat N., Pongtharangkul T., Suphantharika M., Wongkongkatep J. Hydroxypropyl methylcellulose enhances the stability of o/w Pickering emulsions stabilized with chitosan and the whole cells of Lactococcus lactis IO-1. Food Res. Int. 2019;116:559–565. doi: 10.1016/j.foodres.2018.08.074. [DOI] [PubMed] [Google Scholar]
- Rayner M., Marku D., Eriksson M., Sjöö M., Dejmek P., Wahlgren M. Biomass-based particles for the formulation of Pickering type emulsions in food and topical applications. Colloids Surf. A Physicochem. Eng. Asp. 2014;458:48–62. doi: 10.1016/j.colsurfa.2014.03.053. [DOI] [Google Scholar]
- Reque P.M., Brandelli A. Encapsulation of probiotics and nutraceuticals: applications in functional food industry. Trends Food Sci. Technol. 2021;114:1–10. doi: 10.1016/j.tifs.2021.05.022. [DOI] [Google Scholar]
- Rokka S., Rantamäki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur. Food Res. Technol. 2010;231(1):1–12. doi: 10.1007/s00217-010-1246-2. [DOI] [Google Scholar]
- Romyasamit C., Saengsuwan P., Boonserm P., Thamjarongwong B., Singkhamanan K. Optimization of cryoprotectants for freeze-dried potential probiotic Enterococcus faecalis and evaluation of its storage stability. Dry. Technol. 2021:1–10. doi: 10.1080/07373937.2021.1931294. [DOI] [Google Scholar]
- Rosas-Ledesma P., León-Rubio J.M., Alarcón F.J., Moriñigo M.A., Balebona M.C. Calcium alginate capsules for oral administration of fish probiotic bacteria: assessment of optimal conditions for encapsulation. Aquacult. Res. 2012;43(1):106–116. doi: 10.1111/j.1365-2109.2011.02809.x. [DOI] [Google Scholar]
- Saarela M., Virkajärvi I., Alakomi H.-L., Sigvart-Mattila P., Mättö J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int. Dairy J. 2006;16(12):1477–1482. doi: 10.1016/j.idairyj.2005.12.007. [DOI] [Google Scholar]
- Sabatini V., Pellicano L., Farina H., Pargoletti E., Annunziata L., Ortenzi M.A., Cappelletti G. Design of new polyacrylate microcapsules to modify the water-soluble active substances release. Polymers. 2021;13 doi: 10.3390/polym13050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarionpour S. Nanocellulose for stabilization of pickering emulsions and delivery of nutraceuticals and its interfacial adsorption mechanism. Food Bioprocess Technol. 2020;13(8):1292–1328. doi: 10.1007/s11947-020-02481-2. [DOI] [Google Scholar]
- Santivarangkna C., Aschenbrenner M., Kulozik U., Foerst P. Role of glassy state on stabilities of freeze-dried probiotics. J. Food Sci. 2011;76(8):R152–R156. doi: 10.1111/j.1750-3841.2011.02347.x. [DOI] [PubMed] [Google Scholar]
- Sarao L.K., Arora M. Probiotics, prebiotics, and microencapsulation: a review. Crit. Rev. Food Sci. Nutr. 2017;57(2):344–371. doi: 10.1080/10408398.2014.887055. [DOI] [PubMed] [Google Scholar]
- Sehrawat R., Abdullah S., Khatri P., Kumar L., Kumar A., Mujumdar A.S. Role of drying technology in probiotic encapsulation and impact on food safety. Dry. Technol. 2022:1–20. [Google Scholar]
- Sharifi S., Rezazad-Bari M., Alizadeh M., Almasi H., Amiri S. Use of whey protein isolate and gum Arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: enhanced viability of probiotic in Iranian white cheese. Food Hydrocolloids. 2021;113 doi: 10.1016/j.foodhyd.2020.106496. July 2020. [DOI] [Google Scholar]
- Shembekar N., Chaipan C., Utharala R., Merten C.A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip. 2016;16(8):1314–1331. doi: 10.1039/c6lc00249h. [DOI] [PubMed] [Google Scholar]
- Shi W.-J., Tang C.-H., Yin S.-W., Yin Y., Yang X.-Q., Wu L.-Y., Zhao Z.-G. Development and characterization of novel chitosan emulsion films via pickering emulsions incorporation approach. Food Hydrocolloids. 2016;52:253–264. [Google Scholar]
- Shu G., Wang Z., Chen L., Wan H., Chen H. Characterization of freeze-dried Lactobacillus acidophilus in goat milk powder and tablet: optimization of the composite cryoprotectants and evaluation of storage stability at different temperature. Lebensm. Wiss. Technol. 2018;90:70–76. doi: 10.1016/j.lwt.2017.12.013. [DOI] [Google Scholar]
- Šipailienė A., Petraitytė S. Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotic Antimicrobiol. Protein. 2018;10(1):1–10. doi: 10.1007/s12602-017-9347-x. [DOI] [PubMed] [Google Scholar]
- Su J., Cai Y., Tai K., Guo Q., Zhu S., Mao L., Van Der Meeren P. High-internal-phase emulsions (HIPEs) for co-encapsulation of probiotics and curcumin: enhanced survivability and controlled release. Food Funct. 2021;12(1):70–82. doi: 10.1039/d0fo01659d. [DOI] [PubMed] [Google Scholar]
- Sultana M., Chan E.-S., Pushpamalar J., Choo W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022;123:69–86. doi: 10.1016/j.tifs.2022.03.006. [DOI] [Google Scholar]
- Sun H., Li S., Chen S., Wang C., Liu D., Li X. Antibacterial and antioxidant activities of sodium starch octenylsuccinate-based Pickering emulsion films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2020;159:696–703. doi: 10.1016/j.ijbiomac.2020.05.118. [DOI] [PubMed] [Google Scholar]
- Talwalkar A., Miller C.W., Kailasapathy K., Nguyen M.H. Effect of packaging materials and dissolved oxygen on the survival of probiotic bacteria in yoghurt. Int. J. Food Sci. Technol. 2004;39(6):605–611. doi: 10.1111/j.1365-2621.2004.00820.x. [DOI] [Google Scholar]
- Tapia-Hernández J.A., Torres-Chávez P.I., Ramírez-Wong B., Rascón-Chu A., Plascencia-Jatomea M., Barreras-Urbina C.G., Rodríguez-Félix F. Micro- and nanoparticles by electrospray: advances and applications in foods. J. Agric. Food Chem. 2015;63(19):4699–4707. doi: 10.1021/acs.jafc.5b01403. [DOI] [PubMed] [Google Scholar]
- Tavasoli S., Liu Q., Jafari S.M. Development of Pickering emulsions stabilized by hybrid biopolymeric particles/nanoparticles for nutraceutical delivery. Food Hydrocolloids. 2022;124 doi: 10.1016/j.foodhyd.2021.107280. [DOI] [Google Scholar]
- Terpou A., Papadaki A., Lappa I.K., Kachrimanidou V., Bosnea L.A., Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019;11(7) doi: 10.3390/nu11071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh B.M., Smith M., Mekonnen T.H. A nanomaterial-stabilized starch-beeswax Pickering emulsion coating to extend produce shelf-life. Chem. Eng. J. 2022;431 doi: 10.1016/j.cej.2021.133905. [DOI] [Google Scholar]
- Tripathi M.K., Giri S.K. Probiotic functional foods: survival of probiotics during processing and storage. J. Funct.Foods. 2014;9(1):225–241. doi: 10.1016/j.jff.2014.04.030. [DOI] [Google Scholar]
- Vega-Carranza A.S., Cervantes-Chávez J.A., Luna-Bárcenas G., Luna-González A., Diarte-Plata G., Nava-Mendoza R., Pool H. Alginate microcapsules as delivery and protective systems of Bacillus licheniformis in a simulated shrimp's digestive tract. Aquaculture. 2021;540 doi: 10.1016/j.aquaculture.2021.736675. December 2020. [DOI] [Google Scholar]
- Vinderola C.G., Costa G.A., Regenhardt S., Reinheimer J.A. Influence of compounds associated with fermented dairy products on the growth of lactic acid starter and probiotic bacteria. Int. Dairy J. 2002;12(7):579–589. doi: 10.1016/S0958-6946(02)00046-8. [DOI] [Google Scholar]
- Vlasova A.N., Kandasamy S., Chattha K.S., Rajashekara G., Saif L.J. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016;172:72–84. doi: 10.1016/j.vetimm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen L. Impact of a novel nano-protectant on the viability of probiotic bacterium lactobacillus casei k17. Foods. 2021;10(3):1–13. doi: 10.3390/foods10030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Song M., Zhao Z., Chen X., Cai J., Cao Y., Xiao J. Lactobacillus acidophilus loaded pickering double emulsion with enhanced viability and colon-adhesion efficiency. Lebensm. Wiss. Technol. 2020;121 doi: 10.1016/j.lwt.2019.108928. [DOI] [Google Scholar]
- Wilkinson M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: a review. Trends Food Sci. Technol. 2018;78:1–10. doi: 10.1016/j.tifs.2018.05.006. May. [DOI] [Google Scholar]
- Wu C., Sun J., Jiang H., Li Y., Pang J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021;362 doi: 10.1016/j.foodchem.2021.130242. [DOI] [PubMed] [Google Scholar]
- Wu M., Zhou Z., Yang J., Zhang M., Cai F., Lu P. ZnO nanoparticles stabilized oregano essential oil Pickering emulsion for functional cellulose nanofibrils packaging films with antimicrobial and antioxidant activity. Int. J. Biol. Macromol. 2021;190:433–440. doi: 10.1016/j.ijbiomac.2021.08.210. [DOI] [PubMed] [Google Scholar]
- Yadegari A., Fahimipour F., Rasoulianboroujeni M., Dashtimoghadarm E., Omidi M., Golzar H., Tayebi L. In: 10 - Specific Considerations in Scaffold Design for Oral Tissue Engineering. Tayebi L., for O K.B.T.-B., Moharamzadeh D.T.E., editors. 2017. [DOI] [Google Scholar]
- Yao M., Wu J., Li B., Xiao H., McClements D.J., Li L. Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. Food Hydrocolloids. 2017;72:228–236. doi: 10.1016/j.foodhyd.2017.05.033. [DOI] [Google Scholar]
- Ying D.Y., Phoon M.C., Sanguansri L., Weerakkody R., Burgar I., Augustin M.A. Microencapsulated lactobacillus rhamnosus GG powders: relationship of powder physical properties to probiotic survival during storage. J. Food Sci. 2010;75(9):E588–E595. doi: 10.1111/j.1750-3841.2010.01838.x. [DOI] [PubMed] [Google Scholar]
- Yucel Falco C., Geng X., Cárdenas M., Risbo J. Edible foam based on Pickering effect of probiotic bacteria and milk proteins. Food Hydrocolloids. 2017;70:211–218. doi: 10.1016/j.foodhyd.2017.04.003. [DOI] [Google Scholar]
- Zaeim D., Sarabi-Jamab M., Ghorani B., Kadkhodaee R., Tromp R.H. Electrospray-assisted drying of live probiotics in acacia gum microparticles matrix. Carbohydr. Polym. 2018;183:183–191. doi: 10.1016/j.carbpol.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Zafeiri I., Norton J.E., Smith P., Norton I.T., Spyropoulos F. The role of surface active species in the fabrication and functionality of edible solid lipid particles. J. Colloid Interface Sci. 2017;500:228–240. doi: 10.1016/j.jcis.2017.03.085. [DOI] [PubMed] [Google Scholar]
- Zhang T., Xu J., Chen J., Wang Z., Wang X., Zhong J. Protein nanoparticles for Pickering emulsions: a comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021;113:26–41. [Google Scholar]
- Zhang Y., Lin J., Zhong Q. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocolloids. 2016;52:804–810. doi: 10.1016/j.foodhyd.2015.08.020. [DOI] [Google Scholar]
- Zhao M., Wang Y., Huang X., Gaenzle M., Wu Z., Nishinari K., Fang Y. Ambient storage of microencapsulated Lactobacillus plantarum ST-III by complex coacervation of type-A gelatin and gum Arabic. Food Funct. 2018;9(2):1000–1008. doi: 10.1039/C7FO01802A. [DOI] [PubMed] [Google Scholar]
- Zhou S., Han L., Lu K., Qi B., Du X., Liu G., Li Y. Whey protein isolate–phytosterols nanoparticles: preparation, characterization, and stabilized food-grade pickering emulsions. Food Chem. 2022;384 doi: 10.1016/j.foodchem.2022.132486. [DOI] [PubMed] [Google Scholar]
- Zhu J.-Y., Tang C.-H., Yin S.-W., Yang X.-Q. Development and characterization of novel antimicrobial bilayer films based on Polylactic acid (PLA)/Pickering emulsions. Carbohydr. Polym. 2018;181:727–735. doi: 10.1016/j.carbpol.2017.11.085. [DOI] [PubMed] [Google Scholar]
- Zmora N., Zilberman-Schapira G., Suez J., Mor U., Dori-Bachash M., Bashiardes S., Elinav E. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405. doi: 10.1016/j.cell.2018.08.041. e21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.