Chronic Lyme Disease and Post-treatment Lyme Disease
Lyme disease, caused by infection with Borreliella (Borrelia) burgdorferi and transmitted by the bite of the ticks of the Ixodes ricinus complex, is the most common vector-borne disease in the United States and Europe.1 Antibiotic treatment is effective for most patients, but some patients report persisting or relapsing nonspecific symptoms after treatment.2 These symptoms are referred to as post-treatment Lyme disease symptoms (PTLDs) or syndrome (PTLDS), depending on their severity and functional impact.3
Patients with PTLDs are a small subset of what is called “chronic Lyme disease” (CLD) (Figure 1), a term avoided by academic literature and mainstream medical providers, as there are no clear criteria or agreement as to what constitutes “chronic Lyme disease” and the term can refer to very different groups of patients. For example, CLD is defined by one society as a “multisystem illness with a wide range of symptoms and/or signs that are either continuously or intermittently present for a minimum of six months.” CLD has evolved into a “multiple chronic infectious disease syndrome” and patients are diagnosed with multiple infections (Babesia, Ehrlichia, Anaplasma, Rickettsia, Bartonella, Powassan virus, Mycoplasma, Chlamydia, multiple herpesviruses) and poorly defined conditions (“immune dysfunction, inflammation, toxin damage by heavy metals and mold, food and environmental allergies, nutritional and metabolic abnormalities, hormonal imbalances, mitochondrial dysfunction, free radical/oxidative stress, and autonomic system dysfunction”. These diagnoses are based on clinical judgment, together with unvalidated tests and interpretation criteria. A study found that 57.5% of healthy controls could be interpreted as positive using the in-house criteria of a Lyme specialty laboratory.4 Culture assays claiming high positivity have raised serious concerns 5, 6 and could not be replicated.7 A urine antigen assay had false-positive results and the same sample varied from negative to highly positive when testing 5 fractions from each of 10 negative control samples.8 Natural killer cells measurements are unhelpful.9 Practitioners treating CLD often market themselves as integrative, functional, naturopathic, and Lyme-literate. Patients are charged high fees and spend significant amount of money on multiple unproven tests and unvalidated treatments that range from innocuous to highly dangerous.10–16 Once a patient has received the diagnosis of “chronic Lyme disease”, the inclination is to assign any signs and symptoms to it, leading to missed or delayed diagnosis of other medical conditions.17, 18 Patients diagnosed with “chronic Lyme disease” can be reluctant to accept the diagnosis of other diseases, even when this can have dire consequences.14, 18 These patients frequently disagree with the different diagnosis provided, seeking further evaluation and receiving additional antibiotic therapy for Lyme disease.19
Figure 1.
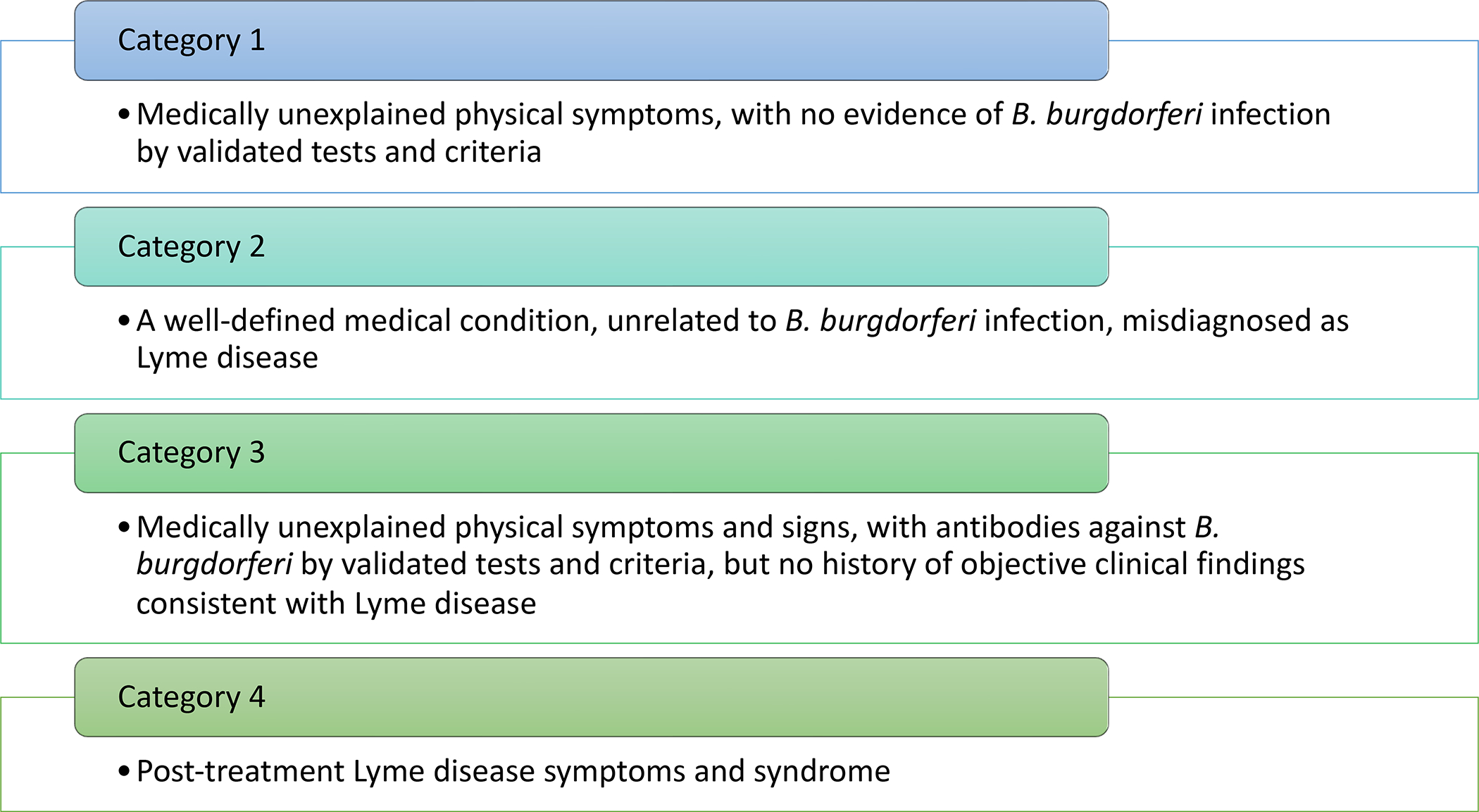
Categories of “Chronic Lyme Disease”. (Data from Feder HM, Jr., Johnson BJ, O’Connell S, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. Oct 4 2007;357(14):1422–30. doi:10.1056/NEJMra072023)
The majority of patients diagnosed with CLD have conditions whose symptoms are misdiagnosed or are patients with medically unexplained physical symptoms (MUPS) who receive the diagnosis based on unproven and/or non-validated laboratory tests and clinical criteria (Figure 2). Studies have shown that 47 to 80% of the patients seeking further evaluation for Lyme disease had no evidence of B. burgdorferi infection.19–26 An alternative diagnosis was made in 15 to 55% (median 34%) of the cases, while MUPS accounted for 19% to 54% (median 32%). Patients with PTLDs accounted for 3 to 15% (median 9%) of the cases.
Figure 2.
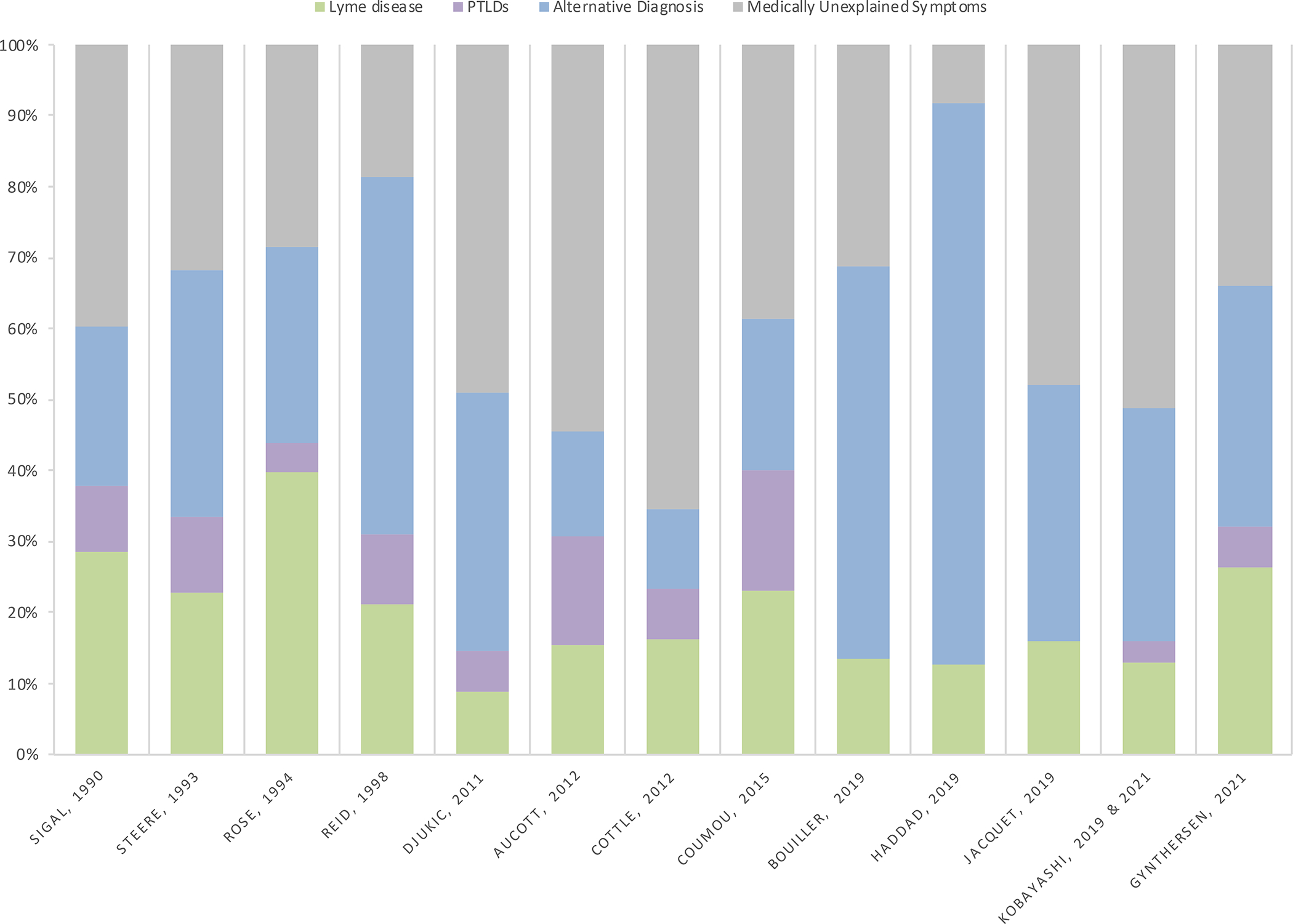
Classification of patients referred for evaluation of possible Lyme disease. Post treatment Lyme disease symptoms (PTLDs).
Patients with MUPS, or persistent physical symptoms, are common across general medicine.27–29 MUPS encompasses a range of conditions, including fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS); with substantial overlap of symptoms between patients.30 These symptoms are caused and maintained by complex interactions between biological, psychological, and social mechanisms.31 A possible mechanism is central sensitization, where maladaptive changes lead to alterations in how pain and other sensory stimuli are processed with the development of chronic symptoms.31 Interestingly, patients with “alternatively diagnosed chronic Lyme syndrome” (another name for CLD) were similar to ME/CFS patients when compared to controls, with no differences in gene expression, B-cell/T-cell receptor profiles and potential viral infections. The CLD diagnosis was due to false-positive results from alternative laboratory tests.32, 33
Post-treatment Lyme Disease Symptoms and Syndrome
The remainder of this chapter will focus on research addressing PTLDs. To fulfill criteria for PTLDs,34 patients must have a documented episode of Lyme disease, have received a recommended course of antibiotic therapy, with resolution or stabilization of the objective manifestation(s) of Lyme disease, have non-specific symptoms (persistent or relapsing) that started within 6 months of the Lyme disease diagnosis and lasted for at least 6 months after completion of antibiotic therapy, and have no other condition that explain the symptoms. Symptoms need to cause a substantial reduction in previous levels of activity to be classified as PTLD syndrome. A standardized approach is very important as it would allow for systematically quantification of the severity level of symptoms in PTLDs patients and allow for comparison between studies of outcomes and other data.3, 35, 36
Most patients who present with defined manifestations of Lyme disease will have a good outcome after antibiotic treatment with recommended regimens,2 returning to their previous level of health. Patients with later manifestations may have a delayed response to therapy, and recovery can take weeks or months.37 Incomplete resolution due to non-reversible damage can occur, as seen in patients with facial palsy and residual facial weakness38. Some patients with Lyme arthritis have persistent synovitis after antibiotic therapy, known as post-infectious Lyme arthritis.39 Treatment failure with current antibiotic regimens (where patients have objective signs of disease after treatment) can occur, but it is very uncommon.
Persisting or relapsing subjective nonspecific complaints, mostly of mild to moderate intensity, occurred in between 0 to 26% of patients with erythema migrans evaluated 6 to 12 months after completion of treatment (Figure 3).36, 40–62 Risk factors for PTLDs include antibiotic treatment delay, symptom severity at the time of treatment, presentation with multiple erythema migrans or with non-erythema migrans manifestations of Lyme disease, and presence of tingling or an abnormal skin sensation at the initial visit.42, 49, 63–65 Other risk factors include a history of stressful events,61, 65 the presence of comorbidities unrelated to Lyme disease,66 older age and female sex.42, 61 Children are less likely to develop PTLDs.45, 51, 52, 56, 62
Figure 3.
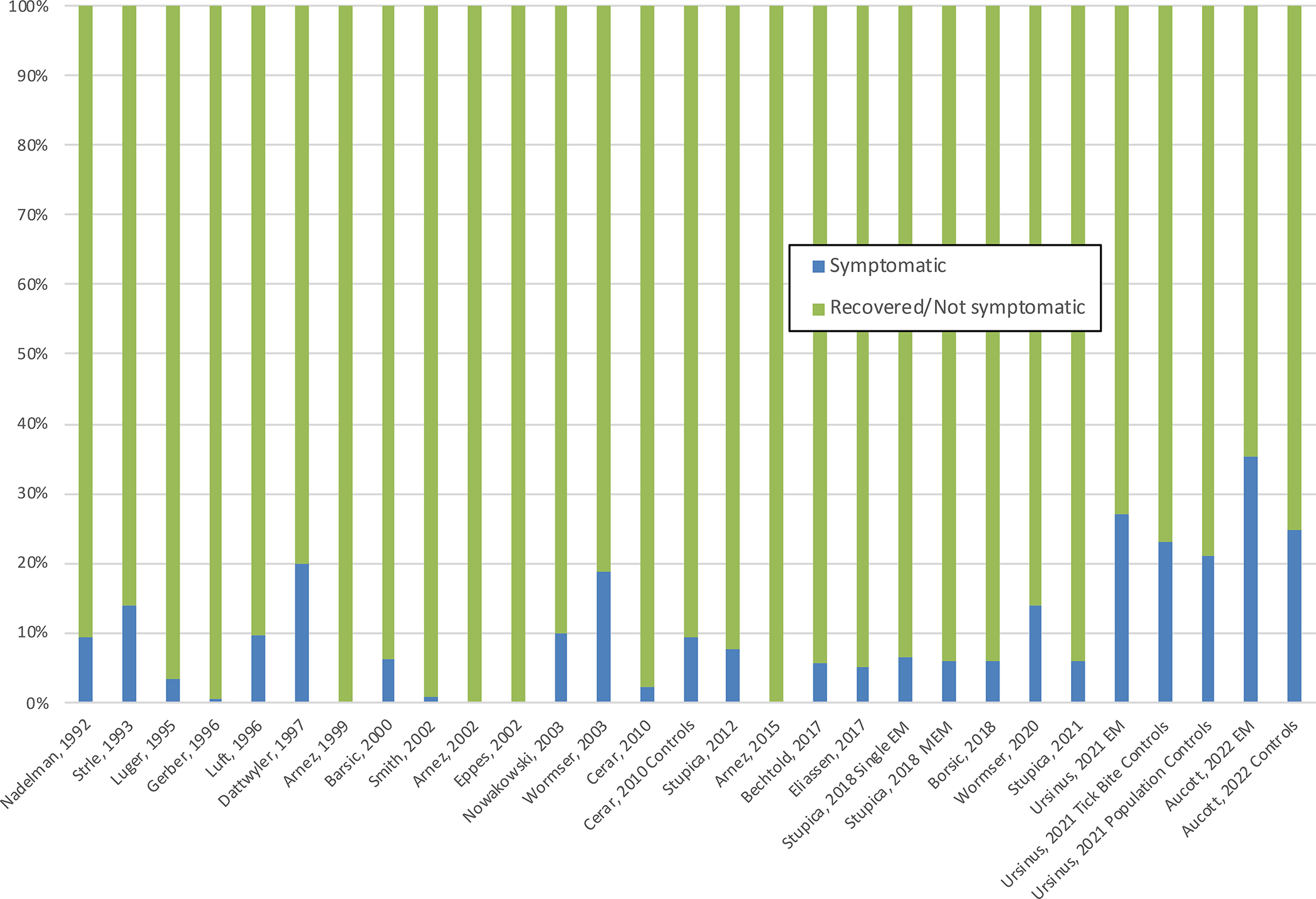
Symptoms after antibiotic therapy in patients with erythema migrans. Erythema migrans (EM). Multiple erythema migrans (MEM)
Possible causes of post-treatment Lyme disease symptoms
The mechanisms underlying PTLDs are not known and are likely to be multifactorial. The pathogenesis of the nonspecific symptoms is likely to differ among individuals (Table 1).67 Possible explanations include both related and unrelated causes.
Table 1.
Possible causes of post-treatment Lyme disease symptoms
| Part of the expected resolution of symptoms after treatment |
| New and preexisting conditions unrelated to Lyme disease |
| Post-infective fatigue syndrome |
| Other tick-borne infections |
| Microbiome changes |
| Metabolome changes |
| Dysregulated immune response/autoimmunity |
| Persistence of B. burgdorferi remnants |
| Persistent infection with B. burgdorferi |
Natural course of response to treatment
For many individuals, these nonspecific symptoms are part of the natural course of the response to the treatment of the infection, and they will continue to improve after completing antibiotic treatment. Studies of patients with erythema migrans show that the proportion of patients reporting symptoms after antibiotic treatment decreases over time (Figure 4).36, 40, 46–48, 68 A prospective study of patients with Lyme disease showed that, regardless of disease stage or severity at diagnosis, their quality-of-life scores increased to just above the United States national average after 3 years of follow-up time. Comorbidities unrelated to Lyme disease were the only factors significantly associated with long-term symptoms or lower quality of life scores.66 Similar results were seen in a study of patients with culture-confirmed erythema migrans evaluated 11–20 years after diagnosis.69
Figure 4.
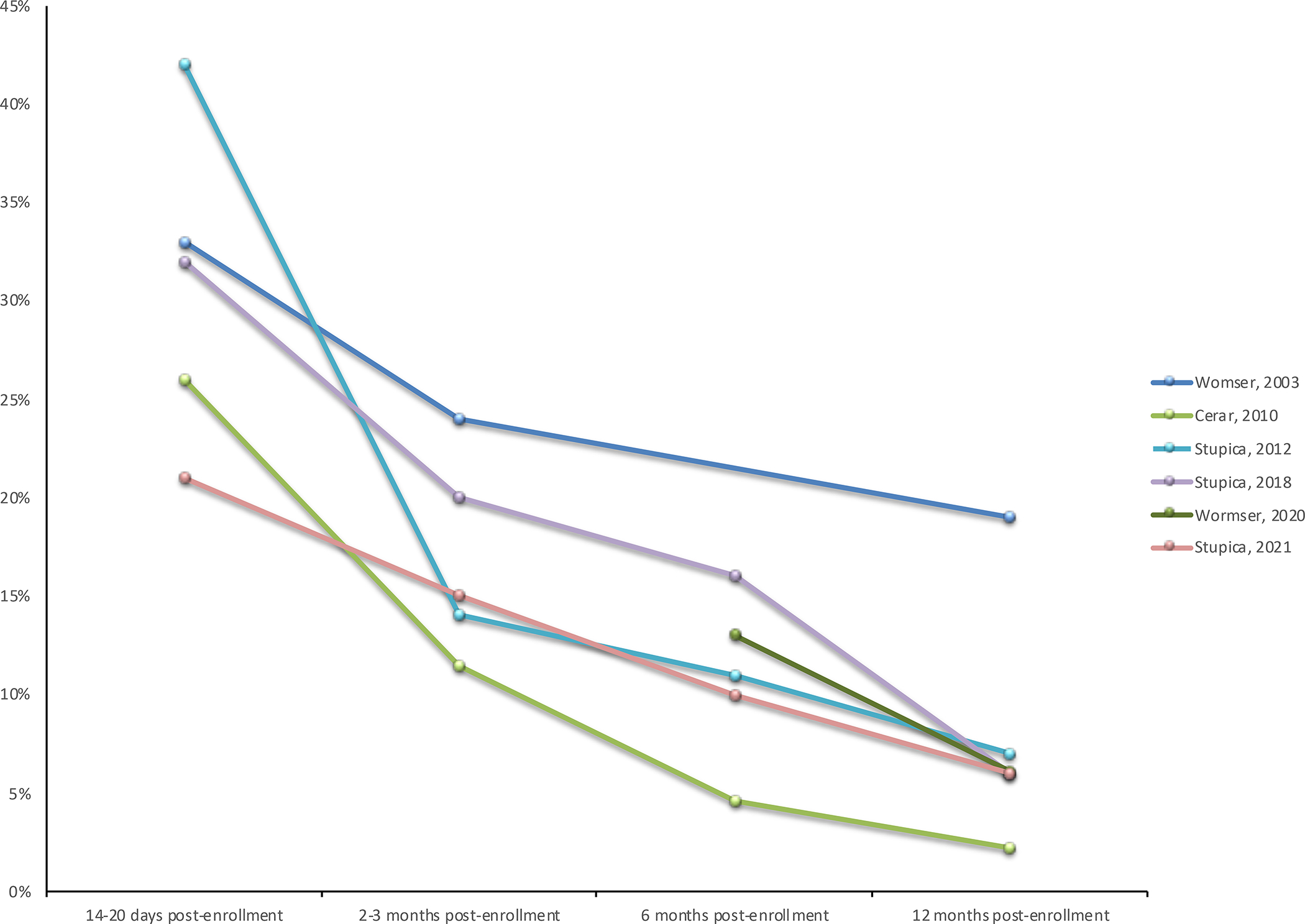
The number of patients reporting symptoms after antibiotic treatment decreases over time.
Post infective fatigue syndrome
Prolonged fatigue after infections is relatively common, can be disabling and persistent, and it is associated with the severity of the acute illness.70–74 The mechanisms triggered during the acute illness that sustain the persistent symptoms are currently unknown. However, prolonged fatigue in patients with early Lyme disease after treatment is not common, with only 3% of patients with erythema migrans having persistent fatigue possibly due to Lyme disease when evaluated 11 to 20 years after treatment.75
Misattribution and other conditions, including background prevalence of nonspecific symptoms and coinfections
Symptoms and signs due to other conditions can be wrongly attributed to Lyme disease, and these patients would be expected to have a less favorable response to specific anti-borrelial therapy. This was shown in a study of patients with disseminated Lyme disease, where 90% of the patients with definite diagnosis had excellent or good outcomes, comparing with only 47% of possible cases.76 Similarly, patients with definite Lyme neuroborreliosis had better long-term outcomes than those with possible Lyme neuroborreliosis. 37, 77, 78
Nonspecific symptoms like fatigue, chronic pain and sleep disorders are common in the general population, often reported together and associated with anxiety and depression.79 Studies where Lyme disease patients and matched controls were followed prospectively showed similar prevalence of symptoms at follow up 36, 41, 46, 47, 68, 80, 81, while two studies showed a higher prevalence of persistent symptoms in a small subset of Lyme disease patients.60, 61 All these studies demonstrate substantial (some more than 20%) background prevalence of nonspecific symptoms in the general population.
Co-infection with other tick-borne pathogens is rare in PTLDs patients.82–84 Patients with concurrent Lyme disease and untreated Babesia infection had increased symptoms in one study 85 but another study showed no difference in symptoms between patients with and without Babesia co-infection.86
Microbiome changes
A study compared patients with PTLDS with healthy controls and intensive care unit patients.87 As expected, intensive care unit controls were distinct from both PTLDS and healthy controls. There was a relative abundance of Blautia sp and Enterobacteriaceae over 10% in PTLDS patients when compared with healthy controls but PTLDS patients had received extensive antibiotic therapy (median of 56 days) and the effect of antibiotics in the gut microbiome can be prolonged.88 A prospective study of patients with Lyme disease before and at intervals after antibiotic therapy will be necessary to assess the validity and relevance of these findings.
Metabolic Changes
The metabolic response was studied in 23 non-PTLDs and 24 PTLDs patients (11 symptoms and 13 syndrome).89 Metabolite classes including glycerophospholipid, bile acid, and acylcarnitine metabolism were altered between the groups. Interestingly, PTLDs patients’ metabolic patterns looked similar at post-treatment and 1-year follow-up, while there were greater differences for the non-PTLDs patients, particularly at the post-treatment timepoint, suggesting the end of treatment as the most informative metabolic biosignature.
Immune dysregulation or maladaptive host responses
Immune dysregulation or maladaptive host responses has been best studied in patients with post-infectious Lyme arthritis 39, which is associated with both host genetic factors and B. burgdorferi factors that together contribute to dysregulated and excessive inflammatory immune responses, increased T-helper 1 and T-helper 17 immune responses, and autoreactive T and B cells. PTLDs differs substantially from post-infectious Lyme arthritis. However, it is possible that immune dysregulation and/or infection-induced autoimmune mechanisms could contribute to symptoms. A few studies considered this question, but no reproducible marker has been identified. An important issue is that, in prospective studies, the number of patients with early Lyme disease who will develop PTLDs is small. Because many variables are being examined with a small number of patients, there is a risk of a type I error. Other reasons include differences in methods and technologies used, and differences in the patient groups studied.
PTLDS patients had higher levels of antibodies against neural proteins than recovered patients or healthy controls90, 91 and higher antibody reactivity frequencies against certain B. burgdorferi antigens 91–93. PTLDS cases had higher levels of anti-lysoganglioside GM1 antibody than controls, but not other antineuronal antibodies 94. Antibodies against endothelial cell growth factor were found more frequently in patients with persistent symptoms in one study 95, but not in another study. 96
Differences in the host response and their association with persistent symptoms have been investigated in the Study of Lyme disease Immunology and Clinical Events (SLICE) cohort 97. One study measured 58 cytokines and chemokines, and six acute-phase markers, on 76 patients with erythema migrans (36 recovered, 29 patients with symptoms-only and 11 PTLDS), and 26 healthy controls. C-C motif chemokine ligand 19 (CCL19), ferritin, fibrinogen, C-X-C motif chemokine ligand (CXCL) 10, CXCL9, C-reactive protein, and serum amyloid A were differentially regulated between Lyme disease and healthy controls at the pretreatment visit. Generalized logistical regressions were used to predict Lyme disease clinical outcome group using these 7 markers. An increase in CCL19 of 0.5 standard deviation for the Lyme disease group at post-treatment visits was associated with PTLDS, and the hypothesis was that elevated CCL19 levels could reflect an ongoing reaction at distal sites to the secondary lymphoid tissue. For comparison, a European study measured 23 cytokines in serum of 45 patients with erythema migrans with at least 1 post-treatment Lyme symptom, compared with 41 recovered patients. In this study, interleukin (IL)-23 levels were higher before therapy, and stayed higher up to 12-month follow up in patients with persistent symptoms,95 a finding not seen in the previous study.97 CCL19 was not found to be significantly different between the groups. A study evaluating serum samples from 19 patients with a history of Lyme disease and objective memory impairment 83 showed increased serum interferon alpha activity in patients when compared with recovered (n=11) and healthy controls (n=20). There were no changes with prolonged antibiotic therapy.91
Blum at al. 98 showed that blood plasmablasts were increased in 32 untreated Lyme patients (7 PTLDS and 25 recovered patients, also part of the SLICE study) and returned to baseline levels by 6 months following antibiotic treatment. As expected, patients with disseminated erythema migrans (10 recovered and 1 PTLDS) had higher plasmablasts numbers compared with those with single erythema migrans (15 recovered and 6 PTLDS). PTLDS patients had significantly lower blood plasmablasts than patients who returned to health at the pretreatment timepoint, and less seroreactivity to surface proteins and peptides from B. burgdorferi. This finding contrast with the studies showing patients with PTLDS have higher levels of autoantibodies and certain B. burgdorferi antigens.90–96
The longitudinal transcriptomic response in blood was studied in 29 individuals with Lyme disease (15 who returned to health, 9 with symptoms only, and 4 PTLDS) and 13 matched controls, part of the SLICE study 99. There were no genes significantly differentially expressed at any single time point between the 15 patients who returned to health and the 13 patients with persistent symptoms. Four differentially expressed genes were identified when all timepoints were combined, and GPR15 was found differentially expressed when comparing patients with resolved Lyme disease with PTLDS at the time of diagnosis visit. A recent publication (also using data from the SLICE study) claims that, based on the projection of the RNA-seq data, cases are separated from controls and stay separate over time, but could not distinguish the patients that would go on to develop post-treatment persistent symptoms.100 Further corroboration of the results will need to wait until the actual dataset for this study is made available to the scientific community.
Persistence of B. burgdorferi remnants
It is possible that leftover B. burgdorferi products may stay trapped and preserved in tissue after infection, playing a role in post-treatment symptoms.101 Deposits of antigens were shown to persist near the cartilage and within joint entheses after antibiotic treatment in mice, and this material was capable of stimulating an inflammatory and immune response in uninfected mice.102 Recently B. burgdorferi peptidoglycan was detected in synovial fluid samples from patients with Lyme arthritis, including patients with post-infectious Lyme arthritis after antibiotic therapy, and its systemic administration elicited acute arthritis in mice.103 Although these observations do not exclude the possibility that these antigens could be produced by residual live bacteria, treatment of patients with post-infectious Lyme arthritis with potent immunosuppressive agents have not led to reemergence of infection.
Persistent infection with B. burgdorferi
The possibility that a persistent infection could be driving PTLDs has been a major concern. Arguments against this hypothesis include studies showing that symptomatic patients were not more likely to be seropositive than recovered patients and that patients did not develop objective manifestations of late Lyme disease. 49, 104 Also, no direct evidence of B. burgdorferi infection was found in these patients (however, B. burgdorferi culture and PCR have low sensitivity in most body fluids from patients with Lyme disease).105
In animal studies, B. burgdorferi DNA and RNA can be detected in tissues for months after antibiotic treatment but is not found by culture in antibiotic-treated animals, while untreated control mice are culture positive, and have significantly higher DNA and RNA copy numbers.106–108 One hypothesis is that some of the spirochetes could become “persisters”. Persistence is a survival mechanism that allows organisms to endure diverse adverse environmental conditions by entering a physiologically dormant state.109 This mechanism is nongenetic and nonheritable (as opposed to acquisition of antibiotic resistance, which does not appear to occur with B. burgdorferi). Resuscitation (exiting the persistent state) is a hallmark of the persistence phenotype, and persister cells will restart proliferating when the adverse condition is removed.110 For B. burgdorferi, persister bacteria have been identified in vitro, where, as expected, B. burgdorferi persisters are able to grow back after removal of the adverse condition.111 The inability to culture bacteria from animals, particularly mice, months after antibiotic therapy is difficult to explain if persisters remained in the tissues of treated animals. One possibility would be that the bacteria is in a viable, but non-culturable (VBNC) state. It is unclear if VBNC actually occurs, or that persisters that do not resuscitate are dead cells.112 There is no convincing data that B. burgdorferi forms true biofilms.113
B. burgdorferi DNA was detected by xenodiagnosis after antibiotic treatment in mice and non-human primate studies, while cultures of tick contents were almost always negative. A clinical study showed that xenodiagnosis using pathogen-free I. scapularis larval ticks was safe; and the procedure, while quite cumbersome, was well tolerated.114 Xenodiagnosis was positive for B. burgdorferi DNA in one of the 10 patients with PTLDS tested. The discussion has been the significance of the detection of borrelial DNA by xenodiagnosis in relation to the presence (or not) of viable spirochetes, when it was not possible to demonstrate the recovery of live spirochetes by culture.115 On the other side, the question is how B. burgdorferi DNA reached the xenodiagnostic ticks if the spirochetes were not alive. The possibility that very sensitive PCR tests could be detecting rare DNA fragments leftover in the skin is unlikely, as all 39 skin biopsy specimens collected 6 months post therapy from the area of erythema migrans lesions were negative using the same assay as the clinical study.116 Another interesting question is how to interpret a negative result in a non-reservoir competent host for B. burgdorferi and what these findings can teach us about borrelial infection in humans. A larger study to assess whether a positive xenodiagnosis correlates with persistence of symptoms in ongoing.
The results from the antibiotic treatment trials
An important question is if PTLDs patients benefit from additional treatment. To answer this question, five randomized, placebo-controlled, double-blind clinical trials have been conducted.82, 83, 117, 118 These studies have shown that prolonged antibiotic treatment offered no sustained benefit, while having potential serious risks.
The first two studies, one for patients who were IgG seropositive for B. burgdorferi at enrollment, and the other for seronegative patients, were published together.82 Participants were randomized to either ceftriaxone 2 g intravenous daily for 30 days, followed by oral doxycycline, 200 mg daily for 60 days (n=64), or matching intravenous and oral placebos (n=65). The studies were stopped early when a planned interim analysis showed it was highly unlikely that a significant difference in treatment efficacy between the groups would be observed. There were no significant differences in quality-of-life scores between the treatment groups at 30, 90, and 180 days in the studies, with about a third of the patients improving, a third worsening and a third unchanged. There were 2 severe adverse events related to treatment.
The next study was the STOP-LD (Study and Treatment of Post Lyme Disease) and enrolled 55 PTLDS patients with persistent significant fatigue.117 Patients were randomized to 28 days of intravenous ceftriaxone 2 g or placebo. There was improvement of fatigue with ceftriaxone therapy at 6 months but no improvement in mental speed or other neurocognitive measures. Exploratory analysis showed that patients who had positive immunoblot, had not received previous intravenous antibiotic therapy, and had less pain were more likely to improve. Three patients in each group discontinued therapy due to side effects, and 4 had to be hospitalized.
The fourth study enrolled PTLDS patients treated with at least 3 weeks of intravenous antibiotic therapy, seropositive by IgG western blot, and objective memory impairment.83 Patients were randomized 2:1 to receive 10 weeks of intravenous ceftriaxone (23 patients) or placebo (14 patients), with 20 patients in the ceftriaxone group and 12 patients in the placebo group completing follow up. Using a model with an aggregate of the six domains of neurocognitive performance measured in the study (chosen by a data-driven selection process, which complicated the interpretation of the results),119 the authors reported a borderline significant small improvement at 12 weeks in the ceftriaxone group. However, both placebo and ceftriaxone groups had similar improvements from baseline when analyzed at 24 weeks. Nine patients discontinued therapy due to side effects. These included three allergic reactions, two thrombus, one staphylococcal infection, and two due to pain. One patient on ceftriaxone underwent cholecystectomy at week 16.
The fifth study is the Persistent Lyme Empiric Antibiotic Study Europe (PLEASE).118 This study, performed in the Netherlands, enrolled 280 participants with persistent symptoms attributed to Lyme disease. About 90% of the patients had received antibiotic treatment, and the median duration of symptoms was more than 2 years. After an initial 2-week treatment with open-label intravenous ceftriaxone, participants were randomized to receive 12 weeks of oral doxycycline with a placebo twice a day, clarithromycin with hydroxychloroquine twice a day, or two placebos twice a day. All groups improved when compared with their initial scores but there were no differences between groups at any time point during follow-up.120 Because all participants received ceftriaxone therapy, it is unknown if the initial improvement was due to the therapy or a placebo effect. The improvement on fatigue was similar to the improvement seen in the intravenous placebo arm of two other studies.121 Regarding safety, 9 patients had a serious adverse event, and 19 patients had an adverse event that led to discontinuation of the study drug.
An interesting analysis, derived from the PLEASE study, examined predictors of symptom improvement.122 Pre-treatment functioning, higher pre-treatment expectancy to improve, and thinking that one had received antibiotics at the end of therapy were positively associated with physical and mental improvement at both end-of-treatment and at follow-up. This highlights the important role of patients’ positive or negative expectancies on symptom course and treatment outcome. This is a well-known issue within the area of placebo (and nocebo) research, and it is important to consider the patient’s expectations of therapeutic benefit as a confounding aspect in clinical trials. Similarly, these considerations apply when evaluating personal reports of the success of alternative therapies and practices. In these contexts, the many variables shown to increase placebo effect are being combined. These include costly doctor’s visits and tests, perceived expertise, complex (and expensive) treatment regimens, priming, social influence and emotional investment, to cite a few.123 How to integrate these multiple variables into therapeutic research is an interesting and challenging topic.
Summary
The controversy regarding the underlying mechanisms of PTLDs, and the increasingly bitter debate over CLD, continues unabated over the past years. If anything, it has become more contentious. For health care providers, it is important that patients be offered the best advice based on current, evidence-based information. Patients treated for Lyme disease can be reassured that most will fully recover after recommended antibiotic therapy,2 and that nonspecific symptom will improve over time. Evaluation of patients with PTLDs and CLD can be quite challenging. Practitioners should carefully review the evidence for the diagnosis of Lyme disease, and not lose sight that symptoms may be due to unrelated conditions. Most importantly is a collaborative approach to the treatment process with the patient, with warmth, empathy, and positive communication. More research on the pathogenesis of PTLDs, particularly biomarkers that would allow for differentiation accordingly to the underlying process, is urgently needed. Such markers could give insights into pathways and lead to valuable treatments. Current evidence shows that prolonged antibiotic therapy, as tested in the randomized placebo-controlled clinical trials, does not offer substantive benefits for PTLDs patients. These studies also showed significant placebo effect and variability of the intensity of subjective symptoms over time. Therefore, interventional studies in this population must have a double-blind, randomized controlled design. There is a critical need for high-quality research to better understand “chronic Lyme disease”, and how to best help this large and heterogenous group of patients.
Synopsis:
Most patients with Lyme disease will fully recover with recommended antibiotic therapy. However, some patients report persisting nonspecific symptoms after treatment, referred to as post-treatment Lyme disease symptoms (PTLDs) or syndrome (PTLDS), depending on the degree which the individual’s symptoms impacts their quality of life. PTLDs occurs in a portion of patients diagnosed with chronic Lyme disease (CLD), a controversial term describing different patient populations, diagnosed based on unvalidated tests and criteria. Practitioners should review the evidence for the Lyme disease diagnosis and not overlook unrelated conditions. Current evidence shows that prolonged antibiotic therapy provides little benefit and carries significant risk. Further research to elucidate the mechanisms underlying persistent symptoms after Lyme disease and to understand CLD is needed.
Key points.
Lyme disease is treated successfully with recommended antibiotics in the majority of cases; however, some patients have persisting nonspecific symptoms after treatment, referred to as post-treatment Lyme disease symptoms (PTLDs) or syndrome (PTLDS).
Chronic Lyme disease (CLD) is a controversial term, with no defined diagnostic criteria, used to describe different patient populations. Patients with PTLDs represent a small portion of CLD patients.
Most CLD patients suffer from other conditions or are patients with medically unexplained physical symptoms and are diagnosed with CLD based on unvalidated tests and criteria.
There is a critical need for more research to elucidate the mechanisms underlying PTLD, together with research into patients who fall under the CLD heading.
Clinical Care Points.
Patients treated for Lyme disease can be reassured that most will fully recover after recommended antibiotic therapy and that nonspecific symptom will improve over time.
Evaluating patients with symptoms after Lyme disease or with the diagnosis of chronic Lyme disease can be challenging. Different factors are likely to play a role in an individual case.
Symptoms and signs due to other conditions can be wrongly attributed to Lyme disease. Practitioners should carefully review the evidence for the diagnosis of Lyme disease, and not lose sight that symptoms may be due to unrelated conditions.
Current evidence shows that prolonged antibiotic therapy for PTLDs provides little benefit and carries significant risk.
Acknowledgments:
Funding for this study was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosures: AM has a patent US 8,926,989 issued; and is an unpaid Scientific Advisor to the Global Lyme Alliance and to the American Lyme Disease Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marques AR, Strle F, Wormser GP. Comparison of Lyme Disease in the United States and Europe. Emerg Infect Dis. Aug 2021;27(8):2017–2024. doi: 10.3201/eid2708.204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clin Infect Dis. Jan 23 2021;72(1):1–8. doi: 10.1093/cid/ciab049 [DOI] [PubMed] [Google Scholar]
- 3.Turk SP, Lumbard K, Liepshutz K, et al. Post-treatment Lyme disease symptoms score: Developing a new tool for research. PLoS One. 2019;14(11):e0225012. doi: 10.1371/journal.pone.0225012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fallon BA, Pavlicova M, Coffino SW, Brenner C. A comparison of lyme disease serologic test results from 4 laboratories in patients with persistent symptoms after antibiotic treatment. Clin Infect Dis. Dec 15 2014;59(12):1705–10. doi: 10.1093/cid/ciu703 [DOI] [PubMed] [Google Scholar]
- 5.Johnson BJ, Pilgard MA, Russell TM. Assessment of new culture method for detection of Borrelia species from serum of lyme disease patients. Research Support, U.S. Gov’t, P.H.S. J Clin Microbiol. Mar 2014;52(3):721–4. doi: 10.1128/JCM.01674-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormser GP, Shapiro ED, Strle F. Studies that report unexpected positive blood cultures for Lyme borrelia - are they valid? Diagn Microbiol Infect Dis. Nov 2017;89(3):178–181. doi: 10.1016/j.diagmicrobio.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques AR, Stock F, Gill V. Evaluation of a new culture medium for Borrelia burgdorferi. J Clin Microbiol. Nov 2000;38(11):4239–41. doi: 10.1128/JCM.38.11.4239-4241.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klempner MS, Schmid CH, Hu L, et al. Intralaboratory reliability of serologic and urine testing for Lyme disease. Am J Med. Feb 15 2001;110(3):217–9. doi: 10.1016/s00029343(00)00701-4 [DOI] [PubMed] [Google Scholar]
- 9.Marques A, Brown MR, Fleisher TA. Natural killer cell counts are not different between patients with post-Lyme disease syndrome and controls. Clin Vaccine Immunol. Aug 2009;16(8):1249–50. doi: 10.1128/CVI.00167-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankl S, Hadar PN, Yakhkind A, Lang AE, Sandsmark DK. Devastating Neurological Injury as a Result of Treatment of “Chronic Lyme Disease”. Mayo Clin Proc. Jul 2021;96(7):2005–2007. doi: 10.1016/j.mayocp.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Auwaerter PG, Bakken JS, Dattwyler RJ, et al. Antiscience and ethical concerns associated with advocacy of Lyme disease. Lancet Infect Dis. Sep 2011;11(9):713–9. doi: 10.1016/S1473-3099(11)70034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantos PM, Shapiro ED, Auwaerter PG, et al. Unorthodox alternative therapies marketed to treat Lyme disease. Clin Infect Dis. Jun 15 2015;60(12):1776–82. doi: 10.1093/cid/civ186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzec NS, Nelson C, Waldron PR, et al. Serious Bacterial Infections Acquired During Treatment of Patients Given a Diagnosis of Chronic Lyme Disease - United States. MMWR Morb Mortal Wkly Rep. Jun 16 2017;66(23):607–609. doi: 10.15585/mmwr.mm6623a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelton A, Giurgea L, Moshgriz M, Siegel M, Akselrod H. A case of Mycobacterium goodii infection related to an indwelling catheter placed for the treatment of chronic symptoms attributed to Lyme disease. Infect Dis Rep. Sep 18 2019;11(2):8108. doi: 10.4081/idr.2019.8108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodlet KJ, Fairman KA. Adverse Events Associated With Antibiotics and Intravenous Therapies for Post-Lyme Disease Syndrome in a Commercially Insured Sample. Clin Infect Dis. Oct 30 2018;67(10):1568–1574. doi: 10.1093/cid/ciy329 [DOI] [PubMed] [Google Scholar]
- 16.Ettestad PJ, Campbell GL, Welbel SF, et al. Biliary complications in the treatment of unsubstantiated Lyme disease. J Infect Dis. Feb 1995;171(2):356–61. doi: 10.1093/infdis/171.2.356 [DOI] [PubMed] [Google Scholar]
- 17.Nelson C, Elmendorf S, Mead P. Neoplasms misdiagnosed as “chronic lyme disease”. JAMA Intern Med. Jan 2015;175(1):132–3. doi: 10.1001/jamainternmed.2014.5426 [DOI] [PubMed] [Google Scholar]
- 18.Strizova Z, Patek O, Vitova L, Horackova M, Bartunkova J. Internet-based self-diagnosis of Lyme disease caused death in a young woman with systemic lupus erythematosus. Joint Bone Spine. Oct 2019;86(5):650–651. doi: 10.1016/j.jbspin.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 19.Reid MC, Schoen RT, Evans J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med. Mar 1 1998;128(5):354–62. doi: 10.7326/0003-4819-128-5-199803010-00003 [DOI] [PubMed] [Google Scholar]
- 20.Sigal LH. Summary of the first 100 patients seen at a Lyme disease referral center. Am J Med. Jun 1990;88(6):577–81. doi: 10.1016/0002-9343(90)90520-n [DOI] [PubMed] [Google Scholar]
- 21.Steere AC, Taylor E, McHugh GL, Logigian EL. The overdiagnosis of Lyme disease. JAMA. Apr 14 1993;269(14):1812–6. [PubMed] [Google Scholar]
- 22.Rose CD, Fawcett PT, Gibney KM, Doughty RA. The overdiagnosis of Lyme disease in children residing in an endemic area. Clinical pediatrics. Nov 1994;33(11):663–8. doi: 10.1177/000992289403301105 [DOI] [PubMed] [Google Scholar]
- 23.Aucott JN, Seifter A, Rebman AW. Probable late lyme disease: a variant manifestation of untreated Borrelia burgdorferi infection. BMC Infect Dis. Aug 1 2012;12:173. doi: 10.1186/14712334-12-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, Higgins Y, Samuels R, et al. Misdiagnosis of Lyme Disease With Unnecessary Antimicrobial Treatment Characterizes Patients Referred to an Academic Infectious Diseases Clinic. Open Forum Infect Dis. Jul 1 2019;6(7)doi: 10.1093/ofid/ofz299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gynthersen RMM, Tetens MM, Orbaek M, et al. Classification of patients referred under suspicion of tick-borne diseases, Copenhagen, Denmark. Ticks and tick-borne diseases. Jan 2021;12(1):101591. doi: 10.1016/j.ttbdis.2020.101591 [DOI] [PubMed] [Google Scholar]
- 26.Kortela E, Kanerva M, Kurkela S, Oksi J, Jarvinen A. Suspicion of Lyme borreliosis in patients referred to an infectious diseases clinic: what did the patients really have? Clin Microbiol Infect. Jul 2021;27(7):1022–1028. doi: 10.1016/j.cmi.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 27.van Westrienen PE, Pisters MF, Veenhof C, de Wit NJ. Identification of patients with moderate medically unexplained physical symptoms in primary care with a five years follow-up. BMC Fam Pract. May 21 2019;20(1):66. doi: 10.1186/s12875-019-0950-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kop WJ, Toussaint A, Mols F, Lowe B. Somatic symptom disorder in the general population: Associations with medical status and health care utilization using the SSD-12. Gen Hosp Psychiatry. Jan – Feb 2019;56:36–41. doi: 10.1016/j.genhosppsych.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 29.Dahli MP, Saltyte-Benth J, Haavet OR, Ruud T, Brekke M. Somatic symptoms and associations with common psychological diagnoses: a retrospective cohort study from Norwegian urban general practice. Fam Pract. Nov 24 2021;38(6):766–772. doi: 10.1093/fampra/cmab038 [DOI] [PubMed] [Google Scholar]
- 30.Chalder T, Willis C. “Lumping” and “splitting” medically unexplained symptoms: is there a role for a transdiagnostic approach? J Ment Health. Jun 2017;26(3):187–191. doi: 10.1080/09638237.2017.1322187 [DOI] [PubMed] [Google Scholar]
- 31.den Boer C, Dries L, Terluin B, et al. Central sensitization in chronic pain and medically unexplained symptom research: A systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res. Feb 2019;117:32–40. doi: 10.1016/j.jpsychores.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 32.Patrick DM, Miller RR, Gardy JL, et al. Lyme Disease Diagnosed by Alternative Methods: A Phenotype Similar to That of Chronic Fatigue Syndrome. Clin Infect Dis. Oct 1 2015;61(7):1084–91. doi: 10.1093/cid/civ470 [DOI] [PubMed] [Google Scholar]
- 33.Bouquet J, Gardy JL, Brown S, et al. RNA-Seq Analysis of Gene Expression, Viral Pathogen, and B-Cell/T-Cell Receptor Signatures in Complex Chronic Disease. Clin Infect Dis. Feb 15 2017;64(4):476–481. doi: 10.1093/cid/ciw767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. Nov 1 2006;43(9):1089–134. doi: 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 35.Aucott JN, Crowder LA, Kortte KB. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int J Infect Dis. Jun 2013;17(6):e443–9. doi: 10.1016/j.ijid.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 36.Wormser GP, McKenna D, Karmen CL, et al. Prospective Evaluation of the Frequency and Severity of Symptoms in Lyme Disease Patients With Erythema Migrans Compared With Matched Controls at Baseline, 6 Months, and 12 Months. Clin Infect Dis. Dec 15 2020;71(12):3118–3124. doi: 10.1093/cid/ciz1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stupica D, Bajrovic FF, Blagus R, et al. Clinical manifestations and long-term outcome of early Lyme neuroborreliosis according to the European Federation of Neurological Societies diagnostic criteria (definite versus possible) in central Europe. A retrospective cohort study. European journal of neurology : the official journal of the European Federation of Neurological Societies. Sep 2021;28(9):3155–3166. doi: 10.1111/ene.14962 [DOI] [PubMed] [Google Scholar]
- 38.Marques A, Okpali G, Liepshutz K, Ortega-Villa AM. Characteristics and outcome of facial nerve palsy from Lyme neuroborreliosis in the United States. Ann Clin Transl Neurol. Jan 2022;9(1):41–49. doi: 10.1002/acn3.51488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lochhead RB, Strle K, Arvikar SL, Weis JJ, Steere AC. Lyme arthritis: linking infection, inflammation and autoimmunity. Nat Rev Rheumatol. Aug 2021;17(8):449–461. doi: 10.1038/s41584-021-00648-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stupica D, Bajrovic FF, Blagus R, et al. Association between statin use and clinical course, microbiologic characteristics, and long-term outcome of early Lyme borreliosis. A post hoc analysis of prospective clinical trials of adult patients with erythema migrans. PLoS One. 2021;16(12):e0261194. doi: 10.1371/journal.pone.0261194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stupica D, Maraspin V, Bogovic P, et al. Comparison of Clinical Course and Treatment Outcome for Patients With Early Disseminated or Early Localized Lyme Borreliosis. JAMA Dermatol. Sep 1 2018;154(9):1050–1056. doi: 10.1001/jamadermatol.2018.2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borsic K, Blagus R, Cerar T, Strle F, Stupica D. Clinical Course, Serologic Response, and Long-Term Outcome in Elderly Patients with Early Lyme Borreliosis. J Clin Med. Dec 2 2018;7(12)doi: 10.3390/jcm7120506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eliassen KE, Hjetland R, Reiso H, Lindbaek M, Tschudi-Madsen H. Symptom load and general function among patients with erythema migrans: a prospective study with a 1-year follow-up after antibiotic treatment in Norwegian general practice. Scand J Prim Health Care. Mar 2017;35(1):75–83. doi: 10.1080/02813432.2017.1288812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bechtold KT, Rebman AW, Crowder LA, Johnson-Greene D, Aucott JN. Standardized Symptom Measurement of Individuals with Early Lyme Disease Over Time. Arch Clin Neuropsychol. Mar 1 2017;32(2):129–141. doi: 10.1093/arclin/acw098 [DOI] [PubMed] [Google Scholar]
- 45.Arnez M, Ruzic-Sabljic E. Azithromycin Is Equally Effective as Amoxicillin in Children with Solitary Erythema Migrans. Pediatr Infect Dis J. Oct 2015;34(10):1045–8. doi: 10.1097/INF.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 46.Stupica D, Lusa L, Ruzic-Sabljic E, Cerar T, Strle F. Treatment of erythema migrans with doxycycline for 10 days versus 15 days. Clin Infect Dis. Aug 2012;55(3):343–50. doi: 10.1093/cid/cis402 [DOI] [PubMed] [Google Scholar]
- 47.Cerar D, Cerar T, Ruzic-Sabljic E, Wormser GP, Strle F. Subjective symptoms after treatment of early Lyme disease. Am J Med. Jan 2010;123(1):79–86. doi: 10.1016/j.amjmed.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 48.Wormser GP, Ramanathan R, Nowakowski J, et al. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. May 6 2003;138(9):697–704. doi: 10.7326/0003-4819-138-9-200305060-00005 [DOI] [PubMed] [Google Scholar]
- 49.Nowakowski J, Nadelman RB, Sell R, et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med. Aug 1 2003;115(2):91–6. doi: 10.1016/s0002-9343(03)00308-5 [DOI] [PubMed] [Google Scholar]
- 50.Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. Mar 19 2002;136(6):421–8. doi: 10.7326/0003-4819-136-6-200203190-00005 [DOI] [PubMed] [Google Scholar]
- 51.Eppes SC, Childs JA. Comparative study of cefuroxime axetil versus amoxicillin in children with early Lyme disease. Pediatrics. Jun 2002;109(6):1173–7. doi: 10.1542/peds.109.6.1173 [DOI] [PubMed] [Google Scholar]
- 52.Arnez M, Pleterski-Rigler D, Luznik-Bufon T, Ruzic-Sabljic E, Strle F. Solitary erythema migrans in children: comparison of treatment with azithromycin and phenoxymethylpenicillin. Wien Klin Wochenschr. Jul 31 2002;114(13–14):498–504. [PubMed] [Google Scholar]
- 53.Barsic B, Maretic T, Majerus L, Strugar J. Comparison of azithromycin and doxycycline in the treatment of erythema migrans. Infection. May–Jun 2000;28(3):153–6. doi: 10.1007/s150100050069 [DOI] [PubMed] [Google Scholar]
- 54.Dattwyler RJ, Luft BJ, Kunkel MJ, et al. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. N Engl J Med. Jul 31 1997;337(5):289–94. doi: 10.1056/NEJM199707313370501 [DOI] [PubMed] [Google Scholar]
- 55.Luft BJ, Dattwyler RJ, Johnson RC, et al. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann Intern Med. May 1 1996;124(9):785–91. doi: 10.7326/0003-4819-124-9-199605010-00002 [DOI] [PubMed] [Google Scholar]
- 56.Gerber MA, Shapiro ED, Burke GS, Parcells VJ, Bell GL. Lyme disease in children in southeastern Connecticut. Pediatric Lyme Disease Study Group. N Engl J Med. Oct 24 1996;335(17):1270–4. doi: 10.1056/NEJM199610243351703 [DOI] [PubMed] [Google Scholar]
- 57.Luger SW, Paparone P, Wormser GP, et al. Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrob Agents Chemother. Mar 1995;39(3):661–7. doi: 10.1128/AAC.39.3.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strle F, Preac-Mursic V, Cimperman J, Ruzic E, Maraspin V, Jereb M. Azithromycin versus doxycycline for treatment of erythema migrans: clinical and microbiological findings. Infection. Mar–Apr 1993;21(2):83–8. doi: 10.1007/BF01710737 [DOI] [PubMed] [Google Scholar]
- 59.Nadelman RB, Luger SW, Frank E, Wisniewski M, Collins JJ, Wormser GP. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. Aug 15 1992;117(4):273–80. doi: 10.7326/0003-4819-117-4-273 [DOI] [PubMed] [Google Scholar]
- 60.Ursinus J, Vrijmoeth HD, Harms MG, et al. Prevalence of persistent symptoms after treatment for lyme borreliosis: A prospective observational cohort study. Lancet Reg Health Eur. Jul 2021;6:100142. doi: 10.1016/j.lanepe.2021.100142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aucott JN, Yang T, Yoon I, Powell D, Geller SA, Rebman AW. Risk of post-treatment Lyme disease in patients with ideally-treated early Lyme disease: A prospective cohort study. Int J Infect Dis. Mar 2022;116:230–237. doi: 10.1016/j.ijid.2022.01.033 [DOI] [PubMed] [Google Scholar]
- 62.Arnez M, Radsel-Medvescek A, Pleterski-Rigler D, Ruzic-Sabljic E, Strle F. Comparison of cefuroxime axetil and phenoxymethyl penicillin for the treatment of children with solitary erythema migrans. Wien Klin Wochenschr. Dec 10 1999;111(22–23):916–22. [PubMed] [Google Scholar]
- 63.Weitzner E, McKenna D, Nowakowski J, et al. Long-term Assessment of Post-Treatment Symptoms in Patients With Culture-Confirmed Early Lyme Disease. Clin Infect Dis. Dec 15 2015;61(12):1800–6. doi: 10.1093/cid/civ735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirsch AG, Poulsen MN, Nordberg C, et al. Risk Factors and Outcomes of Treatment Delays in Lyme Disease: A Population-Based Retrospective Cohort Study. Front Med (Lausanne). 2020;7:560018. doi: 10.3389/fmed.2020.560018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wormser GP, McKenna D, Shaffer KD, Silverman JH, Scavarda C, Visintainer P. Evaluation of selected variables to determine if any had predictive value for, or correlated with, residual symptoms at approximately 12 months after diagnosis and treatment of early Lyme disease. Diagn Microbiol Infect Dis. Jul 2021;100(3):115348. doi: 10.1016/j.diagmicrobio.2021.115348 [DOI] [PubMed] [Google Scholar]
- 66.Wills AB, Spaulding AB, Adjemian J, et al. Long-term Follow-up of Patients With Lyme Disease: Longitudinal Analysis of Clinical and Quality-of-life Measures. Clin Infect Dis. Jun 15 2016;62(12):1546–1551. doi: 10.1093/cid/ciw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feder HM Jr., Johnson BJ, O’Connell S, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. Oct 4 2007;357(14):1422–30. doi: 10.1056/NEJMra072023 [DOI] [PubMed] [Google Scholar]
- 68.Stupica D, Veluscek M, Blagus R, et al. Oral doxycycline versus intravenous ceftriaxone for treatment of multiple erythema migrans: an open-label alternate-treatment observational trial. J Antimicrob Chemother. May 1 2018;73(5):1352–1358. doi: 10.1093/jac/dkx534 [DOI] [PubMed] [Google Scholar]
- 69.Wormser GP, Weitzner E, McKenna D, et al. Long-term assessment of health-related quality of life in patients with culture-confirmed early Lyme disease. Clin Infect Dis. Jul 15 2015;61(2):244–7. doi: 10.1093/cid/civ277 [DOI] [PubMed] [Google Scholar]
- 70.Hickie I, Davenport T, Wakefield D, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. Sep 16 2006;333(7568):575. doi: 10.1136/bmj.38933.585764.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carter BL, Stiff RE, Elwin K, et al. Health sequelae of human cryptosporidiosis-a 12-month prospective follow-up study. Eur J Clin Microbiol Infect Dis. Sep 2019;38(9):1709–1717. doi: 10.1007/s10096-019-03603-1 [DOI] [PubMed] [Google Scholar]
- 72.Furberg M, Anticona C, Schumann B. Post-infectious fatigue following Puumala virus infection. Infect Dis (Lond). Jul 2019;51(7):519–526. doi: 10.1080/23744235.2019.1605191 [DOI] [PubMed] [Google Scholar]
- 73.Morch K, Hanevik K, Rivenes AC, et al. Chronic fatigue syndrome 5 years after giardiasis: differential diagnoses, characteristics and natural course. BMC Gastroenterol. Feb 12 2013;13:28. doi: 10.1186/1471-230X-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. Sep 2021;27(9):1607–1613. doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Nowakowski J. Long-term assessment of fatigue in patients with culture-confirmed Lyme disease. Am J Med. Feb 2015;128(2):181–4. doi: 10.1016/j.amjmed.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 76.Oksi J, Nikoskelainen J, Hiekkanen H, et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: a double-blind, randomized, placebo-controlled, multicenter clinical study. Eur J Clin Microbiol Infect Dis. Aug 2007;26(8):571–81. doi: 10.1007/s10096-007-0340-2 [DOI] [PubMed] [Google Scholar]
- 77.Dersch R, Sommer H, Rauer S, Meerpohl JJ. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. Journal of neurology. Jan 2016;263(1):17–24. doi: 10.1007/s00415-015-7923-0 [DOI] [PubMed] [Google Scholar]
- 78.Kortela E, Kanerva MJ, Puustinen J, et al. Oral Doxycycline Compared to Intravenous Ceftriaxone in the Treatment of Lyme Neuroborreliosis: A Multicenter, Equivalence, Randomized, Open-label Trial. Clin Infect Dis. Apr 26 2021;72(8):1323–1331. doi: 10.1093/cid/ciaa217 [DOI] [PubMed] [Google Scholar]
- 79.Rohrbeck J, Jordan K, Croft P. The frequency and characteristics of chronic widespread pain in general practice: a case-control study. Br J Gen Pract. Feb 2007;57(535):109–15. [PMC free article] [PubMed] [Google Scholar]
- 80.Skogman BH, Croner S, Nordwall M, Eknefelt M, Ernerudh J, Forsberg P. Lyme neuroborreliosis in children: a prospective study of clinical features, prognosis, and outcome. Pediatr Infect Dis J. Dec 2008;27(12):1089–94. doi: 10.1097/INF.0b013e31817fd423 [DOI] [PubMed] [Google Scholar]
- 81.Markowicz M, Kundi M, Stanek G, Stockinger H. Nonspecific symptoms following infection with Borrelia burgdorferi sensu lato: A retrospective cohort study. Ticks and tick-borne diseases. Jan 2022;13(1):101851. doi: 10.1016/j.ttbdis.2021.101851 [DOI] [PubMed] [Google Scholar]
- 82.Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. Jul 12 2001;345(2):85–92. doi: 10.1056/NEJM200107123450202 [DOI] [PubMed] [Google Scholar]
- 83.Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. Mar 25 2008;70(13):992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d [DOI] [PubMed] [Google Scholar]
- 84.Lantos PM, Wormser GP. Chronic coinfections in patients diagnosed with chronic lyme disease: a systematic review. Am J Med. Nov 2014;127(11):1105–1110. doi: 10.1016/j.amjmed.2014.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krause PJ, Telford SR 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. Jun 5 1996;275(21):1657–60. [PubMed] [Google Scholar]
- 86.Wang TJ, Liang MH, Sangha O, et al. Coexposure to Borrelia burgdorferi and Babesia microti does not worsen the long-term outcome of lyme disease. Clin Infect Dis. Nov 2000;31(5):1149–54. doi: 10.1086/317465 [DOI] [PubMed] [Google Scholar]
- 87.Morrissette M, Pitt N, Gonzalez A, et al. A Distinct Microbiome Signature in Posttreatment Lyme Disease Patients. mBio. Sep 29 2020;11(5)doi: 10.1128/mBio.02310-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zimmermann P, Curtis N. Factors Influencing the Intestinal Microbiome During the First Year of Life. Pediatr Infect Dis J. Dec 2018;37(12):e315–e335. doi: 10.1097/INF.0000000000002103 [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald BL, Graham B, Delorey MJ, et al. Metabolic Response in Patients With Post-treatment Lyme Disease Symptoms/Syndrome. Clin Infect Dis. Oct 5 2021;73(7):e2342–e2349. doi: 10.1093/cid/ciaa1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chandra A, Wormser GP, Klempner MS, et al. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun. Aug 2010;24(6):1018–24. doi: 10.1016/j.bbi.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacek E, Fallon BA, Chandra A, Crow MK, Wormser GP, Alaedini A. Increased IFNalpha activity and differential antibody response in patients with a history of Lyme disease and persistent cognitive deficits. J Neuroimmunol. Feb 15 2013;255(1–2):85–91. doi: 10.1016/j.jneuroim.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chandra A, Wormser GP, Marques AR, Latov N, Alaedini A. Anti-Borrelia burgdorferi antibody profile in post-Lyme disease syndrome. Clin Vaccine Immunol. May 2011;18(5):767–71. doi: 10.1128/CVI.00002-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chandra A, Latov N, Wormser GP, Marques AR, Alaedini A. Epitope mapping of antibodies to VlsE protein of Borrelia burgdorferi in post-Lyme disease syndrome. Clin Immunol. Oct 2011;141(1):103–10. doi: 10.1016/j.clim.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fallon BA, Strobino B, Reim S, Stoner J, Cunningham MW. Anti-lysoganglioside and other anti-neuronal autoantibodies in post-treatment Lyme Disease and Erythema Migrans after repeat infection. Brain Behav Immun Health. Feb 2020;2:100015. doi: 10.1016/j.bbih.2019.100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strle K, Stupica D, Drouin EE, Steere AC, Strle F. Elevated levels of IL-23 in a subset of patients with post-lyme disease symptoms following erythema migrans. Clin Infect Dis. Feb 2014;58(3):372–80. doi: 10.1093/cid/cit735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang KS, Klempner MS, Wormser GP, Marques AR, Alaedini A. Association of immune response to endothelial cell growth factor with early disseminated and late manifestations of Lyme disease but not posttreatment Lyme disease syndrome. Clin Infect Dis. Dec 1 2015;61(11):1703–6. doi: 10.1093/cid/civ638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aucott JN, Soloski MJ, Rebman AW, et al. CCL19 as a Chemokine Risk Factor for Posttreatment Lyme Disease Syndrome: a Prospective Clinical Cohort Study. Clin Vaccine Immunol. Sep 2016;23(9):757–66. doi: 10.1128/CVI.00071-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blum LK, Adamska JZ, Martin DS, et al. Robust B Cell Responses Predict Rapid Resolution of Lyme Disease. Front Immunol. 2018;9:1634. doi: 10.3389/fimmu.2018.01634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bouquet J, Soloski MJ, Swei A, et al. Longitudinal Transcriptome Analysis Reveals a Sustained Differential Gene Expression Signature in Patients Treated for Acute Lyme Disease. mBio. Feb 12 2016;7(1):e00100–16. doi: 10.1128/mBio.00100-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clarke DJB, Rebman AW, Bailey A, et al. Predicting Lyme Disease From Patients’ Peripheral Blood Mononuclear Cells Profiled With RNA-Sequencing. Front Immunol. 2021;12:636289. doi: 10.3389/fimmu.2021.636289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wormser GP, Nadelman RB, Schwartz I. The amber theory of Lyme arthritis: initial description and clinical implications. Clin Rheumatol. Jun 2012;31(6):989–94. doi: 10.1007/s10067-012-1964-x [DOI] [PubMed] [Google Scholar]
- 102.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest. Jul 2012;122(7):2652–60. doi: 10.1172/JCI58813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jutras BL, Lochhead RB, Kloos ZA, et al. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc Natl Acad Sci U S A. Jul 2 2019;116(27):13498–13507. doi: 10.1073/pnas.1904170116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kalish RA, Kaplan RF, Taylor E, Jones-Woodward L, Workman K, Steere AC. Evaluation of study patients with Lyme disease, 10–20-year follow-up. Journal of Infectious Diseases. 2001;183(3):453–460. [DOI] [PubMed] [Google Scholar]
- 105.Schutzer SE, Body BA, Boyle J, et al. Direct Diagnostic Tests for Lyme Disease. Clin Infect Dis. Mar 5 2019;68(6):1052–1057. doi: 10.1093/cid/ciy614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. PLoS One. 2014;9(1):e86907. doi: 10.1371/journal.pone.0086907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Embers ME, Hasenkampf NR, Jacobs MB, et al. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS One. 2017;12(12):e0189071. doi: 10.1371/journal.pone.0189071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hodzic E, Imai DM, Escobar E. Generality of Post-Antimicrobial Treatment Persistence of Borrelia burgdorferi Strains N40 and B31 in Genetically Susceptible and Resistant Mouse Strains. Infect Immun. Oct 2019;87(10)doi: 10.1128/IAI.00442-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song S, Wood TK. Are we really studying persister cells? Environ Microbiol Rep. Feb 2021;13(1):3–7. doi: 10.1111/1758-2229.12849 [DOI] [PubMed] [Google Scholar]
- 110.Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. Dec 16 2016;354(6318)doi: 10.1126/science.aaf4268 [DOI] [PubMed] [Google Scholar]
- 111.Wilmaerts D, Windels EM, Verstraeten N, Michiels J. General Mechanisms Leading to Persister Formation and Awakening. Trends Genet. Jun 2019;35(6):401–411. doi: 10.1016/j.tig.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 112.Kim JS, Chowdhury N, Yamasaki R, Wood TK. Viable but non-culturable and persistence describe the same bacterial stress state. Environmental microbiology. Jun 2018;20(6):2038–2048. doi: 10.1111/1462-2920.14075 [DOI] [PubMed] [Google Scholar]
- 113.Marques A, Lemieux J, Hu L. The Widening Gyre: Controversies in Lyme Disease. In: JD R, DS S, eds. Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions and Disease Pathogenesis Caister Academic Press; 2021:685–702:chap 23. [Google Scholar]
- 114.Marques A, Telford SR 3rd, Turk SP, et al. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis. Apr 2014;58(7):937–45. doi: 10.1093/cid/cit939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bockenstedt LK, Radolf JD. Xenodiagnosis for posttreatment Lyme disease syndrome: resolving the conundrum or adding to it? Comment Editorial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Clin Infect Dis. Apr 2014;58(7):946–8. doi: 10.1093/cid/cit942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mosel MR, Rebman AW, Carolan HE, et al. Molecular Microbiological and Immune Characterization of a Cohort of Patients Diagnosed with Early Lyme Disease. J Clin Microbiol. Dec 17 2020;59(1)doi: 10.1128/JCM.00615-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. Jun 24 2003;60(12):1923–30. doi: 10.1212/01.wnl.0000071227.23769.9e [DOI] [PubMed] [Google Scholar]
- 118.Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized Trial of Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N Engl J Med. Mar 31 2016;374(13):1209–20. doi: 10.1056/NEJMoa1505425 [DOI] [PubMed] [Google Scholar]
- 119.Marques A, Shaw P, Schmid CH, et al. Re: A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Prolonged Lyme disease treatment: enough is enough. Neurology. Jan 27 2009;72(4):383–4; author reply 384. doi: 10.1212/01.wnl.0000343855.45704.7c [DOI] [PubMed] [Google Scholar]
- 120.Berende A, Ter Hofstede HJM, Vos FJ, et al. Effect of prolonged antibiotic treatment on cognition in patients with Lyme borreliosis. Neurology. Mar 26 2019;92(13):e1447–e1455. doi: 10.1212/WNL.0000000000007186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wormser GP. Longer-Term Therapy for Symptoms Attributed to Lyme Disease. N Engl J Med. Sep 8 2016;375(10):997. doi: 10.1056/NEJMc1608044 [DOI] [PubMed] [Google Scholar]
- 122.van Middendorp H, Berende A, Vos FJ, Ter Hofstede HHM, Kullberg BJ, Evers AWM. Expectancies as predictors of symptom improvement after antimicrobial therapy for persistent symptoms attributed to Lyme disease. Clin Rheumatol. Oct 2021;40(10):4295–4308. doi: 10.1007/s10067-021-05760-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ashar YK, Chang LJ, Wager TD. Brain Mechanisms of the Placebo Effect: An Affective Appraisal Account. Annu Rev Clin Psychol. May 8 2017;13:73–98. doi: 10.1146/annurev-clinpsy-021815-093015 [DOI] [PubMed] [Google Scholar]


