Abstract
Pseudomonas aeruginosa and Pseudomonas alcaligenes are gram-negative bacteria that secrete proteins using the type II or general secretory pathway, which requires at least 12 xcp gene products (XcpA and XcpP to -Z). Despite strong conservation of this secretion pathway, gram-negative bacteria usually cannot secrete exoproteins from other species. Based on results obtained with Erwinia, it has been proposed that the XcpP and/or XcpQ homologs determine this secretion specificity (M. Linderberg, G. P. Salmond, and A. Collmer, Mol. Microbiol. 20:175–190, 1996). In the present study, we report that XcpP and XcpQ of P. alcaligenes could not substitute for their respective P. aeruginosa counterparts. However, these complementation failures could not be correlated to species-specific recognition of exoproteins, since these bacteria could secrete exoproteins of each other. Moreover, when P. alcaligenes xcpP and xcpQ were expressed simultaneously in a P. aeruginosa xcpPQ deletion mutant, complementation was observed, albeit only on agar plates and not in liquid cultures. After growth in liquid culture the heat-stable P. alcaligenes XcpQ multimers were not detected, whereas monomers were clearly visible. Together, our results indicate that the assembly of a functional Xcp machinery requires species-specific interactions between XcpP and XcpQ and between XcpP or XcpQ and another, as yet uncharacterized component(s).
Many extracellular proteins produced by gram-negative bacteria are secreted by the type II secretion pathway, also called the main terminal branch (MTB) of the general secretory pathway (GSP) (49). Pseudomonas aeruginosa secretes several enzymes and toxins via this pathway (20). These proteins are translocated in two steps across the bacterial cell envelope. After translocation of the signal peptide-bearing exoproteins across the cytoplasmic membrane, a step similar to the Sec-mediated transport of proteins in Escherichia coli (16, 51), the exoproteins are transported from the periplasm across the outer membrane. The latter process requires the products of at least 12 xcp genes (20). The xcpA gene is located between positions 5072694 and 5073566 of the chromosomal map, and xcpPQRSTUVWXYZ are located between positions 3475955 and 3483641 (http://www.pseudomonas.com). Homologs of xcp genes, encoding components of the MTB of the GSP, are present in many other gram-negative bacteria (10, 18, 20) and are now usually referred to as gsp genes. In addition, homologs of several xcp gene products are involved in other macromolecular transport processes, such as the formation of type IV pili, filamentous phage assembly, type III protein secretion, and natural uptake of DNA (23, 32).
Xcp (Gsp) proteins are located in the cell envelope and are proposed to interact and to function as a protein secretion apparatus called the secreton. The only outer membrane component of the secreton is XcpQ (GspD) (2). All the other Xcp proteins are located in or associated with the cytoplasmic membrane. XcpQ (2) and homologs (8, 28, 37, 39, 55) belong to a new family of proteins called secretins, which form multimers in the outer membrane. These multimers may form a channel with a large central cavity through which the exoproteins are likely to pass (7, 43, 46). Moreover, XcpQ has been proposed to interact with bitopic inner membrane protein XcpP (GspC) (3).
It remains unclear how exoproteins, which are at least partially folded before secretion (5, 6, 21, 47, 50), are distinguished from periplasmic proteins. A putative secretion motif involved in this process might be present within the three-dimensional structure of the exoproteins (42, 53, 57). Moreover, recognition of the exoproteins by the Gsp secreton appears to be species specific. Although secretion of an exoprotein expressed in a heterologous host has occasionally been reported (45, 58), it does not generally occur (10, 45), not even with similar enzymes from the closely related bacterial species Erwinia carotovora and Erwinia chrysanthemi (30, 52). The last of these studies also suggested that the XcpP (GspC) and XcpQ (GspD) homologues are involved in this species specificity, since, except for gspC and gspD, every gsp gene of E. chrysanthemi could be replaced by its counterpart from E. carotovora, (41). Moreover, it was demonstrated that GspD from E. chrysanthemi interacts with exoproteins from E. chrysanthemi but not with those from E. carotovora (55).
Recently, the xcp gene cluster of Pseudomonas alcaligenes was cloned and characterized (25). As in P. aeruginosa, the xcp genes of P. alcaligenes are organized in two divergently transcribed operons, xcpPQ and xcpR to -Z. The degree of homology between the xcp gene products of P. alcaligenes and P. aeruginosa ranges from 42 to 82% amino acid identity, which is comparable to the degree of homology between the Gsp proteins of E. chrysanthemi and E. carotovora (41).
The goal of the present study was to determine whether species specificity of secretion between P. aeruginosa and P. alcaligenes exists. Therefore, the ability of these bacteria to secrete exoproteins of each other was tested. We also investigated whether individual Xcp proteins from P. alcaligenes and P. aeruginosa can functionally be exchanged. We demonstrate the species specificity of the XcpP and XcpQ proteins, but, in contrast to what was found for Erwinia, this specificity is not related to substrate recognition.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used are listed in Tables 1 and 2, respectively. Strains were grown at 37°C in Luria broth (LB) or tryptic soy broth (TSB) with agitation or on LB agar plates. Plasmids were introduced into P. aeruginosa and P. alcaligenes by electroporation or by conjugation using pRK2013 as a helper plasmid in triparental matings. Antibiotics were used at the following concentrations (in micrograms per milliliter): ampicillin, 100 (E. coli); carbenicillin, 100 (P. alcaligenes) or 300 (P. aeruginosa); tetracycline, 5 (P. alcaligenes), 15 (E. coli), or 100 (P. aeruginosa); kanamycin, 10 (P. alcaligenes), 25 (E. coli), or 400 (P. aeruginosa); streptomycin, 100 (E. coli) or 1,000 (P. aeruginosa). Isopropyl-β-d-thiogalactopyranoside (IPTG) was added at concentrations up to 2 mM when required.
TABLE 1.
Bacterial strains
| Strain | Relevant characteristics | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Prototroph | Holloway collection |
| DZQ40 | PAO1 ΔxcpP-Z | 1 |
| PAO1ΔPQ | PAO1 ΔxcpPQ | This study |
| PAO1ΔRZ | PAO1 ΔxcpR-Z | V. Chapon-Hervé |
| PAO1ΔP | PAO1 ΔxcpP | 3 |
| PAG2 | PAO1 ΔxcpQ | This study |
| PAG3 | PAO1 ΔxcpRS | This study |
| PAO1ΔT | PAO1 ΔxcpT | V. Chapon-Hervé |
| PAO1ΔX | PAO1 ΔxcpX | 4 |
| KS910-503 | PAO503 xcpY51 | 59 |
| KS902-503 | PAO503 xcpZ5 | 59 |
| PABS1 | PAO1 ΔlipAH | K.-E. Jaeger |
| P. alcaligenes | ||
| Ps93 | Restriction-negative modification-positive strain M-1 mutant | 24 |
| Ps93R | Ps93 xcpR::Kmr | This study |
| E. coli | ||
| CC118(λpir) | Strain to maintain pKNG101 | 31 |
| PC2494 | hsdR thi Δ(lac-proAB) supE (F′ proA+B+lacIqZΔM15 traD36) | Phabagen collectiona |
Utrecht University, Utrecht, The Netherlands.
TABLE 2.
Plasmids
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| pEMBL18, -19 | Apr; phagemid | 14 |
| pUC19 | Apr; ColE1 lacl φ80dlacZ | 60 |
| pMMB67EH, -HE | IncQ Apr; tac promoter; lacIq | 22 |
| pLAFR3 | IncP Tcr | 56 |
| pVDZ′2 | IncP Tcr; lac promoter | 15 |
| pKNG101 | Smr; oriR6K sacBR mobRK2 | 36 |
| pRK2013 | Kmr; ori ColE1; Tra+ Mob+ | 17 |
| pUC4K | Kmr gene block in pUC4 | Pharmacia |
| pPB29 | lasB on an EcoRI-HindIII fragment in pVDZ′2 | This study |
| pJRDlipAB | P. alcaligenes lipAB in pJRD215 | 24 |
| pAX24 | P. aeruginosa xcpP to -Z cluster in pLAFR3 | 19 |
| pUAWB5 | P. aeruginosa xcpPQ on a 5.2-kb BamHI fragment in pUC19 | This study |
| pUAWE6 | P. aeruginosa xcpPRSTUV in pUC19 | 10 |
| pSB10 | P. aeruginosa xcpP in pMMB67HE | 3 |
| pSB96 | 1.88-kb DNA fragment from the P. aeruginosa xcp cluster with an internal xcpPQ deletion in pUC19 | This study |
| pMB4 | P. aeruginosa xcpQ on a 2.9-kb PstI-SmaI fragment in pMMB67HE | This study |
| pAF2 | P. aeruginosa xcpPQ on a 5.2-kb BamHI fragment in pLAFR3 | This study |
| pLAF600 | P. alcaligenes xcpP to -Z cluster in pLAFR3 | 25 |
| pLAF600SB | P. alcaligenes xcpPQ in pLAFR3 | 25 |
| pLAF600BH | P. alcaligenes xcpR to -Z in pLAFR3 | 25 |
| pXA2 | P. alcaligenes xcpR to -V on a 5.3-kb NcoI fragment in pEMBL18 | This study |
| pMPA2 | P. alcaligenes xcpP on a 1.0-kb SalI-EcoRI fragment in pMMB67HE | This study |
| pCK28 | P. alcaligenes xcpQ on a 2.6-kb BsaAI-SalI fragment in pMMB67EH | This study |
| pRVA4 | P. alcaligenes xcpR to -V on a 5.3-kb NcoI fragment in pMMB67HE | This study |
| pMTA3 | P. alcaligenes xcpT on a 1.2-kb SphI-XhoI fragment in pMMB67HE | This study |
| pMXA8 | P. alcaligenes xcpX on a 1.6-kb SphI-StuI fragment in pMMB67HE | This study |
| pMYA3 | P. alcaligenes xcpY on a 1.6-kb XhoI-SphI fragment in pMMB67EH | This study |
| pMZA3 | P. alcaligenes xcpZ on a 1.5-kb SmaI-EcoRI fragment in pMMB67HE | This study |
Tcr, tetracycline resistant; Kmr, kanamycin resistant; Apr, ampicillin resistant; Smr, streptomycin resistant.
Construction of xcp mutant strains.
Plasmid pUAWB5, which contains a 5-kb BamHI fragment, was used to construct a deletion of a 1.9-kb EcoRI fragment in xcpQ. Starting from the 6-kb EcoRI fragment in pUAWE6, a plasmid with a 1.4-kb ScaI-SmaI deletion in xcpR combined with a 1.1-kb AsuII deletion in xcpS was constructed from pEMBL19. The plasmids carrying the deletions were cloned in suicide vector pKNG101. These pKNG101 derivatives were introduced in PAO1, and deletion mutants resulting from double-crossover events were obtained as described previously (12, 36). A 1-kb EcoRI/HindIII DNA fragment containing an internal 500-bp deletion in the xcpP gene and a 881-bp XhoI/EcoRI DNA fragment containing the 3′ end of the xcpQ gene were cloned in tandem in pUC19, yielding pSB96. Plasmid pSB96 was introduced into PAO1, and a double-crossover event, yielding the xcpPQ deletion, was selected after screening for secretion-defective clones on skim milk plates. Secretion in mutants PAG2, PAG3, and PAO1ΔPQ could be restored by introducing plasmids carrying only xcpQ, xcpRS, and xcpPQ, respectively.
To obtain a P. alcaligenes, xcpR mutant, the kanamycin resistance gene of pUC4K was cloned as a HincII fragment into xcpR on plasmid pXA2, which was digested with both SmaI and EcoRV to delete a 0.9-kb fragment of xcpR. The resulting plasmid was introduced into strain Ps93, and colonies resistant to kanamycin but sensitive to carbenicillin, were selected. Defective secretion of lipase was verified on plates containing tributyrin.
SDS-PAGE and immunoblotting.
Cells were harvested at optical densities at 600 nm (OD600) of 3 to 4. Proteins were precipitated from the supernatants with 5% (wt/vol) trichloroacetic acid (final concentration). Cellular and extracellular proteins were solubilized in sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gels contained 11% acrylamide, unless otherwise stated. Immunoblots were incubated with appropriate polyclonal or monoclonal antibodies and subsequently with peroxidase-conjugated secondary antibodies, followed by detection by chemiluminescence (Pierce).
Enzyme assays.
Plates for the detection of protease activity contained 1.5% skim milk. Elastase activity was analyzed on plates with a top layer containing 1% elastin. Quantitative assays for elastase were performed as described previously (10). Plates for the detection of lipase activity contained 1% tributyrin, which was added from a 10× stock solution (10% tributyrin emulsified with 8% gum arabic by short sonication). Quantitative assays for lipase were performed as described by Kordel and collaborators (38). One unit of lipase activity was defined as the amount of enzyme that liberated 1 nmol of p-nitrophenol from p-nitrophenyl palmitate per min. Lipase units were standardized to the activity contained in the supernatant of 1 OD600 unit equivalent of bacterial cell culture.
RESULTS
Heterologous secretion in Pseudomonas species.
Related Pseudomonas species P. aeruginosa and P. alcaligenes were chosen to study heterologous protein secretion. Elastase (LasB) is a P. aeruginosa type II-secreted exoprotein which possesses a strong proteolytic activity. Plasmid pPB29, carrying the elastase structural gene (lasB), was introduced into P. alcaligenes strain Ps93. Immunoblotting revealed that the majority of the elastase produced by P. alcaligenes Ps93(pPB29) was present in the extracellular medium (Fig. 1). This secretion was Xcp dependent, since the majority of elastase produced by an xcpR mutant derivative of Ps93, strain Ps93R, was present in the cellular fraction (Fig. 1). Similar conclusions were reached after plating the strains on skim milk plates. On such plates, no endogenous extracellular protease activity was detected with P. alcaligenes Ps93. However, large halos of degraded milk proteins were visible when strain Ps93(pPB29) was tested, but not when xcpR mutant Ps93R(pPB29) was tested (results not shown). It can thus be concluded that elastase from P. aeruginosa is secreted by the heterologous host P. alcaligenes in an Xcp-dependent manner.
FIG. 1.
Immunoblot demonstrating Xcp-dependent heterologous secretion of elastase by P. alcaligenes. Plasmid pPB29 containing the lasB gene encoding elastase of P. aeruginosa was introduced into P. alcaligenes Ps93 (Xcp+) and into its xcpR mutant derivative Ps93R. Cells (C) and supernatant (S) were separated and analyzed by immunoblotting with an antiserum directed against elastase. Cellular and extracellular proteins corresponding to equal culture volumes were loaded.
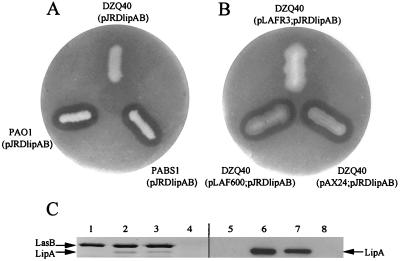
Next, we assessed the ability of P. aeruginosa to secrete a P. alcaligenes Xcp-dependent exoprotein, i.e., lipase. Plasmid pJRDlipAB, containing the P. alcaligenes lipase gene (lipA) and the gene encoding its dedicated chaperone, LipB, was introduced into P. aeruginosa strains PAO1 (wild type), isogenic lipAH mutant PABS1, which is unable to produce the endogenous lipase and its chaperone, and xcpP to -Z deletion mutant DZQ40. The resulting strains were tested for lipase production on plates containing tributyrin as the substrate. For PAO1 and PABS1, but not DZQ40, clear halos revealing extracellular lipase activity were detected (Fig. 2A). When pJRDlipAB was not present in these strains, such halos were not formed (data not shown). After growth of the pJRDlipAB-containing strains in TSB medium and immunoblotting with monoclonal antibodies directed against P. alcaligenes lipase, this lipase was found to be secreted by strains PAO1 and PABS1 but not by DZQ40 (Fig. 2C). The specific lipase activities detected in the supernatant of these strains were 252, 249, and 0 U, respectively. When PAO1 lacking pJRDlipAB was grown in TSB medium, no P. alcaligenes lipase-specific protein could be detected in the supernatant (Fig. 2C) and only weak activity (12 U), due to the endogenous P. aeruginosa lipase, was observed. Hence, we concluded that (i) the high level of lipase activity observed is due to P. alcaligenes lipase and not to the endogenous P. aeruginosa lipase and (ii) P. alcaligenes lipase secretion in P. aeruginosa is Xcp dependent.
FIG. 2.
Secretion of P. alcaligenes lipase by P. aeruginosa. (A and B) Plate assays demonstrating lipase secretion. (A) Plasmid pJRDlipAB, carrying the genes encoding P. alcaligenes lipase and its lipase-specific foldase, was introduced into P. aeruginosa strains PAO1, xcpP to -Z deletion mutant DZQ40, and lipAH deletion mutant PABS1. (B) Plasmid pJRDlipAB was cointroduced with pLAFR3, pAX24 carrying the P. aeruginosa xcpP to -Z cluster, or pLAF600 carrying the P. alcaligenes xcpP to -Z cluster into strain DZQ40. (C) Coomassie brilliant blue-stained gel (lanes 1 to 4) and immunoblot with a monoclonal antibody directed against P. alcaligenes lipase (lanes 5 to 8), using supernatants from P. aeruginosa strains grown in TSB medium. Lanes 1 and 5, PAO1; lanes 2 and 6, PAO1/pJRDlipAB, lanes 3 and 7, PABS1/pJRDlipAB; lanes 4 and 8, D40ZQ/pJRDlipAB. The positions of the P. aeruginosa elastase (LasB) and the P. alcaligenes lipase (LipA) are indicated.
Functional exchange of the Xcp machinery is dependent on growth conditions.
The results of the heterologous secretion experiments demonstrate that the Xcp machineries of P. aeruginosa and P. alcaligenes are not species specific with respect to the recognition of each other's exoproteins. This lack of species specificity was further analyzed in complementation experiments in which xcp genes from both species were exchanged. To study whether the XcpP to -Z proteins from P. alcaligenes (XcpP-Zalc) could assemble into a functional secreton in P. aeruginosa, cosmid pLAF600 carrying the P. alcaligenes xcpP to -Z genes was introduced into the P. aeruginosa xcpP to -Z deletion mutant DZQ40. DZQ40 strains containing cloning vector pLAFR3 and cosmid pAX24, carrying the P. aeruginosa xcpP to -Z gene cluster, were included as controls. Protease plate assays indicated that secretion of elastase was restored efficiently by the P. alcaligenes xcp gene cluster, since the halo size was almost identical to the one of strain DZQ40(pAX24) (Fig. 3A). Comparable results were obtained on plates containing elastin as the elastase substrate (results not shown). The halos observed could have been the result of secretion of only a small fraction of the total amount of elastase produced. To test this possibility, cells were collected after growth on plates and analyzed by immunoblotting with an antiserum directed against elastase. The results showed that only small amounts of elastase accumulated inside the cells of strain DZQ40 containing pLAF600, compared with the amounts inside cells of DZQ40 containing vector pLAFR3 (Fig. 3B), strongly suggesting that the majority of the elastase is secreted by strain DZQ40(pLAF600). The P. alcaligenes xcpA gene, encoding the prepilin peptidase, is not present on pLAF600 but is probably located separate from the xcpP to -Z genes as it is in P. aeruginosa. In line with this, a P. aeruginosa xcpA mutant containing pLAF600 did not show halo formation on skim milk plates (results not shown). These data indicate that the halo observed with strain DZQ40(pLAF600) is due to Xcpalc -dependent secretion of elastase and not to non specific leakage. Finally, strain DZQ40(pLAF600) was able to grow on the selective lipid agar plates described by Kagami et al. (35) (results not shown), indicating that lipase, another Xcp-dependent P. aeruginosa exoprotein, was also secreted. Therefore, the P. alcaligenes secreton is functionally assembled in P. aeruginosa.
FIG. 3.
Complementation of the xcpP to -Z deletion in P. aeruginosa strain DZQ40 by the P. alcaligenes xcpP to -Z cluster. Strain DZQ40 contained either pLAFR3, pAX24 carrying the P. aeruginosa xcp gene cluster, or pLAF600 carrying the P. alcaligenes xcp gene cluster. (A) Plate assay demonstrating protease secretion. (B) Immunoblot showing accumulation of elastase inside the cells. Strains were grown on a plate, resuspended in LB medium in order to measure the cell density, pelleted, and resuspended in sample buffer. Equal amounts of cells were loaded and analyzed with an antiserum directed against elastase (arrow). (C) Coomassie brilliant blue-stained gel showing proteins of culture supernatants. The major protein in the pAX24-labeled lane (arrow) is elastase.
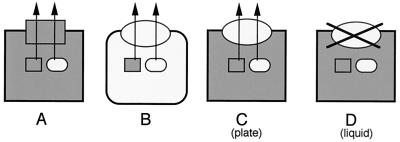
Complementation was also studies after growth of the same set of bacterial strains in liquid medium. Surprisingly, the results differed substantially from those of the plate assays. Compared to that of strain DZQ40(pAX24), the supernatant of strain DZQ40(pLAF600) contained hardly any elastase (Fig. 3C). Similarly, pJRDlipAB-encoded P. alcaligenes lipase was apparently secreted efficiently by P. aeruginosa strain DZQ40 expressing the P. alcaligenes xcp genes from pLAF600 if secretion was assessed on tributyrin plates (Fig. 2B), but not when culture supernatants were analyzed on blots (results not shown). However, when pJRDlipAB was introduced in DZQ40(pAX24), lipase secretion via the P. aeruginosa secreton was revealed under both growth conditions (Fig. 2B and data not shown). In conclusion, these results indicate that efficient functioning of the P. alcaligenes secreton is dependent on (i) the bacterial context in which the secreton is expressed and (ii) the growth conditions, i.e., on a plate or in liquid medium. These different situations are depicted in Fig. 4.
FIG. 4.
Summary showing the dependency of heterologous secretion on the bacterial context in which the xcp genes are expressed and on the growth conditions. Rectangular and oval symbols represent cells, Xcp machineries, or exoproteins from P. aeruginosa and P. alcaligenes, respectively. When xcp genes are expressed in the natural host (A and B), both endogenous and heterologous exoproteins are secreted. However, when the P. alcaligenes xcp genes are expressed in P. aeruginosa, efficient secretion of either P. alcaligenes lipase or P. aeruginosa elastase occurs only when the strain is grown on a plate (C), not in liquid medium (D), possibly because of a secreton assembly problem in the latter conditions.
Formation of P. alcaligenes XcpQ multimers in P. aeruginosa.
The possibility that the xcp genes of P. alcaligenes were not expressed in P. aeruginosa DZQ40 during growth in liquid cultures was investigated by immunoblotting using antisera directed against P. aeruginosa Xcp proteins. The XcpR and XcpY proteins were detected in cell extracts of strain DZQ40(pLAF600), but not in those of strain DZQ40(pLAFR3), after growth both on a plate and in liquid medium (data not shown), demonstrating that xcpR and xcpY from P. alcaligenes, and very likely also the other genes in the xcpR to -Z operon, were expressed under both growth conditions.
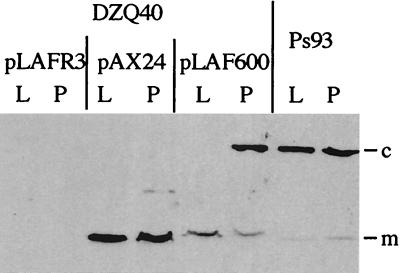
P. aeruginosa XcpQ protein (XcpQaer) forms multimeric ring-shaped structures that dissociate upon heating in SDS-sample buffer (2). In contrast, the XcpQ multimers from P. alcaligenes are heat stable (7). To test the expression and oligomerization of XcpQalc in P. aeruginosa, derivatives of strain DZQ40 containing either pLAFR3, pAX24, or pLAF600 were analyzed by immunoblotting. Protein samples were heated for 10 min at 95°C before loading. After growth of strain DZQ40(pLAF600) on a plate, high-molecular-weight material was detected with the anti-XcpQaer antiserum (Fig. 5), indicating the formation of the stable XcpQalc multimeric complex. Such material was not detected for the other two strains. Some monomeric XcpQalc was also detected. Interestingly, when strain DZQ40(pLAF600) was grown in liquid medium, the heat-stable XcpQalc complex was not formed and only monomeric XcpQalc was detected (Fig. 5). In contrast, when P. alcaligenes was grown in liquid medium, heat-stable XcpQalc multimers were easily detected (Fig. 5).
FIG. 5.
Formation of a heat-stable multimeric complex by P. alcaligenes XcpQ protein. Cellular proteins, heated for 10 min at 95°C prior to SDS-PAGE, were analyzed by immunoblotting with an antiserum directed against P. aeruginosa XcpQ. P. alcaligenes strain Ps93 and derivatives of P. aeruginosa xcpP to -Z deletion mutant DZQ40 containing either pLAFR3, pAX24 carrying the P. aeruginosa xcpP to -Z gene cluster, or pLAF600 carrying the P. alcaligenes xcpP to -Z gene cluster were grown on a plate (P) or in liquid medium (L). Proteins were separated on a gel containing 3 and 9% acrylamide in the stacking and running gels, respectively. c, multimeric complex of P. alcaligenes XcpQ; m, XcpQ monomer.
In conclusion, the xcp genes from P. alcaligenes are expressed from pLAF600 in P. aeruginosa DZQ40 but heat-stable XcpQalc multimers are formed efficiently in this strain only when the bacteria are grown on a plate. In contrast, in the natural host, heat-stable XcpQalc multimers are also formed efficiently in liquid medium.
Complementation of P. aeruginosa xcp mutations by individual P. alcaligenes xcp genes or subsets of these genes.
To study whether P. alcaligenes Xcp proteins can be combined with P. aeruginosa Xcp proteins to form a functional secreton, several subclones carrying xcp genes of P. alcaligenes were introduced in appropriate P. aeruginosa xcp mutants. Efficient complementation was obtained when pLAF600BH, carrying the xcpR to -Z operon of P. alcaligenes, was introduced in xcpR to -Z deletion mutant PAO1ΔRZ. This result was found both on plates (Fig. 6A) and in liquid medium (Fig. 6B and 7). Thus, XcpR-Zalc formed a fully functional mixed secreton with XcpPQaer in P. aeruginosa. The xcpPQ deletion of mutant strain PAO1ΔPQ was as efficiently complemented on plates with pLAF600SB as on plates with pAF2 (pLAF600SB and pAF2 contain the xcpPQ genes of P. alcaligenes and P. aeruginosa, respectively) (Fig. 6A). The proteolytic activity observed was caused by Xcp-dependent secretion since it was not observed when pLAF600SB was introduced in an xcpR mutant (results not shown). Thus, XcpPQalc could form a functional secreton with XcpR-Zaer in P. aeruginosa. In liquid medium, however, the amount of extracellular elastase activity was much less with pLAF600SB than with pAF2, indicating only partial complementation under these growth conditions (Fig. 6B). Again, the lack of full complementation seemed to be related to inefficient formation of heat-stable XcpQalc multimers in P. aeruginosa, since these multimers were hardly detected or not detected after growth of PAO1ΔPQ(pLAF600SB) in liquid medium but were easily detected after growth on plates (results not shown).
FIG. 6.
Complementation of P. aeruginosa xcpPQ and xcpR to -Z deletion mutants by the P. alcaligenes xcpPQ and xcpR to -Z operons. Plasmids pLAF600SB and pLAF600BH carry the P. alcaligenes xcpPQ and xcpR to -Z operon, respectively. Plasmid pAF2 carries the P. aeruginosa xcpPQ operon. (A) Plate assay demonstrating protease activity. (B) Extracellular elastase activity. Samples were incubated with elastin-Congo red for 3 h. The activities are expressed as the amounts of liberated Congo red (OD495) per OD600 unit.
FIG. 7.
Complementation of P. aeruginosa xcp mutations by P. alcaligenes xcp genes. Cellular (C) and supernatant (S) proteins corresponding to equal amounts of culture were analyzed by immunoblotting using an antiserum directed against elastase. The xcpR to -Z mutant PAO1ΔRZ contained either pLAFR3 or pLAF600BH carrying P. alcaligenes xcpR to -Z. The xcpP mutant PAO1ΔP contained either pMMB67HE (pMMB), pSB10 carrying P. aeruginosa xcpP, or pMPA2 carrying P. alcaligenes xcpP. Plasmids pMXA8 and pMZA3, carrying P. alcaligenes xcpX and xcpZ, respectively, were introduced into corresponding mutants PAO1ΔX and KS902-503 (xcpZ5).
Further complementation experiments were performed with individual P. alcaligenes xcp genes, cloned under control of the tac promoter of pMMB67EH/HE (Table 3). With individual genes from the xcpR-Zalc operon, secretion was restored both on skim milk plates (results not shown) and in liquid medium (Fig. 7 and results not shown) in all cases tested. These results show that the Xcp proteins encoded by the xcpR to -Z operons of P. aeruginosa and P. alcaligenes can be combined into a functional secreton. However, introduction of P. alcaligenes xcpP and xcpQ in the corresponding P. aeruginosa mutants did not restore secretion, either on plates (results not shown) or in liquid medium (Fig. 7 and results not shown). Therefore, XcpPalc and XcpQalc cannot replace XcpPaer and XcpQaer, respectively, except when they are expressed simultaneously in bacteria grown on plates (Table 3).
TABLE 3.
Heterologous complementation of P. aeruginosa xcp mutations by P. alcaligenes xcp genes
| P. aeruginosa mutation | P. alcaligenes xcp gene(s) | Complementationa for strains grown:
|
|
|---|---|---|---|
| On a plate | In liquid medium | ||
| ΔxcpP to -Z | xcpP to -Z | + | − |
| ΔxcpPQ | xcpPQ | + | − |
| ΔxcpR to -Z | xcpR to -Z | + | + |
| ΔxcpP | xcpP | − | − |
| ΔxcpQ | xcpQ | − | − |
| ΔxcpRS | xcpR to -V | + | + |
| ΔxcpT | xcpT | + | + |
| ΔxcpX | xcpX | + | + |
| xcpY51 | xcpY | + | + |
| xcpZ5 | xcpZ | + | + |
+, restoration of secretion; −, no restoration.
Species-specific stabilization of XcpP by XcpQ.
The observation that P. aeruginosa xcpP and/or xcpQ deletions were only complemented by P. alcaligenes xcpP and xcpQ when these genes were expressed simultaneously indicates that XcpP and XcpQ interact in a species-specific way. Previously, an interaction between XcpP and XcpQ was suggested by the instability of XcpPaer in P. aeruginosa xcpQ mutant PAG2 (3). Here, we investigated whether XcpQalc could stabilize XcpPaer. Derivatives of strain PAG2 containing either pMMB67HE, pMB4 carrying P. aeruginosa xcpQ, or pCK28 carrying P. alcaligenes xcpQ were grown without IPTG, since the addition of IPTG was lethal for the strains containing pMB4 and pCK28. Immunoblotting showed that the levels of XcpPaer were strongly increased in the presence of XcpQaer but only moderately increased in the presence of XcpQalc (Fig. 8), indicating that XcpQalc is much less efficient than XcpQaer in stabilizing XcpPaer. These results suggest that XcpP is stabilized by a species-specific interaction with XcpQ.
FIG. 8.
Species-specific stabilization of XcpP by XcpQ. Equal amounts of total cell extracts of xcpQ deletion mutant PAG2 containing either pMMB67HE (lane 1), pMB4 carrying P. aeruginosa xcpQ (lane 2), or pCK28 carrying P. alcaligenes xcpQ (lane 3) were analyzed with antisera directed against XcpPaer (A) and XcpQaer (B). Protein samples were heated for 10 min at 95°C and loaded on SDS-PAGE gels containing 11 (A) or 9% (B) acrylamide. Two different start codons in the P. aeruginosa xcpP gene are used as translation start sites, resulting in two distinct XcpP bands (A) (3). No heat-stable XcpQ complex, only monomeric XcpQ, was detected (B).
DISCUSSION
In spite of the conservation of the type II secretion system in many gram-negative bacteria, several studies indicate that the secretion of an exoprotein expressed in a heterologous host does not generally occur (10, 30, 45, 52). For example, very closely related Erwinia species E. chrysanthemi and E. carotovora cannot secrete the cellulases and pectate lyases of each other (30, 52). Such specific recognition suggests the presence of a secretion motif on the exoproteins that is recognized by one or more components of the Gsp machinery. Lindeberg et al. (41) proposed that OutC (GspC) and OutD (GspD) might be the gatekeepers involved in species-specific exoprotein recognition, since, except for OutC and OutD, each Out protein from E. carotovora could substitute for its counterpart from E. chrysanthemi. Similarly, except for PulC (GspC) and PulD (GspD), all Gsp components of Klebsiella oxytoca could be replaced by the corresponding proteins of E. chrysanthemi and E. carotovora (48). Furthermore, Shevchik et al. (55) reported that the Nterminus of OutD might indeed species-specifically bind the exoprotein substrate. However, a chimeric GspD protein, in which the N-terminal domain of K. oxytoca PulD was replaced by the corresponding domain of E. chrysanthemi OutD, supported pullulanase secretion via the K. oxytoca Gsp (Pul) system (26). The same study reported that the C-terminal domain of PulD cannot be replaced by its counterpart from E. chrysanthemi, OutD, without affecting pullulanase secretion. In contrast, Hardie et al. (29) reported that the C-terminal half of E. chrysanthemi OutD could substitute for the C-terminal half of PulD. These contradictory results make it unclear whether and how the GspD homologs might be involved in species-specific recognition of the exoproteins.
Previously, we studied the species specificity of secretion between P. aeruginosa and Pseudomonas putida (10–12). However, despite the presence of an xcp gene cluster in P. putida, it is not clear whether it encodes a functional secreton, since no Xcp-dependent exoproteins were detected in this strain (12). In the present study, we analyzed the species specificity of secretion by P. aeruginosa and P. alcaligenes. Similarly as reported for E. chrysanthemi and E. carotovora, we observed that the individual XcpP (GspC) and XcpQ (GspD) proteins of P. aeruginosa could not be replaced by XcpP and XcpQ from P. alcaligenes, respectively. However, the lack of complementation, in this case, cannot be related to the species specificity of exoprotein recognition by XcpP and/or XcpQ. First, P. aeruginosa elastase and P. alcaligenes lipase were Xcp-dependently secreted when produced in heterologous hosts P. alcaligenes and P. aeruginosa, respectively. Second plate assays showed that introduction of a cosmid carrying the xcpP to -Z genes of P. alcaligenes in a P. aeruginosa xcpP to -Z deletion strain restored Xcp-dependent protein secretion. The failure of XcpPalc and XcpQalc to complement the corresponding P. aeruginosa mutants may therefore be caused by species-specific interactions between these two proteins. Indeed, when P. alcaligenes xcpP and xcpQ were expressed simultaneously under the control of their native promoters, elastase secretion in a P. aeruginosa xcpPQ deletion mutant on skim milk plates was restored. Moreover, unlike XcpQaer, XcpQalc failed to stabilize XcpPaer.
The results of the complementation experiments also indicate that putative interactions of XcpPQ with other known Xcp proteins (XcpR to -Z) are not species specific, at least not between P. aeruginosa and P. alcaligenes. Indeed, XcpPQaer and XcpR-Zalc formed a mixed secretion that supported efficient protein secretion in P. aeruginosa. Similarly, XcpPQalc and XcpR-Zaer apparently formed a functional mixed secreton in P. aeruginosa when the bacteria were grown on plates. Interestingly, complementation of the P. aeruginosa xcpPQ mutant by the P. alcaligenes xcpPQ genes was not efficient when the bacteria were grown in liquid medium. This lack of complementation is again not due to the species specificity of the interactions of XcpPQ with other known Xcp proteins, since the same results were even found when the complete P. alcaligenes xcpP to -Z gene cluster was expressed in the P. aeruginosa xcpP to -Z deletion mutant. The inefficient complementation in liquid medium is not caused by inefficient recognition of the heterologous exoproteins by the secreton under these conditions, since the P. alcaligenes lipase was also not secreted via the Xcpalc secreton in P. aeruginosa in liquid medium. Furthermore, P. aeruginosa elastase was secreted Xcp dependently by P. alcaligenes when grown in liquid medium. Therefore, these results suggest conditional defects in the assembly and/or functioning of the P. alcaligenes secreton in P. aeruginosa. Consistent with this idea, heat-stable multimers of XcpQalc were only found after growth in conditions that supported secretion, whereas only monomers were found when the cells were grown in liquid medium. In conclusion, these data indicate that the assembly of the P. alcaligenes XcpQ complex, and possibly of the complete secretory apparatus, might involve interactions of XcpQalc and/or XcpPalc with additional component(s) that are present in P. alcaligenes but lacking in P. aeruginosa. However, such a putative component(s) is apparently not essential during growth on a plate. Alternatively, such components might be present in P. aeruginosa but the interaction with XcpPalc and/or XcpQalc might be too weak during growth in liquid medium, resulting in inefficient secreton assembly.
A protein possibly required for XcpQ assembly might be a putative PulS homolog. PulS is an outer membrane lipoprotein that protects the XcpQ homolog PulD of K. oxytoca from proteolytic degradation and that is required for insertion of PulD in the outer membrane (28, 29). Although PulD multimers were formed in the absence of PulS, they were composed of PulD degradation products (28). PulS homologs (OutS) have been identified in E. chrysanthemi (55) and E. carotovora (41). Analysis of the whole genome sequence (http://www.pseudomonas.com) did not reveal the existence of a pulS homolog in P. aeruginosa. Nevertheless, a functional homolog of PulS might be present in P. alcaligenes and could be required for efficient assembly of XcpQalc in fast-growth conditions.
Recently, it was shown that two other Gsp proteins (probably) interact with the GspD secretin, i.e., XpsN (GspN) of Xanthomonas campestris (40) and OutB (GspB) of E. chrysanthemi (9). Genes encoding GspN have been identified in the gsp gene clusters of most GSP-containing bacteria, except E. chrysanthemi, P. aeruginosa, and P. alcaligenes (25, 40). Besides E. chrysanthemi, Aeromonas hydrophila (34), E. carotovora (41), and K. oxytoca (13) have been found to contain gspB genes. In A. hydrophila, the gspB (exeB) gene is clustered with the gspA (exeA) gene (34). Both the exeA and exeB gene products, which form a complex, are required for efficient type II secretion in this species (54). Genome sequence analysis did not reveal the presence of a gspA, gspB, or gspN gene in P. aeruginosa. Therefore, the assembly and/or functioning of the P. alcaligenes Xcp machinery in P. aeruginosa may have been affected by the absence of putative GspAalc, GspBalc, and GspNalc homologs.
Several studies indicate the involvement of an additional uncharacterized gene product(s) in type II secretion. For example, in addition to the characterized gsp (out) genes, an uncharacterized region of 4 kb upstream of outS appeared to be required for the reconstitution of the Gsp system of E. chrysanthemi in E. coli (41). This DNA segment might encode factors required for the assembly of the secretion machinery. Hamood et al. (27) described a pleiotropic P. aeruginosa secretion mutant that could not be complemented by xcpA or by the xcpP to -Z gene cluster, indicating the existence of an additional gene(s) required for secretion. Finally, Kagami et al. (35) reported the isolation of suppressors of an xcpT mutation in P. aeruginosa. One class of suppressor mutations mapped outside the xcpP to -Z gene cluster, again indicating that additional gene products may be involved in type II secretion. These uncharacterized gene products might be involved in the biogenesis of cell envelope components other than the secretion itself. It is likely that cell envelope processes, such as peptidoglycan or lipopolysaccharide (LPS) biosynthesis, affect the assembly and/or functioning of the secretion machinery. Consistently, P. aeruginosa strains with defective LPS were affected in the functioning of the Xcp secreton (44). Furthermore, LPS biosynthesis genes are required for type IV pilus biogenesis in Vibrio cholerae, a process that is related to type II protein secretion (33).
In conclusion, one or more of these additional gene products might be involved in Pseudomonas secreton assembly. These additional components might species specifically interact with XcpP and/or XcpQ, since these two components of P. aeruginosa could not efficiently be replaced by their homologs from P. alcaligenes. The construction of XcpP and XcpQ chimeric proteins is under way to identify the domains required for specific interaction between these two proteins and/or with the other, as yet unidentified gene products.
ACKNOWLEDGMENTS
We thank K.-E. Jaeger and V. Chapon-Hervé for providing bacterial strains, and G. Michel and S. Bleves for helpful discussion in the preparation of the manuscript.
This work was supported by EU grant BIO4-CT96-0119.
REFERENCES
- 1.Ball G, Chapon-Hervé V, Bleves S, Michel G, Bally M. Assembly of XcpR in the cytoplasmic membrane is required for extracellular protein secretion in Pseudomonas aeruginosa. J Bacteriol. 1999;181:382–388. doi: 10.1128/jb.181.2.382-388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 3.Bleves S, Gérard-Vincent M, Lazdunski A, Filloux A. Structure-function analysis of XcpP, a component involved in general secretory pathway-dependent protein secretion in Pseudomonas aeruginosa. J Bacteriol. 1999;181:4012–4019. doi: 10.1128/jb.181.13.4012-4019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleves S, Voulhoux R, Michel G, Lazdunski A, Tommassen J, Filloux A. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family) Mol Microbiol. 1998;27:31–40. doi: 10.1046/j.1365-2958.1998.00653.x. [DOI] [PubMed] [Google Scholar]
- 5.Bortoli-German I, Brun E, Py B, Chippaux M, Barras F. Periplasmic disulphide bond formation is essential for cellulase secretion by the plant pathogen Erwinia chrysanthemi. Mol Microbiol. 1994;11:545–553. doi: 10.1111/j.1365-2958.1994.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 6.Braun P, Tommassen J, Filloux A. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol. 1996;19:297–306. doi: 10.1046/j.1365-2958.1996.381908.x. [DOI] [PubMed] [Google Scholar]
- 7.Brok R, van Gelder P, Winterhalter M, Ziese U, Koster A J, de Cock H, Koster M, Tommassen J, Bitter W. The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J Mol Biol. 1999;294:1169–1179. doi: 10.1006/jmbi.1999.3340. [DOI] [PubMed] [Google Scholar]
- 8.Chen L Y, Chen D Y, Miaw J, Hu N T. XpsD, an outer membrane protein required for protein secretion by Xanthomonas campestris pv. campestris, forms a multimer. J Biol Chem. 1996;271:2703–2708. doi: 10.1074/jbc.271.5.2703. [DOI] [PubMed] [Google Scholar]
- 9.Condemine G, Shevchik V E. Overproduction of the secretin OutD suppresses the secretion defect of an Erwinia chrysanthemi outB mutant. Microbiology. 2000;146:639–647. doi: 10.1099/00221287-146-3-639. [DOI] [PubMed] [Google Scholar]
- 10.de Groot A, Filloux A, Tommassen J. Conservation of xcp genes, involved in the two-step protein secretion process, in different Pseudomonas species and other gram-negative bacteria. Mol Gen Genet. 1991;229:278–284. doi: 10.1007/BF00272167. [DOI] [PubMed] [Google Scholar]
- 11.de Groot A, Gerritse G, Tommassen J, Lazdunski A, Filloux A. Molecular organization of the xcp gene cluster in Pseudomonas putida: absence of an xcpX (gspK) homologue. Gene. 1999;226:35–40. doi: 10.1016/s0378-1119(98)00570-8. [DOI] [PubMed] [Google Scholar]
- 12.de Groot A, Krijger J J, Filloux A, Tommassen J. Characterization of type II protein secretion (xcp) genes in the plant growth-stimulating Pseudomonas putida, strain WCS358. Mol Gen Genet. 1996;250:491–504. doi: 10.1007/BF02174038. [DOI] [PubMed] [Google Scholar]
- 13.D'Enfert C, Pugsley A P. Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J Bacteriol. 1989;171:3673–3679. doi: 10.1128/jb.171.7.3673-3679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dente L, Cesareni G, Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deretic V, Chandrasekharappa S, Gill J F, Chatterjee D K, Chakrabarty A M. A set of cassettes and improved vectors for genetic and biochemical characterization of Pseudomonas genes. Gene. 1987;57:61–72. doi: 10.1016/0378-1119(87)90177-6. [DOI] [PubMed] [Google Scholar]
- 16.Douglas C M, Guidi-Rontani C, Collier R J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987;169:4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filloux A, Bally M, Ball G, Akrim M, Tommassen J, Lazdunski A. Protein secretion in gram-negative bacteria: transport across the outer membrane involves common mechanisms in different bacteria. EMBO J. 1990;9:4323–4329. doi: 10.1002/j.1460-2075.1990.tb07881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filloux A, Bally M, Murgier M, Wretlind B, Lazdunski A. Cloning of xcp genes located at the 55 min region of the chromosome and involved in protein secretion in Pseudomonas aeruginosa. Mol Microbiol. 1989;3:261–265. doi: 10.1111/j.1365-2958.1989.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 20.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–198. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 21.Frenken L G, de Groot A, Tommassen J, Verrips C T. Role of the lipB gene product in the folding of the secreted lipase of Pseudomonas glumae. Mol Microbiol. 1993;9:591–599. doi: 10.1111/j.1365-2958.1993.tb01719.x. [DOI] [PubMed] [Google Scholar]
- 22.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 23.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 24.Gerritse G, Hommes R W, Quax W J. Development of a lipase fermentation process that uses a recombinant Pseudomonas alcaligenes strain. Appl Environ Microbiol. 1998;64:2644–2651. doi: 10.1128/aem.64.7.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerritse G, Ure R, Bizoullier F, Quax W J. The phenotype enhancement method identifies the Xcp outer membrane secretion machinery from Pseudomonas alcaligenes as a bottleneck for lipase production. J Biotechnol. 1998;64:23–38. doi: 10.1016/s0168-1656(98)00101-1. [DOI] [PubMed] [Google Scholar]
- 26.Guilvout I, Hardie K R, Sauvonnet N, Pugsley A P. Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J Bacteriol. 1999;181:7212–7220. doi: 10.1128/jb.181.23.7212-7220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamood A N, Ohman D E, West S E, Iglewski B H. Isolation and characterization of toxin A excretion-deficient mutants of Pseudomonas aeruginosa PAO1. Infect Immun. 1992;60:510–517. doi: 10.1128/iai.60.2.510-517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardie K R, Lory S, Pugsley A P. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 29.Hardie K R, Seydel A, Guilvout I, Pugsley A P. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 30.He S Y, Lindeberg M, Chatterjee A K, Collmer A. Cloned Erwinia chrysanthemi out genes enable Escherichia coli to selectively secrete a diverse family of heterologous proteins to its milieu. Proc Natl Acad Sci USA. 1991;88:1079–1083. doi: 10.1073/pnas.88.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 33.Iredell J R, Manning P A. Outer membrane translocation arrest of the TcpA pilin subunit in rfb mutants of Vibrio cholerae O1 strain 569B. J Bacteriol. 1997;179:2038–2046. doi: 10.1128/jb.179.6.2038-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahagirdar R, Howard S P. Isolation and characterization of a second exe operon required for extracellular protein secretion in Aeromonas hydrophila. J Bacteriol. 1994;176:6819–6826. doi: 10.1128/jb.176.22.6819-6826.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagami Y, Ratliff M, Surber M, Martinez A, Nunn D N. Type II protein secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol Microbiol. 1998;27:221–233. doi: 10.1046/j.1365-2958.1998.00679.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 37.Kazmierczak B I, Mielke D L, Russel M, Model P. pIV, a filamentous phage protein that mediates phage export across the bacterial cell envelope, forms a multimer. J Mol Biol. 1994;238:187–198. doi: 10.1006/jmbi.1994.1280. [DOI] [PubMed] [Google Scholar]
- 38.Kordel M, Hofmann B, Schomburg D, Schmid R D. Extracellular lipase of Pseudomonas sp. strain ATCC 21808: purification, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol. 1991;173:4836–4841. doi: 10.1128/jb.173.15.4836-4841.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee H M, Wang K C, Liu Y L, Yew H Y, Chen L Y, Leu W M, Chen D C, Hu N T. Association of the cytoplasmic membrane protein XpsN with the outer membrane protein XpsD in the type II protein secretion apparatus of Xanthomonas campestris pv. campestris. J Bacteriol. 2000;182:1549–1557. doi: 10.1128/jb.182.6.1549-1557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindeberg M, Salmond G P, Collmer A. Complementation of deletion mutations in a cloned functional cluster of Erwinia chrysanthemi out genes with Erwinia carotovora out homologues reveals OutC and OutD as candidate gatekeepers of species-specific secretion of proteins via the type II pathway. Mol Microbiol. 1996;20:175–190. doi: 10.1111/j.1365-2958.1996.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 42.Lu H M, Lory S. A specific targeting domain in mature exotoxin A is required for its extracellular secretion from Pseudomonas aeruginosa. EMBO J. 1996;15:429–436. [PMC free article] [PubMed] [Google Scholar]
- 43.Marciano D K, Russel M, Simon S M. An aqueous channel for filamentous phage export. Science. 1999;284:1516–1519. doi: 10.1126/science.284.5419.1516. [DOI] [PubMed] [Google Scholar]
- 44.Michel G, Ball G, Goldberg J B, Lazdunski A. Alteration of the lipopolysaccharide structure affects the functioning of the Xcp secretory system in Pseudomonas aeruginosa. J Bacteriol. 2000;182:696–703. doi: 10.1128/jb.182.3.696-703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michel L O, Sandkvist M, Bagdasarian M. Specificity of the protein secretory apparatus: secretion of the heat-labile enterotoxin B subunit pentamers by different species of gram-negative bacteria. Gene. 1995;152:41–45. doi: 10.1016/0378-1119(94)00691-k. [DOI] [PubMed] [Google Scholar]
- 46.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley A P. Secretion PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci USA. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peek J A, Taylor R K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci USA. 1992;89:6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Possot O M, Vignon G, Bomchil N, Ebel F, Pugsley A P. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J Bacteriol. 2000;182:2142–2152. doi: 10.1128/jb.182.8.2142-2152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pugsley A P. Translocation of a folded protein across the outer membrane in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:12058–12062. doi: 10.1073/pnas.89.24.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugsley A P, Kornacker M G, Poquet I. The general protein-export pathway is directly required for extracellular pullulanase secretion in Escherichia coli K12. Mol Microbiol. 1991;5:343–352. doi: 10.1111/j.1365-2958.1991.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 52.Py B, Salmond G P C, Chippaux M, Barras F. Secretion of cellulases in Erwinia chrysanthemi and E. carotovora is species-specific. FEMS Microbiol Lett. 1991;79:315–322. [Google Scholar]
- 53.Sauvonnet N, Pugsley A P. Identification of two regions of Klebsiella oxytoca pullulanase that together are capable of promoting beta-lactamase secretion by the general secretory pathway. Mol Microbiol. 1996;22:1–7. doi: 10.1111/j.1365-2958.1996.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 54.Schoenhofen I C, Stratilo C, Howard S P. An ExeAB complex in the type II secretion pathway of Aeromonas hydrophila: effect of ATP-binding cassette mutations on complex formation and function. Mol Microbiol. 1998;29:1237–1247. doi: 10.1046/j.1365-2958.1998.01011.x. [DOI] [PubMed] [Google Scholar]
- 55.Shevchik V E, Robert-Baudouy J, Condemine G. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voulhoux R, Taupiac M-P, Czjzek M, Beaumelle B, Filloux A. Influence of deletions within domain II of exotoxin A on its extracellular secretion from Pseudomonas aeruginosa. J Bacteriol. 2000;182:4051–4058. doi: 10.1128/jb.182.14.4051-4058.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong K R, McLean D M, Buckley J T. Cloned aerolysin of Aeromonas hydrophila is exported by a wild-type marine Vibrio strain but remains periplasmic in pleiotropic export mutants. J Bacteriol. 1990;172:372–376. doi: 10.1128/jb.172.1.372-376.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wretlind B, Pavlovskis O R. Genetic mapping and characterization of Pseudomonas aeruginosa mutants defective in the formation of extracellular proteins. J Bacteriol. 1984;158:801–808. doi: 10.1128/jb.158.3.801-808.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]