Abstract
Z-conformation nucleic acid binding protein 1 (ZBP1), a powerful innate immune sensor, has been identified as the important signaling initiation factor in innate immune response and the multiple inflammatory cell death known as PANoptosis. The initiation of ZBP1 signaling requires recognition of left-handed double-helix Z-nucleic acid (includes Z-DNA and Z-RNA) and subsequent signaling transduction depends on the interaction between ZBP1 and its adapter proteins, such as TANK-binding kinase 1 (TBK1), interferon regulatory factor 3 (IRF3), receptor-interacting serine/threonine-protein kinase 1 (RIPK1), and RIPK3. ZBP1 activated innate immunity, including type-I interferon (IFN-I) response and NF-κB signaling, constitutes an important line of defense against pathogenic infection. In addition, ZBP1-mediated PANoptosis is a double-edged sword in anti-infection, auto-inflammatory diseases, and tumor immunity. ZBP1-mediated PANoptosis is beneficial for eliminating infected cells and tumor cells, but abnormal or excessive PANoptosis can lead to a strong inflammatory response that is harmful to the host. Thus, pathogens and host have each developed multiplex tactics targeting ZBP1 signaling to maintain strong virulence or immune homeostasis. In this paper, we reviewed the mechanisms of ZBP1 signaling, the effects of ZBP1 signaling on host immunity and pathogen infection, and various antagonistic strategies of host and pathogen against ZBP1. We also discuss existent gaps regarding ZBP1 signaling and forecast potential directions for future research.
Keywords: ZBP1, innate immunity, apoptosis, pyroptosis, necroptosis, auto-inflammatory diseases, tumor immunity, signaling transduction, pathogen–host interactions
1. Introduction
Recently, Z-conformation nucleic acid binding protein 1 (ZBP1) has become a rising star and attracts a lot of attention [1]. Initially, ZBP1 is identified as an innate immune sensor to activate the IFN-I response and NF-κB signaling, which powerfully resists pathogen invasion [2,3]. Later research pointed to the great importance of ZBP1 to induce PANoptosis, a crossed inflammatory cell death including pyroptosis, apoptosis, and necroptosis [4]. The ZBP1-mediated PANoptosis helps to remove invasive pathogens and uncontrolled tumor cells. However, ZBP1-mediated excessive or aberrant cell death can also cause adverse inflammation in infected or non-infected contexts. Therefore, ZBP1 signaling is a double-edged sword for host immunity. Pathogens need to antagonize ZBP1 signaling to ensure infection, while host needs to regulate ZBP1 signaling to maintain immune homeostasis. In this review, we systematically elucidated the elaborate ZBP1 signaling mechanism and the antagonistic strategies of host or pathogen, which will lay the foundation for developing highly efficient therapeutics for pathogenic infections, auto-inflammatory diseases, and cancer.
2. The Expression, Structure and Subcellular Location of ZBP1
ZBP1 was originally found to be significantly upregulated in mouse peritoneal tumor stromal cells or macrophages upon stimulation with IFN-γ or LPS [5]. Later studies revealed that ZBP1 holds different expression levels in different cells [4,6]. As an interferon-stimulated gene (ISG), the 5’ promoter region of ZBP1 contains both the IFN-stimulated gene responsive element (ISRE) and IFN-γ activated site (GAS), so ZBP1 can be induced with IFN-I or IFN-II [2,7,8]. Attributed to the heat shock element (HSE) within the ZBP1 promoter region, the expression of ZBP1 also be increased through heat shock transcription factor 1 (HSF1) when subjected to heat stress [9].
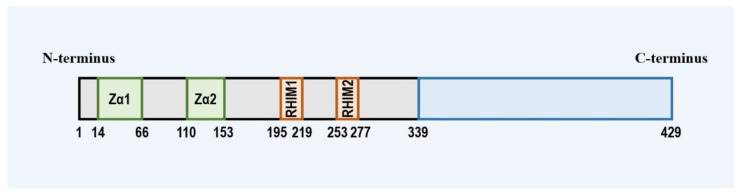
ZBP1 is a member of both the left-handed helical Z-conformation nucleic acid (Z-NA) binding protein family containing the Zα domain and the receptor-interacting serine/threonine protein (RIP) family holding the RIP homotypic interaction motif (RHIM). ZBP1 harbors two N-terminal Z-NA binding domains (ZBD, called Zα1 and Zα2), two intermediate RHIM (RHIM1 and RHIM2), and a C-terminal signal domain (SD) [3,10,11,12] (Figure 1). The N-terminal ZBD enables ZBP1 to sense Z-type or other types of nucleic acid ligands, which is usually critical for the activation of ZBP1 [12,13,14,15]; ZBP1 interaction with other RHIM-containing proteins is the foundation of ZBP1 signaling transduction [3,16,17]. Moreover, the C-terminus SD of ZBP1 is required by the ZBP1-induced IFN-I response [12].
Figure 1.
Illustration of the domain structure of ZBP1. ZBP1 consists of two N-terminal Z-form nucleic acid binding domains, two RHIM domains of the intermediate segment, and the C-terminal domain.
The Zα domains of ZBP1 determine its location in the cell. The initial research on the subcellular localization of ZBP1 indicated that ZBP1 presents the punctate distribution in the cytoplasm and interacts dynamically with stress granules (SGs) and processing bodies (PBs) [18]. SGs and PBs are dynamic non-membrane particles in the cytoplasm used to sequester inactive messenger ribonucleoprotein (mRNP) [19], hinting that ZBP1 is associated with RNA metabolism in cells. Subsequent studies confirmed that not only ZBP1 but also the RNA-editing enzyme ADAR1 and vaccinia virus (VACV) E3L gene-encoded E3 protein (the other two members harboring the Zα domain) all were localized to SGs in stressed cells [20]. In addition, the SGs localization of ZBD proteins depends on the Z-NA sensing of the Zα domain [20]. While the formation mechanism of Z-NA in SGs is unclear, it is sure that the SGs localization of ZBP1 is the result of ZBP1 interaction with the Z-NA [21]. In the process of viral infection, the sensing of ZBP1 to the virus-derived Z-NA also determines its localization. Replication of Influenza A virus (IAV) occurs in the nucleus and produces some incomplete viral gene segments, known as defective viral genomes (DVGs) [22]. The DVGs located in the nucleus likely form virus-derived Z-RNA and are successfully sensed by ZBP1, leading to ZBP1 aggregation and activation in the nucleus [23]. Destruction of ZBP1’s Z-NA binding resulted in the cytoplasmic retention and blocked activation of ZBP1 after IAV infection [23]. Unlike IAV, VACV accumulates the cytoplasmic Z-RNA so that ZBP1 is simply activated in the cytoplasm in response to VACV infection [6]. In summary, ZBP1 shuttles between the cytoplasm and the nucleus and that depends on the location of the nucleic acid ligands recognized by ZBP1.
3. ZBP1 Initiating Innate Immune Signaling
3.1. ZBP1 and IFN-I Response
IFN-I expression is mainly induced by various pattern-recognition receptors (PRRs), including Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), cyclic-GMP-AMP synthase-stimulator of interferon gene (cGAS-STING) [24,25]. IFN-I directly induces the ISGs expression, therefore, it is the key to activating innate immunity and inhibiting pathogenic infection [26,27]. Attributing the promoting effect of IFN-I to the maturation of dendritic cells, IFN-I also acts as a bridge between innate and adaptive immunity [28].
ZBP1 was initially recognized as a sensor for double-stranded DNA (dsDNA). In mouse embryo fibroblasts (MEFs) or L929 cells in response to immunostimulatory dsDNA (including B-DNA, bacterial and viral DNA) overexpressing ZBP1 significantly increased the IFN-I (IFN-α and IFN-β) expression [2,12]. Knockdown of ZBP1 also notably decreased B-DNA or Pseudorabies virus (PRV)-stimulated IFN-β activation in PK-15 cells [29]. The binding of ZBP1 to dsDNA required the D3 domain (a region overlapping the RHIM1 domain) [2]. ZBP1’s dsDNA ligands have no sequence specificity, but longer dsDNA fragments are more likely to activate ZBP1. That is because longer dsDNA act as the scaffold factor, linking many ZBP1 proteins to form ZBP1-dimer or even ZBP1-oligomer complex [12]. The artificially induced dimerization of ZBP1 also resulted in the upregulation of IFN-I genes in the absence of dsDNA [12]. Furthermore, the Zα, Zβ, D3, and SD domains of ZBP1 all were indispensable to the full activation of ZBP1 [2,12]. Activated ZBP1 complex recruit and phosphorylate TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3), then p-IRF3 dimer migrates to the nucleus and finally initiates the transcription of IFN-I [2,12]. In addition, ZBP1 possesses many potential serine/threonine phosphorylation sites in the 338 to 366 amino acids region. In addition, the IFN-I induction was severely weakened for the mutant of ZBP1 (S352A), indicating that the phosphorylation of ZBP1 is vital to its function [12].
ZBP1 acts as both an ISG and the promoting IFN-I-expression gene, which will form the ZBP1-mediated IFN positive feedback loop and provoke a strong innate immune response in dsDNA stimulated cells. However, subsequent studies pointed to the limitation in the ZBP1-dependent induction of IFN-I. Deletion of the ZBP1 did not affect the B-DNA-mediated IRF3 activation and IFN-β production in A549, HepG2 cells, or mice [30,31,32]. On the one hand, similar to the multiple RNA sensor in cells, the cellular DNA sensors are also diverse. For example, cGAS-STING has similar abilities to ZBP1 in strongly recognizing cytoplasmic dsDNA and inducing the IFN transcription [25]. Which represents the cell functional redundancy and ensures effective innate immune responses during pathogenic infection. On the other hand, the regulation of the ZBP1 signaling is very complex. Ectopic ADAR1 or E3 expression powerfully impaired the B-DNA-mediated induction of IFN-β mRNA in MEFs [12]. Additionally, both ADAR1 and E3 had been confirmed to competitively bind Z-RNA with ZBP1 and to block ZBP1-mediated necroptosis via the homotypic Zα domain [6,33,34]. The negative regulation of ZBP1 signaling may be beneficial to cellular immune homeostasis or viral immune escape [35].
3.2. ZBP1 and NF-κB Signaling
NF-κB family (RelA, RelB, c-Rel, p50, IκBα/β/ε, IKKα/β/γ) play a crucial role in regulating inflammation and innate immune [36,37]. NF-κB signaling regulates the expression of proinflammatory cytokines and anti-infection factors, including TNF-α, interleukin-1 (IL-1), IL-6, IL-8, adhesion molecules, cc chemokine ligand 5 (CCL5) [38]. NF-κB signaling also controls other cellular processes, such as cell proliferation, differentiation, and apoptosis [39,40].
The ZBP1-mediated NF-κB signaling is mechanically very similar to TLR3. TLR3 is an innate immune sensor that recognizes pathogen-associated dsRNA. TLR3 activates NF-κB signaling via toll-like receptor adapter molecule 1 (TRIF) [41]. TRIF interacts with receptor-interacting serine/threonine-protein kinase 1 (RIPK1) via the homotypic RHIM domains with ZBP1 [42]. TRIF-RIPK1 interaction recruits transforming growth factor β-activated kinase 1 (TAK1), TAK binding proteins (TAB1 and TAB2), and the IκB kinase (IKK) complex (IKKα, IKKβ, IKKγ). The protein complex promotes IKK activation, IκB phosphorylation, NF-κB nuclear translocation, and induction of antiviral genes [43,44]. ZBP1 also interacts with RIPK1 and mediates similar NF-κB signaling dependent on their shared RHIM domains [3,16]. However, there is one difference between ZBP1-mediated and TLR3-TRIF-mediated NF-κB signaling. Receptor-interacting serine/threonine-protein kinase 1 (RIPK3), the fourth RHIM-containing host protein, blocks TRIF-RIPK1 signaling to NF-κB by competing with RIP1 for binding to the RHIM of TRIF [41]. For ZBP1-RIPK1 signaling to NF-κB, RIPK3 collaborates with RIPK1 to facilitate ZBP1-mediated NF-κB activation [3,16]. The reasons for the differential regulation of RIPK3 need to be further studied to clarify.
4. ZBP1 Initiating Panoptosis Signaling
After suffering from stress or microbial infection, the immune system of cells will initiate programmed cell death (PCD) to maintain homeostasis or obliterate infected cells [45]. Major PCD includes pyroptosis, apoptosis, and necroptosis, and their molecular mechanisms have been elucidated. Apoptosis depends on the activation of the initiator caspases-8 (CASP8), CASP9, CASP10, and the executioner CASP3 and CASP7 [46]. Apoptosis results in the formation of apoptotic bodies and is defined as non-inflammatory cell death [47]. Different from apoptosis, pyroptosis and necroptosis both are characterized by pore-formation and rupture in the plasma membrane [47,48]. Pyroptosis is executed by activated inflammasome to cleave Gasdermin-D (GSDMD) and the pro-IL-1β, pro-IL-18 [49]. The N-terminal pore-forming domain of cleaved GSDMD forms the GSDMD-polymerized membrane pores and pyroptotic cell death [50]. Similar to pyroptosis, canonical necroptosis forms the membrane pores through the RIPK3-mixed lineage kinase domain-like protein (MLKL) necrosome downstream of TNFα-RIPK1 or TLR3/4-TRIF signaling [51,52,53]. The GSDMD pores or MLKL pores both enable intracellular cytokines and damage-associated molecular patterns (DAMPs) to release outside the cell, causing inflammatory cell death [48]. Apoptosis, pyroptosis, and necroptosis are not isolated from each other but are closely linked. The new concept, PANoptosis, is created as an inflammatory PCD pathway and reflects the crosstalk and co-regulation between apoptosis, pyroptosis, and necroptosis [54]. PANoptosis is driven by the PANoptosome complex, the central molecular scaffold to synchronously activate apoptosis, pyroptosis, and necroptosis [55,56].
ZBP1 act as a master regulator of PANoptosis and plays a vital role in initiating the assembly of PANoptosome [54] (Figure 2). ZBP1 PANoptosome consists of ZBP1, RIPK3, MLKL, RIPK1, FAS-associated death domain protein (FADD), CASP8, NLR family pyrin domain containing 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), and CASP1 [57]. The homotypic/heterotypic interactions between ZBP1 PANoptosome members provide a molecular backbone of ZBP1 PANoptosome formation [56]. Upon virus-derived or endogenous Z-NA ligation, ZBP1 binds to the RHIM domains of RIPK3 and RIPK1 [23,58]. Kinase activity-dependent RIPK3 recruits and phosphorylates MLKL to compose the necrosome and implement necroptosis [6,23,51]. For another, RIPK1 binds with FADD via shared death domain (DD) and the kinase activity of RIPK1 is dispensable for RIPK1-FADD interaction. The conserved Death Effector Domain (DED) in FADD and CASP8 makes FADD interact with CASP8, leading to the auto-processing and maturation of CASP8. The activated CASP8 cleaves executioner CASP3/7 to drive extrinsic apoptosis [59,60]. In addition, ZBP1 PANoptosome also further facilitate the assembly and activation of NLRP3 inflammasome [17,61]. Knockout of ZBP1 fully eliminated NLRP3 inflammasome activation and reduced the release of cytokines upon IAV infection [61]. Further, co-deletion of RIPK3 and CASP8 achieved similar results, indicating the close crosstalk between ZBP1-mediated apoptosis, necroptosis, and pyroptosis [61].
Figure 2.
The pattern of ZBP1-initiated PANoptosome assembly. The sensing of ZBP1 Zα for activated ligands allows ZBP1 interaction with RIPK1 and RIPK3 via RHIM domains and forms the core scaffolds of PANoptosome. RIPK3 induces the phosphorylation and membrane translocation of MLKL, which is the hallmark of necroptotic signaling. In addition, the homotypic interaction between RIPK1, FADD, and CASP8 liberates the cleaving activity of CASP8 and CASP3/7 that causes apoptosis. ZBP1-mediated apoptosis and necroptosis signaling promote the assembly and activation of NLRP3 inflammasome. Activated NLRP3 inflammasome matures GSDMD, IL-1β, and IL-18, named pyroptosis.
Much research elucidated some crosstalk between apoptosis, necroptosis, and pyroptosis, which provides a basis for studying the signaling crosstalk in ZBP1-mediated PANoptosis. CASP8 is the key connection between apoptosis and pyroptosis. FADD/CASP8 are necessary for the activation of CASP1 and cell pyroptosis in response to LPS + ATP, C. rodentium or Yersinia stimulation [62,63,64]. Consistent with that, the heterotypic interaction between CASP8-DED and ASC-PYD results in the recruitment of catalytically inactive CASP8 into the inflammasome complex, thereby activating CASP1 [65,66,67,68,69]. Furthermore, FADD/CASP8 also mediate transcriptional priming of the NLRP3 inflammasome under LPS + ATP treatment [62]. The upregulation effect on NLRP3 transcription may be attributed to the CASP8-FADD-RIPK1 complex activated NF-κB signaling [70,71]. In addition, similar to CASP1, CASP8 act as the executioners of pyroptosis to directly cleave and mature GSDMD and IL-1β [72,73,74,75]. Necroptotic signaling became another node to link necroptosis to pyroptosis. MLKL pores collaborate with GSDMD pores or play the compensatory role in the absence of GSDMD pores to promote the release of inflammatory cytokines and DAMPs [75,76]. In macrophages, MLKL pores-mediated potassium efflux also was pointed to upstream signaling of pyroptosis and initiated the activation of NLRP3 inflammasome in a cell-intrinsic manner [77,78].
5. ZBP1 Signaling in Microbial Infection
As a potent innate immune sensor, ZBP1 plays an essential role in immune defense against multiple pathogens infection, including viruses, bacterium, fungi, and parasites (Table 1). For example, ZBP1 detects the incursive virus-derived nucleic acid and destroys the viral replication niche by initiating cell death. In turn, some pathogens have developed diverse tactics to block the ZBP1 signaling to ensure efficient infection.
Table 1.
Programed cell death mediated by ZBP1 during microbial infection.
| Pathogens | Genome or Type | Ligands of ZBP1 | Type of Cell Death | Subcellular Location of ZBP1 | Virulent Factor | Antagonism Mechanism against ZBP1 Signaling | Reference(s) | |
|---|---|---|---|---|---|---|---|---|
| Viruses | MCMV | dsDNA | nascent RNA | Necroptosis | cytoplasm | vIRA | Disrupts ZBP1–RIPK3 interactions | [79,80,81,82,83] |
| HSV-1 | dsDNA | nascent RNA | PANoptosis | cytoplasm | ICP6 | Disrupts ZBP1–RIPK3 interactions | [84,85,86,87,88] | |
| VZV | dsDNA | nascent RNA | Apoptosis | cytoplasm | ORF20 | Disrupts ZBP1–RIPK3 interactions | [89] | |
| VACV | dsDNA | VACV Z-RNA | Necroptosis | cytoplasm | E3 | Interdicts the sensing of ZBP1 for viral Z-RNA | [6,90,91] | |
| IAV | ssRNA (−) |
IAV Z-RNA | PANoptosis | nucleus | [23,61,92] | |||
| WNV | ssRNA (+) | [93] | ||||||
| ZIKV | ssRNA (+) | Necroptosis | [94] | |||||
| SARS-CoV-2 | ssRNA (+) | PANoptosis | ORF8 | Inhibits the IFN-γ-induced expression of ZBP1 | [95,96] | |||
| Bacterium |
Escherichia coli;
Yersinia pseudotuberculosis; F. novicida. |
Gram-negative | PANoptosis | EspL | Cleaves RHIM fragments of all RHIM-containing host proteins | [84,97] | ||
| Fungus | Candida albican; Aspergillus fumigatus | PANoptosis | [98] |
5.1. Herpesviruses
Members of the herpesvirus family are classified as large dsDNA viruses. Based on genome sequence homologies, host or cell tropism, and replication strategies, herpesvirus is divided into alpha-, beta, and gamma-herpesviruses [99].
Cytomegalovirus (CMV) is β-herpesvirus and leads to extensive clinical symptoms, such as scongenital infection, retinitis, and hepatitis. Mouse cytomegalovirus (MCMV) encodes several cell death suppressors including the M38.5-encoded viral mitochondrial inhibitor of apoptosis (vMIA), the M36-encoded viral inhibitor of caspase-8 activation (vICA), and the M45-encoded viral inhibitor of RIP/RHIM activation (vIRA) [100]. The vIRA of MCMV possesses an N-terminal RHIM domain and interplays with RHIM-containing host protein to block the host’s RHIM signaling [79,80] (Figure 3). Compared with the wild-type MCMV (MCMVWT), the M45 mutant MCMV (MCMVM45mutRHIM) infection-induced RIPK3-dependent but RIPK1/TRIF-independent necroptosis and show weaker replication in 3T3-SA or SVEC4-10 cells [81]. Subsequent research confirmed that ZBP1 acts as the initiator of RIPK3-mediated necroptosis, and deletion of ZBP1 or RIPK3 both rescue infected cell viability and MCMVM45mutRHIM virulence [4,81]. The Zα2 of ZBP1 is indispensable for sensing the MCMV-derived nucleic acid ligands, and RHIM1 of ZBP1 is required to interact with RIPK3 [82,83]. Intriguingly, ZBP1-mediated necroptotic signaling requires viral immediate-early protein 3 (IE3), an essential protein required for early and late gene transcription, indicating that MCMV-derived RNA but not DNA became the ligand of ZBP1 [82,83]. The vIRA of MCMVWT reshapes the ZBP1-RIPK3 complex and impedes the necroptotic signaling to maintain viral normal replication [101]. In addition, ZBP1 mediates the IRF3-dependent IFN-β transcription and ISG expression in Human cytomegalovirus (HCMV) infected THF cells [102,103]. Knockdown of ZBP1 impairs the IFN-β transcription and overexpression of ZBP1 markedly inhibits replication of HCMV in THF cells [102].
Figure 3.
DNA viruses developed antagonistic mechanisms against ZBP1 signaling. After sensing virus-derived RNA, ZBP1 triggers necroptosis via the ZBP1-RIPK3-MLKL axis against MCMV, HSV-1, or VACV infection. However, the ZBP1 signaling is blocked by the DNA viruses-encoded virulence factors. At the beginning of the necroptosis signaling, the VACV E3 protein shields the VACV Z-RNA by its Zα domain and abolishes the activation of ZBP1. After that, the homologous RHIM of MCMV vIRA and HSV-1 ICP6 destroys the signaling transduction from ZBP1 to RIPK3.
Herpes simplex virus-1 (HSV-1) and varicella zoster virus (VZV) both belong to the α-herpesvirus. HSV-1 is known to induce cytolytic death and cause orofacial infections or encephalitis [100]. Mice BMDMs infected with HSV-1 form a huge PANoptosome consisting of absent in melanoma 2 (AIM2), ZBP1, pyrin, ASC, CASP1, RIPK3, MLKL, RIPK1, FADD, and CASP8 [84]. The PANoptosome efficiently suppresses HSV-1 replication and saves infected animals from death via driving apoptosis, pyroptosis, and necroptosis in macrophages. AIM2, an inflammasome sensing dsDNA, acts as the initiator of the PANoptosome. Knockout of AIM2 in BMDMs completely abolishes CASP1/3/7/8 cleavage, the release of IL-1β and IL-18, and the phosphorylation of RIPK3 and MLKL. In the AIM2-mediated PANoptosome, ZBP1 and pyrin are placed at the crucial point downstream of AIM2. Because separately deletion of ZBP1 or pyrin in BMDMs only partially weakened the CASP1/3/7/8 cleavage, release of IL-1β and IL-18. Only collective loss of ZBP1 and pyrin in BMDMs prevents the HSV-1-induced cell death-like AIM2−/− BMDMs [84]. Consistently, knockdown of ZBP1 in primary murine microglia and astrocytes relieve inflammatory cytokines, neurotoxic mediators, and cell death, which elucidated a new mechanism of HSV-1-induced and ZBP1-dependent neuropathology of the central nervous system (CNS) [104]. Similar to MCMV infection, Zα2 of ZBP1 recognizes HSV-1-derived nascent RNA and RHIM1 of ZBP1 is required to provoke HSV-1-induced necroptosis [85]. HSV-1 encoded ICP6 also contains RHIM domain and RHIM-dependent interacts with ZBP1-RIPK3 complex to inhibit ZBP1-mediated necroptosis in host cells [85,86,87] (Figure 3). Unexpectedly in HSV-1 infected murine cells, the suppression function of ICP6 on necroptosis is invalid and leads to mice mortality [85,87,88]. Besides that, ZBP1 was demonstrated to interact with HSV-1 ICP0 and inhibit the activation of the ICP0 promoter to suppress HSV-1 replication in HepG2 cells [30]. Different from ICP6 of HSV-1, RHIM-containing ORF20 protein encoded by VZV blocks ZBP1-mediated apoptosis rather than necroptosis via forming stable heteromeric amyloid assemblies with ZBP1 [89].
Epstein–Barr virus (EBV) is a chronic human γ-herpesvirus that persists for life. EBV infection causes an increased risk of cancer and autoimmune diseases [105,106]. However, the efficient elimination of EBV is difficult because of the EBV latency after the initial infection. A new hypothesis holds that ZBP1 is not involved in EBV’s lysis replication but is contribute to the EBV latency [107]. There are two possible pathways by that ZBP1 induces the establishment and maintenance of EBV latency and both depends on suppressive dimethylation of the histone H3 lysine 9 residue (H3K9me2) [107]. The first pathway, ZBP1 senses and binds to the strong Z-DNA prone sequences in multiple promoter regions of the EBV genome, then further locates C-terminal binding protein (CTBP) to the Z-sequences within promoters could induce H3K9 methylation by MYC oncogene protein [107,108]. The other pathway, EBV-produced Z-NA activates ZBP1-RIPK1-mediated NF-κB signaling during the initial stages of infection [16]. The unleash p50 homodimers interact with euchromatic histone lysine methyltransferases (EHMT1) to induce H3K9 methylation and the expression silence of EBV genes in viral NF-κB binding sites [109]. The hypothesis may help the EBV eradication by suppressing ZBP1-mediated virus latency but needs to be confirmed by further experiments.
5.2. Vaccinia Virus (VACV)
VACV belongs to the poxvirus family and has a large linear dsDNA genome and replicates in the cytoplasm. VACV is used as a vaccine against smallpox and is currently being further developed as an efficient vector for novel vaccines against cancer and other infectious diseases [110,111]. The E3 protein of VACV generates cytoplasmic Z-RNA via its C-terminal dsRNA binding domain (dsRBD), and the virus-derived Z-RNA is sensed and bound by Zα2 of ZBP1 [6]. The interaction between E3-Z-RNA-ZBP1 triggers the ZBP1-RIPK3-MLKL axis-dependent necroptosis to restrain viral replication and alleviate clinical symptoms in VACV-infected mice [6,90]. However, the N-terminal Zα domain of E3 competes with ZBP1 for binding the virus-derived Z-RNA so that ZBP1 is separated from the viral Z-RNA and does not interact with RIPK3 to induce necroptosis [6,90] (Figure 3). The functional substitution of ZBP1 Zα1 (E3Zα1ZBP1) or ADAR1 Zα (E3ZαADAR1) for E3 Zα still blocks necroptosis and maintains the normal virulence of the VACV, indicating the equivalence of Zα domain of Z-NA binding family in sensing and binding Z-NA and the significance of E3 Zα as a critical suppressor of ZBP1-induced necroptosis [6,91].
5.3. Influenza A Virus (IAV)
IAV is a negative sense, single-stranded, lytic RNA virus and belongs to the Orthomyxoviridae family. IAV targets and lyses lung epithelial cells and fibroblasts that cause airway and lung dysfunction as well as cardiovascular diseases [112,113]. IAV infection upregulates the expression of ZBP1 relied on the activated RIG-I-MAVS signaling and IFN regulatory factor (IRF1), and induces the TRIM34-mediated K63-linked polyubiquitination of ZBP1 on the K17 position [114,115,116]. The Zα2 of ZBP1 senses the viral Z-RNA derived from the defective viral genomes (DVGs) (some IAV gene segments produced by the IAV RNA polymerase) in the nucleus [15,23,117]. Subsequently, ZBP1 recruits RIPK1, FADD, CASP8, RIPK3, MLKL, NLRP3, ASC, CASP1, and CASP6 to consist of the ZBP1 PANoptosome and execute PANoptosis [61,118,119] (Figure 2). CASP6 of the PANoptosome serves as the scaffold protein to strengthen the interaction between ZBP1 and RIPK3, and its caspase activity is not required [119]. During IAV infection, ZBP1 PANoptosome-mediated necroptosis differs significantly from TNFα signaling. Attributing to the nuclear localization of ZBP1, RIPK3-MLKL necrosome also migrates to the nucleus so that p-MLKL pores firstly rupture the nuclear membrane and secondly plasma membranes [23]. Although ZBP1-mediated PANoptsis significantly suppresses IAV replication, the inflammatory death of pulmonary cells also causes the lethality of infected mice because of the pulmonary dysfunction and inflammation [61,92,120,121]. Therefore, ZBP1-mediated PANoptosis could be a double-edged sword for IAV-infected hosts. In addition, other members of the Orthomyxoviridae family, such as seasonal strains of IAV and IBV but not the Paramyxoviridae family, also produce the nuclear Z-RNA, and whether ZBP1 also mediates similar PANoptosis against other orthomyxoviruses infection requires further verification [23].
5.4. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Since 2019, the Coronavirus disease 2019 (COVID-19) pandemic has led to socioeconomic and psychological distress [122]. COVID-19, caused by SARS-CoV-2, is characterized by acute respiratory distress syndrome (ARDS), multi-organ failure and death [123]. SARS-CoV-2 is a positive sense, single-stranded RNA virus and belongs to β-coronavirus family. High dose infection or late infection with SARS-CoV-2 induce IFN-α/γ responses and the expression of ZBP1 [95,124]. In SARS-CoV-2 infected mice or BMDMs, the additional IFN-β inducing ZBP1 provokes PANoptosis via CASP1/3/7/8, GSDMD, GSDME, MLKL [75,95,125], and deletion of ZBP1 (ZBP1−/−) or Zα2 deficiency of ZBP1 (ZBP1ΔZα2) substantially alleviated the inflammatory cell death and lethality of mice upon treatment with IFN and SARS-CoV-2 infection [95]. The ZBP1-mediated PANoptosis explains why non-early IFN treatment is not clinically helpful in treating SARS-CoV-2 infection [126,127]. In the early stage of infection, the upregulated ZBP1 via IFN-I intervention induces the appropriate death of infected cells which results in a mild inflammatory response to help host clear the invaded SARS-CoV-2. Nonetheless, later administration of IFN induces the ZBP1-mediated massive inflammatory cell death in the lungs and causes inflammatory cytokine storm, lung dysfunction, and increased mortality [95,124]. The retrospective research which showed the increased IFN levels in serum of patients with serious infection compared with those with moderate COVID-19 also supports the above standpoint [128]. Hence, interfering with ZBP1 function could reverse the negative effect of IFN-I clinical treatment and be an efficient therapeutic strategy against SARS-CoV-2 infection. Moreover, ZBP1 also mediated the same PANoptosis against mouse hepatitis virus (MHV) infection, another member of the β-coronavirus family [95].
Genome analysis reveals the potential Z-RNA prone sequences (called flipons) within the coronavirus genome including SARS-CoV, MERS, and SARS-CoV-2. SARS-CoV-2-derived flipons are mainly present in Nsp2, Nsp12, Nsp13, S, and M genes [129]. That flipons are likely to form Z-RNA during viral replication and transcription and thus activate ZBP1-mediated PANoptosis. Moreover, the Nsp13 helicase of coronavirus contains an identified RHIM and a potential Z-flipon helicase (flipase) activity [129]. Thus, coronavirus Nsp13 could strongly suppress Z-RNA-activated immune response via antagonizing ZBP1-RIPK3 signaling or segregating and unwinding of the Z-RNA [4,129]. In addition, more experiments were needed to confirm the above supposes.
5.5. Flaviviruses
Flaviviruses have a positive-sense, single-stranded RNA genome and are generally spread to vertebrate hosts by arthropods. West Nile virus (WNV) and Zika virus (ZIKV) both belong to mosquito-borne flaviviruses and cause encephalitis and severe neurological injuries [130,131]. ZBP1 expression is dramatically increased in the infected murine brain with WNV or ZIKV [132,133]. In WNV-infected mice, lacking ZBP1 leads to elevatory brain viral load, more severe viremia, and significantly higher morbidity and mortality [93]. Transcriptomics analysis shows that apoptosis and pyroptosis pathways are significantly enriched and necroptotic genes (RIP1K, RIPK3, and MLKL) are upregulated in the WNV-infected mice murine brain [134]. Although deletion of ZBP1 inhibited ZBP1-dependent WNV-induced cell death in MEFs, does not cause the viability difference in WNV-infected primary mouse cortical neurons [93]. Similar to WNV infection, ZBP1-RIPK3 also unsuccessfully causes cell death in ZIKV-infected primary mouse cortical neurons [135]. However, ZBP1-RIPK3 effectively provokes necroptosis even though not include apoptosis and pyroptosis in ZIKV-infected human astrocytes, and the necroptosis significantly suppresses viral titers [94]. Hence, flaviviruses may induce ZBP1-dependent cell death only in specific brain cells, such as astrocytes, so that their replication is inhibited. In addition, interestingly, the ZBP1-RIPK1-RIPK3 complex upregulates immune responsive gene 1 (IRG1) to turn cis-aconitate into itaconate in ZIKV-infected primary cortical neurons [135]. Then increased itaconate activates a metabolic state via inhibiting succinate dehydrogenase (SDH) activity and saves the ZIKV-infected mice [135]. That presents a new anti-virus immunometabolic mechanism for ZBP1 in neurons.
5.6. Bacterium
Yersinia pseudotuberculosis (a Gram-negative bacterium) infected or lipopoly-saccharide (LPS, a component of Gram-negative bacteria wall)-treated BMDMs arises apoptosis and pyroptosis via the TRIFosome consisting of TRIF, ZBP1, RIPK1, FADD, CASP8, GSDMD [17]. After TLR4 sensing LPS and translocating to the endosome, adapter protein TRIF recruits ZBP1 and RIPK1 via the shared RHIM domains. The TRIF-ZBP1-RIPK1 complex further binds FADD and CASP8, then CASP8 cleaves CASP3/7 and GSDMD to activate apoptosis and pyroptosis, respectively [17,72]. The ZBD of ZBP1 is unimportant for the TRIFosome assembling, indicating that ZBP1 only serves as a scaffold protein to promote the assembly of TRIFosome [17]. Lacking ZBP1 markedly blocks activation of CASP8 and decreases inflammatory death of macrophages, which are not beneficial for host defense against Yersinia pseudotuberculosis infection [17,136]. ZBP1-mediated necroptosis in macrophages also play a vital role in LPS-induced lung inflammatory injury [97]. TLR4-TRIF may still be the upstream of ZBP1 signaling because of the TLR4-LPS sensing. In addition to necroptosis signaling, ZBP1 of TRIFosome act as a scaffold to promote TRIF-RIPK1 interaction and M1-ubiquitination of RIPK1, which is important for LPS- or dsRNA-activated inflammatory responses by TLR3/4 [137]. Additionally, knockout of ZBP1 mitigates the release of mtDNA, cytokines, and the activation of the NF-κB pathway in macrophages following LPS stimulation, showing that ZBP1 signaling triggers other innate immune pathways [97]. In addition, Francisella novicida (an intracellular Gram-negative bacterium) infected BMDMs form the AIM2 PANoptosome similar to HSV-1 infection [84]. AIM2 functions as the apical sensor to bind the bacterial DNA and initiate the assembly of the PANoptosome [138]. ZBP1 acts as the adapter protein of the AIM2 PANoptosome and is indispensable to provoking PANoptosis [84]. Similar to viruses, some bacterium also develops the capacity to antagonize the ZBP1 signaling. Enteropathogenic Escherichia coli (EPEC) encoded type III secretion system (T3SS) effector EspL has a tripartite cysteine protease motif (Cys47, His131, Asp153) that cleaves within the RHIM domains and degrades the RHIM-containing proteins (including ZBP1, RIPK1, RIPK3, TRIF) [139]. The interfering effect of Espl on RHIM signaling could be an important tactic to ensure EPEC infection.
5.7. Fungi
Candida albicans and Aspergillus fumigatus infection also activate ZBP1-dependent PANoptosis and inflammation in BMDMs [98]. Compared to wild-type BMDMs, ZBP1−/− or ZBP1ΔZα2 BMDMs show significantly attenuated PANoptosis and the release of IL-18 after fungi infection [98]. While it is not clear how ZBP1 is activated during fungal infection, it is expected that ZBP1-mediated PANoptosis may be important in controlling fungi infection via the inflammatory response [140,141].
5.8. Toxoplasma Gondii
Toxoplasma gondii belongs to obligate intracellular parasites and is able to invade all nucleated cells in warm-blooded animals including humans [142]. T. gondii infection upregulates and maintains the ZBP1 expression [143,144]. Deletion of ZBP1 promotes the replication rate of T. gondii in macrophages or mice and reduces host survival against oral challenge, confirming the suppression of ZBP1 on T. gondii infection [145]. But the deletion of ZBP1 does not influences the cell viability, and increases the level of proinflammatory cytokines, such as TNFα, IL-6, and MCP-1 in infected mice or BMDMs, showing ZBP1 is not involved in the inflammatory cell death during T. gondii infection [145,146]. Although BMDMs lacking ZBP1 present lower nitric oxide concentration (NO, an important immune molecule), a recent study confirmed that nitric oxide synthase (iNOS) lacking mice completely resist to T. gondii infection [145,147]. Thus, the relevance of ZBP1 to T. gondii infection could need more effort to illuminate.
6. ZBP1 Signaling in Auto-Inflammatory Diseases
Sensing host-derived nucleic acid ligands causes the spontaneous activation of ZBP1, that is responsible for many auto-inflammatory diseases, such as perinatal lethality, inflammatory bowel disease (IBD), heatstroke and oxidative stress-induced injuries, and acute pancreatitis (AP).
In the setting of pathogens infection, RIPK1 is an important member of the ZBP1-PANoptosome and the key to ZBP1-mediated PANoptosis [23,57]. Paradoxically, various genetic and biochemical approaches have confirmed that the RHIM not kinase activity of RIPK1 disturbs the ZBP1-RIPK3 necrosome and avoids the aberrant necroptosis in the absence of infection (Figure 4). RIPK1−/− mice or RIPK1mR/mR mice expressing RIPK1 with mutated RHIM develop systemic inflammation and early postnatal lethality due to ZBP1-mediated necroptosis in multiple tissues [148,149,150,151]. RIPK1E-KO mice (with the epidermis-specific RIPK1-knockout) suffer from skin inflammation because of ZBP1-dependent keratinocyte necroptosis [149,152]. The dendritic cell (DC)-specific RIPK1−/− mice with loss of RIPK1 kinase activity in other cells, exhibit spontaneous colonic inflammation relied on ZBP1-RIPK3 necrosome [153]. Advanced research indicated that the Zα2 and RHIM1 of ZBP1 are vital for this RIPK1−/− caused spontaneous necroptosis, and endogenous retroviruses (ERV)-derived complementary reads were detected in epidermal RNA [15,58,154] (Figure 4). Besides that, a histone methyltransferase safeguarding genome stability, SETDB1 deficiency in intestinal stem cells (IECs) liberates the ERV and products excessive viral mimicry [155]. The Zα and RHIM1 domains-dependent ZBP1 sense the viral dsRNA to induce necroptosis in IECs, which inevitably destroys the homeostasis of the epithelial barrier and causes inflammatory bowel disease (IBD) [155]. Hence, excluding pathogenic nucleic acid ligands, endogenous ERV-derived dsRNA is also effectively recognized by ZBP1 that induces spontaneous cell death, autoimmune inflammatory diseases, and even death.
Figure 4.
The model for endogenous ZBP1 activation and the host antagonistic mechanisms targeting ZBP1 signaling. The endogenous retroviruses (ERV)-derived dsRNA, released cytoplasmic mitochondria DNA (mtDNA), and Z-RNA enriched in 3’UTR of ISGs mRNA that harbor inverted SINEs or GU-type simple repeats act as the activated ligands of ZBP1 and induce endogenous ZBP1 signaling. The endogenous activation of ZBP1 causes many auto-inflammatory diseases or stimulates tumor immunity. ADAR1-p150 inhibits the ligands sensing of ZBP1 by the Zα domain and RIPK1 blocks the signaling transduction of ZBP1 by its RHIM.
In addition to RIPK1, ADAR1 is another host antagonist protein against ZBP1 signaling. ADAR1 is responsible for the adenosine to inosine (A-to-I) edits of ERV-derived dsRNA and represses abnormal immune responses activated by ERVs [156,157,158,159]. Deletion of ADAR1 or mutant of the Zα domain of ADAR1 causes the accumulation of ERV-derived dsRNA and spontaneous activation of ZBP1 [160,161]. The abnormal activation of ZBP1 causes embryonic lethality, intestinal cell death and skin inflammation similar to RIPK1−/− or RIPK1mR/mR mice [162,163]. ZBP1 plays a dual role in mice with impaired ADAR1 function. On the one hand, ZBP1 induces RIPK3-mediated necroptosis and CASP-8-mediated apoptosis which is MAVS-independent [162,163]. On the other hand, ZBP1 promotes the RIPK1- and RIPK3-independent, but MAVS-dependent IFN-I responses and stimulates interferonopathies [160].
Impaired mitochondrial DNA (mtDNA) is another endogenous ligand to activate ZBP1 (Figure 4). PUMA, NF-κB signaling induced proapoptotic protein, facilitates the rupture of mitochondrial membrane, the cytosolic release of mtDNA, and mtDNA-ZBP1 binding, leading to necroptosis in CASP-inactivated HT-29 cells [164,165,166]. A lack of FADD/CASP8 in IECs leads to ZBP1-dependent IBD, but deletion of PUMA can rescue embryonic death via reducing mtDNA-ZBP1 binding in FADD−/− mice [164,167]. Beyond that, mtDNA-ZBP1 interaction mediated inflammation also is the result of chronic oxidative stress. The chronic low-level oxidative stress-stimulated retinal pigment epithelial (RPE) cells show mtDNA fragmentation and cytosolic release, and the damaged mtDNA is packed into extracellular vesicles (EVs) that transfer to surrounding microglia. The cytoplasmic mtDNA-ZBP1 binding in RPE cells and microglia triggers the TBK1/IRF3-dependent the expression of proinflammatory cytokines, such as IL-1α/β, IL-8, and TNFα, which contributes to the aberrant activation of microglia and ocular pathologies [168]. The ZBP1-mtDNA complex-mediated inflammation in response to oxidative stress also is demonstrated in lung epithelial cells [169].
Intriguingly, the latest research indicated that the ZBP1 without binding to any nucleic acid ligands can also provoke cell death when suffering from heatstroke [9]. Heat stress upregulates ZBP1 via HSF1 and promotes PANoptosome assembly resulting in PANoptosis in BMDMs and circulatory failure, organ injury, and lethality in mice. The Zα2 of ZBP1 is unnecessary but RHIM1 of ZBP1 is pivotal to interact with RIPK3. Lacking ZBP1 or RIPK3 significantly relieved alleviates the fatal injuries from extreme hyperthermia [9].
Moreover, the uncontrolled expression of ZBP1 and following ZBP1-dependent pyroptosis are the main reason for acute pancreatitis (AP) [170]. The tRF3-THr-AGT, an endogenous tRNA-derived small RNAs (tsRNAs), specifically binds the 3’ untranslated regions (3’-UTR) of ZBP1 mRNA to form short dsRNA that causes the degradation of ZBP1 mRNA [170]. In the cellular and animal AP models, the tRF3-THr-AGT is aberrantly decreased resulting in the upregulation of ZBP1, the activation of ZBP1-mediated NLRP3 inflammasome, and the extracellular release of cytokines and DAMPs [170,171]. In addition, further verification is needed to affirm whether the upregulated ZBP1 mediates apoptosis or necroptosis in acinar cells to aggravate AP.
7. ZBP1 Signaling in Tumor Immunity
Although ZBP1-induced aberrant cell death and inflammation are harmful to the host, there is a positive effect in the background of tumorigenesis. Advanced solid tumors generally suffer from necroptosis [172]. However, the tumor cell necroptosis may not be related to the TNFα-RIPK1 pathway [173,174]. In the late breast cancer cells, glucose deprivation causes the cytosolic release of mtDNA through mitochondrial protein NOXA. The ZBP1-mtDNA binding initiates RIPK3-MLKL-dependent tumor necroptosis and blocks metastasis in the lung [174]. In irradiated MC38 tumors, the ZBP1-mediated necroptotic signaling is crucial for anti-tumor immunity. That is not only because of ZBP1-mediated tumor necroptosis but also attribute to mtDNA release via the necroptotic signaling. The cytoplasmic mtDNA activates the cGAS-STING pathway and IFN-I responses. Deletion of ZBP1 inhibited the activation of cGAS-STING signaling and priming of CD8+ T cells in tumor cells with radiation treatment [175]. Mechanistically, the cytoplasmic release of mtDNA induced by ZBP1-necroptosis could be attributed to the translocation of RIPK3-MLKL necrosome to mitochondria. On the one hand, RIPK3 targets and activates the mitochondrial pyruvate dehydrogenase complexes, which require RIPK3-MLKL to be recruited into the mitochondria. Mechanistically, the cytoplasmic release of mtDNA induced by ZBP1-necroptosis could be attributed to the translocation of RIPK3-MLKL necrosome to mitochondria. On the one hand, RIPK3 targets and activates the mitochondrial pyruvate dehydrogenase complexes, which require RIPK3-MLKL to be recruited into the mitochondria [176]. On the other hand, the mitochondria membrane contains phospholipids, including phosphatidylinositol phosphates that anchor MLKL to the plasma membrane and nuclear membrane [23,177,178,179,180]. For these reasons, the hypothesis that RIPK3-MLKL necrosome punch pores in the mitochondrial membrane leading to mtDNA divulgence is possible. Nevertheless, it is worth affirming that the release of mtDNA caused by the ZBP1-mediated necroptotic signaling cascade and subsequent IFN-I responses via the cGAS-STING pathway will shape strong positive feedback of antitumor immunity [175,181].
In addition to mtDNA, the endogenous dsRNA remains the important ligand for ZBP1-mediated tumor immunity. However, the ZBP1 sensing for endogenous dsRNA is still interdicted by ADAR1. The latest research points out that some 3’UTR of ISGs mRNA containing short interspersed nuclear elements (SINEs) or GU-type simple repeats and naturally fold into Z-type dumbbells [161]. Such Z-RNA is segregated by the Zα domain of cytoplasmic ADAR1-p150 isoform or accumulates to activate ZBP1-dependent tumor necroptosis when ADAR1 is depleted or mutated [161] (Figure 4). The deficiency of ADAR1 encouraged ZBP1-mediated PANoptosis and limited tumorigenesis [33] (Figure 4). The antagonism strategy of ADAR1 against ZBP1 is very similar to the E3 of VACV, and all block the recognition of ZBP1 for activated Z-RNA ligands through the twin Zα domains [6,33]. ADAR1’s restriction on ZBP1 signaling complements the mechanism of the oncogenic role of ADAR1, and developing new drugs capable of repressing ADAR1 function or directly activating ZBP1 may be the new strategy for treating cancer [33,161,182].
Through genetic engineering, previous studies have applied ZBP1 to tumor therapy. Various genetic adjuvants enhance vaccine immunogenicity and strengthen adaptive immune responses [183,184,185,186]. Co-inoculation of the plasmid expressing ZBP1 (pZBP1) and the DNA vaccine encoding immunogenic tumor-associated antigens (TAAs) in mice more effectively activated NF-κB and IFN-I signaling and enhanced the TAA-specific cytotoxic CD8+ T lymphocytes (CTLs) and CD4+ Th1 responses, compared to the empty vector plus TAAs vaccine inoculation [187]. Compared to the solely vaccinated mice with pTRP2 (tyrosinase-related protein-2, a highly expressed glycoprotein in human melanomas), the higher percentage of TRP2-specific IFN-γ-producing CD8+ T cells and more durable stronger resistance to B16 melanoma was detected in co-vaccinated mice with pZBP1 and pTRP2 [187]. In addition, modifying oncolytic viruses to express therapeutic genes is a significant tumor immunotherapy strategy [188]. A ZBP1-armed oncolytic vaccinia virus has been created as a fast and effective self-recognition immune enhancement system. The VACV expressing ZBP1 induces T cell infiltration (especially TAA-specific T cells) which establishes a powerful antitumor immune response system and strongly controls tumor volume [189]. Although the above two experiments did not emphasize the relationship between ZBP1 and tumor cell death, the ZBP1-mediated antitumor therapeutic effect has been confirmed in vivo.
8. Concluding Remarks
ZBP1 is another Z-NA binding protein in addition to ADAR1 in mammals. Initially, ZBP1 was shown to be a cytoplasmic DNA sensor that promotes innate immune responses, and subsequent studies confirmed the importance of ZBP1 in PANoptosis initiation. ZBP1 signaling typically relies on the combination of the Zα2 domain with active ligands, such as virus-derived Z-RNA (VACV and IAV) [6,23,161,190]. In addition to sensing invasive pathogens, ZBP1 also binds with endogenous nucleic acid ligands, such as ERV-derived dsRNA and cytoplasmic mtDNA [155,168]. In surprise, endogenous ERV-derived Z-RNA recently has been confirmed to be present in some 3’UTR of ISGs mRNA that contain inverted SINEs or GU-type simple repeats and is sensed by ZBP1 in the absence of ADAR1-p150 [161]. In addition, the histone chaperone inhibitor, CBL0137 can directly induce Z-DNA formation in the LINE-1 element and activate ZBP1 in the presence of ADAR1-p150 [161]. The ZBP1 agonists will be a potential therapeutic avenue for tumors with limited ZBP1 function. However, there is no direct evidence as to whether the cytoplasmic damaged mtDNA detected by ZBP1 belongs to the Z-conformation. Moreover, even without ligands binding function, ZBP1 also acts as an adapter protein of AIM2 or TLR3/4-TRIF to transfer PANoptosis signaling [17,84].
ZBP1-PANoptosome initiates PANoptosis and plays a double-edged sword role in anti-infection and development. ZBP1-mediated PANoptosis efficiently limits the replication of intracellular pathogens, and stimulates the recruitment of immune cells and adaptive immune responses, which is beneficial to host survival [4]. Nonetheless, the excessive cell death activated by ZBP1 also provokes lethal inflammatory cytokine storm, organ dysfunction even host mortality. For instance, ZBP1-dependent PANoptosis restrict the viral loads of IAV and SARS-CoV-2 but caused severe inflammation and lung dysfunction in critical patients [23,95]. ZBP1-mediated brain cell death may be a key cause of the neuropathology during HSV-1 or SARS-CoV-2 infection [104,125]. In addition, the endogenous activation of ZBP1 causes multiple auto-inflammatory diseases. ZBP1 binding with ERV-derived dsRNA activates embryonic lethality in RIPK1−/− mice and colitis in RIPK1−/− IECs [58,155], and binding with leaked mtDNA leads to chronic oxidative stress-induced ocular pathologies [168]. However, the endogenous activation of ZBP1 also has a positive effect in the setting of cancer. ZBP1-PANoptosome promotes tumor death and immune cell infiltration, and limits tumor volume and metastasis [33,174].
Both the pathogen and the host have evolved skills to regulate ZBP1 signaling to ensure pathogen replication or immune homeostasis (Table 1 and Table 2). Relying on the homogenous Zα domains, ADAR1-p150 and VACV E3 segregate the activated Z-RNA of ZBP1 to inhibit ZBP1 signaling [6,33,161]. RIPK1, MCMV M45, HSV-1 ICP6, and VZV ORF20 all block the signaling transduction of ZBP1-RIPK3 via shared RHIM domains [4,85,89,150]. Furthermore, SARS-CoV-2 ORF8 is a new negative regulator that antagonizes the IFN-γ-stimulated expression of ZBP1 [96]. ZBP1 may also play a role for others viruses, and its influence could be antagonized by some unknown virulence factors which need follow-up studies to investigate.
Table 2.
The host proteins regulating the ZBP1 signaling.
| Host Regulatory Factors | Positive(P) or Negative(N) Regulation | Regulatory Mechanisms | Reference(s) |
|---|---|---|---|
| AIM2 | P | Interacts with ZBP1 to drive PANoptosis | [84] |
| TRIM34 | P | Induces the K63-linked polyubiquitination of ZBP1 on the K17 position | [116] |
| CASP6 | P | Strengthens ZBP1–RIPK3 interactions | [119] |
| PUMA | P | Promotes the cytoplasmic release of mtDNA and activation of ZBP1 | [164] |
| ADAR1 | N | Inhibits the ZBP1 sensing for activated Z-RNA | [33,160,161,162,163] |
| RIPK1 | N | Disrupts ZBP1–RIPK3 interactions | [149,150] |
| SETDB1 | N | Holds down the level of ERV-derived dsRNA and activation of ZBP1 | [155] |
| tRF3-Thr-AGT | N | Degrades the mRNA of ZBP1 | [170] |
In this review, we have elucidated the ZBP1-mediated signaling mechanisms of innate immune response and PANoptosis. We also discussed the pathogens and host-developed multiple regulation strategies for ZBP1-mediated signaling; ZBP1 plays a double-effective role in infection and host health. Understanding the impact of ZBP1 signaling on host or pathogens and developing novel positive or negative regulated drugs against ZBP1 signaling will be helpful to anti-infection, alleviation of inflammatory disease, and anti-tumor immunity.
Acknowledgments
The authors would like to thank the anonymous editors and reviewers for their valuable comments and suggestions that helped to improve the quality of this manuscript.
Author Contributions
Conceptualization, Y.H.; writing—original draft preparation, Y.H., B.Y., J.Y., X.S. and D.Z. (Dajun Zhang); writing—review and editing, Y.H., X.Y., W.Y., L.C. and D.Z. (Dengshuai Zhao); supervision, H.Z., K.Z. and X.L.; validation, all authors. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the National Key R&D Program of China (2021YFD1801300); This work was supported by the major science and technology project of Gansu Province (20ZD7NA006-2, 21ZD3NA001); The Research funding from Lanzhou Veterinary Research Institue (CAAS-ZARW202006-03).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuriakose T., Kanneganti T.D. ZBP1: Innate Sensor Regulating Cell Death and Inflammation. Trends Immunol. 2018;39:123–134. doi: 10.1016/j.it.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takaoka A., Wang Z., Choi M.K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K., et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 3.Rebsamen M., Heinz L.X., Meylan E., Michallet M.C., Schroder K., Hofmann K., Vazquez J., Benedict C.A., Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upton J.W., Kaiser W.J., Mocarski E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y., Comella N., Tognazzi K., Brown L.F., Dvorak H.F., Kocher O. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene. 1999;240:157–163. doi: 10.1016/S0378-1119(99)00419-9. [DOI] [PubMed] [Google Scholar]
- 6.Koehler H., Cotsmire S., Zhang T., Balachandran S., Upton J.W., Langland J., Kalman D., Jacobs B.L., Mocarski E.S. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe. 2021;29:1266–1276 e1265. doi: 10.1016/j.chom.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D., Liang Y., Zhao S., Ding Y., Zhuang Q., Shi Q., Ai T., Wu S.Q., Han J. ZBP1 mediates interferon-induced necroptosis. Cell Mol. Immunol. 2020;17:356–368. doi: 10.1038/s41423-019-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao Q., Kundu S., Kleam J., Zhao Z.J., Idell S., Tang H. Enhanced RIPK3 kinase activity-dependent lytic cell death in M1 but not M2 macrophages. Mol. Immunol. 2021;129:86–93. doi: 10.1016/j.molimm.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan F., Cai J., Wu J., Tang Y., Zhao K., Liang F., Li F., Yang X., He Z., Billiar T.R., et al. Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science. 2022;376:609–615. doi: 10.1126/science.abg5251. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz T., Behlke J., Lowenhaupt K., Heinemann U., Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat. Struct. Biol. 2001;8:761–765. doi: 10.1038/nsb0901-761. [DOI] [PubMed] [Google Scholar]
- 11.Rothenburg S., Schwartz T., Koch-Nolte F., Haag F. Complex regulation of the human gene for the Z-DNA binding protein DLM-1. Nucleic Acids Res. 2002;30:993–1000. doi: 10.1093/nar/30.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Choi M.K., Ban T., Yanai H., Negishi H., Lu Y., Tamura T., Takaoka A., Nishikura K., Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. USA. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha S.C., Van Quyen D., Hwang H.Y., Oh D.B., Brown B.A., 2nd, Lee S.M., Park H.J., Ahn J.H., Kim K.K., Kim Y.G. Biochemical characterization and preliminary X-ray crystallographic study of the domains of human ZBP1 bound to left-handed Z-DNA. Biochim. Biophys. Acta. 2006;1764:320–323. doi: 10.1016/j.bbapap.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Ha S.C., Kim D., Hwang H.Y., Rich A., Kim Y.G., Kim K.K. The crystal structure of the second Z-DNA binding domain of human DAI (ZBP1) in complex with Z-DNA reveals an unusual binding mode to Z-DNA. Proc. Natl. Acad. Sci. USA. 2008;105:20671–20676. doi: 10.1073/pnas.0810463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesavardhana S., Malireddi R.K.S., Burton A.R., Porter S.N., Vogel P., Pruett-Miller S.M., Kanneganti T.D. The Zalpha2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J. Biol. Chem. 2020;295:8325–8330. doi: 10.1074/jbc.RA120.013752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser W.J., Upton J.W., Mocarski E.S. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muendlein H.I., Connolly W.M., Magri Z., Smirnova I., Ilyukha V., Gautam A., Degterev A., Poltorak A. ZBP1 promotes LPS-induced cell death and IL-1beta release via RHIM-mediated interactions with RIPK1. Nat. Commun. 2021;12:86. doi: 10.1038/s41467-020-20357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deigendesch N., Koch-Nolte F., Rothenburg S. ZBP1 subcellular localization and association with stress granules is controlled by its Z-DNA binding domains. Nucleic Acids Res. 2006;34:5007–5020. doi: 10.1093/nar/gkl575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov P., Kedersha N., Anderson P. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol. 2019;11:a032813. doi: 10.1101/cshperspect.a032813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng S.K., Weissbach R., Ronson G.E., Scadden A.D.J. Proteins that contain a functional Z-DNA-binding domain localize to cytoplasmic stress granules. Nucleic Acids Res. 2013;41:9786–9799. doi: 10.1093/nar/gkt750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabriel L., Srinivasan B., Kus K., Mata J.F., Joao Amorim M., Jansen L.E.T., Athanasiadis A. Enrichment of Zalpha domains at cytoplasmic stress granules is due to their innate ability to bind to nucleic acids. J. Cell Sci. 2021;134:jcs258446. doi: 10.1242/jcs.258446. [DOI] [PubMed] [Google Scholar]
- 22.Vignuzzi M., Lopez C.B. Defective viral genomes are key drivers of the virus-host interaction. Nat. Microbiol. 2019;4:1075–1087. doi: 10.1038/s41564-019-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T., Yin C., Boyd D.F., Quarato G., Ingram J.P., Shubina M., Ragan K.B., Ishizuka T., Crawford J.C., Tummers B., et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell. 2020;180:1115–1129 e1113. doi: 10.1016/j.cell.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao T.S., Fitzgerald K.A. The cGAS-STING pathway for DNA sensing. Mol. Cell. 2013;51:135–139. doi: 10.1016/j.molcel.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider W.M., Chevillotte M.D., Rice C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laustsen A., Bak R.O., Krapp C., Kjaer L., Egedahl J.H., Petersen C.C., Pillai S., Tang H.Q., Uldbjerg N., Porteus M., et al. Interferon priming is essential for human CD34+ cell-derived plasmacytoid dendritic cell maturation and function. Nat. Commun. 2018;9:3525. doi: 10.1038/s41467-018-05816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L., Fang L., Wang D., Luo R., Cai K., Chen H., Xiao S. Molecular cloning and functional characterization of porcine DNA-dependent activator of IFN-regulatory factors (DAI) Dev. Comp. Immunol. 2010;34:293–299. doi: 10.1016/j.dci.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Pham T.H., Kwon K.M., Kim Y.E., Kim K.K., Ahn J.H. DNA sensing-independent inhibition of herpes simplex virus 1 replication by DAI/ZBP1. J. Virol. 2013;87:3076–3086. doi: 10.1128/JVI.02860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippmann J., Rothenburg S., Deigendesch N., Eitel J., Meixenberger K., van Laak V., Slevogt H., N’Guessan P.D., Hippenstiel S., Chakraborty T., et al. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishii K.J., Kawagoe T., Koyama S., Matsui K., Kumar H., Kawai T., Uematsu S., Takeuchi O., Takeshita F., Coban C., et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 33.Karki R., Sundaram B., Sharma B.R., Lee S., Malireddi R.K.S., Nguyen L.N., Christgen S., Zheng M., Wang Y., Samir P., et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021;37:109858. doi: 10.1016/j.celrep.2021.109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakahama T., Kawahara Y. Deciphering the Biological Significance of ADAR1-Z-RNA Interactions. Int. J. Mol. Sci. 2021;22:11435. doi: 10.3390/ijms222111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim C. How Z-DNA/RNA binding proteins shape homeostasis, inflammation, and immunity. BMB Rep. 2020;53:453–457. doi: 10.5483/BMBRep.2020.53.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilmore T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 39.Guttridge D.C., Albanese C., Reuther J.Y., Pestell R.G., Baldwin A.S., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell Biol. 1999;19:5785–5799. doi: 10.1128/MCB.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel M., Horgan P.G., McMillan D.C., Edwards J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl. Res. 2018;197:43–56. doi: 10.1016/j.trsl.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Meylan E., Burns K., Hofmann K., Blancheteau V., Martinon F., Kelliher M., Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser W.J., Offermann M.K. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 43.Sato S., Sugiyama M., Yamamoto M., Watanabe Y., Kawai T., Takeda K., Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 44.Hsu H., Huang J., Shu H.B., Baichwal V., Goeddel D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/S1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 45.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Opdenbosch N., Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Chen X., Gueydan C., Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank D., Vince J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.H., Zhong C.Q., Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Grootjans S., Vanden Berghe T., Vandenabeele P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017;24:1184–1195. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser W.J., Sridharan H., Huang C., Mandal P., Upton J.W., Gough P.J., Sehon C.A., Marquis R.W., Bertin J., Mocarski E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malireddi R.K.S., Kesavardhana S., Kanneganti T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis) Front. Cell. Infect. Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samir P., Malireddi R.K.S., Kanneganti T.D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis) Front. Cell. Infect. Microbiol. 2020;10:238. doi: 10.3389/fcimb.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., Kanneganti T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021;19:4641–4657. doi: 10.1016/j.csbj.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christgen S., Zheng M., Kesavardhana S., Karki R., Malireddi R.K.S., Banoth B., Place D.E., Briard B., Sharma B.R., Tuladhar S., et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis) Front. Cell. Infect. Microbiol. 2020;10:237. doi: 10.3389/fcimb.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiao H., Wachsmuth L., Kumari S., Schwarzer R., Lin J., Eren R.O., Fisher A., Lane R., Young G.R., Kassiotis G., et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. 2020;580:391–395. doi: 10.1038/s41586-020-2129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ofengeim D., Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 60.Udawatte D.J., Rothman A.L. Viral Suppression of RIPK1-Mediated Signaling. MBio. 2021;12:e0172321. doi: 10.1128/mBio.01723-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuriakose T., Man S.M., Malireddi R.K., Karki R., Kesavardhana S., Place D.E., Neale G., Vogel P., Kanneganti T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016;1:aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gurung P., Anand P.K., Malireddi R.K., Vande Walle L., Van Opdenbosch N., Dillon C.P., Weinlich R., Green D.R., Lamkanfi M., Kanneganti T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Philip N.H., Dillon C.P., Snyder A.G., Fitzgerald P., Wynosky-Dolfi M.A., Zwack E.E., Hu B., Fitzgerald L., Mauldin E.A., Copenhaver A.M., et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc. Natl. Acad. Sci. USA. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang S., Fernandes-Alnemri T., Rogers C., Mayes L., Wang Y., Dillon C., Roback L., Kaiser W., Oberst A., Sagara J., et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vajjhala P.R., Lu A., Brown D.L., Pang S.W., Sagulenko V., Sester D.P., Cridland S.O., Hill J.M., Schroder K., Stow J.L., et al. The Inflammasome Adaptor ASC Induces Procaspase-8 Death Effector Domain Filaments. J. Biol. Chem. 2015;290:29217–29230. doi: 10.1074/jbc.M115.687731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mascarenhas D.P.A., Cerqueira D.M., Pereira M.S.F., Castanheira F.V.S., Fernandes T.D., Manin G.Z., Cunha L.D., Zamboni D.S. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 2017;13:e1006502. doi: 10.1371/journal.ppat.1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Opdenbosch N., Van Gorp H., Verdonckt M., Saavedra P.H.V., de Vasconcelos N.M., Goncalves A., Vande Walle L., Demon D., Matusiak M., Van Hauwermeiren F., et al. Caspase-1 Engagement and TLR-Induced c-FLIP Expression Suppress ASC/Caspase-8-Dependent Apoptosis by Inflammasome Sensors NLRP1b and NLRC4. Cell Rep. 2017;21:3427–3444. doi: 10.1016/j.celrep.2017.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fritsch M., Gunther S.D., Schwarzer R., Albert M.C., Schorn F., Werthenbach J.P., Schiffmann L.M., Stair N., Stocks H., Seeger J.M., et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 69.Man S.M., Tourlomousis P., Hopkins L., Monie T.P., Fitzgerald K.A., Bryant C.E. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J. Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry C.M., Martin S.J. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol. Cell. 2017;65:715–729.e5. doi: 10.1016/j.molcel.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 71.Li W., Cao T., Luo C., Cai J., Zhou X., Xiao X., Liu S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020;104:6129–6140. doi: 10.1007/s00253-020-10614-y. [DOI] [PubMed] [Google Scholar]
- 72.Sarhan J., Liu B.C., Muendlein H.I., Li P., Nilson R., Tang A.Y., Rongvaux A., Bunnell S.C., Shao F., Green D.R., et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demarco B., Grayczyk J.P., Bjanes E., Le Roy D., Tonnus W., Assenmacher C.A., Radaelli E., Fettrelet T., Mack V., Linkermann A., et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 2020;6:eabc3465. doi: 10.1126/sciadv.abc3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orning P., Weng D., Starheim K., Ratner D., Best Z., Lee B., Brooks A., Xia S., Wu H., Kelliher M.A., et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., Shen J., Zhou Y., Shi Z.L., Zhou P., et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen H., Li Y., Wu J., Li G., Tao X., Lai K., Yuan Y., Zhang X., Zou Z., Xu Y. RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ. 2020;27:2568–2585. doi: 10.1038/s41418-020-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gutierrez K.D., Davis M.A., Daniels B.P., Olsen T.M., Ralli-Jain P., Tait S.W., Gale M., Jr., Oberst A. MLKL Activation Triggers NLRP3-Mediated Processing and Release of IL-1beta Independently of Gasdermin-D. J. Immunol. 2017;198:2156–2164. doi: 10.4049/jimmunol.1601757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conos S.A., Chen K.W., De Nardo D., Hara H., Whitehead L., Nunez G., Masters S.L., Murphy J.M., Schroder K., Vaux D.L., et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. USA. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mack C., Sickmann A., Lembo D., Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. USA. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Upton J.W., Kaiser W.J., Mocarski E.S. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J. Biol. Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Upton J.W., Kaiser W.J., Mocarski E.S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sridharan H., Ragan K.B., Guo H., Gilley R.P., Landsteiner V.J., Kaiser W.J., Upton J.W. Murine cytomegalovirus IE3-dependent transcription is required for DAI/ZBP1-mediated necroptosis. EMBO Rep. 2017;18:1429–1441. doi: 10.15252/embr.201743947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maelfait J., Liverpool L., Bridgeman A., Ragan K.B., Upton J.W., Rehwinkel J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017;36:2529–2543. doi: 10.15252/embj.201796476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee S., Karki R., Wang Y., Nguyen L.N., Kalathur R.C., Kanneganti T.D. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597:415–419. doi: 10.1038/s41586-021-03875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo H., Gilley R.P., Fisher A., Lane R., Landsteiner V.J., Ragan K.B., Dovey C.M., Carette J.E., Upton J.W., Mocarski E.S., et al. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis. 2018;9:816. doi: 10.1038/s41419-018-0868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shanmugam N., Baker M., Sanz-Hernandez M., Sierecki E., Gambin Y., Steain M., Pham C.L.L., Sunde M. Herpes simplex virus encoded ICP6 protein forms functional amyloid assemblies with necroptosis-associated host proteins. Biophys. Chem. 2021;269:106524. doi: 10.1016/j.bpc.2020.106524. [DOI] [PubMed] [Google Scholar]
- 87.Huang Z., Wu S.Q., Liang Y., Zhou X., Chen W., Li L., Wu J., Zhuang Q., Chen C., Li J., et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Wang X., Li Y., Liu S., Yu X., Li L., Shi C., He W., Li J., Xu L., Hu Z., et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc. Natl. Acad. Sci. USA. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steain M., Baker M., Pham C.L.L., Shanmugam N., Gambin Y., Sierecki E., McSharry B.P., Avdic S., Slobedman B., Sunde M., et al. Varicella zoster virus encodes a viral decoy RHIM to inhibit cell death. PLoS Pathog. 2020;16:e1008473. doi: 10.1371/journal.ppat.1008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koehler H., Cotsmire S., Langland J., Kibler K.V., Kalman D., Upton J.W., Mocarski E.S., Jacobs B.L. Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3. Proc. Natl. Acad. Sci. USA. 2017;114:11506–11511. doi: 10.1073/pnas.1700999114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim Y.G., Muralinath M., Brandt T., Pearcy M., Hauns K., Lowenhaupt K., Jacobs B.L., Rich A. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Hao Q., Florence J.M., Jung B.G., Kurdowska A.K., Samten B., Idell S., Tang H. Influenza Virus Infection Induces ZBP1 Expression and Necroptosis in Mouse Lungs. Front. Cell. Infect. Microbiol. 2019;9:286. doi: 10.3389/fcimb.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rothan H.A., Arora K., Natekar J.P., Strate P.G., Brinton M.A., Kumar M. Z-DNA-Binding Protein 1 Is Critical for Controlling Virus Replication and Survival in West Nile Virus Encephalitis. Front. Microbiol. 2019;10:2089. doi: 10.3389/fmicb.2019.02089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wen C., Yu Y., Gao C., Qi X., Cardona C.J., Xing Z. RIPK3-Dependent Necroptosis Is Induced and Restricts Viral Replication in Human Astrocytes Infected With Zika Virus. Front. Cell. Infect. Microbiol. 2021;11:637710. doi: 10.3389/fcimb.2021.637710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karki R., Lee S., Mall R., Pandian N., Wang Y., Sharma B.R., Malireddi R.S., Yang D., Trifkovic S., Steele J.A., et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci. Immunol. 2022;7:eabo6294. doi: 10.1126/sciimmunol.abo6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geng H., Subramanian S., Wu L., Bu H.F., Wang X., Du C., De Plaen I.G., Tan X.D. SARS-CoV-2 ORF8 Forms Intracellular Aggregates and Inhibits IFNgamma-Induced Antiviral Gene Expression in Human Lung Epithelial Cells. Front. Immunol. 2021;12:679482. doi: 10.3389/fimmu.2021.679482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Du X.K., Ge W.Y., Jing R., Pan L.H. Necroptosis in pulmonary macrophages mediates lipopolysaccharide-induced lung inflammatory injury by activating ZBP-1. Int. Immunopharmacol. 2019;77:105944. doi: 10.1016/j.intimp.2019.105944. [DOI] [PubMed] [Google Scholar]
- 98.Banoth B., Tuladhar S., Karki R., Sharma B.R., Briard B., Kesavardhana S., Burton A., Kanneganti T.D. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis) J. Biol. Chem. 2020;295:18276–18283. doi: 10.1074/jbc.RA120.015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davison A.J., Eberle R., Ehlers B., Hayward G.S., McGeoch D.J., Minson A.C., Pellett P.E., Roizman B., Studdert M.J., Thiry E. The order Herpesvirales. Arch. Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhowmik D., Zhu F. Evasion of Intracellular DNA Sensing by Human Herpesviruses. Front. Cell. Infect. Microbiol. 2021;11:647992. doi: 10.3389/fcimb.2021.647992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pham C.L., Shanmugam N., Strange M., O’Carroll A., Brown J.W., Sierecki E., Gambin Y., Steain M., Sunde M. Viral M45 and necroptosis-associated proteins form heteromeric amyloid assemblies. EMBO Rep. 2019;20:e46518. doi: 10.15252/embr.201846518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeFilippis V.R., Alvarado D., Sali T., Rothenburg S., Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J. Virol. 2010;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.DeFilippis V.R., Sali T., Alvarado D., White L., Bresnahan W., Fruh K.J. Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B. J. Virol. 2010;84:8913–8925. doi: 10.1128/JVI.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furr S.R., Chauhan V.S., Moerdyk-Schauwecker M.J., Marriott I. A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells. J. Neuroinflamm. 2011;8:99. doi: 10.1186/1742-2094-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farrell P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019;14:29–53. doi: 10.1146/annurev-pathmechdis-012418-013023. [DOI] [PubMed] [Google Scholar]
- 106.Bjornevik K., Cortese M., Healy B.C., Kuhle J., Mina M.J., Leng Y., Elledge S.J., Niebuhr D.W., Scher A.I., Munger K.L., et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 107.Herbert A., Fedorov A., Poptsova M. Mono a Mano: ZBP1’s Love-Hate Relationship with the Kissing Virus. Int. J. Mol. Sci. 2022;23:3079. doi: 10.3390/ijms23063079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tu W.B., Shiah Y.J., Lourenco C., Mullen P.J., Dingar D., Redel C., Tamachi A., Ba-Alawi W., Aman A., Al-Awar R., et al. MYC Interacts with the G9a Histone Methyltransferase to Drive Transcriptional Repression and Tumorigenesis. Cancer Cell. 2018;34:579–595.e8. doi: 10.1016/j.ccell.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Ea C.K., Hao S., Yeo K.S., Baltimore D. EHMT1 protein binds to nuclear factor-kappaB p50 and represses gene expression. J. Biol. Chem. 2012;287:31207–31217. doi: 10.1074/jbc.M112.365601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo Z.S., Lu B., Guo Z., Giehl E., Feist M., Dai E., Liu W., Storkus W.J., He Y., Liu Z., et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer. 2019;7:6. doi: 10.1186/s40425-018-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacobs B.L., Langland J.O., Kibler K.V., Denzler K.L., White S.D., Holechek S.A., Wong S., Huynh T., Baskin C.R. Vaccinia virus vaccines: Past, present and future. Antivir. Res. 2009;84:1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Short K.R., Kroeze E.J.B.V., Fouchier R.A.M., Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014;14:57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 113.Yatim N., Albert M.L. Dying to replicate: The orchestration of the viral life cycle, cell death pathways, and immunity. Immunity. 2011;35:478–490. doi: 10.1016/j.immuni.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Kesavardhana S., Kuriakose T., Guy C.S., Samir P., Malireddi R.K.S., Mishra A., Kanneganti T.D. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J. Exp. Med. 2017;214:2217–2229. doi: 10.1084/jem.20170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuriakose T., Zheng M., Neale G., Kanneganti T.D. IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection. J. Immunol. 2018;200:1489–1495. doi: 10.4049/jimmunol.1701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X., Xiong J., Zhou D., Zhang S., Wang L., Tian Q., Li C., Liu J., Wu Y., Li J., et al. TRIM34 modulates influenza virus-activated programmed cell death by targeting Z-DNA-binding protein 1 for K63-linked polyubiquitination. J. Biol. Chem. 2022;298:101611. doi: 10.1016/j.jbc.2022.101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thapa R.J., Ingram J.P., Ragan K.B., Nogusa S., Boyd D.F., Benitez A.A., Sridharan H., Kosoff R., Shubina M., Landsteiner V.J., et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nogusa S., Thapa R.J., Dillon C.P., Liedmann S., Oguin T.H., 3rd, Ingram J.P., Rodriguez D.A., Kosoff R., Sharma S., Sturm O., et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe. 2016;20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng M., Karki R., Vogel P., Kanneganti T.D. Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense. Cell. 2020;181:674–687.e13. doi: 10.1016/j.cell.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujikura D., Miyazaki T. Programmed Cell Death in the Pathogenesis of Influenza. Int. J. Mol. Sci. 2018;19:2065. doi: 10.3390/ijms19072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Atkin-Smith G.K., Duan M., Chen W., Poon I.K.H. The induction and consequences of Influenza A virus-induced cell death. Cell Death Dis. 2018;9:1002. doi: 10.1038/s41419-018-1035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184:149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rothan H.A., Kumari P., Stone S., Natekar J.P., Arora K., Auroni T.T., Kumar M. SARS-CoV-2 Infects Primary Neurons from Human ACE2 Expressing Mice and Upregulates Genes Involved in the Inflammatory and Necroptotic Pathways. Pathogens. 2022;11:257. doi: 10.3390/pathogens11020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalil A.C., Mehta A.K., Patterson T.F., Erdmann N., Gomez C.A., Jain M.K., Wolfe C.R., Ruiz-Palacios G.M., Kline S., Regalado Pineda J., et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: A double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021;9:1365–1376. doi: 10.1016/S2213-2600(21)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang N., Zhan Y., Zhu L., Hou Z., Liu F., Song P., Qiu F., Wang X., Zou X., Wan D., et al. Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients. Cell Host Microbe. 2020;28:455–464.e2. doi: 10.1016/j.chom.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herbert A., Poptsova M. Z-RNA and the Flipside of the SARS Nsp13 Helicase: Is There a Role for Flipons in Coronavirus-Induced Pathology? Front. Immunol. 2022;13:912717. doi: 10.3389/fimmu.2022.912717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: Review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Musso D., Gubler D.J. Zika Virus. Clin. Microbiol. Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar M., Belcaid M., Nerurkar V.R. Identification of host genes leading to West Nile virus encephalitis in mice brain using RNA-seq analysis. Sci. Rep. 2016;6:26350. doi: 10.1038/srep26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azouz F., Arora K., Krause K., Nerurkar V.R., Kumar M. Integrated MicroRNA and mRNA Profiling in Zika Virus-Infected Neurons. Viruses. 2019;11:162. doi: 10.3390/v11020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lim S.M., van den Ham H.J., Oduber M., Martina E., Zaaraoui-Boutahar F., Roose J.M., van IJcken W.F., Osterhaus A., Andeweg A.C., Koraka P., et al. Transcriptomic Analyses Reveal Differential Gene Expression of Immune and Cell Death Pathways in the Brains of Mice Infected with West Nile Virus and Chikungunya Virus. Front. Microbiol. 2017;8:1556. doi: 10.3389/fmicb.2017.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daniels B.P., Kofman S.B., Smith J.R., Norris G.T., Snyder A.G., Kolb J.P., Gao X., Locasale J.W., Martinez J., Gale M., Jr., et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity. 2019;50:64–76.e4. doi: 10.1016/j.immuni.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weng D., Marty-Roix R., Ganesan S., Proulx M.K., Vladimer G.I., Kaiser W.J., Mocarski E.S., Pouliot K., Chan F.K., Kelliher M.A., et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. USA. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muendlein H.I., Connolly W.M., Magri Z., Jetton D., Smirnova I., Degterev A., Balachandran S., Poltorak A. ZBP1 promotes inflammatory responses downstream of TLR3/TLR4 via timely delivery of RIPK1 to TRIF. Proc. Natl. Acad. Sci. USA. 2022;119:e2113872119. doi: 10.1073/pnas.2113872119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Belhocine K., Monack D.M. Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells. Cell Microbiol. 2012;14:71–80. doi: 10.1111/j.1462-5822.2011.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pearson J.S., Giogha C., Muhlen S., Nachbur U., Pham C.L., Zhang Y., Hildebrand J.M., Oates C.V., Lung T.W., Ingle D., et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat. Microbiol. 2017;2:16258. doi: 10.1038/nmicrobiol.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tomalka J., Ganesan S., Azodi E., Patel K., Majmudar P., Hall B.A., Fitzgerald K.A., Hise A.G. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pathakumari B., Liang G., Liu W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020;130:110550. doi: 10.1016/j.biopha.2020.110550. [DOI] [PubMed] [Google Scholar]
- 142.Lourido S. Toxoplasma gondii. Trends Parasitol. 2019;35:944–945. doi: 10.1016/j.pt.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 143.Pittman K.J., Aliota M.T., Knoll L.J. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genom. 2014;15:806. doi: 10.1186/1471-2164-15-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garfoot A.L., Cervantes P.W., Knoll L.J. Transcriptional Analysis Shows a Robust Host Response to Toxoplasma gondii during Early and Late Chronic Infection in Both Male and Female Mice. Infect. Immun. 2019;87:e00024-19. doi: 10.1128/IAI.00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pittman K.J., Cervantes P.W., Knoll L.J. Z-DNA Binding Protein Mediates Host Control of Toxoplasma gondii Infection. Infect. Immun. 2016;84:3063–3070. doi: 10.1128/IAI.00511-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cervantes P.W., Martorelli Di Genova B., Erazo Flores B.J., Knoll L.J. RIPK3 Facilitates Host Resistance to Oral Toxoplasma gondii Infection. Infect. Immun. 2021;89:e00021-21. doi: 10.1128/IAI.00021-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Z.J., Yu S.M., Gao J.M., Zhang P., Hide G., Yamamoto M., Lai D.H., Lun Z.R. High resistance to Toxoplasma gondii infection in inducible nitric oxide synthase knockout rats. Iscience. 2021;24:103280. doi: 10.1016/j.isci.2021.103280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dillon C.P., Weinlich R., Rodriguez D.A., Cripps J.G., Quarato G., Gurung P., Verbist K.C., Brewer T.L., Llambi F., Gong Y.N., et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin J., Kumari S., Kim C., Van T.M., Wachsmuth L., Polykratis A., Pasparakis M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Newton K., Wickliffe K.E., Maltzman A., Dugger D.L., Strasser A., Pham V.C., Lill J.R., Roose-Girma M., Warming S., Solon M., et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature. 2016;540:129–133. doi: 10.1038/nature20559. [DOI] [PubMed] [Google Scholar]
- 151.Rickard J.A., O’Donnell J.A., Evans J.M., Lalaoui N., Poh A.R., Rogers T., Vince J.E., Lawlor K.E., Ninnis R.L., Anderton H., et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 152.Dannappel M., Vlantis K., Kumari S., Polykratis A., Kim C., Wachsmuth L., Eftychi C., Lin J., Corona T., Hermance N., et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moriwaki K., Park C., Koyama K., Balaji S., Kita K., Yagi R., Komazawa-Sakon S., Semba M., Asuka T., Nakano H., et al. The scaffold-dependent function of RIPK1 in dendritic cells promotes injury-induced colitis. Mucosal Immunol. 2022;15:84–95. doi: 10.1038/s41385-021-00446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Devos M., Tanghe G., Gilbert B., Dierick E., Verheirstraeten M., Nemegeer J., de Reuver R., Lefebvre S., De Munck J., Rehwinkel J., et al. Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J. Exp. Med. 2020;217:e20191913. doi: 10.1084/jem.20191913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang R., Li H., Wu J., Cai Z.Y., Li B., Ni H., Qiu X., Chen H., Liu W., Yang Z.H., et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature. 2020;580:386–390. doi: 10.1038/s41586-020-2127-x. [DOI] [PubMed] [Google Scholar]
- 156.Liddicoat B.J., Chalk A.M., Walkley C.R. ADAR1, inosine and the immune sensing system: Distinguishing self from non-self. Wiley Interdiscip. Rev. RNA. 2016;7:157–172. doi: 10.1002/wrna.1322. [DOI] [PubMed] [Google Scholar]
- 157.Samuel C.E. Adenosine deaminase acting on RNA (ADAR1), a suppressor of double-stranded RNA-triggered innate immune responses. J. Biol. Chem. 2019;294:1710–1720. doi: 10.1074/jbc.TM118.004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chung H., Calis J.J.A., Wu X., Sun T., Yu Y., Sarbanes S.L., Dao Thi V.L., Shilvock A.R., Hoffmann H.H., Rosenberg B.R., et al. Human ADAR1 Prevents Endogenous RNA from Triggering Translational Shutdown. Cell. 2018;172:811–824. doi: 10.1016/j.cell.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen R., Ishak C.A., De Carvalho D.D. Endogenous Retroelements and the Viral Mimicry Response in Cancer Therapy and Cellular Homeostasis. Cancer Discov. 2021;11:2707–2725. doi: 10.1158/2159-8290.CD-21-0506. [DOI] [PubMed] [Google Scholar]
- 160.Jiao H., Wachsmuth L., Wolf S., Lohmann J., Nagata M., Kaya G.G., Oikonomou N., Kondylis V., Rogg M., Diebold M., et al. ADAR1 averts fatal type I interferon induction by ZBP1. Nature. 2022;607:776–783. doi: 10.1038/s41586-022-04878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang T., Yin C., Fedorov A., Qiao L., Bao H., Beknazarov N., Wang S., Gautam A., Williams R.M., Crawford J.C., et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature. 2022;606:594–602. doi: 10.1038/s41586-022-04753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Reuver R., Verdonck S., Dierick E., Nemegeer J., Hessmann E., Ahmad S., Jans M., Blancke G., Van Nieuwerburgh F., Botzki A., et al. ADAR1 prevents autoinflammation by suppressing spontaneous ZBP1 activation. Nature. 2022;607:784–789. doi: 10.1038/s41586-022-04974-w. [DOI] [PubMed] [Google Scholar]
- 163.Hubbard N.W., Ames J.M., Maurano M., Chu L.H., Somfleth K.Y., Gokhale N.S., Werner M., Snyder J.M., Lichauco K., Savan R., et al. ADAR1 mutation causes ZBP1-dependent immunopathology. Nature. 2022;607:769–775. doi: 10.1038/s41586-022-04896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen D., Tong J., Yang L., Wei L., Stolz D.B., Yu J., Zhang J., Zhang L. PUMA amplifies necroptosis signaling by activating cytosolic DNA sensors. Proc. Natl. Acad. Sci. USA. 2018;115:3930–3935. doi: 10.1073/pnas.1717190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaiser W.J., Upton J.W., Long A.B., Livingston-Rosanoff D., Daley-Bauer L.P., Hakem R., Caspary T., Mocarski E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oberst A., Dillon C.P., Weinlich R., McCormick L.L., Fitzgerald P., Pop C., Hakem R., Salvesen G.S., Green D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schwarzer R., Jiao H., Wachsmuth L., Tresch A., Pasparakis M. FADD and Caspase-8 Regulate Gut Homeostasis and Inflammation by Controlling MLKL- and GSDMD-Mediated Death of Intestinal Epithelial Cells. Immunity. 2020;52:978–993.e6. doi: 10.1016/j.immuni.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 168.Saada J., McAuley R.J., Marcatti M., Tang T.Z., Motamedi M., Szczesny B. Oxidative stress induces Z-DNA-binding protein 1-dependent activation of microglia via mtDNA released from retinal pigment epithelial cells. J. Biol. Chem. 2022;298:101523. doi: 10.1016/j.jbc.2021.101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Szczesny B., Marcatti M., Ahmad A., Montalbano M., Brunyanszki A., Bibli S.I., Papapetropoulos A., Szabo C. Mitochondrial DNA damage and subsequent activation of Z-DNA binding protein 1 links oxidative stress to inflammation in epithelial cells. Sci. Rep. 2018;8:914. doi: 10.1038/s41598-018-19216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun B., Chen Z., Chi Q., Zhang Y., Gao B. Endogenous tRNA-derived small RNA (tRF3-Thr-AGT) inhibits ZBP1/NLRP3 pathway-mediated cell pyroptosis to attenuate acute pancreatitis (AP) J. Cell. Mol. Med. 2021;25:10441–10453. doi: 10.1111/jcmm.16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang H., Zhang H., Chen Z., Wang Y., Gao B. Effects of tRNA-derived fragments and microRNAs regulatory network on pancreatic acinar intracellular trypsinogen activation. Bioengineered. 2022;13:3207–3220. doi: 10.1080/21655979.2021.2018880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jiao D., Cai Z., Choksi S., Ma D., Choe M., Kwon H.J., Baik J.Y., Rowan B.G., Liu C., Liu Z.G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018;28:868–870. doi: 10.1038/s41422-018-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patel S., Webster J.D., Varfolomeev E., Kwon Y.C., Cheng J.H., Zhang J., Dugger D.L., Wickliffe K.E., Maltzman A., Sujatha-Bhaskar S., et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 2020;27:161–175. doi: 10.1038/s41418-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baik J.Y., Liu Z., Jiao D., Kwon H.J., Yan J., Kadigamuwa C., Choe M., Lake R., Kruhlak M., Tandon M., et al. ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat. Commun. 2021;12:2666. doi: 10.1038/s41467-021-23004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang Y., Wu M., Cao D., Yang C., Jin J., Wu L., Hong X., Li W., Lu L., Li J., et al. ZBP1-MLKL necroptotic signaling potentiates radiation-induced antitumor immunity via intratumoral STING pathway activation. Sci. Adv. 2021;7:eabf6290. doi: 10.1126/sciadv.abf6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang Z., Wang Y., Zhang Y., He X., Zhong C.Q., Ni H., Chen X., Liang Y., Wu J., Zhao S., et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 2018;20:186–197. doi: 10.1038/s41556-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 177.Dondelinger Y., Declercq W., Montessuit S., Roelandt R., Goncalves A., Bruggeman I., Hulpiau P., Weber K., Sehon C.A., Marquis R.W., et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 178.Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.F., Wang F.S., Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 179.Tan X., Thapa N., Choi S., Anderson R.A. Emerging roles of PtdIns(4,5)P2--beyond the plasma membrane. J. Cell Sci. 2015;128:4047–4056. doi: 10.1242/jcs.175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Naguib A. Following the trail of lipids: Signals initiated by PI3K function at multiple cellular membranes. Sci Signal. 2016;9:re4. doi: 10.1126/scisignal.aad7885. [DOI] [PubMed] [Google Scholar]
- 181.Zhang X., Wu J., Liu Q., Li X., Li S., Chen J., Hong Z., Wu X., Zhao Y., Ren J. mtDNA-STING pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion. Cell Death Dis. 2020;11:1050. doi: 10.1038/s41419-020-03239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Baker A.R., Slack F.J. ADAR1 and its implications in cancer development and treatment. Trends Genet. 2022;38:821–830. doi: 10.1016/j.tig.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lai L., Vodros D., Kozlowski P.A., Montefiori D.C., Wilson R.L., Akerstrom V.L., Chennareddi L., Yu T., Kannanganat S., Ofielu L., et al. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Halwani R., Boyer J.D., Yassine-Diab B., Haddad E.K., Robinson T.M., Kumar S., Parkinson R., Wu L., Sidhu M.K., Phillipson-Weiner R., et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J. Immunol. 2008;180:7969–7979. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- 185.Tse S.W., McKinney K., Walker W., Nguyen M., Iacovelli J., Small C., Hopson K., Zaks T., Huang E. mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol. Ther. 2021;29:2227–2238. doi: 10.1016/j.ymthe.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takeshita F., Tanaka T., Matsuda T., Tozuka M., Kobiyama K., Saha S., Matsui K., Ishii K.J., Coban C., Akira S., et al. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J. Virol. 2006;80:6218–6224. doi: 10.1128/JVI.00121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lladser A., Mougiakakos D., Tufvesson H., Ligtenberg M.A., Quest A.F., Kiessling R., Ljungberg K. DAI (DLM-1/ZBP1) as a genetic adjuvant for DNA vaccines that promotes effective antitumor CTL immunity. Mol. Ther. 2011;19:594–601. doi: 10.1038/mt.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bommareddy P.K., Shettigar M., Kaufman H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018;18:498–513. doi: 10.1038/s41577-018-0014-6. [DOI] [PubMed] [Google Scholar]
- 189.Hirvinen M., Capasso C., Guse K., Garofalo M., Vitale A., Ahonen M., Kuryk L., Vaha-Koskela M., Hemminki A., Fortino V., et al. Expression of DAI by an oncolytic vaccinia virus boosts the immunogenicity of the virus and enhances antitumor immunity. Mol. Ther. Oncolytics. 2016;3:16002. doi: 10.1038/mto.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Balachandran S., Mocarski E.S. Viral Z-RNA triggers ZBP1-dependent cell death. Curr. Opin. Virol. 2021;51:134–140. doi: 10.1016/j.coviro.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]