Abstract
Background
Published randomized controlled trials are underpowered for binary clinical end points to assess the safety and efficacy of renin‐angiotensin system inhibitors (RASi) in adults with COVID‐19. We therefore performed a meta‐analysis to assess the safety and efficacy of RASi in adults with COVID‐19.
Methods and Results
MEDLINE, EMBASE, ClinicalTrials.gov, and the Cochrane Controlled Trial Register were searched for randomized controlled trials that randomly assigned patients with COVID‐19 to RASi continuation/commencement versus no RASi therapy. The primary outcome was all‐cause mortality at ≤30 days. A total of 14 randomized controlled trials met the inclusion criteria and enrolled 1838 participants (aged 59 years, 58% men, mean follow‐up 26 days). Of the trials, 11 contributed data. We found no effect of RASi versus control on all‐cause mortality (7.2% versus 7.5%; relative risk [RR], 0.95; [95% CI, 0.69–1.30]) either overall or in subgroups defined by COVID‐19 severity or trial type. Network meta‐analysis identified no difference between angiotensin‐converting enzyme inhibitors versus angiotensin II receptor blockers. RASi users had a nonsignificant reduction in acute myocardial infarction (2.1% versus 3.6%; RR, 0.59; [95% CI, 0.33–1.06]), but increased risk of acute kidney injury (7.0% versus 3.6%; RR, 1.82; [95% CI, 1.05–3.16]), in trials that initiated and continued RASi. There was no increase in need for dialysis or differences in congestive cardiac failure, cerebrovascular events, venous thromboembolism, hospitalization, intensive care admission, inotropes, or mechanical ventilation.
Conclusions
This meta‐analysis of randomized controlled trials evaluating angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers versus control in patients with COVID‐19 found no difference in all‐cause mortality, a borderline decrease in myocardial infarction, and an increased risk of acute kidney injury with RASi. Our findings provide strong evidence that RASi can be used safely in patients with COVID‐19.
Keywords: acute kidney injury, angiotensin II receptor blockers, angiotensin‐converting enzyme inhibitors, COVID‐19, hypertension, renin‐angiotensin system inhibitors
Subject Categories: Hypertension, Pharmacology, ACE/Angiotension Receptors/Renin Angiotensin System, Clinical Studies
Nonstandard Abbreviations and Acronyms
- ACE2
angiotensin‐converting enzyme 2
- AKI
acute kidney injury
- RASi
renin‐angiotensin system inhibitors
Clinical Perspective.
What Is New?
There was an almost 2‐fold increased risk of acute kidney injurty associated with renin‐angiotensin system inhibitors (RASi) in patients hospitalized with acute COVID‐19 in hospitalized patients (7.0% versus 3.6%; relative risk, 1.82; [95% CI, 1.05–3.16]). The overall event rate was low, but effects were consistent across trials that initiated and those that continued RASi, but was not associated with an increased need for dialysis or mortality at short‐term follow‐up.
What Are the Clinical Implications?
Evidence suggests that patients who are using RASi should continue taking their medication as prescribed; the overall cardiovascular benefits of these drugs are overwhelming, and early alerts of potential increased risk in patients with COVID‐19 have been silenced; similarly, clinicians should not be hesitant to initiate RASi treatment in patients with COVID‐19.
RASi can still be safely used in patients with COVID‐19 while being aware of an increased risk of acute kidney injury in hospitalized patients.
There does not appear to be increased risk of acute kidney injury in outpatients, which is where the vast majority of COVID‐19 is managed, and longer term follow‐up is needed to investigate renal outcomes and whether there may even be benefits of RASi to slow the progression of proteinuric chronic kidney disease in such patients.
Renin‐angiotensin system inhibitors (RASi), including angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), are the most widely prescribed antihypertensive treatments used by hundreds of millions of people worldwide. 1 RASi are not only first‐line agents for the treatment of hypertension but also are the cornerstone for treating conditions such as heart failure, coronary heart disease, diabetes, and chronic kidney disease. It has been suggested that RASi therapy may upregulate the expression of the angiotensin‐converting enzyme 2 (ACE2) receptor, 2 , 3 which is the functional receptor for SARS‐CoV‐2, 4 the virus responsible for the COVID‐19 pandemic. However, ACE2 upregulation has not been consistently demonstrated, 5 nor has it been shown to affect the function of RASi. 6
The BRACE CORONA (blockers of angiotensin receptor and angiotensin‐converting enzyme inhibitors suspension in hospitalized patients with coronavirus infection) randomized trial 7 in patients hospitalized with mild–moderate COVID‐19 suggested that days alive outside of hospital were equivalent in those continuing ACEIs/ARBs compared with those who had therapy suspended. Similarly compared with discontinuation of RASi, the REPLACE COVID (the randomized elimination or prolongation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in coronavirus disease 2019) trial found that continuation of RASi had no effect on a composite global rank score as a marker for COVID‐19 severity. 8 In comparison, the ACE‐COVID trial demonstrated that RASi discontinuation may lead to a more rapid and improved recovery from COVID‐19. 9 Most randomized controlled trials (RCTs) starting ARB therapy have also failed to demonstrate any difference compared with those not randomized to RASi therapy. 10 , 11 , 12 , 13 , 14 , 15 These trials, together with multiple others, are small to moderate in size, with many unable to meet their recruitment targets, and are insufficiently powered to answer questions regarding binary clinical end points or subgroup populations. Animal and observational studies have provided conflicting data, including concerns that RASi‐induced upregulation of ACE2 receptor expression may increase viral cell entry, whereas other studies have suggested that therapies may provide protective benefits 2 , 3 , 16 or have no effect on ACE2 expression. 17 In response to these uncertainties, numerous RCTs have been initiated to determine the short‐term safety of RASi in patients with COVID‐19. International hypertension, cardiology, and nephrology societies have consistently recommended that patients continue RASi therapy during the COVID‐19 pandemic on the basis of the strong and well‐documented evidence on their cardiovascular protective effects, but identified a need for more reliable human data. 18 , 19 , 20 , 21 , 22 We therefore performed a meta‐analysis of RCTs in patients with COVID‐19 to assess the safety and efficacy of RASi therapy compared with controls without RASi at short‐term follow‐up.
Methods
Meta‐Analysis Design and Selection of Trials
Our meta‐analysis and search strategy were reported in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analysis for protocol recommendations. 23 The methods of this review were previously published 24 and will be outlined here in brief. Using the Cochrane Collaboration guidelines, 25 electronic searches of MEDLINE (1996–present), EMBASE (1996–present), the Cochrane Central Register of Controlled Trials (most recent edition), and ClinicalTrials.gov were performed in June 2021 to identify RCTs that meet the inclusion criteria.
Trials with the following criteria were included: (1) RCTs recruiting between March 2020 and June 2021, (2) patients aged ≥18 years; (3) laboratory‐confirmed SARS‐CoV‐2 infection, (4) comparison of patients randomly assigned to RASi versus no RASi therapy (this includes trials that investigate continuation versus cessation of RASi among patients currently treated with RASi and trials that report initiation of RASi versus control in those not currently treated with such therapies), (5) findings reported in English, and (6) oral administration of RASi therapies. Two reviewers (S.R.G. and A.E.S.) independently performed study selection, quality assessment, and data extraction. Data extraction included information regarding study design, participants, methods, interventions, and outcome measures. End points were all‐cause mortality, acute myocardial infarction, congestive cardiac failure, venous thromboembolism, hospitalization, admission to intensive care, mechanical ventilation, hypotension requiring inotropes, and acute kidney injury (AKI; defined according to the Kidney Disease Improving Global Outcomes criteria) 26 at short‐term follow‐up (defined as ≤30 days). Standardized grouped tabular deidentified data were requested from trialists. A quality assessment of each trial was performed by 2 authors (S.R.G. and A.E.S.) using the Cochrane Collaboration risk of bias tool. 25 , 27 Each included trial was approved by an institutional review committee, and the participants gave informed consent.
Statistical Analysis
Trial‐specific outcome data were pooled. For binary outcomes, risk ratios and 95% CIs were estimated. Head‐to‐head meta‐analyses were performed by the Mantel–Haenszel fixed‐effects models, 28 with key results presented using forest plots. A 2‐tailed P value of 5% was used for hypothesis testing. Small study effect was assessed by visual inspection of funnel plots and by formal regression‐based Egger tests. 29 Quantitative heterogeneity has been explored by prespecified subgroup analyses and fitting univariable meta‐regression with the percentage loss to follow‐up as a fixed‐effect covariate. 24 A fixed‐effects analysis was used unless there was significant heterogeneity (as evidenced by I 2 >50% and quantitatively large variation), in which case random‐effects analysis was performed instead. 28 Sensitivity analyses to account for zero and small counts in some trials were performed using the reciprocal of the sample size of the opposite arm. 30 To assess the relative efficacy of ACEIs versus ARBs (versus control), we also fitted a frequentist random‐effects network meta‐analysis. We reported resulting rankograms and P scores 31 : these allow rank treatments on a continuous scale (with a 0–1 range, the higher the better) and are the frequentist analog of the surface under the cumulative ranking curve.
Analyses were conducted using Review Manager 5.3 software (Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration), Comprehensive Meta‐Analysis V3 (Biostat, Englewood, NJ), and the package netmeta in R. 32
The authors declare that all supporting data are available in the article and its supplemental files.
Results
Of 45 articles identified through a systematic search and 23 trials on ClinicalTrials.org, 14 RCTs met the inclusion criteria (Table 1, Figure 1). Of the trials, 11 provided grouped tabular data. A total of 1838 patients with a mean follow‐up of 26 days were enrolled, including sites in Argentina, Austria, Brazil, Canada, France, Germany, Iran, Mexico, the Netherlands, and the United States. Of these, 5 trials evaluated the continuation versus discontinuation of RASi therapies in those already on such therapies (n=1079), and 9 trials involved initiation of RASi in those naïve to therapy (n=759). All 9 trials initiating RASi therapies involved commencement of ARBs (n=5 telmisartan, n=3 losartan, n=1 valsartan).
Table 1.
Characteristics of Included Randomized Controlled Trials of Adults With COVID‐19
| Trial name | Country | Inclusion criteria | Intervention | Control | No. | Follow‐up, d |
|---|---|---|---|---|---|---|
| ACEI‐COVID 9 | Germany; Austria |
|
Continue ACEI/ARB | Discontinue ACEI/ARB | 204 | 30 |
| BRACE CORONA 7 | Brazil |
|
Continue ACEI/ARB | Discontinue ACEI/ARB | 659 | 30 |
| RAAS‐COVID 15 | Canada |
|
Continue ACEI/ARB | Discontinue ACEI/ARB | 46 | 30 |
| REPLACE‐COVID 8 | United States, Canada, Mexico, Sweden, Peru, Bolivia, and Argentina |
|
Continue ACEI/ARB | Discontinue ACEI/ARB | 152 | 5 |
| SWITCH‐COVID | Brazil |
|
Continue ACEI/ARB | Discontinue ACEI/ARB | 18 | 30 |
| ALPS‐COVID IP 14 | United States |
|
Losartan | Placebo | 205 | 28 |
| ALPS‐COVID OP 13 | United States |
|
Losartan | Placebo | 117 | 28 |
| ARB use to minimize progression to respiratory failure 11 | United States |
|
Losartan | Standard care | 31 | 10 |
| COVERAGE‐France | France |
|
Telmisartan | Vitamin supplement | 69 | 14 |
| COVID MED | United States |
|
Losartan | Placebo | 12 | 30 |
| Evaluation of the effect of losartan in COVID‐19 12 | Iran |
|
Losartan | Amlodipine | 80 | 30 |
| PRAETORIAN‐COVID | The Netherlands |
|
Valsartan | Placebo | 23 | 14 |
| STAR‐COVID | Mexico |
|
Telmisartan | Standard care | 64 | 30 |
| Telmisartan for treatment of patients with COVID‐19 10 | Argentina |
|
Telmisartan | Standard care | 141 | 30 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ACEI‐COVID, the stopping ACE‐inhibitors in COVID‐19 trial; ALPS‐COVID OP, angiotensin receptor blocker based lung protective strategy for COVID‐19 outpatient trial; ALPS‐COVIDIP, Angiotensin receptor blocker based lung protective strategy for COVID‐19inpatient trial; ARB, angiotensin II receptor blocker; BP, blood pressure; BRACE CORONA, blockers of angiotensin receptor and angiotensin‐converting enzyme inhibitors suspension in hospitalized patients with coronavirus infection; CAD, coronary artery disease; CCF, congestive cardiac failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVERAGE‐France, randomized trial to evaluate the safety and efficacy of outpatient treatments to reduce the risk of worsening in individuals with COVID‐19 with risk factors; COVID MED, comparison of therapeutics for hospitalized patients infected with SARS‐CoV‐2; PRAETORIAN‐COVID, randomized clinical trial with valsartan for prevention of acute respiratory distress syndrome in hospitalized patients with SARS‐COV‐2 Infection Disease; RAAS‐COVID, renin‐angiotensin aldosterone system inhibitors in COVID‐19; REPLACE COVID, the randomized elimination or prolongation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in coronavirus disease 2019; SOFA, sequential organ failure assessment; STAR‐COVID, telmisartanin respiratory failure due to COVID‐19; and SWITCH‐COVID, switch of renin‐angiotensin system inhibitors in patients with COVID‐19.
Figure 1. Flowchart of study selection methodology.
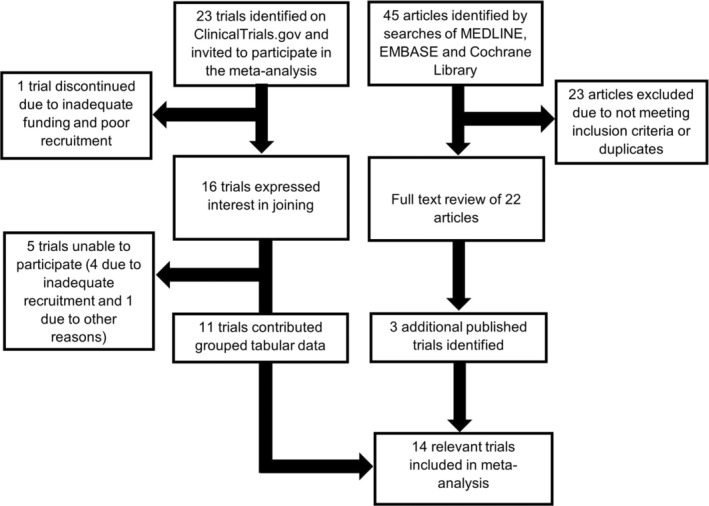
Study Quality
The RCTs were of high quality as assessed by the Cochrane Collaboration risk of bias tool (Table S1, Figure S1). There were 4 placebo‐controlled, double‐blinded RCTs, 9 open‐label trials, and 1 double‐blinded RCT comparing ARB versus amlodipine. Of these, 12 trials were conducted in patients hospitalized with COVID‐19 and 2 trials recruited outpatients. All trials used random sequence generation and were judged as being low risk of selection bias. The double‐blinded trials were judged as being at low risk of allocation concealment and performance biases, whereas the open‐label trials were judged as having moderate risk of these biases. Most trials were at low risk of detection bias and attrition bias, with only 1 trial having a loss to follow‐up of >10%. All trials had a low risk of reporting bias.
Baseline Clinical Characteristics
Baseline clinical characteristics of the intervention and control groups are described in Table 2, indicating comparable profiles. The mean age of the population was 58.8 years, and 57.6% were men. Hypertension was prevalent in 75.5%, diabetes in 28.5%, cardiovascular disease in 10.4%, obesity in 35.8%, and chronic obstructive pulmonary disease in 10.8%. COVID‐19 severity ranged from mild (46.6%) or moderate (44.2%) to severe (9.2%). Of those patients recruited, 21.6% were either current or past smokers.
Table 2.
Baseline Clinical Characteristics of Total Cohort (N=1838)
| Renin‐angiotensin system inhibitors (n=917) | Control (n=921) | |
|---|---|---|
| Mean age, y | 58.6 | 58.9 |
| Sex, n (%) | ||
| Male sex | 526/917 (57.4) | 532/921 (57.8) |
| Female sex | 391/917 (42.6) | 389/921 (42.2) |
| Past medical history, n (%) | ||
| Hypertension | 669/889 (75.3) | 679/897 (75.7) |
| Diabetes | 266/917 (29.0) | 258/921 (28.0) |
| Hypercholesterolemia | 115/329 (35.0) | 94/325 (28.9) |
| Cardiovascular disease | 90/856 (10.5) | 89/867 (10.2) |
| Obesity | 164/451 (36.4) | 159/450 (35.3) |
| Chronic kidney disease | 48/759 (6.3) | 44/763 (5.8) |
| Chronic obstructive pulmonary disease | 64/586 (10.9) | 61/572 (10.7) |
| Smoking, n (%) | ||
| Ever smoked | 109/514 (21.2) | 113/516 (21.9) |
| Nonsmoker | 405/514 (78.8) | 403/516 (78.1) |
| COVID‐19 severity, n (%) | ||
| Mild | 343/722 (47.5) | 324/709 (45.7) |
| Moderate | 311/722 (43.0) | 321/709 (45.3) |
| Severe | 68/722 (9.4) | 64/709 (9.0) |
Cardiovascular disease defined as established coronary artery disease, heart failure, arrythmia, and/or stroke; chronic kidney disease defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2.
Primary Outcome
All‐Cause Mortality
A total of 14 trials provided all‐cause mortality data (n=1838; Figure 2A), with 12 trials reporting a total of 135 deaths. We found no effect of RASi versus control on all‐cause mortality (7.2% versus 7.5%; relative risk [RR], 0.95; [95% CI, 0.69–1.30]; I 2=15%; P=0.73). When analyzed by trial type, there was no significant difference between trials that compared RASi initiation (RR, 0.72; [95% CI, 0.46–1.14]; P=0.16) versus continuation (RR, 1.24; [95% CI, 0.78–1.96]; P=0.36; P=0.28 for subgroup difference; Figure S2). We also found no difference in mortality by placebo control versus open‐label trials, location of trial, or COVID‐19 severity (Figures S3 through S5). In the ARB class, there was no difference between the different drugs (Figure S6). There was no significant publication bias as assessed by Egger regression testing (P=0.86), although inspection of the plot suggested an underrepresentation of trials showing benefit with RASi therapy (Figure S7).
Figure 2. Outcomes at short‐term follow‐up (≤30 days). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 .
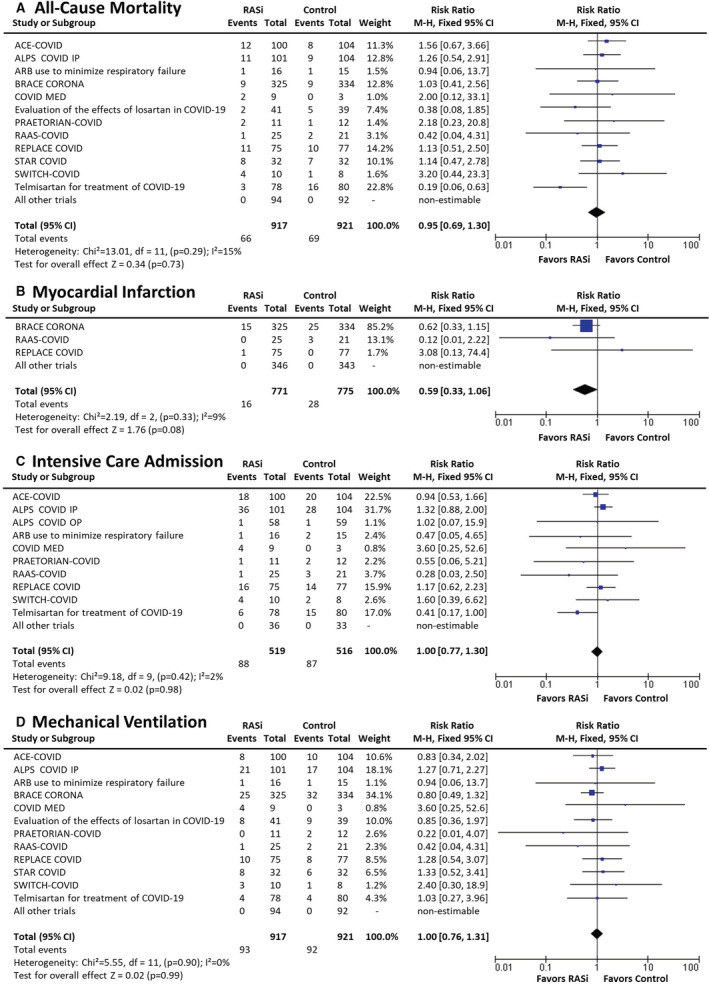
ACEI‐COVID, the stopping ace‐inhibitors in COVID‐19 trial; ALPS‐COVID IP, angiotensin receptor blocker based lung protective strategy for COVID‐19 inpatient trial; ALPS‐COVID OP, angiotensin receptor blocker based lung protective strategy for COVID‐19 outpatient trial; BRACE CORONA, blockers of angiotensin receptor and angiotensin‐converting enzyme inhibitors suspension in hospitalized patients with coronavirus infection; COVERAGE‐France, randomized trial to evaluate the safety and efficacy of outpatient treatments to reduce the risk of worsening in individuals with COVID‐19 with risk factors; COVID MED, comparison of therapeutics for hospitalized patients infected with SARS‐CoV‐2; M‐H indicates Mantel–Haenszel; PRAETORIAN‐COVID, randomised clinical trial with valsartan for prevention of acute respiratory distress syndrome in hospitalised patients with SARS‐COV‐2 infection disease; RAAS‐COVID, renin‐angiotensin aldosterone system inhibitors in COVID‐19 trial; RASi, renin‐angiotensin system inhibitors; REPLACE COVID, the randomized elimination or prolongation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in coronavirus disease 2019; STAR‐COVID, telmisartan in respiratory failure due to COVID‐19; and SWITCH‐COVID, switch of renin‐angiotensin system inhibitors in patients with COVID‐19.
Sensitivity analyses demonstrated that there were no effects on all‐cause mortality across subgroups based on age, sex, or ethnicity (Figures S8 through S10), although there was a nonsignificant trend to increased mortality among the White population with RASi therapy (RR, 1.52; [95% CI, 0.85–2.72]; P=0.16). Analyses accounting for the small counts in some trials also did not change the results (Table S2). There were also no between‐group differences in all‐cause mortality for those on RASi compared with control when stratified by the presence or absence of hypertension, diabetes, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, smoking status, or obesity (Figures S11 through S17). Although the largest trial (BRACE‐CORONA) accounted for a large proportion of participants, an analysis excluding this trial did not change the results (RR, 0.93; [95% CI, 0.66–1.31]). Meta‐regression analysis of trials according to percentage loss to follow‐up demonstrated that trials with a higher loss to follow‐up overestimated mortality benefit with RASi (coefficient, −0.165; [95% CI, −0.281 to −0.050]; P=0.005; Figure S18).
Network meta‐analysis comparing control to ACEIs versus ARBs demonstrated no statistically significant differences between ACEIs and ARBs, but ACEIs were associated with a worse mortality effect with a P score of 0.089 compared with P scores of 0.72 and 0.69 for ARBs and control, respectively (Figures S19 and S20). In particular, we found the RR of ARBs versus ACEIs of 0.60 (95% CI, 0.29–1.23) and the RRs versus placebo for ACEIs and ARBs equal to 1.65 (95% CI, 0.78–3.48) and 0.99 (95% CI, 0.62–1.59), respectively (overall inconsistency I 2=28.3%; test of homogeneity P value=0.15).
Secondary Outcomes
Myocardial Infarction
A total of 10 trials collected acute myocardial infarction outcomes (n=1546; Figure 2B): 3 trials that compared continuation versus discontinuation of RASi in people with preexisting hypertension and/or cardiovascular disease reported a total of 44 events. Pooling of these studies suggest a substantial but nonstatistically significant reduction in acute myocardial infarction with RASi compared with control (2.1% versus 3.6%; RR, 0.59; [95% CI, 0.33–1.06]; I 2=9%; P=0.078).
Coronary Revascularization
Data were collected from 8 trials (n=841), but there were no coronary revascularization events reported in the RASi and control groups.
Cerebrovascular Accidents
A total of 10 trials provided cerebrovascular outcomes (n=1546; Figure S21), with 2 trials reporting events. A total of 8 cerebrovascular events were observed. There was no significant difference in cerebrovascular events with RASi compared with control (0.6% versus 0.4%; RR, 1.62; [95% CI, 0.43–6.15]; I 2=0%; P=0.48).
Congestive Cardiac Failure
A total of 9 trials provided congestive cardiac failure outcomes (n=1341; Figure S22), with 3 trials reporting a total of 41 heart failure events. There were no statistically significant between‐group differences in congestive cardiac failure on RASi compared with control (2.8% versus 3.3%; RR, 0.71; [95% CI, 0.16–3.17]; I 2=60%; P=0.66).
Venous Thromboembolism
Data were available from 9 trials (n=1500; Figure S23), with 3 trials reporting 16 venous thromboembolism events. There was no difference in the rate of thromboembolism between the groups (1.2% versus 0.9%; RR, 1.18; [95% CI, 0.45–3.05]; I 2=0%; P=0.74).
Hospitalization
There were only 2 small outpatient trials 13 , 33 that reported hospitalization rates for COVID‐19 (n=186; Figure S24). A total of 9 hospitalization episodes were observed. There was no significant difference in rates of hospitalization detected between those on RASi compared with control (6.4% versus 3.3%; RR, 1.92; [95% CI, 0.50–7.35]; I 2=0; P=0.34).
Intensive Care Admission
A total of 11 trials collected intensive care admission outcomes (n=1035; Figure 2C), with 10 trials reporting a total of 175 admissions. There was no difference in admission to intensive care between those on RASi compared with control (17.0% versus 16.9%; RR, 1.00; [95% CI, 0.77–1.30]; I 2=2%; P=0.98). Analysis comparing trials that commenced versus those that continued/discontinued RASi also did not demonstrate differences in intensive care admission rates (P=0.91 for subgroup differences; Figure S25).
Mechanical Ventilation
Of the trials, 9 collected outcome data on need for mechanical ventilation (n=1838; Figure 2D), with 6 trials reporting 185 mechanical ventilation events. There was no difference in the rate of mechanical ventilation between people on RASi compared with controls (10.1% versus 10.0%; RR, 1.00; [95% CI, 0.76–1.31]; I 2=0%; P=0.99). Analysis comparing trials that commenced versus those that continued/discontinued RASi also did not demonstrate differences in mechanical ventilation rates (P=0.41 for subgroup differences; Figure S26).
Hypotension Requiring Inotropes
A total of 9 trials measured hypotension requiring inotropes (n=1500; Figure 3A), with 6 trials reporting a total of 127 events requiring inotropes. In the total group, there was no increase in inotrope use between people on RASi compared with no RASi (8.6% versus 8.4%; RR, 1.01; [95% CI, 0.73–1.41]; I 2=0%; P=0.93). However, sensitivity analyses restricted to patients with severe COVID‐19 demonstrated that RASi was associated with a trend to increased risk of hypotension requiring inotropes compared with controls (33.8% versus 20.3%; RR, 1.56; [95% CI, 0.88–2.79]; I 2=0%; P=0.13; Figure S27). Analysis comparing trials that commenced RASi showed a nonsignificant increase in inotrope use compared with those that continued/discontinued RASi (RR, 1.40 [95% CI, 0.82–2.39] versus RR, 0.84 [95% CI, 0.55–1.28], respectively; P=0.15 for subgroup comparison; Figure S28).
Figure 3. Adverse outcomes at short‐term follow‐up (≤30 days). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 .
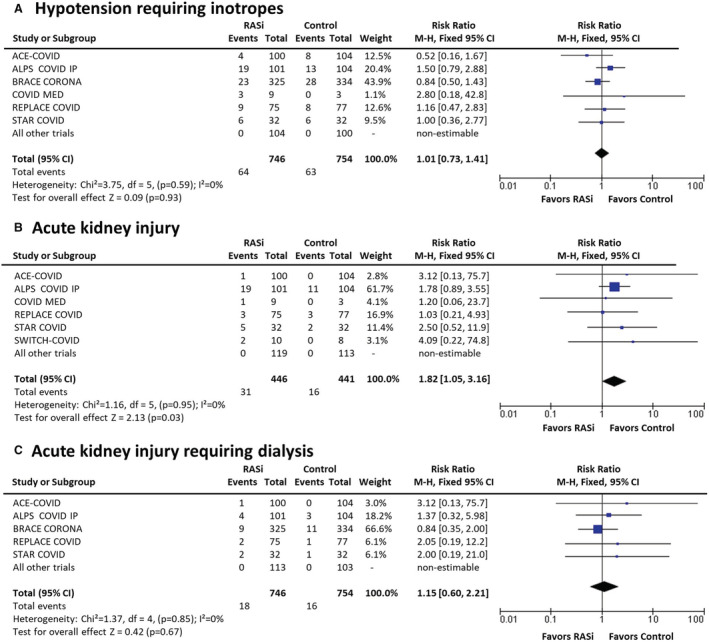
ACEI‐COVID, the stopping ace‐inhibitors in COVID‐19 trial; ALPS‐COVID IP, angiotensin receptor blocker based lung protective strategy for COVID‐19 inpatient trial; ALPS‐COVID OP, angiotensin receptor blocker based lung protective strategy for COVID‐19 outpatient trial; BRACE CORONA, blockers of angiotensin receptor and angiotensin‐converting enzyme inhibitors suspension in hospitalized patients with coronavirus infection; COVERAGE‐France, randomized trial to evaluate the safety and efficacy of outpatient treatments to reduce the risk of worsening in individuals with COVID‐19 with risk factors; COVID MED, comparison of therapeutics for hospitalized patients infected with SARS‐CoV‐2; M‐H indicates Mantel–Haenszel; PRAETORIAN‐COVID, randomised clinical trial with valsartan for prevention of acute respiratory distress syndrome in hospitalised patients with SARS‐COV‐2 infection disease; RAAS‐COVID, renin‐angiotensin aldosterone system inhibitors in COVID‐19 trial; RASi, renin‐angiotensin system inhibitors; REPLACE COVID, the randomized elimination or prolongation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in coronavirus disease 2019; STAR‐COVID, telmisartan in respiratory failure due to COVID‐19; and SWITCH‐COVID, switch of renin‐angiotensin system inhibitors in patients with COVID‐19.
AKI and Need for Dialysis
A total of 9 trials measured AKI outcomes (n=887; Figure 3B); 6 trials of hospitalized patients reported 47 AKI events. Increased AKI (7.0% versus 3.6%; RR, 1.82; [95% CI, 1.05–3.16]; I 2=0%; P=0.033) was noted in the RASi versus control groups. Although the AKI events were low, this effect was consistent across trials that initiated RASi versus those that continued/discontinued RASi (P=0.90 for subgroup differences; Figure S29) and across those with mild, moderate, and severe COVID‐19 (P=0.90 for subgroup differences; Figure S30). There was no statistically significant increase in need for dialysis in the RASi group compared with control (2.4% versus 2.1%; RR, 1.15; [95% CI, 0.60–2.21]; I 2=0%; P=0.67; Figure 3C).
Discussion
In this meta‐analysis of 14 clinical trials in patients with COVID‐19, we found no effect on all‐cause mortality, a trend toward decreased myocardial infarction, and an increased risk of AKI in patients randomly assigned to RASi versus controls. Evidence from RCTs in patient groups without COVID‐19 including those with hypertension and high cardiovascular risk has also indicated an increased risk of AKI from RASi‐based blood pressure (BP) lowering but decreases in vascular events from RASi therapy long term, 34 suggesting that these effects in patients with COVID‐19 may be real. In this analysis, the safety of RASi was seen across other outcomes, including heart failure, stroke, hospitalization, need for intensive care, and use of inotropes or mechanical ventilation. This is consistent with observational studies that suggested there was no adverse effect of renin‐angiotensin system blockade on COVID‐19 severity and outcomes. 16 , 35 , 36 , 37 , 38 The totality of data from this international collaboration provides strong evidence to suggest that RASi can be safely used in patients with COVID‐19 while being aware of an increased risk of AKI, which will better inform public health policy and clinical decision making.
The collective inclusion of data from >1800 patients enabled us to conduct several subgroup analyses. Consistent effects were seen across subgroups. The majority of patients used RASi therapy for the treatment of hypertension, but results in the subgroups with cardiovascular disease and chronic kidney disease were reassuring. Importantly, we were able to demonstrate for the first time that there was no statistically significant difference in ACEI versus ARB use on all‐cause mortality. This suggests that neither the upstream renin‐angiotensin syndrome inhibition by ACEIs nor the downstream inhibition at the receptor level by ARBs influence mortality outcomes in COVID‐19.
We found an almost 2‐fold increased risk of AKI associated with RASi in patients hospitalized with acute COVID‐19 in hospitalized patients, with CIs suggesting a minor to a 4‐fold increase. This risk is a potentially important finding that was unknown before our meta‐analysis. 39 Effects were consistent across trials that initiated and those that continued RASi, 40 , 41 , 42 but were not associated with increased need for dialysis or mortality at short‐term follow‐up. AKI is common in COVID‐19, with proteinuria often seen in those admitted to hospital, 43 although the mechanisms appear to be multicausal. Some studies suggest that SARS‐CoV‐2 can directly infect the renal tubular epithelium through an ACE2‐dependent pathway, 40 , 41 , 42 , 44 whereas others have instead demonstrated acute tubular necrosis, thrombotic microangiopathy, glomerulonephritis, and other intrinsic renal disease. 45 , 46 , 47 Kidney invasion of SARS‐CoV‐2 has been difficult to demonstrate consistently in all studies, and whether it directly leads to AKI is controversial. 48 There have been reports of virus detected in the kidney by different methods,49 but others did not find any such evidence. 48 The kidneys may be particularly susceptible to SARS‐CoV‐2 because of the high ACE2 expression 50 , 51 and coexpression of the cell surface protease facilitating viral cell entry transmembrane serine protease 2 in the proximal tubular cells and tubular progenitor cells. 4 , 52 AKI in COVID‐19 can stem from hypovolemia, hypotension, hypoxia, and inflammation or use of different nephrotoxic medications (eg, nonsteroidal anti‐inflammatory drugs) or their combined effects. 53 It is well recognized that RASi produces reduction in intraglomerular pressure and this can translate into a drop in glomerular filtration rate,54 in particular in patients whose baseline kidney function is compromised. 54 Analyses in patients without COVID‐19 55 , 56 have demonstrated that a decline in glomerular filtration rate associated with intensive BP reduction actually preserves blood flow to the renal tubules, a region highly sensitive to hypoxia and susceptible to acute tubular necrosis with sustained hypoperfusion. 57 Longer term follow‐up is needed to investigate clinical outcomes in patients with a history of COVID‐19 treated with RASi—previous studies in patients without COVID‐19 demonstrated that angiotensin‐converting enzyme inhibition or ARB‐based treatment is associated with lower mortality in the follow‐up after AKI. 58
We also observed a borderline decrease in acute myocardial infarction with continuation of RASi therapy. The results were driven by the BRACE CORONA trial 7 (RR, 0.66; [95% CI, 0.33–1.15]), with the addition of 2 smaller trials further confirming this trend in our meta‐analysis (RR, 0.59; [95% CI, 0.33–1.06]; P=0.078). These 3 trials all compared continuation versus discontinuation of RASi therapy in people with preexisting hypertension and/or cardiovascular disease. The result is unsurprising given the well‐established benefits afforded by RASi therapy in the reduction in cardiovascular mortality, myocardial infarction, and stroke. 59 , 60 One small RCT (n=46) demonstrated that RASi discontinuation increased the incidence of acute heart failure (33% versus 4%; P=0.016), 15 which was consistent with the direction of effect observed in our analysis. The short duration of this analysis did not allow the longer beneficial effects of RASi to be demonstrated. Increased vascular events have been observed with RASi cessation, 61 with continuation leading to avoidance of drug discontinuation syndromes. The benefits of RASi can take months to accrue, but the risks of withdrawal occur more rapidly. 62 Our results support the importance of continuing RASi in people with elevated cardiovascular risk—including patients with COVID‐19—consistent with the recommendations of international guidelines. 18 , 19 , 20 , 21 , 22
There are a number of limitations to the present analysis. Our meta‐analysis focused on binary clinical end points, and benefits on continuous outcomes (eg, length of stay, duration of ventilation) were not assessed. Visual inspection of the all‐cause mortality funnel plot also suggested an underrepresentation of trials showing benefit with RASi therapy. This is likely to arise from poor recruitment leading to trial termination (NCT04329195), inability to participate in this meta‐analysis because of failure to meet predefined recruitment targets for unblinding (NCT04360551, NCT04351581), or provision of only a low number of participants to the analysis (NCT04335786, NCT04328012, NCT04493359). The relatively low event rates and short follow‐up duration of included trials (≤30 days) also prevents robust assessment of long‐term outcomes. The risk profile of patients included in RCTs may also limit the extrapolation of the results to patient groups in clinical practice who are older and more comorbid. The results also do not evaluate the posological discrimination of the ARBs used in each clinical trial. 63 Further research is required to assess the mechanism of AKI associated with RASi, rates of renal recovery, and the benefits of RASi for the treatment of proteinuria in these patients and other longer term outcomes. Nevertheless, this is the largest pooled analysis of RCTs compared with other meta‐analyses that were smaller 64 or included observational studies 65 and represents a major achievement in international collaboration. This is the most highly powered randomized analysis to assess binary clinical end points and the first to directly compare ACEIs versus ARBs.
This first meta‐analysis of RCTs evaluating RASi versus control in patients with COVID‐19 found no difference in all‐cause mortality, a borderline decrease in myocardial infarction, and an increased risk of AKI with RASi. The risk of AKI was consistent across trials that initiated and those that continued RASi. More evidence is needed with longer term follow‐up to establish the clinical implications of this finding.
Conclusion
Early controversies that RASi therapy may upregulate the ACE2 receptor and hence pose safety and efficacy issues in patients with COVID‐19 has resulted in several RCTs to be conducted across the globe to address this issue. Our meta‐analysis including 14 RCTs suggests that RASi can be safely used (continued or initiated) in patients with COVID‐19. In those using RASi, we report a trend toward decreased myocardial infarction, with a potential increased risk of AKI—a finding unknown in patients with COVID‐19 before our meta‐analysis. Our inclusion of several trials also enabled the first direct comparison of ACEIs versus ARBs, but our findings indicate no difference. Overall, this meta‐analysis provides strong evidence that RASi can be used safely in patients with COVID‐19, balancing both the benefits and risks on cardiovascular and renal outcomes, respectively.
Sources of Funding
None.
Disclosures
Dr Gnanenthiran is supported by a postdoctoral fellowship from the Heart Foundation of Australia. Dr Chirinos has recently consulted for Bayer, Sanifit, Fukuda‐Denshi, Bristol‐Myers Squibb, JNJ, Edwards Life Sciences, Merck, NGM Biopharmaceuticals, and the Galway‐Mayo Institute of Technology. He received University of Pennsylvania research grants from National Institutes of Health, Fukuda‐Denshi, Bristol‐Myers Squibb, Microsoft, and Abbott. He is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of heart failure and preserved ejection fraction and for the use of biomarkers in heart failure with preserved ejection fraction. He has received payments for editorial roles from the American Heart Association, the American College of Cardiology, and Wiley. He has received research device loans from Atcor Medical, Fukuda‐Denshi, Uscom, NDD Medical Technologies, Microsoft, and MicroVision Medical. Dr Schutte received consultancy fees from Abbott and speaker honoraria from Servier, Sanofi, Sun Pharmaceuticals, Omron, Takeda, and Novartis. Dr Stergiou received consultancy fees and speaker honoraria from Astra‐Zeneca, Menarini, Novartis, Sanofi, and Servier. Dr Schlaich has received consulting fees and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer‐Ingelheim. Dr Sharma reports receiving support from the Fonds de Recherche Santé Quebec Junior 1 clinician scholar program, Canada Institute for Health Research (grant 175095), Roche Diagnostics, Boeringer‐Ingelheim, Novartis, and Takeda. Dr Poulter has received financial support from several pharmaceutical companies that manufacture blood pressure–lowering agents, for consultancy fees (Servier), research projects and staff (Servier, Pfizer), and for arranging and speaking at educational meetings (AstraZeneca, Lri Therapharma, Napi, Servier, Sanofi, Eva Pharma, Pfizer, Alkem Laboratories, and Glenmark Pharma). He holds no stocks or shares in any such companies. Dr Williams has received honoraria for lectures on hypertension from Servier, Menarini, Pfizer, Boehringer Ingelheim, and Daiichi Sankyo. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S2
Figures S1–S30
Acknowledgments
We thank Stella Galanis, Medical Librarian.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026143
For Sources of Funding and Disclosures, see page 10.
References
- 1. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156 [DOI] [PubMed] [Google Scholar]
- 2. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- 3. Ocaranza MP, Moya J, Barrientos V, Alzamora R, Hevia D, Morales C, Pinto M, Escudero N, García L, Novoa U, et al. Angiotensin‐(1‐9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J Hypertens. 2014;32:771–783. doi: 10.1097/HJH.0000000000000094 [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danser AHJ, Epstein M, Batlle D. Renin‐angiotensin system blockers and the COVID‐19 pandemic: at present there is no evidence to abandon renin‐angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID‐19. J Am Soc Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopes RD, Macedo A, de Barros E, Silva PGM, Moll‐Bernardes RJ, Dos Santos TM, Mazza L, Feldman A, D'Andréa Saba Arruda G, de Albuquerque DC, et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID‐19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen JB, Hanff TC, William P, Sweitzer N, Rosado‐Santander NR, Medina C, Rodriguez‐Mori JE, Renna N, Chang TI, Corrales‐Medina V, et al. Continuation versus discontinuation of renin‐angiotensin system inhibitors in patients admitted to hospital with COVID‐19: a prospective, randomised, open‐label trial. Lancet Respir Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer A, Schreinlechner M, Sappler N, Dolejsi T, Tilg H, Aulinger BA, Weiss G, Bellmann‐Weiler R, Adolf C, Wolf D, et al. Discontinuation versus continuation of renin‐angiotensin‐system inhibitors in COVID‐19 (ACEI‐COVID): a prospective, parallel group, randomised, controlled, open‐label trial. Lancet Respir Med. 2021;9:863–872. doi: 10.1016/S2213-2600(21)00214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duarte M, Pelorosso F, Nicolosi LN, Salgado MV, Vetulli H, Aquieri A, Azzato F, Castro M, Coyle J, Davolos I, et al. Telmisartan for treatment of Covid‐19 patients: an open multicenter randomized clinical trial. EClinicalMedicine. 2021;37:100962. doi: 10.1016/j.eclinm.2021.100962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geriak M, Haddad F, Kullar R, Greenwood KL, Habib M, Habib C, Willms D, Sakoulas G. Randomized prospective open label study shows no impact on clinical outcome of adding losartan to hospitalized COVID‐19 patients with mild hypoxemia. Infect Dis Ther. 2021;10:1323–1330. doi: 10.1007/s40121-021-00453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nouri‐Vaskeh M, Kalami N, Zand R, Soroureddin Z, Varshochi M, Ansarin K, Rezaee H, Taghizadieh A, Sadeghi A, Ahangari Maleki M, et al. Comparison of losartan and amlodipine effects on the outcomes of patient with COVID‐19 and primary hypertension: a randomised clinical trial. Int J Clin Pract. 2021;75:e14124. doi: 10.1111/ijcp.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puskarich MA, Cummins NW, Ingraham NE, Wacker DA, Reilkoff RA, Driver BE, Biros MH, Bellolio F, Chipman JG, Nelson AC, et al. A multicenter phase II randomized clinical trial of losartan on symptomatic outpatients with COVID‐19. EClinicalMedicine. 2021;37:100957. doi: 10.1016/j.eclinm.2021.100957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puskarich MA, Ingraham NE, Merck LH, Driver BE, Wacker DA, Page Black L, Jones AE, Fletcher CV, South AM, Nelson AC, et al. Effect of losartan on hospitalized patients with COVID‐19‐induced lung injury: a randomized clinical trial. JAMA Netw Open. 2022;5:e222735. doi: 10.1001/jamanetworkopen.2022.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma A, Elharram M, Afilalo J, Flannery A, Afilalo M, Tselios C, Ni J, Ezekowitz JA, Cheng MP, Ambrosy AP, et al. A randomized controlled trial of renin‐angiotensin‐aldosterone system inhibitor management in patients admitted in hospital with COVID‐19. Am Heart J. 2022;247:76–89. doi: 10.1016/j.ahj.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Abajo FJ, Rodriguez‐Martin S, Lerma V, Mejía‐Abril G, Aguilar M, García‐Luque A, Laredo L, Laosa O, Centeno‐Soto GA, Ángeles Gálvez M, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang X, Eales J, Scannali D, Nazgiewicz A, Prestes P, Maier M, Denniff M, Xu X, Saluja S, Cano‐Gamez E, et al. Hypertension and renin‐angiotensin system blockers are not associated with expression of angiotensin‐converting enzyme 2 (ACE2) in the kidney. Eur Heart J. 2020;41:4580–4588. doi: 10.1093/eurheartj/ehaa794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. International Society of Hypertension . Statement on COVID‐19. 2020. Available at: https://ish‐world.com/news/a/A‐statement‐from‐the‐International‐Society‐of‐Hypertension‐on‐COVID‐19. Accessed May 6, 2020.
- 19. American College of Cardiology, American Heart Association and Heart Failure Society of America . Patients taking ACE‐i and ARBs who contract COVID‐19 should continue treatment, unless otherwise advised by their physician 2020. Available at: https://www.acc.org/latest‐in‐cardiology/articles/2020/03/17/08/59/hfsa‐acc‐aha‐statement‐addresses‐concerns‐re‐using‐raas‐antagonists‐in‐covid‐19. Accessed May 6, 2020. [Google Scholar]
- 20. European Society of Cardiology Council on Hypertension . Position statement of the ESC Council on Hypertension on ACE‐inhibitors and angiotensin receptor blockers 2020. Available at: https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang. Accessed May 6, 2020.
- 21. European Society of Hypertension . Statement on COVID‐19 2020. Available at: https://www.eshonline.org/spotlights/esh‐stabtement‐on‐covid‐19. Accessed May 6, 2020.
- 22. The Renal Association . UK position statement on COVID‐19 and ACE Inhibitor/Angiotensin Receptor Blocker use. 2020. Available at: https://renal.org/covid‐19/ra‐resources‐renal‐professionals/renal‐association‐uk‐position‐statement‐covid‐19‐ace‐inhibitorangiotensin‐receptor‐blocker‐use. Accessed Oct 6, 2020.
- 23. Liberati A, Altman DF, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gnanenthiran SR, Borghi C, Burger D, Charchar F, Poulter NR, Schlaich MP, Steckelings UM, Stergiou G, Tomaszewski M, Unger T, et al. Prospective meta‐analysis protocol on randomised trials of renin‐angiotensin system inhibitors in patients with COVID‐19: an initiative of the International Society of Hypertension. BMJ Open. 2021;11:e043625. doi: 10.1136/bmjopen-2020-043625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). 2019. [DOI] [PMC free article] [PubMed]
- 26. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 27. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H‐Y, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 28. Hedges LV, Vevea JL. Fixed‐ and random‐effects models in meta‐analysis. Psychol Methods. 1998;3:486–504. doi: 10.1007/978-1-0716-1566-9_3 [DOI] [Google Scholar]
- 29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- 31. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rucker G. Network meta‐analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324. doi: 10.1002/jrsm.1058 [DOI] [PubMed] [Google Scholar]
- 33. ClinicalTrials.gov . Trial of COVID‐19 Outpatient Treatment in Individuals With Risk Factors for Aggravation (COVERAGE France). 2021.
- 34. Blood Pressure Lowering Treatment Trialists' Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant‐level data meta‐analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jung SY, Choi JC, You SH, Kim WY. Association of renin‐angiotensin‐aldosterone system inhibitors with COVID‐19‐related outcomes in Korea: a nationwide population‐based cohort study. Clin Infect Dis. 2020;71(16):2121–2128. doi: 10.1093/cid/ciaa624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–830. doi: 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A, et al. COVID‐19‐associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16:747–764. doi: 10.1038/s41581-020-00356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID‐19). Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su H, Yang M, Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ronco C, Reis T, Husain‐Syed F. Management of acute kidney injury in patients with COVID‐19. Lancet Respir Med. 2020;7(7):738–742. doi: 10.1016/S2213-2600(20)30229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID‐19 pneumonia. J Am Soc Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Braun F, Lutgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, et al. SARS‐CoV‐2 renal tropism associates with acute kidney injury. Lancet. 2020;396:597–598. doi: 10.1016/S0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. May RM, Cassol C, Hannoudi A, Larsen CP, Lerma EV, Haun RS, Braga JR, Hassen SI, Wilson J, VanBeek C, et al. A multi‐center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID‐19). Kidney Int. 2021;100:1303–1315. doi: 10.1016/j.kint.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, Kambham N, Bracamonte E, Houghton D, Ahmed NI, et al. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID‐19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77(1):82–93. doi: 10.1053/j.ajkd.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, et al. COVID‐19‐associated kidney injury: a case series of kidney biopsy findings. Clin J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hassler L, Reyes F, Sparks MA, Welling P, Batlle D. Evidence for and against direct kidney infection by SARS‐CoV‐2 in patients with COVID‐19. Clin J Am Soc Nephrol. 2021;16:1755–1765. doi: 10.2215/CJN.04560421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garreta E, Prado P, Stanifer ML, Monteil V, Marco A, Ullate‐Agote A, Moya‐Rull D, Vilas‐Zornoza A, Tarantino C, Romero JP, et al. A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS‐CoV‐2 infections in human kidney organoids and patient cells. Cell Metab. 2022;34:857–873.e9. doi: 10.1016/j.cmet.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li MY, Lin L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin‐converting enzyme 2 and Angiotensin‐converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423 [DOI] [PubMed] [Google Scholar]
- 52. Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID‐19 outbreak: a study based on single‐cell transcriptome analysis. Intensive Care Med. 2020;46:1114–1116. doi: 10.1007/s00134-020-06026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, Cantaluppi V. Pathophysiology of COVID‐19‐associated acute kidney injury. Nat Rev Nephrol. 2021;1–14(11):751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bakris GL, Weir MR. Angiotensin‐converting enzyme inhibitor‐associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685 [DOI] [PubMed] [Google Scholar]
- 55. Nadkarni GN, Chauhan K, Rao V, Ix JH, Shlipak MG, Parikh CR, Coca SG. Effect of intensive blood pressure lowering on kidney tubule injury: findings from the ACCORD trial study participants. Am J Kidney Dis. 2019;73:31–38. doi: 10.1053/j.ajkd.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Malhotra R, Craven T, Ambrosius WT, Killeen AA, Haley WE, Cheung AK, Chonchol M, Sarnak M, Parikh CR, Shlipak MG, et al. Effects of intensive blood pressure lowering on kidney tubule Injury in CKD: a longitudinal subgroup analysis in SPRINT. Am J Kidney Dis. 2019;73:21–30. doi: 10.1053/j.ajkd.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C. Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl. 2005;99:S46–S51. doi: 10.1111/j.1523-1755.2005.09909.x [DOI] [PubMed] [Google Scholar]
- 58. Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N, Interdisciplinary Chronic Diseases Collaboration . Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med. 2018;178:1681–1690. doi: 10.1001/jamainternmed.2018.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heart Outcomes Prevention Evaluation Study Investigators , Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 60. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 61. Hirakawa Y, Arima H, Webster R, Zoungas S, Li Q, Harrap S, Lisheng L, Hamet P, Mancia G, Poulter N, et al. Risks associated with permanent discontinuation of blood pressure‐lowering medications in patients with type 2 diabetes. J Hypertens. 2016;34:781–787. doi: 10.1097/HJH.0000000000000841 [DOI] [PubMed] [Google Scholar]
- 62. Alharbi FF, Souverein P, de Groot MC, Maitland‐van der Zee AH, de Boer A, Klungel OH. Risk of acute myocardial infarction after discontinuation of antihypertensive agents: a case‐control study. J Hum Hypertens. 2017;31:537–544. doi: 10.1038/jhh.2017.1 [DOI] [PubMed] [Google Scholar]
- 63. Rothlin RP, Duarte M, Pelorosso FG, Nicolosi L, Salgado MV, Vetulli HM, Spitzer E. Angiotensin receptor blockers for COVID‐19: pathophysiological and pharmacological considerations about ongoing and future prospective clinical trials. Front Pharmacol. 2021;12:603736. doi: 10.3389/fphar.2021.603736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kow CS, Ming LC, Hasan SS. Renin‐angiotensin system inhibitor use and the risk of mortality in hospitalized patients with COVID‐19: a meta‐analysis of randomized controlled trials. Hypertens Res. 2021;44:1042–1045. doi: 10.1038/s41440-021-00670-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bavishi C, Whelton PK, Mancia G, Corrao G, Messerli FH. Renin‐angiotensin‐system inhibitors and all‐cause mortality in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. J Hypertens. 2021;39:784–794. doi: 10.1097/HJH.0000000000002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2
Figures S1–S30


