Abstract
Background
Atrial fibrillation (AF) is associated with anatomical and electrical remodeling. Some patients with AF have concomitant sick sinus syndrome and may need permanent pacemaker (PPM) implantation. Association between catheter ablation of AF timing and need for PPM in sick sinus syndrome has not been assessed.
Methods and Results
We used pooled electronic health data to perform retrospective cross‐sectional analysis of 66, 595 patients with AF and sick sinus syndrome to assess the need of PPM implantation temporally, with AF performed divided into earlier within 5 years (group 1), 5 to 10 years (group 2), or beyond 10 years (group 3) of diagnosis. PPM implantation was lowest among those who had catheter ablation within 5 years of sick sinus syndrome diagnosis: group 1 versus group 2 (18.15% versus 27.21%) and group 1 versus group 3 (18.15% versus 27.22%). Interestingly, there was no difference in risk of PPM between group 2 and group 3 (27.21% versus 27.22%; odds ratio [OR], 1.00 [95% CI, 0.85–1.20]).
Conclusions
Even after controlling known risk factors that increase the need for pacemaker implantation, timing of AF ablation was the strongest predictor for need for PPM. Patients adjusted OR of PPM was lower if patients had catheter ablation within 5 years of diagnosis compared with later than 5 years (adjusted OR, 0.64 [95% CI, 0.59–0.70]).
Keywords: atrial fibrillation ablation, atrial fibrillation duration, pacemaker implantation, sick sinus syndrome
Nonstandard Abbreviations and Acronyms
- BBB
bundle‐branch block
- CA
catheter ablation
- PPM
permanent pacemaker
- SND
sinus node dysfunction
- SNOMED‐CT
Systematized Nomenclature for Medicine–Clinical Terms
Clinical Perspective
What Is New?
The combination of atrial fibrillation (AF) and sinus node dysfunction often creates a clinical dilemma for optimal treatment. AF can be associated with symptoms, high heart rates, or presence of cardiomyopathy, but both rate‐ and rhythm‐controlling agents can often worsen sinus node dysfunction in these patients.
What Are the Clinical Implications?
This study has novel data on the timing of catheter ablation of AF and its bearing on the need for a pacemaker. Pacemaker implantation can be the treatment of choice depending on the clinical presentation of the patient. However, the findings of this study support earlier catheter ablation being instrumental in decreasing the need for pacemaker in the patients who have both AF and sinus node dysfunction independent of other risk factors, such as coronary artery disease, congestive heart failure, bundle‐branch block, and hypertension.
Our findings also show that female patients tended to get AF ablation less frequently (especially within 5 years), and had higher need for pacemaker implantation.
Sinus node dysfunction (SND) in conjunction with atrial fibrillation, also referred to as sick sinus syndrome, is the inability of sinoatrial node to incrementally modulate heart rate enough to meet the physiologic need of an individual. The progression of SND can lead to cardiac rhythm disorders that vacillate from complete sinoatrial node arrest with an escape rhythm to various atrial tachyarrhythmias. 1 Atrial arrhythmia, with atrial fibrillation (AF) being the most common one, is present in 40% to 70% of patients at the time of diagnosis of SND. 2 The combination of tachyarrhythmias and bradyarrhythmias constitutes what is also known as “sick sinus syndrome” or “tachy‐brady syndrome.”
AF causes overdrive suppression, which, along with anatomical and electrical remodeling, can predispose individuals to SND. 3 Electrical remodeling is thought to be attributable to proarrhythmic changes with decrease in the atrial effective refractory period as well as changes in sinoatrial conduction time. 4 In persistent AF, there is also a loss of sinoatrial node cell function, loss of atrial muscle mass, and increase in atrial volume. It is well known that duration of AF is an important factor in increasing the persistence of AF.
Bradycardia‐mediated heterogeneity of refractoriness in SND can play a role in AF attributable to atrial premature depolarizations causing protracted pause that may allow for nonsinus focus or multiple foci to take over. Reentry may also occur because of shorter action potential duration of nonsinus foci. 5
Although some patients benefit from permanent pacemaker (PPM) implantation in SND, early catheter ablation (CA) of AF as a tool to temper the progression of SND has not been studied. Elimination of AF may prevent conversion pauses in AF; and with further decrease in the need for medications for rate or rhythm control, sinus node recovery may occur.
The benefit of the CA in the prevention of progression of SND has been consistent in paroxysmal and persistent AF. 6 However, the correlation of the duration of presence of AF before ablation in reverse remodeling or prevention of progression of SND is not well known. Therefore, we used pooled electronic health data to evaluate the difference in rate of PPM implantation if CA was done earlier (within 5 years) or later (≥10 years) after AF diagnosis in patients with SND. We were also interested in how other known risk factors for SND played a role in progression to pacemaker implantation in this study.
Methods
Study Setting
Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Case Western Reserve University at skarim@metrohealth.org.
This was a retrospective cross‐sectional analysis using Explorys (IBM Watson Health, Cleveland, OH), which is a large electronic health record–based pooled deidentified database. This technology platform uses a health data gateway server behind the firewall of participating health care organizations spread over 50 states in the United States. The server collects data from a variety of health information systems (eg, electronic health records, billing systems, and laboratory test systems). The data are then deidentified and passed into the Explorys data grid, which is a private cloud‐based data storage, and are standardized and normalized. This allows researchers relatively easy access to the data, in compliance with the Health Insurance Portability and Accountability Act and Health Information Technology for Economic and Clinical Health Act. 7 , 8 The data are accessed through a secured web‐based interface. The use of Explorys has been validated in multiple fields. 9 The Explorys platform uses the Systematized Nomenclature for Medicine–Clinical Terms (SNOMED‐CT) for medical diagnoses and procedures. For diagnoses, International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM), codes are mapped into the SNOMED‐CT hierarchy. 10 , 11 Institutional review board approval was obtained for the study. Cohorts can further be refined demographically, and comorbid diseases can be extracted.
Patient Selection
Adult subjects (aged between 18 and 90 years) with active electronic health records since 1999 were identified using the search tool in Explorys. Patients with AF were identified by the following SNOMED‐CT diagnosis, “Atrial Fibrillation,” that was inclusive of all types of AF (paroxysmal, persistent, longstanding persistent, and permanent AF). As SND can have a variable presentation, “sinus node dysfunction,” along with any subdiagnosis present, such as “tachy‐brady syndrome,” “chronotropic incompetence,” and “symptomatic sinus bradycardia,” were included. The third inclusion term was “ablation operation for arrhythmia,” which was the designation for AF ablation. Forms for non‐AF ablation and those with diagnoses of arrhythmias other than AF were excluded. The temporal cutoff for AF ablation for this project was within 5 years, 6 to 10 years, or after 10 years after the diagnosis of SND. This defined our initial cohort. Patients who had a pacemaker for the ‘“atrioventricular block’” or patients who had “atrioventricular nodal ablation for AF” were excluded. Patients who had >1 AF ablation within 5 and 10 years of diagnosis were excluded from the analysis.
In our initial cohort using the SNOMED‐CT term “Implantation of Cardiac Pacemaker,” we identified patients who had PPM implanted between 1999 and 2020 who met the other criteria mentioned above. Patients with pacemaker implantation before AF ablation were excluded.
Covariates
We collected cross‐sectional information on patient demographics, such as age, sex, as well as other comorbidities known to be associated with SND, such as hypertension, coronary artery disease (CAD), bundle‐branch block (BBB), and congestive heart failure, by their respective SNOMED‐CT terms. The primary outcome of interest was the PPM implantation after AF ablation within 5 years of diagnosis of AF and SND (group 1), within 5 to 10 years of diagnosis (group 2), and after 10 years of diagnosis (group 3). Secondary analysis of interest in our AF population included studying the correlation of different traditional factors of SND in regard to need for pacemaker among the selected population.
Statistical Analysis
To assess the association between timing of CA after diagnosis of sick sinus syndrome and progression to need for PPM implantation, we divided the whole cohort of patients into those who received a pacemaker and those who did not receive a pacemaker.
The cumulative incidence of PPM implantation was calculated by dividing the number of patients with pacemaker implantation by the total number of patients in each risk group. Categorical variables were presented as numbers and percentages, and were compared using the Pearson χ2 test. Odds ratios (ORs) were presented with 95% CIs. We obtained variables for multiple logistic regression model via entry of all univariate baseline predictors of pacemaker implantation from simple logistic model with a value of P<0.2. We used backward selection method for multiple logistic model.
All statistical analysis was done using SAS version 9.4 (SAS Institute, Cary, NC). Significance was defined as the 2‐tailed value of P<0.05.
Results
A total of 66 595 patients with AF and SND, who had ablation of AF and met the prespecified criteria, were included in the analysis. The cohort was divided into 3 groups, as mentioned above: those with AF ablation within 5 years of diagnosis of AF and SND (group 1), those with AF ablation within 5 to 10 years of diagnosis (group 2), and those who had AF ablation after 10 years of diagnosis (group 3). Baseline characteristics were compared among patients who had PPM versus no PPM implantation (Table 1). Of the total cohort of AF, 44% of patients had paroxysmal AF. Overall, 25% of all patients with AF had PPM during the duration of follow‐up. A total of 16% of the patients with paroxysmal AF had PPM implantation.
Table 1.
Comparison of Characteristics Based on the Need for Pacemaker Implantation of Patients With AF Who Underwent AF Ablation Within 5, 5 to 10, or After 10 Years of Diagnosis of Sick Sinus Syndrome
| Characteristics | Total cohort |
Permanent pacemaker implanted (n=18 250) |
No permanent pacemaker implanted (n=50 345) |
P value |
|---|---|---|---|---|
| Sex | ||||
| Men | 36 355 (53.00) | 9130 (25.11) | 27 225 (74.89) | <0.001 |
| Women | 32 240 (47.00) | 9120 (28.29) | 23 120 (71.71) | |
| Age, y | ||||
| ≥65 | 56 350 (82.15) | 15 870 (28.16) | 40 480 (71.84) | <0.001 |
| <65 | 12 245 (17.85) | 2380 (19.44) | 9865 (80.56) | |
| AF ablation time | ||||
| Within 5 y (group 1) | 4655 (6.79) | 845 (18.15) | 3810 (81.85) | |
| Within 5–10 y (group 2) | 735 (1.07) | 200 (27.21) | 535 (72.79) | |
| After 10 y or no (group 3) | 63 205 (92.14) | 17 205 (27.22) | 46 000 (72.78) | <0.001 |
| Hypertension | ||||
| Yes | 59 260 (86.39) | 16 650 (28.10) | 42 610 (71.90) | <0.001 |
| No | 9335 (13.61) | 1600 (17.14) | 7735 (82.86) | |
| Bundle‐branch block | ||||
| Yes | 9775 (14.25) | 3140 (32.12) | 6635 (67.88) | <0.001 |
| No | 58 820 (85.75) | 15 110 (25.69) | 43 710 (74.31) | |
| Coronary artery disease | ||||
| Yes | 38 460 (56.07) | 11 250 (29.25) | 27 210 (70.75) | <0.001 |
| No | 30 135 (43.93) | 7000 (23.23) | 23 135 (76.77) | |
| Congestive heart failure | ||||
| Yes | 9660 (14.08) | 3025 (31.31) | 6635 (68.69) | <0.001 |
| No | 58 935 (85.92) | 15 225 (25.83) | 43 710 (74.17) | |
Data are given as number (percentage) of each group. AF indicates atrial fibrillation.
Using univariate analysis, patients were found to have higher risk of need for PPM if they were women (28.29% versus 15.11%; OR, 1.18 [95% CI, 1.14–1.22]), aged >65 years (28.16% versus 19.44%; OR, 1.63 [95% CI, 1.55–1.71]), or had hypertension (28.10% versus 17.14%; OR, 1.89 [95% CI, 1.79–2.00]). Need of PPM was higher if patients had preexisting cardiac conditions as BBB (32.12% versus 25.69%; OR, 1.37 [95% CI, 1.31–1.43]), CAD (29.25% versus 23.23%; OR, 1.37 [95% CI, 1.32–1.42]), or congestive heart failure (31.31% versus 25.83%; OR, 1.31 [95% CI, 1.23–1.37]). Patients who had CA within 5 years of diagnosis of AF and SND had a much lower need for PPM compared with patients who had CA within 5 to 10 years of diagnosis or after 10 years of diagnosis: group 1 versus group 2 (18.15% versus 27.21%; OR, 0.59 [95% CI, 0.50–0.71]) and group 1 versus group 3 (18.15% versus 27.22%; OR, 0.59 [95% CI, 0.55–0.64]) (Table 1). Interestingly, there was no difference in risk of PPM among those who had AF ablation within 5 to 10 years compared with those who had it after 10 years (as shown in Figure 1): group 2 versus group 3 (27.21% versus 27.22%; OR, 1.00 [95% CI, 0.85–1.20]).
Figure 1. Forest plot, showing unadjusted odds ratio (OR) of pacemaker implantation in different groups defined by timing of catheter ablation of atrial fibrillation (AF) within 5 years of diagnosis of AF and sinus node dysfunction (group 1), within 5 to 10 years of diagnosis (group 2), and after 10 years of diagnosis (group 3).
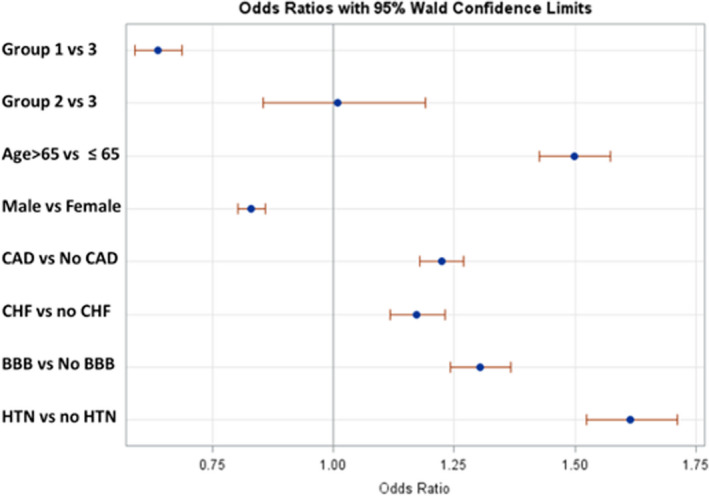
The dots represent the OR, and the horizontal line represents the 95% CI. BBB indicates bundle‐branch block; CAD, coronary artery disease; CHF, congestive heart failure; and HTN, hypertension.
A multivariable binary logistic regression model was built using variables from univariate models for adjustment (Table 2). Results showed even after controlling known risk factors of PPM implantation, patients' adjusted OR (aOR) of PPM was lower if patients had CA within 5 years of AF compared with later than 5 years or if they never had CA (aOR, 0.64 [95% CI, 0.59–0.70]). In adjusted model, risk of pacemaker implantation showed similar trend as univariate model if patients were aged >65 years (aOR, 1.50 [95% CI, 1.42–1.57]), were women (aOR, 1.20 [95% CI, 1.16–1.25]), or had hypertension (aOR, 1.61 [95% CI, 1.52–1.71]), BBB (aOR, 1.30 [95% CI, 1.24–1.37]), CAD (aOR, 1.22 [95% CI, 1.18–1.27]), or congestive heart failure (aOR, 1.17 [95% CI, 1.12–1.23]), as seen in Figure 2.
Table 2.
Logistic Multivariable Analysis of Risk of Progression to Need for Pacemaker Implantation Among Patients Who Had a Diagnosis of AF and SND
| Multivariable model | OR (95% CI) in overall study population | |
|---|---|---|
| Age, y | ≥65 vs <65 | 1.50 (1.43–1.57) |
| Sex | Women vs men | 1.20 (1.16–1.25) |
| Hypertension | Presence vs absence | 1.61 (1.52–1.71) |
| Congestive heart failure | Presence vs absence | 1.17 (1.12–1.23) |
| Coronary artery disease | Presence vs absence | 1.22 (1.18–1.27) |
| Bundle‐branch block | Presence vs absence | 1.30 (1.24–1.37) |
| Time of AF ablation | Within 5 y vs no ablation or >10 y after (group 1 vs 3) | 0.64 (0.59–0.70) |
| Within 10 y vs no ablation or >10 y after (group 2 vs 3) | 1.01 (0.86–1.19) |
AF indicates atrial fibrillation; OR, odds ratio; and SND, sinus node dysfunction.
Figure 2. Forest plot, showing adjusted odds ratio (OR) of pacemaker implantation in different risk groups.
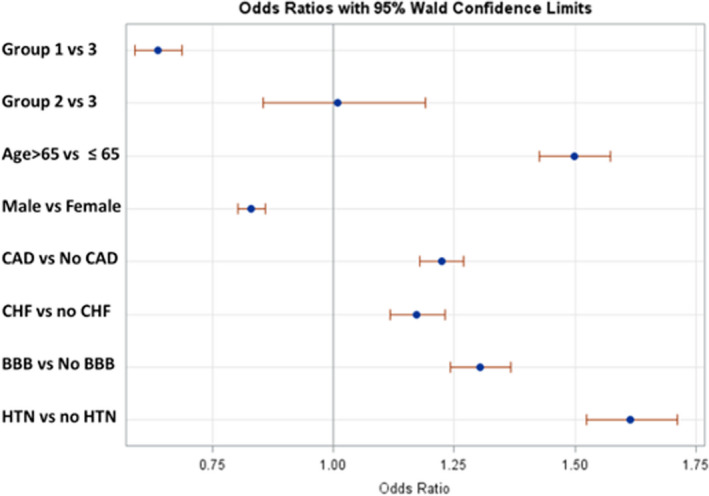
Group numbers were designated by timing of atrial fibrillation (AF) ablation within 5 years of diagnosis of AF and sinus node dysfunction (group 1), within 5 to 10 years of diagnosis (group 2), and after 10 years of data collection (group 3). The dots represent the OR, and the horizontal line represents the 95% CI. BBB indicates bundle‐branch block; CAD, coronary artery disease; CHF, congestive heart failure; and HTN, hypertension.
Discussion
Electronic health record is becoming an increasingly important real‐time data source for clinical research. This study demonstrates the potential use of certain informatics tools to perform large‐scale retrospective studies to address a specific question in a large deidentified patient base.
There is no study to date assessing the timing of AF ablation in relationship to need for pacemaker implantation (serving as a surrogate marker for progressive sinoatrial node dysfunction). We chose the groups empirically on the basis of timing of AF ablation within 5 years, 5 to 10 years, and >10 years of diagnosis of AF and SND. It appears that 5 years is the optimum cutoff in this population to mitigate the need for pacemaker implantation. After 5 years of diagnosis of AF and SND, AF ablation can lead to decreased burden of AF, but there is less likely to be sinoatrial node recovery, leading to higher need for pacemaker implantation.
AF causes metabolic and membrane conduction abnormalities initially by decreasing the atrial effective refractory period and shortening the reentrant circuit wavelength by decreasing the atrial effective refractory period. 12 In animal models, rapid pacing prolongs sinoatrial conduction time and corrects sinus nodal recovery time. 13 These changes may be reversible as it has been demonstrated that both paroxysmal and persistent atrial flutter exhibited an improvement in the sinus node recovery time 3 weeks after CA of atrial arrhythmia. 14 Compared with paroxysmal AF, long‐standing persistent AF leads to hibernating myocardium, anatomical remodeling with irreversible scarring fibrosis, and fatty infiltrate because of altered channel gene expression. 15 , 16 It also leads to atrial stretch and direct damage to sinoatrial node via increased metabolic demand. 17 In our study, we used cumulative duration of AF since diagnosis to provide a temporal focus. The duration of AF since the timing of diagnosis was taken into consideration rather than the historical classification of AF because duration may dictate the changes in atrial substrate with remodeling, as mentioned above. CA in patients with AF relatively early likely decreases the need for pacemakers by 2 modes: electrical reverse remodeling with the elimination of the sinoatrial node suppression and the prolonged sinus pauses as well as reduction or elimination of the use of rate‐ or rhythm‐controlling medications that suppress the sinoatrial node function.
There were specific variables that had a correlation with higher implantation of pacemakers in patients with SND, such as age, sex, hypertension, CAD, heart failure, and conduction system issues at baseline.
Age is an independent risk factor for SND as it leads to progressive degeneration of cells, atrial dilation, and fibrosis of sinoatrial node over time. The risk of SND and thus pacemaker need increases with age expectedly, which is consistent with our findings. In various studies, the risk of SND in the general population has been the same in both sexes. 18 Women historically have a disproportionately lower rate of PPM implantation compared with men, attributed to differential access to health care. 19 However, in the setting of AF overall, women were more likely to have a pacemaker implanted for SND than men. 19 This may be attributable to delay in CA in women compared with their male counterparts.
Hypertension leads to atrial enlargement along with stretching and fibrosis. It also increases the risk for CAD and heart failure leading to SND. 20 , 21 Nondihydropyridine calcium channel blockers and β blockers used for the treatment of hypertension (and AF) increase the risk of SND. 22
CAD may contribute to SND, as the sinoatrial node perfusion may be affected. 23 SND can be seen acutely in inferior myocardial infarction because of ischemia of sinus node, and by stimulation of Bezold‐Jarsich reflex. 24 In one study, patients with sick sinus syndrome who had a history of previous inferior wall myocardial infarction were more likely to have severe stenosis of sinoatrial nodal artery compared with controls without myocardial infarction. 25
Heart failure leads to depressed intrinsic heart rate, prolonged corrected sinus node recovery time, and sinoatrial conduction time attributable to decreased diastolic depolarization rate. 26 Impaired rhythmic spontaneous calcium release from the sarcoplasmic reticulum plays a pivotal role in SND in heart failure. 26 , 27 At the tissue level, sinoatrial node demonstrated exit block, intra‐atrial conduction block, and severe fibrosis. Heart failure therapies or electrolyte abnormalities associated with diuretics can worsen preexisting sick sinus syndrome.
BBB with right BBB or left BBB has a higher propensity for progression of high‐grade heart block. We excluded patients who had a PPM implanted because of complete heart block to prevent any confounding. Primary conduction disease is generally considered to be a degenerative process and can involve the conducting system diffusely, and patients with disease in the bundle branches can develop disease in the sinoatrial node and atrium. Sinus node recovery time, atrial functional refractory periods, and sinoatrial conduction time have been significantly longer in patients with bifascicular blocks, trifascicular blocks, and BBBs compared with those with narrow QRS complex. 28 It is reported that there is an association of atrioventricular conduction disturbances in up to 67% of patients with SND. 29 We did adjust for inherent increased risk factors of SND and thus pacemaker associated if BBB was present at baseline.
Despite adjustment of risk factors that are implicated in the development of SND, as described above, earlier CA for AF remained the single most important modifiable predictor of pacemaker implantation in such patients. Earlier CA within 5 years of diagnosis of AF and SND reduced the risk of progression to need for pacemaker implantation significantly.
Limitations
There are several limitations to this study. As the study is an observational one, the design precludes us from addressing causality. As Explorys provides a population‐level deidentified data set, we could only perform analyses at the population level and did not have access to specific dates, such as diagnosis dates and device implantation dates. We could not establish temporal relationships between onset and duration of the traditional risk factors to the timing of pacemaker implantation. Although it is exceedingly rare, CA of atrial arrhythmias can potentially damage atrial tissue close to sinoatrial node or other parts of the conduction system, leading to need for pacemaker after ablation, but data of individual procedural‐related complications were not available on an individual basis. As data were based on the cross‐sectional prevalence of AF and SND, it was not established whether AF or SND was diagnosed first. This study relies on diagnoses based on SNOMED‐CT codes, so it is not possible to verify the accuracy of diagnoses and thus it is prone to coding errors. Therefore, there is an element of misclassification bias as SND is loosely used interchangeably with other terms. However, the large number of patients in this database would account for small margins of deviations from traditional CA of AF, risk factors, and overall trends in follow‐up. Studies looking at other clinical correlations may not have underlying clinical informatics ontologies and so may not be amenable to this approach.
Conclusions
This study demonstrated a temporal association between ablation of AF and the progression to the need for a pacemaker implantation in patients with AF and SND. The risk of PPM implantation is significantly reduced if AF ablation is performed within 5 years of diagnosis compared with after 5 years after the diagnosis of AF and SND. Thus, earlier AF ablation can significantly decrease the need for pacemaker implantation in those with SND, even after adjusting for traditional risk factors, such as age, sex, hypertension, heart failure conduction system abnormalities, and CAD. A prospective study with close monitoring of patients with AF and SND after varied ablation times since diagnosis should be performed to confirm these findings.
Sources of Funding
None.
Disclosures
None.
This work was presented at Heart Rhythm 2022, April 29 to May 1, 2022, in San Francisco, CA, and published in abstract form (J Am Coll Cardiol. 2022;79[9_Suppl]41. DOI: 10.1016/S0735‐1097[22]01032‐4).
For Sources of Funding and Disclosures, see page 7.
References
- 1. Ferrer MI. The etiology and natural history of sinus node disorders. Arch Intern Med. 1982;142:371–372. doi: 10.1001/archinte.142.2.371 [DOI] [PubMed] [Google Scholar]
- 2. Lamas GA, Lee K, Sweeney M, Leon A, Yee R, Ellenbogen K, Greer S, Wilber D, Silverman R, Marinchak R, et al. The mode selection trial (MOST) in sinus node dysfunction: design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J. 2000;140:541–551. doi: 10.1067/mhj.2000.109652 [DOI] [PubMed] [Google Scholar]
- 3. Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu L‐F, Scavée C, Weerasoriya R, Raybaud F, Macle L, Shah DC, et al. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation. 2003;108:1172–1175. doi: 10.1161/01.CIR.0000090685.13169.07 [DOI] [PubMed] [Google Scholar]
- 4. Daoud EG, Weiss R, Augostini RS, Kalbfleisch SJ, Schroeder J, Polsinelli G, Hummel JD. Remodeling of sinus node function after catheter ablation of right atrial flutter. J Cardiovasc Electrophysiol. 2002;13:20–24. doi: 10.1046/j.1540-8167.2002.00020.x [DOI] [PubMed] [Google Scholar]
- 5. Kezerashvili A, Krumerman AK, Fisher JD. Sinus node dysfunction in atrial fibrillation: cause or effect? J Atr Fibrillation. 2008;1:30. doi: 10.4022/jafib.v1i1.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen YW, Bai R, Lin T, Salim M, Sang CH, Long DY, Yu RH, Tang RB, Guo XY, Yan XL, et al. Pacing or ablation: which is better for paroxysmal atrial fibrillation‐related tachycardia‐bradycardia syndrome? Pacing Clin Electrophysiol. 2014;37:403–411. doi: 10.1111/pace.12340 [DOI] [PubMed] [Google Scholar]
- 7. United States Congress . American Recovery and Reinvestment Act of 2009/Division A/Title XIII e Health Information Technology. 111th Congress, 1st Session, Public Law 111‐115. Washington: GPO . 2009. Print.
- 8. United States Congress . Health Insurance Portability and Accountably Act of 1996. 104th Congress, 1st Session. Public Law 104‐191. Washington: GPO. 1996. Print.
- 9. Winhusen T, Theobald J, Kaelber DC, Lewis D. Medical complications associated with substance use disorders in patients with type 2 diabetes and hypertension: electronic health record findings. Addiction. 2019;114:1462–1470. doi: 10.1111/add.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. SNOMED CT Starter Guide . https://confluence.ihtsdotools.org/display/DOCSTART/SNOMED+CT+Starter+Guide. Accessed February 16, 2021.
- 11. Gaudet‐Blavignac C, Foufi V, Bjelogrlic M, Lovis C. Use of the systematized nomenclature of medicine clinical terms (SNOMED CT) for processing free text in health care: systematic scoping review. J Med Internet Res. 2021;23:e24594. doi: 10.2196/24594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun H, Chartier D, Leblanc N, Nattel S. Intracellular calcium changes and tachycardia‐induced contractile dysfunction in canine atrial myocytes. Cardiovasc Res. 2001;49:751–761. doi: 10.1016/S0008-6363(00)00294-7 [DOI] [PubMed] [Google Scholar]
- 13. Elvan A, Wylie K, Zipes DP. Pacing‐induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling. Circulation. 1996;94:2953–2960. doi: 10.1161/01.CIR.94.11.2953 [DOI] [PubMed] [Google Scholar]
- 14. Sparks PB, Jayaprakash S, Vohra JK, Kalman JM. Electrical remodeling of the atria associated with paroxysmal and chronic atrial flutter. Circulation. 2000;102:1807–1813. doi: 10.1161/01.CIR.102.15.1807 [DOI] [PubMed] [Google Scholar]
- 15. Knoll R, Arras M, Zimmermann R, Schaper J, Schaper W. Changes in gene expression following short coronary occlusions studied in porcine hearts with run‐on assays. Cardiovasc Res. 1994;28:1062–1069. doi: 10.1093/cvr/28.7.1062 [DOI] [PubMed] [Google Scholar]
- 16. Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147 [DOI] [PubMed] [Google Scholar]
- 17. White CW, Holida MD, Marcus ML. Effects of acute atrial fibrillation on the vasodilator reserve of the canine atrium. Cardiovasc Res. 1986;20:683–689. doi: 10.1093/cvr/20.9.683 [DOI] [PubMed] [Google Scholar]
- 18. Jensen PN, Gronroos NN, Chen LY, Folsom AR, Defilippi C, Heckbert SR, Alonso A. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol. 2014;64:531–538. doi: 10.1016/j.jacc.2014.03.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riesenhuber M, Spannbauer A, Rauscha F, Schmidinger H, Boszotta A, Pezawas T, Schukro C, Gwechenberger M, Stix G, Anvari A, et al. Sex differences and long‐term outcome in patients with pacemakers. Front Cardiovasc Med. 2020;7:569060. doi: 10.3389/fcvm.2020.569060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, Oto A, Potpara TS, Steffel J, Marin F, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace. 2017;19:891–911. doi: 10.1093/europace/eux091 [DOI] [PubMed] [Google Scholar]
- 21. Okada R, Kawai S. Histopathology of the conduction system in sudden cardiac death. Jpn Circ J. 1983;47:573–580. doi: 10.1253/jcj.47.573 [DOI] [PubMed] [Google Scholar]
- 22. Richards TR, Tobe SW. Combining other antihypertensive drugs with beta‐blockers in hypertension: a focus on safety and tolerability. Can J Cardiol. 2014;30:S42–S46. doi: 10.1016/j.cjca.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 23. Hudson RE. The human pacemaker and its pathology. Br Heart J. 1960;22:153–167. doi: 10.1136/hrt.22.2.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hatle L, Bathen J, Rokseth R. Sinoatrial disease in acute myocardial infarction. Long‐term Prognosis. Br Heart J. 1976;38:410–414. doi: 10.1136/hrt.38.4.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alboni P, Baggioni GF, Scarfo S, Cappato R, Percoco GF, Paparella N, Antonioli GE. Role of sinus node artery disease in sick sinus syndrome in inferior wall acute myocardial infarction. Am J Cardiol. 1991;67:1180–1184. doi: 10.1016/0002-9149(91)90923-9 [DOI] [PubMed] [Google Scholar]
- 26. Verkerk AO, Wilders R, Coronel R, Ravesloot JH, Verheijck EE. Ionic remodeling of sinoatrial node cells by heart failure. Circulation. 2003;108:760–766. doi: 10.1161/01.CIR.0000083719.51661.B9 [DOI] [PubMed] [Google Scholar]
- 27. Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011 [DOI] [PubMed] [Google Scholar]
- 28. Wyse DG, McAnulty JH, Rahimtoola SH, Murphy ES. Electrophysiologic abnormalities of the sinus node and atrium in patients with bundle branch block. Circulation. 1979;60:413–420. doi: 10.1161/01.CIR.60.2.413 [DOI] [PubMed] [Google Scholar]
- 29. Rosen KM, Loeb HS, Sinno MZ, Rahimtoola SH, Gunnar RM. Cardiac conduction in patients with symptomatic sinus node disease. Circulation. 1971;43:836–844. doi: 10.1161/01.CIR.43.6.836 [DOI] [PubMed] [Google Scholar]


