Abstract
Background
Methamphetamine misuse affects 27 million people worldwide and is associated with cardiovascular disease (CVD); however, risk factors for CVD among users have not been well studied.
Methods and Results
We studied hospitalized patients in California, captured by the Healthcare Cost and Utilization Project database, between 2005 and 2011. We studied the association between methamphetamine use and CVD (pulmonary hypertension, heart failure, stroke, and myocardial infarction). Among 20 249 026 persons in the Healthcare Cost and Utilization Project, 66 199 used methamphetamines (median follow‐up 4.58 years). Those who used were more likely younger (33 years versus 45 years), male (63.3% versus 44.4%), smoked, misused alcohol, and had depression and anxiety compared with nonusers. Methamphetamine use was associated with the development of heart failure (hazard ratio [HR], 1.53 [95% CI, 1.45–1.62]) and pulmonary hypertension (HR, 1.42 [95% CI, 1.26–1.60]). Among users, male sex (HR, 1.73 [95% CI, 1.37–2.18]) was associated with myocardial infarction. Chronic kidney disease (HR, 2.38 [95% CI, 1.74–3.25]) and hypertension (HR, 2.26 [95% CI, 2.03–2.51]) were strong risk factors for CVD among users. When compared with nonuse, methamphetamine use was associated with a 32% significant increase in CVD, alcohol abuse with a 28% increase, and cocaine use with a 47% increase in CVD.
Conclusions
Methamphetamine use has a similar magnitude of risk of CVD compared with alcohol and cocaine. Prevention and treatment could be focused on those with chronic kidney disease, hypertension, and mental health disorders.
Keywords: congestive heart failure, methamphetamine, MINOCA, myocardial infarction, myocardial infarction without coronary artery obstruction, pulmonary hypertension, stroke
Subject Categories: Epidemiology, Risk Factors
Nonstandard Abbreviations and Acronyms
- HCUP
Healthcare Cost and Utilization Project
- MINOCA
myocardial infarction with nonobstructive coronary arteries
- PHTN
pulmonary hypertension
Clinical Perspective.
What Is New?
Methamphetamine use is a growing global phenomenon and an urgent public health crisis.
We found that methamphetamine users have a significantly increased (32%) risk of cardiovascular disease and in particular of developing pulmonary hypertension and heart failure.
Unlike previous studies, we did not demonstrate a significant association between female sex and incidence of methamphetamine‐associated pulmonary hypertension; however, we found that men were more likely to develop myocardial infarction or myocardial infarction with nonobstructive coronary arteries.
What Are the Clinical Implications?
Among methamphetamine users, those with chronic kidney disease, hypertension, and diabetes were most at risk of cardiovascular disease and as such, these modifiable risk factors should be targeted appropriately.
Further studies should elucidate whether specific treatments can be targeted toward improving outcomes in methamphetamine associated cardiovascular disease (particularly pulmonary hypertension and heart failure).
The dual burdens of cardiovascular and mental health disease among methamphetamine users must be addressed by physicians in order to slow this growing global epidemic.
The disordered use of amphetamines and their associated compounds is a growing global health crisis, with the estimated number of annual users exceeding 27 million people around the world in 2017. 1 Methamphetamine in particular, normally consumed in its crystallized form, carries one of the highest burdens of disease associated with drug use in the United States, 2 second only to the abuse of opioids.
Methamphetamine use underlies many cardiovascular disease (CVD) pathologies, 3 most notably cardiomyopathy 4 , 5 and pulmonary hypertension (PHTN). 6 , 7 Its deleterious effect on the cardiovascular system is thought to be multifactorial; a combination of excess catecholamine release leading to hypertension, tachycardia, and coronary vasospasm, as well as the direct cytotoxic effect of increased reactive oxygen species and mitochondrial injury to cardiac myocytes. 8 , 9 , 10
Methamphetamine use has spread beyond its historical epicenter on the West Coast of the United States and in 2017 accounted for over 964 000 dependent users throughout the United States, 11 with a mean age at first use of 21 years old. 12 A recent cross‐sectional study of approximately 1.3 million methamphetamine‐related hospital admissions between 2003 and 2015 showed a huge increase in hospitalization (over 270%) between 2008 and 2015, with the highest frequency affecting the western United States. 13
There remains a paucity of data relating to the incidence and predictors of CVD among amphetamine users. Cessation of methamphetamine use may prevent and abrogate the deleterious effects on cardiovascular health. 14 It is therefore vital that we better understand the disease burden associated with methamphetamine misuse and identify the key factors that lead to its development, in order to identify targets for prevention and treatment in this high‐risk group.
Using a large database of millions of hospitalized patients in California, called the Healthcare Cost and Utilization Project (HCUP), we aim to identify the prevalence and incidence of CVD among methamphetamine users. By comparing users who develop CVD and those who do not, we aim to identify the key risk factors that contribute to a range of CVD pathologies associated with methamphetamine use.
Methods
From 2005 to 2011, data were obtained from patients in HCUP who received care in a hospitalized setting. The database contains information collected as part of billing records; this includes patient demographics, International Classification of Diseases, Ninth Revision (ICD‐9) coded diagnoses, dates of admission and discharge, and follow‐up. We used 3 HCUP data sets: (1) inpatient, (2) emergency department, and (3) ambulatory surgery and services. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NCa) and Stata 14 (StataCorp, College Station, TX). Institutional review board approval for this study was obtained through the University of California, San Francisco. As this was a claims database study, informed consent was waived.
Data transparency and openness: Because we obtained the data for this study from HCUP/Agency for Healthcare Research and Quality and given their data use agreement stipulations, we are unable to make the data available to other parties for analysis. Further information regarding this data set is available at https://www.hcup‐us.ahrq.gov/.
Among 24 358 668 patients recorded in HCUP who had at least 1 hospital visit from 2005 to 2011, we excluded those aged under 14 years (n=2 656 496), those without recorded mortality outcomes (n=174 845), and those who were not California residents (n=686 235). We also excluded those with missing covariates, including no documentation of drug use, (n=6746), and those with preexisting CVD at baseline (n=585 320), resulting in a sample size of 20 249 026 patients (Figure 1). For the cross‐sectional analysis, we included all adult and adolescent (>14 years) patients identified as methamphetamine users in the HCUP cohort (n=66 199). For cohort analysis, we included all adult patients identified in the HCUP who met our inclusion criteria.
Figure 1. Inclusion flow chart.
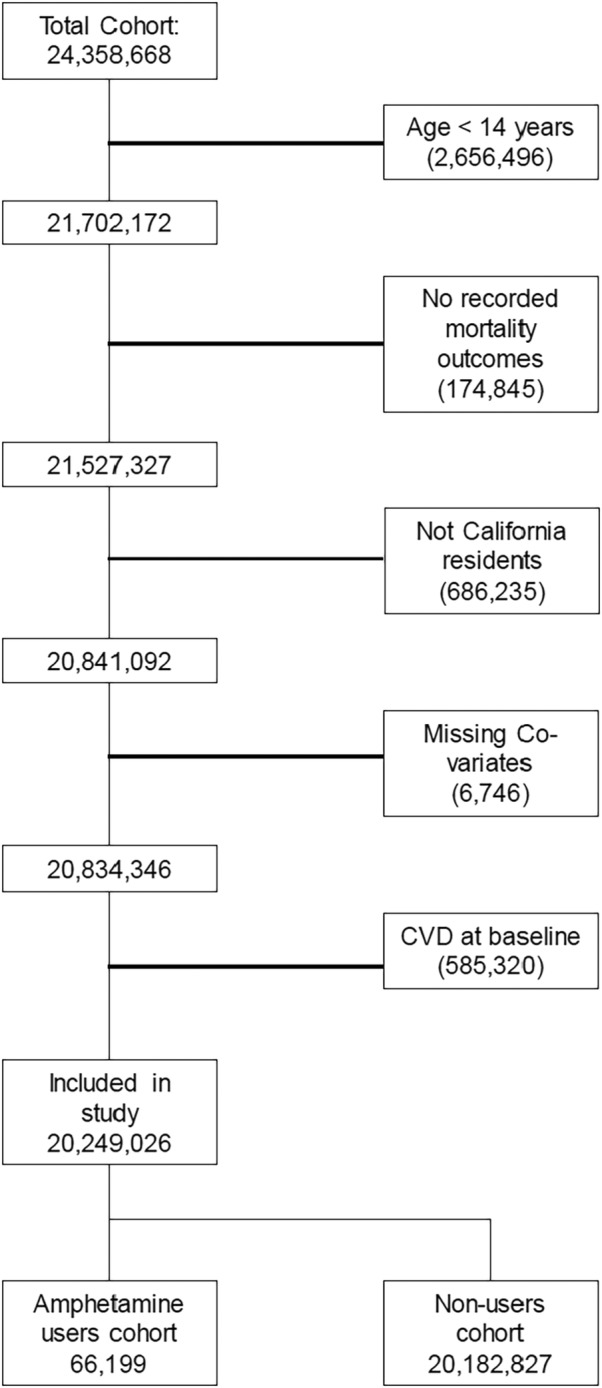
CVD indicates cardiovascular disease.
Patients were indexed at their first health care encounter and methamphetamine use was ascertained based on ICD‐9 coding. Patients not identified as methamphetamine users constituted the referent group. Patients identified as methamphetamine users with preexisting CVD at baseline were excluded from analysis.
Follow‐up began after identification of the initial registered HCUP encounter. The measured outcomes were incident PHTN, stroke, heart failure (HF), myocardial infarction with obstructive coronary artery disease (MI), myocardial infarction with nonobstructive coronary arteries (MINOCA), MI plus MINOCA, and overall CVD burden, defined as a composite of these conditions.
Statistical Analysis
Differences between population means were calculated using unpaired t test for continuous variables and chi‐square test for categorical variables. We used Cox proportional hazards model to assess for the hazards of PHTN, HF, stroke, MI, MINOCA, and MI + MINOCA among methamphetamine users compared with nonusers. Multivariable models were adjusted for age, sex, and race or ethnicity, then additionally for diabetes, hypertension, obesity, hyperlipidemia, chronic kidney disease, depression, smoking status, and cocaine and alcohol abuse.
Results
Prevalence and Correlates of Methamphetamine Use
Among 202 490 246 patients captured by HCUP, followed for a median of 4.6 years (interquartile range 3.0–6.4 years), 66 199 (0.33%) were identified as methamphetamine users. Users were 63.3% male (n=41 922) and were typically younger than nonusers (32.9 ± 11.6 versus 44.9 ± 19.5 years). There was a higher prevalence of coexisting cocaine and alcohol abuse among methamphetamine users (0.33% and 6.5%, respectively) compared with nonusers (0.12% and 0.67% respectively). Methamphetamine users were more likely to be smokers (26.1% versus 3.8%, P<0.001) and have a higher prevalence of chronic depression (11.9 versus 1.9%, P<0.001) and anxiety (4.7 versus 1.5%, P<0.001) than nonusers. In addition, they were more likely than nonusers to be in the 1st income quartile (34.2% versus 24.1%, P<0.001) (Table 1).
Table 1.
Sociodemographic and Clinical Correlates Among Users of Methamphetamines, Nonusers, and the Total Study Population (n, %)
| Total study population (n=20 249 026) | Nonusers (n=20 182 827) | Methamphetamine users (n=66 199) | P value | |
|---|---|---|---|---|
| n (%) | ||||
| Male sex | 9 000 987 (44.4) | 8 959 065 (44.4)* | 41 922 (63.3)* | <0.01 |
| Age (mean, y ± SD) | … | 44.88 ± 19.52 | 32.94 ± 11.63 | <0.01 |
| Race or ethnicity | ||||
| White | 11 347 814 (56.04) | 11 311 240 (56.04) | 36 574 (55.3) | <0.01 |
| Black | 1 483 047 (7.32) | 1 479 127 (7.33) | 3920 (5.9) | <0.01 |
| Hispanic | 5 156 342 (25.46) | 5 134 873 (25.44) | 21 470 (32.4) | <0.01 |
| Asian/Pacific | 1 572 918 (7.77) | 1 570 922 (7.78) | 1996 (3.0) | <0.01 |
| Native American | 30 305 (0.15) | 30 098 (0.15) | 207 (0.3) | <0.01 |
| Other races not specified | 658 599 (3.25) | 656 567 (3.25) | 2032 (3.1) | <0.01 |
| Cocaine abuse | 27 165 (0.13) | 23 449 (0.12) | 3716 (0.33) | <0.01 |
| Alcohol abuse | 140 022 (0.69) | 135 691 (0.67) | 4331 (6.54) | <0.01 |
| Chronic depression | 390 817 (1.93) | 383 470 (1.90) | 7347 (11.10) | <0.01 |
| Anxiety | 296 554 (1.46) | 293 439 (1.45) | 3115 (4.71) | <0.01 |
| Income quartiles | ||||
| First | 4 890 374 (24.15) | 4 867 733 (24.12) | 22 641 (34.20) | <0.01 |
| Second | 5 055 647 (24.97) | 5 036 229 (24.95) | 19 428 (29.35) | <0.01 |
| Third | 5 173 930 (25.55) | 5 159 463 (25.56) | 14 467 (21.85) | <0.01 |
| Fourth | 5 129 075 (25.33) | 5 119 412 (25.37) | 9663 (14.60) | <0.01 |
| Hypertension | 2 732 890 (13.50) | 2 727 149 (13.51) | 5741 (8.67) | <0.01 |
| Smoker | 791 538 (3.91) | 774 249 (3.84) | 17 289 (26.12) | <0.01 |
| Obesity | 503 752 (2.49) | 502 181 (2.49) | 1571 (2.37) | 0.058 |
| Dyslipidemia | 704 687 (3.48) | 703 837 (3.49) | 850 (1.28) | <0.01 |
| Chronic kidney disease | 139 497 (0.69) | 139 298 (0.69) | 199 (0.30) | <0.01 |
| Diabetes | 1 308 196 (6.46) | 1 304 482 (6.46) | 3714 (5.61) | <0.01 |
P value for difference between nonuser and methamphetamine user populations.
Development of CVD Subtypes Among Methamphetamine Users Compared With Nonusers
Unadjusted CVD incidence rates were lower among people who used methamphetamine versus those who did not; however, upon adjustment for age and other risk factors, people who used methamphetamines had a higher risk of incident CVD (Table 2). Methamphetamine users had a 32% increased overall risk of incident CVD (hazard ratio [HR], 1.32 [CI, 1.26–1.38]) compared with nonusers (Table 2). Methamphetamine use was associated with an increased risk of developing each of the studied subtypes of CVD, including HF (HR, 1.53 [CI, 1.45–1.62), MI (HR, 1.19 [CI, 1.08–1.31]), MI or MINOCA (HR, 1.10 [CI, 1.01–1.21]), PHTN (HR, 1.42 [CI, 1.26–1.60]), and stroke (HR, 1.12 [CI, 1.03–1.22]) (Table 2).
Table 2.
Methamphetamine Use and Multivariable Adjusted* Hazards of CVD in HCUP‐CA
| Outcome | Unadjusted incidence rates per 1000‐persons years | HR (95% CI) | |
|---|---|---|---|
| All cardiovascular disease | Methamphetamine users | 6.83 | 1.32 (1.27–1.38) |
| Nonusers | 13.4 | ||
| Heart failure | Methamphetamine users | 4.52 | 1.53 (1.45–1.62) |
| Nonusers | 8.47 | ||
| MI | Methamphetamine users | 1.42 | 1.19 (1.08–1.31) |
| Nonusers | 2.75 | ||
| MINOCA | Methamphetamine users | 0.26 | 0.82 (0.66–1.02) |
| Nonusers | 0.41 | ||
| MI + MINOCA | Methamphetamine users | 1.59 | 1.10 (1.01–1.21) |
| Nonusers | 3.09 | ||
| Pulmonary hypertension | Methamphetamine users | 0.9 | 1.42 (1.26–1.60) |
| Nonusers | 1.63 | ||
| Stroke | Methamphetamine users | 1.7 | 1.12 (1.03–1.22) |
| Nonusers | 4.34 | ||
HCUP‐CA indicates Healthcare Cost and Utilization Project‐California; HR, hazard ratio; MI, myocardial infarction; and MINOCA, myocardial infarction with nonobstructive coronary arteries.
Covariates in the multivariable model included age, sex, race, obesity, hypertension, diabetes, hyperlipidemia, chronic kidney disease, depression, smoking status, health‐payer status, socioeconomic status, and cocaine and alcohol use.
Risk Factors for Development of CVD Outcomes Among Methamphetamine Users
Chronic kidney disease (HR, 2.38 [CI, 1.74–3.25]), hypertension (HR, 2.26 [CI, 2.03–2.51]), diabetes (HR, 1.75 [CI, 1.55–1.97]), and smoking (HR, 1.28 [CI, 1.17–1.40]) were all strongly associated with an increased risk of developing CVD among methamphetamine users (Table 3 , Figure 2A). Although male sex was not a significant risk factor for the development of CVD overall among methamphetamine users (Figure 2A), it was associated with an increased risk of developing MI or MINOCA (HR, 1.73 [CI, 1.37–2.18]) (Figure 2B). Female sex was not found to be associated with increased incidence of any disease subtype in methamphetamine users. Among methamphetamine users, obesity was associated with increased overall risk of CVD (HR, 1.71 [CI, 1.44–2.02]) (Figure 2A), and in particular was associated with PHTN (HR, 2.46 [CI, 1.64–3.67]) (Table 3, Figure 2C) and HF (HR, 1.77 [CI, 1.45–2.16]) (Table 3, Figure 2D ).
Table 3.
Predictors of Cardiovascular Disease among Users of Methamphetamine in HCUP‐CA
| Variable | CVD | HF | PHTN | MI | MI+ MINOCA | Stroke |
|---|---|---|---|---|---|---|
| HR (CI) | ||||||
| Age | 1.07 (1.07–1.08) | 1.07 (1.07–1.08) | 1.08 (1.07–1.09) | 1.09 (1.08–1.10) | 1.08 (1.07–1.09) | 1.07 (1.07–1.08) |
| Sex (male) | 1.09 (0.99–1.20) | 1.08 (0.96–1.22) | 0.93 (0.77–1.20) | 1.73 (1.37–2.18) | 1.44 (1.17–1.77) | 0.94 (0.78–1.13) |
| Race or ethnicity (vs White) | ||||||
| Black | 1.09 (0.93–1.29) | 1.14 (0.937–1.40) | 1.23 (0.79–1.90) | 1.15 (0.80–1.64) | 1.20 (0.87–1.67) | 1.07 (0.76–1.51) |
| Hispanic | 0.90 (0.81–1.01) | 0.93 (0.81–1.06) | 0.77 (0.56–1.05) | 0.86 (0.67–1.10) | 0.85 (0.68–1.07) | 1.01 (0.82–1.25) |
| Asian/Pacific | 1.20 (0.92–1.55) | 0.99 (0.70–1.42) | 1.61(0.85–3.06) | 1.31 (0.75–2.30) | 1.19 (0.69–2.04) | 1.50 (0.93–2.41) |
| Native American | 1.45 (0.78–2.70) | 1.05 (0.44–2.54) | 1.01 (0.14–7.20) | 1.55 (0.38–6.25) | 1.31 (0.33–5.28) | 2.55 (0.95–6.86) |
| Other races not specified | 0.97 (0.73–1.28) | 0.80 (0.55–1.18) | 1.56 (0.82–2.95) | 1.13 (0.63–2.02) | 1.05 (0.60–1.83) | 1.05 (0.60–1.84) |
| Obesity | 1.71 (1.44–2.02) | 1.77 (1.45–2.16) | 2.46 (1.64–3.67) | 1.22 (0.80–1.88) | 1.30 (0.89–1.91) | 1.42 (0.99–2.01) |
| Hypertension | 2.26 (2.03–2.51) | 2.40 (2.11–2.73) | 1.99 (1.49–2.66) | 1.84 (1.46–2.32) | 1.81 (1.46–2.26) | 2.41 (1.97–2.97) |
| Diabetes | 1.75 (1.55–1.96) | 1.85 (1.61–2.13) | 1.71 (1.24–2.34) | 1.73 (1.35–2.32) | 1.74 (1.37–2.20) | 1.83 (1.45–2.30) |
| Dyslipidemia | 1.09 (0.88–1.34) | 0.94 (0.73–1.22) | 1.05 (0.60–1.84) | 1.75 (1.19–2.57) | 1.87 (1.31–2.67) | 1.02 (0.68–1.55) |
| Depression | 0.81 (0.70–0.94) | 0.75 (0.63–0.90) | 0.71 (0.47–1.08) | 0.92 (0.68–1.25) | 0.97 (0.73–1.27) | 0.92 (0.70–1.21) |
| Chronic kidney disease | 2.38 (1.74–3.25) | 3.20 (2.29–4.45) | 4.06 (2.04–8.09) | 1.34 (0.59–3.04) | 1.66 (0.81–3.38) | 1.56 (0.77–3.17) |
| Smoking | 1.28 (1.17–1.40) | 1.26 (1.13–1.41) | 1.29 (1.01–1.65) | 1.25 (1.02–1.53) | 1.27 (1.06–1.54) | 1.16 (0.97–1.40) |
| Cocaine use | 1.14 (0.96–1.35) | 1.17 (0.95–1.44) | 0.83 (0.49–1.41) | 1.62 (1.17–2.23) | 1.45 (1.06–1.98) | 1.03 (0.73–1.46) |
| Alcohol use | 0.91 (0.78–1.07) | 0.91 (0.75–1.11) | 0.87 (0.55–1.36) | 0.79 (0.55–1.13) | 0.80 (0.57–1.12) | 0.91 (0.66–1.24) |
Multivariable‐adjusted HRs are for risk of composite CVD and CVD subtypes: HF, PHTN, MI, MI with or without nonobstructive coronary arteries and stroke, among users of methamphetamine. CVD indicates cardiovascular disease; HCUP‐CA indicates Healthcare Cost and Utilization Project‐California; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; MINOCA, myocardial infarction with nonobstructive coronary arteries; and PHTN, pulmonary hypertension.
Figure 2. Forest plots (hazard ratios or HR [95% CI]) clinical and sociodemographic predictors of cardiovascular disease (CVD) and CVD subtypes among users of methamphetamines.
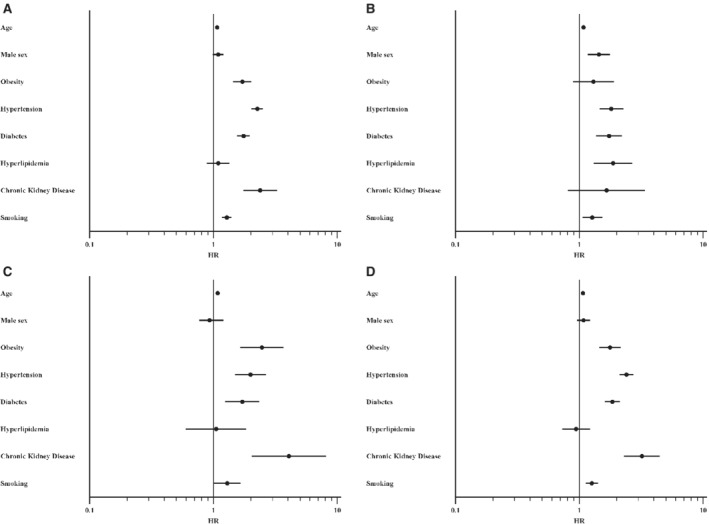
A, CVD; B, myocardial infarction (MI) or MI without obstructive coronary disease; C, pulmonary hypertension; D, heart failure.
Although concurrent cocaine and alcohol use among methamphetamine users was not associated with a significantly increased risk of CVD overall, concomitant cocaine use was associated with increased incidence of MI and/or MINOCA among methamphetamine users (HR, 1.45 [CI, 1.06–2.00]) (Table 3).
Comparison of Incidence of CVD Among Exclusive Methamphetamine Users, Cocaine Users and Alcohol Abusers Compared With Nonusers
There were 58 464 exclusive methamphetamine, 133 968 exclusive alcohol, and 21 726 exclusive cocaine users respectively. Kaplan–Meier curves were constructed from our multivariable models to compare the time to event for development of CVD (measured as a composite of PHTN, HF, stroke, and MI and MINOCA) in populations who used methamphetamines, alcohol or cocaine versus nonusers. In order to isolate the effect of each specific use disorder (methamphetamine, alcohol, and cocaine) we considered patients who had exclusively used each 1 of these substances (Figure 3A , Table 4). People who used cocaine had a 47% (HR, 1.47 [95% CI, 1.40–1.54]) increased risk of developing CVD compared with nonusers (Table 4). Alcohol misusers and methamphetamine users had 28% (HR, 1.28 [95% CI, 1.26–1.31]) and 32% increased risks (HR, 1.32 [95% CI, 1.27–1.38]), respectively (Table 4 , Figure 3A).
Figure 3. Kaplan–Meier plot for time to development of cardiovascular disease.
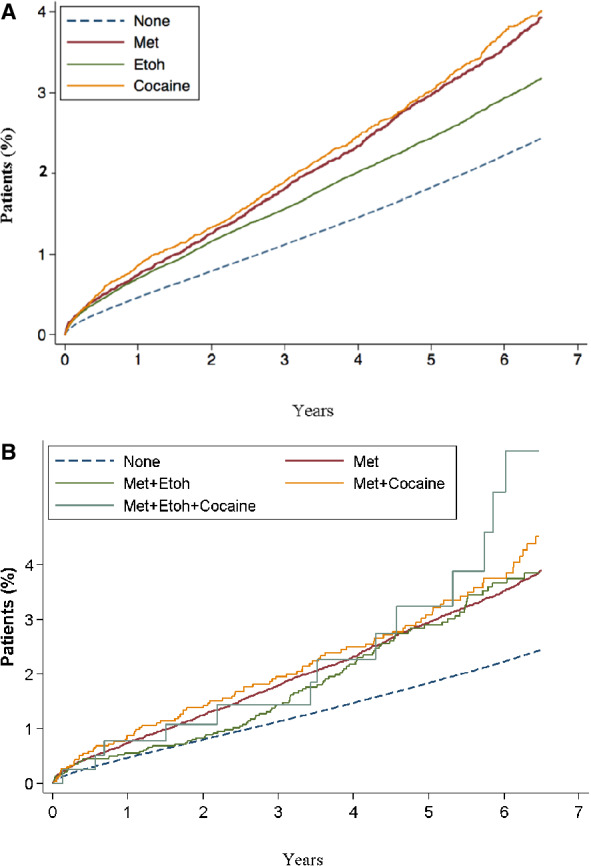
A, comparing patients who exclusively use methamphetamine (Met), alcohol (Etoh), or cocaine and nonusers; B, Comparing patients who use methamphetamine (Met) alone or in combination with alcohol (etoh), with cocaine, with both etoh and cocaine, and nonusers.
Table 4.
Exclusive Methamphetamine, Cocaine, and Alcohol Use and Hazards of Composite Cardiovascular Disease (Referent Group=Nonusers)
| Variable (vs nonusers) | Hazard ratio (adjusted) | CI |
|---|---|---|
| Methamphetamine | 1.32 | 1.27–1.38 |
| Cocaine | 1.47 | 1.40–1.54 |
| Alcohol | 1.28 | 1.26–1.31 |
Additionally, we studied the risk of CVD among users of alcohol or cocaine in combination with methamphetamine in order to detect a potential synergistic deleterious effect of poly‐substance use (Table 5, Figure 3B). We did not demonstrate a significant increased risk of CVD in users of both methamphetamine and alcohol when compared with methamphetamine‐only users (HR, 0.96 [CI, 0.86–1.13]) or in users of both methamphetamine and cocaine when compared with the methamphetamine‐only group (HR, 1.10 [CI, 0.92–1.31]). However, individuals who used all 3 substances (methamphetamine, alcohol, and cocaine) had a higher risk of CVD compared with those using methamphetamines only (Table 5).
Table 5.
Polysubstance Use versus Methamphetamine Use Only and Hazards of Composite Cardiovascular Disease (Referent Group=Users of Methamphetamine Only)
| Variable (vs methamphetamine only) | Hazard ratio (adjusted) | CI |
|---|---|---|
| Methamphetamine and alcohol | 0.96 | 0.82–1.13 |
| Methamphetamine and cocaine | 1.09 | 0.92–1.31 |
| Methamphetamine, alcohol and cocaine | 1.10 | 0.69–1.76 |
Although those that concurrently use all 3 substances had the highest cumulative rates of CVD at 5 years of follow‐up (Figure 3B), this increased risk was not found to be statistically significant when compared with methamphetamine use alone (HR, 1.10 [95% CI, 0.7–1.80]). In addition, we did not find a significantly increased risk of CVD in this group compared with nonusers (HR, 1.52 [95% CI, 0.93–2.48]).
Discussion
Summary of Findings
Our study aimed to define the sociodemographic and clinical factors associated with amphetamine use and to determine the association of methamphetamine use with CVD outcomes. First, in what we believe to be the largest analysis of methamphetamine users and their risk of incident CVD, we found a 32% increase in the development of CVD, comparable in magnitude to that observed in cocaine (47%) and alcohol abuse (28%). In particular, methamphetamine use is strongly associated with an increased risk of the development of PHTN and HF. Second, we found that traditional cardiovascular risk factors strongly predict CVD in methamphetamine users, with chronic kidney disease (HR, 2.38 [CI, 1.74–3.25]), and hypertension (HR, 2.26 [CI, 2.03–2.51]) as the strongest predictors.
Third, we identified several risk factors demonstrated that methamphetamine users were younger and more likely to be male, as compared with the general hospitalized population studied. Methamphetamine users had significantly higher rates of depression and anxiety and were more likely to have a lower socioeconomic status than nonusers. People who concurrent abused multiple substances (methamphetamine, cocaine and alcohol) had a higher rates of CVD over a 5‐year period compared with those abusing methamphetamine alone, suggesting a possible synergistic deleterious effect, although this increase was not found to be statistically significant. This could be due in part to the small sample numbers in our cohort.
Our study did, however, detect a significant increase in the rate of MI and MINOCA among methamphetamine users with concurrent cocaine use compared with those without. This phenomenon could be driven by the combined vasoconstrictive properties of both substances and highlights a subgroup of users at particularly high risk of such events. Finally, although male sex (HR, 1.73) was a significant predictor of MI among methamphetamine users, female sex was not found to be a significant risk predictor for the development of any of the studied CVD outcomes, in contrast to the association between female sex and PHTN demonstrated in previous studies. 15
Comparison With Previous Studies
The first described case of a living patient with methamphetamine‐associated dilated cardiomyopathy was reported in 1989. 15 A 48‐year‐old individual presented with symptoms of breathlessness due to pulmonary edema. Echocardiography and subsequent cardiac catherization revealed severe left ventricular dysfunction in the presence of normal coronary arteries and a negative exercise stress test.
In the intervening decades, retrospective analyses of patients hospitalized with cardiomyopathy further demonstrated the association between methamphetamine use and left ventricular failure, in the absence of coronary artery disease or concomitant drug and alcohol abuse. 16 , 17
A retrospective analysis of a group of patients hospitalized methamphetamine users (n=4407) aimed to determine specific predictors of heart failure. 18 In this prior analysis, investigators studied patients who tested positive for methamphetamine on their toxicology screen during hospital admission over a 2‐year period. Patient demographics, vital signs, echocardiography, and blood samples were compared between amphetamine users with normal versus abnormal B‐type natriuretic peptide. Study investigators reported that methamphetamine users had a higher prevalence of heart failure than the nonuser hospitalized population. In the prior analysis, methamphetamine users who developed HF were more likely male, older, White, former smokers, and to have higher serum creatinine levels. Although our larger study did not confirm the association between male sex and development of HF among methamphetamine users, we found that age, obesity, hypertension, smoking status, and presence of chronic kidney disease were significant risk factors. Our study was a relatively unselected population, representing diverse urban versus rural settings, multiethnic populations, and varied care settings whereas the prior study represented a single‐center experience, possibly accounting for differences in our results and the prior report.
In another recent study, 896 patients with methamphetamine‐associated HF were identified using diagnosis codes, urine toxicology, and natriuretic peptides. 19 Similar to findings in our study, methamphetamine users were more likely to be younger, male, and have higher rates of depression and anxiety than nonusers. Importantly, the prior study also demonstrated that coexisting depression and anxiety were strong predictors of HF readmission among methamphetamine users, whereas concurrent opioid abuse was a predictor of mortality.
Several studies have also highlighted an association between methamphetamine use and incident pulmonary arterial hypertension. A retrospective cohort study of patients with PHTN (n=340) found that 28.9% of patients with a diagnosis of “idiopathic” PHTN had a history of stimulant use (cocaine, amphetamine, or methamphetamine) compared with just 3.8% of patients with pulmonary arterial hypertension with known associated risk factors and 4.3% among those with chronic thromboembolic PHTN. 6 A separate study prospectively compared the clinical characteristics and outcomes of 90 patients with methamphetamine‐associated PHTN with 97 patients with idiopathic PHTN. Investigators found that the methamphetamine group had poorer functional status, right ventricular function, and exercise tolerance at presentation and had a significantly lower 5‐year survival compared with those with idiopathic PHTN. 7
Zhao et al. retrospectively studied the clinical characteristics and outcomes of 50 patients with methamphetamine‐associated pulmonary arterial hypertension and 296 patients with methamphetamine‐associated cardiomyopathy, compared with 356 methamphetamine‐user control patients. 20 Male sex, hypertension, and alcoholism were strongly associated with methamphetamine‐associated cardiomyopathy, whereas female sex was associated with the development of methamphetamine‐associated pulmonary arterial hypertension.
In contrast to this previous study by Zhao et al., we did not find an association between male sex and HF or an association between female sex and the development of PHTN. We did, however, find that male sex was strongly associated with the incidence of MI and MINOCA. It should be noted that the study design described by Zhao et al. allowed for exclusion of patients with known interstitial lung disease, HIV coinfection, and pulmonary embolism (although these accounted for a small number of patients) and would have had a more granular and accurate assessment of PHTN than our current study, which employs ICD codes for outcome definition. Further study is needed to clarify the effect of sex and methamphetamine use in the development of this complex and heterogenous disease.
Strengths and Limitations
Strengths of our study include a longitudinal study design, in a large, relatively unselected population that represents approximately 95% of hospitalizations, ambulatory surgery, or emergency department visits in California. Additional strengths include use of data from a large and ethnically diverse study population. However, several limitations are worthy of discussion. All outcomes and variables were determined using ICD‐9 codes, and therefore our results are subject to the sensitivity and specificity of each organization's diagnoses, as well as our ability to define each of the subtype of CVD. The ICD‐9 codes (304.40–304.42 and 305.70–305.72) used to define methamphetamine use disorder also encompasses misuse of other prescription stimulants. As illicit methamphetamine use comprises the majority of amphetamine use disorder cases, the authors have used this definition throughout the article. In addition, we were able to examine only patients who obtained care from a hospital participating in the HCUP database, introducing possible selection bias. However, HCUP covers the vast majority of California health care data. In addition, factors such as the route of administration and the frequency or duration of amphetamine use could not be obtained from the information available to us. It is possible that the route of drug administration (smoked versus injected) may have an effect on the development of specific CVD subtypes through direct or indirect effects on the vasculature or confer worse clinical outcomes.
Clinical Implications and Future Direction of Study
The recognition of the significant burden of methamphetamine‐associated CVD is vital given the rapidly growing use of methamphetamine and related stimulants worldwide. To our knowledge, we have analyzed the largest studied cohort of methamphetamine users in order to better define the sociodemographic of this cohort and identify those at high risk of developing CVD. Our findings support previous smaller studies that have described the high burden of CVD among methamphetamine users. In contrast to previous work, we did not find any sex differences for methamphetamine use and the development of PHTN or HF. However, we found that traditional risk factors such as age, obesity, hypertension, diabetes, chronic kidney disease, and smoking status were all significantly associated with increased incidence of CVD among users.
Recent investigation has suggested that methamphetamine cessation can prevent or reverse some of its deleterious effects on cardiovascular health. Identification of key risk factors for CVD means that cessation strategies can be targeted to this most at‐risk group.
Further work is also needed to better understand the effect of dose, duration of use, and route of administration of methamphetamine on the cardiovascular system. It is also important that we understand the impact of concomitant HIV and hepatitis B/C infection has on CVD burden among patients with methamphetamine use; of note, we have not demonstrated that polysubstance misuse increases a patient's risk of CVD development beyond the risk of methamphetamine use alone. Finally, more must be done to address the high rate of mental health morbidity among users, especially as this will adversely affect clinical outcome.
Conclusions
Methamphetamine use is associated with the increased incidence of several serious CVD pathologies and thus with epidemics comparable to those known for many decades resulting from cocaine and alcohol abuse. Our study found that methamphetamine users have a significantly increased (32%) risk of CVD and in particular of developing PHTN and HF. Among methamphetamine users, those with chronic kidney disease, hypertension, and diabetes were most at risk. Unlike previous studies, we did not demonstrate a significant association between female sex and incidence of methamphetamine‐associated PHTN, although we found that men were more likely to develop MI or MINOCA. We found a trend for increased risk of CVD among methamphetamine users who also abuse other substances (alcohol and cocaine) though this did not reach significance, possibly due to small sample sizes, and warrants further exploration. Methamphetamine users who also use cocaine are at particular risk of developing MI and MINOCA and should be considered a subgroup where prevention strategies are particularly focused. The dual burdens of cardiovascular and mental health disease among methamphetamine users must be recognized and addressed by physicians in order to slow the rapid pace of growth of this global epidemic.
Sources of Funding
This study was self‐funded.
Disclosures
Dr Gregory Marcus holds research grants from National Institutes of Health, the Patient‐Centered Outcomes Research Institute, Baylis, and Medtronic. Consulting fees from Johnson and Johnson and InCarda. Equity in InCarda. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Crime UNOoDa . World Drug Report 2017 . 2017.
- 2. Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–179. doi: 10.1016/j.drugalcdep.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 3. Kevil CG, Goeders NE, Woolard MD, Bhuiyan MS, Dominic P, Kolluru GK, Arnold CL, Traylor JG, Orr AW. Methamphetamine use and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39:1739–1746. doi: 10.1161/ATVBAHA.119.312461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben‐Yehuda O, Siecke N. Crystal methamphetamine: a drug and cardiovascular epidemic. JACC Heart Fail. 2018;6:219–221. doi: 10.1016/j.jchf.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 5. Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, Alimineti K, Kimata C, Schatz IJ. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. doi: 10.1016/j.amjmed.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 6. Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657 [DOI] [PubMed] [Google Scholar]
- 7. Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, Kudelko K, Liu J, Hsi A, Rupp A, et al. Features and outcomes of methamphetamine‐associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. doi: 10.1164/rccm.201705-0943OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaye S, Darke S, Duflou J, McKetin R. Methamphetamine‐related fatalities in Australia: demographics, circumstances, toxicology and major organ pathology. Addiction. 2008;103:1353–1360. doi: 10.1111/j.1360-0443.2008.02231.x [DOI] [PubMed] [Google Scholar]
- 9. Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine‐induced left ventricular dysfunction. Cardiovasc Res. 2010;87:111–118. doi: 10.1093/cvr/cvq043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen JP. Methamphetamine‐associated acute myocardial infarction and cardiogenic shock with normal coronary arteries: refractory global coronary microvascular spasm. J Invasive Cardiol. 2007;19:E89–E92. [PubMed] [Google Scholar]
- 11. Administration SAaMHS . Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H‐53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2018. [Google Scholar]
- 12. Guerin AA, Kim JH. Age of onset and its related factors in cocaine or methamphetamine use in adults from the United States: results from NHANES 2005‐2018. Int J Environ Res Public Health. 2021;18:12259. doi: 10.3390/ijerph182212259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winkelman TNA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine‐related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1:e183758. doi: 10.1001/jamanetworkopen.2018.3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao SX, Seng S, Deluna A, Yu EC, Crawford MH. Comparison of clinical characteristics and outcomes of patients with reversible versus persistent methamphetamine‐associated cardiomyopathy. Am J Cardiol. 2020;125:127–134. doi: 10.1016/j.amjcard.2019.09.030 [DOI] [PubMed] [Google Scholar]
- 15. Jacobs LJ. Reversible dilated cardiomyopathy induced by methamphetamine. Clin Cardiol. 1989;12:725–727. doi: 10.1002/clc.4960121211 [DOI] [PubMed] [Google Scholar]
- 16. Ito H, Yeo KK, Wijetunga M, Seto TB, Tay K, Schatz IJ. A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clin Cardiol. 2009;32:E18–E22. doi: 10.1002/clc.20367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine‐associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41(7):981–986. doi: 10.1081/clt-120026521 [DOI] [PubMed] [Google Scholar]
- 18. Richards JR, Harms BN, Kelly A, Turnipseed SD. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018;36:1423–1428. doi: 10.1016/j.ajem.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 19. Thomas IC, Nishimura M, Ma J, Dickson SD, Alshawabkeh L, Adler E, Maisel A, Criqui MH, Greenberg B. Clinical characteristics and outcomes of patients with heart failure and methamphetamine abuse. J Card Fail. 2020;26:202–209. doi: 10.1016/j.cardfail.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 20. Zhao SX, Kwong C, Swaminathan A, Gohil A, Crawford MH. Clinical characteristics and outcome of methamphetamine‐associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail. 2018;6:209–218. doi: 10.1016/j.jchf.2017.10.006 [DOI] [PubMed] [Google Scholar]


